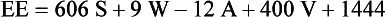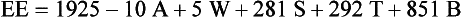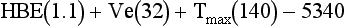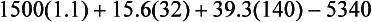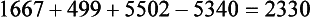Medical Nutrition Therapy for Metabolic Stress
Sepsis, Trauma, Burns, and Surgery
Trauma from motor vehicle accidents, gunshots, stab wounds, falls, and burns are major causes of disability and death. Unintentional injuries and motor vehicle accidents are ranked as the fifth leading cause of death after heart disease, cancer, stroke, and chronic lower respiratory diseases. Injury results in profound metabolic alterations, beginning at the time of injury and persisting until wound healing and recovery are complete. Whether the event involves sepsis (infection), trauma, burns, or surgery, the systemic response is activated. The physiologic and metabolic changes that follow may lead to shock and other negative outcomes (Figure 39-1). Variable responses relate in part to the patient’s age, previous state of health, preexisting disease, type of infection, and presence of multiple organ dysfunction syndrome (MODS).
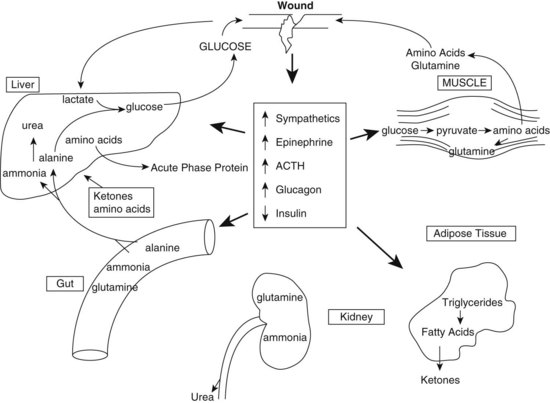
FIGURE 39-1 Neuroendocrine and metabolic consequences of injury, ACTH, adrenocorticotropic hormone. (Reprinted from Lowry SF and Perez JM in Modern Nutrition in Health and Disease, Lippincott Williams & Wilkins, Philadelphia, PA, 2006, 1381-1400.)
Metabolic Response to Stress
The metabolic response to critical illness, traumatic injury, sepsis, burns, or major surgery is complex and involves most metabolic pathways. Accelerated catabolism of lean body or skeletal mass occurs, which clinically results in net negative nitrogen balance and muscle wasting. The response to critical illness, injury, and sepsis characteristically involves both ebb and flow phases. The ebb phase, occurring immediately following injury, is associated with hypovolemia, shock, and tissue hypoxia. Typically decreased cardiac output, oxygen consumption, and body temperature occur in this phase. Insulin levels fall in direct response to the increase in glucagon, most likely as a signal to increase hepatic glucose production (Table 39-1).
TABLE 39-1
Characteristics of Metabolic Phases Occurring After Severe Injury
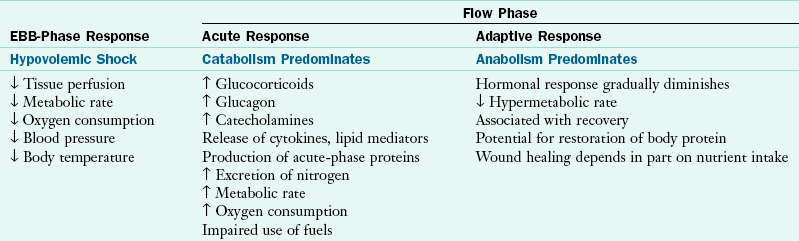
From Enteral nutrition support in critical care, Columbus, OH, 1994, Ross Products Division, Abbott Labs.
Increased cardiac output, oxygen consumption, body temperature, energy expenditure, and total body protein catabolism characterize the flow phase that follows fluid resuscitation and restoration of oxygen transport. Physiologically, a marked increase occurs in glucose production, free fatty acid release, circulating levels of insulin, catecholamines (epinephrine and norepinephrine released by the adrenal medulla), glucagon, and cortisol. The magnitude of hormonal response appears to be associated with the severity of injury.
Hormonal and Cell-Mediated Response
Metabolic stress is associated with an altered hormonal state that results in an increased flow of substrate but poor use of carbohydrate, protein, fat, and oxygen. Counter-regulatory hormones, which are elevated after injury and sepsis, play a role in accelerated proteolysis. Glucagon promotes gluconeogenesis, amino acid uptake, ureagenesis, and protein catabolism. Cortisol, which is released from the adrenal cortex in response to stimulation by adrenocorticotropic hormone secreted by the anterior pituitary gland, enhances skeletal muscle catabolism and promotes hepatic use of amino acids for gluconeogenesis, glycogenolysis, and acute-phase protein synthesis (Table 39-2).
TABLE 39-2
Metabolic Responses During Stress
| Organ | Response |
| Liver | ↑ Glucose production |
| ↑ Amino acid uptake | |
| ↑ Acute-phase protein synthesis | |
| ↑ Trace metal sequestration | |
| Central nervous system | Anorexia Fever |
| Circulation | ↑ Glucose |
| ↑ Triglycerides | |
| ↑ Amino acids | |
| ↑ Urea | |
| ↓ Iron | |
| ↓ Zinc | |
| Skeletal muscle | ↑ Amino acid efflux (especially glutamine), leading to loss of muscle mass |
| Intestine | ↓ Amino acid uptake from both luminal and circulating sources, leading to gut mucosal atrophy |
| Endocrine | ↑ Adrenocorticotropic hormone |
| ↑ Cortisol | |
| ↑ Growth hormone | |
| ↑ Epinephrine | |
| ↑ Norepinephrine | |
| ↑ Glucagon | |
| ↑ Insulin (usually) |
From Michie HR: Metabolism of sepsis and multiple organ failure, World J Surg 20:461, 1996.
After injury or sepsis, energy production increasingly depends on protein. Branched-chain amino acids (BCAAs leucine, isoleucine, and valine) are oxidized from skeletal muscle as a source of energy for the muscle; carbon skeletons are made available for the glucose-alanine cycle and muscle glutamine synthesis. The mobilization of acute-phase proteins, those secretory proteins in the liver that are altered in response to injury or infection, results in rapid loss of lean body mass and an increased net negative nitrogen balance, which continues until the inflammatory response resolves. Breakdown of protein tissue also causes increased urinary losses of potassium, phosphorus, and magnesium. Lipid metabolism is also altered in stress and sepsis. Increased circulation of free fatty acids is thought to result from increased lipolysis caused by elevated catecholamines and cortisol, as well as a marked elevation in the ratio of glucagon to insulin.
Most notable is the hyperglycemia observed during stress. This initially results from a marked increase in glucose production and uptake secondary to gluconeogenesis and elevated levels of hormones, including epinephrine, that diminish insulin release. Stress also initiates the release of aldosterone, a corticosteroid that causes renal sodium retention, and vasopressin (antidiuretic hormone), which stimulates renal tubular water resorption. The action of these hormones results in conservation of water and salt to support the circulating blood volume, noted in Table 39-2.
The response to injury is also regulated by metabolically active cytokines (proinflammatory proteins) such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF), which are released by phagocytic cells in response to tissue damage, infection, inflammation, and some medications. IL-6 is secreted by T cells and macrophages to stimulate the immune response to trauma or other tissue damage leading to inflammation; it has both proinflammatory and anti-inflammatory actions. Cytokines are thought to stimulate hepatic amino acid uptake and protein synthesis, accelerate muscle breakdown, and induce gluconeogenesis. IL-1 appears to have a major role in stimulating the acute-phase response. The vagus nerve helps to regulate cytokine production through a “cholinergic anti-inflammatory pathway,” releasing nicotinic acetylcholine receptor alpha 7 to reduce excessive cytokine activity (Galloswitsch-Puerta and Tracey, 2005).
As part of the acute-phase response, serum iron and zinc levels decrease, and levels of ceruloplasmin increase, primarily because of sequestration and, in the case of zinc, increased urinary zinc excretion. The net effect of the hormonally and cell-mediated response is an increase in oxygen supply and a greater availability of substrates for metabolically active tissues.
Starvation Versus Stress
The metabolic response to critical illness is very different from simple or uncomplicated starvation, in which loss of muscle is much slower in an adaptive response to preserve lean body mass. Stored glycogen, the primary fuel source in early starvation, is depleted in approximately 24 hours. After the depletion of glycogen, glucose is available from the breakdown of protein to amino acids, depicted in Figure 39-2. The depressed glucose levels lead to decreased insulin secretion and increased glucagon. During the adaptive state of starvation, protein catabolism is reduced, and hepatic gluconeogenesis decreases.
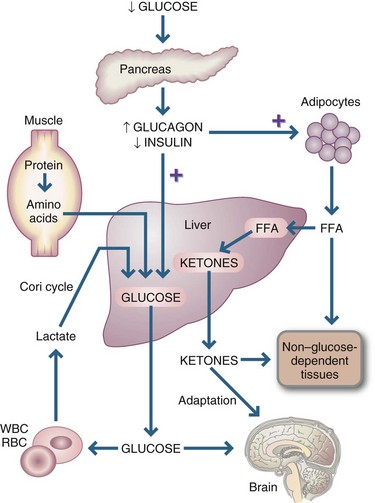
FIGURE 39-2 Metabolic changes in starvation. FFAs, Free fatty acids; RBCs, red blood cells; WBCs, white blood cells. (From Simmons RL, Steed DL: Basic science review for surgeons, Philadelphia, 1992, Saunders.)
Lipolytic activity is also different in starvation and in stress. After 1 week of fasting or food deprivation, a state of ketosis—in which ketone bodies supply the bulk of energy needs, thus reducing the need for gluconeogenesis and conserving body protein to the greatest possible extent—develops. In late starvation, as in stress, ketone body production is increased, and fatty acids serve as a major energy source for all tissues except the glucose-obligated brain, nervous system, and red blood cells.
Starvation is characterized by decreased energy expenditure, diminished gluconeogenesis, increased ketone body production, and decreased ureagenesis. Conversely, energy expenditure in stress is markedly increased, as are gluconeogenesis, proteolysis, and ureagenesis. As discussed, the stress response is activated by hormonal and cell mediators—counter-regulatory hormones such as catecholamines, cortisol, and growth hormone. This mediator activation does not occur in starvation.
Systemic Inflammatory Response Syndrome and Multiple Organ Dysfunction Syndrome
Sepsis and the systemic inflammatory response syndrome (SIRS) often complicate the course of a critically ill patient. The term sepsis is used when a patient has a documented infection and an identifiable organism. Bacteria and their toxins lead to a stronger inflammatory response. Other microorganisms that lead to an inflammatory response include viruses, fungi, and parasites.
Systemic inflammatory response syndrome (SIRS) describes the widespread inflammation that can occur in infection, pancreatitis, ischemia, burns, multiple trauma, hemorrhagic shock, or immunologically mediated organ injury. The inflammation is usually present in areas remote from the primary site of injury, affecting otherwise healthy tissue. Each condition leads to release of cytokines, proteolytic enzymes, or toxic oxygen species (free radicals) and activation of the complement cascade. The American College of Chest Physicians (ACCP)–Society of Critical Care Medicine (SCCM) consensus conference definitions of sepsis are shown in Box 39-1.
A common complication of SIRS is the development of multiple organ dysfunction syndrome (MODS). The syndrome generally begins with lung failure and is followed by failure of the liver, intestines, and kidney in no particular order. Hematologic and myocardial failures usually manifest later; however, central nervous system changes can occur at any time. MODS can be primary as the direct result of injury to an organ from trauma. Examples of primary MODS include pulmonary contusion, renal failure caused by rhabdomyolysis, or coagulopathy from multiple blood transfusions. Secondary MODS occurs in the presence of inflammation or infection in organs remote from the initial injury.
Patients with SIRS and MODS are clinically hypermetabolic and exhibit high cardiac output, low oxygen consumption, high venous oxygen saturation, and lactic acidemia. Patients generally have a strong positive fluid balance associated with massive edema and a decrease in plasma protein concentrations.
Multiple hypotheses have been proposed to explain the development of SIRS or MODS. In some studies, SIRS leading to MODS appears to be mediated by excessive production of proinflammatory cytokines and other mediators of inflammation. The gut hypothesis suggests that the trigger is injury or disruption of the gut barrier function, with corresponding translocation of enteric bacteria into the mesentery lymph nodes, liver, and other organs. Unique gut-derived factors carried in the intestinal lymph but not the portal vein usually lead to acute injury- and shock-induced SIRS and MODS (Deitch et al., 2006).
Shock results in gut hypoperfusion; the hypoperfused gut is a source of proinflammatory mediators. Early gut hypoperfusion causes an ileus or lack of peristalsis in both the stomach and small bowel, and late infections cause further worsening of this gut dysfunction. Early enteral feeding is thought to restore gut function and influence the clinical course. The mechanism for this effect is due to the enhanced functional and structural integrity of the gut (Society of Critical Care Medicine [SCCM] and American Society for Parenteral and Enteral Nutrition [A.S.P.E.N., 2009).
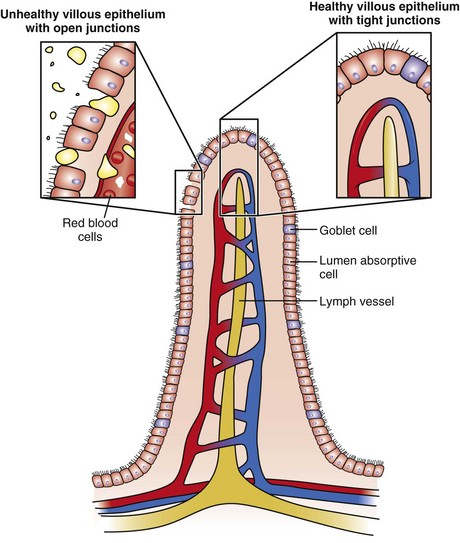
Enteral nutrition (EN) may have a role in maintaining tight junctions between the intraepithelial cells, stimulating blood flow and inducing the release of trophic factors (Figure 39-3). Maintenance of villous height supports the secretory immunocytes that make up the gut-associated lymphoid tissue (Kang & Kudsk, 2007). With central parenteral nutrition (PN), mucosal atrophy and a loss of epithelial barrier function (EBF) may occur. A rise in interferon gamma and decline in IL-10 contribute to the loss of EBF in animal models along with a dramatic decline in the expression of tight junction and adherens junction proteins (Yang et al., 2009). Studies in animals suggest that glutamine added to the parenteral solution may protect the EBF (Nose et al., 2010).
Malnutrition: The Etiology-Based Definition
The historical approach to defining malnutrition in the patient undergoing the stress response has recently been reevaluated. In an effort to provide consistency in its definition, an international group of nutrition support leaders developed an etiology basis for the definition of malnutrition for hospitalized adult patients. This approach focuses on the following three etiologies: starvation-related malnutrition, chronic disease–related malnutrition, or acute disease–related malnutrition (Figure 39-4). The latter category includes those patients experiencing SIRS and MODS and is characterized by a heightened cytokine response which in turn leads to profound losses in fat-free mass. Of note, in this setting, despite adequate provision of nutrition support therapy, repletion of lean body mass cannot occur (Jensen et al., 2009; Jensen et al., 2010).
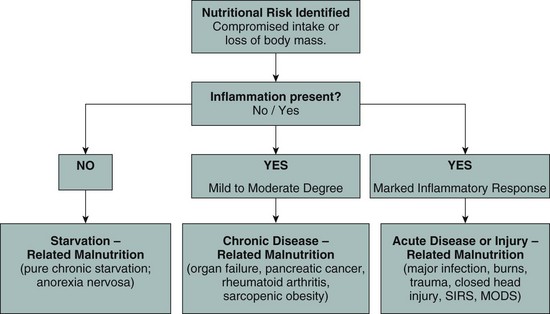
FIGURE 39-4 Diagram of Malnutrition Definitions. (Adapted from Jensen GL et al: Malnutrition syndromes: a conundrum versus continuum, JPEN J Parenter Enteral Nutr 33:710, 2009; and Jensen G et al: Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee, JPEN J Parenter Enteral Nutr 34:156, 2010.)
Medical Nutrition Therapy
The critically ill patient typically enters an intensive care unit (ICU) because of a cardiopulmonary diagnosis, intraoperative or postoperative complication, multiple trauma, burn injury, or sepsis. Traditional methods of assessing nutritional status are often of limited value in the ICU setting. The severely injured patient is usually unable to provide a dietary history. Values for weight may be erroneous after fluid resuscitation, and anthropometric measurements are not easily attainable, nor are they sensitive to acute changes. Hypoalbuminemia reflects severe illness, injury, and inflammation; thus serum albumin should not be used as a marker of nutritional status. Other plasma proteins such as prealbumin and transferrin often drop precipitously, related not to nutrition status but to an inflammation-induced decrease in hepatic synthesis and changes caused by compartmental shifts in body fluid. This is part of the acute-phase response in which secretory and circulating proteins are altered in response to inflammation or injury.
The critical role of physical assessment cannot be overlooked. Loss of lean body mass and accumulation of fluid are common to the ICU patient, and the ability to recognize these, as well as other important physical parameters is essential. In general, assessment focuses on the preadmission, preoperative, or preinjury nutrition status; presence of any organ system dysfunction; the need for early nutrition support therapy; and options that exist for enteral or parenteral access. Care planning should consider these factors. When monitoring critically ill patients, one must focus on laboratory data, not to define or determine nutrition status but to design the nutrition prescription (see Chapter 14).
Because the patient is so ill, oral intake of food or fluid may be severely limited. Therefore some common nutrition diagnoses include:
• Inadequate oral food and beverage intake (requiring other mode of nutrient or fluid administration)
• Inadequate or excessive intake from EN or PN infusion (for body requirements in nonambulatory patient)
• Inappropriate infusion of EN or PN (for example, using PN when EN is possible)
• Inadequate or excessive fluid intake (from intravenous [IV] infusions, nutrient solutions, tube flushes)
• Increased nutrient needs (such as protein for wound healing)
• Excessive carbohydrate intake (as when giving a parenteral solution to a chronically malnourished patient, with potential for refeeding syndrome)
• Abnormal nutrition-related laboratory values
• Altered gastrointestinal (GI) function (such as vomiting, diarrhea, constipation).
Nutrition Support Therapy
Nutrition support therapy incorporates early EN when feasible, appropriate macro- and micronutrient delivery, and glycemic control. Favorable expected outcomes from these practices include reduced disease severity, decreased length of time in the ICU, and decreased infectious morbidity and overall mortality.
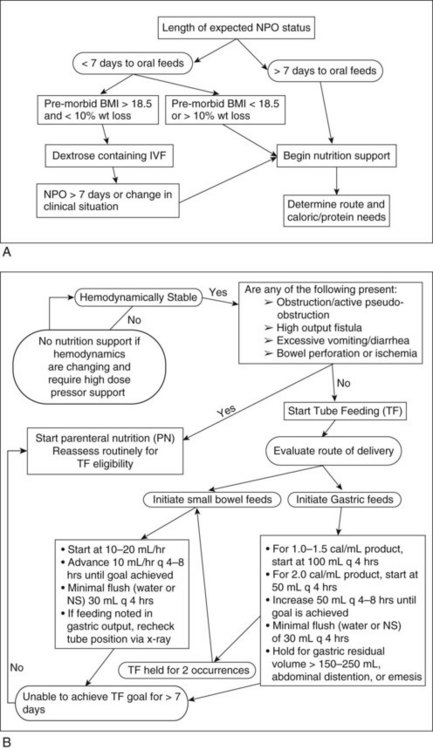
The traditional goals of nutrition support therapy during sepsis and after injury include minimization of starvation, prevention or correction of specific nutrient deficiencies, provision of adequate calories to meet energy needs while minimizing associated metabolic complications, and fluid and electrolyte management to maintain adequate urine output and normal homeostasis (see Pathophysiology and Care Management Algorithm: Hypermetabolic Response).
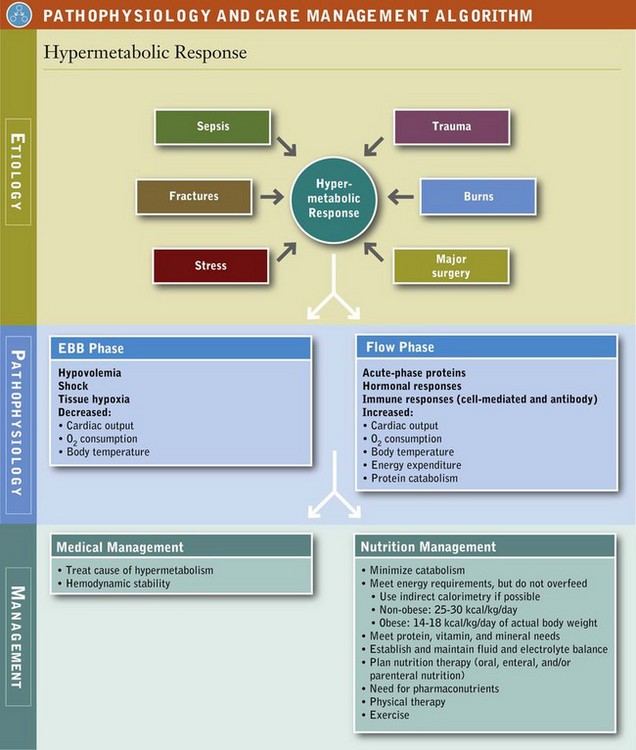
Today, goals focus more on attempting to attenuate the metabolic response to stress, preventing oxidative cellular injury, and modulating the immune response (SCCM and A.S.P.E.N., 2009). The first emphasis of care in the ICU is establishing hemodynamic stability (maintenance of airway and breathing, adequate circulating fluid volume and tissue oxygenation, and acid-base neutrality). It is important to follow the patient’s heart rate, blood pressure, cardiac output, mean arterial pressure, and oxygen saturation to assess hemodynamic stability and whether nutrition support therapy can commence.
Glycemic control and its relationship to improved outcomes has been the focus of extensive study. It is now recognized that more moderate (150-180 mg/dL), rather than tight (80-110 mg/dL), control is associated with positive outcomes in critically ill patients (American Dietetic Association, 2010). Dietitians must recognize the significant contribution of dextrose in PN formulas and its influence on glycemic control.
Nutritional Requirements
Energy: Ideally, indirect calorimetry (IC) should be used to determine energy requirements for critically ill patients. Oxygen consumption is an essential component in the determination of energy expenditure. Septic and trauma patients have substantial increases in energy expenditure associated with the magnitude of injury. IC can be performed serially as a patient’s clinical status changes (Compher et al., 2006); this allows a more accurate assessment of energy requirements during a patient’s stay in the ICU (see Chapter 2). IC is not appropriate for all patients, however, and should be performed and interpreted by experienced clinicians (Compher et al., 2006). High oxygen requirements, the presence of a chest tube, acidosis, and the use of supplemental oxygen are factors that may produce invalid results. In these situations measurement of energy expenditure by IC is not recommended (Malone, 2002).
In the absence of a metabolic cart for IC, energy requirements may be calculated as 25-30 kcal/kg/day (SCCM and A.S.P.E.N., 2009) or by using one of the many published predictive equations (see Chapter 2). Avoidance of overfeeding in the critically ill patient is important. Although adequate energy is essential for metabolically stressed patients, excess calories can result in complications such as hyperglycemia, hepatic steatosis, and excess carbon dioxide production, which can exacerbate respiratory insufficiency or prolong weaning from mechanical ventilation.
The amount of energy to provide critically ill obese patients is of current interest. Improved glycemic control and positive clinical outcomes occur in obese patients provided with 22 kcal/kg/day of ideal weight in conjunction with increased protein (Choban and Dickerson, 2005). There is some debate in practice as to what value should be used for weight in predictive equations. Actual body weight is a better predictor of energy expenditure than ideal body weight in obese individuals (Breen and Ireton-Jones, 2004). A recent evidence analysis review found the Penn State Equation, using actual body weight, to be 70% accurate in predicting energy expenditure if IC is unavailable (Frankenfield et al., 2007).
Research suggests that hypocaloric, high-protein nutrition support therapy or “permissive underfeeding” in critically ill obese patients results in achievement of net protein anabolism and minimizes complications resulting from overfeeding. Dickerson (2005) summarized a review of studies using hypocaloric specialized nutrition support in obese patients in the ICU. Although there is no agreement as to what constitutes hypocaloric feeding, studies suggest that this approximates 14-18 kcal/kg/day of actual body weight or 22 kcal/kg/day of ideal body weight (SCCM and A.S.P.E.N., 2009). More research is needed to validate hypocaloric feeding as the standard approach to nutrition support in obese patients, especially because of the wide variability in body composition (Port and Apovian, 2010).
Protein: Determination of protein requirements is difficult for critically ill patients. Patients typically require 1.2-2 g/kg/day depending on their baseline nutritional status, degree of injury and metabolic demand, and abnormal losses (e.g., through open abdominal wounds or burned skin) (SCCM and A.S.P.E.N., 2009). Administration of excessive amounts of protein will not decrease the characteristic net negative nitrogen balance seen among hypermetabolic patients.
Vitamins, Minerals, and Trace Elements: No specific guidelines exist for the provision of vitamins, minerals, and trace elements in metabolically stressed individuals. Micronutrient needs are elevated during acute illness because of increased urinary and cutaneous losses and diminished GI absorption, altered distribution, and altered carrier protein concentrations. With increased caloric intake there may be an increased need for B vitamins, particularly thiamin and niacin. Catabolism and loss of lean body tissue increase the loss of potassium, magnesium, phosphorus, and zinc. GI and urinary losses, organ dysfunction, and acid-base imbalance necessitate that mineral and electrolyte requirements be determined and adjusted individually. Fluid and electrolytes should be provided to maintain adequate urine output and normal serum electrolytes.
Feeding Strategies: The preferred route for nutrient delivery is an oral diet. However, critically ill patients are often unable to eat because of endotracheal intubation and ventilator dependence. Furthermore, oral feeding may be delayed by impairment of chewing, swallowing, by anorexia induced by pain-relieving medications, or by posttraumatic shock and depression. Patients who are able to eat may not be able to meet the increased energy and nutrient requirements associated with metabolic stress and recovery. They often require combinations of oral nutritional supplements, enteral tube nutrition, and PN. When EN fails to meet nutritional requirements or when GI feeding is contraindicated, PN support should be initiated (Figure 39-5).
Timing and Route of Feeding: EN is the preferred route of feeding for the critically ill patient who cannot eat food and who has good intestinal function. Feedings should be initiated early within the first 24-48 hours of ICU admission and advanced toward goal during the next 48-72 hours (SCCM and A.S.P.E.N., 2009). Intake of 50% to 65% of goal calories during the first week of hospitalization is thought to be sufficient to achieve the clinical benefit of EN. This practice is intended for patients who are hemodynamically stable. In the setting of hemodynamic instability (large volume requirements or use of high-dose catecholamine agents), tube feeding should be withheld until the patient is fully resuscitated or stable to minimize risk of ischemic or reperfusion injury (see Figure 39-5).
Either gastric or small-bowel feedings can be used. Small-bowel feedings are indicated when gastric residuals exceed 250 mL. Nasoenteric or surgically placed feedings tubes can be placed intraoperatively for patients with severe head, major thoracic, or spinal injury; facial injury requiring jaw wiring; proximal gastric or esophageal injuries, and major pancreatic or duodenal injury; and severe trauma with plans for repeated surgeries.
Enteral tolerance should be monitored by assessing level of pain, presence of abdominal distention, passage of flatus and stool, physical examination and, if appropriate, abdominal x-ray examination. Steps to reduce aspiration risk should be implemented, including elevating the head of the bed and use of promotility agents. The cause of diarrhea, when present, should be determined. Patients should be evaluated for intake of hyperosmolar medications and broad-spectrum antibiotics, and should be assessed for infectious diarrhea.
PN is indicated for patients in whom EN is unsuccessful or contraindicated. Supplemental PN is appropriate after 7-10 days of enteral feeding in situations in which goal requirements cannot be met (SCCM and A.S.P.E.N., 2009). For patients with preexisting malnutrition, PN should be used within 5-7 days of surgery and continue into the postoperative period.
Formula Selection: Choosing an enteral product should be based on fluid, energy, and nutrient requirements, as well as GI function. Most standard polymeric enteral formulas can be used to feed the critically ill patient. Some critically ill patients demonstrate intolerance to standard diets because of the fat content of the formula and temporarily require a lower-fat diet or a product containing a higher ratio of medium-chain triglycerides. Several commercially available products are marketed specifically for patients with trauma and metabolic stress. These products typically have higher protein content and a higher ratio of BCAAs or additional glutamine or arginine.
Immune modulating enteral formulations that contain arginine, glutamine, nucleic acids, antioxidants, and ω-3 fatty acids have potential beneficial effects and favorable outcomes for critically ill patients who have undergone GI surgery, as well as for trauma and burn patients. These formulations should not be routinely used for ICU patients with sepsis because they may worsen the inflammatory response (SCCM and A.S.P.E.N., 2009). Insoluble fiber should be avoided in critically ill patients; however, soluble fiber may be beneficial for the hemodynamically stable, critically ill patient who develops diarrhea (SCCM and A.S.P.E.N., 2009). Patients at high risk for bowel ischemia should not receive fiber-containing formulas or diets.
Trauma and the Open Abdomen
Following major abdominal trauma, bowel distention, and states of shock, some patients experience increased intraabdominal pressure leading to hypoperfusion and ischemia of the intestines and other peritoneal and retroperitoneal structures. Abdominal compartment syndrome occurs when there is increased intraabdominal pressure, often following major abdominal trauma or sepsis. This condition has profound consequences, including hemodynamic instability and respiratory, renal, and neurologic abnormalities. Because the abdominal cavity has become too small, management consists of emergent decompressive laparotomy to relieve the intraabdominal pressure (Walker and Criddle, 2003). Closure of the abdomen is not performed and instead a temporary sterile dressing may be applied.
Patients with an open abdomen have severe metabolic alterations, increased loss of fluids, and elevated nutritional requirements. The open abdomen may also be a significant source of protein loss depending on the amount of drainage (Cheatham et al., 2007). There has been some controversy as to whether patients with an open abdomen can be enterally fed. As long as the patient is hemodynamically stable and does not require large-volume fluid resuscitation or increasing doses of pressor agents, enteral feeding should be possible (Byrnes et al., 2010; Collier et al., 2007; Dissanaike et al., 2008). Ideally, a nasojejunal feeding tube should be positioned at the time of surgery to facilitate early EN support therapy.
Management of intestinal fistulas and large draining wounds are also challenging both surgically and nutritionally. These patients have metabolic abnormalities associated with losses of fluid, electrolytes, and nutrients. The priorities for management of intestinal fistulas are to restore blood volume, replace fluid and electrolyte losses, treat sepsis, control fistula drainage, protect the surrounding skin, and provide optimal nutrition support therapy. The use of PN has decreased mortality associated with fistulas and is associated with spontaneous fistula closure; however, these same outcomes are possible with EN if a feeding tube can be placed through or distal to the fistula site. See Chapter 14.
Major Burns
Major burns result in severe trauma. Energy requirements can increase as much as 100% above resting energy expenditure (REE), depending on the extent and depth of the injury (Figure 39-6). Exaggerated protein catabolism and increased urinary nitrogen excretion accompany this hypermetabolism. Protein is also lost through the burn wound exudate. Burn patients are particularly susceptible to infection, and this markedly increases requirements for both energy and protein. Because patients with major burns may develop an ileus and are anorexic, nutrition support therapy can be a real challenge. In children, healing after burn and trauma requires not only restoration of oxygen delivery and adequate calories to support metabolism and repair, but awareness of how children differ from adults in metabolic rate, growth requirements, and physiological response (Cook and Blinman, 2010).
Medical Management
Fluid and Electrolyte Repletion
The first 24 to 48 hours of treatment for thermally injured patients are devoted to fluid resuscitation. Several formulas have been developed to calculate the volume of resuscitation fluid needed. These formulas are based on the physiologic response of the body to thermal injury and are a good starting point for resuscitation. Generally half of the calculated volume for the first 24 hours is given during the first 8 hours following burn injury and the remaining half in the next 16 hours. Urine output is used to titrate the rate of IV fluid replacement.
The volume of fluid needed is based on the age and weight of the patient and the extent of the injury designated by percentage of total body surface area (TBSA) burned. Once resuscitation is complete, ample fluids must be given to cover both maintenance requirements and evaporative losses that continue through open wounds. Evaporative water loss can be estimated at 2 to 3.1 mL/kg of body weight per 24 hours per percent of TBSA burn. Serum sodium, osmolar concentrations, and body weight are used to monitor fluid status. Providing adequate fluids and electrolytes as soon as possible after injury is paramount for maintaining circulatory volume and preventing ischemia.
Wound Management
Wound management depends on the depth and extent of the burn. Current surgical management promotes use of topical antimicrobial agents and biologic and synthetic dressings, early debridement, excision, and grafting. Energy expenditure may be reduced slightly by the practice of covering wounds as early as possible to reduce evaporative heat and nitrogen losses and prevent infection.
Ancillary Measures
Passive and active range of motion exercises should be started early in the hospital to prevent contracture formation. Physical and occupational therapy helps maintain function and prevents muscle wasting and atrophy. A warm environment minimizes heat loss and the expenditure of energy to maintain body temperature. Thermal blankets, heat lamps, and individual heat shields are often used to maintain environmental temperature near 30° C (86° F). Minimizing fear and pain with reassurance from the staff and adequate pain medication can also reduce catecholamine stimulation and help avoid increases in energy expenditure. Treatments such as biofeedback, guided imagery, and good sleep hygiene are helpful. Finally, antacids are given to patients with major burns to prevent formation of stress-related Curling ulcers in the gastric or duodenal mucosa.
Medical Nutrition Therapy
A burn patient has greatly accelerated metabolism and needs increased energy, carbohydrates, proteins, fats, vitamins, minerals, and antioxidants to heal and prevent detrimental sequelae (Chan and Chan, 2009). A healthy liver is also essential. Hepatic acute phase proteins are strong predictors for postburn survival through their roles in gluconeogenesis, glycogenolysis, lipolysis, and proteolysis (Jeschke, 2009).
The goals of nutrition support therapy following major burn injury include provision of adequate calories to meet energy needs while minimizing associated metabolic complications, prevention or correction of specific nutrient deficiencies, and fluid and electrolyte management for adequate urine output and normal homeostasis. Adequate surgical care, infection control, and nutrition should be available as soon as possible after the burn (Dylewski et al., 2010). Delays in admission to an organized burn unit can be detrimental, especially for children, because malnutrition is a common concern.
Achievement of enteral access and provision of a sufficient volume of enteral nutrients early in the hospital course of a critically ill burned patient affords an opportunity to improve the outcome of that patient. Enteral feeding provides a conduit for the delivery of immune stimulants and serves as effective prophylaxis against stress-induced gastropathy and GI hemorrhage. Tube placement beyond the stomach into the small bowel in hypermetabolic, severely ill patients prone to ileus and disordered gut motility may aid delivery of enteral nutrients while reducing risk of aspiration. Placement of enteral tubes during surgery has been practiced at some burn centers in an effort to minimize the length of time a burn patient is without nutrition support therapy. See Box 39-2 for the nutritional care goals for the burned person.
Energy
Increased energy needs of the burned patient vary according to the size of the burn with severe injuries often approaching two times predicted energy expenditure. Once a burn exceeds 50% to 60% TBSA, minimum increases in energy expenditure do not usually occur. Measuring energy expenditure via indirect calorimetry is the most reliable method for assessing energy expenditure in burned patients. Increasing energy requirements by 20% to 30% is necessary to account for energy expenditure associated with wound care and physical therapy. The Ireton-Jones equation is frequently used for assessing energy expenditure in the burned patient because it accounts for burn injury and ventilatory status (Ireton-Jones and Jones, 2002) (Box 39-3).
Additional calories may be required to meet the needs because of fever, sepsis, multiple traumas, or the stress of surgery. Although weight gain may be desirable for the severely underweight patient, this is generally not feasible until acute illness has resolved. Generally, caloric goals should not exceed more than 2 times the REE.
Weight maintenance should be the goal for overweight patients until the healing process is complete. Obese individuals may be at higher risk of wound infection and graft disruption. The energy requirement for the obese burned person is probably more than that calculated when ideal body weight is used but less than that calculated when actual body weight is used. IC is the most accurate method of determining the energy needs of the obese person who is burned.
Protein
The protein needs of burned patients are elevated because of losses through urine and wounds, increased use in gluconeogenesis, and wound healing. Recent evidence promotes the feeding of high amounts of protein. Providing 20% to 25% of total calories as protein of high biologic value is also recommended.
The adequacy of energy and protein intake is best evaluated by monitoring wound healing, graft take, and basic nutrition assessment parameters. Wound healing or graft take may be delayed if weight loss exceeds 10% of the usual weight. An exact evaluation of weight loss may be difficult to obtain because of fluid shifts or edema or because of differences in the weights of dressings or splints. The coordination of weight measurement with dressing changes or hydrotherapy may allow recording of a weight without dressings and splints (Mayes and Gottschlich, 2003). Generally the fluid gained during the resuscitation period is lost within 2 weeks. Trends in weight change can then be identified.
Nitrogen balance often is used to evaluate the efficacy of a nutritional regimen, but it cannot be considered accurate without accounting for wound losses, which is difficult to accomplish in a clinical setting. Nitrogen excretion should begin to decrease as wounds heal or are grafted or covered. However, serum albumin levels usually remain depressed until major burns are healed. Proteins with shorter half-lives such as prealbumin, retinol-binding protein, and transferrin help to assess the resolution of the inflammatory response and the adequacy of nutrition support therapy of burn patients (see Chapter 8).
Micronutrients and Antioxidants
Vitamin needs generally increase for burn patients, but exact requirements have not been established. Supplements may be needed for patients who are eating food; however, most patients who receive tube feeding or PN receive amounts of vitamins in excess of the dietary reference intakes because of the high calorie intake. Vitamin C is involved in collagen synthesis and immune function and may be required in increased amounts for wound healing. Doses of 500 mg twice daily are the routine protocol at some burn centers (Mayes and Gottschlich, 2003). Vitamin A is also an important nutrient for immune function and epithelialization. Provision of 5000 units of vitamin A per 1000 calories of EN is often recommended (Mayes and Gottschlich, 2003).
Electrolyte imbalances that involve serum sodium or potassium are usually corrected by adjusting fluid therapy. Hyponatremia may be seen in patients whose evaporative losses are reduced drastically by the application of dressings or grafts; who have had changes in maintenance fluids; or who have been treated with silver nitrate soaks, which tend to draw sodium from the wound. Restricting the oral consumption of free water and sodium-free fluids may help correct hyponatremia. Hypokalemia often occurs after the initial fluid resuscitation and during protein synthesis. Slightly elevated serum potassium may indicate inadequate hydration.
Depression of serum calcium levels may be seen in patients with burns that involve more than 30% TBSA. Hypocalcemia often accompanies hypoalbuminemia. Calcium losses may be exaggerated if the patient is immobile or being treated with silver nitrate soaks. Early ambulation and exercise should help minimize these losses. Administration of calcium supplements may be necessary to treat symptomatic hypocalcemia.
Hypophosphatemia has also been identified in patients with major burns. This occurs most commonly in patients who receive large volumes of resuscitation fluid along with parenteral infusion of glucose solutions and large amounts of antacids for stress ulcer prophylaxis. Serum levels need to be monitored, and appropriate phosphate supplementation provided. Magnesium levels may also require attention because a significant amount of magnesium can be lost from the burn wound. Supplemental phosphorus and magnesium are often given parenterally to prevent GI irritation.
A depressed serum zinc level has been reported in burn patients, but whether this represents total body zinc nutriture or is an artifact of hypoalbuminemia is unclear, because zinc is bound to serum albumin. Zinc is a cofactor in energy metabolism and protein synthesis. Supplementation with 220 mg of zinc sulfate is appropriate (Mayes and Gottschlich, 2003). The anemia initially seen following a burn is usually unrelated to iron deficiency and is treated with packed red blood cells.
Methods of Nutrition Support Therapy
Methods of nutrition support therapy need to be implemented on an individual basis. Most patients with burns of less than 20% TBSA are able to meet their needs with a regular high-calorie, high-protein oral diet. Often the use of concealed nutrients such as protein added to puddings, milks, and gelatins is helpful because consuming large volumes of foods can be overwhelming to the patient. Patients should have immediate access to food and fluids at the bedside. They should be encouraged to consume calorically dense, high-protein drinks. Involving family and caregivers during mealtimes helps to promote good oral intake. Research is needed to identify ideal timing and forms of nutrition for critically ill infants and children, as this is currently not available (Joffe et al., 2009).
Patients with major burns, elevated energy expenditure, or poor appetites may require tube feeding or PN. Enteral feeding is the preferred method of nutrition support therapy for burn patients, but PN may be necessary with early excision and grafting to avoid the frequent interruptions of tube feeding required for anesthesia. Because ileus is often present only in the stomach, severely burned patients can be successfully fed by tube into the small bowel. PN may be needed for patients with persistent ileus who do not tolerate tube feedings or who have a high risk of aspiration. With careful monitoring, central lines for PN can be maintained through burn wounds. See Chapter 14.
Surgery
The delivery of correctly formulated and safely administered nutritional and metabolic support is a matter of life or death in surgical and critical care units; obese patients have a higher surgical risk (Blackburn et al., 2010). Although surgical morbidity correlates best with the extent of the primary disease and the nature of the operation performed, malnutrition may also compound the severity of complications. A well-nourished patient usually tolerates major surgery better than a severely malnourished patient. Malnutrition is associated with a high incidence of operative complications, morbidity, and death. If a malnourished patient is expected to undergo major upper GI surgery and EN is not feasible, PN should be initiated 5-7 days preoperatively and continued into the postoperative period if the duration of therapy is anticipated to be longer than 7 days (SCCM and A.S.P.E.N., 2009). See Chapter 14.
Medical Nutrition Therapy
The routine practice of ordering that a patient take nothing by mouth (NPO) at midnight prior to surgery has been discontinued in many settings. The American Society of Anesthesiologists historically recommended withholding solids for 6 hours preoperatively and clear liquids for 2 hours prior to induction of anesthesia. This practice was intended to minimize aspiration and regurgitation, but two Cochrane reviews suggest that patients may be allowed to take fluids up until a few hours before surgery without causing increased morbidity (Brady et al., 2003; Brady et al., 2009). The use of a carbohydrate-rich beverage in the preoperative period has been shown to enhance glycemic control; and decrease losses of nitrogen, lean body mass, and muscle strength following abdominal and colorectal surgery (Svanfeldt et al., 2007).
In emergency patients, preoperative fasting is not possible and surgery should be timed according to urgency; patients are treated as if the stomach is full (Søreide and Ljundqvist, 2006).
Postoperative Nutrition Care
Postoperative patients who are critically ill and in the ICU should receive early EN unless there is an absolute contraindication (SCCM and A.S.P.E.N., 2009). This practice following major GI surgery is associated with reduced infection and decreased hospitalization (Lewis et al., 2009). The use of immune-enhanced formulas is associated with a decrease in wound complications in patients who have undergone GI surgery (Mizock, 2010). If oral feeding is not possible or an extended NPO period is anticipated, an access device for enteral feeding should be inserted at the time of surgery. Combined gastrostomy-jejunostomy tubes offer significant advantages over standard gastrostomies because they allow for simultaneous gastric drainage from the gastrostomy tube and enteral feeding via the jejunal tube. Studies are underway to evaluate the effect of the use of fish oil with nutrition therapy to improve surgical outcomes in older adults after major surgery; early results show promise for reducing systemic inflammation, loss of lean muscle, and weight loss (Miller et al., 2010).
The timing of introduction of solid food after surgery depends on the patient’s degree of alertness and condition of the GI tract. A general practice has been to progress over a period of several meals from clear liquids to full liquids and finally to solid foods. However, no physiologic reason exists for solid foods not to be introduced as soon as the GI tract is functioning and a few liquids are tolerated (Lewis et al., 2009). Surgical patients can be fed a regular solid-food diet rather than a clear liquid diet.
American Society for Parenteral and Enteral Nutrition A.S.P.E.N.
http://www.burnsurgery.com/Modules/burnmetabolism/pt2/index_nutrition.htm
http://www.surgical-tutor.org.uk/default-home.htm?core/ITU/nutrition.htm~right
References
American Dietetic Association. Critical illness: glucose control. Evidence-analysis library. Accessed 25 October 2010 from http://www.adaevidencelibrary.com/topic.cfm?cat=4083&auth=1.
A.S.P.E.N. Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patients. JPEN J Parenter Enteral Nutr. 2009;33:277.
Blackburn, GL. Nutrition support in the intensive care unit: an evolving science. Arch Surg. 2010;145:533.
Brady Met al: Preoperative fasting for adults to prevent perioperative complications, Cochrane Database Syst Rev 4:CD004423, 2003.
Brady Met al: Preoperative fasting for preventing perioperative complications in children, Cochrane Database Syst Rev Oct 7(4):CD005285, 2009.
Breen, H, Ireton-Jones, C. Predicting energy needs in obese patients. Nutr Clin Pract. 2004;19:284.
Byrnes, MC, et al. Early enteral nutrition can be successfully implemented in trauma patients with an “open abdomen,”. Am J Surg. 2010;199:359.
Chan, MM, Chan, GM. Nutritional therapy for burns in children and adults. Nutrition. 2009;25:261.
Cheatham, ML, et al. Nitrogen balance, protein loss, and the open abdomen. Crit Care Med. 2007;35:127.
Choban, PS, Dickerson, RN. Morbid obesity and nutrition support: is bigger different? Nutr Clin Pract. 2005;20:480.
Collier, B, et al. Feeding the open abdomen. JPEN J Parenter Enteral Nutr. 2007;31:410.
Compher, C, et al. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881.
Cook, RC, Blinman, TA. Nutritional support of the pediatric trauma patient. Semin Pediatr Surg. 2010;19:242.
Deitch, EA, et al. Role of the gut in the development of injury- and shock-induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520.
Dickerson, RN. Hypocaloric feeding of obese patients in the intensive care unit. Curr Opin Clin Nutr Metabol Care. 2005;8:189.
Dissanaike, S, et al. Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg. 2008;207:690.
Dylewski, ML, et al. Malnutrition among pediatric burn patients: a consequence of delayed admissions. Burns. 2010;36:1185.
Frankenfield, D, et al. Prediction of resting metabolic rate in critically ill adult patients: results of a systematic review of the evidence. J Am Diet Assoc. 2007;107:1552.
Galloswitsch-Puerta, M, Tracey, KJ. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine alpha 7 receptor. Ann NY Acad Sci. 2005;1062:209.
Ireton-Jones, C, Jones, JD. Improved equations for predicting energy expenditure in patients: the Ireton-Jones equations. Nutr Clin Pract. 2002;17:29.
Jensen, GL, et al. Malnutrition syndromes: a conundrum versus continuum. JPEN J Parenter Enteral Nutr. 2009;33:710.
Jensen, G, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34:156.
Jeschke, MG. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med. 2009;15:337.
Joffe A, et al: Nutritional support for critically ill children, Cochrane Database Syst Rev Apr 15(2):CD005144, 2009.
Kang, W, Kudsk, KA. Is there evidence that the gut contributes to mucosal immunity in humans? JPEN J Parenter Enteral Nutr. 2007;31:246.
Lewis, SJ, et al. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569.
Malone, AM. Methods of assessing energy expenditure in the intensive care unit. Nutr Clin Pract. 2002;17:21.
Mayes, T, Gottschlich, MM. Burns and wound healing. In Matarase LE, Gottschlich MM, eds.: Contemporary nutrition support practice: a clinical guide, ed 2, Philadelphia: Saunders, 2003.
Miller, MD, et al. A Trial Assessing N-3 as Treatment for Injury-induced Cachexia (ATLANTIC trial): does a moderate dose fish oil intervention improve outcomes in older adults recovering from hip fracture? BMC Geriatr. 2010;10:76.
Mizock, BA. Immunonutrition and critical illness: an update. Nutrition. 2010;26:701.
Nose, K, et al. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res. 2010;30:67.
Port, AM, Apovian, C. Metabolic support of the obese intensive care unit patient: a current perspective. Curr Opin Clin Nutr Metab Care. 2010;13:184.
Søreide, E, Ljungqvist, O. Modern preoperative fasting guidelines: a summary of the present recommendations and remaining questions. Best Pract Res Clin Anaesthesiol. 2006;20:483.
Svanfeldt, M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342.
Walker, J, Criddle, LM. Pathophysiology and management of abdominal compartment syndrome. Am J Crit Care. 2003;12:367.
Yang, H, et al. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann NY Acad Sci. 2009;1165:338.