CHAPTER 16 Regenerative Endodontics
Overview of Regenerative Dentistry
From a biologic perspective, the goal of endodontics is to prevent or treat apical periodontitis. An optimal way to accomplish this goal is to either maintain pulpal health in cases of pulpal inflammation or to regenerate healthy pulpal tissue in cases of pulpal necrosis. Thus, there is considerable interest in research and clinical studies aimed towards regeneration of a functional and healthy pulp-dentin complex.68,109 This chapter will review the current status of regenerative endodontic procedures with an emphasis on biologic principles and the advantages and limitations of currently available clinical procedures.
The history of dentistry has largely involved the prosthetic replacement of lost or diseased tissues, including endodontic obturation of root canal systems with inert materials (see Chapter 10). In contrast, the goal of regenerative dentistry is to induce biologic replacement of dental tissues and their supporting structures. The potential for regenerative dentistry is in large part due to advancements in biologic therapies that apply growth and differentiation factors which hasten or induce natural biologic regeneration. Many of these concepts have emerged from the burgeoning field of tissue engineering, which emphasizes the spatial assembly of distinct stem cells, growth factors/morphogens, and scaffolds to form a functional tissue or organ.62
Pioneering work supporting the concept of regenerating dental tissues was reported more than 50 years ago when Dr. B.W. Hermann described the application of calcium hydroxide (Ca[OH]2) for vital pulp therapy,47 and Professor Nygaard-Østby evaluated a revascularization method for reestablishing a pulp-dentin complex in permanent teeth with pulpal necrosis (see later).82 Over the last several decades, the scope and clinical application of regenerative dental procedures has continuously advanced to now include guided tissue or bone regeneration (GTR, GBR) procedures and distraction osteogenesis,15,17,86 the application of platelet-rich plasma for bone augmentation,60 Emdogain for regeneration of periodontal tissues,44 recombinant human bone morphogenic protein (rhBMP) for augmentation of bone,34 and preclinical trials on the use of fibroblast growth factor 2 (FGF-2) for periodontal tissue regeneration.118 At the beginning of the 21st century, we are seeing the potential of these therapies for dental practice, including endodontics. For example, pulp, dentin, and enamel can be regenerated using scaffold material and stem cells,29,51,141 and tooth crowns can be regenerated using the primordium of embryonic oral epithelium and adult bone-marrow stem cells.87 Adult bone marrow stem cells are capable of differentiating directly into ameloblasts when mixed with embryonic epithelial cells,49 dissociated cells from tooth germs can be mixed with agar and collagen (i.e., scaffolds) to form crown, root, and periodontal structures in adult hosts,49,71 and postnatal stem cells isolated from extracted wisdom teeth can be used to regenerate tooth roots and periodontal ligaments that can support synthetic crowns.113 Regenerative dental procedures are emerging as a vital, evolving field of dental care.
Overview of Regenerative Endodontics
Regenerative endodontics has been defined as biologically based procedures designed to replace damaged structures such as dentin, root structures, and cells of the pulp-dentin complex.68 Similar to most rapidly evolving fields, the scope and depth of basic research has outpaced the translation of this knowledge into clinical trials and established dental procedures. It is important to review both basic and clinical studies for a full understanding of the scope, limitations, and potential of this field. To understand the implications of preclinical research for clinical practice, it is important to briefly review the development of teeth.
The forming tooth progresses through a succession of morphologic stages—bud, cap, and bell—resulting in the formation of the crown, root, and periodontium (see also Chapter 12). Tooth development is regulated by a series of reciprocal interactions involving both ectodermal and mesenchymal stem cells.119 Ectodermal stem cells differentiate into ameloblasts, and mesenchymal stem cells, also known as ectomesenchymal stem cells owing to their neural crest origin, differentiate into odontoblasts. One important requirement for regeneration of pulp tissues is to obtain stem cells capable of differentiating into odontoblasts.
Stem cells are often viewed as relatively undifferentiated cells capable of self-renewal/expansion (i.e., can divide many times) and able to differentiate into specialized cell types. The range of differentiation (“potency”) is an important property of stem cells and serves as the basis for classifying cells as totipotent—capable of differentiating into any cell type—or pluripotent, multipotent, or unipotent—characterizing more restricted abilities for differentiation. In general, considerable research has focused on totipotent embryonic stem cells that have maximal capacity for differentiation, but ethical, legal, and medical (tissue-rejection) issues can render these cell types unsuitable for clinical development.68 In contrast, postnatal (i.e., adult) stem cells are more readily available, are already applied in clinical practice (e.g., bone marrow transplants, etc.), and when used in an autologous application, will not induce immune responses such as tissue rejection. Many of these stem cells appear to satisfy criteria for multipotency, since they can differentiate into cells that express phenotypic markers for odontoblasts, neurons, and muscle or adipose cells.52
Nakahara has summarized potential methods for regenerating an entire tooth. The first approach, incorporating principles of tissue engineering, involves seeding appropriate stem cells onto scaffolding materials, such as has been used for periodontal regeneration.70 This approach may be controlled by the addition of specific growth factors and/or signaling molecules. The second approach involves replicating the natural developmental processes of embryonic tooth formation. In this approach, artificial tooth germs are transplanted into the bodies of animal hosts where there is enough blood flow to support tissue formation. Although either approach may evolve into a method capable of generating entire teeth, it should be appreciated that the natural development of permanent human teeth takes years to complete. Regenerating an entire tooth from a patient’s own stem cells may not be clinically practical.
One alternative approach would be to regenerate a functional pulp-dentin complex within a patient’s existing permanent tooth. This would restore natural functions, such as formation of replacement dentin and maintenance of tissue immunity and neural sensation, to teeth that otherwise would require endodontic obturation with inert materials or possibly extraction. One key advantage is that the pulp occupies a relatively small volume (about 0.1 to 2 ml) and, compared to complex organs such as the heart or liver, has a relatively simple cytoarchitecture that can be approximated as a core of loose connective tissue surrounded by a layer of odontoblasts attached to dentin. From a tissue-engineering perspective, the dental pulp may be a relatively easier tissue to regenerate.
Preclinical Studies on Regenerative Endodontics
Applying the principles of tissue engineering to the development of regenerative endodontic procedures requires research on the correct spatial assembly of distinct stem cells, growth factors/morphogens, and scaffolds to form a functional pulp-dentin complex.42,62,74 In this section, we will review each of these critical components in turn.
Stem Cells
As described earlier, dental pulp can be viewed as a core of innervated and vascularized loose connective tissue surrounded by a layer of odontoblasts. The major cell type of this core region is the fibroblast. Together with blood vessels, lymphatics, and neurons, this core tissue is embedded in an extracellular matrix consisting of collagen and other fiber types (see also Chapter 12). Pulpal mesenchymal stem cells are thought to be localized in the perivascular region and the cell-rich zone of Hohl adjacent to the odontoblastic layer; both have been proposed to serve as cell sources for replacement odontoblasts.32,106 Thus, several cell types must be developed in order to form this core of loose connective tissue. The regeneration of loose connective tissue has been proposed to be similar to the process of the granulation phase of wound healing, with the local release of angiogenic growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) playing key roles in coordinating angiogenesis and the development of loose connective tissue.8 Interestingly, platelet-rich plasma has about a three- to sixfold increase in VEGF and PDGF and has been reported to increase rates of wound healing.8,81,94,132 Additional studies have demonstrated that the application of recombinant human VEGF substantially increases the degree of revascularization of human dental pulp slices implanted into immunocompromised mice.36 Given the large existing knowledge base on the regeneration of loose connective tissue, recent research has mainly focused on generation of the surrounding layer of odontoblasts.
At least five different types of postnatal mesenchymal stem cells have been reported to differentiate into odontoblast-like cells, including dental pulp stem cells (DPSC),39 stem cells of human exfoliated deciduous teeth (SHED),66 stem cells of the apical papilla (SCAP),113,114 dental follicle progenitor cells (DFPC),67 and bone marrow-derived mesenchymal stem cells (BMMSC).12 Although many of these studies involve human samples collected from patients younger than 25 years of age, it should be noted that multipotent stem cells have been collected from the pulps of patients as old as 41.50 Regenerative procedures may be applicable to at least young and middle-aged patients. To our knowledge, no study has evaluated the formation of odontoblast-like cells from patients older than 50 years of age, and this represents an important gap in knowledge. The identification of a differentiated cell as an odontoblast is not always straightforward, since this cell is similar to osteoblasts in the formation of mineralized nodules and in the expression of several proteins such as dentin sialoprotein (DSP), although DSP levels are nearly 400 times greater in odontoblasts than osteoblasts.131 Measuring only one or two characteristics of a cell might not conclusively identify whether the resulting cell is a true odontoblast. Even among odontoblasts, the phenotype varies in cells located in the apical (squamous shape) versus coronal (tall columnar) pulpal tissue. Importantly, molecular studies have identified many of the genes selectively expressed in odontoblasts,64,89,90 and this knowledge is expected to aid future studies characterizing the conditions necessary for mesenchymal cells of multiple origins to differentiate into true odontoblasts. It is likely that definitive cellular identification depends upon both the morphology of the cell and an assessment of the expression of multiple genes. For accuracy, we will refer to cells that express mineralized nodules and DSP as odontoblast-like.
Growth Factors/Morphogens
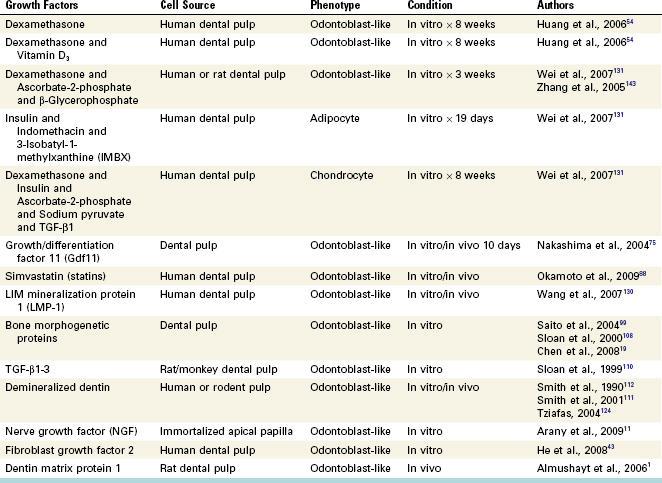
Several growth factors have been evaluated for their ability to trigger the differentiation of selected mesenchymal stem cell populations into odontoblast-like cells. Interestingly, several case studies have reported that patients taking long-term corticosteroids often present with dramatic reduction of the radiographic size of the pulp chamber and up to a fivefold increase in the thickness of the predentin layer.77,136 Although these patients were medically complex patients (e.g., renal failure) taking multiple drugs, the use of corticosteroids appeared to be associated with the observed increased activity of human odontoblasts. More recent studies have extended this general observation by demonstrating that the application of dexamethasone greatly increased the differentiation of human dental pulp cells into odontoblast-like cells.54,131 This was particularly evident when dexamethasone was combined with 1,25-dihydroxyvitamin D3.54 Merely changing the composition of growth factors completely altered the differentiation of these cells, with the same population of cells able to express markers of odontoblasts, chondrocytes, or adipocytes, depending on their exposure to different combinations of growth factors (Fig. 16-1).131 Such findings emphasize the importance of growth factors in guiding the differentiation of these cells. Other studies have evaluated growth factors administered alone or in various combinations for promoting differentiation of odontoblast-like cells (Table 16-1).
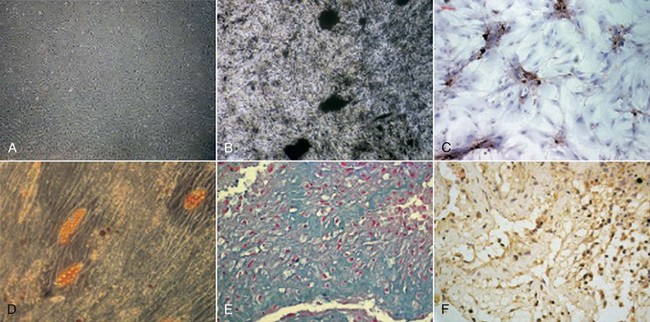
FIG. 16-1 Multilineage differentiation capacity of human dental pulp cells (DPCs). A, DPCs cultured in control medium (×40). Differentiation into odontoblast-like cells was shown by the deposition of a mineralized matrix indicated by von Kossa stain (B; ×100) and by the positive immunostaining of DSP (C; ×200). D, Adipogenic differentiation was shown by the accumulation of neutral lipid vacuoles stainable with Oil Red O (×100). Chondrogenic differentiation was shown by the secretion of cartilage-specific proteoglycans stainable with Alcian blue (E; ×400) and by the positive immunostaining of collagen type II (F; ×400).
(From Wei X, Ling J, Wu L, et al: Expression of mineralization markers in dental pulp cells. J Endod 33:703, 2007.)
Several of the approaches listed in Table 16-1 have immediate clinical implications. First, it is unlikely that a single growth factor will result in maximal differentiation, so combinations of growth factors may be required for evaluation in clinical trials. Related to this point, many of the studied growth factors (e.g., dexamethasone, insulin) are drugs already approved for human use in other medical/dental applications. Second, the demonstration that statins promote the differentiation of an odontoblast-like phenotype suggests that patients clinically taking statins may also have narrowing of the pulp chamber space, similar to the findings previously described for corticosteroids. This would be an important future area of research. Third, clinicians have long used demineralized human bone to augment healing after surgical procedures.95 Demineralized human bone is thought to contain a natural combination of appropriate growth factors and scaffolds, thereby providing an appropriate environment for osteoblast differentiation or function. Extending this concept, several research groups have demonstrated that demineralized human dentin has significant benefit for promoting the differentiation of odontoblast-like cells.* Although human dentin contains many types of noncollagenous proteins, it is notable that transforming growth factor β 1 (TGF-β1) is the only TGF subtype detectable in human dentin.144 Moreover, the application of ethylenediamine tetra-acetic acid (EDTA) strongly exposed immunoreactive TGF-β1 from human dentin, with appreciably smaller activities released after treatment with Ca(OH)2, sodium hypochlorite (NaOCl), mineral trioxide aggregate (MTA), or citric acid.38,122,144 Moreover, dentin contains additional noncollagenous proteins that promote odontoblast differentiation1 or angiogenesis.97 Collectively these findings suggest that future clinical regenerative trials should evaluate whether an EDTA irrigation of the dentinal walls prior to an endodontic regenerative procedure results in improved clinical outcomes.
Scaffolds
An important component of tissue engineering is a physical scaffold.76,104 Tissues are organized as three-dimensional structures, and appropriate scaffolding is necessary to (1) provide a spatially correct position of cell location and (2) regulate differentiation, proliferation, or metabolism. It is known that extracellular matrix molecules control the differentiation of stem cells,16,138 and an appropriate scaffold might selectively bind and localize cells,128 contain growth factors,137 and undergo biodegradation over time.142 Thus, a scaffold is far more than a simple lattice to contain cells.
Scaffolds can be classified as either natural or synthetic. Examples of natural scaffolds include collagen,55,73 glycosaminoglycans, demineralized or native dentin matrix,10,40,55,72,127 and fibrin. From the perspective of focusing on practical clinical applications, platelet-rich plasma (PRP) appears to satisfy several of these criteria. PRP is autologous, fairly easy to prepare in a dental setting, rich in growth factors, degrades over time, and forms a three-dimensional fibrin matrix.8,9,57,85 In addition, these properties may play a role in the reported outcomes of classical revascularization methods applied to patients (see later).
The second major category of scaffolds is based upon synthetic materials. Examples include polylactic acid (PLA), polyglycolic acid (PGA), polylactic-coglycolic acid (PLGA), polyepsilon caprolactone,140 hydroxyapatite/tricalcium phosphate,4 bioceramics,139 titanium (in some143 but not all studies126), and hydrogels such as alginate28,35 or variants of polyethylene glycol (PEG). Moreover, the combination of scaffolds with certain growth factors/morphogens appears to be an important combination for optimal generation of an odontoblast-like cell. This is a critical domain of research for the development of regenerative endodontics as a predictable clinical procedure.
Delivery System
Even with selection of the appropriate cell source, growth factors, and scaffold, the resultant mixture must be delivered in a spatially appropriate fashion into the space of the root canal system. For example, nearly all cells of the body are within 0.1 to 1 mm of a blood vessel in order to maintain adequate diffusion of oxygen and nutrients.37,46 If one were to inject cells along the entire coronal-apical extent of a root canal system, the vast majority of cells would be expected to succumb to tissue hypoxia. One alternative approach would be to inject a cell/scaffold/growth-factor mixture into the apical 1 mm of the root canal system and then “back-fill” the root canal system with a scaffold/growth-factor combination. Since dental pulp can be approximated as a loose connective tissue core surrounded by a layer of odontoblasts, the spatial arrangement of cells and growth factors within the scaffold may be particularly important to promote odontogenesis without having complete calcification of the root canal system. The questions require additional research effort.
Summary of Basic Research on Regenerative Endodontics
Regeneration of a functional pulp-dentin complex relies on the foundation of tissue engineering and can be viewed as a function of the spatially correct delivery of appropriate stem cells and growth factors embedded within a scaffold. Although considerable research has used in vitro cell-culture methods to identify key factors regulating the differentiation of odontoblast-like cells, it should be noted that emerging studies conducted in animal models are quite promising for regeneration of this pulp tissue. A recent preclinical study involved surgical placement of human root tips filled with various human stem cell/growth factor/scaffold combinations into immunocompromised mice.51 This model permits histologic analysis of neovascularization as well as the differentiation and mineralization activity of newly formed odontoblasts. The use of human cells in a mouse model permits histologic confirmation that the resulting odontoblast-like cells were of human origin. These novel findings provide strong evidence that either human SCAP or DPSC cell sources, on a PLGA scaffold, were able to regenerate a vascularized tissue that had histologic evidence for odontoblast-like cell differentiation and the spatially appropriate formation of dentin-like material onto the root canal walls (Fig. 16-2). Although no specific growth factors were added to this mixture, it is important to note that the root canal walls were treated with 17% EDTA, an irrigant known to expose endogenous growth-factor proteins embedded in the dentinal walls.38 This and related studies provide strong impetus for clinical translational research evaluating various potential regenerative endodontic therapies.
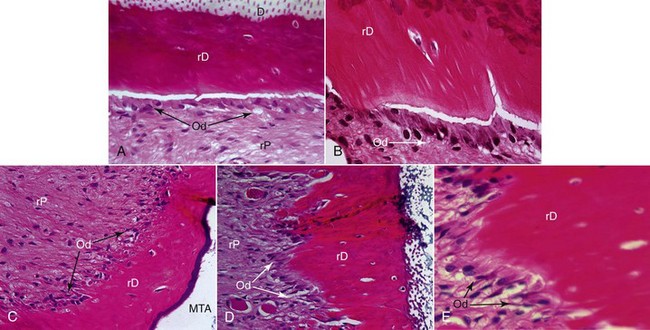
FIG. 16-2 Histologic analysis of a preclinical experiment evaluating regeneration of pulp tissue using dental pulp stem cells (DPSC) implanted with a resorbable polylactic-coglycolic acid (PLGA) scaffold into human root segments that were sealed on the coronal side with mineral trioxide aggregate (MTA) and implanted into immunocompromised mice for 4 months. D, Original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulplike tissue. Green arrows in A indicate rD; blue arrows in A indicate the entrance of blood supply; blue arrow in B and C indicate the thin layer of rD under MTA cement; blue arrows in F and G indicate the junction of D and rD; black arrow in G indicates well-aligned odontoblast-like cells. Scale bars: A = 1 mm; B = 200 µm; C-E = 100 µm; F and G = 50 µm.
(From Huang G, Yamaza T, Shea LD, et al: Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A, 2009 Sep 8. [Epub ahead of print].)
Clinical Studies on Regenerative Endodontics
To date, no published clinical trials have fully incorporated the tissue-engineering concepts described. Instead, studies over the last 50 years have focused on revascularization techniques, which share some features with the principles of regenerative tissue engineering. The key distinction is that in contrast to the focused delivery of cells/growth factors/scaffolds employed in tissue-engineering approaches, revascularization focuses on triggering bleeding into an empty root canal space with the hope that this will trigger a process similar to the role of the blood clot in triggering wound healing in surgical procedures.82 These revascularization case series will be reviewed in this section.
Historical Development of the Field
Nearly 50 years ago, Nygaard-Østby evaluated whether pulp tissue could be regenerated in root canal systems. His approach was based on the known importance of the formation of a blood clot in wound healing82,83 and involved laceration of the periapical tissue with an endodontic file. His first case series reported on nine patients aged 21 to 42 and a total of 17 teeth; there was a 13-day to 3-year follow-up prior to tooth extraction and histologic analysis.82 Some of the cases had vital pulp tissue. In these cases, there was an aseptic access, the pulp was extirpated, and the apex was overinstrumented to evoke bleeding. Then the canals were obturated with Kloroperka N-O paste and a gutta-percha point. In the teeth with necrotic pulp tissue, the procedure was the same as in the teeth with vital pulp tissue, but the canals were irrigated and had interappointment treatment with sulfathiazole and 4% formaldehyde. Teeth were then extracted, and both clinical and histologic findings were reported (Fig. 16-3). In general, apical inflammation from the overinstrumentation resolved by about 2 weeks. At the 1-month postoperative time point, the periodontal ligament tissue was healed. At 10 months, periapical bone was still being formed. The blood clot in the root canal systems gradually was replaced with granulation tissue, and then fibrous connective tissue. However, the growth of this tissue into the root canals was incomplete, and there was also histologic evidence of variable resorption of the dentinal walls along with the deposition of cementum. No newly formed dentin was observed. In his second case series on 47 teeth, histologic evidence of an ingrowth of vascularized fibrous connective tissue was observed in 80% (28 of 35) of teeth with vital pulps but only 8% (1 of 12) of teeth with necrotic pulps.83 Although newly mineralized tissue was evident on some dentinal walls, it appeared to be cementum and not newly formed dentin. This finding of cementum deposition is consistent with recent histologic studies using contemporary revascularization procedures.129 Taken together, most* but not all101 studies of this period suggested that it is possible to create an environment that favors ingrowth of connective tissue into the débrided root canal system that is capable of depositing mineralized tissue on canal walls. However, the extent of tissue ingrowth was limited in most cases to the apical 2 to 3 mm of the root canal system, the mineralized tissue was often described as cementum, and inflammatory elements and/or resorption were also reported. These findings clearly did not replicate the pulp-dentin complex, and research in this area gradually subsided.
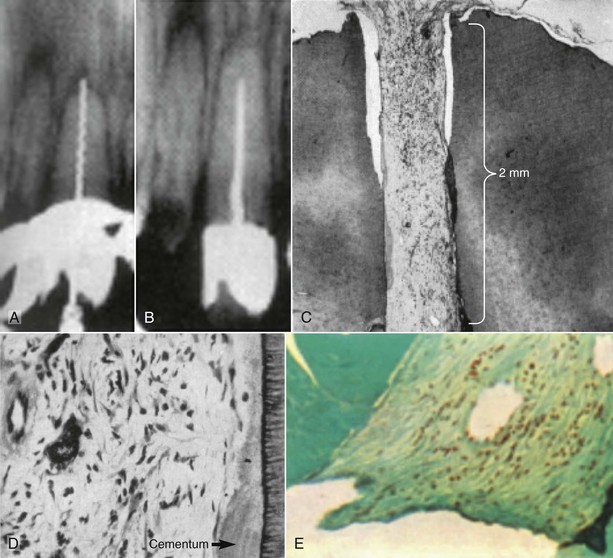
FIG. 16-3 Radiographic and histologic findings from a central incisor with a necrotic pulp, from Nygaard-Østby.82 A, Evidence of a file going beyond the apex. There is also evidence of a radiolucency in the apical area. B, Second radiograph at 14 months, taken shortly before tooth was extracted, showing the short fill. C, Histologic section of same tooth, showing fibrous connective tissue has grown into the apical 2 mm of the tooth. D, Higher magnification (upper right) shows evidence of what appears to be cementum deposition on the canal wall and fibrous connective tissue in the pulp space. E, Evidence of collagen bundles in the canal space.
(From Nygaard-Østby B: The role of the blood clot in endodontic therapy: an experimental histologic study. Acta Odontol Scand 19:323, 1961.)
Clinical Procedures Related to Regenerative Endodontics
Since the report of this original body of research, there have been substantial fundamental advances in our understanding of canal disinfection, coronal microleakage, and the entire field of regenerative medicine. The development of these technologies and a new knowledge base has resulted in a recent surge in the publication of clinical procedures typically applied to immaturely developed (i.e., open apex) permanent teeth with a diagnosis of pulpal necrosis. With these publications has come some controversy regarding the terminology that should be used to describe the clinical procedure and outcomes. There are several procedures designed to treat the incompletely formed root that occur following endodontic procedures. Apexification is defined as a method to induce a calcified barrier in a root with an open apex or the continued apical development of an incompletely formed root in teeth with necrotic pulp tissue.2 This is distinct from revascularization, since apexification does not attempt to regain vital tissue in the canal space. Rather, the outcome of an apexification procedure is establishment of an apical barrier against which an obturating material may be placed. A second term, apexogenesis, is defined as a vital pulp therapy procedure performed to encourage continued physiologic development and formation of the root end.2 An important distinction is that apexogenesis is indicated for teeth in which there has been no loss of vascularity, thus no need to “revascularize” the canal space. Another term used to describe root development is maturogenesis.133 In that the desired outcome of revascularization procedures is to set the stage for physiologic root development, the term that best describes revascularization outcomes.
Revascularization can be broadly defined as the restoration of vascularity to a tissue or organ.5 The use of this term was recently discussed in a letter to the editor of the Journal of Endodontics.53 The authors stated that the term revascularization does not completely address the desired outcomes of regenerative endodontic procedures, because the desired outcome is regeneration of the pulp-dentin complex, rather than just revascularization of the canal space. Further, they suggested that “induced or guided tissue generation and regeneration” would be more appropriate terminology. Others have observed that “Guided tissue regeneration has some merit except that … we do not know what tissue fills the pulp space.”123 Given the lack of clinical and histologic evidence, it is difficult to settle on consistent terminology, and the ongoing development and application of tissue-engineering principles makes this a moving target that is likely to change with development of new clinical procedures. It is important to note that basic research and preclinical studies have identified many of the critical issues for regenerative endodontic procedures, yet case studies published to date continue to use revascularization methods coupled with recent advances in canal disinfection and new materials (e.g., MTA) that minimize the potential for coronal microleakage. Thus, this is a dynamic period of development of this class of procedures.
There have been numerous recent case reports and case series on revascularization of the necrotic pulp space. Investigators and clinicians have used a variety of medicaments to disinfect the canal space. These include a triple antibiotic paste (a 1 : 1 : 1 mixture of ciprofloxacin/metronidazole/minocycline) or variation thereof,14,58,59,92,121 Ca(OH)2 alone or in combination with antibiotics,21,22,59 and formocresol.103
The triple antibiotic paste—because its use has been reported in a number of case reports and case series and because it is less commonly used in dentistry than Ca(OH)2 and formocresol—deserves additional review. The development of the triple antibiotic paste was led in large part by Hoshino and colleagues.48,100 They demonstrated the effectiveness of combinations of antibiotics (and in particular the high efficacy of the combination of ciprofloxacin, metronidazole, and minocycline) in eradicating bacteria from the infected dentin of root canals. Astute practitioners realized that the triple antibiotic paste could be a valuable adjunct for revascularization procedures, since it could be used to create an environment favorable for the ingrowth of vasculature and regenerative cells by reducing or eradicating bacteria in the canal space of teeth with necrotic pulps and incompletely formed apices. The efficacy of the triple antibiotic paste in disinfecting necrotic root canal systems has been demonstrated in a preclinical model.134 In this dog study, 60 teeth were accessed and infected by sealing dental plaque and sterile saline on a cotton pellet into the pulp chamber for 6 weeks. By the end of this period, each premolar was radiographically confirmed to have apical periodontitis. The canals were then sampled at three time points: before and after irrigation with 1.25% NaOCl, and 2 weeks after the delivery of the triple antibiotic paste into the root canal system using a Lentulo spiral. Before irrigation, all of the teeth had positive cultures for anaerobic bacteria, with a mean colony forming unit (CFU) count of 1.7 × 108. After irrigation with 1.25% NaOCl, 10% of the teeth sampled cultured bacteria free. The mean colony-forming unit (CFU) count was 1.4 × 104, or an approximate 10,000-fold reduction in viable bacteria. After dressing with the triple antibiotic paste for 2 weeks, 70% of the teeth sampled cultured bacteria free. The mean CFU count was only 26, which is about another 1000-fold drop in bacteria. This study provides strong support for the effectiveness of the triple antibiotic paste in disinfection of immature teeth with apical periodontitis.
Related dog studies sought to determine how best to create a matrix, or scaffold, onto which stem cells could grow in the canal system.120 Using a protocol similar to that described earlier, immature dog teeth that were infected and confirmed to have developed apical periodontitis were then disinfected with the triple antibiotic paste. At the revascularization step, teeth were randomized into four groups: (1) no further treatment, (2) stimulation of bleeding into the canals, (3) placement of collagen solution in the canals, (4) placement of a mixture of collagen solution and blood. A fifth group of teeth that were not infected were left for natural development. The teeth were radiographically monitored for 3 months, at which time the dogs were sacrificed for histologic examination. In the teeth where nothing was done to create bleeding into the canal, there was evidence of persistent apical radiolucency and a lack of further development in root length and root wall thickness. Histologic sections from these teeth demonstrated persistent apical inflammation and thin root walls. In teeth where a blood clot was formed in the canal system, there was radiographic evidence of resolution of the apical pathosis and an increase in root length and root wall thickness. Histologic sections demonstrated healing of the apical area, including bone and periodontal ligament, ingrowth of tissue into the root canal system, and thickening of the root walls. Findings indicated that there was a trend toward more vital tissue within the canal lumens for roots in which blood clot was formed after disinfection with triple antibiotic paste. In addition, the results suggest the importance of inclusion of a blood clot to improve chances of revascularization. The fibrin polymer of the blood clot likely served as a matrix, or scaffold, for stem cell growth, thus enhancing the likelihood of tissue regeneration. Collectively, these two preclinical studies provide evidence for the efficacy of triple antibiotic paste and suggest the importance of a scaffold in promoting tissue ingrowth.
Prior to reviewing the techniques used for revascularization, it is important to consider the relative advantages and limitations of revascularization compared to other options for treatment of the immature tooth with necrotic pulp (see also Chapter 23). There are several challenges clinicians face when presented with an incompletely formed root in need of endodontic treatment. Because the apex is not fully developed and often has a blunderbuss shape, cleaning and shaping of the apical portion of the root canal system can be difficult. The process is further complicated by the presence of thin, fragile dentinal walls that may be prone to fracture during instrumentation or obturation. In addition, the open apex increases the risk of extruding material into the periradicular tissues. Traditionally an immature tooth with an open apex is treated by apexification, which involves creating an apical barrier to prevent extrusion. In many cases, this entails an involved, long-term treatment with Ca(OH)2, resulting in the formation of a hard-tissue apical barrier.25,26,33,45,115 However, a disadvantage of the traditional apexification procedures is that the short-term98 or long-term6,7 use of Ca(OH)2 can reduce root strength. This finding is consistent with a large case series using the traditional apexification protocol; it showed that a major reason for tooth loss following apexification was root fracture.24 The advent of one-step apexification,23,41 by creation of artificial barriers using materials such as MTA,102,135 has greatly decreased the number of appointments and time to completion. One-step apexification, however, does not generally result in further root development.13,31 A primary advantage of revascularization procedures in these cases is the greater likelihood there will be an increase in root length and root wall thickness.
Overview of Clinical Revascularization Cases
To date, only case reports and case series on endodontic revascularization treatment are available; no randomized controlled clinical trials have been published. Although case series do not provide definitive evidence to support a given treatment modality, they do have the advantage of being conducted in actual patients and thus provide a higher level of evidence than preclinical studies. While techniques for endodontic revascularization have varied in published case reports and case series (Table 16-2 for a summary and Figs. 16-4 to 16-8), there have been some consistent features worth noting. Nearly all reported cases involve patients 8 to 18 years old and teeth with immature apices. Age may be an important issue, since some studies suggest that younger patients have a greater healing capacity or stem cell regenerative potential.3,27,63,69,84 Moreover, the large diameter of the immature (open) apex may foster the ingrowth of tissue into the root canal space and may be indicative of a rich source of mesenchymal stem cells of the apical papilla (SCAP; see Fig. 16-4).56,113,114 These tissues are likely lacerated during the evoked bleeding step and constitute one likely source of mesenchymal stem cells delivered into the root canal space. Another consistent finding reported in nearly every case is the lack of instrumentation of the dentinal walls related to concerns about the potential fracture of these thin, incompletely developed roots. The lack of instrumentation would be expected to have the benefit of avoiding generation of a smear layer that could occlude the dentinal walls or tubules. In addition, NaOCl, alone or in combination with other irrigants, was used to disinfect the canal space. In a majority of cases, a combination of triple antibiotic or NaOCl was left in the canal space for a period of days to weeks, so the disinfection protocol was primarily a chemical method rather than the chemomechanical approach used in conventional nonsurgical endodontic therapy. In most cases, a blood clot formed in the canal. The formation of a blood clot might serve as a protein scaffold, permitting three-dimensional ingrowth of tissue.

FIG. 16-4 A-C, Dissection of an immature permanent tooth indicating the extent of the apical papilla. Note that this structure is likely lacerated during the evoked bleeding step of revascularization cases and thus cells from this structure, including mesenchymal stem cells of the apical papilla (SCAP), are likely to be delivered into the root canal space. Arrow in C denotes junction of apical papilla and dental pulp.
(Courtesy Dr. Michael Henry.)
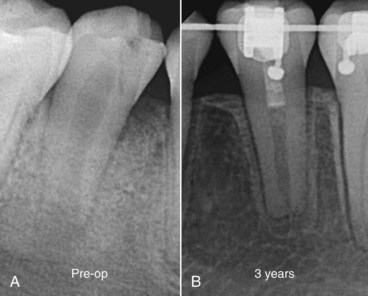
FIG. 16-5 Revascularization case of a 14-year-old female patient with a diagnosis of pulpal necrosis secondary to dens invaginatus. The tooth was isolated, accessed, and irrigated with 2.5% sodium hypochlorite and 2% chlorhexidine, followed by placement of a mixture of ciprofloxacin, metronidazole, and minocycline for 96 days. Upon recall, symptoms had subsided, the tooth was isolated, and the triple antibiotic paste was removed by irrigation. Bleeding was established, and the root canal system was sealed with white mineral trioxide aggregate and a composite restoration.
(Courtesy Dr. Sandra Madison.)
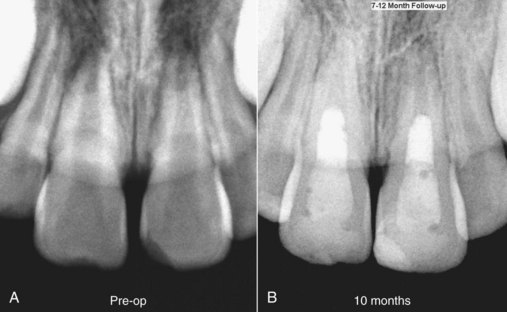
FIG. 16-6 Revascularization case for treating an 8-year-old female patient with a diagnosis of pulpal necrosis secondary to dental trauma (lateral luxation with +2 class fracture of teeth #8 and #9). The teeth were isolated, accessed, and irrigated with 5% sodium hypochlorite and 2% chlorhexidine, followed by placement of a mixture of ciprofloxacin, metronidazole, and minocycline for 21 days. Upon recall, symptoms had subsided, the teeth were isolated, and the triple antibiotic paste was removed by irrigation. Bleeding was established in both teeth, and the root canal systems were sealed with white mineral trioxide aggregate and a composite restoration.
(Courtesy Dr. Alan Law.)
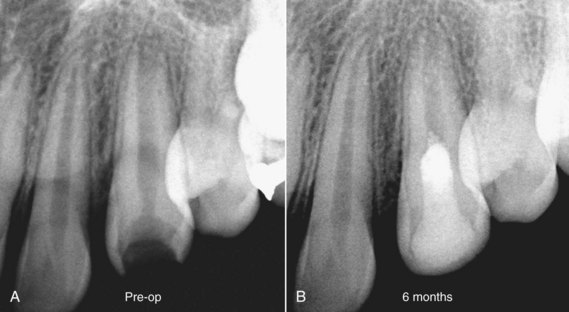
FIG. 16-7 Revascularization case illustrating treatment delivered to a 13-year-old female patient with a diagnosis of pulpal necrosis secondary to caries, with an unspecified prior history of trauma. The tooth was isolated, accessed, and irrigated with 5% sodium hypochlorite and 0.12% chlorhexidine, followed by placement of a mixture of ciprofloxacin, metronidazole, and minocycline for 21 days. Upon recall, the tooth was isolated, and the triple antibiotic paste was removed by irrigation. Bleeding was established in both teeth, and the root canal system was sealed with white mineral trioxide aggregate and a composite restoration.
(Courtesy Dr. Alan Law.)
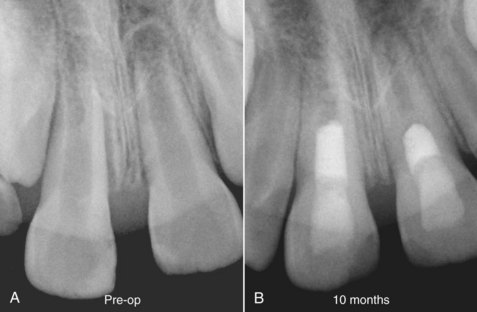
FIG. 16-8 Revascularization case illustrating treatment delivered to a 9-year-old male patient with a diagnosis of pulpal necrosis secondary to trauma, with a class 3 fracture in tooth #8 and a class 2 fracture in tooth #9. The patient reported moderate to severe pain in both teeth. The teeth were isolated, accessed, and irrigated with 5% sodium hypochlorite, followed by placement of a mixture of ciprofloxacin, metronidazole, and minocycline for 55 days. Upon recall, the teeth were isolated, and the triple antibiotic paste was removed by irrigation. Bleeding was established in tooth #9 but not in tooth #8, where CollaCote was placed prior to mineral trioxide aggregate (MTA). The root canal systems were sealed with white MTA and a composite restoration.
(Courtesy Dr. Alan Law.)
Nearly all of these reports noted continued thickening of the root walls and subsequent apical closure. An example of increased root length and thickening of root walls is seen in Fig. 16-6.92 Because of the lack of histology in these clinical cases, it should be recognized that radiographic findings of continued root wall thickness does not necessarily indicate that dentin was formed. Based on histologic results from preclinical studies, it is possible that the radiographic appearance of increased root wall thickness might be due to ingrowth of cementum, bone, or a dentin-like material.* It should also be noted in some of the case reports21,58,59 that although the teeth were nonresponsive to pulp testing, vital tissue was identified in the apical portion of the canal space. In these cases, necrotic tissue was removed until bleeding was observed, then the canals were disinfected with antibiotic paste58,59 or Ca(OH)2.21 One could argue that by leaving vital pulp tissue in the apical segment of the canal, the resulting progression of root formation is more similar to apexogenesis than revascularization. Although the biologic process may differ between the revascularization and apexogenesis cases presented in these case reports, the goals of the procedures are similarly advantageous and significant. In both procedures, there is healing of the periradicular tissues and a progression of root development in a tooth that would otherwise have had a progression of pulpal and periradicular pathosis.
A retrospective study has compared the radiographic changes in 48 revascularization cases to 40 control cases.18 Although published revascularization cases have been treated with varying clinical protocols, they can be grouped by method of canal disinfection—namely, triple antibiotic paste, Ca(OH)2 treatment, or formocresol treatment. This study applied a mathematical image-correction procedure that permitted the comparison of nonstandardized radiographs with subsequent statistical analysis of radiographic outcomes. The percent change in root dimensions was first compared in two negative control groups (nonsurgical root canal treatment [NSRCT] and MTA apexification) predicted to have little to no change in root dimensions. This provides an internal test that the mathematical analysis was appropriate. The results indicate that these two negative control groups had minimal measured changes in root length (Fig. 16-9) or root width (Fig. 16-10), with the anticipated finding that instrumentation with files of greater taper resulted in a slight but detectable loss of apical root width. The results indicated that revascularization treatment with either the triple antibiotic paste or Ca(OH)2 produced significantly greater increases in root length compared with either the MTA or NSRCT control groups. The formocresol group differed only in comparison to the MTA apexification control group. In terms of changes in root width (see Fig. 16-10), treatment with the triple antibiotic paste produced significantly greater increases in dentin wall thickness compared with the MTA and NSRCT control groups. Treatment with Ca(OH)2 or formocresol resulted in significantly greater change in dentinal wall thickness compared with the NSRCT group, but no differences were observed between these medicaments and the MTA apexification group. Finally, the triple antibiotic paste produced significantly greater differences in dentinal wall thickness compared with either the Ca(OH)2 or formocresol groups. In general, the formocresol group showed the smallest improvement in root length and thickness. Secondary analyses indicated that a 12- to 18-month recall is probably the minimal time to judge radiographic evidence of root development, although later time points (36 months) often demonstrate continued root development. Another secondary analysis indicated that the location of Ca(OH)2 placement appeared to be a strong predictor of radiographic outcome. When Ca(OH)2 placement was restricted to the coronal half of the root canal, the increase in root wall thickness was 55%, compared to a 3% increase when it was placed in the apical half of the root canal system. This might be due to residual Ca(OH)2 having a cytotoxic interaction with stem cells. Although prospective randomized clinical trials with standardized radiographic assessment are clearly required, the results of this retrospective study are consistent with a robust outcome of revascularization procedures, particularly when medicated with either a triple antibiotic paste or a Ca(OH)2 medicament. The demonstration of continued root development does not reveal whether this radiopaque material is dentin, cementum, or bone, so given the known reliance of stem cells on an appropriate scaffold and growth factor combination, this will be an important focus for future research efforts.
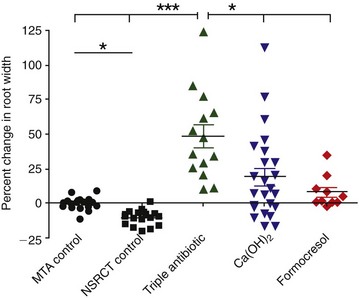
FIG. 16-9 Retrospective analysis of the percentage of change in root length from preoperative image to postoperative image, measured from the cementoenamel junction (CEJ) to the root apex in 40 control patients and in 48 patients following a revascularization procedure. ***P < 0.001 versus mineral trioxide aggregate (MTA) apexification control group (n = 20) and NSRCT control group (n = 20). P < 0.05 versus MTA control group only. Median values for each group are depicted by horizontal line, and individual cases are indicated by the corresponding symbol.
(From Bose R, Nummikoski P, Hargreaves K: A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35:1343, 2009.)
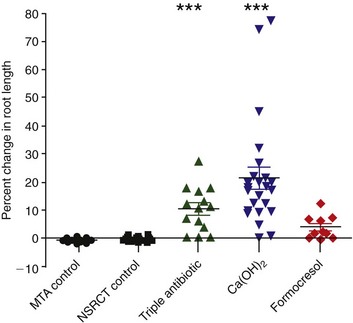
FIG. 16-10 Retrospective analysis of the percentage of change in dentinal wall thickness from preoperative image to postoperative image, measured at the apical third of the root (position of apical third defined in the preoperative image) in 40 control patients and in 48 patients following a revascularization procedure. ***P < 0.001 versus mineral trioxide aggregate (MTA) apexification control group and NSRCT control group. P < 0.05 versus NSRCT control group only. P < 0.05 versus Calcium hydroxide (Ca[OH]2) and formocresol groups. P < 0.05 versus NSRCT control group only.
(From Bose R, Nummikoski P, Hargreaves K: A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35:1343, 2009.)
Example of a Revascularization Protocol
Based on current research, several considerations can be reviewed when considering a protocol for revascularization treatment. The first issue is case selection; the best available evidence indicates that this treatment should be considered for the incompletely developed permanent tooth that has an open apex and is negative to pulpal responsiveness testing. Although the ultimate goal of this approach is to develop a tissue engineering–based method of pulpal regeneration in the fully developed permanent tooth, it should be recognized that current revascularization protocols have not been developed or evaluated for these more challenging cases. Informed consent issues should include the number of appointments (at least two), the possible adverse effects (primarily potential minocycline staining of the crown), the potential lack of response to treatment and alternative treatments, and possible posttreatment symptoms. Clinical staining of the crown and any root structure above the gingival margin appears to be due to the presence of minocycline. This can be minimized by using a delivery system that restricts the drug below the cementoenamel junction (CEJ). When it does occur, it can often be reduced or eliminated by a walking bleach method with sodium perborate. Likely alternative treatments would include MTA apexification, no treatment, or extraction.
At the first appointment (Fig. 16-11, A-E), the treatment alternatives, risks, and potential benefits should be described to the patient and guardian after collecting clinical information and establishing pulpal and periradicular diagnoses. Following informed consent, the tooth is anesthetized, isolated, and accessed. Minimal instrumentation should be accomplished, but the use of a small file to “scout” the root canal system and determine working length is important. If sensation is experienced within the canal system, this may suggest that some residual vital pulp tissue remains.59 The root canal system is copiously and slowly irrigated with 20 ml of NaOCl followed by 20 ml of 0.12% to 2% chlorhexidine (CHX). Since canal disinfection relies considerably on chemical irrigants, it is important to place the needle into the apical third and irrigate using needles with a closed end and side-port vents (e.g., Max-I-Probe needles), together with a slow rate of infusion, to help to reduce any irrigants passing through the open apex. The root canal system is then dried with sterile paper points, and the antimicrobial medicament is delivered into the root canal space. The best available evidence would support the use of either a triple antibiotic paste or Ca(OH)2. Both medicaments have been shown to be effective (see Figs. 16-9 and 16-10). The triple antibiotic paste has the advantage of being a very effective antibiotic combination against odontogenic microorganisms48,100; its efficacy is supported by several case studies.18 This combination is not approved by the U.S. Food and Drug Administration (FDA), however, and carries a potential for minocycline staining of the crown. Alternatively, Ca(OH)2 has the advantage of being widely available and is a commonly used medicament, but it may be cytotoxic to stem cells.14,18,20 After antimicrobial medicament is placed, the tooth is then sealed with a sterile sponge and a temporary filling (e.g., Cavit), and the patient is discharged for 3 to 4 weeks.
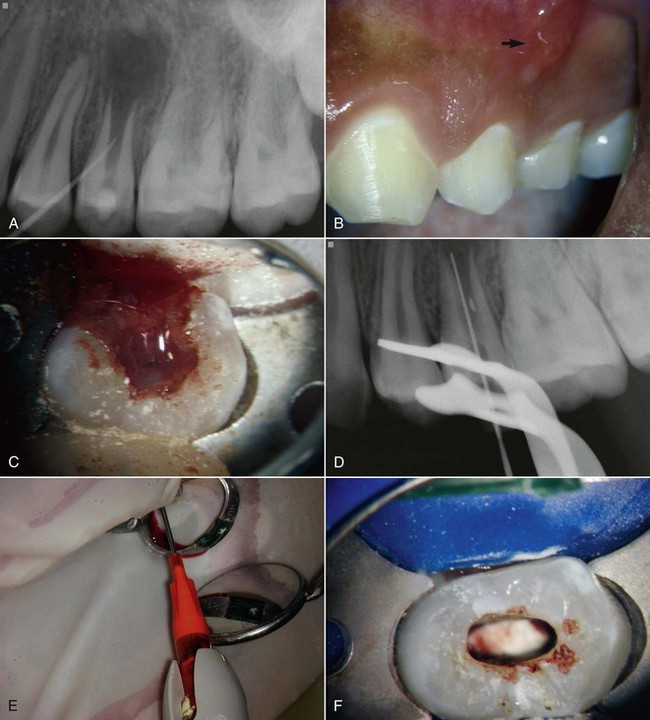
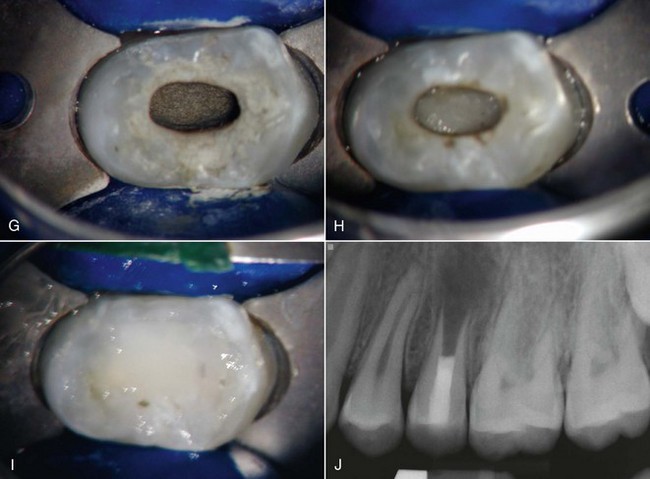
FIG. 16-11 Example of a revascularization protocol. First appointment: Clinical examination revealed a sinus tract (A), soft-tissue swelling that required an incision for drainage (B), and purulent/hemorrhagic exudates upon access (C-D). A working length file was placed into the root canal system, and the tooth was slowly irrigated with 20 ml of 5.25% sodium hypochlorite (NaOCl) and 20 ml of 0.12% chlorhexidine using a Max-I-Probe needle inserted to the apical third. E, The canals were dried and medicated by a triple antibiotic paste (a 1 : 1 : 1 mixture of ciprofloxacin/metronidazole/minocycline) using a Centrix syringe that facilitates delivery below the cementoenamel junction (CEJ). The patient returned 1 month later. The tooth was isolated, accessed, and the medicament removed by slow irrigation with NaOCl. CollaPlug was placed below the CEJ (F) to serve as a matrix to position the mineral trioxide aggregate coronal to the blood clot (G).The tooth was then sealed with a layer of Fuji II (H) and a composite (I-J).
(Courtesy Dr. Anibal Diogenes.)
At the second appointment (see Fig. 16-11, F-J), the patient is evaluated for resolution of any signs or symptoms of an acute infection (e.g., swelling, sinus tract pain, etc.) that may have been present at the first appointment. The antimicrobial treatment is repeated if resolution has not occurred.20,59 In most cases, the acute signs and symptoms have resolved. Since revascularization-induced bleeding will be evoked at this appointment, the tooth should not be anesthetized with a local anesthetic containing a vasoconstrictor. Instead, 3% mepivacaine can be used, which will facilitate the ability to trigger bleeding into the root canal system.93 Following isolation and reestablishment of coronal access, the tooth should be copiously and slowly irrigated with 20 ml NaOCl, possibly together with gentle agitation with a small hand file to remove the antimicrobial medicament. After drying the canal system with sterile paper points, a file is placed a few mm beyond the apical foramen, and the apical tissue is lacerated with bleeding up to 3 mm from the CEJ. A small piece of Colla-Plug may be inserted into the root canal system to serve as a resorbable matrix to restrict the positioning of the MTA. About 3 mm of MTA is then placed, followed by a restoration. A 12- to 18-month recall should be considered as the earliest time point to conduct the clinical examination and evaluate continued radiographic improvement in root development.18
Clinical Measures of Treatment Outcome
The goal of nonsurgical root canal treatment is to maintain or restore the health of periradicular tissues. The goals of revascularization procedures extend beyond those of nonsurgical root canal treatment in that there is an additional objective of regaining vital, functioning tissue capable of supporting continued root development in cases of the immature permanent tooth with a necrotic root canal system. The measures of success for revascularization are not only radiographic evidence of periradicular health but also radiographic and other clinical evidence of functioning vital tissue in the canal space. Radiographic evidence of functioning pulp (or pulplike) tissue would include continued root growth, both in length and wall thickness. These findings have been shown in many published cases. Other measures of the presence of vital, functioning tissue in the canal space include laser Doppler blood flowmetry117; pulp testing involving heat, cold, and electricity91; and lack of signs or symptoms. The ideal clinical outcome is an asymptomatic tooth that does not require retreatment, but to validate that regenerative endodontic techniques are truly effective, nonsubjective vitality-assessment methods are essential.
It is worth noting that the published revascularization cases have shown increased root wall thickness that is limited to the midroot and apical root. There has been no demonstration of increased root thickness in the cervical area, an area shown to be prone to fracture in immature teeth with a history of trauma and subsequent endodontic treatment.24 Future clinical studies should focus on extending revascularization into the cervical area, potentially strengthening this area and decreasing the risk of root fracture.
Summary
The field of regenerative endodontics is rapidly advancing. It is based upon the principles of tissue engineering, namely the spatial delivery of appropriate cells, scaffolds, and growth factors. Similar to most rapidly developing fields, the preclinical area of research has outpaced translational clinical studies. Preclinical studies have identified several mesenchymal stem cell sources capable of differentiating into odontoblast-like cells, as well as candidate scaffolds and growth factors capable of guiding this development. The initial preclinical animal studies indicate that an approach using human stem cells implanted into a human root in an immunocompromised mouse provides a useful model for evaluating combinations of stem cells/scaffolds/growth factors for regenerating the pulp-dentin complex. It is likely that this combined approach of in vitro and in vivo preclinical research will greatly advance our understanding of the conditions necessary to regenerate a functional pulp-dentin complex.
To date, none of the published clinical studies fully engage the concepts of tissue engineering. Instead, these studies are best described as revascularization procedures that attempt to regenerate biologic tissues within the root canal space, without necessarily replicating the pulp-dentin complex. Discussion about the requirements of an appropriate scaffold and growth factor was begun in the 1960s by Nygaard-Østby,82 who had no access to our contemporary instruments, materials, and the entire knowledge base of tissue engineering. Although clinical revascularization procedures do not constitute the ideal regenerative treatment, it is important to note that they do generate a scaffold (fibrin) and growth factors (from platelets and access to proteins embedded in the dentinal walls), and the clinical outcome results in continued radiographic root development of the immature permanent tooth with a diagnosis of pulpal necrosis. Thus, a revascularization procedure provides a very real treatment of high value in cases with an otherwise poor prognosis. Histologic analyses of revascularization procedures conducted in patients82,83 or animals78,96,129 suggests that the increase in root dimensions is often due to deposition of cementum-like material, dentin, or bone.
Future clinical research will likely focus on translating basic research findings into improved regenerative procedures. For example, the formation of a cementum-like material on the dentinal walls may lead to studies evaluating benefits of revascularization procedures for overall tooth resistance to fracture. In addition, it is clear that the multipotent nature of many mesenchymal stem cell types could contribute to the finding of cementum deposition. Controlled differentiation into odontoblasts is an important area of research and amenable to tissue-engineering concepts. The development of delivery systems that permit structural reinforcement of the cervical area (or ideally, the pulp chamber) might provide clinical opportunities to regenerate lost tooth structure, thereby permitting natural teeth to be retained instead of possible fracture or extraction. Finally, the ultimate and long-term goal for regenerative endodontic procedures should be to treat the fully formed permanent tooth. Although this situation is more complex than the immature tooth with an open apex and a ready source of stem cells, it provides the unique potential of saving the natural dentition while restoring the sensory, immunologic, and defensive properties of the pulp-dentin complex.
1. Almushayt A, Narayanan K, Zaki AE, George A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006;13:611.
2. American Association of Endodontists. Glossary of Endodontic Terms, ed 7. Chicago, IL: American Association of Endodontists; 2003.
3. Amler MH. The age factor in human extraction wound healing. J Oral Surg. 1977;35:193.
4. Ando Y, Honda MJ, Ohshima H, Tonomura A, Ohara T, Itaya T, et al. The induction of dentin bridge-like structures by constructs of subcultured dental pulp-derived cells and porous HA/TCP in porcine teeth. Nagoya J Med Sci. 2009;71:51.
5. Andreasen JO, Andreasen FM, Andersson L. Textbook and color atlas of traumatic injuries to the teeth, ed 4. Hoboken, NJ: Wiley-Blackwell; 2007.
6. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134.
7. Andreasen JO, Munksgaard EC, Bakland LK. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent Traumatol. 2006;22:154.
8. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4.
9. Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227.
10. Anneroth G, Bang G. The effect of allogeneic demineralized dentin as a pulp capping agent in Java monkeys. Odontol Revy. 1972;23:315.
11. Arany S, Koyota S, Sugiyama T. Nerve growth factor promotes differentiation of odontoblast-like cells. J Cell Biochem. 2009;106:539.
12. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301.
13. Ballesio I, Marchetti E, Mummolo S, Marzo G. Radiographic appearance of apical closure in apexification: follow-up after 7-13 years. Eur J Paediatr Dent. 2006;7:29.
14. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196.
15. Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35:321.
16. Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219.
17. Block MS, Cervini D, Chang A, Gottsegen GB. Anterior maxillary advancement using tooth-supported distraction osteogenesis. J Oral Maxillofac Surg. 1995;53:561.
18. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009;35:1343.
19. Chen S, Gluhak-Heinrich J, Martinez M, Li T, Wu Y, Chuang HH, et al. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J Biol Chem. 2008;283:19359.
20. Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35:160.
21. Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205.
22. Cotti E, Mereu M, Lusso D. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: report of a case. J Endod. 2008;34:611.
23. Coviello J, Brilliant JD. A preliminary clinical study on the use of tricalcium phosphate as an apical barrier. J Endod. 1979;5:6.
24. Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45.
25. Cvek M. Treatment of non-vital permanent incisors with calcium hydroxide. I. Follow-up of periapical repair and apical closure of immature roots. Odontol Revy. 1972;23:27.
26. Cvek M, Sundstrom B. Treatment of non-vital permanent incisors with calcium hydroxide. V. Histologic appearance of roentgenographically demonstrable apical closure of immature roots. Odontol Revy. 1974;25:379.
27. D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115.
28. Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res. 2002;43:387.
29. Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523.
30. Erausqun J, Muruzabalm J. Evaluation of blood clot after root canal treatment. J Dent Res. 1968;47:34.
31. Felippe WT, Felippe MC, Rocha MJ. The effect of mineral trioxide aggregate on the apexification and periapical healing of teeth with incomplete root formation. Int Endod J. 2006;39:2.
32. Fitzgerald M, Chiego DJJr, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707.
33. Frank AL. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc. 1966;72:87.
34. Fujimura K, Bessho K, Kusumoto K, Ogawa Y, Iizuka T. Experimental studies on bone inducing activity of composites of a telopeptide type I collagen as a carrier for ectopic osteoinduction by rhBMP-2. Biochem Biophys Res Commun. 1995;208:316.
35. Fujiwara S, Kumabe S, Iwai Y. Isolated rat dental pulp cell culture and transplantation with an alginate scaffold. Okajimas Folia Anat Jpn. 2006;83:15.
36. Goncalves SB, Dong Z, Bramante CM, Holland GR, Smith AJ, Nor JE. Tooth slice-based models for the study of human dental pulp angiogenesis. J Endod. 2007;33:811.
37. Gould TR. Ultrastructural characteristics of progenitor cell populations in the periodontal ligament. J Dent Res. 1983;62:873.
38. Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials. 2006;27:2865.
39. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531.
40. Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708.
41. Harbert H. One-step apexification without calcium hydroxide. J Endod. 1996;22:690.
42. Hargreaves KM, Giesler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34:S51.
43. He H, Yu J, Liu Y, Lu S, Liu H, Shi J, et al. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int. 2008;32:827.
44. Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705.
45. Heithersay GS. Stimulation of root formation in incompletely developed pulpless teeth. Oral Surg Oral Med Oral Pathol. 1970;29:620.
46. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177.
47. Hermann BW. [On the reaction of the dental pulp to vital amputation and calxyl capping.]. Dtsch Zahnarztl Z. 1952;7:1446.
48. Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125.
49. Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069.
50. Huang AH, Chen YK, Chan AW, Shieh TY, Lin LM. Isolation and characterization of human dental pulp stem/stromal cells from nonextracted crown-fractured teeth requiring root canal therapy. J Endod. 2009;35:673.
51. Huang G, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan R, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2009 Sep 8. [Epub ahead of print]
52. Huang GT. Pulp and dentin tissue engineering and regeneration: current progress. Regen Med. 2009;4:697.
53. Huang GT, Lin LM. Letter to the editor: comments on the use of the term “revascularization” to describe root regeneration. J Endod. 2008;34:511. author reply 511
54. Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066.
55. Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225.
56. Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645.
57. Ito K, Yamada Y, Nagasaka T, Baba S, Ueda M. Osteogenic potential of injectable tissue-engineered bone: a comparison among autogenous bone, bone substitute (Bio-oss), platelet-rich plasma, and tissue-engineered bone with respect to their mechanical properties and histological findings. J Biomed Mater Res A. 2005;73:63.
58. Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185.
59. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008;34:876.
60. Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71:1654.
61. Kvinnsland I, Heyeraas KJ. Dentin and osteodentin matrix formation in apicoectomized replanted incisors in cats. Acta Odontol Scand. 1989;47:41.
62. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920.
63. Lei L, Liao W, Sheng P, Fu M, He A, Huang G. Biological character of human adipose-derived adult stem cells and influence of donor age on cell replication in culture. Sci China C Life Sci. 2007;50:320.
64. Liu J, Jin T, Chang S, Ritchie HH, Smith AJ, Clarkson BH. Matrix and TGF-beta-related gene expression during human dental pulp stem cell (DPSC) mineralization. In Vitro Cell Dev Biol Anim. 2007;43:120.
65. Matsumiya S, Kitamura M. Histo-pathological and histo-bacteriological studies of the relation between the conditions of sterilization of the interior of the root canal and the healing process of the periapical tissues in experimentally infected root canal treatment. Bull Tokyo dent Coll. 1960;1:1.
66. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807.
67. Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155.
68. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377.
69. Murray PE, Stanley HR, Matthews JB, Sloan AJ, Smith AJ. Age-related odontometric changes of human teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:474.
70. Nakahara T. A review of new developments in tissue engineering therapy for periodontitis. Dent Clin North Am. 2006;50:265.
71. Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227.
72. Nakashima M. Dentin induction by implants of autolyzed antigen-extracted allogeneic dentin on amputated pulps of dogs. Endod Dent Traumatol. 1989;5:279.
73. Nakashima M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch Oral Biol. 1994;39:1085.
74. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod. 2005;31:711.
75. Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, et al. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11). Hum Gene Ther. 2004;15:1045.
76. Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025.
77. Nasstrom K, Forsberg B, Petersson A, Westesson PL. Narrowing of the dental pulp chamber in patients with renal diseases. Oral Surg Oral Med Oral Pathol. 1985;59:242.
78. Nevins A, Finkelstein F, Laporta R, Borden BG. Induction of hard tissue into pulpless open-apex teeth using collagen-calcium phosphate gel. J Endod. 1978;4:76.
79. Nevins A, Wrobel W, Valachovic R, Finkelstein F. Hard tissue induction into pulpless open-apex teeth using collagen-calcium phosphate gel. J Endod. 1977;3:431.
80. Nevins AJ, Finkelstein F, Borden BG, Laporta R. Revitalization of pulpless open apex teeth in rhesus monkeys, using collagen-calcium phosphate gel. J Endod. 1976;2:159.
81. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14:249.
82. Nygaard-Østby B. The role of the blood clot in endodontic therapy: an experimental histologic study. Acta Odontol Scand. 1961;19:323.
83. Nygaard-Østby B. Tissue formation in the root canal following pulp removal. Scand J Dent Res. 1971;79:333.
84. O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95.
85. Ogino Y, Ayukawa Y, Kukita T, Koyano K. The contribution of platelet-derived growth factor, transforming growth factor-beta1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:724.
86. Oh SL, Fouad AF, Park SH. Treatment strategy for guided tissue regeneration in combined endodontic-periodontal lesions: case report and review. J Endod. 2009;35:1331.
87. Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518.
88. Okamoto Y, Sonoyama W, Ono M, Akiyama K, Fujisawa T, Oshima M, et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod. 2009;35:367.
89. Paakkonen V, Bleicher F, Carrouel F, Vuoristo JT, Salo T, Wappler I, et al. General expression profiles of human native odontoblasts and pulp-derived cultured odontoblast-like cells are similar but reveal differential neuropeptide expression levels. Arch Oral Biol. 2009;54:55.
90. Paakkonen V, Vuoristo JT, Salo T, Tjaderhane L. Comparative gene expression profile analysis between native human odontoblasts and pulp tissue. Int Endod J. 2008;41:117.
91. Petersson K, Soderstrom C, Kiani-Anaraki M, Levy G. Evaluation of the ability of thermal and electrical tests to register pulp vitality. Endod Dent Traumatol. 1999;15:127.
92. Petrino JA. Revascularization of necrotic pulp of immature teeth with apical periodontitis. Northwest Dent. 2007;86:33.
93. Petrino JA, Boda K, Shambarger S, Bowles W, McClanahan S. Challenges in regenerative endodontics: a case series. J Endod. 35, 2010. (in press)
94. Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH. Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res. 2008;19:539.
95. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247.
96. Ritter AL, Ritter AV, Murrah V, Sigurdsson A, Trope M. Pulp revascularization of replanted immature dog teeth after treatment with minocycline and doxycycline assessed by laser Doppler flowmetry, radiography, and histology. Dent Traumatol. 2004;20:75.
97. Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013.
98. Rosenberg B, Murray PE, Namerow K. The effect of calcium hydroxide root filling on dentin fracture strength. Dent Traumatol. 2007;23:26.
99. Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205.
100. Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118.
101. Seale NS, Glickman GN. Contemporary perspectives on vital pulp therapy: views from the endodontists and pediatric dentists. Pediatr Dent. 2008;30:261.
102. Shabahang S, Torabinejad M. Treatment of teeth with open apices using mineral trioxide aggregate. Pract Periodontics Aesthet Dent. 2000;12:315.
103. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogenesis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008;34:919.
104. Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148.
105. Sheppard PR, Burich RL. Effects of extra-oral exposure and multiple avulsions on revascularization of reimplanted teeth in dogs. J Dent Res. 1980;59:140.
106. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696.
107. Skoglund A, Tronstad L. Pulpal changes in replanted and autotransplanted immature teeth of dogs. J Endod. 1981;7:309.
108. Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000;45:173.
109. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151.
110. Sloan AJ, Smith AJ. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1-3 in vitro. Arch Oral Biol. 1999;44:149.
111. Smith AJ, Tobias RS, Murray PE. Transdentinal stimulation of reactionary dentinogenesis in ferrets by dentine matrix components. J Dent. 2001;29:341.
112. Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale. 1990;18:123.
113. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79.
114. Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166.
115. Steiner JC, Dow PR, Cathey GM. Inducing root end closure of nonvital permanent teeth. J Dent Child. 1968;35:47.
116. Stramburg T. Wound healing after total pulpectomy in dogs. Odont Revy. 1969;20:147.
117. Strobl H, Gojer G, Norer B, Emshoff R. Assessing revascularization of avulsed permanent maxillary incisors by laser Doppler flowmetry. J Am Dent Assoc. 2003;134:1597.
118. Takayama S, Murakami S, Shimabukuro Y, Kitamura M, Okada H. Periodontal regeneration by FGF-2 (bFGF) in primate models. J Dent Res. 2001;80:2075.
119. Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647.
120. Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680.
121. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007;29:47.
122. Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent. 2007;35:636.
123. Trope M. Reply J Endod. 2008:511.
124. Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38:314.
125. Tziafas D, Alvanou A, Panagiotakopoulos N, Smith AJ, Lesot H, Komnenou A, et al. Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo implantation of dentine matrix components. Arch Oral Biol. 1995;40:883.
126. Tziafas D, Belibasakis G, Veis A, Papadimitriou S. Dentin regeneration in vital pulp therapy: design principles. Adv Dent Res. 2001;15:96.
127. Tziafas D, Kolokuris I, Alvanou A, Kaidoglou K. Short-term dentinogenic response of dog dental pulp tissue after its induction by demineralized or native dentine, or predentine. Arch Oral Biol. 1992;37:119.
128. Vacatello M, D’Auria G, Falcigno L, Dettin M, Gambaretto R, Di Bello C, et al. Conformational analysis of heparin binding peptides. Biomaterials. 2005;26:3207.
129. Wang X, Thibodeau B, Trope M, Lin L, Huang G. Histological characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod. 36, 2010. DOI: 10.1016/j.joen.2009.09.039
130. Wang XY, Zhang Q, Chen Z. A possible role of LIM mineralization protein 1 in tertiary dentinogenesis of dental caries treatment. Med Hypotheses. 2007;69:584.
131. Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33:703.
132. Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: Curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184.
133. Weisleder R, Benitez CR. Maturogenesis: is it a new concept? J Endod. 2003;29:776.
134. Windley W3rd, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439.
135. Witherspoon DE, Ham K. One-visit apexification: technique for inducing root-end barrier formation in apical closures. Pract Proced Aesthet Dent. 2001;13:455.
136. Wysocki GP, Daley TD, Ulan RA. Predentin changes in patients with chronic renal failure. Oral Surg Oral Med Oral Pathol. 1983;56:167.
137. Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955.
138. Yamamura T. Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res. 1985;64(Special issue):530.
139. Yang X, van der Kraan PM, Bian Z, Fan M, Walboomers XF, Jansen JA. Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res. 2009;88:1020.
140. Yang X, Yang F, Walboomers XF, Bian Z, Fan M, Jansen JA. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2009 Jun 25. [Epub ahead of print].
141. Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dent Clin North Am. 2006;50:191.
142. Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695.
143. Zhang W, Walboomers XF, Wolke JG, Bian Z, Fan MW, Jansen JA. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005;11:357.
144. Zhao S, Sloan AJ, Murray PE, Lumley PJ, Smith AJ. Ultrastructural localisation of TGF beta exposure in dentine by chemical treatment. Histochem J. 2000;32:489.