CHAPTER 38 Dentinal Hypersensitivity Management
DENTINAL HYPERSENSITIVITY
Tooth pain and sensitivity are common client complaints in the oral care environment. Several conditions may elicit a pain response, with the nature and extent of pain varying substantially both individually and among persons. Therefore it is critical to assess oral sites of sensitivity using a standardized approach, to identify an appropriate cause and thus manage the problem correctly. Dentinal hypersensitivity is characterized by short, sharp pain arising from exposed dentin that occurs in response to stimuli, typically thermal (both hot and cold), evaporative, tactile, osmotic, or chemical, and that cannot be ascribed to any other form of dental defect or pathology.1,2
Etiology and Nature of Dentinal Hypersensitivity
Tooth development results in the following cementum-to-enamel relationships:
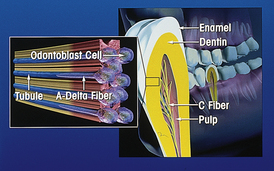
Histologically dentin is composed of numerous thin tubules that transverse from the pulp to the outer dentinal surface. Three types of sensory nerve fibers, known as A-delta fibers, A-beta fibers, and C-fibers, are found to extend 10% to 15% of the distance from the pulpal side of the dentinal tubule to the dentinoenamel junction. Stimulation of these sensory nerve fibers manifests as tooth pain. A-delta fibers are composed of small myelinated fibers that evoke a sensation of well-localized sharp pain and are thought to be responsible for dentinal hypersensitivity. Similarly, A-beta fibers are susceptible to the same types of stimuli but respond more sensitively to electrical stimulation. In contrast to the A-delta and A-beta fibers, stimulation of the unmyelinated C-fibers results in a dull, poorly localized, aching type of pain usually associated with pulpal pain. Thus the activation of specific fibers results in different types of tooth pain.
Hypersensitive dentin has the following characteristics:
 Thin, poorly calcified or breached smear layer (a deposit of salivary proteins, debris from dentifrices and/or other calcified matter that occludes dentinal tubules)
Thin, poorly calcified or breached smear layer (a deposit of salivary proteins, debris from dentifrices and/or other calcified matter that occludes dentinal tubules)In nonsensitive dentin the smear layer covers the opening of the dentinal tubules, or mineral compounds occlude the tubules, reducing the ability of stimuli to induce fluid flow (see section on hydrodynamic theory), and thus stimulate nerve conduction to the pulp. Therefore the loss or removal of a smear layer may result in exposed tubular nerve fibers, leading to a pain response. Nonsensitive dentin is also found to have fewer dentinal tubules present at the surface than sensitive dentin.3 Scanning electron photomicrographs verify that hypersensitive dentin has eight times as many open dentinal tubules and twice the diameter of open tubules as nonsensitive dentin. These findings serve as the basis for treatment options.
Hydrodynamic Theory
Brannstrom was the first to provide evidence to support the widely accepted hydrodynamic theory explaining the pain of dentinal hypersensitivity.4 The hydrodynamic theory proposes that stimuli (e.g., thermal, tactile, or chemical) are transmitted to the pulp surface via movement of fluid or semifluid materials found within the dentinal tubules. This fluid movement acts as a transducing medium that conveys peripheral stimuli to free A-delta nerve endings near the odontoblastic layer of the pulp-dentin interface. Subsequently this reaction is interpreted as tooth pain by the client (Figure 38-1).
For dentinal hypersensitivity, an open dentinal tubule channel must traverse from the exposed dentin surface to a vital pulp. The exposed dentin necessary for such hypersensitivity is most commonly the result of gingival recession or enamel loss along with other causes.5 When gingival recession occurs, cementum is exposed. This exposed layer of cementum is thin and labile and is easily abraded or eroded away, thus offering little protection against sensitivity.6
Causes of Gingival Recession7-22
 Anatomy of the labial plate of the alveolar bone. A thin, fenestrated, or absent labial alveolar bone is a major predisposing factor to recession.7,8 Tooth anatomy9 and tooth position10 also affect the thickness of the labial plate. For example, orthodontic treatment may move the tooth through the buccal plate, predisposing it to recession.
Anatomy of the labial plate of the alveolar bone. A thin, fenestrated, or absent labial alveolar bone is a major predisposing factor to recession.7,8 Tooth anatomy9 and tooth position10 also affect the thickness of the labial plate. For example, orthodontic treatment may move the tooth through the buccal plate, predisposing it to recession. Poor oral hygiene status. Poor oral self-care results in plaque-induced gingival disease, which can progress to attachment loss and result in recession; however, research reveals that even more recession occurs with aggressive oral hygiene.11
Poor oral hygiene status. Poor oral self-care results in plaque-induced gingival disease, which can progress to attachment loss and result in recession; however, research reveals that even more recession occurs with aggressive oral hygiene.11 Acute or chronic trauma. Gingival trauma caused by toothbrushing and/or injury are significant risk factors.12-14 The technique, frequency, duration, and force of brushing and toothbrush filaments have been implicated in recession.15-17 Injury to the gingiva caused by foreign objects18 or damaging habits such as fingernail scratching may also lead to recession.
Acute or chronic trauma. Gingival trauma caused by toothbrushing and/or injury are significant risk factors.12-14 The technique, frequency, duration, and force of brushing and toothbrush filaments have been implicated in recession.15-17 Injury to the gingiva caused by foreign objects18 or damaging habits such as fingernail scratching may also lead to recession. Frenal attachment at the gingival margin. Progressive recession may occur when the fibers of the frenum insert near the gingival margin and cause a tight frenal pull on the gingival tissues during function. Tissue movement resulting from speech and mastication pulls the gingiva from the cementoenamel junction (CEJ), resulting in gingival recession.19
Frenal attachment at the gingival margin. Progressive recession may occur when the fibers of the frenum insert near the gingival margin and cause a tight frenal pull on the gingival tissues during function. Tissue movement resulting from speech and mastication pulls the gingiva from the cementoenamel junction (CEJ), resulting in gingival recession.19 Occlusal trauma. A number of studies performed on human subjects have concluded that occlusal discrepancies appear to be a significant risk factor for attachment loss in subjects with active periodontal disease.20 It is thought that occlusal forces may exceed the resistance threshold of a compromised attachment apparatus, thereby exacerbating a preexisting periodontal lesion21 and thus possibly leading to further recession.
Occlusal trauma. A number of studies performed on human subjects have concluded that occlusal discrepancies appear to be a significant risk factor for attachment loss in subjects with active periodontal disease.20 It is thought that occlusal forces may exceed the resistance threshold of a compromised attachment apparatus, thereby exacerbating a preexisting periodontal lesion21 and thus possibly leading to further recession.Causes of Enamel Loss (see Chapters 14 and 23)
 Attrition. Sites of tooth structure wear are commonly found on the incisal or occlusal surfaces of teeth caused by masticatory forces. Unless malocclusion is involved, it is highly unlikely that attrition is observed at the buccal sites.
Attrition. Sites of tooth structure wear are commonly found on the incisal or occlusal surfaces of teeth caused by masticatory forces. Unless malocclusion is involved, it is highly unlikely that attrition is observed at the buccal sites. Abrasion. Toothbrush variation (stiffness and configuration of the bristles), coupled with force, method, frequency, abrasiveness of toothpaste, and duration of brushing, results in tooth structure loss. When the teeth are brushed, enamel has been found to abrade much more slowly than dentin or cementum. For example, dentin abrades 25 times and cementum 35 times faster than enamel.
Abrasion. Toothbrush variation (stiffness and configuration of the bristles), coupled with force, method, frequency, abrasiveness of toothpaste, and duration of brushing, results in tooth structure loss. When the teeth are brushed, enamel has been found to abrade much more slowly than dentin or cementum. For example, dentin abrades 25 times and cementum 35 times faster than enamel. Erosion. Tooth structure loss caused by a chemical process is most responsible for enamel loss. Intrinsic erosion is caused by acid regurgitation associated with medical and psychologic disorders (e.g., bulimia, acid reflux disease, morning sickness). Extrinsic erosion is a result of dietary factors that contribute to a highly acidic oral environment (e.g., the frequent consumption of acidic, carbonated, or fruit drinks or frequent sugar consumption).23
Erosion. Tooth structure loss caused by a chemical process is most responsible for enamel loss. Intrinsic erosion is caused by acid regurgitation associated with medical and psychologic disorders (e.g., bulimia, acid reflux disease, morning sickness). Extrinsic erosion is a result of dietary factors that contribute to a highly acidic oral environment (e.g., the frequent consumption of acidic, carbonated, or fruit drinks or frequent sugar consumption).23 Abfraction. The ongoing flexion, tension, and compression forces exerted in the cervical area of a tooth from mastication and occlusal trauma can result in cracking and eventual loss of cervical tooth structure.
Abfraction. The ongoing flexion, tension, and compression forces exerted in the cervical area of a tooth from mastication and occlusal trauma can result in cracking and eventual loss of cervical tooth structure.The effects of abrasion and erosion suggest that the loss of enamel and dentin by toothpaste abrasion is considerably increased if there is prior exposure to low-pH fluids, such as acidic juices.23,24 Thus loss of enamel can occur at an accelerated rate under the combined conditions of abrasion, erosion, and abfraction, resulting in exposed dentin.
Additional Causes
Aggressive scaling and root planing, especially after periodontal surgery, can remove layers of protective cementum and dentin, thus exposing tubular dentin and causing sensitivity. One study reported an estimated 73% to 98% prevalence of dentinal sensitivity in periodontal patients, as opposed to 36% in the general population.25
Prevalence and Distribution of Dentinal Hypersensitivity
Reports of dentinal hypersensitivity range from early teens to 70 years of age26; peak incidence occurs at 20 to 40 years of age and is consistent with the incidence and progression of gingival recession.27 However, as an individual ages, the prevalence of dentinal hypersensitivity decreases due to an increase in reparative dentin formation; reduction in pulpal chamber size, vascularity, and pulpal nerve fibers; and dentinal sclerosis (reduction of the dentinal tubule lumen as a result of the deposition of intratubular dentin).
Dentinal hypersensitivity is more prevalent in females than in males.2,27,28 The difference between hypersensitivity in females and in males may be attributed to the more frequent and extensive oral hygiene of females than of males, specifically at buccal sites.29
Dentinal hypersensitivity is most prevalent on the buccal cervical regions of teeth.2,27,30 Similarly, these same sites have a predilection for gingival recession and are the areas where the enamel is the thinnest. Thus gingival recession and loss of enamel appear to be related to the initiation of dentinal hypersensitivity.
The teeth most commonly affected in order of frequency are canines and first premolars, incisors and second premolars, and molars.26-28 Epidemiologic data show that dentinal hypersensitivity is negatively correlated with plaque scores.31 Buccal cervical plaque scores on canines and premolars tend to be lower than at other buccal sites.
Persons with moderate to severe sensitivity exhibit hypersensitivity at the same tooth sites, and there is a greater frequency of left-sided tooth sensitivity in comparison with their right contralateral tooth types. Hence, individuals who are right-handed tend to clean their left-sided teeth more vigorously than their right-sided teeth, contributing to unilateral hypersensitivity.
Diagnosis
Many oral conditions exhibit symptoms similar to dentinal hypersensitivity. Conditions such as chipped or fractured teeth, dental caries, pulpal pathology, or leaking, fractured, or failing restorations require completely different treatment from dentinal hypersensitivity. It is vitally important for the treating practitioner to understand that dentinal hypersensitivity is a diagnosis of exclusion. Therefore a thorough clinical and radiographic examination must be conducted to exclude these conditions and arrive at a differential diagnosis of dentinal hypersensitivity (Box 38-1). For a diagnosis of dentinal hypersensitivity to be made, specific clinical and radiographic criteria must be present.
MANAGEMENT OF DENTINAL HYPERSENSITIVITY
In managing dentinal hypersensitivity, it is essential to identify the condition's cause and risk factors (Box 38-2). Failure to address these conditions can result in inadequate and/or unnecessary therapy.
BOX 38-2 Factors That Contribute to Dentinal Hypersensitivity
Factors that may expose dentin or opening tubules that are already blocked or sealed:
After a cause is established, the client needs to be educated about behaviors that exacerbate their symptoms of dentinal hypersensitivity. If necessary, behavior modification may be discussed (e.g., dietary choices such as avoiding carbonated beverages, acidic foods, and extremes in hot and cold foods; use of a daily fluoride mouth rinse and a low-abrasive, fluoride dentifrice for sensitive teeth) to arrest the hypersensitivity.
Treatment options include self-applied (at-home) desensitizing agents and professionally applied (in-office) desensitizing procedures and surgeries. Desensitizing agents used in treatment are classified by mode of action (Table 38-1): inactivation of the nerve membrane (hyperpolarization) or occlusion of the open dentinal tubules.
TABLE 38-1 Desensitizing Agents and Their Mode of Action
| Nerve inactivator | Potassium nitrate |
|---|---|
| Tubule obtundents | |
| Protein precipitants |
CPP-ACP, Casein phosphopeptide–amorphous calcium phosphate complex.
 Nerve hyperpolarization. Intradental nerves are hyperpolarized by raising their extracellular potassium ion concentration. The sustained hyperpolarized state reduces nerve excitation, and the nerves become insensitive to further stimulation for a finite duration of time. A common example of an agent to use is potassium nitrate.
Nerve hyperpolarization. Intradental nerves are hyperpolarized by raising their extracellular potassium ion concentration. The sustained hyperpolarized state reduces nerve excitation, and the nerves become insensitive to further stimulation for a finite duration of time. A common example of an agent to use is potassium nitrate. Dentinal tubule occlusion. Examples of agents to use include oxalate compounds, strontium chloride, calcium hydroxide, fluorides, silver nitrate, amorphous calcium phosphate, casein phosphopeptide complexes, and hydroxyethyl methacrylate (HEMA).
Dentinal tubule occlusion. Examples of agents to use include oxalate compounds, strontium chloride, calcium hydroxide, fluorides, silver nitrate, amorphous calcium phosphate, casein phosphopeptide complexes, and hydroxyethyl methacrylate (HEMA).Without effective daily oral biofilm control, the desensitizing effects of these agents are limited.
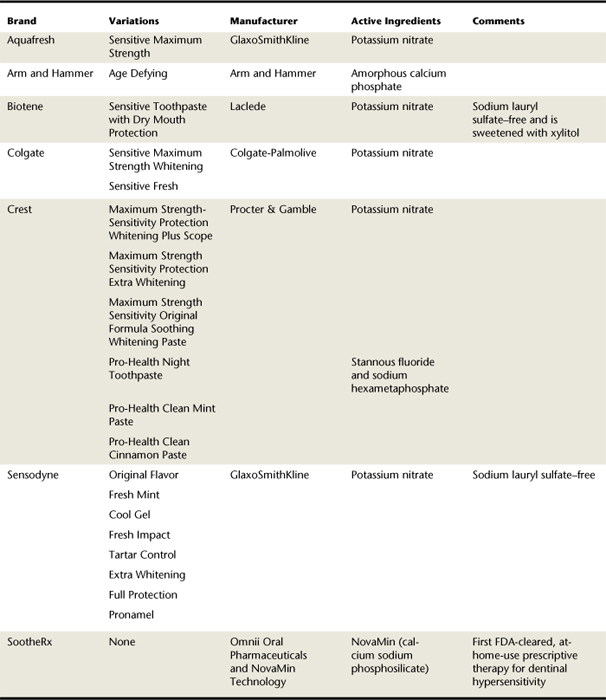
Self-Applied Desensitizing Agents (Table 38-2 and Figure 38-2; see Chapter 23)
Self-applied desensitizing agents should be recommended to manage mild dentinal hypersensitivity. These agents are cost-effective, safe, noninvasive, and simple to use and can be applied at home for convenience. Clients must be informed that regular and continuous application is necessary to manage sensitivity and that the time required to decrease individual sensitivity is variable. Clients may apply a range of desensitizing agents in the form of dentifrices, gels, or rinses as part of their daily self-care regimen at home.
Potassium nitrate is the most common desensitizing agent in over-the-counter dentifrices. At a concentration of 5%, potassium nitrate in conjunction with sodium or monofluorophosphate fluoride significantly reduces symptoms within 2 weeks of daily use. Potassium ions penetrate the length of the dentinal tubule and block repolarization of the nerve ending. Increasing the extracellular potassium ion concentration depolarizes nerve fiber membranes and renders them unable to repolarize (i.e., they are hyperpolarized). Frequent and regular application of a potassium nitrate dentifrice is necessary to avoid recurrence of symptoms, maintain a high abundance of extracellular potassium ions, and maintain the intradental nerves in a hyperpolarized state. Therefore application via a dentifrice is ideal. Moreover, clients can be instructed to dab very small amounts of sensitivity-protection dentifrice on the sensitive area of the tooth at bedtime, which is left overnight.
Self-applied desensitizing agents also are marketed as gels and rinses. The active agents for these products are various fluoride compounds, such as sodium fluoride, sodium silicofluoride, and stannous fluoride. Some dentifrices have the American Dental Association (ADA) Seal of Acceptance for treatment of dentinal hypersensitivity (see Table 38-2). Application of fluoride to exposed dentin leads to the formation of calcium fluoride and other precipitates, reducing the functional radius of the dentinal tubules or blocking the dentinal tubules. Therefore relief can be achieved via the use of fluoride-containing gels and rinses; however, extended periods of use are necessary. It is important for the treating practitioner to inform the client that using products containing potassium nitrate will provide only immediate, short-term relief from dentinal hypersensitivity. Long-term relief requires continued use of fluoride-containing substances to permanently seal off the exposed tubules with calcium fluoride particles.
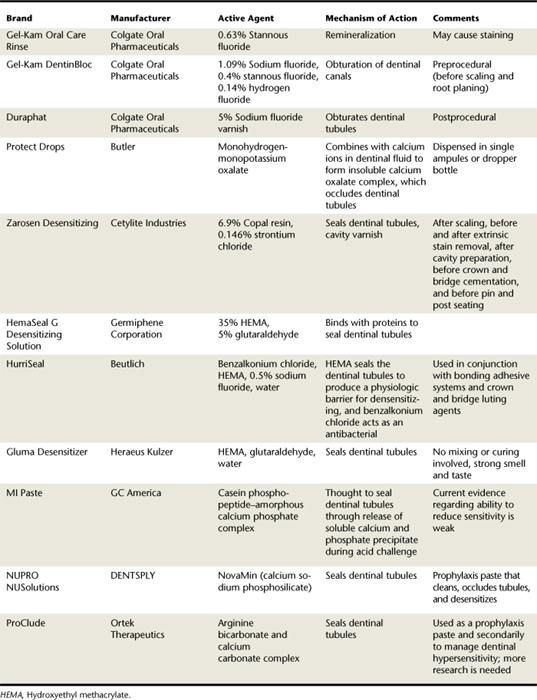
Professionally Applied Desensitizing Agents (Procedure 38-1,Table 38-3, and Figure 38-3)
Although mild hypersensitivity may be managed by using a sensitivity-protection toothpaste twice daily, moderate to severe dentinal hypersensitivity must be treated professionally. Professionally applied agents include varnishes and precipitants, primers containing HEMA, and polymerizing agents. In severe cases, loss of cervical tooth structure often requires restoration with glass ionomer and/or composite resin materials to control hypersensitivity.
Before any desensitizing treatment, hard and soft deposits should be removed from the tooth surfaces. Therapeutic scaling may cause considerable discomfort, in which case teeth should be anesthetized before mechanical treatment.
Varnishes
 5% Sodium fluoride varnish. Fluoride varnishes temporarily occlude dentinal tubules because the material is lost over time. This desensitizing agent is effective for relief of dentinal hypersensitivity (see Chapter 31, discussion of topical fluorides, professionally applied varnishes).
5% Sodium fluoride varnish. Fluoride varnishes temporarily occlude dentinal tubules because the material is lost over time. This desensitizing agent is effective for relief of dentinal hypersensitivity (see Chapter 31, discussion of topical fluorides, professionally applied varnishes).Precipitants
 Oxalates. The efficacy of oxalate-containing agents is unclear. Comparison of the clinical evidence fails to objectively demonstrate the efficacy of oxalate-containing agents because of various experimental designs.
Oxalates. The efficacy of oxalate-containing agents is unclear. Comparison of the clinical evidence fails to objectively demonstrate the efficacy of oxalate-containing agents because of various experimental designs. Calcium phosphate compounds. Burnishing of calcium phosphate into areas of sensitive dentin significantly relieves discomfort. The mechanism of action involves the occlusion of dentinal tubules by forming a calcium phosphate precipitate.32 A randomized, double-blind clinical trial concluded that both amorphous calcium phosphate and a sodium fluoride solution reduced periodontal treatment–induced dentin hypersensitivity by similar amounts.33 However, a double-blind, randomized, placebo-controlled, split-mouth study demonstrated that both amorphous calcium phosphate and its control (water) had statistically similar reductions in dentin hypersensitivity over 3 months.34
Calcium phosphate compounds. Burnishing of calcium phosphate into areas of sensitive dentin significantly relieves discomfort. The mechanism of action involves the occlusion of dentinal tubules by forming a calcium phosphate precipitate.32 A randomized, double-blind clinical trial concluded that both amorphous calcium phosphate and a sodium fluoride solution reduced periodontal treatment–induced dentin hypersensitivity by similar amounts.33 However, a double-blind, randomized, placebo-controlled, split-mouth study demonstrated that both amorphous calcium phosphate and its control (water) had statistically similar reductions in dentin hypersensitivity over 3 months.34 Calcium hydroxide. This desensitizing agent has been used to block dentinal tubules and promote peritubular dentin formation. It also is effective in reducing the permeability of acid-etched dentin and smear layers.
Calcium hydroxide. This desensitizing agent has been used to block dentinal tubules and promote peritubular dentin formation. It also is effective in reducing the permeability of acid-etched dentin and smear layers. Casein phosphopeptide–amorphous calcium phosphate (CPP-ACP). CPP is a protein found in cow's milk and has the ability to stabilize and bind calcium and phosphate ions, thus making them soluble and bioavailable. When applied orally, this nanocomplex has been found to bind to soft tissues, pellicle, oral biofilm, and hydroxyapatite and subsequently releases calcium and phosphate ions when challenged by acid attack. It is thought that this ion release leads to a precipitate which plugs open dentinal tubules. However, a recent study demonstrated that the use of MI Paste (a commercial product that delivers CPP-ACP) had insufficient effectiveness and short-term therapeutic effect in treating hypersensitivity of dentin.35 However, this study was noted to lack an appropriate control group and masking of evaluators.36 Because of its origins, this product should not be used in patients with a milk protein allergy and/or with a sensitivity or allergy to benzoate preservatives. Religious views must also be taken into consideration.
Casein phosphopeptide–amorphous calcium phosphate (CPP-ACP). CPP is a protein found in cow's milk and has the ability to stabilize and bind calcium and phosphate ions, thus making them soluble and bioavailable. When applied orally, this nanocomplex has been found to bind to soft tissues, pellicle, oral biofilm, and hydroxyapatite and subsequently releases calcium and phosphate ions when challenged by acid attack. It is thought that this ion release leads to a precipitate which plugs open dentinal tubules. However, a recent study demonstrated that the use of MI Paste (a commercial product that delivers CPP-ACP) had insufficient effectiveness and short-term therapeutic effect in treating hypersensitivity of dentin.35 However, this study was noted to lack an appropriate control group and masking of evaluators.36 Because of its origins, this product should not be used in patients with a milk protein allergy and/or with a sensitivity or allergy to benzoate preservatives. Religious views must also be taken into consideration.Primers Containing Hydroxyethyl Methacrylate
Although few controlled clinical trials have been conducted on the efficacy of HEMA-containing primers, desensitizing agents containing either 5% glutaraldehyde and 35% HEMA in water or 35% HEMA in water alone are popular.
 5% Glutaraldehyde, 35% HEMA in water. A randomized clinical trial demonstrated that a primer containing 5% glutaraldehyde and 35% HEMA in water was effective in reducing dentinal hypersensitivity after 3 months; however, this treatment was not as effective as 2% sodium fluoride iontophoresis therapy.37 Another study of HEMA-containing desensitizing agents showed reductions in sensitivity that lasted for the entire 6-month trial.38
5% Glutaraldehyde, 35% HEMA in water. A randomized clinical trial demonstrated that a primer containing 5% glutaraldehyde and 35% HEMA in water was effective in reducing dentinal hypersensitivity after 3 months; however, this treatment was not as effective as 2% sodium fluoride iontophoresis therapy.37 Another study of HEMA-containing desensitizing agents showed reductions in sensitivity that lasted for the entire 6-month trial.38Polymerizing Agents
 Glass ionomer cements (GICs). GICs are used in cervical abrasions and abfractions for treatment of dentinal hypersensitivity. The cervical areas of a tooth are etched with 50% citric acid for 30 to 45 seconds, rinsed with water, and dried before GIC placement. GICs are effective in treating hypersensitivity if they cover the affected area.
Glass ionomer cements (GICs). GICs are used in cervical abrasions and abfractions for treatment of dentinal hypersensitivity. The cervical areas of a tooth are etched with 50% citric acid for 30 to 45 seconds, rinsed with water, and dried before GIC placement. GICs are effective in treating hypersensitivity if they cover the affected area. Adhesive resin primers. Adhesive resin primers decrease dentin permeability by occluding the open dentinal tubules. Resin primers come in either a two- or one-bottle system. The product is gently rubbed on the hypersensitive dentin for approximately 30 seconds and air-dried, and the procedure is possibly repeated.
Adhesive resin primers. Adhesive resin primers decrease dentin permeability by occluding the open dentinal tubules. Resin primers come in either a two- or one-bottle system. The product is gently rubbed on the hypersensitive dentin for approximately 30 seconds and air-dried, and the procedure is possibly repeated.Iontophoresis
Iontophoresis involves the delivery of sodium fluoride by passing an electrical current through the cervical dentin. This procedure is based on the principle that similar electromagnetic charges repel each other. When the negative fluorine ions contact the negatively charged electrode and a current is passed through the tooth to the other electrode (which is held by the client, completing the circuit), fluoride ions are pushed into the dentinal tubules, where they react with ions in the hydroxyapatite. Fluorapatite precipitate, an insoluble compound, is formed, thus occluding the tubules.
Use of this technique-sensitive procedure to treat hypersensitive dentin has proponents.39-42 Lack of efficacy reported by others may be the result of the inadvertent passage of current through adjacent gingival tissue rather than through cervical dentin.43 Mild cases of dentinal hypersensitivity may require only a single treatment, whereas in more severe cases two or three applications 1 week apart may be necessary. The procedure requires a special apparatus.
Lasers
Laser therapy is relatively quick, and one treatment drastically reduces or eliminates sensitivity by sealing the dentinal tubules. Dentin treated with laser is harder than untreated dentin. Use of lasers, such as the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, is based on the premise that they cause coagulation and precipitation of plasma proteins in dentinal fluid.44 Use of lasers to treat dentinal hypersensitivity is not well documented in the literature,45,46 and the current high cost of equipment does not yet justify their clinical use.
Restorations
Desensitizing agents either occlude the open tubule or inactivate the nerve. Restorations may be placed to cover exposed dentin and restore tooth anatomy, especially where aesthetics are important. In extreme circumstances it may be necessary to remove the pulp and perform root canal therapy, or extract the tooth. These last two options are indicated for reasons in addition to dentinal hypersensitivity, such as inability to restore the tooth, severe periodontal destruction, overeruption, or aesthetics.
Periodontal Plastic Surgery
Over the years numerous techniques have been developed to surgically correct gingival recession. Procedures range from use of juxtaposed gingiva, guided tissue regeneration, and tissue engineered human fibroblast–derived dermal substitute; however, the most common and predictable procedure for the treatment of Miller Class I and II defects is the subepithelial connective tissue graft. This procedure, which harvests a patient's connective tissue (usually from the palate) and places it on top of the exposed root, has been reported to not only increase patient clinical attachment, but also decrease dentinal sensitivity after 6 months (Box 38-3 and Figure 38-4).47
BOX 38-3 Case Study of Client Treated with a Connective Tissue Graft to Control Dentinal Hypersensitivity
CM came to the practice complaining of severe sensitivity to cold air and fluids around teeth 28 and 29 over the past 5 months, and as a result had avoided toothbrushing or flossing in that area. Periodontal assessment revealed localized erythema, oral biofilm accumulation, and recession of 3 mm and 1 mm on teeth 28 and 29, respectively. In addition, tooth 28 had minimal keratinized tissue (1 mm), extrinsic staining, and cervical abrasion. CM acknowledged a history of aggressive toothbrushing.
CM's care plan included oral self-care instructions, with emphasis on the modified Bass brushing technique with a sensitivity toothpaste, scaling and root planing under a local anesthetic, and use of a soft-bristled toothbrush to improve gingival health before periodontal surgery. A connective tissue graft procedure was performed to provide a thicker gingival biotype buccal to tooth 28, and to achieve root coverage over both premolars. Before surgery, CM reported a Visual Analog Scale (VAS) value of 10 when tooth 28 was subjected to a cold air blast from an air-water syringe.
Six weeks after the surgical procedure was performed, the client's reported VAS value improved to 5 (see Figure 38-4).
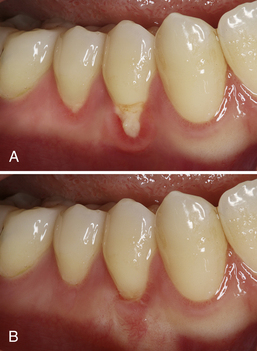
Figure 38-4 Client with severe dentinal hypersensitivity of teeth 28 and 29. A, Before connective tissue graft surgery. B, After connective tissue graft surgery.
(Courtesy Dr. Angela Demeter, Graduate Periodontal Resident, University of British Columbia.)
CLIENT EDUCATION TIPS
 Discuss dietary information, and monitor acidic and sugary fruits and beverages that might contribute to hypersensitivity.
Discuss dietary information, and monitor acidic and sugary fruits and beverages that might contribute to hypersensitivity.LEGAL, ETHICAL, AND SAFETY ISSUES
KEY CONCEPTS
 Assessment of etiology and risk factors is critical in accurately identifying dentinal hypersensitivity.
Assessment of etiology and risk factors is critical in accurately identifying dentinal hypersensitivity. Hypersensitive dentin has the following characteristics: dentinal tubules open to the oral cavity, large and numerous dentinal tubules, and thin, poorly calcified, or breached smear layer (a deposit of salivary proteins, debris from dentifrices, and other calcified matter).
Hypersensitive dentin has the following characteristics: dentinal tubules open to the oral cavity, large and numerous dentinal tubules, and thin, poorly calcified, or breached smear layer (a deposit of salivary proteins, debris from dentifrices, and other calcified matter). Abfraction is damage resulting from the ongoing flexion, tension, and compression forces exerted in the cervical area of a tooth as a result of mastication and occlusal trauma. These forces result in cracking and eventual loss of cervical tooth structure.
Abfraction is damage resulting from the ongoing flexion, tension, and compression forces exerted in the cervical area of a tooth as a result of mastication and occlusal trauma. These forces result in cracking and eventual loss of cervical tooth structure. Dentinal hypersensitivity is characterized by short, sharp pain, arising from exposed dentin, that occurs in response to stimuli, typically thermal (both hot and cold), evaporative, tactile, osmotic, or chemical, and that cannot be ascribed to any other form of dental defect or pathology.
Dentinal hypersensitivity is characterized by short, sharp pain, arising from exposed dentin, that occurs in response to stimuli, typically thermal (both hot and cold), evaporative, tactile, osmotic, or chemical, and that cannot be ascribed to any other form of dental defect or pathology. The hydrodynamic theory proposes that stimuli (i.e., thermal, tactile, or chemical) are transmitted to the pulp surface via movement of the fluid or semifluid materials in the dentinal tubules.
The hydrodynamic theory proposes that stimuli (i.e., thermal, tactile, or chemical) are transmitted to the pulp surface via movement of the fluid or semifluid materials in the dentinal tubules.CRITICAL THINKING EXERCISES
Use Figure 38-5 and the following information to answer the questions about this case.
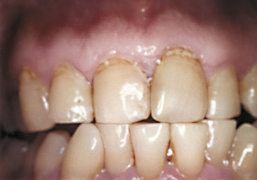
Figure 38-5 Intraoral photo of a young woman. Note accumulation of oral biofilm, gingival recession, cervical abrasion, and attrition.
Chief Complaint: “My teeth are very sensitive when I eat or drink cold foods and beverages.”
Health History: No significant findings
Pharmacologic History: Client takes the following medications:
Clinical Examination Findings:
 Localized recession and cervical abrasion evident on teeth 6, 7, 8, 9, 22, 23, 24, 25, 26, 27, 28, and 29
Localized recession and cervical abrasion evident on teeth 6, 7, 8, 9, 22, 23, 24, 25, 26, 27, 28, and 29 Linear radiolucent areas along the CEJ of 28 and 29 premolar teeth, consistent with the clinically observed posterior cervical abrasion
Linear radiolucent areas along the CEJ of 28 and 29 premolar teeth, consistent with the clinically observed posterior cervical abrasionQuestions: Given the client profile, chief complaint, and examination findings, answer the following questions:
Refer to the Procedures Manual where rationales are provided for the steps outlined in the procedure presented in this chapter.
1. Dowell P., Addy M., Drummer P. Dentine hypersensitivity: aetiology, differential diagnosis and management. Br J Dent. 1985;158:92.
2. Orchardson R., Collins W.J.N. Thresholds of hypersensitive teeth to two forms of controlled stimulation. J Clin Periodontol. 1987;14:68.
3. Absi E.G., Addy M., Adams D. Dentine hypersensitivity: a study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280.
4. Brannstrom M. A hydrodynamic mechanism in the transmission of pain-produced stimuli through dentine. In: Anderson D.J., editor. Sensory mechanisms in dentine. Oxford, England: Pergamon, 1963.
5. Strassler H.E., Drisko C.L., Alexander D.C. Dentin hypersensitivity: its inter-relationship to gingival recession and acid erosion. Suppl Inside Dent. 2008;4:1.
6. Bevenius J., Lindskog S., Hultenby K. The micromorphology in vivo of the buccocervical region of premolar teeth in young adults: a replica study by scanning electron microscopy. Acta Odontol Scand. 1994;52:323.
7. Bernimoulin J., Curilovie Z. Gingival recession and tooth mobility. J Clin Periodontol. 1977;4:107.
8. Lost C. Depth of alveolar bone dehiscences in relation to gingival recession. J Clin Periodontol. 1984;11:583.
9. Olsson M., Lindhe J. Periodontal characteristics in individuals with varying form of upper central incisors. J Clin Periodontol. 1991;18:78.
10. Modheer T., Odenrick L. Post treatment periodontal status of labially erupted maxillary canines. Acta Odontol Scand. 1980;38:253.
11. Mazdyasma S., Stoner J.E. Factors influencing gingival recession in the lower incisor region. J Periodontol. 1980;51:74.
12. Bergstrom J., Eliasson S. Cervical abrasion in relation to toothbrushing and periodontal health. Scand J Dent Res. 1988;96:405.
13. Khocht A., Simon G., Person P., Denepitiya J.L. Gingival recession in relation to history of hard toothbrush use. J Periodontol. 1993;64:900.
14. Sandholm L., Niemi M.I., Ainamo J. Identification of soft tissue brushing lesion: a clinical and scanning electron microscopic study. J Clin Periodontol. 1982;9:397.
15. Hirschfeld I. The toothbrush: its use and abuse—dental items of interest. London: Kimpton; 1939.
16. O'Leary T., Drake R.B., Crump P.P. The incidence of recession in young males. J Periodontol. 1971;42:264.
17. Gillette W.B., van House R.L. Effects of improper oral hygiene procedures. J Am Dent Assoc. 1980;10:476.
18. Jenkins W.M.M., Allan C.J. Guide to periodontics, ed 2. Oxford, England: Wright; 1994.
19. Powell R.N., McEniery T.M. Disparities in gingival height in the mandibular central incisor region of children aged 6-12 years. Community Dent Oral Epidemiol. 1981;9:32.
20. Hallmon W.W., Harrel S.K. Occlusal analysis, diagnosis and management in the practice of periodontics. Periodontol. 2004;34:51. 2000
21. Davies S.J., Gray R.J.M., Linden G.J., James J.A. Occlusal considerations in periodontics. Br Dent J. 2001;191:597.
22. Trott J.R., Love B. An analysis of localised gingival recession in 766 Winnipeg high school students. Dent Pract Dent Rec. 1966;16:209.
23. West N.X., Maxwell A., Hughes J.A., et al. A method to measure clinical erosion: the effect of orange juice consumption on erosion of enamel. J Dent. 1998;26:329.
24. Davis W.B., Winter P.J. The effect of abrasion on enamel and dentine after exposure to dietary acid. Br J Dent. 1980;148:253.
25. Drisko C.H. Dentin hypersensitivity—dental hygiene and periodontal considerations. Int Dent J. 2002;52:385.
26. Fischer C., Fischer R.G., Wennberg A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio De Janeiro, Brazil. J Dent. 1992;20:272.
27. Graf H.E., Galasse R. Morbidity, prevalence and intra-oral distribution of hypersensitive teeth. J Dent Res. 1977;56(Suppl):162.
28. Flynn J., Galloway R., Orchardson R. The incidence of hypersensitive teeth in the west of Scotland. J Dent. 1985;13:230.
29. Dummer P.M., Addy M., Hicks R., et al. The effect of social class on the prevalence of caries, plaque, gingivitis, and pocketing in 11-12 year old children in South Wales. J Dent. 1987;15:185.
30. Jensen A.I. Hypersensitivity controlled by iontophoresis: double blind clinical investigation. J Am Dent Assoc. 1964;68:216.
31. Alexander A.G. A study of the distribution of supra and subgingival calculus, bacterial plaque and gingival inflammation in the mouths of 400 individuals. J Periodontol. 1971;42:21.
32. Tung M.S., Bowen H.J., Derkson G.D., Pashley D.H. Effects of calcium phosphate solutions on dentin permeability. J Endodont. 1993;19:283.
33. Fiocchi M.F., Moretti A.J., Powers J.M., Rives T. Treatment of root sensitivity after periodontal therapy. Am J Dent. 2007;20:217.
34. Yates R., Owens J., Jackson R., et al. A split-mouth placebo-controlled study to determine the effect of amorphous calcium phosphate in the treatment of dentine hypersensitivity. J Clin Periodontol. 1998;25:687.
35. Kowalczyk A., Botulinski B., Jaworska M., et al. Evaluation of the product based Recaldent technology in the treatment of dentin hypersensitivity. Adv Med Sci. 2006;51(Suppl 1):40.
36. Azarpazhooh A., Limeback H. Clinical efficacy of casein derivatives: a systematic review of the literature. J Am Dent Assoc. 2008;139:915.
37. Singal P., Gupta R., Pandit N. 2% Sodium fluoride–iontophoresis compared to a commercially available desensitizing agent. J Periodontol. 2005;76:351.
38. Dondi dall'Orologio G., Malferrari S. Desensitizing effects of Gluma and Gluma 2000 on hypersensitive dentin. Am J Dent. 1993;6:283.
39. Christiansen G.J. Desensitization of cervical tooth structure. J Am Dent Assoc. 1998;129:765.
40. Gangarosa L.P. Iontophoresis in dental practice. Chicago: Quintessence; 1983.
41. Gangarosa L.P. Current strategies for dentist applied treatments in the management of hypersensitive dentine. Arch Oral Biol. 1994;39(Suppl):101.
42. Kerns D., McQuade M., Scheidt M. Effectiveness of sodium fluoride on tooth hypersensitivity with and without iontophoresis. J Periodontol. 1989;60:386.
43. Brough K.M., Anderson D.M., Love J., Overman P.R. The effectiveness of iontophoresis in reducing dentin hypersensitivity. J Am Dent Assoc. 1985;111:761.
44. Goodis H.E., et alWhite J.M., Marshall S.J., Marshall G.W. Measurement of fluid flow through laser-treated dentine. Arch Oral Biol. 1994;39(Suppl):128.
45. Renton-Harper P., Midda M. Nd:YAG laser treatment of dentinal hypersensitivity. Br J Dent. 1992;172:13.
46. Gelskey S.C., White J.M., Pruthi V.K. The effectiveness of the Nd:YAG laser in the treatment of dentinal hypersensitivity. J Can Dent Assoc. 1993;59:377.
47. Bittencourt S., Del Peloso Ribeiro E., Sallum E.A., et al. Comparative 6-month clinical study of a semilunar coronally positioned flap and subepithelial connective tissue graft for the treatment of gingival recession. J Periodontol. 2006;77:174.
48. Kuroiwa M., Kodaka T., Kuroiwa M., Abe M. Dentin hypersensitivity: occlusion of dentinal tubules by brushing with and without an abrasive dentifrice. J Periodontol. 1994;65:291.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..