Acquired Problems of the Newborn
• Summarize the care of the newborn with soft-tissue, skeletal, and nervous system injuries.
• Describe assessment and care of infants with birth trauma.
• Develop a plan of care for a neonate of a mother with diabetes.
• Describe in detail the assessment of a newborn with a suspected infection.
• Formulate nursing diagnoses for the infant and family for common bacterial and viral infections.
• Interpret the evidence available to guide the care of the infant at risk for group B streptococci (GBS) sepsis.
• Review implementation and evaluation of care of infants with infections; include their families.
• Analyze fetal and neonatal effects of maternal substance abuse during pregnancy.
• Describe concerns for fetal and neonatal well-being related to maternal use of caffeine and selective serotonin reuptake inhibitors during pregnancy.
• Describe the assessment and care of a newborn experiencing drug withdrawal (neonatal abstinence syndrome); include the infant’s family.
This chapter deals with acquired problems of the newborn. Acquired problems refer to those conditions resulting from environmental factors rather than genetic circumstances. The focus is on birth trauma, the infant of a mother with diabetes, neonatal infections, effects of maternal substance abuse on the fetus and neonate, and effects of maternal use of caffeine and antidepressant medications during pregnancy.
Birth Trauma
Birth trauma or birth injury refers to physical injury sustained by a neonate during labor and birth. According to the Agency for Healthcare Research and Quality (AHRQ), the incidence of birth injuries in the United States is 1.84 per 1000 live births, excluding preterm and osteogenesis imperfecta births (AHRQ, 2008). Despite improvements in obstetric techniques; increased use of cesarean surgery for births that would be difficult vaginally; and decreased use of forceps, vacuum extraction, and version and extraction; birth injuries still are an important source of neonatal morbidity. Therefore, the clinician should consider the broad range of birth injuries in the differential diagnosis of neonatal clinical disorders (Mangurten, 2006).
The nurse’s contribution to the welfare of the newborn begins with early observation and accurate recording. The prompt reporting of signs that indicate deviations from normal permits early initiation of appropriate therapy. In addition, nurses provide essential support and education to parents whose neonates experience birth injury.
In theory, some birth injuries are avoidable, especially with careful assessment of risk factors and appropriate planning for birth. The use of ultrasonography allows antepartum diagnosis of macrosomia, hydrocephalus, and unusual presentations. Elective cesarean birth can be chosen for some pregnancies to prevent significant birth injury. A small percentage of significant birth injuries are unavoidable despite skilled and competent obstetric care, as in especially difficult or prolonged labor or when the infant is in an abnormal presentation (Mangurten, 2006). Some injuries cannot be anticipated until the specific circumstances occur during birth. Emergency cesarean birth can provide last-minute salvage, but in these circumstances the injury may be truly unavoidable. The same injury can be caused in several ways. For example, a cephalhematoma can result from an obstetric technique such as forceps birth or vacuum extraction or from pressure of the fetal skull against the maternal pelvis.
Many injuries are minor and resolve readily in the neonatal period without treatment. Other traumas require some degree of intervention. A few are considered major trauma and serious enough to be fatal. Major trauma is often the result of instrumentation during birth (forceps or vacuum) and can occur concomitantly with other minor injuries. For example, a neonate who suffers a skull fracture is also likely to have a cephalhematoma (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010; Pressler, 2008).
Several factors predispose an infant to birth injuries. Maternal risk factors include age younger than 16 or older than 35, primigravida, uterine dysfunction that leads to prolonged or precipitate labor, preterm or postterm labor, and cephalopelvic disproportion. Oligohydramnios can increase the likelihood of birth trauma. Injury can result from dystocia caused by fetal macrosomia, multifetal gestation, abnormal or difficult presentation (not caused by maternal uterine or pelvic conditions), and congenital anomalies. Intrapartum events that can result in scalp injury include the use of internal monitoring of fetal heart rate (FHR) and collection of fetal scalp blood for acid-base assessment. Obstetric birth techniques can cause injury. Forceps- or vacuum-assisted birth, version and extraction, and cesarean birth are potential contributory factors. Often more than one factor is present, and multiple predisposing factors can be related to a single maternal condition (Mangurten, 2006; Pressler, 2008; Verklan & Lopez, 2011).
Birth injuries are usually classified according to their etiology (predisposing factors or mechanisms of injury) or anatomically. Table 35-1 is an example of anatomic classification of birth injuries.
TABLE 35-1
ANATOMIC CLASSIFICATION OF BIRTH INJURIES
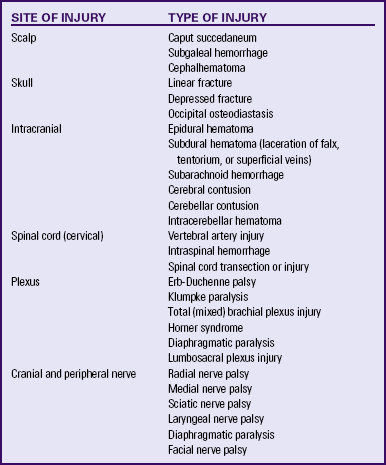
Source: Verklan. M., & Lopez, S. (2011). Neurologic disorders. In S. Gardner, B. Carter, M. Enzman-Hines, & J. Hernandez (Eds.), Merenstein & Gardner’s handbook of neonatal intensive care (7th ed.). St Louis: Mosby.
Soft-Tissue Injuries
Erythema, ecchymoses, petechiae, abrasions, lacerations, and edema of buttocks and extremities can be present. Localized discoloration can appear over presenting or dependent parts. Ecchymoses and edema can appear anywhere on the body and especially on the presenting body part from the application of forceps or vacuum cup. They also can result from manipulation of the infant’s body during birth.
Bruises over the face can be the result of face presentation (Fig. 35-1). In a breech presentation, bruising and swelling can occur over the buttocks or genitalia (Fig. 35-2). The skin over the entire head can be ecchymotic and covered with petechiae caused by a tight nuchal cord. Petechiae, or pinpoint hemorrhagic areas, acquired during birth can extend over the upper portion of the trunk and face. These lesions are benign if they disappear within 2 days of birth and no new lesions appear. Ecchymoses and petechiae can be signs of a more serious disorder, such as thrombocytopenic purpura, if the hemorrhagic areas do not disappear spontaneously in 2 days. To differentiate hemorrhagic areas from skin rashes and discolorations such as mongolian spots, the nurse blanches the skin with two fingers. Because extravasated blood remains within the tissues, petechiae and ecchymoses do not blanch.
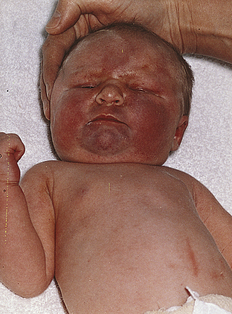
FIG. 35-1 Marked bruising on the entire face of an infant born vaginally after face presentation. Less severe ecchymoses were present on the extremities. Phototherapy was required for treatment of jaundice resulting from the breakdown of accumulated blood. (From O’Doherty, N. [1986]. Neonatology: Micro atlas of the newborn. Nutley, NJ: Hoffmann-La Roche.)
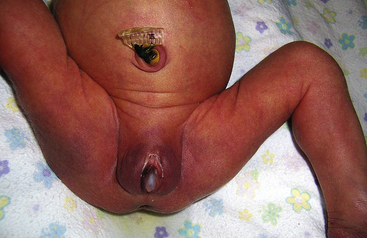
FIG. 35-2 Swelling of the genitals and bruising of the buttocks after a breech birth. Note the position of the infant’s legs. (Courtesy Cheryl Briggs, RNC, Annapolis, MD.)
Forceps injury occurs at the site of application of the instrument. Forceps injury typically has a linear configuration across both sides of the face, outlining the placement of the forceps. The affected areas are kept clean to minimize the risk of secondary infection. These injuries usually resolve spontaneously within several days with no specific therapy.
Accidental lacerations can be inflicted with a scalpel during cesarean birth or with scissors during an episiotomy. These cuts can occur on any part of the body but most often are found on the scalp, buttocks, and thighs. Usually they are superficial, needing only to be kept clean. Butterfly adhesive strips will usually hold together the edges of more serious lacerations. Rarely are sutures needed.
Two of the most commonly occurring birth injuries are subconjunctival (scleral) and retinal hemorrhages. These injuries result from rupture of capillaries caused by increased intracranial pressure (ICP) during birth. They usually clear within 5 days after birth and present no problems; however, parents need reassurance about their presence.
Caput succedaneum and cephalhematoma are commonly seen in neonates, often as the result of pressure on the fetal head pushing through a dilated cervix. These are discussed in Chapter 23.
A more serious injury is subgaleal hemorrhage, which is bleeding into the subgaleal compartment (see Fig. 23-9, C). The subgaleal compartment is a potential space that contains loosely arranged connective tissue; it is located beneath the galea aponeurosis, the tendinous sheath that connects the frontal and occipital muscles and forms the inner surface of the scalp. The injury occurs as a result of forces that compress and then drag the head through the pelvic outlet (Verklan & Lopez, 2011). The bleeding extends beyond bone, often posteriorly into the neck, and continues after birth, with the potential for serious complications such as anemia, hypovolemic shock, or even death. Early detection of the hemorrhage is vital; serial head circumference measurements and inspection of the back of the neck for increasing edema and a firm mass are essential. A boggy scalp, pallor, tachycardia, and increasing head circumference can also be early signs of a subgaleal hemorrhage (Doumouchtsis & Arulkumaran, 2006). Computed tomography (CT) or magnetic resonance imaging (MRI) is useful in confirming the diagnosis. Replacement of lost blood and clotting factors is required in acute cases of hemorrhage. Another possible early sign of subgaleal hemorrhage is a forward and lateral positioning of the infant’s ears because the hematoma extends posteriorly. Monitoring the infant for changes in level of consciousness and a decrease in hematocrit is key to early recognition and management. An increase in serum bilirubin levels can be seen as a result of the breakdown of blood cells within the hematoma.
Skeletal Injuries
The newborn’s immature, flexible skull can withstand a great degree of deformation (molding) before fracture results. Considerable force is required to fracture the newborn’s skull. Two types of skull fractures typically are identified in the newborn: linear fractures and depressed fractures. The location of the fracture and involvement of underlying structures determine its significance. Linear fractures are most common in the parietal bones, require no treatment, and are usually of no clinical significance. Whenever a cephalhematoma or subarachnoid hemorrhage is present, a skull fracture should be suspected (Doumouchtsis & Arulkumaran, 2008).
The soft skull can become indented without laceration of either the skin or the dural membrane. These depressed fractures, or “ping-pong ball” indentations, can occur during difficult births from pressure of the head on the bony pelvis. They also can occur as a result of injudicious application of forceps. A CT scan is done to rule out bone fragments or underlying injury of the brain tissue. Management of depressed skull fractures is controversial; many resolve without intervention. Nonsurgical elevation of the indentation by using a manual breast pump or vacuum extractor has been reported. Surgery can be required in the presence of bone fragments or signs of increased ICP (Doumouchtsis & Arulkumaran, 2008).
The clavicle is the bone most often fractured during birth. Generally the break is in the middle third of the bone (Fig. 35-3). Dystocia, particularly shoulder impaction, is a risk factor for clavicular fracture. Other risk factors include vacuum-assisted birth and birth weight greater than 4000 g. Limited movement of the arm, crepitus over the bone, and the absence of the Moro reflex on the affected side are diagnostic. Except for use of gentle rather than vigorous handling, no accepted treatment for fractured clavicle exists, and the prognosis is good. A sign posted on the bassinet will alert care providers to the need for careful handling. The figure-eight bandage appropriate for an older child should not be used for a newborn.
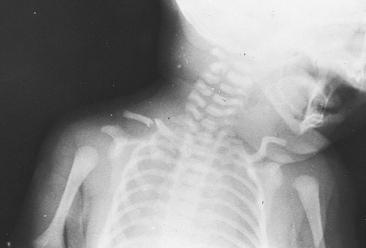
FIG. 35-3 Fractured clavicle after shoulder dystocia. (From O’Doherty, N. [1986]. Neonatology: Micro atlas of the newborn. Nutley, NJ: Hoffmann-La Roche.)
The humerus and femur can be fractured during a difficult birth. Fractures in newborns generally heal rapidly. Immobilization is accomplished with slings, splints, swaddling, and other devices.
The parents need support in handling these infants because they often are fearful of hurting them. Parents are encouraged to practice handling, changing, and feeding the affected neonate under the guidance of nursing staff prior to hospital discharge. This increases their confidence and knowledge and facilitates attachment. A plan for follow-up therapy is developed with the parents so that the times and arrangements for therapy are acceptable to them.
Peripheral Nervous System Injuries
Erb-Duchenne palsy (also called Erb’s palsy or brachial plexus injury) is the most common type of paralysis associated with a difficult birth, occurring at rates of 0.5 to 2 per 1000 live births (Volpe, 2008) (Fig. 35-4). An increased risk of brachial plexus injury occurs with birth weight greater than 4000 g, shoulder dystocia, vaginal breech birth, forceps- or vacuum-assisted birth, maternal diabetes, and a prolonged second stage of labor. Injury to the upper plexus results from stretching or pulling the head away from the shoulder during the difficult birth. The arm hangs limply alongside the body. The shoulder and arm are adducted and internally rotated. The elbow is extended, and the forearm is pronated, with the wrist and fingers flexed; a grasp reflex can be present because finger and wrist movement remains normal (Adams-Chapman & Stoll, 2007).
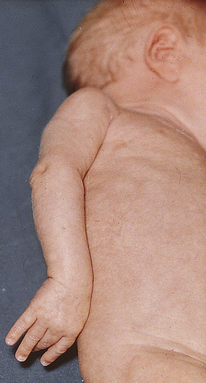
FIG. 35-4 Erb-Duchenne palsy in newborn infant. The Moro reflex was absent in right upper extremity. Recovery was complete. (From O’Doherty, N. [1986]. Neonatology: Micro atlas of the newborn. Nutley, NJ: Hoffmann-La Roche.)
Treatment is by intermittent immobilization across the upper abdomen, proper positioning, and range-of-motion (ROM) exercises. Gentle manipulation and ROM exercises are delayed until about the fifth day to prevent additional injury to the brachial plexus. Immobilization can be accomplished with a brace or splint or by pinning the infant’s sleeve to his or her shirt.
Damage to the lower plexus or Klumpke palsy is less common. With lower arm paralysis the wrist and hand are flaccid, the grasp reflex is absent, and deep tendon reflexes are present; dependent edema and cyanosis can occur in the affected hand. Treatment consists of placing the hand in a neutral position, padding the fist, and gently exercising the wrist and fingers.
If edema or hemorrhage is responsible for the paralysis, the prognosis is good, and recovery can be expected in a few weeks. If laceration of the nerves has occurred and healing does not result in return of function within a few months, surgery can be indicated; however, return of function is variable. Full recovery is expected in 88% to 92% of infants (Volpe, 2008).
Facial paralysis (palsy) (Fig. 35-5) generally is caused by pressure on the facial nerve during birth. Risk factors include a prolonged second stage of labor and forceps-assisted birth. The face on the affected side is flattened and unresponsive to the grimace that accompanies crying or stimulation and the eye will remain open on the affected side. Moreover, the forehead will not wrinkle. Usually the infant’s face appears distorted, especially when crying. Often the condition is transitory, resolving within hours or days of birth. Permanent paralysis is rare.
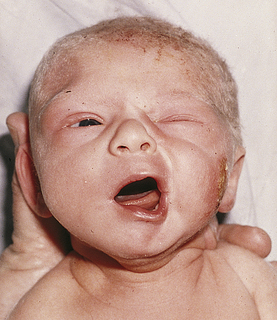
FIG. 35-5 Facial paralysis 15 minutes after forceps birth. Absence of movement on affected side is especially noticeable when infant cries. (From O’Doherty, N. [1986]. Neonatology: Micro atlas of the newborn. Nutley, NJ: Hoffmann-La Roche.)
Treatment involves assistance with feeding, prevention of damage to the cornea of the open eye with the application of artificial tears or taping the eye closed, and supportive care of the parents. Feeding can be prolonged, with the milk flowing out the newborn’s mouth around the nipple on the affected side. The parents will need understanding and sympathetic encouragement while learning how to feed and care for the infant, as well as how to hold and cuddle the baby.
Phrenic nerve injury almost always occurs as a component of brachial plexus injury rather than as an isolated problem. Injury is usually the result of traction on the neck and arm during birth. Injury to the phrenic nerve is usually unilateral, but can be bilateral, and results in diaphragmatic paralysis. Cyanosis and irregular thoracic respirations, with no abdominal movement on inspiration, are characteristic of paralysis of the diaphragm. Babies with diaphragmatic paralysis usually require mechanical ventilatory support, at least for the first few days after birth, and are at risk of developing pneumonia. In the presence of persistent respiratory distress, diaphragmatic pacing or surgical correction can be necessary.
Central Nervous System Injuries
All types of intracranial hemorrhage (ICH) occur in newborns. ICH as a result of birth trauma is more likely to occur in the term, large infant. Risk factors for ICH include primiparity. advanced maternal age, vacuum- or forceps-assisted birth, precipitous or prolonged second stage of labor, and increased fetal size (Limperopoulos, Robertson, Sullivan, Bassan, & du Plessis, 2009). In the newborn, more than one type of hemorrhage frequently occurs.
Subdural hemorrhage (hematoma), a collection of blood in the subdural space, most often is produced by the stretching and tearing of the large veins in the tentorium of the cerebellum, the dural membrane that separates the cerebrum from the cerebellum. When this type of bleeding occurs, the typical history includes a nulliparous mother, with the total labor and birth occurring in less than 2 or 3 hours; a difficult birth involving forceps application; or a large for gestational age (LGA) infant. Subdural hematoma occurs less frequently today because of improvements in obstetric care. However, it is especially serious because of its inaccessibility to aspiration by subdural tap (Askin & Wilson, 2007). Neonates with subdural hemorrhage usually present with apnea, unequal pupils, irritability, tense fontanel, seizures, and even coma (Doumouchtsis & Arulkamaran, 2008).
Subarachnoid hemorrhage, the most common type of ICH, occurs in term infants as a result of trauma and in preterm infants as a result of hypoxia. Small hemorrhages are the most common. Bleeding is of venous origin, and underlying contusion also can occur (Askin & Wilson, 2007).
The clinical presentation of hemorrhage in the term infant can vary considerably. In many infants signs are absent, and hemorrhaging is diagnosed only because of abnormal findings on lumbar puncture (e.g., red blood cells in the cerebrospinal fluid [CSF]). The initial clinical manifestations of neonatal subarachnoid hemorrhage can be the early onset of alternating depression and irritability, with refractory seizures or apnea. Occasionally the infant appears normal initially and then has seizures on the second or third day of life, followed by no apparent after effects.
In general, nursing care of an infant with ICH is supportive and includes monitoring of ventilatory and intravenous (IV) therapy, observation and management of seizures, and prevention of increased ICP. Minimal handling to promote rest and reduce stress should guide nursing care (Askin & Wilson, 2007).
Spinal cord injuries are usually the result of breech births, especially those difficult ones in which version and extraction were used. Brow and face presentations, dystocia, preterm birth, maternal nulliparity, and precipitate birth also have been identified as predisposing factors in these types of injuries. Stretching of the spinal cord, usually by forceful longitudinal traction on the trunk while the head is still firmly engaged in the pelvis, is the most common mechanism of injury. This injury is rarely seen today because cesarean birth is often used for breech presentation (Mangurten, 2006).
Clinical manifestations depend on the severity and location of the injury. High cervical cord injuries are more likely to cause stillbirths or rapid death of the neonate. Lower lesions cause an acute spinal cord syndrome. Common signs of spinal shock include flaccid extremities, diaphragmatic breathing, paralyzed abdominal movements, atonic anal sphincter, and distended bladder.
Therapy is supportive and usually unsatisfactory. Infants who survive present a therapeutic challenge that requires combined treatment from many health care providers: pediatrician, neurologist, neurosurgeon, urologist, orthopedist, nurse, physical therapist, and occupational therapist. Parents need to understand fully the implications of severe injury to the spinal cord and the overwhelming implications it presents for the family. (See the Nursing Process box: Birth Injury.)
Infants of Mothers With Diabetes
No single physiologic or biochemical event can explain the diverse clinical manifestations seen in the infants of mothers with diabetes or infants of mothers with gestational diabetes. A better understanding of maternal and fetal metabolism, resulting in stricter control of maternal diabetes and improved obstetric and neonatal intensive care, has led to a decrease in the perinatal mortality rate in diabetic pregnancy. However, maternal diabetes continues to play a significant role in neonatal morbidity and mortality. Compared to nondiabetic pregnancies, infants born to mothers with diabetes are at an increased risk for complications such as congenital anomalies, macrosomia, birth trauma, perinatal asphyxia, stillbirth, preterm birth, respiratory distress syndrome (RDS), hypoglycemia, hypocalcemia, hypomagnesemia, cardiomyopathy, hyperbilirubinemia, and polycythemia. The degree of risk depends on the severity and duration of maternal disease. For example, women with vascular complications are more likely to have infants who are small for gestational age (SGA). All infants born to mothers with diabetes are at some risk for complications. The likelihood of these complications is reduced when maternal glucose levels are maintained within normal limits during the periconception period and during pregnancy (Cunningham, et al., 2010; Dudley, 2007; Jovanovic & Nakai, 2006).
Pathophysiology
The mechanisms responsible for the problems seen in infants of mothers with diabetes are not fully understood. In early pregnancy, fluctuations in blood glucose levels and episodes of ketoacidosis are believed to cause congenital anomalies. Later in pregnancy, when the mother’s pancreas cannot release sufficient insulin to meet increased demands, maternal hyperglycemia results. Increased amounts of glucose cross the placenta and stimulate the fetal pancreas to release insulin. The combination of the increased supply of maternal glucose and other nutrients and increased fetal insulin results in excessive fetal growth called macrosomia (see later discussion).
Hyperinsulinemia accounts for many of the problems of the fetus or infant. In addition to fluctuating glucose levels,
maternal vascular involvement or superimposed maternal infection adversely affects the fetus. Normally, maternal blood has a more alkaline pH than does the carbon dioxide–rich fetal blood. This phenomenon encourages the exchange of oxygen and carbon dioxide across the placental membrane. When the maternal blood is more acidotic than the fetal blood, such as during ketoacidosis, little carbon dioxide or oxygen exchange occurs at the level of the placenta. The mortality rate for unborn babies resulting from an episode of maternal ketoacidosis may be as high as 50% or more (Lindsay, 2006).
Congenital Anomalies
The incidence of congenital anomalies among mothers with pregestational diabetes is more than three times that of pregnant women who do not have diabetes (Correa, Gilboa, Besser, Botto, Moore, & Hobbs, 2008). Elevated fasting blood glucose levels are correlated with an increased risk for anomalies in women with type 1 and type 2 diabetes (Jovanovic & Nakai, 2006). Gestational diabetes that is diagnosed in mid- to late pregnancy is usually not associated with an increased incidence of congenital anomalies. However, the risk of anomalies is increased in women with gestational diabetes with elevated fasting glucose or A1C levels, especially during early pregnancy (Metzger, Buchanan, Coustan, de Leiva, Dunger, Hadden, et al., 2007). There is an increased incidence of congenital anomalies among women with gestational diabetes with prepregnancy obesity (Correa et al., 2008). In most defects associated with diabetic pregnancies, the structural abnormality occurs before the eighth week after conception. This reinforces the importance of control of blood glucose both before conception and in the early stages of pregnancy.
The most frequently occurring anomalies involve the cardiac, renal, musculoskeletal, and central nervous systems. The incidence of congenital heart lesions is three to five times higher than in the general population (Corrigan, Brazil, & McAuliffe, 2009). Coarctation of the aorta, transposition of the great vessels, and atrial or ventricular septal defects are the most common cardiac anomalies occurring in infants of mothers with diabetes. In the genitourinary system, renal agenesis (failure of the kidney to develop) and obstruction of the urinary tract have been associated with maternal diabetes. Central nervous system (CNS) anomalies include anencephaly, encephalocele, myelomeningocele, and hydrocephalus. The musculoskeletal system can be affected by caudal regression syndrome (sacral agenesis, with weakness or deformities of the lower extremities; malformation and fixation of the hip joints; and shortening or deformity of the femurs). Other defects noted in this population include gastrointestinal atresia and urinary tract malformations (see Chapter 36). Neonatal small left colon syndrome, also called lazy colon syndrome, occurs in up to 50% of infants born to mothers with diabetes (Thigpen, 2007). This syndrome is suspected when the infant fails to pass meconium and has abdominal distention and bile-stained vomitus. Contrast enemas show a greatly diminished caliber of the left colon from the splenic flexure to the anus. The syndrome is transient, with normal bowel function developing early in infancy.
Macrosomia
Despite improvements in the control of maternal blood glucose levels, the incidence of macrosomia is 50% in women with gestational diabetes and 40% in women with type 1 diabetes (Landon, Catalano, & Gabbe, 2007). At birth, the typical LGA infant has a round, cherubic (“tomato” or cushingoid) face, a chubby body, and a plethoric or flushed complexion (Fig. 35-6). The infant has enlarged internal organs (hepatosplenomegaly, splanchnomegaly, cardiomegaly) and increased body fat, especially around the shoulders. The placenta and umbilical cord are larger than average. Because insulin does not cross the blood-brain barrier, the brain is the only organ that is not enlarged. Infants of mothers with diabetes can be LGA but physiologically immature.
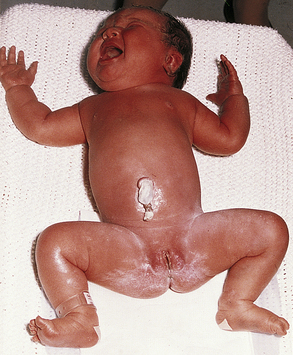
FIG. 35-6 Macrosomia. (From O’Doherty, N. [1986]. Neonatology: Micro atlas of the newborn. Nutley, NJ: Hoffmann-La Roche.)
Insulin has been proposed as the primary growth hormone for intrauterine development. Maternal diabetes results in elevated maternal levels of amino acids and free fatty acids, along with hyperglycemia. As the nutrients cross the placenta, the fetal pancreas responds by producing insulin to match the fuel supply. The resulting accelerated protein synthesis, together with a deposition of excessive glycogen and fat stores, is responsible for the typical macrosomic infant. This is the infant most at risk for the neonatal complications of hypoglycemia, hypocalcemia, hyperviscosity, and hyperbilirubinemia. The excessive amounts of metabolic fuels presented to the fetus from the mother and the consequent fetal hyperinsulinism represent the basic pathologic mechanism in the diabetic pregnancy (Lindsay, 2006).
The excessive shoulder size in these infants often leads to dystocia, particularly because the head may be smaller in proportion to the shoulders than in a nonmacrosomic infant (Esakoff, Cheng, Sparks, & Caughey, 2009). Macrosomic infants, born vaginally or by cesarean after a trial of labor, can incur birth trauma such as clavicle fracture or Erb-Duchenne palsy. Despite increased vigilance in screening, and improvements in ultrasound techniques, the determination of macrosomia can be difficult to make.
Birth Trauma and Perinatal Hypoxia
Birth injury (resulting from macrosomia or method of birth) and perinatal hypoxia occur more often in infants of mothers with diabetes. Examples of birth trauma include cephalhematoma; paralysis of the facial nerve (cranial nerve VII) (see Fig. 35-5); fracture of the clavicle or humerus; brachial plexus paralysis, usually Erb-Duchenne palsy (right upper arm) (see Fig. 35-4); and phrenic nerve paralysis, invariably associated with diaphragmatic paralysis.
Respiratory Distress Syndrome
RDS in infants of mothers with diabetes is a much less common occurrence than in the past because of improved protocols to manage maternal glucose levels and enhanced antepartum fetal surveillance techniques to assess lung maturity. Among infants born to women with well-controlled diabetes who give birth at term, the risk of RDS is similar to that of the general population (Landon et al., 2007). RDS that occurs among infants of mothers with diabetes is more likely to be related to gestational age rather than to maternal diabetes (Cunningham et al., 2010). However, not all pregnant women with diabetes have well-controlled glucose levels. Maternal hyperglycemia can affect fetal lung maturity. In the fetus exposed to high levels of maternal glucose, synthesis of surfactant can be delayed because of the high fetal serum levels of insulin and/or glucose (Weindling, 2009). Fetal lung maturity, as evidenced by a lecithin/sphingomyelin (L/S) ratio of 2:1, is not reassuring if the mother has diabetes mellitus. For the infants of such mothers, an L/S ratio of 3:1 or more or the presence of phosphatidylglycerol (a component of surfactant) in the amniotic fluid is more indicative of adequate lung maturity.
Hypoglycemia
Hypoglycemia (blood glucose levels less than 40 mg/dL in term infants) affects many infants of mothers with diabetes. LGA and preterm infants have the highest risk. After constant exposure to high circulating levels of glucose, hyperplasia of the fetal pancreas occurs, resulting in hyperinsulinemia. Disruption of the fetal glucose supply occurs with the clamping of the umbilical cord, and the neonate’s blood glucose level decreases rapidly in the presence of fetal hyperinsulinism. It can take several days for the newborn to regulate the secretion of insulin in response to a lower postnatal supply of glucose. Hypoglycemia is most common in the macrosomic infant, but the nurse should monitor blood glucose levels in all infants of mothers with known or suspected diabetes.
Asymptomatic or symptomatic hypoglycemia most frequently manifests within the first 1 to 6 hours after birth. Signs of hypoglycemia include jitteriness, apnea, tachypnea, and cyanosis. Many infants with hypoglycemia remain asymptomatic. Significant hypoglycemia can result in seizures. Hypoglycemia is worsened by the presence of hypothermia or respiratory distress.
Hypocalcemia and Hypomagnesemia
Hypocalcemia and hypomagnesemia have been reported to occur in as many as 50% of infants born to mothers with diabetes (Kalhan & Parimi, 2006). A number of these cases are related to hypoxia or prematurity; however, the overall incidence of hypocalcemia is higher than in nondiabetic women. Hypomagnesemia is believed to develop because of maternal renal losses that occur in diabetes. Hypocalcemia is associated with preterm birth, birth trauma, and perinatal asphyxia. Signs of hypocalcemia are similar to those of hypoglycemia, but they occur between 24 and 36 hours of age. Hypocalcemia should be considered if therapy for hypoglycemia is ineffective.
Cardiomyopathy
All infants of mothers with diabetes need careful observation for cardiomyopathy (disease affecting the structure and function of the heart) because an increased heart size is often found in these infants. Cardiomyopathy is more likely to occur in cases of poorly controlled maternal diabetes. Two types of cardiomyopathy can occur: hypertrophic and nonhypertrophic. Clinicians must be alert to identify the type of lesion correctly so that appropriate therapy is instituted. Both types of lesions are associated with respiratory symptoms and congestive heart failure.
Hypertrophic cardiomyopathy (HCM) is characterized by a hypercontractile and thickened myocardium. The ventricular walls are thickened, as is the septum, which in severe cases results in outflow tract obstructions. The mitral valve is poorly functioning. In nonhypertrophic cardiomyopathy (non-HCM), the myocardium is poorly contractile and overstretched. The ventricles are larger, and no outflow obstruction is found. Most infants are asymptomatic, but severe outflow obstruction can cause left ventricular heart failure.
Hyperbilirubinemia and Polycythemia
Infants of mothers with diabetes are at increased risk of developing hyperbilirubinemia. Many infants also are polycythemic. Polycythemia increases blood viscosity, thereby impairing circulation. In addition, this increased number of red blood cells to be hemolyzed increases the potential bilirubin load that the neonate must clear. The excessive red blood cells are produced in extramedullary foci (liver and spleen) in addition to the usual sites in bone marrow; therefore, liver function and bilirubin clearance can be adversely affected. Bruising associated with birth of a macrosomic infant will contribute further to high bilirubin levels.
Nursing Care
Nursing care depends on the neonate’s particular problems. General care of the compromised infant is addressed in Chapter 37. If the maternal blood glucose level was well controlled throughout the pregnancy, the infant may require only monitoring. Because euglycemia (normal blood glucose levels) is not always possible, the nurse must promptly recognize and treat any consequences of maternal diabetes that arise. The most common problems experienced by infants of diabetic mothers that require intervention include birth trauma and perinatal asphyxia; RDS; difficult metabolic transition, including hypoglycemia and hypocalcemia; and congenital anomalies (see previous sections and the Nursing Care Plan: The Infant of the Mother with Pregestational or Gestational Diabetes).
Neonatal Infections
Sepsis (presence of microorganisms or their toxins in blood or other tissues) continues to be one of the most significant causes of neonatal morbidity and mortality. The newborn infant is susceptible to infection. Maternal immunoglobulin M (IgM) does not cross the placenta. IgG levels in term infants are equal to maternal levels; however, in preterm infants the amount of IgG is directly proportional to gestational age (Stoll & Adams-Chapman, 2007). IgA and IgM require time to reach optimal levels after birth. Phagocytosis is less efficient. Serum complement levels are inadequate; serum complement (C1 through C6) is involved in immunologic reactions, some of which kill or lyse bacteria and enhance phagocytosis. Dysmaturity seen with intrauterine growth restriction (IUGR) and preterm and postdate birth further compromises the neonate’s immune system.
Table 35-2 outlines risk factors for neonatal sepsis. Special precautions for preventing infection, as well as prompt recognition when it occurs, are necessary for optimal newborn care. Neonatal infections can be acquired in utero, during labor and birth, during resuscitation, and during the hospital stay.
TABLE 35-2
RISK FACTORS FOR NEONATAL SEPSIS
| SOURCE | RISK FACTORS |
| Maternal | Low socioeconomic status |
| Late or no prenatal care | |
| Poor nutrition | |
| Substance abuse | |
| Recently acquired sexually transmitted infection | |
| Untreated focal infection (urinary tract infection, vaginal, cervical) | |
| Systemic infection | |
| Fever | |
| Intrapartum | Premature rupture of fetal membranes |
| Maternal fever | |
| Chorioamnionitis | |
| Prolonged labor | |
| Premature labor | |
| Use of fetal scalp electrode | |
| Neonatal | Multiple gestation |
| Male | |
| Birth asphyxia | |
| Meconium aspiration | |
| Congenital anomalies of skin or mucous membranes | |
| Metabolic disorders (e.g., galactosemia) | |
| Absence of spleen | |
| Low birth weight | |
| Preterm birth | |
| Malnourishment | |
| Formula feeding | |
| Prolonged hospitalization | |
| Mechanical ventilation | |
| Umbilical artery catheterization or use of other vascular catheters |
Source: Edwards, M. (2006). Postnatal bacterial infections. In R. Martin, A. Fanaroff, & M. Walsh (Eds.), Fanaroff and Martin’s neonatal-perinatal medicine: Diseases of the fetus and infant (8th ed.). Philadelphia: Mosby.
Prenatal acquisition of infection occurs by organisms placentally transferred directly into the fetal circulatory system and transmitted from infected amniotic fluid, such as with herpes simplex virus (HSV), cytomegalovirus (CMV), and rubella. Microorganisms also can ascend from the vagina and pass
through the cervix. The membranes become infected and can rupture. Infection of the fetal skin and the respiratory or gastrointestinal tract can result.
During birth, contact with an infected birth canal can result in generalized or local infection. The upper airway and the gastrointestinal tract are the principal pathways for generalized infections. The conjunctiva and the oral cavity are the usual sites of local infection.
Postnatal infection is sometimes acquired during resuscitation or through the introduction of foreign objects such as indwelling catheters or endotracheal tubes. Nursery–associated infections may be transferred to the infant by the hands of the parents or health care personnel or spread from contaminated equipment. The umbilicus is a receptive site for cutaneous infection leading to sepsis (Edwards, 2006).
Neonatal bacterial infection is classified into two patterns according to the time of presentation. Early-onset or congenital sepsis usually manifests within 24 to 72 hours after birth, progresses more rapidly than later-onset infection, and has a mortality rate between 3% and 50% (Palazzi, Klein, & Baker, 2006). Early-onset infection is usually caused by microorganisms from the normal flora of the maternal vaginal tract, including group B streptococci, Haemophilus influenzae, Listeria monocytogenes, Escherichia coli, and Streptococcus pneumoniae (Venkatesh, Adams, & Weisman, 2011). It is associated with a history of obstetric complications, such as preterm labor, premature rupture of membranes, maternal fever during labor, and chorioamnionitis (Palazzi et al.).
Late-onset sepsis, occurring at approximately 7 to 30 days of age, can include maternally derived infection or health care-associated infection; the offending organisms are usually staphylococci, Klebsiella organisms, enterococci, E. coli, and Pseudomonas, or Candida species (Stoll & Adams-Chapman, 2007). Coagulase-negative staphylococci, considered to be primarily a contaminant in older children and adults, are commonly found to be the cause of septicemia in extremely low-birth-weight (ELBW) and very low-birth-weight (VLBW) infants. Additional infections of concern include methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci, and multidrug-resistant gram-negative pathogens (Stoll, 2007). Bacterial invasion can occur through sites such as the umbilical stump; the skin; mucous membranes of the eye, nose, pharynx, and ear; and internal systems such as the respiratory, nervous, urinary, and gastrointestinal (GI) systems.
Viral infections that are acquired perinatally can cause stillbirth, intrauterine infection, congenital malformations, and acute disease. These pathogens also can cause chronic infection, with subtle manifestations that can be recognized only after a prolonged period. It is important to recognize the manifestations of infections in the neonatal period to treat the acute infection and to prevent health care associated infections in other infants, and to anticipate effects on the infant’s subsequent growth and development.
Fungal infections are of great concern in the immunocompromised or premature infant. Occasionally fungal infections such as thrush are found in otherwise healthy term infants.
Septicemia refers to a generalized infection in the bloodstream. Pneumonia, the most common form of neonatal infection, is one of the leading causes of perinatal death and is caused by many of the same organisms that cause sepsis. Bacterial meningitis affects 1 in 2500 live-born infants (Edwards, 2006). Gastroenteritis is sporadic, depending on epidemic outbreaks. Local infections such as conjunctivitis and omphalitis occur frequently, but incidence rates are unavailable. Infection continues to be a significant factor in fetal and neonatal morbidity and mortality. Sequelae to septicemia include meningitis, disseminated intravascular coagulation (DIC), and septic shock.
Septic shock results from the toxins released into the bloodstream. The most common sign is a decrease in blood pressure, a vital sign often not assessed in the care of the neonate. The infant will often appear gray or mottled and can be noted to have cool extremities. Other signs are rapid, irregular respirations and pulse (similar to septicemia in general).
Care Management
The development of systemic infection in the newborn can be influenced by maternal, peripartum, and neonatal risk factors. Onset within the first 48 hours of life is more often associated with prenatal or perinatal predisposing factors. Onset after 2 or 3 days more frequently reflects disease acquired at or subsequent to birth (See the Nursing Process box: The Infant with Suspected Sepsis.)
The earliest clinical signs of neonatal sepsis are characterized by a lack of specificity. The nonspecific signs include lethargy, poor feeding, poor weight gain, and irritability. The nurse or parent can simply note that the infant is just not doing as well as before. Differential diagnosis can be difficult because signs of sepsis are similar to signs of noninfectious neonatal problems such as anemia or hypoglycemia. Additional clinical and laboratory information and appropriate cultures supplement the findings described. Table 35-3 outlines signs of sepsis.
TABLE 35-3
| SYSTEM | SIGNS |
| Respiratory | Apnea, bradycardia |
| Tachypnea | |
| Grunting, nasal flaring | |
| Retractions | |
| Decreased oxygen saturation | |
| Acidosis | |
| Cardiovascular | Decreased cardiac output |
| Tachycardia | |
| Hypotension | |
| Decreased perfusion | |
| Central nervous | Temperature instability |
| Lethargy | |
| Hypotonia | |
| Irritability, seizures | |
| Gastrointestinal | Feeding intolerance |
| Abdominal distention | |
| Vomiting, diarrhea | |
| Integumentary | Jaundice |
| Pallor | |
| Petechiae | |
| Metabolic | Hypoglycemia |
| Hyperglycemia | |
| Metabolic acidosis | |
| Hematologic | Thrombocytopenia |
| Neutropenia |
Sources: Edwards, M. (2006). Postnatal bacterial infections. In R. Martin, A. Fanaroff, & M. Walsh (Eds.), Fanaroff and Martin’s neonatal-perinatal medicine: Diseases of the fetus and infant (8th ed.). Philadelphia: Mosby.
Laboratory studies are important. Specimens for cultures include blood, CSF, and urine. Fluids such as urine and CSF can be evaluated by counterimmune electrophoresis or latex agglutination to help identify the bacteria. A complete blood cell (CBC) count with differential is performed to determine the presence of bacterial infection or increased or decreased white blood cell count (the latter is an ominous sign). The total neutrophil count, immature to total neutrophil (I/T) ratio, absolute neutrophil count, and C-reactive protein can be used to determine the presence of sepsis. Detection of viral deoxyribonucleic acid (DNA) or antibodies by polymerase chain reaction (PCR) amplification in fluids is also an important diagnostic tool (Edwards, 2006). Antepartum viral infection can now be successfully treated with a number of antiviral medications to decrease viral replication and fetal transmission of disease; neonates can also be treated with antiviral medications such as acyclovir and ganciclovir. Treatment with antibiotics is initiated after blood cultures are obtained in neonates; in high risk infants with significant illness, antiviral or antibiotic treatment can begin once cultures are obtained. Once the pathogen is identified, antibiotic, antiviral, or antifungal therapy can be modified.
Preventive Measures
Virtually all controlled clinical trials have demonstrated that effective hand hygiene is responsible for the prevention of health care acquired infection in nursery units. Nursing is directly or indirectly responsible for minimizing or eliminating environmental sources of infectious agents in the nursery. Measures to be taken include implementing Standard Precautions, carefully and thoroughly cleaning the environment and equipment, frequently replacing used equipment (e.g., changing IV tubing per hospital protocol, cleaning resuscitation and ventilation equipment), and appropriately disposing of excrement and linens. Overcrowding must be avoided in nurseries. Guidelines for space, visitation, and general infection control in areas where newborns receive care have been established and published (American Academy of Pediatrics [AAP] & American College of Obstetricians and Gynecologists [ACOG], 2007).
Specific newborn care procedures are intended to prevent infection. These include the instillation of antibiotic ointment in newborns’ eyes 1 to 2 hours after birth, bathing, and cord care (see Chapter 24).
Curative Measures
Breastfeeding or feeding the newborn breast milk from the mother is encouraged. Breast milk provides protective mechanisms (see Chapter 25). Colostrum contains immunoglobulin A (IgA), which offers protection against infection in the GI tract. Human milk contains iron-binding protein that exerts a bacteriostatic effect on E. coli. Human milk also contains macrophages and lymphocytes. The vulnerability of infants to common mucosal pathogens such as respiratory syncytial virus (RSV) can be reduced by passive transfer of maternal immunity in the colostrum and breast milk. Some evidence indicates that early enteral feedings with human milk (trophic or minimal enteral feedings) can be beneficial in establishing a natural barrier to infection in ELBW and VLBW infants (Anderson, Wood, Keller, & Hay, 2011).
Administering medications safely and correctly, taking precautions when performing treatments, and following isolation procedures are important interventions when a newborn has an infection. Monitoring the IV infusion rate and administering antibiotics are the nurse’s responsibility. If the IV fluid the infant is receiving contains electrolytes, vitamins, or other medications, the nurse should check with the hospital pharmacy before adding antibiotics. The antibiotic (or other medication) can be deactivated or can form a precipitate when combined with other substances. To prevent this from occurring, a secondary line of the prescribed solution is attached with a three-way stopcock at the infusion site.
Care must be taken in suctioning secretions from any newborn’s oropharynx or trachea; the secretions can be infected. Routine suctioning is not recommended and can further compromise the infant’s immune status, as well as cause hypoxia and increase ICP. Isolation procedures are implemented as indicated according to hospital policy. Isolation protocols change rapidly, and the nurse is urged to participate in continuing education and in-service programs to remain up to date.
Transplacental Infections
The occurrence of certain maternal infections during early pregnancy is known to be associated with various congenital malformations and disorders. An acronym that is often used in clinical practice is TORCH, which stands for toxoplasmosis, other (gonorrhea, hepatitis B, syphilis, varicella-zoster virus, parvovirus B19, and HIV), rubella, cytomegalovirus, and herpes simplex virus) (Box 35-1). Additional organisms known to cause congenital infection include enteroviruses and parvovirus, leading some clinicians to suggest the need for a new more comprehensive acronym (Klein, Baker, Remington, & Wilson, 2006). With the advent of newer diagnostic methods, these viral infections can be diagnosed in utero and interventions planned based on the availability of intrauterine treatments.
Toxoplasmosis
Toxoplasmosis is a multisystem disease caused by the protozoan Toxoplasma gondii parasite, commonly found in cats, dogs, pigs, sheep, and cattle, with cats being the definitive host. In the United States risk factors for acquisition of toxoplasmosis include exposure to contaminated soil and consumption of raw or undercooked meats or seafood (oysters, clams, or mussels) (Jones, Dargelas, Roberts, Press, Remington, & Montoya, 2009). Changing cat litter is a known risk for toxoplasmosis. The risk of maternal-fetal transmission of acute infection is approximately 40% with the risk of transmission increasing as the pregnancy progresses. However, the earlier in pregnancy that fetal infection occurs, the greater the severity of congenital disease. Because many women are already seropositive for toxoplasmosis, the overall risk of a primary infection in pregnancy is quite low. The diagnosis of toxoplasmosis in the neonate is supported by elevated levels of cord blood serum IgM.
Most neonates infected with T. gondii in utero are asymptomatic at birth, although many will develop chorioretinitis and signs of CNS involvement such as learning disabilities (Cunningham et al., 2010). For some infected neonates, hydrocephalus is the only clinical sign of the disease (Remington, McLeod, Thulliez, & Desmonts, 2006). Up to 30% of infected infants are born with severe manifestations at birth. Clinical features ascribed to T. gondii infection include three key findings (classic triad) first described by Sabin (1942): hydrocephalus or microcephaly, chorioretinitis, and cerebral calcifications (Cunningham et al.). Severe toxoplasmosis is associated with preterm birth, growth restriction, microcephaly or hydrocephaly, microphthalmos, chorioretinitis, CNS calcification, thrombocytopenia, jaundice, and fever. Petechiae or a maculopapular rash also can be evident.
Infants with congenital toxoplasmosis are treated with pyrimethamine, combined with oral sulfadiazine; folic acid supplement is used to prevent anemia. Treatment should be continued for 1 year (AAP Committee on Infectious Diseases, 2009).
Gonorrhea
The incidence of gonococcal infection in pregnant women ranges from 2.5% to 7.3%. Many women with gonorrhea often have a concurrent Chlamydia trachomatis infection (AAP Committee on Infectious Diseases, 2009). After rupture of membranes, ascending infection can result in orogastric contamination of the fetus. The organism can also invade mucosal surfaces such as the conjunctiva (ophthalmia neonatorum), the rectal mucosa, and the pharynx. Contamination can occur as the infant passes through the birth canal, or it may occur postnatally from an infected adult. Neonatal gonococcal arthritis, septicemia, meningitis, vaginitis, and scalp abscesses also can develop.
Eye prophylaxis (e.g., with 0.5% erythromycin ointment) is administered within the first hour after birth to prevent ophthalmia neonatorum (AAP & ACOG, 2007). Eye prophylaxis alone does not prevent systemic infection; therefore, infants with a gonococcal eye infection should receive one dose of ceftriaxone (Gowen, 2007). Infants with systemic gonococcal infection require hospitalization and 7 days of IV antibiotic therapy. Infants rarely die of overwhelming infection in the early neonatal period. With the prophylactic use of silver nitrate or antibiotics, the incidence of gonococcal conjunctivitis is less than 0.5% (Yudin & Gonik, 2006).
Syphilis
Congenital and neonatal syphilis have reemerged in recent years as significant health problems. Rates of congenital syphilis in the United States increased from 8.2 per 100,000 live births in 2005 to 10.1 per 100,000 live births in 2008 (Su, Berman, Davis, & Weinstock, 2010). It is estimated that for every 100 women diagnosed with primary or secondary disease, 2 to 5 infants will contract congenital syphilis. If syphilis during pregnancy is untreated, approximately 50% of neonates born to these women will have symptomatic congenital syphilis. Treatment failure can occur, particularly when treatment is given in the third trimester; therefore, infants born to women treated within 4 weeks of birth should be investigated for congenital syphilis (Woods, 2009). The following factors have been identified as placing the neonate at high risk for congenital syphilis: lack of or late prenatal care, maternal substance abuse, crack cocaine use in the mother or partner, multiple sexual partners, history of STI, poverty, homelessness, and HIV infection.
The fetus is usually infected in utero by transplacental infection, but infection of the amniotic fluid also can occur. The infant can also contract syphilis during contact with an active genital lesion at birth (Ingall, Sanchez, & Baker, 2006). The risk to the fetus and neonate varies according to the stage of maternal infection, with transmission during primary or secondary syphilis being more common. Untreated maternal disease results in stillbirth in 30% to 40% of cases. Prompt maternal treatment will eliminate most fetal infections; however, delayed treatment or a failure to obtain treatment can result in fetal effects that range from minor anomalies to preterm birth or fetal death. Damage to the fetus depends on when in gestation the infection occurred and the time that has elapsed before treatment. Congenital syphilis infection can be asymptomatic at birth in up to two thirds of infected infants. A portion of these infants will become symptomatic in the first 2 years of life, whereas others may take up to 20 years before displaying the effects of congenital infection (Woods, 2009).
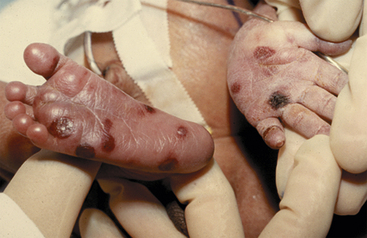
Early congenital syphilis can result in prematurity, hydrops fetalis, and failure to thrive. Hepatosplenomegaly and jaundice are common. Hematologic findings include anemia, leukocytosis, and thrombocytopenia. Characteristic bony lesions occur in the long bones, the cranium, and the spine and include osteochondritis, osteomyelitis, and periostitis. Other findings include snuffles (copious clear mucous discharge from the nose), mucocutaneous lesions, edema, and a copper-colored maculopapular dermal rash first noticeable on the palms of the hands, the soles of the feet, and in the diaper area and around the mouth and the anus by the end of the first week of life in untreated infants (Fig. 35-7). Condylomata (elevated wartlike lesions) can be seen on mucous membranes, moist surfaces, or areas of the body affected by friction. Rough, cracked, mucocutaneous lesions of the lips heal to form circumoral radiating scars known as rhagades. Other involvement results in exfoliation (separation, flaking) of nails and loss of hair. Iritis and choroiditis are characteristic of infection of the eyes. The following can be noted: nephrotic syndrome secondary to renal infection; hepatitis with jaundice, lymphadenopathy, and inflammation of the pancreas, the testes, and the colon; and a pseudoparalysis of the extremities. In some infants, signs of congenital syphilis do not appear until late in the neonatal period. In these newborns, early signs such as poor feeding, slight hyperthermia, and snuffles can be nonspecific (Woods, 2009).
Medical Management: Treatment of the newborn should be carried out when the diagnosis of congenital syphilis is confirmed or suspected or when maternal treatment status is unknown or not well documented. The neonate should be treated when the mother was treated within 1 month of giving birth or does not respond to treatment, when medications other than penicillin were used for the mother, when the mother was treated appropriately but did not have sufficient serologic follow-up to assess response to treatment, and when inadequate neonatal follow-up is anticipated (Duff, Sweet, & Edwards, 2009).
The infant with symptomatic congenital syphilis should have a lumbar puncture, CBC, and long-bone radiography prior to treatment. If the results of these tests are normal, a single intramuscular dose of benzathine penicillin is recommended (Duff et al. 2009). If results are abnormal or there is concern about appropriate follow-up, a 10-day course of IV penicillin or intramuscular (IM) procaine penicillin can be given. If the mother was adequately treated before giving birth and serologic testing of the infant does not show syphilis, generally the infant is not treated with antibiotics. The infant is checked for antibody titer (received from the mother through the placenta) every 2 weeks for 3 months, at which time the test result should be negative. Some physicians recommend antibiotic therapy for asymptomatic or inconclusive cases.
Prognosis: In general, treatment of syphilis is more effective if it begins early rather than late in the course of the disease. However, a recurrence rate of 5% can be expected. Even adequate treatment of congenital syphilis after birth does not always prevent late (5 to 15 years after initial infection) complications. Potential complications include neurosyphilis, deafness, Hutchinson teeth (notched incisors), saber shins, joint involvement, saddle nose (depressed bridge), gummas (soft, gummy tumors) over the skin and other organs, interstitial keratitis (inflammation of the cornea), rhagades, frontal bossing, and mulberry molars (Woods, 2009).
Varicella-Zoster
The varicella-zoster virus, responsible for chickenpox and shingles, is a member of the herpes family. Approximately 90% of women in the childbearing years are immune; therefore the risk of infection in pregnancy is low: 5 per 10,000 pregnancies (Gershon, 2006).
Varicella transmission to the fetus can occur across the placenta when the disease is contracted in the first half of pregnancy, but this is relatively infrequent (about 2%). When transmission to the fetus does occur in the early part of pregnancy, the effects on the fetus include limb atrophy, neurologic abnormalities (hydrocephalus or microcephaly), and eye abnormalities (Gowen, 2007).
When maternal infection occurs in the last 3 weeks of pregnancy, 25% of infants born to these mothers will develop clinical varicella (Gershon, 2006). The severity of the infant’s illness will increase greatly if maternal infection occurred within 5 days before or 2 days after birth (Tan & Koren, 2006). The mortality rate in severe illness is 30% (Gershon).
Seroimmune pregnant women exposed to active chickenpox can be given varicella-zoster immune globulin (VZIG), which does not reduce the incidence of infection but should decrease the effects of the virus on the fetus. The immunoglobulin must be given within 72 hours of exposure to be effective.
Infants born to mothers in whom chickenpox develops between 5 days before birth and 48 hours after should be given VZIG at birth because of the risk of severe disease (Tan & Koren, 2006). Acyclovir can be used to treat infants with generalized involvement and pneumonia (Myers, Seward, & LaRussa, 2007).
Term infants exposed to chickenpox after birth will have a mild or no infection if they are born to immune mothers. In those born to nonimmune mothers, chickenpox can develop, but the course is not usually severe. Experts are divided as to whether this group of infants should receive VZIG. Infants born before 28 weeks gestation are at risk regardless of their mother’s status and probably benefit from VZIG if exposed to chickenpox (AAP Committee on Infectious Diseases, 2009).
Hepatitis B Virus
HBV infection during pregnancy is not associated with an increase in malformations, stillbirths, or IUGR; however, approximately 35% of infected fetuses will be born before term (Baley & Toltzis, 2006). The transmission rate of HBV to the newborn ranges from 70% to 90% when the mother is seropositive for both hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) (AAP Committee on Infectious Diseases, 2009).Transmission occurs transplacentally, serum to serum, and by contact with contaminated urine, feces, saliva, semen, or vaginal secretions during birth. Infants are most frequently infected during birth or in the first few days of life. The rate of transmission is highest when the mother contracts the virus in the third trimester or early in the postpartum period (Bradley, 2006). These mothers will be positive for HBsAg. Transmission can occur through breast milk, but antigens also develop in formula-fed infants at the same or a higher rate, thus breastfeeding is not contraindicated. Diagnosis is made by viral culture of amniotic fluid, as well as the presence of HBsAg and IgM in the cord blood or infants serum.
The majority of infants who become HBsAg positive are symptom free at birth, whereas some show evidence of acute hepatitis with changes in liver function. The mortality rate for full-blown hepatitis is 75%. Infants who become carriers are at high risk for chronic hepatitis, cirrhosis of the liver, or liver cancer even years later (Yudin & Gonik, 2006).
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Approximately 6000 pregnant women infected with HIV give birth each year in the United States. Due to the success of preventive strategies during pregnancy, the incidence of mother-to-child transmission has been reduced to approximately 1% to 2%. Universal HIV testing for all pregnant women allows for early identification and treatment of HIV-positive women during pregnancy, which decreases the risk of transmission to the fetus. Other strategies to prevent neonatal HIV infection include administration of antiviral medications during pregnancy and labor to women who are infected with the virus, administration of antiretrovirals to neonates for 6 weeks, and elective cesarean birth for women with HIV viral loads greater than 1000 copies per ml. In the United States, an additional strategy is the total avoidance of breastfeeding (AAP Committee on Pediatric AIDS, 2008)
Transmission of HIV from the mother to the fetus can occur transplacentally at various gestational ages. The risk of infection in an infant born to an HIV-positive mother (not treated) is approximately 12% to 40%. Transmission most often occurs during birth. Globally, approximately one third to one half of cases of mother-to-child transmission occur through breastfeeding (AAP Committee on Infectious Diseases, 2009).
Diagnosis of HIV infection in the neonate is complicated by the presence of maternal IgG antibodies that cross the placenta after 32 weeks of gestation. The most accurate test for newborns and infants younger than 18 months is the HIV-1 deoxyribonucleic acid (DNA) PCR assay, which is performed on neonatal blood, not cord blood (AAP Committee on Infectious Diseases, 2009). Follow-up testing for infants born to HIV-positive mothers is recommended at several intervals within the first year of life.
Typically the HIV-infected neonate is asymptomatic at birth. Early-onset illness (i.e., virus detected within 48 hours of birth) is attributed to prenatal infection. These infants develop opportunistic infections (Candida and Pneumocystis jiroveci pneumonia) and experience rapid progression of immunodeficiency that often results in death during the first 1 to 2 years of life.
The remainder of infants seroconvert over a period of months to years. By 1 year of life, the vast majority of perinatally infected infants show signs of infection. Some children infected at birth show no signs of disease 8 to 10 years later. The age of onset of symptoms predicts the length of survival.
The presenting signs and symptoms of HIV infection vary from severe immunodeficiency to nonspecific findings such as growth failure, parotitis, and recurrent or persistent upper respiratory tract infections. In the first year of life, lymphadenopathy and hepatosplenomegaly are common. The infant can have fever, chronic diarrhea, chronic dermatitis, interstitial pneumonitis, persistent thrush, and acquired immunodeficiency syndrome (AIDS)–defining opportunistic infections. Common secondary opportunistic infections include pneumonia, candidiasis, CMV, cryptosporidiosis, herpes simplex or herpes zoster, and disseminated varicella.
Although it is rare for an infant to be born with symptoms of HIV infection, all infants born to seropositive mothers should be presumed to be HIV positive until proven otherwise. Management begins by implementing Standard Precautions. Measures should also be taken to protect the infant from further exposure to maternal blood and body fluids. In the United States, breastfeeding is avoided completely if the mother is HIV positive. Regimens for the prevention of HIV transmission include antepartum, intrapartum, and neonatal treatment with highly active retroviral therapy (HAART).
The goal in the administration of antivirals is the suppression of the virus to undetectable concentrations; the available antiviral drugs do not, however, cure the child’s disease. HIV diagnosis in the neonatal period combined with aggressive antibiotic treatment of opportunistic infections has the potential to prolong survival in children (AAP Committee on Infectious Diseases, 2009). Studies of HIV symptoms in children treated in the era of HAART show a significant decrease in the incidence of secondary opportunistic infections (Nesheim, Kapogiannis, Soe, Sullivan, Abrams, Farley, et al., 2007).
Counseling regarding the care of the mothers themselves, the family’s care of the infant, and future pregnancies should be provided. The risk for transmission among members of the same household is minimal. Social services are required in these cases. If the parent chooses to keep the infant, home health care may be arranged. For more information and updated information, parents are referred to the National AIDS Hotline—1-800-342-AIDS.
In the United States, breastfeeding by the HIV-positive mother is contraindicated; however, in developing countries, the risks versus benefits in relation to number of infant deaths attributed to poor sanitary conditions and availability of an appropriate food supply for infants are considered. The World Health Organization (2010) recommends that HIV-positive mothers who are taking antiretroviral medications should breastfeed for at least 12 months. For those who do not have access to ARV therapy, exclusive breastfeeding for 6 months is recommended. After 6 months, complementary foods are introduced and breastfeeding is continued until a safe, nutritional diet without breastmilk can be provided for the infant (WHO, UNICEF, UNFPA, UNAIDS, 2010).
The family must be counseled about vaccinations. All HIV-1–exposed infants should receive routine immunizations. There are specific guidelines for those with confirmed HIV infection (CDC, 2009a). It is usually safe to administer all inactivated vaccines to HIV-1–infected children. In the absence of severe immunosuppression, children with HIV-1 can receive varicella vaccine (CDC, 2009a).
Rubella Infection
Since the rubella vaccination program was begun in 1969, cases of congenital rubella infection have been reduced significantly; however, it is still seen occasionally in the newborn. Vaccination failures, lack of compliance, and the migration of nonimmunized persons result in periodic outbreaks of rubella, also known as German measles.
The risk of a congenitally infected infant varies with the gestational age of the fetus when maternal infection occurs. Abnormalities are most severe if the mother contracts the virus during the first trimester and rare if the disease occurs after that time (Cooper & Alford, 2006).
More than two thirds of infected infants show no apparent symptoms at birth, but sequelae can develop years later. Hearing loss, the most common result, appears to be progressive after birth. Congenital rubella syndrome includes cataracts or glaucoma, hearing loss, and cardiac defects (pulmonary artery stenosis, patent ductus arteriosus, or coarctation of the aorta). Multiple other abnormalities also are present, including IUGR, microphthalmia, hypotonia, hepatosplenomegaly, thrombocytopenic purpura, dermatoglyphic abnormalities, bony radiolucencies, microcephaly, and brain wave abnormalities. Severe
infection can result in fetal death. Delayed effects of infection manifest as thyroid dysfunction, diabetes mellitus, growth hormone deficiency, myocarditis, and glaucoma (Baley & Toltzis, 2006).
Cytomegalovirus Infection
CMV infection during pregnancy can result in miscarriage, stillbirth, or congenital or neonatal cytomegalic inclusion disease (CMID). It is the most common cause of congenital viral infections in humans, occurring in 40,000 newborns in the United States every year. Maternal-fetal transmission of CMV virus occurs in approximately one third of mothers with a primary CMV infection during pregnancy (Edwards, 2006).
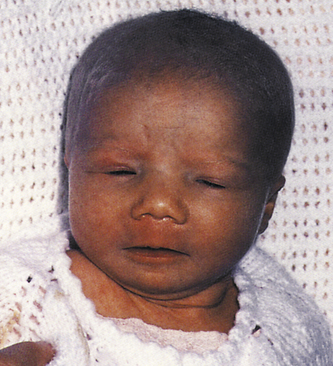
The neonate with classic, full-blown CMID typically displays IUGR and has microcephaly, seizures, hypotonia, and lethargy. The neonate also has a rash, jaundice, and hepatosplenomegaly (Fig. 35-8). Anemia, thrombocytopenia, and hyperbilirubinemia are common (Malm & Engman, 2007). Intracranial, periventricular calcification often is noted on x-ray films. Mortality rates in symptomatic infants are 20% to 30%, with death resulting from hepatic failure, DIC, or secondary bacterial infection (Edwards, 2006). Congenital CMV can cause a variety of neurologic problems such as mental retardation, autism, learning disabilities, hearing loss, visual impairment, or blindness (Malm & Engman, 2007). Most (90%) affected infants are asymptomatic at birth (Kenneson & Cannon, 2007), although there is a 5% to 15% risk that they will develop later sequelae such as hearing loss and learning disabilities (Edwards). Hearing loss can be present at birth or may not be apparent until after the first year of life. The hearing loss is often progressive. Chorioretinitis, microcephaly, mental retardation, and neuromuscular deficits can occur by 2 years of age. Some children are at risk for a defect in tooth enamel, resulting in severe caries (Edwards).
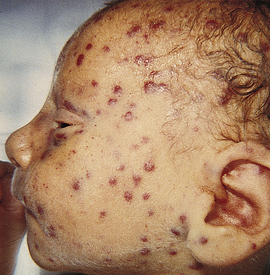
FIG. 35-8 Neonatal cytomegalovirus infection. Typical rash seen in a severely affected infant. (Courtesy David A. Clarke, Philadelphia, PA.)
Elevated levels of cord blood IgM are suggestive of disease. The virus can be isolated from urine or saliva of the newborn. Differential diagnoses include other causes of jaundice, syphilis (positive Venereal Disease Research Laboratory [VDRL] findings), toxoplasmosis (positive Sabin-Feldman dye test result), hemolytic disease of the newborn (positive Coombs’ test reaction), or coxsackievirus infection (positive culture).
CMV can be transmitted through breast milk while the mother has acute CMV infection. CMV infections acquired after birth are often asymptomatic and have no sequelae. Exceptions to this occur in preterm infants in whom postnatal acquisition of CMV can result in pneumonia, hepatitis, thrombocytopenia, and long-term neurologic sequelae.
Treatment of the infected newborn with ganciclovir is effective in decreasing neurologic sequelae, in particular sensorineural hearing loss. There is some evidence that administration of CMV-specific human immunoglobulin to the pregnant woman with primary CMV infection can help protect the fetus (Schleiss, 2008).
Herpes Simplex Virus
HSV infections among newborns are being diagnosed more frequently and are estimated to occur in as many as 1 in 3,000 to 1 in 20,000 births (AAP Committee on Infectious Diseases, 2009). The neonate can acquire the virus through transplacental infection, ascending infection by way of the birth canal, direct contamination during passage through an infected birth canal, or direct transmission from infected personnel or family (Malm, 2009).
Transplacental transmission of HSV infection to the neonate can occur during maternal infection; however, an ascending transcervical infection first involves the intact fetal membranes, causing chorioamnionitis. Transcervical infection can be accelerated by the use of internal fetal monitoring. The scalp electrodes break the fetal skin barrier and increase the risk of infection.
Congenital infection is rare and characterized by in utero destruction of normally formed organs. Affected infants are growth restricted and have skin lesions and scarring. They have severe psychomotor delays, with intracranial calcifications, microcephaly, hypertonicity, and seizures. They have eye involvement, including microphthalmos, cataracts, chorioretinitis, blindness, and retinal dysplasia. Some infants have patent ductus arteriosus, limb anomalies, and recurrent skin vesicles, with a short life expectancy.
HSV is most often transmitted from mother to neonate through viral shedding during passage through the birth canal. The risk of infection during vaginal birth in the presence of genital herpes has not been clearly delineated. It may be as high as 50% with active primary infection at term (Prober, 2008). Primary maternal infections after 32 weeks of gestation have a higher risk for the fetus and newborn than recurrent infections (Baley & Toltzis, 2006). If the mother is shedding HSV virus as a result of reactivated infection, the risk of transmission is only about 2% (AAP Committee on Infectious Diseases, 2009). This is possibly related to passive intrauterine immunity to herpes. Clinical and laboratory features associated with neonatal herpes include maternal primary HSV infection, vaginal birth, preterm birth, neonatal seizures, elevated liver enzymes, vesicular rash, and elevated CSF counts (Caviness, Demmler, & Selwyn, 2008). In approximately 70% of women whose infants have HSV infection, there are no symptoms or history of infection although serologic testing reveals evidence of herpesvirus (Baley & Toltzis, 2006).
Postnatal acquisition of the virus and spread within a nursery have been documented by DNA analysis. Parents have been implicated in neonatal infections. There also is concern regarding symptomatic and asymptomatic shedding among hospital personnel. Nursery personnel with cold sores should practice strict hand hygiene and wear a mask, but no evidence indicates that they should be removed from the nursery unless they have a herpetic whitlow (primary HSV infection of the terminal segment of a finger) (Baley & Toltzis, 2006).
Clinically, neonatal HSV infections are classified as disseminated infection (22%), CNS disease (34%), or localized infection of the skin, eye, or mouth (SEM) (40%) (Baley & Toltzis, 2006). Although the incubation period for HSV is 1 to 7 days, the onset of symptoms varies with the type of infection (Malm, 2009).
Disseminated infections are sepsis-like and can involve virtually every organ system, but primarily the liver, the adrenal glands, and the lungs are involved. By 5 to 11 days of age affected infants show signs of bacterial sepsis or shock. Death often results within 1 week of the onset of symptoms and is related to respiratory failure, pneumonitis, DIC with shock, and CNS complications (Baley & Toltzis, 2006; Malm, 2009).
In CNS disease, blood-borne seeding of the brain results in multiple lesions of cortical hemorrhagic necrosis. It also can occur alone or in association with oral, eye, or skin lesions. Brain involvement usually manifests in the second to fourth weeks of life. Skin lesions are apparent in 60% to 70% of the infants, and the CSF of less than 50% will reveal the virus. The presenting manifestations include lethargy, poor feeding, irritability, and local or generalized seizures. If untreated, the mortality rate in CNS disease approaches 50% with the vast majority of survivors experiencing severe sequelae such as microcephaly and blindness (Baley & Toltzis, 2006).
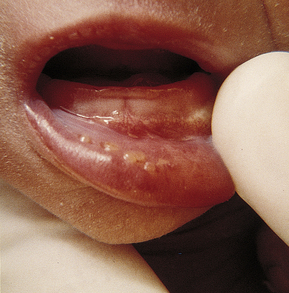
Localized HSV infections most often occur with skin findings or rarely with isolated oral cavity lesions (Fig. 35-9). Without treatment, CNS or disseminated disease develops in 70% of the infants with skin vesicles. Ocular involvement, which can occur alone, can be secondary to either HSV-1 or HSV-2. Ocular disease may not be discovered for months. Microphthalmos, cataracts, optic atrophy, and corneal scarring can result from chorioretinitis, keratitis, and retinal hemorrhage (Baley & Toltzis, 2006).
Gloves should be worn when caregivers are in contact with these infants. The neonate’s eyes, oral cavity, and skin are inspected carefully for the presence of any lesions. Cultures are obtained from the mouth, the eyes, and any possible lesions. Circumcision, if performed, is delayed until the infant is ready to be discharged. The infant can be discharged with the mother if the infant’s cultures are negative for the virus. As long as no suspicious lesions are present on the mother’s breasts, breastfeeding is allowed. For the infant at risk, prophylactic topical eye ointment (vidarabine) is administered for 5 days for prevention of keratoconjunctivitis. No current recommendations exist for prophylactic systemic therapy; each case should be considered individually. Blood, urine, and CSF specimens should be cultured when indicated clinically. If herpetic lesions first occur after 6 weeks of life, the risk of dissemination and severe illness is very low (Baley & Toltzis, 2006).
Therapy includes general supportive measures, as well as treatment with acyclovir. Duration of treatment is 21 days for infants with disseminated or CNS disease and 14 days for the skin-eye-mouth form of the disease (Malm, 2009). Continuing therapy is required in case of recurrence. When there is ocular involvement, ophthalmic ointment should be administered simultaneously (AAP Committee on Infectious Diseases, 2009).
Parvovirus B19
Parvovirus B19 is well known in older children as “fifth disease” or “slapped cheek illness” because of the characteristic facial appearance of the affected child. During pregnancy, infection can result in miscarriage, fetal anemia, hydrops fetalis, IUGR, and stillbirth. Vertical transmission of the parvovirus to the fetus occurs in about one third of maternal infections (de Jong, de Haan, Kroes, Beersma, Oepkes, Walther, et al., 2006) and the overall fetal loss rate after infection is about 5% to 10% (Tolfvenstam & Broliden, 2009).
The virus can be isolated from amniotic fluid, fetal blood, or tissues using DNA PCR assay. Viral load is not predictive of fetal morbidity and mortality (Cunningham et al., 2010).
There are no specific antiviral medications to treat parvovirus B19 infection and there is no vaccine. If a pregnant woman is confirmed with the infection, the fetus should be closely monitored for the development of fetal hydrops using serial ultrasound examinations (Tolfvenstam & Broliden, 2009). Protocols for intrauterine management have not been well developed, but intrauterine transfusion to treat anemia is the only currently accepted therapy (de Jong et al., 2006). There is insufficient evidence about the long-term effects of parvovirus (Cunningham et al., 2010).
Enterovirus
Enteroviruses include poliovirus, coxsackievirus, and echovirus. Nonpolio enteroviruses are among the most common viruses that infect humans. Enteroviral infections in the newborn account for about 10% of all reported cases of enterovirus infection in the United States (Khetsuriani, Lamonte, Oberste, & Pallansch, 2006). Group B coxsackieviruses and echovirus 11 are the serotypes most commonly affecting neonates and can be transmitted transplacentally or through exposure to maternal blood or secretions during birth. The risk of transmission is increased with maternal enterovirus illness around the time of birth and a lack of maternal antibodies to the specific type of enterovirus. Antenatal transmission increases the risk of severe disease and death (Tebruegge & Curtis, 2009).
The onset of infection following perinatal transmission is usually within 1 to 2 weeks after birth. Usual presenting symptoms are fever, irritability, lethargy, and poor feeding; rash, respiratory symptoms and gastrointestinal symptoms also can occur. Clinical manifestations can be severe and include myocarditis, meningitis, respiratory distress, and hepatitis. Deaths are usually due to multiorgan involvement (Tebruegge & Curtis, 2009; Wikswo, Khetsuriani, Fowlkes, Zheng, Penaranda, Verma, et al., 2009).
Currently there is no vaccine or any treatment approved by the U.S. Food and Drug Administration (FDA) for nonpolio enteroviruses. Immunoglobulin is sometimes used to treat symptomatic infants. Pleconaril is under investigation as a treatment for enteroviral infection in neonates and infants (Tebruegge & Curtis, 2009).
Bacterial Infections
GBS infection is a leading cause of neonatal morbidity and mortality in the United States (CDC, 2009d). The practice of giving prophylactic antibiotics to women in labor who are GBS positive has significantly reduced the incidence and severity of early-onset GBS infection in the newborn (Yudin & Gonik, 2006). In the United States, the incidence of early onset neonatal GBS infection is 0.4 per 1000 live births and the incidence of late-onset (after 7 days of age) infection is 0.3 per 1000 live births (CDC, 2009d).
Early-onset GBS infection in the neonate usually occurs in the first 7 days of life but most commonly manifests in the first 24 hours after birth. Risk factors for the development of early-onset GBS include low birth weight, preterm birth, rupture of membranes of more than 18 hours, maternal fever, previous GBS infant, maternal GBS bacteriuria, use of intrauterine fetal monitoring, maternal age less than 20 years, and Hispanic or African-American ethnicity. Early-onset disease usually results from vertical transmission from the birth canal and manifests as systemic infection or respiratory illness that mimics the symptoms of severe respiratory distress. The infant can rapidly develop pneumonia, shock, or meningitis. The mortality rate for early-onset infection is approximately 4%, with higher rates among preterm infants (AAP Committee on Infectious Diseases, 2009; Cunningham et al., 2010).
Late-onset GBS infections occur between 1 week and 3 months of age, with an average age at onset of 24 days. Infection can result from vertical transmission or from health care acquired infection or community exposure. Of infants with late-onset GBS, 30% develop meningitis (Edwards, Nizet, & Baker, 2006). Mortality rates are less than for early-onset infection. Survivors often have neurologic damage (Cunningham et al., 2010).
For newborns with suspected GBS infection, the usual treatment is ampicillin in combination with an aminoglycoside. If GBS is identified as the cause of infection, penicillin G alone can be given (AAP Committee on Infectious Diseases, 2009). Figure 35-10 provides a sample algorithm for the management of a neonate whose mother received intrapartum antibiotics for prevention (IAP) of group B streptococcal disease in the newborn or suspected chorioamnionitis.
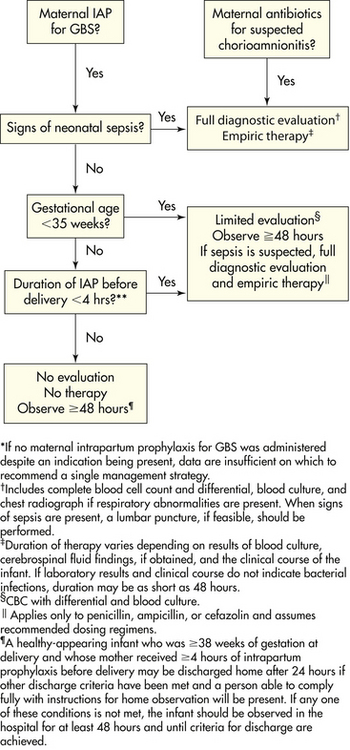
FIG. 35-10 Sample algorithm for management of a newborn whose mother received intrapartum antimicrobial agents for prevention (IAP) of early-onset group B streptococcal disease or suspected chorioamnionitis. This algorithm is not an exclusive course of management. Variations that incorporate individual circumstances or institutional preferences may be appropriate. (From Schrag S., Gorwitz, R., Fultz-Butts, K., & Schuchat, A. [2002]. Prevention of perinatal group B streptococcal disease. Revised guideliness from CDC. MMWR Morbidity and Mortality Weekly Report, 51[RR11],1-22.)
Escherichia coli
E. coli is responsible for approximately 40% of cases of neonatal sepsis and 80% of cases of neonatal meningitis in the United States (AAP Committee on Infectious Diseases, 2009). It is found in the gastrointestinal tract soon after birth and makes up the bulk of human fecal flora. E. coli can cause a variety of neonatal infections, including omphalitis, diarrheal illness, pneumonia, peritonitis, urinary tract infections, and meningitis. Risk factors for the development of E. coli infections in the newborn include preterm birth, low birth weight, maternal infection, prolonged rupture of membranes, and septic or traumatic birth (AAP Committee on Infectious Diseases).
Neonates most often acquire E. coli from the maternal birth canal or rectum during birth, although it also can be hospital acquired through person-to-person transmission or from the hospital environment. Exposure to E. coli can result in no infection, infection without illness, gastroenteritis, or rarely, septicemia. Symptoms can appear within the first 24 hours after birth or several weeks later. Clinical signs of E. coli sepsis are relatively nonspecific and include fever, temperature instability, apnea, cyanosis, jaundice, hepatomegaly, lethargy or irritability, vomiting, abdominal distention, and diarrhea.
The usual treatment for E. coli infection in the newborn is ampicillin or an extended spectrum cephalosporin and an aminoglycoside. Treatment is continued for 1 to 14 days for bacteremia and 21 days for meningitis (AAP Committee on Infectious Diseases, 2009). Use of ampicillin in labor as prophylaxis against GBS disease has not been found to increase the risk of early-onset E. coli infection (Schrag, Hadler, Arnold, Martell-Cleary, Reingold, & Schuchat, 2006) but can result in more virulent E. coli disease resulting from ampicillin-resistant organisms (Bizzaro, Dembry, Baltimore, & Gallagher, 2008).
Staphylococcus aureus
Most staphylococcal infections in the newborn develop within the first few days after birth and involve the skin and soft tissue. Conjunctivitis, presenting with purulent eye discharge, is also a common manifestation of S. aureus infection. Skin lesions are typically small vesicles or pustules that are easily treated with topical antimicrobial agents. The skin lesions can appear as large fragile bullae containing clear or purulent fluid. These bullae rupture easily and leave moist, red, denuded areas of skin. A more severe bullous eruption due to staphylococcal infection is scalded skin syndrome characterized by widespread bullous lesions that easily rupture; fever and irritability are also present (Narendran & Hoath, 2006). S. aureus can cause serious problems such as abscesses, osteomyelitis, endocarditis, and septic arthritis. Breaks in the skin from IV catheters or scalp electrodes present an opportunity for the development of S. aureus abscesses. Colonization of the maternal genital tract with S. aureus is not uncommon although the potential for vertical transmission to the neonate is unclear (Andrews, Schelonka, Waites, Stamm, Cliver, & Moser, 2008). The major sources of colonization by S. aureus are the hands of medical and nursing personnel.
Although most strains of S. aureus are sensitive to semisynthetic penicillins, there are increasing concerns about methicillin-resistant strains (Gorwitz, 2008). MRSA has caused outbreaks of hospital-acquired infection in neonatal intensive care units and hospital nurseries (Fortunov, Hulten, Hammerman, Mason, & Kaplan, 2006).
Listeriosis
Listeriosis, caused by Listeria monocytogenes, is primarily a foodborne infection that can cause maternal and neonatal illness. Globally it is one of the three major causes of neonatal meningitis (Posfay-Barbe & Wald, 2009). The incidence of neonatal listeriosis in the United States is reported to be approximately 8.6 per 100,000 live births (CDC, 2009b). Although it is relatively uncommon and probably underdiagnosed, it occurs more frequently among pregnant women and those who are older or immunocompromised. Mother-to-child transmission occurs transplacentally, through ascending infection, or during birth. Prenatal infection causes chorioamnionitis or endometritis and should be suspected in cases of brown-stained amniotic fluid. It can also cause miscarriage, preterm birth, and stillbirth. Two forms of neonatal listeriosis are recognized: early onset and late onset. Signs of early-onset infection are present at birth or in the first 1 to 2 days. Manifestations of infection are sepsis-like symptoms, acute respiratory distress, pneumonia, and more rarely, meningitis or myocarditis. With severe infection the infant can have granulomatosis infantiseptum which is a widely disseminated granuloma, causing an erythematous rash with pale papules and abscesses in the liver, lungs, brain, kidneys, spleen, adrenal glands, and GI system. (Posfay-Barbe & Wald, 2009). Late-onset listeriosis is more insidious and usually manifests as meningitis. Listeriosis in the newborn is treated with ampicillin and an aminoglycoside (AAP Committee on Infectious Diseases, 2009).
Chlamydia Trachomatis
Chlamydia trachomatis, the most common reportable sexually transmitted infection in the United States, causes neonatal conjunctivitis (25% to 50% of exposed infants) and pneumonia (5% to 20% of exposed infants). Neonatal infection is acquired by newborns in approximately half of all vaginal births by infected mothers and is known to occur in some infants born by cesarean with intact membranes (AAP Committee on Infectious Diseases, 2009). Chlamydial infection in pregnancy has also been suggested as a cause of early and late pregnancy loss, stillbirth, premature labor, and postpartum endometritis (Yudin & Gonik, 2006).
Neonatal chlamydial conjunctivitis (congestion and edema), with minimal discharge, develops within a few days to several weeks after birth and usually lasts 1 to 2 weeks. If untreated, it can progress to chronic follicular conjunctivitis with conjunctival scarring and corneal microgranulations.
Chlamydial pneumonia usually has a gradual onset, between 4 and 11 weeks of age, beginning with rhinorrhea and progressing to tachypnea and coughing (Yudin & Gonik, 2006). Signs may be quite subtle and often go unrecognized. It is speculated that pneumonia can occur as a result of movement of the organism from the conjunctiva into the lower respiratory tract; however, conjunctivitis is not a prerequisite to pneumonia.
Chlamydial conjunctivitis is treated with oral erythromycin or ethylsuccinate for 14 days. The recommended treatment for chlamydial pneumonia is oral azithromycin for 3 days or a 14-day regimen of erythromycin or ethylsuccinate. Erythromycin administration in infants younger than 6 weeks has been associated with an increased risk of infantile hypertrophic pyloric stenosis; therefore, parents should be educated regarding the symptoms of the condition (feeding intolerance, projectile vomiting, and abdominal distention) (AAP Committee on Infectious Diseases, 2009).
Fungal Infections
Candida infections, formerly known as moniliasis, can occur in the newborn. Candida albicans, the organism usually responsible, can cause disease in any organ system. It is a yeastlike fungus (producing yeast cells and spores) that can be acquired during birth from a maternal vaginal infection; by person-to-person transmission; or from contaminated hands, bottles, nipples, or other articles. It usually is a benign disorder in the neonate, often confined to the oral and diaper regions (Wilson & da Cunha, 2007).
Candidal diaper dermatitis appears on the perianal area, inguinal folds, and lower portion of the abdomen. The affected area is intensely erythematous, with a sharply demarcated, scalloped edge, frequently with numerous satellite lesions that extend beyond the larger lesion. The source of the infection is through the GI tract. Treatment is an antifungal ointment, such as nystatin (Mycostatin) or miconazole 2% (Monistat), applied with each diaper change. The infant also can be given an oral antifungal preparation to eliminate any GI source of infection (Wilson & da Cunha, 2007).
Oral candidiasis (thrush, or mycotic stomatitis) is characterized by the appearance of white plaques on the oral mucosa, gums, and tongue. The white patches are easily differentiated from milk curds; the patches cannot be removed and tend to bleed when touched. In most cases, the infant does not seem to be in discomfort from the infection. A few infants seem to have some difficulty swallowing.
Infants who are sick, debilitated, or receiving antibiotic therapy are more susceptible to thrush. Those with cleft lip or palate, neoplasms, and hyperparathyroidism seem to be more vulnerable to mycotic infection.
The objectives of management are to eradicate the causative organism, to control exposure to C. albicans, and to improve the infant’s resistance. Interventions include maintenance of scrupulous cleanliness to prevent reinfection (nursing personnel, parents, others). Careful hand hygiene is always essential. Clean surfaces should be provided for neonates. Proper cleanliness of the equipment and environment is essential. If the infant is breastfeeding, the mother also is treated with a topical antifungal preparation such as nystatin applied to the nipples.
For the infant, topical application of 1 ml nystatin over the surfaces of the oral cavity four times a day, or every 6 hours, is usually sufficient to prevent spread of the disease or prolongation of its course. Several other drugs can be used, including amphotericin B (Fungizone), clotrimazole (Lotrimin, Mycelex), fluconazole (Diflucan), or miconazole (Monistat, Micatin) given intravenously, orally, or topically. To prevent relapse, therapy should be continued for at least 2 days after the lesions disappear. Gentian violet solution can be used in addition to one of the antifungal drugs in chronic cases of oral thrush; however, the former does not treat GI candida and can irritate the oral mucosa.
Infants who are breastfed can acquire thrush from the mother. If the mother is colonized, treatment for mother and infant is recommended. Breastfeeding can continue even if the mother is receiving systemic antifungal medications.
Substance Abuse
Substance abuse during pregnancy is associated with significant fetal and neonatal risks. Other than alcohol and tobacco, cocaine and marijuana are the most commonly used substances by pregnant women. Maternal substance abuse is discussed in Chapter 32.
The adverse effects of exposure of the fetus to drugs are varied. They include transient behavioral changes such as alterations in fetal breathing movements or irreversible effects such as fetal death, IUGR, congenital anomalies, or mental retardation. Critical determinants of the effect of the drug on the fetus include the specific drug, the dosage, the route of administration, the genotype of the mother or fetus, and the timing of the drug exposure. Determining the specific effects of individual drugs on the fetus is made difficult by the common practice of polydrug use, errors or omissions in reporting drug use, and variations in the strength, purity, and types of additives found in street drugs. Maternal conditions such as poverty and malnutrition and comorbid conditions such as STIs further compound the difficulty in identifying the presence and consequences of intrauterine drug exposure. Figure 35-11 shows critical periods in human embryogenesis and the teratogenic effects of drugs. Table 35-4 summarizes the effects of commonly abused substances on the fetus and neonate.
TABLE 35-4
SUMMARY OF NEONATAL EFFECTS OF COMMONLY ABUSED SUBSTANCES
| SUBSTANCE | NEONATAL EFFECTS |
| Alcohol | Fetal alcohol syndrome (FAS): craniofacial anomalies, including short eyelid opening, flat midface, flat upper lip groove, thin upper lip; microcephaly; hyperactivity; developmental delays; attention deficits |
| Alcohol-related birth defects (ARBDs): milder forms of FAS, cardiac anomalies, failure to thrive | |
| Cocaine | Prematurity, small for gestational age, placental or cerebral infarctions hyperactivity, difficult to console, hypersensitivity to noise and external stimuli, reduction in verbal reasoning |
| Heroin | Low birth weight, small for gestational age, neonatal abstinence syndrome (see Table 35-5) |
| Amphetamines | Small for gestational age, prematurity, poor weight gain, lethargy |
| Tobacco | Prematurity, low birth weight, increased risk for sudden infant death syndrome, increased risk for bronchitis, pneumonia, developmental delays |
FIG. 35-11 Critical periods in human embryogenesis.Infant with fetal alcohol syndrome. (From Reed, M., Aranda, J., & Hales, B. [2006]. Developmental pharmacology. In R. Martin, A. Fanaroff, & M. Walsh (Eds.), Fanaroff and Martin's neo natal-perinatal medicine: Diseases of the fetus and infant [8th ed.]. St. Louis: Mosby.)
Physiologic signs of withdrawal have been reported in neonates of mothers who use to excess such drugs as barbiturates, alcohol, opioids, or amphetamines. Prescription opioids such as oxycodone (Percodan, OxyContin) have been identified as increasingly popular drugs of abuse that can cause withdrawal symptoms in neonates. Serious withdrawal reactions can be seen in neonates whose mothers abuse psychoactive drugs. Withdrawal symptoms in the neonate are described as neonatal abstinence syndrome. This syndrome is characterized by symptoms of CNS irritability, respiratory distress, GI dysfunction, and autonomic dysfunction (Table 35-5). Withdrawal symptoms are more severe in newborns exposed to larger amounts of drugs for longer periods of time. The severity of withdrawal is also related to the timing of maternal drug use in relation to birth. Drug use close to the time of birth increases the severity of withdrawal, but delays the onset of symptoms (Fike, 2007).
Tobacco
According to the CDC (2009c), infants born to women who smoke are about 30% more likely to be preterm, weigh on average a half pound less, and have a threefold risk of sudden infant death syndrome (SIDS) as compared with infants born to nonsmokers. When other variables have been controlled for, no clear association has been found between maternal smoking and congenital anomalies (Reed, Aranda, & Hales, 2006).
Nicotine and cotinine, the two pharmacologically active substances in tobacco, are found in higher concentrations in infants whose mothers smoke. These substances can be secreted in breast milk for up to 2 hours after the mother has smoked. Cigarette smoke contains more than 2000 compounds, including carbon monoxide, dioxin, cyanide, and cadmium. Long-term studies show residual effects beyond the neonatal period (Reed et al., 2006). Deficits in growth, in intellectual and emotional development, and in behavior have been documented (Shea & Steiner, 2008). These include poor auditory responsiveness, increased fine motor tremors, hypertonicity, and decreased verbal comprehension.
Increasing concern surrounds secondhand smoke and its potential effects on infants and children. Exposure to secondhand smoke increases the risk of ear infections, respiratory illnesses such as asthma and bronchitis, and SIDS (Best, Committee on Environmental Health, Committee on Native American Child Health, & Committee on Adolescence, 2009; Fleming & Blair, 2007). It is not clear whether the association between smoking and SIDS reflects in utero exposure or passive exposure postnatally, or both.
Smoking cessation during pregnancy greatly decreases the chance of fetal complications; therefore, women should be counseled regarding smoking cessation programs. Mothers and all others should refrain from smoking near the infant.
Alcohol
Alcohol consumption during pregnancy is a growing concern. Alcohol ingestion during pregnancy is associated with both acute short- and long-term effects on the fetus and newborn, which are subsumed under the term fetal alcohol spectrum disorders (FASDs). FASDs include specific conditions such as fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD). FASDs are estimated to occur in as many as 9 to 10 of every 1000 births in the United States. However, the full extent of the problem is unknown and more prevalence studies are needed (Olson, Ohlemiller, O’Connor, Brown, Morris, Damus, & National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effect, 2009).
The quantity of alcohol required to produce fetal effects is unclear, but it is known that infants born to heavy drinkers are at higher risk of congenital abnormalities than those born to moderate drinkers. Alcohol withdrawal can occur in neonates, particularly when maternal ingestion occurs near the time of birth. Signs and symptoms include jitteriness, increased tone and reflex responses, and irritability. Seizures are also common.
Fetal alcohol syndrome (FAS) is based on minimal criteria of signs in each of three categories: prenatal and postnatal growth restriction; CNS malfunctions, including mental retardation; and craniofacial features such as microcephaly, small eyes or short palpebral fissures, thin upper lip, flat midface, and an indistinct philtrum (Box 35-2). Neurologic problems in FAS children include some degree of IQ deficit, attention-deficit disorder, diminished fine motor skills, and poor speech. These children have been shown to lack inhibition, have no stranger anxiety, and lack appropriate judgment skills.
Infants exposed prenatally to alcohol who are affected but do not meet the criteria for FAS can be said to have alcohol-related neurodevelopmental disorders (ARNDs) or alcohol-related birth defects (ARBDs). These disorders run the gamut from learning disabilities and behavioral problems to speech or language problems and hyperactivity. Often these problems are not detected until the child goes to school and learning problems become evident. Similar birth defects can be seen with other disorders, such as fetal hydantoin syndrome (from exposure to antiepileptic drugs); therefore, a careful history is needed.
Predictable abnormal patterns of fetal and neonatal morphogenesis are attributed to severe, chronic alcoholism in women who continue to drink heavily during pregnancy. The pattern of growth deficiency begun in prenatal life persists after birth, especially in the linear growth rate, rate of weight gain, and growth of head circumferences.
Ocular structural anomalies are common findings (Fig. 35-12). Limb anomalies and a variety of cardiocirculatory anomalies, especially ventricular septal defects, pose problems for the child. Box 35-2 outlines physical findings in FAS. Mental retardation (IQ of 79 or less at age 7 years), hyperactivity, and fine motor dysfunction (poor hand-to-mouth coordination, weak grasp) add to the handicapping problems that maternal alcoholism can impose. Genital abnormalities are seen in daughters of alcohol-addicted mothers. Two thirds of newborns with FAS are girls; the cause of this altered fetal sex ratio is unknown. Severe and chronic alcoholism (ethanol toxicity), not maternal malnutrition, is responsible for the severity and consistency of postnatal performance problems (Bandstra & Accornero, 2006). High alcohol levels are lethal to the developing embryo. Lower levels cause brain and other malformations. Long-term prognosis (no studies are available) is discouraging even in an optimal psychosocial environment, when one considers the combination of growth failure and mental retardation.
Heroin
Heroin crosses the placenta and often results in IUGR in exposed infants, although the exact mechanisms of growth inhibition are not clear. There is an increased rate of stillbirths but not of congenital anomalies. Maternal heroin use increases the risk for meconium aspiration, increased neonatal death, microcephaly, neurobehavioral problems, and SIDS (Minozzi, Amato, Vecchi, & Davoli, 2008). If heroin users are placed on methadone maintenance treatment during pregnancy, perinatal outcomes are improved (Wisner, Sit, Reynolds, Altemus, Bogen, Sunder, et al., 2007).
Many of the medical complications attributed to heroin result from prematurity. Other risks include physical dependence in the fetus and the risk of exposure to infections, including hepatitis B and C viruses and HIV. Drug withdrawal in the mother is accompanied by fetal withdrawal, which can lead to fetal death. Maternal detoxification in the first trimester carries an increased risk of miscarriage. Detoxification in pregnancy is not recommended because of possible withdrawal-induced fetal distress. Methadone is often used in pregnancy to treat maternal drug cravings and prevent withdrawal.
Heroin withdrawal (neonatal abstinence syndrome) occurs in the majority of infants born to addicted mothers, usually within the first 12 to 48 hours of life. The signs depend on the length of maternal addiction, the amount of drug taken, and the time of injection before birth. The infant whose mother is taking methadone may not demonstrate signs of withdrawal until a week or so after birth. The symptoms of infants whose mothers used heroin or methadone are similar in nature. Initially the infant can be depressed. The withdrawal syndrome can manifest as a combination of any of the following signs. Most commonly, the infant is jittery and hyperactive. Usually the infant’s cry is shrill and persistent. The infant can yawn or sneeze frequently. The tendon reflexes are increased, but the Moro reflex is decreased. The neonate can exhibit poor feeding and sucking, tachypnea, vomiting, diarrhea, hypothermia or hyperthermia, and sweating. In addition, an abnormal sleep cycle, with absence of quiet sleep and disturbance of active sleep, has been described in these infants (Bandstra & Accornero, 2006).
If withdrawal is not treated, vomiting, diarrhea, dehydration, apnea, and convulsions can develop. Death can follow. Therapy is individualized. Dehydration and electrolyte imbalance are prevented or treated. Usually one of the following drugs is ordered: phenobarbital, methadone, morphine syrup, or diazepam, singly or in combination.
Methadone
Methadone, a synthetic opiate, has been the therapy of choice for heroin addiction since 1965. Methadone crosses the placenta. An increasing number of infants have been born to methadone-maintained mothers, who seem to have better prenatal care and a somewhat better lifestyle than those taking heroin (Bandstra & Accornero, 2006).
Neonatal abstinence syndrome occurs in the majority of neonates born to women taking methadone. Neither the incidence or severity of symptoms has been correlated with methadone dose at delivery (Wisner et al., 2007). Methadone withdrawal resembles heroin withdrawal but tends to be more severe and prolonged. In addition, the incidence of seizures is higher. Seizures usually occur between days 7 and 10. Infants exhibit a disturbed sleep pattern similar to that seen in heroin withdrawal and have a higher birth weight than those in heroin withdrawal, usually appropriate for gestational age. No increased incidence of congenital anomalies is seen.
Late-onset withdrawal occurs at 2 to 4 weeks and can continue for weeks or months. A higher incidence of SIDS also has been reported in these infants (Bandstra & Accornero, 2006). This factor is important for perinatal nurses who coordinate follow-up care for the infant and education for the mother or other caregiver. Pediatric and community health nurses must know about the potential for withdrawal symptoms to occur.
Therapy for methadone withdrawal is similar to that for heroin withdrawal. Buprenorphine, a partial agonist–partial anatagonist synthetic opioid with a long duration of action, has gained acceptance and FDA licensing in the treatment of opioid addiction. Preliminary studies indicate that this drug has advantages over methadone in relation to neonatal outcomes such as severity of neonatal abstinence syndrome and length of hospital stay (Helmbrecht & Thiagarajah, 2008; Kakko, Heilig, & Sarman, 2008).
Methadone is not contraindicated during breastfeeding. Minimal amounts of methadone are transferred to the infant through breast milk. However, infants exposed to methadone are at greater risk for feeding problems (Wisner et al., 2007).
The long-term effects of methadone exposure are primarily related to neurodevelopmental outcomes. These neonates are at increased risk for developmental delays, poor fine motor coordination, lower intelligence, hyperactivity, learning and behavior disorders, and poor social adjustment. Researchers have noted that it is difficult to separate the effects of methadone from other factors in the environment that can influence neurobehavioral outcomes (Bandstra & Accornero, 2006; Helmbrecht & Thiagarajah, 2008).
Marijuana
Marijuana is the most common illicit drug used by pregnant women. Marijuana crosses the placenta, and its use during pregnancy can result in a shortened gestation and a higher incidence of IUGR. A review of studies examining the effects of marijuana during pregnancy found inconsistent results regarding the drug’s effect on birth weight and gestational age (Schempf, 2007). No specific teratogenic effects have been identified. Some investigators have found a higher incidence of meconium staining of the amniotic fluid. Animal models suggest that marijuana smoking during pregnancy can increase carbon monoxide levels in the blood which reduces the amount of oxygen available to the fetus, but this has not been correlated with studies identifying short- or long-term effects on the fetus (Bandstra & Accornero, 2006).
Long-term effects of marijuana exposure can involve deficits in memory, attention, cognitive function, or motor skills (Wisner et al., 2007). Compounding the issue of the effects of marijuana is multidrug use, especially among adolescents, that combines the harmful effects of marijuana, tobacco, alcohol, and cocaine. Long-term follow-up studies on exposed infants are needed.
Cocaine
Cocaine crosses the placenta and is found in breast milk. Considerable controversy exists regarding the effects of cocaine on the fetus and neonate. There is a strong association between maternal cocaine use and both tobacco and marijuana use, which makes the determination of cocaine effects difficult. A large metaanalysis of 15,208 pregnancies did not find an association between illicit drug use and congenital anomalies (van Gelder, Reefhuis, Caton, Werler, Druschel, & Roeleveld, 2009). However, it is possible that habitual cocaine use in pregnancy has negative effects, many of which are too subtle to notice in the newborn and infancy periods. Cocaine is a recognized cause of placental abruption. Infants born to cocaine-abusing mothers show a high rate of perinatal morbidity, IUGR, low birth weight, and preterm birth (Bandstra & Accornero, 2006; Wisner et al., 2007).
Cocaine-dependent neonates do not experience a process of withdrawal as seen in narcotic-exposed infants but rather show neurotoxic effects of the drug. Signs of exposure have some of the same characteristics as those of heroin withdrawal but can be highly varied. There can be an increased risk for SIDS. The effects of prenatal exposure to cocaine on neonatal behavior have been studied extensively. Findings indicate that cocaine-exposed infants have limited ability to habituate to stimuli. As these children enter school, they demonstrate a reduced capacity for verbal reasoning and difficulties maintaining attention (Bandstra & Accornero, 2006).
Methamphetamine
The fetal and neonatal effects associated with maternal use of methamphetamines in pregnancy are not well known. The limited data regarding the risk of congenital anomalies suggest little or no effect on organogenesis. A higher incidence of placental abruption, preterm birth, and IUGR is associated with methamphetamine use during pregnancy (Cunningham et al., 2010; Helmbrecht & Thiagarajah, 2008; Smith, LaGasse, Derauf, Grant, Shah, Arria, et al., 2006). Behavioral changes in infants exposed prenatally to methamphetamines include decreased arousal, increased stress, and alterations in movement (Smith et al., 2006).
Neonatal manifestations of methamphetamine withdrawal have not been clearly identified because of maternal polydrug use. After birth, infants can experience bradycardia or tachycardia that resolves as the drug is cleared from the infant’s system. Lethargy can continue for several months, along with frequent infections and poor weight gain. Emotional disturbances and delays in gross and fine motor coordination can be seen during early childhood.
MDMA/Ecstasy
Increasing use of 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy,” by adolescents and young adults of childbearing age heightens concerns related to fetal and neonatal effects of this substance. This illegal, synthetic psychoactive drug has hallucinogenic and stimulant properties. It alters the activity of neurotransmitters in the brain and causes increased heart rate and blood pressure. Animal studies have demonstrated that MDMA can be toxic to nerve cells containing serotonin, causing long-term damage. MDMA alters the body’s temperature regulatory abilities and in high doses can cause a sharp increase in body temperature (hyperthermia) leading to liver, kidney, and cardiovascular system failure, and death (National Institute on Drug Abuse, 2009). This substance is transferred to the fetus through the placenta, but the effects on the developing fetus are not well substantiated in human studies. It is thought that maternal use of MDMA increases the risk of congenital anomalies in the fetus and that it can cause long-term problems with learning and memory. More research studies are needed to identify the effects of MDMA on the fetus and neonate (Fike, 2007).
Other Drugs of Concern
Caffeine is a mild CNS stimulant that is consumed on a regular basis by more than 85% of adults and children and 68% of pregnant women in the United States (Frary, Johnson, & Wang, 2005). Coffee is the primary source of caffeine for most Americans, although caffeine is also found in tea, chocolate, colas, energy drinks, guarana, and mate (a tea consumed primarily in South America).
The average half-life of caffeine ranges from about 3 to 7 hours with individual variation in metabolism related to genetic and environmental factors. During the second and third trimesters of pregnancy, caffeine metabolism is slowed due to hormonal influences. Caffeine readily crosses the placenta and is distributed to all fetal tissues. Because of immature liver function, fetuses metabolize caffeine very slowly.
Although caffeine has not been implicated as a teratogen in humans, there are concerns about caffeine consumption during pregnancy. The effects of caffeine seem to be dose dependent; that is, the risk increases with greater caffeine consumption. High maternal intake of caffeine (more than 200 to 300 mg/day) increases the risk of miscarriage and low birth weight (Higdon & Frei, 2006; Weng, Odouli, & Li, 2008).
The American Dietetic Association recommends that caffeine intake in pregnant women should not exceed 300 mg/day (Kaiser & Allen, 2008). The March of Dimes more conservatively recommends no more than 200 mg/day for women who are pregnant or are planning to become pregnant (March of Dimes, 2010).
Selective Serotonin Reuptake Inhibitors
Antidepressant medication is the mainstay treatment for maternal depression with selective serotonin reuptake inhibitors (SSRIs) being the first line of pharmacotherapy. Commonly prescribed SSRIs include citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline (Cunningham et al., 2010). The American College of Obstetricians and Gynecologists (2008) published guidelines for the use of psychiatric medications in women who are pregnant and breastfeeding. All medications that have been studied are known to cross the placenta and are transferred in breast milk. Their use should be determined by a risk/benefit analysis of the risks to the fetus/neonate with use of the medication weighed against risks of not treating the mother’s psychiatric condition. Risks of nontreatment include noncompliance with prenatal care, poor nutrition, substance abuse, and interference with maternal-infant bonding (ACOG).
Based on current evidence, the ACOG (2008) has concluded that the absolute risk of any congenital abnormality associated with maternal SSRI use is small. However, certain medications carry greater risk than others. Because of the risk of cardiac defects associated with maternal paroxetine treatment, the ACOG (2008) warns that paroxetine use should be avoided during pregnancy and in women considering pregnancy. There have also been reports linking paroxetine use to other anomalies such as omphalocele, craniosynostosis, and anencephaly.
Fetal exposure to SSRIs predisposes the neonate to a behavioral syndrome resembling neonatal abstinence syndrome. Symptoms include irritability, agitation, tremors, nasal congestion, tachypnea, emesis, and diarrhea. This syndrome is usually mild and lasts for no longer than 2 days. In rare cases the syndrome is severe with seizures, hyperpyrexia, excessive weight loss, and respiratory problems (Cunningham et al., 2010). Persistent pulmonary hypertension in the newborn (PPHN) has been reported in infants who were exposed to maternal SSRIs after 20 weeks of gestation; the risk for PPHN was 6 to 12 per 1000 births (Chambers, Hernandez-Diaz, Van Marter, Werler, Louik, Jones, et al., 2006).
For parents who are concerned about maternal use of psychiatric medications and the fetal or neonatal effects, the nurse can direct them to the following websites: REPROTOX (www.reprotox.org) and TERIS (http://depts.washington.edu/terisweb) (ACOG, 2008).
Care Management
The maternal history is the key to identification of newborns who are at risk because of maternal substance abuse or use of other drugs during pregnancy. Review of the prenatal record can reveal a medical and social history of drug use or abuse and any detoxification treatment that was used. There can also be other factors that contribute to neonatal outcomes and complications. For example, the woman who is addicted to narcotics or cocaine can have infections that compound the risk to the infant, including hepatitis, septicemia, and STIs, including AIDS (Bandstra & Accornero, 2006). In some cases there is no information in the prenatal record to suggest maternal substance abuse when the woman has, in fact, been using one or more substances during pregnancy.
The infant is assessed by means of the guidelines discussed in Chapter 24. The infant’s gestational age and maturity are noted. The infant can have IUGR or be preterm with LBW. In utero exposure to some drugs results in observable malformations or dysmorphism (abnormality of shape). Neonatal behavior can arouse suspicion. Figure 35-13 provides an example of a scoring system for assessing withdrawal symptoms (neonatal abstinence syndrome). Because many women are multidrug users, the newborn initially may exhibit a confusing complex of signs. The nurse often is the first to observe the signs of drug withdrawal in the infant. The nurse’s observations help the health care provider differentiate between signs of neonatal abstinence syndrome and other conditions, such as CNS disorder, sepsis, hypoglycemia, and electrolyte imbalance.
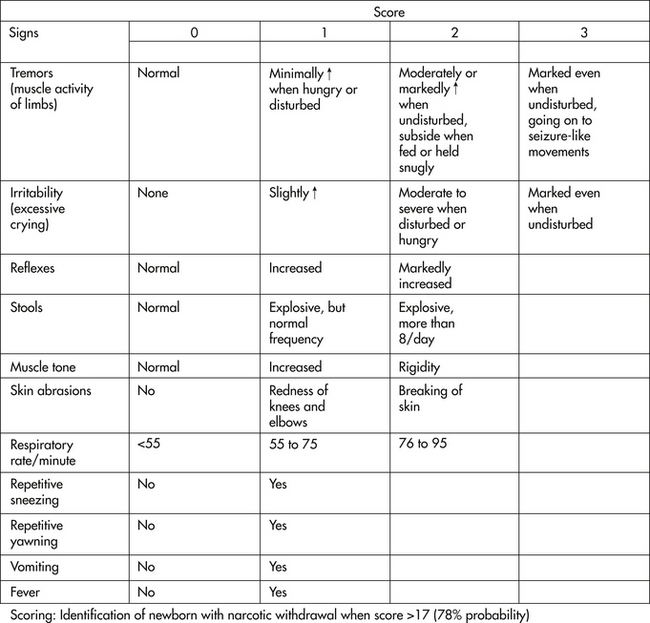
FIG. 35-13 Neonatal Abstinence Scoring System. (Source: Lipsitz, P. [1975]. A proposed narcotic withdrawal score for use with newborn infants: A pragmatic evaluation of its effi cacy. Clinical Pediatrics, 14[6], 592-594.)
Urine or meconium screening can be used to identify substances abused by the mother. Urine screening is most commonly used, but its sensitivity is limited. Initially costly and of limited availability, tests of meconium collected on day 1 or 2 of life have been shown to be sensitive and reliable in detecting the metabolites of several street drugs, including cocaine (López, Bermejo, Tabernero, Cabarcos, Alvarez, & Fernández, 2009).
Nursing Care
Planning for care of the infant born to a substance-abusing mother presents a challenge to the health care team. Parents are included in the planning for the newborn’s care and for the care and support of the mother and her newborn at home. A multidisciplinary approach includes home health or community resource personnel (e.g., regulatory agencies such as child protective services).
Education and social support to prevent the abuse of drugs provide the ideal approach. However, given the scope of the drug abuse problem, total prevention is an unrealistic goal.
Nursing care of the drug-dependent neonate involves supportive therapy for fluid and electrolyte balance, nutrition, infection control, and respiratory care. Swaddling, holding, reducing stimuli, and feeding as necessary can be helpful in easing withdrawal (see the Nursing Care Plan: The Infant Experiencing Drug Withdrawal [Neonatal Abstinence Syndrome]). Specific suggestions for providing care to infants experiencing withdrawal are listed in the Box 35-4.
Pharmacologic treatment is usually based on the severity of withdrawal symptoms, as determined by an assessment tool (see Fig. 35-13). When indicated, medications are given as ordered. Neonatal tincture of opium (0.4 mg/ml of morphine equivalent), phenobarbital, and, less commonly, paregoric may be used to control symptoms. Treatment may be needed for 2 weeks or more.
The issue of breastfeeding in this population is a difficult one. Although breast milk remains the optimal source of nutrition for these infants, care must be taken to avoid exposing the infant to additional drugs through the breast milk. According to the AAP Committee on Drugs (2001), nursing mothers should not ingest drugs of abuse including amphetamine, cocaine, heroin, marijuana, and phencyclidine because they are hazardous to the breastfeeding infant and to the mother.
Drug dependence in the neonate is physiologic, not psychologic. Thus a predisposition to dependence later in life is not believed to be a factor. However, the psychosocial environment in which the infant is raised may create a tendency to addiction.
The mother requires considerable support. Her need for and her abuse of drugs can result in a decreased capacity to cope. The infant’s withdrawal signs and decreased consolability stress her coping abilities even further. Family members also need support as they assist in caring for the infant. Home health care, treatment for addiction, and education are importantconsiderations. Sensitive exploration of the woman’s options for the care of her infant and herself and for future fertility management may help her see that she has choices. This approach helps communicate respect for the new mother as a person who can make responsible decisions.
KEY POINTS
• A small percentage of significant birth injuries can occur despite skilled and competent obstetric care.
• The same birth injury can be caused in several ways.
• The nurse’s primary contribution to the welfare of the neonate begins with early observation, accurate recording, and prompt reporting of abnormal signs.
• Metabolic abnormalities of diabetes mellitus in pregnancy adversely affect embryonic and fetal development.
• Prepregnancy planning and good diabetic control, coupled with strict diabetic control during pregnancy, may prevent the embryonic, fetal, and neonatal conditions associated with pregnancies complicated by diabetes mellitus.
• Infection in the neonate can be acquired in utero, during birth, during resuscitation, and from within the nursery.
• The most common maternal infections during early pregnancy that are associated with various congenital malformations are caused by viruses.
• HIV transmission from mother to infant occurs transplacentally at various gestational ages, perinatally by maternal blood and secretions, and by breast milk.
• The nurse often is the first to observe signs of newborn drug withdrawal.
• Providing high-quality perinatal care to a varied population with multiple conditions is complicated by the special needs of high risk drug-dependent clients.
• Signs and symptoms of withdrawal in an infant vary in time of onset depending on the type and dose of drug involved.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]() .
.