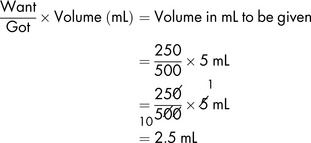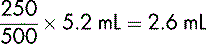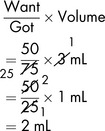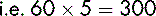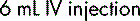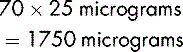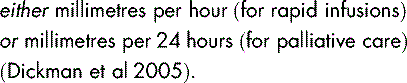Chapter 5 Calculating parenteral doses
small volume
Introduction
Following discussion of some of the essential elements of calculating doses of parenteral drugs, examples of calculations are presented at the different levels of complexity described in Chapter 3. They are presented in the same three ways as in Chapter 4, namely:
All answers are given on p. 195.
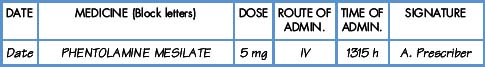
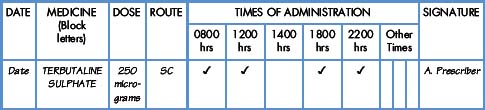
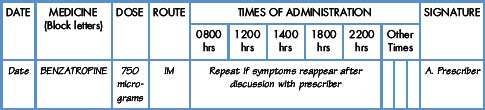
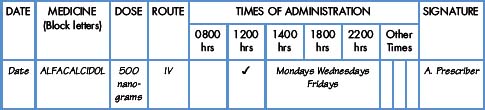
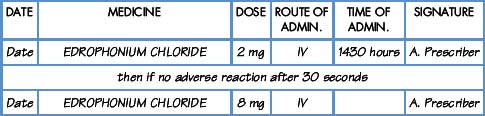
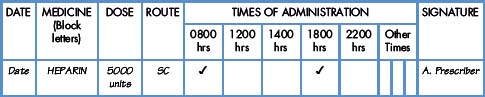
Interpretation of the prescription and the label of a parenteral preparation follows the same principles as a non-parenteral preparation.
Equipment for measuring the dose
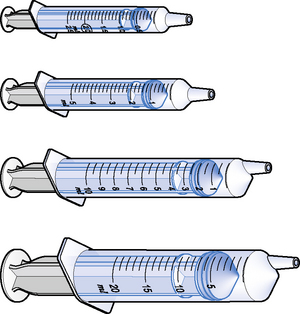
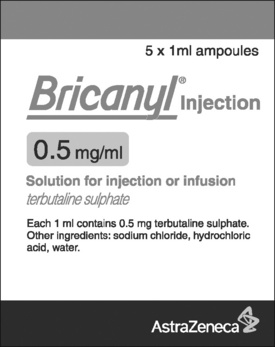
Medicines for injection are presented in either a glass or plastic ampoule (Fig. 5.1), a rubber-capped vial (Fig. 5.2) or a prefilled syringe or a cartridge (Fig. 5.3). The contents are either in the form of a liquid or presented as a powder requiring reconstitution. To aspirate the contents, a syringe and needle of suitable size are selected. Care must be taken to draw up the correct amount into the syringe. It is therefore essential to appreciate the significance of the calibrations on different syringes (Fig. 5.4). The syringe should be held at eye level and air should be expelled. The reading should be taken at the top of the black bung attached to the plunger of the syringe. When drawing up and giving insulin, a syringe (with integral needle), specifically for insulin calibrated in units, will normally be used (Fig. 5.5).
Safety
When administering cytotoxic drugs (and other potentially dangerous compounds), it is essential to follow local safety protocols and procedures.
Overage
When preparing to draw up an injection, it should be noted that ampoules containing a solution for injection always contain more than the nominal volume. For example, a 1 mL ampoule will normally contain a total volume of 1.1 mL. An overage of 0.1 mL is present so as to allow removal of a full 1 mL dose, otherwise it would not be possible to remove 1 mL due to the ‘losses’ of injection solution on the internal surfaces of the ampoule. Larger-volume ampoules contain overages in proportion to the nominal volume.
It follows therefore that it is very important to measure all doses and not simply to draw up the contents of the ampoule. The presence of overages in ampoules is one reason why it is not acceptable to measure doses in terms of, for example, half an ampoule. A 1 mL ampoule with a strength of 10 mg per 1 mL will contain 11 mg in 1.1 mL. Giving a dose of half the contents of the ampoule could introduce a drug error in this case 5.5 mg rather than the required 5 mg. There is no substitute for measuring the dose accurately. It should be noted that labels of Controlled Drugs show the presence of an overage for legal reasons.
Displacement volumes
A number of drugs are unstable in solution (both parenteral and oral) and are therefore supplied in dry powder form. Prior to administration, the powder must be reconstituted using a diluent (or solvent). Water for injections is commonly used but sodium chloride 0.9% solution may be the recommended diluent; on occasion, a specially formulated diluent may be provided. Enough liquid is needed to dissolve (or suspend) the contents to produce a concentration that prevents irritation of body tissues but at the same time is of minimum volume so as to make the injection less painful.
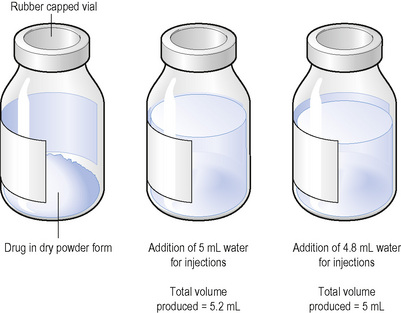
The addition of 5 mL of a diluent to a vial containing 500 mg of a drug in powder form will produce a volume of more than 5 mL due to the presence of the powder (Fig. 5.6). It can be seen in Fig. 5.6 that the powder has added 0.2 mL to the volume of diluent added. The displacement volume (DV) is the volume of liquid displaced by the powder, in this case, 0.2 mL. If the dose to be administered is less than 500 mg, it will be important to take account of the displacement value (Cable 2008).
A dose of 250 mg of a drug is to be administered from the 500 mg vial. The contents of the vial of 500 mg displace 0.2 mL of diluent. It is therefore necessary to add to the vial 5 mL minus 0.2 mL, which equals 4.8 mL, to produce a total volume of solution of 5 mL. The resulting solution contains 500 mg in 5 mL.
To obtain the required dose, the usual formula can then be applied:
If allowance is not made for the DV, measuring and administering a dose of 2.5 mL would result in a drug error. Thus, if 5 mL of a diluent was added (not taking account of the DV), a total volume of 5.2 mL would be produced. The dose of 250 mg would be contained in:
Displacement volumes will normally be found in the product literature. If for some reason the DV is not known, a suitable volume of diluent is added to the vial in the usual way and the powder fully dissolved. The total contents of the vial are measured by drawing into a syringe. The required dose can then be calculated.
It is important to note that all powders (including those for oral administration) intended to be reconstituted before use will also displace a certain volume of diluent. Powder for colistin syrup displaces 22 mL of water when made up to the required volume of 80 mL, i.e. 58 mL (80–22 mL) of water must be added to produce a final volume of 80 mL.
Exercise 5.1
Work out the following taking account of the displacement volume.
A patient is to be administered 500 mg of amoxicillin by intramuscular injection every 8 hours. The displacement volume of amoxicillin is 0.4 mL for a 500 mg vial. If 2.5 mL water for injections is added and a solution made, what is the volume to be administered to give a dose of 500 mg?
How to calculate the quantity to give of small-volume injections
Level 0: Demonstration
Level II: Demonstration
Level II: Exercises
Level II: Further exercises
In the following examples, calculate what you should give the patient.
Level III: Demonstration
Demonstration 5.4
Level III: Further exercises
Syringe driver
Syringe drivers and pumps are small, lightweight, battery-operated, portable devices designed to deliver a steady rate of drugs. They are mostly used to administer medication by the subcutaneous (SC) or the intravenous (IV) routes for symptom management and palliative care (Dickman et al 2005).
There may be a calculation required when filling the syringe. Otherwise, being simply another type of delivery system, the syringe driver presents no more difficulty than calculations by any other route. As much care is required in reading the prescription and the details on the label as when preparing to administer any other medicine. The amount of diluent is dependent on the number and volume of the drugs to be delivered.
Once the syringe is filled with the appropriate amount of drug(s) and diluent, it is attached to an infusion set designed for the purpose. When using the SC route, the infusion set should have a previously attached plastic cannula or ‘butterfly’ needle; for the IV route, the infusion set, once primed, is attached to an already-sited IV cannula. When the syringe is filled, the tubing has been primed and the needle/cannula is in place/attached, the rate the plunger is to advance must be set in accordance with the prescription.
Syringe drivers can be set at:
These settings express the distance travelled by the plunger within a span of time, and not the volume of drug. If both types of syringe drivers are available, great care must be taken when setting up, since a 24-fold error can occur.
It is good practice once the infusion has commenced to check within the first half hour that the plunger is proceeding as intended and that the patient is comfortable. A check thereafter at least every 4 hours should suffice unless local policy dictates otherwise.
Syringe pump
A syringe pump is a computerised device in which the rate of flow is controlled by the speed of the piston attached to the syringe plunger. Small volumes of highly concentrated drugs may be administered intravenously by this method, usually via a 60 mL syringe. The rate is set in millilitres per hour. A syringe pump may be used for the administration of drugs by the IV route or, in palliative care, by the SC or epidural route. The amount to be given and the amount that has been given are displayed on an LCD.
In the interests of patient safety, health authorities are being encouraged to standardise the models in use. Some of the existing devices do not meet the standard set by the MHRA (2009). Choice, maintenance and availability of syringe drivers and pumps need to be carefully considered and budgeted for. The aim is to have a library of devices that can be consistently maintained by medical physics staff and loaned. No ward would ‘own’ devices but would borrow them as required.
Cable C. Why a knowledge of displacement values is important for pharmacists. The Pharmaceutical Journal. 2008;2281:244-245.
Dickman A., Schneider J., Varga J. The syringe driver: continuous subcutaneous infusions in palliative care. Oxford: Oxford Publications, 2005.
MHRA. Devices in practice: a guide for professionals in health and social care. London: Medicines and Healthcare products Regulatory Agency, 2009.