Future Trends in Pain Control
Although local anesthesia remains the backbone of pain control in dentistry, research continues in both medicine and dentistry with the goal of improving all areas of the local anesthetic experience, from that of the administrator to that of the patient. Much of this research has focused on improvements in the area of local anesthesia—safer needles and syringes; more successful techniques of regional nerve block, such as the anterior middle superior alveolar (AMSA) and palatal anterior superior alveolar (P-ASA) (see Chapter 13); and newer drugs, such as articaine HCl. These advances have been discussed in some depth in previous editions of this text and in preceding chapters of this 6th edition: intraosseous anesthesia (see Chapter 15); self-aspirating, pressure, and safety syringes and computer-controlled local anesthetic delivery (C-CLAD) systems (see Chapter 5); and articaine HCl (see Chapter 4). These drugs, devices, and techniques are now a part of the mainstream of pain control in the United States and elsewhere.
Some of the items discussed in previous editions have not progressed into the dental mainstream: the local anesthetics centbucridine and ropivacaine; the ultra-long-acting local anesthetics tetrodotoxin (TTX) and saxitoxin (STX); the topical anesthetic EMLA (eutectic mixture of local anesthetics); and the technique of electronic dental anesthesia (EDA). The reader who is interested in these items is referred to the 5th edition of this textbook.1
Since publication of the 5th edition in 2004, the dental profession in the United States has witnessed the introduction of products and devices that enable the doctor to dramatically decrease the onset time of pulpal anesthesia (the local anesthetic “on” switch), and significantly reduce the duration of the residual soft tissue anesthesia associated with intraoral injections of local anesthetics containing vasopressors (the local anesthetic “off” switch). In addition, recent research appears to demonstrate the usefulness of the infiltration of articaine HCl in providing pulpal anesthesia in the adult mandible.
Increasing the pH of a local anesthetic solution (i.e., buffering) toward a more physiologic pH, although not new in medicine or dentistry, had never been shown to provide consistently reliable clinical results (e.g., shorter onset time, more comfortable injection). Recent changes in the formulation of the buffering solution and in its delivery have greatly increased the effectiveness of this technique.
Residual soft tissue anesthesia—anesthesia of the lips, tongue, chin, or face—oftentimes lasting 5 hours or longer after injection of a vasopressor-containing local anesthetic, is usually unneeded and may, on occasion, present as a potential inconvenience or problem for the patient. Administration of a vasodilating drug into the site of previous local anesthetic administration increases vascular perfusion, allowing the local anesthetic drug to be removed from the site of injection more rapidly, thereby decreasing the duration of residual soft tissue anesthesia.
The inability of the traditional (Halsted approach) inferior alveolar nerve block (IANB) to provide consistently reliable pulpal anesthesia has been a vexing problem for all dentists. The lack of reliable, easy to visualize landmarks and extreme variations in patient anatomy have led to exceedingly high failure rates with this vitally important dental nerve block. Infiltration anesthesia, a mainstay in maxillary anesthesia, did not demonstrate significant clinical success when lidocaine, mepivacaine, and prilocaine were employed by mandibular infiltration in adult patients. However, recent clinical trials of mandibular infiltration with articaine have shown promise.
A current area of clinical research that has demonstrated early promise is the use of intranasally (IN) administered local anesthesia as a means of providing pulpal anesthesia to maxillary teeth without the need for injection (i.e., no needle). Phase II clinical trials comparing IN local anesthetic versus injected local anesthetic have demonstrated significant clinical success in providing pulpal anesthesia bilaterally from the second premolar to the second premolar.
Computer-controlled local anesthetic delivery (C-CLAD) has been included in this chapter on future developments since 1997. Today, C-CLAD has moved into mainstream dentistry. Recent research in this area is reviewed in this chapter.
Buffered Local Anesthetics (The local anesthetic “on” switch)
With the introduction of the first amide local anesthetic (LA), lidocaine HCl, in 1948, providing profound anesthesia of long duration became almost a certainty. Other amides introduced since 1948 include mepivacaine HCl, prilocaine HCl, bupivacaine HCl, etidocaine HCl, and articaine HCl (the latter is considered an amide, although technically it is a hybrid drug, possessing both amide- and ester-type characteristics).
Onset of pulpal anesthesia commonly occurs within 5 to 10 minutes and persists for approximately 60 minutes with articaine HCl, lidocaine HCl, mepivacaine HCl, and prilocaine HCl formulations containing a vasopressor (epinephrine or levonordefrin).
Local anesthetics work. They represent the safest and most effective drugs in medicine for the prevention and management of pain. If deposited in close proximity to a nerve, they will block nerve conduction. However, as good as they are, LAs are not perfect:
• LAs containing a vasopressor sting on injection.
• LAs are associated with a degree of postinjection tissue injury.
• LAs have relatively slow onset.
• LAs do not work as reliably in the presence of infection and inflammation.
These drawbacks can be addressed by buffering the anesthetic solution to a more physiologic pH, which:
• Eliminates the sting on injection
• Reduces tissue injury and postinjection soreness
• Introduces the independent anesthetic effect of carbon dioxide
Reducing Stinging and Postinjection Tissue Injury
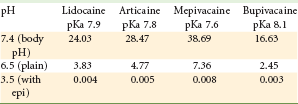
The burning and stinging of acidic injections represent one of the most common complaints in dentistry. LAs containing a vasopressor have a pH of approximately 3.5; “plain” solutions have a pH of approximately 5.9. LA injections that contain epinephrine typically have a very low pH; therefore, a more significant degree of soft tissue injury may be produced by the injection, leading to increased postinjection soreness.
Chemistry and Anesthetic Latency
To achieve anesthesia, two things must happen: (1) the LA must be deposited in close proximity to a nerve, and (2) the LA must diffuse across the nerve membrane to the interior of the nerve, where it blocks sodium channels. The first requirement is met through the injection technique. However, without modification of the solution, the ability of the anesthetic to cross the nerve membrane is dependent on biochemical processes that are out of the practitioner’s control.
Two ionic forms of the LA exist in equilibrium within the anesthetic cartridge: RN (the uncharged, de-ionized, “active” free base form of the drug, which is lipid soluble) and RNH+ (the “charged” or ionized cationic form, which is not lipid soluble). Only the lipid-soluble de-ionized form can cross the nerve membrane. This is described more completely in Chapter 1.
The equilibrium between de-ionized RN and ionized RNH+ is illustrated as follows:
The relative amounts of de-ionized and ionized forms of LA in a dental cartridge are dependent on the pH of the solution, in accordance with the Henderson-Hasselbalch equation. For instance, at a pH of 3.5, 99.996% of lidocaine HCl exists in the non–lipid-soluble ionized (RNH+) form, and only 0.004% is present in the lipid-soluble de-ionized (RN) form. Only the lipid-soluble RN form can cross the nerve membrane. Once within the nerve, the RN picks up an H+ with the resultant RNH+ entering an Na+ channel to block nerve conduction. Only after the body buffers the injected anesthetic solution to a pH closer to the physiologic range (7.35 to 7.45) will enough of the anesthetic enter into the nerve to effectively block nerve conduction. The time that this transformation requires is a key factor in anesthetic latency (e.g., 5 to 10 minute onset for most vasopressor-containing local anesthetic solutions).
Local Anesthesia in the Presence of Infection
Infection represents an additional factor in anesthetic effectiveness. (See Chapter 16 for a more complete description of the effects of infection on LAs and potential solutions to the problem of less effective pain control.) Lower tissue pH at the site of infection makes it extremely difficult for the typical LA injection to provide adequate pulpal anesthesia. Infected tissue is more acidic, making it more difficult for the RN conversion to occur.
Buffering Local Anesthetic Immediately Before Injection
Increasing the pH of a cartridge of lidocaine HCl with epinephrine immediately before administering the injection significantly increases the amount of the active anesthetic form (RN) available: for example, raising the pH of lidocaine HCl from 3.5 to 7.4 produces a 6000-fold increase. This “anesthetic buffering” process results in several clinical advantages, including (1) greater patient comfort during injection; (2) more rapid onset of anesthesia; and (3) decreased postinjection tissue injury. The percentage of RN ions available in local anesthetic solutions at various pH values is shown in Table 20-1.
Introducing Carbon Dioxide via the Buffering Process
When sodium bicarbonate (NaHCO3) solution is mixed with an LA, it interacts with the hydrochloric acid in the LA to create water and carbon dioxide (CO2). The CO2 begins to diffuse out of solution immediately and continues to do so even after the solution has been injected. Catchlove concluded that CO2 in combination with lidocaine HCl potentiates the action of lidocaine HCl by (1) providing a direct depressant effect of CO2 on the axon, (2) concentrating the local anesthetic inside the nerve trunk through ion trapping, and (3) changing the charge of the local anesthetic inside the nerve axon.2 Condouris and Shakalis demonstrated that CO2 possesses an independent anesthetic effect and caused a sevenfold potentiation in anesthetic action.3
Buffering Local Anesthetic in Medicine
Buffering is well known and accepted in medicine where injections of local anesthetic into the skin are considerably more uncomfortable than intraoral injections. Buffering is used frequently in ophthalmology,4 ear nose and throat,5 and dermatology.6 Prefilled cartridges of LA are not used in medicine, so preparation of a buffered solution is relatively simple: the physician adds a volume of NaHCO3 to the local anesthetic solution just before injection. The ratio of LA to bicarbonate in published studies has varied significantly, from 2 : 1 (LA-to-bicarbonate) to 3 : 1, 5 : 1, 6 : 1, 10 : 1, 30 : 1, and 33 : 1, as have their results, from “no positive effect” to “excellent results” to “the formation of a precipitate” within the solution. From these and other studies, it appears that an LA-to-bicarbonate ratio of between 5 : 1 and 10 : 1 provides the greatest opportunity of achieving a more comfortable and more rapid-acting local anesthetic injection.
Buffering Local Anesthetic in Dentistry
The “problem” (as related to buffering) in dentistry is the prefilled local anesthetic cartridge. Because the addition of NaHCO3 must occur within minutes of injection, it is not possible for the local anesthetic manufacturer to produce buffered LA cartridges. Until recently, dentists who attempted to buffer LAs would do so by expelling a volume of LA from the cartridge and replacing it with an equal volume of NaHCO3. This means of buffering led to inconsistent results.7
A recently introduced product (February 2011) provides a means of consistently buffering dental cartridges of LA to a pH ranging between 7.35 and 7.5 (Figs. 20-1 and 20-2).
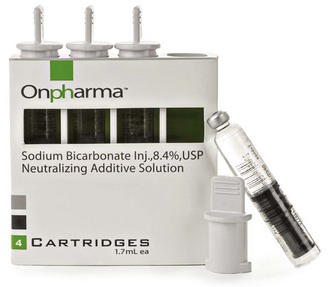
Figure 20-1 Sodium bicarbonate cartridges for buffering local anesthetic solution. (Photo courtesy Onpharma Inc., Los Gatos, Calif.)
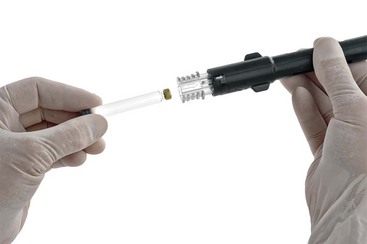
Figure 20-2 Dental local anesthetic cartridge inserted into mixing pen. (Photo courtesy Onpharma Inc., Los Gatos, Calif.)
A prospective, randomized, double-blind, crossover trial (N = 20) compared “standard” LA with epinephrine versus LA with epinephrine buffered toward physiologic pH using sodium bicarbonate.8 Patients served as their own controls, receiving inferior alveolar nerve blocks, once with a standard cartridge of lidocaine 2% with epinephrine 1 : 100,000 (pH ≈3.5), the other time with the same solution buffered with sodium bicarbonate (pH ≈7.4), on two visits separated by at least 2 weeks. The study assessed (1) comfort of injection and (2) speed of onset of pulpal anesthesia.
Seventy-two percent of subjects rated the buffered LA injection as more comfortable than the unbuffered injection, 17% rated them the same, and 11% rated the unbuffered LA as more comfortable (P =.003). When a visual analog scale (VAS) was used to rate pain from “0” (felt nothing) to “10” (worst pain imaginable), 44% of injections with buffered LA were reported to be painless (VAS = 0) versus 6% of injections with traditional LA (P =.004).8
The study also assessed the onset of pulpal anesthesia. Average time to pulpal anesthesia (as determined by electrical pulp testing) was 7 minutes 29 seconds for standard LA and 1 minute 51 seconds for buffered LA (P <.05). Eighty percent of buffered subjects obtained pulpal anesthesia within 2 minutes.8
At this time (January 2012), clinical trials are under way to determine whether buffered local anesthetic solution provides a more profound level of pulpal anesthesia, as seems likely given that approximately 6000 times the RN ionic form of local anesthetic is available to penetrate the nerve membrane.
Developments Since the 5th Edition: Previous editions of this book referenced studies in both medicine and dentistry in which NaHCO3 or hyaluronidase was added to local anesthetic cartridges with extremely variable results.9 The introduction of a stabilized form of NaHCO3 along with use of a delivery device makes addition of NaHCO3 to the dental cartridge with removal of a like volume of LA from the cartridge an easy to accomplish chairside procedure.
Phentolamine Mesylate (The local anesthetic “off” switch)
The local anesthetic armamentarium today consists of drugs that provide a range of durations of pain control, from short-acting drugs (≈30 minutes of pulpal anesthesia) to long-acting drugs that provide pulpal anesthesia up to 7 hours and soft tissue anesthesia up to 12 hours.10 Short-duration drugs provide pulpal anesthesia for approximately 30 minutes and include mepivacaine HCl 3% and prilocaine HCl 4%. The long-duration category consists of bupivacaine HCl 0.5% with epinephrine 1 : 200,000, providing pulpal anesthesia for up to 7 hours (commonly from 90 to 180 minutes) with soft tissue anesthesia for up to 12 hours. It is interesting to note that bupivacaine HCl is a long-acting anesthetic only when administered by nerve block (e.g., inferior alveolar nerve block). It is not nearly as long acting when administered by supraperiosteal (infiltration) injection.
Because the usual length of dental treatment is approximately 44 minutes, the short-duration anesthetics fail to meet the pain control needs of many patients.11 The intermediate-duration category is used most often. With inclusion of a vasopressor (epinephrine or levonordefrin [in North America]), the drugs in this group provide pulpal anesthesia of approximately 60 minutes’ duration. Intermediate-duration drugs include articaine HCl 4% with epinephrine 1 : 100,000 and 1 : 200,000; lidocaine HCl 2% with epinephrine 1 : 50,000 and 1 : 100,000; mepivacaine HCl 2% with levonordefrin 1 : 20,000; and prilocaine HCl 4% with epinephrine 1 : 200,000.
It is pulpal anesthesia that allows a tooth to be treated painlessly. Anesthesia of associated soft tissues (STA) occurs hand-in-hand with pulpal anesthesia. Although necessary for many treatments such as curettage, periodontal surgery, extractions, implants, and subgingival tooth preparation, to be completed painlessly, the duration of STA is considerably longer than that of pulpal anesthesia, averaging 3 to 5 hours in the intermediate-duration group of LAs.
In the mandible, where anesthesia in the adult is usually limited to nerve blocks (inferior alveolar, Gow-Gates), large areas of soft tissue anesthesia develop along with the desired pulpal anesthesia. The anterior two thirds of the tongue, the lower lip, and the cheek are left without sensation for many hours following completion of dental treatment.
Techniques such as periodontal ligament (PDL) injection (also known as intraligamentary injection [ILI]12 and intraosseous [IO] injection)13 provide localized areas of pulpal anesthesia with a minimum of associated soft tissue anesthesia. Anesthesia of the tongue or lip is essentially nonexistent following these injections.
Residual Soft Tissue Anesthesia
Although a long duration of residual STA may be desirable following some dental procedures such as surgery (oral surgical, periodontal, and endodontic), most operative dental care requires profound anesthesia (pulpal) during the relatively brief treatment period while the patient is in the dental chair. Once the traumatic part of the treatment is completed, continued anesthesia of the tissues, hard or soft, is no longer needed. However, the need for effective intraoperative pain control normally mandates the use of a vasoconstrictor-containing local anesthetic.14 Patients commonly are discharged from the dental office with residual numbness to their lips and tongue, which typically persists for an additional 3 to 5 hours.15
Residual STA presents as an inconvenience or embarrassment to the patient, who is unable to function normally for many hours after leaving the dental appointment. In a survey by Rafique and associates16 of patients receiving intraoral local anesthesia, the authors stated that several aspects of the post–local anesthetic experience were disliked by patients, including three major areas—functional, sensory, and perceptual.
Functionally, patients disliked their diminished ability to speak (lisping), to smile (asymmetric), or to drink (liquid runs from the mouth), and their inability to control drooling while still numb. Sensorially, lack of sensation was described as quite discomforting, and the perception that their body was distorted (e.g., swollen lips) was equally unpleasant. For many patients, these sequelae become a significant detriment to their quality of life, making it difficult for them to return to their usual activities for hours after treatment. When the dental appointment concludes at a time approaching a meal—either lunch or dinner—the patient must consider whether to eat while numb or postpone dining until residual STA resolves.
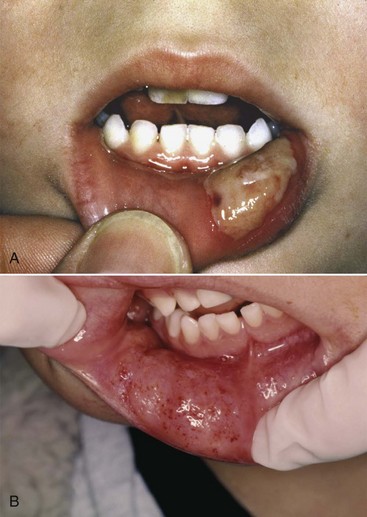
Although not normally a significant problem, residual STA occasionally may lead to self-inflicted injury in any patient. Self-inflicted injury to soft tissues, most commonly the lip or tongue, is more apt to be noted in younger children and in mentally disabled adult and pediatric patients17,18 (Fig. 20-3).
A study of pediatric patients by College and colleagues17 revealed that a significant percentage of inferior alveolar nerve blocks were associated with inadvertent biting of the lips. By age group, the frequency of trauma to the lips was as follows: 18% (<4 yr), 16% (4 to 7 yr), 13% (8 to 11 yr), and 7% (>12 yr) (Table 20-2). This can be explained by the fact that the younger patient will test (by biting) his or her un-numb lip—which hurts—and then will test the still numb side—which doesn’t hurt. Although the adult normally would not proceed beyond this point, the younger child may “play” with this “feeling” and continue to bite ever harder and harder, not realizing the damage that is being inflicted. Mentally handicapped adults are just as likely to incur self-inflicted soft tissue injury. Another group—geriatric patients with dementia—presents a risk of soft tissue injury following LA injection equal to or greater than that of children and mentally challenged adults.
How Local Anesthetics Work—An Overview
When a local anesthetic is deposited close to a nerve, it diffuses into the nerve. When the dental drill stimulates a tooth distal to this site (the area that is “numb”), a nerve impulse is propagated. However, this impulse travels only so far as the area of the nerve where the LA has been deposited. The nerve impulse then dies out, never reaching the patient’s brain. As long as enough LA stays in the nerve, the painful nerve impulse does not reach the brain. This defines the duration of anesthesia produced by the LA drug.
Local anesthetics “stop working” when the volume of LA within the nerve is greater than the volume of LA outside the nerve. The process of diffusion reverses, and the drug begins to leave the nerve and move into the soft tissues surrounding it. Individual nerve fibers are gradually unblocked, causing the patient to tell the doctor that he or she is “starting to feel it again.”
As the drug exits the nerve, it is absorbed into capillaries that carry LA molecules away from the injection site via the venous circulation. The greater the volume of blood flowing through the area where the LA was deposited, the more rapidly this diffusion out of the nerve occurs. This explains why “plain” LAs have a shorter duration of both soft tissue and pulpal anesthesia than those containing a vasopressor. Local anesthetics inherently are vasodilators. Injection of a plain LA increases vascular perfusion at the injection site, allowing entry of a lesser volume of LA into the nerve and more rapid diffusion of the drug back out of the nerve. As a group, plain LAs provide a shorter duration with less profound anesthesia than is provided by anesthetics containing a vasopressor.
The addition of epinephrine or levonordefrin to LA diminishes blood flow into the site of LA deposition. This permits a greater volume of LA to diffuse into the nerve and, because less blood flows through the region, allows LA to remain within the nerve in a higher concentration for a longer time, thus providing a longer duration of more profound anesthesia.
Decreasing the Duration of Residual STA
Increasing the flow of blood through the site in which LA was injected facilitates more rapid diffusion of LA from the nerve into the cardiovascular system, thus decreasing the length of residual STA. Any technique that causes vasodilation can produce this effect.
In the 1980s, the technique known as TENS (transcutaneous electrical nerve stimulation) was successful in shortening the duration of residual STA. TENS is commonly used in sports medicine and in rehabilitation from soft tissue injury.19 Electrodes are placed on the site of injury, and a low-frequency electrical current is delivered to the area (Fig. 20-4). Application of this low-frequency (2.5 Hz) electrical current to an area that has been recently injured is beneficial for the patient in two ways: (1) it acts to increase tissue perfusion produced by capillary and arteriolar dilation, and (2) it simultaneously stimulates the contraction of skeletal muscle. The net effect of these two processes is a pumping action in the area of application of the current. Therapeutically, a 1 hour treatment given at a low frequency helps to decrease edema (skeletal muscle–stimulating effect), and increased perfusion and skeletal muscle stimulation act to “cleanse” the area of tissue injury breakdown products.19 With electrodes placed intraorally around the site where local anesthetic is injected, it was possible to shorten the duration of STA. This technique was short-lived because it was difficult to position the electrodes intraorally and to have them adhere firmly to the moist intraoral mucous membranes.
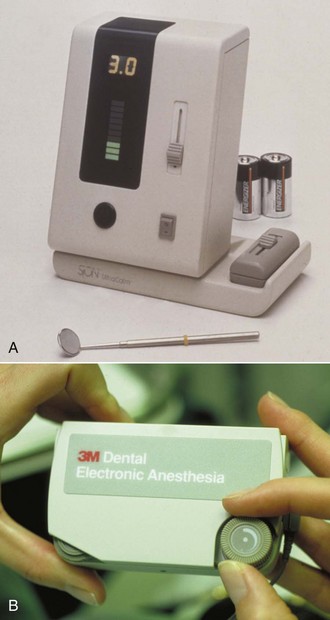
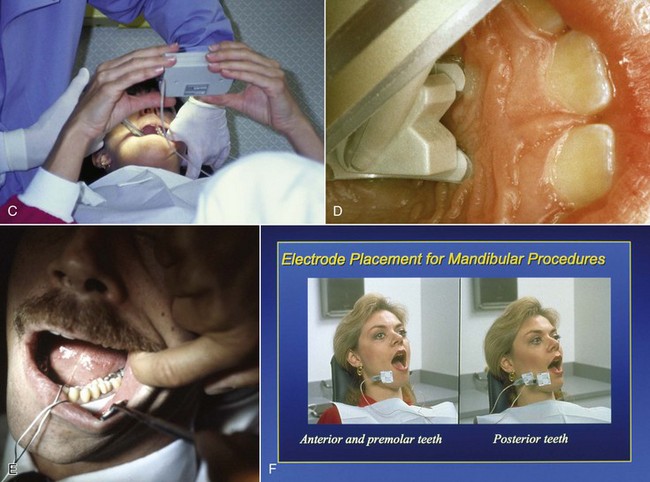
Figure 20-4 A and B, Electronic dental anesthesia (EDA) unit, circa 1980. C and D, Intraoral use of transcutaneous electrical nerve stimulation (TENS) for comfortable administration of local anesthesia and (E and F) for treatment without the need for local anesthesia.
Another approach to the question of how to minimize residual STA consists of injection of a vasodilating drug into the area of prior local anesthetic administration. In theory, this should hasten the redistribution of LA from the nerve into the cardiovascular system (CVS), thereby decreasing the duration of residual STA.
Phentolamine Mesylate
Phentolamine is an α-adrenergic receptor antagonist approved for use by the U.S. Food and Drug Administration (FDA) in 1952 (Fig. 20-5). Approved uses of phentolamine currently include (1) diagnosis of pheochromocytoma, (2) treatment of hypertension in pheochromocytoma,20,21 and (3) prevention of tissue necrosis after norepinephrine extravasation.22,23 An early use of injectable phentolamine involved the management of impotence (erectile dysfunction).24
Phentolamine is a short-acting, competitive antagonist at peripheral α-adrenergic receptors. It antagonizes both α1 and α2 receptors, thus blocking the actions of the circulating catecholamines epinephrine and norepinephrine. Phentolamine also stimulates β-adrenergic receptors in the heart and lungs.
The clinical effects of phentolamine include peripheral vasodilation and tachycardia. Vasodilation results from both direct relaxation of vascular smooth muscle and α blockade. The drug produces positive inotropic and chronotropic effects, leading to an increase in cardiac output. In smaller doses, the positive inotropic effect can predominate and raise blood pressure; in larger doses, peripheral vasodilation can mask the inotropic effect and lower blood pressure. These actions make phentolamine useful in treating hypertension caused by increased circulating levels of epinephrine and norepinephrine, as occurs in pheochromocytoma.
The effects of phentolamine in treating impotence are mediated by α-adrenergic blockade in penile blood vessels. Actions of the drug cause relaxation of the trabecular cavernous smooth muscles and dilation of the penile arteries; this increases arterial blood flow into the corpus cavernosa and subsequently causes an erection.24 Phentolamine is administered IV or IM but can be injected subcutaneously to prevent local tissue necrosis when vasoconstrictor drugs extravasate.23 The pharmacokinetics of phentolamine is largely unknown; 10% of a parenteral dose is excreted in the urine unchanged.
Phentolamine Mesylate for Reversal of Residual STA
Clinical Trials—Adults and Adolescents: An injectable form of phentolamine mesylate (PM) has been formulated to terminate the numbing sensation associated with local anesthesia when it is no longer required. The product, which is available under the proprietary name OraVerse (Septodont Inc. Lancaster, Pa), contains 0.4 mg PM (0.235 mg/mL) packaged in a 1.7 mL dental cartridge22 (Fig. 20-6). In May 2008, the FDA approved phentolamine mesylate, which was marketed in February 2009.25 The dental formulation of phentolamine is approximately  the concentration used in medicine (0.17 mg/mL vs. 5.0 mg/mL).
the concentration used in medicine (0.17 mg/mL vs. 5.0 mg/mL).
Before receiving FDA approval, PM went through a series of clinical trials to demonstrate its safety and efficacy for this new therapeutic indication. Two Phase III, double-blind, randomized, multicenter, controlled studies were conducted.26 One trial studied the safety and efficacy of PM in reversing mandibular STA; the second trial studied the safety and efficacy of PM in reversing maxillary STA. A pediatric Phase II, double-blinded, randomized, multicenter, controlled study was conducted in dental patients, aged 4 to 11 years, who had received 2% lidocaine with 1 : 100,000 epinephrine.27
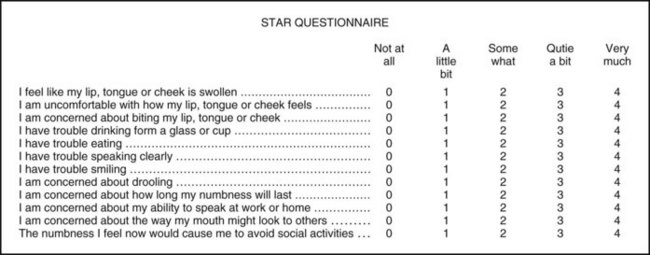
In Phase III trials for this new indication for PM, patients received a local anesthetic containing a vasoconstrictor on one side of the mouth before a restorative or periodontal maintenance procedure was begun. The primary endpoint was elapsed time to the return of normal lip sensation as measured by patient-reported responses to lip palpation. Secondary endpoints included patients’ perceptions of altered function, sensation, and appearance, and functional deficits in smiling, speaking, drinking, and drooling, as assessed by both the patient and an observer blinded to the treatment.26-29 To determine the impact of functional deficits, a patient-reported outcomes questionnaire (Soft Tissue Anesthesia Recovery [STAR]) was developed (Fig. 20-7). In the mandibular study, the time to recovery of tongue sensation was also a secondary endpoint. The dental procedure had to be completed within 60 minutes of the LA injection, and the patient’s lip had to still be numb at that time, or he or she was excluded from the study. All 244 patients randomized in the mandibular study reported lip anesthesia at 1 hour; only 194 reported that their tongue was still numb at this time. The maxillary study enrolled 240 patients.
Patients were randomized to receive one of four local anesthetics: 2% lidocaine + epinephrine 1 : 100,000; 2% mepivacaine + levonordefrin 1 : 20,000; 4% articaine + epinephrine 1 : 100,000; or 4% prilocaine + epinephrine 1 : 200,000. Drugs were randomized using a 6 : 1:1 : 1 ratio based on usage patterns in the United States.
At the conclusion of treatment, the patient received either PM or a control injection. The patient and all investigators were blinded to the treatment assigned. The study drug was administered at the same site, and in the case of PM, the same number of cartridges (one or two) was used as in the previous LA injection(s). The control was a sham injection in which the plastic needle cap attached to the dental syringe containing an empty cartridge was pushed against, but did not penetrate, intraoral soft tissue at the site of the previous LA injection. This sham allowed for a blinded comparison of injection site pain. After receiving PM or the sham injection, all patients were observed for 5 hours for collection of efficacy and safety data, and were monitored for up to 48 hours.
The 5 hour observation and testing period was a primary determinant of the lower age limit (4 years) for patients. It was believed (correctly, it turned out) that younger patients would be unable to cooperate fully with the assessments (see later) required over the 5 hour period of observation.
Lip and Tongue Palpation: All patients were trained in assessing the numbness of their lip. Those in the mandibular protocol were also trained to tap their tongue. The procedure involved light tapping of these soft tissues with the index or middle finger. Research assistants instructed patients that during the study, they would rate the injected side as feeling normal, tingling, or numb, and that they may tap the noninjected side as a comparison. Assessments were made every 5 minutes.
STAR Questionnaire: The STAR questionnaire measures quality of life (see Fig. 20-7). It was developed specifically for these studies to quantify a patient’s perceived clinical benefit derived from reversing soft tissue anesthesia.
Functional Assessment Battery (FAB): The FAB included measurements of smiling, speaking, and drooling, and drinking 3 ounces of water at various time points during the study.28 Each functional assessment was rated as normal or abnormal by a research assistant and by the patient.
Heft-Parker Visual Analog Scale (H-P VAS): The H-P VAS is a 170 mm visual analog scale that contains the following verbal descriptors: none, faint, weak, mild, moderate, strong, intense, and maximum possible.29 Patients were asked to place a mark on the line that corresponded to their current assessment of pain at the injection site and at the procedural site.
Efficacy of Phentolamine Mesylate: Adolescents and Adults.26
In the maxillary trial, the median time to recovery of normal sensation in the upper lip was 50 minutes for PM patients and 132.5 minutes for sham patients, for a reduction in upper lip anesthesia of 82.5 minutes (P <.0001).
In the mandibular trial, the median time to recovery of normal sensation in the lower lip was 70 minutes for PM patients and 155 minutes for sham patients, for a reduction in lower lip anesthesia of 85 minutes (P <.0001).
Within 30 minutes of PM administration, 26.7% of maxillary patients reported return of normal lip sensation as compared with 1.7% in the control group. At 1 hour, 59.2% had normal upper lip sensation versus 11.7% for sham. At 90 minutes, these figures were 75% and 25%, respectively. Upper lip anesthesia persisted beyond 2 hours in 54.2% of sham patients versus 11.6% of PM patients (Fig. 20-8).
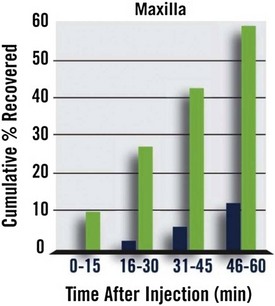
Figure 20-8 Percentage of Patients Reporting Return of Normal Upper Lip Sensation Following Administration of Phentolamine Mesylate (green) or Sham Injection (blue).
In the mandible, within 30 minutes of PM administration, 17.2% of patients reported normal lower lip sensation as compared with 0.8% in the control group. At 1 hour, 41% had normal lower lip sensation versus 7.4% for sham. At 90 minutes, these figures were 70.5% and 13.1%, respectively. Lower lip anesthesia persisted beyond 2 hours in 70.5% of sham patients versus 18.9% of PM patients (Fig. 20-9).
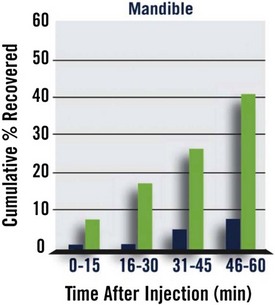
Figure 20-9 Percentage of Patients Reporting Return of Normal Lower Lip Sensation Following Administration of Phentolamine Mesylate (green) or Sham Injection (blue).
The median time to return of normal sensation to the tongue was 60 minutes for PM and 125 minutes for sham-treated patients—a statistically significant (P <.0001) difference of 65 minutes.
Safety of Phentolamine Mesylate: Adolescents and Adults.26
The overall frequency and the nature of adverse events (AEs) reported in the maxillary and mandibular studies appeared similar in nature and frequency. In the maxillary study, a total of 38 patients reported 50 AEs: 32 AEs in 22 patients in the PM group, and 18 AEs in 16 patients in the sham group. In the mandibular study, a total of 63 patients reported 77 AEs: 44 AEs in 34 patients in the PM group, and 33 AEs in 29 patients in the sham group. None of the AEs in either study were serious or rated severe, and no patient was discontinued from the study because of an AE.
Dental patients were administered a dose of 0.2, 0.4, or 0.8 mg of PM. Adverse reactions in which the frequency was greater than or equal to 3% in any PM dose group and was equal to or exceeded that of the control group include diarrhea, facial swelling, increased blood pressure/hypertension, injection site reactions, jaw pain, oral pain, paresthesia, pruritus, tenderness, upper abdominal pain, and vomiting. Most adverse reactions were mild and resolved within 48 hours.30
Safety and Efficacy of Phentolamine Mesylate: Children.31
In a Phase II, double-blind, randomized, multicenter (N = 11), controlled study, pediatric patients between the ages of 4 and 11 years received 2% lidocaine + epinephrine 1 : 100,000 and either PM or sham injection. One hundred fifty-two patients were enrolled and completed the study. A total of 96 patients were included in the PM group and 56 in the sham injection group. Patients received  cartridge of local anesthetic if they weighed more than 15 kg but less than 30 kg, and
cartridge of local anesthetic if they weighed more than 15 kg but less than 30 kg, and  or a full cartridge if they weighed 30 kg or more. Median time to normal lip sensation was evaluated in patients 6 to 11 years of age who were trainable for lip palpation procedures (see earlier). The reduction in median time to normal lip sensation for PM patients (n = 60) was 60 minutes compared with 135 minutes in the sham group (n = 43), representing a reduction in residual STA of 75 minutes (55.6%) for both maxillary and mandibular. Within 1 hour following administration of PM, 61% of patients reported normal lip sensation, but only 21% of patients in the sham injection group reported normal lip sensation. This finding was statistically significant (P <.0001).
or a full cartridge if they weighed 30 kg or more. Median time to normal lip sensation was evaluated in patients 6 to 11 years of age who were trainable for lip palpation procedures (see earlier). The reduction in median time to normal lip sensation for PM patients (n = 60) was 60 minutes compared with 135 minutes in the sham group (n = 43), representing a reduction in residual STA of 75 minutes (55.6%) for both maxillary and mandibular. Within 1 hour following administration of PM, 61% of patients reported normal lip sensation, but only 21% of patients in the sham injection group reported normal lip sensation. This finding was statistically significant (P <.0001).
Among the 152 patients, 35 (23%) reported 37 adverse events with similar frequencies in the PM (20.8%) and sham (26.8%) groups. No deaths or other serious AEs were reported, and all patients completed the study. All but 3 AEs were mild or moderate in severity. One patient in the PM group and 2 in the sham group reported severe AEs: post–dental procedure pain (PM, sham) and injection site pain (sham). All AEs were transient and resolved within the study period.
Clinical Indications for Reversal of Local Anesthesia
Administration of phentolamine mesylate should be a treatment option whenever prolonged STA presents a potential risk (soft tissue injury) or will negatively impact the patient’s lifestyle (e.g., inability to speak or eat). Box 20-1 lists potential candidates for reversal of STA.
Situations that do not usually represent indications for STA reversal involve postsurgical patients in whom prolonged STA is welcomed as a means of preventing breakthrough pain. Further, following local anesthetic administration via the PDL, also known as ILI or IO injection, the extremely localized area of STA associated with these injections precludes the use of PM.
Clinical Use of Phentolamine Mesylate in Dentistry
Phentolamine mesylate is indicated for the reversal of soft tissue anesthesia (i.e., anesthesia of the lip and tongue) and associated functional deficits resulting from intraoral submucosal injection of a local anesthetic containing a vasoconstrictor. Phentolamine mesylate is not recommended for use in children younger than 6 years of age or weighing less than 15 kg (33 lb).30
The recommended dose of phentolamine mesylate is based on the number of cartridges of LA + vasoconstrictor administered. It is administered in an equal volumes, up to a maximum of two cartridges. Phentolamine mesylate is administered at the same location(s) and by the same technique(s) (nerve block or infiltration) used earlier for LA administration.30
Adverse reactions associated with the administration of PM were discussed earlier (safety and adverse reaction discussion). Other potential complications include trismus and paresthesia, both of which are related to the act of injection rather than to the drug itself.
Summary
Phentolamine mesylate (OraVerse) enables the dentist or the dental hygienist (in states or provinces where permitted) to significantly decrease the duration of residual soft tissue anesthesia in patients in whom such numbness may prove to be potentially injurious (children, geriatric patients, and special needs patients) or a negative influence on their quality of life (speaking, eating, negative body image). (Note: As of October 24, 2011, dental hygienists are permitted to administer phentolamine mesylate in the following states: Alaska, Arkansas, California, Hawaii, Idaho, Iowa, Louisiana, Montana, Nevada, New York, North Dakota, Oklahoma, Rhode Island, Tennessee, Utah, and Wisconsin.) Additionally, phentolamine mesylate may be administered following placement of a mandibular implant to aid in the rapid determination of implant impingement on the inferior alveolar nerve.32
Articaine HCL by Buccal Infiltration in the Adult Mandible
Providing effective pain control is one of the most important aspects of dental care. Indeed, patients rate a dentist “who does not hurt” and one who can “give painless injections” as meeting the second and first most important criteria used in evaluating dentists.33 Unfortunately, the ability to obtain consistently profound anesthesia for dental procedures in the mandible has proved extremely elusive. This is even more of a problem when infected teeth are involved, primarily mandibular molars. Anesthesia of maxillary teeth on the other hand, although on occasion difficult to achieve, is rarely an insurmountable problem. Reasons for this include the fact that the cortical plate of bone overlying maxillary teeth is normally thin, thus allowing the local anesthetic drug to diffuse when administered by supraperiosteal injection (infiltration). Additionally, relatively simple nerve blocks, such as posterior superior alveolar (PSA), middle superior alveolar (MSA), anterior superior alveolar (ASA; infraorbital), and anterior middle superior alveolar (AMSA),34 are available as alternatives to infiltration.
It is commonly stated that the significantly higher failure rate for mandibular anesthesia is related to the thickness of the cortical plate of bone in the adult mandible. Indeed it is generally acknowledged that mandibular infiltration is successful when the patient has a full primary dentition.35,36 Once a mixed dentition develops, it is a general rule of teaching that the mandibular cortical plate of bone has thickened to the degree that infiltration might not be effective, leading to the recommendation that “mandibular block” techniques should now be employed.37
A second difficulty with the traditional Halsted approach to the inferior alveolar nerve (i.e., IANB, or “mandibular block”) is an absence of consistent landmarks. Multiple authors have described numerous approaches to this oftentimes elusive nerve.38-40 Indeed, reported failure rates for the IANB are commonly quite high, ranging from 31% and 41% in mandibular second and first molars to 42%, 38%, and 46% in second and first premolars and canines, respectively,9 and 81% in lateral incisors.41
Not only is the inferior alveolar nerve elusive, but studies using ultrasound42 and radiographs43,44 to accurately locate the inferior alveolar neurovascular bundle or the mandibular foramen have revealed that accurate needle location did not guarantee successful pain control.45 The central core theory best explains this problem.46,47 Nerves on the outside of the nerve bundle supply the molar teeth, and nerves on the inside (core fibers) supply the incisor teeth. Therefore the local anesthetic solution deposited near the inferior alveolar nerve may diffuse and block the outermost fibers but not those located more centrally, leading to incomplete mandibular anesthesia.
The difficulty in achieving mandibular anesthesia has, over the years, led to the development of alternative techniques to the traditional (Halsted approach) inferior alveolar nerve block. These have included the Gow-Gates mandibular nerve block,48 the Akinosi-Vazirani closed-mouth nerve block,49 the PDL (intraligamentary) injection,50 intraosseous anesthesia,51 and, most recently, buffered local anesthetics.52 Although all maintain some advantages over the traditional Halsted approach, none is without its own faults and contraindications.
The ability to provide localized areas of anesthesia by infiltration injection without the need for nerve block injections has a number of benefits. Meechan53 has enumerated them as follows: (1) technically simple, (2) more comfortable for patients, (3) can provide hemostasis when needed, (4) in many cases obviates the presence of collateral innervation, (5) avoids the risk of potential damage to nerve trunks, (6) lesser risk of intravascular injection, (7) safer in patients with clotting disorders, (8) reduces risk of needle-stick injury, and (9) preinjection application of topical anesthetic masks needle penetration discomfort.
Mandibular Infiltration
Attempts at mandibular infiltration have been made in the past. In a 1976 study of 331 subjects receiving IANB with lidocaine HCl 2% with epinephrine 1 : 80,000, 23.7% had unsuccessful anesthesia.54 Supplemental infiltration of 1.0 mL of the same drug on the buccal aspect of the mandible proved successful in 70 of the 79 subjects. Of the remaining 9, 7 were successfully anesthetized following additional infiltration of 1.0 mL on the lingual aspect of the mandible.
Yonchak and associates investigated infiltration on incisors and reported 45% success following labial infiltration (lidocaine 2% with 1 : 100,000 epinephrine) and 50% with lingual infiltrations of the same solution for lateral incisors, and 63% and 47% for central incisors on labial and lingual infiltration.55
Meechan and Ledvinka found similar success rates (50%) on central incisor teeth following labial or lingual infiltration of 1.0 mL of lidocaine 2% with 1 : 80,000 epinephrine.56
In 1990 Haas and colleagues compared mandibular buccal infiltrations for canines with prilocaine HCl versus articaine HCl and found no significant differences.57 Success rates were 50% for prilocaine and 65% for articaine (both 4% with epinephrine 1 : 200,000). A second study noted a 63% success rate on mandibular second molars with articaine, and 53% with prilocaine (both 4% with epinephrine 1 : 200,000).58
Recent Findings—Mandibular Infiltration With Articaine HCl
Since the introduction of articaine HCl 4% with epinephrine 1 : 100,000 in the United States in June 2000, numerous anecdotal reports have been received from doctors claiming that they no longer needed to administer the IANB to work in the adult mandible painlessly. They claimed that mandibular infiltration with articaine HCl was uniformly successful. These claims were initially met with skepticism. Over the past 5 years, four well-designed clinical trials have compared infiltration in the adult mandible of articaine HCl 4% with epinephrine 1 : 100,000 versus lidocaine 2% with epinephrine 1 : 100,000 or 1 : 80,000.
Kanaa MD, Whitworth JM, Corbett IP, et al: Articaine and lidocaine mandibular buccal infiltration anesthesia: a prospective randomized double-blind cross-over study, J Endod 32:296–298, 2006.59
Robertson D, Nusstein J, Reader A, et al: The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth, J Am Dent Assoc 138:1104–1112, 2007.60
These first two papers assessed the effectiveness of articaine buccal infiltration administered in lieu of an IANB.
Haase A, Reader A, Nusstein J, et al: Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block, J Am Dent Assoc 139:1228–1235, 2008.61
Kanaa MD, Whitworth JM, Corbett IP, et al: Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block, Int Endod J 42:238–246, 2009.41
These two papers studied the effectiveness of an articaine buccal infiltration as a supplement to the IANB.
These clinically important clinical trials are summarized in the following section.
Kanaa MD, Whitworth JM, Corbett IP, et al: Articaine and lidocaine mandibular buccal infiltration anesthesia: a prospective randomized double-blind cross-over study, J Endod 32:296–298, 2006.59
Design: Infiltrations were administered to 31 subjects in the buccal fold adjacent to the mandibular first molar. A dose of 1.8 mL was administered at a rate of 0.9 mL per 15 seconds. The order of drug administration was randomized, with the second injection administered at least 1 week after the first. The same investigator administered all injections. Electrical pulp testing (EPT) was used to determine pulpal sensitivity. Baseline readings were obtained, and EPT was repeated once every 2 minutes after injection for 30 minutes. If no response (to maximal EPT stimulation of 80 µA) occurred, the number of episodes of no response at maximal stimulation was recorded. The criterion for successful anesthesia was no volunteer response to maximal stimulation on two or more consecutive episodes of testing. (This had been established as the criterion for success in many previous clinical trials.)
Results: The total number of episodes of no sensation on maximal stimulation in first molars over the period of the trial (32 minutes) was greater for articaine (236 episodes) than for lidocaine (129) (P <.001). Twenty (64.5%) subjects experienced anesthetic success following articaine, whereas 12 (38.7%) did so with lidocaine (P <.08). The design of the trial allowed for a maximal possible duration of anesthesia of 28 minutes. Six subjects receiving articaine achieved 28 minutes of anesthesia compared with 2 given lidocaine.
Discussion: The difference between articaine and lidocaine was most obvious toward the end of the study period. The percentage of patients showing no response at maximal stimulation was reduced at all reference points after 22 minutes with lidocaine. With articaine, however, the greatest percentage of nonresponders was noted at the end of the trial (32 minutes).
Conclusion: Overall, 4% articaine with epinephrine was more effective than 2% lidocaine with epinephrine in producing pulpal anesthesia in lower molars following buccal infiltration.
Robertson D, Nusstein J, Reader A, et al: The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth, J Am Dent Assoc 138:1104–1112, 2007.60
Design: A total of 60 blinded subjects randomly received buccal infiltration injections of 1.8 mL of 2% lidocaine with epinephrine 1 : 100,000 and 4% articaine with epinephrine 1 : 100,000 in two separate appointments at least 1 week apart. Each subject served as his/her own control. Sixty infiltrations were administered on the right side, and 60 on the left side. For the second infiltration in each subject, the investigator used the same side randomly chosen for the first infiltration. The teeth chosen for evaluation were the first and second molars and the first and second premolars. The same investigator administered all injections. Before injections were administered, baseline values were determined on the experimental teeth with EPT. A single infiltration was administered buccal to the first mandibular molar, bisecting the approximate location of the mesial and distal roots. The 1.8 mL was deposited over a period of 1 minute. One minute after injection, the first and second molars were pulp tested. At 2 minutes, the premolars were tested. At 3 minutes, the control canine (contralateral side) was tested. This testing cycle was repeated every 3 minutes for 60 minutes. Complete absence of sensation to maximal EPT stimulation on two or more consecutive readings was the criterion for successful anesthesia. The onset of anesthesia was defined as the time at which the first of two consecutive no responses to EPT of 80 occurred.
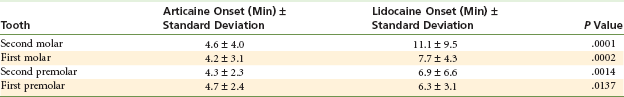
Results: Articaine was significantly better than lidocaine in achieving pulpal anesthesia in each of the four teeth (P <.0001 for all four teeth). Table 20-3 summarizes these findings.
TABLE 20-3
Success Rate in Achieving Pulpal Anesthesia-Articaine versus Lidocaine
| Tooth | Articaine, % Success* | Lidocaine, % Success* |
| Second molar | 75 | 45 |
| First molar | 87 | 57 |
| Second premolar | 92 | 67 |
| First premolar | 86 | 61 |
Modified from Robertson D, Nusstein J, Reader A, et al: The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth, J Am Dent Assoc 138:1104–1112, 2007.
The onset of successful anesthesia was significantly faster for articaine than for lidocaine for all four teeth tested (Table 20-4).
Discussion: The exact mechanism of the increased efficacy of articaine is not known. One theory relates to the 4% concentration of articaine versus the 2% lidocaine solution. However, Potocnik and coworkers found that 4% and 2% articaine formulations were superior to 2% lidocaine in blocking nerve conduction.62 A second theory is that the thiophene ring of articaine enables it to diffuse more effectively than the benzene ring found in other local anesthetics.
Regarding onset of anesthesia, previous studies of lidocaine following IANB found onset times ranging from 8 to 11 minutes for the first molar, and from 8 to 12 minutes for the first premolar.63-68 Articaine provided a more rapid onset of pulpal anesthesia for all tested teeth than for IANB. However, pulpal anesthesia declined steadily over the 60 minute test period. Therefore, if profound pulpal anesthesia is required for 60 minutes, buccal infiltration of 4% articaine with epinephrine 1 : 100,000 will not provide the duration needed because of declining pulpal anesthesia.
Conclusion: Buccal infiltration of the first mandibular molar with 1.8 mL of 4% articaine with epinephrine 1 : 100,000 is significantly better than a similar infiltration of 2% lidocaine with epinephrine 1 : 100,000 in achieving pulpal anesthesia in mandibular posterior teeth. Clinicians should keep in mind that pulpal anesthesia will likely decline slowly over 60 minutes.
Haase A, Reader A, Nusstein J, et al: Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block, J Am Dent Assoc 139:1228–1235, 2008.61
Design: Seventy-three subjects participated in a prospective, randomized, double-blind, crossover study comparing the degree of pulpal anesthesia achieved by means of mandibular buccal infiltration of two anesthetic solutions: 4% articaine with epinephrine 1 : 100,000 and 2% lidocaine with epinephrine 1 : 100,000, following an IANB with 4% articaine with epinephrine 1 : 100,000. Subjects served as their own controls. The side chosen for the first infiltration was used again for the second infiltration. Injections were administered at least 1 week apart. The same investigator administered all injections. An EPT was used to test the first molar for anesthesia in 3 minute cycles for 60 minutes. The IANB was administered over 60 seconds. Fifteen minutes after completion of the IANB, the infiltration was administered buccal to the first mandibular molar, bisecting the approximate location of the mesial and distal roots. The 1.8 mL was deposited over a period of 1 minute. Sixteen minutes after completion of the IANB (1 minute following the infiltration), EPT testing of the first molar was performed. At 3 minutes, the contralateral canine was tested. This cycle was repeated every 3 minutes for 60 minutes. Anesthesia was considered successful when two consecutive EPT readings of 80 were obtained within 10 minutes of the IANB and infiltration injection, and the 80 reading was maintained continuously through the 60th minute.
Results: The articaine formulation was significantly better than the lidocaine formulation with regard to anesthetic success: 88% versus 71% for lidocaine (P <.01), with anesthesia developing within 10 minutes after IANB and buccal infiltration, sustaining the 80 reading on the EPT continuously for the 60 minute testing period.
Discussion: Anesthetic success was significantly better with the 4% articaine formulation than with the 2% lidocaine formulation. Both anesthetic formulations demonstrated a gradual increase in pulpal anesthesia. This is likely a result of the effect of the infiltrations’ overcoming failure or the slow onset of anesthesia following IANB. Therefore, for a maximum effect with the 4% articaine infiltration, a waiting time is required before onset of pulpal anesthesia is achieved. It may be prudent to wait for signs of lip numbness before administering the infiltration. Without an effective IANB, buccal infiltration of articaine alone has a relatively short duration (see previous two citations). A fairly high percentage of patients receiving the articaine infiltration maintained pulpal anesthesia through the 50th minute. Articaine infiltration demonstrated a decline in the incidence of pulpal anesthesia after the 52nd minute. Because most dental procedures require less than 50 minutes for completion, this injection protocol should prove successful for most dental treatments. The 2% lidocaine demonstrated a decline after the 60th minute.
Conclusion: Buccal infiltration of the first molar with a cartridge of 4% articaine with epinephrine 1 : 100,000 resulted in a significantly higher success rate (88%) than was attained with buccal infiltration of a cartridge of 2% lidocaine with epinephrine 1 : 100,000 (71%) after IANB with 4% articaine with epinephrine 1 : 100,000.
Kanaa MD, Whitworth JM, Corbett IP, et al: Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block, Int Endod J 42:238–246, 2009.41
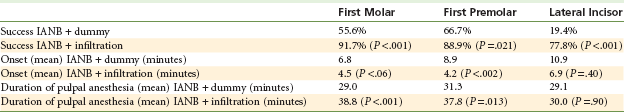
Design: The goal of this study was to compare mandibular tooth anesthesia following lidocaine IANB with and without supplementary articaine buccal infiltration. In this prospective, randomized, double-blind, crossover study, 36 subjects received two IANB injections with 2.2 mL lidocaine 2% with epinephrine 1 : 80,000 over two visits. At one visit, infiltration of 2.2 mL of articaine 4% with epinephrine 1 : 100,000 was administered in the mucobuccal fold opposite the mandibular first molar. At the other visit, a dummy injection was performed. At least 1 week separated the two visits. Pulpal anesthesia of the first molar, the first premolar, and the lateral incisor teeth was assessed with an EPT every 2 minutes for the first 10 minutes, and then at 5 minute intervals for 45 minutes post injection. Successful anesthesia was the absence of sensation on two or more consecutive maximal EPT stimulations. The number of episodes of no response to maximal EPT stimulation was recorded. The onset of pulpal anesthesia was considered the first episode of no response to maximal stimulation (two consecutive readings), whereas the duration of anesthesia was taken as the time from the first of at least two consecutive maximal readings with no response until the onset of more than two responses at less than maximal stimulation, or the end of the 45 minute test period, whichever was sooner.
Results: IANB with supplemental articaine infiltration produced greater success than IANB alone in first molars (33 vs. 20 subjects, respectively; P <.001), premolars (32 vs. 24; P =.021), and lateral incisors (28 vs. 7; P <.001). Additionally, IANB with articaine supplemental infiltration produced significantly more episodes of no response than IANB alone for first molars (339 cases vs. 162, respectively; P <.001), premolars (333 cases vs. 197; P <.001), and lateral incisors (227 cases vs. 63; P <.001) (Table 20-5).
Onset of pulpal anesthesia: In this study, the anesthetic effect for mandibular first molars peaked 25 minutes post injection with the dummy injection versus 6 minutes following articaine infiltration. For first premolars, peak anesthetic effect occurred at 30 minutes post injection versus 8 minutes following articaine infiltration, and for lateral incisors, peak anesthetic effect occurred at 40 minutes post injection versus 20 minutes following articaine infiltration in the first molar region.
Conclusions: IANB injection supplemented with articaine buccal infiltration was more successful than IANB alone for pulpal anesthesia in mandibular teeth. Articaine infiltration increased the duration of pulpal anesthesia in premolar and molar teeth when given in combination with a lidocaine IANB and produced more rapid onset for premolars.
These four clinical trials clearly demonstrate that articaine by mandibular buccal infiltration in the mucobuccal fold by the first mandibular molar can provide more successful anesthesia of longer duration to mandibular teeth when administered alone or as a supplement to IANB.
One thing to consider is that in each of these trials, buccal infiltration of articaine was administered adjacent to the first mandibular molar. The trials demonstrated the effectiveness of articaine in improving pulpal anesthesia success rates in molars and premolars. However, success rates and duration of anesthesia were not improved as significantly in lateral incisors (i.e., teeth at a distance from the site of local anesthetic deposition).
In 2002, Meechan and Ledvinka studied the effects of infiltrating 1.0 mL of 2% lidocaine with 1 : 80,000 epinephrine buccally or lingually to the mandibular central incisor.56 A success rate of 50% was achieved with the buccal or the lingual injection site. However, when the injection dose was split (0.5 mL per site) between buccal and lingual, the success rate increased to a statistically significant 92%.
Jaber and colleagues used a split dose (0.9 mL per site) of 2% lidocaine with 1 : 100,000 epinephrine to confirm this finding.69 For buccal infiltration of 1.8 mL alone, successful anesthesia of the central incisor was 77%, and it was 97% for the split buccal/lingual dose. Investigators also compared articaine 4% with epinephrine 1 : 100,000 versus lidocaine 2% with epinephrine 1 : 100,000 as an anesthetic for infiltration in the anterior mandible and found that articaine was superior to lidocaine in obtaining pulpal anesthesia of the central incisor when infiltrated adjacent to the tooth buccally alone (94%) or in split buccal/lingual injections (97%).
The increased success rate for infiltration in the adult mandibular incisor region is thought to be due to the fact that the cortical plate of bone, both buccal and lingual, is thin and might provide little resistance to infiltration.
Developments Since the 5th Edition: Although anecdotal reports and several studies had hinted at the efficacy of articaine in providing pulpal anesthesia following administration via mandibular infiltration in adults, it had not received serious consideration at the time the 5th edition of this textbook was published in 2004.
Summary and Conclusions
Failure rates for profound pulpal anesthesia following traditional IANB on non–pulpally involved teeth are quite high. This has led to the development of several alternative techniques, including the Gow-Gates mandibular nerve block, the Akinosi-Vazirani closed-mouth mandibular nerve block, periodontal ligament injection, and intraosseous anesthesia. The introduction of the articaine HCl has spurred interest in the use of this local anesthetic by infiltration in the adult mandible.
Initial studies in which articaine was infiltrated in the buccal fold adjacent to the first mandibular molar showed significantly greater success rates compared with lidocaine 2% infiltration (all with epinephrine). Additional studies using articaine mandibular infiltration (by the first molar) as a supplement to IANB (with lidocaine or articaine) revealed the same significant increases. In each of these studies, a full cartridge of local anesthetic (1.8 mL or 2.2 mL) was administered. Future studies are called for to determine the minimal volume of LA solution needed to produce the best clinical result. At this time, the recommendation is to administer a full cartridge of articaine 4% with epinephrine 1 : 100,000 (or 1 : 200,000) in the mucobuccal fold adjacent to the mandibular first molar when treating molars or premolars in the adult mandible.
When mandibular incisors are treated, the recommendation is to administer a split dose of articaine, 0.9 mL in the buccal fold adjacent to the tooth being treated and 0.9 mL on the lingual aspect of the same tooth. Splitting of the LA dose is not effective in the mandibular molar region.
The articaine mandibular infiltration injection can be repeated later in the dental procedure if pulpal anesthesia begins to wane and the patient starts to become sensitive.
An EPT can be used effectively to assess pulpal anesthesia before invasive dental treatment is started.60 Studies have demonstrated that absence of patient response to an 80 reading was an assurance of pulpal anesthesia in vital asymptomatic teeth.70,71 Two consecutive EPT readings at maximal output (80 µA) 2 or 3 minutes apart is almost always indicative of profound anesthesia. Certosimo and Archer reported that patients who had EPT readings of less than 80 experienced pain during operative procedures in asymptomatic teeth.71
Intranasal Local Anesthesia
Absorption of drugs through the nasal mucosa to achieve a systemic effect has a long and varied history. The nares are extremely vascular, so most drugs instilled into them will be absorbed rapidly and distributed systemically (Fig. 20-10). “Snorting a line” of cocaine is an example of illicit use of this route of drug administration. Critical care medicine has used intranasal (IN) drug administration of the central nervous system (CNS)-depressant drug midazolam in the management of status epilepticus in young children.72-74 Lahat and associates compared IN midazolam (0.2 mg/kg) versus intravenous (IV) diazepam (0.3 mg/kg) in 47 children aged 6 months to 5 years.72 Twenty-three of 26 seizures were terminated with IN midazolam—24 of 26 with IV diazepam. The speed at which seizures were terminated was considerably faster with IN midazolam (5.5 minutes) than with IV diazepam (8.0 minutes).
Pediatric dentistry has utilized IN sedation dating as far back as the early 1990s.75-78 The dosage of midazolam most often cited as most effective and safe is 0.2 mg/kg—the same dose employed in critical care medicine for termination of seizures.
Intranasal instillation of local anesthetics has been employed in medicine primarily in the realm of ear nose and throat (ENT) procedures.79,80 Tetracaine, an ester-type local anesthetic, is commonly used to provide a numbing effect before surgical manipulations in the nose. Many patients receiving IN tetracaine have commented on how their upper teeth felt numb, sparking interest in a possible dental application of IN local anesthetic. For dental application, the vasoconstrictor oxymetazoline was added to the tetracaine to enhance effectiveness. Oxymetazoline is the active ingredient in the nasal decongestant spray, Afrin.
In a Phase II double-blind, randomized clinical trial, Ciancio and colleagues compared IN tetracaine with oxymetazoline versus injectable lidocaine 2% with 1 : 100,000 epinephrine in providing pulpal anesthesia bilaterally in the maxilla from first molar to first molar (teeth #3 through #14)81 (Fig. 20-11). Success was defined as the ability to accomplish the dental procedure without the need for rescue medication (injectable local anesthetic) (Fig. 20-12). The tetracaine nasal spray group had a success rate of 88% (22 of 25) versus 93% (14 of 15) in the lidocaine injection group. In the IN group, all failures to achieve adequate pulpal anesthesia occurred on first molars (#3 or #14). Teeth #4 through #13 had 100% success.81

Figure 20-12 Intranasal local anesthesia device. (Photos courtesy St. Renatus, LLC, Ft Collins, Colo.)
Phase III clinical trials have just begun as this text is being written (October 2011).
Computer-Controlled Local Anesthetic Delivery (C-Clad)
During the late 1880s, physicians Sigmund Freud, Carl Koller, and William Halsted were pursuing a common area of clinical research: the development of the drug benzoylmethyl ecognine, more commonly known today as cocaine, for medicinal use and for application as the first local anesthetic.82
Although Freud and Koller were the first to notice the anesthetic effects of cocaine, it was Halsted who introduced cocaine as a local anesthetic in dentistry.83 Using a hypodermic syringe, Halsted demonstrated that interstitial injection of aqueous cocaine resulted in an effective nerve block of the inferior alveolar nerve, and that a small amount of anesthetic solution injected into the trunk of a sensory and motor nerve resulted in blockage of sensory and motor function from the terminal nerve branches. This discovery represented the starting point for local pain control in both dentistry and medicine as we know it today.
This pioneering event relied on the required knowledge of bringing together three separate elements: a drug, a drug delivery instrument, and an anatomic technique. Each of these components had the potential to influence the success or failure of achieving the desired result. The drug delivery instrument commonly known as the hypodermic syringe as used by Halsted was a simple hand-driven mechanical instrument developed in 1853 by the French general surgeon Charles Gabriel Pravas.84 It consisted of a hollow-bore needle connected to a fluid-containing chamber with a sealed plunger. Remarkably, the basic design, mechanics, and manual operation of the Pravaz syringe, invented more than 150 years ago, were virtually identical to those of medical and dental syringes in current use.
What have we learned over the past century about local anesthetic delivery systems? What do clinical data reveal about their common use today? Aside from the noted obvious benefit in providing a convenient means of delivery of a liquid drug, we know (numerous dental studies and consumer surveys have documented this) that the dental syringe predictably invokes fear and anxiety in our patients.85-87 The term trypanophobia (needle phobia) is the extreme and irrational fear of medical and dental procedures involving injection. It is estimated that nearly one in five adults are dental phobics who will avoid, cancel, or fail to appear for required dental treatment because of their fear of the dental injection.88,89
In 1997, a new local anesthetic delivery system was introduced.90 Originally called The Wand (later renamed The CompuDent/Wand; Milestone Scientific, Inc., Livingston, NJ), it represented the first computer-controlled local anesthetic delivery (C-CLAD) system (Fig. 20-13). Within but a few years, C-CLAD technology had helped to redefine our perception and, even more important, the perception of our patients, as to how local anesthesia can and could be achieved.91

Figure 20-13 Early computer-controlled local anesthetic delivery (C-CLAD) devices (1997-2005). (Photo courtesy Milestone Scientific, Livingstone, NJ.)
C-CLAD devices provide clinicians with the ability to precisely control the rate of delivery of the local anesthetic solution.92 In addition, C-CLAD introduced the concept of using a disposable handpiece weighing less than 10 g, allowing the clinician to hold it in a pen-like fashion, greatly increasing tactile control and improving dexterity during injection90 (Fig. 20-14). C-CLAD devices represent a significant advancement for subcutaneous injections and have markedly improved outcomes and experiences for millions of patients over the past decade, helping to mitigate the “fear factor” that has become so closely linked to the dental visit.93-97
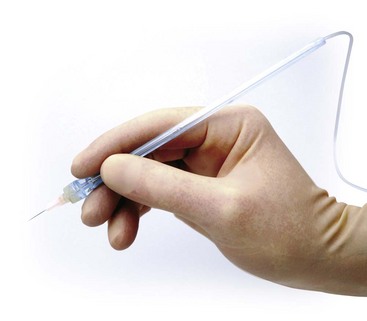
Figure 20-14 Lightweight handpiece for computer-controlled local anesthetic delivery (C-CLAD). (Photo courtesy Milestone Scientific, Livingstone, NJ.)
As a result of this new technology, several new injection techniques were introduced. The first was the anterior middle superior alveolar (AMSA) nerve block (NB), described in 1997 by Friedman and Hochman.96 The AMSA NB achieves maxillary anesthesia of multiple maxillary teeth from a single palatal injection site without the undesired collateral anesthesia to the lip and face. (The AMSA injection is described in Chapter 13.) Subsequently, Friedman and Hochman introduced a technique that they named the palatal-approach anterior superior alveolar (P-ASA) nerve block, in which dental and soft tissue anesthesia of the central and lateral incisors is achieved by a single palatal injection.97 This appears to be the first dental injection that allows practitioners to anesthetize multiple maxillary teeth across the midline during administration of a local anesthetic. (The P-ASA injection is described in Chapter 13.)
A third innovation of this new delivery instrument is related to improving the success rate of the IANB by reducing and/or eliminating needle deflection.98 The IANB injection technique was modified to include use of the bi-rotational insertion technique (BRIT), which is used with the Wand handpiece. Holding the Wand handpiece in a pen-like grasp, the clinician can easily rotate it while simultaneously advancing the needle in a forward direction. This bi-rotational insertion technique has been clinically shown to reduce needle deflection during deep tissue penetration.98,99 Aboushala and associates demonstrated a reduction in missed IANBs and a more rapid onset of anesthesia caused by the increased accuracy of this technique.99
In 2001, Hochman and colleagues advanced the science and understanding of subcutaneous injection fluid dynamics by identifying a predictable method for measuring the precise value of fluid exit-pressure in situ (at the tip of the needle) during drug administration.100 This led to the next significant improvement in C-CLAD technology—the development of an instrument for medical and dental injections capable of controlling all variables of a subcutaneous injection event. This instrument was initially named the CompuFlo (Milestone Scientific).
The CompuFlo technology consists of a C-CLAD device that precisely regulates fluid pressure at the needle tip while a subcutaneous injection or aspiration is performed. The instrument provides the clinician with continuous real-time audible and visual feedback during the injection. The core technology includes a series of mathematical algorithms that function in concert with pressure transducers, allowing instantaneous real-time measurement of fluid exit-pressure at the tip of the needle. This approach to fluid injection dynamics is called dynamic pressure-sensing (DPS) technology, which was developed for the delivery and aspiration of medicaments.101 DPS provides visual and audible in-tissue pressure feedback that helps to (1) identify tissue types for the health care provider; (2) show when certain types of tissue have been penetrated; and (3) ensure that injection of drugs occurs at the precise targeted location. Ghelber and coworkers were the first to publish clinical data related to a medical application for this innovative technology.102 CompuFlo was clinically tested among several human pilot studies involving administration of epidural injections and succeeded in identifying false-positives of anesthesia.103,104 Epidural administration is just one of the many medical and extra-medical applications identified for this sophisticated C-CLAD instrument.
In 2007, CompuFlo technology was applied in dentistry to address an important challenge: performing more predictable single-tooth anesthesia (e.g., the PDL injection). With the decreasing trend of generalized dental caries and the increasing trend toward site-specific treatment of an individual tooth, the use of nerve block anesthesia has become less necessary. Coupled with the unpredictable nature of the IANB, these trends have caused clinicians to look for a more predictable alternative.105-107 To address these findings, the development of a new technology that uses a safer, more predictable approach to performing the PDL injection was pursued.108,109 The STA-Single Tooth Anesthesia system allows dentists to perform a dental injection with real-time feedback, indicating when the needle tip is in the correct location when a dental injection is performed108 (Fig. 20-15).The system incorporates the safety of using dynamic pressure-sensing technology, allowing low-pressure administration of local anesthetic drugs. This same technology allows the easy administration of any traditional injection that may be performed with a manual syringe, in addition to newer dental injections that were developed using C-CLAD instruments (e.g., AMSA, P-ASA, STA-Intraligamentary [PDL] injections).
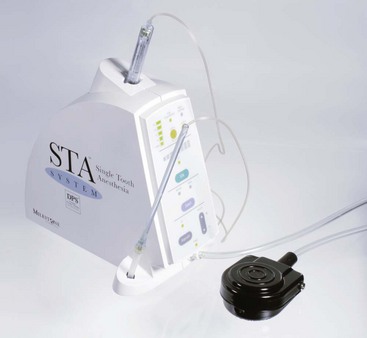
Figure 20-15 STA-Single Tooth Anesthesia computer-controlled local anesthetic delivery (C-CLAD) device incorporates dynamic pressure sensing (DPS) to aid in locating the precise site for periodontal ligament (PDL) injection. (Photo courtesy Milestone Scientific, Livingstone, NJ.)
Over the past decade, numerous clinical trials have been conducted to evaluate the validity and use of this new drug delivery technology for dentistry.110-113 The largest cohort of studies has been related to pain-disruptive behavior in the pediatric dental population.114-121 Two recent publications from Ashkenazi and associates have confirmed several consistent findings, including a measurable reduction in pain-disruptive behavior in children receiving a C-CLAD injection122 and the clinical effectiveness of the PDL injection as a primary injection in primary teeth.123
Ashkenazi and colleagues reported on a study population of 193 children, aged 2 to 13 years, after treating 159 mandibular molars and 48 maxillary molars.123 They reported success rates of 97% mandibular molars and 96% maxillary molars for restorative dentistry treatment when C-CLAD technology was used as the primary dental injection technique. Ashkenazi concluded that pain-disruptive behavior was consistently reported as “relatively non-stressful” for patients, and that a change in the behavior management mode was not required. In essence, dental local anesthesia using low-pressure intrasulcular PDL injection was nondisruptive to these patients. The study concluded that using a C-CLAD device resulted in higher success rates for single-tooth anesthesia, in addition to general absence of pain-disruptive behavior in the pediatric dental patient.
In 2010, Ashkenazi and coworkers published the results of a long-term, controlled clinical study evaluating developmental effects on unerupted permanent teeth following use of a regulated low-pressure PDL injection performed with the STA-System instrument.123 The study population consisted of 78 children (aged 4.1 to 12.8 years) who received STA-Intraligamentary (PDL) injections to 166 primary molar teeth.123 A structured form was designed to include information regarding age at treatment, gender, type of treated tooth, tooth location, type of dental treatment, and type of developmental disturbance(s) present in associated permanent tooth. For each patient, teeth that received conventional dental anesthesia or teeth that were not anesthetized previously served as intrapatient controls. Upon reviewing data collected between 1999 and 2007, Ashkenazi and associates concluded that performing the PDL injection with the use of a low-pressure C-CLAD injection instrument, specifically, the STA-System with dynamic pressure sensing, did not produce damage to the underlying developing permanent tooth bud.123
This finding is important because it represents a new perspective on the PDL injection technique, which contrasts with the long-held position of Brannstrom and colleagues, whose previously published data demonstrated that a PDL injection performed on primary teeth using a hand-controlled high-pressure syringe (e.g., traditional syringe) resulted in developmental disturbances of the underlying tooth bud and had influenced a generation of practitioners.124 Ashkenazi’s findings conclusively demonstrated that use of a precisely regulated low-pressure C-CLAD instrument to perform the PDL injection on primary teeth produced a different outcome based on fluid-pressure differences known between the manual hand-driven syringe and a C-CLAD instrument.123
One can conclude upon reviewing the dental literature that a consensus has been reached over the past 10 years supporting the use of a C-CLAD system to perform dental local anesthesia in the pediatric patient and in the adult patient population. The collective data support the clinical rationale, with the consistent finding of a marked and measurable reduction in pain-disrupted behavior compared with the standard syringe. The following groups have published data supporting this position: Versloot and associates,125 Ram and Kassirer,126 Palm and colleagues,127 Oztas and coworkers,128 Gibson and associates,129 and Allen and colleagues.130
Versloot, Veerkamp, and Hoogstraten compared the behavioral reactions of 125 children, aged 4 to 11 years, who received dental local anesthesia with a traditional syringe compared with a C-CLAD instrument.125 The occurrence of muscle tension, crying, verbal protest, movement, and resistance was scored on the Venham Distress Scale by two independent observers at 15 second intervals. Parents completed the Dental Subscale of the children’s Fear Survey Schedule. Results demonstrated a measurable reduction in overall behavior reactions and patient anxiety when C-CLAD injections were used as compared with the standard syringe. The C-CLAD system demonstrated its greatest effect on low-anxiety children by producing the most positive reaction, making the C-CLAD system useful in treatment of the pediatric patient.
Ram and Kassirer compared the reactions of 138 children, 24 to 38 months old, who received dental local anesthesia of the maxillary incisors by comparing a C-CLAD system with a conventional syringe.126 PDL and P-ASA injection techniques using a C-CLAD instrument were compared with conventional supraperiosteal buccal infiltration performed with a traditional syringe. Behavior reactions related to crying, facial expression, and eye squeeze were independently scored for each injection. Results demonstrated that children receiving the C-CLAD instrument injection displayed better overall behavior than those given supraperiosteal buccal infiltration injection performed with a traditional syringe. The authors concluded that the C-CLAD instrument provided a benefit in treatment of the pediatric patient and recommended its use in this population.
Palm, Kirkegaard, and Poulsen studied the perception of pain and the onset of anesthesia following IANB administered with a C-CLAD device and a traditional dental anesthesia syringe.127 A split-mouth design was used, with each patient serving as his or her own control. Thirty-three patients between the ages of 7 and 18 years were included. All patients were blindfolded, and a sound from the C-CLAD instrument was heard during each injection. Pain ratings were scored on a 10 point Visual Analog Pain-Perception Scale. Results showed that the C-CLAD instrument produced significantly lower pain ratings than were produced by the traditional syringe. Time to onset of anesthesia was similar in this study. Researchers concluded that using a C-CLAD instrument is more effective in reducing subjective pain perception compared with a traditional syringe when IANB is performed.
Oztas, Tezer, Bodur, and Dogan studied 25 children, aged 6 to 10 years, with each child serving as his or her own control.128 Contralateral primary mandibular molars were treated in two separate visits with random use of the C-CLAD device or traditional syringe injection. Pain perception levels for each injection were assessed with the Eland Color Scale. Results of this study show that an overwhelming number of patients preferred the PDL injection performed with the C-CLAD device over IANB injection performed with a traditional syringe.
Jalevik and Klingberg evaluated 20 adolescents in need of bilateral surgical exposure of canines and/or extractions for orthodontic reasons in the maxilla.120 Conventional infiltration anesthesia using a traditional syringe was compared with a palatal injection performed using the C-CLAD device on the same patient. A randomized study design was used to determine the order of injection, and scoring of patient pain perceptions was recorded using a visual analog scale immediately post treatment. Results indicate that the sensation of pain was significantly lower (P <.01) when the C-CLAD device was used than when the traditional syringe was used; patients who reported fear of injection experienced much less pain when receiving a C-CLAD injection (P <.001). Investigators concluded that the C-CLAD device and the palatal injection technique were superior compared with the conventional syringe and injection technique, especially among those who reported fear of injections.
Gibson and associates published results from 62 patients between the ages of 5 and 13 years who required dental local anesthesia.129 Patients were randomly assigned a C-CLAD device or a traditional syringe injection. Pain ratings were recorded, and subjects rated their satisfaction with treatment. Investigators found that C-CLAD injections resulted in significantly fewer disruptive behaviors during initial moments of the injection. Nearly twice as many children receiving a traditional palatal injection were disruptive compared with those receiving a C-CLAD device injection. Disruptive behaviors included significantly increased intervals of crying and disruptive body movements. In addition, five times more patients receiving a traditional palatal injection required restraint compared with patients anesthetized with the C-CLAD device. Gibson and colleagues concluded by stating, “the C-CLAD instrument injection offers a valuable means of reducing the disruptive behavior of children during injections.”129
Allen and coworkers studied 40 preschool patients between 2 and 5 years of age.116 The purpose of the investigation was to evaluate the efficacy of a C-CLAD injection in reducing pain behavior in the preschool-aged child when compared with a traditional syringe. A C-CLAD palatal injection technique was compared with traditional syringe infiltration. Results demonstrated the largest difference related to the need for patient restraint, with only 3% of C-CLAD patients requiring restraint compared with 34% of the intervals for traditional syringe injections. Allen and associates concluded that preschool-aged children anesthetized with the C-CLAD device demonstrated significantly fewer disruptive behaviors when compared with those given a traditional injection regimen. Additionally, even with control applied for the increased duration of the injection, the slower rate of anesthetic delivery of the C-CLAD did appear to reliably reduce pain-related behavior in children. These young children were significantly less likely to cry or to move in a disruptive manner. The authors stated, “most impressively the results showed that none of the preschool-aged children exposed to the C-CLAD instrument required restraint during the initial interval, while nearly half of the children receiving a traditional injection required some type of immediate restraint. These results are important because they demonstrate that the C-CLAD instrument can significantly reduce disruptive behavior in a population of young children who are traditionally more difficult to manage. Reducing disruptive behavior in preschool-aged children is important not only because it creates a more positive experience for the child, but it also creates a more positive experience for the practitioner.”116
Asarch and associates conducted a study of 57 patients between 5 and 13 years of age to evaluate pain-disruptive behavior and each subject’s subjective pain perception when a traditional syringe injection was compared with the C-CLAD system injection.114 IANB injection and palatal and buccal infiltration were the only injections administered, with each subject receiving a single injection. Authors stated the method of use for the C-CLAD device as follows: “A slow rate of administration was used prior to needle insertion. Upon evidence of negative aspiration, the fast-rate of administration was used.” Pain perceptions were rated using a 10 point visual analog scale; pain behavior was evaluated by an external examiner, and subjects rated their overall satisfaction. Results of this study show no significant differences between the C-CLAD device and the traditional syringe. However, two subsequent studies published by three of Asarch’s coauthors116,129 found that Asarch’s study design and methods were suspect and called into question whether injection methods and failure to identify specific injection sites were shortcomings in that study. Subsequently, two published studies were consistent with the findings of other research groups presenting on this topic (i.e., a reduction in pain-disruptive behavior and the ability to reduce fear during a dental injection).116,129
Consistent findings in the dental literature support the use of a C-CLAD device, reporting a significant reduction in general pain perception; a measurable reduction in disruptive pain behavior; and a substantial reduction in the need for restraint in the management of pediatric patients requiring dental local anesthesia.
Most recently, Ferrari and coworkers published data from 60 adult patients receiving a PDL injection and compared the STA-System device versus two other manual syringes: a high-pressure PDL syringe (Ligmaject, IMA Associates, Bloomington, Ind) and a traditional dental syringe.130 EPT was performed on all tested teeth at regular intervals to determine success or failure, and the different instruments and techniques used were compared. In addition to pulp testing, patient subjective pain responses were recorded after treatment. Ferrari reported that the STA-System had 100% success in achieving pulpal anesthesia; in addition, rapid onset of anesthesia was observed. The PDL injection was used as the primary injection for restorative dental care in mandibular teeth in this study. All patients receiving the PDL injection with the STA device reported subjective pain responses of “minimal or no pain.” In contrast, those receiving injections with one of the other two instruments (high-pressure mechanical syringe or conventional syringe) were found to have generally higher pain scores throughout the testing period and required repeated attempts to achieve a successful outcome. Researchers concluded that the STA-System device resulted in more predictable, more reliable, and more comfortable anesthesia than the high-pressure mechanical syringe and/or the conventional dental syringe.130
The STA-System device and its predecessors—The Wand, CompuDent, and the Midwest Comfort Control Syringe—(Dentsply Professional 901 West Oakton Street, Des Plaines, IL 60018) represent a material improvement over the traditional (antiquated) 150-year-old hand-driven manual dental syringe. C-CLAD devices have been shown to enable the dental practitioner to administer a more comfortable and less anxiety-provoking injection. The STA-System instrument adds to those previous advances by introducing dynamic pressure-sensing (DPS) technology that provides continuous real-time injection feedback and identification of specific patient tissues and has been shown to enhance the predictability of the PDL injection130,131 (Fig. 20-16). This is accomplished while adverse tissue reactions are minimized, resulting in a safer, more positive patient and operator experience during administration of dental local anesthetics.123
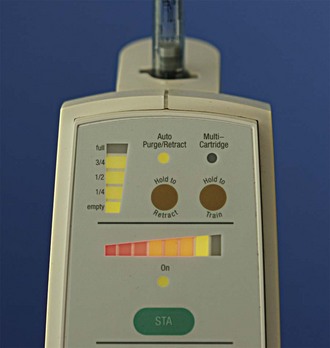
Figure 20-16 Dynamic pressure-sensing (DPS) technology that provides continuous real-time injection feedback and identification of specific patient tissues has been shown to enhance the predictability of the periodontal ligament (PDL) injection. (Photo courtesy Milestone Scientific, Livingstone, NJ.)
Dental local anesthesia delivery has been changed with the introduction of C-CLAD devices. It can be expected that current and future C-CLAD devices will continue to permit clinicians to perform dental injections more successfully and painlessly than in the past. No longer are primary advances in dental local anesthesia concentrated in the areas of pharmacology and pharmacokinetics. Instead, dentistry has entered an era in which advances in local anesthesia are being made by altering the fluid dynamics and physical means by which these drugs are administered to patients. Fifteen years has passed since the first C-CLAD device was introduced into dentistry (The Wand, 1997). It is understood by many in this field that future advances in dental pain control will be derived by improving the drug delivery system, in addition to the drugs used for dental local anesthesia.
Developments Since the 5th Edition: C-CLAD has been a part of this “Future Considerations” chapter ever since the late 1990s. At the present time (January 2012), it is safe to say with confidence that the concept of C-CLAD works (in enabling the LA administrator to provide essentially painless injections to patients). The numbers of dentists and physicians using C-CLAD are growing rapidly.
References
1. Malamed, SF, Future considerations. Handbook of local anesthesia, ed 5. . CV Mosby: St Louis, 2004.
2. Catchlove, RFH. The influence of CO2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972;181:298–309.
3. Condouris, GA, Shakalis, A. Potentiation of the nerve-depressant effect of local anaesthetics by carbon dioxide. Nature. 1964;204:57–58.
4. Metzinger, SE, Rigby, PL, Bailey, DJ, et al. Local anesthesia in blepharoplasty: a new look? South Med J. 1994;87:225–227.
5. Metzinger, SE, Bailey, DJ, Boyce, RG, et al. Local anesthesia in rhinoplasty: a new twist? Ear Nose Throat J. 1992;71:405–406.
6. Stewart, JH, Chinn, SE, Cole, GW, et al. Neutralized lidocaine with epinephrine for local anesthesia, part II. J Derm Surg Oncol. 1990;16:842–845.
7. Whitcomb, M, Drum, M, Reader, A, et al. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1:100,000 epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2010;57:59–66.
8. Malamed, SF, Hersh, E, Poorsattar, S, Falkel, M. Reduction of local anesthetic injection pain using an automated dental anesthetic cartridge buffering system: A randomized, double-blind, crossover study. J Amer Dent Assoc October. 2011.
9. Courtiss, EH, Ransil, BJ, Russo, J. The effects of hyaluronidase on local anesthesia: a prospective, randomized, controlled, double-blind study. Plast Reconstr Surg. 1995;95:876–883.
10. Malamed, SF, Yagiela, JA. Pain control in dentistry. ADA News. 2007. [(supplement) September 15].
11. American Dental Association. 2006 survey of dental practice—characteristics of dentists in private practice and their patients. Chicago: American Dental Association; 2007.
12. Malamed, SF. The periodontal ligament (PDL) injection: an alternative to inferior alveolar nerve block. Oral Surg. 1982;53:117–121.
13. Kleber, CH. Intraosseous anesthesia: implications, instrumentation and techniques. J Am Dent Assoc. 2003;134:487–491.
14. Yagiela, JA. Local anesthetics. In: Yagiela JA, Dowd FJ, Neidle EA, eds. Pharmacology and therapeutics for dentistry. ed 5. St Louis: CV Mosby; 2004:251–270.
15. Hersh, EV, Hermann, DG, Lamp, CJ, et al. Assessing the duration of mandibular soft tissue anesthesia. J Am Dent Assoc. 1995;126:1531–1536.
16. Rafique, S, Fiske, J, Banerjee, A. Clinical trial of an air-abrasion/chemomechanical operative procedure for restorative treatment of dental patients. Caries Res. 2003;37:360–364.
17. College, C, Feigal, R, Wandera, A, et al. Bilateral versus unilateral mandibular block anesthesia in a pediatric population. Pediatr Dent. 2000;22:453–457.
18. Tavares, M, Goodson, JM, Studen-Pavlovich, D, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.
19. Smith, MJ, Hutchins, RC, Hehenberger, D. Transcutaneous neural stimulation use in postoperative knee rehabilitation. Am J Sports Med. 1983;11:75–82.
20. Tuncel, M, Ram, VC. Hypertensive emergencies: etiology and management. Am J Cardiovasc Drugs. 2003;3:21–31.
21. Rhoney, D, Peacock, WF. Intravenous therapy for hypertensive emergencies, part 2. Am J Health-Syst Pharm. 2009;66:1448–1457.
22. Phentolamine, MD. Consult. St Louis: CV Mosby; 2010. [September 3 accessed June 26, 2011].
23. Simons, FE, Lieberman, RL, Read, EJ, Jr., et al. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102:282–287.
24. Zentgraf, M, Ludwig, G, Ziegler, M. How safe is the treatment of impotence with intracavernous autoinjection? Eur Urol. 1989;16:165–171.
25. U.S. Food and Drug Administration (FDA): New dental anesthetic reversal agent receives FDA approval. May 12, 2008.
26. Hersh, E, Moore, P, Papas, A, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate in adolescents and adults. J Am Dent Assoc. 2008;139:1080–1093.
27. Tavares, M, Goodson, JM, Studen-Pavlovich, D, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.
28. DePippo, KL, Holas, MA, Reding, MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Arch Neurol. 1991;49:1259–1261.
29. Heft, M, Parker, S. An experimental basis for revising the graphic rating scale for pain. Pain. 1984;19:153–161.
30. OraVerse prescribing information. San Diego: Novalar Pharmaceuticals, 2009.
31. Tavares, M, Goodson, JM, Studen-Pavlovich, D, et al. Reversal of soft tissue local anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.
32. Froum, SJ, Froum, SH, Malamed, SF. The use of phentolamine mesylate to evaluate mandibular nerve damage following implant placement. Compendium. 2010;31(520):522–528.
33. De St Georges, J. How dentists are judged by patients. Dent Today. 2004;23(96):98–99.
34. Friedman, MJ, Hochman, MN. The AMSA injection: a new concept for local anesthesia of maxillary teeth using a computer-controlled injection system. Quintessence Int. 1998;29:297–303.
35. Oulis, CJ, Vadiakis, GP, Vasilopoulou, A. The effectiveness of mandibular infiltration compared to mandibular block anesthesia in treating primary molars in children. Pediatr Dent. 1996;18:301–305.
36. Sharaf, AA. Evaluation of mandibular infiltration versus block anesthesia in pediatric dentistry. J Dent Child. 1997;64:276–281.
37. Malamed, SF. Local anesthetic considerations in dental specialties. In Malamed SF, ed.: Handbook of local anesthesia, ed 5, St Louis: CV Mosby, 2004.
38. Bennett, CR. Techniques of regional anesthesia and analgesia. In Bennett CR, ed.: Monheim’s local anesthesia and pain control in dental practice, ed 7, St Louis: CV Mosby, 1984.
39. Evers, H, Haegerstam, G. Anaesthesia of the lower jaw. In: Evers H, Haegerstam G, eds. Introduction to dental local anaesthesia, Fribourg. Switzerland: Mediglobe SA, 1990.
40. Trieger, N, New approaches to local anesthesia. Pain control, ed 2. . CV Mosby: St Louis, 1994.
41. Kanaa, MD, Whitworth, JM, Corbett, IP, et al. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42:238–246.
42. Hannan, L, Reader, A, Nist, R, et al. The use of ultrasound for guiding needle placement for inferior alveolar nerve blocks. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:658–665.
43. Berns, JM, Sadove, MS. Mandibular block injection: a method of study using an injected radiopaque material. J Am Dent Assoc. 1962;65:736–745.
44. Galbreath, JC. Tracing the course of the mandibular block injection. Oral Surg Oral Med Oral Pathol. 1970;30:571–582.
45. Reader A, American Association of Endodontists. Taking the pain out of restorative dentistry and endodontics: current thoughts and treatment options to help patients achieve profound anesthesia, Endodontics: Colleagues for Excellence. Winter. 2009.
46. DeJong, RH. Local anesthetics. St Louis: CV Mosby; 1994. [pp 110–111].
47. Strichartz, G. Molecular mechanisms of nerve block by local anesthetics. Anesthesiology. 1976;45:421–444.
48. Gow-Gates, GA. Mandibular conduction anesthesia: a new technique using extraoral landmarks. Oral Surg Oral Med Oral Pathol. 1973;36:321–328.
49. Akinosi, JO. A new approach to the mandibular nerve block. Br J Oral Surg. 1977;15:83–87.
50. Malamed, SF. The periodontal ligament (PDL) injection—an alternative to inferior alveolar nerve block. Oral Surg. 1982;53:117–121.
51. Coggins, R, Reader, A, Nist, R, et al. Anesthetic efficacy of the intraosseous injection in maxillary and mandibular teeth. Oral Surg Oral Med Oral Pathol. 1996;81:634–641.
52. Hanna, MN, Elhassan, A, Veloso, PM, et al. Efficacy of bicarbonate in decreasing pain on intradermal injection of local anesthetics: a meta analysis. Reg Anesth Pain Med. 2009;34:122–125.
53. Meechan, JG. Infiltration anesthesia in the mandible. Dent Clin N Am. 2010;54:621–629.
54. Rood, JP. The analgesia and innervation of mandibular teeth. Br Dent J. 1976;140:237–239.
55. Yonchak, T, Reader, A, Beck, M, et al. Anesthetic efficacy of infiltrations in mandibular anterior teeth. Anesth Prog. 2001;48:55–60.
56. Meechan, JG, Ledvinka, JI. Pulpal anaesthesia for mandibular central incisor teeth: a comparison of infiltration and intraligamentary injections. Int Endod J. 2002;35:629–634.
57. Haas, DA, Harper, DG, Saso, MA, et al. Comparison of articaine and prilocaine anesthesia by infiltration in maxillary and mandibular arches. Anesth Prog. 1990;37:230–237.
58. Haas, DA, Harper, DG, Saso, MA, et al. Lack of differential effect by Ultracaine (articaine) and Citanest (prilocaine) in infiltration anaesthesia. J Can Dent Assoc. 1991;57:217–223.
59. Kanaa, MD, Whitworth, JM, Corbett, IP, et al. Articaine and lidocaine mandibular buccal infiltration anesthesia: a prospective randomized double-blind cross-over study. J Endod. 2006;32:296–298.
60. Robertson, D, Nusstein, J, Reader, A, et al. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138:1104–1112.
61. Haase, A, Reader, A, Nusstein, J, et al. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Am Dent Assoc. 2008;139:1228–1235.
62. Potocnik, I, Tomsic, M, Sketelj, J, et al. Articaine is more effective than lidocaine or mepivacaine in rat sensory nerve conduction block in vitro. J Dent Res. 2006;85:162–166.
63. Vreeland, DL, Reader, A, Beck, M, et al. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod. 1989;15:6–12.
64. Hinkley, SA, Reader, A, Beck, M, et al. An evaluation of 4 percent prilocaine with 1:200,000 epinephrine and 2% mepivacaine with 1:20,000 levonordefrin compared with 2% lidocaine with 1:100,000 epinephrine for inferior alveolar nerve block. Anesth Prog. 1991;38:84–89.
65. Chaney, MA, Kerby, R, Reader, A, et al. An evaluation of lidocaine hydrocarbonate compared with lidocaine hydrochloride for inferior alveolar nerve block. Anesth Prog. 1991;38:212–216.
66. McClean, C, Reader, A, Beck, M, et al. An evaluation of 4% prilocaine and 3% mepivacaine compared with 2% lidocaine (1:100,000 epinephrine) for inferior alveolar nerve block. J Endod. 1993;19:146–150.
67. Ridenour, S, Reader, A, Beck, M, et al. Anesthetic efficacy of a combination of hyaluronidase and lidocaine with epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2001;48:9–15.
68. Steinkruger, G, Nusstein, J, Reader, A, et al. The significance of needle bevel orientation in achieving a successful inferior alveolar nerve block. J Am Dent Assoc. 2006;137:1685–1691.
69. Jaber, A, Whitworth, JM, Corbett, IP, et al. The efficacy of infiltration anesthesia for adult mandibular incisors: a randomized double-blind cross-over trial comparing articaine and lidocaine buccal and buccal plus lingual infiltrations. Br Dent J. 2010;209:E16.
70. Dreven, LJ, Reader, A, Beck, M, et al. An evaluation of the electric pulp tester as a measure of analgesia in human vital teeth. J Endod. 1987;13:233–238.
71. Certosimo, AJ, Archer, RD. A clinical evaluation of the electric pulp tester as an indicator of local anesthesia. Oper Dent. 1996;21:25–30.
72. Lahat, E, Goldman, M, Barr, J, et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321:83–86.
73. Owen, R, Castle, N. Intranasal midazolam. Emerg Med J. 2009;26:217–218.
74. Holsti, M, Sill, BL, Firth, SD, et al. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23:148–153.
75. Fukota, O, Braham, RL, Yanase, H, et al. The sedative effect of intranasal midazolam administration in the dental treatment of patients with mental disabilities. Part 1. The effect of a 0.2 mg/kg dose. J Clin Pediatr Dent. 1993;17:231–237.
76. Fuks, AB, Kaufman, E, Ram, D, et al. Assessment of two doses of intranasal midazolam for sedation of young pediatric dental patients. Pediatr Dent. 1994;16:301–305.
77. Dallman, JA, Ignelzi, MA, Jr., Briskie, DM. Comparing the safety, efficacy and recovery of intranasal midazolam vs. oral chloral hydrate and promethazine. Pediatr Dent. 2001;23:424–430.
78. Lam, C, Udin, RD, Malamed, SF, et al. Midazolam premedication in children: a pilot study comparing intramuscular and intranasal administration. Anesth Prog. 2005;52:56–61.
79. Chadha, NK, Repanos, C, Carswell, AJ. Local anaesthesia for manipulation of nasal fractures: systematic review. J Laryngol Otol. 2009;123:830–836.
80. Jones, TM, Nandapalan, V. Manipulation of the fractured nose: a comparison of local infiltration anaesthesia and topical local anaesthesia. Clin Otolaryngol Allied Sci. 1999;24:443–446.
81. Ciancio S, Ayoub F, Pantera E, et al: Nasal spray for anesthesia of maxillary teeth. Poster presentation at International Association for Dental Research (IADR) General Session, July 14–17, 2010, Barcelona, Spain.
82. Liljestrand, G. The historical development of local anesthesia, vol I, International encyclopedia of pharmacology and therapeutics: local anesthetics. New York: Pergamon Press; 1965. [pp 546–549].
83. Hoffmann-Axtheim, W. History of dentistry. Chicago: Quintessence; 1981.
84. Dobbs, EC. A chronological history of local anesthesia in dentistry. J Oral Ther Pharmacol. 1965;1:546–549.
85. Milgrom, P, Weinstein, B, Kleinknect, R. Treating fearful dental patients. Reston, Va: Reston Publishing; 1985.
86. Naini, FB, Mellow, AC, Getz, T. Treatment of dental fears: pharmacology or psychology. Dental Update. 1999;26:270–276.
87. Dionne, R, Phero, J, Becker, D. Management of pain and anxiety in the dental office. Philadelphia: Saunders; 1985.
88. Agras, S, Sylvester, D, Oliveau, D. The epidemiology of common fears and phobia. Compr Psychiatry. 1979;10:151–156.
89. Kleinknecht, RA, McGlynn, FD, Thorndike, RM, et al. Factor analysis of the dental fear survey with cross validation. J Am Dent Assoc. 1984;108:59–61.
90. Hochman, MN, Chiarello, D, Hochman, CB, et al. Computerized local anesthetic delivery vs. traditional syringe technique: subjective pain response. N Y Dent J. 1997;63:24–29.
91. Clinical Research Associates. Local anesthesia, automated delivery. Clin Res Associates Newsltr. 1999;22:1–2.
92. Friedman, MJ, Hochman, MN. 21st century computerized injection for local pain control. Compend Contin Educ Dent. 1997;18:995–1003.
93. Murphy, D. Ergonomics and the dental care worker. Washington, DC: American Public Health Association; 1998.
94. Yesilyurt, C, Bulut, G, Tasdemir, T. Pain perception during inferior alveolar injection administered with the Wand or conventional syringe. Br Dent J. 2008;205:E10. [discussion 258–259].
95. Krochak, M, Friedman, N. Using a precision-metered injection system to minimize dental injection anxiety. Compend Contin Educ Dent. 1998;19:137–148.
96. Friedman, MJ, Hochman, MN. The AMSA injection: a new concept for local anesthesia of maxillary teeth using a computer-controlled injection system. Quintessence Int. 1998;29:297–303.
97. Friedman, MJ, Hochman, MN. P-ASA block injection: a new palatal technique to anesthetize maxillary anterior teeth. J Esthet Dent. 1999;11:63–71.
98. Hochman, MN, Friedman, MJ. In vitro study of needle deflection: a linear insertion technique versus a bidirectional rotation insertion technique. Quintessence Int. 2000;31:33–39.
99. Aboushala A, Kugel G, Efthimiadis N, et al: Efficacy of a computer-controlled injection system of local anesthesia in vivo, IADR Abstract, 2000. Abstract #2775.
100. Hochman MN, inventor: Pressure/force computer controlled drug delivery system and the like, 2001, U.S. Patent #6,200,289.
101. Hochman MN, inventor: Computer controlled drug delivery system with dynamic pressure sensing, 2006, U.S. Patent #7,618,409.
102. Ghelber, O, Gebhard, R, Vora, S, et al. Utilization of the CompuFlo in determining the pressure of the epidural space: a pilot study. Anesth Analg. 2005;100:S–189.
103. Ghelber, O, Gebhard, R, Szmuk, P, et al. Identification of the epidural space—a pilot study of a new technique. Anesth Analg. 2005;100:S–255.
104. Ghelber, O, Gebhard, RE, Vora, S, et al. Identification of the epidural space using pressure measurement with the CompuFlo injection pump—a pilot study. Reg Anesth Pain Med. 2008;33:346–352.
105. Kaufman, E, Weinstein, P, Milgrom, P. Difficulties in achieving local anesthesia. J Am Dent Assoc. 1984;108:205–208.
106. Roda, RS, Blanton, PL. The anatomy of local anesthesia. Quintessence Int. 1994;25:27–38.
107. Meechan, JG. Intraligamentary anaesthesia. J Dent. 1992;20:325–332.
108. Hochman, MN. Single-tooth anesthesia: pressure sensing technology provides innovative advancement in the field of dental local anesthesia. Compendium. 2007;28:186–193.
109. Hochman MN, inventor: Drug infusion device with tissue identification using pressure sensing. 2008, U.S. Patent #7,449,008.
110. Nicholson, JW, Berry, TG, Summitt, JB, et al. Pain perception and utility: a comparison of the syringe and computerized local injection techniques. Gen Dent. 2001;49:167–173.
111. Fukayama, H. Research for improving local anesthesia method in dentistry. Kokubyo Gakkai Zasshi. 2010;77:169–175.
112. Rosenberg, E. A computer-controlled anesthetic delivery system in a periodontal practice: patient satisfaction and acceptance. J Esthet Restor Dent. 2001;13:25–32.
113. Kudo, M, Ohke, H, Katagiri, K, et al. The shape of local anesthetic injection syringes with less discomfort and anxiety: evaluation of discomfort and anxiety caused by various types of local anesthetic injection syringes in high level trait-anxiety people. J Japan Dent Soc Anesthesiol. 2001;29:173–178.
114. Asarch, T, Allen, K, Petersen, B, et al. Efficacy of a computerized local anesthesia device in pediatric dentistry. Pediatr Dent. 1999;21:421–424.
115. Gibson, RS, Allen, K, Hutfless, S, Beiraghi, S. The Wand vs. traditional injection: a comparison of pain related behaviors. Pediatr Dent. 2000;22:458–462.
116. Allen, KD, Kotil, D, Larzelere, RE, et al. Comparison of a computerized anesthesia device with a traditional syringe in preschool children. Pediatr Dent. 2002;24:315–320.
117. Palm, AM, Kirkegaard, U, Paulsen, S. The Wand versus traditional injection for mandibular nerve block in children and adolescents: perceived pain and time of onset. Pediatr Dent. 2004;26:481–484.
118. Oztas, N, Ulusu, T, Bodur, H, et al. The Wand in pulp therapy: an alternative to inferior alveolar nerve block. Quintessence Int. 2005;36:559–564.
119. Versloot, J, Veerkamp, JSJ, Hoogstraten, J. Computerized anesthesia delivery system vs. traditional syringe: comparing pain and pain-related behavior in children. Eur J Oral Sci. 2005;113:448–493.
120. Jalevik B, Klingberg G: Sensation of pain when using computerized injection technique, the Wand, IADR Pan Federation, September 13–16, 2006. Dublin, Ireland. Abstract # 0070.
121. Ram, D, Kassirer, J. Assessment of a palatal approach-anterior superior alveolar (P-ASA) nerve block with the Wand in paediatric dental patients. Intern J Paediatr Dent. 2006;16:348–351.
122. Ashkenazi, M, Blumer, S, Eli, I. Effective of computerized delivery of intrasulcular anesthetic in primary molars. J Am Dent Assoc. 2005;136:1418–1425.
123. Ashkenazi, M, Blumer, S, Eli, I. Effect of computerized delivery intraligamental injection in primary molars on their corresponding permanent tooth buds. Intern J Paediatr Dent. 2010;20:270–275.
124. Brannstrom, M, Nordenvall, KJ, Hedstrom, KG. Periodontal tissue changes after intraligamentary anesthesia. J Dent Child. 1982;49:417–423.
125. Versloot, J, Veerkamp, JSJ, Hoogstraten, J. Computerized anesthesia delivery system vs. traditional syringe: comparing pain and pain-related behavior in children. Eur J Oral Sci. 2005;113:448–493.
126. Ram, D, Kassirer, J. Assessment of a palatal approach-anterior superior alveolar (P-ASA) nerve block with the Wand in paediatric dental patients. Intern J Paediatr Dent. 2006;16:348–351.
127. Palm, AM, Kirkegaard, U, Paulsen, S. The Wand versus traditional injection for mandibular nerve block in children and adolescents: perceived pain and time of onset. Pediatr Dent. 2004;26:481–484.
128. Oztas, N, Ulusu, T, Bodur, H, et al. The Wand in pulp therapy: an alternative to inferior alveolar nerve block. Quintessence Int. 2005;36:559–564.
129. Gibson, RS, Allen, K, Hutfless, S, et al. The Wand vs. traditional injection: a comparison of pain related behaviors. Pediatr Dent. 2000;22:458–462.
130. Ferrari, M, Cagidiaco, MC, Vichi, A, et al. Efficacy of the computer-controlled injection system STA, the Ligamaject, and the dental syringe for intraligamentary anesthesia in restorative patients. Intern Dent SA. 2010;11:4–12.
131. Hochman, MN, Friedman, MF, Williams, WP, et al. Interstitial pressure associated with dental injections: a clinical study. Quintessence Int. 2006;37:469–476.