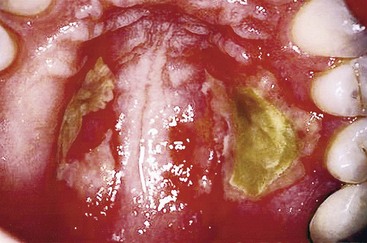
CHAPTER 11 Endodontic Records and Legal Responsibilities
Endodontic Record Excellence
Importance
Endodontic therapy records serve as an important map to document and guide the clinician’s journey down the correct diagnostic and treatment path. Documentation is essential to attaining endodontic excellence.
The dental record must contain sufficient information to identify the patient, support the diagnosis, justify the treatment, and document the course and result of treatment. Records also are fundamental means of communication among health care professionals, designed to protect the patient’s welfare.
Function
Dental records should document the following information:
Patient Information Form
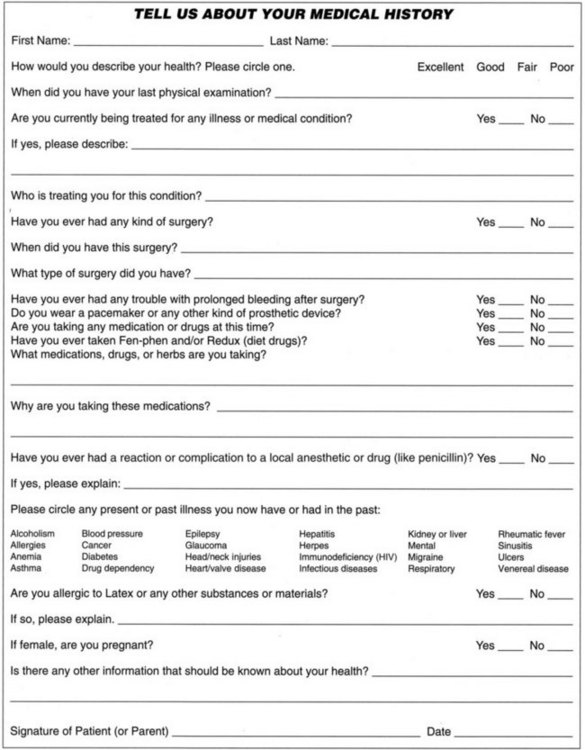
A patient information form provides essential data for patient identification and office communication. The patient’s name; home, business, e-mail addresses; and telephone and fax numbers are needed to contact the patient for scheduling purposes and inquire about postoperative sequelae.144 Location information about the patient’s spouse, relative, or a close friend who can be notified in an emergency is also suggested. In the event the patient is a minor, the responsible parent or guardian should provide the information. Questions about dental insurance and financial responsibility are included on the form to avoid any later misunderstandings and help fulfill federal requirements of the Truth in Lending Law, applicable if four or more installment payments are arranged (whether or not there are interest or late-payment charges).79 Patient information should be updated periodically (Fig. 11-1).
Medical Health History
Past and present health status should be thoroughly reviewed by the clinician before proceeding with treatment so that dental treatment can be safely initiated. Health questionnaires open avenues for discussion about problems of major organ systems and important biochemical mechanisms, such as blood coagulation, allergy, immunocompromised status, need for antibiotic prophylaxis, and disease susceptibility. The clinician may request that the patient be examined by a physician or undergo laboratory testing under medical supervision to determine whether a suspected medical problem may require attention before endodontic therapy proceeds or if drug sensitivity or allergy mandates treatment modifications.29,135,152 Current medications, medical therapy, and the name and location of the treating physician are essential.
Medical histories must be updated periodically (or at least annually or sooner as the need arises). The patient should be asked to review the previous and current medical history (Fig. 11-2). If no changes are necessary, the patient should date and sign the history form. If any changes occurred, the patient should identify each updated medical change, the date, and sign the form where indicated for medical update information. Periodically the patient should provide an entirely new updated form rather than changing data on the old form. Earlier medical histories should be retained in the chart for future reference. If physician communication for treatment occurs, the clinician should record such contacts. In addition, the clinician should verify physician approvals for treatment by fax, with verification of receipt or letter or preferably both, and retain copies in the chart.
Updating the medical history requires the practitioner to be apprised of changes in the patient’s medical condition and any new medications the patient is taking, including over-the-counter or herbal medications and/or supplements. A patient untrained in medical science may not appreciate the fact that new medications may suggest new diseases or changes in existing disease status. For instance, certain regurgitating valvular heart diseases may require antibiotic prophylaxis.269 New medications may also cause a synergistic effect with other medications the patient is using or another treating clinician is prescribing.
Clinicians who pass the buck by claiming, “Oh it was my secretary or my new assistant—I could do nothing about it,” are nonetheless legally liable for their staff’s actions or inactions.270 A clinician exclusively relying on staff members to obtain medical histories in a waiting room full of patients is making a mistake, because the accuracy of the histories must first be checked and then followed up by the clinician. Training and monitoring staff are duties clinicians cannot afford to ignore. President Harry Truman’s sage advice, “The buck stops here,” applies to treating clinicians who overdelegate to nonclinician staff members who lack the requisite dental education for adequate medical history follow-up.
Dental History
The dental history should include past dental difficulties, name and address of current or most recent treating clinician, chief complaint (including duration, frequency, type and intensity of any pain), relevant prior dental treatment, and attitude regarding teeth retention. Positive responses suggest further patient consultation and consideration for obtaining records (by written release) for elucidation from the patient’s previous and/or present treating clinician.188 After receiving positive responses, the clinician may also wish to obtain prior and concurrent treaters’ radiographs for current comparison and notes of any progressive changes (Fig. 11-3).
Diagnostic and Progress Records
Diagnostic and progress records often combine fill-in and check-off types of forms. Fill-in or essay-type forms allow greater latitude of response to a question, resulting in a more detailed description. However, one drawback to these forms is that they are open to oversights unless a clinician is very conscientious in following up with further clinical information.
Using only an essay-type health history is insufficient. Often a patient may not appreciate the significance of important symptoms. A check-off format is efficient and more practical. Forms with questions that reveal pertinent data alert the clinician to medical or dental conditions that warrant further consideration or consultation.29 Moreover, such records can document any missing medical information the patient failed to provide orally. At the end of the check-off portion of the medical history, an essay question allows the patient to provide any omitted pertinent medical information or elaborate on check-marked items.221,231
Electronic Records
Electronic records represent current methodology for recording patient information (see Chapter 28 for details).246 Several safeguards are essential for their use. First, patient confidentiality and security must be protected. Consider using AES encryption with at least 2048 bit key. Be certain that only the clinician and authorized staff view patient data in conformance with the Health Insurance Portability and Accountability Act (HIPAA) security standards, effective April 2005.120 Second, data backup of patient records, including digital radiographs and account ledgers, should be stored off-site. The entire hard drive should be periodically backed up for additional protection.70 Multiple copies of the backup data should be stored in more than one geographic location, and fault protection such as RAID Level 1 or 5 should be installed to prevent data loss.
Radiographs
Radiographs are essential for diagnosis and also serve as additional documentation of the patient’s pretreatment condition. A panographic radiograph is not diagnostically accurate for endodontics and therefore should be used only as a screening device (see Chapter 5).114,219,279 Diagnostic quality periapical plain-film or digital radiographs are essential aids for diagnosis, for working films (e.g., measuring the length of root canals, fitting gutta-percha cones), to verify the final fill, and for follow-up comparisons at recall examinations. Therefore the clinician should retake any radiographs that lack diagnostic quality and retain all radiographs.
Digital radiography is recommended because it increases endodontic efficiency. No developing time is needed, so procedural radiographs can be viewed instantly and approved or retaken with little time in between (no downtime is required to wait for a film to exit the film processor). Newer digital x-rays with improved sensor technology use high-resolution CCD (charged coupling device) chips rather than CMOS (complementary metal-oxide semiconductor) technology, and provide close-up zooming with improved image quality as described in Chapter 29.
Evaluation and Differential Diagnosis
Diagnosis includes evaluating pertinent history of the current problem, clinical examination, pulpal testing, periodontal probe charting, and recorded radiographic results. If therapy is indicated, the reasons should be discussed with the patient in an organized way. When other factors affect the prognosis (e.g., strategic importance or restorability of the tooth), the clinician should consider further consultation with the referring clinician or specialists, including a prosthodontist, periodontist, or both, before initiating endodontic treatment.
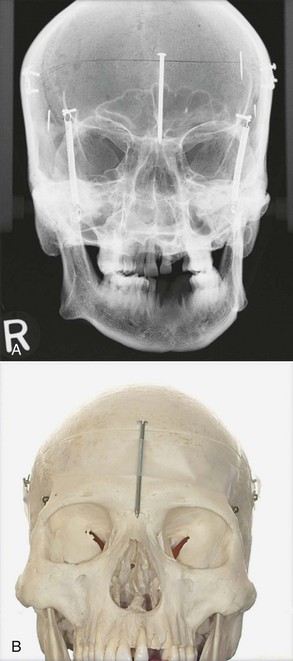
Diagnosis is essential in treatment decisions. Recommending extractions for undiagnosed pain etiology is generally contraindicated. Chronic idiopathic orofacial pain warrants referrals and consultation with other health care professionals, lest wholesale extractions worsen the patient’s dental state and not relieve pain (see Chapter 3).5 For instance, in a long-term follow-up study of patients with chronic facial pain and without recognizable odontologic pathosis, some patients were subsequently diagnosed with maladies ranging from cracked-tooth syndrome to medication-mediated illnesses.5 Accordingly, referral to other specialists is essential, lest a patient be misdiagnosed with idiopathic or atypical facial pain when instead, pain etiology was misdiagnosed. Cone-beam computerized tomography (CBCT) has greatly aided diagnosing pain sources caused by root fractures and incompletely filled, overfilled, or missed extra canals that two-dimensional (2D) periapical imaging may obscure.*
Fig. 11-4, A-B demonstrates the advantage of CBCT three-dimensional (3D) imaging compared to 2D imaging. In A, the nail appears inside the cranium, contrasted with B, which shows the nail taped outside the skull.
Diagnostic Tests
Sound endodontics begins with a proper diagnosis. Otherwise, unnecessary or risky treatment with compromised prognosis follows. Generally, as described in greater detail in Chapter 1, the following endodontic tests should be performed to arrive at a correct and accurate endodontic diagnosis:
Both positive and negative pulpal testing results should be recorded. Juries, peer-review committees, and insurance consultants often disbelieve unrecorded test results. Uncharted test findings may be regarded as never having been done. Consequently, reasonable clinicians should record all testing results, both positive and negative.
Treatment Plan
Treatment records should contain a written plan that includes all aspects of the patient’s oral health. Treatment plans should be coordinated, preferably in writing, with other concurrent or jointly treating clinicians. If an aspect of the patient’s care is not under direct supervision, the clinician is proceeding improperly. Also, the clinician should initiate contact with the other treating clinician. The patient should also be advised of the problem. For instance, endodontic treatment will probably fail if underlying periodontal pathosis is ignored or untreated, so the clinician should assess the patient’s entire dentition, not just a single root canal system. The clinician should also recommend a periodontal consultation to both the patient and referring clinician if periodontal therapy is required. Follow the maxim: If you fail to plan, plan to fail.
Examination
After gathering the dental and medical history, findings obtained from the various phases of the clinical and radiographic examinations are recorded as shown in Fig. 11-5. Lists in each category afford the clinician a systematic format for recording details pertinent to an accurate diagnosis. Appropriate descriptions are circled, followed by the necessary notations in the accompanying spaces. Tabular arrangement allows easier recording and comparison of diagnostic test data acquired from one tooth on different dates or from different teeth on the same visit. A pain intensity index (i.e., 0 to 10) or pain classification (i.e., mild +, moderate ++, severe +++) should be used to differentiate diagnostic test results.
Diagnosis
Careful analysis of accumulated examination data should result in the determination of an accurate pulpal and periapical diagnosis. Clinical conditions and the probable etiologic factors for the presenting problem are circled. Alternative modalities of therapy are considered and analyzed. The recommended treatment plan is circled, followed by a prognostic assessment of the intended therapeutic course.
Patient Consultation
Patients should be advised of each diagnosis and should consent to the treatment plan before therapy is instituted. Consultation should include an explanation of reasonable alternative treatment approaches and rationales and treatment consequences of each alternative therapy, including risks from nontreatment or delayed treatment that may affect the outcome of intended therapy. Such discussion is documented by completing the checklist items.
Treatment
All treatment provided on a given date is documented by placing a check mark (✓) within the designated procedural category. Only the most frequent retreatment procedures are included for tabulation. Descriptions of occasional procedures or explanatory treatment remarks should be entered in writing. A separate dated entry should be made for each patient visit, phone call, e-mail message, and fax communication (e.g., consultations with the patient or other clinicians) or correspondence (e.g., biopsy report, treatment completion letters).
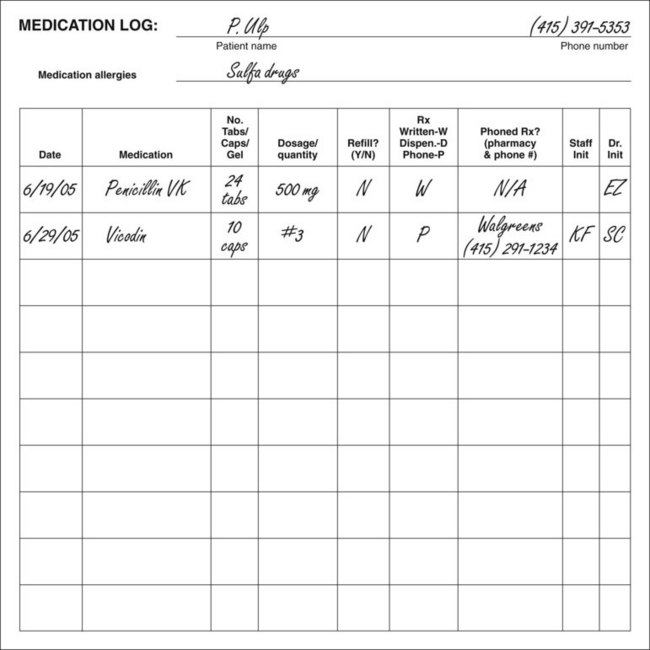
Individual root canal lengths are recorded by (1) circling the corresponding anatomic designation and the method of length determination (e.g., radiograph or electronic measuring device), (2) writing the measurement (in millimeters), and (3) indicating the reference point. For any medication prescribed, refilled, or dispensed, the treatment record should show the date and type of drug (including dose, quantity, and instructions for use) in the treatment table under the heading “Rx.” Fig. 11-6 also gives a sample medication log. Periodic recall intervals, dates, and findings are entered in the spaces provided.
If the scope of the examination or treatment is intentionally limited, such as a screening examination or emergency endodontic therapy, the limited scope of the visit should be recorded. Otherwise, the chart appears as if the examination was superficial and treatment incomplete. If a suspicious apical lesion requires subsequent reevaluation, the clinician should record the future reevaluation date and differential diagnosis (e.g., “Small apical lesion on #28. Reevaluate for changes in 2 months. Also, check for any root fractures.”). If this is not done, it appears (on the chart) as if the clinician ignored a potentially pathologic condition, such as suspected root fracture. A soft-tissue examination (including cancer check) is a standard part of any complete dental examination. E-mail prescriptions and correspondence should be documented with hard copies in the chart unless virtually all treatment records are stored in electronic format.154,246
Informed Consent Form
After endodontic diagnosis, the benefits, risks, treatment plan, and alternatives to endodontic treatment—including any patient refusal of recommended treatment and consequences of refused treatment—should be presented to the patient or the patient’s guardian. This will document acceptance or rejection of treatment recommendations. The patient or guardian, along with a witness (who can be a staff member), should sign and date the consent form. Any informed-consent video should similarly be noted. Subsequent changes in the proposed treatment plan should also be discussed and initialed by the patient to indicate continued acceptance and acknowledge the patient’s understanding of any newly disclosed risks, alternatives, or referrals.
Despite a patient’s confirmed signature on an informed consent form, a jury is free to believe that the patient never read the informed consent form’s content before signing. For instance, the patient may claim it was impossible to have read the consent form, because he or she did not have reading glasses when signing, and no clinician ever explained the form contents. Another frequent factual scenario is when the patient claims the staff stated it was a standard consent form that was a mere procedural formality requiring patient signature, rather than a detailed informed consent form. Consequently, numerous legal cases have been lost at trial on the issue of inadequate informed consent despite a signed patient consent form.
To obviate a patient’s claim that no explanation ever occurred, a patient questionnaire can additionally be used. Patients can be instructed that unless they score 100%, proposed procedures will not be done (because patient understanding is imperative and essential for cooperation, including postoperative care). To be effective, the patient questionnaire should be relatively short and simple (Fig. 11-7). Also, the patient should be given an opportunity to correct any incorrect answers after the clinician reviews with the patient the reasons why the patient answered incorrectly. Note that the majority of answers are false. This precludes a patient from guessing the correct answer by assuming that all test questions are necessarily true.
Treatment Record: Endodontic Chart
Chart
A suggested chart is presented in Fig. 11-8 to facilitate recording of information pertinent to the diagnosis, recommendations, and treatment of the endodontic patient. Systematic acquisition and arrangement of data from the patient questionnaire, along with clinical and radiographic examinations and careful recording of treatment performed, expedites accurate diagnosis and maximizes clinician efficiency. Suggested chart format and use are described in the sections that follow.
General Patient Data
Patient name, address, phone numbers, referring clinician, and chief complaint are printed or typed in the corresponding space at the patient’s initial office visit. The patient’s appointment and financial record are divided into two parts as follows:
Either the clinician or the assistant may complete portions of the diagnosis and treatment sections, but the clinician should review and approve all entries.
Dental History
Chief complaint should include whether the patient is symptomatic at the time of examination and/or recent past. Narrative facts regarding the presenting problem are then recorded. Additional details of the chief complaint obtained during successive questioning are recorded by circling the applicable word within each symptom parameter. The pain intensity index (i.e., 0 to 10) or pain classification (i.e., mild +, moderate ++, severe +++) should be registered alongside the appropriate description. For accurate assessment of the effects of prior dental treatment pertaining to the examination site, a summarized account of such procedures should be documented. In addition, all pretreatment signs and symptoms should be described. It is essential that the patient comprehend all communications with the clinician and staff. If there is any language comprehension difficulty, the clinician should ask the patient whether he or she understands completely the language the clinician is speaking. To verify patient comprehension, the clinician should ask the patient to repeat important information that has been conveyed. A clinician- or patient-provided interpreter may be necessary if language problems exist.57
Medical History
More medical history information is obtained from a patient-administered questionnaire than from the clinician obtaining an oral history solely from the patient.29,231 Maximum information is obtained if the clinician reviews with the patient the written health history form the patient has completed.
Information (e.g., personal physician’s name, address, and phone number; patient’s age; date of last medical examination) is recorded for reference. The clinician can obtain a detailed medical history by completing a survey of common diseases and disorders along with a comprehensive review of corresponding organ systems and assorted pathologic conditions. Specific entities that have affected the patient are circled. Essential remarks regarding these entries (e.g., details of consultations with the patient’s physician) should be documented on an attached blank sheet with dated treatment notes attached to the back of the chart (see Fig. 11-2). A review of the patient’s medical status (including recent or current conditions, treatments, and medications) completes the medical history. Updated medical histories which the patient signs or initials should be done at least annually and at reevaluation visits.
Drug History
A current drug history, both written and oral, is necessary to alert the clinician to any potential for interaction between any newly prescribed drugs and any other medication, supplements or over-the-counter medication the patient is already taking.
In patients aged 57 through 85, 40% potentially are at risk of a major drug reaction—half involving various prescription medications and the other half anticoagulant drug reactions. About half of prescription users also concurrently take over-the-counter medications plus dietary supplements.153,196
Risks of drug interactions or side-effects incidence may not be discovered and/or appreciated in premarket research-controlled drug trials for newly marketed drugs, despite FDA approval. For example, rofecoxib (Vioxx) was withdrawn from the market in September 2004 after increased incidences of myocardial infarcts and strokes manifested in a postmarket research study of 2600 patients. Merck’s voluntary recall followed an FDA researcher’s claim that the FDA tried to ostracize and intimidate him after he raised safety concerns over Vioxx.21 Currently, pharmaceutical manufacturers budget more for marketing than research and development.96 Some drug manufacturers have suppressed publication of adverse research.38,170,272 U.S. Senators Chuck Grassley (R, IA) and Herb Kohl (D, WI) are probing the financial relationships among the drug industries, health care prescribers, and researchers (Senate Bill 2029 [http://thomas.loc.gov]). If their Physician Payments Sunshine Act passes, it would require companies to disclose payments to medical/dental professionals who tout their drugs in seminars or publications.
For newly marketed drugs, the small number of premarket patients studied may lack sufficient statistical power to expose serious side effects that occur infrequently but are nonetheless life threatening.272 For instance, mibefradil (Posicor) was withdrawn from the market after 1 year because it increased plasma concentrations of 25 other coadministered drugs, including erythromycin.196,197 In another example, the popular prescription nighttime heartburn drug, Propulsid (cisapride), was linked to 70 deaths and more than 270 significant negative reactions.263 Other examples include the risk of esophageal cancer with bisphosphonate272 and bisphosphonate-associated osteonecrosis.69,232 In 2008, the FDA added a black box warning label for Levaquin-associated risks of tendonitis (www.levaquin.com/levaquin/medication).
FDA Drug Approval
Until 1962, drug companies were allowed to promote their products for any use so long as they were shown to be “safe” for one use. Manufacturers were marketing drugs with serious side effects for minor conditions and to vulnerable populations, resulting in many injuries and deaths. Ineffective drugs were the rule rather than the exception. When a retrospective review of all drugs was conducted after 1962, the National Academy of Sciences found that fully 80% of the uses for which drugs were being promoted could not be shown to be effective. Since 1962, the FDA has required prescription drug manufacturers to demonstrate safety and efficacy with animal and randomly controlled research trials conducted with FDA-approved protocols. FDA “approval” to market new drugs for specific uses is termed premarket approval (PMA).
In a New Drug Application (NDA) to the FDA, findings from many clinical studies are done to assess a new drug’s efficacy and safety and to gain the FDA’s PMA to market the drug. An NDA judges whether the proposed new drug (1) causes more good than harm (benefit outweighs the risk), (2) is comparatively more effective than already existing marketed drugs, or (3) has unreasonably frequent and/or severe side effects (risk outweighs the benefit).23,257
In 2008, $58 billion in privately funded drug research was approximately twice the basic federal medical research budget and encompassed an estimated 50,000 clinical trials among 2.3 million patients. Medicare drug spending is forecast to grow from 2 billion in 2000 to 153 billion in 2016, a 7550% increase.199 In 2007, one in seven U.S. residents younger than age 65 skipped prescribed medications because of rising prescription cost.81,123
The Pharmaceutical Research and Manufacturers Association of America has created an online database that summarizes clinical study results involving hundreds of prescription medicines. Notwithstanding, the sheer volume of clinical testing has overwhelmed the FDA’s ability to independently assess commercial trials and make all test results public. Deceptive marketing, unreported side effects, and hidden payments to medical/dental researchers highlight the gap between the number of clinical trials conducted and the number published.*
Comprehensive clinical data filed with the FDA for gaining market approval of a new drug (PMA) forms the basis for safety information that accompanies every prescription label.86,88
For the first time, under a new federal law89 effective in 2009, researchers in PMA trials will have to post their basic research results publicly on a federal online registry maintained by the National Library of Medicine. However, new registry regulation will only cover new tests, not tests with poor results done prior to the effective date of the new law. Moreover, findings are not independently FDA verified. Instead, the FDA relies on the manufacturer’s submitted trial data research.
Until September 2007, researchers only had to report the start of a clinical trial and was under no federal legal obligation to report the outcome in the FDA’s registry or in a peer-reviewed journal. Consequently, clinical data on health risks could be downplayed or benefits extolled selectively in any one of 5200 peer-reviewed biomedical journals.254,273
By January 31, 2009, as a voluntary patient guide, the federal registry (www.clinicaltrials.gov) logged 67,000 studies. The results of all drug trials that were ongoing on or after September 27, 2007, must be reported at ClinicalTrials.gov if the products under study have FDA approval. The results for drugs not yet receiving FDA approval do not have to be posted. A deficiency of the current law is that the results of older trials of drugs that were FDA approved before September 27, 2007, and that were no longer the subject of ongoing trials on or after September 27, 2007, do not need to be posted. Such drugs constitute the vast majority of current prescription drugs. The FDA considers much of the data on clinical trials it receives from sponsors—and its own analyses of these data—to be “confidential commercial information.” FDA drug-trial information released to the public is frequently heavily redacted. Redacted disclosure occurs in response to one or multiple requests made under the Freedom of Information Act, but usually only after a prolonged delay. The FDA Amendments Act of 2003 Section 916 institutionalizes this limitation by mandating that the FDA publish on its website key information related to approval of an application for a drug that contains a previously approved active ingredient only after it has received three Freedom of Information Act requests.
In a 2008 University of California, San Francisco (UCSF) study of 164 clinical trials testing 33 different drugs submitted for FDA approval, approximately one in four had yet to be published.216 Virtually all of the unpublished findings were negative. The UCSF study also reported that in 900 FDA filings involving 90 new drugs, more than half the clinical trials were still unpublished 5 years after the drug had FDA approval. UCSF researchers who reviewed the FDA’s regulatory paperwork for dozens of recently FDA-approved (PMA) drugs found that in some clinical trials previously reported to the FDA, when subsequently submitted for publication, conclusions were changed, statistics revised, and outcomes altered to make treatments appear more effective. Among 43 outcomes reported in the FDA filings that did not favor a drug, 20 were never published. In four out of five instances in which the statistical significance of findings were changed from the FDA filing, the published version was more favorable. Positive effects of a new drug were more likely to be submitted for publication than those reporting negative findings. Basic principles of evidence-based practice requires the analysis of all data. Publishing only some results but not others undermines the ability to adequately assess a product’s safety and efficacy, which is FDA’s standard for FDA approval of drugs or devices.
Journals want to sell journals. Companies wish to increase profits. Researchers promote career advancements and seek research funding. Consequently, conflict potentials are pervasive. To offset these temptations, failure to timely report basic data about clinical test results in the federal public registry can subject researchers to fines of $10,000 a day and/or loss of their federal research funding (Public Health Service Act Section 302[J]).86,273
In 2009, the Government Accounting Office (GAO) concluded in GAO’s 2009 High-Risk Series, FDA Oversight of Medical Products259:
New Laws, the complexity of items submitted to FDA for approval, and the globalization of the medical products industry are challenging FDA’s ability to guarantee the safety and effectiveness of drugs, biologic, and medical devices. As a result, the American consumer may not be adequately protected from unsafe and ineffective medical products. FDA needs to improve the data it uses to manage the foreign drug inspection program, do more inspections of foreign establishments that manufacture drugs or medical devices, more systemically review the claims made in drug advertising and promotional material, and ensure that drug sponsors accurately report clinical trial results.
Globalization of clinical research is a relatively recent phenomenon. The number of countries serving as trial sites outside the United States more than doubled in 10 years. Conversely, the proportion of trials conducted in the United States and Western Europe decreased.100 Clinical testing in developing countries help overcome stringent FDA regulatory barriers for FDA drug approval.16
Wide disparities in education, economic and social standing, and health care systems may jeopardize the rights of research participants in foreign countries. Foreign participants may lack understanding of the investigational nature of therapeutic products and/or the use of placebo research participant groups. In some places, financial compensation for research participation may exceed participants’ annual wages. Standards of health care in developing countries may also allow ethically problematic study designs or clinical research trials that would not be allowed in wealthier countries. In one study, only 56% of the 670 researchers surveyed in developing countries reported that their research had been reviewed by a local institutional review board or health ministry.128 In another study, 90% of published clinical trials conducted in China in 2004 did not report ethical review of the protocol, and only 18% adequately discussed informed consent.280
Developing countries will also not realize the benefits of clinical research trials if the drugs being evaluated do not become readily available for host-country research participants after FDA approval is obtained.16 The Declaration of Helsinki includes an expectation that every patient enrolled in a clinical trial should, at the end of the trial, be assured access to the best proven therapy identified in the research study.
Geographically distinct populations can have different genetic profiles; such differences have been shown to be related to the safety and effectiveness of drugs and medical devices. For example, a study of 42 genetic variants associated with pharmacologic response in drug studies showed that more than two thirds had significant differences in frequency between persons of African ancestry and those of European ancestry.105 Genetic diversity is often not considered in study design and interpretation and in the reporting of trial results.
From fiscal 2002 through 2007, the FDA issued 15 warning letters to foreign companies with serious deficiencies, but the agency only reinspected four of the companies, and then only 2 to 5 years later. Decades ago, most pills consumed in the United States were made here. But like other manufacturing operations, drug plants have been moving to Asia because labor, construction, regulatory, and environmental costs are lower there. The world’s growing dependency on Chinese drug manufacturers become apparent in the heparin scare. In 2007, Baxter Intentional and APP Pharmaceuticals split the domestic market for heparin, an anticlotting drug needed for surgery and dialysis. When federal drug regulators discovered that Baxter’s product had been contaminated by Chinese suppliers, the FDA banned Baxter’s product and turned almost exclusively to the one from APP. However, APP also obtained its heparin product from China. Of the 1154 pharmaceutical plants with generic drug application to the FDA in 2007, only 13% were in the United States; 43% were in China, and 39% were in India.259 Drug labels often claim that the pills are manufactured in the United States, but the listed U.S. plants are often the sites where foreign-made drug powders are pounded into pills and packaged.
Under the Prescription Drug User Fee Act, known as PDUFA, pharmaceutical companies pay the FDA to facilitate their PMA applications. Congress provided PDUFA with added power to impose restrictions on the sale of drugs and enforce regulations requiring that postmarketing surveillance studies be timely completed rather than delayed or evaded. Notwithstanding PUDFA, in 2008 the FDA was still missing target dates to act on NDAs because the FDA lacks sufficiently adequate staffing to handle PMA applications.210
Dietary Supplements
The Dietary Supplements Health and Education (DSHE) Act allows dietary supplements to be sold directly to consumers without any oversight or FDA regulation. In response to intense lobbying by the multibillion-dollar dietary supplement industry, Congress in 1994 exempted these products from FDA regulation.153,167,277 Products may contain amounts claimed, but they need not, and nothing can prevent their sale if they do not. For example, an analysis of ginseng167 products showed no ginseng at all in some products. The DSHE Act places the burden of proof for dietary supplements’ safety on the FDA and not the manufacturer. As a result, consumers of herbal supplements must depend on self-regulation within the industry for assurance of product quality, consistency, potency, and purity.134,277 An understaffed and overworked FDA can hardly be expected to be a vigorous enforcer.
An herbal product label can state the way the product is intended to affect “the structure or function” of the body but cannot claim its use for a specific disease. Manufacturers thus use creative language that complies technically with the statute but generally is confusing and/or deceptive.167
A current medication history form should include over-the-counter, herbal, and illicit drugs, many of which have the potential for synergistic or antagonistic interaction with clinician-prescribed drugs.277 Ma-huang, or ephedra, a major component of many weight loss supplements, has been associated with more than 800 adverse health effects, including the death of major league baseball player Steve Bechler. It is an amphetamine-like compound with the potential for overstimulating the central nervous system and heart. Ephedra/ma-huang has been associated with 54 deaths, primarily involving cranial hemorrhage or stroke.28,117 Echinacea increases potential for liver damage when used with steroids. Gingko biloba and feverfew interfere with anticoagulants such as warfarin (Coumadin).53 Dong quai root, willow bark, goldenseal, guarana, horse chestnut, and bilberry tablets/supplements also have antiplatelet properties.242 Ginseng may cause rapid heartbeat and/or high blood pressure in some individuals, as well as coagulation disruption. Vitamin E has antiplatelet properties and inhibits vital clot formation. Gingko and selenium are powerful anticoagulants, three times stronger than vitamin E. In 1998, California investigators found about one third of 260 imported Asian drugs were either spiked with unlisted drugs or contained mercury, lead, or arsenic. In 2009, the FDA warned of 70 weight loss pills containing potentially harmful drugs unlisted on their labels.
A Los Angeles police officer who experienced a crippling stroke after taking an ephedra-based energy supplement, Dymetradine Xtreme, was awarded $4.1 million.20 The retail store owner had read but disregarded 30 journal articles warning of strokes, heart attacks, seizures, and other ailments. The stroke occurred before the FDA banned ephedra from sale in April 2004. Nonetheless, Internet sales proliferated.242
The formulations for herbal supplements—or botanicals, as they are correctly called—such as St. John’s wort and echinacea are often complex, highly variable, and impure. Many are toxic, carcinogenic, or otherwise dangerous. Side effects include blood-clotting abnormalities, high blood pressure, life-threatening allergic reactions, abnormal heart rhythms, exacerbation of autoimmune diseases, and interference with life-saving prescription drugs.
The American Society of Anesthesiologists has warned patients to stop taking herbal supplements at least 2 weeks before surgery to avoid dangerous interactions with anesthesia.
Congress exempted supplements from oversight under a 1994 law that prevents federal regulators from requiring manufacturer’s proof that botanicals are safe or effective, or even that dosage information on the label is correct. In 1999, the FDA allowed manufacturers to make dubious health claims, such as for products to treat conditions such as premenstrual syndrome and acne, although only a few botanicals are proven efficacious for anything.
MedWatch
Newly marketed drugs may not list all potential adverse drug reactions or events because of the limited number of patients in research trials before marketing. Therefore the FDA’s MedWatch program encourages clinicians to report known or suspected drug reactions. MedWatch encourages reporting of serious unexpected adverse drug reactions whose nature and severity are inconsistent with or absent from the drug’s labeled warnings.87-90196 Clinician reporting to the FDA is confidential and voluntary. The patient’s identity need not be disclosed. Clinicians should contact the FDA by telephone at (800) FDA-1088 or fax at (800) FDA-0178 to obtain the FDA Medical Products Reporting Program (MedWatch) form (FDA form #3500). The back portion of the Physicians’ Desk Reference (PDR) contains a MedWatch form. Clinicians can also download the MedWatch reporting form at http://www.fda.gov/medwatch/getforms.htm and report suspected drug interactions, reaction, or adverse product events at http://www.fda.gov/medwatch/report.htm. Other electronic drug databases are available to report and access important drug interaction information.275 For FDA-approved safety-related drug labeling online, see www.fda.gov/MedWatch.
Adverse drug reactions are injuries occurring when drugs are administered at usual doses. These reactions represent the primary focus of regulatory agencies and postmarketing surveillance. An adverse drug event suggests medication prescribing or dispensing errors that compromise patient safety. Unfortunately, clinicians report only between 1% and 5% of adverse drug incidents to the FDA, since clinician reporting is voluntary.150 On the other hand, drug manufacturers must report known adverse drug incidents to the FDA.88
Clinicians should document adverse drug reactions not only in the progress notes but also in the allergy section of the chart in order to assess whether future use of the same drug is contraindicated and to prevent a harmful recurrence. Predictable adverse drug reactions that manifest should also be recorded as consideration for future disuse of the involved particular drug.
Endodontics and Heart Disease
Associations between chronic infections such as periodontal diseases and coronary heart disease (CHD) have been suggested.226 Conversely, supporting evidence linking root canal–filled teeth or teeth with periapical disease to CHD is lacking.94
Abbreviations
Abbreviated records can be frustrating if the clinician is unable to decipher one’s own or other clinicians’ handwritten entries, so the clinician should use standard or easily understood abbreviations. Pencil entries are legally valid, but ink entries are less vulnerable to a plaintiff’s claim of erasure or alteration. A short pencil is better than a long memory; records remember, but patients and clinicians may forget.
A sample of a completed endodontic chart (see Fig. 11-9) illustrates its proper use. Box 11-1 lists a key to the abbreviations.
BOX 11-1 Standard Abbreviation Key
| Ab | = | Antibiotic |
| ABS | = | Abscess |
| access | = | Access cavity |
| AG or AmAL | = | Amalgam |
| AE | = | Air embolus |
| analg. | = | Analgesic |
| apico | = | Apicoectomy |
| B-U | = | Buildup of tooth |
| Bisp | = | Bisphosphate |
| BW | = | Bite wing |
| canal | = | Identify canal that has been cleaned and shaped |
| CBCT | = | Cone-beam computed tomography |
| comp | = | Composite |
| cotton | = | Placed in pulp chamber between treatments |
| CRN | = | Crown |
| Dx | = | Diagnosis |
| ENDO | = | Endodontics |
| EPT | = | Electric pulp test |
| epin | = | Epinepherine |
| final | = | Final file |
| Fx | = | Fracture |
| G.G.B. | = | Gates-Glidden bur |
| G.P. | = | Gutta-percha |
| I & D | = | Incision and drainage |
| I.P. | = | Irreversible puplitis |
| L.A. or local | = | Local anesthetic |
| mm | = | Millimeters |
| O+D | = | Open and drain |
| P.A. | = | Periapical |
| perio | = | Periodontal |
| post | = | Preformed, custom, or transilluminated post |
| pt | = | Patient |
| pulpec | = | Pulpectomy |
| R.D. | = | Rubber dam |
| Rel occ | = | Relieved occlusion |
| resorp. | = | Resorption |
| retro | = | Retrograde procedure |
| S/R | = | Suture removal |
| s.file | = | Serial filing |
| S/D | = | Single insurance or dual coverage |
| sealer | = | Sealer type used |
| sepr | = | Separated |
| sutr | = | Suture |
| SX | = | Symptom |
| tech. | = | Technique for canal obturation |
| temp | = | Temporary restoration |
| test | = | Test file |
| Tx | = | Treatment |
| WNL | = | Within normal limits |
Computerized Treatment Records
Clinicians are increasingly using computerized record storage as described in Chapter 29. To avoid a claim of record falsification, whatever computer system is used should be able to demonstrate that records indicating earlier treatment were not recently falsified. Software technology, such as the “write only read memory” (WORM) system, used to identify computer data tampering, is not foolproof because it cannot detect tampering where an entire disk of recent origin has been substituted for an earlier version. Periodically, a hard copy of computer data should be printed out, hand-initialed, dated in ink, and stored in the chart to provide written verification of the computer records if the office is not a virtual paperless office.246
Health Insurance Portability and Accountability Act
The Department of Health and Human Services’ Security and Electronic Signature Standards rule is part of the Health Insurance Portability and Accountability Act (HIPAA) of 1996. This act emphasizes the safeguarding of clinicians’ health care information and permits a patient to obtain a copy of the requesting patient’s records. Most states have authorized digital electronic signatures as binding for most contracts and orders. Digital signatures with electronic transactions are authorized by the Third Millennium Electronic Commerce Act (also called the Electronic Signature in Global and National Commerce Act).255
Pertinent HIPAA requirements stipulate that:
Record Size
Although there is little harm in recording too much information, there is great danger in recording too little. Standard 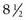 × 11 inch or larger clinical records possess the advantage of providing the treating clinician adequate space for clinical notes.
× 11 inch or larger clinical records possess the advantage of providing the treating clinician adequate space for clinical notes.
Identity of Entry Author
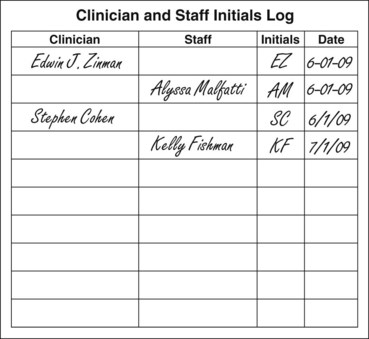
Either a clinician or an auxiliary can chart clinical entries unless state law indicates otherwise. Some states require clinicians and assistants to sign or initial each treatment entry.39 What is important is that the correct clinical information is recorded. Each person who makes a clinical chart entry should record the date and initial the entry. Otherwise, the author’s identity may be forgotten if the individual who recorded the entry is needed in a legal proceeding. For instance, initialing the entry makes it easier to identify the particular clinician or auxiliary who, after the original recording, is now employed elsewhere. A separate log of clinician and staff initials should be created and stored for later comparison to identify the entrant’s initials (Fig. 11-9).
Patient Record Request
Patient requests for record transfers or copies must be honored. It is unethical to refuse to transfer patient records, on patient request, to another treating clinician.10
Moreover, refusing to provide patient records is illegal in some states, subjecting the clinician to discipline and fines should the records not be provided to the patient on written request, even if an outstanding balance is owed.42
Patient Education Pamphlets
Patient education pamphlets may be used in litigation as evidence that a patient was properly informed and given endodontic alternatives but instead chose extraction. Such pamphlets include the American Dental Association’s (ADA’s) Your Teeth Can Be Saved by Endodontic Root Canal Treatment or the American Association of Endodontists’ (AAE’s) Your Guide to Endodontic Treatment, Your Guide to Endodontic Retreatment, and Your Guide to Endodontic Surgery. The clinician should indicate in the patient’s chart which particular pamphlets the patient was provided or which video was shown, since this may prove useful should the patient later deny being informed of endodontic alternatives.
Postoperative Instructions
It is unlikely a patient will remember oral postoperative instructions unless accompanied with written instructions. After endodontic procedures, the patient may be sedated or affected by analgesic drugs. Accordingly, written postoperative instructions are beneficial. Emergency phone numbers to contact the treating clinician should be included on the form. Written instructions reduce postoperative morbidity and pain and improve patient compliance.4 Document that both written and oral postoperative instructions were provided. If treating in close proximity to the inferior alveolar nerve canal (IANC), place a precaution, such as seen in Fig. 11-10, in the postoperative instruction sheet.
Recording Referrals
Every clinician, including the endodontic specialist, has a duty to refer under appropriate circumstances. If consultations with additional experts or specialists become necessary, referrals should be recorded lest they be forgotten or refused. Carbonless, two-part referral cards allow the clinician to provide an original referral slip to the patient while retaining a copy for the patient’s chart. The clinician or staff member should document the fact that the original referral card was given to the patient and record the name of the person who provided the referral card and the date on which it was provided. Document if mailed to the patient. A copy of the referral card should also be sent to the referred doctor. If the patient fails to keep the referral appointment, this copy will provide proof that a referral was made. The clinician should request that the patient and the referred clinician report back if the referral appointment is canceled. Staff should calendar to verify that the referral consultation occurred.
Record Correction
Records must be complete, accurate, legible, and dated. All diagnosis, treatments, and referrals should be recorded. Chart additions may expand, correct, define, modify, or clarify (as long as they are currently dated to indicate a belated entry).
To correct an entry, the clinician should make a line through (but not erase or obscure) the erroneous entry. The correction should then be written on the next available line and dated. Handwriting and ink experts use ink chemical tags, age dating, and infrared technology to prove falsified additions, deletions, or substitutions. If records are proved to be falsified, the clinician may be subject to punitive damages in civil litigation. In addition, the clinician may be subject to license revocation for intentional misconduct.39 Professional liability insurance policies may defend but will usually not indemnify punitive damages if the clinician is found to have committed fraud or deceit.43,45 In addition, insurance carriers may deny renewal of professional liability coverage to a clinician who fraudulently alters dental records.
If an erroneous entry occurs, the clinician should add another entry dated as a late entry to demonstrate later corrected information. Fig. 11-11 gives an example of the proper method of belated record correction.
When patient records have been requested or subpoenaed, it is wise to refrain from examining in detail prior to copying to avoid the temptation to clarify or belatedly add an entry. Alteration of clinical records is a cause of large settlements.
Spoliation
Spoliation is the destruction or significant alteration of evidence, or the failure to preserve property for another’s use as evidence, in pending or reasonably foreseeable litigation under Fed. R. Civ. P. 37(b)(2)(C).78
Spoliation occurs when the wrongdoer alters, changes, or substitutes dental records in an attempt to defeat a civil lawsuit.47,258 It is far easier to defend a dental negligence lawsuit with poor records compared to attempting to justifiably defend altered (falsified) records. A jury and/or a judge will likely conclude the clinician acted with consciousness of guilt for falsifying records compared with excusing staff clerical oversight if records are poorly maintained. Dental records, like all business records, deserve accuracy and completeness.
Questioned document experts utilize ink age dating, examine watermarks, or apply infrared techniques to ascertain substituted pages and additions or deletions in records, including prior erasures, entries underneath whiteouts, and indentations made when one page is overwritten on another page. Infrared technique analyzes these patterns of indentations underneath the altered pages that detect belated second entries. In one case, the defendant clinician claimed a written referral was made to a specialist in 1998, but the patient refused the referral. During litigation, the patient denied the referral and subpoenaed records of the print shop where the specialist’s referral forms were printed. These subpoenaed records proved the carbon copy of the form used for the purported 1998 referral was instead first printed in 2000, which was 2 years after the alleged referral was made (Fig. 11-12, A-B).
In another example, altered records were detected in nine different entries in one medical negligence case involving an undiagnosed malignancy. The physician’s carrier subsequently settled for $1 million. Also, the defendant personally paid a separate fine to the court. As part of the settlement, the defendant authored an article for the Washington Medical Association detailing why records falsification is wrong.17
Records are subjected to (1) audits by insurance carriers for documentation that treatment was performed, (2) review by peer review committees, and (3) subpoena by state licensing boards or agencies for disciplinary proceedings. Accordingly, falsified records expose the clinician not only to civil liability for professional negligence but also to criminal penalties for criminal offenses, such as insurance fraud.47,251,255,258,260
Deliberate record alteration with intent to deceive may also subject the clinician to licensing39 and ethical discipline as well as punitive damages for deceitful misconduct. Insurance carriers may defend but not indemnify a verdict for intentional material alteration of dental records done with intent to deceive.
Digital radiography may have dental advantages, but because the digital images originally may be computer manipulated, they may be legally suspect as altered.137,251 Therefore, hard copies of the digital images may also be printed and dated in ink to show the informational baseline upon which the clinician based diagnostic or therapeutic decisions. This also protects against computer glitches, such as disk drive crashes, electrical power surges, computer viruses, and operator delete errors.
False Claims
Performing or billing for unnecessary endodontic therapy, such as prophylactic endodontic therapy with every crown preparation, subjects the clinician to fraud. In non–pulpal exposure crown preparation, the likely incidence of subsequent endodontic therapy is approximately 3%. Therefore performing prophylactic endodontics on the other 97% of patients represents unnecessary and therefore fraudulent treatment. Excessive treatment also ethically violates the Hippocratic Oath of “primum non nocere” (i.e., “first, do no harm”).
The Federal False Claims Act carries both civil and general penalties of treble damages, fines, and attorneys’ fees for fraudulently billing government programs, such as CHAMPUS (Civilian Health and Medical Program of the Uniformed Services) or Medicare. Fines between $5000 and $10,000 per claim form apply if the U.S. mail was used for a false-claim submission. If any portion of the claimed treatment was fraudulently misrepresented, even if a small minority, a violation results.258 Fraudulent intent need not be conclusively proved. Reckless disregard for the accuracy of submitted data is all that is necessary to obtain a federal criminal conviction or prove civil wrongdoing.252
Legal Responsibilities
Malpractice Prophylaxis: Importance of Records
Good clinicians keep good records. Records represent the single most critical evidence a clinician can present in court as confirmation that an accurate diagnosis, proper planned treatment, and informed consent were provided.
Prevention is the goal of modern dental care. Competent endodontic treatment performed within the requisite standard of care not only saves endodontically treated teeth but also helps prevent a lawsuit for professional negligence. Thus sound and carefully applied endodontic principles protect both patient and clinician. Prudent practices reduce avoidable and unreasonable risks associated with imprudent endodontic diagnosis or therapy.
Standard of Care
Good endodontic practice, as defined by the courts, is the standard of reasonable care legally required to be performed by a reasonably careful clinician. The standard of care does not require perfection; instead, the legal standard is the reasonable degree of skill, knowledge, and care ordinarily possessed and exercised by reasonably careful clinicians under similar circumstances.140
Although the standard of care is flexible to accommodate individual variations in treatment, it is objectively tested based on what a reasonable clinician should do. Reasonable conduct represents a minimum standard required for legal due care. Reasonable care is care based upon reasoning supported by sound science such as evidence-based peer-reviewed literature. Additional precautionary steps that rise above this minimal floor of reasonableness and reach towards the ceiling of ideal care are laudable, but they are not legally mandated. Again, the standard of care does not require perfection; nor does it require ideal endodontics. Nevertheless, prudent clinicians should always strive to achieve a higher level of care than barely or minimally adequate care. Excellent clinicians always strive to do their best and practice endodontics at the highest level. Some have criticized this concept by contending that average care is below average, since 49% of clinicians practice as a minority compared to the majority of clinicians. Careful clinicians should strive to avoid the mediocrity of minimally acceptable care and instead zealously pursue a goal of endodontic excellence.
The written policies and procedures of a clinician, institution, or organization may be used as evidence of standard of care. An expert may consider these the appropriate standard of care in testifying about alleged breaches of standard of care on that issue. For instance, an expert may rely upon AAE’s 1998 position paper on paraformaldehyde-containing root canal filling materials as substandard practice, as well as the fact that all U.S. dental schools teach that toxic paraformaldehyde substances should not be used for adult patients as a root canal obturant.
Health Maintenance Organization Care versus Standard of Care
Reasonably careful or prudent practitioners (not insurance carriers) set the standard of care. Third-party payers may limit reimbursement but should not limit access to quality care. Clinicians have an affirmative duty on behalf of patients to appeal insurance carrier care denial decisions and, in some states, are protected against retaliation.267 The clinician who complies (without protest) with restrictive limitations or denials imposed by a third-party payer when sound judgment dictates otherwise cannot avoid ultimate responsibility for the patient’s care.267
Although dental insurance companies do not set the standard of care, insurance carriers may contractually limit dental benefits. Therefore a clinician is obligated to inform patients of their dental needs, regardless of carrier reimbursement. Patients may then elect to pay out of pocket or decline uninsured services. Informed choice is uninformed if the clinician fails to provide patients with all reasonable options and alternatives.171
If an insurance carrier denies endodontic therapy or limits endodontics to only certain clinical conditions, a prudent clinician must provide informed consent to the patient both legally and ethically that a tooth may be endodontically treated and retained rather than extracted.51,181,233,245,281 The California Dental Association’s Dental Patient Bill of Rights advises patients that “You have the right to ask your clinician to explain all treatment options regardless of coverage or cost.”41
Clinicians may agree to a discounted fee with a health maintenance organization (HMO) carrier but must never discount the quality of care provided. Peer review and the courts recognize only one standard of care. A lower, double, or different standard of care for reduced-fee HMO plans is not legally recognized. Expediency should not be exercised at the expense of quality patient care. Section Three of the ADA Code of Ethics affirms, “Managed care contract obligations do not excuse clinicians from their ethical duty to put the patient’s welfare first.”
An Illinois appellate court held that a clinician may be sued for injuries a patient experiences as a result of the clinician’s failure to disclose a contractual arrangement with the patient’s HMO that creates financial incentives to minimize diagnostic tests and referrals to specialists. The court recognized a distinct cause of action for breach of fiduciary duty for failure to disclose these types of financial incentives.192 In reaching its conclusion, the court cited an American Medical Association (AMA) ethics opinion that stated that a clinician must ensure that a patient is aware of financial incentives through which health insurers limit diagnostic tests and treatment options.
Managed care organizations use a variety of strategies to influence the practice styles of providers. One of the most controversial of these methods is the use of financial incentives designed to limit referrals to specialists. Such financial incentives usually take the form of bonus payments drawn from surpluses in risk pools funded by “withholds.” These funds are deducted from the primary care provider’s base payments or otherwise reserved under contracts in which the care provider bears financial risk. Such bonuses are based on referral limitations.111
Plans that delay treatment approval, resulting in endodontic complications or tooth nontreatability, may be subject to liability.193,245 HMO carriers have not always succeeded in arguing that the 1974 Employee Retirement Income Security Act (ERISA) preempts state law for dental negligence claims against entities who administrate health care benefits to an ERISA plan and shift blame to the clinician.200
Capitation systems have a built-in incentive to undertreat, delay, or discourage treatment and access to care. For the HMO-paid clinician, the patient may be perceived as a threat to profits. Thus capitation creates incentives that can transform clinicians from the patient’s advocate to the patient’s adversary.
Although clinicians seem like double agents serving two masters (i.e., managed care carriers and patients), the law is clear that the clinician must always act in the patient’s best interest, since the clinician owes a fiduciary obligation to the patient. Should the carrier deny requested care, the clinician is legally obligated to appeal the decision to protect the patient’s dental health.267 A patient’s best interest is always paramount, both legally and ethically, to a clinician’s financial interest.97,268
Although profitable to insurance carriers, the success of denying or limiting benefits has created an era of rightfully indignant patients and frustrated clinicians. Consequently, the clinician must guard against patient disappointment with limited insurance benefits by explaining that although insurance carriers determine coverage under their patients’ insurance policy, carriers do not set the standard of care. Regardless of how much or how little is covered by the patient’s insurance carrier, prudent and reasonably careful clinicians set the standard of care. Providing less than necessary care because of insurance carriers’ dictates may explain resulting deficient endodontics but does not excuse such conduct. Moreover, the legal doctrine of informed consent mandates the patient be informed of alternative therapy choices which the patient may pay privately even though the HMO denies approval for alternative therapies. Follow the maxim: Do not x-ray a patient’s pocketbook. Reasonable informed consent is primarily based upon patient choice and not cost alone.
Dental Negligence Defined
Dental negligence is defined as a violation of the standard of care (i.e., an act or omission that a reasonably careful or prudent clinician under similar circumstances would not have done).140 Negligence is equated with carelessness or inattentiveness.145 Malpractice is a lay term for such professional negligence. Dental negligence occurs for two reasons:
One simple test to determine if an adverse outcome results from negligence is to ask the following question: Was the treatment result reasonably avoidable? If the answer is “yes,” it is probably malpractice. If the answer is “no,” it is instead an unfortunate maloccurrence that resulted despite reasonable care attempts.
Not all examples of negligent endodontic treatment are included in this chapter, because the myriad of malpractice incidents far exceeds its scope and length. Rather, only select examples are elucidated for educational purposes.
Locality Rule
The locality rule, which provides for a different standard of care in different communities, is rapidly becoming outdated. Originating in the 19th century, the rule was designed to acknowledge differences in facilities, training, and equipment between rural and urban communities.161,282
The trend across the United States is to move from a locally based standard to a statewide standard, at least for generalists. For endodontists, a national standard of care is applied, since the AAE Board is national in scope.235 Because of nationally published endodontic literature, advances in Internet communication, continuing education courses, and reasonably available transportation for patients, no disparity generally exists between rural and urban endodontic standards. A current exception may be the limited geographic availability of specific cone-beam CT technology such as the Accuitomo or Kodak 9000. Their higher resolution provides superior radiographic endodontic diagnosis compared with 2D imaging and even other CBCT devices.* As CBCT machine pricing is lowered and availability increases, the locality rule may be broadened beyond a local community for CBCT technology. On the other hand, time is critical if an endodontic overfill enters the IANC.206 In such instances, a referral to a distant CBCT facility may be required to assess the need for immediate microsurgical removal or instead obtain at the very least a medical CT in the local geographic area.
A clinician should provide reasonably careful endodontic care to a patient regardless of treatment locality. Rather than focusing on different standards for different communities, more important considerations include knowledge of endodontic advances in the field gained with continuing education to utilize improved diagnostics, instrumentation, and therapeutic interventions.
The locality rule has two major drawbacks. In areas with small populations, clinicians may be reluctant to testify as expert witnesses against other local clinicians. Also, the locality rule allows a small group of clinicians in an area to establish a local standard of care inferior to what the law requires of larger urban areas. Peer-reviewed publications are available to all clinicians in print and online. Clinicians can easily travel great distances to attend continuing education courses or attend webcasts in their own office; to blame technologic ignorance on a clinician’s rural location is inexcusable with modern media, computer technology, and travel ease.
Standards of Care: Generalist versus Endodontist
A general practitioner performing treatment ordinarily performed exclusively by specialists, such as apical endodontic surgery, periodontal surgical grafting, or full bony impaction surgery, will be held to the specialist’s standard of care.103,104 To avoid performing treatment that is below the specialist’s standard, a generalist should refer to a specialist rather than perform procedures that are beyond the general practitioner’s training or competency. The three levels of clinical skill are (1) competency or beginning level, (2) proficiency, and (3) mastery. To err is human. Even specialists may not always know what they believe they know.74,145 Approximately 80% of general practitioners in the United States provide some endodontic therapy. Endodontic expansion into the realm of the generalist can be linked to (1) refinements in root canal preparation and improved obturation (i.e., packing) techniques currently taught in dental schools; (2) continuing education courses; and (3) significant improvements in the armamentarium of instruments, equipment, and materials available to all clinicians.
Ethical Guidelines
The ADA’s Code of Ethics guides conduct that distinguishes the dental profession from a trade by placing patient service paramount and profit secondary.97 Section 5 of the ADA Code of Ethics regarding veracity (“truthfulness”) states that “Professionals have a duty to be honest and trustworthy in their dealings with people.” Accordingly, both ethically and legally,129 clinicians are obligated to communicate truthfully and without deception to patients. Announcements to the public must be truthful so that the average person is not materially misled. Truth in dentistry is the rule and not the exception.97
Section 5.I of the ADA Code of Ethics provides: General Practitioner Announcement … general clinicians who wish to announce the services available in their practices are permitted to announce the availability of those services so long as they avoid any communications that express or imply specialization. Thus clinicians should not misrepresent or overstate the facts of their training and competence in any way that would be false or misleading in any material or significant manner to the public or their peers. General clinicians should avoid false and misleading self-representation regarding endodontic “superiority.” Implying credentials which the generalist lacks misrepresents a general clinician’s endodontic skill and knowledge as equivalent to a board-eligible or board-certified endodontist. A generalist’s 30-year experience may be little more than 1 year’s experience repeated 30 times. Every state has specific laws governing advertising. Consult your state’s law, because each state deals differently with specialty announcements.
Telephone directory listings are organized into categories based on types of procedure, such as endodontics. Generalists must also state in such ads that endodontic services are being provided by general clinicians. The public is entitled to be informed of the distinction between an endodontist specialist versus a generalist, although each provide endodontics. Only a board-eligible or board-certified clinician is entitled to represent that the clinician is an endodontist. With this distinction in mind, the ADA-recognized endodontic specialty organization is entitled the American Association of Endodontists, not the American Association of Endodontics.
Standard of Care for Endodontics
Endodontists set the standard for routine endodontics. Therefore if the endodontist’s standard of care cannot be met, such as the need for microscopy, the generalist should refer the patient to an endodontist.55,103,104,149
Endodontists should not forget their general clinician training. Even though a patient may be referred for a specific procedure or undertaking, the endodontist should not overlook sound biologic principles inherent in the overall treatment. A specialist may also be held liable for relying solely on the information referral card or radiographs of the referring clinician if the diagnosis or therapeutic recommendations prove incorrect, unnecessary, or the referral card lists the wrong tooth for treatment. Fig. 11-13 demonstrates a general clinician’s radiograph showing apparent complete endodontic fill within the canal space, whereas Fig. 11-14 demonstrates an endodontist’s radiograph showing a transported canal. This difference is explained by radiographic quality and angulation. Taking radiographs at different angles improves radiographic accuracy.163,166
FIG. 11-13 A general clinician’s radiograph that ostensibly shows a complete endodontic fill within the root canal space.
Endodontists should not provide rubber-stamp treatment to whatever the clinician refers or recommends. Without performing an independent examination, the endodontist risks misdiagnosis and resulting incorrect treatment. Prevention of misdiagnosis or incorrect treatment requires an accurate medical and dental history preceding a clinical examination (not only of the specific tooth or teeth involved but also of the general oral condition). Obvious problems such as oral lesions, periodontitis, or gross decay should be noted in the chart and the patient advised regarding a referral for further examination, testing, or another specialist’s consultation.
Radiographs from the referring clinician should be reviewed for completeness, clarity, and diagnostic accuracy. An endodontist should expose a new radiograph to verify current status before treatment and only use the referring clinician’s radiograph for historical comparison. Unfortunately, the referring clinician may surreptitiously forward a pretreatment film with the referred patient, rather than the referring clinician’s own posttreatment film depicting a perforation or broken file that necessitated the referral. This may occur whenever an errant clinician attempts to fraudulently conceal negligently performed endodontic treatment in an attempt to shift blame for bungled treatment to the endodontist. In such cases, a valuable lesson is learned when the endodontist exposes independent pretreatment radiographs, rather than relying exclusively on the referring clinician’s radiographs to determine current status of endodontic treatment.
Poor oral hygiene may contribute to periodontal disease. In such cases, endodontic treatment may be compromised unless the associated periodontal condition, along with the tooth being tested endodontically, is brought under periodontal disease control, including any crown-lengthening needs (see Chapter 18 for more information). Referral to a periodontist may be necessary before or contemporaneous with completion of endodontic treatment.
In summary, it is necessary for the endodontist to:
Ordinary Care Equals Prudent Care
Ordinary is commonly understood (outside its legal context) to mean “lacking in excellence” or “being of poor or mediocre quality.”179 As expressed in the context of actions for negligence, however, ordinary care has assumed a technical legal definition somewhat different from its common meaning. The eighth edition of Black’s Law Dictionary describes ordinary care as “that degree which persons of ordinary care and prudence are accustomed to use or employ … that is, reasonable care.”31
In adopting this distinction, the courts have defined ordinary care as “that degree of care which people ordinarily prudent could be reasonably expected to exercise under circumstances of a given case.”83 It has been equated with the reasonable care and prudence exercised by ordinarily prudent clinicians under similar circumstances.107,213 It is not extraordinary or ideal care. Thus ordinary care is not average care but instead equates with prudently careful care. Stated otherwise, average care may be below the average of what all reasonable clinicians should practice.
Although the standard required of a professional cannot be only that of the most highly skilled practitioner, neither can it be limited to the arithmetic-average member of the profession, because those who possess somewhat less than median skill may still be competent and qualified to treat.213 By such an illogical definition of average care, half of all clinicians would automatically fall short of the mark and be negligent as a matter of law. As one court explained, “We are not permitted to aggregate into a common class the quacks, the young men who have not practiced, the old ones who have dropped out of practice, the good, and the very best, and then strike an average between them.”227 Thus the standard of care equates with reasonable and not average care.
Customary Practice versus Negligence
Customary practice may constitute evidence of the standard of care, but it is not the only determinant. Moreover, if the customary practice constitutes negligence, it is not considered reasonable (although or even if customarily practiced by a majority of clinicians).26 Rather, the reasonably careful clinician is the standard of care and not an average or mediocre practitioner. For instance, a majority of clinicians did not perform biologic testing of the dental unit water lines despite ADA recommendations to do so.181,183 However, careful clinicians did follow the ADA’s recommendation (Box 11-2 and Fig. 11-15).
BOX 11-2 Typical Examples of Negligent Customs
Data from American Academy of Orthopaedic Surgeons: Advisory statement, antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 134:895–899, 2003; Simonsen R: Greed and gravy train: is this success? J Esthet Dent 11:287, 1999; Rosenbaum S, Frankford DM, Moore B: Who should determine medical care necessity? N Engl J Med 340:229, 1999; Occupational Safety and Health Administration (OSHA): Occupational Safety and Health Administration Directive, Washington DC, 1999. Available at www.OSHA.90V; Cheung G: Endodontic failures. Int Dent J 146:131, 1996; Lyon TC: Quaternary ammonia compounds: should they be used for disinfection in the dental office? J Dist Columbia Dent Soc 48:10–18, 1973; Kutsch V, Milicich G, Domb W, et al: How to integrate CAMBRA into private practice. J Calif Dent Assoc, 2007; Fontana M, Zero D: Assessing patients’ caries risk. J Am Dent Assoc 137:1231–1239, 2006; Young D, Featherstone J, Roth J, et al: Consensus statement caries management by risk assessment: implementation guidelines to support oral health. J Calif Dent Assoc 35:799–805, 2007; ADA Caries Risk assessment forms. Assertion additional resources. J Am Dent Assoc Jan 12, 2009 update; Zeider S, Ruttimann U, Webber R: Efficacy in the assessment of intraosseous lesions of the face and jaws in asymptomatic patients. Radiology 162:691, 1987; Cotton TP, Geisler TM, Holden DT, et al: Endodontic applications of cone-beam volumetric tomography. J Endod 33:1121–1132, 2007; Kersten DD, Mines P, Sweet M: Use of the microscope in endodontics: results of a questionnaire. J Endod 34:804–807, 2008.
CAMBRA, Caries management by risk assessment; CBCT, cone-beam computed tomography; IANC, inferior alveolar nerve canal.
Merely because a majority of clinicians in a community practice a particular method does not establish it as the standard of care if such practice is unreasonable or imprudent.26 Ultimately, the courts determine what constitutes reasonable practice by considering available dental knowledge and evaluating the relative risks versus benefits of a particular procedure.
Again, the law does not require dental perfection.249 Instead, the legal yardstick by which prudent conduct is measured is what a reasonably prudent clinician should do under the same or similar circumstances, regardless of how many or how few clinicians conform to such standard.
In one case, it was not customary practice in the state of Washington for ophthalmologists to test patients under 40 years old for glaucoma, because the incidence was only 1 in 25,000 patients. Nevertheless, the Supreme Court of Washington state held that the defendant ophthalmologist was negligent as a matter of law, irrespective of customary medical practice, since a negligent custom is no defense.121,175
Little excuse exists for failing to routinely probe and chart periodontal pockets before rendering endodontic therapy, no matter how many other clinicians in the community may fail to do so. The benefit of probing for periodontal disease substantially outweighs the virtually nonexistent risk of conducting this valuable diagnostic procedure. A legal defense likely to invoke a jury’s wrath is to claim that a necessary diagnostic or prophylactic procedure is “too time consuming,” when dental and medical patient health are placed at risk if not done. If the benefit outweighs the risk a reasonable clinician complying with the standard of care should opt for the beneficial diagnostic test or treatment. For instance, the Accuitomo and Kodak 9000 CBCT may prove an invaluable benefit for diagnosing the etiology of tooth or endodontic pain when compared to the relatively low radiation risk. Thus the CBCT diagnostic benefit outweighs the radiation risk if a differential diagnosis can not accurately rule in or rule out a final diagnosis.165
Continuing Education
Continuing education should not add new skills at the expense of compromising or eliminating core clinical values. A prudent practitioner should be cautious adding a new methodology that is counterintuitive to basic biomechanical principles.185 As a general maxim, “Don’t be the first nor the last to adopt new technology.”
Scientific Research Evaluation
In evaluating scientific research, the clinician should remember that epidemiologic data of risk factors are not always equivalent to etiology. Thus epidemiology is not a synonym for etiology. The scientific community has never exclusively relied on epidemiology as the accepted method of evaluating cause-and-effect relationships in an effort to make clinical decisions. Accordingly, clinicians should not consider only one class of data to supply ultimate proof when conducting an evaluation. Instead, clinicians should consider the strength of any study to be related to the presence and contributing cause of other cofactors.108,197,198,205 The unusual characteristics of a particular patient can place the patient at higher risk than the average patient.124
New products and procedures are often introduced faster than epidemiologic and toxicologic studies are implemented to evaluate their potential risks. Academic medical/dental centers are facing severe pressure on their reimbursement for health care services and postgraduate education. This creates the need to examine additional sources of revenue to fund their tripartite mission of education, research, and patient-care delivery. Reduced government spending for research has resulted in proprietary interests (with proprietary goals) funding more research.118 Since 1984, the New England Journal of Medicine’s policy has been to refuse publication of research done by those with financial ties to drug makers. But in 1999, the journal admitted conflicts in nearly half of the drug studies published since 1997.15 The next year, the retiring editor of the New England Journal of Medicine concluded that despite tax-supported privileges and extraordinary pharmaceutical industry profits, the best interests of society were not always served. A public trust more accountable to science was needed.14
Evidenced-Based Endodontics
Although much emphasis is currently placed on evidence-based medicine and dentistry, most clinical practices are not based on data derived from randomized clinical trials.198 It is virtually impossible to conduct a trial to test the validity of every possible patient-management option. Many therapeutic choices are so compelling that a test would be unethical; others are so trivial that a test might not be worth the time or effort. Clinicians do not always practice in conformity to evidence-based research, even when evidence is available from randomized clinical trials. This is because knowing the right answer is only the first step in the process of adopting a new treatment methodology. Many clinicians will not use a new drug simply because supporting research data indicate that it works. Instead, identification of the probable mechanism of action is often a prerequisite before adopting a new treatment. Moreover, the test of time may eventually reveal an adverse effect of a particular drug or device (e.g., defects that were not identified before marketing). Even if the new product’s research proves beneficial, comparative effectiveness studies with other time-proven and tested products are needed to determine a new product’s relative worthiness.108,159,205,239
Nevertheless, clinicians should not avoid using certain drugs if the data supporting their use are overwhelming. When the results of a clinical trial conflict with a widely held mechanistic model, many clinicians doubt research-based evidence.187 However, when the results from well-researched clinical trials become so convincing that the evidence can no longer be ignored, a prudent clinician embraces the new paradigm and discards previously held concepts. Endodontic science advances as disproved older concepts retreat. For example, delaying extractions until after several days of antibiotic use to reduce infection is no longer the accepted practice. Rather, immediate extraction, along with any necessary antibiotics, is the preferred current therapy.4 Another example is one-visit endodontics, which has proven as effective as two-visit endodontics.84,186,202
New Products
New devices that lack definitive research studies should be used cautiously. For instance, high-intensity, wireless, fast-curing lights may generate heat at the wand tip and cause pulpal pathosis. First-generation halogen bulbs require longer composite curing time to achieve polymerization but generate only 400 to 800 mW/cm. Plasma arc bulbs reduce curing time but generate 2000 mW/cm.
Hydron was marketed without long-term clinical testing. Its purported biocompatibility and reduced inflammation portended an improved endodontic drug for obturation. Postmarketing failures resulted from inadequate premarket testing that lacked long-term research. Thus Hydron’s inability to obturate canals with a durable filling material resulted in both clinicians and patients being harmed as postmarket guinea pigs. Hydron’s in vitro research did not match in vivo patients’ experiences.
Other examples include Endocal 10 (formerly Biocalex 6/9), which proved ineffective. Arsenic to promote devitalization of the pulp resulted in local osteonecrosis. Thermafil metal carriers proved difficult to remove and were replaced with plastic carriers. Advance cement was touted as an improved luting cement for retaining permanent restorations. The high incidence of marginal leakage with Advance resulted in its withdrawal from the market in 2000. A multimillion-dollar class action settlement resulted against the manufacturer on behalf of California clinicians with unsealed margins of crowns cemented with Advance luting agent.101
Older devices, over time and with sufficient patient experience, may manifest adverse events. Overheating with ultrasonic tips for post removal has resulted in teeth and tissue loss (Fig. 11-16). Accordingly, despite insufficient label warnings on some ultrasonic devices, ample water coolant, rest periods, and avoidance of prolonged ultrasonic device use are needed for safe post removal.68,71,102
FDA Approval
Despite FDA approval, some drugs are marketed with fraudulent concealment of the number and nature of adverse event incidents.8
FDA Clearance
The applicant must receive FDA approval of its PMA application prior to marketing the device as FDA approved. PMA approval is based on an FDA determination that the PMA contains sufficient valid scientific evidence to assure that the device is safe and effective for its intended uses.
The FDA lists certain products which are exempt from premarket notification requirements under The Food and Drug Administration Modernization Act of 1997 (The Modernization Act),86 which include the following endodontic products:
Negligence Per Se
Compliance with a health safety statute does not conclusively establish due care, because regulations require only minimal care and not (necessarily) prudent care or what the law regards as due care.58,248
Ability to Foresee Unreasonable Risk
Each endodontic procedure has a variable degree of inherent risk. The standard of care requires that the clinician avoid unreasonable risks that may harm the patient. Treatment is deemed negligent when a reasonably careful clinician would have foreseen some unreasonable risk of harm to the patient. Failure to follow the dictates of sound endodontic practice increases the risk of negligently induced deleterious results. Accordingly, prophylactic endodontic practice is designed to prevent foreseeable or reasonably avoidable injury risks. Foreseeability connotes predictability.
It is not necessary that the exact injuries that occur be foreseeable. Nor is it necessary to foresee the precise manner or circumstances under which injuries are inflicted. It is enough that a reasonably prudent clinician would foresee that injuries of the same general type would likely result in the absence of adequate safeguards.213
Informed Consent Principles
In General
The legal doctrine of informed consent requires that the patient be advised of reasonably foreseeable material risks of endodontic therapy, the nature of the treatment, reasonable alternatives, and the consequences of nontreatment.51,141,182 This doctrine is based on the legal principle that individual patients have the right to do with their own bodies as they see fit, including the right to prematurely lose teeth regardless of recommended dental treatment. Once the clinician has informed the patient of the diagnosis, treatment risks, prognosis, and nontreatment risks, as well as recommendations for corrective treatment or alternative therapy, the patient has the right to decide how or if to proceed. An adult of sound mind is entitled to elect to do nothing about existing endodontic disease. Rather than elect corrective treatment, the patient may elect to suffer nontreatment consequences, including present or future tooth loss and/or apical abscess.
To be legally effective, a patient’s consent to treatment involving potentially serious injury complications must include informed consent. Accordingly, a clinician has a fiduciary duty to disclose all material or essential information necessary for the patient to make a decision.51,141,182 The scope of a clinician’s duty to disclose information is measured by the amount of knowledge a reasonable patient requires to make an informed choice. Material information is that disclosure a clinician knows (or should know) that would be regarded as significant by a reasonable person in the patient’s position who must decide whether to accept or reject a recommended endodontic procedure.
If a clinician fails to reasonably disclose information that would make a reasonable person in the patient’s position decline the procedure, the clinician may be liable should an undisclosed risk manifest. Beyond the foregoing minimal disclosure, a clinician must also reveal such additional information as a skilled practitioner of good standing would provide under similar circumstances.
The personal bond between clinician and patient has long been considered an essential element of the therapeutic environment. Information is empowering. Laws requiring informed consent place patients on a closer footing with their clinicians. In addition to the transfer of information in both directions, bonding takes place when the clinician and patient engage in a conversation of length and substance. Canned disclosure displaces this human interaction. One of the surest safeguards clinicians can have against malpractice litigation is the bond of personal relationship forged with their patients.221
Informed Consent
Adequate informed consent requires that a patient be informed of the ABC’s—the alternatives, benefits, and complications—of a proposed treatment, along with the pros and cons of the alternative therapies or options.51,141,182 A patient has a right to be informed of these ABC’s from the treating clinician and not solely from an informed consent form or by a front office staff person who lacks a dental license.
Informed consent must be presented in layperson’s language, so that the patient can appreciate and understand what the treatment risks are.
A patient is not expected to consent to negligent care. Accordingly, informed consent applies only to treatment which complies within the standard of care. If the clinician is reasonably careful during treatment, but an untoward results occurs, the clinician is not liable if the patient was forewarned of the potential risk prior to the treatment.
A reasonably unavoidable risk which manifests during treatment represents a maloccurrence and not malpractice. For example, a small percentage of endodontic failures are due to non-negligent reasons. The clinician should be sensitive to the patient’s feelings of disappointment. When endodontics fails, regardless of cause, it is a 100% failure rate for the patient.
Patient treatment refusals should be documented. Some states require informed refusal disclosures advising the patient about the consequences of refusing treatment or diagnostic modalities.
Consent
Simple consent occurs when the clinician makes the treatment decision unilaterally and obtains the patient’s consent without offering the patient a choice. Conversely, informed consent is a joint decisional process whether to proceed with treatment or not.
Patients are legally empowered to decide for themselves when substantial competing risks are involved with each separate treatment choice. A patient’s therapeutic choice is particularly apt when there is no professional consensus for the best treatment. For instance, different implant systems exist with arguably different risks for each. A decision to proceed with an immediate implant versus waiting for osseointegration before initiating restorations requires that a risk-versus-benefit analysis be provided to the patient. Such decisions should include comparative peer-reviewed longevity research results compared with newer short-term immediate implant research. The benefits of immediate implant placement are attractive to both patient and clinician. To fairly compare researched implant survivability studies, the variables should similarly be controlled for each study, such as whether the implants are placed in native bone, socket size, and shape.
Emergency endodontic treatment often requires that the patient be provided pain relief. Acute pain clouds the patient’s decisional process; palliative treatment provides immediate pain relief so that on a return visit, the patient can rationally decide among treatment choices. With ample time, the patient can rationally decide and compare choices such as completion of endodontics and a crown versus extraction followed by an implant with crown. Also, an extraction which may result in alveolar ridge bone loss and the need for bone grafting are considerations the clinician should explain to the patient.
Patient choices may be influenced by cost, convenience, time off work, travel needs, esthetics, or a myriad of other reasons. The clinician plants the seeds of the decisional tree, but the patient decides which branch of the tree should be followed.
Informed Consent Application
Informed consent is a flexible standard that considers reasonably foreseeable consequences, depending on the clinical situation present both before and during treatment. For instance, a fractured or separated endodontic instrument left in the root canal creates the possibility of root canal failure or impaired success (depending on whether the fracture occurred in the coronal, middle [least favorable], or apical third of the root canal). The clinician must advise the patient of the relative risk of future failure and suggest treatment alternatives to correct the problem. An adequately informed patient can better make an intelligent choice among apicoectomy, referral to an endodontist utilizing microscopy for attempted retrieval, or close radiographic observation at recall visits.55,149,160 In Fig. 11-17, the clinician overinstrumented and perforated the inferior alveolar nerve (IAN), causing both a permanent dysesthesia and paresthesia. The clinician should have immediately referred the patient to an endodontist for attempted gutta-percha retrieval before the endodontic sealant containing eugenol set and caused more deleterious chemical injury to the IAN. If retrieval was unsuccessful, the patient should have been referred to a microsurgeon within 48 hours from occurrence for optimal opportunity to prevent chemical cytotoxic injury to the IAN.206
FIG. 11-17 A, Preoperative radiograph. B, Measuring film without a rubber dam. C, Postoperative radiograph. D, Decompression microsurgery showing protruding “dagger” of bone to right of retractor at inferior border of inferior alveolar nerve, resulting from prior perforation of the mandibular canal. This is probably due to file overinstrumentation.
(Courtesy Anthony Pogrel, DDS, MD.)
Adequate disclosure includes clinical judgment and experience, which assesses current research and applies it to the clinical needs of each patient. Today’s advance may be tomorrow’s retreat if materials, devices, or instruments lacking adequate long-term study of safety and efficacy predictably fail. For instance, the one-component bonding agent is more technique sensitive than its two-component predecessor. This is because the one-component agent contains acetone that desiccates the tooth surface and requires a moist surface to avoid postoperative sensitivity. Long-term testing before marketing would have revealed that the two-step system is more reliable and forgiving.250 Reasonable clinicians do not sacrifice patient safety for speed and profit and then compensate with desensitizing agents. Clinicians should be more sensitive to limited research on new products or devices lest the patient be the injured guinea pig.
Material disclosure concerns whether the patient was provided sufficient information for a reasonable patient to achieve a general understanding of the proposed treatment or procedure. This disclosure includes information concerning any dentally acceptable alternatives, any predictable risks of serious injury, and any likely consequences should the patient refuse the proposed therapy. The standard to be applied is whether a reasonable person in the patient’s position would have consented to the procedure or treatment in question if adequately informed of all significant perils.48 Informed consent applies only to inherent risks of non-negligent treatment, because a patient’s consent to negligent treatment is voidable as being contrary to public policy.253 Accordingly, a patient who refuses necessary diagnostic radiographs should be refused treatment.
If warnings are limited to some risks but not others, some courts have held that the patient can recover for all surgical injuries including warned risks.130
Restorative Informed Consent
Patient choices for restorations may discount longevity and elect instead esthetics. In one study, amalgams lasted 15 years, composites 6 years, and conventional glass isomers 7 years.91 Shorter-term restorations’ longevity increases the frequency of restoration replacement and increased potential for endodontic therapy with each replacement. Nevertheless, it is the patient’s treatment choice after the clinician first explains relative risks, benefits, and alternatives.
Different Schools of Thought
After the clinician advises treatment, if other reasonable clinicians would disagree or if there are other respectable schools of thought on the correct treatment, this material information should be disclosed to the patient.261,281 For example, there may be two schools of thought concerning the optimal treatment for retrofilling and apicoectomy. One school posits that a retrograde with intermediate restorative material (IRM) mineral trioxide aggregate (MTA) is the appropriate procedure, whereas the minority review asserts that retrograde with Super EBA is the preferred treatment.278 One can assume further that an explanation of these two different methods of treatment constitutes material information for the purposes of informed consent. The mere fact that there is a disagreement within the relevant endodontic community does not establish that the selection of one procedure as opposed to the other constitutes negligent endodontic therapy. Because competent endodontists regularly use both procedures, a patient would have a difficult time proving dental negligence (i.e., that the endodontist failed “to have the knowledge and skill ordinarily possessed, and to use the care and skill ordinarily used, by reputable specialists practicing in the same field and in the same or a similar locality and under similar circumstances”). Moreover, neither school of thought possesses long-term clinical research results to prognosticate apical sealant insolubility rates in an 18-year-old patient with an additional 60-year life expectancy.237 Long-term durability depends on an insoluble seal to reduce the risk of bacterial leakage.48,189 Fig. 11-18 represents a preoperative and postoperative radiograph of a successful apicoectomy and retrograde in a 14-year-old.
FIG. 11-18 A, Preoperative panoramic radiograph showing a successful apicoectomy and retrograde seal. B, Postoperative panoramic radiograph showing a successful apicoectomy and retrograde seal.
(Courtesy Edmond Bedrossian, DDS.)
On the other hand, the specialist would have a duty under the above hypothetical circumstances to disclose the two recognized schools of treatment so that the patient could be sufficiently informed to make the final personal decision. An endodontist, being the expert, appreciates the risks inherent in the procedure prescribed, the risks of a decision not to undergo the treatment, and the probability of a successful outcome of the treatment. Once this information has been disclosed, this aspect of the endodontist’s expert function has been performed. The weighing of these risks against the individual subjective fears and hopes of the patient is not an expert skill. Such evaluation and decision constitute a nondental judgment reserved for the patient alone.51,141 In this hypothetical situation, failure to disclose such material information would deprive the patient of the opportunity to weigh the risks. Consequently, the clinician would have failed in the duty of disclosure, which the doctrine of informed consent requires.
When improved technology offers clearly superior results, it no longer becomes a patient choice issue but rather a requisite requirement to fulfill the standard of care. Apical microsurgery with ultrasonic tips for retrofilling exemplifies improved technology and the current standard of practice. Likewise, apical retrogrades should be done with MTA and not with amalgam.48 Finally, microscopic endodontics represents the current standard of care for apical surgery or fractured instrument retrieval.55,136,149,160
Avoiding Patient Claims
If a clinician fails to obtain adequate informed consent, a plaintiff can recover damages (even in the absence of any negligent treatment) should the patient testify that performed treatment would have been refused if the clinician had provided information concerning possible risks. Therefore discussions of treatment risks with the patient must be documented. Informed consent forms are very helpful, although not legally mandated, because a jury may believe that the patient was informed orally. Equally if not more important than consent forms is a chart notation indicating that the clinician discussed informed-consent risks and alternatives (and that the patient understood and accepted this information). Patients may mentally block out frightening information. Trauma and a potent anesthetic agent can create retrograde amnesia. Therefore clinicians must document (in the patient’s chart) any risks, benefits, alternatives, and consequences of nontreatment provided to the alert patient before sedation.
Clinicians should follow only the patient-authorized and consented treatment plan. If an emergency precludes advising treatment risks to the minor patient, lack of informed consent may be defensible as implied consent (because no reasonable parent would refuse necessary, emergency treatment).
The following example demonstrates how a clinician should record any recommended treatment the patient has refused and the reason for refusal:
“Patient refused endodontic referral for consultation with endodontist (Dr. Goodguy) because husband was laid off work last month and cannot afford treatment. After Dr. G. provided explanation, patient states she understands detrimental delay risks.”
Patients may initial any referral refusal on the chart. Although patient signature is not mandatory, it will enhance the clinician’s credibility should the patient later dispute a referral was made.
Reasonable familiarity with a new product or technique is required before it is used. In addition, a patient is entitled to know the clinician’s personal experience with a particular modality because the patient has a right to choose between reasonable alternatives or to seek care from a clinician who has more extensive experience with a particular modality or new product. A clinician who fails to obtain informed consent may be liable for injury caused by a product or instrument. The fact that the clinician followed the manufacturer’s instructions is no defense if the clinician did not provide adequate information concerning a product or instrument risk (to permit the patient to intelligently weigh the disclosed information and choose among treatment options).
Endodontic Informed Consent
If the practitioner’s own statistical experience varies significantly from national statistics, using statistics presented in national literature regarding success rates for endodontic procedures is considered insufficient disclosure and does not fulfill the legal requirements of informed consent (Fig. 11-19).116,236
Among specialists, the reported incidence of treatment complications in endodontics is relatively low. Based on a Southwest Endodontic Society retrospective study, a reasonable endodontist or a practitioner with similar abilities should disclose the following facts to patients233:
Video-Informed Consent
Animated video-informed consent shown to the patient is a dynamic method of providing informed consent. Because the video-informed consent is considered part of the clinician’s records, in the event the patient disputes having ever been advised of (1) the nature of endodontic disease, (2) the availability of endodontic specialists, or (3) the relative indications for nonsurgical versus surgical endodontic care, the videotape can be played back to the jury as proof that the patient was informed. It is doubtful a jury would believe a forgetful patient who admits having previously viewed the videotape, because the videotape refreshes the stream of memories that may have otherwise faded into the unconscious.
A patient viewing the videotape is more likely to understand the informed consent disclosure of a technical procedure. After viewing the videotape and discussing its contents with the clinician, the patient should sign a video consent form to verify that the video was viewed, and all the patient’s questions were answered.
Alternative Technique Choices
Filling the root canal with the lateral compaction method or instead using vertical warm gutta-percha carrier delivery systems, including heat-transfer units for warm gutta-percha, are both currently taught methodologies.160 Each technique has zealous advocates. Both methods are reasonable and acceptable choices, and each method conforms to the standard of care. The choice of techniques changes as new products are introduced and scientific research is conducted. The vertical warm gutta-percha technique promises to deliver improved flow to fill lateral canals and is likely to predominate in the 21st century as the preferred methodology. Nonetheless, lateral compaction has a long-term proven track record, particularly with less-experienced practitioners, and is less technique sensitive.
Referrals to Other Specialists
Every clinician, including specialists, will at some time need to refer a patient to another specialist for treatment to comply with the standard of care required of a reasonably careful clinician.98
Generally, if the referral takes place within the same dental practice, the legal doctrine of respondeat superior (“let the master answer”) may be applicable. Under this doctrine, a clinician is liable for the dental negligence of a person acting as his or her agent, employee, or partner.214 Liability is determined by whether the principal clinician controls the agent clinician’s activities or methodology, regardless of whether such control is actually exercised.
If the referral is made (even within the same physical environment) to an “independent contractor” endodontist who does not diagnose or treat under the direction or supervision of the referring clinician, and that referring clinician has no right of control as to the mode of performing the treatment, the principle of agency and responsibility for the acts of another does not apply.184 The legal test for agency is the principal’s right to control, regardless if the control is exercised over the agent. However, if the independent contractor or referring clinician in the same practice does not provide adequate notice of this independent relationship to the patient, the independent contractor will still be liable under the legal doctrine of ostensible agency. Thus, despite an independent contractor agreement, a patient can legally infer the so-called independent clinician is instead an employee, since neither clinician in the group practice disclosed an independent contractor relationship to the patient.
To ensure that the referred clinician is not considered the agent of the referring clinician within the same facility, fees for the referred clinician should not be set by the referring clinician nor should the fees be divided equally or shared based on some other arrangement. Also, the referred clinician should bill separately and exercise independent diagnostic and therapeutic judgment. The patient should be advised that the referred clinician is independent of the referring clinician. Otherwise, the referred clinician or specialist may be regarded or inferred to be the ostensible agent of the referring clinician in the same office although not intended. The legal test for agency is how the facts or circumstances appear to a reasonable patient, regardless of the clinician’s understanding or written contract intent that the other treating clinician is to be considered an independent contractor. Thus, agency may be actual in fact or, alternatively, ostensible (i.e., implied by circumstantial conduct).142
Surgical versus Nonsurgical Endodontics
Litigation to determine whether nonsurgical or surgical endodontics was the proper treatment choice will not be decided by any one clinician, the ADA, the AAE, or the ablest of judges. Rather, after considering all the evidence and the opinions of experts, a jury of the patient’s peers will decide the disputed matter. Depending on the individual case, the jury may decide that either a combination of nonsurgical and surgical endodontic therapy, rather than one method exclusively, should have been attempted. The jury may also decide that the patient should have been advised of the availability of such alternative therapy. Similarly, the jury may determine that apical surgery should have been done microscopically rather than macroscopically, depending on expert testimony regarding the relative advantages of each method or upon presenting clinical circumstances (e.g., suspected calcification, viewing a separated instrument).
Microscopic Endodontics
Microscopic endodontics improves posture and reduces neck and back fatigue.13 Identification of microfractures in teeth55,149,160,191 and removal of diseased dental tissue are easier and more accurate when done microscopically.
“The operating microscope is an indispensable tool for state-of-the-art endodontic treatment. The specialty practice should not be without a microscope; this instrument is useful in all phases of endodontic treatment from diagnosis to placement of the final restoration.”136
Successful root canal treatment requires the prevention of microorganisms and toxins from the oral flora penetrating through the root canal system into the periapical tissues. Inadequate obturation of the root canal system is the most frequent cause of failure. Either coronal or apical leakage adversely affects success.63,64,241
Failing endodontics that necessitate endodontic disassembly should first be considered for a nonsurgical option because it is less invasive and therefore represents risk reduction. The clinician should first evaluate for coronal leakage, fractures, missed canals, silver point corrosion, and incomplete obturation. Root canal systems can then be recleaned or correctly transported and then thoroughly disinfected, reshaped, and packed in three dimensions to avoid apical surgery by providing instead adequate coronal, lateral, and apical seal nonsurgically.
New Products
Today’s clinician exploring ways to improve the quality and success of endodontic therapy is constantly presented with new dental products and techniques. For prescribing and using drugs or other agents, the ADA’s Principles of Ethics, Section 5D, provide this guideline10:
“Except for formal investigative studies, dentists shall be obliged to prescribe, dispense, or promote only those devices, drugs and other agents whose complete formulae are available to the dental profession. Dentists shall have the further obligation of not holding out as exclusive any device, agent, method or technique if that representation would be false or misleading in any material respect.”
Ethical clinicians should not indiscriminately adopt every new product. Instead, the supporting research should be reviewed, rather than risk the patient’s welfare with inadequately tested or researched products only tested in vitro. Again, “Don’t be the first nor the last to adopt new technology.”
Separated Instruments
Risk reduction of broken files can be accomplished if all hand and rotary nickel and titanium (NiTi) cleaning and shaping files are not resterilized and reused but are discarded after single-tooth use. Efficiency is also increased, because approximately 50% of cutting efficiency is lost after initial use. Episodes of broken instruments will be dramatically reduced and nearly eliminated when files are used correctly and discarded after single-tooth use. Breakage increases sharply when hand or rotary shaping files are reused. Therefore the clinician should discard rotary instruments after a single-use visit. Chair and staff time efficiency, along with improved safety, dictates single-visit use of files.189
The clinician should save broken or defective instruments (e.g., a file that was broken inside a canal) (Fig. 11-20). The instrument manufacturer may be liable because the product was defective, rather than the clinician being liable for dental negligence.215 Electron microscopy spectrographic analysis can determine if manufacturing defects with contaminants caused the break, rather than the clinician excessively stressing the instrument.
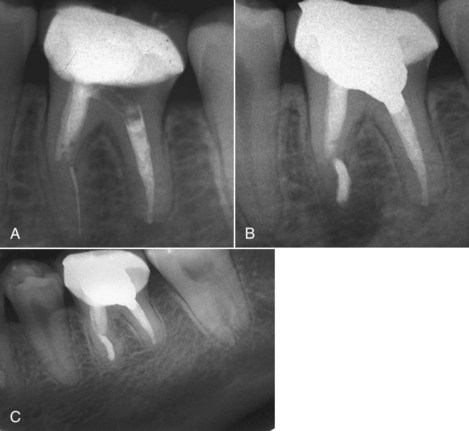
FIG. 11-20 A, Nickel-titanium file broken inside the mesiobuccal canal of the mandibular first molar. B, Due to patient discomfort, the segment was surgically removed and MTA was used as root-filling material. C, Periapical radiograph  years post-treatment shows complete healing.
years post-treatment shows complete healing.
(From Torabinejad M, Walton RE: Endodontics: principles and practice, ed 4, St. Louis, Saunders/Elsevier, 2009.)
Equipment and Supplies
Equipment should be kept in good repair by checking and following the manufacturer’s recommended maintenance schedule. The clinician should carefully read the manufacturer’s instruction warnings on instruments and inform staff of any important points. Infection control in operating dental equipment is mandatory, such as updating and maintaining dental units with check valves to prevent water retraction or suck-back. The clinician should inspect check valves monthly and change clogged valves immediately.152 Water retraction testers, at no charge to the clinician, are available from some manufacturers (A-dec [Newberg, OR], for instance). The clinician or a staff member should also disassemble the unit’s handpiece, run water through the line for a few seconds, and then stop. If a bubble of water is visible at the end of the water hose holes, the check valve is operating properly. If a bubble of water is not visible, water may be sucked back because of an absent or clogged check valve. An absent or clogged check valve is a source of cross-contamination, so it is important for the clinician or staff to perform weekly spore testing of the autoclave and monthly bacteriologic testing of water lines.11 The clinician or staff should consider using filters and should flush dental-unit water lines daily with FDA-approved chemical disinfectants to reduce water tubing biofilm. Some chemical disinfectants claim improved cost effectiveness by increasing handpiece and bur longevity concomitant with water-line purging or with continual use during treatment. Fig. 11-21 shows a student who was burned by an inadequately maintained handpiece that overheated. This case settled for $280,000.
Prescription Drugs
Clinicians should exercise extreme caution when administering or prescribing dangerous drugs. For sedative or narcotic drugs, cautionary directions should be written on prescriptions, and the pharmacist should place these directions on the prescription container as a patient reminder. For example, for the appropriate drugs, the clinician should prepare a prescription rubber stamp or obtain preprinted prescriptions that state the following:
“Do not drive or operate dangerous machinery after taking medication because drowsiness is likely to occur. Alcohol, sedative, or tranquilizing drugs will cause drowsiness if taken in combination with this prescribed drug.”
The ADA and American Medical Association (AMA) provide prescription drug warning pads. Clinicians should document each drug information form provided with the prescription in the patient’s chart.
Overuse of antibiotics risks resistant-strain development and side effects. Studies demonstrate generally no increased therapeutic efficiency of endodontic therapy with antibiotics when performed in the absence of facial swelling.262 Unless persistent infections occur or compelling systemic reasons exist (e.g., uncontrolled diabetes, antibiotic prophylaxis necessary because of mitral valve regurgitation), antibiotics should not be prescribed prophylactically.188 Neither pain nor localized swelling alone justifies antibiotics. However, extraoral swelling, cellulitis, or lymphadenopathy may require surgical drainage, antibiotics, or both. The U.S. Centers for Disease Control and Prevention (CDC) estimates about one third of all antibiotic outpatient prescriptions are unnecessary.9
Product liability may include whether the manufacturer provides adequate hazard warnings for safe use of its product (Fig. 11-22).
Clinician’s Liability for Staff’s Acts or Omissions
A clinician is liable for the acts or omissions of the clinician’s staff under the doctrine of respondeat superior (“let the master answer”). This is termed vicarious liability, which means that the clinician is responsible not because of personal wrongdoing, but because the clinician assumes legal responsibility for the conduct of employees and agents who act in the course and scope of their employment.
The clinician should instruct the staff in advising patients regarding posttreatment complaints. For example, if the staff ignores signs of infection, such as difficulty in swallowing or breathing or elevated temperature, and dismisses the patient’s complaints as normal postoperative swelling, the clinician may be held liable for injury to the patient, such as delayed cellulitis, diagnosis or treatment of Ludwig’s angina, brain abscess, or other serious complications.
A clinician must be cautious when delegating responsibilities and give clear instructions to ensure that staff members properly represent the clinician and the chosen practice methods. Auxiliaries should not be allowed to practice beyond their competency level or license. For example, in states where assistant-placed restorations are legally permissible, the clinician should check any assistant-placed restoration before patient dismissal. Staff members should not make final diagnoses or handle patient clinical complaints without the clinician’s involvement and consultation. Staff should be instructed to ask appropriate questions and relay the patient’s answers to the clinician so the clinician can adequately determine what should be done.
Abandonment
Once endodontic treatment is initiated, the clinician is legally obligated to complete the treatment regardless of the patient’s payment of any outstanding balance. This requirement is posited on the legal premise that any person who attempts to rescue another from harm must reasonably complete the rescue with beneficial intervention unless another rescuer (i.e., clinician) is willing to assume the undertaking.50,158 Another view is that should a patient be placed in a position of danger unless further treatment is performed, the clinician must institute reasonable therapeutic measures to ensure that adverse consequences do not result.174,240
A clinician performing endodontic therapy should have reasonable means of communicating with patients after regular office hours to avoid a claim for abandonment. A recorded message is inadequate if the clinician fails to check for recorded messages frequently. Therefore answering services, pagers, and/or cell phones are required by the standard of care.
If the clinician providing endodontic therapy is away from the office for an extended period, a substitute on-call clinician should be available for any endodontic emergency and to answer patients’ emergency calls. The endodontic treating clinician should arrange in advance for emergency service with a covering clinician. Leaving a name on the office answering machine or with the answering service without first determining the availability of the covering clinician is a mistake.
To avoid an abandonment claim, several prophylactic measures apply:
“Emergency palliative treatment only. Patient advised endodontic treatment of tooth #8 needs to be completed, either here or with another clinician. Explained complications likely if not soon completed, including infection recurrence, tooth loss, or both.”
The patient should also be asked to acknowledge that treatment is limited to the existing emergency by endorsing an informed consent to emergency endodontics statement as follows:
“I agree to emergency endodontic treatment of my tooth #8 and have been advised that (1) emergency treatment is for temporary relief of pain and (2) further root canal treatment of tooth #8 after emergency treatment is necessary to avoid further complications, including, but not limited to, pain, infection, swelling, tooth fracture, abscess, or tooth loss.”
The clinician may discontinue treatment, provided it is not done at a time when the patient’s dental health will be jeopardized (e.g., in the middle of treatment). To discontinue treatment, the clinician should: