CHAPTER 29 Chemotherapy for the Control of Periodontal Diseases
 Discuss indications for chemotherapeutic interventions as adjuncts to mechanical oral biofilm control and nonsurgical periodontal therapy in the prevention and treatment of periodontal and dental diseases.
Discuss indications for chemotherapeutic interventions as adjuncts to mechanical oral biofilm control and nonsurgical periodontal therapy in the prevention and treatment of periodontal and dental diseases.ORAL DISEASE AND ORAL BIOFILM
Initiation and progression of oral diseases are related to the interaction among a susceptible host, a pathogenic agent, and environmental factors. Therefore prevention and control of periodontal diseases and dental caries depend on controlling oral biofilm (pathogenic agent), minimizing risk factors for these diseases, and affecting the host's response. Mechanical disruption of oral biofilm through toothbrushing, tongue brushing, and interdental cleansing, and also through professional care, comprise the backbone of periodontal disease and caries prevention programs. However, recognizing that a large proportion of the North American population demonstrates some level of oral disease, these conventional interventions are not always adequate. Oral chemotherapeutic agents, the incorporation of an effective active ingredient within a delivery system, are used adjunctively to prevent and control oral diseases.
Although total elimination of oral biofilm is unrealistic, a reasonable approach is to prevent disease through methods that reduce the microbial load to a level below the individual's threshold for disease.1 Oral chemotherapeutics contribute to this aim by reducing the number and pathogenic potential of organisms. Dental hygienists, as promoters of oral health, are in a key position to recommend and implement various chemotherapeutic therapies in a shared decision-making relationship with clients.2
PRODUCT SELECTION AND EVALUATION
Because chemotherapeutics present a risk of harm and the potential for misbranding, two major organizations in the United States, the U.S. Food and Drug Administration (FDA) and the American Dental Association (ADA) Council on Scientific Affairs, contribute to ensuring safety and efficacy of oral chemotherapeutics. These bodies and the ADA Seal of Acceptance program (Figure 29-1, A) help oral healthcare providers and their clients make appropriate decisions about product-based interventions.

Figure 29-1 A, American Dental Association Seal of Acceptance. B, Canadian Dental Association Seal of Recognition.
In Canada, similar organizations, such as the Canadian Dental Association (CDA), provide this information. The CDA Seal of Recognition (Figure 29-1, B) program also aims to assist the public and oral health professionals in making informed choices regarding products. For example, manufacturers seeking the CDA Seal of Recognition verify the following:
Food and Drug Administration
The FDA ensures safety and efficacy through federally mandated review and approval of prescription drugs and over-the-counter (OTC) products that make therapeutic claims. The FDA does not supervise the manufacture or importation of herbal remedies. The FDA uses elements from the ADA Seal of Acceptance program (see next section) for approval of chemotherapeutic agents or products for treatment of periodontal diseases. Such approval requires submission of clinical data to support the therapeutic claims of active ingredients. Based on the FDA's assessment, it assigns an OTC active ingredient to one of three categories (Table 29-1).
TABLE 29-1 U.S. Food and Drug Administration Categories for Active Ingredients with Therapeutic Claims
| Category | Description of Assessment |
|---|---|
| Category I | Safe, effective, and not misbranded |
| Category II | Not generally recognized as being safe and effective or is misbranded |
| Category III | Insufficient data to evaluate |
American Dental Association Council on Scientific Affairs
The ADA Council on Scientific Affairs assists oral healthcare providers in the selection and use of chemotherapeutic agents by evaluating new, nonprescription products for safety and efficacy. The ADA Seal of Acceptance (see Figure 29-1, A) is granted to those products demonstrating therapeutic efficacy within stringent guidelines. Products having the Seal also have been approved by the FDA. Specific guidelines for evaluating the effectiveness of oral chemotherapeutics for the prevention and treatment of plaque and gingivitis are listed in Box 29-1.3
BOX 29-1 American Dental Association Council on Scientific Affairs Research Guidelines
Manufacturers who are interested in making therapeutic claims must submit research data to substantiate the effectiveness of the product, and only those products claiming therapeutic value are considered. After evaluation of the product, the Council classifies it as accepted, provisionally accepted, or unaccepted (Table 29-2).
TABLE 29-2 American Dental Association (ADA) Council on Scientific Affairs Classifications
| Classification | Description |
|---|---|
| Accepted | Adequate evidence of safety and effectiveness; may use ADA Seal of Acceptance |
| Provisionally accepted | Reasonable evidence of usefulness and safety, but lacks sufficient documentation; further investigation indicated |
| Unaccepted | Lacks substantial evidence of usefulness and/or has questionable safety |
CHEMICAL ORAL BIOFILM (PLAQUE) CONTROL
Despite widespread use of mechanical oral biofilm removal and technologic advances in oral cleaning devices, several difficulties associated with the conventional approach to care remain. For example, time, dexterity, and motivation required to accomplish mechanical plaque removal are problematic, particularly in difficult access areas such as posterior, interproximal, and lingual regions and in malpositioned teeth. Some oral chemotherapeutic agents have the potential to reduce oral biofilm and improve gingival outcomes beyond those accomplished with mechanical means.4,5 However, oral chemotherapeutics are complex in that there are various active ingredients incorporated into numerous delivery systems that when combined result in differing levels of efficacy.
Many oral chemotherapeutic products have inadequate efficacy levels to recommend their use. Dental hygienists and clients must distinguish between products that have therapeutic benefits versus those limited to having cosmetic effects. Moreover, differences exist among product formulations manufactured in different countries. For example, products available in Canada and the United States having the same name contain different active ingredients or concentrations, causing confusion in advertising and research. These factors complicate the selection of interventions.
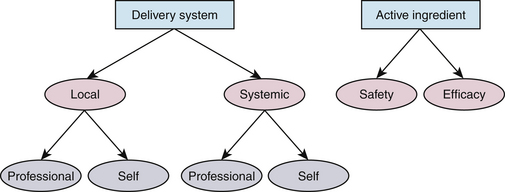
Oral chemotherapeutics have the following two dimensions:
 Delivery system is the mode of application.
Delivery system is the mode of application.
 Active ingredient refers to that agent, chemical, or drug found within a particular delivery system that is responsible for reducing or altering the composition of microbial pathogens (see Chapter 23 discussion on active and inactive ingredients).
Active ingredient refers to that agent, chemical, or drug found within a particular delivery system that is responsible for reducing or altering the composition of microbial pathogens (see Chapter 23 discussion on active and inactive ingredients).Active ingredients are found in various delivery systems; for example, the active ingredient fluoride is available in local delivery systems, such as oral rinses, and also in systemic (ingested) applications, such as fluoridated water and dietary supplements. Furthermore, fluoride is self-applied, as in toothpaste (dentifrice), and also professionally applied, as in fluoride gels, foams, and varnish. Proven efficacy of a specific active ingredient within a particular delivery system does not ensure that this same active ingredient is efficacious in other delivery systems or in different concentrations.
Active ingredients are categorized based on how the agent disables microorganisms (Table 29-3). Of these groups, oral antiseptics have received the most research attention, particularly for OTC and self-care products. However, research on antibiotics shows promising results in systemic and locally delivered applications. Plaque modifying agents, antiadherence agents, and host response modulators have varying contributions to reducing plaque accumulations and controlling periodontal disease, and research into these products, although limited, shows potential. Because oral antiseptics demonstrate very little oral or systemic toxicity or microbial resistance and most have a broad antimicrobial spectrum, they are commonly used in contemporary oral chemotherapeutics. Oral antiseptics exhibit either bactericidal activity, meaning that they kill microbes directly, or bacteriostatic action, which means that the metabolism or reproduction of the microbe is affected.
TABLE 29-3 A Categorization of Active Ingredients in Oral Chemotherapeutics
| Category | Description |
|---|---|
| Antiseptic agents | Usually broad spectrum; kill or prevent propagation of plaque microorganisms |
| Antibiotics | Broad or narrow spectrum; inhibit or kill specific or groups of bacteria, or modulate host inflammatory response |
| Modifying agents | Agents that alter the structure and/or metabolic activity of bacteria |
| Antiadhesives | Products that interfere with the ability of bacteria to attach to the acquired pellicle |
Many antiseptic agents are referred to as being first- or second-generation products. First-generation formulations are distinguished from second-generation products because the latter have some level of substantivity, meaning that the agent has an ability to durably bind with oral tissues and then is released for a prolonged period of time to control bacterial growth. Substantivity has been shown to be a less important characteristic for product efficacy than previously believed, with some first-generation formulations demonstrating significant efficacy.
LOCAL DELIVERY METHODS
Self-Applied Modes of Delivery
Dentifrices
Chemotherapeutics for self-applied delivery systems are typically used more often at home with lower concentrations of active ingredient. The most common of these products is dentifrice. Dentifrice, available mostly as paste and gel, is a vehicle for the local delivery of active ingredients and can have both therapeutic and cosmetic effects (see Chapter 23). Fluoride, the most common active ingredient in dentifrice marketed in the United States and Canada, is a well-established dental caries preventive agent that also has an, albeit less well-documented, effect on periodontally destructive pathogens (see Chapter 31). Regular use of a fluoride dentifrice substantially reduces caries and is considered safe for persons who are able to resist swallowing while toothbrushing. Several types of fluoride dentifrices are available; those with documented safety and anticariogenic efficacy carry the ADA Seal of Acceptance or the CDA Seal of Recognition.
Other active ingredients in dentifrices are available for the reduction of dental caries, oral biofilm, gingivitis, hypersensitivity, and calculus, but each requires additional research evidence to substantiate therapeutic claims. Antimicrobial agents in dentifrices can sometimes present problems of compatibility with other dentifrice components.
Oral Rinses
Oral rinses (mouthwashes) are available for both cosmetic and therapeutic use and in prescription and OTC formulations. Therapeutic uses include oral biofilm reduction, caries prevention, and reductions and control of gingival disease; cosmetic uses include breath freshening, tartar control, and whitening. Few of the oral rinses available have shown convincing evidence of their therapeutic effect. To date, one OTC formulation (Listerine) and a prescription product (Peridex, PerioGard) have earned the ADA Seal for therapeutic efficacy for reducing plaque and gingival measures, and some others have received only FDA approval. Note that the ADA is no longer providing the Seal for prescription products.
Although oral rinses are reportedly well accepted by the public, this has not been established empirically. Approximately half the population uses some type of oral rinse, but not according to manufacturer directions. Clients should be advised to rinse twice daily for 30 seconds with 1 ounce of rinse after mechanical cleansing, but manufacturer instructions should be consulted for specific directions. Oral rinsing is believed to reach the areas of the mouth that are rarely targeted, inaccessible, and often missed by mechanical means. Additional oral health benefits can be gained by adding the use of an ADA-accepted oral rinse to a client's daily oral self-care regimen. Box 29-2 describes ideal properties of an oral rinse. Oral rinses, like dentifrices, have a limited effect on subgingival pathogenic microorganisms because they do not adequately penetrate subgingivally. Although the gel-matrix protects the oral biofilm from penetration of the rinse, some solutions are able to diffuse it.
Oral rinse formulations may include inactive ingredients such as astringent, flavoring agents, ethyl alcohol, sodium, and water.
 Alcohol (10% to 30% by volume) emulsifies the antimicrobial ingredients within the rinse and is not considered in the overall efficacy of the product.
Alcohol (10% to 30% by volume) emulsifies the antimicrobial ingredients within the rinse and is not considered in the overall efficacy of the product.
 Ethanol in oral rinses has not been demonstrated to be carcinogenic; this view is supported by the FDA and the ADA.6
Ethanol in oral rinses has not been demonstrated to be carcinogenic; this view is supported by the FDA and the ADA.6 Alcohol in oral rinses has also been implicated with xerostomia, but this relationship remains unsubstantiated—that is, alcohol is not significantly associated with either the perception of oral dryness or actual reduction in salivary flow.7 Xerostomic individuals do not appear to have an increased dry mouth problem from using alcohol-containing rinses.
Alcohol in oral rinses has also been implicated with xerostomia, but this relationship remains unsubstantiated—that is, alcohol is not significantly associated with either the perception of oral dryness or actual reduction in salivary flow.7 Xerostomic individuals do not appear to have an increased dry mouth problem from using alcohol-containing rinses. Sodium, found in substantial amounts in some oral rinses (e.g., Cepacol, Plax, Viadent), leads to sodium absorption through the oral mucosa from frequent rinsing. People on sodium-restricted diets (e.g., those with hypertension, renal disease, or congestive heart disease) should be aware that some brands of mouthwash may be a significant source of sodium; they should consult their physicians regarding the potential impact.
Sodium, found in substantial amounts in some oral rinses (e.g., Cepacol, Plax, Viadent), leads to sodium absorption through the oral mucosa from frequent rinsing. People on sodium-restricted diets (e.g., those with hypertension, renal disease, or congestive heart disease) should be aware that some brands of mouthwash may be a significant source of sodium; they should consult their physicians regarding the potential impact.Oral Irrigation and Oral Rinse Products
Because oral rinsing is largely ineffective against subgingival microorganisms, oral irrigation has been recommended to counteract these organisms. Oral irrigation refers to both power and manual mechanisms for delivering an active ingredient within a solution or water via an irrigation tip to gingival sulci or periodontal pockets. Whereas one-time professional oral irrigation has limited chemotherapeutic potential, daily self-applied oral irrigation reduces the overall bacterial load by mechanically dislodging oral biofilm.
Home irrigation typically makes use of standard jet tips (Figure 29-3) that deliver a pulsating stream of fluid (often water) with controlled variable pressure and has been shown to be effective as an adjunct to toothbrushing in reducing several gingival parameters.8 In home oral irrigation, the use of water alone is comparable to the use of chemotherapeutic agents.8 Standard tips are unable to access pockets completely; therefore, regardless of the irrigants used, improvements in periodontitis cannot be expected.
When considering home irrigation, the penetration of the irrigant is dependent on the tip design, pocket depth, and individual client access to the site(s). Needle-like tips called cannulas are available and specially designed to be placed below the gingival margin (Figure 29-4). Although the use of such a tip theoretically allows for considerable penetration into deep pockets, it is generally reserved for professional use because these tips require a high level of dexterity and access to the specific disease site. Therefore recommending the use of a cannula with home irrigation for clients' home use should be carefully considered.
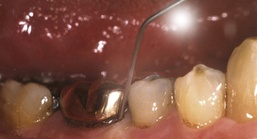
Figure 29-4 Use of a blunt-end cannula for administering product subgingivally
(A, From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders. B, Courtesy Tolmar Inc, Fort Collins, Colorado. Atridox is a registered trademark of Tolmar Inc.)
Professionally Applied Modes of Delivery
Preprocedural Oral Rinse
A preprocedural rinse is the use of a therapeutic oral rinse before professional care to decrease oral microorganisms available to the clinical environment via intraoral procedures that cause aerosols and spatter. Infection control in the oral environment is a concern, as some pathogenic organisms can be suspended in aerosols for considerable periods of time. A preprocedural rinse limits the amount of organisms but does not eliminate them altogether. Either a prescription or an OTC product that has been shown to be therapeutically effective can be used for this purpose. An oral rinse may also be used after professional care, as clients appreciate the provision of a pleasant-tasting rinse before leaving the healthcare facility.
Professionally Applied Oral Irrigation
Professionally applied subgingival irrigation with various antimicrobials has limited clinical value.8 The dental hygienist and client need to carefully weigh the costs (financial and other resources) of delivering professionally applied subgingival irrigation in relation to the questionable clinical outcomes. Although professionally applied chemotherapeutic agents delivered with a cannula are able to reach the base of the periodontal pocket, the following disadvantages exist:
 They are not retained in adequate concentrations for sufficient duration to have significant effects on periodontal disease.
They are not retained in adequate concentrations for sufficient duration to have significant effects on periodontal disease. Gingival crevicular fluid (GCF) is replaced about every 90 seconds, rapidly reducing the concentration of any antimicrobial agent that reaches subgingival organisms.
Gingival crevicular fluid (GCF) is replaced about every 90 seconds, rapidly reducing the concentration of any antimicrobial agent that reaches subgingival organisms.Professional delivery of subgingival irrigation is not recommended as a monotherapy (used independently of other treatment interventions); rather, it should be used in conjunction with scaling and root planing therapy.8 Furthermore, it is necessary to irrigate circumferentially, as lateral dispersion of chemotherapeutics via a cannula is minimal. However, only minimal pressure is required, because low force has been shown to penetrate the pocket adequately.
Controlled-Release Drug Delivery
Self-care behaviors (toothbrushing, flossing, mouth rinsing, and home irrigation) and professional interventions (mechanical debridement and irrigation) become less effective as clinical attachment loss develops and pockets deepen. To address the limitations of these conventional therapies, controlled-release drug delivery methods have been developed.9 Controlled-release drug delivery refers to those intracrevicular devices that are professionally placed to provide drug delivery for sustained periods of time.9
Minimum inhibitory concentration (MIC), a research measurement, is used for describing the lowest concentration of a particular antimicrobial that is able to inhibit overt microbial growth during incubation.10 Selection of controlled-release products should be based on client health, pertinent precautions, number and severity of sites requiring treatment, ease of use, and degree of client-required compliance.
Intracrevicular devices consist of a drug (often an antibiotic) reservoir that controls the rate of drug release and provides a means of sustained administration of the antimicrobial agent directly into the periodontal pocket. In contrast to systemic antibiotic administration (see next section), intracrevicular delivery results in 1000 times the concentration of the drug within the GCF at the diseased site, but only one hundredth of the systemic dose reaches the rest of the body. For all products, the manufacturer's directions are followed.
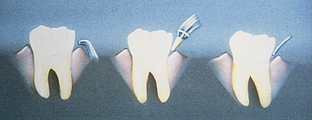
Tetracycline Fiber (Procedure 29-1)
The first controlled-release device approved for treatment of periodontal pockets ≥5 mm was the tetracycline fiber (Actisite), but it is rarely used because of problems with placement and retention and the need for professional removal. It consists of a flexible ethylene vinyl acetate fiber (23 cm long and 0.5 mm in diameter) infused with 12.7 mg of tetracycline hydrochloride, which releases for 10 to 14 days, providing intercrevicular concentrations exceeding that achieved by a systemic tetracycline administration of 250-mg tablets taken four times per day for 10 days, resulting in a total dose of 10,000 mg (Figure 29-5). The fiber is placed in an overlapping pattern within the pocket after debridement. Tetracycline fibers have been shown to improve probing depths, clinical attachment levels, and bleeding on probing and to reduce periodontal pathogens in the sites treated. The fibers are contraindicated in persons with known allergy to tetracycline or cyanoacrylate (in the adhesive used after placement to improve retention) and should not be used by pregnant or breast-feeding women.
Procedure 29-1 PLACEMENT OF CONTROLLED-RELEASE DRUG: TETRACYCLINE FIBER
STEPS
Chlorhexidine Chip (Procedure 29-2)
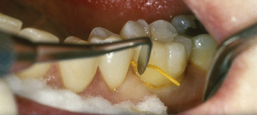
The chlorhexidine chip (PerioChip) is a biodegradable 4 × 5 mm × 0.35 mm thickness of hydrolyzed gelatin material incorporating 2.5 mg of chlorhexidine d-gluconate for insertion into ≤5-mm pockets (Figure 29-6). The amber-colored chip slowly releases and maintains an average GCF concentration of 125 µg of chlorhexidine gluconate/mL over a 7- to 10-day period, exceeding the MIC and inhibiting almost all subgingival bacteria. Suppression of subgingival bacterial flora is evident for up to 11 weeks after placement, resulting in improved pocket depths when used in conjunction with scaling and root planing.
Procedure 29-2 PLACEMENT OF CONTROLLED-RELEASE DRUG: CHLORHEXIDINE CHIP
STEPS
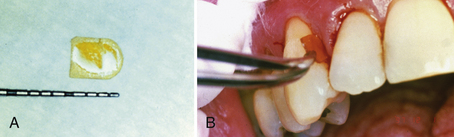
Figure 29-6 A, Chlorhexidine chip size in millimeters. B, Chlorhexidine chip being inserted into periodontal pocket.
One chip per pocket site is placed after scaling and root planing. It is self-retentive once exposed to moisture (GCF) and should not be disturbed by oral care regimens for 1 week. Because the chip self-resorbs in 7 to 10 days, the need for professional removal is eliminated. Placement of a chlorhexidine gluconate chip is contraindicated for clients with the rare allergy to chlorhexidine. The chip can be safely used by pregnant women. Because chlorhexidine is not an antibiotic, there is no potential for the development of bacterial resistance.
Doxycycline Gel (Procedure 29-3)
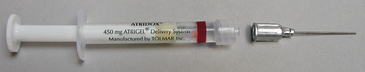
Doxycycline gel (Atridox) consists of 10% doxycycline hyclate in a gel polymer that flows to the pocket base and solidifies on contact with the GCF, providing a controlled release of the antibiotic doxycycline for 7 days. It reaches GCF concentrations of over 1500 mg/mL within hours and remains well above the MIC for most periodontal pathogens for 7 days. The product biodegrades in about 28 days, negating the need for professional removal.
Procedure 29-3 PLACEMENT OF CONTROLLED-RELEASE DRUG: DOXYCYCLINE GEL
STEPS
Doxycycline gel is available as a two-syringe system that is mixed manually and delivered into the ≥5-mm pocket via a cannula (Figures 29-7 and 29-8; see Figure 29-4, B). In clinical studies a periodontal dressing was applied to ensure continued retention, and this is especially recommended when placing the product in shallower pockets and/or with single-rooted teeth. Some studies have demonstrated that doxycycline gel is effective in reducing pocket depths and bleeding on probing and increasing clinical attachment levels when applied as a monotherapy. However, monotherapeutic applications remain controversial, and it is generally recommended that mechanical therapies occur with controlled-release products. Known sensitivity to any drug in the tetracycline family is an absolute contraindication to the administration of doxycycline gel. The product should not be used by pregnant or breast-feeding women.
Metronidazole Gel
Metronidazole gel (Elyzol) is an antibiotic available in a readily flowable, bioresorbable drug delivery system consisting of 25% metronidazole benzoate. It is applied subgingivally in ≥5-mm pockets via a cannula-fitted syringe. It reaches peak concentration in GCF 4 hours after administration and maintains levels above 100 mg/mL for the first 8 hours. The product maintains concentrations exceeding the MIC for anaerobic pathogens susceptible to metronidazole (1 mcg/mL) for approximately 36 hours. Limited research demonstrates this product's clinical efficacy, and it is not available in North America.
Minocycline Microspheres (Procedure 29-4)
Minocycline microspheres (Arestin) consist of minocycline hydrochloride (1 mg) available in North America as a dry powder. The microspheres have been shown to improve pocket depths, particularly in more advanced sites when used in conjunction with scaling and root planning. The dry powder is delivered via a syringelike handle with a narrow tip that is inserted subgingivally to the base of the ≥5-mm pocket and immediately adheres to the periodontal pocket. The product does not set, but rather becomes a sticky paste that is retained within the pocket without periodontal dressing. It well exceeds MIC levels within hours and remains effective against predominant periodontal pathogens for over 20 days while slowly resorbing. This product is contraindicated for clients with a known sensitivity to minocycline or tetracyclines and should not be used by pregnant and breast-feeding women.
Procedure 29-4 PLACEMENT OF CONTROLLED RELEASE DRUG: MINOCYCLINE HYDROCHLORIDE MICROSPHERES
STEP
Photodynamic Disinfection Therapy
Photodynamic disinfection therapy (Periowave) is a novel, two-stage method of inactivating a broad spectrum of subgingival bacteria and potentially damaging enzymes via the following:
This system is recommended as an adjunct to scaling and root planing, with studies showing superior outcomes to conventional mechanical treatments alone. This therapy acts as a short-term topical disinfectant, eradicating harmful microorganisms that may otherwise remain after debridement. Studies have shown this system to reduce most of the subgingival microorganisms, with after-treatment evidence of damaged bacteria and biofilms that are thinner, are less dense, and have fewer channels than control biofilms.
Various delivery systems have obtained regulatory approval or are awaiting approval from the FDA or other national regulatory bodies. Based on efficacy data, these products may be indicated for localized persistent pockets that have not responded to conventional therapies.
SYSTEMIC DELIVERY METHODS
Systemic delivery methods (ingested and then delivered via the bloodstream) include products such as fluoride supplements, fluoridated water (see Chapter 31) and antibiotic medications. Prescription antibiotics delivered systemically for the treatment of periodontitis travel from the bloodstream to the periodontal tissues and eventually reach the GCF and subgingival microflora, albeit in low concentrations. Several antibiotics including doxycycline, penicillins, metronidazole, and clindamycin have been used against gram-negative anaerobic microorganisms to treat periodontitis singularly or in combination. Research has demonstrated positive outcomes with the use of systemic antibiotic therapy on periodontal parameters, particularly in aggressive or unresponsive periodontal disease conditions.11
While systemic administration provides a means of delivering antibiotics to deep periodontal pockets, it is also associated with various contraindications, precautions, and side effects (Box 29-3). Successful systemic administration requires ongoing client adherence to the antibiotic protocol, which is a concern because many clients fail to follow prescriptions. Furthermore, the routine use of antibiotics to treat periodontal diseases is not recommended owing to concerns for developing antibiotic-resistant organisms. Therefore systemic antibiotics are indicated only for clients exhibiting disease progression subsequent to diligent mechanical therapy and for clients with severe, aggressive, and/or acute forms of periodontal disease.
Modulating Host Response
Although bacteria and their byproducts initiate the host's inflammatory response, endogenous enzymes and cytokines (e.g., matrix metalloproteinases [MMPs], tumor necrosis factor-alpha [TNF-α], prostaglandin E2 [PGE2]) are responsible for the degradation of certain proteins including collagen and bone. Therefore researchers are investigating the modulation of host response as an approach to treatment of periodontal diseases.12,13 Host modulation agents can be categorized within three therapeutic approaches: antiproteinases (tetracyclines), anti-inflammatories (nonsteroidal anti-inflammatory drugs [NSAIDs]), and bone-sparing drugs (bisphosphonates).13 Concern over bisphosphonate use is increasing, given that it is a risk factor for osteonecrosis of the mandible (see Chapter 53 on women's health).
Subantimicrobial Systemic Dosage of Doxycycline Hyclate
A subantimicrobial systemic dose refers to the administration of a reduced quantity of a drug for purposes other than the elimination of a pathogenic microorganism—in this case, host modulatory therapy. For example, the antibiotic doxycycline hyclate (Periostat) may be administered in low doses (20 mg twice daily) over long periods of time (6 to 9 months) to inhibit collagenase (which breaks down collagen in the periodontal disease process) as a systemic adjunct to scaling and root planing. Subantimicrobial doses of doxycycline have yielded improved periodontal outcomes over scaling and root planing therapy alone in both nonsmokers and smokers.12,13
In subantimicrobial doses, antibiotics are not antibacterial and no detrimental shifts in the normal periodontal flora or antibiotic resistance have been observed. However, despite the reduced dosages, subantimicrobial doxycycline is contraindicated for clients who are sensitive to tetracyclines. In addition, various other side effects and potential adverse reactions are possible and are described in the literature accompanying the product or on the product's website.
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs block enzymes that promote the inflammatory response, thus reducing inflammation. These drugs have been studied to determine their role in modifying host responses. Some NSAIDs have positive outcomes, particularly surrounding alveolar bone preservation.13 Recently, select NSAIDs have demonstrated this bone-sparing effect without previously associated gastrointestinal implications.
Bone-Sparing Drugs
Used in the treatment of osteoporosis and osteopenia, bisphosphonates are drugs that bind with hydroxyapatite crystals in bone and prevent bone dissolution.13 Although few studies into its therapeutic application for periodontitis have been conducted, some promising results have been demonstrated.13
ACTIVE INGREDIENTS
Dental hygienists need to continuously review the literature related to active ingredients and product safety and efficacy to confidently provide evidence-based information to clients.2,4 Several key active ingredients for the control of plaque and periodontal diseases are available within various delivery systems.
Bis-Biguanides
Bis-biguanides are cationic, broad-spectrum antimicrobials effective for both gram-positive and gram-negative bacteria. Of these, chlorhexidine gluconate (CHG), one of the most widely investigated, is predominantly used in prescription oral rinses, irrigation solutions, and controlled-release products. CHG is considered the gold standard, providing a benchmark for measurement in studies examining the efficacy of other oral rinse active ingredients for plaque and gingivitis reductions. Prescription CHG rinses are CDA accepted for reducing plaque (16% to 45%) and gingivitis (27% to 80%). (Note that the ADA is no longer providing the Seal of Acceptance for prescription products.) (See Chapter 31.)
Action
CHG strongly binds with the bacterial cell membrane and causes the cell to leak and/or disrupt its intracellular components. CHG can interfere with bacterial colonization and cell attachment. CHG is considered a second-generation product in that it has considerable substantivity, binding to oral tissues and remaining active for 8 to 12 hours, which contributes to its superior efficacy. CHG oral rinses (Peridex, Periogard), available only through prescription, typically have a 0.12% concentration. Chlorhexidine is recommended for clients who have problems with plaque and gingivitis control; those with extensive fixed prostheses, those with splinting, orthodontic appliances, dental implants, or overdentures; clients undergoing professional mechanical therapy or in the immediate post–periodontal surgery or post–oral maxillofacial surgery healing phase; clients with acute infection such as necrotizing ulcerative gingivitis (NUG) or necrotizing ulcerative periodontitis (NUP); clients who cannot clean their mouth for a short-term period; and clients with impaired manual dexterity.
Disadvantages
Although there is minimal likelihood that CHG promotes bacterial resistance, several disadvantages exist when it is used as an oral rinse, with dental and tongue staining being the most notable. The brown staining on teeth requires professional removal, and this adverse outcome negates the use of CHG over long periods of time or for clients with anterior restorations with rough or pitted areas because it may stain them permanently. Clients may also note increased calculus deposition. Like many oral rinses, most CHG rinses contain alcohol, and therefore necessary precautions must be observed. An alcohol-free 0.12% CHG rinse is now available by prescription (Sunstar, Inc.).
Administration
The oral rinse formulation of CHG is typically administered in 18- to 20-mg doses for 60 seconds twice daily. It is important that clients be advised to allow at least 30 minutes between rinsing with CHG and toothbrushing to avoid an interaction with the detergent (sodium lauryl sulfate) in toothpaste, which causes a deactivation of CHG. Furthermore, clients should not rinse with water immediately after CHG is used, as this will remove the flavor-masking agents from the oral cavity and increase the medicinal taste.
Phenolic Compounds
Essential Oils
Essential oils (EOs) are a component of plants and contain phenolic compounds. Listerine is a commercially available EO mouth rinse and toothpaste with a combination of three phenolic-derived EOs. Thymol (0.063%), eucalyptol (0.091%), and menthol (0.042%), along with other ingredients, are available in the rinse, which has demonstrated its efficacy in long-term clinical trials. Although EOs are considered to have low substantivity, within the rinse they have been shown to be highly efficacious in plaque and gingivitis reductions, with efficacy comparable to the gold standard, CHG. Long-term clinical trials adhering to ADA guidelines have demonstrated plaque reductions of 56% and gingivitis reductions of 35% when compared with negative (placebo) controls. Listerine is the only OTC oral rinse that has received the ADA Seal of Acceptance for chemotherapeutic control and reduction of bacterial plaque and gingivitis. Almost any client who can control swallowing can benefit from the daily use of Listerine or its generic equivalent.
Action
EO rinse destroys microorganisms by compromising the cell membrane and inhibiting enzyme activity. It prevents bacteria from aggregating, slows bacterial multiplication, reduces the bacterial load overall within the oral cavity, prevents the plaque mass from maturation, and reduces its pathogenicity. It also has anti-inflammatory properties.
Disadvantage
Most concern has surrounded a reportedly sharp taste, but recent formulations are reported to be less intense while maintaining effectiveness.
Administration
Because recent trials show that Listerine mouth rinse approaches the efficacy of CHG in plaque and matches gingivitis reductions without the associated stain, and because it is considered safe when used as directed and produces no changes in bacterial composition, evidence of opportunistic oral pathogens, or antimicrobial resistance, it is an ideal OTC adjunctive home rinse. Clients should be taught to rinse twice daily with 1 ounce (20 mL) for 30 seconds after brushing and cleansing interproximally.
Triclosan
Triclosan, a bisphenol, is considered to be a safe, broad-spectrum antibacterial. Triclosan is predominantly found in dentifrices, but commercially available rinses (0.3% triclosan/2.0% copolymer, Colgate Total, Plax) have, in short-term trials, demonstrated significant reductions in plaque and gingivitis compared with negative (placebo) controls. However, studies have shown that triclosan-based rinses are not as effective as those containing CHG. A reason for the lack of efficacy of triclosan is believed to be its inability to bind with the oral tissues, and therefore triclosan is being placed in combination with other products to increase its retention in the oral cavity. A commercially available toothpaste formulation (Total) delivers 0.3% triclosan via a 2.0% copolymer that increases triclosan substantivity, and it has demonstrated reductions in plaque and gingivitis. Any client who needs help with plaque or gingivitis control can benefit from a dentifrice with triclosan (see Chapter 23).
Halogens and Fluoride (see Chapter 31)
Although the use of fluoride as a caries preventive agent has been well documented, its role in the prevention and control of plaque-induced periodontal disease is less documented. Stannous fluoride (SnF2) is a chemical molecule with a fluoride component in combination with tin. It is believed that the tin enters the bacterial cell and impairs its metabolism, thereby counteracting its growth and adherence properties. SnF2 has been shown to have some antiplaque and gingivitis efficacy, but because of its uncertain clinical value, lack of stability in storage, and increased staining properties, it has questionable application for prevention of gingival disease. Crest Pro-Health Toothpaste contains the Polyfluorite System, which combines SnF2 (for control of gingivitis, plaque, cavities, and tooth sensitivity) and sodium hexametaphosphate (to control stain and tartar formation) in a stable formulation. Other fluoride products have been marketed outside of North America for the control of plaque and gingivitis, including a stable amine/stannous fluoride (AmF/SnF2) solution (Meridol). The AmF component, which is active only against caries, stabilizes the SnF2 component and does not cause side effects (see Chapter 23). It is absorbed by the bacterial cell surface, inhibiting its metabolism and reducing plaque overall. Although some promising research has been conducted, the evidence is inconclusive.
Quaternary Ammonium Compounds
Quaternary ammonium compounds (QACs) are positively charged ions that destroy microorganisms by interacting with the bacterial cell membrane, causing it to become permeable and lose its contents. Both gram-positive and gram-negative bacteria are affected, but QACs are more bactericidal to the former. Like the phenol group, QACs bind well with oral tissues but have low substantivity.
Several QACs available commercially include cetylpyridinium chloride (CPC) (Cepacol 0.05%), which is also available with domiphen bromide (Scope 0.045%) or with benzethonium chloride (Colgate 100 0.05%), and (Crest Pro-Health Rinse 0.07%). CPCs have a history of safety, but overall their efficacy is still considered inconsistent and therefore they have been recommended only for cosmetic use. Crest Pro-Health Rinse (0.07%) has been shown to be effective in reducing plaque and gingivitis; however, long-term trials demonstrating conclusive plaque and gingivitis reductions have not yet been published, and the product has not received the ADA Seal of Acceptance. Brown extrinsic tooth stain can also occur with CPC-containing product use.
Herbal Extracts
Sanguinarine, a benzophenanthridine alkaloid, is an alcohol extract from the root of the plant Sanguinaria canadensis used in concentrations of 0.03% in both oral rinses and toothpaste. Some studies have shown sanguinarine oral rinse formulations to be more effective than placebo on plaque reductions, but the efficacy of sanguinarine with respect to gingival outcomes has been less positive.
Several other naturally sourced products have shown antimicrobial activity, but not in long-term studies with negative controls. “Active ingredients” such as echinacea, goldenseal, povidone-iodine, xylitol, and host proteins (lysozyme, lactoferrin, and lactoperoxidase [LLL]) have also been used in oral health products. For these, long-term studies are lacking or have not demonstrated efficacy in plaque and gingivitis reductions comparable to benchmark controls.
Oxygenating and Oxidizing Agents
Oxygenating agents are media with oxygen added to them (Amosan, Gly-Oxide); oxidizing agents are products that have had an increase in oxidation number forming derivatives of oxygen (e.g., Oxygene, Clo-Syst II). Although these agents negatively affect cell membranes and damage bacteria, long-term studies have not shown beneficial effects on reductions in bacterial plaque and gingivitis when compared with positive controls. Hydrogen peroxide, a common generic oxygenating agent with a history of oral use, continues to be recommended for temporary oral wound cleansing, but chronic use, particularly in higher concentrations, may result in soft-tissue damage and other side effects, and its use in oral self-care programs is not recommended. Hydrogen peroxide is contraindicated in persons who are immunocompromised.
Oxidizing agents (e.g., chlorine dioxide is a common generic) are available in mouth rinse and toothpaste formulations. Product claims are primarily cosmetic, particularly in relieving oral malodor through the neutralization of volatile sulfur compounds in the oral cavity. To date, no evidence supports the use of these products for therapeutic use in the reduction of plaque and gingivitis.
Antibiotics
Antibiotics comprise a group of drugs that inhibit or destroy pathogenic microorganisms including bacteria, and they can possess a broad or narrow spectrum of target organisms. Antibiotics are used in the treatment of periodontitis with both systemic and locally delivered vehicles. Locally delivered modes of application have the advantage of being placed, and therefore concentrated, directly at the disease site without causing systemic negative side effects (see Box 29-3). However, known sensitivities to antibiotics contraindicate their use regardless of the delivery mode.
CLIENT EDUCATION TIPS
 Explain that control of oral biofilm, gingivitis, and periodontitis are primarily addressed through personal and professional mechanical disease control methods; adjunctive chemotherapeutic interventions are introduced as required when mechanical means are insufficient.
Explain that control of oral biofilm, gingivitis, and periodontitis are primarily addressed through personal and professional mechanical disease control methods; adjunctive chemotherapeutic interventions are introduced as required when mechanical means are insufficient. Discuss that evaluation of clinical parameters will be required after introduction of chemotherapeutic intervention. If clinical outcomes are improved, chemotherapeutic therapy may no longer be indicated.
Discuss that evaluation of clinical parameters will be required after introduction of chemotherapeutic intervention. If clinical outcomes are improved, chemotherapeutic therapy may no longer be indicated.LEGAL, ETHICAL, AND SAFETY ISSUES
 Be knowledgeable of current professional literature; make use of systematic reviews and professional guidelines; initiate consultations and make appropriate referrals with other healthcare providers as necessary.
Be knowledgeable of current professional literature; make use of systematic reviews and professional guidelines; initiate consultations and make appropriate referrals with other healthcare providers as necessary. Care plans should be based on comprehensive oral assessment data, topics for educating the client, consideration of adjunctive chemotherapeutic agents, assessment of the client's oral self-care effectiveness, and ongoing reevaluation.
Care plans should be based on comprehensive oral assessment data, topics for educating the client, consideration of adjunctive chemotherapeutic agents, assessment of the client's oral self-care effectiveness, and ongoing reevaluation. Use health, dental, and pharmacologic history to rule out potential allergies or drug interactions with the oral chemotherapy being considered. Instruct clients to keep antimicrobial agents out of the reach of children.
Use health, dental, and pharmacologic history to rule out potential allergies or drug interactions with the oral chemotherapy being considered. Instruct clients to keep antimicrobial agents out of the reach of children. Dental hygienists are gaining limited prescriptive authority in some jurisdictions. Whether it is prescribing systemic agents, subantimicrobial drug dosages, or locally delivered antibiotics and fluorides, all have special considerations, potential side effects and adverse effects, and contraindications.
Dental hygienists are gaining limited prescriptive authority in some jurisdictions. Whether it is prescribing systemic agents, subantimicrobial drug dosages, or locally delivered antibiotics and fluorides, all have special considerations, potential side effects and adverse effects, and contraindications. Failure to prevent or control the progression of periodontal disease can be related to the presence of risk factors, inadequate client education, and inadequate implementation of plaque control measures. Dental hygienists have a legal and ethical responsibility to ensure that clients are completely informed about the link among risk factors, oral self-care, and disease progression.
Failure to prevent or control the progression of periodontal disease can be related to the presence of risk factors, inadequate client education, and inadequate implementation of plaque control measures. Dental hygienists have a legal and ethical responsibility to ensure that clients are completely informed about the link among risk factors, oral self-care, and disease progression. The client record should reveal that the client has been informed of the current disease status and counseled on why and how to implement an effective daily oral self-care program and should describe the client's response or adherence to professional recommendations. Reevaluation of the client's progress and further professional recommendations also should be recorded.
The client record should reveal that the client has been informed of the current disease status and counseled on why and how to implement an effective daily oral self-care program and should describe the client's response or adherence to professional recommendations. Reevaluation of the client's progress and further professional recommendations also should be recorded.KEY CONCEPTS
 Although pathogens in oral biofilm initiate inflammatory periodontal diseases, it is the host's response to the microbial load that causes disease progression.
Although pathogens in oral biofilm initiate inflammatory periodontal diseases, it is the host's response to the microbial load that causes disease progression. Although mechanical removal of oral biofilm via toothbrushes, interdental aides, and other oral physiotherapy devices remains the most widely accepted mechanism for disease control, the host's response to the oral microbial load may necessitate the adjunctive use of antimicrobial agents.
Although mechanical removal of oral biofilm via toothbrushes, interdental aides, and other oral physiotherapy devices remains the most widely accepted mechanism for disease control, the host's response to the oral microbial load may necessitate the adjunctive use of antimicrobial agents. Success of local drug delivery systems in treating periodontal infections is dependent on their ability to deliver the antimicrobial agents to the disease site at the minimum inhibitory concentration for a sufficient duration of time.
Success of local drug delivery systems in treating periodontal infections is dependent on their ability to deliver the antimicrobial agents to the disease site at the minimum inhibitory concentration for a sufficient duration of time. Dentifrices and mouth rinses do not have a therapeutic effect on subgingival pathogenic microorganisms because they do not significantly penetrate the subgingival pocket and because of gingival crevicular fluid flow.
Dentifrices and mouth rinses do not have a therapeutic effect on subgingival pathogenic microorganisms because they do not significantly penetrate the subgingival pocket and because of gingival crevicular fluid flow. Substantivity is the ability of an active ingredient to be retained in the oral cavity and to continue to be released over an extended period of time without losing its potency.
Substantivity is the ability of an active ingredient to be retained in the oral cavity and to continue to be released over an extended period of time without losing its potency. Supragingival irrigation, as a homecare adjunct to conventional mechanical oral hygiene, is of value in the control of oral biofilm and gingivitis.
Supragingival irrigation, as a homecare adjunct to conventional mechanical oral hygiene, is of value in the control of oral biofilm and gingivitis. The clinical benefit of professionally administered subgingival irrigation performed in conjunction with scaling and root planing is limited when compared with scaling and root planing alone.
The clinical benefit of professionally administered subgingival irrigation performed in conjunction with scaling and root planing is limited when compared with scaling and root planing alone. Intracrevicular delivery devices consist of a drug reservoir that controls the rate of drug release directly into the periodontal pocket without the side effects associated with systemic drug administration.
Intracrevicular delivery devices consist of a drug reservoir that controls the rate of drug release directly into the periodontal pocket without the side effects associated with systemic drug administration.CRITICAL THINKING EXERCISES
 Which controlled-release drug delivery system is best suited for this client? Give rationale for your selection.
Which controlled-release drug delivery system is best suited for this client? Give rationale for your selection. What clinical outcomes can be achieved with the use of controlled-release drug delivery? What reevaluation interval should be recommended and why?
What clinical outcomes can be achieved with the use of controlled-release drug delivery? What reevaluation interval should be recommended and why? What homecare instruction should be given to Mr. Thomas, based on the controlled-release drug system used?
What homecare instruction should be given to Mr. Thomas, based on the controlled-release drug system used? What is the average cost of this controlled-release drug therapy? Does it have an insurance code? (See Chapter 28 for codes related to nonsurgical periodontal therapy.)
What is the average cost of this controlled-release drug therapy? Does it have an insurance code? (See Chapter 28 for codes related to nonsurgical periodontal therapy.)ACKNOWLEDGMENT
The authors acknowledge Kim Krust Bray for her past contributions to this chapter.
Refer to the Procedures Manual where rationales are provided for the steps outlined in the procedures presented in this chapter.
1. Gurenlian J.R. The role of dental plaque biofilm in oral health. J Dent Hyg. 2007;SS:4.
2. Asadoorian J. Strategies for incorporating antimicrobial mouthrinses into daily oral care. J Dent Hyg. 2007;SS:26.
3. American Dental Association Council on Scientific Affairs: Acceptance program guidelines: chemotherapeutic products for control of gingivitis, 1999. Available at: www.ada.org/ada/seal/standards/guide_chemo_ging.pdf. Accessed February 5, 2008.
4. DePaola L.G., Spolarich A.E. Safety and efficacy of antimicrobial mouthrinses in clinical practice. J Dent Hyg. 2007;SS:13.
5. Asadoorian J. Oral rinsing. CDHA position paper on commercially available over-the-counter oral rinsing products., Can J Dent Hyg, 40. 2006. 168
6. Claffey N. Essential oil mouthwashes: a key component in oral health management. J Clin Periodontol. 2003;30(Suppl 5):22.
7. Kerr A.R., Katz R.W., Ship J.A. A comparison of the effects of 2 commercially available nonprescription mouthrinses on salivary flow rates and xerostomia. Quintessence Int. 2007;38:440.
8. Research, Science and Therapy Committee, American Academy of Periodontology. Position paper. The role of supra- and subgingival irrigation in the treatment of periodontal diseases., J Periodontol, 76. 2005. 2015
9. Hanes P.J., Purvis J.P. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79.
10. Andrews J.M. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl S1):5.
11. Bidault P., Chandad F., Grenier D. Systemic antibiotic therapy in the treatment of periodontitis. J Can Dent Assoc. 2007;73:515.
12. Preshaw P.M., Hefti A.F., Bradshaw M.H. Adjunctive subantimicrobial dose doxycycline in smokers and nonsmokers with chronic periodontitis. J Clin Periodontol. 2005;32:610.
13. Kirkwood K.L., Cirelli J.A., Rogers J.E., Giannobile W.V. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol. 2007;2000(43):294.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..