CHAPTER 12 Structure and Functions of the Dentin-Pulp Complex
From many perspectives, the pulp is a unique tissue. It is a soft tissue of mesenchymal origin with specialized cells, the odontoblasts, arranged peripherally in direct contact with dentin matrix. The close relationship between odontoblasts and dentin, sometimes referred to as the dentin-pulp complex,115,278,342 is one of several reasons dentin and pulp should be considered as a functional entity made up of histologically distinct elements. In particular, several unique properties are imposed on the pulp by the rigid mineralized dentin in which it is enclosed. It is situated within a low-compliance environment that limits its ability to expand during episodes of vasodilation and increased filtration. Because the pulp is relatively incompressible, the total volume within the pulp chamber cannot be greatly increased (although reciprocal volume changes can occur among arterioles, venules, lymphatics, and extravascular tissue). As a consequence, inflammatory reactions result in an increase in tissue pressure due to a limited increase in fluid volume.141,143 In the pulp, therefore, careful regulation of blood flow is of critical importance.
The dental pulp is in many ways similar to other connective tissues of the body, but its special characteristics deserve serious consideration. Even the mature pulp bears a resemblance to embryonic connective tissue and is therefore a relatively rich source of stem cells (see also Chapter 16). The pulp houses a number of tissue elements, including axons, vascular tissue, connective tissue fibers, ground substance, interstitial fluid, odontoblasts, fibroblasts, immunocompetent cells, and other cellular components. These components respond dynamically to developmental, physiologic (e.g., orthodontic or masticatory forces) or pathologic stimuli. The overall dynamic response pattern plays a key role in whether pulp tissue adapts or undergoes tissue necrosis to these stimuli (see also Chapter 13).
The pulp is supported by a microcirculatory system, and its largest vascular components are arterioles and venules. Unlike most tissues, the pulp lacks a true collateral system and is dependent on relatively few arterioles entering through the apical foramina. The vascular system of the pulp decreases progressively with age.24
The tooth pulp is a unique sensory organ. Because it is encased in a protective layer of dentin, which in turn is covered with enamel, it might be expected to be quite unresponsive to stimulation. However, despite the low thermal conductivity of dentin, the pulp is undeniably sensitive to thermal stimuli, such as ice cream and hot drinks. The unusual mechanism that allows the dentin-pulp complex115,278,342 to function as an exquisitely responsive sensory system is discussed later in the chapter.
After tooth development, the pulp retains its ability to form dentin throughout life. This enables the vital pulp to partially compensate for the loss of enamel or dentin caused by mechanical trauma or disease. How well it serves this function depends on many factors, but the potential for regeneration and repair is as much a reality in the pulp as in other connective tissues of the body.
This chapter brings together what is known about the development, structure, and function of the dentin-pulp complex in the hope that this knowledge will provide a firm biologic basis for clinical decision making.
Development
Embryologic studies have shown that the pulp is derived from the cephalic neural crest. Neural crest cells arise from the ectoderm along the lateral margins of the neural plate and migrate extensively. Those that travel down the sides of the head into the maxilla and mandible contribute to the formation of the tooth germs. The dental papilla, from which the mature pulp arises, starts to develop as ectomesenchymal cells proliferate and condense adjacent to the dental lamina epithelium (Fig. 12-1). It is important to remember this migratory potential of ectomesenchymal cells later in the chapter when reviewing the ability of pulp cells to move into areas of injury and replace destroyed odontoblasts.
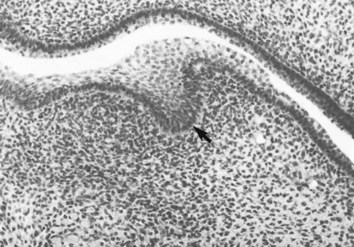
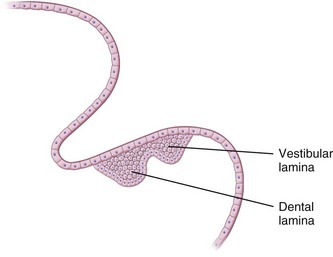
During the sixth week of embryonic life, tooth formation begins as a localized thickening of ectoderm associated with the maxillary and mandibular processes. This activity results in the formation of two horseshoe-shaped structures, one on each process. These structures are termed the primary dental laminae. Each primary dental lamina splits into a vestibular and a dental lamina (Fig. 12-2).
Numerous studies have indicated that the embryonic development of any tissue is promoted by interaction with an adjacent tissue. The complex epithelial-mesenchymal interactions during tooth development have been studied extensively. Cell-to-cell and cell-to-extracellular matrix (ECM) interactions direct the differentiation of ameloblasts and odontoblasts by causing these cells to change gene expression.
Growth factors, often termed signaling molecules, are polypeptides that promote proliferation, migration, and differentiation of a variety of cells. It can be assumed that growth factors are involved in signaling during those epithelial-mesenchymal interactions that regulate tooth initiation, morphogenesis, and cell differentiation.294,298-300,319-321
From the onset of tooth formation, a dental basement membrane (DBM) exists between the inner dental epithelium and the dental mesenchyme. The DBM consists of a thin basal lamina, which is formed by the epithelial cells, and a layer of ECM derived from the dental mesenchyme. The basal lamina is made up of an elastic network composed of type IV collagen, which has binding sites for other basement membrane constituents such as laminin, fibronectin, and heparan sulfate proteoglycans. Laminin binds to type IV collagen and also to receptors on the surface of preameloblasts and ameloblasts. The DBM also contains mesenchyme-derived type I and type III collagen, hyaluronate, heparan sulfate, and chondroitin 4- and 6-sulfates. Certain proteoglycans expressed on the cell surface of the odontoblast function as receptors for these matrix molecules. This permits signals from components of the matrix to influence the migration and differentiation of odontoblasts. The composition of the DBM changes during tooth development, and these alterations appear to modulate the successive steps in odontogenesis. With the differentiation of odontoblasts, type III collagen disappears from the predentin matrix, and fibronectin, which surrounds preodontoblasts, is restricted to the apical pole of mature odontoblasts.196
The initial stage of development of the dental papilla is characterized by proliferative activity beneath the dental lamina at sites corresponding to the positions of the prospective primary teeth. Even before the dental lamina begins to form the enamel organ, a capillary vascular network develops within the ectomesenchyme, presumably to support the increased metabolic activity of the developing tooth buds.
Stages of Development
Although formation of the tooth is a continuous process, as a matter of convenience tooth morphogenesis has been divided into three stages: (1) bud, (2) cap, and (3) bell (Fig. 12-3).186 In the bud stage the epithelial cells of the dental lamina proliferate and produce a budlike projection into the adjacent ectomesenchyme. The cap stage is reached when the cells of the dental lamina have proliferated to form a concavity with a caplike appearance. The outer cells of the cap are cuboidal and constitute the outer enamel epithelium. The cells on the inner or concave aspect of the cap are somewhat elongated and represent the inner enamel epithelium. Between the outer and inner epithelia is a network of cells termed the stellate reticulum because of the branched reticular arrangement of the cellular elements. The rim of the enamel organ (i.e., where the outer and inner enamel epithelia are joined) is termed the cervical loop. As the cells forming the loop continue to proliferate, there is further invagination of the enamel organ into the mesenchyme. During this time, the organ assumes a bell shape, and tooth development then enters the bell stage (Fig. 12-4). During the bell stage, the ectomesenchyme of the dental papilla becomes partially enclosed by the invaginating epithelium.
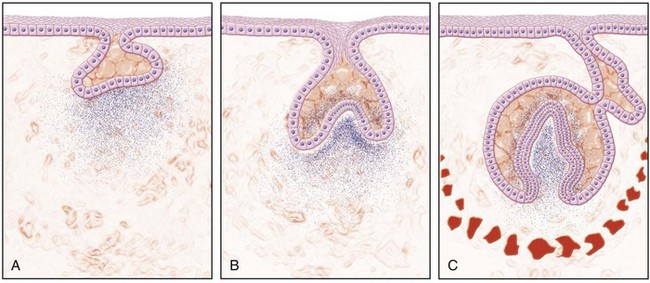
FIG. 12-3 Diagrammatic representation of (A), the bud, (B), the cap, and (C) the bell stages of tooth development.
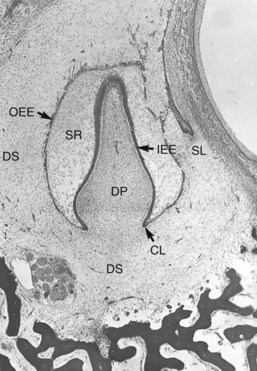
FIG. 12-4 Bell stage of tooth development showing the outer enamel epithelium (OEE), stellate reticulum (SR), inner enamel epithelium (IEE), dental papilla (DP), cervical loop (CL), successional lamina (SL), and dental sac (DS).
The condensed ectomesenchyme surrounding the enamel organ and dental papilla complex forms the dental sac and ultimately develops into the periodontal ligament (see Fig. 12-4). During the bell stage, the primary dental lamina degenerates and is invaded and replaced by mesenchymal tissue. In this way the epithelial connection between the enamel organ and the oral epithelium is severed. The free end of the dental lamina associated with each of the primary teeth continues to grow and forms the successional (secondary) dental lamina. It is from this structure that the tooth germ of the succedaneous tooth arises. As the maxillary and mandibular processes increase in size, the permanent first molar arises from posterior extensions of the dental lamina. After birth, the second and third molar primordia appear as the dental laminae proliferate into the underlying mesenchyme.
The morphogenesis (shape) of the crown is controlled by specialized signaling centers called epithelial enamel knots (EK). The enamel knots are transient clusters of nonproliferative epithelial cells located in the dental epithelium.159,215,353 They express members of molecules belonging to key conserved fibroblast growth factor (Fgf), bone morphogenetic protein (Bmp), Hedgehog (Hh), and Wnt families that are well known to be essential for embryo and organ formation.332 The signaling activity of the enamel knot and expressed molecules such as Fgf4 and Bmp4 regulate cellular processes of the surrounding epithelial and mesenchymal cells, leading to altered proliferation and expression of genes essential for tooth formation, such as transcription factors Mxs1 and Pax9.172,354 The first appearing primary enamel knot (PEK) is present at the tip of the epithelial bud at the bud stage.159 At the cap stage, the PEK is located in the middle part of the anterior-posterior axis of the enamel organ.159,353 The secondary enamel knots (SEK), which appear later at the site of cusp tips, regulate the formation and number of the cusps of the multicusped teeth.159 The recently found tertiary enamel knot (TEK) in turn prevents enamel formation by the underlying ameloblasts and thus controls the formation of the enamel-free areas in animals having these areas, such as mice.215
Development of the Tooth Nerve Supply
Peripheral innervation serves significant roles in pulp biology. The initial innervation of the tooth originates from the sensory trigeminal ganglia. The nerve fibers innervate specific sites in the adult tooth, and as a consequence, the development of dental innervation is not a random event but instead is a strictly spatiotemporally controlled process that is tightly linked to the advancing tooth morphogenesis and dental cell differentiation173,209 (Fig. 12-5). In the well-analyzed mouse mandibular molar, the molar nerve209 from the inferior alveolar nerve branch reaches the tooth at the early bud stage. The nerve follows a narrow mesenchymal pathway under the tooth germ, where it divides into the buccal and lingual branches.173,209,243 During the cap and bell stages, nerve fibers establish a nerve plexus beneath the primary apical foramina. Nerve fibers wait here before they are allowed to enter the dental papilla. This takes place only after the crown shape is being defined by both dentin and enamel appearance in the crown.237,238 The nerve fibers enter the papilla at both the mesial and distal ends, which are the sites of the future mesial and distal root of the molar tooth germ. The nerve fibers innervate the subodontoblastic region, the coronal odontoblast layer, and predentin layer with some fibers terminating in the dentin. The nerve fibers of the nerve plexus also contribute to the innervation of the periodontium.146 Pioneer sympathetic innervation of the dental pulp follows the sensory one.237
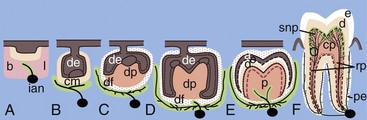
FIG. 12-5 Growth of the sensory trigeminal axons to the dental target areas during tooth formation. The schematic illustration is based on studies on the localization of nerve fibers in the developing mouse molar (A-F).173,237,238 Pioneer dental axons follow a restricted mesenchymal pathway (marked green in B) and grow around the developing tooth germ, termed the mesenchymal dental follicle target field (green in C-F). Nerve fibers penetrate into the dental pulp (p) right after the onset of enamel formation (E) and innervate the major pulp-dentin border target area (marked green in F). a, ameloblasts; b, buccal; cm, condensed dental mesenchyme; cp, coronal pulp; d, dentin; de, dental epithelium; df, dental follicle; dm, dental mesenchyme; dp, dental papilla; e, enamel; ian, inferior alveolar nerve; l, lingual; o, odontobaster; p, dental pulp; pe, periodontium; rp, radicular pulp; snp, subodontoblastic nerve plexus; nerve fibers are marked in black.
(Modified from Kenntunen P, et al: Regularing av utvikling av tannens form og sensorisk nerveforsying er samordent. Nor Tannlaegeforen Tid 119:162–166, 2009.)
Molecular Control of Trigeminal Axon Navigation
There is increasing evidence that the navigation of the growing dental axons involves concerted, redundant signaling activity by molecules of different families. The molecules exerting positive attractive effect on axon guidance or survival are expressed in the mesenchymal pathway or target areas around and within the tooth germ.214 The molecules include neurotrophic factors, which promote survival, differentiation, and maintenance of neurons in developing and adult vertebrate nervous systems. NGF (nerve growth factor), which is essential for dental pulp nerve supply, and GDNF (glial cell line-derived neurotrophic factor) are expressed in the major target area of the developing tooth, namely the dental follicle and pulp-dentin border target areas. In addition, many other molecular families, such as laminins, neuregulin/ErbB, Ephs and Ncam are implicated in the development of the tooth nerve supply.100,214
Repulsive axon guidance molecules in turn, are produced in the areas next to the axon pathways. They control axon navigation by preventing nerve fibers from entering areas that should be avoided. The secreted chemorepellant, semaphorin3A (Sema3A), which is expressed in mesenchymal restriction areas,173 controls dental trigeminal axon fasciculation as well as the timing and patterning of tooth innervation.173 In addition, Sema3A appears to control the site of the nerve fiber penetration to the dental pulp (i.e., through the future roots).216 Because Sema3A expression in mesenchymal restriction areas is controlled by signaling from the dental epithelium, this indicates that tooth innervation development, like the formation of the tooth germ proper, is controlled by local tissue interactions. Of note, Fgfr2b, which is essential to tooth morphogenesis by regulating epithelial proliferation and function of the enamel knot, also controls Sema3A expression and therefore dental axon navigation. Thus, Fgf2b acts as a molecule that integrates tooth morphogenesis and dental trigeminal axon patterning.197
Differentiation of Odontoblasts
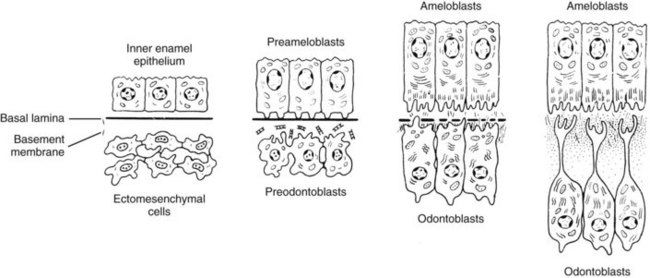
Differentiation of epithelial and mesenchymal cells into ameloblasts and odontoblasts, respectively, occurs during the bell stage of tooth development. This differentiation starts in the region where the cusp tip will develop and proceeds towards the cervical loop. The preameloblasts lining the dental papilla induce differentiation of odontoblasts. Consequently, odontoblast-secreted dentin matrix induces enamel secretion by ameloblasts.
During the bell stage of development, there is still mitotic activity among the relatively immature cells of the inner enamel epithelium in the region of the cervical loop. As they commence to mature into ameloblasts, mitotic activity ceases, and the cells elongate and display the characteristics of active protein synthesis (i.e., an abundance of rough endoplasmic reticulum [RER], a well-developed Golgi complex, and numerous mitochondria).
As the ameloblasts undergo differentiation, changes are taking place across the basement membrane in the adjacent dental papilla because of a coordinated exchange of growth factors and other soluble mediators. Before differentiation of odontoblasts, the dental papilla consists of sparsely distributed polymorphic mesenchymal stem cells with wide intercellular spaces (Fig. 12-6). With the onset of differentiation, a single layer of cells, the presumptive odontoblasts (i.e., preodontoblasts) align themselves along the basement membrane separating the inner enamel epithelium from the dental papilla. These cells stop dividing and elongate into short columnar cells with basally situated nuclei (Fig. 12-7). Several cytoplasmic projections from each of these cells extend toward the basal lamina. At this stage, the preodontoblasts are still relatively undifferentiated.
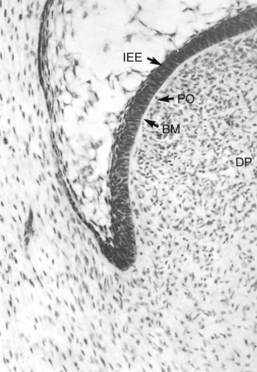
FIG. 12-6 Bell stage shows preodontoblasts (PO) aligned along the basement membrane (BM) separating the inner enamel epithelium (IEE) from the dental papilla (DP).
As the odontoblasts continue to differentiate, they become progressively more elongated and take on the ultrastructural characteristics of protein-secreting cells. Cytoplasmic processes from these cells extend through the DBM toward the basal lamina, and more and more collagen fibrils appear within the ECM. The first formed collagen fibers pass between the preodontoblasts and extend toward the basal lamina to form large, fan-shaped bundles 100 to 200 nm in diameter, often referred to as von Korff fibers. These fibers stain with silver stains and are associated with a high content of proteoglycans. Some smaller collagen fibers, approximately 50 nm in diameter, also pass between the odontoblasts and are thought to arise in the dental papilla subjacent to the odontoblasts.29
A study on collagen gene expression during rat molar tooth development found both types I and III collagen messenger ribonucleic acid (mRNA) in developing odontoblasts.75 Levels of type I collagen mRNA increased with the progression of odontoblast differentiation, whereas type III collagen gene expression decreased as dentinogenesis proceeded. Both type I and type III collagen mRNA were detected in dental pulp mesenchymal cells.
Dentinogenesis first occurs in the developing tooth at sites where the cusp tips or incisal edge will be formed. It is in this region that odontoblasts first reach full maturity and become tall columnar cells, at times attaining a height of about 50 µm (see Fig. 12-7). The width of these cells remains fairly constant at approximately 7 µm. Production of the initial dentin matrix involves the formation, organization, and maturation of collagen fibrils and proteoglycans. As predentin is formed, the odontoblasts commence to move toward the central pulp, depositing predentin at a rate of approximately 4 to 8 µm per day in their wake during early tooth development. Within predentin, a process from each odontoblast becomes accentuated and remains to form the odontoblast process. The dentinal tubules are formed around these cellular processes. Similar to tooth initiation and morphogenesis, dentinogenesis is controlled by epithelial-mesenchymal interactions that are mediated by a combination of EMC and signaling molecules of different families such as BMP and Tgf-β1.197
Root Development
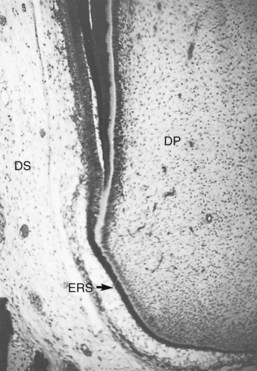
Root development commences after the completion of enamel formation, when the cervical loop does not exist. The stellate reticulum and stratum intermedium cells disappear, and the inner and outer enamel epithelium in the distal part of the cervical loop forms Hertwig’s epithelial root sheath (HERS), composed of two cell layers, the inner and outer enamel epithelium (Fig. 12-8). HERS determines the size and shape of the root or roots of the tooth. As in the formation of the crown, the cells of the inner enamel epithelium induce the adjacent mesenchymal stem cells in the pulp to differentiate into preodontoblasts and odontoblasts. As soon as the layer of dentin matrix mineralizes, gaps appear within the HERS-derived Malassez epithelium, allowing mesenchymal cells from the dental sac to move into contact with the newly formed dentin. These mesenchymal stem cells thereafter differentiate into cementoblasts and deposit cementum matrix on the root dentin. Some cells of the Malassez epithelium persist within the periodontal ligament and are known as the epithelial cell rests of Malassez. These clusters of epithelial cells surrounded by basement membrane appear as a meshlike network around the root. Although the number of these rests gradually decreases with age, it has been shown that at least some of them retain the ability to undergo cell division347 (Fig. 12-9). In later life, if a chronic inflammatory lesion develops within the periapical tissues as a result of pulp disease, proliferation of the epithelial rests may produce a periapical (radicular) cyst.
Accessory Canals in the Root
Occasionally during formation of the root sheath, a break develops in the continuity of the sheath, producing a small gap. When this occurs, dentinogenesis does not take place adjacent to the defect. The result is a small “accessory” canal between the dental sac and the pulp. An accessory canal can become established anywhere along the root, creating a periodontal-endodontic pathway of communication and a possible portal of entry into the pulp if the periodontal tissues lose their integrity. In periodontal disease, the development of a periodontal pocket may expose an accessory canal and allow microorganisms or their metabolic products to gain access to the pulp. Conversely, in the case of teeth with infected, necrotic pulps, the inability to cleanse, shape, and obturate these canals could result in periodontitis (see also Chapter 18).
Dentin
Fully mature dentin is composed of approximately 70% inorganic material, 20% organic material, and 10% water on a weight basis. The main inorganic component of dentin is calcium hydroxyapatite, Ca10(PO4)6(OH)2.204 Organic matrix consists of proteins, the most common of which is type I collagen; there is a minor component of type V collagen.43 The most common noncollagenous proteins include dentin phosphoprotein (DPP), dentin matrix protein 1 (DMP1), dentin sialoprotein (DSP), osteopontin (OPN), osteocalcin, and bone sialoprotein (BSP).43 In addition, dentin contains proteoglycans, small amounts of phospholipids, and numerous growth factors, including bone morphogenetic proteins (BMP), insulin-like growth factors (IGFs), and transforming growth factor beta (TGF-β). These growth factors have significance during demineralization of dentin since they may locally stimulate subsequent stem cell differentiation (see later and Chapter 16). The elasticity of dentin provides flexibility for the overlying brittle enamel and allows the impact of mastication to occur without fracturing enamel.183,184,369 From an engineering perspective, the collagen can be thought of as steel rebar, and the mineralized crystals act as cement; the resulting composite structure has great tensile strength, particularly given its small size. Additionally, the enamel-dentin junction (EDJ) between dentin and enamel is an uneven scalloped border that increases contact and adherence between dentin and enamel, keeping the two hard tissues together during mastication.
Primary, Secondary, and Tertiary Dentin
Primary dentin is produced during tooth development until the teeth have erupted into the oral cavity. It is classified as orthodentin, the tubular form of dentin lacking of cells found in the teeth of all dentate mammals.204 The primary dentin is secreted at a relatively high rate and constitutes the major part of the dentin in the tooth. It is regular in structure and contains dentin tubules that form an S-shaped primary curvature as a result of the directional movement of odontoblasts, which have a cell process that extends from a broad peripheral border towards a narrow, centrally located cell layer. After tooth eruption, the odontoblasts continue to lay down dentin but slightly change their direction, which contributes to bending of the dentinal tubules. It is referred to as secondary dentin and is synthesized at a much lower rate and is less regular in structure than primary dentin. Secondary dentin is deposited during the rest of the tooth’s life. Unlike primary and secondary dentin, tertiary dentin, or reparative or reactionary dentin, is deposited as a result of a pathologic process such caries or occlusal abrasion. Tertiary dentin has been suggested to be secreted by original odontoblasts or in case of their death, by newly differentiated replacement odontoblasts originating from nearby mesenchymal stem cells. The function of the tertiary dentin is to protect the pulp from noxious influences. Tertiary dentin is disorganized in structure compared to primary and secondary dentin.
Mantle Dentin
The first layer of the primary dentin to be deposited is mantle dentin. It is produced by odontoblasts that are not yet fully differentiated. In the adult tooth, mantle dentin is the oldest dentin and is produced adjacent to the enamel in the crown. Odontoblasts first produce the collagen components, which are organized in a collagen fibril network. Electron microscopic analyses have shown that odontoblasts produce small membrane-closed matrix vesicles where precipitation of minerals starts.33 After the rupture of the matrix vesicles, the crystals spread within the collagen matrix and are assumed to act as starting points for the growth of the apatite crystals. In addition to the crown, mantle dentin is also found in the root between cementum and Tomes granular layer. However, this is a controversial finding, and opposing data suggest that a hyaline layer or enameloid is found in the root instead of mantle dentin.
Later during the mineralization of the circumpulpal dentin, the matrix vesicles are no longer detected.205 Mantle dentin can be recognized by the characteristic thick, fan-shaped collagen fibers deposited immediately subjacent to the basal lamina in histologic sections. These fibers run roughly perpendicular to the DEJ. Spaces between the fibers are occupied by smaller collagen fibrils lying more or less parallel with the DEJ or CDJ. Mantle dentin is only 150 µm thick and is slightly less mineralized (about 4% less mineral) than underlying dentin139 and is therefore softer.369 The mineralization of the mantle dentin may differ from mineralization of the rest of the dentin additionally, since dentinphosphoprotein (DPP), which regulates crystal growth in the circumpulpal dentin, does not appear to be present in mantle dentin.246
Circumpulpal Dentin
Circumpulpal dentin is formed after the layer of mantle dentin has been deposited, and it constitutes the major part of primary and secondary dentin. The odontoblasts first deposit the organic matrix, which is composed mainly of collagen fibrils approximately 500 nm in diameter, oriented at right angles to the long axis of the dentinal tubules. These collagen fibrils are closely packed together and form an interwoven network that will be embedded by the subsequent mineral crystals. Calcium is actively transported from the blood vessels by the odontoblasts into the collagen fibril template/network. There is evidence that mineralization of the dentin is under control of dentinphosphoprotein (DPP). DPP is able to bind calcium, since it is highly anionic. Conformational changes of DPP enable it to bind large numbers of calcium ions, which allows the formation and growth of mineral crystals in the collagen matrix network. By regulating the release and concentration of DPP, the odontoblasts may regulate the initiation and speed of dentin mineralization. Hydroxyapatite crystals are deposited on the surface and within the fibrils and continue to grow as mineralization proceeds, resulting in an increased mineral content of the dentin. The circumpulpal dentin is mineralized through calcospherites in the mineralization front between predentin and mineralizing dentin. The mineral crystals are organized in a radiating pattern in calcospherites, the size of which may vary in size and shape in different regions of dentin. As the calcospherites enlarge, they fuse with adjacent calcospherites until the dentin matrix is completely mineralized.
Predentin
Predentin is a 15- to 20-µm unmineralized organic matrix layer of dentin situated between the odontoblast layer and the mineralized dentin.204 Its prime protein constituents include type I and type II collagens.42 Noncollagenous elements consist of several proteoglycans (i.e., dermatan sulfate, heparan sulfate, hyaluronate, keratin sulfate, chondroitin 4-sulfate, chondroitin 6-sulfate),40,117 glycoproteins, glycosaminoglycans (GAGs),83 Gla proteins, and dentin phosphoprotein (DPP)42 DPP is a highly phosphorylated protein that is exclusively found in bone and dentin.290 In dentin, DPP is produced by the odontoblasts and transported to the mineralization front. It is thought to bind to calcium and play a role in mineralization.109,359 Growth factors such as TGF-β, insulin-like growth factor, platelet-derived growth factor, and angiogenic growth factor have also been identified in dentin.294 The presence of growth factors in dentin may have profound clinical significance because resorption of dentin conceivably releases these growth factors into pulp, where they alter biologic functions of this tissue. Dentinal resorption may stimulate formation of tertiary dentin.
Dentinal Tubules
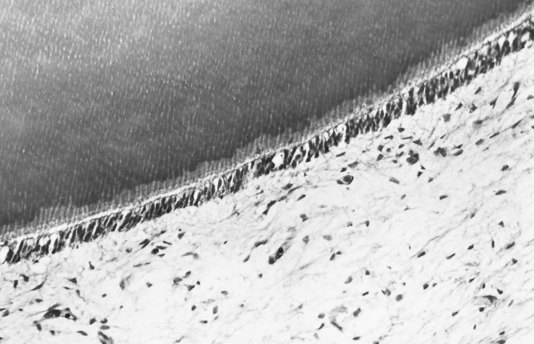
A characteristic of human dentin is the presence of tubules that occupy from 1% (superficial dentin) to 30% (deep dentin) of the volume of intact dentin.107,277 The diameter of tubules vary from 1 µ to 2.5 µm and traverse the entire thickness of dentin from the DEJ or CDJ to the pulp. They are slightly tapered, with the wider portion situated toward the pulp. This tapering is the result of the progressive formation of up to 1 µm of peritubular dentin, which leads to a continuous decrease in the diameter of the lumen of the tubules to a final diameter of about 1 mm.107 No peritubular dentin is present when the tubules reach the predentin and pulp chamber.
In coronal dentin, the tubules have a gentle S shape as they extend from the DEJ to the pulp. The S-shaped curvature is presumably a result of the crowding of odontoblasts as they migrate toward the center of the pulp. As they approach the pulp, the tubules converge because the surface of the pulp chamber has a much smaller area than the surface of dentin along the DEJ. This results in a progressive increase in dentin permeability (discussed later).275-277279
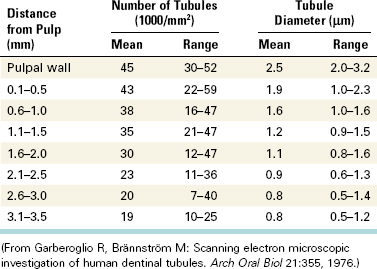
The number and diameter of the tubules has been determined and varies depending on the location in the tooth (Table 12-1).107 The number and diameter of dentinal tubules are similar among rats, cats, dogs, monkeys, and humans, demonstrating a remarkable consistency in mammalian orthodentin.4
TABLE 12-1 Mean Number and Diameter per Square Millimeter of Dentinal Tubules at Various Distances from the Pulp in Human Teeth
Lateral microtubules containing branches of the main odontoblastic processes have been demonstrated by other researchers,167,236 who suggested that they form pathways for the movement of materials between the main processes and the more distant matrix. It is also possible that the direction of the branches influences the orientation of the collagen fibrils in the intertubular dentin.
Near the DEJ, the dentinal tubules ramify into one or more terminal branches236 (Fig. 12-10). This occurs because during the initial stage of dentinogenesis, the differentiating odontoblasts extended several small cytoplasmic processes toward the DEJ, but as the odontoblasts migrated, their processes converged into one major process (see Fig. 12-7).
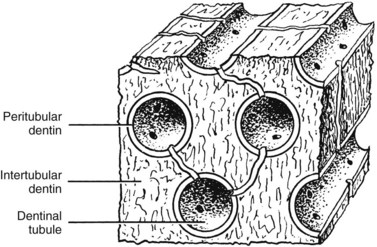
Intertubular Dentin
Intertubular dentin is located between the dentin tubules and constitutes the bulk of dentin (Fig. 12-11). Its organic matrix consists mainly of collagen fibrils having diameters of 50 to 100 nm. These fibrils are oriented approximately at right angles to the dentinal tubules. They are well mineralized and provide tensile strength to dentin.184 Intertubular dentin is found between the DEJ and the mineralization front.204
Intratubular Dentin
Dentin lining the inner walls of tubules is termed intratubular or peritubular dentin (see Fig. 12-11). Intratubular dentin represents a specialized form of orthodentin not found in all mammals. The matrix of peritubular dentin differs from that of intertubular dentin by having relatively fewer collagen fibrils and a higher proportion of sulfated proteoglycans and mineral. Because of its lower content of collagen,183,374 peritubular dentin is harder than intertubular dentin and therefore is more quickly dissolved in acid than intertubular dentin. By preferentially removing peritubular dentin, acid-etching agents used during dental restorative procedures and ethylenediaminetetraacetic acid (EDTA) used in endodontic treatment enlarge the openings of the dentinal tubules, making the dentin more permeable. The absence of intrafibrillar mineral in patients with dentinogenesis imperfecta type II may be responsible for their softer dentin.185
Dentinal Sclerosis
Partial or complete obturation of dentinal tubules may occur as a result of aging or develop in response to persistent stimuli such as attrition of the tooth surface or dental caries. When tubules become filled with mineral deposits, the dentin is termed sclerotic. Dentinal sclerosis is easily recognized in histologic ground sections because of its translucency (due to homogeneity of the dentin). Studies using dyes, solvents, and radioactive ions have shown that sclerosis results in decreased permeability of dentin,92,276,328 so by limiting the diffusion of noxious substances through the dentin, dentinal sclerosis helps shield the pulp from irritation.
One form of dentinal sclerosis is thought to represent an acceleration of intratubular dentin formation. This form appears to be a physiologic process, and in the apical third of the root it develops as a function of age.323,328 Dentinal tubules can also become blocked by the precipitation of hydroxyapatite and whitlockite crystals within the tubules. This type occurs in the translucent zone of carious dentin and in attrited dentin and has been termed pathologic sclerosis.263,328,371
Interglobular Dentin
Interglobular dentin refers to dentin that contains organic matrix that is unmineralized because the growing calcospherites failed to coalesce. This occurs most often in the circumpulpal dentin close to the mantle dentin in the crown and close to the Tomes granular layer in the root. In certain dental anomalies (e.g., vitamin D–resistant rickets, hypophosphatasia), large areas of interglobular dentin are a characteristic feature (Fig. 12-12).108
Dentinal Fluid
Free fluid occupies 1% of superficial dentin but about 22% of the total volume of deep dentin (i.e., near the pulp).277 This fluid is an ultrafiltrate of blood from the pulp capillaries, and its composition not surprisingly resembles plasma in many respects.61 Using calcium ion–specific electrodes, the ionized calcium concentration of dentinal fluid in predentin was two to three times higher than plasma.212 Although it contains plasma proteins, their concentration is only about 10% that of plasma.219 The outward fluid flows between the odontoblasts through the dentinal tubules and is blocked peripherally by enamel on the crown and cementum on the root. It has been shown that the tissue pressure of the pulp is approximately 14 cm H2O (10.3 mm Hg).67 Consequently, a pressure gradient exists between the pulp and the oral cavity that accounts for the slow outward flow of fluid if any dentin becomes exposed. Exposure of the tubules by tooth fracture or during cavity preparation often results in the outward movement of fluid to the exposed dentin surface in the form of tiny droplets.157 Dehydrating the surface of the dentin with compressed air, dry heat, or the application of absorbent paper can accelerate this outward movement of fluid.37-39274 Rapid flow of fluid through the tubules is thought to be a cause of dentin sensitivity.230 The slow outward movement of dentinal fluid is not sufficient to activate the mechanoreceptor nerves responsible for dentin sensitivity. This slow outward fluid flow, about 0.02 ml/sec/mm2, must increase to 1 to 1.5 ml/sec/mm2 before these nerves begin to discharge.230
Bacterial products or other contaminants may be introduced into the dentinal fluid as a result of dental caries, restorative procedures, or growth of bacterial biofilms beneath restorations.37,234,284 Dentinal fluid may thus serve as a sink from which injurious agents can diffuse into the pulp, producing an inflammatory response. Conversely, dentinal fluid may serve as a vehicle for the egress of bacteria from a necrotic pulp into the periradicular tissue. This has particular significance in the apical portion of the root canal system in the development or maintenance of apical periodontitis because bacteria are the key etiologic factor (see Chapter 15). Fortunately, the density of dentinal tubules is much lower in the apical portion of the root compared to the cervical portion, and this anatomic feature may contribute to the success of nonsurgical root canal treatment. In addition, endodontic surgical techniques have been modified to reduce exposure of dentinal tubules during apical root resection by reducing the angle of root resection; as the angle of resection approaches 90 degrees to the long axis of the root, a corresponding decrease is seen in dentinal tubules exposed to the periapical tissue (see Chapter 21 for details).
Dentin Permeability
The permeability of dentin has been well characterized.92,97,274-277,279,282 Dentinal tubules are the major channels for diffusion of material across dentin. Because fluid permeation is proportional to tubule diameter and number, dentin permeability increases as the tubules converge on the pulp (Fig. 12-13). The total tubular surface near the DEJ is approximately 1% of the total surface area of dentin,274,277 whereas close to the pulp chamber, the total tubular surface may be nearly 45%. From a clinical standpoint, it should be recognized that dentin beneath a deep cavity preparation is much more permeable than dentin underlying a shallow cavity when the formation of sclerotic or reparative dentin is negligible.
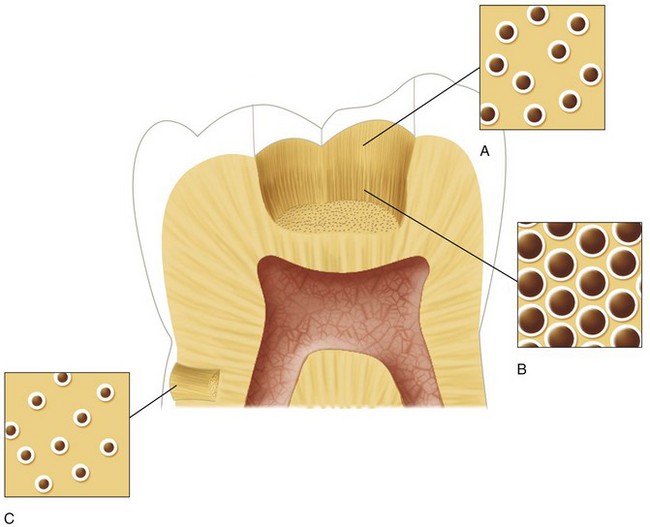
FIG. 12-13 Diagram illustrating the difference in size and density of tubules in the dentinal floor between a shallow (A) and a deep (B) cavity preparation, in coronal dentin versus (C) root dentin.
(Redrawn from Trowbridge HO: Dentin today: research reveals new information about the complexity of dentin. Dentistry 22:22–29, 1982.)
One study97 found that the permeability of radicular dentin is much lower than that of coronal dentin. This was attributed to a decrease in the density of the dentinal tubules from approximately 42,000/mm2 in cervical dentin to about 8000/mm2 in radicular dentin. These investigators found that fluid movement through outer radicular dentin was only approximately 2% that of coronal dentin. The low permeability of outer radicular dentin should make it relatively impermeable to toxic substances such as bacterial products emanating from plaque.
Factors modifying dentin permeability include the presence of odontoblast processes in the tubules and the sheathlike lamina limitans that lines the tubules. Collagen fibers have also been observed in most tubules.71 The functional or physiologic diameter of the tubules is only about 5% to 10% of the actual anatomic diameter (i.e., the diameter seen in microscopic sections).233 This is small enough to trap and remove bacteria from dentinal fluid,234 permitting sterile fluid to enter the pulp chamber.
In dental caries, an inflammatory reaction develops in the pulp long before the pulp actually becomes infected with micororganisms.345 This indicates that bacterial byproducts reach the pulp in advance of the bacteria themselves,19,284 and the early inflammatory response is elicited by the accumulation of bacterial antigens or byproducts into the pulp, not by the bacteria themselves. Dentinal sclerosis (i.e., reparative dentin formation) beneath a carious lesion reduces permeability328 by obstructing the tubules, thus decreasing the delivery of irritants into the pulp.
The cutting of dentin during cavity preparation or root canal therapy produces a microcrystalline debris that coats the dentin and clogs the orifices of the dentinal tubules. This layer of debris is termed the smear layer. Because of the small size of the particles, the smear layer is capable of physically preventing bacteria from penetrating dentin.234,244 Removal of the debris by acid etching or EDTA greatly increases the permeability of the dentin by widening the orifices of the tubules. Consequently, the incidence of pulpal inflammation may be increased significantly if cavities are treated with an acid cleanser unless a cavity liner, base, or dentin-bonding agent is used. Continued pulpal protection will then depend on the durability of the bond.
When the dentin surface of vital and nonvital teeth was exposed to the oral environment for 150 days, bacterial invasion of dentinal tubules occurred more rapidly in the nonvital teeth.244 Presumably this was due to the resistance offered by the outward movement of dentinal fluid and the presence of odontoblast processes in the tubules of vital teeth.280,361,362 Importantly, it has been shown that antibodies or other antimicrobial components are present within the dentinal fluid of teeth with vital pulps.125
Mechanical Properties of Root Dentin
Endodontic therapy has long been criticized for “weakening” teeth, especially roots, possibly making them more prone to fracture. Although some investigators have proposed that endodontically treated root dentin was more “brittle” than normal vital teeth,80 subsequent punch shear tests of endodontically treated teeth showed only a 14% reduction in dentin toughness compared with vital dentin.57 The controversy began when the moisture content of pulpless dog teeth was reported to be 9% less than that of vital controls.137 However, endodontic therapy was shown not to change the Vickers hardness of human root dentin.199 Much of the increased incidence of coronal fractures of endodontically treated teeth can be attributed to the loss of tooth structure produced by preparing adequate access openings.152 Similarly, root fractures in endodontically treated teeth are in part due to physical weakening of roots by creation of post spaces.123,156 In addition, fatigue life studies have shown that dentin from old patients has a much shorter fatigue life than dentin from young teeth.15 The perceived increased incidence of root fractures in endodontically treated teeth may actually reflect a slow, spontaneous reduction in the fracture toughness of old dentin,11 regardless of root canal treatment.
Any occlusal stress applied to the coronal dentin is magnified in the root because it has a smaller volume and cross-sectional area. Collagen in the dentin matrix does not turn over metabolically, so it is never repaired and may accumulate microcracks over decades of function. When collagen is secreted into the forming dentin matrix, a number of noncollagenous proteins are secreted at the same time, some of which bind to collagen. For instance, dentin sialoprotein (DSP),42,116 dentin phosphophoryn (DPP),42,70,75 and matrix metalloproteinases (MMPs)336 are all known to bind to collagen. MMPs such as gelatinase A have been extracted from dentin in their active form.225 More recently, both collagenolytic activity and gelatinolytic activity have been measured in mineralized dentin powder.281 These enzymes may slowly attack the collagenous matrix of dentin, thereby weakening it over many decades. The mineralized dentin surrounding post spaces in roots that had been under function for more than 10 years was shown to be disrupted and fragmented.89 Functional stresses may cause microcracking of dentin in a manner similar to that seen in cortical bone.358 Fatiguing of roots over decades226,247,248 may be responsible for the fractured roots in endodontically treated teeth in older patients. Fatigue life experiments show that old coronal dentin tolerates fewer loading cycles before fracture (Fig. 12-14) than young dentin.15 If this is confirmed in root dentin, and if the mechanism or mechanisms responsible for this increased susceptibility to fatiguing can be identified, then effective interventions may be developed to increase the fatigue life of roots that have been endodontically treated.
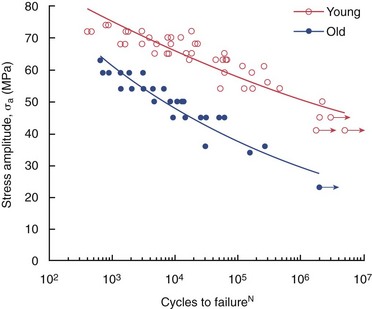
FIG. 12-14 The fatigue life diagram for cyclic loading of coronal dentin using 4-point flexure. The young dentin (average age = 25 years) and old dentin (average age = 62 years) were obtained from fully erupted second and third molars. Data points with arrows indicate specimens that did not fail.
(Courtesy Dr. Dwayne Arola, University of Maryland.)
Morphologic Zones of the Pulp
The Pulp-Dentin Complex
The dental pulp and dentin function as a unit, and the odontoblasts represent a crucial element in this system. The odontoblasts are located in the periphery of the pulp tissue, with extensions into the inner part of dentin. Dentin would not exist unless produced by odontoblasts, and the dental pulp is dependent on the protection provided by the dentin and enamel. Likewise, integrated dynamics of the pulp-dentin complex imply that impacts on dentin may affect the pulpal components, and that disturbances in the dental pulp will in turn affect the quantity and quality of the dentin produced.
Odontoblast Layer
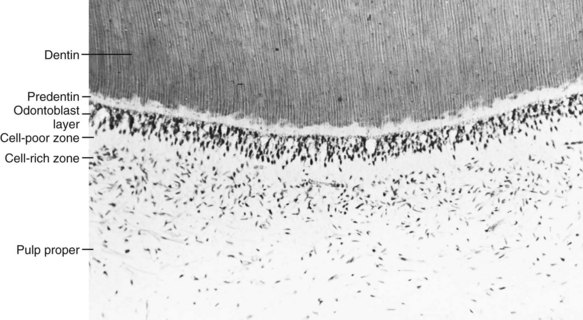
The outermost stratum of cells of the healthy pulp is the odontoblast layer (Figs. 12-15 and 12-16). This layer is located immediately subjacent to the predentin. The odontoblast processes, however, pass on through the predentin into the inner part of dentin. Consequently, the odontoblast layer is actually composed of the cell bodies of odontoblasts. In addition, capillaries, nerve fibers, and dendritic cells may be found among the odontoblasts.
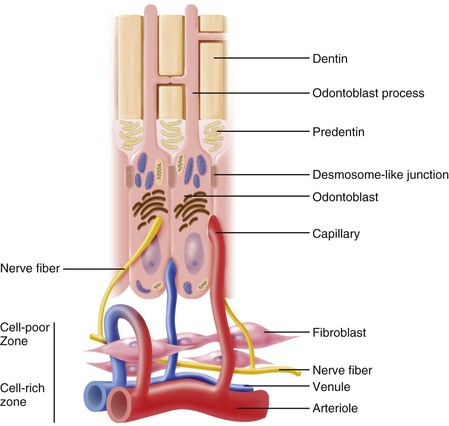
FIG. 12-16 Diagrammatic representation of the odontoblast layer and subodontoblastic region of the pulp.
In the coronal portion of a young pulp that is actively secreting collagen, the odontoblasts assume a tall columnar form.63 The odontoblasts vary in height; consequently, their nuclei are not all at the same level and are aligned in a staggered array, often described as a palisade appearance. This organization makes the layers appear to be three to five cells in thickness even though there is only one actual layer of odontoblasts. Between adjacent odontoblasts there are small intercellular spaces approximately 30 to 40 nm in width. Odontoblast cell bodies are connected by tight and gap junctional complexes.30,63,149 Gap junctions are formed by connexin proteins110 that permit cell-to-cell passage of signal molecules.
The odontoblast layer in the coronal pulp contains more cells per unit area than in the radicular pulp.224 Whereas the odontoblasts of the mature coronal pulp are usually columnar, those in the midportion of the radicular pulp are more cuboidal (Fig. 12-17).63 Near the apical foramen, the odontoblasts appear as a squamous layer of flattened cells. Because fewer dentinal tubules per unit area are present in the root than in the crown of the tooth, the odontoblast cell bodies are less crowded and are able to spread out laterally.224 During maturation and aging, there is a continued ongoing crowding in the odontoblast layer, particularly in the coronal pulp, due to narrowing of the pulp space. Apoptosis of odontoblasts seems to adjust for this limited space during development.235
There are a series of specialized cell-to-cell junctions (i.e., junctional complexes), including desmosomes (i.e., zonula adherens), gap junctions (i.e., nexuses), and tight junctions (i.e., zonula occludens) that connect adjacent odontoblasts. Spot desmosomes located in the apical part of odontoblast cell bodies mechanically join odontoblasts together. The numerous gap junctions provide permeable pathways through which signal molecules can pass between cells (Fig. 12-18) to synchronize secretory activity that produces relatively uniform predentin layers (see Fig. 12-16). These junctions are most numerous during the formation of primary dentin. Gap junctions and desmosomes have also been observed joining odontoblasts to the processes of fibroblasts in the subodontoblastic area. Tight junctions are found mainly in the apical part of odontoblasts in young teeth. These structures consist of linear ridges and grooves that close off the intercellular space. However, tracer studies suggest direct passage of small elements from subodontoblastic capillaries to predentin and dentin between the odontoblasts.330 It appears that tight junctions determine the permeability of the odontoblast layer when dentin is covered by enamel or cementum by restricting the passage of molecules, ions, and fluid between the extracellular compartments of the pulp and predentin.30 During cavity preparation, these junctions are disrupted, thereby increasing dentin permeability.350,351
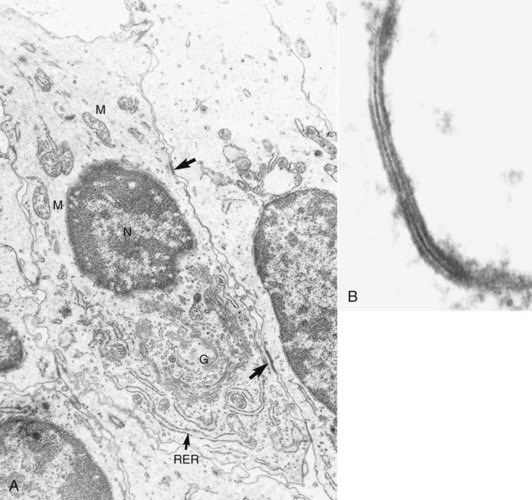
FIG. 12-18 A, Electron micrograph of a mouse molar odontoblast demonstrating gap junctions (arrows), nucleus (N), mitochondria (M), Golgi complex (G), and rough endoplasmic reticulum (RER). B, High magnification of a section fixed and stained with lanthanum nitrate to demonstrate a typical gap junction.
(Courtesy Dr. Charles F. Cox, School of Dentistry, University of Alabama.)
Cell-Poor Zone
Immediately subjacent to the odontoblast layer in the coronal pulp, there is often a narrow zone approximately 40 µm in width that is relatively free of cells (see Fig. 12-15) and hence is called the cell-free layer of Weil. It is traversed by blood capillaries, unmyelinated nerve fibers, and the slender cytoplasmic processes of fibroblasts (see Fig. 12-16). The presence or absence of the cell-poor zone depends on the functional status of the pulp.63 It may not be apparent in young pulps, where dentin forms rapidly, or in older pulps, where reparative dentin is being produced.
Cell-Rich Zone
In the subodontoblastic area, there is a stratum containing a relatively high proportion of fibroblasts compared with the more central region of the pulp (see Fig. 12-15). It is much more prominent in the coronal pulp than in the radicular pulp. Besides fibroblasts, the cell-rich zone may include a variable number of immune cells like macrophages and dendritic cells, but also undifferentiated mesenchymal stem cells.
On the basis of evidence obtained in rat molar teeth, it has been suggested119 that the cell-rich zone forms as a result of peripheral migration of cells populating the central regions of the pulp, commencing at about the time of tooth eruption. Migration of immunocompetent cells out of and into the cell-rich zone has been demonstrated as a result of antigenic challenge.388 Although cell division within the cell-rich zone is rare in normal pulps, the death of the odontoblasts triggers a great increase in the rate of mitosis. Because odontoblasts are postmitotic cells, irreversibly injured odontoblasts are replaced by cells that migrate from the cell-rich zone onto the inner surface of the dentin.96 This mitotic activity is probably the first step in the formation of a new odontoblast layer.73,240-242,320 Studies implicate stem cells as a source for these replacement odontoblasts.326
Pulp Proper
The pulp proper is the central mass of the pulp (see Fig. 12-15). It consists of loose connective tissue and contains the larger blood vessels and nerves. The most prominent cell in this zone is the fibroblast.
Cells of the Pulp
Odontoblast
Because odontoblasts are responsible for dentinogenesis, both during tooth development and aging, the odontoblast is the most characteristic and specialized cell of the dentin-pulp complex. During dentinogenesis, the odontoblasts form dentin and the dentinal tubules, and their presence within the tubules makes dentin a living responsive tissue.
Dentinogenesis, osteogenesis, and cementogenesis are in many respects quite similar, and odontoblasts, osteoblasts, and cementoblasts have many characteristics in common. Each of these cells produces a matrix composed of collagen fibrils, noncollagenous proteins, and proteoglycans that are capable of undergoing mineralization. The ultrastructural characteristics of odontoblasts, osteoblasts, and cementoblasts are likewise similar in that each exhibits a highly ordered RER, a prominent Golgi complex, secretory granules, and numerous mitochondria. In addition, these cells are rich in RNA, and their nuclei contain one or more prominent nucleoli. These are the general characteristics of protein-secreting cells.
The most significant differences among odontoblasts, osteoblasts, and cementoblasts are their morphologic characteristics and the anatomic relationship between the cells and the mineralized structures they produce. Whereas osteoblasts and cementoblasts are polygonal to cuboidal in form, the fully developed odontoblast of the coronal pulp is a tall columnar cell.63,224 In bone and cementum, some of the osteoblasts and cementoblasts become entrapped in the matrix as osteocytes or cementocytes, respectively. The odontoblast, on the other hand, leaves behind a cellular process to form the dentinal tubule, and the cell body resides outside the mineralized tissue. Lateral branches between the major odontoblast processes interconnect167,236 through canaliculi, just as osteocytes and cementocytes are linked together through the canaliculi in bone and cementum. This provides a pathway for intercellular communication and circulation of fluid and metabolites through the mineralized matrix.
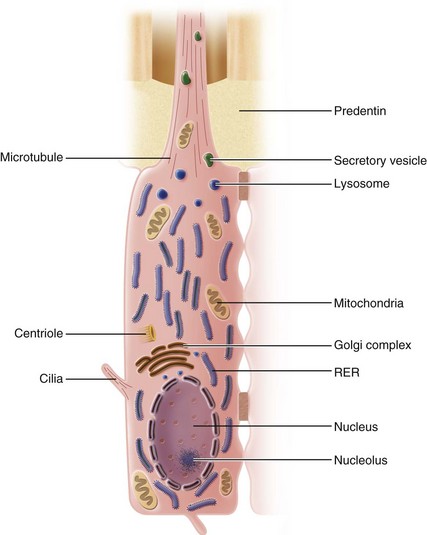
The ultrastructural features of the odontoblast have been the subject of numerous investigations. The cell body of the active odontoblast has a large nucleus that may contain up to four nucleoli (Fig. 12-19). The nucleus is situated at the basal end of the cell and is contained within a nuclear envelope. A well-developed Golgi complex, centrally located in the supranuclear cytoplasm, consists of an assembly of smooth-walled vesicles and cisternae. Numerous mitochondria are evenly distributed throughout the cell body. RER is particularly prominent, consisting of closely stacked cisternae forming parallel arrays that are dispersed diffusely within the cytoplasm. Numerous ribosomes closely associated with the membranes of the cisternae mark the sites of protein synthesis. Within the lumen of the cisternae, filamentous material (probably representing newly synthesized protein) can be observed.
The odontoblast appears to synthesize mainly type I collagen,196,375 although small amounts of type V collagen have been found in the ECM. In addition to proteoglycans40,82,117 and collagen,196,202 the odontoblast secretes dentin sialoprotein42 and phosphophoryn,42,70 a highly phosphorylated phosphoprotein involved in extracellular mineralization.42,75 Phosphophoryn is unique to dentin and is not found in any other mesenchymal cell types.75 The odontoblast also secretes both acid phosphatase and alkaline phosphatase. The latter enzyme is closely linked to mineralization, but the precise role of alkaline phosphatase in dentinogenesis is not completely understood. Acid phosphatase, a lysosomal enzyme, may be involved in digesting material that has been resorbed from predentin matrix.85
In contrast to the active odontoblast, the resting or inactive odontoblast has a decreased number of organelles and may become progressively shorter.63,224 These changes can begin with the completion of root development and eruption when dentin production shifts from primary to secondary dentin.
Odontoblast Process
A dentinal tubule forms around each of the major odontoblastic processes. The odontoblast process occupies most of the space within the tubule and coordinates the formation of peritubular dentin.
Microtubules and microfilaments are the principal ultrastructural components of the odontoblast process and its lateral branches.106,150 Microtubules extend from the cell body out into the process.106,151 These straight structures follow a course that is parallel with the long axis of the cell and impart the impression of rigidity. Although their precise role is unknown, theories as to their functional significance suggest that they may be involved in cytoplasmic extension, transport of materials, or the provision of a structural framework. Occasionally, mitochondria can be found in the process where it passes through the predentin.
The plasma membrane of the odontoblast process closely approximates the wall of the dentinal tubule. Localized constrictions in the process occasionally produce relatively large spaces between the tubule wall and the process. Such spaces may contain collagen fibrils and fine granular material that presumably represents ground substance. The peritubular dentin matrix within the tubule is lined by an electron-dense limiting membrane called the lamina limitans.244,333,372 A narrow space separates the limiting membrane from the plasma membrane of the odontoblast process, except for the areas where the process is constricted.
In restoring a tooth, the removal of enamel and dentin often disrupts odontoblasts.* It would be of considerable clinical importance to establish the extent of the odontoblast processes in human teeth, because with this knowledge, the clinician would be in a better position to estimate the impact of the restorative procedure on the underlying odontoblasts. However, the extent to which the process penetrates into dentin has been a matter of considerable controversy. It has long been thought that the process is present throughout the full thickness of dentin. Although ultrastructural studies using transmission electron microscopy have described the process as being limited to the inner third of the dentin,51,107,333,372 it should be noted that this could possibly be the result of shrinkage occurring during fixation and dehydration. Other studies employing scanning electron microscopy have described the process extending further into the tubule, often as far as the DEJ,121,168,318,381 but it has been suggested that what has been observed in scanning electron micrographs is actually the lamina limitans.333,334,372
In an attempt to resolve this issue, monoclonal antibodies directed against microtubules were used to demonstrate tubulin in the microtubules of the process. Immunoreactivity was observed throughout the dentinal tubule, suggesting that the process extends throughout the entire thickness of dentin.318 However, a study employing confocal microscopy found that odontoblast processes in rat molars do not extend to the outer dentin or DEJ, except during the early stages of tooth development.51 It is likely that the walls of tubules contain many proteins originally derived from odontoblasts that no longer remain at that site. Because dentin matrix does not turn over, these antigens remain fixed in place. From a clinical perspective, it is important to remember that these processes in the tubules represent appendages from living odontoblasts in the pulp, which explains why the dentin must be considered a vital tissue, the destruction of which will affect the pulp.
The odontoblast is considered to be a fixed postmitotic cell in that once it has fully differentiated, it apparently cannot undergo further cell division. If this is indeed the case, the lifespan of the odontoblast coincides with the lifespan of the viable pulp. However, its metabolic activity can be dynamically altered (described under the heading, Pulpal Repair).
Relationship of Odontoblast Structure to Secretory Function
Studies using radiolabeled chemicals have shed a great deal of light on the functional significance of the cytoplasmic organelles of the active odontoblast.375,376 In experimental animals, intraperitoneal injection of a collagen precursor (e.g., 3H-proline) is followed by autoradiographic labeling of the odontoblasts and predentin matrix375 (Fig. 12-20). Rapid incorporation of the isotope in the RER soon leads to labeling of the Golgi complex in the area where the procollagen is packed and concentrated into secretory vesicles. Radiolabeled vesicles can then be followed along their migration pathway until they reach the base of the odontoblast process. Here they fuse with the cell membrane and release their tropocollagen molecules into the predentin matrix by the process of exocytosis.

FIG. 12-20 Autoradiograph demonstrating odontoblasts and predentin in a developing rat molar 1 hour after intraperitoneal injection of 3H-proline.
It is now known that collagen fibrils precipitate from a solution of secreted tropocollagen, and that the aggregation of fibrils occurs on the outer surface of the odontoblast plasma membrane. Fibrils are released into the predentin and increase in thickness as they approach the mineralized matrix. Whereas fibrils at the base of the odontoblast process are approximately 15 nm in diameter, fibrils in the region of the calcification front have attained a diameter of about 50 nm.
Similar tracer studies376 have elucidated the pathway of synthesis, transport, and secretion of the predentin proteoglycans. The protein moiety of these molecules is synthesized by the RER of the odontoblast, whereas sulfation and addition of the GAG moieties to the protein molecules take place in the Golgi complex. Secretory vesicles then transport the proteoglycans to the base of the odontoblast process, where they are secreted into the predentin matrix. Proteoglycans, principally chondroitin sulfate, accumulate near the calcification front. The role of the proteoglycans is speculative, but mounting evidence suggests that they act as inhibitors of calcification by binding calcium. It appears that just before calcification, the proteoglycans are removed, probably by lysosomal enzymes secreted by the odontoblasts.83
Pulp Fibroblast
Fibroblasts are the most numerous cells of the pulp. They appear to be tissue-specific cells capable of giving rise to cells that are committed to differentiation (e.g., odontoblast-like cells) if given the proper signal. These cells synthesize types I and III collagen, as well as proteoglycans and GAGs. Thus they produce and maintain the matrix proteins of the ECM. Because they are also able to phagocytose and digest collagen, fibroblasts are responsible for collagen turnover in the pulp.
Although distributed throughout the pulp, fibroblasts are particularly abundant in the cell-rich zone. The early differentiating fibroblasts are polygonal and appear to be widely separated and evenly distributed within the ground substance. Cell-to-cell contacts are established between the multiple processes that extend out from each of the cells. Many of these contacts take the form of gap junctions that provide for electronic coupling or chemical signaling from one cell to another. In terms of ultrastructure, the organelles of the immature fibroblasts are generally in a rudimentary stage of development, with an inconspicuous Golgi complex, numerous free ribosomes, and sparse RER. As they mature, the cells become stellate in form, and the Golgi complex enlarges, the RER proliferates, secretory vesicles appear, and the fibroblasts take on the characteristic appearance of protein-secreting cells. In addition, collagen fibrils accumulate along the outer surface of the cell body. With an increase in the number of blood vessels, nerves, and collagen fibers, there is a relative decrease in the number of fibroblasts in the pulp.
Many fibroblasts of the pulp are characterized by being relatively undifferentiated. A more modern term for undifferentiated cells is stem cells. Many pulpal cells do seem to remain in a relatively undifferentiated modality (compared with fibroblasts of most other connective tissues128). This perception has been supported by the observation of large numbers of reticulin-like fibers in the pulp. Reticulin fibers have an affinity for silver stains and are similar to the argyrophilic fibers of the pulp. However, in a careful review, it appears that actual reticulin fibers may not be present in the pulp; instead the previously described fibers are actually argyrophilic collagen fibers.17 The fibers apparently acquire a GAG sheath, and it is this sheath that is impregnated by silver stains. In the young pulp, the nonargyrophilic collagen fibers are sparse, but they progressively increase in number as the pulp ages.
Many experimental models have been developed to study wound healing in the pulp, particularly dentinal bridge formation after pulp exposure or pulpotomy. One study96 demonstrated that mitotic activity preceding the differentiation of replacement odontoblasts appears to occur primarily among perivascular fibroblasts.
Pulpal fibroblasts seem to take active part in signaling pathways in the dental pulp. For example, fibroblast growth and synthesis is stimulated by neuropeptides; in turn, fibroblasts produce NGF and proinflammatory cytokines during inflammation.32,380,382 NGF plays an important role not only in development but also in regulating neuronal and possibly odontoblast responses to injury via activation of similar neurotrophin receptors expressed on both cell types (see also Plasticity of Intradental Nerves Fibers in this chapter).380
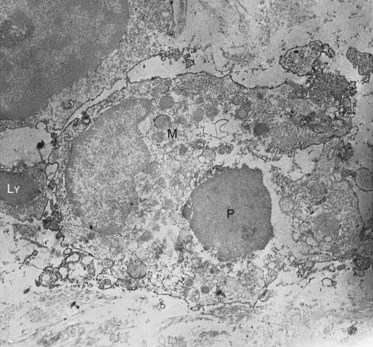
Macrophage
Macrophages are monocytes that have left the bloodstream, entered the tissues, and differentiated into various subpopulations. The different subpopulations can be studied by their antigenic properties in immunohistochemical studies. Many are found in close proximity to blood vessels. A major subpopulation of macrophages is quite active in endocytosis and phagocytosis (Fig. 12-21). Because of their mobility and phagocytic activity, they are able to act as scavengers, removing extravasated red blood cells, dead cells, and foreign bodies from the tissue. Ingested material is destroyed by the action of lysosomal enzymes. Another subset of macrophages participates in immune reactions by processing antigen and presenting it to memory T cells.265 The processed antigen is bound to class II major histocompatibility complex (MHC) molecules on the macrophage, where it can interact with specific receptors present on naïve or memory T cells.124 Such interactions are essential for T cell–dependent immunity. Similar to fibroblasts, macrophages take an active part in the signaling pathways in the pulp. When activated by the appropriate inflammatory stimuli, macrophages are capable of producing a large variety of soluble factors, including interleukin 1, tumor necrosis factor, growth factors, and other cytokines. A recent study has shown that a subset of macrophages express lymphatic markers, indicating a link between macrophages and lymphatic function and development.20
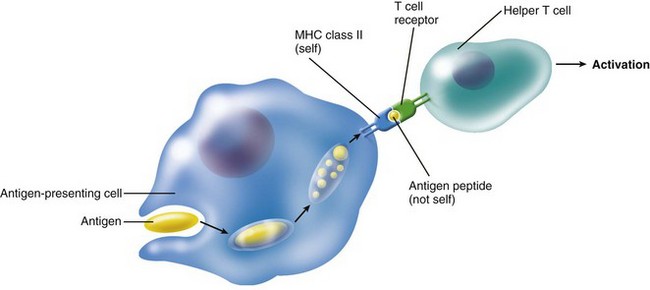
Dendritic Cell
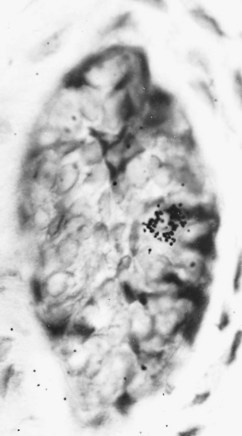
Dendritic cells are accessory cells of the immune system. Similar cells are found in the epidermis and mucous membranes, where they are called Langerhans’ cells.164,264 Dendritic cells are primarily found in lymphoid tissues, but they are also widely distributed in connective tissues, including the pulp303 (Fig. 12-22). These cells are termed antigen-presenting cells and are characterized by dendritic cytoplasmic processes and the presence of class II MHC complexes on their cell surface (Fig. 12-23). In the normal pulp, they are mostly located in the periphery of the coronal pulp close to the predentin but migrate centrally in the pulp after antigenic challenge.388 They are known to play a central role in the induction of T cell–dependent immunity. Like antigen-presenting macrophages, dendritic cells engulf protein antigens and then present an assembly of peptide fragments of the antigens and MHC class II molecules. It is this assembly that T cells can recognize. Then the assembly binds to a T-cell receptor and T-cell activation occurs (Fig. 12-24). Fig. 12-25 shows a cell-to-cell contact between a dendritic-like cell and a lymphocyte.

FIG. 12-22 Class II antigen-expressing dendritic cells in the pulp and dentin border zone in normal human pulp, as demonstrated by immunocytochemistry. D, Dentin; OB, odontoblastic layer.
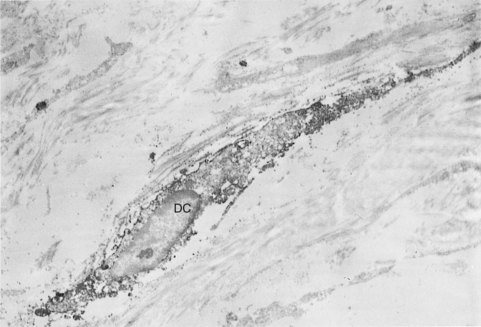
FIG. 12-23 Immunoelectron micrograph of a dendritic-like cell (DC) in the human pulp, showing a dendritic profile with a relatively small amount of lysosomal structures.
Lymphocyte
Hahn et al.124 reported finding T lymphocytes in normal pulps from human teeth. T8 (suppressor) lymphocytes were the predominant T-lymphocyte subset present in these samples. Lymphocytes have also been observed in the pulps of impacted teeth.191 The presence of macrophages, dendritic cells, and T lymphocytes indicates that the pulp is well equipped with cells required for the initiation of immune responses.164,303 B lymphocytes are scarcely found in the normal pulp.
Mast Cell
Mast cells are widely distributed in connective tissues, where they occur in small groups in relation to blood vessels. Mast cells are seldom found in the normal pulp tissue, although they are routinely found in chronically inflamed pulps.303 The mast cell has been the subject of considerable attention because of its dramatic role in inflammatory reactions. The granules of mast cells contain heparin, an anticoagulant, and histamine, an important inflammatory mediator, as well as many other chemical factors.
Metabolism
The metabolic activity of the pulp has been studied by measuring the rate of oxygen consumption and the production of carbon dioxide or lactic acid.27,93-95,127,304 An investigation employed an oxygen-sensitive microelectrode inserted into a rat incisor pulp with a micromanipulator.386 The authors reported that odontoblasts consumed O2 at the rate of 3.2 ± 0.2 ml/min/100 g of pulp tissue.386
Because of the relatively sparse cellular composition of the pulp, the rate of oxygen consumption is low in comparison with that of most other tissues. During active dentinogenesis, metabolic activity is much higher than after the crown is completed. As would be anticipated, the greatest metabolic activity is found in the region of the odontoblast layer, and the lowest is found in the central pulp, where most of the nerves and blood vessels are located.26
In addition to the usual glycolytic pathway, the pulp has the ability to produce energy through a phosphogluconate (i.e., pentose phosphate) shunt type of carbohydrate metabolism,95 a metabolic pathway that permits tissues to function under varying degrees of ischemia. This could explain how the pulp manages to withstand periods of low perfusion resulting from vasoconstriction induced by epinephrine-containing infiltration anesthesia.175
Several commonly used dental materials (e.g., eugenol, zinc oxide and eugenol, calcium hydroxide, silver amalgam) inhibit oxygen consumption by pulp tissue, indicating that these agents may be capable of depressing the metabolic activity of pulpal cells.94,163 One study127 found that application of orthodontic force to human premolars for 3 days resulted in a 27% reduction in respiratory activity in the pulp. This study utilized carbon-14-labeled succinic acid in the medium. As cells metabolize succinic acid, they produce 14CO2 that can be trapped and quantitated by a liquid scintillation counter.127 This technique requires only a few milligrams of tissue.
The Pulpal Interstitium and Ground Substance
The interstitium consists of the interstitial fluid and the interstitial (extracellular) matrix and occupies the extracellular and extravascular space. It is amorphous and generally regarded as a gel rather than a solid. Its constituents are similar in all tissues, but their relative amount varies. The major structural component of the interstitium is collagen (Fig. 12-26). The network of collagen fibers also supports the other components of the interstitium, the proteoglycans, hyaluronan, and elastic fibers. The two former components represent the glycosaminoglycans of the interstitial matrix.
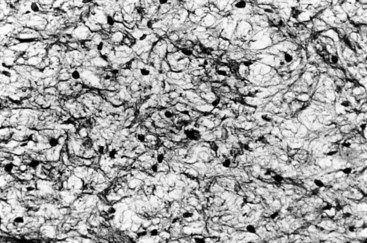
FIG. 12-26 Delicate network of pulpal collagen fibers as demonstrated by the Pearson silver impregnation method.
Because of its content of polyanionic polysaccharides, the interstitium is responsible for the water-holding properties of connective tissues and acts as a molecular “sieve” in regulating the diffusion of substances through this space. The magnitude of the excluded volume has important consequences because the effective protein concentration in the interstitium is higher than the value that would be estimated from fluid volume per se.378
Connective tissue consists of cells and fibers, both embedded in ground substance or extracellular matrix (ECM). Cells that produce connective tissue fibers also synthesize the major constituents of the ECM. Whereas the fibers and cells have recognizable shapes, the ECM is described as being amorphous. It is generally regarded as a gel rather than a solid. Because of its content of polyanionic polysaccharides, the ECM is responsible for the water-holding properties of connective tissues.378
Nearly all proteins of the ECM are glycoproteins.221 Proteoglycans are an important subclass of glycoproteins.117 These molecules support cells, provide tissue turgor, and mediate a variety of cell interactions. They have in common the presence of GAG chains and a protein core to which the chains are linked. Except for heparan sulfate and heparin, the chains are composed of disaccharides. The primary function of GAG chains is to act as adhesive molecules that can bond to cell surfaces and other matrix molecules.
Fibronectin is a major surface glycoprotein that, together with collagen, forms an integrated fibrillary network that influences adhesion, motility, growth, and differentiation of cells. Laminin, an important component of basement membranes, binds to type IV collagen and cell surface receptors.113 Tenascin is another substrate adhesion glycoprotein.
In the pulp, the principal proteoglycans include hyaluronic acid dermatan sulfate, heparan sulfate, and chondroitin sulfate.221 The proteoglycan content of pulp tissue decreases approximately 50% with tooth eruption.202 During active dentinogenesis, chondroitin sulfate is the principal proteoglycan, particularly in the odontoblast and predentin layer, where it is somehow involved with mineralization; with tooth eruption, hyaluronic acid and dermatan sulfate increase, and chondroitin sulfate decreases greatly.
The consistency of a connective tissue (e.g., the pulp) is largely determined by the proteoglycan components of the ground substance. The long GAG chains of the proteoglycan molecules form relatively rigid coils constituting a network that holds water, thus forming a characteristic gel. Hyaluronic acid in particular has a strong affinity for water and is a major component of ground substance in tissues with a large fluid content, such as Wharton’s jelly of the umbilical cord. The water content of the young pulp is very high (approximately 90%), so the ground substance forms a cushion capable of protecting cells and vascular components of the tooth.
Ground substance also acts as a molecular sieve in that it excludes large proteins. Cell metabolites, nutrients, and wastes pass through the ground substance between cells and blood vessels. In some ways, ground substance can be likened to an ion exchange resin, because the polyanionic chains of the GAGs bind cations. In addition, osmotic pressures can be altered by excluding osmotically active molecules. Thus proteoglycans can regulate the dispersion of interstitial matrix solutes, colloids, and water, and (in large measure) they determine the physical characteristics of a tissue, such as the pulp.
Degradation of ground substance can occur in certain inflammatory lesions that have a high concentration of macrophage lysosomal enzymes. Proteolytic enzymes, hyaluronidases, and chondroitin sulfatases of lysosomal and bacterial origin are examples of the hydrolytic enzymes that can attack components of the ground substance. The pathways of inflammation and infection are strongly influenced by the state of polymerization of the ground substance components.
Hyaluronan
Another major structural component of the interstitial matrix is hyaluronan. It is an unbranched, random-coil molecule from repeating nonsulfated disaccharide units and is found in the interstitium as free molecules or bound to cells, possibly via the connection to fibronectin.194 Its large molecular weight together with its protein structure accounts for its unique properties. It has a high viscosity even at low concentration, exhibits exclusion properties, and has a strong affinity for water.
Hyaluronan is one of several types of GAGs in the pulp.203,221 The hyaluronan receptor-1 is expressed on lymphatic vessels and also on immune cells in the dental pulp.20 Hyaluronan is removed from the tissue by the lymphatics and metabolized in the lymph nodes98 and by endothelial cells in the liver.126,285
Elastic Fibers
Elastic fibers comprise an elastin core and a surrounding microfibrillar network and provide elasticity to the tissue.272 The amount of elastin in the interstitial matrix in most tissue is small. There is no evidence for elastic fibers in the matrix in the pulp.126,285
The Inflamed Interstitium
Hyaluronidases, and chondroitin sulfatases of lysosomal and bacterial origin are examples of the hydrolytic enzymes that can attack components of the interstitium. During infection and inflammation, the physical properties of the pulp tissue may then be altered due to production of such degrading enzymes.133,302 In addition to their own damaging effect, they may also pave the way for the deleterious effects of bacterial toxins, increasing the magnitude of the damage.166
The pathways of inflammation and infection are strongly influenced by the particular composition of the interstitium in every tissue and its degradation by either host or microbial enzymes.
Connective Tissue Fibers of the Pulp
Two types of structural proteins are found in the pulp: collagen and elastin. Elastin fibers are confined to the walls of arterioles and, unlike collagen, are not a part of the ECM.
A single collagen molecule, referred to as tropocollagen, consists of three polypeptide chains, designated as either alpha-1 or alpha-2 depending on their amino acid composition and sequence. In the human pulp, the amount of collagen is reported to be 26% to 32% of dry weight in premolars and molars.355 Type I and type III collagen represent the major subtypes of collagen in the pulp, and type I is found in thick striated fibrils throughout the pulp tissue.195,317 The different combinations and linkages of chains making up the tropocollagen molecule have allowed collagen fibers and fibrils to be classified into a number of types:
Type I collagen is synthesized by odontoblasts and osteoblasts; fibroblasts synthesize types I, III, V, and VII collagen.
In collagen synthesis, the protein portion of the molecule is formed by the polyribosomes of the RER of connective tissue cells. The proline and lysine residues of the polypeptide chains are hydroxylated in the cisternae of the RER, and the chains are assembled into a triple-helix configuration in the smooth endoplasmic reticulum. The product of this assembly is termed procollagen, and it has a terminal unit of amino acids known as the telopeptide of the procollagen molecule. When these molecules reach the Golgi complex, they are glycosylated and packaged in secretory vesicles. The vesicles are transported to the plasma membrane and secreted by way of exocytosis into the extracellular milieu, thus releasing the procollagen. Here the terminal telopeptide is cleaved by a hydrolytic enzyme, and the tropocollagen molecules begin aggregating to form collagen fibrils. It is believed that aggregation of tropocollagen is somehow mediated by the GAGs. The conversion of soluble collagen into insoluble fibers occurs as a result of cross linking of tropocollagen molecules. The presence of collagen fibers passing from the dentin matrix between odontoblasts into the dental pulp has been reported in fully erupted teeth.28 Larger collagen fiber bundles are much more numerous in the radicular pulp than in the coronal pulp. The highest concentration of these larger fiber bundles is usually found near the apex (Fig. 12-27). Pulpectomy procedures should engage the pulp with a barbed broach in the region of the apex, since it generally affords the best opportunity to remove this tissue intact.342
Innervation
Pain is a subjective phenomenon involving not only sensory physiologic responses but also emotional, conceptual, and motivational aspects of behavior. The existence of peripheral “nociceptive” (pain-detecting) sensory neurons forms the basis for pain, and pain sensations of varying qualities and intensities are evoked by activation of the intradental nerves innervating teeth. Noxious stimuli in teeth are transmitted in primary afferent neurons located in the trigeminal ganglion via second-order neurons in the brainstem to the brain (Fig. 12-28; see also Chapters 3 and 19). Transmission of sensory information consists of a cascade of events involving input, processing, and sensing,313 so the control of dental pain should be based on an understanding of the origin of pain signals and the complex modulation that may take place locally and at higher levels (see Chapter 19). The sensory system of the pulp appears to be well suited for signaling potential damage to the tooth. The tooth is innervated by a large number of myelinated and unmyelinated axons. The number of axons entering a human premolar may reach 2000 or more, and each axon can arborize to form multiple points of innervation.87,160,161
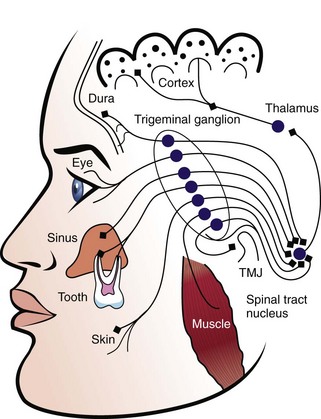
FIG. 12-28 Schematic drawing illustrating the convergence of sensory information from teeth to higher brain centers.
Regardless of the nature of the sensory stimulus (i.e., thermal, mechanical, chemical, electric [e.g., pulp tester]), almost all afferent impulses generated from pulp tissue result in the sensation of pain. However, when the pulp is weakly stimulated by an electric pulp tester under carefully controlled experimental conditions, a nonpainful sensation (i.e., prepain) has been reported.231 Thus not all afferent neurons that innervate the pulp are nociceptors. The innervation of the pulp includes both afferent neurons, which conduct sensory impulses, and autonomic or efferent neurons,154 which provide neurogenic modulation of the microcirculation, inflammatory reactions,136 and perhaps regulate dentinogenesis.48
The sympathetic innervation of teeth derives from the superior cervical ganglion (SCG).10,286 Postganglionic sympathetic nerves travel with the internal carotid nerve, join the trigeminal nerve at the ganglion, and supply teeth and supporting structures via the maxillary and mandibular division of the trigeminal nerve.223 Sympathetic fibers appear with blood vessels at the time the vascular system is established in the dental papilla.101 In the adult tooth pulp, sympathetic fibers form plexuses, usually around pulpal arterioles (Fig. 12-29). Stimulation of these fibers results in constriction of the arterioles and a decrease in blood flow.2,78 The sympathetic neuron terminals contain the classic neurotransmitter, norepinephrine (NE), and neuropeptide Y (NPY) (see Neuropeptides later in this chapter). NPY is synthesized in sympathetic neurons and supplied to terminals by axonal transport. By contrast, NE is mainly produced locally in the terminals. Compared to the sensory nerves, these fibers are most often located in deeper parts of the pulp proper, but fibers have also been found in close relation to odontoblasts.2,155

FIG. 12-29 Histologic section, immunohistologically stained for neuropeptide Y (NPY), shows the distribution of sympathetic nerves in the root pulp of a rat molar. NPY fibers are seen associated with blood vessels.
(Courtesy Dr. Inge Fristad, Department of Clinical Dentistry, University of Bergen.)
The presence of parasympathetic cholinergic nerves in dental tissues has been and is still controversial, although it has been concluded that there is absence of parasympathetic vasodilation in the cat dental pulp.270,307 It has been reported that the neuropeptide, vasoactive intestinal polypeptide (VIP), is localized in the parasympathetic neurons.210,211 The origin of these fibers is uncertain insofar as no form of surgical denervation has resulted in complete loss of VIP-containing fibers from the dental pulp.366
Sensory nerve fibers are usually classified according to their diameter, conduction velocity, and function as shown in Table 12-2. The pulp contains two types of sensory nerve fibers: myelinated (A fibers) and unmyelinated (C fibers). It has been shown that there is some functional overlap between pulpal A and C fibers, since both fiber types can be nociceptors.154,160,161,230,252 The A fibers include both A-beta and A-delta fibers. The A-beta fibers may be slightly more sensitive to stimulation than the A-delta fibers, but functionally these fibers are grouped together in the dental pulp, since both innervate the dentinal tubules, and both are stimulated by dentinal fluid movement (Fig. 12-30). Approximately 90% of the A fibers in dental pulp are A-delta fibers.230 Table 12-3 summarizes the principal characteristics of the main sensory fibers.
FIG. 12-30 Schematic drawing illustrating the location of A and C fibers in the dental pulp. Myelinated A fibers are located in the periphery of the pulp, penetrating the inner part of dentin. Unmyelinated C fibers are located in the deeper part of the pulp proper.
During the bell stage of tooth development, “pioneer” nerve fibers enter the dental papilla following the path of blood vessels.101 Although only unmyelinated fibers are observed in the dental papilla, a proportion of these fibers are probably A fibers that have lost their myelin sheath. Myelinated fibers are the last major structures to appear in the developing human dental pulp.13 The number of nerve fibers gradually increases, and some branching occurs as the fibers near the dentin. During the bell stage very few fibers enter the predentin.101
The sensory nerves of the pulp arise from the trigeminal nerve and pass into the radicular pulp in bundles by way of the foramen in close association with arterioles and venules (Fig. 12-31). Each of the nerves entering the pulp is invested within Schwann cells, and the A fibers acquire their myelin sheath from these cells. With the completion of root development, the myelinated fibers appear grouped in bundles in the central region of the pulp (Fig. 12-32). Most of the unmyelinated C fibers entering the pulp are located within these fiber bundles; the remainder is situated toward the periphery of the pulp (see Fig. 12-30).292 It should be noted that single neurons have been reported to innervate the pulps of multiple teeth in animal studies.145 Assuming similar innervation patterns occur in humans, this finding may partially explain why patients often have difficulty localizing pulpal pain to a specific tooth. An alternative explanation to this clinical observation is that the pulp has a relatively low density of proprioceptors, and thus patients have difficulty identifying the inflamed pulp until the inflammation reaches the periradicular tissue, which is highly innervated with proprioceptors. This is discussed in greater detail in Chapters 3 and 19.
FIG. 12-31 Histologic section, immunohistologically stained for calcitonin gene-related peptide (CGRP), shows distribution of sensory nerves in the apical area of a rat molar. Nerve fibers are seen associated with blood vessels and enter the dental pulp in nerve bundles.
(Courtesy Dr. Inge Fristad, Department of Clinical Dentistry, University of Bergen.)
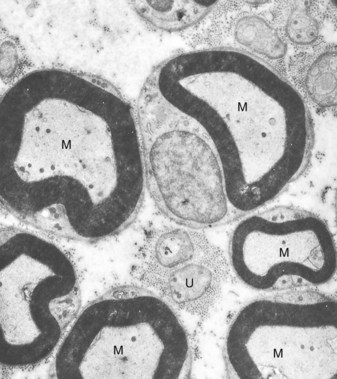
FIG. 12-32 Electron micrograph of the apical pulp of a young canine tooth, showing in cross section myelinated nerve axons (M) within Schwann cells. Smaller, unmyelinated axons (U) are enclosed singly and in groups by Schwann cells.
(Courtesy Dr. David C. Johnsen, School of Dentistry, Case Western Reserve University.)
In the human premolar, the number of unmyelinated axons entering the tooth at the apex reached a maximal number shortly after tooth eruption.160 At this stage, an average of 1800 unmyelinated axons and more than 400 myelinated axons were found, although in some teeth fewer than 100 myelinated axons were present. Five years after eruption, the number of A fibers gradually increased to more than 700. The relatively late appearance of A fibers in the pulp may help to explain why the electric pulp test tends to be unreliable in young teeth, since A fibers are more easily electrically stimulated than C fibers.104
A quantitative study of axons 1 to 2 mm coronal to the root apex of fully developed human canine and incisor teeth160 reported a mean of about 360 myelinated axons in canines and incisors, whereas there were 1600 to 2200 unmyelinated axons. However, this does not reflect the actual number of neurons supporting a single tooth, because multiple branching of the axons may occur in the peripheral tissues. Overall, approximately 80% of the axons were unmyelinated fibers.160,161
The nerve bundles pass upward through the radicular pulp together with blood vessels (see Fig. 12-31). Once they reach the coronal pulp, they fan out beneath the cell-rich zone, branch into smaller bundles, and finally ramify into a plexus of single-nerve axons known as the plexus of Raschkow (Fig. 12-33). Full development of this plexus does not occur until the final stages of root formation.87 It has been estimated that each axon entering the pulp sends at least eight branches to the plexus of Raschkow. There is prolific branching of the fibers in the plexus, producing a tremendous overlap of receptor fields.131,252-254,256 It is in the plexus that the A fibers emerge from their encircling Schwann cells and branch repeatedly to form the subodontoblastic plexus. Finally, terminal axons pass between the odontoblasts as free nerve endings (Figs. 12-34 and 12-35). The extent to which dentin is innervated has been the subject of numerous investigations.45,47,48,52,87,200 With the exception of the innervation of dentinal tubules, discussed later in this chapter, the bulk of dentin is devoid of sensory nerve fibers. This offers an explanation as to why pain-producing agents (e.g., potassium chloride) do not always elicit pain when applied to exposed dentin. Similarly, application of topical anesthetic solutions to dentin does not decrease its sensitivity. A very high concentration of lidocaine solution is needed to block the response of intradental nerves to mechanical stimulation of the dentin.6
FIG. 12-33 Histologic section, immunohistologically stained for calcitonin gene-related peptide (CGRP), shows the distribution of sensory nerves in a rat molar. Nerves enter the coronal pulp in bundles, ramify in a network beneath the odontoblasts (i.e., plexus of Raschkow), before entering between the odontoblasts and the inner part of dentin.

FIG. 12-34 Detailed histologic section, immunohistologically stained for calcitonin gene-related peptide (CGRP), shows the distribution of sensory nerves in the odontoblast layer of a rat molar.
(Courtesy Dr. Inge Fristad, Department of Clinical Dentistry, University of Bergen.)

FIG. 12-35 Unmyelinated nerve fiber (NF) without a Schwann cell covering located between adjacent odontoblasts (O) overlying pulp horn of a mouse molar tooth. Predentin (PD) can be seen at upper right. Within the nerve there are longitudinally oriented fine neurofilaments, microvesicles, and mitochondria.
(From Corpron RE, Avery JK: The ultrastructure of intradental nerves in developing mouse molars. Anat Rec 175:585, 1973.)
One investigator122 studied the distribution and organization of nerve fibers in the dentin-pulp border zone of human teeth. On the basis of their location and pattern of branching, several types of nerve endings were described (Fig. 12-36). Some fibers were found running from the subodontoblastic nerve plexus toward the odontoblast layer. However, these fibers do not reach the predentin; they terminate in extracellular spaces in the cell-rich zone, the cell-poor zone, or the odontoblast layer. Other fibers extend into the predentin and run through a dentinal tubule in close association with an odontoblast process. Most of these intratubular fibers extend into the dentinal tubules for only a few micrometers, but a few may penetrate as far as 100 µm (see Fig. 12-33). The area covered by a single such terminal complex often reaches thousands of square micrometers.122,253
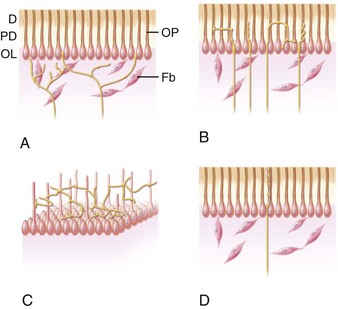
FIG. 12-36 Schematic drawing showing distribution of nerve fibers in the dentin-pulp border zone. A, Fibers running from the subodontoblastic plexus to the odontoblast layer. D, Dentin; Fb, fibroblast; OL, odontoblast layer; OP, odontoblast process; PD, predentin. B, Fibers extending into the dentinal tubules in the predentin. C, Complex fibers that branch extensively in the predentin. D, Intratubular fibers extending into the dentin.
Intratubular nerve endings are most numerous in the area of the pulp horns, where as many as 40% of the tubules may contain fibers.48,200 The number of intratubular fibers decreases in other parts of the dentin, and in root dentin only about 1% of dentinal tubules contain a fiber. This notion has been challenged in a study that stained pulps for protein gene-product 9.5, a specific marker for nerves.218 In that study, root dentin appeared to be as well innervated as coronal dentin. The anatomic relationship between the odontoblast processes and sensory nerve endings has led to much speculation as to the functional relationships between these structures, if any.47 When present, nerve fibers lie in a groove or gutter along the surface of the odontoblast process, and toward their terminal ends, they twist around the process like a corkscrew. The cell membranes of the odontoblast process and the nerve fiber are closely approximated and run closely parallel for the length of their proximity but are not synaptically linked.149
Although it may be tempting to speculate that the odontoblasts and their associated nerve axons are functionally interrelated and that together they play a role in dentin sensitivity, there is a paucity of evidence supporting this hypothesis. If the odontoblast were acting as a classic receptor cell,* it would have chemical, electric, or mechanical communication with the adjacent nerve fiber. However, researchers have been unable to find classical anatomic structures (e.g., synaptic junctions) that could functionally couple odontoblasts and nerve fibers together. With regard to the membrane properties of odontoblasts, it has been reported that the membrane potential of the odontoblast is low (−24 to −30 mV),189,212 and that the cell does not respond to electric stimulation.189,379 It would appear that the odontoblast does not possess the properties of an excitable cell. Further, the sensitivity of dentin is not diminished after disruption of the odontoblast layer.39,201 It is still possible that odontoblasts could modulate neuronal function via alteration in sodium channel activity or the release of paracrine factors that diffuse to the closely approximated nerve terminal.
Another study showed that a reduction in pulpal blood flow induced by stimulation of sympathetic fibers leading to the pulp results in depressed excitability of pulpal A fibers.78 The excitability of C fibers is less affected than that of A fibers by a reduction in blood flow.341
Of clinical interest is the evidence that nerve fibers of the pulp may be resistant to necrosis84,239 because their cell bodies are found in ganglia outside the pulp. Since nerve bundles in general are more resistant to autolysis than other tissue elements, even in degenerating pulps, C fibers might still be able to respond to noxious stimulation. It may be that C fibers remain excitable even after blood flow has been compromised in the diseased pulp, since C fibers are often able to function in the presence of hypoxia.341 This may offer an explanation as to why instrumentation of the root canals of apparently nonvital teeth sometimes elicits pain. On the other hand, histologic studies on nonvital teeth failed to demonstrate high levels of innervation, leading to the suggestion pain may be due to the transfer of noxious chemicals to terminals located in periapical tissues.239
Tissue Injury and Deafferentation
When a peripheral nerve is cut or crushed, an interruption of the afferent input to the CNS occurs, which is called deafferentation. It would be logical to assume that the result of deafferentation would be anesthesia of the formerly innervated area, but occasionally other symptoms may occur, which may surprisingly include pain. Following nerve injury, a dramatic shift in transcription of neuropeptides, receptors, and sodium channels has been documented. The bidirectional contact between the nerve cell and the peripheral target tissue is lost, and the neurons change into a state of either regeneration or neuronal cell death. The impact on neurons in the trigeminal ganglion is dependent on the injury site. A peripheral injury has less effect than a more centrally located one. However, even a small pulp exposure induces neuronal changes, both in the trigeminal ganglion and at the second-order neuronal level in the brainstem.46,367 Because each single-rooted tooth contains about 2000 nerve fibers,160,161 extirpation of the pulp is shown to cause both neurochemical and degenerative changes of their cell bodies in the gasserian (trigeminal) ganglion.120,174,315 The central projection of these nerves to the spinal nucleus of the trigeminal nerve are also affected,343 and there is evidence for transsynaptic changes314,315 that are reflected in the sensory cortex. Even larger responses would be expected from tooth extraction, where both periodontal ligament and pulpal innervation are destroyed.
When an axon is severed peripherally, a complete degeneration of the cell bodies may not always occur.368 Attempted regeneration by axonal sprouting may result in altered expression of various receptors, resulting in sensitivity to norepinephrine (via increased adrenergic receptor activity)261 or acetylcholine (via increased cholinergic receptor activity),72 sensitizing sensory neurons to autonomic activity. In addition, dorsal horn neurons, deprived of their normal sensory input, may begin to respond to other nearby afferents. Thus normal inhibitory influences are reduced, and a widening of the sensory receptive field is produced, which can produce central sensitization (see Chapter 19). Phantom tooth pain is another term often used synonymously with pain following deafferentation. Different reports suggest an incidence of persistent pain following pulpectomy to be in the range of 3% to 6%.222,360
Following tissue injury or tissue inflammation, extensive changes occur in the gene expression of sensory ganglion nerves and, by way of transsynaptic mechanisms, in their central projections.47,253,254,315 One example is the upregulation of inducible gene transcription factors such as c-fos53 and different subsets of sodium channels.114 This is thought to result in alterations in threshold properties and the size of receptor fields. C-fos is not normally expressed in neurons in the brainstem, but chronic pulpitis causes prolonged increases in c-fos expression is some brainstem neurons.
If such changes also occur in humans, they may help to explain why certain patients may complain of vague, poorly described pain for months following endodontic treatment. If their pulpitis caused sprouting of periapical nerves,52-54182 these nerves may have been due to the transport of peripheral signaling molecules to the cell body by way of retrograde axoplasmic flow.45,54 This could induce changes in the expression of many genes, resulting in central senstization8,44 that may require many months to correct.327 Reports of nerve sprouting in human inflamed pulps have been confirmed in different studies.296,297,303 Such reactions might contribute to increases in dentin sensitivity as well as expansion of receptive fields.253,254,256 Sprouting of sympathetic fibers has also been reported,135 but the timing appears to be different. The functional implications and how this is related to pain mechanisms are unknown, but it has been suggested that these reactions are involved in healing and nociception following pulpal inflammation.111,135,136
Pulp Testing
The electric pulp tester delivers a current sufficient to overcome the resistance of enamel and dentin and stimulate the sensory A fibers at the dentin-pulp border zone. Smaller C fibers of the pulp do not respond to the conventional pulp tester because significantly more current is needed to stimulate them.252 Bender et al.18 found that in anterior teeth, the optimal placement site of the electrode is the incisal edge, since the response threshold is lowest at that location and increases as the electrode is moved toward the cervical region of the tooth.
Cold tests using carbon dioxide (CO2) snow or liquid refrigerants and heat tests employing heated gutta-percha or hot water activate hydrodynamic forces within the dentinal tubules, which in turn excite the intradental A fibers. C fibers are generally not activated by these tests unless they produce injury to the pulp. It has been shown that cold tests do not injure the pulp.104 Heat tests have a greater potential to produce injury, but if the tests are used properly, injury is not likely.
Sensitivity of Dentin
The mechanisms underlying dentin sensitivity have been a subject of interest in recent years. How are stimuli relayed from the peripheral dentin to the sensory terminals located in the region of the dentin-pulp border zone? Converging evidence indicates that movement of fluid in the dentinal tubules is the basic event in the arousal of dentinal pain.38,39,249,361,363 It now appears that pain-producing stimuli, such as heat, cold, air blasts, and probing with the tip of an explorer, have in common the ability to displace fluid in the tubules.38,230 This is referred to as the hydrodynamic mechanism of dentin sensitivity. The hydrodynamic theory suggests that dentinal pain associated with stimulation of a sensitive tooth ultimately involves mechanotransduction. Recently, classical mechanotransducers have been recognized on pulpal afferents, providing a mechanistic support to this theory.138 Thus fluid movement in the dentinal tubules is translated into electric signals by receptors located in the axon terminals innervating dentinal tubules. Indeed, there is a positive correlation between rate of fluid flow in the tubules and discharge evoked in intradental nerves,229,230,363 with outward fluid movements producing a much stronger nerve response than inward movements.230
In experiments on humans, brief application of heat or cold to the outer surface of premolar teeth evoked a painful response before the heat or cold could have produced temperature changes capable of activating sensory receptors in the underlying pulp.250,346 The evoked pain was of short duration: 1 or 2 seconds. The thermal diffusivity of dentin is relatively low; yet the response of the tooth to thermal stimulation is rapid, often less than 1 second. Evidence suggests that thermal stimulation of the tooth results in a rapid movement of fluid into the dentinal tubules. This results in activation of the sensory nerve terminal in the underlying pulp. Presumably, heat expands the fluid within the tubules faster than it expands dentin, causing the fluid to flow toward the pulp, whereas cold causes the fluid to contract more rapidly than dentin, producing an outward flow. It is speculated that the rapid movement of fluid across the cell membrane of the axon terminal activates a mechanosensitive receptor, similar to how fluid movement activates hair cells in the cochlea of the ear. All axon terminals have membrane channels through which charged ions pass, and this initial receptor current, if sufficient, can trigger voltage gated sodium channels to depolarize the cell, leading to a barrage of impulses to the brain. Some ion channels are activated by voltage, some by chemicals, and some by mechanical pressure.230,249,253 In the case of pulpal nerve fibers that are activated by hydrodynamic forces, pressure would be transduced, gating mechanosensitive ion channels.
The dentinal tubule is a capillary tube with an exceedingly small diameter.*107 The physical properties of capillarity are significant because fluid force increases as the diameter is reduced. If fluid is removed from the outer end of exposed dentinal tubules by dehydrating the dentinal surface with an air blast or absorbent paper, capillary forces produce a rapid outward movement of fluid in the tubule (Fig. 12-37). According to Brännström,38 desiccation of dentin can theoretically cause dentinal fluid to flow outward at a rate of 2 to 3 mm/sec. In addition to air blasts, dehydrating solutions containing hyperosmotic concentrations of sucrose or calcium chloride can produce pain if applied to exposed dentin.
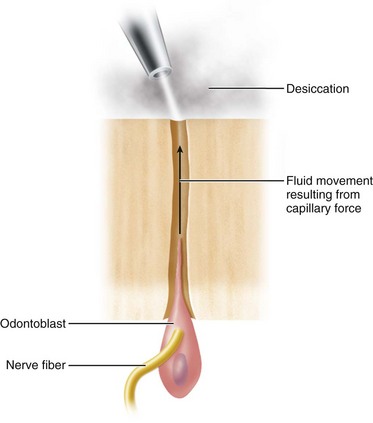
FIG. 12-37 Diagram illustrating movement of fluid in the dentinal tubules resulting from the dehydrating effect of a blast of air from an air syringe.
Investigators have shown that it is the A fibers rather than the C fibers that are activated by hydrodynamic stimuli (e.g., heat, cold, air blasts) applied to exposed dentin.251,256 However, if heat is applied long enough to increase the temperature of the dentin-pulp border by several degrees Celsius, then C fibers may respond, particularly if the heat produces injury. It seems that the A fibers are mainly activated by a rapid displacement of the tubular contents.249 Slow heating of the tooth produced no response until the temperature reached 111° F (43.8° C), at which time C fibers were activated, presumably because of heat-induced injury to the pulp. These C fibers are called polymodal nociceptors because they contain numerous receptors that confer the ability to detect and respond to many different types of stimuli.253,254 Capsaicin, the pungent active ingredient in hot peppers, is known to simulate C fibers and a subset of A-delta fibers.154 Capsaicin activates transient receptor potential, subtype vanilloid 1, (TRPV1).59 The TRPV1 receptor is expressed primarily on a major subclass of nociceptors and responds to heat (>110° F [>43° C]), certain inflammatory mediators, and acid (pH < 6). Thus TRPV1 has been considered a molecular integrator of polymodal noxious stimuli.260 The ability of the TRPV1 antagonist, capsazepine, to inhibit acid-, heat-, and capsaicin-activated trigeminal neurons206 has led to the development of new drugs (i.e., TRPV1 antagonists) for the treatment of pulpal pain. Eugenol is known to activate and ultimately desensitize TRPV1, and this may explain the anodyne action of zinc oxide eugenol temporary restorations.384
It has also been shown that pain-producing stimuli are more readily transmitted from the dentin surface when the exposed tubule apertures are open148,162 and the fluid within the tubules is free to flow outward.162,230,363 For example, acid treatment of exposed dentin to remove the smear layer opens the tubule orifices and makes the dentin much more responsive to stimuli such as air blasts and probing.148,254
Perhaps the most difficult phenomenon to explain is dentinal pain associated with light probing of dentin. Even light pressure of an explorer tip can produce strong forces.* These forces have been shown to mechanically compress dentin and close open tubule orifices with a smear layer that causes sufficient displacement of fluid to excite the sensory receptors in the underlying pulp (Fig. 12-38).55,56 Considering the density of the tubules in which hydrodynamic forces would be generated by probing, thousands of nerve endings would be simultaneously stimulated when a dental explorer is scratched across dentin.
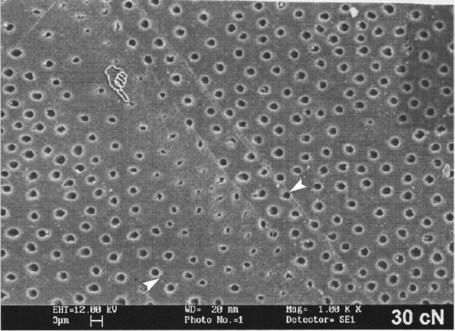
FIG. 12-38 Scanning electron micrograph of a shallow groove (between white arrowheads) created in polished dentin by a dental explorer tine under a force of 30 g (30 cN). Note the partial occlusion of the tubules by smeared matrix.
(From Camps J, Salomon JP, Meerbeek BV, et al: Dentin deformation after scratching with clinically relevant forces. Arch Oral Biol 48:527, 2003.)
Another example of the effect of strong hydraulic forces that are created within the dentinal tubules is the phenomenon of odontoblast displacement. In this reaction, the nuclei and cell bodies of odontoblasts are displaced upward in the dentinal tubules, presumably by a rapid movement of fluid in the tubules produced when exposed dentin is desiccated, as with the use of an air syringe or cavity-drying agents (Fig. 12-39).76,192 Such cellular displacement results in the destruction of odontoblasts, because cells thus affected soon undergo autolysis and disappear from the tubules. Displaced odontoblasts may eventually be replaced by stem cells that migrate from the cell-rich zone of the pulp, as discussed later in the chapter.
The hydrodynamic theory can also be applied to an understanding of the mechanism responsible for hypersensitive dentin.37,39 There is controversy regarding whether exposed dentin is simply sensitive or becomes truly hypersensitive.253,254,256 Growing evidence indicates that new sodium channels responsible for activating nerves are expressed in nerve tissue exposed to inflammation.114,129,293 An increase in the density of sodium channels or their sensitivity may contribute to dentinal hypersensitivity. Hypersensitive dentin is also associated with the exposure of dentin normally covered by cementum or enamel. The thin layer of cementum is frequently lost as gingival recession exposes cementum to the oral environment. Cementum is subsequently worn away by brushing, flossing, or using toothpicks. Once exposed, the dentin may respond to the same stimuli to which any exposed dentin surface responds (e.g., mechanical pressure, dehydrating agents). Although the dentin may at first be very sensitive, within a few weeks the sensitivity usually subsides. This desensitization is thought to occur as a result of gradual occlusion of the tubules by mineral deposits, thus reducing hydrodynamic forces. In addition, deposition of reparative dentin over the pulpal ends of the exposed tubules probably also reduces sensitivity, because reparative dentin is less innervated by sensory nerve fibers.49 However, some hypersensitive dentin does not spontaneously desensitize, so hypersensitivity may be caused by either inflammatory changes in the pulp or mechanical changes in the patency of dentinal tubules.
Currently the treatment of hypersensitive teeth is directed toward reducing the functional diameter of the dentinal tubules to limit fluid movement. Four possible treatment modalities271,348,365 can accomplish this objective:
Dentin sensitivity can be modified by laser irradiation, but clinicians must be concerned about its effect on the pulp.326,336
Neuropeptides
Of immense importance in pulp biology is the presence of neuropeptides in pulpal nerves.45,48,52,327 Pulpal nerve fibers contain neuropeptides such as calcitonin gene–related peptide (CGRP) (see Figs. 12-31, 12-33, 12-34),44 substance P (SP),253,268 neuropeptide Y (see Fig. 12-29), neurokinin A (NKA),14 and VIP.213,365 In rat molars, the largest group of intradental sensory fibers contains CGRP. Some of these fibers also contain other peptides, such as SP and NKA.45,47 Release of these peptides can be triggered by numerous stimuli, including tissue injury,14 complement activation, antigen-antibody reactions,264 or antidromic stimulation of the inferior alveolar nerve.268,269 Once released, vasoactive peptides produce vascular changes that are similar to those evoked by histamine and bradykinin (i.e., vasodilation).269 In addition to their neurovascular properties, SP and CGRP contribute to inflammation and promote wound healing.32,344 The release of CGRP can be modified by sympathetic agonists and antagonists,111,130 offering the promise of using such agonists to treat dental pain. This latter point is important because sympathetic agonists are used by clinicians every day—the vasoconstrictors present in local anesthetic solutions may have direct effects on inhibiting dental nerve activity. Local anesthetic reduction in pain may be due to the actions of both the local anesthetic and the vasoconstrictor on the nerves.129,130 In cats, capsaicin acutely activates and chronically blocks the TRPV1-expressing classes of C and A-delta nociceptors in the pulp.154 In addition, the chronic application of capsaicin ointment to skin has been shown to relieve pain in patients, suggesting that clinical trials evaluating chronic application of capsaicin for treating pulpal or periradicular pain may be of value.
Antidromic stimulation of nerves (i.e., towards the peripheral terminals) simply means that the afferent barrage goes in the opposite direction of the orthograde stimulation (towards the CNS). Normally, sensory nerves are stimulated at their peripheral terminations, and their action potentials then travel toward the brain. In antidromic nerve stimulation, the sensory nerve is usually cut, then the peripheral end of the nerve is electrically stimulated. This causes an action potential to travel backward toward the periphery, which causes release of neuropeptides in the pulp.268 All branches of the nerve also depolarize and release neuropeptides (the so-called axon reflex).306
It has been reported230,268 that mechanical stimulation of dentin produces vasodilation within the pulp, presumably by causing the release of neuropeptides from intradental sensory fibers.268 Electric stimulation of the tooth has a similar effect.142 The pulpal concentrations of CGRP, SP, and NKA are elevated in painful human teeth over healthy control teeth extracted for orthodontic treatment.14 These peptides are also elevated in pulps beneath advancing carious lesions.295,296
Plasticity of Intradental Nerve Fibers
It has become apparent that the innervation of the tooth is a dynamic situation in which the number, size, and cytochemistry of nerve fibers can change because of aging,99,207,327 tooth injury,45,47,48,52 and dental caries.296 For example, in rats, nerve fibers sprout into inflamed tissue surrounding sites of pulpal injury, and the content of CGRP and SP increases in these sprouting fibers.45,47,48,52,54 When inflammation subsides, the number of sprouts decreases. Fig. 12-40 compares the normal distribution of CGRP-immunoreactive sensory fibers in an adult rat molar with those beneath a shallow cavity preparation. The innervational pattern in normal and inflamed teeth is governed by neuronal growth factors. Neurotrophic and target-derived factors regulate neuronal structure, survival, and function and are important for the maintenance of neuronal phenotype characteristics. During development, all dental fibers appear to require nerve growth factor (NGF) and express its receptor, TrkA, at some stages,227 whereas in adult teeth the large trigeminal neurons are potentially dependent only on dental pulp–derived, glial cell line–derived neurotrophic factor (GDNF); the smaller trigeminal neurons remain dependent on NGF.190,289 This suggests that GDNF may function as a neurotrophic factor for the subset of larger nerves in the tooth, which apparently mediate mechanosensitive stimuli, whereas NGF is suggested to support neurons responsible for nociception.243 NGF is the most extensively investigated among the trophic factors.198 Binding of target-derived NGF is dependent on specific TrkA receptors located on the axonal surface, with subsequent internalization and transport to the cell body, where the effects are mediated.
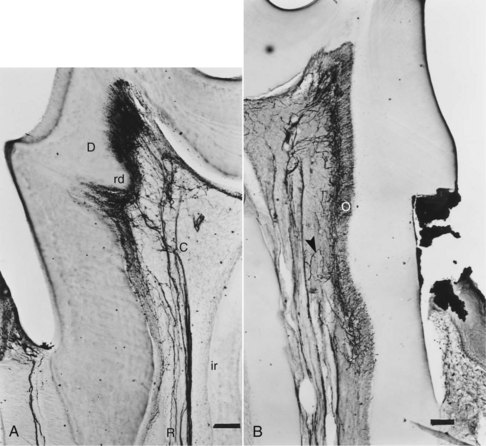
FIG. 12-40 A, Normal distribution of calcitonin gene–related peptide (CGRP)-immunoreactive sensory fibers in adult rat molar. Nerve fibers typically are unbranched in the root (R), avoid interradicular dentin (ir), and form many branches in coronal pulp (C) and dentin (D). Nerve distribution is often asymmetric, with endings concentrated near the most columnar odontoblasts (in this case on the left side of the crown). When reparative dentin (rd) forms, it alters conditions so that dentinal innervation is reduced. B, Shallow class I cavity preparation on the cervical root of a rat molar was made 4 days earlier. Primary odontoblast (O) layer survived, and many new CGRP-immunoreactive terminal branches spread beneath and into the injured pulp and dentin. Terminal arbor can be seen branching (arrowhead) from a larger axon and growing into the injury site. Scale bar: 0.1 mm. A, ×75. B, ×45.
(From Byers MR: Dynamic plasticity of dental sensory nerve structure and cytochemistry. Arch Oral Biol 39[suppl]:13S, 1994.)
Regulation of neural changes during inflammation seems to be a function of NGF expression.50,54 NGF receptors are found on intradental sensory fibers and Schwann cells.47 Evidence indicates that NGF is synthesized by fibroblasts in the coronal subodontoblastic zone (i.e., cell-rich zone), particularly in the tip of the pulp horn.47 Maximal sprouting of CGRP- and SP-containing nerve fibers corresponds to areas of the pulp where there is increased production of NGF.54 Fig. 12-41 shows the expression of NGF-mRNA in a pulp horn subjacent to cavity preparation.
FIG. 12-41 NGF-mRNA is upregulated in the mesial pulp horn 6 hours after cavity preparation.
(From Pimental E: Handbook of growth factors, vol 3, London, Taylor & Francis, 1994).
It has been suggested that neuroimmune interactions take place in the dental pulp, since a coordinated increase of pulpal nerves and immune cells has been demonstrated.164,303 In addition, recruitment of immunocompetent cells has been demonstrated in the dental pulp after electrical tooth stimulation.66,103 Similar responses have been seen in periapical bone in rats with radicular pulpitis.182 Neurogenic inflammation is generally thought to enhance healing, because denervated teeth show poorer healing following pulp exposures than innervated teeth.45,53