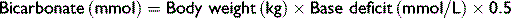Chapter 20 Manifestations and Management of Disease in Neonatal Ruminants
WEAKNESS AND/OR DEPRESSED MENTATION
If weakness has been present since birth, in utero acquired bacterial or viral infections, birth asphyxia and trauma, chronic placental problems, and congenital anomalies should be considered on the list of differential diagnoses. A number of congenital bacterial, fungal, and viral infections that cause abortions and stillbirths may result in the birth of a live, weak neonate. In cattle, brucellosis, salmonellosis, leptospirosis, listeriosis, Escherichia coli, Corynebacterium species, and Aspergillus species may cause placentitis and disease in the newborn. In sheep, in utero infection with chlamydia, Campylobacter, Coxiella, bluetongue virus, and border disease may cause disease in the newborn. Congenital viral infections of neonates are listed in Box 20-1. Clinical manifestations of fetal infections depend on the age of the fetus and the virulence and trophism of the infecting agent (see individual diseases).
Box 20-1 Differential Diagnoses for the Weak or Depressed Large-Animal Neonate
Neonatal calves with storage diseases primarily affecting the nervous system may appear reasonably normal for a short period after birth and then show progressive signs of neurologic dysfunction, including tremors, spasms, depression, recumbency, and coma. Differential diagnoses for weakness and depressed mentation after a period of apparently normal strength and mentation include sepsis, electrolyte and acid-base disturbances, hypoglycemia, and hypothermia. A complete history is obtained, including a detailed description of the delivery process, and complete physical and neurologic examinations are performed. Any signs of trauma, infection, or congenital malformations should be noted. Evaluation of hematologic data and immunoglobulin G (IgG) status, combined with historical and physical examination parameters, results in an assessment of the likelihood of sepsis. Blood glucose, blood gas, and serum electrolyte concentrations should be determined promptly. Blood cultures and cerebrospinal fluid (CSF) analysis are useful for verifying central nervous system (CNS) involvement and targeting antimicrobial therapy.
For collection of fluid from the lumbosacral space, a 20-gauge, 1- to 2-inch needle with a clear hub may be used. A change in resistance is felt when the needle penetrates the dural membranes, and CSF appears in the plastic hub as soon as the subarachnoid space is entered. Approximately 5 to 10 mL of fluid may be removed safely. Urinary reagent strips can be used to rapidly obtain general information on the fluid. If blood is detected, the sample should be spun down after the cytologic examination. Red blood cells contaminating the sample will settle, and the supernatant should be colorless. If hemorrhage occurred before the procedure, the sample remains xanthochromic (yellow). Glucose should be present in “trace” or “+” amounts in the normal sample. Negative values in the adult suggest severe meningitis, but in the neonate may also be caused by profound hypoglycemia. CSF analysis is most useful in determining the presence of septic meningitis. Elevation of the total protein level (>150 mg/dL) and neutrophil count in addition to a positive Gram stain and bacterial culture results in a straightforward diagnosis of bacterial meningitis, and the prognosis is considered poor for the animal.1 Infection in the CNS, however, can be difficult to detect until the process becomes generalized; the lack of positive cultures and Gram stain does not rule out CNS infection. An elevated albumin quotient suggests increased blood-brain permeability and can be seen in both hypoxic-ischemic brain injury and meningitis, but an elevated IgG index indicates increased intrathecal IgG production and is more compatible with a diagnosis of meningitis.
MENINGITIS
Depressed mentation is a common presenting sign in neonates with sepsis. Although bacterial meningitis may occur as a primary entity, it more commonly is a result of generalized septicemia in neonates with failure of passive transfer (FPT). Agents that cause meningitis are the same agents that cause septicemia, most commonly the gram-negative enteric bacteria such as E. coli, Enterobacter species, and Salmonella species. In a review of 32 cases of meningitis in calves by Green and Smith,1 the clinical signs of CNS disturbance observed were lethargy, recumbency, anorexia, loss of suckle reflex, coma, opisthotonos, convulsions, tremor, and hyperesthesia. Leukocytosis and a left shift were evident in 11 of 15 calves (73%). Concurrent metabolic problems were common and included hyperkalemia, respiratory acidosis, hypernatremia, hyponatremia, hypomagnesemia, and hypoglycemia. Analysis of CSF revealed pleocytosis, xanthochromia, turbidity, and high total protein concentration. Cytologically, neutrophils predominated in the CSF in calves with acute disease. Mononuclear cells dominated in calves with chronic disease. Microscopically, bacteria were evident in 10 of 22 (45%) of the antemortem CSF samples, and bacteria were isolated from slightly more than half (11 of 19). All of the calves in this review died.1 In my experience treating calves in a hospital environment, the mortality rate is high; however, aggressive early treatment can be successful. The economics and welfare implications of treating commercial calves in a field setting are questionable. Empiric antimicrobial therapy for meningitis in neonatal calves should include a gram-negative and positive spectrum. Antibiotics enter the CSF predominantly via passive diffusion down a concentration gradient. The major determinant of CSF penetration is lipid solubility. Lipophilic agents diffuse via transcellular pathways; peak concentrations in CSF occur relatively rapidly, and entry into CSF is affected minimally by the presence of inflammation. In contrast, hydrophilic agents enter the CSF through paracellular pathways; their transport depends on the opening of tight junctions, and peak concentrations are relatively delayed.2 Only one report documents the pharmacokinetics of an antimicrobial agent in CSF in calves. Table 20-1 lists CSF-to-blood concentration ratios (penetration) derived from multiple species for a handful of antimicrobial drugs available for use in cattle.
Table 20-1 Cerebrospinal Fluid—to-Blood Concentration Ratios (Penetration) of Antibiotics Available for Treatment of Meningitis in Calves2,3
| Concentration CSF/Concentration Serum (%) | ||
|---|---|---|
| Antimicrobial* | Human | Animals |
| Ampicillin | 13–14 | 8–12 |
| Florfenicol | 46 (calves) | |
| Gentamicin | 0–30 | 21–25 |
| Penicillin | 5–10 | 5–6 |
| Trimethoprim-sulfamethoxazole | <41 | 35–39 |
CSF, Cerebrospinal fluid.
*The list is not conclusive, reflecting the paucity of available data.
In a CSF pharmacokinetic study of florfenicol in calves the maximum concentration of florfenicol attained in CSF was 4.67 ± 1.51 μg/mL following a single intravenous dose of 20 mg/kg. The levels remained above the minimum inhibitory concentration (MIC) for Haemophilus somnus over a 20-hour period.3 This concentration is below the MIC90 for E. coli. Bacteriocidal antibiotics are proposed to be more effective for treatment of meningitis in humans, and it is recommended that the concentration of antibiotic in the CSF should be maintained at 10 times the MIC of the target pathogen.2 Ceftiofur may be used to treat meningitis in calves. In one calf, I measured the concentration of ceftiofur in CSF 28 hours after initiation of treatment with 10 mg/kg twice per day (bid). The concentration of ceftiofur in CSF at this time was 1.27 μg/mL, which happened to be five times the MIC of the E. coli isolated from the CSF of the calf. Unfortunately, owing to the lack of CSF pharmacokinetic data in cattle, antimicrobial treatment of meningitis is an inexact science.
METABOLIC ACIDOSIS
Profound weakness associated with metabolic acidosis is commonly observed in calves with diarrhea and sporadically in kids (“floppy kid syndrome”) and calves without other clinical signs of disease.4,5 Correction of the acidosis by intravenous administration of bicarbonate produces a rapid recovery. An improvement in mentation and strength should be observed within 12 hours; persistent depression is likely to reflect incomplete correction of acidosis, sepsis, hypoglycemia, hypernatremia, or hyponatremia.
HYPOGLYCEMIA
Hypoglycemia is a common sequela to withdrawal of milk for more than 48 hours, especially in cold weather. Affected calves are weak or recumbent but appear to be normally hydrated or minimally dehydrated.6 They are often emaciated and can occasionally have neurologic signs including facial twitches, convulsions, opisthotonus, and coma. They will respond to infusion of 5% glucose, but often this response is temporary, especially in calves with severe malabsorptive disease. It is important to rapidly restore adequate energy intake to ensure resolution of these cases. Starvation and hypothermia resulting from mismothering are common causes of weakness in neonatal lambs. Similarly, weakness, poor body condition, and increased susceptibility to infectious diseases are observed with protein-calorie malnutrition induced by feeding poor-quality or incorrectly mixed milk replacers.7
HYPONATREMIA
Hyponatremia occurs when loss of isotonic fluid through the gastrointestinal tract is replaced by free water or hypotonic solutions. The latter often occurs when too much water is added when making up an oral electrolyte solution. Hyponatremia may also occur when isotonic oral electrolyte solutions are administered to calves with compromised sodium absorption capacity. This may be a result of severe pathologic changes or an inadequate level of agents that facilitate sodium cotransport within the oral electrolyte solution. Hyponatremia results in a fluid shift from the extracellular space to the intracellular compartment along the osmotic gradient, and the resultant swelling of the cells can result in neurologic disturbances, depression, disorientation, and even convulsions.8 Hyponatremia should be considered in calves with serum sodium <132 mmol/L; calves with serum sodium <120 mmol/L have severe hyponatremia.
The goal of therapy is to restore serum sodium levels to >125 mmol/L over the first 6 hours and then to restore to normal levels over 24 hours.8 In hypovolemic calves the initial treatment should be achieved using normal saline, and in normovolemic calves hypertonic saline should be used for the initial treatment, as the administration of large fluid volumes will exacerbate cerebral edema. If the calves are also suspected to be acidotic, this should be corrected with sodium bicarbonate solutions of appropriate tonicity.
The amount of sodium required in the first 6 hours to raise the sodium level to 125 mmol/L can be calculated as follows8:
Calves should then be maintained on a sodium-containing isotonic fluid, such as normal saline or lactated Ringer’s, and treated with oral electrolyte solution as appropriate. The sodium level should be monitored frequently in the first 24 hours because of unknown losses through the gastrointestinal tract as well as unknown kidney function in a severely dehydrated patient.
HYPERNATREMIA
Hypernatremia is defined as a serum sodium concentration over 152 mmol/L (although only levels greater than 170 mmol/L have been associated with nervous dysfunction9). Hypernatremia occurs secondary to improper mixing of oral electrolyte solutions8 or from the use of high—sodium-content milk replacer when there is limited access to fresh water; consequently it is often farm-specific. Rapid development of hypernatremia results in fluid moving from cells into the extracellular fluid and produces cellular dehydration. Neurologic signs include lethargy, weakness, depression, coma, and death. Treatment of hypernatremia involves fluid therapy with a stepwise reduction in serum sodium concentration. A gradual reduction of serum sodium is indicated, as a rapid drop in serum sodium promotes a fluid flux into the brain, exacerbating cerebral edema and resulting in death.8 Intravenous fluids are adjusted to contain concentrations of sodium approximately equal to the patient’s sodium plasma concentration.10 The goal is to reduce plasma sodium by less than 5 mEq/L/day over the first 48 hours by slow excretion through the kidneys. The volume given should be that to provide rehydration and cover maintenance and ongoing losses. The solution may include sodium bicarbonate if the calf is acidotic. Sodium should be added to any oral fluids (e.g., milk replacer) until plasma sodium levels approach normal so that the concentration is approximately equal to the intravenous fluids. Seizures may be observed if the drop in plasma sodium is too rapid. Cerebral edema may be treated with 25% solution of mannitol at 1 g/kg given intravenously (IV) over 30 minutes or an oral solution of glycerin given at 1 g/kg diluted 1:1 with water.
NEUROMUSCULAR AND MUSCULOSKELETAL DISEASE
Primary neuromuscular or musculoskeletal disease should be considered when weakness is not associated with depressed mentation. Weakness associated with micronutrient deficiencies results from myodegeneration (white muscle disease, selenium, and vitamin E) or demyelination (copper, enzootic ataxia). If weakness is detected in one or more limbs immediately after birth, peripheral nerve and muscle damage associated with birth trauma should be ruled out (see Box 20-1). Femoral nerve paralysis may be observed in calves after a “hip lock” dystocia.11 A condition resembling congenital myasthenia gravis has also been described in Brahman calves.12
Nutritional myodegeneration associated with selenium or vitamin E deficiency may produce paresis that is localized (dysphagia) or generalized. Neonatal small ruminants appear to be particularly susceptible. Affected lambs may be unable to rise. Others can stand but may be unable to nurse because they are unable to raise their heads. Diagnosis is based on clinical signs, increased serum creatinine kinase concentration, and reduced whole blood glutathione peroxidase and/or selenium concentrations. (See Chapter 42.) Vitamin E deficiency is observed when pregnant ewes are fed stored forage low in vitamin E; the clinical signs in affected lambs are identical to those of selenium deficiency, but selenium status is adequate. As vitamin E is labile, serum should be harvested quickly after blood collection, frozen, wrapped in aluminum foil, and sent via express mail on ice.
Paraplegia and tetraplegia are commonly associated with spinal cord compression. Compression of the spinal cord in neonates most commonly results from vertebral body malformations, osteomyelitis, or fractures. Generally, vertebral body malformations occur sporadically; genetic, nutritional, and environmental factors have been implicated.13,14 In older calves, underlying metabolic bone disease (copper, vitamin D, or phosphorous deficiency) may increase the propensity for fractures to occur. Osteomyelitis and vertebral body abscess may be sequelae to bacteremia after neonatal septicemia15 or pneumonia.15 The frequent isolation of Arcanobacterium (Actinomyces) pyogenes from vertebral body abscesses in ruminants suggests that chronic respiratory infections is more frequently the source in these species.16,17 Vertebral body abscesses in lambs are occasionally a sequela to infected docking wounds. Leukocytosis and hyperfibrinogenemia are commonly observed in neonates with vertebral body abscesses. In most instances vertebral abscesses do not infiltrate the pachymeninges, so the CSF either is normal or has a mild elevation of protein and/or a mild pleocytosis.15,16
Differential diagnoses for paresis in goat kids include caprine arthritis-encephalitis virus (CAEV) and enzootic ataxia. Enzootic ataxia is also common in lambs. Progressive ataxia and paresis or paralysis is a feature of both diseases. There are two forms of enzootic ataxia (swayback): the neonatal and the delayed types. In the neonatal condition animals are affected at birth; in the delayed type, signs of incoordination appear at 14 to 30 days of age.18 Most affected neonates are afebrile, bright, and alert and will continue to eat if it is physically possible. Enzootic ataxia is associated with low liver copper content and, occasionally, low serum copper concentration.19 It has been proposed that reduction in the activity of the copper-dependent enzyme cytochrome oxidase impairs phospholipid synthesis and subsequently myelin production. Microcytic anemia and increased fragility of bones may be observed in more chronic cases.20 The copper, molybdenum, and sulfur content of the maternal diet should be evaluated and adjustments made for copper deficiency or molybdenum or sulfur excess. (See Chapter 41.)
Goat kids with the neurologic form of CAEV will have mild to moderate fevers and evidence of cerebral involvement. Cerebral signs commonly identified include depression, head tilt, torticollis, and circling.21 Evidence for CAEV would include CSF pleocytosis and increased CSF protein and a positive CAEV (agar gel immunodiffusion [AGID]) test or enzyme-linked immunosorbent assay (ELISA). Both the neurologic form of CAEV and enzootic ataxia carry a poor prognosis.
A complete neurologic examination is an important component of the workup of the weak neonate. In particular, it should be noted whether the weakness is accompanied by signs of depression and diffuse cerebral disease. It should be remembered that strength is preserved if ataxia is caused by cerebellar disease. Limb reflexes should be tested to establish whether components of the spinal reflex pathways are involved in the disease process (sensory nerve, lower motor neuron, neuromuscular junction, muscle). Animals with other types of spinal cord disease (e.g., trauma, vertebral malformations, enzootic ataxia) may also show weakness and ataxia yet appear clinically to have normal cerebral function. Virtually any severe systemic disease such as generalized infection can cause both profound depression and weakness in a neonate without the presence of actual brain pathology. Intermittent signs of severe weakness and depression may be caused by the narcolepsy-cataplexy syndrome. (See Chapter 33.)
RESPIRATORY CONDITIONS
EXAMINATION AND ANCILLARY DIAGNOSTICS
Assessment of the pattern and effort of breathing is a very important part of the examination of the respiratory system. Any obvious abnormal noises associated with respiration should be noted. Inspiratory stridor is often a feature of extrathoracic airway obstruction, and increased abdominal effort on expiration often indicates pulmonary disease causing reduced lung compliance. Absence of cyanosis is not a reliable indicator of adequacy of oxygenation in the neonate, because the partial pressure of oxygen may reach very low levels (<35 to 40 mm Hg) before cyanosis is observed. Fever, cough, and nasal discharge are usually absent in the early stages of pneumonia in the neonate.
Diagnosis of most upper airway disorders can usually be made with a careful physical examination in combination with radiography and/or endoscopy (Box 20-2). An integral part of the diagnostic approach to the neonate with suspected upper airway obstruction is assessment of the lungs for aspiration pneumonia. If the primary upper respiratory problem is not corrected and normal nursing allowed, the pneumonic process will likely persist and become chronic.
Thoracic radiographs are helpful in diagnosing the presence of respiratory disease and in determining the type and extent of pulmonary involvement. Shortly after birth the smaller vessels posterior to the heart and in the caudodorsal lung fields should be clear. The heart, posterior vena cava, and aorta should be clearly defined. When the radiographic appearance of the lung fields is evaluated, the type of infiltrate (interstitial, nodular, alveolar, mixed), severity, and location (diffuse, cranioventral, caudodorsal) should be noted. Other soft-tissue structures (including the heart, vessels, and diaphragm) and bones (ribs, vertebrae, long bones) should also be evaluated. Thoracic radiographs are routinely taken in the standing or recumbent lateral position in calves. Cranioventral consolidation is a common feature of infectious pneumonia in calves. Radiographic changes may either follow or precede changes in clinical condition, and sometimes major changes can occur surprisingly rapidly. Clinical signs of pneumonia frequently resolve much earlier than chest radiographs and hemograms return to normal.
Ultrasonographic evaluation of the thorax is useful for identification of pleural effusion, pulmonary consolidation, pleuritis, and chest wall abscesses and for detecting congenital heart defects.
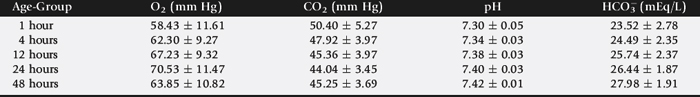
Arterial blood gas concentrations provide a measure of respiratory function. The optimal site for collection of arterial blood samples from neonatal calves is the brachial artery.22 The calf is placed in lateral recumbency, with one hand on the neck and the other pulling the upper leg caudally. The brachial artery is located on the proximomedial aspect of the elbow of the lower limb. The area over the artery is thoroughly scrubbed and the artery stabilized by placing the index and second fingers of one hand above and below the proposed site of puncture. The arterial blood sample is collected using a 25- or 27-gauge ¾-inch needle and a 3-mL syringe.22 Normal arterial blood gas values for neonates of different postnatal and gestational ages are presented in Table 20-2.
Several factors can interfere with accurate interpretation of blood gases in the neonate. First, significant inaccuracies can occur if the blood sample is collected, handled, or measured improperly. The most common artifact is the introduction of room air into the sample, with an artificially increased PaO2, decreased PaCO2, and more alkaline pH resulting. The position of the patient and amount of struggling during sample collection cay also potentially cause transient changes in all blood gas values. The inspired oxygen concentration should also be considered when analyzing arterial blood gas values. With supplemental oxygen, PaO2 is increased variably, depending on the inspired oxygen concentration (FiO2), the amount of pathology present, particularly the extent of right-to-left shunting, and the respiratory rate and tidal volume.
Common patterns of derangement include hypoxemia (PaO2 <70 mm Hg) with low or normal PaCO2 and hypoxemia with hypercapnia (PaCO2 >50 mm Hg). If there is hypercapnia and resulting respiratory acidosis, ventilation is inadequate or pulmonary pathology is severe, impairing diffusion of CO2. Hypoventilation may reflect lack of surfactant in the premature neonate, compromised muscle function (white muscle disease), or neurologic dysfunction with altered chemosensitivity resulting in inappropriate ventilatory responses to changes in blood gas values. Clinical signs must be evaluated along with blood gas analysis if the most appropriate therapy is to be chosen.
Interpretation of blood gas values of venous blood can be very deceptive and should be restricted to evaluation of metabolic conditions (e.g., metabolic acidosis) and not pulmonary gas exchange. To avoid problems associated with regional blood sampling, peripheral venous blood should be taken from a free-flowing jugular vein, because the metabolic status of the head is usually stable.
Transtracheal aspiration provides a sample for both cytologic and microbiologic analysis. (See Chapter 31 for specific details on technique and interpretation.) If the neonate is in respiratory distress, this technique can further compromise the patient. When mycoplasma or chlamydia infection is suspected, the laboratory needs to be notified, as specific media and growth conditions are required to isolate these pathogens. Viral infections are diagnosed directly by viral isolation (cell culture) or indirectly by demonstrating the presence of a virus (polymerase chain reaction [PCR] and fluorescent antibody techniques) or an immunologic response to a virus (seroconversion). Specific tests available for respiratory viral pathogens are discussed in Chapter 31.
UPPER RESPIRATORY TRACT DISORDERS
Conditions that affect pharyngeal and laryngeal function are important, as they predispose to aspiration pneumonia. Dyspneic neonates also have difficulty nursing and are subsequently likely to become malnourished. Congenital defects of the upper respiratory tract include collapsed trachea, stenotic nares, choanal atresia, and epiglottal cyst. Impaired pharyngeal and laryngeal function may result from physical deformation or neuromuscular disorders. Sporadic outbreaks of pharyngeal and laryngeal injuries are often associated with improper application or use of damaged feeding tubes and/oral medication equipment. Compression of the larynx by a retropharyngeal abscess or mass tends to cause inspiratory dyspnea. Edema and necrosis of the larynx may be observed with infectious bovine rhinotracheitis virus infections in neonatal calves.23,24 Fusobacterium necrophorum typically causes necrotic laryngitis in weaned calves but sporadically infects neonates after pharyngeal trauma.25 Partial occlusion of the upper airway induces turbulent airflow and subsequently mucosal edema. Placement of a tracheostomy tube provides an alternate, sometimes lifesaving, airway and rests the inflamed mucosa.
Nutritional myodegeneration and botulism may induce laryngeal paresis. Dysphagia and subsequent aspiration pneumonia are common sequelae of pharyngeal and laryngeal dysfunction. Collapsed trachea is a rare congenital or acquired condition. Clinical signs include an intermittent honking cough, stridor, and dyspnea with mild exercise. There is no stenosis of the trachea; rather a dynamic dorsoventral collapse during inspiration. The caudal cervical and cranial thoracic sections of the trachea in the area of the thoracic inlet are most frequently affected. Acquired tracheal collapse is commonly associated with fractured ribs and compression of the trachea at the thoracic inlet by the subsequent bony callus. Treatment of collapsed trachea in the calf by surgical reconstruction has been attempted, but the prognosis is poor.26-29
RESPIRATORY INFECTION
A number of respiratory disease syndromes may be observed in neonatal calves. Pneumonia in calves less than 3 days of age typically reflects aspiration of milk subsequent to inappropriate feeding practices or pharyngeal dysfunction (white muscle disease). A mixture of gram-positive, gram-negative, and anaerobic bacteria may be introduced into the lungs, inciting a severe inflammatory response necessitating broad-spectrum antimicrobial and antiinflammatory therapy.
Mannheimia hemolytica and Pasteurella multocida infrequently cause pneumonia in calves less than 2 weeks of age. Outbreaks of respiratory disease in this age group may be associated with mixed infections with Mycoplasma bovis or may be secondary to bovine virus diarrhea (BVD) infection. Respiratory disease is most common in calves more than 4 weeks of age, with the peak incidence observed after weaning in intensive calf rearing operations. Bovine respiratory syncytial virus, infectious bovine rhinotracheitis virus, BVD virus, Mycoplasma species infection, and bovine coronavirus may all produce respiratory disease in neonatal calves. Viral infections increase the risk of opportunistic bacterial infections by their immunosuppressive effects and damage to the respiratory epithelium and pulmonary clearance mechanisms. Pleuritis is an uncommon feature of most neonatal respiratory infections but may be a manifestation of a generalized polyserositis with specific pathogens such as mycoplasma infections of ruminant neonates30 and occasionally Pasteurella infections in lambs.31
Environmental risk factors include extremes of temperature, poor ventilation, dust, ammonia, and overcrowding. A number of pathogens capable of causing respiratory disease are shed in milk. These include Mycoplasma species,30,32,33 CAEV,34,35 and Salmonella Dublin. The practice of feeding mastitic milk (hospital milk) to neonates increases the risk of disease transmission. Rapid growth of salmonella in warm milk quickly produces a lethal challenge. Salmonella Dublin is an invasive salmonella serotype host adapted to cattle; calves commonly develop septicemia, and respiratory disease may be the predominant clinical manifestation. Mycoplasma species infection typically produces acute polyserositis; goat kids infected with Mycoplasma mycoides subsp. mycoides (large colony type) are often in pain, febrile, and reluctant to stand and have multiple hot, swollen joints. Approximately 50% of kids develop pneumonia or pleuropneumonia manifested by an increase in respiratory rate and auscultable lung sounds.30 Pasteurizing goat milk at 56° C for 1 hour kills Mycoplasma species and CAEV. Outbreaks of Mycoplasma pneumonia in calves are usually caused by feeding waste milk contaminated with M. bovis. Clinical signs include increased rate and effort of breathing associated with pneumonia, joint and tendon sheath distention reflecting polyserositis and auricular discharge, and a head tilt reflecting otitis media interna.32 Mycoplasma organisms are susceptible to antimicrobial agents that affect DNA, RNA, protein synthesis, or the integrity of the cell membrane. Mycoplasma organisms are not susceptible to agents that interfere with synthesis of folic acid or that act on the cell wall. Tylosin, tetracyclines, erythromycin, tilmicosin, florfenicol, aminoglycosides, and fluoroquinolones have been shown to have activity against one or more Mycoplasma species.36 However, the efficacy of antimicrobial therapy in eliminating the organism is limited, and although animals may recover, chronic infections may persist. Numerous antimicrobials are labeled for the treatment of respiratory disease caused by Pasteurella, Mannheimia, and Hemophilus in cattle. Treatment protocols are reviewed in Chapter 31.
CAEV produces a number of disease syndromes in goats including mastitis, arthritis, encephalitis, and pneumonia. Encephalitis and subclinical respiratory disease typically occur in kids 2 to 4 months of age and occasionally in kids as young as 1 month of age.34,37
NEONATAL APNEA AND IRREGULAR BREATHING PATTERNS
Periods of apnea in the neonate are commonly associated with nonrespiratory factors, including infection, CNS disorders, hypothermia, and metabolic conditions such as hypoglycemia. Seizure activity may be expressed by changes in breathing rate and pattern, and neonatal asphyxia may induce respiratory depression, whether or not cerebral lesions are present.38 Neonatal respiratory distress may also cause apnea resulting from respiratory center depression or diaphragmatic fatigue. There are two mechanisms of apnea: central apnea, resulting from cessation of diaphragmatic activity, and obstructive apnea, resulting from obstruction of the airway, usually at the pharyngeal level.
ABDOMINAL DISTENTION
RUMINAL BLOAT
Ruminal bloat is uncommon in calves less than 5 weeks of age because of the relatively undeveloped state of the neonatal rumen. Causes of ruminal bloat in calves include ruminal putrefaction, obstruction of the cardia or esophagus, and vagal indigestion (Box 20-3).
If milk arrives in the rumen in greater quantities than normal by escaping the esophageal groove, it can be subjected to putrefactive decomposition by proteolytic bacteria. Normally the rumen of neonatal calves has a stable aerobic bacterial population. Anaerobic conditions are rapidly established when appreciable amounts of fermentable substances enter the rumen.39 Clinical signs include diarrhea, poor development, rough haircoat, and recurrent bloat. Reducing the volume of milk fed per feeding, feeding from nipples rather than buckets, and introducing calf starter to promote ruminal development help prevent the condition. A course of oral antibiotics (500 mg oxytetracycline) once daily for 3 or 4 days may help affected calves by killing the putrefactive gut flora.
Bloat is occasionally observed as a complication of severe bronchopneumonia in calves as a consequence of swollenmediastinal lymph nodes compressing the esophagus or compression or inflammation of the vagus.40 Relief of ruminal distention is important for return of ruminal function. Chronic ruminal bloat may be relieved by placement of a ruminal fistula (Buff’s screw trocar). Correct placement of the screw, as described by Dirksen and colleagues,41 reduces the risk of inducing peritonitis. The rumen must be bloated so that it lies firmly against the body wall as the trocar is screwed into place. The site for the trocar is shaved and scrubbed, a small skin incision is made, and the trocar is quickly and forcefully screwed into the belly wall and rumen. After removal of the stylet, the outer rim of the trocar is kept under constant outward tension so that the ruminal wall is held tightly against the parietal peritoneum by the last ridge of the screw. To fix the trocar in this position, gauze soaked in antibiotic should be wrapped around the stem of the trocar between the outer rim and the body wall.41
ABOMASAL ULCERS
Abomasal ulcers are usually asymptomatic in young calves, but if perforation occurs peritonitis and shock rapidly develop. Clinical signs of abomasal ulcers in calves include abdominal distention, pain on abdominal palpation, expiratory grunt, drooling saliva, bruxism, and melena. Less commonly a syndrome of chronic abdominal pain is observed after abomasal perforation.42 Absence of inflammatory changes suggest the gut is unlikely to be perforated or necrotic. Severe hypoproteinemia is common with diffuse peritonitis presumably because of the combination of poor colostral uptake and loss of protein into the abdominal exudate. Obtaining peritoneal fluid from normal calves is difficult; if peritonitis is suspected, collection of abdominal fluid is facilitated by locating pockets of peritoneal fluid via abdominal ultrasound.
Clinically more abomasal ulcers seem to appear during or shortly after a period of weather-induced stress.43 Lilly proposes that this may be associated with higher endogenous cortisol secretion.44 Perforating abomasal ulcers in calves have also been associated with Clostridium perfringens abomasitis,45 copper deficiency, dietary changes, mycotic infections, and abomasal bezoars.
Perforated abomasal ulcers are repaired surgically by a right paracostal approach. The ulcers are commonly located on the midpart of the fundus, on the greater curvature of the abomasum. Prognosis is guarded (40%).43
ABOMASAL DISPLACEMENT
Abomasal displacement is rare in neonatal ruminants. Clinical signs include reduced appetite, poor weight gain, recurrent tympany (left side), and diarrhea. An association of left-sided abomasal displacement with pneumonia in calves suggests that altered vagal function may be involved in the pathogenesis of the condition.46,47 Typically, left-sided abomasal displacement in calves occurs between 6 and 14 weeks of age, but younger calves may be affected. Displacement of the abomasum is diagnosed by auscultation and percussion; affected animals may have a hypochloremic metabolic alkalosis. Correction can be attempted by rolling the calf on its back or via surgery.
ABOMASAL TYMPANY
Acute abdominal distention, colic, depression, and sudden death have been reported in neonatal calves with abomasal ulcers, abomasitis, and abomasal tympany. Possible sequelae to abomasal dilation include abomasal torsion, perforation, and rupture. Numerous causes have been postulated, including dietary changes, in particular the addition of coarse roughage feeds; abomasal bezoars; copper deficiency; and various microorganisms. Roeder and co-workers isolated C. perfringens type A from a group of eight calves affected by this syndrome45 and subsequently experimentally reproduced the disease by intraruminal inoculation of the organism.48Campylobacter species have been incriminated in other studies. Histopathologic evaluation of abomasums from 38 affected calves at necropsy revealed that 31 contained abundant gram-positive bacteria associated with the damaged abomasal mucosa.49Campylobacter-like organisms were demonstrated in nine and C. perfringens in 14 of the 38 cases.49 Studies of range cattle in west central Nebraska and Wyoming suggest subclinical trace mineral deficiencies of copper and/or selenium may be involved in the pathogenesis of the condition in this region.44
Onset of clinical signs is rapid; affected animals become anorectic, depressed, or occasionally restless. Signs of abdominal discomfort including treading on the spot and kicking at the abdomen are observed in approximately half of the cases. On physical examination splashing and metallic sounds are heard on succussion of the distended abdomen, and passage of a stomach tube fails to relieve the distention. Fecal output is reduced, and occasionally melena is observed. Early in the clinical course calves are likely to have a marked metabolic alkalosis; however, rapid deterioration and onset of shock are common and accompanied by metabolic acidosis. Observation of metabolic acidosis carries a poor prognosis.
Management of abomasal tympany requires rapid relief of the abomasal distention. Paracentesis through the right flank often fails to completely drain the abomasum and carries a high risk of inducing peritonitis.50 Kumper51 describes good results with paracentesis using a 14 gauge, 50-mm needle when the calf is turned upside down and the abomasum is deflated by inserting a needle in the highest point of the distended abdominal wall between the umbilicus and xiphoid. Twenty of 21 calves with abomasal tympany were successfully managed without complications using this technique. Repeated paracentesis carries a high risk of inducing peritonitis; if after paracentesis the calf’s condition deteriorates or tympany recurs, a right flank laparotomy is performed to correct a possibly torsed abomasum.51 Intravenous fluids are administered to correct dehydration, electrolyte, and metabolic derangements.
A decreased prevalence of abomasal tympany and ulceration were reported in neonatal calves from herds having a history of these problems after implementation of a C. perfringens vaccination program.44,52
Abomasal bloat is a significant problem in artificially raised lambs. Feeding systems that allow lambs to drink large quantities of milk replacer at infrequent intervals and housing lambs on litter are predisposing factors.53,54 Proliferation of lactobacilli, E. coli, and C. perfringens has been implicated in the disease process.54,55 Fermentation of sugars contained in milk replacer produces carbon dioxide, distending the abomasum.56 Lambs may die within hours from acute abdominal tympany compromising vascular return and respiration. Early treatment of bloated lambs with oral doses of antibiotics is sometimes an effective treatment. Addition of 0.1% formalin (37% formaldehyde) to milk replacer reduces the incidence of the condition.55
INTESTINAL ATRESIA
Intestinal atresia is the most common cause of abdominal distention in calves in the first week of life.42 Typically calves are born normally but develop progressive abdominal distention shortly after birth. Signs of mild colic are occasionally observed. The spiral loop of the ascending colon is usually the site of atresia.57 Other congenital abnormalities may be present (18% of cases).57 Pregnancy diagnosis by palpating the amniotic sac before 40 days of gestation may cause colonic atresia in cattle58; however, an autosomal recessive inheritance in Holstein cattle has recently been proposed.59 Surgical repair by resection of the distended proximal blind end and anastomosis of the proximal segment of intestine to the descending colon has been described, but breeding affected animals is not recommended.57 Long-term survivors are likely to have loose feces and do not tend to grow well.57
INTUSSUSCEPTION
Intussusception occurs most commonly in the jejunum, but the frequency of ileocecal and colon intussusceptions appears higher in calves than in adults.60 Commonly there is a history of diarrhea. Clinical signs may include intermittent colic, absence of feces, and melena; however, these are inconsistent. The inconsistency of clinical signs and inability to perform a rectal examination makes the diagnosis more difficult in calves than in adults.60 Abdominal ultrasound may be useful. The prognosis after surgical correction is strongly influenced by the duration of the condition before correction.
Twisting of the intestinal mass around the cranial root of the mesentery is a rare event but occurs more frequently in calves than in adults.60 Clinically the condition is characterized by a sudden onset of severe colic (kicking at the abdomen, dropping to the ground) that rapidly progresses (abdominal enlargement, tachycardia, tachypnea, reduced or absent fecal passage) to signs of shock and recumbency. Early diagnosis and rapid surgical correction, using either a right paralumbar or ventral midline, together with a supportive fluids approach allow for a good prognosis.
DIARRHEA
Herd management variables that affect the risk of neonatal death losses in housed calves include efficiency of passive transfer, calf nutrition and environmental management (pathogen exposure), calving area sanitation, and cow vaccination status and health. Successful calf rearing is based on good management. A goal of less than 5% death loss from diarrhea is achievable. With neonatal death losses in pasture or range animals it is important to evaluate the dystocia rate, physical management, and nutritional status of the calving and nursing herds; the cleanliness of the calving and nursing areas; the provision of shelter from wind; and any biosecurity risks.
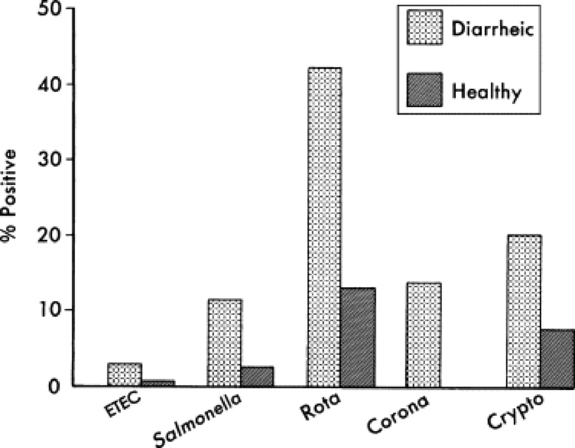
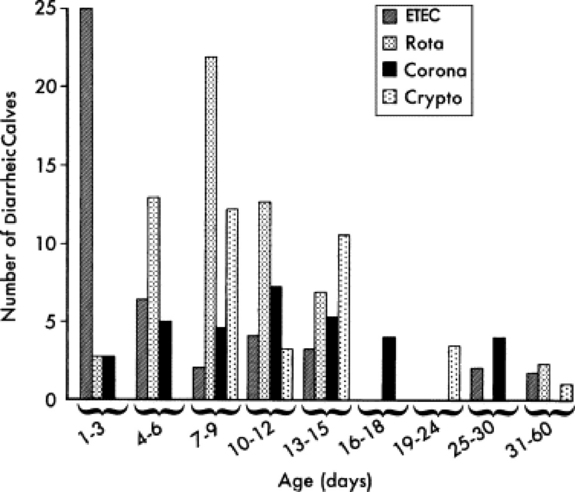
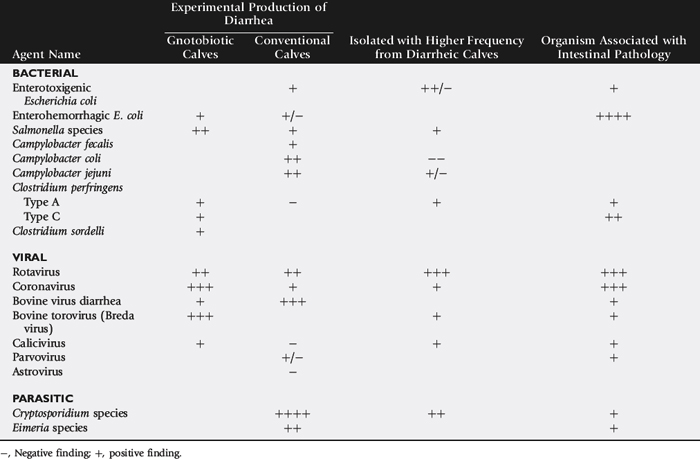
Rotavirus, Cryptosporidium, coronavirus, enterotoxigenic E. coli (ETEC), and Salmonella are recognized as the major pathogens associated with diarrhea in calves (Table 20-3). Rotavirus, Cryptosporidium, coronavirus, and ETEC are common pathogens in beef calves (Fig. 20-1).61-64 Salmonella is more frequently implicated in intensive calf-rearing systems.63-65 Enteropathogenic strains of E. coli are occasionally implicated in calf diarrhea; the true prevalence of disease associated with these strains is unknown because of the lack of routine definitive diagnostic tests. Torovirus has recently been associated with neonatal calf diarrhea in Canada, the United States, and Europe.66-69 BVD is infrequently associated with diarrhea in young calves.70,71 Various other agents have been implicated as causes of neonatal diarrhea, but their importance in the field situation is unknown (see Table 20-3). The incidence of the various etiologic agents varies with the age of the calf, and this is useful in establishing the likelihood that a particular agent is involved (Fig. 20-2). It is usually impossible to make a definitive etiologic diagnosis on clinical grounds. It is possible to detect signs of straining or passage of frank blood and mucus that suggest the presence of colitis, implicating possible Salmonella, coronavirus, BVD, enteropathogenic E. coli (EPEC), or coccidial infection.
Table 20-3 Evaluation of the Pathogenicity of Various Infectious Agents as Gauged by Their Ability to Experimentally Produce Diarrhea in Calves, Field Surveys of the Incidence of Infection in Diarrheic and Healthy Calves, and Similarity in the Distribution of Intestinal Pathology and the Infectious Agent
PATHOGENESIS
Diarrhea can be the result of either increased secretion or decreased absorption. Bacteria such as ETEC and, to some extent, Salmonella cause neonatal diarrhea by secreting enterotoxins that stimulate increased intestinal secretions.72,73 These changes are thought to be mediated by cyclic adenosine monophosphate (AMP) or cyclic guanosine monophosphate (GMP), calmodulin, and changes in protein kinase activity.74,75 The cell’s structure is not affected, but the activity of the membrane pumps is altered, and secretion of chloride, sodium, and potassium is increased.76 Sodium absorption linked to glucose and amino acid transport across the mucosal epithelium is not affected.77,78 Bovine ETEC does not stimulate intestinal bicarbonate secretion.76
Protozoa and enteric viruses cause neonatal diarrhea as a result of the destruction of the absorptive villous epithelial cells.79-82 Diarrhea results because intestinal digestive secretions continue while absorption is impaired.83,84 In rotavirus and coronavirus infections this is further exacerbated by compensatory hyperplasia of the crypt cells. The crypt cells have secretory functions, and their multiplication adds to the secretory load.83 Rotavirus can also stimulate intestinal secretion both at a cellular level and by stimulation of the enteric nervous system. This is associated with the viral nonstructural protein NSP4.85,86
Continued feeding may result in more nutrients presented to the small intestine than the damaged villi can absorb.87,88 Excess nutrients are fermented in the large intestine, promoting bacterial overgrowth74,89 and generation of organic acids and other deleterious compounds. The osmotic effect of the unabsorbed nutrients drags water into the gut and contributes to the diarrhea.83 Marked inflammation is a feature of salmonellosis and clostridiosis. This contributes to diarrhea by increasing mucosal pore size and hydraulic pressures within the intestinal wall, by destroying absorptive cells, and by increasing prostaglandin production, which in turn stimulates secretory mechanisms within the enterocytes.74,83
On an individual animal basis, diarrhea is significant because of fluid and electrolyte losses. As long as the neonate can compensate for these losses it will remain fairly bright and continue to suck. If the losses exceed intake, systemic effects of dehydration (salt and water loss) or acidosis are seen. Fluid is lost preferentially from the vascular compartment,90,91 and cardiovascular collapse results. Acidosis has several causes including fecal loss of bicarbonate, endogenous synthesis of L-lactic acid in response to dehydration and poor tissue perfusion, and D-lactic acid production through bacterial fermentation of undigested or malabsorbed milk within the gastrointestinal tract.92-97 Acidosis contributes to the calf’s malaise by increasing vascular resistance and impairing cardiac function by direct effects and by inhibiting the action of catecholamines. Esophageal groove function may be compromised in acidotic calves, promoting ruminal drinking with the consequences of further production of D-lactic acid in the rumen and subsequent ruminal acidosis.97,98
The neonate becomes depressed, loses its suck reflex, and becomes weak; if the disease progresses, recumbency and coma may develop. One cause of death is believed to be heart failure as a result of myocardial potassium imbalance caused by the combined effects of potassium losses into the gastrointestinal tract and the redistribution of potassium from the cells to extracellular fluid as a result of acidosis.99-101 Hypothermia will also contribute to cardiac failure. In cases of ETEC, Cryptosporidium, rotavirus, and coronavirus infections, correcting the fluid, electrolyte, and acid-base imbalances restores the neonate’s ability to walk and suck. A residual degree of malaise may persist, which can be attributed to inflammation within the gut wall and damage to the integrity of the mucosal barriers, allowing invasion of enteric microbes or their toxins. If malabsorption persists, cachexia can develop—particularly if milk continues to be withheld as part of therapy—and death from malnutrition or hypoglycemia may occur.
Salmonella organisms are invasive and release endotoxins in the systemic circulation. Clostridium organisms produce exotoxins. Both endotoxins and exotoxins have profound systemic effects that are often directly responsible for malaise, microcirculatory failure, and cardiovascular collapse. Correcting fluid and electrolyte disturbances in these infections will aid the neonate but will not overcome the effects of toxemia or bacteremia.
ETIOLOGY
Bacteria
ESCHERICHIA COLI
E. coli are part of the normal flora of the bovine gastrointestinal tract. Pathogenic strains of E. coli possess virulence attributes that are involved in the pathogenesis of disease. Virulence attributes include adhesins, enterotoxins, and cytotoxins. Pathogenic strains of E. coli may be shed by adult cattle with transmission to neonates by the fecal-oral route. Sick neonates amplify environmental contamination via prolific fecal shedding.
ENTEROTOXIGENIC E. COLI
ETEC possess two virulence factors: fimbriae (pili) and enterotoxins. F5 (K99) and/or F41 fimbriae mediate adherence, and thermolabile (LT) and thermostable (STa and STb) enterotoxins stimulate a secretory response by intestinal crypt cells. Although some bovine-origin ETEC produces LT, most strains that cause diarrhea in neonatal calves produce STa heat-stable enterotoxin.102 The STa enterotoxin and F5 antigen are plasmid-mediated virulence factors. Susceptibility to ETEC is age dependent according to the binding specificity of pili antigens to immature enterocytes.103 Disease is typically observed in calves less than 3 days of age; however, concurrent infection with rotavirus may extend this window to 7 to 14 days of age.104,105 Intestinal cells of calves older than 2 days of age acquire natural resistance to F5 adhesion.103 Despite this, F5-positive E. coli organisms have been isolated from healthy 4- to 12-week-old calves and F5-positive ETEC organisms are shed in feces for several weeks after experimental infection of newborn calves.106
ATTACHING AND EFFACING E. COLI AND SHIGA TOXIN–PRODUCING E. COLI
Attaching and effacing E. coli (AEEC) and Shiga toxin—producing E. coli (STEC) have been identified as causes of diarrhea and dysentery in calves.107,108 Disease is mediated by cytotoxic damage to the intestinal mucosa. Lesions may be observed in the ileum, cecum, and colon.109 AEEC (Vero or HeLa toxin—producing) induces a mucohemorrhagic colitis, with petechial or ecchymotic hemorrhages in the wall of the colon and rectum.110-112 E. coli organisms that carry this toxin often belong to O serogroups 5, 26, 111, and 118.111,113 Naturally occurring outbreaks have been reported in 2-day- to 4-week-old calves.114 The most common clinical sign is diarrhea, but dysentery, abdominal pain manifested by bruxism, and dehydration are seen in some cases.
STEC serotypes associated with dysentery in calves include O5:H−, O26:H11, O111:H−, O113:H21.115 These serotypes may produce Shiga toxins—those that are immunologically similar to the Shiga toxin produced by Shigella dysenteriae (Stx1) and those that are immunologically distinct from S. dysenteriae Shiga toxin (Stx2).116 Bovine STEC produces STx1, STx2, or both.117 AEEC, which causes disease and does not produce enterotoxins or Shiga toxin, is referred to as enteropathogenic E. coli (EPEC).
The prevalence of AEEC and STEC in calves and the incidence of disease caused by these strains are not clearly defined, as most diagnostic laboratories do not routinely screen for AEEC and STEC. In a study aimed at determining the clinical significance and prevalence of AEEC in Swiss cattle, fecal swabs of 93 cattle from two farms with calf diarrhea and of 54 cattle from two similar farms without clinical problems were screened for AEEC by PCR assay and colony-blot hybridization. On average, 21% of all cows were positive for AEEC by PCR, without differences between farms with and without diarrhea problems. By contrast, AEEC was detected by PCR in 60% of animals younger than 2 years from farms with diarrhea problems, whereas only 32% of comparable control animals from farms without clinical problems had AEEC.
SALMONELLA
There are over 2200 reported serotypes of Salmonella, yet fewer than 2% of these account for approximately 80% of the disease reported in livestock.118 In cattle, over 95% of Salmonella associated with disease is in serogroups B, C, D, and E. Salmonella induces a wide spectrum of disease in cattle of all ages ranging from inapparent subclinical infections to acute fulminant bacteremia, endotoxemia, and death. The variable manifestations of disease reflect the tissue trophisms of different Salmonella serotypes and the influence of challenge dose and host immunity. Common clinical signs associated with salmonellosis include fever, diarrhea, anorexia, depressed mentation, and dehydration. Many of the clinical signs are associated with endotoxemia. Systemic signs of endotoxemia include, fever, tachypnea, tachycardia, scleral injection, leukopenia or leukocytosis, and weakness. Some serotypes, particularly Salmonella typhimurium, have a tendency to induce severe inflammation of the bowel mucosa, resulting in dysentery and passage of fibrin and mucosal casts. Fluid, electrolyte, and protein loss may progress rapidly and become life-threatening if not corrected. With severe disease animals rapidly become emaciated because of the catabolic state induced by release of tumor necrosis factor alpha (TNF-α). Sequelae occasionally observed after invasive Salmonella infections in neonates include septic osteoarthritis and meningitis.
Immunity to Salmonella changes rapidly during the first 3 months of life. At 2 weeks of age the LD50 for some virulent strains is 105,119 at 6 to 7 weeks is 107, and at 12 to 14 weeks is 10.10,120 In contrast, administration of 1010Salmonella to 24- to 28-week-old calves failed to induce clinical signs of disease.120 The numbers cited reflect the influence of age on immunity but should not be interpreted as absolute. Different age predilections, manifestations of disease, and virulence are observed among Salmonella serotypes and among different strains of the same serotype.121,122 Although adults may serve as carriers and a source of infection of Salmonella Dublin infection in neonates, disease in adults is less common in mature cattle compared with calves. In contrast, S. typhimurium tends to manifest disease in an epidemic manner, causing illness in all age groups.
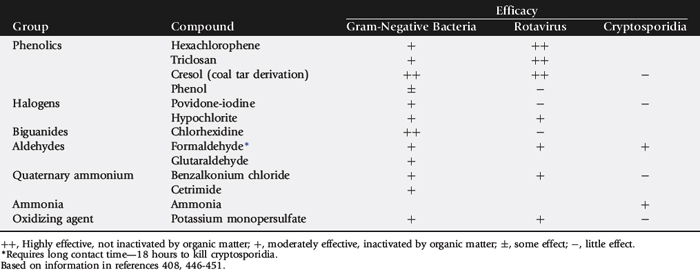
Calves on endemically infected farms are commonly exposed to Salmonella in the first few days of life.123Salmonella exposure may occur via contaminated colostrum or milk; surface contamination of teats and udder, personnel, or equipment; or the environment. Chronically infected carriers may shed 2.5 × 108Salmonella organisms in milk per day (25 kg of milk containing 105Salmonella per milliliter).124 Feeding utensils and personnel often play a significant role in transmitting Salmonella between calves.125 Salmonella infects the salivary glands and is shed in saliva and nasal secretions.126,127 Adequate cleaning and disinfection of feeding and medicating utensils is necessary to remove Salmonella contamination. Salmonella is sensitive to most disinfectants, but removal of contaminating organic debris is imperative, as the activity of disinfectants is reduced by the presence of organic matter.128
CLOSTRIDIA
Although clostridia are not commonly considered a major pathogen causing neonatal calf diarrhea, a number of reports associate clostridial infections with enteritis and abomasitis.
C. perfringens is the most important cause of clostridial enteric disease in calves. Some types of C. perfringens (mainly type A) are consistently recovered from the intestinal tracts of animals and from the environment, whereas others (types B, C, D, and E) are less common in the intestinal tracts of animals and can occasionally be found in the environment in areas where disease produced by these organisms is enzootic.129 Disease is usually precipitated by management factors that lead to the proliferation of the organism within the gastrointestinal tract or attenuated digestion of clostridial toxins within the lumen of the alimentary tract.
C. perfringens type A has been associated with acute hemorrhagic abomasitis in neonatal calves. Clinical signs include acute abdominal distention, colic, depression, and sudden death. Onset of clinical signs is rapid; affected animals become anorectic, depressed, or restless. Signs of abdominal discomfort are observed in approximately half of the cases and include treading on the spot and kicking at the abdomen. On physical examination splashing and metallic sounds are heard on succussion of the distended abdomen; passage of a stomach tube fails to relieve the distention. Fecal output is reduced, and melena may be observed. Gross pathology may include abomasal ulcers, abomasitis, and abomasal tympany.45,48 Trace mineral deficiencies of copper and/or selenium may also be involved in the pathogenesis of the condition.44 A decreased prevalence of abomasal tympany and ulceration was reported in neonatal calves from herds having a history of these problems after implementation of a C. perfringens vaccination program.44,52 Enterotoxemia caused by C. perfringens type A has been described in 2- to 4-month-old calves, with the condition observed more often in beef calves than in dairy calves.130 The disease is characterized by a high case fatality rate, sudden deaths, lesions of necrotic and hemorrhagic enteritis of the small intestine, and, most often, an absence of other clinical signs.131
C. perfringens type B is not commonly associated with neonatal diarrhea in calves. C. perfringens type C infections are most frequently observed in neonates less than 10 days of age.132 Newborn animals are typically most susceptible, perhaps because of ready colonization of the gut by C. perfringens in the absence of well-established normal intestinal flora.129 Alteration of the flora by sudden dietary changes may also be an inciting factor in type C infections. Vigorous, healthy calves develop hemorrhagic, necrotic enteritis and enterotoxemia, often accompanied by evidence of abdominal pain and neurologic signs that may include frenzied bellowing, aimless running, tetany, and opisthotonus. Death may be peracute, occasionally without other clinical signs, but may also follow a clinical course of several days.
CAMPYLOBACTER SPECIES
The clinical significance of Campylobacter species in calf scours is inconclusive. Campylobacter species are part of the normal intestinal flora. Experimental challenge studies have demonstrated the capacity of Campylobacter jejuni to cause enteritis in calves.99,133-135 However, there is a paucity of convincing reports that demonstrate a causal association in naturally occurring cases.
Viruses
Intestinal viruses multiply within enterocytes. As the epithelial cells are destroyed, villous atrophy develops. The various agents cannot be readily separated on clinical grounds. Diarrhea can vary in severity from soft to watery feces.
ROTAVIRUS
Rotaviruses are the most common cause of neonatal diarrhea in calves.136,137 Affected calves are generally 5 days to 2 weeks of age, although disease can occur at 24 hours, particularly in colostrum- deprived calves (see Fig. 20-2).138,139 This age predilection is thought to occur because many cows secrete antirotavirus antibody in their colostrum, which confers local protection against rotavirus attack until antibody levels in milk decline 48 to 72 hours postpartum.140,141 Resistance to infection is not age dependant, but age-dependant resistance to clinical disease has been demonstrated.142 Several possible mechanisms are associated involved in age dependence. Age restriction may be related to immunity, as neutralizing antibodies increase with age and virus exposure. The expression of intestinal mucins and the rate of epithelial cell replacement and fluid absorption are also age dependent and have been shown to affect rotavirus infection and disease expression.143
Rotavirus invades small intestinal villous epithelial cells; the attack is usually self-limiting because of destruction of target cells.82 Enterocytes are lost to the gut faster than they can be replaced from the crypts. The shrunken villi are initially covered by squamous and cuboidal cells from the crypts; the villi gradually regenerate as these differentiate into absorptive columnar epithelium.82,144 Intestinal secretions are increased owing to the compensatory hyperplasia of crypt cells and enterotoxigenic activity of the viral nonstructural protein NSP4.85,86 Both increased secretory load and impaired absorption resulting from villous hypoplasia contribute to the diarrhea. It is thought that virulent strains replicate more quickly and infect a larger area of epithelium. Difference in rotavirus replication rates in the gut and age-dependent differences in the rate of enterocyte loss and natural replacement rate may explain the differences in clinical outcome. Concurrent infection with ETEC has also been shown to cause clinical signs at a later age than with a single infection of either agent alone.145
Rotavirus of calves, lambs, kids, pigs, foals, mice, and children is morphologically identical. Infections are classified by the antigenic properties and/or sequence of the genes encoding the viral capsid proteins. Viral protein (VP) 6 is used to separate them into seven antigenically distinct serogroups, A through F. Rotaviruses from serogroups A, B, and C have been isolated from cattle, and serogroup A is the most common cause of diarrhea in calves. Group B rotaviruses have been isolated from calves and adult cattle; however, there is less information regarding their significance and prevalence in cattle.146-150 Group B rotavirus is more common in lambs than in calves.151 Group C rotavirus has been isolated only from adult cattle.149 The serotype and/or genotype of capsid proteins VP7 and VP4 are also used to differentiate the viruses into a number of G-types (glycoprotein) and P-types (protease sensitive protien).152 A range of both serotypic and genotypic diversity and virulence has been reported within serogroup A.142,153-155 Rotavirus is shed in the feces of infected animals, and transmission is primarily fecal-oral. Clinical signs occur 1 to 3 days after infection and last for 5 to 9 days. Virus excretion commences with the onset of clinical signs and continues for 3 to 7 days.142,156 Adult cows can be subclinically infected and intermittently shed the virus during pregnancy and especially at parturition.157-159 It is likely that this is the most common source of infection, with carrier cows infecting their calves and then these calves infecting other calves.160 Calves from carrier cows have a significantly higher risk of clinical disease, and the birth of calves from known carrier cows has been associated with the beginning of an outbreak. Recovered calves can become reinfected and shed virus.161
The environment may be an important source of infection. Rotaviruses can survive in fresh water for more than 2 weeks at 23° C and for months in water or soil <5° C.162 They are also stable in feces and effluent for up to 9 months and therefore are likely to remain in calving areas from year to year.163
CORONAVIRUS
Bovine coronavirus commonly causes diarrhea in calves 5 days to 1 month of age.63,68,164,165 Disease can occur within 24 hours in colostrum-deprived calves and has also been recorded in calves up to 5 months of age.166 Respiratory infections are common in older calves and may be important in the epizootiology of enteritis.166
Calves may be infected with coronavirus by the oral or respiratory route.156 Fecal shedding commences 3 days after infection and persists for up to a week; nasal shedding can be detected 2 days after infection and persists for 2 weeks. Once infected, calves initially excrete high levels of virus and are potent sources of contamination. Infection persists for weeks in apparently recovered calves, and these excrete low levels of virus for weeks.167 Subclinical infection is common. Disease is more common in the winter months, and coronavirus survives in the environment from year to year.
Calves may be infected by virus shed by persistently infected cows.168 Coronavirus has been detected in the feces of more than 70% of clinically normal cows.159 The rate of virus excretion increases at parturition and in the winter months.157,169 Calves born to carrier animals are at a significantly increased risk for developing diarrhea.157
All BCV isolates are believed to belong to a single serotype.169a Differences in hemagglutination-inhibition characteristics have been used to classify strains as types 1 through 3.169b
The pathology of coronavirus is often more severe than that of rotavirus, resulting in a mucohemorrhagic enterocolitis. The virus infects both the small and large intestine. In the spiral colon there is widespread destruction of the cells of the colonic ridges.79,81 Virus replication occurs in the surface epithelium, especially in the distal half of the villi, resulting in stunting and fusion of the villi. Immature cells replace epithelial cells, and in severe infection there can be areas of complete desquamation. Intestinal secretions continue, and absorption is impaired by reduced surface area. Undigested lactose accumulates in the intestinal lumen, often resulting in a secondary bacterial overgrowth, fermentation, lactate production, and an osmotic imbalance that draws fluid into the intestinal lumen. Most infections are self-limiting because the virus rarely attacks crypt epithelial cells.168 In response to infection the mitotic rate of crypt cells increases, producing immature cells that are more resistant to virus infection and that migrate up the villi to replace the damaged cells.
In experimental challenge studies, diarrhea develops 48 hours after infection. Calves are initially depressed and anorectic for the acute phase and may become dehydrated and pyrexic in a severe infection.168 Severe infections can result in death from dehydration, acidosis, shock, and cardiac failure. Respiratory signs are generally mild. Rhinitis, sneezing, and coughing may occur. Lesions may be found in the lungs, but clinical signs of pneumonia are rare, except when secondary infection occurs.
BOVINE VIRUS DIARRHEA VIRUS
BVD virus occasionally causes diarrhea and thrombocytopenia in young calves outside the confines of the persistently infected disease model.70,71 Colostral antibodies generally protect young calves from BVD infection, but disease may occur as a result of FPT or the introduction of novel BVD strains with new cattle or viral mutation in persistently infected home-grown cattle. BVD is also thought to exacerbate infections caused by other pathogens.170 It has also been implicated in necrotic enteritis, an acute enteritis of 7- to 12-week-old beef calves reported in the United Kingdom.171 Affected calves usually show oral ulcerations, particularly on the hard and soft palates. The buccal papillae are often blunted, and the tips may be ulcerated.172 Some variants of the virus produce intestinal bleeding, petechiation, ecchymosis, or prolonged bleeding from venipuncture sites secondary to thrombocytopenia.70,173-175 Hematologic findings often include leukopenia and thrombocytopenia. The disease must be differentiated from other causes of enteritis that are complicated by bovine papular stomatitis infection. Bovine papular stomatitis is common in neonatal calves. It produces oral lesions that are hyperemic and red, with a central white area of necrosis and often a raised rim of proliferating epithelial cells. These lesions often involve the mucosa around the molars. They are usually of little consequence, and their importance lies in the fact that they may be confused with BVD. One feature that helps identify BVD ulcers is that they lack the zones of epithelial proliferation seen in bovine papular stomatitis.
BOVINE TOROVIRUS (BREDA VIRUS)
Bovine torovirus has been detected worldwide176-179 and has recently been implicated as an important cause of calf diarrhea.66,67 Initially known as Breda virus, it is part of the Coronaviridae family. It has been relatively infrequently reported because it is difficult to recognize by electron microscopy and it cannot as yet be grown in cell culture, which has precluded the development of routine immunospecific diagnostic tests.66 Laboratory studies using PCR testing have implicated it as the sole pathogen isolated in 25% to 30% of fecal samples from calves with diarrhea under 6 weeks of age.66,67 It is also found in the feces and nasal secretions of asymptomatic animals,66,68 suggesting that the epizootiology is likely to be similar to that of rotavirus and coronavirus, with asymptomatic carriers acting as reservoirs of infection within a herd.157 It is mainly a disease of calves less than 3 weeks of age, with diarrhea commencing as early as 1 to 3 days after birth,178,179 but clinical signs have been observed in animals up to 10 months of age.67,180 Clinically it produces mild to moderate diarrhea in calves under both experimental and field conditions.179,181 The virus infects the small and large intestines, affecting differentiating epithelial cells in the crypts of the intestinal villi.179,180 Clinical signs develop 24 to 72 hours after experimental infection.179 It has also been isolated from the respiratory tract of cattle and associated with respiratory signs in calves at 1 month and 4 to 6 months of age.182
Protozoa
CRYPTOSPORIDIUM
Two species of Cryptosporidium have been identified in cattle: Cryptosporidium parvum in the intestine and Cryptosporidium andersoni in the abomasum.188 The two species have morphologically distinct oocysts and differ genetically.189C. andersoni is a parasite of calves postweaning and has not been associated with neonatal diarrhea.
There are several subgenotypes of C. parvum, many of which appear to be host-specific and could represent distinct species.188,190 These genotypes include type 1, which is found in human sources, and type 2, which is considered to be zoonotic and can be isolated from cattle, sheep, and goats.190 Calves generally become infected between 1 and 4 weeks of age and display clinical signs for 4 to 14 days. Animals of all ages can be infected, but diarrhea is mainly associated with calves preweaning.191 Cryptosporidial infections are asymptomatic in cattle older than 4 months of age. C. parvum mainly infects the distal small intestine, but lesions are also found in the cecum and colon and occasionally the duodenum.192 The parasite invades the superficial cells of the mucosa in the intestine but is surrounded by an invagination of the host cell membrane and remains extracytoplasmic. Parasitic invasion of the mucosa leads to epithelial destruction and mild to moderate villous atrophy, with microvillous shortening and destruction. This leads to impaired nutrient digestion and transport and a resulting malabsorption diarrhea.
Affected calves often show no sign other than diarrhea but can show depression, dehydration, and anorexia.193 Pyrexia and tenesmus have been noted.194,195 Variable levels of morbidity have been reported, and mortality is generally low.193,194,196 Other pathogens can be involved and are likely to contribute to the severity of the disease. Affected calves can take 4 to 6 weeks to recover. Cryptosporidiosis occurs less frequently in suckler calves at pasture, but when these calves are affected outbreaks were reported to be more severe than found in dairy calves, with mortality rates up to 30%.188 High mortality rates have been attributed to lack of herd immunity in seasonal calving herds in which the transmission cycle is broken. Neutralizing antibodies in colostrum and milk reduce infectivity by immobilizing the parasite, blocking invasion, inhibiting adhesion to host cells, or having direct cytotoxicity for Cryptosporidium sporozoites.197 High mortality rates have also been associated with concurrent low levels of selenium, inadequate nutrition, presence of concurrent enteric infections, and specific management practices.188
Transmission is fecal-oral, by ingestion of an encysted, sporulated oocyst. Transmission can be direct from host to host, by ingestion of contaminated food or water, and probably mechanically via flies.198 A study of oocyst shedding in experimentally infected neonatal calves demonstrated prepatent and patent periods ranging from 3 to 6 and 4 to 13 days, respectively.199 However, oocyst excretion has been described at as early as 2 days of age, which means that calves are susceptible to infection during or shortly after birth.200 The parasite is capable of autoinfection, sporulating within the intestine and immediately infecting adjacent cells. This can result in protracted clinical illness and relapses. The ability to autoinfect results in huge parasite burdens after very small infective doses. Oocyst excretion has been described at as early as 2 days of age, which means that calves are susceptible to infection during or shortly after birth.200 Calves aged 1 week to 4 months of age are most likely to be actively shedding significant numbers of oocysts, with peak shedding occurring at 1 to 3 weeks of age.191,199-201 Infected calves can shed in excess of 106 oocysts g−1 of feces.199,202C. parvum oocysts have also been isolated from adult cows, with herd prevalence ranging from 7%–100%.191,203-205 Mean shedding intensity reported for adult cows has ranged from 3 to 900 oocysts g−1 of feces.205-207 It is likely that carrier cows are a source of infection of young calves.
The most critical factor affecting environmental oocyst survival is the temperature. Drying of oocysts has been shown to dramatically reduce their viability and infectivity in mice.208,209 Oocysts can enter watercourses and ground water by direct contact with cows or from runoff of rain or irrigation water from pastures and manure storage areas.188,210Cryptosporidium oocysts have been shown to survive in water for at least 12 weeks at 4° C.211 Oocysts are resistant to chlorination of water and most disinfectants.188 They have also been shown to survive in silage.212 Wildlife may be a significant reservoir for C. parvum and may act as a method of amplification and infection in the environment.203,213,214
Cryptosporidia cause diarrhea and sometimes death in 3- to 30-day-old lambs. Protracted infections and mortality are most common in lambs infected in the first few days of life, as age resistance is seen after about 3 weeks of age.92,215-217 Cryptosporidiosis has also been described in goats; it affected 5- to 20-day-old kids, signs lasted from 3 to 7 days, relapses were not uncommon, and there was a moderate mortality rate.218 Cryptosporidia are resistant to all commonly available antimicrobial and anticoccidial agents and most disinfectants and can survive for long periods in the environment.
People working with diarrheic neonates should be warned of the risk of zoonotic disease. An outbreak of cryptosporidiosis has been described in caregivers in a veterinary hospital treating diarrheic calves. Affected people suffered from watery diarrhea, cramping, flatulence, and headache.219 One person became infected as a result of handling soiled clothing.
GIARDIA
Giardia is often found in diarrheic calves in association with other pathogens, but its relevance as a pathogen in its own right is unclear. Several authors have documented cases of diarrhea in which Giardia infection has been implicated as the causative agent either by itself or in conjunction with C. parvum and rotavirus.220-222 Affected calves are at least 2 weeks old, and often older than 1 month of age, with infection often becoming chronic and lasting for several months.200,220,223-225Giardia has a prepatent period of 7 to 8 days, and the delayed interval between birth and infection is likely to relate to high levels of colostral protection against Giardia but low protective levels in milk.226 Many calves were shown to have a poor specific immune response to the infection, accounting for the chronicity of the infection.
The significance of Giardia as a primary pathogen has been questioned by the observation of similar or lower rates of infection in calves with diarrhea compared with asymptomatic calves.200,227 Treatment of affected calves with fenbendazole reduces the duration but not the number of diarrhea episodes.222
COCCIDIOSIS
Thirteen species of Eimeria have been reported in cattle.228 Eimeria bovis and Eimeria zuernii have historically been the most common pathogenic species; however, there are increasing reports of Eimeria alabamensis causing disease.229-231 Transmission is fecal-oral. Infected animals pass unsporulated oocysts in their feces that sporulate and become infective. The sporulated oocysts are protected from the environment by a double cyst wall.232 Moist, temperate, cool conditions favor sporulation, and oocysts can survive for several years. Sporulated oocysts can resist freezing to −8° C for several months but are destroyed by high temperatures and dry conditions within a few weeks.233 Under optimal conditions sporulation can occur within a few days. The prepatent period of the two main pathogenic species is 15 to 20 days, and the patent period is approximately 11 days. E. alabamensis has a prepatent period of only 8 days and a patent period of 5 days.
Calves start shedding at about 1 month of age and shed for 3 to 4 months. E. bovis and E. zuernii schizonts first reproduce in the lower small intestine, then produce second-generation schizonts and gamonts in the cecum and colon, where they attack crypt cells.228 These latter stages induce both local and more extensive lesions.
Outbreaks of disease in calves and lambs are often related to overcrowded and confined conditions. Up to 95% of infections are subclinical, causing decreased growth rates that are often unnoticed.234 Clinical disease can be chronic or acute and is generally found in calves aged 3 weeks to 6 months, although animals 2 years of age or older may be affected. In beef cattle the most common reports of clinical disease are associated with weaning stress.235 Clinical signs may include diarrhea, ill thrift, increased susceptibility to pneumonia, tenesmus, increased mucus in feces, and hematochezia. Pyrexia, dehydration, and anemia may also be observed. The disease is usually self-limiting without reinfection. Chronic disease is often underdiagnosed.234 Calves appear weak and listless, with pasty feces, drooping eyes, and a staring coat. Fecal oocyst count is low or negligible. Disease results from continual reinfection as a result of a heavily contaminated environment.
Nutritional Diarrhea
Producers often express the opinion that scours is caused by calves consuming too much milk. However, there is no documented research in healthy calves to support this. Calves fed 16% to 20% of body weight per day or allowed ad libitum access to milk have not developed problems with diarrhea.236,237 However, in studies in which calves are also infected with enteric pathogens, the diarrhea and depression were exacerbated by feeding normal amounts of whole cow’s milk in the early stages. Villous atrophy as a result of attack by an enteropathogen reduces the ability of the calf to digest nutrients,84,88 and this predisposes to gastrointestinal overload with fermentation of milk in the large intestine. Deliberate underfeeding of healthy calves also predisposes to diarrhea.
Studies in Scotland have shown that poor clotting ability of milk is associated with diarrhea and abdominal distention in calves aged 1 to 3 weeks of age in beef suckler herds.238-240 Milk should clot within 7 minutes when incubated with rennet; the milk from the affected cows took at least 1 hour to clot and in some cases >24 hours. Diarrhea may be a result of the rapid passage of undigested milk through the bowel or secondary to infection by enteric pathogens facilitated by the conditions created in the bowel. Milks with poor clotting ability were shown to have low ultrafilterable calcium levels and low total magnesium levels.240 Calves responded to treatment with 30 mL of 1 molar solution of CaCl administered three times daily by mouth (PO) and relapsed when this treatment was stopped. The majority of the milk samples clotted when 100 μL of 1 mol/L calcium chloride solution was added before the addition of rennet. The exact cause of the impaired clotting ability was not determined. The diet of one group of affected cows was shown to be low in calcium.238,239 After a mineral mixture containing additional calcium was added to the diet of these cows, the clotting time was reduced to ≤12 minutes; treatment of the calves was stopped, and there was no recurrence of clinical symptoms.238
Calves seem to experience more problems with diarrhea on certain milk replacers. One study showed that calves performed well on milk replacers containing soy protein when healthy but that during an outbreak of salmonellosis there was better weight gain and less mortality in calves fed whole milk.241
ESTABLISHING AN ETIOLOGIC DIAGNOSIS
An etiologic diagnosis is useful in selecting specific diagnostic and preventative regimens for bacterial infections. Establishing an etiologic diagnosis for viral infections will allow establishment of specific control methods and development of an appropriate vaccination strategy. Diagnosis of salmonellosis, cryptosporidiosis, and giardiasis can have public health implications. Once an agent has been identified, one of the major problems is in interpretation of whether or not that agent is responsible for diarrhea in the individual or herd, because most agents can also be found in a percentage of normal calves (see Fig. 20-1).
Sample Collection
Appropriate selection of diagnostic specimens is required to achieve a meaningful diagnosis. Best results are obtained when fresh samples and specimens are collected from calves early in the course of disease. When possible, a fresh necropsy is informative, as it provides an opportunity to relate the presence of pathogens to a disease process. This is required to establish causality. The quality of the information gathered is to a large extent determined by the quality of the samples submitted to the diagnostic laboratory. Autolysis and bacterial invasion of gut mucosa begin within 5 minutes of death. Autolysis is a common cause of poor tissue sections for histopathology; this may reflect a prolonged postmortem interval or poor tissue preparation, handling, or transport. To avoid autolysis, formalin needs to distribute into the lumen of intestinal sections; therefore intestinal specimens should be no longer than an inch long and the tissue-to-formalin ratio should be no greater than 1 to 10.
DIAGNOSTIC TESTS
Bacterial Pathogens
ESCHERICHIA COLI
E. coli is a normal inhabitant of the gastrointestinal tract. Isolation of E. coli from fecal samples or gut contents is therefore of no significance unless the isolates are demonstrated to possess virulent attributes that are consistent with the clinical and/or pathologic presentation. Virulence attributes include adhesins, enterotoxins, and cytotoxins. ETEC adheres to enterocytes in the jejunum and ileum.242 On gross pathology, ETEC is associated with fluid-distended loops of bowel without enteritis.243 Calves infected with ETEC have a mild inflammatory reaction in the small intestinal wall and some villous atrophy. In fresh specimens, sheets of gram-negative bacilli can be seen adhering to the small intestinal wall.242 Definitive diagnosis of enterotoxigenicity rests on demonstration of the ability of the E. coli to dilate intestinal loops.244 ETEC can also be identified by the presence of F5 (K99) using antigen-specific immunoassays including latex agglutination,245 ELISA,246 fluorescent antibody,247 slide agglutination,247 and rapid dipstick tests. A potential limitation of immunoassays is the specificity of the antibodies used; strains of ETEC using non-F5 fimbriae will not be detected by these tests.108,248
AEEC and STEC mediate disease by cytotoxic damage to the intestinal mucosa. Diagnosis of E. coli infection may be achieved using phenotypic differentiation of pathogenic strains from nonpathogenic normal flora E. coli via bioassays or immunoassays for toxins and fimbriae. Immunoassays have been developed to identify the presence of Stx1 and Stx2 in feces as a presumptive test for the detection of STEC in cattle feces.249-251 An alternative approach to identify and differentiate ETEC, AEEC, and STEC is to use PCR to identify virulence-associated genes commonly found in these E. coli strains (F5, F41, enterotoxin, intimin, Stx1, and Stx2).117 The significance of STEC, EPEC, and AEEC in bovine enteritis is unknown because of a lack of appropriate assays for routine detection and because of the widespread presence of verotoxin-producing E. coli strains in healthy cattle that complicate the interpretation of detecting fecal shedding in sick animals.252-254 Demonstration of verotoxin in cultures from bovine enteritis is not sufficient to imply a causative association.
CLOSTRIDIUM SPECIES
C. perfringens has been associated with enterotoxemia and hemorrhagic abomasitis in calves.129,131C. perfringens organisms are normal flora of the gastrointestinal tract; therefore isolation of C. perfringens from feces is not in itself diagnostic. Pathogenic strains of C. perfringens produce exotoxins; five of these (alpha-,beta-,epsilon-, and iota-toxins and enterotoxin) are involved in the pathogenesis of disease.129 The complete pathogenesis of enterotoxemia and abomasitis has yet to be completely elucidated. Production of specific toxins can be demonstrated only in a proportion of cases.255 Isolation of toxin-positive C. perfringens from intestinal contents does not confirm a clinical diagnosis of bovine enterotoxemia, because almost as many C. perfringens isolates from normal calves produce toxin and because toxin production cannot be demonstrated in as many as 40% of affected calves.256
A fresh necropsy is required to definitively diagnose clostridial enteritis. Observing many gram-positive bacilli in the mucosa associated with hemorrhagic enteritis is suggestive of clostridial enterotoxemia. Quantitative bacterial counts of intestinal contents at the site of the lesion have proven to be one of the most reliable methods for diagnosing enterotoxemia.131 A C. perfringens count greater than 106/mL of intestinal contents is consistent with a diagnosis of enterotoxemia.131 Demonstrating the presence of C. perfringens toxins or the capacity to produce toxins provides support for the diagnosis. Tests for detecting toxins or the bacteria’s capacity to produce toxins include bioassays, immunoassays, western blot, and PCR assay.257 The basis of the bioassay is to demonstrate protection of mice using antitoxin. C. perfringens enterotoxin is produced during sporulation. In vitro detection of enterotoxin-production capacity of a C. Perfringens isolate using western blot or immunoassays requires sporulation to occur. In vitro techniques to induce sporulation are not 100% efficient, so detection of enterotoxin using these methods is less sensitive than PCR is at detecting the genes required to produce enterotoxin.258
SALMONELLA SPECIES
Salmonellae are capable of causing disease in cattle of all ages. Neonatal infections are common. The classic pathologic lesion is fibrinous or fibrinonecrotic to ulcerative enteritis.259 The severity of lesions is usually greatest in the distal small intestine and proximal large bowel. Hypertrophy of the mesenteric lymph nodes is a common finding.260 Serosal hemorrhages may be observed in the small and large intestines. Septic infarcts in the kidneys and inflammation of the gall bladder are less common findings. Pneumonia is a common finding with Salmonella Dublin infections, and gangrenous necrosis of distal extremities may also be observed.261 Bacteremia is a feature of neonatal salmonellosis and may manifest as osteomyelitis and/or meningitis.
Isolation of Salmonella from feces of calves with diarrhea is consistent with a diagnosis of salmonellosis but in itself does not necessarily establish causality, as Salmonella may be isolated from the feces of apparently healthy calves.262 Isolation of Salmonella from tissues at necropsy is indicative of invasive salmonellosis. A definitive diagnosis of salmonellosis is based on the clinical presentation, pathologic lesions, and isolation of Salmonella from tissues at necropsy.
There are numerous methods for isolating and detecting the presence of Salmonella. These include direct culture, enrichment cultures, PCR, immunoseparation, and immunoassays.
The process of directly inoculating tissues or other samples onto plating media, except in the case of acute infections, is usually nonproductive. Typically, with subclinical infection the number of salmonellae shed in feces is low relative to the high number of other bacteria. Fecal samples should be inoculated into selective-enrichment media for optimal recovery of Salmonella. Selective-enrichment broths are formulated to selectively inhibit other bacteria while allowing Salmonella to multiply to levels that may be detected after plating. Internal organs that are normally sterile do not need to be inoculated onto selective media; rather, they should be inoculated onto nonselective (blood agar) or weakly selective (MacConkey agar) media.
Rapid detection methods have been developed to expedite the detection of salmonella. These methods include electrical conductance and impedance, immunologic techniques, nucleic-acid—based assays, and PCR assay. These methods generally take 24 to 52 hours to screen for or detect and identify salmonella organisms. Most of these tests, and particularly the enzyme-linked immunologic techniques, require 105 cells per milliliter for reliable results. Accordingly all these tests involve a preenrichment stage, and some also involve a selective-enrichment culture.263 When Salmonella is causing disease, clinically affected calves may shed 109 Salmonella organisms per gram of feces.264 Detection of Salmonella in clinical samples when it is the inciting cause of the disease process is not normally difficult when multiple samples are collected from a representative sample of the affected population.
Viral Enteropathogens
Viruses are usually identified by direct examination of the feces, immunoassays, or fluorescent antibody examination of intestinal mucosa. Molecular techniques involving PCR and reverse transcriptase—PCR (RT-PCR) have been described for most pathogens but are not routinely available in all diagnostic laboratories. Electron microscopic examination of feces is not a sensitive means of detecting virus particles, but it has the advantage that many different types of viruses can be detected, including those such as parvovirus that are not recognized as common causes of diarrhea. The recent development of relatively inexpensive immunoassay diagnostic test kits makes these an attractive option; limited test-specific data regarding test sensitivity and specificity limit the application of some of these tests.
CORONAVIRUS
Coronavirus replication occurs in the epithelial cells of the distal half of the villi of the lower small intestine and colon. Infected cells die, slough, and are replaced by immature cells. In the small intestine these changes result in stunting and fusion of adjacent villi, and in the large intestine they lead to atrophy of the colonic ridges. On histopathology the tall columnar epithelial cells are replaced by cuboidal and squamous epithelial cells, and in severe infections there may be areas of complete desquamation.265 Virus is shed in respiratory secretions and feces. There are several methods for detecting bovine coronavirus virus in feces. These include isolation of the virus in cell culture,266 electron microscopy,267 immunoelectron microscopy,166 immunoassays159,246,268-271 and molecular techniques including dot blot hybridization assays272 and RT-PCR assay.273,274 Isolation of bovine coronavirus using cell culture techniques is not often performed in diagnostic laboratories, as the technique is difficult and requires viable virus (fresh samples or shipped on dry ice).275 Electron microscopy has been used as a standard diagnostic method for bovine coronavirus. Although the intact virion of bovine coronavirus is fairly characteristic in appearance, it is not uncommon for the identifying surface projections of the virus to be lost during sample preparation or storage, making it difficult to properly identify virus particles by electron microscopy.
Numerous ELISA assays have been described for the detection of BCV antigen in feces. Several companies have developed commercial kits using this technology. The use of monoclonal antibodies rather than polyclonal antibodies is reported to increase the sensitivity and specificity of bovine coronavirus ELISAs.271 The limit of detection for ELISA assays ranges from 104 to 107 virions per milliliter of feces.
A one-step RT-PCR assay, targeting a 730-bp fragment of the nucleocapsid gene of bovine coronavirus, and a nested PCR assay, targeting a 407-bp fragment of the nucleocapsid gene have been developed to detect bovine coronavirus. Compared with an antigen capture ELISA, the limit of detection for the RT-PCR and nested PCR assays was 105 virions/mL and 103 virions/mL, respectively, compared with 107 virions/mL for the ELISA.276,277
ROTAVIRUS
Bovine rotavirus infects enterocytes of the intestinal villus. Infected cells are predominantly in the distal third to half of the villus. The age at the time of infection influences the distribution of the virus in the gastrointestinal tract and the number of virions shed in feces. In experimental challenge studies, infection of day-old calves resulted in a uniform distribution of virus throughout the small intestine.278 Challenge of 10-day-old calves led to a patchy distribution of the virus with maximal viral load observed in the mid small intestine.278 Villous stunting is more pronounced in young calves.
Methods for detection of rotavirus include cell culture, fluorescent antibody staining, electron microscopy, immunoelectron microscopy, immunoassays, electrophoretic procedures, and RT-PCR assay.146-150,245,246,269,279-281 Bovine rotavirus is difficult to isolate in cell cultures because of the cytotoxic nature of feces and fecal filtrates and because the virus is inconsistent in production of cytopathic effects.280 The fluorescent antibody technique is simple, rapid, and specific; however, rotaviral antigen is usually difficult to detect within 24 to 72 hours after the onset of diarrhea because rotavirus-infected epithelial cells are rapidly shed from the tips of the villi.282 Comparative studies evaluating methods of detecting rotavirus in feces show comparable test results between antigen capture assays (ELISA, latex agglutination) and electron microscopy.245,269,280,283 Direct immunofluorescence testing of fecal samples corresponds well (90%) with electron microscopic examination for rotaviruses when samples are collected during the 24 hours after the onset of diarrhea,284 but have poor agreement (33%) for field specimens submitted to a diagnostic laboratory.280
BOVINE VIRUS DIARRHEA
BVD virus rarely causes diarrhea in neonatal calves.71 Sporadic disease may be observed in persistently infected calves. Pathologic lesions include ulceration of the oral cavity, particularly on the hard and soft palates, and blunting of the buccal papillae.172 Erosions may be observed in the esophagus, and necrosis of Peyer’s patches may be observed in the ileum. Thrombocytopenia has been observed with BVD type II infections. Outbreaks of neonatal disease have been observed with this strain. Petechial and ecchymotic hemorrhages are a feature of this condition.70,174,175 Hematologic findings often include leukopenia and thrombocytopenia.
Several options are available for the detection of BVD; these include virus isolation,285,286 RT-PCR assay,287 immunohistochemistry,288 and antigen capture ELISA.289 Assays; the latter two are used in most commercial laboratories. Maternal antibodies reduce the sensitivity of the ELISA assay in young calves.287
BOVINE TOROVIRUS (BREDA VIRUS)
Bovine torovirus produces cytolytic infections of villi and crypt enterocytes in the small and large intestine.290 Bovine torovirus does not grow in tissue culture, cell culture, or embryonated eggs.291 Therefore the large-scale preparation of reference antisera and antigens for the development of diagnostic tests has been precluded. Torovirus is capable of causing diarrhea in cattle, with disease observed most frequently in calves less than 3 weeks of age.68,179,181,292-294 Like other enteric viruses, bovine torovirus has been detected in feces of normal calves; therefore detection of the virus in feces from diarrheic cattle cannot be interpreted as causal. The lack of diagnostic reagents has limited the study of bovine torovirus, leaving questions about its epidemiology and relative importance in calf diarrhea.67 Diagnostic methods that have been used to detect bovine torovirus include electron microscopy, immunofluorescence, antigen capture ELISA, and RT-PCR assay.67,179
Protozoa
EIMERIA SPECIES
Eimeria species are host-specific. E. bovis affects primarily the mucosa of the cecum and the proximal part of the large intestine, whereas E. zuernii affects the mucosa of the cecum as well as the entire large intestine, including sometimes the rectum.295 The clinical signs of bovine coccidiosis are associated with the final stages of the eimerian life-cycle and commence shortly before oocyst shedding. Gross lesions in the cecum and large intestine range from having semiliquid contents, little or no blood, and few areas of epithelial sloughing to having extensive hemorrhage and large areas of epithelial sloughing and necrosis of the mucosa.295 The serosal surface is often reddened opposite the affected mucosal area, and the submucosa and external muscular layers are often thickened by edema.
Oocysts usually can be recovered 2 to 4 days after the onset of diarrhea.296 Oocysts can be identified microscopically by direct smear, flotation, or centrifugation methods. The oocysts of E. alabamensis are smaller and less distinctive than oocysts of other coccidian but are approximately 4 times larger than cryptosporidia. Oocyst counts of 5000 or more per gram of feces are considered significant in cattle.232 Identification of oocysts in feces is not diagnostic for clinical coccidiosis, because the parasite is frequently detected in small numbers in the feces of healthy cattle.297 When scour problems are being investigated, multiple samples should be collected for oocyst counts to provide an indication as to the level of infection within the group. The potential for discord between clinical signs and fecal shedding limits the diagnostic utility of a single sample from an individual animal.
GIARDIA
Giardia infection is not associated with changes in intestinal villous height or crypt depth. However, transmission electron microscopy has been used to demonstrate a reduction in microvillous surface area.298 Diagnostic methods for detection of Giardia include direct microscopy, immunomagnetic separation, fluorescent antibody staining,221,299 ELISA,300 and PCR assay.301 When direct microscopy is used, fecal samples should be examined within 24 hours of collection. Concentration of trophozoites and cysts via density gradient centrifugation or filtration followed by fluorescent antibody staining is the diagnostic method used in most veterinary epidemiologic studies of Giardia in calves.200,302,303 Other immunoassays and PCR techniques are emerging in human diagnostic laboratories.300,301
CRYPTOSPORIDIA
C. parvum infections are mainly concentrated in the distal small intestine, but lesions may also be found in the cecum and colon, and occasionally in the duodenum.304 The pathologic findings associated with Cryptosporidium are a mild to moderate villous atrophy, villous fusion, and changes in the surface epithelium with infiltration of mononuclear cells and neutrophils in the lamina propria.304
Calves infected with C. parvum usually develop diarrhea in 72 to 96 hours; diarrhea is observed for 8 to 23 days,305 during which oocysts are excreted in feces. Oocysts are stable in feces for many days at room temperature.306 Laboratory methods for the diagnosis of cryptosporidial infections include microscopic examination of fecal smears or fecal preparations, immunoassays, and PCR assay. Cryptosporidia oocysts are small (4 to 6 μm in diameter) and are easily missed on a fecal smear. Because fecal smears do not concentrate the oocysts, this technique is less sensitive than fecal flotation. Concentration of the protozoa is achieved by salt307 or sugar flotation. Special stains may be used to facilitate detection of cryptosporidia during microscopic examination. Differential staining techniques are useful to distinguish Cryptosporidium oocysts from other fecal components (especially some yeasts) of similar size and shape.308-311
A number of immunoassays have been developed for the detection of cryptosporidia. The detection thresholds of the different methods have been reported to be 3 × 105 oocysts/g for a monoclonal antibody—based antigen capture ELISA, compared with 1 × 106 oocysts/g detected by examination of acid-fast stained fecal smears and 1 × 103 oocysts/g detected by indirect immunofluorescence.312 The detection threshold may be further enhanced by using a combination of immunomagnetic separation coupled with immunofluorescent microscopy. With this combination it is possible to detect as few as 10 oocysts/g.313 Several dipstick immunoassays have also been developed. The detection threshold for this technology is reported to be 1 × 103 oocysts/g.314 This technology offers the potential for rapid, cost effective detection of cryptosporidia in fecal specimens.
Molecular techniques have been described for detection and typing of cryptosporidia.190,315 The capacity to differentiate the different genotypes makes this approach useful for epidemiologic studies of cryptosporidia.315
RISK FACTORS FOR NEONATAL CALF DIARRHEA
It is important to identify risk factors, both to set up effective preventive programs and to initiate control in the face of a disease outbreak. The cause of calf diarrhea is multifactorial; consequently it is common for several factors to contribute to the outbreak and perpetuation of disease in a herd.
Dystocia
Dystocia is associated with neonatal calf diarrhea in more intensive beef and dairy systems62,316 and is a risk for preweaning mortality, with over 40% of preweaning deaths occurring in calves born to cows with dystocia.317-319 Dystocia affects the ability of the calf to suckle colostrum, resulting in decreased serum IgG levels; consequently calves that survive dystocia are two to four times more likely to become sick in the first 45 days of life.320-322 Stocking density of preparturient cows, timing of calving, breed, and cow grouping all influence the risk of dystocia.323 Calves that experience dystocia are likely to have edema of the head and tongue, making suckling difficult. They are weak, exhausted, and likely to be recumbent for longer, increasing exposure to fecal pathogens.164 Low- and high-birthweight calves are at greater risk of mortality.318 Small calves experience greatest mortality at parities greater than one, and large calves at first parity.324 There is no direct effect of preparturient nutrition on the subsequent incidence of neonatal calf diarrhea. Increased feed intake precalving will increase calf birthweight but does not increase the risk of dystocia unless cattle become obese.325-327 Weight loss is associated with prolonged labor, increased dystocia, and increased perinatal mortality.327,328
Dam Parity
Calves born to first- and second-parity cows have increased mortality compared with those born to older cows, and the risk of diarrhea in calves born to heifers is 3.9 times greater than in those born to cows.318,329,330 Heifers have an increased risk of dystocia, lower colostrum quality, and inferior mothering ability.320,321,330-332 The stocking density of heifers is often increased before calving to facilitate observation, with the consequence of exposing their calves to a greater environmental pathogen load. These factors are all likely to contribute to increased morbidity, and consequently the percentage of heifers in the herd will affect the risk of diarrhea. Calves from carrier heifers shedding rotavirus and bovine coronavirus are more likely to develop clinical disease than calves born to carrier cows.157 Studies in dairy herds have shown that there is a positive correlation between the number of young stock in the herd, the overall herd size, and herd production with the risk of neonatal calf diarrhea.333-335
Colostrum Management
Many studies have shown that FPT results in increased risk of neonatal calf diarrhea in beef and dairy herds.336-342 Calves are able to absorb immunoglobulins only for a limited time after birth, and the subsequent serum Ig concentration is determined by the perinatal state of the calf, timing of colostrum ingestion, and the mass of immunoglobulin consumed.343 Colostrum also provides local (enteric) immunity, with the major benefits lasting for approximately 3 to 4 days after birth.344-346 After this period, milk contains little immunoglobulin and most colostral antibody has been cleared from the intestine. Colostral antibodies protect against rotaviral infections in the first 4 days of life140,141; in contrast, anti-F5 (K99) E. coli antibody is present in low amounts in unvaccinated cows,347 and enteric E. coli infections are usually seen in very young calves. After 4 days the protective effects of colostrum are primarily a result of systemic antibodies, and there is evidence that these can leak back into the gut and probably give limited long-term protection against diarrhea.348
Colostral quality is affected by colostral volume, genetics, nutrition, parity, and climate. Beef breeds have been shown to have a significantly higher IgG concentration than dairy breeds, and this was attributed to differences in the onset of lactogenesis and colostral volume349; however, studies comparing colostral quality between beef breeds or between dairy breeds have not shown a consistent result.320,321,332,350-355 Beef cows have a significantly smaller prepartum decline in serum IgG compared with dairy cows, but the resulting colostral IgG concentration is higher because of the significantly lower volume produced. However, the combination of low volumes and increased turbidity may limit the calf’s ability to take in sufficient immunoglobulin, especially if the calf is weak. Recent studies in beef cattle have shown an increased incidence of FPT in specific genotypes, with different haplotypes determining receptors for neonatal Ig absorption.356,357 This would indicate that FPT is more prevalent in specific lines of cattle rather than in breeds per se. First- and second-calving beef and dairy cows have a lower colostral immunoglobulin concentration than cows of third parity and above.352,354,358-360 This is reflected in significantly lower mean serum IgG concentration found in calves born to beef heifers and second-parity cows.320,321,330-332
The biggest effect of nutrition on colostral quality is its effect on colostral volume, with increasing volume resulting in dilution of immunoglobulins.359 Severe nutritional restriction has no effect on IgG levels other than an increased concentration associated with a decrease in colostral volume.321,361-365 Similarly, nonlactation period length appears to have little effect, although a prolonged dry period may result in increased volume in mature cows.355,359,366
Passive transfer is also influenced by climate, with both hot and cold extremes leading to decreased immunoglobulin concentration, intake by the calf, and immunoglobulin absorption.339,355,367-371
Beef Cow Herd Management
In beef herds, high stocking rates in the calving area and the use of one calving area are major risks for neonatal calf diarrhea.372,373 The practice of leaving nursing dams and calves with calving cows further increases stocking rates and promotes disease transmission.338 The weather at calving affects both pathogen survival and calf comfort; shelter in the nursing area is associated with decreased mortality resulting from neonatal calf diarrhea.329 Major calf scour pathogens can survive in the environment for months or years in cool, wet conditions, and consequently both the incidence and the mortality from diarrhea increase with prolonged use of the same paddock or a longer calving season, and the incidence of diarrhea increases as the calving season progresses.319,329,330 Adverse weather conditions cause cows to move to shelter and shade, concentrating cows and calves in small contaminated areas. Calves born into a contaminated environment potentially become infected during or shortly after birth and shed enteropathogens even when they remain clinically normal. This further increases the environmental load of infectious agents, infecting adult cattle as well as calves. The outcome of host-pathogen interactions is largely influenced by the pathogen challenge dose and the age of the animal, with clinical disease becoming more common both in younger neonates exposed to higher pathogen numbers (Fig. 20-3).

Fig. 20-3 Cow on a farm with a high incidence of neonatal diarrhea. Note that the mud is so deep around the feeders that it covers the cow’s hocks. As a result the teats will be contaminated by manure and enteric pathogens.
Farms that purchase replacement calves aged less than 4 weeks have an increased mortality from neonatal calf diarrhea.329 Purchased calves may introduce new pathogens, challenging a susceptible population. Stress from transport and arrival at a new location may increase shedding and predispose to clinical disease, increasing the environmentalpathogen load. The risk of pathogen introduction increases when introduced calves from multiple properties are commingled before introduction.
Intensive Calf-Rearing Systems
Intensively reared calves have an increased risk of diarrhea associated with housing, nutrition, and weaning. Variables that have been observed to increase the risk of scours include feeding milk once versus twice a day within 14 days of birth,374 placing preweaned heifers in groups of seven or more, using damp versus dry bedding,374 a male having primary responsibility for the care and feeding of preweaned heifers, calves not receiving hay or other roughages until >20 days old, and feeding mastitic or antibiotic milk versus whole milk.375 Use of individual calf hutches has been reported to increase the risk of scours but to reduce mortality.374
Factors significantly associated with a decreased risk of cryptosporidial infection included ventilation in calf rearing areas, daily addition of bedding, feeding of milk replacer, daily disposal and cleaning of bedding, and use of antibiotics. Postweaning practices that reduced risk of infection included moving animals after weaning, cleaning soiled bedding, and using antibiotics and ionophores as preventive measures. Maternity management factors that reduced risk of infection included using fresh colostrum to feed calves and having a concrete floor in the calving area. General management factors that influenced risk included the total number of dairy cattle, the total number of other species of agricultural animals on the farm, and the distance of the barn water source from the septic system.376 It is likely that many of these factors in all categories will also increase the risk of infection with other enteric pathogens.
HERD STRATEGIES TO PREVENT NEONATAL DIARRHEA
Management practices that reduce the risk of calf scours also promote good health, improve growth rates, and reduce the risk of transmitting other enteric pathogens such as Mycobacterium paratuberculosis. The principles of prevention are as follows:
Minimizing Pathogen Exposure
Enteric pathogens may be shed in large numbers by calving cows, scouring calves, and asymptomatic cohorts, especially those up to 4 months of age. All enteric pathogens can survive in the environment for months or years in moist damp conditions. Other sources of infection include people who have treated or handled infected calves; contaminated water; contaminated colostrum, milk, or solid feed; and equipment that has been used to feed or medicate infected calves.*
MINIMIZING PATHOGEN EXPOSURE IN DAIRY HERDS
Strategies to prevent disease in dairy calves focus on calving cows in a clean environment, removing calves from cows at birth, feeding adequate good-quality colostrum, placing calves in a clean, dry environment separate from other stock, feeding good-quality milk or milk replacer, providing adequate shelter, and providing access to water and high-quality calf pellets. Microbial contamination is an important determinant in the quality of colostrum and milk. Good sanitary practices are required for the harvest, storage, and feeding of both products. Microbial contamination of colostrum compromises passive transfer and in the case of pathogens such as Salmonella may lead to a direct pathogen challenge. Similarly, feeding milk with a high bacteria count increases the risk of diarrhea.
In dairy systems, where calves are reared by hand, the number of young stock on the farm and the incidence of respiratory disease are positive predictors for calf diarrhea.333 Cleanliness of the calving area is important; bedding should be changed between each calving, and large numbers of cows should not be cycled through a few stalls.333 Before calving the udder and the perineum of the cow should be cleaned. The calf should receive adequate colostrum.373 In recent years the use of calf hutches has gained widespread acceptance for managing calves after they have been separated from their dam. This system provides individual isolated housing for each calf. Cleaning is facilitated because the hutches can be moved to new sites between calves. Keeping preweaned calves in groups larger than six puts them at increased risk for diarrhea.335
Cleaning and disinfection after each calf batch plays an important role in reducing contamination in housed calves. The key to decontamination is the physical cleaning. Physical removal of organic contamination through scrubbing is preferred to application of high-pressure sprays, which can aerosolize organisms, allowing dissemination. On smooth, ideal surfaces physical removal of visible contamination by thorough washing with soap and water removes 99% of the microbial load (two logs). However, on typical housing surfaces washing removes only 90% (one log). Application of disinfectant after washing is important to eliminate remaining pathogens and to prevent bacterial pathogens from proliferating. Physical cleaning cannot be replaced by applying disinfectants in larger quantities, as organic material neutralizes most disinfectants. Disinfectant solutions are applied following cleaning. With regard to disinfectants, pathogen elimination is time dependent.380 Other important variables that influence the effectiveness of disinfectants and rate of pathogen reduction include concentration, temperature, pH, and water hardness.
In addition to cleaning between batches, it is important to clean nipple buckets and other feeding utensils between each feed. Separate equipment should be used to administer oral electrolytes and colostrum. Salmonella and coronavirus are shed in saliva and can contaminate equipment used for oral medication. Washing with warm soapy water is required to remove the fat residue left by milk and colostrum handling equipment.
Several microbial characteristics should be considered when disinfecting equipment that comes into contact with calves. Rotavirus is susceptible to sodium hypochlorite but is relatively resistant to many common disinfectants, such as chlorhexidine. Because as a nonenveloped virus it is not affected by soaps, washing with soap alone may actually spread the virus around on the washed surface.381 Coronavirus is an enveloped single-stranded RNA virus and is not as stable in the environment as rotavirus. Because of their envelope, these viruses retain infectiousness better at lower than at higher relative humidity382 and are considerably more sensitive to soaps and common disinfectants than are nonenveloped viruses. Cryptosporidium can autoinfect the original host; consequently, the infectious dose can be exceedingly small. In the environment, cryptosporidia are extremely resistant to most veterinary disinfectants except 5% ammonia, 6% hydrogen peroxide, or 10% formalin.208,383,384 They survive very well in water, requiring 4 to 11 weeks to decline by one log.385 On the other hand, cryptosporidia are susceptible to drying, with oocyst infectivity declining in 1 to 4 days.209
The most readily cleaned surfaces are made of smooth impervious materials such as plastic and varnished wood (Table 20-4). Usually buildings are cleaned and then either disinfected or fumigated.386 Many disinfectants are inactivated by organic matter; viruses, coccidia, and particularly cryptosporidia may be resistant to their action (Table 20-5). Disinfectants may also be toxic and are best applied by personnel wearing rubber gloves and respirators (if indoors). In general, potent phenolics such as cresol (cresylic acid) are very useful for disinfecting dirty surfaces because they are not inactivated by organic matter and are effective against gram-negative organisms and viruses. The phenolics are highly toxic and leave lingering odors. Hypochlorite solutions (5 g of available chlorine per liter) have a broad spectrum of action but are rapidly inactivated by organic matter. They would be useful as a final disinfectant on previously cleaned surfaces. Because hypochlorite is unstable, it is unlikely to leave toxic residues. Iodophors are not very effective against rotavirus, particularly if organic matter is present. Virkon, a newer disinfectant and cleaner containingpotassium monopersulfate as the active ingredient, is effective for all pathogens except cryptosporidia. Normally a 1% solution is used and is prepared by mixing 10 g of powder with 1 L of water. Contact time should be a minimum of 10 minutes. Virkon has the advantage of having a detergent action that facilitates cleaning.
Table 20-4 Ability of Bacteria to Persist on Various Types of Surfaces Found in Farm Buildings
| Total Bacterial Count per 100 cm2 | ||
|---|---|---|
| Material | Uncleaned | Cleaned |
| Brick | 76,000 | |
| Painted wood | 34,000 | |
| Block board | 116,000 | |
| Ply board | 77,000 | 23,000 |
| Fiber board | 57,000 | |
| Chip board | 35,000 | |
| Formica | 29,000 | |
| Polystyrene | 29,000 | |
| Metal | 14,000 | |
| Concrete | 13,000 | |
| Plastic | 16,000 | 100 |
| Varnished wood | 5000 | |
Modified from Morgan-Jones SC: In Collins E, et al: Disinfectants: their use and evaluation of effectiveness, London, 1981, Academic, p 199.
Formaldehyde is one of the few agents that is effective against cryptosporidia. It requires a long contact time and is highly toxic. It is usually used for terminal fumigation in buildings that can be tightly sealed. Formaldehyde gas is best generated by heating paraformaldehyde (5 g/cubic meter of building) in an electrically heated pan at 204° C. Some manufacturers of paraformaldehyde provide pans specially designed for this purpose. The pans should be placed no more than 30 m apart and arranged so that electricity to the pan can be controlled from outside the building. There must be a safety mechanism to ensure that the pan does not overheat and cause a fire. The building must be sealed for at least 24 hours and cannot be entered until it has been thoroughly ventilated. Formaldehyde gas can also be generated by boiling formalin or by adding potassium permanganate to formalin. The latter method generates a violent chemical reaction and carries risks of explosion. Formalin aerosol generators are ineffective.387 After cleaning and disinfection one should allow a rest period for the building to ventilate before reintroducing calves. It is very important that cleaning and disinfection be thorough. Attention should also be given to rodent control because rodents can be a reservoir for Salmonella.388
MINIMIZING PATHOGEN EXPOSURE IN BEEF HERDS
In beef herds, calving areas should be located to take advantage of natural shelter and drainage and rotated from year to year to avoid pathogen buildup.330,372,389,390 Pregnant cattle are moved into a clean paddock no more than 2 weeks before the start of calving. It is best that cows and heifers are managed separately until their calves are at least 1 month old. This gives the opportunity to provide better feed to the heifers and to minimize infection between the groups. Confined, wet, or muddy areas should be avoided for calving cows, and if they need to be used the stocking rate should be decreased. Feed-out areas should be rotated and separated from watering points to encourage dispersal. When pasture for calved cows is limited, supplementary feed should be fed to dry cows to ensure enough freshpasture for calving and nursing cows. Clean water should be available in a trough that is accessible to cows and calves. Chronically sick animals, weak calves, and cows with no milk should be removed from the calving paddock and kept isolated from the herd.
Grazing and reproductive management have a significant impact on pathogen load. Where appropriate to grazing management, calving paddocks should be left vacant during the summer. For producers that manage periparturient cows in smaller calving paddocks to facilitate supervision, the emphasis should be on minimizing the stocking density in the calving paddock by removing cows and calves shortly after parturition.372,389 Alternatively, pregnant cows can be moved away from cows with calves every 1 to 2 weeks or more frequently in large herds. Young calves in the calving paddock will markedly increase the rate of pathogen buildup and the subsequent challenge to newborn calves. Moreover, the increased stocking rate will amplify stress, affecting both transfer of passive immunity and the ability of young calves to rest. Often, calving areas are small because of the perceived need to assist cows with dystocia.390 Well-grown and appropriately fed heifers mated to suitable sires can minimize this need. Beef cows with calves at foot should be moved from the calving paddock into nursing groups that have a maximum age range of 4 weeks and a low stocking rate. Groups should not be mixed until all calves are at least 4 weeks old.372,389,390 Reproductive management influences pathogen load by determining the age spread of the calves. Sick calves amplify environmental contamination. A prolonged calving period leads to a buildup of contamination so that calves born later in the calving period experience an increased pathogen challenge. It is desirable to maintain a calving period that is less than 60 days.
Ensure Adequate Colostral Intake
The principles of colostrum feeding and evaluation are discussed in detail elsewhere (see Chapter 53). In general, colostrum deprivation is seen in 25% to 50% of dairy calves391-393 but is much less common in single suckle beef operations.394 Colostrum deprivation, poor mothering, and early separation of dam and calf are the major causes of failure of transfer in dairy calves. Beef calves are usually mothered well,395-397 and volume of colostrum produced, which is strongly influenced by nutrition, is one limiting factor.361,398,399 Thus adequate colostrum intake is best ensured in dairy calves by assisted sucking of the dam or hand feeding 2 to 3 L of colostrum within 2 to 4 hours of birth. If colostrum is given by stomach tube, 4 L (10% of body weight) should be administered because the efficiency of absorption is reduced. In beef cattle adequate nutrition during late pregnancy is important. The calving area should be carefully monitored, and any calves that fail to suck within 6 hours of birth should be caught and tubed with colostrum. In herds where both dystocia and neonatal disease is a problem, it may be advisable to administer colostrum after calving to all calves that have an assisted birth.
Boosting Specific and Nonspecific Immunity
ENTEROTOXIGENIC E. COLI
The protective efficacy of ETEC bacterins is well documented.400-403 Because ETEC scours occurs during the first 3 days of life, the neonate does not have time to mount a protective immune response to vaccination. Protection is afforded by vaccinating cows in late gestation so as to ensure high concentrations of anti-K99 colostral antibodies. Good maternal management is required to ensure that the calf receives the maternal antibodies. Antipilus antibodies block the adhesion of the pathogen to enterocytes and subsequently prevent disease.401 In general it is recommended that the vaccines are given 6 and 3 weeks before calving. Studies with some vaccines have shown that the vaccine is still effective if the priming dose is given 18 months before calving and boosting is carried out in the second half of gestation.404 Clinical experience in beef farms with severe outbreaks of E. coli F5 (K99) diarrhea indicates that vaccinating cows that are more than 10 days from parturition can give considerable protection against death from ETEC infection.
Products containing monoclonal antibodies against F5 (K99) antigen have been shown to reduce the severity of diarrhea when calves are experimentally challenged a few hours after receiving the product.405 In field situations, however, monoclonal products can have a low efficacy, presumably because a single dose provides only a short period of enteric protection. Antibody supplements are expensive, and vaccination of the dam to boost colostral immunity will usually be more cost-effective. On farms experiencing an outbreak of neonatal diarrhea caused by F5(K99) E. coli, there may be a place for the use of these products until vaccinated cows begin to calve. However, short-term administration (once a day for first 3 days of life) of an antibiotic to which the E. coli is susceptible is also highly effective in preventing diarrhea in herds experiencing outbreaks of ETEC.
SALMONELLA
The successful reduction of Salmonella prevalence in livestock on a national level via implementation of a Salmonella control program emphasizing immunoprophylaxis with modified live and killed Salmonella vaccines indicates the potential benefits that can be derived from the application of effective Salmonella vaccines.406 Salmonella vaccine studies in cattle have focused on Salmonella bacterins and attenuated modified live Salmonella.
There are conflicting reports regarding the efficacy of Salmonella bacterins. The reported efficacy of Salmonella bacterins ranges from good to ineffective.407-415 The overall consensus of these reports is that vaccination of cattle with Salmonella bacterins provides partial protection against Salmonella challenge. In the only reported controlled field trial an autogenous Salmonella bacterin was not found to have any effectiveness.415 Anaphylactic reactions are occasionally reported in cattle vaccinated with Salmonella bacterins. The cause of these reactions is unknown but has been suggested to be associated with the lipopolysaccharide content of these products. Similar allergic-type reactions in humans caused by Salmonella bacterin vaccination during typhoid outbreaks are well documented.416
Several naturally occurring and genetically manipulated attenuated Salmonella strains have been used to immunize cattle against salmonellosis. The most widely tested genetically altered Salmonella mutant vaccines in cattle are the auxotrophic strains. Aromatic amino acid (aro) and purine (pur) auxotrophs of Salmonella are attenuated and have decreased virulence.417-423 Comparative vaccine trials indicate that modified live attenuated Salmonella vaccines provide greater protection against virulent Salmonella challenge than Salmonella bacterins.413,423,424 Vaccination with modified live Salmonella vaccines attenuates the severity of clinical signs and pathologic lesions and reduces Salmonella shedding and mortality.406,419,425
Calves immunized with modified live Salmonella vaccines are protected from homologous and heterologous Salmonella serotypes when challenged within 3 weeks of vaccination.426-428 Live Salmonella vaccines induce transitory T-cell independent nonspecific protection that disappears about 1 month after immunization following clearance of the organisms from the reticuloendothelial system. Thereafter, protection against oral challenge is species- and serotype-specific, with recall of immunity presumably involving specific antigen recognition.429,430
The level of passive protection of calves achieved by feeding colostrum from vaccinated cows is questionable. Several reports suggest that immune colostrum provides passive protection, but others report no protective effect. In trials in which protection was achieved, calves were challenged at 1 week of age; trials in which no protection was observed involved challenging calves at 3 weeks of age, suggesting that the duration of passive immunity associated with colostral transfer is relatively short. Considering that many calves are exposed to Salmonella in the first week of life, colostral protection may be useful.
ROTAVIRUS AND CORONAVIRUS
Bovine coronavirus is associated with several diseases in cattle. All BCV isolates are believed to belong to a single serotype.169a Differences in hemagglutination-inhibition characteristics have been used to classify strains as types 1 through 3.169b There are seven serogroups of rotavirus, with group A accounting for the majority of pathogenic isolates. Members of the group A rotaviruses are further classified according to antigenic and genetic differences in their outer capsid proteins, G and P. Both of these proteins are involved in neutralization of infectivity in vitro and in vivo.433 In the United States eight G serotypes and genotypes and four P serotypes and genotypes have been identified in cattle isolates.153 The genome of rotavirus is composed of 11 gene segments that can be exchanged among isolates when animals are infected by more than one virus at the same time.434 Genetic reassortment can generate new progeny viruses that can evade what was once a protective immune response, thus allowing persistence of rotavirus in susceptible populations.433
Two approaches have been taken with immunoprophylaxis against rotavirus and coronavirus infections in calves. The first approach involves oral vaccination of neonatal calves with a modified live vaccine. Calves begin producing detectable levels of local secretory IgM within 4 to 6 days of vaccination.435 Calves are resistant to challenge from the initial appearance of local IgM antibodies.435 In order to consistently elicit an effective immune response, the vaccine must be administered orally, immediately after birth, and before the calf has nursed because the colostrum of most cows contains virus-neutralizing antibodies that interfere with the vaccine.436 There are conflicting reports of efficacy with these type of vaccines. In double-blind field studies that include vaccinated and nonvaccinated calves the vaccine was not shown to be effective.437 Conversely, when all calves were either vaccinated or not vaccinated in sequential comparisons, morbidity and mortality were significantly reduced.437
The second approach involves intramuscular vaccination of pregnant cows with either modified live vaccine or inactivated viral vaccines to stimulate high levels of specific viral neutralizing antibodies in colostrum and milk during the first several days of the calf’s life. Infectious viral particles are neutralized within the gut lumen, preventing infection of intestinal villous enterocytes. One advantage of passive immunization is the fact that cross-protection between serotypes is less of a problem. This is because vaccination of a mature cow that has had natural rotavirus exposure leads to cross-serotype stimulation of heterotypic antibodies.438 Single serotype vaccination therefore stimulates antibody production to a wide range of rotavirus serotypes, negating the need for multivalent rotavirus vaccines. Passively absorbed anti-bovine rotavirus IgG1 antibodies are transferred to the small intestinal lumen, where they protect against experimental challenge.439 Antigen sensitized maternal lymphocytes also confer partial protection against challenge with virulent bovine rotavirus.440 Colostrum and milk with a high virus-neutralizing antibody titer are highly protective while being consumed by the calf. For example, administering 400 mL of immune colostrum daily to calves from days 2 to 12 reduced the incidence of diarrhea from 41% to 3% in one study.441 The concentration of rotavirus- and coronavirus-neutralizing antibodies in milk of vaccinated cows falls below protective levels by 3 to 7 days after parturition.442-444 Rather than complete protection, the manifestations of passive immunity to bovine rotavirus that are often noted are (1) a delay of a few days in the onset of clinical signs, (2)a reduced severity of clinical signs, and/or (3) a reduction in the length of the period of viral shedding associated with infection.445 Although there are reports of successful field trials involving bovine rotavirus— and bovine rotavirus-coronavirus—vaccinated cows,402,446,447 negative esults have also been reported.448 A common problem with commercial vaccines on the market in the United States and Europe is a lack of vaccine-specific data supporting efficacy claims. Protection correlates with serum titers; independent studies have sometimes failed to demonstrate effective seroconversion with some products.449
Nonspecific Immunity
DIET
The microbial quality of the diet is an important factor in preventing diarrhea. Calves that are fed milk from mastitic quarters or antibiotic-containing milk are at increased risk for diarrhea.335 After fresh colostrum feeding, young calves have less diarrhea if placed on whole cow’s milk rather than on other diets. Pasteurizing surplus colostrum and waste milk reduces the incidence of diarrhea in animals on these diets.450 It is also important to offer a good-quality calf starter from approximately 2 to 3 days of age; at first little will be ingested, so offer small amounts and keep it fresh.
Some producers routinely administer vitamin A to neonatal calves. Many, but not all, studies in children indicate that supplementation can reduce the incidence of diarrhea in areas in which clinical and subclinical vitamin A deficiency is endemic.451 In cattle, vitamin A deficiency is most likely when a diet of unsupplemented straw and grain is fed. Calves born to cows fed good-quality green forage or cattle receiving a vitamin A supplement should not require supplementation—particularly if they received adequate colostrum. Enteric absorption of vitamin A is diminished in calves with cryptosporidiosis, so the systemic route should be used in calves with this type of infection.452
BIOSECURITY
Infectious diseases are often purchased with brought-in stock. Operations that buy calves for rearing purposes should be encouraged to buy from as few sources as possible. Direct purchase from one farm is best; assembling collections of calves through auction markets should be avoided if possible.
TREATMENT OF INDIVIDUAL CALVES
Examination
Physical examination of the diarrheic calf is the first step in establishing therapeutic needs. It is important to determine the presence of any intercurrent disease. Treatment of uncomplicated cases of diarrhea depends on the estimation of dehydration, severity of acidosis, likelihood of intercurrent infection, presence or absence of hypothermia, and hypoglycemia. The severity of dehydration is gauged from the eyeball position and skin tent (Table 20-6). In acute diarrhea the degree of enophthalmus is the most reliable indicator of dehydration, but because the position of the eye within the orbit is also dependent on body fat stores, the skin tent in the cervical region may be the most reliable in calves with chronic diarrhea or cachexia.453 Skin tent can be measured over the eyelids and neck. Best results are obtained when the neck is held straight and the skin of the midneck is tented in the direction of the long axis of the neck to avoid the natural skinfolds that run across the neck. Some have claimed a relationship exists between severity of dehydration and acidosis. However, this has not been borne out in studies of diarrheic calves.454 Instead, acidosis can be gauged from the calf’s sucking or drinking drive, the degree of weakness, and the age of the calf (Fig. 20-4).454,455 Estimation of severity of acidosis either from laboratory or physical findings is very important to the successful therapy of severely depressed calves. Rectal temperature measurement will determine whether or not the calf is hypothermic.
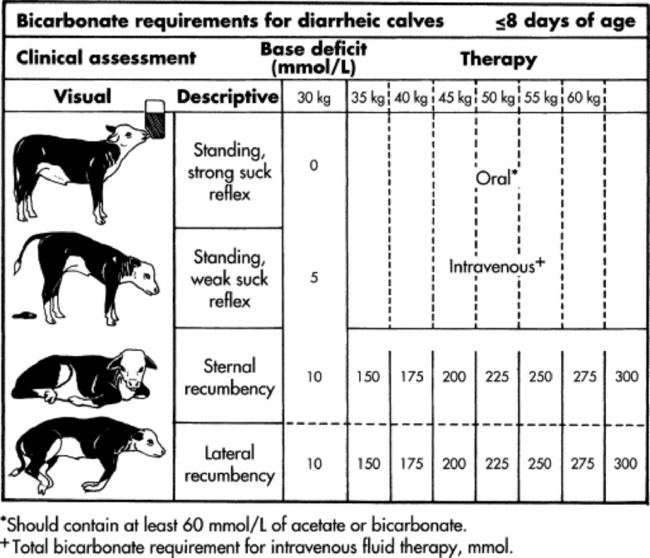
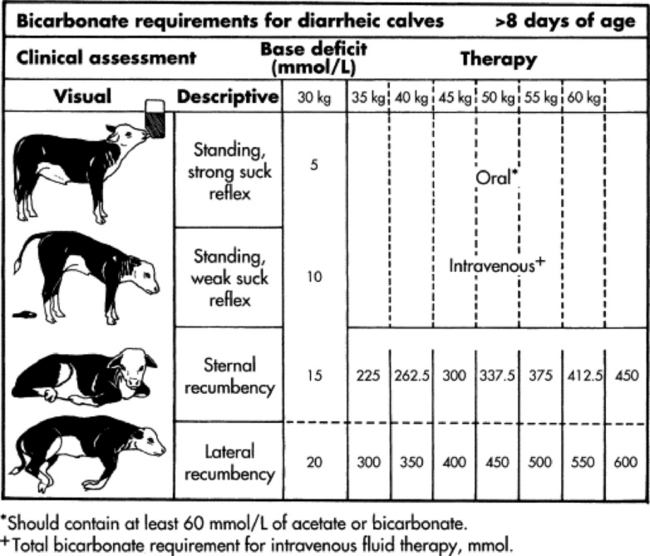
Fig. 20-4 Prediction of severity of metabolic acidosis from body position, strength of suck reflex, and age.
Heart rate is variable in diarrheic calves. Bradycardia (<90 beats/min) is clinically important and may indicate the presence of hypothermia, hypoglycemia, or hyperkalemia. Cardiac arrhythmias occur455 and are usually a result of severe hyperkalemia (K+ above 8 mEq/L) (Fig. 20-5). Hyperkalemic arrhythmia can usually be differentiated from arrhythmia resulting from cardiomyopathy (selenium deficiency) because the heart rate is not elevated. The presence of bradycardia or arrhythmia indicates the need for immediate fluid therapy with bicarbonate-containing solutions to prevent death.
Fig. 20-5 Bradycardia and atrial standstill in a severely hyperkalemic diarrheic calf. Heart rate is 84 beats/min. There is bigeminy, and the T waves are abnormally large. There is only one P wave, and it is not conducted. Serum potassium was 8.9 mmol/L, and sodium 116 mmol/L.
Body condition is usually good at the start of an attack of diarrhea. Poor condition often indicates chronic infection, mismothering, or poor feeding programs, which may be exacerbated by milk withdrawal for therapeutic purposes.
It is important to check for intercurrent infections, which are easily missed even with careful examination. The lungs should be examined for signs of pneumonia; the navel palpated for pain, swelling, and wetness; and the joints checked for signs of distention and lameness. The boundary between calves in which the primary insult is septicemia with a secondary diarrhea and those in which primary diarrhea is complicated by septicemia is blurred. Calves that are recumbent, under a week of age, have lost their suck reflex, or have evidence of intercurrent infection are more likely to be septicemic and require concurrent antibiotic therapy.456 Calves in which septicemia has progressed to produce signs of meningitis (e.g., extended neck with reluctance to flex neck), joint involvement, or ophthalmic signs (congested scleral vessels withhypopyon or iridospasm) have a poor to very poor prognosis. It is best to identify these cases before instituting therapy so that the owner can decide whether treatment is economically feasible.
The laboratory is useful for quantifying metabolic disturbances in diarrheic calves. Blood gas analysis will accurately determine the degree of metabolic acidosis. This is not routinely available to most practitioners. However, it may be worthwhile to make special efforts to get measurements when setting up a fluid therapy protocol for your area or when dealing with cases that fail to respond to treatment. Blood can be collected into a heparinized syringe and the syringe capped, placed in a Styrofoam cup surrounded by ice, and transported 4 hours or more to a laboratory. Alternately, the practice laboratory may have a total CO2 (Harleco) analyzer or access to serum bicarbonate estimation as part of the serum chemistry profile. Total CO2 or bicarbonate is a useful index of the severity of metabolic acidosis. Blood glucose can be readily determined using a hand-held Glucometer.
Fluid Therapy
The most common causes of death in diarrheic calves are dehydration and acidosis.457 The immediate objective in treating depressed diarrheic calves is to restore them to a normal systemic state. In some calves it may also be necessary to correct hypoglycemia or hypothermia, restrict milk intake, or give antibiotics.
The estimated severity of dehydration can be combined with estimates of losses through diarrhea and for the maintenance of essential functions to give the total daily fluid requirement (see Table 20-6). The hydration status of the calf can be estimated from the degree of enophthalmus, the degree of skin tent on the neck, and evaluation of the mucous membranes (see Table 20-6). The volume (L) required to replace the deficit is determined as follows:
The ongoing losses through diarrhea should be estimated from the nature and volume of the diarrhea. Fecal losses can range from 1 to 6 L in diarrheic calves.458 Maintenance requirements have been estimated at 50 to 100 mL/kg/day.6,458 The degree of hydration and the volume of feces passed should be reassessed daily, and the treatment adjusted accordingly. Only 60% to 80% of oral fluids are absorbed, and this needs to be accounted for in the calculation.459
Bicarbonate requirements can be calculated from base deficit values (based on blood gas measurements or estimated from physical findings) as follows:
A chart of bicarbonate requirements for various body weights and base deficit values is available (Table 20-7).
Measurements of serum total carbon dioxide content or bicarbonate are also reliable estimates of bicarbonate requirements.460 Bicarbonate requirements are as follows:
For example a 40-kg calf has a serum bicarbonate or TCO2 of 10 mmol/L. The calf has a bicarbonate deficit of 30 mmol/L − 10 mmol/L = 20 mmol/L, so 40 kg × 20 mmol/L × 0.5 = 400 mmol bicarbonate required to replace existing deficits. Ongoing diarrhea may require additional bicarbonate.
Calves that are unwilling to suck and that are severely depressed are best treated with intravenous fluids. Calves that are only moderately depressed may also be treated with intravenous fluids if the condition is worsening rapidly. Catheterization is easier if a No. 15 scalpel blade is used to nick the skin. If it proves very difficult to catheterize the calf, it can be suspended upside down so that blood will pool and distend the jugular veins. The calf’s neck should be clipped and prepared before inversion, and the calf laid flat as soon as the catheter is placed. It should be possible to place a catheter in less than a minute even in severely dehydrated calves using this technique. Once the catheter is placed, fluids can be administered. If the calf is hypothermic the fluids should be warmed before administration because cold fluids can decrease cardiac output and may kill a critically ill calf. Fluids can be warmed by a number of methods; one convenient technique is to run the fluids through a coil of tubing immersed in a bucket of hot water (check the temperature regularly) on the way to the calf.
Although saline-based fluids are suitable for rehydration (Table 20-8), most severely depressed calves are acidotic, and more consistent recovery is obtained if an alkalizing agent is also used. A wide variety of alkalizing agents is available (lactate, acetate, gluconate), but clinical trials show that only bicarbonate is consistently effective in severely acidotic calves (Fig. 20-6).93,461 Many diarrheic calves require large amounts of bicarbonate to correct their acidosis.91 An isotonic solution (156 mmol/L) of bicarbonate can be readily made by dissolving 13 g of sodium bicarbonate (baking soda) in 1 L of water.462 Sodium bicarbonate solutions can be mixed with saline; there is a possibility that precipitates may form if bicarbonate is mixed with calcium-containing solutions such as Ringer’s.
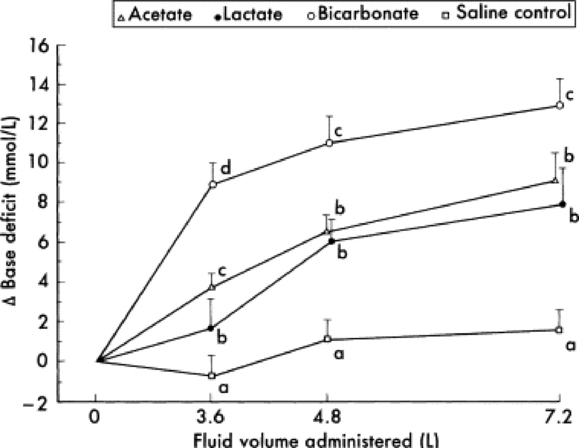
Fig. 20-6 Comparison of the alkalizing effect of various bases in diarrheic calves with severe dehydration and acidosis. At the start of the trial, all calves were at least 8% dehydrated and had a mean blood pH of 7.032 and a base deficit of 18 mmol/L. All calves received a total of 7.2 L of fluid containing 102 mmol/L of saline plus 50 mmol/L of the sodium salt of the respective alkalizing agent. Letters (a, b, c, d) are statistically different (p < .5) from one another at that point.
From Kasari TR, Naylor JM: Clinical evaluation of sodium bicarbonate, sodium L-lactate, and sodium acetate for the treatment of acidosis in diarrheic calves, J Am Vet Med Assoc 187:392, 1985.
Some clinicians may prefer to rehydrate the neonate first and then reconsider the need for bicarbonate if it is not up and sucking within 12 hours of therapy. However, this is time-consuming. It is not always necessary to completely correct acidosis; blood pH from 7.25 to 7.45 has little adverse affect (normal calves have a venous blood pH of 7.34, bicarbonate of 30 mmol/L, and a base excess of 5 mmol/L).
Ideally, dehydration and acidosis should be corrected over a 24-hour period. However, it is unusual to see problems when the fluid and acid-base deficits are corrected over 4 hours, although the calf may continue to improve after this period. Rapid resuscitation techniques include the administration of either hypertonic saline dextran or hypertonic sodium bicarbonate. Hypertonic saline dextran (7.2% saline containing 6% dextran 70) administered at 4 mL/kg of body weight during a 4-minute period concurrently with an isotonic alkalizing oral electrolyte solution is effective in resuscitating dehydrated calves with diarrhea.463,464 Hyperosmotic sodium bicarbonate solutions may also be used to rapidly resuscitate acidotic dehydrated calves.465 The base deficit can be corrected by administering 8.4% sodium bicarbonate IV at a rate of 1 mL/min/kg over 15 minutes.466,467 Hypertonic fluids should always be given concurrently with an isotonic oral electrolyte solution. After 24 hours of appropriate therapy, one would expect the calf to be standing and show a good suck reflex. Persistent depression is usually a sign of uncorrected acidosis or toxemia.
Most diarrheic calves are not markedly hypoglycemic, but glucose supplementation is needed to treat severe hypoglycemia (glucose concentrations <2 mmol/L or <36 mg/dL). Severe hypoglycemia is treated by adding glucose to the intravenous fluids to a final concentration of 2.5% to 5%. Severe hyperkalemia is seen in some dehydrated diarrheic calves, but this responds to rehydration (restores renal perfusion and dilutes out potassium) and correction of acidosis (redistributes potassium into the cells). Nutritional support is not needed if the calf is in good body condition but should be given if the calf is emaciated or if the calf has been deprived of milk for more than 3 days.
There are increased losses of potassium in diarrhea; the significance of this is uncertain, although potassium depletion can result in weakness. Usually there is no need to add potassium to intravenous fluids; the majority of calves respond to infusion of saline and 1.3% sodium bicarbonate. After 12 to 24 hours of intravenous fluid therapy most calves start on oral electrolyte solutions. These usually contain 10 to 30 mmol of potassium per liter. Clinical trials comparing the efficacy of low and high potassium solutions have not been reported.
Once the calf is able to nurse or drink, therapy is usually switched to oral electrolytes. This is also the route of choice for treatment of mildly affected calves on the farm. Calves with weak suck reflexes and calves that are unused to hand-feeding can be administered electrolytes using an esophageal feeder. Oral electrolyte solutions need to supply sufficient sodium to facilitate normalization of extracellular fluid deficits, nutrients to facilitate absorption of sodium from the intestine, alkalizing agents to treat metabolic acidosis, and supplemental energy.468 The first two requirements depend on the coupled active transport of glucose and sodium ions across the brush border membranes of enterocytes, which results in passive absorption of water and other electrolytes.469 This function remains largely intact in calves with ETEC diarrhea, but when there is endothelial damage it may be impaired. Certain amino acids (glycine, L-alanine, L-glutamine) enhance the absorption of sodium and water,469 as do acetate and propionate.470 In order to effectively combat acidosis, oral electrolyte solutions need to contain 50 to 80 mmol of alkalizing agent per liter. Acetate, lactate, citrate, gluconate, and bicarbonate are all used as alkalizing agents. Bicarbonate combines with hydrogen ions directly, whereas the other agents remove hydrogen ions during their metabolism within cells.458 Electrolyte solutions that contain >40 mmol of bicarbonate or citrate per liter have marked adverse effects on milk clotting.471 Bicarbonate raises abomasal pH, whereas citrate binds calcium, and so the presence of either interferes with the normal clotting of milk in the abomasum. Breakdown of abomasal milk clots results in the gradual release of some nutrients into the small intestine. Bicarbonate also reduces milk digestibility. A reduced growth rate was recorded when electrolyte solutions with bicarbonate were fed to milk-fed calves.472 Solutions containing bicarbonate may also alkalize the gastrointestinal tract of milk-fed calves and promote bacterial overgrowth in the small intestine as well as ETEC attachment and toxin production.473 Acetate is the best alkalizing agent to include in electrolytes that are to be fed to calves that are still receiving milk; it has excellent alkalizing ability and does not interfere with milk clotting in the abomasum. Any of the commonly used alkalizing agents are likely acceptable for calves held off milk.471
A wide variety of oral electrolyte preparations are on the market, and different products are suited to different situations. Almost all the products contain water and electrolytes and are suitable to use for rehydration (Table 20-9). Beware of products that are designed for medicating hundreds of liters of water; the final solution is often very dilute (<10 g of electrolytes per liter) and will not rehydrate sick calves. These products are often marketed as “boosters” and “stress relievers.” Glucose and glycine are usually added to oral electrolyte solutions to facilitate sodium absorption. Research in people has shown that adding glycine to solutions containing 110 mmol/L of glucose aids rehydration. It is probable, however, that there is little additional benefit to supplementing solutions that contain more than 200 mmol/L of glucose with glycine. Solutions that contain large amounts of glucose are hyperosmolar and are absorbed more slowly than isotonic solutions, but the differences are too small to be clinically important.474 The ionic composition also affects absorption; mixtures containing sodium chloride and citrate, bicarbonate, or acetate have improved absorption over chloride salts alone. Oral electrolyte solutions are almost completely absorbed in healthy calves, but absorption can be as low as 60% in severe E. coli diarrhea.475
The ability to counteract acidosis varies greatly among oral electrolytes. Some have a net acidifying effect, whereas others alkalize blood (Fig. 20-7). These differences are therapeutically important and are responsible for differences in survival rates among products. Highly alkalizing solutions give the best results. One study showed that it was more important that an electrolyte solution contain bicarbonate than chloride.476 This is particularly important in older calves. Recently there has been interest in adding glutamine to oral electrolyte solutions because it is an important fuel for the gastrointestinal tract and can promote mucosal repair.477-479 However, studies show that oral electrolytes containing glutamine as the sole amino acid are no more effective in diarrheic calves than other well-designed solutions that use glycine as the amino acid.480 Psyllium has been added to some oral electrolyte solutions for a number of perceived benefits, but controlled studies show no clinical advantages, although there may be some moderation of bacterial fermentation within the gastrointestinal tract.481,482
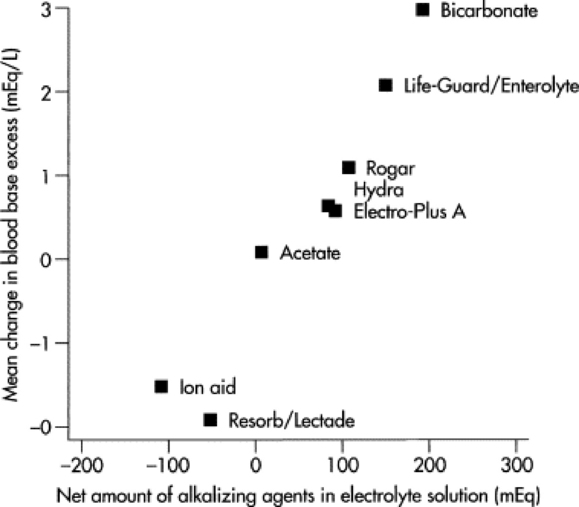
Fig. 20-7 Comparison of the alkalizing abilities of various oral electrolyte solutions. The Y axis shows the mean alkalizing effect after administration of one treatment (1.9 to 2.25 L) of the fluid to healthy calves, and the X axis shows the net amount of alkalizing agents in the solution. Bicarbonate and acetate are experimental solutions. (Life-Guard/Enterolyte is manufactured by SmithKline Beecham Labs, Rogar is Rogar STB’s electrolyte powder, Hydra is manufactured by Vetrepharm, Electro-Plus A by Pitman-Moore, Ion Aid by Syntex, and Re-Sorb by Beecham.)
Modified fromProc 14th World Congr Dis Cattle 1:362, 1986.
The other problem to be considered in the chronically scouring calf is the need for nutritional support. Maintenance metabolizable energy requirements for a 50-kg calf are about 2000 kcal, and 3500 kcal are required to support a weight gain of 0.5 kg/day. These requirements can be met by 3.3 and 5.7 L of whole cow’s milk, respectively. Comparative studies indicate that weight loss in calves fed oral electrolytes are inversely proportional to the energy content of the solutions.483 Assuming a 4-L daily intake and 100% digestibility of oral electrolyte nutrients, regular electrolyte solutions supply between approximately 15% and 25% of energy needs. As a result, diarrheic calves that are held off milk for prolonged periods lose weight484 and can become emaciated. When maintaining body condition is a concern and little milk or solid food is being ingested, then a high-energy oral electrolyte should be fed. Products such as Enterolyte HE provide about 50% of energy requirements if fed twice a day (total intake 4 L) and about 75% if fed three times a day (total intake approximately 6 L). The energy content of various oral electrolyte solutions is presented in Figure 20-8.
Fig. 20-8 Comparison of the energy contents of various oral electrolyte solutions. Milk is also shown for comparative purposes. *ME estimated from water-soluble carbohydrate composition. †ME estimated from glucose and other water soluble carbohydrate composition of as-fed electrolyte solution assuming 1g (cry matter) of carbohydrate = 4kcal. This figure assumes electrolyte solutions are fed to neonatal calves prior to rumen development. (See text for manufacturers.)
MILK WITHDRAWAL
Milk withdrawal can reduce the severity of diarrhea and depression in severe scours. This is because malabsorption exacerbates diarrhea through the osmotic effect of unabsorbed milk nutrients and also promotes bacterial overgrowth and possibly malfermentation with production of organic acids. Milk also has a trophic effect on epithelial cells and maintains higher gastrointestinal tract enzyme activities as well as providing protein for repair of damaged intestinal epithelium.470 In experimental trials, continued feeding of milk maintained weight gain; however, when calves were fed enough milk to fully meet their requirements and the undrunk milk was tube-fed, calves initially had greater inappetence.472,485 Withdrawal of milk without a high-energy alternative can rapidly result in cachexia and malnourishment.472 In many calves, particularly the less severely affected, there is often a considerable degree of residual absorptive capacity; enough to support body weight gain if limited amounts of milk are fed. Milk withdrawal is recommended if the calf is depressed and not interested in sucking. In most cases electrolyte therapy will restore a calf’s vigor and sucking drive within 1 to 2 days. Milk can then be reintroduced in small amounts (e.g., 1 L given two to four times daily). However, forced feeding (by tubing or drenching), dysfunction of the reticular groove reflex, or reflux of abomasal contents may result in ongoing acidosis because of production of D-lactate from fermentation of carbohydrates entering the reticulorumen.98 If the calf is not interested in drinking or gets depressed when reintroduced to milk, a high-energy oral electrolyte preparation can be tried instead. Studies indicate that diarrheic calves have a generalized malabsorption rather than specific lactose intolerance.88 Thus it may be more important to manage calves with milk intolerance by giving smaller amounts of milk in each feed instead of changing carbohydrate source. There is little point in withdrawing milk from calves that remain alert and interested in nursing; it is unlikely to result in clinical improvement. This is particularly likely to be the case when the calf receives whole cow’s milk in frequent small quantities, that is, by natural sucking of the dam.
Role of Antimicrobials
There is some controversy regarding the use of antimicrobials for the treatment of calf scours. Reports questioning the use of antimicrobial therapy cite lack of efficacy, potential for adverse effects, potential for violative residues, and selection for antimicrobial resistance. Conversely, there are reports describing attenuation of clinical disease, reduced pathogen shedding, and lower mortality after the use of antimicrobials to treat scouring calves.
THERAPEUTIC TARGETING
Bacterial pathogens associated with neonatal calf diarrhea include Salmonella and E. coli. During disease outbreaks caused by these pathogens antimicrobial use may be targeted at the specific pathogen. Beneficial responses to antimicrobial therapy have also been reported in field trials involving undifferentiated pathogens.486,487 Calves with diarrhea often have increased coliform bacterial numbers in the small intestine, regardless of cause,488-490 and this colonization is associated with altered small intestinal function, morphologic damage, and increased susceptibility to bacteremia.490 Calves with diarrhea are more likely to have FPT or partial FPT, and this group of calves, in turn, is more likely to be bacteremic.491,492 Blood cultures indicate that gram-negative bacteria account for approximately 80% of bacterial isolates. E. coli is the species most commonly isolated.491,493,494 In a study of 190 recumbent calves on a large calf-raising facility, 31% were determined to be bacteremic; E. coli accounted for 51% of the isolates; other gram-negatives, 25%; gram-negative anaerobes, 5.9%; gram-positive cocci, 11.8%; and gram-positive rods, 5.9%.491
Antimicrobial therapy may therefore be targeted at a specific bacterial enteric pathogen isolated from sick calves or in severely ill calves (as manifested by reduced suckle reflex, >6% dehydration, weakness, inability to stand, or clinical depression) may be used prophylactically to manage the risk of bacteremia. For this application, emphasis should be directed toward gram-negative organisms, particularly E. coli.
ANTIMICROBIAL SUSCEPTIBILITY
Antimicrobial susceptibility testing of fecal isolates is not a good predictor of clinical outcome. Three studies have reported no correlation between in vitro antimicrobial susceptibility of fecal E. coli and Salmonella species isolates and clinical response to antimicrobial treatment.495-497 Antimicrobial efficacy is best evaluated by the clinical response of a number of calves to treatment, with calves randomly assigned to treatment groups, rather than by the results of in vitro antimicrobial susceptibility testing performed on fecal E. coli isolates.498
Antimicrobial susceptibility testing has more clinical relevance for predicting the clinical response to antimicrobial treatment when applied to bacteria isolated from blood or tissues of bacteremic calves because the MIC break points are based on achievable antimicrobial concentrations in human plasma and MIC90 values for human E. coli isolates, which provide a reasonable approximation to achievable MIC values in calf plasma and MIC90 values for bovine E. coli isolates.498 Even within a given herd there will be a diversity of bacteria isolated from bacteremic calves, so the collection of blood cultures and assessment of antimicrobial susceptibility does not necessarily provide information applicable to the next case.
ANTIMICROBIAL SAFETY
A number of antimicrobials have been demonstrated to produce deleterious effects when administered orally to healthy milk-fed dairy calves. The addition to milk replacer powder of procaine penicillin (2 to 60 mg/kg of milk replacer) increased the incidence and duration of diarrhea and decreased growth rate compared with untreated controls in a total of 36 milk-fed calves.499 Penicillin is not labeled for treatment of calf scours and has an inappropriate antimicrobial spectrum to prevent or treat calf scours. Administration of neomycin sulfate (300 mg PO q24h for the first 4 days of life) tended (p = .060) to increase the proportion of calves developing diarrhea (99/233 = 43%) compared with the proportion in an untreated control group (58/174 = 33%).500 Administration of neomycin sulfate (25 mg/kg PO q6h, n = 10), ampicillin trihydrate (12 mg/kg PO q8h, n = 6), or tetracycline hydrochloride (11 mg/kg PO q12h, n = 6) for 5 days increased the occurrence of diarrhea and decreased glucose absorption through unknown mechanisms compared with untreated controls (n = 6).501 Two other studies did not observe adverse side effects in calves administered tetracycline hydrochloride (40 mg PO q12h; 11 mg/kg PO q12h).502,503
EFFICACY OF ORAL ANTIMICROBIAL THERAPY
The response to oral antimicrobial therapy is variable, with many formulations failing to demonstrate a beneficial effect.498 Apramycin administered PO at either 20 or 40 mg/kg significantly decreased mortality.487 Trials with orally administered neomycin reduced the duration of diarrhea but did not reduce mortality.504,505 Similarly, trials with orally administered ampicillin failed to demonstrate a significant reducccction in mortality.506 The results of trials with orally administered trimethoprim have been variable, with no significant improvement in outcome observed in a large field trial507 and a significant reduction in mortality observed in an experimental Salmonella challenge study in which calves were administered 5 mg or trimethoprim per kilogram and 25 mg of sulfadiazine per kilogram PO daily for 5 days.508 Orally administered amoxicillin trihydrate has been demonstrated to reduce mortality and the duration of diarrhea when administered at a dose of 10 mg/kg PO q12h.77,509
In an epidemiologic study of Salmonella in dairy calves conducted in the United States, feeding medicated milk replacer and hay to calves from 24 hours of age to weaning was associated with a reduced risk of Salmonella shedding.510 This observation contradicts an experimental study in which feeding chlortetracycline in milk replacer increased the severity of disease and the rate and duration of Salmonella shedding.511 Similarly in another experimental trial, daily drenching of calves with 50 mg or 100 mg of chlortetracycline failed to alter the excretion pattern or the number of organisms excreted by calves infected orally with 106 S. typhimurium.512
EFFICACY OF PARENTERAL ANTIMICROBIAL THERAPY
Antimicrobial drugs with an appropriate gram-negative spectrum of activity include third-generation cephalosporins (ceftiofur), potentiated penicillins (amoxicillin), trimethoprim-sulfonamide (TMS) combinations, aminoglycosides, sulfonamides, florphenicol, and tetracyclines. There is a paucity of efficacy data to support the use of aminoglycosides, tetracycline, nonpotentiated sulfonamides, and florphenicol.
Ceftiofur has an appropriate antimicrobial spectrum, and therapeutic drug concentrations can be maintained with once-daily dosing. In an S. typhimurium challenge experiment, intramuscular administration of ceftiofur hydrochloride (5 mg/kg q24h for 5 days) reduced the severity of clinical signs and reduced fecal shedding of Salmonella. The MIC of the challenge strain in this experiment was 1 μg/mL, and the therapeutic protocol maintained plasma concentrations above this concentration for the duration of therapy.264
Potentiated sulfonamides have been evaluated in ETEC and Salmonella challenge experiments. Mortality in 2- to 3-week-old calves medicated with trimethoprim-sulfadiazine (in a 1:5 ratio) for 5 days 24 hours after Salmonella Dublin oral challenge was reduced.508 Administration of either sulfadiazine or trimethoprim alone did not reduce mortality.508 Trimethoprim may be used to treat sepsis in neonatal calves, but its half-life rapidly declines as ruminal function develops. In ruminating (6- to 8-week-old) calves, subcutaneous or oral administration of trimethoprim-sulfadiazine leads to high serum levels of sulfadiazine but little or no serum trimethoprim.513
Intramuscular administration of amoxicillin reduced mortality in Salmonella Dublin—challenged calves.514 In a comparative trial of amoxicillin and trimethoprim-sulfadiazine, both drugs were found to have equal efficacy in reducing adverse clinical signs of disease when dosage regimens were based on the MIC of the pathogen.515
The frequency of bacteremia is sufficiently high that treatment of calves with diarrhea that are severely ill (as manifested by reduced suckle reflex, >8% dehydration, weakness, inability to stand, or clinical depression) should include routine treatment against bacteremia, with emphasis on treating potential E. coli bacteremia.498 Parenteral administration of a broad-spectrum β-lactam antimicrobial—ceftiofur (5 mg/kg intramuscularly [IM] q24h), amoxicillin (10 mg/kg IM q12h), or trimethoprim-sulfadiazine (20 mg of sulfadiazine per kilogram with 5 mg of trimethoprim per kilogram IV or IM, q24h for 5 days)—is recommended for treating calves with diarrhea and systemic illness. (Note that these are off-label doses and require an extended meat withholding period.) Antimicrobial therapy is not recommended for calves with diarrhea and no systemic illness (normal appetite for milk or milk replacer, no fever).498
Antiprotozoal Drugs
Drugs reported to have some efficacy against Cryptosporidium in calves include, halofuginone,516-522 paromomycin,523,524 decoquinate,525,526 and β-cyclodextrin.527 Halofuginone is licensed for treatment of calves in Europe and appears to be the most efficacious. The efficacy of decoquinate is questionable, with the only controlled clinical study failing to demonstrate a beneficial therapeutic effect with daily treatment at 2 mg/kg/day.526 A trial of lasalocid for treatment of Cryptosporidium infection has been conducted. Using a toxic dose of 8 mg/kg was found to reduce the shedding of cryptosporidia; however, the calves suffered adverse side effects. At a dose of 0.8 mg/kg, lasalocid was not effective.528 The registered dose for preventing coccidiosis in calves is 1 mg/kg per head per day.
Coccidiosis is uncommon in calves less than 6 weeks of age. In hand-reared calves coccidiostats (lasalocid, amprolium, or decoquinate) may be added to milk replacer. Prophylactic options for beef calves are restricted to coccidiostat medicated pellets (monensin, lasalocid, amprolium, or decoquinate) or water (amprolium or sulfonamides). Therapeutic options include amprolium or sulfonamides such as sulfadimidine.
Both fenbendazole (5 mg/kg PO once daily for 3 days ) or albendazole (20 mg/kg PO once daily for 3 days) have been shown to be effective treatments for Giardia.222,298,529 Because of the high level of subclinically affected animals, all cows and their dams need to be treated, and reinfection is likely to occur unless calves are removed from environmental sources of infection.
Antiinflammatory Drugs
A nonstatistical trend toward decreased morbidity has been reported in a study evaluating the benefits of a single or double injection of flunixin meglumine in scouring calves.530
Probiotics
Probiotics are foods or drugs containing live microbes that are expected to confer beneficial physiologic effects to the host animal through microbial actions. Bacterial and fungal species included in these products include Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium bifidum, Streptococcus salivarius subsp. thermophilus, Aspergillus oryzae, and Candida pintolopesii. General mechanisms of action that have been ascribed to probiotics include competition for receptor sites on the intestinal surface, immune system stimulation, excretion of antimicrobial substances, and competition with pathogens for intraluminal nutrients.531
The number of controlled clinical trials evaluating probiotic formulations in calves is limited. In one report, feeding antimicrobial resistant Streptococcus faecalis to calves reared on an antibiotic-containing diet reduced Salmonella intestinal colonization of calves.532 An improvement in weight gain and a reduction in diarrhea have been reported when calves were fed either 3 × 109 Bifidobacterium pseudolongum or L. acidophilus daily from 1 to 56 days of age or a cell mixture containing 1010 colony forming units (cfu) of Bacillus thermophilum, 1010 cfu of Enterococcus faecium, and 109 cfu of Lactobacillus acidophilus for 28 days.533
Intestinal Protectants
Several products that include intestinal protectants are marketed for treatment of calves with scours. Intestinal protectants include bismuth subsalicylate, kaolin or pectin, and activated charcoal. There are no efficacy data available regarding the use of kaolin in scouring calves. Suggested advantages of bismuth subsalicylate are its neutralization of bacterial toxins and antisecretory effect through its local antiprostaglandin activity.534,535
Prognosis
The prognosis for recovery decreases with the severity of depression. Severe hypothermia and the presence of intercurrent disease are grounds for a guarded prognosis.536-538 An initial examination should be performed. Calves with a primary problem of septicemia are not usually worth treating because of the poor prognosis. The severity of dehydration, hypothermia, and acidosis should be estimated. Recumbent calves are usually treated IV with a saline-based rehydrating fluid (0.9% saline, Ringer’s, lactated Ringer’s, and so on) and isotonic sodium bicarbonate (especially for older and comatose calves). Calves that can suck are treated with oral electrolytes that contain 50 to 80 mmol/L of alkalizing agent. Products that use mainly acetate as the alkalizing agent are best for calves that are still drinking cow’s milk (small quantities, frequently). Any alkalizing agent is likely effective in calves held off milk.
SUMMARY
Calf scours is caused by a variety of infectious agents. At the present time, the need to make a definitive diagnosis of ETEC and Salmonella infections has been established; these diseases can be controlled with antibiotics and prevented by vaccination.539 There are public health implications to the diagnosis of cryptosporidiosis and salmonellosis. New vaccines may help control rotavirus and coronavirus infections. Treatment of diarrhea in neonates is primarily based on correcting dehydration and acidosis through the use of oral and intravenous electrolytes. Only in the case of bacterial infections can direct action be taken against the invading organism, but antibiotics may still be useful in preventing secondary bacteremias. Colostrum feeding will help reduce diarrhea in the first days of life. Management is very important in the control of diarrhea, and because infectious agents are almost always present at some exposure level, the underlying theme is to minimize the level of pathogen exposure and stress on the calf. In approaching a problem of neonatal death losses, the areas to be examined should include calf immunoglobulin status, calf feeding, calf housing, cleanliness of environment, calving area, cow vaccinations, diagnosis of specific infectious agents, and treatment protocols.