Chapter 11 The placenta
The placenta is a complex organ, which originates from the trophoblastic layer of the fertilized ovum. When fully developed it serves as the interface between the mother and the developing fetus carrying out functions that the fetus is unable to perform for itself during intrauterine life. The survival of the fetus depends upon the placenta’s integrity and efficiency.
Early development
Within a few days of fertilization, the zygote (see Ch. 10) develops into a blastocyst, a spherical structure composed of two distinct cell types, the inner cell mass, which will develop into the fetus and an outer ring of trophoblast cells, which will develop into the placenta and membranes. By 8 days, the trophoblasts begin to make human chorionic gonadotrophin (hCG), a hormone that ensures that the endometrium will be receptive to the implanting embryo.
Implantation
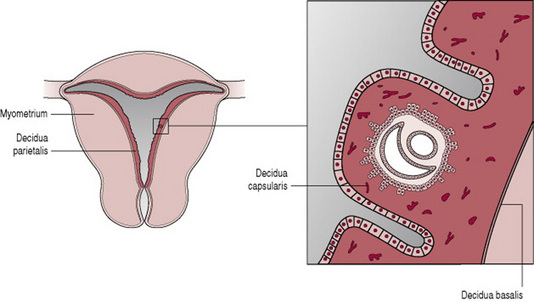
Once the blastocyst makes contact with the endometrium, the trophoblast layer adheres to the endometrial surface and the process of placentation begins (Fig. 11.1). By 10 days, the blastocyst is completely buried in the endometrium, which is now called the decidua.
The trophoblasts have a potent invasive capacity, which if left unchecked would spread throughout the uterus. This potential is moderated by the decidua, which secretes cytokines and protease inhibitors that modulate trophoblast invasion.
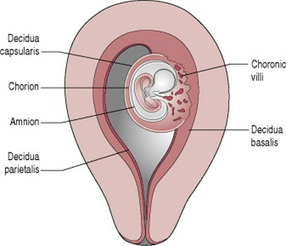
Chorionic villi
Initially the blastocyst appears to be covered by fine, downy hair, which consists of the projections from the trophoblastic layer. These proliferate and branch from about 3 weeks after fertilization, forming the chorionic villi. The villi become most profuse in the area where the blood supply is richest, the decidua basalis. This part of the trophoblast, which is known as the chorion frondosum, eventually develops into the placenta. The portion of decidua surrounding the blastocyst, where it projects into the uterine cavity, is known as the decidua capsularis. The villi under the decidua capsularis gradually degenerate forming the chorion laeve, which is the origin of the chorionic membrane (Fig. 11.2). The remaining decidua is known as the decidua parietalis. As the fetus enlarges and grows to fill the uterus the decidua capsularis thins and disappears and the chorion meets the decidua parietalis on the opposite wall of the uterus.
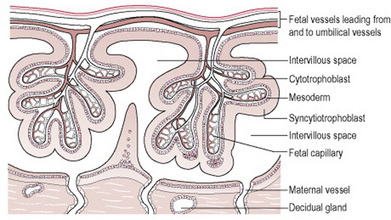
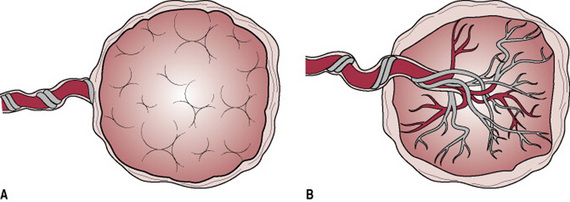
The villi erode the walls of maternal blood vessels as they penetrate the decidua, opening them up to form a lake of maternal blood in which they float. The opened blood vessels are known as sinuses, and the areas surrounding the villi as blood spaces. The maternal blood circulates slowly, enabling the villi to absorb food and oxygen and excrete waste. These are known as the nutritive villi. A few villi are more deeply attached to the decidua and are called anchoring villi.
Each chorionic villus is a branching structure arising from one stem. Its centre consists of mesoderm and fetal blood vessels, and branches of the umbilical artery and vein. These are covered by a single layer of cytotrophoblast cells and the external layer of the villus is the syncytiotrophoblast (Fig. 11.3). This means that four layers of tissue separate the maternal blood from the fetal blood making it impossible for the two circulations to mix unless any villi are damaged.
The mature placenta
The placenta is completely formed and functioning 10 weeks after fertilization. Between 12 and 20 weeks’ gestation, the placenta weighs more than the fetus because the fetal organs are insufficiently developed to cope with the metabolic processes of nutrition. Later in pregnancy, some of the fetal organs, such as the liver, begin to function, so the cytotrophoblast and the syncytiotrophoblast gradually degenerate and this allows easier exchange of oxygen and carbon dioxide.
Functions
The placenta performs a variety of functions for the developing fetus.
Respiration
During intrauterine life, no pulmonary exchange of gases can take place so the fetus must obtain oxygen and excrete carbon dioxide through the placenta. Oxygen from the mother’s haemoglobin passes into the fetal blood by simple diffusion; similarly, the fetus gives off carbon dioxide into the maternal blood.
Nutrition
The fetus needs nutrients for growth and development. For instance, amino acids are required for body building, large quantities of glucose for energy and growth, calcium and phosphorus for bones and teeth, and iron and other minerals for blood formation. These nutrients are actively transferred from the maternal to the fetal blood through the walls of the villi. The placenta is able to select those substances required by the fetus, even depleting the mother’s own supply in some instances.
Water, vitamins and minerals also pass to the fetus. Fats and fat-soluble vitamins (A, D and E) cross the placenta only with difficulty and mainly in the later stages of pregnancy. Some substances, including amino acids, are found at higher levels in the fetal blood than in the maternal blood.
Storage
The placenta metabolizes glucose, stores it in the form of glycogen and reconverts it to glucose as required. It can also store iron and the fat-soluble vitamins.
Excretion
The main substance excreted from the fetus is carbon dioxide. Bilirubin will also be excreted as red blood cells are replaced relatively frequently. There is very little tissue breakdown apart from this and the amounts of urea and uric acid excreted are very small.
Protection
The placenta provides a limited barrier to infection. Few bacteria can penetrate with the exception of the treponema of syphilis and the tubercle bacillus. However, substances including alcohol, some chemicals associated with smoking cigarettes and several types of viruses, such as human cytomegalovirus and rubella are not filtered out. These substances can cross the placental barrier freely and may cause congenital abnormalities.
Although some drugs will cross the placental barrier to the fetus, many will be harmless and others, such as antibiotics administered to a pregnant woman with syphilis, are positively beneficial. However, there are exceptions (see Ch. 49).
Towards the end of pregnancy, antibodies in the form of immunoglobulin G (IgG) are transferred across the placental barrier to the fetus conferring passive immunity on the baby for the first 3 months of extrauterine life. However, it is important to realize that only those antibodies that the mother herself possesses can be passed on.
Endocrine
Human chorionic gonadotrophin (hCG) is produced by the cytotrophoblastic layer of the chorionic villi. Initially it is present in very large quantities, peak levels being achieved between the 7th and 10th week, but these gradually reduce as the pregnancy advances. hCG forms the basis of the many pregnancy tests available, as it is excreted in the mother’s urine. Its function is to stimulate the growth and activity of the corpus luteum.
Oestrogens are growth stimulating hormones, which are secreted in large amounts throughout pregnancy. They are produced by the placenta as the activity of the corpus luteum declines, the fetus providing the placenta with the vital precursors for their production. The amount of oestrogen produced (measured as urinary or serum oestriol) is an index of fetoplacental well-being.
Progesterone is made in the syncytial layer of the placenta in increasing quantities until immediately before the onset of labour when its level falls. It may be measured in the urine as pregnanediol. The main function of progesterone is to act on tissues that have already been receptive to oestrogen.
Human placental lactogen (hPL) has a role in glucose metabolism in pregnancy. It appears to have a connection with the activity of human growth hormone, although it does not itself promote growth. As the level of hCG falls, so the level of hPL rises and continues to do so throughout pregnancy. Monitoring the level of hPL with the intention of assessing placental function has been disappointing in predicting fetal outcome.
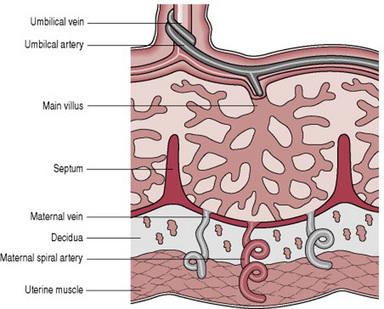
Placental circulation
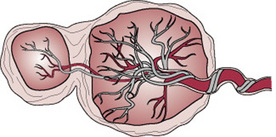
Maternal blood is discharged in a pulsatile fashion into the intervillous space by 80–100 spiral arteries in the decidua basalis. It spurts toward the chorionic plate and flows slowly around the villi, eventually returning to the endometrial veins and the maternal circulation. There are about 150 mL of maternal blood in the intervillous spaces, which is exchanged 3 or 4 times/min.
Fetal blood, low in oxygen, is pumped by the fetal heart towards the placenta along the umbilical arteries and transported along their branches to the capillaries of the chorionic villi where exchange of nutrients takes place between the mother and fetus. Having yielded up carbon dioxide and waste products and absorbed oxygen and nutrients, the blood is returned to the fetus via the umbilical vein (Fig. 11.4).
The membranes
There are two membranes, an outer membrane, the chorion, and an inner membrane, the amnion. As long as the membranes remain intact, they protect the fetus against ascending bacterial infection.
The chorion is a thick opaque friable membrane derived from the trophoblast. It is continuous with the chorionic plate, which forms the base of the placenta and adheres closely to the uterine wall.
The amnion is a smooth tough translucent membrane derived from the inner cell mass. It lines the chorion and the surface of the placenta continuing over the outer surface of the umbilical cord. When first formed the amnion is in contact with the embryo, but 4–5 weeks after conception the amniotic fluid begins to accumulate within it.
Amniotic fluid
Amniotic fluid is a clear alkaline and slightly yellowish liquid contained within the amniotic sac.
Functions
Amniotic fluid distends the amniotic sac allowing for the growth and free movement of the fetus and permitting symmetrical musculoskeletal development. It equalizes pressure and protects the fetus from jarring and injury. The fluid maintains a constant intrauterine temperature, protecting the fetus from heat loss and providing small quantities of nutrients to the fetus. In labour, as long as the membranes remain intact it protects the placenta and umbilical cord from the pressure of uterine contractions. It also aids effacement of the cervix and dilatation of the uterine os, particularly where the presenting part is poorly applied.
Origin
The source of amniotic fluid is thought to be both fetal and maternal. Some fluid is exuded from maternal vessels in the decidua and some from fetal vessels in the placenta. It is secreted by the amnion, especially the part covering the placenta and umbilical cord. Fetal urine also contributes to the volume from the 10th week of gestation onwards. The water in amniotic fluid is exchanged approximately 3-hourly.
Constituents
Amniotic fluid consists of 99% water with the remaining 1% being dissolved solid matter including food substances and waste products. In addition, the fetus sheds skin cells, vernix caseosa and lanugo into the fluid. Abnormal constituents of the liquor, such as meconium in the case of fetal compromise, may give valuable diagnostic information about the condition of the fetus. Aspiration of amniotic fluid for examination is termed amniocentesis.
Research has found that amniotic fluid is a plentiful source of non-embryonic stem cells (De Coppi et al 2007). These cells have demonstrated the ability to differentiate into a number of different cell-types, including brain, liver and bone.
Volume
During pregnancy, amniotic fluid increases in volume as the fetus grows. The volume is greatest at approximately 38 weeks’ gestation, when there is about 1 L. It then diminishes slightly until term, when approximately 800 mL remains.
There are very wide variations in the amount, however if the total amount of fluid exceeds 1500 mL, the condition is known as polyhydramnios and if less than 300 mL, oligohydramnios. Such abnormalities are often associated with congenital malformations of the fetus (see Ch. 46).
The umbilical cord
The umbilical cord, which extends from the fetal surface of the placenta to the umbilical area of the fetus, is formed by the 5th week of pregnancy. It originates from the duct that forms between the amniotic sac and the yolk sac and transmits the umbilical blood vessels.
Functions
The umbilical cord transports oxygen and nutrients to the developing fetus, and removes waste products.
Structure
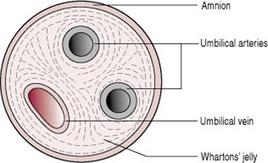
The umbilical cord contains two arteries and one vein (Fig. 11.5), which are continuous with the blood vessels in the chorionic villi of the placenta. The blood vessels are enclosed and protected by Wharton’s jelly, a gelatinous substance formed from mesoderm. The whole cord is covered in a layer of amnion that is continuous with that covering the placenta. There are no nerves in the umbilical cord, so cutting it following the birth of the baby is not painful.
The presence of only two vessels in the cord is sometimes related to abnormalities in the fetus, but may occur without accompanying abnormalities.
Measurements
The cord is approximately 1–2 cm in diameter and 40–50 cm in length. This length is sufficient to allow for the birth of the baby without applying any traction to the placenta.
A cord is considered short when it measures <40 cm. There is no specific agreed length for describing a cord as too long, but the disadvantages of a very long cord are that it may become wrapped round the neck or body of the fetus or become knotted. Either event could result in occlusion of the blood vessels, especially during labour.
Compromise of the fetal blood flow through the umbilical cord vessels can have serious deleterious effects on the health of the fetus and newborn. True knots should always be noted on examination of the cord, but they must be distinguished from false knots, which are lumps of Wharton’s jelly on the side of the cord and are not significant.
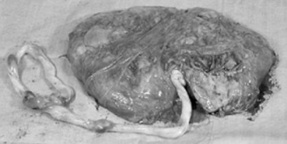
The placenta at term
At term, the placenta is a round flat mass about 20 cm in diameter and 2.5 cm thick at its centre. It weighs approximately one-sixth of the baby’s weight, although this proportion may be affected by the time at which the cord is clamped owing to the varying amounts of fetal blood retained in the vessels.
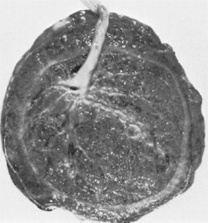
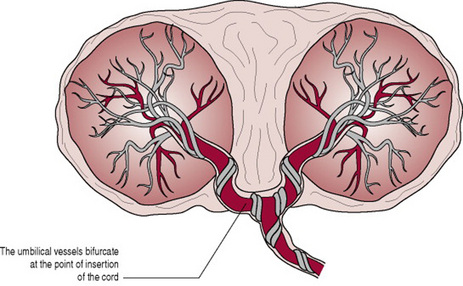
The maternal surface of the placenta is dark red in colour due to maternal blood and because part of the basal decidua will have been separated with it (Fig. 11.6A). The surface is arranged in about 20 cotyledons (lobes), which are separated by sulci (furrows), into which the decidua dips down to form septa (walls). The cotyledons are made up of lobules, each of which contains a single villus with its branches. Sometimes deposits of lime salts may be present on the surface, making it slightly gritty. This has no clinical significance.
The fetal surface of the placenta has a shiny appearance due to the amnion covering it (Fig. 11.6B). Branches of the umbilical vein and arteries are visible, spreading out from the insertion of the umbilical cord, which is normally in the centre. The amnion can be peeled off the surface of the chorion as far back as the umbilical cord, whereas the chorion, being derived from the same trophoblastic layer as the placenta, is continuous with the chorionic plate and cannot be separated from it.
Anatomical variations of the placenta and cord
A succenturiate lobe of placenta is the most significant of the variations in conformation of the placenta. A small extra lobe is present, separate from the main placenta, and joined to it by blood vessels that run through the membranes to reach it (Fig. 11.7). The danger is that this small lobe may be retained in utero after the placenta is delivered, and if it is not removed, it may lead to infection and haemorrhage. Every placenta must be examined for evidence of a retained succenturiate lobe, which can be identified by a hole in the membranes with vessels running to it.
In circumvallate placenta, an opaque ring is seen on the fetal surface of the placenta. It is formed by a doubling back of the chorion and amnion and may result in the membranes leaving the placenta nearer the centre instead of at the edge as usual (Fig. 11.8). This placental variation is associated with prematurity, prenatal bleeding, abruption, multiparity and early fluid loss.
In bipartite placenta, two complete and separate parts are present, each with a cord leaving from it. The bipartite cord joins a short distance from the two parts of the placenta (Fig. 11.9). This is different from the two placentas in a twin pregnancy, where there are also two separate umbilical cords. A tripartite placenta is similar to a bipartite placenta but it has three distinct parts.
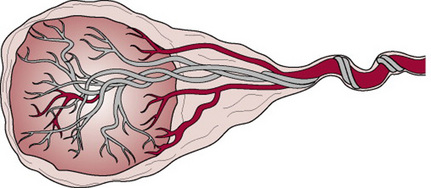
In battledore insertion of the cord, the cord is attached at the very edge of the placenta in the manner of a table tennis bat. It is unimportant unless the attachment is fragile (Fig. 11.10).
A velamentous insertion of the cord, occurs when the cord is inserted into the membranes some distance from the edge of the placenta. The umbilical vessels run through the membranes from the cord to the placenta (Fig. 11.11). If the placenta is normally situated, no harm will result to the fetus, but the cord is likely to become detached upon applying traction during active management of the third stage of labour. However if the placenta is low-lying, the vessels may pass across the uterine os (vasa praevia). In this case, there is great danger to the fetus when the membranes rupture and even more so during artificial rupture, as the vessels may be torn, leading to rapid exsanguination of the fetus. If the onset of haemorrhage coincides with rupture of the membranes, fetal haemorrhage should be assumed and the birth expedited. It is possible to distinguish fetal blood from maternal blood by Singer’s alkali-denaturation test, although, in practice, time is so short that it may not be possible to save the life of the baby. If the baby survives, haemoglobin levels should be estimated after birth.
Apart from the dangers noted above, these varieties of conformation have no clinical significance.
Coad J, Dunstall M. Anatomy and physiology for midwives, 2nd edn. Edinburgh: Churchill Livingstone, 2005.
Chapter 8 of this comprehensive text provides a detailed account of the placenta.
Johnson MH, Everitt BJ. Essential reproduction, 5th edn. Oxford: Blackwell Science, 2000.
Chapter 10 of this volume provides detail about the development of the placenta and helps the reader to a fuller understanding of its function and the interdependence of maternal and fetal systems.
Oats JK, Abraham S. Llewellyn-Jones fundamentals of obstetrics and gynaecology, 8th edn. London: Mosby, 2005.
This book has a section on the placenta (Ch. 3) that the reader may find useful.
Stables D, Rankin J. Physiology in childbearing with anatomy and related biosciences, 2nd edition. Edinburgh: Baillière Tindall, 2004.
The placental hormones are explained in detail in Section 2a and a diagram (12.2) gives a clear visual representation of different forms of placental transfer.
Standring S, Healy J, Johnson D, et al. Gray’s anatomy: The anatomical basis of clinical practice, 39th edn. New York: Churchill Livingstone, 2004.
Section 7 of this authoritative and comprehensive volume provides descriptions of the placenta and its functioning for the interested student.