Obstetric Procedures
Version is the turning of the fetus from one presentation to another. It may be performed externally or internally by the physician.
External Cephalic Version
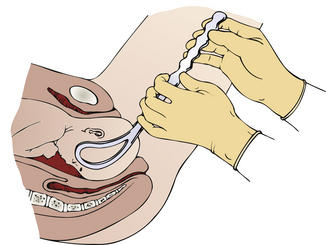
External cephalic version (ECV) is used in an attempt to turn the fetus from a breech or shoulder presentation to a vertex presentation for birth. It may be attempted in a labor and birth setting after 37 weeks of gestation. ECV is accomplished by the exertion of gentle, constant pressure on the abdomen (Fig. 33-6). Before ECV is attempted, ultrasound scanning is done to determine the fetal position; locate the umbilical cord; rule out placenta previa; evaluate the adequacy of the maternal pelvis; and assess the amount of amniotic fluid, the gestational age, and the presence of any anomalies. An NST is performed to confirm fetal well-being, or the FHR and pattern are monitored for a period of time (i.e., 10 to 20 minutes). Informed consent is obtained. A tocolytic agent such as terbutaline often is given to relax the uterus and facilitate the maneuver. Contraindications to ECV include (Thorp, 2009):
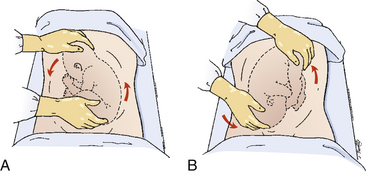
FIG. 33-6 External version of fetus from breech to vertex presentation. This must be achieved without force. A, Breech is pushed up out of pelvic inlet while head is pulled toward inlet. B, Head is pushed toward inlet while breech is pulled upward.
• Evidence of uteroplacental insufficiency
• A nuchal cord (identified by ultrasound)
• Previous cesarean birth or other significant uterine surgery
ECV is most successful in a multiparous woman who has a normal amount of amniotic fluid and whose fetus is not yet engaged in the pelvis (Cunningham et al., 2010). If ECV is not successful, the ACOG recommends that the woman undergo planned cesarean birth (Thorp, 2009).
During an attempted ECV, the nurse continuously monitors the FHR and pattern, especially for bradycardia and variable decelerations; checks the maternal vital signs; and assesses the woman’s level of comfort, because the procedure may cause discomfort. After the procedure is completed, the nurse continues to monitor maternal vital signs and uterine activity, and to assess for vaginal bleeding until the woman’s condition is determined to be stable. FHR and pattern monitoring should continue for at least 1 hour. Women who are Rh negative should receive Rh immune globulin because the manipulation can cause fetomaternal bleeding (Lanni & Seeds, 2007; Thorp, 2009).
Internal Version
With internal version the fetus is turned by the physician, who inserts a hand into the uterus and changes the presentation to cephalic (head) or podalic (foot). Internal version is only rarely used, most often in twin gestations to deliver the second fetus. The safety of this procedure has not been documented; maternal and fetal injury is possible. Cesarean birth is the usual method for managing malpresentation in multifetal pregnancies. The nurse’s role is to monitor the status of the fetus and to provide support to the woman.
Induction of Labor
Induction of labor is the chemical or mechanical initiation of uterine contractions before their spontaneous onset for the purpose of bringing about birth. Labor may be induced either electively or for indicated reasons. The rate of labor induction since 1990 has doubled to a rate of approximately 20% (Martin et al., 2009). It is likely that the rate of elective inductions is increasing more rapidly than the rate of indicated inductions. Additionally, there is concern that elective inductions may increase the risk of cesarean birth especially among primigravid women, and particularly those over the age of 35 (Martin et al.; Thorp, 2009; Wilson, 2007).
Induction of labor is indicated if continuing the pregnancy could be dangerous for either the woman or the fetus, and if no contraindications exist to artificial rupture of the membranes (amniotomy) or augmenting uterine contractions with oxytocin. Prior to labor induction, gestational age should be determined and any potential risks to the maternal-fetal unit evaluated. Women must be fully counseled regarding risks, benefits, and alternatives of labor stimulation methods as part of the process for informed consent (ACOG, 2009; Thorp, 2009). Box 33-8 lists indications and contraindications for labor induction.
An elective induction is one in which labor is initiated without a medical indication. Methods to ripen the cervix (e.g., application of prostaglandins) enhance the likelihood of successful induction and have therefore been a factor in the use of elective induction as an option for managing childbirth rather than waiting for labor to begin spontaneously. Many of these elective inductions are purely for the convenience of the woman or her primary health care provider. At times, however, labor may be electively induced to allay maternal fears and anxieties associated with prior perinatal losses or to ensure that experienced multispecialty personnel are available to handle anticipated maternal or neonatal complications immediately following birth (Battista & Wing, 2007; Moleti, 2009). The two major risks associated with elective labor induction at term are increased rates of cesarean birth and iatrogenic prematurity (Battista & Wing). In order to prevent iatrogenic prematurity, elective induction of labor should not be initiated until the woman reaches 39 completed weeks of gestation (ACOG, 2009; Cherouny, Federico, Haraden, Leavitt Gullo, & Resar, 2005).
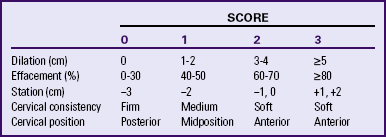
Chemical, mechanical, physical, and alternative methods are used to ripen the cervix and induce labor. Intravenous oxytocin (Pitocin) and amniotomy are the most common methods used in the United States. Success rates for induction of labor are higher when the condition of the cervix is favorable, or inducible. Cervical ripeness is the most important predictor of successful induction. A rating system such as the Bishop score (Table 33-3) can be used to evaluate inducibility. For example, a score of 8 or more on this 13-point scale indicates that the cervix is soft, anterior, 50% or more effaced, and dilated 2 cm or more and that the presenting part is engaged. When the Bishop score totals 8 or more, induction of labor is usually successful (ACOG, 2009; Gilbert, 2011; Moleti, 2009). The Bishop score should be documented prior to the use of methods to ripen the cervix or induce labor.
Cervical Ripening Methods
Chemical Agents: Preparations of prostaglandins E1 (PGE1) and E2 (PGE2) have been shown to be effective when used before induction to “ripen” (soften and thin) the cervix (see the Medication Guides for Prostaglandin E1 and Prostaglandin E2). In some cases, women spontaneously begin laboring after the administration of prostaglandin, thereby eliminating the need to administer oxytocin to induce labor. Additional advantages of prostaglandin use for cervical ripening include decreased oxytocin induction time and a decrease in the amount of oxytocin required for successful induction (Gilbert, 2011). PGE1, although much less expensive and more effective than PGE2 for inducing labor and birth, is associated with a higher risk for uterine tachysystole with abnormal (nonreassuring) fetal heart rate and pattern changes and passage of meconium into the aminotic fluid. Most of these adverse outcomes are associated with higher dose protocols (ACOG, 2009; Battista & Wing, 2007). Although the drug’s manufacturer has acknowledged for several years that PG E1 is effective for cervical ripening and labor induction, it has not yet been approved by the FDA for these uses (Thorp, 2009). PG E2 in the form of a vaginal insert (dinoprostone [Cervidil]), although more expensive than PG E1, has the major advantage of easy removal should adverse reactions, including uterine tachysystole, occur (Moleti, 2009).
Mechanical and Physical Methods: Mechanical dilators ripen the cervix by stimulating the release of endogenous prostaglandins. Balloon catheters (e.g., Foley catheter) can be inserted through the intracervical canal to ripen and dilate the cervix. The catheter balloon is inflated above the internal cervical os with 30 to 50 ml of sterile water. This process results in pressure and stretching of the lower uterine segment and the cervix, as well as the release of endogenous prostaglandins. It is especially helpful for women who cannot receive exogenaut prostaglandins for cervical ripening. The balloon will fall out when cervical dilation reaches approximately 3 cm in about 8 to 12 hours after it is inserted. Evidence supports the insertion of a balloon catheter as a cervical ripening method especially since it is not associated with uterine tachysystole and fetal stress as are the prostaglandin methods (ACOG, 2009; Simpson, 2008). ![]()
Hydroscopic dilators (substances that absorb fluid from surrounding tissues and then enlarge) also can be used for cervical ripening. Laminaria tents (natural cervical dilators made from desiccated seaweed) and synthetic dilators containing magnesium sulfate (Lamicel) are inserted into the endocervix without rupturing the membranes. As they absorb fluid, they expand and cause cervical dilation, and the release of endogenous prostaglandins. These dilators are left in place for 6 to 12 hours before being removed to assess cervical dilation. Fresh dilators
are inserted if further cervical dilation is necessary. Synthetic dilators swell faster than natural dilators and become larger with less discomfort. When compared with prostaglandins, these mechanical methods achieved a lower rate of birth within 24 hours, but caused no change in the cesarean birth rate. Additionally, they were less likely to cause uterine tachysystole with or without changes in the fetal heart rate (ACOG, 2009; Thorp, 2009).
Hydroscopic dilators compare favorably with prostaglandins in terms of their effectiveness in ripening the cervix but are associated with increased discomfort at insertion and during expansion and with a higher incidence of postpartum maternal and newborn infections. They are a reliable alternative when prostaglandins are contraindicated or are unavailable. Nursing responsibilities for women who have dilators inserted include documenting the number of dilators and sponges inserted during the procedure, as well as the number removed, and assessment for urinary retention, rupture of membranes, uterine tenderness or pain, contractions, vaginal bleeding, infection, and fetal distress (Gilbert, 2011).
Amniotic membrane stripping or sweeping is a method of inducing labor through the release of prostaglandins and oxytocin. The procedure involves separation of the membrane from the wall of the cervix and lower uterine segment by inserting a finger into the internal cervical os and rotating it 360 degrees. Membrane stripping seems to work best when the woman is a primigravida at term with an unripe cervix and with the vertex well applied to the cervix. In some studies it has been associated with shorter pregnancies and a decreased likelihood of progressing past 42 weeks of gestation. The procedure is uncomfortable and increases the risk for infection, rupture of membranes, bleeding, and precipitous labor and birth (Simpson, 2008).
Physical methods such as sexual intercourse (prostaglandins in the semen and stimulation of contractions with orgasm), nipple stimulation (release of endogenous oxytocin from the pituitary gland), and walking (gravity applies pressure to the cervix, which stimulates the secretion of endogenous oxytocin) may be used by women to “self-induce” labor in an effort to “get it over with.” Breast (nipple) stimulation has been shown to initiate or enhance labor, especially the latent phase of labor. Although orgasm does stimulate uterine contractions, there is inadequate evidence to support the belief that sexual intercourse enhances cervical ripening (Gilbert, 2011). Ambulation is an effective measure to augment labor (Moleti, 2009).
Alternative Methods: A variety of alternative methods have been used by women to stimulate cervical ripening and the
onset of labor. For example, blue cohosh and castor oil can be used for their labor stimulation effects and black cohosh and evening primrose oil can ripen the cervix. ![]() Nurses must be knowledgeable about these preparations and ask about their use when assessing women during prenatal visits and on admission during labor. Women may accidentally take too much of the preparation or use it incorrectly. Also these preparations may potentiate the effect of pharmacologic methods to stimulate cervical ripening and uterine contractions, thereby increasing the potential for tachysystole and precipitous labor and birth (Gilbert, 2011; Moleti, 2009).
Nurses must be knowledgeable about these preparations and ask about their use when assessing women during prenatal visits and on admission during labor. Women may accidentally take too much of the preparation or use it incorrectly. Also these preparations may potentiate the effect of pharmacologic methods to stimulate cervical ripening and uterine contractions, thereby increasing the potential for tachysystole and precipitous labor and birth (Gilbert, 2011; Moleti, 2009).
Acupuncture has been used effectively to induce labor and has been found, in several studies, to reduce the duration of labor, the use of oxytocin, and the rate of cesarean birth. ![]() Specific points have been identified to stimulate uterine contractions or to facilitate cervical dilation. More than one treatment may be required to establish labor (Gilbert, 2011; Moleti, 2009).
Specific points have been identified to stimulate uterine contractions or to facilitate cervical dilation. More than one treatment may be required to establish labor (Gilbert, 2011; Moleti, 2009).
Amniotomy: Amniotomy (i.e., artificial rupture of membranes [AROM]) can be used to induce labor when the condition of the cervix is favorable (ripe) or to augment labor if progress begins to slow. Labor usually begins within 12 hours of the rupture. Amniotomy can decrease the duration of labor by up to 2 hours, even without oxytocin administration. However, if amniotomy does not stimulate labor, the resulting prolonged rupture may lead to intraamniotic infection. Variable FHR deceleration patterns can occur as a result of cord compression associated with umbilical cord prolapse or decreased aminotic fluid. Once an amniotomy is performed, the woman is committed to labor with an unknown outcome for how and when she will give birth. For this reason, amniotomy often is used in combination with oxytocin induction.
Before the procedure, the woman should be told what to expect. She also should be assured that the actual rupture of the membranes is painless for her and the fetus, although she may experience some discomfort when the Amnihook or other sharp instrument is inserted through the vagina and cervix (see the Procedure box: Assisting with Amniotomy).The presenting part of the fetus should be engaged and well applied to the cervix prior to the procedure to prevent cord prolapse (Battista & Wing, 2007). The woman should also be free of active infection of the genital tract (e.g., herpes) and should be human immunodeficiency virus (HIV) negative. After rupture, the amniotic
fluid is allowed to drain slowly. The color, odor, and consistency of the fluid are assessed (i.e., for the presence or absence of meconium or blood). The time of rupture is recorded.
The woman’s temperature should be checked at least every 2 hours after rupture of membranes, more frequently if signs or symptoms of infection are noted. If her temperature is 38° C or higher, notify the primary health care provider. The nurse assesses for other signs and symptoms of infection, such as maternal chills, uterine tenderness on palpation, foul-smelling vaginal drainage, and fetal tachycardia. Comfort measures, such as frequently changing the woman’s underpads and perineal cleansing, are implemented.
Oxytocin
Oxytocin is a hormone normally produced by the posterior pituitary gland. It stimulates uterine contractions and aids in milk let-down. Synthetic oxytocin (Pitocin) may be used either to induce labor or to augment a labor that is progressing slowly because of inadequate uterine contractions. Oxytocin is used in the majority of all births in the United States. It is also the drug most commonly associated with adverse events during childbirth. The most common errors involving oxytocin administration during labor are dose related (Clark, Simpson, Knox, & Garite, 2009; Mahlmeister, 2008; Simpson & Knox, 2009).
Oxytocin use can present hazards to the mother and fetus. Maternal hazards include placental abruption, uterine rupture, unnecessary cesarean birth due to abnormal (nonreassuring) FHR and patterns, postpartum hemorrhage, and infection. When placental perfusion is diminished by contractions that are too frequent or prolonged, the fetus can experience hypoxemia and acidemia, which eventually results in late decelerations and minimal or absent baseline variability. The goal of oxytocin use is to produce contractions of normal intensity, duration, and frequency while using the lowest dose of medication possible (Simpson & Knox, 2009).
The primary health care provider writes the order for the induction or augmentation of labor with oxytocin. The nurse implements the order by initiating the primary intravenous infusion and administering the oxytocin solution through a secondary line. The nurse’s actions related to assessment and care of a woman whose labor is being induced are guided by hospital protocol and professional standards (see Fig. 33-7 and the Medication Guide: Oxytocin [Pitocin]).
The recommended protocol for administering oxytocin is to begin with a starting dose of 1 milliunit/min and to increase by 1 to 2 milliunits/min no more frequently than every 30 to 60 minutes (Simpson & Knox, 2009). This recommendation is based on research findings related to the pharmacokinetics of oxytocin. ![]() The uterus responds to oxytocin within 3 to 5 minutes of intravenous administration. The half-life of oxytocin (the time required to metabolize and eliminate half the dose) is approximately 10 to 12 minutes. Approximately 40 minutes is required to reach a steady state of oxytocin (the point in time when the rate of oxytocin administered intravenously equals the rate of oxytocin elimination) and for the full effect of a dosage increment to be reflected in more intense, frequent, and longer contractions (Mahlmeister, 2008) (see the Medication Guide: Oxytocin [Pitocin] and Fig. 33-7). Low-dose (physiologic) protocols such as the one described result in less uterine hyperstimulation, decreased fetal compromise, and significantly less use of oxytocin without affecting the duration of labor or cesarean birth rate (Battista & Wing, 2007; Gilbert, 2011).
The uterus responds to oxytocin within 3 to 5 minutes of intravenous administration. The half-life of oxytocin (the time required to metabolize and eliminate half the dose) is approximately 10 to 12 minutes. Approximately 40 minutes is required to reach a steady state of oxytocin (the point in time when the rate of oxytocin administered intravenously equals the rate of oxytocin elimination) and for the full effect of a dosage increment to be reflected in more intense, frequent, and longer contractions (Mahlmeister, 2008) (see the Medication Guide: Oxytocin [Pitocin] and Fig. 33-7). Low-dose (physiologic) protocols such as the one described result in less uterine hyperstimulation, decreased fetal compromise, and significantly less use of oxytocin without affecting the duration of labor or cesarean birth rate (Battista & Wing, 2007; Gilbert, 2011).
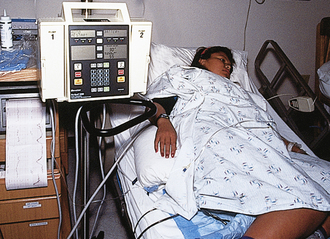
FIG. 33-7 Woman in side-lying position receiving oxytocin. (Courtesy Michael S. Clement, MD, Mesa, AZ.)
High-dose protocols, in which the initial dose of oxytocin is larger and the dosage is increased more rapidly, have been found to result in shorter labors, less forceps-assisted births, and fewer cesarean births due to dystocia. However, high-dose protocols have been associated with more uterine hyperstimulation and more cesarean births related to fetal stress (Battista & Wing, 2007; Gilbert, 2011; Simpson & Knox, 2009). Some practitioners administer oxytocin in 10-minute pulsed infusions rather than as a continuous infusion. This method, which is more like
endogenous secretion of oxytocin than the other approaches, is reported to be effective for labor induction but requires significantly less oxytocin use (Battista & Wing; Gilbert).
Nursing Considerations.: An evidence-based written protocol for the preparation and administration of oxytocin should be established by the obstetric department (physicians, nurses) in each institution. Other safety measures recommended for use of this high-alert drug are using a standard concentration of oxytocin and a standard definition of uterine tachysystole that does not include an abnormal (nonreassuring) FHR or pattern or the woman’s perception of pain. Additionally, standardized treatment of oxytocin-induced uterine tachysystole is recommended (Simpson & Knox, 2009) (see the Emergency box: Uterine Tachysystole with Oxytocin).
There has existed a need for standardizing the definition of excessive uterine contractions. The Eunice Kennedy Shriver National Institute of Child Health and Human Development, along with the ACOG and the Society for Maternal-Fetal Medicine, sponsored a workshop in April 2008 to review definition, interpretation, and research recommendations for intrapartum fetal monitoring. Workshop participants also recommended standardizing definitions regarding uterine contractions for
use in clinical practice. This group defined uterine tachysystole as more than five contractions in 10 minutes, averaged over a 30-minute window. The term tachysystole applies to both spontaneous and stimulated labor. Participants also recommended that use of the terms hyperstimulation and hyperactivity be abandoned because they are not defined (Macones, Hankins, Spong, Hauth, & Moore, 2008).
Augmentation of Labor
Augmentation of labor is the stimulation of uterine contractions after labor has started spontaneously but progress is unsatisfactory. Augmentation is usually implemented for the management of hypotonic uterine dysfunction, resulting in a slowing of the labor process (protracted active phase). Common augmentation methods include oxytocin infusion and amniotomy. Noninvasive methods such as emptying the bladder, ambulation and position changes, relaxation measures, nourishment and hydration, and hydrotherapy should be attempted before initiating invasive interventions. The administration procedure and nursing assessment and care measures for augmenting labor with oxytocin are similar to those used for induction of labor with oxytocin (see the Medication Guide: Oxytocin [Pitocin]).
Some physicians advocate active management of labor, that is, augmentation of ng labor to establish efficient labor with the aggressive use of oxytocin so that the woman gives birth within 12 hours of admission to the labor unit. Advocates of active management believe that intervening early (as soon as a nulliparous labor is not progressing at least 1 cm/hr) with use of higher (pharmacologic) oxytocin doses administered at frequent increment intervals (e.g., a starting dose of 6 milliunits/min with increases of 6 milliunits/min every 15 minutes) shortens labor (Gilbert, 2011).
Additional components of the active management of labor include strict criteria to diagnose that the woman is indeed in active labor with 100% effacement, amniotomy within 1 hour of admission of a woman in labor if spontaneous rupture of the membranes has not occurred, and continuous presence of a personal nurse who provides one-on-one care for the woman while she is in labor. Many U.S. obstetricians emphasize using high-dose oxytocin protocols but do not implement all the other components of active management. At least one review of published studies on the effectiveness of active management of labor protocols concluded that the presence of a personal nurse who provides constant emotional and physical support is the only component associated with shorter labors and lower rates of cesarean birth (Clark et al., 2009; Gilbert, 2011).
The original active management of labor protocols were written for nulliparous women who began laboring spontaneously. However, active management of labor protocols have been implemented by some providers in the United States on women who were not appropriate candidates (Mahlmeister, 2008).
Operative vaginal birth
Operative vaginal births are accomplished with the assistance of forceps or vacuum extractor. Indications and prerequisites for the use of both instruments are similar. The decision to use forceps or vacuum is based on the experience and personal preference of the physician performing the procedure. There are several types of operative vaginal births, defined primarily by the station and position of the fetal head in relationship to the maternal pelvis (Table 33-4) (American Academy of Pediatrics [AAP] & ACOG, 2007). Forceps- and vacuum-assisted vaginal births have been declining. This decline has been attributed to the increasing rate of cesarean birth (Martin et al., 2009).
TABLE 33-4
DEFINITIONS FOR FORCEPS- AND VACUUM-ASSISTED BIRTHS
| Outlet | Fetal scalp is visible on the perineum without manually separating the labia |
| Low | Fetal head is at least at the +2 station |
| Midpelvis | Fetal head is engaged (no higher than 0 station) but above the +2 station |
Source: American Academy of Pediatrics (AAP) & American College of Obstetricians and Gynecologists (ACOG). (2007). Guidelines for perinatal care (6th ed.). Washington, DC: ACOG.
Forceps-Assisted Birth
A forceps-assisted birth is one in which an instrument with two curved blades is used to assist in the birth of the fetal head. The cephalic-like curve of the forceps commonly used is similar to the shape of the fetal head, with a pelvic curve to the blades conforming to the curve of the pelvic axis. The blades are joined by a pin, screw, or groove arrangement. These locks prevent the forceps from compressing the fetal skull (Fig. 33-8). There are several types of forceps-assisted births, defined primarily by the station and position of the fetal head in relationship to the maternal pelvis (see Table 33-4) (AAP & ACOG, 2007).
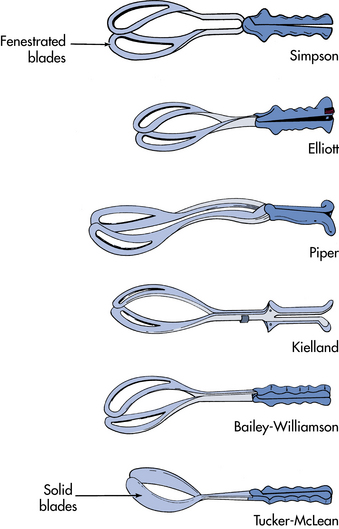
FIG. 33-8 Types of forceps. Piper forceps are used to assist delivery of the head in a breech birth.
Maternal indications for forceps-assisted birth include a prolonged second stage of labor and the need to shorten the second stage of labor for maternal reasons (e.g., maternal exhaustion or maternal cardiopulmonary or cerebrovascular disease) (Nielsen, Galan, Kilpatrick, & Garrison, 2007). Fetal indications include birth of a fetus in distress or in certain abnormal presentations; arrest of rotation; or extraction of the head in a breech presentation. The use of forceps during childbirth has been decreasing, replaced by vacuum extraction or cesarean birth (Nielsen et al.; Thorp, 2009).
Certain conditions are required for a forceps-assisted birth to be successful. The woman’s cervix must be fully dilated to prevent lacerations and hemorrhage. The bladder should be empty. The presenting part must be engaged—vertex presentation is desired. Membranes must be ruptured so that the position of the fetal head can be precisely determined and the forceps can firmly grasp the head during birth (Fig. 33-9). In addition, the size of the maternal pelvis must be assessed as adequate for the estimated fetal head circumference and weight.
Management: Both blades are positioned by the physician, and the handles are locked. Traction is usually applied during contractions. The mother may or may not be instructed to push during contractions, depending on physician preference. If a decrease in the fetal heart rate occurs, the forceps are removed and reapplied.
Nursing Considerations: When a forceps-assisted birth is deemed necessary, the nurse obtains the type of forceps requested by the primary health care provider. The nurse may explain to the mother that the forceps blades fit the same way two tablespoons fit around an egg, with the blades placed in front of the baby’s ears.
After birth the mother should be assessed for vaginal or cervical lacerations, urinary retention, and hematoma formation in the pelvic soft tissues, which may result from blood vessel damage. The infant should be assessed for bruising or abrasions at the site of the blade applications, facial palsy resulting from pressure of the blades on the facial nerve, and subdural hematoma. Newborn and postpartum caregivers should be told that a forceps-assisted birth was performed.
Vacuum-Assisted Birth
Vacuum-assisted birth, or vacuum extraction, is a birth method involving the attachment of a vacuum cup to the fetal head, using negative pressure to assist in the birth of the head (Fig. 33-10, A). It is generally not used to assist birth before 34 weeks of gestation. Indications for its use are the same as those for outlet forceps. Prerequisites for use include a completely dilated cervix, ruptured membranes, engaged head, vertex presentation, and no suspicion of CPD (Cunningham et al., 2010). There are several types of vacuum-assisted births, defined primarily by the station and position of the fetal head in relation to the maternal pelvis (see Table 33-4) (AAP & ACOG, 2007). Advantages of vacuum-assisted compared with forceps-assisted birth are the ease with which the vacuum can be placed and the need for less anesthesia. Also it is far easier to learn the skills necessary to safely use the vacuum than to gain a similar level of skill with forceps (Thorp, 2009).
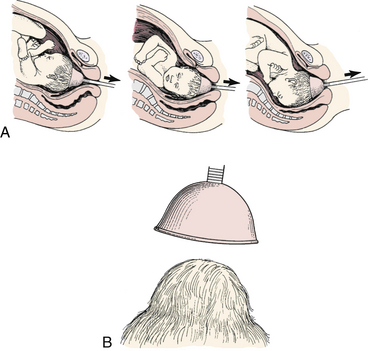
FIG. 33-10 Use of vacuum extraction to rotate fetal head and assist with descent. A, Arrow indicates direction of traction on the vacuum cup. B, Caput succedaneum formed by the vacuum cup.
Management: The vacuum cup is applied to the fetal head by the physician. There are basically two types of vacuum devices in use. One is a self-contained unit, which allows the physician to both position the cup on the baby’s head and generate the desired amount of negative pressure to create a vacuum. When the other type of vacuum device is used, the physician applies the cup to the baby’s head, after which the nurse connects the suction tubing attached to the cup to wall suction or a separate hand pump and generates the amount of pressure requested by the physician. With both devices, a caput develops inside the cup as the pressure is initiated (see Fig. 33-10, B). The woman is encouraged to push as traction is applied by the physician. The vacuum cup is released and removed after birth of the head. If vacuum extraction is not successful, a forceps-assisted or cesarean birth is usually performed.
Risks to the newborn include cephalhematoma, scalp lacerations, and subdural hematoma. Fetal complications can be reduced by strict adherence to the manufacturer’s recommendations for method of application, amount of pressure to be generated, and duration of application. Maternal risks include perineal, vaginal, or cervical lacerations and soft-tissue hematomas.
Nursing Considerations: The nurse’s role for the woman who has a vacuum-assisted birth is primarily one of support person and educator. The nurse can prepare the woman for birth and encourage her to remain active in the birth process by pushing during contractions. The fetal heart rate should be assessed frequently during the procedure. Documentation of the procedure in the medical record is important and is often the nurse’s responsibility (Box 33-9). Neonatal caregivers should be told that the birth was vacuum assisted. After birth, the newborn must be observed for signs of trauma and infection at the application site and for cerebral irritation (e.g., poor sucking or listlessness). The newborn may also be at risk for hyperbilirubinemia and neonatal jaundice as bruising resolves. The parents may need to be reassured that the caput succedaneum usually disappears in 3 to 5 days (see Fig. 33-10, B) (Gilbert, 2011).
Cesarean Birth
Cesarean birth is the birth of a fetus through a transabdominal incision of the uterus. Whether cesarean birth is planned (scheduled) or unplanned, the loss of the experience of giving birth to a child in the traditional manner may have a negative effect on a woman’s self-concept. An effort is therefore made to maintain the focus on the birth of the baby rather than on the operative procedure.
The purpose of cesarean birth is to preserve the life or health of the mother and her fetus. It may be the best choice for birth when evidence exists of maternal or fetal complications. Since the advent of modern surgical methods and care and the use of antibiotics, maternal and fetal morbidity and mortality have decreased. In addition, incisions are usually made into the lower uterine segment rather than in the muscular body of the uterus, thus promoting more effective healing. However, despite these advances, cesarean birth still poses threats to the health of the mother and infant.
The incidence of cesarean births has escalated to 32.3% of live births in 2008, the highest rate ever reported in the United States (Hamilton et al., 2010). Part of the reason for this rise is that a number of common risk factors for cesarean birth are increasing in frequency, especially in developed countries. These factors include fetal macrosomia, advanced maternal age, obesity, gestational diabetes, and multifetal pregnancy (Thorp, 2009). Malpractice concerns are another factor related to the elevated incidence, along with an increase in the number of cesareans done on maternal request, estimated to be 2.5% of all births in the United States (ACOG, 2007; Landon, 2007). An international estimate of the elective cesarean birth rate is much higher compared with the United States, between 4% and 18% (Collard, Diallo, Habinsky, Hentschell, & Vezeau, 2008/2009).
As women age, the likelihood of their having a cesarean birth increases. The rationale for this increase may be related to biologic and medical factors, maternal and physician concerns, and the increased rate of multifetal pregnancies (Martin et al., 2009).
Approaches for managing labor and birth to reduce the rate of cesarean births while increasing the rate of VBAC are presented in Box 33-10. These approaches involve the combined efforts of health care professionals and pregnant women and their families. The type of nursing care given also may influence the rate of cesarean births. A labor management approach that uses one-to-one support and emphasizes ambulation, maternal position changes, relaxation measures, oral fluids and nutrition, hydrotherapy, and nonpharmacologic pain relief supports the physiologic progression of labor, reduces the incidence of dystocia, and increases the likelihood of a spontaneous vaginal birth (Albers, 2007; Hodnett, Gates, Hofmeyr, & Sakala, 2007). Several studies have found that the labor management approach that most consistently reduces cesarean birth rates is continuous, one-on-one, early-onset support of the laboring woman provided by another woman (e.g., doula, relative, friend, nurse, or nurse-midwife). ![]() The greatest reduction in risk for cesarean birth as well as a reduction in the use of epidural analgesia occurs when this woman is a doula, whose role is to spend all of her time providing physical and emotional support to the woman and providing emotional support and encouragement to the woman’s partner (Berghella, Baxter, & Chauhan, 2008; Hodnett et al.; McGrath & Kennell, 2008).
The greatest reduction in risk for cesarean birth as well as a reduction in the use of epidural analgesia occurs when this woman is a doula, whose role is to spend all of her time providing physical and emotional support to the woman and providing emotional support and encouragement to the woman’s partner (Berghella, Baxter, & Chauhan, 2008; Hodnett et al.; McGrath & Kennell, 2008).
Despite these efforts, the rate of cesarean birth is rising and the VBAC rate is decreasing. This decline may be due to reports of VBAC risks including rupture of the uterus, legal pressures, conservative practice guidelines, and debate regarding the relative benefits and risks of repeat cesarean birth versus vaginal birth. The declining trend in VBACs indicates that once a woman has a cesarean birth it is highly (92%) likely that her subsequent births will also be cesarean (Martin et al., 2009).
Indications
Few absolute indications exist for cesarean birth. Today most are performed for conditions that might pose a threat to both the mother and the fetus if vaginal birth occurred, such as placenta previa or placental abruption (Landon, 2007). Box 33-11 lists common indications for cesarean birth.
Elective Cesarean Birth
Elective cesarean birth, sometimes referred to as cesarean on request or cesarean on demand, refers to a primary cesarean birth without medical or obstetric indication. Reasons given for elective cesarean birth include fear of the pain of childbirth and the mistaken belief that the surgery will prevent future problems with pelvic support, bladder and bowel incontinence, or sexual dysfunction. Although some nulliparous women may fear the pain of labor because of no firsthand experience, multiparous women may request a cesarean birth after a previous traumatic vaginal birth (Gardner, 2003). Other women desire an elective cesarean birth because of the convenience of planning a date, or having control and choice about when to give birth (Williams, 2005). At this time evidence is insufficient to recommend elective cesarean birth to prevent urinary or fecal incontinence later in life (Collard et al., 2008/2009; Roberts & Mangan, 2009; Thorp, 2009).
Only limited data are available comparing cesarean births on request with planned vaginal births (ACOG, 2007). In a committee opinion (2007), the ACOG lists potential risks of cesarean birth on request that include a longer hospital stay for the woman, an increased risk of respiratory problems for the baby, and greater complications in subsequent pregnancies, including uterine rupture and placental implantation problems. The ACOG recommends that cesarean birth on request not be performed unless a gestational age of 39 weeks has been accurately determined. The ACOG does not recommend cesarean birth on request for women who desire additional children, because the risks for placenta previa, placenta accreta, and cesarean hysterectomy increase with each cesarean birth (ACOG, 2007). The Society of Obstetricians and Gynaecologists of Canada (SOGC) promotes natural childbirth. The organization does not promote elective cesarean birth but believes that the final decision as to the safest route of childbirth rests with the woman and her health care provider (SOGC, 2004).
Forced Cesarean Birth
A woman’s refusal to undergo cesarean birth when indicated for fetal reasons is often described as a maternal-fetal conflict. Health care providers are ethically obliged to protect the well-being of both mother and fetus; a decision for one affects the other. If a woman refuses a cesarean birth that is recommended because of fetal jeopardy, health care providers must make every effort to find out why she is refusing and provide information that may persuade her to change her mind. If the woman continues to refuse surgery, then health care providers must decide if it is ethical to get a court order for the surgery. Every effort, however, should be made to avoid this legal step.
Surgical Techniques
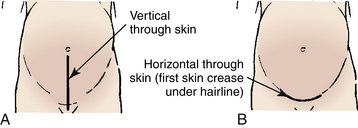
The skin incision will either be vertical, extending from near the umbilicus to the mons pubis or transverse (Pfannenstiel) in the lower abdomen (Fig. 33-11). The transverse incision, sometimes referred to as the “bikini” incision, is performed more often. The type of skin incision is generally determined by the urgency of the surgery and the presence of any prior skin incisions (Landon, 2007). The type of skin incision does not necessarily indicate the type of uterine incision.
The two main types of uterine incision are the low transverse (Fig. 33-12, A) or vertical incision which may be either low or classic (see Fig. 33-12, B and C). Ideally the vertical incision is contained entirely within the lower uterine segment, but extension into the contractile portion of the uterus (e.g., a classic incision) is common (Landon, 2007). Indications for a vertical incision include an underdeveloped lower uterine segment, a transverse lie or preterm breech presentation, certain fetal anomalies such as massive hydrocephalus, and an anterior placenta previa (Landon). Because it is associated with a higher incidence of uterine rupture in subsequent pregnancies than is lower-segment cesarean birth, vaginal birth after a classic uterine incision is contraindicated.
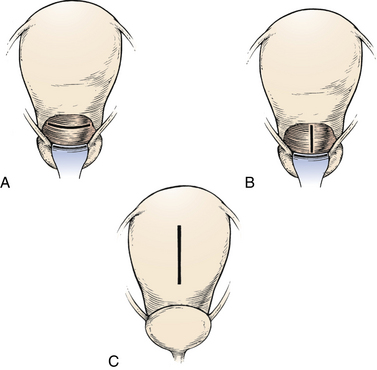
FIG. 33-12 Uterine incisions for cesarean birth. A, Low transverse incision. B, Low vertical incision. C, Classic incision (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
The low transverse uterine incision is performed in more than 90% of cesarean births (see Fig. 33-12, A). Compared with the vertical incision, the transverse incision is preferred because it does not compromise the upper uterine segment, is easier to perform and repair, and is associated with less blood loss. It is also less likely to rupture in subsequent pregnancies (Landon, 2007).
Complications and Risks
Possible maternal complications related to cesarean birth include aspiration, hemorrhage, atelectasis, endometritis, abdominal wound dehiscence or infection, urinary tract infection, injuries to the bladder or bowel, and complications related to anesthesia (Thorp, 2009). The fetus may be born prematurely if the gestational age has not been accurately determined. Fetal asphyxia can occur if the uterus and placenta are poorly perfused as a result of maternal hypotension caused by regional anesthesia (epidural or spinal) or maternal positioning. Fetal injuries (e.g., injuries caused by the scalpel lacerations) can also occur during the surgery. The newborn is more likely to require resuscitation efforts and develop respiratory complications (Roberts & Mangan, 2009; Thorp). In addition to these risks, the woman is at economic risk because the cost of cesarean birth is higher than that of vaginal birth, and a longer recovery period may require additional expenditures.
Anesthesia
Spinal, epidural, and general anesthetics are used for cesarean births. Epidural blocks are popular because women want to be awake for and aware of the birth experience. However, the choice of anesthetic depends on several factors. The mother’s medical history or present condition, such as a spinal injury, hemorrhage, or coagulopathy, may rule out the use of regional anesthesia. Time is another factor, especially if there is an emergency and the life of the mother or infant is at stake. In an emergency, general anesthesia will most likely be used unless the woman already has an epidural block in effect. The woman herself is a factor. Either she may not know all the options or may have fears about having “a needle in her back” or about being awake and feeling pain. She needs to be fully informed about the risks and benefits of the different types of anesthesia so that she can participate in the decision whenever there is a choice.
Scheduled Cesarean Birth
Cesarean birth is scheduled or planned if labor and vaginal birth are contraindicated (e.g., complete placenta previa, active genital herpes, positive HIV status with a high viral load), if birth is necessary but labor is not inducible (e.g., hypertensive states that cause a poor intrauterine environment that threatens the fetus), or if this course of action has been chosen by the primary health care provider and the woman (e.g., a repeat cesarean birth).
Women who are scheduled for a cesarean birth have time to prepare for it psychologically. However, the psychologic responses of these women may differ. Those having a repeat cesarean birth may have disturbing memories of the conditions preceding the initial (primary) cesarean birth and of their experiences in the postoperative recovery period. They may be concerned about the added burdens of caring for the infant and perhaps other children while recovering from surgery. Others may feel glad that they have been relieved of the uncertainty about the date and time of the birth and are free of the pain of labor.
Unplanned Cesarean Birth
The psychosocial outcomes of unplanned or emergency cesarean birth are usually more pronounced and negative when compared with the outcomes associated with a scheduled or planned cesarean birth. Women and their families experience abrupt changes in their expectations for birth, postpartum care, and the care of the new baby at home. This may be an extremely traumatic experience for all.
The woman may approach the procedure tired and discouraged after an ineffective and difficult labor. Fear predominates as she worries about her own safety and well-being and that of her fetus. She may be dehydrated, with low glycogen reserves. Because preoperative procedures must be done rapidly, there is often little time for explanation of the procedures and the operation itself. Because maternal and family anxiety levels are high at this time, much of what is said may be forgotten or misunderstood. The woman may experience feelings of anger or guilt in the postpartum period. Fatigue is often noticeable in these women, and they need much supportive care.
After surgery, counseling strategies that have been implemented by nurses include providing women with opportunities to talk about their birth experience, express feelings about what happened, have their questions answered, address gaps in knowledge or understanding of events, connect the event with emotions and behavior, and talk about future pregnancies. More research is needed to determine how effective these strategies are for these women in influencing their views about the unplanned cesarean birth experience or on future pregnancies (Gamble & Creedy, 2004).
Prenatal Preparation
A discussion of cesarean birth should be included in all childbirth preparation classes. No woman can be guaranteed a vaginal birth, even if she is in good health and no indication of danger to the fetus exists before the onset of labor. Therefore, every woman needs to be aware of and prepared for the possibility of having a cesarean birth.
Childbirth educators should emphasize the similarities and differences between a cesarean and a vaginal birth. In support of the philosophy of family-centered birth, many hospitals have instituted policies that permit fathers and other partners and family members to share in these births as they do in vaginal births. Women who have undergone cesarean birth agree that the continued presence and support of their partners helped them respond more positively to the entire experience. In addition to preparing women for the possibility of cesarean birth, childbirth educators should empower them to believe in their ability to give birth vaginally and to seek care measures during labor that will enhance the progress of their labors and reduce their risk for cesarean birth.
Preoperative Care
Family-centered care is the goal for the woman who is to undergo cesarean birth and for her family. The preparation of the woman for cesarean birth is the same as that for other elective or emergency surgery. The primary health care provider discusses, with the woman and her family, the need for the cesarean birth and the prognosis for the mother and infant. A member of the anesthesia care team assesses the woman’s cardiopulmonary status and describes the options for anesthesia. Women who are scheduled for an elective cesarean are often told to remain NPO (nothing by mouth) for at least 8 hours prior to the surgery (Roberts & Mangan, 2009). Informed consent is obtained for the procedure.
Blood tests are usually done a day or two before a planned cesarean birth or on admission to the labor and birth unit. Laboratory tests commonly ordered include a complete blood cell count and blood type and Rh status. Maternal vital signs and FHR and pattern are assessed according to hospital protocol until the operation begins. Intravenous fluids are started to maintain hydration and to provide an open line for the administration of blood or medications if needed. Other preoperative preparations include making sure that an informed consent form has been signed, inserting a retention (Foley) catheter to keep the bladder empty, and administering prescribed preoperative medications. In addition to medications given to prevent aspiration pneumonia, women may also receive prophylactic antibiotics to prevent postoperative infection. In the rare instance that an abdominal-mons shave or a clipping of pubic hair is ordered by the primary health care provider, it is performed in the operating room just prior to making the incision because shaving can result in injury of the integument thereby increasing the risk for infection. Often, TED hose and SCD boots will be placed on the woman’s legs to prevent blood clot formation. Removal of contact lenses, dentures, nail polish, and jewelry may be optional, depending on hospital policies and the type of anesthesia used. If the woman wears glasses and is going to be awake, the nurse should make sure her glasses accompany her to the operating room so she can see her infant.
During the preoperative preparation, the support person is encouraged to remain with the woman as much as possible to provide continuing emotional support (if this action is culturally acceptable to the woman and support person). The nurse provides essential information about the preoperative procedures during this time. Although the nursing actions may be carried out quickly if a cesarean birth is unplanned, verbal communication, particularly explanations, is important. Silence can be frightening to the woman and her support person. The nurse’s use of touch (if culturally appropriate) can communicate feelings of care and concern for the woman. The nurse can assess the woman’s and her partner’s perceptions about cesarean birth. As the woman expresses her feelings, the nurse may identify a potential for a disturbance in self-concept during the postpartum period that would need to be addressed. If there is time before the birth, the nurse can teach the woman about postoperative expectations and about pain relief, turning, leg exercises, coughing, and deep-breathing measures.
Intraoperative Care
Cesarean births occur in operating rooms in the surgical suite or in the labor and birth unit. Staff members from the labor and birth unit may scrub and circulate during the surgery or these functions may be assumed by members of the hospital’s surgery staff (Fig. 33-13). If possible, the partner, who is dressed appropriately for the operating room, accompanies the mother to the operating room and remains close to her for continued comfort and support. In unplanned cesarean birth, the nurse who cared for the woman during labor should be part of the nursing care team in the operating room if possible.
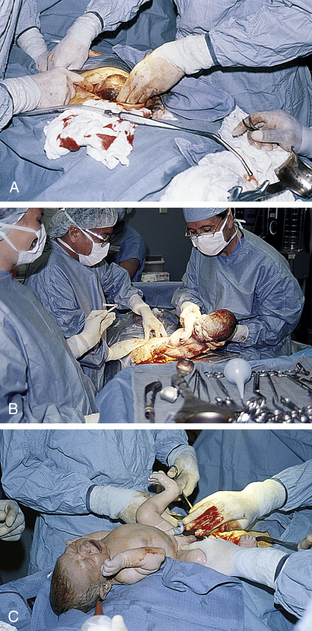
FIG. 33-13 Cesarean birth. A, “Bikini” incision has been made, the muscle layer is separated, the abdomen is entered, and the uterus has been exposed and incised; suctioning of amniotic fluid continues as head is brought up through the incision. Note small amount of bleeding. B, The neonate’s birth through the uterine incision is nearly complete. C, A quick assessment is performed; note extreme molding of head resulting from cephalopelvic disproportion. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
The nurse who is circulating may assist with positioning the woman on the birth (operating) table. It is important to position her so that the uterus is displaced laterally to prevent compression of the inferior vena cava, which causes decreased placental perfusion. This is usually accomplished by placing a wedge under the hip or tilting the table to one side. The woman’s legs should be strapped to the table to ensure proper positioning during the surgery. A retention (Foley) catheter is inserted into the bladder at this time if one is not already in place.
If the partner is not allowed or chooses not to be present, the nurse can stay in communication with him or her and give progress reports whenever possible. If the woman is awake during the birth, the nurse, anesthesia care provider, or both can tell her what is happening and provide support. She may be anxious about the sensations she is experiencing, such as the coldness of solutions used to cleanse the abdomen and pressure or pulling during the actual birth of the infant. She also may be apprehensive because of the bright lights or the presence of unfamiliar equipment and masked and gowned personnel in the room. Explanations can help to decrease the woman’s anxiety.
A nurse from the labor and birth unit usually is present to provide care for the infant. In addition, a pediatrician or a nurse team skilled in neonatal resuscitation may also be present for the surgery because these infants are considered to be at risk until evidence of physiologic stability exists after the birth. A crib with resuscitation equipment is readied before surgery. Personnel who are responsible for care are expert not only in resuscitative techniques, but also in their ability to detect normal and abnormal infant responses (AAP & American Heart Association [AHA], 2006). After birth, if the infant’s condition permits and the mother is awake, the baby can be placed skin-to-skin on the mother or can be given to the woman’s partner to hold (Fig. 33-14). The infant whose condition is compromised is transported after initial stabilization to the nursery for observation and the implementation of appropriate interventions. In some institutions, the partner may accompany the infant; if not, personnel keep the family informed of the infant’s progress and parent-infant contacts are initiated as soon as possible.
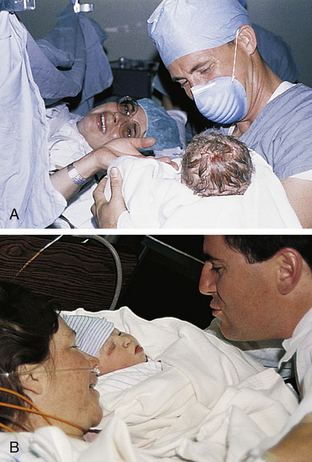
FIG. 33-14 A, Parents and their newborn. The physician manually removes the placenta, suctions the remaining amniotic fluid and blood from the uterine cavity, and closes the uterine incision, peritoneum, muscle layer, fatty tissue, and finally the skin, while the new family shares some time together. B, Parents become better acquainted with their newborn while mother rests after surgery. (Courtesy Marjorie Pyle, RNC, Lifecircle, Costa Mesa, CA.)
If family members cannot accompany the woman during surgery, they are directed to the surgical or obstetric waiting room. The physician then reports on the condition of the mother and infant to the family members after the birth is completed. Family members may be allowed to accompany the infant as she or he is transferred to the nursery, giving them an opportunity to see and admire the new baby.
Immediate Postoperative Care
Once surgery is completed, the mother is transferred to a postanesthesia recovery area. After a cesarean birth, women have postoperative and postpartum needs that must be addressed. They are surgical clients as well as new mothers. Nursing assessments in this immediate postbirth period follow agency protocol and include degree of recovery from the effects of anesthesia, postoperative and postbirth status, and degree of pain. A patent airway is maintained, and the woman is positioned to prevent possible aspiration. Vital signs are taken every 15 minutes for 1 to 2 hours, or until stable. The condition of the incisional dressing and the fundus and the amount of lochia are assessed, as well as the intravenous intake and the urine output through the retention (Foley) catheter. Oxytocin usually is added to at least the first liter of the intravenous infusion to ensure that the fundus remains firmly contracted, thereby reducing blood loss (Roberts & Mangan, 2009). The woman is helped to turn and do coughing, deep-breathing, and leg exercises. Medications for pain relief should be administered before postoperative pain becomes severe.
If the baby is present, the mother and her partner are given some time alone with him or her to facilitate bonding and attachment. Breastfeeding can be initiated if the mother feels like trying. The woman is ready for discharge from the postanesthesia recovery area once her condition is stable and the effects of anesthesia have worn off (i.e., she is alert and oriented and able to feel and move her extremities).
Postoperative or Postpartum Care
The attitude of the nurse and other health care team members can influence the woman’s perception of herself after a cesarean birth. The caregivers should stress that the woman is a new mother first and a surgical client second. This attitude helps the woman perceive herself as having the same problems and needs as other new mothers, while requiring supportive postoperative care.
The woman’s physiologic concerns may be dominated by pain at the incision site and pain resulting from intestinal gas. For the first 24 hours following surgery, pain relief may be provided by epidural opioids, patient-controlled analgesia (PCA) or intravenous or intramuscular injections. The most commonly used analgesics include opioids (e.g., hydromorphone, morphine, nalbuphine) and NSAIDs (e.g., ketorolac [Toradol]). If opioids are used, an antiemetic (e.g., metoclopramide [Reglan]) is often administered either as needed by the woman or around the clock as long as the opioid is used. Palpation of the fundus with the possibility of massage should be performed after an analgesic is given to decrease pain (Roberts & Mangan, 2009). By 24 hours after surgery, women are generally changed to oral analgesics. Other comfort measures such as position changes, splinting of the incision with pillows, and relaxation and breathing techniques (e.g., those learned in childbirth classes) may be implemented (see the Teaching for Self-Management box: Postpartum Pain Relief After Cesarean Birth).
Women are often the best judges of what their bodies need and can tolerate, including the postoperative ingestion of foods and fluids. Some health care providers keep women NPO or allow only “sips and chips” (sips of clear fluids and teaspoons of crushed ice) until bowel sounds return. The diet is then advanced to full liquids. After women are passing flatus they can resume a regular diet (Gilbert, 2011). Because most women have an epidural or spinal anesthetic for surgery, most health care providers allow the early introduction of solid food if desired and tolerated. Women who eat early have been found to require less analgesia, and gastrointestinal problems do not occur (Abrams, Minassian, & Pickett, 2004). Intravenous fluids are usually continued until the woman is tolerating fluids orally. Ambulation and rocking in a rocking chair may relieve gas pains. Avoid gas-forming foods, ice chips, carbonated beverages, and using a straw to drink beverages to help limit gas formation, thereby minimizing the severity of gas pains (see the Teaching for Self-Management box: Postpartum Pain Relief After Cesarean Birth).
Nurses must be alert to a woman’s physiologic needs, managing care to ensure adequate rest and pain relief. Mother-baby care (couplet care) for a cesarean birth mother may have to be modified according to her physical limitations as a surgical client.
Daily care includes perineal care, breast care, and routine hygienic care. The woman may shower after the original incisional dressing is removed, usually on the first postoperative day (if showering is acceptable according to the woman’s cultural beliefs and practices). The indwelling (Foley) catheter usually is also removed on the first postpartum day. The woman is encouraged to be out of bed and ambulating several times each day as soon as the urinary catheter is removed. The nurse assesses the woman’s vital signs, incision, fundus, and lochia according to hospital policies, procedures, or protocols. Breath sounds, bowel sounds, circulatory status of lower extremities, and urinary and bowel elimination patterns also are assessed. It is important to observe maternal emotional status and progress of attachment to her baby.
During the postpartum period, the nurse also can provide care that meets the psychologic and teaching needs of women who have had cesarean births. The nurse can explain postpartum procedures to help the woman participate in her recovery from surgery. The nurse can also help the woman plan care and visits from family and friends that will allow adequate rest periods. Providing information on and assistance with infant care can facilitate adjustment to her role as a mother. With adequate support, these women can benefit from mother-baby care to facilitate attachment and enhance involvement in newborn care. The woman is supported as she breastfeeds her baby by receiving individualized assistance to comfortably hold and position the baby at her breast. Use of the side-lying or football hold positions and supporting the newborn with pillows can enhance comfort and facilitate successful breastfeeding. The partner can be included in infant teaching sessions, and in explanations about the woman’s recovery (Simpson, 2008).
The couple also should be encouraged to express their feelings about the birth experience. Some parents are angry, frustrated, or disappointed that a vaginal birth was not possible. Some women express feelings of low self-esteem or a negative self-image. Others express relief and gratitude that the baby is healthy and safely born. It may be helpful for them to have the nurse who was present during the birth visit and help fill in “gaps” about the experience. Other psychologic and lifestyle concerns that have been reported include depression, feeling limited in activities, and changes in family interactions (Gamble & Creedy, 2004; Roberts & Mangan, 2009).
Discharge after cesarean birth is usually by the third postoperative day. The time is often determined by criteria established by the woman’s insurance carrier or the federal government (e.g., diagnosis-related groups [DRGs]). The Newborn’s and Mother’s Health Protection Act of 1996 provides for a length of stay of up to 96 hours for cesarean births. These criteria may not coincide with the woman’s physical or psychosocial readiness for discharge. Some states have added home care provisions for mothers who meet appropriate criteria for discharge and choose to leave sooner than the allowed length of stay. This policy recognizes that home care is less costly than hospital care and in most cases is more beneficial for recovery.
The nurse provides discharge teaching to prepare women for self-care and newborn care in a limited amount of time while still trying to ensure that the woman is comfortable and able to rest. The nurse must assess the woman’s information needs and coordinate the health care team’s efforts to meet them. Discharge teaching and planning should include information about nutrition; measures to relieve pain and discomfort; exercise and specific activity restrictions; time management that includes periods of uninterrupted rest and sleep; hygiene, breast, and incision care; timing for resumption of sexual activity and contraception; signs of complications (see the Teaching for Self-Management box: Signs of Postoperative Complications After Discharge Following Cesarean Birth); and infant care. The nurse assesses the woman’s need for continued support or counseling to facilitate her emotional recovery from the birth. The woman’s family and friends should be educated regarding her needs during the recovery process, and their assistance should be coordinated before discharge. Referral to support groups (e.g., www.birthrites.org) or to community agencies may be indicated to further promote the recovery process. A postdischarge program of telephone follow-up and home visits can facilitate the woman’s full recovery after cesarean birth.
Trial of Labor
A trial of labor (TOL) is the observance of a woman and her fetus for a reasonable period (e.g., 4 to 6 hours) of spontaneous active labor to assess the safety of vaginal birth for the mother and infant. It may be initiated if the mother’s pelvis is of questionable size or shape or if the fetus is in an abnormal presentation or position. By far the most common reason for a TOL is if the woman wishes to have a vaginal birth after a previous cesarean birth. A woman who has had a previous cesarean birth with a low transverse uterine incision may be a candidate for a TOL. Fetal sonography, maternal pelvimetry, or both may be done before a TOL to rule out CPD. During a TOL, the woman is evaluated for active labor, including adequate contractions, engagement and descent of the presenting part, and effacement and dilation of the cervix.
The nurse assesses maternal vital signs and FHR and pattern and is alert for signs of potential complications. If complications develop, the nurse is responsible for initiating appropriate actions, including notifying the primary health care provider, and for evaluating and documenting the maternal and fetal responses to the interventions. Nurses must recognize that the woman and her partner are often anxious about her health and well-being and that of their baby. Supporting and encouraging the woman and her partner and providing information regarding progress can reduce stress and enhance the labor process and facilitate a successful outcome.
Vaginal Birth After Cesarean
Indications for primary cesarean birth, such as dysfunctional labor, breech presentation, or fetal distress, often are nonrecurring. Therefore, a woman who has had one cesarean birth with a low transverse incision may subsequently become pregnant, experience no contraindications to labor and vaginal birth during the pregnancy, and choose to attempt a VBAC. Box 33-12 lists selection criteria suggested by the ACOG for identifying candidates for VBAC. The overall success rate is approximately 70% to 80% (Landon, 2007). Benefits of VBAC include a shorter maternal hospital stay, less blood loss, fewer infections, and fewer thromboembolic events than with cesarean birth. Risks associated with VBAC include uterine rupture, hysterectomy, operative injury, and neonatal morbidity (ACOG, 2004).
Spontaneous labor with a ripe cervix is more likely to result in a successful VBAC than is labor that has been induced or augmented (ACOG, 2004). Induction or augmentation with oxytocin or prostaglandins increases the risk for uterine rupture, a major concern when a VBAC is attempted. If uterine stimulation is needed, oxytocin is the drug of choice because the risk for rupture is lower (Simpson, 2008). Women most likely to have a successful VBAC are those who are less than 35 years of age, whose fetus weighs less than 4000 g, and whose previous cesarean was performed for some reason other than failure of descent in second stage labor (Thorp, 2009). Women are most often the primary decision makers with regard to choice of birth method. During the antepartal period, the woman should be given information about VBAC and encouraged to choose it as an alternative to repeat cesarean birth, as long as no contraindications exist. VBAC support groups (e.g., www.vbac.com) and prenatal classes can help prepare the woman psychologically for labor and vaginal birth. Women need to believe not only that their efforts during a TOL will be successful but also that they are fully capable of doing what is necessary to give birth vaginally. They must be given the opportunity to discuss their previous labor experience, including feelings of failure and loss of control, and to express concern they may have about how they will manage during their upcoming labor and birth. Not everyone is enthusiastic about TOL and VBAC. After being fully informed about the benefits and risks, more than 25% of potential candidates choose to have a repeat cesarean birth instead (Thorp, 2009).
If a woman chooses TOL, attention should be paid to her psychologic as well as physical needs during the TOL. Anxiety increases the release of catecholamines and can inhibit the release of oxytocin, thus delaying the progress of labor and possibly leading to a repeat cesarean birth. To alleviate such anxiety, the nurse can encourage the woman to use breathing and relaxation techniques and to change positions to promote labor progress. The woman’s partner can be encouraged to provide comfort measures and emotional support. Collaboration among the woman in labor, her partner, the nurse, and other health care providers often results in a successful VBAC. If a TOL does not result in vaginal birth, the woman will need support and encouragement to express her feelings about having another cesarean birth. It is very important that this outcome not be labeled a failed VBAC.
Since 1996, VBAC rates have been decreasing, with the current rate being less than10%. Both medical and nonmedical factors have contributed to this decline. In March 2010, the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH convened a consensus development conference to examine issues related to VBAC. The statement produced by the panel of experts affirmed that TOL is a reasonable option for many women who have had a previous cesarean birth (NIH, 2010a, 2010b).
The experts found, however, that many women who are appropriate candidates for TOL and VBAC do not have access to providers and health care facilities that are able and willing to offer this option. Two surveys of hospital administrators found that 30% of hospitals stopped offering VBAC because they could not meet all the prerequisites specified by ACOG (see Box 33-12). In addition, an ACOG member survey revealed that 30% of respondents stopped offering TOL and performing VBAC because of liability concerns. In order to remove some of the barriers to VBAC, the panel recommended that the VBAC guidelines published by professional associations be reevaluated, malpractice concerns be addressed, and additional research undertaken to better understand the medical and nonmedical factors that influence decision making for women who have had previous cesarean births (NIH, 2010a, 2010b).
Postterm Pregnancy, Labor, and Birth
A postterm pregnancy (also sometimes referred to as a postdate or prolonged pregnancy) is one that extends beyond the end of week 42 of gestation, or 294 days from the first day of the last menstrual period (LMP). The incidence of postterm pregnancy is estimated to be between 4% and 14% (Resnik & Resnik, 2009). Many pregnancies are misdiagnosed as prolonged. The use of first-trimester ultrasound for pregnancy dating has confirmed that the first day of the LMP, traditionally used for pregnancy dating, is much less reliable as a predictor of true gestational age. Therefore, use of the LMP alone for pregnancy dating tends to greatly overestimate the number of postterm gestations (Divon, 2007; Resnik & Resnik).
The exact cause of true postterm pregnancy is still unknown. However, it is clear that the timing of labor is determined by complex interactions among the fetus, the placenta and membranes, the uterine myometrium, and the cervix. For example, congenital primary fetal adrenal hypoplasia and placental sulfatase deficiency cause low estrogen production. Low levels of estrogen may result in a decrease in prostaglandin precursors, thereby preventing normal cervical ripening, reducing the formation of oxytocin receptors in the myometrium, and delaying the onset of labor. Although postterm pregnancy is more common in primiparous women, a woman who experiences one postterm pregnancy is more likely to experience it again in subsequent pregnancies (Divon, 2007; Resnik & Resnik, 2009).
Clinical manifestations of postterm pregnancy include maternal weight loss (more than 3 lb/wk) and decreased uterine size (related to decreased amniotic fluid), meconium in the amniotic fluid, and advanced bone maturation of the fetal skeleton with an exceptionally hard fetal skull (Gilbert, 2011).
Maternal and Fetal Risks
Maternal risks are often related to dysfunctional labor, such as increased risk for perineal injury related to fetal macrosomia. Risk for hemorrhage and infection is higher. Interventions such as induction of labor with prostaglandins or oxytocin, forceps- or vacuum-assisted birth, and cesarean birth are more likely to be necessary. Each of these interventions, of course, carries its own set of risks. The woman also may experience fatigue, physical discomfort, and psychologic reactions such as depression, frustration, and feelings of inadequacy as she passes her estimated date of birth. Relationships with close friends and family members may become strained and the woman’s negative feelings about herself may be projected as feelings of resentment toward the fetus (Gilbert, 2011).
Another complication associated with postterm pregnancy is abnormal fetal growth. Although the risk of having a small for gestational age infant is increased, only 10% to 20% of postterm fetuses are undernourished. Macrosomia (birth weight more than 4000 g) occurs far more often. Macrosomia occurs when the placenta continues to provide adequate nutrients to support fetal growth after 40 weeks of gestation. Macrosomic infants have an increased risk for birth injuries caused by difficult forceps-assisted births and shoulder dystocia (Resnik & Resnik, 2009).
Other fetal risks associated with postterm gestation are related to the intrauterine environment. After 43 to 44 weeks of gestation, the placenta begins to age. Enlarging areas of infarction and increased deposition of calcium and fibrin in its tissue decrease the placenta’s reserve and may affect its ability to oxygenate the fetus. Decreased amniotic fluid (less than 400 mL), oligohydramnios, is the complication most frequently associated with postterm pregnancy. Because of the decreased amount of amniotic fluid, there is a potential for cord compression and resulting hypoxemia (Gilbert, 2011). Other potential complications include meconium-stained amniotic fluid, increased chance of meconium aspiration, and low Apgar scores. Oligohydramnios magnifies the effect of meconium staining. Having less than the normal amount of amniotic fluid available to dilute it makes the meconium thicker and stickier than it would otherwise be (Resnik & Resnik, 2009).
Postmaturity syndrome occurs in about 20% of neonates born following postterm pregnancies. Postmaturity syndrome is characterized by dry, cracked, peeling skin; long nails; meconium staining of skin, nails, and umbilical cord; and perhaps loss of subcutaneous fat and muscle mass (Gilbert, 2011).
Care Management
The management of postterm pregnancy is still controversial. However, because perinatal morbidity and mortality increase greatly after 42 weeks of gestation, pregnancies are usually not allowed to continue after this time. In the United States, most physicians induce labor at 41 weeks of gestation. An alternative approach is to initiate twice-weekly fetal testing at 41 weeks of gestation. The testing generally consists of either a BPP or NST along with an assessment of amniotic fluid volume (see Chapter 26 for discussion of these tests). Evidence is insufficient to determine which of the two management approaches is better (Resnik & Resnik, 2009). ![]()
During the postterm period, the woman is encouraged to assess fetal activity daily, assess for signs of labor, and keep appointments with her primary health care provider (see the Teaching for Self-Management box: Postterm Pregnancy). The woman and her family should be encouraged to express their feelings (e.g., frustration, anger, impatience, fear) about the prolonged pregnancy and helped to realize that these feelings are normal. At times the emotional and physical strain of a postterm pregnancy may seem overwhelming. Referral to a support group or another supportive resource may be needed.
During labor the fetus of a woman with a postterm pregnancy should be continuously monitored electronically for a more accurate assessment of the FHR and pattern. Inadequate fluid volume can lead to compression of the umbilical cord, which results in fetal hypoxia that is reflected in variable or prolonged deceleration patterns. If oligohydramnios is present, an amnioinfusion may be performed to restore amniotic fluid volume to maintain a cushioning of the cord. See Chapter 18 for additional information on amnioinfusion.
Obstetric Emergencies
Meconium Stained Amniotic Fluid
Meconium-stained amniotic fluid indicates that the fetus has passed meconium (first stool) before birth. Meconium-stained amniotic fluid is green. The consistency of the meconium fluid is often described as either thin (light) or thick (heavy), depending on the amount of meconium present. Three possible reasons for the passage of meconium are as follows: (1) it is a normal physiologic function that occurs with maturity (meconium passage being infrequent before weeks 23 or 24, with an increased incidence after 38 weeks) or with a breech presentation; (2) it is the result of hypoxia-induced peristalsis and sphincter relaxation; or (3) it may be a sequel to umbilical cord compression–induced vagal stimulation in mature fetuses.
The major risk associated with meconium-stained amniotic fluid is the development of meconium aspiration syndrome (MAS) in the newborn. MAS causes a severe form of aspiration pneumonia that occurs most often in term or postterm infants who have passed meconium in utero. MAS most likely results from a long-standing intrauterine process, rather than from aspiration immediately following birth as respirations are initiated (Rosenberg, 2007).
Care Management
The presence of a team skilled in neonatal resuscitation is required at the birth of any infant with meconium-stained amniotic fluid. When meconium-stained amniotic fluid is present, the AAP and the AHA Neonatal Resuscitation Program no longer recommends routine suctioning of the newborn’s
mouth and nose on the perineum (after the head is out but before the rest of the baby is born) followed by endotracheal suctioning after birth. Instead, management of a newborn with meconium-stained amniotic fluid is based only on assessment of the baby’s condition at birth. No clinical studies warrant basing tracheal suctioning guidelines simply on meconium consistency (AAP & AHA, 2006). See the Emergency box: Immediate Management of the Newborn with Meconium-Stained Amniotic Fluid for specific interventions.
Shoulder Dystocia
Shoulder dystocia is an uncommon obstetric emergency that increases the risk for fetal and maternal morbidity and mortality during the attempt to accomplish birth vaginally. It is estimated that 0.6% to 1.4% of all vaginal births are complicated by shoulder dystocia (Cunningham et al., 2010). Shoulder dystocia is a condition in which the head is born, but the anterior shoulder cannot pass under the pubic arch. The incidence of shoulder dystocia has increased in recent years, perhaps because of larger birth weights or simply because more attention is now paid to documenting the condition (Cunningham et al.).
Fetopelvic disproportion related to excessive fetal size (more than 4000 g) or maternal pelvic abnormalities may be a cause of shoulder dystocia, although up to half of all cases of shoulder dystocia occur with smaller fetuses (Lanni & Seeds, 2007; Thorp, 2009). Other risk factors for shoulder dystocia include maternal diabetes (risk for macrosomia) and a history of shoulder dystocia with a previous birth. In half of all cases of shoulder dystocia, however, no risk factors are identified (Thorp). Shoulder dystocia cannot be accurately predicted or prevented (Cunningham et al., 2010). Signs that could indicate the presence of shoulder dystocia include slowing of the progress of the second stage of labor and formation of a caput succedaneum that increases in size. The nurse should observe for retraction of the fetal head against the perineum immediately following its emergence (turtle sign), an early sign of shoulder dystocia. External rotation does not occur (Jevitt, 2005; Thorp).
Fetal injuries are usually caused either by asphyxia related to the delay in completing the birth or to trauma from the maneuvers used to accomplish the birth. Complications related to trauma include brachial plexus and phrenic nerve injuries and fracture of the humerus or clavicle. The most serious complication is brachial plexus injury (Erb palsy), which occurs in 10% to 20% of infants born following shoulder dystocia. If brachial plexus injuries are recognized early and treated properly, 80% to 90% heal completely. Therefore, permanent neurologic injury is rare. The major maternal complications associated with shoulder dystocia are postpartum hemorrhage and rectal injuries (Thorp, 2009).
Care Management
Many maneuvers such as suprapubic pressure and maternal position changes have been suggested and tried to free the anterior shoulder, although no particular maneuver has been found to be most effective. Suprapubic pressure can be applied to the anterior shoulder (Fig. 33-15) in an attempt to push the shoulder under the symphysis pubis (Lanni & Seeds, 2007).
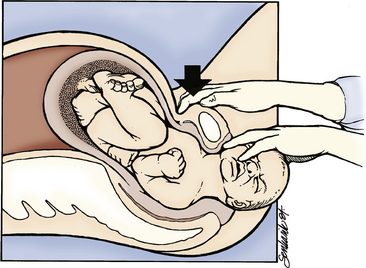
FIG. 33-15 Application of suprapubic pressure (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone).
In the McRoberts maneuver (Fig. 33-16), the woman’s legs are flexed apart, with her knees on her abdomen (Lanni & Seeds, 2007). This maneuver causes the sacrum to straighten, and the symphysis pubis to rotate toward the mother’s head. The angle of pelvic inclination is decreased, which frees the shoulder. Suprapubic pressure can be applied at this time. The McRoberts maneuver is the preferred method when a woman is receiving epidural anesthesia.
FIG. 33-16 McRoberts maneuver. (From Gabbe, S., Niebyl, J., & Simpson, J. [2007]. Obstetrics: Normal and problem pregnancies [5th ed.]. Philadelphia: Churchill Livingstone.)
Having the woman move to a hands-and-knees position (the Gaskin maneuver), a squatting position, or lateral recumbent position also has been used to resolve cases of shoulder dystocia (Jevitt, 2005). However, the Gaskin maneuver requires that the woman be mobile, with no significant loss of motor function caused by regional anesthesia. Additionally, a wide and stable surface must be available (Lanni & Seeds, 2007).
Fundal pressure as a method of relieving shoulder dystocia should be avoided. Its use has been associated with neurologic complications (Gilbert, 2011).
When shoulder dystocia is diagnosed, the nurse should stay calm and immediately call for additional assistance (i.e., extra nurses, anesthesia care provider, and neonatal resuscitation team). The nurse then helps the woman assume the position or positions that may facilitate birth of the shoulders, assists the primary health care provider with these maneuvers and techniques during birth, and documents the maneuvers. The nurse also provides encouragement and support to reduce anxiety and fear.
Newborn assessment should include examination for fracture of the clavicle or humerus as well as brachial plexus injuries and asphyxia (Thorp, 2009). Maternal assessment should focus on early detection of hemorrhage and trauma to the vagina, perineum, and rectum.
Prolapsed Umbilical Cord
Prolapse of the umbilical cord occurs when the cord lies below the presenting part of the fetus. Umbilical cord prolapse may be occult (hidden, rather than visible) at any time during labor whether or not the membranes are ruptured (Fig. 33-17, A and B). It is most common to see frank (visible) prolapse directly after rupture of membranes, when gravity washes the cord in front of the presenting part (see Fig. 33-17, C and D). Contributing factors include a long cord (longer than 100 cm), malpresentation (breech or transverse lie), or an unengaged presenting part.
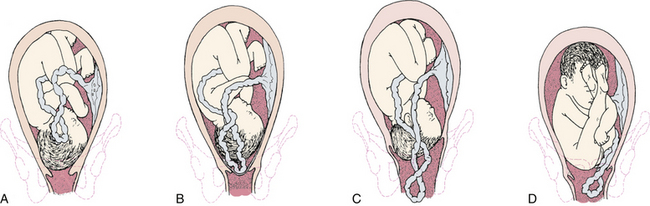
FIG. 33-17 Prolapse of umbilical cord. Note pressure of presenting part on umbilical cord, which endangers fetal circulation. A, Occult (hidden) prolapse of cord. B, Complete prolapse of cord. Note that membranes are intact. C, Cord presenting in front of the fetal head may be seen in vagina. D, Frank breech presentation with prolapsed cord.
If the presenting part does not fit snugly into the lower uterine segment (e.g., as in hydramnios), when the membranes rupture, a sudden gush of amniotic fluid may cause the cord to be displaced downward. Similarly the cord may prolapse during amniotomy if the presenting part is high. A small fetus may not fit snugly into the lower uterine segment; as a result, cord prolapse is more likely to occur.
Care Management
Prompt recognition of a prolapsed umbilical cord is important because fetal hypoxia resulting from prolonged cord compression (i.e., occlusion of blood flow to and from the fetus for more than 5 minutes) usually results in central nervous system damage or death of the fetus. Pressure on the cord may be relieved by the examiner putting a sterile gloved hand into the vagina and holding the presenting part off the umbilical cord (Fig. 33-18, A and B). The woman may also be assisted into a position such as a modified Sims (see Fig. 33-18, C), Trendelenburg, or knee-chest (see Fig. 33-18, D) position, in which gravity keeps the pressure of the presenting part off the cord. If the cervix is fully dilated, a forceps- or vacuum-assisted birth can be performed for the fetus in a cephalic presentation; otherwise, a cesarean birth is likely to be performed. Abnormal (nonreassuring) FHR and pattern (e.g., bradycardia, absent or minimal variability, and variable or prolonged decelerations), inadequate uterine relaxation, and bleeding also can occur as a result of a prolapsed umbilical cord. Indications for immediate interventions are presented in the Emergency box: Prolapsed Umbilical Cord. Ongoing assessment of the woman and her fetus is critical to determine the effectiveness of each action taken. The woman and her family are often aware of the seriousness of the situation; therefore, the nurse must provide support by giving explanations for the interventions being implemented and their effect on the status of the fetus.
FIG. 33-18 Arrows indicate direction of pressure against presenting part to relieve compression of prolapsed umbilical cord. Pressure exerted by examiner’s fingers in A, vertex presentation, and B, breech presentation. C, Gravity relieves pressure when woman is in modified Sims position with hips elevated as high as possible with pillows. D, Knee-chest position.
Rupture of the Uterus
Rupture of the uterus, in which there is complete nonsurgical disruption of all uterine layers, is a rare but very serious obstetric injury that occurs in 1 in 2000 births (Francois & Foley, 2007; Landon, 2007). During labor and birth the major
risk factor for uterine rupture is a TOL for attempted VBAC. Other risk factors include labor induction, multiple prior cesarean births or other types of uterine surgery, multiparity, and trauma (Francois & Foley). The likelihood of uterine rupture depends on both the type and location of the previous uterine scar. Uterine rupture occurs most often with a previous classic incision (Landon).
Uterine dehiscence, sometimes called incomplete uterine rupture, is separation of a prior scar. It may go unnoticed unless the woman undergoes a subsequent cesarean birth or other uterine surgery. The potential for maternal or fetal complications as a result of uterine dehiscence is negligible because separation of a prior scar does not result in hemorrhage (Landon, 2007).
Signs and symptoms vary with the extent of the uterine rupture. The most common finding is an abnormal (nonreassuring) FHR tracing, including variable and late decelerations, bradycardia, and absent or minimal variability. A loss of fetal station may also occur. The woman may experience constant abdominal pain, uterine tenderness, a change in uterine shape, and cessation of contractions (Francois & Foley, 2007). She may also exhibit signs of hypovolemic shock caused by hemorrhage (i.e., hypotension, tachypnea, pallor, and cool, clammy skin). If the placenta separates, the FHR will be absent. Fetal parts may be palpable through the abdomen.
Care Management
Prevention is the best treatment. Women who have had a classic cesarean birth are advised not to labor or attempt vaginal birth in subsequent pregnancies. Those at risk for uterine rupture are assessed closely during labor. Women whose labor is induced with oxytocin or prostaglandin (especially if their previous birth was cesarean) are monitored for signs of uterine tachysystole because this can precipitate uterine rupture. If tachysystole occurs, the oxytocin infusion is discontinued or decreased, and a tocolytic medication may be given to decrease the intensity of the uterine contractions (see the Emergency box: Uterine Tachysystole with Oxytocin). After giving birth, the woman is assessed for excessive bleeding, especially if the fundus is firm and signs of hemorrhagic shock are present.
If rupture occurs, management depends on the severity. A small rupture may be managed with a laparotomy and birth of the infant, repair of the laceration, and blood transfusions, if needed. Hysterectomy may be necessary if the rupture is large and difficult to close or if the woman is hemodynamically unstable (Francois & Foley, 2007).
The nurse’s role can include starting intravenous fluids, transfusing blood products, administering oxygen, and assisting with the preparation for immediate surgery. Supporting the woman’s family and providing information about the treatment are important during this emergency. The associated fetal mortality rate is high (approximately 50% to 70%). Maternal morbidity and mortality also can be substantial (Cunningham et al., 2010). Providing information about spiritual support services or suggesting that the family contact their own support system may be warranted.
Anaphylactoid Syndrome of Pregnancy
Anaphylactoid syndrome of pregnancy (ASP), also known as amniotic fluid embolism, is a rare but devastating complication of pregnancy characterized by the sudden, acute onset of hypoxia, hypotension, or cardiac arrest, and coagulopathy. ASP occurs during labor, during birth, or within 30 minutes after birth. This combination of sudden respiratory and cardiovascular collapse, along with coagulopathy, is similar to that observed in clients with anaphylactic or septic shock. In both conditions, a foreign substance is introduced into the circulation, resulting in disseminated intravascular coagulation, hypotension, and hypoxia (Martin & Foley, 2009).
In ASP the foreign substance that initiates the condition is presumed to be present in amniotic fluid that is introduced into the maternal circulation. However, the exact factor that initiates ASP has not been identified. In the past, particles of fetal debris (e.g., vernix, hair, skin cells, or meconium) found in amniotic fluid were thought to be responsible for initiating the syndrome; however, fetal debris can be found in the pulmonary circulation of most normal laboring women. Also fetal debris is identified in only 78% of women diagnosed with ASP. Although ASP is rare, the mortality rate is 61% or higher (Martin & Foley, 2009). About 50% of the neonates who survive have neurologic impairment (Schoening, 2006).
Maternal risk factors for ASP include advanced age, minority race, placenta previa, preeclampsia, and forceps-assisted or cesarean birth. Other factors commonly associated with the
development of ASP are rapid labor and meconium staining (Cunningham et al., 2010).
Care Management
The immediate interventions for ASP are summarized in the Emergency Box: Anaphylactoid Syndrome of Pregnancy. Care must be instituted immediately. Cardiopulmonary resuscitation is often necessary. If cardiopulmonary arrest occurs, for optimal fetal survival, a perimortem cesarean birth should be accomplished within 5 minutes (Martin & Foley, 2009). The nurse’s immediate responsibility is to assist with the resuscitation efforts.
If the woman survives, she is usually moved to a critical care unit. Additional interventions will likely include replacing blood and clotting factors and maintaining adequate hydration and blood pressure. The woman is usually placed on mechanical ventilation. Invasive hemodynamic monitoring may also be required (see Chapter 31) (Martin & Foley, 2009).
Support of the woman’s partner and family is needed; they will be anxious and distressed. Brief explanations of what is happening are important during the emergency and can be reinforced after the immediate crisis is over. If the woman dies, emotional support and involvement of the perinatal loss support team or other resource for grief counseling is needed. Referral to grief and loss support groups would be appropriate (see Chapter 38). The nursing staff also may need help in coping with feelings and emotions that result from a maternal death.
KEY POINTS
• Preterm labor is uterine contractions with cervical change (e.g., effacement and dilation) that occurs between 20 weeks and 37 weeks of pregnancy; preterm birth is any birth that occurs before the completion of 37 weeks of pregnancy.
• The cause of preterm labor is unknown and is assumed to be multifactorial; therefore, it is not possible to predict with certainty which women will experience preterm labor and birth.
• Because the onset of preterm labor is often insidious and can be mistaken for normal discomforts of pregnancy, nurses should teach all pregnant women how to detect the early symptoms of preterm labor and to call their primary health care provider when symptoms occur.
• Bed rest, a commonly prescribed intervention for preterm labor, has many deleterious side effects and has never been shown to decrease preterm birth rates.
• The best reason to use tocolytic therapy is to achieve sufficient time to administer glucocorticoids in an effort to accelerate fetal lung maturity and reduce the severity of respiratory complications in infants born preterm. Additionally, time is allowed for transport of the woman prior to birth to a center equipped to care for preterm infants.
• If fetal or early neonatal death is expected, the parents and members of the health care team need to discuss the situation before the birth and decide on a management plan that is acceptable to everyone.
• Vigilance for signs of infection is an essential component of the care management for women with preterm PROM.
• Dysfunctional labor results from differences in the normal relations among any of the five factors affecting labor and is characterized by differences in the pattern of progress in labor.
• Obese women are at risk for several pregnancy complications, including venous thromboembolism and cesarean birth. Even routine procedures require more time and effort to accomplish when the client is obese.
• Uterine contractility is increased by the effects of oxytocin and prostaglandin and is decreased by tocolytic agents.
• Cervical ripening using chemical or mechanical measures can increase the success of labor induction.
• Expectant parents benefit from learning about operative obstetrics (e.g., forceps-assisted, vacuum-assisted, or cesarean birth) during the prenatal period.
• The basic purpose of cesarean birth is to preserve the life or health of the mother and her fetus.
• Unless contraindicated, vaginal birth is possible after a previous cesarean birth.
• Labor management that emphasizes one-to-one support of the laboring woman by another woman (e.g., doula, nurse, nurse-midwife) can reduce the rate of cesarean birth and increase the VBAC rate.
• A postterm pregnancy poses a risk to the mother and the fetus.
• Obstetric emergencies (e.g., meconium-stained amniotic fluid, shoulder dystocia, prolapsed cord, rupture of the uterus, and anaphylactoid syndrome of pregnancy) occur rarely but require immediate intervention to preserve the health or life of the mother and fetus or newborn.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()