Anesthetic Agents and Adjuncts
After completion of this chapter, the reader will be able to:
• Classify anesthetic agents and adjuncts based on route of administration, time of administration, principal effect, or chemistry.
• Differentiate agonists, partial agonists, agonist-antagonists, and antagonists based on their action and effect. List anesthetics and adjuncts that can be reversed.
• Apply principles of safe administration of anesthetic agents and adjuncts.
• List anesthetic agents and adjuncts commonly used as preanesthetic medications, and describe their indications, mode of action, effects, adverse effects, and use.
• List injectable anesthetic drugs in common use, and describe their indications, mode of action, effects, adverse effects, and use.
• Describe the effect of ionization, protein binding, lipid solubility, and redistribution on barbiturate pharmacokinetics and pharmacodynamics.
• Define dissociative anesthesia; describe the actions and effects of dissociative anesthetics, and explain ways in which these drugs differ from other injectable anesthetics.
• List the inhalation anesthetic agents in common use, and describe their indications, mode of action, effects, adverse effects, and use.
• Define vapor pressure, partition coefficient, minimum alveolar concentration (MAC), and rubber solubility; and explain the ways in which these properties affect the action and use of inhalant anesthetic agents.
• Describe the uptake, distribution, and elimination of the commonly used inhalation anesthetic agents.
INTRODUCTION TO ANESTHETIC AGENTS AND ADJUNCTS
An anesthetic agent may be defined as any drug used to induce a loss of sensation with or without unconsciousness. The term adjunct is used to describe a drug that is not a true anesthetic but that is used during anesthesia to produce other desired effects such as sedation, muscle relaxation, analgesia, reversal, neuromuscular blockade, or parasympathetic blockade. Because adjuncts are used as a part of balanced anesthesia, they are traditionally included in the study of anesthetic agents. Anesthetic agents and adjuncts may be classified a number of ways.
First, they may be classified based on the route of administration. Inhalant agents are administered from an anesthetic machine into the lower respiratory tree via an endotracheal tube or mask. Injectable agents are injected intravenously, intramuscularly, subcutaneously, intraperitoneally, intralesionally, or into a number of other locations. Oral agents are given by mouth, and topical agents are applied to a body surface such as the skin or mucous membranes.
Another way these agents may be classified is based on the time period at which they are given during the course of an anesthetic procedure. Drugs given before general anesthesia are referred to as preanesthetic medications. Drugs used to induce general anesthesia are referred to as induction agents, and those used to maintain general anesthesia are referred to as maintenance agents.
A third way anesthetic agents and adjuncts may be classified is according to the principal effect. Local anesthetics induce a loss of sensation in a localized area of the body. In contrast, general anesthetics induce a loss of sensation over the entire body, accompanied by unconsciousness. Sedatives and tranquilizers are agents that cause sedation and tranquilization, respectively. Analgesics prevent and control pain. Muscle relaxants decrease muscle tone. Neuromuscular blockers, although infrequently used in general practice, are used to relax or paralyze skeletal muscles during ophthalmic, orthopedic, or other surgeries. Anticholinergic agents are used to decrease effects of parasympathetic nervous system (PNS) stimulation such as bradycardia and excessive salivation. Finally, reversal agents lessen or abolish the effects of other anesthetic agents and are therefore used to “wake” the patient after sedation or anesthesia.
Many of the agents used in anesthesia cause two or more of these effects, depending on the dose and the circumstances under which they are used. For instance, morphine causes sedation and is an excellent analgesic. The injectable drug dexmedetomidine causes moderate to profound sedation, analgesia, and good muscle relaxation when given alone but can be used in combination with other agents to induce general anesthesia. The intravenous (IV) anesthetic propofol induces general anesthesia at higher doses but can be used as a sedative when given as a low-dose constant rate infusion (CRI). As a consequence, classification of these agents based on the principal effect is somewhat arbitrary.
The final way anesthetic agents and adjuncts may be classified is based on their chemistry. For the student, this is perhaps the most useful method of classification because the agents within a given class tend to have similar properties and effects. For this reason, the anesthetic agents and adjuncts discussed herein will be presented this way. Tables 3-1 to 3-4 summarize the principal effects and adverse effects of the anesthetic agents and adjuncts.
TABLE 3-1
Principal Central Nervous System Effects of Anesthetic Agents and Adjuncts
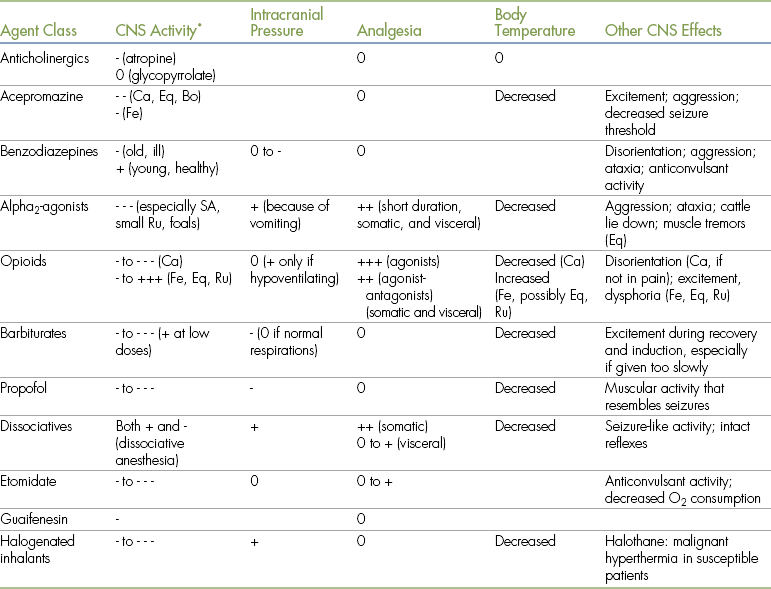
Bo, Bovine; Ca, canine; CNS, central nervous system; Eq, equine; Fe, feline; Ru, ruminants; SA, small animals.
• 0 indicates minimal to no effect.
• - indicates a decrease in the parameter indicated (where - is mild; - - is moderate; - - - is marked).
• + indicates an increase in the parameter indicated (where + is mild; ++ is moderate; +++ is marked).
• Effects of drugs depend on many factors, including species, differences among specific agents within a class, dose, and drug interactions. Therefore quantifications of the principal effects (mild, moderate, marked) are approximations.
• The information regarding opioids includes those of agonists, partial agonists, and agonist-antagonists. When reading this, note that effects of agonists are generally more pronounced than those of agonist-antagonists or partial agonists).
∗- = depression; + = stimulation.
TABLE 3-2
Principal Cardiovascular Effects of Anesthetic Agents and Adjuncts
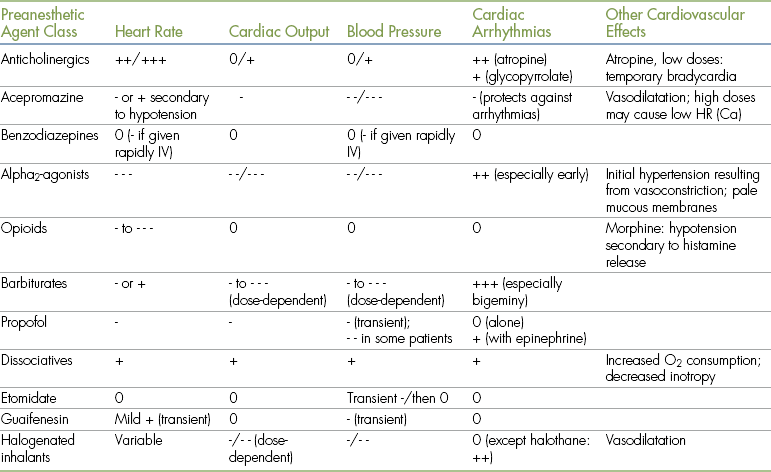
Ca, canine; HR, heart rate; IV, intravenously.
- = depression; + = stimulation.
• 0 indicates minimal to no effect.
• - indicates a decrease in the parameter indicated (where - is mild; - - is moderate; - - - is marked).
• + indicates an increase in the parameter indicated (where + is mild; ++ is moderate; +++ is marked).
• Effects of drugs depend on many factors, including species, differences among specific agents within a class, dose, and drug interactions. Therefore quantifications of the principal effects (mild, moderate, marked) are approximations.
• The information regarding opioids includes those of agonists, partial agonists, and agonist-antagonists. When reading this, note that effects of agonists are generally more pronounced than those of agonist-antagonists or partial agonists).
TABLE 3-3
Principal Respiratory and Gastrointestinal System Effects of Anesthetic Agents and Adjuncts
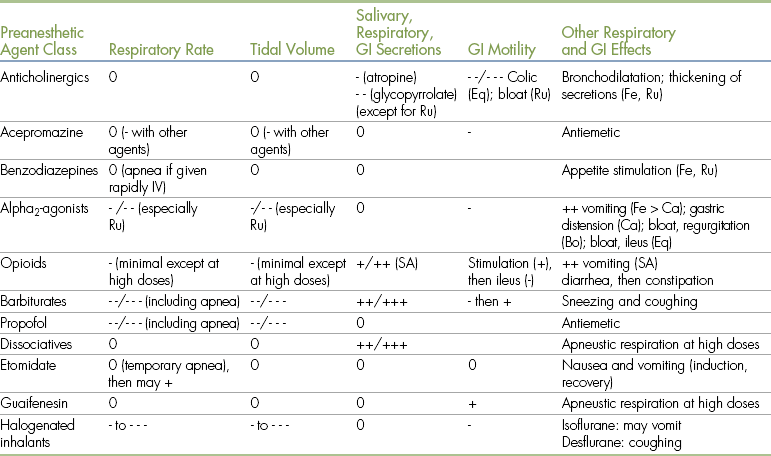
Bo, Bovine; Ca, canine; Eq, equine; Fe, feline; GI, gastrointestinal; IV, intravenously; Ru, ruminants; SA, small animals.
- = depression; + = stimulation.
• 0 indicates minimal to no effect.
• - indicates a decrease in the parameter indicated (where - is mild; - - is moderate; - - - is marked).
• + indicates an increase in the parameter indicated (where + is mild; ++ is moderate; +++ is marked).
• Effects of drugs depend on many factors, including species, differences among specific agents within a class, dose, and drug interactions. Therefore quantifications of the principal effects (mild, moderate, marked) are approximations.
• The information regarding opioids includes those of agonists, partial agonists, and agonist-antagonists. When reading this, note that effects of agonists are generally more pronounced than those of agonist-antagonists or partial agonists).
TABLE 3-4
Other Principal Effects of Anesthetic Agents and Adjuncts
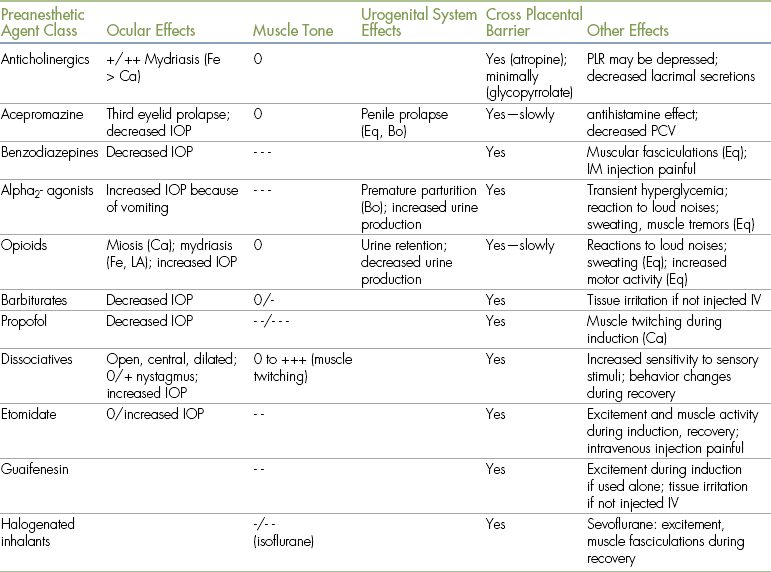
Bo, Bovine; Ca, canine; Eq, equine; Fe, feline; IM, intramuscular; IOP, intraocular pressure; IV, intravenously; LA, large animals; PCV, packed cell volume; PLR, pupillary light reflex; Ru, ruminants; SA, small animals.
- = depression; + = stimulation.
• 0 indicates minimal to no effect.
• - indicates a decrease in the parameter indicated (where - is mild; - - is moderate; - - - is marked).
• + indicates an increase in the parameter indicated (where + is mild; ++ is moderate; +++ is marked).
• Effects of drugs depend on many factors, including species, differences among specific agents within a class, dose, and drug interactions. Therefore quantifications of the principal effects (mild, moderate, marked) are approximations.
• The information regarding opioids includes those of agonists, partial agonists, and agonist-antagonists. When reading this, note that effects of agonists are generally more pronounced than those of agonist-antagonists or partial agonists).
Agonists, Partial Agonists, Mixed Agonist-Antagonists, and Antagonists
Before embarking on a study of anesthetic agents and adjuncts, a general knowledge of pharmacokinetics (the effect the body has on a drug) and pharmacodynamics (the effects a drug has on the body) is necessary. After administration, most drugs are distributed throughout the body by the blood. Each drug binds to specific receptors in one or more “target tissues.” After binding to specific receptors, the drug stimulates the receptor, causing one or more specific effects. In the case of anesthetic agents and adjuncts, the primary target tissue is most often the central nervous system (CNS), and the most common effect is depression and/or stimulation of one or more parts of the CNS.
Anesthetic agents and adjuncts differ according to the degree to which they stimulate target tissue receptors. Agonists bind to and stimulate tissue receptors. Most anesthetics and adjuncts are classified as agonists.
Some drug classes such as the alpha2-adrenergics and opioids include drugs that are classified as antagonists. Antagonists bind to but do not stimulate receptors. These drugs are given after an agonist of the same class to “wake” the patient after anesthesia or sedation. They are therefore called reversal agents because they reverse the effects of the corresponding agonist. Specifically, most antagonists competitively bind to receptors and displace the corresponding agonist, blocking further action.
The opioid class also includes some partial agonists and agonist-antagonists. Partial agonists bind to and partially stimulate receptors. Agonist-antagonists bind to more than one receptor type and simultaneously stimulate at least one and block at least one. Both partial agonists and agonist-antagonists are sometimes used to partially block the effects of pure agonists.
Analgesic Effects of Anesthetics and Adjuncts
When an anesthetic protocol is being chosen for animals that are experiencing pain or that are undergoing a painful procedure, it must be kept in mind that analgesia must be provided before, during, and after the anesthetic event. Many commonly used general anesthetics, including halogenated inhalant agents, propofol, and etomidate, produce unconsciousness but little to no pain control and so are not true analgesics.
As animals do not feel pain while unconscious, general anesthetics indirectly provide analgesia by producing a state of unconsciousness. In other words, when these agents are used, pain is not perceived while the patient is “asleep” but recurs with the return of consciousness. Therefore these agents do not provide adequate pain control at subhypnotic or subanesthetic doses, and their use must be preceded and followed by administration of true analgesics if preoperative or postoperative pain control is necessary.
In contrast, true analgesics such as the opioid agonists produce analgesia regardless of the patient’s level of consciousness. In other words, these agents work whether the patient is “asleep” (unconscious) or “awake” (conscious) and are used to provide analgesia during the pre-, intra-, and post-operative periods.
Using Drugs in Combination
Two or more anesthetic agents and/or adjuncts are often used in combination. Some drugs can be safely mixed in the same syringe, whereas others cannot. Incompatible mixtures can produce a variety of harmful or even fatal adverse effects because of loss of potency, change in chemistry, precipitation of one or more of the drugs, or other untoward interactions. For this reason, the anesthetist must observe some general guidelines when faced with a decision to mix two or more drugs.
With the exception of diazepam (a benzodiazepine tranquilizer), most anesthetic agents and adjuncts are water-soluble. In general, two or more water-soluble drugs can be safely mixed, but a water-soluble drug and a non–water-soluble drug cannot. The sole exception to the rule is the combination of the water-soluble drug ketamine and diazepam, which can be safely mixed and administered unless visible precipitation occurs. Regardless of these guidelines, do not mix two or more drugs unless you have reliable evidence that it is safe to do so. Usually this information can be found in professional publications such as anesthesia textbooks and peer-reviewed journals as well as from experienced anesthetists
Regulatory Considerations for Controlled Substances
Some of the agents covered in this chapter are subject to government (Drug Enforcement Administration [DEA]) regulation regarding purchase, handling, and dispensing. The benzodiazepines, dissociatives, barbiturates, and most opioids fall into this category. Any use of these “controlled substances” necessitates compliance with detailed record-keeping requirements owing to the potential for abuse or theft. In the United States, the Controlled Substances Act assigns each drug to one of five drug schedules according to its potential for abuse. In a similar way, Canadian legislation has classified each opiate as a narcotic, controlled, or prescription drug. Agents classified as narcotics in Canada or as Schedule II substances in the United States cannot be dispensed or drawn into a syringe except under the direct supervision of a licensed veterinarian.
All controlled substances must be kept in a double-locked cabinet, safe, or other secure storage place and must not be left on countertops or in other public areas. After a dose is withdrawn from a bottle containing a controlled substance, the bottle should be immediately returned to locked storage. Usage must be accurately recorded in a drug logbook, and inventory must be periodically checked to ensure that no drug is unaccounted for.
PREANESTHETIC MEDICATIONS
The following agent classes are traditionally classified as preanesthetic medications because they are most commonly administered during the preanesthetic period. These agents are often given alone or in combination as a part of balanced anesthesia.
Reasons for the Use of Preanesthetic Medications
The principal reasons for giving commonly used preanesthetic medications follow. Table 3-5 summarizes these benefits.
TABLE 3-5
Principal Reasons for Giving Preanesthetic Agents
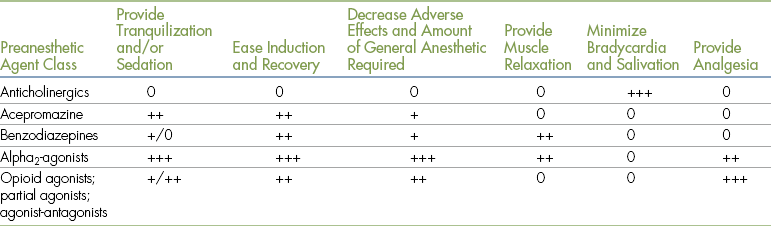
0 = does not have the effect listed
+, ++ or +++ = produces the effect to a slight, moderate or great degree respectively.
1. To calm or sedate an excited, frightened, or vicious animal. Sedation not only enhances patient comfort and reduces anxiety, but also simplifies patient restraint.
2. To minimize adverse effects of concurrently administered drugs. All anesthetic agents and adjuncts cause undesirable adverse effects in addition to their desired action. For example, ketamine causes excessive salivation in some patients, and alpha2-agonists may cause profound bradycardia or heart block. Anticholinergics are sometimes given to decrease these exaggerated parasympathetic effects.
3. To reduce the required dose of concurrently administered agents. For example, administration of a sedative during the preanesthetic period will often decrease the amount of general anesthetic required to produce surgical anesthesia.
4. To produce smoother anesthetic inductions and recoveries. During induction and recovery, patients pass through a period of involuntary excitement. While passing through this stage, they can be dangerous to themselves and to personnel, inflicting bites or experiencing bone fractures or other serious injuries in extreme situations.
5. To decrease pain and discomfort before, during, and after surgery. As noted above, most general anesthetic agents have limited or no analgesic effect, so in many cases adjuncts must be given to provide the necessary level of pain control.
6. To produce muscle relaxation. Muscle relaxation is particularly important during some procedures including orthopedic and ocular surgery. Preanesthetic medications such as benzodiazepines and alpha2-agonists are often given concurrently with general anesthetics to produce the desired amount of muscle relaxation.
Preanesthetic medications also have other uses. For example, tranquilizers are used to calm patients for transport, physical examination, radiographic procedures, and wound treatment. They are helpful in preventing animals from chewing wounds and bandages. The benzodiazepine diazepam stimulates the appetite in cats and ruminants, phenothiazines have antiemetic properties, and many opioids are effective cough suppressants.
The veterinarian will decide which preanesthetic medications to give based on the nature of the procedure, patient need, personal preference, and other factors, as well as the route and time of administration.
Preanesthetic medications are usually given via the IV, intramuscular (IM), or subcutaneous (SC) route, but the onset of action, duration of action, and dose vary with each of these routes. Of these three routes, SC administration is associated with the slowest onset of action and longest duration. IM administration results in a somewhat faster onset (15 to 20 minutes in most cases) and a somewhat shorter duration than SC administration. Patients given sedatives by the IM or SC route should be left undisturbed until peak action is reached because excitement or stimulation can sometimes cause the patient to override the effects of these agents when either of these routes is used. Drugs given intravenously generally act within seconds to a few minutes and have a shorter duration than if given either intramuscularly or subcutaneously but should be administered slowly and cautiously because potency and the potential for adverse effects are increased when drugs are given by this route. In general, IV doses are typically about one half IM or SC doses.
Although some agents such as ketamine can be given by mouth, effects of orally administered drugs are unpredictable. For this reason, this route of administration is usually reserved for patients too aggressive to be handled or for other situations in which it is impossible to inject the drug.
Anticholinergics
Also known as parasympatholytics, anticholinergics are most commonly used to prevent and treat bradycardia and to decrease salivary secretions arising from parasympathetic stimulation. The two anticholinergic agents commonly used in veterinary medicine are atropine and glycopyrrolate. Atropine is a relatively old drug, derived from the deadly nightshade plant, that was used for many years to decrease excessive respiratory secretions and laryngospasm caused by the irritating effects of ether, and the excess secretions caused by the barbiturates. Glycopyrrolate is a newer synthetic quaternary ammonium compound. Both drugs may be given by the IV, IM, SC, or intratracheal (IT) route. When these drugs are used as preanesthetics, the IM and IV routes are most commonly employed. The IT and IV routes are used in emergency situations in which rapid action is essential. Atropine is approved for use in several domestic species, and glycopyrrolate is approved for use in dogs and cats.
Mode of Action and Pharmacology
Acetylcholine is the primary neurotransmitter in the PNS responsible for parasympathetic effects (also referred to as cholinergic effects). The PNS has two types of receptors for acetylcholine. The nicotinic receptors are located on the postganglionic neurons at the junction with the preganglionic neurons, and the muscarinic receptors are located on the target organs (Figure 3-1). Anticholinergics competitively block binding of the neurotransmitter acetylcholine at the muscarinic receptors.
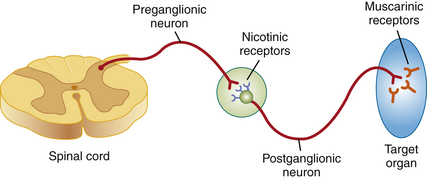
FIGURE 3-1 The parasympathetic nervous system. Preganglionic neuron releases acetylcholine at the nicotinic receptors. Postganglionic neuron releases acetylcholine at the muscarinic receptors of the target organ. Anticholinergics affect only the muscarinic receptors.
The vagus nerve (tenth cranial nerve) provides parasympathetic innervation to numerous target organs including the heart, lungs, gastrointestinal (GI) tract, some secretory glands, and the iris of the eye. During surgery the vagus nerve may be stimulated by endotracheal intubation, traction on visceral organs during abdominal surgery (known as the viscerovagal reflex), and manipulation of the eye during ocular surgery (known as the oculovagal reflex) and by some drugs including the alpha2-agonists, opioids, and common general anesthetics. Increased binding of acetylcholine to the muscarinic receptors caused by vagal stimulation results in observable parasympathetic effects at the target organs, such as bradycardia, bronchoconstriction, excess tear and saliva production, excess production of respiratory system secretions, increased GI motility, and miosis. By blocking the muscarinic receptors, anticholinergics help to reverse and prevent these parasympathetic effects (thus the alternative name, parasympatholytics).
After IM injection, atropine begins to act in about 5 minutes, reaches peak effect in about 10 to 20 minutes, and has a duration of action of 60 to 90 minutes. Glycopyrrolate has a similar onset of action, reaches peak effect in about 30 to 45 minutes, and has a duration of action of 2 to 3 hours, although salivary secretions can be suppressed for up to 7 hours. In order to allow time for peak effect, either agent should be administered intramuscularly at least 20 to 30 minutes before anesthetic induction. When given intravenously, the onset of action of atropine is about 1 minute and peak effect is about 3 to 4 minutes after injection. This makes this drug ideal for treatment of bradycardia in emergency situations such as cardiopulmonary-cerebrovascular resuscitation (CPCR).
Effects on Major Organ Systems
Other Effects
• Reduction of respiratory tract, GI tract, and salivary secretions. Several anesthetic agents, particularly ketamine and thiobarbiturates, induce copious production of saliva. Endotracheal intubation often induces excess airway mucous production, especially in cats. Accumulation of these excessive secretions can lead to airway obstruction.
• Mydriasis. This effect is not commonly seen when dogs are given the usual preanesthetic doses but may be seen in cats. When monitoring patients, the anesthetist must remember that the pupillary light reflex (PLR) may be depressed and therefore unreliable in patients given anticholinergics.
• Reduction of lacrimal secretions. Corneal drying is a risk of general anesthesia because the patient’s eyes remain open. Corneal drying, if severe or prolonged, will result in keratitis and corneal ulceration. For this reason, the corneas of animals receiving anticholinergics should be protected from drying by instilling a 1⁄4- to 1⁄2-inch strip of ophthalmic lubricating ointment in each eye every hour.
• Bronchodilatation. Anticholinergics increase the diameter of bronchioles. This results in increased anatomic dead space, which may put the patient at risk for hypoxemia (low blood oxygen).
Adverse Effects
• Arrhythmias. After IV injection, anticholinergics may induce temporary first- or second-degree atrioventricular (AV) block, followed by sinus tachycardia. Although glycopyrrolate is less arrhythmogenic than atropine, either drug may also induce other cardiac arrhythmias including ventricular arrhythmias. For these reasons, these drugs should be avoided in animals with preexisting rapid heart rates (more than 140 beats per minute [bpm] in dogs, 180 bpm in cats, 60 bpm in horses, and 100 bpm in ruminants) or heart disease (such as congestive heart failure or hyperthyroid-associated cardiomyopathy in cats).
• When given at low doses, atropine may induce a temporary bradycardia. This is because atropine blocks a second type of muscarinic receptor located on presynaptic nerve terminals that normally inhibits acetylcholine release. The atropine-induced blockade of these receptors releases acetylcholine, resulting in bradycardia until the postsynaptic receptors are also blocked.
• Thickening of respiratory and salivary secretions. The use of anticholinergics in cats is associated with the production of thick mucous secretions within the airways. So even though these drugs decrease the volume of the secretions, the increased viscosity may predispose the patient to airway blockage. For this reason, some veterinarians do not use anticholinergics in this species. Anticholinergics are not recommended in ruminants at all except to treat intraoperative bradycardia or for CPCR. This is because these drugs do not decrease the amount of salivary secretions in these species but instead cause the saliva to become thick and ropy, which places these patients at risk for respiratory obstruction.
Use of Anticholinergics
Atropine and glycopyrrolate are still commonly used by veterinary anesthetists, although less so now than in the past. Many modern anesthetic agents do not cause excessive secretions, and bradycardia is well tolerated by most patients. Because of the potential for significant adverse effects, particularly tachycardia, thickening of mucous secretions, decreased tear production, and mydriasis, many authorities question the routine use of these agents. However, anticholinergics are beneficial for some patients (e.g., those with severe bradycardia, heart block, or excessive salivary secretions).
Atropine or glycopyrrolate is often included in preanesthetic and sedative protocols such as the “BAG” and “SuperBAG” protocols (see Chapter 8). Either agent can also be used to prevent adverse effects associated with reversal of nondepolarizing neuromuscular blocking agents. (See Chapter 6 for more information about neuromuscular blocking agents.)
Although the agents are similar, there are subtle differences between atropine and glycopyrrolate. Glycopyrrolate is slightly less likely to induce cardiac arrhythmias, suppresses salivation more effectively than atropine, and only minimally crosses the placental barrier in pregnant animals. For these reasons many practitioners prefer this drug as a preanesthetic despite its greater expense. Atropine is still considered to be the better choice as an emergency treatment for bradycardias associated with cardiopulmonary arrest, however, because of its faster onset.
Veterinary-labeled atropine products are available in two strengths: 0.54 mg/mL (sometimes listed on the label as 1/120 grain/mL) and 15 mg/mL. After calculating a dose, remember to draw the drug of the correct strength into the syringe or an incorrect amount will be administered to the patient. For example, a volume of 0.5 mL of atropine drawn from a solution with a concentration of 15 mg/mL contains nearly 30 times the amount of drug as compared with a solution with a concentration of 0.54 mg/mL.
Tranquilizers and Sedatives
As mentioned in the introduction, a sedative and a tranquilizer are not exactly the same thing. A tranquilizer is a drug that reduces anxiety but does not necessarily decrease awareness and wakefulness. A sedative is a drug that causes reduced mental activity and sleepiness. Most veterinarians use the terms sedative and tranquilizer interchangeably, however. This is because the effects of these drugs often overlap, as most of these drugs produce both effects to some degree. For instance, tranquilizing effects predominate in the drug diazepam, whereas dexmedetomidine has primarily sedative effects.
Three classes of tranquilizers or sedatives are commonly used in veterinary medicine: phenothiazines, benzodiazepines, and alpha2-adrenoceptor agonists (alpha2-agonists). In addition to tranquilization and sedation, some also cause other effects including ataxia and prolapse of the nictitating membrane (also called the third eyelid) (Figure 3-2). Phenothiazines protect against cardiac arrhythmias and are antiemetics. In contrast, alpha2-agonists are analgesics and can produce vomiting in some patients. Both alpha2-agonists and benzodiazepines are good muscle relaxants.
There are some general risks to using these medications of which the technician must be aware. After receiving these drugs, patients should not be left unattended on a table or in a cage with an open door because they can easily fall and be injured. Sedatives relax the tissues in the pharynx, which may cause serious and life-threatening respiratory distress in brachycephalic dogs, especially those that exhibit significant respiratory stridor when awake. There are also risks to personnel and owners. Sedated animals may exhibit unusual behavior such as aggression or may suddenly become aroused and aggressive, particularly if stimulated suddenly (e.g., by pain).
Phenothiazines
Phenothiazines are a group of noncontrolled, water-soluble drugs with somewhat diverse indications. Acepromazine maleate is used in a wide variety of large and small animal species as an anesthetic adjunct. Triflupromazine, a drug used only in humans, is an antipsychotic and antiemetic drug used to treat schizophrenia and vomiting. Chlorpromazine, a drug with similar indications to triflupromazine in human patients, is also used in veterinary patients as an antiemetic but not as an anesthetic adjunct.
Acepromazine maleate (also known as acepromazine or “ace”) is used alone or in combination with other drugs as a preanesthetic in many species to provide sedation, to decrease the dose of general anesthetic required, and to ease induction and recovery. It is also used alone or in combination with opioids to provide a mild to moderate level of sedation and tranquilization for minor procedures including wound treatment, grooming, and noninvasive diagnostic tests such as radiography. It is approved for use in dogs, cats, and horses.
When used as a preanesthetic and sedative, acepromazine is usually administered by the IV or IM route. Although it is also available in oral tablet form for tranquilization and sedation of small animals, this route is seldom used in anesthesia. There is currently no available reversal agent.
Mode of Action and Pharmacology: Acepromazine has a complex mechanism of action that is not fully understood. Major effects include depression of the reticular activating center of the brain and blockage of alpha-adrenergic, dopamine, and histamine receptors. It is metabolized by the liver and crosses the placental barrier slowly. Onset of action is about 15 minutes after IM injection in dogs or IV injection in horses, and peak effect occurs within 30 to 60 minutes. The duration of action is 4 to 8 hours in small animals but may be longer (up to 48 hours) if higher doses are used or if the drug is given to old, sick, or debilitated patients or patients with liver disease. The duration of action in horses is shorter (1 to 3 hours).
Effects on Major Organ Systems:
• Calming, sedation, reluctance to move, and decreased interest in the patient’s surroundings. Sedation is less pronounced in cats than in dogs and horses and is less pronounced than that seen with alpha2-agonists in all species.
• Acepromazine does not provide pain control but does decrease anxiety in patients in pain that are receiving analgesics concurrently.
• Peripheral vasodilatation. This causes hypotension, a reflexive increase in heart rate, and increased heat loss, leading to hypothermia. Acepromazine also directly decreases cardiac output.
• Antiarrhythmic effect. Some anesthetics, such as barbiturates, have a potential to cause cardiac arrhythmias, which may decrease cardiac output. Phenothiazines protect against ventricular arrhythmias.
• Antiemetic effect. Even at very low doses, acepromazine helps prevent vomiting during the anesthetic period. It can also be used to prevent vomiting caused by motion sickness.
• Antihistamine effect. Histamine is a chemical released during an allergic response. Acepromazine prevents the release of histamine and therefore decreases allergic reactions. For this reason, acepromazine should not be used to sedate animals that are to undergo allergy testing.
• Reduction of the seizure threshold. Although it was reported to have antiseizure activity by some researchers, acepromazine is now generally believed to have the ability to induce seizures in patients with epilepsy or seizures from other causes.
• Occasionally acepromazine may induce excitement or aggression rather than sedation. This effect may persist into the postanesthetic period but usually resolves within 48 hours. Owners should be warned that behavioral changes sometimes occur, and care should be used when handling patients returning home within 48 hours of anesthesia.
• Severe hypotension. Hypotension produced by acepromazine is dose-dependent and more pronounced in frightened or excited patients. In animals receiving isoflurane, a significant drop in blood pressure (as much as 20% to 30%) occurs. This contributes to intraoperative hypotension commonly seen in patients given this combination.
• Penile prolapse. Acepromazine has been reported to cause prolapse of the penis in horses and other large animals, which can lead to injury and subsequent permanent paralysis of the retractor penis muscle. For this reason, some veterinarians choose not to use acepromazine in breeding stallions.
• Decreased packed cell volume (PCV). In horses and dogs the PCV drops within 30 minutes, likely because of increased uptake of red blood cells (RBCs) by the spleen.
Use of Acepromazine: Patients should be placed in a quiet location free from stimulation between administration and peak effect, because the sedative effects can be overridden if the patient is stimulated to a sufficient degree.
It has been suggested that the manufacturer’s recommended dose for acepromazine is higher than that actually required for preanesthesia and that the dose should be reduced to minimize the danger of adverse effects. The commonly accepted doses are about 0.05 to 0.1 mg/kg in small animals with a maximum dose of 3 mg in dogs and 1 mg in cats, and 0.03 to 0.05 mg/kg in horses. Higher doses will increase hypotension but not sedation.
The anesthetist should be particularly aware that phenothiazines have increased potency or duration in geriatric animals, neonates, animals with liver or cardiac dysfunction, and generally debilitated patients. Responses to this drug are also species- and breed-dependent. Doses should be reduced 25% in collies and Australian shepherds to minimize the possibility of exaggerated or prolonged sedation. Giant breed dogs, greyhounds, and boxers can be very sensitive to this drug and may experience severe bradycardia and hypotension. Terriers and cats are more resistant to its effects. Severe hypotension and bradycardia are treated with IV fluid therapy and anticholinergics.
In horses, care must be taken to ensure that acepromazine is injected into a vein. Inadvertent injection into the carotid artery can cause severe CNS excitement or depression, seizures, and death. The location of the needle can be checked by the following maneuver. After venipuncture, remove the syringe from the needle. Release digital pressure on the vein. If the needle is in the jugular vein, blood flow from the hub will cease. If it is in an artery, blood flow will continue from the hub despite the release in digital pressure.
Although acepromazine has a relatively low toxicity, severe overdoses require treatment. Hypotension resulting from overdose is worsened by epinephrine and should instead be treated with phenylephrine or norepinephrine.
Benzodiazepines
The benzodiazepines, also referred to as minor tranquilizers, are a group of controlled, reversible drugs most often used in combination with other agents for their muscle relaxant, anticonvulsant, and appetite-stimulating properties. These drugs produce unreliable sedative effects and in dogs, cats, and horses may instead produce dysphoria, excitement, and ataxia, especially when administered to young, healthy animals.
Diazepam, zolazepam (a component of Telazol), and midazolam are the most commonly used anesthetic adjuncts in this class. Although not used for anesthesia, lorazepam is used in dogs as an alternative to diazepam to treat status epilepticus. Of these agents, only zolazepam is licensed for use in animals in the United States and Canada.
Benzodiazepines are commonly administered intramuscularly or intravenously, although diazepam is irritating and poorly absorbed when administered intramuscularly.
Mode of Action and Pharmacology: Benzodiazepines depress the CNS. Although the exact mechanism of action is not known, benzodiazepines exert their primary effects by increasing activity of endogenous gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter in the brain.
The commercially prepared injectable diazepam is mixed with 40% propylene glycol as well as several other ingredients. It is not water-soluble and cannot be mixed with water-soluble drugs except ketamine. Midazolam and zolazepam are water-soluble and so can be used in combination with other water-soluble drugs. Most benzodiazepines have a relatively rapid onset of action (less than or equal to 15 minutes after IM injection for midazolam and zolazepam) and short duration (1 to 4 hours).
Effects on Major Organ Systems:
• Antianxiety and calming effect. Benzodiazepines, unlike phenothiazines, do not cause significant sedation in healthy young animals unless used in combination with other drugs such as ketamine or opioids. Although their effect as a sole sedative in healthy young animals is inadequate, benzodiazepines are much more effective in geriatric or debilitated animals. Diazepam also enhances the sedation and analgesia of other agents, and its relative safety makes it a popular drug for combination protocols.
• The anesthetist should be aware that benzodiazepines, like phenothiazines, have no analgesic effect and when used alone will not be effective in calming animals that are experiencing pain.
• Anticonvulsant activity. To take advantage of this effect, many anesthetists use benzodiazepines in combination with other agents that have a potential to cause seizures, including ketamine and local anesthetics. Benzodiazepines are also preanesthetics of choice for some animals with seizure disorders. Diazepam and lorazepam can be given intravenously or intrarectally, and midazolam can be given intravenously for treatment of seizures.
Cardiovascular and Respiratory Systems:
• At therapeutic doses, benzodiazepines have few effects on the cardiovascular and respiratory systems and therefore have a high margin of safety. Heart rate, blood pressure, and cardiac output are minimally affected. This property makes them particularly useful for anesthesia of high-risk and geriatric animals.
• Skeletal muscle relaxation. Benzodiazepines are excellent skeletal muscle relaxants and often are used to counteract the muscle rigidity seen with agents such as ketamine and etomidate.
• Potentiation of general anesthetics. Premedication with diazepam decreases the requirements of many general anesthetics including the inhalant agents.
• Appetite stimulation in cats and ruminants. Diazepam and midazolam are commonly used as appetite stimulants in cats, and may also be effective appetite stimulants in many other species.
• The primary adverse effects of these drugs involve the CNS. Animals, especially those that are young and healthy, receiving these drugs alone may actually become more difficult to control. Dogs may become disoriented and excited, and cats may become dysphoric or aggressive. Horses may have muscle fasciculations, and animals of any large species may become ataxic or recumbent.
• Effect on neonates: Benzodiazepines cross the placenta and may cause CNS depression in neonates delivered by cesarean section.
• Adverse effects specific to diazepam: Diazepam is painful and poorly absorbed when administered intramuscularly. Diazepam should be given by slow IV injection, because if given rapidly it can cause pain, bradycardia, hypotension, and apnea. Cats receiving oral diazepam have been reported to develop hepatic failure within 5 to 11 days of commencing therapy.
Use of Benzodiazepines: Diazepam is not water-soluble and is therefore not physically compatible with most other agents. It should not be mixed in a single syringe with atropine, acepromazine, barbiturates, or opioids because a precipitate may form. Although not recommended by the manufacturer, diazepam is often combined in the same syringe with ketamine. Although this seldom results in adverse events, this combination should not be used if a precipitate forms and should not be stored in syringes or other plastic containers because diazepam is very soluble in plastic and over time is absorbed by syringes, IV bags, and IV tubing.
Diazepam and midazolam are both commonly used in combination with other agents to induce anesthesia. Although it is not possible to induce anesthesia in a healthy animal through the use of these drugs alone, they are an effective supplement to other agents. In particular, the combination of ketamine and diazepam has gained wide acceptance as a safe and effective IV induction agent in small animals and horses. When this combination is used in small animals, midazolam may be substituted for diazepam. Diazepam may also be administered concurrently with opioids, propofol, or thiopental (provided separate syringes are used) to achieve safe, smooth induction of high-risk patients. If diazepam is given in combination with opioids, thiopental, or propofol, an IV catheter should be used and a saline flush given after each drug.
Diazepam and midazolam are light-sensitive and for this reason are often provided in brown glass vials. If in a clear glass container, these drugs should be stored away from light.
Benzodiazepines are classified as controlled drugs in Canada and the United States. Some potential for human abuse and theft exists, so these drugs must be stored in a locked cabinet, and appropriate records must be kept in compliance with DEA requirements.
Midazolam has some advantages over diazepam. It is water-soluble and therefore can be mixed with other preanesthetic and anesthetic agents. It is less irritating to tissues and is also more reliably absorbed after IM or SC injection. Like diazepam, midazolam causes unreliable sedation in dogs when used alone but is an excellent sedative in swine and some exotics including ferrets, rabbits, and birds.
In dogs, small mammals, and birds, midazolam is usually used in combination with ketamine and/or opioids for sedation and intubation. It can also be administered before induction of anesthesia with thiopental, propofol, or etomidate to increase muscle relaxation and reduce adverse effects.
Zolazepam is available only mixed with tiletamine in the combination product Telazol. This powdered product requires reconstitution with sterile water and is discussed more in the section covering dissociative anesthetics on p. 82.
The benzodiazepine antagonist flumazenil can be administered to reverse the effects of benzodiazepines, although at the present time this drug is not commonly used in practice because of high expense and the very low incidence of complications seen with these agents.
Benzodiazepine agents are used for a variety of purposes in veterinary medicine other than anesthesia and seizure control. For instance, oral diazepam is sometimes used to modify undesirable behavior such as inappropriate urination in cats.
Alpha2-Adrenoceptor Agonists
Alpha2-adrenoceptor agonists (also written as alpha2-agonists or α2-agonists) are a group of noncontrolled agents used alone and in combination with other anesthetics and adjuncts in both large and small animal patients for sedation, analgesia, and muscle relaxation. They are commonly given before minor procedures such as radiography, wound treatment, or bandaging and subsequently reversed with an alpha2-adrenoceptor antagonist. This allows the patient to be sequentially sedated and “awakened” so that it can be sent home a short time after completion of the procedure. Xylazine (Rompun, AnaSed), dexmedetomidine (Dexdomitor), detomidine (Dormosedan), and romifidine (Sedivet) are members of this class of drugs. Medetomidine (Domitor), a predecessor to dexmedetomidine, was recently discontinued in the United States. These drugs are most often administered intramuscularly or intravenously. SC injection is less reliable and not recommended.
Mode of Action and Pharmacology: Alpha2-agonists act on alpha2-adrenergic receptors (also called alpha2-adrenoceptors) of the sympathetic nervous system (SNS) both within the CNS and peripherally, causing a decrease in the release of the neurotransmitter norepinephrine. When these drugs are combined with other tranquilizers or analgesic agents, the result tends to be additive or synergistic (supraadditive) in nature.
The SNS has several different types of receptors (alpha, beta, and dopaminergic). In general, stimulation of the SNS is associated with the “fight-or-flight response.” For this reason, the CNS depression induced by alpha2-agonists does not at first glance seem logical. This paradoxic effect occurs, however, because stimulation of the alpha2-receptors does not cause the fight-or-flight response but instead causes sedation, analgesia, bradycardia, hypotension, and hypothermia. These unique effects make these particular SNS stimulants useful sedatives and analgesics.
Alpha2-agonists are metabolized in the liver, and the metabolites are excreted in the urine. Adequate hepatic and renal function are therefore important requirements for any animal receiving these drugs.
The onset and duration of action are similar with all currently available alpha2-agonists. After injection, sedation occurs rapidly (within 5 to 15 minutes of IV injection or 15 to 30 minutes of IM injection) and lasts about 1 to 2 hours in most cases. Complete recovery takes about 2 to 4 hours if the drug is not reversed.
Effects on Major Organ Systems:
• Alpha2-agonists are potent sedatives. Sedation is dose-dependent and can be profound in small animals, small ruminants, and foals. In horses, effects include a lowered head (“knees-to-nose” position), relaxed facial muscles, and a drooping lower lip. When the drugs are combined with other agents, sedation may be sufficient for minor or even major surgical procedures (referred to as “standing sedation”).
• Unlike phenothiazines and benzodiazepines, alpha2-agonists also provide analgesia. When combined with other analgesics and sedatives, they may provide sufficient analgesia to allow surgical procedures to be performed. Although the sedative effect of alpha2-agonists may last for several hours, the analgesia may be short-lived (approximately 20 minutes for xylazine) and should be supplemented with another agent, typically an opioid, if a prolonged effect is required.
• Alpha2-agonists have a significant effect on the cardiovascular system. After injection, there is an early dose-dependent vasoconstriction that results in a brief period of hypertension and reflex bradycardia, during which the mucous membranes may look pale. Various cardiac arrhythmias including first- and second-degree AV block may also occur during this time (see p.147 for a discussion of these arrhythmias). These effects are more pronounced when the drug is given intravenously. This phase is followed by a decrease in cardiac output, hypotension, and a further drop in heart rate owing to decreased sympathetic tone.
• Muscle relaxation. These drugs are useful adjuncts to general anesthetics for procedures in which muscle relaxation is necessary.
• Increased effects of other anesthetics. When these agents are given as a preanesthetic, or concurrently with general anesthetics, the required doses of the anesthetics are substantially reduced (e.g., up to an 80% reduction of thiopental and a 50% reduction of propofol and inhalant agents).
• Vomiting. Many cats and some dogs vomit within a few minutes of receiving these agents. Because affected patients may have decreased swallowing reflexes, the airway must be protected to prevent aspiration of stomach contents by placing the head in a dependent position as soon as retching is noted. Dexmedetomidine is less likely to cause vomiting than xylazine.
• Hyperglycemia. Alpha2-agonists reduce the secretion of insulin by the pancreas, causing transient hyperglycemia, which is not harmful to the animal but may confound the interpretation of blood samples collected during this period.
• Hypothermia. Alpha2-agonists decrease thermoregulation and shivering, leading to hypothermia.
Adverse Effects: Alpha2-agonists have considerable potential for adverse effects. These are reported most commonly when the drugs are given by the IV route and include the effects discussed in the following paragraphs.
• Temporary behavior changes. Patients that are excited before administration may become agitated and aggressive when touched, and any patient may move or startle in response to loud noises. Horses may experience muscle tremors and may kick in response to loud noises. Cattle frequently lie down. Excessive doses given intravenously may cause falling from ataxia and sedation.
• Alpha2-agonists cause profound cardiovascular depression. Dramatic decreases in heart rate (e.g., down to 30 to 50 bpm in dogs), blood pressure, and cardiac output with a resultant decrease in tissue perfusion can occur with these agents especially when given at high doses. Because of these serious effects, the use of alpha2-agonists should be avoided in animals that are debilitated or that have cardiovascular disease.
• Respiratory system depression varies from animal to animal and is more severe when alpha2-agonists are given with other drugs. Some animals show respiratory depression of sufficient magnitude to negatively affect ventilation. Brachycephalic dogs and horses with upper respiratory obstruction may become dyspneic. As a general rule, alpha2-agonists should not be administered to animals showing signs of respiratory disease.
• Increased urination. Alpha2-agonists may increase urination because they interfere with the release of antidiuretic hormone.
• GI effects. Dogs may develop gaseous distension of the stomach that is visible on radiographs and may need to be relieved. Cattle may salivate, bloat, and regurgitate stomach contents. Rarely, horses may develop gas, colic, or intestinal bloat.
• Premature parturition. Xylazine causes increased intrauterine pressure in cattle and has the potential to cause abortion in the last trimester.
• Accidental intraarterial injection in horses can result in excitement, seizures, and collapse.
• Alpha2-agonists can be absorbed through skin abrasions and mucous membranes, and as little as 0.1 mL of dexmedetomidine can cause hypotension and sedation in humans. Hospital employees handling these agents should ensure that any of the drugs spilled on human or animal skin is immediately washed off.
Use of Alpha2-Agonists: Careful monitoring of vital signs is always essential for patients receiving these drugs, all of which should be used with caution. All members of this class can be given in standard doses to young, healthy patients but should be avoided in geriatric, diabetic, pregnant, pediatric, or sick patients.
To reduce the incidence of bradycardia, some veterinarians give atropine or glycopyrrolate as a premedication. However, this is not always effective and may in fact increase the workload of the heart and myocardial oxygen consumption. If used, anticholinergics must be administered 10 to 20 minutes before these agents are given, or bradycardia may worsen and other arrhythmias may develop. Anticholinergics should also be avoided with alpha2-agonist–ketamine combinations, because prolonged tachycardia can occur. If bradycardia occurs, the best treatment is administration of an appropriate reversal agent.
Xylazine: Xylazine has been available for several decades and is the first widely used alpha2-agonist in both large and small animal species. It is supplied as a 2% solution (20 mg/mL) for small animal use and as a 10% solution (100 mg/mL) for equine use. Therefore to avoid a serious overdose, it is very important to look carefully at the label before drawing up a dose.
For several decades xylazine has been frequently used alone or in various combinations with ketamine, opioids, and other agents in dogs, cats, and other small animals. It has now been largely replaced by dexmedetomidine in these species. It is still used in large animals as a preanesthetic and sedative, in combination with butorphanol for minor procedures, and in combination with ketamine and guaifenesin (a combination known as “triple drip”) to produce total IV anesthesia. Cattle have a much lower tolerance for xylazine, requiring only about one tenth the dose of horses.
Dexmedetomidine: Dexmedetomidine (Dexdomitor) is currently the most commonly used alpha2-agonist in dogs and cats. It is closely related to medetomidine, which was recently discontinued in the United States. Medetomidine is a mixture of two molecules that are mirror images of each other (enantiomers). The dextrorotatory enantiomer is a molecule that rotates the plane of polarized light to the right, and a levorotatory molecule rotates it to the left. Dexmedetomidine is the dextrorotatory enantiomer of medetomidine and produces the sedative and analgesic effects we associate with this drug, whereas levomedetomidine (the levorotatory enantiomer) has no sedative or analgesic activity. Dexmedetomidine does not contain the inactive enantiomer and so has approximately twice the potency of medetomidine. In 2008 a new product approved for use in both dogs and cats containing only dexmedetomidine (Dexdomitor) was introduced in the United States.
Because dexmedetomidine is relatively new, at the time of this writing, experience with the use of this drug is limited. However, because it is the active form of medetomidine, current evidence suggests that it can be used in much the same way. Its use is summarized in the following paragraphs.
Dexmedetomidine has greater potency and fewer adverse effects than xylazine. It is supplied as a 0.5-mg/mL solution for sedation and analgesia in dogs and cats and as a preanesthetic in dogs and is marketed along with a corresponding antagonist atipamezole (Antisedan). These two products are packaged such that equivalent volumes of the two drugs can be used to sequentially sedate and wake a patient for short or minor diagnostic, surgical, or therapeutic procedures. Dexmedetomidine is labeled for IM use in dogs and cats and IV use only in dogs.
The manufacturer recommends that dexmedetomidine dose be determined according to body surface area in dogs, so that smaller patients receive relatively more and larger patients receive relatively less. Charts based on this dosage scheme are supplied with the drug, with progressively increasing doses listed for patients of various body weights for “sedation/analgesia in dogs,” and “preanesthesia in dogs.” Low doses of dexmedetomidine given as a preanesthetic are generally more effective than acepromazine in reducing general anesthetic requirements and do not cause as profound a degree of hypotension when given with isoflurane.
Like xylazine, dexmedetomidine is also used in combination with other agents. For instance, one combination in widespread use is dexmedetomidine (10 mcg/kg) and butorphanol (0.2 mg/kg). The two drugs can be mixed in the same syringe and given intramuscularly. Dexmedetomidine can also be mixed with other opioids or with ketamine.
Animals should be left in a quiet environment for 10 to 15 minutes after injection to allow the drug to take maximum effect. If a short, minimally invasive procedure is planned (such as an ear flush, bandage change, or radiography), the sedation provided by dexmedetomidine-butorphanol and other combinations may be adequate without a general anesthetic. The sedation can be supplemented with a local block if minor surgery such as suturing a laceration or removal of a superficial skin tumor is planned. Although these patients are usually too awake for intubation, some veterinarians choose to provide supplemental oxygen by facemask. Caution should be exercised when dexmedetomidine combinations are used for minor operations, because sudden arousal has been reported even in heavily sedated patients.
If the sedated animal is to undergo more extensive surgery, an injectable or inhalant general anesthetic (e.g., thiopental, propofol, or isoflurane) can be given. The dose of general anesthetic required will be significantly less than that required for a nonsedated animal, and caution must be exercised to avoid overdosage.
Pain may be associated with IM injection of dexmedetomidine. Do not give anticholinergics with high doses of dexmedetomidine because paradoxic bradycardia may result.
The parent drug medetomidine has been used extensively in many other species, including cats, horses, and exotic animals.
Detomidine: Detomidine is used in horses to produce sedation, analgesia, and muscle relaxation. This drug is similar to xylazine, producing similar beneficial and adverse effects, but has approximately twice the duration of action. It is commonly given with butorphanol to produce standing sedation. It is also used to provide analgesia for colic pain.
Alpha2-Antagonists
Yohimbine, tolazoline, and atipamezole are alpha2-antagonists that can be used to reverse the effects of alpha2-agonists. Because of the adverse cardiovascular effects and long duration of sedation that can occur after administration of alpha2-agonists, use of an antagonist is often desirable. Yohimbine and tolazoline are used to reverse the effects of xylazine in dogs, cats, ruminants, horses and exotic species. Atipamezole is used to reverse the effects of dexmedetomidine in dogs, cats, and exotic species.
Mode of Action and Pharmacology: These agents work by displacing the agonist from the alpha2-receptors. Alpha2-antagonists preferentially bind to the receptors, thus reversing the effect of the corresponding agonist.
Effects: The major effect of alpha2-antagonists is reversal of the sedative and cardiovascular effects of the corresponding agonist.
Adverse Effects: Alpha2-antagonists have few adverse effects at clinical doses but can have significant adverse effects if too much is given. Doses should be based on the amount of the agonist that was given and the length of time since agonist administration and should be reduced if more than 30 minutes have elapsed. Adverse effects are neurologic, cardiovascular, and GI and include excitement, muscle tremors, hypotension, tachycardia, salivation, and diarrhea. Because of the potential for tachycardia, these agents should not be given when anticholinergics have also been given.
Use of Alpha2-Antagonists: Alpha2-antagonists will reverse all of the clinical effects of alpha2-agonists, both detrimental (bradycardia) and beneficial (analgesia). To maintain analgesia it may be necessary to administer another analgesic agent just before the reversal. The anesthetist must also realize that alpha2-antagonists do not reverse the effects of other drugs given concurrently (e.g., dissociatives, opioids, general anesthetics). Because the duration of these drugs is generally short, resedation may occur, in which case an additional dose may be necessary.
The dose of these drugs is expressed as a ratio of the agonist dose to the antagonist dose. In other words, an agonist-antagonist ratio of 10:1 means that if 0.1 mg/kg of the agonist was given, then the correct dose for the antagonist is 0.01 mg/kg (one tenth the agonist dose). An agonist-antagonist ratio of 2:1 means that 0.1 mg of the agonist per kilogram should be followed by 0.05 mg of the antagonist per kilogram (half the agonist dose). When given intravenously, alpha2-antagonists should be given slowly.
Tolazoline: Tolazoline is a nonspecific alpha-antagonist (it binds to both alpha1 and alpha2 receptors) primarily used to reverse the sedative and cardiovascular effects of xylazine in ruminants. The dose is calculated using a 1:10 agonist-antagonist ratio (0.1 mg of xylazine per kilogram is reversed with 1 mg of tolazoline per kilogram).
Yohimbine: Yohimbine is primarily used to reverse sedative and cardiovascular effects of xylazine in dogs, cats, horses, and exotic species. The calculated dose is based on a 10:1 agonist-antagonist ratio in dogs and horses and a 2:1 ratio in cats. (In a dog, 1 mg of xylazine per kilogram is reversed with 0.1 mg of yohimbine per kilogram.)
Atipamezole: Atipamezole (Antisedan) is packaged as a specific antagonist for dexmedetomidine and medetomidine. Atipamezole is labeled for IM injection only and should be given by this route unless IV administration is necessary for emergency resuscitation. The dose of atipamezole is based on an agonist-antagonist ratio of about 1:10. As the formulation of atipamezole is 10 times more concentrated than dexmedetomidine (atipamezole 5 mg/mL, dexmedetomidine 0.5 mg/mL), equal volumes of the two drugs should be administered to dogs. Cats are more sensitive to the effects of atipamezole, so about half of this dose should be used in this species (an agonist-antagonist ratio of 1:5). Because of marked side effects such as hypersalivation and CNS excitement, atipamezole should not be given intravenously in cats. Reversal of effects typically occurs 5 to 10 minutes after IM injection.
Opioids
Opioids are derivatives of opium, an extract of a species of poppy called Papaverum somniferum. Opioids include both naturally derived compounds called opiates and synthesized compounds.
This is a versatile class of drugs used for analgesia, sedation, and, when combined with other agents, anesthetic induction. Opioids are classified as agonists, partial agonists, agonist-antagonists, or antagonists, depending on their predominant effects.
Commonly used opioids include the agonists morphine, hydromorphone, oxymorphone, fentanyl, and meperidine; the partial agonist buprenorphine; the agonist-antagonists butorphanol and nalbuphine; and the antagonist naloxone; as well as etorphine and carfentanil, two agonists used for wild animal capture. With the exception of the antagonists and nalbuphine, opioids are controlled drugs and include several that are Class II (including morphine, hydromorphone, oxymorphone, and fentanyl). They may be administered by a wide variety of routes including IV, IM, SC, oral, rectal, and transdermal, as well as subarachnoid and epidural routes (two different types of “spinal injections”). They have a wide safety margin and can be used on both healthy and debilitated patients. Information on the use of opioids as induction agents is found in Chapters 8, 9, and 10, and detailed information on specific opioid agents and their use for analgesia is presented in Chapter 7.
Mode of Action and Pharmacology
Opioids produce similar effects as natural chemicals present in the body called endogenous opioid peptides, which include β-endorphin, dynorphins and enkephalins. Although the mode of action of opioids is not completely understood, it has been the subject of a great deal of research over the past several decades that has yielded much information. Although opioid receptors are found on neurons throughout the body, the analgesic and sedative effects are chiefly the result of their action on receptors located in the brain and spinal cord.
Three major types of opioid receptors have been identified: mu (μ), kappa (κ), and delta (δ), each of which has two or more subtypes. This variety of receptors produces a wide spectrum of effects because each opioid agent differs in its action at each of these sites and therefore in its overall effects on the body.
Opioid agonists exert their effects primarily by binding to and stimulating the mu and kappa receptors and are the best drugs available for moderate and severe pain. Partial agonists only partially stimulate the opioid receptors. Agonist-antagonists do not stimulate the mu receptors, but instead stimulate the kappa receptors. Pure antagonists bind to but do not stimulate mu or kappa receptors. They are therefore called reversal agents, because they displace agonists from the receptors and block their effect. These antagonists have no clinical effect on their own but are used to reverse the effects of the pure agonists, partial agonists, and mixed agonist-antagonists.
With few exceptions, opioids have a relatively short duration of action (1⁄2 to 3 hours). Exceptions are buprenorphine, which has a significantly longer duration (6 to 8 hours), and morphine, which has a duration of 6 to 8 hours in horses.
Effects on Major Organ Systems
• Opioid agents may cause CNS depression or excitement. The exact effect depends on the dose, route, agent used, species, patient temperament, and pain status.
• In dogs the predominant effect is sedation, which tends to be greater with agonists and milder with partial agonists and agonist-antagonists. Most dogs exhibit CNS depression within 60 seconds of IV administration and 15 minutes after IM administration. If high doses of opioid agonists are given (particularly to a sick animal), a sleeplike state called narcosis may be produced.
• In contrast, cats, horses, and ruminants exhibit CNS stimulation that is more pronounced with pure agonists. This may include bizarre behavior patterns, including excitement, increased motor activity, or dysphoria (a state characterized by anxiety or restlessness), particularly if the drug is given intravenously. For this reason, some opioids (e.g., morphine) must be used at low doses in these species and IV injection is avoided. Dogs that are not in pain may show a similar reaction (e.g., whining and barking) after opioid administration, particularly if given by rapid IV injection or if a tranquilizing agent is not used concurrently.
• Analgesia. Opioids have long been considered to be the most effective agents for the treatment of pain. The degree of analgesia varies among members of the class: pure agonists such as morphine and hydromorphone are more effective for treatment of severe pain than the partial agonists such as buprenorphine or the mixed agonist-antagonists such as butorphanol. Opioids are particularly useful when included in premedication for patients undergoing surgery for painful conditions (e.g., repair of fractures).
• Because most commonly used general anesthetics have limited analgesic properties (e.g., isoflurane, sevoflurane, propofol, and barbiturates), the analgesic effect of opioids remains one of the chief indications in veterinary patients.
• Although these drugs have the potential to decrease respiratory rate and tidal volume, this effect is often minimal in the absence of preexisting CNS depression. Some dogs pant after administration of opioid agonists. This is because of a direct effect on the thermoregulatory center of the brain, which mistakenly interprets normal body temperature as being elevated.
Other Effects
• Changes in pupil size. Opioids cause miosis in dogs and mydriasis in cats, ruminants, and horses.
• Changes in body temperature. Dogs become hypothermic as a result of resetting of the thermoregulatory center in the hypothalamus and panting. Cats become hyperthermic for unknown reasons.
• Increased responsiveness to noise. Some patients will startle in reaction to loud noises, requiring caution to ensure that the patient does not fall off a table or out of an open cage.
Adverse Effects
Many of the adverse effects manifest as an exaggeration of the effects discussed previously.
• A serious potential side effect of opioids is their tendency to depress respiration, particularly when used with tranquilizing agents. Although this effect is not seen at low dose rates (such as those used for pain control), opioids given at high dose rates may cause a decrease in both respiratory rate and tidal volume, resulting in decreased blood oxygen levels (Pao2) and increased carbon dioxide levels (Paco2). This effect is particularly noticeable if the opioid is given in combination with another drug that is a respiratory depressant, such as dexmedetomidine or isoflurane. Respiratory depression is dose-dependent for many opioid agents, meaning the effect is more pronounced at high doses. Other opioids (e.g., butorphanol) exhibit a “ceiling effect” in that high doses cause no more respiratory depression than do low doses.
• Opioids can cause several adverse GI effects, including salivation and vomiting in small animal patients. Initially many agents also cause an increase in peristaltic movement, resulting in diarrhea, vomiting, and flatulence. Pretreatment with atropine or acepromazine usually moderates this effect. After the initial stimulation of peristalsis, a prolonged period of GI stasis may occur, resulting in constipation. GI stasis or ileus is of particular concern in horses, as it can predispose them to developing colic. Because of the emetic effect of some opioid agents (particularly morphine), these drugs should be avoided in animals in which vomiting would be detrimental (e.g., animals with GI obstruction).
• Physical dependence (addiction). Prolonged use of some opioid agents can lead to physical dependence. Human patients given morphine develop a tolerance for its effects in approximately 2 to 3 weeks. Not all opioids are addictive: those with minimal or antagonistic activity at mu receptors (e.g., butorphanol) have less potential for causing physical dependence.
• Morphine and meperidine may cause facial swelling and hypotension after rapid IV administration because of histamine release.
• Opioids tend to increase intraocular and intracranial pressure because of their tendency to depress ventilation, which in turn increases Paco2, and therefore should be avoided or used cautiously in patients with head trauma and other CNS disorders.
• Drug interactions. Some opioids, particularly meperidine, may cause a potentially fatal reaction known as serotonin syndrome when given to animals receiving monoamine oxidase inhibitors such as selegiline or tricyclic antidepressants such as clomipramine. Although the incidence of this reaction in veterinary patients is unknown, these combinations should be avoided until future studies clarify the risk.
Use of Opioids
Opioid agonists, partial agonists, and agonist-antagonists are used in many ways in veterinary anesthesia. They are a common component of preanesthetic protocols. For high-risk patients, some anesthetists prefer to use an opioid such as morphine or hydromorphone as the sole preanesthetic agent. More commonly, however, opioids are mixed with a tranquilizer (such as acepromazine, diazepam, or dexmedetomidine) and/or an anticholinergic (atropine or glycopyrrolate) and given during the preanesthetic period. Many combinations are used; see Protocols 8-1 and 8-2 in Chapter 8 for common combinations used in small animals.
Ideally, these combinations should be chosen on the basis of individual patient need and drawn up into a syringe immediately before use. Some practices prepare these mixtures in advance and administer a set dose by IM or SC injection according to patient weight. This is more convenient than individual preparation, but there is some risk of inappropriate treatment, particularly if the patient is geriatric or debilitated or has significant organ dysfunction (e.g., liver disease).
Opioids are also used to prevent and treat postoperative pain (see Chapter 7 for a complete discussion of analgesia) and are often used in combination with a tranquilizer to achieve a state of profound sedation and analgesia termed neuroleptanalgesia.
Neuroleptanalgesia
Neuroleptanalgesia is a state of profound sedation and analgesia induced by the simultaneous administration of an opioid and a tranquilizer. Neuroleptanalgesia is commonly used for procedures that require significant CNS depression and analgesia but not general anesthesia.
Opioids commonly used for neuroleptanalgesia include morphine, buprenorphine, butorphanol, and hydromorphone. Tranquilizers that may be combined with opioids include acepromazine, diazepam, midazolam, xylazine, and dexmedetomidine. Virtually any combination of these drugs may be used according to the veterinarian’s preference.
Animals given neuroleptanalgesics generally lie quietly in lateral or sternal recumbency (adult horses stand quietly) but can be aroused by sufficient noise or surgical stimulation. Neuroleptanalgesics are commonly used to induce sedation in patients undergoing minor procedures including wound treatment, porcupine quill removal, and diagnostic procedures such as endoscopy or radiography. They are also used for induction of general anesthesia in dogs. Although this type of induction is slow and may cause some respiratory depression and bradycardia, it is safe for most patients, especially if the patient is subsequently intubated and ventilation is assisted.
Neuroleptanalgesics are generally not suitable for routine induction of anesthesia in healthy young dogs, because the level of sedation is not sufficient to permit intubation in these patients unless supplemented with an inhalation agent such as isoflurane or sevoflurane given by mask. However, neuroleptanalgesics may have a profound effect in high-risk or debilitated dogs (including those with hepatic, renal, and CNS disorders) and are a useful and safe alternative to propofol, barbiturate, or ketamine induction in these animals. Neuroleptanalgesics are seldom used to induce anesthesia in cats because of unacceptable side effects (excitement, mania).
The two drugs are often mixed in the same syringe (as long as diazepam is not included), although they may be injected separately. They are given intramuscularly or by slow IV injection and may be administered after pretreatment with anticholinergics, although this is not required, provided the heart rate is carefully monitored. If bradycardia is excessive, the anticholinergic can be administered later.
Neuroleptanalgesics provide a wide margin of safety in most patients, but care must be taken to administer them slowly intravenously, because if they are rapidly injected, CNS stimulation may occur. The anesthetist must also be prepared to intubate and ventilate the patient if necessary because respiratory depression may be profound.
Several procedures have been described for induction of anesthesia with neuroleptanalgesics. These include the following:
• Administration of an anticholinergic and tranquilizer intramuscularly 15 minutes before slow IV injection of the opioid.
• Administration of an anticholinergic intramuscularly, followed 15 minutes later by slow IV administration of the tranquilizer-opioid mixture. If diazepam is selected as the tranquilizing agent, the opioid should be given first, followed 1 to 2 minutes later by diazepam, because if diazepam is given before the opioid agent has taken effect, excitement may be seen. In young dogs, acepromazine offers more reliable sedation than diazepam and can be given at the same time as the opioid.
• Alternating small doses of hydromorphone or fentanyl and diazepam by slow IV injection until the animal is adequately anesthetized. Two syringes are used because these drugs cannot be mixed together.
When the procedure is over, the opioid in neuroleptanalgesic combinations can be reversed with naloxone. However, the tranquilizer component may or may not be reversible, depending on whether or not a specific antagonist is available. A detailed discussion of opioid reversal follows.
Opioid Antagonists
One distinct advantage of using opioids is their reversibility, which allows undesirable effects to be antagonized in situations in which the patient is in danger, or allows the anesthetist to “wake” or partially wake the patient after sedation with these agents. The opioid antagonist naloxone hydrochloride is commonly used to reverse the CNS and respiratory depression caused by administration of opioid agonists, partial agonists, or agonist-antagonists. It is administered by IM or slow IV injection and can be used in dogs, cats, horses, and exotic mammals. Although not used in domestic animals, naltrexone is a longer-lasting antagonist that is used in wild animals to reverse the effects of potent opioids such as etorphine.
Agonist-antagonists such as butorphanol can be also be used to partially reverse the effects of pure agonists. Realize that opioid antagonists are effective in reversing opioid agents only and cannot be used to reverse the effects of phenothiazines, benzodiazepines, or other nonopioid agents.
Mode of Action and Pharmacology
Although, as with many other anesthetics and adjuncts, the exact mechanism of action of naloxone is not known, it is believed to competitively bind to the mu, kappa, and sigma receptors. Naloxone acts within 2 minutes of IV administration and 5 minutes of IM administration and has a duration of action of 30 to 60 minutes, and in some cases may last longer.
Effects and Adverse Effects
The primary effect of naloxone is reversal of both the desirable and undesirable effects of an agonist, partial agonist, or agonist-antagonist, including sedation, dysphoria, panting, respiratory depression, hypotension, bradycardia, GI effects, and analgesia. The action of opioid antagonists is often dramatic, causing the patient to appear nearly unaffected shortly after administration. The respiratory depression caused by buprenorphine may be unresponsive, however, because this agent tightly binds to the mu receptors and is not easily displaced.
Adverse effects are rare at clinical doses, although sudden loss of analgesic effects resulting from routine use may precipitate excitement, anxiety, and SNS stimulation, resulting in tachycardia and cardiac arrhythmias. Total reversal of analgesia can be avoided by using an agonist-antagonist such as butorphanol that has some analgesic effect.
Use of Opioid Antagonists
Reversal of opioid effects through the use of an antagonist is not necessary for routine anesthesia. However, the technique is extremely useful in emergencies, after an overdose, or to reverse opioid effects after neuroleptanalgesia for nonpainful procedures. Opioid antagonists are also helpful in reviving neonates delivered by a cesarean section if the dam was given opioids. One drop of naloxone placed under the tongue of each puppy or kitten is usually sufficient to reverse the respiratory depression caused by fentanyl, morphine, or other opioid agonists.
In cases where the duration of action of the drug being reversed is longer than the duration of naloxone, signs of CNS or respiratory depression recur (a phenomenon referred to a renarcotization). In these cases, additional doses may be needed.
INJECTABLE ANESTHETICS
Injectable anesthetics are drugs characterized by their ability to produce unconsciousness when given alone. They do not provide all the effects of general anesthesia (such as analgesia and muscle relaxation), however. Consequently these drugs must be used with other agents to produce the complete spectrum of effects of general anesthesia.
Injectable anesthetic agents include propofol, barbiturates, and etomidate, each of which is used to induce anesthesia. Through use of repeat boluses, or a CRI, propofol is also used to maintain general anesthesia. Barbiturates are used in both small and large animals. Propofol and etomidate are used only in small animals, small ruminants, and neonates of any species, but not in adult large animals because of the high expense of these agents.
Although they do not produce unconsciousness when given alone, dissociative agents are included in this discussion of injectable anesthetics because they are frequently used in combination with tranquilizers and opioids to produce general anesthesia.
With few exceptions, these agents are administered by IV injection to effect, instead of giving the entire calculated dose at once. “To effect” means that the drug is given in small boluses until the desired level of anesthesia is reached. More about this technique can be found in Chapter 8.
Barbiturates
The barbiturates are a large class of controlled drugs, developed during the 1930s through the 1950s, that were commonly used general anesthetics for many decades because of their low cost, ease of use, and relative safety for healthy animals. Although these drugs are still used for certain indications, in recent years their use has decreased with the development of newer injectable agents such as propofol, and inhalant agents such as isoflurane and sevoflurane. Despite this decrease in popularity, there is a large body of knowledge about these agents based on the experience of more than seven decades of widespread use. Therefore the anesthetist must have a general knowledge of barbiturates not only to use them safely, but also as a point of reference against which other injectable anesthetics can be compared. In fact, some references still classify all injectable anesthetics as either barbiturates or nonbarbiturates.
Classes of Barbiturates
All barbiturates in clinical use are derivatives of barbituric acid. They are placed into subclasses based on their duration of action (ultra–short-acting, short-acting, intermediate-acting, and long-acting). Two subclasses of barbiturates are used to induce general anesthesia in veterinary patients. The ultra–short-acting barbiturates thiopental sodium and methohexital are used to induce anesthesia primarily in dogs, cats, and horses, and the short-acting barbiturate pentobarbital is used to induce and maintain general anesthesia in laboratory animals. Most intermediate- and long-acting barbiturates are no longer used as anesthetics, although the long-acting barbiturate phenobarbital is commonly used as a sedative and anticonvulsant.
Barbiturates are also classified based on their chemical structure as oxybarbiturates (which include phenobarbital, pentobarbital, and methohexital) and thiobarbiturates (which include the ultra–short-acting agents thiopental and thiamylal).
Action and Pharmacodynamics of Barbiturates
Although the precise mechanism of action of these drugs is not fully known, all barbiturates act on the neurons in a similar manner to the inhibitory neurotransmitter GABA. This action depresses nerve impulses in the cerebral cortex, resulting in CNS depression and a loss of consciousness. This effect is terminated when the agent leaves the brain and is either metabolized, excreted, or redistributed elsewhere in the body. To understand the differences in the potency and onset and duration of action of specific barbiturates, it is necessary to have some knowledge of the pharmacodynamics of these drugs.
Ionization: All barbiturates exist in both ionized (polar) and nonionized (nonpolar) forms. Only the nonionized molecules can pass through cell membranes. When the blood pH is normal (7.4), barbiturates penetrate to the expected extent and consequently have the expected effects. When the blood pH is lower (a condition called acidosis), more of the drug is nonionized, causing increased diffusion into the brain. A normal dose of barbiturates may produce an exaggerated response in these animals. Therefore the dose of barbiturates required to anesthetize acidotic animals may be significantly less than that required to anesthetize healthy animals.
Protein Binding: Barbiturates travel in the blood bound to plasma proteins and freely circulating. Only the free (unbound) molecules of barbiturate are able to enter the brain to induce anesthesia because the molecules bound to proteins are unable to cross cell membranes. In hypoproteinemic animals (that is, those with total plasma protein less than 3 g/dL) there is less plasma protein to bind barbiturate molecules and more barbiturate in the active, unbound form. The potency of barbiturates within the body is therefore increased in animals with low plasma protein levels. Doses of barbiturates suitable for healthy animals may cause prolonged unconsciousness or death in these patients.
Lipid Solubility: The tendency of a drug to dissolve in fats, oils, or lipids is referred to as lipid solubility (also called partition coefficient) and is related to the ability to penetrate the fatty layer of the cell membranes. Highly lipid-soluble drugs (ultra–short-acting agents) pass into the brain cells more quickly, causing a faster onset of action as compared with drugs with low lipid solubility.
Lipid solubility is also related to duration of action. Drugs with high lipid solubility are rapidly removed from the brain by a process known as tissue redistribution. Drugs with moderate lipid solubility (the short-acting agents) are largely metabolized by the liver, a process that takes longer than redistribution; and drugs with low lipid solubility (the long-acting agents) are primarily excreted by the kidneys, a process that takes even longer.
Redistribution: As mentioned, highly lipid-soluble ultra–short-acting agents such as thiopental sodium and methohexital are removed from the brain by a process known as redistribution. This process occurs because of the way in which these drugs are distributed to various tissues based on blood flow. After IV administration, absorption is most rapid in tissues with very high blood flow, known as the vessel-rich group (the CNS, heart, liver, kidney, and endocrine tissues), which make up only about 10% of total body weight but receive about 75% of the total blood flow. Absorption is less rapid in muscle, which makes up about 50% of the body weight but receives only 20% of the blood flow, and slowest in fat, which makes up about 20% of body weight but receives a meager 5% of the blood flow (Figure 3-3).
FIGURE 3-3 A comparison of blood flow and tissue mass. Note that some tissues make up a small proportion of total body weight but receive a relatively large proportion of total blood flow (e.g., approximately 10% of total body weight and approximately 75% of total blood flow in the case of the vessel-rich group), whereas other tissues make up a relatively large proportion of total body weight but receive much less blood flow (e.g., approximately 20% of total body weight and approximately 5% of total blood flow in the case of fat).
The process of redistribution can be illustrated by using the example of administration of the drug thiopental sodium, the most commonly used drug in this class. Within seconds of IV injection, thiopental is dispersed throughout the body via the bloodstream. Large amounts of the drug rapidly reach the brain because of the excellent blood supply this organ receives. Thiopental is highly lipid-soluble, and its entry into brain tissue is enhanced by the high lipid content of the brain. The rapid absorption of thiopental into the brain causes the animal to lose consciousness within 30 seconds of injection.
Once the thiopental concentration in the blood falls below that in the brain tissue, the drug begins to leave the brain and reenter the circulation, where it is redistributed to muscle, fat, and other body tissues. Thiopental leaves the brain because it, like all drugs, diffuses from areas of high concentration to areas of low concentration. The animal shows signs of recovery (often within 10 to 15 minutes) as the concentration in the brain decreases, although the drug is still present in other tissues.
Over the next several hours, thiopental is gradually released from the muscle and fat and is eliminated from the body by liver metabolism and excretion of the metabolites in urine.
Because thiopental persists in muscle and fat for up to several hours and then is slowly metabolized over time, repeat administration can saturate the tissues. When this happens, drug levels may persist in the brain because the muscle and fat are “full” and unable to accommodate additional drug. This effect causes prolonged recovery. Therefore it is inadvisable to administer these drugs on a continuous or repeated basis.
The other commonly used barbiturates have different pharmacodynamics. Like thiopental, the ultra–short-acting agent methohexital has a short duration because of redistribution, but this drug is metabolized much more rapidly than thiopental. Consequently, repeat doses of methohexital can be given with minimal concern about prolonged recovery.
The long-acting barbiturate phenobarbital has low lipid solubility and is consequently slow to act, but effects are sustained because a decrease in brain levels depends primarily on excretion by the kidneys, a relatively lengthy process.
The short-acting barbiturate pentobarbital has intermediate lipid solubility when compared with thiopental and phenobarbital. It takes several minutes to reach its full effect and has a duration of action that is shorter than that of phenobarbital but longer than that of thiopental, because brain levels decrease as the drug is metabolized by the liver—which occurs faster than kidney excretion but more slowly than redistribution.
Use of Barbiturates
The convenience, relative safety, low cost, and rapid induction associated with the ultra–short-acting barbiturates led to their once common use in small and large animal anesthesia. Although their popularity has waned, thiopental and methohexital are both still used as induction agents to allow endotracheal intubation. After intubation, anesthesia is most often maintained with an inhalation anesthetic such as isoflurane or sevoflurane. Thiopental may also be used as the sole anesthetic agent for brief procedures, and methohexital can be used to maintain anesthesia for longer procedures with repeat boluses or a CRI. Regardless of the specific drug used, endotracheal intubation is strongly advised for all patients anesthetized with barbiturates, even for brief periods, to prevent aspiration of fluid or vomitus and to allow the anesthetist to support ventilation if necessary.
Effects on Major Organ Systems
• Barbiturates cause cardiac depression, particularly shortly after IV injection. It has been shown in the dog that barbiturates cause a dose-dependent decrease in cardiac output and blood pressure that may be profound in patients that are already anesthetized.
• In addition, thiopental stimulates the PNS and the SNS, causing autonomic imbalances, and also increases the heart’s sensitivity to the action of epinephrine, particularly in patients that are excited or stressed. This may lead to cardiac arrhythmias including ventricular premature complexes (VPC), tachycardia or bradycardia, and AV heart block.
• Barbiturates commonly depress the respiratory system, reducing respiratory rate and tidal volume and in some cases causing respiratory arrest. After IV administration of thiopental for anesthetic induction, a brief period of apnea is common, especially after rapid injection or administration of high doses. This occurs because of direct depression of the respiratory center in the medulla, the area of the brain that controls respiration. In the conscious animal the respiratory center responds to rising levels of carbon dioxide in arterial blood (Paco2) by sending impulses to the muscles that initiate inspiration. Barbiturates cause the respiratory center to become relatively insensitive to increased Paco2. Breathing resumes when the concentration of the drug in the plasma and brain decreases or when decreased blood oxygen stimulates breathing. Most healthy patients are not adversely affected by a brief period of apnea, but the other vital signs should be closely monitored to ensure that perfusion and oxygenation are maintained. Mucous membranes should remain pink, the heartbeat should be regular, the pulse should be strong, and oxygen saturation as measured by a pulse oximeter should be normal.
• During anesthesia induced with pentobarbital, the most common effect on respiration is a persistent reduction in tidal volume (that is, shallow breaths). The effect may be short-lived, or the patient’s respirations may appear shallow throughout the anesthetic period. Patients with reduced tidal volume are predisposed to respiratory acidosis and poor tissue oxygenation, particularly if a barbiturate is used as the sole anesthetic agent and the patient does not receive oxygen supplementation.
Other Effects
• Barbiturate induction is often accompanied by sneezing, laryngospasm, and coughing owing to increased salivation. Premedication with an anticholinergic minimizes these effects. Barbiturates also reduce GI motility at first, and then can increase it. Muscle relaxation with these drugs is not complete.
Adverse Effects
• During the induction period, it is not uncommon for cardiac arrhythmias such as VPC to occur because of the combined effect of barbiturates, epinephrine, and hypoxia. A condition termed bigeminy in which each normal beat is followed by a single VPC is particularly common (Figure 3-4). These cardiac arrhythmias are not clinically significant in most patients; however, cardiac arrest has been reported, especially in animals that are stressed during induction. For these reasons, barbiturates should be used with caution in animals with known cardiac disease because a dose of barbiturate that is well tolerated by a healthy heart may severely stress a diseased heart.
FIGURE 3-4 Ventricular bigeminy (cat). Note that normal complexes (the odd numbered complexes) alternate with ventricular premature complexes, which are early, are wider, and have a different shape than the normal complexes. 25 mm/sec; 1 cm/mV. (From Bonagura JD, Twedt DC: Kirk’s current veterinary therapy XIV, St Louis, 2009, Elsevier.)
• Adverse effects on the heart can be minimized by ensuring that barbiturates are given slowly and that dilute concentrations are used (e.g., 2% or 2.5%). Preoxygenating a patient for 3 to 5 minutes before inducing anesthesia with a barbiturate also helps to reduce the adverse cardiac effects of these drugs. If cardiac arrhythmias occur during induction, bagging the patient two or three times is often helpful to ensure adequate oxygenation.
• Respiratory depression is related to dose and speed of administration and in some cases may be profound. If apnea persists for more than 1 to 2 minutes after administration, patients must be immediately intubated and bagged every 15 seconds to 1 minute until spontaneous breathing resumes.
• Respiratory depression of neonates is also a potential problem when barbiturates are used in cesarean deliveries. Barbiturates, like most anesthetic agents, readily cross the placenta and enter the fetal circulation and may interfere with the neonates’ ability to breathe immediately after delivery. In fact, the newborn animal is more susceptible than the mother to the effect of barbiturates, and respiration may be completely inhibited by doses of barbiturates that are inadequate to anesthetize the dam.
• In addition to the cardiovascular and respiratory problems previously outlined, barbiturates may exhibit exaggerated and potentially dangerous potency in some animals. Classes of patients that are particularly sensitive to barbiturates include sighthounds, critically ill animals, and, as previously mentioned, hypoproteinemic and acidotic animals.
• Effect on sighthounds. Because of the redistribution of thiopental, prolonged recovery is common in very thin animals, including sighthounds (e.g., afghan hounds, whippets, salukis, borzoi, and greyhounds), especially if more than one dose is given. This is because the low fat stores in these patients quickly become saturated, preventing redistribution of the drug. Therefore drug levels in the brain remain high. Hepatic metabolism of barbiturates may also be slow in sighthounds, further delaying clearance from the body. For these reasons, thiopental is not recommended for use in sighthounds, although owing to rapid metabolism, methohexital is considered relatively safe in these breeds.
• Effect on critically ill animals. Animals with hepatic or renal disease and animals with hypothermia may exhibit prolonged recovery from thiopental and other barbiturates because metabolism and excretion are delayed. Barbiturates also show greater potency in animals that have hypotension or shock because blood flow to nonessential tissues (such as fat) is decreased, and blood flow to the brain and heart is increased. Thus the normal redistribution of these agents from the brain to body fat stores does not occur.
• Tissue irritation and barbiturate sloughs. Use of concentrations greater than 2.5% is toxic to capillary muscles and can cause thrombophlebitis. Barbiturate solutions are strongly alkaline (pH 10 to 11) and may cause significant tissue damage if injected perivascularly, particularly if the concentration of barbiturate exceeds 2.5%. Perivascular injection of concentrated barbiturate solutions may be followed within 48 hours by local swelling, pain, necrosis, and even tissue sloughing. The animal may chew at the area, thereby increasing tissue damage. Tissue sloughs are slow to heal and usually result in permanent scarring.
• A dilute solution of barbiturate should be used to avoid tissue irritation (in the case of thiopental 2% to 2.5%, although up to 10% is used in horses to decrease the volume that needs to be injected for induction of anesthesia). As an additional precaution, barbiturates are often administered through an IV catheter or winged infusion set. If perivascular injection occurs, the area should be immediately infiltrated with saline (in a volume at least equal to the volume of barbiturate injected) to dilute the barbiturate and reduce its irritating effect; 1 to 2 mL of 2% lidocaine without epinephrine may be added to the saline. The lidocaine causes vasodilatation, absorption of the barbiturate, and neutralization of the drug.
• Although uncommon, inadvertent intraarterial injections of thiopental must also be avoided, as they may cause vasoconstriction, pain, and tissue necrosis.
• Excitement during induction or recovery. Perivascular injection or excessively slow administration of some barbiturates may result in stage II excitement during induction. This occurs because the amount of barbiturate reaching the brain is insufficient to induce stage III anesthesia. In this case additional drug must be administered immediately until the patient reaches surgical anesthesia. The use of barbiturates, particularly pentobarbital, is also associated with a high incidence of excitement during the recovery period. Paddling and vocalization are commonly seen but may be relieved by administration of IV diazepam. Use of preanesthetic medications is helpful in reducing the incidence and severity of excitement in the recovery period.
• Interaction with other drugs. Barbiturates enhance the neuromuscular blocking effect of muscle relaxants (see Chapter 6). Chronic administration of barbiturates (e.g., phenobarbital given daily to an epileptic dog or cat) increases the activity of some hepatic enzymes. This may lead to more rapid clearance and therefore shorter duration of effect of drugs that are metabolized in the liver such as diazepam and opioids. Concurrent administration of the antibiotic chloramphenicol may increase the effects of pentobarbital and phenobarbital.
Commonly Used Barbiturate Drugs
Thiopental: Thiopental is an ultra–short-acting thiobarbiturate used for anesthetic induction of small animals and horses and can also be used as the sole anesthetic for brief procedures. Onset of action after injection is rapid (30 to 60 seconds), and duration of anesthesia is brief (10 to 15 minutes). Complete recovery usually occurs within 1 to 2 hours.
Thiopental is supplied as crystalline powder in multidose vials. Sterile water, normal saline, or 5% dextrose in water is added to the vial to make a 2% (20 mg/mL) or 2.5% (25 mg/mL) solution for small animals and a 5% (50 mg/mL) solution for large animals. All vials should be labeled with the percent concentration and date of reconstitution, because thiopental is unstable in solution and once reconstituted has a maximum shelf life of 1 week if refrigerated and 3 days at room temperature. Care should be taken to avoid injecting air into prepared solutions, because this may cause premature precipitation of the barbiturate. The solution should not be used if a precipitate is present.
The dose of thiopental used for induction varies depending on concurrent use of other agents and the depth of anesthesia required. Doses are commonly reduced by up to 80% in debilitated animals or in animals that have been heavily sedated.
Whether given alone or in combination with other drugs, thiopental is always given “to effect.” Repeated administration is cumulative, and recovery can be greatly prolonged. For this reason it is not advisable to use this agent for anesthetic maintenance.
One method for IV induction with thiopental is described in Procedure 8-3. Another strategy is to give the antiarrhythmic drug lidocaine (2 mg/kg) intravenously, immediately followed by thiopental to reduce the incidence of arrhythmias. This technique is not suitable in cats because of their sensitivity to lidocaine. Thiopental can also be given at a dose of 8 mg/kg, after an IV injection of 0.2 mg/kg diazepam or midazolam (using separate syringes). Finally, thiopental can be mixed with propofol and given in the same syringe.
Methohexital: Methohexital is an ultra–short-acting methylated oxybarbiturate similar to thiopental in that the onset of action is rapid (15 to 60 seconds) and the duration of anesthesia is brief (5 to 10 minutes). In fact, animals induced with methohexital and subsequently connected to an anesthetic machine may show signs of recovery before the inhalation anesthetic has time to take effect. The rapid induction achieved with this agent is useful when anesthetizing a patient with a full stomach, because the anesthetist can intubate rapidly, decreasing the risk of aspiration of vomitus.
As with thiopental, methohexital is provided as a powder that must be reconstituted. A 1%-2.5% solution is commonly used in small animals. The reconstituted drug has a shelf life of at least 6 weeks when reconstituted with sterile water for injection, and does not require refrigeration. Methohexital is significantly more expensive than thiopental. The cost of thiopental required to anesthetize a 10-kg dog at the time of this writing is approximately $1 to $2, whereas the cost of methohexital is approximately $10 to $12.
Methohexital is administered in a manner similar to that of thiobarbiturates. In the premedicated patient, one third to one half of the calculated dose is given intravenously over 10 seconds. One injection is usually sufficient to allow intubation, but additional drug should be given in 30 seconds if an adequate plane of anesthesia is not reached. Further delay will result in a poor induction because of the rapid redistribution of the drug.
Methohexital may be used in sighthounds, because unlike thiopental it does not produce prolonged recoveries. Liver metabolism of methohexital is much faster than that of other barbiturates; therefore repeated administration is not cumulative.
Although methohexital is a useful drug for veterinary anesthesia, it should be used with caution. Methohexital can cause profound respiratory depression, and the lethal dose is only two to three times the anesthetic dose. Methohexital is also associated with excitement and seizures during induction (if given too slowly) or during recovery. Premedication with a tranquilizer helps to prevent seizures and is always recommended. Postoperative seizures may be controlled with IV diazepam. Animals with preexisting CNS disease, including epilepsy, should not receive methohexital.
Pentobarbital: Pentobarbital is a short-acting barbiturate used in the treatment of status epilepticus. In recent years, propofol has become more widely used for this purpose owing to fewer adverse effects and smoother recoveries. Pentobarbital is also administered by intraperitoneal injection to produce general anesthesia in rodents (see Chapter 11); however, its potency and duration of effect are variable when given by this route. Before halogenated inhalant anesthetics were available, this drug was commonly used as the sole anesthetic agent for procedures such as ovariohysterectomy in the dog. Because of significant respiratory depression, difficulty controlling anesthetic depth, rough inductions and recoveries, and other adverse effects, the use of this agent for major surgical procedures is now rare in veterinary practice.
When treating status epilepticus, pentobarbital should be given at a rate of 3 to 15 mg/kg intravenously to effect, until the seizures stop and heavy sedation is achieved. It should be given very slowly in small doses, waiting several minutes before giving additional boluses. This agent must be used with caution because it has a narrow margin of safety; the euthanasia dose in healthy animals is only 60 mg/kg, which is approximately double the dose used for surgical anesthesia.
The onset of action is 30 to 60 seconds after IV injection, although the full effect may not be seen for several minutes. Animals anesthetized with pentobarbital will initially appear unable to raise the head. As more pentobarbital is given, the jaw and tongue become completely relaxed, but a pedal reflex is still present, indicating a light plane of anesthesia. As the animal achieves a moderate depth of anesthesia, the pedal reflex becomes sluggish. If the pedal reflex is lost, the animal likely requires intubation and respiratory support. Additional drug can be given to prolong anesthesia; however, repeated doses may be associated with excitement during the recovery period and with an extended recovery time. The anesthetic effect lasts 30 minutes to 2 hours, depending on dose and species. Pentobarbital is supplied as a 5% solution (50 mg/mL).
Propofol
Propofol is an ultra–short-acting nonbarbiturate injectable anesthetic with a wide margin of safety and is given intravenously for induction and short-term maintenance of general anesthesia in small animals, small ruminants, exotic animals, and neonates of any domestic species. Since its introduction to the veterinary market over a decade ago, its popularity has gradually increased, and now it is the most commonly used ultra–short-acting injectable agent in small animals for brief procedures or for anesthetic induction before intubation and maintenance with inhalant agents.
Propofol is also administered as an IV bolus followed by a CRI to treat status epilepticus in dogs and cats that do not respond to diazepam and phenobarbital.
Mode of Action and Pharmacology
Propofol has a chemical structure unlike that of other anesthetic or preanesthetic agents. It is minimally water-soluble and is available in an egg lecithin, glycerin, and soybean oil aqueous emulsion at a concentration of 10 mg/mL. Although this agent has a milky appearance, it is the sole exception to the general rule that milky liquids should never be given intravenously.
Although the mode of action is not completely understood, propofol appears to affect GABA receptors in a similar manner to barbiturates. Propofol has a rapid onset and short duration of action because it is highly fat-soluble. Propofol is rapidly taken up by vessel-rich tissues such as the brain, heart, liver, and kidneys but is very quickly redistributed to muscle and fat (see p. 71 for a discussion of redistribution) and is subsequently rapidly metabolized. This accounts for the rapid recovery and minimal residual sedative effects seen even after repeated injections.
The onset of action is about 30 to 60 seconds, and the duration of action is 5 to 10 minutes, with complete recovery in 20 minutes (dogs) and 30 minutes (cats).
Effects on Major Organ Systems
• Propofol is a potent respiratory depressant. High doses or rapid injection may cause significant respiratory depression, including apnea. To lessen the respiratory depression associated with the use of propofol, the anesthetist should give the initial bolus gradually over 1 to 2 minutes, carefully titrate the dose to effect, and monitor respiratory rate and depth carefully during the first few minutes after injection.
Other Effects
• Some dogs may exhibit muscle twitching during induction. This reaction should not be interpreted as an indicator of inadequate anesthetic depth.
• Propofol results in good muscle relaxation.
• Because metabolism of propofol is rapid, propofol is relatively safe and effective in animals with liver or kidney disease.
• Propofol is an appetite stimulant at low doses.
Adverse Effects
• Transient excitement and muscle tremors are seen occasionally during induction, especially if injection is slow or the animal has not been premedicated. Paddling, muscle twitching, nystagmus, and opisthotonus (hyperextended head and front legs) can occur and may resemble seizures. This response can be treated with diazepam if persistent.
• Hypotension is often of short duration in animals with normal cardiovascular function, but in some patients it may be significant and prolonged. For this reason, propofol should be given cautiously to animals with preexisting hypotension, such as patients in shock or those that have blood loss or dehydration.
• Apnea is sometime seen, particularly after rapid injection or high doses. If apnea lasts more than 1 minute or if pulse oximetry shows oxygen saturation to be less than 95%, the patient should be intubated and ventilated. The best approach is prevention of hypoxemia by delivering oxygen to the patient (by face mask) for 3 to 5 minutes before inducing anesthesia with propofol.
• Based on experience in people, propofol is known to cause pain on IV injection but does not produce the tissue damage after perivascular injection seen with barbiturates.
• When given repeat doses, cats can develop Heinz body production, diarrhea, and anorexia and can experience slow recoveries.
• Animals of some breeds including sighthounds may have prolonged recovery if maintained with propofol for longer than 30 minutes.
Use of Propofol
Propofol should be given intravenously slowly, over a period of 1 to 2 minutes until the desired anesthetic depth is reached (see Case Studies 3-1 and 3-2). IM injection may cause mild sedation and ataxia but does not induce anesthesia because the drug is metabolized too rapidly.
One effective induction method is to give one quarter of the calculated dose slowly intravenously every 30 seconds until the desired plane of anesthesia is reached. The dose of propofol needed for a patient and the duration of anesthesia depend on the type of premedication used. Do not give propofol too slowly, however, as this can cause paradoxic excitement, making the patient difficult to handle. Propofol is highly protein bound and therefore should be used with caution in patients with significant hypoproteinemia.
Propofol boluses can be given repeatedly every 3 to 5 minutes or as required to maintain anesthesia in dogs and cats up to 20 minutes. Alternatively, propofol can be delivered by CRI. In this procedure, a low dose of propofol (0.2 to 0.4 mg/kg/min) is continuously administered to the patient with a syringe pump (Figure 3-5) or through an IV line. This method allows the anesthetist to precisely control the depth of anesthesia at a stable plane for up to several hours. Intubation and oxygen administration are advisable for patients unless the period of anesthesia is anticipated to be very brief.
FIGURE 3-5 This syringe pump (also known as a syringe driver) is being used to administer a CRI of propofol from a 5-mL syringe. The syringe is attached to microbore tubing that has been preloaded with propofol.
In dogs, recovery from propofol anesthesia is rapid and smooth, even after multiple injections. Because of the rapid recovery seen with this agent, it is useful for patients that need to be released immediately after surgery. Dogs that have received propofol may appear completely recovered within 20 minutes of the final dose. Cats recover within about 30 minutes after a single injection but may experience longer recoveries after multiple injections owing to the fact that they metabolize propofol more slowly.
Administration of tranquilizers decreases the dose of propofol required by as much as 75% and facilitates IV injection in fractious animals. Some premedications, however, may prolong recovery time.
Handling and Storage
One significant disadvantage of propofol is the poor storage characteristics of this agent. Because the product contains soybean oil, egg lecithin, and glycerol, it will support bacterial growth. Ampules and bottles should be handled in a strictly aseptic manner, and the manufacturer
recommends that unused product be discarded within 6 hours of opening to avoid contamination. (Unopened, the shelf life is approximately 3 years.) Some authorities suggest that unused propofol can be stored up to 24 hours, provided that sterile technique is used for opening, dispensing, and storing the product. Refrigeration may be preferable to storage at room temperature. Unfortunately, detailed studies on the storage characteristics of propofol have not been published, but the incidence of infection from contaminated drug appears to be low.
The cost of propofol induction in dogs is approximately four times that of ketamine-diazepam and two times that of thiopental.
Dissociative Anesthetics
In the late 1950s the introduction of the injectable anesthetic phencyclidine made available a new class of injectable anesthetic drugs called the dissociative anesthetics. Although phencyclidine is not used in veterinary medicine because of its abuse potential, its derivative, ketamine hydrochloride, is used alone to induce dissociative anesthesia in cats for minor procedures. It is also commonly used in combination with a variety of tranquilizers and opioids to induce general anesthesia in a wide variety of species, and it is given in subanesthetic doses by CRI to provide analgesia. Ketamine may also be given orally in feral cats to facilitate restraint.
Tiletamine hydrochloride, another dissociative agent, is combined with the benzodiazepine zolazepam in a proprietary product called Telazol. This product is administered intravenously or intramuscularly, alone or in combination with other agents, to produce sedation and anesthesia. Both ketamine and Telazol are controlled.
Mode of Action and Pharmacology
The mechanism of action of the dissociative anesthetics is complex. Unlike most general anesthetics, which cause general CNS depression, dissociative anesthetics cause disruption of nerve transmission in some parts of the brain and selective stimulation in others. Dissociative agents also inhibit NMDA (N-methyl-d-aspartate) receptors in the CNS that are responsible for “windup.” Windup is an exaggerated response to low-intensity pain stimuli that results in worsening of postoperative pain. This NMDA inhibition is believed to be responsible for the analgesic and many of the other primary effects of these drugs (see Chapter 7).
The unusual combination of actions result in a distinctive trancelike state termed dissociative anesthesia (so called because it dissociates various regions of the brain), in which the animal appears awake but immobile and unaware of its surroundings (Figure 3-6). Dissociative anesthesia is described in the section on effects on major organ systems.

FIGURE 3-6 A cat in dissociative anesthesia. Note the central, dilated pupils, open eyes, and muscle rigidity. The patient appears awake but unaware of its surroundings.
Peak action of ketamine is about 1 to 2 minutes after IV injection, and about 10 minutes after IM injection. The duration of effect is about 20 to 30 minutes. A higher dose increases duration but does not increase anesthetic effect. Dissociatives are redistributed and metabolized by the liver; they may also be excreted unchanged in the urine and so should be used cautiously in patients with liver or kidney disease. As with barbiturates, redistribution is largely responsible for the initial decrease in effects.
Effects on Major Organ Systems
• The primary effect on the CNS is dissociative anesthesia. Dissociatives do not induce stage III anesthesia. Characteristics of dissociative anesthesia include the following:
• Cataleptoid state. Catalepsy is a state in which a patient does not respond to external stimuli and has muscle rigidity in which the limbs will remain in the position in which they are placed. In people this state may be drug induced or may occur with conditions including schizophrenia and epilepsy. A cataleptoid state (the suffix oid meaning “like”) is similar to catalepsy but with a variable degree of muscle rigidity.
• Intact reflexes: Palpebral, corneal, and pedal reflexes, pupillary light reflex (PLR), and laryngeal and swallowing reflexes remain intact. Because reflex activity is preserved even at moderate anesthetic depth, it may be difficult to determine anesthetic depth in patients under dissociative anesthesia. Anesthetic depth in patients that show signs of purposeful movement is likely inadequate, whereas in those with very depressed respiration it is likely excessive. When using these agents, the anesthetist may find it challenging to determine where an individual patient lies between these two extremes.
• Ocular effects: Unlike conventional anesthesia, dissociative anesthesia does not usually result in partial closure of the eyelids or eyeball rotation. The eye normally remains fully open, with a central and dilated pupil. Ophthalmic lubricant should be applied hourly to prevent corneal drying.
• Normal or increased muscle tone. In some animals muscle tone is retained, whereas in others it is increased, almost to the point of rigidity. The animal may assume a stiff posture, with outstretched front limbs and extended neck. During recovery or light anesthesia, spontaneous, random movements of the head and neck may be seen. This is in marked contrast to the muscle relaxation that is seen with most other anesthetics. The concurrent use of a tranquilizing agent such as diazepam, acepromazine, dexmedetomidine, or xylazine helps prevent excessive muscle rigidity, improves ease of intubation, and produces a state more characteristic of general anesthesia.
• Analgesia. Because of inhibition of the NMDA receptor, dissociative agents provide significant analgesia to the skin and limbs (somatic analgesia) but limited visceral analgesia (that is, involving the organs). A patient given a dissociative anesthetic may be able to perceive pain but is unable to respond to it. It is the veterinarian’s obligation to ensure that pain is not perceived, through the use of supplementary anesthetic agents and/or analgesics.
• Amnesia. Human patients anesthetized with dissociative agents do not recall the procedure afterward, even though they do not appear unconscious at the time.
• Sensitivity to sensory stimuli. Animals anesthetized with these agents may show marked sensitivity to sound, light, and other sensory stimuli. These agents are used in combination with other drugs, particularly diazepam, to avoid excitement and improve muscle relaxation.
• Unlike most other anesthetics, dissociative anesthetics do not decrease heart rate or decrease cardiac output in patients with normal heart function. In fact, most animals exhibit increased heart rate, cardiac output, and mean arterial blood pressure owing to stimulation of the SNS. These cardiovascular effects are not usually harmful to the animal but can be if the patient has preexisting heart disease (e.g., cats with hyperthyroidism or cardiomyopathy), because cardiac work and oxygen consumption increase.
• Dissociative anesthetics do not affect the respiratory system in the same way as most other anesthetics. Respiratory rate and tidal volume may change, but respiratory depression is usually insignificant at usual doses. At higher doses, animals exhibit apneustic respiration, a breathing pattern in which there is a pause for several seconds at the end of the inspiratory phase, followed by a short, quick expiratory phase. These animals appear to hold their breath.
Adverse Effects
• Animals recovering from dissociative anesthesia often show an exaggerated response to touch, light, or sound, and seizure-like activity may be observed. Reduction of light, sound, and other stimuli or administration of benzodiazepines may reduce this activity. Because CNS stimulation is seen with dissociatives, these drugs are contraindicated in animals with a history of epilepsy or other seizure disorders. Dissociative anesthetics should be avoided in animals that have ingested strychnine, street drugs, organophosphates, and other toxins that affect the CNS. Dissociative anesthetics should also be used with caution in animals undergoing procedures involving the neurologic system, including cerebrospinal fluid (CSF) taps and myelograms, because such animals are at increased risk for postoperative seizures immediately after these procedures.
• Some animals recovering from dissociative anesthesia may attempt to paw their faces or demonstrate other bizarre behavior, possibly resulting from hallucinations. Recovering patients should be closely monitored in the hospital to prevent self-injury. Personality changes that can persist for several days have been reported in animals after recovery from ketamine anesthesia. Fortunately, these usually resolve spontaneously after a few days or weeks.
• Dissociative anesthetics may induce nystagmus, a repetitive side-to-side motion of the eyeball. Ketamine-induced nystagmus is more commonly seen in cats than in dogs. This condition is harmless and resolves on recovery from anesthesia.
• Dissociative agents are felt to decrease inotropy—an effect that is normally counteracted by SNS stimulation. This decrease in the strength of heart muscle contraction can become problematic in patients with preexisting heart disease, leading to sudden development of congestive heart failure, even if the heart disease is not evident. For this reason it is important to screen patients for preexisting heart disease (such as hypertrophic cardiomyopathy in cats caused by hyperthyroidism), as these patients are at increased risk for serious complications if given dissociative agents.
• Dissociatives also slightly increase the risk that cardiac arrhythmias will develop in response to epinephrine release.
• Although this is not usually a problem at conventional doses, overdoses may cause severe respiratory depression or respiratory arrest.
• Dissociatives also significantly increase salivation and respiratory tract secretions. Care must be taken to ensure that the airway is protected to prevent aspiration. Anticholinergics can be used to control these signs but may further predispose the patient to cardiac arrhythmias.
Other Adverse Effects: Dissociative anesthetics have many other effects, including the following:
• Tissue irritation. Ketamine and tiletamine are irritating to tissues, and many animals show transitory pain after IM injection. However, these agents do not cause tissue necrosis.
• Increased intracranial and intraocular pressure. Dissociative anesthetics are contraindicated in patients with cranial trauma, conditions that cause elevated CSF pressure, and some ocular surgeries.
Use of Dissociative Anesthetics
Unlike barbiturates, propofol, and etomidate, dissociatives may be given by either the IM or IV route. The versatility and wide margin of safety of dissociatives has led to their widespread acceptance in veterinary anesthesia, particularly for use in cats and horses. When given in combination with a tranquilizer (e.g., diazepam, midazolam, xylazine, dexmedetomidine, acepromazine, or zolazepam), they are useful for brief procedures, such as castrations, or as a means of induction before intubation and inhalation anesthesia. They are also useful for chemical restraint of intractable cats, allowing examinations and minor treatments without endangering hospital personnel. In dogs, dissociative-tranquilizer combinations (ketamine-diazepam or tiletamine-zolazepam) are also commonly used as induction agents. These agents are also used in combination with tranquilizers in an extremely wide variety of large animal and exotic species for sedation, immobilization, and anesthesia. In addition to the uses listed above, ketamine is an NMDA antagonist–class analgesic used in combination with other analgesics for pain control.
One limitation of dissociatives is the lack of an effective reversal agent.
Ketamine: Ketamine is among the most commonly used agents in North American small animal practice and can be used to anesthetize not only cats and dogs but also birds, horses, and exotic species. Currently, ketamine is licensed only for use in cats and subhuman primates.
Ketamine is most commonly supplied as a 100-mg/mL solution. In 1999 ketamine was classified as a Schedule III controlled substance in the United States. It is presently classified as a prescription drug in Canada.
Ketamine has a rapid onset of action after IV or IM administration. This is a result of its high lipid solubility, which allows quick entry into brain tissue. Cats may lose their righting reflex within 90 seconds of IV administration and within 2 to 4 minutes of an IM injection of ketamine. IV administration has several advantages over IM administration, including more rapid induction and recovery and a lower dose. The anesthetist must ensure that the dose of ketamine is correct for the route of administration used: if the IM dose is inadvertently given by the IV route, serious overdose and death could result. Ketamine is given only by the IV route in dogs because IM ketamine may cause excitement and seizure activity. In cats, the primary use of IM injections is for restraint of fractious animals, in which IV injections are problematic.
Ketamine can also be administered orally to fractious cats, although this constitutes extra-label use and requires the owner’s informed consent. For a 5-kg cat, 100 mg of ketamine are drawn into a syringe, and an open-ended feline urethral catheter is attached. This is inserted through the cage bars, and the drug is squirted into the cat’s mouth. The oral ketamine takes effect in 5 to 10 minutes. If the eyes are accidentally sprayed with ketamine, they should be flushed with saline after the animal is anesthetized.
Although ketamine can be administered repeatedly to maintain anesthesia, this should be done with caution. After repeated injections, large amounts of the drug accumulate in the tissues, increasing the risk of seizure activity during recovery and significantly prolonging recovery.
Recovery from ketamine anesthesia normally occurs within 2 to 6 hours in healthy patients, depending on the dose given and the administration route. Dogs appear to have faster recoveries than cats, probably because of differences in ketamine metabolism and excretion. Elimination of ketamine depends on hepatic metabolism in the dog, but in the cat the drug is primarily excreted through the kidney. It follows that ketamine should be used with caution in dogs with hepatic disease and in cats with compromised renal function or urinary obstruction.
Ketamine is commonly used in combination with acepromazine, benzodiazepines, alpha2-agonists, and other agents to produce a wide variety of mixtures. Although most of these mixtures will be covered in the species chapters, it is fitting to mention diazepam and ketamine, which is one of the most commonly used combinations in small and large animal practice.
Ketamine-Diazepam Combination: A mixture of ketamine and diazepam is popular for IV induction of cats and dogs (including sighthounds) and is formulated by combining equal volumes of diazepam (5 mg/mL) and ketamine (100 mg/mL). The drugs are commonly mixed in the same syringe, although there is a small risk of precipitate formation. After premedication with an opioid and/or tranquilizer, ketamine-diazepam is given intravenously at a dose of 1 mL/20 lb (9.1 kg). This is equivalent to doses of 0.28 mg of diazepam per kilogram and 5.5 mg of ketamine per kilogram. The animal loses consciousness within 30 to 90 seconds and remains sufficiently deeply anesthetized for intubation or minor surgery for 5 to 10 minutes. This is followed by a 30- to 60-minute recovery period.
This combination of ketamine and diazepam has several advantages, including minimal cardiac depression, good muscle relaxation, superior recovery, and some analgesia. Respiratory depression, however, may be greater than that seen with ketamine alone.
Ketamine-diazepam does not work well when given by the IM route in either cats or dogs, because diazepam is poorly absorbed after IM injection. However, midazolam is well absorbed and may be used in place of diazepam for minor procedures (midazolam 0.28 mg/kg and ketamine 5.5 mg/kg).
Tiletamine: Tiletamine is a dissociative agent with effects similar to those of ketamine. Tiletamine is sold only in combination with zolazepam, which is a benzodiazepine closely related to diazepam. The use of zolazepam in combination with tiletamine reduces the risk of seizures during recovery and helps promote skeletal muscle relaxation. The product (Telazol) is sold as a powder, which contains 50 mg of each drug per milliliter. It is reconstituted with sterile water and is stable for 4 days at room temperature and 14 days if refrigerated. Telazol is a Class III controlled substance in the United States.
Tiletamine-zolazepam is used alone or in combination with other tranquilizers and with ketamine for immobilization, sedation, restraint, and anesthetic induction in a wide variety of domestic and exotic species. It is useful as an induction agent in healthy dogs and cats, particularly in animals with aggressive temperaments. It can be used alone (after premedication) or supplemented with inhalation anesthetics. The dose used is frequently lower than that recommended by the manufacturer.
The combination of tiletamine and zolazepam is similar in effect to ketamine-diazepam but offers the following advantages:
• Tiletamine appears to cause less pronounced apneustic respiration than ketamine. However, respiratory depression may be significant, particularly if a high dose is used or tiletamine is used in combination with other sedatives or anesthetics.
• Tiletamine-zolazepam may be administered intramuscularly, intravenously, or subcutaneously, although currently in the United States it is approved only for IM use in dogs and cats.
• Tiletamine-zolazepam is effective in many species of wildlife, and in some species it is the drug of choice for capture and immobilization.
Many reflexes are maintained throughout tiletamine-zolazepam anesthesia (including the palpebral, corneal, laryngeal, and pedal reflexes), and depth of anesthesia may be difficult to judge. As with ketamine, there is some analgesia, but visceral analgesia is inadequate for major abdominal surgery unless supplemented with other agents. Tachycardia and cardiac arrhythmias may be present in light anesthesia, and cardiac output is significantly reduced at high doses (more than 20 mg/kg IM). Like ketamine, tiletamine induces a marked increase in salivation and respiratory secretions unless the patient is premedicated with an anticholinergic.
One disadvantage of tiletamine-zolazepam is the long and difficult recoveries seen in some animals. As with ketamine, ataxia and increased sensitivity to stimuli are commonly observed during the recovery period. Tremors, muscle rigidity, seizure activity, and hyperthermia can also be seen, especially in dogs given tiletamine-zolazepam at labeled doses by the IM route. IV diazepam administration may be helpful in affected animals. In cats, recovery may be prolonged (up to 5 hours after IM injection), particularly if high doses are administered. Because the drug is metabolized by the liver and excreted via the kidneys, prolonged recovery should be expected in animals with liver or kidney dysfunction.
Tiletamine-zolazepam should be avoided in patients with an American Society of Anesthesiologists (ASA) physical status class of P3 or greater and in animals with CNS signs, hyperthyroidism, cardiac disease, pancreatic or renal disease, pregnancy, glaucoma, or penetrating eye injuries.
Etomidate
Etomidate is a noncontrolled, sedative-hypnotic imidazole drug that is occasionally used for induction of anesthesia in dogs, cats, and exotics. Because of its minimal effect on the cardiovascular and respiratory systems, etomidate is very useful in high-risk patients. However, it is not routinely used owing to its high cost and significant adverse effects including pain on IV injection, nausea, and vomiting. This ultra–short-acting nonbarbiturate drug has a wide therapeutic index, is noncumulative, and has a duration of action that is about 3 to 5 minutes but that is somewhat dependent on the dose.
Mode of Action and Pharmacology
Although the mode of action of etomidate is not completely understood, it appears to augment action of the inhibitory neurotransmitter GABA in a similar manner to barbiturates and propofol. The duration of effect is short because, like propofol, the drug is redistributed away from the brain and rapidly metabolized.
Effects on Major Organ Systems
• Anesthesia with etomidate is characterized by hypnosis but little analgesia. The drug decreases brain oxygen consumption, maintains brain perfusion better than most other injectable agents, and has anticonvulsant properties. It is therefore a good choice for patients with brain trauma or those undergoing brain or spinal surgery.
• This drug minimally affects respiratory rate and tidal volume; however, a brief period of apnea may be seen after induction. Although it crosses the placental barrier, it is rapidly eliminated and causes little neonatal respiratory depression and so is a good choice for cesarean sections (C-sections).
Adverse Effects
Although etomidate has a wide margin of safety, it has the following potential adverse effects:
• IV injection is reported to be painful. Some authors recommend administration through a running IV fluid line to decrease pain. Perivascular injection may be associated with the development of sterile abscesses.
• Rapid injection of etomidate may cause RBC hemolysis in cats owing to the propylene glycol vehicle. This is clinically insignificant unless the cat has an extremely low hematocrit or the drug is given repeatedly.
• Adrenal cortical function may be depressed for several hours after etomidate administration, decreasing levels of cortisol (a natural cortisone-like hormone). This is not harmful unless the drug is given for several hours or repeated over several days. For this reason, CRI is not recommended to sedate or anesthetize patients with serious illnesses.
• Nausea, vomiting, and involuntary excitement may occur during induction or recovery, particularly in patients that have not been adequately premedicated.
Use of Etomidate
Etomidate is administered only by the IV route. As with other induction agents, it is given to effect, starting with one quarter to one half of the calculated dose, depending on how rapid an induction is desired. Adverse effects including vomiting and muscle contractions are minimized by premedication with an opioid or diazepam (0.5 mg of diazepam per kilogram given intravenously 30 seconds before etomidate). Some authors also recommend premedication with dexamethasone to counteract suppression of the adrenal gland.
Like propofol, etomidate can be administered in repeated boluses for short-term maintenance of anesthesia.
Guaifenesin
Guaifenesin (GG) (previously known as glyceryl guaiacolate ether, or GGE) is a noncontrolled muscle relaxant that is commonly given to large animals to increase muscle relaxation, facilitate intubation, and ease induction and recovery. It is not an anesthetic or analgesic by itself and so is usually given in combination with other agents.
Mode of Action and Pharmacology
Although the mechanism of action is not fully known, GG is felt to block nerve impulses in the CNS.
Effects of Guaifenesin
GG causes skeletal muscle relaxation, including the pharyngeal and laryngeal muscles, but minimally affects the diaphragm. It affects the cardiovascular and respiratory systems minimally, causing transient mildly decreased blood pressure and tidal volume and mildly increased respiratory rate and GI motility.
Adverse Effects
At therapeutic doses, few adverse effects are seen. Excessive doses can cause muscle rigidity and apneustic breathing. GG is irritating to the tissues, so IV injection can cause thrombophlebitis, and perivascular injection may lead to tissue reaction. Concentrated solutions of this agent above 7% in ruminants and above 12% in horses can cause RBC hemolysis. Therefore a 5% or 10% solution in dextrose is preferred, and GG should be used with caution in anemic patients.
Use of Guaifenesin
GG is usually given as a part of an anesthetic induction protocol in combination with ketamine (after premedication with an alpha2-agonist or acepromazine). GG is also used to maintain anesthesia for short periods of time (less than 1 hour) in horses as part of a total IV anesthetic mixture commonly known as “triple drip” (GG combined with ketamine and an alpha2-agonist, typically xylazine).
It is administered rapidly intravenously after premedication until the patient shows signs of ataxia (“knuckling” at the fetlock in horses; ruminants will sway or even lie down), after which the induction agent is given. Recovery is usually smooth.
Guaifenesin should not be used without premedication or as the sole agent, because excitement is likely to be seen during induction and large doses will be required for recumbency, which increases the risk of side effects. Sedation and analgesia are inadequate for surgery when this agent is used alone.
The 5% solution is prepared by adding 50 g of guaifenesin to 50 g of medical grade dextrose, which is dissolved in 1 L of very hot sterile water. If a precipitate develops, it can be dissolved by rewarming the solution.
