Chapter 18 Integumentary System
The integumentary system consists of the skin and its appendages: sweat glands, nails, hairs, sebaceous glands, and arrector muscles of hairs. The system also includes the mammary glands and teeth.
Development of Skin
The integument or skin consists of two layers (epidermis and dermis) that are derived from two different germ layers (Fig. 18-1): the ectoderm and mesoderm.
• The epidermis is a superficial epithelial tissue that is derived from surface ectoderm.
• The dermis is a deep layer composed of dense connective tissue that is derived from primordial connective tissue (mesenchyme).
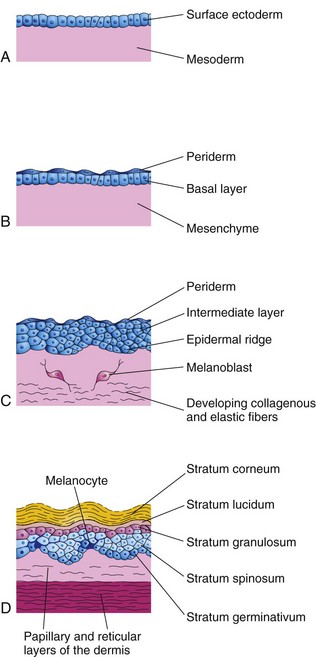
Figure 18–1 Successive stages of skin development. A, At 4 weeks. B, At 7 weeks. C, At 11 weeks. The cells of the periderm continually undergo keratinization and desquamation. Exfoliated peridermal cells form part of the vernix caseosa. D, Newborn infant. Note the position of the melanocytes in the basal layer of the epidermis. Their processes extend between the epidermal cells to supply them with melanin.
Ectodermal (epidermal) and mesenchymal (dermal) interactions involve mutual inductive mechanisms. The embryonic skin at 4 to 5 weeks consists of a single layer of surface ectoderm overlying the mesoderm (Fig. 18-1A).
Epidermis
The primordium of the epidermis is the surface ectoderm (Fig. 18-1A). The cells in this layer proliferate and form a layer of squamous epithelium, the periderm, and a basal layer (Fig. 18-1B). The cells of the periderm continually undergo keratinization and desquamation and are replaced by cells arising from the basal layer. The exfoliated peridermal cells form part of a white, greasy substance—vernix caseosa—that covers the fetal skin. Later, the vernix contains sebum, the secretion from the sebaceous glands in the skin (Fig. 18-2). The vernix protects the developing skin from constant exposure to amniotic fluid containing urine.
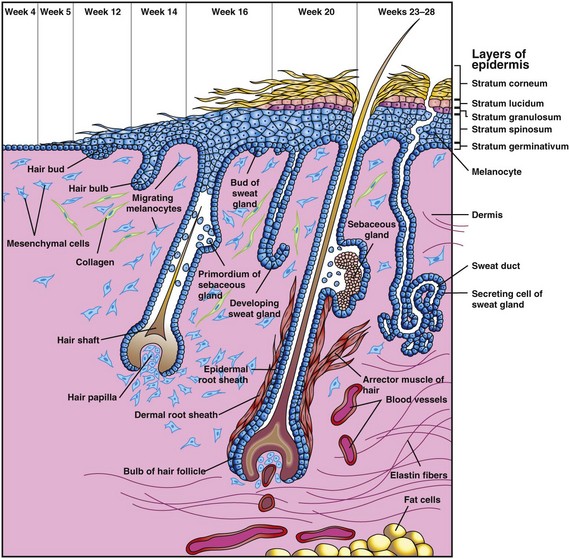
Figure 18–2 Successive stages in the development of a hair and its associated sebaceous gland and arrector muscle. Note also the successive stages in the development of a sweat gland.
The basal layer of the epidermis becomes the stratum germinativum (Fig. 18-1D), which produces new cells that are displaced into the more superficial layers. By 11 weeks, cells from the stratum germinativum have formed an intermediate layer (Fig. 18-1C). Replacement of the peridermal cells continues until approximately the 21st week; thereafter, the periderm disappears and the stratum corneum forms from the stratum lucidum (Fig. 18-1D). Proliferation of the cells in the stratum germinativum also produces epidermal ridges, which extend into the developing dermis. These ridges begin to appear in embryos of 10 weeks and are permanently established by the 17th week. The pattern of epidermal ridges that develops on the surface of the palms of the hands and the soles of the feet is determined genetically and constitutes the basis for examining fingerprints (dermatoglyphics) in criminal investigations and medical genetics. Abnormal chromosome complements affect the development of ridge patterns; for example, infants with Down syndrome have distinctive ridge patterns on their hands and feet that are of diagnostic value.
Late in the embryonic period, neural crest cells migrate into the mesenchyme in the developing dermis and differentiate into melanoblasts (Fig. 18-1B and C). Later, these cells migrate to the dermoepidermal junction and differentiate into melanocytes (Fig. 18-1D). The melanocytes begin producing melanin before birth and distribute it to the epidermal cells. After birth, increased amounts of melanin are produced in response to ultraviolet light. The relative content of melanin in the melanocytes accounts for the different colors of skin. Molecular studies indicate that melanocyte-stimulating hormone cell surface receptor and melanosomal P-protein determine the degree of pigmentation by regulating tyrosinase levels and activity.
Dermis
The dermis develops from the mesenchyme underlying the surface ectoderm (Fig. 18-1A and B). The mesenchyme that differentiates into the connective tissue of the dermis originates from the somatic layer of the lateral mesoderm and from the dermatomes of the somites. By 11 weeks, the mesenchymal cells have begun to produce collagenous and elastic connective tissue fibers (Fig. 18-1C). As the epidermal ridges form, the dermis projects into the epidermis, forming dermal papillae. Capillary loops develop in some of the dermal ridges and provide nourishment for the epidermis. Sensory nerve endings form in other ridges.
The developing afferent nerve fibers apparently play an important role in the spatial and temporal sequence of dermal ridge formation. The blood vessels in the dermis differentiate from the mesenchyme. As the skin grows, new capillaries grow out from the primordial vessels (angiogenesis). Some capillaries acquire muscular coats through the differentiation of myoblasts developing in the surrounding mesenchyme, and they become arterioles, arteries, venules, and veins. By the end of the first trimester, the blood supply of the fetal dermis is well established.
Disorders of Keratinization
Ichthyosis is a general term for a group of skin disorders resulting from excessive keratinization (keratin formation). The skin is characterized by dryness and fish-skin–like scaling, which may involve the entire body surface (Fig. 18-3). A harlequin fetus results from a rare keratinizing disorder that is inherited as an autosomal recessive trait. The skin is markedly thickened, ridged, and cracked. Most of the affected infants die during the first week of life. A collodion infant is covered at birth by a thick, taut membrane that resembles collodion or parchment. This membrane cracks with the first respiratory efforts and begins to fall off in large sheets. Complete shedding of membranes may take several weeks, occasionally leaving normal-appearing skin.
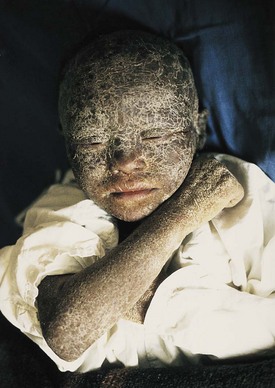
Figure 18–3 A child with epidermolytic hyperkeratosis. This condition is characterized by severe hyperkeratosis from the time of birth. It has an autosomal dominant inheritance pattern.
(Courtesy of Dr. Joao Carlos Fernandes Rodrigues, Servico de Dermatologia, Hospital de Desterro, Lisbon, Portugal.)
Angiomas of Skin
Angiomas of the skin are vascular anomalies in which some transitory primordial blood vessels persist. Similar lesions that are composed of lymphatics are called cystic lymphangiomas, or cystic hygromas (see Chapter 14). True angiomas are benign tumors of endothelial cells, usually composed of solid or hollow cords; the hollow cords contain blood. Various terms are used to describe angiomatous anomalies (“birthmarks”). Nevus flammeus denotes a flat, pink or red, flame-like blotch that often appears on the posterior surface of the neck. A port wine stain, or hemangioma, is a larger, darker angioma than a nevus flammeus and is nearly always anterior or lateral on the face, neck, or both.
Glands of Skin
Two kinds of glands, sebaceous glands and sweat glands, are derived from the epidermis and grow into the dermis.
Sebaceous Glands
Most sebaceous glands develop as buds from the sides of the developing epidermal root sheaths of the hair follicles (Fig. 18-2). The glandular buds grow into the surrounding connective tissue and branch to form the primordia of the alveoli and their associated ducts. The central cells of the alveoli break down, forming an oily secretion called sebum; this is released into the hair follicle and passes to the surface of the skin, where it mixes with desquamated peridermal cells to form vernix caseosa. Sebaceous glands, independent of the hair follicles (e.g., in the glans penis and labia minora), develop in a similar manner as buds from the epidermis.
Sweat Glands
Eccrine sweat glands develop as epidermal downgrowths—cellular buds—into the underlying mesenchyme (Fig. 18-2). As a bud elongates, its end coils to form the primordium of the secretory part of the gland. The epithelial attachment of the developing gland to the epidermis forms the primordium of the sweat duct. The central cells of the primordial ducts degenerate, forming a lumen. The peripheral cells of the secretory part of the gland differentiate into myoepithelial and secretory cells (Fig. 18-2). The myoepithelial cells are believed to be specialized smooth muscle cells that assist in expelling sweat from the glands. Eccrine sweat glands begin to function shortly after birth.
Apocrine sweat glands develop from downgrowths of the stratum germinativum of the epidermis that give rise to the hair follicles. As a result, the ducts of these glands open into the upper part of the hair follicles, superficial to the openings of the sebaceous glands. They begin to secrete sweat during puberty.
Development of Hairs
Hairs begin to develop during the 9th to 12th weeks, but they do not become easily recognizable until approximately the 20th week (Fig. 18-2). Hairs are first recognizable on the eyebrows, upper lip, and chin. A hair follicle begins as a proliferation of the stratum germinativum of the epidermis and extends into the underlying dermis. The hair bud soon becomes a club-shaped hair bulb. The epithelial cells of the hair bulb constitute the germinal matrix, which later produces the hair. The hair bulb is soon invaginated by a small mesenchymal hair papilla (Fig. 18-2). The peripheral cells of the developing hair follicle form the epidermal root sheath, and the surrounding mesenchymal cells differentiate into the dermal root sheath. As cells in the germinal matrix proliferate, they are pushed toward the surface, where they keratinize to form hair shafts. The hair grows through the epidermis on the eyebrows and the upper lip by the end of the 12th week.
The first hairs—lanugo (downy hair)—are fine, soft, and lightly pigmented. Lanugo begins to appear toward the end of the 12th week and is plentiful by 17 to 20 weeks. These hairs help to hold the vernix on the skin. Lanugo is replaced during the perinatal period by coarser hairs that persist over most of the body. In the axillary and pubic regions, the lanugo is replaced at puberty by even coarser terminal hairs. In males, similar coarse hairs also appear on the face and often on the chest.
Melanoblasts migrate into the hair bulbs and differentiate into melanocytes. The melanin produced by these cells is transferred to the hair-forming cells in the germinal matrix several weeks before birth. The relative content of melanin accounts for different hair colors. Arrector muscles of hairs, small bundles of smooth muscle fibers, differentiate from the mesenchyme surrounding the hair follicle and attach to the dermal root sheath and the papillary layer of the dermis (Fig. 18-2). The arrector muscles are poorly developed in the hairs of the axilla and in certain parts of the face. The hairs forming the eyebrows and the cilia forming the eyelashes have no arrector muscles.
Albinism
In generalized albinism, an autosomal recessive trait, the skin, hairs, and retina lack pigment; however, the iris usually shows some pigmentation. Albinism occurs when the melanocytes do not produce melanin because of a lack of the enzyme tyrosinase. In localized albinism—piebaldism—an autosomal dominant trait, there is a lack of melanin in patches of skin, hair, or both.
Development of Nails
Toenails and fingernails begin to develop at the tips of the digits at approximately 10 weeks (Fig. 18-4). Development of the fingernails precedes that of the toenails by approximately 4 weeks. The primordia of the nails appear as thickened areas, or fields, of epidermis at the tip of each digit. Later, these nail fields migrate onto the dorsal surface (Fig. 18-4A), carrying their innervation from the ventral surface. The nail fields are surrounded laterally and proximally by folds of epidermis—nail folds. Cells from the proximal nail fold grow over the nail field and keratinize to form the nail plate (Fig. 18-4B). At first, the developing nail is covered by superficial layers of epidermis called eponychium (Fig. 18-4C). These layers degenerate, exposing the nail, except at its base, where it persists as the cuticle. The fingernails reach the fingertips at approximately 32 weeks; the toenails reach the toe tips at approximately 36 weeks.
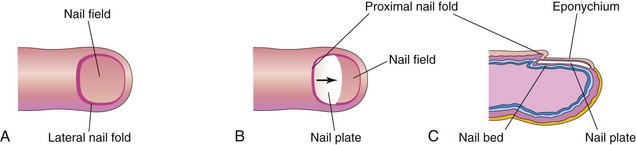
Figure 18–4 Successive stages in the development of a fingernail. A, The first indication of a nail is a thickening of the epidermis, the nail field, at the tip of the finger. B, As the nail plate develops, it slowly grows toward the tip of the finger. C, The fingernail normally reaches the end of the digit by 32 weeks.
Development of Mammary Glands
Mammary glands are modified and highly specialized types of sweat glands. Mammary buds begin to develop during the sixth week as solid downgrowths of the epidermis into the underlying mesenchyme (Fig. 18-5C). These changes occur in response to an inductive influence from the mesenchyme. The mammary buds develop from mammary crests, which are thickened strips of ectoderm extending from the axillary to the inguinal regions (Fig. 18-5A). The mammary crests appear during the fourth week but normally persist only in the pectoral area where the breasts develop (Fig. 18-5B). Each primary mammary bud soon gives rise to several secondary mammary buds that develop into the lactiferous ducts and their branches (Fig. 18-5D and E). Canalization of these buds is induced by maternal sex hormones entering the fetal circulation. This process continues until late gestation and by term, 15 to 20 lactiferous ducts have formed. The fibrous connective tissue and fat of the mammary gland develop from the surrounding mesenchyme.
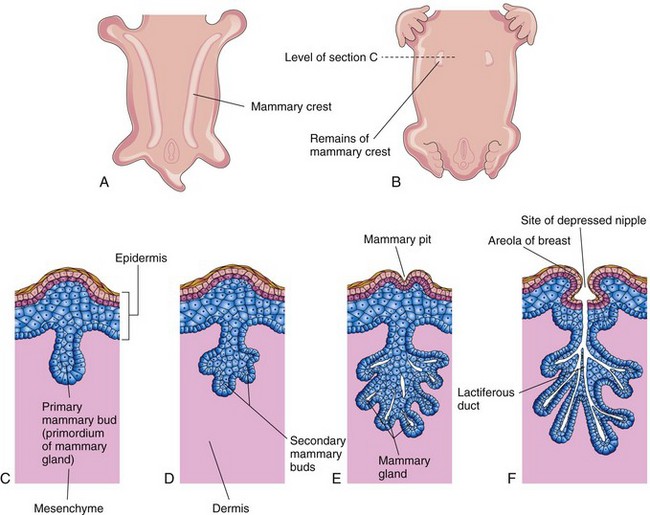
Figure 18–5 Development of the mammary glands. A, Ventral view of an embryo at approximately 28 days, showing mammary crests. B, Similar view at 6 weeks, showing the remains of these crests. C, Transverse section of a mammary crest at the site of a developing mammary gland. D to F, Similar sections, showing successive stages of breast development between the 12th week and birth.
During the late fetal period, the epidermis at the site of origin of the primordial mammary gland becomes depressed, forming a shallow mammary pit (Fig. 18-5E). The nipples are poorly formed and depressed in newborn infants. After birth, the nipples usually rise from the mammary pits. At birth, the main lactiferous ducts are formed (Fig. 18-5F); the mammary glands remain underdeveloped until puberty. The mammary glands develop similarly and are of the same structure in both sexes. In females, the glands enlarge rapidly during puberty, mainly because of fat and other connective tissue development in the breasts. Growth of the duct system also occurs because of the increased levels of circulating estrogens.
Gynecomastia
The rudimentary mammary glands in males normally undergo no postnatal development. Gynecomastia refers to excessive development of male mammary tissue. It occurs in most newborn males because of stimulation of the mammary glands by maternal sex hormones. This effect disappears in a few weeks. During mid-puberty, approximately two thirds of boys have varying degrees of hyperplasia of the breasts. Approximately 80% of males with Klinefelter syndrome have gynecomastia (see Chapter 19).
Supernumerary Breasts and Nipples
An extra breast (polymastia) or nipple (polythelia), an inheritable condition, occurs in approximately 1% of the female population. Supernumerary nipples are also relatively common in males; they are often mistaken for moles. Less commonly, supernumerary breasts or nipples appear in the axillary or abdominal regions of females. In these positions, the nipples or breasts arise from extra mammary buds that develop along the mammary crests.
Development of Teeth
Two sets of teeth normally develop: the primary dentition, or deciduous teeth, and the secondary dentition, or permanent teeth. Teeth develop from the oral ectoderm, mesenchyme, and neural crest cells. The enamel is derived from the ectoderm of the oral cavity; all other tissues differentiate from the surrounding mesenchyme and neural crest cells. Expression of homeobox MSX and Dlx genes and BMP in the migrating neural crest cells, as well as in the ectoderm and mesenchyme, is essential for the initiation of tooth development. Wnt/β-catenin signaling also regulates many stages of tooth development.
Odontogenesis (tooth development) is initiated by the inductive influence of the neural crest mesenchyme on the overlying ectoderm. The first tooth buds appear in the anterior mandibular region; later tooth development occurs in the anterior maxillary region and progresses posteriorly in both jaws. Tooth development continues for years after birth (Table 18-1). The first indication of tooth development is a thickening of the oral epithelium, a derivative of the surface ectoderm seen during the sixth week. These U-shaped bands—dental laminae—follow the curves of the primordial jaws (Figs. 18-6A and 18-7A).
Table 18–1 The Order and Usual Time of Eruption of Teeth and the Time of Shedding of Deciduous Teeth
| TOOTH | USUAL ERUPTION TIME | SHEDDING TIME |
|---|---|---|
| Deciduous | ||
| Medial incisor | 6-8 mo | 6-7 yr |
| Lateral incisor | 8-10 mo | 7-8 yr |
| Canine | 16-20 mo | 10-12 yr |
| First molar | 12-16 mo | 9-11 yr |
| Second molar | 20-24 mo | 10-12 yr |
| Permanent | ||
| Medial incisor | 7-8 yr | |
| Lateral incisor | 8-9 yr | |
| Canine | 10-12 yr | |
| First premolar | 10-11 yr | |
| Second premolar | 11-12 yr | |
| First molar | 6-7 yr | |
| Second molar | 12 yr | |
| Third molar | 13-25 yr |
Data from Moore KL, Dalley AF, Agur AMR: Clinically Oriented Anatomy, 6th ed. Baltimore, Williams & Wilkins, 2010.
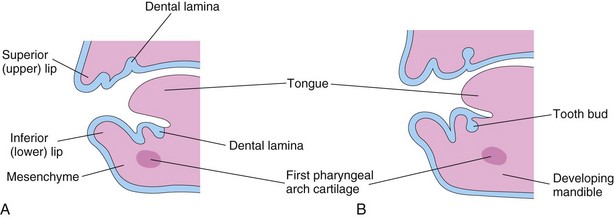
Figure 18–6 Sagittal sections through the developing jaws, showing early development of the teeth. A, Early in the sixth week, the dental laminae are evident. B, Later in the sixth week, tooth buds arise from the laminae.
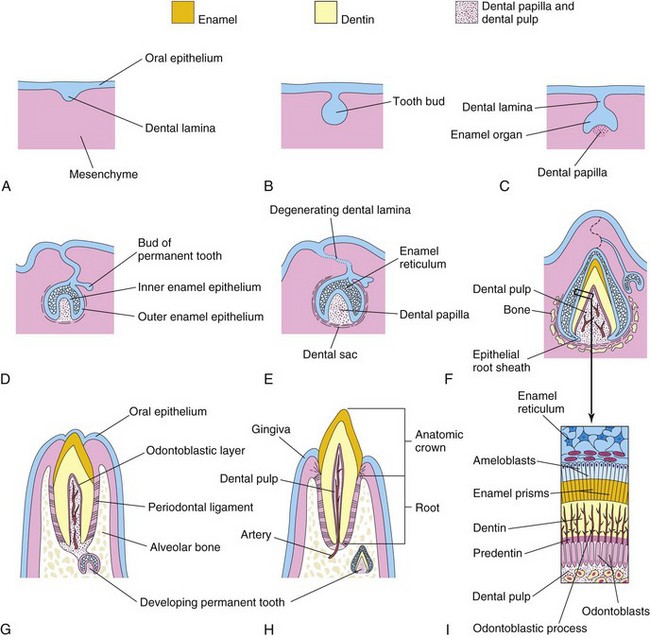
Figure 18–7 Sagittal sections, showing successive stages in the development and eruption of an incisor tooth. A, At 6 weeks, showing the dental lamina. B, At 7 weeks, showing the tooth bud developing from the dental lamina. C, At 8 weeks, showing the cap stage of tooth development. D, At 10 weeks, showing the early bell stage of a deciduous tooth and the bud stage of a permanent tooth. E, At 14 weeks, showing the advanced bell stage of tooth development. Note that the connection (dental lamina) of the tooth to the oral epithelium is degenerating. F, At 28 weeks, showing the enamel and dentin layers. G, At 6 months postnatally, showing early tooth eruption. H, At 18 months postnatally, showing a fully erupted deciduous incisor tooth. The permanent incisor tooth now has a well-developed crown. I, Section through a developing tooth, showing ameloblasts (enamel producers) and odontoblasts (dentin producers).
Bud Stage of Tooth Development
Each dental lamina develops 10 centers of proliferation from which tooth buds grow into the underlying mesenchyme (Figs. 18-6B and 18-7B). These buds develop into the deciduous teeth, which are shed during childhood (Table 18-1). There are 10 tooth buds in each jaw, one for each deciduous tooth. The tooth buds for the permanent teeth begin to appear at approximately 10 weeks from deep continuations of the dental laminae (Fig. 18-7D). The permanent molars have no deciduous predecessors; they develop as buds from posterior extensions of the dental laminae. The tooth buds for the permanent teeth appear at different times, mostly during the fetal period. The buds for the second and third permanent molars develop after birth.
Cap Stage of Tooth Development
As each tooth bud is invaginated by the mesenchyme—the primordium of the dental papilla and dental follicle—the bud becomes cap-shaped (Fig. 18-7C). The ectodermal part of the developing tooth, the enamel organ, eventually produces enamel. The internal part of each cap-shaped tooth, the dental papilla, is the primordium of the dental pulp. Together, the dental papilla and enamel organ form the tooth germ (primordial tooth). The outer cell layer of the enamel organ is the outer enamel epithelium, whereas the inner cell layer lining the “cap” is the inner enamel epithelium (see Fig. 18-7D).
The central core of loosely arranged cells between the layers of enamel epithelium is the enamel reticulum or stellate reticulum (Fig. 18-7E). As the enamel organ and dental papilla of the tooth develop, the mesenchyme surrounding the developing tooth condenses to form the dental sac, a vascularized capsular structure (Fig. 18-7E). The dental sac is the primordium of the cement and periodontal ligament. The cement is the bone-like, rigid connective tissue covering the root of the tooth. The periodontal ligament is derived from neural crest cells. It is a specialized vascular connective tissue that surrounds the root of the tooth, separating it from and attaching it to the alveolar bone (Fig. 18-7G).
Bell Stage of Tooth Development
As the enamel organ differentiates, the developing tooth becomes bell-shaped (Figs. 18-7D and 18-8). The mesenchymal cells in the dental papilla adjacent to the inner enamel epithelium differentiate into odontoblasts, which produce predentine and deposit it adjacent to the epithelium. Later, the predentine calcifies and becomes dentin. As the dentin thickens, the odontoblasts regress toward the center of the dental papilla; however, their cytoplasmic processes—odontoblastic processes—remain embedded in the dentin (Fig. 18-7F and I). Enamel is the hardest tissue in the body. It overlies the yellowish dentin, the second hardest tissue in the body, and protects it from being fractured.
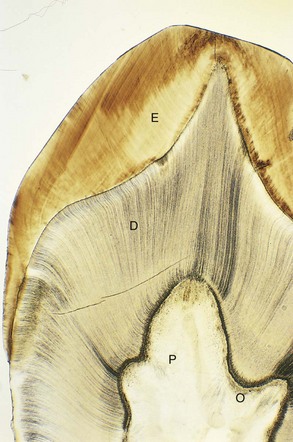
Figure 18–8 Photomicrograph (×17) of a section of the crown and neck of a tooth. Observe the enamel (E), dentin (D), dental pulp (P), and odontoblasts (O).
(From Gartner LP, Hiatt JL: Color Textbook of Histology, 2nd ed. Philadelphia, WB Saunders, 2001.)
Cells of the inner enamel epithelium differentiate into ameloblasts, which produce enamel in the form of prisms (rods) over the dentin. As the enamel increases, the ameloblasts regress toward the outer enamel epithelium. The root of the tooth begins to develop after dentin and enamel formation is well advanced. The inner and outer enamel epithelia come together in the neck region of the tooth, where they form a fold, the epithelial root sheath (Fig. 18-7F). This sheath grows into the mesenchyme and initiates root formation. The odontoblasts adjacent to the epithelial root sheath form dentin that is continuous with that of the crown. As the dentin increases, it reduces the pulp cavity to a narrow root canal through which the vessels and nerves pass. The inner cells of the dental sac differentiate into cementoblasts, which produce cement that is restricted to the root. Cement is deposited over the dentin of the root and meets the enamel at the neck of the tooth.
As the teeth develop and the jaws ossify, the outer cells of the dental sac also become active in bone formation. Each tooth soon becomes surrounded by bone, except over its crown. The tooth is held in its alveolus (bony socket) by the strong periodontal ligament, a derivative of the dental sac (Fig. 18-7G and H). Some fibers of this ligament are embedded in the cement; other fibers are embedded in the bony wall of the alveolus. The periodontal ligament is located between the cement of the root and the bony alveolus.
Tooth Eruption
As the deciduous teeth develop, they begin a continuous slow movement toward the oral cavity (Fig. 18-7F and G). The mandibular teeth usually erupt before the maxillary teeth, and girls’ teeth usually erupt sooner. A child’s dentition contains 20 deciduous teeth. As the root of the tooth grows, its crown gradually erupts through the oral epithelium. The part of the oral mucosa around the erupted crown becomes the gingiva (gum). Usually, eruption of the deciduous teeth occurs between 6 and 24 months after birth (see Table 18-1). The mandibular medial incisors—or central incisors—usually erupt 6 to 8 months after birth, but this process may not begin until 12 or 13 months in some children. Despite this, all 20 deciduous teeth are usually present by the end of the second year in healthy children
The permanent teeth develop in a manner similar to that described for deciduous teeth. As a permanent tooth grows, the root of the corresponding deciduous tooth is gradually resorbed by osteoclasts. Consequently, when the deciduous tooth is shed, it consists only of the crown and the cervical, or uppermost, part of the root. The permanent teeth usually begin to erupt during the sixth year and continue to appear until early adulthood (Fig. 18-9; see also Table 18-1).
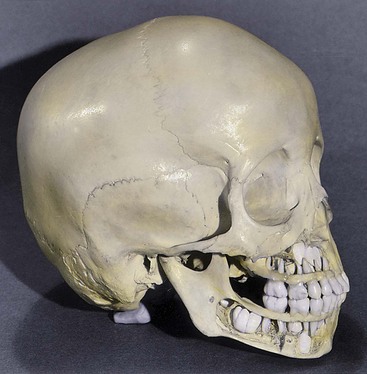
Figure 18–9 Cranium (skull) of a 4-year-old child. Bone has been removed from the jaws to show the relation of the developing permanent teeth to the erupted deciduous teeth.
Enamel Hypoplasia
Defective enamel formation causes pits, fissures, or both in the enamel. These defects result from temporary disturbances of enamel formation. Various factors may injure ameloblasts (source of enamel), such as nutritional deficiency, tetracycline therapy, and infectious diseases. Rickets arising during the critical period of permanent tooth development (6-12 weeks) is the most common known cause of enamel hypoplasia. Rickets is a disease affecting children who are deficient in vitamin D.
Variations of Tooth Shape
Abnormally shaped teeth are relatively common. Occasionally there are spherical masses of enamel—enamel pearls—attached to the tooth (Fig. 18-10E). They are formed by aberrant groups of ameloblasts. In other cases, the maxillary lateral incisor teeth may have a slender, tapered shape (peg-shaped incisors). Congenital syphilis affects the differentiation of the permanent teeth, resulting in incisors with central notches.
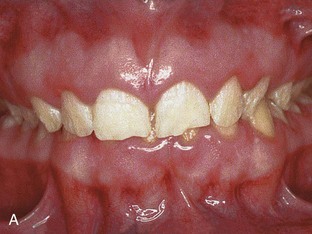
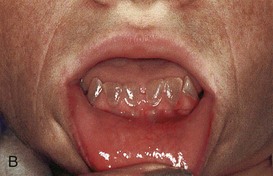
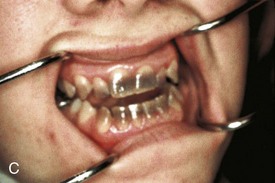
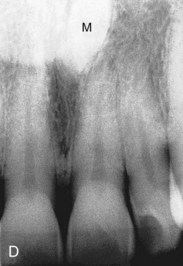
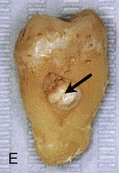
Figure 18–10 Common tooth anomalies. A, Amelogenesis imperfecta. B, Dentinogenesis imperfecta. C, Tetracycline-stained teeth. D, Midline supernumerary tooth (M, mesiodens), located near the root apex of the central incisor. E, Molar tooth with an enamel pearl (arrow).
(A, Courtesy of Dr. Blaine Cleghorn, Faculty of Dentistry, Dalhousie University, Halifax, Nova Scotia, Canada; B to D, Courtesy of Dr. Steve Ahing, Faculty of Dentistry, University of Manitoba, Winnipeg, Manitoba, Canada.)
Numeric Abnormalities of Teeth
One or more supernumerary teeth may develop, or the normal number of teeth may not form (Fig. 18-10D). Supernumerary teeth usually develop in the area of the maxillary incisors and may disrupt the position and eruption of normal teeth. The extra teeth commonly erupt posterior to the normal teeth. In partial anodontia, one or more teeth are absent. Congenital absence of one or more teeth is often a familial trait. In total anodontia, no teeth develop; this very rare condition is usually associated with congenital ectodermal dysplasia (disorders involving tissues that are ectodermal in origin).
Macrodontia
Macrodontia (a single large tooth) is a condition caused by the union of two adjacent tooth germs. The crowns of the two teeth can be partially or completely fused. The same applies to roots. Occasionally, a tooth bud divides or two buds partially fuse to form fused teeth. This condition is commonly observed in the mandibular incisors of the primary dentition, but can also occur in the permanent dentition.
Amelogenesis Imperfecta
In amelogenesis imperfecta, the tooth enamel is soft and friable because of hypocalcification, and the teeth are yellow to brown color (Fig. 18-10A). The teeth are covered with only a thin layer of abnormally formed enamel through which the color of the underlying dentin is visible, giving the teeth a darkened appearance. This autosomal dominant condition affects approximately 1 in 7000 newborn infants.
Dentinogenesis Imperfecta
Dentinogenesis imperfecta is relatively common in white children (Fig. 18-10B). In affected children, the teeth are brown to gray-blue, with an opalescent sheen. This is caused by failure of the odontoblasts to differentiate normally, producing poorly calcified dentin. Both deciduous and permanent teeth are usually involved. The enamel tends to wear down rapidly, exposing the dentin. This anomaly is inherited as an autosomal dominant trait.
Discolored Teeth
Foreign substances incorporated into the developing enamel and dentin discolor the teeth. The hemolysis (liberation of hemoglobin) associated with hemolytic disease of the newborn (Chapter 8) may produce blue to black discoloration of the teeth. All tetracyclines are extensively incorporated into the teeth. The critical period of risk is from approximately 14 weeks of fetal life to the 10th postnatal month for deciduous teeth, and from approximately 14 weeks of fetal life to the 16th postnatal year for permanent teeth. Tetracyclines produce brownish-yellow discoloration (mottling) and enamel hypoplasia because they interfere with the metabolic processes of the ameloblasts (Fig. 18–10C). The enamel is completely formed on all but the third molars by approximately 8 years of age. For this reason, tetracyclines should not be administered to pregnant women or to children younger than 8 years.
Clinically Oriented Questions
1. An infant was reportedly born without skin. Is this possible? If so, could such an infant survive?
2. A dark-skinned person presented with patches of white skin on the face, chest, and limbs. He even had a white forelock. What is this condition called, and what is its developmental basis? Is there any treatment for these skin defects?
3. Some boys have enlarged breasts at birth. Is this an indication of abnormal sex development? Some boys develop breasts during puberty. Why might this occur?
4. A girl developed a breast in the axilla during puberty. She also had extra nipples on her chest. What is the embryologic basis for these anomalies?
5. An infant was born with two teeth. Would these be normal teeth? Is this a common occurrence? Are they usually extracted?