CHAPTER 31 Caries Management
Fluoride, Chlorhexidine, Xylitol, and Amorphous Calcium Phosphate Therapies
 Distinguish between ingested fluorides and topical fluorides (self-applied and professionally applied) in dental caries management.
Distinguish between ingested fluorides and topical fluorides (self-applied and professionally applied) in dental caries management. Differentiate between acute and chronic fluoride toxicity including causes, signs and symptoms, and management.
Differentiate between acute and chronic fluoride toxicity including causes, signs and symptoms, and management. Discuss xylitol for dental caries management in terms of action, indications, and therapeutic dosage.
Discuss xylitol for dental caries management in terms of action, indications, and therapeutic dosage.Caries Management1-12
To determine the caries risk for an individual, the number and severity of risk factors present are evaluated (see Chapter 16 for caries risk assessment). The clinician also considers the existing protective factors and uses clinical judgment to determine degree of risk.
Caries management is aimed at restoring and maintaining a balance between protective factors and pathologic factors and reducing dental caries transmission (see Chapter 16, Figure 16-5). Caries management involves the following:
 Bacterial infection control; suppressing the infectious agents, Streptococcus mutans and Lactobacillus acidophilus, by using mechanical and chemical interventions (e.g., toothbrushing, interdental cleaning, chlorhexidine gluconate, xylitol)
Bacterial infection control; suppressing the infectious agents, Streptococcus mutans and Lactobacillus acidophilus, by using mechanical and chemical interventions (e.g., toothbrushing, interdental cleaning, chlorhexidine gluconate, xylitol) Remineralizing early noncavitated carious lesions by enhancing salivary flow and using professionally applied and self-applied fluorides and amorphous calcium phosphate (ACP) and casein phosphopeptide (CPP) to promote remineralization
Remineralizing early noncavitated carious lesions by enhancing salivary flow and using professionally applied and self-applied fluorides and amorphous calcium phosphate (ACP) and casein phosphopeptide (CPP) to promote remineralization Avoiding using the sharp explorer tip on demineralized tooth surfaces to prevent further tooth surface damage; rather, using other caries detection systems such as laser fluorescence and transillumination
Avoiding using the sharp explorer tip on demineralized tooth surfaces to prevent further tooth surface damage; rather, using other caries detection systems such as laser fluorescence and transillumination Surgically removing carious lesions that are beyond remineralization, and restoring the teeth with minimally invasive techniques and materials
Surgically removing carious lesions that are beyond remineralization, and restoring the teeth with minimally invasive techniques and materialsIncreasing protective factors involves strategies such as the use of fluorides; ACP containing dentifrices, prophylaxis pastes, and gum; CPP-ACP paste and gum; sealants, xylitol-containing gum, and mints; baking soda rinses and gum; and agents to increase salivary flow and therefore availablility of calcium and phosphate ions for remineralization. Decreasing pathologic factors and transmission involves strategies such as client education, oral hygiene instruction, reduction in intake of fermentable carbohydrates, and use of 0.12% chlorhexidine gluconate oral rinse and/or xylitol-containing products (which interferes with the metabolism and proliferation of S. mutans).
After determining the caries risk of an individual, the clinician educates the client about the caries process and risk versus preventive factors and makes recommendations based on test results, responses to questions, and clinical observation. Box 16-9 provides caries management principles for high-risk individuals; Table 31-1 identifies target areas for caries management. The client’s compliance with professional recommendations is assessed 3 to 6 months after the initial visit. If bacterial levels were moderate or high at the initial visit, bacterial testing is repeated to see if the infection is controlled. Recommendations should be modified or reinforced based on bacterial results and client compliance.
TABLE 31-1 Target Areas for Caries Management
| Target Area | Strategy |
|---|---|
| Tooth | Sealants, in-office and home fluorides, amorphous calcium phosphate (ACP) |
| Diet | Control consumption and frequency of sugar intake; increase consumption of xylitol-containing products to about 5-10 g/day with exposure throughout the day |
| Acid in mouth | Baking soda rinses; enhance saliva with salivary substitutes and xylitol-containing and ACP-containing gum and mints) |
| Low saliva flow | Increase consumption of xylitol-containing and ACP-containing gum and mints; use saliva-stimulating drugs (e.g., 20-30 mg of pilocarpine per day by prescription) |
| Bacteria | Good oral hygiene instruction; power toothbrushes; 0.12% chlorhexidine gluconate rinse; increase consumption of xylitol-containing gum and mints |
| Caries in family members or significant other | Control vertical and horizontal disease transmission by treating cariogenic bacterial infections in family members too |
FLUORIDE THERAPIES
See Chapter 16 for a discussion of fluoride therapies.
Fluoride is a naturally occurring nutrient that in the right concentrations can decrease dental caries risk and has a reparative (remineralizing) effect. Fluoride may be delivered via drinking water, foods, beverages, supplements, or professionally applied or self-applied techniques (Table 31-2).
 Fluoridated water and prescription supplements are ingested and delivered via the bloodstream and topically.
Fluoridated water and prescription supplements are ingested and delivered via the bloodstream and topically. Topical fluoride products (self-applied by clients in a nonprescription form, self-applied by clients in a prescription form, and professionally applied prescription products) are delivered for variable amounts of time to exposed crown and root surfaces and then expectorated.
Topical fluoride products (self-applied by clients in a nonprescription form, self-applied by clients in a prescription form, and professionally applied prescription products) are delivered for variable amounts of time to exposed crown and root surfaces and then expectorated.Fluoride Delivered via Water System or Supplements
Some fluorides are delivered via the community water supply or school-based water system, in the form of supplements (drops, lozenges, or tablets), and in foodstuffs (naturally occurring and in additives.) In the United States the most common systemic fluoride delivery is via the community water supply. By 2010, approximately 75% of the U.S. population will reside in communities where the water supply is fluoridated.
Community Water Fluoridation9
The notion of fluoridating the community water supply grew out of a need to address the prevalence of dental caries in the United States in the 1940s. At that time the population was not exposed to the myriad fluoride-containing products to which the population is exposed today. One of the great disease prevention measures of all time, community water fluoridation, is a nearly ideal public health intervention because the system has the following characteristics:
As a public health measure, community water fluoridation calls for testing of the community water supply to determine the naturally occurring level of fluoride. The level of fluoride is then adjusted through fluoridation or defluoridation to the accepted level of 0.7 to 1.2 parts per million (ppm), depending on the average maximum daily air temperature of the area. Defluoridation is necessary if the naturally occurring level of fluoride in the water exceeds the recommended level, putting the public at risk for dental fluorosis10 (chronic fluoride toxicity).
Antifluoridationists
Although the benefits of community water fluoridation are well documented, some individuals oppose this public health measure and actively seek to prevent or reverse water fluoridation programs. Antifluoridationists have a range of arguments for why community water supplies should not be fluoridated. They associate fluoride ingestion with an increased risk for certain systemic diseases and conditions (e.g., congenital anomalies, bone fractures, Alzheimer’s disease, cancer) and view mandated fluoride ingestion as a conspiracy by the government, the healthcare industry, and others. These individuals cite a variety of other reasons for their antifluoride stance, including cost, freedom of choice, and a violation of individual and religious rights. Antifluoridationists have been effective in blocking community fluoride programs in many areas.
Food and Beverages
The public is exposed to a variety of fluoride-containing foodstuffs that become part of the client’s overall exposure to topical and systemic fluoride. There is considerable variation in the amount of fluoride in products routinely ingested; thorough questioning is necessary to document this information during client assessment procedures (see Chapter 10Table 10-2Figure 10-4).
Infants primarily ingest breast milk, cow’s milk, and milk-based and soy-based formulas. Fluoride levels are generally low in human breast milk (<0.01 ppm) and cow’s milk (0.05 ppm). When the formula industry recognized that milk-based formula might be reconstituted with fluoridated water, many voluntarily reduced the fluoride content in powdered formula. Formula packaging should be consulted to determine fluoride levels in the prepared powder. Ready-to-use soy-based formulas have more fluoride than do milk-based products (0.30 ppm).10
Beverages prepared from natural ingredients may be a systemic and topical source of fluoride. Raw tea leaves are high in fluoride content and contain as much as 400 ppm of fluoride. Brewed tea contains an average of 3 ppm of fluoride. This is a risk factor for dental fluorosis in cultures where children consume tea on a daily basis during tooth development (e.g., Asian Indians and Asians).10
The fluoride level in processed beverages and bottled waters varies considerably. For example, fluoride content in fruit juices and carbonated beverages ranges from <0.1 to 6.7 ppm. Differences in fluoride content in processed beverages is attributed to the variations in the fluoride levels of the water used to prepare these products.10 Although there is also variation in the fluoride content of bottled waters, these beverages generally have low fluoride concentration. Bottled waters are marketed as distilled, drinking, mineral, or natural spring waters and may be carbonated or noncarbonated. Given that consumption of tap water among U.S. children has declined and consumption of other beverages has grown, it is becoming increasingly difficult to assess clients’ fluoride exposure via fluid intake.
Other foodstuffs such as seafood products, processed baby food, and chicken may also contain substantial levels of fluoride. Prepared foods are generally processed in urban areas with fluoridated water and become additional sources of fluoride for individuals living in both fluoridated and nonfluoridated communities. Internationally, other foodstuffs are used as vehicles for delivering fluoride. In countries such as Switzerland, Jamaica, and France, fluoride is incorporated into table salt.
Prescription Supplements
Fluoride supplements in the form of drops, lozenges, or tablets can provide systemic and topical (if tablet or lozenge is sucked or chewed) fluoride to children residing in nonfluoridated communities (communities without water fluoridation or having well water with low or undetectable levels of fluoride) (Figure 31-1). The goal of supplementation is to have children in nonfluoridated communities reach a similar level of caries reduction as children living in fluoridated communities. However, considerable debate remains regarding this issue because of the following factors:
 Fluoride supplementation increases risk for dental fluorosis; the risk has increased over the years owing to clients’ exposure to multiple sources of fluoride.
Fluoride supplementation increases risk for dental fluorosis; the risk has increased over the years owing to clients’ exposure to multiple sources of fluoride. Prescriptive dosages of fluoride supplements may be improper, and supplements may be prescribed inadvertently by both dentists and pediatricians.
Prescriptive dosages of fluoride supplements may be improper, and supplements may be prescribed inadvertently by both dentists and pediatricians. Fluoride supplements may be recommended for clients who use well water as their primary water source before the well water is tested to determine natural fluoride levels.
Fluoride supplements may be recommended for clients who use well water as their primary water source before the well water is tested to determine natural fluoride levels.The use of prenatal fluoride supplements is not recommended for pregnant women because permanent teeth do not develop in utero.
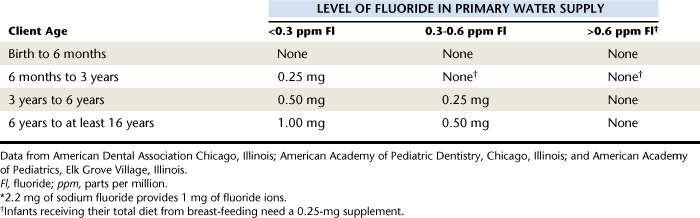
After an evaluation of these factors, the American Dental Association (ADA) Council on Scientific Affairs published a recommended supplementation schedule (Table 31-3). Fluoride supplementation recommendations are based on client age and the level of fluoride in the primary water source. When supplementation is considered, assessment procedures should include questions about the client’s primary water source (e.g., fluoridated community water supply, nonfluoridated community water supply, well water, filtered water) and exposure to other systemic fluoride sources (see the discussion of the patient dental history in Chapter 10).
When supplementation is recommended, it should be in consultation with the child’s pediatrician or primary medical care provider. This will prevent a child from receiving duplicate prescriptions for fluoride supplements. Given that many pediatric dentists recommend that children be seen in general dental offices as infants, there is an increased risk that a child could be given prescriptions by more than one provider. Table 31-4 describes various types of fluoride supplements and indications for their use.
TABLE 31-4 Consideration for the Use of Prescription Fluoride Supplements
| Available Forms of Fluoride Supplements | Recommended Method of Ingestion | Indications for Use |
|---|---|---|
| Drops | Swallowed, then avoid milk products for 1 hour as calcium may interfere with the bioavailability of fluoride. | |
| Tablets | Chewed, then avoid milk products for 1 hour as calcium may interfere with the bioavailability of fluoride. | Children from 2-16 years old who ingest nonfluoridated and underfluoridated water and are at an elevated risk for dental caries. |
| Lozenges | Retained in oral cavity until dissolved, then avoid milk products for 1 hour as calcium may interfere with the bioavailability of fluoride. | Children from 2-16 years old who ingest nonfluoridated or underfluoridated water and are at an elevated risk for dental caries. |
Data from Levy SM, Kiritsy MC, Warren JJ: Sources of fluoride intake in children, J Public Health Dent 55:39, 1995; Newburn E: Fluorides and dental caries, Springfield, Ill, 1986 Charles C Thomas; Tate WH, Chan J: Fluoride concentration in bottled and filtered waters, Gen Dent 42:362, 1994.
Risk of Chronic Fluoride Toxicity (Dental Fluorosis)10
Dental fluorosis is the hypomineralization of the enamel that results from the chronic ingestion of fluoride that exceeds optimal levels. Dental fluorosis is detected by clinical evaluation and classified by degree (Table 31-5). Mild dental fluorosis is often difficult to identify and requires careful assessment and good lighting. Although some consider fluorosis primarily to be an aesthetic concern, in its most severe state the enamel may actually break down. This alteration in the enamel occurs during tooth formation and is therefore a risk only during the preeruptive stages of tooth development. Dental fluorosis is associated with chronic fluoride toxicity and can occur only as a result of systemic ingestion of fluoride during tooth development (Figure 31-2).
TABLE 31-5 Classification of Degree of Dental Fluorosis
| Grade of Fluorosis | Description |
|---|---|
| Normal | None |
| Questionable | A few white flecks or white spots |
| Very mild | Small, opaque, paper-white areas involving less than 25% of the surface |
| Mild | White opacities are more extensive but do not involve as much as 50% of the surface |
| Moderate | All enamel surfaces affected; frequent brown staining |
| Severe | Discrete or confluent pitting; brown stains are widespread; all enamel surfaces affected |
Adapted from Newburn E: Fluorides and dental caries, Springfield, Ill, 1986, Charles C Thomas.
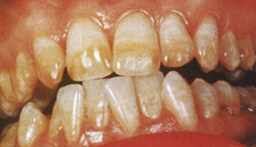
(From Ibsen OAC, Phelan JA: Oral pathology for the dental hygienist, ed 5, St Louis, 2009, Saunders.)
Clients cannot develop fluorosis as a result of topical fluoride exposure, even if the topical exposure is excessive (high-concentration fluoride at frequent intervals). The only way a client can develop dental fluorosis from topical fluoride is by swallowing the product during periods of tooth development, that is, changing the mechanism of fluoride action from topical to systemic. Fluorosis risk increases with ingestion of low-concentration fluoride products such as fluoride-containing dentifrices or mouth rinses. These products are of concern because they are used unsupervised by young children, have an appealing taste and appearance, are often swallowed, and may be stored within reach of small children. The administration of professionally applied, high-concentration fluoride products may initially appear to be a risk factor for dental fluorosis. Scientific evidence confirms that fluoride ingestion occurs as the result of topical treatments and that plasma levels of fluoride are elevated after the treatment. Even though fluoride concentration in professionally administered products is high, the infrequent application of these products does not result in clinically evident enamel disturbances.
Ingested Fluoride—Issues for Consideration
 Community water fluoridation remains a successful public health approach and is the cornerstone of a fluoride protocol and caries management program. This systemic fluoride vehicle is available to all individuals residing in fluoridated communities, regardless of socioeconomic background or ability to access other fluoride therapies. (Consult lists of fluoridated communities available from the Department of Health.)
Community water fluoridation remains a successful public health approach and is the cornerstone of a fluoride protocol and caries management program. This systemic fluoride vehicle is available to all individuals residing in fluoridated communities, regardless of socioeconomic background or ability to access other fluoride therapies. (Consult lists of fluoridated communities available from the Department of Health.) Debate continues regarding need for additional systemic supplementation (based on the multiple sources of fluoride children receive from processed foodstuffs, prepared beverages, and naturally occurring fluoride).
Debate continues regarding need for additional systemic supplementation (based on the multiple sources of fluoride children receive from processed foodstuffs, prepared beverages, and naturally occurring fluoride). Many households use bottled water as their primary water source. Bottled waters have low fluoride concentrations and typically do not reveal fluoride content. A few bottled water manufacturers identify fluoride content on product labels. Other companies are adding fluoride and using the added fluoride as a marketing tool (e.g., “nursery water with fluoride”).
Many households use bottled water as their primary water source. Bottled waters have low fluoride concentrations and typically do not reveal fluoride content. A few bottled water manufacturers identify fluoride content on product labels. Other companies are adding fluoride and using the added fluoride as a marketing tool (e.g., “nursery water with fluoride”). It has become common for individuals to attach water filtration devices (reverse osmosis, bubbling ozone, activated charcoal, and so on) to household water taps. Many of these water filtration systems remove significant amounts of fluoride from the water.
It has become common for individuals to attach water filtration devices (reverse osmosis, bubbling ozone, activated charcoal, and so on) to household water taps. Many of these water filtration systems remove significant amounts of fluoride from the water. Persons who use well water as their primary water sources should have the water tested to determine fluoride level. Without this information, it is impossible to prescribe fluoride supplements accurately. In addition, consideration may be given to how dental hygienists and other dental providers can facilitate water testing by providing clients with testing kits and submitting specimens for testing.
Persons who use well water as their primary water sources should have the water tested to determine fluoride level. Without this information, it is impossible to prescribe fluoride supplements accurately. In addition, consideration may be given to how dental hygienists and other dental providers can facilitate water testing by providing clients with testing kits and submitting specimens for testing. Some topical preparations, especially fluoride-containing dentifrices and oral rinses, may be swallowed, causing the client to receive both a topical and systemic exposure to fluoride. Children using fluoride products must be monitored to decrease risk for acute and chronic fluoride toxicity.
Some topical preparations, especially fluoride-containing dentifrices and oral rinses, may be swallowed, causing the client to receive both a topical and systemic exposure to fluoride. Children using fluoride products must be monitored to decrease risk for acute and chronic fluoride toxicity.Ingested fluoride delivery, especially community water fluoridation, has a track record of success in reducing caries incidence and prevalence. Just as with other client care decisions, the scientific literature provides evidence to substantiate changes in ingested fluoride protocols.
Topical Fluoride11-21
See Chapter 16.
Topical fluorides are delivered into the oral cavity in three primary forms:
 Self-applied by clients in the form of nonprescription products available over the counter (OTC) (recommend those that carry the ADA Seal of Acceptance or the Canadian Dental Association [CDA] Seal of Recognition)
Self-applied by clients in the form of nonprescription products available over the counter (OTC) (recommend those that carry the ADA Seal of Acceptance or the Canadian Dental Association [CDA] Seal of Recognition)Typically, self-applied topical fluoride agents for at-home use are lower in fluoride concentration than those that are applied professionally:
Because fermentable carbohydrate challenges create daily opportunities for enamel demineralization, the frequent use of low-potency fluoride products is recommended for the daily management of dental caries (Table 31-6).
TABLE 31-6 Vehicles for Delivering Topical Fluorides
| Vehicle | Fluoride Concentration | Typical Frequency of Application |
|---|---|---|
| Water | Varies with water supply | Varies with drinking |
| Fluoride toothpaste | 250-1500 ppm | Twice daily |
| Fluoride rinses | 230-1000 ppm | Once daily |
| High-potency fluoride solutions | 10,000 ppm | Typically biannually, but varies according to caries risk |
| Fluoride gels | 4000-12,300 ppm | Varies with potency and caries risk (two to four applications annually) |
| Fluoride varnishes | 1000-22,600 ppm | Varies according to caries risk (two to four applications annually) |
Adapted from Ten Cate JM, van Loveren C: Fluoride mechanisms, Dent Clin North Am 43:713, 1999.
Self-Applied Dentifrices14
See Chapter 23. Other than fluoride consumed in drinking water, dentifrices are the most widely used fluoride-containing preparations. Dentifrices that carry the ADA Seal of Acceptance or the CDA Seal of Recognition provide sufficient concentrations of fluoride to facilitate enamel remineralization when applied two to three times daily (Figure 31-3). Scientific literature provides clinical evidence to substantiate the use of a fluoridated dentifrice with this frequency. Studies done to compare caries reduction indicate that brushing once a day with a fluoridated toothpaste results in a 21% reduction in dental caries, whereas brushing three times a day results in 45% fewer caries.

Figure 31-3 Sample over-the-counter dentifrices that have the American Dental Association Seal of Acceptance for dental caries prevention as well as other benefits. A, Crest ProHealth. B, Colgate Total.
(A, courtesy Procter & Gamble, Cincinnati, Ohio. B, courtesy Colgate Oral Pharmaceuticals, New york, New York.)
The majority of commercial dentifrices available in the United States contain 0.1% fluoride or 1000 ppm. Some manufacturers produce higher-strength dentifrices that contain 1500 ppm fluoride; others have marketed a pediatric toothpaste with lower levels of fluoride ranging from 250 to 550 ppm. It is important for the dental hygienist to recommend products that have undergone clinical evaluation and have documented caries-preventive ability.
Special consideration must be given when recommending fluoridated dentifrices for children under 6 years of age. The primary concern is that young children swallow dentifrice because they enjoy the taste and are not capable of (or efficient at) expectorating dentifrice from their mouths. Only 5% of children younger than 2½ years of age expectorate after brushing, and only 32% of 2½- to 4-year-olds expectorate.11 This inability to expectorate results in the ingestion of an agent designed for topical use and increases dental fluorosis risk. When recommending dentifrices for young children, it is important to involve the client’s parent or caregiver and emphasize the following:
 Delay in introduction of fluoridated toothpaste until child is 2 years of age (although this recommendation may be modified by the ADA to a smear of toothpaste for some children after 6 months of age)
Delay in introduction of fluoridated toothpaste until child is 2 years of age (although this recommendation may be modified by the ADA to a smear of toothpaste for some children after 6 months of age) Limiting toothpaste used to a smear, a pea-sized amount (0.25 mL), or enough paste to cover one quarter of the toothbrush head (depending on the age of the child).
Limiting toothpaste used to a smear, a pea-sized amount (0.25 mL), or enough paste to cover one quarter of the toothbrush head (depending on the age of the child). Avoidance of higher-concentration dentifrices and other fluoride products with children under 6 years of age
Avoidance of higher-concentration dentifrices and other fluoride products with children under 6 years of ageToothpaste is the most widely used at-home, topical fluoride vehicle, but various other products are available for those clients whose caries risk warrants the use of additional agents (Table 31-7).
Self-Applied Mouth Rinses and Gels14
Fluoride rinses and gels may be used in addition to fluoride-containing dentifrice to manage dental caries. The majority of the rinses and gels are of low- to mid-range potency and as such are administered with higher frequency. Research suggests that these fluoride products reduce caries by 30% to 35%. Table 31-7 identifies some rinses and gels available to clients. Clients should be provided with verbal and written instructions regarding home fluoride use. Care must be taken to keep oral products out of the reach of children.
Daily Fluoride Mouth Rinses
Low-potency, high-frequency fluoride rinses (0.05%) are available OTC; they have a fluoride concentration that equates to 230 ppm (Figure 31-4). These products are used daily as an adjunct to brushing with a fluoride dentifrice. Clients should be educated to use 5 mL (1 teaspoon) to 10 mL (2 teaspoons) of the rinse, to vigorously swish the rinse in the oral cavity for 1 minute, and then to thoroughly expectorate the rinse. Because there is a risk for young children to swallow fluoride rinses, this product is not recommended for children under 6 years of age. For the same reason, fluoride rinses should be stored out of the reach of young children. Low-potency, high-frequency 0.044% sodium fluoride (NaF) in an acidulated phosphate fluoride (APF) solution (e.g., OrthoWash Daily Rinse by 3M ESPE), 0.4% stable stannous fluoride (SnF2), and 0.2% neutral NaF rinses that equate to 200 ppm are available by prescription only. These are indicated for the following:
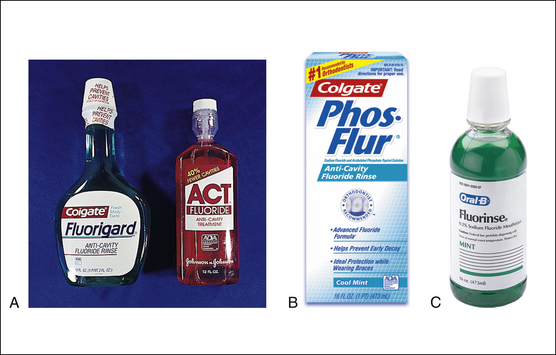
Figure 31-4 A and B, Sample of over-the-counter 0.05% sodium fluoride rinses with the American Dental Association Seal of Acceptance. C, Prescription-only 0.2% sodium fluoride rinse (for once-weekly use).
(A and B, Courtesy Colgate Oral Pharmaceuticals, New York, New York; and Chattem, Inc, Chattanooga, Tennessee. C, Courtesy Procter & Gamble, Cincinnati, Ohio.)
The 0.63% SnF2 rinse (e.g., PerioMed by 3M ESPE) is diluted per manufacturers directions and swished in the mouth twice daily for up to 4 weeks after nonsurgical periodontal therapy as an antimicrobial, to manage dentinal hypersensitivity, and to protect against root caries.
Weekly Fluoride Mouth Rinses
High-potency, low-frequency NaF rinses (0.2%) are used for weekly rinsing, equate to about 910 ppm, and are typically used in school-based programs for children who do not reside in communities with water fluoridation. School-based programs are effective because they are administered by school personnel, are closely supervised, are performed as part of a class schedule, and result in good compliance. Young children rinse with 5 mL (1 teaspoon); older children and adults rinse with 10 mL. After 1 minute of vigorous rinsing, the product is expectorated.
It may be used at home and is the highest concentration home fluoride rinse. Up to 55% caries reduction can be expected from weekly use.
Daily Fluoride Gels and Pastes
Fluoride gels are marketed as a 0.4% SnF2 product at 1000 ppm available OTC 1.1% neutral NaF gel, 1.1 NaF toothpaste with 5% potassium nitrate, and 1.1% NaF toothpaste at 5000 ppm available by prescription only (Figure 31-5). These products are designed for daily use and are brushed on the teeth for 1 minute. If a product does not have a built-in cleaning system, then it is used after toothbrushing with a conventional fluoride dentifrice. Manufacturer instructions are as follows:
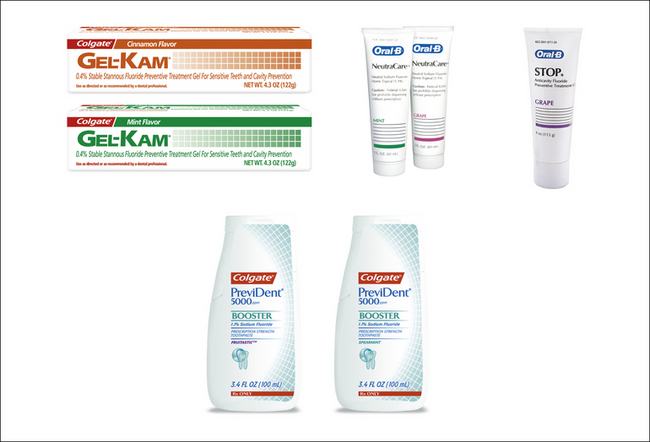
Figure 31-5 Examples of 0.4% stable stannous fluoride gels and pastes, and 1.1% sodium fluoride prescription toothpaste.
(Colgate products courtesy Colgate Oral Pharmaceuticals, New York, New York; Oral-B products courtesy Procter & Gamble, Cincinnati, Ohio.)
This daily procedure is recommended for persons with the following:
Because there is a risk for young children to swallow fluoride gels, these products are not recommended for children under 6 years of age. Although there are a very limited number of studies documenting their efficacy, the ADA Council on Scientific Affairs approved OTC SnF2 gels. In addition, the U.S. Food and Drug Administration (FDA) approved SnF2 gels for sale OTC because they contain the same fluoride concentration as conventional dentifrices. Stannous gels do not contain abrasives and should not be substituted for dentifrices that achieve pellicle and extrinsic stain removal.
Neutral and acidulated NaF gels (5000 ppm) are available without abrasives and with abrasives (marketed as prescription dentifrices). These products have gained widespread use for clients with special needs. Careful client education is required when these products are recommended for unsupervised home use. The products should be used as directed in a custom tray or brushed on the teeth for 1 minute and then expectorated. Clients should be reminded that these products are available by prescription owing to their moderate levels of fluoride and therefore should be carefully stored out of the reach of children. Neutral NaF preparations are indicated if the client has porcelain, composite, titanium, or sealant materials placed in the mouth; acidulate fluorides are contraindicated when these materials are present.
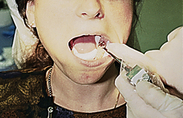
Professionally Applied Fluoride (In-Office Administration)6,13-20
Professionally applied topical fluorides solutions and gels (prescription agents) have been used successfully (Table 31-8). Although fluoride foams are also in use, little evidence exists to support their efficacy; more research is needed before they can be used with the same confidence as gels. See Figure 31-6 for additional product examples.
Solutions, Gels, and Foams
Professionally applied fluoride products are typically delivered using a disposable tray technique, are one of the last procedures performed in the appointment sequence, and are administered by licensed dental professionals. Three high-potency, low-frequency topical fluoride systems have been approved by the FDA for in-office use. These systems contain 9000 to 25,000 ppm of fluoride and are manufactured in the form of solutions, gels, or foams, as follows:
These high-potency, low-frequency fluoride systems have a caries reduction rate of approximately 30%. Of the three products, the 1.23% APF gel system is the most widely researched and used. The 2.0% neutral NaF gel is the second most widely used and is recommended when it is inappropriate to use an acidulated product (when client has tooth-colored restorations, titanium implants, sealants, and/or dentinal hypersensitivity). The foam system lacks research to support efficacy. The 8.0% SnF2 system is rarely used owing to its limited availability and lack of stability in aqueous solution. In addition, this product has poor client acceptance because of taste, tissue irritation, gingival sloughing, and extrinsic tooth staining.
Client Selection
(Table 31-9). Although client selection criteria for this procedure will continue to change based on research evidence, key issues must be considered during identification of clients who will benefit from professionally applied fluoride therapy:
 Client’s fluoride history, for example, fluoride level in the primary water supply, overall exposure to fluoride.
Client’s fluoride history, for example, fluoride level in the primary water supply, overall exposure to fluoride. Demonstrated risk for developing caries. Box 31-1 summarizes the caries risk factors that make children and adults candidates for professionally applied topical fluoride treatments.
Demonstrated risk for developing caries. Box 31-1 summarizes the caries risk factors that make children and adults candidates for professionally applied topical fluoride treatments. Presence of newly erupted teeth (studies indicate that teeth are most susceptible to caries formation during the first 2 years after eruption).
Presence of newly erupted teeth (studies indicate that teeth are most susceptible to caries formation during the first 2 years after eruption). Age of the client. Extreme care should be used if in-office fluoride treatment is administered to a child under 6 years of age. Inadvertent swallowing of fluoride and the inability to effectively expectorate place the client at risk for ingesting high-potency fluoride. This could result in acute fluoride toxicity. Older adults may present the same risks as the child client. Fluoride varnish therapy is a better choice for young children at moderate to high risk for dental caries.
Age of the client. Extreme care should be used if in-office fluoride treatment is administered to a child under 6 years of age. Inadvertent swallowing of fluoride and the inability to effectively expectorate place the client at risk for ingesting high-potency fluoride. This could result in acute fluoride toxicity. Older adults may present the same risks as the child client. Fluoride varnish therapy is a better choice for young children at moderate to high risk for dental caries. Physical or cognitive disabilities (e.g., inability to expectorate or understand the importance of refraining from swallowing).
Physical or cognitive disabilities (e.g., inability to expectorate or understand the importance of refraining from swallowing).TABLE 31-9 Clinical Recommendations for the Use of Professionally Applied Topical Fluoride
| Caries Risk Level | Age | Recommendation |
|---|---|---|
| Low | ||
| No incipient or cavitated primary or secondary carious lesions during the last 3 years and no factor that may increase caries risk∗ | All age groups | May not receive additional benefitFluoridated water and fluoride toothpastes may provide adequate caries prevention |
| Moderate | ||
| No incipient or cavitated primary or secondary carious lesions during the last 3 years, but at least one factor that may increase caries risk∗ | <6 years of age | Fluoride varnish applications at 6-month intervals |
| Either of the following:
No incipient or cavitated primary or secondary carious lesions during the last 3 years, but at least one factor that may increase caries risk∗
|
6 years of age or older | Fluoride varnish or fluoride gel applications at 6-month intervals |
| High | ||
| <6 years of age | Fluoride varnish applications at 3- to 6-month intervals | |
| 6 years of age or older |
∗ Factors increasing risk of developing caries also may include, but are not limited to, high titers of cariogenic bacteria, poor oral hygiene, prolonged nursing (bottle or breast), poor family dental health, developmental or acquired defects, genetic abnormality of teeth, many multisurface restorations, chemotherapy or radiation therapy, drug or alcohol abuse, irregular dental care, cariogenic diet, active orthodontic treatment, presence of exposed root surfaces, restoration overhangs and open margins, and physical or mental disability with inability to perform proper oral healthcare
† On the basis of findings from population studies, groups with low socioeconomic status have been found to have increased risk of developing caries. In children too young for their risk to be based on caries history, low socioeconomic status should be considered as a caries risk factor.
‡ Medication-, radiation-, or disease-induced xerostomia.
Adapted from American Dental Association Council on Scientific Affairs: Professionally applied topical fluoride: evidence-based clinical recommendations, J Dent Educ 71:393, 2007.
BOX 31-1 Client Selection for Professionally Applied Fluoride Treatments—Risk Factors∗
∗ Individuals who are at low risk for dental caries may have no additional benefit from professionally applied fluoride therapy, particularly if they use a fluoride toothpaste and drink fluoridated water.
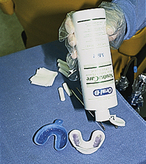
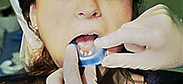
Tray Selection
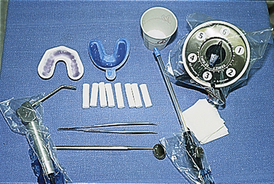
Fluoride gel and foam products may be applied with a variety of techniques; however, the use of stock trays is most common (Figure 31-7). Advantages of the tray technique for professionally applied fluoride treatments are as follows:

(Courtesy Dental Hygienist News, funded by an educational grant from Procter & Gamble, Cincinnati, Ohio, and published by Harfst Associates, Inc, Troy, Michigan.)
Fluoride delivery trays are marketed by several manufacturers. Tray design and fit are critical to optimize the anticaries efficacy of the treatment and prevent ingestion of these high-potency products. When selecting trays for fluoride delivery, criteria include the ability of the tray to create a distal dam to prevent flow of fluoride out the posterior border, anatomic arch fit, anterior vertical coverage, posterior vertical coverage, ease of use, and tray features that promote client comfort.
Product Selection
Once the need for a professionally applied topical fluoride treatment is determined, the dental hygienist selects the type of high-potency fluoride for this procedure. Issues to consider when selecting a product include the following:
 Fluoride gel versus foam products. Evolution of in-office fluoride products went from solutions to gels to thixotropic gels to foams. Gels were initially developed for ease of application and use of fluoride trays. However, cellulose added to gels to increase viscosity inhibited the fluoride to flow into protected areas. To overcome this problem, thixotropic gels were developed; these gels flow under pressure but remain viscous when not under pressure. Both professionally applied APF and neutral NaF products are available in thixotropic gels.
Fluoride gel versus foam products. Evolution of in-office fluoride products went from solutions to gels to thixotropic gels to foams. Gels were initially developed for ease of application and use of fluoride trays. However, cellulose added to gels to increase viscosity inhibited the fluoride to flow into protected areas. To overcome this problem, thixotropic gels were developed; these gels flow under pressure but remain viscous when not under pressure. Both professionally applied APF and neutral NaF products are available in thixotropic gels. Professional-strength foam-based products were developed to address the risks associated with using high-potency, low-frequency fluoride products. With foam products, 75% less fluoride is used, dramatically reducing the risk of acute fluoride toxicity. APF foam products deliver the same fluoride concentration as APF gels, but limited research exists to support their use over the gel system. Both professionally applied APF and neutral NaF are available in foam-based products.
Professional-strength foam-based products were developed to address the risks associated with using high-potency, low-frequency fluoride products. With foam products, 75% less fluoride is used, dramatically reducing the risk of acute fluoride toxicity. APF foam products deliver the same fluoride concentration as APF gels, but limited research exists to support their use over the gel system. Both professionally applied APF and neutral NaF are available in foam-based products. APF versus neutral NaF products. Efficacy of APF products has been documented in controlled clinical trials. Neutral NaF products were developed because repeated exposure to APF products may etch restorative and preventive materials that contain porcelain, composite, titanium, glass, or similar materials (e.g., tooth-colored restorative materials, filled sealants).
APF versus neutral NaF products. Efficacy of APF products has been documented in controlled clinical trials. Neutral NaF products were developed because repeated exposure to APF products may etch restorative and preventive materials that contain porcelain, composite, titanium, glass, or similar materials (e.g., tooth-colored restorative materials, filled sealants).
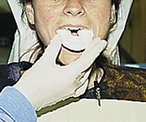
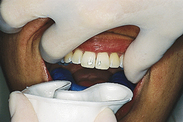
Procedures for Application Using the Tray Technique
The procedural steps for professionally applied topical fluoride begin with the client and product selection as outlined in the previous section. Sequence for administration using the tray technique is described in Procedure 31-1.
Procedure 31-1 PROFESSIONALLY APPLIED TOPICAL FLUORIDE USING THE TRAY TECHNIQUE FOR IN-OFFICE FLUORIDE TREATMENT (GEL OR FOAM)
EQUIPMENT
| Mouth mirror | Timer |
| Cotton forceps | Saliva ejector |
| Fluoride tray(s) | 2 × 2 gauze |
| Cotton rolls | Tissues |
| 1.23% acidulated phosphate fluoride (APF) or 2.0% sodium fluoride gel | |
| Air syringe |
STEPS
Varnish6,14-20
Fluoride varnishes are lacquers containing 5% NaF in a colophony/rosin base. The varnish system enables the delivery of an increased concentration of fluoride to the outer surface of the tooth and also prolongs fluoride exposure on the tooth surface for safe and effective caries control (Figure 31-19). In the United States the FDA has approved these products as medical devices for use only as cavity liners and for the treatment of hypersensitive teeth. The FDA considers caries prevention a drug claim. For the FDA to approve varnishes as a caries prevention agent, manufacturers would have to conduct clinical trials that meet ADA standards and submit evidence that this product should be approved as a therapeutic, anticaries agent.
Because of numerous clinical studies and successful use of fluoride varnishes in other countries, fluoride varnishes dispensed in unit doses have become a standard of care, albeit off-label, in the following situations:
European studies that evaluated the ability of fluoride varnish to reduce caries on permanent teeth reported reductions of 18% to 77%. A preliminary U.S. study that assessed the ability of fluoride varnish to remineralize initial carious lesions in primary teeth reported an 80% reversal of lesions. Both the ADA and the American Academy of Pediatric Dentistry support the use of fluoride varnish within a caries prevention program.
The following fluoride varnishes are available in the United States (see Figure 31-8):
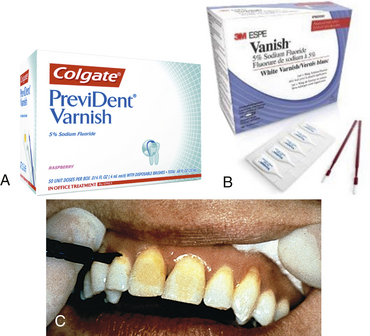
Figure 31-19 A and B, Examples of unit-dose 5% sodium fluoride varnish. C, Fluoride varnish on central incisors.
(A, Courtesy Colgate Oral Pharmaceuticals, New York, New York. B, Courtesy 3M ESPE, St Paul, Minnesota.)
Varnish products have a higher concentration of fluoride than gels or foams, but because varnish is painted on as a thin layer, an overall smaller amount of fluoride is used (<7 mg of varnish versus 30 mg of gel for a child).
Safety of Fluoride Varnish
To date there are no documented incidents of acute or chronic fluoride toxicity as a result of using fluoride varnish. The rapid drying characteristic of fluoride varnishes seems to prevent ingestion and to minimize risk for a toxic dose. The release of fluoride after placement peaks early and then drops dramatically. Plasma levels of fluoride after varnish applications are similar to those experienced after the use of an OTC fluoridated toothpaste. Ingestion of high doses of fluoride is reduced with varnish use. The varnish breaks down over a period of several days, so ingestion occurs slowly over a period of time, reducing the likelihood of acute fluoride toxicity. Because the varnish contains colophony/rosin, it should not be used on persons with a known sensitivity to this material. Fluoride varnish also should not be used in the presence of ulcerative gingivitis, stomatitis, or large open lesions.6
Frequency of Fluoride Varnish Application.6
Some providers support application every 3 to 6 months, determined by the client’s caries risk. Procedure 31-2 outlines the steps for fluoride varnish therapy. Application is safe, effective, and fast, and client acceptance is satisfactory.
Procedure 31-2 PROFESSIONALLY APPLIED SODIUM FLUORIDE VARNISH USING THE PAINT-ON TECHNIQUE
STEPS
Professionally Applied Fluorides: Issues and Controversies
The scientific literature highlights the following key issues and controversies regarding use of professionally applied fluoride products:
 Use of an SnF2-APF combination dual-rinse system as a substitute for a professionally applied topical fluoride delivered via the tray technique. This two-part system is marketed as a 0.31% APF solution and a 1.64% SnF2 solution. The two solutions are used one at a time or mixed together. The total recommended rinsing time is 2 minutes, and the fluoride exposure is 1500 to 3000 ppm. Some dental professionals choose this procedure because it takes less time and equipment than the tray technique or paint-on varnish technique, because it is less labor intensive, and because clients find it more tolerable than fluoride trays.
Use of an SnF2-APF combination dual-rinse system as a substitute for a professionally applied topical fluoride delivered via the tray technique. This two-part system is marketed as a 0.31% APF solution and a 1.64% SnF2 solution. The two solutions are used one at a time or mixed together. The total recommended rinsing time is 2 minutes, and the fluoride exposure is 1500 to 3000 ppm. Some dental professionals choose this procedure because it takes less time and equipment than the tray technique or paint-on varnish technique, because it is less labor intensive, and because clients find it more tolerable than fluoride trays. Despite its use in some offices, there are no rigorous studies to substantiate the use of a dual, sequential fluoride rinse system. Contemporary standards of practice require that dental hygienists make client care decisions based on scientific evidence. The SnF2-APF combination dual rinse does not meet this standard.
Despite its use in some offices, there are no rigorous studies to substantiate the use of a dual, sequential fluoride rinse system. Contemporary standards of practice require that dental hygienists make client care decisions based on scientific evidence. The SnF2-APF combination dual rinse does not meet this standard. Marketing and use of professionally applied APF gel and foam products for 1 minute rather than 4 minutes. Many manufacturers have successfully marketed 1-minute 1.23% APF gel and foam products. These products have become popular owing to the reduced time required for administration. No clinical studies of caries inhibition substantiate leaving the gel in contact with the teeth for just 1 minute. Fluoride uptake by the enamel is limited when fluoride contact is reduced to 1 minute. In addition, there is no difference in the concentration of products marketed as 1-minute products versus those marketed as 4-minute products. No scientific evidence substantiates a reduction in fluoride exposure time. Limited data exist to clearly support the delivery of fluoride via foam.
Marketing and use of professionally applied APF gel and foam products for 1 minute rather than 4 minutes. Many manufacturers have successfully marketed 1-minute 1.23% APF gel and foam products. These products have become popular owing to the reduced time required for administration. No clinical studies of caries inhibition substantiate leaving the gel in contact with the teeth for just 1 minute. Fluoride uptake by the enamel is limited when fluoride contact is reduced to 1 minute. In addition, there is no difference in the concentration of products marketed as 1-minute products versus those marketed as 4-minute products. No scientific evidence substantiates a reduction in fluoride exposure time. Limited data exist to clearly support the delivery of fluoride via foam. Whether a professional fluoride treatment is required after extrinsic stain removal (selective polishing) procedures. Many commercially available prophylaxis pastes contain fluoride. There is no existing clinical evidence to suggest that fluoride-containing pastes improve caries protection. These pastes should not be used in place of professionally applied fluoride therapy. Some clinical evidence supports that fluoride lost when enamel is removed during selective polishing may be replaced by the fluoride in prophylaxis pastes. Although this may be the case, additional studies are needed to determine if the ingredients in prophylaxis pastes restrict the bioavailability of the fluoride. Before adjusting clinical protocols, dental hygienists should consult the literature for evidence that such a change is warranted.
Whether a professional fluoride treatment is required after extrinsic stain removal (selective polishing) procedures. Many commercially available prophylaxis pastes contain fluoride. There is no existing clinical evidence to suggest that fluoride-containing pastes improve caries protection. These pastes should not be used in place of professionally applied fluoride therapy. Some clinical evidence supports that fluoride lost when enamel is removed during selective polishing may be replaced by the fluoride in prophylaxis pastes. Although this may be the case, additional studies are needed to determine if the ingredients in prophylaxis pastes restrict the bioavailability of the fluoride. Before adjusting clinical protocols, dental hygienists should consult the literature for evidence that such a change is warranted.Risk of Acute Fluoride Toxicity21
Acute toxicity from the rapid ingestion of a topical fluoride agent within a very short period of time may yield a mild systemic reaction (stomach upset) to death. Many factors influence the toxicity of fluoride compounds (e.g., route of administration, client age and weight, and rate of absorption). When fluoride is swallowed, it reacts with stomach acids; the reaction product is hydrogen fluoride (HF). HF is very irritating, and initial abdominal reactions can occur after a relatively small amount of higher concentration product is swallowed.
Symptoms
Initial symptoms of acute fluoride toxicity are nausea, gastrointestinal pain, and vomiting. If sufficient quantities of fluoride are ingested, these initial symptoms may be followed by muscular weakness and spasms that occur as a result of fluoride combining with blood calcium ions. Toxic doses of fluoride affect major body systems as follows:
Emergency Management
The initial treatment goal for fluoride toxicity is to reduce the amount of fluoride available for absorption from the gastrointestinal tract. Depending on the amount of fluoride ingested, initial emergency response in the dental office should be followed by medical treatment. Box 31-2 outlines treatment procedures for acute fluoride toxicity.
BOX 31-2 Management of Acute Fluoride Toxicity
The amount of fluoride that will cause a toxic reaction or result in a lethal dose is based on a variety of factors described earlier. Terms used to describe how much fluoride can be tolerated by a client are the safely tolerated dose (STD) and the certainly lethal dose (CLD):
Prevention of Fluoride Toxicity
Although fluoride products have evident therapeutic benefits, their use and storage must be monitored. In the home setting, caution must be taken regarding the use and storage of even low-dose fluoride products. Oral care professionals must educate clients (and parents or caregivers) regarding the safe use of fluorides. In addition, dental professionals must exercise extreme caution when using high-potency prescription products that contain the highest concentrations of fluoride available. To safely use professional-strength fluoride products, it is essential that the dental hygienist carefully select clients for this therapy and know the following:
CHLORHEXIDINE AS AN ANTIBACTERIAL FOR DENTAL CARIES,1,22,23
Chlorhexidine gluconate has been used as an antibacterial for the management of both dental caries and periodontal diseases (see Chapter 29). A broad-spectrum antibacterial agent, it works by opening up the cell membranes of the bacteria. In the United States, only 0.12 % chlorhexidine gluconate is available by prescription as a mouth rinse (Figure 31-20); it is effective against S. mutans as well as periodontal pathogens.
To reduce S. mutans infection and caries transmission in persons with active caries, the 2002 Caries Management Risk Assessment Consensus Statement recommends that 10 mL of 0.12 % chlorhexidine mouth rinse be used at bedtime for 1 minute, once daily for a 2-week period every 2 to 3 months.1 In high–bacterial-challenge individuals, this therapy needs to be continued for approximately 1 year and monitored by bacterial assessment. Use of this compound affects taste, causes extrinsic tooth stain, and increases calculus formation, and compliance is often poor. Because chlorhexidine loses its effectiveness in the presence of sodium lauryl sulfate or fluoride, this therapy should be used about 30 minutes after toothbrushing with fluoride and/or sodium lauryl sulfate–containing dentifrices.
XYLITOL AS AN ANTIBACTERIAL FOR DENTAL CARIES1,24
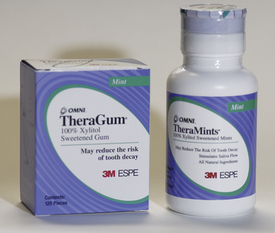
Xylitol is a noncariogenic sweetener that looks and tastes like sucrose. It inhibits attachment and transmission of S. mutans, reduces plaque biofilm formation, stimulates salivary secretion, and can be delivered through chewing gum, mints, breath sprays, toothpaste, or lozenges as an effective anticaries therapeutic measure (Figure 31-21). Xylitol is not fermented by cariogenic bacteria and therefore decreases S. mutans infection and caries transmission.1 For a therapeutic dose, chewing gum, lozenges, or mints must contain 1.55 g of xylitol and must be used at least four to five times daily throughout the day. Xylitol use can reduce transmission of S. mutans from mother to child. Pregnant women who are infected with S. mutans are encouraged to use xylitol as directed during the last months of pregnancy and during the first 3 months postpartum to reduce the risks of vertical caries transmission to their infants. Use of xylitol within a family also can help prevent horizontal transmission among siblings. Diabetics and the elderly can also safely use xylitol products to control caries.
AMORPHOUS CALCIUM PHOSPHATE25,26
ACP contains the same minerals as in the hydroxyapatite crystals of tooth enamel. Researchers have found that calcium and phosphate ions in ACP will seek out areas of demineralization and do the following:
ACP-containing products are not a substitute for fluoride therapy, but rather can be used in conjunction with fluoride therapy for clients at risk for caries. ACP is activated on contact with salivary ions and protease enzymes that catalyze remineralization. Because of the affinity of calcium and phosphate ions with fluoride, fluoride uptake is increased.
Although more research is needed, several suggested benefits of ACP include the following:
 Formation of a new hydroxyapatite coating on the tooth that is larger in crystal formation and more resistant to acid attack than the enamel crystal
Formation of a new hydroxyapatite coating on the tooth that is larger in crystal formation and more resistant to acid attack than the enamel crystalProfessionally applied and OTC self-applied products formulated with ACP are available. Self-applied ACP products include dentifrices (Arm and Hammer Enamel Care with Liquid Calcium, Mentadent Replenishing White). Professionally applied products with ACP include tooth whitening agents (Zoom 2-Day White, Night White with ACP), prophylaxis paste (Enamel Pro), and 5% NaF Varnish (Enamel ProVarnish).
CASEIN PHOSPHOPEPTIDES–AMORPHOUS CALCIUM PHOSPHATE27,28 (Figure 31-22)
Recaldent is a complex of ACP and CPP that precipitates calcium and phosphate ions to help remineralize teeth. Because casein is derived from cow’s milk, it is contraindicated in people with milk allergies.
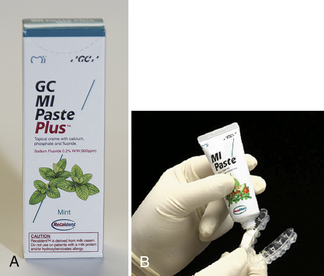
Figure 31-22 A, Example of professionally administered casein phosphopeptide–amorphous calcium phosphate product. B, Product can be delivered as a prophylaxis paste or via a tray.
(B, Courtesy GC America, Inc, Alsip, Illinois.)
Self-applied products with Recaldent include Trident White Chewing Gum. Professionally applied products with CPP-ACP include MI Paste and MI Paste Plus.
CPP-ACP–containing products are not a substitute for fluoride therapy, but rather can be used in conjunction with fluoride therapy for clients at risk for caries. ACP and CPP-ACP products enhance the effects of fluoride and provide a supersaturated environment of calcium and phosphate for remineralization. ACP helps to repair microscopic enamel defects, resulting in enamel luster. More rigorous clinically based studies on these products are needed. (See Chapters 27 and 38 for ACP, CPP-ACP, and other products that contain calcium and phosphate ions being used and researched in prophylaxis pastes and to control dentinal hypersensitivity.)
SODIUM BICARBONATE
Sodium bicarbonate (baking soda) neutralizes acids produced by acidogenic bacteria and has antibacterial properties. It can be delivered in toothpaste or in a solution for individuals with low saliva flow.1
CARIES MANAGEMENT PLAN1-8,29-32
For the dental hygienist to design a preventive care plan, all clients must have a dental home by age 12 months. Then careful consideration must be given to the information gathered during risk assessment (see Chapter 16). This information assists in determining client risk for dental caries and recommending preventive strategies that will address client needs (see Chapter 16Table 16-1Table 16-2Box 16-1Box 16-2Box 16-3Box 16-4Box 16-5Box 16-6Box 16-7Box 16-8Box 16-9Box 16-10Figure 16-5Figure 16-6Figure 16-7Figure 16-8Figure 16-9). Dental sealant placement is also considered as part of the overall caries management plan (see Chapter 32).
Evaluation of exposure to fluoride requires careful questioning of the client. With young clients the need for fluoride supplementation is based on an analysis of exposures to fluoride and consultation with other dental and medical providers. Special attention should be paid to whether the client is receiving too much fluoride.
As teeth erupt the clinician must be alert to the presence of dental fluorosis. Should the dental hygienist identify even mild areas of fluorosis, every attempt should be made to determine its cause(s). Regardless of dental caries status, the use of a fluoridated toothpaste is the cornerstone of any caries prevention plan. Need for additional preventive measures is based on clinical judgment and presence of new demineralized areas. Should the client exhibit new demineralized areas, the dental hygienist must consider whether the continued-care interval is appropriate (if more frequent preventive visits are required), if the client is using a fluoridated dentifrice at least two times a day, and whether additional professionally applied or self-applied topical fluoride products are indicated.
Depending on the age of the client, number of caries risk factors, ability of the client to modify risk factors, and number of demineralized areas, the dental hygienist may decide that a modification in professional care is needed. This may involve more-frequent preventive appointments or more-frequent application of in-office fluoride gels or varnish. An increase in the number of preventive dental visits is dependent on the client’s compliance with this service.
The dental hygienist may also decide that an adjustment in self-applied topical fluoride products is the best approach for managing an increase in the number of demineralized areas. Self-applied products are less expensive, do not require a visit to the dental care setting, facilitate the delivery of low-dose, frequent-use fluorides, and address the demineralization dynamic on a daily basis. Table 31-7 identifies some self-applied products that may be recommended based on client risk in addition to a fluoridated dentifrice.
One approach may be to supplement the use of a fluoridated dentifrice with an additional fluoride vehicle, starting with a low-concentration, frequent-use product (e.g., 230 ppm/0.05% oral rinse). This adjustment would be followed by a reassessment visit in 2 to 3 months. Should additional demineralized areas be present, the dental hygienist may recommend a higher-concentration frequent-use, self-applied product.
To control the infection, the use of 0.12% chlorhexidine mouth rinse is recommended for individuals with caries activity—that is, individuals with existing decay and individuals with high levels of S. mutans in the mouth. The underlying rationale suggesting the 1-minute use of ½-ounce chlorhexidine rinses for a 2-week period every 2 to 3 months in a caries-active client is to eliminate the infectious microorganisms that initiate the caries process and to prevent their transmission either vertically or horizontally to others. Daily therapeutic doses of xylitol are also used to control the bacterial infection that leads to caries. The intent is to treat the infection as well as the result of the infection (which historically has been the placement of a restoration).
CLIENT EDUCATION TIPS
 Explain the role of diet, fluorides, oral biofilm removal, saliva, amorphous calcium phosphate, antibacterial mouth rinses, xylitol, and sealants as methods of preventing and controlling tooth decay.
Explain the role of diet, fluorides, oral biofilm removal, saliva, amorphous calcium phosphate, antibacterial mouth rinses, xylitol, and sealants as methods of preventing and controlling tooth decay. Explain the significance of using a therapeutic dose of 1.55 g xylitol at least four to five times spread throughout the day; explain how to select over-the-counter xylitol products.
Explain the significance of using a therapeutic dose of 1.55 g xylitol at least four to five times spread throughout the day; explain how to select over-the-counter xylitol products. Discuss that caries is a Streptococcus mutans infection that can be transmitted from parent to child (vertical transmission) or person to person (horizontal transmission).
Discuss that caries is a Streptococcus mutans infection that can be transmitted from parent to child (vertical transmission) or person to person (horizontal transmission). Emphasize the role of frequent-use, low-concentration fluoride-containing products (dentifrices and oral rinses) in the repair of demineralized areas.
Emphasize the role of frequent-use, low-concentration fluoride-containing products (dentifrices and oral rinses) in the repair of demineralized areas. Teach parents and caregivers that they are critical partners in the management of dental caries in children under 6 years of age.
Teach parents and caregivers that they are critical partners in the management of dental caries in children under 6 years of age.LEGAL, ETHICAL, AND SAFETY ISSUES
 Document recommendations regarding self-applied products in client record, including information regarding type of product, frequency of use, safe use, and storage.
Document recommendations regarding self-applied products in client record, including information regarding type of product, frequency of use, safe use, and storage. Work in collaboration with other oral care professionals to develop a response plan in the event of an acute fluoride overdose.
Work in collaboration with other oral care professionals to develop a response plan in the event of an acute fluoride overdose. Have a clear understanding of the amount of professional-strength fluoride that is administered and how it relates to certainly lethal dose (CLD) and safely tolerated dose (STD).
Have a clear understanding of the amount of professional-strength fluoride that is administered and how it relates to certainly lethal dose (CLD) and safely tolerated dose (STD).KEY CONCEPTS
 Dental caries involve an interaction among pathologic factors and protective factors. Pathologic factors include acidogenic bacteria (Streptococcus mutans and Lactobacillus), low saliva flow due to salivary gland dysfunction or the use of many medications, and fermentable carbohydrates in the diet. Protective factors include calcium, phosphate, proteins, and fluoride in the saliva; normal salivary flow; and antibacterial agents if needed.
Dental caries involve an interaction among pathologic factors and protective factors. Pathologic factors include acidogenic bacteria (Streptococcus mutans and Lactobacillus), low saliva flow due to salivary gland dysfunction or the use of many medications, and fermentable carbohydrates in the diet. Protective factors include calcium, phosphate, proteins, and fluoride in the saliva; normal salivary flow; and antibacterial agents if needed. Caries management is aimed at restoring and maintaining a balance between protective factors and pathologic factors. Saliva plays a key role in that it neutralizes acids and provides minerals and proteins that protect the teeth.
Caries management is aimed at restoring and maintaining a balance between protective factors and pathologic factors. Saliva plays a key role in that it neutralizes acids and provides minerals and proteins that protect the teeth. The dental hygienist evaluates the number and the severity of risk factors before planning a caries management program.
The dental hygienist evaluates the number and the severity of risk factors before planning a caries management program. After determining the caries risk of an individual, the clinician provides the client with educational materials about the caries process and makes recommendations based on assessment results, responses to questions, and clinical observation.
After determining the caries risk of an individual, the clinician provides the client with educational materials about the caries process and makes recommendations based on assessment results, responses to questions, and clinical observation. Caries management involves treating the bacterial infection that causes caries with chlorhexidine and xylitol; remineralizing early noncavitated carious lesions by enhancing salivary flow and using fluorides; protecting tooth surfaces by using sealants, fluorides, and amorphous calcium phosphate; decreasing the frequency of fermentable carbohydrates, especially between meals; and surgically removing carious lesions that are beyond hope of remineralization and restoring the teeth with minimally invasive techniques and materials.
Caries management involves treating the bacterial infection that causes caries with chlorhexidine and xylitol; remineralizing early noncavitated carious lesions by enhancing salivary flow and using fluorides; protecting tooth surfaces by using sealants, fluorides, and amorphous calcium phosphate; decreasing the frequency of fermentable carbohydrates, especially between meals; and surgically removing carious lesions that are beyond hope of remineralization and restoring the teeth with minimally invasive techniques and materials. Chlorhexidine is used as a 60-second mouth rinse (10 mL once daily for a 2-week period every 2 to 3 months). In individuals with high bacterial challenge, this therapy will need to be continued for approximately 1 year and monitored by bacterial assessment.
Chlorhexidine is used as a 60-second mouth rinse (10 mL once daily for a 2-week period every 2 to 3 months). In individuals with high bacterial challenge, this therapy will need to be continued for approximately 1 year and monitored by bacterial assessment. Demineralization is an issue from the time the primary dentition erupts into the oral cavity until death or the permanent teeth are prematurely lost.
Demineralization is an issue from the time the primary dentition erupts into the oral cavity until death or the permanent teeth are prematurely lost. Community water fluoridation is the cornerstone of ingested and topical fluoride delivery; fluoridated dentifrices play a similar role in topical fluoride delivery.
Community water fluoridation is the cornerstone of ingested and topical fluoride delivery; fluoridated dentifrices play a similar role in topical fluoride delivery. Incidence of chronic fluoride toxicity (dental fluorosis) has increased; it is the dental hygienist’s responsibility to assess systemic fluoride exposure.
Incidence of chronic fluoride toxicity (dental fluorosis) has increased; it is the dental hygienist’s responsibility to assess systemic fluoride exposure. Various evidence-supported self-applied dentifrices, rinses, and gels are available. Consumers must be educated in their selection and use as needed.
Various evidence-supported self-applied dentifrices, rinses, and gels are available. Consumers must be educated in their selection and use as needed.CRITICAL THINKING EXERCISES
ACKNOWLEDGMENT
The authors acknowledge Jeanne Maloney and Anne Miller for their past contributions to this chapter.
Refer to the Procedure Manual where rationales are provided for the steps outlined in the procedures presented in this chapter.
1. Featherstone J.D., Adair S.M., Anderson M.H., et al. Caries management by risk assessment: consensus statement, April 2002. J Calif Dent Assoc. 31(257), 2003.
2. Featherstone J.D.B. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887.
3. Featherstone J.D.B. Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol. 1999;27:31.
4. Ramos-Gomez F.J., Crall J., Gansky S.A., et al. Caries risk assessment appropriate for the age 1 visit (infants and toddlers). J Calif Dent Assoc. 2007;35:687.
5. Young D.A., Featherstone J.D., Roth J.R., et al. Caries management by risk assessment: implementation guidelines. J Calif Dent Assoc. 2007;35:799.
6. Association of State and Territorial Dental Directors: Fluoride varnish: an evidence-based approach. Available at: http://www.kdheks.gov/ohi/download/Flvarnishpaper.pdf. Accessed October 9, 2008.
7. Featherstone J.D., Domejean-Orliaguet S., Jenson L., et al. Caries risk assessment in practice for age 6 through adult. J Calif Dent Assoc. 2007;35:703.
8. Domejean-Orliaguet S., Gansky S.A., Featherstone J.D. Caries risk assessment in an educational environment. J Dent Educ. 2006;70:1346.
9. Horowitz H.S. Decision-making for national programs of community fluoride use. Community Dent Oral Epidemiol. 2000;28:321.
10. Pendrys D.G. Risk of enamel fluorosis in nonfluoridated and optimally fluoridated populations: considerations for dental professionals. J Am Dent Assoc. 2000;131:746.
11. Warren D.P., Chan J.T. Topical fluorides: efficacy, administration, and safety. Gen Dent. 1997;45:134.
12. Adair SM, Bowen WH, Burt BA Recommendations for using fluoride to prevent and control dental caries in the United States, Atlanta, 2001, Centers for Disease Control and Prevention.
13. American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Dent Educ. 2007;71:393.
14. Marinho V.C., Higgins J.P., Logan S., Sheiham A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 4, 2003. CD002782
15. Marinho V., Higgins J.P., Logan S., Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 3, 2002. CC002279
16. Weintraub J.A., Ramos-Gomez F., Jue B., et al. Fluoride varnish efficacy in preventing early childhood caries. J Dent Research. 2006;85:172.
17. Hiiri A., Ahovuo-Saloranta A., Nordblad A., Makela M. Pit and fissure sealants versus fluoride varnishes for preventing dental decay in children and adolescents. Cochrane Database Syst Rev. 4, 2006. CD003067
18. Tung M.S., Hwang J., Malerman R., McHale W.A. Reactivity of varnish containing calcium, phosphate and fluoride salts. J Dent Res. 2007;86(Spec Issue A):1078.
19. Moberg Sköld U., Petersson L.G., Lith A., Birkhed D. Effect of school-based fluoride varnish programmes on approximal caries in adolescents from different caries risk areas. Caries Res. 2005;39:273.
20. Weintraub J.A., Ramos-Gomez F., Jue B., et al. Fluoride varnish efficacy in preventing early childhood caries. J Dent Res. 2006;85:172.
21. Whitford G.M. Acute and chronic fluoride toxicity. J Dent Res. 1992;71:1249.
22. Tweetman S., Petersson L.G. Comparison of the efficacy of three different chlorhexidine preparations in decreasing the levels of mutans streptococci in saliva and interdental plaque. Caries Res. 1998;32:113.
23. Lopex L., Berkowitz R., Spiekerman C., Weinstein P. Topical antimicrobial therapy in the prevention of early childhood caries: a follow-up report. Pediatr Dent. 2002;24:204.
24. Soderling E., Pienihakkinene P., Tnovuo J. Influence of maternal xylitol consumption on acquisition of mutans streptococci by infants. J Dent Res. 2000;79:82.
25. Tung M.S., Eichmiller F.C. Amorphous calcium phosphates for tooth mineralization. Compend Contin Educ Dent. 2005;25(Suppl):1.
26. Tung M.S., O Farrell T.J., Liu D.W. Remineralization by amorphous calcium phosphate compounds. J Dent Res. 1993;72:1738.
27. Reynolds E.C. Remineralization of enamel subsurface lesions by casein phosphopeptide–stabilized calcium phosphate solutions. J Dent Res. 1997;76:158.
28. Shen P., Cai F., Nowicki A., et al. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide–amorphous calcium phosphate. J Dent Res. 2001;80:2066.
29. Jenson L., Brideny A.W., Featherstone J.D.B., et al. Clinical protocols for caries management by risk assessment. J Calif Dent Assoc. 2007;35:714.
30. American Dental Association (ADA): ADA statement on early childhood caries, 2004. Available at: www.ada.org/prof/resources/positions/statements/caries.asp. Accessed August 9, 2008.
31. American Association of Public Health Dentistry: First oral health assessment policy, 2004. Available at: www.aaphd.org/default.asp?page=FirstHealthPolicy.htm. Accessed August 9, 2008.
32. American Academy of Pediatric Dentistry: Policy on the dental home, 2004. Available at: www.aapd.org/media/Policies_Guidelines/P_DentalHome.pdf. Accessed August 9, 2008.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..