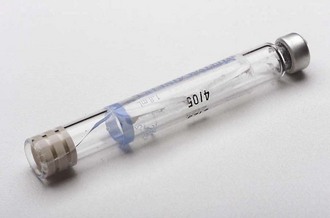
The Cartridge
The dental cartridge is a glass cylinder containing the local anesthetic drug, among other ingredients. In the United States and in many other countries, the glass cylinder itself can hold 2 mL of solution; however, as prepared today, the dental cartridge contains approximately 1.8 mL of local anesthetic solution. Local anesthetic products manufactured by Septodont (Lancaster, PA) list their volume as 1.7 mL (although in actuality they contain approximately 1.76 mL of local anesthetic solution). In other countries, notably the United Kingdom and Australia, the prefilled dental cartridge contains approximately 2.2 mL of local anesthetic solution; some countries including France and Japan have 1-mL dental cartridges (Fig. 7-1).
The dental cartridge is, by common usage, referred to by dental professionals as a carpule. The term carpule is actually a registered trade name for the dental cartridge prepared by Cook-Waite Laboratories, which introduced it into dentistry in 1920.
In recent years, local anesthetic manufacturers in some countries (but not as of yet in North America) have introduced a local anesthetic cartridge composed of plastic.1 Plastic cartridges have several negative features, primarily leakage of solution during injection, the requirement for considerable force to be applied to the plunger of the syringe (e.g., periodontal ligament [PDL], nasopalatine),1 and the fact that the plunger does not “glide” down the plastic cartridge as smoothly as it does down the glass cartridge, leading to sudden spurts of administration of local anesthetic, which can produce pain in the patient. Another problem with plastic cartridges is the fact that they are permeable to air. Exposure to oxygen leads to more rapid degradation of the vasoconstrictor in the cartridge and to a shorter shelf-life.2
Components
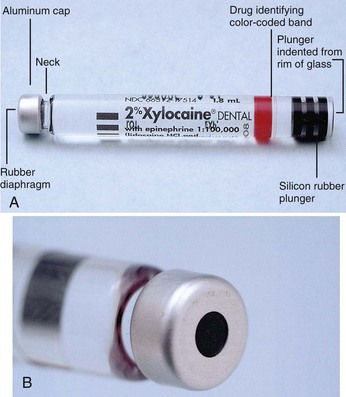
The prefilled 1.8-mL dental cartridge consists of four parts (Fig. 7-2):
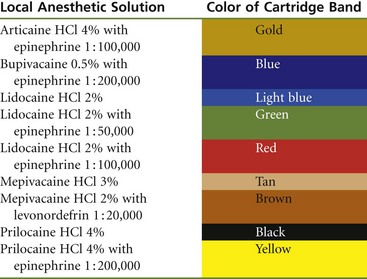
The stopper (plunger, bung) is located at the end of the cartridge that receives the harpoon of the aspirating syringe. The harpoon is embedded into the silicone (non–latex-containing) rubber plunger with gentle finger pressure applied to the thumb ring of the syringe. The plunger occupies a little less than 0.2 mL of the volume of the entire cartridge. Until recently, the stopper was sealed with paraffin (wax) to produce an airtight seal against the glass walls of the cartridge. Glycerin was added in channels around the stopper as a lubricant, permitting it to traverse the glass cylinder more easily. Today, most local anesthetic manufacturers treat the stopper with silicone, eliminating both the paraffin and the glycerin. “Sticky stoppers” (stoppers that do not move smoothly down the glass cartridge) are infrequent today. Recent years have seen a move toward the use of a uniform black rubber stopper in all local anesthetic drug combinations. Virtually gone are the color-coded red, green, and blue stoppers that aided in identification of the drug. When black stoppers are used, a color-coding band, required by the American Dental Association (ADA) as of June 2003 for products to receive the ADA Seal of Approval, is found around the glass cartridge (Table 7-1).
TABLE 7-1
Color-Coding of Local Anesthetic Cartridges, as per American Dental Association Council on Scientific Affairs
In an intact dental cartridge (Fig. 7-3), the stopper is slightly indented from the lip of the glass cylinder. Cartridges whose plungers are flush with or extruded beyond the glass of the cylinder should not be used. This problem is discussed later in this chapter (see “Problems”).
An aluminum cap is located at the opposite end of the cartridge from the rubber plunger. It fits snugly around the neck of the glass cartridge, holding the thin diaphragm in position. It is silver colored on most cartridges.
The diaphragm is a semipermeable membrane through which the needle penetrates into the cartridge. When properly prepared, the perforation of the needle is centrically located and round, forming a tight seal around the needle. Improper preparation of the needle and cartridge can produce an eccentric puncture with ovoid holes leading to leakage of the anesthetic solution during injection. The diaphragm is a semipermeable membrane that allows any solution in which the dental cartridge is immersed to diffuse into the cartridge, thereby contaminating the local anesthetic solution.
Persons with latex allergy may be at increased risk when administered a local anesthetic through a glass cartridge.3 However, a recent literature review by Shojaei and Haas stated that although the possibility of an allergic reaction precipitated by latex in the dental local anesthetic cartridge does exist, “there are no reports of studies or cases in which a documented allergy was due to the latex component of cartridges for dental anesthesia.”4
In recent years, latex-free dental cartridges have been introduced.
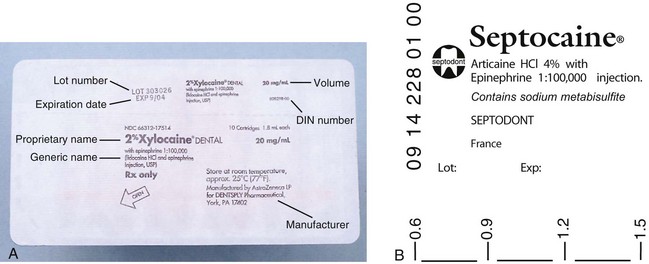
A thin Mylar plastic label applied to all cartridges (Fig. 7-4) serves to (1) protect the patient and the administrator in the event that the glass cracks, and (2) provide specifications about the enclosed drug. In addition, some manufacturers include a volume indicator on their labels, making it easier for the administrator to deposit precise volumes of anesthetic (see Fig. 7-4).
Cartridge Contents
The composition of the solution found in the dental cartridge varies depending on whether a vasopressor is included (Table 7-2).
TABLE 7-2
Composition of Local Anesthetic Solution
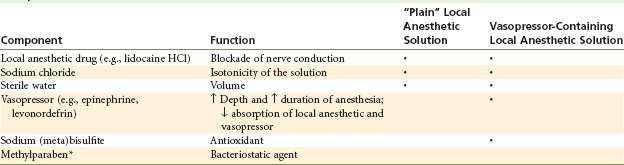
*Methylparaben is no longer included in single-use dental cartridges of local anesthetic; however, it is found in ALL multidose vials of injectable drugs.
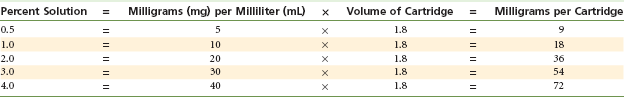
The local anesthetic drug is the raison d’être for the entire dental cartridge. It interrupts the propagated nerve impulse, preventing it from reaching the brain. The drug contained within the cartridge is listed by its percent concentration. The number of milligrams of the local anesthetic drug can be calculated by multiplying the percent concentration (e.g., 2% = 20 mg/mL) by the volume: 1.8 (United States) or 2.2 (United Kingdom) (the number of milliliters in the cartridge). Thus a 1.8-mL cartridge of a 2% solution contains 36 mg (Table 7-3). The local anesthetic drug is stable and is capable of being autoclaved, heated, or boiled without breaking down. However, other components of the cartridge (e.g., vasopressor drug, cartridge seals) are more labile and are easily destroyed.
A vasopressor drug is included in most anesthetic cartridges to enhance safety and the duration and depth of action of the local anesthetic. The pH of dental cartridges containing vasopressors is lower (more acidic) than that of cartridges not containing vasopressors (pH of 3.5 [3.3 to 4.0] vs. 6.5). Because of this pH difference, plain local anesthetics have a somewhat more rapid onset of clinical action and are more comfortable (less “burning” on injection).5-7
Cartridges containing vasopressors also contain an antioxidant, most often sodium (meta)bisulfite. Sodium bisulfite prevents oxidation of the vasopressor by oxygen, which can be trapped in the cartridge during manufacture or can diffuse through the semipermeable diaphragm (or the walls of a plastic cartridge) after filling. Sodium bisulfite reacts with oxygen before the oxygen is able to destroy the vasopressor. When oxidized, sodium bisulfite becomes sodium bisulfate, having an even lower pH. The clinical relevance of this lies in the fact that increased burning (discomfort) is experienced by the patient on injection of an “older” cartridge of anesthetic with vasopressor compared with a fresher cartridge. Allergy to bisulfites must be considered in the medical evaluation of all patients before local anesthetic is administered8,9 (see Chapter 10).
Sodium chloride is added to the cartridge to make the solution isotonic with the tissues of the body. In the past, isolated instances have been reported in which local anesthetic solutions containing too much sodium chloride (hypertonic solutions) produced tissue edema or paresthesia, sometimes lasting for several months, after drug administration.10 This is no longer a problem.
Distilled water is used as a diluent to provide the volume of solution in the cartridge.
A significant change in cartridge composition in the United States and in many other countries was the removal of methylparaben, a bacteriostatic agent. A ruling by the Food and Drug Administration (FDA) mandated the removal of methylparaben from dental local anesthetic cartridges manufactured after January 1, 1984. Methylparaben possesses bacteriostatic, fungistatic, and antioxidant properties. It and related compounds (ethyl-, propyl-, and butylparaben) are commonly used as preservatives in ointments, creams, lotions, and dentifrices. In addition, paraben preservatives are found in all multiple-dose vials of drugs. Methylparaben is commonly used in a 0.1% concentration (1 mg/mL). Its removal from local anesthetic cartridges was predicated on two facts. First, dental local anesthetic cartridges are single-use items meant to be discarded and not reused. Therefore inclusion of a bacteriostatic agent is unwarranted. Second, repeated exposure to paraben has led to reports of increased allergic reactions in some persons.11,12 Responses have been limited to localized edema, pruritus, and urticaria. Fortunately, to date there has not been a systemic allergic reaction to a paraben. Removal of methylparaben has further decreased an already minimal risk of allergy to local anesthetic drugs.
Care And Handling
Local anesthetics are marketed in vacuum-sealed tin containers of 50 cartridges and in blister packs of (usually) 10 cartridges. Although no manufacturer makes any claim of sterility about the exterior surface of the cartridge, bacterial cultures taken immediately on opening a container usually fail to produce any growth. Therefore it seems obvious that extraordinary measures related to cartridge sterilization are unwarranted. Indeed, the glass dental cartridge should not be autoclaved. The seals on the cartridge cannot withstand the extreme temperatures of autoclaving, and the heat-labile vasopressors are destroyed in the process. Plastic cartridges cannot be autoclaved.
Most commonly today, local anesthetics are marketed in cardboard boxes of approximately 50 cartridges. Within the box are 5 sealed units of 10 cartridges each (Fig. 7-5), called blister packs. Kept in this container until use, cartridges remain clean and uncontaminated.
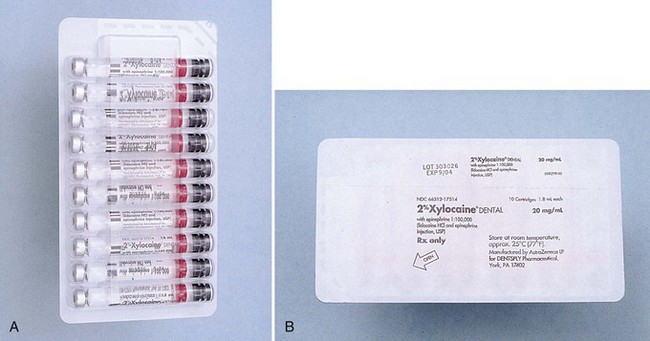
Figure 7-5 A, Ten local anesthetic cartridges are contained in a sealed “blister pack.” B, Back of blister pack contains information about the drug.
Local anesthetic cartridges should be stored in their original container, preferably at room temperature (e.g., 21° C to 22° C), and in a dark place. There is no need to “prepare” a cartridge before use. The doctor or assistant should insert it into the syringe. However, many doctors feel compelled to somehow “sterilize” the cartridge. When this urge strikes, the doctor should apply an alcohol wipe moistened with undiluted 91% isopropyl alcohol or 70% ethyl alcohol to the rubber diaphragm (Fig. 7-6).
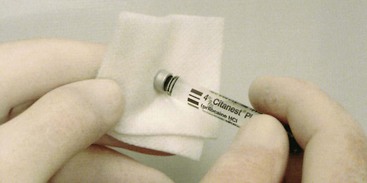
Figure 7-6 Preparing local anesthetic cartridge for use by wiping the rubber diaphragm with alcohol.
If a clear plastic cartridge dispenser is used, 1 day’s supply of cartridges should be placed with the aluminum cap and diaphragm facing downward. Several (two or three) sterile dry 2 × 2-inch gauze wipes are placed in the center of the dispenser and are moistened with (not immersed in) 91% isopropyl alcohol or 70% ethyl alcohol. No liquid alcohol should be present around the cartridges. Before the syringe is loaded, the aluminum cap and the rubber diaphragm are rubbed against the moistened gauze.
Cartridges should not be permitted to soak in alcohol or other sterilizing solutions because the semipermeable diaphragm allows diffusion of these solutions into the dental cartridge, thereby contaminating it. Therefore, it is recommended that cartridges be kept in their original container until they are to be used.
Cartridge warmers are unnecessary. Indeed, occasionally they may produce problems. Overheating the local anesthetic solution can lead to (1) discomfort for the patient during injection, and (2) the more rapid degradation of a heat-labile vasopressor (producing a shorter duration of anesthesia with more burning on injection). It has been demonstrated that after the warmed glass cartridge is removed from the cartridge warmer and is placed in a metal syringe with the solution forced through a fine metal needle, its temperature has decreased almost to room temperature.2,5,13,14
Cartridge warmers, designed to maintain anesthetic solutions at “body temperature,” are not needed and cannot be recommended. Local anesthetics in cartridges maintained at room temperature (20° C to 22° C) do not cause the patient any discomfort on injection into tissues, nor do patients complain of the solution being too cold.14 On the other hand, warmed local anesthetic solutions at 27° C or above have a much greater incidence of being described as too hot or burning on injection.13
Local anesthetic cartridges should not be left exposed to direct sunlight because some contents may undergo accelerated deterioration. The primary clinical effect of this will be destruction of the vasopressor, with a corresponding decrease in the duration of clinical action of the anesthetic solution.
Included in every package of local anesthetic is an important document: the drug package insert. It contains valuable information about the product such as dosages, warnings, precautions, and care and handling instructions. All persons involved in the handling or administration of local anesthetics should review this document periodically (Fig. 7-7).
Problems
Occasionally problems develop with dental cartridges of local anesthetics. Although most are minor, producing slight inconvenience to the drug administrator, others are more significant and might prove harmful to the patient:
Bubble in the Cartridge
A small bubble of approximately 1 to 2 mm diameter (described as “BB”-sized) frequently is found in the dental cartridge. It is composed of nitrogen gas, which was bubbled into the local anesthetic solution during its manufacture to prevent oxygen from being trapped inside the cartridge, potentially destroying the vasopressor. The nitrogen bubble may not always be visible in a normal cartridge (Fig. 7-8, A).
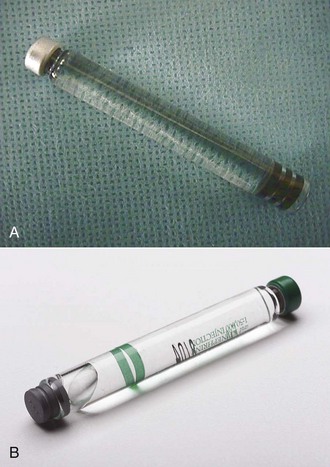
Figure 7-8 A, Normal cartridge with no bubble or a small BB-sized bubble. Note that the rubber stopper is indented from the glass rim. B, Local anesthetic cartridge with an extruded stopper and a large bubble caused by freezing.
A larger bubble, which may be present with a plunger that is extruded beyond the rim of the cartridge, is the result of freezing of the anesthetic solution (Fig. 7-8, B). Such cartridges should not be used, because sterility of the solution cannot be assured. Instead, these cartridges should be returned to their manufacturer for replacement.
Extruded Stopper
The stopper can become extruded when a cartridge is frozen and the liquid inside expands. In this case, the solution no longer can be considered sterile and should not be used for injection. Frozen cartridges can be identified by the presence of a large (>2 mm) bubble, along with an extruded stopper.
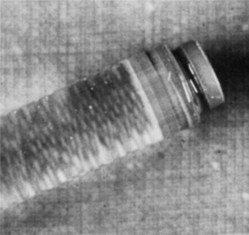
An extruded stopper with no bubble is indicative of prolonged storage in a chemical disinfecting solution and diffusion of the solution into the cartridge. Shannon and Wescott demonstrated that alcohol enters a cartridge through the diaphragm in measurable amounts within 1 day if the diaphragm is immersed in alcohol.15 Local anesthetic solutions containing alcohol produce an uncomfortable burning on injection. Alcohol in sufficiently high concentration is a neurolytic agent capable of producing long-term paresthesia. The greatest concentration of alcohol reported to date in a dental cartridge has been 8% (Fig. 7-9), which is not likely to produce significant long-term injury.16
Antirust tablets should not be used in disinfectant solutions. The sodium nitrate (or similar agent) that they contain is capable of releasing metal ions, which have been related to an increased incidence of edema after local anesthetic administration.17
It should be remembered that small quantities of sterilizing solution can diffuse into a dental cartridge with no visible movement of the plunger. Care always must be taken in storage of local anesthetic cartridges.
Burning on Injection
A burning sensation on injection of anesthetic solution may be the result of one of the following:
During the few seconds immediately after deposition of a local anesthetic solution, the patient may complain of a slight sensation of burning. This normal reaction is caused by the pH of the local anesthetic solution; it lasts a second or two, until the anesthetic takes effect, and is noted mainly by sensitive patients when receiving local anesthetics containing epinephrine or levonordefrin.
A more intense burning on injection is usually the result of diffusion of disinfecting solution into the dental cartridge and its subsequent injection into the oral mucous membranes. Although burning most often is a mere annoyance, the inclusion of disinfecting agents such as alcohol in dental cartridges can lead to more serious sequelae, such as postinjection paresthesia and tissue edema.15,16
Overheating of the solution in a cartridge warmer may produce burning on injection. The (Christmas tree) bulb-type cartridge warmer most often is at fault in this regard. Unless local anesthetic cartridges are unusually cold, there is little justification for use of a cartridge warmer. Local anesthetic solutions injected at room temperature are well tolerated by tissues and patients.
Use of a vasopressor-containing local anesthetic solution may be responsible for the sensation of burning on injection. The addition of a vasopressor and an antioxidant (sodium bisulfite) lowers the pH of the solution to approximately 3.5, making it significantly more acidic than solutions not containing a vasopressor (pH about 6.5).5-7,17 Patients are more likely to feel the burning sensation with these solutions. A further decrease in the pH of the local anesthetic solution results as sodium bisulfite is oxidized to sodium bisulfate. This response can be minimized by careful checking of the expiration date on all cartridges before use. Conversely, increasing the pH of the anesthetic solution has the effect of making local anesthetic administration more comfortable for the patient.17-19
Sticky Stopper
The “sticky stopper” has become a rarity today, with the inclusion of silicone as a lubricant and the removal of paraffin as a sealant in the cartridge. In cases where paraffin is still used, difficulty in advancing the stopper may occur because the paraffin hardens on colder days. Using cartridges at room temperature minimizes this problem; using silicone-coated stoppers eliminates it. Plastic cartridges are associated with this problem to a greater degree than glass cartridges.
Corroded Cap
The aluminum cap on a local anesthetic cartridge can be corroded if immersed in disinfecting solutions that contain quaternary ammonium salts, such as benzalkonium chloride (e.g., “cold” sterilizing solution). These salts are electrolytically incompatible with aluminum. Aluminum-sealed cartridges should be disinfected in 91% isopropyl alcohol or 70% ethyl alcohol. Cartridges with corroded caps must not be used. Corrosion may be easily distinguished from rust, which appears as a red deposit on an intact aluminum cap.
Rust on the Cap
Rust found on a cartridge indicates that at least one cartridge in the tin container has broken or leaked. The “tin” container (actually steel dipped in molten tin) rusts, and the deposit comes off on the cartridges. Cartridges containing rust should not be used. If any cartridge contains rust a dented cap, or a visible crack (Fig. 7-10), all cartridges in the container must be carefully checked before use. With the introduction of nonmetal packaging, rust is rarely seen.
Leakage During Injection
Leakage of local anesthetic solution into the patient’s mouth during injection occurs if the cartridge and the needle are prepared improperly and the needle puncture of the diaphragm is ovoid and eccentric. Properly placed on the syringe after the cartridge is inserted, the needle produces centric perforation of the diaphragm, which tightly seals itself around the needle. When pressure is applied to the plunger during injection, all of the solution is directed into the lumen of the needle. If the cartridge is placed in a breech-loading syringe after the needle, an eccentric ovoid perforation may occur; with pressure on the plunger, some solution is directed into the lumen of the needle, while some may leak out of the cartridge between the needle and the diaphragm and run into the patient’s mouth (see Fig. 5-24). When the safety syringe is used, it is necessary to insert the cartridge after the needle has been attached; however, because the cartridge slides directly into the syringe, not from the side, leakage during injection is rarely a problem. Verbal and written communications from doctors using plastic cartridges indicate that the occurrence of leakage appears to be considerably greater when they are used.
The plastic dental cartridge does not withstand the application of injection pressure as well as the traditional glass cartridge. Meechan and associates applied pressures equal to those achieved during PDL injection with both glass and plastic local anesthetic cartridges.1 Leakage of anesthetic occurred in 1.4% of glass cartridges, whereas leakage was noted in 75.1% of plastic cartridges.
Broken Cartridge
The most common cause of cartridge breakage is the use of a cartridge that has been cracked or chipped during shipping. Dented metal containers and damaged boxes should be returned to the supplier immediately for exchange. If a broken cartridge is found in a container, all remaining cartridges must be examined for hairline cracks or chips. Two areas that must be examined carefully are the thin neck of the cartridge where it joins the cap (see Fig. 7-10) and the glass surrounding the plunger (Fig. 7-11). Subjecting a cracked cartridge to the pressure of injection often causes the cartridge to shatter or “explode.” If this occurs inside the patient’s mouth, serious sequelae may result from ingestion of glass. It is essential to suction the patient’s mouth thoroughly and consult with a physician or emergency department staff about follow-up therapy before discharging this patient. The addition of a thin Mylar plastic label to the glass cartridge minimizes such injury. Additionally, if the aluminum “cap” on the cartridge is damaged, the cartridge should not be used, because the underlying glass may have been damaged as well (see Fig. 7-10).
Plastic cartridges do not fracture when subjected to PDL injection pressures.1
Excessive force used to engage the aspirating harpoon in the stopper has resulted in numerous cases of shattered cartridges. Although they have not broken in the patient’s mouth, injury to dental personnel has been reported. Hitting the thumb ring of the syringe in an attempt to engage the harpoon in the rubber stopper should be avoided. If this technique is essential to embed the harpoon in the rubber plunger (as it is with the plastic safety syringe), one hand should be used to cover the entire exposed glass face of the cartridge (Fig. 7-12). Proper preparation of the armamentarium (see Chapter 9) minimizes this problem.
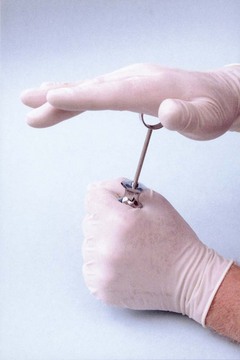
Figure 7-12 If force is necessary to embed the harpoon in the rubber plunger, the glass face of the syringe should be covered with the hand.
Breakage also can occur as a result of attempting to use a cartridge with an extruded plunger. Extruded plungers can be forced back into the cartridge only with difficulty, if at all. Cartridges with extruded plungers should not be used.
Syringes with bent harpoons may cause cartridges to break (see Fig. 5-26). Bent needles that are no longer patent create a pressure buildup within the cartridge during attempted injection (see Fig. 5-25). No attempt should be made to force local anesthetic solution from a dental cartridge against significant resistance.
Recommendations
1. Dental cartridges must never be used on more than one patient.
2. Cartridges should be stored at room temperature.
3. It is not necessary to warm cartridges before use.
4. Cartridges should not be used beyond their expiration date.
5. Cartridges should be checked carefully for cracks, chips, and the integrity of the stopper and cap before use.
References
1. Meechan, JG, McCabe, JF, Carrick, TE. Plastic dental anaesthetic cartridges: a laboratory investigation. Br Dent J. 1990;169:254–256.
2. Meechan, JG, Donaldson, D, Kotlicki, A. The effect of storage temperature on the resistance to failure of dental local anesthetic cartridges. J Can Dent Assoc. 1995;61:143–144. [147–148].
3. Sussman, GL, Beezhold, DH. Allergy to latex rubber. Ann Intern Med. 1995;122:143–146.
4. Shojaei, AR, Haas, DA. Local anesthetic cartridges and latex allergy: a literature review. J Can Dent Assoc. 2002;68:10622–10626.
5. Jeske, AH, Blanton, PL. Misconceptions involving dental local anesthesia. Part 2, Pharmacology. Tex Dent J. 2002;119:4310–4314.
6. Wahl, MJ, Schmitt, MM, Overton, DA, Gordon, MK. Injection of bupivacaine with epinephrine vs. prilocaine plain. J Am Dent Assoc. 2002;133:111652–111656.
7. Wahl, MJ, Overton, DA, Howell, J, et al. Pain on injection of prilocaine plain vs. lidocaine with epinephrine: a prospective double-blind study. J Am Dent Assoc. 2001;132:101396–101401.
8. Seng, GF, Gay, BJ. Dangers of sulfites in dental local anesthetic solutions: warning and recommendations. J Am Dent Assoc. 1986;113:769–770.
9. Perusse, R, Goulet, JP, Turcotte, JY. Contraindications to vasoconstrictors in dentistry. Part II. Hyperthyroidism, diabetes, sulfite sensitivity, cortico-dependent asthma, and pheochromocytoma. Oral Surg. 1992;74:5687–5691.
10. Nickel, AA. Paresthesia resulting from local anesthetics. J Oral Maxillofac Surg. 1984;42:52–79.
11. Wurbach, G, Schubert, H, Pillipp, I. Contact allergy to benzyl alcohol and benzyl paraben. Contact Dermatitis. 1993;28:3187–3188.
12. Klein, CE, Gall, H. Type IV allergy to amide-type anesthetics. Contact Dermatitis. 1991;25:145–148.
13. Volk, RJ, Gargiulo, AV. Local anesthetic cartridge warmer-first in, first out. Ill Dent J. 1984;53:292–294.
14. Rogers, KB, Fielding, AF, Markiewicz, SW. The effect of warming local anesthetic solutions prior to injection. Gen Dent. 1989;37:6496–6499.
15. Shannon, IL, Wescott, WB. Alcohol contamination of local anesthetic cartridges. J Acad Gen Dent. 1974;22:20–21.
16. Oakley J: Personal communications, 1985.
17. Moorthy, AP, Moorthy, SP, O’Neil, R. A study of pH of dental local anesthetic solutions. Br Dent J. 1984;157:11394–11395.
18. Crose, VW. Pain reduction in local anesthetic administration through pH buffering. J Ind Dent Assoc. 1991;70:224–225.
19. Hanna, MN, Elhassan, A, Veloso, PM, et al. Efficacy of bicarbonate in decreasing pain on intradermal injection of local anesthetics: a meta-analysis. Reg Anesth Pain Med. 2009;34:122–125.