CHAPTER 10 Obturation of the Cleaned and Shaped Root Canal System
Importance of Effectively Sealing the Root Canal System
Success in endodontic treatment was originally based on the triad of débridement, thorough disinfection, and obturation with all aspects equally important. At present, successful root canal treatment is based on broader principles. These include diagnosis and treatment planning; knowledge of anatomy and morphology; the traditional concepts of débridement, thorough disinfection, and obturation; and the coronal restoration. A meta-analysis of factors influencing the efficacy of primary root canal treatment found that the following four factors influenced success: the absence of a pretreatment periapical lesion, root canal fillings with no voids, obturation to within 2.0 mm of the apex, and an adequate coronal restoration.214
In an early radiographic study of success and failure, Ingle and colleagues145 indicated that 58% of treatment failures were due to incomplete obturation. Unfortunately, teeth that are poorly obturated are often poorly prepared. Procedural errors such as loss of length, canal transportation, perforations, loss of coronal seal, and vertical root fracture may have occurred and have been shown to adversely affect the apical seal.341
Since the classic study by Ingle and colleagues, great emphasis has been placed on developing materials and techniques for obturating the radicular space. Various experimental methods have been used to assess microleakage after obturation, including radioisotopes,83 dyes,151 bacteria,56 proteins,202 endotoxins,56 glucose penetration,225 and computerized fluid filtration.309 These methodologies have employed a variety of in vitro conditions and experimental periods that often produce conflicting results. Fortunately, clinical success rates after endodontic treatment are high despite the varied conditions, materials, and techniques employed.62,179,256 Circumstantial evidence indicates that the cleaning and shaping procedures provide an aseptic environment, and with this elimination of the etiology for pathosis the method of obturation becomes less critical.
A primate study of infected teeth with apical periodontitis demonstrated nonhealing in 28% of the teeth with no bacteria after cleaning and shaping whereas the presence of bacteria after cleaning and shaping resulted in 79% being classified as not healed.98 When no bacteria were present, healing occurred regardless of the quality of the obturation. When bacteria were present at the time of obturation, there was a correlation between the quality of obturation and nonhealing. Results emphasized the role of bacteria in apical pathosis and the importance of cleaning and shaping procedures.
In a controlled animal study,253 periapical lesions were created by removing the pulp and leaving the teeth open to the oral cavity. In the control group the canals were cleaned and shaped before obturation with gutta-percha and a resin sealer. The teeth of the experimental group were cleaned and shaped as in the control group but left unobturated. At 190 days the animals were killed and histological evaluations were performed. There was no difference in the healing between the instrumented and obturated teeth and the instrumented and unobturated teeth, emphasizing the importance of cleaning and shaping in eliminating bacteria. Although obturation may not influence the short-term success rates, results may be different in long-term studies if coronal leakage were to occur.
At present there is no effective method for determining whether cleaning and shaping procedures have been effective. The criteria of clean dentinal filings and/or enlargement beyond the first file to bind at working length proved to be unreliable.326 Although the length of preparation has been emphasized, the irregular canal diameter (the forgotten dimension) may be a more significant factor in success and failure.146 Evidence indicates canals are often underprepared in the apical one third.55 Historically, culturing has been employed and obturation delayed until a “negative” culture was obtained. In contemporary endodontic treatment culturing has been abandoned during routine care.209 With vital pulp tissue, bacteria are not a major concern. In necrotic cases the organisms involved in the disease process are primarily facultative or obligate anaerobes that may not grow in culture. Molecular microbiologic techniques (polymerase chain reaction) have demonstrated that a variety of organisms are present that do not grow in culture.288,289 The role these organisms play in the disease process is not well understood. The reader is referred to Chapter 15 for a fuller discussion of this vexing issue.
The process of cleaning and shaping determines both the degree of disinfection and the ability to obturate the radicular space. Obturation is therefore a reflection of the cleaning and shaping and is evaluated on the basis of length, taper, density, level of gutta-percha removal, and the coronal seal (adequate provisional restoration) (Fig. 10-1). It is not possible to assess the quality of the seal established during obturation with a radiograph, and it is important to remember that no material or technique prevents leakage.2,122 Indeed, obtaining an impervious seal may not be feasible because of the porous tubular structure of dentin2 and canal irregularities.
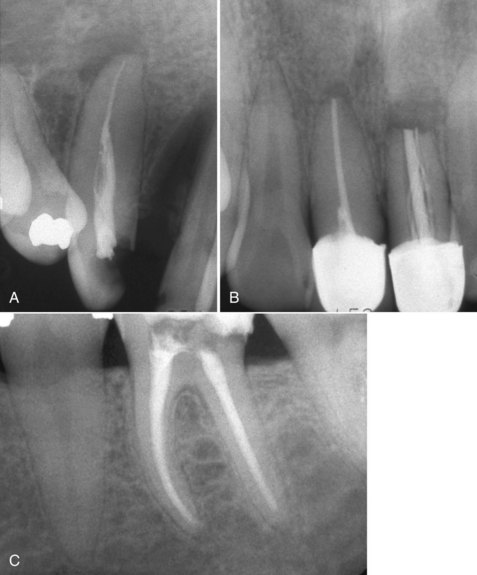
FIG. 10-1 Examples of inadequate obturation. A, Maxillary right canine with adequate length but lacking density and no coronal seal. Central incisor is filled to adequate length but obturation exhibits voids. B, Maxillary central incisors. Maxillary right central incisor exhibits a lack of density and taper. Maxillary left central incisor has voids and unfilled canal space. C, Mandibular left first molar with adequate obturation; provisional restoration shows poor adaptation on the distal because of the failure to remove caries.
The primary etiology of pulpal and periradicular pathosis is, as discussed in Chapter 15, bacterial.155,205 Pulpal remnants, necrotic tissue, bacteria, and bacterial by-products remaining in the inaccessible areas of a cleaned and shaped canal system could initiate and/or perpetuate a lesion because the host defense mechanisms are unable to remove them. Studies indicate that root canal systems cannot be completely cleaned and disinfected.132,287,326,344 Obturation of the radicular space is necessary to eliminate leakage. Obturation reduces coronal leakage and bacterial contamination, seals the apex from the periapical tissue fluids, and entombs the remaining irritants in the canal.81
Coronal leakage has also been demonstrated to contribute to treatment failure.244,315 Maintaining an effective coronal seal and placing an appropriate restoration, as discussed in Chapter 22 should be considered an essential component of successful endodontic treatment.134 Investigators suggest that it is more prudent to use a final restorative material versus a temporary material to prevent leakage.319 One study244 reported that good postendodontic restorations resulted in significantly more successful cases when compared with good endodontics (80% vs. 75.7%) and poor restorations resulted in significantly more periradicular inflammation cases when compared with poor endodontics (30.2% vs. 48.6%). The success rate for a good restoration and good endodontics was 91% compared with a success rate of 18% with the poor endodontic treatment and a poor restoration. In combination with technically good restorations the success rate was 81%. With poor restorations the success rate was 71%. Technically poor endodontics combined with either good restorations or poor restorations had significantly lower success rates of 56% and 57%, respectively. The radiographic quality of the endodontic treatment was significantly more important than the technical quality of the coronal restoration when the periapical status of endodontically treated teeth was evaluated. Investigators12 noted that the prognosis for endodontically treated posterior teeth restored with crowns was enhanced sixfold. Thus, the ability to deliver combined high-quality endodontic and restorative treatment is a major factor in good clinical outcomes.
Using histologic and microbiologic techniques, investigators evaluated 39 teeth that were without proper restorations for at least 3 months and exposed to caries and the oral environment.246 Thirty-four specimens were without radiographically discernible periradicular pathosis. Lesions were detected on five roots. Stainable bacteria were found in abundance at the orifice and in dentinal tubules but were absent midroot and apically in 37 roots. Inflammatory cell infiltrates were absent or sparse in 32 teeth whereas 7 teeth exhibited distinct inflammation. Despite pathosis involving five roots the results indicated that well-obturated root canals are resistant to bacterial penetration when exposed to the oral environment. Findings in this study were consistent with an observational study finding that coronal leakage was not a significant factor in root canal failure, using matched pairs of teeth for analysis.247
Three-dimensional obturation of the radicular space is essential to long-term success. The canal system should be sealed apically, coronally, and laterally. Various methods have been advocated for obturation. Unfortunately, all materials and techniques result in some degree of leakage.346 Although a poorly obturated canal and leakage are correlated, radiographic evaluation of obturation does not correlate well with leakage.124,164 An adequate radiographic appearance of the obturation may not correlate with an adequate seal (Fig. 10-2).90 Variation in radiographic interpretation by the clinician, the overlying osseous structures, and the lack of uniformity in the obturation materials are significant variables.29,86,87,164
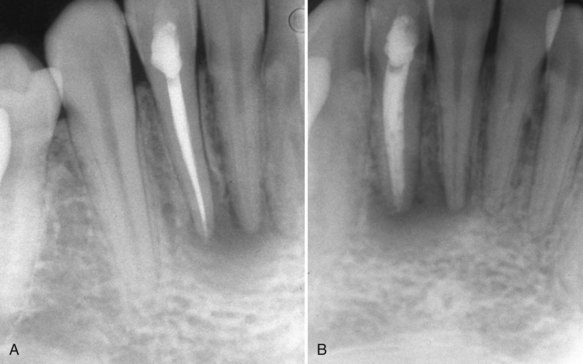
FIG. 10-2 A, Posttreatment radiograph of a mandibular left lateral incisor with ostensibly adequate obturation. B, Angled view reveals voids.
In a prospective study the Toronto group100 evaluated success and failure of endodontic treatment at 4 to 6 years after completion of treatment. Teeth were treated by using flared preparation and vertical compaction of warm gutta-percha or step-back preparation and lateral compaction. Differences were noted with the adequacy of the fill and the treatment technique. Adequate length had a higher success rate (87%) when compared with inadequate length (77%). The flared preparation and vertical compaction had a higher success rate (90%) when compared with step-back preparation and lateral compaction (80%). This study also agreed with previous studies291,302 indicating preexisting apical pathosis as a factor reducing a favorable prognosis and highlighted the obturation technique as a factor influencing success and failure.291
Historical Perspectives
The achievement of a “hermetic seal” is often cited as a major goal of root canal treatment. According to accepted dictionary definitions, the word hermetic means sealed against the escape or entry of air—or made airtight by fusion or sealing. However, root canal seals are commonly evaluated for fluid leakage—a parameter used to praise or condemn obturation materials and techniques. This occurs both apically and coronally. Somehow the term hermetic has crept into endodontic nomenclature in a manner probably quite similar to the invention of an airtight seal. A god of wisdom, learning, and magic in ancient Egypt, Thoth, better known as Hermes Trismegistus (Hermes thrice greatest), is credited with this invention.270 His significant contribution to civilization allowed the preservation of oils, spices, aromatics, grains, and other necessities in previously porous, earthenware vessels. A simple wax seal of the vessel walls helped to create the “hermetic seal.” Endodontically speaking, the term hermetic is inappropriate; instead, terms such as fluid-tight, fluid-impervious, or bacteria-tight seals are more contemporary.
In 1924 Hatton129 indicated, “Perhaps there is no technical operation in dentistry or surgery where so much depends on the conscientious adherence to high ideals as that of pulp canal filling.” The essence of this statement had been significantly influenced by years of trial and error in both the techniques and materials used to obturate the prepared root canal system. Much of the frustration and challenge that emanated from this concern, however, was due to the lack of development in root canal preparation techniques coupled with indictments of the “focal infection” craze of that era.144
Before 1800, root canal filling, when done, was limited to gold. Subsequent obturations with various metals, oxychloride of zinc, paraffin, and amalgam resulted in various degrees of success and satisfaction.166 In 1847 Hill developed the first gutta-percha root canal filling material known as “Hill’s stopping.”166 The preparation, which consisted principally of bleached gutta-percha and carbonate of lime and quartz, was patented in 1848 and introduced to the dental profession. In 1867 Bowman made claim (before the St. Louis Dental Society) of the first use of gutta-percha for canal filling in an extracted first molar.10
References to the use of gutta-percha for root canal obturation before the turn of the twentieth century were few and vague. In 1883 Perry claimed that he had been using a pointed gold wire wrapped with some soft gutta-percha (the origin of the present-day core carrier technique?).234 He also began using gutta-percha rolled into points and packed into the canal. The points were prepared by cutting base plate gutta-percha into slender strips, warming them with a lamp, laying them on his operating case, and rolling them with another flat surface (a contemporary technique still used by a few to custom roll a large cone?). Perry then used shellac warmed over a lamp and rolled the cones into a point of desired size, based on canal shape and length. Before placing the final gutta-percha point, he saturated the tooth cavity with alcohol; capillary attraction let the alcohol run into the canal, softening the shellac so that the gutta-percha could be packed (the forerunner of a chemical-softening technique?).
In 1887 the S.S. White Company began to manufacture gutta-percha points.159 In 1893 Rollins introduced a new type of gutta-percha to which he added vermilion.328 Because vermilion is pure oxide of mercury and therefore dangerous in quantity, many people justifiably criticized this technique.
With the introduction of radiographs for the assessment of root canal obturations, it became painfully obvious that the canal was not cylindrical, as earlier imagined, and that additional filling material was necessary to fill the observed voids. At first, hard-setting dental cements were used, but these proved unsatisfactory. It was also thought that the cement used should possess strong antiseptic action, hence the development of many phenolic or formalin-type paste cements. The softening and dissolution of the gutta-percha to serve as the cementing agent, through the use of rosins, was introduced by Callahan in 1914.52 Subsequently a multitude of various pastes, sealers, and cements were created in an attempt to discover the best possible sealing agent for use with gutta-percha.
Over the past 70 to 80 years the dental community has seen attempts to improve on the nature of root canal obturation with these cements and with variations in the delivery of gutta-percha to the prepared canal system. During this era the impetus for these developments was based heavily on the continued belief in the concept of focal infection, elective localization, the hollow-tube theory, and the concept that the primary cause for failure of root canal treatment was the apical percolation of fluids, and microorganisms, into a poorly obturated root canal system.83,252 From this chronological perspective of technical and scientific thought this chapter clarifies and codifies contemporary concepts in the obturation of the cleaned and shaped root canal system.
Timing of Obturation
Factors influencing the appropriate time to obturate a tooth include the patient’s signs and symptoms, status of the pulp and periradicular tissue, the degree of difficulty, and patient management.
Vital Pulp Tissue
At present the consensus is that one-step treatment procedures are acceptable when the patient exhibits a completely or partially vital pulp.318 Removal of the normal or inflamed pulp tissue and performance of the procedure under aseptic conditions should result in a successful outcome because of the relative absence of bacterial contamination. Obturation at the initial visit also precludes contamination as a result of leakage during the period between patient visits.
Elective root canal treatment for restorative reasons can be completed in one visit provided the pulp is vital, to some degree, and time permits. Obturation of patients whose condition is urgent depends on the pretreatment diagnosis. When pain occurs as the result of irreversible pulpitis, obturation can occur at the initial visit because removal of the vital tissue will generally resolve the patient’s pain.
Necrotic Pulp Tissue
Patients who present with pulp necrosis with or without asymptomatic periapical pathosis (chronic apical periodontitis, chronic apical abscess, condensing osteitis) may be treated in one visit, based on the best available information. When patients present with acute symptoms caused by pulp necrosis and acute periradicular abscess, obturation is generally delayed until the patient is asymptomatic. However, more than 20 years ago, investigators demonstrated that cases with soft-tissue swelling could be completed in one visit with appropriate endodontic treatment, incision for drainage, and a regimen of antibiotics.296 Management of these patients, however, may be more difficult should problems persist or become worse after the completion of treatment.
During the 1970s there was concern about the timing of obturation. Performing endodontic treatment in one visit was controversial. Conventional wisdom suggested that patients would have a higher incidence of posttreatment pain; however, studies demonstrated that the incidence of pain was not increased in patients who were treated in one appointment versus those treated in multiple appointments.107,210,217,220,249,294
In contrast to teeth with vital pulp tissue, teeth exhibiting pulp necrosis frequently exhibit bacterial contamination and may require a different approach to treatment.318 Sjögren and colleagues290 raised questions regarding the long-term prognosis of teeth exhibiting necrotic pulp tissue and apical periodontitis treated in a single visit. In their clinical study the authors thoroughly instrumented 55 infected teeth with apical pathosis, using only 0.5% sodium hypochlorite (NaOCl) [Editor’s note: Today, stronger concentrations of NaOCl are more commonly used. The reader is referred to Chapters 9 and 15 for a fuller discussion of this issue.] Before obturation, cultures were obtained, using advanced anaerobic bacteriologic techniques. After cleaning and shaping, bacteria could be detected in 22 teeth. Complete healing occurred in 94% of cases that yielded a negative culture, whereas the rate of successful treatment of teeth with positive cultures before obturation was 68%, a statistically significant difference.
However, other investigators were unable to confirm Sjögren’s results, in a study of 39 patients with periapical lesions exhibiting both positive and negative canal cultures at the time of obturation. Twenty-one teeth were treated in one visit and 18 in two visits with an interappointment dressing of calcium hydroxide. Periapical healing was observed over a period of up to 4.5 years. Complete radiographic healing occurred in 81% of the cases in the one-visit group and in 71% of the cases in the two-visit group.236 A randomized control trial comparing the difference in success between one- and two-visit procedures, using a standardized protocol, demonstrated no significant differences in success at 12 months in 63 patients.230 A 2-year clinical and radiographic study demonstrated no statistical difference in healing between one- and two-visit treatments; however, negative cultures at obturation produced 80% success compared with 44% when cultures were positive. In addition, 52% of teeth with positive cultures were classified as uncertain with regard to healing compared with 7% of the negative culture group.206
In a prospective clinical study, investigators evaluated the effect of calcium hydroxide as an interappointment dressing on the periapical healing of lesions associated with necrotic pulps in 73 patients. Thirty-six teeth were endodontically treated in one visit. Thirty-one teeth were treated in two visits with calcium hydroxide as an intracanal medicament. Periapical healing increased with the length of the observation period. In both treatment groups the success rate exceeded 90%, and no statistically significant difference existed between the two groups.327
Controlled laboratory studies support the use of calcium hydroxide as an antimicrobial agent before obturation of teeth with pulp necrosis. Two studies156,157 evaluated periapical healing of infected root canals in dogs. After inducing periapical pathosis the teeth were treated by immediate obturation or with calcium hydroxide for 1 week before obturation. Results of the radiographic156 examination at 6 months indicated complete healing was similar for the one-visit (35.3%) and calcium hydroxide (36.8%) groups. The calcium hydroxide group, however, had fewer failed cases (15.8% vs. 41.2%) and more improved cases (47.4% vs. 23.5%) when compared with the one-visit group.157 In histologic evaluations the calcium hydroxide group had significantly less inflammation than the one-visit group. One study also evaluated periapical healing of infected teeth in dogs after immediate obturation or with prior calcium hydroxide treatment for 7 or 14 days. Results indicated both calcium hydroxide groups were superior to the one-visit group, with the 14-day calcium hydroxide group being superior to the 7-day group.139
Other investigators overinstrumented and overextended teeth 45 minutes after extraction. After the procedures, teeth exhibiting vital pulps and no periradicular disease were without bacteria. Teeth exhibiting periradicular pathosis before extraction demonstrated a high percentage of bacteria at the root apices. The organisms were found to remain firmly attached to resorptive lacunae, indicating the instrumentation/obturation procedures were unable to eliminate the organisms.121
In general, obturation can be performed after cleaning and shaping procedures when the canal can be dried and the patient is not experiencing swelling. An exception is the presence or persistence of exudation from the canal. Obturation of a canal that cannot be dried is contraindicated.
A few studies suggest that complete cleaning and shaping should be accomplished and calcium hydroxide placed as an antimicrobial and temporary obturant in necrotic cases that cannot be treated in one visit290 because investigators noted that bacteria in instrumented, unfilled canals can multiply and reach their pretreatment numbers in 2 to 4 days.50
Procedural concerns also dictate the time of obturation. Difficult cases may require more time for preparation and can be managed more uneventfully in multiple appointments. Patients may require multiple short appointments because of medical conditions, their psychologic state of mind, and fatigue.
Length of Obturation
One of the controversies in endodontics that remains unresolved is the apical limit of root canal treatment and obturation. Early studies identified the dentinocemental junction as the apical limit for obturation.70,117,118,221,292 However, this histologic landmark cannot be determined clinically, and it has been found to be irregular within the canal. The dentinocemental junction may be several millimeters higher on the mesial canal wall when compared with the distal wall. In addition, the dentinocemental junction does not coincide with the narrowest portion of the canal or apical constriction. The reader is referred to Chapter 7 for more information about this anatomy.
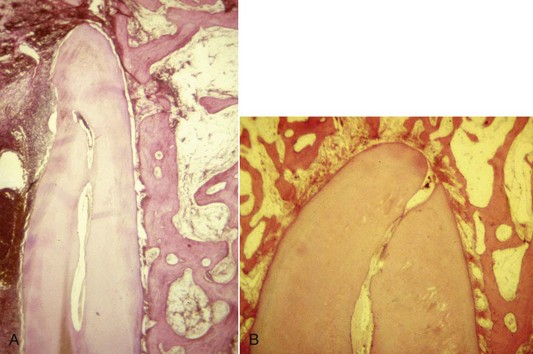
Traditionally, the apical point of termination has been approximately 1 mm from the radiographic apices as determined by radiographs. Kuttler174 noted that the apical anatomy consists of the major diameter of the foramen and the minor diameter of the constriction (Fig. 10-3), with the apical constriction identified as the narrowest portion of the canal. The average distance from the foramen to the constriction was found to be 0.5 mm, with the foramen varying in distance from the apex up to 2.5 mm. Kuttler also noted that the foramen-to-constriction distance increases with age because of cementum deposition. Supporting this finding, other investigators found that the location of the foramen was not at the apex. Deviations occurred in 92% of the roots and averaged 0.6 mm.48 Still another investigator noted in 92% of examined teeth that the apical constriction was between 0.5 and 1 mm from the apex.61 One study noted the average apex-to-constriction distance was 0.9 mm and that 95% of the constrictions were between 0.5 and 1 mm in diameter88; this study also noted that the classic apical anatomy described by Kuttler was present in only 46% of the teeth. Other variations identified were the tapering constriction, the multiconstriction, and the parallel constriction. Other investigators examined 230 roots of permanent teeth stereomicroscopically and with radiographs.30 Results of this study indicated a deviation of the foramen from the apex in 76% of the roots with microscopy and 57% with radiography; the mean distance was 1 mm.
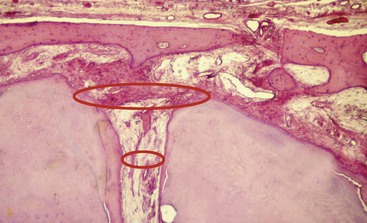
FIG. 10-3 Histologic section of a root apex, demonstrating anatomy of the classic foramen and constriction.
A later study found that no foramina coincided with the long axis of the root, with the distance ranging from 0.2 to 3.8 mm (Fig. 10-4).121 Root resorption is an additional factor in length determination. Resorption is more common with necrosis and apical bone resorption, and this can result in loss of the constriction (Fig. 10-5).154,195 On the basis of these findings it appears that canals filled to the radiographic apex reflect an overextension of the obturating material.
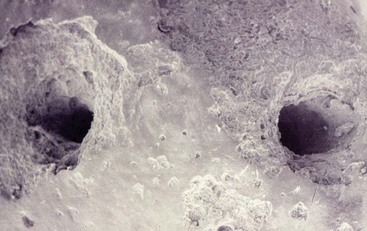
FIG. 10-5 Scanning electron microscopy of a tooth exhibiting a necrotic pulp and apical pathosis and resorption.
A study by the Toronto group99 on the prognosis of retreatment identified perforation, pretreatment periradicular disease, and adequate length of the root canal filling as factors significantly influencing success and failure. The authors speculated that canals filled more than 2 mm short harbored necrotic tissue, bacteria, and irritants that when retreated could be cleaned and sealed. The success rate for negotiating the apical unfilled canal was 74%.
Controversy also exists regarding the role accessory canals play in success and failure (Fig. 10-6). For example, one scanning electron microscopy (SEM) study of the apical anatomy of each tooth group except the third molars noted no pattern for foraminal openings120; the number of accessory canals ranged from 1 to 16. Although lateral canals can be associated with pathosis, one study indicates that accessory canals are common but play little role in periradicular pathosis (Fig. 10-7).17
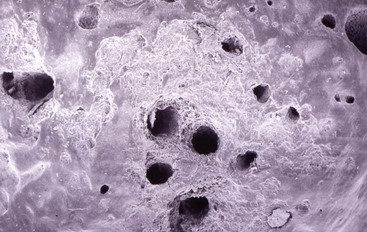
FIG. 10-6 Scanning electron microscopy of the apex of an extracted tooth that was removed because of pulp necrosis. Note the multiple accessory foramina and resorption.
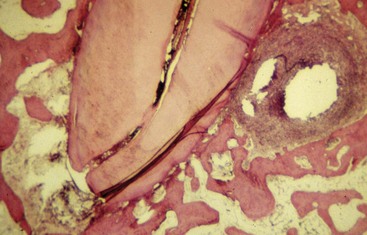
FIG. 10-7 Histologic section of a mesial root of a mandibular molar with a lateral canal present and associated lesion. Will the lesion resolve after the removal of the main canal contents, or will the lesion persist because of necrotic pulpal remnants in the lateral canal? The question remains unanswered.
Further, other studies demonstrated that it is not possible to completely débride the canal space regardless of the technique or irrigant.177,326 Investigators329 noted that these anatomic structures are only demonstrated. Often, smaller apical accessory canals remain unfilled or partially filled.322 Accessory/lateral canals are often obturated by chance and only serendipitously identified on the posttreatment radiograph (Fig. 10-8).
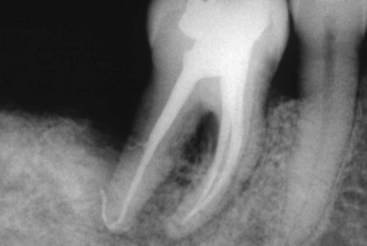
FIG. 10-8 Posttreatment radiograph of a mandibular right first molar with a lateral canal associated with the distal root.
Investigators compared obturation of lateral canals, using six obturation techniques in resin blocks.85 All techniques were able to obturate lateral canals with sealer. Warm vertical compaction, carrier-based thermoplastic gutta-percha, continuous wave compaction, and vertically compacted high-temperature gutta-percha filled lateral canals with gutta-percha significantly better than lateral compaction or warm lateral compaction. The use of sealer enhanced the ability of the gutta-percha to obturate the lateral canals.85
The importance of length control in obturation relates to extrusion of materials. Studies indicate that extrusion decreases the prognosis for complete regeneration.27,105,271,272,291,293,301 One study evaluated the quality of root canal treatment in an American population.47 Periapical disease was evident in 4.1% of all teeth and 31.3% of root-filled teeth, and the study noted that a periapical pathosis was found with 43% of the teeth with overfills. In another study of 1000 cases, investigators found that overfilling resulted in a failure rate of 37%. This was four times greater than for cases filled short.301 A third study found that, in necrotic cases, better success was achieved when the procedures terminated at or within 2 mm of the radiographic apex.348 Obturation shorter than 2 mm from the apex or past the apex resulted in a 20% lower success rate. For vital cases, termination between 2 and 3 mm was acceptable. Other investigators found that teeth obturated less than 2 mm from the apex had a higher success rate when compared with cases obturated more than 2 mm from the apex.229
On the basis of biologic and clinical principles, instrumentation and obturation should not extend beyond the apical foramen. This was demonstrated in one study that histologically evaluated 41 human root-filled teeth from 36 patients.248 In six cases exhibiting overfills, histologic examination revealed severe inflammation.
Whereas the guideline of 1 mm from the radiographic apex remains rational when using radiographs, the point of apical termination of the preparation and obturation remains empiric. The use of an apex locator in conjunction with radiographs and sound clinical judgment makes this decision more logical. The need to compact the gutta-percha and sealer against the apical dentin matrix (constriction of the canal) is necessary to prevent extrusion of materials into the periapical tissues. Deciding where the apical constriction of the canal lies is based on the clinician’s basic knowledge of apical anatomy, tactile sensation, radiographic interpretation, apex locators, apical bleeding, and (if not anesthetized) the patient’s response.
Preparation for Obturation
During the cleaning and shaping process, organic pulpal materials and inorganic dentinal debris accumulate on the canal wall, producing an amorphous irregular smear layer (Fig. 10-9),22,198,227 as shown in a study noting that the smear layer is superficial, with a thickness of 1 to 5 µm,198 and this superficial debris can be packed into the dentinal tubules to various distances.1

FIG. 10-9 Scanning electron microscopy of a prepared canal wall. The tubules are covered with a smear layer of organic and inorganic material.
In cases of necrosis this layer may also be contaminated with bacteria and their by-products. For example, one study found that bacteria can extend 10 to 150 µm into the dentinal tubules of necrotic teeth.273 Another study noted that capillary action and fluid dynamics play a role in packing debris into the tubules3 and yet another investigation noted a mean penetration of 479 µm after a 28-day incubation period.232
The smear layer is not a complete barrier to bacteria but may act as a physical barrier, decreasing bacterial penetration into tubules. This was illustrated by a study demonstrating that removal of the smear layer permitted colonization of the dentinal tubules at a significantly higher rate when compared with leaving the smear layer in place.84
The smear layer may also interfere with adhesion and penetration of sealers into dentinal tubules.335 Evidence indicates that sealer penetration into dentinal tubules does not occur when the smear layer is present.106,226 For example, one study found that removal of the smear layer permitted Roth 811 (Roth International, Ltd., Chicago, IL), Calciobiotic root canal sealer (CRCS; Coltène/Whaledent, Cuyahoga Falls, OH), and Sealapex (SybronEndo, Orange, CA) to penetrate to between 35 and 80 µm, whereas the presence of the smear layer obstructed tubular penetration of all sealers.171 Further, other studies found that smear layer removal increased bond strength and reduced microleakage in teeth obturated with AH-26 (DENTSPLY Maillefer, Ballaigues, Switzerland).91,108 Another investigation found that a combination of smear layer removal, AH-26 as the sealer, and vertical compaction of gutta-percha had a cumulative effect in reducing leakage.310
There does not appear to be a consensus on removing the smear layer before obturation.59,274,282 The advantages and disadvantages of the smear layer remain controversial; however, growing evidence supports removal of the smear layer before obturation.143,282 The organic debris present in the smear layer might constitute a substrate for bacterial growth,227 and it has been suggested that the smear layer prohibits sealer contact with the canal wall and permits leakage.25 Bacterial penetration in the presence of a smear layer in canals obturated with thermoplasticized gutta-percha and sealer has been shown to be significantly higher than with smear layer removal before obturation.227 An additional consideration is the presence of viable bacteria that remain in the dentinal tubules and use the smear layer for sustained growth and activity.40 Removal of the smear layer introduces the possibility of reinfecting the dentinal tubules if leakage occurs.274 However, one study demonstrated that bacteria present before obturation are not viable after obturation.81
The smear layer may also interfere with the action of irrigants used as disinfectants.222 When the smear layer is not removed, it may slowly disintegrate and dissolve around leaking obturation materials, or it may be removed by bacterial by-products such as acids and enzymes.274
The smear layer may interfere with the adhesion and penetration of root canal sealers. It also may prevent gutta-percha penetration during thermoplastic techniques.123 Significant tubular penetration of gutta-percha and sealers has been shown with thermoplasticized obturations123 and with dentin-bonded composite resins.181 Removal of the smear layer also enhances the adhesion of sealers to dentin and tubular penetration.181,219,274,333 Root canal filling materials adapt better to the canal walls after smear layer removal.82,219,333-335
One investigation examined the penetration depth of three different root canal sealers into the dentinal tubules with and without the smear layer. Scanning electron microscopy of extracted single-rooted human teeth obturated by lateral compaction of gutta-percha, using AH Plus (DENTSPLY Maillefer), Apexit (Ivoclar Vivadent, Schaan, Liechtenstein), and Roth 811, demonstrated that the smear layer prohibited the sealers from penetrating dentinal tubules. Smear layer removal allowed the penetration of all sealers to occur to various depths.168 Another study found that removal of the smear layer reduced both coronal and apical leakage regardless of the sealer tested.66
Another study examined the smear layer and the passage of bacteria through and around obturating materials,64 using human maxillary incisors obturated with gutta-percha and AH-26. The teeth were exposed to standardized bacterial suspensions containing Fusobacterium nucleatum, Campylobacter rectus, and Peptostreptococcus micros for a period of 60 days, using a leakage model employing upper and lower chambers. Results indicated that 60% of the samples in which the smear layer was not removed demonstrated bacterial leakage. There was no leakage in specimens from which the smear layer was removed.
One study reported no difference in the apical and middle thirds of canals irrigated with 17% ethylenediaminetetraacetic acid (EDTA), using either the traditional syringe or a newer irrigation device, the Quantec-E irrigation system (SybronEndo).276
An additional method for removing the smear layer involves sonic and ultrasonic instruments. In early studies of ultrasonic instrumentation, investigators noted the technique was effective in removing the smear layer.72 Another investigator also demonstrated smear layer removal with ultrasonication and NaOCl.54 One study compared the cleaning efficacy of short-term sonic and ultrasonic passive irrigation with 5.25% NaOCl after hand instrumentation in the apical 3 to 6 mm of maxillary molar root canals.254 Passive sonic or ultrasonic irrigation for 30 seconds resulted in significantly cleaner canals than hand filing alone, and ultrasonic irrigation produced significantly cleaner canals than irrigation. However, other studies found ultrasonication and NaOCl to be ineffective in removing the smear layer.21,325
A new method for removing the smear layer employs the use of a mixture of a tetracycline isomer, an acid, and a detergent (MTAD) (BioPure; DENTSPLY Tulsa Dental Specialties, Tulsa, OK) as a final rinse to remove the smear layer.312 MTAD removed most of the smear layer; however, some organic components of the smear layer remained on the surface of the root canal walls. The effectiveness of MTAD in completely removing the smear layer was enhanced when low concentrations of NaOCl were used as an intracanal irrigant before the use of MTAD as the final rinse. Further studies demonstrated that MTAD was superior to NaOCl in antimicrobial action.277,278 Another study showed that MTAD was effective in killing Enterococcus faecalis at 200-fold dilution, which was more potent than NaOCl because it ceased being active when diluted 32-fold; EDTA had no antimicrobial activity.313 One investigator found MTAD to be less toxic than eugenol, 3% H2O2, Ca(OH)2 paste, 5.25% NaOCl, chlorhexidine gluconate (Peridex), and EDTA.354 Other investigators found no significant difference in flexural strength and modulus of elasticity in dentin bars exposed to MTAD, indicating no alteration in the physical properties of the dentin, and noted that teeth treated with the MTAD protocol for clinical use (20 min of 1.3% NaOCl/5 min of MTAD) may not need any additional dentin conditioning before the application of bonding agents.193
After the completion of cleaning and shaping procedures, removal of the smear layer is generally accomplished by irrigating the canal with 17% disodium EDTA and 5.25% NaOCl (Fig. 10-10).22 Chelators remove the inorganic components, leaving the organic tissue elements intact. NaOCl is necessary for removal of the remaining organic components. Fifty percent citric acid has also been shown to be an effective method for removing the smear layer,20,131 as has tetracycline.16,131
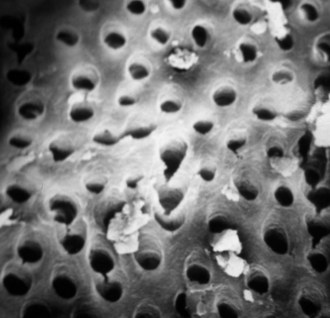
FIG. 10-10 Scanning electron microscopy of the canal wall after removal of the smear layer with 17% EDTA and 5.25% sodium hypochlorite.
Chelating agents were introduced to endodontic treatment by Nygaard-Ostby in 1957 for treatment of calcified narrow root canals.216 EDTA is the chelating solution customarily used in endodontic treatment. It is available in both liquid and paste forms with common concentrations between 15% and 17%.143 A detergent is frequently added to the liquid to decrease surface tension, to increase the cleaning ability, and to enhance the bactericidal action of the solution.323 The effectiveness of EDTA is related to time of application, the pH, and the concentration.208,216
Demineralization results in increased dentin permeability119 because of the removal of the smear layer and plugs and enlargement of the tubules. It appears that the tubular enlargement is due to selective removal of the peritubular dentin.141 The action of chelators and acids appears to be more effective in the coronal and middle thirds of the root and is reduced apically.143,188 This reduced activity may be a reflection of canal size.172 This is a clinical concern because of the more irregular structure of dentin in the apical third. Another investigation demonstrated marked variations in the apical portion of the root,204 including accessory root canals, areas of resorption and repaired resorptions, pulp stones, irregular or absent primary tubules, irregular secondary dentin, and cementum-like tissue lining the apical root canal wall. The variable structure of the apical region of human teeth presents challenges to the use of endodontic obturation techniques requiring adhesives, because this may influence the dentin bonding ability in the apical region.204
EDTA appears to be biocompatible when used clinically216; however, irreversible decalcification of periapical bone and neuroimmunologic disturbances have been noted.269 Extrusion of both NaOCl and EDTA in clinical treatment should be avoided.130,228,306
The recommended time for removal of the smear layer is 1 to 5 minutes.53,143,264 The small particles of the smear layer are primarily inorganic with a high surface-to-mass ratio that facilitates removal by acids and chelators. Investigators have found that a 1-minute exposure to 10 ml of EDTA was adequate to remove the smear layer and that a 10-minute exposure caused excessive removal of both peritubular and intratubular dentin.53
The use of EDTA in combination with NaOCl is recommended300,307 and may enhance the cleaning188,349 and antimicrobial effects of these solutions when compared with using them alone.49
The Ideal Root Canal Filling
Various endodontic materials have been advocated for obturation of the radicular space. Most techniques employ a core material and sealer. Regardless of the core material a sealer is essential to every technique and helps achieve a fluid-tight seal.
The American Association of Endodontists’ Guide to Clinical Endodontics96 outlines contemporary endodontic treatment. Nonsurgical root canal treatment of permanent teeth “involves the use of biologically acceptable chemical and mechanical treatment of the root canal system to promote healing and repair of the periradicular tissues.” The process is accomplished under aseptic conditions with rubber dam isolation. Regarding obturation, the guide states, “Root canal sealers are used in conjunction with a biologically acceptable semi-solid or solid obturating material to establish an adequate seal of the root canal system.” In this area the guidelines indicate that “Paraformaldehyde-containing paste or obturating materials have been shown to be unsafe. Root canal obturation with paraformaldehyde-containing materials is below the standard of care for endodontic treatment” (Fig. 10-11). Chapter 11 gives further information about this issue.
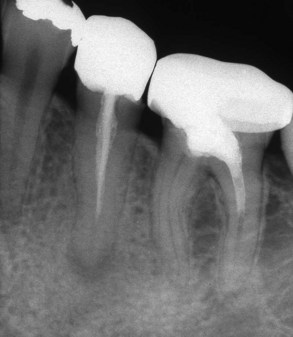
FIG. 10-11 A periapical radiograph of a mandibular left second premolar and first molar, demonstrating Sargenti paste root canal treatment. In addition to the toxic material, the technique often accompanies inadequate cleaning and shaping procedures.
Assessment of nonsurgical treatment is based primarily on the posttreatment radiographic examination. The radiographic criteria for evaluating obturation include the following categories: length, taper, density, gutta-percha and sealer removal to the facial cementoenamel junction in anterior teeth and to the canal orifice in posterior teeth, and an adequate provisional restoration or definitive (Fig. 10-12).
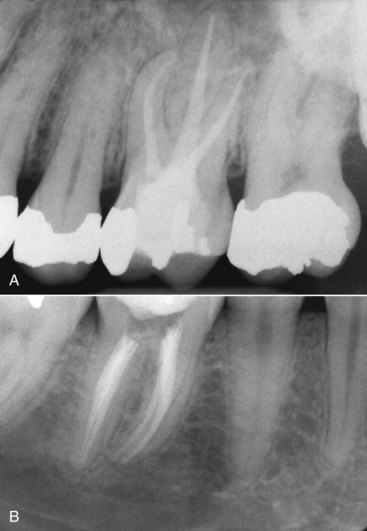
FIG. 10-12 A, Posttreatment radiograph of a maxillary right first molar, demonstrating adequate length, density, and taper. B, Posttreatment radiograph of a mandibular right first molar with an adequate obturation.
Quality assurance is accomplished through a careful evaluation of treatment procedures. Only by this approach can deficiencies be identified and corrected. Although the anatomy and morphology of the radicular space vary tremendously, the obturated root canal should reflect the original canal shape. Procedural errors in preparation, such as loss of length, ledging, apical transportation, apical perforation, stripping perforation, and separated instruments, may not be correctable. Errors in obturation, such as length, voids, inadequate removal of obturation materials, and temporization, may be correctable.
Radiographic interpretation may vary among clinicians because of differences in radiopacity in root canal sealer/cements, constituents in specific brands of gutta-percha, interpretation of voids in vivo versus in vitro,353 the overlying bony anatomy, radiographic angulation, and the limited two-dimensional view of the obturated root canal or canals.
An often overlooked aspect in the assessment of root canal obturation is the density of the apical portion of the fill.122 The apical third of the canal may be filled with a sea of root canal cement and a single master cone or poorly compacted mass of previously softened gutta-percha. Radiographically, the apical third of the canal appears less radiodense. An ill-defined outline to the canal wall is evident, along with obvious gaps or voids in the filling material or its adaptation to the confines of the canal. Because of the use of highly radiopaque root canal sealers/cements, the apical portion may be filled only with sealer, giving the clinician the false impression of a dense, three-dimensional obturation with gutta-percha.
Root canal sealers vary in radiopacity.243,305 Some contain silver particles or significant amounts of barium sulfate to enhance their radiopacity. Although these components may enhance visualization of anatomic structures such as lateral canals, it is important to realize they do not increase the sealing ability of the sealer and the quality of the obturation. They may also give the impression that a canal is well obturated when voids are masked by the density of the sealer. It is erroneous to claim that obturations with highly radiopaque sealers are better than those made with less radiopaque materials. This type of comparison and claim to superiority are both unfounded and unwarranted. The radiographic appearance or aesthetic appearance of the obturated canal system should be secondary to meticulous cleaning and shaping. Although assessment of the root canal obturation is based on radiographic findings, root canal sealers do not have to be highly radiopaque to be effective.
Types of Sealers
Root canal sealers are necessary to seal the space between the dentinal wall and the obturating core interface. Sealers also fill voids and irregularities in the root canal, lateral and accessory canals, and spaces between gutta-percha points used in lateral condensation. Sealers also serve as lubricants during the obturation process. Grossman115 outlined the properties of an ideal sealer (Box 10-1). At present no sealer satisfies all the criteria.
Sealers should be biocompatible and well tolerated by the periradicular tissues.297 All sealers exhibit toxicity when freshly mixed; however, their toxicity is greatly reduced on setting.176 Sealers are resorbable when exposed to tissues and tissue fluids.13 Tissue healing and repair generally appear unaffected by most sealers, provided there are no adverse breakdown products of the sealer over time.35,41-43,45 Breakdown products from the sealers may have an adverse effect on the proliferative capability of periradicular cell populations.113 As a result, sealers should not be placed routinely in the periradicular tissues as part of an obturation technique.176 Although an osteogenic response has been observed,138,295,303,316 the ability of these sealers to sustain a high pH over time has been questioned.170
The most popular sealers are zinc oxide–eugenol formulations, calcium hydroxide sealers, glass ionomers, and resins. Regardless of the sealer selected, all exhibit toxicity until they have set. For this reason, extrusion of sealers into the periradicular tissues should be avoided (Fig. 10-13).
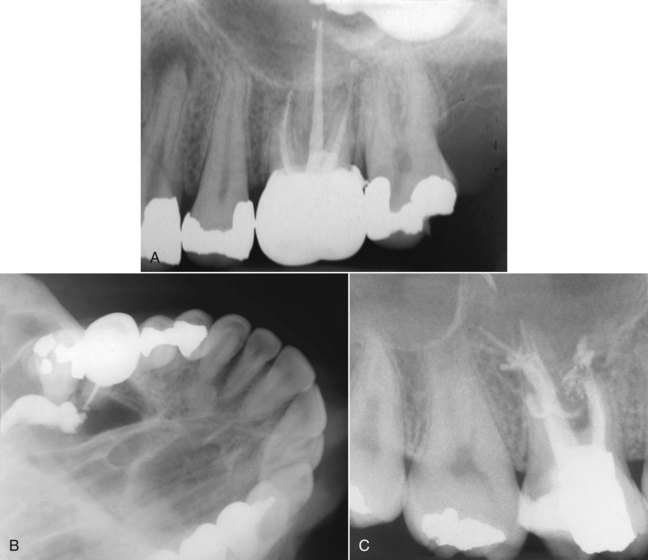
FIG. 10-13 A, Extrusion of sealer evident on the posttreatment radiograph of a maxillary first molar. The separated lentulo spiral in the mesiobuccal root indicates a possible method of sealer placement. B, Maxillary occlusal film demonstrates that the sealer is located in the maxillary sinus. Correction by nonsurgical techniques is not possible. C, Maxillary right first molar with extrusion of the sealer and gutta-percha.
Zinc Oxide and Eugenol
Zinc oxide–eugenol sealers have a history of successful use over an extended period of time. Zinc oxide–eugenol sealers will absorb if extruded into the periradicular tissues.13 They exhibit a slow setting time,6 shrinkage on setting, solubility,158,235 and they can stain tooth structure.75,320 An advantage to this sealer group is antimicrobial activity.4,15,133,203
An early zinc oxide–eugenol sealer was introduced by Rickert and Dixon.245 This powder/liquid sealer contained silver particles for radiopacity. Although it was possible to demonstrate the presence of lateral and accessory canals the sealer had the distinct disadvantage of staining tooth structure if not completely removed. Marketed as Pulp Canal Sealer (SybronEndo) and Pulp Canal Sealer EWT (extended working time), this sealer is popular with clinicians using thermoplastic techniques. Procosol (Procosol, Inc., Philadelphia, PA) is a modification of Rickert’s formula in which the silver particles have been removed (zinc oxide, hydrogenated resin, bismuth subcarbonate and barium sulfate; liquid eugenol).
Grossman116 modified the formulation and introduced a nonstaining formula in 1958 (Box 10-2). This is the formulation in Roth’s Sealer (Roth International). Tubli-Seal (SybronEndo) is a catalyst/base zinc oxide–eugenol sealer that is convenient to mix but has a faster setting time when compared with the liquid/powder sealers. Tubli-Seal EWT provides an extended working time. Wach’s sealer (Balas Dental, Chicago, IL) contains Canada balsam, which gives the material a sticky or tacky property that softens the gutta-percha into a more homogeneous mass when used with lateral compaction.
Calcium Hydroxide Sealers
Calcium hydroxide sealers were developed for therapeutic activity. It was thought that these sealers would exhibit antimicrobial activity and have osteogenic–cementogenic potential. Unfortunately, these actions have not been demonstrated. Solubility is required for release of calcium hydroxide and sustained activity. This is inconsistent with the purpose of a sealer. Calciobiotic root canal sealer (CRCS) is a zinc oxide–eugenol sealer with calcium hydroxide as one ingredient. Sealapex (SybronEndo) is a catalyst/base system. The base contains zinc oxide, calcium hydroxide, butyl benzene, sulfonamide, and zinc stearate. The catalyst contains barium sulfate and titanium dioxide as radiopacifiers in addition to resin, isobutyl salicylate, and aerosol R 972. Apexit and Apexit Plus (Ivoclar Vivadent, Schaan, Liechtenstein) consist of an activator (disalicylate, bismuth hydroxide/bismuth carbonate, and fillers) and a base (calcium hydroxide, hydrated colophonium, and fillers).
Noneugenol Sealers
Developed from a periodontal dressing, Nogenol (GC America, Alsip, IL) is a root canal sealer without the irritating effects of eugenol. The base contains zinc oxide, barium sulfate, and bismuth oxychloride.
Glass Ionomer Sealers
The glass ionomers have been advocated for use in obturation because of their dentin-bonding properties. Ketac-Endo (3M ESPE, Minneapolis, MN) enables adhesion between the material and the canal wall.105 It is also difficult to properly treat the dentinal walls in the apical and middle thirds with preparatory bonding agents to receive the glass ionomer sealer. A disadvantage of glass ionomers is that they must be removed if retreatment is required.189 This sealer has minimal antimicrobial activity.133
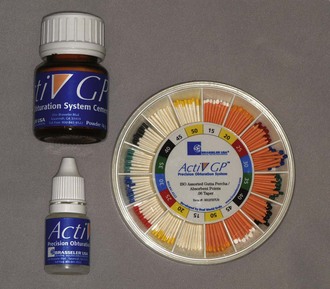
Activ GP (Brasseler USA, Savannah, GA) consists of a glass ionomer–impregnated gutta-percha cone with a glass ionomer external coating and a glass ionomer sealer (Fig. 10-14). Available in .04 and .06 tapered cones, the sizes are laser verified to ensure a more precise fit. This single cone technique is designed to provide a bond between the dentinal canal wall and the master cone (monoblock). A bacterial leakage study comparing Activ GP/glass ionomer sealer, Resilon/Epiphany, and gutta-percha (GP)/AH Plus demonstrated no statistically significant differences at 65 days.103
Resin
Resin sealers have a long history of use, provide adhesion, and do not contain eugenol. AH-26 is a slow-setting epoxy resin that was found to release formaldehyde when setting.167,298 AH Plus is a modified formulation of AH-26 in which formaldehyde is not released (Fig. 10-15).182 The sealing abilities of AH-26 and AH Plus appear comparable.79 AH Plus is an epoxy-bis-phenol resin that comes in two tubes. It exhibits a working time of approximately 4 hours.
FIG. 10-15 AH Plus sealer is a resin formulation.
(Courtesy DENTSPLY, Konstanz, Germany. All rights owned by and used with permission from DENTSPLY International, Inc.)
EndoREZ (Ultradent Products, South Jordon, UT) is a methacrylate resin with hydrophilic properties. When used with EndoREZ resin-coated gutta-percha cones the dual cure EndoREZ sealer bonds to both the canal walls and the core material.
Diaket, a polyvinyl resin (3M ESPE), consists of a powder composed of bismuth phosphate and zinc oxide and a liquid consisting of dichlorophen, triethanolamine, propionyl-acetophenone, and copolymers of vinyl acetate, vinyl chloride, and vinylisobutyl ether. The material appears to be biocompatible.212
Other resin-based sealers, Epiphany (Pentron Clinical Technologies, Wallingford, CT) and RealSeal (SybronEndo), have been introduced for use with a new core material, Resilon (Pentron Clinical Technologies). Advocates of these sealers propose that they bond to the canal wall and to the core material to create a “monoblock.” One study indicated that the bond strength to dentin can be influenced by the irrigant used. Water and chlorhexidine decreased the bond strength compared with NaOCl, NaOCl/EDTA, and NaOCl/MTAD. The use of EDTA and MTAD did not improve the bond strength compared with NaOCl alone.324
Silicone Sealers
RoekoSeal (Coltène/Whaledent, Germany) is a polyvinylsiloxane that has been reported to expand slightly on setting.
GuttaFlow (Coltène/Whaledent) is a cold flowable matrix that is triturated. It consists of gutta-percha added to RoekoSeal (Fig. 10-16). The material is provided in capsules for trituration. The technique involves injection of the material into the canal, followed by placement of a single master cone. The material provides a working time of 15 minutes and it cures in 25 to 30 minutes. Evidence suggests that the material fills canal irregularities with consistency355 and is biocompatible,36,95 but the setting time is inconsistent and may be delayed by final irrigation with sodium hypochlorite.36 Sealing ability appears comparable to other techniques in some studies and inferior in others.38,169,207,225
Bioceramic
Bioceramic (BC) sealer is composed of zirconium oxide, calcium silicates, calcium phosphate monobasic, calcium hydroxide, and various filling and thickening agents. The material is available in a premixed syringe with calibrated intracanal tips. As a hydrophilic sealer it utilizes moisture within the canal to complete the setting reaction and it does not shrink on setting. It is biocompatible and exhibits antimicrobial properties during the setting reaction. The manufacturer advocates expressing the sealer into the coronal one third to one half of the canal and then seating the master gutta-percha cone.
Medicated Sealers
Sealers containing paraformaldehyde are strongly contraindicated in endodontic treatment (Fig. 10-17). Although the lead and mercury components may have been removed from these zinc oxide–eugenol formulations over time, the severely toxic paraformaldehyde content has remained a constant. These sealers are not approved by the U.S. Food and Drug Administration10 and are unacceptable under any circumstances in clinical treatment because of the severe and permanent toxic effects on periradicular tissues.275 A paste containing 6.5% paraformaldehyde as well as lead and mercury was advocated for use by Sargenti258-260 and originally marketed as N-2. Lead has been reported in distant organ systems when N-2 is placed within the radicular space.223 In another study the investigators reported the same results regarding systemic distribution of the paraformaldehyde component of N-2.31 Removal of the heavy metals resulted in a new formulation: RC2B. Other paraformaldehyde sealers include Endomethasone, SPAD, and Reibler’s paste. The toxic in vivo effects of these materials on the pulp and periapical tissues have been demonstrated over time.67,213
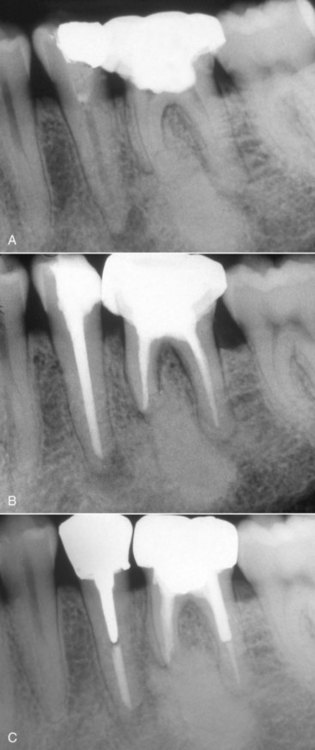
FIG. 10-17 Patient treated with Sargenti paste in her mandibular left second premolar and first molar. A, Pretreatment radiograph exhibits an osteolytic response associated with the premolar and a proliferative response associated with the molar. B, Posttreatment radiograph of the teeth. C, One year follow-up radiograph exhibiting osseous regeneration apical to the second premolar and appropriate restorative treatment.
In addition to the toxic nature of the material, clinicians employing the material place it with a lentulo spiral. Overextension has resulted in osteomyelitis and paresthesia.97,165 One clinician reported irreversible neurotoxicity, manifested as dysesthesia, in cases where paraformaldehyde pastes were forced through the apical foramen into the periapical tissues.165 The reader is referred to Chapter 11 for further discussion about this harmful material and technique.
Sealer Placement
Various methods of sealer placement have been advocated, including the master cone, lentulo spirals, files and reamers, and ultrasonics. Investigators compared sealer placement using a file rotated counterclockwise, the lentulo spiral (Fig. 10-18), an ultrasonic file, and coating the master gutta-percha cone.336 Placement did not differ with the various techniques; however, the investigators noted the most variation in sealer coating was in the apical area.336 Another study compared sealer placement with a K-type file, the lentulo spiral, and using the master cone in curved canals. Results demonstrated no significant differences in the techniques after obturation; no technique covered more than 62.5% of the canal wall surface.125 Other investigators found that ultrasonics produced the best sealer distribution when used circumferentially.299 These findings were supported by another study that found ultrasonic placement to be superior to manual techniques.1
The method of obturation does not seem to affect the sealer distribution on the canal wall in the apical portion of the canal; however, lateral compaction results in better distribution in the mid-coronal areas when compared with warm vertical compaction.343 Another well-controlled study reported that none of five obturation techniques evaluated resulted in uniform sealer distribution along the entire length of the core obturation material.142 Evidence indicates that the method of obturation affects the sealer penetration into tubules. This was exemplified by a study finding that thermoplastic techniques produced deeper sealer penetration into tubules.78 Removal of the smear layer enhances sealer penetration into the dentinal tubules.77
Core Materials
Although a variety of core materials have been used in conjunction with a sealer/cement, the most common method of obturation involves gutta-percha as a core material. Regardless of the obturating technique, emphasis should be placed on the process of cleaning and shaping the canal. The materials and techniques described do not routinely provide for an impervious seal of the canal system; all materials leak to some extent.2 The choice of obturation techniques depends on the unique circumstances each case provides.
The properties of an ideal obturation material were outlined by Grossman115 (Box 10-3). Historically, a variety of materials have been employed to obturate the root canal space. Solids, semisolid materials, and pastes have been employed. A common solid material was the silver cone.
Silver Cones
Jasper148 introduced cones made of silver, which he claimed produced the same success rate as gutta-percha and were easier to use. The rigidity provided by the silver cones made them easy to place and permitted more predictable length control; however, their inability to fill the irregularly shaped root canal system permitted leakage (Fig. 10-19). When silver points contact tissue fluids or saliva, they corrode.39 The corrosion products have been found to be cytotoxic and produced pathosis or impeded periapical healing.271
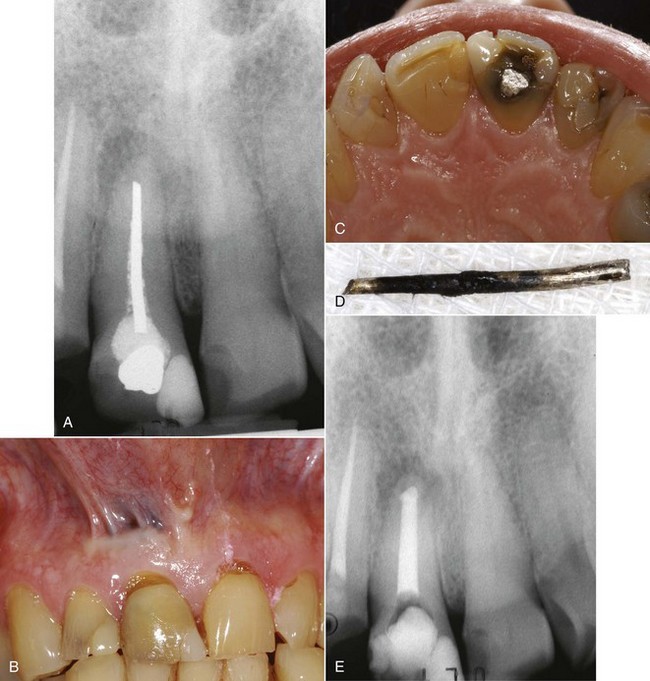
FIG. 10-19 Silver cones are advocated for ease of placement and length control. A, Radiograph of a facial maxillary right central incisor obturated with a silver cone. B, Tissue discoloration indicating corrosion and leakage. C, Lingual view indicates coronal leakage. D, Corroded silver cone removed from the tooth. E, Posttreatment radiograph of the tooth.
With the introduction of rigid silver cones it became possible to easily place them to length. This resulted in clinicians often failing to properly clean and shape the canal before obturation. Treatment failures were the result of leakage and failure to remove the irritants from the root canal system. The use of silver cones today is considered to be below the standard of care in contemporary endodontic practice. For further information about this issue, the reader is referred to Chapter 11.
Gutta-Percha
Gutta-percha is the most popular core material used for obturation. Major advantages to gutta-percha are its plasticity, ease of manipulation, minimal toxicity, radiopacity, and ease of removal with heat or solvents. Disadvantages include its lack of adhesion to dentin and, when heated, shrinkage on cooling. Gutta-percha is the trans isomer of polyisoprene (rubber) and exists in two crystalline forms (α and β).112 In the unheated β phase the material is a solid mass that is compactable. When heated the material changes to the α phase and becomes pliable and tacky and can be made to flow when pressure is applied. A disadvantage to the α phase is that the material shrinks on setting.267
Gutta-percha cones consist of approximately 20% gutta-percha, 65% zinc oxide, 10% radiopacifiers, and 5% plasticizers.104 Attempts have been made to make gutta-percha more antimicrobial by the addition of materials such as iodoform,63 calcium hydroxide,190 chlorhexidene,192 and tetracycline.201 The clinical effectiveness of adding these materials has not been demonstrated.
Unlike rubber, room temperature gutta-percha cannot be compressed or made to flow. Compaction results in transmission of forces to the material and the canal wall equally and may result in root fracture. Gutta-percha can be made to flow if it is modified by either heat or solvents such as chloroform. This permits adaptation to the irregularities of the canal walls.
The α form of gutta-percha melts when heated above 65° C. When cooled extremely slowly, the α form will recrystallize. Routine cooling results in the recrystallization of the β form. Although the mechanical properties for the two forms are the same, when α-phase gutta-percha is heated and cooled it undergoes less shrinkage, making it more dimensionally stable for thermoplasticized techniques. The use of α-phase gutta-percha for obturation has increased as thermoplastic techniques have become more common.
Gutta-percha cones are available in standardized and nonstandardized (conventional) sizes. The nonstandard nomenclature refers to the dimensions of the tip and body (Fig. 10-20). A fine-medium cone has a fine tip with a medium body. Standardized cones are designed to match the taper of stainless steel and nickel–titanium instruments (Figs. 10-21 and 10-22). A size 40/04 has a tip of 0.4 mm and a taper of 0.04 mm per millimeter. Unfortunately uniformity in manufacturing is not present, and the actual cone size varies.111,197

FIG. 10-20 Nonstandard gutta-percha cones: extra fine, fine fine, fine, medium fine, fine medium, medium, large, and extra large.
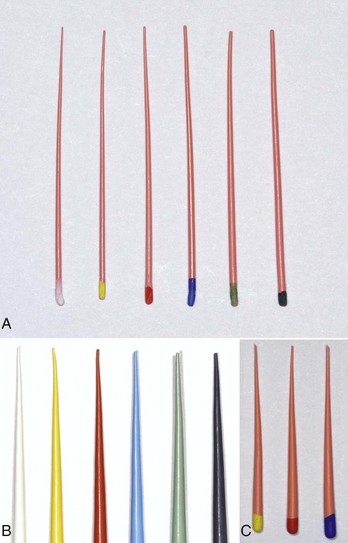
FIG. 10-21 A, Standard gutta-percha cone sizes #15 to #40. B, Standard cones #.06, taper sizes #15 to #40. C, Standard cones Protaper F1, F2, F3.
Although the points cannot be heat sterilized, a study found that gutta-percha points can be sterilized before use by placing the cones in 5.25% NaOCl for 1 minute. This study also found that 2% glutaraldehyde, 2% chlorhexidine, and 70% ethyl alcohol were not effective in killing Bacillus subtilis spores.287
Activ GP
Activ GP (Brasseler USA) consists of gutta-percha cones impregnated on the external surface with glass ionomer (see Fig. 10-14). Single cones are used with a glass ionomer sealer. Available in .04 and .06 tapered cones, the sizes are laser verified to ensure a more precise fit. The single cone technique is designed to provide a bond between the dentinal canal wall and the master cone. A bacterial leakage study comparing Activ GP/glass ionomer sealer, Resilon/Epiphany, and gutta-percha/AH Plus demonstrated no statistically significant differences in leakage at 65 days.103
Resilon
The resin-based obturation systems Epiphany (Pentron Clinical Technologies), RealSeal (SybronEndo), and Resinate (Obtura Spartan, Earth City, MO) have been introduced as alternatives to gutta-percha (Figs. 10-23 and 10-24). Resilon is a high-performance industrial polyurethane that has been adapted for dental use.

FIG. 10-23 Epiphany system (Pentron Clinical Technologies, Wallingford, CT) with the primer, thinning resin, sealant, and standard Resilon points.
(Courtesy SybronEndo, Orange, CA.)

FIG. 10-24 Resilon #.02, #.04, and #.06 tapered points and a thermoplastic plug for use in the Obtura II system (Obtura Spartan, Earth City, MO).
The resin sealer bonds to a Resilon core, and attaches to the etched root surface. The manufacturer claims that this forms a “monoblock” (Fig. 10-25). With traditional techniques there is a gutta-percha–sealer interface and a tooth–sealer interface. With Resilon the resin sealer bonds to both the canal wall and the cone. Whether a monoblock is achievable remains controversial.242,308 An in-depth review article on the subject of monoblocks indicates that with current materials and techniques, the monoblock has yet to be achieved.308
The system resembles gutta-percha and can be placed by lateral compaction, warm lateral or vertical compaction, or thermoplastic injection. It consists of a resin core material (Resilon) composed of polyester, difunctional methacrylate resin, bioactive glass, radiopaque fillers, and a resin sealer. Resilon is nontoxic, nonmutagenic, and biocompatible. The core material is available in nonstandard and standard cones and pellets for use in thermoplastic techniques (see Fig. 10-24).
After cleaning and shaping procedures an appropriate master cone is placed into the prepared canal and a radiograph/image is exposed to verify the apical position. Because NaOCl may affect the bond strength of the primer, EDTA should be the last irrigant used before rinsing the canal with sterile water, saline, or chlorhexidine.
After drying the canal a self-etch primer (sulfonic acid–terminated functional monomer, 2-hydroxyethyl methacrylate [HEMA], water, and polymerization initiator) is used to condition the canal walls and prepare them for bonding to the resin sealant (resin matrix of bisphenol A-glycidyl methacrylate [Bis-GMA], ethoxylated Bis-GMA, urethane dimethacrylate [UDMA], and hydrophilic difunctional methacrylates and fillers [70%] of calcium hydroxide, barium sulfate, barium glass, bismuth oxychloride, and silica). Two or three drops are placed in the canal with a pipette, a syringe, or a paper point that wicks the material to the working length. The excess primer is removed, the resin sealer is dispensed onto a mixing slab, and the viscosity is adjusted with the thinning resin. The sealer is applied with a paper point, Resilon point, or lentulo spiral. The canal is then obturated by lateral compaction, warm vertical compaction, or thermoplastic injection. The sealer takes approximately 25 minutes to set, so it is recommended that the coronal surface of the material be light cured for 40 seconds.
When using the System B (SybronEndo) for warm vertical compaction, the temperature setting should be 150° C at a power of 10. With the Obtura II thermoplasticized injection system (Obtura Spartan) the temperature settings vary depending on the needle tip employed. For the 25-gauge needle a 160° C setting is selected, for the 23-gauge needle a 140° C setting is used, and for the 20-gauge needle the setting that is recommended is 120° C to 130° C.
Resilon appears to be comparable to gutta-percha in its ability to seal the radicular space.19 Investigators evaluated coronal leakage of Resilon, using Streptococcus mutans and E. faecalis in roots that were filled by lateral and vertical compaction techniques with gutta-percha and AH-26 or Resilon and Epiphany sealer.283 Resilon showed significantly less coronal leakage when compared with gutta-percha. In another study, investigators used a dog model to assess the ability of Resilon or gutta-percha and AH-26 to prevent apical periodontitis in teeth inoculated with microorganisms. Results indicated periapical inflammation in 18 of 22 roots (82%) obturated with gutta-percha and AH-26 whereas the Resilon group exhibited periapical inflammation in 4 of 21 roots (19%).284 Still another study demonstrated that teeth filled with Resilon were more resistant to fracture than roots filled with gutta-percha and AH-26 sealer.311 More recent evidence suggests that Resilon does not strengthen roots.114,338
Resilon appears to be biocompatible. Implantation in the subcutaneous tissues of rats demonstrated fibrous encapsulation and negligible inflammation at 60 days.34
One retrospective study compared the success and failure rates between obturation with gutta-percha and Kerr Pulp Canal Sealer and obturation with Resilon or Epiphany, with recall examination between 12 and 25 months. Statistical analysis indicated that the results were indistinguishable.71 Another study demonstrated that 82 randomly selected clinical cases treated with Resilon produced success rates at 1 year that were comparable to cases treated with gutta-percha.69
Custom Cones
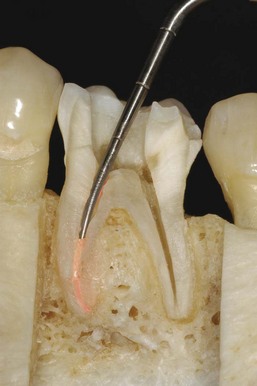
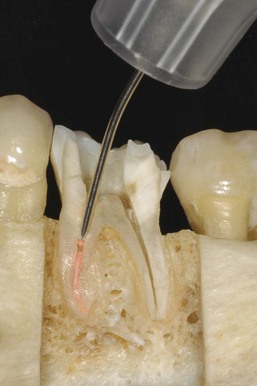
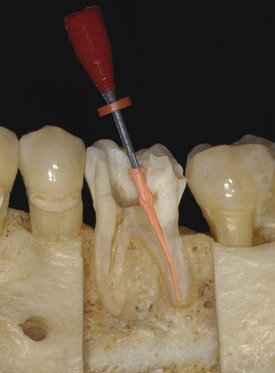
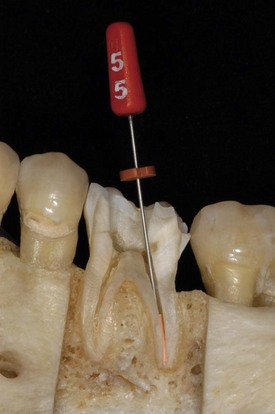
When the apical foramen is open or a canal is large a custom cone may need to be fabricated (Fig. 10-26). This permits the adaptation of the cone to the canal walls, reduces the potential for extrusion of the core material, and may improve the seal.24,160 The technique involves selection of a master cone and fitting that cone 2 to 4 mm short of the prepared length with frictional resistance. The cone is grasped with locking cotton pliers or a hemostat so that it can be placed into the canal in the same spatial relationship each time. The cone is removed and the tip is softened in chloroform, eucalyptol, or halothane for 1 or 2 seconds. Only the outer superficial portion of the cone is softened. The central core of the cone should remain semirigid. The cone is then placed into the canal and gently tamped to length. The process can be repeated until an adequate impression of the canal is obtained at the prepared length. A radiograph is exposed to verify proper fit and position. An alternative to solvents is softening with heat.161
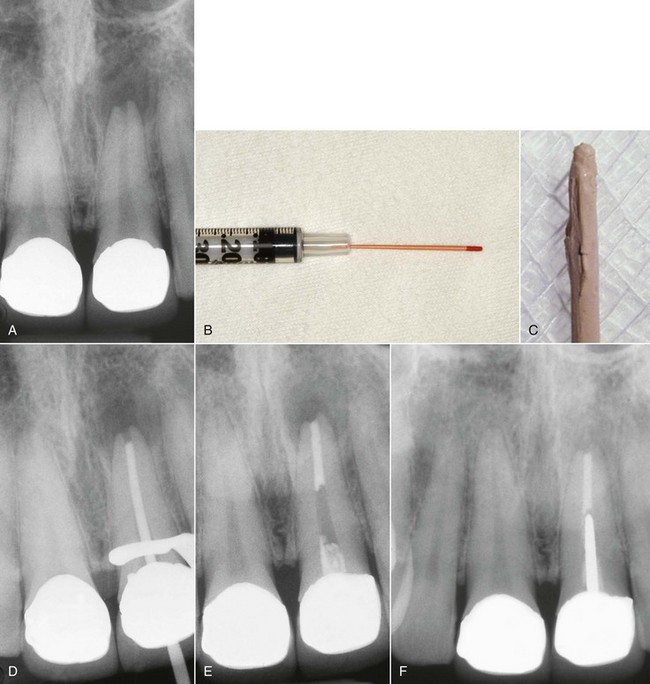
FIG. 10-26 Apical root resorption often results in an open apex requiring fabrication of a custom cone. A, Pretreatment radiograph of the maxillary left central incisor with pulp necrosis and chronic apical periodontitis. Apical root resorption is present. B, In fabricating a custom master cone a gutta-percha point is fit several millimeters short before softening in solvent and tamping into place. C, Softening the apical 2 to 3 mm in chloroform that has been placed in a tuberculin syringe. D, The completed custom cone represents an impression of the apical portion of the canal. E, The posttreatment radiograph with post space prepared. F, A 1-year follow-up radiograph demonstrating osseous regeneration.
Large canals may necessitate fabricating a large master cone before canal adaptation. This can be accomplished by heating several large gutta-percha cones and rolling the mass between two glass slabs until an appropriate size is obtained (Fig. 10-27). A spatula may also be used to shape the cone.
Methods of Obturation
To date little evidence exists to support one method of obturation as being superior to another and the influence of treatment technique on success/failure has yet to be determined.11,215 The prospective Toronto studies have suggested that warm vertical compaction may be superior to lateral compaction; however, definitive evidence is lacking.76,231
Lateral Compaction
Lateral compaction is a common method for obturation (Fig. 10-28).51 The technique can be used in most clinical situations and provides for length control during compaction.109 A disadvantage is that the technique may not fill canal irregularities347 as well as warm vertical compaction or thermoplastic techniques.345 The procedure can be accomplished with any of the acceptable sealers.
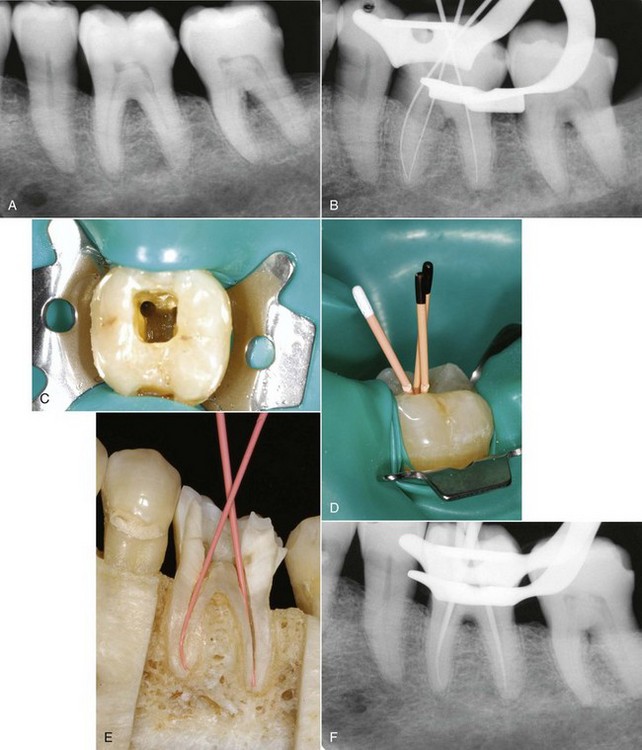
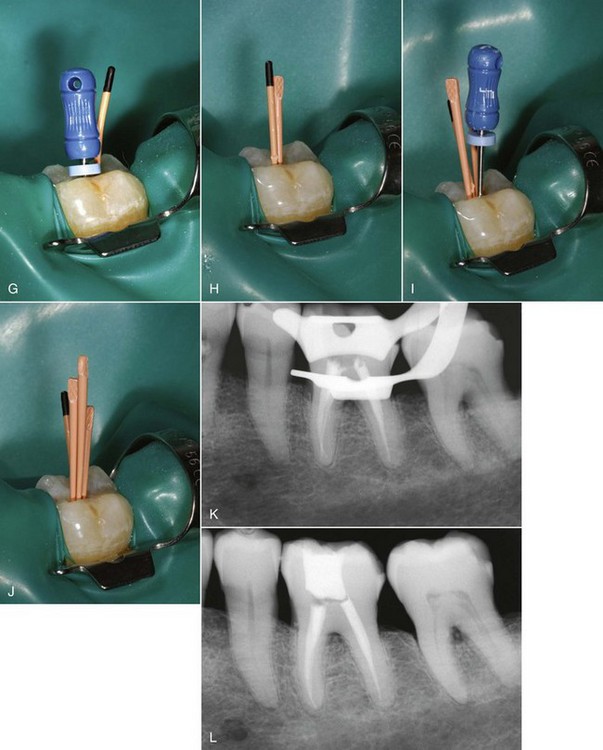
FIG. 10-28 Left first mandibular molar. A, Pretreatment radiograph. B, Working length radiograph. C, Coronal access opening, demonstrating the prepared mesiobuccal canal. D, Standardized master cones with coronal reference marked. E, Standard master cones fit to length as they exhibit minimal taper and permit deeper penetration of the spreader. F, Master cone radiograph.G, Finger spreader in place. H, Fine-medium accessory cone placed in the space created by the spreader. I, Finger spreader placed in preparation, creating space for additional accessory cones. J, Additional cones are placed until the spreader does not penetrate past the coronal one third of the canal. The cones are then removed at the orifice with heat, and the coronal mass is vertically compacted with a plugger. K, Interim radiograph may be exposed to assess the quality of obturation. L, Posttreatment radiograph demonstrating adequate length, density, and taper. The gutta-percha is removed to the level of the orifice, and a coronal seal has been established with an adequate provisional restoration.
After canal preparation a standard cone is selected that has a diameter consistent with the prepared canal diameter at the working length. Standard cones generally have less taper when compared with conventional cones and will permit deeper spreader penetration.233 An alternative is to adapt an appropriately tapered nonstandard cone by cutting small increments from the tip. This “master cone” is measured and grasped with forceps so that the distance from the cone tip to the forceps is equal to the prepared length. A reference point on the cone can be made by pinching the cone. The cone is placed in the canal, and if an appropriate size is selected, there will be resistance to displacement or “tug back.” If the cone is loose it can be adapted by removing small increments from the tip. If the master cone fails to go to the prepared length a smaller cone can be selected. Devices are available to cut cones accurately at a predetermined length (Tip Snip; SybronEndo). When the cone extends beyond the prepared length a larger cone must be adapted or the existing cone shortened until there is resistance to displacement at the corrected working length.
The master cone placement is confirmed with a radiograph. The canal is irrigated and dried with paper points. Sealer is applied to the canal walls, and a spreader is prefitted so as to allow it to be inserted to within 1.0 to 2.0 mm from working length. Appropriate accessory points are also selected to closely match the size of the spreader. The correlation between spreader size and nonstandard cones is variable,44,357 and in small curved canals there does not appear to be a difference in the quality of obturation with nonstandard cones when compared with standard cones.135,321
Finger spreaders provide better tactile sensation and are less likely to induce fractures in the root when compared with the more traditional D-11T hand spreader.74,183,184 In addition to the type of spreader, forces applied, and amount of dentin removed, spreader size may be a factor in root fracture, with large sizes inducing more stress.238 Spreaders made from nickel–titanium are available and provide increased flexibility,28 reduce stress,89 and provide deeper penetration when compared with stainless steel instruments.152,268,339 The spreader should fit to within 1 to 2 mm of the prepared length, and when introduced into the canal with the master cone in place, it should be within 2 mm of the working length.7 There appears to be a correlation between establishing a seal and spreader penetration.7,281
After placement the spreader is removed by rotating it back and forth as it is withdrawn. An accessory cone is placed in the space vacated by the instrument. The process is repeated until the spreader no longer goes beyond the coronal one third of the canal. The excess gutta-percha is removed with heat and the coronal mass is compacted with an appropriate plugger. Only light pressure is required during lateral compaction because the gutta-percha is not compressible, and because as little as 1.5 kg of pressure is capable of fracturing the root (Fig. 10-29). In addition to the force applied, investigators have noted that removal of dentin during preparation is a significant factor in root fracture.337
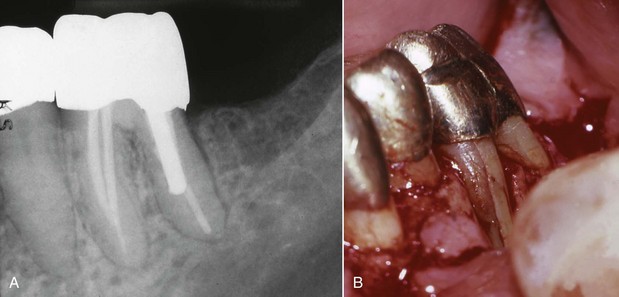
FIG. 10-29 Vertical root fractures can occur with excessive compaction forces. A, Follow-up radiograph of a mandibular left first molar. A deep isolated periodontal probing defect was associated with the buccal aspect of the mesiobuccal root. B, Flap reflection revealed a vertical root fracture.
A disadvantage to lateral compaction is that the process does not produce a homogeneous mass. The accessory and master cones are laminated and remain separate. It is hoped that the space between each of the cones is filled with sealer.
The excess gutta-percha in the chamber is then seared off and vertically compacted with a heated plugger at the orifice or approximately 1 mm below the orifice in posterior teeth. Warm vertical compaction of the coronal gutta-percha enhances the seal.351 In anterior teeth the desired level is the cementoenamel junction on the facial surface.
An alternative to lateral compaction with finger spreaders is ultrasonics.18 For example, one study found that the technique produced adequate obturation and a 93% clinical success rate.356
Another study used ultrasonic-energized files in a warm lateral compaction technique and found that the amount of gutta-percha by weight increased by 33% with two applications of ultrasonics when compared with lateral compaction.80 Unfortunately, investigators found that the mean internal temperature rise was 29° C at the 6-mm level with external heat generation exceeding the safe limit of 10° C.302
Warm Vertical Compaction
Schilder266 introduced warm vertical compaction as a method of filling the radicular space in three dimensions. Preparation requirements for the technique include preparing a canal with a continuously tapering funnel and keeping the apical foramen as small as possible.
The armamentarium includes a variety of pluggers and a heat source. Schilder pluggers come in a variety of sizes (#8 = 0.4 mm, # = 0.5 mm, etc., for sizes #9, #
= 0.5 mm, etc., for sizes #9, # , #10, #
, #10, # , #11, #
, #11, # , #12) with increasing diameter. The instruments are marked vertically at 5-mm intervals. Various ISO standardized instruments are also available (Fig. 10-30).
, #12) with increasing diameter. The instruments are marked vertically at 5-mm intervals. Various ISO standardized instruments are also available (Fig. 10-30).
FIG. 10-30 Various pluggers are manufactured for compacting warm gutta-percha. A, ISO standard spreader. B, ISO standard plugger. C, Obtura S Kondensers (C, courtesy Obtura Spartan, Earth City, MO). D, Closeup of Obtura S-Kondensers.
The technique involves fitting a master cone short of the corrected working length (0.5 to 2 mm) with resistance to displacement (Fig. 10-31). This ensures that the cone diameter is larger than the prepared canal. Nonstandard cones that closely replicate the canal taper are best because they permit the development of hydraulic pressure during compaction. After the adaptation of the master cone it is removed and sealer is applied. The cone is placed in the canal and the coronal portion is removed with heat. A heated spreader or plugger is used to remove portions of the coronal gutta-percha and soften the remaining material in the canal. The Touch ’n Heat (SybronEndo) (Fig. 10-32), EI DownPak (EI/Hu-Friedy, Chicago, IL), and System B (SybronEndo) (Fig. 10-33) are alternatives to applying heat with a flame-heated instrument because they permit temperature control. A plugger is inserted into the canal and the gutta-percha is compacted, forcing the plasticized material apically. The process is repeated until the apical portion has been filled. The coronal canal space is back-filled, using small pieces of gutta-percha. The sectional method consists of placing 3- to 4-mm sections of gutta-percha approximating the size of the canal into the root, applying heat, and compacting the mass with a plugger.
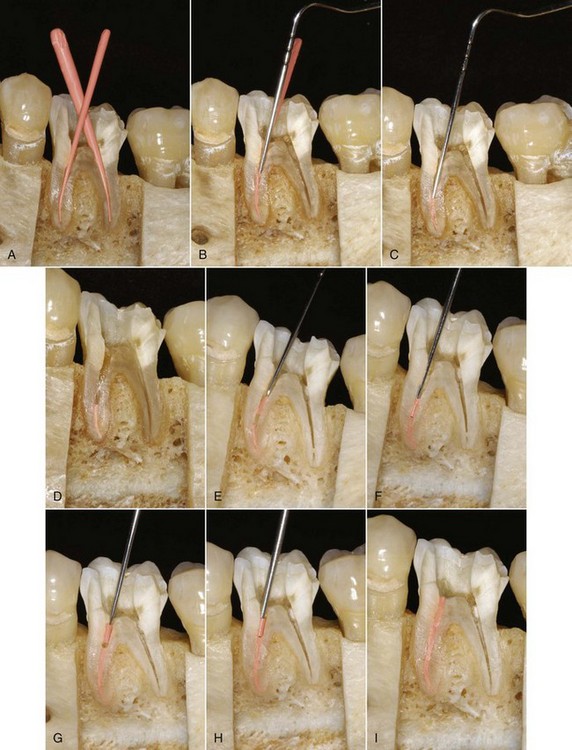
FIG. 10-31 Warm vertical compaction of gutta-percha employs heat and various condensers. A, Nonstandard cones are selected and fit short of the prepared length because they more closely replicate the prepared canal. B, Heated pluggers or spreaders are used to apply heat to the master cone and remove the excess coronal material. C, A room temperature plugger is used to compact the heated gutta-percha. D, Apical compaction is complete. E, A gutta-percha segment is placed in the canal, and heat is applied. F, The heated segment is compacted. G, The process is repeated for the coronal portion of the canal by placing and heating a segment of gutta-percha. H, A plugger is again used to compact the heated material. I, Completed obturation.
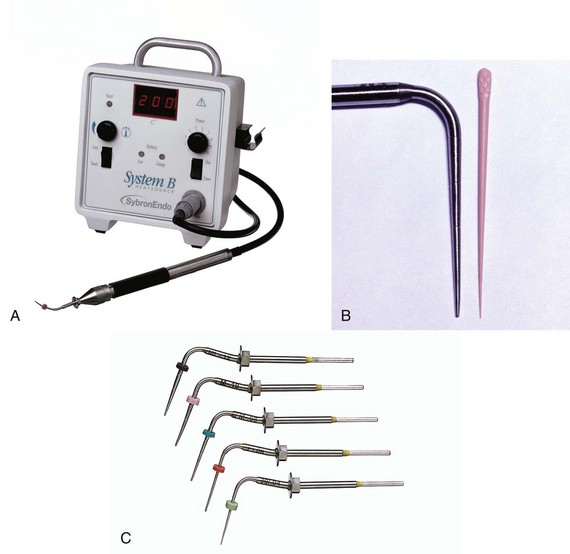
FIG. 10-33 Continuous wave obturation uses the System B unit. A, The System B unit. B, System B plugger with a nonstandard cone of similar taper. C, System B pluggers.
(Courtesy SybronEndo, Orange, CA.)
One study33 measured temperature changes in the canal with warm vertical compaction. The maximal temperatures occurred coronally and decreased apically. The authors reported that the maximal temperature in the canal was 118° C 8 mm from the apex. At 0 to 2 mm from the apex the maximal temperature decreased to 44° C. Another study compared root surface temperatures for warm vertical obturation using the System B heat source, the Touch ’n Heat device, and a flame-heated carrier in maxillary and mandibular incisors and premolars 2 mm below the cementoenamel junction. System B and the Touch ’n Heat produced a surface temperature rise that was less than 10° C for all maxillary incisors and premolar teeth. The Touch ’n Heat produced a greater than 10° C rise in mandibular incisors. The flame-heated carrier produced temperature changes greater than 10° C in all experimental teeth. Because the critical level of root surface heat required to produce irreversible bone damage is believed to be greater than 10° C the findings suggest that warm vertical compaction with the System B should not damage supporting periodontal structures; however, caution should be exercised with the Touch ’n Heat and flame-heated carriers.180
The potential for vertical root fracture is also present with warm vertical compaction.32 The forces developed appear to be equal to lateral compaction. Investigators compared warm vertical compaction and lateral compaction as a function of time. Results indicated that the forces developed with the two techniques were not significantly different. In a follow-up study, the mean value for wedging with warm vertical compaction was 0.65 ± 0.07 kg, whereas for lateral compaction it was 0.8 ± 0.1 kg.
Warm thermoplastic techniques have the advantage of producing movement of the plasticized gutta-percha, filling irregularities and accessory canals better than lateral compaction.85,342 This was illustrated in a study that reported a correlation between the quality of adaptation and the depth of heat application and canal size. Heat application close to the apical extent of the preparation produced the best results, and adaptation was better in small canals when compared with wide canals.341 However, thermoplasticized techniques result in more extrusion of obturation materials.175 There appeared to be no consistent differences between the techniques in sealing the canal space.342
Advantages of warm vertical compaction include filling of canal irregularities and accessory canals. Disadvantages include a slight risk of vertical root fracture because of compaction forces, less length control than with lateral compaction, and the potential for extrusion of material into the periradicular tissues. Warm vertical compaction is difficult in curved canals, where the rigid pluggers are unable to penetrate to the necessary depth. To allow the rigid carriers to penetrate within 4 to 5 mm of the apex, the canals must be enlarged and tapered more, in comparison with the lateral compaction technique; however, excessive removal of tooth structure weakens the root.
Continuous Wave Compaction Technique
A variation of warm vertical compaction is the continuous wave compaction technique.46 The increasing use of nickel–titanium rotary preparation techniques and the fabrication of standard cones for files of greater taper have resulted in more clinicians using thermoplasticizing techniques. The manufacturing of cones to mimic the tapered preparation permits the application of greater hydraulic force during compaction when appropriately tapered pluggers are used. The continuous wave compaction technique employs an electric heat carrier, the System B unit, and #.04, #.06, #.08, #.10, and #.12 tapered stainless steel pluggers, each with a tip diameter of 0.5 mm (see Fig. 10-33). The #.06 tapered plugger approximates the fine nonstandard gutta-percha cone, the #.08 plugger the fine-medium cone, the #.10 plugger the medium cone, and the #.12 plugger the medium-large cone. Pluggers are consistent with the ProFile GT instruments (DENTSPLY Tulsa Dental Specialties), and Autofit gutta-percha cones (SybronEndo) are also available.
The electric heat source permits a variable temperature setting. The recommended temperature setting for the System B unit is 200° C. One study evaluated internal and external temperature changes with the System B unit with varied tips and temperature settings of 200° C, 250° C, and 300° C. At 6 mm from the apex, the System B unit set at 300° C with the fine-medium plugger produced the highest mean internal temperature (74° C). The authors noted that the external temperature setting never exceeded the critical 10° C rise with any temperature setting or tip configuration.302 This was confirmed in another study that measured temperature changes 2 mm apical to the cementoenamel junction and 1.5 mm from the apex. Results indicated that temperature changes apically were negligible. The mean change near the cementoenamel junction was 4.1° C.322
Another study reported that obturation temperature elevations produced during obturation with System B were significantly less (P < 0.001) than with warm vertical compaction. An elevation of external root surface temperature by more than 10° C was noted with vertical compaction.285 Investigators measured the root surface temperatures while using the System B heat source at various temperature settings from 250° C to 600° C. Results indicated that the highest temperature occurred 5 mm from the apex, and this was the only site that exceeded the 10° C rise. On the basis of this study a temperature setting of 250° C or greater may be potentially hazardous.101 For example, investigators using a thermocouple and simultaneous infrared analysis of temperatures found that the root surface temperature averaged 13.9° C whereas the infrared technology indicated a 28.4° C rise at the same sites.200
After selecting an appropriate master cone, a plugger is prefitted to fit within 5 to 7 mm of the canal length (Fig. 10-34). Placing the plugger deeper into the canal may enhance the flow of gutta-percha.5 The point of plugger binding should be noted because once the instrument reaches this point the hydraulic forces on the gutta-percha will decrease and forces on the root will increase. There appears to be a correlation between the depth of the heated plugger relative to the working length and the quality of obturation and filling of canal irregularities.37,153,345 Increasing the temperature settings does not seem to increase the effectiveness of obturation.153
The System B unit is set to 200° C in the touch mode. The plugger is inserted into the canal orifice and activated to remove excess coronal material. Compaction is initiated by placing the cold plugger against the gutta-percha in the canal orifice. Firm pressure is applied and heat is activated with the device. The plugger is moved rapidly (1 to 2 s) to within 3 mm of the binding point (Fig. 10-35). The heat is inactivated while firm pressure is maintained on the plugger for 5 to 10 seconds. After the gutta-percha mass has cooled a 1-second application of heat separates the plugger from the gutta-percha, and it is removed. The pluggers are designed to heat from the tip to their shank, which decreases the potential for dislodging the compacted mass and prevents a second application of heat to the material. Confirmation that the apical mass is still present in the canal can be established with hand pluggers. Two hand instruments are manufactured with tip diameters of 0.4 and 0.9 mm and 0.7 and 1.4 mm. It should be noted that with the continuous wave technique the heat source is placed only to within 5 to 7 mm from the tip of the gutta-percha; the apical portion of the gutta-percha remains essentially a single cone technique as the heat transfer does not take place in the apical 2 to 5 mm of the gutta-percha.265
In ovoid canals, where the canal configuration may prevent the generation of hydraulic forces, an accessory cone can be placed alongside the master cone before compaction. With type II canals the master cones are placed in both canals before compaction. A hand plugger is used to stabilize the cone in one canal while the other is being obturated.
Filling the space left by the plugger can be accomplished by a thermoplastic injection technique (Obtura II or Ultrafil 3D [Coltène/Whaledent], Calamus [DENTSPLY Tulsa Dental Specialties], Elements [SybronEndo, Orange, CA], or HotShot [Discus Dental, Culver City, CA]) (Fig. 10-36)150 or by fitting an accessory cone into the space with sealer, heating it, and compacting by short applications of heat and vertical pressure.
Warm Lateral Compaction
Lateral compaction of gutta-percha provides for length control, which is an advantage over thermoplastic techniques. The Endotec II device (Medidenta) provides the clinician with the ability to employ length control while incorporating a warm gutta-percha technique (Fig. 10-37). Investigators demonstrated that the Endotec II produced a fusion of the gutta-percha into a solid homogeneous mass.147 One study evaluated three thermoplasticized filling techniques and lateral compaction, using a bacterial metabolite model, and found the Endotec to be superior to lateral compaction alone, lateral thermocompaction, and the Ultrafil 3D.162 The use of warm lateral compaction with the Endotec demonstrated an increased weight of gutta-percha mass, by 14.63%, when compared with traditional lateral compaction.187 Another study found a 24% increase in weight with warm lateral compaction when using the System B device.211 Using the Endotec II, one investigation reported a statistically significantly better ability of warm vertical and warm lateral compaction techniques versus cold lateral compaction to reproduce artificially produced canal irregularities in a split-tooth model.68 Another group of investigators used the EndoTwinn (Hu-Friedy), an instrument for warm lateral compaction, in a similar experiment. The EndoTwinn instrument also possesses the ability to vibrate the electronically heated tip. They reported that warm lateral compaction, using both heat and vibration, and warm vertical compaction of gutta-percha provided statistically better replication of defects than cold lateral compaction.173 EI, a subsidiary of Hu-Friedy, has now introduced the EI DownPak (Fig. 10-38), a variation of the original EndoTwinn that can be used with either warm lateral or warm vertical compaction techniques. Other investigators compared the stress generated with lateral compaction and warm lateral compaction, using the Endotec, and found that the warm lateral compaction technique created less stress during obturation.196 An additional concern is the heat generated by the technique. Evaluation of the effects of warm lateral and warm vertical compaction on periodontal tissues demonstrated that neither technique produced heat-related damage.57

FIG. 10-38 The EI DownPak device for heat softening and vibrating gutta-percha
(Courtesy EI/Hu-Friedy, Chicago, IL).
The warm lateral compaction technique involves adapting a master cone in the same manner as with traditional lateral compaction. An appropriate-size Endotec II tip is selected. Endotec II tips are available in various taper and tip diameters. The sizes consist of #.02/20 and #.02/40. The device is activated and the tip is inserted beside the master cone to within 2 to 4 mm of the apex, using light pressure. The tip is rotated for 5 to 8 seconds and removed. An unheated spreader can be placed in the channel created to ensure adaptation and then an accessory cone is placed. The process is continued until the canal is filled.
Thermoplastic Injection Techniques
Heating of gutta-percha outside the tooth and injecting the material into the canal is an additional variation of the thermoplastic technique (Fig. 10-39). The Obtura III (Fig. 10-40), Calamus (Fig. 10-41), Elements (Fig. 10-42), HotShot (Fig. 10-43), and Ultrafil 3D (Fig. 10-44) are available devices. The Obtura II system heats the gutta-percha to 160° C, whereas the Ultrafil 3D system employs a low-temperature gutta-percha that is heated to 90° C.
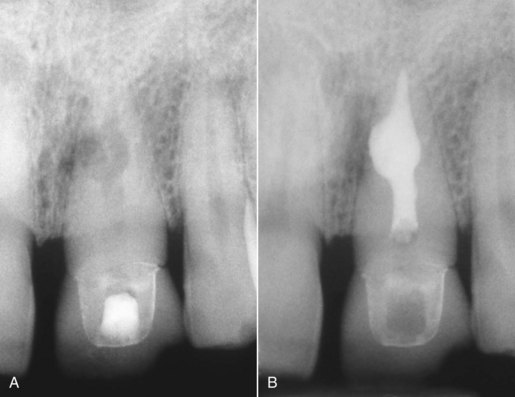
FIG. 10-39 Thermoplastic techniques are often used in cases with significant canal irregularities. A, A pretreatment radiograph of a maxillary central incisor exhibiting internal resorption. B, Posttreatment radiograph demonstrates a dense obturation of the resorptive defect with gutta-percha.
FIG. 10-40 Obtura III unit with silver tips, gutta-percha plugs, and cleaning solution (Obtura Spartan, Earth City, MO).
FIG. 10-41 The Calamus thermoplastic unit (DENTSPLY Tulsa Dental Specialties, Tulsa, OK) for heating and injecting gutta-percha.
FIG. 10-42 The Elements obturation unit (SybronEndo, Orange, CA) for injecting and compacting gutta-percha. Note the System B heat source.

FIG. 10-43 The battery-powered HotShot unit (Discus Dental, Culver City, CA) for heating and injecting gutta-percha.
FIG. 10-44 The Ultrafil 3D system consists of an injection syringe, gutta-percha cannulas, and heating unit (Coltène/Whaledent, Cuyahoga Falls, OH).
Obtura III
The Obtura III system (Obtura Spartan, Earth City, MO) consists of a hand-held “gun” that contains a chamber surrounded by a heating element into which pellets of gutta-percha are loaded (see Fig. 10-40). Silver needles (varying gauges of 20, 23, and 25) are attached to deliver the thermoplasticized material to the canal. The control unit allows the operator to adjust the temperature and thus the viscosity of the gutta-percha. At 6 mm from the apex a study found that the highest internal temperature with the Obtura II was 27° C.302
Canal preparation is similar for other obturation techniques. The apical terminus should be as small as possible to prevent extrusion of gutta-percha. The technique requires the use of sealer, and once the canal is dried, the canal walls are coated with sealer, using the last file used to length or a paper point. Gutta-percha is preheated in the gun, and the needle is positioned in the canal so that it reaches within 3 to 5 mm of the apical preparation. Gutta-percha is then gradually, passively injected by squeezing the trigger of the “gun.” The needle backs out of the canal as the apical portion is filled. Pluggers dipped in alcohol are used to compact the gutta-percha. A segmental technique may also be used, in which 3- to 4-mm segments of gutta-percha are sequentially injected and compacted. In either case, compaction should continue until the gutta-percha cools and solidifies to compensate for the contraction that takes place on cooling.
The difficulties with this system include lack of length control. Both overextension and underextension are common results. To overcome this drawback, a hybrid technique may be used, in which the clinician begins filling the canal by the lateral compaction technique. When the master cone and several accessory cones have been placed so that the mass is firmly lodged in the apical portion of the canal, a hot plugger is introduced, searing the points off approximately 4 to 5 mm from the apex. Light vertical compaction is applied to restore the integrity of the apical plug of gutta-percha. The remainder of the canal is then filled with thermoplasticized gutta-percha injected as previously described.
Investigators studied, at 3, 6, and 12 months posttreatment, the success rate of 236 teeth obturated with the Obtura system. Results indicated that 96% of the cases were successful, with the highest success rate being in teeth filled flush with the apex (97%) when compared with overextensions (93%) and filling short (93%).304 Another study compared lateral compaction with Thermafil (DENTSPLY Tulsa Dental Specialties) and Obtura II in root canal models and found that the Obtura II produced the best adaptation to the canal walls.332 Other investigators found that continuous wave obturation with the Obtura II backfill initially produced a better bacterial seal when compared with lateral compaction, using bilaterally matched teeth and an anaerobic bacterial leakage model.147
Ultrafil 3D
Ultrafil 3D (Coltène/Whaledent) is a thermoplastic gutta-percha injection technique involving gutta-percha cannulas, a heating unit, and an injection syringe (see Fig. 10-44). The system employs three types of gutta-percha cannulas. The Regular Set is a low-viscosity material that requires 30 minutes to set. The Firm Set is also a low-viscosity material but differs in that it sets in 4 minutes. The manufacturer recommends compaction after the initial set with both materials. Endoset has a higher viscosity and does not flow as well. It is recommended for techniques employing compaction and sets in 2 minutes. The heater is preset at 90° C and does not require adjustment.
Each cannula has a 22-gauge stainless steel needle that measures 21 mm in length. The needles can be precurved. Cannulas can be disinfected but are not designed for heat sterilization procedures. Heating time varies, but for a cold unit it takes 10 to 15 minutes. In a warm heater the recommended time is 3 minutes. After removing the cannula from the heater the needle should be placed on the hot part of the heater for several seconds. The gutta-percha remains able to flow for 45 to 60 seconds depending on the viscosity.
Calamus
The Calamus flow obturation delivery system (DENTSPLY Tulsa Dental Specialties) is a thermoplastic device equipped with a cartridge system with 20- and 23-gauge needles (see Fig. 10-41). The unit permits control of temperature and also the flow rate. Pluggers are also available for use with the system. The 360 degree activation switch allows great tactile sensation during use.
Elements
The Elements obturation unit (SybronEndo) consists of a System B heat source and plugger as well as a handpiece extruder for delivering thermoplastic gutta-percha or RealSeal from a disposable cartridge (see Fig. 10-42). The cartridges come with 20-, 23-, and 25-gauge needles for gutta-percha and 20- and 23-gauges for RealSeal.
HotShot
The HotShot delivery system (Discus Dental) is a cordless thermoplastic device that has a heating range from 150° C to 230° C (see Fig. 10-43). The unit is cordless and can be used with either gutta-percha or Resilon. Needles are available in 20, 23, and 25 gauges.
GuttaFlow
GuttaFlow (Coltène/Whaledent) consists of a cold, flowable matrix that consists of polydimethylsiloxane matrix filled with very finely ground gutta-percha (see Fig. 10-16). The material is provided in capsules for trituration in an amalgamator. The technique involves injection of the material into the canal and placing a single master cone to length. The material provides a working time of 15 minutes and it cures in 25-30 minutes. Evidence suggests that the material fills canal irregularities with consistency355 and is biocompatible,36,95 but the setting time is inconsistent and may be delayed by final irrigation with sodium hypochlorite.36 Sealing ability appears comparable to other techniques in some studies and inferior in others.38,169,207,225
Carrier-Based Gutta-Percha
Thermafil, Profile GT Obturators, GT Series X Obturators, and ProTaper Universal Obturators
Thermafil (DENTSPLY Tulsa Dental Specialties) was introduced as a gutta-percha obturation material with a solid core (Fig. 10-45). Originally manufactured with a metal core and a coating of gutta-percha, the carrier was heated over an open flame. The technique was popular because the central core provided a rigid mechanism to facilitate the placement of the gutta-percha. Advantages included ease of placement and the pliable properties of the gutta-percha. Disadvantages were that the metallic core made placement of a post challenging and retreatment procedures were difficult. In addition, the gutta-percha was often stripped from the carrier, leaving the carrier as the obturating material in the apical area of the canal.
Changes in the carrier systems include the development of a plastic core coated with α-phase gutta-percha (Fig. 10-46) and a heating device that controls the temperature (Fig. 10-47). Obturators are designed to correspond to the ISO standard file sizes, variable tapered nickel–titanium rotary files, and the ProFile GT and GT Series X nickel–titanium rotary files (DENTSPLY Tulsa Dental Specialties) (see Fig. 10-46). Size verifiers are available to aid in selection of the appropriate carrier and should fit passively at the corrected working length (see Fig. 10-45).
As with all techniques, a sealer is required. Grossman formulation sealers and resin sealers consistent with AH-26 and AH Plus are acceptable; however, Tubli-Seal and Wach’s paste are not recommended.
Removal of the smear layer is strongly recommended (see Chapter 9) and has been shown to enhance the seal with Thermafil.25 After drying the canal a light coat of sealer is applied and a carrier is marked, set to the predetermined length. This is accomplished by using the millimeter calibration markings on the carrier shaft. Markings are made at 18, 19, 20, 22, 24, 27, and 29 mm. Gutta-percha on the shaft that may be obscuring the calibration rings can be removed with a surgical blade or knife. The carrier is disinfected with 5.25% NaOCl for 1 minute and rinsed in 70% alcohol.
The carrier is then placed in the heating device. When the carrier is heated to the appropriate temperature the clinician has approximately 10 seconds to retrieve it and insert it into the canal (Fig. 10-48). This is accomplished without rotation or twisting. Evidence suggests that the insertion rate affects the obturation. The fill length and obturation of irregularities increase with increasing insertion rates.186 A rapid insertion rate enhances obturation.186
The position of the carrier is verified radiographically. The gutta-percha is allowed 2 to 4 minutes to cool before resecting the coronal portion of the carrier, which can be several millimeters above the canal orifice. This is accomplished by applying stabilizing pressure to the carrier and cutting the device with an inverted cone, round bur, or a specially designed Prepi bur (DENTSPLY Tulsa Dental Specialties). Heated instruments are not recommended for this process because this may result in displacement.
Vertical compaction of the coronal gutta-percha can be accomplished. When necessary, gutta-percha can be added, heat softened, and compacted. An advantage to this technique is the potential for movement of gutta-percha into lateral and accessory canals (Fig. 10-49)340; however, extrusion of material beyond the apical extent of the preparation is a disadvantage.65,73,175

FIG. 10-49 Apical obturation of accessory canals by the Thermafil technique.
(Courtesy DENTSPLY Tulsa Dental Specialties, Tulsa, OK. All rights owned by and used with permission from DENTSPLY International, Inc.)
Pro-Post drills (DENTSPLY Tulsa Dental Specialties) are recommended if post space is required for restoration of the tooth. The unique eccentric cutting tip keeps the instrument centered in the canal while friction softens and removes the gutta-percha and plastic carrier.
When retreatment is necessary the plastic carrier has a groove along its length to provide an access point for placement of a file. Chloroform and hand files can be used to remove the gutta-percha surrounding the carrier. Rotary #.04 and #.06 nickel–titanium files may also be used to remove the obturation materials. Retreatment rotary nickel–titanium files are available in three different sizes to facilitate removal of gutta-percha and the carrier.
The plastic carriers are composed of two nontoxic materials. Sizes #20 to #40 are manufactured from a liquid crystal plastic. Sizes #40 to #90 are composed of polysulfone polymer. Both have similar physical characteristics, with the polysulfone carriers being susceptible to dissolution in chloroform.
Successfil
Successfil (Coltène/Whaledent) is a carrier-based system associated with Ultrafil 3D (Fig. 10-50); however, the gutta-percha used in this technique comes in a syringe. Carriers (titanium or radiopaque plastic) are inserted into the syringe to the measured length of the canal. The gutta-percha is expressed on the carrier, with the amount and shape determined by the rate of withdrawal from the syringe. Sealer is lightly coated on the canal walls, and the carrier with gutta-percha is placed in the canal to the prepared length. The gutta-percha can be compacted around the carrier with various pluggers depending on the canal morphology. This is followed by severing of the carrier slightly above the orifice with a bur.
SimpliFill
SimpliFill (LightSpeed Technology, San Antonio, TX/Discus Dental) is gutta-percha or Resilon manufactured for use after canal preparation with LightSpeed instruments (Fig. 10-51). The carrier has an apical plug with 5 mm of gutta-percha. The technique involves fitting a carrier that is consistent with the master apical rotary file (LightSpeed Technology/Discus Dental) to within 1 to 3 mm of the prepared length (Fig. 10-52). The apical gutta-percha plug can be modified by clipping the end in 1-mm increments to obtain an appropriate fit if the plug is too small. Once the cone is fitted it is withdrawn and sealer is applied to the canal walls. AH Plus is recommended. The SimpliFill carrier is slowly advanced to the prepared length. This may require firm pressure. With the plug at the corrected working length the handle is quickly rotated a minimum of four complete terms in a counterclockwise direction to separate the shaft from the apical gutta-percha. The coronal space can then be filled with gutta-percha, using lateral compaction or the warm thermoplastic technique. When using lateral compaction it is recommended that the first cone be the same size as the SimpliFill carrier. This sectional technique is efficient, and leakage potential is similar to that of other common techniques.257
Thermomechanical Compaction
McSpadden introduced an instrument, the McSpadden Compactor, with flutes similar to a Hedström file but in reverse. When activated in a slow-speed handpiece the instrument would generate friction, soften the gutta-percha, and move it apically. Rotary compactors similar in design have been developed and advocated. To increase flexibility the instrument is available in nickel–titanium.
The technique requires fitting a master cone short of the prepared length and applying sealer. A compactor is selected on the basis of the size of the canal and inserted alongside the gutta-percha cone 3 to 4 mm from the prepared length. The handpiece is activated and the gutta-percha is heated by the friction of the rotating bur. The pliable mass is compacted apically and laterally as the device is withdrawn from the canal.
Advantages include simplicity of the armamentarium, the ability to fill canal irregularities,128,163,191,261,262 and time. Disadvantages include possible extrusion of material, instrument fracture,218 gouging of the canal walls, the inability to use the technique in curved canals, and heat generation.23,102,126,199,262,263 Microseal condensers (SybronEndo) are a variation of this product.
Solvent Techniques
Gutta-percha can be plasticized with solvents such as chloroform, eucalyptol, and xylol. Disadvantages include shrinkage caused by evaporation, voids, the inability to control the obturating material, and irritation of periradicular tissues. The Callahan and Johnston technique involved dissolving gutta-percha in chloroform and placing the mixture into the canal with a syringe.52 A gutta-percha cone was then softened and placed into the canal; the mass hardened as the solvent evaporated. Unfortunately, shrinkage occurred with the evaporation process.241 The techniques using solvents have been abandoned and replaced with materials and methods that do not exhibit shrinkage.
Pastes
Pastes fulfill some of the criteria outlined by Grossman115 and can adapt to the complex internal canal anatomy; however, the flow characteristic can result in extrusion or incomplete obturation. The inability to control the material is a distinct disadvantage, and when extrusion occurs it can be corrected only by surgical intervention. In addition, pastes are sometimes used as a substitute for complete cleaning and shaping procedures, and the addition of paraformaldehyde results in severe toxicity.
Immediate Obturation
Apical barriers may be necessary in cases with immature apical development, cases with external apical root resorption, and cases where instrumentation extends beyond the confines of the root. Dentin chips, calcium hydroxide, demineralized dentin, lyophilized bone, tricalcium phosphate, hydroxyapatite, and collagen have been advocated for placement as a barrier in canals exhibiting an open apex. The barriers are designed to permit obturation without extrusion of materials into the periradicular tissues but are often incomplete and do not seal the canal.255
Dentin chips appear to confine materials to the canal space during instrumentation/obturation and may encourage development of a biologic seal.94,255 Enhanced healing, minimal inflammation, and apical cementum deposition have been noted histologically.224 A concern with this technique is contamination of the dentin with bacteria, because investigators found that infected dentin adversely affected healing.139
Calcium hydroxide has also been extensively used as a common apical barrier. Calcium hydroxide has been shown to induce an apical barrier in apexification procedures. Calcifications similar to dentin plugs have been noted at the apical foramen.239 Calcium hydroxide has the advantage of being free of bacterial contamination and may provide a better, although imperfect, apical seal.330
Immature teeth exhibiting pulp necrosis or teeth with apical resorption traditionally were treated with calcium hydroxide to establish an apical barrier (apexification) before obturation. Studies have demonstrated that teeth treated with calcium hydroxide for prolonged periods are more susceptible to fracture.9,251 Immediate obturation is an alternative to apexification. An apical barrier material should confine obturation materials to the canal space94 and enhance healing by inducing cementum and bone formation.137,224,239 Mineral trioxide aggregate (MTA) (ProRoot MTA; DENTSPLY Tulsa Dental Specialties) has been successfully employed as an apical barrier material before obturation (Fig. 10-53).279

FIG. 10-53 Mineral trioxide aggregate is available as ProRoot MTA. This material is advocated for use in apexification, repair of root perforations, repair of root resorption, root-end fillings, and pulp capping.
(Courtesy DENTSPLY Tulsa Dental Specialties, Tulsa, OK. All rights owned by and used with permission from DENTSPLY International Inc.)
After the cleaning and shaping procedures the canal is dried and MTA is placed. The material is compacted into the apical portion of the root to form a barrier. After the material sets, gutta-percha can then be compacted without extrusion (Fig. 10-54). Other investigators found that hand compaction of MTA provided better adaptation to the canal walls with fewer voids when compared with ultrasonic placement.8 Another investigation reported that ultrasonic placement of a 4-mm apical plug of MTA improved the seal352 and that placement of a composite resin as the obturation material enhanced the seal and strengthened the root.178
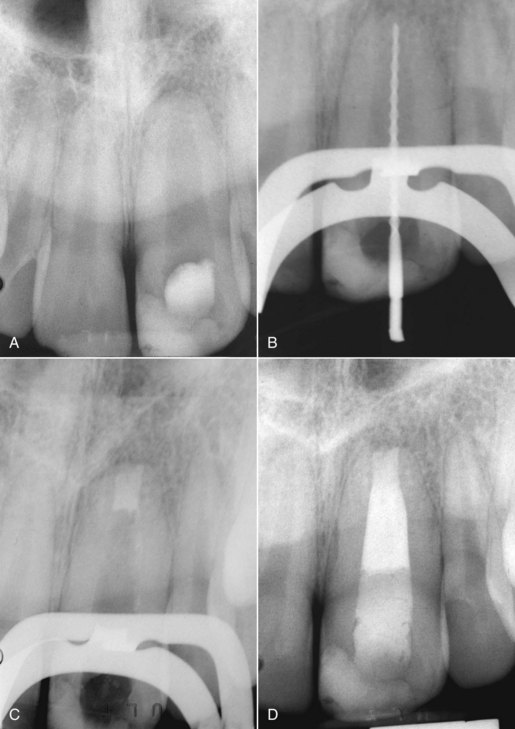
FIG. 10-54 Immediate obturation employs a barrier technique to prevent extrusion when the apex is open. This case involves a maxillary left central incisor with pulp necrosis caused by trauma. A, Pretreatment radiograph demonstrates a large canal with an open apex. B, Working length is established and the canal is prepared. C, Mineral trioxide plug is placed. D, The canal is obturated with gutta-percha.
MTA is sterile, biocompatible, and capable of inducing hard tissue formation.92,140 The technique has been shown to be clinically successful and can be accomplished quickly, eliminating the need for numerous visits and possible coronal recontamination during the months required for apexification.110,185
Clinical evidence supports this technique. In a study comparing calcium hydroxide with mineral trioxide aggregate in 15 children, each having paired immature teeth with necrotic pulps, two teeth treated with Ca(OH)2 exhibited pathosis on recall examination whereas all the teeth treated with MTA were clinically and radiographically successful.93 A prospective clinical study found the success rate of MTA barriers and immediate obturation in 43 cases to be 81%.286 In another study, 85% of 20 teeth with immature apical development were considered healed and 5% were considered healing after immediate barrier placement.136 The time required for healing is comparable to traditional apexification with Ca(OH)2 and treatment time is reduced.240
Another option for treating immature teeth with pulp necrosis involves regenerative techniques including revascularization (see also Chapter 16).127,317 Advantages include continued increasing thickness of the canal walls, apical root development, and apical closure.280 The technique involves copious irrigation, minimal canal preparation, and the use of an antibiotic paste as an interim medication. At a subsequent visit bleeding is induced in the canal to induce a clot that is covered with MTA. When the MTA is set a definitive restoration can be placed to ensure a coronal seal.14
Coronal Orifice Seal
No matter which technique is used to obturate the canals, coronal microleakage can occur within a short time through seemingly well-obturated canals,56,314 potentially causing infection of the periapical area. Early research efforts focused on the quality of the seal in the apical part of the root canal system to prevent percolation of apical fluids. However, contemporary research efforts have identified the importance of maintaining a coronal seal to prevent bacterial leakage.149 Leakage studies indicate that the coronal seal can be enhanced by the application of supplemental restorative materials over the canal orifice237,250,331 and by placing a definitive coronal restoration as soon as is feasible.244
Cavit (Premier Dental Products, Plymouth Meeting, 3M Espe Dental Products) has traditionally been advocated as an acceptable material. One study demonstrated that placement of 3.5 mm of Cavit or SuperEBA cement (Bosworth, Skokie, IL) decreased bacterial leakage by 85% and 65%, respectively, when compared with unsealed controls, which all leaked at 45 days.237 In an animal study in which the access openings were left open for 8 months, placing a dentin-bonded composite resin or IRM in the orifice decreased periapical inflammation from 89% for teeth without orifice plugs to 39% with plugs.350 Another study demonstrated in a dog model that MTA placed in the orifice decreased inflammation in teeth inoculated with bacteria.194
Another method to retard leakage through the obturated canal, should failure of the coronal restoration occur, is to cover the floor of the pulp chamber with a lining of bonded material after removal of excess gutta-percha and sealer to the canal oriface.26,58,181 This results in the formation of a hybrid layer with microtags of resin in the tubules. A resin-modified glass ionomer cement is placed approximately 1 mm thick over the floor of the pulp chamber and polymerized with a curing light. Investigators found that this procedure resulted in none of the experimental canals showing bacterial leakage at 60 days.60
1. Aguirre AM, el-Deeb ME, Aguirre R. The effect of ultrasonics on sealer distribution and sealing of root canals. J Endod. 1997;23:759.
2. Ainley JE. Fluorometric assay of the apical seal of root canal fillings. Oral Surg Oral Med Oral Pathol. 1970;29:753.
3. Aktener BO, Cengiz T, Piskin B. The penetration of smear material into dentinal tubules during instrumentation with surface-active reagents: a scanning electron microscopic study. J Endod. 1989;15:588.
4. al-Khatib ZZ, Baum RH, Morse DR, Yesilsoy C, Bhambhani S, Furst ML. The antimicrobial effect of various endodontic sealers. Oral Surg Oral Med Oral Pathol. 1990;70:784.
5. Alicia Karr N, Baumgartner JC, Marshall JG. A comparison of gutta-percha and Resilon in the obturation of lateral grooves and depressions. J Endod. 2007;33:749.
6. Allan NA, Walton RC, Schaeffer MA. Setting times for endodontic sealers under clinical usage and in vitro conditions. J Endod. 2001;27:421.
7. Allison DA, Michelich RJ, Walton RE. The influence of master cone adaptation on the quality of the apical seal. J Endod. 1981;7:61.
8. Aminoshariae A, Hartwell GR, Moon PC. Placement of mineral trioxide aggregate using two different techniques. J Endod. 2003;29:679.
9. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134.
10. FDA explains status of N2 material Anonymous J Am Dent Assoc 123 1992 236
11. Aqrabawi JA. Outcome of endodontic treatment of teeth filled using lateral condensation versus vertical compaction (Schilder’s technique). J Contemp Dent Pract. 2006;7:17.
12. Aquilino SA, Caplan DJ. Relationship between crown placement and the survival of endodontically treated teeth. J Prosthet Dent. 2002;87:256.
13. Augsburger RA, Peters DD. Radiographic evaluation of extruded obturation materials. J Endod. 1990;16:492.
14. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196.
15. Barkhordar RA. Evaluation of antimicrobial activity in vitro of ten root canal sealers on Streptococcus sanguis and Streptococcus mutans. Oral Surg Oral Med Oral Pathol. 1989;68:770.
16. Barkhordar RA, Watanabe LG, Marshall GW, Hussain MZ. Removal of intracanal smear by doxycycline in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:420.
17. Barthel CR, Zimmer S, Trope M. Relationship of radiologic and histologic signs of inflammation in human root-filled teeth. J Endod. 2004;30:75.
18. Baumgardner KR, Krell KV. Ultrasonic condensation of gutta-percha: an in vitro dye penetration and scanning electron microscopic study. J Endod. 1990;16:253.
19. Baumgartner G, Zehnder M, Paqué F. Enterococcus faecalis type strain leakage through root canals filled with gutta-percha/AH Plus or Resilon/Epiphany. J Endod. 2007;33:45.
20. Baumgartner JC, Brown CM, Mader CL, Peters DD, Shulman JD. A scanning electron microscopic evaluation of root canal debridement using saline, sodium hypochlorite, and citric acid. J Endod. 1984;10:525.
21. Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18:605.
22. Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13:147.
23. Beatty RG, Vertucci FJ, Hojjatie B. Thermomechanical compaction of gutta-percha: effect of speed and duration. Int Endod J. 1988;21:367.
24. Beatty RG, Zakariasen KL. Apical leakage associated with three obturation techniques in large and small root canals. Int Endod J. 1984;17:67.
25. Behrend GD, Cutler CW, Gutmann JL. An in-vitro study of smear layer removal and microbial leakage along root-canal fillings. Int Endod J. 1996;29:99.
26. Belli S, Zhang Y, Pereira PN, Pashley DH. Adhesive sealing of the pulp chamber. J Endod. 2001;27:521.
27. Bergenholtz G, Lekholm U, Milthon R, Heden G, Odesjo B, Engstrom B. Retreatment of endodontic fillings. Scand J Dent Res. 1979;87:217.
28. Berry KA, Loushine RJ, Primack PD, Runyan DA. Nickel–titanium versus stainless-steel finger spreaders in curved canals. J Endod. 1998;24:752.
29. Beyer-Olsen EM, Orstavik D. Radiopacity of root canal sealers. Oral Surg Oral Med Oral Pathol. 1981;51:320.
30. Blaskovic-Subat V, Maricic B, Sutalo J. Asymmetry of the root canal foramen. Int Endod J. 1992;25:158.
31. Block RM, Lewis RD, Hirsch J, Coffey J, Langeland K. Systemic distribution of N2 paste containing 14C paraformaldehyde following root canal therapy in dogs. Oral Surg Oral Med Oral Pathol. 1980;50:350.
32. Blum JY, Esber S, Micallef JP. Analysis of forces developed during obturations: comparison of three gutta-percha techniques. J Endod. 1997;23:340.
33. Blum JY, Parahy E, Machtou P. Warm vertical compaction sequences in relation to gutta-percha temperature. J Endod. 1997;23:307.
34. Bodrumlu E, Muglali M, Sumer M, Guvenc T. The response of subcutaneous connective tissue to a new endodontic filling material. J Biomed Mater Res B Appl Biomater. 2008;84:463.
35. Boiesen J, Brodin P. Neurotoxic effect of two root canal sealers with calcium hydroxide on rat phrenic nerve in vitro. Endod Dent Traumatol. 1991;7:242.
36. Bouillaguet S, Wataha JC, Tay FR, Brackett MG, Lockwood PE. Initial in vitro biological response to contemporary endodontic sealers. J Endod. 2006;32:989.
37. Bowman CJ, Baumgartner JC. Gutta-percha obturation of lateral grooves and depressions. J Endod. 2002;28:220.
38. Brackett MG, Martin R, Sword J, et al. Comparison of seal after obturation techniques using a polydimethylsiloxane-based root canal sealer. J Endod. 2006;32:1188.
39. Brady JM, del Rio CE. Corrosion of endodontic silver cones in humans: a scanning electron microscope and x-ray microprobe study. J Endod. 1975;1:205.
40. Brannstrom M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984;3:35.
41. Briseno BM, Willerhausen B. Root canal sealer toxicity on human gingival fibroblasts. III Calcium hydroxide–based sealers. J Endod. 1992;18:110.
42. Briseno BM, Willershausen B. Root canal sealer cytotoxicity on human gingival fibroblasts. I. Zinc oxide–eugenol-based sealers. J Endod. 1990;16:383.
43. Briseno BM, Willershausen B. Root canal sealer cytotoxicity on human gingival fibroblasts. II. Silicone- and resin-based sealers. J Endod. 1991;17:537.
44. Briseno Marroquin B, Wolter D, Willershausen-Zonnchen B. Dimensional variability of nonstandardized greater taper finger spreaders with matching gutta-percha points. Int Endod J. 2001;34:23.
45. Brodin P, Roed A, Aars H, Orstavik D. Neurotoxic effects of root filling materials on rat phrenic nerve in vitro. J Dent Res. 1982;61:1020.
46. Buchanan L. Continuous wave of condensation technique. Endod Prac. 1998;1:7.
47. Buckley M, Spangberg LS. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:92.
48. Burch JG, Hulen S. The relationship of the apical foramen to the anatomic apex of the tooth root. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1972;34:262.
49. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35.
50. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321.
51. Cailleteau JG, Mullaney TP. Prevalence of teaching apical patency and various instrumentation and obturation techniques in United States dental schools. J Endod. 1997;23:394.
52. Callahan JR. Rosin solution for the sealing of the dental tubuli as an adjuvant in the filling of root canals. J Allied Dent Soc. 1914;9:110.
53. Calt S, Serper A. Smear layer removal by EGTA. J Endod. 2000;26:459.
54. Cameron JA. The synergistic relationship between ultrasound and sodium hypochlorite: a scanning electron microscope evaluation. J Endod. 1987;13:541.
55. Card SJ, Sigurdsson A, Orstavik D, Trope M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J Endod. 2002;28:779.
56. Carratu P, Amato M, Riccitiello F, Rengo S. Evaluation of leakage of bacteria and endotoxins in teeth treated endodontically by two different techniques. J Endod. 2002;28:272.
57. Castelli WA, Caffesse RG, Pameijer CH, Diaz-Perez R, Farquhar J. Periodontium response to a root canal condensing device (Endotec). Oral Surg Oral Med Oral Pathol. 1991;71:333.
58. Chailertvanitkul P, Saunders WP, MacKenzie D. Coronal leakage in teeth root-filled with gutta-percha and two different sealers after long-term storage. Endod Dent Traumatol. 1997;13:82.
59. Chailertvanitkul P, Saunders WP, MacKenzie D. The effect of smear layer on microbial coronal leakage of gutta-percha root fillings. Int Endod J. 1996;29:242.
60. Chailertvanitkul P, Saunders WP, Saunders EM, MacKenzie D. An evaluation of microbial coronal leakage in the restored pulp chamber of root-canal treated multirooted teeth. Int Endod J. 1997;30:318.
61. Chapman C. A microscopic study of the apical region of human anterior teeth. J Br Endod Soc. 1969;3:52.
62. Chen SC, Chueh LH, Hsiao CK, Tsai MY, Ho SC, Chiang CP. An epidemiologic study of tooth retention after nonsurgical endodontic treatment in a large population in Taiwan. J Endod. 2007;33:226.
63. Chogle S, Mickel AK, Huffaker SK, Neibaur B. An in vitro assessment of iodoform gutta-percha. J Endod. 2005;31:814.
64. Clark-Holke D, Drake D, Walton R, Rivera E, Guthmiller JM. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J Dent. 2003;31:275.
65. Clinton K, Van Himel T. Comparison of a warm gutta-percha obturation technique and lateral condensation. J Endod. 2001;27:692.
66. Cobankara FK, Adanr N, Belli S. Evaluation of the influence of smear layer on the apical and coronal sealing ability of two sealers. J Endod. 2004;30:406.
67. Cohler CM, Newton CW, Patterson SS, Kafrawy AH. Studies of Sargenti’s technique of endodontic treatment: short-term response in monkeys. J Endod. 1980;6:473.
68. Collins J, Walker MP, Kulild J, Lee C. A comparison of three gutta-percha obturation techniques to replicate canal irregularities. J Endod. 2006;32:762.
69. Conner DA, Caplan DJ, Teixeira FB, Trope M. Clinical outcome of teeth treated endodontically with a nonstandardized protocol and root filled with Resilon. J Endod. 2007;33:1290.
70. Coolidge ED. Anatomy of root apex in relation to the treatment problems. J Am Dent Assoc. 1929;16:1456.
71. Cotton TP, Schindler WG, Schwartz SA, Watson WR, Hargreaves KM. A retrospective study comparing clinical outcomes after obturation with Resilon/Epiphany or gutta-percha/Kerr sealer. J Endod. 2008;34:789.
72. Cunningham WT, Martin H. A scanning electron microscope evaluation of root canal debridement with the endosonic ultrasonic synergistic system. Oral Surg Oral Med Oral Pathol. 1982;53:527.
73. Da Silva D, Endal U, Reynaud A, Portenier I, Orstavik D, Haapasalo M. A comparative study of lateral condensation, heat-softened gutta-percha, and a modified master cone heat-softened backfilling technique. Int Endod J. 2002;35:1005.
74. Dang DA, Walton RE. Vertical root fracture and root distortion: effect of spreader design. J Endod. 1989;15:294.
75. Davis MC, Walton RE, Rivera EM. Sealer distribution in coronal dentin. J Endod. 2002;28:464.
76. de Chevigny C, Dao TT, Basrani BR, et al. Treatment outcome in endodontics: the Toronto study—phase 4: initial treatment. J Endod. 2008;34:258.
77. de Deus G, Gurgel Filho ED, Ferreira CM, Coutinho Filho T. [Intratubular penetration of root canal sealers]. Pesquisa Odontologica Brasileira [Braz Oral Res]. 2002;16:332.
78. De Deus GA, Gurgel-Filho ED, Maniglia-Ferreira C, Coutinho-Filho T. The influence of filling technique on depth of tubule penetration by root canal sealer: a study using light microscopy and digital image processing. Aust Endod J. 2004;30:23.
79. De Moor RJ, De Bruyne MA. The long-term sealing ability of AH 26 and AH Plus used with three gutta-percha obturation techniques. Quintessence Int. 2004;35:326.
80. Deitch AK, Liewehr FR, West LA, Patton WR. A comparison of fill density obtained by supplementing cold lateral condensation with ultrasonic condensation. J Endod. 2002;28:665.
81. Delivanis PD, Mattison GD, Mendel RW. The survivability of F43 strain of Streptococcus sanguis in root canals filled with gutta-percha and Procosol cement. J Endod. 1983;9:407.
82. Diamond A, Carrel R. The smear layer: a review of restorative progress. J Pedod. 1984;8:219.
83. Dow PR, Ingle JI. Isotope determination of root canal failure. Oral Surg Oral Med Oral Pathol. 1955;8:1100.
84. Drake DR, Wiemann AH, Rivera EM, Walton RE. Bacterial retention in canal walls in vitro: effect of smear layer. J Endod. 1994;20:78.
85. DuLac KA, Nielsen CJ, Tomazic TJ, Ferrillo PJJr, Hatton JF. Comparison of the obturation of lateral canals by six techniques. J Endod. 1999;25:376.
86. Dummer PM, Kelly T, Meghji A, Sheikh I, Vanitchai JT. An in vitro study of the quality of root fillings in teeth obturated by lateral condensation of gutta-percha or Thermafil obturators. Int Endod J. 1993;26:99.
87. Dummer PM, Lyle L, Rawle J, Kennedy JK. A laboratory study of root fillings in teeth obturated by lateral condensation of gutta-percha or Thermafil obturators. Int Endod J. 1994;27:32.
88. Dummer PM, McGinn JH, Rees DG. The position and topography of the apical canal constriction and apical foramen. Int Endod J. 1984;17:192.
89. Dwan JJ, Glickman GN. 2-D photoelastic stress analysis of NiTi and stainless steel finger spreaders during lateral compaction. J Endod. 1995;21:221.
90. Ebert J, Pawlick H, Petschelt A. Relation berween dye penetration and radiographic assessment of root canal fillings in vitro. Int Endod J. 1996;29:198.
91. Economides N, Liolios E, Kolokuris I, Beltes P. Long-term evaluation of the influence of smear layer removal on the sealing ability of different sealers. J Endod. 1999;25:123.
92. Economides N, Pantelidou O, Kokkas A, Tziafas D. Short-term periradicular tissue response to mineral trioxide aggregate (MTA) as root-end filling material. Int Endod J. 2003;36:44.
93. El-Meligy OA, Avery DR. Comparison of apexification with mineral trioxide aggregate and calcium hydroxide. Pediatr Dent. 2006;28:248.
94. ElDeeb ME, Thuc-Quyen NT, Jensen JR. The dentinal plug: its effect on confining substances to the canal and on the apical seal. J Endod. 1983;9:355.
95. Eldeniz AU, Mustafa K, Orstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329.
96. American Association of Endodontists Guide to clinical endodontics vol. 4 2004 American Association of Endodontists Chicago
97. Erisen R, Yucel T, Kucukay S. Endomethasone root canal filling material in the mandibular canal: a case report. Oral Surg Oral Med Oral Pathol. 1989;68:343.
98. Fabricius L, Dahlen G, Sundqvist G, Happonen RP, Moller AJ. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur J Oral Sci. 2006;114:278.
99. Farzaneh M, Abitbol S, Friedman S. Treatment outcome in endodontics: the Toronto Study. Phases I and II: orthograde retreatment. J Endod. 2004;30:627.
100. Farzaneh M, Abitbol S, Lawrence HP, Friedman S. Treatment outcome in endodontics—the Toronto Study. Phase II: initial treatment. J Endod. 2004;30:302.
101. Floren JW, Weller RN, Pashley DH, Kimbrough WF. Changes in root surface temperatures with in vitro use of the system B HeatSource. J Endod. 1999;25:593.
102. Fors U, Jonasson E, Berquist A, Berg JO. Measurements of the root surface temperature during thermo-mechanical root canal filling in vitro. Int Endod J. 1985;18:199.
103. Fransen JN, He J, Glickman GN, Rios A, Shulman JD, Honeyman A. Comparative assessment of Activ GP/glass ionomer sealer, Resilon/Epiphany, and gutta-percha/AH Plus obturation: a bacterial leakage study. J Endod. 2008;34:725.
104. Friedman CE, Sandrik JL, Heuer MA, Rapp GW. Composition and physical properties of gutta-percha endodontic filling materials. J Endod. 1977;3:304.
105. Friedman S, Lost C, Zarrabian M, Trope M. Evaluation of success and failure after endodontic therapy using a glass ionomer cement sealer. J Endod. 1995;21:384.
106. Gencoglu N, Samani S, Gunday M. Dentinal wall adaptation of thermoplasticized gutta-percha in the absence or presence of smear layer: a scanning electron microscopic study. J Endod. 1993;19:558.
107. Genet JM, Hart AA, Wesselink PR, Thoden van Velzen SK. Preoperative and operative factors associated with pain after the first endodontic visit. Int Endod J. 1987;20:53.
108. Gettleman BH, Messer HH, ElDeeb ME. Adhesion of sealer cements to dentin with and without the smear layer. J Endod. 1991;17:15.
109. Gilhooly RM, Hayes SJ, Bryant ST, Dummer PM. Comparison of lateral condensation and thermomechanically compacted warm alpha-phase gutta-percha with a single cone for obturating curved root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:89.
110. Giuliani V, Baccetti T, Pace R, Pagavino G. The use of MTA in teeth with necrotic pulps and open apices. Dent Traumatol. 2002;18:217.
111. Goldberg F, Gurfinkel J, Spielberg C. Microscopic study of standardized gutta-percha points. Oral Surg Oral Med Oral Pathol. 1979;47:275.
112. Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha. II. The history and molecular chemistry of gutta-percha. Oral Surg Oral Med Oral Pathol. 1974;37:954.
113. Granchi D, Stea S, Ciapetti G, Cavedagna D, Pizzoferrato A. Endodontic cements induce alterations in the cell cycle of in vitro cultured osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:359.
114. Grande NM, Plotino G, Lavorgna L, et al. Influence of different root canal-filling materials on the mechanical properties of root canal dentin. J Endod. 2007;33:859.
115. Grossman L. Endodontics, 11 ed. Philadelphia: Lea & Febiger; 1988.
116. Grossman L. An improved root canal cement. J Am Dent Assoc. 1958;56:381.
117. Grove CJ. Why root canals should be filled to the dentinocemental junction. J Am Dent Assoc. 1931;18:314.
118. Grove CJ. Why root canals should be filled to the dentinocemental junction. J Am Dent Assoc. 1930;17:293.
119. Guignes P, Faure J, Maurette A. Relationship between endodontic preparations and human dentin permeability measured in situ. J Endod. 1996;22:60.
120. Gutierrez JH, Aguayo P. Apical foraminal openings in human teeth: number and location. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:769.
121. Gutierrez JH, Brizuela C, Villota E. Human teeth with periapical pathosis after overinstrumentation and overfilling of the root canals: a scanning electron microscopic study. Int Endod J. 1999;32:40.
122. Gutmann JH, Hovland EJ. Problems in root canal obturation. In: Gutmann J, Dumsha T, Lovdahl P, Hovland E, editors. Problem Solving in Endodontics. ed 2. St. Louis, MO: Mosby; 1997:92-116.
123. Gutmann JL. Adaptation of injected thermoplasticized gutta-percha in the absence of the dentinal smear layer. Int Endod J. 1993;26:87.
124. Gutmann JL. Clinical, radiographic, and histologic perspectives on success and failure in endodontics. Dent Clin North Am. 1992;36:379.
125. Hall MC, Clement DJ, Dove SB, Walker WAIII. A comparison of sealer placement techniques in curved canals. J Endod. 1996;22:638.
126. Hardie EM. Further studies on heat generation during obturation techniques involving thermally softened gutta-percha. Int Endod J. 1987;20:122.
127. Hargreaves KM, Giesler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:S51.
128. Harris GZ, Dickey DJ, Lemon RR, Luebke RG. Apical seal: McSpadden vs lateral condensation. J Endod. 1982;8:273.
129. Hatton EH. Changes produced in the pulp and periapical regions, and their relationship to pulp-canal treatment and to systemic disease. Dental Cosmos. 1924;66:1183.
130. Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 2. Root-canal-filling materials. Int Endod J. 2003;36:147.
131. Haznedaroglu F, Ersev H. Tetracycline HCl solution as a root canal irrigant. J Endod. 2001;27:738.
132. Heard F, Walton RE. Scanning electron microscope study comparing four root canal preparation techniques in small curved canals. Int Endod J. 1997;30:323.
133. Heling I. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod. 1996;22:257.
134. Heling I, Gorfil C, Slutzky H, Kopolovic K, Zalkind M, Slutzky-Goldberg I. Endodontic failure caused by inadequate restorative procedures: review and treatment recommendations. J Prosthet Dent. 2002;87:674.
135. Hembrough MW, Steiman HR, Belanger KK. Lateral condensation in canals prepared with nickel titanium rotary instruments: an evaluation of the use of three different master cones. J Endod. 2002;28:516.
136. Holden DT, Schwartz SA, Kirkpatrick TC, Schindler WG. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J Endod. 2008;34:812.
137. Holland GR. Periapical response to apical plugs of dentin and calcium hydroxide in ferret canines. J Endod. 1984;10:71.
138. Holland R, de Souza V. Ability of a new calcium hydroxide root canal filling material to induce hard tissue formation. J Endod. 1985;11:535.
139. Holland R, de Souza V, Murata SS, et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J. 2001;12:109.
140. Holland R, de Souza V, Nery MJ, Otoboni Filho JA, Bernabe PF, Dezan Junior E. Reaction of dogs’ teeth to root canal filling with mineral trioxide aggregate or a glass ionomer sealer. J Endod. 1999;25:728.
141. Hottel TL, el-Refai NY, Jones JJ. A comparison of the effects of three chelating agents on the root canals of extracted human teeth. J Endod. 1999;25:716.
142. Hugh CL, Walton RE, Facer SR. Evaluation of intracanal sealer distribution with 5 different obturation techniques. Quintessence Int. 2005;36:721.
143. Hulsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003;36:810.
144. Hunter W. The role of sepsis and antisepsis in medicine. Lancet. 1911:79.
145. Ingle JI, Beveridge E, Glick D, Weichman J. The Washington Study. In: Ingle I, Taintor JF, editors. Endodontics. Philadelphia: Lea & Febiger; 1994:1-53.
146. Iqbal MK, Ku J. Instrumentation and obturation of the apical third of root canals: addressing the forgotten dimension. Compend Contin Educ Dent. 2007;28:314.
147. Jacobsen EL, BeGole EA. A comparison of four root canal obturation methods employing gutta-percha: a computerized analysis of the internal structure. Endod Dent Traumatol. 1992;8:206.
148. Jasper E. Adaptation and tolerance of silver point canal filling. J Dent Res. 1941;4:355.
149. Jenkins S, Kulild J, Williams K, Lyons W, Lee C. Sealing ability of three materials in the orifice of root canal systems obturated with gutta-percha. J Endod. 2006;32:225.
150. Johnson BT, Bond MS. Leakage associated with single or multiple increment backfill with the Obtura II gutta-percha system. J Endod. 1999;25:613.
151. Johnson WT, Zakariasen KL. Spectrophotometric analysis of microleakage in the fine curved canals found in the mesial roots of mandibular molars. Oral Surg Oral Med Oral Pathol. 1983;56:305.
152. Joyce AP, Loushine RJ, West LA, Runyan DA, Cameron SM. Photoelastic comparison of stress induced by using stainless-steel versus nickel–titanium spreaders in vitro. J Endod. 1998;24:714.
153. Jung IY, Lee SB, Kim ES, Lee CY, Lee SJ. Effect of different temperatures and penetration depths of a System B plugger in the filling of artificially created oval canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:453.
154. Kaffe I, Tamse A, Littner MM, Schwartz I. A radiographic survey of apical root resorption in pulpless permanent teeth. Oral Surg Oral Med Oral Pathol. 1984;58:109.
155. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germfree and conventional laboratory rats. J South Calif Dent Assoc. 1966;34:449.
156. Katebzadeh N, Hupp J, Trope M. Histological periapical repair after obturation of infected root canals in dogs. J Endod. 1999;25:364.
157. Katebzadeh N, Sigurdsson A, Trope M. Radiographic evaluation of periapical healing after obturation of infected root canals: an in vivo study. Int Endod J. 2000;33:60.
158. Kazemi RB, Safavi KE, Spangberg LS. Dimensional changes of endodontic sealers. Oral Surg Oral Med Oral Pathol. 1993;76:766.
159. Keane HC. A century of service to dentistry. Philadelphia: SS White Dental Manufacturing; 1944.
160. Keane KM, Harrington GW. The use of a chloroform-softened gutta-percha master cone and its effect on the apical seal. J Endod. 1984;10:57.
161. Kerezoudis NP, Valavanis D, Prountzos F. A method of adapting gutta-percha master cones for obturation of open apex cases using heat. Int Endod J. 1999;32:53.
162. Kersten HW. Evaluation of three thermoplasticized gutta-percha filling techniques using a leakage model in vitro. Int Endod J. 1988;21:353.
163. Kersten HW, Fransman R, Thoden van Velzen SK. Thermomechanical compaction of gutta-percha. I. A comparison of several compaction procedures. Int Endod J. 1986;19:125.
164. Kersten HW, Wesselink PR, Thoden van Velzen SK. The diagnostic reliability of the buccal radiograph after root canal filling. Int Endod J. 1987;20:20.
165. Kleier DJ, Averbach RE. Painful dysesthesia of the inferior alveolar nerve following use of a paraformaldehyde-containing root canal sealer. Endod Dent Traumatol. 1988;4:46.
166. Koch CRE, Thorpe BLT a history of dentistry vols. 2 and 3 1909 National Art Publishing Company Fort Wayne, IN
167. Koch MJ. Formaldehyde release from root-canal sealers: influence of method. Int Endod J. 1999;32:10.
168. Kokkas AB, Boutsioukis A, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod. 2004;30:100.
169. Kontakiotis EG, Tzanetakis GN, Loizides AL. A 12-month longitudinal in vitro leakage study on a new silicon-based root canal filling material (Gutta-Flow). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:854.
170. Kontakiotis EP. pH of root canal sealers containing calcium hydroxide. Int Endod J. 1996;29:202.
171. Kouvas V, Liolios E, Vassiliadis L, Parissis-Messimeris S, Boutsioukis A. Influence of smear layer on depth of penetration of three endodontic sealers: an SEM study. Endod Dent Traumatol. 1998;14:191.
172. Krell KV, Johnson RJ, Madison S. Irrigation patterns during ultrasonic canal instrumentation. I. K-type files. J Endod. 1988;14:65.
173. Kulild J, Lee C, Dryden J, Collins J, Feil P. A comparison of 5 gutta-percha obturation techniques to replicate canal defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e28.
174. Kuttler Y. Microscopic investigation of root apexes. J Am Dent Assoc. 1955;50:544.
175. Kytridou V, Gutmann JL, Nunn MH. Adaptation and sealability of two contemporary obturation techniques in the absence of the dentinal smear layer. Int Endod J. 1999;32:464.
176. Langeland K. Root canal sealants and pastes. Dent Clin North Am. 1974;18:309.
177. Langeland K, Liao K, Pascon EA. Work-saving devices in endodontics: efficacy of sonic and ultrasonic techniques. J Endod. 1985;11:499.
178. Lawley GR, Schindler WG, Walker WAIII, Kolodrubetz D. Evaluation of ultrasonically placed MTA and fracture resistance with intracanal composite resin in a model of apexification. J Endod. 2004;30:167.
179. Lazarski MP, Walker WAIII, Flores CM, Schindler WG, Hargreaves KM. Epidemiological evaluation of the outcomes of nonsurgical root canal treatment in a large cohort of insured dental patients. J Endod. 2001;27:791.
180. Lee FS, Van Cura JE, BeGole E. A comparison of root surface temperatures using different obturation heat sources. J Endod. 1998;24:617.
181. Leonard JE, Gutmann JL, Guo IY. Apical and coronal seal of roots obturated with a dentine bonding agent and resin. Int Endod J. 1996;29:76.
182. Leonardo MR, Bezerra da Silva LA, Filho MT, Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221.
183. Lertchirakarn V, Palamara JE, Messer HH. Load and strain during lateral condensation and vertical root fracture. J Endod. 1999;25:99.
184. Lertchirakarn V, Palamara JE, Messer HH. Patterns of vertical root fracture: factors affecting stress distribution in the root canal. J Endod. 2003;29:523.
185. Levenstein H. Obturating teeth with wide open apices using mineral trioxide aggregate: a case report. SADJ. 2002;57:270.
186. Levitan ME, Himel VT, Luckey JB. The effect of insertion rates on fill length and adaptation of a thermoplasticized gutta-percha technique. J Endod. 2003;29:505.
187. Liewehr FR, Kulild JC, Primack PD. Improved density of gutta-percha after warm lateral condensation. J Endod. 1993;19:489.
188. Lim TS, Wee TY, Choi MY, Koh WC, Sae-Lim V. Light and scanning electron microscopic evaluation of Glyde File Prep in smear layer removal. Int Endod J. 2003;36:336.
189. Loest C, Trope M, Friedman S. Follow-up of root canals obturated with glass ionomer and epoxy resin root canal sealer. J Endod. 1993;19:201.
190. Lohbauer U, Gambarini G, Ebert J, Dasch W, Petschelt A. Calcium release and pH-characteristics of calcium hydroxide plus points. Int Endod J. 2005;38:683.
191. Lugassy AA, Yee F. Root canal obturation with gutta-percha: a scanning electron microscope comparison of vertical compaction and automated thermatic condensation. J Endod. 1982;8:120.
192. Lui JN, Sae-Lim V, Song KP, Chen NN. In vitro antimicrobial effect of chlorhexidine-impregnated gutta percha points on Enterococcus faecalis. Int Endod J. 2004;37:105.
193. Machnick TK, Torabinejad M, Munoz CA, Shabahang S. Effect of MTAD on flexural strength and modulus of elasticity of dentin. J Endod. 2003;29:747.
194. Mah T, Basrani B, Santos JM, et al. Periapical inflammation affecting coronally-inoculated dog teeth with root fillings augmented by white MTA orifice plugs. J Endod. 2003;29:442.
195. Malueg LA, Wilcox LR, Johnson W. Examination of external apical root resorption with scanning electron microscopy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:89.
196. Martin H, Fischer E. Photoelastic stress comparison of warm (Endotec) versus cold lateral condensation techniques. Oral Surg Oral Med Oral Pathol. 1990;70:325.
197. Mayne JR, Shapiro S, Abramson II. An evaluation of standardized gutta-percha points. I. Reliability and validity of standardization. Oral Surg Oral Med Oral Pathol. 1971;31:250.
198. McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1:238.
199. McCullagh JJ, Biagioni PA, Lamey PJ, Hussey DL. Thermographic assessment of root canal obturation using thermomechanical compaction. Int Endod J. 1997;30:191.
200. McCullagh JJ, Setchell DJ, Gulabivala K, et al. A comparison of thermocouple and infrared thermographic analysis of temperature rise on the root surface during the continuous wave of condensation technique. Int Endod J. 2000;33:326.
201. Melker KB, Vertucci FJ, Rojas MF, Progulske-Fox A, Belanger M. Antimicrobial efficacy of medicated root canal filling materials. J Endod. 2006;32:148.
202. Messing JJ. An investigation of the sealing properties of some root filling materials. J Br Endod Soc. 1970;4:18.
203. Mickel AK, Nguyen TH, Chogle S. Antimicrobial activity of endodontic sealers on Enterococcus faecalis. J Endod. 2003;29:257.
204. Mjor IA, Smith MR, Ferrari M, Mannocci F. The structure of dentine in the apical region of human teeth. Int Endod J. 2001;34:346.
205. Moeller AJR. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475.
206. Molander A, Warfvinge J, Reit C, Kvist T. Clinical and radiographic evaluation of one- and two-visit endodontic treatment of asymptomatic necrotic teeth with apical periodontitis: a randomized clinical trial. J Endod. 2007;33:1145.
207. Monticelli F, Sadek FT, Schuster GS, et al. Efficacy of two contemporary single-cone filling techniques in preventing bacterial leakage. J Endod. 2007;33:310.
208. Morgan LA, Baumgartner JC. Demineralization of resected root-ends with methylene blue dye. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:74.
209. Morse DR. The endodontic culture technique: an impractical and unnecessary procedure. Dent Clin North Am. 1971;15:793.
210. Mulhern JM, Patterson SS, Newton CW, Ringel AM. Incidence of postoperative pain after one-appointment endodontic treatment of asymptomatic pulpal necrosis in single-rooted teeth. J Endod. 1982;8:370.
211. Nelson EA, Liewehr FR, West LA. Increased density of gutta-percha using a controlled heat instrument with lateral condensation. J Endod. 2000;26:748.
212. Nencka D, Walia H, Austin BP. Histologic evaluation of the biocompatibility of Diaket. J Dent Res. 1995;74:101.
213. Newton CW, Patterson SS, Kafrawy AH. Studies of Sargenti’s technique of endodontic treatment: six-month and one-year responses. J Endod. 1980;6:509.
214. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature. 2. Influence of clinical factors. Int Endod J. 2008;41:6.
215. Ng YL, Mann V, Rahbaran S, Lewsey J, Gulabivala K. Outcome of primary root canal treatment: systematic review of the literature. 1. Effects of study characteristics on probability of success. Int Endod J. 2007;40:921.
216. Nygaard-Ostby B. Chelation in root canal cleansing and widening of root canals. Odontol Tidskr. 1957;65:3.
217. O’Keefe EM. Pain in endodontic therapy: preliminary study. J Endod. 1976;2:315.
218. O’Neill KJ, Pitts DL, Harrington GW. Evaluation of the apical seal produced by the McSpadden compactor and the lateral condensation with a chloroform-softened primary cone. J Endod. 1983;9:190.
219. Oksan T, Aktener BO, Sen BH, Tezel H. The penetration of root canal sealers into dentinal tubules: a scanning electron microscopic study. Int Endod J. 1993;26:301.
220. Oliet S. Single-visit endodontics: a clinical study. J Endod. 1983;9:147.
221. Orban B. Why root canals should be filled to the dentinocemental junction. J Am Dent Assoc. 1930;17:1086.
222. Orstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142.
223. Oswald RJ, Cohn SA. Systemic distribution of lead from root canal fillings. J Endod. 1975;1:59.
224. Oswald RJ, Friedman CE. Periapical response to dentin filings: a pilot study. Oral Surg Oral Med Oral Pathol. 1980;49:344.
225. Ozok AR, van der Sluis LW, Wu MK, Wesselink PR. Sealing ability of a new polydimethylsiloxane-based root canal filling material. J Endod. 2008;34:204.
226. Pallares A, Faus V, Glickman GN. The adaptation of mechanically softened gutta-percha to the canal walls in the presence or absence of smear layer: a scanning electron microscopic study. Int Endod J. 1995;28:266.
227. Pashley DH. Smear layer: overview of structure and function. Proc Finn Dent Soc. 1992;88:215.
228. Pashley EL, Birdsong NL, Bowman K, Pashley DH. Cytotoxic effects of NaOCl on vital tissue. J Endod. 1985;11:525.
229. Peak JD, Hayes SJ, Bryant ST, Dummer PM. The outcome of root canal treatment: a retrospective study within the armed forces (Royal Air Force). Br Dent J. 2001;190:140.
230. Penesis VA, Fitzgerald PI, Fayad MI, Wenckus CS, BeGole EA, Johnson BR. Outcome of one-visit and two-visit endodontic treatment of necrotic teeth with apical periodontitis: a randomized controlled trial with one-year evaluation. J Endod. 2008;34:251.
231. Peng L, Ye L, Tan H, Zhou X. Outcome of root canal obturation by warm gutta-percha versus cold lateral condensation: a meta-analysis. J Endod. 2007;33:106.
232. Perez F, Rochd T, Lodter JP, Calas P, Michel G. In vitro study of the penetration of three bacterial strains into root dentine. Oral Surg Oral Med Oral Pathol. 1993;76:97.
233. Pérez Heredia M, Clavero González J, Ferrer Luque CM, González Rodríguez MP. Apical seal comparison of low-temperature thermoplasticized gutta-percha technique and lateral condensation with two different master cones. Med Oral Patol Oral Cir Bucal. 2007;12:E175.
234. Perry SG. Preparing and filling the roots of teeth. Dental Cosmos. 1883;25:185.
235. Peters DD. Two-year in vitro solubility evaluation of four gutta-percha sealer obturation techniques. J Endod. 1986;12:139.
236. Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660.
237. Pisano DM, DiFiore PM, McClanahan SB, Lautenschlager EP, Duncan JL. Intraorifice sealing of gutta-percha obturated root canals to prevent coronal microleakage. J Endod. 1998;24:659.
238. Piskin B, Aydin B, Sarikanat M. The effect of spreader size on fracture resistance of maxillary incisor roots. Int Endod J. 2008;41:54.
239. Pitts DL, Jones JE, Oswald RJ. A histological comparison of calcium hydroxide plugs and dentin plugs used for the control of gutta-percha root canal filling material. J Endod. 1984;10:283.
240. Pradhan DP, Chawla HS, Gauba K, Goyal A. Comparative evaluation of endodontic management of teeth with unformed apices with mineral trioxide aggregate and calcium hydroxide. J Dent Child (Chic). 2006;73:79.
241. Price WA. Report of laboratory investigations on the physical properties of root canal filling materials and the efficiency of root canal fillings blocking infection from sterile tooth structure. J Natl Dent Assoc. 1918;5:1260.
242. Raina R, Loushine RJ, Weller RN, Tay FR, Pashley DH. Evaluation of the quality of the apical seal in Resilon/Epiphany and gutta-percha/AH Plus-filled root canals by using a fluid filtration approach. J Endod. 2007;33:944.
243. Rasimick BJ, Shah RP, Musikant BL, Deutsch AS. Radiopacity of endodontic materials on film and a digital sensor. J Endod. 2007;33:1098.
244. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28:12.
245. Rickert U, Dixon C: The control of root surgery. Transactions of the 8th International Dental Congress, Section IIIA, No. 9. 20:1458, 1933.
246. Ricucci D, Bergenholtz G. Bacterial status in root-filled teeth exposed to the oral environment by loss of restoration and fracture or caries—a histobacteriological study of treated cases. Int Endod J. 2003;36:787.
247. Ricucci D, Grondahl K, Bergenholtz G. Periapical status of root-filled teeth exposed to the oral environment by loss of restoration or caries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:354.
248. Ricucci D, Langeland K. Apical limit of root canal instrumentation and obturation. 2. A histological study. Int Endod J. 1998;31:394.
249. Roane JB, Dryden JA, Grimes EW. Incidence of postoperative pain after single- and multiple-visit endodontic procedures. Oral Surg Oral Med Oral Pathol. 1983;55:68.
250. Roghanizad N, Jones JJ. Evaluation of coronal microleakage after endodontic treatment. J Endod. 1996;22:471.
251. Rosenberg B, Murray PE, Namerow K. The effect of calcium hydroxide root filling on dentin fracture strength. Dent Traumatol. 2007;23:26.
252. Rosenow EC. Studies on elective localization: focal infection with special reference to oral sepsis. J Dent Res. 1919;1:205.
253. Sabeti MA, Nekofar M, Motahhary P, Ghandi M, Simon JH. Healing of apical periodontitis after endodontic treatment with and without obturation in dogs. J Endod. 2006;32:628.
254. Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning efficacy of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod. 2003;29:674.
255. Safavi K, Horsted P, Pascon EA, Langeland K. Biological evaluation of the apical dentin chip plug. J Endod. 1985;11:18.
256. Salehrabi R, Rotstein I. Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. J Endod. 2004;30:846.
257. Santos MD, Walker WAIII, Carnes DLJr. Evaluation of apical seal in straight canals after obturation using the Lightspeed sectional method. J Endod. 1999;25:609.
258. Sargenti A. Endodontic course for the general practitioner, ed 3. Bruxelles, Belgium: EES; 1965.
259. Sargenti A. The endodontic debate ends? CDS Rev. 1977;70:28.
260. Sargenti A. The Sargenti N-2 method. Dent Surv. 1978;54:55.
261. Saunders EM. The effect of variation in thermomechanical compaction techniques upon the quality of the apical seal. Int Endod J. 1989;22:163.
262. Saunders EM. In vivo findings associated with heat generation during thermomechanical compaction of gutta-percha. 1. Temperature levels at the external surface of the root. Int Endod J. 1990;23:263.
263. Saunders EM. In vivo findings associated with heat generation during thermomechanical compaction of gutta-percha. 2. Histological response to temperature elevation on the external surface of the root. Int Endod J. 1990;23:268.
264. Scelza MF, Teixeira AM, Scelza P. Decalcifying effect of EDTA-T, 10% citric acid, and 17% EDTA on root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:234.
265. Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha. Part IV. A thermal profile of the warm gutta-percha packing procedure. Oral Surg Oral Med Oral Pathol. 1981;51:544-551.
266. Schilder H. Filling root canals in three dimensions. Dent Clin North Am. 1967;Nov:723.
267. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha. V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg Oral Med Oral Pathol. 1985;59:285.
268. Schmidt KJ, Walker TL, Johnson JD, Nicoll BK. Comparison of nickel–titanium and stainless-steel spreader penetration and accessory cone fit in curved canals. J Endod. 2000;26:42.
269. Segura JJ, Calvo JR, Guerrero JM, Sampedro C, Jimenez A, Llamas R. The disodium salt of EDTA inhibits the binding of vasoactive intestinal peptide to macrophage membranes: endodontic implications. J Endod. 1996;22:337.
270. Seltzer S Endodontology: Biologic Considerations in Endodontic Practice ed 2 1988 Lea & Febiger Philadelphia
271. Seltzer S, Green DB, Weiner N, DeRenzis F. A scanning electron microscope examination of silver cones removed from endodontically treated teeth. Oral Surg Oral Med Oral Pathol. 1972;33:589.
272. Seltzer S, Soltanoff W, Smith J. Biologic aspects of endodontics. V. Periapical tissue reactions to root canal instrumentation beyond the apex and root canal fillings short of and beyond the apex. Oral Surg Oral Med Oral Pathol. 1973;36:725.
273. Sen BH, Piskin B, Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6.
274. Sen BH, Wesselink PR, Turkun M. The smear layer: a phenomenon in root canal therapy. Int Endod J. 1995;28:141.
275. Serper A, Ucer O, Onur R, Etikan I. Comparative neurotoxic effects of root canal filling materials on rat sciatic nerve. J Endod. 1998;24:592.
276. Setlock J, Fayad MI, BeGole E, Bruzick M. Evaluation of canal cleanliness and smear layer removal after the use of the Quantec-E irrigation system and syringe: a comparative scanning electron microscope study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:614.
277. Shabahang S, Pouresmail M, Torabinejad M. In vitro antimicrobial efficacy of MTAD and sodium hypochlorite. J Endod. 2003;29:450.
278. Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis–contaminated root canals of extracted human teeth. J Endod. 2003;29:576.
279. Shabahang S, Torabinejad M. Treatment of teeth with open apices using mineral trioxide aggregate. Pract Periodontics Aesthet Dent. 2000;12:315.
280. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34:919.
281. Shahi S, Zand V, Oskoee SS, Abdolrahimi M, Rahnema AH. An in vitro study of the effect of spreader penetration depth on apical microleakage. J Oral Sci. 2007;49:283.
282. Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007;33:96.
283. Shipper G, Orstavik D, Teixeira FB, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J Endod. 2004;30:342.
284. Shipper G, Teixeira FB, Arnold RR, Trope M. Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or Resilon. J Endod. 2005;31:91.
285. Silver GK, Love RM, Purton DG. Comparison of two vertical condensation obturation techniques: Touch ‘n Heat modified and System B. Int Endod J. 1999;32:287.
286. Simon S, Rilliard F, Berdal A, Machtou P. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J. 2007;40:186.
287. Siqueira JFJr, da Silva CH, Cerqueira M, das D, Lopes HP, de Uzeda M. Effectiveness of four chemical solutions in eliminating Bacillus subtilis spores on gutta-percha cones. Endod Dent Traumatol. 1998;14:124.
288. Siqueira JFJr, Rocas IN. Exploiting molecular methods to explore endodontic infections. 1. Current molecular technologies for microbiological diagnosis. J Endod. 2005;31:411.
289. Siqueira JFJr, Rocas IN. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J Clin Microbiol. 2005;43:3314.
290. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis [erratum appears in Int Endod J 31:148, 1998]. Int Endod J. 1997;30:297.
291. Sjögren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498.
292. Skillen WG. Why root canals should be filled to the dentinocemental junction. J Am Dent Assoc. 1930;17:2082.
293. Smith CS, Setchell DJ, Harty FJ. Factors influencing the success of conventional root canal therapy: a five-year retrospective study. Int Endod J. 1993;26:321.
294. Soltanoff W. A comparative study of the single-visit and the multiple-visit edodontic procedure. J Endod. 1978;4:278.
295. Sonat B, Dalat D, Gunhan O. Periapical tissue reaction to root fillings with Sealapex. Int Endod J. 1990;23:46.
296. Southard DW, Rooney TP. Effective one-visit therapy for the acute periapical abscess. J Endod. 1984;10:580.
297. Spangberg L. Biological effects of root canal filling materials. 7. Reaction of bony tissue to implanted root canal filling material in guinea pigs. Odontologisk Tidskrift. 1969;77:133.
298. Spangberg LS, Barbosa SV, Lavigne GD. AH 26 releases formaldehyde. J Endod. 1993;19:596.
299. Stamos DE, Gutmann JL, Gettleman BH. In vivo evaluation of root canal sealer distribution. J Endod. 1995;21:177.
300. Stewart GG. A scanning electron microscopic study of the cleansing effectiveness of three irrigating modalities on the tubular structure of dentin. J Endod. 1998;24:485.
301. Swartz DB, Skidmore AE, Griffin JAJr. Twenty years of endodontic success and failure. J Endod. 1983;9:198.
302. Sweatman TL, Baumgartner JC, Sakaguchi RL. Radicular temperatures associated with thermoplasticized gutta-percha. J Endod. 2001;27:512.
303. Tagger M, Tagger E, Kfir A. Release of calcium and hydroxyl ions from set endodontic sealers containing calcium hydroxide. J Endod. 1988;14:588.
304. Tani-Ishii N, Teranaka T. Clinical and radiographic evaluation of root-canal obturation with Obtura II. J Endod. 2003;29:739.
305. Tanomaru-Filho M, Jorge EG, Guerreiro Tanomaru JM, Goncalves M. Radiopacity evaluation of new root canal filling materials by digitalization of images. J Endod. 2007;33:249.
306. Tanomaru Filho M, Leonardo MR, Silva LA, Anibal FF, Faccioli LH. Inflammatory response to different endodontic irrigating solutions. Int Endod J. 2002;35:735.
307. Tatsuta CT, Morgan LA, Baumgartner JC, Adey JD. Effect of calcium hydroxide and four irrigation regimens on instrumented and uninstrumented canal wall topography. J Endod. 1999;25:93.
308. Tay FR, Pashley DH. Monoblocks in root canals: a hypothetical or a tangible goal. J Endod. 2007;33:391.
309. Tay KC, Loushine BA, Oxford C, et al. In vitro evaluation of a Ceramicrete-based root-end filling material. J Endod. 2007;33:1438.
310. Taylor JK, Jeansonne BG, Lemon RR. Coronal leakage: effects of smear layer, obturation technique, and sealer. J Endod. 1997;23:508.
311. Teixeira FB, Teixeira EC, Thompson JY, Trope M. Fracture resistance of endodontically treated roots using a new type of filling material. J Am Dent Assoc. 2004;135:646.
312. Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233.
313. Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003;29:400.
314. Torabinejad M, Ung B, Kettering JD. In vitro bacterial penetration of coronally unsealed endodontically treated teeth. J Endod. 1990;16:566.
315. Tronstad L, Asbjornsen K, Doving L, Pedersen I, Eriksen HM. Influence of coronal restorations on the periapical health of endodontically treated teeth. Endod Dent Traumatol. 2000;16:218.
316. Tronstad L, Barnett F, Flax M. Solubility and biocompatibility of calcium hydroxide–containing root canal sealers. Endod Dent Traumatol. 1988;4:152.
317. Trope M. Regenerative potential of dental pulp. J Endod. 2008;34:S13.
318. Trope MB, Burgenholtz G. Microbial basis for endodontic treatment: can a maximal outcome be achieved in one visit? Endod Topics. 2002;1:40.
319. Uranga A, Blum JY, Esber S, Parahy E, Prado C. A comparative study of four coronal obturation materials in endodontic treatment. J Endod. 1999;25:178.
320. van der Burgt TP, Mullaney TP, Plasschaert AJ. Tooth discoloration induced by endodontic sealers. Oral Surg Oral Med Oral Pathol. 1986;61:84.
321. VanGheluwe J, Wilcox LR. Lateral condensation of small, curved root canals: comparison of two types of accessory cones. J Endod. 1996;22:540.
322. Venturi M, Prati C, Capelli G, Falconi M, Breschi L. A preliminary analysis of the morphology of lateral canals after root canal filling using a tooth-clearing technique. Int Endod J. 2003;36:54.
323. Von der Fehr F, Nygaard-Ostby B. Effect of EDTAC and sulfuric acid on root canal dentine. Oral Surg Oral Med Oral Pathol. 1963;16:199.
324. Wachlarowicz AJ, Joyce AP, Roberts S, Pashley DH. Effect of endodontic irrigants on the shear bond strength of epiphany sealer to dentin. J Endod. 2007;33:152.
325. Walker TL, del Rio CE. Histological evaluation of ultrasonic debridement comparing sodium hypochlorite and water. J Endod. 1991;17:66.
326. Walton RE. Histologic evaluation of different methods of enlarging the pulp canal space. J Endod. 1976;2:304.
327. Weiger R, Rosendahl R, Lost C. Influence of calcium hydroxide intracanal dressings on the prognosis of teeth with endodontically induced periapical lesions. Int Endod J. 2000;33:219.
328. Weinberger BW. An Introduction to the History of Dentistry. St. Louis, MO: Mosby; 1948.
329. Weine FS, Buchanan LS. Controversies in clinical endodontics. 1. The significance and filling of lateral canals. Compend Contin Educ Dent. 1996;17:1028.
330. Weisenseel JAJr, Hicks ML, Pelleu GBJr. Calcium hydroxide as an apical barrier. J Endod. 1987;13:1.
331. Welch JD, Anderson RW, Pashley DH, Weller RN, Kimbrough WF. An assessment of the ability of various materials to seal furcation canals in molar teeth. J Endod. 1996;22:608.
332. Weller RN, Kimbrough WF, Anderson RW. A comparison of thermoplastic obturation techniques: adaptation to the canal walls. J Endod. 1997;23:703.
333. Wennberg A, Orstavik D. Adhesion of root canal sealers to bovine dentine and gutta-percha. Int Endod J. 1990;23:13.
334. White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by endodontic filling materials. II. J Endod. 1987;13:369.
335. White RR, Goldman M, Lin PS. The influence of the smeared layer upon dentinal tubule penetration by plastic filling materials. J Endod. 1984;10:558.
336. Wiemann AH, Wilcox LR. In vitro evaluation of four methods of sealer placement. J Endod. 1991;17:444.
337. Wilcox LR, Roskelley C, Sutton T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. J Endod. 1997;23:533.
338. Williams C, Loushine RJ, Weller RN, Pashley DH, Tay FR. A comparison of cohesive strength and stiffness of Resilon and gutta-percha. J Endod. 2006;32:553.
339. Wilson BL, Baumgartner JC. Comparison of spreader penetration during lateral compaction of .04 and .02 tapered gutta-percha. J Endod. 2003;29:828.
340. Wolcott J, Himel VT, Powell W, Penney J. Effect of two obturation techniques on the filling of lateral canals and the main canal. J Endod. 1997;23:632.
341. Wu MK, Fan B, Wesselink PR. Leakage along apical root fillings in curved root canals. I. Effects of apical transportation on seal of root fillings. J Endod. 2000;26:210.
342. Wu MK, Kast’akova A, Wesselink PR. Quality of cold and warm gutta-percha fillings in oval canals in mandibular premolars. Int Endod J. 2001;34:485.
343. Wu MK, Ozok AR, Wesselink PR. Sealer distribution in root canals obturated by three techniques. Int Endod J. 2000;33:340.
344. Wu MK, van der Sluis LW, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218.
345. Wu MK, van der Sluis LW, Wesselink PR. A preliminary study of the percentage of gutta-percha–filled area in the apical canal filled with vertically compacted warm gutta-percha. Int Endod J. 2002;35:527.
346. Wu MK, Wesselink PR. Endodontic leakage studies reconsidered. I. Methodology, application and relevance. Int Endod J. 1993;26:37.
347. Wu MK, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J. 2001;34:137.
348. Wu MK, Wesselink PR, Walton RE. Apical terminus location of root canal treatment procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:99.
349. Yamashita JC, Tanomaru Filho M, Leonardo MR, Rossi MA, Silva LA. Scanning electron microscopic study of the cleaning ability of chlorhexidine as a root-canal irrigant. Int Endod J. 2003;36:391.
350. Yamauchi S, Shipper G, Buttke T, Yamauchi M, Trope M. Effect of orifice plugs on periapical inflammation in dogs. J Endod. 2006;32:524.
351. Yared GM, Dagher FB, Machtou P. Influence of the removal of coronal gutta-percha on the seal of root canal obturations. J Endod. 1997;23:146.
352. Yeung P, Liewehr FR, Moon PC. A quantitative comparison of the fill density of MTA produced by two placement techniques. J Endod. 2006;32:456.
353. Youngson CC, Nattress BR, Manogue M, Speirs AF. In vitro radiographic representation of the extent of voids within obturated root canals. Int Endod J. 1995;28:77.
354. Zhang W, Torabinejad M, Li Y. Evaluation of cytotoxicity of MTAD using the MTT–tetrazolium method. J Endod. 2003;29:654.
355. Zielinski TM, Baumgartner JC, Marshall JG. An evaluation of Guttaflow and gutta-percha in the filling of lateral grooves and depressions. J Endod. 2008;34:295.
356. Zmener O, Banegas G. Clinical experience of root canal filling by ultrasonic condensation of gutta-percha. Endod Dent Traumatol. 1999;15:57.
357. Zmener O, Hilu R, Scavo R. Compatibility between standardized endodontic finger spreaders and accessory gutta-percha cones. Endod Dent Traumatol. 1996;12:237.