CHAPTER 33 Pharynx
The pharynx is a 12–14 cm long musculomembranous tube shaped like an inverted cone. It extends from the cranial base to the lower border of the cricoid cartilage (the level of the sixth cervical vertebra), where it becomes continuous with the oesophagus. The width of the pharynx varies constantly because it is dependent on muscle tone, especially of the constrictors: at rest the pharyngo-oesophageal junction is closed as a result of tonic closure of the upper oesophageal sphincter, and during sleep muscle tone is low and the dimensions of the pharynx are markedly decreased (which may give rise to snoring and sleep apnoea). The pharynx is limited above by the posterior part of the body of the sphenoid and the basilar part of the occipital bone, and it is continuous with the oesophagus below. Behind, it is separated from the cervical part of the vertebral column and the prevertebral fascia which covers longus colli and longus capitis by loose connective tissue in the retropharyngeal space above and the retrovisceral space below.
The muscles of the pharynx are three circular constrictors and three longitudinal elevators. The constrictors may be thought of as three overlapping cones which arise from structures at the sides of the head and neck and pass posteriorly to insert into a midline fibrous band, the pharyngeal raphe. The arterial supply to the pharynx is derived from branches of the external carotid artery, particularly the ascending pharyngeal artery, but also from the ascending palatine and tonsillar branches of the facial artery, the maxillary artery (greater palatine and pharyngeal arteries and the artery of the pterygoid canal) and dorsal lingual branches of the lingual artery. The pharyngeal veins begin in a plexus external to the pharynx, receive meningeal veins and a vein from the pterygoid canal, and usually end in the internal jugular vein. Lymphatic vessels from the pharynx and cervical oesophagus pass to the deep cervical nodes, either directly or through the retropharyngeal or paratracheal nodes. The motor and sensory innervation is principally via branches of the pharyngeal plexus.
The pharynx lies behind, and communicates with, the nasal, oral and laryngeal cavities via the nasopharynx, oropharynx and laryngopharynx respectively (Figs 33.1, 33.2). Its lining mucosa is continuous with that lining the pharyngotympanic tubes, nasal cavity, mouth and larynx.
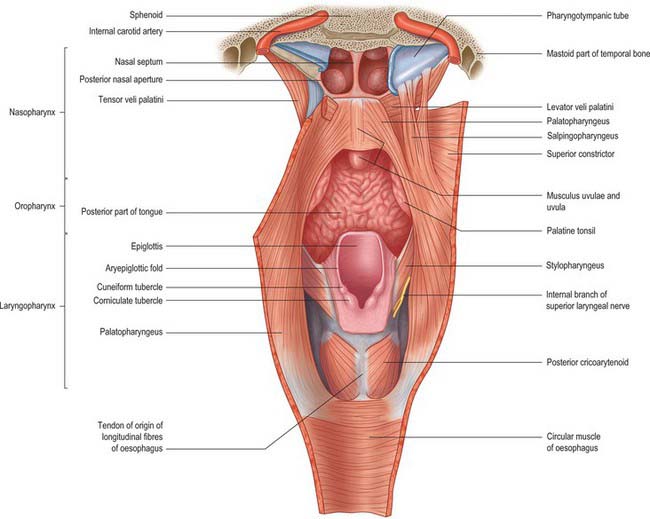
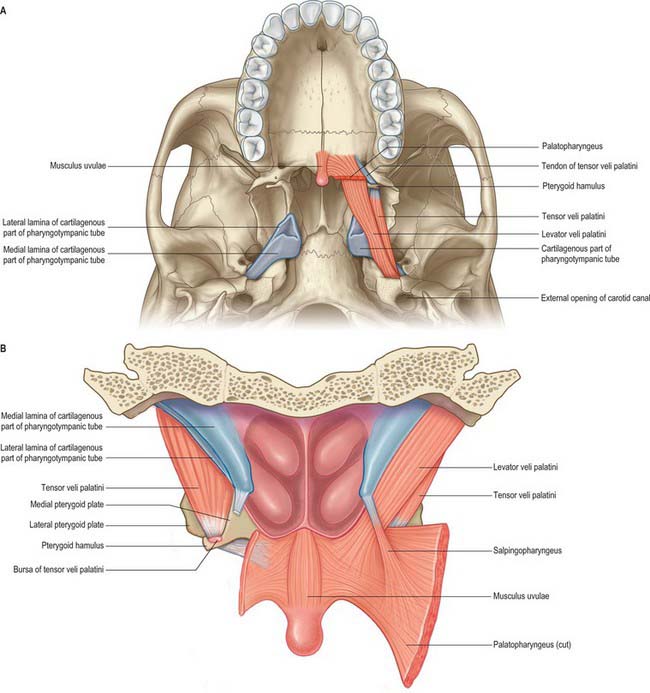
Fig. 33.1 The nasopharynx, oropharynx and laryngopharynx, exposed by cutting the median pharyngeal raphe and reflecting the constrictor muscles laterally on either side, posterior view.
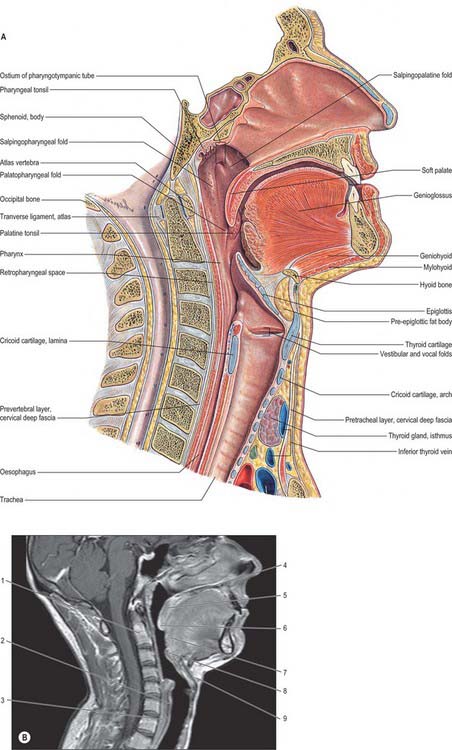
Fig. 33.2 A, Sagittal section through the head and neck, including the nasal and oral cavities but excluding the intracranial region (from Sobotta 2006). B, Corresponding MRI, also includes the posterior cranial fossa, cerebellum and cervical spinal cord. 1. Dens of axis. 2. Lamina of cricoid cartilage. 3. Oesophagus. 4. Hard palate. 5. Soft palate. 6. Uvula. 7. Pharyngeal part of tongue. 8. Epiglottis. 9. Hyoid bone.
(B by courtesy of Dr Roger JS Chinn.)
NASOPHARYNX
BOUNDARIES
The nasopharynx lies above the soft palate and behind the posterior nares, which allow free respiratory passage between the nasal cavities and the nasopharynx (Figs 33.1, 33.2). The nasal septum separates the two posterior nares, each of which measures approximately 25 mm vertically and 12 mm transversely. Just within these openings lie the posterior ends of the inferior and middle nasal conchae. The nasopharynx has a roof, a posterior wall, two lateral walls and a floor. These are rigid (except for the floor, which can be raised or lowered by the soft palate) and the cavity of the nasopharynx is therefore never obliterated by muscle action, unlike the cavities of the oro- and laryngopharynx. The nasal and oral parts of the pharynx communicate through the pharyngeal isthmus which lies between the posterior border of the soft palate and the posterior pharyngeal wall. Elevation of the soft palate and constriction of the palatopharyngeal sphincter close the isthmus during swallowing.
The roof and posterior wall form a continuous concave slope that leads down from the nasal septum to the oropharynx. It is bounded above by mucosa overlying the posterior part of the body of the sphenoid, and further back by the basilar part of the occipital bone as far as the pharyngeal tubercle. Further down, the mucosa overlies the pharyngobasilar fascia and the upper fibres of the superior constrictor, and behind these, the anterior arch of the atlas. A lymphoid mass, the adenoid, lies in the mucosa of the upper part of the roof and posterior wall in the midline.
The lateral walls of the nasopharynx display a number of important surface features. On either side each receives the opening of the pharyngotympanic tube (also termed the auditory or Eustachian tube), situated 10–12 mm behind and a little below the level of the posterior end of the inferior nasal concha (Fig. 33.2). The tubal aperture is approximately triangular in shape, and is bounded above and behind by the tubal elevation which consists of mucosa overlying the protruding pharyngeal end of the cartilage of the pharyngotympanic tube. A vertical mucosal fold, the salpingopharyngeal fold, descends from the tubal elevation behind the aperture (Fig. 33.2) and covers salpingopharyngeus in the wall of the pharynx (see Fig. 33.4); a smaller salpingopalatine fold extends from the anterosuperior angle of the tubal elevation to the soft palate in front of the aperture. As levator veli palatini enters the soft palate it produces an elevation of the mucosa immediately around the tubal opening (see Fig. 33.4). A small variable mass of lymphoid tissue, the tubal tonsil, lies in the mucosa immediately behind the opening of the pharyngotympanic tube, and further behind the tubal elevation there is a variable depression in the lateral wall, the lateral pharyngeal recess (fossa of Rosenmüller). The floor of the nasopharynx is formed by the nasal, upper surface of the soft palate.
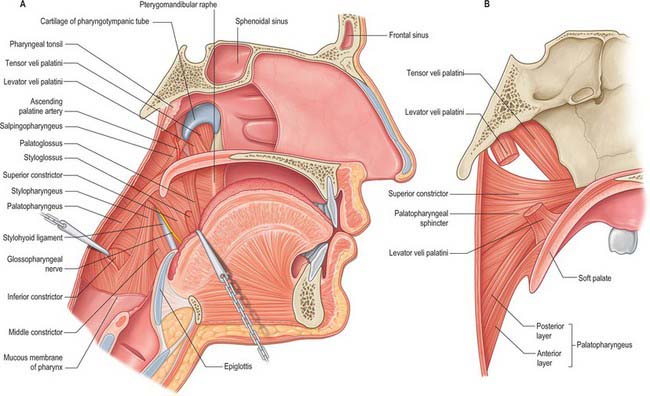
Fig. 33.4 A, Interior of the pharynx, exposed by removal of the mucous membrane, sagittal section. The bodies of the cervical vertebrae have been removed, the cut posterior wall of the pharynx retracted dorsolaterally and palatopharyngeus reflected dorsally to show the cranial fibres of the inferior constrictor. The dorsum of the tongue has been pulled ventrally to display a part of styloglossus in the angular interval between the mandibular and the lingual fibres of origin of the superior constrictor. B, Muscles of the left half of the soft palate and adjoining part of the pharyngeal wall, sagittal section.
MICROSTRUCTURE OF NON-TONSILLAR NASOPHARYNX
The nasopharyngeal epithelium anteriorly is ciliated, pseudostratified respiratory in type with goblet cells (see Fig. 2.2). The ducts of mucosal and submucosal seromucous glands open onto its surface. Posteriorly, the respiratory epithelium changes to non-keratinized stratified squamous epithelium which continues into the oropharynx and laryngopharynx. The transitional zone between the two types of epithelium consists of columnar epithelium with short microvilli instead of cilia. Superiorly, this zone meets the nasal septum; laterally, it crosses the orifice of the pharyngotympanic tube; and it passes posteriorly at the union of the soft palate and the lateral wall. Numerous mucous glands surround the tubal orifices.
INNERVATION
Much of the mucosa of the nasopharynx behind the pharyngotympanic tube is supplied by the pharyngeal branch of the pterygopalatine ganglion which traverses the palatovaginal canal with a pharyngeal branch of the maxillary artery.
ADENOID OR PHARYNGEAL TONSIL
The adenoid, or pharyngeal tonsil, is a median mass of mucosa-associated lymphoid tissue (MALT) situated in the roof and posterior wall of the nasopharynx (Fig. 33.3). At its maximal size (during the early years of life) it is shaped like a truncated pyramid, often with a vertically oriented median cleft, so that its apex points towards the nasal septum and its base to the junction of the roof and posterior wall of the nasopharynx.
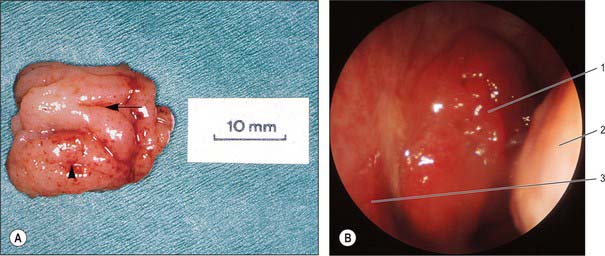
Fig. 33.3 A, Nasopharyngeal tonsil following adenoidectomy by curettage. The rostral surface is to the left; surface folds radiate forwards from a median recess (arrow). In this example, the impression left by contact with the left Eustachian cushion is evident laterally (arrowhead). B, Transnasal endoscopic view of adenoid. 1. Adenoid (in posterior naris). 2. Inferior concha (posterior view). 3. Posterior end of nasal septum.
(A, Specimen provided by Professor MJ Gleeson, School of Medicine, King’s College London; B, by courtesy of Mr Simon A Hickey.)
The free surface of the pharyngeal tonsil is marked by folds that radiate forwards and laterally from a median blind recess, the pharyngeal bursa (bursa of Luschka), which extends backwards and up. The recess is present in the fetus and the young, but only occasionally present in the adult, and marks the rostral end of the embryological notochord. The number and position of the folds and of the deep fissures which separate them vary. A median fold may pass forwards from the pharyngeal bursa towards the nasal septum, or instead a fissure may extend forwards from the bursa, dividing the nasopharyngeal tonsil into two distinct halves which reflect its paired developmental origins (Fig. 33.3).
The prenatal origins of the nasopharyngeal tonsil are described on page 604. After birth it initially grows rapidly, but usually undergoes a degree of involution and atrophy from the age of 8–10 years (although hypoplasia may still occur in adults up to the seventh decade). Relative to the volume of the nasopharynx, the size of the tonsil is largest at 5 years, which may account for the frequency of nasal breathing problems in preschool children, and the incidence of adenoidectomy in this age group.
Vascular supply and lymphatic drainage
The arterial supply of the pharyngeal tonsil is derived from the ascending pharyngeal and ascending palatine arteries, the tonsillar branches of the facial artery, the pharyngeal branch of the maxillary artery and the artery of the pterygoid canal (see Figs 33.5, 33.6). In addition, a nutrient or emissary vessel to the neighbouring bone, the basisphenoid artery, which is a branch of the inferior hypophysial arteries, supplies the bed of the pharyngeal tonsil and is a possible cause of persistent postadenoidectomy haemorrhage in some patients.
Numerous communicating veins drain the pharyngeal tonsil into the internal submucous and external pharyngeal venous plexuses. They emerge from the deep lateral surface of the tonsil and join the external palatine (paratonsillar) veins, and pierce the superior constrictor either to join the pharyngeal venous plexus, or to unite to form a single vessel that enters the facial or internal jugular vein; they may also connect with the pterygoid venous plexus.
Microstructure of the pharyngeal tonsil
The adenoid is covered laterally and inferiorly mainly by ciliated respiratory epithelium which contains scattered small patches of non-keratinized stratified squamous epithelium. Its superior surface is separated from the periosteum of the sphenoid and occipital bones by a connective tissue hemicapsule. The fibrous framework of the tonsil, consisting of a mesh of collagen type III (reticular) fibres supporting a lymphoid parenchyma similar to that in the palatine tonsil, is anchored to the hemicapsule.
The nasopharyngeal epithelium lines a series of mucosal folds around which the lymphoid parenchyma is organized into follicles and extrafollicular areas. Internally, the tonsil is subdivided into four to six lobes by connective tissue septa, which arise from the hemicapsule and penetrate the lymphoid parenchyma. Seromucous glands lie within the connective tissue, and their ducts extend through the parenchyma to reach the nasopharyngeal surface.
Functions of the pharyngeal tonsil
The pharyngeal tonsil forms part of the circumpharyngeal lymphoid ring (Waldeyer’s ring), and therefore presumably contributes to the defence of the upper respiratory tract. The territories served by its lymphocytes are uncertain, but may include the nasal cavities, nasopharynx, pharyngotympanic tubes and the middle and inner ears.
Adenoidectomy
Surgical removal of the adenoid is commonly performed to clear nasopharyngeal obstruction and as part of the treatment of chronic secretory otitis media. A variety of methods are employed, including suction diathermy, suction microdebridement and, most commonly, blind curettage. When using the latter, it is important to avoid hyperextension of the cervical spine as this throws the arch of the atlas into prominence and may result in damage to the prevertebral fascia and anterior spinal ligaments, with resultant infection and cervical instability. Extreme lateral curettage can result in damage to the tubal orifice and excessive bleeding because the vasculature is denser laterally. Removal of the adenoid in children can result in an impairment in the ability of the soft palate to close the pharyngeal isthmus fully (velopharyngeal insufficiency, VPI), causing excessive nasality of speech.
PHARYNGOTYMPANIC TUBE
The pharyngotympanic tube (see Fig. 36.7) connects the tympanic cavity to the nasopharynx and allows the passage of air between these spaces in order to equalize the air pressure on both aspects of the tympanic membrane. It is about 36 mm long and descends anteromedially from the tympanic cavity to the nasopharynx at an angle of approximately 45° with the sagittal plane and 30° with the horizontal (these angles increase with age and elongation of the skull base). It is formed partly by cartilage and fibrous tissue and partly by bone.
The cartilaginous part, which is approximately 24 mm long, is formed by a triangular plate of cartilage, the greater part of which is in the posteromedial wall of the tube. Its apex is attached by fibrous tissue to the circumference of the jagged rim of the bony part of the tube, and its base is directly under the mucosa of the lateral nasopharyngeal wall, forming a tubal elevation (torus tubarius; Eustachian cushion) behind the pharyngeal orifice of the tube (Fig. 33.2; see Fig. 30.1). The upper part of the cartilage is bent laterally and downwards, producing a broad medial lamina and narrow lateral lamina. In transverse section it is hook-like and incomplete below and laterally, where the canal is composed of fibrous tissue. The cartilage is fixed to the cranial base in the groove between the petrous part of the temporal bone and the greater wing of the sphenoid, and ends near the root of the medial pterygoid plate. The cartilaginous and bony parts of the tube are not in the same plane, the former descending a little more steeply than the latter. The diameter of the tube is greatest at the pharyngeal orifice, least at the junction of the two parts (the isthmus), and increases again towards the tympanic cavity.
The bony part, approximately 12 mm long, is oblong in transverse section, with its greater dimension in the horizontal plane. It starts from the anterior tympanic wall and gradually narrows to end at the junction of the squamous and petrous parts of the temporal bone, where it has a jagged margin for the attachment of the cartilaginous part. The carotid canal lies medially.
The mucosa of the pharyngotympanic tube is continuous with the nasopharyngeal and tympanic mucosae. It is lined by a ciliated columnar epithelium and is thin in the osseous part but thickened by mucous glands in the cartilaginous part. Near the pharyngeal orifice there is a variable, but sometimes considerable, lymphoid mass called the tubal tonsil.
At birth the pharyngotympanic tube is about half its adult length, it is more horizontal and its bony part is relatively shorter but much wider. The pharyngeal orifice is a narrow slit, level with the palate and without a tubal elevation.
Salpingopharyngeus is attached to the inferior part of the cartilaginous tube near its pharyngeal opening. Posteromedially are the petrous part of the temporal bone and levator veli palatini, which arises partly from the medial lamina of the tube. Anterolaterally, tensor veli palatini separates the tube from the otic ganglion, the mandibular nerve and its branches, the chorda tympani nerve and the middle meningeal artery. Some fibres of tensor veli palatini are attached to the lateral lamina of the cartilage and to the fibrous part, and these fibres are sometimes referred to as dilator tubae. The pharyngotympanic tube is opened during deglutition but the mechanism is uncertain: dilator tubae, aided by salpingopharyngeus, may be responsible. Levator veli palatini elevates the cartilaginous part of the pharyngotympanic tube, and so might allow passive opening by releasing tension on the cartilage.
Arteries to the pharyngotympanic tube arise from the ascending pharyngeal branch of the external carotid artery, and from the middle meningeal artery and the artery of the pterygoid canal, which are both branches of the maxillary artery. The veins of the pharyngotympanic tube usually drain to the pterygoid venous plexus.
The pharyngotympanic tube is innervated by filaments from the tympanic plexus and from the pharyngeal branch of the pterygopalatine ganglion. The tympanic plexus ramifies on the promontory in the middle ear cavity and is formed by the tympanic branch of the glossopharyngeal nerve and caroticotympanic nerves of sympathetic origin (see also Ch. 36).
OROPHARYNX
BOUNDARIES
The oropharynx extends from below the soft palate to the upper border of the epiglottis (Figs 33.1, 33.2). It opens into the mouth through the oropharyngeal isthmus, demarcated by the palatoglossal arch, and faces the pharyngeal aspect of the tongue. Its lateral wall consists of the palatopharyngeal arch and palatine tonsil (see Fig. 30.3). Posteriorly, it is level with the bodies of the second, and upper part of the third, cervical vertebrae (Fig. 33.2).
SOFT PALATE
The soft palate is a mobile flap suspended from the posterior border of the hard palate, sloping down and back between the oral and nasal parts of the pharynx (Figs 33.2, 33.4). The boundary between the hard and soft palate is readily palpable and may be distinguished by a change in colour, the soft palate being a darker red with a yellowish tint. The soft palate is a thick fold of mucosa enclosing an aponeurosis, muscular tissue, vessels, nerves, lymphoid tissue and mucous glands; almost half its thickness is represented by numerous mucous glands which lie between the muscles and the oral surface of the soft palate. The latter is covered by a stratified squamous epithelium, while the nasal surface is covered with a ciliated columnar epithelium. In most individuals, two small pits, the fovea palatini, may be seen, one on each side of the midline: they represent the orifices of ducts from some of the minor mucous glands of the palate. In its usual relaxed and pendant position, the anterior (oral) surface of the soft palate is concave, and has a median raphe. The posterior aspect is convex and continuous with the nasal floor, the anterosuperior border is attached to the posterior margin of the hard palate, and the sides blend with the pharyngeal wall. The inferior border is free, and hangs between the mouth and pharynx. A median conical process, the uvula, projects downwards from its posterior border (see Fig. 30.3). Taste buds are found on the oral aspect of the soft palate.
The anterior third of the soft palate contains little muscle and consists mainly of the palatine aponeurosis. This region is less mobile and more horizontal than the rest of the soft palate and is the chief area acted upon by tensor veli palatini.
A small bony prominence, produced by the pterygoid hamulus, can be felt just behind and medial to each upper alveolar process, in the lateral part of the anterior region of the soft palate. The pterygomandibular raphe (a tendinous band between buccinator and the superior constrictor) passes downwards and outwards from the hamulus to the posterior end of the mylohyoid line. When the mouth is opened wide, this raphe raises a fold of mucosa that indicates the internal, posterior boundary of the cheek; it is an important landmark for an inferior alveolar nerve block.
Palatine aponeurosis
A thin, fibrous, palatine aponeurosis composed of the expanded tendons of the tensor veli palatini muscles strengthens the soft palate. It is attached to the posterior border and inferior surface of the hard palate behind any palatine crests, and extends medially from behind the greater palatine foramina. It is thick in the anterior two-thirds of the soft palate but very thin further back. Near the midline it encloses the musculus uvulae. All the other palatine muscles are attached to the aponeurosis.
Palatoglossal and palatopharyngeal arches
The lateral wall of the oropharynx presents two prominent folds, the palatoglossal and palatopharyngeal folds (anterior and posterior pillars of the fauces respectively) (see Fig. 30.3). The palatoglossal arch, the anterior fold, runs from the soft palate to the side of the tongue and contains palatoglossus. The palatopharyngeal arch, the posterior fold, projects more medially and passes from the soft palate to merge with the lateral wall of the pharynx; it contains palatopharyngeus. A triangular tonsillar fossa (tonsillar sinus), lies on each side of the oropharynx between the diverging palatopharyngeal and palatoglossal arches, and contains the palatine tonsil.
Vascular supply
The arterial supply of the soft palate is usually derived from the ascending palatine branch of the facial artery. Sometimes this is replaced or supplemented by a branch of the ascending pharyngeal artery which descends forwards between the superior border of the superior constrictor and levator veli palatini, and accompanies the latter to the soft palate. The veins of the soft palate usually drain to the pterygoid venous plexus.
Innervation
General sensation from most of the soft palate is carried by branches of the lesser palatine nerve (a branch of the maxillary nerve) and from the posterior part of the palate by pharyngeal branches from the glossopharyngeal nerve and from the plexus around the tonsil (formed by tonsillar branches of the glossopharyngeal and lesser palatine nerves). The special sensation of taste from taste buds in the oral surface of the soft palate is carried in the lesser palatine nerve: the taste fibres initially travel in the greater petrosal nerve (a branch of the facial nerve) and pass through the pterygopalatine ganglion without synapsing. The lesser palatine nerve also carries the secretomotor supply to most of the mucosa of the soft palate, via postganglionic branches from the pterygopalatine ganglion. Postganglionic secretomotor parasympathetic fibres may pass to the posterior parts of the soft palate from the otic ganglion (which receives preganglionic fibres via the lesser petrosal branch of the glossopharyngeal nerve). Postganglionic sympathetic fibres run from the carotid plexus along arterial branches supplying the palate.
Uvulopalatopharyngoplasty
The pharyngeal airway is kept patent in the patient who is awake by the combined dilating action of genioglossus, tensor veli palatini, geniohyoid and stylohyoid, which act to counter the negative pressure generated in the lumen of the pharynx during inspiration. The tone in the muscles is reduced during sleep, and is also affected by alcohol and other sedatives, hypothyroidism and a variety of neurological disorders. If the dilator muscle tone is insufficient, the walls of the pharynx may become apposed. Intermittent pharyngeal obstruction may cause snoring, and complete obstruction may cause apnoea, hypoxia and hypercarbia which lead to arousal and sleep disturbance.
Surgical techniques involving reduction in the length of the soft palate, removal of the tonsils and plicating of the tonsillar pillars can be used to raise the intrinsic dilating tone in the pharyngeal wall and to reduce the bulk of (and to stiffen) the soft palate. This will reduce the tendency of the soft palate to vibrate and generate noise during periods of incipient collapse of the pharynx. An alternative treatment is to deliver air to the pharynx at above atmospheric pressure via a closely fitting facemask, thus inflating the pharynx and countering its tendency to collapse.
PALATINE TONSIL
The right and left palatine tonsils form part of the circumpharyngeal lymphoid ring. Each tonsil is an ovoid mass of lymphoid tissue situated in the lateral wall of the oropharynx (Fig. 33.5; see Figs 30.3, 30.5). Size varies according to age, individuality and pathological status (tonsils may be hypertrophied and/or inflamed). It is therefore difficult to define the normal appearance of the palatine tonsil. For the first 5 or 6 years of life the tonsils increase rapidly in size. They usually reach a maximum at puberty, when they average 20–25 mm in vertical, and 10–15 mm in transverse, diameters, and they project conspicuously into the oropharynx. Tonsillar involution begins at puberty, when the reactive lymphoid tissue begins to atrophy, and by old age only a little tonsillar lymphoid tissue remains.
The long axis of the tonsil is directed from above, downwards and backwards. Its medial, free, surface usually presents a pitted appearance. The pits, 10–20 in number, lead into a system of blind-ending, often highly branching, crypts which extend through the whole thickness of the tonsil and almost reach the connective tissue hemicapsule. In a healthy tonsil the openings of the crypts are fissure-like and the walls of the crypt lumina are collapsed so that they are in contact with each other. The human tonsil is polycryptic. The branching crypt system reaches its maximum size and complexity during childhood. The mouth of a deep tonsillar cleft (intratonsillar cleft, recessus palatinus) opens in the upper part of the medial surface of the tonsil. It is often erroneously called the supratonsillar fossa, and yet it is not situated above the tonsil but within its substance. The mouth of the cleft is semilunar, curving parallel to the convex dorsum of the tongue in the sagittal plane. The upper wall of the recess contains lymphoid tissue which extends into the soft palate as the pars palatina of the palatine tonsil. After the age of 5 years this embedded part of the tonsil diminishes in size. There is a tendency for the whole tonsil to involute from the age of 14 years, and for the tonsillar bed to flatten out. During young adult life, a mucosal fold, the plica triangularis, stretches back from the palatoglossal arch down to the tongue. It is infiltrated by lymphoid tissue and frequently represents the most prominent (anteroinferior) portion of the tonsil. It rarely persists into middle age.
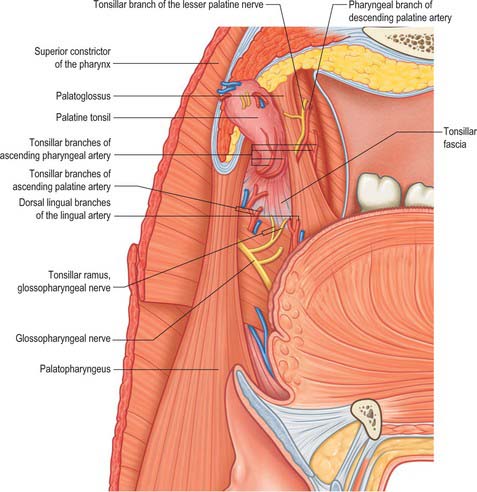
The lateral or deep surface of the tonsil spreads downwards, upwards and forwards. Inferiorly, it invades the dorsum of the tongue, superiorly, the soft palate, and, anteriorly, it may extend for some distance under the palatoglossal arch. This deep, lateral aspect is covered by a layer of fibrous tissue, the tonsillar hemicapsule. The latter is separable with ease for most of its extent from the underlying muscular wall of the pharynx, which is formed here by the superior constrictor, and sometimes by the anterior fibres of palatopharyngeus, with styloglossus on its lateral side (Fig. 33.5). Anteroinferiorly, the hemicapsule adheres to the side of the tongue and to palatoglossus and palatopharyngeus. In this region, the tonsillar artery, a branch of the facial artery, pierces the superior constrictor to enter the tonsil, accompanied by venae comitantes (Fig. 33.5). An important and sometimes large vein, the external palatine or paratonsillar vein, descends from the soft palate lateral to the tonsillar hemicapsule before piercing the pharyngeal wall. Haemorrhage from this vessel from the upper angle of the tonsillar fossa may complicate tonsillectomy. The muscular wall of the tonsillar fossa separates the tonsil from the ascending palatine artery, and, occasionally, from the tortuous facial artery itself, which may lie near the pharyngeal wall at the lower tonsillar level. The glossopharyngeal nerve lies immediately lateral to the muscular wall of the tonsillar fossa (Figs 33.4A, 33.5): it is at risk if the wall is pierced, and is commonly temporarily affected by oedema following tonsillectomy. The internal carotid artery lies approximately 25 mm behind and lateral to the tonsil. In some individuals the styloid process may be elongated and deviate towards the tonsillar bed.
Microstructure of the palatine tonsil
Each tonsil is a mass of lymphoid tissue associated with the oropharyngeal mucosa and fixed in its position, unlike most other examples of mucosa-associated lymphoid tissue. It is covered on its oropharyngeal aspect by non-keratinized stratified squamous epithelium. The whole of the tonsil is supported internally by a delicate meshwork of fine collagen type III (reticulin) fibres which are condensed in places to form more robust connective tissue septa that also contain elastin. These septa partition the tonsillar parenchyma, and merge at their ends with the dense irregular fibrous hemicapsule on the deep aspect of the tonsil and with the lamina propria on the pharyngeal surface. Blood vessels, lymphatics and nerves branch or join within the connective tissue condensations. The hemicapsule forms its lateral boundary with the oropharyngeal wall, and with the mucosa which covers its highly invaginated free surface.
The 10–20 crypts formed by invagination of the free surface mucosa are narrow tubular epithelial diverticula which often branch within the tonsil and frequently are packed with plugs of shed epithelial cells, lymphocytes and bacteria, which may calcify. The epithelium lining the crypts is mostly similar to that of the oropharyngeal surface, i.e. stratified squamous, but there are also patches of reticulated epithelium, which is much thinner, and which has a complex structure that is of great importance in the immunological function of the tonsil.
Reticulated epithelium lacks the orderly laminar structure of stratified squamous epithelium. Its base is deeply invaginated in a complex manner so that the epithelial cells, with their slender branched cytoplasmic processes, provide a coarse mesh to accommodate the infiltrating lymphocytes and macrophages. The basal lamina of this epithelium is discontinuous. Although the oropharyngeal surface is unbroken, the epithelium may become exceedingly thin in places, so that only a tenuous cytoplasmic layer separates the pharyngeal lumen from the underlying lymphocytes. Epithelial cells are held together by small desmosomes, anchored into bundles of keratin filaments. Interdigitating dendritic cells (antigen-presenting cells, APCs) are also present. The intimate association of epithelial cells and lymphocytes facilitates the direct transport of antigen from the external environment to the tonsillar lymphoid cells, i.e. reticulated epithelial cells are functionally similar to the microfold (M) cells of the gut. The total surface area of the reticulated epithelium is very large because of the complex branched nature of the tonsillar crypts, and has been estimated at 295 cm2 for an average palatine tonsil.
There are four lymphoid compartments in the palatine tonsils. Lymphoid follicles, many with germinal centres, are arranged in rows roughly parallel to neighbouring connective tissue septa. Their size and cellular content varies in proportion to the immunological activity of the tonsil. The mantle zones of the follicles, each with closely packed small lymphocytes, form a dense cap, always situated on the side of the follicle nearest to the mucosal surface. These cells are the products of B-lymphocyte proliferation within the germinal centres. Extrafollicular, or T-lymphocyte, areas contain a specialized microvasculature including high endothelial venules (HEVs), through which circulating lymphocytes enter the tonsillar parenchyma. The lymphoid tissue of the reticulated crypt epithelium contains predominantly IgG- and IgA-producing B lymphocytes (including some mature plasma cells), T lymphocytes and antigen-presenting cells. There are numerous capillary loops in this subsurface region.
Vascular supply and lymphatic drainage
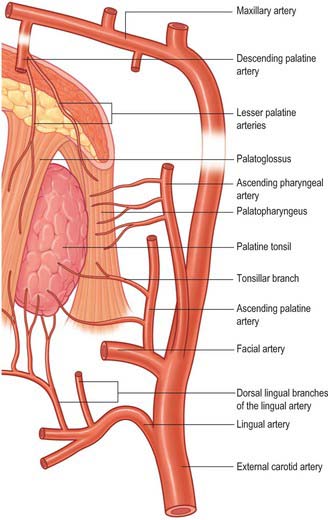
The arterial blood supply to the palatine tonsil is derived from branches of the external carotid artery (Figs 33.5, 33.6). Three arteries enter the tonsil at its lower pole. The largest is the tonsillar artery, which is a branch of the facial, or sometimes the ascending palatine, artery. It ascends between medial pterygoid and styloglossus, perforates the superior constrictor at the upper border of styloglossus, and ramifies in the tonsil and posterior lingual musculature (Figs 35.5, 33.6). The other arteries found at the lower pole are the dorsal lingual branches of the lingual artery, which enter anteriorly, and a branch from the ascending palatine artery, which enters posteriorly to supply the lower part of the palatine tonsil. The upper pole of the tonsil also receives branches from the ascending pharyngeal artery, which enter the tonsil posteriorly, and from the descending palatine artery and its branches, the greater and lesser palatine arteries. All of these arteries enter the deep surface of the tonsil, branch within the connective tissue septa, narrow to become arterioles and then give off capillary loops into the follicles, interfollicular areas and the cavities within the base of the reticulated epithelium. The capillaries rejoin to form venules, many with high endothelia, and the veins return within the septal tissues to the hemicapsule as tributaries of the pharyngeal drainage. The tonsillar artery and its venae comitantes often lie within the palatoglossal fold, and may haemorrhage if this fold is damaged during surgery.
Unlike lymph nodes, the palatine tonsils do not possess afferent lymphatics or lymph sinuses. Instead, dense plexuses of fine lymphatic vessels surround each follicle and form efferent lymphatics which pass towards the hemicapsule, pierce the superior constrictor, and drain to the upper deep cervical lymph nodes directly (especially the jugulodigastric nodes) or indirectly through the retropharyngeal lymph nodes. The jugulodigastric nodes are typically enlarged in tonsillitis, when they project beyond the anterior border of sternocleidomastoid and are palpable superficially 1–2 cm below the angle of the mandible: when enlarged, they represent the most common swelling in the neck.
Innervation
The tonsillar region is innervated by tonsillar branches of the maxillary and glossopharyngeal nerves (Fig. 33.5). The fibres from the maxillary nerve pass through, but do not synapse in, the pterygopalatine ganglion; they are distributed through the lesser palatine nerves and form a plexus (the circulus tonsillaris) around the tonsil together with the tonsillar branches of the glossopharyngeal nerve. Nerve fibres from this plexus are also distributed to the soft palate and the region of the oropharyngeal isthmus. The tympanic branch of the glossopharyngeal nerve supplies the mucous membrane lining the tympanic cavity. Infection, malignancy and postoperative inflammation of the tonsil and tonsillar fossa may therefore be accompanied by pain referred to the ear.
Tonsillectomy
Surgical removal of the pharyngeal tonsils is commonly performed to prevent recurrent acute tonsillitis or to treat airway obstruction by hypertrophied or inflamed palatine tonsils. Occasionally, the tonsil may be removed to treat an acute peritonsillar abscess, which is a collection of pus between the superior constrictor and the tonsillar hemicapsule. Many methods have been employed, the commonest being dissection in the plane of the fibrous hemicapsule followed by ligation or electrocautery to the vessels divided during the dissection. The nerve supply to the tonsil is so diffuse that tonsillectomy under local anaesthesia is performed successfully by local infiltration rather than by blocking the main nerves. Surgical access to the glossopharyngeal nerve may be achieved by separating the fibres of superior constrictor.
Waldeyer’s ring
Waldeyer’s ring is a circumpharyngeal ring of mucosa-associated lymphoid tissue which surrounds the openings into the digestive and respiratory tracts. It is made up anteroinferiorly by the lingual tonsil, laterally by the palatine and tubal tonsils, and posterosuperiorly by the nasopharyngeal tonsil and smaller collections of lymphoid tissue in the inter-tonsillar intervals.
LARYNGOPHARYNX
BOUNDARIES
The laryngopharynx is situated behind the entire length of the larynx (known clinically as the hypopharynx) and extends from the superior border of the epiglottis, where it is delineated from the oropharynx by the lateral glossoepiglottic folds, to the inferior border of the cricoid cartilage, where it becomes continuous with the oesophagus (Figs 33.1, 33.2). The laryngeal inlet lies in the upper part of its incomplete anterior wall, and the posterior surfaces of the arytenoid and cricoid cartilages lie below this opening.
A small piriform fossa lies on each side of the laryngeal inlet, bounded medially by the aryepiglottic fold and laterally by the thyroid cartilage and thyrohyoid membrane. Branches of the internal laryngeal nerve lie beneath its mucous membrane. At rest, the laryngopharynx extends posteriorly from the lower part of the third cervical vertebral body to the upper part of the sixth. During deglutition it may be elevated considerably by the hyoid elevators.
The obliquely sloping inlet of the larynx lies in the anterior part of the laryngopharynx and is bounded above by the epiglottis, below by the arytenoid cartilages of the larynx, and laterally by the aryepiglottic folds (Fig. 33.1). Below the inlet, the anterior wall of the laryngopharynx is formed by the posterior surface of the cricoid cartilage.
PHARYNGEAL FASCIA
The two named layers of fascia in the pharynx are the pharyngobasilar and buccopharyngeal fascia. The fibrous layer that supports the pharyngeal mucosa is thickened above the superior constrictor to form the pharyngobasilar fascia (see Fig. 33.9). It is attached to the basilar part of the occipital bone and the petrous part of the temporal bone medial to the pharyngotympanic tube, and to the posterior border of the medial pterygoid plate and the pterygomandibular raphe. Inferiorly, it diminishes in thickness, but is strengthened posteriorly by a fibrous band attached to the pharyngeal tubercle of the occipital bone which descends as the median pharyngeal raphe of the constrictors. This fibrous layer is really the internal epimysial covering of the muscles and their aponeurotic attachment to the base of the skull. The thinner, external part of the epimysium is the buccopharyngeal fascia, which covers the superior constrictor and passes forwards over the pterygomandibular raphe to cover buccinator.
PHARYNGEAL TISSUE SPACES
Pharyngeal tissue spaces can be subdivided into peripharyngeal and intrapharyngeal spaces. The anterior part of the peripharyngeal space is formed by the submandibular and submental spaces, posteriorly by the retropharyngeal space and laterally by the parapharyngeal spaces. The retropharyngeal space is an area of loose connective tissue which lies behind the pharynx and anterior to the prevertebral fascia, extending upwards to the base of the skull and downwards to the retrovisceral space in the infrahyoid part of the neck. Each parapharyngeal space passes laterally around the pharynx and is continuous with the retropharyngeal space. However, unlike the retropharyngeal space, it is a space which is restricted to the suprahyoid region. It is bounded medially by the pharynx, laterally by the pterygoid muscles (where it is part of the infratemporal fossa) and by the sheath of the parotid gland, superiorly by the base of the skull, and inferiorly by suprahyoid structures, particularly the sheath of the submandibular gland. An intrapharyngeal space potentially exits between the inner surface of the constrictor muscles and the pharyngeal mucosa. Infections in this space are either restricted locally or spread through the pharynx into the retropharyngeal or parapharyngeal spaces. The peritonsillar space is an important part of the intrapharyngeal space: it lies around the palatine tonsil between the pillars of the fauces. Infections in the intratonsillar space usually spread up or down the intrapharyngeal space, or through the pharynx into the parapharyngeal space.
Tissue spaces between the layers of cervical fascia are described in Chapter 28: tissue spaces around the larynx are described in Chapter 34.
SPREAD OF INFECTION
Infection that spreads into the parapharyngeal space will produce pain and trismus. There may be swelling in the oropharynx which extends up to the uvula, displacing it to the contralateral side, and dysphagia. Posterior spread from the parapharyngeal space into the retropharyngeal space will produce bulging of the posterior pharyngeal wall, dyspnoea and nuchal rigidity. Involvement of the carotid sheath may produce symptoms caused by thrombosis of the internal jugular vein and cranial nerve symptoms involving the glossopharyngeal, vagus, cranial accessory and hypoglossal nerves. If the infection continues to spread unchecked, mediastinitis will ensue. A virulent infection in the retropharyngeal space may spread through the prevertebral fascia into the underlying prevertebral space: infection in this tissue space may descend into the thorax and even below the diaphragm, and results in chest pain, severe dyspnoea and retrosternal discomfort.
Pharyngeal infection from mucosa-associated lymph tissues such as the palatine tonsil, or as a result of a penetrating injury (e.g. from an ingested foreign body), may result in the spread of infection into the tissue spaces of the neck adjacent to the pharynx. This is an extremely dangerous situation because there is potential for rapid spread throughout the neck and, more dangerously, to the superior mediastinum, to cause overwhelming life-threatening infection.
PARAPHARYNGEAL SPACE TUMOURS
Tumours that develop in the parapharyngeal tissue space may remain asymptomatic for some time. When they do present, it may be with a diffuse pattern of symptoms, reflecting the effects of compression on the lower cranial nerves, e.g. dysarthria, resulting from impairment of tongue movements secondary to hypoglossal nerve damage; dysphagia, with overspill and aspiration of ingested material into the airway, resulting from loss of sensory information from the territory of the pharyngeal plexus nerves; motor dysfunction of the pharynx and larynx, resulting from loss of motor innervation via the pharyngeal plexus and the recurrent laryngeal branch of the vagus to the intrinsic muscles of the larynx.
MUSCLES OF THE SOFT PALATE AND PHARYNX
The muscles of the soft palate and pharynx are levator veli palatini, tensor veli palatini, palatoglossus, palatopharyngeus, musculus uvulae, salpingopharyngeus, stylopharyngeus, and the superior, middle and inferior constrictors.
Levator veli palatini
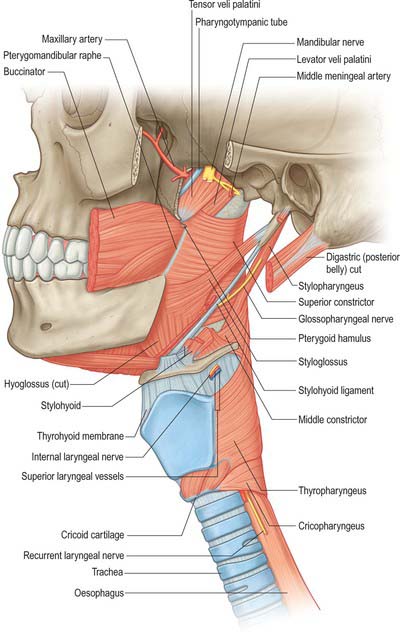
Levator veli palatini arises by a small tendon from a quadrilateral roughened area on the medial end of the inferior surface of the petrous part of the temporal bone, in front of the lower opening of the carotid canal (Figs 33.4, 33.7, 33.8, 33.9). Additional fibres arise from the inferior aspect of the cartilaginous part of the pharyngotympanic tube and from the vaginal process of the sphenoid bone. At its origin the muscle is inferior rather than medial to the pharyngotympanic tube and only crosses medial to it at the level of the medial pterygoid plate. It passes medial to the upper margin of the superior constrictor and anterior to salpingopharyngeus. Its fibres spread in the medial third of the soft palate between the two strands of palatopharyngeus to attach to the upper surface of the palatine aponeurosis as far as the midline, where they interlace with those of the contralateral muscle. Thus, the two levator muscles form a sling above and just behind the palatine aponeurosis.
The blood supply of levator veli palatini is derived from the ascending palatine branch of the facial artery and the greater palatine branch of the maxillary artery.
Levator veli palatini is innervated from the cranial part of the accessory nerve via the pharyngeal plexus.
The primary role of the levator veli palatini muscles is to elevate the almost vertical posterior part of the soft palate and pull it slightly backwards: during swallowing, the soft palate is elevated so that it touches the posterior wall of the pharynx, separating the nasopharynx from the oropharynx. By additionally pulling on the lateral walls of the nasopharynx posteriorly and medially, the levator veli palatini muscles also narrow that space. The muscle has little or no effect on the pharyngotympanic tube, although it might allow passive opening.
Tensor veli palatini
Tensor veli palatini arises from the scaphoid fossa of the pterygoid process and posteriorly from the medial aspect of the spine of the sphenoid bone (Figs 33.4, 33.7, 33.8, 33.9; see Fig. 30.6). Between these two sites it is attached to the anterolateral membranous wall of the pharyngotympanic tube (including its narrow isthmus where the cartilaginous medial two-thirds meets the bony lateral one-third). Some fibres may be continuous with those of tensor tympani. Inferiorly, the fibres converge on a delicate tendon that turns medially around the pterygoid hamulus to pass through the attachment of buccinator to the palatine aponeurosis and the osseous surface behind the palatine crest on the horizontal plate of the palatine bone. There is a small bursa between the tendon and the pterygoid hamulus.
The muscle is thin and triangular and lieslateral to the medial pterygoid plate, pharyngotympanic tube and levator veli palatini. Its lateral surface contacts the upper and anterior part of medial pterygoid, the mandibular, auriculotemporal and chorda tympani nerves, the otic ganglion and the middle meningeal artery.
The blood supply of tensor veli palatini is derived from the ascending palatine branch of the facial artery and the greater palatine branch of the maxillary artery.
The motor innervation of tensor veli palatini is derived from the mandibular nerve via the nerve to medial pterygoid, and reflects the development of the muscle from the first branchial arch.
Acting together, the tensor veli palatini muscles tauten the soft palate, principally its anterior part, and depress it by flattening its arch. Acting unilaterally, the muscle pulls the soft palate to one side. Although contraction of both muscles will slightly depress the anterior part of the soft palate, it is often assumed that the increased rigidity aids palatopharyngeal closure. However, it is now believed that a primary role of the tensor is to open the pharyngotympanic tube, for example during deglutition and yawning. In this way, the muscle equalizes air pressure between the middle ear and nasopharynx.
Palatoglossus
Palatoglossus is narrower at its middle than at its ends (Fig. 33.4). Together with its overlying mucosa it forms the palatoglossal arch or fold (see Fig. 30.3). It arises from the oral surface of the palatine aponeurosis where it is continuous with its contralateral fellow. It extends forwards, downwards and laterally in front of the palatine tonsil to the side of the tongue. Some of its fibres spread over the dorsum of the tongue, others pass deeply into its substance to intermingle with fibres of the intrinsic transverse muscle.
Palatoglossus receives its blood supply from the ascending palatine branch of the facial artery and from the ascending pharyngeal artery.
Palatopharyngeus
Palatopharyngeus and its overlying mucosa form the palatopharyngeal arch (see Fig. 30.3). Within the soft palate, palatopharyngeus is composed of two fasciculi that are attached to the upper surface of the palatine aponeurosis; they lie in the same plane but are separated from each other by levator veli palatini (Figs 33.4, 33.8). The thicker, anterior fasciculus arises from the posterior border of the hard palate as well as the palatine aponeurosis, where some fibres interdigitate across the midline. The posterior fasciculus is in contact with the mucosa of the pharyngeal aspect of the palate, and joins the posterior band of the contralateral muscle in the midline. The two layers unite at the posterolateral border of the soft palate, and are joined by fibres of salpingopharyngeus. Passing laterally and downwards behind the tonsil, palatopharyngeus descends posteromedial to and in close contact with stylopharyngeus, to be attached with it to the posterior border of the thyroid cartilage. Some fibres end on the side of the pharynx, attached to pharyngeal fibrous tissue, and others cross the midline posteriorly, decussating with those of the contralateral muscle. Palatopharyngeus thus forms an incomplete internal longitudinal muscular layer in the wall of the pharynx.
Passavant’s muscle (palatopharyngeal sphincter)
The existence of Passavant’s muscle remains controversial. It has been described as a part of the superior constrictor and palatopharyngeus muscles. An alternative view holds that it is a distinct palatine muscle that arises from the anterior and lateral parts of the upper surface of the palatine aponeurosis, lies lateral to levator veli palatini, blends internally with the upper border of the superior constrictor, and encircles the pharynx as a sphincter-like muscle (Figs 33.4, 33.8). Whatever its origin, when it contracts, it forms a ridge (Passavant’s ridge) when the soft palate is elevated. The change from columnar, ciliated, ‘respiratory’ epithelium to stratified, squamous epithelium that takes place on the superior aspect of the soft palate occurs along the line of attachment of the palatopharyngeal sphincter to the palate. The muscle is hypertrophied in cases of complete cleft palate.
Palatopharyngeus receives its arterial supply from the ascending palatine branch of the facial artery, the greater palatine branch of the maxillary artery and the pharyngeal branch of the ascending pharyngeal artery.
Musculus uvulae
Musculus uvulae arises from the posterior nasal spine of the palatine bone and the superior surface of the palatine aponeurosis, and lies between the two laminae of the aponeurosis (Figs 33.1, 33.8). It runs posteriorly above the sling formed by levator veli palatini and inserts beneath the mucosa of the uvula. The two sides of the muscle are united along most of its length.
The blood supply of musculus uvulae is derived from the ascending palatine branch of the facial artery and the descending palatine branch of the maxillary artery.
The nerve supply to musculus uvulae is derived from the cranial part of the accessory nerve via the pharyngeal plexus.
Salpingopharyngeus
Salpingopharyngeus arises from the inferior part of the cartilage of the pharyngotympanic tube near its pharyngeal opening and passes downwards within the salpingopharyngeal fold to blend with palatopharyngeus (Figs 33.1, 33.4A, 33.8).
Salpingopharyngeus receives its arterial supply from the ascending palatine branch of the facial artery, the greater palatine branch of the maxillary artery and the pharyngeal branch of the ascending pharyngeal artery.
Stylopharyngeus
Stylopharyngeus is a long slender muscle, cylindrical above and flat below. It arises from the medial side of the base of the styloid process, descends along the side of the pharynx, and passes between the superior and middle constrictors to spread out beneath the mucous membrane (Figs 33.4, 33.9; see Fig. 30.6). Some fibres merge into the constrictors and the lateral glossoepiglottic fold, while others join fibres of palatopharyngeus and are attached to the posterior border of the thyroid cartilage. The glossopharyngeal nerve curves round the posterior border and the lateral side of stylopharyngeus, and passes between the superior and middle constrictors to reach the tongue.
Superior constrictor
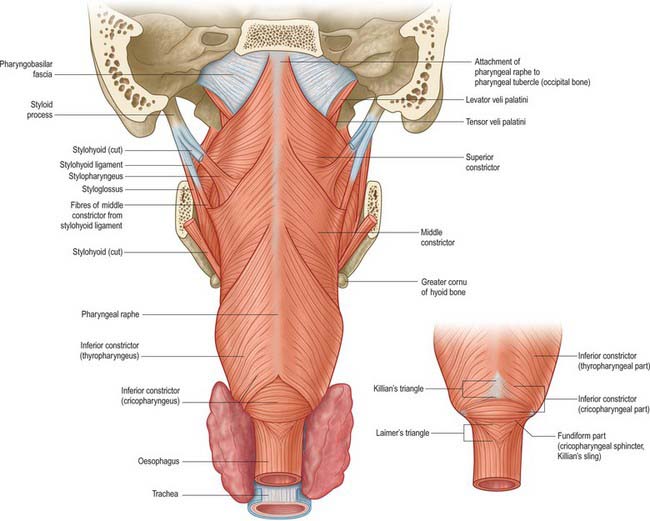
The superior constrictor is a quadrilateral sheet of muscle and is thinner than the other two constrictors. It is attached anteriorly to the pterygoid hamulus (and sometimes to the adjoining posterior margin of the medial pterygoid plate), the posterior border of the pterygomandibular raphe, the posterior end of the mylohyoid line of the mandible, and, by a few fibres, to the side of the tongue (Figs 33.1, 33.4, 33.5, 33.7, 33.9; see Fig. 30.6). The fibres curve back into a median pharyngeal raphe which is attached superiorly to the pharyngeal tubercle on the basilar part of the occipital bone.
The upper border of the superior constrictor is separated from the cranial base by a crescentic interval which contains levator veli palatini, the pharyngotympanic tube and an upward projection of pharyngobasilar fascia. The lower border is separated from the middle constrictor by stylopharyngeus and the glossopharyngeal nerve (Fig. 33.8). Anteriorly, the pterygomandibular raphe separates the superior constrictor from buccinator, and, posteriorly, the superior constrictor lies on the prevertebral muscles and fascia, from which it is separated by the retropharyngeal space. The ascending pharyngeal artery, pharyngeal venous plexus, glossopharyngeal and lingual nerves, styloglossus, middle constrictor, medial pterygoid, stylopharyngeus and the stylohyoid ligament all lie laterally, and palatopharyngeus, the tonsillar capsule and the pharyngobasilar fascia lie internally.
The arterial supply of the superior constrictor is derived mainly from the pharyngeal branch of the ascending pharyngeal artery and the tonsillar branch of the facial artery.
Middle constrictor
The middle constrictor is a fan-shaped sheet attached anteriorly to the lesser cornu of the hyoid and the lower part of the stylohyoid ligament (the chondropharyngeal part of the muscle), and to the whole of the upper border of the greater cornu of the hyoid (the ceratopharyngeal part) (Figs 33.4A, 33.7, 33.9; see Fig. 30.6). The lower fibres descend deep to the inferior constrictor to reach the lower end of the pharynx; the middle fibres pass transversely and the superior fibres ascend and overlap the superior constrictor. All fibres insert posteriorly into the median pharyngeal raphe.
The glossopharyngeal nerve and stylopharyngeus pass through a small gap between the middle and superior constrictors, and the internal laryngeal nerve and the laryngeal branch of the superior thyroid artery pass between the middle and inferior constrictors. The prevertebral fascia and longus colli and longus capitis are posterior, the superior constrictor, stylopharyngeus and palatopharyngeus are internal, and the carotid vessels, pharyngeal plexus of nerves and some lymph nodes are lateral. Near its hyoid attachment, the middle constrictor lies deep to hyoglossus, from which it is separated by the lingual artery.
The arterial supply of the middle constrictor is derived mainly from the pharyngeal branch of the ascending pharyngeal artery and the tonsillar branch of the facial artery.
Inferior constrictor
The inferior constrictor is the thickest of the three constrictor muscles, and is usually described in two parts, thyropharyngeus and cricopharyngeus (Figs 33.4A, 33.7, 33.9; see Fig. 30.6). Thyropharyngeus arises from the oblique line of the thyroid lamina, a strip of the lamina behind this, and by a small slip from the inferior cornu. Some additional fibres arise from a tendinous cord that loops over cricothyroid. Cricopharyngeus arises from the side of the cricoid cartilage between the attachment of cricothyroid and the articular facet for the inferior thyroid cornu. Some authors have described cricopharyngeus as consisting of a superficial upper oblique portion, the pars oblique, and a lower, deeper, transverse portion, the pars fundiformis. The upper part attaches to the median raphe while the lower part forms a circular band that lacks a median raphe. The area demarcated by the pars oblique and pars fundiformis of cricopharyngeus is termed Killian’s dehiscence (or Killian’s triangle). A second triangular area, Laimer’s triangle, can be identified beneath cricopharyngeus between the longitudinal fibres of the oesophagus as they pass laterally on either side to attach to the cricoid cartilage: only the circular muscle of the oesophagus forms the wall here. Both triangles are postulated to be sites of weakness in the wall of the pharynx and oesophagus, and are therefore areas where diverticula could potentially form. Both cricopharyngeus and thyropharyngeus spread posteromedially to join the contralateral muscle. Thyropharyngeus is inserted into the median pharyngeal raphe, and its upper fibres ascend obliquely to overlap the middle constrictor; however, cricopharyngeus blends with the circular oesophageal fibres around the narrowest part of the pharynx.
The buccopharyngeal fascia is external, the prevertebral fascia and muscles are posterior, the thyroid gland, common carotid artery and sternothyroid are lateral, and the middle constrictor, stylopharyngeus, palatopharyngeus and the fibrous lamina are internal. The internal laryngeal nerve and laryngeal branch of the superior thyroid artery reach the thyrohyoid membrane by passing between the inferior and middle constrictors. The external laryngeal nerve descends on the superficial surface of the muscle, just behind its thyroid attachment, and pierces its lower part. The recurrent laryngeal nerve and the laryngeal branch of the inferior thyroid artery ascend deep to its lower border to enter the larynx.
The arterial supply of the inferior constrictor is derived mainly from the pharyngeal branch of the ascending pharyngeal artery and the muscular branches of the inferior thyroid artery.
Both parts of the inferior constrictor are usually supplied by the cranial part of the accessory nerve from the pharyngeal plexus. Although controversial, available evidence in humans suggests that cricopharyngeus is also supplied by the recurrent laryngeal nerve and the external branch of the superior laryngeal nerve.
Thyropharyngeus constricts the lower part of the pharynx. Cricopharyngeus is the main component of the upper oesophageal sphincter, or pharyngoesophageal high-pressure zone, the other parts being thyropharyngeus and the proximal cervical oesophagus. (The extent to which the lower fibres of thyropharyngeus and the upper fibres of the oesophageal musculature are involved in closing the upper end of the oesophagus appears to depend on the physiological state, whereas cricopharyngeus always participates in closure.) The upper oesophageal sphincter is defined manometrically as a region of elevated intraluminal pressure, 2–4 cm long, located at the junction of the hypopharynx and cervical oesophagus.
Cricopharyngeus contains about 40% of endomysial connective tissue, much of which is elastic, but it lacks muscle spindles. It contains both slow-twitch type I and fast-twitch type II fibres, a structural arrangement that underpins the various functions of the upper oesophageal sphincter, i.e. maintaining constant basal tone, yet being able to relax and contract rapidly during swallowing, belching and vomiting. The tonic activity of cricopharyngeus between swallows prevents influx of air during inspiration and tracheobronchial aspiration and pharyngeal reflux of oesophageal contents during oesophageal peristalsis. For further reading, see Lang and Shaker (2000).
Hypopharyngeal diverticula
The pharyngeal mucosa that lies between cricopharyngeus and thyropharyngeus is relatively unsupported by pharyngeal muscles and is called the dehiscence of Killian. A delay in the relaxation of cricopharyngeus, which can occur when the swallowing mechanism becomes discoordinated, generates a zone of elevated pressure adjacent to the mucosa in the dehiscence. The result is the development of a pulsion diverticulum (a pouch of prolapsing mucosa), which breaches the thin muscle wall adjacent to the sixth cervical vertebra and expands, usually a little to the left side, into the parapharyngeal potential space. This may trap portions (or all) of the passing food bolus, resulting in regurgitation of old food, aspiration pneumonia, halitosis and weight loss. Treatment may involve open excision or inversion of the pouch to prevent it filling, coupled with division of the circular fibres of cricopharyngeus, to prevent the build-up of pressure in the region and recurrence of the pouch.
PHARYNGEAL PLEXUS
Almost all of the nerve supply to the pharynx, whether motor or sensory, is derived from the pharyngeal plexus, which is formed by the pharyngeal branches of the glossopharyngeal and vagus nerves with contributions from the superior cervical sympathetic ganglion. The plexus lies on the external surface of the pharynx, especially on the middle constrictor. Filaments from the plexus ascend or descend external to the superior and inferior constrictors before branching within the muscular layer and mucosa of the pharynx.
The pharyngeal branch of the vagus supplies all the muscles of the pharynx (excluding stylopharyngeus, which is supplied by the glossopharyngeal nerve) and of the soft palate (excluding tensor veli palatini, which is supplied by the mandibular division of the trigeminal via the nerve to medial pterygoid). It emerges from the upper part of the inferior vagal ganglion and consists mainly of filaments derived from the cranial accessory nerve: almost all the neuronal cell bodies are in the nucleus ambiguus. The nerve passes between the external and internal carotid arteries to reach the upper border of the middle pharyngeal constrictor, and subsequently divides into numerous filaments which contribute to the pharyngeal plexus. It also gives off a minute filament, the ramus lingualis vagi, which joins the hypoglossal nerve as it curves round the occipital artery.
ANATOMY OF SWALLOWING (DEGLUTITION)
Swallowing involves a series of activities that occur within a matter of seconds. Traditionally described as a reflex, the process is more properly regarded as a programmed motor behaviour. Swallowing is initiated when food or liquid stimulates sensory nerves in the oropharynx. In a 24-hour period, an average person will swallow between 600 and 1000 times, but, of these, only some 150 will relate to feeding; the remainder occur to clear continuously produced saliva and are less frequent at night.
Eating and drinking are basic human pleasures, and problems associated with swallowing can impact dramatically upon the quality of life. Swallowing disorders are usually symptoms of other complex diseases: an inability to swallow may adversely affect nutritional status and therefore indirectly exacerbate the underlying disease. Aside from the risk of asphyxiation through choking, swallowing disorders can also be a direct cause of morbidity and mortality as a result of aspiration of food, liquid or possibly refluxed gastric acid contents, causing bacterial infection or tissue damage.
Swallowing in the adult human has traditionally been studied in relation to swallowing solid or liquid food carried out on command. For descriptive purposes, the process has been divided into four phases: oral preparatory, oral transit/transfer, pharyngeal and oesophageal. However, the boundaries between these phases are not entirely clear, thus, for example, the demarcation between the first and second phases is defined primarily by convention. Moreover, although it has long been thought that the oral and pharyngeal stages of swallowing were clearly separated, some authors consider that the initiation of the swallow entails both oral and pharyngeal movements, especially when swallowing solid food rather than saliva or other liquids.
Food is reduced in the mouth to a consistency suitable for swallowing and is formed into a cohesive bolus (oral preparatory phase). The bolus is delivered to the oropharynx (oral transit/transfer phase), transported down the pharynx past the airways and through the upper oesophageal sphincter (pharyngeal phase), and then transported down the oesophagus to the stomach (oesophageal phase). The oral preparatory and oral transit/transfer phases are voluntary and under cortical control, whereas the pharyngeal and oesophageal phases are involuntary and controlled by the brainstem. Airway protection is vital during the pharyngeal phase.
ORAL PREPARATORY PHASE
In the oral preparatory phase, the essential action is chewing: the mandible is moved by the action of the jaw elevators and depressors (see Ch. 31), and the food is reduced by the grinding action of the teeth and simultaneously mixed with saliva. The lips are maintained as a tight labial seal by the contraction of orbicularis oris: buccinator performs a similar function for the cheeks. In this way, the sulci are closed, the vestibule normally remains empty, and any food that enters the vestibule is returned to the oral cavity proper. Buccinator also keeps the cheeks taut, ensuring that they are kept clear of the occlusal surfaces of the teeth during chewing: loss of the nerve supply to buccinator as a result of damage to the facial nerve results in painful and repeated lacerations of the cheeks.
The soft palate is depressed during this phase and premature spillage of food is common. Spillage occurs because the soft palate is not in continuous contact with the posterior part of the tongue, as was once thought (Hiiemae & Palmer 1999). Bolus formation appears to involve several cycles of food being transported from the anterior to the posterior part of the tongue through the palatoglossal and palatopharyngeal arches until a bolus accumulates on the oropharyngeal surface of the tongue (retrolingual loading). Throughout this phase, the lateral and rotatory tongue movements that deliver the food to the teeth for grinding and reduction are crucial for normal bolus formation. If effective tongue movements do not occur, chewing will be compromised. The end of this phase of swallowing is marked by the tongue holding the bolus of food that has been formed against the hard palate in readiness for transport to the posterior part of the oral cavity.
ORAL TRANSIT/TRANSFER PHASE
In the oral transit/transfer phase, the bolus formed in the oral preparatory stage is finally transported through the palatoglossal and palatopharyngeal arches into the oropharynx.
Genioglossus raises both the tongue tip and the part of the tongue immediately behind the tip. The soft palate is then fully lowered by contraction of palatoglossus and palatopharyngeus, and the posterior part of the tongue is simultaneously elevated: the apposed soft palate and tongue form a tight seal that helps to prevent premature entry of the bolus into the pharynx. Orbicularis oris and buccinator remain contracted, so keeping the lips and cheeks taut and the bolus of food central in the oral cavity. The bolus is accommodated in a shallow midline gutter that forms along the dorsum of the tongue, probably as a result of the co-contraction of the styloglossi and the genioglossi, aided by the superior longitudinal and transverse fibres of the intrinsic muscles.
The mandible is elevated and the mouth is closed. The floor of the mouth and the anterior and middle portions of the tongue are elevated, by co-contraction of the suprahyoid group of muscles (mylohyoid, digastric, geniohyoid and stylohyoid): the effectiveness of the suprahyoid muscles is increased as they contract against a fixed mandible (the mouth does not have to be closed to swallow, but it is much harder to swallow if it is open). Contraction of stylohyoid elevates the more posterior parts of the tongue and empties the longitudinal gutter. At the same time, the tongue flattens, probably as a result of the contraction of hyoglossus and some of the intrinsic lingual muscles, especially the vertical fibres. The elevated, flattened tongue pushes the bolus against the hard palate, and the sides of the tongue seal against the maxillary alveolar processes, helping to move the bolus further posteriorly. Contraction of styloglossus and mylohyoid completes the elevation of the posterior part of the tongue. At the same time, the posterior oral seal relaxes and the posterior tongue moves forward: the overall effect is of a cam-like action of the tongue, sweeping or squeezing the bolus towards the pillars of the fauces, finally delivering it to the oropharynx where the pharyngeal aperture is initially increased and then closed.
PHARYNGEAL PHASE
The delivery of the bolus to the oropharynx triggers the pharyngeal phase of the swallow. This phase, which is involuntary and the most critical stage of swallowing, involves the pharynx changing from being an air channel (between the posterior nares and laryngeal inlet) to a food channel (from the fauces to the upper end of the oesophagus). The airway is protected from aspiration during swallowing by hyolaryngeal elevation, and by resetting respiratory rhythm so that airflow ceases briefly as the bolus passes through the hypopharynx: the total time that elapses from the bolus triggering the pharyngeal phase to the re-establishment of the airway is barely 1 second.
The nasopharynx is sealed off from the oropharynx by activation of the superior pharyngeal constrictor and contraction of a subset of palatopharyngeal fibres to form a variable, ridge-like structure (Passavant’s ridge) against which the soft palate is elevated. From an evolutionary perspective, this ridge represents the remnant of a sphincter which encircled a more highly placed larynx: a high laryngeal position is the norm in other mammals and in the human infant (see below), but not in the human adult. Interestingly, the pharyngeal ridge becomes hypertrophic in an infant with a cleft palate, presumably in an attempt to produce a seal to the nasal airway. Ineffective velopharyngeal closure may result in nasal regurgitation of food.
The airway is sealed at the laryngeal inlet by closure of the glottis (see Ch. 34), and the epiglottis is retroflexed over the laryngeal aditus as a result of passive pressure from the base of the tongue and active contraction of the aryepiglottic muscles. The conventional view that laryngeal closure during swallowing occurs from inferior to superior, i.e the vocal folds adduct first and the epiglottis covers the arytenoids and glottis last, has been challenged by studies using simultaneous electromyography and fibreoptic endoscopic evaluation of swallowing, FEES, which have reported that the aryepiglottic folds close before vocal fold adduction during a swallow. It is probably reasonable to assume that the sequence of events that close the glottis may alter according to the type of swallow and consistency of the bolus. What is not in dispute is that the hyoid bone and larynx are raised and pulled anteriorly by the suprahyoid muscles and the longitudinal muscles of the pharynx, so that the laryngeal inlet is brought forward under the bulge of the posterior tongue, i.e. out of the path of the bolus. This action helps expand the hypopharyngeal space and relax the upper oesophageal sphincter, which is also raised by several centimetres. The bolus passes over the reflected anterior surface of the epiglottis and is swept through the laryngopharynx to the upper oesophageal sphincter.
The sequential contraction of the three pharyngeal constrictor muscles is often assumed to be the driving force propelling the bolus towards the oesophagus. However, evidence that the head of the bolus moves faster than the wave of pharyngeal contraction suggests that, at least in some situations, the kinetic energy imparted to the bolus as it is expelled from the mouth into the oropharynx may be sufficient to carry it through the pharynx. This energy is generated by pressure gradients created within the pharynx by the tongue driving force, the hypopharyngeal suction pump, and the ‘stripping action’ of the pharyngeal constrictors.
The tongue driving force, or the tongue thrust pressure force, is a positive pressure that squeezes the bolus towards the laryngopharynx. It is generated by the upward movement of the tongue pressing the bolus against the contracting pharyngeal wall and requires a tight nasopharyngeal seal (created by elevation of the soft palate). There is a view that the tongue driving force is the most important factor responsible for moving the bolus down the pharynx. The hypopharyngeal suction pump is caused by the elevation and anterior movement of the hyoid and larynx, which creates a negative pressure in the laryngopharynx, drawing the bolus towards the oesophagus, aided by a more negative pressure inside the oesophagus. The pharyngeal constrictors generate a positive pressure wave behind the bolus. Their sequential contraction may facilitate clearance (‘stripping’) of the pharyngeal walls and piriform sinuses: if this is so, residues that remain in the valleculae must reflect inadequate tongue force generation at the end of the oral phase of swallowing.
Gag reflex
Traditionally, the stimulus for triggering a swallow has been regarded as contact with the posterior wall of the pharynx, since this is usually where the gag reflex is triggered (see p. 283 and Fig. 19.12). However, it has become clear that many regions of the oropharynx, when appropriately stimulated by the presence of food or liquid, are capable of triggering a swallow, although some regions are more sensitive than others, e.g. the area over the palatoglossal arches. Moreover, there appears to be little relationship between a functioning gag reflex and the ability to swallow normally. Individuals with a reduced or absent gag reflex can swallow safely; conversely, the presence of a brisk and clear gag reflex is not always associated with the ability to swallow normally.
OESOPHAGEAL PHASE
The third, or oesophageal, stage begins after the relaxation of the upper oesophageal sphincter has allowed the bolus to enter the oesophagus. Sequential waves of contractions of the oesophageal musculature subsequently propel the bolus down to the lower oesophageal sphincter, which opens momentarily to admit the bolus to the stomach. The oesophageal phase of swallowing is much more variable than the other phases, and lasts between 8 and 20 seconds.
SWALLOWING PATTERN GENERATOR
The patterning and timing of striated muscle contraction during swallowing are generated at a brainstem level in a network of neural circuits which collectively form a central swallowing pattern generator (SPG). Experimental neurophysiological and tracer studies in animals have shown that the SPG includes several brainstem motor nuclei and two groups of interneurones in the dorsal and ventral medulla, a dorsal swallowing group (DSG) and a ventral swallowing group (VSG). The DSG is located in the nucleus of the solitary tract and possibly in the adjacent reticular formation, and contains neurones that generate the swallowing pattern, probably triggered by convergent information from both cortical and peripheral inputs. The afferent feedback from the branches of the superior laryngeal nerve which innervate the valleculae, epiglottis and supraglottic part of the larynx, and which relay through the nucleus of the solitary tract, facilitates laryngeal closure during swallowing (Jafari et al 2003). The VSG is in the ventrolateral medulla above the nucleus ambiguus and contains neurones that act as ‘switches’, distributing the swallowing drive to motoneurone pools in the trigeminal, facial and hypoglossal nuclei and in the nucleus ambiguus.
The patterns of activation in the smooth muscle of the lower part of the oesophagus are generated locally in intramural plexuses driven by vagal autonomics.
Supramedullary influence on swallowing
Techniques including transcranial magnetic stimulation, cortical evoked potentials, positron emission tomography and functional magnetic resonance imaging (fMRI) have all been exploited in the study of volitional swallowing in humans. Thus far, these studies have shown that numerous cortical and subcortical regions are recruited in swallowing, although, as yet, the functional contributions made by each region have not been established, and it is not known how they influence the SPG. The most consistent cortical activation occurs in the primary motor and somatosensory cortices. Perhaps not surprisingly, the greatest area of fMRI activation in the primary motor cortex occurs over the portion of the precentral gyrus where the face, tongue and pharynx are represented: there is evidence for functional asymmetry between the right and left oral sensorimotor cortices (Martin et al 2004). The activity of the SPG is coordinated with other medullary reflexes, e.g. it is difficult to elicit a swallow when the cortical masticatory centres are stimulated.
SWALLOWING IN THE NEONATE
In the adult, the tip of the epiglottis is significantly lower than the inferior edge of the soft palate. In the neonate, the larynx is high in the neck and the epiglottis may extend above the soft palate so that the laryngeal airway is in direct continuity with the posterior nares (Fig. 33.10): a potential space is therefore formed between the soft palate above, the epiglottis behind and the tongue anteroinferiorly. In other mammals with an oropharyngeal anatomy similar to that of the human infant, up to 14 cycles of tongue movement or oral phases cause the accumulation of food in this space. Subsequent emptying of the space is a single event followed by movement of the bolus down the oesophagus. The ratio of accumulation cycles to swallow events in the human neonate is approximately 1.5 : 1, which is lower than in other mammals, but still implies some temporary accumulation. In the case of a liquid bolus, accumulated material may be passed laterally to the epiglottis through the piriform fossae rather than over the flexed epiglottis, although it is not known whether this happens in the human infant.
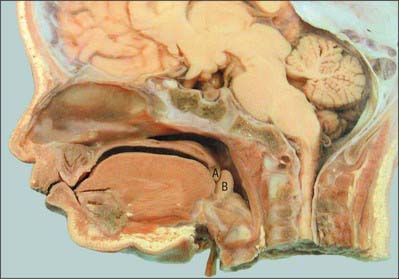
Fig. 33.10 Sagittal section of the head of a neonate. Note the relatively high position of the larynx, the opening being at the level of the soft palate A, B, epiglottis.
(By permission from Berkovitz BKB, Holland GR, Moxham BJ 2002 Oral Anatomy, Embryology and Histology, 3rd edn. Edinburgh: Mosby.)
Swallow safety is critical at all ages. In the neonate, where coordination of suckling, swallowing and breathing is not fully developed, the potential risk of airway obstruction and/or aspiration of ingested milk or other material during swallowing is reduced because the intranarial larynx prevents the bolus from entering the larynx before and after a swallow (see Ch. 34). The change towards adult anatomy and coordination of the phases of swallowing starts a few months after birth. Differential growth in length of the human pharynx causes the larynx to take up its low adult position and the epiglottis to lose contact with the soft palate: the larynx reaches its final position around the time of puberty. The adult anatomy does not allow any significant accumulation of food to occur immediately anterior to the epiglottis, which means that the transport of food through the fauces has to bear a 1 : 1 relationship to pharyngeal and oesophageal transit. Moreover, the lowered larynx compromises the previously protected airway: the hyoid and larynx are therefore raised and pulled forwards during a swallow in order to minimize the risk of deglutitive aspiration.
PHARYNGOSTOMY AND EPIGLOTTOPEXY
Loss of control of the pharyngeal phase of swallowing, e.g. due to neurological disease or ablative head and neck surgery, may result in aspiration of food, especially fluids, leading to pneumonia. This problem may be addressed surgically by pharyngostomy or epiglottopexy. In pharyngostomy, a fine bore feeding tube is passed into the lower oesophagus or stomach via the nose or anterior abdominal wall. Alternatively, a tube may be passed through the cervical skin, fascia and platysma directly into the piriform fossa. This is achieved by passing a curved forcep into the piriform fossa and pushing it laterally, displacing the contents of the carotid sheath and tenting up the platysma and cervical skin from the inside. By cutting down on to the forcep, it is possible to grasp the feeding tube and pull it into the piriform fossa prior to feeding it on into the oesophagus. The tract formed by such a puncture epithelializes and may be used for long-term alimentation. In epiglottopexy, the neck and the pharynx are opened to expose the laryngeal inlet, the aryepiglottic folds are denuded of mucosa to encourage their adhesion, and the epiglottis is sutured down to the aryepiglottic folds to shield the laryngeal inlet. The resultant compromise of the airway can be offset by the creation of an alternative airway via a tracheostomy. The pharyngeal wall and cervical skin are reconstituted by suturing.
Bluestone CD. Anatomy and physiology of the Eustachian tube. Cummings CW, et al, editors. Otolaryngology: Head and Neck Surgery, 3rd edn., vol 4. St Louis: Mosby, 1998;3003-3025.
Bonnington A, Mahon M, Whitmore I. A histological study of the cricopharyngeus muscle in man. J Anat. 1988;156:27-37.
Brownlow H, Whitmore I, Willan PLT. A quantitative study of the histochemical and morphometric characteristics of the human cricopharyngeus muscle. J Anat. 1989;166:67-75.
The above two references provide an account of the detailed anatomy of cricopharyngeus in relation to its function as the main part of the upper oesophageal sphincter..
Freelander E. Blood supply of the human levator and tensor veli palatini muscles. Clin Anat. 1992;5:34-44.
Graney DO, Retruzzelli GJ, Myers EW. Anatomy. Cummings CW, et al, editors. Otolaryngology: Head and Neck Surgery, 3rd edn., vol 2. St Louis: Mosby, 1998;1327-1348.
Provides a concise account of the anatomy of the pharynx, highlighting features of clinical relevance..
Gray SD, Pinborough-Zimmerman J. Velopharyngeal incompetence. In: Cummings CW, et al. Otolaryngology: Head and Neck Surgery, vol 5: Pediatric Otolaryngology. 3rd edn. St Louis: Mosby; 1998:174-187.
Harris ML, Julyan P, Kulkarni B, et al. Mapping metabolic brain activation during human volitional swallowing: a positron emission tomography study using [18F]fluorodeoxyglucose. J Cereb Blood Flow Metab. 2005;25:520-526.
Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14:31-42.
Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550:287-304.
Lang IM, Shaker R. An overview of the upper esophageal sphincter. Curr Gastroenterol Rep. 2000;2:185-190.
Martin RE, MacIntosh BJ, Smith RC, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428-2443.
Sade J, editor. Basic Aspects of the Eustachian Tube and Middle Ear Disease. Geneva: Kugler & Ghedini, 1989.
Thexton A. Some aspects of swallowing. In: Harris M, Edgar M, Meghji S. Clinical Oral Science. Oxford: Wright; 1998:150-166.
Thexton AJ, Crompton AW. The control of swallowing. Linden RWA, editor. The Scientific Basis of Eating. Taste and Smell, Salivation, Mastication and Swallowing and their Dysfunctions. Frontiers of Oral Biology Series vol 9. Basel: Karger; 1998:168-222.
Wood-Jones I. The nature of the soft palate. J Anat. 1940;77:147-170.
Describes the structure of the soft palate and its movements during swallowing..