CHAPTER 13 Pulpal Reactions to Caries and Dental Procedures
The dental pulp is a very dynamic tissue that responds to external stimuli in a variety of different ways. However, there are certain unique features about the dental pulp response that distinguish it from other connective tissues in the body. The pulp’s exposure to dental caries, a very prevalent chronic infectious disease, its encasement in an unyielding environment after complete tooth maturation, and the scarcity of collateral circulation render it susceptible to injury and complicate its regeneration. Moreover, the pulp is endowed with a rich neurovascular supply that promotes the effects of inflammation and may lead to rapid degeneration and necrosis, a condition considered very serious in any tissue in the body. The treatment of dental caries and other tooth abnormalities involves the cleaning and shaping of the enamel and dentin, the hardest tissues in the body, thus adding to the irritation of the pulp. In this chapter, the response of the pulp to all these variables will be discussed, and recent advances in our understanding of dental procedures and their effects on the pulp will be presented.
Pulpal Reaction to Caries
Dental caries is a localized, destructive, and progressive infection of dentin; if left unchecked, caries can result in pulpal necrosis and potential tooth loss. Both bacterial byproducts and products from the dissolution of the organic and inorganic constituents of dentin mediate the effects of dental caries on the pulp. Three basic reactions tend to protect the pulp against caries: (1) a decrease in dentin permeability, (2) tertiary dentin formation, and (3) inflammatory and immune reactions. These responses occur concomitantly, and their robustness is highly dependent on the aggressive nature of the advancing lesion.
In the advancing infection front of the carious lesion, multiple intrinsic and extrinsic factors are released that are stimulatory to subjacent pulpal parenchyma. Bacterial metabolites such as acids have been thought to be initiators of pulpal reactions, yet the buffering capacity of dentin fluid likely attenuates the pH before it can directly effect a deleterious response, except when the remaining dentin thickness is minimal.214 When relatively unhindered access to pulpal tissue is present, both bacterial metabolites and cell wall components induce inflammation. In initial to moderate lesions, current evidence suggests that acidic byproducts of the carious process act indirectly by degrading the dentin matrix, thereby liberating bioactive molecules previously sequestered during dentinogenesis. Once liberated, these molecules once again assume their role in dentin formation, this time stimulatory for tertiary dentinogenesis.216 This theory is supported by the findings that demineralized dentin matrix implanted at the site of pulpal exposure can induce dentinogenesis.243 Furthermore, placement of purified dentin matrix proteins on exposed dentin or exposed pulp stimulates tertiary dentin formation, indicating that these molecules can act directly or across intact dentin.217,242
Recent evidence offers several candidate molecules that are stimulatory for reparative dentinogenesis. Heparin-binding growth factor, transforming growth factor (TGF)-β1, TGF-β3, insulin-like growth factors (IGF)-1 and -2, platelet-derived growth factor, and angiogenic growth factors have been shown to be stimulatory for dentinogenesis in vitro. The TGF-β superfamily in particular seems to be important in the signaling process for odontoblast differentiation as well as primary and tertiary dentinogenesis. As the predominant isoform, TGF-β1 is equally distributed in the soluble and insoluble fractions of dentin matrix.39 During the carious dissolution of dentin, it is believed that the soluble pool of TGF-β1 can diffuse across intact dentin while the insoluble pool is immobilized on insoluble dentin matrix and serves to stimulate odontoblasts, much like membrane-bound TGF-βs during odontogenesis.214
Despite the research interest in tertiary dentinogenesis, it is neither the first nor necessarily the most effective pulpally mediated defense against invading pathogens. A combination of an increased deposition of intratubular dentin and the direct deposition of mineral crystals into the narrowed dentin tubules to decrease dentin permeability is the first defense to caries and is called dentin sclerosis. It occurs by a combination of increased deposition of intratubular dentin and tubule occlusion by precipitated crystals. This results in an effective decrease in dentin permeability that occurs in a relatively short period of time. Classic studies have noted that while sclerosis is observed in the dentin of disease-free and attrition-free teeth, there is a 95% increase in the incidence in carious teeth.222 In vitro studies with cultured tooth slices implicate TGF-β1 as a central player in the increased deposition of intratubular dentin.213 The deposition of whitlockite crystals in the tubular lumen most likely results from a similar stimulation of vital associated odontoblasts, possibly in combination with precipitation of mineral released during the demineralization process140,239 (Fig. 13-1).
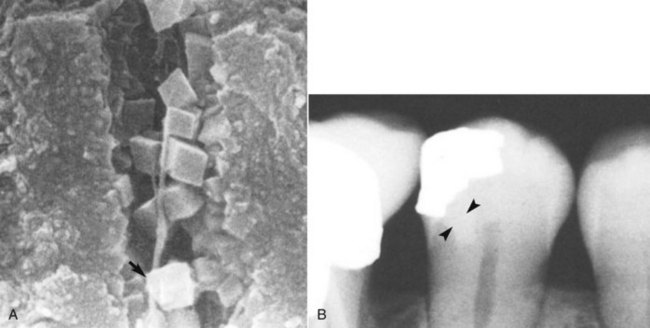
FIG. 13-1 A, Whitlockite crystals occlude the dentinal tubules in sclerotic dentin. B, Dentinal sclerosis is radiographically apparent beneath a deep class II lesion.
(A from Yoshiyama M, Masada J, Uchida A, Ishida H: Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res 68:1498–1502, 1989.)
The formation of tertiary dentin occurs over a longer period than does that of sclerotic dentin, and its resultant character is highly dependent on the stimulus. Mild stimuli activate resident quiescent odontoblasts, whereupon they elaborate the organic matrix of dentin. This type of tertiary dentin is referred to as reactionary dentin and can be observed when initial dentin demineralization occurs beneath the noncavitated enamel lesion.135 Mediators present during the carious process induce a focal upregulation of matrix production by resident odontoblasts. The resultant dentin is similar in morphology to physiologic dentin and may only be apparent due to a change in the direction of the new dentinal tubules (Fig. 13-2). In the aggressive lesion, the carious process may prove cytocidal to subjacent odontoblasts and require repopulation of the disrupted odontoblast layer with differentiating progenitors. The appearance of the resultant matrix is a direct reflection of the differentiation state of the secretory cells. This accounts for the heterogeneity of reparative dentin, where the morphology can range from organized tubular dentin to more disorganized irregular fibrodentin. Fibrodentin, owing to its irregular configuration and tissue inclusions, is more permeable than physiologic dentin252 (Fig. 13-3).
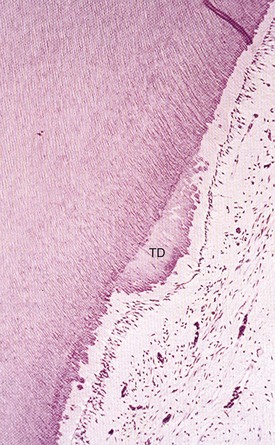
FIG. 13-2 Reactionary dentinogenesis (TD); note the tubular morphology and the discontinuity of the tubules at the interface of secondary and reactionary dentin. Resident odontoblasts are still present.
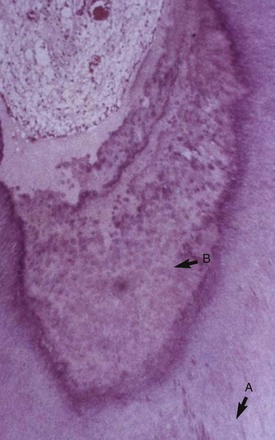
FIG. 13-3 Reparative dentin; the strong stimulus of the impinging infection is cytocidal for odontoblasts. The resultant dentin is irregular with soft-tissue inclusions.
Although dentin can provide a physical barrier against noxious stimuli, the pulpal immune response provides humoral and cellular challenges to invading pathogens. In the progressing carious lesion, the host immune response increases in intensity as the infection advances. It has been shown that titers of T helper cells, B-lineage cells, neutrophils, and macrophages are directly proportional to lesion depth in human teeth.107 The disintegration of large amounts of dentin, however, is not necessary to elicit a pulpal immune response. This is supported by the observation that a pulpal inflammatory response can be seen beneath noncavitated lesions and noncoalesced pits and fissures.33
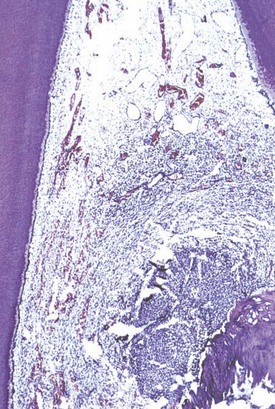
The early inflammatory response to caries is characterized by the focal accumulation of chronic inflammatory cells (Fig. 13-4). This is mediated initially by odontoblasts and later by dendritic cells. As the most peripheral cell in the pulp, the odontoblast is positioned to encounter foreign antigens first and initiate the innate immune response. Pathogen detection in general is accomplished via specific receptors called pattern recognition receptors (PRRs).109 These receptors recognize pathogen-associated molecular patterns (PAMPs) on invading organisms and initiate a host defense through the activation of the nuclear factor (NF)-κB pathway.91 One class of the PAMP recognition molecules is the toll-like receptor family (TLRs). Odontoblasts have been shown to have increased expression of certain TLRs in response to bacterial products. Under experimental conditions, odontoblast expression of TLR3, 5, and 9 was increased in response to lipoteichoic acid, whereas lipopolysaccharide increased TLR2 expression.59,166 Once the odontoblast TLR is stimulated by a pathogen, proinflammatory cytokines, chemokines, and antimicrobial peptides are elaborated by the odontoblast, resulting in recruitment and stimulation of immune effector cells as well as direct bacterial killing.67

FIG. 13-4 The early pulpal response to caries is represented by a focal accumulation of chronic inflammatory cells. Note that peripheral to the inflammation, the pulpal parenchyma is relatively unaffected.
Many cells constitutively produce chemokines at low levels. Unstimulated odontoblasts express genes coding for CCl2, CXCL12, and CXCL14, three genes known to code for factors chemotactic for immature dendritic cells.40 They also produce CCL26, a natural antagonist for CCR1, CCR2, and CCR5, which are chemokines normally produced by monocytes and dendritic cells.264 Stimulation with bacterial cell wall constituents has been shown to upregulate the production of several chemokines, suggesting that odontoblasts sense pathogens and express factors that recruit immune effector cells40,136 (Fig. 13-5). These data suggest a scenario whereby stimulated odontoblasts express high levels of chemokines such as interleukin (IL)-8 (CXCL8) that act in concert with the release of formerly sequestered TGF-β1 from carious dentin, the result of which is a focal increase in dendritic cell numbers, with additional release of chemotactic mediators.68 The subsequent influx of immune effector cells is composed of lymphocytes, macrophages, and plasma cells. This cellular infiltrate is accompanied by localized capillary sprouting in response to angiogenic factors, as well as coaggregation of nerve fibers and HLA-DR-positive dendritic cells.257,258
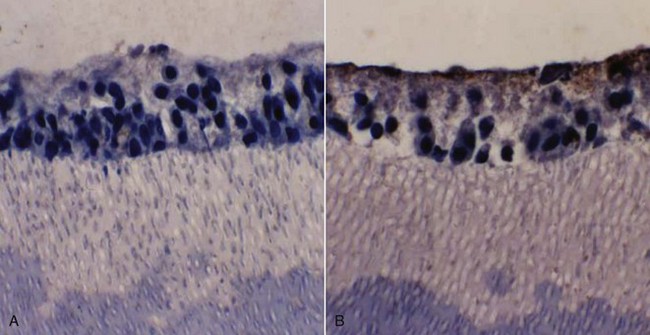
FIG. 13-5 Odontoblasts exposed to lipopolysaccharide (LPS) in an in vitro culture model express interleukin 8 (IL-8), evidenced by immunostaining with anti-IL-8 antibodies.
As the carious lesion progresses, the density of the chronic inflammatory infiltrate, as well as that of dendritic cells in the odontoblast region, increases. Pulpal dendritic cells are responsible for antigen presentation and stimulation of T lymphocytes. In the uninflamed pulp, they are scattered throughout the pulp. With caries progression, they aggregate initially in the pulp and subodontoblastic regions, then extend into the odontoblast layer, and eventually migrate into the entrance to tubules beside the odontoblast process259 (Fig. 13-6). There are two distinct populations of dendritic cells that have been identified in the dental pulp. CD11c+ are found in the pulp/dentin border and subjacent to pits and fissures. F4/80+ dendritic cells are concentrated in the perivascular spaces in the subodontoblastic zone and inner pulp.264 CD11c+ dendritic cells express toll-like receptors 2 and 4 and are CD205 positive. F4/80+ dendritic cells have migratory ability. As they migrate from the central pulp, they increase in size and become CD86 positive. The close spatial relationship between odontoblasts and dendritic cells under the carious lesion have led to speculation that dendritic cells may play a role in odontoblast differentiation and/or secretory activity in the immune defense and dentinogenesis. Pulpal Schwann cells have also been shown to produce molecules in response to caries, which is indicative of the acquisition of the ability for antigen presentation.
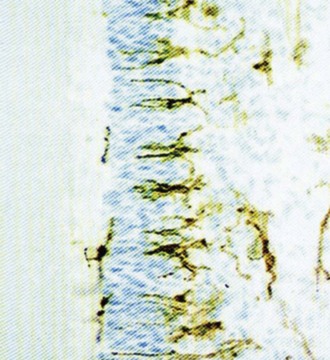
FIG. 13-6 Dental caries stimulates the accumulation of pulpal dendritic cells in and around the odontoblastic layer.
(Courtesy Mats Jontell.)
Evidence suggests that odontoblasts also play a role in the humoral immune response to caries. Immunoglobulin (Ig)G, IgM, and IgA have been localized in the cytoplasm and cell processes of odontoblasts in human carious dentin, suggesting that these cells actively transport antibodies to the infection front.171 In the incipient lesion, antibodies accumulate in the odontoblast layer and with lesion progression can be seen in the dentinal tubules. Eventually this leads to a focal concentration of antibodies beneath the advancing lesion.170
In the most advanced phase of carious destruction, the humoral immune response is accompanied by immunopathologic destruction of pulpal tissue. In animal studies where monkeys were hyperimmunized to bovine serum albumin (BSA), there was an observed increase in pulpal tissue destruction subsequent to antigenic challenge across freshly cut dentin.20 These findings support the contention that antigen-antibody complex formation, in addition to various products of the inflammatory cascade, gives rise to a nonspecific response that, while designed to rid the body of pathogens, effects destruction of parenchymal tissues as well.
Neurogenic Mediators
Neurogenic mediators are involved in the pulpal response to irritants and like immune components; they can mediate pathology as well as the healing response (for a review see Ref. 43). External stimulation of dentin causes the release of proinflammatory neuropeptides from pulpal afferent nerves.35 Substance P, calcitonin gene-related peptide (CGRP), neurokinin A (NKA), NKY, and vasoactive intestinal peptide are released and affect vascular events such as vasodilatation and increased vascular permeability. This results in a net increase in tissue pressure that can progress to necrosis in extreme and persistent circumstances. Stimulation of sympathetic nerves in response to the local release of mediators such as norepinephrine, neuropeptide Y, and adenosine triphosphate (ATP) has been shown to alter pulpal blood flow. Both receptor field studies and anatomic studies have shown sprouting of afferent fibers in response to inflammation.35
Neuropeptides can act to modulate the pulpal immune response. It has been demonstrated that substance P acts as a chemotactic and stimulatory agent for macrophages and T lymphocytes. The result of this stimulation is increased production of arachidonic acid metabolites, stimulation of lymphocytic mitosis, and production of cytokines. CGRP demonstrates immunosuppressive activity, which is evidenced by a decrease in H2O2 production by macrophages and a diminution of class II antigen presentation and lymphocyte proliferation.
Substance P and CGRP are mitogenic for pulpal and odontoblast-like cells and thereby initiate and propagate the pulpal healing response.237 CGRP has been shown to stimulate the production of bone morphogenic protein by human pulpal cells. The result of such stimulation has been postulated to induce tertiary dentinogenesis.37
As the carious lesion approximates the pulp, there is an acute exacerbation of the precedent chronic inflammation. This is characterized by an influx of neutrophils. The accumulation of inflammatory cells becomes marked when the infection front reaches tertiary dentin.128,129,200 In the presence of severe pulpal inflammation, focal microabscesses form and eventually coalesce, leading to progressive pulpal necrosis (Fig. 13-7).
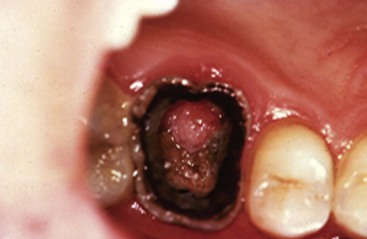
Pulpal exposure in primary and immature permanent teeth can lead to a proliferative response, or hyperplastic pulpitis. Exuberant inflammatory tissue proliferates through the exposure and forms a “pulp polyp” (Fig. 13-8). It is presumed that a rich blood supply coupled with ample lymphatic and oral drainage allows this proliferative response. Conventional root canal therapy or progressive vital pulp therapy is indicated.
Correlation between Clinical Symptoms and Actual Pulpal Inflammation
From a clinical perspective, it would be most helpful to the clinician to be able to diagnose pulpal conditions from a profile of the patient’s presenting symptoms. If symptoms are not conclusive, a number of objective tests should aid the clinician in reaching a definitive diagnosis of the pulpal pathologic status. In actuality, such combinations of subjective and objective findings are frequently insufficient in reaching definitive diagnosis of the status of the dental pulp. This is particularly true in cases of vital inflamed pulp, where it is difficult for the clinician to determine clinically whether the inflammation is reversible or irreversible.
Many clinicians rely on painful symptoms to determine the status of the pulp. Several studies have examined this question in some detail. A number of classic studies were performed in which the subjective and objective clinical findings related to carious teeth were recorded prior to extracting the teeth and examining them histologically. The underlying hypothesis in these studies was that the more severe the clinical symptoms, the more intense pulpal inflammation and destruction was evident histologically. The findings of these studies revealed that in the vital pulp, clinical symptoms generally did not correlate with gross histomorphologic findings.90,150,209 Furthermore, carious pulp exposure was associated with severe inflammatory response or liquefactive necrosis, regardless of symptoms (Fig. 13-9). These histologic changes ranged in extent from being present only at the site of the exposure to deep into the root canals.209 In a few studies, prolonged or spontaneous severe symptoms were associated with chronic partial or total pulpitis or pulp necrosis.57,209 However, in these as well as other studies, it was common to find cases with histologic evidence of severe inflammatory responses, including partial necrosis, but little or no clinical symptoms—the so-called painless pulpitis.57,90,150,209 Based on these studies and more recent data, it was reported that the incidence of painless pulpitis that leads to pulp necrosis and chronic periradicular periodontitis is about 40% to 60% of pulpitis cases.149
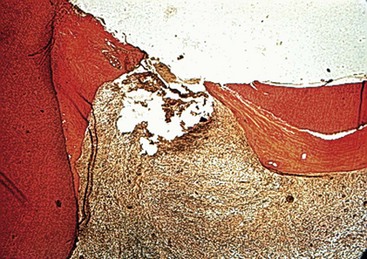
FIG. 13-9 Histologic photomicrograph of a molar with carious pulp exposure. The exposure had been capped but had failed, and the patient presented with symptoms. The photomicrograph shows an area of necrosis and extensive inflammation throughout the coronal pulp.
(Courtesy Dr. Larz Spangberg, University of Connecticut.)
Objective clinical findings are essential for determining the vitality of the pulp and whether the inflammation has extended into the periapical tissues. Lack of response to electric pulp testing is generally indicative that the pulp has become necrotic.202,209 Thermal pulp testing is valuable in reproducing a symptom of thermal sensitivity and allowing the clinician to assess the reaction of the patient to a stimulus and the duration of the response. However, pulp testing cannot determine the degree of pulpal inflammation.57,209 These studies show that irreversible pulpal inflammation can be diagnosed with some certainty only in cases where, in addition to being responsive to pulp testing, the pulp develops severe spontaneous symptoms. Pulp necrosis could be predictably diagnosed by a consistent negative response to pulp tests, preferably to both cold and electrical tests to avoid false responses.190,191 Pulp necrosis could be verified by a test cavity and/or lack of hemorrhagic pulp tissue upon access preparation. It should be noted, however, that the latter sign should be assessed cautiously. Occasionally, the pulp space is very small, such as in older individuals with calcified canals, and hemorrhage upon access to the pulp may not be clinically appreciable. Conversely, cases with pulp necrosis and acute periapical infections may have hemorrhagic purulent drainage through the large pulp space upon access preparation, particularly after initial instrumentation.
The lack of correlation between the histologic status of the pulp and clinical symptoms may be explained by recent advances in the science of pulp biology. In the last few decades, studies have shown that numerous molecular mediators may act in synchrony to initiate, promote, and/or modulate the inflammatory response in the dental pulp. Without the use of very specialized staining techniques, the nature and quantity of these inflammatory mediators cannot be determined from histologic analysis. Many of these molecular mediators tend to reduce the pain threshold, either directly by acting on peripheral nerve cells or through promoting the inflammatory process. Thus a number of these mediators were shown to be elevated in human pulp diagnosed with painful pulpitis. These mediators include prostaglandins47,204; the vasoactive amine, bradykinin134; tumor necrosis factor alpha (TNF-α)123; neuropeptides such as substance P,29 CGRP, and NKA11; and catecholamines.167 In fact, it was even shown that when patients have painful pulpitis, the crevicular fluid related to the affected teeth has significantly increased neuropeptides compared to the levels in contralateral teeth.11 In another study, trained volunteers had an incisor stimulated with a constant current threefold the threshold value for 90 seconds.10 This resulted in a significant increase in crevicular matrix metalloproteinase 8 (MMP8), one of the collagenases involved in tissue destruction.
It has also been determined that peripheral opioid receptors are present in the dental pulp,108 and these could play a role in why many cases with irreversible pulpitis are asymptomatic. As noted before, carious teeth are frequently not associated with significant symptoms. However, they still have a significant amount of inflammation. The pulp in teeth with mild to moderate caries has increased neuropeptide Y61 and its Y1 receptor,62 compared to normal teeth. Neuropeptide Y is a sympathetic nervous system neurotransmitter and is thought to act as a modulator of neurogenic inflammation. Likewise, the levels of vasoactive intestinal peptide (VIP), although not its receptor VPAC1, seemed to increase in the pulp of moderately carious teeth.60
With recent advances in molecular biology, efficient simultaneous detection of hundreds of molecular mediators by their gene expression has become a reality. Current research seeks to examine which genes are specifically expressed or upregulated in the pulp in response to the carious lesion. In this regard, preliminary studies have shown that various cytokines and other inflammatory mediators are upregulated underneath a carious lesion in a manner that correlates with the depth of caries.147 Gene microarrays have been used in several studies to obtain an accurate mapping of candidate genes that show elevated expression in inflamed pulp, the odontoblastic cell layer.148,176,177 Development of more accurate chairside diagnostic methods is potentially feasible, particularly sampling from crevicular fluid, dentinal fluid, or the pulp directly. For this reason, more research is needed to determine the key mediators that would predict survival or degeneration of the dental pulp in difficult diagnostic cases.
Dentin Hypersensitivity and Its Management
Dentin hypersensitivity represents a special situation in which a significant, usually chronic, pulpal problem arises that does not seem to be associated with irreversible pulpal pathosis in the majority of cases. Dentin hypersensitivity is characterized by short, sharp pain arising from exposed dentin in response to stimuli—typically thermal, evaporative, tactile, osmotic, or chemical—that cannot be ascribed to any other form of dental defect or pathology.94 Facial root surfaces in canines, premolars, and molars are particularly affected, especially in areas of periodontal attachment loss. Dentin hypersensitivity may be related to excessive abrasion during tooth brushing, periodontal disease, or erosion from dietary or gastric acids2,3,44 and may be increased following scaling and root planning.44,251 The dentin is hypersensitive most likely due to the lack of protection by cementum, loss of smear layer, and the hydrodynamic movement of fluid in dentinal tubules.4,31 The degree of inflammation in the pulp in cases of dentin hypersensitivity is not well characterized because the condition is usually not severe enough to warrant tooth extraction or endodontic therapy. However, patent dentinal tubules are present in areas of hypersensitivity261 (Fig. 13-10) and may result in increased irritation and localized reversible inflammation of the pulp at the sites involved.
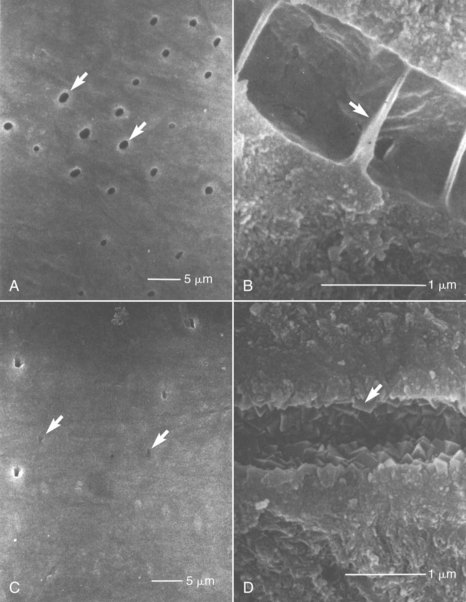
FIG. 13-10 A, Scanning electron microscope (SEM) image of an exposed dentin surface of a hypersensitive area. A large proportion of dentinal tubules (arrows) are seen to be open. B, SEM image of a fractured dentinal tubule of a hypersensitive area. The lumen of the dentinal tubule is partitioned by membranous structures (arrow). C, SEM image of exposed dentin surface of a naturally desensitized area. The lumens of dentinal tubules (arrows) are mostly occluded, and the surface is extremely smooth. D, SEM image of a fractured dentinal tubule of a naturally desensitized area. Rhombohedral platelike crystals of 0.1 to 0.3 µm (arrow) are present.
(From Yoshiyama M, Masada J, Uchida A, Ishida H: Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res 68:1498, 1989.)
The application of neural modulating agents such as potassium nitrate146 or tubule blocking agents such as strontium chloride, oxalates, or dentin bonding agents (Fig. 13-11)4,184 usually alleviates the condition, at least temporarily. However, the placement of passive molecules or crystals may provide only temporary relief, so there has been a need to provide biocompatible materials that bond to the root surface to provide a more lasting solution. One such material was a calcium sodium phosphosilicate bioactive glass,141 which was developed into a commercial product (SootheRx, NovaMin Technology Inc, Alachua, FL). Another product uses a combination of a calcium oxalate and an acid-etched bonding material to seal the dentinal tubules (BisBlock, Bisco Inc, Schaumberg, IL). A concern has been raised that the acidic pH during etching may cause dissolution of the oxalate crystals, thus interfering with the effectiveness of the material.256 However, a recent study found that BisBlock and two other products (Seal&Protect, DENTSPLY DeTrey GmbH, Konstanz, Germany, and Vivasens, Ivoclar Vivadent AG, Schaan, Liechtenstein) were effective several weeks after treatment, compared to placebo.180 In the long term, the development of smear layer, such as from tooth brushing, dentin sclerosis, reactionary dentin, and the blockage of tubules with large endogenous macromolecules are all thought to reduce the problem183 (illustrated by Mechanism of Dentin Hypersensitivity animation on the Expert Consult Site).

FIG. 13-11 Smear layer treated with 30% dipotassium oxalate for 2 minutes plus 3% monopotassium and monohydrogen oxalate for 2 minutes. Dentin surface is completely covered with calcium oxalate crystals (×1900).
(From Pashley DH, Galloway SE: The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol 30:731, 1985.)
Pulpal Reactions to Local Anesthetics
An intact pulpal blood flow is critical for maintaining the health of the dental pulp. Because the dental pulp is enclosed in a rigid chamber and supplied by few arterioles through the apical foramina, it cannot benefit from collateral circulation or volumetric changes that compensate for changes in blood flow in other soft tissues. Furthermore, reduction in blood flow has the compounding effect of reducing the clearance of large molecular-weight toxins or waste products,185 thus causing irreversible pulpal pathosis. Vasoconstrictors are added to local anesthetics to enhance the duration of anesthesia. However, vasoconstrictors in local anesthetics could negatively impact the health of the pulp if they reduce blood flow, particularly if the pulp is inflamed preoperatively. Earlier studies have documented that vasoconstrictors in local anesthetics do reduce pulpal blood flow in experimental animals when administered by infiltration and nerve block118 (Fig. 13-12), and that this effect was more severe with periodontal ligament injections117 (Fig. 13-13). More recently, clinical trials were conducted in which subjects were given infiltration of different local anesthetics with or without epinephrine at a concentration of 1:100,000, and the pulpal blood flow was measured by laser Doppler flowmetry. In groups that received the epinephrine, there were consistently significant reductions in pulpal blood flow,5,46,160 even if the infiltration was palatal to maxillary premolars.193 Interestingly in one study, the reduction in pulpal blood flow with epinephrine infiltration was more than the reduction in gingival blood flow and did not return to baseline values after 1 hour of injection.5 Similar reductions in pulpal blood flow were reported when inferior alveolar nerve block injections of lidocaine and 1:100,000 or 1:80,000 epinephrine were administered.169 It is important to note a limitation of studies using laser Doppler flowmetry: a large proportion of the signal measured may be from sources other than the dental pulp.192,219 Thus the monitoring of minor changes in pulpal blood flow must be interpreted with caution, particularly if the rubber dam or a similar barrier was not used.89 Human studies on the effects of periodontal ligament or intraosseous injections on pulpal blood flow are unavailable, but from animal studies, it is probable that these supplemental anesthetic techniques cause more severe reduction or even transient cessation of pulpal blood flow. It was also shown that intraosseous injection of Depo-Medrol (a corticosteroid) in patients with symptomatic irreversible pulpitis causes significant reduction of prostaglandin E2 in the pulp 1 day after administration, indicating that this route of injection results in significant permeation into the pulpal tissues.104 Taken together, these findings suggest that local anesthesia may compromise the inflamed pulp’s ability to recover from inflammation, particularly if it is severely inflamed, if the tooth is subjected to extensive restorative procedures, or if the anesthetic is delivered via a periodontal ligament or an intraosseous route. However, it is important to realize that this hypothesis should be supported or refuted by prospective randomized clinical trials.
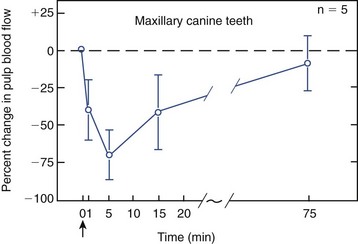
FIG. 13-12 Effects of infiltration anesthesia (i.e., 2% lidocaine with 1:100,000 epinephrine) on pulpal blood flow in the maxillary canine teeth of dogs. There is a drastic decrease in pulpal blood flow soon after the injection. Arrow indicates the time of injection. Bars depict standard deviation.
(From Kim S, Edwall L, Trowbridge H, Chien S: Effects of local anesthetics on pulpal blood flow in dogs. J Dent Res 63:650, 1984.)
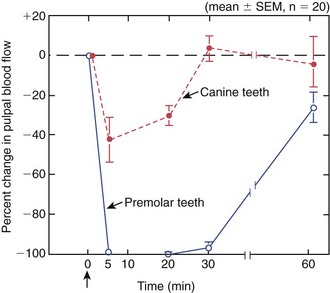
FIG. 13-13 Effects of ligamental injection (i.e., 2% lidocaine with 1:100,000 epinephrine) on pulpal blood flow in the mandibular canine and premolar teeth of dogs. Injection was given in the mesial and distal sulcus of premolar teeth. Injection caused total cessation of pulpal blood flow that lasted about 30 minutes in the premolar teeth. Arrow indicates time of injection.
(From Kim S: Ligamental injection: a physiological explanation of its efficacy. J Endod 12:486, 1986.)
Intrapulpal anesthesia is often used as a last resort when pulpal anesthesia is insufficient during root canal therapy. The effect of intrapulpal anesthesia on the pulp in these cases is not considered, since the pulp will be removed. However, occasionally a pulpotomy is performed to maintain pulpal vitality, such as in children, where the tooth has an immature apex. One study has shown that intrapulpal anesthesia can be used in these cases, with no clinical differences on follow-up of over 24 weeks between the groups that did or did not receive intrapulpal anesthesia, and when given, in the groups where the anesthetic contained or did not contain epinephrine.233
Pulpal Reactions to Restorative Procedures
A voluminous body of literature exists on the effects of restorative procedures on the dental pulp. This topic understandably has been important for practicing dentists for many years. Restorative procedures are performed primarily to treat an infectious disease—dental caries—which itself causes significant irritation of the pulp. They may also be performed to help restore missing teeth, correct developmental anomalies, address fractures, cracks, or failures of previous restorations, or a myriad of other abnormalities. One key requirement of a successful restorative procedure is to cause minimal additional irritation of the pulp so as not to interfere with normal pulpal healing. When pulp vitality is to be maintained during a restorative procedure, a provisional diagnosis of reversible pulpitis (rather than irreversible pulpitis) must preexist. Therefore, it would be most desirable to perform a minimally traumatic restorative procedure that would not potentially convert the diagnosis to irreversible pulpitis. As was discussed previously, irreversible pulpitis may present clinically with severe spontaneous postoperative pain, but it may also be asymptomatic, leading to the quiet demise of the pulp. The additive effects of restorative procedures are particularly critical in borderline cases, such as those of mildly symptomatic teeth with deep caries but no pulp exposure. There are still many factors whose influence on the response of the dental pulp to cumulative effects of caries, microleakage, restorative procedures, and materials is not well understood. It is generally accepted that the effects of pulpal insults, be they from caries, restorative procedures, or trauma, are cumulative. That is, with each succeeding irritation, the pulp has a diminished capacity to remain vital.
As a part of informed consent, the clinician is often faced with the task of outlining possible risks of restorative treatment. The question of the fate of pulps beneath single unit metal-ceramic (MC) crowns or MC bridge abutments was addressed.45 Patients who had received either treatment from 1981 to 1989 at the Prince Philip Dental Hospital in Hong Kong were randomly selected and invited to attend a recall appointment that involved both clinical and radiographic examinations. One hundred twenty-two teeth with preoperatively vital pulps treated with single unit MC crowns and 77 treated as bridge abutments were examined. The mean observation period was 169 months for the former and 187 months for the latter. Pulpal necrosis had occurred in 15.6% of the teeth treated with single unit crowns after 10 years, while 32.5% of the pulps in the bridge retainer groups had become necrotic. There was a significantly higher percentage of pulpal necrosis in anterior teeth that served as bridge abutments (54.5% of anterior abutment teeth examined). In general, however, the available evidence indicates that the effects of dental procedures on the pulp depend on several key factors discussed in the following sections.
Degree of Pretreatment Pulp Inflammation
As stated previously, the dental pulp is compromised in its ability to respond to external irritants because (1) it is enclosed in a noncompliant environment, and (2) it lacks sufficient collateral circulation. Thus the more severely the pulp is inflamed, the less will be its ability to respond to further irritation, such as in the form of restorative procedures.130
Most research studies designed to evaluate the effects of restorative procedures (or materials) on the pulp are conducted on human or experimental animal teeth with normal pulp. Furthermore, many of the animal research projects have been performed on anesthetized animals without the use of local anesthesia, which as stated previously, reduces pulpal blood flow. Therefore, the results of these studies may not reveal the true effects of these procedures when the pulp is already inflamed by the carious lesion and pulpal blood flow is reduced by local anesthetic injection. Classic experiments have documented that different levels of irritation induce different degrees of inflammation in the pulp,154 and more severe inflammation may take longer to heal.155 In a study that evaluated the response of the pulp to capping procedures as a function of duration of exposure, it was shown that the pulp responds favorably to exposures of up to 24 hours after exposure, but not as favorably after longer periods of exposure to oral environment.52 It may be that the longer exposure periods lead to the formation of a bacterial biofilm that is difficult for the pulpal immune responses to eliminate. This is relevant in cases of aseptic mechanical exposures or teeth where the pulp is exposed by traumatic injuries for a brief duration. In these cases, the pulp usually responds favorably to vital pulp therapy procedures. Models of standardized pulpal inflammation with chronic caries are not commonly used in determining the effects of dental procedures. Older clinical studies show an unfavorable long-term outcome of capping cases with carious pulp exposures,17,95 but newer studies in which MTA was used show more favorable results in these cases.16,26
In the absence of severe spontaneous symptoms or pulp exposure, the clinician currently cannot accurately determine the degree of preoperative pulpal inflammation. Thus, every effort should be made to minimize added irritation during restorative procedures, because it is possible that excessive irritation could convert the inflammatory status of the pulp from a reversible to an irreversible condition.
Degree of Physical Irritation Caused by Procedure
The physical irritation of the pulp during restorative procedures, such as from heat, desiccation or vibration, may adversely affect the dental pulp.
Heat
Restorative procedures such as cavity preparation, crown preparation, or curing of resins during direct fabrication of provisional restorations234 may cause significant increases in pulpal temperatures. It has been shown using primate models that an intrapulpal temperature rise of 10° C causes irreversible pulp pathosis in 15%, and a 20° C rise caused pulp abscess formation in 60% of teeth evaluated.262 A number of other older studies documented burns or severe inflammation in the pulp when cavity or crown preparations were performed without coolants (Figs. 13-14, 13-15, 13-16). However, a study, in which gradual controlled heat application over a large area of the intact occlusal surface of human unanesthetized teeth was employed, failed to corroborate these earlier findings.12 In this study, an increase of intrapulpal temperature of about 11° C, followed for 2 to 3 months of evaluation, did not induce any clinical or histologic changes in the pulp of any of the teeth evaluated. Heat increase in rat pulp tissue to 42° C in vitro raised heat shock protein-70, which is known to be tissue protective, and caused changes in alkaline phosphatase and gap junction proteins that were reversed to normalcy a few hours later.7 By contrast, in another study, heat applied in deep cavity preparations (prepared atraumatically in human teeth) caused histologic changes that were dependent on the proximity of the heat source to the pulp.168 It was common in that study to see loss of odontoblasts or their aspiration into the dentinal tubules. In cases where the cavity floor was less than 0.5 mm from the pulp, areas of coagulation necrosis could be seen, although the patients remained asymptomatic for the 1-month duration of the study. The measurement of heat in the tooth being prepared, in areas other than the site of tooth preparation, occasionally shows reduction in temperature,80 presumably because of the poor conductive properties of dentin and the cooling effect of compressed air from the high-speed handpiece. Furthermore, cavity and crown preparations include a number of other irritating stimuli, such as desiccation, severance of odontoblastic processes, vibration, and smearing of bacterial irritants onto the surface of dentin. Taken together, these findings suggest that the transient increase in temperature to levels relevant to modern dental procedures on its own may not be the culprit in inducing pulpal changes. Rather, the synergistic application of excessive heat with other irritation factors and its proximity to the pulp may induce pathologic changes.
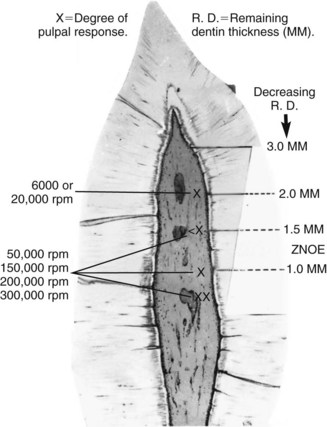
FIG. 13-14 With adequate water and air spray coolant, the same cutting tools, and a comparable remaining dentin thickness, the intensity of the pulpal response with high-speed techniques (i.e., decreasing force) is considerably less traumatic than with lower-speed techniques (i.e., increasing force).
(From Stanley HR, Swerdlow H: An approach to biologic variation in human pulpal studies. J Prosthet Dent 14:365, 1964.)
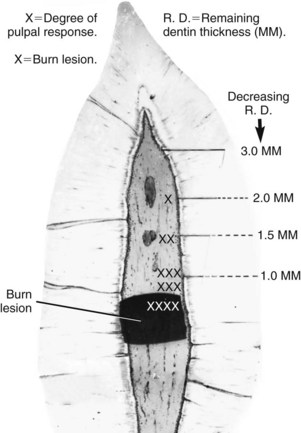
FIG. 13-15 Without adequate water coolant, larger cutting tools (e.g., No. 37 diamond point) create typical burn lesions within the pulp when the remaining dentin thickness becomes less than 1.5 mm.
(From Stanley HR, Swerdlow H: An approach to biologic variation in human pulpal studies. J Prosthet Dent 14:365, 1964.)
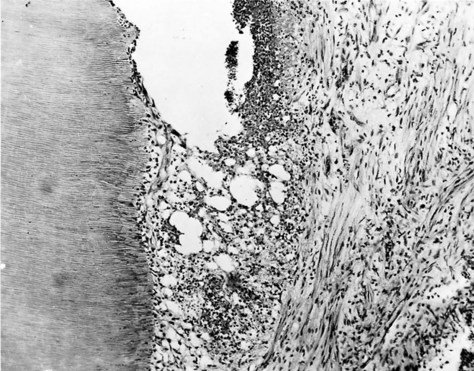
FIG. 13-16 Burn lesion with necrosis and expanding abscess formation in a 10-day specimen. Cavity preparation dry at 20,000 rpm, with remaining dentin thickness 0.23 mm.
(From Swerdlow H, Stanley HR Jr: Reaction of the human dental pulp to cavity preparation. I. Effect of water spray at 20,000 rpm. J Am Dent Assoc 56:317, 1958.)
Desiccation
Desiccation during cavity and crown preparation has long been known to cause aspiration of odontoblastic nuclei into dentinal tubules and pulpal inflammation.30 One study showed that as little as 30 seconds of continuous air drying of class V cavities in human molars with uninflamed pulp caused significant displacement of odontoblastic nuclei, pulp inflammation, and even areas of necrosis related to the areas that were dried.51 However, another study showed that the effects of desiccation are transient, in that within 7 to 30 days there is autolysis of the aspirated cells and formation of reactionary dentin.32 The pulp in cases with aspirated odontoblasts, following desiccation for 1 minute, was not sensitive to clinical scraping with an explorer. The sensitivity was restored with rehydration of the cavities and was increased in other cases where pulp inflammation was induced by microbial contamination.138 In this study, despite the lack of sensitivity in desiccated cavities, neural elements were seen histologically to be pushed into the tubules like the odontoblastic nuclei. The disruption of the odontoblastic layer and peripheral neural elements in the pulp with desiccation was also observed in a rat model using axonal transport of radioactive protein.36
Biologic and Chemical Irritation
Dental caries is clearly an infectious disease in which microorganisms and their virulence determinants constantly irritate the pulp, even at the early stages long before pulp exposure.33 However, despite the elimination of visible caries during cavity preparation, the cavity floor is undoubtedly left with some contamination by caries bacteria. Whereas the rubber dam should be used with any cavity preparation to prevent cavity contamination with salivary microorganisms, the use of water coolants allows the cavity to be contaminated with bacteria from water lines. Concerns about residual cavity contamination prompted some to use cavity disinfection with caustic chemicals. Chemicals such as hydrogen peroxide, sodium hypochlorite, or calcium hydroxide solutions have been proposed for this purpose, although they may exert a toxic effect.50 An earlier study showed that the amounts of residual bacteria following adequate restoration are not significant.151 Once dentin is exposed, there is constant outward flow of dentinal fluid that minimizes the inward flow of any noxious agents.252 This may aid in the reduction of irritation from residual microbial factors in dentinal tubules.
In contemporary practice, most chemical irritation during restorative procedures results from the application of etching agents, especially strong acids in the form of total dentin etch, particularly if capping of exposed pulp is performed.81,179 Etching is performed to remove the smear layer, promote physical adhesion of bonding agents to dentin by forming resin tags in the dentinal tubules, and promote permeation of the newer unfilled resin primers into the unmineralized surface layer of collagen to form the so-called hybrid layer.
If the cavity is relatively superficial and is adequately sealed with a restorative resin, then etching of dentin is probably not detrimental to the pulp, because of the narrow diameter of dentinal tubules and their low density in peripheral dentin.28 In fact, one study documented that histologic evidence of bacteria in human cavities restored with composite was significantly less if the cavity had been etched with phosphoric acid than if it were etched with 17% ethylenediamine tetra-acetic acid (EDTA) or not etched.159 Pulpal inflammation in this study was not correlated with the etching treatments but with bacterial presence; in cases of etching with phosphoric acid, if bacteria were also present, severe pulpal inflammation and necrosis could be seen.
In recent years, self-etching formulations have become popular because they eliminate the separate etching step involved in total-etch procedures. Some have speculated that the bonding of self-etching systems may be poorer than total-etch systems because of the weaker acidity of the acidic primers of self-etching systems compared to total-etch systems.28 However, studies have shown no significant differences between the two adhesive systems in long-term in vitro bond strength, postoperative sensitivity,188 long-term in vivo degradation,125 or long-term in vitro bond strength.9 One clinical study showed no differences between the two systems with respect to bacterial leakage and the inflammatory response in the pulp.164 The most important variable that affected the pulp in this study was the amount of bacterial leakage with either system.
Other factors that may contribute to pulpal irritation during resin placement from chemical/biologic irritants include unpolymerized monomer and polymerization shrinkage. Higher concentrations of monomeric resin components were shown to exert an inhibitory effect on T-lymphocytes and spleen cells113 and monocytes/macrophages133,196,197,208 in vitro. These components may leach directly into the pulp in deep cavities and cause chemical irritation.98 Shrinkage during polymerization of composites may induce internal stresses on dentin and create voids that allow microleakage. Shrinkage of resins is estimated to range from 0.6% to 1.4% and should be minimized during placement by incremental curing and possibly starting the restoration with flowable resins.28
In summary, the available evidence indicates that chemicals involved in modern restorative procedures may irritate the pulp if placed directly on an exposure or if there is microbial leakage along the tooth/restoration interface.
Proximity of Restorative Procedures to Dental Pulp and Surface Area of Dentin Exposed
It has been known for several decades that as the carious lesion progresses towards the pulp, particularly when the remaining dentin thickness (RDT) is less than 0.5 mm, there is an increasingly severe pulpal reaction, with a greater likelihood of the pulp undergoing irreversible pathosis.201 The diameter and density of dentinal tubules increase closer to the pulp (Fig. 13-17). Based on the dentinal tubule density at the dentin-enamel junction (DEJ) (about 65,000/mm2) and the pulp (about 15,000/mm2),74,78 it was estimated that the area occupied by tubule lumina at the DEJ was 1% of the total surface area at the DEJ and 22% at the pulp.183 Thus it is not surprising that several studies have shown that pulpal inflammation in response to restorative procedures increases with the reduction in RDT.1,53,158 A recent study examined the differential effects on the rat pulp of the preparation method, remaining dentin thickness, coolant, drill speed, conditioning with EDTA, and filling materials.157 Subsequent to the cavity preparations, a tooth slice was obtained and maintained ex vivo as an organ culture for up to 2 weeks. The results showed that the remaining dentin thickness was the most important factor on pulpal injury.
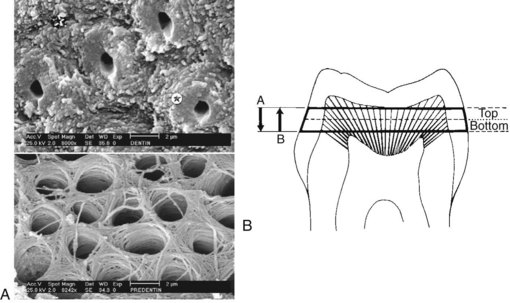
FIG. 13-17 Convergence of tubules toward the pulp. A, Periphery of the dentin. Most surface area is occupied by intertubular dentin ( ), with a few tubules surrounded by hypermineralized peritubular dentin (
), with a few tubules surrounded by hypermineralized peritubular dentin ( ). B, Near the pulp, the increase in tubule diameter has occurred largely at the expense of the peritubular dentin. This substrate has a high protein content. As the remaining dentin is made thinner (from A-B), the permeability of the dentin increases because both diameter and density of dentinal tubules are increased.
). B, Near the pulp, the increase in tubule diameter has occurred largely at the expense of the peritubular dentin. This substrate has a high protein content. As the remaining dentin is made thinner (from A-B), the permeability of the dentin increases because both diameter and density of dentinal tubules are increased.
(Bouillaguet S: Biological risks of resin-based materials to the dentin-pulp complex. Crit Rev Oral Biol Med 15:47–60, 2004.)
With the passage of time following cavity preparation, there is reduction in the permeability of RDT.186 This may be due to rapid deposition of reactionary dentin, the migration of large proteins into the tubules, and/or the diminution of tubule diameter as dentin becomes more sclerotic. Using a primate model, one study showed that the basic rate of secondary dentin deposition is about 0.8 µm/day, and this rate increases to an average of 2.9 µm/day following restorative procedures. Interestingly, dentin deposition was more rapid next to shallow cavities than deep cavities in one study,254 but another study showed that total reactionary dentin deposited was thicker in deeper and wider cavities.156
Clinically it has been observed that postoperative sensitivity is common with many restorative procedures. In one study, it was shown that following resin composite restorations on patients, postoperative sensitivity was related to the depth of the cavity but not to the presence or absence of liners or bases.244
In addition to the depth and/or width of a large cavity preparation, a crown preparation exposes more dentinal tubules to microbial or chemical irritation. During crown fabrication, there are added irritation factors, such as length of time of the preparation, impression techniques, and imperfect adaptation of temporary restorations, leading to microleakage during the temporization period. Because of the precise engineering requirements of some restorations, some providers may be inclined to reduce the coolant during crown preparation steps such as finalizing the finishing lines. However, crown preparations without coolants have been shown to dramatically reduce pulpal blood flow in an animal model (Fig. 13-18). There are few studies available on the effects of modern crown and bridge techniques on the pulp. However, some long-term outcome studies have documented that the incidence of pulp necrosis following crown placement ranges from 10% to 18%.70,245 As noted earlier, a more recent paper of work done in the 1980s in Hong Kong showed these figures to be much larger in bridge retainers compared to single crowns, reaching a value of up to 54% in anterior teeth.45
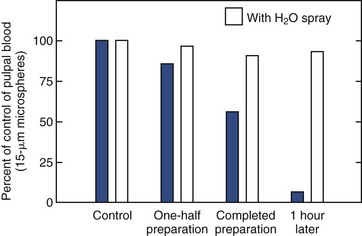
FIG. 13-18 Effects of crown preparation in dogs, with and without water and air spray (at 350,000 rpm) on pulpal blood flow. Tooth preparation without water and air spray caused substantial decrease in pulpal blood flow, whereas that with water and air spray caused insignificant changes in the flow.
Permeability of Dentin and Odontoblastic Layer Between Area Being Restored and Pulp
The permeability of dentin plays an important role in the ingress of potential irritants to the pulp. It is clear from research done in the last 3 decades that dentin is not uniformly permeable, and that permeability depends on factors such as the location within the same tooth, the age of the patient, and the presence of pathologic conditions such as dental caries. Fundamentally, the permeability of dentin depends on the collective sum of permeability of individual tubules at a particular site in the tooth. The tubular diameter increases from about 0.6 to 0.8 µm close to the DEJ to about 3 µm at the pulp.86 Given that bacterial cells are about 0.5 to 1 µm in diameter, it is evident that in deep cavity preparations, particularly when total-etch procedures are employed, bacteria can migrate through the remaining dentin into the pulp.
With age, the width of peritubular dentin increases, causing reduction in tubular lumen or sclerosis. Caries causes demineralization in superficial dentin, which is associated with remineralization and the formation of caries crystals within the tubules of inner undemineralized dentin (Fig. 13-19). This causes a decrease in permeability in dentin subjacent to the carious lesion184 and could be considered a protective mechanism, since it may delay the progress of the carious lesion.
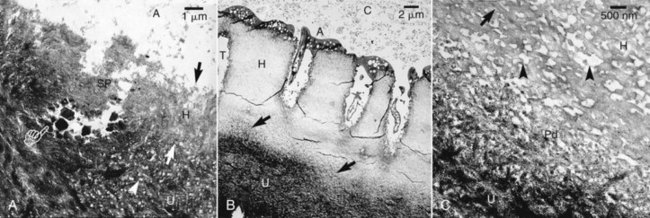
FIG. 13-19 Transmission electron micrographs (TEM) of undemineralized specimens of resin-bonded caries-affected dentin. A, Stained TEM of undemineralized specimens following the application of the self-etch ABF system to caries-affected dentin. The hybrid layer (H, between arrows) was about 3 µm thick, and the underlying undemineralized dentin (U) was highly porous (arrowhead). The dentinal tubule was covered with a smear plug (SP) and was partially obliterated with large caries crystals (pointer). (A), filled adhesive. B, Stained section of the total etch Single Bond adhesive bonded to caries-affected dentin. A hybrid layer (H) between 15 and 19 µm thick could be seen, with a partially demineralized zone (open arrows) above the undemineralized caries affected dentin (U). (T), dentinal tubule. (C), composite. C, Higher magnification of the basal part of the unusually thick hybrid layer (H) shown in (A). Banded collagen fibrils (open arrow) were separated by unusually wide and porous interfibrillar spaces (open arrowheads). A partially demineralized zone (Pd) was present along the demineralization front. This zone was not seen in phosphoric-acid-etched sound dentin (B). (U), undemineralized caries-affected dentin.
(From Yoshiyama M, Tay FR, Doi J, et al: Bonding of self-etch and total-etch adhesives to carious dentin. J Dent Res 81:556, 2002.)
It has been reported that the odontoblastic cell layer itself may contribute to the reduction of permeability to large molecules such as bacterial proteins or toxins. Experiments were conducted in which large protein markers were allowed to permeate the odontoblastic layer in experimental animals before and after cavity preparation. It was shown that irritation from cavity preparation increased the odontoblastic permeability only at the site of the cavity preparation.106,240 In addition to the physical barrier to permeability and the production of reactionary or reparative dentin, the odontoblastic layer may in fact contribute to the host response of the dental pulp by expressing important inflammatory mediators136,178 or recognize bacteria through toll-like receptors.27,59,67,112
Patient Age
It was reported that resting pulpal blood flow (PBF), as well as the changes in PBF in response to cold application, decrease with age.100 Age may also be associated with reduction in pulpal neuropeptides.75 However, a recent animal study failed to show any differences between young and old pulp in the regenerative capacity of odontoblast-like cells and in the presence of cells positive for class II major histocompatibility complex, heat shock protein 25, or nestin, when subjected to cavity preparation.116 Thus in humans with advancing age, the net result of the ability of the pulp to cope with external stimulation or irritation is not clear.
Pulpal Reactions to Restorative Materials
The effects of restorative materials on the dental pulp have been investigated and seem to relate directly to the permeability of the associated dentin. The degree of dentin permeability, however, is often variable and is governed by several factors including age and caries status.230 As noted before with respect to restorative procedures and bacterial ingress, perhaps the most important variable in dentin permeability to restorative materials is the thickness of dentin between the floor of the cavity preparation and the pulp.1
Unbound components of resin materials and preparative agents such as acid etchants can affect the subjacent pulp by inducing an inflammatory response.77,195 The indirect effects of desiccation and/or demineralization of dentin, as well as direct effects of the material itself when in contact with pulpal tissue, mediate this inflammatory response. Studies have shown that certain cytotoxic components of resin monomers (e.g., triethylene glycol dimethacrylate and 2-hydroxyethyl methacrylate) readily penetrate dentin.72 Similarly, eugenol and components of Ledermix (triamcinolone and demeclocycline) have been shown to pass through dentin into the subjacent pulp.97,99 In vivo data show that these chemicals have an effect on the pulp, but the effect seems to be short lived and, in the absence of bacteria, reversible.21
The mechanisms whereby restorative materials exert an injurious effect on the dental pulp are varied. Evidence exists that supports direct and, in some instances, prolonged cytotoxicity, stimulation of hypersensitivity reactions, or impairment of the host immune response to bacteria. Some of the components of resin restorations are released at cytotoxic levels after polymerization is completed, leading to chronic stimulation and a resultant prolonged inflammatory response.71 Furthermore, even subtoxic concentrations of certain agents are capable of eliciting allergic reactions in humans.98 Primates hyperimmunized with BSA showed significant pulpal damage with repeated antigenic challenge in class V cavity preparations, suggesting a role for antigen-antibody complex–mediated hypersensitivity in tissue destruction.24 In a separate study, exposure to dentin primers elicited a delayed-type hypersensitivity reaction in guinea pigs.115 These studies taken together present a compelling argument for immune-mediated pulpal tissue damage subsequent to exposure to restorative materials. Foreign body reactions have also been described in pulps containing extruded globules of resin material.102,103 Histologic examination of such pulps shows macrophages and giant cells surrounding the resin particles. Lastly, resin monomers have been shown to decrease the activity of immunocompetent cells in a dose-dependent manner in in vitro functional assays.113 Although all of these effects are documented, their extent and therefore morbidity on the dental pulp is speculative and doubtless does not act solely to effect pulpal demise. As previously noted, most restorative materials are placed adjacent to pulps that are previously compromised by bacterial insult; disease, débridement, and restoration of the tooth have cumulative effects on the dental pulp.
Although pulpal irritation is largely considered to be a negative sequela, the irritant potential of certain restorative materials is central to their usefulness in restorative dentistry. Calcium hydroxide is one of the oldest and most widely used medicaments for stimulation of dentinal bridge formation subsequent to microscopic or gross pulpal exposure. The low-grade pulpal irritation that it induces is important for dentinal bridge formation in exposures.55,207 The degree of inflammation is dependent on the preparation of calcium hydroxide used. Aqueous suspensions of calcium hydroxide applied to exposed pulps causes superficial necrosis of pulpal tissue followed by tissue displaying low-grade inflammation. Within 30 days, the tissue subjacent to the necrotic zone has reorganized and resumed normal architecture. Hard-setting calcium hydroxide preparations are effective in eliciting dentinal bridge formation with a much smaller to nonexistent necrotic zone.92 This is preferable in vital pulp therapies such as the Cvek pulpotomy, where maintenance of the maximum amount of vital pulp tissue is desirable, and the extent of pulpal inflammation is minimal54 (Fig. 13-20). The irritation potential of calcium hydroxide across intact dentin is dependent on factors such as the remaining dentin thickness and permeability. Application of calcium hydroxide to intact dentin appears to induce sclerosis by promoting crystal precipitation within the tubules, accompanied by reductions in permeability.153
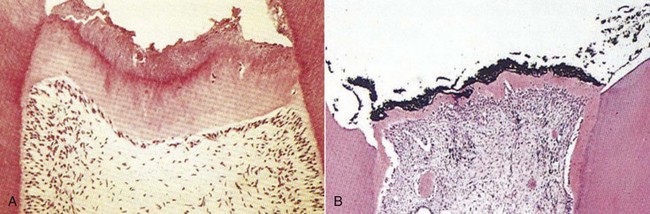
FIG. 13-20 Calcium hydroxide produces an inflammatory response that stimulates dentinal bridge formation. The dentin bridge forms lower in the tooth with calcium hydroxide paste (A) versus hard-setting calcium hydroxide (B).
In addition to the direct chemical effects of restorative materials, there are indirect factors that contribute to pulpal irritation. The technique sensitivity of certain materials predisposes them to faulty bonds to tooth structure that can translate to dentin hypersensitivity, recurrent disease, and pulpal inflammation or necrosis. Much attention has been given to the interface created between resin-bonded materials and dentin. During the etching process, the more highly mineralized peritubular dentin is preferentially dissolved, leaving free collagen fibrils and opening lateral tubular branches.82,152 Applied resin infiltrates the exposed collagen mesh, creating a layer 5 to 10 µm thick referred to as the hybrid layer.161 This layer, along with the resin permeating exposed tubules, forms the bond between the resin and dentin. If the preparation is too dry, the collagen fibrils collapse, and the resin cannot effectively permeate the mesh, which results in a defective bond. The optimal degree of hydration of the preparation surface can vary from material to material, so resin restoration placement is technique sensitive. This same principle is applicable to the practice of bonding fractured tooth fragments where the segment has become dehydrated while outside of the mouth. Current protocols recommend rehydration of the segment prior to bonding, thus increasing the mechanical and presumably the microbial seal.69 This is particularly important with a complicated crown fracture where the pulpal protection by intact dentin is absent.
Some restorative materials rely on their medicinal properties as well as their ability to seal a cavity preparation. Materials containing zinc oxide and eugenol (ZOE) fall into this category. ZOE is used for a variety of purposes in dentistry, largely because of its anesthetic and antiseptic properties. It has been shown to block transmission of action potentials in nerve fibers and to suppress nerve excitability in the pulp when applied to deep excavations.238 In addition, ZOE has good adaptation to dentin and inhibits bacterial growth on cavity walls. These properties have made this a favored material for temporary fillings but not long-term restorations; ZOE temporaries have been shown to leak after only a few weeks in situ.263
Direct Pulp Capping with Mineral Trioxide Aggregate
Direct capping of pulp exposures is indicated in pulps that were previously healthy and exposed by trauma or dental restorative procedures.229 Although calcium hydroxide has historically been the preferred dressing agent on mechanically exposed pulps, the use of mineral trioxide aggregate (MTA) has recently been proposed, even on carious pulp exposures.16,26 Prospective animal studies and human case reports have evaluated the ability of MTA to allow for the formation of a reparative dentin bridge and maintain continued pulp vitality.66,73,121 Although the results are generally favorable, one concern is of tooth discoloration if the gray MTA formulation is used on anterior teeth.
In a recent investigation, white MTA was compared to gray MTA in the capping of direct mechanical pulp exposures created in dogs’ teeth.182 No significant differences were found in the healing response to either material. At 1 week, none of the capped pulps showed necrosis close to the exposure site, and odontoblast-like cells were observed at the periphery and under the calcified bridge. At 2 weeks, almost all of the specimens from both types of MTA showed complete calcified bridge formation just below the exposure site. In a recent clinical study, MTA was used as a pulp capping material for carious pulp exposures.26 Forty patients aged 7 to 45 were diagnosed with reversible pulpitis and had caries removed using a caries-detection dye and sodium hypochlorite for hemostasis. The treatment was performed in two visits to allow the MTA to set up and to confirm pulp sensibility to pulp tests in the second visit. Success was determined radiographically, with subjective symptoms, pulp testing with cold, and continued root formation on immature teeth. Outcomes were measured over a period of up to 9 years postoperatively and showed an overall success of 97%, with all the teeth in the immature root group showing success. Within the parameters of these studies, it seems that white MTA is a suitable capping agent for pulp exposures of healthy or reversibly inflamed pulps. It must be emphasized, however, that not only must the pulp capping agent be biocompatible and hopefully stimulate the formation of reparative dentin, but the prevention of bacterial ingress by the placement of a well-sealed restoration must also be provided.
Use of Hemostatic Agents and Disinfectants on Direct Pulp Exposures
As noted before, the use of direct capping procedures in an attempt to maintain the vitality of an exposed dental pulp has been a controversial area of pulp biology. In particular, the impact of preoperative diagnosis and the effects of various dental medicaments and materials on the long-term prognosis of exposed pulps have been questioned.53,95,179,215,221 With regard to the former, it has generally been accepted that carious pulp exposures offer poor therapeutic opportunities for continued pulp vitality with direct capping techniques.17,95 In the case of the latter, there is still debate as to whether the toxicity of medicaments and materials placed on vital pulp tissues determines outcome, or if their ability to seal the cavity from further bacterial ingress is of prime importance. It is likely a combination of the two, as was shown in the recent clinical trial with MTA mentioned before.26 Another factor in the prognosis of direct pulp caps is the ability to control hemorrhage at the exposure site.221 Given the difficulty in creating a bacteria-free operating environment during tooth preparation, the ideal hemostatic agent also would have the ability to kill bacteria.
A recent study compared the effects of two hemostatic/disinfectant agents on the healing of experimental pulp exposures created in human third molar teeth and capped with calcium hydroxide.211 Pulp exposures were made in 45 maxillary wisdom teeth scheduled for extraction for orthodontic reasons. Teeth were randomly assigned to receive hard-setting calcium hydroxide pulp caps after a 30-second surface treatment with one of three agents: 0.9% saline, 2% chlorhexidine, or 5.25% sodium hypochlorite. The teeth were restored with class I bonded resin restorations, extracted after 7, 30, or 90 days, and processed for routine histologic evaluation. Although the 7-day saline specimens showed slightly less inflammatory response, there were no statistically significant differences between the groups with respect to all dependent measures over the course of the study. Complete healing was seen in 88% of all specimens at 90 days.
The pulps in these teeth were previously uninjured, and the exposures were made in a clean environment. Therefore, it seems that a mechanical exposure of a healthy pulp created during cavity preparation could be disinfected with either 2% chlorhexidine or 5.25% sodium hypochlorite (full concentration is now 6.0%), capped with a hard-setting calcium hydroxide formulation, and expected to have a favorable prognosis for healing.
Pulpal Reactions to Laser Procedures
Numerous studies have been published on the effect of using lasers on enamel, dentin, and pulp (see also Chapter 8). Laser use on hard tissue has been a popular area of research because of the potential benefits of efficiency, reduced sensitivity, disinfection, and precision. There are several different types of laser technologies available that depend on the wavelength, active medium, emission mode, delivery system, power output, and duration of application. The main types available in dentistry today are shown in Fig. 13-21. The CO2 laser is historically the oldest type used on soft tissues and thus has been the most studied. It has the longest wavelength (10,600 nm). It cannot be delivered in an optic fiber, thus must be used in a hollow-tubelike wave guide in continuous gated-pulse mode. This means that the operator does not feel a solid resistance when using this laser. Er:YAG, Nd:YAG, or Ho:YAG lasers all have an active medium of a solid crystal of yttrium-aluminum-garnet, which is impregnated in erbium, neodymium, or holmium, respectively. Er:YAG has a wavelength of 2940 nm, delivered using a solid optic fiber. It has a high affinity for water and hydroxyapatite, thus can be used for removal of caries and cutting dentin with coolant. It can also be used on soft tissue. Ho:YAG laser has a wavelength of 2120 nm and has high affinity to water but not to tooth structure, thus is used primarily for soft-tissue surgery. Nd:YAG laser is also delivered fibroptically, has a wavelength of 1064 nm, and has been used extensively in dentistry because it has high affinity for water and pigmented tissues and offers good hemostasis, thus is used extensively in surgery.48 In addition, there are lasers with low power output, such as HeNe, or helium neon (632 nm), and GaAlAs, or gallium-aluminum-arsenide (diode; semiconductor) (720-904 nm) lasers, which have been used in laser Doppler flowmetry and in treating dentin hypersensitivity.119
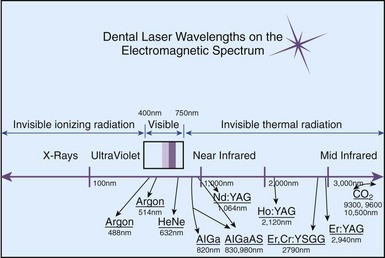
FIG. 13-21 Currently available dental wavelengths on the electromagnetic spectrum. Note all of the wavelengths are nonionizing.
(From Coluzzi DJ: An overview of laser wavelengths used in dentistry. Dent Clin North Am 44:753, 2000.)
Lasers in the Prevention, Diagnosis, and Treatment of Caries
Laser irradiation of deep susceptible pits and fissures may reduce the incidence of dental caries. Once caries develops, some lasers may be effective in removing the carious lesion and sparing undemineralized dentin because of their differential absorption by water and hydroxyapatite. Furthermore, if caries exposes the pulp in young teeth, particularly those with immature apex, lasers may be able to effectively excise coronal infected pulp in pulpotomy because of their hemostatic and antibacterial properties. All these potential uses prompted a large number of investigations on the effectiveness of lasers in these applications.
Some clinicians have proposed using lasers to enhance adhesion of pit and fissure sealants, but this was shown to not add any advantage following acid etching—a necessity for adhesion.143 Laser fluorescence is used by the DIAGNOdent and DIAGNOdent pen, which are laser devices that have been introduced for the diagnosis of noncavitated caries. Although these devices initially showed some promising results,144 more recent work suggests that they are best used as adjunctive devices to radiography and visual examination.6,49,126
Laser ablation of superficial carious lesions may be more conservative than bur preparation. A controlled clinical trial supported this tenet when free-running pulsed Nd:YAG laser was used to ablate superficial pit and fissure caries in third molars scheduled for extraction88 (Fig. 13-22). In this study, there were no histologic differences in the pulp response between the two groups.
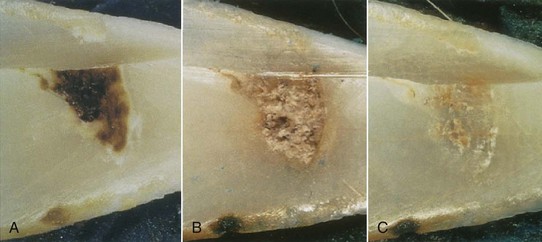
FIG. 13-22 A, Dental caries. B, Pulsed Nd:YAG ablation byproducts (160 mJ, 10 Hz). C, Debris removed with acid etch and polishing. Enamel surface was faceted for reflection spectroscopy.
(From Harris DM, White JM, Goodis H, et al: Selective ablation of surface enamel caries with a pulsed Nd:YAG dental laser. Lasers Surg Med 30:342, 2002.)
From the perspective of effects on the pulp, most laser applications employed in cutting or modifying cavities in dentin or acting directly on the pulp tissue are important. Earlier studies showed reduced permeability of dentin in vitro with a XeCl excimer laser (a laser with a relatively short wavelength of 308 nm in the ultraviolet range).220 The apparent fusion of tubules in superficial layers of dentin was shown to occur with CO2, Nd:YAG, and Er:YAG lasers in vitro.255 The pulpal responses to Nd:YAG and CO2 lasers were not favorable. It was shown that Nd:YAG and, to a lesser degree, CO2 lasers may be associated with charring and significant inflammation in the pulp compared with Er:YAG laser235,255 (Fig. 13-23). More recently it was reported that water cooling was as necessary for laser ablation as it is for high-speed bur preparations.41 Studies have shown that Er:YAG laser appears to induce similar responses in the pulp to those seen with high-speed bur preparations at the level of light microscopy analysis.65,231,232,255 However, the findings with electron microscopy were different. It was reported that whereas shallow cavities ablated in rat molars using Er:YAG lasers did not show changes from baseline using light microscopy, transmission electron microscopy (TEM) showed disruption and degeneration of pulpal peripheral nerve endings and of myelin sheath in the immediate postoperative period (Fig. 13-24).101 This may explain the reduced sensitivity that accompanies laser cavity preparations. Thus in summary, it does not appear that the use of lasers provides predictable advantages in cavity preparation compared with traditional methods at this time.
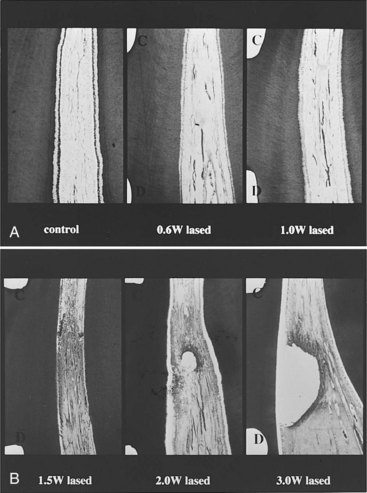
FIG. 13-23 A-B, Histopathologic picture of Nd:YAG laser specimen with an increasing power. There is a direct relationship between the degree of pathologic changes and the increasing power of the laser. In fact, 1.5 W and the greater power cause permanent damage to the pulp.
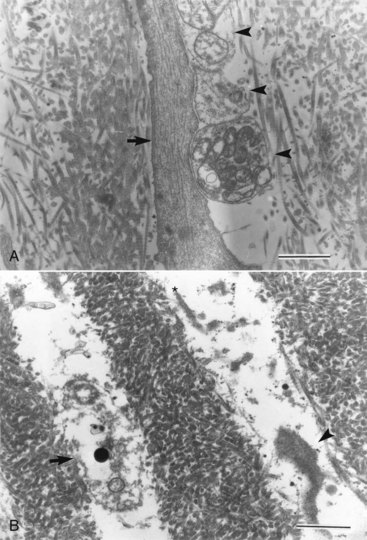
FIG. 13-24 A, Control; normal odontoblastic process (arrow) and a few nerve terminals (arrowheads) are seen in a dental tubule of rat upper first molar. B, Six hours after Er:YAG laser irradiation; disrupted cell membrane of a nerve terminal that contains some granular vesicles (arrow) and shrinkage of an odontoblastic process (arrowhead) are noted in dentinal tubules just under the ablated area. Asterisk indicates the irradiated side (transmission electron micrograph, ×13,700; bar = 1 µm).
(From Inoue H, Izumi T, Ishikawa H, Watanabe K: Short-term histomorphological effects of Er:YAG laser irradiation to rat coronal dentin-pulp complex. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:246, 2004.)
For pulpotomy procedures, such as in primary teeth, permanent teeth with immature apex, or pulps that are exposed due to fracture and treated promptly, the lasers (particularly CO2 lasers) may be useful in achieving precise surgical excision of coronal pulp and immediate hemostasis. A controlled clinical study showed that CO2 laser pulpotomy was comparable to traditional methods in experimental pulpotomies of primary teeth scheduled for extraction for orthodontic reasons.63 However, an animal study in which CO2 and Nd:YAG lasers were compared revealed poor response with both lasers compared with calcium hydroxide.114 A more recent animal study using Er:YAG laser showed that the results are dependent on the power settings, in that lower energy delivered with this laser produced favorable results.120
Lasers in the Treatment of Dentin Hypersensitivity
Earlier studies have shown effectiveness ranging from 5% to 100% of low-output lasers on dentin hypersensitivity.119 One author reported a reduction of hypersensitivity in 73% of mild cases, 19% of moderate cases, and 14% of severe cases after 4 months, using a GaAlAs laser.119 Low-output lasers do not have any effects on the morphology of enamel or dentin, but they are thought to cause transient reduction in action potential mediated by pulpal C-fibers, but not Aδ-fibers,253 although this finding was not consistent.174 Nd:YAG lasers have also been used in dentin hypersensitivity. Because of the higher power output, these lasers cause superficial occlusion of dentinal tubules of up to 4 µm,142 in addition to action potential blockage within the pulp in vitro or in experimental animals.173,174 However, a placebo-controlled clinical trial has shown that both Nd:YAG and placebo caused significant reduction in dentin hypersensitivity for up to 4 months postoperatively but were not different from each other.137 Therefore, the collective available evidence does not support the use of lasers for treating dentin hypersensitivity. Other more conservative and economical therapies are recommended.
Use of Lasers as a Protective Measure for Dentin
A recent clinical study has proposed that if lasers are used to prepare cavities or used on prepared dentin after traditional cavity preparation with burs, this would protect the dentin because its permeability and bacterial content may decrease. In this study, two patients were scheduled to have six teeth extracted during orthodontic treatment.79 In the teeth that had laser irradiation (GaA1As laser, lambda = 660 nm, power of 30 mW and energy dose of 2 J/cm2) and were examined with TEM after 28 days, the odontoblastic process had increased contact with the extracellular matrix, and the collagen fibrils appeared more organized than those of the control group (traditional bur preparation only). The study concluded that laser irradiation accelerates the recovery of the dental tissues in the predentin region.
Pulpal Reactions to Cavity Preparation Using Air Abrasion Techniques
Air abrasion is another modality that has been adopted for conservative, nonpainful cavity preparations. Aluminum oxide particles are forced in a rapid stream onto the tooth structure and simultaneously evacuated from the field. An earlier study evaluated the effects of air abrasion in class V preparations using two different particle sizes (25 and 50 µm) and two different forces (80 and 160 psi) on the dental pulp of dogs. The findings showed that histologically, higher pressures and smaller particles yielded significantly fewer pulpal effects than the high-speed treated teeth, whereas lower pressures and larger particles were not significantly different.132 Air abrasion cavity preparations were more recently shown to be equivalent to high-speed bur preparations in microleakage studies if both techniques are followed by acid etching.250 Because of the lack of tactile control, the use of air abrasion is limited to specific situations such as shallow caries or pediatric applications.
Pulpal Reactions to Vital Bleaching Techniques
Vital bleaching techniques employ the use of strong oxidizing agents, namely 10% carbamide peroxide and hydrogen peroxide, to bleach enamel of teeth with vital pulp. There have been concerns about the potential for pulpal irritation during these procedures because of the long duration that the chemicals are in contact with the teeth, particularly if dentin with open tubules or cracks are present. An earlier study identified the toxicity of these chemicals on cell cultures in vitro.84 Histologic or histochemical analysis of the pulp following bleaching for up to 2 weeks showed minor inflammatory changes in the pulp of the bleached teeth that were reversible.8,76 One clinical report documented that if 16% carbamide peroxide was used, gingival irritation was evident; however, no changes in pulp vitality or in symptoms were noted. Even in patients who develop posttreatment symptoms, these tend to be reversible and could be prevented by treating the teeth with fluorides and by correcting restorative pretreatment deficiencies.163 Clinical symptoms are likely to be due to increases in neuropeptides in the pulp. A recent report showed that light- and laser-activated bleaching systems increase substance P expression in the pulp to significant levels.42 An earlier clinical trial showed that vital bleaching using 10% carbamide peroxide in a custom tray for 6 weeks was safe for the pulp health for up to 10 years post treatment, although the bleaching effectiveness may decline with time.203
Pulpal Reactions to Periodontal Procedures
In an intact nontraumatized tooth, the dental pulp maintains its healthy existence throughout life because it is insulated from microbial irritation in the oral cavity (see also Chapter 15). Periodontal disease causes attachment loss, which exposes the root surface to the oral cavity. Occasionally, pulpal inflammation secondary to severe periodontitis is observed.131 Some reports have described bacterial infiltration through dentinal tubules of exposed root surface, causing mild inflammatory changes in the pulp (Fig. 13-25).129 However, in a case report of 25 teeth that were extracted in a patient with extensive periodontal disease, none of the pulp tissue had significant inflammatory changes.236 It is much more common for pulp necrosis or failed healing of periradicular lesions to present clinically with signs of periodontal disease than for periodontal disease to cause pulpal pathosis. Primary endodontic, secondary periodontal pathosis is particularly evident if a perforation occurs in the pulp chamber or coronal third of the root during endodontic treatment and is not promptly treated, as happens in cases of vertical root fractures or congenital tooth defects such as palatal groove defect. Thus it is more likely for microbial irritants to move outward from a necrotic pulp to cause periodontal breakdown than for them to move inward from a periodontal pocket to cause irreversible pathosis in the pulp. The reasons for this observation are not fully understood. However, if the assumption is that bacteria may migrate through patent dentinal tubules in these situations, as is shown in Fig. 13-25, then it may be that the outward dentinal fluid flow in teeth with vital pulp contributes to the resistance to ingress of bacteria in sufficient amounts to cause a clinically significant disease process. Once the pulp degenerates, dentinal fluid flow no longer exists. Thus microbial irritants from the pulp may promote pocket formation and periodontal bone loss,110 and the prognosis of endodontic and periodontal treatment may be related,111 since the microbial factors may pass across dentin more readily.
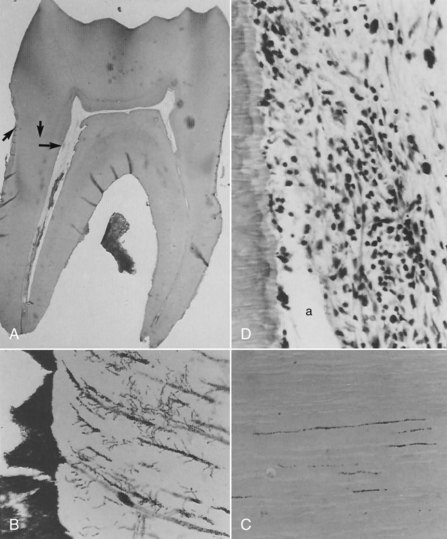
FIG. 13-25 Mandibular first molar of 49-year-old female, no pain. Clinically caries free. Calculus, periodontal disease, and bone loss from two thirds to three fourths of root. A, Note narrowness of pulp chamber seen throughout all serial sections (×8.5). B, Bacterial plaque and adjacent dentinal tubules with bacteria (oblique arrows in A) (×400). C, Farthest penetration of bacteria in dentinal tubules (vertical arrow in A) (×400). D, Where dentinal tubules invaded by bacteria terminate in the pulp (horizontal arrow in A), a small but dense accumulation of lymphocytes and macrophages can be seen. a, Artifact, pulp tissue torn away from dentin during processing (×400).
(From Langeland K: Tissue response to dental caries. Endod Dent Traumatol 3:149–171, 1987.)
Periodontal scaling and root planing may result in removal of cementum and exposure of dentin to the oral cavity. Frequently this treatment results in dentin hypersensitivity, as discussed previously. In theory, periodontal disease and its treatment should be associated with increased incidence of pulpal pathosis. In an older study, investigators induced periodontal disease in a primate model (using ligatures) and compared the effects on the pulp of periodontal disease with or without scaling.22 In teeth with periodontal disease, mild chronic inflammatory changes were observed in 29% of the teeth and could be seen in areas of the pulp related to bone loss. One of 40 teeth developed pulp necrosis. In teeth that received scaling, a similar percentage of 32% developed the same mild inflammation, but none developed pulp necrosis. In a later clinical study,23 52 periodontitis patients who had 672 teeth with vital pulp were followed up and maintained every 3 to 6 months for 4 to 13 years (mean 8.7 years). Of those teeth, 255 were bridge abutments. The results showed a significantly higher chance of pulpal complications in teeth that were bridge abutments than teeth that were not abutments (15% versus 3%; P < 0.01). Considering that both types of teeth had similar degrees of periodontal disease, the authors concluded that prosthodontic treatment is associated with pulpal involvement more frequently than periodontal disease and its treatment. Another histologic analysis of 46 teeth with varying degrees of periodontal disease and coronal restorations reached a similar conclusion.56 Furthermore, two comprehensive reviews of the topic concluded that although the potential exists for periodontal disease and its treatment to cause pulpal pathosis, particularly if large lateral or accessory canals are exposed, this occurrence is rare.87,205
Mechanical Irritants: Orthodontic Movement
The most conspicuous pulpal change observed in response to orthodontic forces is hemodynamic. Both human and animal studies have confirmed that both lateral and intrusive forces result in an increase in pulpal blood flow.127,165,172 Furthermore, blood flow alterations are not confined to the tooth in active movement. Observed increases in blood flow are seen in teeth adjacent to the focus of movement forces, implying that directed forces on one tooth could shunt blood to proximal vessels supplying other oral structures including teeth. If orthodontic forces are extreme, circulatory interruptions can occur and result in pulpal necrosis.34
Biochemical, biologic, and histologic studies of the effects of orthodontic movement have confirmed that metabolic as well as inflammatory changes can result. The dental pulp tissue respiration rate is depressed after short-term application of orthodontic force.83 Biochemical and molecular markers confirm that apoptosis and necrosis of pulpal cells is also increased subsequent to movement.189 However, it was shown that the inflammatory mediators IL-1α and TNF-α show minor increase in the pulp during orthodontic movement, compared to the increase in periodontal tissues.25 Histologic examination of pulps in teeth subjected to intrusive forces showed vascular congestion and dilatation as well as vacuolization of the odontoblastic layer.224-226 Most if not all of these effects are due to circulatory changes, and the consensus is that they are transient, provided the movement forces are not excessive.124 It was recently shown, however, that pulp necrosis of teeth that had suffered trauma prior to undergoing orthodontic treatment was significantly increased, compared to teeth that had either of these conditions alone—particularly in lateral incisors and when the traumatized tooth had pulp canal obliteration.18,19
Pulpal Reactions to Orthodontic Surgery
It has been known for decades that osteotomies in the maxilla or mandible may cause disruption in the blood supply to teeth in the area of the surgery, with resultant inflammation and/or necrosis.15,122,162,187 Occasionally the teeth affected show postoperative manifestations common with traumatic injuries, such as pulp canal obliteration.247 Animal studies have shown that if a safe distance of 5 to 10 mm is maintained between the site of the surgery and the teeth, minimal disruption occurs.58,260 Using laser Doppler flowmetry, a number of studies have documented actual reduction in PBF immediately following maxillary Le Fort I osteotomy,175,198,206 particularly if segmental osteotomy is performed.64 In most cases, the blood flow is regained within months of the surgery. Recently a modification of the Le Fort I osteotomy technique was described in which the Le Fort I sectioning was combined with a horseshoe palatal osteotomy to spare any disruption to the descending palatine artery.85 An examination of PBF of maxillary teeth using laser Doppler flowmetry, as well as the responsiveness to electric pulp testing, showed significant differences between the two surgical techniques in the postoperative recovery values (Fig. 13-26). In cases where the surgery did not disrupt the palatine artery, the PBF in the anterior teeth consistently increased without disruption in the postoperative period.
FIG. 13-26 A, Postoperative change of the mean pulpal blood flow in the upper incisors of the two groups. Pre, Before the operation; d, day(s) after the operation; M, months after the operation; bars, SD; *, P < 0.05; **, P < 0.01. B, The postoperative change in the percentages of teeth (upper incisors) with positive pulpal sensibility in the two groups. Pre, Before the operation; d, day(s) after the operation; M, months after the operation; *, P < 0.05.
(From Harada K, Sato M, Omura K: Blood-flow and neurosensory changes in the maxillary dental pulp after differing Le Fort I osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:12–17, 2004.)
It should also be noted that occasionally teeth are traumatized during endotracheal intubation for surgery that requires general anesthesia, when the surgery itself is not related to the jaws or teeth.212
Biomechanical Irritation: Parafunctional Habits
Occlusal loading of teeth affects deformation to varying degrees.86 Enamel is largely resistant to flexure, but the underlying dentin demonstrates considerable elastic and viscoelastic characteristics. As a result, defects in enamel secondary to cracks, decay, and/or restorative preparation allow cuspal flexure, with subsequent pulpal responses presumably due to dentinal fluid flow secondary to compression and microleakage.93 The magnitude of the pulpal response is dictated by the degree and chronicity of dentinal deformation.
Multiple factors influence the degree of tooth deformation during occlusal loading. Investigators have noted that preparation geometry has a direct impact on cuspal flexure. The width of the occlusal isthmus relative to the faciolingual dimension of the tooth, as well as the ablation of marginal ridges, directly impact on the degree of cuspal flexure.246,199,181 MOD preparations have been shown to effect a 50% reduction in cuspal stiffness and resistance to fracture. Physical properties of the restorative material can also play a part in cuspal flexure. Studies have shown that polymerization shrinkage of certain resin composites can induce an inward deflection of cusps, with resultant stresses on tooth structure.248,249
Symptomology from cuspal flexure can result from two primary sources. It has been theorized that cuspal flexure results in dentin deformation, thus promoting dentinal fluid flow that activates nerve endings in the odontoblastic layer of the tooth. This is supported in part by an in vitro study that found that dentinal fluid flow could be induced by occlusal loading of restored teeth.93 A second source of pulpal pain is bacterial microleakage created by a gap at the restoration/dentin interface that is repeatedly opened during cycles of occlusal loading. If repeated cuspal flexure gives rise to a crack, dentinal exposure to bacteria and their byproducts is even greater. It is likely that in vivo, both dentinal fluid flow and bacterial access to dentinal surfaces work together to produce inflammation that is often manifested by thermal and biting sensitivity for the patient. In the instance of parafunctional habits, this is often combined with concussive periodontal forces to the periodontium that induce acute periradicular periodontitis, mobility, and radiographic changes.
Dentinal cracks expose tubules unoccluded by a smear layer and therefore offer a direct portal to the subjacent pulp. When dentinal tubules are freely exposed, there is an outward flow of dentinal fluid driven by relatively high pulpal tissue pressures. Dentinal fluid is composed of proteins such as fibrinogen and serum albumin, which can coagulate and effectively block the tubule lumen, thereby limiting fluid egress and resultant dentin hypersensitivity. This phenomenon can occur within 2 days. As this serves as a short-term protective mechanism for the pulp, dentinal sclerosis and tertiary dentin formation ultimately can provide greater protection for the pulp and ablation of symptoms. Clinical interventions include the application of materials that occlude the tubules and extracoronal restorations to prevent the propagation of cracks.
Pulpal Reactions to Implant Placement and Function
Osseointegrated implants are now a common option for the replacement of missing teeth. The placement of implants requires multifaceted preoperative radiographic techniques, including intraoral, tomographic, cephalometric, and panoramic imaging.241 This assures that implant placement fully rests in bone and does not compromise neighboring structures, including teeth. The lack of attention to (1) the three-dimensional anatomy of the site of implant placement and (2) the orientation of neighboring teeth may lead to the implant perforating the root and devitalizing the pulp.145,228
It is usually recommended that implants not be placed directly at a site where a periradicular lesion, particularly one with signs of purulence, exists; microbial irritants may interfere with osseointegration.194 However, recent data suggest that immediate implant placement in sites that have been adequately débrided is successful.38
Case reports have also claimed that teeth with periradicular lesions may reduce the success of neighboring implants, even if adequate endodontic treatment is performed.227 To address this issue, a study was reported in which implants were placed to replace premolars in dogs, and periradicular lesions were then induced. Some were treated nonsurgically or with combined surgical and nonsurgical treatment.210 The results showed that the presence of treated or untreated periradicular lesions did not affect the long-term osseointegration of implants that were already osseointegrated. Following adequate endodontic treatment, complete resolution of periapical lesions that also involve neighboring implants has been reported.139,223 Furthermore, implants that are not mobile but have apical periimplant lesions have been successfully treated with local débridement and “implant-apex resection” procedures.13
Regenerative Potential of the Dental Pulp
Recent studies and case reports suggest that the dental pulp has great regenerative potential, particularly in the immature permanent tooth (see also Chapter 16).14,105 This has been attributed to the store of stem cells resident in the dental papillae of developing teeth.218 Mesenchymal stem cells (MSCs) have been found in a variety of tissues and have proven to be pluripotential. Stem cells from the apical papilla (SCAP) of immature teeth have likewise been identified and have been shown to develop into dentinogenic cells, given the proper stimulation.96,218 The results of these studies, coupled with case reports of continued root development subsequent to apical periodontitis in young teeth, suggest the potential for bioroot engineering in the future.96
1. About I, Murray PE, Franquin JC, Remusat M, Smith AJ. Pulpal inflammatory responses following non-carious class V restorations. Oper Dent. 2001;26:336.
2. Absi EG, Addy M, Adams D. Dentine hypersensitivity–the effect of toothbrushing and dietary compounds on dentine in vitro: an SEM study. J Oral Rehabil. 1992;19:101.
3. Addy M, Pearce N. Aetiological, predisposing and environmental factors in dentine hypersensitivity. Arch Oral Biol. 1994;39(Suppl):33S.
4. Ahlquist M, Franzen O, Coffey J, Pashley D. Dental pain evoked by hydrostatic pressures applied to exposed dentin in man: a test of the hydrodynamic theory of dentin sensitivity. J Endod. 1994;20:130.
5. Ahn J, Pogrel MA. The effects of 2% lidocaine with 1:100,000 epinephrine on pulpal and gingival blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:197.
6. Aljehani A, Yang L, Shi XQ. In vitro quantification of smooth surface caries with DIAGNOdent and the DIAGNOdent pen. Acta Odontol Scand. 2007;65:60.
7. Amano T, Muramatsu T, Amemiya K, Kubo K, Shimono M. Responses of rat pulp cells to heat stress in vitro. J Dent Res. 2006;85:432.
8. Anderson DG, Chiego DJJr, Glickman GN, McCauley LK. A clinical assessment of the effects of 10% carbamide peroxide gel on human pulp tissue. J Endod. 1999;25:247.
9. Armstrong SR, Vargas MA, Fang Q, Laffoon JE. Microtensile bond strength of a total-etch 3-step, total-etch 2-step, self-etch 2-step, and a self-etch 1-step dentin bonding system through 15-month water storage. J Adhes Dent. 2003;5:47.
10. Avellan NL, Sorsa T, Tervahartiala T, Mantyla P, Forster C, Kemppainen P. Painful tooth stimulation elevates matrix metalloproteinase-8 levels locally in human gingival crevicular fluid. J Dent Res. 2005;84:335.
11. Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;35:30.
12. Baldissara P, Catapano S, Scotti R. Clinical and histological evaluation of thermal injury thresholds in human teeth: a preliminary study. J Oral Rehabil. 1997;24:791.
13. Balshi SF, Wolfinger GJ, Balshi TJ. A retrospective evaluation of a treatment protocol for dental implant periapical lesions: long-term results of 39 implant apicoectomies. Int J Oral Maxillofac Implants. 2007;22:267.
14. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196.
15. Banks P. Pulp changes after anterior mandibular subapical osteotomy in a primate model. J Oral Maxillofac Surg. 1977;5:39.
16. Barrieshi-Nusair KM, Qudeimat MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006;32:731.
17. Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26:525.
18. Bauss O, Rohling J, Rahman A, Kiliaridis S. The effect of pulp obliteration on pulpal vitality of orthodontically intruded traumatized teeth. J Endod. 2008;34:417.
19. Bauss O, Rohling J, Sadat-Khonsari R, Kiliaridis S. Influence of orthodontic intrusion on pulpal vitality of previously traumatized maxillary permanent incisors. Am J Orthod Dentofacial Orthop. 2008;134:12.
20. Bergenholtz G, Ahlstedt S, Lindhe J. Experimental pulpitis in immunized monkeys. Scand J Dent Res. 1977;85:396.
21. Bergenholtz G, Cox CF, Loesche WJ, Syed SA. Bacterial leakage around dental restorations: its effect on the dental pulp. J Oral Pathol. 1982;11:439.
22. Bergenholtz G, Lindhe J. Effect of experimentally induced marginal periodontitis and periodontal scaling on the dental pulp. J Clin Periodontol. 1978;5:59.
23. Bergenholtz G, Nyman S. Endodontic complications following periodontal and prosthetic treatment of patients with advanced periodontal disease. J Periodontol. 1984;55:63.
24. Bergenholtz G, Warfvinge J. Migration of leukocytes in dental pulp in response to plaque bacteria. Scand J Dent Res. 1982;90:354.
25. Bletsa A, Berggreen E, Brudvik P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur J Oral Sci. 2006;114:423.
26. Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139:305.
27. Botero TM, Shelburne CE, Holland GR, Hanks CT, Nor JE. TLR4 mediates LPS-induced VEGF expression in odontoblasts. J Endod. 2006;32:951.
28. Bouillaguet S. Biological Risks of resin-based materials to the dentin-pulp complex. Crit Rev Oral Biol Med. 2004;15:47.
29. Bowles WR, Withrow JC, Lepinski AM, Hargreaves KM. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. J Endod. 2003;29:265.
30. Brannstrom M. Dental and Pulpal response. III. Application of an air stream to exposed dentin. Long observation periods. Acta Odontol Scand. 1960;18:234.
31. Brannstrom M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984;3:35.
32. Brannstrom M. The effect of dentin desiccation and aspirated odontoblasts on the pulp. J Prosthet Dent. 1968;20:165.
33. Brannstrom M, Lind PO. Pulpal response to early dental caries. J Dent Res. 1965;44:1045.
34. Butcher EO, Taylor AC. The effects of denervation and ischemia upon the teeth of the monkey. J Dent Res. 1951;30:265.
35. Byers MR, Narhi MV. Dental injury models: experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4.
36. Byers MR, Narhi MV, Mecifi KB. Acute and chronic reactions of dental sensory nerve fibers to cavities and desiccation in rat molars. Anat Rec. 1988;221:872.
37. Calland JW, Harris SE, Carnes DLJr. Human pulp cells respond to calcitonin gene-related peptide in vitro. J Endod. 1997;23:485.
38. Casap N, Zeltser C, Wexler A, Tarazi E, Zeltser R. Immediate placement of dental implants into debrided infected dentoalveolar sockets. J Oral Maxillofac Surg. 2007;65:384.
39. Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42:219.
40. Caux C, Vanbervliet B, Massacrier C, et al. Regulation of dendritic cell recruitment by chemokines. Transplantation. 2002;73:S7.
41. Cavalcanti BN, Lage-Marques JL, Rode SM. Pulpal temperature increases with Er:YAG laser and high-speed handpieces. J Prosthet Dent. 2003;90:447.
42. Caviedes-Bucheli J, Ariza-Garcia G, Restrepo-Mendez S, Rios-Osorio N, Lombana N, Munoz HR. The effect of tooth bleaching on substance P expression in human dental pulp. J Endod. 2008;34:1462.
43. Caviedes-Bucheli J, Munoz HR, Azuero-Holguin MM, Ulate E. Neuropeptides in dental pulp: the silent protagonists. J Endod. 2008;34:773.
44. Chabanski MB, Gillam DG. Aetiology, prevalence and clinical features of cervical dentine sensitivity. J Oral Rehabil. 1997;24:15.
45. Cheung GS, Lai SC, Ng RP. Fate of vital pulps beneath a metal-ceramic crown or a bridge retainer. Int Endod J. 2005;38:521.
46. Chng HS, Pitt Ford TR, McDonald F. Effects of prilocaine local anaesthetic solutions on pulpal blood flow in maxillary canines. Endod Dent Traumatol. 1996;12:89.
47. Cohen JS, Reader A, Fertel R, Beck M, Meyers WJ. A radioimmunoassay determination of the concentrations of prostaglandins E2 and F2alpha in painful and asymptomatic human dental pulps. J Endod. 1985;11:330.
48. Coluzzi DJ. An overview of laser wavelengths used in dentistry. Dent Clin North Am. 2000;44:753.
49. Costa AM, Bezzerra AC, Fuks AB. Assessment of the accuracy of visual examination, bite-wing radiographs and DIAGNOdent on the diagnosis of occlusal caries. Eur Arch Paediatr Dent. 2007;8:118.
50. Costa CA, Edwards CA, Hanks CT. Cytotoxic effects of cleansing solutions recommended for chemical lavage of pulp exposures. Am J Dent. 2001;14:25.
51. Cotton WR. Pulp response to an airstream directed into human cavity preparations. Oral Surg Oral Med Oral Pathol. 1967;24:78.
52. Cox CF, Bergenholtz G, Fitzgerald M, et al. Capping of the dental pulp mechanically exposed to the oral microflora–a 5 week observation of wound healing in the monkey. J Oral Pathol. 1982;11:327.
53. Cox CF, Hafez AA, Akimoto N, Otsuki M, Suzuki S, Tarim B. Biocompatibility of primer, adhesive and resin composite systems on non-exposed and exposed pulps of non-human primate teeth. Am J Dent. 1998;11:S55.
54. Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod. 1978;4:232.
55. Cvek M, Granath L, Cleaton-Jones P, Austin J. Hard tissue barrier formation in pulpotomized monkey teeth capped with cyanoacrylate or calcium hydroxide for 10 and 60 minutes. J Dent Res. 1987;66:1166.
56. Czarnecki RT, Schilder H. A histological evaluation of the human pulp in teeth with varying degrees of periodontal disease. J Endod. 1979;5:242.
57. Dummer PM, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. 1980;13:27.
58. Duran S, Guven O, Gunhan O. Pulpal and apical changes secondary to segmental osteotomy in the mandible–an experimental study. J Craniomaxillofac Surg. 1995;23:256.
59. Durand SH, Flacher V, Romeas A, et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176:2880.
60. El Karim IA, Lamey PJ, Ardill J, Linden GJ, Lundy FT. Vasoactive intestinal polypeptide (VIP) and VPAC1 receptor in adult human dental pulp in relation to caries. Arch Oral Biol. 2006;51:849.
61. El Karim IA, Lamey PJ, Linden GJ, Awawdeh LA, Lundy FT. Caries-induced changes in the expression of pulpal neuropeptide Y. Eur J Oral Sci. 2006;114:133.
62. El Karim IA, Lamey PJ, Linden GJ, Lundy FT. Neuropeptide Y Y1 receptor in human dental pulp cells of noncarious and carious teeth. Int Endod J. 2008;41:850.
63. Elliott RD, Roberts MW, Burkes J, Phillips C. Evaluation of the carbon dioxide laser on vital human primary pulp tissue. Pediatr Dent. 1999;21:327.
64. Emshoff R, Kranewitter R, Brunold S, Laimer K, Norer B. Characteristics of pulpal blood flow levels associated with non-segmented and segmented Le Fort I osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:379.
65. Eversole LR, Rizoiu I, Kimmel AI. Pulpal response to cavity preparation by an erbium, chromium:YSGG laser-powered hydrokinetic system. J Am Dent Assoc. 1997;128:1099.
66. Faraco IMJr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163.
67. Farges JC, Keller JF, Carrouel F, et al. Odontoblasts in the dental pulp immune response. J Exp Zoolog B Mol Dev E. 2008.
68. Farges JC, Romeas A, Melin M, et al. TGF-beta1 induces accumulation of dendritic cells in the odontoblast layer. J Dent Res. 2003;82:652.
69. Farik B, Munksgaard EC, Andreasen JO, Kreiborg S. Drying and rewetting anterior crown fragments prior to bonding. Endod Dent Traumatol. 1999;15:113.
70. Felton D. Long-term effects of crown preparation on pulp vitality. J Dent Res. 1989;68:1009. (abstract 1139)
71. Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dent Mater. 1990;6:282.
72. Fitzgerald M, Hanks CT. In vivo study of resin diffusion through intact vital human dentin (abstract). J Dent Res. 1997;76:305.
73. Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491.
74. Fosse G, Saele PK, Eide R. Numerical density and distributional pattern of dentin tubules. Acta Odontol Scand. 1992;50:201.
75. Fried K. Changes in pulpal nerves with aging. Proc Finn Dent Soc. 1992;88:517.
76. Fugaro JO, Nordahl I, Fugaro OJ, Matis BA, Mjor IA. Pulp reaction to vital bleaching. Oper Dent. 2004;29:363.
77. Fujitani M, Inokoshi S, Hosoda H. Effect of acid etching on the dental pulp in adhesive composite restorations. Int Dent J. 1992;42:3.
78. Garberoglio R, Brannstrom M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355.
79. Godoy BM, Arana-Chavez VE, Nunez SC, Ribeiro MS. Effects of low-power red laser on dentine-pulp interface after cavity preparation. An ultrastructural study. Arch Oral Biol. 2007;52:899.
80. Goodis HE, Schein B, Stauffer P. Temperature changes measured in vivo at the dentinoenamel junction and pulpodentin junction during cavity preparation in the Macaca fascicularis monkey. J Endod. 1988;14:336.
81. Gwinnett AJ, Tay F. Early and intermediate time response of the dental pulp to an acid etch technique in vivo. Am J Dent. 1998;11:S35.
82. Gwinnett AJ, Tay FR, Pang KM, Wei SH. Quantitative contribution of the collagen network in dentin hybridization. Am J Dent. 1996;9:140.
83. Hamersky PA, Weimer AD, Taintor JF. The effect of orthodontic force application on the pulpal tissue respiration rate in the human premolar. Am J Orthod. 1980;77:368.
84. Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res. 1993;72:931.
85. Harada K, Sato M, Omura K. Blood-flow and neurosensory changes in the maxillary dental pulp after differing Le Fort I osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:12.
86. Hargreaves K. Seltzer and Bender’s the dental pulp, ed 2. Chicago: Quintessence; 2010.
87. Harrington GW, Steiner DR, Ammons WF. The periodontal-endodontic controversy. Periodontol 2000. 2002;30:123.
88. Harris DM, White JM, Goodis H, et al. Selective ablation of surface enamel caries with a pulsed Nd:YAG dental laser. Lasers Surg Med. 2002;30:342.
89. Hartmann A, Azerad J, Boucher Y. Environmental effects on laser Doppler pulpal blood-flow measurements in man. Arch Oral Biol. 1996;41:333.
90. Hasler JE, Mitchell DF. Painless pulpitis. J Am Dent Assoc. 1970;81:671.
91. Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758.
92. Heys DR, Cox CF, Heys RJ, Avery JK. Histological considerations of direct pulp capping agents. J Dent Res. 1981;60:1371.
93. Hirata K, Nakashima M, Sekine I, Mukouyama Y, Kimura K. Dentinal fluid movement associated with loading of restorations. J Dent Res. 1991;70:975.
94. Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808.
95. Horsted P, Sandergaard B, Thylstrup A, El Attar K, Fejerskov O. A retrospective study of direct pulp capping with calcium hydroxide compounds. Endod Dent Traumatol. 1985;1:29.
96. Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645.
97. Hume WR. An analysis of the release and the diffusion through dentin of eugenol from zinc oxide-eugenol mixtures. J Dent Res. 1984;63:881.
98. Hume WR, Gerzia TM. Bioavailability of components of resin-based materials which are applied to teeth. Crit Rev Oral Biol Med. 1996;7:172.
99. Hume WR, Kenney AE. Release of 3H-triamcinolone from Ledermix. J Endod. 1981;7:509.
100. Ikawa M, Komatsu H, Ikawa K, Mayanagi H, Shimauchi H. Age-related changes in the human pulpal blood flow measured by laser Doppler flowmetry. Dent Traumatol. 2003;19:36.
101. Inoue H, Izumi T, Ishikawa H, Watanabe K. Short-term histomorphological effects of Er:YAG laser irradiation to rat coronal dentin-pulp complex. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:246.
102. Inoue T, Miyakoshi S, Shimono M. The in vitro and in vivo influence of 4-META/MMA-TBB resin components on dental pulp tissues. Adv Dent Res. 2001;15:101.
103. Inoue T, Miyakoshi S, Shimono M. Dentin/pulp adhesive resin interface. Biological view from basic science to clinic. In: Shimono M MT, Suda H, Takahashi K, editors. Proceedings of the International Conference on Dentin/Pulp Complex 1995. Chiba, Japan, Chicago: Quintessence; 1996:217.
104. Isett J, Reader A, Gallatin E, Beck M, Padgett D. Effect of an intraosseous injection of Depo-Medrol on pulpal concentrations of PGE2 and IL-8 in untreated irreversible pulpitis. J Endod. 2003;29:268.
105. Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185.
106. Izumi T, Inoue H, Matsuura H, et al. Changes in the pattern of horseradish peroxidase diffusion into predentin and dentin after cavity preparation in rat molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:675.
107. Izumi T, Kobayashi I, Okamura K, Sakai H. Immunohistochemical study on the immunocompetent cells of the pulp in human non-carious and carious teeth. Arch Oral Biol. 1995;40:609.
108. Jaber L, Swaim WD, Dionne RA. Immunohistochemical localization of mu-opioid receptors in human dental pulp. J Endod. 2003;29:108.
109. Janeway CAJr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197.
110. Jansson L, Ehnevid H, Lindskog S, Blomlof L. Relationship between periapical and periodontal status. A clinical retrospective study. J Clin Periodontol. 1993;20:117.
111. Jansson L, Sandstedt P, Laftman AC, Skoglund A. Relationship between apical and marginal healing in periradicular surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:596.
112. Jiang HW, Zhang W, Ren BP, Zeng JF, Ling JQ. Expression of toll-like receptor 4 in normal human odontoblasts and dental pulp tissue. J Endod. 2006;32:747.
113. Jontell M, Hanks CT, Bratel J, Bergenholtz G. Effects of unpolymerized resin components on the function of accessory cells derived from the rat incisor pulp. J Dent Res. 1995;74:1162.
114. Jukic S, Anic I, Koba K, Najzar-Fleger D, Matsumoto K. The effect of pulpotomy using CO2 and Nd:YAG lasers on dental pulp tissue. Int Endod J. 1997;30:175.
115. Katsuno K, Manabe A, Itoh K, et al. A delayed hypersensitivity reaction to dentine primer in the guinea-pig. J Dent. 1995;23:295.
116. Kawagishi E, Nakakura-Ohshima K, Nomura S, Ohshima H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006;326:111.
117. Kim S. Ligamental injection: a physiological explanation of its efficacy. J Endod. 1986;12:486.
118. Kim S, Edwall L, Trowbridge H, Chien S. Effects of local anesthetics on pulpal blood flow in dogs. J Dent Res. 1984;63:650.
119. Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol. 2000;27:715.
120. Kimura Y, Yonaga K, Yokoyama K, Watanabe H, Wang X, Matsumoto K. Histopathological changes in dental pulp irradiated by Er:YAG laser: a preliminary report on laser pulpotomy. J Clin Laser Med Surg. 2003;21:345.
121. Koh ET, Ford TR, Kariyawasam SP, Chen NN, Torabinejad M. Prophylactic treatment of dens evaginatus using mineral trioxide aggregate. J Endod. 2001;27:540.
122. Kohn MW, White RPJr. Evaluation of sensation after segmental alveolar osteotomy in 22 patients. J Am Dent Assoc. 1974;89:154.
123. Kokkas AB, Goulas A, Varsamidis K, Mirtsou V, Tziafas D. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factor-alpha gene expression in human pulp. Int Endod J. 2007;40:198.
124. Konno Y, Daimaruya T, Iikubo M, et al. Morphologic and hemodynamic analysis of dental pulp in dogs after molar intrusion with the skeletal anchorage system. Am J Orthod Dentofacial Orthop. 2007;132:199.
125. Koshiro K, Inoue S, Tanaka T, et al. In vivo degradation of resin-dentin bonds produced by a self-etch vs. a total-etch adhesive system. Eur J Oral Sci. 2004;112:368.
126. Kuhnisch J, Bucher K, Henschel V, Hickel R. Reproducibility of DIAGNOdent 2095 and DIAGNOdent Pen measurements: results from an in vitro study on occlusal sites. Eur J Oral Sci. 2007;115:206.
127. Kvinnsland S, Heyeraas K, Ofjord ES. Effect of experimental tooth movement on periodontal and pulpal blood flow. Eur J Orthod. 1989;11:200.
128. Langeland K. Tissue Changes in the dental pulp. An experimental histologic study. Odontol Tidskr. 1957;65:1.
129. Langeland K. Tissue response to dental caries. Endod Dent Traumatol. 1987;3:149.
130. Langeland K, Dowden WE, Tronstad L, Langeland LK. Human pulp changes of iatrogenic origin. Oral Surg Oral Med Oral Pathol. 1971;32:943.
131. Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria, and pulpal histopathology. Oral Surg Oral Med Oral Pathol. 1974;37:257.
132. Laurell KA, Carpenter W, Daugherty D, Beck M. Histopathologic effects of kinetic cavity preparation for the removal of enamel and dentin. An in vivo animal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:214.
133. Lefebvre CA, Wataha JC, Bouillaguet S, Lockwood PE. Effects of long-term sub-lethal concentrations of dental monomers on THP-1 human monocytes. J Biomater Sci Polym Ed. 1999;10:1265.
134. Lepinski AM, Haegreaves KM, Goodis HE, Bowles WR. Bradykinin levels in dental pulp by microdialysis. J Endod. 2000;26:744.
135. Lesot H, et al. Epigenetic signals during odontoblast differentiation. ADV Dent Res. 2001;15:8-13.
136. Levin LG, Rudd A, Bletsa A, Reisner H. Expression of IL-8 by cells of the odontoblast layer in vitro. Eur J Oral Sci. 1999;107:131.
137. Lier BB, Rosing CK, Aass AM, Gjermo P. Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Periodontol. 2002;29:501.
138. Lilja J, Nordenvall KJ, Branstrom M. Dentin sensitivity, odontoblasts and nerves under desiccated or infected experimental cavities. A clinical, light microscopic and ultrastructural investigation. Swed Dent J. 1982;6:93.
139. Lin S, Mayer Y. Treatment of a large periradicular lesion of endodontic origin around a dental implant with enamel matrix protein derivative. J Periodontol. 2007;78:2385.
140. Linde A, editor. Noncollagenous proteins and proteoglycans in dentinogenesis. Boca Raton: CRC Press, 1984.
141. Litkowski LJ, Hack GD, Sheaffer HB, Greenspan DC: Occlusion of dentin tubules by 45S5 Bioglass. Bioceramics 10, Proceedings of the 10th International Symposium on Ceramics in Medicine, Paris, France, Oct 1997;411.
142. Liu HC, Lin CP, Lan WH. Sealing depth of Nd:YAG laser on human dentinal tubules. J Endod. 1997;23:691.
143. Lupi-Pegurier L, Bertrand MF, Muller-Bolla M, Rocca JP, Bolla M. Comparative study of microleakage of a pit and fissure sealant placed after preparation by Er:YAG laser in permanent molars. J Dent Child (Chic). 2003;70:134.
144. Lussi A, Hibst R, Paulus R. DIAGNOdent: an optical method for caries detection. J Dent Res. 2004;83:C80. Spec No C
145. Margelos JT, Verdelis KG. Irreversible pulpal damage of teeth adjacent to recently placed osseointegrated implants. J Endod. 1995;21:479.
146. McCormack K, Davies R. The enigma of potassium ion in the management of dentine hypersensitivity: is nitric oxide the elusive second messenger? Pain. 1996;68:5.
147. McLachlan JL, Sloan AJ, Smith AJ, Landini G, Cooper PR. S100 and cytokine expression in caries. Infect Immun. 2004;72:4102.
148. McLachlan JL, Smith AJ, Bujalska IJ, Cooper PR. Gene expression profiling of pulpal tissue reveals the molecular complexity of dental caries. Biochim Biophys Acta. 2005.
149. Michaelson PL, Holland GR. Is pulpitis painful? Int Endod J. 2002;35:829.
150. Mitchell DF, Tarplee RE. Painful pulpitis; a clinical and microscopic study. Oral Surg Oral Med Oral Pathol. 1960;13:1360.
151. Mjor IA. Bacteria in experimentally infected cavity preparations. Scand J Dent Res. 1977;85:599.
152. Mjor IA, Ferrari M. Pulp-dentin biology in restorative dentistry. Part 6: reactions to restorative materials, tooth-restoration interfaces, and adhesive techniques. Quintessence Int. 2002;33:35.
153. Mjor IA, Finn SB, Quigley MB. The effect of calcium hydroxide and amalgam on non-carious, vital dentine. Arch Oral Biol. 1961;3:283.
154. Mjor IA, Tronstad L. Experimentally induced pulpitis. Oral Surg Oral Med Oral Pathol. 1972;34:102.
155. Mjor IA, Tronstad L. The healing of experimentally induced pulpitis. Oral Surg Oral Med Oral Pathol. 1974;38:115.
156. Murray PE, About I, Lumley PJ, Smith G, Franquin JC, Smith AJ. Postoperative pulpal and repair responses. J Am Dent Assoc. 2000;131:321.
157. Murray PE, Smith AJ, Garcia-Godoy F, Lumley PJ. Comparison of operative procedure variables on pulpal viability in an ex vivo model. Int Endod J. 2008;41:389.
158. Murray PE, Smith AJ, Windsor LJ, Mjor IA. Remaining dentine thickness and human pulp responses. Int Endod J. 2003;36:33.
159. Murray PE, Smyth TW, About I, Remusat R, Franquin JC, Smith AJ. The effect of etching on bacterial microleakage of an adhesive composite restoration. J Dent. 2002;30:29.
160. Musselwhite JM, Klitzman B, Maixner W, Burkes EJJr. Laser Doppler flowmetry: a clinical test of pulpal vitality. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:411.
161. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265.
162. Nanda R, Legan HL, Langeland K. Pulpal and radicular response to maxillary osteotomy in monkeys. Oral Surg Oral Med Oral Pathol. 1982;53:624.
163. Nathanson D. Vital tooth bleaching: sensitivity and pulpal considerations. J Am Dent Assoc. 1997;128(Suppl):41S.
164. Nayyar S, Tewari S, Arora B. Comparison of human pulp response to total-etch and self-etch bonding agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e45.
165. Nixon CE, Saviano JA, King GJ, Keeling SD. Histomorphometric study of dental pulp during orthodontic tooth movement. J Endod. 1993;19:13.
166. Nomiyama K, Kitamura C, Tsujisawa T, et al. Effects of lipopolysaccharide on newly established rat dental pulp-derived cell line with odontoblastic properties. J Endod. 2007;33:1187.
167. Nup C, Rosenberg P, Linke H, Tordik P. Quantitation of catecholamines in inflamed human dental pulp by high-performance liquid chromatography. J Endod. 2001;27:73.
168. Nyborg H, Brannstrom M. Pulp reaction to heat. J Prosthet Dent. 1968;19:605.
169. Odor TM, Pitt Ford TR, McDonald F. Adrenaline in local anaesthesia: the effect of concentration on dental pulpal circulation and anaesthesia. Endod Dent Traumatol. 1994;10:167.
170. Okamura K. Histological study on the origin of dentinal immunoglobulins and the change in their localization during caries. J Oral Pathol. 1985;14:680.
171. Okamura K, Maeda M, Nishikawa T, Tsutsui M. Dentinal response against carious invasion: localization of antibodies in odontoblastic body and process. J Dent Res. 1980;59:1368.
172. Olgart L, Gazelius B, Sundstrom F. Intradental nerve activity and jaw-opening reflex in response to mechanical deformation of cat teeth. Acta Physiol Scand. 1988;133:399.
173. Orchardson R, Peacock JM, Whitters CJ. Effects of pulsed Nd:YAG laser radiation on action potential conduction in nerve fibres inside teeth in vitro. J Dent. 1998;26:421.
174. Orchardson R, Whitters CJ. Effect of HeNe and pulsed Nd:YAG laser irradiation on intradental nerve responses to mechanical stimulation of dentine. Lasers Surg Med. 2000;26:241.
175. Ozturk M, Doruk C, Ozec I, Polat S, Babacan H, Bicakci AA. Pulpal blood flow: effects of corticotomy and midline osteotomy in surgically assisted rapid palatal expansion. J Craniomaxillofac Surg. 2003;31:97.
176. Paakkonen V, Ohlmeier S, Bergmann U, Larmas M, Salo T, Tjaderhane L. Analysis of gene and protein expression in healthy and carious tooth pulp with cDNA microarray and two-dimensional gel electrophoresis. Eur J Oral Sci. 2005;113:369.
177. Paakkonen V, Vuoristo JT, Salo T, Tjaderhane L. Comparative gene expression profile analysis between native human odontoblasts and pulp tissue. Int Endod J. 2008;41:117.
178. Palosaari H, Wahlgren J, Larmas M, et al. The expression of MMP-8 in human odontoblasts and dental pulp cells is down-regulated by TGF-beta1. J Dent Res. 2000;79:77.
179. Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates [published erratum appears in Am J Dent 1998 Jun;11(3):148]. Am J Dent. 1998;11:S45.
180. Pamir T, Dalgar H, Onal B. Clinical evaluation of three desensitizing agents in relieving dentin hypersensitivity. Oper Dent. 2007;32:544.
181. Panitvisai P, Messer HH. Cuspal deflection in molars in relation to endodontic and restorative procedures. J Endod. 1995;21:57.
182. Parirokh M, Asgary S, Eghbal MJ, et al. A comparative study of white and grey mineral trioxide aggregate as pulp capping agents in dog’s teeth. Dent Traumatol. 2005;21:150.
183. Pashley DH. Dynamics of the pulpo-dentin complex. Crit Rev Oral Biol Med. 1996;7:104.
184. Pashley DH. In vitro simulations of in vivo bonding conditions. Am J Dent. 1991;4:237.
185. Pashley DH. The influence of dentin permeability and pulpal blood flow on pulpal solute concentrations. J Endod. 1979;5:355.
186. Pashley DH, Kepler EE, Williams EC, Okabe A. Progressive decrease in dentine permeability following cavity preparation. Arch Oral Biol. 1983;28:853.
187. Pepersack WJ. Tooth vitality after alveolar segmental osteotomy. J Oral Maxillofac Surg. 1973;1:85.
188. Perdigao J, Geraldeli S, Hodges JS. Total-etch versus self-etch adhesive: effect on postoperative sensitivity. J Am Dent Assoc. 2003;134:1621.
189. Perinetti G, Varvara G, Festa F, Esposito P. Aspartate aminotransferase activity in pulp of orthodontically treated teeth. Am J Orthod Dentofacial Orthop. 2004;125:88.
190. Peters DD, Baumgartner JC, Lorton L. Adult pulpal diagnosis. I. Evaluation of the positive and negative responses to cold and electrical pulp tests. J Endod. 1994;20:506.
191. Petersson K, Soderstrom C, Kiani-Anaraki M, Levy G. Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod Dent Traumatol. 1999;15:127.
192. Polat S, Er K, Akpinar KE, Polat NT. The sources of laser Doppler blood-flow signals recorded from vital and root canal treated teeth. Arch Oral Biol. 2004;49:53.
193. Premdas CE, Pitt Ford TR. Effect of palatal injections on pulpal blood flow in premolars. Endod Dent Traumatol. 1995;11:274.
194. Quirynen M, Gijbels F, Jacobs R. An infected jawbone site compromising successful osseointegration. Periodontol 2000. 2003;33:129.
195. Qvist V, Stoltze K, Qvist J. Human pulp reactions to resin restorations performed with different acid-etch restorative procedures. Acta Odontol Scand. 1989;47:253.
196. Rakich DR, Wataha JC, Lefebvre CA, Weller RN. Effect of dentin bonding agents on the secretion of inflammatory mediators from macrophages. J Endod. 1999;25:114.
197. Rakich DR, Wataha JC, Lefebvre CA, Weller RN. Effects of dentin bonding agents on macrophage mitochondrial activity. J Endod. 1998;24:528.
198. Ramsay DS, Artun J, Bloomquist D. Orthognathic surgery and pulpal blood flow: a pilot study using laser Doppler flowmetry. J Oral Maxillofac Surg. 1991;49:564.
199. Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15:512.
200. Reeves R, Stanley HR. The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surg Oral Med Oral Pathol. 1966;22:59.
201. Reeves R, Stanley HR. The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surg Oral Med Oral Pathol. 1966;22:59.
202. Reynolds RL. The determination of pulp vitality by means of thermal and electrical stimuli. Oral Surg Oral Med Oral Pathol. 1966;22:231.
203. Ritter AV, Leonard RHJr, St Georges AJ, Caplan DJ, Haywood VB. Safety and stability of nightguard vital bleaching: 9 to 12 years post-treatment. J Esthet Restor Dent. 2002;14:275.
204. Rodd HD, Boissonade FM. Substance P expression in human tooth pulp in relation to caries and pain experience.[In Process Citation.]. Eur J Oral Sci. 2000;108:467.
205. Rotstein I, Simon JH. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontol 2000. 2004;34:165.
206. Sato M, Harada K, Okada Y, Omura K. Blood-flow change and recovery of sensibility in the maxillary dental pulp after a single-segment Le Fort I osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:660.
207. Schroder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64:541. Spec No
208. Schuster GS, Caughman GB, Rueggeberg FA, Lefebvre CA, Cibirka R. Alterations in cell lipid metabolism by glycol methacrylate (HEMA). J Biomater Sci Polym Ed. 1999;10:1121.
209. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. 1963;16:846.
210. Shabahang S, Bohsali K, Boyne PJ, Caplanis N, Lozada J, Torabinejad M. Effect of teeth with periradicular lesions on adjacent dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:321.
211. Silva AF, Tarquinio SB, Demarco FF, Piva E, Rivero ER. The influence of haemostatic agents on healing of healthy human dental pulp tissue capped with calcium hydroxide. Int Endod J. 2006;39:309.
212. Simon JH, Lies J. Silent trauma. Endod Dent Traumatol. 1999;15:145.
213. Sloan AJ, Smith AJ. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1-3 in vitro. Arch Oral Biol. 1999;44:149.
214. Smith AJ. Pulpal responses to caries and dental repair. Caries Res. 2002;36:223.
215. Smith AJ. Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J Dent Educ. 2003;67:678.
216. Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 2001;12:425.
217. Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. Preliminary studies on the in vivo morphogenetic properties of dentine matrix proteins. Biomaterials. 1990;11:22.
218. Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166.
219. Soo-ampon S, Vongsavan N, Soo-ampon M, Chuckpaiwong S, Matthews B. The sources of laser Doppler blood-flow signals recorded from human teeth. Arch Oral Biol. 2003;48:353.
220. Stabholz A, Rotstein L, Neev J, Moshonov J. Efficacy of XeCl 308-nm Excimer laser in reducing dye penetration through coronal dentinal tubules. J Endod. 1995;21:266.
221. Stanley HR. Pulp capping: conserving the dental pulp–can it be done? Is it worth it? Oral Surg Oral Med Oral Pathol. 1989;68:628.
222. Stanley HR, Pereira JC, Spiegel E, Broom C, Schultz M. The detection and prevalence of reactive and physiologic sclerotic dentin, reparative dentin and dead tracts beneath various types of dental lesions according to tooth surface and age. J Oral Pathol. 1983;12:257.
223. Steiner DR. The resolution of a periradicular lesion involving an implant. J Endod. 2008;34:330.
224. Stenvik A. Pulp and dentine reactions to experimental tooth intrusion. (A histologic study–long-term effects). Rep Congr Eur Orthod Soc. 1969:449.
225. Stenvik A, Mjor IA. Pulp and dentine reactions to experimental tooth intrusion. A histologic study of the initial changes. Am J Orthod. 1970;57:370.
226. Stenvik A, Mjor IA. The effect of experimental tooth intrusion on pulp and dentine. Oral Surg Oral Med Oral Pathol. 1971;32:639.
227. Sussman HI. Endodontic pathology leading to implant failure–a case report. J Oral Implantol. 1997;23:112.
228. Sussman HI. Tooth devitalization via implant placement: a case report. Periodontal Clin Investig. 1998;20:22.
229. Swift EJJr, Trope M. Treatment options for the exposed vital pulp. Pract Periodontics Aesthet Dent. 1999;11:735.
230. Tagami J, Hosoda H, Burrow MF, Nakajima M. Effect of aging and caries on dentin permeability. Proc Finn Dent Soc. 1992;88(Suppl 1):149.
231. Takamori K. A histopathological and immunohistochemical study of dental pulp and pulpal nerve fibers in rats after the cavity preparation using Er:YAG laser. J Endod. 2000;26:95.
232. Tanabe K, Yoshiba K, Yoshiba N, Iwaku M, Ozawa H. Immunohistochemical study on pulpal response in rat molars after cavity preparation by Er:YAG laser. Eur J Oral Sci. 2002;110:237.
233. Teixeira LS, Demarco FF, Coppola MC, Bonow ML. Clinical and radiographic evaluation of pulpotomies performed under intrapulpal injection of anaesthetic solution. Int Endod J. 2001;34:440.
234. Tjan AH, Grant BE, Godfrey MF3rd. Temperature rise in the pulp chamber during fabrication of provisional crowns. J Prosthet Dent. 1989;62:622.
235. Tokita Y, Sunakawa M, Suda H. Pulsed Nd:YAG laser irradiation of the tooth pulp in the cat: I. Effect of spot lasing. Lasers Surg Med. 2000;26:398.
236. Torabinejad M, Kiger RD. A histologic evaluation of dental pulp tissue of a patient with periodontal disease. Oral Surg Oral Med Oral Pathol. 1985;59:198.
237. Trantor IR, Messer HH, Birner R. The effects of neuropeptides (calcitonin gene-related peptide and substance P) on cultured human pulp cells. J Dent Res. 1995;74:1066.
238. Trowbridge H, Edwall L, Panopoulos P. Effect of zinc oxide-eugenol and calcium hydroxide on intradental nerve activity. J Endod. 1982;8:403.
239. Tsatsas BG, Frank RM. Ultrastructure of the dentimal tubular substances near the dentino-enamel junction. Calcif Tissue Res. 1972;9:238.
240. Turner DF, Marfurt CF, Sattelberg C. Demonstration of physiological barrier between pulpal odontoblasts and its perturbation following routine restorative procedures: a horseradish peroxidase tracing study in the rat. J Dent Res. 1989;68:1262.
241. Tyndall AA, Brooks SL. Selection criteria for dental implant site imaging: a position paper of the American Academy of Oral and Maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:630.
242. Tziafas D. Basic mechanisms of cytodifferentiation and dentinogenesis during dental pulp repair. Int J Dev Biol. 1995;39:281.
243. Tziafas D, Kolokuris I. Inductive influences of demineralized dentin and bone matrix on pulp cells: an approach of secondary dentinogenesis. J Dent Res. 1990;69:75.
244. Unemori M, Matsuya Y, Akashi A, Goto Y, Akamine A. Composite resin restoration and postoperative sensitivity: clinical follow-up in an undergraduate program. J Dent. 2001;29:7.
245. Valderhaug J, Jokstad A, Ambjornsen E, Norheim PW. Assessment of the periapical and clinical status of crowned teeth over 25 years. J Dent. 1997;25:97.
246. Vale WA. Cavity Preparation. Ir Dent Rev. 1956;2:33.
247. Vedtofte P. Pulp canal obliteration after Le Fort I osteotomy. Endod Dent Traumatol. 1989;5:274.
248. Versluis A, Douglas WH, Cross M, Sakaguchi RL. Does an incremental filling technique reduce polymerization shrinkage stresses? J Dent Res. 1996;75:871.
249. Versluis A, Tantbirojn D, Douglas WH. Distribution of transient properties during polymerization of a light-initiated restorative composite. Dent Mater. 2004;20:543.
250. von Fraunhofer JA, Adachi EI, Barnes DM, Romberg E. The effect of tooth preparation on microleakage behavior. Oper Dent. 2000;25:526.
251. von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Suppl 3):173.
252. Vongsavan N, Matthews RW, Matthews B. The permeability of human dentine in vitro and in vivo. Arch Oral Biol. 2000;45:931.
253. Wakabayashi H, Hamba M, Matsumoto K, Tachibana H. Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med. 1993;13:605.
254. Wennberg A, Mjor IA, Heide S. Rate of formation of regular and irregular secondary dentin in monkey teeth. Oral Surg Oral Med Oral Pathol. 1982;54:232.
255. Wigdor H, Abt E, Ashrafi S, Walsh JTJr. The effect of lasers on dental hard tissues. J Am Dent Assoc. 1993;124:65.
256. Yiu CK, King NM, Suh BI, et al. Incompatibility of oxalate desensitizers with acidic, fluoride-containing total-etch adhesives. J Dent Res. 2005;84:730.
257. Yoshiba K, Yoshiba N, Iwaku M. Class II antigen-presenting dendritic cell and nerve fiber responses to cavities, caries, or caries treatment in human teeth. J Dent Res. 2003;82:422.
258. Yoshiba N, Yoshiba K, Iwaku M, Ozawa H. Immunohistochemical localizations of class II antigens and nerve fibers in human carious teeth: HLA-DR immunoreactivity in Schwann cells. Arch Histol Cytol. 1998;61:343.
259. Yoshiba N, Yoshiba K, Nakamura H, Iwaku M, Ozawa H. Immunohistochemical localization of HLA-DR-positive cells in unerupted and erupted normal and carious human teeth. J Dent Res. 1996;75:1585.
260. Yoshida S, Oshima K, Tanne K. Biologic responses of the pulp to single-tooth dento-osseous osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:152.
261. Yoshiyama M, Masada J, Uchida A, Ishida H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J Dent Res. 1989;68:1498.
262. Zach L, Cohen G. Pulp response to externaly applied heat. Oral Surg Oral Med Oral Pathol. 1965;19:515.
263. Zaia AA, Nakagawa R, De Quadros I, et al. An in vitro evaluation of four materials as barriers to coronal microleakage in root-filled teeth. Int Endod J. 2002;35:729.
264. Zhang J, Kawashima N, Suda H, Nakano Y, Takano Y, Azuma M. The existence of CD11c+ sentinel and F4/80+ interstitial dendritic cells in dental pulp and their dynamics and functional properties. Int Immunol. 2006;18:1375.