Medical Nutrition Therapy for Renal Disorders
Physiology and Function of the Kidneys
The main function of the kidney is to maintain the balance of fluids, electrolytes, and organic solutes. The normal kidney performs this function over a wide range of fluctuations in sodium, water, and solutes. This task is accomplished by the continuous filtration of blood with alterations in secretion and reabsorption of this filtered fluid. The kidney receives 20% of cardiac output, filtering approximately 1600 L/day of blood and producing 180 liters of fluid called ultrafiltrate. Through active processes of reabsorbing certain components and secreting others, composition of this ultrafiltrate is changed into the 1.5 L of urine excreted in an average day.
Each kidney consists of approximately 1 million functioning nephrons (Figure 36-1), consisting of a glomerulus connected to a series of tubules. Tubules consist of different segments: the proximal convoluted tubule, loop of Henle, distal tubule, and collecting duct. Each nephron functions independently and contributes to the final urine, although all are under similar control and coordination. If one segment of a nephron is destroyed, that complete nephron is no longer functional.
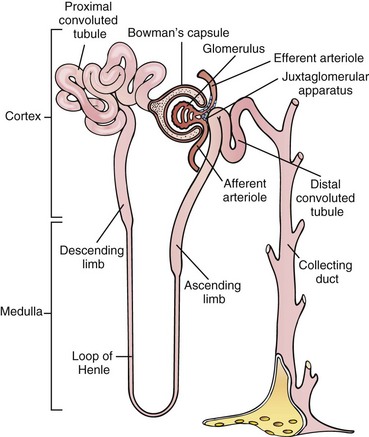
FIGURE 36-1 The nephron. (Modified from Thibodeau GA, Patton KT: The human body in health and disease, ed 4, St Louis, 2005, Mosby.)
The glomerulus is a spherical mass of capillaries surrounded by a membrane, Bowman’s capsule. The glomerulus produces the ultrafiltrate, which is then modified by the next segments of the nephron. Production of ultrafiltrate is mainly passive and relies on the perfusion pressure generated by the heart and supplied by the renal artery.
The tubules reabsorb the vast majority of components that compose the ultrafiltrate. Much of this process is active and requires a large expenditure of energy in the form of adenosine triphosphate (ATP). The tubule is a unique structure; differences in permeability between the various segments and hormonal responses allow the tubule to produce the final urine, which can vary widely in concentration of electrolytes, osmolality, pH, and volume. Ultimately, this urine is funneled into common collecting tubules and into the renal pelvis. The renal pelvis narrows into a single ureter per kidney, and each ureter carries urine into the bladder, where it accumulates before elimination.
The kidney has almost unlimited ability to regulate water homeostasis. Its ability to form a large concentration gradient between its inner medulla and outer cortex allows the kidney to excrete urine as dilute as 50 mOsm or as concentrated as 1200 mOsm. Given a daily fixed solute load of approximately 600 mOsm, the kidney can get rid of as little as 500 mL of concentrated urine or as much as 12 L of dilute urine. Control of water excretion is regulated by vasopressin (antidiuretic hormone [ADH]), a small peptide hormone secreted by the posterior pituitary. An excess of relative body water, indicated by a low osmolality, leads to prompt shut-off of all vasopressin secretion. Likewise, a small rise in osmolality brings about marked vasopressin secretion and water retention. However, the need to conserve sodium sometimes leads to a sacrifice of the homeostatic control of water for the sake of volume. See Focus On: Syndrome of Inappropriate Antidiuretic Hormone.
The minimum urinary volume capable of eliminating a relatively fixed 600 mOsm of solute is 500 mL, assuming that the kidney is capable of maximum concentration. Urinary volume of less than 500 mL/ day is called oliguria; it is impossible for such a small urine volume to eliminate all of the daily waste.
The majority of the solute load consists of nitrogenous wastes, primarily the end products of protein metabolism. Urea predominates in amount, depending on the protein content of the diet. Uric acid, creatinine (Cr), and ammonia are present in small amounts. If normal waste products are not eliminated appropriately, they collect in abnormal quantities in the blood, known as azotemia. The ability of the kidney to adequately eliminate nitrogenous waste products is defined as renal function. Thus renal failure is the inability to excrete the daily load of wastes.
The kidney also performs functions unrelated to excretion. One of these involves the renin-angiotensin mechanism, a major control of blood pressure. Decreased blood volume causes cells of the glomerulus (the juxtaglomerular apparatus) to react by secreting renin, a proteolytic enzyme. Renin acts on angiotensinogen in the plasma to form angiotensin I, which is converted to angiotensin II, a powerful vasoconstrictor and a potent stimulus of aldosterone secretion by the adrenal gland. As a consequence, sodium and fluid are reabsorbed, and blood pressure is returned to normal.
The kidney also produces the hormone erythropoietin (EPO), a critical determinant of erythroid activity in the bone marrow. Deficiency of EPO is the primary cause of the severe anemia present in chronic renal disease.
Maintenance of calcium-phosphorus homeostasis involves the complex interactions of parathyroid hormone (PTH); calcitonin; active vitamin D; and three effector organs: the gut, kidney, and bone. The role of the kidney includes production of the active form of vitamin D—1,25-dihydroxycholecalciferol (1,25-[OH]2D3)—as well as elimination of both calcium and phosphorus. Active vitamin D promotes efficient absorption of calcium by the gut and is one of the substances necessary for bone remodeling and maintenance. Active vitamin D also suppresses PTH production, which is responsible for mobilization of calcium from bone (see Chapter 25).
Renal Diseases
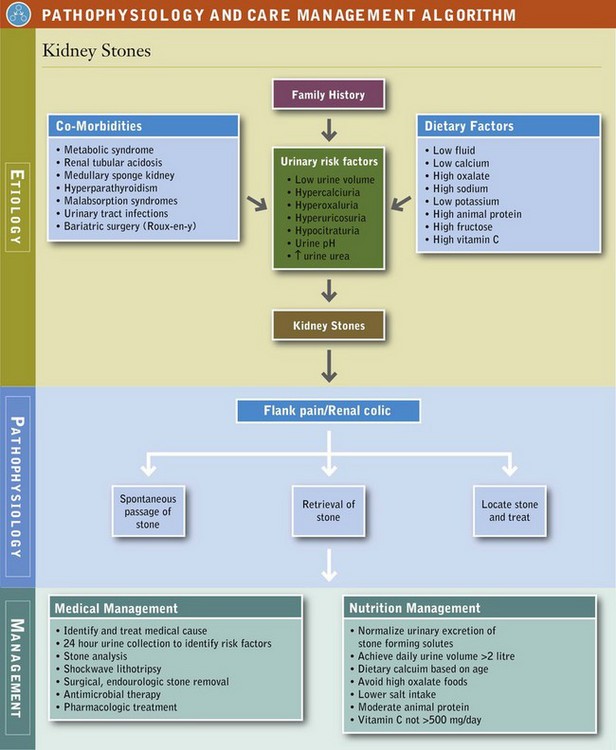
The manifestations of renal disease are significant. They can be ordered by degree of severity: (1) kidney stones, (2) acute kidney injury (AKI), (3) chronic kidney disease (CKD), and (4) end-stage renal disease (ESRD) (National Kidney Foundation, 2002). Objectives of nutritional care depend on the abnormality being treated.
Kidney Stones (Nephrolithiasis)
Nephrolithiasis, the presence of kidney stones, is a significant health problem in the United States. It is characterized by frequent occurrences between the ages of 30 and 50, predominance in males, and a high recurrence rate. The risk doubles in those with a family history of kidney stones; stone formers often have first-degree relatives with kidney stones. Increased frequency of obesity, diabetes, and metabolic syndrome have resulted in increasing rates of nephrolithiasis among women, decreasing the male/female ratio from 1.7 : 1 to 1.3 : 1 (Zilberman, 2010). One Canadian study found significant differences in occurrence rates among ethnic groups, with highest rates in those of Arabic and West Indian descent and the lowest rates among those of East Asian or African descent (Mente, 2007). However, low urine volume is the single most important risk factor for nephrolithiasis.
Pathophysiology
Kidney stone formation is a complex process that consists of saturation; supersaturation; nucleation; crystal growth or aggregation; crystal retention; and stone formation in the presence of promoters, inhibitors, and complexors in urine. A typical metabolic evaluation is described in Table 36-1.
Calcium stones are the most common: 60% of stones are calcium oxalate, 10% calcium oxalate and calcium phosphate, and 10% calcium phosphate. Other stones are 5% to 10% uric acid, 5% to 10% struvite, and 1% cystine.
Obese stone formers excrete increased amount of sodium, calcium, uric acid, and citrate, and have lower urine pH. Obesity is the strongest predictor of stone recurrence in first-time stone formers. As body weight increases, the excretion of calcium, oxalate, and uric acid also increases. Patients with higher body mass index (BMI) have a decrease in ammonia excretion and impaired hydrogen ion buffering (Li et al., 2009). With increasing BMI, uric acid stones become more dominant than calcium oxalate stones, especially in men (Eisner, 2010b).
Uric acid stones are common in the presence of type 2 diabetes. Hyperinsulinemia may also contribute to the development of calcium stones by increasing urinary calcium excretion (Maalouf et al., 2010b). Weight control may be considered one of the preventive modalities and in stone formers, a BMI of 18 to 25 kg/m2 is recommended.
With malabsorptive bariatric procedures such as Roux-en-Y gastric bypass (RYGB), urolithiasis is higher than in obese controls, probably because of the increased prevalence of hyperoxaluria and hypocitraturia in RYGB patients (Maalouf et al., 2010a). However, restrictive gastric surgery (i.e., gastric banding or sleeve gastrectomy) is not associated with increased risk of kidney stones (Semins et al., 2010).
Agents added intentionally or unintentionally to food or drug products have led to the appearance of new types of stones containing melamine and indinavir (Zilberman, 2010). See Table 36-2.
TABLE 36-2
Causes and Composition of Renal Stones
| Pathogenetic Causes | Composition of Stone |
| Hypercalciuria, hyperoxaluria, hyperuricosuria, or hypocitraturia | Calcium oxalate |
| Primary hyperparathyroidism | Calcium oxalate |
| Cystinuria | Cystine |
| Infection | Struvite |
| Acid urine pH Hyperuricosuria |
Uric acid Uric acid |
| Renal tubular acidosis Alkaline urine pH |
Calcium phosphate Calcium phosphate |
Modified from: Asplin JR: Evaluation of a kidney stone patient, Seminars in Nephrol 28(2):99, 2008.
Calcium Stones: One third to one half of patients with calcium stones are hypercalciuric. Hypercalciuria describes a value of calcium in excess of 300 mg (7.5 mmol) per day in men, 250 mg (6.25 mmol) per day in women, or 4 mg (0.1 mmol)/kg/day for either in random urine collections of outpatients on unrestricted diets. Causes of hypercalciuria may include primary hyperparathyroidism, sarcoidosis, excess vitamin D intake, hyperthyroidism, glucocorticoid use, or renal tubular acidosis (RTA).
Idiopathic hypercalciuria (IH) seems to have a genetic basis. IH can be triggered by an excessive dietary calcium intake, increased intestinal absorption of calcium that may or may not be vitamin D–mediated, decreased renal tubular reabsorption of calcium, or prolonged bed rest. Increased gut absorption of calcium is noted in essentially all patients with IH. However, urinary calcium is higher than normal at any level of net calcium absorption, suggesting that some of the urine calcium is derived from the bone. When challenged with a very-low-calcium diet, the loss of more calcium in the urine than is in the diet results in abnormal bone mineral wasting. Patients with IH tend toward negative phosphorus balance even on normal intakes. The defective phosphate metabolism may lead to increased 1,25(OH)2D3 levels, and increased intestinal calcium absorption.
Bone loss can be high in patients with IH in whom low calcium intake exaggerates bone loss from increased net acid excretion (NAE). For decades low-calcium diets were recommended to reduce the hypercalciuria in these stone-formers. However, chronic prolonged calcium restriction, deficient calcium intake, and increased losses from hypercalciuria decrease bone mineral density at the spine and cortical sites. Thus vertebral fracture risk increases fourfold among urolithiasis patients in comparison with the general population.
Undesirable bone resorption may be enhanced by a high protein intake of nondairy origin. An inadequate calcium with high protein intake induces metabolic acidosis, increases calcium excretion, and lowers urinary pH. This acid load inhibits the renal reabsorption of calcium. A reduction in nondairy animal protein may be recommended; see Clinical Insight: Urinary pH—How Does Diet Affect It?
Calcium supplements do not have the same protective effect as dietary calcium. A trial of combined calcium–vitamin D supplementation to prevent bone loss and fractures led to higher rates of stone formation in women (Jackson et al., 2006). If taken as a supplement, timing is important. Calcium supplements taken with meals increase urinary calcium and citrate but decrease urinary oxalate; thus the increase in citrate and decrease in oxalate counterbalance the effects of elevated urinary calcium. Therefore, if used by patients who cannot tolerate dairy products because of lactose intolerance, allergies, or preference, calcium supplements should be taken with meals. Urine calcium should be measured prior to starting the supplement and afterward to see the effect; if urine calcium increases, patients should increase fluid intake to dilute the urine concentration of calcium.
Patients may select 700-800 mg of calcium from dairy choices and consume the rest from nondairy foods to make up the total needed per day, according to age group and dietary reference intake (DRI) recommendations. Calcium should be taken in divided doses, choosing a source with each meal to maximize oxalate binding. Any low-fat dairy choices are good options.
Oxalate Stones: Hyperoxaluria (>40 mg of oxalate in urine per day) plays an important role in calcium stone formation and is observed in 10% to 50% of recurrent stone formers. Primary hyperoxaluria is a feature of an autosomal-recessive genetic defect of a hepatic enzyme that results in overproduction of oxalate and a urinary oxalate concentration three to eight times normal. Multiple stones occur in these children, causing renal failure and early death.
Patients with inflammatory bowel diseases or gastric bypass often develop hyperoxaluria because of fat malabsorption. The bile acids produced during the digestive process normally are reabsorbed in the proximal gastrointestinal (GI) tract, but when this fails to occur, bile salts and fatty acids increase colonic permeability to oxalate. The unabsorbed fatty acids also bind calcium to form soaps, decreasing availability of calcium in a soluble form. With less calcium available to bind oxalate in the gut and prevent its absorption, serum oxalate and thus urinary oxilate levels increase.
Urinary oxalate also comes from endogenous synthesis, proportional to lean body mass. Ascorbic acid accounts for 35% to 55%, and glyoxylic acid accounts for 50% to 70% of urinary oxalate. In patients with CKD, excessive vitamin C intake may lead to stone formation. Oxalate synthesis is not increased with a high protein diet (Knight et al., 2009). Because pyridoxine acts as a cofactor in the conversion of glyoxylate to glycine, its deficiency could increase endogenous oxalate production.
The bioavailability of food oxalate and urine oxalate are affected by salt forms of oxalate, food processing and cooking methods, meal composition, and the presence of Oxalabacter formigenes in the GI tract (Massey, 2007). Stone-forming patients who lack this bacteria have significantly higher urinary oxalate excretion and stone episodes compared with patients colonized with the bacteria (Hatch and Freel, 2008).
Administration of Oxalobacter formigenes as enteric-coated capsules significantly reduces urine oxalate in patients with primary hyperoxaluria. Dietary advice for reducing urinary oxalate should include both use of this probiotic and reduction of dietary oxalate and simultaneous consumption of calcium-rich food or supplement to reduce oxalate absorption (Massey, 2007) (Box 36-1).
Uric Acid Stones: Uric acid is an end product of purine metabolism from food, de novo synthesis, and tissue catabolism. Approximately half of the purine load is from endogenous sources and is constant. Exogenous dietary sources provide the other half, accounting for the variation in urinary uric acid. The solubility of uric acid depends on urine volume, the amount excreted, and urine pH (Table 36-3). Uric acid stones form when urine is supersaturated with undissociated uric acid, which occurs at urinary pH less than 5.5.
TABLE 36-3
Effect of Urine pH on Stone Formation
| pH | State of Urate | Likely Stone Development |
| <5.5 | Undissociated urate | Uric acid stones |
| 5.5-7.5 | Dissociated urate | Calcium oxalate stones |
| >7.5 | Dissociated urate | Calcium phosphate stones |
The most important feature in uric acid stone formers is low urine pH resulting from increased NAE and impaired buffering caused by reduced urinary ammonium excretion. The former can be a result of low intake of alkali-producing foods or increased consumption of acid-producing foods. See Clinical Insight: Urine pH—How Does Diet Affect It?
Inflammatory bowel disease results in chronically acidic urine, usually from dehydration. GI bicarbonate loss from diarrhea may predispose these patients to uric acid stones. Uric acid stones are also associated with lymphoproliferative and myeloproliferative disorders, with increased cellular breakdown that releases purines and thus increases uric acid load. Diabetes, obesity, and hypertension appear to be associated with nephrolithiasis; diabetes is a common factor in uric acid stone development (Lieske et al., 2006). Besides diabetes management for patients with uric acid lithiasis and hyperuricosuric calcium oxalate stones, dietary purines should also be restricted.
Meat, fish, and poultry are rich in purines and acid ash, and thus should be used in moderation. Foods specifically high in purines should be avoided, including organ meats, anchovies, herrings, sardines, meat-based broth, and gravy (see Box 40-3 in Chapter 40). Dietary noncompliance or persistence of hyperuricosuria warrants use of medication such as allopurinol. Uric acid stones are the only stones amenable to dissolution therapy by urine alkalinization to a pH of 6 to 6.5. Potassium citrate has been used as the therapy of choice. Sodium bicarbonate increases urinary monosodium urate and calcium and should not be used.
Cystine Stones: Cystine stones represent 1% to 2% of urinary calculi and are caused by homozygous cystinuria. Cystine stones affect approximately 1 in 15,000 persons in the United States. Whereas normal individuals daily excrete 20 mg or less of cystine in their urine, stone-forming cystinuric patients excrete more than 250 mg/day. Cystine solubility increases when urine pH exceeds 7; therefore an alkaline urine pH must be maintained 24 hours per day, even while the patient sleeps. This is almost always achieved with the use of medication. Fluid intake of more than 4 L daily is recommended to prevent cystine crystallization. Lower sodium intake may be useful in reducing cystine in the urine.
Melamine and Indinavir Stones: Kidney stones, ARF, and death have been reported in young children who received melamine-contaminated infant formula. Melamine is an organic base synthesized from urea. When added to liquid milk or milk powder, it deceptively increases the protein content. Melamine precipitates in the distal renal tubules, forming crystals and sandlike stones. Hydration and urine alkalinization help with stone passage.
The treatment of human immunodeficiency virus infection with protease inhibitors has led to the appearance of another previously unknown urinary calculus: indinavir. Hypocitraturia is universal in all patients with indinavir stones as well as decreased solubility in a low urine volume with a low pH. These stones are soft, gelatinous, radiolucent, and are not amenable to basket removal or ureteroscopy. Intravenous (IV) hydration and temporary cessation of indinavir should be the first choice of treatment (Zilberman, 2010).
Struvite Stones: Struvite stones are composed of magnesium ammonium phosphate and carbonate apatite. They are also known as triple-phosphate or infection stones. Unlike most urinary stones, they occur more commonly in women than in men, at a ratio of 2 : 1. They form only in the presence of bacteria such as Pseudomonas, Klebsiella, Proteus mirabilis, and Urealyticum that carry urease, a urea-splitting enzyme. Urea breakdown results in ammonia and carbon dioxide (CO2) production, thus raising urine pH and the level of carbonate. Struvite stones grow rapidly to large staghorn calculi in the renal pelvic area. The mainstay of treatment is extracorporeal shockwave lithotripsy (ECSWL) with adjunctive culture-specific antimicrobial therapy that uses urease inhibitors. The goal is to eliminate or prevent urinary tract infections by regularly screening and monitoring urine cultures.
Medical Management
Uric acid and struvite stones are the only type amenable to dissolution therapy in the form of ECSWL. Shockwave lithotripsy and endourologic techniques have almost replaced the open surgical procedures of stone removal of 20 years ago. Struvite stones are also treated with adjunctive culture-specific antimicrobial therapy that uses urease inhibitors. Management strategies are now aimed at kidney stone prevention (Asplin, 2008).
Medical Nutrition Therapy
After corrective treatment, nutrition assessment is needed to determine risk factors for stone recurrence. The risk in both men and women rises with increasing urine calcium and oxalate and decreases with increasing citrate and urine volume. There is a continuum of risk related to increasing urinary calcium and urinary oxalate. (Curhan and Taylor, 2008). Because urine chemistries change from day to day based on changes in the environment and diet, two 24-hour urine specimens are needed based on a usual diet, one during a week day and one on the weekend. Specific medical nutrition therapy (MNT) is then based on comprehensive metabolic evaluations. Nutrition counseling and metabolic monitoring can be quite effective (Table 36-4).
TABLE 36-4
Recommendations for Diet and 24-Hour Urine Monitoring in Kidney Stone Disease
| Diet Component | Intake Recommendation | 24-Hour Urine |
| Protein | Normal intake: avoid excess | Monitor urinary urea |
| Calcium | Normal intake:1000 mg if age < 50 years; 1200 mg if age > 50 years Divide intake between three or more eating sessions |
Calcium < 150 mg/L (<3.75 mmol/L) |
| Oxalate | Avoid moderate- to high-oxalate foods initially; further restrict if necessary | Oxalate < 20 mg/L (<220 µmol/L) |
| Fluid | 2.5 L or more; assess type of fluids consumed; provide guidelines | Volume > 2 L/day |
| Purines | Avoid excessive protein intake; avoid specific high-purine foods | Uric acid < 2 mmol/L (<336 mg/L) |
| Vitamin C | < 500 mg/day | Monitor urinary oxalate |
| Vitamin D; cod liver oil | Supplements not recommended | |
| Vitamin B6 | 40 mg or more per day reduces risk. No recommendation made | |
| Sodium | <100 mmol/day | Monitor urinary sodium |
From: Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones, Kidney Internat 73:489, 2008.
When a patient passes a stone, it should be determined if it is a new stone or a preexisting one and advisement given accordingly (Asplin, 2008). The effectiveness of any MNT should be monitored with evaluation of subsequent 24-hour urine collections. This gives the nutritionist and patient a measure of the effect of dietary changes. Once diet therapy is initiated, the goal is to prevent new stones from forming and preexisting stones from growing. See Pathophysiology and Care Management Algorithm: Kidney Stones.
Fluid and Urine Volume: A low urine volume is by far the most common abnormality noted on metabolic evaluation of stone formers, and its correction with a high fluid intake should be the focus in all types of kidney stones. The objective is to maintain urinary solutes in the undersaturated zone to inhibit nucleation; this increases urine volume and reduces solute load. The goal is the amount of urine flow rather than a specified fluid intake. High urine flow rate tends to wash out any formed crystals, and a urine volume of 2 to 2.5 L/day should prevent stone recurrence. Fluid intake should change based on different rates of extrarenal fluid loss that affect rate of urine flow.
Achieving a urine volume of 2 to 2.5 L/day usually requires an intake of 250 mL of fluid at each meal, between meals, at bedtime, and when arising to void at night. Hydration during sleep hours is important to break the cycle of the “most-concentrated” morning urine. Half of this daily 2.5 L should be taken as water. Even higher fluid intake, perhaps as much as 3 L/day, may be necessary to compensate for any GI fluid loss, excessive sweating from strenuous exercise, or an excessively hot or an excessively dry environment, such as a commercial airplane cabin.
Cranberry juice acidifies urine and is useful in the treatment of struvite stones. Black currant juice increases urinary citrate and oxalate and, because of its urine alkalinizing effect, may prevent the occurrence of uric acid stones.
Tea, coffee, beer, and wine have been associated with reduced risk of stone formation. The oxalate content of tea brewed from regular black or green tea is 300-1500 µmol/L. Because of the high oxalate content of tea, it should be taken with generous amounts of added milk; milk appears to reduce oxalate absorption by binding it in the gut lumen as calcium oxalate, making it less absorbable. Herbal teas have much lower oxalate content of 31-75 µmol/L and are an acceptable alternative (Massey, 2007). Soft drinks and colas that contain phosphoric acid should be avoided because of their urine acidifying effect.
Animal Protein: Epidemiologic studies find a correlation between improved standard of living, high animal protein intake, and the rising incidence of kidney stones. Meat, fish, poultry eggs, cheese, and grains are the primary contributors of acid; see Clinical Insight: Urine pH—How Does Diet Affect It? An adequate-calcium, low animal–protein, low-salt diet (less than 4 g) reduces oxalate excretion more than a traditional low oxalate diet (Nouvenne et al., 2009).
Oxalate: Because much less oxalate than calcium exists in urine (ratio 1 : 5), changes in oxalate concentration have a greater effect than changes in urinary calcium. However, oxalate absorption, which is 3% to 8% of the amount in food, is affected by the amount of dietary calcium. On very low calcium intakes of less than 200 mg/day, oxalate absorption rises; it falls when a higher calcium (1200 mg) is ingested.
Dietary counseling to reduce oxalate absorption is beneficial for stone-forming individuals who have large intakes of high-oxalate foods and who excrete more than 30 mg (350 µmol) of oxalate per day. The American Dietetic Association recommends restriction of oxalate to approximately 60 mg/day. To keep the diet plan simple, the patient is told to avoid those foods that are high in oxalate as the first step.
When these foods are avoided, other foods eaten will often only add up to the 50-60 mg, the daily dietary target. In addition, the patient is advised to add calcium to each meal to bind oxalate. The total calcium intake for the day can be divided between at least three meals or as many eating occasions as possible. It takes 150 mg of calcium to bind 100 mg of oxalate. Patients should include approximately 150 mg calcium in each meal, such as that found in 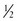 cup milk, ice cream, pudding, yogurt, or
cup milk, ice cream, pudding, yogurt, or 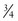 oz cheese (Marcason, 2006; Massey, 2007).
oz cheese (Marcason, 2006; Massey, 2007).
Potassium: Stone formers often have a low to normal potassium intake and high sodium intake. Potassium intake is inversely related to the risk of kidney stones. Estimation of fruit and vegetable intake should be included in the metabolic evaluation. Stone formers should be encouraged to increase the potassium in their diets by choosing low-oxalate fruit and vegetables many times throughout the day (Domrongkitchaiporn et al., 2006; see Appendix 56). See Box 36-1.
Magnesium: Magnesium is a low-molecular-weight inhibitor that forms soluble complexes with oxalate. Like calcium, it inhibits oxalate absorption and may have a role to play in hyperoxaluric patients.
Phosphate: Excess urine phosphate contributes to calcium phosphate stone risk, but it is not as important a risk factor as urinary pH, which determines how much phosphate will be in the form of hydrogen phosphate (HPO4) (Asplin, 2008). Calcium phosphate stones tend to occur in pregnant women in the second and third trimester of pregnancy.
Sodium: The daily amount of sodium chloride in modern diets reaches excessive levels of up to 10 g/day. The amount of sodium in the urine and hypercalciuria are directly correlated because sodium and calcium are reabsorbed at common sites in the renal tubule. Because the risk for nephrolithiasis is significantly higher in hypertensive individuals compared with normotensive individuals, sodium intake should be lowered to less than 2300 mg/day in patients with hypercalciuria (Asplin, 2008; Nouvenne et al, 2009; Straub and Hautmann, 2005). Consumption of a diet modeled on the Dietary Approaches to Stop Hypertension diet reduces the risk for kidney stones (Taylor et al., 2009). See Appendix 33.
Citrate: Citrate inhibits urinary stones by forming a complex with calcium in urine. Thus less calcium is available to bind urinary oxalate, which helps prevent the formation of calcium oxalate or calcium phosphate stones. Distal RTA is an acidosis accompanied by hypokalemia. RTA, malabsorption syndrome with enteric hyperoxaluria, and excessive meat intake (lower urine pH) are associated with decreased urinary citrate levels.
Many citrate-containing beverages have been tested for their effect on urine. Several diet sodas contain moderate amounts of citrate and malate; malate increases the total alkali load delivered, which augments citraturia (Eisner, 2010a). One commercial sports drink tested in non–stone formers increased urine citrate as much as 170 mg/day, but many sports drinks contain too much fructose and do not increase urinary citrate (Goodman, 2009).
Hypocitraturia is most commonly idiopathic, but may also be caused by acidosis accompanied by hypokalemia, malabsorption syndrome with enteric hyperoxaluria, excessive meat intake, and acid ash (Zuckerman and Assimos, 2009). Half of recurrent calcium stone formers have hypocitraturia (urinary citrate of <300 mg/day), predominantly of dietary origin. Normal daily urinary citrate level should be more than 640 mg/day. Long-term lemonade or lime or lemon juice therapy in hypocitraturic stone formers results in increased urinary citrate levels and decreased stone formation rate (Kang et al., 2007). Mineral water, with its magnesium and bicarbonate content, raises urine pH and stone inhibition.
Fructose: Fructose intake has increased approximately 2000% during the past 30 years from a widespread increase in the use of high-fructose corn syrup in foods. Fructose may increase urinary excretion of calcium and oxalate. It is the only carbohydrate known to increase the production of uric acid and its urinary excretion. Fructose may also increase insulin resistance, which is associated with low urine pH. Fructose intake has been positively associated with the risk of incident kidney stones (Taylor, 2008). Increased fruit and vegetable consumption is recommended to increase potassium intake, but because of the fructose content of fruit, there should be even more emphasis on vegetables (Asselman and Verkoelen, 2008).
Vitamins: Health benefits from 500 mg of ascorbic acid or more are not substantiated. Thus individuals at risk for calcium oxalate stones are wise to not exceed 500 mg/day (Massey et al., 2005; Moyad et al., 2009). Vitamin B6 in the form of pyridoxal phosphate is a required cofactor in oxalate metabolism; marginal B6 status should be avoided. Using 2 to 10 mg/day of vitamin B6 may reduce urinary oxalate in some calcium oxalate stone formers.
Acute Kidney Injury (Acute Renal Failure)
Pathophysiology
Acute kidney injury (AKI), formerly acute renal failure (ARF), is characterized by a sudden reduction in glomerular filtration rate (GFR), the amount of filtrate per unit in the nephrons, and altered ability of the kidney to excrete the daily production of metabolic waste. AKI can occur in association with oliguria (decreased output of urine) or normal urine flow, but it typically occurs in previously healthy kidneys. Duration varies from a few days to several weeks. The causes of AKI are numerous, and can occur simultaneously (Table 36-5). These causes are generally classified into three categories: (1) Inadequate renal perfusion (prerenal), (2) diseases within the renal parenchyma (intrinsic), and (3) urinary tract obstruction (postrenal).
Medical Management
The ratio of blood urea nitrogen (BUN) to Cr can be used diagnostically to assess the location of damage to the kidney. Depending on where the insult occurs, BUN is increased because of poor filtration, and is more actively reabsorbed. In this situation, with a BUN/Cr ratio greater than 20 : 1, damage is prerenal (before the kidney). When damage is intrinsic (within the kidney), the BUN/Cr ratio decreases to less than 10 : 1. Generally, if careful attention is directed at diagnosing and correcting the prerenal or obstructive causes, AKI is short lived and requires no particular nutritional intervention.
Intrinsic AKI can result from causes listed in Table 36-5; of these, a prolonged episode of ischemia leading to ischemic acute tubular necrosis is the most devastating. Typically patients develop this illness as a complication of an overwhelming infection, severe trauma, surgical accident, or cardiogenic shock. The clinical course and outcome depends mainly on the underlying cause. Patients with AKI caused by drug toxicity generally recover fully after they stop taking the drug. On the other hand, the mortality rate associated with ischemic acute tubular necrosis caused by shock is approximately 70%. Typically these patients are highly catabolic, and extensive tissue destruction occurs in the early stages. Hemodialysis (HD) is used to reduce the acidosis, correct the uremia, and control hyperkalemia.
If recovery is to occur, it generally takes place within 2 to 3 weeks after the insult is corrected. The recovery (diuretic) phase is characterized first by an increase in urine output and later by a return of waste elimination. During this period dialysis may still be required, and careful attention must be paid to fluid and electrolyte balance and appropriate replacement.
Medical Nutrition Therapy
Nutritional care in AKI is particularly important because the patient not only has uremia, metabolic acidosis, and fluid and electrolyte imbalance, but also usually suffers from physiologic stress (e.g., infection or tissue destruction) that increases protein needs. The problem of balancing protein and energy needs with treatment of acidosis and excessive nitrogenous waste is complicated and delicate. In the early stages of AKI the patient is often unable to eat. Mortality in AKI is high, especially among those who are malnourished (Strejc, 2005). Early attention to nutritional support and early dialysis improves patient survival.
At the onset of AKI, depending on severity, some patients can be treated with medical management, other patients require renal replacement therapy (RRT) with standard HD or peritoneal dialysis (PD) to remove wastes and fluids until kidney function returns. In significant AKI, a patient in the intensive care unit (ICU) may require continuous treatments, rather than periodic dialysis. Continuous renal replacement therapy (CRRT) is the broad category term that includes a whole host of modalities. Most often used are continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodialysis (CVVHD), which use a small ultrafiltration membrane to produce an ultrafiltrate that can be replaced by parenteral nutrition (PN) fluids. This treatment allows parenteral feeding without fluid overload. Less often used are continuous arteriovenous hemofiltration, continuous arteriovenous hemodialysis, and continuous venovenous hemodiafiltration. These modalities differ in the type of blood access used, as well as type of filtration (diffuse versus convective, or both).
Protein: The amount of protein recommended is influenced by the underlying cause of AKI and the presence of other conditions. A range of recommended levels can be found in the literature, from 0.5-0.8 g/kg for nondialysis patients to 1-2 g/kg for dialyzed patients. With CRRT protein losses are high, and estimated protein needs increase to 1.5-2.5 g/kg. As the patient’s overall medical status stabilizes and improves, metabolic requirements decrease. During this stable period before renal function returns, a minimum protein intake of 0.8-1 g/kg of body weight should be given. This remains dependent on the patient’s overall status and comorbidities and should be evaluated individually.
Energy: Energy requirements are determined by the underlying cause of AKI and comorbidity. Energy needs can be measured at the bedside by indirect calorimetry in most ICUs (see Chapter 2). If this equipment is not available, calorie needs should be estimated at 30 to 40 kcal/kg of dry body weight per day. Excessive calorie intake can lead to excess CO2 production, depressing respiration (see Chapter 35). If PD or CRRT with a glucose containing solution is used, the amount of glucose absorbed can add significantly to the daily energy intake and should be calculated. Large intakes of carbohydrate and fat are needed to prevent the use of protein for energy production. For patients who receive PN, high concentrations of both carbohydrate and lipid can be administered to fulfill these needs as long as respiratory status is monitored.
A high-calorie, low-protein diet may be used in cases in which dialysis or hemofiltration is unavailable. In addition to the usual dietary sources of refined sweets and fats, special high-calorie, low-protein, and low-electrolyte formulas have been developed to augment the diet. However, care must be taken with these products because hyperglycemia is not uncommon as a result of glucose intolerance, and additional insulin is often needed
Fluid and Sodium: During the early (often oliguric) phase of AKI, meticulous attention to fluid status is essential. Ideally fluid and electrolyte intake should balance the net output. With negligible urine output, significant contributions to total body water output include emesis and diarrhea, body cavity drains, and skin and respiratory losses. If fever is present, skin losses can be excessive; whereas if the patient is on humidified air, almost no losses occur. Because of the numerous IV drugs, blood, and blood products necessitated by the underlying disease, the challenge in managing patients at this point becomes how to cut fluid intake as much as possible while providing adequate protein and energy.
Sodium is restricted, based on decreased urinary production. In the oliguric phase when the sodium output is very low, intake should be low as well, perhaps as low as 20 to 40 mg/day. However, limiting sodium is often impossible because of the requirement for many IV solutions (including IV antibiotics, medications for blood pressure, and PN). The administration of these solutions in electrolyte-free water in the face of oliguria quickly leads to water intoxication (hyponatremia). For this reason, all fluid above the daily calculated water loss should be given in a balanced salt solution.
Potassium: Most of the excretion of potassium and the control of potassium balance are normal functions of the kidney. When renal function is impaired, potassium balance should be scrutinized carefully. In addition to dietary sources, all body tissues contain large amounts of potassium; thus tissue destruction can lead to tremendous potassium overload. Potassium levels can shift abruptly and need to be monitored frequently. Potassium intake needs to be individualized according to serum levels (see Appendices 36 and 56). The primary mechanism of potassium removal during AKI is dialysis. Control of serum potassium levels between dialysis administrations rely mainly on IV infusions of glucose, insulin, and bicarbonate, all of which serve to drive potassium into cells. Exchange resins such as sodium polystyrene sulfonate (Kayexalate), which exchange potassium for sodium in the GI tract, can be used to treat high potassium concentrations, but for many reasons these resins are less than ideal. Table 36-6 summarizes MNT for AKI.
TABLE 36-6
Summary of Medical Nutrition Therapy for Acute Kidney Injury
| Nutrient | Amount |
| Protein | 0.8-1 g/kg IBW increasing as GFR returns to normal; 60% should be HBV protein |
| Energy | 30-40 kcal/kg of body weight |
| Potassium | 30-50 mEq/day in oliguric phase (depending on urinary output, dialysis, and serum K+ level); replace losses in diuretic phase |
| Sodium | 20-40 mEq/day in oliguric phase (depending on urinary output, edema, dialysis, and serum Na+ level); replace losses in diuretic phase |
| Fluid | Replace output from the previous day (vomitus, diarrhea, urine) plus 500 mL |
| Phosphorus | Limit as necessary |
GFR, Glomerular filtration rate; HBV, high biologic value; IBW, ideal body weight; K+, potassium; Na+, sodium.
Chronic Kidney Disease
A wide range of kidney lesions are characterized by a slow, steady decline in renal function. A number of the diseases discussed earlier lead to renal failure in some patients, whereas other patients have a benign course without loss of renal function. It is unclear why some patients remain stable with chronic kidney disease (CKD) for many months to years while others progress rapidly to renal failure and dialysis. The nature of this progressive loss of function has been the subject of an enormous amount of basic and clinical research during the past several decades and the subject of several excellent reviews (Remuzzi et al., 2006; Wenjun et al., 2009).
Pathophysiology
Once approximately one half to two thirds of kidney function has been lost, regardless of the underlying disease, progressive further loss of kidney function ensues. This is true even in diseases in which the underlying cause has been eliminated completely, such as in vesicoureteral reflux, cortical necrosis of pregnancy, or analgesic abuse. It is thought that, in response to a decreasing GFR, the kidney undergoes a series of adaptations to prevent this decline. Although in the short term this leads to improvement in filtration rate, in the long term it leads to an accelerated loss of nephrons and progressive renal insufficiency (Remuzzi et al., 2006). The nature of these adaptations involves a change in the hemodynamic characteristics of the remaining glomeruli, specifically leading to increased glomerular pressure. Factors that increase glomerular pressure tend to accelerate this process, whereas factors that decrease glomerular pressure tend to alleviate it.
Diabetes is the leading risk factor for CKD followed by hypertension. The National Kidney Foundation (NKF) divides CKD into five stages related to the estimated GFR (eGFR) (Table 36-7). Stages 1 and 2 are early stages with markers like proteinuria, hematuria, or anatomic issues. Stages 3 and 4 are considered advanced stages. Stage 5 results in death unless dialysis or transplantation is initiated.
Medical Management
The prevalence of CKD is now estimated at approximately one in nine adults in the United States, or 20 million Americans. This estimated prevalence of CKD is 11% of the population. Many states now urge clinical laboratories reporting serum Cr to also report the patient’s eGFR, the rate at which the kidneys are filtering wastes. The formula, which takes into account the patient’s sex, age, race, and Cr, is more accurate than the older Cockcroff-Gault formula sometimes used to calculate Cr clearance (Rigalleau et al., 2006). Patients having their eGFR calculated who have a low number do not necessarily have CKD. They must have repeated values over 3 months apart that are consistently low.
An online eGFR calculator can be found at the NKF site at http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm. With screening tools like the calculated eGFR and a greater awareness of the progressive nature of CKD, more attention has focused on its social, medical, and financial effects. For example, CKD is strongly linked with cardiovascular disease; see Clinical Insight: Chronic Kidney Disease and Heart Disease—A Deadly Union.
Medical Nutrition Therapy
With each level of CKD, a different nutritional therapy may be proposed. The primary objectives of MNT are to manage the symptoms associated with the syndrome (edema, hypoalbuminemia, and hyperlipidemia), decrease the risk of progression to renal failure, and maintain nutritional stores. Patients are primarily treated with statins to correct hyperlipidemia, low-sodium diets, and diuretics (Appel, 2006). Patients with an established severe protein deficiency who continue to lose protein may require an extended time of carefully supervised nutritional care. The diet should attempt to provide sufficient protein and energy to maintain a positive nitrogen balance and to produce an increase in plasma albumin concentration and disappearance of edema. In most cases, sufficient intake from carbohydrate and fats are needed to spare protein for anabolism.
Some of the more common nutrition diagnoses in the CKD population include:
Depending on the nutrition diagnosis, MNT interventions are adjusted for various intakes of minerals, protein, and fluids.
Protein: The recommended dietary protein level for CKD patients has changed over time. Historically, these patients received diets high in protein (up to 1.5 g/kg/day) in an attempt to increase serum albumin and prevent protein malnutrition. However, studies have shown that a reduction of protein intake to as low as 0.8 mg/kg/day may decrease proteinuria without adversely affecting serum albumin. Dietary protein has been championed as a factor that increases glomerular pressure and thus leads to accelerated loss of renal function. Numerous studies in experimental models of moderate renal insufficiency demonstrate a significant decline in this process with protein restriction. Clinical studies demonstrate a role for protein restriction in the management of patients with mild to moderate renal insufficiency to preserve renal function. To allow for optimal protein use, 50% to 60% of the protein should be from sources of high biologic value (HBV). The description for HBV protein has been expanded to include proteins with a high protein digestibility corrected amino acid score (PDCAAS) (see Chapter 3).
A large multicenter trial, Modification of Diet in Renal Disease, attempted to determine the role of protein, phosphorus restriction, and blood pressure control in the progression of renal disease. Thus the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD) developed recommendations for the management of patients with progressive renal disease or pre-ESRD. Those recommendations for dietary protein intake in progressive renal failure are 0.8 g/kg/day with 60% HBV for patients whose GFR is greater than 55 mL/min, and 0.6 g/kg/day with 60% HBV for patients whose GFR is 25 to 55 mL/min.
The NKF’s Kidney Dialysis Outcome Quality Initiative (KDOQI) panel suggests that patients whose GFR is less than 25 mL/min and who have not yet begun dialysis should be maintained on 0.6 g/kg/day of protein and 35 kcal/kg/day. If patients cannot maintain an adequate caloric intake on this protein recommendation, their protein intake should be increased to 0.75 g/kg/day. In both cases approximately 50% of the protein should be of HBV.
The potential benefits of protein restriction in the patient with moderate renal insufficiency must be weighed against the potential hazards of such treatment (i.e., protein malnutrition). If protein is restricted, careful monitoring and anthropometric studies should be carried out periodically as directed by the KDOQI guidelines.
Systemic hypertension, which aggravates the progressive loss of renal function, must be well controlled to produce benefits from protein restriction. Also important in the control of the progression of renal failure in people with diabetes is good blood glucose control. In a national multicenter trial, the Diabetes Control and Complications Trial showed that blood glucose control was more important than protein restriction in delaying the onset of renal failure in individuals who have diabetes (see Chapter 31).
Energy: Energy intake should be approximately 35 kcal/kg/day for adults to spare protein for tissue repair and maintenance.
Sodium: Edema, the most clinically apparent manifestation, indicates total body sodium overload. Additionally, because of low oncotic pressure from hypoalbuminemia, the volume of circulating blood may be reduced. Attempts to severely limit sodium intake or to use diuretics constantly may cause marked hypotension, exacerbation of coagulopathy, and deterioration of renal function. Therefore control of edema in this group of diseases should be with dietary intake of 2-3 g of sodium daily. The use of elastic full-length support hose may also be beneficial.
Potassium: Variability in disease states, individual intakes, and use of medications that may decrease potassium, such as diuretics, make potassium management possible. Many patients in early stage CKD take potassium-wasting diuretics and require supplementation. When urine output drops below 1 L/day, these same patients may require a potassium restriction as the kidney is no longer able to excrete all potassium ingested. This typically occurs rather late in stage 4 CKD.
Phosphorus: The importance of controlling phosphate in patients with early stage disease is often overlooked. Serum phosphorous levels elevate at the same rate as eGFR decreases. Early initiation of phosphate reduction therapies is advantageous for delaying hyperparathyroidism and bone disease. Unfortunately, patients are often asymptomatic during the early phase of hyperparathyroidism and hyperphosphatemia; they may not attend to their modified diets or understand the need to take phosphate binders with meals.
Those with an eGFR of less than 60 should be evaluated for renal bone disease, and benefit from phosphorus restriction. Ongoing monitoring of patient’s phosphorus and use of phosphate binders is recommended. The diet is typically modified to allow no more than 1000 mg of phosphates daily, a limit that allows approximately 1-2 dairy foods per day. Because of the decrease in protein intake, the control of phosphorus is somewhat easier to manage. Patients who are in later stages of CKD, and intolerant of red meats because of uremic taste alterations, are often able to substitute milk foods for meat and still maintain a limited phosphate intake.
Lipids: The important consequence of dyslipidemia is cardiovascular disease. Pediatric patients with frequently relapsing or resistant nephrotic syndrome are at particular risk for premature atherosclerosis. Certain lipid-lowering agents in combination with a cholesterol-lowering diet can reduce total cholesterol, low-density lipoprotein cholesterol, and triglycerides in these patients (see Chapter 34). Lowering protein intake in adult patients may also lower fat and cholesterol intake from animal sources.
Diseases of the Tubules and Interstitium
To a great extent, the functions of the kidney tubules make them susceptible to injury. The enormous energy requirements and expenditures of the tubules for active secretion and reabsorption often leave this part of the kidney particularly vulnerable to ischemic injuries. Many toxic drugs can destroy or damage various segments of the tubules. The high-solute concentration generated in the medullary interstitium exposes it to damage from oxidants and precipitation of calcium-phosphate product (extraosseous calcification) and favors the sickling of red blood cells in sickle cell anemia. Indeed, a wide variety of diseases or disorders of the tubules and interstitium exist. They share common manifestations and can be considered together with respect to nutritional management.
Chronic interstitial nephritis can occur as a result of analgesic abuse, sickle cell disease, diabetes mellitus, or vesicoureteral reflux, and manifests primarily as inability to concentrate the urine and as mild renal insufficiency. A hereditary disorder of the interstitium, medullary cystic disease, also presents with this picture. Dietary management consists of adequate fluid intake, which can require several liters of extra fluid. This is generally quite well tolerated by the patient, except when intercurrent illness occurs.
Fanconi syndrome is characterized by an inability to reabsorb the proper amount of glucose, amino acids, phosphate, and bicarbonate in the proximal tubule, thus causing urinary excretion of these substances. Adults with this syndrome present with acidosis, hypokalemia, polyuria, or osteomalacia, whereas children present with polyuria, growth retardation, rickets, and vomiting. No specific medical treatment is available to treat Fanconi syndrome; therefore dietary treatment is the main form of management. Replacement therapy usually consists of large volumes of water and dietary supplements of bicarbonate, potassium, phosphate, calcium, and vitamin D.
Renal tubular acidosis (RTA), a defect in tubular handling of bicarbonate, can be caused by either a defect in the distal tubule (type 1) or a proximal tubular defect (type 2). Distal RTA leads to severe osteomalacia, kidney stones, or even nephrocalcinosis (calcification of the kidney). Distal RTA is treated with small amounts of bicarbonate, 70 to 100 mEq/day, with complete resolution of disease manifestations. Isolated proximal RTA in the adult is a benign disease, which is often made worse with bicarbonate and therefore should not be treated.
Pyelonephritis, a bacterial infection of the kidney, does not require extensive dietary management. However, in chronic cases the use of cranberry juice to reduce bacteriuria is useful (Kontiokari et al., 2005; Jepson, 2008). Concentrated tannins or proanthocyanidins in cranberry juice and blueberry juice may inhibit the adherence of Escherichia coli bacteria to the epithelial cells of the urinary tract.
Glomerular Diseases
The functions of the glomerulus that are important with respect to disease are production of an adequate ultrafiltrate and prevention of certain substances from entering this ultrafiltrate.
Pathophysiology
Nephritic syndrome incorporates a group of diseases characterized by inflammation of the capillary loops of the glomerulus. These acute glomerulonephritides are sudden in onset, brief, and may proceed to complete recovery, development of chronic nephrotic syndrome, or ESRD. The primary manifestation of these diseases is hematuria (blood in the urine), a consequence of the capillary inflammation that damages the glomerular barrier to blood cells. The syndrome is also characterized by hypertension and mild loss of renal function. The most common presentation follows a streptococcal infection and is usually, although not always, self-limiting. Other causes include primary kidney diseases such as immunoglobulin A nephropathy (IgA); hereditary nephritis; and secondary diseases such as systemic lupus erythematosus (SLE), vasculitis, and glomerulonephritis (GN) associated with endocarditis, abscesses, or infected ventriculoperitoneal shunts.
Nephrotic Syndrome
Nephrotic syndrome comprises a group of diseases that derive from a loss of the glomerular barrier for protein. Large urinary protein losses lead to hypoalbuminemia with consequent edema, hypercholesterolemia, hypercoagulability, and abnormal bone metabolism. More than 95% of the cases of nephrotic syndrome stem from three systemic diseases: (1) diabetes mellitus, (2) SLE, and (3) amyloidosis; and from four diseases that are primarily of the kidney: (1) minimum change disease (seen only with electron microscopy), (2) membranous nephropathy, (3) focal glomerulosclerosis, and (4) membranoproliferative glomerulonephritis. Although renal function can deteriorate during the course of these diseases, it is not a consistent feature.
End-Stage Renal Disease
End-stage renal disease (ESRD) reflects the kidney’s inability to excrete waste products, maintain fluid and electrolyte balance, and produce hormones. As renal failure slowly progresses, the level of circulating waste products eventually leads to symptoms of uremia (see Pathophysiology and Care Management Algorithm: Chronic Kidney Disease and End-Stage Renal Disease). Uremia is a clinical syndrome of malaise, weakness, nausea and vomiting, muscle cramps, itching, metallic taste in the mouth, and neurologic impairment that is brought about by an unacceptable level of nitrogenous wastes in the body.
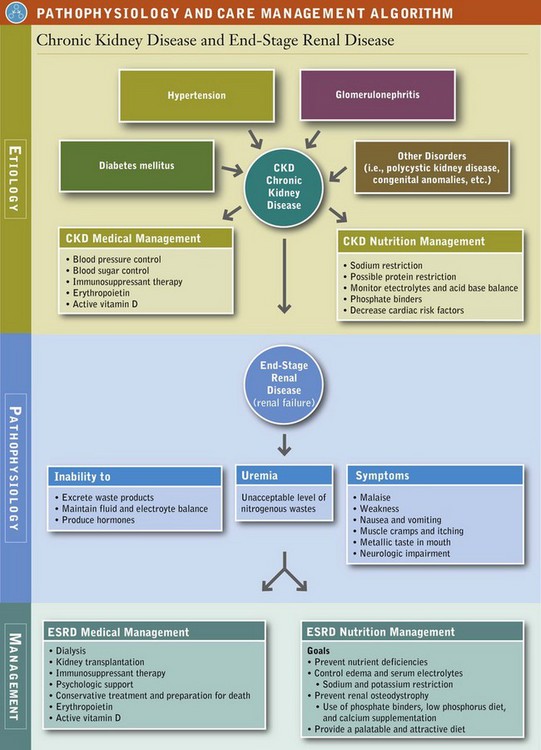
Pathophysiology
ESRD can result from a wide variety of different kidney diseases. Currently 90% of patients reaching ESRD have chronic (1) diabetes mellitus, (2) hypertension, or (3) glomerulonephritis. The manifestations are somewhat nonspecific and vary by patient. No reliable laboratory parameter corresponds directly with the beginning of symptoms. However, as a rule of thumb, BUN of more than 100 mg/dL and Cr of 10 to 12 mg/dL are usually quite close to this threshold.
Medical Treatment
Once the patient progresses from stage 4 to stage 5 CKD, options for treatment for ESRD include dialysis, transplantation, or medical management progressing to death. Patients do best if they have some control and choice over their options.
Dialysis
Patients may choose to dialyze in an outpatient dialysis facility or they may prefer HD at home using either conventional daily or nocturnal dialysis. They may choose PD and have a choice of continuous ambulatory peritoneal dialysis (CAPD) or continuous cyclic peritoneal dialysis (CCPD), or combinations of the two. Patients, families, and their physicians together evaluate the therapy that best meets the patient’s needs. Factors that come into account in this decision are availability of family or friends to assist with therapy, type of water supply to the home, capability of the patient or involved family (including eyesight and ability to perform sterile technique), previous abdominal surgeries, membrane characteristics of the individual’s peritoneal membrane, body size, cardiac status, presence of poor vascular access, desire to travel, and a host of other considerations.
HD requires permanent access to the bloodstream through a fistula created by surgery to connect an artery and a vein (Figure 36-2). If the patient’s blood vessels are fragile, an artificial vessel called a graft may be surgically implanted. Large needles are inserted into the fistula or graft before each dialysis and removed when dialysis is complete. Temporary access through subclavian catheters is common until the patient’s permanent access can be created or can mature; however, problems with infection make these catheters undesirable.
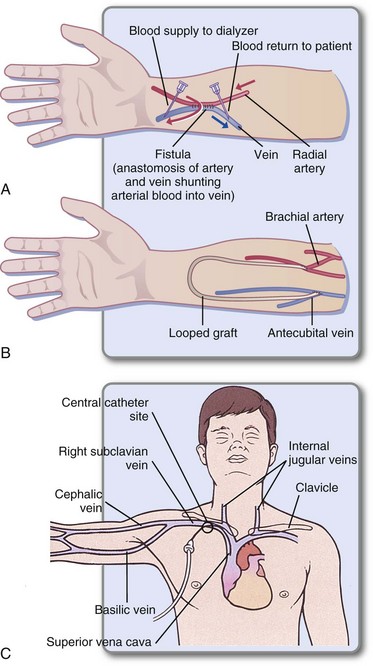
FIGURE 36-2 Types of access for hemodialysis. A, Arteriovenous fistula. B, Artificial loop graft. C, Subclavian catheter (usually temporary). (From Lewis SL et al: Medical-surgical nursing: assessment and management of clinical problems, ed 7, St Louis, 2007, Mosby.)
The hemodialysis (HD) fluid and electrolyte content is similar to that of normal plasma. Waste products and electrolytes move by diffusion, ultrafiltration, and osmosis from the blood into the dialysate and are removed (Figure 36-3) (Himmelfarb and Ikizler, 2010). Outpatient HD usually requires treatment of 3 to 5 hours three times per week in a dialysis unit (Figure 36-4). Newer therapies can shorten the duration of treatment by increasing its frequency. Patients on these more frequent dialysis therapies have lower mortality rates, approaching that of transplantation. Patients on daily dialysis at home typically have treatments lasting from 2 to 3.5 hours 5 to 6 days a week, whereas some home dialysis patients receive nocturnal dialysis 3 to 6 times a week for 8 hours, while they sleep.
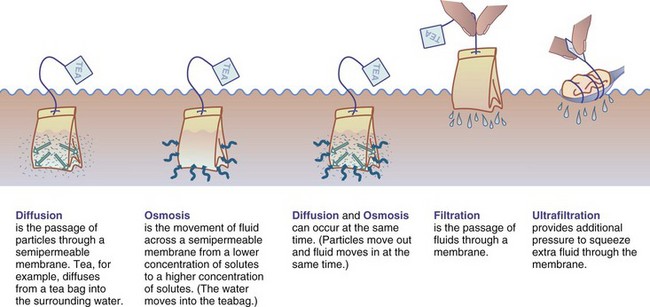
FIGURE 36-3 Dialysis: how it works. (Modified from Core curriculum for the dialysis technician: a comprehensive review of hemodialysis, AMGEN, Inc.)
Peritoneal dialysis (PD) makes use of the body’s own semipermeable membrane, the peritoneum. A catheter is surgically implanted in the abdomen and into the peritoneal cavity. Dialysate containing a high-dextrose concentration is instilled into the peritoneum, where diffusion carries waste products from the blood through the peritoneal membrane and into the dialysate; water moves by osmosis. This fluid is then withdrawn and discarded, and new solution is added.
Several types of PD exist. In CAPD, the dialysate is left in the peritoneum and exchanged manually, by gravity. Exchanges of dialysis fluid are done four to five times daily, making it a 24-hour treatment (Figure 36-5). In CCPD, patient treatments are done at night by a machine that does the exchanges. During the day these patients may keep a single dialysate exchange in the peritoneal cavity for extended periods (called a long dwell), perhaps the entire day. Several combinations of CAPD and CCPD are possible and are referred to here as PD.
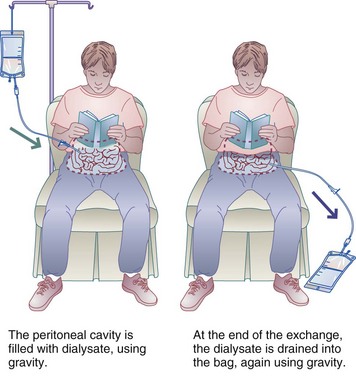
FIGURE 36-5 Continuous ambulatory peritoneal dialysis; 20-minute exchanges are given four to five times daily every day.
Advantages of PD are avoidance of large fluctuations in blood chemistry, longer residual renal function, and the ability of the patient to achieve a more normal lifestyle. Complications include peritonitis, hypotension that requires fluid and sodium replacement, and weight gain. Tissue weight gain is experienced by most patients as a result of absorbing 400 to 800 calories per day from the glucose in the dialysate. This may be desirable in patients who are underweight, but eventually dietary intake or activity have to be modified to account for the energy absorbed from dialysate. Icodextrin (Extraneal, Baxter) is a long-chain, nonabsorbable sugar available for long dwell times. It offers superior fluid removal (ultrafiltration) without excess dextrose absorption. This can be useful for patients with diabetes and those with excessive tissue weight gain, but can cause other complications and is costly.
Evaluation of Dialysis Efficacy
Kinetic modeling is a method for evaluating the efficacy of dialysis that measures the removal of urea from the patient’s blood over a given period. This formula, often called Kt/V (where K is the urea clearance of the dialyzer, t is the length of time of dialysis, and V is the patient’s total body water volume), should ideally produce a result higher than 1.4 per HD, or 3.2 per week. These calculations are somewhat complex and are typically calculated using a computer program. A more accurate method for determining adequacy of HD is the eKt/V where e stands for equilibrated and takes into account the amount of time it takes for urea to equilibrate across cell membranes after dialysis has stopped. An acceptable eKt/V is 1.2 or greater.
Another method to determine effective dialysis treatment is the urea reduction ratio (URR), which looks at the reduction in urea before and after dialysis. The patient is considered well dialyzed when a 65% or greater reduction in the serum urea occurs during dialysis. Unlike Kt/V, this calculation can be done quickly at the patient’s bedside by the practitioner. The method for calculating the efficacy of PD is somewhat different, but a weekly Kt/V of 2 is the goal. The Kt/V can be altered by several patient- and dialysis-associated variables. The calculations for Kt/V can also be used to determine the patient’s protein-nitrogen appearance (PNA) rate, which is a simplified nitrogen balance test in the dialysis patient. The PNA values should be between 0.8 and 1.4. Patients on short daily HD and nocturnal HD require different calculations to estimate their Kt/V.
Medical Nutrition Therapy
Goals of medical nutrition therapy in the management of ESRD are intended to:
1. Prevent deficiency and maintain good nutrition status (and, in the case of children, growth) through adequate protein, energy, vitamin, and mineral intake (Table 36-8).
TABLE 36-8
Nutrient Requirements of Adults with Renal Disease Based on Type of Therapy
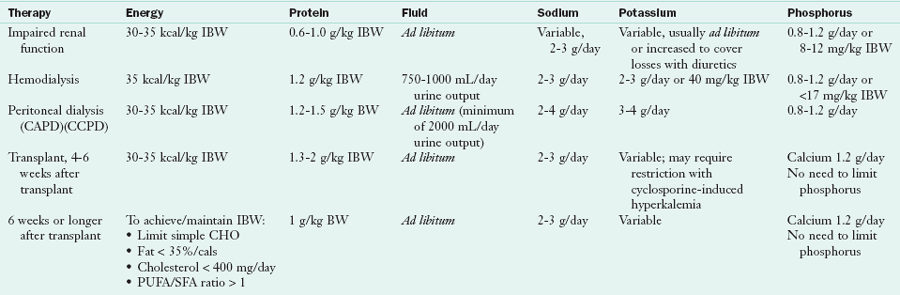
CAPD, Continuous ambulatory peritoneal dialysis; CCPD, continuous cyclical peritoneal dialysis; IBW, ideal body weight; PUFA, polyunsaturated fat; SFA, saturated fat.
Modified from National Kidney Foundation: DOQI clinical practice guidelines for nutrition in chronic renal failure, Am J Kidney Dis 35(suppl 2), 2000; Wiggins K: Guidelines for nutrition care of renal patients, ed 3, Chicago, 2002, American Dietetic Association.
2. Control edema and electrolyte imbalance by controlling sodium, potassium, and fluid intake.
3. Prevent or retard the development of renal osteodystrophy by controlling calcium, phosphorus, vitamin D, and PTH.
4. Enable the patient to eat a palatable, attractive diet that fits his or her lifestyle as much as possible.
5. Coordinate patient care with families, dietitians, nurses, and physicians in acute care, outpatient, or skilled nursing facilities.
6. Provide initial nutrition education, periodic counseling and long-term monitoring of patients.
Table 36-9 presents a guide for teaching patients about their blood values and control of their disease. Because dialysis is done at home or in an outpatient unit, all most all patients with ESRD assume responsibility for their own diets. Most long-term patients know their diets very well (Figure 36-6), having been instructed many times by renal dietitians at their dialysis units.
TABLE 36-9
Guide to Blood Values in End-Stage Renal Disease Patients
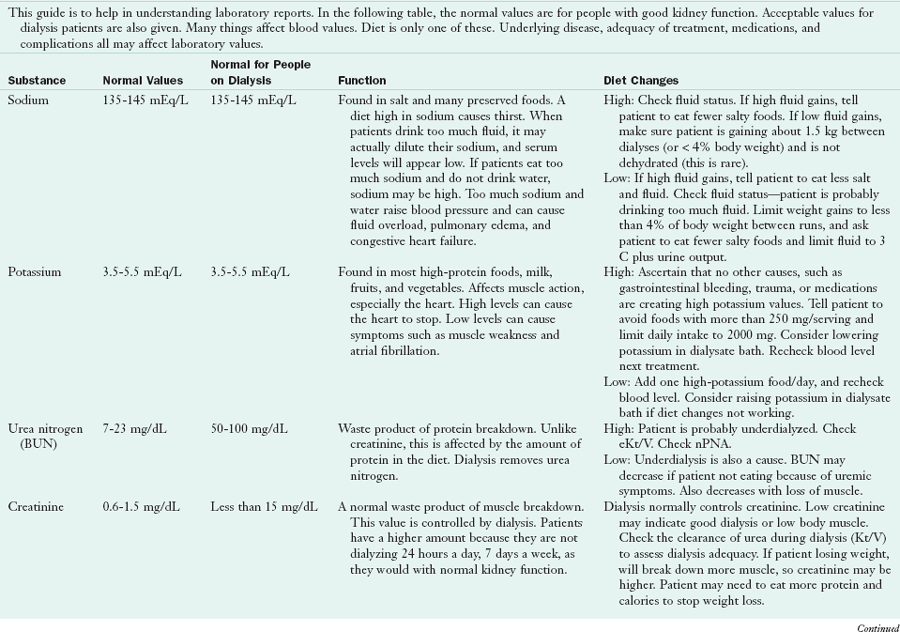


BUN, Blood urea nitrogen; CO2, carbon dioxide; DHT, dihydrotachysterol; EPO, erythropoietin; IV, intravenous; N/A, not applicable; nPNA, normalized protein nitrogen appearance; PTH, parathyroid hormone; URR, urea reduction ratio.
Developed by Katy G. Wilkens, MS, RD, Northwest Kidney Centers, Seattle, Washington.
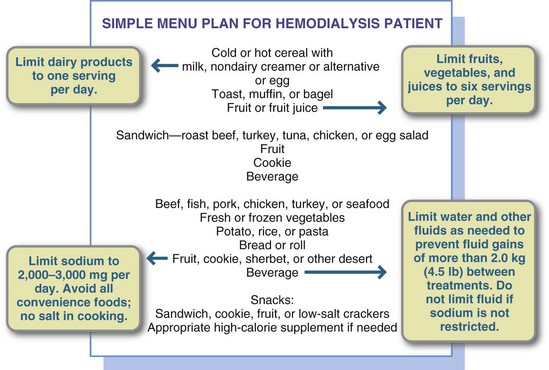
FIGURE 36-6 A simple menu plan for a patient on hemodialysis. The diet should allow for less than 4% fluid weight gain between dialyses.
Protein
Dialysis is a drain on body protein, so protein intake must be increased accordingly. Protein losses of 20 to 30 g can occur during a 24-hour PD, with an average of 1 g/hour. Those receiving PD need 1.2 to 1.5 g/kg of body weight. At least 50% should be HBV protein. Patients who receive HD three times per week require a daily protein intake of 1.2 g/kg of body weight. Patients on dialysis who have low albumin levels have much higher mortality rates; thus emphasis is placed on adequate protein intake. Serum BUN and serum Cr levels, uremic symptoms, and weight should be monitored; and the diet should be adjusted accordingly.
In renal failure, prealbumin, which is metabolized by the kidney, is not a good nutritional marker, as values are routinely elevated. Albumin is a limited indicator of protein nutriture, but is routinely used in evaluating ESRD patients’ nutritional status. Federal mandates require intervention at levels below 4 g/dL. However, because of the complexity of either acute or chronic inflammation, albumin remains predictive of poor survival in ESRD. Hypoalbuminemia is multifactorial and may be related to poor nutrition, inflammation, or comorbid disease. When interpreting albumin values, it is important to know the laboratory’s methodology for measuring serum albumin, because different laboratory techniques give different results in renal failure (see Table 36-9).
Most patients find it challenging to consume adequate protein because uremia itself causes taste aberrations, notably to red meats. Some patients cannot even tolerate the smell of meat cooking. Often this protein aversion makes it difficult to achieve recommended high biological value protein intake. Patients may tolerate eggs, tofu, and “white” meats better. They can also use spices to hide the taste of meats, or serve animal proteins cold, to minimize the urea taste. Nutritional supplements may be helpful in some patients, and, occasionally, the phosphate restriction may need to be lifted to allow the consumption of dairy products to meet protein needs. As with all the nutritional parameters, meeting patient needs must be individualized.
Energy
Energy intake should be adequate to spare protein for tissue protein synthesis and to prevent its metabolism for energy (Byham-Gray, 2006). Depending on the patient’s nutrition status and degree of stress, between 25 and 40 kcal/kg of body weight should be provided, with the lower amount for transplantation and PD patients and the higher level for the nutritionally depleted patient. Tools have been developed to allow the renal dietitian to assess the quality of the patient’s nutrition status. The Subjective Global Assessment (Box 6-5) technique has been modified in this population to recognize the basic physiologic and immunologic changes in ESRD.
Fluid and Sodium Balance
The kidney’s ability to handle sodium and water in ESRD must be assessed frequently through measurement of blood pressure, edema, fluid weight gain, serum sodium level, and dietary intake. The vast majority of dialysis patients need to restrict sodium and fluid intakes. Excessive sodium intake is responsible for increased thirst, increased fluid gain, and resultant hypertension. Even those patients who do not experience these symptoms but produce minimal amounts of urine will benefit from a reduced sodium intake to limit their thirst and prevent large intradialytic fluid gains.
In the patient who is maintained on HD, sodium and fluid intake are regulated to allow for a weight gain of 4 to 5 lb (2 to 3 kg) from increased fluid in the vasculature between dialyses. The goal is a fluid gain of less than 4% of body weight. A sodium intake of 87 to 130 mEq (2 to 3 g) daily and a limit on fluid intake (usually about 750 mL/day plus the amount equal to the urine output) is usually sufficient to meet these guidelines. Only fluids that are liquid at room temperature are included in this calculation. The fluid contained in solid foods is not included in the 750 mL limit. Solid foods in the average diet contribute approximately 500 to 800 mL/day of fluid. This fluid in solid food is calculated to approximately replace the 500 mL/day net insensible water loss.
An 86- to 130-mEq (2- to 3-g) sodium diet requires no salt in cooking; no salt at the table; no salted, smoked, or cured meat or fish; and no salted snack foods, canned soups, or high-sodium convenience foods. In today’s increasing convenience food–oriented marketplace, it is estimated that 75% to 90% of sodium intake is consumed in convenience foods, with only 10% to 25% added to foods in cooking or at the table. It is important to remember that the most effective way to reduce the renal patient’s thirst and fluid intake is to decrease sodium intake. It is salt intake that drives fluid consumption. Appendix 37 gives the details of a low-sodium meal plan. Many physicians and dietitians believe it is unethical to restrict fluid in patients who are not on a restricted sodium diet, because feelings of thirst become overwhelming to the patient with a high sodium intake.
When educating about fluid balance, the health care provider must teach the patient how to deal with thirst without drinking. Sucking on a few ice chips, cold sliced fruit, or sour candies or using artificial saliva are all good suggestions. In approximately 15% to 20% of patients, hypertension is not alleviated even after meticulous attention to fluid and water balance. In these patients hypertension is usually perpetuated by a high level of renin secretion and requires medication for control.
Although most patients with ESRD retain sodium, a small number may lose it. Examples of conditions with a salt-losing tendency are polycystic disease of the kidney, medullary kidney disease, chronic obstructive uropathy, chronic pyelonephritis, and analgesic nephropathy. To prevent hypotension, hypovolemia, cramps, and further deterioration of renal function, extra sodium may be required. A diet for these types of patients may contain 130 mEq (3 g) or more of sodium per day, which is the amount in a normal diet without added salt. Adding salt or salty foods can satisfy the need for extra sodium. The number of patients who require this higher sodium intake is small, but these patients exemplify the need for individual consideration of the diet prescription and a thorough understanding of the patient’s underlying disease and present diet.
Patients receiving frequent dialysis treatments, either daily PD, short daily, or daily nocturnal dialysis may have higher allowance for sodium and fluid, based on an individual evaluation of their dry weight and laboratory values.
Potassium
Potassium usually requires restriction, depending on the serum potassium level, urine output, medications, and the frequency of HD. The daily intake of potassium for most Americans is 75 to 100 mEq (3 to 4 g). This is usually reduced in ESRD to 60 to 80 mEq (2.3 to 3.1 g) per day and is reduced for the anuric patient on dialysis to 51 mEq (2 g) per day. Some patients (i.e., those on high-flux dialysis or with increased dialysis times or frequencies such as PD, short daily or nocturnal) will be able to tolerate higher intakes. Again, a close monitoring of the patient’s laboratory values, K+ content of the dialysate, and dietary intake is essential.
The potassium content of foods is listed in Box 7-1 in Chapter 7, and in Appendixes 36 and 56. When counseling HD patients on a low-potassium diet, one should take care to point out that some low-sodium foods contain potassium chloride as a salt substitute rather than sodium chloride. Nutrition labels for products such as salt substitutes, LiteSalt, and low-sodium herb mixtures must all be checked carefully to be sure they do not contain dangerous levels of potassium. Low-sodium soy sauces, low-sodium soups, and other special dietary products may need particular review by a trained professional. Reviewing such practices not only with the patient but with other people who may be cooking for the patient, such as a church group or neighbors who may use salt substitutes is advisable.
When a thorough diet history does not reveal the reason for elevated serum potassium, other nondietetic sources of potassium should be researched. Examples include poor dialysis adequacy or missed dialysis treatments, too high a concentration of potassium in the dialysate bath, very elevated blood sugar in patients with diabetes, acidosis, constipation, significant GI bleeding, some medications, blood transfusions, major trauma, chemotherapy, or radiation therapy. Occasionally, blood samples are handled improperly, resulting in hemolysis and falsely elevated potassium levels.
Phosphorus
More than 99% of excess phosphate is excreted in the urine. However, as GFR decreases, phosphorus is retained in the plasma. Because of the large molecular weight of the phosphate molecule, it is not easily removed by dialysis and patients experience net “gain” of about one half of the phosphate they consume daily. Phosphate intake is lowered by restricting dietary sources to 1200 mg/day or less. The difficulty in implementing the phosphorus restriction comes from the necessity for a high-protein diet. High protein foods, such as meats, contain high levels of phosphorus in the form of ATP. Additionally, other sources of protein—dairy products, nuts, and legumes—are also high in phosphorus. Thus high phosphorus foods cannot be eliminated without restricting protein, creating a challenge to balance intake with dietary intervention alone.
The American diet, which contains highly processed foods, has resulted in increases in both the types and amounts of phosphorus available for absorption, making compliance with a phosphorus restriction more difficult. Naturally occurring phosphate in food is only approximately 60% absorbed. Commonly used phosphate additives such as trisodium phosphate, disodium phosphate, and dicalcium phosphate are nearly 100% absorbed, making the processed diet a likely contributor to elevated phosphorus levels. Dietary intervention should focus on a balance of limiting dairy, nuts, beans, and processed foods while still encouraging enough HBV proteins to meet dietary needs.
Because dietary restrictions alone are not adequate to control serum phosphorus, nearly all patients who undergo dialysis require phosphate-binding medications. Phosphate binders such as calcium carbonate, calcium acetate, sevelamer carbonate and lanthanum carbonate are routinely used with each meal or snack to bind to phosphorus in the gut. These medications bind excess dietary phosphate and transport it through the GI tract for elimination, thus preventing its absorption into the blood. Side effects of taking these medications over long periods are common. Some may cause GI distress, diarrhea, or gas. Severe constipation, leading to intestinal impaction is a potential risk of excessive use of some types of phosphate binders; occasionally this may lead to perforation of the intestine resulting in peritonitis or death. Common medications are listed in Table 36-10.
TABLE 36-10
Common Medications and Nutritional Supplements for Patients with End-Stage Renal Disease
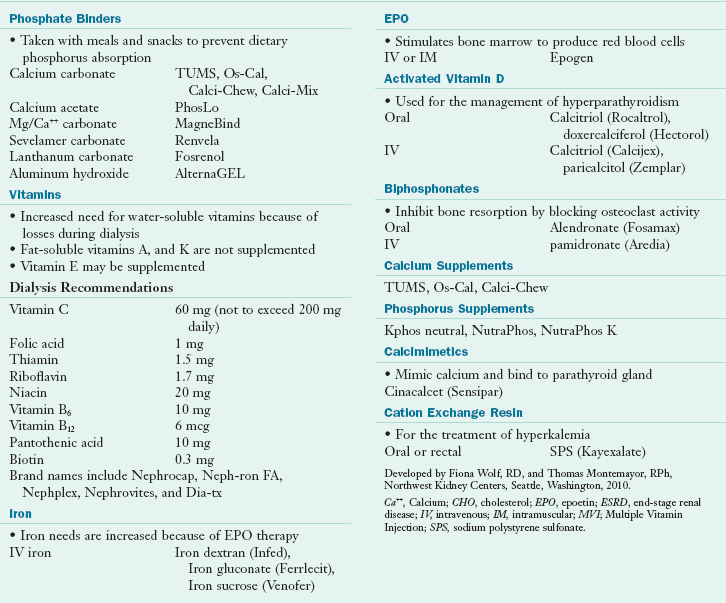
Ca++, Calcium; CHO, cholesterol; EPO, epoetin; ESRD, end-stage renal disease; IV, intravenous; IM, intramuscular; MVI; Multiple Vitamin Injection; SPS, sodium polystyrene sulfonate.
Developed by Fiona Wolf, RD, and Thomas Montemayor, RPh, Northwest Kidney Centers, Seattle, Washington, 2010.
Calcium and Parathyroid Hormone
In ESRD, the body’s ability to maintain phosphorus-calcium balance is complicated by calcium and PTH controls. As GFR decreases, the serum calcium level declines for several reasons. First, decreased ability of the kidney to convert to inactive vitamin D to its active form, 1,25-(OH)2D3 leads to poor GI absorption of calcium. Second, the need for serum calcium increases as serum phosphate levels increase. Both of these causes lead to hypertrophy of the parathyroid gland, which is responsible for calcium homeostasis. The resultant oversecretion of PTH increases resorption of bone to provide a calcium source.
The resulting metabolic bone disease, renal osteodystrophy, is essentially one of four types: (1) osteomalacia, (2) osteitis fibrosa cystica, (3) metastatic calcification, or (4) adynamic bone disease. With a deficit of calcium available from dietary absorption, the low calcium level triggers the release of PTH from the parathyroid glands. This acts to increase release of calcium from the bones by stimulating osteoclast activity. This can lead to osteomalacia or bone demineralization, as a result of lack of osteoblast stimulation to replace lost calcium in the bones.
Ongoing low calcium causes the parathyroid glands to continue producing PTH in an attempt to elevate serum calcium levels. In time this leads to secondary hyperparathyroidism, where even the baseline production of PTH by these enlarged glands is enough to cause severe bone demineralization (osteitis fibrosis cystica), which is characterized by dull, aching bone pain.
Even though the serum calcium level is elevated in response to PTH, serum phosphate concentration remains high as the GFR falls lower. If the product of serum calcium multiplied by the serum phosphate level is greater than 70, metastatic calcification is imminent. Metastatic calcification occurs when calcium phosphate, removed from bones, is deposited in nonbone cells. This extraskeletal calcification may develop in joints, soft tissue, and vessels.
Calciphylaxis occurs when calcium phosphate is deposited in wound tissues with resultant vascular calcification, thrombosis, nonhealing wounds, and gangrene. It is frequently fatal. Clinical management aims to keep the calcium × phosphorus product below 55 by preventing transient elevations in serum phosphate concentration (National Kidney Foundation, 2003). Phosphorus intake must be controlled to as great a degree as possible to avoid aggravation of the delicate situation posed by hyperparathyroidism, phosphate retention, vitamin D deficiency, and hypocalcemia in renal failure.
Many patients on dialysis suffer from hypocalcemia, despite calcium supplementation. Because of this, the routine drug of choice is active vitamin D, 1,25-(OH)2D3, available as calcitriol (Rocaltrol and Calcijex). Analogs such as doxercalciferol (Hectorol) and paricalcitol (Zemplar) are also effective in lowering PTH and raising calcium levels, but with less enhancement of gut absorption of calcium than the 1,25 forms. Other mechanisms for controlling PTH include the medication cinacalcet (Sensipar), a calcimimetic or calcium-imitating drug, which binds to sites on the parathyroid gland and gives the gland a false impression that calcium levels are elevated. The drug is effective in suppressing PTH production and may also lower calcium levels dramatically, with significant consequences. Overall, close monitoring is essential.
In more extreme cases of hyperparathyroidism, use of surgical excision of portions of the parathyroid glands can be used in an attempt to restore balance. This creates the risk of low PTH, and can lead to adynamic (low turnover) bone disease, characterized by decreased levels of bone turnover and suppression of both osteoclasts and osteoblasts. This condition is unique to ESRD, in which oversuppression of the parathyroid gland and too much active vitamin D lead to decreased bone formation and fragile bones with very little matrix. Usually diagnosed by a low PTH level, this disease results in a high risk of nonhealing fractures. Oversuppression of the parathyroid gland by use of vitamin D or its analogs can mimic this as well.
Overall the dietary balance of phosphorus, the use of phosphate binders and vitamin D analogs, the removal of phosphate by dialysis, and intense monitoring of laboratory values all contribute to bone management in ESRD.
Lipid
Atherosclerotic cardiovascular disease is the most common cause of death among patients maintained on long-term dialysis (Bennett et al., 2006). This appears to be a function of both underlying disease (e.g., diabetes mellitus, hypertension, nephrotic syndrome) and a lipid abnormality common among patients with ESRD. The patient with ESRD typically has an elevated triglyceride level with or without an increase in cholesterol. The lipid abnormality likely represents both increased synthesis of very-low-density lipoprotein and decreased clearance as well as increased reliance on animal-based proteins.
Treatment of hyperlipidemia with diet or pharmacologic agents remains controversial. Epidemiologic evidence demonstrating increased incidence of atherosclerotic coronary disease is balanced by studies that demonstrate that patients with clearly defined clinical evidence of atherosclerosis at the initiation of dialysis are at no increased risk for a cardiovascular event. Although routine treatment appears unwarranted, a good case can be made for dietary and pharmacologic treatment of patients with ESRD with underlying lipid disorders and evidence of atherosclerosis. Lipid-lowering drugs, including most statins, may have a significant effect on better management (see Chapter 34).
On the other hand, low cholesterol levels can be a significant predictor of mortality in ESRD. They can indicate poor oral intake, and may be a useful tool in diagnosing malnutrition. Use of lipid-lowering drugs should be monitored and cut back if needed in these patients, particularly if they are underweight or suffering from malnutrition.
Iron and Erythropoietin
The anemia of chronic renal disease is caused by an inability of the kidney to produce EPO, the hormone that stimulates the bone marrow to produce red blood cells, an increased destruction of red blood cells secondary to circulating uremic waste products; and blood loss with dialysis or blood sampling. A synthetic form of EPO, recombinant human EPO (rHuEPO), is used to treat this form of anemia. Clinical trials have demonstrated a dramatic improvement in correcting anemia and restoring a general sense of well being (Locatelli F, 2006).
Use of EPO increases red blood cell production 2.5-fold. Almost always accompanying the rise in hematocrit is an increased need for iron that requires IV supplementation. Oral iron alone is not effective in maintaining adequate iron stores in patients who take EPO. Unless a documented allergic reaction exists, almost all patients taking EPO require periodic IV or intramuscular iron. For patients who are allergic to IV iron, several better-tolerated forms are now available as iron dextran (Infed), iron gluconate (Ferrlecit), and iron sucrose (Venofer).
Serum ferritin is an accurate indicator of iron status in renal failure. Patients who have received several transfusions and who are storing extra iron may have high serum ferritin levels of 800 to 5000 ng/mL (a normal level is 68 ng/mL for women and 150 ng/mL for men; see Appendix 30). In patients who are receiving EPO, ferritin should be kept above 300 ng/mL but below 800 ng/mL. When ferritin values fall below 100 ng/mL, IV iron is usually given. The percent of transferrin saturation is another useful indicator of iron status in these patients and should be between 25% to 30%.
Vitamins
Water-soluble vitamins are rapidly lost during dialysis. In general, ascorbic acid and most B vitamins are lost through dialysate at approximately the same rate they would have been lost in the urine (depending on the type and duration of treatment), with the exception of folate, which is highly dialyzable. Patients who still produce urine may be at increased risk of loss of water-soluble vitamins. Folate is recommended to be supplemented at 1 mg/day based on extra losses. Because vitamin B12 is protein-bound, losses of this B vitamin during dialysis are minimal. Altered metabolism and excretory function, as well as drug administration, also may alter vitamin levels. Little is known about GI absorption of vitamins in uremia, but it may be significantly decreased. Uremic toxins may interfere with the activity of some vitamins, such as inhibition of phosphorylation of pyridoxine and its analogs.
Another cause of decreased vitamin intake in uremia is the restriction of dietary phosphorus and potassium. Water-soluble vitamins are usually abundant in high-potassium foods such as citrus fruits and vegetables and high-phosphorus foods such as milk. Diets for patients on dialysis tend to be low in folate, niacin, riboflavin, and vitamin B6. With frequent episodes of anorexia or illness, vitamin intake is decreased further. Whereas levels of water-soluble vitamins decrease as a result of dialysis, replacement of fat-soluble vitamins is not usually required in renal disease.
Several vitamin supplements that fit the needs of the uremic patient or the dialysis patient are now available by prescription: Nephrocaps, Nephplex, Dialyvite, and Renal Caps. An over-the-counter supplement containing the vitamin B complex and vitamin C is often used and can be less expensive than a prescription, but additional supplements of folic acid and pyridoxine may be needed.
Niacin has been found to be helpful in lowering phosphate levels in ESRD patients. It interferes with the sodium-phosphate pump in the GI lumen, causing decreased transport of phosphate, and thus works with a different mechanism than phosphate binders, (Cheng, S, 2006, 2008). It is usually dosed once daily, increasing compliance.
Nutrition Support in End-Stage Renal Disease
Patients with ESRD who require enteral tube feeding often do quite well on standard formulas used for most tube-fed patients (see Chapter 14). Patients should receive standard formulas before they try a “specialty” formula because the former are usually less expensive and typically have lower osmolality than the specific renal products. If electrolyte or fluid concerns arise, patients can be changed to one of the formulas now available that are specifically designed for renal patients: Nepro or Novasource Renal. If patients are receiving renal products only, they may develop problems with a low phosphorus or potassium levels as these products are often used in conjunction with oral intake.
Parenteral Nutrition
PN in ESRD is similar to PN used for other malnourished patients with respect to protein, carbohydrates, and fat, but differs in use of vitamins and minerals. Most researchers agree that vitamin needs for ESRD during PN differ from normal requirements; however, they do not agree on recommendations for individual nutrients. Folate, pyridoxine, and biotin should be supplemented. Vitamin A should not be provided parenterally unless retinol-binding protein is monitored because it is elevated in patients with ESRD. Because there is currently no parenteral vitamin that is specifically designed for patients with renal failure, a standard vitamin preparation is usually administered. Little information related to parenteral trace mineral supplementation is available. Because most trace minerals, including zinc, chromium, and magnesium, are excreted in the urine, a close monitoring of these minerals in the serum seems to be appropriate.
Intradialytic Parenteral Nutrition
Malnourished patients with chronic renal failure who are on HD have easy access to PN because of the direct blood access required by the dialysis therapy itself. Intradialytic PN (IDPN) can be administered if necessary without additional invasive procedures or need for a separate port. It is typically administered through a connection to the venous side of the extracorporeal circuit during dialysis. Because of the high blood flow rate achieved through use of the surgically created fistula and the high blood pump speeds that are attained, hypertonic glucose and protein can be administered without danger of phlebitis. Lipids may also be administered. Reimbursement issues are complex because IDPN is a supplemental feeding that requires the patient to have at least a functioning GI tract and only supplies an average of 1000 calories every HD treatment (Table 36-11).
TABLE 36-11
Regimen for Intradialytic Parenteral Nutrition Administered During Hemodialysis Therapy

*Additional volume may include insulin and vitamins.
†3.4 kcal/gm because of hydration in IDPN solution.
Developed by Katy G. Wilkens, MS, RD, Northwest Kidney Centers, Seattle, Wash.
Complications are similar to those encountered in usual PN, with the exception of postdialysis hypoglycemia caused by the abrupt ending of the glucose supply. To avoid this problem, glucose administration typically is tapered up and down during the first and last half hour of the 3- to 5-hour treatment. Insulin is given often, and can be given in the bag of the dextrose–amino acid solution, so that the patient does not become hypoglycemic if the infusion must be stopped. Blood sugar levels are typically monitored during the therapy. Some patients may benefit from a complex carbohydrate snack toward the end of the treatment to avoid posttreatment rebound hypoglycemia. Amino acid losses through the dialysate average approximately 10%.
Another method of nutrition support in PD patients is called intraperitoneal nutrition (IPN) using a peritoneal dialysate solution that contains amino acids instead of dextrose. Typically one bag of this solution is used per day. Some patients experience side effects from this treatment. Reimbursement issues are significant.
End-Stage Renal Disease in Patients with Diabetes
Because renal failure is a complication of diabetes, approximately 40% to 50% of all new patients starting dialysis have diabetes (Zhang et al., 2005). The need to control blood sugar in these patients requires specialized diet therapy. The diet for diabetes management (see Chapter 31) can be modified for the patient on dialysis. In addition, the diabetic patient on dialysis often has other complications such as retinopathy, neuropathy, gastroparesis, and amputation, all of which can place this patient at high nutritional risk. The NKF has established guidelines for managing diabetes in the presence of CKD (National Kidney Foundation, 2007; Nelson and Tuttle, 2007), which can be accessed at www.kidney.org.
Increased osmolality caused by high serum levels of glucose may cause water and potassium to be pulled out of cells, with resultant hyperkalemia. Interpretation of commonly used laboratory values will change when a diabetic patient develops renal failure, because the hemoglobin A1c is affected by the shortened half life of red blood cells in uremia (Rigalleau et al., 2006).
Chronic Kidney Disease and End-Stage Renal Disease in Children
Although CKD may occur in children at any age, from the newborn infant to the adolescent, it is a relatively uncommon diagnosis. Causal factors in children include congenital defects, anatomic defects (urologic malformations or dysplastic kidneys), inherited disease (autosomal-recessive polycystic kidney disease), metabolic disorders that eventually result in renal failure (cystinosis or methylmalonic aciduria), or acquired conditions or illnesses (untreated kidney infections, physical trauma to kidneys, exposure to nephrotoxic chemicals or medications, hemolytic anemia due to Escherichia coli 0157 ingestion, or glomerular nephritis).
As with all children, the major concern is to promote normal growth and development. Without aggressive monitoring and encouragement, the child with renal failure rarely meets his or her nutritional requirements. If the renal disease is present from birth, nutrition support needs to begin immediately to avoid losing the growth potential of the first few months of life. Growth in children with CKD is usually delayed. Although no specific therapy ensures normal growth, factors capable of responding to therapy include metabolic acidosis, electrolyte depletion, osteodystrophy, chronic infection, and protein-calorie malnutrition. Energy and protein needs for children with chronic renal disease are at least equivalent to the DRIs for normal children of the same height and age. If nutrition status is poor, energy needs may be even higher to promote weight gain and linear growth.
Feeding by tube is required in the presence of poor intake, particularly in the critical growth period of the first 2 years of life. Gastrostomy tubes are almost always placed in these children to enhance nutritional intake and facilitate growth. PN is rarely initiated unless the GI tract is nonfunctional. For the nutritional requirements of children with renal failure see the Kidney Disease Outcome Quality Initiative at www.kdoqi.org.
Control of calcium and phosphorus balance is especially important for maintaining good growth. The goal is to restrict phosphorus intake while promoting calcium absorption with the aid of 1,25-(OH)2D3. This helps prevent renal osteodystrophy, which can cause severe growth retardation. Use of calcium carbonate formulations to supplement the dietary intake enhances calcium intake while binding excess phosphorus. Persistent metabolic acidosis is often associated with growth failure in infancy. In chronic acidosis the titration of acid by the bone causes calcium loss and contributes to bone demineralization. Bicarbonate may be added to the infant formula to counteract this effect.
Restriction of protein in pediatric diets is controversial. The so-called “protective” effect on kidney function must be weighed against the clearly negative effect of possible protein malnutrition on growth (National Kidney Foundation, 2009). The recommended dietary allowance for protein for age is usually the minimum amount given.
Each child’s diet should be adjusted to his or her food preferences, family eating patterns, and biochemical needs. This is often not an easy task. In addition, care must be taken not to place too much emphasis on the diet to avoid its becoming a manipulative tool and an attention-getting device. Special encouragement, creativity, and attention are required to help the child with CKD consume the necessary energy. When possible, CCPD is given intermittently during the day and continuously at night as it allows liberalization of the diet. The child is more likely to meet nutritional requirements with fewer dietary restrictions and therefore experience better growth. See www.kidney.org for further tips on pediatric renal nutrition support.
Other treatments that help renal disease in children include the use of rHuEPO and recombinant deoxyribonucleic acid–produced human growth hormone. EPO is usually started when the child’s serum hemoglobin falls below 10 g/dL, with a goal of maintaining hemoglobin between 11 and 12 g/dL. Correction of anemia with the use of rHuEPO may increase appetite, intake, and feeling of well being, but it has not been found to affect growth, even with seemingly adequate nutrition support.
Medical Nutrition Therapy for Transplantation
The nutritional care of the adult patient who has received a transplanted kidney is based mainly on the metabolic effects of the required immunosuppressive therapy. Medications typically used for the long term include azathioprine (Imuran), corticosteroids (e.g., prednisone), calcineurin inhibitors (cyclosporine A, Gengraf, SangCya, Sandimmune], tacrolimus [Prograf, F506]), sirolimus (Rapamune), mycophenolate mofetil (CellCept), and mycophenolic acid (Myfortic). Corticosteroids are associated with accelerated protein catabolism, hyperlipidemia, sodium retention, weight gain, hyperglycemia, osteoporosis and electrolyte disturbances. Calcineurin inhibitors are associated with hyperkalemia, hypertension, hyperglycemia, and hyperlipidemia. The doses of these medications used after transplantation are decreased over time until a “maintenance level” is reached.
During the first 6 weeks after surgery, a high-protein diet is often recommended (1.2-1.5 g/kg ideal body weight [IBW]) with an energy intake of 30-35 kcal/kg IBW, to prevent negative nitrogen balance. A moderate sodium restriction of 2-3 g/day during this period minimizes fluid retention and helps to control blood pressure (Keven, K. 2006). After recovery, protein intake should be decreased to 1 g/kg IBW, with calorie intake providing sufficient energy to maintain or achieve an appropriate weight for height. A balanced low-fat diet aids in lowering cardiac complications (National Kidney Foundation, 2009). Sodium intakes are individualized based on fluid retention and blood pressure (see Appendix 37).
Hyperkalemia warrants a temporary dietary potassium restriction. Following transplantation, many patients exhibit hypophosphatemia and mild hypercalcemia caused by bone resorption; this is associated with persistent hyperparathyroidism and the effects of steroids on calcium, phosphorus, and vitamin D metabolism. The diet should contain adequate amounts of calcium and phosphorus (1200 mg of each daily) and cholecalciferol (vitamin D3, 2000 IU daily). Supplemental phosphorus may also be necessary to correct hypophosphatemia.
Hydration must also be monitored closely after transplantation. Because most kidney recipients required a fluid restriction while on dialysis, they need to be reminded of the importance of maintaining fluid intake after transplant. Typically, patients are encouraged to drink 2 L/day, but their overall needs depend on their urine output.
The majority of transplant recipients have elevated serum triglycerides or cholesterol for a variety of reasons. Intervention consists of medications, calorie restriction for those who are overweight, cholesterol intake limited to less than 300 mg/day, and limited total fat (see Chapter 34). In patients with glucose intolerance, limiting carbohydrates and a regular exercise regimen are appropriate. Tissue weight gain with resultant obesity is common after transplantation. Medication side effects, fewer dietary restrictions, and the lack of physical exercise can all contribute to post-transplant weight gain.
Education of Patients with End-Stage Renal Disease
For effective intervention, it is important to look at the long-range goals for educating the patient with ESRD about his or her nutritional needs. The average patient survives on dialysis 7 to 10 years. Patients with a relatively benign diagnosis may look forward to life spans of 20 to 40 years, particularly if they receive kidney transplants as a part of their treatment. The challenge for the dietitian is educating the patient with a chronic disease who will be primarily responsible for implementing the nutritional recommendations for the rest of his or her life. Thus intervention for ESRD and that for diabetes share many similarities.
Exchange lists are not always used in educating the patient about a renal diet. Rather a booklet, The National Renal Diet available from the American Dietetic Association (see “Useful Websites” later in this chapter), provides information about food sources of nutrients, adapting patients’ usual intakes to meet requirements based on their laboratory values, and reducing the intake of certain foods when blood values rise. A sample diet for the patient receiving dialysis can be found in Appendix 36.
Counseling for Patients with End-Stage Renal Disease
It is incumbent on the registered dietitian (RD) to develop a long-standing rapport with the patient and family and to serve as an ally to help them make the best nutritional choices over an extended period. Understanding the burdens of a complex, challenging, ever-changing diet suggests the transfer of information to the renal patient and family in a workable, flexible, and easily understood manner. Skills for this task are just as challenging, if not more so, than maintaining a patient’s iron status or keeping the patient at a good body weight. Empathy and use of techniques such as motivational interviewing or cognitive behavioral therapy are essential tools.
Coordination of Care in End-Stage Renal Disease
The position of the RD in the care of dialysis patients is unique because it is a federally mandated position. So, too, is the RD’s place on the mandated interdisciplinary health care team that exists within each dialysis unit. The team approach is an important aspect of all health care; however, its importance is magnified in the dialysis team, which consists of the patient, renal nurse, renal social worker, nephrologist, and renal dietitian. Care of these complex, long-term ESRD patients requires the skill and compassion of each member of the health care team working together (Unruh et al., 2005). Advanced levels of practice are available for dietitians who wish to be certified as renal RDs; they can be certified through examination by the American Dietetic Association, and become a Board Certified Specialist in Renal Nutrition.
Emergency Diets for Dialysis Patients
When power outages, flooding, storms, hurricanes, or earthquakes threaten a community, they threaten the most vulnerable people in that community. Patients on HD require power and water sources to do their own treatments at home. If they travel to a dialysis unit, they need access to transportation. Because of poor outcomes in recent natural disasters, the federal government’s ESRD program has specified that patients and care givers must be familiar with alternative nutritional therapies when dialysis is not available because of a natural or manmade emergency. Box 36-2 demonstrates the type of nutrient and practical information that must be considered when patients may be without dialysis for days, or because they must be evacuated to a site that cannot meet their urgent nutritional needs.
Medical Management (Conservative Treatment) or Palliative Care
The decision to forego or discontinue dialysis and opt for end-of-life care is a difficult and emotional one. Factors such as religious practices, age, quality of life, and comorbid disease all play a role. Patients who are poor candidates for dialysis or transplantation may benefit from a low-protein, low-sodium diet to minimize physical symptoms, such as shortness of breath and uremia. Palliative care can be offered with the goal of balancing patient wishes for food choice with these complex side effects.
American Dietetic Association and the National Renal Diet
National Institute of Diabetes and Digestive and Kidney Diseases
www.niddk.nih.gov/health/kidney/kidney.htm
Kidney Disease Outcome Quality Initiative
Nationwide End-Stage Renal Network
References
Appel, GB. Improved outcomes in nephrotic syndrome. Cleve Clin J Med. 2006;73:161.
Asplin, JR. Evaluation of a kidney stone patient. Semin Nephrol. 2008;28:99.
Asselman, M, Verkoelen, CF. Fructose intake as a risk factor for kidney stone disease. Kidney Int. 2008;73:139.
Bennett, SJ, et al. Nutrition in chronic heart failure with coexisting chronic kidney disease. J Cardiovasc Nurs. 2006;21:56.
Berardi, JM, et al. Plant based dietary supplement increases urinary pH. J Int Soc Sports Nutr. 2008;5:20.
Byham-Gray, LD. Weighing the evidence: energy determinations across the spectrum of kidney disease. J Ren Nutr. 2006;16(1):17.
Cheng, S, et al. A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1131.
Cheng, S, Coyne, DW. Niacin and niacinamide for hyperphosphatemia in patients undergoing dialysis. Int Urol Nephrol. 2006;38:171.
Curhan, GC, Taylor, EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73:489.
Domrongkitchaiporn, S, et al. Hypocitraturia in recurrent calcium stone formers: focusing on urinary potassium excretion. Am J Kidney Dis. 2006;48:546.
Eisner, BH, et al. Citrate, malate and alkali content in commonly consumed diet sodas: implications for nephrolithiasis treatment. J Urol. 2010;183:2419.
Eisner, BH, et al. Relationship between body mass index and quantitative 24-hour urine chemistries in patients with nephrolithiasis. Urology. 2010;75:1289.
Goodman, JW, et al. Effect of two sports drinks on urinary lithogenicity. Urol Res. 2009;37:41.
Hatch, M, Freel, RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol. 2008;28:143.
Himmelfarb, J, Ikizler, TA. Hemodialysis. N Engl J Med. 2010;363:1833.
Jackson, RD, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669.
Jepson, R. Review: cranberry products may prevent urinary tract infection in women with recurrent infections. Evid Based Nurs. 2008;11:74.
Kang, DE, et al. Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol. 2007;177:1358.
Keven, K, et al. The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplant Proc. 2006;38:1323.
Kiwull-Schone, H, et al. Food composition and acid-base balance: alimentary alkali depletion and acid load in herbivores. J Nutr. 2008;138:431S.
Knight, J, et al. Increased protein intake on controlled oxalate diets does not increase urinary oxalate excretion. Urol Res. 2009;37:63.
Kontiokari, T, et al. Cranberry juice and bacterial colonization in children—a placebo-controlled randomized trial. Clin Nutr. 2005;24:1065.
Li, WM, et al. Association of body mass index and urine pH in patients with urolithiasis. Urol Res. 2009;37:193.
Lieske, JC, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case controlled study. Am J Kidney Dis. 2006;48:897.
Locatelli, F, et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant. 2006;21:991.
Maalouf, NM, et al. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183:1026.
Maalouf, NM, et al. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5:1277.
Marcason, W. Where can I find information on the oxalate content of foods? J Am Diet Assoc. 2006;106:627.
Massey, LK, et al. Ascorbate increases human oxaluria and kidney stone risk. J Nutr. 2005;135:1673.
Massey, LK. Food oxalate: Factors affecting measurement, biological variation, and bioavailability. J Am Diet Assoc. 2007;107:1191.
Mente, A, et al. Ethnic differences in relative risk of idiopathic nephrolithiasis in north America. J Urol. 2007;178:1992.
Minich, D, et al. Acid-alkaline balance: role in chronic disease and detoxification. Alter Ther. 2007;13:62.
Moyad, MA, et al. Vitamin C with metabolites reduce oxalate levels compared to ascorbic acid: a preliminary and novel clinical urologic finding. Urol Nurs. 2009;29:95.
National Kidney Foundation. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update. Am J Kidney Dis. 2009;53(Suppl 2):S1–S124.
National Kidney Foundation, Kidney Dialysis Outcome Quality Initiative (KDOQI) clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl 3):S1–S202. These guidelines, as well as other KDOQI guidelines, can be accessed online at. www.kdoqi.org
National Kidney Foundation, K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. These guidelines, as well as all other KDOQI guidelines, can be accessed online at. www.kdoqi.org
National Kidney Foundation. KDOQI. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(Suppl 2):S1–S180.
Nelson, RG, Tuttle, KR. The new KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and CKD. Blood Purif. 2007;25:112.
Nouvenne, A, et al. Effects of a low-salt diet on idiopathic hypercalcuria in calcium-oxalalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2009;91:365.
Remer, T, et al. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791.
Remuzzi, G, et al. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288.
Rigalleau, V, et al. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism. 2006;55:108.
Semins, MJ, et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology. 2010;76:826.
Straub, M, Hautmann, RE. Developments in stone prevention. Curr Opin Urol. 2005;15:119.
Strejc, JM. Considerations in the nutritional management of patients with acute renal failure. Hemodial Int. 2005;9:135.
Taylor, EN, Curhan, GC. Fructose consumption and the risk of kidney stones. Kidney Int. 2008;73:207.
Taylor, EN, et al. DASH-style diet associates with reduced risk of kidney stones. J Am Soc Nephrol. 2009;20:2253.
Unruh, ML, et al. Choices for healthy outcomes in caring for end-stage renal disease (CHOICE) study. Am J Kidney Dis. 2005;46:1107.
Wenjun, Ju, et al. Renal gene and protein expression signatures for prediction of kidney disease progression. Am J Pathol. 2009;174:2073.
Zhang, R, et al. Kidney disease and the metabolic syndrome. Am J Med Sci. 2005;330:319.
Zilberman, DE, et al. The impact of societal changes on patters of urolithiasis. Curr Opin Urol. 2010;20:148.
Zuckerman, JM, Assimos, DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009;11:134.

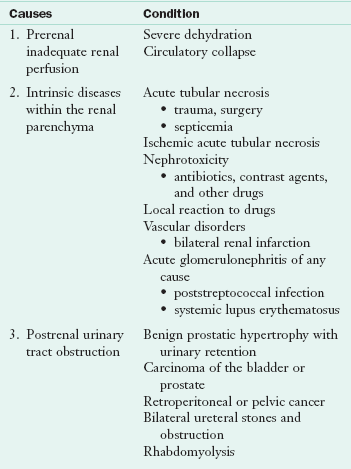
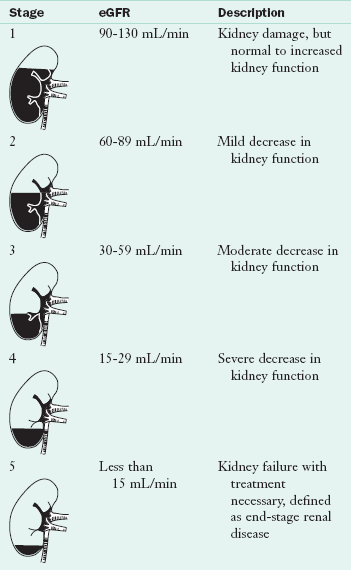
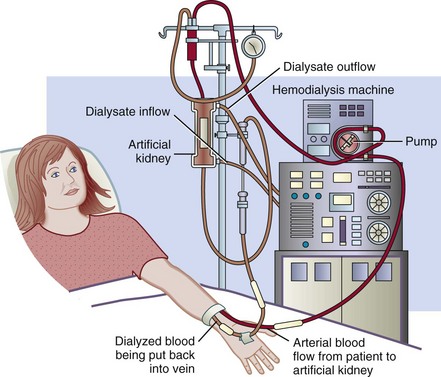
 cup unsalted or rinsed canned fish or poultry
cup unsalted or rinsed canned fish or poultry cup cottage cheese
cup cottage cheese can Ensure Plus, Boost Plus or Nepro
can Ensure Plus, Boost Plus or Nepro English muffin or bagel
English muffin or bagel -cup serving of green beans, summer squash, corn, beets, carrots, or peas
-cup serving of green beans, summer squash, corn, beets, carrots, or peas cup serving of berries, cherries, applesauce, canned pears or canned pineapple
cup serving of berries, cherries, applesauce, canned pears or canned pineapple cup Ensure Plus, Boost Plus or Nepro
cup Ensure Plus, Boost Plus or Nepro cup serving of milk, half and half, soy, or rice milk
cup serving of milk, half and half, soy, or rice milk