Immunological tests
Clinical and laboratory tests of an immunological nature are valuable in the diagnosis and management of certain skin diseases. Patch tests are helpful in the investigation of contact dermatitis, serum immunoglobulin (Ig) E tests or prick tests are sometimes of use in atopic disease, and immunofluorescent studies on biopsied skin (or with serum) are essential in the diagnosis of bullous disorders and in some other conditions such as connective tissue diseases (e.g. lupus erythematosus) or vasculitis.
Skin prick tests
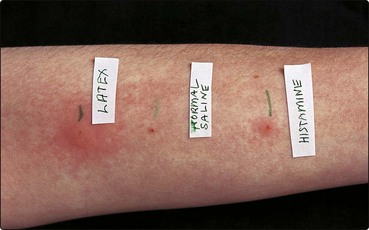
Skin prick testing detects immediate (type I) hypersensitivity. The reaction is mediated by the antigen-triggered IgE-mediated release of vasoactive substances from skin mast cells (p. 11). Small drops of commercially prepared antigen solutions are placed on marked areas on the forearm and lightly pricked into the skin using separate blunt lancets. The stratum corneum of the epidermis is punctured by gently pressing the blunt lancet perpendicular to the skin surface through the test solution. It is important to develop a prick testing technique that is reproducible. Food allergens are often tested by pricking the fresh food and then the skin (prick to prick testing). The sites are inspected at 15 min, and a positive result is regarded, by convention, as one showing a wheal of 3 mm or greater (Fig. 1). Patients should have stopped antihistamines 48 h before the test. Prick tests are used to demonstrate allergy to aeroallergens (e.g. house dust mite) or to foods (e.g. hen’s egg or peanut), and contact urticaria to latex (p. 120). A positive test correlates well with a positive allergen-specific IgE test (usually an enzyme-linked immunosorbent assay (ELISA); the radioallergosorbent test (RAST) is no longer used). The risk of anaphylaxis is very small, but resuscitation facilities, including adrenaline (epinephrine) for intramuscular injection, antihistamines and oxygen are mandatory.
Patch testing
The epicutaneous patch test detects cell-mediated (type IV) hypersensitivity (p. 11). It is very helpful in the investigation of contact dermatitis. Commercially prepared allergens are available in the correct concentration for testing, usually in petrolatum (or sometimes water) as a diluent. Details of the procedure are shown in Figure 2.
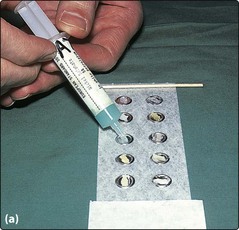
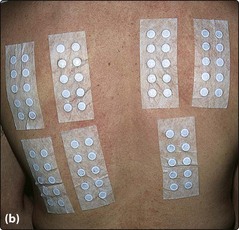
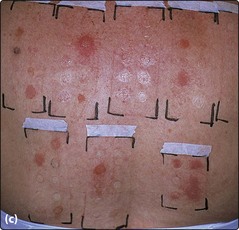
Fig. 2 Patch testing methodology.
Small amounts of the test substances are applied to the 8-mm-diameter aluminium discs on adhesive tape (Finn chambers) that are used for patch testing. The exact selection of test substances depends on the clinical problem, the site of the dermatitis, the environmental contacts and the patient’s occupation.
A ‘standard series’ of 38 substances is applied to every patient, with additional allergens as necessary. A record sheet is kept. The patches are fixed to the upper back, left on for 2 days and then removed after marking the top margin of each text strip with adhesive tape or with a marker pen.
Numerous allergic-positive patch test sites are shown. A positive allergic response is manifest by a localized eczema reaction, which is scored according to the following convention:
?+ doubtful: faint erythema only
+ weak: erythema, maybe papules
++ strong: vesicles, infiltration
IR irritant (of various types, but often showing a glazed circumscribed area, frequently with increased skin markings).
The test sites are read for a second time at 4 days, as positive reactions commonly do not appear until this time. The results are interpreted in the light of the clinical situation: a positive reaction is not always relevant to the current skin problem. Common allergens and their sources are shown on page 34.
Immunofluorescence
Immunofluorescence, either direct (on the patient’s skin) or indirect (using the patient’s serum reacted with an animal substrate) (Fig. 3), is helpful in making a diagnosis in the autoimmune blistering diseases (Table 1). Bullous disorders such as pemphigoid (Fig. 4) and pemphigus (Fig. 5, p. 78) are characterized by the deposition of organ-specific autoantibodies (usually IgG) in the skin and, less easily demonstrated, by the presence of these autoantibodies in the serum. Dermatitis herpetiformis (Fig. 6) and other conditions such as leucocytoclastic vasculitis or lupus erythematosus often show the deposition of immunoglobulin or complement components in the patient’s skin (negative indirect immunofluorescence).
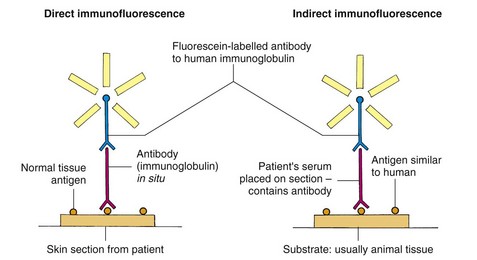
In direct immunofluorescence, usually done on perilesional skin, the antibodies or complement components are detected by reacting the freshly cut skin sections with an antibody directed against the specific immunoglobulin or complement fraction and labelled with a fluorescent marker, which is visualized with a fluorescence microscope. The indirect method is a two-step procedure that involves the use of cut sections of an animal substrate (e.g. monkey oesophagus) or human skin. The patient’s diluted serum (containing the putative antibody) is placed on this section, incubated for an hour or so and then revealed using a fluorescein-tagged antihuman immunoglobulin antibody that is demonstrated by examining with ultraviolet radiation. Human skin, split at the dermoepidermal junction by saline incubation, may be used as a substrate to distinguish variants of pemphigoid, when deposition of the antibody on the epidermal or dermal side of the split can be diagnostic.
Table 1 Immunofluorescence in bullous disease
| Bullous disorder | Direct immunofluorescence (skin) | Indirect immunofluorescence (serum) |
|---|---|---|
| Bullous pemphigoid | Linear IgG/C3 at BMZ in 80% | Linear IgG at BMZ in 75% (IgA/IgM in 25%) |
| Pemphigus vulgaris | Intercellular epidermal IgG/C3 in 100% (IgA/IgM in 20%) | Intercellular IgG in 80% (the antibody titre reflects disease activity) |
| Dermatitis herpetiformis | Granular IgA deposition at dermal papilla (100%) | Absent |
| Linear IgA disease | Linear IgA at BMZ in 80% (IgG/IgM/C3 in 10%) | Linear IgA at BMZ found in some cases |
BMZ, basement membrane zone.
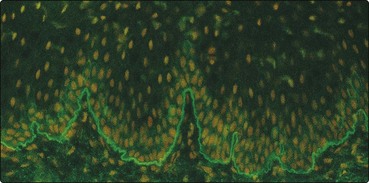
Indirect immunofluorescence demonstrates a linear band of IgG antibodies along the basement membrane zone (BMZ) using monkey oesophagus as substrate. These antibodies are directed against bullous pemphigoid antigens (MW 230 and 180 kDa), proteins located within the adhesion complex of the hemidesmosomes and synthesized by basal keratinocytes.
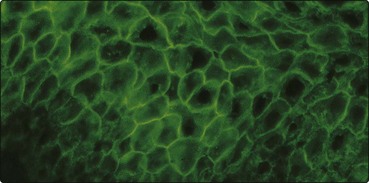
Direct immunofluorescence demonstrates IgG antibodies, directed against desmoglein 3 (MW 130 kDa), a desmosomal cadherin involved in mediating epidermal intercellular adhesion, showing up in a chicken-wire pattern throughout the epidermis.
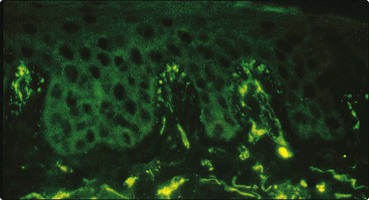
Fig. 6 Dermatitis herpetiformis.
Direct immunofluorescence reveals the deposition of IgA in a granular pattern at the dermal papillae. This is diagnostic for dermatitis herpetiformis (p. 78), although it is unlikely that the eruption is solely due to the presence of this IgA.
Immunological tests
 Prick tests reveal type I (IgE-mediated) hypersensitivity and can demonstrate airborne (e.g. house dust mite), food and latex allergies.
Prick tests reveal type I (IgE-mediated) hypersensitivity and can demonstrate airborne (e.g. house dust mite), food and latex allergies.
 Patch tests detect type IV (cell-mediated) hypersensitivity and are helpful in investigating contact dermatitis, e.g. in hand eczema.
Patch tests detect type IV (cell-mediated) hypersensitivity and are helpful in investigating contact dermatitis, e.g. in hand eczema.
 Direct immunofluorescence demonstrates immunoglobulin and complement deposition in the skin and is very useful in the diagnosis of bullous disorders, e.g. pemphigoid or pemphigus.
Direct immunofluorescence demonstrates immunoglobulin and complement deposition in the skin and is very useful in the diagnosis of bullous disorders, e.g. pemphigoid or pemphigus.
 Indirect immunofluorescence uses an animal substrate to detect antibodies in a patient’s serum. It is often positive in pemphigus and pemphigoid, and sometimes in linear IgA disease, but is negative in dermatitis herpetiformis.
Indirect immunofluorescence uses an animal substrate to detect antibodies in a patient’s serum. It is often positive in pemphigus and pemphigoid, and sometimes in linear IgA disease, but is negative in dermatitis herpetiformis.
 Autoantibodies in bullous pemphigoid (BP) are against BP antigens of molecular weight (MW) 230 and 180 kDa and, in pemphigus vulgaris, against desmoglein 3 antigen (MW 130 kDa). The autoantigen, if any, in dermatitis herpetiformis is not currently known.
Autoantibodies in bullous pemphigoid (BP) are against BP antigens of molecular weight (MW) 230 and 180 kDa and, in pemphigus vulgaris, against desmoglein 3 antigen (MW 130 kDa). The autoantigen, if any, in dermatitis herpetiformis is not currently known.