Obstetric Critical Care
• Discuss factors that have contributed to the development of the specialty of critical care obstetrics.
• Describe conditions that may place a pregnant woman in a critically ill state.
• Examine factors that affect the provision of obstetric critical care when a pregnant woman becomes critically ill.
• Describe significant cardiovascular, pulmonary, and hematologic alterations during pregnancy that affect critical care for the pregnant woman.
• Describe the parameters measured and state normal values for pulmonary artery and arterial pressure monitoring.
• Identify treatment strategies based on interpretation of hemodynamic profiles.
• Identify physiologic alterations of pregnancy that affect stabilization and treatment of the pregnant woman who has undergone trauma.
• Describe immediate assessment and stabilization measures for the pregnant victim of trauma.
• Compare components of the primary and secondary surveys for the pregnant woman who has undergone trauma.
• Discuss inclusion of the components of family-centered maternity care for the critically ill pregnant woman.
• Examine the effect of maternal death on families and nursing staff members who cared for the woman.
Obstetric and critical care units are equally challenged whenever presented with the multiple, complex needs of a critically ill pregnant woman and her fetus. Optimal outcome for mother and fetus depends on: (1) swift recognition of severe complications, and (2) delivery of critical care therapies adjusted for the physiologic alterations of pregnancy. Fetal effects of therapies also must be considered. Management of the critically ill pregnant woman includes assessing maternal and fetal status continuous, selecting therapeutic interventions appropriate for mother and fetus, and carefully choosing the timing of birth.
Providing critical care and hemodynamic monitoring for the seriously ill pregnant woman has developed slowly in many institutions because of two factors: (1) obstetric nurses and physicians, expert in the care of pregnant women, can feel threatened by pressure transducers, alarms, hemodynamic monitoring, and ventilators; and (2) critical care nurses and physicians, expert in hemodynamic monitoring and mechanical ventilation, can feel threatened by the pregnant uterus, labor, birth, the fetus, and fetal monitoring. The result is that few institutions have been able to provide optimal care whenever a sudden, acute, life-threatening complication has occurred in a pregnant woman.
Obstetric Intensive Care Unit
Critical care obstetrics evolved as a subspecialty of perinatal medicine in response to the need for optimal care for the critically ill pregnant woman and her fetus. This subspecialty prepares the obstetric team, which has in-depth knowledge of pregnancy, to use critical care techniques in the management of the critically ill pregnant woman and her fetus. Some centers have developed obstetric intensive care units (OBICUs) so that specific equipment and individuals with special training and expertise in obstetric care and critical care are available to provide this care.
Complications may develop during pregnancy that are so severe and life threatening that optimal maternal and fetal outcome, and many times survival, depends on the woman receiving critical care that meets her specific needs. Maternal adaptations that are normal for the pregnancy state alter physiologic status and make the pregnant woman hemodynamically different from the nonpregnant woman.
Before the development of OBICUs, care for the critically ill pregnant woman was usually provided in an ICU for adults, where management modalities were based on hemodynamic values that are normal for the nonpregnant individual but resulted in less than desirable outcomes for pregnant women. Research in some of the first OBICUs reported on physical and hemodynamic differences during the pregnant state. Normal hemodynamic values for pregnancy were identified. Maternal and fetal outcomes improved when management of care was based on the enhanced hemodynamic state that accompanies normal pregnancy and care was provided in the specialized units.
Anyone providing care for pregnant women may encounter the pregnant woman with a life-threatening complication and be challenged to recognize the need for immediate, critical care and to provide such care. This care may be delivered in a variety of ways. Problems can be decreased by developing a viable plan to provide care for the critically ill pregnant woman, adequately educating nursing, medical, and ancillary staff, providing necessary equipment at the bedside, and encouraging frequent consultation between the obstetric and critical care units.
The most practical, efficient, and economic method to provide care for the critically ill obstetric client who requires invasive hemodynamic monitoring, mechanical ventilation, or both depends primarily on the numbers of pregnant women cared for annually and the referral patterns in a specific facility. The ideal method to provide care for the pregnant woman is a specially trained team of obstetricians and obstetric nurses in an OBICU, augmented by anesthesiologists, pulmonologists, cardiologists, and intensivists. A larger tertiary center is more likely to have this type of unit because the census of pregnant women cared for annually in the referral center would support development of the service. OBICUs are often small and may consist of one bed. Admissions may be limited only to the very sickest women and may not include all women eligible for a bed in the OBICU.
However, even in many large, tertiary centers, the number of truly critically ill pregnant women is not large enough to warrant such an investment in equipment and training of medical and nursing staff. In these centers, collaborative practice between obstetrics and intensive care has been successful in providing the best care for these women (Zeeman, 2006). If the woman remains pregnant, the optimal place for her is the labor and birth unit, with a critical care nurse in attendance. The American College of Obstetricians and Gynecologists (ACOG, 2009) reports that 75% of admissions to the ICU occur in the postpartum period, after consideration of the fetal condition and management decisions regarding birth are no longer a concern. However, there are still situations before birth in which the ICU is the best site for this collaborative care. In this instance, obstetric practitioners serve a vital role in monitoring the woman as well as assessing the fetus. The institution must develop policies and procedures so that care is provided where it is most advantageous. Some institutions provide dual training for selected nurses (i.e., an ICU nurse receives advanced education in obstetrics or an obstetric nurse receives advanced education in intensive care nursing).
In still other institutions, an obstetric service may be established to provide care during the low risk pregnancy. The plan for the smaller unit or the low risk unit may be for the nursing and medical team to recognize the critical illness immediately, stabilize the woman, and initiate measures for transport to the tertiary center and/or the OBICU. The Standards and Guidelines for Professional Nursing Practice in the Care of Women and Newborns (Association of Women’s Health, Obstetric and Neonatal Nurses [AWHONN], 2009) includes guidelines for the care of the pregnant woman requiring critical care. Obstetric clients requiring critical care are at risk for undesirable pregnancy outcomes because of the severity of complications, including decreased oxygen transport and multiple system organ failure and the possibility of long-term residual physical effects of the illness.
Equipment and Expertise
The usual equipment for a labor, birth, and recovery suite is mandatory for an ICU or surgical suite where a woman may be admitted for care. Staff and equipment for neonatal resuscitation must also be available. The usual equipment for intensive care, including hemodynamic monitoring and mechanical ventilation, is equally necessary whenever the critically ill woman is in the labor and birth unit.
A severe complication of pregnancy may prompt the need for obstetric critical care with hemodynamic monitoring or mechanical ventilation. See Box 31-1 for a list of complications that indicate a need for critical care.
The number of pregnant women who need obstetric critical care has increased in recent years as the result of many factors: women are surviving childhood illnesses because of advances in pediatric care which include the development of pediatric ICUs; improved surgical procedures for infants with congenital defects, such as cardiac lesions; and advanced knowledge in pediatric care for children with chronic health problems, including diabetes, cystic fibrosis, and pulmonary disorders. The desire to become a parent is not eliminated by a chronic health problem, and numerous women each year risk their lives to have a baby. Many women have had successful pregnancies after kidney transplants (Davison & Bailey, 2003), after thoracic organ transplant such as heart, lung and heart and lung (Wu, Wilt & Restaino, 2007), and after liver transplants (Christopher, Al-Chalabi, Richardson, Muiesan, Rela, Heaton, et al., 2006). Most transplant clients do very well with pregnancy, provided the new organ is functioning well. Another reason for the increase in critically ill pregnant women is that more are waiting to become pregnant at a later age, thus increasing the occurrence of heart disease and other age-related factors.
Other pregnant women need critical care because of trauma resulting from motor vehicle crashes or violence and battering. These account for most of the traumatic injuries during pregnancy. There has been an increase in the number of women with respiratory compromise as a result of the H1N1 flu outbreak because pregnant women are more susceptible to the virus and also become sicker than women in the same age-group who are not pregnant. Pregnant women were at increased risk for severe complications such as pneumonia, acute respiratory distress syndrome (ARDS), and even death from the 2009 H1N1 influenzavirus (ACOG, 2009).
Since 2004, there has been little new information written about the care of critically ill pregnant women and critical care obstetrics in the United States. Admission of an obstetric client to the intensive care unit occurs in approximately 1% to 3% of pregnant women requiring critical care services (40,000 to 120,000/year) (ACOG, 2009). Zeeman (2006) reported that the most common diagnosis for admission to an OBICU is severe preeclampsia with complications including refractory pulmonary edema, refractory oliguria, hypertensive crisis, severe hemorrhage or disseminated intravascular coagulation (DIC), and renal failure. Massive hemorrhage or DIC is reported as the second most common reason for admission. Reports from other developed countries report similar incidences of ICU admission with regard to frequency and diagnosis (Anwari, Butt, & Al-Dar, 2004; Baskett & O’Connell, 2009; Keizer, Zwart, Meerman, Harinck, Feuth, & Roosmalen, 2006; Saravanakumar, Davies, Lewis, & Cooper, 2008; Selo-Ojeme, Omosaiye, Battacharjee, & Kadir, 2005).
Conditions that classify the parturient as critically ill and indicate the need for a pulmonary artery catheter include the following (ACOG, 1992):
• Sepsis with refractory hypotension or oliguria
• Unexplained or refractory pulmonary edema, congestive heart failure, or oliguria
• Severe preeclampsia with pulmonary edema or refractory oliguria
• Intraoperative or intrapartum cardiovascular decompensation
• Massive blood loss or volume replacement needs
• Chronic disease, particularly when associated with labor or major surgery
The identification of women needing OBICU services merits careful consideration. It is important not to visualize just the “sickest client scenario”—that dramatic case, never to be forgotten. Instead the typical picture of a critically ill pregnant woman is one with severe preeclampsia, whose condition has worsened and now has headache, high blood pressure (BP), oliguria, and low platelets. Or the woman may have been referred from a level I center with a preexisting cardiac lesion exacerbated by pregnancy that has gradually deteriorated from class I to class III cardiac disease. Her presenting complaint may be “feeling extremely tired.”
Physiologic Changes in Pregnancy
The normal physiologic adaptations that accompany pregnancy and produce profound hemodynamic changes are the primary factors that make the pregnant woman a different type of critical care client and merit a separate critical care facility. Knowledge of the effects of the physiologic alterations during pregnancy is essential for optimal critical care management. Normal maternal alterations during pregnancy affect the major systems: cardiovascular, pulmonary, renal, and hematologic (Norwitz, Robinson, & Malone, 2004).
Cardiovascular Changes
The cardiovascular system changes dramatically during pregnancy. Because it is a state of high flow and low resistance, pregnancy is hemodynamically similar to early sepsis. Hypervolemia is the result of the influence of estrogen and progesterone on aldosterone, which produces an increase in circulating blood volume. The expansion in maternal blood volume begins with a 22% increase by 8 weeks of gestation and progresses to a maximum increase of 45% by 32 to 34 weeks of gestation. This represents an increase of approximately 1570 ml for a singleton gestation and includes a 40% to 50% increase in plasma volume and a 20% to 30% increase in red blood cell mass. This disproportional increase results in a state of hemodilution. An increase in total body water of 6 to 8 L in the extravascular compartment, accompanied by an accumulation of 500 to 900 mEq of sodium, also occurs during pregnancy. Heart rate (HR) increases 20% (10 to 15 beats/min) with the major increase in the third trimester, and stroke volume increases to accommodate the increased circulating volume. See Table 31-1 for a summary of cardiovascular adaptations.
TABLE 31-1
CARDIOVASCULAR CHANGES DURING PREGNANCY
| PARAMETER | CHANGE |
| Blood volume | 40%-50% increase |
| Plasma volume | 40%-50% increase (1200-1300 ml) |
| Red blood cell mass | 20%-30% increase (250-450 ml) |
| Heart | Displaced to the left and upward |
| Point of maximal intensity (PMI) | Fourth intercostal space and lateral |
| Rate | 20% increase (10-15 beats/min) |
| Sounds | Exaggerated splitting first sound |
| Systolic murmur usually present | |
| Third sound present | |
| Stroke volume | 32% increase by 20-24 wks |
| Cardiac output | Increases by 30%-50% |
| 22% increase by 28 wks | |
| 43% increase by term | |
| Increases during labor | |
| ≤3 cm, 17% increase | |
| 4-7 cm, 23% increase | |
| ≥8 cm, 34% increase |
Source: Norwitz, E., Robinson, J., & Malone, F. (2004). Pregnancy-induced physiologic alterations. In G. Dildy, M. Belfort, G. Saade, J. Phelan, G. Hankins, & S. Clark (Eds.), Critical care obstetrics (4th ed.). Malden, MA: Blackwell Science.
Colloid Osmotic Pressure
Colloid osmotic pressure (COP) is the gradient controlling whether fluid remains inside the capillary or moves into the interstitial space. The force to keep the fluid inside the vessel is the pulling pressure of the colloids, or proteins, present in the plasma. The most important plasma proteins are albumin, globulin, and fibrinogen. Pregnancy produces a decrease in COP values resulting from the hemodilutional state that reduces the concentration of plasma proteins. Severe preeclampsia usually produces renal damage, with a subsequent loss of proteins in the urine, further reducing the COP. The force exerted to push fluids through the membrane is the capillary hydrostatic pressure and is measured as the pulmonary capillary wedge pressure (PCWP). Colloid osmotic (oncotic) values in pregnancy are shown in Table 31-2. Though not routinely evaluated and not available in many institutions, knowledge of this key concept is helpful in understanding one of the basic differences between pregnant and nonpregnant women. Pulmonary edema tends to develop in pregnant women at a lower PCWP than in those who are not pregnant and have a normal COP.
TABLE 31-2
COLLOID OSMOTIC (ONCOTIC) PRESSURE VALUES
| Nonpregnant | 25.4 ± 2.3 mm Hg |
| Pregnant, antepartum | 22.4 ± 0.54 mm Hg |
| Pregnant, postpartum | 15.4 ± 2.1 mm Hg |
| Preeclampsia, antepartum | 17.9 ± 0.68 mm Hg |
| Preeclampsia, postpartum | 13.7 ± 0.46 mm Hg |
The lower the COP and the higher the PCWP, the more likely it is for pulmonary edema to develop, as reflected by a lower COP to PCWP gradient. The gradient is the difference between the COP and the PCWP. COP to PCWP gradient values are as follows: nonpregnant, 14.5 ± 2.5; pregnant, 10.5 ± 2.7.
Pulmonary edema is more likely to develop in pregnancy. For example, the normal PCWP is 6 to 10 mm Hg during pregnancy and 4 to 9 mm Hg in the nonpregnant state (Clark, Cotton, Lee, Bishop, Hill, Southick, et al., 1989). With a PCWP of 8, calculations of the COP-PCWP gradient show:
• Nonpregnant woman: COP of 25 – PCWP of 8 = 17 mm Hg
• Pregnant woman with severe preeclampsia: COP of 13 – PCWP of 8 = 5 mm Hg
Lower COP to PCWP gradients during pregnancy are usually caused by lower COP values occurring during pregnancy.
Respiratory Changes
Anatomic and physiologic alterations during pregnancy are necessary to adequately oxygenate the mother and fetus. A relative hyperventilation of pregnancy begins in the first trimester and increases 42% by term. Respiratory rate increases only slightly. Tidal volume and minute ventilations increase approximately 50% by term to meet increased oxygen consumption needs. The enlarging uterus pushes the diaphragm upward approximately 4 to 7 cm, reducing lung volume. Compensation is necessary to meet increased ventilatory demands, and the transverse diameter of the thorax increases 2 to 4 cm as the rib cage flares out. The functional residual capacity decreases 25% because more of the inhaled air is used, resulting in decreased reserve. Pregnant women become short of breath easily, as seen in walking or light exercise. This can become critical if the oxygen demands of the pregnant woman increase. The hyperventilation of pregnancy is associated with a resting arterial carbon dioxide tension of less than 30 mm Hg. Maternal alkalosis is prevented by the compensatory decrease in serum bicarbonate of about 4 mEq/L, from 26 to 22 mEq. During gestation respiratory acidosis and metabolic acidosis develop more rapidly than in the nonpregnant state.
Normal arterial blood gas (ABG) values for pregnancy reflect a chronic state of compensated respiratory alkalosis, represented by a right shift in the oxyhemoglobin dissociation curve caused by the increased levels of 2,3-diphosphoglycerate from the high progesterone and estrogen levels present. Normal ABG values for pregnant women as compared to those for nonpregnant women are listed in Table 13-3, p. 299.
Hematologic Changes
Pregnancy is a hypercoagulable state, as preparation is made for the blood loss that accompanies childbirth.Table 13-3, p. 299, gives a summary of alterations that enhance coagulation.
Bleeding and clotting times remain unchanged even when hypervolemia and hemodilution are present. The critically ill pregnant woman is at increased risk for thrombus formation whenever hemoconcentration develops, as occurs with severe preeclampsia or dehydration.
Systemic Vascular Resistance
Systemic vascular resistance (SVR) is a measure of the tension required for the ejection of blood into the circulation (afterload). To describe the physiologic relations between pressure and flow, measurements are made by the following formula as a ratio of pressure to flow:
where MAP is mean arterial pressure (in millimeters of mercury), CVP is central venous pressure (in millimeters of mercury), and CO is cardiac output (in liters per minute).
Vasodilation of arterial vessels, a result of hormonal influences, and development of the uteroplacental circulation result in a decrease in SVR of 20% to 25% and a decrease in pulmonary vascular resistance of 40% during pregnancy. Blood pressure, especially diastolic pressure, decreases during pregnancy, reaching the nadir at 24 to 32 weeks of gestation, with a gradual return to prepregnancy values by term (Blackburn, 2007).
Hemodynamic Monitoring
Anatomic and Physiologic Characteristics of Circulation
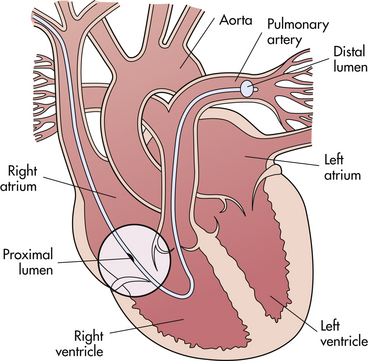
An in-depth knowledge of normal functioning and hemodynamics of the cardiovascular system is the basis for understanding hemodynamic monitoring; therefore, a review of these functions is included. The purpose of the cardiopulmonary system is to deliver oxygenated blood to the tissues throughout the body and to remove waste products through the dynamics of normal circulation as follows (Fig. 31-1).
Deoxygenated blood flows from the capillaries into the veins and the right side of the heart through the superior vena cava, draining the upper part of the body, and from the inferior vena cava, draining the lower part of the body. Venous blood flows into the right atrium, a holding chamber for the right side of the heart. When the atrium is filled, the tricuspid valve opens, and blood flows through this valve into the right ventricle.
When the right ventricle is filled, its muscles (myocardium) contract, and blood is ejected through the pulmonic valve into the pulmonary artery. Blood is pushed through the pulmonary artery and its branches to the capillary beds (pulmonary beds) in both lungs. Gas exchange occurs in the capillary beds as carbon dioxide is released and oxygen enters the circulation across the alveolar membrane.
Oxygenated blood drains from the pulmonary beds into the pulmonary veins, two from each lung, into the left atrium, the holding chamber for the left side of the heart. When the left atrium is filled, the mitral valve opens, and blood flows through this valve into the left ventricle. After the left ventricle is filled, the muscles of the left ventricle contract, and the oxygenated blood is ejected through the aortic valve into the aorta and then pumped throughout the systemic arterial circulation so that oxygen is supplied to the organs and tissues of the body.
Synchronization by the electrical conduction system of the myocardium causes the right and left atrial and ventricular contractions to occur simultaneously. The period of the cardiac cycle when both ventricles are relaxed and filling is termed diastole, and the period of the cardiac cycle when both ventricles contract is termed systole.
Left ventricle contractions must generate enough force to pump blood throughout the systemic circulation. Force required for right ventricular work is less because the right ventricle has to exert only enough force for blood to flow through the pulmonary circulation. Therefore, the left ventricle is referred to as the hemodynamic ventricle, and the left side of the heart is referred to as the hemodynamic heart. Hemodynamic monitoring with a pulmonary artery catheter provides left heart values, and during a critical illness, values of left heart function are more significant than are values of right heart function (Fig. 31-1).
Cardiac Output
Cardiac output (CO) is the volume of blood ejected from the left ventricle in 1 minute; it is measured in liters per minute. CO is the product of stroke volume (SV), the volume of blood ejected from the left ventricle during one cardiac cycle, and HR; thus CO = HR × SV. Because HR and SV increase during pregnancy, CO increases. The normal prepregnancy range of CO is 3 to 5 L/min. CO increases 40% to 50% during pregnancy, to produce a normal CO in the last trimester of 6 to 7 L/min at rest. Labor produces an additional 40% increase in CO because of catecholamine release in response to pain perception and the shunting of blood from the placental-fetal unit with uterine contractions, for a CO range during labor of 8 to 10 L/min. CO increases further after birth because of significant hemodynamic fluctuations that reflect the net effect of blood loss at birth and the autotransfusion with approximately 1000 ml of blood that occurs after the uterus is emptied. Soon after birth the accumulated 6 to 8 L of extravascular fluid is mobilized into the intravascular compartment. The large increase in CO remains for 7 to 10 days after birth in the healthy woman but will continue longer if the usual diuresis fails to occur because of a complication.
Positional Changes
Maternal CO in the last trimester is position dependent. Clark and colleagues (1991) showed the effect of maternal position on CO output (Table 31-3).
TABLE 31-3
CARDIAC OUTPUT IN RELATION TO MATERNAL POSITION
| Knee-chest | 6.9 L/min (±2.1) |
| Right lateral | 6.8 L/min (±1.3) |
| Left lateral | 6.6 L/min (±1.4) |
| Sitting | 6.2 L/min (±2.0) |
| Supine | 6.0 L/min (±1.4) |
| Standing | 5.4 L/min (±2.0) |
The right or left lateral recumbent position provides the optimal CO for the critically ill pregnant woman. Whenever maternal CO is decreased, compensatory mechanisms are initiated. The first compensatory response is to shunt blood away from the peripheral circulation to the central circulation, to save the heart and brain. Peripheral circulation includes circulation to the skin, the renal system, the gastrointestinal system, the lung beds, and the reproductive system. Enhancing maternal CO, therefore, enhances fetal perfusion.
Cardiac Output Determinants
The four determinants of CO are reflected in the calculation for cardiac output (CO = HR × SV) because SV is the result of preload, afterload, and contractility.
Preload is defined as the volume of blood in the ventricles at the end of diastole; it is determined by intraventricular pressure and volume. Right preload is assessed by right atrial or CVP, and left preload is assessed by PCWP. The volume of blood in the ventricles stretches the myocardial muscle fibers and produces the intraventricular pressure. Measurements are made at end diastole, the time immediately preceding systole when the ventricles reach maximal stretch because of the extra amount of blood delivered into the ventricles when the tricuspid and mitral valves snap closed.
Right preload reflects the blood circulating through the right side of the heart, and left preload reflects the amount of blood circulating in the left side of the heart. The two sides of the heart are not equal when cardiac or pulmonary complications are present; therefore, they are measured separately.
Preload must be adequate to maintain CO, and plotting of CO against preload gives a cardiac function curve (Fig. 31-2). As preload increases, CO increases up to the point of failure. The cardiac function curve shows that a heart in failure requires a higher preload than the healthy heart to produce the same CO. Bedside manipulations of preload are possible with continuous hemodynamic monitoring to determine effects on CO. A low preload can be increased by the administration of fluids, including crystalloids, colloids, or blood, and by positioning with legs elevated. A high preload can be decreased by the administration of a vasodilator or diuretic or by phlebotomy and upright positioning.
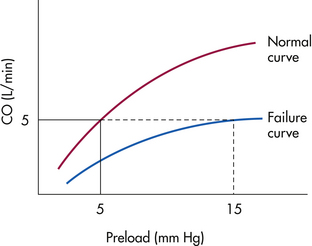
FIG. 31-2 Relation of preload to cardiac output. Ventricular function (Starling) curve for heart, showing both normal function and during failure.
Afterload is defined as the ventricular wall tension during systole, or the resistance the blood meets as blood is ejected from the ventricles. Afterload is dependent on the end-diastolic radius of the ventricle, the aortic pressure, and the thickness of the ventricle wall. As afterload increases, CO decreases. Bedside manipulation of afterload is possible to achieve optimal cardiac output (Fig. 31-3). Right afterload is assessed by the pulmonary vascular resistance (PVR), and left afterload is assessed by the SVR. Arterial BP measurement does not give as accurate an indication of left ventricular work as the SVR but is used clinically as reflecting left afterload. Therefore, control of the woman’s blood pressure is used to control left afterload.
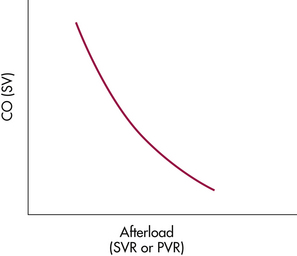
FIG. 31-3 Relation of afterload to cardiac output when preload is maintained constant. As afterload increases, cardiac output decreases.
Afterload must be adequate for circulation and CO; extremes of afterload may decrease CO. Increased left afterload occurs with hypertensive disease caused by the systemic arterial vasoconstriction. Increased right afterload occurs with pulmonary hypertension resulting from vasoconstriction in the pulmonary circulation.
Increased afterload can be corrected by the administration of vasodilator drugs. Hydralazine is commonly used as the first-line drug for hypertension. It is an arterial vasodilator that is usually effective and safe for mother and fetus. As an added bonus, the majority of obstetric personnel are comfortable with its use. However, others believe that the calcium channel blockers (such as nifedipine, orally) and the beta-blockers (such as labetalol) cause less hypotension (Magee & von Dadelszen, 2009) (see Table 27-5, p. 666). Sodium nitroprusside is rarely used in pregnancy because it produces cyanide as a metabolite; fetal cyanide toxicity must be a concern if this drug is administered during pregnancy. Of paramount importance is the correction of hypovolemia before administration of any antihypertensive to prevent acute hypotension.
Severe vasodilation and loss of arterial resistance with a decreased afterload impedes venous return of blood to the right side of the heart and decreases CO. This situation is seen with septic shock. Decreased afterload can be corrected by fluid administration to fill the vascular space or by the administration of vasopressor medications such as dopamine or dobutamine (Martin & Foley, 2009). Contractility (inotropic state of the heart) is defined as the force and velocity of ventricular contractions when preload and afterload are held constant. Contractility is governed by the Frank-Starling law, which states that the greater the length of the muscle fibers before contraction, the greater will be the contraction of the fibers, up to the point of failure. Fiber length is related to the maximal stretch of preload, because as more blood volume enters the ventricles, the more the fibers in the myocardium stretch to accommodate the increased volume.
Decreased contractility results in decreased CO. The first response to correct this problem is bedside manipulation to optimize both preload and afterload. If this fails to increase CO to the desired level, medications to increase myocardial contractility are indicated. Inotropic drugs such as dopamine hydrochloride or dobutamine are administered. Digitalis therapy also may be necessary.
Heart rate is the fourth determinant of cardiac output. The rate at which the ventricles fill and contract affects CO. Extremes of HR may decrease CO.
Sustained tachycardia can decrease CO as a result of myocardial ischemia or the decreased time for adequate filling of the ventricles during diastole and shortened systolic ejection times. The cause, such as fever, hypoxia, pain, hypovolemia, or hyperthyroidism, should be determined and treated. Drugs to correct tachycardia are seldom necessary for obstetric clients. However, propranolol, digoxin, or calcium channel blockers such as verapamil are effective agents to decrease HR if needed.
Bradycardia can compromise CO when the number of ventricular contractions per minute that occur are inadequate to deliver the circulating volume needed to perfuse and oxygenate the body. If treatment is necessary for this problem, atropine or cardiac pacing is used.
Invasive Hemodynamic Monitoring
Invasive hemodynamic monitoring provides continuous measurements of preload, afterload, myocardial contractility, and HR in the critically ill pregnant woman so that therapeutic manipulations may be made quickly at the bedside in response to changes in client status. Monitoring may be done by use of a pulmonary artery catheter (PAC) (a balloon-tipped, multilumen catheter) or by CVP.
Although few adequate clinical studies demonstrate the benefit of pulmonary artery catheterization for the critically ill client, most critical care bedside clinicians use the PAC to direct their therapy modalities. They believe that use of the PAC does improve outcomes in selected critically ill clients (Fujitani & Baldisseri, 2005).
Use of a PAC allows the management of care to be based on immediate recognition of changes in hemodynamic values from the left ventricle. Immediate information is obtained, calculations are made, and management is adjusted quickly as needed. Results of therapeutic strategies can be calculated and evaluated (ACOG, 1992). The continuous hemodynamic measurements obtained will reinforce therapies in use or show that therapy should be changed. Use of a PAC in combination with an arterial pressure catheter and a pulse oximeter provides adequate data to assess the cardiac, fluid, and pulmonary status continuously. Indications for the use of invasive hemodynamic monitoring are the same in obstetrics as in any other area of medicine.
It is essential to evaluate risks and benefits of any procedure before its use, especially because there are risks associated with invasive techniques. The information obtained from hemodynamic monitoring is essential for management of critical, complex cases and is unavailable by other means; thus the benefits outweigh the risks in most cases. The overall complication rate in the obstetric population is low, approximately 1%. This low rate of complications is related to three factors: (1) the pregnant woman usually needs the device because of an acute event, (2) the duration of use is usually short, and (3) the majority of pregnant women requiring its use are young and healthy before the acute event.
It is essential that meticulous attention be paid to each step and detail of all procedures to decrease problems with the technique itself. The rate of complications associated with PAC insertion decreases as the experience level of the clinician increases. Therefore, only properly trained personnel should insert catheters for invasive hemodynamic monitoring (Martin & Foley, 2009).
Although the PAC remains the gold standard for measuring hemodynamic status in the critically ill client, transesophageal echocardiography (TEE) is sometimes used as a noninvasive bedside method for assessing the hemodynamic status of nonpregnant adults. A few studies in obstetric clients have found that CO measured with TEE correlates well with CO measured by PAC. More research is needed, but TEE may prove useful in the care of critically ill pregnant women (Martin & Foley, 2009).
Pulmonary Artery Catheter
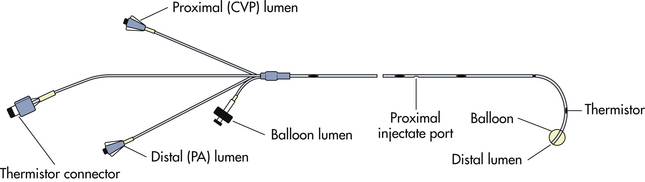
A PAC, commonly called the Swan-Ganz catheter, provides continuous measurements of pulmonary artery pressure (PAP) and right atrial pressure (RAP) or CVP. Intermittent measurements of PCWP and CO also are possible. The standard flow-directed thermodilution PAC has three lumina and a thermistor connector (Fig. 31-4). The distal lumen or port is located in the pulmonary artery after insertion. It is connected to a transducer with a heparinized pressure line to measure a continuous PAP when the balloon is deflated and intermittent PCWP when the balloon is inflated. A continuous flush of 3 ml/hr of heparinized solution maintains patency of the lumen.
The proximal lumen or port exits approximately 30 cm from the tip of the catheter and is located in the right atrium after insertion. This port also is connected to a transducer with a heparinized line and can be used to measure continuous RAP, which is comparable to the CVP, or to administer fluid or drugs. Both the proximal and distal lumina of the catheter can be used to withdraw blood samples for laboratory studies.
The balloon lumen ends in a small latex balloon, located 0.5 inch from the tip of the PAC. Inflation of the balloon is used to assist in the insertion of the catheter and to obtain PCWP readings.
The thermodilution port is connected to a thermistor, a temperature sensor, located 5 cm proximal to the tip of the PAC. The thermistor continuously measures the temperature of the blood in the pulmonary artery.
Other types of PACs are available in addition to the standard PAC. Some have an extra right atrial port for intracardiac infusions. The small size of the ports makes them more effective for the administration of vasopressors or drugs such as antibiotics, than for the rapid administration of large volumes of blood or fluids. A fiberoptic PAC includes a sensor that can continuously measure the hemoglobin saturation of mixed venous blood, the Svo2, in the pulmonary artery and is useful in cases of decreased oxygen transport, such as with preeclampsia and eclampsia.
Venous Access
The internal or external jugular vein or the subclavian vein is most commonly used for venous access for invasive hemodynamic monitoring during pregnancy. The right internal jugular vein is usually the preferred site because it offers the shortest and most direct entry into the right heart (Martin & Foley, 2009). Access with femoral or antecubital veins is not used as frequently for pregnant women because of the greater difficulty in positioning the catheter when this route is accessed. Furthermore, with use of a vein in the inguinal area, birth of the infant at a critical time can limit access to and manipulation of the catheter.
The flow-directed PAC is usually inserted at the bedside after preparations are complete. Meticulous attention to detail is critical when the equipment is prepared. Flushing the pressure tubing to eliminate any air, establishing a zero reference point, and both zeroing and calibrating the pressure transducer are done carefully before insertion. See the Procedure box that explains how to set up a pressure line and how to zero-reference and calibrate a pressure transducer. Box 31-2 describes nursing care during insertion and continuing care for a woman with a PAC.
Hemodynamic monitoring systems have three major components: (1) a pressure transducer that converts physiologic pressures into electrical energy, (2) an amplifier to magnify the volume of the signal being measured, and (3) a monitor screen to display in digital and graphic form the converted physiologic signal (Fig. 31-6).
Waveforms and Pressure Readings
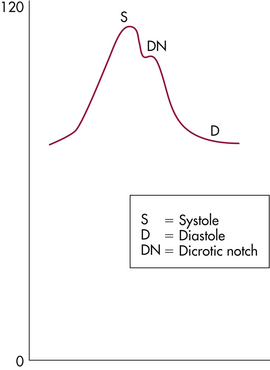
The basis of interpretation of assessments obtained by hemodynamic monitoring is to understand the relation to cardiovascular status of the waveforms observed on the monitor screen and the pressure readings obtained.
Specific chambers in the heart have different pressures that are reflected by changing waveform patterns, and placement of the PAC is evaluated through the pressure and waveform changes that appear on the monitor screen to reflect the position of the catheter. Figure 31-7 displays pressure waveforms in different chambers of the heart.
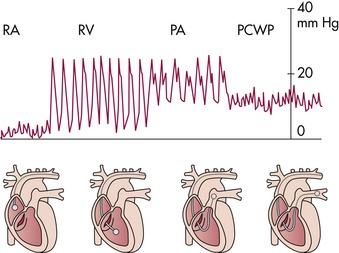
FIG 31-7 Pressure waveform in relation to catheter position from right atrium (RA), to right ventricle (RV), to pulmonary artery (PA), to pulmonary capillary wedge pressure (PCWP).
Because the right atrium is a holding chamber with relatively small muscle mass, the waveform pattern for this chamber has a low amplitude. Right atrium pressures reflect intravascular volume and compliance of the right ventricle. Mean right atrium pressures in pregnancy are relatively low, 0 to 7 mm Hg.
When the catheter is inserted into the right ventricle, pressures change, and a distinct spiking waveform appears. The low-amplitude waveform of the right atrium converts to a high-amplitude waveform with distinct systolic and diastolic components in the right ventricle, with a baseline pressure of 0 mm Hg. Right ventricular pressures are measured as systolic and diastolic. Normal right ventricle systolic pressure is 18 to 30 mm Hg, and the normal diastolic pressure is 0 to 7 mm Hg.
The catheter is then advanced into the pulmonary artery. This is reflected by a different spiking waveform with baseline pressures greater than 0 mm Hg. Pulmonary artery systolic pressures are equal to the systolic pressures in the right ventricle, but pulmonary artery diastolic pressures abruptly increase to the range of 6 to 10 mm Hg.
The catheter advances through the pulmonary artery as far as possible and becomes “wedged” in the vessel. This wedging is reflected by a distinct waveform of a dampened tracing with respiratory variation. This relatively low amplitude reflects the low pressures in the capillary beds of the lungs.
The PCWP is obtained when the balloon is inflated, with all pressures from the right side of the heart obstructed, so that the distal port now reads pressures from the left side of the heart across the lungs, because there are no valves in the pulmonary circulation. The PCWP measures left atrial filling pressures or left-sided preload. During right ventricular diastole, the pulmonic valve is closed, with the mitral valve open; the diastolic PAP is measured. In the absence of a problem, such as mitral valve disease or pulmonary edema, PAP diastolic readings reflect PCWP or left ventricular preload. The diastolic PAP is therefore used clinically to reflect left preload (Darovic, 2002). See the Procedure box that explains how to obtain a PCWP reading.
The normal PCWP during pregnancy is 6 to 10 mm Hg. Pressures higher than 20 mm Hg are usually caused by abnormal left ventricular performance, such as left ventricular failure, mitral valve stenosis, or fluid volume overload. Lower than normal readings are seen usually with hypovolemia (see Fig. 31-7).
Box 31-3 describes nursing measures for managing selected problems that may arise with a PAC.
Arterial Pressure Catheter
Percutaneous arterial catheterization, in which a Teflon intravenous catheter, usually 20 gauge, is placed in an artery and connected to a hemodynamic monitor by a pressure line, provides
continuous measurements of the systolic, diastolic, and mean arterial blood pressures. The arterial pressure catheter produces a waveform and provides access for ABG sampling and analysis.
Indications for use of this type of hemodynamic monitoring include situations in which frequent and accurate BP measurements are needed, such as the administration of potent drugs (e.g., dopamine to treat septic shock or nitroprusside to treat severe hypertensive disease), or when frequent ABG determinations are needed. Characteristics of desirable arteries for an arterial line are: (1) a vessel that has a diameter large enough for accurate measurement of pressure without occlusion of the artery by the catheter, (2) adequate collateral circulation, (3) ease of access to the site for care, and (4) a site not prone to infection. The most common vessels used in the pregnant woman are the radial, axillary, and pedal arteries, in that order.
In preparation for insertion of the arterial line into the radial artery, explain the procedure to the woman in simple terms. Obtain her informed consent and perform Allen’s test to confirm collateral circulation into the hand by demonstrating a patent ulnar artery. Box 31-4 describes the steps to perform Allen’s test.
The most common risk associated with an intraarterial line is infection. More serious but less common risks include hemorrhage, thrombus formation, and embolization. The heparinized continuous flush solution of the pressure line helps prevent thrombus formation. Once the line is in place, a transparent occlusive dressing is applied. The transducer is then rezeroed, and an armboard is used to prevent movement of the wrist.
The hemodynamic monitor displays an arterial waveform and the systolic, diastolic, and MAPs for the health care team to interpret. Normal versus abnormal waveforms must be recognized at the bedside, and immediate intervention must be available if indicated. The normal waveform should include the following (Fig. 31-8):
A blood pressure taken with a sphygmomanometer should be ascertained periodically to verify accuracy of the hemodynamic monitor.
Continuing assessments and documentation include the following:
Pressure Lines
All pressure lines use a specialized high-pressure tubing to transmit the physiologic signal to the transducer and monitor. The pressure tubing is rigid to prevent the dampening or absorption of pressure itself, so that the physiologic pressures are transmitted directly from the catheter tip to the transducer through the fluid that fills the length of the tubing. The line includes a continuous flushing mechanism to ensure patency (see Fig. 31-5).
Data Collection
Continuous measurements of the CVP and PAPs and intermittent PCWPs are obtained from the PAC. CO is also calculated intermittently by the use of a PAC with the thermodilution technique (see the Procedure box that explains how to make and record hemodynamic assessments). Electrocardiographic (ECG) monitoring permits continuous evaluation of HR and rhythm. Newer ECG monitors with sensitive leads connected to the chest wall also monitor respiratory rate. Systemic arterial BP can be evaluated by a manual or an automatic sphygmomanometer or by an arterial pressure line. Placement of an arterial
pressure line also permits ready access for frequent arterial blood sampling and analysis, especially for ABGs.
The PCWP is reported as a mean value, determined by the average of its maximal and minimal deflections on the monitor screen or oscilloscope. Reading values from the oscilloscope is usually adequate for clinical management at the bedside; however, for complex cases, strip chart recordings are recommended (see the Procedure box on p. 745 that explains how to make and record hemodynamic assessments).
When the thermodilution procedure to calculate CO in a pregnant woman is performed, the injectate should be chilled to obtain an accurate reading because of the high COs normal for pregnancy (Wallace & Winslow, 1993). The thermistor at the tip of the PAC measures the speed with which the blood temperature cools and returns to normal after the chilled fluid is injected. The time required for the temperature changes is computed, and a CO readout is given. See the Procedure box that describes the thermodilution procedure for measuring CO.
Oxygenation
Oxygen delivery to and use by the peripheral tissues must be adequate. Determination of oxygen transport is essential in the care of the critically ill pregnant woman. Oxygen delivery is directly proportional to CO; if CO decreases 50%, oxygen delivery decreases 50%. Conversely, increasing CO 50% doubles oxygen delivery. Oxygen delivery also can be improved by increasing the hemoglobin value, which can be accomplished by the administration of red blood cells.
Sao2 Monitoring: Oxygen is transported to the tissues in two ways: dissolved in plasma and bound to hemoglobin. The oxygen dissolved in plasma (Pao2) makes up only 1% to 2% of the total oxygen content, whereas the oxygen bound to hemoglobin (Sao2) makes up 98% to 99% of the total oxygen content. Results of arterial blood gas determination reflect the dissolved oxygen according to the Pao2 value. The Pao2 reflects the partial pressure that oxygen exerts when it is dissolved in blood; it is
measured in millimeters of mercury. The normal Pao2 for the pregnant state is 100 to 106 mm Hg. The adequacy of maternal oxygenation for delivery to the fetus can be evaluated by the usual methods of fetal well-being assessment such as electronic fetal monitoring or biophysical profile. The partial pressure of the oxygen tension is important because circulation transports the higher Pao2 blood to organs with a lower Pao2, and gas exchange occurs as gases move from the higher concentration to the lower concentration. The higher tension (Pao2) pushes the oxygen molecule off the hemoglobin molecule through the cell membrane so that tissues receive a supply of oxygen.
The oxygen that cells can use is the oxygen molecules bound to hemoglobin (Sao2). Each molecule of hemoglobin has four binding sites for oxygen. When all four sites are bound, an oxyhemoglobin molecule results. The saturation of hemoglobin with oxygen (Sao2) is evaluated with the pulse oximeter (Sao2 monitoring). The normal range for Sao2 is 95% to 100%, and normal Sao2 values for pregnancy are 97% to 100%. Critically ill pregnant women benefit from continuous Sao2 monitoring.
Svo2 Monitoring: The percentage of saturation of hemoglobin with oxygen in mixed venous and arterial blood is reflected as an Svo2 value. Svo2 monitoring involves insertion of a fiberoptic PAC that is connected to a bedside microprocessor to give a continuous Svo2 value. Mixed venous blood saturation reflects the balance between oxygen delivery and oxygen use. It reflects tissue perfusion, the variation in oxygen requirements for different organs, and the affinity of hemoglobin to accept and then release oxygen. The normal value of Svo2 is 60% to 80%. Values less than 60% are interpreted as abnormally low.
Fiberoptic PACs and bedside microprocessors provide the technology to plot the mixed venous blood oxygen saturation continuously. Plotting of mixed venous oxygen content is used as an early warning system because values may decrease before any other evidence of hemodynamic instability is seen (Fujitani & Baldisseri, 2005).
Use of pulse oximetry permits evaluation of the Sao2, arterial blood saturation. Arterial blood usually has a saturation of 90% or greater. Because of the shape of the oxyhemoglobin dissociation curve, fluctuations at the high levels of oxygen tension, 90% and higher, are reflected by a small change in arterial oxygen saturation (Sao2). However, the lower levels of oxygen tension in mixed venous blood (40% or less) produce a linear relation between saturation and tension, resulting in the Svo2 becoming a sensitive alarm to detect physiologic instability.
Arterial blood analysis evaluates pulmonary oxygen exchange and ventilation but does not evaluate overall adequacy of oxygen delivery to the peripheral tissues. However, mixed venous blood analysis (Svo2) reflects the end product of supply and demand and is used to evaluate overall adequacy of oxygen delivery to the peripheral tissues. This technique provides additional data and can be especially useful to support or change management strategies when used in conjunction with thermodilution CO calculations.
Continuous monitoring of Svo2 is recommended for titration of vasoactive or inotropic drugs, during adjustments of positive end-expiratory pressure, evaluation of fluid administration, and routine care of critically ill pregnant women.
Central Venous Pressure Lines
Before the development of the flow-directed PAC in the early 1970s, CVP lines were used for the critically ill. Two primary problems with the use of CVP lines became apparent. First, CVP monitoring provides right-sided heart information only, the right preload. When a cardiac or pulmonary complication is present, right- and left-sided values are not equal. CVP monitoring gives no information about left ventricular function. Second, changes in CVP values occur much later with left ventricular dysfunction. This type of monitoring does not permit rapid assessment of the effects of treatment modalities. Additionally, several studies reported disparities in the relationship between CVP and PCWP in preeclampsia (Martin & Foley, 2009). Considering that the risks for insertions are similar, when invasive hemodynamic monitoring is required, use of the PAC is preferred.
Interpretation of Hemodynamic Data
Data obtained with hemodynamic monitoring techniques include values that reflect blood volume in the pulmonary and systemic circulations, which profoundly affect cardiac performance, in the following ways:
• Right atrial pressure or CVP indicates right end-diastolic pressure and reflects right preload.
• PAP: PVR is the calculation for right afterload. Clinically, mean PAP reflects right afterload.
• Diastolic PAP approximates PCWP and reflects left preload.
• PCWP indicates left end-diastolic pressure and reflects left preload.
• CO refers to the volume of blood ejected from the left ventricle in liters per minute.
• SVR is the calculation for left afterload; arterial BP reflects left afterload.
Hemodynamic profiles provide valuable data for evaluating the status of the woman and changing treatment strategies as needed. Normal hemodynamic values in pregnancy to be used for calculations in the cases described in the following material are shown in Table 31-4. Table 31-5 compares normal nonpregnant hemodynamic values with normal values during pregnancy.
TABLE 31-4
NORMAL HEMODYNAMIC VALUES IN PREGNANCY
| Right atrial pressure (RAP) or central venous pressure (CVP) | 0-7 mm Hg |
| Pulmonary artery pressure (PAP) | 18-30 mm Hg (systolic) |
| 6-10 mm Hg (diastolic) | |
| Pulmonary capillary wedge pressure (PCWP) | 6-10 mm Hg |
| Cardiac output (CO) | 6-7 L/min |
| Systemic vascular resistance (SVR) | 1210 ± 266 |
| Pulmonary vascular resistance (PVR) | 78 ±22 |
TABLE 31-5
CENTRAL HEMODYNAMIC NORMAL VALUES
| PARAMETER | NONPREGNANT | PREGNANT |
| CO (L/min) | 4.3 ± 0.9 | 6.2 ± 1 |
| HR (beats/min) | 71 ± 10 | 83 ± 10 |
| SVR (dynes/cm/sec-5) | 1530 ± 520 | 1210 ± 266 |
| PVR (dynes/cm/sec-5) | 119 ± 47 | 78 ± 22 |
| PCWP (mm Hg) | 6.3 ± 2.1 | 7.5 ± 1.8 |
| CVP (mm Hg) | 3.7 ± 2.6 | 3.6 ± 2.5 |
| Left ventricular work index | 41 ± 8 | 48 ± 6 |
Source:Clark, S., et al. (1989). Central hemodynamic assessment of normal term pregnancy. American Journal of Obstetrics and Gynecology, 16(6), 1439-1442.
Oliguria is diagnosed if urine output is less than 20 to 30 mL for 2 consecutive hours. It may indicate severe renal dysfunction (Roberts & Funai, 2009). If oliguria is not corrected with fluid challenge, the woman is diagnosed as having oliguria refractory to conservative therapy.
Refractory oliguria caused by preeclampsia may be associated with three different hemodynamic subsets: hypovolemia, hypervolemia, and renal artery spasm. Treatment strategies for correction of oliguria are different for each hemodynamic subset. Noninvasive assessments do not differentiate the type of treatment that would be appropriate for the pregnant woman. Recognition of which hemodynamic subset has produced the refractory oliguria is obtained only by the use of a PAC. Failure to use a PAC when refractory oliguria is present can result in inappropriate treatment.
Examples of three different pregnant women with preeclampsia complicated by refractory oliguria are included in Box 31-5 to demonstrate how useful hemodynamic monitoring is in determining the appropriate care for the individual woman with this complication. These three women, all with severe preeclampsia with similar noninvasive assessment findings, illustrate the need for use of a PAC when the pregnant woman with hypertensive disease and oliguria fails a fluid challenge test. Noninvasive parameters do not reflect volume status. Completely different treatment modalities to correct oliguria are indicated for each of these three women.
Pulmonary Edema: One of the most common uses of the PAC during pregnancy is the differentiation of cardiogenic (heart failure or hydrostatic) pulmonary edema from noncardiogenic (permeability or lung failure) pulmonary edema (Mabie, 2004). Optimal therapies for the two types of pulmonary edema are dramatically different; however, the correct
diagnosis can be determined only by evaluation of the hemodynamic profile.
Cardiogenic or heart failure pulmonary edema develops as a result of left ventricular failure or acute fluid overload. The PCWP is elevated because of the increased volume of fluid in the pulmonary circulation. Therapy is focused on improvement of myocardial contractility with an inotropic drug, decreasing left afterload, if elevated, with an arterial vasodilator and reducing preload to a normal range with a diuretic drug. This type of pulmonary edema usually responds to therapy within a few hours, and a normal PCWP is restored.
Pulmonary edema also may result from damage to the pulmonary alveolar capillary membrane by numerous factors, the most common being sepsis. Disturbance of membrane permeability results in the leakage of both protein and fluid into the pulmonary interstitium and alveoli, in the presence of normal cardiac function and ventricular filling pressures (Mabie, 2004). The PCWP is normal. Alveolar membranes require days to heal, and ARDS may develop if the source of injury is not found and eradicated. The focus of therapy is to maintain the PCWP in the low-normal range to minimize transudation of protein and fluid into the lung and to eliminate the source of injury.
When radiography reveals pulmonary edema, evaluation of the hemodynamic profile is the only way to correctly diagnose the type of pulmonary edema present, as illustrated in the following two hemodynamic profiles (refer to Table 31-4 for the normal values):
The high wedge pressure reflects a high left preload and correlates with cardiogenic pulmonary edema. Treatment includes an inotropic drug such as dobutamine, a vasodilator such as hydralazine if hypertension is present, and a diuretic such as furosemide (Lasix) because CO is adequate.
All readings are in the normal range, although pulmonary edema is present. This correlates with noncardiogenic pulmonary edema. A diuretic should not be administered. Preload is in the low-normal range. Reducing the preload with a diuretic drug could decrease CO and jeopardize the woman’s status. Instead, sepsis should be suspected, and treatment should focus on antibiotic therapy and elimination of foci of infection, continuing assessments, and support of vital systems while lung membranes heal.
Trauma During Pregnancy
Trauma continues to be a common complication during pregnancy because of the continuation of usual activities by the majority of pregnant women in the United States.
Significance
Approximately 8% of pregnancies have been reported to be complicated by physical trauma (Brown, 2009). As pregnancy progresses, the risk of trauma seems to increase because more cases of trauma are reported in the third trimester than earlier in gestation. Most maternal injuries are a result of motor vehicle accidents and falls. Other sources of trauma include intimate partner violence, assaults, and suicide attempts (Martin & Foley, 2009).
Trauma is the leading nonobstetric cause of maternal mortality (Brown, 2009). Motor vehicle accidents account for more than 50% of maternal trauma incidents. About 50% of fetal deaths are associated with maternal trauma, and most of these are due to motor vehicle accidents. Maternal death caused by trauma is usually the result of head injury or hemorrhagic shock. Fetal death usually occurs as a result of maternal death or because of placental abruption (abruptio placentae). Fortunately the majority of trauma injuries during pregnancy are minor and have no effect on pregnancy outcome. However, each case of trauma during pregnancy must be evaluated carefully because pregnancy can mask signs of severe injury.
Multisystem trauma during pregnancy is usually the result of a serious motor vehicle crash, especially if the woman is not wearing a seat belt with a shoulder harness and is ejected from the vehicle. To improve chances of survival for mother and fetus, pregnant women should wear properly positioned restraints at all times when in a motor vehicle (see Fig. 15-17) (Cunningham, Leveno, Bloom, Hauth, Rouse, & Spong, 2010).
The effect of trauma on pregnancy is influenced by the length of gestation, type and severity of the trauma, and degree of disruption of uterine and fetal physiologic features. Trauma increases the incidence of preterm labor and birth, placental abruption, and fetal or neonatal death (Martin & Foley, 2009). Other common fetal effects of trauma include premature rupture of membranes (PROM), fetomaternal transfusion, skull injuries, and hypoxia because of maternal respiratory compromise. Trauma results in fetal death more often than in maternal death (Gilbert, 2011).
Special considerations for mother and fetus are necessary when trauma occurs during pregnancy because of the physiologic alterations that accompany pregnancy and because of the presence of the fetus. Fetal survival depends on maternal survival; therefore, the pregnant woman must receive immediate stabilization and appropriate care for optimal fetal outcome.
Maternal Physiologic Characteristics
Optimal care for the pregnant woman after trauma is dependent on understanding the physiologic state of pregnancy and its effects on trauma. The pregnant woman’s body will exhibit responses different from those of a nonpregnant person to the same traumatic insults. Because of the different responses to injury during pregnancy, management strategies must be adapted for appropriate resuscitation, fluid therapy, positioning, assessments, and most other interventions. Significant maternal adaptations and the relation to trauma are summarized in Table 31-6.
TABLE 31-6
MATERNAL ADAPTATIONS DURING PREGNANCY AND RELATION TO TRAUMA
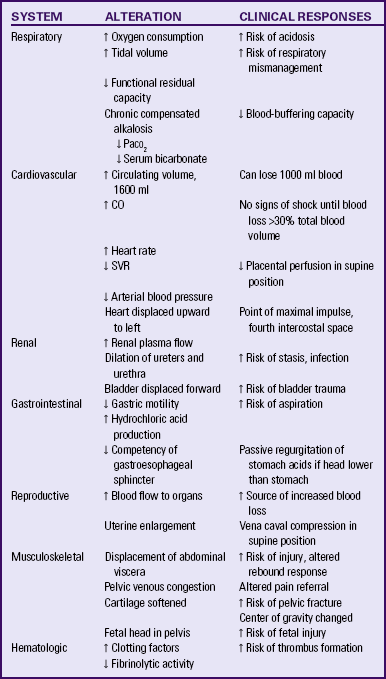
CO, Cardiac output; Paco2, arterial partial pressure of carbondioxide; SVR, systemic vascular resistance.
The uterus and bladder are confined to the bony pelvis during the first trimester of pregnancy and are at reduced risk for injury in cases of abdominal trauma. After pregnancy progresses beyond the fourteenth week, the uterus becomes an abdominal organ, and the risk for injury in cases of abdominal trauma increases. During the second and third trimesters, the distended bladder becomes an abdominal organ and is at increased risk for injury and rupture. Bowel injuries occur less often during pregnancy because of the protection provided by the enlarged uterus.
The elevated levels of progesterone that accompany pregnancy relax smooth muscle and profoundly affect the gastrointestinal tract. Gastrointestinal motility decreases, with a resultant increased time required for gastric emptying, whereas the production of hydrochloric acid increases in the last trimester, and the gastroesophageal sphincter relaxes (Norwitz et al., 2004). Airway management of the unconscious pregnant woman is of critical importance.
A pregnant woman has decreased tolerance for hypoxia and apnea because of her decreased functional residual capacity and increased renal loss of bicarbonate. Acidosis develops more quickly in the pregnant than in the nonpregnant state.
CO increases 44% to 50% over prepregnancy values and is position dependent in the third trimester. Because of compression of the inferior vena cava and descending aorta by the pregnant uterus, CO will decrease dramatically if the woman is placed in the supine position. The supine position must be avoided, even in women with cervical spine injuries. It is a primary priority that lateral uterine displacement be accomplished without any head movement. As soon as the neck is immobilized, the stretcher should be tilted laterally.
Circulating blood volume increases 40% to 50% during gestation, and pregnant women can tolerate a 1000 ml blood loss readily without demonstrating clinical signs. Hemodynamic instability that indicates the need for transfusion may not be apparent until blood loss exceeds 1500 ml (Martin & Foley, 2009). Clinical signs of hemorrhage do not appear until after a 30% loss of circulating volume occurs. Although HR increases with pregnancy, a maternal HR greater than 100 beats/min should be considered abnormal.
Fetal Physiologic Characteristics
Perfusion of the uterine arteries, which provide the primary blood supply to the uteroplacental unit, depends on adequate maternal arterial pressure, because these vessels lack autoregulation. Therefore maternal hypotension decreases uterine and fetal perfusion. Maternal shock results in splanchnic and uterine artery vasoconstriction, which decreases blood flow and oxygen transport to the fetus. Electronic fetal monitoring (EFM) tracings can assist in the evaluation of maternal status after trauma. EFM tracings reflect fetal cardiac responses to hypoxia and hypoperfusion, including tachycardia or bradycardia, minimalor absent baseline variability, and late decelerations.
Careful monitoring of fetal status assists greatly in maternal assessment, because the fetal monitor tracing works as an “oximeter” of internal maternal well-being. Hypoperfusion may be present in the pregnant woman before the onset of clinical signs of shock. The EFM tracings show the first signs of maternal compromise, such as when maternal HR, BP, and color appear normal, yet the EFM printout shows signs of fetal hypoxia (Murray, 2007).
Mechanisms of Trauma
Blunt abdominal trauma is most commonly the result of motor vehicle crashes but also may be the result of battering or falls. Maternal and fetal mortality and morbidity rates are directly correlated with whether the mother remains inside the vehicle or is ejected. Maternal death is usually the result of a head injury or exsanguination from a major vessel rupture. Serious retroperitoneal hemorrhage after lower abdominal and pelvic trauma is reported more frequently during pregnancy. Serious maternal abdominal injuries are usually the result of splenic rupture or liver or renal injury.
When the mother survives, placental abruption is the most common cause of fetal death (Gilbert, 2011). Placental separation is thought to be a result of deformation of the elastic myometrium around the relatively inelastic placenta. Shearing of the placental edge from the underlying decidua basalis results and is worsened by the increased intrauterine pressure resulting from the impact. It is critical that all pregnant victims be carefully evaluated for signs and symptoms of placental abruption after even minor blunt abdominal trauma.
Pelvic fracture may result from severe injury and may produce bladder trauma or retroperitoneal bleeding with the two-point displacement of pelvic bones that usually occurs. One point of displacement is commonly at the symphysis pubis, and the second point is posterior, because of the structure of the pelvis. Careful evaluation for clinical signs of internal hemorrhage is indicated.
Direct fetal injury as a complication of trauma during pregnancy most often involves the fetal skull and brain. Most commonly this injury accompanies maternal pelvic fracture in late gestation, after the fetal head becomes engaged. When the force of the impact is great enough to fracture the maternal pelvis, the fetus will often sustain a skull fracture. Evaluation for fetal skull fracture or intracranial hemorrhage is indicated.
Uterine rupture as a result of trauma is rare, occurring in less than 1% of severe cases. Rupture is more likely in a previously scarred uterus. When uterine rupture occurs, it is usually associated with a direct blow delivered with substantial force (Cunningham et al., 2010). Fetal death is common with traumatic uterine rupture. However, maternal death occurs less than 10% of the time, and when it occurs, it is usually the result of massive injuries sustained from an impact severe enough to rupture the uterus.
Penetrating Abdominal Trauma
Bullet wounds are the most frequent cause of penetrating abdominal injury, followed by stab wounds. When the uterus sustains penetrating wounds, the fetus is more likely than the mother to be seriously injured. The enlarged uterus may protect other maternal organs, particularly the bowel, but the fetus is more vulnerable (Cunningham et al., 2010; Martin & Foley, 2009).
Numerous factors determine the extent and severity of maternal and fetal injury from a bullet wound, including size and velocity of the bullet, anatomic region penetrated, angle of entry, path of the bullet, organs damaged, gestational age, and exit wound. Once the bullet enters the body, it may ricochet several times as it encounters organs or bone, or it may sever a large blood vessel. During the second half of pregnancy, the fetus usually sustains a direct injury from the bullet. Gunshot wounds require surgical exploration to determine the extent of injury and repair damage as needed.
Stab wounds are limited by the length and width of the penetrating object and are usually confined to the pathway of the weapon. Maternal and fetal injury is less if the stab wound is located in the upper abdomen and from movement of the penetrating object from above the head downward toward the abdomen than from movement of the penetrating object from the ground upward toward the lower abdomen. Stab wounds usually require surgical exploration to clean out debris, determine extent of injury, and repair damage.
Thoracic Trauma
Thoracic trauma is reported to produce 25% of all trauma deaths. Pulmonary contusion results from nearly 75% of blunt thoracic trauma and is a potentially life-threatening condition. Pulmonary contusion can be difficult to recognize, especially if flail chest also is present or if there is no evidence of thoracic injury. Pulmonary contusion should be suspected in cases of thoracic injury, especially after blunt acceleration or deceleration trauma, such as that occurring when a rapidly moving vehicle crashes into an immovable object.
Penetrating wounds into the chest can result in pneumothorax or hemothorax. This type of injury is usually caused by a vehicular crash that results in impalement by the steering column or a loose article in the vehicle that became a projectile with the force of impact. Stab wounds into the chest also may occur as a result of violence.
Immediate Stabilization
Immediate priorities for stabilization of the pregnant woman after trauma should be identical to those of the nonpregnant trauma client. Pregnancy should not result in any restriction of the usual diagnostic, pharmacologic, or resuscitative procedures or maneuvers (American Academy of Pediatrics [AAP] & ACOG, 2007). The initial response of many trauma team members when caring for the pregnant woman is to assess fetal status first because of the concern for a healthy neonate. Instead, the trauma team should follow a methodical evaluation of maternal status to ensure complete assessment and stabilization of the mother. Fetal survival depends on maternal survival, and stabilization of the mother improves the chance of fetal survival.
Primary Survey
The systematic evaluation begins with a primary survey and the initial ABCDs of resuscitation: establishment of and maintaining an airway, ensuring adequate breathing, maintenance of an adequate circulatory volume, and defibrillation. If defibrillation is needed, the paddles need to be placed one rib interspace higher than usual because the heart is displaced slightly by the enlarged uterus.
Increased oxygen needs during gestation necessitate a rapid response. The presence of a cervical spine injury is always assumed.
Once an airway is established, assessment should focus on adequacy of oxygenation. The chest wall is observed for movement. If breathing is absent, ventilations and endotracheal intubation are initiated. Supplemental oxygen should be administered with a tight-fitting, nonrebreathing face mask at 10 to 12 L/min to maintain adequate oxygen availability to the fetus. The chest wall is assessed for penetrating chest wound or flail chest. Breathing with a flail chest will be rapid and labored; chest wall movements will be uncoordinated and asymmetric; crepitus from bony fragments may be palpated.
Rapid placement of two large-bore (14- to 16-gauge) intravenous lines is necessary in the majority of seriously injured clients. It is important to place the lines while veins are still distended. Cardiac arrest during the immediate stabilization period is usually the result of profound hypovolemia, necessitating massive fluid resuscitation. Infusion of crystalloids such as Ringer’s solution or normal saline solution should be given as a 3:1 ratio; that is, 3 ml of crystalloid replacement to 1 ml of the estimated blood loss is given over the first 30 to 60 minutes of acute resuscitation (ACOG, 2006). Because of the 50% increase in blood volume during pregnancy, published formulas for nonpregnant adults used for estimating crystalloid and blood replacement to counter blood loss must be adjusted upward for pregnancy.
Replacement of red blood cells and other blood components is anticipated, and blood is drawn for type, crossmatch, complete blood cell count, and platelet count. Infusion of type-specific whole blood or packed red blood cells is usually necessary to improve fetal oxygenation status and to replace blood loss. During an extreme emergency, type O Rh-negative blood may be administered without matching.
Vasopressor drugs to restore maternal arterial BP should be avoided if possible until volume replacement is administered. Although vasopressor agents result in decreased perfusion to the uterus, they should be given if needed for successful resuscitation of the mother (Lu & Curet, 2007).
After 20 weeks of gestation venous return to the heart is best accomplished by positioning the uterus to one side to eliminate the weight of the uterus compressing the inferior vena cava or the descending aorta. This facilitates efforts to establish the forward flow of blood through resuscitation and stabilization. If a lateral position is not possible because of resuscitative efforts or cervical spine immobilization, the uterus can be manually deflected, or a wedge should be inserted underneath one side of the backboard or stretcher.
Signs of bleeding may be more difficult to recognize in the pregnant woman because a 30% to 35% loss of maternal blood volume may produce only a minimal change in maternal MAP. Hypovolemia can be detrimental for the fetus because the vascular bed of the uterus is a low-resistance system that depends on adequate maternal arterial pressure to maintain uterine and fetal perfusion. Maternal hypovolemia can be fatal for the fetus (Friese & Wojciehoski, 2005).
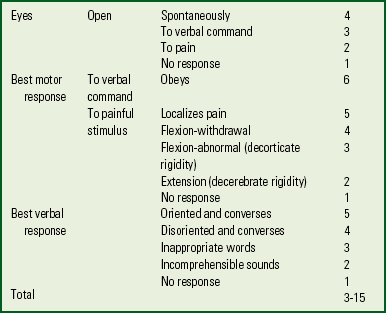
Establishing a baseline neurologic status (level of consciousness, pupil size and reactivity) is essential. The Glasgow Coma Scale is commonly used at the scene of the accident to help determine the extent of the head injury. The scale is simple and easy to use (Box 31-6).
Secondary Survey
After immediate resuscitation and successful stabilization measures, a more detailed secondary survey of the mother and fetus should be accomplished. A complete physical assessment including all body systems is performed (Box 31-7).
The maternal abdomen should be evaluated carefully because a large percentage of serious injuries involve the uterus, intraperitoneal structures, and the retroperitoneum. The pregnant woman’s stomach is assumed to be full. A nasogastric tube can be used to empty the stomach to help prevent acid aspiration syndrome. An empty stomach facilitates respiratory efforts. The uterus should be evaluated for evidence of gross deformity, tenderness, or contractions (ACOG, 2006).
The greatest clinical concern after vehicular crashes is placental abruption because up to 40% of these women have an abruption (Brown, 2009). Assessments should focus on recognition of this complication, with careful evaluation of fetal monitor tracings, uterine tenderness, labor, or vaginal bleeding. Ultrasound examination may be performed to determine gestational age, viability of the fetus, and placental location. However, ultrasound studies cannot exclude placental abruption. Most cases of abruption that occur as a result of trauma are associated with relatively minor injuries (Cunningham et al., 2010; Martin & Foley, 2009).
If trauma is the result of a penetrating wound, the woman should be completely undressed and carefully examined for all entrance and exit wounds. Ultrasonography and computed tomography (CT) scan should be performed to assess for the likelihood of intraabdominal bleeding. Peritoneal lavage can be performed on hemodynamically stable women if ultrasound and CT findings do not provide a clear diagnosis. Under direct visualization, the peritoneum is incised, and a peritoneal dialysis catheter is positioned. If aspiration yields free-flowing blood, the test is considered positive, and a laparotomy is warranted (Cunningham et al., 2010; Martin & Foley, 2009).
Exploratory laparotomy is necessary after a gunshot wound to explore the abdominal cavity for organ damage and to repair any damage, with careful examination of all organs, the entire bowel, and posterior vessels. If uterine injury is found, a careful evaluation of the risks and benefits of cesarean birth is quickly accomplished. A cesarean birth is desirable if the fetus is alive and near term and may be necessary for the preterm fetus because of the high incidence of fetal injury in these cases. The fetus usually tolerates surgery and anesthesia if adequate uterine perfusion and oxygenation are maintained. Tetanus prophylaxis guidelines are not changed by pregnancy.
Trauma may affect numerous systems in the maternal body and may affect more than the pregnancy. External signs of maternal trauma should suggest the possibility of internal trauma. Back and neck pain suggest spine injury, abrasions on the chest suggest chest injury, and limb pain and malposition suggest limb fractures. If head injury results in nonresponsiveness, suspect spinal, thoracic, and abdominal injuries. Hypovolemic shock can occur with internal hemorrhage, fracture of long bones, ruptured liver or spleen, hemothorax, or arterial dissection.
Once immediate stabilization is achieved, obstetric clients with severe trauma and massive blood loss that merits vigorous fluid resuscitation will usually benefit from the use of hemodynamic monitoring with a fiberoptic pulmonary catheter to determine cardiac and pulmonary function more precisely and to calculate volume and oxygen needs. The hemodynamic profile permits precise fluid resuscitation volumes. Oxygenation status calculations, including oxygen content, delivery, and consumption, will demonstrate which fluids are needed to enhance oxygen transport. Precise determinations can help prevent the potential sequelae of too little or too much fluid administration. With massive trauma, this care may be best provided in a regional trauma center with the obstetric team working closely with the trauma team, the obstetric team providing the obstetric care while the trauma team provides the trauma care.
All female trauma victims of childbearing age should be considered pregnant until proven otherwise. Determination of the health history and a history of the events preceding the trauma are important components of care. If the pregnant woman was involved in a vehicular crash, it should be determined whether she was the driver or a passenger and if she was ejected from the vehicle or used a restraining device and remained within the vehicle.
The physical examination should be performed in a systematic manner (see Box 31-7).
Electronic Fetal Monitoring
External FHR and contraction monitoring is recommended after blunt trauma in a viable gestation for a minimum of 4 hours, regardless of injury severity. Fetal monitoring should be initiated soon after the woman is stable (Cunningham et al., 2010; Martin & Foley, 2009). Continuous EFM may show early signs of placental abruption, including a change in baseline rate, loss of accelerations, or the presence of late decelerations, especially when accompanied by absent or minimal variability. The external device to monitor uterine activity, the tocodynamometer, is unable to measure pressures, and the pattern made with this device shows the frequency and duration of contractions only. Palpation is required to evaluate the intensity of contractions and the uterine resting tone. It is important to palpate between contractions to verify that the uterus is well relaxed. If the uterus does not relax between contractions, placental abruption could be present.
The exact duration of FHR and contraction monitoring required after blunt abdominal trauma is not known. Monitoring should be continued indefinitely if uterine contractions, abnormal (nonreassuring) FHR characteristics, vaginal bleeding, uterine tenderness or irritability, serious maternal injury, or ruptured membranes are present. Most physicians recommend continuous monitoring for at least 24 hours. Most abruptions develop soon after the traumatic event, although in rare cases abruption has developed days afterward (Cunningham et al., 2010; Martin & Foley, 2009).
Fetal-Maternal Hemorrhage
The potential for fetal-maternal hemorrhage exists after trauma. Hemorrhage can lead to fetal anemia, distress, or even death. If the pregnant trauma victim is Rh negative, fetal-maternal hemorrhage can result in sensitization and hemolytic disease of the neonate. The Kleihauer-Betke assay is often performed in women following blunt abdominal trauma to estimate the amount of fetal blood within the maternal circulation. Because most cases have less than 30 ml of hemorrhage, however, Kleihauer-Betke test results seldom alter management (Cunningham et al., 2010; Martin & Foley, 2009). Usually the routine administration of 300 mcg (one ampule) of Rho(D) immunoglobulin is sufficient to protect almost all pregnant trauma clients negative for Rho(D) from isoimmunization.
Ultrasound
Ultrasonography after trauma is not as sensitive as EFM for diagnosing placental abruption. Ultrasound may be useful to help establish gestational age, locate the placenta, evaluate cardiac activity (to determine whether the fetus is alive), and determine amniotic fluid volume. Ultrasound may also be used to evaluate the presence of intraabdominal fluid that would suggest the presence of intraabdominal hemorrhage.
Radiation Exposure
If the pregnant woman has sustained serious injuries, any necessary radiographic examination should be performed, regardless of fetal exposure. If radiographic examination would be performed for the nonpregnant trauma victim, it also should be performed for the pregnant woman. Abdominal or pelvic CT scanning can be used to visualize extraperitoneal and retroperitoneal structures and the genitourinary tract. Radiation exposure of less than 5 rads has not been associated with fetal abnormalities or pregnancy loss, and the radiation level associated with abdominal or pelvic CT scans is far below this amount (Martin & Foley, 2009). Blunt head trauma and loss of consciousness necessitate skull films and CT assessment with neurosurgical consultation. Magnetic resonance imaging also can be safely used to assess injuries because it does not produce ionizing radiation (Martin & Foley, 2009).
Perimortem Cesarean Birth
In the presence of multisystem trauma, perimortem cesarean birth may be indicated. Removal of the stressor of pregnancy early in the process of resuscitation may increase the chance for maternal survival. Fetal survival is unlikely if cesarean birth is accomplished more than 20 minutes after maternal cardiac arrest. Therefore, to facilitate resuscitative efforts, a cesarean birth should be performed after 4 minutes of resuscitative efforts if there is no evidence of a maternal pulse (Martin & Foley, 2009). It should be emphasized that this procedure is rarely successful.
Family-centered Obstetric Critical Care
Obstetric critical care should include the components of family-centered maternity care because the critically ill woman also is experiencing pregnancy and birth.
Family-centered critical care includes the following components:
• Open visitation so that spouse, significant other, or family members are present and provide support during labor, birth, and the postpartum period.
• Facilitation of parent-infant contact and attachment. The neonate should be brought to the bedside frequently if the condition permits. When the infant’s condition does not permit leaving the neonatal intensive care unit (NICU), pictures of the infant should be placed within focus of the mother’s eyes. Staff and family can talk about the infant and keep her updated on the infant’s condition.
• Sibling visitation, reassuring the mother that the older child is included in the experience.
• Family visitation as the mother desires. This can be arranged around necessary care procedures. The critically ill mother needs this support, perhaps even more than the healthy mother does.
Obstetric nurses have become accustomed to family-centered maternity care. When the critically ill mother receives care in an OBICU, there seem to be fewer problems for inclusion of the components of family-centered care than when the mother has to be transported to the adult ICU, probably because the obstetric staff is accustomed to this care and the nursery is more readily available to the labor and birth unit than to the ICU. Obstetric and critical care nurses can work together to include the components of family-centered care. Many ICUs are adopting the family-centered care philosophy, which ranges from open visitation to more inclusion of the family in the woman’s care—depending mainly on her condition (Henneman & Cardin, 2002).
Never assume that the mother is too ill to see and hold her infant. Encourage this and observe her body language to determine how much contact with the infant she desires. If perinatal loss occurs as a result of severe illness, provide grief support. Initiate all the components of grief support as usual for the institution. Burial plans can be delayed until the mother’s condition improves so that her concerns are addressed (see Chapter 38).
Maternal Death
It is extremely rare for a woman to die in childbirth, but it does happen. The yearly occurrence of maternal deaths in the United States is 12.7 per 100,000 live births (Xu, Kochanek, Murphy, & Tejada-vera, 2010). The leading causes of maternal death are embolism and preeclampsia. Older women (≥35) and those without prenatal care are at greatest risk. A higher mortality ratio exists in African-American women compared with Caucasian women. The father and extended family who are faced with mourning not only the death of a wife and mother but also the death of a baby have a particularly difficult time. Conversely, the father may be faced with parenting a baby without a surviving mother. Death of a mother disrupts the family structure and leaves the father with the care of a baby when he is greatly distressed. Thus the father and extended family, especially other children and grandparents, need supportive grief counseling at the time of death and after discharge for them to heal after such a devastating loss.
The nursing care of families at this time is similar to that described in Chapter 38. Options need to be offered, memories made, and mementos obtained and held for the family until they are ready for them. These families are at risk for complicated bereavement and altered parenting of the surviving baby and other children in the family. Referral to social services to help the family mobilize support systems and for counseling can help combat problems before they develop. Such a referral may be beneficial not only at the time of the loss but also in the future.
The emotional toll that a maternal death can take on the nursing and medical staff also must be addressed. Guilt, anger, fear, sadness, and depression are common responses to a maternal death. The staff may want to review the situation surrounding the events, the chart, and their responses in the forum of a morbidity-mortality review and a critical incident debriefing to help in coping with the feelings and emotions that result after a maternal death. Attending memorial or funeral services may benefit staff and family.
KEY POINTS
• The numbers of critically ill pregnant women are increasing, paralleling improvements in pediatric and neonatal care.
• Any nurse providing care for pregnant women may encounter the critically ill pregnant woman and must be able to initiate appropriate care.
• Nurses must recognize early signs of severe complications and expedite the institution’s plan for the critically ill pregnant woman to receive necessary complex care.
• The institutional plan may consist of actual implementation of critical care procedures in a labor and birth unit or stabilization and preparation for transport to a tertiary center or adult ICU, with obstetric consultation after transport.
• Normal physiologic alterations during pregnancy produce profound hemodynamic changes. The critical care team should base care on knowledge of normal hemodynamic values for the pregnant state.
• Pregnancy is not a contraindication for hemodynamic monitoring.
• Invasive hemodynamic monitoring is associated with potential complications. However, the benefits usually outweigh the risks when the woman is critically ill.
• Pulmonary artery catheterization provides continuous information about left ventricular function; this information is not available from any other method.
• Use of a PAC in combination with an arterial pressure catheter and pulse oximeter provides adequate data to assess continuously both cardiac and pulmonary status.
• The fiberoptic PAC provides a continuous Svo2, which is a sensitive marker of physiologic instability because it reflects overall adequacy of oxygen delivery to the tissues.
• The active roles assumed by most pregnant women today place them at risk for vehicular crashes, falls, violence, and other injuries.
• Pregnancy does not limit or restrict resuscitative, diagnostic, or pharmacologic treatment after trauma.
• Fetal survival depends on maternal survival. After trauma the first priority, before consideration of fetal concerns, is resuscitation and stabilization of the mother.
• Optimal care for the pregnant victim of trauma depends on knowledge of the physiologic state of pregnancy.
• Minor trauma can be associated with major complications for the pregnancy, including placental abruption, fetomaternal hemorrhage, preterm labor and birth, and fetal death.
• Trauma from accidents is the most common cause of death in women of childbearing age.
• Care of the critically ill pregnant woman should include the components of family-centered childbirth.
• Death of a woman in childbirth is rare; it disrupts family structure, and the family needs supportive grief counseling. Medical and nursing staff also can benefit from debriefing and counseling sessions.
![]() Audio Chapter Summaries Access an audio summary of these Key Points on
Audio Chapter Summaries Access an audio summary of these Key Points on ![]()