CHAPTER 67 Large intestine
OVERVIEW
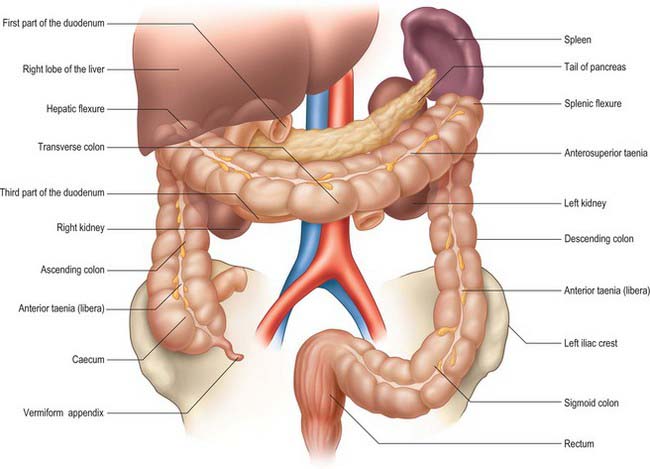
The large intestine extends from the ileocaecal valve to the anus. Broadly speaking, it lies in a curve which tends to form a border around the loops of small intestine that are located centrally within the abdomen (Figs 67.1, 67.2). The large intestine begins in the right iliac fossa as the caecum, from which the vermiform appendix arises. The caecum becomes the ascending colon which passes upwards in the right lumbar region and hypochondrium to the inferior aspect of the liver where it bends to the left forming the hepatic flexure (right colic flexure) and becomes the transverse colon. This loops across the abdomen with an anteroinferior convexity until it reaches the left hypochondrium, where it curves inferiorly to form the splenic flexure (left colic flexure) and becomes the descending colon, which proceeds through the left lumbar and iliac regions to become the sigmoid colon in the left iliac fossa. The sigmoid colon descends deep into the pelvis and becomes the rectum which ends in the anal canal at the level of the pelvic floor. The large intestine is approximately 1.5 m long in adults, although there is considerable variation in its length. Its calibre is greatest near the caecum and gradually diminishes to the level of the sigmoid colon. The rectum is largest in calibre in its lower third and forms the rectal ampulla above the anal canal.
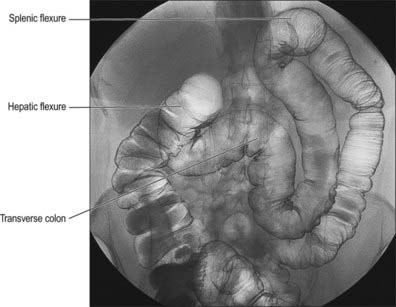
Fig. 67.2 Appearance of the abdominal colon on double contrast barium enema examination demonstrating the transverse colon, hepatic and splenic flexures.
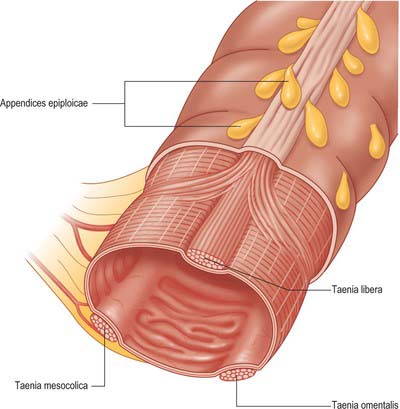
The large intestine differs from the small intestine in several ways: it has a greater calibre; for most of its course it is more fixed in position; its longitudinal muscle, though a complete layer, is concentrated into three longitudinal bands, taeniae coli, in all but the distal sigmoid colon and rectum; small adipose projections, appendices epiploicae, are scattered over the free surface of the whole colon (they tend to be absent from the caecum, vermiform appendix and rectum); the colonic wall is puckered into sacculations (haustrations), which may partly be due to the presence of the taeniae coli, and which may be demonstrated on plain radiographs as incomplete septations arising from the bowel wall.
The large intestine develops as a fully mesenteric organ. However, after the rotation of the gut tube in utero, large portions of it come to lie adherent to the retroperitoneum, which means that some parts of the colon are fixed within the retroperitoneum, and other parts are suspended by a mesentery within the peritoneal cavity. Those portions of the colon within the retroperitoneum are separated from other retroperitoneal structures by a thin layer of connective tissue which forms an avascular field during surgical dissection, but which offers little or no barrier to the spread of disease within the retroperitoneum.
The caecum may be within the retroperitoneum, but more frequently is suspended by a short mesentery. The ascending colon is usually a retroperitoneal structure although the hepatic flexure may be suspended by a mesentery. The transverse colon emerges from the retroperitoneum on a rapidly elongating mesentery and lies, often freely mobile, in the upper abdomen. The transverse mesocolon shortens to the left of the upper abdomen and may become retroperitoneal at the splenic flexure. Occasionally the splenic flexure is suspended by a short mesentery. The descending colon is retroperitoneal usually to the level of the left iliac crest. As the colon enters the pelvis it becomes increasingly more mesenteric again at the origin of the sigmoid colon, although the overall length of the sigmoid mesentery is highly variable. The distal sigmoid colon lies on a rapidly shortening mesentery as it approaches the pelvis; by the level of the rectosigmoid junction the mesentery has all but disappeared, so that the rectum enters the pelvis as a retroperitoneal structure. The caecum and proximal ascending colon are often more mobile on a longer mesentery in the neonate and infant than they are in the adult.
The mesenteries of the colon consist of visceral peritoneum enclosing connective and adipose tissues which envelop the vessels, nerves and lymphatics as they course from the retroperitoneum. Where the colonic mesenteries lie in contact with the retroperitoneum, the (potential) space between the retroperitoneum and mesentery is referred to as the subperitoneal space, which allows free tracking of pathological processes in either direction.
EXTERNAL APPEARANCE
The haustrations of the colon are often absent in the caecum proximal to the origin of the ascending colon and are often relatively sparse in the ascending and proximal transverse colon. In these regions the taeniae coli are usually thin and occupy only a small percentage of the circumference of the colon. There are few if any appendices epiploicae on the serosal surface of the caecum, and only a limited number on the surface of the ascending colon. The haustrations become more pronounced from the middle of the transverse colon to the distal portion of the descending colon: the sigmoid colon is often characterized by marked sacculation. The width of the taeniae coli remains fairly constant throughout the length of colon but the number of appendices epiploicae usually increases, becoming in the sigmoid colon where they can be large in the obese individual. The taeniae are located in fairly constant positions beneath the serosal surface of the colon except in the transverse colon. They are oriented anteriorly on the anti-mesenteric aspect of the colon opposite the midline of the mesenteric attachment (taenia libera), posterolaterally (taenia omentalis) and posteromedially (taenia mesocolica) midway between the taenia libera and the mesentery (Fig. 67.3). In the caecum and descending colon, which are partly retroperitoneal structures, the posterolateral taenia is often obscured from view by the peritoneal reflection onto the colonic wall. In the transverse colon, the taeniae are rotated through 90° as a consequence of the mobility and dependent position of this part of the colon, thus anterior becomes inferior, posteromedial becomes posterior and posterolateral becomes superior. The taeniae coli broaden to occupy more of the circumference of the sigmoid colon in its distal portion and by the level of the rectosigmoid junction they form distinct anterior and posterior bands. These bands subsequently unite to form a complete longitudinal muscle covering for the rectum which therefore has no external sacculation. The rectum also lacks serosal appendices epiploicae.
INTERNAL APPEARANCE
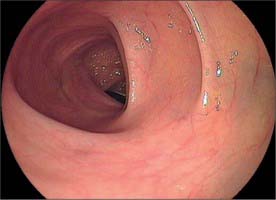
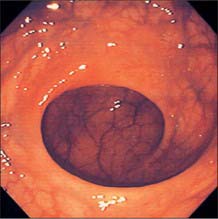
Throughout its length, the internal aspect of the colon is characterized by the presence of haustrations. These infoldings of the wall consist of mucosa and submucosa, and may partially span the lumen, but they never form a complete, circumferential ring. The pattern of the haustrations and appearance of the colonic mucosa help the clinician appreciate the level reached during flexible endoscopic examinations of the colon. In the portion of the caecum where haustrations occur, the three longitudinal taeniae coli converge to form a characteristic ‘trefoil’ pattern on the caecal wall (Fig. 67.4). Elsewhere, the wall of the lower pole of the caecum is usually devoid of haustrations, although a spiral mucosal pattern is often seen in the region of the appendix orifice (see Fig. 67.19). The upper caecum and ascending colon possess shallow but long haustrations which may extend across one-third of the lumen (Fig. 67.5). This pattern is most pronounced in the transverse colon where the long haustrations often confer a triangular appearance to the cross section of the lumen when viewed along the axis (such as at colonoscopy) (Fig. 67.6). The wall of the colon is thinnest in the region of the caecum and ascending colon and is most at risk of perforation during therapeutic endoscopic procedures. The haustrations of the descending and sigmoid colon tend to be thicker and shorter than those of the transverse colon, and this gives a more circular cross-section to the lumen. The overall luminal diameter is often smallest in the descending colon (Fig. 67.7). During endoscopy, the pattern of the submucosal vessels becomes more conspicuous in the sigmoid colon (Fig. 67.8). The mobility of the sigmoid colon on its mesentery means that shorter lengths of colon tend to be visible during endoscopy than anywhere else in the colon. The haustrations of the rectum tend to form consistent and recognizable folds: the pattern of the submucosal vessels is the most pronounced in the colon being of largest calibre with multiple visible veins (Fig. 67.9). Distinct veins are usually visible during endoscopy, and they are most marked above the anorectal junction.
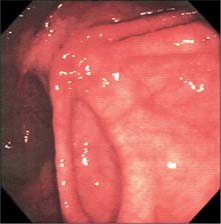
Fig. 67.4 Endoscopic appearance of the caecum. The characteristic trefoil appearance of the confluence of the three taeniae is usually obvious.
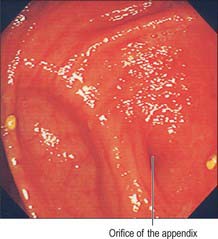
Fig. 67.19 Endoscopic appearance of the orifice of the appendix. The orifice varies from a small depression to an obvious lumenal structure.
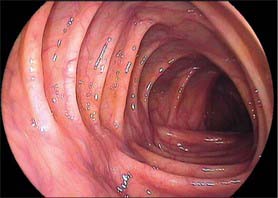
Fig. 67.6 Endoscopic appearance of the transverse colon. Note the characteristic triangular appearance of the haustrations when viewed collectively (see also Fig. 67.10C).
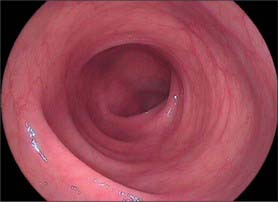
Fig. 67.7 Endoscopic appearance of the descending colon. The lumen tends to look rather more featureless than the more proximal colon.
RADIOGRAPHIC APPEARANCES
Cross-sectional imaging of the colon can be performed with computerized tomography (CT) and magnetic resonance imaging (MRI). On axial imaging the colon may be filled with particulate faeces and air (Fig. 67.10). The wall in normal individuals is thin. The caecum and ascending colon often contain faecal residue and are easily identified in the right retroperitoneum. The transverse colon may contain faeces or gas, but lies in a variable position suspended by its mesentery. The descending colon in the left retroperitoneum is frequently collapsed and contains little faecal residue.
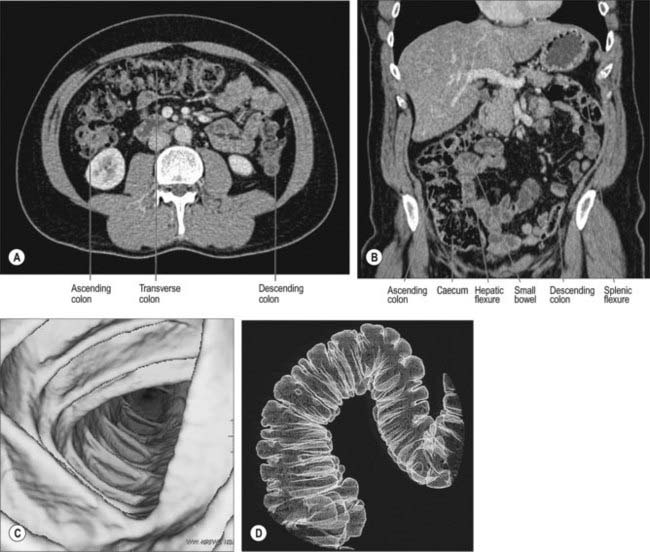
Fig. 67.10 Appearance of the colon on multislice computerized tomographic examination. A, Axial CT. B, Coronal reformat showing normal calibre and distribution of the abdominal colon (ascending, transverse and descending). C, Volume-rendering of the colonic wall using the axial data set to produce virtual colonoscopic views, showing the triangular lumen of the transverse colon. D, Volume-rendering of the air-filled colon using the axial data set to give an image similar to a double contrast barium enema demonstrating haustrations.
(A and B by courtesy of Dr Louise Moore, Chelsea and Westminster Hospital.) (C and D by courtesy of GE Worldwide Medical Systems.)
The volume data sets produced by modern multislice CT can now produce virtual colonoscopic mucosal images in the distended and cleaned colon, and surface-rendered images of the internal surface of the bowel.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
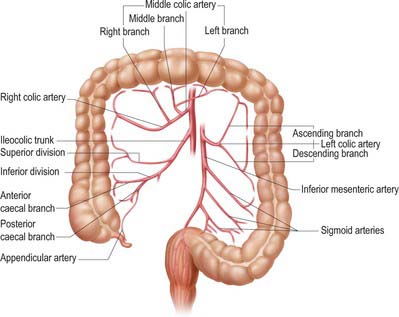
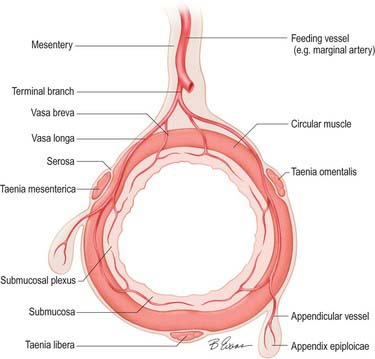
The arterial supply of the large intestine is derived from both the superior and inferior mesenteric arteries (Fig. 67.11). The caecum, appendix, ascending colon and right two-thirds of the transverse colon (derived from the midgut) are supplied from ileocolic, right colic and middle colic branches of the superior mesenteric artery. The left part of the transverse, descending and sigmoid colon, rectum and upper anal canal (hindgut derivatives) are supplied predominantly from the inferior mesenteric artery via the left colic, sigmoid and superior rectal arteries, with small contributions from branches of the internal iliac artery (the middle and inferior rectal arteries). The larger unnamed branches of these vessels ramify between the muscular layers of the colon which they supply. They subdivide into smaller submucosal rami and enter the mucosa. The terminal branches divide into vasa brevia and vasa longa which either enter the colonic wall directly or run through the subserosa for a short distance before crossing the circular smooth muscle to give off branches to the appendices epiploicae (Fig. 67.12).
Arterial anastomoses and the marginal artery of the colon
The arterial supply to the large intestine has less profuse anastomoses and ‘cross-over’ of arterial territories than the small intestine but there are several areas and anastomoses which provide for some degree of anatomical collateral supply to areas of the large intestine.
The marginal artery (of Drummond) of the colon is the name given to the vessel which lies closest to and parallels the wall of the large intestine. It is formed by the main trunks, and the arcades arising from, the ileocolic and right colic, middle colic and left colic arteries. It is most apparent in the ascending, transverse and descending colons (Fig. 67.13). The sigmoid colon has little if any marginal artery. Anastomoses form between the main terminal branches which run parallel to the colon within the mesentery and give rise to vasa recta and vasa brevia to supply the colon. The marginal artery in the region of the splenic flexure may be absent or of such small calibre as to be of little clinical relevance. It may hypertrophy significantly when one of the main visceral arteries is compromised, e.g. following stenosis or occlusion of the inferior mesenteric artery, and it then provides a vessel of collateral supply.
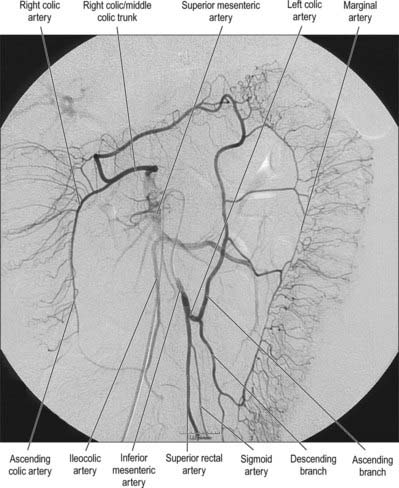
Fig. 67.13 The marginal artery running parallel to the colon and anastomosing with the branches of the superior mesenteric artery supplying the right side of the colon, digital subtraction arteriogram.
(By courtesy of Dr J Jackson, Hammersmith Hospital, London.)
The arc of Riolan is the name given to the arterial anastomosis which is sometimes present in the mesentery between the right side of the transverse colon and the upper descending colon. When present it is formed from a large branch of the middle colic artery which runs parallel and posterior to the middle colic artery in the transverse mesocolon and anastomoses with an ascending branch of the left colic artery close to its origin in the root of the mesentery. This provides a direct communication between the superior and inferior mesenteric arteries.
Colonic vascular occlusion
The marginal artery of the colon may become massively dilated when there is chronic, progressive occlusion of the superior mesenteric artery, because under these conditions it is required to supply the majority of the midgut (except the proximal portion which is supplied by collateral vessels from the coeliac artery). Occlusion of the aorta or common iliac arteries may also result in dilatation of the marginal and inferior mesenteric arteries, which become an important collateral supply to the legs via dilated middle rectal vessels arising from the internal iliac artery.
Occlusion of the inferior mesenteric artery does not always result in irreversible ischaemia of the descending and sigmoid colon, because the marginal artery of the colon usually receives an adequate supply from the left branch of the middle colic artery. Moreover, the sigmoid arteries may be supplied by the superior rectal artery, which anastomoses with the middle and inferior rectal arteries. When ischaemia does occur, it is usually maximal in the proximal descending colon because this region is furthest from the collateral arterial supplies.
Veins
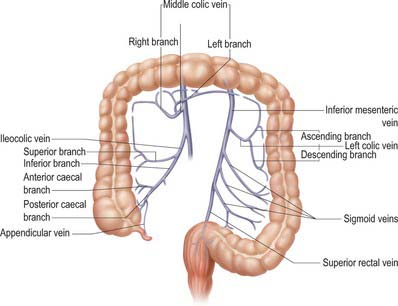
The venous drainage of the large intestine is primarily into the hepatic portal vein via the superior mesenteric and inferior mesenteric veins, although a small amount of drainage from the rectum occurs via middle rectal veins into the internal iliac veins and via inferior rectal veins into the pudendal vein. Those parts of the colon derived from the midgut (caecum, appendix, ascending colon and right two-thirds of the transverse colon) drain into colic branches of the superior mesenteric vein, while hindgut derivatives (left part of the transverse, descending and sigmoid colon, rectum and upper anal canal) drain into the inferior mesenteric vein (Fig. 67.14).
Lymphatics
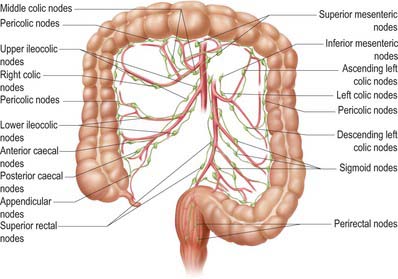
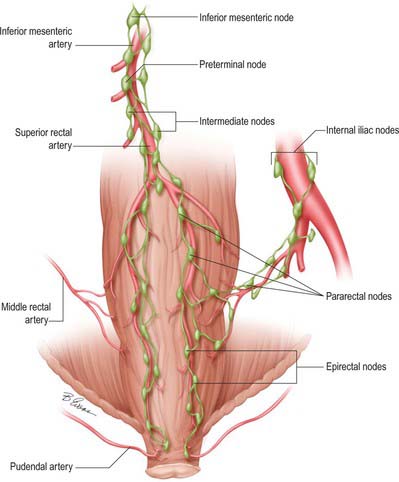
Lymphatic vessels of the caecum, ascending and proximal transverse colon drain ultimately into lymph nodes related to the superior mesenteric artery, while those of the distal transverse colon, descending colon, sigmoid colon and rectum drain into nodes following the course of the inferior mesenteric artery (Fig. 67.15). In cases where the distal transverse colon or splenic flexure is predominantly supplied by vessels from the middle colic artery, the lymphatic drainage of this area may be predominantly to superior mesenteric nodes.
Colic nodes
Lymph nodes related to the colon form four groups, namely epicolic, paracolic, intermediate colic and preterminal colic nodes. Epicolic nodes are minute nodules on the serosal surface of the colon, sometimes in the appendices epiploicae. Paracolic nodes lie along the medial borders of the ascending and descending colon and along the mesenteric borders of the transverse and sigmoid colon. Intermediate colic nodes lie along the named colic vessels (the ileocolic, right colic, middle colic, left colic, sigmoid and superior rectal arteries). Preterminal colic nodes lie along the main trunks of the superior and inferior mesenteric arteries and drain into para-aortic nodes at the origin of these vessels. These are commonly referred to as the highest nodes of the territory which they drain.
Lymph node clearance in colorectal cancer resections
Radical lymphadenectomy during resection for colorectal cancer requires removal of the highest possible lymph node draining the area of colon in which the tumour is located. In cases of cancer involving the rectum and sigmoid colon, this usually involves resection of the preterminal colic nodes of the inferior mesenteric artery and thus ligation of the inferior mesenteric artery at its root or just below the origin of the left colic artery. A detailed description of the classification of the lymph nodes with regard to the site of the primary tumour within the colon has been suggested by the Japanese Society for the Cancer of the Colon and Rectum.
INNERVATION
The colon and rectum are innervated by the sympathetic and parasympathetic systems (see Ch. 60; see Figs 60.2–60.4).
The sympathetic supply to the caecum, appendix, ascending colon and right two-thirds of the transverse colon originates in the fifth to the 12th thoracic spinal segments and are conveyed to the coeliac and superior mesenteric plexuses via the greater and lesser splanchnic nerves where they and synapse on ganglionic neurones. Postganglionic axons travel along branches of the superior mesenteric artery, and are distributed to the colon from periarterial plexuses. The parasympathetic supply is derived from the vagus nerve via the coeliac and superior mesenteric plexuses.
The sympathetic supply of the left third of the transverse colon, the descending and sigmoid colon, rectum and upper anal canal originates in the first and second lumbar spinal segments. The fibres are distributed via the lumbar splanchnic nerves through the abdominal aortic and the inferior mesenteric plexuses and via the sacral splanchnic nerves through the superior and inferior hypogastric plexuses. Postganglionic axons reach the gut wall via periarterial plexuses on branches of the inferior mesenteric artery. The parasympathetic supply travels via the pelvic splanchnic nerves (nervi erigentes) from cell bodies in the second to the fourth sacral spinal segments. Those distributed to the rectum and upper anal canal run through the inferior and superior hypogastric plexuses to branches of the inferior mesenteric artery. Some of those distributed to the descending and sigmoid colon run via these plexuses; however, a large number of fibres pass directly through the retroperitoneal tissues to the splenic flexure, descending and sigmoid colon independently of the inferior mesenteric artery.
The ultimate distribution within the wall of the large intestine is similar to the small intestine. The colic sympathetic nerves are motor to the ileocaecal valve musculature and inhibitory to mural muscle in the colon and rectum. Some fibres are vasoconstrictor to the colic vasculature. Parasympathetic nerves are secretomotor to colorectal glands, motor to the colic and rectal musculature and inhibitory to the internal anal sphincter. Afferent impulses mediating sensations of distension are carried by visceral afferent fibres which travel with the parasympathetic nerves; pain impulses pass in visceral afferents travelling with the sympathetic and parasympathetic nerves supplying the rectum and the upper part of the anal canal.
CAECUM, VERMIFORM APPENDIX AND ASCENDING COLON
CAECUM
The caecum is a large blind pouch lying in the right iliac fossa below the ileocaecal valve, continuous proximally with the distal ileum and distally with the ascending colon. The blind-ending vermiform appendix usually arises on its medial side at the level of the ileal opening. Its average axial length is 6 cm and its breadth 7.5 cm. It rests posteriorly on the right iliacus and psoas major, with the lateral cutaneous nerve of the thigh interposed. Posteriorly lies the retrocaecal recess which frequently contains the vermiform appendix. The anterior abdominal wall is immediately anterior to the caecum except when it is empty, in which case the greater omentum and some loops of the small intestine may be interposed. Usually the caecum is entirely covered by peritoneum, but occasionally this is incomplete posterosuperiorly where it lies over the iliac fascia only separated from it by loose connective tissue.
In early fetal life the caecum is usually short, conical and broad based, with an apex turned superomedially towards the ileocaecal junction. As the fetus grows, the caecum increases initially in length more than in breadth, still with the apex turned superomedially; in the later stages of intrauterine growth, the proximal part of the caecum widens so that the caecum is still a conical shape, but the apex (from which the appendix arises) comes to point inferomedially. This infantile form persists throughout life in only a very small percentage of individuals. Occasionally the originally conical caecum becomes quadrate as a result of the outgrowth of a saccule on each side of the anterior taenia: the saccules are of equal size and the appendix arises from the depression between them instead of from the apex of a cone. In the normal adult form, the right saccule grows more rapidly than the left, forming a new ‘apex’. The original apex, with the appendix attached, is pushed towards the ileocaecal junction (Fig. 67.16).
The process of fluid and electrolyte reabsorption starts in the caecum but takes place largely in the ascending and transverse colon. The distensible nature and ‘sac-like’ morphology of the caecum permit storage of large volumes of semi-liquid chyme that enters from the small bowel via the ileocaecal valve. The large resting diameter of the caecum makes it most liable to distension during periods of increased intracolonic pressure and thus it is the most vulnerable portion of the large intestine to perforation secondary to dilatation (obstructed or otherwise).
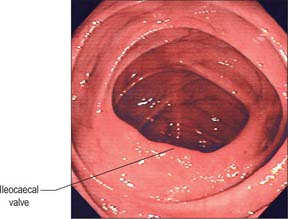
Ileocaecal valve
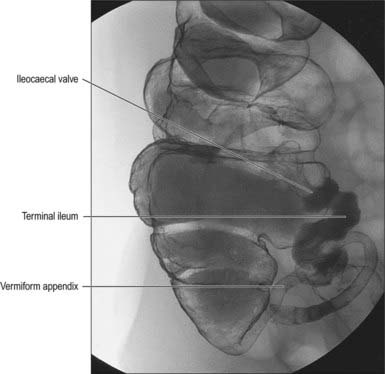
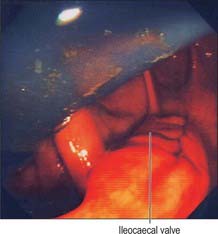
The ileum opens into the posteromedial aspect of the large intestine at the junction of the caecum and colon via the ileocaecal ‘valve’. The orifice has two flaps which project into the lumen of the large intestine (Fig. 67.17). The precise shape and form of the valve varies; in the cadaveric caecum the flaps are often semilunar, but in life they can often be seen to form a rosette or trefoil pattern of mucosa. The upper flap, approximately horizontal, is attached to the junction of the ileum and colon; the lower flap is longer and more concave, and is attached to the junction of the ileum and caecum. At their ends the flaps fuse, continuing as narrow membranous ridges, the frenula of the valve. The orifice may appear in many different shapes depending on the state of contraction or distension of the caecum; it is commonly either a slit or an oval.
The margin of the ileocaecal valve is formed by a reduplication of the intestinal mucosa and circular muscle. Longitudinal muscle fibres are partly reduplicated as they enter the valve, but the more superficial fibres and the peritoneum continue from the small to the large intestine without interruption. The valve may prevent reflux of chyme from the caecum to the ileum, and may slow the passage of ileal contents into the caecum when the circular muscle of the valve is contracted by sympathetic stimulation. Although circular and longitudinal muscle layers of the terminal ileum continue into the valve, there is little evidence that it constitutes a true functional sphincter.
The ileal valvular surfaces are covered with villi and have the structure of the small intestinal mucosa, whereas their caecal surfaces have no villi but display numerous orifices of tubular glands peculiar to the colonic mucosa.
Caecal volvulus
Because the caecum and ascending colon may possess a mesentery to a variable degree, it is possible for the caecum (and lower portion of the ascending colon) to rotate about their mesenteric attachment (mesentero-axial volvulus) such that the lower pole of the caecum comes to lie in the left or right upper quadrant of the abdomen. The fixed apex of this rotation is formed by the attachment of the caecal mesentery to the retroperitoneum. Volvulus occurs most commonly in those individuals where a large portion of the ascending colon lies on a mesentery and the common origin of the caecal and ascending colic mesentery is narrow. It is extremely unlikely in individuals where the caecum and ascending colon possess a short broadly attached mesentery. True isolated volvulus of the caecum is extremely rare.
VERMIFORM APPENDIX
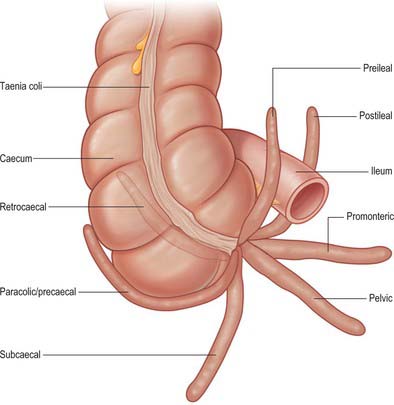
The vermiform appendix is a narrow, vermiform (worm-like) tube which arises from the posteromedial caecal wall, approximately 2 cm below the end of the ileum. It may occupy one of several positions (Fig. 67.18). The commonest positions seen in clinical practice are retrocaecal or retrocolic (behind the caecum or lower ascending colon respectively), pelvic, or descending (when the appendix hangs dependently over the pelvic brim, in close relation to the right uterine tube and ovary in females). Other positions, including subcaecal (below the caecum), and pre- or post-ilial (anterior or posterior to the terminal ileum respectively), are occasionally seen, especially when there is a long appendicular mesentery which allows greater mobility.
The three taeniae coli on the ascending colon and caecum converge on the base of the appendix, and merge into its longitudinal muscle. The anterior caecal taenia is usually distinct and can be traced to the appendix, which affords a guide to its location intra-operatively. The appendix varies from 2–20 cm in length: it is often relatively longer in children and may atrophy and shorten after mid-adult life. It is connected by a short mesoappendix to the lower part of the ileal mesentery. This fold is usually triangular, extending almost to the appendicular tip along the whole viscus.
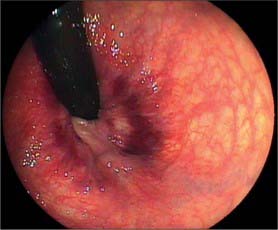
The lumen of the appendix is small and opens into the caecum by an orifice lying below and slightly posterior to the ileocaecal opening. The orifice is sometimes guarded by a straight mucosal fold forming an asymmetrical ‘valve’ which gives the appendix orifice the appearance of a ‘strung bow’ (Fig. 67.19). This fold tends to lie parallel to the medial wall of the caecum and ileocaecal valve and an imaginary ‘arrow’ placed in the bow usually points in the direction of the ileocaecal valve; this is a useful sign during colonoscopic examinations. The lumen may be widely patent in early childhood but is often partially or wholly obliterated in the later decades of life.
Microstructure
The layers of the wall of the appendix are essentially those of the rest of the large intestine (see below) but some features are notably different and are described here. The serosa forms a complete covering, except along the mesenteric attachment. The longitudinal muscular fibres form a complete layer of uniform thickness, except over a few small areas where both muscular layers are deficient, so that the serosa and submucosa are in contact. At the base of the appendix, the longitudinal muscle thickens to form rudimentary taeniae that are continuous with those of the caecum and colon.
The submucosa typically contains many large lymphoid aggregates that extend from the mucosa and obscure the muscularis mucosae layer, which consequently is discontinuous. These aggregates also cause the mucosa to bulge into the lumen of the appendix, so that it narrows irregularly (Fig. 67.20). The mucosa is covered by a columnar epithelium as it is elsewhere in the large intestine, and the epithelium that overlies the mucosal lymphoid tissue contains M cells. Glands (crypts) are similar to those of the colon but are fewer in number and so are less densely packed. They penetrate deep into the lymphoid tissue of the mucosal lamina propria. The submucosal lymphoid tissue frequently exhibits germinal centres within its follicles, indicative of B-cell activation, as occurs in secondary lymphoid tissue elsewhere. Lymphoid follicles are absent at birth but accumulate over the first 10 years of life to become a prominent feature of the appendix. In adults, the normal layered structure of the appendix is lost and the lymphoid follicles atrophy and are replaced by collagenous tissue, and in the elderly, the appendix may be filled with fibrous scar tissue.
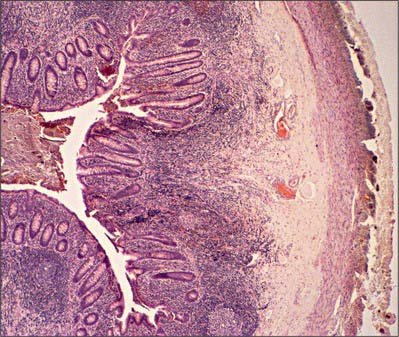
Fig. 67.20 Low-power micrograph of the appendix in transverse section, showing part of its circumference and a faecal pellet lodged in its lumen. Lymphoid tissue (basophilic staining) occupies much of the mucosa between crypts, and part of the submucosa. The muscularis externa and outermost serosal layer are seen at the right of the field.
Acute appendicitis
The genesis of acute appendicitis varies between individuals. It may follow obstruction of the lumen, when it is a consequence of increased intraluminal pressure and retention of (infected) contents which allows acute suppuration to occur. The size of the orifice of the appendix in some individuals may contribute to the risk that this will happen. Appendicoliths may be visible on plain radiographs in 7–15% of the normal population: in those patients with acute abdominal pain, their presence indicates a high risk of appendicitis being present. The increased size of the orifice and lumen at the extremes of life may be a reason why acute appendicitis is relatively uncommon in these age groups. Acute appendicitis may also present as a primary suppuration of the tissues of the appendix itself: the reduction in appendicular lymphoid tissue that occurs in later life may be another reason why the disease is infrequent in the elderly. Although the appendix is well supplied by arterial anastomoses at its base, the appendicular artery is an end artery from the midpoint upwards and its close proximity to the wall makes it susceptible to thrombosis during episodes of acute inflammation. This may render the distal appendix ischaemic and explains the frequency of gangrenous perforation seen in the disease.
The appendix and overlying visceral peritoneum are innervated by sympathetic and parasympathetic nerves from the superior mesenteric plexus. Visceral afferent fibres carrying sensations of distension and pressure mediate the symptoms of ‘pain’ felt during the initial stages of appendicular inflammation. In keeping with other structures derived from the midgut, these sensations are poorly localized initially, and referred to the central (periumbilical) region of the abdomen. It is not until parietal tissues adjacent to the appendix become involved in any inflammatory process that somatic nociceptors are stimulated, and there is an associated change in the nature and localization of pain. The caecum and proximal ascending colon share a common innervation with the appendix (sympathetic and parasympathetic nerves via the superior mesenteric plexus), which means that early inflammation in the caecum (typhlitis) results in similar visceral pain symptoms to those experienced in appendicitis.
ASCENDING COLON
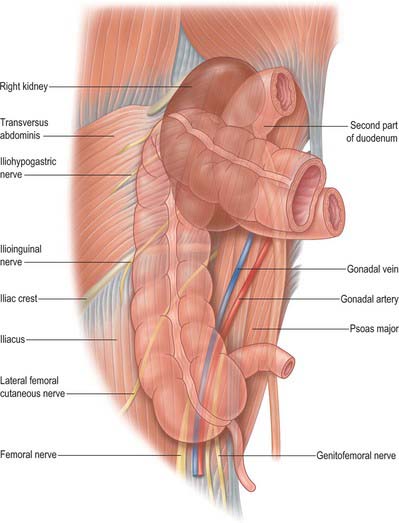
The ascending colon is approximately 15 cm long and narrower than the caecum. It ascends to the inferior surface of the right lobe of the liver, on which it makes a shallow depression, and then turns abruptly forwards and to the left, at the hepatic flexure. It is a retroperitoneal structure covered anteriorly and on both sides by peritoneum. Its posterior surface is separated by loose connective tissue from the iliac fascia, the iliolumbar ligament, quadratus lumborum, the aponeurosis of transversus abdominis, and the anterior peri-renal fascia inferolateral to the right kidney. The lateral femoral cutaneous nerve, usually the fourth lumbar artery, and sometimes the ilioinguinal and iliohypogastric nerves, lie posteriorly as they cross quadratus lumborum. Laterally the peritoneum forms the lateral paracolic gutter and medially the medial paracolic gutter (Fig. 67.21). The ascending colon possesses a narrow mesocolon for part of its course in up to one-third of cases. Anteriorly it is in contact with loops of ileum, the greater omentum and the anterior abdominal wall.
Hepatic flexure
The hepatic flexure forms the junction of the ascending and transverse colon as the latter turns down, forwards and to the left. It is variable in position and usually has a less acute angle than the splenic flexure. The anterior surface of the lower pole of the right kidney is posterior, the right lobe of the liver is superior and anterolateral (Fig. 67.22), the descending (second) part of the duodenum is medial and the fundus of the gallbladder is anteromedial. The posterior aspect of the hepatic flexure is not covered by peritoneum and the gut wall is in direct contact with pararenal fascia. The hepatic flexure is often covered in the greater omentum which may be attached to the anterior surface both of the upper ascending colon and the proximal (right) end of the transverse colon.
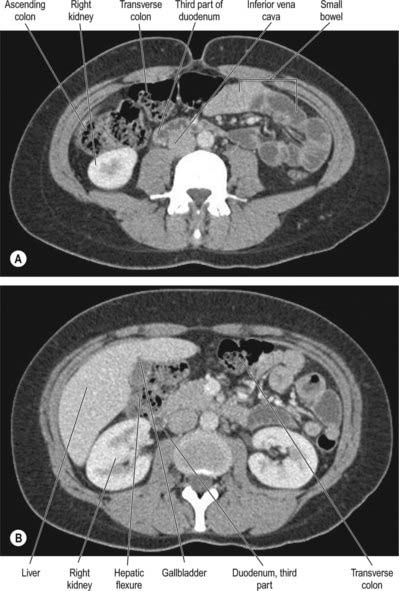
Fig. 67.22 Axial CT images of the ascending colon obtained (A) at the level of the mid ascending colon and (B) at the level of the hepatic flexure, showing the relationship of the ascending colon and hepatic flexure to the right lobe of the liver, the right and left kidneys, the second part of the duodenum, and the gallbladder (supplied by Dr Louise Moore, Chelsea and Westminster Hospital, London).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
Superior mesenteric artery
The arteries to the caecum, appendix and ascending colon are somewhat variable, but all are derived from the superior mesenteric artery via the ileocolic and right colic (when present) arteries (see Figs 67.11, 67.13).
Ileocolic artery
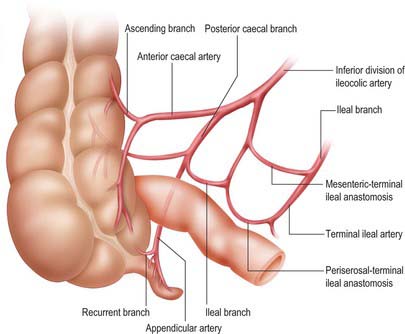
The ileocolic artery arises from the right side of the superior mesenteric artery in its upper half in the root of the mesentery. It descends to the right beneath the parietal peritoneum towards the caecum and crosses anterior to the right ureter, gonadal vessels and psoas major to enter the right iliac fossa. Although the terminal distribution varies, the artery usually divides into a superior division which runs superolaterally towards the ascending colon where it anastomoses with the right colic artery (or right branch of the middle colic artery), and an inferior division which anastomoses with the final ileal branch of the distal superior mesenteric artery. The inferior division approaches the superior border of the ileocolic junction. It usually gives rise to the following branches: ascending colic (which passes up on the ascending colon to anastomose with the superior division), anterior caecal, posterior caecal (which usually gives rise to the appendicular artery) and ileal (which ascends to the left on the lower ileum, supplies it and anastomoses with the last ileal branch of the superior mesenteric artery (Fig. 67.23). The caecum is supplied almost entirely from the ileocolic artery.
The ileocolic artery is the most prominent vessel in the lower right colic mesentery and traction on the caecum in the direction of the anterior superior iliac crest will readily cause the vessel to ‘tent’ the mesentery due to its direct course towards the caecum. This allows easy identification of the vessel during laparoscopic surgery.
The appendicular artery descends behind the terminal ileum to enter the mesoappendix a short distance from the appendicular base (Fig. 67.23). Here it gives off a recurrent branch, which anastomoses at the base of the appendix with a branch of the posterior caecal artery: the anastomosis is sometimes extensive. The main appendicular artery approaches the tip of the organ, at first near to, and then in the edge of, the mesoappendix. The terminal part of the artery lies on the wall of the appendix and may be thrombosed in appendicitis, which results in distal gangrene or necrosis. Accessory arteries are common, and many individuals possess two or more arteries of supply to the appendix.
The arcades of the terminal ileal artery provide a collateral supply to the caecum via anastomoses with the ileal branch of the ileocolic artery.
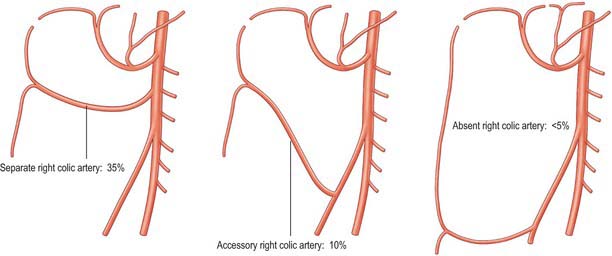
Right colic artery
The right colic artery is a small vessel that is highly variable in its anatomy (Fig. 67.24). Most commonly it arises as a common trunk with the middle colic artery. Alternatively it may arise as a separate branch from the right side of the superior mesenteric artery, or from the ileocolic artery (when it is referred to as an accessory right colic artery), and occasionally it may be absent. From its origin in common with the middle colic artery it passes towards the ascending colon, deep to the parietal peritoneum and anterior to the right gonadal vessels, right ureter and psoas major. Sometimes it arises more superiorly and crosses the second part of the duodenum and inferior pole of the right kidney. Near the colon it divides into a descending branch, which anastomoses with the ileocolic artery, and an ascending branch which anastomoses with the right branch of the middle colic artery. These form the marginal artery in the area of the hepatic flexure from which vessels are distributed to the upper third of the ascending colon, and the right side of the transverse colon.
Veins
Venous blood from the wall of the caecum, appendix and ascending colon drains into mesenteric arcades and subsequently into segmental veins, which accompany their respective arteries and tend to follow variations in arterial drainage. The segmental veins drain into the superior mesenteric vein, which lies to the right of the mesenteric artery.
Superior mesenteric vein
The superior mesenteric vein receives one or more middle colic veins, the right colic vein and the ileocolic vein and drains into the portal vein (see Figs 67.31, 66.12).
Ileocolic vein
The tributaries of the ileocolic vein follow a broadly similar pattern to the branches of the ileocolic artery, and are formed from superior and inferior tributaries. The vein ascends alongside the ileocolic artery beneath the peritoneum of the ileocaecal mesentery and drains into the superior mesenteric vein. The inferior tributary receives appendicular veins (via the posterior caecal or ileocolic veins) and anterior and posterior caecal and ileal veins, and the superior tributary drains the ascending colic veins.
Ascending tributaries of the ileocolic and right colic veins accompany their respective arteries into the root of the mesentery and drain via the ileocolic vein into the superior mesenteric vein.
Lymphatics
Lymphatic vessels originate from both anterior and posterior aspects of the colon and drain into nodes located along the branches of the ileocolic and the right colic arteries. Lymphatic drainage from the distal ascending colon and hepatic flexure may be predominantly to the nodes of the right colic artery. The lymphatic anastomoses are rich and the preterminal nodes for both routes of drainage are the ileocolic nodes, located close to the superior mesenteric artery (Fig. 67.15).
Lymphatic vessels in the appendix are numerous and there is abundant lymphoid tissue in its walls. From the body and apex of the appendix, 8–15 vessels ascend in the mesoappendix, occasionally interrupted by one or more nodes. They unite to form three or four larger vessels which run into the lymphatic vessels that drain the ascending colon, and end in the inferior and superior nodes of the ileocolic chain.
INNERVATION
The sympathetic supply to the caecum, appendix and ascending colon originates in the intermediolateral grey matter of the fifth to the 12th thoracic spinal segments. Preganglionic axons travel via the greater and lesser splanchnic nerves to the coeliac and superior mesenteric plexuses where they synapse on neurones in the coeliac and superior mesenteric ganglia; postganglionic axons form periarterial plexuses along the branches of the superior mesenteric artery, whence they are distributed to the walls of the colon.
The parasympathetic supply to the caecum, appendix and ascending colon is derived from the vagus nerve via the coeliac and superior mesenteric plexuses.
Referred pain
The appendix and overlying visceral peritoneum are innervated by sympathetic and parasympathetic nerves from the superior mesenteric plexus. Visceral afferent fibres carrying sensations of distension and pressure mediate the symptoms of ‘pain’ felt during the initial stages of appendicular inflammation. In keeping with other structures derived from the midgut, these sensations are poorly localized initially, and referred to the central (periumbilical) region of the abdomen. It is not until parietal tissues adjacent to the appendix become involved in any inflammatory process that somatic nociceptors are stimulated, and there is an associated change in the nature and localization of pain. The caecum and proximal ascending colon share a common innervation to the appendix (sympathetic and parasympathetic nerves via the superior mesenteric plexus); early inflammation in the caecum (typhlitis) results in similar visceral pain symptoms to appendicitis.
TRANSVERSE COLON
The transverse colon is approximately 50 cm long, and extends from the hepatic flexure in the right lumbar region across into the left hypochondriac region, where it curves posteroinferiorly below the spleen as the splenic flexure. It is highly variable in length and position, as may be confirmed by radiological assessment, but it often describes an inverted arch, with its concavity directed posteriorly and superiorly. Near the splenic flexure an abrupt U-shaped curve may descend lower than the main arch. The posterior surface at the hepatic flexure is devoid of peritoneum and is attached by loose connective tissue to the front of the descending part of the duodenum and the head of the pancreas. The transverse colon from here to the splenic flexure is almost completely invested by peritoneum. It is suspended from the anterior border of the body of the pancreas by the transverse mesocolon which is attached from the inferior part of the right kidney, across the second part of the duodenum and pancreas, to the inferior pole of the left kidney. The transverse colon hangs down between the flexures to a variable extent, and sometimes reaches the pelvis. Above it are the liver and gallbladder, the greater curvature of the stomach and the body of the spleen. The transverse colon is usually attached to the greater curvature of the stomach by the gastrocolic ligament, which is in continuity with the greater omentum, lying anteriorly and extending inferiorly. Behind and below the transverse colon lie the descending part of the duodenum, the head of the pancreas, the upper end of the small bowel mesentery, the duodenojejunal flexure and loops of the jejunum and ileum.
The transverse mesocolon permits considerable mobility of the transverse colon: occasionally the colon may be interposed between the liver and the diaphragm (Chilaiditi syndrome), and may be mistaken for free intraperitoneal gas.
SPLENIC FLEXURE
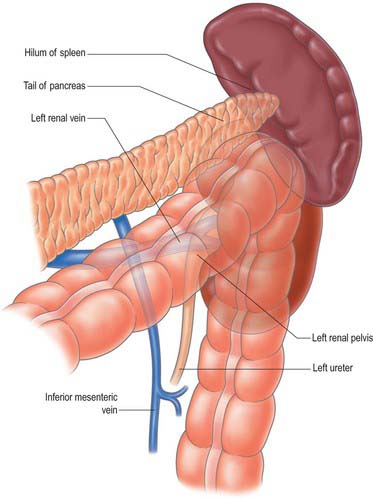
The splenic flexure forms the junction of the transverse and descending colon and lies in the left hypochondrium, inferomedial to the lower pole of the spleen (Fig. 67.25). It is anterior to the pancreatic tail, and also to the left kidney, from which it is separated by the anterior perirenal fascia. The splenic flexure often adopts a very acute angle such that the end of the transverse colon overlaps the beginning of the descending colon; there may be peritoneal and omental adhesions between the two structures. It lies more superiorly and posteriorly than the right hepatic flexure; the visceral peritoneum covering its lateral aspect is often attached to the diaphragm at the level of the 10th and 11th ribs by the phrenicocolic ligament, which lies below the anterolateral pole of the spleen. Its position with respect to the spleen in variable: it usually lies directly inferomedial to the lower pole, forming the colic impression, but it may lie anterior to the splenic hilum, and even a little above. In these cases, the visceral peritoneum is often adherent to the splenic capsule or hilar connective tissue; inadvertent, downwards traction on the splenic flexure during surgery may tear the capsule or hilar vessels. It may lie ‘low’ in the splenorenal recess with no direct attachment to the splenic capsule.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
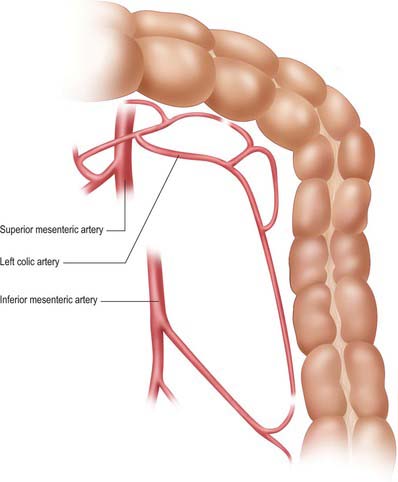
The proximal two-thirds of the transverse colon is supplied by the superior mesenteric artery via the middle colic artery (Fig. 67.11). The distal third is usually supplied by the ascending branch of the left colic artery via the marginal artery of the colon, although this is somewhat variable.
Middle colic artery
The middle colic artery usually arises from the anterolateral aspect of the superior mesenteric artery, either separately or as a common trunk with the right colic artery, just inferior to the uncinate process of the pancreas and anterior to the third part of the duodenum. Initially it passes inferiorly, then turns to run anteriorly and superiorly within the root of the transverse mesocolon, just to the right of the midline, and usually divides into a right and left branch. The right branch anastomoses with the right colic artery, and the left branch anastomoses with the left colic artery. The arterial arches thus formed lie 3 or 4 cm from the transverse colon, which they supply. Sometimes the middle colic artery divides into three or more branches within the transverse mesocolon, in which case the most lateral branches form the arterial anastomoses. An accessory or rarely a replaced middle colic artery may arise from the dorsal pancreatic, hepatic, inferior mesenteric or left colic arteries (Fig. 67.26). In the latter two instances the left half or more of the transverse colon will derive its arterial supply from the inferior mesenteric artery.
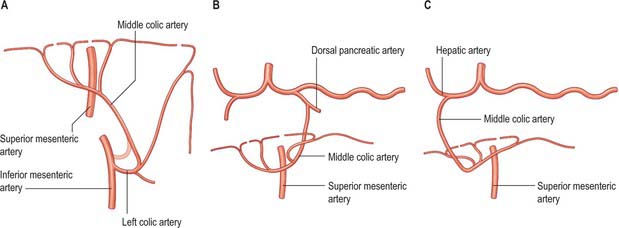
Fig. 67.26 Variants in the origin of the middle colic artery: only replaced arteries have been illustrated for simplicity. Accessory arteries are more common in each case than complete replacement of the origin. A, Left colic artery or inferior mesenteric artery (<5%). B, The dorsal pancreatic artery (<5%). C, Hepatic artery (<5%).
Veins
Superior mesenteric vein
The superior mesenteric vein receives one or more middle colic veins and the ileocolic vein (see Figs. 67.31, 66.12). The right colic vein is often absent or drains as a tributary to the right branch of the middle colic vein.
Lymphatics
Lymph vessels drain into nodes along the middle colic arteries and then into the superior mesenteric nodes. The predominant lymphatic drainage of the splenic flexure is usually via nodes along the left colic artery which drain into the inferior mesenteric nodes: this arrangement is dependent on the arterial supply to the distal third of the transverse colon.
INNERVATION
The proximal two-thirds of the transverse colon is innervated by sympathetic and parasympathetic nerves via the superior mesenteric plexus. The distal third usually receives a sympathetic supply from the inferior mesenteric plexus and a parasympathetic supply that is derived partly from the inferior mesenteric plexus and partly from retroperitoneal fibres which travel in the pelvic splanchnic nerves that arise from neurones in the second, third and fourth sacral spinal segments. The sympathetic nerves are inhibitory to mural muscle in the transverse colon; some fibres are vasoconstrictor to the colic vasculature. Parasympathetic nerves are secretomotor to colic glands and motor to the colic musculature.
DESCENDING COLON
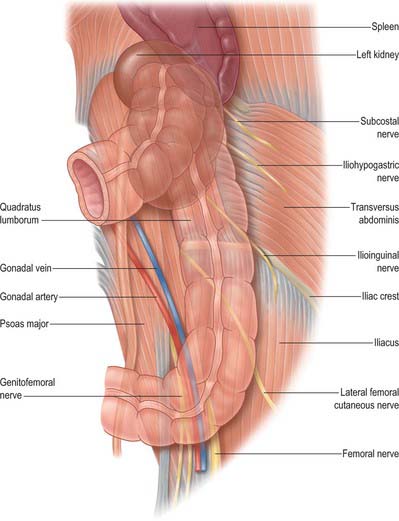
The descending colon is approximately 25 cm long. It descends through the left hypochondrium and lumbar region, initially following the lateral border of the lower pole of the left kidney, and then descending in the angle between psoas major and quadratus lumborum to the iliac crest. It then curves inferomedially, lying anterior to iliacus and psoas major, to become the sigmoid colon below the level of the iliac crest (inlet of the lesser pelvis). It lies on the retroperitoneum covered anteriorly and on both sides by peritoneum. Its posterior surface is separated by loose connective tissue from the anterior peri-renal fascia inferolateral to the left kidney, the aponeurosis of transversus abdominis, quadratus lumborum, iliacus and the most superolateral part of psoas major. The subcostal vessels and nerves, iliohypogastric, ilio-inguinal, lateral femoral cutaneous, femoral and genitofemoral nerves, and the fourth lumbar artery (usually), all lie posterior to the descending colon (Fig. 67.27). Loops of jejunum lie anteriorly. If the anterior abdominal walls are relaxed, the most inferior part of the descending colon may be directly palpated transabdominally. The descending colon is smaller in calibre, more deeply placed, and more frequently covered posteriorly by peritoneum, than the ascending colon.
SIGMOID COLON
The sigmoid colon begins below the pelvic inlet and ends at the rectum. Characteristically it forms a mobile loop which normally lies in the lesser pelvis, but its length and form are the most variable of all colonic segments. It is usually completely invested in peritoneum and is attached to the lower posterior abdominal wall and the posterior wall of the false pelvis by the fan-shaped sigmoid mesocolon. The root of the sigmoid mesocolon has an inverted ‘V’ attachment to the posterior abdominal wall.
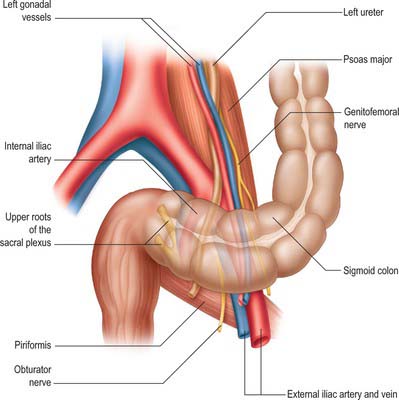
The sigmoid colon initially descends over the iliac crest into the false pelvis but thereafter its position is extremely variable. It may remain folded and principally in contact with, or adherent to, the peritoneum overlying iliacus, or it may cross the pelvic cavity between the rectum and bladder in males, or the rectum and uterus in females, and it may even reach the right pelvic wall. If long, the sigmoid loop may rise out of the pelvis into the abdominal cavity and lie in contact with loops of ileum. The sigmoid loop ends in a relatively constant position lying just to the left of the midline at the level of the third sacral body, where it bends inferiorly and is continuous with the rectum. The sigmoid loop is fixed at its junctions with the descending colon and rectum but quite mobile between them. Its relations are therefore variable (Fig. 67.28). Laterally are the left external iliac vessels, the obturator nerve, ovary or vas deferens and the lateral pelvic wall; posteriorly lie the left external and internal iliac and gonadal vessels, ureter, piriformis and sacral plexus; anteroinferiorly lie the bladder in males, or uterus and bladder in females; superiorly and to the right the sigmoid colon is in contact with loops of the ileum. The gonadal vessels and ureter lie in a distinct fascial plane which is the inferior extension of the anterior and posterior peri-renal fascia in the lower retroperitoneum and which is separate from the thin fascial coverings of the mesosigmoid. The plane between the two layers can be recognized during surgical dissection of the mesentery of the sigmoid colon because it does not contain the numerous small vessels which are often present in the loose connective tissue surrounding the ureter and gonadal vessels.
The position and shape of the sigmoid colon vary according to the length of the colon; the length and mobility of its mesocolon; the degree of distension (when distended the sigmoid colon rises into the abdominal cavity, and it sinks again into the lesser pelvis when empty); the condition of the rectum, bladder and uterus (the sigmoid colon tends to rise when these are distended, and to fall when they are empty). The length and diameter of the sigmoid colon vary in different races.
SIGMOID VOLVULUS
Rotation or volvulus may occur around the mesenteric attachment of the sigmoid colon, similar to the rotation of the caecum around its mesentery. Only the sigmoid colon is affected, because the descending colon rarely possesses sufficient mesentery to become involved in the rotation. Rotation usually involves at least 270°. Volvulus is more common when the length of the sigmoid colon increases, since this is associated with a ‘fan-like’ mesentery with a short retroperitoneal attachment. Volvulus does not occur in individuals where the sigmoid colon is short or where the mesentery is short and its attachment runs over the brim of the pelvis. The combination of anatomical features predisposing to sigmoid volvulus is most commonly found in sub-Saharan Africans and chronically institutionalized patients.
DIVERTICULAR DISEASE
The development of acquired diverticula occurs commonly in the sigmoid colon, particularly in white Caucasian populations. Their location appears to be related to the underlying anatomy of the wall of the colon. Thus, diverticula commonly occur midway between the anti-mesenteric and lateral taeniae, i.e. where the colonic wall is potentially weak, not only because the circular muscle lacks the support of the longitudinal muscle, but also because it is traversed here by arteries as they access the submucosal vascular plexus. The predilection of the diverticula for the sigmoid colon probably relates to causative factors rather than intrinsic differences in the structure of its walls. In marked contrast, congenital diverticula may occur anywhere throughout the colon, and are often found adjacent to the mesenteric attachment of the colon.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The arterial supply of the descending colon is from the inferior mesenteric artery via its left colic branch, which also anastomoses with the marginal artery of the colon (in the region of the splenic flexure), and the sigmoid arteries (in the region of the junction with the sigmoid colon) (Figs 67.29-67.31).
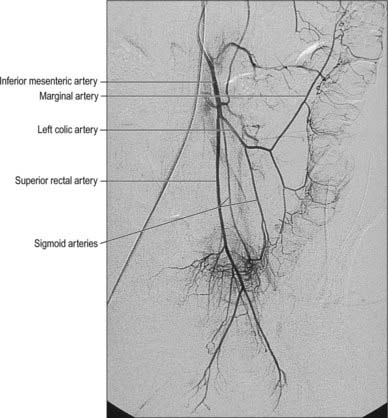
Fig. 67.29 Digital subtraction arteriogram showing the inferior mesenteric artery and its branches.
(By kind permission from Dr Adam Mitchell, Charing Cross Hospital, London.)
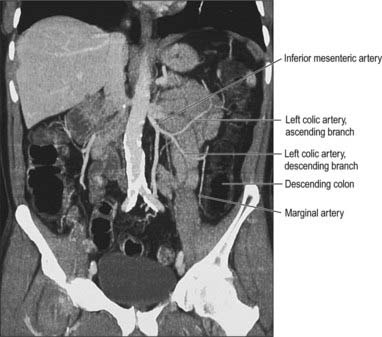
Fig. 67.30 The vascular supply of the descending colon from the inferior mesenteric artery via the ascending and descending branches of the left colic artery, coronal reformat CT.
(By kind permission from Dr Louise Moore, Chelsea and Westminster Hospital, London.)
Inferior mesenteric artery
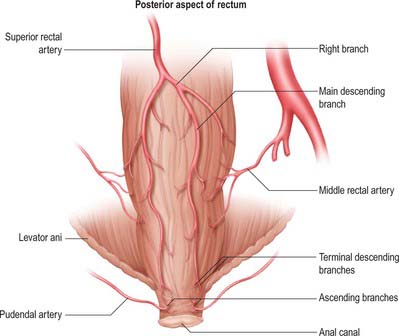
The inferior mesenteric artery is usually smaller in calibre than the superior mesenteric artery. It arises from the anterior or left anterolateral aspect of the aorta at about the level of the third lumbar vertebra, 3 or 4 cm above the aortic bifurcation and below the third part of the duodenum. It descends in the root of the left colic mesentery initially anterior and then to the left of the aorta, crosses the origin of the left common iliac artery medial to the left ureter, and then enters, and continues in, the root of the sigmoid mesocolon as the superior rectal artery. The inferior mesenteric vein lies on its lateral side in its distal course. The principal branches of the inferior mesenteric artery are the left colic, sigmoid and superior rectal arteries (see Figs 67.11, 67.29).
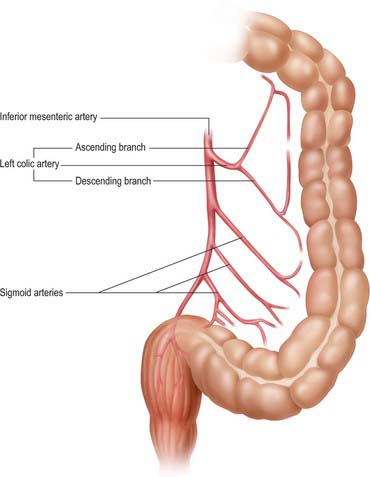
Left colic artery
The left colic artery arises from the inferior mesenteric artery shortly after its origin, ascends within the left colic mesentery anterior to the left psoas major, and bifurcates into ascending and descending branches (this division may occur soon after its origin). The artery or its branches cross the left ureter and gonadal vessels. The ascending branch passes anterior to the left kidney and anastomoses with the left branch of the middle colic artery in the subperitoneal space within the transverse mesocolon. The descending branch passes laterally in the retroperitoneum and approaches the descending colon, where it forms part of the marginal artery and anastomoses with the highest sigmoid artery. The arterial arches thus formed supply the distal third of the transverse and the descending colon. Occasionally an accessory, or rarely a replaced, left colic artery may originate from the trunk of the superior mesenteric artery or the first jejunal branches. When present, it runs laterally in the upper left colic mesentery just below the level of the duodenojejunal junction to supply the upper descending colon and form part or all of the marginal artery and the arterial anastomosis in the region of the distal transverse colon (Fig. 67.32). The left colic artery may itself give rise to an accessory left middle colic artery. Occasionally the left colic artery gives rise to a branch shortly after its origin which ascends in the mesentery and anastomoses directly with a similar descending branch of the left branch of the middle colic artery (the arc of Riolan).
Sigmoid (inferior left colic) arteries
There are between two and five sigmoid arteries, which are branches of the inferior mesenteric artery. They descend obliquely within the subperitoneal space in the sigmoid mesentery, anterior to the left psoas major, ureter and gonadal vessels. They supply the distal descending colon and sigmoid colon, and anastomose superiorly with the left colic artery and inferiorly with the superior rectal artery. Unlike the small intestine, arterial arcades do not form until the arteries are close to the wall of the colon, when small branches arise which supply the sigmoid colon directly. The formation of a true marginal artery is less pronounced in the region of the sigmoid colon than in the region of the descending colon. A significant space often exists in the mesentery between the highest sigmoid artery and the descending branch of the left colic artery: this is a useful guide to the arterial territories during surgical dissection.
In the region of the splenic flexure the marginal artery receives contributions from the left branch of the middle colic artery which anastomoses with an ascending branch of the left colic artery to supply the upper descending colon. The descending branch of the left colic artery anastomoses with upper branches of the highest sigmoid artery to supply the descending colon. The origin of the primary arterial supply for the splenic flexure and distal third of the transverse colon is usually via the left colic artery but may be from the left branch of the middle colic artery. The marginal artery in the region of the splenic flexure may be absent or of such small calibre as to be of little clinical relevance. It may, however, hypertrophy significantly when one of the main visceral arteries is compromised, e.g. following stenosis or occlusion of the inferior mesenteric artery, and it then provides a vessel of collateral supply.
Vascular ligation in left colonic resections
During resection of the distal descending and sigmoid colon, ligation of the inferior mesenteric artery close to its origin preserves the bifurcation of the left colic artery. The arterial supply to the proximal descending colon is maintained via the anastomosis between the ascending branch of the left colic artery and the left branch of the middle colic artery. Less radical resection, involving ligation of the left colic artery close to its bifurcation, may interfere with or obliterate this supply and render the descending colon more likely to become ischaemic. The same is true for ligation of the left colic vein. If the inferior mesenteric vein is ligated, then the bifurcation of the vein forms the route of venous drainage for the descending colon to the middle colic vein territory. Ligation of the branches separately will impair the venous drainage.
Veins
Inferior mesenteric vein
The inferior mesenteric vein drains the rectum, sigmoid, descending and distal transverse colon (Fig. 67.33; see also Fig. 67.14). It begins as the superior rectal vein, from the rectal plexus, through which it connects with middle and inferior rectal veins. The inferior mesenteric vein lies to left of the inferior mesenteric artery, ascends deep to the peritoneum and anterior to the left psoas major and may cross the testicular or ovarian vessels or ascend medial to them. It usually lies lateral to or occasionally posterior to the duodenojejunal flexure and the peritoneal fold directly lateral to the flexure is a useful guide to locating the vein surgically. It usually drains into the splenic vein, but occasionally drains into the confluence of the splenic and superior mesenteric veins or directly into the superior mesenteric vein. If a duodenal or paraduodenal fossa exists, the vein is usually in its anterior wall. The inferior mesenteric vein receives tributaries from several sigmoid veins, the middle and the left colic veins.
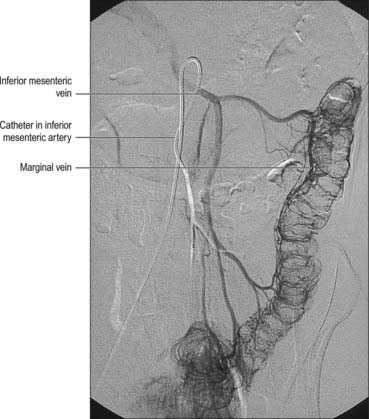
Fig. 67.33 The inferior mesenteric vein and its tributaries, digital subtraction arteriogram.
(By kind permission from Dr Adam Mitchell, Charing Cross Hospital, London.)
Left colic vein
The left colic vein is formed from several tributaries including ascending and descending branches which correspond to the equivalent arteries. These tributaries may not form a discrete vein until they drain into the inferior mesenteric vein, and occasionally there may be two distinct veins which both run into the inferior mesenteric vein. The left colic vein usually lies more superiorly in the left colon mesentery than its equivalent artery, and has a shorter course because the inferior mesenteric vein lies more laterally than the inferior mesenteric artery.
Several sigmoid veins drain the sigmoid colon and run superiorly alongside their respective arteries to drain into the inferior mesenteric vein.
INNERVATION
The sympathetic supply to the left third of the transverse colon and the descending and sigmoid colon originates in the first and second lumbar spinal segments, and runs in the upper lumbar splanchnic nerves: preganglionic axons synapse on neurones in the abdominal aortic and the inferior mesenteric plexuses, whence postganglionic axons reach the gut wall via periarterial plexuses on branches of the inferior mesenteric artery. These axons are inhibitory to the mural muscle of the colon and some fibres are vasoconstrictor to the colic vasculature.
The parasympathetic supply is via the pelvic splanchnic nerves. Some fibres run through the inferior and superior hypogastric plexuses and along branches of the inferior mesenteric artery, but many more pass directly through the retroperitoneal tissues, independent of the inferior mesenteric artery, to reach the splenic flexure and descending and sigmoid colon. Parasympathetic nerves are secretomotor to colic glands and motor to the colic musculature. Afferent impulses mediating sensations of distension are carried by visceral afferent fibres which travel with the parasympathetic nerves.
RECTUM
The rectum is continuous with the sigmoid colon at the level of the third sacral vertebra and terminates at the upper end of the anal canal. It descends along the sacrococcygeal concavity as the sacral flexure of the rectum, initially inferoposteriorly and then inferoanteriorly, to join the anal canal by passing through the pelvic diaphragm. The anorectal junction is 2–3 cm in front of and slightly below the tip of the coccyx, which is opposite the apex of the prostate in males. From this level the anal canal passes inferiorly and posteriorly from the lower end of the rectum. The posterior bend is termed the perineal flexure of the rectum and the angle it forms with the upper anal canal is the anorectal angle. The rectum also deviates in three lateral curves: upper, convex to the right; middle (the most prominent), convex to the left; lower, convex to the right. Both ends of the rectum are in the median plane (Fig. 67.34).
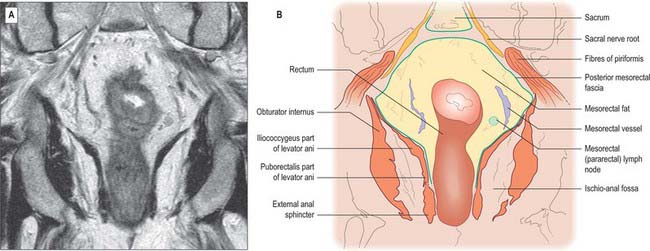
Fig. 67.34 A, Coronal T2-weighted MRI of the rectum. B, Line diagram illustrating main features in A.
Although variable in absolute length, a common landmark used in clinical practice to define the rectum is a length of 15 cm above the external anal margin. The initial diameter is similar to that of the sigmoid colon, but more inferiorly it becomes dilated as the rectal ampulla. The rectum differs from the sigmoid colon in having no sacculations or appendices epiploicae. The taeniae blend approximately 5 cm above the rectosigmoid junction, forming two wide muscular bands which descend anteriorly and posteriorly in the rectal wall. These then fuse to form an encircling layer of longitudinal muscle, which invests the entire length of the rectum. At the rectal ampulla a few strands of the anterior longitudinal fibres pass forwards to the perineal body as the musculus recto-urethralis. In addition, two fasciculi of smooth muscle may pass anteroinferiorly from the anterior surfaces of the bodies of the second and third coccygeal vertebrae to blend with the longitudinal muscle fibres on the posterior wall of the anal canal, forming the rectococcygeal muscles.
The upper third of the rectum is covered by peritoneum on its anterior and lateral aspects. It is related anteriorly to the sigmoid colon or loops of ileum if these lie in the pelvis, otherwise it is related to the urinary bladder in males, or the cervix and body of the uterus in females. The middle third of the rectum is covered by peritoneum only on its anterior aspect. The peritoneum is reflected superiorly onto the urinary bladder in males to form the rectovesical pouch, or onto the posterior vaginal wall in females to form the recto-uterine pouch (pouch of Douglas). The level of this reflection is higher in males; the rectovesical pouch is approximately 7.5 cm (about the length of the index finger) from the anorectal junction. In females the recto-uterine pouch is approximately 5.5 cm from the anorectal junction. In the male neonate, peritoneum extends on to the front of the rectum as far as the lower limit of the prostate. Superiorly the peritoneum is firmly attached to the muscle layer of the sigmoid colon by fibrous connective tissue, but as it descends onto the rectum it is more loosely attached by fatty connective tissue, which allows for considerable expansion of the upper half of the rectum (Figs 67.35, 67.36).
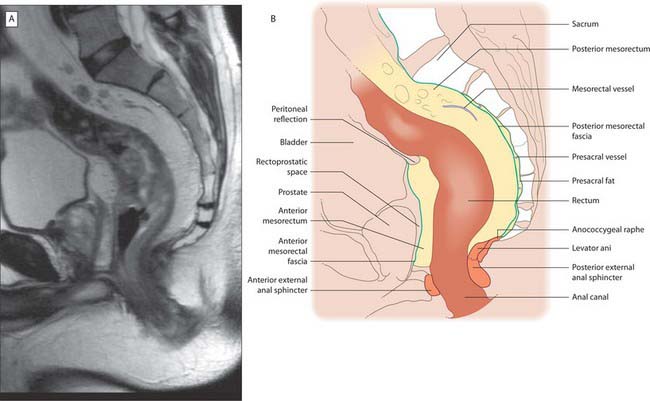
Fig. 67.35 A, Sagittal T2-weighted MRI of the rectum in a male. B, Line diagram illustrating main features in A.
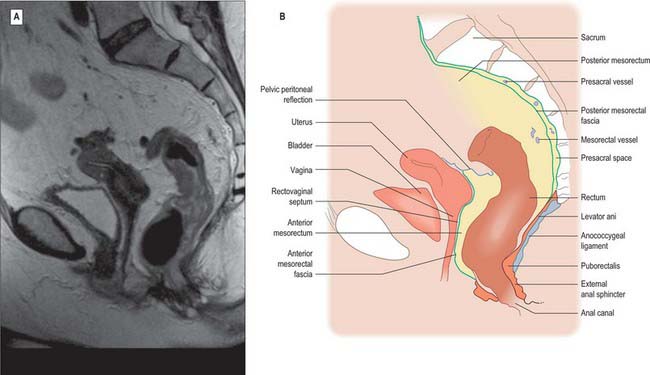
Fig. 67.36 A, Sagittal T2-weighted MRI of the rectum in a female. B, Line diagram illustrating main features in A.
There are no haustra in the rectum. When empty the mucosa forms a number of longitudinal folds in its lower part which become effaced during distension. The rectum commonly has three permanent semi-lunar transverse or horizontal folds (although the number can vary), which are most marked in rectal distension (Fig. 67.9). Two forms of horizontal fold have been recognized. One consists of the mucosa, a circular muscle layer and part of the longitudinal muscle, and is marked externally by an indentation. The other is devoid of longitudinal muscle and has no external marking. The most superior fold at the beginning of the rectum may be either on the left or right and occasionally encircles the rectal lumen. The middle fold is largest and most constant. It lies immediately above the rectal ampulla, projecting from the anterior and right wall just below the level of the anterior peritoneal reflection; the circular muscle is more marked in this fold than in the others. The most inferior and variable fold is found on the left below the middle fold. Sometimes a fourth fold is found on the left a little above the middle fold.
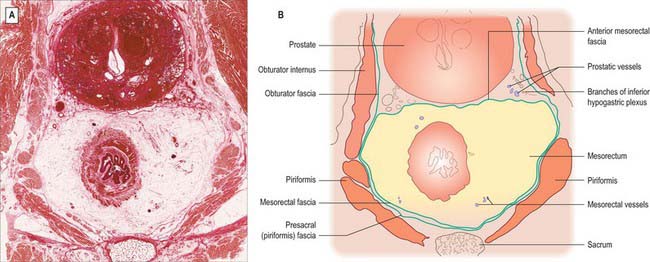
MESORECTUM, RECTAL FASCIAE AND ‘SPACES’
The mesorectum (mesentery of the rectum) and its contents are intimately related to the rectum down to the level of levator ani (Figs 67.37-67.39). It is a distinct compartment derived from the embryological hindgut. It contains the superior rectal artery and its branches, the superior rectal vein and its tributaries, the lymphatic vessels and nodes that lie along the superior rectal artery, branches from the inferior mesenteric plexus which descend to innervate the rectum, and loose adipose connective tissue.
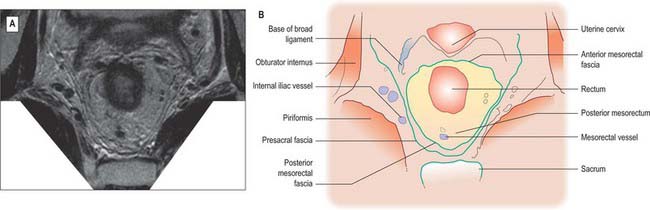
Fig. 67.37 A, Axial T2-weighted MRI of the upper rectum in a female. B, Line diagram illustrating main features in A.
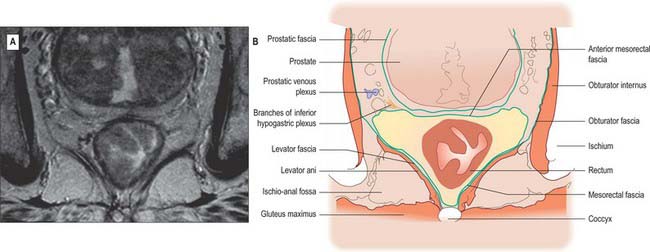
Fig. 67.38 A, Axial T2-weighted MRI of the mid rectum below the peritoneal reflection in a male. B, Line diagram illustrating main features in A.
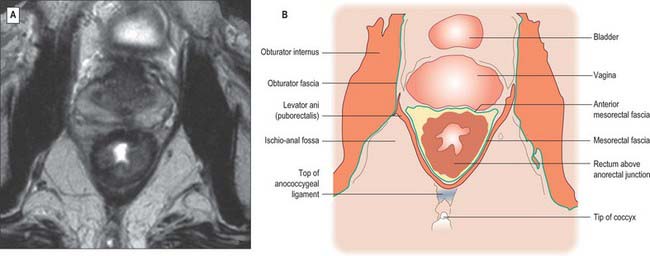
Fig. 67.39 A, Axial T2-weighted MRI of the low rectum below the peritoneal reflection in a female. B, Line diagram illustrating main features in A.
The mesorectum is enclosed by mesorectal fascia, a distinct covering derived from the visceral peritoneum that is also called the visceral fascia of the mesorectum, fascia propria of the rectum or the presacral wing of the hypogastric sheath. The fascia bounds the mesorectum posteriorly and thus lies anterior to the retrorectal space and the pre-sacral fascia. The mesorectal fascia is surrounded by a very thin layer of loose areolar tissue which separates it from the posterior and lateral walls of the true pelvis. Superiorly, the mesorectal fascia blends with the connective tissue bounding the sigmoid mesentery. Laterally, it extends around the rectum and mesorectum and becomes continuous with a denser condensation of fascia anteriorly. In males this anterior fascia is known as the rectovesical fascia of Denonvillier, and in females it forms the fascia of the rectovaginal septum.
On MRI scanning, the mesorectum appears as a fat-containing envelope in which vessels are depicted as low signal due to signal void produced by blood flow. Lymph nodes appear as high signal ovoid structures. Small nerves within the mesorectum are not visualized, but interlacing connective tissue within the mesorectum can be seen as low signal intensity strands. The mesorectal fascia is demonstrated on axial views as a low signal layer that surrounds the mesorectum, and which corresponds to the distinct condensation of fascia seen on histological sections containing the mesorectum (Brown et al 1999). Identification of the involvement of this layer by malignant tumours of the rectum on MRI scanning may help plan preoperative radiotherapy, and predict the chance of successful surgical resection.
Anterolaterally, branches of the inferior hypogastric plexus and branches of the middle rectal artery and veins run into the mesorectum. They are ensheathed by fascia and are sometimes jointly referred to as the ‘lateral rectal ligaments’. The number and calibre of the middle rectal vessels are highly variable; they may be very small or even absent (Sato & Sato 1991). The ‘lateral ligaments’ are not seen on MRI or CT scanning, and only appear as an identifiable structure with surgical traction on the rectum. The fascia of the ‘ligaments’ is flimsy and they probably play very little role in support of the rectum.
The parietal fascia that covers levator ani and the muscles of the side-wall of the pelvis forms a denser condensation of fascial tissue overlying the sacrum (Fig. 67.40).
RELATIONS OF THE RECTUM
Posterior to the rectum and mesorectum, and separated from them by the presacral fascia, in the median plane are the lower three sacral vertebrae, coccyx, median sacral vessels, and the lowest portion of the sacral sympathetic chain. Laterally, the upper part of the rectum is related to the pararectal fossa and its contents (sigmoid colon or terminal ileum), while below the peritoneal reflection lie piriformis, the anterior rami of the lower three sacral and coccygeal nerves, the sympathetic trunk, lower lateral sacral vessels, and coccygei and levatores ani. Anteriorly, above the level of the peritoneal reflection, lie loops of sigmoid colon or terminal ileum (if these lie in the pelvis), otherwise the rectum is related to the upper parts of the base of the bladder in males, or to the cervix/body of the uterus and upper vagina in females. The lower parts of the base of the bladder, the seminal vesicles, vas deferens, terminal parts of the ureters, and the prostate in males (Figs 67.38, 67.39), or the lower part of the vagina in females, lie below the reflection. In females, the rectovaginal septum is composed of a condensation of connective tissue which is continuous with the connective tissue of the outer layers of the rectal and vaginal walls. In postmenopausal females, and following childbirth, the connective tissue of the rectovaginal septum may atrophy or be thinned, reducing the support for the anterior rectal and posterior vaginal walls.
RECTAL PROLAPSE
Prolapse of the rectum involving all layers of the rectal wall may occur: the precise aetiology is not known. During prolapse, the mid and upper rectum descends towards the pelvic floor on straining. This descent tends to occur within the lumen of the rectum as a form of intussusception, and may be a consequence of the large diameter of the lower rectal ampulla and relative fixation of the anorectal junction and anal canal. The upper mesorectum is formed by relatively loose adipose connective tissue, and so relatively little tissue actually fixes the mid and upper rectum in position within the pelvis. The ‘lateral ligaments’ are composed mainly of vascular structures and offer only limited support against rectal descent. Chronic enlargement of the anorectal space bound by levatores ani commonly occurs in patients suffering rectal prolapse, but it is thought to be a consequence rather than a cause of the prolapse. During prolapse, the recto-uterine pouch in females also descends with the anterior rectal wall, and in extreme cases may become everted through the anus between the layers of rectal wall. This rarely happens to the rectovesical pouch in males.
RECTOCELE
When the rectovaginal septum is grossly effaced, especially in postmenopausal females, the pressure of defecation transmitted forward along the sacral flexure of the rectum can eventually cause bulging of the rectal wall into the posterior vagina, and, in extreme cases, through the vaginal introitus. Failure of support of the rectum and perineum by the puborectalis and pubovaginalis muscles contributes to this prolapse by allowing descent of the posterior perineum during straining.
MESORECTAL EXCISION IN RECTAL CANCER
An important concept in the oncological treatment of adenocarcinoma of the rectum is the integrity of the rectum and its mesorectal tissue. The epirectal and pararectal lymphatic tissues are located throughout the mesorectum. If the mesorectum is divided or disrupted during surgical excision of rectal carcinoma, involved nodes may be left in situ, which may predispose to local recurrence of tumour (Heald et al 1982). Oncological excision of the rectum involves mobilization of the rectum in the plane formed by the mesorectal fascia posterolaterally around the mesorectum. This plane allows the rectum and mesorectum to be removed as a whole without disruption of the presacral fascia and its underlying venous plexus. It is continuous with the plane between the mesentery surrounding the origin of the superior rectal artery and the presacral fascia over the sacral promontory, which is opened during mobilization of the origin of the inferior mesenteric artery prior to ligation. The mesorectal plane extends laterally and anteriorly, disrupted only by small branches of the middle rectal vessels, and is continuous with the rectovesical or rectovaginal fascia, which provides a similar oncological plane of dissection. Since the inferior hypogastric nerves are closely related to the plane of the mesorectal fascia in its upper third, dissection must spare the nerves if a degree of pelvic autonomic disturbance (bladder dysfunction and erectile dysfunction in males) is to be avoided following surgery. Complete excision of the rectum – total mesorectal excision – involves mobilization of this plane to the level of levatores ani. During the excision it is important to follow the sacral curve of the rectum to prevent entry into the presacral fascia or anococcygeal ligament with subsequent bleeding from presacral vessels.
ABDOMINOPERINEAL EXCISION IN RECTAL CANCER
The posterior mesorectum is thinnest at its lowest extremity, where the mesorectal fascia becomes continuous with the periserosal tissues which condense on the outer muscle layer as it passes inside the fibres of puborectalis and it lies directly on the fascia of levator ani (endopelvic fascia) (see Fig. 63.4). Tumours penetrating through the rectal wall at this point may therefore lie in extremely close proximity to the mesorectal margin. Conventional dissection in the mesorectal plane as far as the pelvic floor may open this fascial space close to the tumour and abdominoperineal excision of the rectum should be conducted through the ischio-anal fossa, vertically through levator ani outside the puborectalis sling and into the mesorectal plane well above the area of contact with the pelvic floor to avoid opening this potential tumour plane.
LOCAL EXCISION OF THE RECTUM
Full thickness excision of lesions of the rectal wall is possible in those parts of the rectum lying in the extraperitoneal compartment, i.e. below the peritoneal reflections. The mesorectal adipose tissue contains any leakage of contents and provides support to the closure of the defect in the rectal wall. Since only the lower third of the rectum is wholly extraperitoneal, full thickness local excisions of the rectum should be avoided on the anterior wall of the middle third and anterolateral walls of the upper third of the rectum. The height to which such excision can be performed anteriorly in females is lower than in males, because the lower level of the peritoneal reflection makes entry into the peritoneal cavity more likely.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The principal arterial supply to the upper two-thirds of the rectum is the superior rectal artery. Branches of the middle rectal artery provide some additional supply to the middle third, and ascending branches of the inferior rectal artery supply the distal third. The median sacral artery (the terminal midline branch of the aorta), contributes a small branch which enters the posterior wall of the anorectal junction on the sacrorectal fascia (Fig. 67.41).
Superior rectal artery
The superior rectal artery is the principal continuation of the inferior mesenteric artery (Fig. 67.31). It descends into the pelvis in the sigmoid mesocolon, crosses the left common iliac vessels and passes over the sacral promontory usually just to the left of the midline. It is straddled by the inferior hypogastric nerves on either side. It lies anterior to the upper sacral vertebrae and passes into the upper mesorectum, then descends, initially in the midline, and divides into two branches at the level of the third sacral vertebra. These lie initially posterolateral, then lateral, to the rectal wall as they descend one on each side of the rectum. Terminal branches pierce the muscle wall from the level of the upper mesorectum to enter the rectal submucosa, where they anastomose with ascending branches of the inferior rectal arteries.
Middle rectal arteries
The middle rectal arteries arise either directly from the anterior division of the internal iliac artery or from the inferior vesical artery (vaginal artery in females). They enter the mesorectum anterolaterally in the ‘lateral rectal ligaments’, and are frequently either absent or may be very small in calibre (Sato & Sato 1991). When present, they provide an arterial supply to the muscle of the mid and lower rectum, but form only poor anastomoses with the superior and inferior rectal arteries.
Inferior rectal arteries
The inferior rectal arteries are terminal branches of the internal pudendal arteries. They cross the ischio-anal fossa to enter the upper anal canal laterally where they branch. They supply the internal and external anal sphincters, the anal canal and the perianal skin. Ascending branches pass superiorly in the submucosal plain, and anastomose with the terminal branches of the superior rectal artery.
Veins
Rectal venous plexus
The rectal venous plexus surrounds the rectum, and connects anteriorly with the vesical plexus in males or the uterovaginal plexus in females. It consists of an internal part, beneath the rectal and anal epithelium, and an external part outside the muscular wall. In the anal canal the internal plexus displays longitudinal dilatations, connected by transverse branches in circles immediately above the anal valves. The dilatations are most prominent in the left lateral, right anterolateral and right posterolateral sectors. The internal plexus drains mainly to the superior rectal vein but connects widely with the external plexus. The inferior portion of the external plexus is drained by the inferior rectal vein into the internal pudendal vein, the middle portion by one or more middle rectal veins into the internal iliac vein, and its superior part by the superior rectal vein, which drains into the inferior mesenteric vein. Communication between portal and systemic venous systems is thus established in the rectal plexus.
Superior rectal veins
The superior rectal veins are formed from the internal rectal plexus. The tributaries of the superior rectal vein ascend in the rectal submucosa as about six vessels of considerable size which pierce the rectal wall approximately 7.5 cm above the anus. The branches unite to form the superior rectal vein, which runs along the superior rectal artery in the root of the mesorectum and mesosigmoid, crosses the left common iliac vessels with the superior rectal artery, and continues upwards as the inferior mesenteric vein to the left of the midline (see Figs 67.14 and 67.43A).
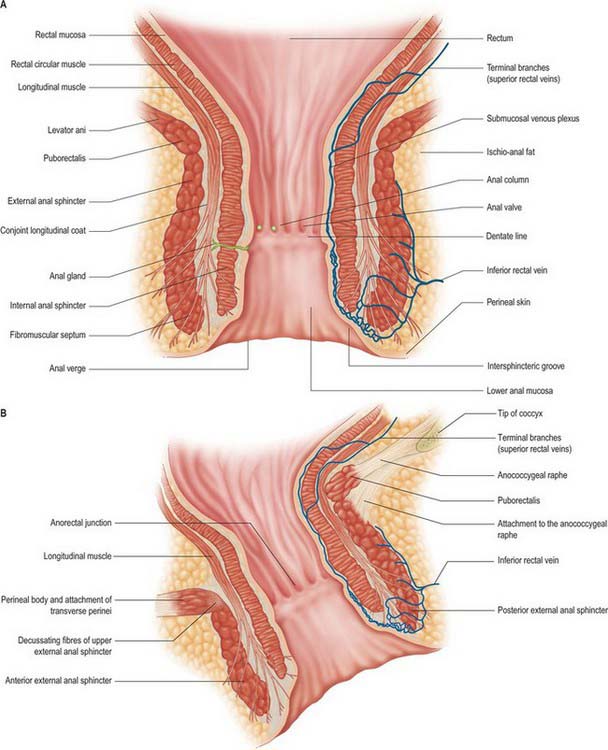
Fig. 67.43 A, Coronal section through the anal canal: glandular and vascular structures are shown unilaterally for clarity. B, Sagittal section through the anal canal: the anal canal is angled posteriorly and so anterior sphincteric structures appear to be lower than posterior structures. The glandular, vascular and fibromuscular structures and the conjoint longitudinal coat have been omitted for clarity.
Lymphatics
Lymphatics draining the rectum and the upper anal canal above the level of the dentate line pass superiorly, initially through the rectal wall, and then as a fine network over the surface of the rectum, before draining into nodes which lie in the loose adipose connective tissue of the mesorectum. Epirectal nodes lie very close to the outer fibres of the rectal longitudinal muscle while pararectal nodes lie within the mesorectum, a variable distance from the rectal wall. The overall direction of drainage is upwards along the branches of the superior rectal artery. Drainage to intermediate groups occurs up to the nodes near the origin of the inferior mesenteric artery (Fig. 67.42).
INNERVATION
The sympathetic supply to the rectum and upper anal canal originates in the first and second lumbar spinal segments. The fibres are distributed via the lumbar splanchnic nerves through the inferior mesenteric plexuses and via the sacral splanchnic nerves through the superior and inferior hypogastric plexuses: postganglionic axons reach the gut wall via periarterial plexuses on branches of the superior and middle rectal arteries, and are inhibitory to the muscle of the rectal wall.
The preganglionic parasympathetic supply to the rectum and upper anal canal is mediated via the pelvic splanchnic nerves, which travel through the inferior and superior hypogastric plexuses and along the nerve plexuses on the branches of the inferior mesenteric artery. Parasympathetic nerves are secretomotor to the colorectal glands, and motor to the rectal musculature, causing relaxation of the internal anal sphincter. Afferent impulses mediating sensations of distension are carried by visceral afferent fibres which travel with the parasympathetic nerves. Pain impulses pass in visceral afferents travelling with the sympathetic and parasympathetic nerves that supply the rectum and the upper part of the anal canal.
The role of rectal innervation in anorectal function and continence is described below.
ANAL CANAL
The anal canal begins at the anorectal junction and ends at the anal verge (Figs 67.43-67.45). It is angulated in relation to the rectum because the pull of the sling-like puborectalis produces the anorectal angle. It lies 2–3 cm in front of and slightly below the tip of the coccyx, which is opposite the apex of the prostate in males. The anal verge is marked by a sharp turn where the squamous epithelium which lines the lower anal canal becomes continuous with the skin of the perineum. The pigmentation of skin around the anal verge approximately corresponds to the extent of the external sphincter. Identification of the anal verge may be difficult, particularly in males in whom the perineum may ‘funnel’ upwards into the lower anal canal, however, the characteristic puckering of the external epithelium caused by the penetrating fibres of the conjoint longitudinal layer makes a useful landmark. The functional anal canal is represented by a zone of high pressure which roughly equates to the anatomical canal. The anal canal consists of an inner epithelial lining, a vascular subepithelium, the internal and external anal sphincters and fibromuscular supporting tissue as well as dense neuronal networks of both autonomic and somatic origin. It is between 2.5 and 5 cm long in adults although the anterior wall is slightly shorter than the posterior. It is usually shorter in females. At rest it forms an oval slit in the anteroposterior plane rather than a circular canal: the arrangement of the external anal sphincter and its attachments to the perineal body and coccyx create sites of maximum pressure within the anal canal in the anterior and posterior midline.
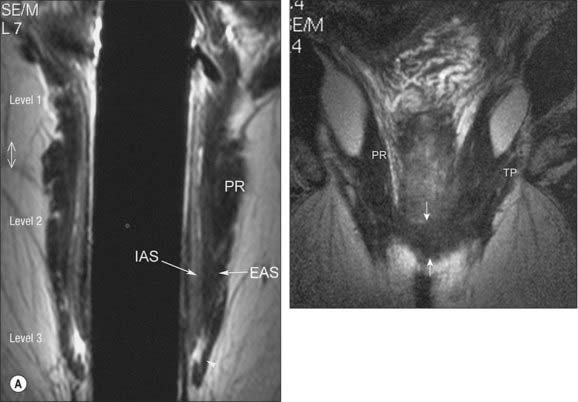
Fig. 67.44 A, Mid-coronal MRI endocoil image of the anal canal. B, Anterior coronal MRI endocoil section in a woman showing the transverse perineii (TP) joining the external anal sphincter anteriorly (between arrows). EAS, external anal sphincter; IAS, internal anal sphincter; PR, puborectalis.
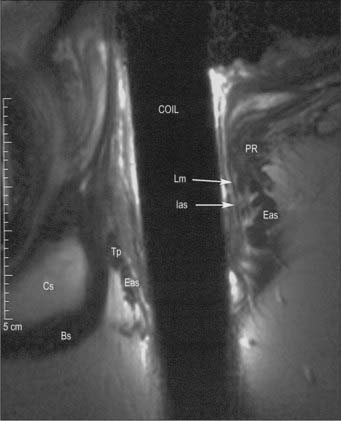
Fig. 67.45 MRI endocoil mid sagittal view of the anal canal in a man. Cs, corpus spongiosus; Tp, transverse perineii; Eas, external anal sphincter; Lm, longitudinal muscle; Ias, internal anal sphincter; PR, puborectalis; Bs, bulbospongiosus.
Anteriorly, the middle third of the anal canal is attached by dense connective tissue to the perineal body, which separates it from the membranous urethra and penile bulb in males or from the lower vagina in females. Laterally and posteriorly, the anal canal is surrounded by loose adipose tissue within the ischio-anal fossae; this offers a potential pathway for the spread of perianal sepsis from one side to the other (see Ch. 63). Posteriorly, the anal canal is attached to the coccyx by the anococcygeal ligament, a midline fibroelastic structure which may possess some skeletal muscle elements, and which runs between the posterior aspect of the middle portion of the external sphincter and the coccyx. Just above this is the raphe of the levator ani ‘plate’, the fusion of the two halves of iliococcygeus, which merges anteriorly with puborectalis. Between these two structures is a potential ‘postanal’ space.
The ischial spines may be palpated laterally by an examining finger in the upper anal canal. The pudendal nerves pass over the ischial spines at this point and pudendal nerve motor terminal latency may be measured digitally using a modified electrode worn on the examining glove.
LINING OF THE ANAL CANAL
The upper portion of the anal canal is lined by columnar epithelium similar to that of the rectum. It contains secretory and absorptive cells with numerous tubular glands or crypts. The subepithelial tissues are mobile and relatively distensible and possess profuse submucosal arterial and venous plexuses. Terminal branches of the superior rectal vessels pass downwards towards the anal columns. The submucosal veins drain into the submucosal rectal venous plexus and also through the fibres of the upper internal anal sphincter into an intermuscular venous plexus.
There are 6–10 vertical folds, the anal columns, in the mid anal canal. They tend to be obvious in children but less well-defined in adults. Each column contains a terminal radicle of the superior rectal artery and vein: the vessels are largest in the left-lateral, right-posterior and right-anterior quadrants of the wall of the canal where the subepithelial tissues expand into three ‘anal cushions’ (Fig. 67.46). The cushions help to seal the anal canal, to maintain continence to flatus and fluid, and are also important in the pathogenesis of haemorrhoids. The lower ends of the columns may form small crescentic folds, called the anal valves, between which lie small recesses referred to as anal sinuses. The anal valves and sinuses together form the dentate (or pectinate) line. About six anal glands open into small depressions, anal crypts, in the anal valves. The glands are branched and lined by stratified columnar epithelium. Cystic dilatations in the glands may extend through the internal sphincter and even into the external sphincter (Seow-Cheon & Ho 1994).
The mucosa below the dentate line is smooth, and termed the pectin. It is non-keratinized stratified squamous epithelium which lacks sweat and sebaceous glands and hair follicles, but contains numerous somatic nerve endings. It extends down to the intersphincteric groove, a depression at the lower border of the internal sphincter. The canal below the intersphincteric groove is lined by hair-bearing, keratinizing, stratified epithelium which is continuous with the perianal skin. The submucosa in this region contains profuse arterial and venous plexuses and has more connective tissue than the upper canal. It may be tethered to fibres of the conjoint longitudinal layer in the region of the intersphincteric groove.
The junction between the columnar and squamous epithelia is referred to as the anal transition zone (ATZ). It is variable in height and position and often contains islands of squamous epithelium extending up into the columnar mucosa; it is not the same as the dentate line which is an indistinct line observed in the anal canal. Nerve endings including thermoreceptors exist in the submucosa around the upper ATZ; they probably play a role in continence by providing a highly specialized ‘sampling’ mechanism to identify the contents of the lower rectum when the upper anal canal relaxes to allow rectal contents to come into contact with the upper anal canal epithelium (Duthie & Gairns 1960).
The well-defined muscularis mucosae of the rectum continues into the upper canal. Fibres from the longitudinal muscle pass through the internal sphincter and surround the submucosal venous plexus before turning upwards to merge with the muscularis mucosae and form the musculus submucosae ani.
Haemorrhoids represent abnormal enlargement of the anal cushions. The partial drainage of the venous plexus into the intermuscular plane may play a role in this chronic engorgement as a result of obstruction of the venous flow during prolonged straining and defecation. Since the subepithelial vascular cushions of the upper, mid and lower anal canal form a continuous plexus, the differentiation of haemorrhoids into ‘internal’ and ‘external’ is somewhat arbitrary. The laxity of the upper anal canal submucosa is probably responsible for the fact that internal haemorrhoids may form more easily, although engorgement of the plexus deep to the lower anal epithelium may also occur. Since the epithelium in the lower canal is well supplied with sensory nerve endings, acute distension or invasive treatment to haemorrhoids in this area causes profound discomfort, whereas invasive or destructive therapy with relatively few symptoms is possible in the upper canal because the latter is lined with insensate columnar mucosa.
Acute, transient, vertical breaks in the anal epithelium are not uncommon. The development of chronic, non-healing fissures is less common. They are more likely to develop in the anterior, and particularly the posterior, midline of the anus, possibly because stress within the anal canal is concentrated in these regions by the arrangement of the fibres of the external sphincter: the relative paucity of the arterial supply in these regions probably contributes to poor healing. Persistent hypertonicity of the anal sphincter is a primary pathogenic factor, possibly developing as a consequence of disordered local reflex mechanisms between the lower rectum and internal anal sphincter.
Cryptoglandular sepsis and fistula in ano
Infection of the anal glands is the main cause of anal sepsis and cryptogenic fistula formation (Parks et al 1976). Because many of these glands extend into the intersphincteric plane, so-called cryptoglandular sepsis may readily spread into the same plane. The commonest route of drainage of this sepsis is downwards between the internal and external anal sphincters, appearing beneath the skin at the anal verge. Rupture or drainage of the sepsis at this point will form a fistula in ano in the intersphincteric plane. Less commonly, the infection drains outwards from the intersphincteric plane and passes through the fibres of the external anal sphincter, possibly along the spreading septa of the conjoint longitudinal coat, and appears in the perianal skin outside the external anal sphincter as a transphincteric fistula. If the sepsis does not originate in the intersphincteric plane, drainage is likely to be beneath the anal mucosa alone and a submucous fistula forms. Sepsis within the ischio-anal fossa surrounding the lower anal canal can spread with relative ease because the connective tissue is mostly loose adipose tissue. Pus may spread posteriorly, behind the lower anal canal into the contralateral ischio-anal fossa, because the lower anal canal lacks any ligamentous attachments (Parkes 1961).
MUSCLES OF THE ANAL CANAL
The anal canal is encircled by internal and external anal sphincters, separated by the longitudinal layer, and has connections superiorly to puborectalis and the transverse perineii (Fig. 67.47).
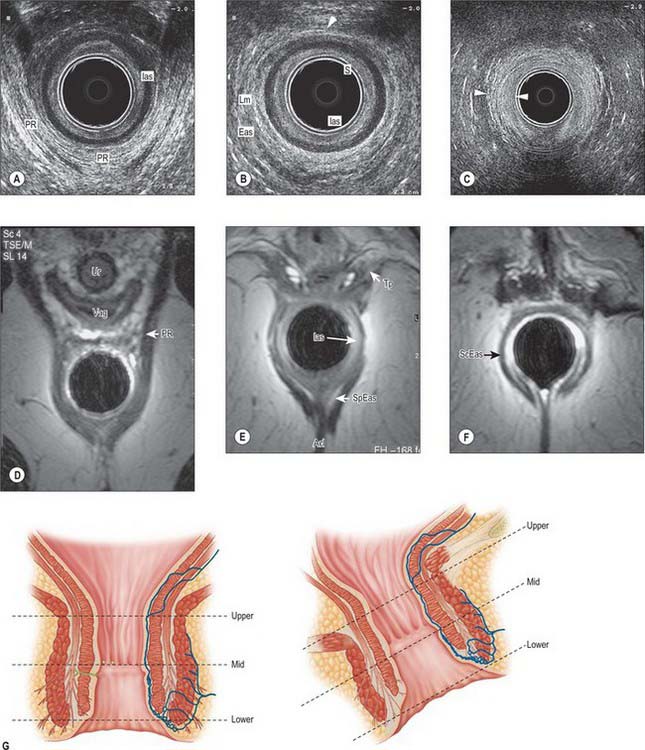
Fig. 67.47 A–C, Axial views of the anal canal at three levels on endoanal ultrasound in a woman. The endoanal ultrasound probe is the central black structure. A, Upper anal canal. The ‘U’ shape of puborectalis (PR) is visible. Ias, internal anal sphincter. B, Middle anal canal. The external anal sphincter (Eas) is now a complete ring anteriorly (arrowhead). Lm, longitudinal muscle; S, subepithelial tissues. C, Lower anal canal. Below the termination of the internal anal sphincter, the longitudinal layer extends through the subcutaneous external anal sphincter (between arrowheads). D–F, MRI of the anal canal. D, At upper anal canal level, the sling of puborectalis (PR) extends anteriorly to the pubic bones. Vag, vagina; Ur, urethra. E, At mid anal canal level, the transverse perineii (Tp) fuse into the external anal sphincter anteriorly. The superficial (middle) external anal sphincter (SpEas) is attached either side of the anococcygeal ligament (Acl). Ias, internal anal sphincter. F, Low anal canal level, below the internal anal sphincter. ScEas, subcutaneous (lower) part of the external anal sphincter. G, Key for levels of the anal canal.
Internal anal sphincter
The internal anal sphincter is a well-defined ring of obliquely orientated smooth muscle fibres that is continuous with the circular muscle of the rectum, and which terminates at the junction of the superficial and subcutaneous components of the external sphincter. Its thickness varies between 1.5 and 3.5 mm, depending upon the height within the anal canal and whether the canal is distended. It is usually thinner in females and becomes thicker with age. It may also be thickened in disease processes such as rectal prolapse and chronic constipation. The lower portion of the sphincter is crossed by fibres from the conjoint longitudinal coat which pass into the submucosa of the lower canal.
The internal anal sphincter is supplied from the terminal branches of the superior rectal vessels and branches of the inferior rectal vessels.
The internal anal sphincter is supplied by the sympathetic and parasympathetic systems by fibres that extend down from the lower rectum. Sympathetic fibres originate in the lower two lumbar spinal segments, are distributed via the inferior hypogastric plexus, and cause contraction of the sphincter. Parasympathetic fibres originate in the second to fourth sacral spinal segments, are distributed via the inferior hypogastric plexus, and cause relaxation of the sphincter.
External anal sphincter
The external anal sphincter is an oval tube-shaped complex of striated muscle, composed mainly of type 1 (slow twitch) skeletal muscle fibres, which are well suited to prolonged contraction. It has been described as consisting of deep, superficial and subcutaneous parts but the external anal sphincter should be considered as a single functional and anatomical entity although the upper middle and lower thirds show different features and attachments. Endoanal ultrasound and magnetic resonance imaging reveal that the uppermost fibres blend with the lowest fibres of puborectalis. In the upper third some of these upper fibres decussate anteriorly into the superficial transverse perineal muscles and posteriorly some fibres are attached to the anococcygeal raphe. The majority of the fibres of the middle third of the external anal sphincter surround the lower part of the internal sphincter. The middle third is attached anteriorly to the perineal body and posteriorly to the coccyx via the anococcygeal ligament: some fibres from each side of the sphincter decussate in these areas to form a commissure in the anterior and posterior midline. The fibres of the lower third lie below the level of the internal anal sphincter and are separated from the lowest anal epithelium by submucosa.
The length and thickness of the external anal sphincter varies between the sexes. In females, the anterior portion tends to be shorter, the wall may be slightly thinner, and the tube may take the form of an asymmetrical cone (Rociu et al 2000) and the transverse perineii and bulbospongiosus fuse with the external sphincter in the lower part of the perineum. In males, the external sphincter is more anular and is separate from the central point of the perineum into which the transverse perineii and bulbospongiosus fuse, so that there is a surgical plane of cleavage between the external sphincter and perineum (Fig. 67.48).
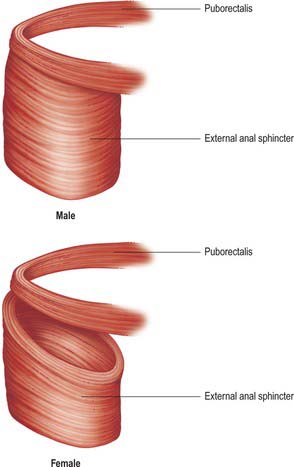
Fig. 67.48 Typical anatomy of male and female external anal sphincters. Puborectalis is shown in isolation with the external anal sphincter viewed from the superolateral aspect. The anterior portion of the external anal sphincter is typically shorter and thinner in females.
The external anal sphincter is supplied from the terminal branches of the inferior rectal vessels with a small contribution from the median sacral artery.
The external anal sphincter is innervated mainly by the inferior rectal branch of the pudendal nerve (anterior divisions of the second, third and fourth sacral spinal nerves). It may also receive some direct supply via fibres which leave the ventral rami of these nerves as they exit the sacral foramina and run beneath the fascia over levator ani to reach the anorectal junction.
FIBROMUSCULAR STRUCTURES OF THE ANAL CANAL
Longitudinal layer and conjoint longitudinal coat
The longitudinal layer is situated between the internal and external sphincters. It contains a fibromuscular layer, the conjoint longitudinal coat, and the intersphincteric space with its connective tissue components (Lunniss & Phillips 1992). The longitudinal layer has muscular and fibroelastic components. The muscular element is formed by fusion of striated muscle fibres from puboanalis, which is the innermost part of puborectalis, with smooth muscle from the longitudinal muscle of the rectum. Endoanal ultrasound and magnetic resonance imaging demonstrate either bundles or incomplete sheets of muscle extending down between the sphincters in the upper canal in both sexes: in men these often end just above the lower border of the internal sphincter. The layer then becomes completely fibroelastic, and splits into septa running between bundles of the subcutaneous external sphincter to terminate in the perianal skin. The area bounded by these septa is generally referred to as the perianal space. The most peripheral of the septa extend between the fibres of the external sphincter into the ischio-anal fat. The most central septa pass through the fibres of the internal sphincter to reach the anal lining and may help to form the intersphincteric groove. The conjoint longitudinal coat is innervated by autonomic fibres that share an origin with the fibres that innervate the internal sphincter.
Other fibromuscular structures
A layer of smooth muscle, yellow elastic fibres, and collagenous connective tissue is found in the anal submucosa, inferior to the anal sinuses. It is derived mainly from strands of the conjoint longitudinal coat, which descend inwards between the fibres of the internal sphincter. Some of the strands end by turning outwards around the lower edge of the internal sphincter to rejoin the main longitudinal layer. Most continue obliquely downwards and insert into the dermis below the intersphincteric groove. These attachments may help to form the corrugations seen in the perianal skin. The septa end in a honeycomb-like arrangement of fibres, which prevents easy distension of the lowest anal lining. This may explain the severe pain produced by pus or blood which collects here, since small volumes of fluid rapidly produce high pressure within the subepithelium.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The arterial supply to the anal canal is derived from terminal branches of the superior rectal artery, the inferior rectal branch of the pudendal artery and branches of the median sacral artery. The supply to the anal canal lining is not distributed uniformly. The arterial supply of the anterior and, more particularly, the posterior, midline epithelia is less good than that lining the lateral portions of the canal (Klosterhalfen et al 1989), which is relevant to the perpetuation of chronic anal fissures.
Veins
The venous drainage of the upper anal canal mucosa, internal anal sphincter and conjoint longitudinal coat passes via the terminal branches of the superior rectal veins into the inferior mesenteric vein. The lower anal canal and external sphincter drain via the inferior rectal branch of the pudenal vein into the internal iliac vein.
Lymphatics
Lymphatics from the upper anal mucosa, internal anal sphincter and conjoint longitudinal coat drain upwards into the submucosal and intramural lymphatics of the rectum. The lower anal canal epithelium and external anal sphincter lymphatics drain downwards via perianal plexuses into vessels that drain into the external inguinal lymph nodes. The lymphatics of puborectalis drain into the internal iliac lymph nodes. This arrangement has considerable importance for tumours of the lower rectum and upper anal canal. If the tumour is confined to the tissue of origin, malignant spread is confined to the mesorectal lymph nodes. However, if the tumour involves puborectalis or tissues associated with the external anal sphincter, the possibility of lymph node spread to these other groups may require radical excision and much more extensive surgery.
INNERVATION
The anal canal is innervated by both autonomic and somatic nervous systems. Preganglionic sympathetic fibres originate in the lower two lumbar segments, run via the lumbar sympathetic chain into the upper two sacral sympathetic ganglia, and into the inferior hypogastric plexus via the sacral splanchnic nerves. Parasympathetic fibres originate in the second to fourth sacral spinal segments and also run to the inferior hypogastric plexus via the pelvic splanchnic nerves. From the inferior hypogastric plexus both sympathetic and parasympathetic fibres run together in nerves of the ‘pelvic plexus’ and enter the lower rectal wall via the ‘lateral ligaments’ of the rectum and anorectal junction via the endopelvic fascia over levator ani. They are distributed to the submucosal and myenteric plexuses where most fibres terminate on interstitial cells (of Cajal).
The autonomic supply to the anus innervates the upper anal mucosa (rich in thermoreceptors), the internal anal sphincter and the conjoint longitudinal coat. Sympathetic fibres cause relaxation of the lower rectal muscle but contraction of the conjoined longitudinal coat and internal anal sphincter fibres via α-adrenergic receptors. Parasympathetic fibres cause relaxation of the internal sphincter via muscarinic receptors which stimulate release of nitric oxide from intramural nitrergic neurones (Brading & Ramalingam 2006).
The external anal sphincter and lower half of the anal mucosa and perianal skin (which are both rich in nocioceptors and pressure receptors) receive a somatic innervation from the anterior divisions of the second, third and fourth sacral spinal nerves which is mainly distributed via the inferior rectal branch of the pudendal nerve. There may also be some direct supply via fibres which leave the ventral rami of the second to fourth sacral nerves as they exit the sacral foramina and run beneath the fascia over levator ani to reach the anorectal junction (because some somatic reflexes are not abolished by division of the pudendal nerves).
Referred pain
Pain in the lower two thirds of the anus is usually felt with a high degree of acuity and is well localized to the perineum and anal canal itself.
Reflexes, control of anorectal function and continence
Local reflex arcs exist within the anorectal intramural plexuses and also within the sacral segments of the spinal cord a spinal centre for co-ordination of anorectal function has not yet been identified. These reflex arcs involve both autonomic and somatic elements because the co-ordination of continence and the initiation of defecation requires that the lower rectum, internal and external anal sphincters act together.
The internal anal sphincter often relaxes following distension of the rectum (Recto-Anal Inhibitory Reflex or ‘RAIR’), suggesting that local reflex pathways exist between the lower rectal sensory fibres and sphincteric motor fibres. This relaxation also occurs on stimulation of somatic sensory nerves, even after the rectum has been excised completely, and is presumably mediated via proprioceptors present in the pelvic floor and an additional reflex pathway via the sacral spinal segments. Integrating the sensory input from the anal canal so as to control the activity of the anal musculature occurs at many levels in the nervous system, including the spinal cord, brain stem, thalamus and cortex. Neural activity not only monitors and regulates defecation, but also other more subtle behaviours within the rectum and anal canal, such as the separation of faeces from rectal gas; local adjustments to faecal consistency and quantities; self-cleansing movements in the rectum and anal canal; coordination with other actions of the perineal and abdominal muscles.
To keep the anal canal closed, the pressure within the anal canal has to be higher than that in the rectum. The resting pressure in the canal is maintained mainly by tonic activity of the internal sphincter as a result of sympathetic tone: sudden increases of intrarectal pressure, e.g. during coughing or exertion, are compensated for by rapid contraction of the external sphincter and puborectalis via spinal reflexes. Angulation of the anorectal junction and the ‘flap valve’ theory is no longer considered important in continence since dynamic imaging of the process of defecation (proctography) has demonstrated that there is a wide range of angulation which does not correlate with function. The internal sphincter does not close the canal completely; the gap (approximately 7 mm) is sealed by the distensible, vascular subepithelial tissues.
Defecation
Defecation is a conscious physiological act in response to feeling the need to pass stool in the rectum: it requires coordinated relaxation of the pelvic floor muscles and anal sphincter. The dynamics have been studied using pressure measurements in the colon, rectum and anus (Herbst et al 1997), and by various imaging techniques including fluoroscopy, ultrasonography and magnetic resonance imaging (Kruyt et al 1991). The process is initiated by mass colonic contractions which drive faeces into the rectum. Rectal distension occurs progressively; there is little increase in intra-rectal pressure because the rectal musculature relaxes as a result of either local intramural or spinal reflexes mediated via sympathetic and parasympathetic fibres. The RAIR is probably involved in allowing the descent of rectal contents into the upper anal canal where it can be ‘sampled’ by contact with the mucosal receptors (particularly thermoreceptors). Defecation may be deferred by conscious contraction of the external anal sphincter until contractions cease and retrograde rectal peristalsis moves stool out of the distal rectum, so that the sensation to defecate passes off. Initiation of defecation involves conscious relaxation of the pelvic floor muscles and external sphincter, so that the pelvic floor descends slightly allowing the anorectal angle to straighten. Reflex relaxation of the internal anal sphincter and conjoint longitudinal coat allows the anal canal to open. Abdominal muscle contraction will aid expulsion from the rectum, but continuing mass colonic contractions push more faeces down into the rectum, so that the entire left colon may be emptied.
Anorectal incontinence
Anal incontinence is common and may occur for a variety of reasons. Abnormally high rectal pressures, as occur in severe diarrhoea, may overcome a normal sphincter, and autonomic disorders may produce abnormal motor activity or loss of normal sensation, so that there is no awareness of the presence of stool. Damage to the anal sphincter may result from vaginal delivery due to stretch, instrumentation or surgical procedures. Atrophy of the external sphincter may occur secondary to pudendal nerve damage during delivery (and may also occur in diabetics, neurological disease and the elderly) A damaged sphincter will not be able to overcome normal fluctuations of intrarectal pressures, or there may be a combination of damage and rectal dysfunction resulting in anal incontinence.
MICROSTRUCTURE
The layers of tissue in the large intestinal wall (see Fig. 67.3; Fig. 67.49) resemble those in the small intestine (Ch. 66), except that villi and circular folds are absent and the glands (crypts) are longer.
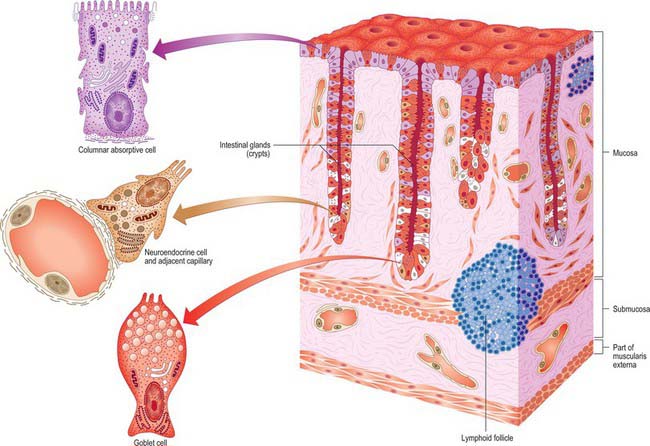
Fig. 67.49 The microstructure of the colonic wall and its epithelial cells. Note the aggregations of lymphocytes (blue) and undifferentiated epithelial cells (white).
Mucosa
The mucosa is pale, smooth, and, in the colon, raised into numerous crescent-shaped folds between the sacculi. In the rectum it is thicker, darker, more vascular, and more loosely attached to the submucosa.
Epithelium
The luminal surface of all but the anorectal junction is lined by columnar cells, mucous (goblet) cells, and occasional microfold (M) cells that are restricted to the epithelium overlying lymphoid follicles. Columnar and mucous cells are also present in the intestinal glands (crypts) which additionally contain stem cells and neuroendocrine cells. In general, the glands lack Paneth cells, but some may be present in the caecum.
Columnar (absorptive) cells are the most numerous of the epithelial cell types. They are responsible for ion exchange and other transepithelial transport functions including water resorption, particularly in the colon. Although there is some variation in their structure, they all bear apical microvilli, which are shorter and less regular than those on enterocytes in the small intestine. All cells have typical junctional complexes around their apices, and these limit extracellular diffusion from the lumen across the intestine wall.
Mucous cells have a similar structure to those of the small intestine, but are more numerous. They are outnumbered by absorptive cells for most of the length of the colon, but they are equally frequent towards the rectum, where their numbers increase further.
Microfold cells are similar to those of the small intestine: they are flattened or cuboidal cells with long, blunt microfolds rather than typical microvilli, and they are restricted to epithelium overlying lymphoid follicles.
Stem cells are the source of the other epithelial cell types in the large intestine. They are located at or near the bases of the intestinal glands, where they divide by mitosis. They provide cells that migrate towards the luminal surface of the intestine: their progeny differentiate, undergo apoptosis and are shed after approximately 5 days.
Intestinal glands (crypts)
The crypts are narrow perpendicular tubular glands which are longer, more numerous and closer together than those of the small intestine. Their openings give a cribriform appearance to the mucosa in surface view. The glands are lined by low columnar epithelial cells, mainly goblet cells, between which are columnar absorptive cells and neuroendocrine cells. Epithelial stem cells at their bases give rise to all three cell types.
Lamina propria
The lamina propria is composed of connective tissue that supports the epithelium. It forms a specialized pericryptal myofibroblast sheath around each intestinal gland. Solitary lymphoid follicles within the lamina propria are most abundant in the caecum, appendix and rectum, but are also present scattered along the rest of the large intestine. They are similar to those of the small intestine; efferent lymphatic vessels originate within them. Lymphatic vessels are absent from the lamina propria core between crypts.
Muscularis externa
The muscularis externa has outer longitudinal and inner circular layers of smooth muscle. The longitudinal fibres form a continuous layer but, with the exception of the uniform outer muscle layer of most of the appendix, macroscopically these are aggregated as longitudinal bands or taeniae coli (see Figs 67.3 and 67.12). Between the taeniae coli the longitudinal layer is much thinner, less than half the circular layer in thickness. The circular fibres form a thin layer over the caecum and colon, and are aggregated particularly in the intervals between the sacculi. In the rectum they form a thick layer and in the anal canal they form the internal anal sphincter. There is an interchange of fascicles between circular and longitudinal layers, especially near the taeniae coli. Deviation of longitudinal fibres from the taeniae to the circular layer may, in some instances, explain the haustration of the colon.
Serosa
The serosa or visceral peritoneum is variable in extent. Along the colon the peritoneum forms small fat-filled appendices epiploicae which are most numerous on the sigmoid and transverse colon but generally absent from the rectum. Subserous loose connective tissue attaches the peritoneum to the muscularis externa.
Brading A, Ramalingam T. Mechanisms controlling normal defecation and the potential effects of spinal cord injury. Prog Brain Res. 2006;152:345-358.
Broden B, Snellman B. Procidentia of the rectum studied with cineradiography. A contribution to the discussion of causative mechanisms. Dis Colon Rectum. 1968;11:330-347.
Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211(1):215-222.
Buschard K, Kjaeldgaard A. Investigations and analysis of the positions, fixation, length and embryology of the vermiform appendix. Acta Chir Scand. 1973;139:293-298.
Duthie HL, Gairns FW. Sensory nerve-endings and sensation in the anal region of man. Br J Surg. 1960;47:585-595.
Fenlon HM. Virtual colonoscopy. Br J Surg. 2002;89(1):1-3.
Describes and reviews the use of multislice CT to image the colon..
Fisher DFJr, Fry WJ. Collateral mesenteric circulation. Surg Gynecol Obstet. 1987;164(5):487-492.
Reviews collateral mesenteric circulations that develop during disease processes..
Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg. 1982;69:613-616.
Original description of the mesorectal plane and its relevance to the surgical excision of rectal tumours..
Herbst F, Kamm MA, Morris GP, Britton K, Woloszko J, Nicholls RJ. Gastrointestinal transit and prolonged ambulatory colonic motility in health and faecal incontinence. Gut. 1997;41:381-389.
Jackson JE. Vascular anatomy of the gastrointestinal tract. In: Butler P, Mitchell AWM, Ellis H. Applied Radiological Anatomy. Cambridge: Cambridge University Press, 1999.
Japanese Society for the Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma. Tokyo: Kanehara, 1997.
Describes the topography of colonic mesenteric lymph nodes with particular reference to radical excision for carcinoma..
Klosterhalfen B, Vogel P, Rixen H, Mittermayer C. Topography of the inferior rectal artery: a possible cause of chronic primary anal fissure. Dis Colon Rectum. 1989;32:43-52.
A detailed postmortem angiographic study demonstrating the arrangement of anal arterial supply..
Kruyt RH, Delemarre JB, Doornbos J, Vogel HJ. Normal anorectum: dynamic MR imaging anatomy. Radiology. 1991;179(1):159-163.
Describes the MR anatomy of the anorectum in relation to surrounding structures and the anorectal angle at rest, during perineal contraction, and during straining, in asymptomatic subjects..
Lunniss PJ, Phillips RK. Anatomy and function of the anal longitudinal muscle. Br J Surg. 1992;79:882-884.
Murphy JM, Maibaum A, Alexander G, Dixon AK. Chilaiditi’s syndrome and obesity. Clin Anat. 2000;13(3):181-184.
Oliphant M, Berne AS, Meyers MA. The subperitoneal space of the abdomen and pelvis: planes of continuity. Am J Roentgenol. 1996;167(6):1433-1439.
Parkes AG. The pathogenesis and treatment of fistula in ano. Br Med J. 1961;1:463-469.
An early, full description of the relationship between the anatomy of anal glands and cryptoglandular sepsis..
Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63(1):1-12.
A classification of anal fistulas based on the pathogenesis of the disease and the normal muscular anatomy of the pelvic floor..
Rociu E, Stoker J, Eijkemans MJ, Lameris JS. Normal anal sphincter anatomy and age- and sex-related variations at high-spatial-resolution endoanal MR imaging. Radiology. 2000;217:395-401.
Sato K, Sato T. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg Radiol Anat. 1991;13(1):17-22.
Seow-Choen F, Ho JM. Histoanatomy of anal glands. Dis Colon Rectum. 1994;37:1215-1218.
Silverstein FE, Tytgat GNJ. Atlas of Gastrointestinal Endoscopy, 2nd edn. London: Gower Medical Publishing, 1991.
Yamaguchi S, Kuroyanagi H, Milson JW, Sim R, Shimada H. Venous anatomy of the right colon: precise structure of the major veins and gastrocolic trunk in 58 cadavers. Dis Colon Rectum. 2002;45:1337-1340.