Analgesia
After completion of this chapter, the reader will be able to:
• Define pain, nociception, physiologic pain, pathologic pain, neuropathic pain, idiopathic pain, visceral pain, somatic pain, preemptive analgesia, pain scale, and multimodal therapy.
• List the main steps of the pain pathway.
• List the benefits of multimodal analgesia.
• List the consequences of untreated pain.
• Explain how primary hyperalgesia (peripheral hypersensitivity) develops.
• Explain how secondary hyperalgesia (central hypersensitivity) develops.
• List common surgical and medical conditions that are considered to be painful.
• Describe how to recognize and assess pain-associated behaviors in animals.
• List the routes by which analgesic drugs are commonly administered.
• List the adverse effects of opioid drugs.
• Explain the mechanism of action of nonsteroidal antiinflammatory drugs.
• List the adverse effects of nonsteroidal antiinflammatory drugs.
• Describe the procedure for application of a fentanyl patch.
• List two examples of multimodal therapy.
• Describe nursing care that relieves discomfort in hospitalized patients.
The veterinary team has the unique responsibility of assessing and treating animal pain. The role of the veterinary technician, either as anesthetist or patient caregiver, in the provision of analgesia cannot be overstated. The technician forms a vital part of the team through his or her understanding of pain physiology, pain-associated behaviors, pain assessment tools, analgesic drug pharmacology, and communication with the veterinarian-in-charge (VIC) regarding the welfare of patients. Pain assessment is currently considered to be an essential part of every patient evaluation, regardless of presenting complaint. Under the currently accepted standard of practice, provision of analgesia is mandatory for patients that are deemed to be in pain or that undergo painful procedures, including surgery for any reason. Clients expect, request, or sometimes even demand pain control for their pets. Consequently, failure to provide effective pain control not only results in patient discomfort but may evoke anger, could damage the doctor’s reputation, or, in extreme circumstances, could result in disciplinary action by a Veterinary Medical Licensing Board.
Pain is a complex phenomenon that has been defined as an aversive sensory and emotional experience that elicits protective motor actions (such as a dog trying to bite when given an injection), results in learned avoidance (the same dog exhibits fear the next time it is taken to the vet for vaccine boosters), and may modify species-specific behavior traits, including social behavior. This experience is different for every individual animal, although within a given species some physiologic and behavioral responses will be similar (e.g., dogs in pain will often seek attention from their owners, whereas cats are more likely to hide). If left untreated, pain can negatively affect a patient’s behavior, physiology, metabolism, and immune system, causing poor performance, weight loss, and increased susceptibility to infection.
PHYSIOLOGY OF PAIN
Detection by the nervous system of the potential for or the actual occurrence of tissue injury is called nociception. This serves to protect the animal from painful or noxious stimuli. The protective sensation of pain that occurs when there is no or minimal tissue injury is referred to as physiologic pain, or “ouch” pain (e.g., the pain you would feel that would warn you if you touched something sharp, hot, or chemically noxious). Pain that occurs after tissue injury is referred to as pathologic pain (e.g., the pain you would feel after a bone is broken). It is usually helpful to classify pathologic pain based on duration as either acute (hours) or chronic (days to years).
Pathologic pain is often classified based on the mechanism, origin, and severity of pain, as this may help determine treatment options. The mechanism of pain can be inflammation (e.g., after trauma or surgery), nerve injury (neuropathic), or cancer, or the pain can be idiopathic (of no identifiable cause). Pain can originate from organs, in which case it is visceral pain (e.g., pleuritis or colic), or from the musculoskeletal system, in which case it is somatic pain. Somatic pain can be divided into superficial (i.e., skin) and deep (i.e., joints, muscles, bones) pain. Some diseases or surgeries may result in more than one of these types of pain—for example, abdominal surgery has components of somatic pain (skin and abdominal wall incisions) and visceral pain (organ manipulation and surgery). Pain severity is often classified as none, mild, moderate, or severe, although more involved classifications exist (see the discussion of pain assessment tools later in this chapter).
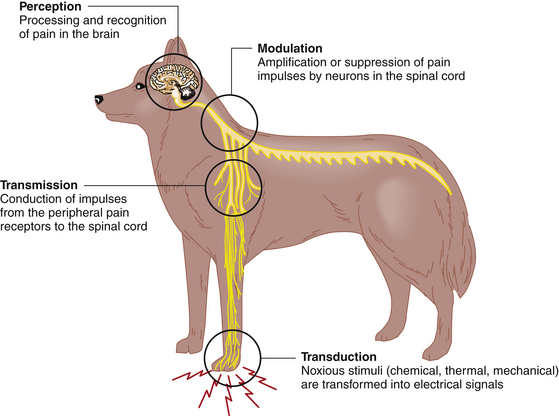
Nociception, or the pain pathway, consists of four main steps (Figure 7-1). The first step is the transformation of noxious thermal, chemical, or mechanical stimuli into electrical signals called action potentials by peripheral A-delta and C nerve fibers, and is called transduction. These sensory impulses are then conducted to the spinal cord, a process known as transmission. In the spinal cord, where the A-delta and C fibers terminate, the impulses can be altered by other neurons, which either amplify or suppress them. The general term to cover both possibilities is modulation. The final step is perception, in which the impulses are transmitted to the brain, where they are processed and recognized. At each level of the pain pathway different receptors are involved in the nociceptive process, allowing the pain management team to choose drugs that target a specific receptor (e.g., nonsteroidal antiinflammatory drugs [NSAIDs], opioids, local anesthetics, or corticosteroids inhibit transduction) (Box 7-1). A goal of pain management, particularly severe pain, is to target two or more of these receptors. This pain management strategy is called multimodal therapy and is preferable to using a single analgesic because lower dosages can be used, which decreases adverse effects and improves safety.
CONSEQUENCES OF UNTREATED PAIN
Pathologic pain that goes untreated has undesirable consequences for the patient.
• Pain produces a catabolic state, which may lead to wasting.
• Pain suppresses the immune response, predisposing to infection and increasing hospitalization time and cost.
• Pain promotes inflammation, which delays wound healing.
• Anesthetic risk is increased because higher doses of anesthetic drugs are required to maintain a stable plane of anesthesia.
• Pain causes patient suffering, which is also stressful for owners and caregivers.
Tissue damage results in constant stimulation of the nerves involved in the nociceptive process. This constant noxious stimulation of the central nervous system (CNS) can alter the function of neurons and receptors involved in the pain pathway. The ability of the CNS to change in this way can result in hypersensitivity in both acute and chronic pain. Neurons in the periphery (limbs, organs) and central neurons (spinal cord) can be affected. Peripheral tissue trauma results in the release of substances called mediators from damaged cells and also attracts inflammatory cells, which also release mediators. These mediators combine to form what is called a “sensitizing soup” that lowers the threshold of the peripheral pain receptors, thus increasing their sensitivity. This peripheral hypersensitivity, referred to as primary hyperalgesia, manifests clinically as an area close to the site of tissue injury that, if stimulated with a normally non-noxious stimulus, is painful. Centrally, in the spinal cord, neurons that are stimulated by constant nociceptive input from the periphery become hyperexcitable and sensitive to low-intensity stimuli that would not normally elicit a pain response. This phenomenon is called central nervous system hypersensitivity and is also referred to as “windup.” Clinically this may manifest as an area of hypersensitivity that is farther away from the initial injury, and is known as secondary hyperalgesia. A receptor that is activated in windup is the N-methyl-d-aspartate (NMDA) receptor. Drugs such as ketamine can block this receptor.
Pain also causes physiologic changes. Neuroendocrine changes that occur in response to pain include release of adrenocorticotropic hormone (ACTH); elevation in cortisol, norepinephrine, and epinephrine; and decrease in insulin. These changes result in a catabolic state, which, in addition to the fact that patients in pain may be reluctant or unable to eat, leads to wasting.
Sympathetic stimulation leads to vasoconstriction, increased myocardial work, and increased myocardial oxygen consumption, predisposing the patient to arrhythmias. Skeletal muscle blood flow tends to increase, whereas blood flow to the gastrointestinal and urinary tracts decreases.
The stress response caused by tissue injury (including surgery) is important for immediate survival but if left unchecked can lead to increased morbidity and mortality. An extreme form of stress, distress, occurs when stressors such as pain negatively affect the animal’s physiology and behavior. An animal that is enduring pain is therefore suffering.
The quality of life of a patient in pain should be a concern of every veterinary professional and owner. Although not always easy to define, high quality of life requires that several basic needs be met. Representing these basic needs, the “five freedoms” can help those caring for animals assess quality of life (Box 7-2). As freedom from pain is a necessary element of a high quality of life, assessment of pain and provision of analgesia and comfort should be goals of treatment of veterinary patients.
SIGNS OF PAIN IN ANIMALS
Animals cannot verbally express their pain. Pain recognition therefore relies on a sound understanding of normal animal physiology and behavior and stress-related or behavioral changes that may be indicative of painful conditions. A thorough, detailed history may also be helpful in assessing pain, because the owner is more likely to be aware of behaviors that may be masked during a visit to the hospital or when the veterinarian is at a farm or stable. An understanding of the degree of pain that is expected and associated with specific diseases and surgeries is also helpful.
There is no single physiologic parameter that is a specific indicator of pain. Common pain-related physiologic changes are presented in Box 7-3; however, note that there are other causes for each change. It is important to avoid projecting complex human emotions onto animals (anthropomorphizing); however, the pain pathways and therefore pain perceptions in animals and people have much in common. Therefore our personal experiences can help us determine whether a disease or procedure is likely to be painful or not.
Many disease processes and surgical procedures are associated with moderate to severe pain (see Box 7-4 for some examples). Pain management can be initiated for these types of patients when they are presented or before elective surgery. The administration of pain medication before pain occurs reduces the overall requirement for analgesics and the duration of analgesic administration postoperatively compared with waiting until after surgery to start analgesic therapy. Providing analgesia before tissue injury is called preemptive analgesia. Preemptive analgesia is most commonly achieved by adding an analgesic to the premedication before anesthetizing a patient for surgery. Preemptive analgesia also helps to prevent windup, which, through changes in the CNS from central sensitization, can lead to pain that lasts longer than anticipated and is more difficult to manage.
Behavioral responses to pain vary depending on the species, age, breed, and temperament of the patient and the nature, duration, and severity of pain. Younger patients are less likely to tolerate pain and more likely to vocalize, whereas older animals may be more stoic and are more likely to become aggressive. Cattle are generally much more stoic than other species. Within a species certain breeds may have higher or lower pain thresholds (e.g., calm draught horses versus excitable thoroughbreds; large breed versus toy breed dogs; Labradors versus Siberian huskies and greyhounds). Individuals within a breed will also respond differently to pain. It is important to recognize that nonspecific behavioral indicators of pain may be present with painful as well as nonpainful conditions.
Assessment of pain-associated behavior is often best achieved by using a staged process. It may be useful to observe patient behavior when people are not present (e.g., via remote camera or one-way window), although this is more difficult to do in a busy hospital setting and requires special equipment or facilities. Many animals change their behavior when a human observer is watching the patient, when a person interacts verbally with a patient, and finally when the patient undergoes physical examination. A thorough assessment of pain-related behavior can therefore be time-consuming, although decreasing the time devoted to the assessment may result in subtle changes being overlooked. It is important to remember that animals may mask pain-related behavior in the presence of people, owing either to a protective response to avoid looking like prey or to socialization instincts. Different species often respond differently when they are in pain; cats tend to hide, dogs tend to seek attention from their owners, and herd or flock animals tend to separate from the other animals.
Changes in gait and level of activity such as lameness, limping, stiffness, and reluctance to move are all indicators of limb or joint pain. Development of exercise intolerance or a decrease in performance may also indicate the presence of limb pain. Arthritic dogs may not walk normally, whereas cats with arthritis show a reluctance to jump as high or as often as before. Horses will shift weight more frequently and point, rotate, or hang their limbs. Cows may arch their backs. A reluctance to lie down or a constant shifting of position is an indicator of thoracic or abdominal pain. Patients may stand, sit, or adopt a prayer position rather than lie down, eventually becoming exhausted. Pain may not consistently be present but can be elicited by an event such as palpation or manipulation of a joint or forced activity (e.g., a horse that is not lame at the walk but is when it is encouraged to trot).
Vocalization is frequently associated with pain. Dogs tend to whine, growl, whimper, and groan and may snarl or bite if manipulation is painful. Cats are more likely to groan, growl, or purr. They may also bite or hiss if palpation causes pain. Large animals do not vocalize as commonly; however, groaning and grunting are associated with pain in these species. Horses may bite or kick in response to pain or may try to flee. In small animals, particularly dogs, vocalization is common in the immediate postoperative period and may be caused by emergence delirium or pain. Emergence delirium can be differentiated from pain by the fact that it lasts for a short time and responds to sedation.
Facial expressions, appearance, and attitude may be altered in patients that are in pain. Common facial expressions encountered in dogs in pain include a glazed or fixed stare. Cats may squint and have a furrowed brow. Horses and cattle will have an altered head carriage and may also curl their lips. Cattle will grind their teeth. Animals in pain do not typically groom themselves and may appear unkempt. Hospitalized patients may sit staring at the back of the cage, or stand at the back of the stall. Such patients may be unaware of their surroundings and not interested in interacting, often appearing stuporous, or may be unwilling to move because of pain. Cats in pain will attempt to hide by moving as far from view as possible, a form of escape behavior, and will often become unresponsive to people. Aggression is most commonly observed with severe acute pain that is elicited on palpation. (See Figure 7-2 for examples of pain-related behaviors.)
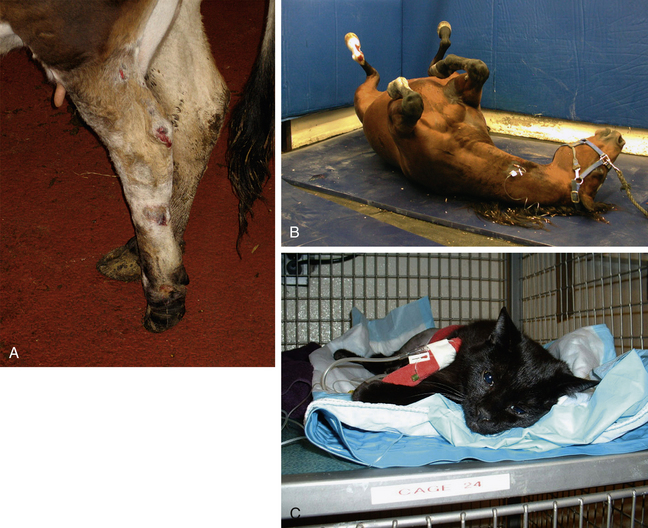
FIGURE 7-2 Pain behaviors. A, Signs of locomotor pain in a cow. This cow had a chronic wound of the hock joint and was three-legged lame. B, Signs of abdominal pain in a horse. This horse with severely painful colic was violently thrashing in its stall and causing injury to itself. C, General signs of pain in a cat. This cat suffered multiple trauma (fractured femur and ruptured bladder) from being hit by a car. Note the laterally recumbent posture, the vacant stare with dilated pupils, and the lack of interest in the photographer. (B courtesy the OSU Equine Section.)
PAIN ASSESSMENT TOOLS
Several pain assessment tools have been adapted from human medicine, evaluated, and used to quantify pain and response to therapy in animals. Most of these tools require evaluator training for consistently meaningful results to be obtained.
The pain assessment tools that have been developed for use in animals include verbal rating scales, simple descriptive scales, numeric rating scales, visual analogue scales, and comprehensive scales.
Verbal rating and simple descriptive scales (Figure 7-3) rate pain as absent, mild, moderate, or severe. They are quick and easy to use. These scales are not well suited to chronic or subtle changes, as they are not very detailed in their approach to assessing pain.
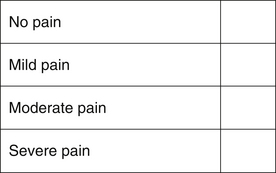
FIGURE 7-3 Simple descriptive scale. The observer makes an overall assessment of the animal’s pain, and places an X in the box next to the descriptor.
Numeric rating scales assign point values to various categories, such as physiologic parameters, locomotor activity, and behavior (Figure 7-4). Each category is subdivided with different descriptors, which are awarded different point scores. After the assessment the points are totaled, with a higher score indicating an animal in more pain. This type of scale can never include every possible pain-related behavior or measurable parameter, so the scales are usually simplified or designed for specific types of pain (e.g., a numeric rating scale for horses undergoing castration).
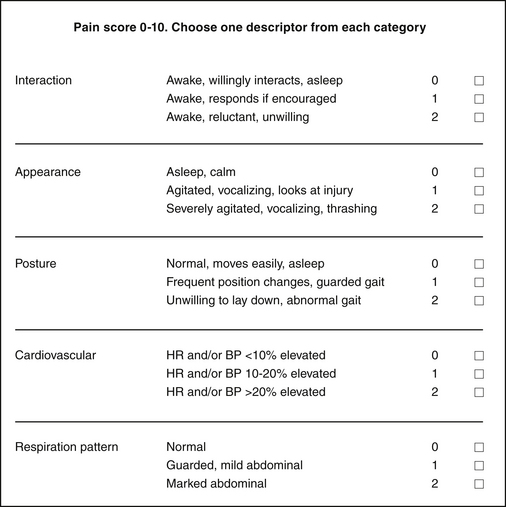
FIGURE 7-4 Numeric pain scale for use in small animal patients. The observer assigns a score for each category. Observations are performed before the animal is physically touched (e.g., to take a pulse rate). The overall score is used as a guide as to when to treat—for example, the veterinarian in charge may request that analgesics be administered if the pain score is ≥4—or as an assessment of response to treatment (i.e., a score that decreases after therapy).
A visual analogue scale consists of a ruler where the left end of the line equates to no pain, and the right end of the line to the worst pain imaginable for the specific disease or surgical procedure (Figure 7-5). The assessor places a mark (usually an X) on the ruler corresponding to the level of pain the assessor feels the animal is experiencing.

FIGURE 7-5 Visual analogue scale. The assessor places a mark (usually an X or a small vertical line) on the ruler corresponding to the level of pain the assessor feels the animal is experiencing.
Veterinary teaching hospitals often have their own pain assessment form that typically combines different types of pain assessment tools in order to assess pain and progression of signs in response to treatment (Figure 7-6).
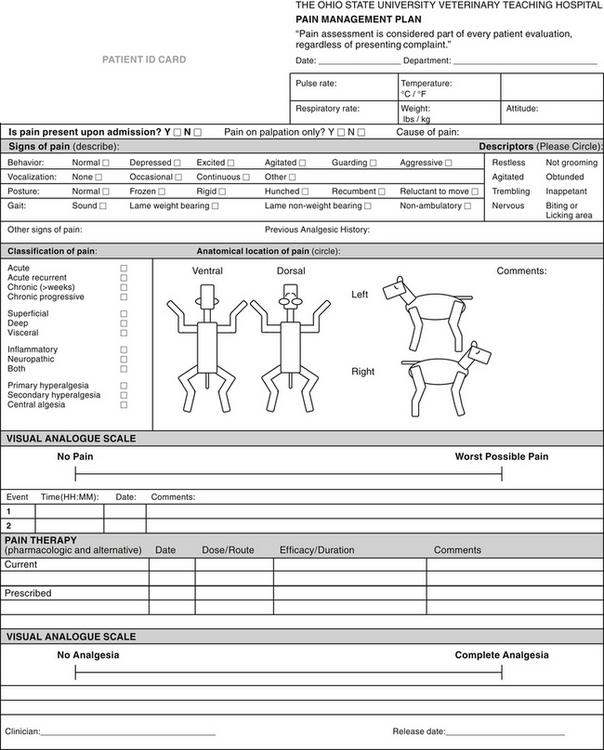
FIGURE 7-6 The Ohio State pain assessment form. Note that there is a visual analogue scale to assess level of pain as well as degree of analgesia after therapy. Complex pain assessment forms or scales are useful for evaluating pain that is subtle, chronic, or difficult to treat. The main drawback of this type of pain assessment tool is that it is time-consuming.
Each type of assessment has its limitations. The least complex scales allow quicker assessment but are not as effective at detecting subtle changes. The common purpose of these tools is to help determine how much pain the animal is in and to assess response to treatment. Pain scales are more accurate when observers are trained and when the same observer performs all assessments on a given patient.
Assessing Response to Therapy
Animals undergoing major surgery need to be assessed hourly or possibly more frequently in the first few hours of the postoperative period, whereas patients with diseases associated with chronic pain such as arthritis require less frequent evaluation (monthly or at a longer interval). Effective analgesic treatment causes pain-associated behaviors to gradually recede. Hospitalized patients rest and sleep more easily and assume normal body positions and postures when asleep and awake. Appetite returns to normal, and appearance will improve as grooming behaviors also return to normal. When awake, animals will be more likely to interact with caregivers rather than ignore them or try to escape. Performance animals will return to form. Figure 7-7 shows examples of patients that seem comfortable after surgery.
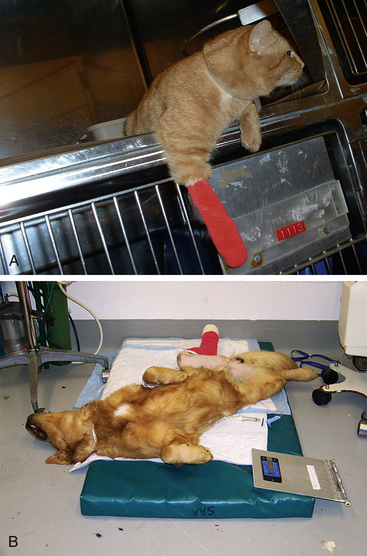
FIGURE 7-7 Signs of comfort after surgery. A, One day after surgery for a forelimb wound this cat was playful and interactive. Analgesia consisted of oral buprenorphine and oral meloxicam. B, One hour after a tibial plateau leveling osteotomy, this golden retriever was sleeping on its back and seemed very comfortable. Analgesia consisted of a preoperative morphine epidural and postoperative intravenous carprofen.
Pain assessment scores will decrease if analgesic therapy is effective, although it is almost impossible to remove all pain that an animal experiences. Thus the aim is a reduction in the pain score and a more comfortable patient rather than the production of a pain-free patient.
PERIOPERATIVE PAIN MANAGEMENT
Preemptive analgesia and multimodal therapy are key to providing successful perioperative analgesia. Multiple receptors and mechanisms have been identified that are responsible for pain and the development of windup. An analgesic plan for moderate to severe pain should make use of several drugs, each having a different mechanism of action. The benefits of multimodal analgesic therapy are that each individual drug dose is reduced, overall anesthetic drug requirement is reduced, and therefore the risk of toxicity and adverse effects is decreased.
Management of perioperative pain begins in the preoperative period. Premedication (see Chapter 3) offers an opportunity to administer analgesia before surgery (i.e., preemptively). Animals that are in pain before surgery will be in pain after surgery and should be treated for the anticipated severity of postoperative pain. Analgesics can be administered as part of the anesthetic premedication if they also provide or enhance sedation (opioids, alpha2-adrenoceptor agonists, ketamine). Another commonly used modality to provide preemptive analgesia in small animals is the application of a transdermal fentanyl patch at least 6 to 12 hours in advance of anticipated pain. NSAIDs are typically administered preoperatively in large animal patients and can be administered to small animal patients preoperatively when intravenous (IV) formulations are available and approved for this use.
PHARMACOLOGIC ANALGESIC THERAPY
Pain control in the surgical patient should be available at every stage of hospitalization and treatment: during the preanesthetic period, the surgical procedure itself, the immediate postoperative period, the remainder of the hospital stay, and, if necessary, after the patient’s return home.
The choice of analgesic is governed by the severity and type of pain and the animal’s general condition. The veterinarian also selects the route of delivery, which may include injection (subcutaneous [SC], intramuscular [IM], IV, intraarticular, epidural, local infiltration), oral administration, or transdermal patch.
Pharmacologic analgesia can be achieved through a variety of agents, including opioids, NSAIDs, alpha2-agonists, ketamine, and local anesthetics.
Opioid Agents
Opioids provide analgesia through their action on opioid receptors in both the spinal cord and brain, as well as in some peripheral tissues, such as the synovial membranes of joints. Opioid agonist drugs acting centrally inhibit perception in the brain and central sensitization in the spinal cord.
Doses, routes, and adverse effects of opioids used for postoperative analgesia are given in Table 7-1.
TABLE 7-1
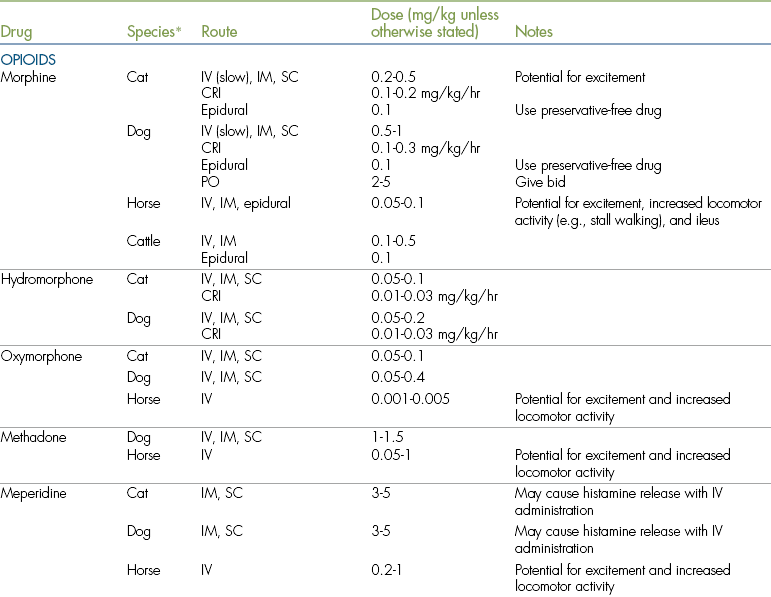
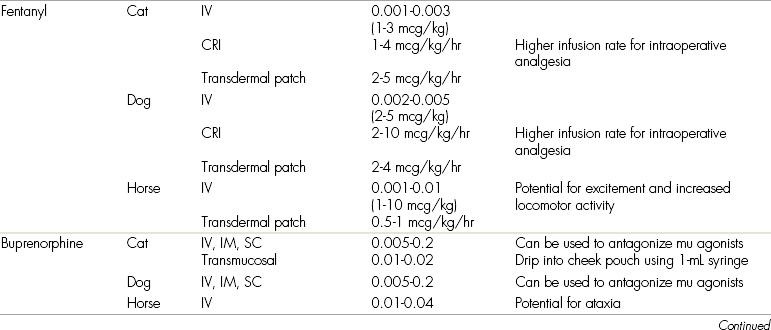
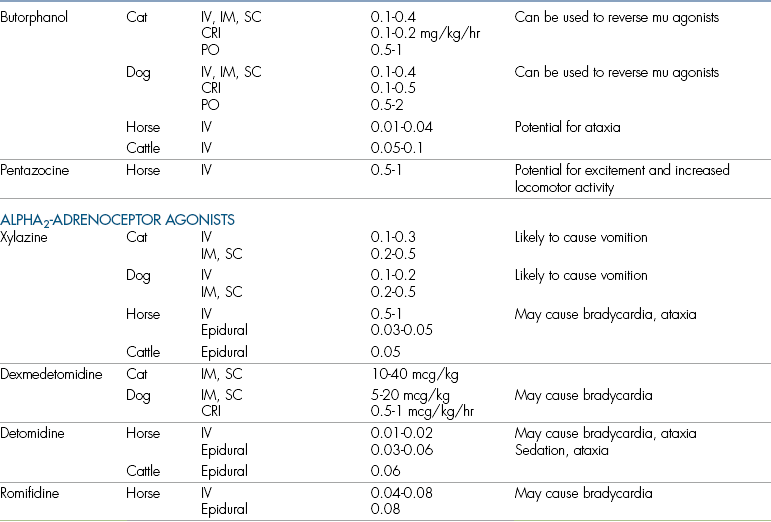
COX, Cyclooxygenase; CRI, constant rate infusion; GI, gastrointestinal; IM, intramuscular; IV, intravenous; NSAID, nonsteroidal antiinflammatory drug; NMDA, N-methyl-d-aspartate; PO, by mouth; SC, subcutaneous.
∗Where a species is not listed, no data are available for that specific drug.
As outlined in Chapter 3, opioids have many uses in veterinary anesthesia, including the following:
• Opioids are commonly included in injectable premedications, often in combination with a tranquilizer such as acepromazine or dexmedetomidine. When used preemptively, they diminish windup. The analgesic effect of these preanesthetic mixtures is generally gone by 2 to 4 hours after administration, however, and is usually inadequate to prevent or control even moderate pain after surgery.
• At higher doses, opioids can be used in combination with tranquilizers to induce a state of potent sedation (neuroleptanalgesia) that offers considerable analgesia throughout the surgical period and for some time after surgery. (See the discussions of neuroleptanalgesia in Chapter 3 and standing chemical restraint in Chapter 9.)
• Opioids can be used on their own or in combination with other agents (e.g., alpha2-agonists, NSAIDs, and local anesthetics) to provide postoperative pain control (see later).
Individual opioids vary in their potency, duration, and adverse effects. Mu opioid receptor agonists (morphine, fentanyl, hydromorphone, oxymorphone, methadone, and meperidine) are generally considered to produce the most potent analgesic effects but are equally likely to induce unwanted adverse effects. They are used for moderate to severe pain. Partial mu agonists (buprenorphine, nalbuphine) and agonist-antagonists (nalbuphine, butorphanol) are less potent analgesics, and the degree of sedation is also less pronounced, as are adverse effects on the cardiovascular and respiratory systems. Because of their weaker analgesic effect, these agents should be reserved for use as preanesthetics and for treatment of mild to moderate pain.
In addition to their analgesic properties, many opioids cause some degree of sedation and relieve anxiety. If used as sole agents and at high doses, some may induce excitement in patients that are awake, particularly cats and horses.
Common gastrointestinal effects are characterized by an initial increase in gastrointestinal activity, including nausea and vomiting in cats and dogs, and defecation. This is followed by a relative slowdown of the gastrointestinal tract with the development of ileus, colic in horses, and constipation.
Opioids are metabolized in the liver. Animals with liver disease may have impaired drug metabolism, and these agents should be given at reduced doses.
Opioid Agents Used for Moderate to Severe Pain
The potent opioid analgesics most commonly used in veterinary medicine are morphine, oxymorphone, hydromorphone, methadone, and fentanyl.
Morphine: Morphine was the first opioid agent used in human and veterinary medicine and is still extensively used in veterinary practice as a preanesthetic and an analgesic. It is a pure agonist with affinity for both mu and kappa opioid receptors (see Chapter 3). Morphine can be used in cats, dogs, and horses; however, as the likelihood for excitement or dysphoria is higher in cats and horses, it may be prudent to use lower doses initially in these species. Restlessness is seen in some dogs and horses (which manifests as an increase in locomotor activity) shortly after morphine administration, especially in the absence of pain. In addition, there is conflicting evidence as to whether morphine is an effective analgesic in horses when given systemically. For this reason morphine is more commonly administered to horses in combination with other drugs or administered epidurally.
Morphine is an inexpensive and effective option for treatment of moderate to severe pain in most patients. It is effective in treating both visceral and somatic pain and can be given by several routes, including slow IV, IM, SC, intraarticular, and epidural routes and by spinal injection. Morphine is also available in regular or sustained release tablets for oral use, although efficacy of oral morphine varies considerably among patients.
When morphine is given intravenously, it must not be injected too rapidly, particularly in dogs, as rapid injection may cause release of histamine (characterized by a fall in blood pressure, flushing, and pruritus). One technique for use in dogs is to draw up a loading dose of 0.1 to 0.5 mg/kg, which is given slowly intravenously (over 5 minutes) and repeated until the patient appears free of pain or adverse effects occur. Morphine can also be administered intravenously by constant rate infusion (CRI). With this technique, morphine contained in a syringe is slowly administered through an IV catheter by means of a syringe pump at a rate of 0.05 to 0.3 mg/kg/hr.
IM administration of morphine (and other opioids) results in a slightly longer duration of action than IV administration and avoids the risk of hypotension, although IM injection appears to be somewhat painful. SC injections cause less patient discomfort (although SC injections have a slightly slower onset of effect). Also, the incidence of excitement, dysphoria, and vomiting in cats is lower when morphine is given subcutaneously rather than intramuscularly.
Although morphine provides potent analgesia and sedation, it is associated with several undesirable adverse effects. In cats and dogs there is initial gastrointestinal stimulation characterized by vomiting, salivation, and defecation. The incidence of vomiting can be reduced by pretreatment with acepromazine (0.02 mg/kg IM, 15 to 20 minutes before the morphine is given). In horses morphine may cause ileus and colic. Morphine also has the potential to cause severe respiratory depression, although this is less common in animals than in human patients. Excitement (particularly in cats given doses greater than 0.1 mg/kg), bradycardia, panting, increased intraocular pressure, increased intracranial pressure, urinary retention, miosis (in dogs), mydriasis (in cats), hypothermia, and hyperthermia (cats) are also encountered in some patients after morphine administration. Fortunately, these adverse effects are seldom a significant problem in patients in pain that are treated with analgesic doses. Morphine also reduces cardiac afterload and is sometimes used in dogs with congestive heart failure, especially if sedation is desirable.
Morphine has a strong tendency to cause physical dependence (addiction) in humans. It is therefore classified as a Schedule II drug in the United States and is designated as a narcotic in Canada.
Oxymorphone: Oxymorphone is a pure opioid agonist with a greater analgesic potency and sedative effect than morphine, fewer adverse effects, and a longer duration of analgesia (4 hours). Despite these advantages, use of oxymorphone in veterinary practice is somewhat limited by its high cost.
Oxymorphone has a lesser tendency to induce vomiting in cats and dogs than morphine does. Also, unlike morphine, it does not induce histamine release and therefore does not decrease blood pressure. For this reason, it is preferred to morphine in patients with trauma, which may be in shock, or at increased risk for developing hypotension.
Oxymorphone may cause respiratory depression in some animals, especially in animals under inhalation anesthesia. Paradoxically, it also induces panting in many dogs, which may make some procedures (such as thoracic radiography) difficult. Oxymorphone causes some animals to become hyperresponsive to sound, and affected animals are easily startled by sudden noises. Bradycardia is also a potential adverse effect of this drug that can be prevented by pretreatment with atropine or glycopyrrolate.
Oxymorphone can be administered by many routes, including the IV, IM, SC, and epidural routes. Oxymorphone may be given rapidly intravenously to dogs, but rapid IV administration may cause excitement in cats. A tranquilizer such as dexmedetomidine, acepromazine, or diazepam may be used concurrently to prevent excitement and supplement the sedative effect of oxymorphone. Oxymorphone mixed with sterile saline and administered by IV drip is a safe analgesic agent in very sick or debilitated animals. Oxymorphone can be mixed with acepromazine (at a dose of 0.05 to 0.1 mg of acepromazine per kilogram, not to exceed 3 mg, and 0.2 mg of oxymorphone per kilogram) and used to induce anesthesia. Oxymorphone and diazepam (IV, administered alternately with separate syringes) can also be used to induce anesthesia with less risk of hypotension or decreased cardiac contractility than oxymorphone-acepromazine.
Oxymorphone is used infrequently in large animal patients because of cost and lack of information about adverse effects and analgesic efficacy.
Oxymorphone is classified as a Schedule II drug in the United States and as a narcotic in Canada.
Hydromorphone: Hydromorphone is an opioid agonist with slightly less potency than oxymorphone and with a similar duration of effect. Hydromorphone is much less expensive than oxymorphone. It can be given via the IV, IM, and SC routes to both cats and dogs at a dose of 0.05 to 0.2 mg/kg. Unlike morphine, it is not associated with histamine release and has less potential to cause excitement in cats. Otherwise, adverse effects are similar to those seen with morphine: respiratory depression, bradycardia, vomiting, panting, excessive sedation, and excitement (seen especially at doses greater than 0.2 mg/kg).
Like oxymorphone, hydromorphone can be used as a premedication (at 0.1 to 0.2 mg/kg IM, alone or in combination with a tranquilizer), as an analgesic (at 0.05 to 0.2 mg/kg IM or SC for dogs and cats, repeated every 4 to 6 hours), or as an induction agent for high-risk patients (0.05 to 0.1 mg of hydromorphone per kilogram by slow IV injection followed by 0.05 to 0.2 mg of diazepam per kilogram intravenously). It is also given by the epidural route (0.03 to 0.1 mg) and has a similar effect to morphine.
Hydromorphone is used infrequently in large animal patients because of lack of information about adverse effects and analgesic efficacy.
Hydromorphone is classified as a Schedule II drug in the United States and as a narcotic in Canada.
Methadone: Another synthetic opioid, methadone has similar characteristics to oxymorphone and hydromorphone with the exception that it has the lowest likelihood of causing vomiting in cats and dogs. This drug is also an antagonist at the NMDA receptor (see later), which may make it a favorable choice for treating pain when central sensitization is present or is likely to develop.
Fentanyl: Among the most potent analgesics known, fentanyl has a rapid onset and short duration of effect in small animals (onset approximately 2 minutes, duration of effect approximately 20 to 30 minutes after IV injection). It is most commonly administered by continuous IV drip or by means of a transdermal patch (see following section). In small animal patients, fentanyl and midazolam or diazepam drawn into separate syringes can be used as induction agents. When fentanyl is given intravenously, a loading dose of 1 to 5 mcg/kg is typically administered, followed by a CRI of up to 10 mcg/kg/hr. It can also be given by IM, SC, or epidural injection.
Fentanyl can induce profound sedation, bradycardia, and respiratory depression in human patients, and like oxymorphone it may cause panting or increased sensitivity to sound. Adverse effects of fentanyl are further discussed in the section on transdermal patches later in the chapter.
Fentanyl was formerly sold in combination with the tranquilizer droperidol, in the injectable neuroleptanalgesic Innovar-Vet; however, this combination is no longer commercially available. Fentanyl is sold in combination with the tranquilizer fluanisone (under the name Hypnorm) in some countries.
Fentanyl (including fentanyl patches) is a Schedule II drug in the United States and is classified as a narcotic in Canada.
Meperidine or pethidine: A synthetic opioid, meperidine has been extensively used in veterinary medicine as an analgesic and preanesthetic agent. Although meperidine is classified as a pure opioid agonist, its analgesic properties are less potent than those of the other pure agonists, and it is by comparison short acting. Meperidine is usually administered by SC injection, because IM injection may be painful and rapid IV injection may cause severe hypotension, excitement, and seizure-like activity.
Meperidine has a wide safety margin and causes less respiratory depression and gastrointestinal stimulation than morphine. It decreases salivary and respiratory secretions, a property that may be helpful in preanesthesia. Like morphine, it may cause histamine release in some patients, and pretreatment with histamine blockers may be advisable before IV injection.
In the past, meperidine was commonly used alone as a postoperative analgesic at a dose rate of 2 to 5 mg/kg IM or SC. Unfortunately, its analgesic effect is weak and of short duration, particularly in cats. For this reason it is no longer considered to be suitable for postoperative analgesia in animals, and in recent years it has been superseded by other opioids, particularly agonist-antagonists, buprenorphine, and butorphanol.
Despite its weak analgesic properties in animals, meperidine is still a useful drug for preanesthetic mixtures in combination with atropine and low doses of acepromazine. When administered with a tranquilizer (usually diazepam or acepromazine), meperidine also provides effective neuroleptanalgesia in puppies. Meperidine is also used in conjunction with injectable NSAIDs such as ketoprofen because it confers analgesia during the 30 to 60 minutes before the NSAID takes effect.
Meperidine is rarely used to provide analgesia in large animal patients.
Meperidine is a Schedule II drug in the United States and is classified as a narcotic in Canada.
Butorphanol: A synthetic opioid with agonist and antagonist properties, butorphanol was first used in veterinary medicine as a cough suppressant. Butorphanol is widely used as a preanesthetic or sedative (at a dose of 0.1 to 0.4 mg/kg SC or IM or in mixtures with dexmedetomidine or acepromazine) and is also an effective postoperative analgesic for mild to moderate visceral pain.
Butorphanol is a mixed agonist-antagonist agent that stimulates kappa receptors and antagonizes or blocks mu receptors (see Chapter 3). As such, it is not as effective as the pure agonists for treating severe pain, especially orthopedic pain. Butorphanol is, however, an effective and safe treatment for mild to moderate visceral pain, especially cranial abdominal pain, in both dogs and cats. It is also extensively used in horses and ruminants.
Butorphanol is available in several concentrations, including 0.5 mg/mL, 2 mg/mL, and 10 mg/mL. Injectable butorphanol may be given via the IV, IM, or SC route. It is potentially toxic to the spinal cord, which limits its use for epidural injection. Tablets are available for long-term administration (at a dose of 0.4 to 1 mg/kg tid) and may be dispensed for postoperative pain.
The duration of analgesia provided by butorphanol may be short (as little as 1 hour in dogs after IM or SC injection). To avoid frequent readministration, butorphanol can be given as a CRI (in IV fluids or by syringe pump) at a dose of 0.1 to 0.2 mg/kg/hr, after a loading dose of 0.2 to 0.4 mg/kg.
Butorphanol produces less sedation, dysphoria, and respiratory depression than most opioids. Although moderate doses of butorphanol may cause some respiratory depression, higher doses do not depress respiration further (a phenomenon known as the ceiling effect). Heart rate, blood pressure, and cardiac output may be decreased after the administration of butorphanol; however, the effect is less than that of morphine, and pretreatment with atropine is not usually required. Panting, vomiting, and histamine release are seldom seen with this drug.
Butorphanol can be used as an antagonist to partially reverse respiratory depression and sedation induced by mu agonist opioids such as morphine or fentanyl, although the anesthetist must be aware that it will also partially reverse the analgesic effect of these drugs. The dose used for reversal is 0.2 to 0.4 mg/kg, administered intravenously to effect in increments of 0.01 to 0.05 mg/kg every 3 to 5 minutes. The antagonistic effect of butorphanol is less predictable and less potent than that of naloxone and can be overridden by subsequent administration of high doses (two to three times the normal dose) of morphine.
Since 1997, butorphanol has been classified as a Schedule IV drug in the United States. In Canada it is classified as a controlled drug.
Buprenorphine: Buprenorphine is a partial mu agonist. It stimulates mu receptors, producing some analgesia, but is less effective than morphine and other pure agonists.
Buprenorphine can be given by the IV, IM, or epidural route. It has a delayed onset of action (15 minutes intravenously and 40 minutes intramuscularly) but provides a longer duration of analgesia than other opioids (authorities suggest as little as 6 hours or as long as 12 hours after IM injection and 18 to 24 hours after epidural injection). The injectable formulation of buprenorphine can be administered orally to cats; the pH of the feline mucosa allows excellent absorption via this route. Like butorphanol, buprenorphine does not provide adequate analgesia for severe pain (such as orthopedic pain), but it is useful for mild to moderate pain. It is commonly used to provide analgesia for rodents and other species used in research, as well as postoperative analgesia for dogs and cats.
Like butorphanol, buprenorphine can be used to reverse the sedation and respiratory depression induced by mu agonist opioids while maintaining some analgesic effect. Its effectiveness as a reversal agent is not as dramatic as butorphanol’s because of the delay in its onset of action.
Buprenorphine at high doses may induce respiratory depression, which is difficult to reverse with naloxone. Analeptic agents such as doxapram may be somewhat effective in correcting buprenorphine-induced respiratory depression. Intubation and assisted ventilation are necessary in some patients because there is a potential for carbon dioxide retention and increased intracranial pressure.
Although buprenorphine has little sedative effect on its own, it can prolong sleep times when given with other agents.
Buprenorphine is a Schedule V drug in the United States and is unavailable in Canada. It is relatively expensive and is sometimes provided in ampules that are inconveniently large for small animal patients. Buprenorphine can, however, be transferred to sterile vials for use on multiple patients.
Nalbuphine: Nalbuphine is a kappa agonist and mu antagonist like butorphanol, but its antagonist properties are greater. It is currently the only injectable opioid agent for veterinary use that is not classified as a controlled drug in the United States. It is a weak analgesic and sedative and can be used as a reversal agent for opioid agonists. Bradycardia, respiratory depression, and sedation are uncommon with this agent.
Use of Opioid Injections to Treat Postoperative Pain
Opioids can be administered by a variety of routes for prevention or treatment of postoperative pain. In many practices, opioids are given by IM or SC injection, preferably before the animal regains consciousness from anesthesia. Injections may be repeated as necessary to prolong the analgesic effect (see Table 7-1 for information on dose and duration).
From the standpoint of analgesia, one major disadvantage of opioid agents is their relatively short duration of effect when given via the SC or IM route. Morphine offers 2 to 3 hours of analgesia for severe pain and 4 to 6 hours for moderate or mild pain; hydromorphone, 2 to 4 hours; oxymorphone, 1.5 to 5 hours; meperidine, less than 1 hour; fentanyl, approximately 20 minutes; and butorphanol, 1 to 2 hours in dogs and up to 4 hours in cats. Repeat injections can be given but are expensive, require hospitalization, and create “peak-and-trough” blood levels instead of a constant, effective blood concentration.
A second disadvantage of opioid use is the potential for adverse effects such as respiratory depression, bradycardia, excitement (usually exhibited as apprehension, hypersalivation, and mydriasis), excessive sedation, panting, increased sensitivity to sound, urinary retention, and gastrointestinal effects. These adverse effects are seldom severe when analgesic dosages are used. However, it is probably advisable to avoid opioid use or to use with caution in high-risk patients such as those with hypotension, hepatic disease, preexisting respiratory difficulties, CNS disorders such as head injuries or increased intracranial pressure, or altered bowel motility.
Both disadvantages of opioids—their short duration and potential for adverse effects—may be partially overcome by giving these drugs by routes other than IM or SC injection. Alternative routes for opioid administration include IV infusion, epidural injection, transdermal patches, and intraarticular administration. When used by these alternative routes, opioids may produce effective, economical, and long-acting analgesia with minimal adverse effects. However, use of opioids by these routes constitutes off-label use in many animal species, and informed consent should be obtained from the animal’s owner.
Intravenous Infusion of Opioids
Morphine, fentanyl, oxymorphone, hydromorphone, methadone, and butorphanol can be given intravenously by CRI. This is sometimes the only method of analgesic delivery that is effective in constant, unremitting pain. Animals are given an initial loading dose (e.g., 0.1 mg of morphine per kilogram intravenously every 3 to 5 minutes) until the desired effect is achieved. The same dose is then given over 4 hours through a constant flow of IV fluids. At its most elaborate, IV infusion consists of an automated infusion pump or syringe pump. Practices that lack this type of specialized equipment can deliver opioid analgesics continuously in IV fluids by adding an opioid directly to the bag of fluids or burette, making sure to rotate the bag several times for adequate mixing (see Table 7-1 for doses). Patients must be frequently monitored for signs of inadequate pain control (in which case the rate of administration should be increased) or excessive sedation, dysphoria, respiratory depression, bradycardia, panting, and other signs that indicate an overdose. If these signs are present, the rate of administration should be decreased. The duration of pain control (for morphine) is 30 minutes past the discontinuation of the IV fluids. If morphine is inadequate to control pain, lidocaine also can be added to the fluids and infused at 5 to 20 mcg/kg/min. For severe pain, morphine, lidocaine, and ketamine can be co-infused (see later).
Intraarticular Use of Opioids
Opioids may be given by the intraarticular route, particularly after elbow or stifle surgery. In this technique, 0.1 to 0.3 mg/kg of morphine is diluted in a volume of saline equivalent to 1 mL/10 kg body weight and instilled into the joint with a sterile catheter immediately after closure of the joint capsule. The morphine can also be combined with a local anesthetic such as 0.5% bupivacaine (at a dose of 1 mL/4.5 kg). This technique provides 8 to 12 hours of postoperative analgesia.
Epidural Use of Opioids
With the instillation of a small dose of an opioid or other analgesic into the epidural space at the lumbosacral junction, it is possible to achieve excellent analgesia of the hind limbs, abdomen, caudal thorax, pelvis, and tail. Currently, morphine is the drug most commonly used for epidural analgesia. Oxymorphone is more expensive, butorphanol is less effective and may have some spinal toxicity, and local anesthetics such as lidocaine may impair movement, urination, and defecation and may cause a sympathetic blockade if the drug diffuses too far cranially. Occasionally, morphine and local anesthetics are used epidurally in combination. Morphine is also sometimes combined with an alpha2-agonist for epidurals in large animal patients.
Morphine given by the epidural route offers more profound analgesia for a longer time than when given by IM or SC injection. The analgesia is not sufficient for a surgical procedure unless supplemented with general anesthesia; however, it is an excellent means of achieving postoperative pain relief. Epidural morphine has a direct, long-lasting effect on the pain receptors in the spinal cord, but it does not reach high concentrations in the bloodstream because of low fat solubility. Adverse effects such as sedation (dogs), excitement (cats), respiratory depression, and nausea are therefore rare.
To achieve preemptive analgesia for postoperative pain, epidural morphine should be given after induction but before the surgical procedure. Epidural morphine is significantly less effective when administered in the postoperative period. Preservative-free morphine is preferred, because the preservatives typically found in morphine preparations (formaldehyde and phenol) are potentially neurotoxic.
The technique for epidural morphine administration is similar to epidural administration of lidocaine (see Procedure 6-3). Normally the animal is anesthetized or deeply sedated and is positioned in sternal recumbency with the head slightly elevated and the hind limbs pulled forward to open the lumbosacral space. An epidural puncture is performed. Once it has been determined that the needle is in the epidural space, morphine is injected over 30 seconds. Currently, the recommended dose for epidural morphine is 0.1 mg/kg in dogs and 0.05 to 0.1 mg/kg in cats. Ideally, a single-dose vial of preservative-free morphine should be used, diluted with sterile saline to a volume of 0.3 mL/kg. The recommended maximum volume that can be injected is 0.45 mL/kg. Onset of analgesia is approximately 20 to 60 minutes after injection, and analgesia lasts 6 to 24 hours. If more prolonged analgesia is required, an epidural catheter can be used to instill morphine into the epidural space over a longer period (hours to days).
Although epidural analgesia is regarded as a safe procedure, it should not be undertaken in animals with septicemia, local infections in the lumbosacral space, bleeding disorders, spinal trauma, or neurologic disease of the spinal cord. It is relatively difficult to administer epidural anesthetics to obese animals. Epidural hematomas and abscesses may result from improper needle placement or unsterile technique. Urinary retention may occur in the first 24 hours after surgery, and the bladder should be monitored closely in all patients that have received epidural analgesics. Urinary catheterization may be necessary in some patients. Pruritus, delayed respiratory depression, sedation, vomiting, and nausea have been reported in human patients but are uncommon in dogs. These symptoms, if they occur, can be treated with naloxone hydrochloride (0.01 mg/kg IV). Animals that have received epidural morphine should be repositioned every 2 to 4 hours because normal sensation may be absent. Failure to reposition animals may result in pulmonary atelectasis or prolonged pressure on superficial nerves, leading to temporary or permanent loss of function.
Transdermal Use of Opioids
Transdermal patches containing fentanyl are another convenient option for long-term opioid administration. Fentanyl patches have been used for several years in the treatment of severe pain in human patients. The analgesic effect of a fentanyl patch is thought to be comparable to that of IM oxymorphone, but the duration of analgesia is considerably longer.
A “patch” consists of a reservoir of fentanyl enclosed in plastic. The patch is applied to the clipped skin of the animal and is left in place for several days (Procedure 7-1, p. 230). Patches come in different sizes that deliver different amounts of fentanyl each hour. A 12.5-mcg/hr patch is useful in animals weighing less than 4 kg. The 25-mcg/hr patch is used in cats and in dogs that weight less than 7 kg. A 50-mcg/hr patch is used in dogs weighing 7 to 20 kg; a 75-mcg/hr patch is used in dogs weighing 20 to 30 kg; and dogs that weigh more than 30 kg receive a 100-mcg/hr patch. Large animal patients may require several patches for effective plasma levels of fentanyl to be reached. Patches should not be cut or trimmed because this will cause erratic drug release and possible human exposure. In patients showing signs of inadequate pain control 24 hours after patch application, a second patch or another analgesic agent can be added.
Because fentanyl is relatively slowly absorbed through the skin, there is a delay of 4 to 12 hours in cats and 12 to 24 hours in dogs before therapeutic blood levels are achieved. To achieve preemptive analgesia, apply the patch at least 6 hours before the start of anesthesia in cats and at least 12 hours before the start of surgery in dogs. If application of the patch is delayed until after surgery, it is necessary to provide the patient with another opioid (e.g., morphine, hydromorphone, or oxymorphone) or NSAID such as meloxicam, ketoprofen, or carprofen until the patch takes effect. Butorphanol or buprenorphine should not be used concurrently with a fentanyl patch because either may partially block the opioid receptors, reducing the analgesic effect. Procedure 7-1 demonstrates how to apply a patch.
Many types of patients benefit from a fentanyl patch, including postoperative patients (e.g., after onychectomy, orthopedic procedures, or abdominal surgery) and those that have trauma, burns, cancer, or painful abdominal conditions such as pancreatitis.
Recent studies have shown considerable variation among animals in the concentration of fentanyl absorbed from a transdermal patch. One study showed that a 50-mcg/hr patch in dogs delivered as little as 13.7 and as much as 49.8 mcg/hr. Patients should be observed for signs of breakthrough pain (which may indicate a low plasma fentanyl concentration) and supplemented with morphine, oxymorphone, hydromorphone, or an NSAID as required.
Excessively high plasma fentanyl concentrations may develop in some patients. If this occurs, the most common signs are ataxia and sedation in dogs and dysphoria and disorientation in cats. Affected cats appear fearful or excited, are hypersensitive to sound, and may have widely dilated pupils. Panting is also a problem in some animals. Treatment, if necessary, consists of removing the patch and/or giving a narcotic antagonist (e.g., naloxone or butorphanol).
There have been some reports of death caused by respiratory failure when human patients self-administered more than one patch at a time, but respiratory depression is apparently uncommon in veterinary patients with fentanyl patches. Respiratory depression may be seen in trauma patients, particularly animals with CNS signs. Other adverse effects reported in human patients include constipation, physical dependence, muscle rigidity, miosis, mood changes, bradycardia, and bronchoconstriction. Use of fentanyl patches is not recommended in human patients with respiratory disease, increased intracranial pressure, impaired consciousness, bradycardia, pulmonary disease, hepatic or renal dysfunction, or brain tumors; these recommendations may also hold true for animals. Some patients may exhibit a mild transient dermatitis at the patch site after removal, and delayed hair regrowth at the patch site is common.
Transdermal patches may release excessive amounts of fentanyl if they are heated, and fentanyl overdoses have been reported in human beings who lie under electric blankets while wearing a patch. It is therefore suggested that fentanyl patches be avoided in animals with fevers and that patch contact with hot water bottles and other external sources of heat be avoided.
There is some concern regarding the potential for abuse by adult people or ingestion of a patch by a child. For this reason, the manufacturer does not support the use of fentanyl patches in animals. Some veterinarians address this concern by using the patch only on hospitalized animals or by carefully selecting and educating owners before discharging an animal that is wearing a patch.
Nonsteroidal Antiinflammatory Drugs
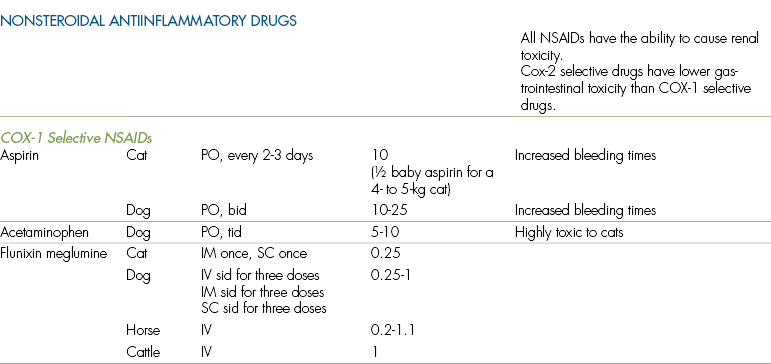
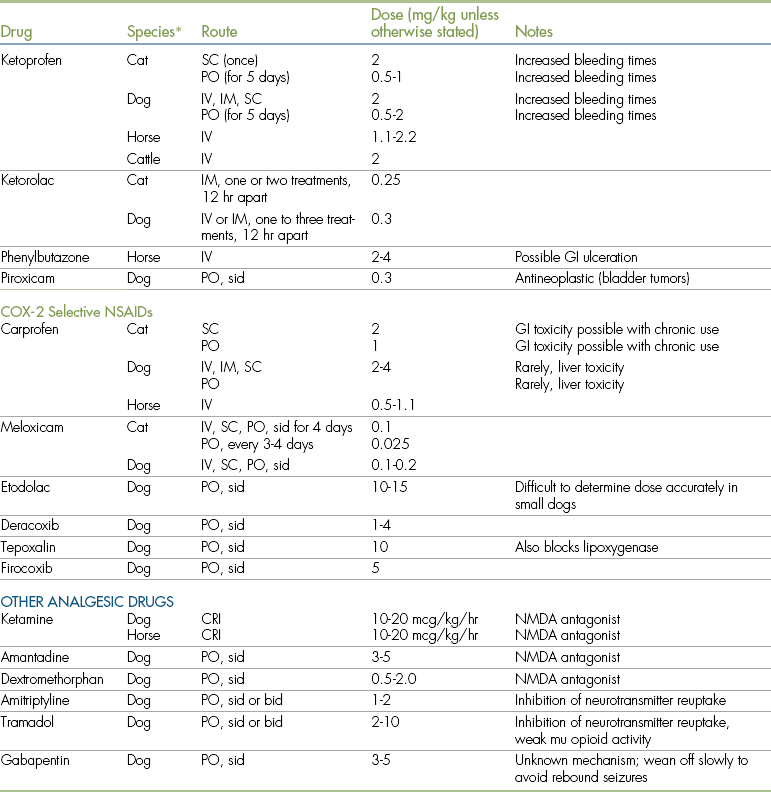
NSAIDs, also called nonsteroidal antiinflammatory analgesics or NSAAs, are a large group of agents that have been used for many years to control minor pain in human beings and animals. The NSAID group includes such common drugs as acetylsalicylic acid (aspirin) and acetaminophen, and newer agents such as carprofen, meloxicam, etodolac, ketoprofen, tolfenamic acid, firocoxib, and deracoxib. Dose and toxicity information for individual NSAID agents are summarized in Table 7-1.
Traditionally veterinarians have thought that NSAIDs are not potent enough to treat anything other than mild postoperative pain. However, newer and more powerful NSAIDs such as ketoprofen, meloxicam, and carprofen are increasingly used for postoperative analgesia after procedures as diverse as ovariohysterectomy and fracture repair. NSAIDs are also useful for treatment of dental pain, panosteitis, osteoarthritis, meningitis, mastitis, and other painful medical conditions because of their strong antiinflammatory action.
Mechanism of Action
NSAIDs have several beneficial effects on animal patients, including the following:
• All NSAIDs appear to be effective analgesics for somatic (musculoskeletal) pain. Some NSAIDs such as aspirin have little efficacy against visceral (organ-related) pain, whereas others such as ketoprofen and carprofen are potent analgesics with both somatic and visceral activity. All NSAIDs require approximately 30 to 60 minutes to achieve full analgesic effect, regardless of the route of administration.
• Many NSAIDs have potent antiinflammatory properties. This, combined with their analgesic effect, is the basis for the widespread use of drugs such as aspirin and carprofen in the treatment of osteoarthritis, panosteitis, hypertrophic osteodystrophy, and muscular pain.
The clinical effects of NSAIDs stem chiefly from their inhibition of prostaglandin synthesis. Prostaglandins (often abbreviated PGs) are a group of extremely potent chemicals that are normally present in all body tissues and are involved in the mediation of pain and inflammation following tissue injury. Prostaglandins are also responsible for a variety of homeostatic (“housekeeping”) processes, including maintenance of normal gastrointestinal, reproductive, renal, and ophthalmologic function. Most NSAIDs prevent pain and inflammation by inactivating the enzyme cyclooxygenase (COX), which catalyzes one of the steps in the production of prostaglandins. There are two important COX isoenzymes that vary in importance from tissue to tissue. COX-1 is normally present (or constitutive) in most tissues, whereas COX-2 is constitutive in some, such as the kidney, reproductive organs, and eyes. COX-2 is inducible (i.e., not normally present, but is produced under certain circumstances), particularly during tissue damage and inflammation. Inhibition of both COX isoenzymes, but particularly COX-2, has been linked to analgesic effects. Most NSAIDs inhibit both COX-1 and COX-2, although the ratio of COX-1 to COX-2 inhibitory effects of individual NSAIDs vary considerably. Drugs that are COX-2 selective (carprofen, meloxicam, deracoxib) or specific (firocoxib) are less likely to interfere with intestinal barrier function and produce gastrointestinal ulceration; however, all NSAIDs have the potential to be nephrotoxic.
The relative effect of an NSAID on these enzymes will determine both the analgesic potency and the severity and type of adverse effects after the administration of that particular drug (see the separate section on adverse effects).
Although some NSAIDs are active against prostaglandins in peripheral tissues only, others (e.g., acetaminophen and ketorolac) exert their effects mainly on prostaglandin synthesis in brain tissue and are therefore said to be “central acting.” Some agents (ketoprofen, meloxicam) appear to exert their effects both centrally and in the peripheral tissues.
As a group, NSAIDs are well absorbed orally, and many are available in tablet or liquid form. Recently, potent injectable NSAIDs have also become available. Injectable NSAIDs can be given at the end of surgery to provide 24 hours of pain relief. Some NSAIDs, for example, carprofen, can also be used before surgery in selected patients to achieve preemptive analgesia. If long-term analgesia is required, injections can be repeated in some cases, or tablets can be dispensed.
All NSAIDs are eliminated by metabolism and conjugation within the liver, followed by renal or biliary elimination. The NSAID group of drugs is unusual in that there is significant variation in duration of effect between species. For example, the plasma half-life of aspirin is 1 hour in the horse, 8 hours in the dog, and 38 hours in the cat. The prolonged half-life of aspirin in the cat is a result of the low levels of the enzyme glucuronyl transferase (one of the enzymes that metabolizes salicylate NSAIDs, such as aspirin) in that species. There is also significant variation among species in the toxicity of particular NSAIDs. For example, acetaminophen is extremely toxic in cats but is a useful agent in dogs. Similarly, ibuprofen is considered to be safe for use in humans but has significant toxicity in dogs and cats. Because of this variation, the safety of any NSAID in one species does not imply that it can be used with impunity in all species (see Table 7-1 for dosages and cautions for specific agents). In particular, it cannot be assumed that dosages and administration schedules that are appropriate for dogs can be safely used in cats.
NSAIDs have some advantages over opioids: they are not subject to the storage, handling, and record-keeping regulations that govern narcotics; they have little abuse potential; and they are effective when given orally. Unlike opioids, NSAIDs have a negligible effect on the cardiovascular and respiratory systems. NSAIDs also do not depress the CNS and therefore lack the sedative effect of opioids. When used in healthy young to middle-aged patients according to label directions, they provide effective and safe relief for mild to moderate pain. For some applications, their analgesic effect appears to be superior to that of butorphanol or meperidine.
Adverse Effects
Unfortunately, NSAIDs as a group have significant potential for toxicity in small animal patients. Most people who work in veterinary hospitals are aware of the toxicity of acetaminophen in cats. A single 325-mg capsule may cause acute hepatotoxicosis within 4 hours of ingestion because of the formation of toxic metabolites within the liver. Many NSAIDs are safe for use in healthy animals but can have serious toxic effects in animals that are dehydrated or hypotensive.
Many of the adverse effects of NSAIDs are attributable to the fact that they reduce not only the production of the prostaglandins that mediate pain, inflammation, and fever, but also those that are beneficial. Pharmaceutical companies have attempted to formulate NSAIDs that will prevent the production of harmful prostaglandins while preserving the production of beneficial prostaglandins. This can be achieved if the NSAID inhibits the enzyme COX-2 (which is active in damaged or inflamed tissues and synthesizes the prostaglandins that cause pain) but does not affect COX-1 (which synthesizes the prostaglandins that help maintain normal physiologic functions such as protection of the gastric mucosa and modulation of blood flow to the kidney). In theory, it is possible to produce NSAIDs that have more than 1000-fold specificity for COX-2 over COX-1 and are therefore extremely safe for use in terms of their gastrointestinal adverse effects. However, the drugs currently available do not have this degree of specificity, and the COX-2 isoenzyme is important for normal function in some organs, such as the kidney and reproductive tract. Additionally confusing, an agent that has pronounced specificity for COX-2 in one species does not necessarily show the same specificity in another species.
One example of a beneficial prostaglandin that is adversely affected by many NSAIDs is prostacyclin, which is normally present within the stomach mucosa and helps reduce gastric acid secretion and promote mucous production. When prostacyclin levels are reduced by the administration of an NSAID, gastric acid secretion increases and mucous production decreases, which sometimes leads to the production of stomach ulcers. Up to 50% of dogs treated with aspirin have mild stomach ulceration within a few days of treatment, which may result in vomiting, gastrointestinal bleeding, and inappetence, but more often is not clinically apparent. Occasionally, animals with gastrointestinal ulceration resulting from NSAID use may undergo a sudden episode of life-threatening hemorrhage. In dogs, ulcerogenic potential appears to be high for ketoprofen, naproxen, ibuprofen, flunixin, piroxicam, and meclofenamic acid, and use of these agents for prolonged periods (over 5 days) is associated with a high incidence of adverse effects. Meloxicam, carprofen, and etodolac have less ulcerogenic activity in dogs and are preferred for long-term use, as in dogs with osteoarthritis.
In an effort to avoid gastrointestinal problems in human beings and animals receiving NSAIDs, pharmaceutical companies have prepared enteric-coated or buffered formulations. Enteric coating does not appear to reliably reduce the toxicity of these drugs; however, buffered formulations may have reduced toxicity. It is also helpful to administer oral NSAIDs with a meal to dilute the drug that is present in the stomach. In susceptible patients, it may be advisable to use gastrointestinal protectants such as sucralfate suspension (at a dose of 0.25 to 0.5 g by mouth [PO] tid in cats, 0.5 to 1 g PO tid in dogs) in conjunction with an NSAID to prevent or treat gastrointestinal effects. Sucralfate forms a proteinaceous complex that adheres to damaged gastric mucosa, preventing further injury. Sucralfate should be administered on an empty stomach at the same time as the NSAID. Another helpful gastrointestinal protectant is the synthetic prostaglandin misoprostol, which is given orally at a dose of 2 to 4 mg/kg tid. Histamine-2 (H2)–receptor antagonists such as famotidine or ranitidine are also helpful in treatment of stomach ulcers but should not be given at the same time as sucralfate, which requires an acid environment to work.
Another potential adverse effect of NSAIDs is renal toxicity. A beneficial prostaglandin, PGE2, normally maintains adequate blood flow within the kidney. In anesthetized animals and other animals that are prone to hypotension (such as trauma patients), PGE2 plays a vital role in maintaining renal blood flow. By blocking synthesis of PGE2, NSAIDs have the potential to decrease renal blood flow in these patients, leading to renal hypoxia. Dogs are apparently very susceptible to development of renal failure when blood pressure decreases, and there are several reports of acute renal failure after the administration of NSAIDs during anesthesia. To avoid the risk of renal damage in anesthetized patients, the use of NSAIDs should be postponed until after anesthesia, and preemptive or intraoperative use is not advised unless the patient is receiving intraoperative IV fluids and arterial blood pressure monitoring is available. Fortunately, NSAID-induced renal insufficiency is usually reversible (in young, healthy patients) with the administration of IV fluids. It is much more difficult to reverse in geriatric patients with preexisting renal failure. It is a prudent practice to screen geriatric patients for renal disease before anesthesia (by determining values for blood urea nitrogen, creatinine, and/or urine specific gravity) and to avoid NSAIDs in patients with decreased renal function.
Another potential adverse effect of NSAIDs is impaired platelet aggregation, which can lead to prolonged bleeding times. This effect may be beneficial in some circumstances (e.g., by lowering the risk of stroke in human patients who regularly take aspirin). However, there is a potential for increased bleeding in patients that are given NSAIDs before or during surgery. As with the potential for renal toxicity, this concern can be minimized by postponing the use of NSAID agents until after surgery has been completed. If preemptive use of an NSAID is indicated, carprofen can be used (in the dog), because it has been shown to have less renal toxicity and platelet-inhibiting effect than some other NSAID agents.
Liver damage appears to be associated with the use of NSAID agents in some patients. Carprofen has been extensively studied in this regard, and although the incidence of liver disease is small, this is a recognized adverse effect of this drug. Hepatocellular toxicosis appears to be most common in Labrador retrievers and may be evident as soon as 2 weeks after initiation of treatment. Monitoring bile acid levels appears to be a sensitive method of detecting early signs of toxicity.
NSAIDs may antagonize the action of several drugs commonly prescribed for cardiac disease and hypertension, including angiotensin-converting enzyme (ACE) inhibitors, and some diuretics.
As with most drugs, there is great variation among individual patients in the potency, duration, and adverse effects produced by NSAIDs. When used for postoperative pain control, NSAIDs are safe to use in well-hydrated young to middle-aged animals with normal renal and hemostatic function. NSAIDs should be used with care or avoided entirely in dehydrated patients and in animals with coagulopathies or liver or kidney dysfunction. Because of the potential for gastrointestinal ulceration, these agents should be avoided in patients with gastrointestinal disorders and in patients that are receiving corticosteroids (which also contribute to ulcer formation). Animals that have low blood pressure, congestive heart failure, or hemostatic disorders such as thrombocytopenia are generally high-risk candidates for NSAID therapy. Patients with trauma should not receive NSAIDs unless they are in stable condition with no indication of hemorrhage, they are receiving IV fluids, and no surgery is anticipated in the next 48 hours. For some patients (e.g., geriatric patients and patients with renal disease) NSAIDs should be used only in conjunction with IV fluids and blood pressure monitoring. Opioids appear to be a safer therapeutic option in these patients.
Other Analgesic Agents
Opioids and NSAIDs are the mainstays of postoperative pain control; however, other agents may be useful in some circumstances. These include local anesthetics, alpha2-adrenergic agonists, and ketamine.
Local Anesthetics
Local anesthetic agents have long been used to allow surgical procedures to be performed in conscious animals, but their use in preventing or treating postoperative pain is relatively recent. The presence of local anesthetic blocks sodium channels, which prevents transduction and transmission of noxious stimuli into nerve impulses peripherally (local blocks) as well as centrally (if administered by epidural). Local anesthetic can be sprayed or injected at the site of an injury or a surgical site or infiltrated around nerves supplying the affected area. They can also be used to desensitize an entire region, as with epidural administration or IV infusion. Local anesthetics have many advantages, including complete anesthesia of the affected area, low toxicity (when given at the appropriate dose), and rapid onset of action. Unfortunately, the duration of action is relatively short, and the danger of CNS and cardiac toxicity limits repeated use. The use of local anesthetics for pain control is discussed in detail in Chapter 6.
Alpha2-Adrenoceptor Agonists
Although alpha2-adrenoceptor agonists such as xylazine and dexmedetomidine provide good analgesia by activating alpha2-adrenergic receptors both centrally and in the periphery, their use for pain control in small animals is limited by three factors: (1) the short duration of their analgesic effect (in the case of xylazine, 30 to 60 minutes, and for dexmedetomidine, 30 to 90 minutes); (2) the profound sedative effect of these agents; and (3) the potential for serious adverse effects (respiratory depression, vomiting, bradycardia, heart block, and hypotension, which may be exacerbated by opioids). It is difficult to determine the quality or duration of analgesia in some patients because the sedative effect of these drugs remains even after the analgesic effect has worn off. In dogs and cats, these agents should be used only for young to middle-aged, healthy patients. However, when used in low doses (e.g., xylazine at 0.1 mg/kg IV, IM, SC; and dexmedetomidine at 0.001 to 0.005 mg/kg IV, IM, SC), these agents appear to potentiate the effect of opioids and may contribute to the quality of analgesia in the postoperative period. Butorphanol and dexmedetomidine in combination appear to provide effective analgesia and sedation for minor clinical procedures.
Recently, alpha2-adrenoceptor agonists have been shown to produce significant analgesia when administered by the epidural route (alone or in combination with opioids and other agents). Dexmedetomidine (0.005 mg/kg) can be added to morphine to prolong the duration of epidural analgesia.
The analgesic effect of xylazine is antagonized by yohimbine, and the analgesic effect of dexmedetomidine is antagonized by atipamezole.
Alpha2-adrenoceptor agonists (xylazine, detomidine, romifidine) are commonly administered to horses to provide sedation, muscle relaxation, and analgesia. The degree of sedation provided is typically less than seen in small animals, and horses typically remain standing although they may become ataxic. Analgesia is adequate for moderately to severely painful diseases or procedures. Cardiovascular adverse effects such as bradyarrhythmias (including second-degree atrioventricular block), initial hypertension, and ultimately hypotension are commonly seen. Heavy sedation should be induced cautiously in horses with preexisting upper airway stridor, as relaxation of the upper airway and pharyngeal muscles, along with congestion of the nares and nasal passages, may lead to respiratory obstruction in these patients. Alpha2-adrenoceptor agonists cause decreased gut motility, which may lead to gas distension and colic postoperatively. Alpha2-adrenoceptor antagonists (yohimbine, atipamezole) can be used to reverse these unwanted effects; however, analgesia and sedation will also be reversed. Use of these agents may even result in excitement.
Ketamine
Ketamine is a dissociative injectable anesthetic (see Chapter 3) that has become popular as an adjunct to more potent analgesics (opioids, local anesthetics, alpha2-agonists) because it blocks the NMDA receptors in the CNS at the level of the spinal cord. Antagonism of the NMDA receptors is important in preventing central sensitization, or windup. The dose of ketamine needed to antagonize these receptors is much lower than that required to induce anesthesia. Ketamine can be administered as IV boluses (0.5 mg/kg) or as a CRI (10 to 15 mcg/kg/hr). Ketamine alone does not typically provide sufficient analgesia; therefore it is most commonly administered in conjunction with other drugs. A commonly used approach to provide intraoperative analgesia for painful orthopedic surgery in healthy dogs is to co-infuse morphine, lidocaine, and ketamine, or MLK (see Table 7-1 and Procedure 7-2, p. 231).
Ketamine should be avoided or used with extreme caution in patients with hypertrophic cardiomyopathy or in cats with compromised renal function. Adverse effects of ketamine are dose related and rarely seen at analgesic dosages but may include tachycardia, increased blood pressure, increased intraocular and intracranial pressure, seizures and postoperative delirium, and salivation.
Orally administered NMDA antagonists include amantadine and dextromethorphan.
Corticosteroids
These drugs (e.g., prednisone, dexamethasone) have strong antiinflammatory properties, which, as with the NSAIDs, act by decreasing prostaglandin activity. They should not be used concurrently with NSAIDs, as both drug classes are ulcerogenic. Other long-term adverse effects include immunosuppression and development of hyperadrenocorticism.
Tramadol
Tramadol is a nonopiate drug that is given orally and has activity at the mu receptor. It is useful as a postoperative alternative to opioids once a patient has resumed eating (usually 12 to 24 hours after surgery) and can be prescribed for continuation of analgesic therapy at home. An additional mechanism of tramadol is inhibition of norepinephrine and serotonin reuptake, which also promotes analgesia. Tramadol should not be administered with other norepinephrine and serotonin reuptake inhibitors (e.g., amitriptyline).
Tranquilizers
Although acepromazine, diazepam, and other tranquilizers are not considered to be analgesics, they may potentiate the effect of opioids in some patients. Possible explanations for this include the fact that pain appears to be intensified in anxious patients, and drugs that cause CNS depression also alter pain perception by the brain. Animals that have received adequate analgesia but are restless may become calmer after administration of acepromazine (0.01 to 0.05 mg/kg SC, IM, or IV) or diazepam (0.2 mg/kg IV). Tranquilizers are also useful in cats and horses that show excitement after opioid administration. Because tranquilizers have no analgesic effect, they should not be used as a substitute for opioids or other analgesic agents. Acepromazine should be used with caution in patients with blood loss, dehydration, or low blood pressure.
Multimodal Therapy
Because there are several mechanisms by which pain is produced, it is often helpful to use more than one type of analgesic to relieve pain. Multimodal therapy (also known as combination or balanced analgesia) may be more successful than treatment with any single agent, because pain perception is affected at several points along the pain pathway and different mechanisms are targeted. For example, it has been shown in human patients that the use of piroxicam (an NSAID) and buprenorphine (an opioid) together provides analgesia that is superior to that with use of either agent alone. The concurrent use of NSAIDs with opioids may allow a 20% to 50% reduction in the opioid dose.
One familiar example of combination therapy is a mixture of acetaminophen and codeine, which is an effective oral treatment for moderate to severe pain in the dog. When given orally at a dose rate of 10 mg of acetaminophen per kilogram and 0.5 to 1 mg of codeine per kilogram every 6 to 12 hours, the combination is safe in healthy dogs for up to 5 days. If necessary, the codeine can be supplemented up to 4 mg/kg. Constipation and sedation are common adverse effects of this drug combination, and the diet should be supplemented with a fiber source such as bran or psyllium. Acetaminophen-codeine should never be given to cats and should also be avoided in dogs with hepatic disease.
Opioids and NSAIDs may be given to a patient simultaneously or at different times. For example, a fentanyl patch may be applied to a cat and a dose of meloxicam given at the same time to provide analgesia during the lag time when the patch has not yet taken effect. Alternatively, a dog undergoing an orthopedic operation can be premedicated with morphine (0.2 to 0.3 mg/kg IM) followed by administration of an injectable NSAID (such as meloxicam or carprofen) at the end of operation and followed up with an NSAID given orally for 3 days. This type of “balanced analgesia” allows the use of relatively modest doses of analgesics with a low risk of adverse effects, yet achieves effective pain relief in many patients.
Co-infusion of MLK is another type of combination therapy that is easily administered in IV fluids during surgery. Benefits include multimodal analgesia provided by three different mechanisms of action, and decreased inhalant anesthetic requirement. Problems encountered with MLK include delayed recovery from anesthesia and decreased accuracy of administration, especially with smaller patients. Delayed recovery can be avoided by decreasing the infusion rate until the patient is awake. Use of infusion pumps or burettes increases accuracy of delivery to smaller patients.
See Protocols 7-1 to 7-5 for examples of multimodal perioperative analgesia therapies.
HOME ANALGESIA
There are several options for pain relief in dogs and cats discharged from the hospital. Fentanyl patches can be used sequentially for a period of up to several months in patients with chronic pain (e.g., cancer). Meloxicam, carprofen, etodolac, and other NSAIDs are commonly prescribed for long-term therapy of osteoarthritis and other chronic painful conditions. Oral morphine is available as a sustained-release tablet that is effective when given to dogs or cats twice daily, beginning with a low dose and gradually increasing the dose as needed. Tylenol with codeine (dogs only) and butorphanol are also available in tablet form and are suitable for treatment of mild to moderate chronic pain. Tramadol is also commonly prescribed for patients to take at home.
NURSING CARE
Patient discomfort can also be reduced through conscientious nursing care, including keeping the animal and its cage or stall clean and dry, affording ample opportunity for defecation and urination (including bladder expression or catheterization if necessary), providing comfortable bedding and quiet surroundings, and gently reassuring the patient. The patient should be positioned so that it does not lie on a surgery site or traumatized area. Some patients benefit from being turned every 2 to 3 hours. Anxious patients may benefit from having a blanket or toy from home with them. Unconscious animals may require the application of ophthalmic ointment to prevent corneal
drying. Treatments and monitoring should be scheduled so that the patient is not disturbed unnecessarily.
NONPHARMACOLOGIC THERAPIES
Acupuncture and transcutaneous electric nerve stimulation may effectively treat pain by stimulating the release of endogenous opioids (endorphins). Other nonpharmacologic methods of pain control that may be effective in some situations include massage therapy, application of cold (for acute injuries) or heat (for chronic injuries), physiotherapy, laser therapy, magnetic therapy, and homeopathic or herbal remedies such as Bach flower remedies. Generally these methodologies are used in conjunction with and as an adjunct to pharmacologic therapy. The effectiveness of some of these therapies has not been demonstrated in controlled studies.
KEY POINTS
1. The veterinary technician forms a vital part of the veterinary care-giving team. Through an understanding of pain physiology, pain-associated behaviors, pain assessment tools, analgesic drug pharmacology, and communication skills, the technician contributes significantly to the comfort and welfare of patients.
2. Pain assessment is an essential part of every patient evaluation, regardless of presenting complaint.
3. Pain is a complex, individual experience that has been defined as an aversive sensory and emotional experience that elicits protective motor actions, results in learned avoidance, and may modify species-specific behavior traits, including social behavior.
4. Untreated pain can negatively affect a patient’s behavior, physiology, metabolism, and immune system, causing poor performance, weight loss, delayed wound healing, increased susceptibility to infection, and patient suffering.
5. Physiologic pain occurs in response to a noxious stimulus where there is no or minimal tissue injury, and is a protective mechanism.
6. Pathologic pain occurs after a noxious stimulus that results in tissue injury, and can be classified based on origin, duration, and severity.
7. The pain pathway consists of the following four components: transduction, transmission, modulation, and perception.
8. Peripheral hypersensitivity or primary hyperalgesia is caused by the presence of inflammation.
9. Central hypersensitivity or secondary hyperalgesia (“windup”) is caused by changes to neurons in the spinal cord.
10. The practice of administering analgesics before surgery to decrease analgesic requirements and minimize central nervous system sensitization is called preemptive analgesia.
11. The practice of administering several analgesic drugs that work via different receptor mechanisms is called multimodal analgesia.
12. Pain assessment tools can be used to assess pain and response to analgesic therapy.
13. Opioid analgesics such as morphine, oxymorphone, hydromorphone, methadone, and fentanyl are opioid receptor agonists and are the most effective drugs for treating acute pain.
14. Potential side effects of the mu-agonist opioids are sedation, respiratory depression, vomition, defecation, gastrointestinal ileus, pruritus, excitement, hyperthermia, and dysphoria.
15. The partial agonist opioid buprenorphine has a long duration of action but may not provide sufficient analgesia for severe acute pain. It has been shown to provide good analgesia in rodents.
16. Agonist-antagonist opioid drugs such as butorphanol have a short duration of action and are not as effective at treating severe pain, but have fewer side effects and are used extensively in large animals.
17. Nonsteroidal antiinflammatory drugs decrease inflammation by inhibiting prostaglandin synthesis and are commonly used to provide analgesia postoperatively and in patients with less severe or inflammatory pain.
18. The side effects of NSAIDs include liver and renal toxicity, increased bleeding times, and gastrointestinal ulceration.
19. Local anesthetics provide analgesia via their sodium channel blocking activity and can be administered locally, epidurally, or, in the case of lidocaine, as a constant rate infusion.
20. Alpha2-adrenoceptor agonists are effective analgesics; however, because of their side effects in small animals and ruminants, they are more commonly used for this purpose in horses.
21. Ketamine antagonizes NMDA receptors in the spinal cord, preventing central sensitization.
22. Corticosteroids have potent antiinflammatory activity and, because they act by the same mechanism as NSAIDs, should not be used concurrently with drugs of that class.
23. Tramadol is a nonopiate drug with activity at the mu receptor and is given orally, commonly as part of an analgesic plan for the patient at home.
24. Providing appropriate nursing care to the hospitalized animal is an important part of ensuring that a patient is comfortable when its pain is being treated.
25. Nonpharmacologic therapies, such as acupuncture, may effectively treat pain by stimulating the release of endorphins.
REVIEW QUESTIONS
1. Idiopathic pain is defined as:
a. Pain that is caused by cancer
b. Pain that is of unknown cause
2. Pathologic pain is defined as:
a. Pain that is caused by cancer
b. Pain that is of unknown cause
3. An ovariohysterectomy, which involves surgically incising the skin and abdominal wall and excising the uterus and ovaries, has the following components of pain:
4. The process by which thermal, mechanical, or chemical noxious stimuli are converted into electrical signals called action potentials is:
5. In the spinal cord, pain impulses can be altered by neurons that either suppress or amplify nerve impulses. This process is known as:
6. Where in the pain pathway does secondary sensitization or “windup” occur?
7. Which of the following statements regarding multimodal analgesic therapy is true?
a. The dose of each drug is decreased when several drugs are used.
b. Multiple pain receptor mechanisms are targeted by one drug.
c. One pain receptor mechanism is targeted by several drugs.
8. Which of the following anesthetic plans includes multimodal analgesic therapy?
a. Dexmedetomidine, sevoflurane
b. Acepromazine, ketamine, isoflurane
9. Which one of the following analgesic plans targets three different pain receptor mechanisms?
a. Morphine IM, fentanyl CRI, lidocaine nerve block
b. Morphine IM, fentanyl CRI, bupivacaine nerve block
10. Treating pain does not improve wound healing.
11. Administering analgesics before tissue injury is known as:
12. Which of the following is not a potential side effect of opioid administration in cats and dogs?
13. What is the mechanism of action of nonsteroidal antiinflammatory drugs?
a. They block sodium channels.
b. They are alpha2-receptor agonists.
14. Which of the following is not a potential side effect of NSAID administration?
15. A pain scale can be used to assess pain as well as response to analgesic therapy.