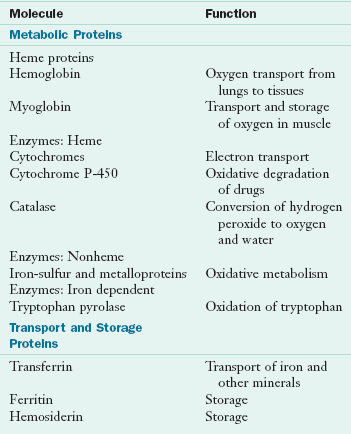
Micronutrients: Minerals
The mineral nutrients most are traditionally divided into macrominerals (≥100 mg/day required) and microminerals or trace elements (<15 mg/day required). Studies of patients receiving long-term total parenteral nutrition (TPN) have helped to determine the essentiality of ultratrace elements that are necessary in microgram quantities each day. Mineral nutrients are recognized as essential for human function, even though specific requirements have not been established for a few of them.
Mineral Composition of the Body
Minerals represent approximately 4% to 5% of body weight, or 2.8 to 3.5 kg in adult women and men, respectively. Approximately 50% of this weight is calcium, and another 25% is phosphorus, existing as phosphates. Almost 99% of the calcium and 70% of the phosphates are found in bones and teeth. The five other essential macrominerals (magnesium, sodium, potassium, chloride, and sulfur) and the 11 established microminerals (iron, zinc, iodide, selenium, manganese, fluoride, molybdenum, copper, chromium, cobalt, and boron) constitute the remaining 25%. The ultratrace elements such as arsenic, aluminum, tin, nickel, vanadium, and silicon, provide a negligible amount of weight.
Macrominerals exist in the body and food chiefly in the ionic state. Sodium, potassium, and calcium form positive ions (cations), whereas other minerals exist as negative ions (anions). The latter include chlorine as chloride, sulfur as sulfate, and phosphorus as phosphates. Minerals also exist as components of organic compounds such as phosphoproteins, phospholipids, metalloenzymes, and other metalloproteins such as hemoglobin.
With the exception of heme iron, minerals are usually absorbed in the ionic state. Therefore minerals that remain bound to organic molecules (chelated) or remain as inorganic complexes after the digestion usually cannot be absorbed and are not bioavailable. However, a few minerals may be absorbed better in a chelated form when they are properly bound to an amino acid in a covalent bond (e.g., selenomethionine). Unabsorbed minerals are excreted in the feces. Once a mineral is absorbed at the brush border of the intestinal epithelial cells, each must transfer through the cytosol and be transported across the basolateral membrane into the blood, usually by an active transport mechanism. If the mineral is not transported across the basolateral membrane, it remains in the intestinal cell bound to proteins. For example, calcium ions bind to calbindins, iron to intestinal ferritin, and zinc to metallothionein; if not transported into the blood, they are excreted when the intestinal cells die and slough off into the intestinal lumen. Such mechanisms may have evolved to protect the body against the potential toxicity of excessive absorption.
Bioavailability also is equated with absorption of a mineral element after its digestion from food and before its use in tissue and cells. Several factors can affect bioavailability of ingested minerals. Low bioavailability may also result from the formation of soaps, from calcium and magnesium binding to free fatty acids in the lumen in fat malabsorption, or from precipitation when one of a pair of ions (e.g., calcium, which combines with phosphates) is present in the lumen in a very high concentration. Mineral-mineral interactions also can result in depressed absorption of elements or reduced bioavailability. For example, the absorption of zinc is typically reduced by nonheme iron supplementation; excessive intake of zinc reduces the absorption of copper; and excessive intake of calcium may reduce the absorption of manganese, zinc, and iron.
Many organic molecules in foods influence bioavailability, either by enhancing absorption or inhibiting absorption. Examples of inhibitors include the binding by phytates and oxalates of calcium and other divalent cations. Enhancers include ascorbate for nonheme iron or the hemoglobin protein for iron. Vegetarians tend to consume foods with higher quantities of many of the inhibiting factors, but they typically also ingest more ascorbic acid, an enhancer. In addition, the bioavailability of elements may be influenced by many physiologic factors such as gastric acidity, homeostatic adaptations, and stress. NDOs fermented by intestinal bacteria stimulate the intestinal absorption and retention of calcium, magnesium, zinc, and iron.
Certain minerals generally have a low bioavailability from foods (e.g., iron, chromium, manganese), whereas others have a high bioavailability (e.g., sodium, potassium, chloride, iodide, fluoride). Calcium and magnesium have a medium bioavailability.
Problem Minerals in the U.S. Diet
A few minerals, such as calcium and iron, continue to be consumed in less than optimal amounts by a large percentage of people in the United States. The intakes of magnesium, zinc, and possibly a couple of other trace minerals are also generally insufficient in the population. In the last decade fortification of foods, especially of ready-to-eat cereals, have improved intakes of iron and zinc but not calcium (Heaney and Rafferty, 2009); the mean intakes still do not meet DRI levels.
Calcium
Calcium, the most abundant mineral in the body, makes up approximately 1.5% to 2% of the body weight and 39% of total body minerals. Approximately 99% of the calcium exists in the bones and teeth. The calcium in teeth, unlike bone, cannot be mobilized back to the blood; the minerals of erupted teeth are fixed. The remaining 1% of calcium is in the blood and extracellular fluids and within the cells of all tissues, where it regulates many important metabolic functions. Figure 3-26 illustrates the pathways of calcium metabolism. Bone is a dynamic tissue that returns calcium and other minerals to the extracellular fluids and blood on demand. Bone also takes up calcium and other minerals from the blood when they are consumed.
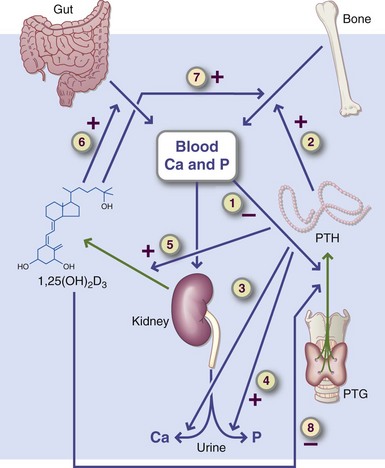
FIGURE 3-26 Pathways of calcium metabolism. The regulation of calcium metabolism involves intestinal absorption (gut), blood calcium, and phosphate concentrations, bone, the kidneys—which produce the hormonal form of vitamin D (1,25[OH]2 D3)—and the parathyroid glands, which secrete parathyroid hormone (PTH). Steps 1 through 8 are specific regulation points. A low serum calcium or high serum phosphate level stimulates PTH secretion (step 1) through negative feedback.
Absorption, Transport, Storage, and Excretion: Calcium is absorbed by all parts of the small intestine, but the most rapid absorption after a meal occurs in the more acidic (pH <7) duodenum. Absorption is slower in the remainder of the small bowel because of the alkaline pH, but the amount of calcium absorbed is actually greater in the lower segments of the small intestine, including the ileum. Small amounts of calcium can also be absorbed in the colon. Only approximately 30% of ingested calcium is absorbed by adults, but a few individuals may absorb as little as 10% and some (rarely) as much as 60% of ingested calcium. Late in life, bone retention of calcium from food and supplements is limited unless sufficient vitamin D or a bone-conserving drug is available.
Calcium is absorbed by two mechanisms: active transport, which operates predominantly at low luminal concentrations of calcium ions, and passive transport or paracellular transfer, which operates at high luminal concentrations of calcium ions. The active transport mechanism, mainly in the duodenum and proximal jejunum, has limited capacity. It is controlled through the action of 1,25(OH)2D3. This vitamin-hormone increases calcium uptake at the brush border of the intestinal mucosal cell by also stimulating the production of calcium-binding proteins (calbindins) and other mechanisms. The role of calbindins in the intestinal absorbing cells is to store calcium ions temporarily after a meal and ferry them to the basolateral membrane for the final step of absorption. The calcium-binding proteins bind two or more calcium ions per protein molecule.
The second absorption mechanism, which is passive, nonsaturable (with no limit), and independent of vitamin D, occurs along the entire length of the small intestine. When large amounts of calcium are consumed in a single meal (e.g., from a dairy food or a supplement), much of the calcium is absorbed by this passive route. The active transport mechanism is more important when calcium intakes are well below recommended intakes and body requirements are not being met.
Numerous factors influence the bioavailability and absorption of calcium within the gut lumen. The greater the need and the smaller the dietary supply, the more efficient is absorption. Increased needs during growth, pregnancy, lactation, calcium-deficient states, or exercise resulting in high bone density enhance calcium absorption. Low vitamin D intake or inadequate exposure to sunlight reduces calcium absorption, especially among older adults. In addition, the efficiency of skin production of vitamin D by older adults is lower than that of younger people. Aging is also characterized by achlorhydria, which results in less gastric acidity and reduced calcium absorption.
Calcium is absorbed only if it is present in an ionic form. Thus calcium is best absorbed in an acidic medium; the hydrochloric acid secreted in the stomach, such as that secreted during a meal, increases calcium absorption by lowering the pH in the proximal duodenum. This also applies to calcium supplements; therefore taking a calcium supplement with a meal improves absorption, especially in older adults. Lactose enhances calcium absorption. Even in adults with lactose intolerance, lactose probably improves calcium absorption.
Calcium is not absorbed if it is precipitated by another dietary constituent such as oxalate or if it forms soaps with free fatty acids. Oxalic acid (oxalate) in rhubarb, spinach, chard, and beet greens forms insoluble calcium oxalate in the digestive tract (see Chapter 36). For example, only 5% of the calcium in spinach is absorbed. Phytic acid (phytate) combines with calcium to form calcium phytate, which is insoluble and cannot be absorbed. These unabsorbed forms of calcium are excreted in the feces as calcium oxalates and calcium soaps.
Dietary fiber may decrease calcium absorption, but this may only be a problem for those who consume more than 30 g/day. Lower intakes of fiber have little effect on calcium availability. Medications can affect bioavailability or increase calcium excretion, both of which may contribute to bone loss. With fat malabsorption, calcium absorption is decreased because of the formation of calcium–fatty acid soaps. Calcium absorption does not seem to be affected by the amount of phosphate in the diet unless the intake of phosphate is excessively high or by the calcium/phosphorus ratio.
Renal Excretion: Approximately 50% of the ingested calcium is excreted in the urine each day, but an almost equivalent amount is also secreted into the intestine and joins unabsorbed calcium in the feces. Calcium resorption from the renal tubules occurs by transport mechanisms similar to those in the small intestine. Urinary calcium excretion varies throughout the life cycle, but it is typically low during periods of rapid skeletal growth. At menopause calcium excretion increases greatly, but in postmenopausal women treated with estrogen, less calcium is excreted. After approximately 65 years of age, calcium excretion decreases, most likely because of decreased intestinal absorption of calcium. In general, urinary calcium levels correlate well with calcium intake. A high sodium intake contributes to lower renal resorption of calcium and higher urinary calcium losses.
Skin Losses: Dermal losses of calcium occur in the form of skin exfoliation and sweat. The amount of calcium lost in sweat is approximately 15 mg/day. Strenuous physical activity with sweating increases the loss, even in persons with a low calcium intake.
Serum Calcium: Total serum calcium consists of three distinct fractions: free, or ionized, calcium; complexes between calcium and anions such as phosphate and citrate; and calcium that is protein bound with albumin. Serum albumin binds between 70% and 90% of the calcium that is protein bound. Ionized calcium (Ca2+) is regulated and equilibrates rapidly with protein-bound calcium in blood. The serum ionized calcium concentration is controlled primarily by PTH, although other hormones have minor roles in its regulation. These other hormones include calcitonin, vitamin D, estrogens, and others.
Total serum calcium level is maintained within a narrow range of 8.8 to 10.8 mg/dL, of which the ionized calcium concentrations range from 4.4 to 5.2 mg/dL because hypocalcemia and hypercalcemia have significant physiologic effects. Serum levels of calcium are highest early in life, gradually decrease throughout life, and reach the lowest levels during the older years. Several factors affect the relative distribution of calcium in blood serum or plasma. One of these is pH; the ionized calcium fraction is higher in acidosis and lower in alkalosis. Total calcium changes concurrently with changes in plasma protein levels; however, the ionized fraction usually remains within normal limits. The strict regulation of ionized calcium makes it a useful diagnostic tool in assessing parathyroid gland function, monitoring kidney disease, and monitoring sick neonates for whom hypocalcemia could be life threatening.
Regulation of Serum Calcium: Calcium in bones is in equilibrium with calcium in the blood. PTH plays the major role in maintaining serum calcium, as noted previously. When the blood calcium concentration falls below this level, PTH stimulates the transfer of exchangeable calcium from the bone into the blood. At the same time, PTH promotes renal tubular resorption of calcium, and it indirectly stimulates increased intestinal absorption of calcium by increasing kidney production of vitamin D (1,25[OH]2D3) (see Figure 3-26).
Other hormones—such as glucocorticoids, thyroid hormones, and sex hormones—also have important roles in calcium homeostasis. Glucocorticoid excess leads to bone loss, particularly of trabecular bone, as a result of impaired calcium absorption through both active and passive mechanisms. Thyroid hormones (T4 and T3) may stimulate bone resorption; chronic hyperthyroid conditions result in loss of compact and trabecular bone. In women normal bone balance requires serum estrogen concentrations to be within normal limits. The rapid decrease of the serum estrogen concentration during menopause is a major factor contributing to bone resorption. Treating postmenopausal women with estrogen slows the rate of bone resorption; bone reabsorption is also inhibited by testosterone.
Functions: Adequate dietary calcium is needed to permit optimal gains in bone mass and density in the prepubertal and adolescent years. These gains are especially critical for girls because the accumulated bone may provide additional protection against osteoporosis in the years after menopause. Peak calcium retention by girls has been shown to occur in the prepubertal and early pubertal periods and is influenced by race, with black girls having significantly higher retention rates (Wigertz et al., 2005).
Postmenopausal women need to obtain sufficient amounts of calcium to maintain bone health and suppress PTH, which increases later in life in most individuals, perhaps as a result of inadequate calcium in the diet. Additional amounts of calcium are recommended to meet the needs of pregnancy and lactation, infancy, childhood, and adolescence.
In addition to its function in building and maintaining bones and teeth, calcium also has numerous critical metabolic roles in cells in all other tissues. However, compared with the significant needs of the skeleton, only small amounts of calcium are required for all other cellular and extracellular functions.
The transport functions of cell membranes are influenced by calcium, which affects membrane stability in poorly understood ways. Calcium also influences the transmission of ions across membranes of cell organelles, the release of neurotransmitters at synaptic junctions, the function of hormones, and the release or activation of intracellular and extracellular enzymes.
Calcium is required for nerve transmission and regulation of heart muscle function. The proper balance of calcium, sodium, potassium, and magnesium ions maintains skeletal muscle tone and controls nerve irritability. A significant increase in the serum calcium level can cause cardiac or respiratory failure, whereas a decrease results in tetany of skeletal muscles. In addition, calcium ions play a critical role in smooth muscle contractility.
Ionized calcium initiates the formation of a blood clot by stimulating the release of thromboplastin from blood platelets. Calcium ions also serve as required cofactors for several enzymatic reactions, including the conversion of prothrombin to thrombin, which aids in the polymerization of fibrinogen to fibrin and the final step in blood clot formation.
High dietary calcium intakes are associated with decreased prevalence of overweight and obesity. The mechanism for this affect appears to be related to (1) depression of the PTH and 1,25 hydroxy vitamin D, which leads to inhibition of lipogenesis and increased lipolysis; and (2) increased excretion of fecal fat caused by soaps formation (Heaney and Rafferty, 2009) (Table 3-25).
TABLE 3-25


AI, Adequate intake; DRI, dietary reference intake; RDA, recommended dietary allowance; TPN, total parenteral nutrition.
From Institute of Medicine, The Food and Nutrition Board: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, Washington, DC, 2001, National Academies Press; Institute of Medicine, Food and Nutrition Board: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids, Washington, DC, 2000b, National Academy Press; and Institute of Medicine, Food and Nutrition Board: Dietary reference intakes for calcium and vitamin D, Washington, DC, 2011, National Academy Press.
Dietary Reference Intakes: The IOM, Food and Nutrition Board (2010) has recently set the RDA for calcium, based on estimates of requirements of both genders throughout the life cycle. The tolerable UL has also been established for this nutrient. During several periods of the female life cycle, calcium intake is critical: prepuberty and adolescence, postmenopause, and during pregnancy and lactation (Kovacs, 2005). In a study of adolescent girls, calcium intakes of 1300 mg or more each day were necessary for maximum calcium retention by the body’s skeleton. Abrams (2005) noted that calcium supplementation was helpful to children and adolescents and that catch-up mineralization was possible later in puberty if intakes were adequate.
Food Sources and Intakes: Cow’s milk and dairy products are the most concentrated sources of calcium. Dark green leafy vegetables such as kale, collards, turnip greens, mustard greens, and broccoli; almonds; blackstrap molasses; the small bones of sardines and canned salmon; and clams and oysters are good sources of calcium. Soybeans also contain ample amounts. Oxalic acid limits the availability of calcium in rhubarb, spinach, chard, and beet greens. Fortified foods (orange juice, soy, nut, grain or rice milks) contain as much calcium as cow’s milk. Many bottled waters and energy bars have calcium and sometimes vitamin D added. Tofu prepared by calcium precipitation is also a source of calcium. Table 3-26 shows the calcium content of selected foods.
TABLE 3-26
Calcium Content of Selected Foods
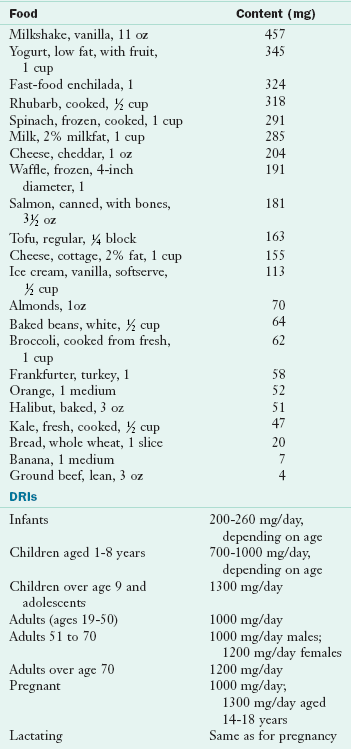
AI, Adequate intake; DRI, dietary reference intake.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18. Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w301.pdf; accessed 2011.
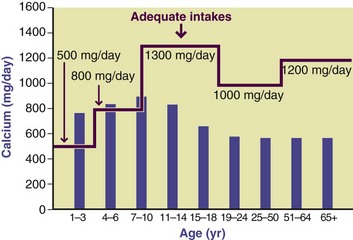
Calcium supplements are now commonly used to increase intake. The most common form is calcium carbonate, which is relatively insoluble, particularly at a neutral pH. Although it has less calcium than calcium carbonate by weight, calcium citrate is much more soluble and would be suitable for patients with a lack of hydrochloric acid in the stomach (achlorhydria). In patients with achlorhydria, the efficiency of calcium absorption is greatly decreased because of the higher pH of the stomach contents; however, calcium absorption is increased by the consumption of a meal, which improves the solubility of calcium ions because of the increased gastric acidity. The selection of the most appropriate calcium supplement depends on several factors, including physical and chemical properties, interactions with other medications being taken concurrently, current medical conditions, and age. Beginning at the age of 11 years, median dietary calcium intakes in the United States are considerably less than the AIs (Figure 3-27). Therefore calcium intakes of Americans are insufficient for the critical ages of bone deposition in both genders, as well as being inadequate at other critical stages.
Deficiency: The development of peak bone mass requires adequate amounts of calcium and phosphorus, vitamin D, and other nutrients. Compared with adulthood, greater amounts of calcium and phosphate are required for skeletal development; therefore AIs of these minerals and others have a significant effect on peak bone mass development until the time of puberty and throughout adolescence. After adolescence, bone gains may still occur, but the amounts of calcium required decrease. Vitamin D status may or may not be a problem, depending on the intakes of calcium and phosphorus. When calcium intake is well below the recommended amount, PTH is released; a persistent elevation may contribute to low bone mass. Calcium and vitamin D intakes of many older women are inadequate.
Hogan (2005) suggested that epidemic obesity and subsequent dieting may have a detrimental effect on bone status, leading to osteoporosis. An inadequate intake of calcium, in addition to an inadequate intake of vitamin D, may contribute to osteomalacia, colon cancer, and hypertension. Dietary Approaches to Stop Hypertension studies show that adequate dietary intakes of calcium, magnesium, potassium, and other micronutrients from low-fat dairy foods, fruits, and vegetables can substantially reduce blood pressure in those with hypertension or prevent its development.
Toxicity: A very high intake of calcium (>2000 mg/day) may lead to hypercalcemia. This can be exacerbated by high intakes of vitamin D. Such toxicity may lead to excessive calcification in soft tissues, especially the kidneys, and may be life-threatening. In addition, long-term high intakes of calcium may lead to increased bone fractures in older adults, perhaps because of high bone remodeling rates that lead to exhaustion of osteoblast (Klompmaker, 2005).
High intakes of calcium may also interfere with the absorption of other divalent cations such as iron, zinc, and manganese. Therefore supplements of certain minerals should be taken at different times. Another effect of excessive calcium intake is constipation, common among older women who take calcium supplements.
Physical Immobility: Prolonged bed rest or periods of weightlessness during space travel promote significant calcium losses in response to a lack of tension or gravity on the bones. Older individuals who require a prolonged recovery with limited activity, such as those with hip fractures or other illnesses, also have increased calcium losses. Physical activity, especially weight-bearing exercise, promotes bone health.
Phosphorus
Phosphorus ranks second to calcium in abundance in human tissues. Approximately 700 g of phosphorus exists in adult tissues, and approximately 85% is present in the skeleton and teeth as calcium phosphate crystals. The remaining 15% exists in the metabolically active pool in every cell in the body and in the extracellular fluid compartment. Almost 50% of the inorganic phosphate is present in serum as free ions (i.e., H2PO4− and H2PO42−). Smaller percentages are bound to protein (≈10%) or complexed (≈40%).
The serum inorganic phosphorus level is closely maintained by PTH at 3 to 4 mg/l00 mL in adults, but it is not as closely regulated as the serum calcium level. Normal blood concentrations in infants are higher. In older adults serum phosphate concentrations are typically lower; hypophosphatemia (<2.5 mg/dL) may be common among older adults. Phosphorus balance is illustrated in Figure 3-28.
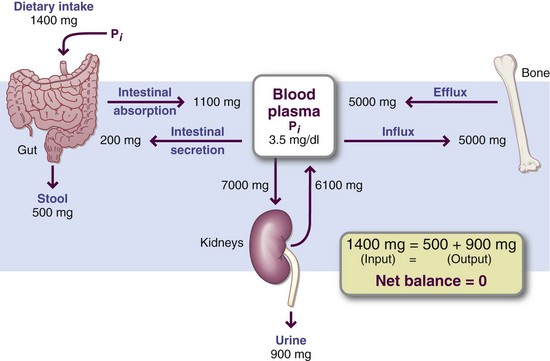
FIGURE 3-28 Phosphorus balance is maintained primarily by the amount of phosphate absorbed versus the amount excreted by the kidneys and intestine. Bone is the major storage site for phosphate, as it is for calcium. The metabolic pathways share many similarities with the calcium pathways.
Absorption, Transport, Storage, and Excretion: The relative amounts of inorganic and organic phosphates in the diet vary with the food or supplement consumed. Regardless of the form, most phosphates are absorbed in the inorganic state. Organically bound phosphate is hydrolyzed in the lumen of the intestine and released as inorganic phosphate, primarily through the action of pancreatic or intestinal phosphatases. Bioavailability depends on the form of the phosphate and the pH. The acidic milieu of the most proximal portion of the duodenum is important in maintaining phosphorus solubility and therefore bioavailability. In vegetarian diets the major portion of the phosphorus exists as phytate, which is poorly digested. Humans do not have the phytase enzyme; however, intestinal bacteria have the enzyme needed to hydrolyze phosphates. The yeast used in making bread contains a phytase, which releases phosphate.
In general, the efficiency of phosphate absorption is 60% to 70% in adults, twice as high as that of calcium. Phosphate absorption is also much more rapid than that of calcium. Peak absorption of phosphates occurs approximately 1 hour after ingestion of a meal; calcium enters the blood 3 to 4 hours after a meal.
The primary route of phosphorus excretion is renal, which also is the primary site of phosphate regulation. Major determinants of urinary phosphorus loss are an increased intake of phosphate, an increase in phosphate absorption, and the plasma phosphorus concentration. Other factors contributing to increased urinary phosphate loss are hyperparathyroidism, acute respiratory or metabolic acidosis, diuretic use, and the expansion of extracellular volume. If PTH levels are high, the urinary route excretes additional phosphate. Starvation or chronic undernutrition typically contributes to most of the alterations in metabolism that result in hypophosphatemia and renal losses of phosphate. According to Berndt and Kumar (2009), long-term regulation of phosphorus homeostasis may be controlled by hormones such as the vitamin D endocrine system and PTH as well as and the phosphatonins (FGF-23, sFRP-4, MEPE). Endogenous fecal phosphate excretion can also contribute to phosphorus homeostasis by eliminating excessive phosphate when PTH levels are elevated and phosphate load in the blood or tissues is excessively high. Reduced phosphate excretion is associated with dietary phosphorus restriction; increased plasma insulin, thyroid hormone, growth hormone, glucagon, or glucocorticoids; metabolic or respiratory alkalosis; and extracellular volume contraction.
Functions: As phosphates, phosphorus participates in numerous essential functions of the body. DNA and RNA are based on phosphate. The major cellular form of energy, ATP, contains high-energy phosphate bonds, as do creatinine phosphate and PEP. Cyclic adenosine monophosphate (cAMP) acts as a secondary signal within cells following peptide hormone activation of many membrane receptors. As part of phospholipids, phosphorus is present in every cell membrane in the body. Numerous phospholipid molecules also act as secondary messengers within the cytosol. Phosphorylation-dephosphorylation reactions control various steps in the activation or deactivation of cytosolic enzymes by kinases or phosphatases. Total intracellular concentrations of phosphate (but not ionic concentrations) are much higher than extracellular concentrations because phosphorylated compounds do not cross cell membranes easily and are trapped within the cell.
The phosphate buffer system is important in intracellular fluid and the kidney tubules, where phosphate functions in the excretion of hydrogen ion. Filtered phosphate reacts with secreted hydrogen ions, releasing sodium in the process. In turn, the sodium can be resorbed under the influence of aldosterone. Finally, phosphate ions combine with calcium ions to form hydroxyapatite, the major inorganic molecule in teeth and bones. Bone mineral, but not tooth mineral, provides phosphate ions via homeostatic regulation of serum calcium by PTH.
Dietary Reference Intakes: DRIs for phosphorus are somewhat lower than those for calcium for all age groups. Tolerable ULs are also established.
Food Sources and Intakes: In general, good sources of protein are also good sources of phosphorus. Meat, poultry, fish, and eggs are excellent sources. Milk and milk products are good sources, as are nuts and legumes, cereals, and grains. Phosphorus is bound to serine, threonine, and tyrosine in proteins. In the outer coating of cereal grains, particularly wheat, phosphorus exists in the form of phytic acid, which can form a complex with some minerals to create insoluble compounds. In conventional breads phytic acid is converted to the soluble form of orthophosphate during the leavening process. However, in the unleavened breads commonly eaten in the Middle East, the availability of practically all minerals is much lower. Table 3-27 and Appendix 36 list the phosphorus content of selected foods.
TABLE 3-27
Phosphorus Content of Selected Foods
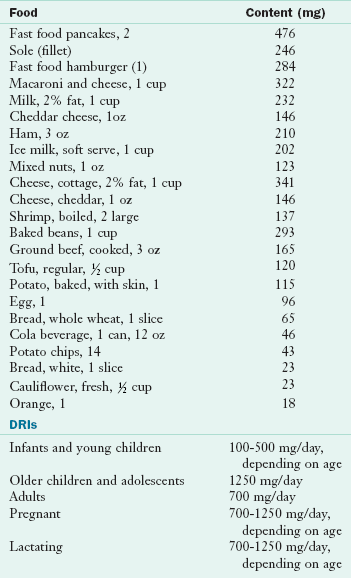
DRI, Dietary reference intake.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, retrieved 2005, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w305.pdf; accessed 1-14-11.
The average intakes of phosphorus by adults in the United States are approximately 1300 mg/day for men and 1000 mg/day for women. More than 60% of phosphorus comes from milk, meat, poultry, fish, and eggs. Cereals and legumes provide another 20%. Less than 10% is derived from fruits and their juices; tea, coffee, vegetable oils, and spices supply only small amounts of phosphorus. The amount provided by food additives to such products as meats, cheeses, dressings, beverages, and bakery products can be significant.
Deficiency: Phosphate deficiency is rare. It could develop in individuals who are taking phosphate binders for renal disease or in older adults because of poor intake in general. The widespread, ultimately fatal consequences of severe phosphorus depletion reflect its ubiquitous roles in body functions. Symptoms result from decreased synthesis of ATP and other organic phosphate molecules. Neural, muscular, skeletal, hematologic, renal, and other abnormalities occur.
Because phosphorus is so widely available from foods, including processed foods and soda types of soft drinks, a dietary inadequacy is unlikely. Clinical phosphate depletion and hypophosphatemia can result from long-term administration of glucose or TPN without sufficient phosphate, excessive use of phosphate-binding antacids, hyperparathyroidism, or treatment of diabetic acidosis. It may develop in those who have alcoholism, with or without decompensated liver disease. Premature infants who are fed unfortified human milk may also develop hypophosphatemia.
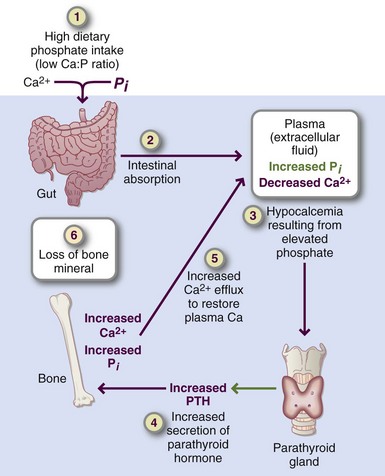
Toxicity: A persistently high concentration of PTH may result after chronic consumption of a low-calcium, high-phosphorus diet, called nutritional secondary hyperparathyroidism. PTH levels in blood that result from this diet typically remain within a high normal range (Figure 3-29). This persistently high PTH contributes to increased bone turnover, reduction of bone mass and density, and even fragility fractures because of excessive resorption and thinning of trabecular plates at bone sites throughout the skeleton. Individuals with a low calcium/phosphorous ratio benefit from increasing calcium intake from foods or supplements. Adequate calcium intake reduces the serum PTH concentration and may inhibit bone loss. The persistently high PTH level contributes to the limited bone mineralization during growth; this yields inadequate peak bone mass accumulation and the loss of bone mass.
Magnesium
After potassium, magnesium is the second-most abundant intracellular cation in the body. The adult human body contains approximately 20 to 28 g of magnesium, of which approximately 60% is found in bone, 26% in muscle, and the remainder in soft tissues and body fluids. Gender differences in the body content of magnesium begin before puberty. Magnesium in bone is present in exchangeable and nonexchangeable pools. Magnesium ions in the bone fluid compartment are much more exchangeable than magnesium ions that have become part of the crystal lattice. Normal serum levels are usually in the range of 1.5 to 2.1 mEq/L (0.75 to 1.1 mmol/L). Approximately half the magnesium in plasma is free; approximately one third is bound to albumin; and the remainder is complexed with citrate, phosphate, or other anions. Magnesium homeostasis is governed by intestinal absorption and renal excretion. No hormone is known to have a major role in the control of serum magnesium.
Absorption, Transport, Storage, and Excretion: The efficiency of absorption of magnesium varies widely from 35% to 45%. Magnesium may be absorbed along the length of the small intestine, but most absorption occurs in the jejunum. Like other divalent cation minerals, the entry of magnesium from the gut lumen occurs by two mechanisms: a carrier-facilitated process and simple diffusion. A saturable facilitated mechanism operates at low intraluminal concentrations, whereas paracellular movement across the mucosa predominates throughout the length of the small bowel when intraluminal concentrations are high. The efficiency of absorption varies with the magnesium status of the individual, the amount of magnesium in the diet, and the composition of the diet as a whole. Vitamin D has little or no effect on magnesium absorption.
Serum magnesium concentration is remarkably constant. Maintenance of these constant values depends on absorption, excretion, and transmembranous cation flux rather than on hormonal regulation. Once in the cells, magnesium is bound mainly to protein and energy-rich phosphates. The magnesium balance is illustrated in Figure 3-30.
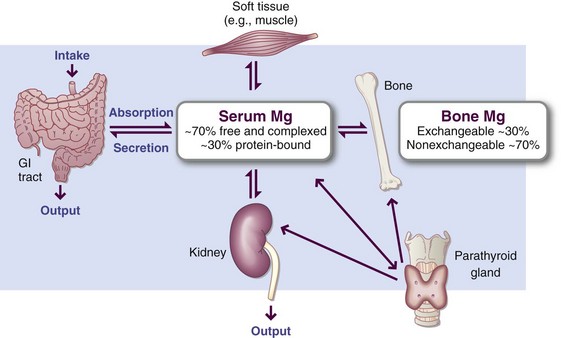
FIGURE 3-30 Magnesium balance is maintained largely by gastrointestinal absorption and renal excretion.
The kidneys control magnesium balance by conserving magnesium efficiently, particularly when intake is low. Supplementing a normal intake increases urinary excretion while serum levels remains stable. Low dietary intake of magnesium results in reduced urinary excretion of magnesium. To allow nursing mothers to meet the increased needs for magnesium, urinary excretion of the mineral tends to decrease during lactation. Renal resorption varies inversely with that of calcium.
Functions: The major function of magnesium is to stabilize the structure of ATP in ATP-dependent enzyme reactions. Magnesium is a cofactor for more than 300 enzymes involved in the metabolism of food, synthesis of fatty acids and proteins, phosphorylation of glucose in the glycolytic pathway, and promoting transketolase reactions. Magnesium is important in the formation of cAMP, the first cytosolic messenger to be identified as a mechanism for transmitting messages from outside the cells in response to hormones, local hormonelike factors, or other molecules.
Magnesium plays a role in neuromuscular transmission and activity, working in concert with and against the effects of calcium, depending on the system involved. In a normal muscle contraction, calcium is a stimulator, and magnesium is a relaxant. Magnesium acts as a physiologic calcium-channel blocker. High magnesium intakes are associated with greater bone density. The reactivity of vascular and other smooth muscle cells depends on the ratio of calcium to magnesium in the blood.
Magnesium also plays a role in learning and memory. A new product, magnesium-L-threonate, leads to the enhancement of learning, working memory, and short and long-term memory in all ages of rats (Slatsky et al., 2010). Although it is too early to extrapolate to humans, this is an exciting area of research. Magnesium depletion has been detected in persons with migraine headaches, severe asthma, dysmenorrhea, leg cramps, diabetes mellitus, chronic renal failure, nephrolithiasis, osteoporosis, aplastic osteopathy, and heart and vascular disease (Guerrera et al., 2009; Musso, 2009).
Large doses of magnesium can result in central nervous system depression, anesthesia, or even paralysis, especially in patients with renal insufficiency. Thus patients with renal problems should not be given magnesium supplements.
Dietary Reference Intakes: The RDA for magnesium was increased in 1997; different recommendations were made for boys and girls beginning at puberty. ULs were also established, as were AIs for infants.
Food Sources and Intakes: Magnesium is abundant in many foods. Good sources are seeds, nuts, legumes, and milled cereal grains, as well as dark green vegetables, because magnesium is an essential constituent of chlorophyll. Milk is a moderately good source of magnesium, especially because milk and other dairy products are so widely consumed. Tofu prepared by magnesium precipitation (check the label) is a good source.
Fish, meat, oranges, apples, and bananas, are poor sources of magnesium. Diets high in refined foods, meat, and dairy products are usually lower in magnesium than diets rich in vegetables and unrefined grains (Table 3-28). Magnesium is lost during the processing of foods such as sugar; after refining wheat cereals, it is not generally replaced as enrichment.
TABLE 3-28
Magnesium Content of Selected Foods

AI, Adequate intake; DRI, dietary reference intake; RDA, recommended dietary allowance.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w304.pdf; accessed 1-14/11.
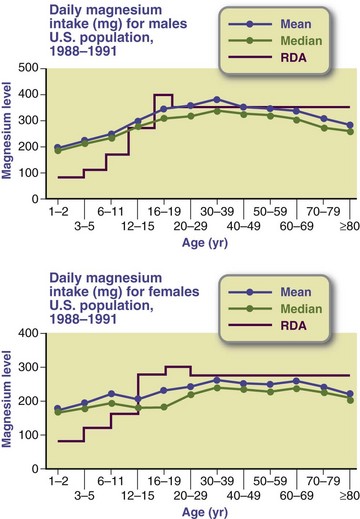
The most commonly consumed food sources include milk, bread, coffee, ready-to-eat cereals, beef, potatoes, and dried beans and lentils. Americans have had median intakes of magnesium well below the RDAs, with older adults having the lowest intakes (Figure 3-31). This trend is implicated in development of diseases such as osteoporosis and diabetes (He et al., 2006). High intakes of calcium, protein, vitamin D, and alcohol all increase the requirements for magnesium; physical or psychologic stress may also increase magnesium needs.
Deficiency: Although rare, severe magnesium deficiency symptoms include tremors, muscle spasms, personality changes, anorexia, nausea, and vomiting. Tetany, myoclonic jerks, athetoid movements, convulsions, and coma have also been reported. Hypocalcemia and hypokalemia typically occur first, combined with impairment of the individual’s responsiveness to PTH and sodium retention.
The effects of severe magnesium depletion on bone metabolism include decreased PTH secretion by the parathyroid glands, very low serum PTH, impaired responsiveness of bone and kidneys to PTH, decreased serum 1,25(OH)2D3, vitamin D resistance, altered hydroxyapatite crystal formation, impaired bone growth in young patients, or the development of osteoporosis in seniors. With continued depletion of magnesium, PTH concentrations drop even further. Intravenous administration of magnesium reverses the clinical signs and symptoms within a short time.
Moderate depletion of magnesium apparently is prevalent in older populations in Western nations (Leenhardt et al., 2005). Such deficiencies are typically precipitated by low dietary intakes, especially in individuals who avoid consuming dark green leafy vegetables, milk, and other good sources of magnesium. An increased loss of electrolytes, especially potassium, or a shift in electrolyte balance also triggers a moderate magnesium deficiency. Conditions and situations that may cause acute deficiencies include renal disease, diuretic therapy, malabsorption, hyperthyroidism, pancreatitis, protein insufficiency, diabetes, parathyroid gland disorders, postsurgical stress, and vitamin D–resistant rickets. Magnesium deficiency has also been linked to insulin resistance and metabolic syndrome because magnesium is required for carbohydrate metabolism (He et al., 2006).
Magnesium status is difficult to determine from serum measurements of magnesium because the total serum magnesium level remains constant within a wide range of intake levels. Leukocyte magnesium contents are much more sensitive to nutritional status, which makes them a superior marker. Urinary excretion of magnesium (and often of potassium) is less in those with a magnesium deficiency than in those with sufficient magnesium, suggesting that those with magnesium deficiencies have greater retention of magnesium and improved tissue magnesium status throughout the body.
Attention has focused on the interrelationships of magnesium and other electrolytes, particularly potassium, and related effects. For example, a low magnesium intake contributes to hypertension along with inadequate intakes of potassium and calcium. Oral magnesium supplementation may lower systolic and diastolic blood pressure significantly. Low magnesium intakes have also been associated with coronary heart disease, myocardial infarction, and osteoporosis.
Toxicity: Although excess magnesium can inhibit bone calcification, excesses from dietary sources and supplements are unlikely to result in toxicity. However, the ULs for magnesium from supplements or pharmacologic agents were established in 1998. The only cases of toxicity that have been reported involve smelter workers who inhale or otherwise ingest toxic levels of magnesium dust.
Sulfur
Although sulfur has long been studied as a mineral, it functions almost entirely as a component of organic molecules. Sulfur exists in the body as a constituent of three amino acids—cystine, cysteine, and methionine—and as part of organic molecules in all cells and extracellular compartments, such as connective tissue. The tertiary structure of proteins is attributable in part to covalent bonding between cysteine residues in which the SH groups are oxidized to form disulfide bridges. These bridges also provide the three-dimensional structural modifications necessary for the activity of some enzymes, insulin, and other proteins. Sulfhydryl groups of proteins also participate in diverse cellular reactions. For example, the poisonous effects of arsenic are caused by its ability to bind sulfhydryl groups of enzymes. The sulfur of cysteine binds to iron-sulfur clusters in electron transfer proteins involved in basic, life-sustaining processes, such as photosynthesis, nitrogen fixation, and oxidative phosphorylation.
Glutathione, a tripeptide-containing cysteine, acts as a donor of reducing equivalents for the reduction of hydrogen peroxide and organic peroxides by GSH-Px. In the broadest sense, sulfur can be considered an antioxidant. Sulfur is as a component of heparin, an anticoagulant found in liver and tissues, and as chondroitin sulfate in bone and cartilage. Sulfur is also an essential component of three vitamins—thiamin, biotin, and pantothenic acid (Brosnan and Brosnan, 2009).
Sulfur is also part of the molecule, S-adenosylmethionine. The transmethylation pathway within cells, especially in the liver, converts methionine to homocysteine while transferring the methyl group to other molecules. This pathway is linked to the metabolism of other important molecules such as cysteine, adenine (a nucleoside), and polyamines.
Sulfur-containing amino acids regulate lipid metabolism (Oda, 2006). Taurine, a sulfur-containing amino acid made by liver cells, is used to conjugate bile acids before secretion. Nonhepatic cells use sulfate bound to an organic donor for the synthesis of iron-sulfur proteins. In addition, structural molecules within cells like proteoglycans contain sulfated monosaccharide (glucose and galactose) residues.
The metabolism of sulfur-containing amino acids generates inorganic acids, especially sulfate anions, in substantial amounts. These sulfates are thought to combine with calcium ions in the glomerular ultrafiltrate, thereby reducing the renal tubular resorption of calcium. This mechanism may explain as much as 50% of the calcium loss associated with protein-induced hypercalciuria, which develops after consumption of meals rich in animal proteins—proteins that are rich in sulfur.
Methionine and cysteine provide almost 100% of the sulfur in the human diet. Food sources of sulfur include meat, poultry, fish, eggs, dried beans, broccoli, and cauliflower. Sulfur deficiency or toxicity is highly unlikely. Excess inorganic sulfur generated as a result of hepatic or renal metabolism is excreted in the urine as sulfates. There are no DRIs for sulfur.
Microminerals/Trace Elements
Numerous microminerals or trace elements are present in minute amounts in body tissues and are essential for optimum human growth, health, and development. The functions of and deficiency symptoms produced by trace elements are subtle and difficult to identify, partly because many of these effects occur at the cellular or subcellular level. For example, iron deficiency eventually results in a type of anemia that is easy to identify. The cellular effects cannot be identified as easily but may actually be more harmful to the individual.
The knowledge of the various functions of trace and ultratrace minerals continues to grow. DRIs and ULs have been established for nine essential trace elements: chromium, copper, iodine, iron, manganese, molybdenum, selenium, zinc, and fluoride. DRIs for five potentially essential trace elements—arsenic, boron, nickel, silicon, and vanadium—have not yet been published. No DRI exists for cobalt, just for cobalt-containing vitamin B12 (cobalamin).
General Characteristics
Trace elements exist in two forms: as charged ions or bound to proteins. Each element has different chemical properties that become critical in its functional role in cells or extracellular compartments. In blood and other tissue and cellular fluids, the trace elements do not exist in the free ionic state; they are typically bound to transporting or holding proteins. Fluoride ions become bound in the hydroxyapatite crystals of bones and teeth.
Functions: Many enzymes require small amounts of one or more trace metals for full activity. Metals function in enzyme systems by participating directly in the catalyzed reaction, by combining with substrates to form complexes on which enzymes act, by forming metalloenzymes that bind substrates, by combining with reaction end products, or by maintaining quaternary structures. Minute concentrations of trace minerals affect the whole body through interactions with the enzymes or hormones that regulate masses of substrate. This ability is amplified if, in turn, the substrate has some regulatory function. Trace minerals may also interact with DNA to control the transcription of proteins important for the metabolism of that particular trace mineral.
Food Sources: Compared with other sources, foods of animal origin are generally superior sources of trace elements because concentrations of the elements tend to be higher and the metals more available for absorption. Seafood in particular is usually rich in nearly all micronutrients except manganese, which is more readily available from plant sources. The trace element content of many plants depend on the minerals content of the soil; in addition, trace elements are not distributed evenly in wheat grains, and the germ and outer layers that contain major amounts of most minerals are removed to a large extent by the milling process. However, the small quantities of minerals that remain in white flour are more biologically available than those in whole-wheat flour, which are in complexes with or bound by molecules in the inner layer such as phytate and fiber. Unless the pH is lowered during product production, these minerals remain unavailable.
Iron
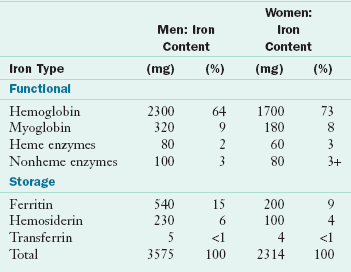
Iron has been recognized as an essential nutrient for more than a century. Nutritional iron deficiency and iron deficiency anemia remain far too common in the twenty-first century given the wide availability of iron-rich foods (see Chapter 33). Indeed, iron-deficiency anemia is the world’s most common nutritional deficiency disease. Many advances have been made in the study of iron metabolism and iron deficiency, but questions persist about mechanisms regulating absorption and iron balance. The adult human body contains iron in two major pools: (1) functional iron in hemoglobin, myoglobin, and enzymes; and (2) storage iron in ferritin, hemosiderin, and transferrin. Healthy adult men have approximately 3.6 g of total body iron, whereas women have approximately 2.4 g (Table 3-29). Adult women store much lower amounts of iron than do men. Iron is highly conserved; approximately 90% is recovered and reused every day and the rest is excreted, primarily in bile. If dietary iron is not available to meet this 10% gap, iron deficiency results.
Two concerns about iron nutritional status predominate: the incidence of iron-deficiency anemia and the role of excessive iron intake in coronary heart disease and cancer. Because of food fortification and the use of iron supplements by so many individuals, high iron intakes by men and postmenopausal women may be contributing to the risk of these chronic diseases. In fact, a study of older adults replete with iron in the Framingham Heart Study cohort concluded that increased iron stores are a liability (Fleming et al., 2001).
Absorption, Transport, Storage, and Excretion: Dietary iron exists as heme iron, found in hemoglobin, myoglobin, and some enzymes; and as nonheme iron, found predominantly in plant foods, but also in some animal foods as nonheme enzymes and ferritin. Heme iron is absorbed across the brush border of intestinal absorbing cells after it is digested from animal sources. After heme enters the cytosol, the ferrous iron is enzymatically removed from the ferroporphyrin complex. The free iron ions combine immediately with apoferritin to form ferritin in the same way that free nonheme iron combines with apoferritin.
Ferritin is an intracellular store, a “ferry” that carries bound iron from the brush border to the basolateral membrane of the absorbing cell. The final step of absorption by which iron ions are moved into the blood involves an active transport mechanism. At this point, it is the same for heme and nonheme iron (Figure 3-32). The absorption of heme iron is affected only minimally by the composition of meals and GI secretions. Heme iron represents only 5% to 10% of the dietary iron in a mixed diet, but absorption may be as high as 25%, compared with only 5% for nonheme iron. Because vegans consume only plant foods, sufficient amounts of nonheme iron must be ingested and absorbed to meet body requirements or supplements would be needed.
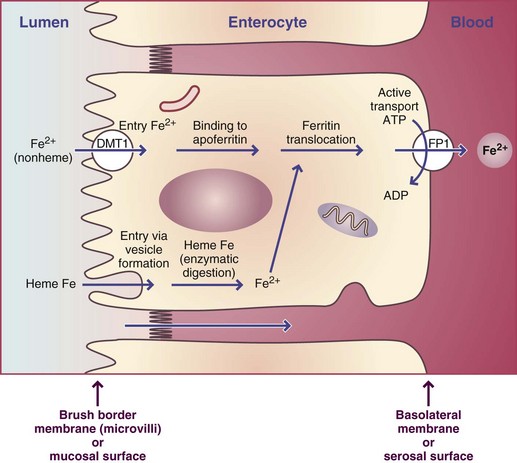
FIGURE 3-32 Intestinal absorption of iron from heme and nonheme sources by an intestinal absorbing cell, or enterocyte. Enterocytes contain two membranes: the brush border membrane and the basolateral membrane. The entry step of nonheme iron at the brush border membrane is different from that of heme iron. Heme iron enters by vesicle formation around the heme, whereas nonheme iron (ionic iron) enters by facilitated diffusion down a concentration gradient. Absorbed ions combine with apoferritin to form ferritin complexes that move across the cell by diffusion to the basolateral membrane for the exit step of absorption by active transport. The iron of heme iron is enzymatically removed, and these ions exit at the basolateral membrane by an unknown mechanism.
ADP, Adenosine diphosphate; ATP, adenosine triphosphate.
Three steps of absorption also precede the entry of nonheme iron into the circulation. Nonheme iron must be digested from plant sources and enter the duodenum and upper jejunum in a soluble, ionized form if it is to be transferred across the brush border. The acid of gastric secretions enhances the solubility and changes iron to the ionic state—either as ferric (+3 oxidation state) or ferrous (+2 oxidation state) iron—within the gut luminal contents. Iron in the reduced, ferrous state is preferred for the entry step of absorption. The brush border iron transporter, divalent metal transporter 1 (DMT1), transports ferrous iron. Ferric iron may be reduced by a brush border enzyme, ferric reductase, for absorption. As chyme moves down the duodenum, pancreatic and duodenal secretions increase the pH of the contents to 7, at which point most ferric iron is precipitated unless it has been chelated. However, ferrous iron is significantly more soluble at a pH of 7, so these ions remain available for absorption in the remainder of the small intestine.
The efficiency of nonheme iron absorption seems to be controlled by the intestinal mucosa, which allows certain amounts of iron to enter the blood from the cytosolic ferritin pool according to the body’s needs. A small peptide hormone known as hepcidin is the main iron regulatory hormone. Production in the liver is responsive to liver iron levels, inflammation, hypoxia, and anemia. Its major action is to act on the mucosa cell and inhibit iron absorption. Therefore chronic inflammation can lead to decreased iron absorption from production of hepcidin (Muñoz et al., 2009).
Other signals from the body to the absorbing cells may be transferrin saturation, or the percentage of iron bound to transferrin (Figure 3-33). Normally transferrin saturation is 30% to 35% in healthy, iron-consuming individuals. The percentage can vary greatly, depending on iron intake and bioavailability. A low percentage (e.g., 15%) of the total iron-binding capacity (TIBC) of transferrin would stimulate the absorbing cells to transport iron by the exit step at the basolateral membrane to the blood. Conversely, if the iron concentration in the body is excessive, absorbing cells would be downregulated, and less iron would be absorbed. The latter situation occurs during iron overloads to protect the body against toxicity.
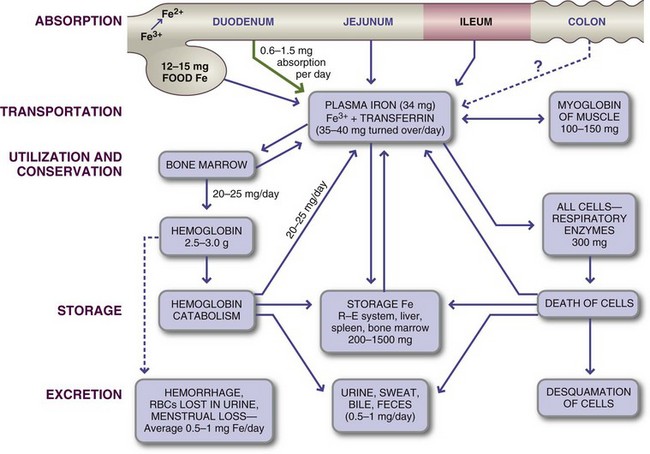
FIGURE 3-33 Iron metabolism in adults. Most iron is absorbed from the duodenum and jejunum, after which it is transported as plasma iron or bound to transferrin.
RBCs, Red blood cells; R-E system, reticuloendothelial system.
The life span of an intestinal absorbing cell is approximately 3 to 6 days. During this time the cell emerges from the crypt after cell division, passes up the villus to the tip, and eventually sloughs off as a dead cell. During the early life of the individual cell, signals resulting from the saturation percentage of circulating transferrin are sent to the young cells to adjust their number of receptors for transferrin (e.g., to increase iron absorption in a state of iron deficiency). Other cells formed before or after may have different numbers of receptors, depending on the nutritional supply of iron.
In individuals who persistently consume inadequate levels of iron, especially women in their childbearing years, the number of receptors may consistently be upregulated to maximize iron absorption. The efficiency of iron absorption by adults with normal hemoglobin values averages 5% to 15% of the iron, heme and nonheme, from food and supplements. Although absorption may be as high as 50% in those with iron deficiency anemia, this level is not common. Most women with an iron deficiency, but not anemia, probably have absorption efficiencies of 20% to 30%. From 2% to 10% of nonheme iron in vegetables is absorbed, and from 10% to 30% of iron heme and nonheme from animal sources is typically absorbed.
Several factors affect the intestinal absorption of iron. The efficiency of iron absorption is determined to some extent by the foods from which it is derived or with which it is consumed. Ascorbic acid, the most potent enhancer of iron absorption, reduces ferric to ferrous iron and forms a chelate with iron that remains soluble at the alkaline pH of the lower small intestine. Other food molecules, such as sugars and sulfur-containing amino acids, may also enhance iron entry by forming chelates with ionic iron. In addition, animal proteins from beef, pork, veal, lamb, liver, fish, and chicken enhance absorption. The substance responsible for this improved absorption—the meat-fish-poultry (MFP) factor—remains unknown, but specific amino acids or dipeptide digestion products may enhance iron absorption.
Although the iron content of human milk is very low, it is highly bioavailable because of the presence of milk lactoferrin, which enhances iron absorption. Infants retain more iron from human milk than from cow’s milk or infant formulas because of the presence of lactoferrin in breast milk. Whey protein (lactalbumin), which constitutes a greater percentage of the total protein in human milk than in cow’s milk, may also improve iron absorption.
The degree of gastric acidity enhances solubility and therefore bioavailability of iron derived from foods. Therefore achlorhydria, hypochlorhydria, or administration of alkaline substances such as antacids can interfere with nonheme iron absorption by not permitting the solubilization of iron in gastric and duodenal fluids. Gastric secretions also seem to increase the absorption of heme iron.
Certain physiologic states such as pregnancy and growth that involve increased blood formation stimulate iron absorption. In addition, more iron is absorbed during iron deficiency states because of adaptive mechanisms that enhance nonheme iron absorption.
Foods with high phytate content have low iron bioavailability, but whether phytate is the cause is not clear. Oxalates can inhibit absorption. Tannins, which are polyphenols, in tea also reduce nonheme iron absorption. On the other hand, the presence of an adequate amount of calcium helps to remove phosphate, oxalate, and phytate that would otherwise combine with iron and inhibit its absorption.
The availability of iron from various compounds used for food enrichment or as supplements varies widely according to their chemical composition. Although iron in the ferrous form is most readily absorbed, not all ferrous compounds are equally available. Ferrous pyrophosphate is used frequently in products such as breakfast cereals because it does not add a gray color to the food; however, this compound and others such as ferrous citrate and ferrous tartrate are poorly absorbed. Iron is usually added to baby foods in an elemental form, the absorbability of which depends on the iron particle size. Increased intestinal motility decreases iron absorption by decreasing contact time and rapidly removing the chyme from the area of highest intestinal acidity. Poor fat digestion leading to steatorrhea also decreases iron absorption and the absorption of other cations.
Transport: Iron (nonheme) is transported, bound to transferrin (see Figure 3-33), from the intestinal absorbing cells to various tissues to meet their needs. It rarely exists in the free ionic state in serum.
Storage: Between 200 and 1500 mg of iron is stored in the body as ferritin and hemosiderin; 30% is in the liver, 30% is in the bone marrow, and the rest is in the spleen and muscles. Up to 50 mg/day can be mobilized from storage iron, 20 mg of which is used in hemoglobin synthesis; see estimates in Table 3-29. The amounts of circulating ferritin in blood correlate closely with total body iron stores, which makes this measurement valuable for evaluation of iron status.
Intestinal Excretion: Iron is lost from the body only through bleeding and in very small amounts through defecation, sweat, and the normal exfoliation of hair and skin. Most of the iron lost in the feces could not be absorbed from food. The remainder comes from bile and the cells exfoliated from the GI epithelium. Almost no iron is excreted in the urine. Daily iron loss is approximately 1 mg for men and slightly less for nonmenstruating women. The loss of iron accompanying menstruation averages approximately 0.5 mg/day. However, wide variations exist among individuals, and menstrual losses of more than 1.4 mg of iron daily have been reported in approximately 5% of normal women.
Functions: The functions of iron relate to its ability to participate in oxidation and reduction reactions. Chemically, iron is a highly reactive element that can interact with oxygen to form intermediates with the potential of damaging cell membranes or degrading DNA. Iron must be tightly bound to proteins to prevent these potentially destructive oxidative effects.
Iron metabolism is complex because this element is involved in so many aspects of life, including red blood cell function, myoglobin activity, and the roles of numerous heme and nonheme enzymes. Because of its oxidation-reduction (redox) properties, iron has a role in the blood and respiratory transport of oxygen and carbon dioxide, and it is an active component of the cytochromes (enzymes) involved in the processes of cellular respiration and energy (ATP) generation. Iron is also involved in immune function and cognitive performance; this underscores the importance of preventing iron deficiency anemia throughout the world.
Hemoglobin, which is present in red blood cells, is synthesized in immature cells in bone marrow. Hemoglobin works in two ways: the iron-containing heme combines with oxygen in the lungs; and the heme releases the oxygen in tissues, where it picks up carbon dioxide and then releases it in the lungs after its return from the tissues. Myoglobin, also a heme-containing protein, serves as an oxygen reservoir within muscle. Table 3-30 lists the major iron molecules in the body and their functions.
Oxidative production of ATP within the mitochondria involves many heme and nonheme iron-containing enzymes. The cytochromes, present in nearly all cells, function in the mitochondrial respiratory chain in the transfer of electrons and the storage of energy through the alternate oxidation and reduction (redox) of iron (Fe2+ to and from Fe3+). Numerous water-insoluble drugs and endogenous organic molecules are transformed by the iron-containing cytochrome P-450 system in the liver into more water-soluble molecules that can be secreted in the bile and eliminated. Ribonucleotide reductase, the rate-limiting enzyme involved in DNA synthesis, is also an iron enzyme. Although these vital enzymes represent only a small portion of the total iron in the body, a severe decrease in their concentrations can have long-term consequences. Other enzymes, including several in the brain, also require iron.
An adequate iron intake is essential for the normal function of the immune system. Iron overloads and deficiencies result in changes in the immune response. Iron is required by bacteria; therefore an iron overload (especially intravenously) may result in an increased risk of infection. Iron deficiency affects humoral and cellular immunity. Concentrations of circulating T-lymphocytes decrease in individuals with an iron deficiency, and the mitogenic response is typically impaired. Natural killer (NK) cell activity also decreases. Production of interleukin-1 is reduced in iron-deficient animals, and depressed interleukin-2 production has been reported.
Two iron-binding proteins—transferrin (in blood) and lactoferrin (in breast milk)—seem to protect the body against infection by withholding iron from microorganisms that need it for proliferation. Iron is used by brain cells for normal function in people of all ages. Iron is involved in the function and synthesis of neurotransmitters and possibly myelin. The detrimental effects of early iron deficiency anemia in children can persist for many years. For example, declines have been found between the scholastic performance, sensorimotor competence, attention, learning, and memory of children with anemia. Iron supplementation in children with iron deficiency anemia has been found to improve learning, as indicated by achievement test scores (Beard, 2001). Changes in iron metabolism occur in Alzheimer disease and other disorders.
Dietary Reference Intakes: DRIs have been established for iron. The RDA for men and postmenopausal women is 8 mg/day. The RDA for women of childbearing age (to replace iron loss from menstruation and provide for iron stores sufficient to support a pregnancy) is 18 mg/day. For teenage boys (ages 14 to 18) the iron RDA is 11 mg/day. Full-term infants are born with a reserve supply of iron from placental transfer during gestation, but normal-term infants still require adequate iron from food sources and fortified milk products during the first year of life. Premature infants have limited iron stores because they lack most of the iron and other trace minerals that are normally transferred during the last trimester of pregnancy. The need for iron to support rapid growth in premature infants becomes apparent at approximately 2 to 3 months of age. The RDAs for ages 1 year and older are (variably) 7, 8, or 10 mg/day until adolescence begins at age 14. Figure 3-34 shows the physiologic requirements for iron in relation to age. Requirements are highest during infancy and adolescence. Iron needs among males decrease after the adolescent growth spurt, whereas the iron needs of their female counterparts continue to be high until the menopause. Iron allowances increase during pregnancy from 15 to 27 mg/day, but not during lactation, although many lactating women are told to continue taking their iron supplements.
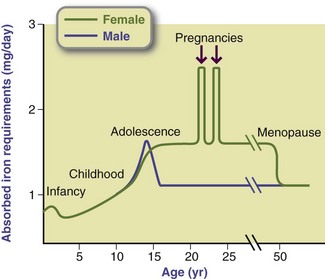
FIGURE 3-34 The absorbed iron requirement for various ages. The greatest requirements for iron occur during infancy. During childhood, requirements are the same for boys and girls. During the adolescent growth spurt, iron needs increase and are greater for boys than girls. However, because of menstruation, the requirements after adolescence remain high for females but decrease for males.
Food Sources and Intakes: By far the best source of dietary iron is liver, followed by seafood, kidney, heart, lean meat, and poultry. Dried beans and vegetables are the best plant sources. Some other foods that provide iron are egg yolks, dried fruits, dark molasses, whole-grain and enriched breads, wine, and cereal. Old-fashioned iron skillets used for cooking add to the total iron intake. Table 3-31 presents the iron content of selected foods.
TABLE 3-31
Iron Content of Selected Foods
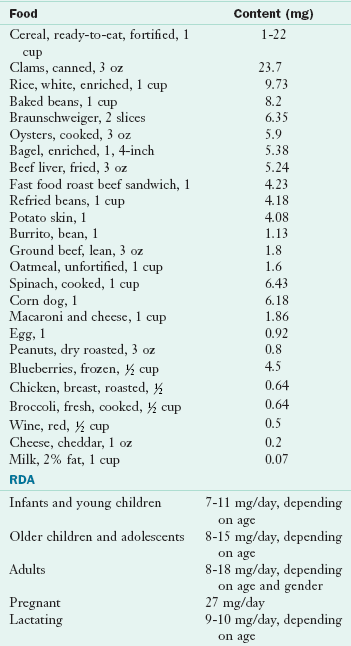
RDA, Recommended dietary allowance.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w303.pdf; accessed 2011.
The availability of iron derived from food is important in the consideration of dietary sources. For example, only 50% or less of the iron in whole-grain cereals and in some green vegetables is available in a usable form. Corn is a notoriously poor source of iron; milk and milk products are practically devoid of iron. When dietary intake focuses primarily on these foods, anemia levels can be high. Vegetarian or vegan women can obtain enough iron from their plant-based diet, but they must consume sufficient amounts of moderately iron-rich foods, such as legumes and dried fruits. Soy products are typically good sources of both iron and zinc.
Iron fortification of cereals, flours, and bread has added significantly to the total iron intake of the U.S. population. Fortified cereals are a substantial source of iron for infants and children, as well as for adolescents and adults. Concern about potential iron overloading from fortified breakfast foods has been raised because analyzed values of iron content may be considerably greater than labeled values. The foods that supply the greatest amount of iron in the U.S. diet include ready-to-eat cereals fortified with iron; bread, cakes, cookies, doughnuts, and pasta (all fortified with iron); beef; dried beans and lentils; and poultry.
Whereas the median iron intakes of most women are lower than the RDA, the median intakes of men generally exceed the RDA. An adequate diet containing meats and other animal sources typically has high iron content, containing approximately 6 mg of iron per 1000 kcal. Therefore the average omnivorous woman of childbearing age consuming 2000 kcal takes in only 12 mg of iron, or approximately 67% of the RDA of 18 mg/day. This intake level meets the needs of almost no menstruating woman. However, iron intakes totaling much less than 12 mg/day place women at more serious risk for developing deficiency anemia. Women with high daily iron losses compensate with an increased rate of absorption. Even with this adaptation, insufficient stores of iron typically exist and the risk of anemia remains high.
Deficiency: Iron deficiency, the precursor of iron deficiency anemia, is the most common of all nutritional deficiency diseases. In the United States and worldwide, iron deficiency anemia is prevalent among children and women of childbearing age. The groups considered to be at greatest risk for iron deficiency anemia are infants younger than 2 years of age, adolescent girls, pregnant women, and older adults. Pregnant teenagers are frequently at high risk because of poor eating habits and continuing growth. Women in their childbearing years who are iron deficient benefit from either a diet rich in iron-containing foods or supplements.
The final stages of iron deficiency include hypochromic, microcytic anemia. Anemia may be corrected by providing high-dose supplements in the form of ferrous sulfate or ferrous gluconate until blood parameters return to normal. To prevent worsening of the iron deficiency, individuals should be counseled regarding a diet that is appropriately rich in iron.
Iron deficiency can be caused by injury, hemorrhage, or illness (e.g., blood loss from hookworms, GI diseases that interfere with iron absorption). Iron deficiency may also be aggravated by an unbalanced diet containing insufficient iron, protein, folate, and vitamin C. Anemia typically develops because of an inadequate amount of dietary iron or faulty iron absorption.
Female athletes, especially cross-country runners and others involved in endurance sports, often have an iron deficiency at some point in their training if they are not taking iron supplements or do not have diets high in iron. The source of the additional iron losses in those with athletic amenorrhea may be through the gut; losses may increase during the stressful conditions of training. It seems that without supplementation, the greater the intensity of training, the lower the iron levels become in women.
Toxicity: The major cause of iron overload is hereditary hemochromatosis, whereas transfusion iron overload is rare. The latter may be seen in individuals with sickle cell disease or thalassemia major who require transfusions for their anemia. Iron overload is linked to a distinct gene that favors excessive iron absorption if the iron is available in the diet. Both are linked to decreased hepcidin levels (Nemeth and Ganz, 2009). The characteristic chemical parameters of iron overload are listed in Box 3-7.
Frequent blood transfusions or long-term ingestion of large amounts of iron can lead to abnormal accumulation of iron in the liver. Saturation of tissue apoferritin with iron is followed by the appearance of hemosiderin, which is similar to ferritin but contains more iron and is very insoluble. Hemosiderosis is an iron storage condition that develops in individuals who consume abnormally large amounts of iron or in those with a genetic defect resulting in excessive iron absorption. If the hemosiderosis is associated with tissue damage, it is called hemochromatosis. See Chapter 33.
Iron supplements may not be beneficial for postmenopausal women and older men because of increased risks for heart disease and cancer; iron contributes to an environment that favors oxidation of LDL cholesterol, arterial vessel damage, and other adverse effects. In addition, excessive iron may help generate free radicals that attack cellular molecules, thereby increasing the number of potentially carcinogenic molecules within cells. These potential adverse iron-disease linkages need to be confirmed.
Zinc
Zinc is abundantly distributed throughout the human body, second only to iron among trace elements. The human body has approximately 2 to 3 g of zinc, with the highest concentrations in the liver, pancreas, kidney, bone, and muscles. Other tissues with high concentrations include parts of the eye, prostate gland, spermatozoa, skin, hair, fingernails, and toenails. Zinc is primarily an intracellular ion, functioning in association with more than 300 different enzymes of various classes. Even though zinc is abundant in the cytosol, virtually all of it is bound to proteins, but it is in equilibrium with a small ionic fraction.
The most readily available form of zinc occurs in animal flesh, particularly red meats and poultry. Meat intake is frequently low among preschoolers, usually displaced by cereal foods, milk, and milk products that children tend to prefer. This observation led to the fortification of infant and children’s foods, especially cereals, with zinc. Milk is a good source of zinc, but high intakes of calcium from milk may interfere with the absorption of iron and zinc (see the bioavailability discussion. The phytates from whole grains in unleavened breads may limit zinc absorption in some populations. According to the WHO, zinc deficiency is one of the 10 major factors contributing to disease in developing countries (Shrimpton et al., 2005). Deficiencies are less likely to be a problem in Western nations, where breads, breakfast foods, and other cereal-based foods are made primarily from refined grains and are typically fortified.
Absorption, Transport, Storage, and Excretion: Zinc absorption and excretion are controlled by poorly understood homeostatic mechanisms. The mechanism of absorption involves two pathways. A saturable carrier mechanism operates most efficiently at low zinc intakes when luminal zinc concentrations are low; a passive mechanism works when zinc intakes and luminal concentrations are high. Solubility of zinc in the gut lumen is critical. Zinc ions are generally bound to amino acids or short peptides in the lumen, released at the brush border for absorption via the carrier mechanism (hZIPI family). The entry step of absorption across the brush border is followed by the binding of zinc ions to metallothionein and other proteins within the cytosol of the absorbing cell. Metallothionein carries the zinc (via transcellular movement) to the basolateral border for the exit step from the absorbing cell to the blood. The exit step occurs by active transport because the blood concentration of zinc is significantly greater than the cytosolic ion concentration. The process of zinc absorption is illustrated in Figure 3-35.
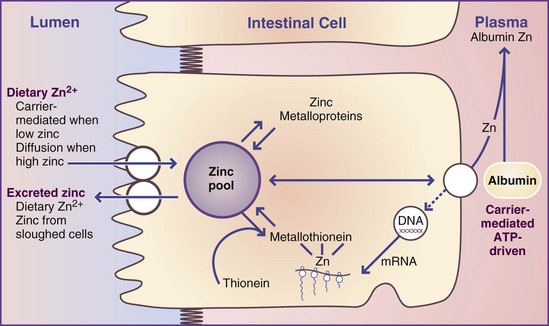
FIGURE 3-35 Model for zinc absorption showing the relationship between metallothionein and cysteine-rich intestinal protein.
ATP, Adenosine triphosphate; DNA, deoxyribonucleic acid; mRNA, messenger ribonucleic acid.
Zinc absorption is affected not only by the level of zinc in the diet but also by the presence of interfering substances, especially phytates. After the consumption of zinc in a meal, the serum zinc level rises and then decreases in a dose-response pattern. A protein-rich diet promotes zinc absorption by forming zinc–amino acid chelates that present zinc in a more absorbable form. Zinc absorption is slightly higher during pregnancy and lactation. Absorbed zinc is taken up from the portal circulation initially by the liver, but most of the zinc is subsequently redistributed to other tissues. Impaired absorption is associated with a variety of intestinal diseases such as Crohn disease or pancreatic insufficiency.
Several dietary factors affect zinc absorption. Phytate decreases zinc absorption, but other complexing agents (e.g., tannins) do not. Copper and cadmium compete for the same carrier protein; thus they reduce zinc absorption. High calcium or iron intakes reduce zinc absorption and balance. Folic acid may also reduce zinc absorption when zinc intake is low. On the other hand, high doses of zinc can impair absorption of iron from ferrous sulfate, the form in vitamin and mineral supplements. Dietary fiber may also interfere with zinc absorption, but the significance is unclear. Zinc absorption may be enhanced by glucose or lactose and by soy protein consumed alone or mixed with beef. Red table wine also increases zinc absorption, probably because of its congeners. Like iron, zinc is better absorbed from human milk than from cow’s milk.
Transport in Blood: Albumin is the major plasma carrier of zinc. The amount of zinc transported in the blood depends not on zinc but also on the availability of albumin. Some zinc is transported by transferrin and by α2-macroglobulin. Most zinc in the blood is localized in erythrocytes and leukocytes. Plasma zinc is metabolically active and fluctuates in response to dietary intake and physiologic factors such as injury or inflammation. Levels drop by 50% in the acute phase of a response to an injury, probably because of the sequestering of zinc by the liver.
Intestinal Excretion: Excretion of zinc in normal individuals is via the feces. When zinc is administered intravenously, approximately 10% of the dose appears in the intestine within 30 minutes. However, increased urinary excretion has been reported in starvation, nephrosis, diabetes, alcoholism, hepatic cirrhosis, and porphyria. Plasma and urine concentrations of zinc-binding cysteine and histidine, and other urinary metabolites, may have a role in increasing zinc losses in these patients.
Functions: Zinc has structural, catalytic, and regulatory functions in the cell, primarily as an intracellular ion (Tuerk and Fazel, 2009). Zinc plays important structural roles as components of several proteins. It also functions in association with more than 300 different enzymes, in reactions involving either synthesis or degradation of carbohydrates, lipids, proteins, and nucleic acids. It also functions as an intracellular signal in brain cells where it is stored in specific synaptic vesicles and is fundamental to normal central nervous system function (Bitanihirwe and Cunningham, 2009). In addition, zinc is involved in the stabilization of protein and nucleic acid structure and the integrity of subcellular organelles, as well as in transport processes, immune function, and expression of genetic information.
Metallothionein is the most abundant, nonenzymatic zinc-containing protein. This low-molecular-weight protein is rich in cysteine and exceptionally high in metals, among which are zinc and lesser amounts of copper, iron, cadmium, and mercury. The biologic role of metallothionein is not clear, but it does have a function in zinc absorption. Metallothionein may function as an intracellular reservoir that can donate zinc ions to other proteins, or it may have a redox role that reduces oxidative stress, especially in cells with high stress. Thus metallothionein may have a role in the detoxification of metals as well as in their absorption.
Zinc is abundant in the nucleus, where it stabilizes RNA and DNA structure, and is required for the activity of RNA polymerases important in cell division. Zinc also functions in chromatin proteins involved in transcription and replication, and it protects against age-related macular degenerative disease. Although widely touted to cure or prevent common colds, zinc gluconate lozenges or nasal sprays are not very effective.
Dietary zinc causes an increase in bone mass. Zinc appears in the crystalline structure of bone, in bone enzymes, and at the zone of demarcation. It is needed for adequate osteoblastic activity, formation of bone enzymes such as alkaline phosphatase, and calcification (see Table 3-25). Beta-alanyl-histidine (carnosine) is a zinc compound that stimulates bone formation intensively and restores bone loss from aging, skeletal unloading, aluminum bone toxicity, calcium and vitamin D deficiency, adjuvant arthritis, estrogen deficiency, diabetes, and fracture healing (Yamaguchi, 2010). Such new zinc compounds may become adjuvant therapy for osteoporosis and other disorders.
Dietary Reference Intakes: The zinc DRI for adolescent and adult males is 11 mg/day. Because of the lower body weight of adolescent and adult women, their DRI is 8 to 9 mg/day. The DRI for preadolescents is estimated to be 8 mg/day. The DRIs for infants are 2 mg/day for the first 6 months and 3 mg/day for the second 6 months of life.
Food Sources and Intakes: For most Americans, most daily intake of zinc is provided by meat, fish, poultry, ready-to-eat breakfast cereals fortified with zinc, and milk and milk products. Oysters are especially high in zinc, and other shellfish, liver, whole-grain cereals, dry beans, and nuts are all good sources (Table 3-32). Soy products may also be fairly good sources of zinc. In general, zinc intake correlates well with protein intake.
TABLE 3-32
Zinc Content of Selected Foods
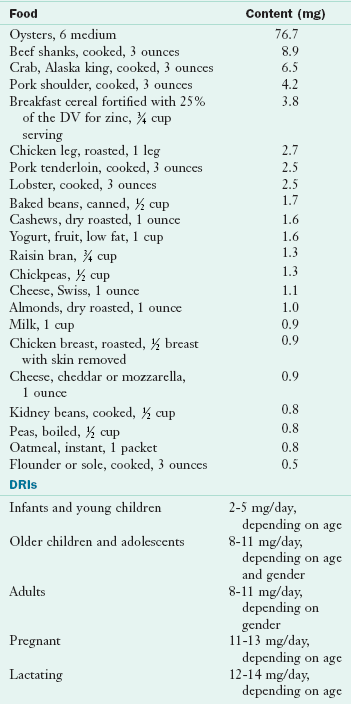
DRI, Dietary reference intake.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w309.pdf; accessed 1-14-11.
The zinc content of typical diets of adults in Western countries ranges between 10 and 15 mg/day; women consume less than men because of lower energy intakes. The zinc density of the American adult’s diet is approximately 5.6 mg/l000 kcal.
Deficiency: The clinical signs of zinc deficiency were first described as short stature, hypogonadism, mild anemia, and low plasma zinc level. This deficiency was caused by a diet high in unrefined cereals and unleavened breads, which contain high levels of fiber and phytate, which chelate with zinc in the intestine and prevent absorption. Additional symptoms of zinc deficiency include hypogeusia (decreased taste acuity), delayed wound healing, alopecia, and diverse forms of skin lesions. Acquired zinc deficiency may occur as the result of malabsorption, starvation, or increased losses via urinary, pancreatic, or other exocrine secretions. Patients with alcoholism may have altered zinc metabolism. Pregnant women and older adults are also at increased risk for deficiency. Low-dose zinc supplementation may improve measures of poor zinc status.
Acrodermatitis enteropathica, an autosomal-recessive disease characterized by zinc malabsorption, results in eczematoid skin lesions (Figure 3-36), alopecia, diarrhea, bacterial and yeast infections, and even death if left untreated. Symptoms generally first develop during weaning from human milk to cow’s milk. Researchers are working to identify the genetic basis of inherited nutritional deficiencies such as acrodermatitis enteropathica; the hZIP4 gene is involved. Zinc, biotin, protein, or essential fatty acid deficiency should be considered when there are systemic signs of failure to thrive (Gehrig and Dinulos, 2010).
FIGURE 3-36 Cutaneous manifestations of zinc deficiency. (From Callen WBS et al: Color atlas of dermatology, Philadelphia, 1993, Saunders.)
Zinc deficiency results in various immunologic defects. Severe deficiency is accompanied by thymic atrophy, lymphopenia, reduced lymphocyte proliferative response to mitogens, a selective decrease in T-helper cells, decreased NK cell activity, anergy, and deficient thymic hormone activity. Even mild zinc deficiency can reduce immune function, such as impaired interleukin-2 production. Supplementation with zinc may improve immune status, but more studies are needed. Moderate zinc deficiency is associated with anergy and diminished NK cell activity but not with thymic atrophy or lymphopenia. Box 3-8 summarizes the clinical manifestations of human zinc deficiency. Similarities between patients with sickle cell anemia and zinc deficiency suggest the possibility of a secondary zinc deficiency in those with the anemia.
Problems caused by low zinc intakes seem to be increasing, partly because of the low bioavailability of zinc. Athletes may also have an increased risk for developing zinc deficiency. Physical activity may increase mobilization of zinc from bone stores for cellular needs (e.g., for the synthesis of zinc-metalloenzymes). Finally, patients on long-term parenteral nutrition who do not receive zinc will show signs of deficiency; requirements have been estimated to be 3 mg/day in patients without GI losses and a mean of 12 mg/day in patients with diarrhea and fistula losses (Jeejeebhoy, 2009).
Toxicity: Oral ingestion of toxic amounts of zinc (100 to 300 mg/day) is rare, but the UL for zinc in adults is 40 mg/day. Excessive zinc supplementation has long been known to interfere with copper absorption. A major form of zinc toxicity develops in patients receiving hemodialysis for renal failure, with contamination of dialysis fluids from the adhesive plastic used on the dialysis coils or from galvanized pipes. The toxic syndrome in these patients is characterized by anemia, fever, and central nervous system disturbances. Zinc sulfate in amounts of 2 g/day or more may cause GI irritation and vomiting. Inhalation of zinc fumes during welding may be toxic, but exposure to fumes can be prevented with proper precautions.
Fluoride
Fluoride is a natural element found in nearly all drinking water and soil, although the fluoride content varies greatly throughout the world. For example, some well water has much more fluoride than other water, so families who use well water need to monitor fluoride levels periodically to make sure that levels are not in the toxic range. Although fluoride is not considered an essential element, this anion is known to be important for the health of bones and teeth. The average skeleton contains 2.5 mg of fluoride.
Functions: Fluoride is considered important, if not essential, because of its benefits for tooth enamel. Its incorporation into enamel produces more stable apatite crystals (Robinson et al., 2004). Fluoride also acts as an antibacterial agent in the oral cavity, serving as an enzyme inhibitor. Fluoride has no known requirement in human metabolic pathways.
The prevalence of dental caries has decreased by 50% in recent decades because of fluoridation of drinking water and the use of topical fluorides. The prevalence of dental caries has also decreased in communities without fluoridated water. This decrease probably results from the use of fluoridated toothpaste, topical fluoride applications, and use of fluoridated water used in food processing, all of which provide fluoride for incorporation into teeth. Soft drinks typically are prepared with fluoridated waters at bottling plants in urban areas.
Fluoride substitutes for the hydroxyl group on the lattice structure of calcium phosphate salts (i.e., hydroxyapatites) of the bones and teeth to form fluoroapatite, which is harder and less readily resorbed than hydroxyapatite (Chachra et al., 2008). After fluoridation, bone tissue formed at high fluoride blood levels is not healthy; it is subject to fractures from too tight binding of the fluoroapatite crystals compared with the hydroxyapatite in unfluoridated bone.
Dietary Reference Intakes: The AIs for fluoride were established for the first time in 1997. AIs for adult men and women are 4 and 3 mg/day, respectively. Depending on age, the AIs range from 2 to 3 mg/day for children and adolescents and from 0.7 to 1 mg/day for young children between the ages of 1 and 8 years. For comparison, an 8-oz glass of fluoridated water (1 ppm or 1 mg/L) provides approximately 0.2 mg of fluoride. ULs have also been established for fluoride.
Food Sources and Intakes: The major dietary sources of fluoride are drinking water and processed foods that have been prepared or reconstituted with fluoridated water. Seafood also is high in fluoride, but the content in freshwater fish is lower than that of saltwater fish. Soups and stews made with fish and meat bones also provide substantial fluoride, as do beef liver and mechanically deboned meat and fowl. Although fluorides exist in fruits and vegetables, the amounts in most foods are not significant.
The amount in tea leaves can be substantial, depending on the brewing strength. One cup of tea may contain as much as 1 mg of fluoride. Fluoridated toothpastes are also a source of fluoride; calcium carbonate–based fluoride toothpastes are effective in reducing caries and provide oral calcium as well (Lynch and Cate, 2005). The standard recommendation is 1 ppm for fluoridated community water supplies. Children who drink fluoridated water typically consume more total fluoride than those who do not. Intakes higher than 2 mg raise concerns about mild fluorosis, which has been reported in a few U.S. communities.
Deficiency: Because no known metabolic function exists for fluoride, fluoride cannot have a true deficiency that results in disease. Fortuitous binding in hydroxyapatite crystals, especially from fluoridated water supplies, reduces dental caries, but it does not seem to have any effect on reducing osteoporotic fractures.
Toxicity: A mild fluorosis can develop from daily doses of 0.1 mg/kg (i.e., greater than approximately 2 to 3 ppm of fluoride in the drinking water). The resulting discoloration of the teeth, or mottling, has no adverse effect except cosmetically. However, higher intakes lead to tooth flaking and more serious dental effects. Some evidence suggests that fluoride intakes are increasing among toddlers and young children because of the proliferating sources of fluoride. When drinking water contained less fluoride, the average intake was lower. Even the highest values did not exceed the recommendation of 0.08 mg/kg daily. Intakes of fluoride by young children may vary greatly from the use of dentifrices, fluoridated water, bottled drinks, and other sources. Some children may ingest total amounts of fluoride that exceed the optimum intake level (0.05 to 0.07 mg/kg daily), possibly causing dental fluorosis.
Copper
Copper, a normal constituent of blood, is another established essential micronutrient. Recent interest in copper has increased because of the many tissue-related functions. Concentrations of copper are highest in the liver, brain, heart, and kidney. Muscle contains a low level of copper, but, because of its large mass, skeletal muscle contains almost 40% of all the copper in the body. Recent investigations have increased the understanding of the physiologic roles of copper, copper homeostasis, and copper needs throughout the life cycle.
Absorption, Transport, Storage, and Excretion: Copper absorption, transport, storage and excretion are highly controlled (Kaplan and Lutsenko, 2009; Lalioti et al., 2009). Copper absorption occurs in the small intestine. Entry at the mucosal surface is by facilitated diffusion, and exit across the basolateral membrane is primarily by active transport; facilitated transfer may also occur. Competition between copper ions and other divalent cations exists at each step. Within the intestinal absorbing cells, copper ions are bound to metallothionein with greater affinity than zinc or other ions; the amount of copper absorbed may be regulated by the amount of metallothionein in the mucosal cells. Net absorption of copper varies from 25% to 60%. Low absorption efficiencies help to regulate the retention of copper in the body; therefore the percentage of absorption decreases with increased intake. Fiber and phytate intakes may slightly inhibit copper absorption.
Copper does not exist as a free ion in the body. Approximately 90% of the copper in serum is incorporated into ceruloplasmin, a functional enzyme at the erythrocyte-forming cells of the bone marrow. The remaining 10% is bound loosely to albumin, transcuprein, and other proteins; free amino acids; and possibly histidine. Serum copper and immunoreactive ceruloplasmin levels tend to be higher in women than in men. Serum copper concentration is greatest in the neonate, decreasing gradually during the first year of life.
Copper is transported bound to albumin, which serves as a temporary storage site for copper. In the liver copper binds to metallothionein. It serves as a storage form of copper and is incorporated into ceruloplasmin and secreted into the plasma for the transport of copper to cells. Copper is also secreted from the liver as a component of bile, its major route of excretion. Once in the GI tract, copper becomes part of the pool that may be resorbed or excreted, depending on bodily needs. Biliary excretion increases in response to excessive intakes of copper but may not be able to keep up with intake, sometimes allowing it to reach toxic levels.
Small amounts of copper are found in urine, sweat, and menstrual blood. Copper can be conserved by the kidney if necessary when substantial amounts are filtered through the glomeruli and resorbed in the tubules.
The interaction of copper with other nutrients negates the fallacy that taking vitamin and mineral supplements in amounts in excess of the recommended levels of consumption is good. In amounts of 150 mg/day, zinc induces copper deficiency by overwhelming the capacity of metallothionein in intestinal absorbing cells. High ascorbic acid intake (1500 mg/day) also reduces blood concentrations of copper, which may decrease the role of ceruloplasmin in red cell formation.
Functions: Copper is a component of many enzymes, and symptoms of copper deficiency are attributable to enzyme failures. Copper in ceruloplasmin has a well-documented role in oxidizing iron before it is transported in the plasma. Lysyl oxidase, a copper-containing enzyme, is essential in the lysine-derived cross-linking of collagen and elastin, connective tissue proteins with great tensile strength. Through the involvement of copper-containing electron transport proteins, copper also has roles in mitochondrial energy production. As part of copper-containing enzymes such as SOD, copper protects against oxidants and free radicals and promotes the synthesis of melanin and catecholamines. Other functions of copper-containing enzymes have not yet been completely defined.
Dietary Reference Intakes: An RDA of 900 mcg/day (0.9 mg/day) for adults of both genders has been established for copper (IOM, Food and Nutrition Board, 2001). Adolescents need 890 mcg. Copper intakes should range between 200 and 220 mcg/day for infants and between 340 and 440 mcg for young children. Premature infants are born with low copper reserves and may require additional dietary copper during their first few months of life.
Food Sources and Intakes: Copper is distributed widely in foods, including animal products (except for milk), and most diets provide between 0.6 and 2 mg/day. Foods high in copper are shellfish (oysters), organ meats (liver, kidney), muscle meats, chocolate, nuts, cereal grains, dried legumes, and dried fruits (Table 3-33).
TABLE 3-33
Copper Content of Selected Foods
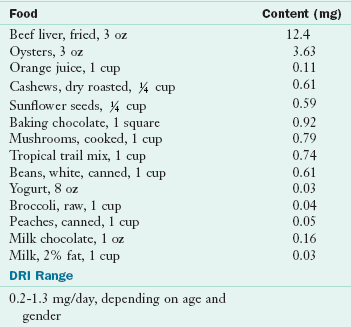
DRI, Dietary reference intake.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w312.pdf; accessed 1-14-11.
In general, fruits and vegetables contain little copper. Cow’s milk, a poor source of copper, contains 0.015 to 0.18 mg/L, whereas the copper in human milk is well absorbed and ranges from 0.15 to 1.05 mg/L. Infants fed cow’s milk may be at risk for copper deficiency because of its low copper content.
Copper intakes of individuals in several age categories in the United States have been consistently below recommended amounts, with adolescent girls consuming only approximately 50% of the recommended intakes. Typically the copper content of drinking water is not considered in diet surveys. The amount of copper in water from copper pipes is considered insignificant. Copper intakes may be low in U.S. diets because until recently, ready-to-eat cereals typically were not fortified with copper as they were for iron and zinc. Another reason for the potential existence of low copper intakes is the inaccuracy associated with short-term assessments of dietary copper.
Deficiency: Serum copper and ceruloplasmin levels are useful biomarkers to assess copper status in populations. More sensitive indicators of copper status such as copper-containing enzymes in blood cells are needed (Harvey et al., 2009). Copper deficiency is characterized by anemia, neutropenia, and skeletal abnormalities, especially demineralization. Other changes may also develop, including subperiosteal hemorrhages, hair and skin depigmentation, and defective elastin formation. The failure of erythropoiesis, as well as cerebral and cerebellar degeneration, may lead to death. Neutropenia and leukopenia are the best early indications of copper deficiency in children.
Classic cases of copper deficiency were reported among infants who were poorly nourished, had diarrhea, and were fed diluted cow’s milk. Other cases of deficiency have been reported. Premature infants are likely to have copper deficiency unless given a supplement because most of the copper is normally transferred across the placenta during the last few months of a full-term pregnancy. Because diets in developing countries continue to be low in copper, pregnancy outcomes need to be monitored (Pathak and Kapil, 2004).
Copper is stored in the liver; deficiency develops slowly as stores become depleted. Deficiencies have not been reported in otherwise healthy humans consuming a varied diet. Low serum copper, ceruloplasmin, and SOD levels provide supportive evidence of copper deficiency, but these markers are not sensitive to marginal copper status. Bone changes, osteoporosis, metaphyseal spur formation, and soft tissue calcification in infants receiving prolonged TPN may resolve with copper supplementation. The only signs of copper deficiency found in adults are neutropenia and microcytic anemia; deficiency is very rare because copper accumulates in the liver throughout life in most individuals.
Wilson disease is an autosomal-recessive disorder of copper metabolism and liver dysfunction (Schilsky, 2009). Menkes syndrome, also known as kinky-hair syndrome, is a sex-linked recessive defect caused by at least 160 identified gene mutations (Møller et al., 2009). The syndrome results in copper malabsorption, increased urinary copper loss, and abnormal intracellular copper transport, all of which cause an abnormal distribution of copper among organs and within cells. Affected infants have retarded growth, defective keratinization and pigmentation of the hair, hypothermia, degenerative changes in aortic elastin, abnormalities of the metaphyses of long bones, and progressive mental deterioration. These infants typically do not survive the first few months of life. Many of the features of this disorder result from interference with the cross-linking of collagen and elastin, steps that require one or more copper enzymes. Brain tissue is practically devoid of cytochrome C oxidase, and a marked accumulation of copper occurs in the intestinal mucosa, although serum copper and ceruloplasmin levels remain very low. Many connective tissue defects occur in patients with Menkes syndrome.
Decreased plasma copper levels develop in patients with malabsorption diseases such as celiac disease, tropical sprue, protein-losing enteropathies, and nephrotic syndrome. Like zinc, low copper intakes may also contribute to reduced immune responses in otherwise healthy individuals.
Toxicity: Copper toxicity from food consumption is considered impossible. Excessive supplementation or copper salts used in agriculture may lead to liver cirrhosis and abnormalities in red blood cell formation.
Ceruloplasmin concentrations increase during pregnancy and with the use of oral contraceptives. Serum copper concentrations in pregnant women are approximately twice those in women who are not pregnant. Serum and biliary copper concentrations can be elevated in acute or chronic infections, liver disease, and pellagra. The physiologic significance of these elevations is not known.
Any chronic liver disease that interferes with the excretion of bile may contribute to the retention of copper. Primary biliary cirrhosis, as well as mechanical obstruction of the bile ducts, contributes to a progressive rise in liver copper content.
Ultratrace Minerals
Ultratrace minerals such as iodine, selenium, manganese, molybdenum, chromium, and a few other nonessential minerals are found in the body in small quantities; their amounts are typically measured in micrograms. Each of these elements has one or more essential roles. Because of their small quantities in human tissues, special analytic instrumentation and ultraclean laboratories are necessary for the routine analysis or experimental work relating to the ultratrace minerals.
Iodine
Iodine deficiency in the United States and many Western nations has practically been eliminated with the iodinization of salt. However, people living in many mountainous areas of the world and a few low-lying delta regions still have low iodine intakes because of the low content in soil used for cultivating crops. Others living in lowlands may have high goitrogen consumption that reduces iodine use by the thyroid gland. The body normally contains 20 to 30 mg of iodine, with more than 75% in the thyroid gland and the rest distributed throughout the body, particularly in the lactating mammary gland, gastric mucosa, and blood.
Absorption, Transport, Storage, and Excretion: Iodine is absorbed easily as iodide. In the circulation iodine exists freely and protein bound, but the bound iodide predominates. Excretion is primarily via urine, but small amounts are found in the feces as a result of biliary secretion.
Functions: Dietary iodine is needed for the synthesis of thyroid hormones. Iodine is stored in the thyroid gland, where it is used in the synthesis of triiodothyronine (T3) and thyroxine (T4). Uptake of iodide ions by the thyroid cells may be inhibited by goitrogens (substances that exist naturally in foods). Thyroid hormone is degraded in target cells and the liver, and the iodine is highly conserved under normal conditions. Selenium is important in iodine metabolism because of its presence in one enzyme responsible for forming active T3 from thyroglobulin stored in the thyroid gland.
Dietary Reference Intakes: An iodine intake of 150 mcg/day has been suggested as sufficient for all adults and adolescents. The RDA for pregnant and lactating women increases to 220 mcg and 290 mcg, respectively. The AI is 110 mcg for infants up to 6 months of age and 130 mcg for older infants. The RDA for children is between 90 and 120 mcg and increases with age or body size. Urinary iodine, serum thyroxine, or thyroid-stimulating hormone levels are useful biomarkers of iodine status (Ristic-Medic et al., 2009). See Chapter 32.
Food Sources and Intakes: Iodine exists in variable amounts in food and drinking water. Seafood, such as clams, lobsters, oysters, sardines, and other saltwater fish, is the richest source. Saltwater fish contain 300 to 3000 mcg/kg of flesh; freshwater fish contain 20 to 40 mcg/kg, but they are still good sources. The iodine content of cow’s milk and eggs is determined by the iodides available in the diet of the animal; the iodide content of vegetables varies according to the iodine content of the soil in which they grow. Iodine also enters the food chain through iodophors, which are used as disinfectants in dairy processing, coloring agents, and dough conditioners. These sources add significant amounts of iodine to the food supply. Table 3-34 lists the iodine content of various foods.
TABLE 3-34
Iodine Content of Selected Foods
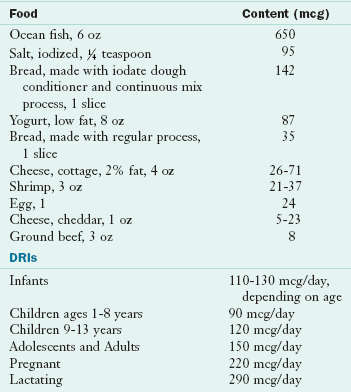
DRI, Dietary reference intake.
From:
(1) U.S. Department of Agriculture: Composition of foods, USDA Handbook No. 8 Series, Washington, DC, 1976-1986, Agricultural Research Service, The Department.
(2) Medline Plus. Website http://www.nlm.nih.gov/medlineplus/ency/article/002421.htm, accessed 1-14-11.
The best way to obtain an AI of iodine is to use iodized salt (approximately 60 mcg of iodine per gram of salt) in food preparation. Sea salt naturally contains variable amounts of iodine and only approximately one-tenth the level of iodized salt. More than 50% of the table salt sold in the United States is iodized; however, iodized salt is not used in processed foods. Mandatory iodinization has been adopted by many nations, including Canada, but is not legally required in the United States, where iodine deficiency is now very rare. The use of iodized salt should still be advocated in certain areas to prevent goiter.
The Total Diet Study of the FDA showed that median adult iodine intakes from 1982 to 1991 ranged from 130 to 140 mcg/day for women and 182 to 204 mcg/day for men. The median iodine intake for male and female teenagers was even higher. Intakes of iodine in the United States seem adequate for most people because of iodinization of salt and the use of iodofors. Vegans consume iodine in seaweed or kelp tablets; some individuals may have excessive iodine intakes.
Deficiency: An estimated two billion people worldwide remain at risk for iodine deficiency. Most are in developing countries, especially those who do not have seafood as part of their diets. These individuals may have a moderate iodine deficiency, even when obvious goiter, a severe condition, is not evident. In children, iodine deficiency is associated with poor cognition. Use of iodized salt or the oral administration of a single dose of iodized oil and weekly iodine supplements are effective. Use of iodized salt should be encouraged during pregnancy, especially through the end of the second trimester.
Very low iodine intakes are associated with the development of endemic or simple goiter, which is an enlargement of the thyroid gland (Figure 3-37). Deficiency may be nearly total, especially in mountainous areas and regions of high goitrogen intakes, or relative, subsequent to an increased need for thyroid hormones (e.g., by females during adolescence, pregnancy, and lactation).
FIGURE 3-37 Goiter caused by iodine deficiency. (From Swartz MH: Textbook of physical diagnosis history and examination, ed 3, Philadelphia, 1998, Saunders.)
Although many countries have worked to eliminate iodine deficiency, goiter may affect as many as 200 to 300 million people worldwide (Kusic and Jukic, 2005). In some countries goiter is so common that it is regarded as a normal physical feature. In the United States the prevalence rate of goiter for all ages is 1.9/1000 persons. The rate is higher in women than in men and in older individuals than in younger ones.
Goitrogens, which exist naturally in foods, can also cause goiter by blocking uptake of iodine from the blood by thyroid cells. Foods containing goitrogens include cabbage, turnips, rapeseeds (from rape plants), peanuts, cassava, sweet potatoes, kelp, and soybeans. Goitrogens are inactivated by heating or cooking.
Severe iodine deficiency during gestation and early postnatal growth results in cretinism in infants, characterized by mental deficiency, spastic diplegia or quadriplegia, deaf mutism, dysarthria, a characteristic shuffling gait, shortened stature, and hypothyroidism. Less severe variations of this syndrome also exist, manifesting as moderate retardation in intellectual or neuromotor maturation. In some areas, use of iodine supplementation has been found to improve cognitive function in school-aged children who are mildly deficient (Gordon et al., 2009). The WHO has increased the recommended iodine intake during pregnancy from 200 to 250 mcg/day; in regions where less than 90% of households are using iodized salt, iodine supplementation should be used in pregnancy and infancy (Zimmerman, 2009).
Toxicity: Even though iodine intakes have a wide margin of safety, tolerable ULs have been established (IOM, Food and Nutrition Board, 2001). Adults have a UL of 1100 mcg/day, and young children have a UL of 200 to 300 mcg/day. In some cases goiter develops slowly as a consequence of long-term iodine intakes that are much higher than physiologic requirements. The role of excessive iodine in thyroid disease or disorder is not clear. Today the level of iodine in foods is not considered a significant public health problem in the United States or Canada. The level of iodine in most American diets is appropriate for good health. For small groups of people with underlying thyroid pathologic conditions, excessive iodine in the diet may result in hypothyroidism, goiter formation, or hyperthyroidism (Mussig et al., 2006).
Selenium
A narrow dietary intake range exists for selenium, below which deficiency occurs and above which toxicity develops. Only in China have these extremes been shown to relate to the selenium content of the soil. A dietary intake of approximately 40 mcg of selenium per day seems to be necessary to maintain glutathione peroxidase (GSH-Px), an enzyme containing selenium (Schrauzer and Surai, 2009). GSH-Px, discovered to be a selenoenzyme in the early 1970s, is considered the major active form of selenium in tissues, although other selenium proteins have since been discovered. Tissue levels are influenced by dietary intake and reflect the geochemical environment. Regions of North America identified as low in selenium content are the Northeast, Pacific, Southwest, and coastal plains of the southeastern region of the United States, as well as north central and eastern Canada. The lowest selenium content of soil exists in a few regions of China, especially Keshan, where severe selenium deficiency was first reported in a human population in 1979. Other areas with low selenium content include parts of Finland and New Zealand.
Absorption, Transport, Storage, and Excretion: Absorption of selenium, which occurs in the upper segment of the small intestine, is more efficient under conditions of deficiency. Increased intake frequently results in increased excretion of selenium in the urine. Selenium status is assessed by measuring selenium or GSH-Px in serum, platelets, and erythrocytes or in whole blood. Erythrocyte selenium measurement is an indicator of long-term intake. Selenium is transported bound to albumin initially and subsequently to α2-globulin.
Functions: Selenium, as selenomethionine or selenocysteine, exists in several proteins that are widely distributed in the body. Cellular GSH-Px has been found in almost all cells and extracellularly in serum and milk. GSH-Px acts with other antioxidants to reduce cellular peroxides and free radicals in general into water and other harmless molecules. This family of enzymes may help provide a reserve of selenium in proteins that can be drawn on when needed. Many but not all of the pathologic changes caused by selenium deficiency reflect inadequate levels of GSH-Px enzymes.
Phospholipid hydroperoxide GSH-Px is found in lipid-soluble fractions of the cell and has roles in lipid and eicosanoid metabolism. Type 1 iodothyronine 5′-deiodinase, an enzyme capable of converting T4 to T3, is a selenoprotein. Moderate selenium intakes (40 mcg/day) seem adequate to maintain activities of these deiodinases. However, high intakes (350 mcg/day) are associated with depressed T3 levels. GSH-Px enzymes have also been shown to be critical in several endocrine systems (Beckett and Arthur, 2005).
Selenoprotein P may act as a free radical scavenger or a transporter of selenium. Selenium is used in the synthesis of these molecules in the anionic form. In the molecules, selenium is covalently bound, as is sulfur, which it typically replaces in some of these molecules. The antioxidant effects of selenium and vitamin E may reinforce each other by the overlap of their protective actions against oxidative damage. These two antioxidant nutrients may participate in other cooperative activities that help maintain healthy cells. GSH-Px acts in the cytosol and the mitochondrial matrix, whereas vitamin E exerts its antioxidative actions within cell membranes.
The GSH-Px reaction step is illustrated in Figure 3-38. Other selenium-dependent enzymes exist in mammalian systems, but less is known about their requirements for selenium. The selenium-containing enzymes may have an antioxidative role in preventing cancer. Many other selenoproteins have been identified, but their functions have not yet been elucidated.
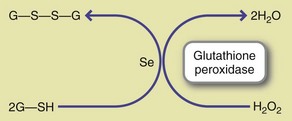
FIGURE 3-38 Enzymatic reaction catalyzed by the selenium-containing enzyme, glutathione peroxidase. Selenium is a prosthetic form of the enzyme that removes highly reactive hydrogen peroxide from within cells by converting it to water while simultaneously converting two molecules of reduced glutathione to oxidized glutathione.
Dietary Reference Intakes: The RDAs for selenium are 55 mcg/day for women, men, and adolescents (ages 14 to 18), whereas the RDAs for children range from 20 to 30 mcg/day. The AIs for infants is 15 to 20 mcg/day. The RDA during pregnancy is 60 mcg, and the RDA during lactation is 70 mcg/day. Requirements for selenium may increase with a high consumption of unsaturated fatty acids because of the need for higher antioxidant activity. However, low-dose chronic exposure to selenium may be widespread in some populations; the upper safe limit may actually be set too high (Vinceti et al., 2009).
Food Sources and Intakes: No comprehensive table of the selenium content of foods has been published. The selenium concentration in foods depends on the selenium content of the soil and water where the food was grown. Improvements in analytic techniques have resulted in changes being made to many previously published data of the selenium content of foods during the last few decades (Table 3-35).
TABLE 3-35
Selenium Content of Selected Foods
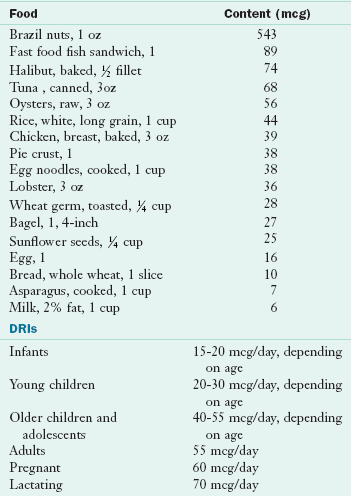
DRI, Dietary reference intake.
From U.S. Department of Agriculture, Agricultural Research Service: Nutrient Database for Standard Reference, Release 18, Data Laboratory home page: http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/nutrlist/sr18w317.pdf; accessed 2011.
Major food sources of selenium are Brazil nuts, seafood, kidney, liver, meat, and poultry. Highest intake in the United States is from animal flesh foods. Grains vary in selenium content, depending on where they are grown. Fruits and vegetables are low in selenium content.
Selenium content and GSH-Px activity in human breast milk are influenced directly by maternal intake and by the form of selenium consumed. Plasma selenium concentrations of infants fed unsupplemented formula are lower than those of infants fed supplemented formula or human milk. Selenium fortification of infant formulas has been shown to improve the status of infants.
Deficiency: Despite a wide range of selenium intakes from food, selenium deficiency is rare in populations throughout the world. Selenium deficiency takes years to develop when food intake is adequate. Severe selenium deficiency in a population has only been reported in China. Keshan disease, a cardiomyopathy that mainly affects children and women, was first observed in the Keshan province of China. Since its discovery, supplementation programs in Keshan have totally eradicated the disease. However, in individuals with established disease, the response to supplementation is poor, probably because of other factors contributing to myopathy; a viral infection combined with selenium deficiency has been suggested (Beck et al., 1995).
The second selenium deficiency disease, discovered in Mongolia, is known as Kashan-Beck disease and is common in preadolescent and adolescent children. These two diseases occur in areas where the soil content of selenium is very low. This disease may also have a viral component combined with the selenium deficiency. Illness initially involves symmetric stiffness, swelling, and often pain in the interphalangeal joints of the fingers, followed by generalized osteoarthritis. Here iodine deficiency may be another risk factor.
Patients with some cancers have been shown to have low serum selenium levels, although the underlying mechanisms for this correlation have not been established. One possible explanation lies in the possible failure of GSH-Px to scavenge free radicals efficiently in dividing cells. In addition, patients with cirrhosis have low plasma selenium concentrations, which may predispose them to cancer (Latavayova et al., 2006).
Selenium deficiency has been reported in malnourished patients receiving long-term TPN. Supplementation results in improved serum selenium levels, platelet GSH-Px activity, and reduced clinical symptoms. Selenium deficiency should no longer be a problem in patients receiving long-term TPN or enteral nutrition because these solutions now include a trace element supplement.
Toxicity: Indicators of selenium toxicity have been reported in China and Australia. Signs of toxicity (selenosis) include skin and nail changes, tooth decay, and nonspecific GI and neurologic abnormalities. Research also suggests that excessive intakes may be mutagenic or genotoxic, a point that is important to note for pregnant women. In addition, the long-term effects of selenium supplementation on the thyroid hormones have not been clearly identified (Combs et al., 2009). In countries such as New Zealand, where both selenium and iodine levels tend to be low, supplementation with selenium alone will not improve thyroid function; iodine is also required (Thomson et al., 2009).
Manganese
Manganese deficiency in humans was first reported in 1972, and its essentiality in humans is well established. Chronic exposure to excessive manganese levels can result in psychiatric and motor disturbances, referred to as manganism (Yin et al., 2010). A healthy balance is required.
Absorption, Transport, Storage, and Excretion: Manganese is absorbed throughout the small intestine. Iron and cobalt compete for common binding sites for absorption. Men absorb less manganese than women, a difference noted by iron status and plasma ferritin levels. Heme iron has no influence on manganese status, but diets high in nonheme iron can be associated with lower serum manganese values, higher urinary manganese losses, and lower activity of manganese-containing enzymes.
Manganese is transported bound to a macroglobin, transferrin, and transmanganin. Excretion occurs mainly in the feces after secretion into the intestine via the bile. The cytoplasmic iron exporter ferroportin (Fpn) functions as a manganese exporter; manganese exposure promotes Fpn protein expression, which reduces manganese accumulation and its related cytotoxicity (Yin et al., 2010). Antioxidants may also play a role in the excretion of toxic levels of manganese.
Functions: Manganese is associated with the formation of connective and skeletal tissues, growth, and reproduction. The 10 to 20 mg of manganese contained in the adult human body tends to be concentrated predominantly in mitochondria. Manganese activates many enzymes, especially in conjunction with magnesium. Manganese is essential for proper metabolism of amino acids, proteins and lipids.
Manganese is a component of many enzymes, including glutamine synthetase, pyruvate carboxylase, and mitochondrial SOD (MnSOD). It catalyzes detoxification of free radicals and may protect against some types of cancer. MnSOD converts superoxide anion into hydrogen peroxide but this must be detoxified by GSH-Px. If that does not occur, hydrogen peroxide forms a hydroxyl radical with iron, which is detrimental for patients with cirrhosis who have an altered SOD gene (Nahon et al., 2009).
Dietary Reference Intakes: The AIs for manganese are 2.3 mg/day for men and 1.8 mg/day for women. For children 9 years of age and older the AIs are 1.9 to 2.2 mg/day for boys and 1.6 mg/day for girls. For children younger than 9 the AIs are 1.2 to 1.5 mg/day, depending on their age.
Food Sources and Intakes: The manganese content of foods varies greatly. The richest sources are whole grains, legumes, nuts, and tea. Fruits and vegetables are moderately good sources. Relatively high amounts exist in instant coffee and tea.
Animal tissues, seafood, and dairy products are poor sources. Human milk is relatively low in manganese. Intakes are often low for adolescent girls.
Deficiency: Manganese deficiency, although rare, affects reproductive capacity, pancreatic function, and aspects of carbohydrate metabolism. Symptoms of deficiency are weight loss, transient dermatitis, occasionally nausea and vomiting, a change in hair color, and slow hair growth. In addition, there is a correlation between low blood manganese and convulsions (Gonzalez-Reyes et al., 2007).
Animal studies have established that manganese is necessary for reproduction. Sterility develops in both sexes; striking skeletal abnormalities and ataxia characterize the offspring of mothers who are manganese deficient. Lower blood manganese levels may be associated with fetal intrauterine growth retardation and lower birth weight in humans (Wood, 2009).
Toxicity: Manganese toxicity has developed in miners as a result of absorption of manganese through the respiratory tract. The excess, which accumulates in the liver and central nervous system, produces Parkinson-like symptoms. Manganese intake in excess leads to neurotoxicity, impairing energy metabolism and causing cell death (Puli et al., 2006). This is especially true for dopaminergic neurons.
Toxicity has also been reported in patients receiving TPN with added manganese. Symptoms include headaches, dizziness, and abnormal magnetic resonance imaging results and hepatic dysfunction (Masumoto et al., 2001).
The ULs of manganese from diet have been difficult to establish. Red wine may be a source of relatively high levels of metal ions, leading to a very high target hazard quotient for those individuals consuming at least 250 mL per day for many years (Hague et al., 2008). The implications for manganese require further study.
Chromium
A biologic role for chromium was proposed in 1954 but not accepted until 1977. Patients receiving parenteral nutrition exhibited abnormalities of glucose metabolism that were reversed with chromium supplementation. The low concentrations of chromium in food, body tissues, and body fluids require careful, appropriate analytic techniques, and new standard reference materials for accurate measurements.
Absorption, Transport, Storage, and Excretion: As with other minerals, organic and inorganic forms of chromium are absorbed differently. Organic chromium is readily absorbed but quickly passes out of the body. Less than 2% of the trivalent chromium consumed is absorbed. Chromium absorption is increased by oxalate and is higher in iron-deficient animals than in animals with adequate iron, suggesting that it shares some similarities with the iron absorption pathway. With dietary intakes of 40 mcg or more per day, chromium absorption reaches and remains at a plateau; at such high intakes, urinary excretion increases to maintain balance.
The type of dietary carbohydrate consumed modifies absorption from chromium chloride. Starch rather than sugar increases absorption. Absorption is also greater from chromium picolinate than from chromium chloride, where absorption is 2% or less.
Chromium and iron are carried by transferrin; however, albumin is also capable of assuming this role if iron transferrin saturation is high. In addition, α- and β-globulins and lipoproteins can also bind chromium.
Primarily the kidney excretes inorganic chromium, with small amounts being excreted through hair, sweat, and bile. Organic chromium is excreted through bile. Strenuous exercise, physical trauma, or an increased intake of simple sugar results in increased chromium excretion.
Functions: Chromium potentiates insulin action and influences carbohydrate, lipid, and protein metabolism. It may have a beneficial effect on serum triglyceride levels. Although the chemical nature of the relationship between chromium and insulin activity has not been clearly identified, a possible chromium-NA (chromium polynicotinate) complex has been identified. Chromium may regulate the synthesis of a molecule that potentiates insulin action; this glucose tolerance factor (GTF) is controversial. However, chromium supplementation alone or combined with vitamins C and E minimizes oxidative stress; it also improves glucose metabolism in type 2 diabetes mellitus patients (Lai, 2008). Another possible role for chromium, similar to that of zinc, is in the regulation of gene expression.
Dietary Reference Intakes: The AIs recommended for chromium range from 25 to 35 mcg/day for males 9 years of age and older and 21 to 25 mcg/day for females of the same age. Depending on the age of the child, 11 to 15 mcg/day has been established for children 1 to 8 years of age.
Food Sources and Intakes: Precise assessment of chromium in foods is difficult; biologically available chromium and inorganic chromium cannot be distinguished from each other. Analyses done before 1980 must be considered with caution because determinations were flawed by contamination and analytic problems.
Brewer’s yeast, oysters, liver, and potatoes have high chromium concentrations; seafood, whole grains, cheeses, chicken, meats, and bran have medium chromium concentrations. The refining of wheat removes chromium with the wheat germ and the bran; refining sugar fractionates the chromium into the molasses portion. Dairy products, fruits, and vegetables are low in chromium. Table 3-36 and Appendix 52 present the chromium content of selected foods.
TABLE 3-36
Chromium Content of Selected Foods

AI, Adequate intake; DRI, dietary reference intake.
From:
(1) Anderson RA, Bryden NA, Polansky MM: Dietary chromium intake, Biol Trace Elem Res 32:117, 1992.
(2) Chromium Fact Sheet. http://ods.od.nih.gov/factsheets/chromium/; accessed 1-14-11.
Usual chromium intakes range between 25 and 35 mcg/day for women and men, respectively. Chromium intakes are not assessed in the USDA, National Health and Nutrition Examination Survey, or Total Diet Study surveys because of inadequate methodology. Human breast milk contains 3 to 8 nmol/L of chromium, which is lower than the recommended intake for infants.
Deficiency: Chromium deficiency results in insulin resistance and a few lipid abnormalities, which can be ameliorated by chromium supplementation. Insufficient chromium may be consumed by some Americans; true deficiency is more likely to occur in populations with very low intakes. Some epidemiologic studies suggest low tissue levels of chromium in patients with diabetes. However, they recommend long-term clinical trials to assess safety of chronic chromium supplementation before it is used in these patients. Claims that the ingestion of high doses of chromium consumed as chromium picolinate improves strength, body composition, endurance, or other characteristics of physical fitness are controversial, with some studies supporting these claims and others not.
Toxicity: Chromium toxicity from food has not been reported. Chromium picolinate taken as a supplement in high doses by athletes and power lifters has resulted in some adverse effects, primarily skin lesions. An increase risk of cancer has been identified in China in a population that was exposed to high chromium levels in drinking water (Smith and Steinmaus, 2009).
Molybdenum
Molybdenum has been established as an essential micronutrient, particularly because of its requirement in the enzyme xanthine oxidase (see Table 3-25). Interrelationships among molybdenum, copper, and sulfate absorption in livestock and between molybdenum intake and copper excretion in humans and animals have been demonstrated. Individuals receiving long-term TPN have displayed symptoms of molybdenum deficiency, including mental changes and abnormalities of sulfur and purine metabolism.
Absorption, Transport, Storage, and Excretion: Molybdenum, which is found in minute amounts in the body, is readily absorbed from the stomach and small intestine, with the rate of absorption being higher in the proximal small intestine than in the distal small intestine. As with other minerals, molybdenum is absorbed by two mechanisms: carrier-mediated and passive diffusion. Molybdenum is excreted primarily in the urine. Excretion rather than absorption is the homeostatic mechanism. Some molybdenum is also excreted in the bile.
Functions: Xanthine oxidase, aldehyde oxidase, and sulfite oxidase, all enzymes that catalyze oxidation-reduction reactions, require a prosthetic group containing molybdenum (Schwarz et al., 2009). Sulfite oxidase is important to the degradation of cysteine and methionine and catalyzes the formation of sulfate from sulfite. Whether molybdenum is involved in the response of some asthmatics to sulfites is not known. Genetic sulfite oxidase deficiency is a fatal disorder of cysteine metabolism. Clinical symptoms include severe brain damage with mental retardation, dislocation of the lens, and increased urinary output of sulfate.
Dietary Reference Intakes: The RDAs for molybdenum throughout the life cycle range from 43 to 45 mcg/day for adolescents and adults. Depending on the child’s age, RDAs range from 17 to 34 mcg/day for children.
Food Sources and Intakes: Molybdenum is distributed widely in commonly consumed foods such as legumes, whole-grain cereals, milk and milk products, and dark green leafy vegetables. Estimated intakes, as determined by the Total Diet Study of the FDA, ranged from 50 mcg/day in infants to 80 and 126 mcg/day for 14- to 16-year-old girls and boys, respectively. These intakes were found to decrease slowly over the lifetime.
Deficiency: Molybdenum deficiency has not been established in humans other than patients treated with TPN. Symptoms of molybdenum deficiency include mental changes and abnormalities of sulfur and purine metabolism.
Toxicity: An excessive molybdenum intake of 10 to 15 mg/day is associated with a goutlike syndrome (Nielsen, 2009). However, no good biomarkers are available to assess the presence of molybdenum excess accurately. Nonetheless, plasma molybdenum does seem to reflect molybdenum intake.
Boron
The essentiality of boron for humans has not yet been established, but its essentiality for plants and animals is widely accepted. Boron, an ultratrace element, is obtained from foods such as sodium borate, and it is rapidly and almost completely (90%) absorbed. The highest concentrations of boron are found in bone, spleen, and thyroid, although it is present in all other tissues of the body.
Functions: Boron is associated with cell membranes and in plants is involved with the functional efficiency of cell membranes. Response to boron deprivation is enhanced when other nutrients that alter membrane functions are also deficient. Boron apparently binds to the active site of some enzymes, reducing their ability to function. Boron is also thought to compete with some enzymes for the coenzyme NAD.
Boron influences the activity of many metabolic enzymes and the metabolism of such nutrients as calcium, magnesium, and vitamin D (Devirian and Volpe, 2003). Evidence from animal studies shows that boron deprivation affects two major organs: the brain and bone. Boron deficiency alters brain composition and function and reduces bone composition, structure, and strength. Because of the role of boron in bone, studies in humans have focused on its potential role in the development of osteoporosis. Boron may have actions similar to estrogens on bone (Nielsen, 2009). Boron is also required for normal reproduction and healthy immune response. Other roles of boron in humans have not been well studied.
Food Sources and Intakes: Foods that are good sources of boron include plant foods, especially noncitrus fruits, vegetables, nuts, and legumes. Wine, cider, and beer are other good sources of boron.
Deficiency and Toxicity: Symptoms of severe boron deficiency have not been established (Nielsen, 2009). No toxicity level has been identified.
Cobalt
Most of the cobalt in the body exists with vitamin B12 stores in the liver. Only one enzyme has an established specific requirement for cobalt. Blood plasma contains approximately 1 mcg of cobalt per 100 mL.
Absorption, Transport, Storage, and Excretion: Cobalt may share part of the same intestinal transport mechanism as iron. Absorption is higher in patients with deficient iron intake, portal cirrhosis with iron overload, and idiopathic hemochromatosis. The major route of cobalt excretion is the urine; small amounts are excreted via feces, sweat, and hair.
Functions: The well-known essential role of cobalt is as a component of vitamin B12 (cobalamin). This vitamin is essential for the maturation of red blood cells and the normal function of all cells. Methionine aminopeptidase, an enzyme involved in the regulation of translation (i.e., of DNA to RNA), is the only enzyme in humans known to have an established requirement of this trace element.
Dietary Reference Intakes: The dietary requirement for cobalt is expressed in terms of vitamin B12. Approximately 2 to 3 mcg of vitamin B12 is needed daily.
Food Sources and Intakes: Cobalt exists in foods; however, only microorganisms are able to synthesize vitamin B12. Ruminant animals obtain cobalamin as the result of a symbiotic relationship with the microorganisms of their GI tract. The microorganisms of monogastric species such as humans have an extremely limited capacity for synthesis; therefore humans must obtain vitamin B12—and thus cobalt—from animal foods such as organ and muscle meats. In some circumstances ordinary bacterial contamination of foods of vegetable origin may supply the minute required amounts of this vitamin. Strict vegetarians who avoid all animal products may develop vitamin B12 deficiency, either after 3 to 6 years or not at all.
Deficiency: A cobalt deficiency develops only in relation to a vitamin B12 deficiency. Insufficient vitamin B12 causes a macrocytic anemia. A genetic defect limiting vitamin B12 absorption results in pernicious anemia, which is treated appropriately with massive doses of the vitamin.
Toxicity: A high intake of inorganic cobalt (existing freely from cobalamin) in animal diets produces polycythemia (an overproduction of red blood cells), hyperplasia of bone marrow, reticulocytosis, and increased blood volume.
The information on the microminerals known to be required by humans is summarized in Table 3-36.
Other Trace Elements
Several other trace elements of uncertain essentiality exist, including aluminum, lithium, nickel, silicon, tin, and vanadium. A few other ultratrace elements, including arsenic, may be added to this list in the future. They are classified as ultratrace elements because of their very low quantities in human tissues. Requirements remain undefined for all of these elements because of their uncertain essentiality. The ultratrace elements continue to be enigmas because of their uncertain roles in human function. It has long been established that these elements exist in human tissues, especially in the skeleton, because of their abundance on the earth’s surface, but the essentiality of any of these in humans remains questionable (Nielsen, 2009).
American Society for Bone and Mineral Research
Dietary Guidelines for Americans
http://www.cnpp.usda.gov/dietaryguidelines.htm
http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=3&tax_subject=256&topic_id=1342&level3_id=5140
http://www.fda.gov/Food/default.htm
National Institute of Medicine
References
Abrams, SA. Calcium supplementation during childhood: long-term effects on bone mineralization. Nutr Rev. 2005;63:251.
Agarwal, R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4:1523.
Alberts, B, et al, Cell chemistry and biosynthesis, membrane structure, energy conversion, mitochondria and chloroplasts, Molecular Biology of the Cell. ed 4. New York: Garland Science; 2002.
Allen, LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008;29:20S.
American Dietetic Association. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109:1266.
American Dietetic Association. Position of the American Dietetic Association: fat replacers. J Am Diet Assoc. 2005;105:266.
American Dietetic Association. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2008;108:1716.
Andrés, E, et al. Clinical aspects of cobalamin deficiency in elderly patients: epidemiology, causes, clinical manifestations, and treatment with special focus on oral cobalamin therapy. Eur J Intern Med. 2007;18:456.
Artaza, JN, et al. Vitamin D and the cardiovascular system. Clin J Am Soc Nephrol. 2009;4:1515.
Baddini, F, et al. Conjugated linoleic acid (CLA): effect modulation of body composition and lipid profile. Nutr Hosp. 2009;24:422.
Beal, MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:189S.
Beard, JL. Iron biology in immune function, muscle metabolism, and neuronal functioning. J Nutr. 2001;131:568.
Beck, M, et al. Rapid genomic evolution of a non-virulent coxsackievirus B3—in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433.
Beckett, GJ, Arthur, JR. Selenium and endocrine systems. J Endocrinol. 2005;184:455.
Berger, MM. Vitamin C requirements in parenteral nutrition. Gastroenterology. 2009;137:70S.
Berndt, T, Kumar, R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda). 2009;24:17.
Bitanihirwe, BK, Cunningham, MG. Zinc: the brain’s dark horse. Synapse. 2009;63:1029.
Bjerrum, JT, et al. Metabonomics in ulcerative colitis: diagnostics, biomarker identification, and insight into the pathophysiology. J Proteome Res. 2010;9:954.
Block, RJ, Mitchel, HH. The correlation of the amino acid composition of proteins with their nutritive value. Nutr Abstr Rev. 1946;16:249.
Booth, SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89.
Brigelius-Flohe, R. Induction of drug metabolizing enzymes by vitamin E. J Plant Physiol. 2005;162:797.
Brosnan, JT, Brosnan, ME. The sulfur-containing amino acids: an overview. J Nutr. 2009;136:1636S.
Bruce, SJ, et al. Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J Agric Food Chem. 2010;58:2055.
Calvo, MS, et al. Vitamin D fortification in the United States and Canada: current stats and data needs. Am J Clin Nutr. 2004;80(6 Suppl):1710S.
Chachra, D, et al. Fluoride and mineralized tissues. Crit Rev Biomed Eng. 2008;36:183.
Churrucal, I, et al. Conjugated linoleic acid isomers: differences in metabolism and biological effects. Biofactors. 2009;35:105.
Clayton, PT. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis. 2006;29:317.
Combs, GF, et al. Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr. 2009;89:1808.
Cummings, JH, et al. Prebiotic digestion and fermentation. Am J Clin Nutr. 2001;73:415S.
Dakshinamurti, K. Biotin–a regulator of gene expression. J Nutr Biochem. 2005;16:419.
Denisova, NA, Booth, SL. Vitamin K and sphingolipid metabolism: evidence to date. Nutr Rev. 2005;63:111.
Devirian, TA, Volpe, SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43:219.
Dong, LM, et al. Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA. 2008;299:2423.
Elder, SJ, et al. Vitamin K contents of meat, dairy, and fast foods in the U.S. diet. J Agric Food Chem. 2006;54:463.
Eussen, SJ, et al. Vitamins B2 and B6 and genetic polymorphisms related to one-carbon metabolism as risk factors for gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2010;19:28.
Feskanich, D, et al. Vitamin A intake and hip fracture among postmenopausal women. JAMA. 2002;287:47.
Fleming, DJ, et al. Iron status of the free-living, elderly Framingham Heart Study cohort: an iron-replete population with a high prevalence of elevated iron stores. Am J Clin Nutr. 2001;73:638.
Freemantle, E, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75:213.
Frikke-Schmidt, H, Lykkesfeldt, J. Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol. 2009;104:419.
Gdynia, HJ, et al. Severe sensorimotor neuropathy after intake of highest dosages of vitamin B6. Neuromuscul Disord. 2008;18:156.
Gehrer, S, et al. Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)—a prospective study. Obes Surg. 2010;20:447.
Gehrig, KA, Dinulos, JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107.
Gerss, J, Kopcke, W. The questionable association of vitamin E supplementation and mortality—inconsistent results of different meta-analytic approaches. Cell Mol Biol. 2009;55:1111S.
Gilani, GS, et al. Need for accurate and standardized determination of amino acids and bioactive peptides for evaluating protein quality and potential health effects of foods and dietary supplements. JAOAC Int. 2008;91:894.
Gogia, S, Sachdev, HS. Neonatal vitamin A supplementation for prevention of mortality and morbidity in infancy: systematic review of randomized controlled trials. BMJ. 2009;338:919.
Goldberger, J, et al. A study of the diet of nonpellagrous and pellagrous households. JAMA. 1918;71:944.
Gonzalez-Reyes, RE, et al. Manganese and epilepsy: a systematic review of the literature. Brain Res Rev. 2007;53:332.
Gordon, RC, et al. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr. 2009;90:1264.
Grant, WB, Holick, MF. Benefits and requirement of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94.
Gropper, SS, et al. Advanced Nutrition and Human Metabolism, ed 4. Stamford, Conn.: Wadsworth; 2005. [p 584].
Guerrera, MP, et al. Therapeutic uses of magnesium. Am Fam Physcian. 2009;80:157.
Hague, T, et al. Determination of metal ion content of beverages and estimation of target hazard quotients: a comparative study. Chm Cent J. 2008;2:13.
Harvey, LJ, et al. Methods of assessment of copper status in humans: a systematic review. Am J Clin Nutr. 2009;89:2009S.
He, K, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675.
Heaney, RP, Rafferty, K. Preponderance of the evidence: an example from the issue of calcium intake and body composition. Nutr Rev. 2009;67:32.
Heimen, KA, et al. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J Am Acad Nurse Pract. 2009;21:295.
Hermann, W, et al. Enhanced bone metabolism in vegetarians—the role of vitamin B12 deficiency. Clin Chem Lab Med. 2009;47:1381.
Hogan, SL. The effects of weight loss on calcium and bone. Crit Care Nurs Q. 2005;28:269.
Holick, MF, Chen, TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S.
Holick, MF. Vitamin D deficiency. Med Prog. 2007;357:266.
Holick, MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062.
Holick, MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academies Press; 2000.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press; 2000.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrates, fiber, fat, protein and amino acids (macronutrients). Washington, DC: National Academies Press; 2002.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2010.
Jacques, PF, et al. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Opthalmol. 2005;123:517.
Jeejeebhoy, K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137:7S.
Kamanna, VS, et al. Niacin: an old drug rejuvenated. Curr Atheroscler Rep. 2009;11:45.
Kamanna, VS, Kashyap, ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B.
Kaplan, JH, Lutsenko, S. Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem. 2009;284:25461.
Kaur B, et al: Vitamin C supplementation for asthma. Cochrane Databse Syst Rev 2009 Jan 21;(1):CD000993.
Kharbanda, KK. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155.
Kile, ML, Ronnenberg, AG. Can folate intake reduce arsenic toxicity? Nutr Rev. 2008;66:349.
Kim, H. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorder—focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. 2005;20:309.
Kirkland, JB. Niacin and carcinogenesis. Nutr Cancer. 2003;46:110.
Klompmaker, TR. Lifetime high calcium intake increases osteoporotic fracture risk in old age. Med Hypotheses. 2005;65:552.
Kovacs, CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105.
Kris-Etherton, PM, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179.
Kruizenga, HM, et al. Effectiveness and cost-effectiveness of early screening and treatment of malnourished patients. Am J Clin Nutr. 2005;82:1082.
Kusic, Z, Jukic, T. History of endemic goiter in Croatia: from severe iodine deficiency to iodine sufficiency. Coll Antropol. 2005;29:9.
Lai, MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43:191.
Lalioti, V, et al. Molecular mechanisms of copper homeostasis. Front Biosci. 2009;4:4878.
Lanska, DJ. Historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handbook Clin Neurol. 2009;95:445.
Latavayova, L, et al. Selenium: from cancer prevention to DNA damage. Toxicology. 2006;227:1.
Leenhardt, F, et al. Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. J Agric Food Chem. 2005;53:98.
Li, F, et al. Cell Life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide. Curr Med Chem. 2006;13:883.
Lin, PH, et al. Factors influencing dietary protein sources in the PREMIER trial population. J Am Diet Assoc. 2010;110:291.
Livesey, G, et al. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258S.
Ludwig, DS. The glycemic index: physiological mechanisms relating to obesity, diabetes and cardiovascular disease. JAMA. 2002;287:2414.
Lynch, RJ, Cate, JM. The anti-caries efficacy of calcium carbonate-based fluoride toothpastes. Int Dent J. 2005;55:175.
Maiese, K, et al. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446.
Malik, S, Kashyap, ML. Niacin, lipids, and heart disease. Curr Cardiol Rep. 2003;5:470.
Mancuso, M, et al. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr Drug Targets. 2010;11:111.
Masumoto, K, et al. Manganese intoxication during intermittent parenteral nutrition: report of two cases. J Parenter Enter Nutr. 2001;25:95.
McCowen, KC, Bistrian, BR. Essential fatty acids and their derivatives. Curr Opin Gastroenterol. 2005;21:207.
McKiernan, F, et al. Relationships between human thirst, hunger, drinking, and feeding. Physiol Behav. 2008;94:700.
McNulty, PH, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102:2040.
Meck, WH, Williams, CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385.
Micha, R, Mozaffarin, D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5:335.
Mingrone, G. Carnitine in type 2 diabetes. Ann NY Acad Sci. 2004;1033:99.
Muñoz, M, et al. An update on iron physiology. World J Gastroenterol. 2009;15:4617.
Musso, CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41:357.
Møller, LB, et al. Molecular diagnosis of Menkes disease: genotype-phenotype correlation. Biochimie. 2009;91:1273.
Mussig, K, et al. Iodine-induced thyrotoxicosis after ingestion of kelp-containing tea. J Gen Intern Med. 2006;21:666.
Nahon, P, et al. Myeloperoxidase and superoxide dismutase 2 polymorphisms comodulate the risk of hepatocellular carcinoma and death in alcoholic cirrhosis. Hepatology. 2009;50:1484.
Nemeth, E, Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78.
Nielsen, FH. Is boron nutritionally relevant? Nutr Rev. 2009;66:183.
Nieves, JW. Osteoporosis: the role of micronutrients. Am J Clin Nutr. 2005;81:1232S.
Oda, H. Functions of sulfur-containing amino acids in lipid metabolism. J Nutr. 2006;136:1666S.
Omdahl, JL, et al. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr. 2002;22:139.
Osterhues, A, et al. Shall we put the world on folate? Lancet. 2009;374:959.
Parks, S, Johnson, MA. Living in low-latitude regions in the United States does not prevent poor vitamin D status. Nutr Rev. 2005;63:203.
Pathak, P, Kapil, U. Role of trace elements zinc, copper and magnesium during pregnancy and it outcome. Indian J Pediatr. 2004;71:1003.
Penry, JT, Manore, MM. Choline: an important micronutrient for maximal endurance-exercise performance? Int J Sport Nutr Exerc Metab. 2008;18:191–203.
Peterlik, M Cross, HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290.
Pettifor, JM. Rickets and vitamin D deficiency in children and adolescents. Endocrinol Metab Clin North Am. 2005;34:537.
Pietras, SM, et al. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806.
Puli, S, et al. Signaling pathways mediating manganese-induced toxicity in human glioblastoma cells (u87). Neurochem Res. 2006;31:1211.
Riccardi, G, et al. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:269S.
Ristic-Medic, D, et al. Methods of assessment of iodine status in humans: a systematic review. Am J Clin Nutr. 2009;89:2052S–2069S.
Rivas, CI, et al. Vitamin C transporters. J Physiol Biochem. 2008;64:357.
Roberfroid, MB. Introducing inulin-type fructans. Br J Nutr. 2005;93(Suppl 1):S13.
Roberfroid, MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137:2493S.
Robinson, C, et al. The effect of fluoride on the developing tooth. Caries Res. 2004;38:268.
Romieu, I. Nutrition and lung health. Int J Tuberc Lung Dis. 2005;9:362.
Rufer, AC, et al. Structural insight into function and regulation of carnitine palmitoyltransferase. Cell Mol Life Sci. 2009;66:2489.
Said, ZM, et al. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am J Physiol Cell Physiol. 2008;294:1192.
Schlemmer, U, et al. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res. 2009;53:330S.
Schilsky, ML. Wilson disease: Current status and the future. Biochimie. 2009;91:1278.
Scholz-Ahrens, KE, et al. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73:459S.
Schrauzer, GN, Surai, PF. Selenium in human and animal nutrition: resolved and unresolved issues. Crit Rev Biotechnol. 2009;29:2.
Schwarz, G, et al. Molybdenum cofactors, enzymes and pathways. Nature. 2009;460:839.
Shaw, GM, et al. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology. 2009;20:714.
Shelton, RC, et al. Therapeutic options for treatment-resistant depression. CNS Drugs. 2010;24:131.
Shrimpton, R, et al. Zinc deficiency: what are the most appropriate interventions? BMJ. 2005;330:347.
Simon, KC, et al. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16:133.
Slatsky, I, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165.
Smith, AH, Steinmaus, CM. Health effects of arsenic and chromium in drinking water: recent human findings. Ann Rev Public Health. 2009;30:107.
Sokol, RJ. Antioxidant defenses in metal-induced liver damage. Semin Liver Dis. 2001;16:39.
Sommer, A. Vitamin A deficiency and clinical disease: an historical overview. Nutrition. 2008;138:1835.
Stahl, W, et al. Non-antioxidant properties of carotenoids. Biol Chem. 2002;383:553.
Stevenson, M, et al. Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health Technol Assess. 2009;13:iii.
Stiff, L. How should the effectiveness of a nutrition intervention addressing suboptimal intake of vitamin D be evaluated? J Am Diet Assoc. 2009;109:2120.
Tazoe, H, et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59:251S.
Thomson, CD, et al. Selenium and iodine supplementation: effect on thyroid function of older New Zealanders. Am J Clin Nutr. 2009;90:1038.
Tobacman, JK. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ Health Perspect. 2001;109:983.
Traber, MG. Vitamin E and K interactions—a 50-year-old problem. Nutr Rev. 2008;66:624.
Tuerk, MJ, Fazel, N. Zn deficiency. Curr Opin Gastroenterol. 2009;25:136.
United States Department of Agriculture. Dietary guidelines for Americans. http://www.health.gov/dietaryguidelines/dga2005/document/, 2005.
Varela-Moreiras, G, et al. Cobalamin, folic acid, and homocysteine. Nutr Rev. 2009;67:S69.
Vinceti, M, et al. Risk of chronic low-dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health. 2009;24:231.
Weinberg, PD. Analysis of the variable effect of dietary vitamin E supplements on experimental atherosclerosis. Plant Physiol. 2005;162:823.
Welch, G, et al. Evaluation of clinical outcomes for gastric bypass surgery: results from a comprehensive follow-up study. Obes Surg. 2011;21:18–28.
Wertz, PW. Essential fatty acids and dietary stress. Toxicol Ind Health. 2009;25:279.
Wigertz, K, et al. Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr. 2005;81:895.
Winkels, RM, et al. Bioavailability of food folates is 80% of that of folic acid. Am J Clin Nutr. 2007;85:465.
Wood, RJ. Manganese and birth outcome. Nutr Rev. 2009;67:416.
Wright, AJ, et al. Comparison of (6 S)-5-methyltetrahydrofolic acid v. folic acid as the reference folate in longer-term human dietary intervention studies assessing the relative bioavailability of natural food folates: comparative changes in folate status following a 16-week placebo-controlled study in healthy adults. Br J Nutr. 2009;26:1.
Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem. 2010;338:241.
Yin, Z, et al. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190.
Zeisel, S, da Costa, KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615.
Zempleni, J, et al. Biotin. Biofactors. 2009;35:36.
Zimmerman, MB. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr. 2009;89:668S.