Endocrine and Metabolic Disorders in Pregnancy
• Differentiate the types of diabetes mellitus and their respective risk factors in pregnancy.
• Compare insulin requirements during pregnancy, postpartum, and with lactation.
• Identify maternal and fetal risks or complications associated with diabetes in pregnancy.
• Develop a plan of care for the pregnant woman with pregestational or gestational diabetes.
• Explain the effects of hyperemesis gravidarum on maternal and fetal well-being.
• Discuss the management of the woman with hyperemesis gravidarum in the hospital and at home.
• Explain the effects of thyroid disorders on pregnancy.
• Compare the management of a pregnant woman with hyperthyroidism with one who has hypothyroidism.
• Discuss care management for the woman with phenylketonuria during the perinatal period.
• Examine the effects of maternal phenylketonuria on pregnancy outcome.
This chapter discusses the care of women for whom pregnancy represents a significant risk because it is superimposed on an endocrine or metabolic disorder. Specific disorders covered in this chapter include diabetes mellitus, hyperemesis gravidarum, hyper- and hypothyroidism, and phenylketonuria. Providing safe and effective care for women with these disorders and their fetuses is a challenge. Although unique needs related to the specific endocrine or metabolic condition are present, these women also experience the feelings, needs, and concerns associated with a normal pregnancy. The primary objective of nursing care is to achieve optimal outcomes for both the pregnant woman and the fetus. With the active participation of well-motivated women in the treatment plan and careful management from a multidisciplinary health care team, positive outcomes are often possible.
Diabetes Mellitus
Around the world the incidence of diabetes mellitus is increasing at a rapid rate. In 2009 an estimated 23.6 million people in the United States (7.8% of the total population) have diabetes. Of these, 5.7 million are undiagnosed (National Center for Chronic Disease Prevention and Health Promotion, 2009). In the United States, experts predict a marked increase in the number of women with preexisting diabetes who will become pregnant (Moore & Catalano, 2009). Diabetes mellitus is currently the most common endocrine disorder associated with pregnancy, occurring in approximately 4% to 14% of pregnant women (Gilbert, 2011). The perinatal mortality rate for well-managed diabetic pregnancies, excluding major congenital malformations, is approximately the same as for any other pregnancy (Landon, Catalano, & Gabbe, 2007). The key to an optimal pregnancy outcome is strict maternal glucose control before conception, as well as throughout the gestational period. Consequently, for women with diabetes, much emphasis is placed on preconception counseling.
Pregnancy complicated by diabetes is still considered high risk. It is most successfully managed by a multidisciplinary approach involving the obstetrician, perinatologist, internist or endocrinologist, ophthalmologist, nephrologist, neonatologist, nurse, nutritionist or dietitian, and social worker, as needed. A favorable outcome requires commitment and active participation by the pregnant woman and her family.
Pathogenesis
Diabetes mellitus refers to a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both (American Diabetes Association [ADA], 2008). Insulin, produced by the beta cells in the islets of Langerhans in the pancreas, regulates blood glucose levels by enabling glucose to enter adipose and muscle cells, where it is used for energy. When insulin is insufficient or ineffective in promoting glucose uptake by the muscle and adipose cells, glucose accumulates in the bloodstream, and hyperglycemia results. Hyperglycemia causes hyperosmolarity of the blood, which attracts intracellular fluid into the vascular system, resulting in cellular dehydration and expanded blood volume. Consequently the kidneys function to excrete large volumes of urine (polyuria) in an attempt to regulate excess vascular volume and to excrete the unusable glucose (glycosuria). Polyuria, along with cellular dehydration, causes excessive thirst (polydipsia).
The body compensates for its inability to convert carbohydrate (glucose) into energy by burning proteins (muscle) and fats. However, the end products of this metabolism are ketones and fatty acids, which, in excess quantities, produce ketoacidosis and acetonuria. Weight loss occurs as a result of the breakdown of fat and muscle tissue. This tissue breakdown causes a state of starvation that compels the individual to eat excessive amounts of food (polyphagia).
Over time, diabetes causes significant changes in the microvascular and macrovascular circulations. These structural changes affect a variety of organ systems, particularly the heart, the eyes, the kidneys, and the nerves. Complications resulting from diabetes include premature atherosclerosis, retinopathy, nephropathy, and neuropathy.
Diabetes may be caused either by impaired insulin secretion, when the beta cells of the pancreas are destroyed by an autoimmune process, or by inadequate insulin action in target tissues at one or more points along the metabolic pathway. Both of these conditions are commonly present in the same person, and determining which, if either, abnormality is the primary cause of the disease is difficult (ADA, 2008). For additional information on diabetes, visit the American Diabetes Association’s website at www.diabetes.org.
Classification
The current classification system includes four groups: type 1 diabetes, type 2 diabetes, other specific types (e.g., diabetes caused by genetic defects in beta cell function or insulin action, disease or injury of the pancreas, or drug-induced diabetes), and gestational diabetes mellitus (GDM) (ADA, 2008; Moore & Catalano, 2009). Almost 90% of all pregnant women with diabetes have GDM (Gilbert, 2011). Of the women with pregestational diabetes, the majority (65%) have type 2 diabetes (Chan & Johnson, 2006).
Type 1 diabetes includes cases that are caused primarily by pancreatic islet beta cell destruction and that are prone to ketoacidosis. People with type 1 diabetes usually have an abrupt onset of illness at a young age and an absolute insulin deficiency. Type 1 diabetes includes cases thought to be caused by an autoimmune process, as well as those for which the cause is unknown (ADA, 2008; Landon et al., 2007).
Type 2 diabetes is the most prevalent form of the disease and includes individuals who have insulin resistance and usually relative (rather than absolute) insulin deficiency. Specific causes of type 2 diabetes are unknown at this time. Type 2 diabetes often goes undiagnosed for years because hyperglycemia develops gradually and is often not severe enough for the client to recognize the classic signs of polyuria, polydipsia, and polyphagia. Most people who develop type 2 diabetes are obese or have an increased amount of body fat distributed primarily in the abdominal area. Other risk factors for the development of type 2 diabetes include aging, a sedentary lifestyle, family history and genetics, puberty, hypertension, and prior gestational diabetes. Type 2 diabetes often has a strong genetic predisposition (ADA, 2008; Moore & Catalano, 2009).
Pregestational diabetes mellitus is the label sometimes given to type 1 or type 2 diabetes that existed before pregnancy.
Gestational diabetes mellitus (GDM) is any degree of glucose intolerance with the onset or first recognition occurring during pregnancy. This definition is appropriate whether or not insulin is used for treatment or the diabetes persists after pregnancy. It does not exclude the possibility that the glucose intolerance preceded the pregnancy or that medication might be required for optimal glucose control. Women experiencing gestational diabetes should be reclassified 6 weeks or more after the pregnancy ends (ADA, 2008; Moore & Catalano, 2009).
White’s Classification of Diabetes in Pregnancy
Dr. Priscilla White, a physician who worked with pregnant women with diabetes during the 1940s, developed a classification system specifically for use with this group of women (Table 29-1). White’s system was based on age at diagnosis, duration of illness, and presence of vascular disease (Landon et al., 2007; Moore & Catalano, 2009). Her classification system has been modified through the years but is still frequently used to assess both maternal and fetal risk. Women in classes A through C generally have good pregnancy outcomes as long as their blood glucose levels are well controlled. Women in classes D through T, however, usually have poorer pregnancy outcomes because they have already developed the vascular damage that often accompanies long-standing diabetes.
TABLE 29-1
WHITE’S CLASSIFICATION OF DIABETES IN PREGNANCY (MODIFIED)
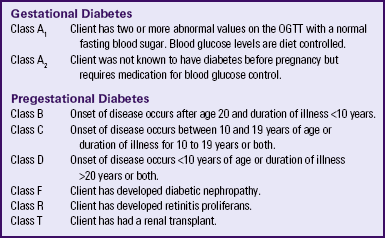
OGTT, Oral glucose tolerance test.
Sources: Landon, M., Catalano, P., & Gabbe, S. (2007). Diabetes mellitus complicating pregnancy. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone; Moore, T., & Catalano, P. (2009). Diabetes in pregnancy. In R. Creasy, R. Resnik, J. Iams, C. Lockwood, & T. Moore (Eds.), Creasy and Resnik’s maternal-fetal medicine: Principles and practice (6th ed.). Philadelphia: Saunders.
Metabolic Changes Associated with Pregnancy
Normal pregnancy is characterized by complex alterations in maternal glucose metabolism, insulin production, and metabolic homeostasis. During normal pregnancy, adjustments in maternal metabolism allow for adequate nutrition for the mother and the developing fetus. Glucose, the primary fuel used by the fetus, is transported across the placenta through the process of carrier-mediated facilitated diffusion, meaning that the glucose levels in the fetus are directly proportional to maternal levels. Although glucose crosses the placenta, insulin does not. Around the tenth week of gestation the fetus begins to secrete its own insulin at levels adequate to use the glucose obtained from the mother. Therefore, as maternal glucose levels rise, fetal glucose levels are increased, resulting in increased fetal insulin secretion.
During the first trimester of pregnancy the pregnant woman’s metabolic status is significantly influenced by the rising levels of estrogen and progesterone. These hormones stimulate the beta cells in the pancreas to increase insulin production, which promotes increased peripheral use of glucose and decreased blood glucose, with fasting levels being reduced by approximately 10% (Fig. 29-1, A). At the same time, an increase in tissue glycogen stores and a decrease in hepatic glucose production occur, which further encourage lower fasting glucose levels. As a result of these normal metabolic changes of pregnancy, women with insulin-dependent diabetes are prone to hypoglycemia during the first trimester.
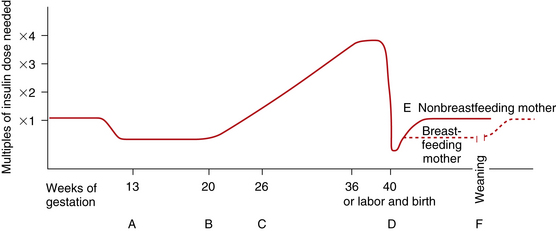
FIG. 29-1 Changing insulin needs during pregnancy. A, First trimester: Insulin need is reduced because of increased insulin production by pancreas and increased peripheral sensitivity to insulin; nausea, vomiting, and decreased food intake by mother and glucose transfer to embryo or fetus contribute to hypoglycemia. B, Second trimester: Insulin needs begin to increase as placental hormones, cortisol, and insulinase act as insulin antagonists, decreasing insulin’s effectiveness. C, Third trimester: Insulin needs may double or even quadruple but usually level off after 36 weeks of gestation. D, Day of birth: Maternal insulin requirements decrease drastically to approach prepregnancy levels. E, Breastfeeding mother maintains lower insulin requirements, as much as 25% less than those of prepregnancy; insulin needs of nonbreastfeeding mother return to prepregnancy levels in 7 to 10 days. F, Weaning of breastfeeding infant causes mother’s insulin needs to return to prepregnancy levels.
During the second and third trimesters, pregnancy exerts a “diabetogenic” effect on the maternal metabolic status. Because of the major hormonal changes, decreased tolerance to glucose, increased insulin resistance, decreased hepatic glycogen stores, and increased hepatic production of glucose occur. Rising levels of human chorionic somatomammotropin, estrogen, progesterone, prolactin, cortisol, and insulinase increase insulin resistance through their actions as insulin antagonists. Insulin resistance is a glucose-sparing mechanism that ensures an abundant supply of glucose for the fetus. Maternal insulin requirements gradually increase from approximately 18 to 24 weeks of gestation to approximately 36 weeks of gestation. Maternal insulin requirements may double or quadruple by the end of the pregnancy (see Fig. 29-1, B and C).
At birth, expulsion of the placenta prompts an abrupt drop in levels of circulating placental hormones, cortisol, and insulinase (see Fig. 29-1, D). Maternal tissues quickly regain their prepregnancy sensitivity to insulin. For the nonbreastfeeding mother the prepregnancy insulin-carbohydrate balance usually returns in approximately 7 to 10 days (see Fig. 29-1, E). Lactation uses maternal glucose; therefore, the breastfeeding mother’s insulin requirements remain low during lactation. On completion of weaning the mother’s prepregnancy insulin requirement is reestablished (see Fig. 29-1, F).
Pregestational Diabetes Mellitus
Approximately 2 per 1000 pregnancies are complicated by preexisting diabetes. Women who have pregestational diabetes mellitus may have either type 1 or type 2 diabetes, which may be complicated by vascular disease, retinopathy, nephropathy, or other diabetic sequelae. Type 2 is a more common diagnosis than type 1. Almost all women with pregestational diabetes are insulin dependent during pregnancy. According to White’s classification system, these women fall into classes B through T (see Table 29-1).
The diabetogenic state of pregnancy imposed on the compromised metabolic system of the woman with pregestational diabetes has significant implications. The normal hormonal adaptations of pregnancy affect glycemic control, and pregnancy may accelerate the progress of vascular complications.
During the first trimester, when maternal blood glucose levels are normally reduced and the insulin response to glucose is enhanced, glycemic control is improved. The insulin dose for the woman with well-controlled diabetes may have to be reduced to prevent hypoglycemia. Nausea, vomiting, and cravings typical of early pregnancy result in dietary fluctuations that influence maternal glucose levels and may also necessitate a reduction in the insulin dose.
Because insulin requirements steadily increase after the first trimester, the insulin dose must be adjusted accordingly to prevent hyperglycemia. Insulin resistance begins as early as 14 to 16 weeks of gestation and continues to rise until it stabilizes during the last few weeks of pregnancy.
Preconception Counseling
Preconception counseling is recommended for all women of reproductive age who have diabetes because it is associated with less perinatal mortality and fewer congenital anomalies (Moore & Catalano, 2009). Under ideal circumstances, women with pregestational diabetes are counseled before the time of conception to plan the optimal time for pregnancy, establish glycemic control before conception, and diagnose any vascular complications of diabetes. However, estimates indicate that less than 20% of women with diabetes in the United States participate in preconception counseling (Landon et al., 2007).
The woman’s partner should be included in the counseling to assess the couple’s level of understanding related to the effects of pregnancy on the diabetic condition and of the
potential complications of pregnancy as a result of diabetes. The couple should also be informed of the anticipated alterations in management of diabetes during pregnancy and the need for a multidisciplinary team approach to health care. Financial implications of diabetic pregnancy and other demands related to frequent maternal and fetal surveillance should be discussed. Contraception is another important aspect of preconception counseling to assist the couple in planning effectively for pregnancy.
Maternal Risks and Complications
Although maternal morbidity and mortality rates have improved significantly, the pregnant woman with diabetes remains at risk for the development of complications during pregnancy. Poor glycemic control around the time of conception and in the early weeks of pregnancy is associated with an increased incidence of miscarriage. Women with good glycemic control before conception and in the first trimester are no more likely to miscarry than women who do not have diabetes (Moore & Catalano, 2009).
Poor glycemic control later in pregnancy, particularly in women without vascular disease, increases the rate of fetal macrosomia. Macrosomia has been defined in several different ways, including a birth weight more than 4000 to 4500 g, birth weight greater than the 90th percentile, and estimates of neonatal adipose tissue. Macrosomia occurs in approximately 40% of pregestational diabetic pregnancies and in up to 50% of pregnancies complicated by GDM (Landon et al., 2007; Moore & Catalano, 2009). Infants born to women with diabetes tend to have a disproportionate increase in shoulder, trunk, and chest size. Because of this tendency the risk of shoulder dystocia is greater in these babies than in other macrosomic infants. Women with diabetes therefore face an increased likelihood of cesarean birth because of failure of fetal descent or labor progress or of operative vaginal birth (birth involving the use of episiotomy, forceps, or vacuum extractor) (Landon et al.; Moore & Catalano).
Women with preexisting diabetes are at risk for several obstetric and medical complications. In general, the risk of developing these complications increases with the duration and severity of the woman’s diabetes. In one study the rates of preeclampsia, preterm birth, cesarean birth, and maternal mortality were much higher in women with preexisting diabetes than in women who did not have this disease. Approximately a third of women who have had diabetes for more than 20 years, for example, develop preeclampsia. Women with nephropathy and hypertension in addition to diabetes are also increasingly likely to develop preeclampsia. The rate of hypertensive disorders in all types of pregnancies complicated by diabetes is 15% to 30%. Chronic hypertension occurs in 10% to 20% of all pregnant women with diabetes, and in up to 40% of those women who have preexisting renal or retinal vascular disease (Moore & Catalano, 2009).
Hydramnios (polyhydramnios) frequently develops during the third trimester of pregnancy in women with diabetes. Its cause is unknown. One theory is that hydramnios in women with diabetes is caused by an increased glucose concentration in amniotic fluid resulting from maternal and fetal hyperglycemia. The complications most frequently associated with hydramnios (usually defined as an amniotic fluid index [AFI] greater than 24 to 25 cm) are placental abruption (abruptio placentae), uterine dysfunction, and postpartum hemorrhage (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010).
Infections are more common and more serious in pregnant women with diabetes than in pregnant women without the disease. Disorders of carbohydrate metabolism alter the body’s normal resistance to infection. The inflammatory response, leukocyte function, and vaginal pH are all affected. Vaginal infections, particularly monilial vaginitis, are more common. Urinary tract infections (UTIs) are also more prevalent. Infection is serious because it causes increased insulin resistance and may result in ketoacidosis.
Ketoacidosis (accumulation of ketones in the blood resulting from hyperglycemia and leading to metabolic acidosis) occurs most often during the second and third trimesters, when the diabetogenic effect of pregnancy is the greatest. When the maternal metabolism is stressed by illness or infection, the woman is at increased risk for diabetic ketoacidosis (DKA). DKA can also be caused by poor client compliance with treatment or the onset of previously undiagnosed diabetes (Moore & Catalano, 2009). The use of beta-mimetic drugs such as terbutaline for tocolysis to arrest preterm labor or corticosteroids given to enhance fetal lung maturation may also contribute to the risk for hyperglycemia and subsequent DKA (Cunningham et al., 2010; Iams, Romero, & Creasy, 2009).
DKA may occur with blood glucose levels barely exceeding 200 mg/dl, as compared with 300 to 350 mg/dL in the nonpregnant state. In response to stress factors such as infection or illness, hyperglycemia occurs as a result of increased hepatic glucose production and decreased peripheral glucose use. Stress hormones, which act to impair insulin action and further contribute to insulin deficiency, are released. Fatty acids are mobilized from fat stores to enter the circulation. As they are oxidized, ketone bodies are released into the peripheral circulation. The woman’s buffering system is unable to compensate, and metabolic acidosis develops. The excessive blood glucose and ketone bodies result in osmotic diuresis with subsequent loss of fluid and electrolytes, volume depletion, and cellular dehydration. DKA is a medical emergency. Prompt treatment is necessary to prevent maternal coma or death. Ketoacidosis occurring at any time during pregnancy can lead to intrauterine fetal death. The incidence of DKA has decreased in recent years. Currently it affects only about 1% of pregnant women with diabetes (Cunningham et al., 2010). The rate of intrauterine fetal demise (IUFD) with DKA, formerly approximately 35%, is 10% or less (Moore & Catalano, 2009) (Table 29-2).
TABLE 29-2
DIFFERENTIATION OF HYPOGLYCEMIA (INSULIN SHOCK) AND HYPERGLYCEMIA (DIABETIC KETOACIDOSIS)
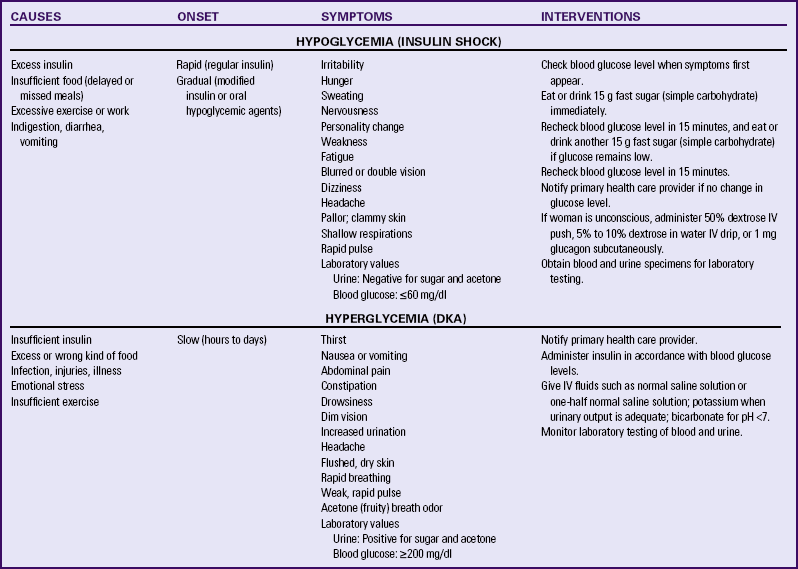
The risk of hypoglycemia (a less than normal amount of glucose in the blood) is also increased. Early in pregnancy, when hepatic production of glucose is diminished and peripheral use of glucose is enhanced, hypoglycemia occurs frequently, often during sleep. Later in pregnancy, hypoglycemia may also result as insulin doses are adjusted to maintain euglycemia (a normal blood glucose level). Women with a prepregnancy history of severe hypoglycemia are at increased risk for severe hypoglycemia during gestation. Mild to moderate hypoglycemic episodes do not appear to have significant damaging effects on fetal well-being (see Table 29-2).
Fetal and Neonatal Risks and Complications
From the moment of conception the infant of a woman with diabetes faces an increased risk of complications that may occur during the antepartum, intrapartum, or neonatal periods. Infant morbidity and mortality rates associated with diabetic pregnancy are significantly reduced with strict control of maternal glucose levels before and during pregnancy.
Despite the improvements in care of pregnant women with diabetes, IUFD (sometimes called stillbirth) remains a major concern. Approximately 2% to 5% of all fetal deaths occur in women whose pregnancies are complicated by preexisting diabetes. Hyperglycemia, ketoacidosis, congenital anomalies, infections, and maternal obesity are thought to be reasons for fetal death. In the third trimester, fetal acidosis is the most likely cause of fetal death (Paidas & Hossain, 2009).
The most important cause of perinatal loss in diabetic pregnancy is congenital malformations, which account for 30% to 50% of all perinatal loss (Lindsay, 2006). The incidence of congenital malformations is related to the severity and duration of the diabetes. Hyperglycemia during the first trimester of pregnancy, when organs and organ systems are forming, is the main cause of diabetes-associated birth defects. Anomalies commonly seen in infants affect primarily the cardiovascular system, the central nervous system (CNS), and the skeletal system (Cunningham et al., 2010; Moore & Catalano, 2009) (see Chapter 35).
The fetal pancreas begins to secrete insulin at 10 to 14 weeks of gestation. The fetus responds to maternal hyperglycemia by secreting large amounts of insulin (hyperinsulinism). Insulin acts as a growth hormone, causing the fetus to produce excess stores of glycogen, protein, and adipose tissue and leading to increased fetal size, or macrosomia. Birth injuries are more common in infants born to mothers with diabetes compared with mothers who do not have diabetes, and macrosomic fetuses have the highest risk for this complication. Common birth injuries associated with diabetic pregnancies include brachial plexus palsy, facial nerve injury, humerus or clavicle fracture, and cephalhematoma. Most of these injuries are associated with difficult vaginal birth and shoulder dystocia (Moore & Catalano, 2009). Hypoglycemia at birth is also a risk for infants born to mothers with diabetes (for further discussion of neonatal complications related to maternal diabetes, see Chapter 35).
Care Management
When a pregnant woman with diabetes initiates prenatal care, a thorough evaluation of her health status is completed. At the initial visit a complete physical examination is performed to assess the woman’s health status. In addition to the routine prenatal examination, specific efforts are made to assess the effects of the diabetes, specifically retinopathy, nephropathy, peripheral and autonomic neuropathy, peripheral vascular, and cardiac involvement (Gilbert, 2011) (see the Nursing Process box).
In addition to routine prenatal laboratory tests, baseline renal function may be assessed with a 24-hour urine collection for total protein excretion and creatinine clearance. Urinalysis and culture are performed to assess for the presence of a urinary tract infection (UTI), which is common in diabetic pregnancy. Because of the risk of coexisting thyroid disease, thyroid function tests may also be performed (see later discussion of thyroid disorders). The glycosylated hemoglobin A1c level may be measured to assess recent glycemic control. With prolonged hyperglycemia, some of the hemoglobin remains saturated with glucose for the life of the red blood cell (RBC). Therefore, a test for glycosylated hemoglobin provides a measure of glycemic control over time, specifically over the previous 4 to 6 weeks. Hemoglobin A1c levels greater than 6 indicate elevated glucose during the previous 4 to 6 weeks (Gilbert, 2011). Fasting blood glucose or random (1 to 2 hours after eating) glucose levels may be assessed during antepartum visits (Fig. 29-2). Self-monitoring blood glucose records may also be reviewed.
FIG. 29-2 A, Clinic nurse collects blood to determine glucose level. B, Nurse interprets glucose value displayed by monitor. (Courtesy Dee Lowdermilk, UNC Ambulatory Care Clinics, Chapel Hill, NC.)
Because of her high risk status, a woman with diabetes is monitored much more frequently and thoroughly than other pregnant women. During the first and second trimesters of pregnancy, her routine prenatal care visits will be scheduled every 1 to 2 weeks. In the last trimester she will likely be seen one or two times each week. In the past, routine hospitalization for management of the diabetes, such as insulin dose changes, was common. With the availability of improved home glucose monitoring and the growing reluctance of third-party payers to reimburse for hospitalization, pregnant women with diabetes are now generally managed as outpatients. Some client and family education and maternal and fetal assessment may be performed in the home, depending on the woman’s insurance coverage and care provider preference.
Achieving and maintaining constant euglycemia, with plasma glucose levels in the range of 65 to 95 mg/dl preprandially and no higher than 130 to 140 mg/dl when measured 1 hour postprandially (Table 29-3), is the primary goal of medical therapy (Moore & Catalano, 2009). Euglycemia is achieved through a combination of diet, insulin, and exercise. Providing the woman with the knowledge, skill, and motivation she needs to achieve and maintain excellent blood glucose control is the primary nursing goal.
TABLE 29-3
TARGET BLOOD GLUCOSE LEVELS DURING PREGNANCY
| TIME OF DAY | TARGET PLASMA GLUCOSE LEVEL (mg/dl) |
| Premeal or fasting | >65 but <95 |
| Postmeal (1 hr) | <130-140 |
| Postmeal (2 hr) | <120 |
Sources: Landon, M., Catalano, P., & Gabbe, S. (2007). Diabetes mellitus complicating pregnancy. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone; Moore, T., & Catalano, P. (2009). Diabetes in pregnancy. In R. Creasy, R. Resnik, J. Iams, C. Lockwood, & T. Moore (Eds.), Creasy and Resnik’s maternal-fetal medicine: Principles and practice (6th ed.). Philadelphia: Saunders.
Achieving euglycemia requires commitment on the part of the woman and her family to make the necessary lifestyle changes, which can sometimes seem overwhelming. Maintaining tight blood glucose control necessitates that the woman follow a consistent daily schedule. She must get up and go to bed, eat, exercise, and take insulin at the same time each day. Blood glucose measurements are taken frequently to determine how well the major components of therapy (diet, insulin, and exercise) are working together to control blood glucose levels. The pregnant woman with diabetes should wear a medical identification bracelet at all times and carry insulin, syringes, and sources of fast sugar (simple carbohydrate) with her whenever she is away from home.
Because the woman with diabetes is at increased risk for infections, eye problems, and neurologic changes, foot care and general skin care are important. A daily bath that includes good perineal care and foot care is important. For dry skin, lotions, creams, or oils can be applied. Tight clothing should be avoided. Shoes or slippers that fit properly should be worn at all times and are best worn with socks or stockings. Feet should be inspected regularly; toenails should be cut straight across, and professional help should be sought for any foot problems. Extremes of temperature should be avoided.
Diet: The woman with pregestational diabetes has usually had nutritional counseling regarding management of her diabetes. However, because pregnancy produces special nutritional concerns and needs, the woman must be educated to incorporate these changes into dietary planning. For the woman who has “controlled” her diabetes for several years the changes in her insulin and dietary needs mandated by pregnancy may be difficult. Nutritional counseling is usually provided by a registered dietitian.
Dietary management during diabetic pregnancy must be based on blood (not urine) glucose levels. The diet is individualized to allow for increased fetal and metabolic requirements, with consideration of such factors as prepregnancy weight and dietary habits, overall health, ethnic background, lifestyle, stage of pregnancy, knowledge of nutrition, and insulin therapy. The dietary goals are to provide weight gain consistent with a normal pregnancy, to prevent ketoacidosis, and to minimize wide fluctuation of blood glucose levels.
For nonobese women, dietary counseling based on preconception body mass index (BMI) is 30 to 35 kcal/kg/day (Cunningham et al., 2010). In contrast, for obese women with a BMI greater than 30, experts recommend that the caloric intake total 25 kcal/kg/day (Moore & Catalano, 2009). The average diet includes 2200 calories (first trimester) to 2500 calories (second and third trimesters). Total calories may be distributed among three meals and one evening snack or, more commonly, three meals and two or three snacks. Meals should be eaten on time and never skipped. Going more than 4 hours without food intake increases the risk for episodes of hypoglycemia. Snacks must be carefully planned in accordance with insulin therapy to prevent fluctuations in blood glucose levels. A large bedtime snack of at least 25 g of carbohydrate with some protein or fat is recommended to help prevent hypoglycemia and starvation ketosis during the night (Moore & Catalano).
The ideal diet is composed of 55% carbohydrate, 20% protein, and 25% fat, with less than 10% as saturated fat (Cunningham et al., 2010) (see the Teaching for Self-Management box: Dietary Management of Diabetic Pregnancy). Simple carbohydrates are limited. Complex carbohydrates that are high in fiber content are recommended because the starch and protein in such foods help regulate the blood glucose level by more sustained glucose release (Gilbert, 2011; Moore & Catalano, 2009).
Exercise: Although studies have shown that exercise enhances the use of glucose and decreases insulin need in women without diabetes, data regarding exercise in women with pregestational diabetes are limited. Any prescription of exercise during pregnancy for women with diabetes should be given by the primary health care provider and should be monitored closely to prevent complications. Regular exercise may be contraindicated in women with diabetes who also have uncontrolled hypertension, advanced retinopathy, or severe autonomic or peripheral neuropathy (Gilbert, 2011).
When exercise is prescribed by the health care provider as part of the treatment plan, specific instructions are given to the woman. Aerobic exercise with resistance training for at least 30 minutes most days of the week is the best type of exercise (Gilbert, 2011). Other exercises that may be recommended are non–weight-bearing activities such as arm exercises or use of a recumbent bicycle. The best time for exercise is after meals, when the blood glucose level is rising. To monitor the effect of insulin on blood glucose levels the woman can measure blood glucose before, during, and after exercise.
Insulin Therapy: Adequate insulin is the primary factor in the maintenance of euglycemia during pregnancy, thus ensuring proper glucose metabolism of the woman and fetus. Insulin requirements during pregnancy change dramatically as the pregnancy progresses, necessitating frequent adjustments in the dose. In the first trimester, from weeks 3 to 7 of gestation, insulin requirements are increased followed by a decrease between weeks 7 and 15 of gestation. The commonly prescribed dose is 0.7 units/kg in the first trimester for women with type 1 diabetes. During the second and third trimesters, because of insulin resistance, the dose must be increased significantly to maintain target glucose levels. Insulin requirements normally plateau after 35 weeks of gestation and often drop significantly after 38 weeks (Moore & Catalano, 2009).
For the woman with type 1 pregestational diabetes who has typically been accustomed to one injection per day of intermediate-acting insulin, multiple daily injections of mixed insulin are a new experience. The woman with type 2 diabetes previously treated with oral hypoglycemics is faced with the task of learning to self-administer injections of insulin. The nurse is instrumental in the education and support of women with pregestational diabetes in regard to insulin administration and adjustment of the insulin dose to maintain euglycemia (see the Teaching for Self-Management box: Self-Administration of Insulin and Box 29-1).
Since 1982, most insulin preparations have been produced by inserting portions of deoxyribonucleic acid (DNA) (“recombinant DNA”) into special laboratory-cultivated bacteria or yeast cells. The cells then produce synthetic human insulin (Humulin), which is less likely to cause antibody formation than animal-derived (beef or pork) insulin. More recently, insulin products called insulin analogs, in which the structure differs slightly from human insulin, have been produced. This small alteration in insulin structure results in changes in the onset and peak of action of the medication. The most commonly used insulin preparations include rapid-acting, short-acting, intermediate-acting, and long-acting (Landon et al., 2007) (Table 29-4). Mixtures of short- and intermediate-acting insulins in several proportions are also available.
TABLE 29-4
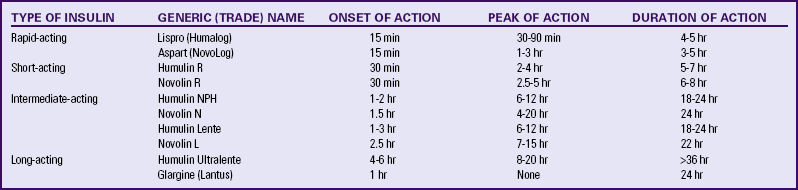
Source: Landon, M., Catalano, P., & Gabbe, S. (2007). Diabetes mellitus complicating pregnancy. In S. Gabbe, J. Niebyl, & J. Simpson (Eds.), Obstetrics: Normal and problem pregnancies (5th ed.). Philadelphia: Churchill Livingstone.
Lispro (Humalog) and aspart (NovoLog) are commonly prescribed rapid-acting insulins with a shorter duration of action than regular insulin. Advantages of rapid-acting insulins include convenience because they are injected immediately before mealtime, less hyperglycemia after meals, and fewer hypoglycemic episodes in some people. Because their effects last only 3 to 5 hours, most clients require a longer-acting insulin in addition to the rapid-acting insulin to maintain optimal blood glucose levels (Landon et al., 2007; Moore & Catalano, 2009) (see Table 29-4).
Glargine (Lantus) is a long-acting insulin lasting approximately 24 hours. Small amounts of glargine insulin are slowly released, with no pronounced peak. This preparation is most often used with women who have insulin-resistant diabetes (type 2) requiring high doses of long-acting insulin. Glargine insulin is combined with a rapid-acting insulin to prevent hypoglycemia. Concerns associated with this medication include the need for monitoring for nocturnal hypoglycemia (Moore & Catalano, 2009) and a possible increase in the progression of retinopathy in some women (Landon et al., 2007) (see Table 29-4).
Most women with insulin-dependent diabetes are managed with two or three injections per day (Landon et al., 2007). Usually two thirds of the daily insulin dose, with intermediate-acting and short-acting insulin combined in a 2:1 ratio, is given before breakfast. The remaining one third, again a combination of longer- and short-acting insulin, is administered in the evening before dinner. To reduce the risk of hypoglycemia during the night, separate injections often are administered, with short-acting insulin given before dinner followed by longer-acting insulin at bedtime. An alternative insulin regimen that works well for some women is to administer short-acting insulin before each meal and longer-acting insulin at bedtime (Moore & Catalano, 2009).
Continuous subcutaneous insulin infusion (CSII) systems are increasingly used during pregnancy. The insulin pump is designed to mimic more closely the function of the pancreas in secreting insulin (Fig. 29-3). This portable battery-powered device is worn, similar to a pager, during most daily activities. The pump infuses regular insulin at a set basal rate and has the capacity to deliver up to four different basal rates in 24 hours. It also delivers bolus doses of insulin before meals to control postprandial blood glucose levels. A fine-gauge plastic catheter is inserted into subcutaneous tissue, usually in the abdomen, and attached to the pump syringe by connecting tubing. The subcutaneous catheter and connecting tubing are changed every 2 to 3 days, although the infusion tubing can be left in place for several weeks without local complications. Although the insulin pump is convenient and generally provides good glycemic control, complications such as pump failure, precipitation of insulin inside the pump mechanism, abscess formation, and poor uptake from the infusion site can still occur. Therefore, use of the insulin pump requires a knowledgeable, motivated woman; skilled health care providers; and prompt 24-hour availability of emergency assistance (Moore & Catalano, 2009).
Monitoring Blood Glucose Levels: Blood glucose testing at home with a glucose reflectance meter is considered the standard of care for monitoring blood glucose levels during pregnancy. It provides the most important tool available to the woman to assess her degree of glycemic control. Most of the newer reflectance meters are calibrated to provide plasma (rather than whole blood) glucose values. Plasma glucose values are 10% to 15% lower than those measured in whole blood from the same sample (Moore & Catalano, 2009).
To perform blood glucose monitoring a drop of blood is obtained by means of a fingerstick and placed on a test strip. After a specified amount of time the glucose level is displayed by the meter (see the Teaching for Self-Management box: Self-Testing of Blood Glucose Level). Blood glucose levels are routinely measured at various times throughout the day, such as before breakfast, lunch, and dinner; 2 hours after each meal; at bedtime; and in the middle of the night. When any readjustment in the insulin dose or diet is made, more frequent measurement of blood glucose is warranted. If nausea, vomiting, or diarrhea occurs, or if any infection is present, the woman will be asked to monitor her blood glucose levels more closely than usual.
Target levels of blood glucose during pregnancy are lower than nonpregnant values (see Table 29-3). Acceptable fasting levels are generally between 65 and 95 mg/dl, and 1-hour postprandial levels should be less than 130 to 140 mg/dl (Moore & Catalano, 2009). Two-hour postprandial levels should be less than 120 mg/dl (Landon et al., 2007). The woman should be told to report episodes of hypoglycemia (less than 60 mg/dl) and hyperglycemia (more than 200 mg/dl) immediately to her health care provider so that adjustments in diet or insulin therapy can be made.
Pregnant women with diabetes are much more likely to develop hypoglycemia than hyperglycemia. Most episodes of mild or moderate hypoglycemia can be treated with oral intake of 15 g of simple carbohydrate (fast sugar) (see the Teaching for Self-Management box: Treatment for Hypoglycemia). If severe hypoglycemia occurs, in which case the woman experiences a decrease in or loss of consciousness or an inability to swallow, she will require a parenteral injection of glucagon or intravenous (IV) glucose. Because hypoglycemia can develop rapidly, and because impaired judgment can be associated with even moderate episodes, family members, friends, and work colleagues must be able to recognize signs and symptoms quickly and initiate proper treatment if necessary.
Hyperglycemia is less likely than hypoglycemia to occur, but it can rapidly progress to DKA, which is associated with an increased risk of fetal death (Cunningham et al., 2010; Moore & Catalano, 2009). Women and family members should be particularly alert for signs and symptoms of hyperglycemia, especially when infections or other illnesses occur (see the Teaching for Self-Management box: What to Do When Illness Occurs).
Urine Testing: Urine testing for glucose is not beneficial during pregnancy. Because of the lowered renal threshold for glucose, the degree of glycosuria does not accurately reflect the blood glucose level. Urine testing for ketones, however, continues to have a place in diabetic management. Monitoring for urine ketones may detect inadequate caloric or carbohydrate intake or skipped meals or snacks. Testing may also be performed when illness occurs, or when the blood glucose level is greater than 200 mg/dl (Gilbert, 2011).
Complications Requiring Hospitalization: Occasionally, hospitalization is necessary to regulate insulin therapy and stabilize glucose levels. Infection, which can lead to hyperglycemia and DKA, is an indication for hospitalization, regardless of gestational age. Hospitalization during the third trimester for close maternal and fetal observation may be indicated for women whose diabetes is poorly controlled. In addition, women with diabetes are 10% to 20% more likely than women who do not have diabetes to also have preexisting hypertension or develop preeclampsia, which may necessitate hospitalization (Moore & Catalano, 2009).
Fetal Surveillance: Diagnostic techniques for fetal surveillance are often performed to assess fetal growth and well-being. The goals of fetal surveillance are to detect fetal compromise as early as possible and to prevent intrauterine fetal death or unnecessary preterm birth.
Early in pregnancy the estimated date of birth is determined. A baseline sonogram is obtained during the first trimester to assess gestational age. Follow-up ultrasound examinations are usually performed during the pregnancy (as often as every 4 to 6 weeks) to monitor fetal growth, estimate fetal weight, and detect hydramnios, macrosomia, and congenital anomalies.
Because the fetus of a woman with diabetes is at increased risk for neural tube defects (e.g., spina bifida, anencephaly, microcephaly), measurement of maternal serum alpha-fetoprotein is performed between 15 and 20 weeks of gestation (ideally between 16 and 18 weeks of gestation) (Wapner, Jenkins, & Khalek, 2009). This assessment is often performed in conjunction with a detailed ultrasound study to examine the fetus for neural tube defects.
Fetal echocardiography may be performed between 20 and 22 weeks of gestation to detect cardiac anomalies, especially in women who had less-than-desirable glucose control early in pregnancy, as demonstrated by a hemoglobin A1c level above 6% at the first prenatal visit (Gilbert, 2011; Moore & Catalano, 2009). Some practitioners repeat this fetal surveillance test at 34 weeks of gestation. Doppler studies of the umbilical artery may be performed in women with vascular disease to detect placental compromise.
The majority of fetal surveillance measures are concentrated in the third trimester, when the risk of fetal compromise is greatest. The goals of antepartum testing during the third trimester are to prevent IUFD and maximize the opportunity for the woman to safely give birth vaginally. Pregnant women should be taught how to make daily fetal movement counts, beginning at 28 weeks of gestation (see Chapter 26) (Moore & Catalano, 2009).
Biophysical testing (nonstress testing, contraction stress testing, or biophysical profile) once or twice weekly to evaluate fetal well-being, is typically begun around 34 weeks of gestation. This testing should begin around 28 weeks in women who have poor glucose control or significant hypertension (Moore & Catalano, 2009) (see Chapter 26).
Determination of Birth Date and Mode of Birth: The optimal time for birth is between 38.5 and 40 weeks of gestation, as long as good metabolic control is maintained and parameters of antepartum fetal surveillance remain within normal limits. Reasons to proceed with birth before term include poor metabolic control, worsening hypertensive disorders, fetal macrosomia, or fetal growth restriction (Moore & Catalano, 2009).
Many practitioners plan for elective labor induction between 38 and 40 weeks of gestation. To confirm fetal lung maturity an amniocentesis should be performed when birth will occur before 38.5 weeks of gestation. For the pregnancy complicated by diabetes, fetal lung maturation is best predicted by the amniotic fluid phosphatidylglycerol (greater than 3%). If the fetal lungs are still immature, birth should be postponed until 40 weeks of gestation as long as fetal assessment test results remain reassuring. After that time, however, the benefits of conservative management are outweighed by the increasing risk of fetal compromise if the pregnancy is allowed to continue. Birth, despite poor fetal lung maturity, may be necessary when testing suggests fetal compromise or worsening maternal condition, such as deteriorating renal function or severe preeclampsia (Moore & Catalano, 2009).
Although vaginal birth is expected for most women with pregestational diabetes, the cesarean rate for these women ranges from 50% to 80% (Cunningham et al., 2010). The American College of Obstetricians and Gynecologists (ACOG) recommends that cesarean birth be considered when the estimated fetal weight is expected to be greater than 4500 g in an attempt to reduce the risk of shoulder dystocia. This recommendation appears to result in a small improvement in neonatal outcome (Moore & Catalano, 2009). Fetal distress and induction failures before term also contribute to the high rate of cesarean birth in these women (Gilbert, 2011).
Intrapartum
During the intrapartum period the woman with pregestational diabetes must be monitored closely to prevent complications related to dehydration, hypoglycemia, and hyperglycemia. An IV line is inserted for infusion of a maintenance fluid. Initially this infusion may be normal saline or lactated Ringer’s solution. The IV fluid will be changed to one containing 5% dextrose during active labor to provide the energy (calories) necessary for the woman to accomplish the work and manage the stress of labor and birth. Most commonly, insulin is administered by continuous infusion, piggybacked into the main IV line. Only rapid- or short-acting insulin may be administered intravenously. Determinations of blood glucose levels are made every hour, and fluids and insulin are adjusted to maintain the blood glucose level at less than 140 mg/dl (Landon et al., 2007). Maintaining this target glucose level is essential because hyperglycemia during labor can cause metabolic problems in the neonate, particularly hypoglycemia.
During labor continuous fetal heart monitoring is necessary. The woman should assume an upright or side-lying position during bed rest in labor to prevent supine hypotension because of a large fetus or polyhydramnios. Labor (spontaneous or induced) is allowed to progress as long as expected rates of cervical dilation and fetal descent are maintained, and fetal well-being is evident. Failure to progress may indicate a macrosomic infant and cephalopelvic disproportion, necessitating a cesarean birth. The woman is observed and treated during labor for complications of diabetes such as hyperglycemia, ketosis, and ketoacidosis. During second-stage labor, shoulder dystocia may occur with birth of a macrosomic infant (see Chapter 33). A neonatologist, pediatrician, or neonatal nurse practitioner will likely be present at the birth to initiate assessment and neonatal care.
If a cesarean birth is planned, it should be scheduled in the early morning to facilitate glycemic control. Women should take their full dose of insulin the night before surgery. No morning insulin is given on the day of surgery, and the woman is given nothing by mouth. Epidural anesthesia is recommended because hypoglycemia can be detected earlier if the woman is awake. After surgery, glucose levels should be monitored carefully. Generally sliding scale insulin is used to control blood glucose levels until the woman resumes a regular diet (Moore & Catalano, 2009).
Postpartum
During the first 24 hours postpartum, insulin requirements decrease substantially because the major source of insulin resistance, the placenta, has been removed. Women with type 1 diabetes may require only one third to one half of their last pregnancy insulin dose on the first postpartum day, provided that they are eating a full diet (Landon et al., 2007). These women who give birth by cesarean may require an IV infusion of glucose and insulin until they resume a regular diet (Moore & Catalano, 2009). Several days after birth may be required to reestablish carbohydrate homeostasis (see Fig. 29-1, D and E). Blood glucose levels are carefully monitored in the postpartum period and the insulin dose is adjusted, often using a sliding scale. The woman who has insulin-dependent diabetes must realize the importance of eating on time even if the baby needs feeding or other pressing demands exist. Women with type 2 diabetes often require only 30% to 50% of their pregnancy insulin dose in the postpartum period (Moore & Catalano).
Possible postpartum complications include preeclampsia or eclampsia, hemorrhage, and infection. Hemorrhage is a possibility if the mother’s uterus was overdistended (hydramnios, macrosomic fetus) or overstimulated (oxytocin induction). Postpartum infections such as endometritis are more likely to occur in women with diabetes than in women who do not have diabetes.
Mothers are encouraged to breastfeed. In addition to the advantages of maternal satisfaction and pleasure, breastfeeding has an antidiabetogenic effect for the children of women with diabetes and for women with gestational diabetes (Lindsay, 2006; Moore & Catalano, 2009). This effect is important because a child born to a mother with type 2 diabetes has a 70% chance of also developing type 2 diabetes later in life. In addition, children who were exposed to hyperglycemia prenatally have an increased risk for obesity in childhood (Gilbert, 2011).
Insulin requirements in breastfeeding women may be one half of prepregnancy levels because of the carbohydrate used in human milk production. Because glucose levels are lower than normal, breastfeeding women are at increased risk for hypoglycemia, especially in the early postpartum period and after breastfeeding sessions, particularly after late-night nursing (Gilbert, 2011; Moore & Catalano, 2009). Breastfeeding mothers with diabetes may be at increased risk for mastitis and yeast infections of the breast. The insulin dose, which is decreased during lactation, must be recalculated at weaning (see Fig. 29-1, F).
The mother may have early breastfeeding difficulties. Poor metabolic control may delay lactogenesis and contribute to decreased milk production (Moore & Catalano, 2009). Initial contact with and opportunity to breastfeed the infant may be delayed for mothers who gave birth by cesarean or if infants are placed in neonatal intensive care units or special care nurseries for observation during the first few hours after birth. Support and assistance from nursing staff and lactation specialists can facilitate the mother’s early experience with breastfeeding and encourage her to continue.
The new mother needs information about family planning and contraception. Although family planning is important for all women, it is essential for the woman with diabetes so as to safeguard her own health and to promote optimal outcomes in future pregnancies. The risks and benefits of contraceptive methods should be discussed with the mother and her partner before discharge from the hospital. Barrier methods are often recommended as safe, inexpensive options that have no inherent risks for women with diabetes. The intrauterine device (IUD) may also be used without concerns about an increased risk of infection (Landon et al., 2007).
Use of oral contraceptives by women with diabetes is controversial because of the risk of thromboembolic and vascular complications and the effect on carbohydrate metabolism. In non-smoking women who are less than 35 years old and do not have vascular disease, combination low-dose oral contraceptives be prescribed. Progestin-only oral contraceptives also be used because they affect minimally, if at all, carbohydrate metabolism (Cunningham et al., 2010). Close monitoring of blood pressure and lipid levels is necessary to detect complications (Landon et al., 2007).
Opinion is divided about the use of long-acting parenteral progestins, such as Depo-Provera. Some health care providers recommend their use, particularly in women who are noncompliant with daily dosing oral contraceptives. In contrast, other health care providers believe this method may adversely affect glycemic control.
Transdermal (patch) and transvaginal (vaginal ring) administration are newer contraceptive methods, particularly effective in women who prefer weekly or every-third-week dosing, respectively. For women weighing more than 90 kg the contraceptive failure rate with transdermal administration is higher than in normal-weight women. Therefore, this method would be contraindicated in obese women. In addition, women who choose the patch as their contraceptive method should have no risk factors for cardiovascular or thromboembolic disease (Cunningham et al., 2010).
The risks associated with pregnancy increase with the duration and severity of diabetes. In addition, pregnancy may contribute to the vascular changes associated with diabetes. This information needs to be thoroughly discussed with the woman and her partner. Sterilization is often recommended for the woman who has completed her family, who has poor metabolic control, or who has significant vascular problems.
Gestational Diabetes Mellitus
GDM complicates approximately 3% to 9% of all pregnancies (Moore & Catalano, 2009) and accounts for more than 90% of all cases of diabetic pregnancy (Landon et al., 2007). According to White’s classification system, these women fall into classes A1 and A2 (see Table 29-1). GDM is more likely to occur among Hispanic, Native American, Asian, and African-American women than in Caucasians and is likely to recur in future pregnancies; the risk for development of overt diabetes in later life is also increased (Moore & Catalano). This tendency is especially true of women whose GDM is diagnosed early in pregnancy or who are obese (Landon et al.). Classic risk factors for GDM include maternal age older than 25 years, previous macrosomic infant, previous unexplained IUFD, previous pregnancy with GDM, strong immediate family history of type 2 diabetes or GDM, obesity (weight more than 90 kg), and fasting blood glucose greater than 140 mg/dl or random blood glucose greater than 200 mg/dl. Women at high risk for developing GDM should have glucola screening at the first prenatal visit and again at 24 to 28 weeks of gestation if the initial screen is negative (Landon et al.).
GDM is usually diagnosed during the second half of pregnancy. As fetal nutrient demands rise during the late second and the third trimesters, maternal nutrient ingestion induces greater and more sustained levels of blood glucose. At the same time, maternal insulin resistance is also increasing because of the insulin-antagonistic effects of the placental hormones, cortisol, and insulinase. Consequently, maternal insulin demands rise as much as threefold. Most pregnant women are capable of increasing insulin production to compensate for insulin resistance and to maintain euglycemia. When the pancreas is unable to produce sufficient insulin or the insulin is not used effectively, however, gestational diabetes can result.
Fetal Risks
No increase in the incidence of birth defects has been found among infants of women who develop gestational diabetes after the first trimester because the critical period of organ formation has already passed by that time (Moore & Catalano, 2009). However, Anderson and associates (2005) found that women who were obese before conception (BMI more than 30 kg/m2) and developed gestational diabetes were at greater risk to give birth to infants with CNS defects.
Screening for Gestational Diabetes Mellitus
All pregnant women not known to have pregestational diabetes should be screened for GDM by history, clinical risk factors, or laboratory screening of blood glucose levels. Based on history and clinical risk factors, some women are at low risk for the development of GDM. Therefore, glucose testing for this low risk population is not cost effective (ADA, 2008). This group includes normal-weight women younger than 25 years of age who have no family history of diabetes, are not members of an ethnic or a racial group known to have a high prevalence of the disease, and have no history of abnormal glucose tolerance or adverse obstetric outcomes usually associated with GDM (ADA).
The screening test (glucola screening) most often used consists of a 50-g oral glucose load followed by a plasma glucose measurement 1 hour later. The woman need not be fasting. A glucose value of 130 to 140 mg/dl is considered a positive screen and should be followed by a 3-hour (100-g) oral glucose tolerance test (OGTT). The OGTT is administered after an overnight fast and at least 3 days of unrestricted diet (at least 150 g of carbohydrate) and physical activity. The woman is instructed to avoid caffeine because it will increase glucose levels and to abstain from smoking for 12 hours before the test. The 3-hour OGTT requires a fasting blood glucose level, which is drawn before giving a 100-g glucose load. Blood glucose levels are then drawn 1, 2, and 3 hours later. The woman is diagnosed with gestational diabetes if two or more values are met or exceeded (Moore & Catalano, 2009) (Fig. 29-4).
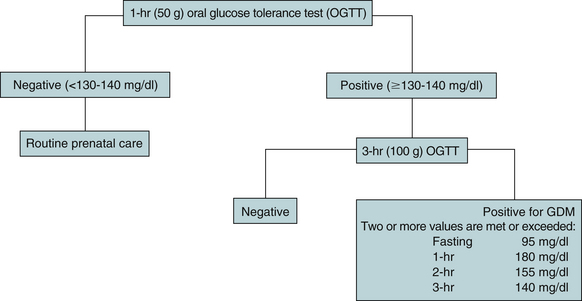
FIG. 29-4 Screening and diagnosis for gestational diabetes. (Sources: American Diabetes Association. [2008]. Position statement: Diagnosis and classification of diabetes mellitus. Diabetes Care, 31[Suppl.], S55-S60; Moore, T., & Catalano, P. [2009]. Diabetes in pregnancy. In R. Creasy, R. Resnik, J. Iams, C. Lockwood, & T. Moore [Eds.], Creasy and Resnik’s maternal-fetal medicine: Principles and practice [6th ed.]. Philadelphia: Saunders.)
Nursing diagnoses and expected outcomes of care for women with GDM are basically the same as those for women with pregestational diabetes except that the time frame for planning may be shortened with GDM because the diagnosis is usually made later in pregnancy (see the Nursing Care Plan).
Care Management
When the diagnosis of gestational diabetes is made, treatment begins immediately, allowing little or no time for the woman and her family to adjust to the diagnosis before they are expected to participate in the treatment plan. With each step of the treatment plan the nurse and other health care providers should educate the woman and her family, providing detailed and comprehensive explanations to ensure understanding, participation, and adherence to the necessary interventions. Potential complications should be discussed, and the need for maintenance of euglycemia throughout the remainder of the pregnancy reinforced. Knowing that gestational diabetes typically disappears when the pregnancy is over may be reassuring for the woman and her family.
As with pregestational diabetes, the aim of therapy in women with GDM is strict blood glucose control. Fasting blood glucose levels should range from 65 to 95 mg/dl, and 1-hour postprandial blood glucose levels should be less than 130 to 140 mg/dl (Moore & Catalano, 2009).
Diet: Dietary modification is the mainstay of treatment for GDM. The woman with GDM is placed on a standard diabetic diet. The usual prescription is 30 kcal/kg/day based on a normal preconception weight. For obese women the usual prescription is up to 25 kcal/kg/day, which translates into 1500 to 2000 kcal/day. Carbohydrate intake is restricted to approximately 50% of caloric intake (Moore & Catalano, 2009). Dietary counseling by a registered dietician is recommended.
Exercise: Several studies have examined the benefits of exercise in women with GDM. Three randomized trials found that exercise improved cardiovascular fitness without improving pregnancy outcome. Another study found that exercise decreased the need for insulin in overweight women with GDM (Cunningham et al., 2010). ![]()
Monitoring Blood Glucose Levels: Blood glucose monitoring is necessary to determine whether euglycemia can be maintained by diet and exercise. Women are instructed to monitor their blood sugar daily. The frequency and timing of blood glucose monitoring should be individualized for each woman. A typical schedule for monitoring blood glucose is on rising in the morning, after breakfast, before and after lunch, after dinner, and at bedtime (Moore & Catalano, 2009). Women with GDM usually perform self-monitoring at with additional monitoring at the clinic or office visit.
Medications for Controlling Blood Glucose Levels: Up to 20% of women with GDM will require insulin during the pregnancy to maintain adequate blood glucose levels, despite compliance with the prescribed diet. In contrast to women with insulin-dependent diabetes, women with gestational diabetes are initially managed with diet and exercise alone. If fasting plasma glucose levels are greater than 95 mg/dl or 2-hour postprandial levels are greater than 120 mg/dl, then insulin therapy is begun (Cunningham et al., 2010) (see Table 29-3). Glyburide, an oral hypoglycemic agent, is being used more frequently with women with GDM instead of insulin. The fact that only minimal amounts of glyburide cross the placenta to the fetus makes it a good drug for use during pregnancy. It has also been used in women with type 2 diabetes who required large amounts of insulin to achieve glucose control with smaller insulin doses. Studies have shown that glyburide should be taken at least
30 minutes (preferably 1 hour) before a meal so that its peak effect covers the 2-hour postprandial blood glucose level. Because episodes of hypoglycemia can occur between meals, women taking glyburide should always carry with them sources of fast sugar (Moore & Catalano, 2009). Women with diabetes who are unable or unwilling to take insulin by injection or are cognitively impaired also may be candidates for glyburide use.
Fetal Surveillance: No standard recommendation has been formulated for fetal surveillance in pregnancies complicated by GDM. Women whose blood glucose levels are well controlled by diet are at low risk for fetal complications. Limited antepartum fetal testing is performed in women with gestational diabetes as long as their fasting and 2-hour postprandial blood glucose levels remain within normal limits and they have no other risk factors. Women with hypertension, a prior IUFD, or suspected macrosomia or those who require insulin for blood glucose control may have twice-weekly nonstress testing beginning at 32 weeks of gestation (Landon et al., 2007). In general, women with GDM can continue pregnancy until 40 weeks of gestation and the spontaneous onset of labor. However, fetal growth should be monitored carefully because the risk for macrosomia as the pregnancy approaches 40 weeks of gestation is apparently increased (Landon et al.).
Intrapartum
During the labor and birth process, blood glucose levels are monitored hourly to maintain levels at 80 to 120 mg/dl (Moore & Catalano, 2009). Levels within this range will decrease the incidence of neonatal hypoglycemia. Infusing rapid-acting insulin intravenously may be necessary during labor to maintain blood glucose levels within this range. However, it is usually possible to maintain excellent glucose control in women with Class A1 GDM during labor by simply avoiding dextrose intravenous fluids (Gilbert, 2011; Moore & Catalano, 2009). Although gestational diabetes is not an indication for cesarean birth, this procedure may be necessary in the presence of preeclampsia or macrosomia.
Postpartum
Most women with GDM will return to normal glucose levels after childbirth. However, the recurrence risk for GDM in the next pregnancy is 35% to 75%. In addition, women who have had GDM have a 35% to 60% risk for developing type 2 diabetes mellitus within the next twenty years (Gilbert, 2011). Assessment for carbohydrate intolerance with a 75-g OGTT should be performed at 6 to 12 weeks postpartum and a random or fasting blood glucose level should be checked each year (Cunningham et al., 2010; Gilbert). Obesity is a major risk factor for the later development of diabetes. Women with a history of GDM, particularly those who are overweight, should be encouraged to make lifestyle changes that include weight loss and exercise to reduce this risk (Gilbert). Children born to women with GDM are also at risk for becoming obese in childhood or adolescence (Lindsay, 2006).
Hyperemesis Gravidarum
Nausea and vomiting complicate as many as 80% of all pregnancies beginning typically at 4 weeks of gestation. These symptoms are usually confined to the first 20 weeks of gestation (Kelly & Savides, 2009). Although nausea and vomiting are distressing, they are typically benign, with no significant metabolic alterations or risks to the mother or fetus. The cause of nausea and vomiting in pregnancy is not well understood, although it may involve relaxation of the smooth muscle of the stomach and increasing levels of estrogen, progesterone, and human chorionic gonadotropin (hCG). Pregnancies complicated by nausea and vomiting generally have a more favorable outcome than those without these symptoms (Gordon, 2007).
When vomiting during pregnancy becomes excessive enough to cause weight loss, electrolyte imbalance, nutritional deficiencies, and ketonuria, the disorder is termed hyperemesis gravidarum. This disorder occurs in approximately 0.5% of all live births. Hyperemesis gravidarum usually begins during the first trimester, but approximately 10% of women with the disorder continue to have symptoms throughout the pregnancy (Kelly & Savides, 2009). It has been associated with women who are nulliparous, have increased body weight, have a history of migraines (Davis, 2004), have a multiple gestation, have gestational trophoblastic disease, or are carrying a fetus with a chromosomal abnormality such as triploidy or trisomy 21 (Kelly & Savides). For unknown reasons, women carrying a female fetus are more likely than those carrying a male fetus to develop hyperemesis (Cunningham et al., 2010; Kelly & Savides). A family history of hyperemesis may also be present (Gilbert, 2011). In some cases, there is also an interrelated psychologic component (Cunningham et al.).
Complications accompanying severe hyperemesis gravidarum include esophageal rupture and deficiencies of vitamin K and thiamine with resulting Wernicke encephalopathy (CNS involvement) (Cunningham et al., 2010; Kelly & Savides, 2009). Fetal and neonatal complications include small-for-gestational-age fetuses, low birth weight, prematurity, and 5-minute Apgar scores less than 7 (Kelly & Savides).
Etiology
The cause of hyperemesis gravidarum remains obscure. Several theories have been proposed as to the cause, although none of them adequately explains the disorder. Hyperemesis gravidarum may be related to high levels of estrogen or hCG and may be associated with transient hyperthyroidism during pregnancy. Gastric dysrhythmias, esophageal reflux, and reduced gastric motility may also contribute to the development of hyperemesis gravidarum (Kelly & Savides, 2009).
Psychosocial factors may play a part in the development of hyperemesis gravidarum for some women. Ambivalence toward the pregnancy and increased stress may be associated with this condition (Davis, 2004). Conflicting feelings regarding prospective motherhood, body changes, and lifestyle alterations may contribute to episodes of vomiting, particularly if these feelings are excessive or unresolved. Women with associated psychosocial factors usually improve dramatically while in the hospital but may resume vomiting after discharge (Cunningham et al., 2010).
Clinical Manifestations
The woman with hyperemesis gravidarum usually has significant weight loss and dehydration. She may have dry mucous membranes, decreased blood pressure (BP), increased pulse rate, and poor skin turgor. She is frequently unable to keep down even clear liquids taken by mouth. Laboratory tests may reveal electrolyte imbalances.
Care Management
Whenever a pregnant woman has nausea and vomiting, the first priority is a thorough assessment to determine the severity of the problem. In most cases the woman should be told to come immediately to the health care provider’s office or to the emergency department because the severity of the illness is often difficult to determine by telephone conversation.
The assessment should include frequency, severity, and duration of episodes of nausea and vomiting. If the woman reports vomiting, then the assessment should also include the approximate amount and color of the vomitus. Other symptoms such as diarrhea, indigestion, and abdominal pain or distention also are identified. The woman is asked to report any precipitating factors relating to the onset of her symptoms. Any pharmacologic or nonpharmacologic treatment measures used should be recorded. Prepregnancy weight and documented weight gain or loss during pregnancy is important to note.
The woman’s weight and vital signs are measured, and a complete physical examination is performed, with attention to signs of fluid and electrolyte imbalance and nutritional status. The most important initial laboratory test to be obtained is a determination of ketonuria. Other laboratory tests that may be ordered are a urinalysis, a complete blood cell count, electrolytes, liver enzymes, and bilirubin levels. These tests help rule out the presence of underlying diseases such as gastroenteritis, pyelonephritis, pancreatitis, cholecystitis, peptic ulcer, and hepatitis (Cunningham et al., 2010). Because of the recognized association between hyperemesis gravidarum and hyperthyroidism, thyroid levels may also be measured (Nader, 2009).
Psychosocial assessment includes asking the woman about anxiety, fears, and concerns related to her own health and the effects on pregnancy outcome. Family members should be assessed both for anxiety and in regard to their role in providing support for the woman.
Initial Care
Initially the woman who is unable to keep down clear liquids by mouth will require IV therapy for correction of fluid and electrolyte imbalances. In the past, women requiring IV therapy were admitted to the hospital. Today, however, they may be, and often are, successfully managed at home, even if on enteral therapy. Medications may be used if nausea and vomiting are uncontrolled. Frequently prescribed drugs include pyridoxine (vitamin B6), doxylamine (Unisom), promethazine (Phenergan), and metoclopramide (Reglan) (Kelly & Savides, 2009). Other antiemetic medication options include prochlorperazine (Compazine) and ondansetron (Zofran) (Gordon, 2007). Chlorpromazine (Thorazine) given rectally may be effective in difficult to treat cases (Kelly & Savides). Corticosteroids (methylprednisolone [Medrol] or hydrocortisone) may be prescribed, although there is little evidence that their use is effective (Cunningham et al., 2010). Lastly, enteral or parenteral nutrition may be used for women who are nonresponsive to other medical therapies (Kelly & Savides).
Nursing care of the woman with hyperemesis gravidarum involves implementing the medical plan of care, whether this care is given in the hospital or home setting. Interventions may include initiating and monitoring IV therapy, administering drugs and nutritional supplements, and monitoring the woman’s response to interventions. The nurse observes the woman for any signs of complications such as metabolic acidosis (secondary to starvation), jaundice, or hemorrhage and alerts the physician should these occur. Monitoring includes assessment of the woman’s nausea, retching without vomiting, and vomiting, given that these symptoms, although related, are separate. Intake and output, including the amount of emesis, should be accurately measured and recorded. Oral hygiene while the woman is receiving nothing by mouth, and after episodes of vomiting, helps allay associated discomforts. Assistance with positioning and providing a quiet, restful environment that is free from odors may increase the woman’s comfort.
Once the vomiting has stopped, feedings are started in small amounts at frequent intervals. In the beginning, limited amounts of oral fluids and bland foods such as crackers, toast, or baked chicken are offered. The diet is progressed slowly as tolerated by the woman until she is able to consume a nutritionally sound diet. Because sleep disturbances may accompany hyperemesis gravidarum, promoting adequate rest is important. The nurse can assist in coordinating treatment measures and periods of visitation to provide opportunity for rest periods.
Follow-up Care
Most women are able to take nourishment by mouth after several days of treatment. They should be encouraged to eat small, frequent meals and to eat foods that sound appealing, often nongreasy, dry, sweet, and salty foods. In many instances, women discover that foods they normally like have no appeal
at all during this time see the Teaching for Self-Management box: Diet for Hyperemesis for more suggestions to Many pregnant women find exposure to cooking odors nauseating. Having other family members cook may lessen the woman’s nausea and vomiting, even if only temporarily. The woman is counseled to contact her health care provider immediately if the nausea and vomiting recur.
The woman with hyperemesis gravidarum needs calm, compassionate, and sympathetic care, with recognition that the manifestations of hyperemesis can be physically and emotionally debilitating to her and stressful for her family. Irritability, tearfulness, and mood changes are often consistent with this disorder. Fetal well-being is a primary concern of the woman. The nurse can provide an environment conducive to discussion of concerns and assist the woman in identifying and mobilizing sources of support. The family should be included in the plan of care whenever possible. Their participation may help alleviate some of the emotional stress associated with this disorder.
Thyroid Disorders
Hyperthyroidism in pregnancy is rare, occurring in approximately 1 of every 1000 to 2000 pregnancies (Cunningham et al., 2010). In 90% to 95% of pregnant women, hyperthyroidism is caused by Graves’ disease (Nader, 2009). Clinical manifestations of hyperthyroidism include heat intolerance, diaphoresis, fatigue, anxiety, emotional lability, and tachycardia. Many of these symptoms also occur with pregnancy; thus the disorder can be difficult to diagnose. Signs that may help differentiate hyperthyroidism from normal pregnancy changes include weight loss, goiter, and a pulse rate greater than 100 beats/min (Nader). Laboratory findings include elevated free thyroxine (T4) and triiodothyronine (T3) levels and greatly suppressed thyroid-stimulating hormone (TSH) levels (Cunningham et al.; Nader). Moderate and severe hyperthyroidism must be treated during pregnancy. Untreated or inadequately treated women have an increased risk of miscarriage, preterm birth, and giving birth to stillborn infants or infants with goiter, hyperthyroidism, or hypothyroidism. Most neonates born to women with hyperthyroidism, however, will have normal thyroid function. Women with hyperthyroidism are at increased risk for developing severe preeclampsia and heart failure (Cunningham et al.; Nader).
The primary treatment of hyperthyroidism during pregnancy is drug therapy. The medication most often prescribed in the United States is propylthiouracil (PTU). The usual starting dose is 100 to 150 mg every 8 hours, with higher doses required for some women. Women generally show clinical improvement within 2 weeks of beginning therapy, but the medication requires 6 to 8 weeks to reach full effectiveness. During therapy the woman’s free T4 levels are measured monthly, and the results are used to taper the drug to the smallest effective dose to prevent development of unnecessary fetal or neonatal hypothyroidism. In many women the medication can be discontinued by 32 to 36 weeks of gestation. PTU readily crosses the placenta and may cause fetal hypothyroidism, which is characterized by goiter, bradycardia, and intrauterine growth restriction (IUGR) (Mestman, 2007; Nader, 2009).
PTU is well tolerated by most women. Maternal side effects include pruritus, skin rash, drug-related fever, hepatitis, bronchospasm, and a lupus-like syndrome. The most severe side effect is agranulocytosis, which occurs rarely and usually develops only in older women and in those taking high doses of PTU. Symptoms of agranulocytosis are fever and unexpected sore throat, which should be reported immediately to the health care provider, and the woman should stop taking the PTU. Leukopenia of a transient and benign nature may occur as a result of PTU therapy (Cunningham et al., 2010; Nader, 2009); liver toxicity is another rare but serious complication (Cunningham et al.). Beta-adrenergic blockers such as propranolol (Inderal) or atenolol (Tenormin) may be used in severe hyperthyroidism to control maternal symptoms, especially heart rate. Long-term use of these medications is not recommended because of the potential for IUGR, bradycardia, and hypoglycemia (Nader).
After birth, women taking PTU who choose to breastfeed should be informed that the medication is not significantly concentrated in breast milk and does not appear to adversely affect the neonate’s thyroid function. The woman should take her antithyroid medication just after breastfeeding, thus allowing a 3- to 4-hour period before nursing again (Nader, 2009).
Radioactive iodine must not be used in diagnosis or treatment of hyperthyroidism in pregnancy because therapeutic doses given to treat maternal thyroid disease may also destroy the fetal thyroid (Cunningham et al., 2010). In severe cases, surgical treatment of hyperthyroidism, subtotal thyroidectomy, can be performed during pregnancy. Surgery is best performed during the second trimester of pregnancy, although it can be performed during the first or third trimester if necessary. Surgery is usually reserved for women with severe disease, those for whom drug therapy proves toxic, and those who are unable to follow the prescribed medical regimen. Risks associated with the surgery are hypoparathyroidism, recurrent laryngeal nerve paralysis, and anesthesia-related complications (Nader, 2009).
Hypothyroidism
Hypothyroidism occurs in 2 to 3 pregnancies per 1000. Because severe hypothyroidism is often associated with infertility and an increased risk of miscarriage, it is not often seen during pregnancy (Cunningham et al., 2010). Although iodine deficiency is rare in the United States, it is a common cause of maternal, fetal, and neonatal hypothyroidism in the world (Nader, 2009). Adult hypothyroidism is usually caused by glandular destruction by autoantibodies, most commonly because of Hashimotos thyroiditis. Characteristic symptoms of hypothyroidism include weight gain, lethargy, decrease in exercise capacity, and cold intolerance. Women who are moderately symptomatic can also develop constipation, hoarseness, hair loss, brittle nails, and dry skin. Laboratory values in pregnancy include elevated levels of TSH, with or without low T4 levels (Nader).
Pregnant women with untreated hypothyroidism are at increased risk for miscarriage, preeclampsia, gestational hypertension, placental abruption, preterm birth, and stillbirth. Infants born to mothers with hypothyroidism may also be of low birth weight (Cunningham et al., 2010; Nader, 2009). These outcomes can be improved with early treatment (Nader).
Thyroid hormone supplements are used to treat hypothyroidism. Levothyroxine (e.g., thyroxine [Synthroid]) is most often prescribed during pregnancy. The usual beginning dosage is 0.1 to 0.15 mg per day, with adjustment by 25 to 50 mcg every 4 to 6 weeks as necessary based on the maternal TSH level (Cunningham et al., 2010; Nader, 2009). The aim of drug therapy is to maintain the TSH level at the lower end of the normal range for pregnant women. Women with little or no functioning thyroid tissue will require higher doses of levothyroxine. Also, as pregnancy progresses, increased doses of thyroid hormone are usually required. This increased demand during pregnancy is probably related to increased estrogen levels (Cunningham et al.; Nader).
The fetus depends on maternal thyroid hormones until approximately 18 weeks of gestation, when fetal production begins. Normal maternal thyroxine levels early in pregnancy are important for proper fetal brain development. Studies have shown that even mild maternal hypothyroidism during the first trimester has been associated with long-term neuropsychologic damage in their children. More research needs to be conducted on this topic (Mestman, 2007).
Nursing Care
Education of the pregnant woman with thyroid dysfunction is essential to promote compliance with the plan of treatment. Important points to discuss with the woman and her family include the disorder and its potential effect on her, her family, and her fetus; the medication regimen and possible side effects; the need for continuing medical supervision; and the importance of compliance.
The woman often needs assistance from the nurse in coping with the discomforts and frustrations associated with symptoms of the disorder. For example, the woman with hyperthyroidism who has nervousness and hyperactivity along with weakness and fatigue may benefit from suggestions to channel excess energies into quiet diversional activities such as reading or crafts. Discomfort associated with hypersensitivity to heat (hyperthyroidism) or cold intolerance (hypothyroidism) can be minimized by appropriate clothing and regulation of environmental temperatures, and by avoiding temperature extremes.
Nutritional counseling with a registered dietitian may provide guidance in selecting a well-balanced diet. The woman with hyperthyroidism who has increased appetite and poor weight gain and the hypothyroid woman who has anorexia and lethargy need counseling to ensure adequate intake of nutritionally sound foods to meet both maternal and fetal needs.
Maternal Phenylketonuria
Phenylketonuria (PKU), a recognized cause of mental retardation, is an inborn error of metabolism caused by an autosomal recessive trait that creates a deficiency in the enzyme phenylalanine hydrolase. Absence of this enzyme impairs the body’s ability to metabolize the amino acid phenylalanine, found in all protein foods. Consequently, toxic accumulation of phenylalanine in the blood occurs, which interferes with brain development and function. Individuals with this disorder also have hypopigmentation of hair, eyes, and skin because phenylalanine inhibits melanin production. PKU affects 1 in every 10,000 to 15,000 Caucasian newborns (Cunningham et al., 2010).
PKU was the first inborn error of metabolism to be universally screened for in the United States. Since 1961, all newborns have been tested soon after birth for this disorder. Prompt diagnosis and therapy with a phenylalanine-restricted diet significantly decreases the incidence of mental retardation (Aminoff, 2009). The special diet should be followed indefinitely because individuals who do not continue phenylalanine restriction have been reported to have significantly lower IQs (Cunningham et al., 2010).
The keys to the prevention of fetal anomalies caused by PKU are the identification of women in their reproductive years with the disorder and dietary compliance for women who are diagnosed. Screening for undiagnosed homozygous maternal PKU at the first prenatal visit may be warranted, especially in individuals with a family history of the disorder, with low intelligence of uncertain origin, or who have given birth to microcephalic infants. Ideally women with PKU begin dietary phenylalanine restriction before conception and continue it throughout pregnancy. The dietary modification normally excludes all high-protein foods such as meat, milk, eggs, and nuts, as well as wheat products. Phenylalanine levels are monitored at least once and preferably twice a week throughout pregnancy (Gilbert, 2011). Experts recommend that maternal phenylalanine levels be less than 6 mg/dl for a least 3 months before conception and range between 2 and 6 mg/dl throughout pregnancy. These levels are associated with a decrease in fetal sequelae (Cunningham et al., 2010; Gilbert). High maternal phenylalanine levels are associated with microcephaly, mental retardation, and congenital heart defects in their children (Aminoff, 2009; Cunningham et al.). Ultrasound examinations are used for fetal surveillance beginning in the first trimester. A spontaneous vaginal birth is anticipated.
Women with PKU should be advised against breastfeeding because their milk will contain a high concentration of phenylalanine (Aminoff, 2009). If these women choose to breastfeed despite the risk, their phenylalanine blood levels must be monitored closely (Lawrence & Lawrence, 2005).
KEY POINTS
• In pregnant women with pregestational diabetes, lack of glycemic control before conception and in the first trimester of pregnancy may be responsible for fetal congenital malformations.
• For pregnant women who have diabetes and are insulin dependent, insulin requirements increase as the pregnancy progresses and may quadruple by term as a result of insulin resistance created by placental hormones, insulinase, and cortisol. After birth, levels decrease dramatically; breastfeeding affects insulin needs.
• Poor glycemic control before and during pregnancy in women who have diabetes can lead to maternal complications such as miscarriage, infection, and dystocia (difficult labor) caused by fetal macrosomia.
• Careful glucose monitoring, insulin administration when necessary, and dietary counseling are used to create a normal intrauterine environment for fetal growth and development in the pregnancy complicated by diabetes mellitus.
• Because GDM is asymptomatic in most cases, all women who do not have pregestational diabetes undergo routine screening by history, clinical risk factors, or glucola administration during pregnancy.
• The woman with hyperemesis gravidarum may have significant weight loss and dehydration. Management focuses on restoring fluid and electrolyte balance and preventing recurrence of nausea and vomiting.
• Thyroid dysfunction, hyperthyroidism or hypothyroidism during pregnancy requires close monitoring of thyroid hormone levels to regulate therapy and prevent fetal insult.
• High levels of phenylalanine in the maternal bloodstream cross the placenta and are teratogenic to the developing fetus. Damage can be prevented or minimized by dietary restriction of phenylalanine before and during pregnancy.
![]() Audio Chapter Summaries Access an audio summary of the Key Points on
Audio Chapter Summaries Access an audio summary of the Key Points on ![]()
