Chapter 1 Introduction
Chapter contents
Evolution and classification
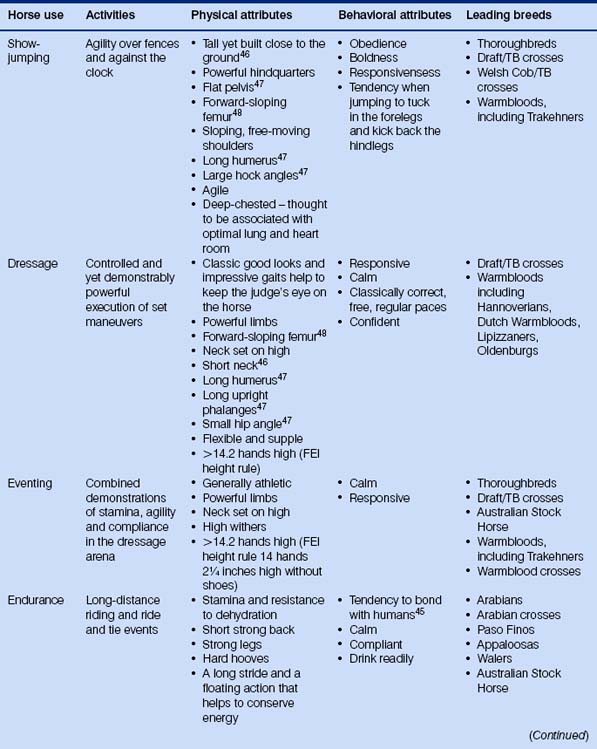
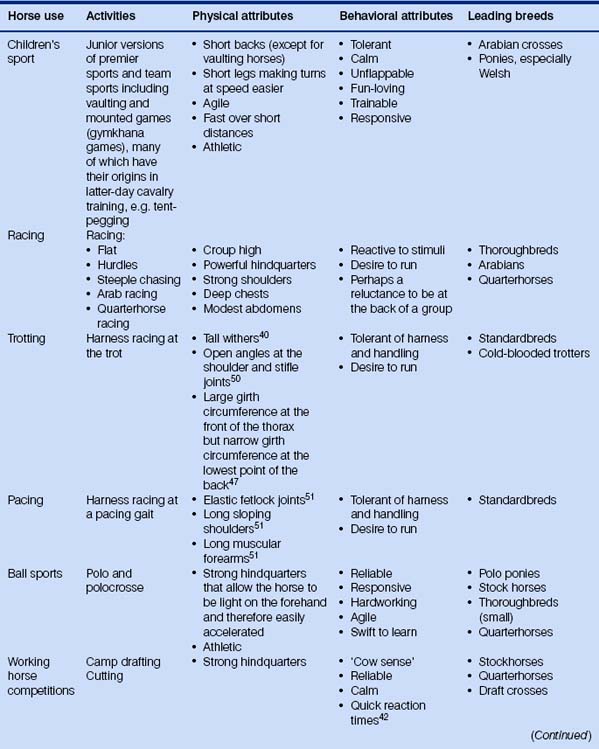
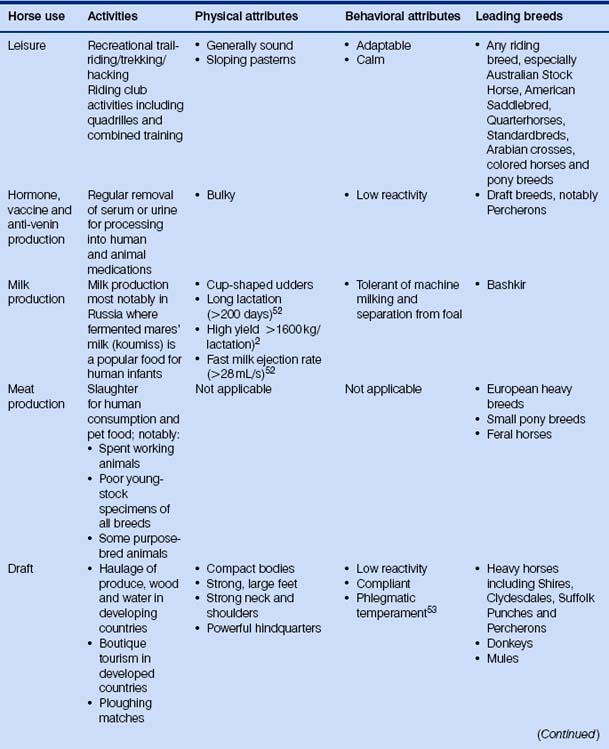
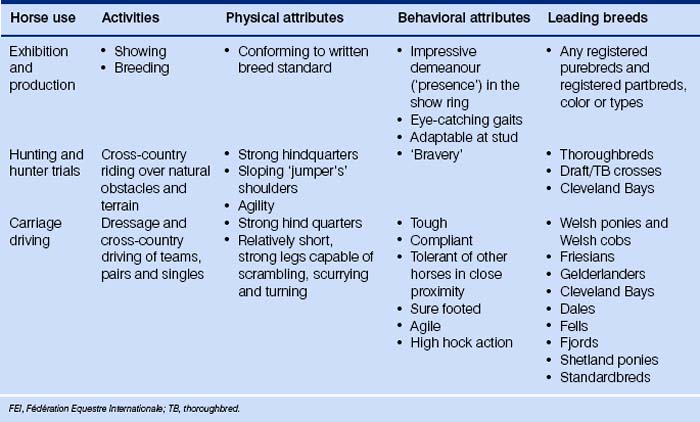
Because the evolution of the horse has been dealt with many times elsewhere, I shall spare the reader detailed descriptions of three-toed forest-dwelling dog-like creatures. Suffice it to say that we have been left with a most remarkable animal that can exploit impoverished grazing niches by foraging on very poor fibrous material and digesting it quicker than ruminants. Various anatomical, physiological and behavioral features of the modern horse mean that it is useful to humans in many ways (Fig. 1.1).

Figure 1.1 Humans and horses have had a long association. From depictions in cave paintings such as in Lascaux, France (A), we know that horses were originally hunted as a source of food, but since then the relationship has been developed through teamwork (B) and companionship (C).
((C) Courtesy of Francis Burton.)
Evolutionary background
A plains feeder that does not disperse and defend territories individually or in pairs as foragers of richer resources tend to, the horse has a long nose that allows it to graze while maintaining surveillance above the sward. As an animal without horns or antlers it relies largely on caution, speed and agility as its chief means of self-preservation. A social herbivore that capitalizes on companions for added safety, mutual comfort and probably enhanced food detection, this is a creature that is likely to feel insecure when isolated. The social skills of horses account for some of the species’ pre-adaptation to the domestic context.
To digest material ruminants generally ignore, the horse needs a large fermentation chamber, the cecum. Obliged to carry this voluminous digestive vat, the successful horse must therefore have tremendous muscular power to shift its necessary and considerable bulk from rest to top speed in the event of danger, so it has developed the ability to use minimal physical effort to rest while standing. With its small stomach this ‘trickle feeder’ is obliged to forage frequently and has not evolved to eat and then ruminate in one spot. Instead it eats and moves and eats and moves. Restricting movement and imposing periods of fasting are therefore likely to be more profound insults to equids than to members of many other species.
Classification of equids
Prior to the beginning of domestication, in the late Pleistocene, long-term geographic isolation of equid populations occurred.1 This led to the distinct species that exist today (Table 1.1). True horses (Equus caballus) occupied the Eurasian lowlands north of the great mountain ranges, while the asses occupied the arid zones of Asia. Crosses with asses, zebras and onagers are possible but the hybrids are normally sterile.
Despite their sometimes great morphological differences, all the breeds, groups and types of domestic horses belong to one species, E. caballus. Populations such as ‘mustangs’ and ‘brumbies’ found roaming in North America and Australia are feral representatives of this species. Within the E. caballus the occurrence of isolated environmental niches has given rise to the types we see among modern horses. Equine remains found in the Siberian permafrost suggest the presence of two distinct types of E. caballus: a small, heavily set type and a finer-boned ‘Oriental’ type of up to 14.3 hands (150 cm). The latter, less sturdy version was probably swifter and most likely the forebear of our modern ‘hot-blooded’ animals (so named because of their adapted suitability to hotter climes), the Arabians and Thoroughbreds. Reactivity and athleticism are the core characteristics of the hot-bloods that have led to their being favored as race, performance and sports horses. To respond rapidly to pivotal stimuli such as the opening of the starting stalls and the impact of the whip, racehorses have been selected for heightened reactivity to environmental changes. It seems likely that this is why they are over-represented in surveys of abnormal behaviors in stables, behaviors that are seen by many as responses to aversive stimuli. The least reactive equids are the so-called ‘cold-bloods’, the heavy horses and solid ponies. In between the two groups are the ‘warmbloods’. These can be described as crosses between hot and cold bloods and are exemplified by European performance horse breeds such as the Hannoverian. The domestication process is ongoing. It is important to recognize that selection for reactivity and speed in modern racehorses and extraordinary gaits in dressage horses may run counter to durability and rideability, respectively.
Equus caballus and Equus przewalskii
Mitochondrial deoxyribonucleic acid (mtDNA) studies suggest that a divergence occurred as long ago as a million years,2,3 between the modern horse (E. caballus) and its more numerous and diverse predecessors. The link between them was thought to be the pony-shaped Mongolian Wild Horse, Przewalski’s Horse (E. przewalskii). Apart from Caspian ponies that appear to be polymorphic in the diploid number (some having 64 chromosomes while others have 66),4 E. caballus has 64 chromosomes. Equus przewalskii, on the other hand, has 66 chromosomes in the diploid state. However, the recent publication of the horse genome5 has shown that E. caballus is not only related to E. przewalskii, they are essentially the same species.
Table 1.1 Classification of equidae
| Common name | Species | Diploid chromosome number |
|---|---|---|
| Przewalski’s horse ([Mongolian] wild horse) | Equus przewalskii | 66 |
| Horse (domestic) | Equus caballus | 64 |
| African wild ass (and domestic donkey) | Equus asinus | 62 |
| Nubian wild ass | Equus asinus africanus | |
| Somali wild Ass | Equus asinus somalicus | |
| Asian ass | Equus hemionus | 56 |
| Mongolian wild ass | Equus hemionus hemionus | |
| Onager | Equus hemionus onager | |
| Indian wild ass | Equus hemionus khur | |
| Kiang | Equus hemionus kiang | |
| Grevy’s zebra | Equus grevyi | 46 |
| Common zebra | Equus burchelli | 44 |
| Chapman’s zebra | Equus burchelli antiquorum | |
| Grant’s zebra | Equus burchelli boehmi | |
| Selous’s zebra | Equus burchelli selousi | |
| Mountain zebra | Equus zebra | 32 |
| Cape mountain zebra | Equus zebra zebra | |
| Hartman’s zebra | Equus zebra hartmannae |
This explains the similarities in their serum proteins and their blood groups and why cross-breeding between E. caballus and E. przewalskii produces fertile offspring. Geneticists explain that a single fusion mutation in the haploid state (32) could have brought about their differences. Similar intra-species chromosomal dimorphism occurs in the Asian ass (Equus hemionus).6
Subsequent isolation of the E. caballus and E. przewalskii gene pools could perhaps have arisen because of capricious selectivity on the part of Caballus stallions (see Ch. 11) and the retreat of Przewalski herds into regions of the steppe uninhabited by humans.7 While outnumbered by the similarities between the two species, the differences are fascinating. For example, the striking bistability of hock joints that allows a very rapid switch from one extreme position to the other is much more marked in domestic horses than in Przewalski horses and, for that matter, zebras.8
Przewalski horses became extinct in the wild in the 1950s.9 However, from a nucleus of 11 foundation animals, they survive today in captivity and in successfully re-introduced wild populations, e.g. in Mongolia.10,11 The survival story of the Przewalski horse is an extraordinary one, and we are indeed fortunate to be able to study them in a variety of contexts. It is fascinating that we now have data to refute the notion that Przewalski horses are socially more aggressive than domestic horses.12 However, studies of their behavior should be treated with some caution since they have emerged from a shallow gene pool that has been filtered through 20 generations of captivity. Therefore before assuming that present-day Przewalski horses behave as true wild horses, we should bear in mind that some would regard them as survivors of the first stages of domestication. In this text, I have selected examples of feral horse behavior rather than Przewalski horse behavior as benchmarks for what is normal. Discussions of such free-ranging behavior appear in each of the subsequent chapters.
Changing roles
Although initially horses found themselves in the human domain as a food source, their subsequent roles are nearly as varied as those of the other most domesticated species, the dog, and include providing power, leisure and companionship.
Domestication
French and Spanish cave paintings from around 15 000 years ago, depicting hunting for food and hides, represent the earliest record of human use of horses.13 While early horses would eventually provide their keepers with unprecedented mobility and power, horse herding probably had its origins in the consumption of horseflesh.14 Hunters favor meat, such as that from horses, that has a high glycogen content. As a source of dietary sugar, this facilitates endurance, which is important for members of hunting cultures. Horses were regularly consumed in favor of other large herbivores. In the Paleolithic, our ancestors may have hunted horses by driving them over cliffs and into traps and pitfalls. They continued to do so for thousands of years to the extent that they had a pivotal effect on population numbers.15 It is thought that, along with climatic changes, this exploitation contributed to the extinction of wild horses in North and South America. This may be why wild horse remains elsewhere dating from periods since 9000 years ago are rare.16 Interestingly, it seems the ‘true horse’ (E. caballus) survived in Eurasia but with patchy distribution17 despite, or perhaps even because of, this predation.
Archaeological evidence of horse domestication dates from 4000 bc (late Neolithic or Bronze Age) in the Eurasian Steppes of the Ukraine.18 Ancient middens used as dumping grounds for the bony remains of human meals, most notably in a place called Dereivka on the steppes north of the Black Sea, have proved an especially rich source of domestication data. This is where river-valley agricultural economies evolved, relying on the use of stone enclosures to trap large numbers of game. The numbers involved in a draft often exceeded the immediate demand for consumption and prompted the maintenance of surviving herd members, to be slaughtered at a later stage.
From studies of the bone remains found in middens, the first pastoral species seems to have been bovine. A colder climatic shift that affected this region around 3500 bc is thought to have favored equids rather than other domestic or wild herbivores because, being large and long-legged, they were better able to forage in snow. The prevalence of horses in Ukrainian communities underwent a dramatic rise at this time, with horse bones comprising 74% of non-human bones at Dereivka.19 Evidence of domestication at this site includes this dramatic rise and the predominance of colt skeletons. Analysis of the dental remains of horses in these sites suggests that humans consumed more males than females and that the age range of these animals did not show a normal distribution.
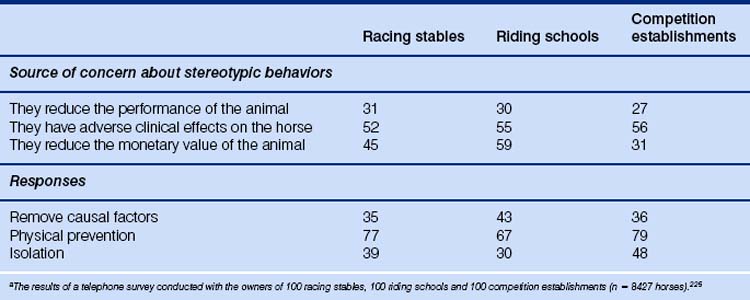
Some authors15,20 maintain that the relatively high number of colts indicates domestication rather than hunting, which would be more likely to harvest older animals, more often mares. Other workers suggest that the vast majority of horses at this time were hunted by stalking (Fig. 1.2).14 Perhaps the age distribution of horse teeth most closely matches what one would expect as remnants of such activity, but it is worth remembering that assessing age by dentition is an imprecise science.21
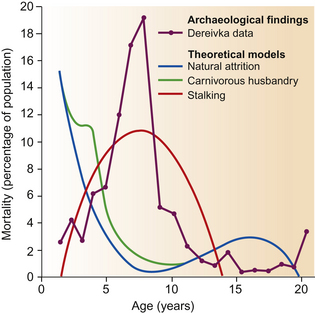
Figure 1.2 Graph of age distribution of remains of 151 horses found at Dereivka, Ukraine. In free-ranging horses death occurs most commonly in the very young and very old (natural attrition model). In populations raised for meat (carnivorous husbandry), the curve is skewed by preferential consumption of 2- to 3-year-olds. Meanwhile, selective hunting of prime-age adults (stalking) produces a different pattern, with a sharp central peak. The age distribution of horse teeth found at Dereivka most closely matches the stalking model.
(Reproduced by permission of Marsha Levine and Antiquity Publications Ltd, from Levine M, The Problems of Horse Domestication in Antiquity 64:727–740.)
The issue here seems to pivot on whether there is any reason for members of a domesticated population to be selected for consumption at 5–8 years of age. A more traditional farming approach would be to harvest the surplus while they were younger and their flesh was reasonably tender. Although one might also ask why hunters would target animals in a wild group that were undoubtedly in their prime and therefore most fleet of foot, it seems likely that they would be more abundant than adolescents or elderly horses.
The reported preponderance of what we regard as male type canine teeth seems to be crucial here. Could it be that small canines as found in many modern mares were erroneously identified as the teeth of stallions?
Horse remains at Dereivka have been characterized as being from large-headed, short and heavy-set animals,18 a description strikingly reminiscent of the Przewalski horse. When the skull of a male equid was removed from a ritual burial site shared by two dogs and anthropomorphic figurines, it aroused considerable excitement in archaeological circles. Dating from the Copper Age, about 4300 bc, the ritualized nature of the burial suggests profound domestication. Interestingly, in the same grave, excavations revealed antler tines that seemed to have been crafted into the cheek-pieces of a crude bit (Fig. 1.3). By facilitating the comparison of the teeth of modern domesticated and feral horses with those of the so-called Dereivka cult stallion, electron-microscopic analysis of anterior premolar tooth wear has revealed bevels and fractures consistent with the use of a bit.22 Even though the first bit was not as robust and therefore as damaging as modern hardware, the damage incurred was regarded as being consistent with 300 hours of bridled control. However, before relying too heavily on such comparisons, it is worth considering ways in which the evidence may have been skewed. Freshly domesticated horses may have been very headstrong and early equitation unrefined. Notwithstanding this minor point, the Dereivka stallion is regarded as the first ridden horse and is thought to have predated the invention of the wheel by at least 500 years.23 The domestication of horses on the steppes may have been repeated in other places. Recent mitochondrial DNA evidence24 indicates that a large number of founders were recruited over an extended period. This ‘multiple origins’ scenario implies that horses may have been independently captured from diverse wild populations and then bred in captivity as the wild populations disappeared.24 This suggests that what spread from that equine cradle was not necessarily the horses themselves but the innovation and technology of taming them. That said, the proposed dates for the emergence of bits, saddles and stirrups are still hotly debated.
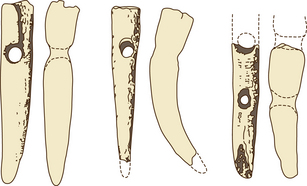
Figure 1.3 Artifact from what is believed to be an early bit fashioned from antlers.19 A rope would have been held in place in the mouth by the cheek-pieces.
(Reproduced by permission of David Anthony, Dorcas Brown and Antiquity Publications Ltd, from The Origins of Horseback Riding in Antiquity 65:22–38.)
Although it seems likely that meat production was the first reason for the domestication of horses, their prevailing impact was linked to their use in riding and haulage. When handling an orphan foal, early horse farmers would have appreciated the horse’s ability to pull and the fact that controlling the body depended on controlling the head. A natural progression would have seen the development of harnesses for traction and headcollars for control. It may be that too much emphasis has been placed on the significance of bit wearing as the necessary step that took the use of horses beyond the dining table. It is implied that horses could not have been controlled from behind by anything less than a bit. This is something of a moot point since it precludes riding with bitless bridles. Equally, horses could have been used for non-riding purposes such as traction of non-wheeled haulage devices, or fallen prey for that matter.
Early farmers had to have some means of preventing the usual migratory dispersal of horses but men on foot, even if they were accompanied by trained dogs, would be no match for galloping horses.25 Only when riding was attempted could horse herding become efficient. Furthermore, while using horses is a matter of taming the individuals as required, breeding them is far more technically demanding.26 The difficulty of preventing domestic mares from dispersing in search of stallions whenever they were in estrus may have been the reason domesticating the horse took such a long time. For these reasons, horses were domesticated after cattle, pigs, sheep and goats, which were all easier to herd and contain.
Although the horse was one of the last mammals to be domesticated, it has had more impact than most on humans, not least in terms of the dispersion of culture and language.16,23 Beginning with human migration across the deep steppes of the Ukraine that could not support large human populations prior to the emergence of riding, horses facilitated the spread of human genes. Because riders move two or three times faster and farther than pedestrians, exploitable territories expanded sixfold. At the same time grassland subsistence became more predictable, reliable and productive, and decisive military advantages were secured over more static neighbors.23 As a consequence, human social groups grew tenfold, conflicts over resources escalated and patterns of trade and theft expanded in range and variety. While riding was established as the prime means of hunting, exploring and herding, the additional use of horses for milk and blood is likely to have peaked at the same time. One should not overlook the usefulness of horses in hauling fallen game, firewood, ploughs, produce and physically challenged members of the group. Such was the importance of the horse in the early dispersal of Copper Age humans that horses became one of the chief tradable items.
The emergence of equestrian cultures is also likely to have brought with it an increase in hit-and-run warfare (Fig. 1.4). This suggestion is borne out by the effective use of the horse by native Americans in the 1800s, which resulted in the unprecedented rise in conflicts and accumulation of wealth leading to the tribal supremacy of the Sioux, Comanche and Apache.27

Figure 1.4 Horse being ridden by a stunt rider as a demonstration of use in combat. Equitation improved a warrior’s usefulness and chances of survival.
(Reproduced with permission of Gerard Naprous.)
Interestingly it was not until approximately 2000 bc that horses arrived in the Middle East and displaced asses and ass-onager hybrids as the favored draft animals for battle carts from which arrows could be fired. A similar displacement took place in mid 19th century Africa when colonists’ demand for the social status inferred by using apparently superior riding animals prompted the importation of often poorly adapted horses while the local equid, the quagga (Equus quagga), was hunted to extinction by English and Boer farmers.28
A new wave of horse-mediated military success emerged when the horse’s speed and maneuverability were fully appreciated. In the first place, chariots, as mobile platforms for archers, provided the means of delivering flanking blows to infantrymen,29 then came the complete development of cavalry warfare. Many authors have discussed the introduction of modern equestrian techniques30,31 and the development of bits, saddles and stirrups19,32,33 and the extent to which each lent a military advantage. The invention of the saddle that shifted the rider’s weight bilaterally to the lumbar musculature and subsequently the stirrup that facilitated the use of weapons by mounted warriors are thought to have been major steps in the culture of mounted combat. With the increasing sophistication of equine-dependent power that led to the emergence of jousting tournaments and ultimately haute école dressage (Fig. 1.5), noblemen came to be judged by, among other things, the quality and quantity of horseflesh in their possession.34 The role of the horse in colonial success was acknowledged by Cortes after the Spanish invasion of Mexico, when he noted that (next to God) he owed his victory to the horses.

Figure 1.5 An etching showing the development of haute école training, as archived by Le Baron D’Eisenberg.
(From L’Art de Monter à Cheval, 1737.)
The management of warhorses was often chronicled but the emphasis in these accounts seems to have been upon their training, nutrition35,36 and medical care37 rather than their housing. The use of horses by military and invading nations has been marked by considerable wastage. There is evidence that Roman equids in Britain sustained injuries consistent with poor living conditions and gross overwork.38 Horses continued to serve in battle up until the end of the Second World War, when the Polish cavalry suffered horrific losses from the guns of Russian tanks.
Current status
Horses are regarded as a familiar part of many agricultural idylls but their role as farm animals is now in question. If we compare the life of a stabled riding horse to that of other farm species, some differences are obvious. Unlike most cows, pigs and sheep, horses are generally not kept with a view to productivity in terms of meat or milk and therefore are often on limited food rations. Because they cannot be regarded as production animals, horses are more often these days described as a ‘companion animal species’. Unfortunately they do not fit terribly well into this category either, since they do not share living space with humans as do true companion animals such as cats, dogs and caged birds. Their contact with humans is largely restricted to being groomed, fed and ridden.
The key roles of the horse in human activity changed tremendously during the 20th century. The overarching fields in which horses are now used include recreational and social purposes, breeding, sport and competition and to some extent meat production.39 Huge reductions in the use of horses in military, agricultural and transportation activities have been matched, in developed countries, by increased equine numbers for sport and leisure.40–42
Prevalent modern breeds are often used in more than one activity (Table 1.2), and this may help to account for the retention of some diversity. Different breeds are thought to have different temperaments that reflect their original purpose (Fig. 1.6). Happily, horse breed societies have tended to avoid the sort of intense line-breeding that characterizes the bloodlines of many purebred dogs,43 and very few populations are inbred. Notable exceptions are the horses of the Namib Desert, Namibia44 and Blue Arabians from the USA.45 In-hand showing as a sole means of selecting breeding animals removes many performance indicators from the selection process in breeding systems and is therefore short-sighted.
Figure 1.6 Breeds seem to have different tendencies when faced with a potential threat.
(Permission from Paul McGreevy.)
While donkeys are still used by the working classes in many parts of the world, the horse is now a status symbol in Western cultures, a luxury item or an emotional focus. Interestingly, this has done nothing to guarantee its welfare, since many owners of luxury items fail to budget for their maintenance. Despite the emergence of equine insurance policies that have helped to remedy the shortfall, many owners find themselves financially embarrassed, e.g. when invoiced for colic surgery. Financial circumstances have considerable influence on the way horses are managed, as indeed does the value of the horse itself. Sometimes the value of a horse can prove an impediment to optimal welfare.54
Although many owners keep their horses in the best possible conditions that money allows,42 sometimes it seems that when performance is less critical, management standards may drop and welfare may be affected. Ignorance and economies may combine to jeopardize the welfare of hobby-horses. This is not to suggest that high-performance animals always enjoy the best welfare, since, for example, intensive management of racehorses involves some of the greatest alterations in time budgets, some of which can reflect profoundly compromised welfare. It is interesting to contrast the management of a Thoroughbred stallion kept in a mahogany trimmed loosebox with brass fittings near Newmarket, on the one hand, with a child’s pony grazing on impoverished land in suburban Dublin. The relative absence of confinement of the latter may well enhance its welfare.
While private owners report increasingly emotional attachments to their horses,55 large numbers of people work in the equine sector for little or no financial reward. With the shift of horse use from military and utilitarian services, the demographics of horse ownership changed, with women forming the majority.56 It has been suggested that female horse owners are more affectionate than males.57 That said, there are interesting reports of an increasing proportion of male riders and an increasing number of older riders.42 Equestrian sports remain the only Olympic events in which men and women compete on equal terms.
In developed countries, horses kill more humans than any other animal.58 On the other hand, while there are no published data on the health benefits of horse ownership, there is convincing evidence that riding is a therapeutic activity.59 Equine practitioners do well to consider the profundity of many human–horse bonds, especially at the time of euthanasia.60 However, while some cultures have come to regard horses as companion animals, others have retained the pragmatism that leads them to consume young horses surplus to breeding requirements or those too old to be of use as breeders or as a means of conveyance.39
Noting the human tendency to forget to appreciate horses for what they are, Rollin61 highlights our ‘unfortunate skill in putting square pegs into round holes’. Compared with close relatives such as zebras that have only very rarely been put to work (Fig. 1.7), horses can be manipulated to behave in many ways and persuaded to cope with a tremendous variety of uses and abuses. The behavioral flexibility of horses is fundamental to their utility in the domestic context. It allows them to tolerate negative reinforcement more than other domestic species but also explains why they are subject to tremendous abuse by clumsy and ignorant handlers (see Ch. 13).

Figure 1.7 Lord Rothschild and his team of four zebras (E. burchelli), circa 1900.
(Reproduced with permission. Copyright Natural History Museum, London.)
The explosion in the popularity of riding for leisure and in first-time horse ownership has led some veterinarians to bemoan the welfare of some present-day equids, e.g. inner city ponies. Ignorance is a considerable force to be reckoned with. I have encountered novice owners who believed their horses could thrive on remarkable diets, including rabbit food and, on one occasion, cat food.
The horse’s future
Information technology should facilitate the education of horse owners and, one hopes, liberate their thinking. The impact of ethology on horse welfare has already been recognized,62 and there is a growing demand for enlightened approaches to horse handling as evidenced by the success of the modern ‘horse whisperers’.63–66 Unfortunately, for some exponents the emphasis seems to be to placed on shows and the introduction of jargon rather than on education. It is hoped that ethologists will continue to demystify the language of showmen and shamans so that communication with horses can be enhanced wholesale.67 The growing influence of equitation science suggests that an evidence-based approach to horse-training and riding will ultimately prevail. (Equitation science is considered in detail in Chapter 13.)
The practice of identifying and logging the performance of non-racing sport horses has the potential to enhance the quality and, it is hoped, the depth of the gene pool. For that reason it is particularly good to note the re-emergence of the UK’s National Equine Database.
The introduction of novel reproductive technologies such as artificial insemination should allow horse breeders to capitalize on genetic material from overseas in the same way that shuttle stallions, flown in from the Northern hemisphere, have liberated Thoroughbred breeders in the Southern hemisphere. It is anticipated that the publication of the horse genome5 and the production of cloned horses68 (Fig. 1.8) will become significant landmarks in the history of domestic horse breeding.

Figure 1.8 Paris Texas, one of the world’s first cloned horses.
(Texas A&M University. Reproduced with permission of Eric Palmer.)
Since it has been shown that crib-biting is associated with a given gene array,69 it may be that there will emerge a drive to breed from animals that can cope with the stressors of intensive management. Geneticists may also help to improve fertility, one of the most pressing topics in Thoroughbred circles. Globally, the annual reproduction rate for Thoroughbreds is approximately 50%.40 This compares poorly with other single-offspring species such as cattle, in which 85% is normal.40 This disturbing trend is certainly not helped by a rise in the rate of twinning, an unfit gene that is passed from one generation to the next almost exclusively by veterinary intervention.
It seems unlikely that, since it has been the single breeding objective for the past two centuries, speed on the track can undergo much further improvement. Mirroring the current focus of human exercise physiologists, the role of lactic acid clearance as a limiting factor in racehorse performance continues to attract considerable attention.70 It may be that selection of genotypes capable of accelerated lactic acid metabolism may reduce winning times in the longer classic races such as the St Leger and the Oaks.40,71 Markers of speed may be identified by genome mapping studies but these are likely to combine a number of features, including cardiac output and maximal oxygen consumption.
While there may be initiatives to identify the genes of horses that cope best with intensive management and do so without displaying stereotypies, it is hoped that the technology can be harnessed to improve horse welfare rather than simply to select for tolerance. The worldwide web offers a unique platform for information on the identification and treatment of common ailments and the showcasing of best practice in stable management and evidence-based approaches to training, i.e. equitation science.
Although grazing land will become harder to come by, stabling is likely to evolve to meet more closely the behavioral needs of horses. We may yet identify key elements of established management protocols that can have a deleterious effect on our horses.72,73 Although these days it is common for owners and trainers to regard the risk of injury in sport horses as being great enough to outweigh the benefits of being turned out,39 evidence is steadily accumulating to fortify the case for increased opportunities for spontaneous exercise and social contact.74
The welfare of riding-school horses is likely to improve as technological advances make it easier to detect those of a novice rider’s signals that are particularly confusing and unhelpful. In the UK, electronic riding machines have been developed with all of the paces of a horse (Fig. 1.9). Their predictable action allows the rider to learn, for instance, how to rise at the trot and even jump a fence.75 With sensors to detect and respond to the rider’s signals, the simulator reduces the time required for novices to learn to ride.76 It is not difficult to see how this technology may advance. It could be programmed to behave less predictably, to be more or less responsive or to demonstrate typical equine conflict behaviors in response to confusing commands.77 One could even speculate that the welfare of those dependable riding-school horses that always carry novice riders will be of less concern in the future. Currently balance is acquired over weeks of crude education, for example, staying on board by pulling on the reins and therefore the mouth. The numbing effect this has on the horse’s mouth reflects the discomfort it must cause. This inelegant and inhumane use of sentient beings in human education may disappear with the growing use of mechanical alternatives.

Figure 1.9 A mechanical horse simulator.
(Reproduced with permission of Racewood Equestrian Simulators.)
Digitization of the interaction of elite riders with their mounts may provide for a quantum improvement in the teaching of equitation. Measurement of tensions and pressures applied by the handler’s hands, seat and legs can be correlated to observed behavior of the animal and provide an indication of the magnitude of stimulation required to be applied by the handler to modify the behavior of the animal. Logging devices of this sort will also provide teachers with some quantitative means of assessing the handling of an animal under the control of a student. It has been proposed that technologies that decipher the complex physical interactions between riders and horses will facilitate remote coaching and the development of biofeedback systems that permit the use of training templates that can be optimized for each horse–rider dyad.78
Stable management
Humans can control horses most effectively by stabling them. This imposes limits on the extent to which horses can meet their behavioral needs and can have profound effects on welfare. Equine behavior undergoes considerable modification when animals are removed from pastured (or feral) environments and placed in a stable.
Traditional stable management
The horses sent over to the British Isles by the Roman conquerors would have included a variety of heavy, ‘cold-blooded’ and less weather-tolerant, ‘hot-blooded’ animals. Archaeological evidence79 suggests that they were all small by modern standards. Evidence of how Roman horses were housed exists but is rather cryptic, with remnants often being limited to urine and manure staining on chalk beds and teeth in feeding channels. However, a site at Hod Hill in Dorset80 appears to have been particularly well studied. Two types of stable division were noted, and the pattern of chalk-compression and the extent to which hooves had mixed chalk with manure gave the investigators an impression of how the animals were secured within the building. In one type of compartment, measuring 3.6 × 3.3 m, three horses might have been tethered to the wall, their dung falling into a 2-m channel to the rear. This channel also permitted access by grooms. The other compartment appeared to have accommodated six horses that again were tethered facing the wall. Measuring 3.6 × 5.5 m, this house comprised a central dunging passage that was communal to both rows of horses.
Information regarding accommodation for military horses since that time is limited. Smith81 indicates that the British Army billeted horses in lines and did not have stables until a program of building barracks and stables began in 1792. Of these stables little is known except that they were not well ventilated and therefore precipitated considerable losses from glanders and other respiratory diseases.81 Coach horses from the same era were confined to tie-stalls rather than individual boxes that were reserved for saddle horses.
Modern stable management
Just as domestic horses are put to a number of uses, so they are housed for a number of reasons and in stables of varying design. The most common issues around the ‘whys’ and ‘hows’ of intensive horse management are considered below.
Reasons for stabling horses
Horse owners throughout the world house their animals for a number of reasons. Among these, the most important is often the need to spare limited grazing areas from damage underfoot, especially in regions of and in times of heavy precipitation. The danger of disease associated with exposure, such as ‘rain-scald’ and ‘mud-fever’, in these regions can be reduced by providing field shelters and appropriate prophylaxis. Therefore, the economics of pasture management can prevail over horse welfare when owners consider stabling their horses for the winter.
By stabling their horses, owners eliminate food-stealing by dominant conspecifics and can have more control over food quality and intake. Food and water intakes are easier to monitor, with an eye toward early detection of disease, in the stable rather than the field. During the winter, time spent mucking-out and bedding a horse can be offset by the time saved by not having to groom a thick layer of mud off the horse prior to exercise. Clipping, made more feasible by the fact that the horse is sheltered, adds to this advantage since clipped horses sweat less in response to heavy work than their counterparts in a full winter coat and are therefore easier to clean.
Stabling may be favored by the owners of horses that are difficult to catch when at pasture. The limit on kinetic activity that is imposed by stabling is considered advantageous by trainers of performance horses since they can more easily control the amount of daily exercise taken by their charges. Post-inhibitory rebound in locomotory behavior after periods of confinement74 may give trainers the impression that horses want to work.
Health advantages may accrue from stabling since injuries can be more effectively rested, worms can be better controlled and flies are less numerous than at pasture. Apparent advantages for the horses of being stabled include shelter (from sun, wind, rain and flies), freedom from bullying and a reduced physical requirement to work (e.g. forage) for food. This book should help readers decide whether these benefits outweigh the costs paid by the horse as it loses choice and control over social and ingestive activities.
Management practices
The time of year and the seasonal nature of some sports such as hunting and eventing influence the way in which horses are managed. Traditionally during the summer, while gymkhana ponies undergo a peak in their use because children are on school holidays, hunters are ‘let down’ at grass.
Feeding
Horses are notoriously wasteful grazers (see Ch. 8). Many farmers remark that, in wet weather, when one considers the damage done by its unforgiving hooves, a horse is equivalent to the activity of five bovine mouths. The reluctance of owners to allow this damage means that time spent by horses at pasture in temperate climates is often limited during the winter months. While sparing the horses the pain of disorders such as mud fever and dermatophilosis, this intervention often has a deleterious impact on their behavior and nutrition.
Traditionally, horses are fed long fiber from a haynet or hayrack. This is intended to reduce wastage from contamination with urine or feces and possibly to decrease the likelihood of endoparasite transmission. However, there is growing evidence that this unnatural foraging position can have a deleterious effect on the efficacy of the mucociliatory escalator in clearing the upper airways of inhaled particles (Fig. 1.10), especially those inhaled from dried foodstuffs (which are to some extent unnatural in themselves).82 Similarly, haynets reduce the space available in the stable, may increase the risk of the horse becoming snared (e.g. by trapping its hoof), and elevate the forage so increasing its potential as a source of ocular foreign bodies. It is also argued that feeding from a net or rack may adversely affect muscles and nerves in the neck.83 Perhaps this is why, when financial considerations are almost insignificant relative to the value and maintenance of performance (e.g. in the majority of racing yards84) horses are fed roughage, whether it be hay, haylage or silage, from the stable floor.

Figure 1.10 Dust plumes from a haynet as a horse forages. The position of the food and its contamination with fungal spores are unnatural. The harmful effects on respiratory health are well recognized.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
There are marked seasonal variations in feeding practices according to the weather and the availability of pasture. We do well to remember that the incidence of colic follows a similar pattern, with changes from fresh grass to a dried diet being associated with rises in various enteropathies, especially impactions. The use of concentrated feeds and the periods of fasting with which they are associated have been linked to unwelcome consequences, including increases in gastric acidity that can result in rapid ulceration.85 Compared with those who ride mainly for competitive reasons, those who ride mainly for pleasure are less likely to feed cereal-based foods according to the manufacturer’s recommendations during summer, when grass is available.86
Bedding
For a variety of reasons, including availability, ease of disposal and tradition, straw remains the favored bedding for horses.86 Although it raises concerns for some about the risk of impaction and chronic obstructive pulmonary disease (COPD), straw seems to have the added advantage of being the bedding preferred by horses themselves.87 It may be worth noting that straw is regularly used as horse food in developing countries.
Depth of various bedding types that is needed to maximize their appeal to horses has not been studied. However, it has been noted that horses lie down on deep litter bedding rather more than do horses at grass or on daily mucked-out beds.88 The common practice of providing limited bedding to save money can affect choice by reducing the extent to which the horse can comfortably lie down. Houpt89 notes that if bedding is present but inadequate, horses tend to lie down as soon as more bedding is supplied.
Whereas horses have evolved to be very sociable animals, many owners feel that their charges should be given solitary quarters to make bullying less likely. At pasture, horses choose their affiliates and will spend time interacting with them, for example, while playing, mutually grooming,63 or settling reasonably minor disputes. On a stable yard, managers tend to dictate the distribution of horses with efficiency of service in mind rather than the associations established between pairs of horses at pasture. It is a shame that there are so few data on the effects of separating horses from preferred companions, or exposing them to agonistic approaches from neighbors whom they would normally avoid.
Watering
While some military horses are still communally watered on a three-times-daily basis, modern textbooks of stable management rarely commend the limited availability of water. However, the maxim that food should not be given before work and that water should not be offered after food probably represents a considerable limitation on the choice that stabled horses can exercise in terms of drinking behavior.90
Stable design
There are few publications on the design of stables, and these have been based largely on extrapolations of recommendations for shelter and ventilation of agricultural species. The Universities Federation for Animal Welfare (UFAW)91 states that for a horse of average size – 500–600 kg bodyweight and 1.5–1.6 m (15–16 hands) – the loosebox should be at least 4 × 4 m and preferably 4 × 5 m. Ensminger92 states that except for foaling mares and for stallions, there is no advantage in having box stalls larger than 3.6 m square. Evans et al93 note that the popular size is 3.6 × 3.6 m and that 3 × 3 m is adequate for young horses but suggests that the more time the horse spends in the stall, the larger the stall should be. There are several differences between the housing of horses compared with that of other farm animals. Horses usually have individual living areas, and a much greater labor input per animal is present in stables than in farm-animal housing. A survey of racing stables in the Southwest of England found that floor space varied between 8.76 m2 and 21.8 m2 with a median of 12.1 m2 per horse.94 This compares favorably with the floor space provided for other agricultural species per unit of body size.95
While box designs have been suggested by agricultural engineers and by horse-lore, there appear to be no recommendations in management texts about the amount of time that the occupants of these quarters should spend within them on a daily basis. There are a number of reasons for protracted confinement, including inclement weather, locomotory illness and isolation of contagious pathogens. Episodes of enforced confinement are popularly noted as being contemporaneous with the onset of stereotypies. Given that Evans93 advocates the provision of more space for animals that are turned out less regularly, it could be argued that the design of all stables should meet the needs of the worst-case scenario that precipitates withdrawal of the daily turn-out period.
The traditional layout of a stable-yard includes the quadrangular enclosure of a central lawned area by boxes whose doors and windows face inwards. Walkways in many yards pass close to these portals and expose stabled horses to the unpredictable and arousing movement of humans, feed-buckets and conspecifics. Therefore, it has been suggested that regimented geometrical accommodation of this sort makes relaxation difficult for the occupants.63
Despite similar problems with the distracting effects of activity in the passageways and others to do with air hygiene, barn-style housing (in which horses are housed individually, under one roof, in pens made largely from bars and grilles), which facilitates communication between neighboring horses, is increasing in popularity. Studies on the effect of increasing visual contact with conspecifics on weaving96,97 (see Ch. 5) suggest that whether a stable, rather than a tie-stall, is acceptable depends on its walls more than its size in that isolation is more of a problem than confinement.89 Group housing of young horses allows more normal social development98 and reduces the risk of stereotypies99,100 when compared with individual housing.
Modern stable management brings with it a number of disadvantages for both owners and horses. For the owner, the disadvantages of keeping their animals in stables rather than at grass revolve around time and money. Time commitments in stable management include bedding and feeding as well as having to exercise the confined horse on a more regular basis than its grazing equivalent in order to maintain a given level of fitness. The disadvantages of stabling from the horse’s perspective are considered later.
Behavior
When practitioners are asked to comment on abnormal or unwelcome behaviors, they must first of all appreciate the range of normal behaviors.
Normal behavior in stabled horses
Domestication affects behavior,101 not least because it limits the amount of space in which stock is able to range. This limitation can be effective from day one of life, since many foals, especially valued Thoroughbreds, are born indoors.102 Interestingly it has been suggested that myopia may be a consequence for horses that spend too much time in stables103 (this is discussed further in Ch. 2).
The changes in equine behavior associated with confinement and limited choice merit particular consideration. From the horse’s perspective, the ways in which stabling can compromise feeding, social and kinetic behavior and indeed health are considered below.
Feeding behavior
Because behavior is a response to an organism’s environment, the more restrictive an environment is, the more limited are the choices available to the organism. It is possible that where choice is limited or eliminated, welfare may be compromised.104 Choice allows animals to perform the behaviors that are important to them,105 although the choices they make are not exclusively in the direction of their own welfare.106 While the debate about the importance of environmental choice in the welfare of animals continues, there appears to be a number of ways in which modern stable management limits choice.
Choice is certainly reduced in feeding behavior when horses are stabled. Feeding behavior in stables seems to show the most marked difference from that at pasture since concentrated rations may be consumed more rapidly than a pure forage diet (Fig. 1.11). While the feral or pastured horse may spend 70% of its day foraging, stabled horses on ‘complete diets’ may spend only 10% of their time feeding.107 These diets for competition or maintenance can be eaten in less than 2 hours and have removed the feeding behavior of stabled horses even further from its evolutionary origins. In addition, modern diets for the stabled horse can be monotonous. There is compelling evidence that giving stabled horses variety in their forage ration and distributing various forages throughout the stable can help to bring their time budgets closer to normal.108
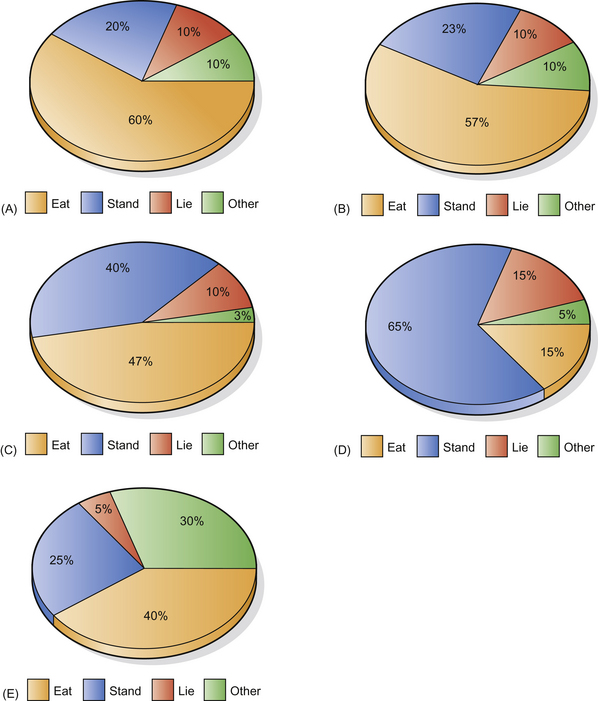
Figure 1.11 Time budgets for horses in a variety of environments: (A) free-ranging Camargue horses, averaged over a year; (B) eight horses housed as a group with ad libitum hay and straw; (C) three horses in individual stables given ad libitum hay and straw and able to see and touch each other; (D) horses fed restricted fiber in stables where they cannot touch each other and only see each other over stable doors; (E) six crib-biters fed ad libitum hay and able to see and touch each other. NB: Crib-biting accounts for 20% of these horses’ ‘other’ behaviors.
(Charts (A)–(D) reproduced with permission of M Kiley-Worthington from The Behaviour of Horses in Relation to Management and Training.)
The time at which food is made available often adheres to a strict regime, which may have variable implications for gastrointestinal function. The designation of such feeding times may address the convenience of the operator rather than the needs of the horse. Horses have not been observed to fast voluntarily for more than 3–4 hours109 but spells of this sort are imposed on many stabled animals.
It has been shown that after 2–3 weeks of continuous access to a single feed, ponies stabilize their bodyweights, consuming 2–3% of their bodyweight in dry matter in a 24-hour period.110 Despite this finding in support of ad libitum feeding of housed equids, stable managers prefer to control intake, since it is known that to do otherwise is to risk temperamental volatility and metabolic disorders, e.g. laminitis.
There is considerable anecdotal evidence for the effects of diet on behavior in the horse,1,111 but little has been done to determine the relative importance of factors such as energy, protein and fiber levels. (The relationship between diet and behavior is discussed in more detail in Chapter 8.)
It has been argued that the sleep pattern of a species can be used as an index of adaptation.112 It is therefore interesting that, while drowsing accounts for 8% of the resting time of stabled horses, this figure has been shown to rise to 14% at pasture.113 The concomitant dietary differences between intake at grass and in the stable may play a role in this phenomenon since a preliminary study in ponies has shown that, when oats replaced hay in the diet, total rest time increased.114 This merits further scrutiny (see Ch. 10). Although the number of feeds per day can affect behavior via factors such as disturbances and arousal,115 it could be that restlessness in horses peaks when stabled animals are fed small amounts of forage and after consuming them are left with little to do in so-called vacuum periods.
Social behavior
The importance of social behavior to stabled horses has yet to be fully quantified with consumer demand studies. However, anecdotes that describe horses performing operant responses, such as undoing bolts with their lips to escape from the stable (Fig. 1.12) may indicate that housed individuals will perform work in order to return to their conspecifics.

Figure 1.12 (A–D) Irish Draft Horse undoing the bolt of his stable door. (E) After a chain was secured across the threshold to prevent his escape, he moved his hay so that he could watch activities on the yard while eating.
It is known that frightened riding horses may bolt in a bid to return to their fieldmates, apparently because of an innate preference for the presence of equine company.116 There are considerable data to suggest that isolation can be aversive.117–120 When the choice of equine neighbors in a stable yard is dictated by the manager, bonded affiliates may be separated while individuals with mutually low tolerance may be housed next to one another. Disruption of an established social structure in this way may be associated with heightened aggression, especially at times of concentrated food delivery.121
Generally, the opportunities for social interaction with conspecifics, favored or otherwise, are minimized by the individual housing of horses. While olfactory, auditory and visual communication can often occur between stabled neighbors, tactile communication, which is of considerable importance in groups of horses,122,123 is rarely possible in most stable designs.94,115 Similarly, interactions such as mutual fly-swatting cannot be performed by isolated horses. These concerns are of particular importance when tie-stalls are considered, with evidence to suggest that they do not sufficiently cater for horses’ needs to perform social, recumbent resting and allogrooming behaviors.124
Stables of all sizes when designed for individual horses have the potential to conflict with many of their occupants’ survival instincts.13 While imposing the vulnerability of isolation and an unhelpful concentration of excretory products that may attract predation, they prevent detection of predators and escape. Until we cost out the value of resources or the absence of threats to safety and comfort, it behooves us to consider these impositions from the horses’ perspective. For example, while stallions and occasionally geldings create stud-piles of feces in the stable, eliminative behavior in the stable differs markedly from that in the paddock, since the occupants cannot easily avoid contact with their urinary and fecal waste. Because fresh layers of bedding regularly prompt urination in male equids, some consider this a form of marking.63 So, the extent to which bedding is managed to meet human rather than equine needs bears consideration but it is only one feature of the stabled horse’s world that represents a removal of choice. In many ways horses attempt to meet their behavioral needs despite the shortcomings of intensive environments but may fail to do so because of management routines.
Kinetic behavior
Among the prevailing features that arise in intensively managed horses is restricted space. The physical limitations imposed on a horse by stabling mean that kinetic behavior is more difficult to perform than it is at pasture. For example, it has been estimated that an average sized horse needs a 6-m span to roll from one side to another, so many stabled horses are unable to perform this most basic of maintenance behaviors. The relative lack of space either may prevent the stabled horse from choosing to roll or may precipitate casting. Confinement within looseboxes is common and is an important limitation on the amount of exercise that a horse can take through choice. After periods of confinement, horses show post-inhibitory rebound74,125 that may explain why unwanted behaviors during training occur more frequently among stabled horses than those at pasture.116
It has been suggested53 that voluntary kinesis indicates that, rather than being simply a substratum for most behavior, locomotory activity has a motivation of its own.74 However, the possibility of motivation for spontaneous locomotion is a contentious area because there is often difficulty in eliminating motivation to perform other behaviors for which locomotion is a prerequisite, e.g. intrinsic exploration.127
Although unacquainted horses from different yards may be mixed on a national and international scale for equestrian competition, isolation is often practiced when new horses arrive on a yard, in a bid to control the spread of pathogens. Should such animals perform an unwelcome behavior pattern, they will often remain in isolation, since stereotypies are traditionally regarded as contagious.
Space is certainly restricted when horses are housed, particularly in the case of tie-stalls. The extent to which stabled horses can see conspecifics is restricted. In a traditional loosebox, this can often be achieved only by looking over the stable door, while many tie-stalls restrict visibility further by having tall dividers between neighboring pens. This view is a contentious one. Marsden128 maintains that the behavior of horses kept in tie-stalls is similar to that when they are kept at pasture. This was based on the observation that, in tie-stalls, horses showed no significant increase in the time they spent performing abnormal behavior. The proximity of neighboring horses was thought to facilitate social behavior more than is the case in individual looseboxes. It may be that the small sample size (n = 4) could have resulted in the inadvertent selection of horses that were especially unreactive and not predisposed to perform stereotypies. This work did, however, indicate that stalled horses spent significantly less time lying down and moving, with significantly more time standing in stalls than at pasture. Subsequent studies of pregnant mares in tie-stalls have shown their time budgets to be similar to those of free-range horses.129 It would be interesting to see whether the mares’ gravid state influenced this outcome. There is a danger in assuming that, even though horses do not choose to be so confined, tie-stalls somehow meet horses’ behavioral needs. Jones & McGreevy130 have described a framework for judging the impact of and ethical justification for various interventions imposed on horses in the domestic context.
Health
The continual proximity to feces and urine may be aversive to a stable’s occupant and may also have an impact on health since humidity and airborne pathogen viability may rise, especially if air-changes per hour are insufficient.94 An unnatural foraging posture, e.g. eating from a haynet for extended periods rather than from the ground, may compromise the function of the ciliary escalator in the respiratory tract131 and precipitate pulmonary disease. Rhabdomyolysis132 and chronic degenerative joint disease133 are often exacerbated by the physical restriction that accompanies extended periods of stabling. A stable with insufficient or uncomfortable bedding may limit the willingness of its occupant to lie down.134 This is thought to be associated with the horse’s working life being cut short.135 Stabling has also been implicated as a predisposing cause of one of the most dreaded and frequently fatal equine disorders, colic.136
Conclusion
The five freedoms (Box 1.1) have been established as a means of judging the extent to which the needs of domestic and captive animals are met by human carers.137,138 If we apply this model of good animal welfare to horses, we can highlight areas in which wellbeing may be compromised. For example, we see many ways in which they are denied the freedom to express their normal behavior in a stable. When one considers the social nature of equids, one quickly appreciates the impediment that the isolation of single-horse housing represents to normal equine behavior. Therefore, while it has many advantages for the horse owner, the stable should be viewed not simply as a source of physical confinement but also as a limitation on behavioral choice.
To analyze all the factors likely to influence the welfare of farm animals, consider whether the animal has:
1. freedom from thirst, hunger and malnutrition – by providing access to fresh water and a diet to maintain full health and vigor
2. freedom from physical and thermal discomfort – by providing a suitable environment, including shelter and a comfortable resting area
3. freedom from pain, injury and disease – by prevention or rapid diagnosis and treatment
4. freedom to express most patterns of normal behavior – by providing sufficient space, proper facilities and company of the animal’s own kind
5. freedom from fear and distress – by ensuring conditions that avoid mental suffering
Abnormal behavior in stabled horses
Most changes in ingestive, eliminative and social behaviors that follow a horse’s move from an extensive to an intensive management system are usually of little concern to the animal’s owner. However, behavioral changes that are directly deleterious to the stable or the usefulness and value of its occupant are regarded as abnormal. Tradition maintained that it was a form of disobedience when stabled horses did not behave as their owners required. Such miscreants were said to have ‘vices’ whether they ate their feces, bit their grooms or refused to lie down.135 Far from elucidating the reasons for these unwelcome behaviors, this umbrella term tended to put the blame on the horses themselves, as if they had malicious intent.
In 1912, a Captain Moseley, writing in the Veterinary Record, described one such ‘vice’, crib-biting, as ‘a form of mental masturbation’. However, while still saddled with a label that implied viciousness, the same behavior in 1959 was recognized by Summerhays139 as being induced by confinement. Applied ethology has developed since then and serves to examine shortfalls in management that may have prompted such behaviors. Behaviors that have been described as vices140 are now better categorized as redirected behaviors, learned behaviors, physical problems, stereotypies or the consequence of inappropriate amounts of stimulation.141
Displacement and redirected behaviors
Displacement behaviors are regarded as responses that are inappropriate for the current situation. Of course, this rather arrogantly fails to acknowledge that observers may not always know what is appropriate and inappropriate from the horse’s perspective. Displacement behaviors are recognized in situations that involve behavioral conflict (see Ch. 13). For example, when a ridden horse is prevented from moving forward for longer than it can readily tolerate, it may start bending its neck laterally in an apparent attempt to groom its flank even though self-grooming is not the most appropriate response to restraint mediated through the bit. In the stabled horse, a similar self-grooming response as a result of frustration may be the kernel of stereotypic self-mutilation.
When a behavior (e.g. an act of aggression) is directed away from the primary target and toward another, less appropriate object, it is said to be redirected.141 The term must be used with caution since it requires that the observer has correctly identified the primary target. For example, horses that eat their bedding material are thought by some to be performing a redirected behavior that meets their physiological needs for dietary fibre.110 Others regard this as a form of coprophagia if the bedding is soiled, but since the remedies advocated involve increasing the provision of roughage (which redirects the behavior back to its evolutionary target), the distinction is descriptive rather than functional. Coprophagia is regarded as an adaptive behavior in foals142–144 but as being an ethopathy in older individuals.53
Another common example of redirected behavior in equine husbandry systems is wood-chewing, which is regarded as an ingestive behavior that would be more readily directed toward grass or other palatable fiber were it available.110 That said, it is known that horses at pasture will chew wood,145–147 which implies that our understanding of the causes of this behavior is incomplete. It is possible that the ingestion of small quantities of bark may be adaptive as a means of acquiring micronutrients.110
Both redirected and displacement behaviors are subject to the rules of operant conditioning (see Ch. 4) and may be reinforced to become established unwelcome responses. For example, many horses paw at the ground or at the stable door prior to being fed. Although this may be a response that would be appropriate for a free-ranging horse that is being kept from its forage by a temporary barrier, such as snow, some would see the targeting of the door as a redirection of the behavior. If the horse is fed during or shortly after pawing the door, and is therefore rewarded, it will be more likely to paw the door in future.
Over-stimulation or under-stimulation of behavioral systems
While lack of stimulation is often cited as a cause of anomalous behavior,50,148 including apathy, the opposite also merits consideration in the ontogeny of unusual behavioral strategies linked to housing. Kiley-Worthington88 indicates that too much noise, excitement or exercise can cause over-stimulation, as can the presence of too many horses and humans to mix with socially. Raised levels of arousal in these horses may make them generally more reactive when being handled in the stable.63 Similarly, inappropriate levels of stimulation are thought to cause psychopathologies such as hyperphagia nervosa53 and polydipsia nervosa.149
Learned behavior
Learning in companion animals is often not recognized and is therefore regularly misinterpreted. This often involves so-called superstitious learning whereby a response is acquired as a result of its accidental link with a reinforcer.150 Misinterpretation arises because the handlers are unaware of the cues that their presence or actions represent to the animal. Chapter 4 is designed to allow readers to become conversant with learning theory and to understand why the term superstitious learning is virtually redundant, given that animals are learning all the time. Recognizing ways in which animals learn associations unanticipated by their handlers is a cornerstone of behavior therapy. A bizarre example is a case of psychogenic colic that was associated with a demand for human company.151
Another example is learned aggression to humans that can evolve as a result of inappropriate associations between agonistic posturing and the unwitting delivery of a food reward. Thus, arrival of the handler prompts hunger-motivated head-threatening behavior that is reinforced by the consequent acquisition of food as the human retreats. This agonistic behavior escalates when punished directly by the handler since his eventual retreat becomes a more valued goal.152 Other learned responses, including conflict behaviors that arise during horse–human interactions, are considered in Chapter 13.
Physical problems
Problems that arise as a direct consequence of the dimensions of the stable, such as ‘refusal to lie down’, have been blamed on some deficiency on the part of the occupants rather than the designers of the accommodation. Certainly, the area available influences resting behavior.153 Other examples of physical problems include habitually getting cast, catching hips on the doorway (often exacerbated by learned fear that prompts faster, though not necessarily better judged, exits through doorways) and getting feet caught in haynets.
Stereotypic behaviors
Stereotypic behavior is characterized by being repetitive, relatively invariant and apparently functionless.154 Stereotypies are heterogeneous in their causes and their forms. Caged tigers that repeatedly pace up and down in their enclosures raise public concern about welfare in zoos. On a less exotic scale, similar behaviors are performed by horses and ponies. Historically known in horse-lore as ‘stable vices’ and given specific descriptive labels such as box-walking (stall-walking in the USA), weaving, wind-sucking and crib-biting, these largely irreversible behavior changes tended to cause more embarrassment than concern. Despite this, questions regarding stereotypies (as these unwelcome behaviors are correctly described) have been fielded by behaviorists and vets for decades. In recent times, equine stereotypies have received considerable scientific attention.
Most lay authors on the subject tend to use the blanket term ‘boredom’ to explain how these behaviors arise, and the remainder imply that it is the fault of the horses themselves. However, the days of dismissive attitudes to behavioral anomalies in horses and ponies would appear to be numbered. Therefore, while there are still those who regard the ‘private life’ of their stabled horses as being unimportant as long as it does not cause poor performance, others have begun to question the merits of traditional stable management that pushes the horse beyond its limits of adaptation.
Historical accounts of stereotypic behavior
Stereotypic behavior is not described in the earliest text on horsemanship and stable management155 but archaeologists have used erosion on the incisors of equine skulls as an indicator of crib-biting and therefore domestication. Donkey teeth gathered from what are thought to be ancient sites of worship in Syria have shown signs of wear that have been likened to the erosion one finds in crib-biters.156 The use of incisor wear to identify Paleolithic horses as crib-biters is not without its critics.157 The current prevalence of crib-biting in donkeys is negligible and, while it is possible that crib-biting was more common in the past and has been selected out of the population to some extent, it seems strange that three of the five donkeys in this site would have this stereotypy. The use of incisor erosion as a means of confirming that the bearer is or was a crib-biter remains controversial because pre-purchase evidence of erosion is cited in instances of litigation when the vendor fails to declare the behavior.
This link between crib-biting and domestication is made in the belief that stereotyped behavior occurs only in animals that have experienced captivity. Furthermore, the assumption that this wear is invariably the result of an oral stereotypy is not without hazards since sandy soils and particular species of grass may produce similar erosion on the labial surface and the tables of the incisors.
As well as crib-biting and a number of the other so-called stable vices, weaving is described in a number of antique texts on equine husbandry.158–160
Characteristics of stereotypic behaviors
Stereotypic behaviors are not recognized in free-living feral horses and are not purely a product of domestication since they are also reported in captive examples of wild equids such as the onager mountain zebra161 and Przewalski horses.162 In the horse, these behaviors have therefore been linked to a number of management practices. A number of equine stereotypies have been identified,88 including:
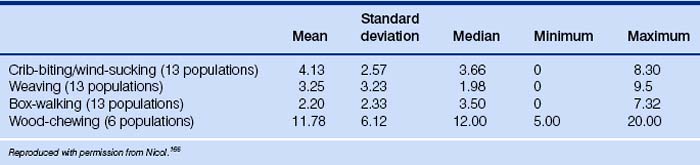
This is an exhaustive list and includes a number of behaviors that are not morphologically invariant and therefore might be regarded as redirected behaviors by other authors. Other behaviors in the above list are difficult to define because changes in form may accompany aging of the stereotypy, e.g. weaving may change from a side-to-side pacing to a stationary head-swing.163 More than one stereotypy may be performed by an individual horse, e.g. a box-walker may well be seen weaving on occasions.164 Indeed if a horse has one stereotypy, it has a greater chance of having a second than do normal horses.165 The results of 13 studies into the prevalence of stereotypies and 6 into that of wood-chewing appear in Table 1.3.166
Table 1.3 Reported prevalence (%) of stereotypies and wood-chewing from studies published between 1993 and 1998166
Since many stereotypies are popularly regarded as being transmissible by mimicry134 and some are associated with health and performance problems, horses exhibiting them are often isolated. Some (such as box-walking, weaving and wind-sucking) must be declared at auction and tend to lower the value of affected animals. These are dealt with more thoroughly in Chapters 5 and 8.
The functional significance of stereotypies
There has been much recent debate about the functional significance of stereotypies performed by captive domestic animals.167–169 One influential theory is that stereotypies enable animals to cope with stress.170–173 However, experimental studies to examine the effects of preventing animals from performing stereotypies in order to assess the validity of the stress-coping hypothesis have produced equivocal results.174–178 Nevertheless, transient decreases in heart rates have been demonstrated in association with bouts of crib-biting (Fig. 1.13).179,180 Furthermore, efforts to prevent the performance of equine stereotypies are linked to some increases in physiological stress parameters.177,178 It has also been suggested that crib-biters may have higher basal sympathetic activity because they have been found to have higher overall mean heart rate.180

Figure 1.13 Horse crib-biting on metal substrate. Although horses prefer to grasp wood rather than metal (perhaps because it cushions their teeth), they will crib-bite on metal if no other substrate is available. This helps to demonstrate a considerable behavioral need to perform the stereotypy.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Stereotypies could help a horse cope with suboptimal environments or bring direct and immediate rewards that make the behaviors intrinsically gratifying. Endorphins have been implicated as a possible source of reinforcement for crib-biting181 because opioid antagonists can reduce crib-biting by 84%, suggesting that at least one of the perceived benefits of crib-biting (from the horse’s perspective) is mediated by opioid receptors at some point. However, because resting behavior in crib-biters was also significantly increased by opioid antagonists, it may be that the reduction in crib-biting was linked to a generalized sedative effect. The effects of opioid antagonists on weaving are extremely variable, with reports of both decreases182,183 and increases.178 While it is possible that weaving may also be opioid mediated, further work with larger numbers of weavers would be required before there can be a clearer understanding of the mechanisms involved.178 Recent studies on the brain of stereotypic horses indicate altered dopamine activity.184
A further suggestion is that a given stereotypy may retain a function within the motivational system from which it is derived.88 Thus, an oral stereotypy such as crib-biting may provide a route to normal feeding and digestive activity within an environment that severely limits normal forage intake (e.g. an intensive training program characterized by the provision of high concentrate: minimal roughage diets).
Persevering with non-functional behaviors or previously trained but currently unrewarded responses is a characteristic of stereotypic animals.185,186 There is evidence that stereotypic horses may have developed a general inability to suppress non-functional behavior.187 While this may have important repercussions for the trainability of stereotypic horses, it should also be borne in mind when equine scientists select subjects for studies of learning.
Management factors and abnormal behaviors in stabled horses
It is clear that the prevalence of crib-biting and weaving is greater in Thoroughbreds than in other breeds.188,189 The prevalence of box-walking, wind-sucking/crib-biting and weaving in UK Thoroughbred populations has been estimated at 1.1%, 4.2% and 2.8%, respectively (n = 1033)190 and in Italy at 2.5%, 2.4% and 2.5% (n = 1035).191 Data from 4468 UK Thoroughbred horses in training showed the total prevalence of all three of these stereotypies to be 10.8%, similar to the prevalence of lameness.192 Estimates of the combined prevalence of all stereotypies are more difficult; for example, when lip-licking, pawing, tail-swishing, head-tossing, head-nodding and box-kicking were included, the prevalence on some yards reached 26%.88 However, it remains difficult to dissect the effects of management and breed because Thoroughbreds are generally raced and therefore managed intensively.
In other species, cage-design,193 isolation-rearing194 and food-deprivation195 have been implicated as proximate causes of stereotypic behavior. Arousal, generated by frustrated motivation, is a possible shared underlying cause,196 although others emphasize the possible heterogeneity of the cause and effect of different stereotypies.154 Despite much work on farm and laboratory species, the proximate causes of stereotypic and redirected behavior in the horse have yet to be verified. However, the possible causal factors associated with oral and locomotory stereotypies are discussed in Chapters 8 and 5, respectively.
The role of genes and environment
Although they have failed to control for variability in management factors, studies into the role that heritability plays in stereotypy frequency have consolidated the view that certain family groups are more likely than others to demonstrate stereotypic behavior.107,191,197 More recently, it has been demonstrated that crib-biting at least is a canine oral stereotypy associated with certain gene arrays.198 Meanwhile a growing branch of the literature identifies the importance of management factors that might frustrate motivation in the horse.115,166 In horses bred for Flat racing, these include the amount and type of forage, the bedding type, the number of horses on the yard and the amount of communication that is possible between neighboring horses.115 A parallel study in dressage and eventing horses demonstrated that the amount of time spent in the stable correlated with the likelihood of stereotypies being reported.199
Although feeding practices tend to have a greater effect than housing practices on the incidence of abnormal behavior,128 a single causative factor is rarely to blame. For example, wood-chewing increased when high-protein rations were fed, because of a concomitant reduction in the total fiber content,109 and when exercise was withdrawn.146 Many authors have suggested other possible causes of anomalous behaviors, including factors associated with weaning, social contact, crowding, feeding, housing and/or training practices.88,200
Some authors135,164 emphasize the importance of mimicry rather than environmental deficits, believing that exposure to a stereotypic neighbor may increase the likelihood of stereotypy development or performance. Such social influences, known in voles,201 may also affect stereotypy levels in horses,202 but the evidence that horses can learn by observation is so far rather limited203–206 Even if observational learning is not involved, we should not rule out the possibility that having a stereotypic neighbor may simply increase the arousal of observing horses and therefore predispose them to developing stereotypic behavior.
An exhaustive study of abnormal behavior on Thoroughbred studs gathered data on more than 11 000 horses, which suggested that, as a result of emancipation (where abnormal behaviors become detached from their initiating causes and thus less responsive to environmental enrichment), the prevalence of stereotypies tends to rise with age (Fig. 1.14).192 Therefore, it seems likely that few horses are ever ‘cured’ of these behaviors. This implies that once these behavioral anomalies are established, they persist to an extent, despite attempts to improve any potentially causative deficiencies in the horse’s management (Fig. 1.15). The continual reinforcement of stereotypic behaviors contributes to their resistance to therapy. That said, in one study effective treatment of crib-biting was offered as a possible reason (along with euthanasia) for the largest percentage prevalence in crib-biting rates being found in foals.207
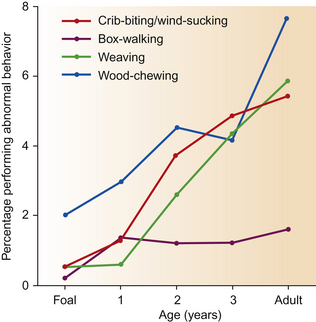
Figure 1.14 The percentage of Thoroughbreds reported as showing stereotypic and redirected behaviors from a sample population of 1023 foals, 746 yearlings, 1001 2-year-olds, 711 3-year-olds and 6250 adults.

Figure 1.15 Two horses crib-biting at pasture. Since they have one another’s company and optimal foraging opportunities they provide a useful example of emancipation.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Fundamentally the possibility of emancipation means that it is better to implement environmental enrichment prophylactically rather than therapeutically. Notwithstanding its effects in maintaining normal behavior in youngstock,208 it is also likely to increase learning ability209 while at the same time promoting musculoskeletal health.210
The process of emancipation also means that stereotypic foals are better research subjects than mature stereotypers. Studies on cohorts of foals from neonates to 4-year-olds have yielded fascinating results. For example, time-related patterns for the development of oral behaviors differ from those of locomotory behaviors.207 This seems to emphasize that a single process such as ‘boredom’ could never fully explain the full spectrum of equine stereotypies. The results of these and other recent studies on oral and locomotory stereotypies are discussed in more detail in Chapters 8 and 5, respectively.
The future holds tremendous promise for sympathetic and productive understanding of equine stereotypies and the responsibility we must take for their emergence and effective management.
Management of stereotypies
Detailed therapeutic approaches to individual stereotypic behaviors will be considered in later parts of this book, but it is worth noting some overarching principles that apply to many of these ethopathies. Of all of the anomalous behavior patterns in horses, stereotypies seem to have proved most insoluble to horse owners. Work on other species by different groups169,174,211–213 often appears contradictory and has highlighted heterogeneity in stereotypies.167 Since the study of equine stereotypies is still in its infancy and because information from other species can be applied only with caution, it is perhaps not surprising that hippologists have found vices so mysterious and frustrating and have met with so little success when attempting to modify these behaviors.135
As the data demonstrating increasing prevalence with age suggest, stereotypic behaviors can become emancipated from their initiating causes.214 Therefore, while stereotypies may arise in response to adverse management, they may persist in more enriched environments. Despite this, owners of stereotypic horses often feel responsible for their charges’ behavioral anomalies. The behaviors are therefore unwelcome not least because they are a potential source of embarrassment to owners.
Stereotypic horses are considered undesirable for many reasons. Generally diminished performance in stereotypers has been identified by some authors,88,215 while health problems associated with these behaviors have been highlighted by others.116,134,216–219 Furthermore, it has been suggested that stereotypic horses in tie-stalls spend less time interacting with neighboring horses.129 Prevention of crib-biting, involving physical or surgical approaches,1,134,220–224 is less commonly attempted presumably because of considerable variation in rates of success. Certainly surgery is falling out of favor as the causes of stereotypies become better understood.
There is recent empirical evidence that observational learning can occur in horses, but this is so far confined to a simple, following response, quite unrelated to the complex and covert oral maneuvers required in, say, crib-biting. Nevertheless, the belief that behaviors can be learnt or copied continues to affect the management of stereotypic horses. Physical prevention of the behaviors is the prevalent response, while isolation of affected horses from other horses is also common (Table 1.4). If these behaviors are not as functionless as assumed, e.g. if they constitute a coping response to a suboptimal environment, the common practice of preventing stereotypic behavior may be of welfare significance. Conversely, managing stereotypic animals so that they are subjected to minimal frustration, e.g. by feeding and exercising before other horses on the yard, is very helpful.
The need to prevent stereotypies for aesthetic and occasional health reasons has prompted searches for permanent cures. A remedy that is effective for every crib-biter remains elusive (but readers are directed to Chapter 8 for a discussion of current humane approaches). The resourcefulness of horses in satisfying their motivation to perform this behavior seems to overwhelm humans’ ability to physically prevent established forms of the behavior. In the light of evidence of brain chemistry differences in affected horses,184 physical and surgical prevention attempts seem especially crude.
Environmental enrichment, often mentioned in the context of captive exotic species, is no less important in the horse. Turning stabled horses out to pasture offers a dramatic illustration of the effects of enrichment. However, for some owners this is not an option. While many owners reach for the blanket term ‘boredom’ to describe the problems faced by the stabled horse, some are beginning to explore ways to increase complexity of stables.226 For example, using a chain in place of the stable door is a surprisingly effective means of increasing the visual stimuli that enter a stable (see Fig. 1.12E). The key with any environmental enrichment is to ensure that the intervention is relevant and unlikely to provide a focus for simple redirection of abnormal behaviors, an outcome that is quite unsatisfactory. By studying this book, and most notably Chapters 5 and 8, readers will be well placed to design enrichment strategies that are likely to meet the behavioral needs of all horses, especially those that exhibit under-stimulation and frustrated motivation.
The web site http://research.vet.upenn.edu/HavemeyerEquineBehaviorLabHomePage/ReferenceLibraryHavemeyerEquineBehaviorLab/LabPublications contains a tremendous amount of information and advice on the behavior and management of stallions. Before attempting to modify the sexual responses of stallions, e.g. for semen collection purposes, readers are advised to visit this site and study the research papers it offers.
Introduction to evaluating behavior problems
Many behaviors labeled abnormal are simply normal but unwelcome. It is the veterinarian’s job to distinguish normal and learned behaviors from those with organic origins. Owners and grooms generally know their horses better than the consulting clinician and therefore it is important to keep one’s counsel until a thorough case history has been taken.
Care should be taken to translate horsey jargon when taking a history. Various terms may be used to describe the same behavior: for example, depending on the observer, a reactive horse may be variously described as sharp, keen, fizzy or flighty.11,227 A survey of horses described by their owners as ‘head-shakers’ identified some that were simply nodding their heads.228 Depending on their professional background, equestrian industry stakeholders seem to have different criteria when identifying horses in behavioral conflict.229 That said, there is evidence that even among a large group of amateurs the use of a human personality inventory achieves high inter-correlations in the ranking of horses, with especially strong agreement being achieved for neuroticism and extraversion.230 The reliability of these assessments increases the legitimacy of everyday use of psychological terms to describe animal behavior.230 It is also encouraging to note that when confined to the use of objective descriptive definitions, riders score horses’ temperaments with significant agreement and that their scores correlate significantly with objective measures from temperament tests.231
Although clients tend to be impressed by shortcuts in history-taking, experience shows this tempting path to be more hazardous than worthwhile. Acquiring only half the salient facts means one is half as likely to emerge with a correct diagnosis and successful therapy. More than dog and cat owners, horse owners tend to seek advice from each other before calling out the veterinarian, so it is often the case that horse-lore has been applied unsuccessfully in the past. At the end of a complete case history, one should have all the benefit of hindsight and therefore make intelligent suggestions based on what is known of previous attempts to remedy the problem. Given the priority that most owners give to ridden horse behavior and the putative link between activities under-saddle and those outside work,232 behavioral practitioners who do not themselves ride may unfortunately lack some authority in equine behavior therapy consultations.
How to take a case history
Behavioral consultations can be considered under two main headings; the background and management details, which constitute a behavioral profile, and the unwanted behavior. Although entirely a matter for personal preference, it seems good practice to establish details of the horse’s management before exploring details of the unwanted behavior. This is because, having acquired a picture of the way the horse sees its world, one can better understand the motivation it may have to deploy unwelcome counter strategies. Box 1.2 gives an example of factors constituting a typical behavioral profile.
Early history and education
Since what age has horse been in present ownership?
Is it still used for this purpose?
If there has been a change, why?
Circumstances at early rearing (numbers, ages and gender of companions, housing, exposure to humans)?
Methods of training? (primarily operant vs classical)a
Do related horses have similar problems?
Do other horses that were raised or trained at the same yard have similar problems?
Environment
a See Chapter 13.
Because such potentially dangerous behaviors as bucking and rearing can be both expressions of joie de vivre and evasions from pain, veterinarians undertaking behavioral consultations should begin by performing physical examinations to eliminate somatic causes before embarking on a program of behavior therapy. This book explores the normal behavior of horses in considerable depth so that practitioners are equipped to consider the adaptive significance of many signs that present as behavioral problems. Horses that shy provide a useful example in that they should undergo ophthalmological examinations that are complemented by information regarding the frequency and variability of shying, and the spatial relationships of objects at which they tend to shy.
Problem behaviors, especially those under-saddle, can be acquired as a result of unfortunate experiences but may also reflect ongoing pain. To some extent it is a shame that the veterinary profession is called upon only as a last resort when a horse starts refusing jumps it is capable of clearing. As a result, lower limb pain is probably under-diagnosed as a cause of such problems.233 The application of various gadgets and escalated coercion often come before a full clinical examination.
Recognition of pain ranks as an extremely important feature of the examination in a clinical case with behavioral manifestations. Houpt234 suggests that a visual appraisal may help to indicate chronic pain, with the affected horse typically having a loose lip but clenched masseter muscles. Meanwhile, there may be a facial expression suggestive of ‘concern’, with decreased mobility of the eyes, puckering of the eyelids, backward inclination of the ears233 and dilation of the nostrils.53 As social herbivores, horses might not be expected to demonstrate signs of pain more overt than teeth-grinding, since to do so may single them out for predation. However, it is proposed that when vocalization accompanies pain, it generally takes the form of a squeal if the pain is of somatic origin and a grunt if visceral.233 While reduced locomotion and postural attempts to shift weight away from the seat of pain can be spotted from a distance, guarding of muscle groups may be appreciated during palpation.
Although the role of neuropathic pain is now recognized in the etiology of headshaking (see Ch. 3),235 it remains unclear why some horses headshake most when being ridden in certain ways, e.g. with a flexed poll. Sometimes the role of pain in emergent unwelcome behavior is not revealed without analgesics. While one horse that is slow to leave from and quick to return to its yard may have become overly bonded to a companion, another may refuse to leave the yard until placed on a course of anti-inflammatory drugs. The latter would be one of those horses that have learned that being ridden, especially on resilient surfaces, is reliably painful and therefore should be avoided.
In behavioral terms, donkeys are not small horses. Behavioral peculiarities in the donkey are sometimes of clinical significance. Compared with horses, donkeys give fewer overt signs of pain, tending instead to lie down or stand still with their heads lowered. Sweating and hyperpnoea are more common responses to abdominal pain than rolling and flank-kicking (Jane French, personal communication). Even during intestinal strangulation or rupture, donkeys rarely lie down and roll but instead show little more than inappetance, a mildly elevated heart rate and a vague reluctance to move.236
Because behavior modification relies on being able to redirect motivation that has prompted unwanted behaviors, it pays to clarify the specifics of any behavior that seems to have departed from the norm. For example, if the animal is described as aggressive, considerably more information is necessary before the motivation to demonstrate agonistic behaviors can be understood. One should establish whether the horse is aggressive to other horses, other domestic species, humans or all of these. Aggression toward specific targets merits further questioning. For example, aggression toward humans can be investigated further with questions such as:
• Under what circumstances is the horse aggressive?
• Does the horse avoid personnel or is it actively aggressive?
• Does the horse target particular individuals or types of people (male, female, large, small) or all people?
The unwanted behavior and the way it has evolved to its current form need to be clearly defined (Box 1.3). Given the apparent inconsistencies in the terminology and interpretation of equine behavior,237 practitioners are commended to equine ethograms designed to establish standard nomenclature for equid behavior.238 That said, there remains a need for a working horse ethogram that offers robust definitions on which the majority of experienced observers can agree.229 Because of the possibility of inconsistent labeling of behaviors,192,235 direct observation or video material is certainly preferable to an oral account of the behavior. Problems in the ridden horse can be fully appreciated only if the patient is ridden saddled and unsaddled and, if feasible and safe, with and without a bit, preferably on more than one occasion by different riders.
• Description of what specifically happens
• Where does the behavior occur?
• When does the behavior occur?
• What was the owner’s reaction?
• What advice has the owner received to date?
• What problems has the owner encountered with any attempts at correction?
• Has there been any change in the frequency or appearance of the problem?
• What will be done with the horse if its behavior does not improve?
With a clear picture of the ontogeny and motivation of the unwelcome behavior, the development of an effective, tailored strategy to remove any reinforcement of the behavior and redirect the horse’s motivation is likely to emerge.239–242 This is behavior modification. Although a number of case studies are presented in this book, readers may find more exhaustive accounts of behavior modification elsewhere (for a series of 60 cases, see Why does my horse…?54). Even when consistently applied, the results of behavior modification programs are rarely immediate in the horse, because of its innate resistance to extinction,88 but they are usually impressive. There have often been years of inconsistent and inappropriate handling which leave habits that must be surmounted before the slate is clean enough for fresh lessons to be learned. It is therefore worth reminding owners that, because horses have excellent memories, ‘quick fixes’ are generally a rarity in equine behavior therapy. Behaviors with their origins in fear are especially prone to re-emerge in response to historic triggers. Some apparently expeditious solutions may mask pain, so they should be used with tremendous caution and only after the role of discomfort has been assessed.
In the following chapters, we will explore the variety of innate and learned behaviors seen in horses. Given this information, the success of behavior therapy depends largely on the versatility of the practitioner and the compliance of the owner.
References
1. Waring GH. Horse behaviour: the behavioural traits and adaptations of domestic and wild horses including ponies. Park Ridge, NJ: Noyes, 1983.
2. Trifonov VA, Stanyon R, Nesterenko AI, et al. Multidirectional cross-species painting illuminates the history of karyotypic evolution in Perissodactyla. Chromosome Res. 2008;16(1):89–107.
3. Piras FM, Nergadze SG, Poletto F, et al. Phylogeny of horse chromosome 5q in the genus Equus and centromere repositioning. Cytogenet Genome Res. 2009;126(1–2):165–172.
4. Hatami-Monagazah H, Pandit RV. A cytogenetic study of the Caspian pony. Journal of Reproduction and Fertility. 1979;57:331–333.
5. Wade CM, Giulotto E, Sigurdsson S, et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326(5954):865–867.
6. Ryder OA. The chromosomes of a rare equine: Equus hemionus kulan. Zoonooz. 1977;50:15.
7. Budiansky S. The nature of horses – exploring equine evolution, intelligence and behavior. New York: Free Press, 1997.
8. Alexander RMcN, Trestik CL. Bistable properties of the hock joint of horses. J Zool. 1989;218:383–391.
9. Mohr E. The Asiatic wild horse. London: Allen, 1971.
10. Boyd L, Bandi N. Reintroduction of Takhi, Equus ferus przewalskii, to Hustai National Park, Mongolia: time budget and synchrony of activity pre- and post-release. Appl Anim Behav Sci. 2002;78(2–4):87–102.
11. King SRB. Home range and habitat use of free-ranging Przewalski horses at Hustai National Park, Mongolia. Appl Anim Behav Sci. 2002;78(2–4):103–113.
12. Christensen JW, Zharkikh T, Ladewig J, Yasinetskaya N. Social behaviour in stallion groups (Equus przewalskii and Equus caballus) kept under natural and domestic conditions. Appl Anim Behav Sci. 2002;76(1):11–20.
13. Goodwin D. The importance of ethology in understanding the behaviour of the horse. The role of the horse in Europe. Equine Vet J Suppl. 1999:15–19.
14. Levine MA. Social evolution and horse domestication. Oxbow Monograph. 1993;33:135–141.
15. Bokonyi S. Evolution of domesticated animals. London: Longman, 1984.
16. Clutton-Brock J. Horse power: a history of the horse and donkey in human societies. In Cambridge, MA. Harvard University Press; 1992.
17. Forsten A. Horse diversity through the ages. Biol Rev. 1989;64:279–304.
18. Bibikova VI. Studies on ancient domestic horses in Eastern Europe. Bjulleten Moscovskogo Obshchestva Ispytatelei Prirody, Otdel Biologicheskii. 1967;22:106–118.
19. Telegin DY, ed. Dereivka: a settlement and cemetery of Copper Age horse keepers on the middle Dneiper [Russian]. Pyatkovsky VK, transl; Mallory JP, ed. Oxford: British Archaeological Reports International Series; 287; 1986.
20. Bibikova VI. A study of the earliest domestic horses of Eastern Europe, parts 1 and 2, reprinted in ‘Dereivka: a settlement and cemetery of Copper Age horse keepers on the middle Dneiper’. Ed: Telegin DY. 1986. Oxford: British Archaeological Reports International Series 1986; 287:135–162.
21. Richardson JD, Cripps PJ, Hillyer MH, et al. An evaluation of the accuracy of ageing horses by their dentition: a matter of experience. Vet Rec. 1995;137(4):88–90.
22. Anthony DW, Brown DR. The origins of horseback riding. Antiquity. 1991;65:22–38.
23. Anthony D, Telegin DY, Brown D. The origin of horseback riding. Scientific American. 1991;12:44–48.
24. Vila C, Leonard JA, Gotherstrom A, et al. Widespread origins of domestic horse lineages. Science. 2001;291(5503):412.
25. Anthony DW. The domestication of the horse. In: Meadow RH, Oerpmann HP, eds. Equids in the ancient world, vol II. Wiesbaden: Rechart Verlag; 1991:250–277.
26. Levine MA. Domestication, breed diversification and early history of the horse. Havemeyer Workshop on Horse Behavior and Welfare, Holar, Iceland 2002; 64–69.
27. Ewers JC. The horse in Blackfoot Indian culture. Bureau of American Ethnology Bulletin. 1955:159.
28. Lawrence EA. Horses in society. In: Rowan AN, ed. Animals and people sharing the world. Hanover, NH: University Press of New England; 1988:95–115.
29. McMiken DF. Ancient origins of horsemanship. Equine Vet J. 1990;22:73–78.
30. Schulman AR. Egyptian representations of horsemen and riding in the new kingdom. J Near East Studies. 1957;16:263–271.
31. Moorey PRS. Pictorial evidence for the history of horse riding in Iraq before the Kassite period. Iraq. 1970;32:36–50.
32. Hancar F. Das pferd in prähistorisches and frühen historisches Zeit. Weiner Beitrage zur Kulturgeschichte and Linguistik, 11. Vienna: Harold; 1955.
33. Azzaroli A. An early history of horsemanship. Leiden: EJ Brill, 1985.
34. Cohen E. Animals in medieval perceptions, the image of ubiquitous other. In: Manning A, Serpell J. Animals and human society, changing perspectives. London: Routledge, 1994.
35. Probst GF. The Kikkuli text on the training of horses (ca. 1350 bc). Lexington: University of Kentucky, King Library Press, 1977.
36. Anderson JK. Ancient Greek horsemanship. Berkeley: University of California Press, 1961.
37. Cohen C, Sivan D. The Ugaritic hippiaric texts: a critical edition. New Haven, CT: American Oriental Society, 1983.
38. Hyland A. Equus, the horse in the Roman world. London: Batsford, 1990.
39. Endenburg N. Perceptions and attitudes toward horses in European societies. The role of the horse in Europe. Equine Vet J Suppl. 1999;28:38–41.
40. Cunningham P. The genetics of Thoroughbred horses. Scientific American. 1991;5:56–62.
41. Mellor DJ, Love S, Reeves MJ, et al. A demographic approach to equine disease in the northern UK through a sentinel practice network. Epidemiol Sante Anim. 1997:31–32.
42. Robinson IH. The human–horse relationship: how much do we know? The role of the horse in Europe. Equine Vet J Suppl. 1999;28:42–45.
43. McGreevy PD. Breeding for Quality of Life. Anim Welf. 2007;16:125–128.
44. Goergen R. Life on the edge. New Scientist. 2001;2305:30–33.
45. Cothran EG, van Dyk E, van der Merwe FJ. Genetic variation in the feral horses of the Namib Desert, Namibia. J S Afr Vet Med Assoc. 2001;72(1):18–22.
46. Clayton HM. Performance in equestrian sports. In: Back W, Clayton HM. Equine locomotion. London: WB Saunders, 2001.
47. Holstrom M, Magnusson LE, Philipsson J. Variation in conformation of Swedish warmblood horses and conformational characteristics of elite sport horses. Equine Vet J. 1990;22:186–193.
48. Holstrom M. The effects of conformation. In: Back W, Clayton HM. Equine Locomotion. London: WB Saunders, 2001.
49. Hayes KN. Temperament tip-offs. Horse and Rider. 1998;37:46–51.
50. Magnusson LE. Studies on the conformation and the related traits of standardbred trotters in Sweden. PhD thesis, Swedish University of Agricultural Sciences, Skara, 1985.
51. Sellet LC, Albert WW, Groppel JL. Forelimb kinematics of the Standardbred pacing gait. Proc Equine Nutr Physiol. 1981:210–215.
52. Akhatova IA. A system for breeding horses for dairy purposes. Zootekhniya. 1995;10:13–15.
53. Fraser AF. The behaviour of the horse. London: CAB International, 1992.
54. McGreevy PD. Why does my horse …? London: Souvenir Press, 1996.
55. Lagoni L, Butler C, Hetts S. The human–animal bond and grief. Philadelphia: WB Saunders, 1994.
56. Digard J-P. Research in the social science of horses: why? And how? The role of the horse in Europe. Equine Vet J Suppl. 1999;28:56–57.
57. Brown D. Personality and gender influences on human relationships with horses and dogs. In: Anderson RK, Hart BL, Hart LA. The pet connection: its influence on our health and quality of life. Minneapolis: Center to Study Human–Animal Relationships and Environments, University of Minnesota; 1984:216–223.
58. Hawson LA, McLean AN, McGreevy PD. The roles of equine ethology and applied learning theory in horse-related human injuries. J Vet Behav. 2010;5(6):324–338.
59. Burch MR, Bustad LK, Duncan SL. The role of pets in therapeutic programmes. In: Robinson IH, ed. The Waltham book of human–animal interaction: benefits and responsibilities of pet ownership. Oxford: Pergamon/Elsevier; 1995:55–69.
60. Brackenridge SS, Shoemaker RS. The human–horse bond and client bereavement in equine practice. Equine Practice. 1996;18:19–22.
61. Rollin BE. Equine welfare and emerging social ethics. Animal Welfare Forum: Equine Welfare. J Am Vet Med Assoc. 2000;216(8):1234–1237.
62. Ewbank R. Contribution of ethology to clinical interpretation of the horse’s welfare. Equine Vet J. 1985;17(1):2–3.
63. Rees L. The horse’s mind. London: Stanley Paul, 1984.
64. Dorrance T. True unity. Tuscarora, NB: Give-it-a-go Enterprises, 1987.
65. Parelli P. Natural horsemanship. Colorado Springs, CO: Western Horseman, 1995.
66. Hunt R. Think Harmony with Horses. Fresno, CA: Pioneer, 1995.
67. McGreevy PD, Oddie C, Burton FL, McLean AN. The horse–human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet J. 2009;181(1):12–18.
68. Galli C, Lagutina I, Crotti G, et al. Pregnancy: A cloned horse born to its dam twin. Nature. 2003;424:635.
69. Wade CM. Mapping cryptic human genetic disorders in the dog and horse. 32nd Annual Lorne Genome Conference 2011 Feb 13th–15th. Mantra Erskine Beach Resort, Lorne, Victoria, Australia: 2011; 60.
70. Fregin GF, Thomas DP. Cardiovascular response to exercise: a review. In: Snow DH, Persson SGB, Rose RJ. Equine exercise physiology. Cambridge: Burlington, 1983.
71. Gaffney B, Cunningham PE. Estimation of genetic trend in racing performance of Thoroughbred horses. Nature. 1988;322(6166):722–724.
72. Gable AA. Metabolic bone disease: problems of terminology. Equine Vet J. 1988;20:4–6.
73. Jeffcott LB. Osteochondroses in the horse – searching for the key to pathogeneses. Equine Vet J. 1991;23:331–338.
74. Freire R, Buckley P, Cooper JJ. Effects of different forms of exercise on post-inhibitory rebound and unwanted behaviour in stabled horses. Equine Vet J. 2009;41:1–6.
75. Galloux P, Richard N, Dronka T, et al. Analysis of equine gait using three dimensional accelerometers fixed on the saddle. Equine Vet J Suppl. 1994;17:44–47.
76. Arabian AK, Clayton HM. Modeling of locomotion. In: Back W, Clayton HM. Equine locomotion. London: WB Saunders; 2001:365–371.
77. Herr HM, McMahon TA. A galloping horse model. Int J Robotics Res. 2001;20(1):26–37.
78. McGreevy PD. The advent of equitation science. Vet J. 2007;174:492–500.
79. Walker RE. Roman veterinary medicine. In: Toynbee J, ed. Animals in Roman life and art. London: Thames & Hudson, 1973.
80. Toynbee J. Animals in Roman life and art. London: Thames & Hudson, 1973. p 343
81. Smith F, The early history of veterinary literature, London: Reprinted. JA Allen, 1976;vol 3p. 17.
82. Holcombe SF, Jackson C, Gerber V, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet J. 2001;33(3):244–249.
83. Hintz HF. Hay racks vs feeding hay on the stall floor. Equine Practice. 1997;19:5–6.
84. Townson J, Dodd VA, Brophy PO. A survey and assessment of racehorse stables in Ireland. Irish Vet J. 1995;48:364–372.
85. Murray MJ, Eichorn ES. Effects of intermittent feed deprivation, intermittent feed deprivation with ranitidine administration, and stall confinement with ad libitum access to hay on gastric ulceration in horses. Am J Vet Res. 1996;11:1599–1603.
86. Harris PA. Review of equine feeding and stable management practices in the UK concentrating on the last decade of the 20th century. The role of the horse in Europe. Equine Vet J Suppl. 1999:46–54.
87. Mills DS, Eckley S, Cooper JJ. Thoroughbred bedding preferences, associated behaviour differences and their implications for equine welfare. Anim Sci. 2000;70(1):95–106.
88. Kiley-Worthington M. The behaviour of horses: in relation to management and training. London: JA Allen, 1987.
89. Houpt KA. Equine welfare. In recent advances in companion animal behavior problems. Ithaca, NY: International Veterinary Information Services; 2001. A0810. 1201
90. Hinton MH. On the watering of horses – a review. Equine Vet J. 1978;10:27–31.
91. UFAW (Scott WN, ed). The care and management of farm animals. London: Baillière Tindall; 1978.
92. Ensminger ME. Horses and horsemanship. Danville, IL: Interstate, 1969.
93. Evans JW, Borton A, Hintz HF, van Vleck LD. The horse. San Francisco: WH Freeman, 1969.
94. Jones RD, McGreevy PD, Robertson A, et al. Survey of the designs of racehorse stables in the South-west of England. Equine Vet J. 1987;19:454–457.
95. Sainsbury DWB, Sainsbury P. Livestock health and housing, 2nd edn. London: Baillière Tindall, 1979.
96. Cooper JJ, McDonald L, Mills DS. The effect of increasing visual horizons on stereotypic weaving: implications for the social housing of stabled horses. Appl Anim Behav Sci. 2000;69:67–83.
97. Mills DS, Davenport K. The effect of a neighbouring conspecific versus the use of a mirror for the control of stereotypic weaving behaviour in the stabled horse. Anim Sci. 2002;74:95–101.
98. Søndergaard E, Ladewig J. Group housing exerts a positive effect on the behaviour of young horses during training. Appl Anim Behav Sci. 2004;87:105–118.
99. Nicol CJ, Badnell-Waters AJ. Suckling behaviour in domestic foals and the development of abnormal oral behaviour. Anim Behav. 2005;70:21–29.
100. Nicol CJ, Badnell-Waters AJ, Bice R, et al. The effects of diet and weaning method on the behaviour of young horses. Appl Anim Behav Sci. 2005;95:205–221.
101. Ratner SC, Boice R. Effects of domestication on behaviour. In: Hafez ESE, ed. The behaviour of domestic animals. London: Baillière Tindall; 1975:3–19.
102. Rossdale PD. Modern stud management in relation to the oestrus cycle and fertility of Thoroughbred mares. Equine Vet J. 1968;2:65–72.
103. Harman AM, Moore S, Hoskins R, Keller P. Horse vision and an explanation of the visual behaviour originally explained by the ‘ramp retina’. Equine Vet J. 1999;31(5):384–390.
104. Wiepkema PR. Umwelt and animal welfare. In: Baxter SH, Baxter MR, MacCormack JAC. Farm animal housing and welfare. The Hague: Martinus Nijhoff; 1983:45–51.
105. Dawkins MS. The current status of preference tests in the assessment of animal welfare. In: Baxter SH, Baxter MR, MacCormack JAC. Farm animal housing and welfare. The Hague: Martinus Nijhoff; 1983:20–26.
106. Duncan IJH. An ethological approach to the welfare of farm animals. In: Proceedings 19th International Ethological Conference, Toulouse, France, 1985: 295–305.
107. Marsden MD. An investigation of the heredity of susceptibility of stereotypic behaviour pattern (stable vices) in the horse. Equine Vet J. 1995;27(6):415.
108. Thorne JB, Goodwin D, Kennedy MJ, et al. Foraging enrichment for individually housed horses: Practicality and effects on behaviour. Appl Anim Behav Sci. 2005;94:149–164.
109. Ralston SL, van den Brock G, Baile CA. Feed intake patterns and associated blood glucose free fatty acid and insulin changes in ponies. J Anim Sci. 1979;49:838–845.
110. Ralston SL. Feeding behaviour. Vet Clin North Am Equine Pract: Behav. 1986;2:609–621.
111. McBane S. Behaviour problems in horses. London: David & Charles, 1987. p 304
112. Ruckebusch Y. The hypnogram as an index of adaptation of farm animals to changes in their environment. Appl Anim Ethol. 1975;2:3–8.
113. Dallaire A. Rest behaviour. Vet Clin North Am Equine Pract: Behav. 1986;2:591–607.
114. Dallaire A, Ruckebusch Y. Sleep and wakefulness in the housed pony under different dietary conditions. Can J Compar Med. 1974;38:65–71.
115. McGreevy PD, Cripps PJ, French NP, et al. Management factors associated with stereotypic and redirected behaviour in the Thoroughbred horse. Equine Vet J. 1995;27:86–91.
116. Summerhays RS. The problem horse. London: JA Allen, 1975. p 93
117. Houpt KA, Hintz HF, Pagan JD. The responses of mares and their foals to brief separation. J Anim Sci. 1981;53:129.
118. Houpt KA, Hintz HF, Butler WR. A preliminary study of two methods of weaning foals. Appl Anim Behav Sci. 1984;12:1877–1881.
119. McCall CA, Potter GD, Kreider JL. Locomotor, vocal and other behavioural responses to varying methods of weaning foals. Appl Anim Behav Sci. 1985;14:27–35.
120. Mal ME, Friend TH, Lay DC, et al. Physiological responses of mares to short-term confinement and isolation. J Equine Vet Sci. 1991;11:96–102.
121. Keiper RR. Social structure. Vet Clin North Am Equine Pract: Behav. 1986;2:465–484.
122. Feh C, de Mazieres J. Grooming at a preferred site reduces heart rate in horses. Anim Behav. 1993;46:1191–1194.
123. Dougherty DM, Lewis P. Generalization of a tactile stimulus in horses. J Exp Anal Behav. 1993;59:521–528.
124. Zeitler-Feicht MH, Buschmann S. [Are standing stalls for horses acceptable today with regard to animal welfare?] (German). Pferdeheilkunde. 2002;18(5):431.
125. Houpt K, Houpt TR, Johnson JL, et al. The effect of exercise deprivation on the behaviour and physiology of straight-stall confined pregnant mares. Anim Welfare. 2001;10(3):257–267.
126. Rivera E, Benjamin S, Nielsen B, et al. Behavioral and physiological responses of horses to initial training: the comparison between pastured versus stalled horses. Appl Anim Behav Sci. 2002;78(2–4):235–252.
127. Nicol CJ. Behavioural responses of laying hens following a period of spatial restriction. Anim Behav. 1987;35:1709–1719.
128. Marsden MD. Feeding practices have greater effect than housing practices on the behaviour and welfare of the horse. Livestock Environment IV, 4th International Symposium of the American Society of Agricultural Engineers, University of Warwick, Coventry, 1993:314–318.
129. Flannigan G, Stookey JM. Day-time time budgets of pregnant mares housed in tie stalls: a comparison of draft versus light mares. Appl Anim Behav Sci. 2002;78(2–4):125–143.
130. Jones B, McGreevy PD. Ethical equitation: applying a cost-benefit approach. J Vet Behav. 2010;5:196–202.
131. Racklyeft DJ, Love DN. Influence of head posture on the respiratory tract of healthy horses. Aust Vet J. 1990;67:402–405.
132. Rose RJ. The TG Hungerford Vade Mecum series for domestic animals. Sydney, Australia: Number 1: Horses, p 24.Post-graduate Committee in Veterinary Science, 1983.
133. Richardson DW. Degenerative joint disease. In: Robinson NE, ed. Current therapy in equine medicine. Philadelphia: WB Saunders; 1992:137–140.
134. Schramm U. The trouble with horses. Bury St Edmunds, Suffolk: JA Allen, 1988. p 124
135. Hayes MH. Veterinary notes for horse-owners. London: Stanley Paul, 1968.
136. Fraser A, Manolson F. Fraser’s horse book. London: Pan, 1979.
137. Farm Animal Welfare Council – second report on priorities for research and development in farm animal welfare. MAFF, Tolworth; 1993.
138. Webster AJF. Animal welfare: a cool eye towards Eden. London: Blackwell Science, 1994.
139. Summerhays RS. The problem horse. London: JA Allen, 1959.
140. Hamilton S. The vice squad: conquering your horse’s behavioural quirks. Equus. 1980;32:68–71.
141. Fraser AF, Broom DM. Farm animal behaviour and welfare. London: Baillière Tindall, 1990.
142. Francis-Smith K, Wood-Gush DGM. Coprophagia as seen in Thoroughbred foals. Equine Vet J. 1977;9:155–157.
143. Crowell-Davis SL, Houpt KA. Coprophagia by foals: effects of age and possible functions. Equine Vet J. 1985;17:17–19.
144. Crowell-Davis SL, Caudle AB. Coprophagy by foals: recognition of maternal faeces. Appl Anim Behav Sci. 1989;24:267–272.
145. Crowell-Davis SL, Houpt KA, Carnevale JM. Feeding and drinking behaviour of mares and foals with free access to pasture and water. J Anim Sci. 1985;60:883–889.
146. Krzak WE, Gonyou HW, Lawrence LM. Wood-chewing by stabled horses: diurnal pattern and effects of exercise. J Anim Sci (USA). 1991;69:1053–1058.
147. McCall CA. Wood-chewing by horses. Equine Practice. 1993;15:35–36.
148. Bush K. The problem horse – an owner’s guide. Marlborough, Wilts, UK: Crowood Press, 1992. p 160
149. Buntain BJ, Coffmann JR. Polyuria and polydipsia in a horse induced by psychogenic salt consumption. Equine Vet J. 1981;13:266–268.
150. Lieberman DA. Learning: behavior and cognition. Pacific Grove, CA: Brooks/Cole, 1993.
151. Murray MJ, Crowell-Davis S. Psychogenic colic in a horse. J Am Vet Med Assoc. 1985;186:381–383.
152. Beaver B. Aggressive behaviour problems. Vet Clin North Am Equine Pract: Behav. 1986;2:609–621.
153. Zeitler-Feicht MH, Prantner V. Recumbence resting behaviour of horses in loose housing systems with open yards. Arch Tierzucht. 2000;43(4):327–335.
154. Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037.
155. Xenopohon. The art of horsemanship (400 bc). Morgan MH, transl. London: JA Allen; 1999.
156. Clutton-Brock J, Davies S. More donkeys from Tell Brak. Iraq. 1993;55:209–221.
157. Bahn PG. Crib-biting: tethered horses in the Palaeolithic? World Archaeology. 1980;12:212–217.
158. Lawson A. The modern farrier. London: Newcastle, Mackenzie & Dent, 1828. 47
159. Youatt W. The horse. London: Charles Knight, 1846. 448–451
160. Lupton JI. Evils of modern stables. In Mayhew’s illustrated horse management. London: WH Allen; 1884.
161. Holzapfel M. Uber Bewegungsstereotypien bei gehalten Sangetieren; 2. Mitteilung: das Weben der Pferde. Z Tierpsychol. 1939:60–72.
162. Boyd LE. Behaviour problems of equids in zoos. Vet Clin North Am Equine Pract: Behav. 1986;2:653–664.
163. Meyer-Holzapfel M. Abnormal behavior in zoo animals. In: Fox MW, ed. Abnormal behavior in animals. Philadelphia: WB Saunders; 1968:476–503.
164. Sambraus HH, Rappold D. Crib-biting and wind-sucking in horses. Pferdeheilkunde. 1991;7:211–216.
165. Mills DS, Alston RD, Rogers V, Longford NT. Factors associated with the prevalence of stereotypic behaviour amongst thoroughbred horses passing through auctioneer sales. Appl Anim Behav Sci. 2002;78(2–4):115–124.
166. Nicol CJ. Stereotypies and their relation to management. In: Harris PA, Gomarsall GM, Davidson HPB, Green RE, eds. Proceedings of the BEVA specialist days on behaviour and nutrition, 1999:11–14.
167. Mason GJ. Forms of stereotypic behaviour. In: Lawrence AB, Rushen J. Stereotypic animal behaviour: fundamentals and applications to welfare. London: CAB International, 1993.
168. Broom DM. Animal welfare: concepts and measurements. J Anim Sci. 1991;69:4167–4175.
169. Cooper JJ, Nicol CJ. Stereotypic behaviour affects environmental preference in bank voles (Clethrionomys glareolus). Anim Behav. 1991;41:971–977.
170. Fentress JC. Dynamic boundaries of patterned behaviour: interaction and self-organization. In: Bateson PPG, Hinde RA. Growing points in ethology. Cambridge: Cambridge University Press; 1976:135–167.
171. Levine MA, Weinberg J, Ursin H. Definition of the coping process and statement of the problem. In: Ursin H, Baade E, Levine S. Psychobiology of stress: a study of coping men. New York: Academic Press; 1978:3–21.
172. McBride G. Adaptation and welfare at the man–animal interface. In: Wodzickka M, Edey TN, Lynch JJ. Reviews in rural science IV. Armidale, NSW: University of New England, 1980.
173. Wood-Gush DGM, Stolba A, Miller C. Exploration in farm animals and animal husbandry. In: Archer J, Birke LIA. Exploration in animals and humans. London: Van Nostrand Reinhold; 1983:198–209.
174. Kennes D, de Rycke PH. Influences of performance of stereotypies on plasma corticosterone and leucocyte levels in the bank vole (Clethrionomys glareolus). In: Proceedings of the International Congress on Applied Ethology in Farm Animals, 1988:238–240.
175. Terlouw EMC, Lawrence AB, Ladewig J, et al. Relationship between plasma cortisol and stereotypic activities in pigs. Behav Processes. 1991;25:133–153.
176. Würbel H, Stauffacher M. Prevention of stereotypy in laboratory mice: effects on stress physiology and behaviour. Physiol Behav. 1996;59(6):1163–1170.
177. McGreevy PD, Nicol CJ. Behavioural and physiological consequences associated with the short-term prevention of crib-biting in horses. Physiol Behav. 1998;65(1):15–23.
178. McBride SD, Cuddeford D. The putative welfare-reducing effects of preventing equine stereotypic behaviour. Anim Welfare. 2001;10(2):173–189.
179. Lebelt D, Zanella AJ, Unshelm J. Physiological correlates associated with cribbing behaviour in horses: changes in thermal threshold, heart rate, plasma beta-endorphin and serotonin. Equine Vet J Suppl. 1998;27:21–27.
180. Minero M, Canali E, Ferrante V, Verga M, Ödberg FO. Heart rate and behavioural responses of crib-biting horses to two acute stressors. Vet Rec. 1999;145(15):430–433.
181. Gillham SR, Dodman NH, Shuster L, et al. The effect of diet on cribbing behaviour and plasma ß-endorphin in horses. Appl Anim Behav Sci. 1994;41:147–153.
182. Dodman NH, Shuster L, Court MH, Dixon R. Investigation into the use of narcotic antagonists in the treatment of a stereotypic behavior pattern (crib-biting) in the horse. Am J Vet Res. 1987;48:311–319.
183. McBride S. A comparison of physical and pharmacological treatments for stereotypic behaviour in the horse. In: Duncan IJH, Widowski TM, Haley DB. Proceedings of the 30th International Congress for the International Society of Applied Ethology. Guelph, Canada: CSAW, 1996.
184. McBride SD, Hemmings A. Altered mesoaccumbens and nigro-striatal dopamine physiology is associated with stereotypy development in a non-rodent species. Behav Brain Res. 2005;159:113–118.
185. Garner JP, Mason GJ, Broadbent H. Cage stereotypy in Blue tits (Parus caeruleus) and Marsh tits (Parus palustris) is associated with a deficit in the inhibition of non-functional behaviour. In: Veissier I, Boissy A. Proceedings of the 32nd International Congress of the ISAE. Clermont-Ferrand, France: INRA; 1998:56.
186. Garner JP, Meehan CL, Mench JA. Stereotypic parrots fail the same psychiatric task as stereotypic autists and schizophrenics. In: Garner JP, Mench JA, Heekin S. Proceedings of the 35th International Congress of the ISAE. Davis, CA: Center For Animal Welfare, University of California at Davis, 2001.
187. Parker M, Redhead ES, Goodwin D, McBride SD. Impaired instrumental choice in crib-biting horses (Equus caballus). Behav Brain Res. 2008;191:137–140.
188. Luescher UA, McKeown DB, Dean H. A cross-sectional study on compulsive behaviour (stable vices) in horses. Equine Vet J Suppl. 1998;27:14–18.
189. Redbo I, Redbo-Tortensson P, Ödberg FO, et al. Factors affecting behavioural disturbances in racehorses. Anim Sci. 1998;66:475–481.
190. Prince D. Stable vices. In: McBane S, ed. Behaviour problems in horses. London: David & Charles, 1987.
191. Vecchiotti G, Galanti R. Evidence of heredity of cribbing, weaving and stall-walking in Thoroughbred horses. Livestock Production Sci. 1986;14:91–95.
192. McGreevy PD. The functional significance of stereotypies in the stabled horse. PhD thesis. University of Bristol, UK; 1995.
193. Ödberg FO. The jumping stereotypy in the bank vole (Clethrionomys glareolus). Biol Behav. 1986;11:130–143.
194. Morgan MJ. Effects of post-weaning environment on learning in the rat. Anim Behav. 1973;21:429–442.
195. Appleby MC, Lawrence AB. Food restriction as a cause of stereotypic behaviour in tethered gilts. J Anim Production. 1987;45:103–110.
196. Duncan IJH, Rushen J, Lawrence AB. Conclusions and implications for animal welfare. In: Lawrence AB, Rushen J. Stereotypic animal behaviour: fundamentals and applications to welfare. London: CAB International, 1993.
197. Hosoda T. On the heritability of susceptibility to windsucking in horses. Jpn J Zootechnical Sci. 1950;21:25–28.
198. Dodman NH, Karlsson EK, Moon-Fanelli A, Galdzicka M, et al. A canine chromosome 7 locus confers compulsive disorder susceptibility. Mol Psych. 2010;15:8–10.
199. McGreevy PD, French NP, Nicol CJ. The prevalence of abnormal behaviours in dressage, eventing and endurance horses in relation to stabling. Vet Rec. 1995;137:36–37.
200. Luescher UA, McKeown DB, Halip J. Reviewing the causes of obsessive-compulsive disorders in horses. Equine Pract Vet Med. 1991;5(91):527–530.
201. Cooper JJ, Nicol CJ. Neighbour effects on the development of locomotor stereotypies in bank voles (Clethrionomys glareolus). Anim Behav. 1994;47:214–216.
202. Houpt KA, McDonnell SM. Equine stereotypies. The Compendium of Continuing Education for the Practicing Veterinarian. 1993;15:1265–1272.
203. Baer KL, Potter GD, Friend TH, Beaver BV. Observation effects on learning in horses. Appl Anim Ethol. 1983;11:123–129.
204. Baker AEM, Crawford BH. Observational learning in horses. Appl Anim Behav Sci. 1986;15:7–13.
205. Clarke J, Nicol CJ, Jones R, McGreevy PD. Effects of observational learning on food selection in horses. Appl Anim Behav Sci. 1996;50:177–184.
206. Krueger K, Heinze J. Horse sense: social status of horses (Equus caballus) affects their likelihood of copying other horses’ behaviour. Anim Cogn. 2008;3:431–439.
207. Waters AJ, Nicol CJ, French NP. Factors influencing the development of stereotypic and redirected behaviours in young horses: findings of a four year prospective epidemiological study. Equine Vet J. 2002;34(6):572–579.
208. Heleski CR, Shelle AC, Nielsen BD, Zanella AJ. Influence of housing on weanling horse behavior and subsequent welfare. Appl Anim Behav Sci. 2002;78(2–4):297–308.
209. Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behaviour. Behav Brain Res. 1996;78:57–65.
210. Bell RA, Nielsen BD, Waite K, et al. Daily access to pasture turnout prevents loss of mineral in the third metacarpus of Arabian weanlings. J Anim Sci. 2001;79(5):1142–1150.
211. Keiper RR. Causal factors of stereotypies in caged birds. Anim Behav. 1969;17:114–117.
212. Dantzer R, Mormede P. De-arousal properties of stereotypic behaviour: evidence from pituitary-adrenal correlates in pigs. Appl Anim Ethol. 1983;10:233–244.
213. Cronin GM, Wiepkema PR, Van Ree JM. Endogenous opioids are involved in abnormal stereotyped behaviour in tethered sows. Neuropeptides. 1985;6:527.
214. Cooper JJ, Ödberg FO. The emancipation of stereotypies with age. In: Appleby MC, Horrell RI, Petherick JC, Rutter SM. Applied Animal behaviour: past, present and future. Hertfordshire: UFAW; 1991:142.
215. Straiton EC. The TV vet horse book. Suffolk: Farming Press, 1973. p 193
216. Steele DG. El Cribbing (pica) y la aerofagia en los pura sangre. Ganaderia. 1960;18:74–78.
217. Karlander S, Mansson J, Tufvesson G. Buccostomy as a method of treatment for aerophagia (wind-sucking) in the horse. Nordisk Veterinär Medicin. 1965;17:455–458.
218. Hachten W. Cribbing treatment. The Equine Athlete. 1995;8:20–21.
219. Malamed R, Berger J, Bain MJ, et al. Retrospective evaluation of crib-biting and windsucking behaviours and owner-perceived behavioural traits as risk factors for colic in horses. Equine Vet J. 2010;42(8):686–692.
220. Baker GJ, Kear-Colwell J. Aerophagia (windsucking) and aversion therapy in the horse. Proc Am Assoc Equine Practitioners. 1974;20:127–130.
221. Hamm D. A new surgical procedure to control crib-biting. Proc Am Assoc Equine Practitioners. 1977;23:301–302.
222. Firth EC. Bilateral ventral accessory neurectomy in windsucking horses. Vet Rec. 1980;106:30–32.
223. Hakansson A, Franzen P, Petersson H. Comparison of two surgical methods for treatment of crib-biting in horses. Equine Vet J. 1992;24:494–496.
224. Broom DM, Kennnedy MJ. Stereotypies in horses: their relation to welfare and causation. Equine Vet Ed. 1993;5:151–154.
225. McBride SD, Long L. Management of horses showing stereotypic behaviour, owner perception and the implications for welfare. Vet Rec. 2001;148(26):799–802.
226. Goodwin D, Davidson HPB, Harris P. Foraging enrichment for stabled horses: effects on behaviour and selection. Equine Vet J. 2002;34(7):686–691.
227. Mills DS. Personality and individual differences in the horse, their significance, use and measurement. Equine Vet J Suppl. 1998;27:10–13.
228. Taylor K, Cook S, Mills DS. A case-controlled study investigating health, management and behavioural features of horses commonly described as headshakers. Havemeyer Workshop on Horse Behavior and Welfare, 2002: 16–20.
229. Nyman S, Björk J, Puronne C, Roepstorff L. How is conflict in the horse evaluated by different categories of horse professionals? In: Hartmann E, Blokhuis MZ, Fransson C, Dalin G, eds. Proceedings of the 6th International Equitation Science Symposium. Uppsala, Sweden, 2010; 10.
230. Morris PH, Gale A, Duffy K. Can judges agree on the personality of horses? Personality and Individual Differences. 2002;33(1):67–81.
231. Visser EK, van Reenen CG, Rundgren M, et al. Responses of horses in behavioural tests correlate with temperament assessed by riders. Equine Vet J. 2003;35(2):176–183.
232. McLean AN. The mental processes of the horse and their consequences for training. University of Melbourne, 2005. PhD thesis
233. Casey R. Recognising the importance of pain in the diagnosis of equine behaviour problems. In: Harris PA, Gomarsall GM, Davidson HPB, Green RE, eds. Proceedings of the BEVA specialist days on behaviour and nutrition, 1999: 25–28.
234. Houpt KA. Domestic animal behaviour for veterinarians and animal scientists, 3rd edn. London: Manson, 1998.
235. Taylor KD, Cook S, Mills DS. A case-controlled study investigating health, management and behavioural features of horses commonly described as headshakers. Ippologia. 2001;12:29–37.
236. Taylor TS, Matthews NS. Mammoth asses – selected behavioural considerations for the veterinarian. Appl Anim Behav Sci. 1998;60(2–3):283–289.
237. Houpt KA, Rudman R. Foreword to special issue on equine behaviour. Appl Anim Behav Sci. 2002;78(2–4):83–85.
238. McDonnell SM. The equid ethogram: a practical field guide to horse behavior. Lexington, KY: The Blood Horse, 2003.
239. McCall CA. A review of learning behavior in horses and its application in horse training. J Anim Sci. 1990;68:75–81.
240. Mills DS. Applying learning theory to the management of the horse: the difference between getting it right and getting it wrong. Equine Vet J Suppl. 1998;27:44–48.
241. Cooper JJ. Comparative learning theory and its application in the training of horses. Equine Vet J Suppl. 1998;27:39–43.
242. Barakat C, McGreevy PD. How cribbing takes hold. Equus. 2002;297:34–43.