Pharmacology of Vasoconstrictors
All clinically effective injectable local anesthetics are vasodilators, the degree of vasodilation varying from significant (procaine) to minimal (prilocaine, mepivacaine) and also possibly with both the injection site and individual patient response. After local anesthetic injection into tissues, blood vessels (arterioles and capillaries primarily) in the area dilate, resulting in increased perfusion at the site, leading to the following reactions:
1. An increased rate of absorption of the local anesthetic into the cardiovascular system, which in turn removes it from the injection site (redistribution)
2. Higher plasma levels of the local anesthetic, with an attendant increase in the risk of local anesthetic toxicity (overdose)
3. Decrease in both the depth and duration of anesthesia because the local anesthetic diffuses away from the injection site more rapidly
4. Increased bleeding at the site of treatment as a result of increased perfusion
Vasoconstrictors are drugs that constrict blood vessels and thereby control tissue perfusion. They are added to local anesthetic solutions to oppose the inherent vasodilatory actions of the local anesthetics. Vasoconstrictors are important additions to a local anesthetic solution for the following reasons:
1. By constricting blood vessels, vasoconstrictors decrease blood flow (perfusion) to the site of drug administration.
2. Absorption of the local anesthetic into the cardiovascular system is slowed, resulting in lower anesthetic blood levels.1,2 Table 3-1 illustrates levels of local anesthetic in the blood with and without a vasoconstrictor.1
TABLE 3-1
Effects of Vasoconstrictor (Epinephrine 1 : 200,000) on Peak Local Anesthetic Levels in Blood
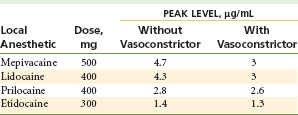
Data from Cannall H, Walters H, Beckett AH, Saunders A: Circulating blood levels of lignocaine after peri-oral injections, Br Dent J 138:87–93, 1975.
3. Local anesthetic blood levels are lowered, thereby decreasing the risk of local anesthetic toxicity.
4. More local anesthetic enters into the nerve, where it remains for longer periods, thereby increasing (in some cases significantly,3 in others minimally4) the duration of action of most local anesthetics.
5. Vasoconstrictors decrease bleeding at the site of administration; therefore they are useful when increased bleeding is anticipated (e.g., during a surgical procedure).5,6
The vasoconstrictors commonly used in conjunction with injected local anesthetics are chemically identical or similar to the sympathetic nervous system mediators epinephrine and norepinephrine. The actions of the vasoconstrictors so resemble the response of adrenergic nerves to stimulation that they are classified as sympathomimetic, or adrenergic, drugs. These drugs have many clinical actions besides vasoconstriction.
Sympathomimetic drugs also may be classified according to their chemical structure and mode of action.
Chemical Structure
Classification of sympathomimetic drugs by chemical structure is related to the presence or absence of a catechol nucleus. Catechol is orthodihydroxybenzene. Sympathomimetic drugs that have hydroxyl (OH) substitutions in the third and fourth positions of the aromatic ring are termed catechols.
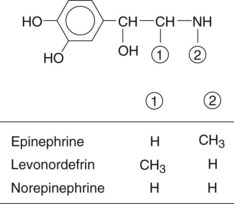
If they also contain an amine group (NH2) attached to the aliphatic side chain, they are then called catecholamines. Epinephrine, norepinephrine, and dopamine are the naturally occurring catecholamines of the sympathetic nervous system. Isoproterenol and levonordefrin are synthetic catecholamines.
Vasoconstrictors that do not possess OH groups in the third and fourth positions of the aromatic molecule are not catechols but are amines because they have an NH2 group attached to the aliphatic side chain.
| Catecholamines | Noncatecholamines |
| Epinephrine | Amphetamine |
| Norepinephrine | Methamphetamine |
| Levonordefrin | Ephedrine |
| Isoproterenol | Mephentermine |
| Dopamine | Hydroxyamphetamine |
| Metaraminol | |
| Methoxamine | |
| Phenylephrine |
Felypressin, a synthetic analog of the polypeptide vasopressin (antidiuretic hormone), is available in many countries as a vasoconstrictor. As of the time of this writing (November 2011), felypressin is not available in the United States.
Modes of Action
Three categories of sympathomimetic amines are known: direct-acting drugs, which exert their action directly on adrenergic receptors; indirect-acting drugs, which act by releasing norepinephrine from adrenergic nerve terminals; and mixed-acting drugs, with both direct and indirect actions (Box 3-1).1-3
Adrenergic Receptors
Adrenergic receptors are found in most tissues of the body. The concept of adrenergic receptors was proposed by Ahlquist in 1948 and is well accepted today.7 Ahlquist recognized two types of adrenergic receptor, termed alpha (α) and beta (β), based on inhibitory or excitatory actions of catecholamines on smooth muscle.
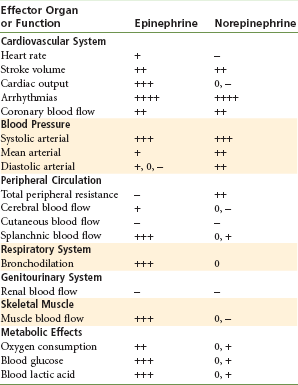
Activation of α receptors by a sympathomimetic drug usually produces a response that includes contraction of smooth muscle in blood vessels (vasoconstriction). Based on differences in their function and location, α receptors have since been subcategorized. Whereas α1 receptors are excitatory-postsynaptic, α2 receptors are inhibitory-postsynaptic.8
Activation of β receptors produces smooth muscle relaxation (vasodilation and bronchodilation) and cardiac stimulation (increased heart rate and strength of contraction).
Beta receptors are further divided into β1, and β2:β1 are found in the heart and small intestines and are responsible for cardiac stimulation and lipolysis; β2, found in the bronchi, vascular beds, and uterus, produce bronchodilation and vasodilation.9
Table 3-2 illustrates the differences in varying degrees of α and β receptor activity of three commonly used vasoconstrictors.
TABLE 3-2
Adrenergic Receptor Activity of Vasoconstrictors

Relative potency of drugs is indicated as follows: +++, high, ++, intermediate, and +, low.
From Jastak JT, Yagiela JA, Donaldson D: Local anesthesia of the oral cavity, Philadelphia, 1995, WB Saunders.
Table 3-3 lists the systemic effects, based on α and β receptor activity, of epinephrine and norepinephrine.
Release of Catecholamines
Other sympathomimetic drugs, such as tyramine and amphetamine, act indirectly by causing the release of the catecholamine norepinephrine from storage sites in adrenergic nerve terminals. In addition, these drugs may exert direct action on α and β receptors.
The clinical actions of this group of drugs therefore are quite similar to the actions of norepinephrine. Successively repeated doses of these drugs will prove to be less effective than those given previously because of depletion of norepinephrine from storage sites. This phenomenon is termed tachyphylaxis and is not seen with drugs that act directly on adrenergic receptors.
Dilutions of Vasoconstrictors
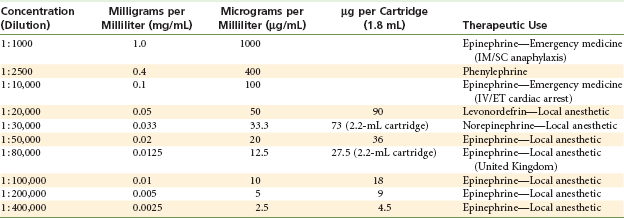
The dilution of vasoconstrictors is commonly referred to as a ratio (e.g., 1 to 1000 [written 1 : 1000]). Because maximum doses of vasoconstrictors are presented in milligrams, or more commonly today as micrograms (µg), the following interpretations should enable the reader to convert these terms readily:
• A concentration of 1 : 1000 means that 1 g (1000 mg) of solute (drug) is contained in 1000 mL of solution.
• Therefore, a 1 : 1000 dilution contains 1000 mg in 1000 mL or 1.0 mg/mL of solution (1000 µg/mL).
Vasoconstrictors, as used in dental local anesthetic solutions, are much less concentrated than the 1 : 1000 dilution described in the preceding paragraph. To produce these more dilute, clinically safer, yet effective concentrations, the 1 : 1000 dilution must be diluted further. This process is described here:
• To produce a 1 : 10,000 concentration, 1 mL of a 1 : 1000 solution is added to 9 mL of solvent (e.g., sterile water); therefore 1 : 10,000 = 0.1 mg/mL (100 µg/mL).
• To produce a 1 : 100,000 concentration, 1 mL of a 1 : 10,000 concentration is added to 9 mL of solvent; therefore 1 : 100,000 = 0.01 mg/mL (10 µg/mL).
The milligram per milliliter and µg per milliliter values of the various vasoconstrictor dilutions used in medicine and dentistry are shown in Table 3-4.
The genesis of vasoconstrictor dilutions in local anesthetics began with the discovery of adrenalin in 1897 by Abel. In 1903, Braun suggested using adrenalin as a chemical tourniquet to prolong the duration of local anesthetics.10 Braun recommended the use of a 1 : 10,000 dilution of epinephrine, ranging to as dilute as 1 : 100,000, with cocaine in nasal surgery (a highly vascular area). It appears at present that an epinephrine concentration of 1 : 200,000 provides comparable results, with fewer systemic side effects. The 1 : 200,000 dilution, which contains 5 µg/mL (or 0.005 mg/mL), has become widely used in both medicine and dentistry and is currently found in articaine, prilocaine, lidocaine (though not in North America), etidocaine, and bupivacaine. In several European and Asian countries, lidocaine with epinephrine concentrations of 1 : 300,000 and 1 : 400,000 is available in dental cartridges.
Although it is the most used vasoconstrictor in local anesthetics in both medicine and dentistry, epinephrine is not an ideal drug. The benefits to be gained from adding epinephrine (or any vasoconstrictor, for that matter) to a local anesthetic solution must be weighed against any risks that might be present. Epinephrine is absorbed from the site of injection, just as is the local anesthetic. Measurable epinephrine blood levels are obtained, and these influence the heart and blood vessels. Resting plasma epinephrine levels (39 pg/mL) are doubled after administration of one cartridge of lidocaine with 1 : 100,000 epinephrine.11 Elevation of epinephrine plasma levels is linearly dose dependent and persists from several minutes to a half-hour.12 Contrary to a previously held position that intraoral administration of usual volumes of epinephrine produced no cardiovascular response, and that patients were more at risk from endogenously released epinephrine than they were from exogenously administered epinephrine,13,14 recent evidence demonstrates that epinephrine plasma levels equivalent to those achieved during moderate to heavy exercise may occur after intraoral injection.15,16 These are associated with moderate increases in cardiac output and stroke volume (see the following section). Blood pressure and heart rate, however, are minimally affected at these dosages.17
In patients with preexisting cardiovascular or thyroid disease, the side effects of absorbed epinephrine must be weighed against those of elevated local anesthetic blood levels. It is currently thought that the cardiovascular effects of conventional epinephrine doses are of little practical concern, even in patients with heart disease.12 However, even following usual precautions (e.g., aspiration, slow injection), sufficient epinephrine can be absorbed to cause sympathomimetic reactions such as apprehension, tachycardia, sweating, and pounding in the chest (palpitation)—the so-called epinephrine reaction.18
Intravascular administration of vasoconstrictors and their administration to sensitive individuals (hyperresponders), or the occurrence of unanticipated drug–drug interactions, can however produce significant clinical manifestations. Intravenous administration of 0.015 mg of epinephrine with lidocaine results in an increase in the heart rate ranging from 25 to 70 beats per minute, with elevations in systolic blood from 20 to 70 mm Hg.12,19,20 Occasional rhythm disturbances may be observed, and premature ventricular contractions (PVCs) are most often noted.
Other vasoconstrictors used in medicine and dentistry include norepinephrine, phenylephrine, levonordefrin, and felypressin. Norepinephrine, lacking significant β2 actions, produces intense peripheral vasoconstriction with possible dramatic elevation of blood pressure, and is associated with a side effect ratio nine times higher than that of epinephrine.21 Although it is currently available in some countries in local anesthetic solutions, the use of norepinephrine as a vasopressor in dentistry is diminishing and cannot be recommended. The use of a mixture of epinephrine and norepinephrine is to be absolutely avoided.22 Phenylephrine, a pure α-adrenergic agonist, theoretically possesses advantages over other vasoconstrictors. However, in clinical trials, peak blood levels of lidocaine were actually higher with phenylephrine 1 : 20,000 (lidocaine blood level = 2.4 µg/mL) than with epinephrine 1 : 200,000 (1.4 µg/mL).23 The cardiovascular effects of levonordefrin most closely resemble those of norepinephrine.24 Felypressin was shown to be about as effective as epinephrine in reducing cutaneous blood flow.5
Epinephrine remains the most effective and the most used vasoconstrictor in medicine and dentistry.
Pharmacology of Specific Agents
The pharmacologic properties of the sympathomimetic amines commonly used as vasoconstrictors in local anesthetics are reviewed. Epinephrine is the most useful and represents the best example of a drug mimicking the activity of sympathetic discharge. Its clinical actions are reviewed in depth. The actions of other drugs are compared with those of epinephrine.
Epinephrine
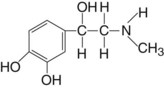
Chemical Structure
Epinephrine as the acid salt is highly soluble in water. Slightly acid solutions are relatively stable if they are protected from air. Deterioration (through oxidation) is hastened by heat and the presence of heavy metal ions. Sodium bisulfite is commonly added to epinephrine solutions to delay this deterioration. The shelf life of a local anesthetic cartridge containing a vasoconstrictor is somewhat shorter (18 months) than that of a cartridge containing no vasoconstrictor (36 months).
Source
Epinephrine is available as a synthetic and is also obtained from the adrenal medulla of animals (epinephrine constitutes approximately 80% of adrenal medullary secretions). It exists in both levorotatory and dextrorotatory forms; the levorotatory form is approximately 15 times as potent as the dextrorotatory form.
Mode of Action
Epinephrine acts directly on both α- and β-adrenergic receptors; β effects predominate.
Systemic Actions
Myocardium: Epinephrine stimulates β1 receptors of the myocardium. There is a positive inotropic (force of contraction) and a positive chronotropic (rate of contraction) effect. Both cardiac output and heart rate are increased.
Pacemaker Cells: Epinephrine stimulates β1 receptors and increases the irritability of pacemaker cells, leading to an increased incidence of dysrhythmias. Ventricular tachycardia (VT) and premature ventricular contractions (PVCs) are common.
Coronary Arteries: Epinephrine produces dilation of the coronary arteries, increasing coronary artery blood flow.
Blood Pressure: Systolic blood pressure is increased. Diastolic pressure is decreased when small doses are administered because of the greater sensitivity to epinephrine of β2 receptors compared with α receptors in vessels supplying the skeletal muscles. Diastolic pressure is increased with larger epinephrine doses because of constriction of blood vessels supplying the skeletal muscles caused by α receptor stimulation.
Cardiovascular Dynamics: The overall action of epinephrine on the heart and cardiovascular system is direct stimulation:
These actions lead to an overall decrease in cardiac efficiency.
The cardiovascular responses of increased systolic blood pressure and increased heart rate develop with the administration of one to two dental cartridges of a 1 : 100,000 epinephrine dilution.25 Administration of four cartridges of 1 : 100,000 epinephrine will bring about a slight decrease in diastolic blood pressure.
Vasculature: The primary action of epinephrine is on smaller arterioles and precapillary sphincters. Blood vessels supplying the skin, mucous membranes, and kidneys primarily contain α receptors. Epinephrine produces constriction in these vessels. Vessels supplying the skeletal muscles contain both α and β2 receptors, with β2 predominating. Small doses of epinephrine produce dilation of these vessels as a result of β2 actions. β2 receptors are more sensitive to epinephrine than are α receptors. Larger doses produce vasoconstriction because α receptors are stimulated.
Hemostasis: Clinically, epinephrine is used frequently as a vasoconstrictor for hemostasis during surgical procedures. Injection of epinephrine directly into surgical sites rapidly produces high tissue concentrations, predominant α receptor stimulation, and hemostasis. As epinephrine tissue levels decrease over time, its primary action on blood vessels reverts to vasodilation because β2 actions predominate; therefore it is common for some bleeding to be noted at about 6 hours after a surgical procedure. In a clinical trial involving extraction of third molars, postsurgical bleeding occurred in 13 of 16 patients receiving epinephrine with their local anesthetic for hemostasis, whereas 0 of 16 patients receiving local anesthetic without vasoconstrictor (mepivacaine plain) had bleeding 6 hours post surgery.26 Additional findings of increased postsurgical pain and delayed wound healing were noted in the epinephrine-receiving group.26
Respiratory System: Epinephrine is a potent dilator (β2 effect) of bronchiole smooth muscle. It is an important drug for management of more refractory episodes of bronchospasm (e.g., status asthmaticus).
Central Nervous System: In usual therapeutic dosages, epinephrine is not a potent central nervous system (CNS) stimulant. Its CNS-stimulating actions become prominent when an excessive dose is administered.
Metabolism: Epinephrine increases oxygen consumption in all tissues. Through β action, it stimulates glycogenolysis in the liver and skeletal muscle, elevating blood sugar levels at plasma epinephrine concentrations of 150 to 200 pg/mL.25 The equivalent of four dental local anesthetic cartridges of 1 : 100,000 epinephrine must be administered to elicit this response.27
Termination of Action and Elimination: The action of epinephrine is terminated primarily by its reuptake by adrenergic nerves. Epinephrine that escapes reuptake is rapidly inactivated in the blood by the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO), both of which are present in the liver.28 Only small amounts (approximately 1%) of epinephrine are excreted unchanged in the urine.
Side Effects and Overdose: The clinical manifestations of epinephrine overdose relate to CNS stimulation and include increasing fear and anxiety, tension, restlessness, throbbing headache, tremor, weakness, dizziness, pallor, respiratory difficulty, and palpitation.
With increasing levels of epinephrine in the blood, cardiac dysrhythmias (especially ventricular) become more common; ventricular fibrillation is a rare but possible consequence. Dramatic increases in both systolic (>300 mm Hg) and diastolic (>200 mm Hg) pressures may be noted and have led to cerebral hemorrhage.29 Anginal episodes may be precipitated in patients with coronary artery insufficiency. Because of the rapid inactivation of epinephrine, the stimulatory phase of the overdose (toxic) reaction usually is brief. Vasoconstrictor overdose is discussed in greater depth in Chapter 18.
Clinical Applications
• Management of acute allergic reactions
• Management of refractory bronchospasm (status asthmaticus)
• Management of cardiac arrest
• As a vasoconstrictor, for hemostasis
• As a vasoconstrictor in local anesthetics, to decrease absorption into the cardiovascular system
• As a vasoconstrictor in local anesthetics, to increase depth of anesthesia
• As a vasoconstrictor in local anesthetics, to increase duration of anesthesia
Availability in Dentistry: Epinephrine is the most potent and widely used vasoconstrictor in dentistry. It is available in the following dilutions and drugs:
| Epinephrine Dilution | Local Anesthetic (generic) |
| 1 : 50,000 | Lidocaine |
| 1 : 80,000 | Lidocaine (lignocaine) (United Kingdom) |
| 1 : 100,000 | Articaine |
| Lidocaine | |
| 1 : 200,000 | Articaine |
| Bupivacaine | |
| Etidocaine† | |
| Lidocaine | |
| Mepivacaine* | |
| Prilocaine | |
| 1 : 300,000 | Lidocaine* |
| 1 : 400,000 | Articaine* |
Maximum Doses: The least concentrated solution that produces effective pain control should be used. Lidocaine is available with two dilutions of epinephrine—1 : 50,000 and 1 : 100,000—in the United States and Canada, and with 1 : 80,000, 1 : 200,000, and 1 : 300,000 dilutions in other countries. The duration of effective pulpal and soft tissue anesthesia is equivalent with all forms. Therefore it is recommended (in North America) that the 1 : 100,000 epinephrine concentration be used with lidocaine when extended pain control is necessary. Where 1 : 200,000 or 1 : 300,000 epinephrine is available with lidocaine, these concentrations are preferred for pain control.30
The dosages in Table 3-5 represent recommended maximums as suggested by this author and others.31 They are conservative figures but still provide the dental practitioner with adequate volumes to produce clinically acceptable anesthesia. The American Heart Association as far back as 1964 stated that “the typical concentrations of vasoconstrictors contained in local anesthetics are not contraindicated in patients with cardiovascular disease so long as preliminary aspiration is practiced, the agent is injected slowly, and the smallest effective dose is administered.”32 In 1954 the New York Heart Association recommended that maximal epinephrine doses be limited to 0.2 mg per appointment.33 In the following years, the American Heart Association recommended the restriction of epinephrine in local anesthetics when administered to patients with ischemic heart disease.34
TABLE 3-5
Recommended Maximum Dosages of Epinephrine

*Maximum epinephrine dose of 0.2 mg or 200 µg per appointment.
†Maximum recommended dose of 0.04 or 40 µg per appointment.
‡Actual maximum volume of administration is limited by the dosage of local anesthetic drug.
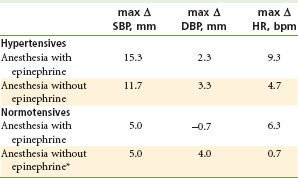
More recently, the Agency for Healthcare Research and Quality (AHRQ) reviewed the published literature on the subject of the effects of epinephrine in dental patients with high blood pressure.35 The report reviewed six studies that evaluated the effects of dental treatment (extraction of teeth) in hypertensive patients when they received local anesthetics with and without epinephrine. Results suggest that hypertensive subjects undergoing an extraction experience small increases in systolic blood pressure and heart rate associated with the use of a local anesthetic containing epinephrine. These increases associated with the use of epinephrine occur in addition to increases in systolic and diastolic blood pressures and heart rate associated with undergoing the procedure without epinephrine that are larger for hypertensive than for normotensive patients. No adverse outcomes were reported among any of the subjects in the studies included in the review, and only one report of an adverse event associated with the use of epinephrine in local anesthetic in a hypertensive patient was identified in the literature (Table 3-6).35
TABLE 3-6
Means of Maximum Changes from Baseline for Blood Pressure and Heart Rate*
DBP, Diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
*Unweighted mean of subject means reported in three studies.
Data from Cardiovascular effects of epinephrine in hypertensive dental patients: summary, evidence report/technology assessment number 48. AHRQ Publication Number 02-E005, Rockville, Md. March 2002, Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/epcsums/ephypsum.htm
In cardiovascularly compromised patients, it seems prudent to limit or avoid exposure to vasoconstrictors, if possible. These include poorly controlled American Society of Anesthesiologists Physical Status classification system (ASA) 3 and all ASA 4 and greater cardiovascular risk patients. However, as stated, the risk of epinephrine administration must be weighed against the benefits to be gained from its inclusion in the local anesthetic solution. Can clinically adequate pain control be provided for this patient without a vasoconstrictor in the solution? What is the potential negative effect of poor anesthesia on endogenous release of catecholamines in response to sudden, unexpected pain?
The use of vasoconstrictors for cardiovascularly compromised patients is reviewed in greater depth in Chapter 20.
Hemostasis: Epinephrine-containing local anesthetic solutions are used, via infiltration into the surgical site, to prevent or to minimize hemorrhage during surgical and other procedures. The 1 : 50,000 dilution of epinephrine is more effective in this regard than less concentrated 1 : 100,000 or 1 : 200,000 solutions.36 Epinephrine dilutions of 1 : 50,000 and 1 : 100,000 are considerably more effective in restricting surgical blood loss than are local anesthetics without vasoconstrictor additives.26
Clinical experience has shown that effective hemostasis can be obtained with concentrations of 1 : 100,000 epinephrine. Although the small volume of 1 : 50,000 epinephrine required for hemostasis does not increase a patient’s risk, consideration always should be given to use of the 1 : 100,000 dilution, especially in patients known to be more sensitive to catecholamines. These include hyperresponders on the Bell-shaped curve, as well as ASA 3 or 4 risk cardiovascularly compromised individuals and geriatric patients.
Norepinephrine (Levarterenol)
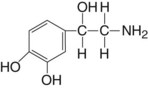
Chemical Structure
Norepinephrine (as the bitartrate) in dental cartridges is relatively stable in acid solutions, deteriorating on exposure to light and air. The shelf life of a cartridge containing norepinephrine bitartrate is 18 months. Acetone-sodium bisulfite is added to the cartridge to retard deterioration.
Source
Norepinephrine is available in both synthetic and natural forms. The natural form constitutes approximately 20% of the catecholamine production of the adrenal medulla. In patients with pheochromocytoma, a tumor of the adrenal medulla, norepinephrine may account for up to 80% of adrenal medullary secretions. It exists in both levorotatory and dextrorotatory forms; the levorotatory form is 40 times as potent as the dextrorotatory form. Norepinephrine is synthesized and is stored at postganglionic adrenergic nerve terminals.
Mode of Action
The actions of norepinephrine are almost exclusively on α receptors (90%). It also stimulates β actions in the heart (10%). Norepinephrine is one fourth as potent as epinephrine.
Systemic Actions
Myocardium: Norepinephrine has a positive inotropic action on the myocardium through β1 stimulation.
Pacemaker Cells: Norepinephrine stimulates pacemaker cells and increases their irritability, leading to a greater incidence of cardiac dysrhythmias (β1 action).
Coronary Arteries: Norepinephrine produces an increase in coronary artery blood flow through a vasodilatory effect.
Heart Rate: Norepinephrine produces a decrease in heart rate caused by reflex actions of the carotid and aortic baroreceptors and the vagus nerve after a marked increase in both systolic and diastolic pressures.
Blood Pressure: Both systolic and diastolic pressures are increased, systolic to a greater extent. This effect is produced through the α-stimulating actions of norepinephrine, which lead to peripheral vasoconstriction and a concomitant increase in peripheral vascular resistance.
Cardiovascular Dynamics: The overall action of norepinephrine on the heart and cardiovascular system is as follows:
Vasculature: Norepinephrine, through α stimulation, produces constriction of cutaneous blood vessels. This leads to increased total peripheral resistance and increased systolic and diastolic blood pressures.
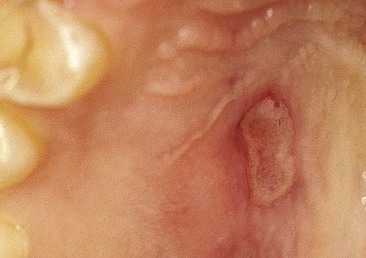
The degree and duration of ischemia noted after norepinephrine infiltration into the palate have led to soft tissue necrosis (Fig. 3-1).
Respiratory System: Norepinephrine does not relax bronchial smooth muscle, as does epinephrine. It does, however, produce α-induced constriction of lung arterioles, which reduces airway resistance to a small degree. Norepinephrine is not clinically effective in the management of bronchospasm.
Central Nervous System: Similar to epinephrine, norepinephrine does not exhibit CNS-stimulating actions at usual therapeutic doses; its CNS-stimulating properties are most prominent after overdose. Clinical manifestations are similar to those of epinephrine overdose (p. 43) but are less frequent and usually are not as severe.
Metabolism: Norepinephrine increases the basal metabolic rate. Tissue oxygen consumption is also increased in the area of injection. Norepinephrine produces an elevation in the blood sugar level in the same manner as epinephrine, but to a lesser degree.
Termination of Action and Elimination: The action of norepinephrine is terminated through its reuptake at adrenergic nerve terminals and its oxidation by MAO. Exogenous norepinephrine is inactivated by COMT.
Side Effects and Overdose: Clinical manifestations of norepinephrine overdose are similar to but less frequent and less severe than those of epinephrine. They normally involve CNS stimulation. Excessive levels of norepinephrine in the blood produce markedly elevated systolic and diastolic pressures with increased risk of hemorrhagic stroke, headache, anginal episodes in susceptible patients, and cardiac dysrhythmias.
Extravascular injection of norepinephrine into tissues may produce necrosis and sloughing because of intense α stimulation. In the oral cavity, the most likely site to encounter this phenomenon is the hard palate (see Fig. 3-1). Norepinephrine should be avoided for vasoconstricting purposes (e.g., hemostasis), especially on the palate. An increasing number of authorities have stated that norepinephrine should not be used at all with local anesthetics.30,37
Clinical Applications: Norepinephrine is used as a vasoconstrictor in local anesthetics and for the management of hypotension.
Availability in Dentistry: In the United States, norepinephrine is no longer available in local anesthetic solutions used in dentistry. In the past, it was included with the local anesthetics propoxycaine and procaine in a 1 : 30,000 concentration. In other countries, norepinephrine is included with lidocaine (Germany) and mepivacaine (Germany) or as the combination of norepinephrine and epinephrine with lidocaine (Germany) or tolycaine (Japan).21
Maximum Doses: When given, norepinephrine should be used for pain control only, there being no justification for its use in obtaining hemostasis. It is approximately 25% as potent a vasopressor as epinephrine and therefore is used clinically as a 1 : 30,000 dilution.
Recommendations of the International Federation of Dental Anesthesiology Societies (IFDAS) suggest that norepinephrine be eliminated as a vasoconstrictor in dental local anesthetics, a statement with which this author wholeheartedly agrees.30
Levonordefrin
Chemical Structure
Levonordefrin is freely soluble in dilute acidic solutions. Sodium bisulfite is added to the solution to delay its deterioration. The shelf life of a cartridge containing levonordefrin-sodium bisulfite is 18 months.
Source
Levonordefrin, a synthetic vasoconstrictor, is prepared by the resolution of nordefrin into its optically active isomers. The dextrorotatory form of nordefrin is virtually inert.
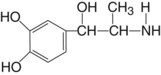
Mode of Action
It appears to act through direct α receptor stimulation (75%) with some β activity (25%), but to a lesser degree than epinephrine. Levonordefrin is 15% as potent a vasopressor as epinephrine.
Systemic Actions
Levonordefrin produces less cardiac and CNS stimulation than is produced by epinephrine.
Respiratory System: Some bronchodilation occurs, but to a much smaller degree than with epinephrine.
Termination of Action and Elimination: Levonordefrin is eliminated through the actions of COMT and MAO.
Side Effects and Overdose: These are the same as with epinephrine, but to a lesser extent. With higher doses, additional side effects include hypertension, ventricular tachycardia, and anginal episodes in patients with coronary artery insufficiency.
Maximum Doses: Levonordefrin is considered one sixth (15%) as effective a vasopressor as epinephrine; therefore it is used in a higher concentration (1 : 20,000).
For all patients, the maximum dose should be 1 mg per appointment; 20 mL of a 1 : 20,000 dilution (11 cartridges).*
In the concentration at which it is available, levonordefrin has the same effect on the clinical activity of local anesthetics as does epinephrine in 1 : 50,000 or 1 : 100,000 concentration.
Phenylephrine Hydrochloride
Chemical Structure
Phenylephrine is quite soluble in water. It is the most stable and the weakest vasoconstrictor employed in dentistry.
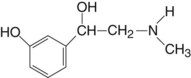
Mode of Action
Direct α receptor stimulation occurs (95%). Although the effect is less than with epinephrine, duration is longer. Phenylephrine exerts little or no β action on the heart. Only a small portion of its activity results from its ability to release norepinephrine. Phenylephrine is only 5% as potent as epinephrine.
Systemic Actions
Heart Rate: Bradycardia is produced by reflex actions of the carotid–aortic baroreceptors and the vagus nerve. Cardiac dysrhythmias are rarely noted, even after large doses of phenylephrine.
Cardiovascular Dynamics: Overall, the cardiovascular actions of phenylephrine are as follows:
Respiratory System: Bronchi are dilated but to a lesser degree than with epinephrine. Phenylephrine is not effective in treating bronchospasm.
Metabolism: Some increase in the metabolic rate is noted. Other actions (e.g., glycogenolysis) are similar to those produced by epinephrine.
Termination of Action and Elimination: Phenylephrine undergoes hydroxylation to epinephrine, then oxidation to metanephrine, after which it is eliminated in the same manner as epinephrine.
Side Effects and Overdose: CNS effects are minimal with phenylephrine. Headache and ventricular dysrhythmias have been noted after overdose. Tachyphylaxis is observed with long-term use.
Clinical Applications: Phenylephrine is used as a vasoconstrictor in local anesthetics, for the management of hypotension, as a nasal decongestant, and in ophthalmic solutions to produce mydriasis.
Availability in Dentistry: Phenylephrine was used with 4% procaine in a 1 : 2500 dilution (no longer available in dental cartridges).
Felypressin
Source
Felypressin is a synthetic analog of the antidiuretic hormone vasopressin. It is a nonsympathomimetic amine, categorized as a vasoconstrictor.
Mode of Action
Felypressin acts as a direct stimulant of vascular smooth muscle. Its actions appear to be more pronounced on the venous than on the arteriolar microcirculation.38
Systemic Actions
Pacemaker Cells: Felypressin is nondysrhythmogenic, in contrast to the sympathomimetic amines (e.g., epinephrine, norepinephrine).
Coronary Arteries: When administered in high doses (greater than therapeutic), it may impair blood flow through the coronary arteries.
Vasculature: In high doses (greater than therapeutic), felypressin-induced constriction of cutaneous blood vessels may produce facial pallor.
Central Nervous System: Felypressin has no effect on adrenergic nerve transmission; thus it may be safely administered to hyperthyroid patients and to anyone receiving MAO inhibitors or tricyclic antidepressants.
Uterus: It has both antidiuretic and oxytocic actions, the latter contraindicating its use in pregnant patients.
Side Effects and Overdose: Laboratory and clinical studies with felypressin in animals and humans have demonstrated a wide margin of safety.39 The drug is well tolerated by the tissues into which it is deposited, with little irritation developing. The incidence of systemic reactions to felypressin is minimal.
Clinical Applications: Felypressin is used as a vasoconstrictor in local anesthetics to decrease their absorption and increase their duration of action.
Availability in Dentistry: Felypressin is employed in a dilution of 0.03 IU/mL (International Units) with 3% prilocaine in Japan, Germany, and other countries. It is not available as a vasoconstrictor in local anesthetics in North America.
Maximum Doses: Felypressin-containing solutions are not recommended for use where hemostasis is necessary because of its predominant effect on the venous rather than the arterial circulation.40
Selection of a Vasoconstrictor
Two vasoconstrictors are available in local anesthetic solutions in North America: epinephrine and levonordefrin.
In the selection of an appropriate vasoconstrictor, if any, for use with a local anesthetic, several factors must be considered: the length of the dental procedure, the need for hemostasis during and after the procedure, the requirement for postoperative pain control, and the medical status of the patient.
Length of the Dental Procedure
The addition of any vasoactive drug to a local anesthetic prolongs the duration (and depth) of pulpal and soft tissue anesthesia of most local anesthetics. For example, pulpal and hard tissue anesthesia with 2% lidocaine lasts approximately 10 minutes; the addition of 1 : 50,000, 1 : 80,000, 1 : 100,000, or 1 : 200,000 epinephrine increases this to approximately 60 minutes. The addition of a vasoconstrictor to prilocaine, on the other hand, does not significantly increase the duration of clinically effective pain control. Prilocaine 4%, after nerve block injection, provides pulpal anesthesia of about 40 to 60 minutes’ duration. (Infiltration injection with prilocaine 4% provides approximately 10 to 15 minutes of pulpal anesthesia.) The addition of a 1 : 200,000 epinephrine concentration to prilocaine increases this slightly (to about 60 to 90 minutes).41
Average durations of pulpal and hard tissue anesthesia expected from commonly used local anesthetics with and without vasoconstrictors are shown in Table 3-7.
TABLE 3-7
Average Durations of Pulpal and Hard Tissue Anesthesia
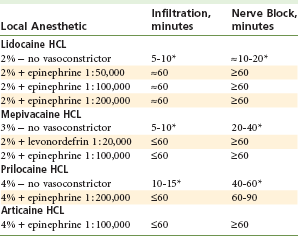
*Indicates duration of pulpal anesthesia usually inadequate to provide pain control for a typical 48-minute procedure.
The typical dental patient is scheduled for a 1-hour appointment. The duration of actual treatment (and the desirable duration of profound pulpal anesthesia) is 47.9 minutes (standard deviation [SD] 14.7 minutes) in a general dentistry office, whereas in the offices of dental specialists, treatment time is 39.1 minutes (SD 19.4 minutes).42
For routine restorative procedures, it might be estimated that pulpal anesthesia will be required for approximately 40 to 50 minutes. As can be seen in Table 3-7, it is difficult to achieve consistently reliable pulpal anesthesia without inclusion of a vasoconstrictor (see minutes marked with asterisks in Table 3-7).
Requirement for Hemostasis
Epinephrine is effective in preventing or minimizing blood loss during surgical procedures. However, epinephrine also produces a rebound vasodilatory effect as the tissue level of epinephrine declines. This leads to possible bleeding postoperatively, which potentially interferes with wound healing.26
Epinephrine, which possesses both α and β actions, produces vasoconstriction through its α effects. Used in a 1 : 50,000 concentration, and even at 1 : 100,000 (but to a lesser extent), epinephrine produces a definite rebound β effect once α-induced vasoconstriction has ceased. This leads to increased postoperative blood loss, which, if significant (not usually the case in dentistry), could compromise a patient’s cardiovascular status.
Phenylephrine, a longer-acting, almost pure α-stimulating vasoconstrictor, does not produce a rebound β effect because its β actions are minimal. Therefore because it is not as potent a vasoconstrictor as epinephrine, hemostasis during the procedure is not as effective; however, because of the long duration of action of phenylephrine compared with that of epinephrine, the postoperative period passes with less bleeding. Total blood loss is usually lower when phenylephrine is used. Phenylephrine is not included in any dental local anesthetic formulation.
Norepinephrine is a potent α stimulator and vasoconstrictor that has produced documented cases of tissue necrosis and sloughing. Norepinephrine cannot be recommended as a vasoconstrictor in dentistry because its disadvantages outweigh its advantages. Other more or equally effective vasoconstrictors are available that do not possess the disadvantages of norepinephrine.43,44
Felypressin constricts the venous circulation more than the arteriolar circulation and therefore is of minimal value for hemostasis.
Vasoconstrictors must be deposited locally into the surgical site (area of bleeding) to provide hemostasis. They act directly on α receptors in the vascular smooth muscle. Only small volumes of local anesthetic solutions with vasoconstrictor are required to achieve hemostasis (i.e., just enough to produce ischemia at the site).
Medical Status of the Patient
Few contraindications are known to vasoconstrictor administration in the concentrations in which they are found in dental local anesthetics. For all patients, and for some in particular, the benefits and risks of including the vasopressor in the local anesthetic solution must be weighed against the benefits and risks of using a plain anesthetic solution.45-47 In general, these groups consist of the following:
• Patients with more significant cardiovascular disease (ASA 3 and 4)*
• Patients with certain noncardiovascular diseases (e.g., thyroid dysfunction, diabetes, sulfite sensitivity)
• Patients receiving MAO inhibitors, tricyclic antidepressants, and phenothiazines
In each of these situations, it is necessary to determine the degree of severity of the underlying disorder to determine whether a vasoconstrictor may be safely included or should be excluded from the local anesthetic solution. It is not uncommon for medical consultation to be sought to aid in determining this information.
Management of these patients is discussed in depth in Chapters 10 and 20. Briefly, however, it may be stated that local anesthetics with vasoconstrictors are not absolutely contraindicated for the patient whose medical condition has been diagnosed and is under control through medical or surgical means (ASA 2 or 3 risk), and if the vasoconstrictor is administered slowly, in minimal doses, after negative aspiration has been ensured.
Patients with a resting blood pressure (minimum 5-minute rest) of greater than 200 mm Hg systolic or greater than 115 mm Hg diastolic should not receive elective dental care until their more significant medical problem of high blood pressure is corrected. Patients with severe cardiovascular disease (ASA 3 or 4 risk) may be at too great a risk for elective dental therapy, for example, a patient who has had a recent (within the past 6 months) acute myocardial infarction with significant myocardial damage; a patient who has been experiencing anginal episodes at rest on a daily basis, or whose signs and symptoms are increasing in severity (preinfarction or unstable angina); or a patient whose cardiac dysrhythmias are refractory to antiarrhythmic drug therapy.45 Epinephrine and other vasoconstrictors can be administered, within limits, to patients with mild to moderate cardiovascular disease (ASA 2 or 3). Because felypressin has minimum cardiovascular stimulatory action and is nondysrhythmogenic, it is the recommended drug for the ASA 3 or 4 cardiovascular risk patient. Epinephrine also is contraindicated in patients exhibiting clinical evidence of the hyperthyroid state.46 Signs and symptoms include exophthalmos, hyperhidrosis, tremor, irritability and nervousness, increased body temperature, inability to tolerate heat, increased heart rate, and increased blood pressure. Minimal dosages of epinephrine are recommended as a vasoconstrictor during general anesthesia when a patient (in any ASA category) is receiving a halogenated anesthetic (halothane, isoflurane, sevoflurane, or enflurane). These inhalation (general) anesthetics sensitize the myocardium such that epinephrine administration is frequently associated with the occurrence of ventricular dysrhythmias (PVCs or ventricular fibrillation). Felypressin is recommended in these situations; however, because of its potential oxytocic actions, felypressin is not recommended for pregnant patients. Once the impaired medical status of the patient is improved (e.g., ASA 4 becomes ASA 3), routine dental care involving the administration of local anesthetics with vasoconstrictors is indicated.
Patients being treated with MAO inhibitors may receive vasoconstrictors within the usual dental dosage parameters without increased risk.47,48 Patients receiving tricyclic antidepressants are at greater risk for the development of dysrhythmias with epinephrine administration. It is recommended that when epinephrine is administered to these patients, its dose be minimal. Administration of levonordefrin or norepinephrine is absolutely contraindicated in patients receiving tricyclic antidepressants.49 Large doses of vasoconstrictor may induce severe (exaggerated) responses.
Local anesthetic formulations with vasoconstrictors also contain an antioxidant (to delay oxidation of the vasoconstrictor). Sodium bisulfite is the most frequently used antioxidant in dental cartridges. It prolongs the shelf life of the anesthetic solution with vasoconstrictor to approximately 18 months. However, sodium bisulfite renders the local anesthetic considerably more acidic than the same solution without a vasoconstrictor. Acidic solutions of local anesthetics contain a greater proportion of charged cation molecules (RNH+) than of uncharged base molecules (RN). Because of this, diffusion of the local anesthetic solution into the axoplasm is slower, resulting in (slightly) delayed onset of anesthesia when local anesthetics containing sodium bisulfite (and vasoconstrictors) are injected.
Vasoconstrictors are important additions to local anesthetic solutions. Numerous studies have demonstrated conclusively that epinephrine, when added to short- or medium-duration local anesthetic solutions, slows the rate of absorption, lowers the systemic blood level, delays cresting of the peak blood level, prolongs the duration of anesthesia, intensifies the depth of anesthesia, and reduces the incidence of systemic reactions.18 In modern dentistry, adequate pain control of sufficient clinical duration and depth is difficult to achieve without inclusion of vasoconstrictors in the local anesthetic solution. Unless specifically contraindicated by a patient’s medical status (ASA 4 or above) or by the required duration of treatment (short), inclusion of a vasoconstrictor should be considered routinely. When these drugs are used, however, care always must be taken to avoid unintended intravascular administration of the vasoconstrictor (as well as the local anesthetic) through multiple aspirations and slow administration of minimum concentrations of both the vasoconstrictor and the local anesthetic.
References
1. Moore, PA, Hersh, EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54:587–599.
2. Finder, RL, Moore, PA. Adverse drug reactions to local anesthesia. Dent Clin North Am. 2002;46:447–457.
3. Brown, G. The influence of adrenaline, noradrenaline vasoconstrictors on the efficacy of lidocaine. J Oral Ther Pharmacol. 1968;4:398–405.
4. Cowan, A. Further clinical evaluation of prilocaine (Citanest), with and without epinephrine. Oral Surg Oral Med Oral Pathol. 1968;26:304–311.
5. Carpenter, RL, Kopacz, DJ, Mackey, DC. Accuracy of Doppler capillary flow measurements for predicting blood loss from skin incisions in pigs. Anesth Analg. 1989;68:308–311.
6. Myers, RR, Heckman, HM. Effects of local anesthesia on nerve blood flow: studies using lidocaine with and without epinephrine. Anesthesiology. 1989;71:757–762.
7. Ahlquist, RP. A study of adrenotropic receptors. Am J Physiol. 1948;153:586–600.
8. Hieble, JP. Adrenoceptor subclassification: an approach to improved cardiovascular therapeutics. Pharmaceut Acta Helvet. 2000;74:63–71.
9. Smiley, RM, Kwatra, MM, Schwinn, DA. New developments in cardiovascular adrenergic receptor pharmacology: molecular mechanisms and clinical relevance. J Cardiothorac Vasc Anesth. 1998;12:10–95.
10. Braun, H. Uber den Einfluss der Vitalitat der Gewebe auf die ortlichen und allgemeinen Giftwirkungen localabaesthesierender Mittel, und uber die Bedeutung des Adrerenalins fur die Lokalanasthesie. Arch Klin Chir. 1903;69:541–591.
11. Tolas, AG, Pflug, AE, Halter, JB. Arterial plasma epinephrine concentrations and hemodynamic responses after dental injection of local anesthetic with epinephrine. J Am Dent Assoc. 1982;104:41–43.
12. Jastak JT, Yagiela JA, Donaldson D, eds. Local anesthesia of the oral cavity. Philadelphia: WB Saunders, 1995.
13. Holroyd, SV, Requa-Clark, B. Local anesthetics. In Holroyd SV, Wynn RL, eds.: Clinical pharmacology in dental practice, ed 3, St Louis: Mosby, 1983.
14. Malamed, SF. Handbook of local anesthesia, ed 5. St Louis: Mosby; 2004.
15. Cryer, PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med. 1980;303:436–444.
16. Yagiela, JA. Epinephrine and the compromised heart. Orofac Pain Manage. 1991;1:5–8.
17. Kaneko, Y, Ichinohe, T, Sakurai, M, et al. Relationship between changes in circulation due to epinephrine oral injection and its plasma concentration. Anesth Prog. 1989;36:188–190.
18. de Jong, RH. Uptake, distribution, and elimination. In: de Jong RH, ed. Local anesthetics. St Louis: Mosby, 1994.
19. Huang, KC. Effect of intravenous epinephrine on heart rate as monitored with a computerized tachometer. Anesthesiology. 1990;73:A762.
20. Narchi, P, Mazoit, J-X, Cohen, S, Samii, K. Heart rate response to an IV test dose of adrenaline and lignocaine with and without atropine pretreatment. Br J Anaesth. 1991;66:583–586.
21. Malamed, SF, Sykes, P, Kubota, Y, et al. Local anesthesia: a review. Anesth Pain Control Dent. 1992;1:11–24.
22. Lipp, M, Dick, W, Daublander, M. Examination of the central venous epinephrine level during local dental infiltration and block anesthesia using tritium marked epinephrine as vasoconstrictor. Anesthesiology. 1988;69:371.
23. Stanton-Hicks, M, Berges, PU, Bonica, JJ. Circulatory effects of peridural block. IV. Comparison of the effects of epinephrine and phenylephrine. Anesthesiology. 1973;39:308–314.
24. Robertson, VJ, Taylor, SE, Gage, TW. Quantitative and qualitative analysis of the pressor effects of levonordefrin. J Cardiovasc Pharmacol. 1984;6:529–935.
25. Clutter, WE, Bier, DM, Shah, SD, Cryer, PE. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980;66:94–101.
26. Sveen, K. Effect of the addition of a vasoconstrictor to local anesthetic solution on operative and postoperative bleeding, analgesia, and wound healing. Int J Oral Surg. 1979;8:301–306.
27. Meechan, JG. The effects of dental local anaesthetics on blood glucose concentration in healthy volunteers and in patients having third molar surgery. Br Dent J. 1991;170:373–376.
28. Lefkowitz, RJ, Hoffman, BB, Taylor, P. Neurohumoral transmission: the autonomic and somatic motor nervous system. In Brunton LL, Lazo JS, Parker KL, eds.: Goodman and Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill Companies, 2006.
29. Campbell, RL. Cardiovascular effects of epinephrine overdose: case report. Anesth Prog. 1977;24:190–193.
30. Jakob, W. Local anaesthesia and vasoconstrictive additional components. Newslett Int Fed Dent Anesthesiol Soc. 1989;2:1.
31. Bennett, CR. Monheim’s local anesthesia and pain control in dental practice, ed 7. St Louis: Mosby; 1983.
32. Management of dental problems in patients with cardiovascular disease: report of a working conference jointly sponsored by the American Dental Association and American Heart Association. J Am Dent Assoc. 1964;68:333–342.
33. Use of epinephrine in connection with procaine in dental procedures: report of the Special Committee of the New York Heart Association, Inc., on the use of epinephrine in connection with procaine in dental procedures. J Am Dent Assoc. 1955;50:108.
34. Kaplan EL, ed. Cardiovascular disease in dental practice. Dallas: American Heart Association, 1986.
35. Cardiovascular effects of epinephrine in hypertensive dental patients: summary, evidence report/technology assessment number 48. AHRQ Publication Number 02-E005, March 2002, Agency for Healthcare Research and Quality, Rockville, Md. Available at http://www.ahrq.gov/clinic/epcsums/ephypsum.htm.
36. Buckley, JA, Ciancio, SG, McMullen, JA. Efficacy of epinephrine concentration in local anesthesia during periodontal surgery. J Periodontol. 1984;55:653–657.
37. Kaufman, E, Garfunkel, A, Findler, M, et al. Emergencies evolving from local anesthesia. Refuat Hapeh Vehashinayim. 2002;19:13–18. [98].
38. Altura, BM, Hershey, SG, Zweifach, BW. Effects of a synthetic analogue of vasopressin on vascular smooth muscle. Proc Soc Exp Biol Med. 1965;119:258–261.
39. Sunada, K, Nakamura, K, Yamashiro, M, et al. Clinically safe dosage of felypressin for patients with essential hypertension. Anesth Prog. 1996;43:408–415.
40. Newcomb, GM, Waite, IM. The effectiveness of local analgesic preparations in reducing haemorrhage during periodontal surgery. J Dent. 1972;1:37–42.
41. Epstein, S. Clinical study of prilocaine with varying concentrations of epinephrine. J Am Dent Assoc. 1969;78:85–90.
42. American Dental Association. 2009 survey of dental practice. Chicago: American Dental Association; February 2010.
43. van der Bijl, P, Victor, AM. Adverse reactions associated with norepinephrine in dental local anesthesia. Anesth Prog. 1992;39:37–89.
44. Hirota, Y, Hori, T, Kay, K, Matsuura, H. Effects of epinephrine and norepinephrine contained in 2% lidocaine on hemodynamics of the carotid and cerebral circulation in older and younger adults. Anesth Pain Control Dent. 1992;1:343–351.
45. Goulet, JP, Perusse, R, Turcotte, JY. Contraindications to vasoconstrictors in dentistry. Part I. Cardiovascular diseases. Oral Surg Oral Med Oral Pathol. 1992;74:579–686.
46. Goulet, JP, Perusse, R, Turcotte, JY. Contraindications to vasoconstrictors in dentistry. Part II. Hyperthyroidism, diabetes, sulfite sensitivity, cortico-dependent asthma, and pheochromocytoma. Oral Surg Oral Med Oral Pathol. 1992;74:587–691.
47. Goulet, JP, Perusse, R, Turcotte, JY. Contraindications to vasoconstrictors in dentistry. Part III. Pharmacologic interactions. Oral Surg Oral Med Oral Pathol. 1992;74:592–697.
48. Verrill, PJ. Adverse reactions to local anaesthetics and vasoconstrictor drugs. Practitioner. 1975;214:380–387.
49. Jastak JT, Yagiela JA, Donaldson D, eds. Local anesthesia of the oral cavity. Philadelphia: WB Saunders, 1995.
*Maximum volume for administration may be limited by the dose of the local anesthetic.
*The ASA Physical Status Classification System is discussed in depth in Chapter 10.