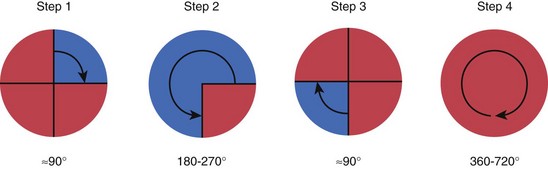
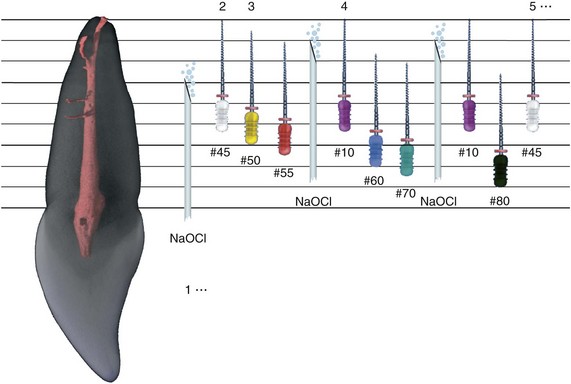
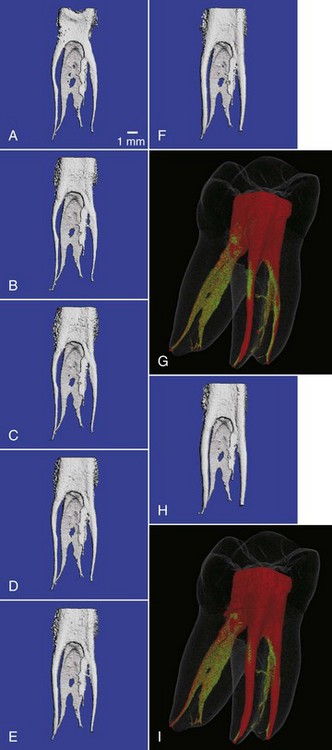
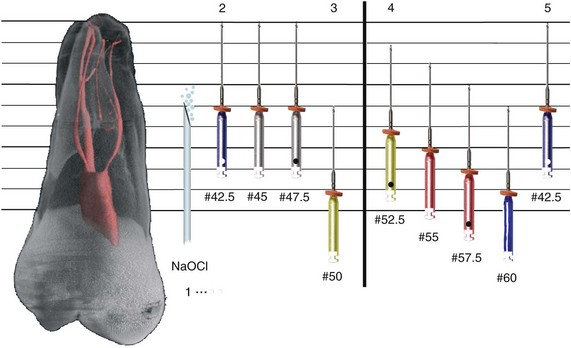
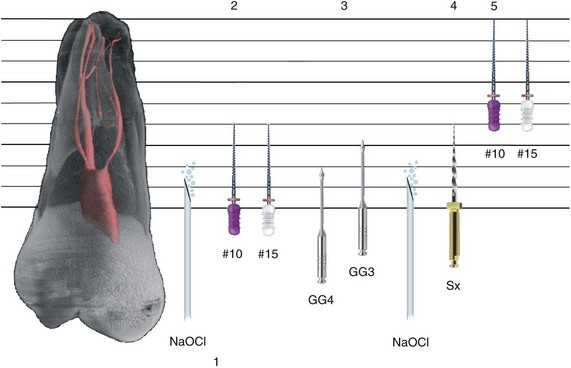
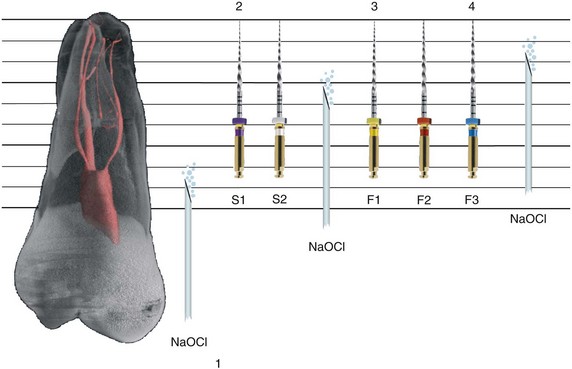
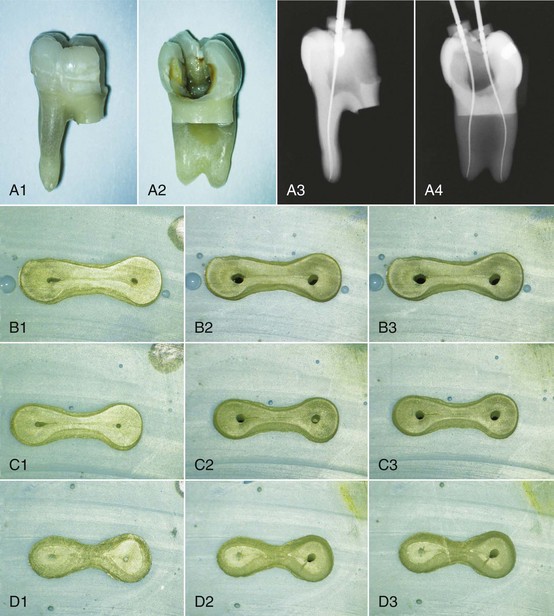
1. Abbott PV. Assessing restored teeth with pulp and periapical diseases for the presence of cracks, caries and marginal breakdown. Aust Dent J. 2004;49:33.
2. Abbott PV. The periapical space–a dynamic interface. Aust Endod J. 2002;28:96.
3. Abbott PV, Heijkoop PS, Cardaci SC, Hume WR, Heithersay GS. An SEM study of the effects of different irrigation sequences and ultrasonics. Int Endod J. 1991;24:308.
4. Abou-Rass M, Frank AL, Glick DH. The anticurvature filing method to prepare the curved root canal. J Am Dent Assoc. 1980;101:792.
5. Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod. 1981;7:376.
6. Abou-Rass M, Patonai FJJr. The effects of decreasing surface tension on the flow of irrigating solutions in narrow root canals. Oral Surg Oral Med Oral Pathol. 1982;53:524.
7. Abou-Rass M, Piccinino MV. The effectiveness of four clinical irrigation methods on the removal of root canal debris. Oral Surg Oral Med Oral Pathol. 1982;54:323.
8. Ahmad M. Effect of ultrasonic instrumentation on Bacteroides intermedius. Endod Dent Traumatol. 1989;5:83.
9. Ahmad M, Pitt Ford TR, Crum LA, Wilson RF. Effectiveness of ultrasonic files in the disruption of root canal bacteria. Oral Surg Oral Med Oral Pathol. 1990;70:328.
10. Al-Jadaa A, Paqué F, Attin T, Zehnder M. Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: impact of canal location and angulation. Int Endod J. 2009;42:59.
11. Al-Majed I, Murray JJ, Maguire A. Prevalence of dental trauma in 5-6- and 12-14-year-old boys in Riyadh, Saudi Arabia. Dental Traumatology. 2001;17:153.
12. Al-Omari MA, Dummer PM, Newcombe RG. Comparison of six files to prepare simulated root canals, Part 1. Int Endod J. 1992;25:57.
13. Al-Omari MA, Dummer PM, Newcombe RG, Doller R. Comparison of six files to prepare simulated root canals. Part 2. Int Endod J. 1992;25:67.
14. Al-Sudani D, Al-Shahrani S. A comparison of the canal centering ability of ProFile, K3, and RaCe Nickel-titanium rotary systems. J Endod. 2006;32:1198.
15. Alapati SB, Brantley WA, Nusstein JM, Daehn GS, Svec TA, Powers JM, et al. Vickers hardness investigation of work-hardening in used NiTi rotary instruments. J Endod. 2006;32:1191.
16. Alapati SB, Brantley WA, Svec TA, Powers JM, Mitchell JC. Scanning electron microscope observations of new and used Nickel-titanium rotary files. J Endod. 2003;29:667.
17. Albrecht L, Baumgartner J, Marshall J. Evaluation of apical debris removal using various sizes and tapers of ProFile GT files. J Endod. 2004;30:425.
18. Allison DA, Weber CR, Walton RE. The influence of the method of canal preparation on the quality of apical and coronal obturation. J Endod. 1979;5:298.
19. Alodeh MH, Doller R, Dummer PM. Shaping of simulated root canals in resin blocks using the step-back technique with K-files manipulated in a simple in/out filling motion. Int Endod J. 1989;22:107.
20. Alodeh MHA, Dummer PMH. A comparison of the ability of K-files and Hedstrom files to shape simulated root canals in resin blocks. Int Endod J. 1989;22:226.
21. American National Standards Institute: ADA specification No. 28 for Root Canal Files, 1981.
22. Amir FA, Gutmann JL, Witherspoon DE. Calcific metamorphosis: a challenge in endodontic diagnosis and treatment. Quintessence Int. 2001;32:447.
23. Anderson DN, Joyce AP, Roberts S, Runner R. A comparative photoelastic stress analysis of internal root stresses between RC Prep and saline when applied to the Profile/GT rotary instrumentation system. J Endod. 2006;32:224.
24. Anderson MA, Price JW, Parashos P. Fracture resistance of electropolished rotary nickel-titanium endodontic instruments. J Endod. 2007;33:1212.
25. Arias A, Azabal M, Hidalgo JJ, Macorra JC. Relationship between postendodontic pain, tooth diagnostic factors and apical patency. J Endod. 2009;35:189.
26. Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J. 2007;52:S64.
27. Attin T, Buchalla W, Zirkel C, Lussi A. Clinical evaluation of the cleansing properties of the noninstrumental technique for cleaning root canals. Int Endod J. 2002;35:929.
28. Aydin C, Inan U, Yasar S, Bulucu B, Tunca YM. Comparison of shaping ability of RaCe and Hero Shaper instruments in simulated curved canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e92.
29. Backman CA, Oswald RJ, Pitts DL. A radiographic comparison of two root canal instrumentation techniques. J Endod. 1992;18:19.
30. Bahia MG, Melo MC, Buono VT. Influence of cyclic torsional loading on the fatigue resistance of K3 instruments. Int Endod J. 2008;41:883.
31. Baker N, Liewehr F, Buxton T, Joyce A. Antibacterial efficacy of calcium hydroxide, iodine potassium iodide, Betadine, and Betadine scrub with and without surfactant against E. faecalis in vitro. Oral Surg Oral Med Oral Pathol. 2004;98:359.
32. Barbakow F. The LightSpeed system. Dent Clin North Am. 2004;48:113.
33. Barbakow F, Lutz F. The ‘Lightspeed’ preparation technique evaluated by Swiss clinicians after attending continuing education courses. Int Endod J. 1997;30:46.
34. Barbosa FO, Gomes JA, de Araujo MC. Influence of sodium hypochlorite on mechanical properties of K3 nickel-titanium rotary instruments. J Endod. 2007;33:982.
35. Barbosa FOG, Gomes JACP, Araujo MCP. Influence of sodium hypochlorite on mechanical properties of K3 nickel-titanium rotary instruments. J Endod. 2007;33:982.
36. Barbosa SV, Spångberg LSW, Almeida D. Low surface tension calcium hydroxide solution is an effective antiseptic. Int Endod J. 1994;27:6.
37. Barnard D, Davies J, Figdor D. Susceptibility of Actinomyces israelii to antibiotics, sodium hypochlorite and calcium hydroxide. Int Endod J. 1996;29:320.
38. Barnett F, Godick B, Tronstad L. Clinical suitability of a sonic vibratory endodontic instrument. Endod Dent Traumatol. 1985;1:77.
39. Basmadjian-Charles CL, Farge P, Bourgeois DM, Lebrun T. Factors influencing the long-term results of endodontic treatment: a review of the literature. Int Dent J. 2002;52:81.
40. Basrani B, Santos JM, Tjäderhane L, Grad H, Gorduysus O, Huang J, et al. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240.
41. Basson NJ, Tait CM. Effectiveness of three root canal medicaments to eliminate Actinomyces israelii from infected dentinal tubules in vitro. SADJ. 2001;56:499.
42. Baumgartner JC, Mader CL. A scanning electron microscope evaluation of four root canal irrigation regimes. J Endod. 1987;13:147.
43. Behrend GD, Cutler CW. An in-vitro study of smear layer removal and microbial leakage along root-canal fillings. Int Endod J. 1996;29:99.
44. Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334.
45. Bergenholtz G, Lekholm U, Milthon R, Engstrom B. Influence of apical overinstrumentation and overfilling on re-treated root canals. J Endod. 1979;5:310.
46. Bergenholtz G, Lindhe J. Effect of soluble plaque factors on inflammatory reactions in the dental pulp. Scand J Dent Res. 1975;83:153.
47. Bergenholtz G, Spångberg L. Controversies in endodontics. Crit Rev Oral Biol Med. 2004;15:99.
48. Bergmans L, Van Cleynenbreugel J, Beullens M, Wevers M, Van Meerbeek B, Lambrechts P. Progressive versus constant tapered shaft design using NiTi rotary instruments. Int Endod J. 2003;36:288.
49. Bergmans L, Van Cleynenbreugel J, Beullens M, Wevers M, Van Meerbeek B, Lambrechts P. Smooth flexible versus active tapered shaft design using NiTi rotary instruments. Int Endod J. 2002;35:820.
50. Bergmans L, Van Cleynenbreugel J, Wevers M, Lambrechts P. Mechanical root canal preparation with NiTi rotary instruments: rationale, performance and safety. Status report for the American Journal of Dentistry. Am J Dent. 2001;14:324.
51. Berutti E, Angelini E, Rigolone M, Migliaretti G, Pasqualini D. Influence of sodium hypochlorite on fracture properties and corrosion of ProTaper rotary instruments. Int Endod J. 2006;39:693.
52. Berutti E, Cantatore G, Castellucci A, et al. Use of Nickel-titanium rotary PathFile to create the glide path: comparison with manual preflaring in simulated root canals. J Endod. 2009;35:408.
53. Berutti E, Marini R. A scanning electron microscopic evaluation of the debridement capability of sodium hypochlorite at different temperatures. J Endod. 1996;22:467.
54. Best S, Watson P, Pilliar R, Kulkarni GGV, Yared G. Torsional fatigue and endurance limit of a size 30.06 ProFile rotary instrument. Int Endod J. 2004;37:370.
55. Bhaskar SN. Oral surgery–oral pathology conference No. 17, Walter Reed Army Medical Center. Periapical lesions–types, incidence, and clinical features. Oral Surg Oral Med Oral Pathol. 1966;21:657.
56. Bjørndal L, Darvann T. A light microscopic study of odontoblastic and non-odontoblastic cells involved in tertiary dentinogenesis in well-defined cavitated carious lesions. Caries Res. 1999;33:50.
57. Blum JY, Cohen A, Machtou P, Micallef JP. Analysis of forces developed during mechanical preparation of extracted teeth using Profile NiTi rotary instruments. Int Endod J. 1999;32:24.
58. Blum JY, Machtou P, Ruddle C, Micallef JP. Analysis of mechanical preparations in extracted teeth using ProTaper rotary instruments: value of the safety quotient. J Endod. 2003;29:567.
59. Boessler C, Paqué F, Peters OA. The effect of electropolishing on torque and force during simulated root canal preparation with ProTaper shaping files. J Endod. 2009;35:102.
60. Boessler C, Peters OA, Zehnder M. Impact of lubricant parameter on rotary instrument torque and force. J Endod. 2007;33:280.
61. Bonaccorso A, Schäfer E, Condorelli GG, Cantatore G, Tripi TR. Chemical analysis of Nickel-titanium rotary instruments with and without electropolishing after cleaning procedures with sodium hypochlorite. J Endod. 2008;34:1391.
62. Bonaccorso A, Tripi TR, Rondelli G, Condorelli GG, Cantatore G, Schäfer E. Pitting corrosion resistance of Nickel-titanium rotary instruments with different surface treatments in seventeen percent ethylenediaminetetraacetic acid and sodium chloride solutions. J Endod. 2008;34:208.
63. Booth JR, Scheetz JP, Lemons JE, Eleazer PD. A comparison of torque required to fracture three different nickel-titanium rotary instruments around curves of the same angle but of different radius when bound at the tip. J Endod. 2003;29:55.
64. Boutsioukis C, Lambrianidis T, Kastrinakis E. Irrigant flow within a prepared root canal using various flow rates: a Computational Fluid Dynamics study. Int Endod J. 2009;42:144.
65. Brännström M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984;3:35.
66. Brantley WA, Luebke NH, Luebke FL, Mitchell JC. Performance of engine-driven rotary endodontic instruments with a superimposed bending deflection: V. Gates Glidden and Peeso drills. J Endod. 1994;20:241.
67. Braun A, Kappes D, Kruse F, Jepsen S. Efficiency of a novel rinsing device for the removal of pulp tissue in vitro. Int Endod J. 2005;38:8.
68. Briseno Marroquin B, El-Sayed MAA, Willershausen-Zönnchen B. Morphology of the physiological foramen: I. Maxillary and mandibular molars. J Endod. 2004;30:321.
69. Briseno Marroquin B, Pistorius A, Willershausen-Zonnchen B. Canal transportation caused by a new instrumentation technique and three standard techniques. J Endod. 1996;22:406.
70. Brown DC, Moore BK, Brown CEJr, Newton CW. An in vitro study of apical extrusion of sodium hypochlorite during endodontic canal preparation. J Endod. 1995;21:587.
71. Bryant ST, Dummer PMH, Pitoni C, Bourba M, Moghal S. Shaping ability of .04 and .06 taper ProFile rotary nickel-titanium instruments in simulated root canals. Int Endod J. 1999;32:155.
72. Bryant ST, Thompson SA, al-Omari MA, Dummer PM. Shaping ability of ProFile rotary nickel-titanium instruments with ISO sized tips in simulated root canals: Part 1. Int Endod J. 1998;31:275.
73. Bryant ST, Thompson SA, al-Omari MA, Dummer PM. Shaping ability of ProFile rotary nickel-titanium instruments with ISO sized tips in simulated root canals: Part 2. Int Endod J. 1998;31:282.
74. Buchanan LS. The standardized-taper root canal preparation–Part 2. GT file selection and safe handpiece-driven file use. Int Endod J. 2001;34:63.
75. Buehler WH, Gilfrich JV, Wiley RC. Effect of low temperature phase changes on the mechanical properties of alloys near composition NiTi. J Appl Phys. 1963;34:282.
76. Bui T, Mitchell J, Baumgartner JC. Effect of electropolishing ProFile nickel-titanium rotary instruments on cyclic fatigue resistance, torsional resistance, and cutting efficiency. J Endod. 2008;34:190.
77. Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 2007;33:782.
78. Busslinger A, Sener B, Barbakow F. Effects of sodium hypochlorite on nickel-titanium Lightspeed instruments. Int Endod J. 1998;31:290.
79. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307.
80. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res. 1981;89:321.
81. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35.
82. Cailleteau JG, Mullaney TP. Prevalence of teaching apical patency and various instrumentation and obturation techniques in United States dental schools. J Endod. 1997;23:394.
83. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28:17.
84. Camara AC, Aguiar CM, Poli de Figuereido JA. Assessment of the deviation after biomechanical preparation of the coronal, middle, and apical thirds of root canals instrumented with three HERO rotary systems. J Endod. 2007;33:1460.
85. Cameron JA. Factors affecting the clinical efficiency of ultrasonic endodontics: a scanning electron microscopy study. Int Endod J. 1995;28:47.
86. Cameron JA. The effect of ultrasonic endodontics on the temperature of the root canal wall. J Endod. 1988;14:554.
87. Cameron JA. The use of ultrasonics in the removal of the smear layer: a scanning electron microscope study. J Endod. 1983;9:289.
88. Cameron JA. The use of ultrasound in the cleaning of root canals: a clinical report. J Endod. 1982;8:472.
89. Card SJ, Sigurdsson A, Ørstavik D, Trope M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J Endod. 2002;28:779.
90. Carver K, Nusstein J, Reader A, Beck M. In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. J Endod. 2007;33:1038.
91. Charles TJ, Charles JE. The ‘balanced force’ concept for instrumentation of curved canals revisited. Int Endod J. 1998;31:166.
92. Cheung GS. Instrument fractures: mechanisms, removal of fragments, and clinical outcomes. Endod Topics. 2009;16:1.
93. Cheung GS, Darvell BW. Low-cycle fatigue of NiTi rotary instruments of various cross-sectional shapes. Int Endod J. 2007;40:626.
94. Cheung GS, Shen Y, Darvell BW. Does electropolishing improve the low-cycle fatigue behavior of a nickel-titanium rotary instrument in hypochlorite? J Endod. 2007;33:1217.
95. Cheung GS, Shen Y, Darvell BW. Effect of environment of low-cycle fatigue of a nickel-titanium instrument. J Endod. 2007;33:1433.
96. Cheung GS, Stock CJ. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endod J. 1993;26:334.
97. Chow TW. Mechanical effectiveness of root canal irrigation. J Endod. 1983;9:475.
98. Chugal N, Clive JM, Spångberg LSW. Endodontic infection: some biologic and treatment factors associated with outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:81.
99. Chugal NM, Clive JM, Spångberg LS. A prognostic model for assessment of the outcome of endodontic treatment: effect of biologic and diagnostic variables. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:342.
100. Civjan S, Huget EF, DeSimon LB. Potential applications of certain nickel-titanium (nitinol) alloys. J Dent Res. 1975;54:89.
101. Clark-Holke D, Drake D, Walton RE, Rivera E, Guthmiller JM. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J Dent. 2003;31:275.
102. Coldero LG, McHugh S, MacKenzie D, Saunders WP. Reduction in intracanal bacteria during root canal preparation with and without apical enlargement. Int Endod J. 2002;35:437.
103. Cunningham CJ, Senia ES. A three-dimensional study of canal curvatures in the mesial roots of mandibular molars. J Endod. 1992;18:294.
104. Cunningham WT, Joseph SW. Effect of temperature on the bactericidal action of sodium hypochlorite endodontic irrigant. Oral Surg Oral Med Oral Pathol. 1980;50:569.
105. Cunningham WT, Martin H. A scanning electron microscope evaluation of root canal debridement with the endosonic ultrasonic synergistic system. Oral Surg Oral Med Oral Pathol. 1982;53:527.
106. Cunningham WT, Martin H, Pelleu GBJr, Stoops DE. A comparison of antimicrobial effectiveness of endosonic and hand root canal therapy. Oral Surg Oral Med Oral Pathol. 1982;54:238.
107. Cvek M, Nord CE, Hollender L. Antimicrobial effect of root canal debridement in teeth with immature root. A clinical and microbiologic study. Odontol Rev. 1976;27:1.
108. Cymerman JJ, Jerome LA, Moodnik RM. A scanning electron microscope study comparing the efficacy of hand instrumentation with ultrasonic instrumentation of the root canal. J Endod. 1983;9:327.
109. Czonstkowsky M, Wilson EG, Holstein FA. The smear layer in endodontics. Dent Clin North Am. 1990;34:13.
110. Dahlén G, Samuelsson W, Molander A, Reit C. Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol Immunol. 2000;15:309.
111. Dakin HD. On the use of certain antiseptic substances in the treatment of infected wounds. Br Med J. 1915;2:318.
112. Dalton BC, Ørstavik D, Phillips C, Pettiette M, Trope M. Bacterial reduction with nickel-titanium rotary instrumentation. J Endod. 1998;24:763.
113. Daugherty DW, Gound TG, Comer TL. Comparison of fracture rate, deformation rate, and efficiency between rotary endodontic instruments driven at 150 rpm and 350 rpm. J Endod. 2001;27:93.
114. Davis RD, Marshall JG, Baumgartner JC. Effect of early coronal flaring on working length change in curved canals using rotary nickel-titanium versus stainless steel instruments. J Endod. 2002;28:438.
115. De-Deus G, Namen F, Galan J, Zehnder M. Soft chelating irrigation protocol optimizes bonding quality of Resilon/Epiphany root fillings. J Endod. 2008;34:703.
116. DeFiore PM, Genov KA, Komaroff E, Li Y, Lin L. Nickel-titanium rotary instrument fracture: a clinical practice assessment. Int Endod J. 2006;39:700.
117. Dental root-canal instruments—Part 1: Files, reamers, barbed broaches, rasps, paste carriers, explorers and cotton broaches. Geneva: International Organization for Standardization, 1992.
118. DeNunzio MS, Hicks ML, Pelleu GBJr, Kingman A, Sauber JJ. Bacteriological comparison of ultrasonic and hand instrumentation of root canals in dogs. J Endod. 1989;15:290.
119. Dietz DB, Di Fiore PM, Bahcall JK, Lautenschlager EP. Effect of rotational speed on the breakage of nickel-titanium rotary files. J Endod. 2000;26:68.
120. Discus Dental. LightSpeed Technique Guide. http://www.discusdental.com/endo/inst_technique_guide.php. accessed 2/20, 2009
121. Drake DR, Wiemann AH. Bacterial retention in canal walls in vitro: effect of smear layer. J Endod. 1994;20:78.
122. Dumani A, Yoldas O, Isci A, Köksal F, Kayar B, Polat E. Disinfection of artificially contaminated Resilon cones with chlorhexidine and sodium hypochlorite at different time exposures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e82.
123. Dummer PMH, McGinn JH, Rees DG. The position and topography of the apical canal constriction and apical foramen. Int Endod J. 1984;17:192.
124. Economides N, Liolios E, Kolokuris I, Beltes P. Long-term evaluation of the influence of smear layer removal on the sealing ability of different sealers. J Endod. 1999;25:123.
125. Eggert C, Peters O, Barbakow F. Wear of nickel-titanium LightSpeed instruments evaluated by scanning electron microscopy. J Endod. 1999;25:494.
126. El Ayouti A, Weiger R, Löst C. Frequency of overinstrumentation with an acceptable radiographic working length. J Endod. 2001;27:49.
127. Ercan E, Ozekinci T, Atakul F, Gül K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J Endod. 2004;30:84.
128. Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101.
129. Eriksson JH, Sundstrom F. Temperature rise during root canal preparation–a possible cause of damage to tooth and periodontal tissue. Swed Dent J. 1984;8:217.
130. Esposito M, Grusovin M, Kakisis I, Coulthard P, Worthington H: Interventions for replacing missing teeth: treatment of perimplantitis. Cochrane Database Syst Rev 16:CD004970, 2008.
131. Evanov C, Liewehr F, Buxton TB, Joyce AP. Antibacterial efficacy of calcium hydroxide and chlorhexidine gluconate irrigants at 37 degrees C and 46 degrees C. J Endod. 2004;30:653.
132. Evans JT, Simon JHS. Evaluation of the apical seal produced by injected thermoplasticized gutta-percha in the absence of smear layer and root canal sealer. J Endod. 1986;12:101.
133. Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221.
134. Fairbourn DR, McWalter GM, Montgomery S. The effect of four preparation techniques on the amount of apically extruded debris. J Endod. 1987;13:102.
135. Falk KW, Sedgley CM. The influence of preparation size on the mechanical efficacy of root canal irrigation in vitro. J Endod. 2005;31:742.
136. Fava LR. The double-flared technique: an alternative for biomechanical preparation. J Endod. 1983;9:76.
137. FDA explains status of N2 material. J Am Dent Assoc. 1992;123:236.
138. Friedman S. Management of post-treatment endodontic disease: a current concept of case selection. Aust Endod J. 2000;26:104.
139. Friedman S. Prognosis of initial endodontic therapy. Endod Topics. 2002;2:59.
140. Friedman S, Abitbol T, Lawrence HP. Treatment outcome in endodontics: The Toronto study. Phase 1: initial treatment. J Endod. 2003;29:787.
141. Gabel WP, Hoen M, Steiman HR, Pink FE, Dietz R. Effect of rotational speed on nickel-titanium file distortion. J Endod. 1999;25:752.
142. Gambarini G. Cyclic fatigue of nickel-titanium rotary instruments after clinical use with low- and high-torque endodontic motors. J Endod. 2001;27:772.
143. Gambarini G. Rationale for the use of low-torque endodontic motors in root canal instrumentation. Endod Dent Traumatol. 2000;16:95.
144. Gambarini G. Shaping and cleaning the root canal system: a scanning electron microscopic evaluation of a new instrumentation and irrigation technique. J Endod. 1999;25:800.
145. Gambarini G, Gerosa R, DeLuca M, Garala M, Testarelli L. Mechanical properties of a new and improved nickel-titanium alloy for endodontic use: an evaluation of file flexibility. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:798.
146. Gambarini G, Grande NM, Plotino G, Somma F, Garala M, DeLuca M, et al. Fatigue resistance of engine-driven rotary nickel-titanium instruments produced by new manufacturing methods. J Endod. 2008;34:1003.
147. Garala M, Kuttler S, Hardigan P, Steiner-Carmi R, Dorn S. A comparison of the minimum canal wall thickness remaining following preparation using two nickel-titanium rotary systems. Int Endod J. 2003;36:636.
148. Gettleman BH, Messer HH, ElDeeb ME. Adhesion of sealer cements to dentin with and without the smear layer. J Endod. 1991;17:15.
149. Gluskin AH, Brown DC, Buchanan LS. A reconstructed computerized tomographic comparison of Ni-Ti rotary GT files versus traditional instruments in canals shaped by novice operators. Int Endod J. 2001;34:476.
150. Goerig AC, Michelich RJ, Schultz HH. Instrumentation of root canals in molar using the step-down technique. J Endod. 1982;8:550.
151. Goldberg F, Araujo JA. Comparison of three instruments in the preparation of curved root canals. Endod Dent Traumatol. 1997;13:265.
152. Goldberg F, Massone EJ. Patency file and apical transportation: an in vitro study. J Endod. 2002;28:510.
153. Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigating solutions for endodontics: a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1981;52:197.
154. Goldman M, Goldman LB, Cavaleri R, Bogis J, Lin PS. The efficacy of several endodontic irrigating solutions: a scanning electron microscopic study: Part 2. J Endod. 1982;8:487.
155. Gomes BP, Montagner F, Berber VB, et al. Antimicrobial action of intracanal medicaments on the external root surface. J Dent. 2009;37:76.
156. Gomes BP, Souza SF, Ferraz CC, et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267.
157. Gonzalez-Rodrguez MP, Ferrer-Luque CM. A comparison of Profile, Hero 642, and K3 instrumentation systems in teeth using digital imaging analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:112.
158. Goodman A, Reader A, Beck M, Melfi R, Meyers W. An in vitro comparison of the efficacy of the step-back technique versus a step-back/ultrasonic technique in human mandibular molars. J Endod. 1985;11:249.
159. Grande NM, Plotino G, Pecci R, Bedini R, Malagnino VA, Somma F. Cyclic fatigue resistance and three-dimensional analysis of instruments from two nickel-titanium systems. Int Endod J. 2006;39:755.
160. Grandini S, Balleri P, Ferrari M. Evaluation of Glyde File Prep in combination with sodium hypochlorite as a root canal irrigant. J Endod. 2002;28:300.
161. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J. 2003;36:411.
162. Green D. A stereomicroscopic investigation of the root apices of 400 maxillary and mandibular anterior teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1956;9:1224.
163. Green D. Stereomicroscopic study of 700 root apices of maxillary and mandibular posterior teeth. Oral Surg Oral Med Oral Pathol. 1960;13:728.
164. Grigoratos D, Knowles J, Ng YL, Gulabivala K. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. Int Endod J. 2001;34:113.
165. Guelzow A, Stamm O, Martus P, Kielbassa AM. Comparative study of six rotary nickel-titanium systems and hand instrumentation for root canal preparation. Int Endod J. 2005;38:743.
166. Gulabivala K, Stock CJ, Lewsey JD, Ghori S, Ng YL, Spratt DA. Effectiveness of electrochemically activated water as an irrigant in an infected tooth model. Int Endod J. 2004;37:624.
167. Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31:166.
168. Gutiérrez JH, Brizuela C, Villota E. Human teeth with periapical pathosis after overinstrumentation and overfilling of the root canals: a scanning electron microscopic study. Int Endod J. 1999;32:40.
169. Haapasalo HK, Sirén EK, Waltimo TMT, Ørstavik D, Haapasalo MPP. Inactivation of local root canal medicaments by dentine: an in vitro study. Int Endod J. 2000;22:126.
170. Haapasalo M, Endal U, Zandi H, Coil J. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005;10:77.
171. Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66:1375.
172. Haapasalo M, Udnaes T, Endal U. Persistent, recurrent, and acquired infection of the root canal system post-treatment. Endod Topics. 2004;6:29.
173. Haikel Y, Serfaty R, Bateman G, Senger B, Allemann C. Dynamic and cyclic fatigue of engine-driven rotary nickel-titanium endodontic instruments. J Endod. 1999;25:434.
174. Haikel Y, Serfaty R, Wilson P, Speisser JM, Allemann C. Mechanical properties of nickel-titanium endodontic instruments and the effect of sodium hypochlorite treatment. J Endod. 1998;24:731.
175. Hancock HH, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579.
176. Hänni S, Schmidlin PR, Müller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36:100.
177. Hasselgren G. Where shall the root filling end? NY State Dent J. 1994;60:34.
178. Hata G, Uemura M, Kato AS, Imura N, Novo NF, Toda T. A comparison of shaping ability using ProFile, GT file, and Flex-R endodontic instruments in simulated canals. J Endod. 2002;28:316.
179. Hayashi Y, Yoneyama T, Yahata Y, et al. Phase transformation behaviour and bending properties of hybrid nickel-titanium rotary endodontic instruments. Int Endod J. 2007;40:247.
180. Heard F, Walton RE. Scanning electron microscope study comparing four root canal preparation techniques in small curved canals. Int Endod J. 1997;30:323.
181. Heling I, Chandler NP. Antimicrobial effect of irrigant combinations within dentinal tubules. Int Endod J. 1998;31:8.
182. Herold KS, Johnson BR, Wenckus CS. A scanning electron microscopy evaluation of microfractures, deformation and separation in EndoSequence and Profile Nickel-Titanium rotary files using an extracted molar tooth model. J Endod. 2007;33:712.
183. Hess W. Formation of root canals in human teeth. J Natl Dent Assoc. 1921;3:704.
184. Hilt BR, Cunningham CJ, Shen C, Richards N. Torsional properties of stainless-steel and nickel-titanium files after multiple autoclave sterilizations. J Endod. 2000;26:76.
185. Hsieh YD, Gau CH, Kung Wu SF, Shen EC, Hsu PW, Fy E. Dynamic recording of irrigation fluid distribution in root canals using thermal image analysis. Int Endod J. 2007;40:11.
186. Huang TY, Gulabivala K, Ng YL. A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. Int Endod J. 2008;41:60.
187. Hübscher W, Barbakow F, Peters OA. Root canal preparation with FlexMaster: assessment of torque and force in relation to canal anatomy. Int Endod J. 2003;36:883.
188. Hübscher W, Barbakow F, Peters OA. Root canal preparation with FlexMaster: canal shapes analysed by micro-computed tomography. Int Endod J. 2003;36:740.
189. Hülsmann M, Gressmann G, Schäfers F. A comparative study of root canal preparation using FlexMaster and HERO 642 rotary Ni-Ti instruments. Int Endod J. 2003;36:358.
190. Hülsmann M, Hahn W. Complications during root canal irrigation–literature review and case reports. Int Endod J. 2000;33:186.
191. Hülsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003;36:810.
192. Hülsmann M, Peters OA, Dummer PMH. Mechanical preparation of root canals: shaping goals, techniques and means. Endod Topics. 2005;10:30.
193. Hülsmann M, Schade M, Schäfers F. A comparative study of root canal preparation with HERO 642 and Quantec SC rotary Ni-Ti instruments. Int Endod J. 2001;34:538.
194. Huque J, Kota K, Yamaga M, Iwaku M, Hoshino E. Bacterial eradication from root dentine by ultrasonic irrigation with sodium hypochlorite. Int Endod J. 1998;31:242.
195. Huttula AS, Tordik PA, Imamura G, Eichmiller FC, McClanahan SB. The effect of ultrasonic post instrumentation on root surface temperature. J Endod. 2006;32:1085.
196. Iqbal MK, Banfield B, Lavorini A, Bachstein B. A comparison of LightSpeed LS1 and LightSpeed LSX root canal instruments in apical transportation and length control in simulated root canals. J Endod. 2007;33:268.
197. Isom TL, Marshall JG, Baumgartner JC. Evaluation of root thickness in curved canals after flaring. J Endod. 1995;21:368.
198. Izu KH, Thomas SJ, Zhang P, Izu AE, Michalek S. Effectiveness of sodium hypochlorite in preventing inoculation of periapical tissue with contaminated patency files. J Endod. 2004;30:92.
199. Javaheri HH, Javaheri GH. A comparison of three Ni-Ti rotary instruments in apical transportation. J Endod. 2007;33:284.
200. Jensen SA, Walker TL, Hutter JW, Nicoll BK. Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canals. J Endod. 1999;25:735.
201. Jiang J, Zuo J, Hurst IR, Holliday LS. The synergistic effect of peptidoglycan and lipopolysaccharide on osteoclast formation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:738.
202. Johal S, Baumgartner J, Marshall J. Comparison of the antimicrobial efficacy of 1.3% NaOCl/BioPure MTAD to 5.25% NaOCl/15% EDTA for root canal irrigation. J Endod. 2007;33:48.
203. Johnson E, Lloyd A, Kuttler S, Namerow K. Comparison between a novel nickel-titanium alloy and 508 Nitinol on the cyclic fatigue life of ProFile 25/.04 rotary instruments. J Endod. 2008;34:1406.
204. Jou Y-T, Karabuchak B, Levin J, Liu D. Endodontic working width: current concepts and techniques. Dent Clin North Am. 2004;48:323.
205. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340.
206. Karagöz-Küçükay I, Ersev H, Engin-Akkoca E, Kucukay S, Gursoy T. Effect of rotational speed on root canal preparation with Hero 642 rotary Ni-Ti instruments. J Endod. 2003;29:447.
207. Katebzadeh N, Sigurdsson A, Trope M. Radiographic evaluation of periapical healing after obturation of infected root canals: an in vivo study. J Endod. 2000;33:60.
208. Keate KC, Wong M. Comparison of endodontic file tip quality. J Endod. 1990;16:486.
209. Kell T, Arzarpazhooh A, Peters OA, El-Mowafy O, Thompson B, Basrani B. Torsional profiles of new and used 20/.06 GT series X and GT rotary endodontic files. J Endod submitted. 2009.
210. Kerekes K, Tronstad L. Morphometric observations on root canals of human anterior teeth. J Endod. 1977;3:24.
211. Kerekes K, Tronstad L. Morphometric observations on root canals of human premolars. J Endod. 1977;3:74.
212. Kerekes K, Tronstad L. Morphometric observations on the root canals of human molars. J Endod. 1977;3:114.
213. Knowles KI, Hammond NB, Biggs SG, Ibarrola JL. Incidence of instrument separation using LightSpeed rotary instruments. J Endod. 2006;32:14.
214. Koch KA, Brave DG. Real World Endo Sequence file. Dent Clin North Am. 2004;48:159.
215. Korzen BH, Krakow AA, Green DB. Pulpal and periapical tissue responses in conventional and monoinfected gnotobiotic rats. Oral Surg Oral Med Oral Pathol. 1974;37:783.
216. Kramkowski TR, Bahcall J. An in vitro comparison of torsional stress and cyclic fatigue resistance of ProFile GT and ProFile GT Series X rotary Nickel-titanium files. J Endod. 2009;35:404.
217. Kuah H-G, Lui J-N, Tseng PS The effect of EDTA with and without ultrasonics on removal of the smear layer N.-N. C J Endod 35 2009 393
218. Kuhn G, Jordan L. Fatigue and mechanical properties of nickel-titanium endodontic instruments. J Endod. 2002;28:716.
219. Kuttler Y. Microscopic investigation of root apexes. J Am Dent Assoc. 1955;50:544.
220. Kuyk JK, Walton RE. Comparison of the radiographic appearance of root canal size to its actual diameter. J Endod. 1990;16:528.
221. Kyomen SM, Caputo AA, White SN. Critical analysis of the balanced force technique in endodontics. J Endod. 1994;20:332.
222. Lalonde ER. A new rationale for the management of periapical granulomas and cysts: an evaluation of histopathological and radiographic findings. J Am Dent Assoc. 1970;80:1056.
223. Larsen CM, Watanabe I, Glickman GN, He J. Cyclic fatigue analysis of a new generation of Nickel titanium rotary instruments. J Endod. 2009;35:401.
224. Lee SJ, Wu M-K, Wesselink PR. The effectiveness of syringe irrigation and ultrasonics to remove debris from simulated irregularities within prepared root canal walls. Int Endod J. 2004;37:672.
225. Leeb J. Canal orifice enlargement as related to biomechanical preparation. J Endod. 1983;9:463.
226. Leseberg DA, Montgomery S. The effects of Canal Master, Flex-R, and K-Flex instrumentation on root canal configuration. J Endod. 1991;17:59.
227. Li UM, Lee BS, Shih CT, Lan WH, Lin CP. Cyclic fatigue of endodontic nickel titanium rotary instruments: static and dynamic tests. J Endod. 2002;28:448.
228. Lim SS, Stock CJ. The risk of perforation in the curved canal: anticurvature filing compared with the stepback technique. Int Endod J. 1987;20:33.
229. Lin LM, Rosenberg PA, Lin J. Do procedural errors cause endodontic treatment failure? J Am Dent Assoc. 2005;136:187.
230. Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: part 3. The antibacterial effect of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29:565.
231. Linsuwanont P, Parashos P, Messer HH. Cleaning of rotary nickel-titanium endodontic instruments. Int Endod J. 2004;37:19.
232. Löe H, Schiott C. The effect of mouth rinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodont Res. 1970;5:79.
233. Lottanti S, Gautschi H, Sener B, Zehnder M. Effects of ethylenediamine tetraacetic, etidronic and peracetic acid irrigation on human root dentine and the smear layer. Int Endod J. 2009;42:335.
234. Loushine RJ, Weller RN, Hartwell GR. Stereomicroscopic evaluation of canal shape following hand, sonic, and ultrasonic instrumentation. J Endod. 1989;15:417.
235. Love RM, Chandler NP, Jenkinson HF. Penetration of smeared or nonsmeared dentine by Streptococcus gordonii. Int Endod J. 1996;29:2.
236. Love RM, McMillan MD, Park Y, Jenkinson HF. Coinvasion of dentinal tubules by Porphyromonas gingivalis and Streptococcus gordonii depends upon binding specificity of streptococcal antigen I/II adhesin. Infect Immun. 2000;68:1359.
237. Luebke NH, Lausten LL, Brantley WA, Mitchell JC. Torsional performance of nickel-titanium Gates Glidden drills with an applied bending deflection (Abstract). J Dent Res. 2001;80:259.
238. Lui J-N, Kuah H-G, Chen N-N. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. J Endod. 2007;33:472.
239. Lumley PJ. A comparison of dentine removal using safety or conventional Hedstrom files. Endod Dent Traumatol. 1997;13:65.
240. Lumley PJ, Walmsley AD, Laird WR. Streaming patterns produced around endosonic files. Int Endod J. 1991;24:290.
241. Lumley PJ, Walmsley AD, Walton RE, Rippin JW. Cleaning of oval canals using ultrasonic or sonic instrumentation. J Endod. 1993;19:453.
242. Lundy T, Stanley H. Correlation of pulpal histopathology and clinical symptoms in human teeth subjected to experimental irritation. Oral Surg. 1969;27:187.
243. Lussi A, Imwinkelried S, Stich H. Obturation of root canals with different sealers using non-instrumentation technology. Int Endod J. 1999;32:17.
244. Lussi A, Messerli L, Hotz P, Grosrey J. A new non-instrumental technique for cleaning and filling root canals. Int Endod J. 1995;28:1.
245. Lussi A, Nussbacher U, Grosrey J. A novel noninstrumented technique for cleansing the root canal system. J Endod. 1993;19:549.
246. Lussi A, Portmann P, Nussbacher U, Imwinkelried S, Grosrey J. Comparison of two devices for root canal cleansing by the noninstrumentation technology. J Endod. 1999;25:9.
247. Madison S, Krell KV. Comparison of ethylenediamine tetraacetic acid and sodium hypochlorite on the apical seal of endodontically treated teeth. J Endod. 1984;10:499.
248. Manzur A, González A, Pozos A, Silva-Herzog D, Friedman S. Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: a randomized clinical trial. J Endod. 2007;33:114.
249. Marais JT. Cleaning efficacy of a new root canal irrigation solution: a preliminary evaluation. Int Endod J. 2000;33:320.
250. Marais JT, Williams WP. Antimicrobial effectiveness of electro-chemically activated water as an endodontic irrigation solution. Int Endod J. 2001;34:237.
251. Marending M, Lutz F, Barbakow F. Scanning electron microscope appearances of Lightspeed instruments used clinically: a pilot study. Int Endod J. 1998;31:57.
252. Marending M, Paqué F, Fischer J, Zehnder M. Impact of irrigant sequence on mechanical properties of human root dentin. J Endod. 2007;33:1325.
253. Marshall FJ, Pappin JB. A crown-down pressureless preparation root canal enlargement technique. Technique Manual. Portland OR: Oregon Health Sciences University; 1980.
254. Marsicovetere ES, Burgess JO, Clement DJ, del Rio CE. Torsional testing of the LightSpeed nickel-titanium instrument system. J Endod. 1996;22:681.
255. Martin H. Ultrasonic disinfection of the root canal. Oral Surg Oral Med Oral Pathol. 1976;42:92.
256. Martinho FC, Gomes BP. Quantification of endotoxins and cultivable bacteria in root canal infection before and after chemomechanical preparation with 2.5% sodium hypochlorite. J Endod. 2008;34:268.
257. Martins CR, Bahia MG, Buono VT, Horizonte B. The effect of sodium hypochlorite on the surface characteristics and fatigue resistance of ProFile nickel-titanium instruments. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:99.
258. Mayer BE, Peters OA, Barbakow F. Effects of rotary instruments and ultrasonic irrigation on debris and smear layer scores: a scanning electron microscopic study. Int Endod J. 2002;35:582.
259. Mazur A, Gonzalez AM, Pozos A, Silva-Herzog D, Friedman S. Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: a randomized clinical trial. J Endod. 33, 2007.
260. McCann JT, Keller DL, LaBounty GL. Remaining dentin/cementum thickness after hand or ultrasonic instrumentation. J Endod. 1990;16:109.
261. McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1:238.
262. McGill S, Gulabivala K, Mordan N, Ng YL. The efficacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen ‘bio-molecular film’ from an ex vivo model. Int Endod J. 2008;41:602.
263. Merrett SJ, Bryant ST, Dummer PM. Comparison of the shaping ability of RaCe and FlexMaster rotary Nickel-titanium systems in simulated canals. J Endod. 2006;32:960.
264. Mickel AK, Chogle S, Liddle J, Huffacker K, Jones JJ. The role of apical size determination and enlargement in the reduction of intracanal bacteria. J Endod. 2007;33:21.
265. Miyai K, Ebihara A, Hayashi Y, Doi H, Suda H, Yoneyama T. Influence of phase transformation on the torsional and bending properties of Nickel-titanium rotary endodontic instruments. Int Endod J. 2006;39:119.
266. Mize SB, Clement DJ, Pruett JP, Carnes DLJr. Effect of sterilization on cyclic fatigue of rotary nickel-titanium endodontic instruments. J Endod. 1998;24:843.
267. Mizutani T, Ohno N, Nakamura H. Anatomical study of the root apex in the maxillary anterior teeth. J Endod. 1992;18:344.
268. Mohammadi Z. Sodium hypochlorite in endodontics: an update review. Int Dent J. 2008;58:329.
269. Molander A, Dahlen G. Evaluation of the antibacterial potential of tetracycline or erythromycin mixed with calcium hydroxide as intracanal dressing against Enterococcus faecalis in vivo. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:744.
270. Möller AJ. Microbiological examination of root canals and periapical tissues. Methodological studies. Odontol Tidskr. 1966;74:1.
271. Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475.
272. Möller AJ, Fabricius L, Dahlén G, Sundqvist G, Happonen RP. Apical periodontitis development and bacterial response to endodontic treatment. Experimental root canal infections in monkeys with selected bacterial strains. Eu J Oral Sci. 2004;112:207.
273. Mullaney TP. Instrumentation of finely curved canals. Dent Clin North Am. 1979;23:575.
274. Nagy CD, Bartha K, Bernath M, Verdes E, Szabo J. A comparative study of seven instruments in shaping the root canal in vitro. Int Endod J. 1997;30:124.
275. Nair P, Pajorola G, Schroeder H. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg. 1996;81:93.
276. Nair PN. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39:249.
277. Nair PN, Sjögren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580.
278. Nair PN, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term post-treatment follow-up. Int Endod J. 1993;26:225.
279. Natkin E, Oswald RJ, Carnes LI. The relationship of lesion size to diagnosis, incidence, and treatment of periapical cysts and granulomas. Oral Surg Oral Med Oral Pathol. 1984;57:82.
280. Nguy D, Sedgley C. The influence of canal curvature on the mechanical efficacy of root canal irrigation in vitro using real-time imaging of bioluminescent bacteria. J Endod. 2006;32:1077.
281. Nielsen BA, Baumgartner JC. Comparison of the EndoVac system to needle irrigation of root canals. J Endod. 2007;33:611.
282. Norrington DW, Ruby J, Beck P, Eleazer PD. Observations of biofilm growth on human dentin and potential destruction after exposure to antibiotics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:526.
283. Nygaard-Østby B. Chelation in endodontic therapy: ethylenediamineacetate acid for cleansing and widening of root canals. Odontol Tidskr. 1957;65:3.
284. O’Hoy PY, Messer HH, Palamara JE. The effect of cleaning procedures on fracture properties and corrosion of NiTi files. Int Endod J. 2003;36:724.
285. Oehlers FA. Dens invaginatus. I. Variations of the invagination process and associated anterior crown forms. Oral Surg Oral Med Oral Pathol. 1957;10:1024.
286. Olgart L, Bergenholtz G. The dentine-pulp complex: responses to adverse influences. In Bergenholtz G, Hørsted-Bindslev P, Reit C, editors: Textbook of Endodontology, ed 1, Oxford: Blackwell-Munksgaard, 2003.
287. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142.
288. Ørstavik D, Kerekes K, Molven O. Effects of extensive apical reaming and calcium hydroxide dressing on bacterial infection during treatment of apical periodontitis: a pilot study. Int Endod J. 1991;24:1.
289. Ounsi HF, Salameh Z, Al-Shalan T, et al. Effect of clinical use of the cyclic fatigue resistance of ProTaper Nickel-titanium rotary instruments. J Endod. 2007;33:737.
290. Paqué F, Barbakow F, Peters OA. Root canal preparation with Endo-Eze AET: changes in root canal shape assessed by micro-computed tomography. Int Endod J. 2005;38:456.
291. Paqué F, Ganahl D, Peters OA. Effects of root canal preparation with 6 different nickel-titanium instruments on apical geometry assessed by micro computed tomography. J Endod. 2009. accepted for publication
292. Paqué F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. Int Endod J. 2006;39:18.
293. Paqué F, Musch U, Hülsmann M. Comparison of root canal preparation using RaCe and ProTaper rotary Ni-Ti instruments. Int Endod J. 2005;38:8.
294. Park H. A comparison of Greater Taper files, ProFiles, and stainless steel files to shape curved root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:715.
295. Parris J, Wilcox L, Walton R. Effectiveness of apical clearing: histological and radiographical evaluation. J Endod. 1994;20:219.
296. Pashley DH. Smear layer: overview of structure and function. Proc Finn Dent Soc. 1992;88(Suppl 1):215.
297. Pashley DH. Smear layer: physiological considerations. Oper Dent Suppl. 1984;3:13.
298. Patino PV, Biedma BM, Liebana CR, Cantatore G, Bahillo JG. The influence of a manual glide path on the separation of NiTi rotary instruments. J Endod. 2005;31:114.
299. Peters LB, Wesselink PR. Periapical healing of endodontically treated teeth in one and two visits obturated in the presence or absence of detectable microorganisms. Int Endod J. 2002;35:660.
300. Peters LB, Wesselink PR, Moorer WR. The fate and the role of bacteria left in root dentinal tubules. Int Endod J. 1995;28:95.
301. Peters OA. Current challenges and concepts in the preparation of root canal systems: a review. J Endod. 2004;30:559.
302. Peters OA, Barbakow F. Dynamic torque and apical forces of ProFile .04 rotary instruments during preparation of curved canals. Int Endod J. 2002;35:379.
303. Peters OA, Barbakow F, Peters CI. An analysis of endodontic treatment with three nickel-titanium rotary root canal preparation techniques. Int Endod J. 2004;37:849.
304. Peters OA, Boessler C, Zehnder M. Effect of liquid and paste-type lubricants on torque values during simulated rotary root canal instrumentation. Int Endod J. 2005;38:223.
305. Peters OA, Kappeler S, Bucher W, Barbakow F. Engine-driven preparation of curved root canals: measuring cyclic fatigue and other physical parameters. Aust Endod J. 2002;28:11.
306. Peters OA, Kappeler S, Bucher W, Barbakow F. Maschinelle Aufbereitung gekrümmter Wurzelkanäle: Messaufbau zur Darstellung physikalischer Parameter. Schw Monatsschr Zahnmed. 2001;111:834.
307. Peters OA, Peters CI, Schönenberger K, Barbakow F. ProTaper rotary root canal preparation: assessment of torque and force in relation to canal anatomy. Int Endod J. 2003;36:93.
308. Peters OA, Peters CI, Schönenberger K, Barbakow F. ProTaper rotary root canal preparation: effects of canal anatomy on final shape analysed by micro CT. Int Endod J. 2003;36:86.
309. Peters OA, Roelicke JO, Baumann MA. Effect of immersion in sodium hypochlorite on torque and fatigue resistance of nickel-titanium instruments. J Endod. 2007;33:589.
310. Peters OA, Schönenberger K, Laib A. Effects of four NiTi preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221.
311. Pettiette MT, Delano EO, Trope M. Evaluation of success rate of endodontic treatment performed by students with stainless-steel K-files and nickel-titanium hand files. J Endod. 2001;27:124.
312. Pineda F, Kuttler Y. Mesiodistal and buccolingual roentgenographic investigation of 7,275 root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1972;33:101.
313. Plotino G, Grande NM, Sorci E, Malagnino VA, Somma F. A comparison of cyclic fatigue between used and new Mtwo NiTi rotary instruments. Int Endod J. 2006;39:716.
314. Plotino G, Grande NM, Sorci E, Malagnino VA, Somma F. Influence of a brushing working stroke on the fatigue life of NiTi rotary instruments. Int Endod J. 2007;40:45.
315. Podbielski A, Spahr A, Haller B. Additive antimicrobial activity of calcium hydroxide and chlorhexidine on common endodontic bacterial pathogens. J Endod. 2003;29:340.
316. Ponti TM, McDonald NJ, Kuttler S, Strassler HE, Dumsha TC. Canal-centering ability of two rotary file systems. J Endod. 2002;28:283.
317. Portenier I, Haapasalo H, Ørstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against Enterococcus faecalis by dentin, dentin matrix, type-I collagen, and heat-killed microbial whole cells. J Endod. 2002;28:634.
318. Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxyapatite and bovine serum albumin. Int Endod J. 2001;34:184.
319. Portenier I, Lutz F, Barbakow F. Preparation of the apical part of the root canal by the LightSpeed and step-back techniques. Int Endod J. 1998;31:103.
320. Portenier I, Waltimo T, Ørstavik D, Haapasalo H. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod. 2006;32:138.
321. Pruett JP, Clement DJ, Carnes DL. Cyclic fatigue testing of nickel-titanium endodontic instruments. J Endod. 1997;23:77.
322. Radcliffe CE, Potouridou L, Qureshi R, Habahbeh N, Qualtrough A, Worthington H, et al. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int Endod J. 2004;37:438.
323. Ram Z. Effectiveness of root canal irrigation. Oral Surg Oral Med Oral Pathol. 1977;44:306.
324. Rangel S, Cremonese R, Bryant S, Dummer PM. Shaping ability of RaCe rotary nickel-titanium instruments in simulated root canals. J Endod. 2007;31:460.
325. Rapisarda E, Bonaccorso A, Tripi TR, Fragalk I, Condorelli GG. The effect of surface treatments of nickel-titanium files on wear and cutting efficiency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:363.
326. Rapisarda E, Bonaccorso A, Tripi TR, Guido G. Effect of sterilization on the cutting efficiency of rotary nickel- titanium endodontic files. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:343.
327. Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28:12.
328. Ray JJ, Kirkpatrick TC, Rutledge RE. Cyclic fatigue of EndoSequence and K3 rotary files in a dynamic model. J Endod. 2007;33:1469.
329. Reynolds MA, Madison S, Walton RE, Krell KV, Rittman BR. An in vitro histological comparison of the step-back, sonic, and ultrasonic instrumentation techniques in small, curved root canals. J Endod. 1987;13:307.
330. Ricucci D, Bergenholtz G. Bacterial status in root-filled teeth exposed to the oral environment by loss of restoration and fracture or caries–a histobacteriological study of treated cases. Int Endod J. 2003;36:787.
331. Ricucci D, Gröndahl K, Bergenholtz G. Periapical status of root-filled teeth exposed to the oral environment by loss of restoration or caries. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:354.
332. Ricucci D, Langeland K. Apical limit of root canal instrumentation and obturation, part 2. A histological study. Int Endod J. 1998;31:394.
333. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM. In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982;8:200.
334. Roane JB. Principles of preparation using the balanced force technique. In: Hardin J, editor. Clark’s clinical dentistry. Philadelphia, USA: JB Lippincott Co, 1991.
335. Roane JB, Powell SE. The optimal instrument design for canal preparation. J Am Dent Assoc. 1986;113:596.
336. Roane JB, Sabala CL, Duncanson MGJr. The “balanced force” concept for instrumentation of curved canals. J Endod. 1985;11:203.
337. Ruddle C. Cleaning and shaping the root canal system. In Cohen S, Burns RC, editors: Pathways of the Pulp, ed 8, St. Louis MO: Mosby, 2002.
338. Ruff ML, McClanahan SB, Babel BS. In vitro antifungal efficacy of four irrigants as a final rinse. J Endod. 2006;32:331.
339. Sabeti M, Simon JH, Slots J. Cytomegalovirus and Epstein-Barr virus are associated with symptomatic periapical pathosis. Oral Microbiol Immunol. 2003;18:327.
340. Sabeti M, Slots J. Herpesviral-bacterial coinfection in periapical pathosis. J Endod. 2004;30:69.
341. Sabins RA, Johnson JD, Hellstein JW. A comparison of the cleaning ability of short-term sonic and ultrasonic passive irrigation after hand instrumentation in molar root canals. J Endod. 2003;29:674.
342. Safavi E, Spångberg LSW, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990;16:207.
343. Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D. Bacterial penetration along different root canal filling materials in the presence or absence of smear layer. Int Endod J. 2008;41:32.
344. Sarkar NK, Redmond W, Schwaninger B, Goldberg AJ. The chloride corrosion behaviour of four orthodontic wires. J Oral Rehab. 1983;10:121.
345. Sattapan B, Nervo GJ, Palamara JEA, Messer HH. Defects in rotary nickel-titanium files after clinical use. J Endod. 2000;26:161.
346. Sattapan B, Palamara JEA, Messer HH. Torque during canal instrumentation using rotary nickel-titanium files. J Endod. 2000;26:156.
347. Saunders WP, Saunders EM. Comparison of three instruments in the preparation of the curved root canal using the modified double-flared technique. J Endod. 1994;20:440.
348. Saunders WP, Saunders EM. Effect of noncutting tipped instruments on the quality of root canal preparation using a modified double-flared technique. J Endod. 1992;18:32.
349. Saunders WP, Saunders EM. Influence of smear layer on the coronal leakage of Thermafil and laterally condensed gutta-percha root fillings with a glass ionomer sealer. J Endod. 1994;20:155.
350. Saunders WP, Saunders EM. The effect of smear layer upon the coronal leakage of gutta-percha root fillings and a glass ionomer sealer. Int Endod J. 1992;25:245.
351. Sayin TC, Cehreli ZC, Deniz D, Akcay A, Tuncel B, Dagli F, et al. Time-dependent decalcifying effects of endodontic irrigants with antibacterial properties. J Endod. 2009;35:280.
352. Schaeffer MA, White RR, Walton RA. Determining the optimal obturation length: a meta-analysis of literature. J Endod. 2005;31:271.
353. Schäfer E. Effect of physical vapor deposition on cutting efficiency of nickel-titanium files. J Endod. 2002;28:800.
354. Schäfer E. Effects of four instrumentation techniques on curved canals: a comparison study. J Endod. 1996;22:685.
355. Schäfer E. Root canal instruments for manual use: a review. Endod Dent Traumatol. 1997;13:51.
356. Schäfer E, Diey C, Hoppe W, Tepel J. Roentgenographic investigation of frequency and degree of canal curvatures in human permanent teeth. J Endod. 2002;28:211.
357. Schäfer E, Florek H. Efficiency of rotary nickel-titanium K3 instruments compared with stainless steel hand K-Flexofile. Part 1. Shaping ability in simulated curved canals. Int Endod J. 2003;36:199.
358. Schäfer E, Schulz-Bongert U, Tulus G. Comparison of hand stainless steel and nickel-titanium rotary instrumentation: a clinical study. J Endod. 2004;30:432.
359. Schäfer E, Vlassis M. Comparative investigation of two rotary nickel-titanium instruments: ProTaper versus RaCe. Part 2. Cleaning effectiveness and shaping ability in severely curved root canals of extracted teeth. Int Endod J. 2004;37:239.
360. Schäfer E, Vlassis M. Comparative investigation of two rotary nickel-titanium instruments: ProTaper vs. RaCe. Part 1: Shaping ability in simulated canals. Int Endod J. 2004;37:229.
361. Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18:269.
362. Schirrmeister J, Strohl C, Altenburger MJ, Wrbas K-T, Hellwig E. Shaping ability and safety of five different rotary nickel-titanium instruments compared with stainless steel hand instrumentation in simulated curved root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:807.
363. Schrader C, Ackermann M, Barbakow F. Step-by-step description of a rotary root canal preparation technique. Int Endod J. 1999;32:312.
364. Schrader C, Peters OA. Analysis of torque and force during step-back with differently tapered rotary endodontic instruments in vitro. J Endod. 2005;31:120.
365. Schrader C, Sener B, Barbakow F. Evaluating the sizes of LightSpeed instruments. Int Endod J. 1998;31:295.
366. Sedgley C, Nagel A, Hall D, Applegate B. Influence of irrigant needle depth in removing bioluminescent bacteria inoculated into instrumented root canals using real-time imaging in vitro. Int Endod J. 2005;38:97.
367. Seidberg BH, Schilder H. An evaluation of EDTA in endodontics. Oral Surg Oral Med Oral Pathol. 1974;37:609.
368. Sen BH, Safavi KE, Spångberg LS. Antifungal effects of sodium hypochlorite and chlorhexidine in root canals. J Endod. 1999;25:235.
369. Sen BH, Wesselink PR, Türkün M. The smear layer: a phenomenon in root canal therapy. Int Endod J. 1995;28:141.
370. Serene TP, Adams JD, Saxena A. Nickel-titanium instruments: applications in endodontics. St. Louis: Ishiaku EuroAmerica; 1995.
371. Shabahang S, Aslanyan J, Torabinejad M. The substitution of chlorhexidine for doxycycline in MTAD: the antibacterial efficacy against a strain of Enterococcus faecalis. J Endod. 2008;34:288.
372. Shabahang S, Pouresmail M, Torabinejad M. In vitro antimicrobial efficacy of MTAD and sodium hypochlorite. J Endod. 2003;29:450.
373. Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29:576.
374. Shabalovskaya S, Anderegg J, van Humbeed J. Critical overview of Nitinol surfaces and their modifications for medical applications. Acta Biomater. 2008;4:447.
375. Shadid DB, Nicholls JI, Steiner JC. A comparison of curved canal transportation with balanced force versus LightSpeed. J Endod. 1998;24:651.
376. Shen Y, Cheung GS, Bian Z, Peng B. Comparison of defects in ProFile and ProTaper systems after clinical use. J Endod. 2006;32:61.
377. Silvaggio J, Hicks ML. Effect of heat sterilization on the torsional properties of rotary nickel-titanium endodontic files. J Endod. 1997;23:731.
378. Siqueira JFJr. Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34:1.
379. Siqueira JFJr, Batista MM, Fraga RC, de Uzeda M. Antibacterial effects of endodontic irrigants on black-pigmented gram-negative anaerobes and facultative bacteria. J Endod. 1998;24:414.
380. Siqueira JFJr, Lima KC, Magalhaes FA, Lopes HP, de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J Endod. 1999;25:332.
381. Siqueira JFJr. Endodontic culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:365.
382. Siqueira JFJr, Araujo MC, Garcia PF, Fraga RC, Dantas CJ. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod. 1997;23:499.
383. Siqueira JFJr, Rocas IN, Favieri A, Lima KC. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J Endod. 2000;26:331.
384. Siqueira JFJr, Rocas IN, Santos SR, Lima KC, Magalhaes FA, de Uzeda M. Efficacy of instrumentation techniques and irrigation regimens in reducing the bacterial population within root canals. J Endod. 2002;28:181.
385. Sirén E, Haapasalo M, Waltimo TM, Ørstavik D. In vitro antibacterial effect of calcium hydroxide combined with chlorhexidine or iodine potassium iodide on Enterococcus faecalis. Eur J Oral Sci. 2004;112:326.
386. Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31:669.
387. Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297.
388. Sjögren U, Figdor D, Spångberg L, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119.
389. Sjögren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498.
390. Sjögren U, Sundqvist G. Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surg Oral Med Oral Pathol. 1987;63:366.
391. Solovyeva AM, Dummer PM. Cleaning effectiveness of root canal irrigation with electrochemically activated analyte and catholyte solutions: a pilot study. Int Endod J. 2000;33:494.
392. Sonntag D, Delschen S, Stachniss V. Root-canal shaping with manual and rotary Ni-Ti files performed by students. Int Endod J. 2003;36:715.
393. Sonntag D, Guntermann A, Kim SK, Stachniss V. Root canal shaping with manual stainless steel files and rotary Ni-Ti files performed by students. Int Endod J. 2003;36:246.
394. Sonntag D, Peters OA. Effect of prion decontamination protocols on nickel-titanium rotary surfaces. J Endod. 33, 2007.
395. Southard DW, Oswald RJ, Natkin E. Instrumentation of curved molar root canals with the Roane technique. J Endod. 1987;13:479.
396. Spanaki-Voreadi AP, Kerezoudis NP, Zinelis S. Failure mechanism of ProTaper Ni-Ti rotary instruments during clinical use: fractographic analysis. Int Endod J. 2006;39:171.
397. Spångberg L. Instruments, materials, and devices. In Cohen S, Burns RC, editors: Pathways of the Pulp, ed 7, St. Louis, MO: Mosby, 1998.
398. Spångberg L, Engström B, Langeland K. Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856.
399. Spångberg LSW, Haapasalo M. Rationale and efficacy of root canal medicaments and root filling material with emphasis on treatment outcome. Endod Topics. 2002;2:35.
400. Spili P, Parahos P, Messer HH. The impact of instrument fracture on outcome of endodontic treatment. J Endod. 2005;31:845.
401. Stamos DE, Squitieri ML, Costas JF, Gerstein H. Use of ultrasonics in single-visit endodontic therapy. J Endod. 1987;13:246.
402. Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9:498.
403. Stein TJ, Corcoran JF, Zillich RM. Influence of the major and minor foramen diameters on apical electronic probe measurements. J Endod. 1990;16:520.
404. Stenman E, Spångberg LS. Root canal instruments are poorly standardized. J Endod. 1993;19:327.
405. Suter B, Lussi A, Sequiera P. Probability of removing fractured instruments from root canals. Int Endod J. 2005;38:112.
406. Svec T, Powers J. Effects of simulated clinical conditions on nickel-titanium rotary files. J Endod. 1999;25:759.
407. Svec TA, Powers JM. A method to assess rotary nickel-titanium files. J Endod. 2000;26:517.
408. Tan BT, Messer HH. The quality of apical canal preparation using hand and rotary instruments with specific criteria for enlargement based on initial apical file size. J Endod. 2002;28:658.
409. Tasman F, Cehreli ZC, Ogan C, Etikan I. Surface tension of root canal irrigants. J Endod. 2000;26:586.
410. Tay F, Pashley D, Loushine R, Doyle M, Gillesp R, King N. Ultrastructure of smear layer-covered intraradicular dentin after irrigation with BioPure MTAD. J Endod. 2006;32:218.
411. Taylor JK, Jeansonne BG, Lemon RR. Coronal leakage: effects of smear layer, obturation technique, and sealer. J Endod. 1997;23:508.
412. Tepel J. Experimentelle Untersuchungen über die maschinelle Wurzelkanalaufbereitung. Berlin, Germany: Quintessenz Verlags-GmbH; 2000.
413. Thompson SA. An overview of nickel-titanium alloys used in dentistry. Int Endod J. 2000;33:297.
414. Thompson SA, Dummer PM. Shaping ability of Hero 642 rotary nickel-titanium instruments in simulated root canals: Part 2. Int Endod J. 2000;33:255.
415. Thompson SA, Dummer PM. Shaping ability of Lightspeed rotary nickel-titanium instruments in simulated root canals. Part 1. J Endod. 1997;23:698.
416. Thompson SA, Dummer PM. Shaping ability of LightSpeed rotary nickel-titanium instruments in simulated root canals. Part 2. J Endod. 1997;23:742.
417. Thompson SA, Dummer PM. Shaping ability of ProFile.04 Taper Series 29 rotary nickel-titanium instruments in simulated root canals. Part 1. Int Endod J. 1997;30:1.
418. Thompson SA, Dummer PM. Shaping ability of ProFile.04 Taper Series 29 rotary nickel-titanium instruments in simulated root canals. Part 2. Int Endod J. 1997;30:8.
419. Timpawat S, Sripanaratanakul S. Apical sealing ability of glass ionomer sealer with and without smear layer. J Endod. 1998;24:343.
420. Torabinejad M. Passive step-back technique. A sequential use of ultrasonic and hand instruments. Oral Surg Oral Med Oral Pathol. 1994;77:402.
421. Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233.
422. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658.
423. Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of the smear layer. J Endod. 2003;29:170.
424. Torabinejad M, Walton R. Principles and practice of endodontics, ed 4. St. Louis: Saunders; 2008.
425. Tripi TR, Bonaccorso A, Condorelli GG. Cyclic fatigue of different nickel-titanium endodontic rotary instruments. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;1002:e106.
426. Tripi TR, Bonaccorso A, Tripi V, Condorelli GG, Rapisarda E. Defects in GT rotary instruments after use: an SEM study. J Endod. 2001;27:782.
427. Troian CH, So MV, Figuereido JA, Oliveira EP. Deformation and fracture of RaCe and K3 endodontic instruments according to the number of uses. Int Endod J. 2006;39:616.
428. Tronstad L, Asbjørnsen K, Døving L, Pedersen I, Eriksen HM. Influence of coronal restorations on the periapical health of endodontically treated teeth. Endod Dent Traumatol. 2000;16:218.
429. Trope M. The vital tooth–its importance in the study and practice of endodontics. Endod Topics. 2003;5:1.
430. Turpin YL, Cagneau F, Vulcain JM. Impact of two theoretical cross-sections on torsional and bending stresses of nickel-titanium instrument models. J Endod. 2000;26:414.
431. Turpin YL, Chagneau F, Bartier O, Cathelineau G, Vulcain JM. Impact of torsional and bending inertia on root canal instruments. J Endod. 2001;27:333.
432. Uitto VJ, Haapasalo M, Laakso T, Salo T. Degradation of basement membrane (Typ IV) collagen by proteases from some anaerobic microorganisms. Oral Microbiol Immunol. 1988;3:97.
433. Ullmann C, Peters OA. Effect of cyclic fatigue on static fracture loads in ProTaper nickel-titanium rotary instruments. J Endod. 2005;31:183.
434. Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod. 2004;30:110.
435. v. d. Sluis LW, Gambarini G, Wu M-K, Wesselink PR. The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. Int Endod J. 2006;39:472.
436. v. d. Sluis LW, Wu M-K, Wesselink PR. A comparison between a smooth wire and a K-file in removing artificially placed dentine debris from root canals in resin blocks during ultrasonic irrigation. Int Endod J. 2005;38:593.
437. v. d. Sluis LW, Wu M-K, Wesselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from human root canals prepared using instruments of varying taper. Int Endod J. 2005;38:764.
438. v. d. Sluis LW, Wu M-K, Wesselink PR. The evaluation of removal of calcium hydroxide paste from an artificial standardized groove in the apical root canal using different irrigation methodologies. Int Endod J. 2007;40:52.
439. v. d. Sluis LW, Versluis M, Wu M-K, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415.
440. Vaudt J, Bitter K, Neumann K, Kielbassa AM. Ex vivo study on root canal instrumentation of two rotary nickel-titanium systems in comparison to stainless steel hand instruments. Int Endod J. 2009;42:22.
441. Verdelis K, Eliades G, Oviir T, Margelos J. Effect of chelating agents on the molecular composition and extent of decalcification at cervical, middle and apical root dentin locations. Endod Dent Traumatol. 1999;15:164.
442. Viana AC, Gonzalez BM, Buono VT, Bahia MG. Influence of sterilization on mechanical properties and fatigue resistance of Nickel-titanium rotary endodontic instruments. Int Endod J. 2006;39:709.
443. Vianna ME, Gomes BP. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009.
444. Vianna ME, Horz HP, Conrads G, Zaia AA, Souza-Filho FJ, Gomes BP. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol Immunol. 2007;22:411.
445. Walia HM, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod. 1988;14:346.
446. Walker A. A definite and dependable therapy for pulpless teeth. J Am Dent Assoc. 1936;23:1418.
447. Walmsley AD, Williams AR. Effects of constraint on the oscillatory pattern of endosonic files. J Endod. 1989;15:189.
448. Walsch H. The hybrid concept of NiTi rotary instrumentation. Dent Clin North Am. 2004;48:183.
449. Waltimo T, Ørstavik D, Sirén E, Haapasalo M. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J. 1999;32:421.
450. Waltimo TM, Sen BH, Meurman JH, Ørstavik D, Haapasalo MP. Yeasts in apical periodontitis. Crit Rev Oral Biol Med. 2003;14:128.
451. Waltimo TM, Sirén EK, Torkko HL, Olsen I, Haapasalo MP. Fungi in therapy-resistant apical periodontitis. Int Endod J. 1997;30:96.
452. Walton RE. Endodontic considerations in the geriatric patient. Dent Clin North Am. 1997;41:795.
453. Ward JR, Parashos P, Messer HH. Evaluation of an ultrasonic technique to remove fractured rotary nickel-titanium endodontic instruments from root canals: clinical cases. J Endod. 2003;29:764.
454. Weber CD, McClanahan SB, Miller GA, Diener-West M, Johnson JD. The effect of passive ultrasonic activation of 2% chlorhexidine or 5.25% sodium hypochlorite irrigant on residual antimicrobial activity in root canals. J Endod. 2003;29:562.
455. Weiger R, Bruckner M, El Ayouti A, Löst C. Preparation of curved root canals with rotary FlexMaster instruments compared to Lightspeed instruments and NiTi hand files. Int Endod J. 2003;36:483.
456. Weiger R, El Ayouti A, Löst C. Efficiency of hand and rotary instruments in shaping oval root canals. J Endod. 2002;28:580.
457. Weine FS, Healey HJ, Gerstein H, Evanson L. Pre-curved files and incremental instrumentation for root canal enlargement. J Can Dent Assoc. 1970;36:155.
458. West JD, Roane JB. Cleaning and shaping the root canal system. In Cohen S, Burns RC, editors: Pathways of the pulp, ed 7, St. Louis, MO: Mosby, 1998.
459. Williamson AE, Sandor AJ, Justman BC. A comparison of three Nickel-titanium rotary systems, EndoSequence, ProTaper Universal, and ProFile GT for canal-cleaning ability. J Endod. 2009;35:107.
460. Wolcott S, Wolcott J, Ishley D, et al. Separation incidence of ProTaper rotary instruments: a large cohort clinical evaluation. J Endod. 2006;32:1139.
461. Wu M-K, Barkis D, Roris A, Wesselink PR. Does the first file to bind correspond to the diameter of the canal in the apical region. Int Endod J. 2002;35:264.
462. Wu M-K, Dummer PM, Wesselink PR. Consequences of and strategies to deal with residual post-treatment root canal infection. Int Endod J. 2006;39:343.
463. Wu M-K, R’Oris A, Barkis D, Wesselink PR. Prevalence and extent of long oval canals in the apical third. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:739.
464. Wu M-K, v. d. Sluis LW, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218.
465. Wu M-K, Wesselink PR. A primary observation on the preparation and obturation of oval canals. Int Endod J. 2001;34:137.
466. Wu M-K, Wesselink PR. Efficacy of three techniques in cleaning the apical portion of curved root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:492.
467. Wu M-K, Wesselink PR. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. Int Endod J. 1993;26:37.
468. Wu M-K, Wesselink PR, Walton RE. Apical terminus location of root canal treatment procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:99.
469. Wu M-K, v. d. Sluis LWM, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218.
470. Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9:137.
471. Yao JH, Schwartz SA, Beeson TJ. Cyclic fatigue of three types of rotary nickel-titanium files in a dynamic model. J Endod. 2006;32:55.
472. Yared G. Canal preparation using only one Ni-Ti rotary instrument: preliminary observations. Int Endod J. 2008;41:339.
473. Yared GM, Bou Dagher FE. Influence of apical enlargement on bacterial infection during treatment of apical periodontitis. J Endod. 1994;20:535.
474. Yared GM, Bou Dagher FE, Machtou P. Cyclic fatigue of ProFile rotary instruments after clinical use. Int Endod J. 2000;33:204.
475. Yared GM, Bou Dagher FE, Machtou P. Cyclic fatigue of Profile rotary instruments after simulated clinical use. Int Endod J. 1999;32:115.
476. Yared GM, Bou Dagher FE, Machtou P. Failure of ProFile instruments used with high and low torque motors. Int Endod J. 2001;34:471.
477. Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod. 1995;21:513.
478. Yguel-Henry S, Vannesson H, von Stebut J. High precision, simulated cutting efficiency measurement of endodontic root canal instruments: influence of file configuration and lubrication. J Endod. 1990;16:418.
479. Yun HH, Kim SK. A comparison of the shaping abilities of 4 nickel-titanium rotary instruments in simulated root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:228.
480. Zamany A, Safavi K, Spångberg L. The effect of chlorhexidine as an endodontic disinfectant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:578.
481. Zehnder M. Root canal irrigants. J Endod. 2006;32:389.
482. Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:756.
483. Zehnder M, Lehnert B, Schönenberger K, Waltimo T. Spüllösungen und medikamentöse Einlagen in der. Zahnmedizin. Schw Monatsschr Zahnmed. 2003;113:756.
484. Zehnder M, Schicht O, Sener B, Schmidlin P. Reducing surface tension in endodontic chelator solutions has no effect on their ability to remove calcium from instrumented root canals. J Endod. 2005;31:590.
485. Zehnder M, Schmidlin PR, Sener B, Waltimo TM. Chelation in root canal therapy reconsidered. J Endod. 2005;31:817.
486. Zeltner M, Peters OA, Paqué F. Temperature changes during ultrasonic irrigation with different inserts and modes of activation. J Endod. 2009;35:573.
487. Zinelis S, Magnissalis EA, Margelos J, Lambrianidis T. Clinical relevance of standardization of endodontic files dimensions according to the ISO 3630-3631 specification. J Endod. 2002;28:367.
488. Zmener O, Banegas G. Comparison of three instrumentation techniques in the preparation of simulated curved root canals. Int Endod J. 1996;29:315.
489. Zuolo ML, Walton RE. Instrument deterioration with usage: Nickel-titanium versus stainless steel. Quintessence Int. 1997;28:397.