Infertility
• List common causes of infertility.
• Discuss the psychosocial impact of infertility.
• Identify common diagnoses and treatments for infertility.
• Identify reproductive alternatives for infertile couples.
• Examine the various ethical and legal considerations of assisted reproductive therapies for infertility.
This chapter addresses infertility, associated tests, and common therapies. The available alternatives and the psychosocial implications of infertility are discussed.
Incidence
Infertility is a serious medical concern that affects quality of life and is a problem for 10% to 15% of reproductive-age couples (American Society for Reproductive Medicine [ASRM], 2010a; Nelson, Marshall, Trussell, Stewart, Nelson, Cates, et al., 2007). The term infertility implies subfertility, a prolonged time to conceive, as opposed to sterility, which means inability to conceive. Normally a fertile couple has approximately a 20% chance of conception in each ovulatory cycle. Primary infertility applies to a woman who has never been pregnant; secondary infertility applies to a woman who has been pregnant.
The prevalence of infertility is relatively stable among the overall population but increases with the age of the woman, particularly in those older than 40 years (Lobo, 2007). Probable causes include the trend toward delaying pregnancy until later in life, when fertility decreases naturally due to ovulatory dysfunction and the accumulated damage from diseases such as endometriosis and tubal infection (ASRM, 2010a). There is some controversy regarding whether there has been an increase in male infertility, or whether male infertility is being more readily identified because of improvements in diagnosis.
Diagnosis and treatment of infertility require considerable physical, emotional, and financial investment over an extended period. Men as well as women can experience emotional vulnerability (Burns, 2007). However, women have more stress from tests and treatments, and place greater importance on having children. In contrast, men express distress in their partner’s suffering and the resulting changes in their partnership and sexual relationship (Wischmann, Scherg, Strowitzki, & Verres, 2009).
Same-sex couples desire pregnancy and parenthood for the same reasons as do heterosexual couples, but may feel unaccepted and marginalized in health care settings. Lesbian women are at risk for higher general stress, which can be compounded by the invasive nature of fertility treatments (Weisz, 2009).
In the United States, feelings connected with infertility are many and complex. The origins of some of these feelings are myths, superstitions, misinformation, or magical thinking about the causes of infertility. Other feelings arise from the medicalization of reproduction and from a perception of being “different” from others. The attitude, sensitivity, and caring nature of those who are involved in the assessment of infertility lay the foundation for the clients’ ability to cope with the subsequent therapy and management. Couples seeking infertility treatments can benefit from instruction in stress management and coping skills (Cousineau & Domar, 2007). Health team members also must respect affected individuals’ and couples’ desires in choosing to stop treatment and to select other alternatives, such as adoption (see Clinical Reasoning box).
Factors Associated with Infertility
Many factors, both male and female, contribute to normal fertility. A normally developed reproductive tract in both the male and female partner is essential. Normal functioning of an intact hypothalamic-pituitary-gonadal axis supports gametogenesis—the formation of sperm and ova. The life spans of the sperm and the ovum are short. Although sperm remain viable in the female’s reproductive tract for 48 hours or more, probably only a few retain fertilization potential for more than 24 hours. Ova remain viable for about 24 hours, but the optimal time for fertilization may be no more than a few hours (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010); thus timing of intercourse becomes critical.
An alteration in one or more of these structures, functions, or processes results in some degree of impaired fertility. Causes of impaired fertility are sometimes difficult to assign to either the male or female. In general, about 20% of couples will have unexplained or idiopathic causes of infertility. Among the 80% of couples who have an identifiable cause of infertility, about 40% are related to factors in the female partner, 40% are related to factors in the male partner, and 20% are related to factors in both partners (Lobo, 2007; Nelson et al., 2007). Boxes 9-1 and 9-2 list factors affecting female and male infertility.
Infertility also may be caused by something as simple as poor timing or inadequate frequency of intercourse. The couple should be taught about the menstrual cycle and the ways to detect ovulation (see Chapters 4 and 8).
Female Infertility
Congenital or Developmental Factors
Congenital factors rarely cause impaired fertility. If the woman has abnormal external genitals, surgical reconstruction of abnormal tissue and construction of a functional vagina may permit normal intercourse. Vaginal and uterine anomalies and their surgical repair vary from individual to individual. If a functional uterus can be reconstructed, pregnancy may be possible.
Hormonal and Ovulatory Factors
Anovulation may be primary or secondary (see Chapter 6). Primary anovulation may be caused by a pituitary or hypothalamic hormone disorder or an adrenal gland disorder, such as congenital adrenal hyperplasia. It is usually seen in adolescents. Secondary anovulation, usually seen in young to midlife women, is relatively common and is caused by the disruption of the hypothalamic-pituitary-ovarian axis. In amenorrheic states and instances of anovulatory cycles, hormone studies usually reveal the problem.
Although relatively rare, amenorrhea after the discontinuation of oral contraceptives is seen more frequently in women with histories of menstrual dysfunction before initiation of contraceptive use. Because most women resume menstruating within 6 months, the workup should be delayed until that time in the absence of other symptoms.
Occasionally women experience menopause before they are 40 years old. In a vast majority of cases of early menopause, the ovaries do not respond to ovulation-inducing drugs. Obesity and related metabolic disorders, such as polycystic ovary syndrome (PCOS), and eating disorders also contribute to anovulation (Balen & Anderson, 2007; Leddy, Jones, Morgan & Schulkin, 2009; Malik, 2009).
An increased prolactin level may cause anovulation and amenorrhea, in the same way it does during lactation. Hyperprolactinemia can be a side effect of drugs, such as phenothiazine, opiates, diazepam, reserpine, methyldopa, and tricyclic antidepressants. Prolactin may also be elevated as a result of physical stressors such as surgery, cranial lesions, or injury, or severe emotional stress. Benign pituitary adenoma, which is diagnosed through sophisticated radiographic techniques or computed tomography (CT) scan, may also cause hyperprolactinemia.
Cancer treatments involving radiation and chemotherapy can decrease or halt ovarian function (Pauli, Berga, Shang, & Session, 2009).
Age-Related Infertility: The rate of fertility declines dramatically after the age of 35. By 40, the total number of ovarian follicles diminishes and the quality of remaining eggs is poor. Abnormalities of oocytes are thought to be the primary reason for age-associated infertility, followed by cumulative factors such as endometriosis, infection, metabolic disease, and smoking (ASRM, 2008).
Tubal/Peritoneal Factors
The motility of the tube and its fimbriated end may be reduced or absent as a result of infections, adhesions, scarring, or tumors. Chlamydial infection impairs tubal function and impedes fertility. In rare instances one tube may be congenitally absent. One tube may be relatively shorter than the other, which is often associated with an abnormally developed uterus.
Inflammation within the tube or involving the exterior of the tube or the fimbriated ends represents a major cause of impaired fertility. Tubal adhesions resulting from pelvic infections (e.g., ruptured appendix, sexually transmitted infections [STIs]) may impair fertility. When infection with purulent discharge heals, scar tissue adhesions form. In the process, the tube can be blocked anywhere along its length. It can be closed off at the fimbriated end, or it can be distorted and kinked by adhesions. Adhesions may permit the tiny sperm to pass through
the tube but may prevent a fertilized egg from completing the journey into the intrauterine cavity. This results in an ectopic pregnancy that can completely destroy the tube and be life threatening. In other cases, adhesion of the tubes to the ovary or the bowel can be caused by endometriosis (see Chapter 6). Endometriosis is more commonly seen in women who delay childbearing until they are older than 30 years. Women who have a first-degree relative with a history of endometriosis also have a slightly higher risk.
Uterine Factors
Abnormalities of the uterus are more common than might be expected. Minor developmental anomalies of the uterus are fairly common; major anomalies occur rarely. Hysterosalpingography may reveal müllerian malformations of the uterine cavity, such as bicornuate or septate uterus (Fig. 9-1) (Chalazonitis, Tzovara, Laspas, Porfyrdis, Ptohis, & Tsimitselis, 2009). Endometrial and myometrial tumors (e.g., polyps or myomas) may also be revealed by x-ray studies of infertile women. These anomalies can affect implantation and the maintenance of a pregnancy.
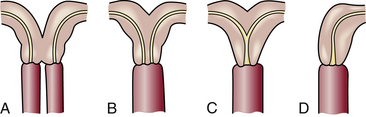
FIG. 9-1 Abnormal uterus. A, Complete bicornuate uterus with vagina divided by a septum. B, Complete bicornuate uterus with normal vagina. C, Partial bicornuate uterus with normal vagina. D, Unicornuate uterus.
Asherman syndrome (uterine adhesions or scar tissue) is characterized by hypomenorrhea. The adhesions prevent normal cyclic endometrial proliferation necessary for implantation. This can result from surgical interventions such as too vigorous curettage (scraping) after an elective abortion or miscarriage.
Endometritis (inflammation of the endometrium) may result from any of the causes of infection of the cervix or uterine tubes (e.g., Chlamydia). Women who have numerous sexual partners are more susceptible to endometrial infection than are women in monogamous relationships.
Vaginal-Cervical Factors
Vaginal fluid is acidic (pH of 4 to 5), whereas cervical mucus is normally alkaline (pH of 7 or more). Ejaculation should place the sperm at or near the cervical os. The alkalinity of cervical mucus helps support sperm and permits the ascending transportation of sperm around the time of ovulation.
Endocervical mucus normally obstructs or plugs the cervix, acting as a barrier against infection, until increasing estrogen levels cause the mucus to become clear, thin, and nutritionally supportive of sperm. This change occurs around the time of ovulation and lasts approximately 48 to 72 hours. The amount of cervical mucus and its characteristics are influenced by the hormone estrogen (see Teaching for Self-Management box, p. 173 in Chapter 8).
Vaginal-cervical infections (e.g., bacterial vaginosis) cause an increased pH (decreased acidity) of the vaginal fluid, plus the presence of white blood cells. Inflammation from vaginal infection often destroys or drastically reduces the number of viable motile sperm before they enter the cervical canal (Mania-Pramanik, Kerkar, & Salvi, 2009). The amount of mucus and its physical changes are influenced by the presence of blood, pathogenic bacteria, and irritants such as an intrauterine contraceptive device (IUD) or a polyp. Severe emotional stress, antibiotic therapy, and diseases such as diabetes mellitus alter the acidity of mucus.
Some infertile women develop sperm antibodies. The production of antibodies by one member of a species against something that is commonly found within that species is termed isoimmunization. Sperm may be immobilized or agglutinated within the cervical mucus, rendering them incapable of migration into the uterus (see Postcoital Test later in this chapter).
Male Infertility
Male infertility can be caused by structural and hormonal disorders such as undescended testes, hypospadias, varicocele (varicose veins of the scrotum), low testosterone levels, or previous vasectomy, all of which can cause azoospermia (no sperm cells produced) or oligospermia (few sperm cells produced). Mumps, especially after adolescence, can result in permanent damage to the testes.
Male infertility also may be caused by some of the same health issues that affect women, such as nutrition, endocrine disorders, genetic disorders, psychologic disorders, and STIs (ASRM, 2010a). Male obesity can lead to decreased semen quality (Shayeb & Bhattacharya, 2009). Exposure to hazards in the workplace such as radiation also can affect sperm production; exposure of the scrotum to high temperatures can both decrease and cause abnormal sperm production. Cancer treatments can decrease production or quality of sperm (Pauli et al., 2009).
Substance abuse can be a major factor in male infertility. Alcohol consumption can cause erectile problems (impotence). In addition, cigarette smoking has been associated with abnormal sperm, a decreased number of sperm, and chromosome damage. Heroin and marijuana use may depress the number and motility of sperm and increase the percentage of abnormally formed sperm. Amyl nitrate, butyl nitrate, ethyl chloride, and methaqualone (used to prolong orgasm) cause changes in spermatogenesis. Heroin, methadone, selective serotonin reuptake inhibitors (SSRIs), and barbiturates decrease libido. Monoamine oxidase inhibitors (MAOIs), a class of antidepressants, adversely affect spermatogenesis. In addition, some antihypertensives may cause impotence.
Male fertility declines slowly after age 40 years; however, no cessation of sperm production occurs analogous to menopause in women.
Care Management
The nurse assists in the assessment by obtaining data relevant to fertility through interview and physical examination. The database must include information to identify whether infertility is primary or secondary. Religious, cultural, and ethnic data are noted (Box 9-3).
Much of the data needed to investigate impaired fertility are of a sensitive, personal nature. Obtaining these data may be viewed as an invasion of privacy. The tests and examinations are occasionally painful and intrusive and can take the romance out of lovemaking. A high level of motivation is needed to endure the investigation.
Many couples have already visited various physicians and have read extensively on the subject. Their previous infertility experiences and knowledge should be explored and recorded.
Because multiple factors involving both partners are common, the investigation of impaired fertility is conducted systematically and simultaneously for male and female partners. Both partners must be interested in the solution to the problem. The medical investigation requires time (3 to 4 months) and considerable financial expense (Box 9-4), and it causes emotional distress and strain on the couple’s interpersonal relationship (ASRM, 2010b). Preparatory and concurrent counseling support is recommended (Burns, 2007) (see Nursing Process box).
Investigation of impaired fertility begins for the woman and the man with a complete history and physical examination. A complete general physical examination is followed by a specific assessment of the reproductive tract. Laboratory data are assembled. Data from routine urine and blood tests are obtained along with other diagnostic tests.
Assessment of Female Infertility
Diagnostic Tests for Female Infertility: Several examinations and tests for impaired fertility in the woman include the basic infertility survey, which involves evaluation of the cervix, uterus, tubes, and peritoneum; detection of ovulation; assessment of immunologic compatibility; and evaluation of psychogenic factors (Nelson et al., 2007). The nurse can alleviate some of the anxiety associated with diagnostic testing by explaining to clients the timing and rationale for each test (Table 9-1). Test findings that are favorable to fertility are summarized in Box 9-5.
TABLE 9-1
| TEST OR EXAMINATION | TIMING (MENSTRUAL CYCLE DAYS) | RATIONALE |
| Hysterosalpingogram | 7-10 | Late follicular, early proliferative phase; will not disrupt a fertilized ovum; may open uterine tubes before time of ovulation |
| Sonohysterogram | 7-10 | Same as hysterosalpingogram |
| Postcoital test | 1-2 days before ovulation | Ovulatory late proliferative phase; look for normal motile sperm in cervical mucus |
| Sperm immobilization antigen-antibody reaction | Variable, ovulation | Immunologic test to determine sperm and cervical mucus interaction |
| Assessment of cervical mucus | Variable, ovulation | Cervical mucus should have low viscosity, high spinnbarkeit |
| Ultrasound diagnosis of follicular collapse | Ovulation | Collapsed follicle is seen after ovulation |
| Serum assay of plasma progesterone | 20-25 | Midluteal midsecretory phase; check adequacy of corpus luteal production of progesterone |
| Basal body temperature | Chart entire cycle | Elevation occurs in response to progesterone, documents ovulation |
| Endometrial biopsy | 21-27 | Late luteal, late secretory phase; check endometrial response to progesterone and adequacy of luteal phase |
| Sperm penetration assay | After 2 days but no more than 1 week of abstinence | Evaluation of ability of sperm to penetrate an egg |
| Hysteroscopy | Variable | Direct visualization of inside of uterus, via cervix |
| Laparoscopy | Variable | Direct visualization of outside of uterus, ovaries, and tubes, via abdomen |
Couples should be cautioned that everything can be normal and conception still may not occur. In addition, pregnancy may still occur even with poor test results.
Detection of Ovulation: All infertile women should have ovulatory function assessed, because a history of monthly menstruation is inadequate to conclude that ovulation is occurring and is optimal for conception.
Documentation of time of ovulation is important in the investigation of impaired fertility. Direct proof of ovulation is pregnancy or the retrieval of an ovum from the uterine tube. Several indirect or presumptive methods for detection of ovulation include assessment of basal body temperature (BBT) and cervical mucus characteristics, as well as endometrial biopsy and pelvic ultrasound examination. A serum progesterone level may be obtained in the latter half of the menstrual cycle to determine
the presence of sufficient amounts to accommodate implantation and maintain pregnancy. Occurrence of mittelschmerz and midcycle spotting provides unreliable presumptive evidence of ovulation.
Hormone Analysis: Hormone analysis is performed to assess endocrine function of the hypothalamic-pituitary-ovarian axis when menstrual cycles are absent or irregular, or to assess the woman’s ovarian reserve. Determination of blood levels of prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), progesterone, and the thyroid hormones may be necessary to diagnose the cause of irregular or absent menstrual cycles. The clomiphene citrate challenge test (CCCT) may be performed to determine ovarian reserves: fewer eggs means less responsiveness to FSH.
Ultrasonography: Abdominal or transvaginal ultrasound is used to assess pelvic structures (Fig. 9-2). This procedure is used to visualize pelvic tissues for a variety of reasons (e.g., to identify abnormalities such as fibroid tumors and ovarian cysts, to verify follicular development and maturity, and to assess thickness of the endometrium around the time of ovulation). Sonohysterography uses fluid infused into the uterus via the cervix to help define the uterine cavity and the depth of the uterine lining, using vaginal ultrasound.
Hysterosalpingography: Radiographic (x-ray) film examination allows visualization of the uterine cavity and tubes after the instillation of radiopaque contrast material through the cervix (Fig. 9-3). It is possible to see abnormalities of the uterus such as congenital defects or defects produced by submucous myomas and endometrial polyps. Distortions of the uterine cavity or uterine tubes can be a result of current or past pelvic inflammatory disease (PID). Scar tissue and adhesions from inflammatory processes can immobilize the uterus and tubes, kink the tubes, and surround the ovaries.
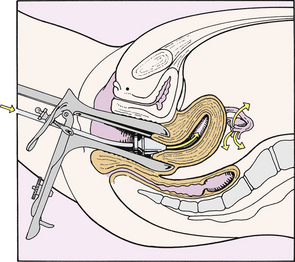
FIG. 9-3 Hysterosalpingography. Note that contrast medium flows through intrauterine cannula and out through the uterine tubes.
Hysterosalpingography is scheduled 2 to 5 days after menstruation to avoid flushing a potential fertilized ovum out through a uterine tube into the peritoneal cavity. Endometrial blood vessels are closed at this time, and all menstrual debris has been discharged. This decreases the risk of embolism or of forcing menstrual debris into the peritoneal cavity.
Referred shoulder pain may occur during this procedure. The referred pain is indicative of subphrenic irritation from the contrast media if it is spilled out of the patent uterine tubes. The discomfort can be managed with position change and mild analgesics. Pain usually subsides within 12 to 14 hours. Women with blocked tubes may have cramping for up to 48 hours.
Hysteroscopy: Hysteroscopy uses a flexible scope threaded through the cervix to directly view the uterine cavity. This is the gold standard for evaluation of leiomyomas (fibroids) and adhesions that might impair implantation (Thomson, Abbott, Deans, Kingston, & Vancaillie, 2009).
Timed Endometrial Biopsy: Endometrial biopsy is scheduled after ovulation, during the luteal phase of the menstrual cycle. Late in the menstrual cycle, 2 to 3 days before expected menses, a small cannula is introduced into the uterus, and a small portion of the endometrium is removed for histologic evaluation. To assess the response of the endometrium to progesterone production, the tissue is dated with respect to expected normal menstrual development. Tissue that is “out of phase” with expected development signifies either abnormal function of the corpus luteum or abnormal response of the endometrium.
Findings favorable to fertility include endometrial tissue that shows no signs of tuberculosis, polyps, or inflammatory conditions and that reflects secretory changes normally seen in the presence of adequate luteal (progesterone) phase.
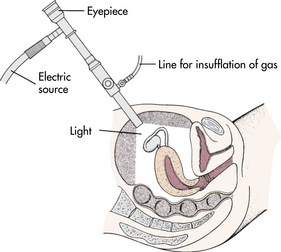
Laparoscopy: Laparoscopy is useful to view the pelvic structures intraperitoneally, outside the uterus, which may reveal endometriosis, pelvic adhesions, tubal occlusion, leiomyomas (fibroids), or polycystic ovaries. Performed early in the menstrual cycle, under either general or local anesthesia, a small endoscope is inserted through a small incision in the anterior abdominal wall. Cold fiberoptic light sources allow superior visualization of the internal pelvic structures (Fig. 9-4). A needle is inserted, and carbon dioxide gas is pumped into the peritoneum to elevate the abdominal wall from the organs, thereby creating an empty space that permits visualization and exploration with the laparoscope. If tubal patency is being assessed, a cannula is used to instill a dye contrast medium through the cervix.
After surgery deflation of most gas is done by direct expression. Trocar and needle sites are closed with a single subcuticular absorbable suture or skin clip, and an adhesive bandage is applied. Referred shoulder pain or subcostal discomfort usually lasts only 24 hours and is relieved with a mild analgesic.
Assessment of Male Infertility
Semen Analysis: The basic test for male infertility is the semen analysis. A complete semen analysis, study of the effects of cervical mucus on sperm forward motility and survival, and evaluation of the sperm’s ability to penetrate an ovum provide basic information. Semen is collected by ejaculation into a clean container or a plastic sheath that does not contain a spermicidal agent. The specimen is usually collected by masturbation after 2 to 5 days of abstinence from ejaculation. The semen is taken to the laboratory in a sealed container within 2 hours of ejaculation. Avoid exposure to excessive heat or cold. Commonly accepted values based on the World Health Organization (WHO) criteria for semen characteristics are given in Box 9-6. If results are in the fertile range, no further sperm evaluation is necessary. If results are not within this range, the test is repeated. If subsequent results are still in the subfertile range, further evaluation is needed to identify the problem (Nelson et al., 2007).
Seminal deficiency can be attributable to one or more of a variety of factors. The male is assessed for these factors: hypopituitarism; nutritional deficiency; debilitating or chronic disease, including obesity and metabolic disease (Hammoud, Gibson, Peterson, Meikle, & Carrell, 2008); trauma; exposure to environmental hazards such as radiation and toxic substances; use of tobacco, alcohol, and marijuana; gonadotropic inadequacy; and obstructive lesions of the epididymis and vas deferens. Congenital absence of the vas deferens can occur more frequently in men with the gene for cystic fibrosis. Genetic testing may reveal other reproductive problems. Hormone analyses are done for testosterone, gonadotropin, FSH, and LH. The sperm penetration assay and other alternative tests may be used to evaluate the ability of sperm to penetrate an egg. In addition, testicular biopsy may be warranted.
Assessment of the Couple
Postcoital Test: The postcoital test (PCT) is one method used to test for adequacy of coital technique, cervical mucus, antisperm antibodies, sperm, number and quality, and degree of sperm penetration through cervical mucus. Intercourse is synchronized with the expected time of ovulation (as determined from evaluation of BBT, cervical mucus changes, and usual length of menstrual cycle or use of LH detection kit to determine LH surge). It is performed only in the absence of vaginal infection. The test is performed within several hours after ejaculation of semen into the vagina. A specimen of cervical mucus obtained from the cervical os is examined under a microscope. The quality of mucus and the number of forward-moving sperm are noted. A PCT with no agglutination, good mucus, and motile sperm is associated with fertility. Although the PCT has been a traditional test for identifying cervical factor infertility, evidence is lacking that the PCT is a valid clinical tool and thus is not necessary for most couples (Nelson et al., 2007).
Couples may experience some difficulty abstaining from intercourse for 2 to 4 days before expected ovulation and then having intercourse with ejaculation on schedule. Sex on demand may strain the couple’s interpersonal relationship. A problem may arise if the expected day of ovulation occurs when facilities or the physician is unavailable (such as over a weekend or holiday).
The management of clients with infertility problems includes psychosocial, nonmedical, medical, and surgical interventions. Assisted reproductive therapies may be indicated. Nursing interventions are an important aspect of care (see Nursing Care Plan).
Psychosocial
Infertility is recognized as a major life stressor that can affect self-esteem; relations with the spouse, family, and friends; and careers. Individuals experiencing infertility are at risk for distress, anxiety, anger, lowered self-esteem, isolation, marital dysfunction, and grief. The distress of infertility and its treatment can exacerbate preexisting mental conditions (Burns, 2007). Couples often need assistance in separating their concepts of success and failure related to treatment for infertility
from personal success and failure. Recognizing the significance of infertility as a loss and resolving these feelings are crucial to putting infertility into perspective, even if treatment is successful (ASRM, 2010b; Nelson et al., 2007; Paterno, 2008).
Nurses can help couples express and discuss their feelings as honestly as possible. Ventilation may help couples to unburden themselves of negative feelings. A meta-analysis found that psychologic interventions alone can improve some couples’ chances of becoming pregnant (Hammerli, Znoj, & Barth, 2009).
Psychologic responses to a diagnosis of infertility may tax a couple’s giving and receiving of physical and sexual closeness. Sexual dysfunction may manifest as dyspareunia, decreased libido, unrealistic or rigid routines, decreased body image, depression, and ambivalence (Burns, 2007). Couples sometimes report orgasmic dysfunction or midcycle erectile disorders.
To be able to deal comfortably with a couple’s sexuality, nurses must be comfortable with their own sexuality so that they can better help couples understand why the private act of lovemaking must be shared with health care professionals. Nurses need up-to-date factual knowledge about human sexual practices and must be accepting and nonjudgmental of the preferences and activities of others (including same-sex couples). They need skills in interviewing and in therapeutic use of self, sensitivity to the nonverbal cues of others, and knowledge regarding each couple’s sociocultural and religious beliefs. Gender-neutral and inclusive language, as well as pictures and brochures depicting all types of families, including same-sex couples, establishes a tone of respect and safety in the health care setting (Gay and Lesbian Medical Association [GLMA], 2006).
The woman or couple facing infertility exhibits behaviors of the grieving process that are associated with other types of loss (Table 9-2). The loss of one’s genetic continuity with the generations to come leads to a loss of self-esteem, to a sense of inadequacy as a woman or a man, to a loss of control over one’s destiny, and to a reduced sense of self. The investigative process leads to a loss of spontaneity and control over the couple’s marital relationship and sometimes to a loss of control over progress toward career and life goals. All people do not have all the reactions described, nor can it be predicted how long any reaction will last for an individual.
TABLE 9-2
Nursing Actions in Response to Behavior Associated with Impaired Fertility
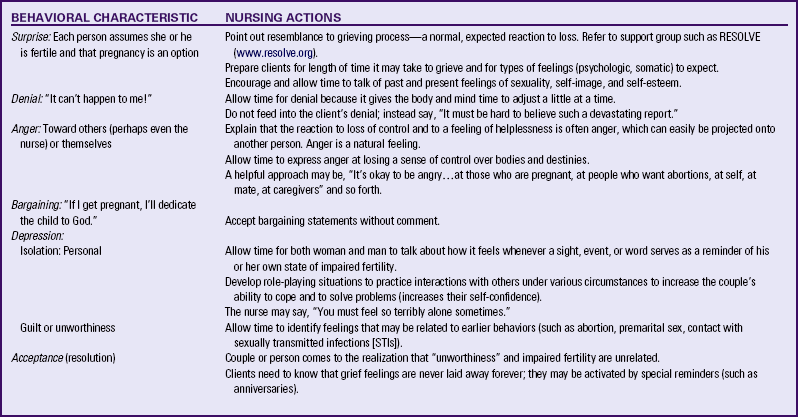
Sources: American Society for Reproductive Medicine (ASRM). (2010b). Frequently asked questions: The psychological component of infertility. Available at www.asrm.org. Accessed June 7, 2010; Resolve. (2010). Emotional aspects of infertility. Available at www.resolve.org/support-and-services/managing-infertility-stress/emotional-aspects.html. Accessed June 7, 2010.
The support systems of the couple with impaired fertility must be explored. This exploration should include persons available to assist, their relationship to the couple, their ages, their availability, and the available cultural or religious support (ASRM, 2010b). Individuals undergoing infertility evaluation and treatment should be encouraged to share this information with all providers of health care, including mental health practitioners (Burns, 2007).
If the couple conceives, nurses must be aware that the concerns and problems of the previously infertile couple may not be over. Many couples are overjoyed with the pregnancy; however, some are not. Some couples rearrange their lives, sense of selves, and personal goals within their acceptance of their infertile state. The couple may feel that those who worked with them to identify and treat impaired fertility expect them to be happy with the pregnancy. Some couples may be shocked to find that they feel resentment because the pregnancy, once a cherished dream, now necessitates another change in goals, aspirations, and identities. The normal ambivalence toward pregnancy may seem to be a retreat from the original choice to become parents. Reactions of couples range from dealing with the feelings of being overwhelmed to worrying about miscarriage to thinking about choosing to abort the pregnancy. If the couple chooses to continue with the pregnancy, they will need the care other expectant couples need. The couple may need extra preparation to adjust to realities of pregnancy, labor, and parenthood because they developed fantasies about idealized childbearing when they thought it was beyond their reach (Hammarberg, Fisher, & Wynter, 2008). A history of impaired fertility is considered to be a risk factor for pregnancy. A higher level of anxiety, noted in women with assisted pregnancies, could be a risk factor for postpartum depression; therefore, ongoing supportive psychologic therapy should be encouraged (Monti, Agostini, Fagandini, Paterlini, La Sala, & Blickstein, 2008).
If the couple does not conceive, they are assessed regarding their desire to be referred for help with adoption, therapeutic intrauterine insemination, other reproductive alternatives, or choosing a child-free state. The couple may find a list of agencies, support groups, and other resources in their community helpful such as ASRM (www.asrm.org) and RESOLVE (www.resolve.org).
Nonmedical
Simple changes in lifestyle may be effective in the treatment of subfertile men. Only water-soluble lubricants should be used during intercourse because many commonly used lubricants contain spermicides or have spermicidal properties. High scrotal temperatures may be caused by daily hot-tub bathing or saunas, or some sports, in which the testes are kept at temperatures too high for efficient spermatogenesis. It must be remembered that these conditions lead only to lessened fertility and should not be used as a means of contraception.
Treatment is available for women who have immunologic reactions to sperm (agglutination in the PCT). The use of condoms during genital intercourse for 6 to 12 months will reduce female antibody production in most women who have elevated antisperm antibody titers. After the serum reaction subsides, condoms are used at all times except at the expected time of ovulation. Approximately one third of couples with this problem conceive by following this course of action.
Changes in nutrition and habits may increase fertility for men and women. For example, a well-balanced diet, exercise, decreased alcohol intake, not smoking or abusing drugs, and stress management may be effective. Because obesity is associated with infertility as well as poorer outcomes for assisted reproduction techniques (ART), infertility treatment may be deferred until the body mass index (BMI) is less than 30 kg/m2, or 35 kg/m2 if older than 37 years of age, with no other risk factors (Balen & Anderson, 2007). Women who are overweight or obese have a reduced chance of pregnancy following in vitro fertilization (IVF) (Maheshwari, Stofberg, & Bhattacharya, 2007) and a significantly greater risk of spontaneous abortion following infertility treatment as compared to women with an optimal BMI (Wang, Davies, & Norman, 2002). Women wishing to conceive need to understand that even modest weight loss (5%-10%) may be sufficient to increase their chances of achieving a successful pregnancy.
Herbal Alternative Measures
Most herbal remedies have not been proven clinically to promote fertility or to be safe in early pregnancy. Women should take these only when prescribed by a physician, nurse-midwife, or nurse practitioner who has expertise in herbology. Relaxation, osteopathy, stress management (e.g., aromatherapy, yoga), and nutritional and exercise counseling have increased pregnancy rates in some women. Herbal remedies that reportedly promote fertility in general include red clover flowers, nettle leaves, dong quai, St John’s wort, chasteberry, antioxidants, and false unicorn root (Dennehy, 2006; Weed, 1986). ![]() Vitamin C, calcium, and magnesium may promote fertility and conception. Vitamins E and C, glutathione, and coenzyme Q10 are antioxidants that have proven beneficial effects for male infertility (Sheweita, Tilmisany, & Al-Sawaf, 2005). Herbs to avoid while trying to conceive include licorice root, yarrow, wormwood, ephedra, fennel, goldenseal, lavender, juniper, flaxseed, pennyroyal, passionflower, wild cherry, cascara, sage, thyme, and periwinkle (Sampey, Bourque, & Wren, 2004).
Vitamin C, calcium, and magnesium may promote fertility and conception. Vitamins E and C, glutathione, and coenzyme Q10 are antioxidants that have proven beneficial effects for male infertility (Sheweita, Tilmisany, & Al-Sawaf, 2005). Herbs to avoid while trying to conceive include licorice root, yarrow, wormwood, ephedra, fennel, goldenseal, lavender, juniper, flaxseed, pennyroyal, passionflower, wild cherry, cascara, sage, thyme, and periwinkle (Sampey, Bourque, & Wren, 2004).
Herbals and nutritional supplements can complement fertility treatments. Two systematic reviews found that acupuncture improves the success rates of in vitro fertilization outcomes (Anderson, Haimovici, Ginsburg, Schust, & Wayne, 2007; Cheong, Ng, & Ledger, 2008). ![]() N-acetylcysteine, an antioxidant amino acid, potentiates clomiphene therapy in women with PCOS, resulting in increased pregnancies (Badawy, State, & Abdelgawad, 2007).
N-acetylcysteine, an antioxidant amino acid, potentiates clomiphene therapy in women with PCOS, resulting in increased pregnancies (Badawy, State, & Abdelgawad, 2007).
Medical
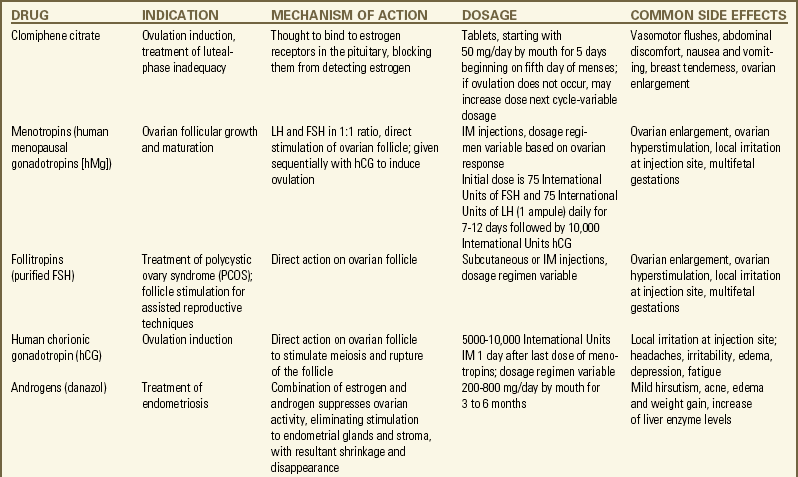
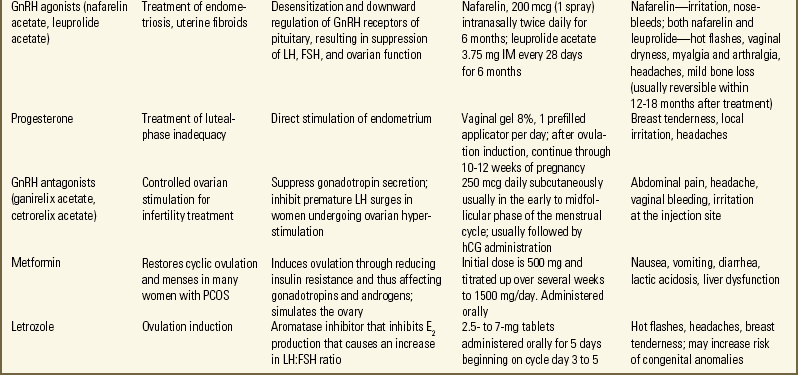
Pharmacologic therapy for female infertility is often directed at treating ovulatory dysfunction either by stimulating ovulation or by enhancing ovulation so that more oocytes mature. The most common medications include clomiphene citrate, human menopausal gonadotropin (hMG), FSH, human chorionic gonadotropin (hCG), and gonadotropin-releasing hormone (GnRH) (Lobo, 2007; Nelson et al., 2007). Metformin (an insulin sensitizing agent) or dexamethasone (a steroid) can potentiate clomiphene for anovulatory cycling women who have polycystic ovarian disease (Agency for Healthcare Research and Quality [AHRQ], 2008; Brown, Farquhar, Beck, Boothroyd, & Hughes, 2009). Bromocriptine is used to treat anovulation associated with hyperprolactinemia. TSH (Synthroid) is indicated if the woman has hypothyroidism. The Medication Guide describes common medications used for treating female infertility.
These medications are extremely potent and require daily monitoring with ovarian ultrasonography and monitoring of estradiol levels to prevent ovarian hyperstimulation syndrome, a potentially serious illness. The prevalence of multiple pregnancies with the use of these medications is greater than 25%.
Ovarian stimulation therapy is then used with timed intercourse, or intrauterine insemination if tubal blockage or poor sperm quality is suspected (Bensdorp, Cohlen, Heineman, & Vanderchove, 2007; Cantineau & Cohlen, 2007).
The woman who has low estrogen levels may be a candidate for conjugated estrogens and medroxyprogesterone. A hypoestrogenic condition may result from a high stress level or decreased percentage of body fat as a result of an eating disorder (e.g., anorexia nervosa) or excessive exercise. Hydroxyprogesterone supplementation with vaginal suppositories or intramuscular injection is used to treat luteal phase defects (ASRM, 2010a). The nurse may encounter other medications as well. In the presence of adrenal hyperplasia, prednisone, a
glucocorticoid, is taken orally. Treatment of endometriosis may include progesterones, combined oral contraceptives, or GnRH agonists (Lobo, 2007; Nelson et al., 2007) (see Medication Guide). The androgen danazol, an endometriosis treatment, is no longer considered effective for treating unexplained subfertility (Hughes, Brown, & Tiffin, 2007). Infections are treated with appropriate antimicrobial formulations.
TABLE 9-3
Assisted Reproductive Therapies
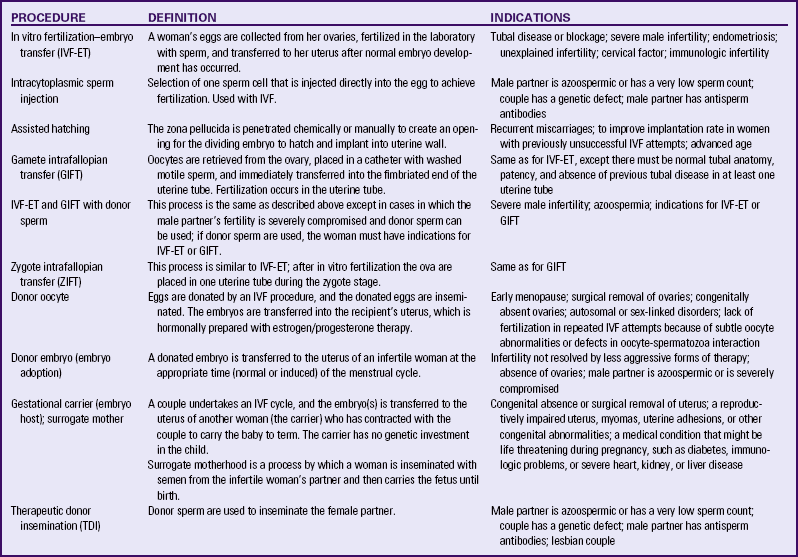
Data from American Society for Reproductive Medicine (ASRM). (2010a). Frequently asked questions about infertility. Available at www.asrm.org. Accessed June 7, 2010; Van Voorhis, B. (2006). Outcomes from assisted reproductive technology. Obstetrics and Gynecology, 107(1), 183-200; Pauli, S., Berga, S., Shang, W., & Session, D. (2009). Current status of the approach to assisted reproduction. Pediatric Clinics of North America, 56(3), 467-488.
Drug therapy may be indicated for male infertility. Problems with the thyroid or adrenal glands are corrected with appropriate medications. Infections are identified and treated promptly with antimicrobials. FSH, hMG, and clomiphene may be used to stimulate spermatogenesis in men with hypogonadism (Attia & Al-Inany, 2007).
The primary care provider is responsible for informing clients fully about the prescribed medications. However, the nurse must be ready to answer clients’ questions and to confirm their understanding of the drug, its administration, potential side effects, and expected outcomes. Because information varies with each drug, the nurse must consult the medication package inserts, pharmacology references, the physician, and the pharmacist as necessary.
Surgical
A number of surgical procedures can be used for problems causing female infertility. Ovarian tumors must be excised. Whenever possible, functional ovarian tissue is left intact. Scar tissue adhesions caused by chronic infections may cover the ovary. These adhesions usually necessitate surgery to free and expose the ovary so that ovulation can occur.
Hysterosalpingography, using an oil-soluble contrast medium to flush the tubes, is useful for identification and treatment of tubal obstruction (Johnson, Vanderchove, Lilford, Harada, Hughes, Luttjeboer, et al., 2007). The passage of contrast medium may clear tubes of mucous plugs, straighten kinked tubes, or break up adhesions within the tubes (caused by salpingitis). The procedure may stimulate cilia in the lining of the tubes to facilitate transport of the ovum. It also may aid healing as a result of the bacteriostatic effect of the iodine within the contrast medium.
During laparoscopy, delicate adhesions may be divided and removed, and endometrial implants may be destroyed by electrocoagulation or laser (Zarei, Al-Ghafri, & Tulandi, 2009). Laparotomy and even microsurgery may be required to do extensive repair of the damaged tube. Prognosis is dependent on the degree to which tubal patency and function can be restored.
Reconstructive surgery (e.g., the unification operation for bicornuate uterus) often improves a woman’s ability to conceive and carry the fetus to term. Laparoscopic or hysteroscopic surgical removal of tumors or fibroids involving the endometrium or uterus often improves the woman’s chance of conceiving and maintaining the pregnancy to viability (Luciano, 2009). Surgical treatment of uterine tumors or maldevelopment that results in successful pregnancy usually requires birth by cesarean surgery near term gestation because the uterus may rupture as a result of weakness of the area of reconstructive surgery.
Chemocautery (destruction of tissue with chemicals) or thermocautery (destruction of tissue with heat, usually electrical) of the cervix, cryosurgery (destruction of tissue by application of extreme cold, usually liquid nitrogen), or conization (excision of a cone-shaped piece of tissue from the endocervix) is effective in eliminating chronic inflammation and infection. However, when the cervix has been deeply cauterized or frozen, or when extensive conization has been performed, extreme limitation of mucus production by the cervix may result. Sperm migration may be difficult or impossible because of the absence of a mucus bridge from the vagina to the uterus. Therapeutic intrauterine insemination may be necessary to carry the sperm directly through the internal os of the cervix.
Surgical procedures also may be used for problems causing male infertility. Surgical repair of varicocele has been relatively successful in increasing sperm count but not fertility rates. Microsurgery to reanastomose (restore tubal continuity) the sperm ducts after vasectomy can restore fertility.
Assisted Reproductive Therapies
Although remarkable developments have occurred in reproductive medicine, assisted reproductive therapies (ARTs) account for less than 1% of all U.S. births (Wright, Chang, Jeng, Macaluso, & CDC, 2008) and less than 3% of infertility treatment (ASRM, 2010a). They are associated with many ethical and legal issues (Box 9-7). The lack of information or misleading information about success rates and the risks and benefits of treatment alternatives prevents couples from making informed decisions. Nurses can provide information so that couples have an accurate understanding of their chances for a successful pregnancy and live birth. In 2005, the success rate for pregnancy with ART transfer procedures was 42%, whereas the success rate for live births was 35% (Wright, et al.). Nurses also can provide anticipatory guidance about the moral and ethical dilemmas regarding the use of ARTs.
Table 9-3 summarizes the following ART procedures and their possible indications.
In Vitro Fertilization–Embryo Transfer: In vitro fertilization–embryo transfer (IVF-ET) is a common approach for women with blocked or absent uterine tubes or with unexplained infertility and for men with very low sperm counts. About 99% of all ARTs use this procedure (Van Voorhis, 2006). Ovarian stimulation using pharmacologic therapy results in multiple mature ova, which are collected at midcycle via intravaginal needle aspiration. The ova are fertilized with sperm in vitro (in a dish) for up to 6 days, then transferred to the uterus using ultrasound guidance (AHRQ, 2008). If sperm are not available via ejaculation, they can be retrieved via needle from the testes or the epididymis (Proctor, Johnson, van Peperstraten, & Phillipson, 2008).
Intracytoplasmic sperm injection (ICSI) is a micromanipulation technique that makes it possible to achieve fertilization even with few or poor-quality sperm by introducing sperm beneath the zona pellucida directly into the egg. ICSI offers the opportunity to enhance the chances of fertilization in cases of a severe male factor (i.e., poor sperm quality) (Pauli et al., 2009; Van Voorhis, 2006). Another micromanipulation option is assisted hatching. In some instances, the zona pellucida is thick or tough and the embryo cannot break through or “hatch” through this coating in the blastocystic phase of development. An infrared laser is used to create a hole in the zona pellucida so that the embryo can break through and implant. This procedure is recommended for couples that have had previous IVF failures (AHRQ, 2008).
Preimplantation genetic diagnosis (PGD) is a form of early genetic testing designed to eliminate embryos with serious genetic defects before implantation through one of the ARTs and to avoid future termination of the pregnancy for genetic reasons. Micromanipulation allows removal of a single cell from a multicellular embryo for genetic study (i.e., embryo biopsy). Couples must be counseled about their options and choices, as well as the implications of their choices, when genetic analysis is considered. For example, the transfer of only embryos that are free from abnormalities can increase the implantation rate and decrease the miscarriage rate and may increase the likelihood of the birth of a healthy infant (Kulieve & Verlinsky, 2008; Pauli et al., 2009).
To minimize the risks of multiple pregnancies, guidelines recommend only two embryos be transferred into women, unless they are older than 37 (Practice Committees of ASRM and the Society for Assisted Reproductive Technology, 2009).
Ovarian tissue, oocytes, or embryos can be cryopreserved for later use (Wallberg, Keros, & Hovatta, 2009).
Success rates for pregnancy and for live births vary widely from center to center. Each couple’s physical status and age factor into their individual chances for pregnancy as well as whether the embryos are fresh or frozen and are from eggs of the woman or a donor (Van Voorhis, 2006). Costs vary by treatment and by region of the country: one cycle of IVF-ET averages $12,400 (ASRM, 2010a).
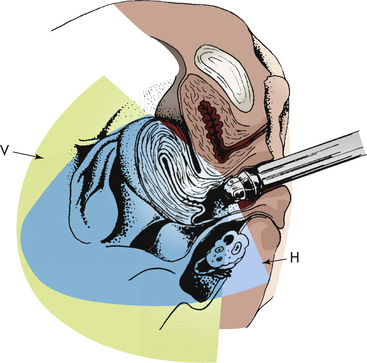
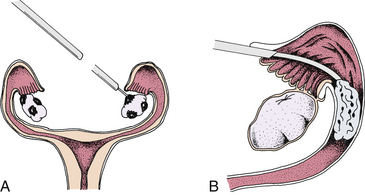
Gamete Intrafallopian Transfer: Gamete intrafallopian transfer (GIFT) is similar to IVF-ET. GIFT requires women to have at least one normal uterine tube. Ovulation is induced as in IVF-ET, and the oocytes are aspirated from follicles via laparoscopy (Fig. 9-5, A). Semen is collected before laparoscopy, and sperm are capacitated by the same technique used for IVF-ET. The ova and sperm are then transferred to one uterine tube (Fig. 9-5, B), permitting natural fertilization and cleavage. Less than 1% of all ARTs use this technique (ASRM, 2010a).
Zygote Intrafallopian Transfer: Zygote intrafallopian transfer (ZIFT) is similar to GIFT except that in ZIFT, after in vitro fertilization the ova are placed in the uterine tube during the zygote stage. ZIFT accounts for less than 1% of all ART procedures (ASRM, 2010a).
Complications: Other than the established risks associated with ovarian stimulation, invasive procedures and general anesthesia, few risks are associated with IVF-ET, GIFT, and ZIFT. The more common transvaginal needle aspiration requires only local or intravenous analgesia. Evidence of increased risk of congenital malformations associated with ART is mixed: the anomalies may be related to the underlying cause of infertility (Pauli et al., 2009). In addition, the underlying cause of infertility, such as severe male infertility factor, may be passed on to the offspring. Infertility treatment increases the risk of placental problems (AHRQ, 2008). Multiple gestations are more likely and are associated with increased risks for both the mother and fetuses (Wright et al., 2008). Ectopic pregnancies occur more often, and these carry a significant maternal risk. In addition, psychologic, financial, and emotional stresses are common.
Oocyte Donation: Women who have ovarian failure or oophorectomy, who have a genetic defect, or who fail to achieve pregnancy with their own oocytes may be eligible for the use of donor oocytes. Oocyte donation is usually done by women who are younger than 35 years and healthy, and who are recruited and paid to undergo ovarian stimulation and oocyte retrieval. The donor eggs are then fertilized in the laboratory with the male partner’s sperm. The recipient woman undergoes hormonal stimulation to allow development of the uterine lining. Embryos are then transferred. The psychosocial issues are similar to those in therapeutic donor insemination. Historically the courts have upheld the gestational mother as the legal mother. It is expected that the egg donor will have no rights or responsibilities in relation to the offspring.
Embryo Donation: On occasion a couple decides that they do not want their frozen embryos, and they release these for “adoption” by other infertile couples. Infertility centers are struggling to develop guidelines and protocols to address the various legal and ethical issues associated with these procedures. Extensive medical testing of both partners who wish to release the embryos is required as well.
Surrogate Mothers/Embryo Hosts: Surrogate motherhood can be achieved by two methods. The first is for the surrogate mother to be inseminated with semen from the infertile woman’s partner and to carry the baby until the birth. The baby is then formally adopted by the infertile couple. A less common method is to retrieve an ovum from the infertile woman, fertilize it with her partner’s sperm, and place it into the uterus of a surrogate, who becomes an embryo host or gestational carrier. These interventions raise considerable legal and ethical issues that require extensive counseling of couples and the women who choose to become surrogates.
Therapeutic Donor Insemination: Therapeutic donor insemination (TDI), previously referred to as artificial insemination by donor, is used when the male partner has no sperm or a very low sperm count (less than 20 million motile sperm per milliliter), the couple has a genetic defect, or the male partner has antisperm antibodies. Couples need to be counseled extensively regarding the mutuality of their decision, their ability (particularly of the male partner) to grieve the loss of a biologic child, and long-term issues relating to parenting the child conceived through TDI (Van Voorhis, 2006). Couples also must be aware of the legal status of TDI in their state.
In TDI, donor semen is subjected to laboratory testing to reduce the possibility of life-threatening illnesses for the recipient and her fetus, as well as for factors that could jeopardize the woman’s future fertility or compromise the chance of the success of the procedure. No increase in maternal or perinatal complications occurs with TDI; the same frequencies of anomalies (about 5%) and obstetric complications (between 5% and 10%) that accompany natural insemination (through sexual intercourse) apply also to TDI.
The intrauterine insemination procedure is done in the physician’s office or clinic, usually the day after the woman has an LH surge. The sperm are injected via catheter through the cervix, and placed high in the uterine cavity. The woman lies flat for a few minutes and then can get up and resume her usual activities.
Assuming normal female fertility, intrauterine TDI at or about the time of ovulation has resulted in pregnancy in as many as 70% of cases. If pregnancy has not occurred within six cycles of well-timed insemination, further investigation of the female partner is warranted. The couple must know that there is no guarantee of pregnancy and that the miscarriage rate is approximately the same as in a control population.
Adoption
Couples may choose to build their family through adoption of children who are not their own biologically. With increased availability of birth control and abortion and increasing numbers of single mothers keeping their babies, however, the adoption of Caucasian infants is extremely limited. Minority infants and infants with special needs, older children, and foreign adoptions are other options.
Most adults assume that they will be able to have children of their own. The discovery that they are unable to do so is often accompanied by feelings of inferiority, doubts about masculinity or femininity, and feelings of guilt or blame in relation to the partner. These feelings and frustrations, combined with the anxiety of waiting for pregnancies, feelings of loss, and the multiple medical procedures to investigate infertility, plus the legal uncertainties and potential financial considerations surrounding adoption, create many challenges for the adoptive couple preparing for parenthood.
Couples who decide to adopt a child have decided that being a parent and having a child is more important than the actual process of birthing the baby. The birth process is a very small aspect of becoming a parent. Prospective parents can become so focused on attempting to become pregnant with their own genetic child that they don’t see alternate ways of creating a family and parenting a child. The question couples who are considering adoption need to answer is, “What is important to you—that you become parents or that you go through the experience of pregnancy and birth?” Nurses should have information on options for adoption available for couples or refer them to community resources for further assistance (ASRM, 2010b).
KEY POINTS
• Infertility is the inability to conceive and carry a child to term gestation when the couple has chosen to do so.
• Infertility affects between 10% and 15% of otherwise healthy adults. Infertility increases as the woman ages, especially after age 40 years.
• In the United States, about 20% of infertility causes are unexplained; of that 80% in which causative factors are known, about 40% are related to female causes, 40% are related to male causes, and 20% are attributable to both male and female causes.
• Common etiologic factors of infertility include decreased sperm production, ovulation disorders, tubal occlusion, and endometriosis. Obesity in either partner is receiving increasing attention as a cause of infertility.
• The investigation of infertility is conducted systematically and simultaneously for male and female partners.
• The couple’s relationship dynamics, sexuality, and ability to cope with the psychologic and emotional effects caused by diagnostic procedures and treatment of infertility must be considered in the plan of care. Ongoing support is recommended.
• Most infertility cases are treated with conventional medical and surgical therapies; less than 3% are treated with in vitro fertilization.
• Reproductive alternatives for family building include IVF-ET, GIFT, ZIFT, oocyte donation, embryo donation, TDI, surrogate motherhood, and adoption.
![]() Audio Chapter Summaries Access an audio summary of the Key Points on
Audio Chapter Summaries Access an audio summary of the Key Points on ![]()