Chapter 61Osteoarthritis
Joints are highly differentiated structures composed of a number of connective tissues including bone, articular cartilage, and periarticular soft tissues, all of which contribute to normal joint function and undergo changes in structure and metabolism in disease.1,2 From the point of view of joint diseases, perhaps the most important of these components is articular or hyaline cartilage, composed principally of a precisely organized arrangement of collagens and proteoglycans. This tissue is responsible for the load-distributing functions of the joint, and, in health, cartilaginous surfaces glide over one another in a virtually frictionless manner, even when under substantial load. In joint trauma or osteoarthritis (OA), the normal structure and function of articular cartilage are deranged, leading to biochemical, structural, and biomechanical abnormalities in all joint tissues. If the trauma is uncorrected, the result is progressive joint destruction, a process likened to organ failure in other body systems. Joint disease is a particularly prevalent cause of lameness and as such is an expensive equine health problem.3-5 An understanding of the biology and pathobiology of joints enables the clinician better to diagnose joint disease and to provide appropriate treatment and prevention recommendations.
Structure and Function of Normal Joints
Synovium and Synovial Fluid
The synovium is a vascular connective tissue lining the inner surface of the joint and consists of the cells of the synovial intima and a subsynovial stroma; the latter is composed of various amounts of fibrous, areolar, and fatty tissues. The synovium covers all articular surfaces, excluding articular cartilage and localized areas of bone. However, synovium is not uniform throughout the joint, and dense connective tissue may be found in its place in areas predisposed to trauma. Because the synovial lining bears neither true epithelium nor conventional basement membrane separating the joint cavity from the synovial vasculature, no true synovial membrane exists. Rather the intima and subsynovial tissues comprise a structural and functional continuum that acts as a macromolecular sieve.
The synovial intima is lined by a diverse population of synoviocytes, which have been classified according to their ultrastructure and with the use of specific antisera.6-8 The three cell types are type A cells, of macrophage origin; fibroblast-derived type B cells; and type C cells, which appear to be an intermediate between A and B forms.7,9-11 The most abundant are the type B cells, which synthesize a variety of important macromolecules, including collagen and hyaluronan.12,13 The viscosity of synovial fluid is largely the result of the concentration and degree of polymerization of hyaluronan, which serves a vital function in soft tissue lubrication. Type A cells, comprising only 10% to 20% of the lining cells, are predominately phagocytic. However, apparently some overlap in function between the two principal cell types exists.14,15 Importantly, synoviocytes synthesize a variety of soluble mediators implicated in the pathogenetic events of OA, including cytokines (e.g., interleukin-1 [IL-1]),16-18 eicosanoids (e.g., prostaglandin E2),19,20 and proteinases.21 That the synovial lining is capable of expressing these substances supports a role for the synovium in the pathogenesis of OA.
Deep to the synovial lining, the subsynovial region possesses a rich blood supply that is essential to generating synovial fluid, facilitates the exchange of nutrients and metabolic wastes of the synovium, and provides the sole source of nutrition to adult articular cartilage. Because of the specialized structure and functions of the synovial lining and subsynovial stroma, synovial blood flow is subject to a complex regulatory system involving extrinsic control and locally produced factors such as angiotensin II, endothelin-1, and nitric oxide.2
Periarticular Soft Tissues
The periarticular soft tissues include muscles, tendons, ligaments, and joint capsule. Muscles effect movement and, via complex reflexes, are vital to providing joint stability and protecting the joint from supraphysiological excursion. Muscle mass is more abundant near joints with a wide range of movement, such as the shoulder and the hip, and less so around joints that move in a single plane. Tendons serve as a bridge between muscle and bone, and ligaments provide stability between bones composing a joint. Tendons and ligaments are of similar, although not identical, composition, consisting mainly of water, an organized array of collagen bundles (predominantly type I), and a sparse population of fibroblasts. Ligaments contain more elastin fibers and have greater elasticity than tendons.22 Importantly, the molecular composition of these tissues responds to physical stimuli, and immobilization elicits catabolic events leading to tissue weakening.23 The composition of capsular structures parallels that of ligaments. Indeed, some ligaments can be recognized only as hypertrophied portions of the joint capsule. The fundamental role of the capsule is to provide stability; however, its specific nature varies with anatomical location and joint position. For example, the caudal capsule of the human knee is lax in flexion but exerts an important stabilizing force when the joint is in extension.24
Subchondral Bone
Although subchondral bone is histologically and biochemically similar to bone in other locations, the organization of the subchondral plate is specific. The plate is thinner than cortical bone found at other locations, and its Haversian systems are oriented parallel to the joint surface rather than parallel to the long axis of the bone.25 Similarly the organization of subchondral cancellous bone varies between joints, reflecting predominant biomechanical forces and adaptation to exercise.26,27 The deformability of the subchondral cortical and epiphyseal trabecular bone exceeds that of the diaphyseal cortical shaft by many times and has the important function of force attenuation. As such, possibly the bone stiffening (sclerosis) observed in OA contributes to disease progression.28,29
Articular Cartilage
Cartilage is the principal working tissue of the joint and allows simultaneous motion and weight-bearing with negligible friction. Cartilage covers the subchondral plate of bones composing the joint, to which the cartilage is firmly attached. Its thickness varies between joints and at different locations within them. Cartilage is composed of water, collagen, and proteoglycans that are present in respective proportions of 65% to 80%, 10% to 30%, and 5% to 10% of its wet weight. Chondrocytes account for less than 2% of its volume in most species. In adults, cartilage is avascular, alymphatic, and aneural; thus cartilage is nourished mainly via the synovial fluid (see the following discussion). Because articular cartilage is aneural, lesions restricted to cartilage are nonpainful, and the innervation of the underlying bone and adjacent periarticular soft tissues is responsible for providing information on joint position.
Cartilage possesses a number of zones or layers including the following:
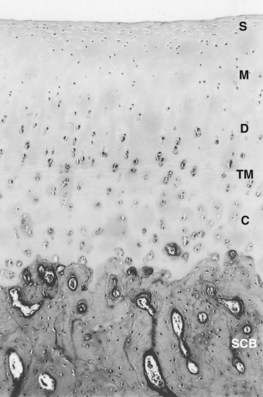
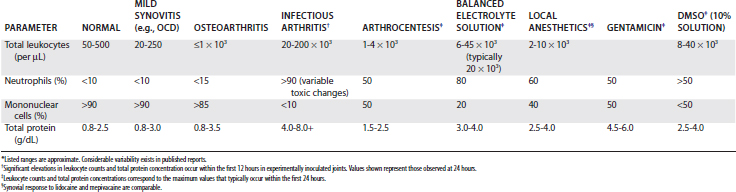
Fig. 61-1 Photomicrograph illustrating regional organization of mammalian cartilage. The four main zones from the articular surface to the subchondral plate include the superficial (tangential) (S), the middle (transitional) (M), the deep (radial) (D), and the calcified (C) zones. The deep and calcified zones are separated by the tidemark (TM). Pericellular matrix regions, distinguishable by their histological and ultrastructural differences and located at progressively greater distances from chondrocyte lacunae, are the pericellular, territorial, and interterritorial regions (×50). SCB, Subchondral bone.
The latter two zones are separated by an irregular line, visible on standard histological preparations, called the tidemark, the specific function of which is unclear.30 The density of chondrocytes in the matrix varies with depth from the articular surface, as does the macromolecular composition of the matrix surrounding the chondrocytes. These regional differences can be identified histologically and have been designated as the pericellular, territorial, and interterritorial regions.
The unique functional properties of articular cartilage are reflected in its biochemistry. Articular cartilage is composed of an abundant, specialized extracellular matrix maintained by the aforementioned sparse population of chondrocytes (Figure 61-2). Its water content varies with age but may be as high as 80%.31 This water is freely exchangeable with that in the synovial fluid and is maintained in the matrix in the form of a gel, with matrix collagens and proteoglycans. Water movement is believed to be pivotal to the capacity of cartilage to absorb and distribute compressive load and for its lubrication.

Fig. 61-2 Organization of the major extracellular matrix components in articular cartilage. The principal collagen of cartilage is type II, and a network of these fibrils provides much of the tensile strength of the tissue. Aggrecan is composed of a linear protein with three globular domains (G1 to G3) to which are attached numerous glycosaminoglycan chains of chondroitin sulfate (CS) and keratan sulfate (KS) (see also Figure 61-4). Supramolecular aggregates are formed by the noncovalent interaction of aggrecan with hyaluronan (HA) and stabilized by link protein (Link). The negatively charged glycosaminoglycans (CS and KS) attract several times their weight in water, and this proteoglycan-water composite is responsible for the compressive stiffness of cartilage. Cartilage also has a number of minor proteoglycans and collagens (e.g., decorin and dermatan sulfate [DS], the functions of which are not fully characterized). Fragments of aggrecan, remaining bound to hyaluronan, are depicted to illustrate the effects of proteolytic activity in cartilage.
(From Koopman WJ, editor: Arthritis and allied conditions: a textbook of rheumatology, ed 13, vol 1, Baltimore, 1997, Williams & Wilkins.)
Collagens
The collagens of articular cartilage differ from those found in most other locations in the body. Several collagens, fibrillar and nonfibrillar, are present in this tissue and are thought to provide cartilage with structural support. These proteins also interact with other matrix components to contribute to cartilage architecture and function.32-34 Collagen fibrils are oriented parallel to the joint surface in the superficial zone and act as a protective layer, whereas larger, radially oriented fibrils in the deeper layers anchor the cartilage to the underlying articular end plate.
Type II collagen is the most abundant in cartilage, accounting for about 90% of the fibrillar network and half of the dry weight of cartilage.2,35 Type II collagen consists of three identical amino acid chains arranged in a triple helix, is less soluble, possesses a higher proportion of hydroxylysine residues, and is more richly glycosylated than type I collagen.36,37 Unlike type I, which typically forms fibers, type II collagen is organized in the form of fibrils that are composed of molecules aligned with a 25% overlap or quarter stagger (Figure 61-3). This structure is stabilized by chemical bonds between specific amino acids in each chain, called hydroxypyridinium cross-links.38 Fibrils are not uniform in size throughout the matrix; they tend to be larger in the middle and deep zones of the matrix, which reflects regional biomechanical demands.39 This protein is arranged in arcades, which form the three-dimensional network or skeleton of the cartilage matrix. Type II collagen is produced by the chondrocytes, and whereas significant degradation and resynthesis of fibrils occur during growth and development, limited turnover occurs in adults.40,41

Fig. 61-3 Schematic representation of collagen fibril organization and its proteolytic degradation in cartilage. Cartilage collagen is arranged in fibrils of cross-linked, triple-helical molecules overlapping at regular intervals. Collagenases cleave intact helical collagen and produce characteristic “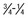 ” fragments. Other proteases (e.g., stromelysin) degrade collagen in nonhelical regions.
” fragments. Other proteases (e.g., stromelysin) degrade collagen in nonhelical regions.
(From Koopman WJ, editor: Arthritis and allied conditions: a textbook of rheumatology, ed 13, vol 1, Baltimore, 1997, Williams & Wilkins.)
Minor collagens are present in modest amounts in cartilage, and the specific roles of these collagens in its structure and function have yet to be defined fully. Type XI is a fibrillar collagen that is found within type II fibrils. Its function is unclear, but likely it plays a role in type II collagen fibril assembly and organization because a mutation in the type XI gene in mice leads to a disorganized matrix, with abnormally thick collagen fibrils.42 Type VI is a microfibrillar collagen that may act as a bridge between fibrillar collagen and other matrix components.43,44 So-called fibril-associated small collagens include collagens IX, XI, and XIV. Type IX collagen molecules bound covalently to the surface of type II fibrils may serve to stabilize the latter.45 Types XII and XIV collagen also are associated with fibrillar collagen, but the specific functions have yet to be identified.
Proteoglycans
By definition, proteoglycans are composite molecules consisting of protein and glycosaminoglycan (polysaccharide) components. This definition is broad because some of the aforementioned minor collagens (e.g., type IX) have a single glycosaminoglycan side chain and thus can be designated as proteoglycans. A number of proteoglycans are found in articular cartilage. Aggrecan, the largest and most abundant, has a well-defined function in the extracellular matrix; however, the specific roles of the smaller proteoglycans remain to be characterized fully.
Aggrecan is the primary proteoglycan of articular cartilage that interacts with hyaluronan to form aggregates (see Figures 61-2 and 61-3; Figure 61-4). The individual or monomeric form of this molecule consists of a linear core protein interrupted by three globular domains. The first of these globular domains is designated G1, exists at the amino-terminal portion of the molecule, and is the site at which the proteoglycan attaches to hyaluronan. As many as 100 aggrecan monomers may be attached to the same hyaluronan chain to form supramolecular aggregates of micrometer dimensions (see Figure 61-2).46 The interaction of aggrecan with hyaluronan is noncovalent but is stabilized by a link protein that binds to the G1 domain and hyaluronan with equal affinity.47 Equine link protein was characterized and is similar to that found in human cartilage.48 The specific functions of the G2 and G3 domains are unclear; however, because the G3 domain is present in only about one third of the aggrecan monomers in adult cartilage, it is unlikely that it plays a pivotal role in the extracellular matrix.49
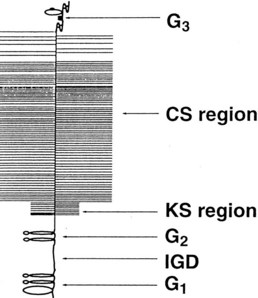
Fig. 61-4 Schematic representation of an aggrecan monomer. Proteolytic cleavage of the molecule in vivo usually first occurs in the G1 to G2 interglobular domain (IGD). The specific sites involved vary among the enzymes implicated in the process. Keratan sulfate (KS) and chondroitin sulfate (CS) regions are positioned on the periphery of the molecule.
(From Koopman WJ, editor: Arthritis and allied conditions: a textbook of rheumatology, ed 13, vol 1, Baltimore, 1997, Williams & Wilkins.)
In the region between the second and third globular domains, glycosaminoglycan chains of variable length and composition are attached radially to the protein core (see Figure 61-4). Immediately adjacent to the G2 domain is a region rich in keratan sulfate, and this portion of the proteoglycan, detectable by monoclonal antibodies, has served as a tissue marker of matrix turnover.50 Farther peripherally on the core protein is the chondroitin sulfate–rich region, where up to 100 chondroitin sulfate chains may be found attached radially to the core protein. These chondroitin sulfate chains vary in length, which is the main reason for heterogeneity in the size of aggrecan. Importantly, these glycosaminoglycan chains contain numerous carboxyl and sulfate groups, so that aggrecan is highly negatively charged and can bind up to 50 times its weight in water.46,51,52 This highly hydrated matrix gives cartilage its compressive stiffness and ability to dissipate load.
Matrix Proteins
Cartilage, like other connective tissues, contains a number of noncollagenous proteins, many of which are proteoglycans. Among the best characterized of the small proteoglycans are decorin, biglycan, lumican, and fibromodulin, all of which are similar in molecular organization. These proteins have been shown to interact with a number of matrix constituents, including cartilage collagens, and in many cases these interactions involve a number of different collagens and appear to regulate a variety of metabolic processes.46,52 For example, decorin and fibromodulin inhibit fibrillogenesis of type II collagen, a process that may regulate the size of collagen fibrils in the matrix.53 Some of these small matrix proteins also may contribute to the antiadhesive properties of articular cartilage.54,55
Cartilage also contains a number of small proteins that are neither collagens nor proteoglycans,56,57 and most are involved in interactions with a variety of matrix molecules and chondrocytes. For example, anchorin is found on the surface of chondrocytes and within the cell membrane and has a high affinity for type II collagen fibrils. These properties suggest that anchorin may act as a mechanoreceptor, providing chondrocytes with information on changes in stresses experienced by the matrix. Fibronectin is a minor component of cartilage that is thought to contribute to matrix assembly, via interactions with chondrocytes and elements of the extracellular matrix. Fibronectin fragments are present in elevated quantities in OA and may contribute to catabolic events in affected cartilage.58,59 Cartilage oligomeric matrix protein (COMP), also known as thrombospondin-5, is abundant in articular cartilage and is formed by the association of five identical subunits. COMP is most abundant in the proliferative cell layer of growth cartilage, where it is thought to regulate cell growth.
Chondrocytes
Studies of cartilage metabolism contradict the seemingly inert histological appearance of this relatively acellular tissue. Despite the fact that chondrocytes represent a small percentage of the volume of cartilage, they are responsible for extracellular matrix synthesis, including all the collagens and proteoglycans. They are also capable of elaborating a variety of proteolytic enzymes effecting degradation of matrix macromolecules. The rate of turnover of the various matrix components is not uniform. At least a portion of the proteoglycan pool is renewed at a relatively rapid rate, whereas the rate of collagen turnover is minimal.60-62 Chondrocyte metabolism is influenced by intrinsic and extrinsic mechanical influences. For example, cyclic loading and alterations in matrix pressure, as a result of changes in solute content, materially influence proteoglycan synthetic rates.63,64 Thus the maintenance of the cartilage matrix involves the chondrocyte-mediated processes of synthesis and degradation, and the cartilage loss in OA appears to be attributable to a disequilibrium in favor of matrix degradation.
Nutrition
Unlike the cartilage of growing animals, in which articular cartilage receives some blood supply from subchondral vasculature, adult articular cartilage contains no blood vessels. As a result, the chondrocyte exists under relatively hypoxic and acidic conditions, with an extracellular pH typically 7.1 to 7.2.65 Nutrients migrate from subsynovial vessels to the synovial fluid and subsequently penetrate the dense connective tissue matrix of the cartilage, while metabolic wastes are simultaneously cleared in the opposite direction. The density of the matrix appears not to hamper diffusion because molecules as large as hemoglobin (65 kDa) can penetrate normal articular cartilage.66 The highly charged proteoglycans contained in the matrix do not inhibit the diffusion of small, uncharged molecules.67 Entry of solutes into the matrix occurs by simple diffusion or may be facilitated by compression-relaxation cycles. Intermittent loading of cartilage is vital to its health, as is evidenced by the deleterious effects of immobilization.68
Joint Lubrication
Although several mechanisms for cartilage-on-cartilage lubrication have been hypothesized, two main systems are accepted: a hydrostatic or weeping system that functions at high loads, and a boundary system that functions at low loads.69 Hydrostatic lubrication of opposing cartilaginous surfaces is effected by a thin film of water liberated from the matrix during cartilage compression. Because little movement of water can occur from cartilage to the subchondral bone, most is squeezed from the opposing cartilages onto the surface, immediately peripheral to the zone of impending contact.70 With the release of compressive force, the cartilage expands, and water is drawn back into the matrix.
Whereas hydrostatic mechanisms function well under relatively heavy loads, boundary lubrication occurs under low-load conditions. Boundary lubrication is accomplished by specialized materials including lubricin71 (a glycoprotein of synovial origin) and hyaluronan. These molecules bind to opposing articular cartilage surfaces and prevent the direct contact of these surfaces under low loads. Coefficients of friction were unchanged after hyaluronidase treatment of synovial fluid, suggesting that hyaluronan has no place in cartilage-on-cartilage lubrication.72,73 However, others have found that hyaluronan actually does function as a boundary lubricant.74,75
Articular soft tissues require lubrication because they contribute most of the frictional resistance to joint movement. Indeed, the energy requirement for the stretching of articular soft tissues is 100 times that of the frictional resistance of opposing cartilage surfaces.76 The synovium is lubricated by a thin film of synovial fluid, rich in hyaluronan, its principal boundary lubricant.77
Intraarticular Volume and Pressure
Intraarticular volume varies and is influenced by joint position (see Chapter 66). Specifically, volume and pressure are respectively minimal and maximal near the extremes of flexion and extension.78-81 This effect is exacerbated in horses with synovial effusion, providing a physiological rationale for diagnostic flexion tests in equine lameness examinations. Moreover, the pointing of an equine limb in which there is joint effusion likely parallels the observation that in the human knee there is a maximum of intraarticular volume (and minimum of intraarticular pressure and pain) at 30 degrees of flexion.82,83
Whereas intraarticular pressure varies during movement, pressure within a normal joint is subatmospheric at rest.84,85 As a result, the normal synovial cavity is merely a potential space, the surfaces of which are coated with a thin film of synovial fluid to reduce friction during movement. Although the mechanisms by which this negative pressure occurs remain unclear, the phenomenon contributes measurably to joint stability.86 Lack of familiarity with the physiological concept of negative intraarticular pressure in normal joints results in the common misconception that the sound of air being aspirated into a joint during arthrocentesis heralds a dry or diseased joint.
Biomechanical Considerations
Articular cartilage remains healthy despite being regularly subjected to considerable normal and shear forces during normal activities. A number of mechanisms exist to facilitate this phenomenon, including the transmission of forces to surrounding tissues by periarticular soft tissues, the incongruity of cartilage surfaces, and the inherent compliance of cartilage and subchondral bone. Indeed, the capacity for considerable elastic deformation permits normal cartilage to withstand compressive stresses considerably greater than those of body weight alone.87,88 Nonetheless, cartilage is subject to mechanical breakdown after supraphysiological stresses, and loads exceeding 25 kg/cm2 are reported to result in matrix damage.89 Apparently these loads occur in specific areas of cartilage under a variety of clinical circumstances, such as the cartilage degeneration that accompanies the incongruent articular surfaces of a poorly reduced or unstable intraarticular fracture.
At the tissue level, the ability of cartilage matrix to resist compression and shear is a function of the interaction of collagen, aggrecan, and tissue fluid. Aggrecan can absorb many times its weight in water, but its complete hydration is restricted by the collagen network. Thus a balance exists between the internal swelling pressure exerted by the association of water with aggrecan (Gibbs-Donnan ionic equilibrium) and the tensile forces of the collagen fibrils. Cartilage under load undergoes a two-phase (viscoelastic) deformation.90,91 Initially rapid bulk movement of water from the matrix and compression of collagen occur. Subsequently, a time-dependent compression occurs, known as the creep phase, in which water flows through the matrix at a slower rate.
These mechanical phenomena were studied in experiments evaluating the mechanical properties of cartilage after the selective depletion of specific matrix components. The tensile strength of cartilage is a function of its type II collagen content because strength is reduced in collagen-depleted tissues but is unaffected by proteoglycan removal.92,93 Proteoglycans (mainly aggrecan) provide the matrix with its compressive stiffness and protect the collagen network from mechanical damage. Trypsin-treated (proteoglycan-depleted) specimens lose the ability to rebound from compressive load and have reduced stiffness.94
At rest, opposing articular surfaces are not completely congruent, but when loaded, articular cartilage contact increases, which serves to distribute stress and increase joint stability. This may be a physiological reason why cartilage tends to be somewhat thicker in less congruous joints, such as the hip and stifle.95 Although cartilage is designed to withstand compressive stress, its ability to act as a shock absorber is finite, largely because it receives its nutrition by diffusion and as a result is of limited thickness. Because its ability to absorb load is limited, cartilage must transmit load to the underlying subchondral bone. As such the articular ends of most bones are flared (less force per unit area) and deform under physiological load to absorb stress.96 Noteworthy is that the stiffness of the subchondral bone is attributable not only to the cancellous trabeculae but also to the extracellular fluid content. This was demonstrated in an experiment where subchondral stiffness of canine femoral heads was reduced by 30% after fluid decompression by drilling.97 When the subchondral bone is unable to accommodate loading, so-called adaptive remodeling failure occurs, in which repetitive subchondral deformation causes trabecular microfractures, which may or may not be accompanied by changes in articular cartilage. Fortunately, when occurring at an acceptable rate, trabecular microfractures undergo a reparative response leading to an orientation of subchondral bone that provides improved strength and shock absorption capacity.98 Articular surfaces are protected by stress distribution mechanisms beyond those of cartilage and bone. For example, muscles absorb a large proportion of the force experienced during impact loading, leaving the remainder to be cushioned by cartilage and bone. Fine-tuned neuromuscular reflexes are required for this system to work effectively, and small failures in these reflex arcs lead to insufficient attenuation of impact loading, which may lead to degenerative changes in cartilage and subchondral bone.99
Osteoarthritis
Etiopathogenesis
OA has been defined as an essentially noninflammatory disorder of movable joints, characterized by degeneration and loss of articular cartilage and the development of new bone on joint surfaces and margins.36,100 As in people, equine OA is probably not a single disease but reflects a common response of joint tissues to a number of potential causes. Unfortunately the specific contributions and interactions of various mechanical and biological factors contributing to development of OA lesions remain unclear.
Three pathogenetic mechanisms are hypothesized for OA.100 The first involves a fundamentally defective cartilage, with abnormal biomechanical properties. In this pathway a biomechanically flawed matrix fails under normal loading. In people a type II collagen defect exemplifies this primary form of OA.101,102 OA attributed to inherently defective cartilage matrix components has not yet been identified in the horse.
A second proposed pathogenetic pathway of OA involves physical changes in the subchondral bone.28,29 Because articular cartilage is too thin to be an effective shock absorber, impact loading must be attenuated by periarticular soft tissues, muscles, and subchondral bone. Although substantially stiffer than cartilage or joint capsule, cancellous subchondral bone is considered an important shock attenuator. Thus in this hypothesis of OA pathogenesis, normal mechanical stresses result in microfractures of the subchondral and epiphyseal trabecular bone. However, when occurring at an excessive frequency, these fractures exceed the rate at which optimal healing and remodeling of the subchondral trabeculae can occur. Bone accretion with healing of these microfractures increases the density of the subchondral plate and adjacent trabeculae, with a concomitant reduction in the ability to absorb repetitive physiological loads. The resulting increase in bone stiffness leads to a state in which the bone-cartilage unit fails to deform normally under load, and the cartilage experiences supraphysiological stresses, resulting in mechanical damage. Subsequent events are those outlined in the following discussion of the third pathogenetic mechanism of OA.
To date, a cause-and-effect relationship between subchondral bone plate thickening and cartilage degeneration remains to be established. The hypothesis that subchondral bone and cartilage degeneration are related is supported by the demonstration of microfractures of the subchondral plate and more distant trabeculae in arthritic specimens.103 Moreover, in mice with OA, cartilage degenerates over areas of sclerotic bone but remains intact over areas of normal bone density.104 However, mathematical models predict that even with considerable increases in subchondral bone stiffness, cartilage stresses are only modestly increased.105 Collectively these data indicate that subchondral sclerosis contributes to the osteoarthritic process but is probably not a prerequisite to initiate articular cartilage destruction.106
The third and most popular hypothesis of the pathogenesis of OA is based on the concept of mechanical forces causing damage to healthy cartilage.36,52,100,107 Matricial or cellular injury by these forces results in metabolic alterations of chondrocytes, leading to the release of proteolytic enzymes that cause cartilage fibrillation and breakdown of the proteoglycan network. Cartilage is remarkably resistant to shear forces but is relatively susceptible to repetitive impact trauma. In people, repetitive trauma is an acknowledged predisposing factor to OA in athletes (e.g., metacarpophalangeal joints of boxers) and certain occupations (e.g., shoulder joints of jack-hammer operators). Of many potential causes, repeated microtrauma (use trauma) is probably the most common pathogenetic factor in equine OA, and the correlation of lesions at defined sites in horses participating in specific sports supports this hypothesis.
Role of the Synovium
Although conventional concepts of OA emphasize the direct and predominant involvement of cartilage and bone in OA development, it is increasingly recognized that the synovium contributes to the central pathophysiological event of cartilage matrix depletion. Recent investigations in several species have shown that synoviocytes are a rich source of a variety of inflammatory mediators and degradative enzymes implicated in cartilaginous degeneration, including prostaglandins,19,20,108-111 cytokines,112-114 and matrix metalloproteinases (MMPs).113-116 These laboratory data are supplemented by the identification of increased levels of these and other inflammatory mediators in the synovial fluid of horses with naturally occurring or experimentally induced synovitis.117-124 Experiments using synovially conditioned culture media, or coincubation of synovial tissues with cartilage, support a role of the synovial membrane in cartilage degradation.125,126 Recent experiments indicate that synovial macrophages are important contributors to the inflammatory and degradative responses in affected joints, effects mediated by a combination of IL-1 and tumor necrosis factor–α (TNF-α) (see Cytokines discussion).127 Nonetheless, determining the specific role of the synovium in OA is hampered by the fact that both chondrocytes and synoviocytes are a rich source of the pertinent mediators and enzymes. Thus precise characterization of the relative quantitative and temporal contributions of cartilage and synovium to lesion development has not yet been accomplished.
Role of the Chondrocyte
Of all joint tissues, articular cartilage shows the greatest aberration from normal during disease development, and it is generally considered that metabolic changes in chondrocytes play a primary role in the pathophysiological events of cartilage loss. In normal joints, chondrocytes are responsible for maintaining a balance between matrix degradation and repair, and this equilibrium is maintained by a complex interaction between chondrocytes, cytokines, and mechanical stimuli.128 In OA, a disruption of this homeostatic state develops, in which catabolic processes predominate. Although proteoglycan synthesis is greater than normal early in the disease, the rate of matrix digestion is sufficient that the result is a net loss of matrix. With this imbalance toward matrix depletion, cartilage mass is progressively lost, and the viscoelastic properties of the remaining tissue become insufficient to withstand normal loads. Subsequently, cartilage fissuring and separation occur (Figure 61-5). The ultimate result is generalized cartilage loss and secondary remodeling of bone and articular soft tissues (Figure 61-6).
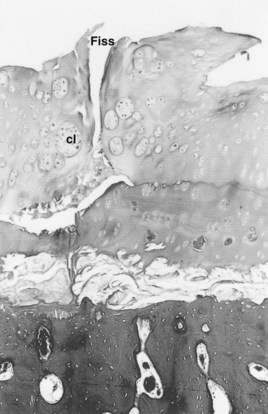
Fig. 61-5 Photomicrograph illustrating the pathological changes associated with cartilage matrix degeneration. Loss of matrix proteoglycans alters the biomechanical properties and ultimately leads to fissures (Fiss) in the cartilage, in this case a full-thickness fissure. Chondrocyte clones (cl) represent the abortive healing attempts of chondrocytes (×50).
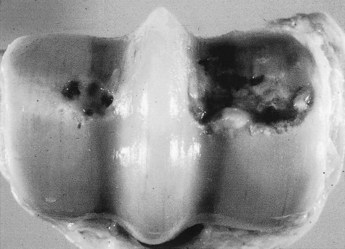
Fig. 61-6 Post mortem specimen of the distal aspect of an equine third metacarpal bone illustrating partial and full-thickness cartilage loss. Although osteoarthritis affects all articular tissues, degeneration of extracellular matrix of cartilage and its subsequent loss are considered the central events in the disease. Note also the wear lines on the articular cartilage.
A number of studies indicate that an important initial biochemical change in OA is the loss of aggregating proteoglycans. Up-regulation of chondrocyte proteoglycan synthesis is insufficient to offset enhanced degradation, so that the concentration in the matrix progressively decreases. In addition to a reduced quantity of proteoglycan, the quality of molecules remaining in the matrix, and newly synthesized replacements, appears to be altered.129,130 Collagen degradation accompanies proteoglycan loss and is manifested by surface fibrillation (Figure 61-7). The loss of collagen and changes in collagen fibril size contribute to weakening of the matrix and may account for the increased water content in early cartilage lesions.131-133
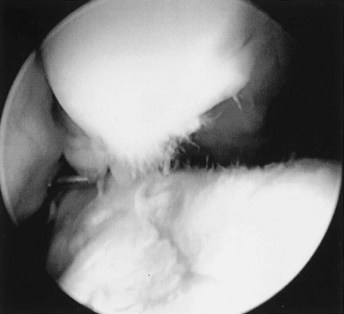
Fig. 61-7 Arthroscopic endophotograph of the middle carpal joint of a Standardbred racehorse with a large osteochondral fragment of the third carpal bone (top) and concomitant lesions of the intermediate carpal bone (bottom). Cartilage fibrillation is substantial, and its evaluation is greatly facilitated by fluid distention of the joint. Fibrillation indicates damage to the collagenous network of the extracellular matrix.
Whereas degradation of articular cartilage may occur by the action of a number of mediators, including oxygen-derived free radicals,134-137 proteolytic enzymes synthesized by chondrocytes are thought to be the major mediators of matrix depletion. Proteinases are classified according to the catalytic mechanism into four main groups, including aspartic proteinases, cysteine proteinases, serine proteinases, and metalloproteinases. Members of each class are synthesized by chondrocytes or synoviocytes and may contribute to cartilage degradation. However, the MMPs and related enzymes apparently are the most active in OA.36,52,138-140 Chondrocytes have surface receptors that respond to mechanical stress, and physical disruptions of cell-matrix associations can negatively influence chondrocyte synthetic activites.141
Matrix-Degrading Enzymes
MMPs are considered to play a major role in cartilage matrix degradation in OA because this group of proteinases is capable of digesting all major components of the extracellular matrix. The relative contributions of these proteolytic enzymes to the overall process remain to be firmly established; however, a wealth of evidence implicates them in cartilage loss. Specifically, MMPs are synthesized by synoviocytes and chondrocytes21,52,142-144 and are present in increased concentrations in diseased cartilage,145,146 and the topographical distribution and concentration of MMPs in cartilage are correlated with the histological severity of lesions.146-148 Several types of MMPs are expressed by articular tissues, and these are classified as collagenases, stromelysins, gelatinases, membrane-type metalloproteinases, and other MMPs.144 In addition to other substrates, collagenases degrade intact, helical type II collagen. Stromelysins cleave partially degraded collagen, proteoglycans, and other minor proteins in cartilage. The gelatinases have a diverse range of substrates, including partially degraded type II collagen and types X and XI collagen and elastin. Like collagenases, membrane-type 1 MMP is also capable of digesting fibrillar collagen and a number of other matrix components. MMPs are secreted in an inactive or latent form and require activation through proteolytic cleavage. A variety of enzymes including trypsin, chymotrypsin, plasmin, kallikrein, cathepsin B, and certain MMPs themselves are capable of such cleavage.52,138,148 The classification and general properties of MMPs are summarized in Table 61-1.
TABLE 61-1 Matrix Metalloproteinases Implicated in Cartilage Matrix Degradation
| PROTEINASE* | MMP | CARTILAGE SUBSTRATES |
|---|---|---|
| Collagenases | ||
| Interstitial collagenase (collagenase)† | MMP-1‡ | Collagens II and X (not IX and XI), denatured type II, aggrecan, link protein |
| Neutrophil collagenase† | MMP-8 | Collagen II, aggrecan, link protein |
| Collagenase 3†§ | MMP-13‡ | Collagens II, IV, IX, X; aggrecan; fibronectin |
| Stromelysins | ||
| Stromelysin 1† | MMP-3‡ | Aggrecan, fibronectin; denatured collagen II; collagens IV, IX, X, XI; procollagens; link protein; decorin; elastin; laminin |
| Stromelysin 2† | MMP-10 | Same as for stromelysin 1 |
| Gelatinases | ||
| Gelatinase A (72 kD)† | MMP-2‡ | Denatured collagen II, collagens X and XI, elastin |
| Gelatinase B (92 kD)† | MMP-9‡ | Aggrecan, fibronectin, collagens IX and XI, procollagens, link protein, decorin, elastin |
| MT-MMPs | ||
| MT1-MMP† | MMP-14 | Aggrecan, collagen II, denatured collagen II, fibronectin, laminin |
| Others | ||
| Matrilysin (PUMP)† | MMP-7 | Aggrecan |
| Stromelysin-3 | MMP-11 | Proteoglycan, denatured collagen II, fibronectin, laminin |
| Macrophage metalloelastase | MMP-12 | Elastin |
| Novel MMP | MMP-19 | Denatured collagen II, collagen IV, aggrecan, fibronectin, laminin |
MMP, Matrix metalloproteinase, MT(1)-MMP, membrane-type (1) matrix metalloproteinase; PUMP, putative metalloproteinase.
* All except membrane-type 1 MMP are inhibited by some or all of the tissue inhibitors of metalloproteinases 1 to 3.
† Expressed by chondrocytes. All are expressed in synovium.
‡ MMPs characterized in the horse.
§ MMP-13 expression is relatively weak in equine synovium.
Several members of the ADAM (a disintegrin and metalloproteinase) family of enzymes were shown to be expressed by chondrocytes.149,150 Many of the ADAM enzymes are proteinases and are structurally and functionally related to MMPs. Importantly, certain members of this group cleave aggrecan at a specific site in the interglobular (G1 to G2) domain, resulting in aggrecan fragments identical to those found in the tissues and synovial fluids of osteoarthritic animals. These proteinases have been termed aggrecanase and share many similarities to typical MMPs, including the inactivation with conventional MMP inhibitors and being inhibited by tissue inhibitor of matrix metalloproteinase–1 (TIMP-1).151 Two forms of aggrecanase have been implicated in OA, and both are ADAMTS enzymes (ADAM with thrombospondin type 1 motifs).152,153 (The thrombospondin subunits of the protein appear to be critical for the binding and digestion of aggrecan.154) ADAMTS4 and ADAMTS5 (aggrecanases 1 and 2, respectively) are related enzymes but demonstrate important differences in their regulation by cytokines. For example, although ADAMTS4 is induced by cytokines and ADAMTS5 appears to be constitutively expressed, both are implicated in aggrecan degradation in OA.155 Aggrecanase is considered pivotal in proteoglycan degradation. Indeed, some contend that aggrecanase is the principal mediator of proteoglycan depletion in OA.140
In healthy cartilage, the activity of proteolytic enzymes is controlled by a number of mechanisms, one of which is naturally occurring inhibitory proteins. The most important of these inhibitors are the TIMPs.52,137,144,156 Synthesized by synoviocytes, chondrocytes, and endothelial cells, TIMPs inactivate MMPs by binding to them in a 1 : 1 noncovalent complex,157 and these inhibitors are hypothesized to be critical to the longevity of the extracellular matrix of cartilage.148,158 TIMP exists in at least four forms, the first three of which (TIMP-1, TIMP-2, TIMP-3) are expressed by chondrocytes. Interestingly, each is subject to somewhat different regulatory mechanisms.159,160 The important role of TIMPs in cartilage matrix health is supported by the observation that imbalances in the ratio of MMP to TIMP synthesis in cartilage are important determinants of the rate of matrix degradation.148,161
Cytokines
Cytokine is a general term to describe a broad array of small regulatory proteins produced by a variety of cells in the body. In joints, these mediators exist in a complex balance of activities that regulate the metabolism of the synovial membrane, bone, and articular cartilage in health and disease (Table 61-2).162,163 Numerous cytokines are involved in articular metabolism, and they possess one or more proinflammatory (catabolic), antiinflammatory (regulatory), or anabolic functions. Important in OA are the proinflammatory cytokines, such as IL-1 and TNF-α. Chondrocyte receptors for IL-1 and TNF-α are up-regulated in osteoarthritic cartilage, and the activation of these receptors has several deleterious effects on chondrocyte metabolism.164,165
TABLE 61-2 General Classification of Cytokines and Their Actions on Cartilage Metabolism
| CATEGORY OF CYTOKINE | EXAMPLES | ACTIONS |
|---|---|---|
| Catabolic (pro-inflammatory) cytokines | IL-1, TNFα | |
| Modulatory (regulatory) cytokines* | IL-4, IL-6, IL-10, IL-13 | |
| Anabolic cytokines (growth factors) | IGF-1, TGF-β, bFGF |
IL, Interleukin; TNF, tumor necrosis factor; MMP, matrix metalloproteinase; PGE2, prostaglandin E2; TIMP, tissue inhibitor of matrix metalloproteinase; IRAP, interleukin-1 receptor antagonist protein; IGF, insulin-like growth factor; bFGF, basic fibroblast growth factor.
* Regulatory cytokines can have mixed actions (e.g., IL-6 amplifies IL-1 effects on MMP synthesis but induces TIMP synthesis).
A wealth of recent research suggests that IL-1 is the most important of the proinflammatory cytokines in OA. Early studies using cartilage organ culture provided data supporting a role for IL-1 in cartilage matrix degradation,166 which were supplemented by the identification of elevated levels of this cytokine in synovial fluids of affected patients, including horses.119,120 IL-1 is involved in the destruction of the extracellular matrix and formation of the functionally inadequate repair tissue in arthritic cartilage. IL-1 decreases the synthesis of proteoglycans and type II collagen and induces the synthesis and secretion of proteolytic enzymes that degrade these matrix macromolecules.167-177 Decreased synthesis of matrix macromolecules occurs in cartilage exposed to IL-1 concentrations substantially less than those required to stimulate matrix degradation.169 Catabolism is further promoted by inhibition of the synthesis of MMP inhibitors such as TIMP.178 In addition, IL-1 stimulates the synthesis of prostaglandin E2 and nitric oxide, the effects of which are outlined in the following discussion.179-183 IL-1 may also contribute to the proliferative events in OA. Osteophytosis may be caused, at least in part, by the stimulation of osteoblast-like cells by IL-1.184 Perhaps the most compelling evidence supporting the involvement of IL-1 in OA is the protective effect of IL-1 receptor antagonist protein, which blocks many of the catabolic events typical of IL-1 in vitro. This naturally occurring competitive antagonist of IL-1 was shown to be protective for OA-like lesions in arthritis models.185-187
TNF-α is another proinflammatory cytokine that was implicated in the development of osteoarthritic lesions and was found in elevated concentrations in inflamed and arthritic joints.188-191 Like IL-1, this cytokine stimulates the synthesis of matrix-degrading enzymes175 and inhibits chondrocyte synthesis of proteoglycan and collagen.176 TNF-α appears to be less potent than IL-1192; however, the effects of IL-1 and TNF-α are potentiated when combined.193 TNF-α appears to stimulate the synthesis of IL-1.194 Experiments with adenoviral transfers of the gene expressing an endogenous inhibitor of nuclear factor κB (IκBα) indicate that a substantial proportion of synovially derived inflammatory mediators and degradative enzymes are regulated by the nuclear factor κB (NFκB) pathway.195
The degradative effects of certain cytokines, including IL-1 and TNF-α, are balanced by inhibitory cytokines (e.g., IL-4, IL-10, and IL-13). Moreover, opposing effects on matrix synthesis are induced by other cytokines, also known as growth factors (e.g., insulin-like growth factor and basic fibroblast growth factor) (see Table 61-2). Using these antiinflammatory and inhibitory cytokines to control the osteoarthritic process is an active area of research.
Nitric Oxide
Nitric oxide is another mediator of the pathophysiological events in OA. This highly reactive, cytotoxic free radical is a byproduct of the oxidation of l-arginine to citrulline, catalyzed by a group of enzymes called nitric oxide synthases (NOSs), which produce large amounts of the mediator when cells expressing this enzyme are activated by mediators such as endotoxins and cytokines.182,183,196 Early evidence for the involvement of nitric oxide in rheumatic diseases was the observation that nitrite, a stable end product of nitric oxide, was found in elevated concentrations in the synovial fluid and serum of people with rheumatoid arthritis.197 Subsequently, it has been shown that osteoarthritic cartilage spontaneously produces nitric oxide.198-200 Nitric oxide may mediate the inhibition of chondrocyte synthetic activities that occur in OA. Proteoglycan and type 2 collagen synthesis are inhibited under conditions conducive to nitric oxide formation.201-203 Thus, like other inflammatory mediators, inducible NOS is stimulated by IL-1 and TNF-α and requires NFκB for its expression.204
Nitric oxide also is hypothesized to mediate, in part, the augmented expression and activation of MMPs,205,206 as well as the reduced synthesis of the natural IL-1 receptor antagonist protein,199 and is reported to be an important inducer of chondrocyte apoptosis.207 However, the specific role of nitric oxide in IL-1–induced cartilage matrix depletion is controversial. Early laboratory studies revealed that the MMP activity in IL-1– and TNF-α–stimulated cartilage cultures was enhanced by adding substrates favoring nitric oxide formation (nitric oxide donors), and this effect was blocked by NOS inhibitors.205,206 Conversely, cytokine-mediated induction of MMP expression can occur independently of stimulation by nitric oxide,208 and cytokine-stimulated cartilage explants cultured in the presence of nitric oxide inhibitors had rates of proteoglycan depletion comparable with controls.209 Nonetheless, nitric oxide remains an important area of study, because in animal models of inflammatory arthritis and OA, using compounds that directly or indirectly inhibit NOS activity reduces the severity of lesions.210-213
Prostaglandins
Prostaglandins are found in elevated concentrations in inflamed joints,188,214 and although the specific effects of prostaglandins on joint metabolism are unclear, it is widely held that prostaglandin E2 contributes to the lesions of OA. Prostaglandin E2 causes synovial inflammation and may contribute to cartilage matrix depletion215,216 and the erosion of cartilage and bone.217 Certain data indicate that prostaglandins may actually modulate the release of metalloproteinases, such as collagenases and stromelysins.218,219 Conversely, increasing evidence suggests that cytokine and MMP expression in articular cells is regulated by E-series prostaglandins.20,220 The net effect of this regulation is unclear because, like corticosteroids, prostaglandin E2 appears to inhibit TIMP synthesis and MMP synthesis.221 Moreover, some of the effects of prostanoids may be indirect, acting by promoting the synthesis of other proteins that have unique influences on cartilage metabolism.222 Thus although prostanoids are a factor in the signs and certain of the pathophysiological processes of OA, the specific role in regulating cartilage depletion and the interactions with other mediators of cartilage lesion development requires elucidation.
Clinical Evaluation of Joint Disease
Joint Pain
Traumatic arthritis and OA may be the most common cause of lameness in equine athletes of all types. Unfortunately, there is a weak correlation between the magnitude of pain and the severity of articular damage observed.223-225 The hallmark of OA is articular cartilage degeneration, a process occurring in a tissue devoid of sensory innervation. As a result, lameness is typically attributed to involvement of periarticular soft tissues and bone, the former being relatively richly innervated. In capsular and ligamentous tissues, unmyelinated sensory nerve fibers conduct painful sensations from widely distributed free nerve endings.226,227 With joint inflammation, these receptors exhibit increased sensitivity. Specifically the threshold for these receptors is reduced by inflammatory mediators such as prostaglandins, and increased receptor activity accompanies physiological joint excursions.228 Although the severity of soft tissue changes and lameness are related,229,230 horses with substantial periarticular fibrosis occasionally demonstrate less than the expected degree of lameness. Studies of joint capsule innervation in arthritic specimens revealed that with time degeneration of neurons is common, which provides a potential reason for the less than expected magnitude of pain in some horses having clearly demonstrable changes in periarticular soft tissues.
Bone and periosteum also contribute to pain observed in horses with OA. The periosteum is well innervated, and the periosteal disruption that accompanies the development of periarticular osteophytes is a source of joint pain.231 The subchondral plate and epiphyseal trabecular bone make variable contributions to clinical signs. For example, many, but not all, horses with subchondral cystic lesions demonstrate lameness.232-234 Inconsistent lameness among horses with similar radiological signs parallels the weak correlation between pain and radiological findings of early OA in people.235,236 Osseous receptor stimulation often accompanies joint movements that cause elevations in intramedullary pressure. People with OA of the hip have elevated intraosseous pressure,237 which in some people responds favorably to cortical fenestration. Elevation in intramedullary pressure occurs with flexion or extension of equine joints and is a likely source of articular pain in some horses. For example, both simulated effusion and metatarsophalangeal joint flexion increase intramedullary pressure in the third metatarsal bone.238 The concept also is supported by the clinical observation of a favorable response to transcortical decompression in horses with lameness related to osseous cystlike lesions.
Magnetic resonance imaging (MRI) has been used to try to characterize osseous lesions associated with pain in people with OA. For example, one study demonstrated a correlation between knee pain and the presence of poorly marginated areas of increased signal intensity (T2-weighted images).239 These areas of increased signal intensity, corresponding to fluid, have been termed “marrow edema.” Unfortunately, the fluid comprising them has not been identified in correlative histological examinations.240-242
Local Signs
Limited range of motion is a common feature of equine OA and is probably caused by a combination of factors, including guarding from pain, synovial effusion and edema, and progressive periarticular fibrosis. Synovial edema and proliferation and pain are probably the main causes of reduced range of motion in horses with early OA, whereas fibrosis is important in chronically affected horses. The specific mechanisms causing periarticular fibrosis are unclear. However, cytokines and neuropeptides are likely to contribute to fibrosis, given the mitogenic effects on fibroblasts of these substances.243-245
Effusion is a common feature of OA and is manifested in joints in the distal aspect of equine limbs as visible or palpable distention of joint pouches. Leakage of protein into the synovial space, because of increased permeability in the capillary endothelium and intercellular spaces of the synovium, which is not matched by compensatory increases in lymphatic clearance, leads to a progressively increased colloid osmotic pressure and augmented synovial fluid volume. Although mild effusion enhances nutrient exchange in the joint,246 severe effusion results in progressively elevated intraarticular pressure that ultimately destabilizes the joint and causes pain, stiffness, and a reduced range of motion. Increased permeability of the synovium to cells and proteins varies with the degree of synovial inflammation and is reflected in cytological findings in synovial fluid samples.
Synovial Fluid Changes
Reduced viscosity of synovial fluid is a frequent finding in horses with OA, particularly in horses with active synovitis. Reduced viscosity has been attributed to a reduced concentration, or depolymerization, of synovial fluid hyaluronan. Substantial reductions in hyaluronan concentration have been documented in the synovia of horses with chronic traumatic arthritis247; however, considerable variability exists. In a study comparing the hyaluronan concentration in normal horses with those having lameness that could be eliminated by intraarticular analgesia, normal horses had a mean hyaluronan concentration approximately 50% higher than that of horses with synovitis. However, the variability between horses was sufficient that this difference was not statistically significant.248 Clinical determinations of hyaluronan concentration are not routine because of this variability and the technically involved procedures required for the quantitative determination of hyaluronan. The mucin clot test is a relatively simple, semiquantitative test of hyaluronan quantity and quality, but it is not particularly sensitive. Therefore the quality of hyaluronan is often determined clinically by assessing viscosity on gross inspection of synovial fluid obtained during arthrocentesis.
Because increases in cell numbers and protein concentration in OA are not dramatic, cytological evaluation of synovial fluid is not used routinely diagnostically. Cytological analysis of synovial fluid is most useful in identifying and monitoring infection and untoward postinjection reactions. Approximate values for cell count and total protein concentrations under a variety of clinical situations are given in Table 61-3. Total protein concentration varies between joints, tending to be considerably higher in the larger, more proximal joints, such as the scapulohumeral joint.
Role of Radiography/Radiology
Radiography has long been the traditional means of assessing the structural changes of OA (see Chapter 15). Radiography has the advantages of availability, convenience, relative safety, and economy. Indeed, it is standard practice for many veterinarians to localize lameness to a particular area of the limb and subsequently to obtain radiographs in an attempt to identify changes to support a diagnosis of OA. Despite the advantages of superior contrast resolution and options for postprocessing image enhancement that accompany advances in digital radiography, the technique still lacks sensitivity and is of limited value in identifying horses with incipient or focal lesions. Radiology does, however, have some merit in characterizing changes in bone that accompany chronic OA and can be useful in adding confidence to the diagnosis of established disease. Nevertheless, it should be recognized that radiologically undetectable performance-limiting lesions occur in horses.257,258
Radiological findings tend to witness past events in the pathological process and do not consistently reflect ongoing processes. Additionally, in horses, as has been long accepted in people, a lack of correlation exists between lameness or reduced performance and specific osseous structural changes evident radiologically.259-263 A lack of correlation with arthroscopically evident degeneration and radiological findings is also common.264-267 In addition to this underlying fundamental biological dichotomy, precise quantification of radiological findings is hampered by difficulties in precisely duplicating conditions from one radiographic examination to the next. Positioning, degree of weight bearing, and radiographic technique contribute measurably to results. Nonetheless, largely because of its aforementioned advantages, radiology remains a principal method of evaluating horses with joint disease.
The radiological features of OA mirror the pathological changes occurring in the affected joint (Figure 61-8). Initially, joint space narrowing, subchondral increased radiopacity (sclerosis), and osteophytosis occur. With time, subchondral radiolucent defects (lysis), osteochondral fragmentation, and eventually ankylosis may develop (Box 61-1). In horses substantial differences appear between specific joints respecting the relative degree of these changes. For example, radiologically evident changes tend to be less dramatic and appear later in the disease process in the metacarpophalangeal and metatarsophalangeal joints than in many other articulations.
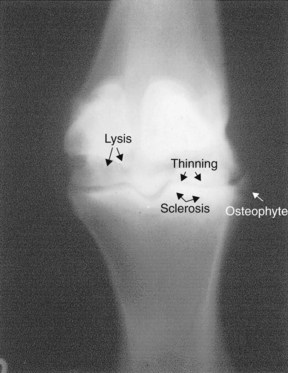
Fig. 61-8 Dorsopalmar radiographic image of the metacarpophalangeal joint of a horse demonstrating radiological changes of advanced osteoarthritis. There is periarticular osteophytosis, joint-space thinning, and subchondral bone increased radiopacity (sclerosis) and radiolucency (lysis).
BOX 61-1 Radiological Features of Osteoarthritis
| Radiological Feature | Pathogenetic Mechanism* |
| Periarticular osteophytosis | Endochondral ossification occurring at bony margins of unknown cause. Possible repair attempt modulated by altered cytokine milieu. |
| (Asymmetrical) Joint space thinning | Cartilage degeneration and loss.† Usually at areas of weight bearing or high stress. May be absent when focal cartilage loss occurs. |
| Subchondral increased radiopacity | Deposition of new bone as a response to changes in force transmission and from healing of trabecular microfractures. Corresponds to areas of maximum stress. Clinically significant sclerosis often corresponds to full-thickness cartilage loss. |
| Subchondral radiolucency | Less common change of uncertain pathogenesis. Possibly pressure necrosis from synovial fluid gaining access to subchondral plate via fissures, or related to pressure necrosis from trauma to bone. |
| Osteochondral bodies | Disintegration of joint surfaces or fractured osteophytes. May represent inciting lesions (e.g., osteochondral fracture). |
| Advanced remodeling/ankylosis | Articular response to advanced degeneration. Environment more consistent with fracture than synovial joint. |
* Specific pathophysiological mechanisms and reasons for disproportionate representation of changes among and between joints remain unclear.
† Seldom used as a marker of disease progression because of problems with technical aspects of radiographic positioning and focal-film distance.
Other Imaging Modalities
Increasingly available are other imaging modalities for assessing joint diseases. Among them are nuclear scintigraphy, MRI, and ultrasonography.
Nuclear Scintigraphy
Although more commonly used to detect nondisplaced, incomplete fractures and to identify stress-related bone damage in performance horses, nuclear scintigraphy can provide useful information in selected horses with OA (see Chapter 19). Whereas radiography (and related techniques, including computed tomography) provides considerable anatomical detail of osseous changes in affected joints, nuclear scintigraphy yields current physiological information on bone metabolism. The main disadvantages of scintigraphy are its relatively poor resolution (isotopes produce much fewer gamma rays than the number of x-rays generated by a cathode tube) and a lack of specificity because bone responds to most insults by increasing turnover. Owing to the unavailability of a cartilage-specific agent, the most common approach to the scintigraphic study of OA is using the delayed bone phase images, obtained 3 to 4 hours after injection of the radiopharmaceutical. Currently used agents are the bisphosphonates, which bind to the microcrystals of hydroxyapatite in bone. Areas of increased bone modeling have enhanced localization of the radiopharmaceutical, as long as an adequate blood supply exists.
It has long been known that bone-seeking isotopes accumulate rapidly in the bone of OA joints.268 The most intense radiopharmaceutical uptake typically occurs in the subchondral bone and at the osteochondral junctions of osteophytes, although temporal variation in the anatomical distribution of uptake may occur, as was illustrated in an animal OA model.269 Evidence exists that scintigraphy is useful in predicting the progression of OA in the human knee,270 and scintigraphy apparently may prove useful in diagnosing preclinical joint disease, that is, before the appearance of radiological abnormalities.271 Conversely, bone phase images may be normal in chronic OA joints if the rate of bone turnover returns to normal.
In the equine athlete, scintigraphy has proved useful to document a number of sport-specific lesions among different types of equine athletes.271-273 Unlike the human digit, the size of the bones in the equine skeleton is sufficient that relatively precise anatomical localization of lesions is often possible. Moreover, nuclear scintigraphy has the advantage of allowing a survey of all joints in a single examination. Importantly, as for radiography, close correlation between lameness and scintigraphic findings does not always exist.271,274
Magnetic Resonance Imaging
MRI involves detecting alternating electrical current produced when hydrogen protons, predominately found in fat and water, are subjected to pulsed electromagnetic fields applied in a specific manner (see Chapter 21). The rate at which protons change orientation varies among soft tissues and fluids of different composition, which allows the considerable tissue discrimination possible with MRI.
MRI is assuming a growing role in assessing joint disease in people and has a number of compelling advantages over many of the existing imaging modalities. Specifically, MRI has the capacity to provide noninvasive, high-resolution, three-dimensional (tomographic) images of all joint components. Importantly, advances in equipment and improved imaging sequences allow direct evaluation of articular cartilage, rather than the indirect assessment obtained using conventional radiography. Specifically, high-field MRI can be used to evaluate cartilage morphology, determine tissue volume, and evaluate cartilage composition. In human patients, detailed analysis of cartilage structure, including the detection of subtle cartilage lesions, is now possible using current sequences and protocols.275 Cartilage loss is a hallmark of OA, and assessment of cartilage volume using MRI has been shown to be both sensitive and reliable.276,277 In the future, quantitative assessments of cartilage volume and composition may prove useful to monitor disease progression and therapeutic responses. Specialized techniques, including the use of contrast agents (e.g., gadopentetate dimeglumine), enable the assessment of glycosaminoglycan content in joint cartilage. To date, the technique of delayed gadolinium-enhanced MRI of cartilage has proved useful in monitoring the influence of exercise on cartilage health and for assessing the effects of a number of interventions on matrix composition.275 Optimizing MRI as a diagnostic and monitoring tool requires sophisticated (high-field) equipment and considerable experience for proper image acquisition and interpretation.
Developments in low-field magnets and motion correction software adaptable to the standing horse have made possible diagnostic MRI of the equine distal limb; however, these systems are unsuitable for evaluating cartilage in the manner described previously.278-280
To date, MRI studies of equine joints have been largely limited to anatomical and correlative studies of cadaver specimens.281-284 In one of the few published reports of using the technique to investigate OA in the horse, post mortem magnetic resonance images of the metacarpophalangeal joint correlated well with arthroscopic and necropsy findings.285 Widespread use of MRI in horses is limited by the availability, expense, and the tunnel configuration of currently available equipment; however, it holds considerable promise as a diagnostic and monitoring tool in the future.
Ultrasonography
Ultrasonographic evaluation of diseased joints represents an additional diagnostic tool that has enjoyed increased use lately (see Chapter 17). Ultrasonography initially was used to assess chronic proliferative synovitis in the metacarpophalangeal joint.286,287 More recently its use has expanded to include most appendicular (and some axial) skeletal joints.
The principal benefit of ultrasonographic examination over conventional radiography is its superiority in demonstrating soft tissue abnormalities such as thickened synovial and capsular tissues and damaged intraarticular and periarticular ligaments. With experience and an appropriate knowledge of the regional anatomy and acoustic principles, it is also possible to identify and localize accumulations of synovial or other fluids. Although the ultrasound beam cannot penetrate the cortex, surface characteristics of bone can be evaluated, including periarticular osteophytes and enthesophytes, osteochondral fragments, and irregularities in the subchondral plate. Ultrasonographic features of arthritic cartilage include thinning, loss of sharp contours, and changes in echogenicity. Inherent limitations of ultrasonography preclude its use in areas where the tissue overlying the joint of interest is too voluminous and for structures having a shape or orientation that is not conducive to ultrasonographic evaluation. The basic principles and techniques of ultrasonographic examination of equine joints have been thoroughly reviewed.288