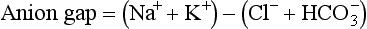Clinical
Water, Electrolytes, and Acid-Base Balance
The volume, composition, and distribution of body fluids have profound effects on cell function. A stable internal environment is maintained through a sophisticated network of homeostatic mechanisms that are focused on ensuring that water intake and water loss are balanced. Protein-energy malnutrition, disease, trauma, and surgery can disrupt fluid, electrolyte, and acid-base balance and alter the composition, distribution, or amount of body fluids. Even small changes in pH, electrolyte concentrations, and fluid status can adversely affect cell function. If these derangements are not corrected, severe consequences or death can ensue (Bartelmo and Terry, 2008.)
Body Water
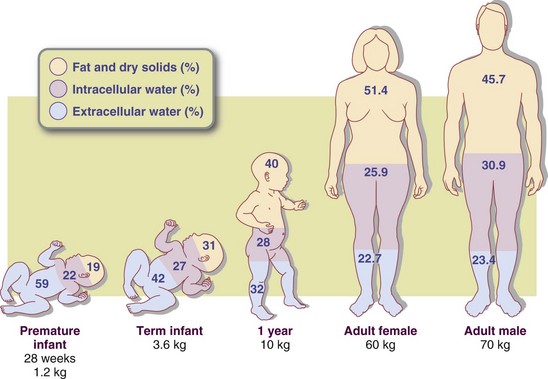
Water is the largest single component of the body. At birth, water accounts for approximately 75% to 85% of total body weight; this proportion decreases with age and adiposity. Water accounts for 60% to 70% of total body weight in the lean adult but only 45% to 55% in the obese adult. Metabolically active cells of the muscle and viscera have the highest concentration of water; calcified tissue cells have the lowest. Total body water is higher in athletes than in nonathletes and decreases with age and diminished muscle mass (Figure 7-1). Although the proportion of body weight accounted for by water varies with age and body fat, there is little day-to-day variation in the percentage of body water in the individual.
Functions
Water makes solutes available for cellular reactions. It is a substrate in metabolic reactions and a structural component, providing form to cells. Water is essential for the processes of digestion, absorption, and excretion. It also plays a key role in the structure and function of the circulatory system and acts as a transport medium for nutrients and all body substances.
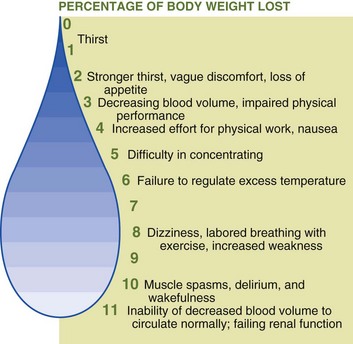
Water maintains the physical and chemical constancy of intracellular and extracellular fluids and has a direct role in maintaining body temperature. Evaporation of perspiration cools the body during warm weather, preventing or delaying hyperthermia. Loss of 20% of body water (dehydration) may cause death; loss of only 10% may lead to damage to essential body systems (Figure 7-2). Healthy adults can live up to 10 days without water, and children can live up to 5 days, whereas one can survive for several weeks without food.
Distribution
Intracellular water (ICW) is contained within cells and accounts for two thirds of total body water. Extracellular water in plasma, lymph, secretions, and spinal fluid equals one third of total body water or 20% of body weight. Extracellular fluid is the water and dissolved substances in the plasma, lymph, spinal fluid, and secretions; this includes the interstitial fluid, which is the fluid between and around the cells in tissues. While the distribution of body water varies under different circumstances, the total amount in the body remains relatively constant. Water consumed during the day through intake of foods and beverages is balanced by water lost through urination, perspiration, feces, and respiration. Edema is the abnormal accumulation of fluid in the intercellular tissue spaces or body cavities.
Water Balance
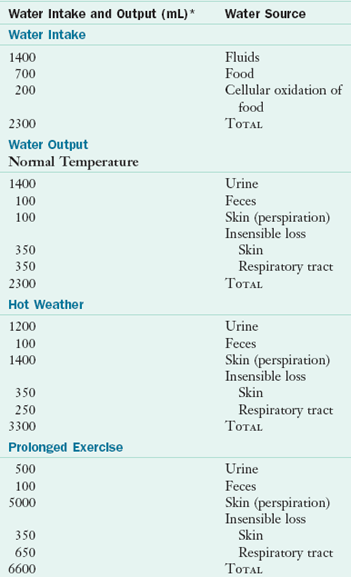
Shifts in water balance can have adverse consequences. Therefore homeostatic regulation by the gastrointestinal (GI) tract, kidneys, and brain keeps body water content fairly constant. The amount of water taken in daily is approximately equivalent to the amount lost (Table 7-1).
TABLE 7-1
*Average values.
Modified from Guyton AC: Textbook of medical physiology, ed 9, Philadelphia, 1996, Saunders.
Hormonal Regulation
Changes in cellular water content are sensed by baroreceptors in the central nervous system that provide feedback to the hypothalamus, close to the centers that regulate the antidiuretic hormone, vasopressin. Increased serum osmolality or decreased blood volume lead to its release, signaling the kidneys to conserve water. When vascular baroreceptors are stimulated by decreased extracellular fluid volume, the kidneys release renin to produce angiotensin II (the renin-angiotensin system). Angiotensin II has several functions, including stimulation of vasoconstriction and the thirst centers.
Water Intake
The sensation of thirst is a powerful signal to consume fluids. In fact, it controls water intake in healthy individuals. Both cellular dehydration and decreased extracellular fluid volume play a role in stimulating thirst. Sensitivity to thirst is decreased in older individuals, leading to higher risk for water deficits and ensuing dehydration.
Water is ingested as fluid and as part of food (Table 7-2). The oxidation of foods in the body also produces metabolic water as an end product. The oxidation of 100 g of fat, carbohydrate, or protein yields 107, 55, or 41 g of water, respectively, for a total of approximately 200 to 300 mL/day from consumption of the usual diet. When water cannot be ingested via the GI system it must be administered intravenously in the form of salt (saline) solutions, which closely resemble the electrolyte content of body fluids; dextrose solutions; parenteral nutrition; or in blood or plasma as transfusions. Water is absorbed rapidly because it moves freely through membranes by diffusion. This movement is controlled mainly by osmotic forces generated by inorganic ions in solution in the body (see Clinical Insight: Osmotic Forces).
Water Intoxication
Water intoxication occurs as a result of water intake in excess of the body’s ability to excrete water. The increased intracellular fluid volume is accompanied by osmolar dilution. The increased volume of intracellular fluid causes the cells, particularly the brain cells, to swell, leading to headache, nausea, vomiting, muscle twitching, blindness, and convulsions with impending stupor. If left untreated, water intoxication can be fatal. Water intoxication is not commonly seen in normal, healthy individuals. It may be seen in endurance athletes who consume large amounts of electrolyte-free beverages during events, individuals with psychiatric illness, or as a result of water drinking contests (Goldman, 2009; Rogers and Hew-Butler, 2009).
Water Elimination
Water loss normally occurs through the kidneys as urine and through the GI tract in the feces (measurable, sensible water loss), as well as through air expired from the lungs and water vapor lost through the skin (nonmeasurable, insensible water loss) (see Table 7-1). The kidney is the primary regulator of sensible water loss. Under normal conditions the kidneys have the ability to adjust to changes in body water composition by either decreasing or increasing water loss in the urine. Natural diuretics are substances in the diet that increase urinary excretion, such as alcohol, caffeine and some herbs.
Insensible water loss is continuous and usually unconscious. High altitude, low humidity, and high temperatures can increase insensible fluid loss through the lungs and through sweat. Athletes can lose 3 to 4 lb from fluid loss when exercising in a temperature of 80° F and low humidity or even more at higher temperatures.
The GI tract can be a major source of water loss. Under normal conditions the water contained in the 7 to 9 L of digestive juices and other extracellular fluids secreted daily into the GI tract is almost entirely reabsorbed in the ileum and colon, except for approximately 100 mL that is excreted in the feces. Because this volume of reabsorbed fluid is approximately twice that of the blood plasma, excessive GI fluid losses through diarrhea may have serious consequences, particularly for very young and very old individuals.
Fluid loss through diarrhea is responsible for thousands of children’s deaths in developing countries. Oral rehydration therapy with a simple mixture of water, sugar, and salt is highly effective in reducing the number of deaths if instituted early. Other abnormal fluid losses may occur as a result of emesis, hemorrhage, fistula drainage, burn and wound exudates, gastric and surgical tube drainage, and the use of diuretics.
When water intake is insufficient or water loss is excessive, healthy kidneys compensate by conserving water and excreting more concentrated urine. The renal tubules increase water reabsorption in response to the hormonal action of vasopressin. However, the concentration of the urine made by the kidneys has a limit: approximately 1400 mOsm/L. Once this limit has been reached, the body loses its ability to excrete solutes. The ability of the kidneys to concentrate urine may be compromised in older individuals or in young infants, resulting in increased risk of developing dehydration or hypernatremia, especially during illness.
Signs of dehydration include headache, fatigue, decreased appetite, light-headedness, poor skin turgor (although this may be present in well-hydrated older persons), skin tenting on the forehead, concentrated urine, decreased urine output, sunken eyes, dry mucous membranes of the mouth and nose, orthostatic blood pressure changes, and tachycardia (Armstrong, 2005). In a dehydrated person the specific gravity, a measure of the dissolved solutes in urine, increases above the normal levels of 1.010 where the urine becomes remarkably darker (Cheuvront et al, 2010.) High ambient temperature and dehydration adversely affect exercise performance; changes may be mediated by serotonergic and dopaminergic alterations in the central nervous system (Maughan et al., 2007). Fluids of appropriate composition in appropriate amounts are essential. See Clinical Insight: Water Requirements—When Eight Cups Is Not Enough.
Electrolytes
Electrolytes are substances that dissociate into positively and negatively charged ions (cations and anions) when dissolved in water. Electrolytes can be simple inorganic salts of sodium, potassium, or magnesium or complex organic molecules; they play a key role in a host of normal metabolic functions (Table 7-3). One milliequivalent (mEq) of any substance has the capacity to combine chemically with 1 mEq of a substance with an opposite charge. For univalent ions (e.g., Na+) 1 millimole (mmol) equals 1 mEq; for divalent ions (e.g., Ca++) 1 mmol equals 2 mEq (see Appendix 3 for conversion guidelines).
TABLE 7-3
Normal Electrolyte Concentration of Serum
| Electrolyte | Normal Range |
| Cations | |
| Sodium | 136-145 mEq/L |
| Potassium | 3.5-5 mEq/L |
| Calcium | 4.5-5.5 mEq/L (9-11 mg/dL) |
| Magnesium | 1.5-2.5 mEq/L (1.8-3 mg/dL) |
| Anions | |
| Chloride | 96-106 mEq/L |
| CO2 (content) | 24-28.8 mEq/L |
| Phosphorus (inorganic) | 3-4.5 mg/dL (1.9-2.85 mEq/L as HPO42−) |
| Sulfate (as S) | 0.8-1.2 mg/dl (0.5-0.75 mEq/L as SO22−) |
| Lactate | 1.8 mEq/L (6-16 mg/dL) |
| Protein | 6 g/dl (14-18 mEq/L); depends on albumin level |
CO2, Carbon dioxide; HPO42, monohydrogen phosphate; SO22, sulfate.
The major extracellular electrolytes are sodium, calcium, chloride, and bicarbonate (HCO3−). Potassium, magnesium, and phosphate are the major intracellular electrolytes. These elements, which exist as ions in body fluids, are distributed throughout all body fluids. They maintain physiologic body functions, including osmotic equilibrium, acid-base balance, and intracellular and extracellular concentration differentials. Changes in either intracellular or extracellular electrolyte concentrations can have a major effect on bodily functions. The sodium-potassium adenosine triphosphatase (Na/K ATPase) pump closely regulates cellular electrolyte contents by actively pumping sodium out of cells in exchange for potassium. Other electrolytes follow ion gradients.
Calcium
Although approximately 99% of the body’s calcium (Ca++) is stored in the bone, the remaining 1% has important physiologic functions. Ionized calcium within the vascular compartment is a cation with a positive charge. Approximately half of the calcium found in the intravascular compartment is bound to the serum protein albumin. Thus when serum albumin levels are low, total calcium levels decrease because of hypoalbuminemia. The corrected calcium formula, often used in renal disease is
The binding ability of calcium and its ionized content in blood have implications for normal homeostatic mechanisms. Blood tests for calcium levels often measure both total and ionized calcium levels. This is because ionized (or free, unbound) calcium is the active form of calcium and is not affected by hypoalbuminemia. In healthy adults, normal levels for serum total calcium are approximately 8.5 to 10.5 mg/dL, whereas normal levels for ionized calcium are 4.5 to 5.5 mEq/L.
Ionized calcium levels are inversely altered by changes in acid-base balance; as serum pH rises, calcium binds with protein, leading to decreased ionized calcium levels. As pH is lowered, the opposite occurs. Because calcium has an important role in cardiac, nervous system, and skeletal muscle function, both hypocalcemia and hypercalcemia can become life-threatening.
Functions
Calcium is found in bones as part of the compound hydroxyapatite. Outside of the bone, calcium is a second messenger in responding to changes in intracellular calcium content following binding of hormones or proteins to the cell surface (the first messenger). Calcium is also an important factor in regulating cell electroconductivity and in blood clotting.
Calcium content is carefully regulated by the actions of parathyroid hormone (PTH), calcitonin, vitamin D, and phosphorus. When serum calcium levels are low, PTH causes release of calcium from the bones and stimulates increased absorption from the GI tract. Calcitonin works in the opposite direction, shutting off bone calcium release and decreasing GI absorption. Vitamin D stimulates while phosphorus inhibits calcium absorption in the GI tract.
Absorption and Excretion
Approximately 20% to 60% of dietary calcium is absorbed and is tightly regulated because of the need to maintain steady serum calcium levels in the face of fluctuating intake. The ileum is the most important site of calcium absorption. Calcium is absorbed via passive transport and through a vitamin D–regulated transport system. See Chapter 3.
The kidney is the main site of calcium excretion. The majority of serum calcium is bound to proteins and not filtered by the kidneys; only approximately 100 to 200 mg is excreted in the urine in normal adults.
Sodium
Sodium (Na+) is the major cation of extracellular fluid. Normal serum concentration is 136 to 145 mEq/L. Secretions such as bile and pancreatic juice contain substantial amounts of sodium. Approximately 35% to 40% of the total body sodium is in the skeleton; however, most of it is only slowly exchangeable with that in body fluids. Contrary to common belief, sweat is hypotonic and contains a relatively small amount of sodium.
Functions
As the predominant ion of the extracellular fluid, sodium thus regulates both extracellular and plasma volume. Sodium is also important in neuromuscular function and maintenance of acid-base balance. Maintenance of serum sodium levels is vital, because severe hyponatremia can lead to seizures, coma and death.
Extracellular sodium concentrations are much higher than intracellular levels (normal serum sodium is approximately 135 mEq/L whereas intracellular levels are approximately 10 mEq/L). The Na/K ATPase pump is an active transport system that works to keep sodium outside the cell through exchange with potassium. The Na/K ATPase pump requires carriers for both sodium and potassium along with energy for proper function. Exportation of sodium from the cell is the driving force for facilitated transporters that import glucose, amino acids, and other nutrients into the cells.
Absorption and Excretion
Sodium is readily absorbed from the intestine and carried to the kidneys, where it is filtered and returned to the blood to maintain appropriate levels. The amount absorbed is proportional to the intake in healthy adults.
Approximately 90% to 95% of normal body sodium loss is through the urine; the rest is lost in feces and sweat. Normally the quantity of sodium excreted daily is equal to the amount ingested. Sodium excretion is maintained by a mechanism involving the glomerular filtration rate, the cells of the juxtaglomerular apparatus of the kidneys, the renin-angiotensin-aldosterone system, the sympathetic nervous system, circulating catecholamines, and blood pressure.
Sodium balance is regulated in part by aldosterone, a mineralocorticoid secreted by the adrenal cortex. When blood sodium levels rise, the thirst receptors in the hypothalamus stimulate the thirst sensation. Ingestion of fluids returns sodium levels to normal. Under certain circumstances, sodium and fluid regulation can be disrupted, resulting in abnormal blood sodium levels. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is characterized by concentrated, low-volume urine and dilutional hyponatremia as water is retained. SIADH can result from central nervous system disorders, pulmonary disorders, tumors, and certain medications. See Chapter 36.
Estrogen, which is slightly similar to aldosterone, also causes sodium and water retention. Changes in water and sodium balance during the menstrual cycle, during pregnancy, and while taking oral contraceptives are partially attributable to changes in progesterone and estrogen levels.
Dietary Reference Intakes
Actual minimum requirements for sodium are not known but have been estimated to be as low as 200 mg/day. Estimated adequate intakes (AIs) for sodium were published in the 2004 Dietary Reference Intakes (Institute of Medicine, 2004). The mean daily salt intake in Western societies is approximately 10 to 12 g (4 to 5 g of sodium) per capita, far in excess of the estimated minimum requirements and even in excess of the AIs for sodium of 1.2 to 1.5 g per day, depending on age, with lower amounts recommended for the elderly (Table 7-4).
TABLE 7-4
Dietary Reference Intakes for Sodium, Potassium, and Chloride Daily Intake

UL, Tolerable upper intake level.
Institute of Medicine, Food and Nutrition Board: Dietary reference intakes for water, potassium, sodium, chloride, and sulfate, Washington, DC, 2004, National Academies Press.
Approximately 3 g of the daily salt intake exists naturally in foods, 3 g is added during processing, and 4 g is added by the individual. Increased reliance on restaurants, fast food, and commercially prepared convenience foods has contributed to this high per capita salt and thus sodium intake.
Healthy kidneys are usually able to excrete excess sodium intake; however, there is concern about persistent excessive sodium intake, which has been implicated in development of hypertension. See Chapter 34. In addition to its role in hypertension, excessive salt intake has been associated with increased urinary calcium excretion (Teucher and Fairweather-Tait, 2003) (see Chapter 36) and some cases of osteoporosis (He and MacGregor, 2010). The dietary reference intakes (DRI) give an upper limit of 2.3 g of sodium per day (or 5.8 g sodium chloride per day), given the potential role of sodium in hypertension (Joint National Committee, 2003).
Sources
The major source of sodium is sodium chloride, or common table salt, of which sodium constitutes 40% by weight. Protein foods generally contain more naturally existing sodium than do vegetables and grains, whereas fruits contain little or none. The addition of table salt, flavored salts, flavor enhancers, and preservatives during food processing accounts for the high sodium content of most convenience and fast-food products. For instance,  cup of frozen vegetables prepared without salt contains 10 mg of sodium, whereas
cup of frozen vegetables prepared without salt contains 10 mg of sodium, whereas  cup of canned vegetables contains approximately 260 mg of sodium. Similarly, 1 ounce of plain meat contains 30 mg of sodium, whereas 1 ounce of luncheon meat contains approximately 400 mg of sodium. The larger-portion sizes that are being offered by dining establishments to consumers are increasing the sodium intake even more.
cup of canned vegetables contains approximately 260 mg of sodium. Similarly, 1 ounce of plain meat contains 30 mg of sodium, whereas 1 ounce of luncheon meat contains approximately 400 mg of sodium. The larger-portion sizes that are being offered by dining establishments to consumers are increasing the sodium intake even more.
Magnesium
The adult human body contains approximately 24 g of magnesium, which is the second most prevalent intracellular cation. Approximately half of the body’s magnesium is located in bone, whereas another 45% resides in soft tissue; only 1% of the body’s magnesium content is in the extracellular fluids (Rude, 2000). Normal serum magnesium levels are approximately 1.7 to 2.5 mEq/L; however, approximately 70% of serum magnesium is free or ionized. The remainder is bound to proteins and is not active.
Function
Magnesium (Mg++) is an important cofactor in many enzymatic reactions in the body and is also important in bone metabolism as well as central nervous system and cardiovascular function. Many of the enzyme systems regulated by magnesium are involved in nutrient metabolism and nucleic acid synthesis, leading to the body’s need to carefully regulate magnesium status. As with calcium, severe hypomagnesemia or hypermagnesemia can have life-threatening sequelae.
Intakes of Mg++, potassium, fruits, and vegetables have been associated with higher alkaline status and a subsequent beneficial effect on bone health; enhanced mineral-water consumption may be an easy, inexpensive way to reduce the onset of osteoporosis (Wynn et al., 2010). See Clinical Insight: Urinary pH—How Does Diet Affect It? in Chapter 36.
Absorption and Excretion
Approximately one third of ingested magnesium is absorbed. Although magnesium absorption occurs throughout the GI tract, absorption is optimized in the ileum and distal jejunum through both passive and active mechanisms. Magnesium absorption is regulated to maintain serum levels; if levels drop, more is absorbed and if levels increase, less is absorbed. The kidney is the major regulator of magnesium excretion.
Sources
Magnesium is found in a wide variety of foods, making an isolated magnesium deficiency unlikely in otherwise healthy individuals. Highly processed foods tend to have lower magnesium content, whereas green leafy vegetables, legumes, and whole grains are good sources. The high magnesium content of vegetables helps to alleviate some concerns about the potential for phytate binding.
Phosphorus
Phosphorus is an important constituent of the intracellular fluid and in its role in ATP is vital in energy metabolism. In addition, phosphorus is important in bone metabolism. Approximately 80% of the body’s phosphorus is found in bones. Phosphorus is found in the body as phosphate—the terms are often used interchangeably. Normal levels for serum phosphorus are between 2.4 and 4.6 mg/dL.
Functions
Large amounts of free energy are released when the phosphate bonds in ATP are split. In addition to this role, phosphorus is vital for cellular function in phosphorylation and dephosphorylation reactions, as a buffer in acid-base balance, and in cellular structure as part of the phospholipids membrane. Because of the vital role that phosphorus plays in energy production, severe hypophosphatemia can be a life-threatening event.
Absorption and Excretion
Phosphorus absorption is fairly efficient and is related to intake at most intake levels. The kidney is the major site of phosphorus excretion.
Potassium
Potassium (K+), the major cation of intracellular fluid, is present in small amounts in extracellular fluid. The normal serum potassium concentration is 3.5 to 5 mEq/L.
Functions
With sodium, potassium is involved in maintaining a normal water balance, osmotic equilibrium, and acid-base balance. In addition to calcium, it is important in the regulation of neuromuscular activity. Concentrations of sodium and potassium determine membrane potentials in nerves and muscle. Potassium also promotes cellular growth. The potassium content of muscle is related to muscle mass and glycogen storage; therefore, if muscle is being formed, an adequate supply of potassium is essential. Potassium has an integral role in the Na/K ATPase pump. Both hypokalemia and hyperkalemia can have devastating cardiac implications.
Absorption and Excretion
Potassium is readily absorbed from the small intestine. Approximately 80% to 90% of ingested potassium is excreted in the urine; the remainder is lost in the feces. The kidneys maintain normal serum levels through their ability to filter, resorb, and excrete potassium under the influence of aldosterone. Ionized potassium is excreted in place of ionized sodium through the renal tubule exchange mechanism.
Sources
As a rule, fruits, vegetables, fresh meat, and dairy products are good sources of potassium. Box 7-1 categorizes select foods according to their potassium content.
Dietary Reference Intakes
The AI level for potassium for adults is 4700 mg/day. No upper limit has been set. Potassium intake is inadequate in a as many as 50% of adult Americans. The reason for the poor potassium intakes is simply inadequate consumption of fruits and vegetables. Insufficient potassium intakes have been linked to hypertension and cardiac arrhythmia.
Acid-Base Balance
An acid is any substance that tends to release hydrogen ions in solution, whereas a base is any substance that tends to accept hydrogen ions in solution. The hydrogen ion concentration [H+] determines acidity. Because the magnitude of hydrogen ion concentration is small compared with that of other serum electrolytes, acidity is more readily expressed in terms of pH units. A low blood pH indicates a higher hydrogen ion concentration and greater acidity, whereas a high pH value indicates a lower hydrogen ion concentration and greater alkalinity.
Acid-base balance is the dynamic equilibrium state of hydrogen ion concentration. Maintaining the arterial blood pH level within the normal range of 7.35 to 7.45 is crucial for many physiologic functions and biochemical reactions. Regulatory mechanisms of the kidneys, lungs, and buffering systems enable the body to maintain the blood pH level despite the enormous acid load from food consumption and tissue metabolism. A disruption of the acid-base balance occurs when acid or base losses or gains exceed the body’s regulatory capabilities, or when normal regulatory mechanisms become ineffective. These regulatory disturbances may develop in association with certain diseases, toxin ingestion, shifts in fluid status, and certain medical and surgical treatments (Table 7-5). If a disrupted acid-base balance is left untreated, multiple detrimental effects ranging from electrolyte abnormalities to death can ensue.
TABLE 7-5
Four Major Acid-Base Imbalances
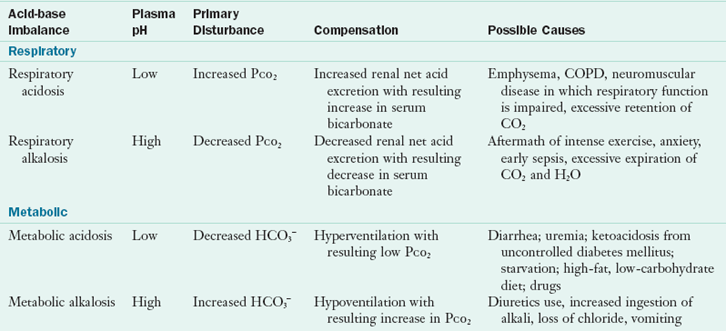
CO2, Carbon dioxide; COPD, chronic obstructive pulmonary disease; H2O, water; HCO3−, bicarbonate; PCO2, carbon dioxide pressure.
Acid Generation
Acids are introduced exogenously through the ingestion of food, acid precursors, and toxins. They are also generated endogenously through normal tissue metabolism. Fixed acids such as phosphoric and sulfuric acids are produced from the metabolism of phosphate-containing substrates and sulfur-containing amino acids, respectively. Organic acids such as lactic and keto acids typically accumulate only during exercise, acute illness, or fasting. Carbon dioxide (CO2), a volatile acid, is generated from the oxidation of carbohydrates, amino acids, and fat. Under normal conditions, the body is able to maintain normal acid-base status through a wide range of acid intake from foods. See Clinical Insight: Urinary pH—How Does Diet Affect It? in Chapter 36 for the acid and alkaline effects of foods.
Regulation
Various regulatory mechanisms maintain the pH level within very narrow physiologic limits. At the cellular level, buffer systems composed of weak acids or bases and their corresponding salts minimize the effect on pH of the addition of a strong acid or base. The buffering effect involves formation of a weaker acid or base in an amount equivalent to the strong acid or base that has been added to the system (Figure 7-3).
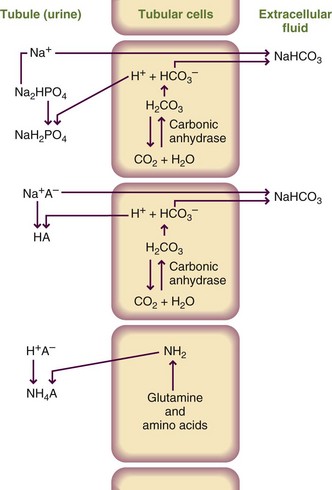
FIGURE 7-3 Generation of sodium bicarbonate and clearance of hydrogen ion concentration by the three buffer systems that function in the kidney. HA, Any acid in the body.
Proteins and phosphates are the primary intracellular buffers, whereas the HCO3− and carbonic acid (H2CO3) system is the primary extracellular buffer. The acid-base balance is also maintained by the kidneys and lungs. The kidneys regulate hydrogen ion (H+) secretion and HCO3− resorption. The lungs control alveolar ventilation by altering either the depth or rate of breathing. In turn, changes in breathing alter the amount of carbon dioxide expired.
Acid-Base Disorders
Acid-base disorders can be differentiated based on whether they have metabolic or respiratory causes. The evaluation of acid-base status requires analysis of serum electrolytes and arterial blood gas (ABG) values (Table 7-6). Metabolic acid-base imbalances result in changes in HCO3− (i.e., base) levels, which are reflected in the total carbon dioxide (TCO2) portion of the electrolyte profile. TCO2 includes HCO3−, H2CO3, and dissolved carbon dioxide; however, all but 1 to 3 mEq/L is in the form of HCO3−. Thus, for ease of interpretation, TCO2 should be equated with HCO3−. Respiratory acid-base imbalances result in changes in the partial pressure of dissolved carbon dioxide (PCO2). This is reported in the ABG values in addition to the pH, which reflects the overall acid-base status.
TABLE 7-6
Normal Arterial Blood Gas Values
| Clinical Test | ABG Value |
| pH | 7.35-7.45 |
| PCO2 | 35-45 mm Hg |
| PO2 | 80-100 mm Hg |
| HCO3− | 22-26 mEq/L |
| O2 saturation | >95% |
ABG, Arterial blood gas; HCO3−, bicarbonate; O2, oxygen; PCO2, carbon dioxide pressure; PO2, oxygen pressure.
Metabolic Acidosis
Metabolic acidosis results from increased generation or accumulation of acids or loss of base (i.e., HCO3−) in the extracellular fluids. Simple, acute metabolic acidosis results in a low blood pH, or acidemia. Examples of metabolic acidosis include diabetic ketoacidosis, lactic acidosis, toxin ingestion, uremia, and excessive HCO3− loss via the kidneys or intestinal tract. Multiple deaths have previously been attributed to lactic acidosis caused by administration of parenteral nutrition devoid of thiamin. In patients with metabolic acidosis, the anion gap is calculated to help determine cause and appropriate treatment. The anion gap is a measurement of the interval between the sum of “routinely measured” cations minus the sum of the “routinely measured” anions in the blood.
in which Na− is sodium, K+ is potassium, Cl− is chloride, and HCO3− is bicarbonate. Normal is 12 to 14 mEq/L.
Anion gap metabolic acidosis occurs when a decrease in HCO3− concentration is balanced by increased acid anions other than chloride. This causes the calculated anion gap to exceed the normal range of 12 to 14 mEq/L. This normochloremic metabolic acidosis may develop in association with the following conditions, represented by the acronym MUD PILES (Wilson, 2003):
Nongap metabolic acidosis occurs when a decrease in HCO3− concentration is balanced by an increase in chloride concentration, resulting in a normal anion gap. This hyperchloremic metabolic acidosis, may develop in association with the following, represented by the acronym USED CARP) (Wilson, 2003):
Metabolic Alkalosis
Metabolic alkalosis results from the administration or accumulation of HCO3− (i.e., base) or its precursors, excessive loss of acid (e.g., during gastric suctioning), or loss of extracellular fluid containing more chloride than HCO3− (e.g., from villous adenoma or diuretic use). Simple, acute metabolic alkalosis results in a high blood pH, or alkalemia. Metabolic alkalosis may also result from volume depletion; decreased blood flow to the kidneys stimulates reabsorption of sodium and water, increasing HCO3− reabsorption. This condition is known as contraction alkalosis. Alkalosis can also result from severe hypokalemia (serum potassium concentration <2 mEq/L). As potassium moves from the intracellular to the extracellular fluid, hydrogen ions move from the extracellular to the intracellular fluid to maintain electroneutrality. This process produces intracellular acidosis, which increases hydrogen ion excretion and HCO3− reabsorption by the kidneys.
Respiratory Acidosis
Respiratory acidosis is caused by decreased ventilation and consequent carbon dioxide retention. Simple, acute respiratory acidosis results in a low pH, or acidemia. Acute respiratory acidosis can occur as a result of sleep apnea; asthma; aspiration of a foreign object; or acute respiratory distress syndrome, also known as adult respiratory distress syndrome. Chronic respiratory acidosis is associated with obesity hypoventilation syndrome, chronic obstructive pulmonary disease or emphysema, certain neuromuscular diseases, and starvation cachexia.
Respiratory Alkalosis
Respiratory alkalosis results from increased ventilation and elimination of carbon dioxide. The condition may be mediated centrally (e.g., from head injury, pain, anxiety, cerebrovascular accident, or tumors) or by peripheral stimulation (e.g., from pneumonia, hypoxemia, high altitudes, pulmonary embolism, congestive heart failure, or interstitial lung disease). Simple, acute respiratory alkalosis results in a high pH, or alkalemia.
Compensation
When an acid-base imbalance occurs, the body attempts to restore the normal pH by developing an opposite acid-base imbalance to offset the effects of the primary disorder, a response known as compensation. For example, the kidneys of a patient with a primary respiratory acidosis (decreased pH) compensate by increasing HCO3− reabsorption, thereby creating a metabolic alkalosis. This response helps to increase the pH. Similarly, in response to a primary metabolic acidosis (decreased pH), the lungs compensate by increasing ventilation and carbon dioxide elimination, thereby creating a respiratory alkalosis. This compensatory respiratory alkalosis helps to increase pH.
Respiratory compensation for metabolic acid-base disturbances occurs quickly—within minutes. In contrast, renal compensation for respiratory acid-base imbalances may take 3 to 5 days to be maximally effective. Compensation does not always occur; and when it does, it is not completely successful (i.e., does not result in a pH of 7.4). The pH level still reflects the underlying primary disorder. It is imperative to distinguish between primary disturbances and compensatory responses because treatment is always directed toward the primary acid-base disturbance and its underlying cause. As the primary disturbance is treated, the compensatory response corrects itself. Predictive values for compensatory responses are available to differentiate between primary acid-base imbalances and compensatory responses (Whitmire, 2002). Clinicians may also use tools such as clinical algorithms.
The Beverage Institute Hydration Calculator
http://www.weather.com/outlook/health/fitness/tools/hydration
The Merck Manual of Diagnosis and Therapy
http://www.merckmanuals.com/professional/index.html
The Weather Channel—Hydration Calculator
http://www.weather.com/outlook/health/fitness/tools/hydration
References
Armstrong, LE. Hydration assessment techniques. Nutr Rev. 2005;63:S40.
Bartelmo, J, Terry, DP. Fluids and Electrolytes Made Incredibly Easy, ed 4. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
Cheuvront, SN, et al. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr. 2010;92:565.
Goldman, MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res Rev. 2009;61:210.
He, FJ, MacGregor, GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52:363.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, DC: National Academies Press; 2004.
Joint National Committee (JNC): The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, NIH Pub No 03-5233, 2003.
Maughan, RJ, et al. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26:604S.
Rogers, IR, Hew-Butler, T. Exercise-associated hyponatremia: overzealous fluid consumption. Wilderness Environ Med. 2009;20:139.
Rude, RK. Magnesium. In: Stipanuk MH, ed. Biochemical and physiological aspects of human nutrition. Philadelphia: Saunders, 2000.
Teucher, B, Fairweather-Tait, S. Dietary sodium as a risk factor for osteoporosis: where is the evidence? Proc Nutr Soc. 2003;62:859.
Whitmire, SJ. Fluids, electrolytes, and acid-base balance. In Matarese LE, Gottschlich MM, eds.: Contemporary nutrition support practice: a clinical guide, ed 2, Philadelphia: Saunders, 2002.
Wilson, RF. Acid-base problems. In Tintinalli JE, Krome RL, Ruiz E, eds.: Emergency medicine: a comprehensive study guide, ed 6, New York: McGraw-Hill, 2003.
Wynn, E, et al. Postgraduate symposium: positive influence of nutritional alkalinity on bone health. Proc Nutr Soc. 2010;69:166.
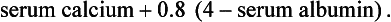
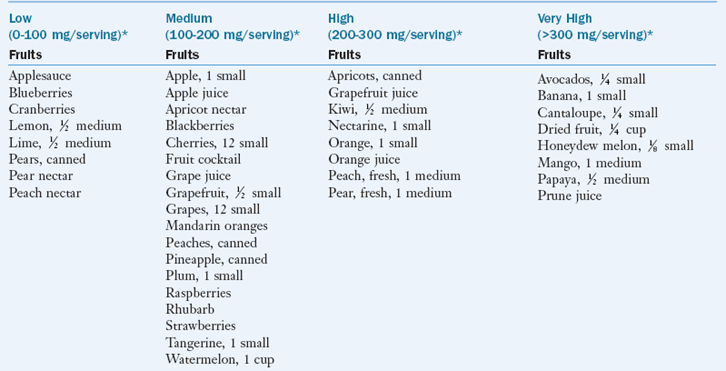
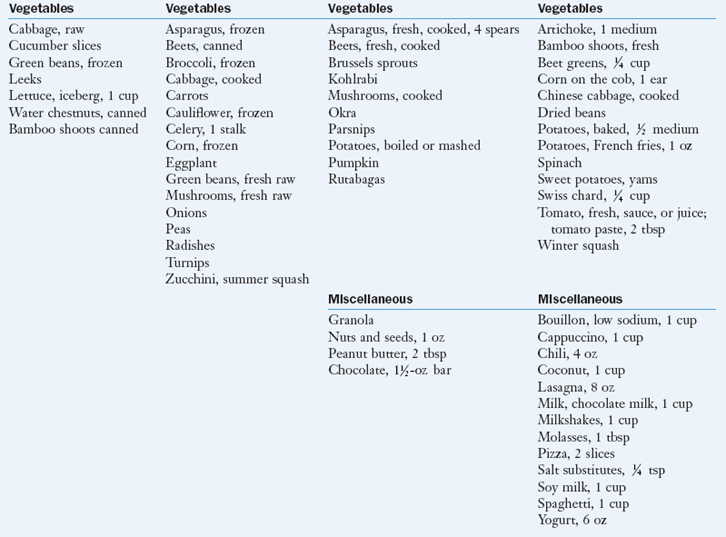
 cup unless otherwise specified.
cup unless otherwise specified.