Chapter 8 Ingestive behavior
Chapter contents
The transition from milk to solids
Foals will nibble at blades of grass from their first day of life,1–3 often targeting grass on raised ground, such as on banks, because this overcomes the need (in horse foals rather than pony foals) to spread their forelegs.4 As foals grow, they gradually increase their exploration (Fig. 8.1A) and intake of solids5 and learn to flex their knees to access vegetation on the ground (Fig. 8.1B). The phase of playing with grass and, for that matter, drinking-water is very transient, often lasting less than a day.6 However, a rapid increase in time spent grazing does not occur until approximately 4 months of age5,7 (Fig. 8.2).

Figure 8.1 Foals (A) explore a variety of foods and (B) then develop ways of prehending them.
((A) Courtesy of Kate Ireland; (B) courtesy of Francis Burton.)
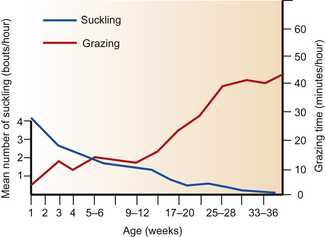
Figure 8.2 Mean time foals spend grazing and the number of bouts of nursing prior to weaning.
(After Tyler.5)
Consuming a variety of non-grass substrates, including clay, bark, twigs, leaves and humus has been noted in many adult equids, with the suggestion that in moderation this response may be adaptive and provide necessary trace elements8 or material that may facilitate gut motility. Learning about food substrates can have a profound effect on biological fitness. Indeed the availability of appropriate nutrients can have an impact not only on individual success but also on herd composition. For example in a study of a herd of horses that underwent a period of nutritional stress, male offspring had higher neonatal mortality rates in nutritionally poor years than in good years.9
Avoiding poisonous plants and selecting grass rather than other items occur concurrently between the ages of 4 and 6 weeks.10 Foals generally feed when their mothers are feeding.2 In a domestic setting some may acquire some of their dam’s concentrate feed by stealth, while others are offered their own concentrate feed from birth or are given concentrate diets just prior to weaning to encourage good growth.
Although foals need a balanced diet to support adequate bone formation, regular concentrated feed should be restricted, since it may contribute to problems of gastric acidity.11 Furthermore, because the rate of developing stereotypic and redirected behavior is greatest during the first 9 months of life and is related to feeding concentrates,11 great care should be taken in selecting diets for nursing mares and weanlings. Diets that most frequently cause stereotypic responses in adult crib-biters should be avoided. These include acidic diets and those that can be ingested without much chewing.
Nicol et al12 compared the behavior of foals fed from one month of age on a starch-and-sugar-based diet with foals fed an isoenergetic fat-and-fiber-based diet. During weaning, the foals fed fat and fiber appeared more settled and less distressed than their counterparts fed the starch-and-sugar-based diet, cantering less frequently and for shorter periods. During temperament tests, they also seemed calmer and more inquisitive than the foals fed the starch-and-sugar-based diet. For example, they were more willing to approach and investigate a novel object, less likely to walk away from unfamiliar personnel and significantly quicker to cross a novel groundsheet.
Grazing becomes more efficient as juveniles mature.13 Juveniles also learn to avoid areas used by adults for elimination purposes.14 Using the upper lip to isolate the selected plants, horses pass them as a bundle into the teeth and break them away using a backwards jerk of the head. Several bundles may be taken into the mouth before a bout of chewing begins.4
Animals seem to learn about food selection more quickly when in groups than when alone,15 possibly reflecting an ability to learn which foods to select through social facilitation. Although several studies have failed to demonstrate observational learning related to the acquisition of food,16–18 at least one19 showed that horses that observed a demonstration of an approach to feeding learned the general location of the food and therefore had a shorter latency to approach the food than control horses.Thus, stimulus enhancement may improve learning efficiency, through the transfer of information from experienced to inexperienced grazers.20
Observational learning studies in horses have tended to use mature subjects that are unaffiliated to one another. This represents an important possible flaw in experimental design since these may be among the least likely of all horses to be receptive to socially transmitted information. In contrast, one would predict that young foals at pasture would be more likely to use social models to learn correct food selection if their dams were effective demonstrators, because they are inexperienced foragers in a social group. Hypotheses that support this view generally assume that the greatest transfer of information occurs between a mare and her offspring. Moreover, lessons do not have to be learned by direct observation. Although yet to be demonstrated in horses, work in other species has indicated that ‘taints’ in a mother’s milk as a result of her diet may provide a mechanism for such exchange.20
Foals between 1 and 6 weeks of age display exploratory grazing which, unlike typical adult grazing, involves mouthing plants rather than ingesting them. Although it is likely that a combination of mechanisms is involved in learning food selection, one of the most likely involves coprophagy (ingesting feces). Often seen after pawing at fecal material, coprophagy by foals peaks during the exploratory period. The dam’s feces are the preferred or only fecal substrate that is explored in this way.10,21 Old feces are rarely consumed.8 As a feeding-exploratory behavior, coprophagy may be a method of learning about the gustatory and olfactory features of plants consumed by the dam. The significance of this activity in learning is supported by the coinciding decline in the foal’s interactions with toxic plant species during this time.10 In this way it appears that horses may circumvent the sort of observation learning typical of predatory species such as felids, by modifying their behavior in response to information from the gastrointestinal tract being transmitted to the brain.22 Coprophagy may have additional purposes,23 such as the acquisition of intestinal microbes and possibly deoxycholic acid, which has been found in the feces of lactating rats and is thought to play a role in the deposition of myelin.24 For these putative reasons, hand-reared foals should be given the opportunity to perform coprophagy. It is unlikely to expose the foal to viable forms of endoparasite. By contrast, coprophagy is rarely seen in adults, except in those on fiber-restricted diets (see p. 198).25
Once valuable food sources have been located, they will be revisited after a lull that allows regrowth. Spatial memory of food patches has been demonstrated in horses,26 and it has been suggested that this is an adaptive feature for grazers that allows them to take short-cuts through their environment.27 Seasonal variation in the use of home ranges (see Ch. 5) supports the view that such memory may be retained from one year to the next.
Food selection and rejection
Preferences and aversions allow horses to obtain the correct nutrition for their current needs, while preventing the intake of numerous toxic plants. Post-ingestive consequences seem to influence short-term diet selection based chiefly on a substrate’s apparent energy content.28 Because nutrients are used at different rates, they are required in different amounts in the diet. Feedback from the liver and gastrointestinal tract informs the brain of the body’s current balance between nutrient supply and demand.29 Wood-Gush30 suggests that a ‘specific hunger’ for a given nutrient ensues, and the horse will then select foods that alleviate this. However, a counter argument proposes that horses eat for pleasure, and correction of specific deficiencies is improbable due to a lack of specific regulatory systems.31 This may help to explain why horses and, possibly even more so, ponies show a lack of nutritional wisdom when foraging on substrates that elicit metabolic catastrophes, e.g. laminitis.
In a study of ponies’ voluntary feeding responses to different diets, meal size increased and meal frequency decreased as diets were increasingly diluted with undigestible fiber32 (Fig. 8.3). Although ponies responded to energy dilution by increasing voluntary food intake to maintain energy intake when the diet had 25% sawdust added, increases were at a rate inadequate to maintain energy intake when the diet had 50% sawdust added.33

Figure 8.3 Pony being offered diets of different dilutions. Visits made to each bucket broke infrared beams and were subsequently logged to demonstrate that, to an extent, horses eat for kilojoules not volume.
(Photograph courtesy of Katherine Houpt.)
To select different foods, equids must be able to differentiate, and this is achieved through sensory analysis. Using innate taste preferences or aversions alone would be undesirable since it could result in ingesting inappropriate material.15,33 Therefore, prior to being prehended by horses, plants are distinguished on features of their visual appearance, including leaf shape and color, as well as their odor.33 The importance of familiar odors in selecting food is hinted at when respiratory disorders are sufficiently profound to interfere with the horse’s ability to recognize its food. Olfaction plays a particularly significant role in the way horses avoid areas contaminated with equine feces.14,21 Although the rate of grazing is often sustained,32,34 selectivity is hampered when the horse is grazing at night, as visual assessment of plants is impaired. This is believed to contribute to a reduction in soluble carbohydrate intake at night.35 Once taken into the mouth, the taste and texture of plant matter provide further sensory information. More detailed differentiation between food items is facilitated by cognitive feedback that allows the visual and olfactory features of a food item to be integrated with its taste.20
To differentiate beneficial from detrimental feeds, horses must relate the post-ingestive consequences of ingesting a food item to the sensory stimuli that food provided before deglutition. A learned aversion can result from ingesting a food that contains a toxin, or an excess of a nutrient, such as protein, that then acts as a toxin.33 If toxins are present, neurons in the brainstem are stimulated, causing the animal to feel discomfort.29 Affective feedback can then integrate information about the taste of a food to the post-ingestive consequences of that food. When this is combined with the cognitive processes that integrate taste and smell, an aversion to that food is formed and committed to memory so that the food will be rejected in future.29
Strong aversions to novel foods can be formed when illness immediately follows ingestion of a novel food. Houpt et al36 also showed that an aversion could be formed to a novel food that caused illness, even when the novel food was given simultaneously with a familiar food. In sheep, aversions to novel foods can occur even if the post-ingestive consequences are not experienced until 12 hours after the food was tasted and ingested.37 This is also seen in carnivores. However, in the horse, if the discomfort is delayed by as little as 30 minutes, the ability to link the discomfort to the causative food is significantly reduced.36 A possible reason for the difference between horses and carnivores is that the latter tend to eat large sporadic meals, usually from a single source, and can therefore relate post-ingestive consequences to the last food consumed, even after a long delay.36 In a natural situation, horses spend most of each day grazing, and consume a variety of plants. It is therefore difficult to identify the cause of discomfort after only a short delay, as more food has since been ingested.
Grazing
Grazing is the preferred means of ingestion in adult horses, but browsing is also adopted when grass becomes particularly scarce.38–40 Although, like horses, donkeys graze, they tend to select coarser grasses and demonstrate greater agility as they exercise their preference for browsing. Because of their motivation to select a variety of forage, donkeys are in greater danger of ingesting poisonous plants than horses are. Horses generally prefer legumes and grasses to shrubs and herbs41,42 but there is apparently no correlation between gross energy of a given species of forage and the amount voluntarily consumed. Within legumes, horses are reported to prefer alfalfa pasture.43 When grazing, horses prefer young growth and select leaf material rather than stems,15,44 presumably because it is higher in carbohydrates. Studies on the preferences shown by horses for different grass species are rarely consistent and perhaps reflect variation in fiber and sugar content as the plants mature. However, timothy and white clover are often cited as preferred species.41,45,46
Horses spend much more time than cattle feeding on short grass.47 Compared with cattle and sheep, horses maintain their levels of food intake even at low sward heights.13 That said, dense leafy herbage is generally preferred because it provides the maximum intake per bite.48 Apart from grasses, herbs and browse, horses will also eat bark,49 roots,50 soil,51 acorns and aquatic plants.5
Typical domestic horse pastures have a patchy appearance not seen in free-ranging contexts.52 Selective grazing of feeding areas and the preferential deposition of potassium-rich manure in latrine areas (see Ch. 9) combine to form well-defined lawns and roughs that are typical of horse-sick pastures.53 Adult horses tend to avoid the long growth that characterizes latrine areas.54,4 Once this pattern has emerged, it persists and can lead to as little as 10% of the pasture being grazed.44 The avoidance response is attributed to the fecal content of latrine areas, urine being less repugnant.44,55 This may be an anti-endoparasite strategy that relies on the presence of alternative grazing opportunities. Therefore, because horses kept at exceptionally high stocking rates will graze near feces in the roughs and will also defecate in the lawns, the risk of parasite infection is increased under such circumstances.56 Indeed ponies can develop serious helminth infections within 20 days of being released onto a small pasture.57
Equids coexist with bovids of similar body size in many ecosystems. Although the grasses usually selected by horses have a higher fiber content than those selected by cattle, they have variable fiber content.44 Ponies have been shown to have lower digestible organic matter intake than cattle and deer.58 They compensate for this with a greater total dry matter intake.59 Time spent by cattle ruminating is occupied with grazing in horses.
While grasses preferred by horses have a variable soluble carbohydrate content, they have significantly higher water contents and lower sodium concentrations than non-preferred forages.45 Notwithstanding their tendency to develop mosaics of lawns and roughs (prized by some ecologists as a form of structural diversity), horses are recognized as economical grazers of marginal land and can be combined with goats and sheep to make the best use of pastures in poor condition.60,61 Horses are helpful in integrated pasture management because, compared with cattle, they graze closer to the ground and use the most productive plant communities and plant species (especially graminoids) to a greater extent.
Taste tests
Studies on immature horses have provided useful data on innate preferences. When given a choice between tap water and sucrose solution (at concentrations from 1.25–10 g/100 mL), foals preferred the sweet solution.62,63 With salty (NaCl) and sour (acetic acid) flavorants, the foals rejected solutions of higher concentrations (of 0.63 g/100 mL and 0.16 mL/100 mL respectively).62 Water made bitter by the addition of quinone hydrochloride at concentrations greater than 0.16 mL/100 mL was also rejected. Although bitterness is commonly linked to toxins,29 some adult horses have been shown to be extremely tolerant of it, and this accounts for its variable effectiveness as a taste deterrent – for example, to prevent wood-chewing.64 Even in foals, taste preferences can vary among individuals. For example, both Randall et al62 and Hawkes et al63 found that individual subjects rejected sucrose.
Through sampling and operant (trial-and-error) conditioning, horses can learn which foods should be ingested to meet given needs. In combination with nutritional wisdom,65 this may account for selection and ingestion of soils that contained more iron and copper than surrounding samples.51
Flexibility in dietary intake is desirable since it allows horses to adapt to fluctuations in the availability of vegetation. When the availability of preferred food items is limited, horses are quick to reach a compromise between plant quality and quantity to meet their dry matter requirements.35,61 Therefore, horses may ingest poisonous plants when they are more prevalent than safe forage or when they are dried and less detectable.66,67 Because horses differ individually in their ability to graze selectively,64 observing individuals as they graze a mixed sward may be worthwhile since it can help to predict their ability to avoid eating toxic plants.68
Natural influences on food intake
Social factors
Among the strongest influences on a free-ranging horse’s feeding behavior are the size of the group and the horse’s rank within the group.52 Leaders play a critical role in the timing of grazing,23 and social facilitation is an important stimulant of grazing.69 When grazing as a group, horses tend to distance themselves from conspecifics other than their preferred associates.70 It is likely that although they are often dispersed,4 herd members use subtle visual signals to communicate while grazing. The subtlety of such signals may account for the ability of horses to detect discrete cues in handlers.
The caution exercised by all horses when sampling novel potential foods is appropriate since horses do not regurgitate readily.20 In some circumstances, horses can be very reluctant to eat new foods. Such neophobia is most common in horses that are currently consuming a well-balanced diet.33 Neophobia can be overcome through social facilitation. If they are less neophobic than mature conspecifics, foals may be more able to learn preferences from experienced foragers.10
Lactation and seasons
Time spent grazing by mares increases with lactation, most notably in the first 3 weeks after parturition.71 Notwithstanding the energy demands of milk production, mares have been found to have a slight tendency to forage more than stallions in all seasons.72
Because the duration of the photoperiod and equine appetite, as measured by voluntary food intake, are similar, a causal relationship has been suggested. Physiological responses to photoperiodic change are not immediate, sometimes occurring after a delay of up to 8 weeks.73 Free-ranging horses take advantage of seasonal increases in food availability by increasing their intake and accumulating body fat.61 In a study of Przewalski horses under semi-natural conditions (12 mares in a 44 ha enclosure), feeding activity accounted for 40% of total activity in summer and 62% in spring.74 The fresh weight of single stools defecated by grazing ponies peaks in mid-summer.75 As the quality and availability of forage declines, a peak in food-searching activity occurs in autumn.76 In winter the continued decline in availability is accompanied by a trough in the level of general activity.74,77
Biting flies have a strong influence on the feeding behavior of horses at pasture.78 During hot summers many activities, including feeding, are shifted to night-time.74,79 Heavy rain also tends to inhibit grazing activity.71
Time-budgeting
Although the only true wild horses (E. przewalskii) are in or from captive populations, we can use their behavior and that of feral E. caballus as guidelines for what could be regarded as normal equine behavioral organization. Of the major groups of behaviors, those that occupy most of a free-ranging horse’s day are searching for choice grazing spots and ingesting forage. Equine feeding control mechanisms appear to have evolved to maintain a high gut-fill.78
Horses spend an average of 16–17 hours a day grazing,80 but when forage is scarce the grazing period may exceed 19 hours81 and horses increase their bite-frequency.82 Horses devote much less time to chewing than ruminants, such as sheep,83 but compensate for this by ingesting more food per unit of bodyweight per day. Peaks in foraging occur in the early morning and late afternoon1 with bouts lasting from 30 minutes to 4 hours.84 Both the morning and afternoon grazing bouts may be extended when forage is scarce.85 Nocturnal ingestion naturally punctuates periods of drowsing and sleep,86 and for this reason stable managers seeking to reduce the behavioral changes inherent to stabling should leave horses with sufficient forage for the whole night.
Time-budgets for feral horses7 (see Fig. 1.11) are similar to those of domestic horses at grass,87 with a similar diurnal rhythm of foraging.88 Night feeding accounted for 23% of total feeding time in pastured fillies.89 The suggestion is that foals may learn normal feeding time-budgets from their dams,2 and this has led to some debate about the extent to which orphan foals develop normal feeding behavior.90,91
While prehension may amount to 30 000 bites per day,78 chewing rates range from 1.0 to 1.7 per second92 with faster rates being achieved by reduced displacement of the lower jaw.93 The type of food consumed affects the number of chews and therefore the time taken before deglutition. For example, per unit of weight, hay requires four times as many chews as oats and therefore takes approximately four times as long to consume.94 It has been estimated that a horse foraging exclusively on high-fiber substrates may chew 57 000 times per day, but that this can be almost halved under moderately intensive stable conditions (e.g. a 500 kg horse on 5 kg of forage and 7.8 kg of concentrate).95 This has a profound impact on salivation which, in horses, occurs chiefly in association with mastication rather than in anticipation of a meal (Table 8.1). Up to 100 L per day has been estimated as the saliva production of a 500 kg horse on a very dry hay diet.94 Conversely, the consequence of feeding less fiber is that there is less chewing and that the horse generates less saliva.96 Since the buffering effects of bicarbonate are diminished under these circumstances, gastric acidity rises and the risk of gastric ulceration increases.97
In stabled ponies fed ad libitum, the number and frequency of feeding bouts are lowest between 1700 and 0800 hours.87 Experimentally, satiety has been mimicked in ponies by intragastric infusions.98 Infusions of glucose, vegetable oil and socka floc (cellulose) stimulated satiety, but in a time-dependent fashion: glucose caused immediate reduction in intake, vegetable oil took a few hours to work and the cellulose caused a reduction only in 24-hour intake.98 The interpretation of this was that gastrointestinal cues play very little part in the immediate control of intake, but that metabolic cues related to caloric availability controlled intake by regulating the size and/or frequency of meals. Interestingly a sodium chloride infusion reduced intake, not by stimulating satiety but by causing malaise. The ponies developed a learned aversion to the nasogastric tube after receiving the sodium chloride, whereas after the other infusions they actually seemed to welcome the tubing process (Sarah Ralston, personal communication 2002).
Human influences on food intake
Timing and content
In the domestic situation, food is concentrated to give performance horses readily digested energy resources that can therefore be consumed more rapidly than less energy-dense (more natural) forages. In a bid to reduce the chances of colic, it is usual to restrict access to concentrated food immediately before and after strenuous exercise. It is not clear whether this traditional practice is effective. The effect of exercise itself is interesting since, compared with non-exercised animals, exercised horses modify their grazing behavior by taking fewer, but larger, bites.99
Providing long fiber such as hay in nets and raised feeders to prevent wastage by soiling in the bedding confounds the horse’s natural grazing posture. It has been suggested that some horses demonstrate their motivation to graze when they displace forage from these devices onto the stable floor.100 That said, the most profound imposed impediment to natural behaviors is the increase in concentrated parts of the diet relative to feral menus and the accompanying reduction in fibrous components.
Bulky foods that make a considerable contribution to gut-fill and thermal load are avoided in racehorses because they are thought to compromise lung-volume and racing performance. Furthermore, fiber and the saliva that must be swallowed with it add to the non-functional weight that the horse must carry. Hay has also fallen out of favor with some horse-keepers because of its role in the etiology of chronic obstructive pulmonary disease95 and the suggestion that attempts to reduce its allergen content by soaking may decrease its nutritive value.101,102 All of these factors mean that horses are often denied the opportunity to fulfill their behavioral needs to forage to maintain gut-fill, even though their nutritional needs may be met. Because horses evolved to be trickle feeders, the stomach of an adult horse is relatively small (9–15 L) and inelastic.94 It empties within about 20 minutes, and its rate of emptying is a function of the physical qualities of the current meal. Restrictions on feeding behavior, and especially the provision of discrete meals, lead to digestive anomalies and behavioral frustration.
The physical form of food is known to influence the rate of chewing103 and the energy104 required for ingestion. The addition of chaff, a traditional means of increasing the time taken to consume concentrated feeds, works simply by increasing the total forage content of the ration. High-energy hay replacers, such as haylage, have been associated with the development of wood-chewing in foals.11 Haylage is often fed in restricted quantities and as such may result in the redirection of oral behavior due to increased motivation to forage because of decreased gut-fill105 or other feedback mechanisms.106
Food presented as highly compounded pellets may be less attractive to horses than softer substrates.107 Palatability can increase motivaiton to defend food and can play a signfiicant role in some cases of food-related aggression (Fig. 8.4). While foodstuffs are dried to make them easier to store and handle, this may also make their flavors less accessible and reduce their palatability. In the case of hay, some horses learn to remedy this dryness by dunking mouthfuls in water, either because moisture makes it more pleasing to the palate, or makes it easier to swallow,4,25 or less painful to swallow if there is concurrent dental pain (Caroline Hahn, personal communication 2002).
In the free-ranging state, the primary function of movement within a home range is to select habitat that allows horses to maximize their intake of high-quality food.108,109 Similarly, the tremendous variability in the shape and quality of pastures offered to horses in domestic contexts influences the amount of locomotion required to forage on them optimally (although it has been estimated that horses take 10 000 steps per day) when grazing (Katherine Houpt, personal communication 2002). Pasture shapes of equal length and breadth evoke more even grazing than do rectangular paddocks.110 When exposed to a new pasture, horses rapidly detect patches of their preferred grasses and concentrate foraging activities within them.92 Fertilizers that encourage leaf rather than stem growth increase the intake per bite.111
Free-ranging horses regularly consume soil,77 but it is not clear what elements they are seeking when they do so. It has been suggested that sodium, iron and copper supplementation are among the benefits of this activity.51,112 Voluntary salt intake is noted to increase in stabled horses when they are not exercised.113 This is surprising because generally exercise increases the voluntary food intake of horses offered food ad libitum114 and salt consumption could be expected to rise to reflect salt losses through sweating. However, the oral occupation that salt-licking offers to stabled horses with unmet oral needs should not be overlooked here. Ponies fed an all-concentrate diet spend more time licking salt than those on a hay diet.115 Occasionally, the use of saltlicks may become excessive and result in polydipsia (see subsection on drinking, p. 204).116
Variety
Although strong seasonal variation may occur in diet quality, the grass content of diets rarely falls below 80% in open-range situations.77 When offered a choice of edible plants, horses tend to select grasses to meet their immediate energy needs and then seem to select more forbs in a form of supplementation.117 Schafer118 suggests that Icelandic horses have been observed selecting medicinal plants among the surrounding grasses. He proposes that this is helpful in a bid to ‘avoid worm infections’ and he offers the consumption of chestnut leaves as another example of pharmacognosy, suggesting that this causes a demonstrable improvement in vigor.118
Most stabled horses are provided with a single forage,119 so they have no opportunity to blend substrates to suit their individual needs. The effects of such monotony are as yet unclear, but it seems plausible that providing multiple forages may improve the welfare of stabled horses by enriching their environments and allowing them to perform highly motivated foraging behavior patterns.120 Further, it is proposed that this approach may reduce the chances of intestinal obstruction by decreasing the amount of straw that stabled horses consume (Fig. 8.5).120 That said, in the absence of any better forage source, horses in developing countries are commonly fed large quantities of straw with no apparent ill-effects on gut motility (Caroline Hahn, personal communication 2002). This is presumably because the gut flora of horses fed straw have had an opportunity to adapt to the robust nature of such a substrate.
Behavior associated with ingestion
Locomotion is an integral part of grazing behavior, and horses cover large areas because they seldom take more than two mouthfuls in one spot before moving on to the next.21,31,41,46 Because stabled horses are generally offered forage in one site, they are not driven to move between food sources. Although the extent to which they can move in a stable is limited, it would be useful to increase locomotion if we are seeking to mimic free-ranging feeding strategies. As horses travel between food items in a pasture, they accelerate to a maximum foraging velocity by increasing both the length and frequency of strides.121 On sloping pasture the dedicated use of certain routes becomes apparent with the formation of tracks that facilitate grazing. The spacing of the tracks depends on the incline.122 Increasingly, pasture management protocols are considering the locomotion of horses at grass, since it is believed that requiring movements between feeding and watering stations in a paddock mimics free-ranging behavior and has health benefits. For example, the spaced provision of food, water and mineral licks is being used to force unshod horses to traverse gravel in a bid to toughen their hooves.
Submersion of the muzzle may be required when horses feed on aquatic vegetation.4 Meanwhile, pawing may occur as horses forage for roots under soil50 or grass under snow that is too deep to be pushed aside with their muzzles.77 Pawing is also reported in horses as a means of breaking ice over watering holes.118
While vocalization and walking occur more often before than after feeding,34 presumably as a result of anticipation, a significant elevation in heart rate occurs in ponies both before and during feeding.123 Meanwhile, in horses fed large meals episodically, there is a transient post-prandial hypovolemia124 and an increase in blood flow to the feet.125 Although their function is obscure, these changes are thought to be of clinical significance in the etiology of laminitis and may relate to the persistent increase in hoof wall temperature for 16–40 hours after vasodilation.126 As an aside, it may be that lack of locomotion between mouthfuls of food is a contributory factor in the emergence of laminitis.
Although increased surveillance is a benefit of social living for prey animals, such as the horse, vigilance by individuals within a group always persists. The rate of looking-up during grazing and drinking23 and social spacing are important anti-predator strategies that are mathematically related to herd size. For example, when the number of Thoroughbred yearlings in experimental groups at pasture was increased from 2 to 12, the mean distance between them increased from 5 m to as much as 50 m.127
Social facilitation strongly influences behaviors associated with grazing in the horse.69 This is illustrated by the finding that locomotion as an adjunct to grazing is significantly greater for a lone horse than for those in a group.127 Meanwhile, the duration of grazing bouts increases linearly as the group size increases up to four.127 This could reflect competition among grazing horses or possibly decreased anxiety as a result of increased predator vigilance.
A study of stabled Shetland ponies, showed that social facilitation was crucial for the stability of time budgets and that visual, more than olfactory or auditory, contact with conspecifics facilitated feeding behavior.128 However, the presence of conspecifics is not equally helpful for all members of a group, since when feeding from a shared trough, in the presence of high-ranking pen-mates, subordinate horses are less likely to be disturbed if solid partitions between feeding bays are used. Stable designs that give horses the choice of visual contact or privacy (Fig. 8.6) are an appropriate response to these findings. The use of computer-controlled feeding stations may help to reduce disturbance of lower-ranked members of a group, although attention must be paid to the design of feeding bays so that entering and leaving are facilitated.129,130 Some horses learn to guard the entrance to automated feeding bays even when it is not their turn to enter and thus they can deprive herdmates of access to food.

Figure 8.6 Partial barriers between stalls allow horses to choose between visual contact with neighbors or privacy. This simple form of environmental enrichment can help to reduce distress in stabled animals.
(Photograph courtesy of Centennial Parklands Equestrian Complex, Sydney, NSW, Australia.)
Strangely, fluctuations in the R–R intervals that are common features of equine heart function and possibly represent respiratory sinus arrhythmia are absent during feeding periods.131 Perhaps there is an increase in vagal tone associated with gastrointestinal activity that means that feeding has an effect on autonomic activity in the same way that crib-biting bouts are associated with reductions in heart rate.132
The relationship between nutrition and behavior
This relationship is fascinating. For example, there is a report that a group of horses in New Zealand with deteriorating ‘behavior and manners’ responded to parenteral administration of vitamin E-selenium.133 Horses on pasture, however, have higher plasma serotonin and tryptophan concentrations than those in stalls fed grain (unpublished data from Sarah Ralston, personal communication 2002). Many equestrians report that horses and especially ponies become ‘hot’, ‘fizzy’ or ‘corned-up’ – in other words, more reactive and less tractable – when fed more oats and less hay. This may reflect a shift in the glycemic peak that merits further scientific study. As mentioned above, there is evidence that replacing starch and sugars with fat and fiber may diminish spontaneous locomotion and reactivity to a variety of novel stimuli.12,134,135 Those who report ‘not resting properly’ (restlessness) as one of the more common undesirable behavior patterns in sport horses136 should consider the role of over-nutrition in the emergence of the problem.
Paradoxically, changing the diet of ponies from hay to oats has been reported to cause a transient augmentation of total sleep time with increases in both slow-wave and REM sleep.137 However, after 4 days sleep patterns in these animals had returned to normal. It may be that the post-prandial physiological changes in the lower limbs of these animals as a result of the dietary imposition, resulted in spending more time in recumbency, and that sleep was the consequence of the physiological stimulation that also resulted from the novel carbohydrate load. Fasting is associated with similar transient increases in both slow-wave and REM sleep.86
It is possible that some diets may better equip horses to cope with stressors associated with competition and fatigue.135 A crossover study demonstrated that foals coped better during weaning if they were allowed access to a supplement in which starch and sugar were replaced by fat and fiber, before separation from the dams. Similar amelioration of stress-related behaviors, such as vocalization, has been noted in foals supplemented with zinc.135
Dysphagia
Reasons for dysphagia can include trigeminal nerve injuries that may cause the mouth to gape.4 Difficulty in swallowing is also noted in white muscle disease, botulism and some cases of poisoning. Dental problems may cause refusal of hard dry foods, irregular chewing and tilting the head while chewing. Food dropping may also be associated with dysfunction of the facial nerve. Horses with esophagitis may lower the neck and extend the head, while those with stomatitis may loll out their tongues and those with choke often show repeated arching of the neck. While involuntary chewing and tongue flicking have been described as symptoms of Yellow-star thistle poisoning,4 bruxism (tooth grinding) is generally regarded as a sign of generalized low-grade discomfort, e.g. as a result of gastric ulcers.138
Anorexia and hypophagia
In normal horses the reasons for failing to meet nutritional requirements are often related to social consequences or distracting arousal. Subordinate members of a group may be bullied away from a limited supply of food, especially if it is not dispersed appropriately. In addition, horses that eat slowly may miss out if peers can consume more than their share of the ration. Horses may also fail to feed when distressed by a move to a new environment, or the departure of a favored affiliate, or by the arousal resulting from activities on the yard, such as the arrival of conspecifics.84
When horses are given an equal opportunity to feed, disease is the primary cause of a reduced food intake. For example, febrile states are a common finding by practitioners called to acutely inappetant horses. Pain may not only reduce appetite but also affect behaviors associated with eating. For example, an individual may be less likely to approach food and defend its rations. In donkeys such changes in behavior can mark the onset of estrus but nevertheless should be taken very seriously because they may be the only indication of illness that may be grave but masked by the species’ apparent tolerance of discomfort.139 Feeding responses to disease in the donkey are important, since anorexia, a frequent consequence of pain, can lead to hyperlipemia and death.140
While dental erosion can lead to inadequate mastication, dysphagia and food being lost from the mouth during mastication (so-called ‘quidding’141), buccal pain can even cause reluctance to approach food. With horses that present with weight-loss, time spent observing them as they interact with a variety of foods can be extremely helpful in diagnosing the problem. For example, this may help to determine the extent of quidding versus inappetance or the preference a horse may have developed for soft or moistened food. Intriguingly, patterns of feeding behavior are altered markedly as a legacy of dysautonomia.142
Among the best approaches to improving food consumption in a sick horse, one should consider:
• warming the food to release volatile flavorants – this works best if the horse has encountered that sort of warmed food when healthy
• dampening the food to reduce the effort involved in chewing (this is especially helpful if the horse is experiencing oral pain), or adding dissolved attractive components to lubricate the passage of food and facilitate deglutition
• feeding small amounts at a time in a bid to mimic the trickle effect, and avoid the horse being overwhelmed with food to sustain a healthy interest in the food
• feeding within sight (but not physical contact) of an affiliate that is eating.
Hyperphagia
The prodigious appetite of horses is well recognized, and it is suggested that the ability of oropharyngeal stimuli to override metabolic or gastrointestinal feedback within a single feeding session contributes to the horse’s susceptibility to colic and metabolic disorders.143 The rate of eating and size of meal in ponies is negatively correlated with pre-prandial plasma glucose concentrations.87 Gorging and bolting of food may therefore occur after a period of food restriction and can lead to esophageal obstruction.138 Because they precipitate excess acidity, periods without food can rapidly lead to severe gastric ulceration.144 Clearly, therefore, management regimens that facilitate trickle rather than episodic feeding are desirable.
A rapacious appetite is sometimes accompanied by inadequate mastication that may compromise the horse’s ability to buffer gastric acidity and digest its food. Veterinarians are rarely consulted about horses that are eating too rapidly, since it is rarely a sign of ill-health. However, there are clear behavioral reasons for this to occur, and owners should consider the role of current or even previous competition from other horses as among the most likely. Feeding more fiber to increase the sense of gut-fill prior to the presentation of discrete concentrated meals is the best approach to these cases. Furthermore, increasing the fiber content of concentrated meals themselves by introducing chaff is more effective than attempts to thwart ingestion by the use of physical obstacles such as bricks within the feeding device.
Practitioners should be aware that over-feeding of laminitic ponies (that through no fault of their own are depreciating rapidly in market value with age) may sometimes continue contrary to veterinary advice to ensure that euthanasia ensues and the insured value can be realized.
Wood-chewing and bed-eating
Food restriction is a common feature of stable management because of the appeal of concentrated foods that can be consumed rapidly, often in less than 3 hours. Although when offered food constantly, ponies consumed 80% of their daily intake in an average of 10 separate meals,87 most owners provide feed at frequencies and times that suit them rather than their horses’ gastrointestinal function. This often leads to the imposition of fasting at night.145 Food and fiber restriction may prompt horses to consume straw provided as bedding, and this has been linked to impaction colic.146
Bark chewing is a common feature of horses on irrigated pastures that have less fiber content than natural pastures (Fig. 8.7).49 That said, it is also seen in horses grazing on pastures that appear entirely adequate, leading some veterinarians to blame trace element deficiencies. Once fiber, vitamin and mineral deficiencies have been ruled out, bark chewing can be considered normal. Sometimes referred to as a normal vestige of browsing behavior, wood-chewing is important because, although it is not sufficiently invariant to be classed as a stereotypy, it may be associated with or precede the development of crib-biting.147 The tendency to chew wood can be reduced by increasing the fiber content of the ration. While helping to normalize gut function, this intervention can also save stables from rapidly being destroyed by their occupants. Horses can ingest approximately 1.5 kg of timber per day.148 This may help to explain why it is one of the most troubling behaviors for stable managers and may be more likely than less destructive responses to be spotted and reported when stakeholders are asked to comment on unwelcome behaviors in stabled horses.

Figure 8.7 Although wood-chewing is regarded by some as unwelcome, many ethologists consider it a normal analogue of bark-chewing, a normal feature of the repertoire of free-ranging horses.
(Photograph courtesy of Francis Burton.)
It is accepted that horses fed low-forage diets spend significantly more time chewing wood than horses fed hay.106,149 Further, Krzak et al113 reported that wood-chewing increased when the stomachs of horses were at their emptiest. Lignophagia is particularly unwelcome because it can cause intestinal obstruction.150 It is suggested that fiber restriction reduces hindgut pH and that this leaves horses fundamentally unsated, but it also has the effect of eliciting abnormal oral behaviors.106 While my own studies have shown an increase in intraspecific aggression in horses on so-called ‘complete’ diets, others have noted an increased nervousness as well as wood-chewing in ponies deprived of hay.151 When forage is in short supply, many owners report increased aggression among horses at pasture. Along with biting the stable, wood-chewing was the main oral behavior that was reduced by giving stabled horses non-therapeutic oral doses of virginiamycin.106 A suggested mechanism for this phenomenon involves the reduction of fermentative acidosis in the hindgut by changing the population of hindgut flora. The remedial elevation of a horse’s hindgut pH with antimicrobials may be an expensive alternative to increasing its fiber intake. Studies of crib-biters (and weavers) have failed to show an effect of virginiamycin on the frequency of stereotypy152. It is possible that early reports of its effect on oral behaviors were simply reflecting its significant effect on the palatability of rations that had been supplemented with virginiamycin. Less palatable feeds are generally associated with reduced crib-biting.
Fiber restriction has been associated with the ingestion of a variety of unusual substrates (so-called pica), including shavings and sawdust (in bedding), hair153 (from the manes and tails of other horses) and feces.84 That said, foals mouth and chew many of these items as a normal part of object play.6
Coprophagy
In all foals coprophagia is a normal behavior with a variety of suggested functions (see above). In free-ranging horses, there are rare reports of foals and their dams consuming old fecal material as a consequence of food shortage.51 Fiber-restricted rations,8,154 frustration (which probably results from the same deficit) and underfeeding in general84 can lead to an adult horse consuming its own feces or those of a conspecific. The digestible energy content of equine fecal material is sufficient to suggest that this substrate may help to nourish a horse.84
There are anecdotal reports of horses consuming the blood and even the flesh of freshly dead rabbits, some of which are said to have been killed by the horses themselves.155 It is interesting to contemplate the sort of nutritional deficit that could generate the motivation to perform this behavior. Generally speaking, practitioners should investigate the possibility of a dietary deficiency and screen blood biochemistry before labeling an unusual craving ‘depraved’.
Geophagia
Occasionally, horses lick and chew at soil but it is not yet clear whether they do so to resolve nutritional deficiencies or because they simply enjoy the activity.51 Involuntary soil ingestion occurs mainly during grazing, because soil adheres to vegetation.156 Incisor erosion may sometimes result and may even mimic damage caused by crib-biting. Sand colic is associated with the ingestion of some soil types either during eating or drinking.83 In many cases, horses reject, from their mouths, roots that contain appreciable amounts of soil and grit. This serves as evidence that their teeth and buccal lining are sensitive and should caution us against assuming that, until habituation has occurred, bits do not cause at least some discomfort.
Oral stereotypies
Licking and crib-whetting
Although rarely reported as problems by owners, licking and crib-whetting are sometimes seen as appetitive behaviors before crib-biting or may become stereotypic in their own right. The approach to their management follows that of other redirected and stereotypic oral activities.
Crib-biting and wind-sucking
A crib-biting horse repeatedly seizes fixed objects with its incisor teeth and pulls back while making a characteristic grunting noise that signifies the passage of air into the esophagus. A wind-sucker achieves the same characteristic neck posture and grunt without holding onto any fixed object. It is believed that crib-biters may become wind-suckers – for example, if no substrate is available or if this component of the behavior is punished.157 Horses that merely hold onto a fixed object without grunting are said to show grasping.158 These behavior patterns have been linked to various forms of ill-health, including tooth wear, colic and a failure to maintain bodyweight. Radiography of horses as they were crib-biting challenged the traditional view that crib-biters actively ingest air, because there was no movement of the tongue as one would expect in true swallowing.159 Instead, each horse showed an explosive distension of the proximal esophagus (Fig. 8.8) that prompted no peristalsis. Much of the air exited the proximal esophagus between crib-bites by returning through the cranial esophageal sphincter into the pharynx. This may explain why tympanitic colic (abdominal pain associated with wind or flatulence) is not seen in all crib-biting horses.
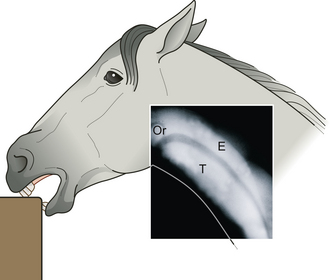
Figure 8.8 Composite image from three stills captured during fluoroscopy of the cranial half of a horse’s neck during a bout of crib-biting. Air is visible in the esophagus (E), trachea (T) and oropharynx (Or).
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Risk factors
Epidemiological studies have identified husbandry factors that are associated with peaks in the prevalence of abnormal behavior in racehorses.119,149 These management factors include small amounts of daily forage (less than 6.8 kg) and stable designs that limit the amount of communication possible between neighboring horses. This association is somewhat predictable because one would expect horses to behave normally if they have plenty of food and company. However, when interpreting the results of surveys, it is important not to arrive at cause-and-effect conclusions. Although cross-sectional studies have revealed and quantified the association between certain management practices and stereotypic behavior, it must be remembered that all such studies are essentially retrospective.
Further light has been shed on the causal factors influencing the development of stereotypic behaviors by a 4-year prospective study of a population of 225 young Thoroughbred and part-Thoroughbred horses.11 Dynamic cohorts were followed for between 1 and 4 years, with each foal being observed directly during the pre-weaning period, at weaning and at regular intervals post-weaning. The study found that crib-biting was initiated by 10.5% of this population at a median age of just 20 weeks (Fig. 8.9).
After weaning, youngsters given concentrate feed have a significantly greater risk of developing crib-biting than those not given concentrate, while those fed on hay replacers (such as silage or haylage) rather than hay have a significantly greater risk of developing wood-chewing.11
The strong effect of weaning is of considerable importance. Weaning is a stressful time for the juvenile, and abrupt weaning has been implicated as a source of emotional anxiety, because of numerous management changes at weaning time.160–162 These often include:
• withdrawal of opportunities to suckle
• breaking of the mare–foal bond
• introduction to drinking water (in one study of 15 foals, only 7 were observed to drink water before weaning2)
Although the use of creep feeds may benefit the foal nutritionally, they may compromise the health of the gastric mucosa163 and do little to meet the behavioral needs of non-nutritive suckling. After traditional total and abrupt weaning, which involves permanent, sudden separation from the mare, foals frequently attempt to redirect suckling behavior toward the genital regions of conspecifics. It is believed that the thwarted motivation to suckle at this time may contribute to the emergence of crib-biting and wood-chewing in some individuals.
Crib-biting may also originate from specific dietary problems in young horses. For example, foals that received concentrates after weaning were four times more likely to develop crib-biting than those that did not.11 It has been suggested that normal gut motility and transit times in crib-biting horses may depend on physical flushing by saliva associated with their crib-biting behavior.164,165 Furthermore, crib-biting may increase the flow of alkaline saliva and reduce gastric acidity146 associated with feeding concentrates.166 There is some evidence that the volumes of saliva produced by crib-biting are sufficient only to have a soothing, rather than a flushing or buffering effect on the gastrointestinal tract.167 Compared with the company of other distressed freshly weaned peers, the presence of calm, grazing horses may help to reduce the distress of weaning for paddock-weaned foals, even if they have been abruptly separated from their dams.11 Because links between distress and gastric ulcers have been proposed in foals168,169 and established in other species,170,171 strategies that help to reduce the emotional impact of weaning are likely to be of benefit at a somatic level.
The relationship between oral stereotypies and gastrointestinal health
The significance of gastric ulceration in intensively managed (food-restricted) horses is now widely recognized, with 82% of racehorses172 and 51% of Thoroughbred foals under the age of 3 months173 showing lesions.
Epidemiological risk factors for oral stereotypies that relate to diet (such as small amounts of daily forage) could have the effect of increasing gastric acidity.116,153 Saliva is the natural buffer to excess gastric acidity; however, in horses, its production depends on pressure being exerted on the parotid salivary gland, primarily as an adjunct to chewing. If insufficient time is spent grazing or eating forage, horses may produce saliva of insufficient volume and quality to buffer their stomach contents. It has therefore been proposed that crib-biting may originate in an attempt by the horse to produce additional saliva.144 This is supported by there being a trend towards increased water consumption by crib-biters compared with normal horses.174 The fact that the attempt may not always be successful may be one reason why the biting behavior develops stereotypic characteristics.
The putative links between weaning and crib-biting have been explored because of the causative role of concentrate feeding in the emergence of gastric ulcers.164 Furthermore, because the original eliciting causes of the stereotypy are more apparent in youngsters, which have been exposed to fewer variables than older animals, a cohort of young horses was recruited to explore this hypothesis.164 After an initial assessment of behavior, and endoscopic examination of the gastric lining, horses were randomly allocated to antacid or control diets. Initially, the stomachs of crib-biting foals were significantly more ulcerated, eroded and inflamed than the stomachs of normal foals. However, the use of antacid diets resulted in a significant improvement in the condition of their stomachs. Crib-biting behavior declined in all foals (a finding that challenges the concept of emancipation) but the decline was especially marked in those maintained on the antacid diet.164 These results are of great interest, because they indicate humane approaches to preventing and treating oral stereotypies in young horses. That said, we should be careful not to oversimplify the etiology of crib-biting.
Physiological associations with crib-biting
Plasma cortisol concentrations in crib-biters are higher than in normal horses under a variety of treatments,175 which suggests that they are particularly susceptible to stress. Crib-biters are often regarded as being less able than normal horses to maintain bodyweight. Although this is sometimes blamed on incisor erosion (Fig. 8.10), it more commonly arises because they are occupied with performing their stereotypy and therefore they rest less than normal horses.176 The catabolic effects of sustained elevations in circulating concentrations of cortisol may also contribute to this failure to thrive. This may be an important cost for crib-biting horses as they expend energy that would otherwise have been conserved at rest. The tendency for crib-biters to spend less time eating is also likely to contribute to a relative energy deficit and consequent unthriftiness when on a critical plane of nutrition.

Figure 8.10 Erosion on the tables of the mandibular incisors of a crib-biter.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
A significant reduction in oro-cecal motility has been reported in crib-biters prevented from eating and crib-biting.166 This suggests that normal gut function in these animals is affected by oral activity. However, horses on ‘nutritionally complete’ diets, with no forage component, showed an association between crib-biting and increased total gut-transit time.176 These data suggest that eating and crib-biting may be partial substitutes for each other and that there is a triangular relationship between diet, gut activity and crib-biting. Certainly, in terms of their diurnal distribution, eating and crib-biting tend to occur together (Fig. 8.11). Studies of gut mobility are important because increased total gut transit times may predispose horses to simple colonic obstruction and distension colic.177
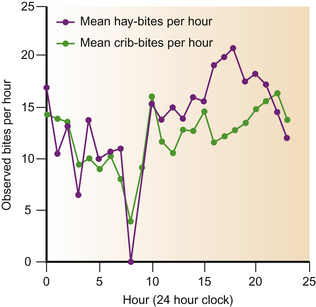
Figure 8.11 Diurnal rhythm in the bite rates for crib-biting and eating hay (from 64 days of observation). Horses were stabled with ad libitum hay. Between 0830 hours and 0930 hours they were turned out in paddocks.
The consequences of preventing crib-biting are interesting because they help to clarify the possible function of oral stereotypies. Studies using purpose-built projection-free looseboxes have compared short-term deprivation of crib-biting with the temporary withdrawal of forage. Intriguingly, neither deprivation of food nor of opportunities for crib-biting alone was associated with a plasma stress response.165,178 However, when the horses were deprived of both food and the opportunity to crib-bite, they showed a significant rise in the plasma stress response. This exposes the link between foraging and crib-biting and explains why restricted feeding is a management factor associated with an increase in abnormal behavior. There was also a significant reduction in oro-cecal motility recorded in crib-biters deprived of food and opportunities for crib-biting, which implies that normal gut function in these animals depends on their being given ad libitum access to food and to suitable crib-biting substrates. Crib-biters deprived of the opportunity to stereotype ate more than normal horses, suggesting that they had greater oral needs.
The suggestion that crib-biters underwent relative gut stasis when deprived of food and the opportunity to crib-bite, should be borne in mind when owners elect to stable their horses overnight with a haynet that will be emptied by morning. Methods of making horses work harder to gather their forage ration, e.g. by using haynets with especially small holes (so-called restrictor nets), can protract feeding times and may therefore have a beneficial effect on gut function as well as time-budgeting in general. However, this may not be beneficial for all individuals since it is possible that, for some, the frustration associated with working for forage may represent an additional stressor.
Beyond proximate gastrointestinal effects, crib-biting may bring more subtle rewards that could account for its persistence in the absence of gastric insults. Dopamine may activate basal ganglia motor systems to reinforce crib-biting via a reward mechanism. It has been suggested that stress stimulates the release of endorphins, triggering excessive dopaminergic activity within the striatum.179 In rats, the release of dopamine by endorphins has been shown to depend on activation of NMDA receptors.180 When 9 crib-biting horses were treated with the NMDA receptor antagonist dextromethorphan (1 mg/kg i.v.) – the active ingredient in most cough mixtures – eight showed a reduced cribbing rate compared with baseline.181 This pathway certainly merits further investigation so that we can better understand the way in which crib-biting may be self-reinforcing and therefore prone to emancipation. There is evidence that emancipation may reflect increased activity within the mesoaccumbens dopamine pathway182 that may manifest as a change in motivation.
Treatment of oral stereotypies
Many owners resort to physical restriction in an attempt to prevent the performance of abnormal oral behaviors.165 One of the most common methods involves the use of a tight collar (Fig. 8.12) designed to make crib-biting so uncomfortable or painful to perform that the horse stops. The effect of these collars was studied in a group of 12 horses fitted with the collars for 24 hours.178 After removal of the collars, the horses performed crib-biting at a higher rate than before the collars were fitted. This post-inhibitory rebound demonstrates that the motivation to crib-bite increases during periods of physical prevention.

Figure 8.12 Collar used to make crib-biting uncomfortable or less gratifying.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Despite welfare concerns, surgical responses to crib-biting continue to be developed. For example, laser-assisted removal of 10 cm of the ventral branch of the spinal accessory nerve and 34-cm sections of the paired omohyoideus and sternothyrohyoideus muscles is reported to result in elimination of the behavior in 10 horses that were followed up for a minimum of 7 months.183 This intervention presumably makes the distension of the proximal esophagus difficult and therefore makes the behavior less gratifying.
From rebound studies, it is argued that the reason collars, electric shock ‘treatments’ and surgical removal of neck muscles or nerves, often prove unsuccessful is because the motivation to crib-bite in horses is sustained.
The relationship between gut acidity and the incidence of oral activities such as crib-biting, grasping and wind-sucking105,164,184 is becoming clearer and has prompted the development of novel feeding practices, especially regimens that are likely to be particularly useful at weaning. Similarly, the addition of dietary antacids reduces the intensity and frequency of the response in emancipated crib-biters, especially post-feeding.184 Unfortunately we have no direct evidence to date that shows that resolution of gastric ulceration reduces the frequency of crib-biting, but nevertheless it is prudent to check crib-biters for gastric ulcers. The extent to which an individual horse should be allowed to crib-bite (e.g. on tailor-made surfaces that are cushioned to reduce incisor wear) depends on its colic history. High-risk animals should be managed in ways that minimize the primary need to crib-bite. Moreover, owners of all crib-biters should maximize periods at pasture and, where it is necessary to supplement feed, the fiber component of the total diet. By maintaining a high meal frequency they can give their horses the chance to emulate trickle feeding and thus reduce gastric acidity.
Drinking
Nursing foals rarely drink water, relying on milk for their supply of fluids.2,52 The youngest age at which foals have been confirmed to drink water is 3 weeks.2 Drinking in weaned animals is related to ambient temperature, water availability and lactational status.2
Movement to water is usually, although not exclusively, made by the entire herd.2 In pastured horses, trips to water most commonly occur in the afternoon.2 Horses submerge their lips below the surface of the water to drink (Fig. 8.13) and generate a pressure gradient by movement of the tongue in combination with swallowing at the approximate rate of once per second.4 The importance of the pressure gradient was hinted at by reports of the post-operative behavior of horses that underwent the outdated buccostomy approach to crib-biting and wind-sucking. Such horses were often said to take 2 or 3 days to learn to drink with fistulae in their cheeks. Although it was assumed that these horses were struggling to create a pressure gradient within their altered mouths, we should not discount the contemporaneous buccal pain as a likely post-operative side-effect that would have made drinking uncomfortable.
Hydration levels depend on voluntary fluid intake and are affected by exertion, ambient temperature, humidity, gut-fill, the speed of water conservation responses, such as antidiuretic hormone release, and the accessibility of transcellular reserves, such as those in the gastrointestinal tract.
Although horses in the exceptionally hostile heat of the Namib Desert have been noted to consume an average of 30 L per day,185 those in a cool environments may drink much less.186 The water intake of stabled horses is in the range 2–4 L/kg of dry-matter food consumed,187 the variation being a reflection of the variable amount of chewing, and therefore salivation, required for dry hay versus, for example, cereals.94 While water intake for working horses is also related to exercise and can send daily requirements up to 90 L,188 for free-ranging horses, trips to water may vary in their frequency according to the location of forage sources and the environmental temperature.2 Horses usually visit their water source at least once per day, whereas those foraging farther from their water source may schedule visits up to 72 hours apart.189 Some Namib horses can survive for 100 hours without water.185
Drinking is a social activity undertaken by the group as a whole and it is usually completed within 30 minutes.50 Competition for fresher, unmuddied water means that the higher-ranking members of a band tend to drink first, as do the more dominant bands.4 Most commonly undertaken in daylight, especially toward dusk, visits to water involve increased exposure to predation and the chance of encountering other bands. Swift departures from watering holes, which reduce the opportunity for unwelcome interactions, have been remarked upon.190
For the owners of performance horses, voluntary fluid intake can prove pivotal to success. Although intake is known to vary with the supply of water, the individual characteristics of a water source (such as its flavor) and the behavior of conspecifics, the pattern of drinking is generally tailored to meet homeostatic demands. In winter, very cold water can be aversive and decrease voluntary water intake to the extent of dehydration and even colic, whereas offering horses a choice of chilled water and ambient warm water in hot weather produces no preferences or qualitative differences in drinking behavior.191
Despite fears articulated in lay texts of inappropriate flushing of undigested food particles into the small intestine, most equine scientists seem to agree that, as long as water has not been withheld for an extended period, a horse can safely drink before, during and after feeding.188 Thirst tends to increase after feeding,188 and large meals tend to cause a rapid and short-lived decrease in plasma volume.192 So, rather than hampering digestion, drinking may actually facilitate it. When food and water are freely available 89% of drinking occurs within a period from 10 minutes before to 30 minutes after feeding of concentrates.193 The flushing effect is of negligible consequence because, when the stomach is full, water tends to pass along the lesser curvature, leaving the food largely undisturbed194,195 and, in any case, almost all digestion in the horse is post-gastric.94 By the same token we should accept that the provision of dry foods is unnatural, and for this reason the habit of some horses to dunk their hay should be regarded as a behavioral need and certainly not something to be prevented or discouraged.
As long as it has no harmful effects on gut motility,196 intermittent water delivery is not thought to have a negative impact on psychological well-being of stabled horses.197 The method by which water is supplied can affect both drinking behavior and fluid balance in the horse. For example, a preference for buckets over pressure valve bowls and a failure to maintain fluid balance in some horses using the latter system have been demonstrated.198 This may have harmful effects on horses that have an increased requirement for water. Therefore if a horse is normally watered using an automatic system, supplementation with water from a bucket may help to ensure rehydration after exertion.
Hygiene is of concern, since automatic water bowls and buckets may become contaminated with food or fecal material.199 Regardless of the receptacle from which horses drink, stable managers sometimes report reduced water intake after the use of potent agents to clean water containers. This is because, even after thorough rinsing, some of the stronger detergents, disinfectants and soaps can leave traces of unpleasant smells and tastes.
Changes in routine, such as those that prevail in international travel and many competitive contexts, can disturb the natural tendency of horses to drink at around feeding time. Until compensation is effected, which can take up to 7 days, the welfare and performance of horses disturbed in this way can be compromised.200
Reluctance to drink can be a neophobic response in that many horses and most donkeys139 are suspicious of novel odors and flavors in their drinking water. Having evolved to occupy a home range, horses are unlikely to have to encounter new water sources in nature and are innately wary of contaminants. Many equestrian competitors counter this neophobia by using flavorants, such as molasses or peppermint cordial, in the drinking water at home so that the same ingredient can be added to water offered at competitions.201 Equine hospitals should consider the same approach in anticipation of elective surgery. It seems that simply offering a variety of familiar flavors may enhance overall consumption, perhaps as a product of exploratory behavior.201 Meanwhile, horses that consistently prefer drinking muddy to clean water may be attempting to remedy a mineral deficiency.25
Inadequate water intake has been identified as a cause of impaction colic202 and should certainly be avoided when horses are required to make the transition between pasture and stabling.95 Horses affected by tetanus are sometimes seen to immerse their muzzle into water without being able to consummate the behavior by drinking. Meanwhile some normal donkeys are said to refuse to drink from troughs if the water level is so low that they have to put their head so far into the trough that they can no longer see around them.139 Notwithstanding the normal reluctance on the part of horses to drink water from novel sources or via novel receptacles, any suspicion of adipsia should prompt immediate action, including monitoring of water intake and drinking behavior as well as inspection of the water source to eliminate possible contaminants.84
Because horses drink in response to falls in plasma volume and increases in plasma osmolality (experimental increases in plasma osmolality over a threshold of 3% prompt drinking193), any factor that alters these parameters can cause thirst.187 Where furosemide (frusemide) has been administered – for example, to prevent exercise-induced pulmonary hemorrhage – horses can be expected to show increased thirst and to crave salt.203
Pathologies that lead to polydipsia and polyuria are dealt with thoroughly in other texts. Before a behavioral cause is proposed, polydipsic horses should be assessed for evidence of various pathologies that increase demand for water, including diarrhea, diabetes and Cushing’s disease. Stereotypic interaction with water may not necessarily involve increased water consumption, because water may be splashed and dribbled rather than simply drunk. It has been associated with frustrating environments but, paradoxically, in tie-stalled mares it has been reported only in those given continuous access to water.197,199 Excessive water intake in the absence of medication or disease has been noted as a consequence of stereotypic salt-licking115 but primary psychogenic polydipsia is more common.187 When seen in association with feeding in stabled horses, polydipsia should not be confused with a salivation deficit or a product of thwarted oral motivation, as reported in horses fed scheduled feeds from automated devices.187
Summary of Key Points
• Food selection allows horses to adjust their intake of nutrients to suit their current situation, while avoiding poisonous alternatives.
• A horse is born with innate dietary preferences which generally include:
• There is individual variation in preferences and aversions.
• Horses may learn from conspecifics and personal experience which foods to select.
• Concentrated feeds are associated with reduced saliva production and increased gastric acidity.
• Periods without food are associated with increased gastric acidity and risk of gastric ulceration.
• Lack of forage is the most important management factor linked with the development of stereotypic behaviors in cross-sectional epidemiological studies.
• Lack of forage and the provision of concentrate feed are important causal factors that precede the development of oral stereotypies in young horses in prospective epidemiological studies.
• It does not appear easy for horses to copy novel behavior patterns, which suggests that imitation of stereotypies is unlikely.
• Crib-biting is associated with disorders of the digestive system. There is a significant association between stomach ulceration and crib-biting in foals.
• Dietary treatments that reduce the incidence and severity of oral stereotypies offer humane avenues of treatment and prevention.
• The way in which water is supplied to horses can affect both drinking behavior and fluid balance.
Case study
A 4-month-old Warmblood colt was spotted repeatedly grasping at the top of a gatepost and occasionally licking it while the rest of the group, including its dam, idled in the same corner of the paddock. On closer scrutiny the owners found no evidence of wood-chewing and did not detect a grunting sound coincident with the behavior. The foal was not receiving creep feed but the mare, the highest-ranking adult in the group, was receiving concentrated feed every evening. None of the four adult horses in the paddock showed similar behaviors.
Video surveillance was used for this case. Because of its repetitive nature, the invariantly arched neck posture and the appetitive licking associated with it, the behavior was diagnosed as an early form of stereotypic crib-biting.
The grasping behavior was regarded with concern because of the likelihood of its becoming stylized into crib-biting and the probability of its becoming emancipated after weaning, so measures were taken to reduce management factors associated with its appearance. All gateposts in the paddock were coated very generously with a proprietary taste deterrent. The fence-line was protected with a single line of electrified wire. The sward in the paddock was low, so hay was fed to the group on a daily basis. It was delivered to the centre of the enclosure rather than being placed near the gateway where concentrates had previously been offered. This was intended to break down any association between eating and reaching for the gatepost. The hay was placed on the ground in the short term to avoid there being any uprights in close proximity to the feeding horses and to normalize their posture.
Hay from three farms was sourced, and samples of each were offered in seven separate piles, which allowed the five horses a pile each and two forage sources to choose from if displaced. Alternating the source of the hay in adjacent piles meant that the mare and foal had a choice of forages. When the pair were brought in for supervised supplementary feeding of the dam, it became clear that she was dropping a considerable amount of grain while chewing. Great care was taken to ensure that no dropped grain was left lying around for the foal to consume. Meanwhile, the mare received dental treatment that resolved the quidding.
Before weaning was attempted, a mare-and-filly-foal dyad was located by means of an advertisement in a local newspaper and brought to the paddock. The foals bonded extremely well and one month later the visiting mare was removed. Two weeks later the resident mare was removed and the colt was observed carefully for signs of the grasping response. None was seen. The pair of weanlings were kept at pasture throughout the winter. Ad libitum hay feeding was maintained and small amounts of concentrated food with a dietary antacid supplement were introduced very gradually. Although the amount fed to the youngsters was small it was nonetheless divided into three feeds to reduce its effect on gastric pH. Four years later the colt’s behavior remains normal. The owners have not challenged him with traditional feeding regimens and intend to keep the time he spends in the stable to a minimum. None of the dam’s subsequent foals have shown signs of similar behaviors.
References
1. Tyler SJ. The behaviour and social organisation of the New Forest ponies. Anim Behav Monogr. 1972;5:85–196.
2. Crowell-Davis SL, Houpt KA, Carnevale J. Feeding drinking behaviour of mares and foals with free access to pasture and water. J Anim Sci. 1985;60(4):883–889.
3. Weaver LT. Milk and neonatal gut: comparative lessons to be learnt. Equine Vet J. 1996;18(6):427–429.
4. Waring GH. Horse behavior. Park Ridge, NJ: Noyes, 1983.
5. Tyler SJ. The behaviour and social organisation of the New Forest ponies. Dissertation, University of Cambridge, UK, 1969.
6. McDonnell SM, Poulin A. Equid play ethogram. Appl Anim Behav Sci. 2001;78(2–4):263–295.
7. Boy V, Duncan P. Time-budgets of Camargue horses, I: Developmental changes in the time-budgets of foals. Behaviour. 1979;71:187–202.
8. Crowell-Davis SL. Developmental behavior. Vet Clin North Am Equine Pract: Behav. 1986;2(3):573–590.
9. Monard AM, Duncan P, Fritz H, Feh C. Variations in the birth sex ratio and neonatal mortality in a natural herd of horses. Behav Ecol Sociobiol. 1997;41(4):243–249.
10. Marinier SL, Alexander AJ. Coprophagy as an avenue for foals of the domestic horse to learn food preferences from their dams. J Theor Biol. 1995;173:121–124.
11. Waters AJ, Nicol CJ, French NP. Factors influencing the development of stereotypic and redirected behaviours in young horses: findings of a four year prospective epidemiological study. Equine Vet J. 2002;34(6):572–579.
12. Nicol CJ, Badnell-Waters AJ, Bice R, et al. The effects of diet and weaning method on the behaviour of young horses. Appl Anim Behav Sci. 2005;95:205–221.
13. Mesochina P, Peyraud JL, Duncan P, et al. [Grass intake by growing horses at pasture: a test of the effects of the horses’ age and sward biomass] [French]. Ann Zootech. 2000;49(6):505–515.
14. Ödberg FO, Francis-Smith K. A study of eliminative and grazing behaviour—the use of the field by captive horses. Equine Vet J. 1976;8(4):147–149.
15. Forbes JM. Voluntary food intake and diet selection in farm animals. In Wallingford, Oxon. CAB International; 1995.
16. Baer KL, Potter GD, Friend TH, Beaver BV. Observation effects on learning in horses. Appl Anim Ethol. 1983;11:123–129.
17. Baker AEM, Crawford BH. Observational learning in horses. Appl Anim Behav Sci. 1986;15:7–13.
18. Lindberg AC, Kelland A, Nicol CJ. Effects of observational learning on acquisition of an operant response in horses. Appl Anim Behav Sci. 1999;61(3):187–199.
19. Clarke JV, Nicol CJ, Jones R, McGreevy PD. Effects of observational learning on food selection in horses. Appl Anim Behav Sci. 1996;50:177–184.
20. Provenza FD, Cincotta RP. Foraging as a self-organisational learning process; accepting adaptability at the expense of predictability. In: Hughes RN, ed. Diet selection. Oxford: Blackwell Scientific, 1993.
21. Francis-Smith K, Wood-Gush DGM. Coprophagia as seen in Thoroughbred foals. Equine Vet J. 1977;9:155–157.
22. McLean AN. Cognitive abilities – the result of selective pressures on food acquisition? Appl Anim Behav Sci. 2001;71:241–258.
23. Crowell-Davis SL, Houpt KA. Coprophagy by foals: effect of age and possible functions. Equine Vet J. 1985;17(1):17–19.
24. Moltz H, Lee TM. The maternal pheromone of the rat: identity and functional significance. Physiol Behav. 1981;26:301–306.
25. McGreevy PD. Why does my horse …? London: Souvenir Press, 1996.
26. McCall CA, Potter GD, Friend TH, Ingram RS. Learning abilities in yearling horses using the Hebb-Williams closed field maze. J Anim Sci. 1981;53(4):928–933.
27. Nicol CJ. Farm animal cognition. J Anim Sci. 1996;62(3):375–391.
28. Cairns MC, Cooper JJ, Davidson HPB, Mills DS. Ability of horses to associate orosensory characteristics of foods to their post-ingestive consequences in a choice test. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland, 2002: 5–9.
29. Forbes JM. Dietary Awareness. Appl Anim Behav Sci. 1998;57:287–297.
30. Wood-Gush DGM. Elements of ethology. New York: Chapman & Hall, 1983.
31. Fraser AF. The behaviour of the horse. Wallingford, Oxon: CAB International, 1992.
32. Laut JE, Houpt KA, Hintz HF, Houpt TR. The effects of caloric dilution on meal patterns and food intake of ponies. Physiol Behav. 1985;35(4):549–554.
33. Forbes JM. Natural feeding behaviour and feed selection. In: van der Heide D, Huismam EA, Karis E, Osse JWM, Verstegen MWA. Regulation of feed intake. Wallingford, Oxon: CAB International, 1999.
34. Houpt KA, O’Connell MF, Houpt TA, Carbonaro DA. Nighttime behaviour of stabled and peri-parturient ponies. Appl Anim Behav Sci. 1986;15:103–111.
35. Duncan P. Horses and grasses—the nutritional ecology of equids and their impact on the Camargue. New York: Springer, 1992.
36. Houpt KA, Zahorik DM, Swartzman-Andert JA. Taste aversion learning in horses. J Anim Sci. 1990;68:2340–2344.
37. Burritt EA, Provenza FD. Ability of lambs to learn with a delay between food ingestion and consequences given meals containing novel and familiar foods. Appl Anim Behav Sci. 1991;32:179–189.
38. Fraser AF, Brownlee A. Veterinary ethology and grass sickness in horses. Vet Rec. 1974;95(9):448.
39. Hansen RM. Foods of free-roaming horses in Southern New Mexico. J Range Manag. 1976;29(4):437.
40. Putman RJ, Pratt RM, Ekins JR, Edwards PJ. Food and feeding behaviour of cattle and horses in the New Forest Hampshire. J Appl Ecol. 1987;24:369–380.
41. Archer M. Preliminary studies on the palatability of grasses, legumes and herbs to horses. Vet Rec. 1971;89:236.
42. Collery L. Observations of equine animals under farm and feral conditions. Equine Vet J. 1974;6:170.
43. Merritt TL, Washko JB, Swain RH. Pasture preferences of horses. Mimeo: Pennsylvania State University, 1969. H-69–1
44. Archer M. Grazing patterns of horses. Br Vet J. 1977;133:98.
45. Archer M. The species preferences of grazing horses. J Br Grassland Soc. 1973;28:123.
46. Houpt KA, Wolski TR. Domestic animal behavior for veterinarians and animal scientists. Ames: Iowa State University Press, 1982.
47. Menard C, Duncan P, Fleurance G, et al. Comparative foraging and nutrition of horses and cattle in European wetlands. J Appl Ecol. 2002;39(1):120–133.
48. Stobbs TH. The effect of plant structure on the intake of tropical pastures, II: Differences in sward structure, nutritive value and bite size of animals grazing Setaraia anceps and Chloris gayana at various stages of growth. Aust J Agric Res. 1973;24:821–829.
49. Keenan DM. Bark chewing by horses grazed on irrigated pasture. Aust Vet J. 1986;63(7):234–235.
50. Feist JD, McCullough DR. Behaviour patterns and communication in feral horses. Z Tierpsychol. 1976;41:337–371.
51. McGreevy PD, Hawson LA, Habermann TC, Cattle SR. Geophagia in horses: a short note on 13 cases. Appl Anim Behav Sci. 2001;71:119–215.
52. Carson K, Wood-Gush DGM. Equine Behaviour, II: A review of the literature on feeding, eliminative and resting behaviour. Appl Anim Ethol. 1983;10:179–190.
53. DiPietro JA, Ewert KE, Todd KS. Ivermectin treatment of horses: effect on the distribution of lawns and roughs in horse pastures. Vet Parasitol. 1993;48(1/4):241–246.
54. Edwards PJ, Hollis S. The distribution of excreta on New Forest grassland used by cattle ponies and deer. J Appl Ecol. 1982;19(3):953–964.
55. Archer M. Studies on producing and maintaining balanced pastures for studs. Equine Vet J. 1978;10(1):54–59.
56. Medica DL, Hanaway MJ, Ralston SL, Sukhdeo MVK. Grazing behaviour of horses on pasture: predisposition to strongylid infection. J Equine Vet Sci. 1996;16(10):421–427.
57. Round MC. Some aspects of naturally acquired helminthiasis of horses. Equine Vet J. 1971;3:31–37.
58. van Wieren SE. Do large herbivores select a diet that maximizes short-term energy intake rate? Forest Ecol Manage. 1996;88(1/2):149–156.
59. Dulphy JP, Jouany JP, Theriez M. [Comparison of the ability of different species of domestic herbivores to ingest and digest forages distributed in feeding troughs] (French). Ann Zootech. 1994;43(1):11–32.
60. Somlo R, Bonvissuto G, Shriller A, et al. [Effect of range condition on the seasonal diets of herbivores and on multiple grazing in the western mountains and plateaus of Patagonia] (Spanish). Revista Argentina de Produccion Animal. 1994;3/4:187–207.
61. Gudmundson O, Dyrmundsson OR. Horse grazing under cold and wet conditions; a review. Livestock Prod Sci. 1994;40(1):57–63.
62. Randall RP, Schurg WA, Church DC. Response of horses to sweet, salty, sour and bitter solutions. J Anim Sci. 1978;47:51–55.
63. Hawkes J, Hedges M, Daniluk P, et al. Feed preferences of ponies. Equine Vet J. 1985;17:20–22.
64. Marinier SL, Alexander AJ. Selective grazing behavior in horses: development of methodology and preliminary use of tests to measure individual grazing ability. Appl Anim Behav Sci. 1991;30(3–4):203–221.
65. Kiley-Worthington M. The behaviour of horses in relation to management and training. London: JA Allen, 1987.
66. Colon JL, Jackson CA, del Piero F. Hepatic dysfunction and photodermatitis secondary to alsike clover poisoning. Compend Contin Educ Practicing Vet. 1996;18(9):1022–1026.
67. McBarron EJ. Poisonous plants – handbook for farmers and graziers. Department of Agriculture. New South Wales: Inkata Press, 1983.
68. Marinier SL, Alexander AJ. Use of field observations to measure individual grazing ability in horses. Appl Anim Behav Sci. 1992;33(1):1–10.
69. Rifa H. Social facilitation in the horse (Equus caballus). Appl Anim Behav Sci. 1990;25:167–176.
70. Hansen DK, Rouquette FM, Jr., Florence MJ, et al. Grazing behavior of yearling horses, I: Time spent grazing different forages. Forage Research in Texas, Texas A & M University, 1987. 17–21
71. Martin-Rosset W, Doreau M, Cloix J. [Activities of a herd of draught brood mares and their foals on pasture] (French). Ann Zootech. 1978;27(1):33–45.
72. Duncan P. Time-budgets of Camargue horses, II: Time-budgets of adult horses and weaned sub-adults. Behaviour. 1980;72:26–49.
73. Fuller Z, Cox JE, Argo CM. Photoperiodic entrainment of seasonal changes in the appetite, feeding behaviour, growth rate and pelage of pony colts. Anim Sci. 2001;72(1):65–74.
74. Berger A, Scheibe KM, Eichhorn K, et al. Diurnal and ultradian rhythms of behaviour in a mare group of Przewalski horse (Equus ferus przewalskii), measured through one year under semi-reserve conditions. Appl Anim Behav Sci. 1999;64(1):1–17.
75. Choung CC, Kang MS, Ha SC. Grazing behaviour of Cheju native ponies. [Korean] Korean J Anim Sci. 1994;36(4):428–433.
76. Gill J. Circadian pattern of motor activity in horses in dependence of age and season [Polish]. Medycyna Weterynaryjna. 1992;48(4):183–185.
77. Salter RE, Hudson RJ. Feeding ecology of feral horses in Western Alberta. J Range Management. 1979;32(3):221–225.
78. Mayes E, Duncan P. Temporal patterns of feeding behaviour in free-ranging horses. Behaviour. 1986;96(1/2):105–129.
79. Kaseda Y. Characteristics of the horse tracks on the slopes of Misaki pasture in Toi Cape [Japanese]. Jpn J Zootech Sci. 1983;51(9):642–648.
80. Gallagher JR, McMeniman NP. Grazing behaviour of horses in S.E. Queensland pastures. Recent advances in animal nutrition in Australia 1989. Armidale, Australia: Department of Biochemistry, Microbiology and Nutrition, University of New England, 1989. 11A
81. Rubenstein DI. Behavioural ecology in island feral horses. Equine Vet J. 1981;13:27–34.
82. Rogalski M. Behaviour of the horse at pasture [Polish]. Kon Polski. 1970;5:26–27.
83. Dulphy JP, Martin-Rosset W, Dubroeucq H, et al. Compared feeding patterns in ad libitum intake of dry forages by horses and sheep. Livestock Prod Sci. 1997;52(1):49–56.
84. Ralston SL. Feeding behavior. Vet Clin North Am Equine Pract. 1986;2(3):609–621.
85. Arnold GW. Comparison of the time budgets and circadian patterns of maintenance activities in sheep, cattle and horses grouped together. Appl Anim Behav Sci. 1984;13(1–2):19–30.
86. Dallaire A. Rest behavior. Vet Clin North Am Equine Pract: Behav. 1986:591–607.
87. Ralston SL, van den Broek G, Baile CA. Feed intake and associated blood glucose, free fatty acid and insulin changes in ponies. J Anim Sci. 1979;49(3):838–845.
88. Kern D, Bond J. Eating patterns of ponies fed ad libitum. J Anim Sci. 1972;35:285.
89. Doreau M, Martin-Rosset W, Petit D. [Nocturnal feeding activities of horses at pasture] (French). Ann Zootech. 1980;29(3):299–304.
90. Glendinning SA. A system of rearing foals on an automatic calf-feeding machine. Equine Vet J. 1974;6:12–16.
91. Houpt KA, Hintz HF. Some effects of maternal deprivation on maintenance behaviour, spatial relationships and responses to environmental novelty in foals. Appl Anim Ethol. 1983;9:221–230.
92. Okuda Y, Ngata Y, Kubo K, et al. Grazing behaviour and heart rate of young Thoroughbreds on pasture. Bull Equine Res Inst Jpn. 1980;17:8–20.
93. Ruckebusch Y, Vigroux P, Candau M. Analyse du comportement alimentaire chez les equides. CR Journée d’Etude CEREOPA, Paris. 1976:69–72.
94. Harris PA. How understanding the digestive process can help minimise digestive disturbances due to diet and feeding practices. In: Harris PA, Gomarsall G, Davidson HPB, Green R, eds. Proceedings of the BEVA Specialist days on Behaviour and Nutrition. Equine Vet J 1999; 45–49.
95. Cuddeford D. Why feed fibre to the performance horse today? In: Harris PA, Gomarsall G, Davidson HPB, Green R, eds. Proceedings of the BEVA Specialist days on Behaviour and Nutrition. Equine Vet J 1999; 50–54.
96. Meyer H, Coenen M, Gurer C. Investigations of saliva production and chewing in horses fed various feeds. In: Proceedings of the 9th Equine Nutrition and Physiology Symposium, East Lansing, Michigan, 1985:38–41.
97. Pagan JD. Gastric ulcers in horses: a widespread but manageable disease. World Equine Vet Rev. 1997;2:28–30.
98. Ralston SL, Baile CA. Effects of intragastric loads of xylose, sodium chloride and corn oil on feeding behaviour of ponies. J Anim Sci. 1983;56(2):302–308.
99. Duren SE, Dougherty CT, Jackson SG, Baker JP. Modification of ingestive behaviour due to exercise in yearling horses grazing orchardgrass. Appl Anim Behav Sci. 1989;22(3–4):335–345.
100. Heleski CR, Shelle AC, Nielsen BD, Zanella AJ. Influence of housing on weanling horse behaviour and subsequent welfare. Appl Anim Behav Sci. 2002;78(2–4):297–308.
101. Warr EM, Petch JL. Effects of soaking hay on its nutritional quality. Equine Vet Educ. 1992;5:169–171.
102. Blackman M, Moore-Colyer MJS. Hay for horses: the effect of three different wetting treatments on dust and nutrient contents. Anim Sci. 1998;66:745–750.
103. Bergero D, Nardi S. [Eating time of some feeds for saddle horses reared in Italy] (Italian). Obiettivi e Documenti Veterinaria. 1996;17(1):63–67.
104. Vernet J, Vermorel M, Martin-Rosset W. Energy cost of eating long hay, straw and pelleted food in sport horses. Anim Sci. 1995;61(3):581–588.
105. Nicol CJ. Stereotypies and their relationship to management. In: Harris PA, Gomarsall G, Davidson HPB, Green R, eds. Proceedings of the BEVA Specialist days on Behaviour and Nutrition. Equine Vet J 1999; 11–14.
106. Johnson KG, Tyrell J, Rowe JB, Pethick DW. Behavioural changes in stabled horses given nontherapeutic levels of virginiamycin. Equine Vet J. 1998;30(2):139–143.
107. Hintz HF, Loy RC. Effects of pelleting on the nutritive value of horse rations. J Anim Sci. 1966;25:1059.
108. Duncan P. Determinants of the use of habitat by horses in a Mediterranean wetland. J Anim Ecol. 1983;52(1):93–109.
109. Crane KK, Smith MA, Reynolds D. Habitat selection patterns of feral horses in south-central Wyoming. J Range Manage. 1997;50(4):374–380.
110. Kusunose R, Hatakeyama H, Ichikawa F, et al. Behavioural studies on yearling horses in the field environment, 3: Effects of the pasture shape on the behaviour of horses. Bull Jpn Equine Res Inst. 1987;24:1–5.
111. Stobbs TH. The effect of plant structure on the intake of tropical pastures, I: Variation in the bite size of grazing cattle. Aust J Agric Res. 1973;24:809–819.
112. Salter RE, Pluth DJ. Determinants of mineral lick utilization by feral horses. Northwest Sci. 1980;54:109–118.
113. Krzak WE, Gonyou HW, Lawrence LM. Wood chewing by stabled horses: diurnal pattern and effects of exercise. J Anim Sci. 1991;69:1053–1058.
114. Sasimowski E, Budzynski M, Jelen B, et al. [Observations on the behaviour of horses in an open stable and the fodder consumption when offered ad libitum] (Polish). Prace I Materialy Zootechniczne. 1979;20:69–86.
115. Willard JG, Willard JC, Baker JP. Dietary influences on feeding behaviour in ponies. J Anim Sci. 1973;37:227.
116. Buntain BJ, Coffmann JR. Polyuria and polydipsia in a horse induced by psychogenic salt consumption. Equine Vet J. 1981;13:266–268.
117. Olson-Rutz KM, Marlow CB, Hansen K, et al. Packhorse grazing behaviour and immediate impact on timberline meadow. J Range Manage. 1996;49(6):546–550.
118. Schafer M. The Language of the horse. London: Kaye & Ward, 1975.
119. McGreevy PD, Cripps PJ, French NP, et al. Management factors associated with stereotypic and redirected behaviour in the Thoroughbred horse. Equine Vet J. 1995;27:86–91.
120. Goodwin D, Davidson HPB, Harris P. Foraging enrichment for stabled horses: effects on behaviour and selection. Equine Vet J. 2002;34(7):686–691.
121. Shipley LA, Spalinger DE, Gross JE, et al. The dynamics and scaling of foraging velocity and encounter rate in mammalian herbivores. Funct Ecol. 1996;10(2):234–244.
122. Kaseda Y. Seasonal changes in time spent grazing and resting of Misaki horses (Japanese). Jpn J Zootech Sci. 1980;54(7):464–469.
123. Youket RJ, Carnevale JM, Houpt KA, Houpt TR. Humoral, hormonal, and behavioural correlates of feeding in ponies: the effects of meal frequency. J Anim Sci. 1985;61:1103–1110.
124. Clarke LL, Ganjam VK, Fitchenbaum B, et al. Effect of feeding on renin angiotensin-aldosterone system in the horse. Am J Physiol. 1988;254:R524–R530.
125. Hoffman KL, Wood AKW, Griffiths KA, et al. Postprandial arterial vasodilation in the equine distal thoracic limb. Equine Vet J. 2001;33(3):269–273.
126. Pollitt CC, Davies CT. Equine laminitis: its development coincides with increased sublamellar blood flow. Equine Vet J Suppl. 1998;26:125–132.
127. Kusunose R, Hatakeyama H, Ichikawa F, et al. Behavioural studies on yearling horses in field environments, 2: Effects of group size on the behaviour of horses. Bull Jpn Equine Res Inst. 1986;23:1–6.
128. Sweeting MP, Houpt CE, Houpt KA. Social facilitation of feeding and time budgets in stabled ponies. J Anim Sci. 1985;60(2):369–374.
129. Pirkelmann H. [Behaviour of horses at electronic controlled concentrate feeding stations] (German). Ktbl-Schrift. 1991;344:116–127.
130. Fleege G. [Behaviour of horses held in groups with individual feeding] (German). Ktbl-Schrift. 1991;344:128–139.
131. Matsui K, Sugano S, Sawasaki T. Relationship between diurnal variation in R–R interval and feeding behaviour in the horse. J Equine Sci. 1994;5(4):131–135.
132. Minero M, Canali E, Ferrante V, et al. Heart rate and behavioural responses of crib-biting horses to two acute stressors. Vet Rec. 1999;145(15):430–433.
133. Dewers HF. A possible vitamin E-responsive condition in adult horses. NZ Vet J. 1981;29(5):83–84.
134. Holland JL, Kronfield DS, Meacham TN. Behaviour of horses is affected by soy lecithin and corn oil in the diet. J Anim Sci. 1996;74(6):1252–1255.
135. Kronfeld D, Holland J, Hoffman R, Harris P. Dietary influences on behaviour and stress. The role of the horse in Europe. Equine Vet J Suppl. 1999;28:64.
136. Lopez Olivia M, Gonzalez G, Corbeira P, et al. [Undesirable behaviours in sport horses subject to the rigors of management, training and competition] (Spanish). Revistas de Medicina Veterinaria. 1999;80(2):125–128.
137. Dallaire A, Ruckebusch Y. Sleep patterns in the housed pony under different dietary conditions. Can J Comp Med. 1974;38:65–71.
138. Eades SC, Booth AJ, Hansen TO, et al. The gastrointestinal and digestive system. In: Higgins AJ, Wright IM. The equine manual. London: WB Saunders; 1995:453–539.
139. Taylor TS, Matthews NS. Mammoth asses – selected behavioural considerations for the veterinarian. Appl Anim Behav Sci. 1998;60(2–3):283–289.
140. Harris PA, Frape DL, Jeffcott LB, et al. Equine nutrition and metabolic diseases. In: Higgins AJ, Wright IM. The equine manual. London: WB Saunders; 1995:123–186.
141. Naylor JM, Freeman DE, Kronfield DS. Alimentation of hypophagic horses. Compend Cont Ed Pract Vet. 1984:S93–S99.
142. Doxey DL, Tothill S, Milne EM, Davis Z. Patterns of feeding and behaviour in horses recovering from dysautonomia (grass sickness). Vet Rec. 1995;137(8):181–183.
143. Ralston SL. Regulation of feed intake in the horse in relation to gastrointestinal disease. Pferdeheilkunde Sonderausgabe. 1992:15–18.
144. Murray MJ, Eichorn ES. Effects of intermittent feed deprivation, intermittent feed deprivation with ranitidine administration and stall confinement with ad libitum access to hay on gastric ulceration in horses. Am J Vet Res. 1996;57(11):1599–1603.
145. Doreau M. [Feeding behaviour of horses in stables] (French). Ann Zootech. 1978;27(3):291–302.
146. Greet TRC, Rossdale PD. The digestive system. In: Hayes MH, Rossdale PD. Veterinary notes for horse owners. London: Stanley Paul; 1987:5–24.
147. Nicol CJ. Understanding equine stereotypies. The role of the horse in Europe. Equine Vet J Suppl. 1999:56–57.
148. Houpt KA. Oral vices of horses. Equine Pract. 1982;4(4):16–25.
149. Redbo I, Redbo-Torstensson P, Ödberg FO, et al. Factors affecting behavioural disturbances in race-horses. Anim Sci. 1998;66:475–481.
150. Green P, Tong JMJ. Small intestinal obstruction associated with wood chewing in two horses. Vet Rec. 1988;123:196–198.
151. Haenlein GFW, Holdren RD, Yoon YM. Comparative responses of horses and sheep to different physical forms of alfalfa hay. J Anim Sci. 1966;25:740–743.
152. Freire R, Clegg HA, Buckley P, et al. The behavioural and physiological effects of virginiamycin in the diets of stereotypic horses. Vet Rec. 2008;163:413–417.
153. Willard JG, Willard JC, Wolfram SA, Baker JP. Effect of diet on cecal pH and feeding behaviour of horses. J Anim Sci. 1977;45:87–93.
154. Nagata Y. Effects of various degrees of size and hardness of complete pelletized feed on feeding behaviour of horses. Exp Reprod Equine Health Lab (Japan). 1971;8:72.
155. McDonnell S. Carnivorous horses. The Horse October 2002, article 3832.
156. Herlin AH, Andersson J. Soil ingestion in farm animals: a review. Report—Department of Agricultural Biosystems and Technology, Swedish University of Agricultural Sciences 1996; 105:35.
157. Sambraus HH, Rappold D. Crib-biting and wind-sucking in horses. Pferdeheilkunde. 1991;7:211–216.
158. Owen RR. Crib-biting and wind-sucking—that equine enigma. In: Hill CSG, Grunsell FWG. The veterinary annual 1982. Bristol: Wright Scientific Publications; 1982:156–168.
159. McGreevy PD, Richardson JD, Nicol CJ, Lane JG. A radiographic and endoscopic study of horses performing an oral stereotypy. Equine Vet J. 1995;27:92–95.
160. McCall CA, Potter GD, Kreider JL. Locomotor, vocal and other behavioural responses to varying methods of weaning foals. Appl Anim Behav Sci. 1985;14(1):27–35.
161. McCall CA, Potter GD, Kreider JL, Jenkins WL. Physiological responses in foals weaned by abrupt or gradual methods. J Equine Vet Sci. 1987;7(6):368–374.
162. Apter RC, Householder DD. Weaning and weaning management of foals: a review and some recommendations. J Equine Vet Sci. 1996;16(10):428–435.
163. Nicol CJ, Davidson HPD, Harris PA, et al. Study of crib-biting and gastric inflammation and ulceration in young horses. Vet Rec. 2002;151(22):658–662.
164. McGreevy PD, Nicol CJ. Prevention of crib-biting: a review. Equine Vet J Suppl. 1998;27:35–38.
165. McGreevy PD, Nicol CJ. Physiological and behavioural consequences associated with short-term prevention of crib-biting in horses. Physiol Behav. 1998;65:15–23.
166. Nadeau JA, Andrews FM, Mathew AG, et al. Evaluation of diet as a cause of gastric ulcers in horses. Am J Vet Res. 2000;61:784–790.
167. Moeller BA, McCall CA, Silverman SJ, McElhenney WH. Estimation of saliva production in crib-biting and normal horses. J Equine Vet Sci. 2008;28:85–90.
168. Borrow HA. Duodenal perforations and gastric ulcers in foals. Vet Rec. 1993;132(12):297–299.
169. Furr MO, Murray MJ, Ferguson DC. The effects of stress on gastric ulceration, T3, T4, reverse T3 and cortisol in neonatal foals. Equine Vet J. 1992;24(1):37–40.
170. Tarara EB, Tarara RP, Suleman MA. Stress-induced gastric ulcers in vervet monkeys (Cercopithecus aethiops): the influence of life history factors, Part II. J Zoo Wildlife Med. 1995;26(1):72–75.
171. Henrotte JG, Aymard N, Allix M, Boulu RG. Effect of pyridoxine and magnesium on stress-induced gastric ulcers in mice selected for low or high blood magnesium levels. Ann Nutr Metabol. 1995;39(5):285–290.
172. Vatistas NJ, Snyder JR, Carlson G, et al. Cross-sectional study of gastric ulcers of the squamous mucosa in Thoroughbred racehorses. Equine Vet J. 1999;29:34–39.
173. Murray MJ, Schusser GF, Pipers FS, Gross SJ. Factors associated with gastric lesions in Thoroughbred horses. Equine Vet J. 1998;28(5):368–374.
174. McGreevy PD. The functional significance of stereotypies in the stabled horse. PhD thesis, University of Bristol, 1995.
175. Pell S, McGreevy PD. The prevalence of abnormal and stereotypic behaviour in Thoroughbreds in Australia. Aust Vet J. 1999;77(10):678–679.
176. McGreevy PD, Webster AJF, Nicol CJ. A study of the digestive efficiency, behaviour and gut transit times of crib-biting horses. Vet Rec. 2001;148:592–596.
177. Hillyer MH, Taylor FGR, Proudman CJ, et al. Case control study to identify risk factors for simple colonic obstruction and distension colic in horses. Equine Vet J. 2002;34(5):455–463.
178. McGreevy PD, Nicol CJ. The effect of short term prevention on the subsequent rate of crib-biting in Thoroughbred horses. Equine Vet J Suppl. 1998;27:30–34.
179. Dodman NH, Shuster L, Court MH, Dixon R. Investigation into the use of narcotic antagonist in the treatment of a stereotypic behavior pattern (crib-biting) in the horse. Am J Vet Res. 1997;48:311–319.
180. Dourmap N, Costentin J. Involvement of the glutamate receptors in the striatal enkephalin-induced dopamine release. Eur J Pharmacol. 1994;253:R9–R11.
181. Rendon RA, Shuster L, Dodman NH. The effect of the NMDA receptor blocker, dextromethorphan, on cribbing in horses. Pharmacol Biochem Behav. 2001;68(1):49–51.
182. McBride SD, Hemmings A. Altered mesoaccumbens and nigro-striatal dopamine physiology is associated with stereotypy development in a non-rodent species. Behav Brain Res. 2005;159:113–118.
183. Delacalle J, Burba DJ, Tetens J, Moore RM. YAG laser-assisted modified Forssell’s procedure for treatment of cribbing (crib-biting) in horses. Vet Surg. 2002;31(2):111–116.
184. Mills DS, MacLeod CA. The response of crib-biting and windsucking in horses to dietary supplementation with an antacid mixture. Ippologia. 2002;13(2):1–9.
185. Goergen R. Life on the edge. New Scientist. 2001;2305:30–33.
186. Tasker JB. Fluid and electrolyte studies in the horse, III: Intake and output of water, sodium and potassium in normal horses. Cornell Vet. 1967;57:649–657.
187. Houpt KA. Thirst of horses: the physiological and psychological causes. Equine Pract. 1987;9:28–30.
188. Hinton H. On the watering of horses—a review. Equine Vet J. 1978;10:27–31.
189. Bannikov AG. [Special natural conditions of the biotope of the Przewalski wild horse and some biological features of this species]. (Russian). Equus. 1961;I:13–21.
190. Ganskopp D, Vavra M. Habitat use by feral horses in the northern sagebrush. J Range Manage. 1986;39(3):207–212.
191. McDonnell SM, Kristula MA. No effect of drinking water temperature (ambient vs. chilled) on consumption of water during hot summer weather in ponies. Appl Anim Behav Sci. 1996;49(2):159–163.
192. Houpt KA, Perry PJ, Hintz HF, Houpt TR. Effect of meal frequency on fluid balance and behaviour in ponies. Physiol Behav. 1988;42(5):401–407.
193. Sufit E, Houpt KA, Sweeting M. Physiological stimuli of thirst and drinking patterns in ponies. Equine Vet J. 1985;17(1):12–16.
194. Richardson C, Cassell’s new book of the horse, London: Waverley, 1911;vol 4
195. Linton RG. Animal nutrition and dietetics. Edinburgh: Green, 1927.
196. Mansmann RA, Woodie B. Equine transportation problems and some preventives: a review. J Equine Vet Sci. 1995;15:141–144.
197. McDonnell SM, Freeman DA, Cymbaluk NF, et al. Behavior of stabled horses provided continuous or intermittent access to drinking water. Am J Vet Res. 1999;60(11):1451–1456.
198. Nyman S, Dahlborn K. Effect of water supply method and flow rate on drinking behavior and fluid balance in horses. Physiol Behav. 2001;73(1–2):1–8.
199. Freeman DA, Cymbaluk NF, Schott HC, et al. Clinical, biochemical, and hygiene assessment of stabled horses provided continuous or intermittent access to drinking water. Am J Vet Res. 1999;60:1445–1450.
200. Welford D, Mills D, Murphy K, Marlin D. The effect of changes in management scheduling on water intake by the Thoroughbred horse. The role of the horse in Europe. Equine Vet J Suppl. 1999;28:71–72.
201. Murphy K, Wishart S, Mills D. The acceptability of various flavoured solutions by Thoroughbred horses. The role of the horse in Europe. Equine Vet J Suppl. 1999;28:67.
202. Frape D. Equine nutrition and feeding, 2nd edn. Oxford: Blackwell Science, 1998.
203. Houpt KA. Equine welfare. In: Houpt KA, ed. Recent advances in companion animal behavior problems. Ithaca, NY: International Veterinary Information Services, 2001. A0810. 1201