CHAPTER 23 Dentifrices
PURPOSE OF A DENTIFRICE
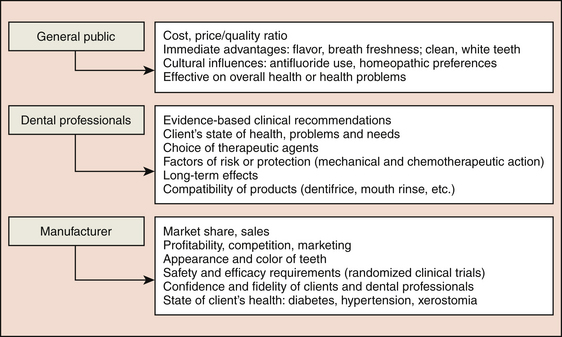
Clients look to the dental hygienist to recommend a daily use dentifrice that will meet the clients’ unique oral health needs. A dentifrice or toothpaste is a substance used with a toothbrush or other oral hygiene device to clean the teeth, tongue, and gingiva and to deliver cosmetic and therapeutic agents to the teeth and oral environment. A dentifrice can yield the following types of effects:
 Cosmetic effect—Prevents or removes stains, inhibits supragingival calculus formation, whitens teeth, freshens breath, controls oral malodor
Cosmetic effect—Prevents or removes stains, inhibits supragingival calculus formation, whitens teeth, freshens breath, controls oral malodor Therapeutic effect—Prevents or reverses dental caries; reduces gingivitis, oral biofilm, or dentinal hypersensitivity1
Therapeutic effect—Prevents or reverses dental caries; reduces gingivitis, oral biofilm, or dentinal hypersensitivity1Toothpastes that carry the American Dental Association (ADA) Seal of Acceptance or the Canadian Dental Association Seal of Recognition have been shown through rigorous research to be safe and therapeutically effective (antiplaque, antigingivitis, anticaries, and anti–dentinal hypersensitivity). The ADA Seal has also been awarded to dentifrices that safely and effectively remove tooth stain. (See the discussion of product selection and evaluation in Chapter 29.)
CHOOSING A DENTIFRICE
Dentifrice is selected to meet particular client needs, but the array of dentifrices on the market can be confusing to the consumer as well as the professional. Manufacturers develop dentifrices for cleaning dentures; nonfoaming to low-foaming dentifrices for use with power toothbrushes and children; ingestible dentifrices for persons with special needs; and dentifrices free of preservatives, sodium lauryl sulfate (SLS), fluoride, dyes, peroxide, and artificial flavors for persons who may be allergic or who desire a “natural” product. There are also dentifrices that contain bioavailable fluorides2 to strengthen or remineralize tooth enamel or exposed roots and amorphous calcium phosphate (ACP) to remineralize teeth and add luster.3 Others have antibacterial ingredients such as chlorhexidine gluconate (CHG), triclosan4 with copolymer, or stannous fluoride (SnF2) to control gingivitis and oral biofilm; and some contain fluoride, ACP, potassium nitrate, and/or strontium chloride to reduce dentinal hypersensitivity. Dentifrices have been formulated for persons with health problems such as diabetes (with sweeteners that have no effect on blood sugar), xerostomia (with salivary enzymes, lubricant, and salivary enhancers), recurrent aphthous ulcers (SLS-free), and hypertension (sodium bicarbonate–free or sodium chloride–free).
TOOTHPASTE TUBE CONTAMINATION
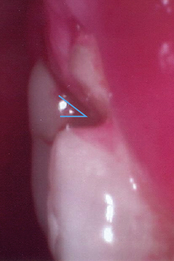
The orifice of the tube can be a source of cross-contamination. Transmission of bacteria responsible for oral and systemic disease is possible when family members’ toothbrushes come into contact with the neck of the same tube of dentifrice. Each family member should have his or her own tube of toothpaste to prevent cross-contamination, to control infection among people living in the same household, and to meet his or her unique oral care needs (Figure 23-1).
FORMS OF DENTIFRICES
Dentifrices come in powders, liquid gels, gels, pastes, and gel-paste combinations (Figure 23-2). A fluoridated liquid gel dentifrice is effective in caries prevention, as it reaches both the interproximal surfaces and deep grooves of the teeth.5,6
COMPONENTS OF DENTIFRICES (FIGURE 23-3 and TABLE 23-1)7-9
Toothpastes are complex formulas of therapeutic, active, and inactive ingredients that must be compatible to be effective. An active ingredient is an additive that produces a therapeutic or beneficial effect on either the hard or soft tissues. Some authors use the terms active ingredient and therapeutic ingredient interchangeably; however, in order for an active ingredient to be therapeutic, it must improve oral health status in a safe end effective way—for example, fluoride for caries control; triclosan-copolymer for control of gingivitis and oral biofilm; desensitizing agents such as sodium fluoride (NaF) and SnF2 to achieve desensitization and caries control; and potassium chloride and potassium citrate to control dentinal hypersensitivity. An active ingredient may be beneficial but still not be therapeutic (e.g., pyrophosphate zinc systems to inhibit calculus formation). An inactive ingredient is an additive that is necessary to make the formulation thick, hold together, clean efficiently, or have a particular color or flavor for consumer appeal. Listing specific ingredients on the packaging of oral care products would make it possible to meet client needs and avoid risks of allergies and intolerances. Active, therapeutic, and inactive ingredients found in dentifrices are discussed in the following sections.
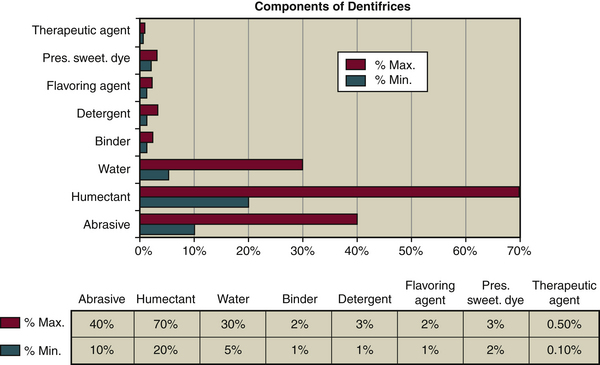
Figure 23-3 Components of dentifrices and percentage by weight. Pres., Preservative; Sweet., sweetener.
Abrasives
Abrasive agents are used to clean and polish teeth to a smooth, lustrous surface; they establish the abrasive capacity of the dentifrice (see Tables 23-1, 23-2, and 23-3 and Figure 23-4). If abrasive capacity is too low, the abrasive agent is less effective in removing the soft deposits and stains. If it is too high, it will increase abrasion of tooth structure and restored tooth surfaces. A client who toothbrushes without dentifrice must brush longer, as there is no abrasive agent on the toothbrush to help remove soft deposits and stains adequately.1 Dentrifice powders, gels, and pastes contain abrasive agents.1-9
TABLE 23-1 Components of Dentifrices
| Components | Examples | Quantity |
|---|---|---|
| Cleansing and polishing agents (10%-40%) | Calcium carbonate, sodium bicarbonate, calcium pyrophosphate, dicalcium phosphate dihydrate, anhydrous dicalcium phosphate, hydrated aluminium oxides, insoluble calcium metaphosphate (IMP), silica, silicates and dehydrated silica gels, synthetic amorphous silicates in gel form, complex salt of synthetic amorphous aluminium silicate, magnesium carbonate | NA |
| Humectants (20%-70%) | Glycerin, sorbitol, mannitol, propylene glycol, vegetable oils, synthetic cellulose | NA |
| Water (5%-30%) | Distilled water, deionized water, spring water | NA |
| Binders (1%-2%) | Hydrophilic organic colloids such as alginates and synthetic cellulose derivatives (e.g., cellulose gum, carboxymethylcellulose), glycerol, glycerin mineral colloids, polyethylene glycol (PEG)Natural products: carrageenan, carbomer, xanthan gum, seaweed colloids or algae, agar-agar | NA |
| Detergents (1%-3%) | Sodium lauryl sulfate, N-lauryl sodium sarcosinate, sodium cocomonoglyceride sulfonate, cocamidopropyl betaine or betaine de cocamidopropyl, steareth-30, sodium monoglyceride sulfate, ethionates of fatty acid | NA |
| Flavoring agents or aromatizers (1%-2%) | Essential oils, menthol, noncariogenic artificial sweetener | NA |
| Preservatives (2%-3%)∗ | Alcohols, sodium benzoates, formaldehydes, dichlorinated phenols, methylparaben, ethylparaben | NA |
| Sweeteners (2%-3%)∗ | Noncariogenic artificial sweetener, sorbitol, glycerin, sodium saccharin, sodium cyclamate, xylitol | NA |
| Dyes or coloring agents (2%-3%)∗ | Vegetable coloring, titanium dioxide | NA |
| Therapeutic agents (0.10%-0.50%)∗ | % minimal | |
| Desensitizing Agents | ||
| Antigingivitis and Oral Biofilm Reduction Agents | ||
| Active ingredients but not considered therapeutic | Anticalculus Agents | |
| Other ingredients | NA |
ADA, American Dental Association; CDA, Canadian Dental Association; NA, not applicable; PVM/MA, polyvinylmethoxyethylene and maleic acid.
∗ Total for preservatives, sweeteners, and dyes.
† Italic typeface denotes acceptance by the ADA or CDA.
TABLE 23-3 Variables Influencing Dentifrice Abrasiveness
| Particle size (grit) | The larger the particles, the more they wear on dental surfaces (see Figure 23-5). |
| Particle shape | The more irregular the shape, the more dental surfaces are worn and abraded. A round particle is less detrimental to the tooth (see Figure 23-5). |
| Particle hardness | The harder the particles, the more the dental surfaces are abraded (see Figure 23-10). |
| pH level | The more acidic and abrasive, the more the dentifrice increases tooth surface mineral loss, particularly if dentin or cementum is exposed (see Figure 23-6 and Table 23-9). |
| Quantity of glycerin and water in dentifrice | The higher the level of glycerin in a dentifrice, the higher its level of abrasiveness, as the dissolution of insoluble materials is reduced. The greater the amount of water in a dentifrice, the more soluble particles can dissolve, making them less abrasive to dental surfaces (see Table 23-2). |
Data from Lavoie F, Dubreuil N: Variables associated with the choice of a dentifrice. In press.
Figure 23-4 Size and shape of particles.
(Courtesy Comité Dentifrice, Collège François-Xavier-Garneau, Quebéc, Canada.)
The three common dentifrice abrasives are phosphates, carbonates, and silicas.
 Some silicas mechanically clean the teeth, and some thicken the dentifrice. Silicas are chemically compatible with NaF and MFP. Silicas are nonreactive and therefore are frequently used as abrasives in toothpaste.
Some silicas mechanically clean the teeth, and some thicken the dentifrice. Silicas are chemically compatible with NaF and MFP. Silicas are nonreactive and therefore are frequently used as abrasives in toothpaste.In 1812 Mohs created a 10-point scale of mineral hardness. With this scale the hardness of materials can be rated, with 1 being the softest and 10 being the hardest. The hardness of abrasive agents found in dentifrices and the hardness of a tooth are compared in Figure 23-5.
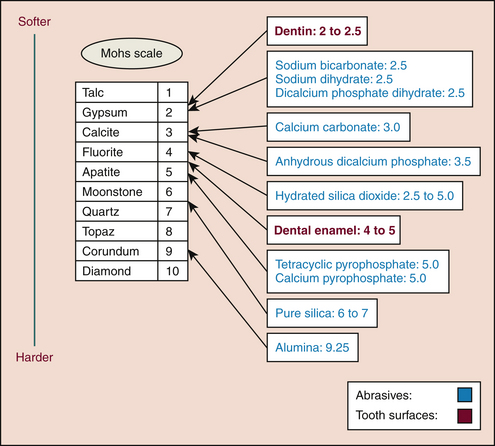
Figure 23-5 Comparison of hardness scales, abrasives, and dental surfaces.
(Courtesy Nadia Dubreuil. Adaptation of Mohs Scale [1812]).
Mohs Hardness Scale is useful for understanding abrasiveness of cleaning and polishing agents. For example, the threshold of 2 to 4 is important because it is equal to the hardness of cementum or dentin, often exposed because of gingival recession. Dentifrices whose level of abrasiveness is ≤2 are recommended to avoid tooth structure loss on exposed roots. Children can use a more abrasive dentifrice when their tooth enamel is mature (hardness level of 4 to 5). A dentifrice containing alumina is efficient for stain removal but has a higher risk for damaging tooth surfaces because its hardness level is higher than that of the tooth’s enamel. Even hard agents can be made safe by varying their particle size and shape in the manufacturing process.
Humectants
A humectant is a substance used to retain moisture, prevent air-drying, and ensure a chemically and physically stable product. Low concentrations of synthetic cellulose are used as a humectant; high concentrations are used as binders to stabilize the gel or liquid gel dentifrice formula.10 Other humectants include propylene glycol (PEG) and glycerin.
Water
The list of dentifrice ingredients seldom specifies the type of water used (deionized or distilled water) despite the fact that it represents 20% to 30% of the composition of the dentifrice (see Figure 23-3).
Preservatives
Because the risk of microorganism contamination is omnipresent, preservatives such as dichlorinated phenol, sodium benzoate, methylparaben, trisodium phosphate, and alcohols are added to inhibit mold and bacterial growth and prolong shelf life.
Binders
Substances such as sodium carrageenan, xanthan gum, alginates, and synthetic cellulose derivatives (carboxymethylcellulose) are used as thickeners and prevent liquid and solid ingredients from separating in pastes and gels. Gel formulas contain more binders than pastes. “Natural” dentifrice manufacturers tend to replace the oil-based products such as PEG with plant-based products such as algae and agar-agar.
Detergents
Foaming agents or detergents such as sodium lauryl sarcosinate, SLS, or cocamidopropyl betaine, called surfactants, are popular in toothpastes to lower surface tension to loosen debris and stain. SLS10 can contribute to recurrent aphthous ulcers in some people. If such intolerance occurs, it is preferable to use a dentifrice containing cocamidopropyl betaine such as Sensodyne Pronamel or Colgate Luminous. SLS neutralizes the effects of CHG; therefore clients using a dentifrice containing SLS should wait half an hour before using a CHG-containing oral rinse.10,11
Flavoring and Sweetening Agents
Ingredients are added to provide a refreshing flavor and aftertaste and to mask the taste of unpleasant chemicals compounds. Typical flavoring agents include oils of spearmint, peppermint, wintergreen, or cinnamon; bubble gum and fruit flavors; and menthol. Certain flavors, such as cinnamon, can cause a burning sensation, tissue sloughing, contact stomatitis, intolerances, or allergic reactions.
Sorbitol and xylitol are sweeteners that contribute to a pleasant taste.
Colorants
Dyes, such as vegetable coloring, make the product attractive; tartrazine in some dentifrices may produce an allergic reaction, especially in persons hypersensitive to aspirin.
Therapeutic Agents and Active Ingredients
Ingredients added for specific preventive, treatment, or beneficial purposes are referred to as therapeutic agents or active ingredients. For example, CHG is available in different concentrations and is used to control dental plaque formation and gingivitis.12-14 GUM Gingidex dentifrice contains 0.06% CHG; GUM dental floss contains 0.12% CHG,15 and Mirafluor dental floss contains 0.4% CHG. Others products such as GUM Paroex dentifrice contain 0.12% CHG, and some prescription oral rinses contain 0.12% CHG to control gingivitis and plaque.14 These products vary by country. For a dentifrice to be considered therapeutic, the manufacturer must follow strict pharmaceutical standards with regard to the quantity and bioavailability of therapeutic agents used, their safety, and their efficacy.16 The main therapeutic and active agents found in dentifrices follow.14-17
Fluoride
Fluoride plays a key role in keeping the remineralization-demineralization process in favor of remineralization.12 Common fluorides found in daily-use dentifrice formulas include SnF2, NaF, MFP, and SnF2-sodium hexametaphosphate. A dentifrice with 0.24% NaF has an efficacy equivalent to a dentifrice containing 0.76% MFP. These two concentrations differ because the agents do not have the same molecular weight. Fluoride levels in dentifrices vary among countries. In Europe dentifrices may contain from 250 ppm18 to 10,000 ppm of fluoride.19 In North America the levels are between 400 ppm (for children) and 5000 ppm of fluoride. Most products contain about 1000 ppm. Fluoride neutralizes the antibacterial effect of CHG; therefore a client brushing with a fluoride-containing oral care product should wait for 30 minutes before using a CHG-containing product.12
Xylitol
Xylitol, a sugar alcohol and sugar substitute derived from fruits, mushrooms, and birch bark, has anticaries and antiplaque properties. Streptococcus mutans cannot metabolize xylitol; therefore their acids that demineralize tooth structure are decreased. Xylitol in a therapeutic dose of 1.55 g (minimum of 5 g used daily in the oral cavity) decreases S. mutans levels and plaque biofilm and its adhesion to the tooth.
Amorphous Calcium Phosphate
Other minerals, such as calcium and phosphates, may be added for the remineralization of tooth structure, as mild abrasive agents for tooth cleaning, as lubricants, and for enhancement of ambient calcium and phosphate in the saliva.
Desensitizing agents (see Chapter 38)
Chemotherapeutic agents may be added to do the following:
 Mechanically block dentinal tubules—for example, NovaMin 5%, a calcium sodium phosphosilicate (in Oravive, SoothRx, and DenShield); SnF2 0.4%; strontium chloride 10% (Sensodyne Original14); or high levels of fluoride (10,000 ppm) such as in Elmex 1.25% (12,500 ppm), an amine fluoride (AmF) that remineralizes enamel.
Mechanically block dentinal tubules—for example, NovaMin 5%, a calcium sodium phosphosilicate (in Oravive, SoothRx, and DenShield); SnF2 0.4%; strontium chloride 10% (Sensodyne Original14); or high levels of fluoride (10,000 ppm) such as in Elmex 1.25% (12,500 ppm), an amine fluoride (AmF) that remineralizes enamel. Chemically prevent the depolarization of nerve fibers in the tooth (transmission of nervous influx), as potassium nitrate or potassium citrate does.
Chemically prevent the depolarization of nerve fibers in the tooth (transmission of nervous influx), as potassium nitrate or potassium citrate does. Reducing dentinal hypersensitivity. After tooth bleaching or mechanical oral debridement, use of a 5000-ppm fluoride product is efficient in reducing dentinal hypersensitivity (Oral-B NeutraCare with 1.1% NaF or Colgate PreviDent 5000 ppm Sensitive 1.1% NaF and 5% potassium nitrate.)14 A low abrasive sensitivity-control dentifrice and meticulous oral biofilm control should also be recommended.
Reducing dentinal hypersensitivity. After tooth bleaching or mechanical oral debridement, use of a 5000-ppm fluoride product is efficient in reducing dentinal hypersensitivity (Oral-B NeutraCare with 1.1% NaF or Colgate PreviDent 5000 ppm Sensitive 1.1% NaF and 5% potassium nitrate.)14 A low abrasive sensitivity-control dentifrice and meticulous oral biofilm control should also be recommended.Triclosan
Triclosan, a bisphenol, is a broad-spectrum antimicrobial agent that has antiplaque and antigingivitis properties. Polyvinylmethoxyethylene and maleic acid (PVM/MA) copolymer (Gantrez) is added to the triclosan to increase its duration in the mouth (substantivity) and hence its antibacterial effect. A dentifrice formulation (Colgate Total) with triclosan, PVM/MA copolymer and NaF has the ADA Seal of Acceptance for its safety and efficacy as an anticaries, antiplaque, antigingivitis, and anticalculus dentifrice. In North America, triclosan with copolymer PVM/MA (Gantrez), used in Colgate Total, is available as an antigingivitis, antiplaque ingredient.14 Triclosan slows periodontal disease progression in 3- to 5-mm periodontal pockets.11 (Note that in some countries, triclosan is regarded as an active ingredient with restricted use.4 The long-term safety of this ingredient for the microbial ecosystem and general health is unknown.)
Chlorhexidine Gluconate
CHG is an efficacious ingredient for the treatment of gingivitis. Although it cannot be found in over-the-counter dentifrices in North America, a CHG mouthwash is available by prescription. CHG-containing dentifrice is available in Europe.
Sodium Hexametaphosphate, Tetrapotassium Pyrophosphate, Gantrez, Zinc Chloride, and Zinc Citrate
Chlorites, derivatives of sodium, phosphate, and stabilized SnF2, are accepted by the ADA14 as supragingival calculus-inhibiting agents (see Table 23-1). Disodium pyrophosphate, tetrasodium pyrophosphate, and tetrapotassium pyrophosphate inhibit the mineralization of biofilm before it is transformed into supragingival calculus. Zinc chloride and zinc citrate prevent or break down calculus formation. Pyrophosphate and zinc chloride also have minor abrasive properties.
Dentifrice formulations with zinc citrate or pyrophosphates are very effective as antitartar toothpastes. Extrinsic stain removal ingredients or whitening agents work mechanically with the help of abrasives such as silicate or alumina. However, the dental hygienist must be cautious in recommending toothpastes containing alumina because this abrasive is harder than enamel and cementum, and if the particle size is large and irregular, the toothpaste can increase tooth surface lost. A dentifrice formulation with SnF2–sodium hexametaphosphate (Crest Pro-Health) has the ADA Seal of Acceptance for its safety and efficacy as an anticaries, antiplaque, antigingivitis, antistain, and anti–dentinal hypersensitivity dentifrice. It also has anticalculus benefits. Note that the ADA Seal is not given for anticalculus properties because calculus does not cause disease.
Hydrogen Peroxide
Hydrogen peroxide or carbamide peroxide can remove stains, chemically whiten teeth, assist in the control of oral malodor, and have antigingivitis properties. If the percentage of hydrogen peroxide is higher than 6% in toothpaste,20 it can damage soft tissues14-21 and cause dental erosion.21 If the percentage of hydrogen peroxide in the dentifrice is <1%, the product is considered safe.
Sodium Bicarbonate
Baking soda, a mild abrasive, has been shown to neutralize acids produced by acidogenic bacteria that cause demineralization, effectively control extrinsic staining, reduce oral malodor, and have a mild antibacterial effect. It can be combined with hydrogen peroxide for tooth whitening and fluoride for an anticaries effect. It can be delivered for persons at extreme risk for caries (high risk plus dry mouth or special needs) in gum or toothpaste or in a solution for individuals with low saliva flow.
CONCEPT OF BIOAVAILABILITY
Bioavailability occurs when the therapeutic agent is stable during storage and biologically active when used in the mouth to achieve the desired therapeutic effect.2 It corresponds to the proportion of the therapeutic agent available in a pharmaceutical substance that will produce the desired effect when used as recommended. Some manufacturers use cocamidopropyl betaine as a detergent instead of SLS to increase the bioavailability of fluoride ions. For example, Colgate-Palmolive Canada (Colgate Luminous) or GlaxoSmithKline (Sensodyne Pronamel) use this detergent. The percentage of available fluoride ions can be lower in the case of a dentifrice containing 1000 ppm of fluoride, such as NaF (Table 23-4). This difference could be attributed mainly to the following three elements:
TABLE 23-4 Comparison of Bioavailability among Dentifrices
| Product | Fluoride | Bioavailability of Fluoride (ppm and %) |
|---|---|---|
| Colgate Cavity Protection | 1450 ppm MFP/NaF | 839 ppm = 57.8% |
| Crest Cavity Protection | 1100 ppm NaF | 951 ppm = 86.5% |
| Colgate Total | 1450 ppm NaF | 1024 ppm = 70.6% |
| Elmex Sensitive | 1400 ppm AMF | 1022 ppm = 73.0% |
| Solidox | 1000 ppm SnF2 | 577 ppm = 57.7% |
AMF, Amine fluoride; MFP, sodium monofluorophosphate; NaF, sodium fluoride, SnF2, stannous fluoride.
Some manufacturers, such as Meridol, Homéodent, and Colgate, purport to have more fluoride in their toothpaste by mixing fluorides such as MFP, NaF, and AmF with SnF2 to makes the dentifrice more effective against caries. However, bioavailability is not proportional to the quantity of fluoride contained in the toothpaste. As an example, Colgate Cavity Protection contains 1450 ppm MFP/NaF and has only 839 ppm fluoride (57.8%) available.2
DENTIFRICE: PREVENTIVE MEASURE OR RISK FACTOR?
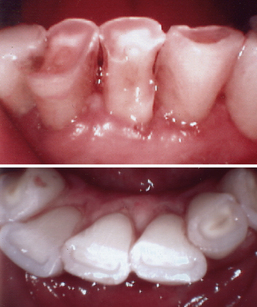
A dentifrice can prevent or control an oral disease or condition when it provides a therapeutic function.1 It can also be a risk factor if it causes dentin hypersensitivity, erosion, or abrasion (Figures 23-6, 23-7, 23-8, and 23-9). Therefore dentifrices must be selected to meet the needs of each client. For example, a client with root exposures must be given advice regarding abrasiveness, role of pH, and insoluble materials contained in dentifrices.
Insoluble and Soluble Materials
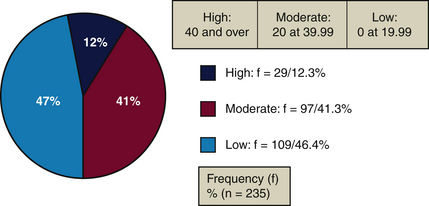
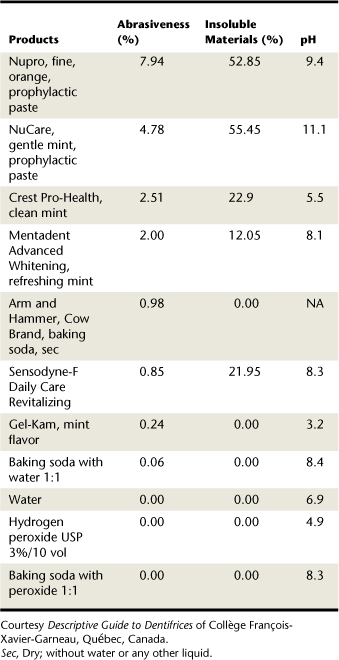
Dentifrices contain insoluble and water-soluble ingredients.1 Ingredients in toothpaste that cannot dissolve in water are insoluble materials. Ingredients that dissolve in water are soluble materials, such as sodium bicarbonate. Insoluble abrasives such as silica remain intact in water. Insoluble ingredients can increase a dentifrice’s abrasiveness (Figure 23-10 and Table 23-5).
TABLE 23-5 Insoluble Materials and Dentifrices
| Example | Insoluble Materials | |
|---|---|---|
| High: ≥40 | Pearl Drops Whitening Toothpaste, mint | 42.80% |
| Moderate: 20-39.99 | GUM Whitening Plus | 20.09% |
| Low: 0-19.99 | Prospec MI Paste, Recaldent, mint | 11.35% |
Courtesy Descriptive Guide to Dentifrices of Collège François-Xavier-Garneau, Québec, Canada.
For some dentifrices the level of insoluble materials is <20%, making them gentle on tooth surfaces.22 For others the insoluble material level is 30% to 55% (Figure 23-11). Colgate Gel-Kam Fruit and Berry flavor dentifrice contains no insoluble materials, has 0% abrasiveness, and has a pH of 3.6.
Advantages of High Abrasive Levels (>2%)
In a client without root exposure but with heavy quantities of oral biofilm, a more-abrasive dentifrice will remove biofilm and acquire pellicle faster than a less-abrasive agent. Fortunately, the acquired pellicle forms quickly and can protect enamel against erosion.12-22
Disadvantages of High Abrasive Levels
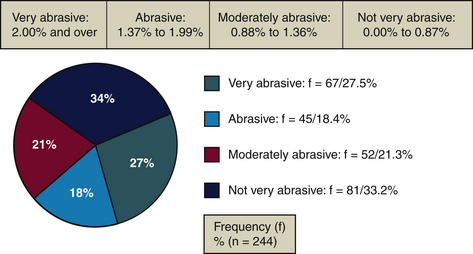
A dentifrice with a high abrasive level can increase abrasion in a client with exposed root surfaces and can cause dentinal hypersensitivity23 (see Figure 23-11 and Table 23-6). A dentifrice with low abrasiveness is recommended for persons with esthetic restorations and/or titanium implants to avoid damaging the surface. Smooth intact restorative materials do not retain bacteria easily.24
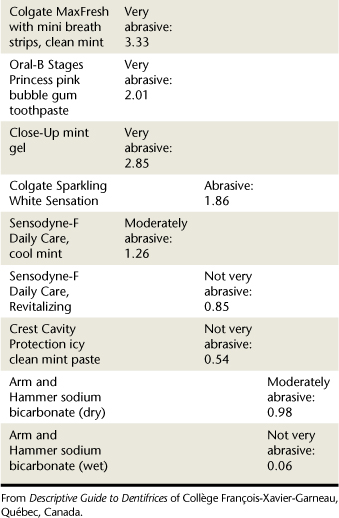
TABLE 23-6 Abrasiveness Scale and Dentifrices9-32
| Examples | Abrasiveness | |
|---|---|---|
| Very abrasive | Healthy Mouth Tea Tree oil | 5.17% |
| Abrasive | Crest MultiCare Whitening, neat squeeze dispenser | 1.90% |
| Moderately abrasive | Colgate Total, long-lasting antibacterial protection | 1.20% |
| Not very abrasive | Aquafresh Whitening Advanced Freshness, mint | 0.65% |
Courtesy Descriptive Guide to Dentifrices of Collège François-Xavier-Garneau, Québec, Canada.
Abrasive Scales Used to Evaluate Dentifrices
Abrasiveness Scale
According to the abrasiveness scale developed by Desautels and used by the team of researchers at the College François-Xavier-Garneau (Québec, Canada),1,32 about 33% of commercially available dentifrices are “not very abrasive”(<0.87%). Approximately 54% of dentifrices fall below 2 on the Abrasiveness Scale,9 meaning they do not risk damaging dentin or exposing cementum. About 28% of dentifrices are very abrasive (>2%). Dentifrices such as Jasön Healthy Mouth (5.17%) and Nature’s Gate (4.72%) are more abrasive than enamel (Mohs Hardness Score of 4 to 5) and can damage tooth structures.
Relative Dentin Abrasivity Scale
Abrasiveness of most dentifrices is determined by the universally used Relative Dentin Abrasivity (RDA) Scale (Table 23-7). The higher the score, the more abrasive the dentifrice. Unfortunately, there is no link among abrasiveness scales because research methodologies differ. Consequently, research findings cannot always be compared.
TABLE 23-7 Relative Dentin Abrasivity (RDA) Scale
| RDA Score | Level |
|---|---|
| 0-70 | Low abrasive: safe for cementum, dentin, and enamel |
| 70-100 | Medium abrasive: safe for enamel, dangerous for cementum and dentin |
| 100-150 | High abrasive: dangerous for cementum, dentin, and enamel |
| 150-250 | Very high abrasive: harmful limit, damaging for teeth |
| 250 and over | Not recommended |
DENTIFRICE pH
The potential of the hydrogen molecule (pH) of a substance is measured on a scale from 1 to 14. Level 1 is very acidic, 7 is neutral, and 14 is basic (alkaline). The pH of a dentifrice can be beneficial or detrimental to dental structures while interfering in the demineralization-remineralization process. Decay caused by acids from the fermentation of sugars by S. mutans occurs at a pH of 6.5 on cementum and dentin,2-25 at a pH of 5.5 on enamel (hydroxyapatite),2-25 and at a pH of 4.5 on fluorapatite enamel.26 The majority of dentifrices have a neutral pH, but some products have an acidic pH.
Low or Acidic pH
Advantages for Tooth Enamel
Acidity of a dentifrice promotes the formation of fluorapatite by facilitating the incorporation of fluoride ions into the enamel crystals. Fluorapatite crystals are characteristically larger, more stable, and less acid soluble.27-28 Therefore a low pH is a desirable characteristic in fluoride toothpastes.
Disadvantages in the Case of Root Exposure or Titanium Implants
Low pH contributes to erosion of tooth structure. Because demineralization of dentin and cementum occurs at a pH of 6.52-25, the practitioner must pay attention to the client with root exposures in order to avoid tooth mineral loss and dentinal hypersensitivity. Low pH can tarnish titanium implants.24
Neutral and Basic pH
Advantages for Teeth and Mucous Membrane
Because of similarities to healthy saliva, a neutral or basic pH is less irritating for soft tissues and will not demineralize teeth.
Disadvantages for Teeth and Gums
Neutral or basic pH levels promote the mineralization of biofilm (calculus formation), which in turn supports the retention of biofilm and extrinsic stains.
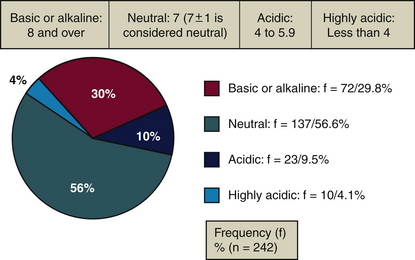
More than 86% of dentifrices (Figure 23-12 and Table 23-8) have a neutral or basic pH.23 The pH of acidic or highly acidic dentifrices (14% of dentifrices) is below the critical threshold of demineralization. A dentifrice with a pH under 6.5 demineralizes and weakens exposed root surfaces. A combination of dentifrice acidity and abrasiveness further increases the loss of dental substance.21-28 According to the ADA,14 dentifrices bearing the Seal of Acceptance contain a safe level of abrasives, but the organization makes no mention of pH levels. For example, Crest Pro-Health,9 which carries the ADA Seal of Acceptance,14 has a pH of 5.5; some believe that this pH might risk root surface demineralization in persons with gingival recession, yet it is important for fluoride uptake.1,9,23 More research is needed on dentifrice pH and its role in tooth remineralization and demineralization.
TABLE 23-8 pH Measurements and Dentifrices
| Example | pH | |
|---|---|---|
| Basic (alkaline) (≥8) | Tom’s of Maine with propolis and myrrh, spearmint | 9.9 |
| Neutral (7 ± 1) | Colgate Total Advanced Fresh, Gel | 7.0 |
| Acidic (4-5.9) | Crest Pro-Health, clean mint | 5.5 |
| Highly acidic (<4) | OmniiGel Natural | 3.2 |
Courtesy Comité Dentifrice, Collège François-Xavier-Garneau, Québec, Canada.
Thus, pH levels are relevant in the analysis of dentifrices. Loss of dental substance of chemical origin (erosion) can be increased further by mechanical actions such as toothbrush abrasion. Table 23-9 provides a comparison of various dental products. Persons who experience dietary acid or gastroesophageal reflux should wait 60 minutes28,29 before toothbrushing to minimize loss of tooth structure. If waiting is not an option, the client should first rinse with water, fluoride-containing mouthwash, water and baking soda solution, or milk before toothbrushing.22 Persons who have cancer, chronic xerostomia, or chronic vomiting from bulimia or pregnancy and persons who take antidepressants, antiparkinsonian medications, antihistamines, or antihypertensives that cause chronic xerostomia14 need the same approach.
RECOMMENDING DENTIFRICES TO CLIENTS
A comparison of commercial dentifrices, professionally dispensed dentifrices, and professionally applied dental products is important in order to recommend and use available products with confidence.1-9 For example, no commercially available dentifrice is as abrasive as Nupro Fine prophylaxis paste, which is 7.9 on the abrasiveness scale and very alkaline as well. Products such as water, hydrogen peroxide, and sodium bicarbonate have no abrasiveness and contain no insoluble materials; however, they differ in pH. Water is neutral, peroxide is acidic with a pH of 4.9, and sodium bicarbonate is basic. Thus, a client who uses peroxide-containing toothpaste daily increases risk of tooth surface erosion over time. As for sodium bicarbonate, its relatively high solubility contributes to its low level of abrasiveness.1 It can abrade dentin if used dry, as its particles are very irregular. Certain dentifrices contain micronized abrasive particles to reduce abrasiveness. (The finer the particle size or grit, the less abrasive the material, even if the material scores very high on the Mohs Hardness Scale.)
LOSS OF TOOTH STRUCTURES
Saliva plays several roles: lubrication, predigestion (contains ptyalin), immunity, and buffering capacity to neutralize mouth acids. Therefore quality and quantity of saliva affect the demineralization and remineralization process. Medications that cause xerostomia, an individual’s condition (stress, fatigue, and dehydration), time of the day (saliva is more abundant in the morning and at mealtimes), and certain health problems (Sjogren’s syndrome, radiotherapy in the salivary gland region) influence the quantity and quality of saliva as well.
Loss of dental substance may occur by erosion, abrasion, abfraction, and attrition and has diverse causes such as dentifrice used, frequency of brushing, toothbrush filament hardness, pressure during brushing, the direction of the brush strokes, choice of manual or power toothbrush, the surface substrate being brushed (dentin, cementum, or enamel), and an insufficient amount of saliva.10,30
 Erosion: Dissolution of organic and inorganic tooth structure as a result of chemical agents (see Figure 23-6)
Erosion: Dissolution of organic and inorganic tooth structure as a result of chemical agents (see Figure 23-6) Abrasion: Pathologic tooth wear caused by a foreign substance that is harder than the tooth structure (see Figure 23-7)
Abrasion: Pathologic tooth wear caused by a foreign substance that is harder than the tooth structure (see Figure 23-7) Abfraction: Cervical tooth structure loss of noncariogenic origin caused when the tooth is subjected to a high occlusal load—that is, the occlusal stress is high enough to cause cervical cracking and mineral loss of tooth structure (see Figure 23-8)
Abfraction: Cervical tooth structure loss of noncariogenic origin caused when the tooth is subjected to a high occlusal load—that is, the occlusal stress is high enough to cause cervical cracking and mineral loss of tooth structure (see Figure 23-8) Attrition: Loss of tooth structure on surfaces resulting from tooth-to-tooth contact (proximal or biting surfaces) from normal (chewing) or pathologic (bruxing or clenching) friction with adjacent or opposite teeth (see Figure 23-9)
Attrition: Loss of tooth structure on surfaces resulting from tooth-to-tooth contact (proximal or biting surfaces) from normal (chewing) or pathologic (bruxing or clenching) friction with adjacent or opposite teeth (see Figure 23-9)COMPARISON OF METHODS TO EVALUATE DENTIFRICE ABRASIVENESS
Dentifrice abrasiveness is not easily compared because of the various methods used to evaluate abrasiveness.31-33 Manufacturers use different protocols and laboratory products, making direct comparisons among dentifrices difficult.32 Therefore one can only compare dentifrices from the same manufacturer1 or resort to an independent laboratory that conducts testing using the same protocol (Figure 23-13).
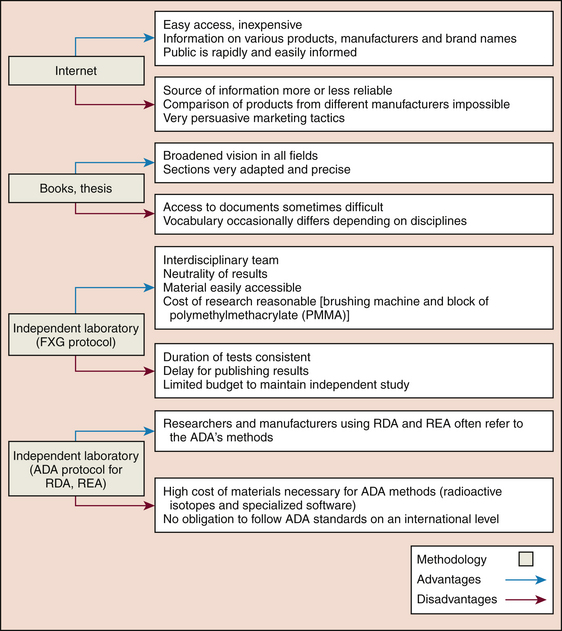
Figure 23-13 Methodologies: advantages and disadvantages. ADA, American Dental Association; RDA, Radioactive Dentin Abrasivity; REA, Radioactive Enamel Abrasivity.
(Courtesy France Lavoie.)
An interdisciplinary team representing dental hygiene, chemistry, physics, and biology independently studied the abrasiveness, the pH, and the insoluble materials found in dentifrices using the Collège François-Xavier-Garneau protocols.1,9,32 This team’s findings have been reported in this chapter.
CLIENT EDUCATION TIPS
 Explain importance of the daily use of dentifrices with fluoride and amorphous calcium phosphate in the remineralization process.
Explain importance of the daily use of dentifrices with fluoride and amorphous calcium phosphate in the remineralization process. Understand that dentifrices, depending on the one used, can be a risk factor or a therapeutic agent for clients. The American Dental Association Seal of Acceptance and the Canadian Dental Association Seal of Recognition are designed to help the consumer and dental hygienist make informed oral care product choices.
Understand that dentifrices, depending on the one used, can be a risk factor or a therapeutic agent for clients. The American Dental Association Seal of Acceptance and the Canadian Dental Association Seal of Recognition are designed to help the consumer and dental hygienist make informed oral care product choices. Balance risk of dental caries with risk of future dental fluorosis on permanent teeth in young or disabled children who cannot expectorate.
Balance risk of dental caries with risk of future dental fluorosis on permanent teeth in young or disabled children who cannot expectorate. Analyze the effects of combining over-the-counter fluoride and ingested fluoride to avoid dental fluorosis and acute fluoride toxicity.
Analyze the effects of combining over-the-counter fluoride and ingested fluoride to avoid dental fluorosis and acute fluoride toxicity. Demonstrate the quantities of fluoride dentifrices recommended for different age groups. Avoid fluoridated dentifrice until children are 2 years of age, then use minimal quantity (pea size or smear on the toothbrush head) for young children18,19 with primary teeth. A young child risks acute poisoning if he swallows a complete tube of fluoride toothpaste.8 There are established tables that make it possible to determine the maximum tolerated dose (MTD) and the lethal dose (LD) according to the weight and age of the individual8 (see Chapter 31). Keep oral healthcare products out of young children’s reach.
Demonstrate the quantities of fluoride dentifrices recommended for different age groups. Avoid fluoridated dentifrice until children are 2 years of age, then use minimal quantity (pea size or smear on the toothbrush head) for young children18,19 with primary teeth. A young child risks acute poisoning if he swallows a complete tube of fluoride toothpaste.8 There are established tables that make it possible to determine the maximum tolerated dose (MTD) and the lethal dose (LD) according to the weight and age of the individual8 (see Chapter 31). Keep oral healthcare products out of young children’s reach.LEGAL, ETHICAL, AND SAFETY ISSUES
 Assess client’s health, dental, and pharmacologic histories to make sure there are no conditions, allergies, or medications that would contraindicate a particular dentifrice recommendation.
Assess client’s health, dental, and pharmacologic histories to make sure there are no conditions, allergies, or medications that would contraindicate a particular dentifrice recommendation. Make recommendations based on a client’s assessed needs and expectations and product evidence. Use the American Dental Association (ADA) Seal of Acceptance or the Canadian Dental Association (CDA) Seal of Recognition as a guide to help make product recommendations.
Make recommendations based on a client’s assessed needs and expectations and product evidence. Use the American Dental Association (ADA) Seal of Acceptance or the Canadian Dental Association (CDA) Seal of Recognition as a guide to help make product recommendations. Document recommendations in client’s record, including the product, frequency, dosage, and reasons for use. Confirm, with client’s signature, that the client understands these recommendations and agrees to the regimen.
Document recommendations in client’s record, including the product, frequency, dosage, and reasons for use. Confirm, with client’s signature, that the client understands these recommendations and agrees to the regimen. Review client’s self-care regimen regularly; offer advice based on evolving research evidence to reach optimal oral health.
Review client’s self-care regimen regularly; offer advice based on evolving research evidence to reach optimal oral health. Make sure that products recommended to clients have been accepted by the ADA14 or CDA17 and contain ingredients that have been approved by the U.S. Food and Drug Administation,16 Health Canada,4 or other regulatory organization. In 2007 about 30 dentifrices were taken off the market because of the presence of dangerous amounts of toxic ingredients and high levels of harmful bacteria. It is therefore of utmost importance to consult these organizations regularly via their websites.
Make sure that products recommended to clients have been accepted by the ADA14 or CDA17 and contain ingredients that have been approved by the U.S. Food and Drug Administation,16 Health Canada,4 or other regulatory organization. In 2007 about 30 dentifrices were taken off the market because of the presence of dangerous amounts of toxic ingredients and high levels of harmful bacteria. It is therefore of utmost importance to consult these organizations regularly via their websites.KEY CONCEPTS
 The majority of manufacturers produce dentifrices effective against calculus, gingivitis and oral biofilm, dentinal hypersensitivity, or a combination of the above. Studies to date indicate that these products make toothpastes more therapeutically efficacious but more abrasive than first-generation products.
The majority of manufacturers produce dentifrices effective against calculus, gingivitis and oral biofilm, dentinal hypersensitivity, or a combination of the above. Studies to date indicate that these products make toothpastes more therapeutically efficacious but more abrasive than first-generation products. When developing a care plan, it is necessary to verify the dentifrice and the quantity and frequency with which it is to be used. Evaluate whether the product meets client needs, taking into consideration root exposure, erosion, abrasion, dental caries, stains, calculus, and brushing habits (e.g., pressure applied, soft- or stiff-bristled toothbrush, brushing method).
When developing a care plan, it is necessary to verify the dentifrice and the quantity and frequency with which it is to be used. Evaluate whether the product meets client needs, taking into consideration root exposure, erosion, abrasion, dental caries, stains, calculus, and brushing habits (e.g., pressure applied, soft- or stiff-bristled toothbrush, brushing method). Variables about level of abrasion, the pH of dentifrices, and the insoluble materials must be considered when recommending dentifrices to clients. Various sources of references exist, such as the American Dental Association,14 Radioactive Dentin Abrasivity, and Radioactive Enamel Abrasivity, but the Descriptive Guide to Dentifrices considers all of these variables, making it useful for comparison of dentifrices.
Variables about level of abrasion, the pH of dentifrices, and the insoluble materials must be considered when recommending dentifrices to clients. Various sources of references exist, such as the American Dental Association,14 Radioactive Dentin Abrasivity, and Radioactive Enamel Abrasivity, but the Descriptive Guide to Dentifrices considers all of these variables, making it useful for comparison of dentifrices.CRITICAL THINKING EXERCISES
Role-play each of the following case scenarios. One person should be the clinician and one the client. Analyze each scenario by noting the following:
What information should be given to the client (or caregiver) to ensure safe, effective dentifrice use?
Example: A 3-year-old child with healthy teeth, no composite restorations, a thin biofilm at the lingual surface of the mandibular molars, healthy eating habits, and good oral hygiene. The child brushes twice daily with a dentifrice for children with 0.24% NaF, which completely covers bristles of a small-headed toothbrush. Parental supervision of brushing occurs once daily.
Clinical Findings: None except for biofilm on molars
Therapeutic Agent: Fluoride is used as primary prevention against caries. The quantity of dentifrice is equal to the size of a grain of rice to reduce the risk of fluorosis.
Abrasiveness: Low-abrasiveness dentifrice is used for primary teeth, because in this case the child has only a thin biofilm
pH: Slightly acidic or neutral to promote fluoroapatite formation (the more acidic a dentifrice, the more it demineralizes, allowing for a greater remineralization and incorporation of fluoride onto dental structures).
Scenario 1: An 8-year-old boy with one area of decalcification (white spot lesion), two untreated carious areas, no sealants, generalized gingival redness, materia alba, and oral biofilm. Daily he chews one package of sugarless chewing gum (nine pieces), and he has variable eating habits, including two glasses of regular cola (pH 3).
Daily Oral Care Regimen: Rapid brushing once daily with whichever dentifrice is available at home
Scenario 2: A 22-year-old woman with four dental sealants, two composite restorations, two areas of gingival recession on maxillary first premolars causing no sensitivity, a slight supragingival calculus, moderate gingivitis , and generally good eating habits.
Daily Oral Care Regimen: Uses dental floss once weekly, brushes twice daily using a power toothbrush and a small amount of dentifrice because of too much foam. Choice of dentifrice depends on price.
Scenario 3: A 58-year-old smoker (one pack of cigarettes per day) with generalized moderate tobacco stains, periodontitis (four sites at 4 PSR), heavy subgingival calculus, and occasional bleeding on probing.
Daily Oral Care Regimen: brushes once or twice daily with a dentifrice chosen by his spouse. Never uses an interdental cleaning aid.
Refer to the Procedures Manual where rationales are provided for the steps outlined in the procedures presented in this chapter.
1. Comité Dentifrice Collège François-Xavier-Garneau, Lavoie F., Feeney N. Evaluation of toothpastes and of variables associated with the choice of a product. CDHA. 2007;41:42.
2. Newby C.S., Creeth J.E., Rees G.D., Schemehorn B.R. Surface microhardness changes, enamel fluoride uptake, and fluoride availability from commercial toothpastes. J Clin Dent. 2006;17:4.
3. Lynch R.J.M. Calcium glycerophosphate and caries: a review of the literature. Int Dent J. 2004;54:310.
4. Health Canada: Health Canada/Santé Canada website. Available at: www.hc-sc.gc.ca/cps-spc/person/cosmet/prohibited_f.html. Accessed February 27, 2008.
5. Silva M.F., Giniger M.S., Zhang Y.P., Devisio W. The effect of a triclosan/copolymer/fluoride liquid dentifrice on interproximal enamel remineralization and fluoride uptake. J Am Dent Assoc. 2004;135:1023.
6. Josiak MT, Fisher SW, Schemehorn BR: Comparison of enamel fluoride uptake and fluoride release from liquid and paste dentifrices. The 32nd Annual Meeting and Exhibition of the AADR, March 12-15, 2003, Indiana University, Bloomington, Indiana.
7. Mahieu V, Moucheron C: La chimie des produits cosmétiques, Université Libre de Bruxelles. Available at: www.ulb.ac.be. Accessed October 28, 2007.
8. Wilkins E. Clinical practice of the dental hygienist, ed 10. Philadelphia: Lippincott Williams and Wilkins, 2008.
9. Lavoie F., Dubreuil N., Bourassa L. Descriptive guide to dentifrices. Québec: Collège François-Xavier-Garneau, 2007.
10. Harris N.M., Garcia-Godoy F. Primary preventive dentistry, ed 6. Upper Saddle River, NJ: Pearson Prentice Hall; 2004.
11. Schiffner U. Contrôle chimique de la plaque. Revue mensuelle Suisse Odontostomatologie. 2000;110:836.
12. Zero D.T. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health. 6(Suppl 1), 2006. Available at: www.biomedcentral.com. Accessed October 8, 2007.
13. Paraskevas S. Randomized controlled clinical trials on agents used for chemical plaque control. Int J Dent Hyg. 2005;3:162.
14. American Dental Association (ADA). ADA/PDR guide to dental therapeutics, ed 4. Chicago: ADA; 2006.
15. Imai P.H. The effects of flossing with a chlorhexidine solution on interproximal gingivitis: a randomized controlled trial. Can J Dent Hyg. 2008;42:8.
16. U.S. Food and Drug Administration (FDA): U.S. FDA website. Available at: www.fda.gov. Accessed October 7, 2007.
17. Canadian Dental Association (CDA): CDA econnaissance de l’ADC. Available at: www.cda-adc.ca. Accessed October 28, 2007.
18. Sixou J.-L., Bailleul-Forrestier I., Dajean-Trutaud S., Vaysse F. Recommandations sur la prescription des fluorures de la naissance à l’adolescence. J Odonto-Stomatologie Française. 2004;11:157.
19. Les médicaments de prévention de la carie dentaire. Available at: www.automedication.fr. Accessed October 28, 2007.
20. La Commission Européenne se prononce sur les produits blanchissants au peroxyde d’hydrogène: Une évaluation du Comité scientifique des produits de consommation (CSPC) de la Commission européenne. Available at: www.blanchiment-dentaire.com/?q=node/76. Accessed February 27, 2008.
21. Price R.B.T., Sedarous M., Hiltz G. Le pH des produits de blanchiment des dents. J Can Dent Assoc. 2000;66:421. Available at: www.cda-adc.ca/jadc
22. Zero D.T., Lussi A. Érosion—facteurs chimiques et biologiques importants pour le praticien dentaire. Int Dent J. 2005;55:285.
23. Comité Dentifrice, Collège François-Xavier-Garneau, Québec, 2007.
24. Hossain A., Okawa S., Miyakawa O. Effect of toothbrushing on titanium surface: an approach to understanding surface properties of brushed titanium. Dent Mater. 2006;22:346.
25. Addy M. Brossage des dents, usure dentaire et hyperesthésie dentaire—existe-t-il un lien? Int Dent J. 2005;4:261.
26. Mount G.J. La fin des traitements invasifs de la carie? No more invasive treatments for caries. Cahiers de l’ADF. 2001;11:10.
27. Daniel S.J., Harfst S.A., Wilder R.S. Mosby’s dental hygiene: concepts, cases, and competencies, ed 2. St Louis: Mosby; 2008.
28. Barbour M.E. The role of erosion, abrasion and attrition in tooth wear. J Clin Dent. 2006. Spec Issue:88
29. Zero D.T., Hara A.T., Kelly S.A., et al. Evaluation of desensitizing test dentifrice using an in situ erosion remineralization model. J Clin Dent. 2006;17:112.
30. Wiegand A., Lemmrich F., Attin T. Influence of rotating-oscillating, sonic and ultrasonic action of power toothbrushes on abrasion of sound and eroded dentine. J Periodont Res. 2006;41:221.
31. Imfeld T. Confusion autour des valeurs RDA des pâtes dentifrices. Dimensions Revue de Suisse Dental Association. 2006;2:6.
32. Désautels P., Labrèche H. Abrasion relative des dentifrices. Un dentifrice pour chacun. J Dentaire du Québec. 1994;31:461.
33. Lutz F., Imfeld T. Relative dentin (RDA) and relative enamel abrasion (REA) of toothpastes and prophylaxis pastes. Compend Contin Educ Dent. 2002;23:61.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..