Labor and Birth Complications
• Differentiate between preterm birth and low birth weight.
• Identify major risk factors associated with preterm labor.
• Analyze current interventions to prevent spontaneous preterm birth.
• Discuss the use of tocolytics and antenatal glucocorticoids in preterm labor.
• Evaluate the effects of prescribed bed rest on pregnant women and their families.
• Design a nursing care plan for women with preterm premature rupture of the membranes (preterm PROM).
• Explain the challenge of caring for obese women during labor and birth.
• Summarize the nursing care for a trial of labor, the induction and augmentation of labor, forceps- and vacuum-assisted birth, cesarean birth, and vaginal birth after a cesarean birth (VBAC).
• Explain the care of a woman with postterm pregnancy.
• Discuss obstetric emergencies and their appropriate management.
![]() http://evolve.elsevier.com/Lowdermilk/MWHC/
http://evolve.elsevier.com/Lowdermilk/MWHC/
Animations
Audio Key Points
Critical Thinking Exercise
NCLEX Review Questions
Spanish Guidelines
When complications arise during labor and birth, risk for perinatal morbidity and mortality increases. Some complications are anticipated, especially if the woman is identified to be at high risk during the antepartum period; other complications are unexpected or unforeseen. It is crucial for nurses to understand the normal birth process to prevent and detect deviations from normal labor and birth and to promptly implement nursing measures when complications arise. Optimal care of the laboring woman, fetus, and family experiencing complications is possible only when the nurse and other members of the obstetric team use their knowledge and skills in a concerted effort to provide competent and compassionate care. This chapter focuses on the problems of preterm labor and birth, dystocia, obesity, postterm pregnancy, and obstetric emergencies.
Preterm Labor and Birth
Preterm labor is defined as cervical changes and uterine contractions occurring between 20 and 37 weeks of pregnancy. Preterm birth is any birth that occurs before the completion of 37 weeks of pregnancy (Iams & Romero, 2007). More than 33% of infant mortality is associated with prematurity. In 2008 the preterm birth rate for all races in the United States was 12.3%, an increase of 20% since 1990 (Hamilton, Martin, & Ventura, 2010).
About 75% of all preterm births in the United States are termed late preterm because they occur between 34 and 36 weeks of gestation. The steady increase in the preterm birth rate has been attributed to the rise in the rate of late preterm births, which has increased 25% since 1990. Late preterm infants are at increased risk for early death and long-term health problems when compared with infants who are born full term. Although late preterm babies do experience significant problems, the great majority of infant deaths and the most serious morbidity and mortality occur among the 16% of all preterm infants who are born before 32 weeks of gestation (very preterm birth). The very preterm birth rate is approximately 2%, representing only a slight increase from the rate in 1981, which was 1.81% (Iams, Romero, & Creasy, 2009; Martin, Hamilton, Sutton, Ventura, Menacker, Kirmeyer, et al., 2009).
In the United States the incidence of preterm birth has been increasing for the three largest racial and ethnic groups. Non-Hispanic black women have the highest rate. Hispanic woman have the next highest, and non-Hispanic white women have the lowest rate of preterm birth. The extremely preterm birth (less than 28 weeks of gestation) rate has decreased for non-Hispanic black women, but the rate is still three times higher than the rates for non-Hispanic white and Hispanic women (Martin et al., 2009). The incidence of preterm birth in developed countries has also been increasing, mainly due to more late preterm births and multifetal gestations. An increased rate of assisted reproductive technologies and more births to women older than the age of 35, which increases the risk for twinning, has led to the rise in multifetal gestations. More willingness on the part of health care providers to proceed to birth when maternal or obstetric conditions threaten the health of the mother or fetus after 32 to 34 weeks of gestation also contributes to the rise in preterm births (Iams et al., 2009).
Preterm Birth Versus Low Birth Weight
Although they have distinctly different meanings, the terms preterm birth or prematurity and low birth weight are often interchanged. Preterm birth describes length of gestation (i.e., less than 37 weeks regardless of the weight of the infant), whereas low birth weight describes only weight at the time of birth (i.e., 2500 g or less). Because birth weight was far easier to determine than gestational age, in many settings and publications low birth weight was used as a substitute term for preterm birth. Preterm birth, however, is a more dangerous health condition for an infant because less time in the uterus correlates with immaturity of body systems. Low-birth-weight babies can be, but are not necessarily, preterm; low birth weight can be caused by conditions other than preterm birth, such as intrauterine growth restriction (IUGR), a condition of inadequate fetal growth not necessarily correlated with initiation of labor. Pregnant women who have various complications of pregnancy that interfere with uteroplacental perfusion, such as gestational hypertension or poor nutrition, may give birth to a baby at term who is low birth weight because of IUGR. However, infants born at a preterm gestation can weigh more that 2500 g at birth. Today, thanks to advances in pregnancy dating, outcomes related to gestational age can increasingly be distinguished from outcomes related to birth weight (Iams et al., 2009).
Preterm births are divided into two categories: spontaneous and indicated. Spontaneous preterm births occur following an early initiation of the labor process and comprise nearly 75% of all preterm births in the United States. Conditions such as preterm labor with intact membranes, preterm premature rupture of membranes (preterm PROM), cervical insufficiency, or amnionitis often result in preterm birth (Iams et al., 2009). Box 33-1 lists risk factors for the development of spontaneous preterm labor.
Indicated preterm births occur as a means to resolve maternal or fetal risk related to continuing the pregnancy. About 25% of all preterm births in the United States are indicated because of medical or obstetric conditions that affect the mother, the fetus, or both. An increase in the number of indicated preterm births accounts for much of the recent rise in late preterm births (Iams et al., 2009). Box 33-2 lists common causes of indicated preterm births.
The remainder of this section deals with spontaneous preterm labor and birth.
Predicting Spontaneous Preterm Labor and Birth
Major risk factors associated with preterm labor and birth are a history of preterm birth, current multifetal pregnancy, cervical or uterine abnormalities, bleeding after the first trimester of pregnancy, and a low or a high maternal body mass index (BMI). Non-white race (especially non-Hispanic black), low socioeconomic and educational status, living with chronic stress, intimate partner violence, lack of social support, smoking, substance abuse, and physically demanding working conditions also have been identified as risk factors (Bowers, Curran, Freda, Krening, Poole, Slocum, et., al., 2008; Iams et al., 2009). A study by nurse researchers found that perceived levels of stress measured at 28 weeks of gestation in black women experiencing preterm labor were higher in those who gave birth prematurely than in those whose pregnancies reached term (Gennaro, Shults, & Garry, 2008). In addition, the risk of preterm birth appears to be genetically related. Relatives of women who were born prematurely or gave birth prematurely also have an increased risk for spontaneous preterm birth. Research has found links between preterm birth and singleton pregnancies conceived through assisted reproductive technologies (ART) (Bowers et al.; Iams et al.).
Researchers have developed many risk scoring systems in an attempt to determine which women might go into labor prematurely. No risk scoring system has been very successful in lowering the preterm birth rate, however, because at least 50% of all women who ultimately give birth prematurely have no identifiable risk factors (Iams & Romero, 2007; Iams et al., 2009). Therefore, it is important that all women be educated about prematurity, not only in early pregnancy but also in the preconception period. Unless all women are included in prevention efforts, a widespread reduction of preterm birth rates cannot be expected (Maloni & Damato, 2004).
Biochemical Marker
Fetal fibronectin has been studied extensively and is marketed in the United States as a diagnostic test for preterm labor. Fetal fibronectin is a glycoprotein “glue” found in plasma and produced during fetal life. The test is performed by collecting fluid from the woman’s vagina using a swab during a speculum examination. Fetal fibronectin normally appears in cervical and vaginal secretions early in pregnancy, and then again in late pregnancy.
The presence of fetal fibronectin during the late second and early third trimesters of pregnancy may be related to placental inflammation, which is thought to be one cause of spontaneous preterm labor. The presence of fetal fibronectin is not very sensitive as a predictor of preterm birth, however. Before 35 weeks of gestation, a positive fetal fibronectin test predicts preterm birth only about 25% of the time. The test’s sensitivity may be better earlier in pregnancy. In one study, the fetal fibronectin test predicted 65% of preterm births occurring before 28 weeks when it was performed between 22 and 24 weeks. Often the test is used to predict who will not go into preterm labor because preterm labor is very unlikely to occur in women with a negative result. Use of the fetal fibronectin test in women who are at low risk for preterm birth as a screening tool is not recommended (Iams et al., 2009).
Cervical Length
Another possible predictor of preterm labor is endocervical length. Changes in cervical length occur before uterine activity, so cervical measurement can identify women in whom the labor process has begun. However, because preterm cervical shortening occurs over a period of weeks, neither digital nor ultrasound cervical examination is very sensitive at predicting imminent preterm birth (Iams et al., 2009). Women whose cervical length is greater than 30 mm are unlikely to give birth prematurely even if they have symptoms of preterm labor (Iams & Romero, 2007; Iams et al.).
Causes of Preterm Labor and Birth
Infection is the only factor definitely shown to cause preterm labor. When bacterial cervical or urinary tract infections are present, the risk of preterm birth increases. Thus early, continuous, and comprehensive prenatal care, which can detect and treat infections, is essential in dealing with this aspect of preterm birth prevention. Evidence has demonstrated a link between periodontal infection and preterm labor and birth as a result of the release of prostaglandins by the causative pathogens. Although research is ongoing, recommendations for all pregnant women should include regular dental care before and during pregnancy, oral assessment as part of prenatal care, and strict oral hygiene measures (e.g., brushing teeth, using dental floss, rinsing with baking soda and water after vomiting) (Bowers et al., 2008; Werner and Lavigne, 2004).
Another proposed cause of preterm labor and birth is bleeding at the site of placental implantation in the uterus in the first or second trimester of pregnancy. The resulting uteroplacental ischemia or hemorrhage at the decidual layer of the placenta may somehow activate the preterm labor process. Intrauterine inflammation is associated with infection, uterine vascular compromise, and decidual hemorrhage, and may contribute to preterm labor. Maternal and fetal stress, uterine overdistention, allergic reaction, and a decrease in progesterone are other factors that may play a part in initiating preterm labor. It is becoming increasingly clear that preterm labor is caused by multiple pathologic processes that eventually result in uterine contractions, cervical changes, and membrane rupture (Iams, et al., 2009; Romero & Lockwood, 2009).
Two research studies suggest that recurrent preterm birth can be prevented in some women by administering prophylactic progesterone supplementation. ![]() In one study, women were given vaginal suppositories daily; in the other, women received weekly intramuscular injections of 17-alpha hydroxyprogesterone caproate. In both studies the risk of recurrent preterm birth was reduced by about one third. Exactly how progesterone works to prevent recurrent preterm birth is unclear, so more study is necessary. It is also important to note that prophylactic supplemental progesterone administration is recommended only for women who have previously given birth prematurely (Meis & Society for Maternal-Fetal Medicine, 2005; Romero & Lockwood, 2009).
In one study, women were given vaginal suppositories daily; in the other, women received weekly intramuscular injections of 17-alpha hydroxyprogesterone caproate. In both studies the risk of recurrent preterm birth was reduced by about one third. Exactly how progesterone works to prevent recurrent preterm birth is unclear, so more study is necessary. It is also important to note that prophylactic supplemental progesterone administration is recommended only for women who have previously given birth prematurely (Meis & Society for Maternal-Fetal Medicine, 2005; Romero & Lockwood, 2009).
Sociodemographic factors such as poverty, low educational level, lack of social support, smoking, little or no prenatal care, intimate partner violence, and stress are thought to contribute to the 50% of preterm births that may be preventable (Gennaro & Hennessy, 2003; Iams et al., 2009). If prenatal care programs are to be effective in reducing the rate of preterm labor and birth, they must address these sociodemographic factors and develop strategies to attract all women to participate, including those at high risk for preterm labor. Addressing the factors that contribute to preterm labor and birth can produce significant results (Maloni & Damato, 2004).
Care Management
Because all pregnant women must be considered at risk for preterm labor, nursing assessment for factors that contribute to this risk begins early in pregnancy and continues throughout the prenatal period. The onset of preterm labor is often insidious and can be easily mistaken for normal discomforts of pregnancy. Nursing diagnoses, expected outcomes of care, and evidence-based interventions are established for each woman based on her assessment findings (see the Nursing Care Plan).
Prevention
Primary prevention strategies that address risk factors associated with preterm labor and birth are less costly in human and financial terms than the high-tech and often lifelong care required by preterm infants and their families. Programs aimed at health promotion and disease prevention that encourage healthy lifestyles for the population in general and women of childbearing age in particular should be developed. Preconception counseling and care for women, especially those with a history of preterm birth, may identify correctable risk factors and provide a means to encourage women to participate in health promoting activities. Smoking cessation, for example, has been shown to prevent preterm labor and birth (Freda, 2006; Iams et al., 2009). Many interventions intended to prevent spontaneous preterm birth have been recommended in the past and are still often prescribed. However, some of these interventions have not been shown to reduce the rate of preterm birth. Ongoing research is needed, especially since our understanding of the pathophysiology of preterm birth is increasing (Iams et al.).
Early Recognition and Diagnosis
Although preterm birth is often not preventable, early recognition of preterm labor is still essential to implement interventions that have been demonstrated to reduce neonatal morbidity and mortality. ![]() These interventions include transfer of the mother prior to birth to a hospital equipped to care for her preterm infant, giving antibiotics in labor to prevent neonatal group B streptococci infection, and administering glucocorticoids to the woman in labor in order to prevent or reduce neonatal morbidity and mortality from health problems including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis (Iams & Romero, 2007).
These interventions include transfer of the mother prior to birth to a hospital equipped to care for her preterm infant, giving antibiotics in labor to prevent neonatal group B streptococci infection, and administering glucocorticoids to the woman in labor in order to prevent or reduce neonatal morbidity and mortality from health problems including respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis (Iams & Romero, 2007).
Although maternal transport helps ensure a better health outcome for the mother and the baby, it also has a downside. Women may be transported to tertiary centers far from home, making visits by family and friends difficult and increasing the anxiety levels of the woman and her family. Attention to the needs of the woman and her family before, during, and after the transport is essential to comprehensive nursing care.
Because more than half of preterm births occur in women without obvious risk factors, it is essential that all pregnant women be taught the symptoms of preterm labor (Box 33-3). The nurse caring for women in a prenatal setting should use modalities that are known to be successful for teaching pregnant women about how to recognize these symptoms and then assess for these symptoms at each prenatal visit. Women also must be taught the significance of these symptoms of preterm labor and what to do should they occur (see the Teaching for Self-Management box: What to Do If Symptoms of Preterm Labor Occur).
In particular, client education regarding any symptoms of uterine contractions or cramping between 20 and 27 weeks of gestation must emphasize that these symptoms are not just normal discomforts of pregnancy, but indications of possible preterm labor (Fig. 33-1). Waiting too long to see a health care provider could result in inevitable preterm birth without sufficient time to administer antenatal corticosteroids (i.e., medication given to accelerate fetal lung maturity) and to transfer the woman to a hospital capable of providing care for her preterm infant.

FIG. 33-1 A nurse teaching a couple signs and symptoms of preterm labor. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
The diagnosis of preterm labor is based on three major diagnostic criteria:
• Gestational age between 20 and 37 weeks
• Uterine activity (e.g., contractions)
• Progressive cervical change (e.g., effacement of 80%, or cervical dilation of 2 cm or greater)
If the presence of fetal fibronectin is used as another diagnostic criterion, a sample of cervical and vaginal secretions for testing should be obtained before an examination for cervical changes because the lubricant used to examine the cervix can reduce the accuracy of the test for fetal fibronectin. The presence of vaginal bleeding or ruptured membranes, or a history of intercourse within the past 24 hours can also reduce the accuracy of the test results.
The pregnant woman at 30 weeks with an irritable uterus but no documented cervical change is not in preterm labor, though she should be carefully evaluated during follow-up care to determine whether she has progressed to active preterm labor (e.g., effacement, dilation, or both). Misdiagnosis of preterm labor can lead to inappropriate use of pharmacologic agents that can be dangerous to the health of the woman, the fetus, or both (Bowers et al., 2008).
Lifestyle Modifications
Activity Restriction: Activity restriction, including bed rest and limited work, is a commonly prescribed intervention for the prevention of preterm birth. Bed rest, however, is not a benign intervention, and no evidence has been published in the literature to support the effectiveness of this intervention in reducing preterm birth rates ![]() (Iams et al., 2009). In fact, the American College of Obstetricians and Gynecologists (ACOG) (2003) states in its practice bulletin on management of preterm labor that bed rest should not be routinely recommended. Research indicates that bed rest causes adverse physical effects, including risk of thrombus formation, muscle atrophy, osteoporosis, and cardiovascular deconditioning (Iams & Romero, 2007). In many instances these symptoms are not resolved by 6 weeks postpartum (Maloni & Park, 2005). Additionally, bed rest affects women and their families psychologically, emotionally, socially, and financially. Box 33-4 lists adverse effects of bed rest.
(Iams et al., 2009). In fact, the American College of Obstetricians and Gynecologists (ACOG) (2003) states in its practice bulletin on management of preterm labor that bed rest should not be routinely recommended. Research indicates that bed rest causes adverse physical effects, including risk of thrombus formation, muscle atrophy, osteoporosis, and cardiovascular deconditioning (Iams & Romero, 2007). In many instances these symptoms are not resolved by 6 weeks postpartum (Maloni & Park, 2005). Additionally, bed rest affects women and their families psychologically, emotionally, socially, and financially. Box 33-4 lists adverse effects of bed rest.
Restriction of Sexual Activity: Restriction of sexual activity is frequently recommended for women at risk for preterm birth. This intervention has not been shown to be effective at preventing preterm birth. However, sexual abstinence has not been studied in women with specific risk factors for preterm birth, such as a short cervix. Therefore, more research is indicated (Iams et al., 2009). If, however, symptoms of preterm labor occur after sexual activity, then that activity may need to be curtailed until 37 weeks of gestation.
Home Care: Women who are at high risk for preterm birth commonly are told that it would be best for them to “take it easy” at home for weeks or months. Many health care providers now recommend only modified bed rest because strict bed rest has not been shown to be effective at preterm birth prevention. Home care of the woman at risk for preterm birth is a challenge, however, for the nurse, who must assist the woman and her family in dealing with the many difficulties faced by families in which one member is unable to fulfill usual role responsibilities.
The woman’s environment can be modified for convenience by using tables and storage units around her bed to keep essential items within reach (e.g., cell phone, television, radio, MP3 player, CD player, computer with Internet access, snacks, books, magazines, newspapers, and items for hobbies) (Fig. 33-2). Ensuring that the bed or couch is near a window and the bathroom is also helpful. Covering the bed with an eggcrate mattress can relieve discomfort. Women often find that a daily schedule of smaller, more frequent meals, activities (e.g., paying bills, planning and helping with meal preparation, hobbies), limited naps, and hygiene and grooming (e.g., shower, dressing in street clothes, applying makeup) reduces boredom and helps them maintain control and normalcy. See the Teaching for Self-Management box: Coping with Activity Restriction on p. 663 for more information.

FIG. 33-2 Woman at home on restricted activity for preterm labor prevention. Note how she has arranged her daytime resting area so that needed items are close at hand. (Courtesy Amy Turner, Cary, NC.)
Also see the Teaching for Self-Management box: Activities for Children of Women on Activity Restriction for additional ideas and suggestions. With modified bed rest, women are usually allowed bathroom privileges for toileting and showering and can be up to the table for meals.
Suppression of Uterine Activity
Tocolytics are medications given to arrest labor after uterine contractions and cervical change have occurred. Tocolytic therapy usually will not prolong the pregnancy long enough for further fetal growth or maturation to occur; rather, the goal is to delay birth long enough to institute interventions that reduce neonatal morbidity and mortality (Iams et al., 2009). Maternal and fetal contraindications to tocolytic therapy are listed in Box 33-5. Box 33-6 describes nursing care for women receiving tocolytic therapy.
Selecting the appropriate tocolytic medication requires consideration of each drug’s effectiveness, risks, and side effects. Currently only one medication, (ritodrine [Yutopar]), in the United States has been approved by the U.S. Food and Drug Administration (FDA) for the purpose of arresting preterm labor. It is very rarely used, however, because of adverse reactions associated with its stimulation of beta-receptors. Drugs marketed for other purposes, such as treatment of asthma or hypertension, or as antiinflammatory or analgesic agents, are used on an “off-label” basis (i.e., drugs known to be effective for a specific purpose, although not specifically developed and tested for this purpose) to suppress preterm labor (Iams et al., 2009). Important contraindications exist to the use of all tocolytics (see Box 33-5).
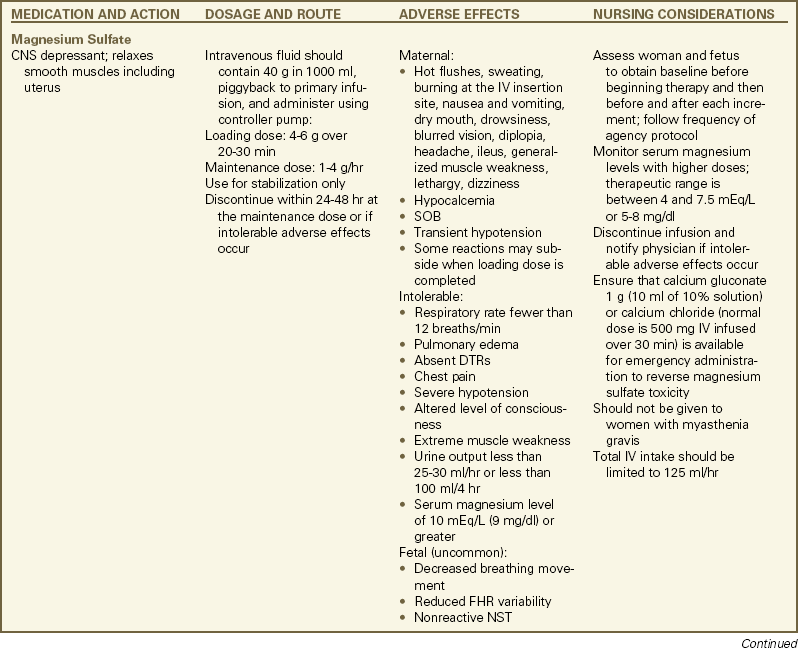
Magnesium sulfate is the most commonly used tocolytic agent because maternal and fetal or neonatal adverse reactions are less common than with the beta-adrenergic agonists. Clinicians are familiar with its use as a treatment of preeclampsia and believe it is safer to use when compared with the beta-adrenergic agonists. Evidence for its effectiveness as a tocolytic is weak, however (Grimes & Nanda, 2006). ![]() Magnesium sulfate apparently promotes relaxation of smooth muscles by competing with calcium in cells (Iams & Romero, 2007; Rideout, 2005). Magnesium sulfate is administered intravenously. It may be a good choice for use in women in whom other tocolytic agents are contraindicated (see the Medication Guide: Tocolytic Therapy for Preterm Labor) (Iams & Romero; Iams et al., 2009).
Magnesium sulfate apparently promotes relaxation of smooth muscles by competing with calcium in cells (Iams & Romero, 2007; Rideout, 2005). Magnesium sulfate is administered intravenously. It may be a good choice for use in women in whom other tocolytic agents are contraindicated (see the Medication Guide: Tocolytic Therapy for Preterm Labor) (Iams & Romero; Iams et al., 2009).
Beta2-adrenergic agonists (e.g., ritodrine and terbutaline [Brethine]) have been widely used in the past as tocolytics. They have many maternal and fetal adverse reactions, however, including beta1-stimulated cardiopulmonary (e.g., tachycardia) effects and beta2-stimulated metabolic (e.g., hyperglycemia) effects. Therefore, beta2-adrenergic agonists are increasingly being replaced by medications that are safer and have fewer adverse reactions. They should not be used in women with known or suspected heart disease, severe preeclampsia or eclampsia, pregestational or gestational diabetes, or hyperthyroidism (Iams et al., 2009; Iams & Romero, 2007) Use of beta2 -adrenergic agonists is also contraindicated in women with migraine headaches (Gilbert, 2011).
Terbutaline, the most commonly administered beta-adrenergic agonist used for tocolysis, works by relaxing uterine smooth muscle as a result of stimulation of beta2-receptors in the uterine smooth muscle. A single dose of terbutaline given subcutaneously may help diagnose preterm labor. In one study, women whose contractions persisted or recurred after a single injection of terbutaline were more likely to actually be in preterm labor than those whose contractions ceased. Terbutaline is often given subcutaneously to facilitate maternal transfer to a tertiary center or to initiate tocolytic therapy while another agent with a slower onset of action is administered concurrently. Additionally, a subcutaneous injection of 0.25 mg may be given to suppress uterine tachysystole during labor induction or augmentation or to suppress contractions prior to cesarean birth. Long-term oral or subcutaneous administration (e.g., terbutaline pump) as maintenance therapy to suppress preterm labor has not been proven to be effective at reducing prematurity or neonatal morbidity (Bowers et al., 2008; Gilbert, 2011; Iams & Romero, 2007; Iams et al., 2009) (see the Medication Guide: Tocolytic Therapy for Preterm Labor). ![]()
Nifedipine (Adalat, Procardia), a calcium channel blocker, is another tocolytic agent that can suppress contractions. It works
by inhibiting calcium from entering smooth muscle cells, thus reducing uterine contractions. Because of its ease of administration and low incidence of significant maternal and fetal side effects, nifedipine’s use is increasing. The drug is rapidly absorbed after oral administration. Maternal side effects, which include headache, flushing, dizziness, and nausea, are generally mild and relate primarily to hypotension and reflex tachycardia that occurs with administration. The decrease in blood pressure that occurs may be helpful for women who are also diagnosed with gestational hypertension or preeclampsia. However, at least one myocardial infarction has been reported in a healthy young woman who received a second dose of nifedipine. Concerns regarding adverse fetal effects have been reduced. Safety is achieved by following recommended dosages, avoiding concurrent use with magnesium sulfate, and maintaining maternal blood pressure, thereby preserving effective uteroplacental perfusion. Administering both nifedipine and magnesium sulfate can cause skeletal muscle blockade. Additionally, nifedipine should not be given along with or immediately following a beta2—adrenergic agonist (Iams & Romero, 2007; Iams et al., 2009) (see the Medication Guide: Tocolytic Therapy for Preterm Labor).
Indomethacin (Indocin), a nonsteroidal antiinflammatory drug (NSAID), has been shown in some trials to suppress preterm labor by blocking the production of prostaglandins. Serious maternal side effects are uncommon and indomethacin is usually well tolerated. However, three serious fetal or neonatal side effects have caused major concerns about its use as a tocolytic. These side effects include constriction of the ductus arteriosus, oligohydramnios, and neonatal pulmonary hypertension. Therefore, limiting the use of indomethacin to a short duration of treatment in women with preterm labor at less than 32 weeks of gestation is recommended (Iams & Romero, 2007; Iams et al., 2009) (see the Medication Guide: Tocolytic Therapy for Preterm Labor).
Promotion of Fetal Lung Maturity
Antenatal glucocorticoids, given as intramuscular injections to the mother to accelerate fetal lung maturity by stimulating fetal surfactant production, are now considered one of the most effective and cost-efficient interventions for preventing morbidity and mortality associated with preterm labor. Antenatal glucocorticoids have been shown to significantly reduce the incidence of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and death in neonates, without increasing the risk of infection in either mothers or newborns (Mercer, 2009a). The National Institutes of Health (NIH) consensus panel recommended that all women between
24 and 34 weeks of gestation be given a single course of antenatal glucocorticoids when preterm birth is threatened, unless evidence indicates that glucocorticoids will have an adverse effect on the mother or birth is imminent. In general, women who are candidates for tocolytic therapy are also candidates for antenatal glucocorticoids (Mercer). The regimen for administration of antenatal glucocorticoids is given in the Medication Guide: Antenatal Glucocorticoid Therapy with Betamethasone or Dexamethasone.
Management of Inevitable Preterm Birth
Labor that has progressed to a cervical dilation of 4 cm or more is likely to lead to inevitable preterm birth. If birth appears imminent, preparations to care for a small, immature neonate should be made. Women in preterm labor may rapidly progress to birth and a very small fetus may be born through a partially dilated cervix. Also malpresentation (e.g., breech presentation) occurs much more frequently in preterm than in term fetuses. Therefore, nurses must be prepared to handle the emergency birth of a preterm infant, from either cephalic or breech presentation, without the woman’s primary health care provider being present. Personnel skilled at neonatal resuscitation should be present at the time of birth. Equipment, supplies, and medications used for neonatal resuscitation should be gathered in advance and prepared for immediate use. If birth occurs in a hospital that is not prepared to provide continuing care for a preterm neonate, plans should be made for transfer of the baby to a higher level of care as soon as possible.
Fetal and Early Neonatal Loss
Preterm birth or the presence of congenital anomalies or genetic disorders incompatible with life are major reasons for intrauterine fetal demise (stillbirth) or early neonatal death. In many of these situations the parents will have already been told that the fetus has died or that the baby has a condition that is incompatible with life and will most likely die very soon after birth. Sometimes, however, the fetal death will be unexpected, diagnosed only after the woman has been admitted to the labor and birth unit. Whatever the case, labor and birth nurses must be prepared to provide sensitive care to these women and their families.
If fetal or early neonatal death is expected, the parents and members of the health care team need to discuss the situation before the birth and decide on a management plan that is acceptable to everyone. Despite counseling about the likelihood of a poor outcome, some parents want “everything possible”, including cesarean birth for an abnormal fetal heart rate (FHR) tracing, to be done for the baby. If such intervention is not desired, usually the FHR will not be monitored during labor.
Another major decision is whether to attempt neonatal resuscitation, and to what lengths resuscitation should go. Sometimes the feasibility of neonatal resuscitation cannot be determined until the baby’s size and physical appearance have been assessed. If the baby is too small, too immature, or too malformed for effective resuscitation, comfort care can be provided instead. The baby is kept warm and comfortable, either at the mother’s bedside or in the nursery, depending on the parents’ desires, until death occurs. Parents can choose to view and hold the baby as they wish.
After the birth, the woman should be given the opportunity to decide if she wants to stay on the maternity unit or be moved to another hospital unit. She may prefer to be away from the sound of crying babies and exposure to other families who have had healthy infants. However, postpartum care and grief support may not be as good on another hospital unit where the staff is not experienced in postpartum and bereavement care.
Whether death occurs in utero or after birth, parents are faced with the same needs. See Chapter 38 for additional information on dealing with families experiencing a perinatal loss.
Premature Rupture of Membranes
Premature rupture of membranes (PROM) is the spontaneous rupture of the amniotic sac and leakage of amniotic fluid beginning before the onset of labor at any gestational age. Preterm premature rupture of membranes (preterm PROM) (i.e., membranes rupture before the completion of 37 weeks of gestation) is responsible for about one third of all preterm births (Mercer, 2007). Preterm PROM most likely results from pathologic weakening of the amniotic membranes caused by inflammation, stress from uterine contractions, or other factors that cause increased intrauterine pressure. Infection of the urogenital tract is a major risk factor associated with preterm PROM (Mercer, 2007; 2009b). PROM or preterm PROM are diagnosed after the woman reports either a sudden gush of fluid or a slow leak of fluid from the vagina.
Chorioamnionitis is the most common maternal complication of preterm PROM, making it a major complication of pregnancy (see later discussion). Other less common but serious maternal complications include placental abruption, sepsis, and death (Mercer, 2007; 2009b). Fetal complications from preterm PROM are primarily related to intrauterine infection, cord prolapse, umbilical cord compression associated with oligohydramnios, and placental abruption. Another possible fetal complication when preterm PROM occurs prior to 20 weeks of gestation is pulmonary hypoplasia (Mercer, 2009b).
Care Management
Management of PROM is determined for each woman based on an assessment of the estimated risk of maternal, fetal, and neonatal complications if pregnancy is allowed to continue or immediate labor and birth are attempted. At term, because infection is the greatest maternal, fetal, and neonatal risk, birth is the best option. Labor will most likely be induced if it does not begin spontaneously soon after PROM occurs (Mercer, 2009b).
Preterm PROM is often managed expectantly or conservatively if the risks to the fetus and newborn associated with preterm birth are considered to be greater than the risks of infection. Women with preterm PROM may be hospitalized in an attempt to prolong pregnancy and allow additional time for fetal maturation unless intrauterine infection, significant vaginal bleeding, placental abruption, preterm labor, or fetal compromise occurs (Mercer, 2009b). Nursing support of the woman and her family is critical at this time. They are often anxious about the health of the baby and the woman may fear that she was responsible in some way for the membrane rupture. The nurse can reassure the woman that in most cases the cause of PROM is unknown (Gilbert, 2011). Other nursing interventions include encouraging expression of feelings and concerns, providing information, and making referrals as needed.
Conservative management of preterm PROM includes fetal assessment by nonstress test (NST) and biophysical profile (BPP). The woman should also be taught how to assess her fetus using daily fetal movement counts (DFMC) because a slowing of fetal movement has been shown to be a precursor to severe fetal compromise. (See Chapter 26 for further discussion of these tests.) In addition, the woman will be monitored for signs of labor, placental abruption, and the development of intrauterine infection. Antenatal glucocorticoids will be administered to women who are less than 32 weeks of gestation because they have been proven to decrease the risk of several neonatal complications. In addition, a 7-day course of broad-spectrum antibiotics (e.g., ampicillin, erythromycin) will be administered to treat or prevent intrauterine infection (Mercer, 2007; 2009b).
Vigilance for signs of infection is a major part of the nursing care and client education after preterm PROM. The woman must be taught how to keep her genital area clean and that nothing should be introduced into her vagina. Signs of infection (e.g., fever, foul-smelling vaginal discharge, maternal and fetal tachycardia) should be reported to the primary health care provider immediately (see the Teaching for Self-Management
box: The Woman with Preterm Premature Rupture of Membranes). If chorioamnionitis develops, labor will be induced. Should preterm labor occur, tocolytic medications may be administered in an attempt to gain time for transporting the woman to a hospital capable of providing care to a preterm infant or for antenatal corticosteroids or antibiotics to reach effective levels (Gilbert, 2011; Mercer, 2007).
Chorioamnionitis
Chorioamnionitis, bacterial infection of the amniotic cavity, is a major cause of complications for both mothers and newborns at any gestational age. It occurs in 0.5% to 10% of pregnancies. Other terms for this condition include clinical chorioamnionitis, amnionitis, intrapartum infection, amniotic fluid infection, and intra-amniotic infection. Chorioamnionitis is usually diagnosed by the clinical findings of maternal fever, maternal and fetal tachycardia, uterine tenderness, and foul odor of amniotic fluid. (Duff, Sweet, & Edwards, 2009).
Chorioamnionitis most often occurs after membranes rupture or labor begins, as organisms that are part of the normal vaginal flora ascend into the amniotic cavity. Many of the risk factors for chorioamnionitis are associated with a long labor, such as prolonged membrane rupture, multiple vaginal examinations, and use of internal FHR and contraction monitoring modes (Duff et al., 2009). Other risk factors include young maternal age, low socioeconomic status, nulliparity, and preexisting infections of the lower genital tract (Duff, 2007).
Women with chorioamnionitis can develop bacteremia. They are also more likely to have dysfunctional labor, which can result in the need for cesarean birth (see later discussion). If cesarean birth is necessary, wound infection or pelvic abscess are complications that can occur. Neonatal risks include pneumonia, bacteremia, and sepsis. Death is more likely to occur in preterm than in term infants (Duff, 2007; Duff et al., 2009). An association between chorioamnionitis and long-term neurologic development in the newborn, including cerebral palsy, has been reported (Duff et al.).
In order to prevent maternal and neonatal complications, prompt treatment with intravenous broad-spectrum antibiotics and birth of the fetus are necessary. Ampicillin or penicillin and gentamicin are the antibiotics most often used to treat chorioamnionitis during labor. After cesarean birth, an antibiotic that provides coverage for anaerobic organisms, such as clindamycin (Cleocin) or metronidazole (Flagyl) should be added. Antibiotics can usually be discontinued soon after birth (Duff, 2007).
The increased use of intrapartum antibiotic prophylaxis during labor in women who are group B streptococci positive has decreased the incidence of chorioamnionitis. Other measures that have proven to be effective in decreasing the frequency of chorioamnionitis are active management of labor (see later discussion) and induction of labor, rather than expectant management, following rupture of membranes at term (Duff et al., 2009).
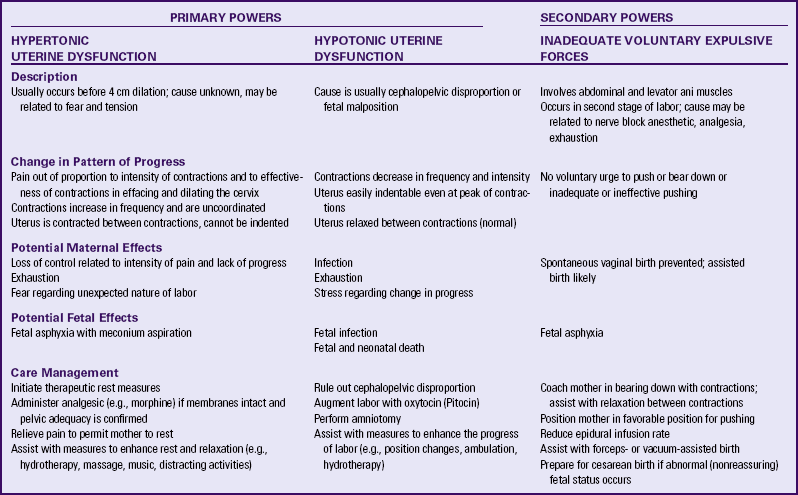
Dysfunctional Labor (Dystocia)
Dysfunctional labor (dystocia) is defined as a long, difficult, or abnormal labor caused by various conditions associated with the five factors affecting labor. It is estimated that dysfunctional labor occurs in approximately 8% to 11% of all births and it is the most common indication for cesarean birth (Gilbert, 2011). Dysfunctional labor is responsible for approximately 60% of all primary cesarean births in the United States (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010). It can be caused by any of the following:
• Ineffective uterine contractions or maternal bearing-down efforts (the powers)
• Alterations in the pelvic structure (the passage)
• Fetal causes, including abnormal presentation or position, anomalies, excessive size, and number of fetuses (the passenger)
• Maternal position during labor and birth
• Psychologic responses of the mother to labor related to past experiences, preparation, culture and heritage, and support system
These five factors are interdependent. In assessing the woman for an abnormal labor pattern, the nurse must consider the way in which these factors interact and influence labor progress. Dysfunctional labor is suspected when there is an alteration in the characteristics of uterine contractions, a lack of progress in the rate of cervical dilation, or a lack of progress in fetal descent and expulsion.
Gilbert (2011) cited several factors that seem to increase a woman’s risk for dysfunctional labor including the following:
• Uterine abnormalities (e.g., congenital malformations; overdistention, as with multiple gestation; or polyhydramnios)
• Malpresentations and positions of the fetus
• Cephalopelvic disproportion (CPD) (or fetopelvic disproportion [FPD])
• Uterine overstimulation with oxytocin
• Maternal fatigue, dehydration and electrolyte imbalance, and fear
• Administration of an analgesic too early in labor or use of continuous epidural analgesia
Abnormal Uterine Activity
Abnormal uterine activity can be further described as being hypertonic or hypotonic. Contractions may be frequent and painfully strong with hypertonic uterine activity, but ineffective at promoting cervical effacement and dilation. With hypotonic uterine activity, the rise in uterine pressure generated during contractions is insufficient to promote cervical effacement and dilation (Gilbert, 2011).
Hypertonic Uterine Dysfunction
The woman experiencing hypertonic uterine dysfunction, or primary dysfunctional labor, often is an anxious first-time mother who is having painful and frequent contractions that are ineffective in causing cervical dilation or effacement to progress. These contractions usually occur in the latent phase of first stage labor (cervical dilation of less than 4 cm) and are usually uncoordinated. The force of the contractions may be in the midsection of the uterus rather than in the fundus; therefore, the uterus is unable to apply downward pressure to push the presenting part against the cervix. The uterus may not relax completely between contractions.
Women with hypertonic uterine dysfunction may be exhausted and express concern about loss of control because of the intense pain they are experiencing and the lack of progress. Therapeutic rest, which is achieved with a warm bath or shower and the administration of an analgesic such as morphine, to inhibit uterine contractions, reduce pain, and encourage sleep, is usually prescribed to manage hypertonic uterine dysfunction. In the absence of pain, zolpidem (Ambien) may be used to facilitate rest and sleep. After a 4- to 6-hour rest, these women are likely to awaken in active labor with a normal uterine contraction pattern (Battista & Wing, 2007; Gilbert, 2011).
Hypotonic Uterine Dysfunction
The second and more common type of uterine dysfunction is hypotonic uterine dysfunction, or secondary uterine inertia. The woman initially makes normal progress into the active phase of first stage labor but then the contractions become weak and inefficient or stop altogether. The uterus is easily indented, even at the peak of contractions. Intrauterine pressure (IUP) during the contraction (usually less than 25 mm Hg) is insufficient for progress of cervical effacement and dilation. CPD and malposition are common causes of this type of uterine dysfunction.
A woman with hypotonic uterine dysfunction may become exhausted and be at increased risk for infection. Management usually consists of ruling out CPD and assessing the FHR and pattern, characteristics of amniotic fluid if membranes are ruptured, and maternal well-being. An intrauterine pressure catheter (IUPC) may be inserted to accurately evaluate uterine activity. If findings are normal, labor augmentation measures may be implemented (e.g., ambulation, hydrotherapy, rupture of membranes, nipple stimulation, oxytocin infusion).
Secondary Powers
Secondary powers, or bearing-down efforts, are compromised when large amounts of analgesia are given. Anesthesia may also block the bearing-down reflex and, as a result, alter the effectiveness of voluntary bearing-down efforts. Exhaustion resulting from lack of sleep or long labor, and fatigue resulting from inadequate hydration and food intake reduce the effectiveness of the woman’s voluntary bearing-down efforts. Maternal position can work against the forces of gravity and decrease the strength and efficiency of the contractions. Table 33-1 summarizes the characteristics of dysfunctional labor.
Abnormal Labor Patterns
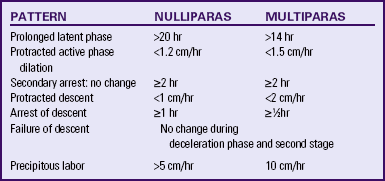
Six abnormal labor patterns were identified and classified by Friedman (1989) according to the nature of the cervical dilation and fetal descent. These patterns include, (1) prolonged latent phase, (2) protracted active phase dilation, (3) secondary arrest: no change, (4) protracted descent, (5) arrest of descent, and (6) failure of descent. Table 33-2 further describes these abnormal labor patterns. These patterns may result from a variety of causes, including ineffective uterine contractions, pelvic contractures, CPD, abnormal fetal presentation or position, early use of analgesics, nerve block analgesia or anesthesia, and anxiety and stress. Progress in either the first or the second stage of labor can be protracted (prolonged) or arrested (stopped). Abnormal progress can be identified by plotting cervical dilation and fetal descent on a labor graph (partogram) at various intervals after the onset of labor and comparing the resulting curve with the expected labor curve for a nulliparous or multiparous labor. If a woman exhibits an abnormal labor pattern, the primary health care provider should be notified.
Health care providers must be careful when diagnosing a labor pattern as prolonged and when intervening based on this diagnosis. Cesario (2004) found that although the average length of labor today is similar to that found by Friedman, a wider range of normal occurs. Parameters to determine if labor is progressing normally may have to be expanded. Criteria defining the differences between false, latent, and active labor should be established. Using hospital admission areas to evaluate a woman’s labor status is helpful in preventing the premature implementation of labor interventions such as administration of systemic opioid analgesics or induction of epidural analgesia or anesthesia. If a woman is found to be in false (prelabor) or latent (early) labor, she can be sent home if she lives near the hospital or remain in the admissions area until labor becomes active. Only when they are in active labor should women be admitted to the labor and birth unit.
Maternal morbidity and mortality from uterine rupture, infection, severe dehydration, and postpartum hemorrhage are higher for women experiencing dysfunctional labor. The fetus is at increased risk for hypoxia. A long and difficult labor also can have an adverse psychologic effect on the mother, father, and family.
Precipitous Labor
Precipitous labor is defined as labor that lasts less than 3 hours from the onset of contractions to the time of birth. This abnormal labor pattern occurs in approximately 2% of all births in the United States. Precipitous birth alone is not usually associated with significant maternal or infant morbidity or mortality (Battista & Wing, 2007).
Precipitous labor may result from hypertonic uterine contractions that are tetanic in intensity. Conditions often associated with this type of uterine contractions include placental abruption, an excessive number of uterine contractions, and recent cocaine use (Battista & Wing, 2007). Maternal complications can include uterine rupture, lacerations of the birth canal, anaphylactoid syndrome of pregnancy (amniotic fluid embolism), and postpartum hemorrhage. Fetal complications include hypoxia caused by decreased periods of uterine relaxation between contractions and, in rare instances, intracranial trauma related to rapid birth (Cunningham et al., 2010).
Women who have experienced precipitous labor often describe feelings of disbelief that their labor began so quickly, alarm that their labor progressed so rapidly, panic about the possibility they would not make it to the hospital in time to give birth, and finally, relief when they arrived at the hospital. In addition, women have expressed frustration when nurses did not believe them when they reported their readiness to push. Progress can be so rapid in some women that they may have difficulty remembering the details of their childbirth. They should be provided with an opportunity to discuss their labor and birth with caregivers.
Alterations in Pelvic Structure
Pelvic dystocia can occur whenever there are contractures of the pelvic diameters that reduce the capacity of the bony pelvis, including the inlet, the midpelvis, outlet, or any combination of these planes. Pelvic contractures may be caused by congenital abnormalities, maternal malnutrition, neoplasms, or lower spinal disorders. An immature pelvic size predisposes some adolescent mothers to pelvic dystocia. Pelvic deformities also may be the result of automobile or other accidents or trauma.
Soft-Tissue Dystocia
Soft-tissue dystocia results from obstruction of the birth passage by an anatomic abnormality other than that involving the bony pelvis. The obstruction may result from placenta previa (low-lying placenta) that partially or completely obstructs the internal cervical os. Other causes, such as leiomyomas (uterine fibroids) in the lower uterine segment, ovarian tumors, and a full bladder or rectum, may prevent the fetus from entering the pelvis. Occasionally cervical edema occurs during labor when the cervix is caught between the presenting part and the symphysis pubis or when the woman begins bearing-down efforts prematurely, thereby inhibiting complete dilation. Sexually transmitted infections (e.g., human papillomavirus) can alter cervical tissue integrity and thus interfere with adequate effacement and dilation.
Fetal Causes
Dystocia of fetal origin may be caused by anomalies, excessive fetal size (macrosomia), malpresentation, malposition, or multifetal pregnancy. Complications associated with dystocia of fetal origin include neonatal asphyxia, fetal injuries or fractures, and maternal vaginal lacerations. Although spontaneous vaginal birth is possible in these instances, a forceps-assisted, vacuum-assisted, or cesarean birth often is necessary.
Anomalies
Gross ascites, large tumors, open neural tube defects (e.g., myelomeningocele), and hydrocephalus are examples of fetal anomalies that can cause dystocia. The anomalies affect the relationship of the fetal anatomy to the maternal pelvic capacity, with the result that the fetus is unable to descend through the birth canal.
Cephalopelvic Disproportion
Cephalopelvic disproportion (CPD), also called FPD, is disproportion between the size of the fetus and the size of the mother’s pelvis. With CPD the fetus cannot fit through the maternal pelvis to be born vaginally. Although CPD is often related to excessive fetal size, or macrosomia (i.e., 4000 g or more), the problem in many cases is malposition of the fetal presenting part rather than true CPD (Battista & Wing, 2007). Fetal macrosomia is associated with maternal diabetes mellitus, obesity, multiparity, or the large size of one or both parents. If the maternal pelvis is too small, abnormally shaped, or deformed, CPD may be of maternal origin. In this case, the fetus may be of average size or even smaller. CPD cannot be accurately predicted (Battista & Wing).
Malposition
The most common fetal malposition is persistent occipitoposterior position (i.e., right occipitoposterior [ROP] or left occipitoposterior [LOP]; see Chapter 16), occurring in approximately 15% of all labors during the latent phase of the first stage of labor. About 5% of all fetuses are in this position at birth (Gilbert, 2011). Labor, especially the second stage, is prolonged. The woman typically complains of severe back pain from the pressure of the fetal head (occiput) pressing against her sacrum. Box 33-7 identifies suggested measures to relieve back pain and encourage rotation of the fetal occiput to an anterior position, which will facilitate birth (Gilbert; Stremler, Hodnett, Petryshen, Stevens, Weston, & Willan, 2005). Evidence provides ![]() support for encouraging women whose fetus is in an occiput posterior position to assume and maintain a Sims position on the same side as the fetal spine as much as possible during labor. There is limited evidence to support the commonly used hands-and-knees position (Ridley, 2007).
support for encouraging women whose fetus is in an occiput posterior position to assume and maintain a Sims position on the same side as the fetal spine as much as possible during labor. There is limited evidence to support the commonly used hands-and-knees position (Ridley, 2007).
Malpresentation
Malpresentation (the fetal presentation is something other than cephalic or head first) is another commonly reported complication of labor and birth. Breech presentation is the most common form of malpresentation, occurring in 3% to 4% of all labors (Lanni & Seeds, 2007). The three types of breech presentation are frank breech (hips flexed, knees extended), complete breech (hips and knees flexed), and footling breech (when one foot [single footling] or both feet [double footling] present before the buttocks) (Gilbert, 2011) (Fig. 33-3). Breech presentations are associated with multifetal gestation, preterm birth, fetal and maternal anomalies, hydramnios, and oligohydramnios. High rates of breech presentation are also noted in fetuses with certain genetic disorders (e.g., trisomies 13, 18, and 21; Potter’s syndrome [renal agenesis]; and myotonic dystrophy). Fetuses with neuromuscular disorders have a high rate of breech presentation, perhaps because they are less capable of movement within the uterus. Diagnosis is made by abdominal palpation (e.g., Leopold maneuvers) and vaginal examination and usually confirmed by ultrasound scan (Lanni & Seeds, 2007; Thorp, 2009).
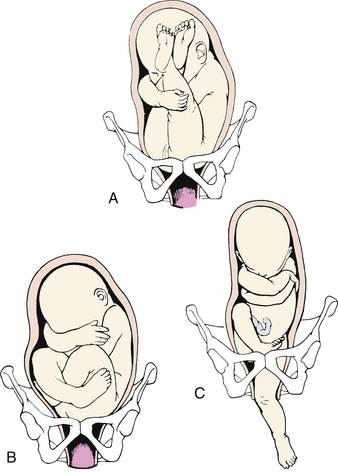
FIG. 33-3 Breech presentation. A, Frank breech. B, Complete breech. C, Single footling breech. (From Gilbert, E. [2011]. Manual of high risk pregnancy & delivery [5th ed.]. St. Louis: Mosby.)
During labor the descent of the fetus in a breech presentation may be slow because the breech is not as effective a dilating wedge as is the fetal head. There is risk of prolapse of the cord if the membranes rupture in early labor. The presence of meconium in amniotic fluid is not necessarily a sign of fetal distress because it results from pressure on the fetal abdominal wall as it traverses the birth canal. Assessment of FHR and pattern should be used to determine whether the passage of meconium is an expected finding associated with breech presentation or is an abnormal (nonreassuring) sign associated with fetal hypoxia. The heart tones of fetuses (FHTs) in a breech position are best heard at or above the umbilicus.
Vaginal birth is accomplished by mechanisms of labor that manipulate the buttocks and lower extremities as they emerge from the birth canal (Fig. 33-4). Risks associated with vaginal birth from a breech presentation include prolapse of the umbilical cord (especially in single or double footling breech presentations) and trapping of the after-coming fetal head (especially with preterm infants). Safe vaginal birth from a breech presentation is largely dependent on the experience, judgment, and skill of the health care provider who assists the birth. Criteria for attempting a vaginal birth from a breech presentation are (Thorp, 2009):
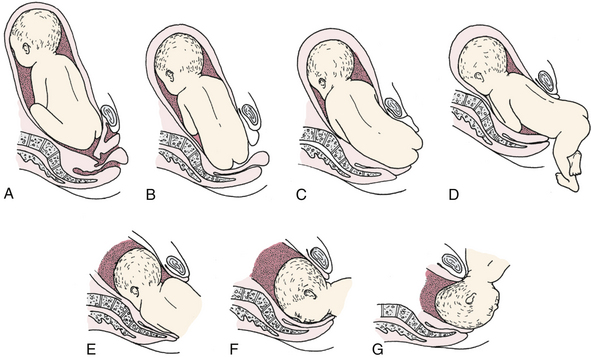
FIG. 33-4 Mechanism of labor in breech presentation. A, Breech before onset of labor. B, Engagement and internal rotation. C, Lateral flexion. D, External rotation or restitution. E, Internal rotation of shoulders and head. F, Face rotates to sacrum when occiput is anterior. G, Head is born by gradual flexion during elevation of fetal body.
• Frank or complete breech presentation
• Estimated fetal weight between 2000 and 3800 g
External cephalic version (ECV) (see later discussion) may be tried to turn the fetus to a vertex presentation. If the attempt at ECV is unsuccessful, the woman usually gives birth by cesarean (Gilbert, 2011).
Face and brow presentations are uncommon and are associated with fetal anomalies, pelvic contractures, and CPD. Spontaneous vaginal birth is possible if the fetus flexes to a vertex presentation, although forceps often are used. Cesarean birth is indicated if the presentation persists, if fetal distress occurs, or if labor stops progressing.
Cesarean birth is usually necessary for a fetus in a transverse lie (i.e., shoulder) presentation, although ECV may be attempted after 36 to 37 weeks of gestation (Thorp, 2009).
Multifetal Pregnancy
Multifetal pregnancy is the gestation of twins, triplets, quadruplets, or more infants. Multiple gestations now account for more than 3% of all live births in the United States (Malone & D’Alton, 2009). The increasing number of twin gestations has been attributed to the use of fertility-enhancing medications and procedures and the older age of childbearing women. When compared with younger women, those age 35 years and older are naturally more likely to have a multifetal pregnancy. The rate of triplet and higher-order multiple pregnancies has been steadily declining since the all-time high rate of 193.5 per 100,000 in 1998. This decrease has been attributed to refinements in the treatments used for infertility (Cleary-Goldman, Chitkara, & Berkowitz, 2007; Malone & D’Alton; Martin et al., 2009).
Multiple births are associated with more complications (e.g., dysfunctional labor) than single births. The higher incidence of fetal and newborn complications and higher risk of perinatal mortality primarily stem from the birth of low birth weight infants resulting from preterm birth or IUGR (or both), in part related to placental dysfunction and twin-to-twin transfusion. Fetuses can experience distress and asphyxia during the birth process as a result of cord prolapse and the onset of placental separation with the birth of the first fetus. As a result, the risk for long-term problems such as cerebral palsy is higher among infants who were part of a multiple birth.
In addition, fetal complications such as congenital anomalies and abnormal presentations can result in dysfunctional labor and an increased incidence of cesarean birth. For example, in only 40% to 45% of all twin pregnancies do both fetuses present in the vertex position, the most favorable for vaginal birth. In 35% to 40% of the pregnancies, one twin may present in the vertex position and the other in a breech or transverse lie presentation (Malone & D’Alton, 2009).
The health status of the mother may be compromised by an increased risk for hypertension, anemia, and hemorrhage associated with uterine atony, placental abruption, and multiple or adherent placentas. Duration of the phases and stages of labor may vary from the duration experienced with singleton births.
Teamwork and planning are essential components of the management of childbirth in multiple pregnancies, especially those of higher-order multiples. The nurse plays a key role in coordinating the activities of many highly skilled health care professionals. Early detection and management of the maternal, fetal, and newborn complications associated with multiple births are essential to achieve a positive outcome for mother and babies. Maternal positioning and active support are used to enhance labor progress and placental perfusion. Stimulation of labor with oxytocin, epidural anesthesia, internal or external version, and forceps and vacuum assistance may be used to accomplish the vaginal birth of twins. Cesarean birth is almost always performed with higher-order multiple births. Each infant will have its own team of health care providers present at the birth. Emotional support that includes expression of feelings and full explanations of events as they occur and of the status of the mother and the fetuses and newborns is important to reduce the anxiety and stress the mother and her family experience.
Position of the Woman
The functional relationship among the uterine contractions, the fetus, and the mother’s pelvis are altered by the maternal position. In addition, the position can provide a mechanical advantage or disadvantage to the mechanisms of labor by altering the effects of gravity and the body-part relationships that are important to the progress of labor. For example, the lateral position facilitates rotation from an occiput posterior position more effectively than the hands-and-knees position. Upright positions such as sitting and squatting for birth enhance fetal descent during bearing-down efforts, shorten the second stage of labor, reduce pain and perineal trauma, and decrease the need for forceps or vacuum assistance to facilitate vaginal birth (see chapter 19) (Roberts & Hanson, 2007).
Discouraging maternal movement or restricting labor to the recumbent or lithotomy position may compromise progress. The incidence of dysfunctional labor in women confined to these positions is increased, resulting in a greater need for augmentation of labor or forceps-assisted, vacuum-assisted, or cesarean birth.
Psychologic Responses
Hormones and neurotransmitters released in response to stress (e.g., catecholamines) can cause dysfunctional labor. Sources of stress vary for each woman, but pain and the absence of a support person are two factors often related to dysfunctional labor. Confinement to bed and restriction of maternal movement can be a source of psychologic stress that compounds the physiologic stress caused by immobility in the unmedicated laboring woman. When anxiety is excessive, it can inhibit cervical dilation and result in prolonged labor and increased pain perception. Anxiety also causes increased levels of stress-related hormones (e.g., beta-endorphin, adrenocorticotropic hormone, cortisol, and epinephrine). These hormones act on the smooth muscles of the uterus. Increased levels can cause dysfunctional labor by reducing uterine contractility.
Care Management
Risk assessment is a continuous process in the laboring woman. By reviewing the woman’s past labor or labors and observing her physical and psychologic responses to the current labor, any factors that might contribute to dysfunctional labor should be identified. Nursing diagnoses, expected outcomes of care, and interventions are then established for each woman based on assessment findings. Many interventions for dysfunctional labor (e.g., ECV, cervical ripening, induction or augmentation of labor, and operative procedures [forceps- or vacuum-assisted birth, cesarean birth]) are implemented collaboratively with other members of the health care team. Commonly performed interventions are discussed in detail in the Obstetric Procedures section (see the Nursing Process box: Dysfunctional Labor).
When providing care for a woman who is experiencing labor or birth complications, all members of the health care team are responsible for complying with professional standards of care.
Obesity
Excessive weight is an increasingly serious problem for children, adolescents, and adults living in affluent nations, including the United States, and pregnant women are no exception. The BMI is used to define obesity. Persons with a BMI of 30 or greater kg/m2 are considered obese, whereas those with a BMI of 40 kg/m2 or greater are classified as morbidly obese (Cunningham et al., 2010).
Obese women are at risk for several pregnancy complications, including venous thromboembolism and cesarean birth. In addition to having an increased risk for cesarean birth in general, obese women are also more likely to require emergency cesarean birth. As the woman’s BMI increases, the risk of developing these complications also rises (Cunningham et al., 2010; Walters & Taylor, 2009, 2010).
Care Management
Nursing care of obese women during labor and birth is challenging for a number of reasons. Sometimes standard furniture such as beds, chairs, and operating tables is simply not large enough to accommodate the woman’s size. Extra-large furniture may not fit through a standard doorway, so room renovation may be necessary. Some hospitals have created rooms specifically designed to accommodate obese clients (Fig. 33-5). Continuous external FHR and contraction monitoring may be extremely difficult if not impossible to perform. Special equipment, such as extra-large blood pressure cuffs, is necessary to properly assess the woman’s condition.
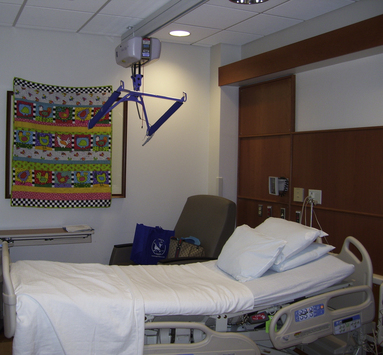
FIG. 33-5 Room specifically designed to accommodate obese pregnant clients. Note lift attached to ceiling for use in transferring women from the bed to chairs or stretchers. (Courtesy Dee Lowdermilk, Chapel Hill, NC).
Even routine procedures will require more time and effort to accomplish when a woman is obese. This is extremely worrisome, given that obesity is a risk factor for emergency cesarean birth, when time is often of the essence. Establishing intravenous access, for example, may require multiple attempts, sometimes by multiple persons. Mobility is often a problem. Moving the woman from a labor room to the operating room and transferring her from a bed to the operating table may require the assistance of additional personnel or special equipment, especially if
regional anesthesia is already in effect. If it is not, surgery may be further delayed by anesthetic complications, such as difficulty establishing an epidural or spinal block or accomplishing endotracheal intubation.
Postoperatively, obese women are at increased risk for blood clot formation. In the immediate recovery period, use of thromboembolic stockings (TED hose) and sequential compression device (SCD) boots help to decrease the chance for clot formation. Some women may also be given heparin prophylactically for clot prevention (Cunningham et al., 2010). Women should also be encouraged to get out of bed and begin ambulating as soon as possible.
Keeping the incision clean and dry to prevent wound infection and promote healing is another postoperative challenge. Many obese women have a pannus (large roll of abdominal fat) that overlies a lower abdominal transverse skin incision made just above the pubic area. The pannus causes the area to remain moist, which encourages infection development. Women should be taught to wash the incision with soap and water several times a day, drying the area well afterward. Sutures or staples used to close the skin incision are generally left in place longer than usual in order to avoid possible wound disruption when they are removed. Sometimes the skin and subcutaneous layers of the incision are left open to heal by secondary intention to avoid possible dehiscence. If this course of action is chosen, the woman and other family members must be taught to do dressing changes and wound care.