CHAPTER 34 Larynx
The larynx is an air passage, a sphincter and an organ of phonation, and extends from the tongue to the trachea. It projects ventrally between the great vessels of the neck and is covered anteriorly by skin, fasciae and the hyoid depressor muscles. Above, it opens into the laryngopharynx and forms its anterior wall; below, it continues into the trachea (see Fig. 33.2). It is mobile on deglutition. At rest, the larynx lies opposite the third to sixth cervical vertebrae in adult males; it is somewhat higher in children and adult females. In infants between 6 and 12 months, the tip of the epiglottis (the highest part of the larynx) lies a little above the junction of the dens and body of the axis vertebra. Until puberty, male and female larynges are similar in size. After puberty, the male larynx enlarges considerably in comparison with that of the female: all the cartilages increase in both size and weight, the thyroid cartilage projects in the anterior midline of the neck, and its sagittal diameter nearly doubles. The male thyroid cartilage continues to increase in size until 40 years of age, after which no further growth occurs.
SKELETON OF THE LARYNX
The skeletal framework of the larynx is formed by a series of cartilages interconnected by ligaments and fibrous membranes, and moved by a number of muscles (Figs 34.1-34.3). The hyoid bone is attached to the larynx: it is usually regarded as a separate structure with distinctive functional roles, and is described on page 436. The laryngeal cartilages are the single thyroid, cricoid and epiglottic cartilages, and the paired arytenoid, cuneiform, corniculate and tritiate cartilages.
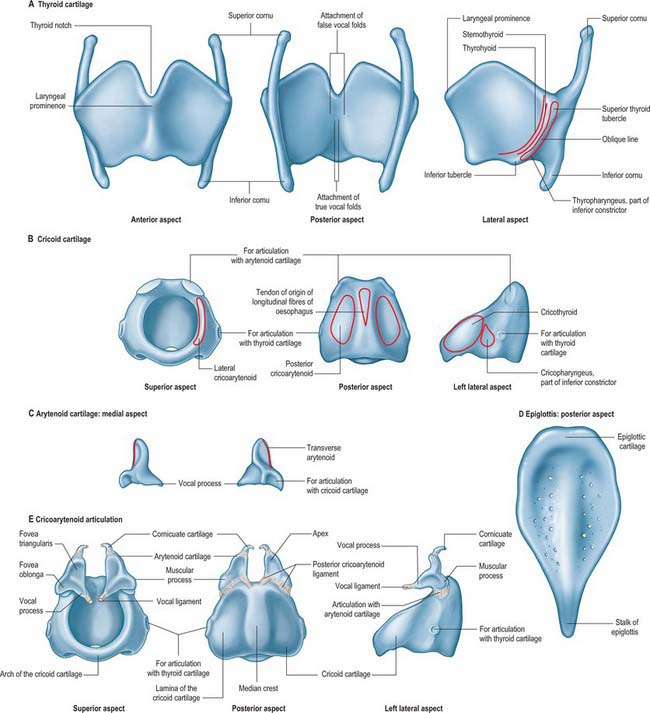
Fig. 34.3 Cartilages of the larynx: thyroid (A), cricoid (B), arytenoid (C), epiglottis (D), cricoarytenoid joint (E). The attachments of the false vocal folds (vestibular ligaments) (above) and the true vocal folds (vocal ligaments) (below) are shown in A, posterior aspect. Note the pitted surface of the epiglottis (D).
In relation to the surface anatomy of the larynx, the levels of the laryngeal cartilages worth noting are: C3 (level of body of hyoid and its greater cornu); C3–4 junction (level of upper border of thyroid cartilage and bifurcation of common carotid artery); C4–5 junction (level of thyroid cartilage); C6 (level of cricoid cartilage).
The corniculate, cuneiform, tritiate and epiglottic cartilages and the apices of the arytenoid are composed of elastic fibrocartilage, with little tendency to calcify. The thyroid, cricoid and the greater part of the arytenoid cartilages consist of hyaline cartilage and may undergo mottled calcification as age advances, starting about the 25th year in the thyroid cartilage and somewhat later in the cricoid and arytenoids. By the 65th year, these cartilages commonly appear patchily dense in radiographs.
EPIGLOTTIS
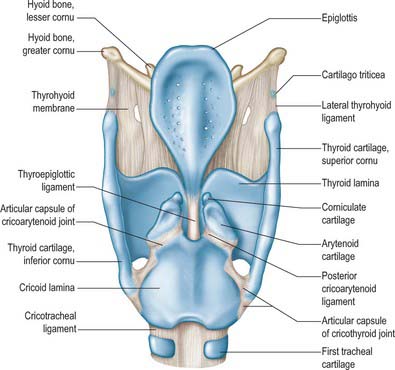
The epiglottis is a thin leaf-like plate of elastic fibrocartilage which projects obliquely upwards behind the tongue and hyoid body, and in front of the laryngeal inlet (Figs 34.2, 34.3; see Fig. 34.5). Its free end, which is broad and round, and occasionally notched in the midline, is directed upwards. Its attached part, or stalk (petiolus), is long and narrow and is connected by the elastic thyroepiglottic ligament to the back of the laryngeal prominence of the thyroid cartilage just below the thyroid notch. Its sides are attached to the arytenoid cartilages by aryepiglottic folds (which contain the aryepiglottic muscle). Its free upper anterior, or lingual, surface is covered by mucosa (the epithelium is non-keratinized stratified squamous), which is reflected onto the pharyngeal aspect of the tongue and the lateral pharyngeal walls as a median glossoepiglottic, and two lateral glossoepiglottic, folds. There is a depression, the vallecula, on each side of the median fold. The lower part of its anterior surface, behind the hyoid bone and thyrohyoid membrane, is connected to the upper border of the hyoid by an elastic hyoepiglottic ligament, and separated from the thyrohyoid membrane by adipose tissue, which constitutes the clinically important preepiglottic space. The smooth posterior, or laryngeal, surface is transversely concave and vertically concavo-convex, and is covered by ciliated respiratory mucosa: its lower projecting part is called the tubercle. This surface forms the oblique anterior wall of the laryngeal vestibule. The cartilage is posteriorly pitted by small mucous glands (Fig. 34.3D) and is perforated by branches of the internal laryngeal nerve and fibrous tissue, which means that the posterior surface of the epiglottis is in continuity through these perforations with the pre-epiglottic space.
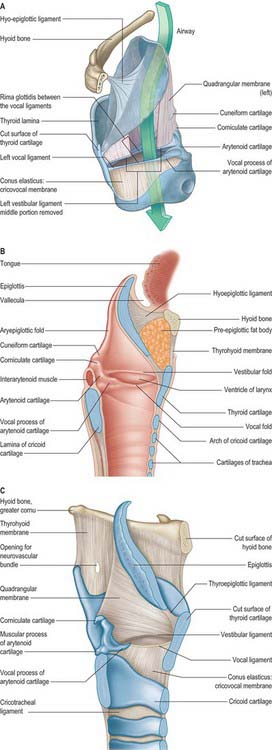
Fig. 34.5 A and B, Sagittal sections of the left side of the larynx, showing the laryngeal membranes (A) and the interior aspect (B) of the left half of the larynx. C, The quadrangular membrane viewed from the left side.
(A, From Drake, Vogl and Mitchell 2005.)
Functions of the epiglottis
During swallowing, the hyoid bone moves upwards and forwards, and the epiglottis is bent posteriorly as a result of passive pressure from the base of the tongue and active contraction of the aryepiglottic muscles. Though the epiglottis is not essential to swallowing, which can occur with minimal aspiration even if the epiglottis is destroyed by disease, it diverts food and liquids away from the laryngeal inlet and into the lateral food channels. It is not essential for respiration or phonation.
THYROID CARTILAGE
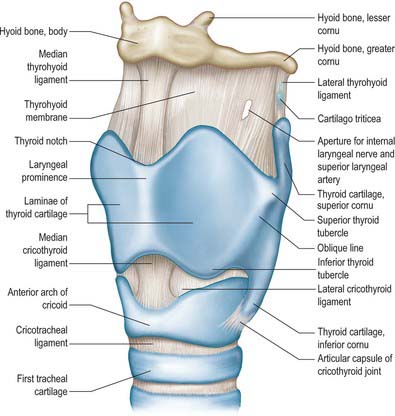
The thyroid cartilage is the largest of the laryngeal cartilages (Figs 34.1-34.3). It consists of two quadrilateral laminae with anterior borders that fuse along their inferior two-thirds at a median angle to form the subcutaneous laryngeal prominence (‘Adam’s apple’). This projection is most distinct at its upper end, and is well marked in men but scarcely visible in women. Above, the laminae are separated by a V-shaped superior thyroid notch or incisure. Posteriorly, the laminae diverge, and their posterior borders are prolonged as slender horns, the superior and inferior cornua. A shallow ridge, the oblique line, curves downwards and forwards on the external surface of each lamina: it runs from the superior thyroid tubercle lying a little anterior to the root of the superior cornu, to the inferior thyroid tubercle on the inferior border of the lamina. Sternothyroid, thyrohyoid and thyropharyngeus (part of the inferior pharyngeal constrictor) are attached to the oblique line, usually as little more than a tendon (Fig. 34.3A).
The internal surface of the lamina is smooth. Above and behind, it is slightly concave and covered by mucosa. The thyroepiglottic ligament, the paired vestibular and vocal ligaments, the thyroarytenoid, thyroepiglottic and vocalis muscles, and the stalk of the epiglottis are all attached to the inner surface of the cartilage, in the angle between the laminae. The true vocal folds lie 6–9 mm below the median thyroid notch. The superior border of each lamina is concave behind and convex in front, and the thyrohyoid membrane is attached along this edge (Figs 34.1, 34.2). The inferior border of each lamina is concave behind and nearly straight in front, and the two parts are separated by the inferior thyroid tubercle. Anteriorly, the thyroid cartilage is connected to the cricoid cartilage by the anterior (median) cricothyroid ligament, which is a thickened portion of the cricothyroid membrane.
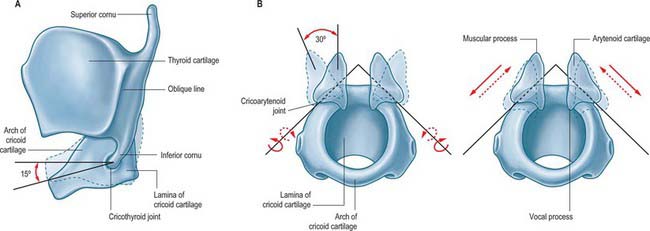
The anterior border of each thyroid lamina fuses with its partner at an angle of approximately 90° in men and approximately 120° in women. The shallower angle in men is associated with the larger laryngeal prominence, the greater length of the vocal cords, and the resultant deeper pitch of the voice. The posterior border is thick and rounded and receives fibres of stylopharyngeus and palatopharyngeus. The superior cornu, which is long and narrow, curves upwards, backwards and medially, and ends in a conical apex to which the lateral thyrohyoid ligament is attached. The inferior cornu is short and thick, and curves down and slightly anteromedially. On the medial surface of its lower end there is a small oval facet for articulation with the side of the cricoid cartilage: this facet is variable and is well defined only sometimes.
During infancy, a narrow, rhomboidal, flexible strip, the intra-thyroid cartilage, lies between the two laminae, and is joined to them by fibrous tissue.
CRICOID CARTILAGE
The cricoid cartilage is attached below to the trachea, and articulates with the thyroid cartilage and the two arytenoid cartilages by synovial joints. It forms a complete ring around the airway, the only laryngeal cartilage to do so (Fig. 34.3B). It is smaller, but thicker and stronger, than the thyroid cartilage, and has a narrow curved anterior arch, and a broad, flatter posterior lamina.
Cricoid arch
The cricoid arch is vertically narrow in front (5–7 mm in height), and widens posteriorly towards the lamina. Cricothyroid is attached to the external aspect of its front and sides, and cricopharyngeus (part of the inferior pharyngeal constrictor) is attached behind cricothyroid. The arch is palpable below the laryngeal prominence, from which it is separated by a depression containing the resilient cricothyroid membrane. The inferior border of the cartilage is nearly horizontal and is circular in outline, whereas the upper border is more elliptical.
Cricoid lamina
The cricoid lamina is approximately quadrilateral in outline, and 2–3 cm in vertical dimension. It bears a posterior median vertical ridge that creates posterior concavities on either side. The two fasciculi of the longitudinal layer of oesophageal muscle fibres (muscularis externa) are attached by a tendon to the upper part of the ridge. Posterior cricoarytenoid attaches to a shallow depression on either side of the ridge.
A discernible circular synovial facet, facing posterolaterally, sometimes marks the junction of the lamina and arch: it indicates the site where the cricoid articulates with the inferior thyroid cornu. The inferior border of the cricoid is horizontal, and joined to the first tracheal cartilage by the cricotracheal ligament (Fig. 34.1). The superior border runs obliquely up and back, and gives attachment anteriorly to the thick median part of the cricothyroid membrane, and laterally to the membranous parts of the cricothyroid membrane (Fig. 34.1) and lateral cricoarytenoid. The posterosuperior aspect of the lamina presents a shallow median notch, on each side of which is a smooth, oval, convex facet, directed upwards and laterally, for articulation with the base of an arytenoid cartilage.
The internal surface of the cricoid cartilage is smooth and lined by mucosa.
Subglottic stenosis
Congenital malformation of the cricoid cartilage may result in severe narrowing of the subglottic airway and respiratory obstruction, which, in severe cases, is present from birth. It is the third most common congenital disorder of the larynx. Acquired subglottic stenosis is more common and is the result of trauma and scarring following prolonged endotracheal intubation for the purposes of ventilation of premature babies on intensive care units.
ARYTENOID CARTILAGE
The paired arytenoid cartilages articulate with the lateral parts of the superior border of the cricoid lamina (Figs 34.2, 34.3). Each is pyramidal, and has three surfaces, two processes, a base and an apex. The posterior surface, which is triangular, smooth and concave, is covered by transverse arytenoid. The anterolateral surface is convex and rough, and bears, near the apex of the cartilage, an elevation from which a crest curves back, down and then forwards to the vocal process. The lower part of this arcuate crest separates two depressions (foveae). The upper is triangular (fovea triangularis), and the vestibular ligament is attached to it. The lower is oblong (fovea oblonga), and vocalis and lateral cricoarytenoid are attached to it. The medial surface is narrow, smooth and flat, and is covered by mucosa: its lower edge forms the lateral boundary of the intercartilaginous part of the rima glottidis. The base is concave, with a smooth surface for articulation with the lateral part of the upper border of the cricoid lamina. Its round, prominent lateral angle, or muscular process, projects backwards and laterally: it gives attachment to posterior cricoarytenoid behind, and lateral cricoarytenoid in front. The vocal ligament is attached to its pointed anterior angle (vocal process), which projects horizontally forward. The apex curves backwards and medially and articulates with the corniculate cartilage.
CORNICULATE CARTILAGES
The corniculate cartilages are two conical nodules of elastic fibrocartilage which articulate with the apices of the arytenoid cartilages, prolonging them posteromedially (Fig. 34.3E). They lie in the posterior parts of the aryepiglottic mucosal folds, and are sometimes fused with the arytenoid cartilages.
CUNEIFORM CARTILAGES
The cuneiform cartilages are two small, elongated, club-like nodules of elastic fibrocartilage, one in each aryepiglottic fold anterosuperior to the corniculate cartilages, and are visible as whitish elevations through the mucosa (see Fig. 34.5).
TRITIATE CARTILAGES (CARTILAGO TRITICEA)
The tritiate cartilages are two small nodules of elastic cartilage, situated one on either side above the larynx within the posterior free edge of the thyrohyoid membrane, about halfway between the superior cornu of the thyroid cartilage and the tip of the greater cornu of the hyoid bone (Figs 34.1, 34.2). Their functions are unknown, although they may serve to strengthen this connection.
CALCIFICATION OF LARYNGEAL CARTILAGES
The thyroid, cricoid, and most of the arytenoid cartilages consist of hyaline cartilage, and may therefore become calcified. This process normally starts at about 18 years of age. Initially it involves the lower and posterior part of the thyroid cartilage, and subsequently spreads to involve the remaining cartilages, calcification of the arytenoid cartilage starting at its base. The degree and frequency of calcification of the thyroid and cricoid cartilages appear to be less in females. There is some evidence to suggest that a predilection for tumour invasion may be enhanced by calcification of the laryngeal cartilages.
The tip and upper portion of the vocal process of the arytenoid cartilage consists of non-calcifying, elastic cartilage. This may have considerable functional significance: the vocal process may bend at the elastic cartilage during adduction and abduction, and the two arytenoid cartilages will contact mainly at their ‘elastic’ superior portions during adduction.
JOINTS
CRICOTHYROID JOINT
The joints between the inferior cornua of the thyroid cartilage and the sides of the cricoid cartilage are synovial. Each is enveloped by a capsular ligament strengthened posteriorly by fibrous bands (Figs 34.1, 34.2, 34.4). Both capsule and ligaments are rich in elastin fibres. The primary movement at the joint is rotation around a transverse axis which passes transversely through both cricothyroid joints. The effect of this rotation is to move the cricoid and thyroid cartilages relative to one another in such a way as to bring together the lamina of the thyroid cartilage and the arch of the cricoid cartilage (‘closing the visor’). There is some controversy as to which cartilage moves, but it seems most likely that the cricoid cartilage rotates to a greater extent. When the joint is in a neutral position, the ligaments are slack and the cricoid can glide, to a limited extent, in different directions on the thyroid cornua. The effect of these movements is to lengthen the vocal folds, provided the arytenoid cartilages are stabilized at the cricoarytenoid joint. This may also increase vocal fold tension.
CRICOARYTENOID JOINT
The cricoarytenoid joints are a pair of synovial joints between the facets on the lateral parts of the upper border of the lamina of the cricoid cartilage and the bases of the arytenoids. Each joint is enclosed by a capsular ligament and strengthened by a ligament that, although traditionally called the posterior cricoarytenoid ligament, is largely medial in position (Figs 34.1-34.4).
The cricoid facets are elliptical, convex and obliquely directed laterally, anteriorly and downwards. The long axes of the two facets intersect posteriorly at an angle of about 50°. Two movements occur at this joint. The first is rotation of the arytenoid cartilages at right angle to the long axis of the cricoid facet (dorso-medio-cranial to ventro-latero-caudal), which, because of its obliquity, causes each vocal process to swing laterally or medially, thereby increasing or decreasing the width of the rima glottidis. This movement is sometimes referred to as a rocking movement of the arytenoid cartilages. There is also a gliding movement, by which the arytenoids approach or recede from one another, the direction and slope of their articular surfaces imposing a forward and downward movement on lateral gliding. The movements of gliding and rotation are associated, i.e. medial gliding occurs with medial rotation and lateral gliding with lateral rotation, resulting in adduction or abduction of the vocal folds respectively. When viewed from above, foreshortening can give the illusion that the arytenoid cartilages are rotating about their vertical axes, but the shape of the facets prevents such movement occurring. The posterior cricoarytenoid ligaments limit forward movements of the arytenoid cartilages on the cricoid cartilage. It has been suggested that the ‘rest’ position of the cricoarytenoid ligament is a major determinant of the position of a denervated vocal cord.
ARYTENOCORNICULATE JOINTS
Synovial or cartilaginous joints link the arytenoid and corniculate cartilages.
INNERVATION OF THE CRICOTHYROID, CRICOARYTENOID AND ARYTENOCORNICULATE JOINTS
The cricothyroid, cricoarytenoid and arytenocorniculate joints are innervated by branches of the recurrent laryngeal nerves, which arise either independently or from branches of the nerve to the laryngeal muscles. The capsules of the laryngeal joints contain numerous lamellated (Pacinian) corpuscles, Ruffini corpuscles and free nerve endings.
SOFT TISSUES
The skeletal framework of the larynx is joined to surrounding structures by extrinsic membranes. It is also interconnected by intrinsic ligaments and fibroelastic membranes, of which the thyrohyoid, quadrangular and cricothyroid membranes and the conus elasticus are the most significant. The thyrohyoid membrane is external to the larynx, whereas the paired quadrangular membranes, the cricothyroid membrane and the conus elasticus are internal. The named ligaments are the median (anterior) cricothyroid ligament, the hyoepiglottic and thyroepiglottic ligaments and the cricotracheal ligament.
EXTRINSIC LIGAMENTS AND MEMBRANES
Thyrohyoid membrane
The thyrohyoid membrane is a broad, fibroelastic layer attached below to the superior border of the thyroid cartilage lamina and the front of its superior cornua, and above to the superior margin of the body and greater cornua of the hyoid (Figs 34.1, 34.2, 34.5). It thus ascends behind the concave posterior surface of the hyoid, separated from its body by a bursa which facilitates the ascent of the larynx during swallowing. Its thicker part is the median thyrohyoid ligament. The more lateral, thinner, parts are pierced by the superior laryngeal vessels and internal laryngeal nerves (Fig. 34.1). Externally, it is in contact with thyrohyoid and omohyoid and the body of the hyoid bone. Its inner surface is related to the lingual surface of the epiglottis and the piriform fossae of the pharynx. The round, cord-like, elastic lateral thyrohyoid ligaments form the posterior borders of the thyrohyoid membrane, and connect the tips of the superior thyroid cornua to the posterior ends of the greater hyoid cornua (Fig. 34.1).
Hyo- and thyroepiglottic ligaments
The epiglottis is attached to the hyoid bone and thyroid cartilage by the extrinsic hyoepiglottic and intrinsic thyroepiglottic ligaments respectively.
Cricotracheal ligament
The cricotracheal ligament unites the lower border of the cricoid to the first tracheal cartilage, and is thus continuous with the perichondrium of the trachea (Fig. 34.1).
INTRINSIC LIGAMENTS AND MEMBRANES
The fibroelastic membrane of the larynx lies within the cartilaginous skeleton of the larynx, beneath the laryngeal mucosa (Fig. 34.5). It forms a discontinuous sheet separated on both sides of the larynx by a horizontal cleft between the vestibular and vocal ligaments. Its upper part, the quadrangular membrane, extends between the arytenoid cartilages and the sides of the epiglottis. Its lower part, the cricothyroid membrane and conus elasticus, connects the thyroid, cricoid and arytenoid cartilages.
Quadrangular membrane
Each quadrangular membrane passes from the lateral margin of the epiglottis to the ipsilateral arytenoid cartilage. It is often poorly defined, especially in its upper portion. The upper and lower borders of the membrane are free. The upper border slopes posteriorly to form the aryepiglottic ligament, which constitutes the central component of the aryepiglottic fold. Posteriorly, it passes through the fascial plane of the oesophageal suspensory ligament, and helps to form the median corniculopharyngeal ligament which extends into the submucosa adjacent to the cricoid cartilage. This ligament may exert vertical traction on the tissues of the laryngopharynx. The cuneiform cartilages lie within the aryepiglottic folds. The lower border of the quadrangular membrane forms the vestibular fold.
Cricothyroid membrane and the conus elasticus
The cricothyroid ligament is composed mainly of elastic tissue. It consists of two parts: the cricothyroid membrane below and the conus elasticus above.
Cricothyroid membrane and median (anterior) cricothyroid ligament
The cricothyroid membrane passes upwards from the upper border of the cricoid cartilage to the lower border of the thyroid cartilage. Anteriorly, it is thickened to form the median (anterior) cricothyroid ligament, which is broader below and narrower above.
In part or in whole, the conus elasticus has been variously described as the cricovocal membrane, cricothyroid ligament or lateral cricothyroid ligament. The conus elasticus is thinner than the anterior cricothyroid ligament. It arises beneath the cricothyroid membrane on both sides from the inner surface of the cricoid cartilage, near its lower margin, and passes upwards beneath the lower border of the thyroid cartilage. It is attached anteriorly to the inner surface of the angle of the thyroid cartilage (just below its midpoint) and posteriorly to the tip of the vocal process of the arytenoid cartilage. Between these attachments, the upper edge of the conus elasticus is free, horizontally aligned and thickened to form the vocal ligament, which lies within the mucosa-covered vocal fold. It is covered internally by mucosa and externally by lateral cricoarytenoid and thyroarytenoid. The conus elasticus derives its name from the cone or funnel shape produced by the superior and medial curving of its walls between its inferior and superior attachments. This is said to maximize efficient flow of air towards the rima glottidis during phonation.
LARYNGEAL CAVITY
The laryngeal cavity extends from the laryngeal inlet (from the pharynx) down to the lower border of the cricoid cartilage, where it continues into the trachea (Figs 34.5, 34.6). The walls of the cavity are formed of the fibroelastic membranes described above and are lined with mucous membrane which folds over the free edges of these fibroelastic membranes within the larynx. On either side, the continuity of the fibroelastic membrane is interrupted between the upper and lower folds.
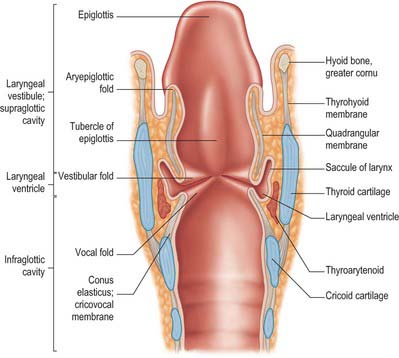
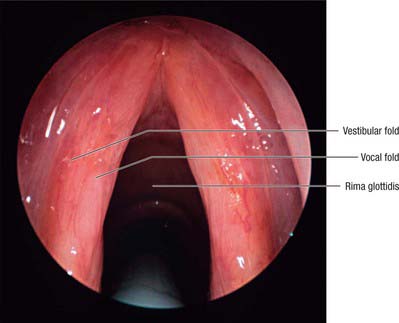
The folds project into the lumen of the cavity and divide it into upper and lower parts, separated by a middle portion between the two sets of folds leading into the laryngeal ventricle. The upper folds are the vestibular (ventricular or false vocal) folds; the median aperture which they guard is the rima vestibuli. The lower pair are the (true) vocal folds (or vocal cords), and the fissure between them is the rima glottidis or glottis. The true vocal folds are the primary source of phonation, whereas the vestibular folds normally do not contribute directly to sound production. The clinical term supraglottis refers to the part of the larynx that lies above the glottis and comprises the laryngeal inlet formed of the laryngeal surface of the epiglottis and arytenoid cartilages and the laryngeal aspects of the aryepiglottic folds, the laryngeal vestibule (introitus) and the vestibular folds.
MICROSTRUCTURE OF THE LARYNX
The laryngeal mucosa is continuous with that of the pharynx above and the trachea below. It lines the entire inner surface of the larynx, including the ventricle and saccule, and is thickened over the vestibular folds, where it is the chief component. Over the vocal folds it is thinner, and is firmly attached to the underlying vocal ligaments. It is loosely adherent to the anterior surface of the epiglottis, but firmly attached to its anterior surface and the floor of the valleculae. On the aryepiglottic folds it is reinforced by a considerable amount of fibrous connective tissue, and it adheres closely to the laryngeal surfaces of the cuneiform and arytenoid cartilages.
The laryngeal epithelium is mainly a ciliated, pseudostratified respiratory epithelium where it covers the inner aspects of the larynx, including the posterior, laryngeal surface of the epiglottis, and it provides a mucociliary clearance mechanism shared with most of the respiratory tract (see Ch. 57). However, the vocal folds are covered by non-keratinized, stratified squamous epithelium where they contact each other: this important variation protects the tissue from the effects of the considerable mechanical stresses that act on the surfaces of the vocal folds. The exterior surfaces of the larynx, which merge with the laryngopharynx and oropharynx (including the anterior, lingual surface of the epiglottis and the aryepiglottic folds), are subject to the abrasive effects of swallowed food, and are therefore covered by non-keratinized, stratified squamous epithelium.
The laryngeal mucosa has numerous mucous glands, especially over the epiglottis, where they pit the cartilage, and along the margins of the aryepiglottic folds anterior to the arytenoid cartilages, where they are known as the arytenoid glands. Many large glands in the saccules of the larynx secrete periodically over the vocal folds during phonation. The free edges of these folds are devoid of glands, and their stratified epithelium is vulnerable to drying and requires the secretions of neighbouring glands: hoarseness as a result of excessive speaking is due to partial temporary failure of this secretion. The epithelial surfaces are ridged and this may help retain the lubricating secretions over the surfaces of the edges of the folds. Poorly lubricated folds offer increased resistance to airflow, which means that higher subglottal pressures are needed to initiate phonation. Taste buds, like those in the tongue, occur on the posterior epiglottic surface, aryepiglottic folds and less often in other laryngeal regions.
UPPER PART
The upper part of the laryngeal cavity contains the laryngeal inlet (aditus), the aryepiglottic fold and the laryngeal vestibule (introitus).
Laryngeal inlet (aditus)
The upper part of the laryngeal cavity is entered by the laryngeal inlet (aditus laryngis), the aperture between the larynx and pharynx. This faces backwards and somewhat upwards, because the anterior wall of the larynx is much longer than the posterior (and slopes downwards and forwards in its upper part because of the oblique inclination of the epiglottis). The inlet is bounded anteriorly by the upper edge of the epiglottis, posteriorly by the transverse mucosal fold between the two arytenoids (posterior commissure), and on each side by the edge of a mucosal ridge, the aryepiglottic fold, that runs between the side of the epiglottis and the apex of the arytenoid cartilage. The midline groove between the two corniculate tubercles is termed the interarytenoid notch.
Aryepiglottic fold
The aryepiglottic fold contains ligamentous and muscular fibres. The ligamentous fibres represent the free upper border of the quadrangular membrane (Fig. 34.5). The muscle fibres are continuations of the oblique arytenoids. The posterior part of the aryepiglottic fold contains two oval swellings, one above and in front, the other behind and below, that mark the positions of the underlying cuneiform and corniculate cartilages respectively. They are separated by a shallow vertical furrow which is continuous below with the opening of the laryngeal ventricle.
Laryngeal vestibule (introitus)
Vestibule is a clinical term that denotes the space between the laryngeal inlet and vestibular folds. It is wide above, narrow below, and higher anteriorly than posteriorly. Its anterior wall is formed by the posterior surface of the epiglottis, the lower part of which (epiglottic tubercle) bulges backwards a little. Its lateral walls, which are higher in front and shallow behind, are formed by the medial surfaces of the aryepiglottic folds. Its posterior wall consists of the interarytenoid mucosa above the ventricular folds.
MIDDLE PART
The middle part of the laryngeal cavity is the smallest, and extends from the rima vestibuli above to the rima glottidis below. On each side it contains the vestibular folds, the ventricle and the saccule of the larynx.
Vestibular folds and ligaments
The narrow vestibular ligament represents the thickened lower border of the quadrangular membrane (Fig. 34.5). It is fixed in front to the thyroid angle below the epiglottic cartilage and behind to the anterolateral surface of the arytenoid cartilage above its vocal process. With its covering of mucosa, it is termed the vestibular (ventricular or false vocal) fold (Figs 34.5, 34.6). The presence of a loose vascular mucosa lends the vestibular folds a pink appearance in vivo, as they lie above and lateral to the vocal cords.
Ventricle (sinus) of the larynx
The laryngeal ventricle is a slit between the vestibular and vocal cords (Figs 34.5, 34.6). It opens into a fusiform recess on each side of the larynx and extends upwards into the laryngeal wall lateral to the vestibular fold, opening into the saccule.
Saccule of the larynx
The saccule is a pouch which ascends forwards from the ventricle, between the vestibular fold and thyroid cartilage, and occasionally reaches the upper border of the cartilage (Fig. 34.6). It is conical, and curves slightly backwards; 60–70 mucous glands, sited in the submucosa, open onto its luminal surface. The orifice of the saccule is guarded by a delicate fold of mucosa, the ventriculosaccular fold.
The saccule has a fibrous capsule that is continuous below with the vestibular ligament. It is covered medially by a few muscular fasciculi from the apex of the arytenoid cartilage which pass forwards between the saccule and vestibular mucosa into the aryepiglottic fold, and laterally it is separated from the thyroid cartilage by the thyroepiglottic muscle. The latter compresses the saccule, expressing its secretion onto the vocal cords, which lack glands, to lubricate and protect them against desiccation and infection. Saccules occasionally protrude through the thyrohyoid membrane.
Laryngoceles and saccular cysts
Laryngoceles and saccular cysts are air- or fluid-filled enlargements of the saccule. A laryngocele is a herniation of the saccular mucosa. The aetiology is uncertain: repeated sustained high transglottal pressures (such as in trumpet playing) may be a possible cause of acquired symptoms, and some cases may be the result of congenital enlargement of the saccule. Growth of a laryngocele is constrained by the surrounding tissues, and so it expands upwards into the paraglottic space anterior to the piriform fossa, and superiorly to expand the aryepiglottic fold and reach the vallecula (internal laryngocele). It can extend to the thyrohyoid membrane, which it may pierce to form an external laryngocele, and where it may be palpable in the neck. Symptoms include hoarseness, stridor and dysphagia. The laryngeal saccule can also become pathologically enlarged as a result of obstruction of the ventricular aditus by inflammation, scarring, or compression by a tumour: an expanding mucus-filled cyst forms as the glandular secretions accumulate. These fluid-filled saccular cysts can expand in a similar direction to a laryngocele and may also pierce the thyrohyoid membrane. In addition to hoarseness and stridor, acute respiratory obstruction may occur, especially in the young, if the contents of the cyst become infected.
Vocal folds (cords) and ligaments
The free thickened upper edge of the conus elasticus forms the vocal ligament. It stretches back on either side from the mid level of the thyroid angle to the vocal processes of the arytenoids. When covered by mucosa, it is termed the vocal fold or vocal cord (cord is the preferred clinical term) (Figs 34.5, 34.6). The vocal folds form the anterolateral edges of the rima glottidis and are concerned with sound production. Each fold consists of five layers, namely mucosa, lamina propria (three layers) and the vocalis muscle (Fig. 34.7).
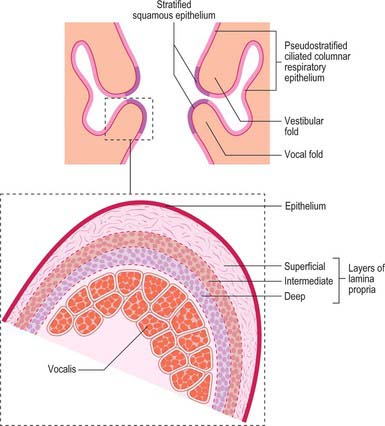
Fig. 34.7 Coronal view of the laryngeal cavity, showing the distribution of the mucous membrane in the laryngeal cavity; the inset shows the structure of the true vocal folds.
The mucosa overlying the vocal ligament is thin and attached to the underlying lamina propria by a basement membrane. It lies directly on the ligament, and so the vocal fold appears pearly white in vivo. The lamina propria consists of three layers. The most superficial consists of loose collagen and elastic fibres, and is only loosely attached to the underlying vocal ligament, an arrangement that produces a potential space (Reinke’s space) that extends along the length of the free margin of the vocal ligament and a little way onto the superior surface of the cord: oedema fluid readily collects here in disease. The intermediate layer consists of elastic fibres, and the deep layer is formed of collagen fibres; these two layers collectively form the vocal ligament. Fibres of the vocalis muscle form the fifth layer of the vocal folds. The site where the vocal folds meet anteriorly is known as the anterior commissure, and is the region where fibres of the vocal ligament pass through the thyroid cartilage to blend with the overlying perichondrium, forming Broyle’s ligament. Since Broyle’s ligament contains blood vessels and lymphatics, it represents a potential route for the escape of malignant tumours from the larynx.
Reinke’s oedema
The mucous membrane is loosely attached throughout the larynx, and can accommodate considerable swelling which may compromise the airway in acute infections. At the edge of the true vocal folds the mucosal covering is tightly bound to the underlying ligament so that oedema fluid does not pass between the upper and lower compartments of the vocal cord mucosa. Any tissue swelling above the vocal cord exaggerates the potential space deep to the mucosa (Reinke’s space), causing accumulation of extracellular fluid and flabby swelling of the vocal cords (Reinke’s oedema). The oedema can persist because of the poor lymphatic drainage in this region of the larynx. Vocal abuse may initiate such changes, but the condition is nearly always confined to smokers.
Vocal cord nodules
Vocal fold nodules are chronic lesions of the vocal folds and develop most commonly as the result of persistent overuse of the voice which has caused an increase in vocal fold tension and a more forceful adduction. They normally develop at the point of maximum contact of the vocal folds, i.e. at the junction of the anterior third and the posterior two-thirds of the vocal ligament. Excessive trauma at this point, for example when singing with poor technique or forcing the voice, initially produces subepithelial haemorrhage or bruising; in time this results in pathological changes such as subepithelial scarring (‘singer’s nodes’ or ‘clergyman’s nodes’). Nodules increase vocal fold mass and affect vocal fold closure: the persistent posterior glottal opening causes hoarseness, a breathy voice, reduced vocal intensity and an inability to produce higher frequencies of vibration. These changes can cause a cycle in which increasing vocal effort is required by way of compensation, and this exacerbates the problem.
Rima glottidis
The rima glottidis or glottis is the fissure between the vocal cords anteriorly and the arytenoid cartilages posteriorly (see Figs 34.8, 34.9). It is bounded behind by the mucosa that passes between the arytenoid cartilages at the level of the vocal cords. The glottis is customarily divided into two regions, an anterior intermembranous part, which makes up about three-fifths of its anteroposterior length and is formed by the underlying vocal ligament, and a posterior intercartilaginous part formed by the vocal processes of the arytenoid cartilages. It is the narrowest part of the larynx, having an average sagittal diameter in adult males of 23 mm, and in adult females of 17 mm: its width and shape vary with the movements of the vocal cords and arytenoid cartilages during respiration and phonation (see p. 590).
LOWER PART
The lower part of the laryngeal cavity, the subglottis or infraglottic cavity, extends from the vocal cords to the lower border of the cricoid. In transverse section it is elliptical above and wider and circular below, and is continuous with the trachea. Its walls are lined by respiratory mucosa, and supported by the cricothyroid ligament above and the cricoid cartilage below. The walls of this part of the laryngeal cavity are said to be exponentially curved, a feature that may serve to accelerate the airflow towards the glottis with the minimum loss of energy.
LARYNGOSCOPIC EXAMINATION
The laryngeal inlet, the structures around it, and the cavity of the larynx can all be inspected using fibreoptic endoscopy, through either the mouth or nasopharynx. The epiglottis is seen foreshortened, but its tubercle is visible. From the epiglottic margins the aryepiglottic folds can be traced posteromedially and the cuneiform and corniculate elevations recognized. The pink vestibular folds and pearly white vocal cords are visible and, when the rima glottidis is wide open, the anterior arch of the cricoid cartilage, the tracheal mucosa and cartilages may be seen (Fig. 34.8). The piriform fossae can also be inspected.
LARYNGEAL OBSTRUCTION AND TRAUMA
The mucosa of the upper larynx is highly sensitive, and contact with foreign bodies excites immediate coughing. Large foreign bodies may obstruct the laryngeal inlet or rima glottidis and suffocation may ensue. Smaller articles may enter the trachea or bronchi, or lodge in the laryng-eal ventricle and cause reflex closure of the glottis with subsequent suffocation. Inflammation of the upper larynx, e.g. secondary to infection or the effects of smoke inhalation, may swell the mucosa by effusion of fluid into the loose submucous tissue (oedema of the supraglottis). The effusion neither involves nor extends below the vocal cords, because the mucosa here is bound directly to the vocal ligaments and there is no submucous tissue. Laryngotomy below the vocal cords through the cricothyroid ligament, or tracheotomy below the cricoid cartilage, may be necessary to restore a free airway.
Suicidal wounds are usually made through the thyrohyoid membrane, damaging the epiglottis, superior thyroid vessels, external and internal carotid arteries and internal jugular veins. Less frequently, these wounds are inflicted above the hyoid, so that the lingual muscles and lingual and facial vessels are damaged.
THE INFANT LARYNX
The infant larynx differs markedly from its adult counterpart. Although it is about one-third adult size, it is proportionately larger. Its lumen is short and funnel-shaped and disproportionately narrower than that of the adult. It lies higher in the neck than the adult larynx (see Fig. 33.10). At rest, the upper border of the infant epiglottis is at the level of the second or third cervical vertebra; when the larynx is elevated, it reaches the level of the first cervical vertebra. This high position enables an infant to use its nasal airway to breathe while suckling. The epiglottis is X-shaped, with a furled petiole, and the laryngeal cartilages are softer and more pliable than in the adult larynx (which may predispose to airway collapse in inspiration, leading to the clinical picture of laryngomalacia). The thyroid cartilage is shorter and broader and lies closer to the hyoid bone in the neonate, which means that the thyrohyoid ligament is relatively short. Neither the superior notch nor the laryngeal prominence are as marked as they are in the adult. The cricoid cartilage is the same shape as in the adult. The arytenoid cartilages are more prominent and the aryepiglottic folds are disproportionally large. The vocal cords are 4–4.5 mm long, which is relatively shorter than in either childhood or the adult. The ventricle of the larynx is small, whereas the saccule of the larynx is often considerably larger than it is in adult life.
The mucosa of the supraglottis is more loosely attached than it is in the adult larynx and it exhibits multiple submucosal glands. Inflammation of the supraglottis will therefore rapidly result in gross oedema. The mucosa is also lax in the subglottis, the narrowest part of the infant larynx (3.5 mm in diameter in neonates). Swelling at this point rapidly results in severe respiratory obstruction because of the disproportionally narrower lumen. Unlike in the adult, the neonatal subglottic cavity extends posteriorly as well as inferiorly, which is an important consideration when passing an endotracheal tube.
By about the third year, sexual differences become apparent: the larynx is larger in boys, although the angle between the thyroid laminae is more pronounced in girls. At puberty these changes increase, and there is greater enlargement of the male larynx.
PARALUMENAL SPACES
A number of potential spaces lie between the laryngeal cartilages and the ligaments and membranes that support them. The three main spaces are the pre-epiglottic, the paraglottic and the subglottic spaces. Their precise definition, and the extent to which they communicate with one another, remain controversial. They are not closed compartments and so their existence does not preclude the spread of tumours. An awareness of the anatomy of these spaces, and the potential pathways of spread of tumours from them, has significantly influenced the surgical approach to disease in this region.
PRE-EPIGLOTTIC SPACE
Its name implies that the pre-epiglottic space lies anterior to the epiglottis; in reality, it also extends beyond the lateral margins of the epiglottis, an arrangement that gives it the form of a horseshoe. The space is primarily filled with adipose tissue and does not appear to contain any lymph nodes.
The upper boundary is formed by the weak hyoepiglottic membrane, strengthened medially as the median hyoepiglottic ligament. The anterior boundary is the thyrohyoid membrane, strengthened medially as the median thyrohyoid ligament, and the lower boundary is the thyroepiglottic ligament, continuous laterally with the quadrangular membrane behind. The greater cornu of the hyoid bone forms its upper lateral border. Inferolaterally, the pre-epiglottic space is in continuity with the paraglottic space, from where it is often invaded by the laryng-eal saccule. It is also in continuity with the mucosa of the laryngeal surface of the epiglottis via multiple perforations in the cartilage of the epiglottis. Malignancies of the laryngeal surface of the epiglottis may invade the fat and areolar tissue of the pre-epiglottic space through these perforations.
PARAGLOTTIC SPACE
The paraglottic space is a region of adipose tissue that contains the internal laryngeal nerve, the laryngeal ventricle, and all or part of the laryngeal saccule. It is bounded laterally by the thyroid cartilage and thyrohyoid membrane, superomedially by the quadrangular membrane, inferomedially by the conus elasticus, and posteriorly by the piriform fossa. The lower border of the thyroid cartilage is inferior, and the paraglottic space is continuous inferiorly with the space between the cricoid and thyroid cartilages. Anteroinferiorly, there are deficiencies in the paramedian gap at the side of the anterior cricothyroid ligament, and posteroinferiorly, adipose tissue extends towards the cricothyroid joint. Some authorities exclude thyroarytenoid from the paraglottic space. Superiorly, the space is usually continuous with the pre-epiglottic space, although the two spaces may be separated by a fibrous septum.
Supraglottic tumours may spread into the paraglottic space and reach the subglottis, or extend beyond the limits of the larynx. Ventricular tumours may obstruct mucus outflow from the saccule and cause its expansion within the paraglottic space to form a saccular cyst: the tumour itself may also spread transglottically, and thereby fix the vocal cord either by invasion of the cricoarytenoid joint or by damaging the recurrent laryngeal nerve. Fixation of the vocal cord is a good indicator of a tumour within the paraglottic space. The proximity of the mucosa at the piriform fossa makes its removal in surgery mandatory for such disease.
MUSCLES
The muscles of the larynx may be divided into extrinsic and intrinsic groups. The extrinsic muscles connect the larynx to neighbouring structures and are responsible for moving it vertically during phonation and swallowing. They include the infrahyoid strap muscles, thyrohyoid, sternothyroid and sternohyoid, and the inferior constrictor muscle of the pharynx. Two of the three elevator muscles of the pharynx, stylopharyngeus and palatopharyngeus, are also connected directly to the thyroid cartilage, mainly to the posterior aspect of the thyroid laminae and cornua.
The role of the extrinsic muscles during respiration appears to be variable: the larynx has been seen to rise, descend or barely move during inspiration. The extrinsic muscles can affect the pitch and the quality of the voice by raising or lowering the larynx, and geniohyoid elevates and anteriorly displaces the larynx, particularly during deglutition.
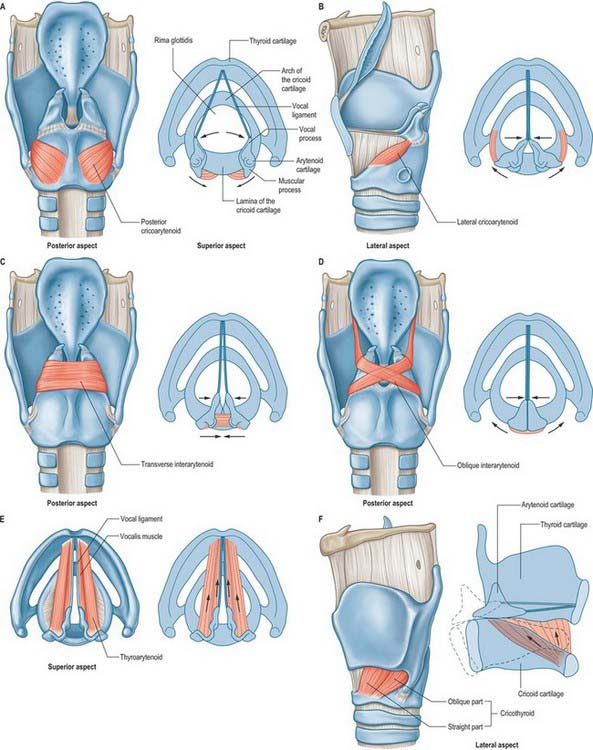
The intrinsic muscles are the cricothyroid, posterior and lateral cricoarytenoid, transverse and oblique arytenoid, aryepiglotticus, thyroarytenoid and its subsidiary part, vocalis, and thyroepiglotticus: all are confined to the larynx in their attachments, and all but the transverse arytenoid are paired (Fig. 34.9). Whereas most of the intrinsic muscles lie internally, under cover of the thyroid cartilage or the mucosa, the cricothyroids appear on the outer aspect of the larynx.
The intrinsic laryngeal muscles may be placed in three groups according to their main actions. The posterior and lateral cricoarytenoids and oblique and transverse arytenoids vary the dimensions of the rima glottidis. The cricothyroids, posterior cricoarytenoids, thyroarytenoids and vocales regulate the tension of the vocal ligaments. In reality, the obliquity of the cricoarytenoid facets means that some functional overlap between these two muscle groups is inevitable. Thus, alterations in the dimensions of the rima glottidis will produce small changes in vocal fold length, and shortening the vocal folds will also result in a degree of adduction. The oblique arytenoids, aryepiglottic and thyroepiglottic muscles modify the laryngeal inlet. Bilateral pairs of muscles usually act in concert with each other.
Neuromuscular spindles have been found in all human laryngeal muscles, the maximum number (23) being found in the transverse arytenoid. The control of phonation requires very considerable neuromuscular coordination, and effective proprioception would appear to be essential to this capacity. The mass of muscle related to adduction far outweighs that related to abduction. In this context, it is of interest to note that histological examination of normal larynges revealed evidence of some degenerative changes in posterior cricoarytenoid, the single muscle associated with abduction, but none in the remaining muscles.
INTRINSIC MUSCLES
Oblique arytenoid and aryepiglotticus
The oblique arytenoids lie superficial to the transverse arytenoid, and are sometimes considered to be part of it. They cross each other obliquely at the back of the larynx, each extending from the back of the muscular process of one arytenoid cartilage to the apex of the opposite one. Some fibres continue laterally round the arytenoid apex into the aryepiglottic fold, forming the aryepiglottic muscle.
Oblique arytenoid receives its blood supply from the laryngeal branches of the superior and inferior thyroid arteries.
The oblique arytenoids and aryepiglottic muscles act as a sphincter of the laryngeal inlet by adducting the aryepiglottic folds, and approximating the arytenoid cartilages to the tubercle of the epiglottis. Their poor development limits their capacity to act as a sphincter of the inlet. The oblique interarytenoids are weak adductors of the vocal folds, and may be more effective in this action than the transverse interarytenoids because of the superior mechanical advantage.
Transverse (inter) arytenoid
Transverse arytenoid is a single, unpaired muscle deep to the oblique interarytenoids (Fig. 34.9C). It bridges the gap at the back of the larynx between the two arytenoid cartilages and fills their posterior concave surfaces. It is attached to the back of the muscular process and adjacent lateral border of both arytenoids.
Transverse arytenoid receives its blood supply from the laryngeal branches of the superior and inferior thyroid arteries.
Transverse arytenoid is innervated by the recurrent laryngeal nerves. It also receives branches from the internal laryngeal nerve, although there is debate as to whether these branches contain any distinct motor input. The nerves form a dense, but highly variable, plexus.
Transverse arytenoid pulls the arytenoid cartilages towards each other, closing the posterior, intercartilaginous, part of the rima glottidis, such as occurs during a whisper (Fig. 34.9C). This action is accomplished by drawing the arytenoids upwards to slide along the sloping shoulders of the cricoid lamina, without rotation.
Posterior cricoarytenoid
Posterior cricoarytenoid arises from the posterior surface of the cricoid lamina (Fig. 34.9A). Its fibres ascend laterally and converge to insert on the upper and posterior surfaces of the muscular process of the ipsilateral arytenoid cartilage. The highest fibres run almost horizontally, the middle obliquely, and the lowest are almost vertical: some reach the anterolateral surface of the arytenoid cartilage. An additional strip of muscle, ceratocricoid, is occasionally seen in relation to the lower border of posterior cricoarytenoid, arising from the cricoid cartilage and inserting onto the posterior aspect of the inferior cornu of the thyroid cartilage.
Posterior cricoarytenoid receives its blood supply from the laryngeal branches of the superior and inferior thyroid arteries.
The posterior cricoarytenoids are the only laryngeal muscles that open the glottis. They rotate the arytenoid cartilages laterally around an axis that passes through the cricoarytenoid joints, producing separation of the vocal processes and the attached vocal cords (Fig. 34.9A). They also pull the arytenoids backwards, assisting the cricothyroids to lengthen the vocal cords. The most lateral fibres draw the arytenoid cartilages laterally, and so the rima glottidis becomes triangular when the posterior cricoarytenoid muscles contract. The posterior cricoarytenoids are active in the production of unvoiced sounds.
Lateral cricoarytenoid
Lateral cricoarytenoid is attached anteriorly to the upper border of the cricoid arch. It ascends obliquely backwards to be attached to the front of the muscular process of the ipsilateral arytenoid cartilage (Fig. 34.9B).
Lateral cricoarytenoid receives its blood supply from the laryngeal branches of the superior and inferior thyroid arteries.
Lateral cricoarytenoid is innervated by one to six branches of the anterior terminal division of the recurrent laryngeal nerve, with the most frequent pattern consisting of three branches. In the majority of cases, these branches arise over the muscle itself.
Lateral cricoarytenoid rotates the arytenoid cartilage in a direction opposite to that of posterior cricoarytenoid, and so closes the rima glottidis (Fig. 34.9B). As it does so, it brings the tips of the vocal processes together, closing the ligamentous part of the rima glottidis, an action known as medial compression. The action of lateral cricoarytenoid in adducting the vocal folds is therefore distinct and complementary to that of the interarytenoid muscles. Contraction of lateral cricoarytenoid also results in shortening and relaxing of the vocal folds.
Cricothyroid
Cricothyroid is attached anteriorly to the external aspect of the arch of the cricoid cartilage (Fig. 34.9F). Its fibres pass backwards and diverge into two groups, a lower ‘oblique’ part which slants backwards and laterally to the anterior border of the inferior cornu of the thyroid, and a superior ‘straight’ part which ascends more steeply backwards to the posterior part of the lower border of the thyroid lamina. The medial borders of the paired cricothyroids are separated anteriorly by a triangular gap occupied by the median cricothyroid ligament.
Cricothyroid is supplied by the cricothyroid artery, a branch of the superior thyroid artery, which crosses high on the cricothyroid ligament to communicate with its contralateral fellow.
Unlike the other intrinsic muscles of the larynx, cricothyroid is innervated by the external branch of the superior laryngeal nerve, and not by the recurrent laryngeal nerve.
Cricothyroid lengthens and affects tension in the vocal folds. It does this by shortening the space between the inferior border of the thyroid cartilage and the cricoid cartilage, an action that increases the distance between the tip of the vocal process of the arytenoid cartilage and the posterior surface of the thyroid lamina. Rotation occurs at the cricothyroid joint: it remains unclear which cartilage rotates to a greater extent, although the consensus appears to be that the predominant rotation is by the cricoid. At the same time, the posterior part of cricothyroid pulls the thyroid cartilage forwards, and this gliding action also lengthens the vocal folds.
Thyroarytenoid and vocalis
Thyroarytenoid is a broad, thin muscle, lying lateral to the vocal fold, conus elasticus, laryngeal ventricle and saccule (Fig. 34.9E). It is attached anteriorly to the lower half of the angle of the thyroid cartilage, and to the cricothyroid ligament. Its fibres pass backwards, laterally and upwards to the anterolateral surface of the arytenoid cartilage. The lower and deeper fibres form a band which, in coronal section, appears as a triangular bundle attached to the lateral surface of the vocal process and to the inferior impression on the anterolateral surface of the arytenoid cartilage. This bundle, the vocalis muscle, is parallel with, and just lateral to, the vocal ligament. It is said to be thicker behind than in front, because many deeper fibres start from the vocal ligament and do not extend to the thyroid cartilage. (An alternative view is that all its fibres loop and intertwine as they pass from the thyroid to the arytenoid cartilage.) A few fibres extend along the wall of the ventricle from the lateral margin of the arytenoid cartilage to the side of the epiglottis. Superior thyroarytenoid, an inconstant small muscle, lies on the lateral surface of the main mass of thyroarytenoid; when present, it extends obliquely from the thyroid angle to the muscular process of the arytenoid cartilage.
Thyroarytenoid receives its arterial blood supply from the laryngeal branches of the superior and inferior thyroid arteries.
All parts of thyroarytenoid are supplied by the recurrent laryngeal nerve. It also receives a communicating branch from the external laryngeal nerve, although it is not clear whether such branches carry motor or sensory fibres.
The thyroarytenoids draw the arytenoid cartilages towards the thyroid cartilage, thereby shortening and relaxing the vocal ligaments. At the same time, they rotate the arytenoids medially to approximate the vocal folds and so aid closure of the rima glottidis. Relaxation of the posterior parts of the vocal ligaments by the vocalis muscles, combined with tension in the anterior parts of the ligaments, is responsible for raising the pitch of the voice. Vocalis can change the timbre of the voice by affecting the mass of the vocal cords.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
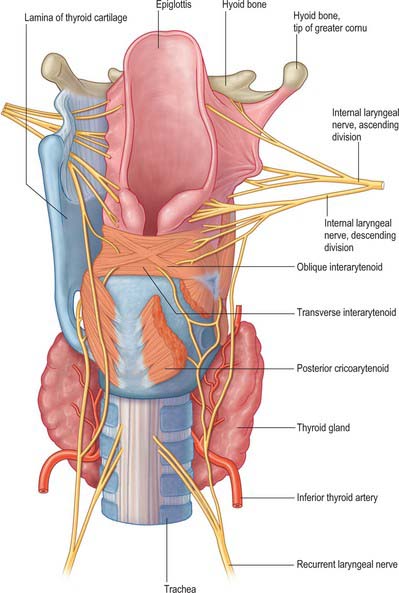
The blood supply of the larynx is derived mainly from the superior and inferior laryngeal arteries (Fig. 34.10). Rich anastomoses exist between the corresponding contralateral laryngeal arteries and between the ipsilateral laryngeal arteries. The superior laryngeal arteries supply the greater part of the tissues of the larynx, from the epiglottis down to the level of the vocal cords, including the majority of the laryngeal musculature. The inferior laryngeal artery supplies the region around cricothyroid, while its posterior laryngeal branch supplies the tissue around posterior cricoarytenoid.
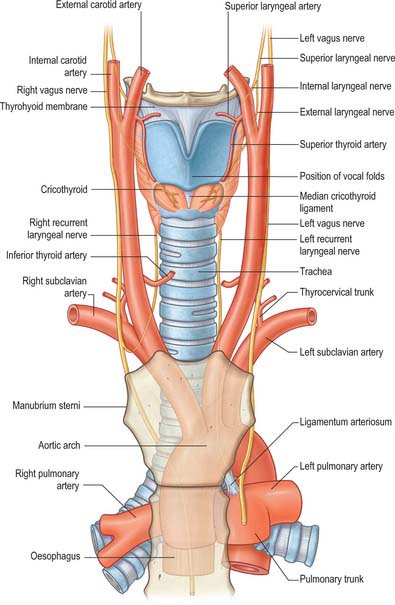
Fig. 34.10 Anterior view of the blood supply and innervation of the larynx.
(From Drake, Vogl and Mitchell 2005.)
Superior laryngeal artery
The superior laryngeal artery is normally derived from the superior thyroid artery, a branch of the external carotid artery, as this artery passes down towards the upper pole of the thyroid gland. However, sometimes it arises directly from the external carotid artery between the origins of the superior thyroid and lingual arteries. The superior laryngeal artery runs down towards the larynx, with the internal branch of the superior laryngeal nerve lying above it. It enters the larynx by penetrating the thyrohyoid membrane and divides into a number of branches which supply the larynx from the tip of the epiglottis down to the inferior margin of thyroarytenoid. It anastomoses with its contralateral fellow and with the inferior laryngeal branch of the inferior thyroid artery.
Cricothyroid artery
The cricothyroid artery arises from the superior thyroid artery and may contribute to the supply of the larynx. It follows a variable course either superficial or deep to sternothyroid. If superficial, it may be accompanied by branches of the ansa cervicalis, and if deep, it may be related to the external laryngeal nerve. It can anastomose with the artery of the opposite side and with the laryngeal arteries.
Inferior laryngeal artery
The inferior laryngeal artery is smaller than the superior laryngeal artery. It is a branch of the inferior thyroid artery, which arises from the thyrocervical trunk of the subclavian artery. It ascends on the trachea with the recurrent laryngeal nerve, enters the larynx at the lower border of the inferior constrictor, just behind the cricothyroid articulation, and supplies the laryngeal muscles and mucosa. The inferior laryngeal artery anastomoses with its contralateral fellow and with the superior laryngeal branch of the superior thyroid artery.
A posterior laryngeal artery of variable size has been described as a regular feature that arises as an internal branch of the inferior thyroid artery.
Superior and inferior laryngeal veins
Venous return from the larynx occurs via superior and inferior laryngeal veins which run parallel to the laryngeal arteries and are tributaries of the superior and inferior thyroid veins respectively. The superior thyroid vein drains into the internal jugular vein, and the inferior thyroid vein usually into the left brachiocephalic vein.
LYMPHATIC DRAINAGE
The vocal cords, with their firmly bound mucosa and paucity of lymphatics, provide a clear demarcation between the upper and lower areas of the larynx. Above the vocal cords, the lymph vessels draining the supraglottic part of the larynx accompany the superior laryngeal artery, pierce the thyrohyoid membrane, and end in the upper deep cervical lymph nodes, often bilaterally. The supraglottic lymphatics also communicate with those at the base of the tongue. Below the vocal cords, some of the lymph vessels pass through the conus elasticus to reach the prelaryngeal (Delphian) and/or pretracheal and paratracheal lymph nodes, while others accompany the inferior laryngeal artery and join the lower deep cervical nodes.
Spread of supra- and subglottic tumours
The upper deep cervical lymph nodes act as pathways of spread for malignant tumours of the supraglottic larynx: up to 40% of these tumours will have undergone such spread at the time of clinical presentation. The glottis is very poorly endowed with lymphatic vessels: 95% of malignant tumours confined to the glottis will present with a change in voice or airway obstruction but will not show signs of spread to adjacent lymph nodes at presentation. Tumours of the subglottic larynx will often spread to the paratracheal lymph node chain prior to clinical presentation. However, the presenting symptoms may be voice change and airway obstruction rather than a mass in the neck, because the paratracheal lymph nodes occupy a deep-seated position in the root of the neck and so their enlargement may remain occult.
INNERVATION
The larynx is innervated by the internal and external branches of the superior laryngeal nerve, the recurrent laryngeal nerve and sympathetic nerves (Figs 34.10, 34.11). Conventionally, the internal laryngeal nerve is described as sensory, the external laryngeal nerve as motor, and the recurrent laryngeal nerve as mixed. The internal laryngeal nerve is sensory down to the vocal cords, the recurrent laryngeal nerve is sensory below the vocal cords, and there is overlap between the territories innervated by the two nerves at the vocal cords themselves. All the intrinsic muscles of the larynx are supplied by the recurrent laryngeal nerve except for cricothyroid, which is supplied by the external laryngeal nerve. However, a number of anastomoses between these three nerves have been described, with varying estimates of their incidence. The majority occur on the posterior surface of the larynx, forming what has been described as a laryngeal plexus to parallel the pharyngeal plexus. Their precise nature and function is unclear, but since some are thought to convey motor fibres, it is reasonable to assume that functions commonly ascribed to the three laryngeal nerves may be more complex than the conventional descriptions imply, and this may have potential clinical implications.
The detailed course of the vagus in the neck is described on page 458.
Superior laryngeal nerve
The superior laryngeal nerve arises from the middle of the inferior vagal ganglion, and in its course receives one or more communications from the superior cervical sympathetic ganglion: most frequently, the connection is with the external laryngeal nerve. The superior laryngeal nerve divides into two branches – a smaller, external and a larger, internal branch – approximately 1.5 cm below the ganglion: rarely, both branches may arise from the ganglion.
Internal laryngeal nerve
The internal laryngeal nerve passes forwards approximately 7 mm before piercing the thyrohyoid membrane, usually at a higher level than the superior thyroid artery. It splits into superior, middle and inferior branches on entering the larynx. The superior branch supplies the mucosa of the piriform fossa. The large middle branch is distributed to the mucosa of the ventricle, specifically the quadrangular membrane, and therefore probably conveys the afferent component of the cough reflex. The inferior ramus is mainly distributed to the mucosa of the ventricle and subglottic cavity. On the medial wall of the piriform fossa, descending branches give twigs to the interarytenoid muscle and share a number of communicating branches with the recurrent laryngeal nerve.
External laryngeal nerve
The external laryngeal nerve continues downwards and forwards on the lateral surface of the inferior constrictor, to which it contributes some small branches. Indeed, in approximately 30% of cases, the nerve is located within the fibres of the constrictor muscle. It passes beneath the attachment of sternothyroid to the oblique line of the thyroid cartilage and supplies cricothyroid. A communicating nerve continues from the posterior surface of cricothyroid, crosses the piriform fossa and enters thyroarytenoid, where it anastomoses with branches from the recurrent laryngeal nerve. It has been suggested that these communicating branches may provide both additional motor components to thyroarytenoid and sensory fibres to the mucosa in the region of the subglottis. An anastomosis between the external and internal laryngeal nerves has also been described in some cases.
The close relationship of the external laryngeal nerve to the superior thyroid artery puts the nerve at potential risk when the artery is clamped during thyroid lobectomy. The external laryngeal nerve is potentially at risk where it is either particularly close to the artery (in some 20% of cases), or where, instead of crossing the superior thyroid vessels approximately 1 cm or more above the superior pole of the gland, it actually passes below this point (in some 20% of cases).
Recurrent laryngeal nerve
The upper part of the recurrent laryngeal nerve has a close but variable relationship to the inferior thyroid artery: it may pass in front of, behind, or parallel to, the artery. The nerve enters the larynx by passing either deep to (two-thirds of cases) or between (one-third of cases) the fibres of cricopharyngeus at its attachment to the lateral aspect of the cricoid cartilage. It supplies cricopharyngeus as it passes. At this point, the nerve is in intimate proximity to the posteromedial aspect of the thyroid gland. The main trunk divides into two or more branches, usually below the lower border of the inferior constrictor, although branching may occur higher up. The anterior branch is mainly motor and is sometimes called the inferior laryngeal nerve (although this term is best avoided), while the posterior branch is mainly sensory. The anterior branch of the recurrent laryngeal nerve ascends posterior to the cricothyroid joint and its ligament, and is usually covered by fibres of posterior cricoarytenoid at this point. It bends over the joint, continuing forward over the lateral cricoarytenoid muscle before terminating within thyroarytenoid.
The anterior branch of the recurrent laryngeal nerve first innervates posterior cricoarytenoid by one or more branches, then innervates interarytenoid and lateral cricoarytenoid, and terminates in thyroarytenoid, which it also supplies.
The recurrent laryngeal nerve forms several anastomoses with the superior laryngeal nerves (Fig. 34.11). The posterior branch of the recurrent laryngeal nerve ascends deep to posterior cricoarytenoid to join the descending branch of the internal laryngeal nerve. In addition, the ansa Galeni that lies on the interarytenoid muscles forms a direct connection between the recurrent and internal laryngeal nerves (Fig. 34.11), while there is also a complex anastomosis within and over the posterior surface of the interarytenoid muscles; and, less frequently, anastomoses on the cricoid lamina and thyroarytenoid that also form connections with the internal laryngeal nerve.
The recurrent laryngeal nerve does not always lie in a protected position in the tracheo-oesophageal groove, but may be slightly anterior to it (more often on the right), and it may be markedly lateral to the trachea at the level of the lower part of the thyroid gland. On the right the nerve is as often anterior to, or posterior to, or intermingled with, the terminal branches of the inferior thyroid artery. On the left the nerve is usually posterior to the artery, though occasionally it lies anterior to it. The nerve may supply extralaryngeal branches to the larynx which arise before it passes behind the inferior thyroid cornu.
Outside its capsule the thyroid gland has a distinct covering of pretracheal fascia which splits into two layers at the posterior border of the gland. One layer covers the entire medial surface of its lobe; at, or just above, the isthmus it is conspicuously thickened to form the lateral ligament of the thyroid gland, which attaches the gland to the trachea and the lower part of the cricoid cartilage. The other layer is posterior; it passes behind the oesophagus and pharynx and is attached to the prevertebral fascia (p. 439). In this way, a compartment is formed on each side of the midline, lateral to the trachea and oesophagus: the recurrent laryngeal nerve and terminal parts of the inferior thyroid artery lie in the fat of this compartment. The nerve may be lateral or medial to the lateral ligament of the thyroid gland, and sometimes may be embedded in it.
An very rare anomaly that is of relevance to laryngeal pathology and surgery is the so-called ‘non-recurrent’ laryngeal nerve, where the right recurrent laryngeal nerve arises directly from the vagus nerve trunk high up in the neck and enters the larynx close to the inferior pole of the thyroid gland. Only the right side is affected, and it is always associated with an abnormal origin of the right subclavian artery from the aortic arch on the left side. If unrecognized, a non-recurrent laryngeal nerve may be susceptible to injury during surgery. It may also potentially be compressed by small tumours of the thyroid gland (p. 462).
Vagal nerve lesions and recurrent laryngeal nerve paralysis
Unilateral complete palsy of the recurrent laryngeal nerve is more common on the left side, presumably because the nerve is longer on this side. There is isolated paralysis of all the laryngeal muscles on the affected side except cricothyroid, which is innervated by the external laryngeal nerve. The patient may be asymptomatic or have a hoarse, breathy voice and there will be a loss of ability to manipulate pitch. The hoarseness may be permanent or may become less severe with time as the contralateral cord develops the ability to hyperadduct and appose the paralysed cord and thus close the glottis during phonation and coughing, although this is seldom enough to restore full voice quality.
Clinically, the position of the vocal cord in the acute phase after section of the recurrent laryngeal nerve is very variable. Stridor is more common after bilateral lesions, but by no means the rule; indeed, the cords may be sufficiently abducted that there is little problem with airway obstruction, although the voice is always weaker in this situation. The cords are more widely separated in chronic lesions, which means that the voice is weaker but the upper airway is more secure. Atrophy and fibrosis of paraglottic muscles probably affect the position of paralysed vocal cords in chronic lesions to a greater degree than variations in the strength of the apposing adductor and abductor muscle groups.
For many years the conventional wisdom was that movements of abduction were affected to a greater degree than those of adduction when the recurrent laryngeal nerve was partially lesioned (Semon’s law). This was attributed to the presumed segregation of the axons supplying the laryngeal abductor muscles within the recurrent laryngeal nerve. However, studies of human nerves have failed to support this idea, and have shown that axons destined for particular laryngeal muscles are randomly distributed within the recurrent laryngeal nerve. It seems more likely that the relative sparing of abduction in these lesions is a consequence of the weak abduction produced by crico-thyroid, which is spared. The difficulty of predicting the effect of partial lesions of the recurrent laryngeal nerve may reflect the variable patterns of anastomosis between the laryngeal nerves.
Paralysis of all laryngeal musculature (including cricothyroid) suggests a lesion proximal to the inferior (nodose) ganglion that involves the superior laryngeal nerve as well as the recurrent laryngeal nerve. The affected cord is paralysed and lies in the so-called ‘cadaveric’ position halfway between abduction and adduction. If the lesion is unilateral, the voice is weak and hoarse, but if it is bilateral, phonation is almost absent, the vocal pitch cannot be altered, and the cough is weak and ineffective.
There is debate as to the effect of lesions of the external laryngeal nerve. Complete section is most likely during the ligation of the vessels forming the vascular pedicle of the thyroid gland during thyroid lobectomy, and characteristically causes breathiness and mild hoarseness accompanied by reduced pitch and loudness in bilateral lesions; these effects may not be noticeable when lesions are unilateral.
Damage to the internal laryngeal nerve causes loss of mechanoreceptive and proprioceptive sensation from the larynx. Unilaterally, this produces a feeling that something is stuck in the throat. Bilaterally, it will result in aspiration and can cause dysphagia, with a risk of choking.
ANATOMY OF SPEECH
The principal biological function of the larynx is to act as a sphincter controlling the entry of foreign bodies into the airways and regulate airflow during ventilation. Thus, the larynx is opened widely during ventilation and is closed tightly during swallowing. The larynx can also close tightly during exertion, effort closure, to regulate thoracic and abdominal pressure during activities such as defecation or parturition, or to fix the thorax to increase mechanical advantage when using the arms to lift objects. The importance of the latter activity should not be underestimated: one of the many problems reported by patients who have undergone a laryngectomy is a loss of power in their arms when trying to lift heavy objects.
The larynx is much more than a simple valve that opens and closes. In addition to its sphincteric functions, its location means that it is perfectly placed to act as a sound source, or voice, that forms the basis of nearly all sounds in human speech. The musculoskeletal structure of the larynx is under exquisite neuromuscular control, allowing it to modify the expiratory stream to produce highly complex patterns of sound of varying loudness, frequency and duration. The ability to execute these complex movements depends largely on specific areas of the cerebral hemispheres which are involved in the motor aspects of language, such as speech and writing, and sensory manifestations of language, including reading and understanding the spoken word.
OVERVIEW OF SPEECH PRODUCTION
All speech requires an input of energy. For all sounds in Western European languages, and most sounds in other languages, this energy takes the form of a pulmonary expiration. This continuous airflow is converted into a vibration within the larynx by a mechanism called phonation, in which the vocal folds vibrate periodically, interrupting the column of air as it leaves the lungs and converting it into a series of discrete puffs of air. Speech sounds that are produced by vocal fold vibration in this way are said to be voiced. Speech sounds that are produced without vocal fold vibration are termed unvoiced sounds.
The larynx is an inadequate sound source: the laryngeal ‘buzz’ that is produced by phonation is very quiet and cannot be varied sufficiently to produce the complex range of sounds that is human speech. Amplification and modification of the sound occur in the supralaryngeal vocal tract, which may be considered as a 17 cm long tube, narrow at the larynx and broadening out proximally as it passes through the pharynx, and oral and nasal cavities. This tube acts as a passive amplifier of the sound. (The analogy here is of a megaphone: cupping the hands round the lips lengthens the vocal tract and increases the volume of speech.) The supralaryngeal vocal tract modifies the basic vibration of the larynx by altering its geometry, length and calibre: it provides a series of resonators that can dampen or amplify certain sound frequencies and can transiently interrupt the exhaled air flow and modify it to produce speech. This process is known as articulation. The range of sounds that the human vocal tract is capable of producing is very wide, although any one human language will employ a subset of these sounds to convey meaning.
MUSCULAR CONTROL OF THE AIRSTREAM
Normal vegetative ventilation involves rhythmic movements of the thoracic cage that are produced by intercostal muscles and the diaphragm and a number of accessory muscles in the neck, arm and abdomen that have one attachment to some part of the thoracic cage. The thorax is capable of responding mechanically to widely varying demands for oxygen. From a tidal volume at rest of 500 mL and a respiratory rate of 12 per min, ventilation can increase in fit individuals during vigorous exercise to tidal volumes of 4.5 L and respiratory rates of 20–25.
Normal ventilatory patterns are considerably modified during speech, reflecting the special demands that speech places upon ventilation. The main source of energy for the production of speech sounds is a pulmonary expiration, although other mechanisms are possible. In order that speech is produced, sufficient pressure has to be generated beneath the vocal folds. This subglottal pressure (the difference between the air pressures above and below the vocal folds) has to be sustained above a minimum level throughout an utterance. It sets the vocal folds into vibration if the sound is to be voiced or generates airflow for an unvoiced sound. The minimum subglottal pressure needed for speech production is 7 cmH2O, and this increases when loud sounds are produced or when sounds are stressed.
The need to generate sufficient sustained pressure means that speech ventilation is markedly non-rhythmical. At the onset of an utterance, inspiration is typically 1.5 L, which is deeper than for normal quiet ventilation, ensuring that sufficient air is taken in to maintain adequate subglottal pressure for the duration of the utterance. Inspiration is also quicker, 0.5 sec rather than 2–3 sec. Expiration is much longer than normal, perhaps lasting up to 30 sec, reflecting the fact that the vocal tract is more constricted at the larynx to ensure that pauses for further inspirations are made at suitable points in an utterance. At the end of the first inspiration during running speech, lung volumes do not fully return to resting levels. Conversational speech normally takes place at a higher range of lung capacities than operate in normal quiet ventilation.
The non-rhythmical pattern during speech requires greater inspiratory effort, and for most people it involves a greater use of the diaphragm, usually in combination with the abdominal muscles that are attached to the lower ribs (they stabilize the costal attachments of the diaphragm and increase the effectiveness of its action) (see p. 1011). However, the main differences are seen in expiration. Speech takes place at higher lung volumes, which means that greater recoil forces are stored in the elastic tissues of the lungs and the ribcage. The generation of subglottal pressure is the product of these elastic recoil forces and the muscular forces generated by the expiratory muscles. At the onset of an utterance, unrestrained recoil forces would generate excessive subglottal pressures that would be wasteful of air, and hence energy, and would affect the loudness of speech. Conversely, towards the end of an utterance, as recoil forces decline, subglottal pressure would fall without additional muscular exertion. Therefore, early in an utterance, inspiratory muscles, particularly the external intercostals and parasternal parts of the internal intercostals, continue to contract, relaxing slowly to counteract the effects of excessive passive elastic recoil. As the recoil forces decline below the point where they can maintain the minimum subglottal pressure needed for phonation, expiratory muscles contract to maintain subglottal pressure as lung air volume nears its resting expiratory level. The main muscles involved are the costal parts of the internal intercostals and the subcostal and transversus thoracis muscles. Their actions are aided by contraction of the anterior abdominal muscles to compress the abdomen. Accessory muscles such as latissimus dorsi may also come into play, but normally these accessory muscles are only active at the end of a very long or loud utterance, or in patients whose ventilatory function is compromised.
Though subglottal pressure tends to remain fairly constant during an utterance, it rises when sounds are stressed, and falls during the production of unvoiced sounds when the larynx is less constricted. At these times, compensatory mechanisms to ensure that pressure is maintained are required: the precise mechanisms have yet to be elucidated, but the internal intercostal muscles have been implicated. The anterior abdominal muscles are also active in singing, shouting and in attempts to speak without the pause necessary for inspiration. Contrary to popular belief, the diaphragm plays little part in the regulation of expiratory force. Unlike the intercostal muscles, the diaphragmatic musculature is sparsely supplied with muscle spindles, and therefore control of the diaphragm is poorly regulated: minute changes can be effected more successfully using the intercostal and anterior abdominal muscles.
Though the expiratory airflow from the lungs is the source of energy for most speech sounds, other sources of airflow are also used. The larynx can be used to generate airflow. The sound /p/ is produced by closing the larynx and then raising it with the lips closed, using the larynx like a piston. Opening the lips then releases a puff of air. This is called an ejective, and is an example of egressive glottalic airflow. Ingressive glottalic airflow is also possible, though less effective; examples are not found in English, but do occur in some African and native American languages. A third kind of airflow is velaric, in which the back of the tongue is raised against a lowered soft palate and the vocal tract is closed anterior to that point, either at the lips or with the tongue against the hard palate. This produces a click and is rare. A non-linguistic example in English is the sound made in encouraging a horse. Ingressive pulmonary airflow, such as is found in a groan or a gasp, is theoretically possible, but none of the world’s languages employ this as a source of airflow.
After removal of the larynx, e.g. following laryngeal cancer, patients can be taught to swallow air, store it in a segment of the oesophagus and then use it as the energy source to produce egressive oesophageal airflow (oesophageal speech). Speech in these circumstances tends to have a belching quality and may be badly phrased. Laryngectomy patients always produce phrases that are shorter than normal, and so prostheses incorporating valves and surgical shunts are often inserted to provide a larger egressive airstream by diverting air from the respiratory tract into the oesophagus.
PHONATION
The default position of the rima glottidis is open, to maintain patency of the airway during respiration. In quiet respiration, the anterior intermembranous part of the rima glottidis is triangular when viewed from above. Its apex is anterior and its base posterior, and it is represented by an imaginary line approximately 8 mm long connecting the anterior ends of the arytenoid vocal processes. The intercartilaginous part between the medial surfaces of the arytenoids is rectangular as the two vocal processes lie parallel to each other. During forced respiration, the rima glottidis is widened and the vocal cords are fully abducted to increase the airway. The arytenoid cartilages rotate laterally, and this moves their vocal processes apart, and converts the rima glottidis into a diamond shape in which both intermembranous and intercartilaginous parts are triangular. The greatest width of the rima glottidis is at the point of the attachments of the vocal cords to the vocal processes.
During speech the true vocal folds vibrate to act as a source of sound for subsequent speech. There have been a number of theories to explain the mechanism that produces this vibration, but these are now only of historic interest. The aerodynamic–myoelastic theory is generally accepted as the mechanism underlying vocal fold vibration, although it does not account for all aspects of phonation.
At the onset of an utterance, during an expiration, the true vocal folds are adducted: the lateral cricoarytenoids and interarytenoids bring together both the intermembranous and intercartilaginous parts of the glottis, actions which either close the glottis completely or reduce the space between the vocal folds to a linear chink. The mucous membrane covering the interarytenoid muscles, the interarytenoid fold, intrudes into the larynx when these muscles adduct the arytenoids, and so aids closure of the intercartilaginous part of the rima glottidis. The vocal cords are also tensed, an essential prerequisite for vibration. These actions cause a build-up of subglottal pressure that continues until a point is reached when the muscular force of adduction is no longer sufficient to resist the rising pressure, and the vocal folds are forced open a little, releasing air into the supralaryngeal vocal tract. The subglottal pressure falls when the subglottic and supraglottic cavities become continuous and the vocal folds begin to close. Two mechanisms bring about closure. If adductive tension is sustained, then the vocal folds will close. In addition, rapid closure is aided by a physical process, the Bernoulli effect. The forcing of air from a region of high to low pressure through a narrow space causes an increase in the kinetic energy of the molecules at the edge of the space. The effect of this is to lower pressure in the space between the folds at the level of the folds themselves, and this negative pressure simply sucks the folds together because they are mobile. This causes a rise once more in the subglottal pressure and the cycle is repeated. The effect is to cause the release of a series of puffs of air into the supralaryngeal vocal tract at a frequency of many times per second, which is perceived as a sound of a particular frequency (Figs 34.12, 34.13).
Fig. 34.12 Video montage of the normal phonatory cycle, obtained using a rigid fibre endoscope with stroboscopic illumination.
(By courtesy of Professor Paul Carding.)
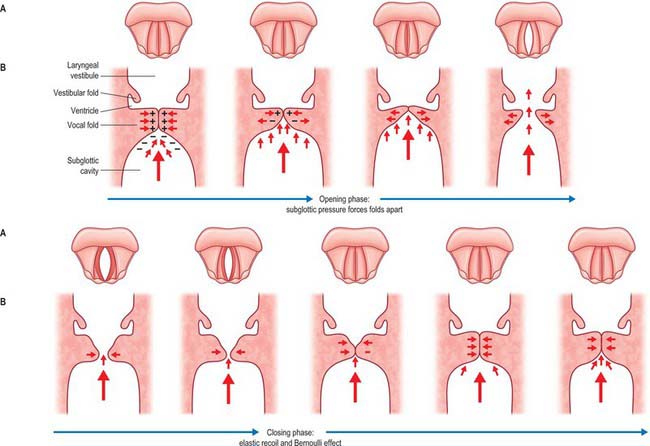
Fig. 34.13 Position of the vocal folds during a cycle of vocal fold vibration (phonatory cycle) in superior (A) and coronal (B) views.
The source of energy for the vibration does not come from the larynx itself. The vocal folds do not behave like the prongs of a tuning fork: their predominant motion is in the horizontal plane at right angles to the movement of the air column, and there is little vertical movement. The energy is derived from the motion of the air generated by the muscular and recoil forces in the thorax, and the larynx is simply chopping that column into a series of segments.
The aerodynamic–myoelastic theory does not explain how vocal fold vibration is sustained, nor does it account for the very obvious muco-undulatory component of vocal fold vibration that is visible when the larynx is viewed stroboscopically. Without further input of energy the vibration of the vocal folds as described above would not be sustained but would be damped and gradually diminish. For vibration to continue there has to be additional energy input. The analogy here is with a child on a swing. For the motion to continue either the parent has to push the child at an appropriate point in the cycle, or the child has to sustain the motion for themselves by swinging their legs at the crucial point in the swing cycle. In the case of phonation, the source of this additional energy is unclear. It may come from the inertia of the air column itself, i.e. once the vocal folds close, the air column will continue moving upwards because inertia creates a negative pressure above the folds. Alternatively, the energy could come from the manner in which the folds open and close. As the subglottal pressure rises, the lower portion of the fold opens first and the upper edge of the fold is last to open, and when the subglottal pressure falls, the folds close from the bottom edge. It has been suggested that this non-uniform closure creates different shapes within the glottis that may result in differing negative pressures at different phases of the cycle. It also produces a vertical wave-like motion in the folds, termed the muco-undulatory component. The analogy here is with a flag blowing in the wind, and it reflects the differing stiffnesses of the various layers of the vocal folds described above. This vertical component will impart a negligible amount of energy to the air column, but it is likely to impart harmonics to the basic laryngeal vibration.
The sound that results from the process of phonation has three characteristics: a frequency that is perceived as pitch, an intensity that is perceived as loudness, and a timbre perceived as voice quality.
The fundamental frequency of the human voice is determined by the resting length of the vocal cords and varies with age and sex. The frequency range of human speech is from 60 to 500 Hz, with an average of approximately 120 Hz in males, 200 Hz in females and 270 Hz in children. The mechanism of frequency alteration is not entirely clear. An increase in subglottal pressure will cause the frequency of phonation to rise. However, during an utterance, subglottal pressure appears to remain fairly constant, which suggests that the mechanism of frequency alteration resides in intrinsic changes within the vocal folds. Variations in frequency (pitch changes) during an utterance are determined by the complex interrelationships between length, tension and thickness of the vocal cords: one of these variables cannot be altered without affecting the other two parameters to some extent. Gross changes to the vocal cords demonstrate the effects of these variables. Inflamed and swollen vocal cords are much thicker than normal and result in a hoarse voice. At puberty, growth of the thyroid cartilage in males lengthens the vocal cords and lowers the fundamental frequency, and the voice ‘breaks’ as a result. During panic, the vocal cords may be tensed, which means that the cry for help is a high-pitched squeak.
Pitch is increased by increasing the length of the vocal folds, as may be confirmed during direct endoscopic examination of the larynx. At first sight this may seem counterintuitive, but, as the vocal cords are lengthened, there will be a consequent thinning and change in tension. Although an analogy is often drawn between the vocal cords and vibrating strings, a better analogy is a rubber band: if a rubber band is lengthened, the tension will increase, but the thickness will decrease. The vocal cords may be lengthened by up to 50% of their resting length. It is likely that the initial pitch setting is achieved by action of the cricothyroids, and that fine adjustments can then be made using the vocales. Paralysis of both cricothyroids, which is usually associated with loss of the neurones that are distributed via the superior laryngeal nerve (as a result of damage to the vagal nuclei in brainstem stroke), results in permanent hoarseness and inability to vary the pitch of the voice. It is important to remember that once the vocal cords are set in motion they will deviate from their original setting as they vibrate. Auditory feedback of the sounds produced is used to make minute compensatory adjustments to length, tension and thickness in order to maintain a constant pitch.
Any lengthening of the vocal cords tends to thin them. The thickness can be increased by the vocalis part of thyroarytenoid: as vocalis shortens, it will relax the vocal cords and at the same time increase their thickness. Changes in the tension of the vocal cords are produced by the same muscles that change their length, namely cricothyroid, posterior cricoarytenoid and vocalis, probably acting isometrically.
The mechanism by which loudness is increased is the subject of less debate. Loud sounds are produced by increasing subglottal pressure. This is achieved, in turn, by changing the opening quotient of the glottis (the ratio of the time spent in the open phase of the cycle to the total cycle time). In normal speech, this ratio is usually around 0.5, but in very loud speech it can fall to 0.3.
Timbre or voice quality refers to the harsh or mellow quality of the voice. At high volume the voice tends to be harsher, especially in untrained voices: higher-frequency components predominate because higher subglottal pressures are needed to sustain the increased volume. This can be overcome to an extent by increasing the airflow rather than the pressure.
A fundamental distinction in speech needs to be made between voiced and unvoiced sounds: nearly all languages make this distinction. Voicing has been described above. In unvoiced sounds, the vocal folds are not vibrating and will usually be opened under the action of posterior cricoarytenoid. The energy from the airstream is then used by other parts of the vocal tract to generate sound, normally by constricting or stopping the airflow. However, phonation is not an all-or-nothing process, but is subject to considerable modification and adjustment. In modal voice, i.e. speaking using habitual pitch, forces acting on the vocal folds are moderate, pressures are sustained, and air is conserved. However, phonation can occur when the vocal folds are more open than usual, resulting in breathy phonation with more air escaping per phonatory cycle than usual. Some languages in South Asia exploit the difference between breathy and non-breathy sounds, whereas in spoken English, a breathy voice is simply recognized as a feature of some speakers. At the other end of the spectrum is vocal creak, in which the vocal folds are more closed than normal. Different speakers will habitually employ different laryngeal settings which contribute to their particular voiced quality. In whispering, the intramembranous part of the glottis is closed, but the intercartilaginous part remains open, which produces a characteristic Y-shaped glottis and a greater loss of air at each phonatory cycle.
The main function of the larynx is to act as a sound source, but it can also function in speech as an airstream generator and as an articulator.
ARTICULATION
The sound produced by the phonation is not a pure tone because several harmonics at multiples of the fundamental frequency are also generated. Harmonics give a note of a particular frequency its defining characteristics. An ‘A’ played on an oboe or violin is immediately recognizable because of the different harmonics generated by the design of the instrument. The harmonic spectra of individual voices differ and will also vary depending upon the mode of phonation adopted. In the human vocal tract, the fundamental frequency and its harmonics are transmitted to the column of air which extends from the vocal cords to the exterior, mainly through the mouth. Part of the airstream can also be diverted through the nasal cavities when the soft palate is depressed to allow air into the nasopharynx. The supralaryngeal vocal tract acts as a selective resonator whose length, shape and volume can be varied by the actions of the muscles of the pharynx, soft palate, fauces, tongue, cheeks and lips; the relative positions of the upper and lower teeth, which are determined by the degree of opening and protrusion or retraction of the mandible; and alterations in the tension of the walls of the column, especially in the pharynx. Thus, the fundamental frequency (pitch) and harmonics produced by the passage of air through the glottis are modified by changes in the supralaryngeal vocal tract.
Harmonics may be amplified, or dampened. The fundamental frequency and its associated harmonics may also be raised or lowered by appropriate elevation or depression respectively of the hyoid bone and the larynx as a unit by the selective actions of the extrinsic laryngeal muscles. Effectively, these movements shorten or lengthen the resonating column, and to some extent also alter the geometry of the walls of the air passages. Analysis of the human voice shows that it has a very similar pattern of harmonics for all fundamental frequencies, determined by the vocal tract acting as a selective filter and resonator. This maintains a constant quality of voice without which intelligibility would be lost (recorded speech played back without its harmonics is completely unintelligible). Each human voice is unique: it has been suggested that the unique frequency spectrum of each individual voice could be used for personal identification.
During articulation the egressive airstream is given a rapidly changing specific quality by the articulatory organs, the lips, oral cavity, tongue, teeth, palate, pharynx, and nasal cavity. The discipline of phonetics primarily deals with the way in which speech sounds are produced, and consequently with the analysis of the mode of production of speech sounds by the vocal apparatus. In order to analyse the way in which the articulators are used in different speech sounds, words are broken down into units called phonemes, which are defined as the minimal sequential contrastive units used in any language.
The human vocal tract can produce many more phonemes than are employed in any one language. Not all languages have the same phonemes, and within the same language, the phonemes can vary in different parts of the same country and in other countries where that language is also spoken. Reproducing phonemes that are not used in native speech is difficult because such phonemes require unfamiliar positioning of the speech organs. A native speaker of any language can quickly recognize the origins of anyone attempting to use their language as a second language. The second-language speaker will usually speak it with an accent characteristic of their own first language because they are using the familiar configurations of their vocal tract for each phoneme instead of the correct positioning.
PRODUCTION OF VOWELS
All vowel sounds require phonation by vibration of the vocal cords. Each vowel sound has its own characteristic higher harmonics (frequency spectrum) that exhibit peaks of energy at certain frequencies. These energy peaks are always higher multiples of the fundamental frequencies and are called formants. Formants are the result of the combined effects of phonation, selective resonance of the vocal tract, and the properties of the head as a radiator of sound. The sounds of the different vowels are determined by the shape and size of the mouth, and the positions of the tongue and lips are the most important variables. The tongue may be placed high or low (close and open vowels), or further forwards or back (front and back vowels) and the lips may be rounded or spread.
PRODUCTION OF CONSONANTS
The production of consonants always involves some degree of constriction of the vocal tract. There are many more consonants than vowels, and, in general, consonants cannot be combined to produce syllables. The classification of consonants is complex and beyond the remit of this book: what follows is a summary (for fuller details, a textbook of phonetics should be consulted).
Consonants may be classified of the basis of where the constriction occurs, termed the place of articulation; the degree or extent of constriction, termed the manner of articulation; the shape of the constriction, termed the stricture; and whether or not there is vibration of the vocal folds, when consonants are described as voiced or unvoiced respectively.
Consonants may also be classified as labial, dental, alveolar, velar, uvular, pharyngeal or glottic, depending upon whether the point of maximum constriction occurs at the level of the lips, teeth, bony ridge behind the teeth, palate, uvula or pharynx (Fig. 34.14). Different parts of the tongue can be used in combination with the above places of articulation. Phoneticians divide the tongue into the tip, anterior edge, the front part of the dorsum, the centre and back parts of the remaining dorsum, and a most posterior part (the root). These divisions bear no obvious relationship to the anatomical landmarks on the tongue, but they are useful in describing the part of the dorsum of the tongue that contacts other areas of the mouth. Similarly, the manner of articulation can vary from a complete closure to a slight narrowing. An actual closure of the vocal tract is called a stop. A narrowing that is sufficient to produce turbulence of the air in the vocal tract, and which is perceived as a rustling sound, is termed a fricative. Approximants involve a degree of closure insufficient to produce turbulence but with closure greater than that for a vowel. Nasals involve a stoppage in the oral cavity with the soft palate lowered to allow airflow through the nose, and, unlike stops, they can be sustained. Stricture describes the shape of the constriction, e.g. a lateral consonant involves depression of the sides of the tongue, while a grooved consonant is produced by grooving the dorsum of the tongue. Consonants can be produced with the vocal folds vibrating, when they are termed voiced, or without vocal fold vibration, in which case they are termed unvoiced.
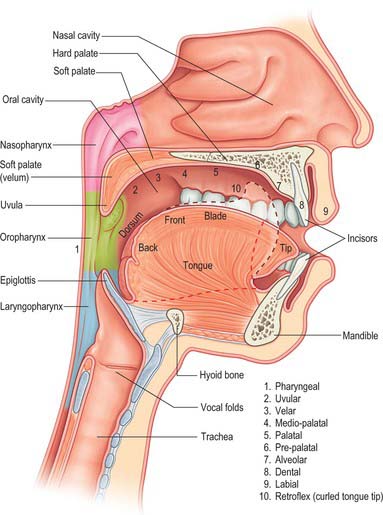
Fig. 34.14 Sagittal view of the left side of the head, showing the supralaryngeal vocal tract, the articulators and places of articulation. The broken line indicates the tongue position during retroflexion (10).
The best way to illustrate these classificatory systems in operation is by contrasting the production of different consonant pairs in which only one or two parameters have been changed. The /p/ of peat, the /b/ of beat and the /m/ of meat are all bilabial stops, meaning that they are produced by bringing together the lips. The /b/ and the /m/ are voiced but the /p/ is not. The contrast between the /b/ and the /p/ is in the differing way in which the airstreams are produced. The /b/ is produced with an egressive pulmonary airflow, while the /p/ is produced with the glottis closed, hence unvoiced, and the glottis is then raised using the larynx as a piston to compress the air in the supra-laryngeal vocal tract prior to the stop being released. The /m/ differs from the other two stops in being a nasal in which the soft palate is lowered to allow air to escape through the nasal cavity, and unlike the other two stops it can be sustained as in a sound of approval, e.g. encouraging a horse to move. Bilabial stops can be contrasted with the labiodental fricatives /f/ of feet and the /v/ of veal, both of which are produced by retracting the lower lip beneath the upper teeth. Neither involves a complete closure but both produce a significant constriction of the vocal tract with audible turbulence: the /f/ is unvoiced, while the /v/ is voiced. The sh sound (/∫/) in ship is also a fricative involving a grooving of the tongue and is associated with significant audible turbulence; it may be contrasted with the lateral approximant /l/ in law, in which the sides of the tongue are lowered. In this case it is the nature of the stricture which is different and the degree of closure, e.g. in the case of the approximant /l/, closure is insufficient to produce turbulence. The sh sound in ship can be compared to the /k/ in keen, where the position of the tongue is different: in /k/ the tongue blade contacts the soft palate, while in sh the tongue tip or the blade contact the postalveolar region. The most dramatic example of the difference between voiced and unvoiced sounds may be appreciated if the /s/ sound in sip is compared with the /z/ in zip. If the larynx is loosely palpated while making a sustained unvoiced ‘ssssss’ sound, no vibration is felt, but if the ‘ssssss’ is commuted into a prolonged voiced ‘zzzzzz’, then vibration in the larynx should be readily detectable. The position of the tongue and other articulators is exactly the same for both /s/ and /z/, the difference between them is the presence or absence of phonation.
Atkinson M, McHanwell S. Basic Medical Science for Speech & Language Therapy Students. London: Whurr Publishers, 2002.
Berkovitz BKB, Moxham BJ, Hickey S. The anatomy of the larynx. In: Ferlito A, editor. Diseases of the Larynx. London: Chapman & Hall; 2000:25-44.
Bosma JF, Bartner MA. Ligaments of the larynx and the adjacent pharynx and esophagus. Dysphagia. 1993;8:23-28.
Clark J, Yallop C. An Introduction to Phonetics and Phonology, 2nd edn. Oxford: Blackwell, 1995.
A comprehensive introduction to all aspects of phonetics and phonology..
Durham FC, Harrison TS. The surgical anatomy of the superior laryngeal nerve. Surg Gynecol Obstet. 1962;118:33-44.
Erkki A, Pitkanen R, Suominen H. Observations on the structure and the biomechanics of the cricothyroid articulation. Acta Otolaryngol (Stockh). 1987;103:117-126.
Friedman M, Toriumi DM, Grybauskas V, Katz A. Nonrecurrent laryngeal nerves and their clinical significance. Laryngoscope. 1986;96:87-90.
Kirchner JA, Carter D. Intralaryngeal barriers to the spread of cancer. Acta Otolaryngol (Stockh). 1987;103:503-513.
Maranillo E, Leon X, Orus C, Quer M, Sanudo J-R. Variability in nerve patterns of the adductor muscle group supplied by the recurrent laryngeal nerve. Laryngoscope. 2005;115:358-362.
This paper and that by Sanudo et al (1999) below describe variations in the innervation of the human larynx and discuss the possible functions and clinical significance of the variations described..
Munir Turk L, Hogg DA. Age changes in the human laryngeal cartilages. Clin Anat. 1993;6:154-162.
Provides data concerning the distribution of areas of calcification that occur with age within the cartilages of the larynx..
Pracy R. The infant larynx. J Laryngol Otol. 1983;97:933-947.
Draws attention to features that differ from the adult condition and that may have clinical significance..
Reidenbach MM. Normal topography of the conus elasticus. Anatomical bases for the spread of laryngeal cancer. Surg Radiol Anat. 1995;17:107-111.
Reidenbach MM. The periepiglottic space: topographic relations and histological organisation. J Anat. 1996;188:173-182.
Reidenbach MM. The paraglottic space and transglottic cancer: anatomic considerations. Clin Anat. 1996;9:244-251.
Reidenbach MM. Subglottic region: normal topography and possible clinical implications. Clin Anat. 1998;11:9-21.
The four papers by Reidenbach are based on serial reconstruction of sections derived from plastinated material..
Sanudo J-R, Maranillo E, León X, Mirapeix R-M, Orús C, Quer M. An anatomical study of anastomoses between the laryngeal nerves. Laryngoscope. 1999;109:983-987.
Sato I, Shimada K. Arborization of the inferior laryngeal nerve and internal nerve on the posterior surface of the larynx. Clin Anat. 1995;8:379-387.
Discusses the possible clinical significance of connections between the inferior (recurrent) laryngeal nerve and the internal laryngeal nerve..
Welsh LW, Welsh JJ, Rizzo TA. Laryngeal spaces and lymphatics: current anatomic concepts. Ann Otol Rhinol Laryngol Suppl. 1983;105:19-31.
Describes the ‘tissue spaces’ and lymphatic drainage of the larynx and their importance in determining the route of spread of tumours..