Special Techniques
After completion of this chapter, the reader will be able to:
• Define or explain the terms local anesthesia, sensory neuron, motor neuron, infiltration, line block, nerve block, ring block, splash block, epidural anesthesia, cauda equina, tidal volume, respiratory minute volume, atelectasis, controlled ventilation, assisted ventilation, manual ventilation, mechanical ventilation, and sympathetic blockade.
• List the advantages and disadvantages associated with the use of local anesthetic agents.
• Describe the ways in which local anesthetic agents may be used, including topical, infiltration, regional, intraarticular, epidural, and intravenous administration.
• Outline the methods for performing a nerve block and a line block, and list clinical situations in veterinary practice in which these blocks are used.
• Describe the technique for performing an epidural block, and give examples of clinical situations in which this block could be used.
• Explain the risks involved and the adverse effects that may be seen with the use of local anesthetic agents.
• Explain the difference between assisted and controlled ventilation.
• Describe the techniques of manual, mechanical, periodic, and intermittent mandatory ventilation and their application to anesthesia.
• List the indications for the use of neuromuscular blocking agents and the hazards associated with their use.
• Describe the differences between the two classes of neuromuscular blocking agents, including mode of action and reversibility.
The anesthetic agents and techniques used for routine procedures on most veterinary patients are described in Chapters 8, 9, and 10. In addition, one or more specialized techniques such as local anesthesia, mechanical ventilation, and/or the use of neuromuscular blocking agents may be indicated for a patient. This chapter describes these techniques and indicates the circumstances in which they may be useful.
LOCAL ANESTHESIA
The term local anesthesia (also referred to as local analgesia, because local anesthesia blocks pain transmission) can be defined as the use of a chemical agent on sensory neurons to produce a disruption of nerve impulse transmission, leading to a temporary loss of sensation.
Local anesthesia is an effective, practical, and inexpensive means of producing anesthesia when the patient is tractable, when general anesthesia is undesirable or of high risk, or when the means to deliver it safely are unavailable. The advantages of local anesthesia include low cardiovascular toxicity, low cost, excellent pain control in the immediate postoperative period, and minimal patient recovery time. Local anesthetics are therefore commonly used in ruminants for obstetric and abdominal procedures, often without sedation. Local anesthesia is frequently used to complement standing sedation in horses. Although local anesthesia is less commonly used than general anesthesia in canine and feline patients, it may be a viable alternative in some patients. The choice between local anesthesia and general anesthesia is made by the veterinarian on the basis of such factors as the temperament, age, species, and physical status of the patient; cost; the nature of the operation to be performed; and the anesthetist’s skill in performing the local anesthesia procedure.
Local anesthetics are also used in conjunction with general anesthesia to enhance pain control during and after surgery. The dose of the general anesthetic required may be significantly reduced because of the excellent analgesia provided by the local anesthetic.
Local Anesthetic Agents
Many local anesthetic agents are available. These agents vary in strength, duration of effect, and method of use (Table 6-1). Lidocaine, bupivacaine, mepivacaine, and procaine are the agents most commonly used for skin infiltration and application to mucous membranes. Tetracaine and proparacaine are reserved chiefly for ophthalmic use.
TABLE 6-1
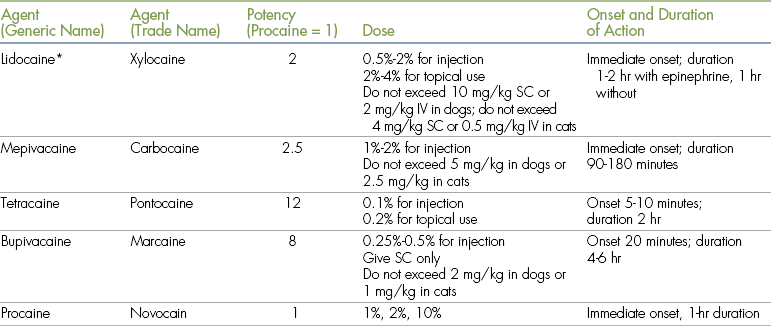
IV, Intravenously; SC, subcutaneously.
∗Sodium bicarbonate (8.4%, 1 mEq/mL) is used as follows: add 0.8 mL bicarbonate to 10 mL of 2% lidocaine or 0.08 mL of bicarbonate to 20 mL of 0.5% bupivacaine.
From Skarda RT: Local and regional analgesia. In Short CE: Principles and practices of veterinary anesthesia, Baltimore, 1987, Williams & Wilkins.
Of the various local anesthetic agents available, lidocaine and bupivacaine are most commonly used in veterinary medicine. Lidocaine is administered at a concentration of 0.5% to 2%, and bupivacaine as a 0.25% or 0.5% solution. Both agents can be diluted with sterile saline (not water) if a lower concentration is desired. Bupivacaine has a slower onset of action (20 minutes) and a longer duration (6 hours) compared with lidocaine (almost immediate onset, duration 1 to 2 hours).
Characteristics of Local Anesthetics
Local anesthetics differ from general anesthetics in several important respects:
• Local anesthetics are not general anesthetics. The term general anesthetic is reserved for those drugs such as inhalation anesthetics, propofol, ketamine, or barbiturates that primarily affect neurons in the brain. Like general anesthetics, local anesthetics exert their effect on neurons, but the target is the peripheral nervous system and spinal cord. Because local anesthetics normally do not affect the brain, they have no sedative effect. The patient remains fully conscious unless other agents such as tranquilizers, neuroleptanalgesics, or general anesthetics are used.
• If the appropriate dose and route of administration are used, local anesthetics have relatively few effects on the cardiovascular or respiratory systems. In contrast, preanesthetics and general anesthetics may have significant cardiovascular and respiratory effects. For this reason, local anesthesia may be preferable to general anesthesia in certain high-risk patients. However, local anesthetics are not without risk of toxicity, and the anesthetist should use caution to avoid overdosage, especially in small patients.
• Whereas general anesthetics are widely distributed throughout the body, local anesthetics primarily exert their effects in the area closest to the site of injection. Effective use of local anesthetics requires precise placement of the drug immediately adjacent to the target nerve. The veterinarian or technician performing the procedure must be familiar with the technique involved for each type of nerve block. For example, it is possible to block the sensory nerve of a tooth in a dog to perform a dental procedure; however, accurate and detailed knowledge of the neuroanatomy of the oral cavity is necessary. Local anesthetics are relatively ineffective in areas where drug diffusion is impeded by fat, bone, cartilage, fascia, tendon, and other connective tissues, as well as the presence of inflammation and infection.
• Unlike general anesthetics, local anesthetics are not normally transferred across the placenta to the fetus. For this reason, local anesthesia is used for cesarean sections and obstetric manipulations, particularly in ruminants, and also in small animal patients when general anesthesia is considered high risk.
Mechanism of Action
The peripheral nervous system and spinal cord are made up of many types of neurons. The primary targets of local anesthetic drugs are the neurons that convey sensations (i.e., pain, heat, cold, and pressure) from the skin, muscles, and other peripheral tissues to the brain. These neurons (called sensory neurons) are affected by even small amounts of local anesthetic, provided the drug is deposited in proximity to the neuron. Local anesthetics result in antagonism, or blockade, of sodium channels. When the sodium channels of a neuron are blocked, the neuron cannot generate electrical impulses. A local anesthetic drug therefore acts as a membrane stabilizer, stopping the process of nerve depolarization. The result is a loss of nerve conduction. Reversal of this effect occurs as the drug is absorbed into the local circulation. Local anesthetics are then redistributed to the liver, where they are metabolized.
Another type of neuron (called a motor neuron) conveys impulses from the brain to muscle fibers and is responsible for initiating and controlling voluntary movements. Motor neurons are also sensitive to the effect of local anesthetics, and administration of a local anesthetic may cause temporary paresis (weakness) or paralysis (loss of voluntary movement) in the area served by the affected motor neurons.
Loss of sensation and loss of motor ability are seen concurrently. For example, use of a local anesthetic near the terminal end of the spinal cord (called an epidural block) will result in loss of sensation and voluntary movement to all areas innervated by the affected sensory and motor neurons. The patient sensations that are lost include (in order of loss) pain, cold, warmth, touch, joint sensation, and deep pressure in the caudal abdomen and pelvic limbs. After an epidural block, the patient will be unable to move the pelvic limbs, and the muscles will appear relaxed.
Local anesthetics also affect the neurons of the autonomic nervous system. These neurons convey impulses between the brain and the blood vessels and internal organs (including the heart). If these neurons are exposed to local anesthetics, there may be a temporary loss of function. This is most important in the sympathetic nervous system, and the loss of function of these neurons is called a sympathetic blockade. The main effect in the peripheral tissues is vasodilation, resulting in flushing and increased skin temperature of the affected area. If severe, vasodilation may cause blood pressure to fall, leading to hypotension. Sympathetic blockade can also be seen after epidural blocks with lidocaine and other local anesthetics, because sympathetic ganglia adjacent to the vertebrae are affected. If sympathetic blockade occurs within the thoracic spinal cord (as may occur if local anesthetic is allowed to diffuse into the thoracic spinal canal), sympathetic innervation to the heart may be blocked, resulting in bradycardia and impaired ventricular contractions, which are undesirable.
Route of Administration of Local Anesthetics
Local anesthetic techniques are often used in conjunction with neuroleptanalgesics, tranquilizers, or other injectable medications. This helps to ensure adequate restraint, allowing accurate and safe injection of the local anesthetic and preventing patient movement during the surgical procedure.
Local anesthetics can be administered by a variety of routes, including topical application, infiltration (injection), or introduction into a joint, nerve plexus, vein, or the epidural space.
Topical Use
Local anesthetics such as lidocaine are usually ineffective when applied directly to intact skin because the drug molecules are unable to penetrate the epidermis and reach the dermis, where the peripheral nerves are located. However, local anesthetics can be used for topical analgesia in some clinical situations:
• Ethyl chloride sprayed on intact skin provides partial, short-term (less than 3 minutes) analgesia by significantly cooling the skin. It is occasionally used for skin biopsies and other superficial procedures. There is some risk of frostbite if a large area is sprayed.
• A cream formulation containing a eutectic mixture of 2.5% lidocaine and 2.5% prilocaine (EMLA cream) can be used to desensitize intact skin for superficial minor procedures such as catheterization. A thick layer of cream is applied to intact shaved skin in the area to be anesthetized and covered with an occlusive dressing for 10 minutes. Duration of effect is 1 to 2 hours. EMLA cream is available in 5-g or 30-g tubes and in single-dose anesthetic discs. It should not be applied to the eyes or to inflamed or broken skin. The patient should be prevented from licking treated areas.
• Wounds or open surgical sites (e.g., lateral ear resections, dewclaw removals) can be treated with topical anesthetic sprays such as 10% lidocaine, or by direct application of 0.25% bupivacaine. Sterile gauze sponges soaked with a mixture of local anesthetic and saline can also be placed on an open surgery site. The use of sprays or soaked gauze sponges is called a splash block. The efficacy of this technique has not been well established, but it appears to be most effective if the surgical field is relatively dry, with minimal bleeding. Care must be taken to avoid local anesthetic overdose, which is a particular concern with small patients. The dose of lidocaine given by this route should not exceed 4 mg/kg for the dog (2 mg/kg for the cat). For bupivacaine the dose should not exceed 2 mg/kg in dogs and 0.5 mg/kg in cats.
• Bupivacaine can be instilled through a chest tube placed during thoracic surgery. Local anesthetic administration should be delayed until the patient is awake, because instillation of bupivacaine into the chest cavity of an anesthetized patient may cause cardiac arrhythmias.
• Local anesthetics can be absorbed by mucous membranes, including the conjunctiva, nose, mouth, larynx, and lining of the urethra. They can be administered as topical sprays, drops, or ointment applied to these areas. For example, lidocaine spray is used to desensitize the larynx and prevent laryngospasm in cats. Another example of topical use of local anesthetics is the application of 0.5% proparacaine or tetracaine to the surface of the eye. This procedure desensitizes the cornea and conjunctiva, allowing procedures such as conjunctival scraping or tonometry. Gel containing local anesthetic (lidocaine, tetracaine, or amethocaine) can be applied to a urinary catheter to ease the catheterization process. In each case, analgesia of the mucous membrane results within 60 to 90 seconds and allows procedures to be performed with less discomfort to the patient. Analgesia lasts for 10 to 15 minutes.
Although topical anesthetics are useful in some situations, they generally offer less pain relief and shorter duration of effect than local anesthetics given by infiltration. Lidocaine patches are available that are applied to the skin and are effective for management of wound or incisional pain.
Infiltration
Local anesthetics may be infiltrated (injected) into tissues, preferably in proximity to the target nerve. The local anesthetic can be given intradermally, subcutaneously, or between muscle planes. Infiltration techniques are used to provide analgesia for surgery involving superficial tissues, including skin biopsies, removal of small skin tumors, and repair of minor lacerations.
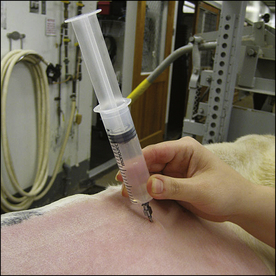
The procedure used for infiltration of local anesthetics is relatively simple. The area must be clipped and a skin antiseptic applied in a manner similar to surgical preparation. This prevents inadvertent contamination of the tissues with skin bacteria when the local anesthetic is injected. A small needle (23 or 25 gauge in small animal and equine patients, 20 or 22 gauge in ruminants) is often used to prevent tissue damage and allow for more precise placement of the drug. The amount of the drug to be injected varies with the location and procedure used and may be as little as 0.1 mL or as much as several milliliters in a dog, or several to tens of milliliters in large animal patients. The onset of analgesia is usually 3 to 5 minutes after the injection of lidocaine.
Before surgery commences it is advisable to test the effectiveness of the block by gently pricking the skin with a 22-gauge needle. If sensation is still present, the anesthetist should wait several minutes longer and consider repeating the injection or using another anesthetic protocol if the block does not take effect.
The infiltration of local anesthetics is not universally effective. Deep tissues such as muscles are unlikely to be affected when only superficial neurons are blocked. In addition, obstacles such as scar tissue or fibrous tissue, fat, edema, and hemorrhage impede diffusion of local anesthetic. Local anesthetics are also relatively ineffective when injected into inflamed or infected areas. In areas of active inflammation, tissue pH is acidic, resulting in rapid inactivation of the drug. For this reason and also for the prevention of contamination of other tissue, local anesthetics should not be infiltrated into infected tissues.
Once the local anesthetic reaches the neuron, the duration of effect depends on the type of drug being used and the rate of absorption by local blood vessels (see Table 6-1). This in turn depends on whether epinephrine is used with the local anesthetic. Lidocaine, the drug most commonly used for local injection, may be purchased with or without epinephrine. The concentration of epinephrine used is 0.01 mg/mL of lidocaine.
Epinephrine is added to lidocaine for the following two reasons:
1. Epinephrine causes constriction of blood vessels in the area of the injection. This decreases the rate of drug absorption and thereby prolongs the effect of the lidocaine by approximately 50%.
2. By causing vasoconstriction, epinephrine reduces the concentration of local anesthetic that enters the circulation at any given time, thereby reducing toxicity of the drug. This is most effective for short-acting drugs such as lidocaine and less helpful for long-acting drugs such as bupivacaine.
Lidocaine without epinephrine may be preferred to lidocaine with epinephrine in some situations. A solution containing epinephrine should not be used at an incision site because it may impair tissue perfusion and healing. Lidocaine with epinephrine should not be used on the ears, tail, or digits, because circulation to these areas may be compromised. Epinephrine also increases the risk of ventricular arrhythmias (particularly in animals anesthetized with halothane) and should be used with caution in animals with known cardiac disease. Lidocaine without epinephrine is used for intravenous techniques.
Local anesthetic injections may be painful in the awake patient. Some anesthetists add sodium bicarbonate (one tenth the volume of local anesthetic) to decrease pain on injection.
Two techniques are commonly used for infiltration of local anesthetics: nerve blocks and line blocks.
Nerve Blocks: A nerve block is achieved by injecting local anesthetic in proximity to a nerve to desensitize a particular anatomic site. One familiar example is the use of Novocain in human dentistry. Nerve blocks are used commonly in anesthesia of large animals and also may be used in small animals provided the location of the target nerve is known exactly. Reference texts contain illustrations that indicate the location of nerve blocks for different species and particular areas of the body. Before a nerve block is performed, the nerve is palpated to determine its location, although this may not always be possible if the nerve is small.
Once the location of the nerve supplying the surgical site is known, the procedure is straightforward. After the skin is clipped and prepared for surgery, a small amount of local anesthetic is injected immediately adjacent to the nerve. Lidocaine, bupivacaine, or a 1:1 mixture of the two can be used to take advantage of the shorter onset of action of lidocaine and the longer duration of action of bupivacaine. The drug diffuses through the tissues to reach the target nerve. Caution should be used to avoid injecting directly into the nerve because temporary or permanent loss of nerve function can occur. Also, avoid intravenous injection of the local anesthetic, which may cause unwanted central nervous system and cardiovascular effects. To avoid intravenous injection, aspirate before injecting a local anesthetic. If blood appears in the syringe, the needle should be withdrawn and the location of the injection changed.
Clinical situations in which nerve blocks can be used include the following:
• Lameness examinations in horses
• Cornual blocks for dehorning cattle
• Paravertebral blocks for abdominal surgery or cesarean sections in cattle (Procedure 6-1, p. 197)
• Dental blocks in dogs and cats (infraorbital, mental, mandibular, and maxillary nerve blocks)
• Intercostal nerve blocks in animals undergoing chest surgery
• Infiltration of nerves during amputation of a limb (0.5 mL of 0.5% bupivacaine, injected into and around the nerve during the operation)
• Nerve block to provide analgesia for declawing cats (Procedure 6-2, p. 199)
Specialty textbooks and journal articles provide complete descriptions of these and other nerve blocks. Regardless of the technique used, it is important to allow sufficient time for the tissues to absorb the drug (15 to 20 minutes) before the surgical or dental procedure is started. When properly performed, these blocks not only decrease the amount of general anesthetic required, but also provide excellent short-term analgesia after the procedure.
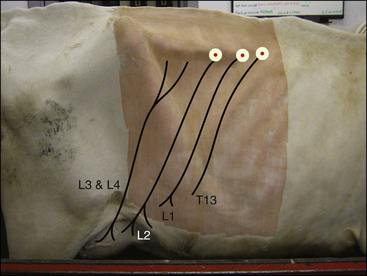
Line blocks: Often the veterinarian will perform an operation on an area of tissue that is served by numerous small nerves. In this situation, a line block consisting of a continuous line of local anesthetic can be placed in the subcutaneous or subcuticular tissues immediately proximal to the target area (Figure 6-1, A). If the line of local anesthetic completely encircles an anatomic part, such as a digit or teat, it is called a ring block.
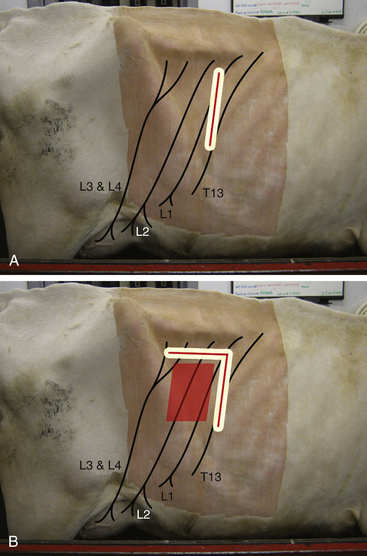
FIGURE 6-1 Location for performing a line block or L-block in a cow for standing flank laparotomy. A, Line for infiltrating local anesthetic. The surgical incision is made along the line where anesthesia has been infiltrated. B, The line can be extended in an inverted L shape to anesthetize the caudal part of the surgical field. The surgical incision is made anywhere in the box outlined to the right of the L.
Line blocks should be positioned between the target area and the spinal cord because this will block the sensory neurons most effectively. As with nerve blocks, the area is clipped and the skin prepared. A line block is placed by inserting the needle along the proposed line of infiltration, then gradually withdrawing the needle while simultaneously injecting a small amount of local anesthetic. If several injections are made, the needle should be inserted into a desensitized area of skin to avoid patient discomfort. Care should be taken to avoid inserting the needle into contaminated tissue (e.g., an infected wound).
Line blocks and ring blocks are used extensively in food animal and equine surgery, particularly in cattle. Examples include teat surgery and wound repair. A particular type of line block, called an L-block, is used for laparotomy in ruminants (see Figure 6-1, B).
Intraarticular Administration
In certain circumstances, local anesthetics can be injected directly into a joint. For example, bupivacaine has been shown to provide significant analgesia when injected into the stifle joint at the conclusion of stifle arthroscopy and surgery. A dose of 0.4 mL of 0.5% bupivacaine per kilogram, diluted with sterile saline (not water) to a volume sufficient to fill the joint, has been recommended. The drug is injected immediately after closure of the joint capsule.
Regional Anesthesia
Regional anesthesia is a technique whereby a local anesthetic is injected into a major nerve plexus or in proximity to the spinal cord. This results in the blockage of nervous impulses to and from a relatively large area, such as an entire limb or the caudal portion of the body. Examples of regional anesthesia in veterinary medicine include paravertebral, epidural, spinal (intrathecal), and brachial plexus blocks.
Paravertebral Anesthesia (Ruminants Only): This is an alternative to a line block for standing laparotomy in cattle. The dorsal and ventral branches of spinal nerves T13-L2 are blocked, along with L3-L4 if anesthesia of the paralumbar fossa is required (e.g., for flank cesarean section) (see Procedure 6-1).
Advantages include provision of a wide, uniform area of anesthesia and a shorter time required to perform the block. Disadvantages include the increase in technical difficulty, hindlimb weakness if L3 and L4 are blocked, and the scoliosis (lateral curvature of the spine) commonly seen with this technique, which may make closure of the incision more challenging.
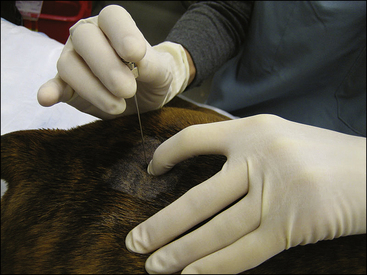
Epidural Anesthesia: Epidural anesthesia is a regional anesthesia technique that is commonly used in both large and small animal patients. The procedure is not difficult (Procedure 6-3, p. 200) and, once mastered, allows the anesthetist to reliably block sensation and motor control of the rear, abdomen, pelvis, tail, pelvic limbs, and perineum. The technique is useful for tail amputation, anal sac removal, perianal surgery, urethrostomies, obstetric manipulations, cesarean sections, and some rear limb operations. Epidural anesthesia is most commonly used in three classes of patients:
1. Ruminants, in which procedures such as replacement of a vaginal prolapse can be undertaken with epidural anesthesia alone or in combination with a sedative. Epidural procedures are also useful to prevent straining during obstetric procedures, including cesarean sections.
2. Debilitated small animal patients in which general anesthesia is problematic but that may tolerate sedation and a lidocaine or bupivacaine epidural block. One common example is cesarean sections.
3. Patients requiring profound pain control after surgical procedures involving the hind limbs, pelvis, or caudal abdomen. For example, morphine or lidocaine epidural blocks are useful for animals undergoing surgical repair of a fractured femur.
The choice of drug used for epidural anesthesia is governed by the reason for the epidural: if immobility and anesthesia for surgery are required, a local anesthetic such as 2% lidocaine or 0.5% bupivacaine can be used at a dose of 1 mL/5 kg of body weight. Duration of effect is 1 to 2 hours for lidocaine and up to 6 hours for bupivacaine. If the main objective is epidural analgesia to ensure postoperative pain control, an opioid such as morphine is used instead (see Chapter 7). Opioids and local anesthetics such as lidocaine can also be mixed together and delivered epidurally. Opioids are generally associated with fewer unwanted side effects than lidocaine or bupivacaine (less risk of sympathetic blockade and hypotension, unlikely to cause motor blockade, and therefore less ataxia) but may cause pruritus and urinary retention in some patients. Opioids may also be combined with alpha2-agonists for epidural analgesia in horses and cattle.
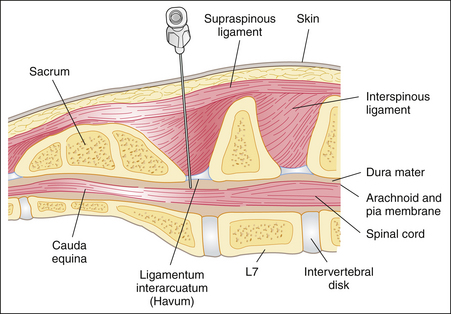
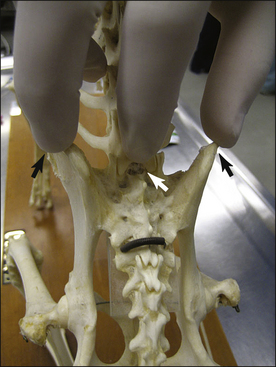
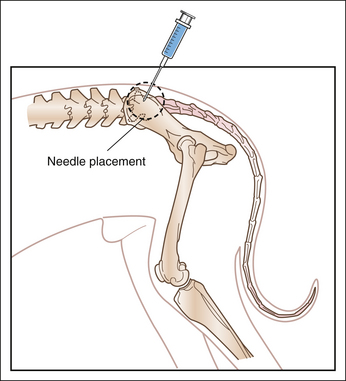
Anatomic considerations: To understand the technique used for epidural anesthesia, the anesthetist must be familiar with the anatomy of the terminal spinal cord region (see Procedure 6-3, Figure 5). The spinal cord is made up of sensory, motor, and autonomic neurons and is surrounded by three membrane layers: the pia mater, arachnoid, and dura mater. The subarachnoid space, which is the area between the arachnoid and the pia mater, is filled with cerebrospinal fluid. This fluid surrounds the entire spinal cord and communicates with the cerebrospinal fluid in the ventricles of the brain. The spinal cord and its membrane layers are encased within the spinal canal. This canal consists of bony vertebrae (cervical, thoracic, lumbar, sacral, and coccygeal) that protect the spinal cord from injury. Several ligaments also protect the vertebral canal, including the supraspinous ligament (which lies directly under the skin), the ligamentum flavum (also called the ligamentum interarcuatum), and the interspinous ligament (see Procedure 6-3, Figure 5). The neurons that supply the tissues exit from the spinal cord at regular intervals, emerging between the vertebrae and ultimately ending in the skin and other tissues. The spinal cord itself terminates in a group of neurons collectively called the cauda equina.
When epidural anesthesia is performed, local anesthetic is deposited in the epidural space, between the dura mater and the vertebrae. This is a potential space that is often filled with fat. Spinal nerves pass through the epidural space as they exit through the intervertebral foramina and are affected by local anesthetics and other drugs deposited in this space. In dogs the location of the block is between the last lumbar vertebra (L7) and the sacrum (see Procedure 6-3, Figure 5). When properly performed, injection of local anesthetic into this area is unlikely to damage the spinal cord because the cord normally ends at the sixth or seventh lumbar vertebra (L6 or L7). In cats the spinal cord extends farther caudally (as far as S1), and there is a slight risk of entering the subarachnoid space when performing epidural anesthesia.
Epidural anesthesia must be differentiated from spinal anesthesia, which is commonly performed in human patients. In spinal anesthesia the local anesthetic is injected into the subarachnoid space, where it mixes with the cerebrospinal fluid. Inadvertent injection of local anesthetic into the subarachnoid space is more common in the cat than in the dog.
Because local anesthetics block not only sensory nerves (including those that transmit pain sensation) but also motor neurons, an animal that has undergone epidural anesthesia may be unable to walk until the block wears off. Opioids, on the other hand, have minimal effect on motor neurons, and movement of the legs and tail is usually unimpaired after morphine epidural analgesia.
Intravenous Regional Anesthesia (Bier Block): Intravenous regional anesthesia is used to provide short-term (less than 1 hour) of local anesthesia to an extremity. When a Bier block is performed, a calculated amount of lidocaine (bupivacaine is not used, as it is more cardiotoxic when given intravenously) is injected into the distal segment of a superficial vein after a tourniquet has been applied proximal to the vein (Procedure 6-4, p. 203).
Systemic Administration
Lidocaine can also be administered by constant rate infusion to healthy anesthetized animals to reduce the dose of general anesthetic or analgesic required for painful operations and to prevent cardiac arrhythmias. The dose used is 50 to 75 mcg/kg/min in dogs and horses, and 25 mcg/kg/min in cats.
Adverse Effects of Local Anesthetics
The use of local anesthetics is not without risk, and several adverse effects have been reported, including the following:
1. Motor neurons may also be affected, and the patient may lose voluntary motor control of the affected body part. The loss of motor function to the limbs typically results in recumbency. This may be inconvenient (e.g., for a standing procedure in a cow) or undesirable and dangerous (e.g., a horse that falls and thrashes as it repeatedly tries unsuccessfully to stand).
2. If local anesthetic is injected into a nerve, temporary or permanent loss of function may result. Direct injection into a nerve should be avoided, except for animals undergoing an amputation.
3. Tissue irritation may occur after the injection of some local anesthetics. Some veterinarians prefer mepivacaine instead of other local anesthetics because it appears to cause less tissue irritation.
4. Paresthesia, an abnormal sensation of tingling, pain, or irritation, may be apparent during recovery from local anesthesia. (Human patients also experience this tingling sensation—for example, during recovery from “numbing” of the oral cavity with Novocain.) Animals should be monitored during recovery because they may chew or otherwise traumatize affected areas and may require chemical or physical restraint or the use of E-collars (cats and dogs).
5. Human and animal patients may exhibit allergic reactions to local anesthetics, usually in the form of a skin rash or hives. Anaphylaxis is also occasionally seen. Local anesthetics should not be used in patients in which an allergic reaction to these drugs has been previously observed.
6. Systemic toxicity may occur, particularly if a local anesthetic is inadvertently given intravenously without the use of a tourniquet. Systemic toxicity may be seen even if the drug is placed in the subcutaneous tissues, if a large amount of local anesthetic is injected. The most common signs of systemic toxicity originate in the central nervous system. The first sign of systemic toxicity is usually sedation, followed by nausea, restlessness, muscle twitching, hyperexcitability, seizures, respiratory depression, and, eventually, coma. Treatment of central nervous system signs may include intravenous or intrarectal diazepam (0.2 to 0.4 mg/kg) and administration of oxygen. Cardiovascular effects may also be observed, because of the direct effect of local anesthetics on the heart. Intravenous injection of lidocaine may inhibit the conduction of electrical impulses within the heart muscle and decrease the force of cardiac contractions. This is undesirable when local anesthesia is performed but makes this drug useful in the treatment of some types of ventricular arrhythmias. Bupivacaine is more cardiotoxic than lidocaine. The dose of lidocaine given subcutaneously to dogs, cows, and horses should not exceed 10 mg/kg and in cats should not exceed 4 mg/kg to avoid systemic toxicity. The smallest possible dose should be used. If the drug is given intravenously, the dose of lidocaine should not exceed 4 mg/kg in dogs, 2 mg/kg in large animals, and 0.5 mg/kg in cats. The dose of bupivacaine given subcutaneously should not exceed 2 mg/kg in dogs and 1 mg/kg in cats. For the average (4-kg) cat, the maximum dose of 2% (20 mg/mL) lidocaine is 0.8 mL (16 mg) if given subcutaneously and 0.1 mL (2 mg) if given intravenously. For 0.5% (5 mg/mL) bupivacaine, the maximum subcutaneous dose for a 4-kg cat is 4 mg (0.8 mL). Bupivacaine should not be given by intravenous injection. Dilution of the calculated dose of lidocaine or bupivacaine with sterile saline is helpful in small patients because it increases the volume and decreases the concentration of the solution to be injected.
7. Epidural or spinal injection may rarely traumatize the spinal cord or cauda equina, particularly if the animal is struggling during placement of the needle. Inflammation and fibrosis have been reported after epidural infiltration of local anesthetics containing preservatives. In addition, myelitis (spinal cord inflammation) and meningitis (inflammation of the pia mater, arachnoid, or dura mater) may occur if asepsis is not maintained.
8. If local anesthetics are permitted to infiltrate into the cranial portion of the spinal cord, serious toxicity and even death may occur. If the local anesthetic reaches the midthoracic vertebrae, innervation of the intercostal muscles may be blocked, interfering with normal respiration. If local anesthetic diffuses as far forward as the cervical spinal cord, the phrenic nerve may be affected. This nerve innervates the diaphragm, and loss of function may result in respiratory paralysis. When epidural anesthesia is performed, care should be taken to keep the patient’s head elevated to avoid gravitational flow of the anesthetic into the anterior spinal canal around the thoracic and cervical spinal cord. The anesthetist should be prepared to intubate and artificially ventilate the lungs of any patient undergoing epidural anesthesia, because this may be necessary if intercostal and phrenic nerve function is impaired.
9. Diffusion of local anesthetic into the cervical and thoracic spinal cord may also affect sympathetic nerves supplying the heart and blood vessels, resulting in a sympathetic blockade with symptoms of bradycardia, decreased cardiac output, and hypotension. If blood pressure measurement is unavailable, careful monitoring of the capillary refill time, heart rate, and pulse strength will alert the anesthetist to a fall in blood pressure. Treatment consists of intravenous fluid administration at a rate of 20 mL/kg over a 15- to 20-minute period.
ASSISTED AND CONTROLLED VENTILATION
The anesthetist may be called on to assist or control patient ventilation during any period of general anesthesia. In assisted ventilation, the anesthetist ensures that an increased volume of air or, more commonly, oxygen and anesthetic gases is delivered to the patient, although the patient initiates each inspiration. In controlled ventilation, the anesthetist delivers all of the air that is required by the patient, and the patient does not make spontaneous respiratory efforts. The anesthetist controls the respiratory rate and the volume and pressure of gas that the animal breathes.
Any procedure by which the anesthetist assists or controls the delivery of oxygen and anesthetic gas to the patient’s lungs may be termed positive pressure ventilation (PPV). Whether achieved by bagging the patient (applying pressure to the reservoir bag with the pop-off valve fully or partially closed) or by using a mechanical ventilator, PPV is intended to ensure that the animal receives adequate oxygen and is able to exhale adequate amounts of carbon dioxide. This is a concern in veterinary anesthesia because the patient’s own ventilation efforts may be inadequate to achieve these objectives.
Ventilation in the Awake Animal
To understand the use of PPV in anesthesia, it is necessary to review the mechanics of normal breathing and the reasons why they may be ineffective in the anesthetized animal. Ventilation is the physical movement of air (and anesthetic gases in an anesthetized patient) into and out of the lungs and upper respiratory passageways. Ventilation has two parts: an active phase (inhalation) and a passive phase (exhalation). Inhalation is initiated by the respiratory center in the brain and is normally triggered by an increased level of carbon dioxide in the arterial blood (Paco2). As Paco2 rises above a threshold level (approximately 40 mm Hg), the respiratory center initiates the active inspiratory phase by stimulating the intercostal muscles and diaphragm to move, expanding the thorax. This creates a negative pressure (partial vacuum) within the chest, which causes the lungs to expand. As the lungs expand, air moves through the breathing passages and into the alveoli. When the lungs reach an adequate volume, nerve impulses feed back to the respiratory center, signaling the brain to stop the active phase of respiration. The intercostal muscles and diaphragm then relax, and exhalation takes place as the lungs deflate. Exhalation is passive, which means that no active muscle movement occurs (with the exception of exhalation during vigorous exercise, which has an active component). During exhalation, the carbon dioxide level in the blood begins to rise again, and after a short pause the respiratory center responds by initiating another inspiration.
Normally, exhalation lasts approximately twice as long as inspiration. For example, in an animal breathing 20 times per minute, each inspiration will last approximately 1 second and each exhalation will last approximately 2 seconds.
The amount of air that passes into or out of the lungs in a single breath is the tidal volume (VT). Animals that are breathing deeply have a relatively large VT, whereas animals that have shallow breathing or that are panting have a relatively small VT. Normal VT in the awake animal is 10 to 15 mL/kg.
The respiratory rate or frequency is the number of breaths that occur in 1 minute. The respiratory minute volume is the total amount of air that moves into and out of the lungs in 1 minute. This value can be found by multiplying the average VT by the respiratory rate.
Ventilation in the Anesthetized Animal
Ventilation in the anesthetized animal differs significantly from normal ventilation just described. These differences include the following:
• Tranquilizers and general anesthetics may decrease the responsiveness of the respiratory center in the brain to carbon dioxide. This means that inspiration does not occur as often in the anesthetized animal as in the healthy awake animal, despite the fact that the carbon dioxide level may be significantly elevated. This explains the observation that a respiratory rate of 8 to 20 breaths per minute is normal in cats and dogs under inhalation anesthesia, whereas the same animal would be expected to have a respiratory rate of 15 to 30 breaths per minute when awake.
• Tranquilizers and general anesthetics relax the intercostal muscles and diaphragm, causing them to expand less than they normally do during the inspiratory phase. Because the chest does not expand fully, the VT is reduced. The anesthetist may become aware of the reduced VT by noting that the reservoir bag does not collapse significantly during the inhalation phase (i.e., the volume of gas inhaled is relatively small). Because VT and respiratory rate are decreased, respiratory minute volume is also decreased.
As the amount of air entering and leaving the lungs in the anesthetized animal may be considerably reduced compared with the healthy awake animal, the anesthetist must be aware of the following potential problems:
• Hypercarbia. Paco2 may rise in the anesthetized patient, because carbon dioxide produced by the body is not eliminated as rapidly as in the awake animal. As the blood carbon dioxide level rises, carbon dioxide combines with water molecules in the bloodstream to form bicarbonate ions (HCO3−) and hydrogen ions (H+). The accumulation of hydrogen ions causes the pH of circulating blood to fall, leading to respiratory acidosis. Blood pH in the healthy awake animal is 7.38 to 7.42, whereas in the anesthetized animal, blood pH may be as low as 7.20.
• Hypoxemia. If the anesthetized animal is breathing room air, Pao2 may fall below normal values as a result of the decreased respiratory minute volume. Less oxygen enters the lungs, and therefore less is available to be absorbed into the blood.
• Atelectasis. Because VT is reduced, the alveoli do not expand as fully as normal on inspiration. The alveoli in some sections of the lung, particularly those that are lower in the animal’s body, may partially collapse (a condition called atelectasis).
Some patients are at increased risk for having or developing these problems. Predisposing factors for hypercarbia, hypoxemia, and atelectasis include the following:
• Prolonged anesthesia (more than 90 minutes).
• Administration of neuromuscular blocking agents (see following section).
• Preexisting lung disease such as pneumonia.
• Surgical procedures involving the chest or diaphragm. These animals may have preexisting cardiovascular or pulmonary disease and are at significant risk for cardiovascular collapse or respiratory arrest if conventional anesthesia with unassisted ventilation is attempted.
• Species differences. Horses in particular are prone to the problems listed previously, regardless of physical status. Adult ruminants also tend to hypoventilate and become hypercarbic.
The anesthetist has several ways of compensating for these effects. The Pao2 can be elevated to normal levels (and, in fact, often above normal levels) if the patient is supplied with adequate oxygen. This is easily achieved because animals connected to anesthetic machines normally receive close to 100% oxygen. The anesthetized patient connected to an anesthetic machine is unlikely to have a reduced Pao2 unless a problem such as pulmonary edema or upper airway obstruction is present.
It is more difficult to prevent atelectasis or an increase in Paco2 and resulting respiratory acidosis. In each of these situations, it may be advisable to take active steps to assist or control patient ventilation.
Types of Controlled Ventilation
The following are two ways to control patient ventilation in the anesthetized patient:
1. The patient’s lungs are ventilated by the anesthetist using the anesthetic breathing system; this technique is referred to as manual ventilation. A common term that is also used to indicate manual ventilation is bagging. In this type of PPV, the lungs are filled with oxygen by the pressure of gas entering the airways as the anesthetist squeezes the reservoir bag with the pop-off valve fully or partially closed. Exhalation is passive and occurs when the positive pressure is discontinued and the pop-off valve is opened fully, allowing the lungs to empty. For most patients, periodic bagging (one or two breaths every 2 to 5 minutes) is adequate to expand the lungs and reduce atelectasis. However, some patients require bagging throughout the anesthetic period, which is referred to as intermittent mandatory ventilation.
2. The patient’s lungs are ventilated by a ventilator; this technique is called mechanical ventilation. In this type of PPV, the lungs are filled with oxygen by the pressure of gas from a special apparatus called a ventilator. As with manual ventilation, exhalation is passive and occurs when the positive pressure is discontinued. Ventilators used in anesthesia are not usually used to periodically ventilate patients, but are most commonly used to provide intermittent mandatory ventilation.
Whether the patient’s lungs are to be ventilated by manual or mechanical means, the patient must first be intubated and connected to an anesthetic machine. Ventilating the patient’s lungs through an ordinary anesthesia mask does not deliver adequate amounts of oxygen to the lungs and may cause the stomach to fill with air, increasing the risk of regurgitation.
Manual Ventilation
Manual ventilation can be performed on a periodic basis (one or two breaths every 2 to 5 minutes) on any anesthetized patient. These periodic breaths, also called “sighs,” help expand collapsed alveoli and reverse atelectasis. For the patient’s lungs to be manually ventilated, the pop-off valve is closed and the reservoir bag is compressed until the lungs are inflated. When pressure on the reservoir bag is released, exhalation can occur. The anesthetist must use caution to ensure that the pressure used is not excessive: the patient’s chest should rise only to the same extent as with normal awake respiration. When normal lungs are ventilated, the pressure manometer reading should not exceed 20 cm H2O in small animal patients and 40 cm H2O in large animal patients. The bag should be squeezed for 1 to 1.5 seconds. Excessive or prolonged pressure may damage lung tissue and impede venous return to the heart.
For some patients, respiratory depression is so severe that periodic bagging does not provide the necessary level of ventilation, placing increased demand on the anesthetist’s time and attention. Animals with preexisting heart or lung disease or diaphragmatic hernias may go into respiratory arrest immediately after induction. Other patients continue to breathe under anesthesia, but their VT is small (shallow breaths) and/or the respiratory rate is less than 6 breaths/min. These patients require intermittent mandatory ventilation. Ventilation can be assisted starting immediately after intubation, at which point the anesthetist should use the reservoir bag to superimpose positive pressure on the animal’s own spontaneous breathing efforts. For many patients it is adequate to give a few larger than normal breaths manually then to connect the patient to a ventilator or to initiate manual ventilation. The initial large VT depresses the animal’s urge to breathe by lowering blood carbon dioxide levels. Once the patient is connected to a ventilator or is undergoing appropriate manual ventilation, the patient usually stops spontaneous breathing efforts within 1 minute. Initially, the assisted ventilation rate should be 8 to 20 respirations per minute, depending on patient size. If after 3 to 5 minutes the patient still makes spontaneous breathing efforts, it may be necessary to use a neuromuscular blocking agent, which paralyzes the muscles of respiration (see the following section).
Once control has been established, a ventilation rate of 6 to 12 breaths/min is usually adequate. A pressure of 15 to 20 cm H2O is recommended in small animals (30 to 40 cm H2O in large animals), unless the chest is open, in which case higher pressures may be required depending on the degree to which the lungs are packed off by the surgeon to better expose the surgical field. When the reservoir bag is squeezed, the inspiratory time should be 1 to 1.5 seconds. Expiratory time should be at least twice as long as inspiratory time. The pop-off valve must be closed when the reservoir bag is squeezed; however, it should be opened briefly every two to three breaths to allow gas to escape from the circuit. The anesthetist must allow the airway pressure to return to zero during expiration so that cardiopulmonary function can normalize (improving venous return of blood to the heart and increasing stroke volume).
When assisting or controlling ventilation, the anesthetist may find it difficult to evaluate the adequacy of the ventilation efforts. Pulse oximetry and end-tidal capnography are valuable aids (see Chapter 5). For example, if the pulse oximeter reading is 95% or less in a patient undergoing manual ventilation, the patient may need more frequent ventilation or ventilation at a greater pressure or volume.
When the surgical procedure is nearing completion, the anesthetist must transition the patient from the intermittent mandatory breaths given by the ventilator, to spontaneous breathing. This is known as “weaning” the animal off the ventilator and is accomplished by turning off the anesthetic (and nitrous oxide if it is being used) while continuing to ventilate the patient’s lungs with oxygen. If a neuromuscular blocking agent has been used, it should be reversed, if necessary. The anesthetist should gradually reduce the rate of inspirations to approximately two to four per minute while observing the animal for evidence of spontaneous breathing. When this is seen, the patient’s ventilation can then be assisted by squeezing a small amount of air from the reservoir bag with each inspiration. Eventually, the animal will regain the ability to maintain a normal rate and VT, and ventilation assistance can be discontinued. This may take several minutes to reestablish, particularly in older, hypothermic, or debilitated patients and in patients in which ventilation has been controlled for a long time. Warming the patient or stimulating the patient by pinching the toe pads or gently rubbing the thorax and abdomen (small animal), or twisting an ear (large animal), may help the patient regain spontaneous respiratory movements.
Mechanical Ventilation
Mechanical ventilation is similar to intermittent mandatory manual ventilation in many respects. In mechanical ventilation, however, the patient’s breathing is controlled by a ventilator, rather than by hand compression of the reservoir bag. When a ventilator is connected to the breathing circuit, it functionally replaces the reservoir bag and becomes a part of the breathing circuit. The ventilator automatically compresses a bellows, which forces oxygen and anesthetic gas into the patient’s airways via an endotracheal tube.
Many types of ventilators can be used with a veterinary anesthesia machine, and they vary in the number and complexity of the controls (Figure 6-2). The basic design is a bellows inside a housing, which is attached to the reservoir bag port of a circle breathing system. The bellows is compressed at a specified rate and a specified volume by a driving gas. Most ventilators have a double gas circuit pattern, with one circuit providing oxygen and anesthetic for the patient, and the other circuit containing a separate gas (oxygen or air) to drive the bellows. Ventilators are available for either large animal or small animal use. In both cases the scavenger should be attached to the exhaust port of the ventilator.

FIGURE 6-2 Ventilator control panel of a Hallowell small animal anesthesia ventilator. From right to left, the controls are the on-off switch with indicator light; respiratory rate knob; inspiratory flow knobs (smaller silver knob for fine control, larger black knob for gross control); I:E ratio knob (controls ratio of inspiration to expiration); inspiratory hold button (allows the anesthetist to hold a breath on inflation); and the pressure limit knob (allows the anesthetist to set the maximum pressure within the breathing system on inspiration; an alarm will sound if this pressure is reached).
Depending on the type of ventilator used, the anesthetist may choose to deliver gases on inspiration according to a pressure cycle, volume cycle, or time cycle. A pressure-cycled ventilator (such as the Bird Mark 7 Respirator) will supply air until the pressure reaches a preset level. A time-cycled ventilator (such as the Small Animal Ventilator by Drager) supplies air according to a set inspiratory time. A volume-cycled type (such as the Ohio Metomatic) delivers a preset VT regardless of the pressure required. In volume-cycled ventilators, the anesthetist must adjust the volume of gas to be delivered on inspiration (usually 10 to 15 mL/kg, less if respiration is to be assisted rather than controlled).
After connecting the ventilator to the breathing circuit, the anesthetist should closely observe the patient to ensure that the chest rises with each inspiration. Respiratory rate is usually 6 to 12 breaths/min. Duration of inspiration is set at 1 to 1.5 seconds, and duration of expiration should be 2 to 6 seconds with an inspiratory/expiratory ratio of 1:2 to 1:3. If a pressure-cycled ventilator is used, a pressure setting of 12 cm to 20 cm is normally selected. These settings may be varied to adapt to the special needs of selected patients. For example, a dog with gastric dilation–volvulus or a horse with colon torsion may need higher airway pressures to deliver a minimal effective VT. Obese cats and dogs often require normal rates but higher airway pressures to overcome the thoracic effects of obesity.
Like manual ventilation, mechanical ventilation is particularly indicated for patients with compromised respiration. It is not normally necessary in healthy anesthetized patients, in which periodic manual bagging (once every 5 minutes) is usually sufficient. Mechanical ventilation is particularly helpful in animals undergoing a thoracotomy or other lengthy operation, in which manually providing intermittent mandatory ventilation would be difficult for the anesthetist.
Technical Consideration for Intermittent Mandatory Ventilation of Anesthetized Patients
Rebreathing versus Non-rebreathing Systems
Intermittent mandatory manual ventilation can be performed using either a rebreathing or a non-rebreathing system. Non-rebreathing systems usually lack a manometer, and the anesthetist must use sight and touch to determine the optimal VT.
In-Circle versus Out-of-Circle Vaporizers
Patients connected to a circle system with an in-circle nonprecision vaporizer can be bagged every 5 minutes to assist ventilation. However, it is advisable to turn the vaporizer setting to zero before bagging the patient, to prevent sudden vaporization of large amounts of anesthetic as a result of the increased flow of carrier gas through the vaporizer. Mandatory controlled ventilation with an in-circle vaporizer is difficult and may be dangerous, unless the vaporizer is turned down or off or end-tidal anesthetic agent monitoring is available. Because of the increased gas flow associated with controlled ventilation and the lack of back pressure compensation in these vaporizers, the patient may receive excessive amounts of vaporized anesthetic.
In contrast, it is feasible to use mandatory manual or mechanical ventilation on patients connected to a circle system with an out-of-circle precision vaporizer. If the vaporizer is compensated for gas flow and back pressure, it is not necessary to turn the concentration setting to zero during controlled ventilation. However, if mandatory manual ventilation is used, it may be advisable to reduce the precision vaporizer setting, or the patient’s level of anesthesia may become too deep because of increased delivery of anesthetic to the lungs. The anesthetist should closely monitor the patient and adjust the vaporizer setting according to the patient’s anesthetic depth.
Risks of Controlled Ventilation
Controlled ventilation, whether by manual ventilation with a reservoir bag or mechanical ventilation with a ventilator, has the potential to damage the animal’s lungs if performed incorrectly.
• Excessive airway pressure may rupture alveoli, causing pneumothorax and/or pneumomediastinum.
• Cardiac output may be decreased if positive pressure is maintained throughout the respiratory cycle (during expiration and inspiration).
• If the ventilation rate is too high, excessive amounts of carbon dioxide may be exhaled, leading to respiratory alkalosis, which (if severe) can cause cerebral vasoconstriction and decreased cerebral blood flow.
• Controlled ventilation is generally more efficient at delivering anesthetic gas, even from a precision out-of-circle vaporizer. A ventilator will thus deliver more inhalant anesthetic to the patient, which may lead to exacerbation of side effects such as hypotension and increased central nervous system depression. Conversely, ventilators are often used in large animal patients as anesthetic delivery devices to maintain a smoother plane of anesthesia than sometimes occurs with spontaneous breathing.
• Mechanical ventilation is not intended to relieve the anesthetist of the necessity for patient monitoring. The anesthetist must closely monitor all animals in which ventilation is controlled or assisted to ensure that patient anesthetic depth and vital signs are maintained within acceptable limits.
NEUROMUSCULAR BLOCKING AGENTS
Neuromuscular blocking agents (also called muscle-paralyzing agents) are often used in human anesthesia, but they have found only limited use in veterinary practice. Paralysis of the muscles of respiration is undesirable in conventional veterinary anesthesia; however, it is useful in the following situations:
• Patients that require mechanical ventilation. The use of neuromuscular blocking agents prevents spontaneous inspiratory efforts by the patient and allows more rapid and complete control of ventilation. This is particularly useful for thoracic or diaphragmatic surgery.
• Orthopedic surgery. Neuromuscular blocking agents provide excellent muscle relaxation, which is helpful in orthopedic procedures.
• Ophthalmic surgery. Neuromuscular blocking agents prevent movement of the eyeball and cause it to remain in a central rather than ventral position, which facilitates intraocular surgery.
• Cesarean sections. Neuromuscular blocking agents provide abdominal muscle relaxation and are not transferred across the placenta to the fetus.
• Neuromuscular blocking agents may be useful in facilitating difficult intubation (e.g., intubating animals with laryngospasm) because they allow rapid control of the airway without coughing or gagging.
• Occasionally, muscle-paralyzing agents are used in “balanced anesthesia” techniques. In balanced anesthesia, different drugs provide the three components of general anesthesia (unconsciousness, muscle relaxation, and analgesia). Instead of using a high dose of a single agent such as thiopental to induce general anesthesia, a balanced technique will include low doses of multiple agents (often an analgesic, a muscle-paralyzing agent, and an unconsciousness-inducing agent). The aim is to induce general anesthesia with a minimum of cardiovascular, respiratory, and other side effects.
Neuromuscular blocking agents should be administered only after the patient is unconscious and respiration has been controlled by means of intermittent mandatory manual or mechanical ventilation. These agents should be considered to be an adjunct to, rather than a replacement for, anesthesia with other agents. Use of neuromuscular blocking agents in a conscious animal is inhumane because these agents have no tranquilizing, analgesic, or anesthetic properties. The patient given only these drugs will be fully conscious and have normal sensitivity to pain, but will be unable to move or otherwise resist the surgeon’s efforts. Control of respiration is also essential after the administration of these drugs because the respiratory muscles will be paralyzed, making it impossible for the patient to breathe on its own.
Neuromuscular blocking agents act by interrupting normal transmission of impulses from motor neurons to the muscle synapse. The site of action is the nerve-muscle junction, where acetylcholine is released by the neurons in proximity to the muscle end plate. There are two ways in which muscle-paralyzing agents may disrupt nervous transmission, and neuromuscular blocking agents are classified as depolarizing or nondepolarizing according to which of the two mechanisms applies.
Depolarizing agents, such as succinylcholine, cause a single surge of activity at the neuromuscular junction, which is followed by a period in which the muscle end plate is refractory to further stimulation. Animals given these agents may show spontaneous muscle twitching followed by paralysis. Succinylcholine has a fast onset (20 seconds) but a short duration of effect and is useful for rapid intubation. Potential adverse effects of succinylcholine include hyperkalemia and cardiac arrhythmias.
Nondepolarizing agents, such as gallamine, pancuronium, atracurium besylate, and cisatracurium, act by blocking the receptors at the end plates. As their classification suggests, they do not cause an initial surge of activity at the neuromuscular junction, and spontaneous muscle movements are not seen. Potential adverse effects of these agents include histamine release, hypotension or hypertension, tachycardia, and ventricular arrhythmias.
Concurrent use of inhalant anesthetics increases the potency of neuromuscular blocking agents. Animals that have undergone recent treatments with organophosphate insecticides also show an increased susceptibility to neuromuscular blocking agents. Other drugs, including corticosteroids, barbiturates, furosemide and other diuretics, anticancer drugs, epinephrine, tetracycline, and aminoglycoside antibiotics such as gentamicin, have been shown to affect the potency of neuromuscular blocking agents.
Muscle-paralyzing agents are normally given by slow intravenous injection. The dose required varies among patients and with the anesthetic protocol used. Most agents take effect within 2 minutes, and the duration of paralysis is approximately 10 to 30 minutes (although this varies considerably, depending on the agent used). If more prolonged paralysis is required, repeated doses can be given. Alternatively, some agents may be given by constant intravenous infusion.
Regardless of the agent used, only voluntary (skeletal) muscles are affected. These agents do not affect the involuntary muscles, including cardiac muscle and the smooth muscle of the intestine and bladder. Skeletal muscles are affected in a predictable order: facial and neck paralysis is seen first, followed by paralysis of the tail, limbs, and abdominal muscles. The intercostal muscles and diaphragm are affected last.
Anesthetic depth may be difficult to assess in animals that have been given muscle-paralyzing agents because of the inhibition or absence of normal reflex responses and the absence of jaw tone. Heart rate and blood pressure may give some indication of anesthetic depth. If salivation, tongue curling, or lacrimation is seen, the patient is likely not deeply anesthetized enough for surgery.
Animals given muscle-paralyzing agents cannot blink and are predisposed to corneal drying. An ophthalmic lubricant must be used. The anesthetist must also monitor the patient for hypothermia, which is a common side effect resulting from the decreased muscle tone seen in patients given these agents. Hypothermia can slow the metabolism of the agents and delay recovery from anesthesia.
Nondepolarizing agents should be reversed with an anticholinesterase agent, even if the effects appear to be wearing off. Reversal agents have no effect on depolarizing agents. The most commonly used reversal agents are edrophonium, neostigmine, and pyridostigmine. The patient should be maintained at a light anesthetic depth until the reversal agent has taken effect. After reversal of muscle-paralyzing agents, signs of returning muscle function include diaphragmatic movements, eye rotation, an active palpebral reflex, and increasing jaw tone. Sometimes the effect of the reversal agent wears off before the muscle-paralyzing agent is eliminated, in which case respiratory support may be required.
Reversal agents may have undesirable side effects such as bradycardia and increased bronchial and salivary secretions and should be given only after pretreatment with atropine or glycopyrrolate.
KEY POINTS
1. Local anesthesia is the use of a chemical agent on sensory and motor neurons to produce a temporary loss of pain sensation and movement. Because of low patient toxicity, low cost, and minimal recovery time, local anesthesia may be preferred to general anesthesia in some patients. Disadvantages include lack of patient restraint, risk of overdose in smaller patients, and technical difficulties.
2. If sufficient quantity of local anesthetic reaches the sympathetic ganglia, sympathetic blockade may result. This causes flushing, increased skin temperature, and, occasionally, hypotension and bradycardia.
3. Local anesthetics have many topical uses, including application to the conjunctiva or the epithelium of the respiratory or urogenital tracts.
4. Local anesthetics may be injected in proximity to a peripheral nerve, blocking sensation from the tissues served by the nerve. Surgical preparation of the area is necessary before injection of a local anesthetic. Epinephrine is commonly added to the lidocaine to delay absorption of the local anesthetic agent from the site, but epinephrine must not be used at peripheral locations where blood supply may be compromised.
5. Epidural anesthesia is achieved by injecting local anesthetic in the epidural space, between the dura and the vertebrae. In dogs and cats, the injection is performed between the last lumbar vertebra and the sacrum. This technique is useful for surgical procedures in patients that are debilitated and in patients that require profound analgesia of the caudal abdomen, limbs, or pelvis.
6. Intravenous injection of local anesthetics may be useful for distal limb surgery, including amputation.
7. Local anesthetics may be harmful if injected into a nerve. They may also cause temporary paresthesia, which may result in self-mutilation.
8. Adverse systemic effects of local anesthesia include sedation, hyperexcitability, respiratory depression, and sympathetic blockade. Toxicity may be avoided by limiting the amount of lidocaine administered to the patient (a maximum of 10 mg/kg in dogs when given subcutaneously) and avoiding intravenous injection of bupivacaine.
9. Controlled or assisted ventilation may be used to deliver oxygen and anesthetic to the patient. Either a mechanical ventilator or manual bagging may be used. These procedures are particularly useful in patients with poor respiratory function. Controlled or assisted ventilation helps prevent the development of hypercarbia, respiratory acidosis, and pulmonary atelectasis.
10. Intermittent mandatory manual ventilation can be achieved by gently squeezing the reservoir bag at a rate of 8 to 12 breaths/min and a pressure of 15 to 20 cm H2O. Inspiration time should be 1 to 1.5 seconds, and expiratory time should be at least 2 to 3 seconds. Manual ventilation is challenging and potentially dangerous to the patient if a nonprecision vaporizer is used.
11. Mechanical ventilators may be incorporated into either a rebreathing or a non-rebreathing system. Depending on the type of ventilator used, the anesthetist may control the pressure or volume of gas to be delivered, the respiratory rate, and the length of inspiration and expiration.
12. If controlled ventilation is used, the anesthetist must use caution to avoid excessive expansion of the alveoli, continuous positive pressure, and excessive ventilation rates.
13. Neuromuscular blocking agents may be useful in some anesthetic procedures to allow relaxation of voluntary muscles. They should never be used as the sole anesthetic agent.
14. Neuromuscular blocking agents may be depolarizing or nondepolarizing in their action. Nondepolarizing agents may be reversed by the administration of neostigmine or edrophonium.
15. Neuromuscular blocking agents may cause systemic effects such as hypothermia and respiratory failure. Mechanical or manual ventilation must be available when these agents are used.
16. Many drugs, including isoflurane, halothane, aminoglycoside antibiotics, organophosphates, and diuretics, may alter the potency of neuromuscular blocking agents.
REVIEW QUESTIONS
1. In the healthy awake animal, the main stimulus to breathe is the result of:
a. Excess oxygen concentration in the blood
b. Excess carbon dioxide concentration in the blood
2. In the healthy awake animal, exhalation lasts at least __ times as long as inhalation.
3. The normal VT in an awake animal is __ mL/kg.
4. In the anesthetized animal that is breathing room air, the anesthetist may expect to see:
a. An increase in the Paco2 and a decrease in the Pao2
b. A decrease in the Paco2 and an increase in the Pao2
5. When used in a line block, a local anesthetic agent will have a direct effect on the:
6. Local anesthetics block transmission of nerve impulses from:
7. Local anesthetic agents work because:
a. They mechanically block nerve impulse transmission
b. They interfere with the movement of sodium ions
8. When a local anesthetic is injected around a single major nerve, the procedure is referred to as a(n):
9. Epinephrine may be mixed with a local anesthetic agent to prolong the effects of the drug.
10. When performing an epidural, one must be aware that the spinal cord in a cat may extend as far caudally as:
11. The maximum subcutaneous dose of lidocaine for a dog is ___ mg/kg.
12. When performing intravenous regional anesthesia (Bier block), one should use lidocaine:
13. The term atelectasis refers to:
14. What is the most common acid-base abnormality in anesthetized patients?
15. When intermittent mandatory manual ventilation is applied to a patient that is connected to a circle system with a precision vaporizer, it is customary to:
a. Increase the vaporizer setting
b. Decrease the vaporizer setting
c. Disconnect the patient from the circle system before starting manual ventilation
d. The lungs of patients that are connected to a circle system should not be manually ventilated.
16. Which of the following can be used to monitor anesthetic depth in a patient that has been given a neuromuscular blocking agent?
17. A neuromuscular blocking agent not only will paralyze skeletal muscle, but also will give some analgesia.
18. When an animal is given a __ neuromuscular blocking drug, an initial surge of muscle activity may be seen before there is paralysis of the muscles.
19. The muscle type that is most affected by neuromuscular blocking agents is:
20. Both depolarizing and nondepolarizing drugs can be reversed.
For the following questions, more than one answer may be correct.
21. Problems that may result from excessive controlled ventilation may include:
22. Local anesthetic agents such as lidocaine or proparacaine work well when applied:
23. Factors that may interfere with the action of local anesthetic agents include:
24. Clinical signs of systemic toxicity from a local anesthetic agent may include:
25. The effects that could result from an epidural anesthetic if the drug reached the thoracic and cervical spinal cord include: