Chapter 39 Care in the third stage of labour
Introduction
The period from the birth of the baby until expulsion of the placenta and membranes is known as the third stage of labour. During this phase of the birthing process, the mother and child meet face to face for the first time. It is a time of great importance, when the actions of those present can have a long-term effect on developing family relationships and successful breastfeeding. It is also a time when the placenta will separate from the uterine wall, descend into the lower uterine segment and be expelled together with the membranes.
The skill and expertise of the midwife continue to be needed to support this special time between mother, baby and family whilst monitoring successful completion of the childbirth process.
Traditionally, this period of childbirth has been regarded as ‘hazardous’ because of the risk of excessive bleeding; haemorrhage is a major cause of maternal death in the world (Khan et al 2006). However, currently within the UK, a very small number of women die as a result of excessive bleeding (Lewis 2007). This low rate of haemorrhage has been attributed to the prophylactic or routine use of active management during the third stage of labour for all women.
Active management is a package of care which includes the administration of an oxytocic drug, early clamping and cutting of the cord, and the speedy delivery of the placenta, usually by controlled cord traction (NICE 2007).
While the benefits of active management cannot be questioned for women at risk of postpartum haemorrhage, its indiscriminate use for women at low risk experiencing normal birth has been challenged (Harris 2001, Soltani 2008). Active management is not without risk and the component of active management which reduces blood loss has still not been clearly identified. A more targeted approach for active management has therefore been suggested, rather than its indiscriminate use for all women (Harris 2001, Soltani 2008), as currently there is insufficient evidence to support a clear recommendation.
An alternative to active management is expectant management, also called ‘passive’, ‘physiological’, or ‘natural’ management. This is a package of care where there is no active intervention in the normal physiological processes. It is characterized by activity on the part of the woman in birthing the placenta and membranes herself; the midwife’s role is one of ‘watchful waiting’.
The role of the midwife during the third stage of labour is:
In achieving this, midwives need to have an understanding of the physiology of the third stage and be able to develop partnerships with women to achieve successful delivery of the placenta and membranes with the appropriate rather than indiscriminate use of intervention.
It is suggested that women often are not given information about the third stage and do not choose how it will be managed. If women are to benefit from controlling their birth experience, then this should include the third stage of labour. A discussion should ideally take place antenatally and include the benefits and limitations of both active and expectant management. Following the discussion, the woman’s choice should be recorded clearly in her notes.
The midwife needs to pay careful attention to offering women clear information, which should be as value-free as possible.
Some midwives struggle in offering women this choice for a variety of reasons.
If women are to have a real choice, then consideration needs to be given to educating and supporting midwives in clinical practice and in developing a reflective analytical approach to discussions about the third stage which take place between midwives and women.
Physiology of the third stage
The third stage of labour is not really a stage at all. It is an extension of what has gone before (that is, the process of giving birth) and what will happen afterwards (the control of bleeding and the return of the uterus to its non-pregnant state). During labour, the uterine muscles contract and retract under the influence of naturally produced oxytocin. These muscles continue to contract and retract during the third stage to expel the placenta and membranes. The control of bleeding is brought about by the same physiological processes.
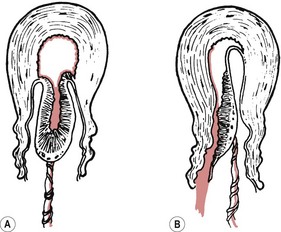
Separation of the placenta usually begins with the contraction that delivers the baby’s trunk and is completed with the next one or two contractions. As the body of the baby is delivered, there is a marked reduction in the size of the uterus because of the powerful contraction and retraction which take place. The placental site therefore greatly diminishes in size. Initially, placental separation was thought to be brought about by the bursting of decidual sinuses under pressure and the subsequent forming of a retroplacental blood clot which tore the septa of the spongiosa layer of the decidua basalis, detaching the placenta from the uterine wall (Brandt 1933). However, Dieckmann et al (1947) and more recently Herman et al (1993) suggest separation is caused by the active placental site uterine wall thickening and reducing in size, causing the placenta to ‘shear off’. Krapp et al (2000, 2003) describe three phases to the third stage of labour (Fig. 39.1). These three phases have now been widely accepted as describing the process of placental detachment and expulsion.
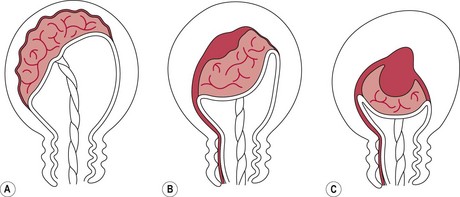
Figure 39.1 Phases in the third stage of labour. A. Latent phase: characterized by a thick placenta-free wall and thin placental site wall. B. Detachment phase: characterized by a gradual thickening of the uterine wall over the site of placental attachment. This process can be monophasic (a constant shearing-off movement) or multiphasic (which is characterized by pauses between phases of active detachment). C. Expulsion phase: the uterine wall is uniformly thickened and drives the placenta into the lower segment for expulsion.
The mean length of the third stage is calculated to be approximately 6 minutes (365 ± 270 seconds) (see Fig. 39.2).
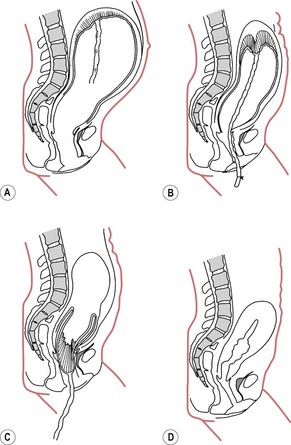
Figure 39.2 The mechanism of placental separation. A. The placenta before the child is born. B. The placenta partially separated immediately after the birth of the child. C. The placenta completely separated. D. The placenta expelled and the uterus strongly contracted and retracted.
Cord clamping
If the umbilical cord remains intact during the third stage, blood can pass to and from the infant until cord pulsation has ceased. The amount of blood gained or lost by the baby will depend on its position, with the potential for a net gain of 80 mL (Yao & Lind 1974). It is suggested that if the cord is clamped early, the resulting extra fetal blood retained in the placenta prevents it from being so tightly compressed by the uterus. As a result, contraction and retraction of the uterus may be less effective, and maternal blood loss increased, leading to a greater retroplacental blood clot being formed. Botha (1968) does not consider the formation of a retroplacental blood clot a physiological process. Rather, it occurs as a result of this intervention. Late cord clamping has also been associated with benefits for the infant (Hutton & Hassan 2007) (Box 39.1).
Detachment of the membranes begins in the first stage of labour, when separation occurs around the internal os. In the third stage, complete separation takes place assisted by the weight of the descending placenta, peeling them from the uterine wall.
During the process of separation, descent and expulsion of the placenta, a number of clinical signs may be seen.
Presentation of the placenta at the vulva
During the expulsive phase, the placenta may appear at the vagina in one of two ways (Fig. 39.3).
Schultze
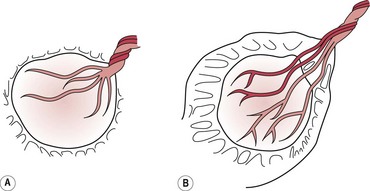
The placenta appears fetal surface first, like an inverted umbrella with the membranes trailing behind. Any blood lost during the third stage will collect on the maternal surface of the placenta and be encased by the membranes. Over 80% of placentae are delivered in this way (Akiyama et al 1981).
Matthews Duncan
Less commonly, the placenta slips from the vagina sideways and the maternal surface appears at the vulva first. Midwives often use the term ‘dirty Duncan’ for this type of presentation because more bleeding is seen vaginally – blood escapes immediately from the placental site because it is not encased in the membranes. This is often associated with slower separation of the placenta and ragged membranes.
Control of bleeding
Following placental expulsion, several mechanisms come into play to control bleeding from maternal sinuses at the site of placental attachment.
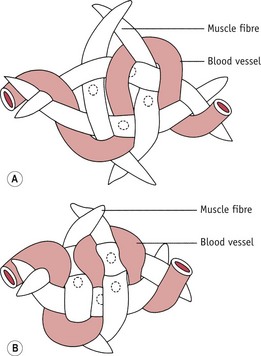
Figure 39.4 How the blood vessels run between the interlacing muscle fibres of the uterus. A. Muscle fibres relaxed and blood vessels not compressed. B. Muscle fibres contracted, blood vessels compressed and bleeding arrested.
Any factor that interferes with the normal physiological processes can influence the outcome of the third stage of labour (see Box 39.2). This includes a variety of complications of pregnancy and childbirth as well as the actions of individual midwives. Oxytocic drugs given prior to and during the third stage of labour also influence events. A woman’s ability to avoid complications will also be based on her general health and by avoiding predisposing factors, such as anaemia, ketosis, exhaustion, and hypotonic uterine action.
Management of the third stage of labour
Commonly, midwives describe two ways of managing the third stage: active management and expectant management. However, difficulties remain in defining what these terms mean, as midwives practise both methods in a variety of different ways (Harris 2005). The most commonly described form of each management will be outlined here with discussion about where variation may take place. The woman and her midwife will have discussed options for the third stage during the antenatal period and again during labour and made a decision over which management she would like.
Expectant management
Expectant management is one of ‘watchful anticipation’ and draws upon the normal physiological processes to bring about expulsion of the placenta and membranes. The woman is active during this process and the midwife’s role is a passive one involving close observation and encouragement.
Principles of expectant management
Positioning the baby after birth
Whichever position a woman chooses to give birth in, the newborn infant will be placed either on the bed/floor covering between the woman’s legs or on the woman’s abdomen, depending on her choice. Early skin-to-skin contact is advantageous in maintaining the infant’s temperature, in promoting successful breastfeeding and in supporting development of mother–infant attachment (Moore et al 2007). The midwife then steps back to leave the woman and her family to experience undisturbed the powerful first meeting with their new baby, while continuing to observe the wellbeing of the infant and maternal vaginal blood loss.
When to cut the cord
There is some debate about when the umbilical cord should be clamped and cut. The potential benefits and risks to the mother and infant of delayed cord clamping (McDonald & Middleton 2008) are being considered alongside the routine use of active management which normally incorporates immediate clamping and cutting of the cord (NICE 2007, WHO 2007). In accordance with the principles of non-intervention in expectant management, Inch (1985) suggests that ideally the cord should be left intact until the placenta and membranes are completely expelled, as this enables compaction and compression of the placenta and retraction of muscle fibres to occur unhindered. There may also be beneficial effects of continued delivery of oxygenated blood to the newborn infant via the cord (Hutchon 2006), particularly in those born prematurely or asphyxiated. In a study of premature infants conducted by Kinmond et al (1993), a 30-second delay in cord clamping with the infant held 20 cm below the introitus was associated with improved outcome for the baby. A more recent study in term neonates has linked a delay in cord clamping of 2 minutes with significantly higher mean corpuscular volume, ferritin and total body iron stores in the infant up to 6 months of age (Chaparro et al 2006). Early cord clamping has also been associated with fetomaternal transfusion, of particular importance in women who are rhesus negative (Lapido 1972). Enkin et al (2000) suggest that free bleeding of the cut end of the severed umbilical cord reduces the risk of fetomaternal transfusion. Placental cord drainage has also been associated with a reduction in the length of the third stage of labour (Soltani et al 2005).
If the cord is short, this may prevent a woman from holding her baby. In these circumstances, the woman may choose to have the cord clamped and cut once it has stopped pulsating (approximately 5–10 minutes after the baby is born).
Position of the woman during the third stage of labour
Usually, whichever position a woman gives birth in, she will choose to sit once the baby is born. This allows her the opportunity to touch, hold and examine her baby. Skin-to-skin contact and breastfeeding should be encouraged as this aids separation and expulsion of the placenta through endogenous oxytocin release.
Detection of separation and descent of the placenta
As the uterus begins to contract again, the woman will usually indicate this and may have an urge to bear down. The midwife may also notice abdominal changes; the fundus rises up and becomes more globular. The separated placenta may be seen as a bulge, similar to a full bladder, just above the symphysis pubis, with the well-contracted uterus sitting above it. In addition, a gush of blood per vagina and cord lengthening may occur. There is no necessity to palpate the abdomen unless there is cause for concern or the midwife suspects there may be some delay. Encouraging the woman to adopt an upright position at this time will lead to rapid expulsion of the placenta and membranes. Care needs to be taken when assisting the woman to move into another position, as she will have the baby in her arms. Standing, squatting and sometimes using a toilet, bucket or bedpan can be used.
Delivery of the placenta and membranes
The placenta is delivered by maternal effort. Normally, the woman in an upright position will feel the placenta as it descends to the pelvic floor, which triggers an urge to push, or the placenta will just fall out under the influence of gravity.
The midwife’s role is to let the woman know what is happening, to encourage her to adopt an upright position, and to advise her to listen to what her body is saying (to push or bear down if she wants to). A flat hand placed across the lower abdomen may assist the woman to birth the placenta, as the counterpressure compensates for poor muscle tone. The placenta and membranes are then delivered either onto the bed/floor or into a bedpan/toilet/bucket. If the membranes trail behind, they can be eased out of the vagina by turning the placenta to make a rope of the membranes, by applying gentle traction on the membranes with the fingers (usually in an up-and-down motion), or by asking the woman to cough. Once the placenta is completely expelled, the time is noted, to calculate the length of the third stage for recording later. The midwife then palpates the abdomen to ensure the uterus is well contracted and there is no excessive bleeding. The placenta and membranes can then be checked in front of the parents if they wish and any cord blood taken if the woman is rhesus negative.
It is suggested by midwives that this process normally takes between 5 and 15 minutes but can take up to an hour (Harris 2005). Whilst some authors suggest the length of the third stage is longer with expectant management than active management (Prendiville et al 1988, Rogers et al 1998), other authors looking specifically at ultrasound images suggest there is no difference (Herman et al 1993, Krapp et al 2000). If physiologically the length of the third stage is similar for both active and expectant management, perhaps time differences may be attributed to the actions of either the midwife or the woman. It has been noted that the use of an upright posture following separation appears to reduce blood loss and the length of the third stage in expectant management (Rogers et al 1998).
If there is delay in placental delivery, a number of actions can be taken. Odent (1998) suggests gently pressing upwards on the abdominal wall just above the pubic bone (with the woman lying on her back). If the cord does not move, then placental separation can be confirmed. Emptying the bladder, changing the woman’s position and encouraging the woman to walk a short way may assist in placental delivery. Encouraging a woman to blow into an empty bottle has also been described (Fry 2007). According to NICE (2007), an expectant management is prolonged if not completed by 60 minutes.
Active management
Active management is an intervention in the normal physiological processes. The administration of oxytocic drugs at the end of the second stage of labour combined with early cord clamping and controlled cord traction are used to bring about delivery of the placenta and membranes. The woman for the most part is a passive participant in this process.
History of active management
Active intervention in the third stage of labour is not new but did not become popular until after the isolation of ergometrine (Dudley & Moir 1935) and the development of synthetic oxytocin (Syntocinon) (du Vigneaud & Tippett 1954). Initially, ergometrine was used as a treatment for postpartum haemorrhage (PPH), and it was then given following the third stage to prevent PPH. Syntometrine® (a mixture of Syntocinon and ergometrine) was marketed in the 1960s (Embrey et al 1963) as a uterotonic drug, administered at the birth of the baby’s anterior shoulder and followed by cord traction. This package of care became known as active management and its popularity quickly spread. It has been so successful that it has become normal practice throughout the UK, irrespective of the degree of risk.
Uterotonic drugs
Uterotonic drugs (drugs that make the uterus contract) are used during the third stage in three ways, as:
While the benefits of oxytocic drugs in controlling atonic postpartum haemorrhage are recognized, their routine use in preventing the problem has been the subject of much debate and various clinical trials. A systematic review of four studies comparing active management with an expectant or physiological approach supports the prophylactic use of active management in a hospital birth situation (Prendiville et al 2000). The implications for home birth are less clear (Enkin et al 2000). The review concluded that there was an overall reduction in maternal blood loss of less than 100 mL in women having an active third stage of labour over expectant management (mean weighted difference −79.33 mL; 95% confidence interval −94.29 to −64.37) (Prendiville et al 2000). The review also highlights that certain uterotonic drugs have been associated with raised blood pressure, nausea, vomiting and headaches (Prendiville et al 2000). Higher rates of retained placenta in active management have also been reported, along with more serious complications such as postpartum eclampsia and cardiac disorders (Begley 1990, WHO 1997). It has been suggested that Syntocinon replace Syntometrine® as the drug of choice in active management as some of the complications above have been associated with the ergometrine component of Syntometrine® (McDonald et al 1993, NICE 2007).
Critics of these research studies highlight a number of factors that may have influenced the results achieved.
Lack of skill in expectant management among midwives
Three out of four studies were conducted in hospitals where active management was the norm (Gyte 1994). Whilst the latest study was conducted at Hinchingbrooke (Rogers et al 1998), where expectant management was said to be more common, statistics were not available as to the rate of expectant management before the trial began.
Difficulty in defining what constitutes excessive blood loss
It is well recognized that blood loss estimation is inaccurate, with high loss often being underestimated (Razvi et al 1996). In addition, as the reduced loss associated with active management has become the norm, midwives may interpret the slightly higher blood loss rate in expectant management as abnormal. Currently, a postpartum haemorrhage is defined as a blood loss in excess of 500 mL. In some countries this is 1000 mL, with evidence suggesting that healthy women appear to cope well with the loss of such amounts (Bais et al 2004, McDonald 2007). If this more generous definition had been used in the Hinchingbrooke study, no statistically significant difference in PPH rates would have been found between expectant and active management approaches (Rogers et al 1998). This may add weight to the growing evidence that for low-risk women an expectant management approach may not significantly increase blood loss following birth, making it a realistic option.
More recently it has been suggested that whilst oxytocics may appear to reduce blood loss at delivery in the short term, when the action wears off on the postnatal ward, the blood will be lost then (Kashanian et al 2008, Wickham 1999). Wickham (1999) observed that following active management, women often experienced a heavy blood loss when going to the bathroom for the first time, and Kashanian et al (2008) confirmed this, noting statistically significant higher blood loss in the ‘fourth stage of labour’ (see below) in women having active management. This may suggest that the use of uterotonics during the third stage merely delays blood loss until a time when it is less likely to be noticed. It is possible that women are supposed to lose blood at this time as they no longer require such a high circulating blood volume to supply the placental bed, and the haemodilution of pregnancy may support a woman’s ability to cope with this. Further studies are required to look at what constitutes normal blood loss following childbirth and the implications of actively reducing it.
Variation in practice
When comparing research protocols of published trials, no consensus can be reached on a definition of what constitutes active and expectant management, which implies that there is variation in practice (Begley 1990, Prendiville et al 1988, Rogers et al 1998, Thilaganathan et al 1993). Gyte (1994) suggests that a ‘piecemeal’ approach, a combination of active and expectant management techniques, was used by a significant number of midwives within the Bristol trial. Inter and intra third stage practice variation among midwives has subsequently been reported (Harris 2005) as has variation in third stage policies (Winter et al 2007), intra and inter country variation in third stage care (Festin et al 2003), and variation in management between maternity care providers (Tan et al 2008). This highlights the difficulty in evaluating the results of comparative studies where variation in practice could have occurred.
Logue (1990) looked at PPH rates among doctors and midwives and found considerable variation, with some individuals having consistently much higher rates of PPH than others. She suggests that when managing the third stage, ‘more conservative and patient operators show the lowest PPH rates compared with the more impatient and heavy-handed who show the highest rates’ (Logue 1990:S11). This implies that the action or inaction of practitioners may have a direct impact on the outcome of the third stage and requires further exploration. This is supported in the literature by reference to the potential dangers of ‘fundal fiddling’ and inappropriate cord traction leading to uterine inversion (McDonald 2009).
Current options
Currently, the following uterotonic drugs are available to manage the third stage of labour:
Syntocinon
This is a synthetically produced form of oxytocin, given either intravenously (5 IU) or intramuscularly (5–10 IU). It can be given at crowning of the head, with delivery of the anterior shoulder or shortly after the birth. Intramuscular Syntocinon acts within 2–3 minutes of administration. It is considered the oxytocic drug of choice for active management (NICE 2007). Whilst Syntometrine® remains the most effective oxytocic in reducing blood loss (McDonald et al 2004), it may cause nausea, vomiting and hypertension, making Syntocinon, which has fewer side-effects, an appropriate alternative. Syntocinon becomes inactive at high temperatures.
Intramuscular Syntometrine® 1 mL
This is usually given with the birth of the anterior shoulder or shortly after. Syntometrine® contains ergometrine 500 mcg and oxytocin 5 units in 1 mL. The oxytocin fraction induces strong, rhythmic contraction of the muscle fibres of the upper segment of the uterus within 2–3 minutes of administration. Its effect lasts for approximately 5–15 minutes (Baskett 1999). This rapid-acting, short-duration component is designed to initiate strong uterine action, which is sustained by the action of the ergometrine fraction, which will induce a strong, non-physiological spasm of uterine muscle within 6–8 minutes (Sorbe 1978). The effect of ergometrine is maintained for approximately 60–90 minutes (Baskett 1999). Because of the spasm-inducing properties of ergometrine there is a theoretical risk of retained placenta and therefore the midwife should aim to deliver the placenta before ergometrine takes effect. Syntometrine® becomes inactive at high temperatures.
Intramuscular ergometrine 500 mcg.
This will cause a strong, sustained uterine contraction. If intramuscular ergometrine is administered instead of Syntometrine®, it is given a little earlier, at the crowning of the head, as it takes longer to act, 6–8 minutes. The World Health Organization (WHO) (1997, 2007) do not support its use for routine management in the third stage owing to its effect on blood pressure. The conclusion of a recent review of ergot alkaloids in the third stage suggests that while these drugs are effective, other drugs such as oxytocin and prostaglandins may be preferable (Liabsuetrakul et al 2007).
Prostaglandins
There is currently some interest in exploring the use of a prostaglandin E1 analogue (misoprostol) for management of the third stage. Misoprostol (in doses of 400–600 mg) can be given orally or rectally, needs no equipment to administer and does not become inactive at high temperatures. This makes it an ideal therapy for countries in the developing world where refrigeration and health services are limited. However, it is associated with shivering and transient pyrexia. In a systematic review of prostaglandins for preventing PPH (Gülmezoglu et al 2007), findings suggested that neither intramuscular prostaglandins nor misoprostol was as effective at preventing PPH as were injectable uterotonics.
Nipple stimulation
A simple alternative to parenteral oxytocics for the third stage of labour is nipple stimulation, which, according to Irons et al (1994), tends to reduce the length of the third stage and the amount of blood loss. This was a small study, however, and larger trials are needed.
Principles of active management
Currently, active management is recommended for all women, though those at low risk who request expectant management should be supported in their choice (ICM/FIGO 2003, NICE 2007, RCM 2008).
Positioning the baby after birth
Where the infant is placed at birth will depend on the position that the woman chooses for birth. As discussed previously, there are significant benefits for both mother and child of early skin-to-skin contact. There is also a need to provide a safe, warm, draught-free environment.
When to give the uterotonic
Traditionally, it has been recommended that during active management a uterotonic drug is administered either at the birth of the baby’s anterior shoulder or shortly after the birth of the baby (if a midwife is alone). Midwives have identified that this may occur before or after the cord is clamped and cut (Harris 2005).
When to cut the cord
Traditionally, midwives were advised to clamp and cut the umbilical cord as soon as possible after the birth of the baby to prevent an excess of placental blood being forced into the infant’s circulation under the influence of the administered oxytocic. This was considered to have the potential to cause hypervolaemia and hyperbilirubinaemia in the neonate. Clamping occurred before or shortly after the administration of the uterotonic. However, evidence now suggests there is a benefit to delaying cord clamping for 2–3 minutes and that any subsequent polycythaemia in the neonate appears to be benign (Hutton & Hassan 2007).
Position of the woman during the third stage of labour
As with expectant management, irrespective of the position in which a woman gives birth, she will usually choose to sit once the baby is born.
Detection of separation and descent of the placenta
In active management it is normally the midwife who detects the first uterine contraction following the baby’s birth by placing a hand gently on the woman’s abdomen and waiting for the uterus to rise up and contract beneath it. Midwives are often warned at this time of the dangers of fundal ‘fiddling’, which may lead to partial separation of the placenta, with the potential for excessive bleeding to occur. Although cord traction as described by Spencer (1962) should be commenced as soon as the uterus contracts, Levy & Moore (1985) suggest waiting until signs of separation are present. This latter study found no significant difference in the incidence of postpartum haemorrhage, or the length of the third stage, between those who started controlled cord traction as soon as the uterus contracted and those who waited for signs of separation. However, the rate of postpartum haemorrhage appeared to be significantly higher when the midwife unsuccessfully used controlled cord traction without awaiting signs of separation.
Signs of separation and descent
As the uterus contracts and the placenta separates, the fundus rises up and becomes more globular. The separated placenta may be seen as a bulge, similar to a full bladder, just above the symphysis pubis, with the well-contracted uterus sitting above it. Combined with the abdominal findings, the midwife may notice that there is a gush of blood per vagina and that the cord lengthens. The woman may experience pain at this time and also feel the urge to bear down as the placenta enters the vagina.
Delivery of the placenta and membranes
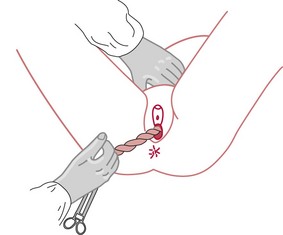
The placenta is delivered by cord traction with the woman in a sitting/semi-recumbent position. The midwife either wraps her fingers around the cord or uses a clamp to apply downward sustained traction until the placenta appears at the vulva. When the placenta becomes visible, traction is applied upwards (following the curve of Carus) to extract the placenta from the vagina (Spencer 1962). The placenta is delivered into the midwife’s hands or into a bowl placed close to the introitus. Care is taken with the membranes, which may trail behind. Some midwives ask women to cough gently, particularly if the membranes appear caught in the cervix.
Whilst applying cord traction, some midwives place a hand above the symphysis pubis and push the uterus upwards (known as ‘guarding of the uterus’) (Fig. 39.5). This is said to prevent uterine inversion. However, there is currently no evidence available to support this practice. Some midwives use this hand as counterpressure when applying cord traction and others suggest it provides valuable information on descent, as the placenta can be felt beneath the hand, moving down into the vagina (Harris 2005).
In some units, women are encouraged to deliver the placenta by maternal effort (see expectant management).
Following placental delivery, the length of the third stage is noted, for recording later. The midwife palpates the abdomen to ensure the uterus is well contracted and notes vaginal blood loss.
Examination of the placenta and membranes
Whatever management, the placenta and membranes are carefully and systematically examined as soon after delivery as possible so that, if incomplete, action can be taken immediately. The examination is to determine completeness, and to detect any abnormalities, which may suggest problems in the neonate.
Initially, the placenta is held up by the cord to view the membranes; a discrete hole, which the baby passed through, may be seen. Sometimes membranes are ragged and every attempt should be made to piece them together to ensure completeness.
The placenta is placed on a flat surface and thoroughly examined in a good light. The amnion is stripped from the chorion up to the umbilical cord to confirm that both membranes are present. The maternal surface is wiped clear of blood clots and carefully examined to ensure all the cotyledons are present. Any areas of infarction (firm whitish patches) are noted and the placental edge examined for blood vessels running into the membranes. These vessels may track back to the placenta (an erratic vessel) or go to an accessory lobe in the membranes (a succenturiate lobe). If a vessel ends at a hole in the membranes (Fig. 39.6), a succenturiate lobe may have been left behind in the uterus and the woman will need referral to an obstetrician.
The cord is examined, noting its insertion and length (though this is no longer measured) and the number of umbilical vessels. Usually the cord insertion is central and the length is approximately 50 cm. Occasionally only one umbilical artery is present; this is associated with congenital anomalies, especially renal agenesis (absence of kidneys). The paediatrician would need to be informed and a detailed examination of the newborn requested.
The placenta is usually weighed; weight at term is normally about one-sixth of the baby’s birthweight. Early cord clamping increases the placental weight as it contains a greater residual blood volume.
Finally, any blood loss collected is measured and added to the estimated amount of loss which has soaked into linen and pads. Particular care is needed in estimating losses in excess of 300 mL, when amounts are often underestimated (Levy & Moore 1985), with the level of error increasing with the amount lost.
The findings are recorded in the mother’s notes. Immediate referral to a doctor is made if it is thought that a piece of placental tissue has been retained.
Care following birth (the fourth stage)
Following delivery of the placenta and membranes, the midwife palpates the woman’s abdomen to ensure the uterus is well contracted, assesses vaginal blood loss and examines the woman for any soft tissue damage which may require repair. The midwife makes the mother comfortable. This is an ideal time for the midwife to share the couple’s delight in their baby and encourage any questions, and affords an excellent opportunity for health education to facilitate parent–baby attachment. Most women will enjoy early contact with their baby and there is evidence that this early and unhurried contact significantly affects maternal emotional wellbeing when measured 6 weeks postnatally (Ball 1994). The father, too, usually wishes to share this time with his family and should be encouraged to do so. This must be given priority over the many routine procedures (Sheridan 2010). Sensitivity is required in caring for women who appear to show little interest in their baby at birth.
Women who plan to breastfeed should be encouraged to do so soon after birth, usually within the first hour. At this time the baby usually displays a strong urge to suck and a successful feed benefits both mother and baby. Early feeding is associated with ongoing breastfeeding success and the release of endogenous oxytocin, which stimulates uterine contractions and helps to maintain haemostasis (see Ch. 43).
Ongoing care includes regular examination of the woman’s abdomen to ensure the uterus remains contracted and observation of the lochia. The woman is encouraged to pass urine, as a full bladder predisposes to a relaxed uterus and heavy blood loss. If this occurs, the bladder is emptied, and then the midwife can massage the fundus of the uterus to stimulate a contraction.
Observation of the infant will include colour, respirations, and general activity. The umbilical cord is checked to ensure the cord clamp is firmly in place and that there is no bleeding. Care is taken to ensure that the baby does not become chilled; body temperature can be maintained by skin-to-skin contact or warm wrapping and cuddling by the parents. The midwife should remain in the birthing room for at least 1 hour after delivery, whether at home or in hospital.
Records
A complete and accurate account of labour must be recorded, and must be sufficiently comprehensive to enable other carers to have a clear picture of events, thus facilitating communication and avoiding discontinuity of care (NMC 2009).
Statute requires that a birth notification form be completed. This is normally undertaken by the midwife and sent, within 36 hours of the birth, to the medical officer of the district in which the birth took place (NMC 2004).
The placenta at term
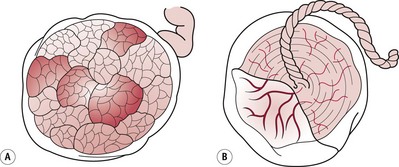
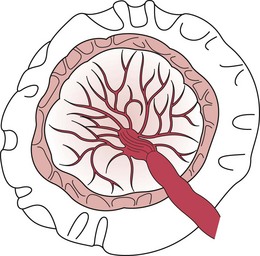
At term, the placenta is flat and round or oval in shape. It is 18–20 cm in diameter and about 2.5 cm thick in the centre, becoming thinner towards the edges. Its weight is about one-sixth of the weight of the fetus and it is usually situated on the anterior or posterior wall of the uterine cavity, near the fundus. The placenta has two surfaces, maternal and fetal. The maternal surface is attached to the uterine decidua, is deep red in colour and divided by deep grooves or sulci into about 15–20 irregular lobes. These lobes (called cotyledons) contain masses of chorionic villi. On examination after birth, a thin greyish layer, part of the basal decidua, can be seen (Fig. 39.7). It may feel gritty due to the presence of lime salts. The fetal surface lies adjacent to the fetus and has a pearly white appearance as it is covered with amnion. From the insertion of the cord, which is usually situated centrally, blood vessels can be seen radiating to the periphery, like the roots of a tree. These vessels give off branches which penetrate into the substance of the placenta, each cotyledon having its own supply of fetal blood. In the centre of each cotyledon there is a main branch of the umbilical artery and vein.
Abnormalities of the placenta
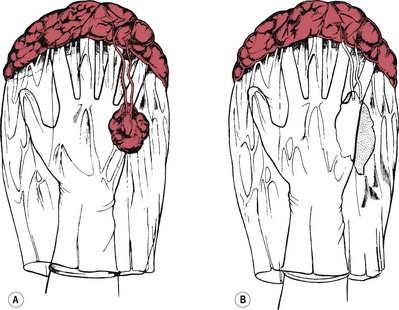
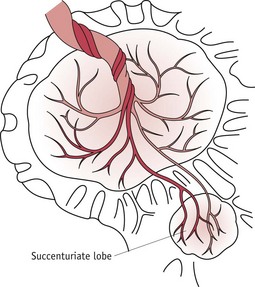
Succenturiate lobe
A succenturiate lobe (Fig. 39.8) is a small portion or lobe of placenta which is separated from the main body. This is formed from some of the villi of the chorionic membrane which have continued to develop instead of becoming atrophied. It is attached to the main placenta by blood vessels which pass through the membrane. A succenturiate lobe may be retained in the uterus after the placenta has been expelled; and can cause postpartum haemorrhage and sepsis. When there is a small hole in the fetal membranes with placental vessels running towards it, a retained succenturiate lobe may be suspected and the woman should be referred to an obstetrician for assessment.
Circumvallate placenta
In this type of placenta the chorion is attached not to the edge of the placenta, but to the fetal surface at some distance from the edge (Fig. 39.9). A thickened ring of membrane is seen on the fetal surface.
Placenta accreta
This is a placenta which becomes abnormally adherent to the uterine muscle over the whole or part of its surface. It is very rare.
Infarcts
Red or white patches are sometimes seen on the maternal surface of the placenta. These are caused by localized death of placental tissue due to interference with the blood supply.
Infarcts are red at an early stage of their development; they later become white and appear as patches of white fibrous tissue. They may be seen occasionally in any placenta, but they are often associated with pre-eclampsia.
Fetal membranes
There are two fetal membranes: the chorion and amnion.
The chorion
This is the outer membrane, continuous with the edge of the placenta and derived from the trophoblast. It is opaque, thick but friable, and roughened by tiny pieces of decidua adherent to it. It lines the uterine cavity.
The amnion
This is the inner membrane, derived from the inner cell mass. It is smooth, transparent and the stronger membrane of the two. The two membranes lie over each other, but can be separated; the amnion may be stripped back to the insertion of the cord. The amnion secretes amniotic fluid or liquor amnii, which at term measures 1000–1500 mL.
Umbilical cord
The umbilical cord (Fig. 39.10), or funis, connects the placenta to the fetus. The cord is about 50 cm long and 2 cm thick. It is composed of a jelly-like substance known as Wharton’s jelly; this is a primitive connective tissue, primary mesenchyme. The cord is covered externally by amnion. It supports and protects three blood vessels: one large umbilical vein carrying oxygenated blood to the fetus; and two umbilical arteries winding around the vein, carrying deoxygenated blood from the fetus to the placenta. The absence of a vessel may be associated with fetal abnormalities. The cord has a spiral twist; this torsion gives a certain amount of protection from pressure.
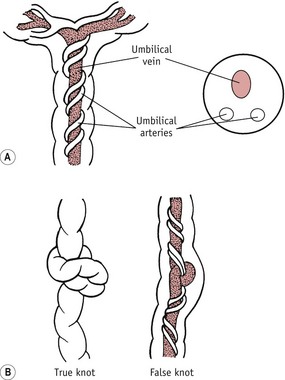
Figure 39.10 A. The umbilical cord in side view and cross-section, showing the one umbilical vein with the two umbilical arteries twisting spirally around it. The vein and arteries lie in Wharton’s jelly; the cord is enclosed within the amnion. B. True and false knots.
The function of the cord ceases as pulmonary respiration is established shortly after birth. Lacking a blood supply, the cord becomes dead tissue and quickly atrophies, as do the internal structures continuous with it. It can provide access for bacteria to enter the body, therefore care needs to be taken to keep it dry until the cord stump separates (see Ch. 41).
Abnormalities of the umbilical cord
The cord may be too short (which may cause delay during labour), or too long, when there is a risk of cord prolapse. Occasionally it is very thick or very thin; in either case, great care is required in tying the cord and subsequently watching for haemorrhage. Rarely, a piece of fetal intestine may protrude into the cord; the possibility of this abnormality will be suspected if the cord is swollen close to the umbilicus, the size of the swelling depending on the amount of intestine which has protruded (see Ch. 48). Knots are caused by movements of the fetus before birth, the baby slipping through a loop of the cord. False knots may be due to the blood vessels being longer than the actual cord, and so doubling back upon themselves in the Wharton’s jelly, or to irregularities and the formation of nodes.
Abnormalities of insertion
The cord may be attached to one side of the placenta (an eccentric insertion) or to the margin of the placenta (a battledore insertion), or the vessels of the cord may break up and run into the membrane before reaching the placenta (a velamentous insertion) (Fig. 39.11). This is particularly dangerous if the unprotected blood vessels should lie near the internal os. This very rare condition is called vasa praevia (vessels in advance of the fetus). Should a blood vessel so situated be compressed when the membranes rupture, the fetus will suffer hypoxia. If such a vessel should actually rupture, blood will be lost from fetal vessels in the membrane. Such fetal haemorrhage is dangerous and could lead to stillbirth.
Conclusion
The third stage of labour is an important period for mother and baby, when the importance of their first meeting cannot be overestimated. The midwife supports this special time while monitoring successful delivery of the placenta and membranes. Currently, women appear to have little choice about how the third stage is managed, with active management being used indiscriminately. If midwives are to offer women a choice for the third stage, skills in both active and expectant management are required with a body of knowledge which enables the detection of those women who deviate from the norm, in whom active management may be the most appropriate form of care. In this way, women at low risk may be spared unnecessary intervention in the normal process of giving birth.