Later Stages of Development
Adolescence: The Early Permanent Dentition Years
Adolescence is a sexual phenomenon, the period of life when sexual maturity is attained. More specifically, it is the transitional period between the juvenile stage and adulthood, during which secondary sexual characteristics appear, the adolescent growth spurt takes place, fertility is attained, and profound physiologic changes occur. All these developments are associated with the maturation of the sex organs and the accompanying surge in secretion of sex hormones.
This period is particularly important in dental and orthodontic treatment because the physical changes at adolescence significantly affect the face and dentition. Major events in dentofacial development that occur during adolescence include the exchange from the mixed to the permanent dentition, an acceleration in the overall rate of facial growth, and differential growth of the jaws.
Initiation of Adolescence
The first events of puberty occur in the brain, and although considerable research progress has been made in this area, the precise stimulus for their unfolding remains unknown. For whatever reason, apparently influenced both by an internal clock and external stimuli, brain cells in the hypothalamus begin to secrete substances called releasing factors. Both the cells and their method of action are somewhat unusual. These neuroendocrine cells look like typical neurons, but they secrete materials in the cell body, which are carried by cytoplasmic transport down the axon toward a richly vascular area at the base of the hypothalamus near the pituitary gland (Figure 4-1). The substances secreted by the nerve cells pass into capillaries in this vascular region and are carried the short distance to the pituitary by blood flow. It is unusual in the body for the venous return system to transport substances from one closely adjacent region to another, but here the special arrangement of the vessels seems made to order for this purpose. Accordingly, this special network of vessels, analogous to the venous supply to the liver but on a much smaller scale, is called the pituitary portal system.
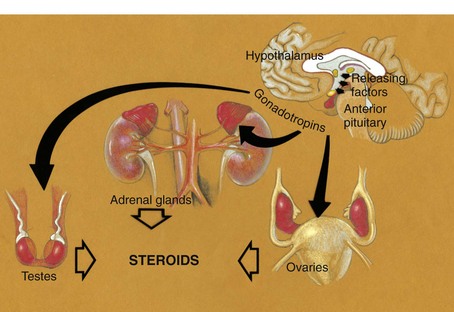
FIGURE 4-1 Diagrammatic representation of the cascade of endocrine signals controlling sexual development. Releasing factors from the hypothalamus are carried via the pituitary portal circulation to the anterior pituitary gland, where they initiate the release of pituitary gonadotropic hormones. These in turn stimulate cells in the testes, ovaries, and adrenals, which secrete the steroid sex hormones.
In the anterior pituitary, the hypothalamic releasing factors stimulate pituitary cells to produce several related but different hormones called pituitary gonadotropins. Their function is to stimulate endocrine cells in both the adrenal glands and the developing sex organs to produce sex hormones. In every individual a mixture of male and female sex hormones is produced, and it is a biologic fact, as well as an everyday observation, that there are feminine males and masculine females. Presumably this represents the balance of the competing male and female hormones. In the male, different cell types in the testes produce both the male sex hormone testosterone and the female sex hormones. A different pituitary gonadotropin stimulates each of these cell types. In the female, the pituitary gonadotropins stimulate secretion of estrogen by the ovaries, and later progesterone by the same organ. In the female, male sex hormones are produced in the adrenal cortex, stimulated by still another pituitary hormone, and possibly some female hormones are produced in the male adrenal cortex.
Under the stimulation of the pituitary gonadotropins, sex hormones from the testes, ovaries, and adrenal cortex are released into the bloodstream in quantities sufficient to cause development of secondary sexual characteristics and accelerated growth of the genitalia. The increasing level of the sex hormones also causes other physiologic changes, including the acceleration in general body growth and shrinkage of lymphoid tissues seen in the classic growth curves described in Chapter 2. Neural growth is unaffected by the events of adolescence, since it is essentially complete by age 6. The changes in the growth curves for the jaws, general body, lymphoid, and genital tissues, however, can be considered the result of the hormonal changes that accompany sexual maturation (Figure 4-2).
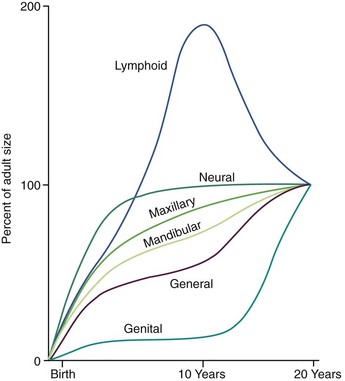
FIGURE 4-2 Growth curves for the maxilla and mandible shown against the background of Scammon’s curves. Note that growth of the jaws is intermediate between the neural and general body curves, with the mandible following the general body curve more closely than the maxilla. The acceleration in general body growth at puberty, which affects the jaws, parallels the dramatic increase in development of the sexual organs. Lymphoid involution also occurs at this time.
The system by which a few neurons in the hypothalamus ultimately control the level of circulating sex hormones may seem curiously complex. The principle, however, is one utilized in control systems throughout the body and also in modern technology. Each of the steps in the control process results in an amplification of the control signal, in a way analogous to the amplification of a small musical signal between the signal source and speakers of a stereo system. The amount of pituitary gonadotropin produced is 100 to 1000 times greater than the amount of gonadotropin-releasing factors produced in the hypothalamus, and the amount of sex hormones produced is 1000 times greater than the amount of the pituitary hormones themselves. The system, then, is a three-stage amplifier. Rather than being a complex biologic curiosity, it is better viewed as a rational engineering design. A similar amplification of controlling signals from the brain is used, of course, in all body systems.
Timing of Puberty
There is a great deal of individual variation, but puberty and the adolescent growth spurt occur on the average nearly 2 years earlier in girls than in boys (Figure 4-3). Why this occurs is not known, but the phenomenon has an important impact on the timing of orthodontic treatment, which must be done earlier in girls than in boys to take advantage of the adolescent growth spurt. Because of the considerable individual variation, however, early-maturing boys will reach puberty ahead of slow-maturing girls, and it must be remembered that chronologic age is only a crude indicator of where an individual stands developmentally. The stage of development of secondary sexual characteristics provides a physiologic calendar of adolescence that correlates with the individual’s physical growth status. Not all the secondary sexual characteristics are readily visible, of course, but most can be evaluated in a normal fully clothed examination, such as would occur in a dental office.
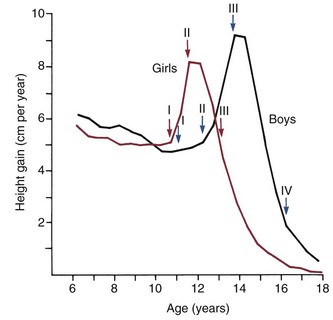
FIGURE 4-3 Velocity curves for growth at adolescence, showing the difference in timing for girls and boys. Also indicated on the growth velocity curves are the corresponding stages in sexual development (see text). (From Marshall WA, Tanner JM. Puberty. In: Falkner F, Tanner JM, eds. Human Growth, vol 2. 2nd ed. New York: Plenum Publishing; 1986.)
Adolescence in girls can be divided into three stages, based on the extent of sexual development. The first stage, which occurs at about the beginning of the physical growth spurt, is the appearance of breast buds and early stages of the development of pubic hair. The peak velocity for physical growth occurs about 1 year after the initiation of stage I, and coincides with stage II of development of sexual characteristics (see Figure 4-3). At this time, there is noticeable breast development. Pubic hair is darker and more widespread, and hair appears in the armpits (axillary hair).
The third stage in girls occurs 1 to 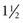 years after stage II and is marked by the onset of menstruation. By this time, the growth spurt is all but complete. At this stage, there is noticeable broadening of the hips with more adult fat distribution, and development of the breasts is complete.
years after stage II and is marked by the onset of menstruation. By this time, the growth spurt is all but complete. At this stage, there is noticeable broadening of the hips with more adult fat distribution, and development of the breasts is complete.
The stages of sexual development in boys are more difficult to specifically define. Puberty begins later and extends over a longer period—about 5 years compared with 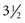 years for girls (see Figure 4-3). In boys, four stages in development can be correlated with the curve of general body growth at adolescence.
years for girls (see Figure 4-3). In boys, four stages in development can be correlated with the curve of general body growth at adolescence.
The initial sign of sexual maturation in boys usually is the “fat spurt.” The maturing boy gains weight and becomes almost chubby, with a somewhat feminine fat distribution. This probably occurs because estrogen production by the Leydig cells in the testes is stimulated before the more abundant Sertoli cells begin to produce significant amounts of testosterone. During this stage, boys may appear obese and somewhat awkward physically. At this time also, the scrotum begins to increase in size and may show some increase or change in pigmentation.
At stage II, about 1 year after stage I, the spurt in height is just beginning. At this stage, there is a redistribution and relative decrease in subcutaneous fat, pubic hair begins to appear, and growth of the penis begins.
The third stage occurs 8 to 12 months after stage II and coincides with the peak velocity in gain in height. At this time, axillary hair appears and facial hair appears on the upper lip only. A spurt in muscle growth also occurs, along with a continued decrease in subcutaneous fat and an obviously harder and more angular body form. Pubic hair distribution appears more adult but has not yet spread to the medial of the thighs. The penis and scrotum are near adult size.
Stage IV for boys, which occurs anywhere from 15 to 24 months after stage III, is difficult to pinpoint. At this time, the spurt of growth in height ends. There is facial hair on the chin and the upper lip, adult distribution and color of pubic and axillary hair, and a further increase in muscular strength.
The timing of puberty makes an important difference in ultimate body size, in a way that may seem paradoxical at first: the earlier the onset of puberty, the smaller the adult size, and vice versa. Growth in height depends on endochondral bone growth at the epiphyseal plates of the long bones, and the impact of the sex hormones on endochondral bone growth is twofold. First, the sex hormones stimulate the cartilage to grow faster, and this produces the adolescent growth spurt. But the sex hormones also cause an increase in the rate of skeletal maturation, which for the long bones is the rate at which cartilage is transformed into bone. The acceleration in maturation is even greater than the acceleration in growth. Thus during the rapid growth at adolescence, the cartilage is used up faster than it is replaced. Toward the end of adolescence, the last of the cartilage is transformed into bone, and the epiphyseal plates close. At that point, of course, growth potential is lost and growth in height stops.
This early cessation of growth after early sexual maturation is particularly prominent in girls. It is responsible for much of the difference in adult size between men and women. Girls mature earlier on the average and finish their growth much sooner. Boys are not bigger than girls until they grow for a longer time at adolescence. The difference arises because there is slow but steady growth before the growth spurt, and so when the growth spurt occurs, for those who mature late, it takes off from a higher plateau. The epiphyseal plates close more slowly in males than in females, and therefore the cutoff in growth that accompanies the attainment of sexual maturity is also more complete in girls.
The timing of puberty seems to be affected by both genetic and environmental influences. There are early- and late-maturing families, and individuals in some racial and ethnic groups mature earlier than others. As Figure 4-4 shows, Dutch boys are about 5 cm taller than their American counterparts at age 10, and it is likely that both heredity and environment play a role in producing that considerable difference. In girls, it appears that the onset of menstruation requires the development of a certain amount of body fat. In girls of a slender body type, the onset of menstruation can be delayed until this level is reached. Athletic girls with low body fat often are slow to begin their menstrual periods, and highly trained female athletes whose body fat levels are quite low may stop menstruating, apparently in response to the low body fat levels.
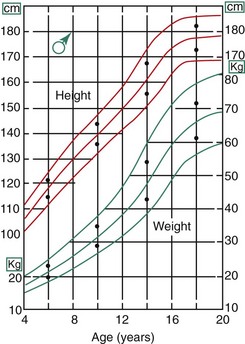
FIGURE 4-4 Height and weight curves for boys in the United States, showing means ± 2 standard deviations. Note the black dots on the graphs at ages 6, 10, 14, and 16. The upper dot shows median height and weight for boys in the Netherlands, the lower one shows median height and weight for boys in the United States. Note that at all ages, the Dutch boys are larger and heavier than their U.S. counterparts—at age 10 the height difference is nearly 5 cm (2 inches). This is a dramatic illustration of how growth is affected by racial, ethnic, national, and other variables.
Seasonal and cultural factors also can affect the overall rate of physical growth. For example, everything else being equal, growth tends to be faster in spring and summer than in fall and winter, and city children tend to mature faster than rural ones, especially in less developed countries. Such effects presumably are mediated via the hypothalamus and indicate that the rate of secretion of gonadotropin-releasing factors can be influenced by external stimuli.
In the description above, the stages of adolescent development were correlated with growth in height. Fortunately, growth of the jaws usually correlates with the physiologic events of puberty in about the same way as growth in height (Figure 4-5). There is an adolescent growth spurt in the length of the mandible, though not nearly as dramatic a spurt as that in body height, and a modest though discernible increase in growth at the sutures of the maxilla. The cephalocaudal gradient of growth, which is part of the normal pattern, is dramatically evident at puberty. More growth occurs in the lower extremity than in the upper, and within the face, more growth takes place in the lower jaw than in the upper. This produces an acceleration in mandibular growth relative to the maxilla and results in the differential jaw growth referred to previously. The maturing face becomes less convex as the mandible and chin become more prominent as a result of the differential jaw growth.
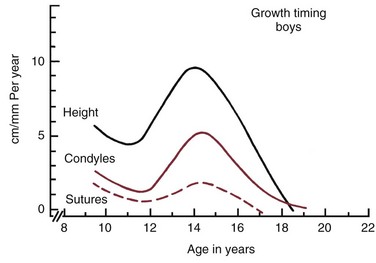
FIGURE 4-5 On average, the adolescent spurt in growth of the jaws occurs at about the same time as the spurt in height, but it must be remembered that there is considerable individual variation. (Data from Woodside DG. In: Salzmann JA, ed. Orthodontics in Daily Practice. Philadelphia: JB Lippincott; 1974.)
Although jaw growth follows the curve for general body growth, the correlation is not perfect. Longitudinal data from studies of craniofacial growth indicate that a significant number of individuals, especially among the girls, have a “juvenile acceleration” in jaw growth that occurs 1 to 2 years before the adolescent growth spurt (Figure 4-6).1 This juvenile acceleration can equal or even exceed the jaw growth that accompanies secondary sexual maturation. In boys, if a juvenile spurt occurs, it is nearly always less intense than the growth acceleration at puberty.
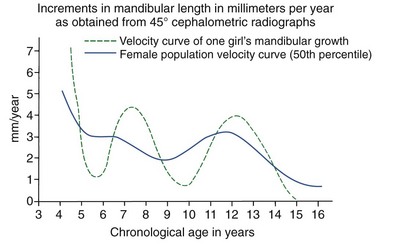
FIGURE 4-6 Longitudinal data for increase in length of the mandible in one girl, taken from the Burlington growth study in Canada, demonstrates an acceleration of growth at about 8 years of age (juvenile acceleration) equal in intensity to the pubertal acceleration between ages 11 and 14. Changes of this type in the pattern of growth for individuals tend to be smoothed out when cross-sectional or group average data are studied. (From Woodside DG. In: Salzmann JA, ed. Orthodontics in Daily Practice. Philadelphia: JB Lippincott; 1974.)
Recent research has shown that sexual development really begins much earlier than previously thought.2 Sex hormones produced by the adrenal glands first appear at age 6 in both sexes, primarily in the form of a weak androgen (dehydroepiandrosterone [DHEA]). This activation of the adrenal component of the system is referred to as adrenarche. DHEA reaches a critical level at about age 10 that correlates with the initiation of sexual attraction. It is likely that a juvenile acceleration in growth is related to the intensity of adrenarche and not surprising that a juvenile acceleration is more prominent in girls because of the greater adrenal component of their early sexual development.
This tendency for a clinically useful acceleration in jaw growth to precede the adolescent spurt, particularly in girls, is a major reason for careful assessment of physiologic age in planning orthodontic treatment. If treatment is delayed too long, the opportunity to utilize the growth spurt is missed. In early-maturing girls, the adolescent growth spurt often precedes the final transition of the dentition, so that by the time the second premolars and second molars erupt, physical growth is all but complete. The presence of a juvenile growth spurt in girls accentuates this tendency for significant acceleration of jaw growth in the mixed dentition. For many girls, if they are to receive orthodontic treatment while they are growing rapidly, the treatment must begin during the mixed dentition rather than after all succedaneous teeth have erupted.
In slow-maturing boys, on the other hand, the dentition can be relatively complete while a considerable amount of physical growth remains. In the timing of orthodontic treatment, clinicians have a tendency to treat girls too late and boys too soon, forgetting the considerable disparity in the rate of physiologic maturation.
Growth Patterns in the Dentofacial Complex
Growth of the Nasomaxillary Complex
As we have noted in the preceding chapters, growth of the nasomaxillary area is produced by two basic mechanisms: (1) passive displacement, created by growth in the cranial base that pushes the maxilla forward, and (2) active growth of the maxillary structures and nose (Figure 4-7). Because the push from behind decreases greatly as the cranial base synchondroses close at about age 7, most of the growth after that time (i.e., during the time period when most orthodontic treatment is done) is due to active growth at the maxillary sutures and surfaces.
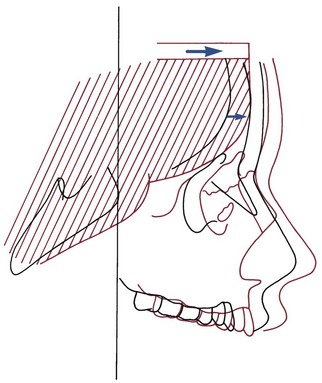
FIGURE 4-7 Diagrammatic representation of a major mechanism for growth of the maxilla: Structures of the nasomaxillary complex are displaced forward as the cranial base lengthens and the anterior lobes of the brain grow in size. (Redrawn from Enlow DH, Hans MG. Essentials of Facial Growth. Philadelphia: WB Saunders; 1996.)
The effect of surface remodeling must be taken into account when active growth of the maxilla is considered. Surface changes can either add to or subtract from growth at the sutures by surface apposition or resorption, respectively. In fact, the maxilla grows downward and forward as bone is added in the tuberosity area posteriorly and at the posterior and superior sutures, but the anterior surfaces of the bone are resorbing at the same time (Figure 4-8). For this reason, the distance that the body of the maxilla and the maxillary teeth are carried downward and forward during growth is greater by about 25% than the forward movement of the anterior surface of the maxilla. This amount of surface remodeling that conceals the extent of relocation of the jaws is even more prominent when rotation of the maxilla during growth is considered (see the following sections).
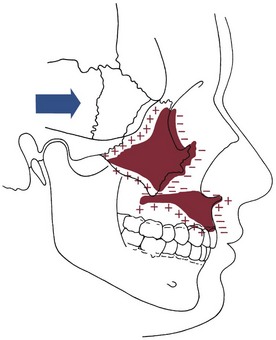
FIGURE 4-8 As the maxilla is translated downward and forward, bone is added at the sutures and in the tuberosity area posteriorly, but at the same time, surface remodeling removes bone from the anterior surfaces (except for a small area at the anterior nasal spine). For this reason, the amount of forward movement of anterior surfaces is less than the amount of displacement. In the roof of the mouth, however, surface remodeling adds bone, while bone is resorbed from the floor of the nose. The total downward movement of the palatal vault, therefore, is greater than the amount of displacement. (Redrawn from Enlow DH, Hans MG. Essentials of Facial Growth. Philadelphia: WB Saunders; 1996.)
The nasal structures undergo the same passive displacement as the rest of the maxilla. However, the nose grows more rapidly than the rest of the face, particularly during the adolescent growth spurt. Nasal growth is produced in part by an increase in size of the cartilaginous nasal septum. In addition, proliferation of the lateral cartilages alters the shape of the nose and contributes to an increase in overall size. On average, nasal dimensions increase at a rate about 25% greater than growth of the maxilla during adolescence, but growth of the nose is extremely variable, as a cursory examination of any group of people will confirm.
Mandibular Growth
Growth of the mandible continues at a relatively steady rate before puberty. On the average, as Table 4-1 shows, ramus height increases 1 to 2 mm per year and body length increases 2 to 3 mm per year. These cross-sectional data tend to smooth out the juvenile and pubertal growth spurts, which do occur in growth of the mandible (see previous discussion).
TABLE 4-1
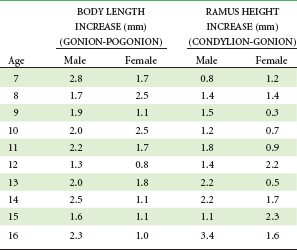
Data from Riolo ML, et al. An Atlas of Craniofacial Growth. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development; 1974.
One feature of mandibular growth is an accentuation of the prominence of the chin. At one time, it was thought that this occurred primarily by addition of bone to the chin, but that is incorrect. Although small amounts of bone are added, the change in the contour of the chin itself occurs largely because the area just above the chin, between it and the base of the alveolar process, is a resorptive area. The increase in chin prominence with maturity results from a combination of forward translation of the chin as a part of the overall growth pattern of the mandible and resorption above the chin that alters the bony contours.
An important source of variability in how much the chin grows forward is the extent of growth changes at the glenoid fossa. If the area of the temporal bone to which the mandible is attached moved forward relative to the cranial base during growth, this would translate the mandible forward in the same way that cranial base growth translates the maxilla. However, this rarely happens. Usually, the attachment point moves straight down, so that there is no anteroposterior displacement of the mandible, but occasionally it moves posteriorly, thus subtracting from rather than augmenting the forward projection of the chin.3 In both the patients shown in Figure 4-9, for instance, there was an approximate 7 mm increase in length of the mandible during orthodontic treatment around the time of puberty. In one of the patients, the temporomandibular (TM) joint did not relocate during growth and the chin projected forward 7 mm. In the other patient, the TM joint moved posteriorly, resulting in only a small forward projection of the chin despite the increase in mandibular length.
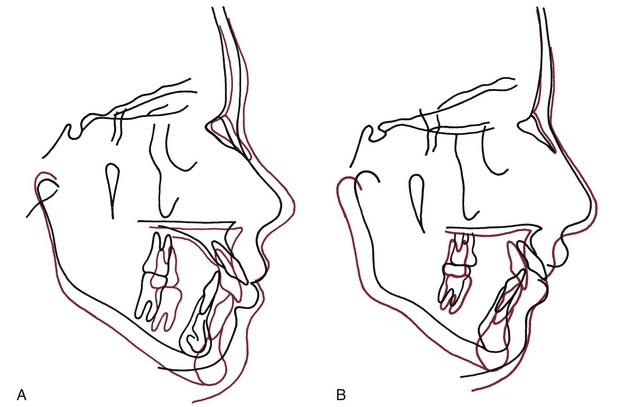
FIGURE 4-9 Cephalometric tracings showing growth in two patients during the orthodontic correction of moderate Class II malocclusion (superimposed on sphenoethmoid triad in cranial base). A, Changes from age 11 years 10 months to age 14 years 11 months. In this patient, approximately 7 mm of mandibular growth was expressed entirely as forward movement of the chin, while the area of the temporomandibular (TM) joint remained in the same anteroposterior position relative to the cranial base. B, Changes in another patient from age 11 years 8 months to age 15 years 0 months. This patient also had approximately 7 mm of mandibular growth, but the TM joint area moved downward and backward relative to the cranial base, so that much of the growth was not expressed as forward movement of the chin. (Courtesy Dr. V. Kokich.)
Timing of Growth in Width, Length, and Height
For the three planes of space in both the maxilla and mandible, there is a definite sequence in which growth is “completed” (i.e., declines to the slow rate that characterizes normal adults). Growth in width is completed first, then growth in length, and finally growth in height.
Growth in width of both jaws, including the width of the dental arches, tends to be completed before the adolescent growth spurt and is affected minimally if at all by adolescent growth changes (Figure 4-10). For instance, intercanine width is more likely to decrease than increase after age 12.4 There is a partial exception to this rule, however. As the jaws grow in length posteriorly, they also grow wider. For the maxilla, this affects primarily the width across the second molars, and if they are able to erupt, the third molars in the region of the tuberosity as well. For the mandible, both molar and bicondylar widths show small increases until the end of growth in length. Anterior width dimensions of the mandible stabilize earlier.
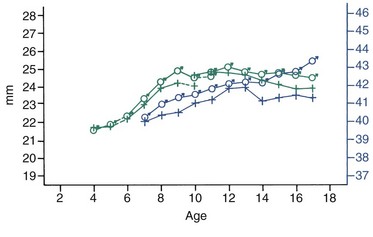
FIGURE 4-10 Average changes in mandibular canine and molar widths in both sexes during growth. Molar widths are shown in blue, canine widths in green. (From Moyers RE, et al. Standards of Human Occlusal Development. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development; 1976.)
Growth in length and height of both jaws continues through the period of puberty. In girls, the maxilla grows slowly downward and forward to age 14 to 15 on the average (more accurately, by 2 to 3 years after first menstruation), then tends to grow slightly more almost straight forward (Figure 4-11).5 In both sexes, growth in vertical height of the face continues longer than growth in length, with the late vertical growth primarily in the mandible. Increases in facial height and concomitant eruption of teeth continue throughout life, but the decline to the adult level (which for vertical growth is surprisingly large [see the following section]) often does not occur until the early twenties in boys, somewhat earlier in girls.
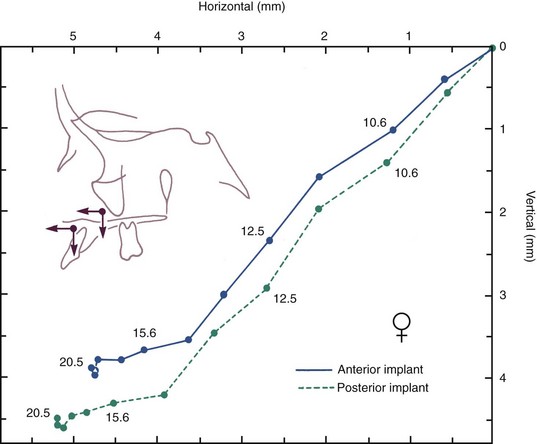
FIGURE 4-11 Mean growth tracks of anterior and posterior maxillary implants relative to the cranial base and its perpendicular, in a group of Danish girls. The two tracks are shown with their origins superimposed to facilitate comparison. Note that the posterior implant moves down and forward more than the anterior one, with growth continuing into the late teens at a slow rate. (Courtesy Dr. B. Solow.)
Rotation of Jaws During Growth
Implant Studies of Jaw Rotation
Until longitudinal studies of growth using metallic implants in the jaws were carried out in the 1960s, primarily by Björk and coworkers in Copenhagen (see Chapter 2), the extent to which both the maxilla and mandible rotate during growth was not appreciated. The reason is that the rotation that occurs in the core of each jaw, called internal rotation, tends to be masked by surface changes and alterations in the rate of tooth eruption. The surface changes produce external rotation. Obviously, the overall change in the orientation of each jaw, as judged by the palatal plane and mandibular plane, results from a combination of internal and external rotation.
The terminology for describing these rotational changes is itself confusing. The descriptive terms used here, in an effort to simplify and clarify a complex and difficult subject, are not those Björk used in the original papers on this subject6 or exactly the same as the Copenhagen group suggested later.7 See Table 4-2 for a comparison of terms.
TABLE 4-2
Terminology: Rotational Changes of the Jaws
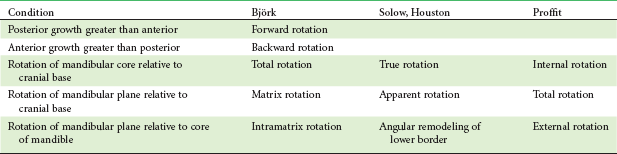
Proffit: Total rotation = internal rotation − external rotation.
Björk: Matrix rotation = total rotation − intramatrix rotation.
Solow: Apparent rotation = true rotation − angular remodeling of lower border.
It is easier to visualize the internal and external rotation of the jaws by considering the mandible first. The core of the mandible is the bone that surrounds the inferior alveolar nerve. The rest of the mandible consists of its several functional processes (Figure 4-12). These are the alveolar process (bone supporting the teeth and providing for mastication), the muscular processes (the bone to which the muscles of mastication attach), and the condylar process, the function in this case being the articulation of the jaw with the skull. If implants are placed in areas of stable bone away from the functional processes, it can be observed that in most individuals, the core of the mandible rotates during growth in a way that would tend to decrease the mandibular plane angle (i.e., up anteriorly and down posteriorly). This can occur either by rotation around the condyle or rotation centered within the body of the mandible (Figure 4-13). By convention, the rotation of either jaw is considered “forward” and given a negative sign if there is more growth posteriorly than anteriorly.8 The rotation is “backward” and given a positive direction if it lengthens anterior dimensions more than posterior ones, bringing the chin downward and backward.
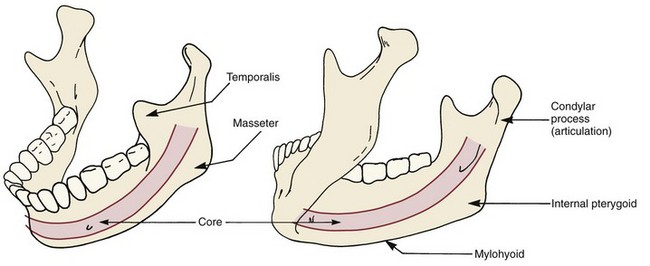
FIGURE 4-12 The mandible can be visualized as consisting of a core of bone surrounding the inferior alveolar neurovascular bundle and a series of functional processes: the alveolar process, serving the function of mastication; the muscular processes, serving as muscle attachments; and the condylar process, serving to articulate the bone with the rest of the skull.
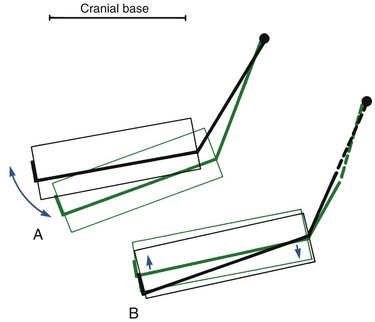
FIGURE 4-13 Internal rotation of the mandible (i.e., rotation of the core relative to the cranial base) has two components: A, Rotation around the condyle, or matrix rotation. B, Rotations centered within the body of the mandible, or intramatrix rotation. (Redrawn from Björk A, Skieller V. Eur J Orthod 5:1-46, 1983.)
One of the features of internal rotation of the mandible is the variation between individuals, ranging up to 10 to 15 degrees. The pattern of vertical facial development, discussed in more detail later, is strongly related to the rotation of both jaws. For an average individual with normal vertical facial proportions, however, there is about a 15-degree internal rotation from age 4 to adult life. Of this, about 25% results from rotation at the condyle and 75% results from rotation within the body of the mandible.
During the time that the core of the mandible rotates forward an average of 15 degrees, the mandibular plane angle, representing the orientation of the jaw to an outside observer, decreases only 2 to 4 degrees on the average. The reason that the internal rotation is not expressed in jaw orientation, of course, is that surface changes (external rotation) tend to compensate. This means that the posterior part of the lower border of the mandible must be an area of resorption, while the anterior aspect of the lower border is unchanged or undergoes slight apposition. Studies of surface changes reveal exactly this as the usual pattern of apposition and resorption (Figure 4-14).
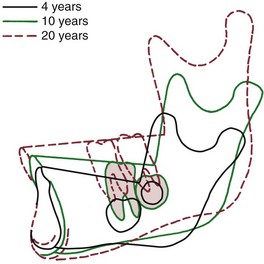
FIGURE 4-14 Superimposition on implants for an individual with a normal pattern of growth, showing surface changes in the mandible from ages 4 to 20 years. For this patient, there was a 19-degree internal rotation but only a 3-degree change in the mandibular plane angle. Note how the dramatic remodeling (external rotation) compensates for and conceals the extent of the internal rotation. (From Björk A, Skieller V. Eur J Orthod 5:1-46, 1983.)
It is less easy to divide the maxilla into a core of bone and a series of functional processes. The alveolar process is certainly a functional process in the classic sense, but there are no areas of muscle attachment analogous to those of the mandible. The parts of the bone surrounding the air passages serve the function of respiration, and the form–function relationships involved are poorly understood. If implants are placed above the maxillary alveolar process, however, one can observe a core of the maxilla that undergoes a small and variable degree of rotation, forward or backward (Figure 4-15).9 This internal rotation is analogous to the rotation within the body of the mandible.
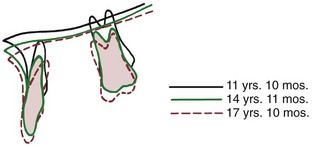
FIGURE 4-15 Superimposition on implants in the maxilla reveals that this patient experienced a small amount of backward internal rotation of the maxilla (i.e., down anteriorly). A small amount of forward rotation is the more usual pattern, but backward rotation occurs frequently. (From Björk A, Skieller V. Am J Orthod 62:357, 1972.)
At the same time that internal rotation of the maxilla is occurring, there also are varying degrees of remodeling of the palate. Similar variations in the amount of eruption of the incisors and molars occur. These changes amount, of course, to an external rotation. For most patients, the external rotation is opposite in direction and equal in magnitude to the internal rotation, so that the two rotations cancel and the net change in jaw orientation (as evaluated by the palatal plane) is zero (see Figure 3-19). Until the implant studies were done, rotation of the maxilla during normal growth had not been suspected.
Although both internal and external rotation occur in everyone, variations from the average pattern are common. Greater or lesser degrees of both internal and external rotation often occur, altering the extent to which external changes compensate for the internal rotation.10 The result is moderate variation in jaw orientation, even in individuals with normal facial proportions. In addition, the rotational patterns of growth are quite different for individuals who have what are called the short face and long face types of vertical facial development.
Individuals of the short face type, who are characterized by short anterior lower face height, have excessive forward rotation of the mandible during growth, resulting from both an increase in the normal internal rotation and a decrease in external compensation. The result is a nearly horizontal palatal plane, a low mandibular plane angle and a large gonial angle (Figure 4-16). A deep bite malocclusion and crowded incisors usually accompany this type of rotation (see following sections).
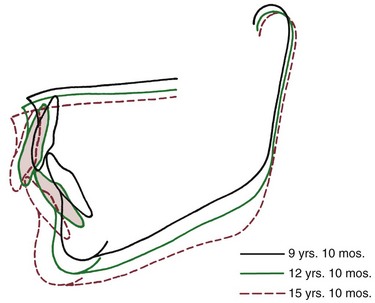
FIGURE 4-16 Cranial base superimposition shows the characteristic pattern of forward mandibular rotation in an individual developing in the “short face” pattern. The forward rotation flattens the mandibular plane and tends to increase overbite. (From Björk A, Skieller V. Am J Orthod 62:344, 1972.)
In long face individuals, who have excessive lower anterior face height, the palatal plane rotates down posteriorly, often creating a negative rather than the normal positive inclination to the true horizontal. The mandible shows an opposite, backward rotation, with an increase in the mandibular plane angle (Figure 4-17). The mandibular changes result primarily from a lack of the normal forward internal rotation or even a backward internal rotation. The internal rotation, in turn, is primarily centered at the condyle. This type of rotation is associated with anterior open bite malocclusion and mandibular deficiency (because the chin rotates back as well as down). Backward rotation of the mandible also occurs in patients with abnormalities or pathologic changes affecting the temporomandibular joints. In these individuals, growth at the condyle is restricted. The interesting result in three cases documented by Björk and Skieller was backward rotation centered in the body of the mandible, rather than the backward rotation at the condyle that is seen in individuals of the classic long face type.11 Jaw orientation changes in both the backward-rotating types, however, are similar, and the same types of malocclusions develop.
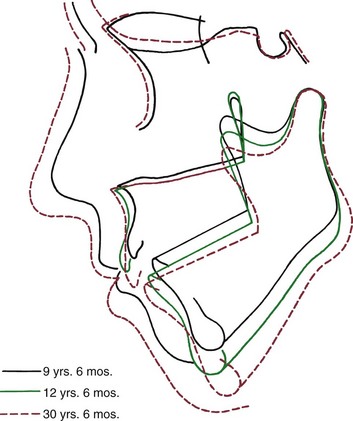
FIGURE 4-17 The pattern of jaw rotation in an individual with the “long face” pattern of growth (cranial base superimposition). As the mandible rotates backward, anterior face height increases, there is a tendency toward anterior open bite, and the incisors are thrust forward relative to the mandible. (From Björk A, Skieller V. Eur J Orthod 5:29, 1983.)
Interaction Between Jaw Rotation and Tooth Eruption
As we have discussed, growth of the mandible away from the maxilla creates a space into which the teeth erupt. The rotational pattern of jaw growth obviously influences the magnitude of tooth eruption. To a surprising extent, it can also influence the direction of eruption and the ultimate anteroposterior position of the incisor teeth.
The path of eruption of the maxillary teeth is downward and somewhat forward (see Figure 4-11). In normal growth, the maxilla usually rotates a few degrees forward but frequently rotates slightly backward. Forward rotation would tend to tip the incisors forward, increasing their prominence, while backward rotation directs the anterior teeth more posteriorly than would have been the case without the rotation, relatively uprighting them and decreasing their prominence. Movement of the teeth relative to the cranial base obviously could be produced by a combination of translocation as the tooth moved along with the jaw in which it was embedded, and true eruption, movement of the tooth within its jaw. As Figure 4-18 shows, translocation contributes about half the total maxillary tooth movement during adolescent growth.
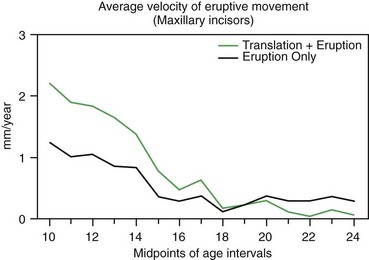
FIGURE 4-18 The average velocity of continued eruption (movement of the incisors relative to implants in the maxilla) and translocation (movement away from the cranial base) of maxillary incisors in Danish girls, from a mixed longitudinal sample. Note that movement of the teeth away from the cranial base is due to a combination of eruption and translocation as the jaw grows, and that small changes due to eruption continue after growth has essentially stopped. (Redrawn from Solow B, Iseri H. Maxillary growth revisited: an update based on recent implant studies. In: Davidovitch Z, Norton LA, eds. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Boston: Harvard Society for Advancement of Orthodontics; 1996.)
The eruption path of mandibular teeth is upward and somewhat forward. The normal internal rotation of the mandible carries the jaw upward in front. This rotation alters the eruption path of the incisors, tending to direct them more posteriorly than would otherwise have been the case (Figure 4-19). Because the internal jaw rotation tends to upright the incisors, the molars migrate further mesially during growth than do the incisors, and this migration is reflected in the decrease in arch length that normally occurs (Figure 4-20). Since the forward internal rotation of the mandible is greater than that of the maxilla, it is not surprising that the normal decrease in mandibular arch length is somewhat greater than the decrease in maxillary arch length.
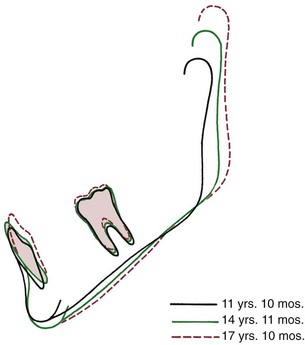
FIGURE 4-19 Superimposition on mandibular implants shows the lingual positioning of the mandibular incisors relative to the mandible that often accompanies forward rotation during growth. (From Björk A, Skieller V. Am J Orthod 62:357, 1972.)
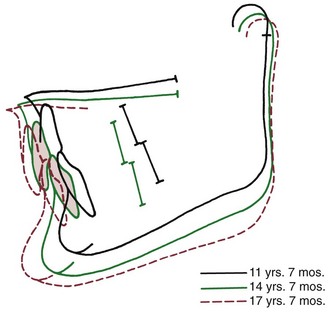
FIGURE 4-20 Cranial base superimposition for a patient with the short face pattern of growth. As the mandible rotates upward and forward, the vertical overlap of the teeth tends to increase, creating a deep bite malocclusion. In addition, even though both the upper and lower teeth do move forward relative to cranial base, lingual displacement of incisors relative to the maxilla and mandible increases the tendency toward crowding. (From Björk A, Skieller V. Am J Orthod 62:355, 1972.)
Note that this explanation for the decrease in arch length that normally occurs in both jaws is different from the traditional interpretation that emphasizes forward migration of the molar teeth. The modern view places relatively greater importance on lingual movement of the incisors and relatively less importance on the forward movement of molars. In fact, the same implant studies that revealed the internal jaw rotation also confirmed that changes in anteroposterior position of the incisor teeth are a major influence on arch length changes.
Given this relationship between jaw rotation and incisor position, it is not surprising that both the vertical and anteroposterior positions of the incisors are affected in short face and long face individuals. When excessive rotation occurs in the short face type of development, the incisors tend to be carried into an overlapping position even if they erupt very little, thus the tendency for deep bite malocclusion in short face individuals (Figure 4-21). The rotation also progressively uprights the incisors, displacing them lingually and causing a tendency toward crowding. In the long face growth pattern, on the other hand, an anterior open bite will develop as anterior face height increases unless the incisors erupt for an extreme distance. The rotation of the jaws also carries the incisors forward, creating dental protrusion.
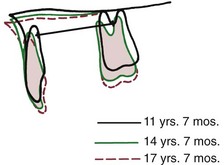
FIGURE 4-21 Superimposition on the maxilla reveals uprighting of the maxillary incisors in the short face growth pattern (same patient as Figure 4-20). This decreases arch length and contributes to progressive crowding. (From Björk A, Skieller V. Am J Orthod 62:355, 1972.)
This interaction between tooth eruption and jaw rotation explains a number of previously puzzling aspects of tooth positioning in patients who have vertical facial disproportions and is a key to understanding the growth pattern in affected individuals.
Maturational and Aging Changes
Maturational changes affect both the hard and soft tissues of the face and jaws as slow growth continues in adult life, with changes in jaw relationships and greater long-term changes in the soft tissues. There are important aging effects on the teeth, their supporting structures, and the dental occlusion itself.
Facial Growth in Adults
Although some anthropologists in the 1930s had reported small amounts of growth continuing into middle age, it was generally assumed until the late twentieth century that growth of the facial skeleton ceased in the late teens or early twenties. In the early 1980s, Behrents12 succeeded in recalling over 100 individuals who had participated in the Bolton growth study in Cleveland in the 1930s and late 1940s, more than 40 years previously. Only a few had ever had orthodontic treatment. While they were participants in the study, the growth of these individuals had been carefully evaluated and recorded, by both measurements and serial cephalometric films. The magnification in the radiographs was known precisely, and it was possible to obtain new radiographs more than 4 decades later with known magnification, so that precise measurements of facial dimensions could be made.
The results were surprising but unequivocal: facial growth had continued during adult life (Figure 4-22). There was an increase in essentially all of the facial dimensions, but both size and shape of the craniofacial complex altered with time. Vertical changes in adult life were more prominent than anteroposterior changes, whereas width changes were least evident, and so the alterations observed in the adult facial skeleton seem to be a continuation of the pattern seen during maturation. In a point of particular interest, an apparent deceleration of growth in females in the late teens was followed by a resumption of growth during the twenties. It appears that a woman’s first pregnancy often produces some growth of her jaws. Although the magnitude of the adult growth changes, assessed on a millimeters per year basis, was quite small, the cumulative effect over decades was surprisingly large (Figure 4-23).
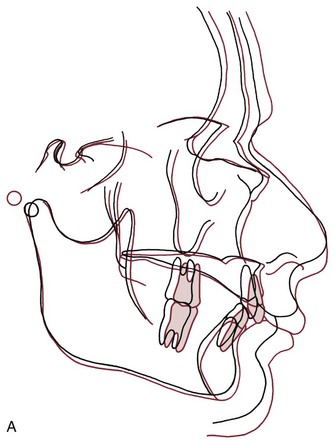
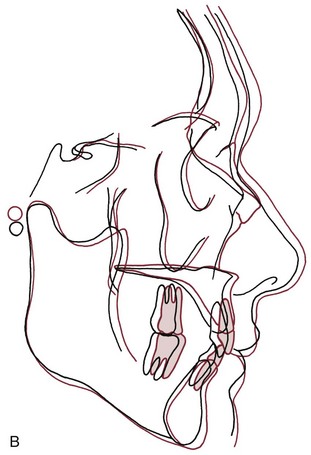
FIGURE 4-22 Growth changes in adults. A, Changes in a male from age 37 (black) to age 77 (red). Note that both the maxilla and mandible grew forward, and the nose grew considerably. B, Growth changes in a woman between age 34 (black) and 83 (red). Note that both jaws grew forward and somewhat downward, and that the nasal structures enlarged. (From Behrents RG. A Treatise on the Continuum of Growth in the Aging Craniofacial Skeleton. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development; 1984.)
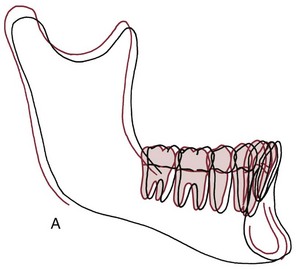
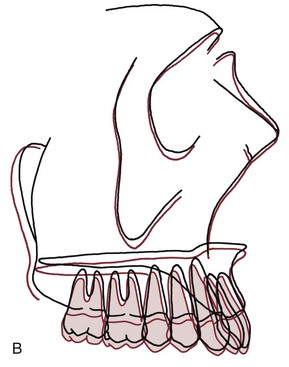
FIGURE 4-23 Growth changes in adults. A, Mean dimensional changes in the mandible for males in adult life. It is apparent that the pattern of juvenile and adolescent growth continues at a slower but ultimately significant rate. B, The mean positional changes in the maxilla during adult life, for both sexes combined. Note that the maxilla moves forward and slightly downward, continuing the previous pattern of growth. (From Behrents RG. A Treatise on the Continuum of Growth in the Aging Craniofacial Skeleton. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development; 1984.)
The data also revealed that rotation of both jaws continued into adult life, in concert with the vertical changes and eruption of teeth. Because implants were not used in these patients, it was not possible to precisely differentiate internal from external rotation, but it seems likely that both internal rotation and surface changes did continue. In general, males showed a net rotation of the jaws in a forward direction, slightly decreasing the mandibular plane angle, whereas females had a tendency toward backward rotation, with an increase in the mandibular plane angle. In both groups, compensatory changes were noted in the dentition, so that occlusal relationships largely were maintained.
Both a history of orthodontic treatment and loss of multiple teeth had an impact on facial morphology in these adults and on the pattern of change. In the smaller group of patients who had orthodontic treatment many years previously, Behrents noted that the pattern of growth associated with the original malocclusion continued to express itself in adult life. This finding is consistent with previous observations of growth in the late teens but also indicates how a gradual worsening of occlusal relationships could occur in some patients long after the completion of orthodontic treatment.
As expected, changes in the facial soft tissue profile were greater than changes in the facial skeleton. The changes involved an elongation of the nose (which often became significantly longer during adult life), flattening of the lips, and an augmentation of the soft tissue chin. A knowledge of soft tissue changes during aging is important in planning modern orthodontic treatment, and this is discussed further in Chapter 6.
In the light of Behrents’ findings, it seems clear viewing facial growth as a process that ends in the late teens or early twenties is not correct. It is correct, however, to view the growth process as one that declines to a basal level after the attainment of sexual maturity, continues to show a cephalocaudal gradient (i.e., more mandibular than maxillary changes in adult life), and affects the three planes of space differently. Growth in width is not only the first to drop to adult levels, usually reaching essential completion by the onset of puberty, but the basal or adult level observed thereafter is quite low.13 Anteroposterior growth continues at a noticeable rate for a longer period, declining to basal levels only after puberty, with small but noticeable changes continuing throughout adult life. Vertical growth, which had previously been observed to continue well after puberty in both males and females, continues at a modest level far into adult life. Although most of the skeletal change occurs between adolescence and mid-adulthood,14 skeletal growth comes much closer to being a process that continues throughout life than most observers had previously suspected.
Changes in Facial Soft Tissues
An important concept is that changes in facial soft tissues not only continue with aging, they are much larger in magnitude than changes in the hard tissues of the face and jaws.
The change of most significance for orthodontists is that the lips, and the other soft tissues of the face, sag downward with aging (Figure 4-24). The result is a decrease in exposure of the upper incisors and an increase in exposure of the lower incisors, both at rest and on smile (Figures 4-25 and 4-26). With aging, the lips also become progressively thinner, with less vermilion display (Figure 4-27). A recent study of individuals followed longitudinally in the Michigan growth study reported that in Americans of European descent, the upper lip lengthened by an average of 3.2 mm and thinned 3.6 mm between adolescence and mid-adulthood. This continued until late adulthood, with a further average mean lengthening and thinning of 1.4 mm.14
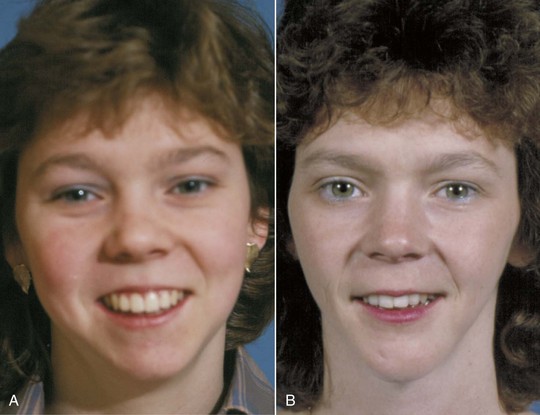
FIGURE 4-24 Maxillary incisor exposure on smile at age 15 (A) and age 25 (B). An important characteristic of facial aging is the downward movement of the lips relative to the teeth, so that the maxillary incisors have progressively decreased exposure over time after adolescent growth is completed. (From Proffit WR, White RP, Sarver DM. Contemporary Treatment of Dentofacial Deformity. St. Louis: Mosby; 2003.)
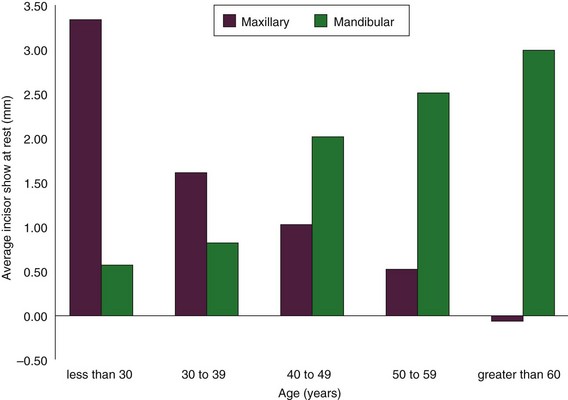
FIGURE 4-25 Incisor display at rest as a function of age. With aging, both men and women show less of their upper incisors and more of their lower incisors, so display of upper incisors is a youthful characteristic. (Redrawn from Vig RG, Brundo GC. Kinetics of anterior tooth display. J Prosthet Dent 39:502-504, 1978.)

FIGURE 4-26 Incisor exposure on smile at the completion of orthodontic treatment at age 30 (A) and 20 years later, at age 50 (B). Note that downward movement of the facial soft tissues continues, so that the lower incisors are seen more prominently with increasing age.
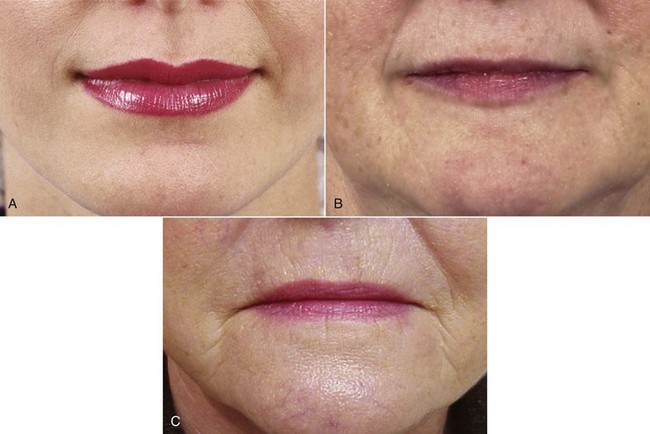
FIGURE 4-27 A decrease in the fullness of the lips is an obvious sign of aging. A, Age 20. B, Age 40. C, Age 70.
Since exposure of all the upper incisors and a small amount of gingiva on smile is both youthful appearing and esthetic, it is important to remember in orthodontic treatment that the vertical relationship of the lip to the teeth will change after adolescence. In fact, leaving the upper incisors somewhat more exposed than the ideal adult relationship is necessary in treatment of an adolescent, if this relationship is to be ideal later in life (Figure 4-28).
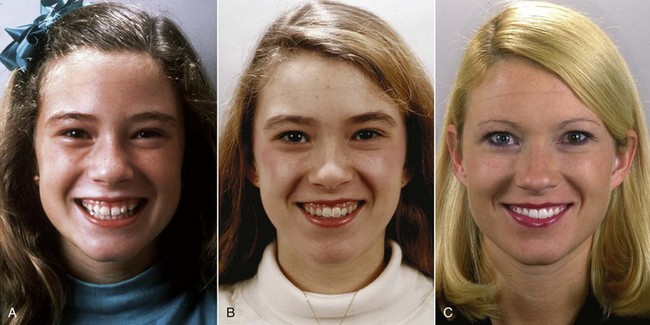
FIGURE 4-28 Because lip height increase and the facial soft tissues move downward relative to the teeth with increasing age, what looks like excessive exposure of teeth and gingiva on smile at age 12 (A) appears to be less excessive at age 14 (B) and has totally disappeared at age 24 (C). She received no treatment between ages 12 and 24.
Changes in Alignment and Occlusion
The alveolar bone bends during heavy mastication, allowing the teeth to move relative to each other (see Chapter 9 for more details). With a coarse diet, not only did occlusal wear reduce the height of the crowns, but also the width of teeth was reduced as interproximal wear occurred. When this type of interproximal wear occurs, spaces do not open up between the posterior teeth, although some spacing may develop anteriorly. Instead, the permanent molars migrate mesially, keeping the contacts reasonably tight even as the contact points are worn off and the mesiodistal width of each tooth decreases. The result in many primitive populations was a reduction in arch circumference of 10 mm or more after completion of the permanent dentition at adolescence.
In modern populations, there is a strong tendency for crowding of the mandibular incisor teeth to develop in the late teens and early twenties, no matter how well aligned the teeth were initially. Mild crowding of the lower incisors tends to develop if the teeth were initially well aligned, or initially mild crowding becomes worse. These changes appear as early as age 17 to 18 in some individuals and as late as the mid-twenties in others. Three major theories to account for this crowding have been proposed:
1. Lack of “normal attrition” in the modern diet. As noted in Chapter 1, primitive populations tend to have a much smaller prevalence of malocclusion than contemporary populations in developed countries. If a shortening of arch length and mesial migration of the permanent molars is a natural phenomenon, it would seem reasonable that crowding would develop unless the amount of tooth structure was reduced during the final stages of growth. Raymond Begg, a pioneer Australian orthodontist, noted in his studies of the Australian aborigines that malocclusion is uncommon but large amounts of interproximal and occlusal attrition occurred (Figure 4-29).15 He concluded that in modern populations the teeth became crowded when attrition did not occur with soft diets, and advocated widespread extraction of premolar teeth to provide the equivalent of the attrition he saw in aborigines. More recent observations have shown that when Australian aborigines change to a modern diet, as they did during the twentieth century, occlusal and interproximal wear all but disappears. Late crowding rarely develops,16 although periodontal disease does become a major problem. It has been observed in other population groups that late crowding may develop even after premolars are extracted and arch length is reduced by modern orthodontic treatment. Thus the Begg theory, though superficially attractive, does not explain late crowding.
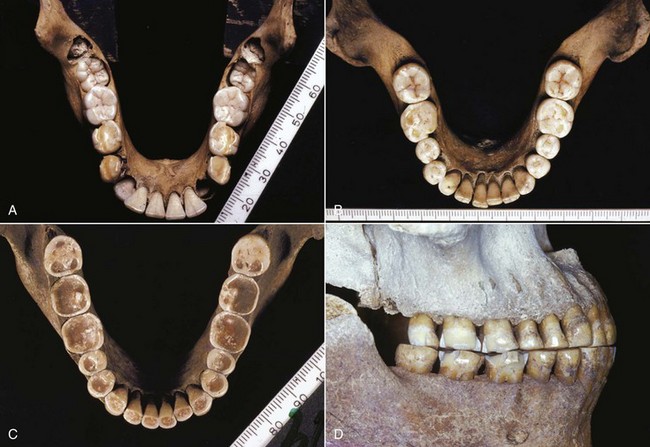
FIGURE 4-29 Australian aboriginal mandibles for a child approximately at dental age 8 (A), an adolescent at approximately dental age 14 (B), and an adult of indeterminate age (C and D). Note the increasing attrition of the teeth in the younger specimens and the severe attrition of the adult’s teeth, with interproximal, as well as occlusal, wear. Arch length in this population shortened by 1 cm or more after adolescence because of the extensive interproximal wear. (Specimens from the Begg Collection, University of Adelaide, Adelaide, Australia; Courtesy Professor W. Sampson.)
2. Pressure from third molars. Late crowding develops at about the time the third molars should erupt. In most individuals, these teeth are hopelessly impacted because the jaw length did not increase enough to accommodate them via backward remodeling of the ramus (Figure 4-30). It has seemed entirely logical to dentists and patients that pressure from third molars with no room to erupt is the cause of late incisor crowding. It is difficult to detect such a force, however, even with modern instrumentation that should have found it if it exists.17 In fact, late crowding of lower incisors can and often does develop in individuals whose lower third molars are congenitally missing. There is some evidence that incisor crowding may be lessened by early removal of second molars, which presumably would relieve pressure from third molars, but pressure from third molars clearly is not the total explanation either.18
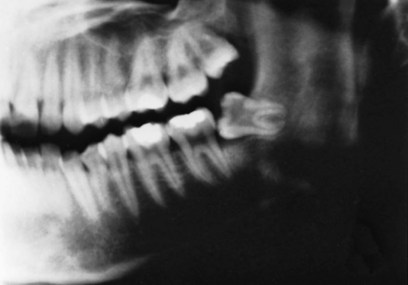
FIGURE 4-30 It seems reasonable that a horizontally impacted third molar would provide pressure against the dental arch, but it is highly unlikely that there is enough pressure from this source to cause the crowding of mandibular incisors that often develops in the late teens.
3. Late mandibular growth. As a result of the cephalocaudal gradient of growth discussed in Chapter 2, the mandible can and does grow more in the late teens than the maxilla. Is it possible that late mandibular growth somehow causes late mandibular incisor crowding? If so, how? Björk’s implant studies have provided an understanding of why late crowding occurs and how it indeed relates to the growth pattern of the jaw.
The position of the dentition relative to the maxilla and mandible is influenced by the pattern of growth of the jaws, a concept explored in some detail in previous sections. When the mandible grows forward relative to the maxilla, as it usually does in the late teens, the mandibular incisor teeth tend to be displaced lingually, particularly if forward rotation is also present (as it would be in short face individuals). This can be seen most clearly when the mandibular growth is excessive (Figure 4-31), but a milder version of the same uprighting occurs in almost everyone.
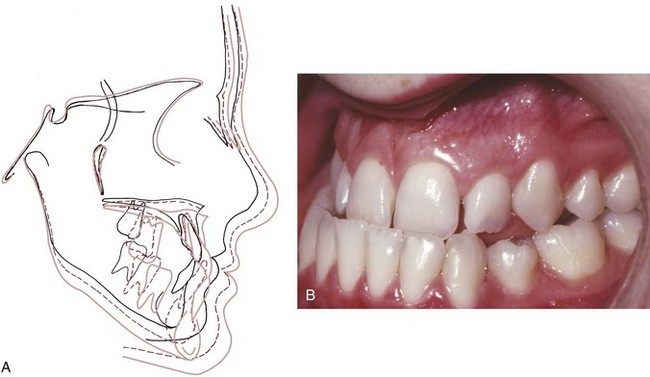
FIGURE 4-31 In this patient with a prolonged pattern of excessive mandibular growth (A), the lower incisors were increasingly tipped lingually as the mandible grew forward and were noticeably retroclined (B) by the end of adolescent growth. This is a more obvious demonstration of what often happens under normal circumstances, when a small amount of late mandibular growth occurs in the late teens after maxillary growth stops. Late mandibular growth is a major cause of the mandibular incisor crowding that frequently develops at that time.
In patients with a tight anterior occlusion before late mandibular growth occurs, the contact relationship of the lower incisors with the upper incisors must change if the mandible grows forward. In that circumstance, one of three things must happen: (1) the mandible is displaced distally, accompanied by a distortion of temporomandibular joint function and displacement of the articular disc; (2) the upper incisors flare forward, opening space between these teeth; or (3) the lower incisors displace distally and become crowded.
All three of these phenomena have been reported. The second response, flaring and spacing of the maxillary incisors, is rarely seen. Posterior displacement of a “trapped mandible” can happen and may occasionally be related to myofascial pain and dysfunction, but despite the claims of some occlusion theorists, this too seems to be quite rare. Distal displacement of the lower incisors, with concomitant crowding and a decrease in the lower intercanine distance, is the usual response.
It is not even necessary for the incisors to be in occlusal contact for late crowding to develop. This also occurs commonly in individuals who have an anterior open bite and backward, not forward, rotation of the mandible (see Figure 4-20). In this situation, the rotation of the mandible carries the dentition forward, thrusting the incisors against the lip. This creates light but lasting pressure by the lip, which tends to reposition the protruding incisors somewhat lingually, reducing arch length and causing crowding.
The current concept is that late incisor crowding almost always develops as the mandibular incisors (and perhaps the entire mandibular dentition) move distally relative to the body of the mandible late in mandibular growth. This sheds some light on the possible role of the third molars in determining whether crowding will occur and how severe it will be. If space were available at the distal end of the mandibular arch, it might be possible for all the mandibular teeth to shift slightly distally, allowing the lower incisors to upright without becoming crowded. But impacted third molars at the distal end of the lower arch would prevent the posterior teeth from shifting distally, and if differential mandibular growth occurred, their presence might guarantee that crowding would develop. In this case, the lower third molars could be the “last straw” in a chain of events that led to late incisor crowding. As noted previously, however, late incisor crowding occurs in individuals with no third molars at all, so the presence of these teeth is not the critical variable. Instead, the extent of late mandibular growth is. The more your mandible grows after other growth has essentially stopped, the greater the chance your lower incisors will become crowded, and this is true both in those who had orthodontic treatment and those who did not.19
Aging Changes in Teeth and Supporting Structures
At the time a permanent tooth erupts, the pulp chamber is relatively large. As time passes, additional dentin slowly deposits on the inside of the tooth, so that the pulp chamber gradually becomes smaller with increasing age (Figure 4-32). This process continues relatively rapidly until the late teens, at which time the pulp chamber of a typical permanent tooth is about half the size that it was at the time of initial eruption. Because of the relatively large pulp chambers of young permanent teeth, complex restorative procedures are more likely to result in mechanical exposures in adolescents than in adults. Additional dentin continues to be produced at a slower rate throughout life, so in old age the pulp chambers of some permanent teeth are all but obliterated.
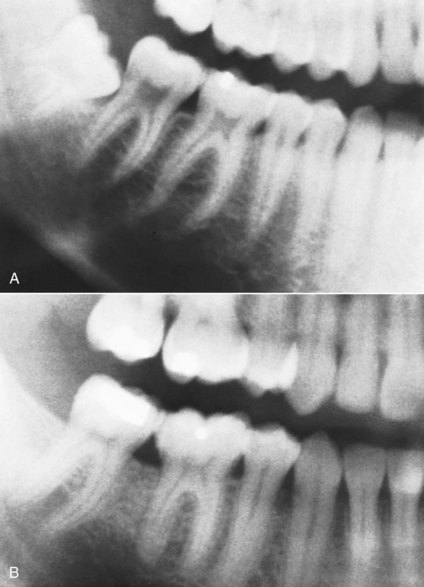
FIGURE 4-32 The size of the pulp chambers of permanent teeth decreases during adolescence then continues to fill in more slowly for the rest of adult life. A, Age 16. B, Age 26.
Maturation also brings about greater exposure of the tooth outside its investing soft tissues. At the time a permanent first molar erupts, the gingival attachment is high on the crown. Typically, the gingival attachment is still well above the cementoenamel junction when any permanent tooth comes into full occlusion, and during the next few years more and more of the crown is exposed. This relative apical movement of the attachment (in normal circumstances) results more from vertical growth of the jaws and the accompanying eruption of the teeth than from downward migration of the gingival attachment. As we have noted previously, vertical growth of the jaws and an increase in face height continue after transverse and anteroposterior growth have been completed. By the time the jaws all but stop growing vertically in the late teens, the gingival attachment is usually near the cementoenamel junction. In the absence of inflammation, mechanical abrasion, or pathologic changes, the gingival attachment should remain at about the same level almost indefinitely. In fact, however, most individuals experience some pathology of the gingiva or periodontium as they age, and so further recession of the gingiva is common.
At one time, it was thought that “passive eruption” (defined as an actual gingival migration of the attachment without any eruption of the tooth) occurred. It now appears that as long as the gingival tissues are entirely healthy, this sort of downward migration of the soft tissue attachment does not occur. What was once thought to be apical migration of the gingiva during the teens is really active eruption, compensating for the vertical jaw growth still occurring at that time (Figure 4-33).
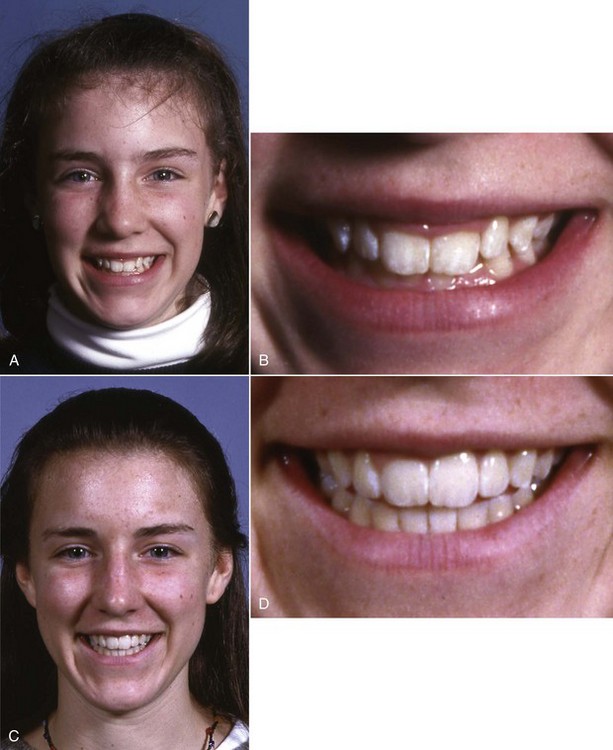
FIGURE 4-33 The increasing crown height of permanent teeth during adolescence was once thought to result from a downward migration of the gingival attachment but now is recognized to occur mostly from tooth eruption in response to vertical growth. A and B, Age 10. C and D, Age 16.
Both occlusal and interproximal wear, often to a severe degree, occurred in primitive people eating an extremely coarse diet. The elimination of most coarse particles from modern diets has also largely eliminated wear of this type. With few exceptions (tobacco chewing is one), wear facets on the teeth now indicate bruxism, not what the individual has been eating.
References
1. Anderson, DL, Thompson, GW, Popovich, F. Interrelationship of dental maturity, skeletal maturity, height and weight from age 4 to 14 years. Growth. 1975;39:453–462.
2. Sisk, CL, Foster, DL. The neural basis of puberty and adolescence. Nature Neurosci. 2004;7:1040–1047.
3. Agronin, KJ, Kokich, VG. Displacement of the glenoid fossa: a cephalometric evaluation of growth during treatment. Am J Orthod Dentofac Orthop. 1987;91:42–48.
4. Bishara, SE, Jakobsen, JR, Treder, J, et al. Arch width changes from 6 weeks to 45 years of age. Am J Orthod Dentofac Orthop. 1997;111:401–409.
5. Solow, B, Iseri, H. Maxillary growth revisited: an update based on recent implant studies. In: Davidovitch Z, Norton LA, eds. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Boston: Harvard Society for Advancement of Orthodontics, 1996.
6. Björk, A. The use of metallic implants in the study of facial growth in children: method and application. Am J Phys Anthropol. 1968;29:243–254.
7. Solow, B, Houston, WJ. Mandibular rotations: concept and terminology. Eur J Orthod. 1988;10:177–179.
8. Björk, A, Skieller, V. Normal and abnormal growth of the mandible: a synthesis of longitudinal cephalometric implant studies over a period of 25 years. Eur J Orthod. 1983;5:1–46.
9. Björk, A, Skieller, V. Postnatal growth and development of the maxillary complex. In: McNamara JA, ed. Factors Affecting Growth of the Midface. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development, 1976.
10. Houston, WJ. Mandibular growth rotations—their mechanisms and importance. Eur J Orthod. 1988;10:369–373.
11. Björk, A, Skieller, V. Contrasting mandibular growth and facial development in long face syndrome, juvenile rheumatoid arthritis and mandibulofacial dysostosis. J Craniofac Genet Dev Biol. 1985;1(suppl):127–138.
12. Behrents, RG. A Treatise on the Continuum of Growth in the Aging Craniofacial Skeleton. Ann Arbor, Mich: University of Michigan Center for Human Growth and Development; 1984.
13. Harris, EF. A longitudinal study of arch size and form in untreated adults. Am J Orthod Dentofac Orthop. 1997;111:419–427.
14. Pecora, NG, Beccetti, T, McNamara, JA, Jr. The aging craniofacial complex: a longitudinal cephalometric study from late adolescence to late adulthood. Am J Orthod Dentofac Orthop. 2008;134:496–506.
15. Begg, PR. Stone age man’s dentition. Am J Orthod. 1954;40:298–312. [373-383, 462-475, 517-531].
16. Corruccini, RS. Australian aboriginal tooth succession, interproximal attrition and Begg’s theory. Am J Orthod Dentofac Orthop. 1990;97:349–357.
17. Southard, TE, Southard, KA, Weeda, LW. Mesial force from unerupted third molars. Am J Orthod Dentofac Orthop. 1991;99:220–225.
18. Richardson, ME. Late lower arch crowding: the aetiology reviewed. Dent Update. 2002;29:234–238.
19. Jonsson, T, Magnusson, TE. Crowding and spacing in the dental arches: long-term development in treated and untreated subjects. Am J Orthod Dentofac Orthop. 2010;138:384.e1–384.e7. [discussion 384-386].