Emergency Nursing
When you have completed this chapter, you will be able to:
1 List the components in an emergency care station/crash cart.
2 Discuss standard triage protocols used in evaluation of a small animal trauma patient.
3 Discuss possible secondary complications of trauma and critical illness, including pain, DIC, shock, and cardiopulmonary arrest; and their recommended treatment or resuscitation protocols.
4 Discuss small animal blood donors, blood types, and transfusion protocols.
5 List the objectives of monitoring critical care patients and discuss the approach to patient monitoring based on the principles of triage and body system anatomy.
6 List common emergencies in small animal veterinary medicine.
7 List equipment, supplies, and medications needed to respond to equine emergencies.
8 List minimal data collected during assessment of equine emergency patients.
9 Describe initial management, assessment, diagnostic, and treatment procedures for common equine emergencies.
10 Describe procedures for placement and maintenance of IV catheters in equine patients.
11 Discuss considerations in development and implementation of an equine fluid therapy plan in emergency situations.
12 Describe indications for use of blood and blood products and procedure for their use.
SMALL ANIMAL EMERGENCY NURSING
THE EMERGENCY CARE STATION AND RESUSCITATION AREA
Animals frequently come to the veterinarian with emergent and often life-threatening injuries or illnesses. Such demands require that the veterinary facility be set up in a manner in which quick assessment and immediate therapies are possible. Every veterinary practice should contain a centrally located emergency care station and resuscitation area devoted to crisis management. This area should be designed to facilitate rapid triage and treatment. It should be easy to access and have adequate space to accommodate multiple staff members responding to a patient emergency. Emergency drugs and equipment should be stored within easy reach and in designated areas. Equipment and drug inventory of the emergency care station should be checked at each shift change and following each use to ensure that all items are in working order and in adequate supply.
As a minimum, this area should have a source of oxygen, a suction unit, adequate electrical capability, and sufficient lighting. The emergency care station should have a sufficient number of electrical outlets to supply monitoring equipment without the use of excessive extension cords, which can be clumsy, unsafe, and impede the movements of staff. Standard fluorescent lighting can be augmented by well-positioned overhead surgery or examination lights.
In many veterinary practices, oxygen is supplied via anesthetic equipment. Whereas an anesthetic machine provides a familiar means of ventilation and access to sedation (if needed), it can also be a source of catastrophe when errors of anesthetic depth or pop-off valve closure occur. If an anesthetic machine is used, waste anesthetic gas scavenging systems should be available. To prevent the potential problems associated with anesthetic equipment, an oxygen source with a flowmeter and Ambu bag (Figure 33-1) is preferred. Ambu bags are especially useful because they are an easily transported, easily stored, and inexpensive source of artificial ventilation.

FIGURE 33-1 An endotracheal tube connected to an Ambu bag and oxygen source provides an ideal means to supply 100% oxygen and manual assisted ventilation
Many small practices have suction units with adjustable suction pressure, which are used in surgery (Figure 33-2). The emergency area should have its own suction unit designated and supplied with a variety of suction tips. Suction equipment is often used in the emergency area to clear the airway or endotracheal tube of fluid or debris (mucus, blood, exudates, vomitus, etc.).

FIGURE 33-2 A suction unit similar to those used in a surgery suite should be centrally located and used in emergencies to clear airways of fluid and debris
CRASH CART
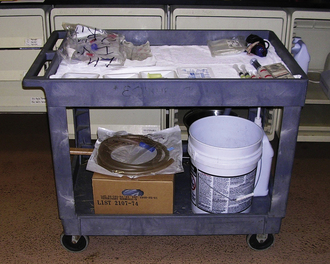
An integral part of preparation for an emergency is the “crash cart” (Figure 33-3). This can be a fishing tackle box with necessary items or a large cart on wheels with multiple drawers. A tool storage cart available at hardware stores can work quite well. The crash cart should be located at the emergency station and contain necessary items for treating patients that are medically unstable. Additional crash carts may be placed in select locations throughout the hospital, if needed (i.e., operating room or dental suite). Basic supplies contained in the crash cart should include items necessary to establish an airway, venous access, emergency drugs, and a dose chart.

FIGURE 33-3 An effective “crash cart” is easily accessible and spacious enough to contain an array of emergency supplies
Following each use and at every shift change, the contents of the crash cart should be checked and restocked. The function of all battery or electrical items should be checked and replaced or recharged as necessary. Drug expiration dates should be checked regularly, and expired drugs should be discarded.
Crash cart airway supplies should include at least one laryngoscope with a small- and large-size blade (Figure 33-4). The laryngoscope battery and bulb light should be checked at each shift to make sure that the equipment is in working order. Various sizes of clean endotracheal tubes and stylets should be placed in well-marked and organized places so that the appropriate tube can be located rapidly during an emergency. It is also helpful to maintain supplies needed to secure the tube in place once the animal has been intubated. This would include tie gauze, or other tube-securing material, and a clean empty syringe to inflate the cuff. Endotracheal tube cuffs should be checked at least once a week to ensure that they are functional and that no leak is present. This can be accomplished by inflating the endotracheal tube cuff underwater and observing the cuff for leaking air bubbles. Tubes with leaking cuffs should be discarded.
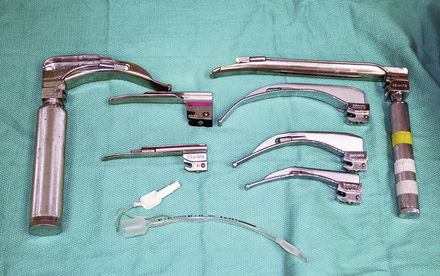
FIGURE 33-4 A variety of laryngoscope blade sizes and configurations are needed to assist with endotracheal intubation of dogs and cats
In larger crash carts, additional equipment to assist with airway control should be kept on hand. Sponge forceps may be used to clear the airway with gauze or to remove a foreign body without getting bitten. Transtracheal cannula and tracheostomy tubes may be useful in cases where normal per oral intubation is not possible because of facial trauma or upper airway obstruction (tumor, foreign body, or severe trauma). Tracheal cannulae are attached to an oxygen source and inserted into the trachea between cartilage rings. Some items used for tracheal cannulae include large-gauge through-the-needle catheters, large-gauge needles (attached to an extension set and 6-ml syringe adapter), or a modified macrodrip IV fluid set (Figure 33-5). In some situations, a small-diameter polypropylene catheter may be passed through the mouth into the trachea and used as a cannula to administer oxygen or as a guide (stylet) to direct an endotracheal tube. This procedure may be helpful until a surgical tracheostomy can be performed or until airway obstruction can be otherwise resolved. As previously noted, an Ambu bag or anesthetic machine should be located close to the crash cart so that assisted ventilation can start immediately after the animal is intubated.

FIGURE 33-5 A macrodrip IV fluid set can be fashioned for use as an emergency tracheal cannula until more appropriate equipment can be located
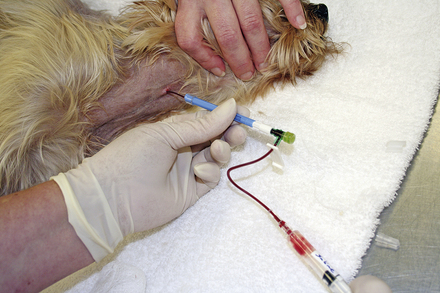
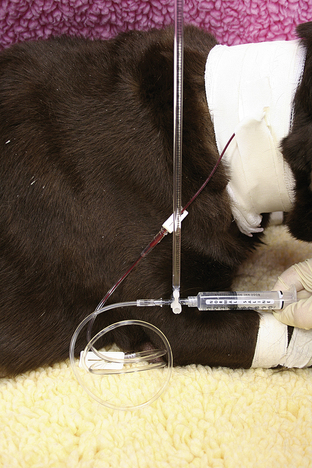
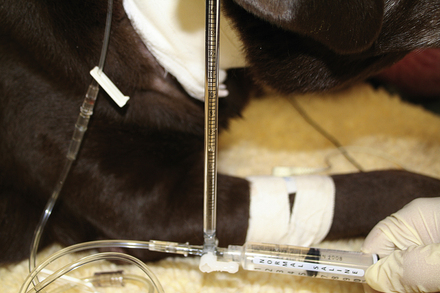
Items to establish venous access should be kept in the crash cart for use in crisis patients who do not already have an IV catheter or who need additional venous access. A selection of various sizes of IV catheters should be well stocked and organized. In addition, bone marrow needles or intramedullary catheters are desirable for small patients (Figures 33-6 and 33-7). Bone marrow catheters are ideal for puppies, kittens, and exotic pets because the bone marrow cavity connects to the vascular system and small patients often have fragile or inaccessible veins. Commercially available bone marrow catheters, spinal needles, or 18- to 20-gauge hypodermic needles can be used for this purpose. Porous tape and gauze bandage material for stabilization of any vascular line will also be necessary and should be included in the crash cart inventory. Hair clippers and solutions for aseptic skin preparation should be within easy reach. Commonly used IV fluid solutions (i.e., lactated Ringer's, 0.9% saline solution), synthetic colloids (i.e., hetastarch), hypertonic saline, and a pressure infusion bag should be kept close at hand for emergency fluid resuscitation.
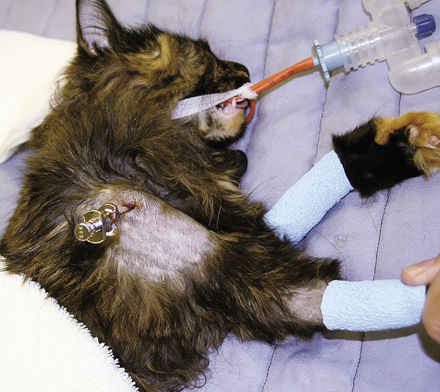
FIGURE 33-7 A bone marrow catheter is shown inserted into the humerus of a cat after repeated attempts at IV catheterization failed
A selection of emergency drugs, especially those used in the treatment of cardiopulmonary arrest, should be kept in the crash cart. Drug bottles should be well labeled and kept in specific and consistent locations within the cart to facilitate their use during an emergency.
Cardiopulmonary resuscitation (CPR) and emergency drug selection are discussed in detail later in this chapter. During emergencies, drug dose and administration errors may be prevented if the veterinary staff is familiar with the location of the drugs and with drug concentrations. If space allows, it is helpful to have various sizes of sterile syringes with the needles already attached to facilitate rapid drug administration. During CPR attempts, some drugs may be administered via the endotracheal tube (see administration routes under the discussion of CPR). This requires using a catheter of some sort (i.e., red rubber catheter cut to an appropriate length or a similar size polypropylene urinary catheter). Two such catheters should be on hand (one for larger-sized animals and one for small animals). During a crash scenario, remembering and calculating doses can take time and introduce errors. At the same time, estimated doses may be dangerously inadequate or overzealous. Emergency situations rarely allow time for individual dose calculations for each patient. Therefore some sort of centrally located drug dose chart should be posted at the emergency care station with a smaller version kept with drugs in the crash cart.
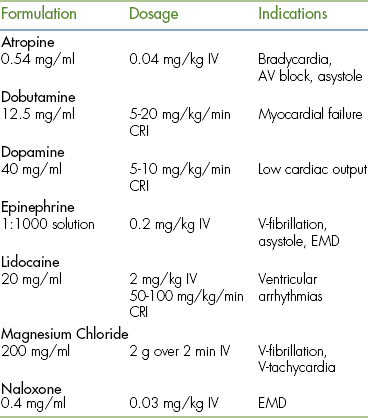
Commonly used emergency drugs include atropine, epinephrine, lidocaine, and naloxone (Table 33-1). Computer drug calculation programs are available that generate an emergency drug card that can be printed for all high-risk patients. Charts and drug cards allow team members to quickly read the appropriate drug volume based on the species and body weight.
Electrical defibrillation is indicated in the treatment of some cardiac arrhythmias (primarily ventricular fibrillation). Electrical defibrillators are available through many medical equipment distributors and may be combined with an electrocardiogram (ECG) monitor. This equipment should be located at the central emergency care station and used by experienced staff and veterinarians. Special training is required for the safe use of electrical defibrillators.
Depending on space, other items, such as surgical packs for emergency procedures, may also be stored in the crash cart. Common procedures performed at the emergency station include venous cutdown, thoracotomy for open chest CPR, thoracic drain placement, and tracheostomy (Figure 33-8). Sterile instruments and drapes for these procedures should be available in the emergency area (if not in the crash cart). Basic bandaging and splinting supplies, irrigation fluids, and sterile water-soluble lube (for clipping around and lavaging open wounds) should also be available.
LABORATORY
Next to the emergency care station, equipment should be close at hand to obtain baseline examination and laboratory parameters. Often these parameters include but are not limited to temperature, pulse, respiration, mucous membrane color, capillary refill time (CRT), blood pressure, ECG, and oxygen saturation via a pulse oximeter. Basic laboratory data, often referred to as “quick assessment tests” (QATs), may include packed-cell volume (PCV), total plasma solids, blood glucose, blood lactate, blood urea nitrogen (BUN), and urine specific gravity. Cage-side laboratory data can be obtained using “dip stick” test strips, glucometers, or point-of-care analyzers. Point-of-care testing equipment is available for coagulation assessments, arterial blood gas analysis, and basic serum biochemistries. Automated blood analyzers (Figure 33-9) allow for multiple blood parameters to be assessed quickly and repeated later for comparison.

FIGURE 33-9 Automated blood analyzers are available for assessment of biochemical, arterial blood gas, and coagulation parameters (i-Stat, Symbiotics 3000)
All veterinary hospitals should also have a basic laboratory area with a microscope set up to view blood smears or cytology samples. Blood smear examination can provide important diagnostic clues that may aid the emergency clinician (including red cell morphology, relative white blood cell numbers, and platelet count estimates).
Commercial test kits can also be kept at hand for rapid detection of toxin exposure (i.e., ethylene glycol) and infectious disease status (i.e., canine parvovirus, feline retroviruses, and heartworm disease). Rapid blood typing and crossmatching kits are available for pretransfusion testing. Other useful equipment, such as an ultrasound unit, may also be kept near the emergency triage area.
FLUID THERAPY
Fluid therapy is a valuable asset in the treatment of critically ill animals. Although fluid therapy is commonly used in veterinary hospitals, there are often questions concerning which fluids are appropriate and what volumes should be delivered. It is helpful to think of fluid therapy as expanding the animal's blood or plasma volume. This volume expansion lasts for a variable period of time depending upon the fluid administered and the animal's condition. Common reasons for providing fluid support in critically ill pets include:
3. Maintaining IV access and delivering other medications
4. Treatment of shock or hypoproteinemia
Crystalloid fluids are isotonic fluids consisting primarily of water with sodium or glucose. They are used for volume expansion and rapidly redistribute into the extracellular space. Only approximately 25% of crystalloid fluids remain in the vascular space after 1 hour. Crystalloid fluids are inexpensive and readily available in most practices. Examples of commonly used crystalloid solutions include 0.9% saline, lactated Ringer's solution (LRS), Normosol-R, and Plasma-Lyte. Colloid solutions are also used to expand vascular volume. They contain high-molecular-weight particles, which remain in the vascular space for longer periods of time. The hemodynamic effects of most colloids are similar to plasma and last longer than crystalloid fluids. Colloids can be used as single-agent therapy or in conjunction with crystalloid fluids. Use of colloids can reduce the volume of crystalloid solution required in some animals. These agents are used in a variety of critical care cases. One disadvantage of using colloids is the additional expense incurred. Examples of synthetic colloids include hydroxyethyl starch (hetastarch).
The route of fluid administration is an important aspect of fluid therapy. Subcutaneous fluid administration is popular because it is quick and easy and can be used during home management or outpatient management of some animals. However, fluid absorption via this route may be slow and unpredictable. For this reason, overreliance on subcutaneous fluid therapy should be avoided. The IV route is often the best route to administer fluids in critical animals.
Large-bore catheters can be placed in peripheral or central veins to increase the veterinarian's ability to administer fluid and medications to sick animals. In some critically ill animals, (especially puppies and kittens), venous access may be difficult to obtain. If venous access is limited, the intraosseous route can be used by using purpose-made intramedullary catheters, stylet needles, or bone marrow needles. Once the animal is volume expanded, peripheral veins may be more accessible for IV catheterization.
This fluid therapy plan should be adapted to the needs of each individual case and based on solid patient monitoring. In planning fluid therapy, veterinarians often consider an emergency phase, a replacement phase, and a maintenance phase. Emergency fluid therapy in the treatment of shock (i.e., “emergency phase”) is outlined elsewhere in this chapter. Replacement fluid therapy is intended to restore fluid balance to dehydrated animals. The volume of fluid to be replaced is calculated by estimating the percent dehydration and multiplying this number by the body weight in kilograms. The product of these numbers equals the replacement fluid volume in liters. For example, a 20-kg dog estimated to be 7% dehydrated would need 1.4 L of fluid (0.07 × 20 kg = 1.4 L). The rate of fluid replacement is dependent on clinical signs and the rate of fluid loss. If the animal has become acutely dehydrated, the volume may be replaced over 6 to 8 hours. If the loss has been chronic, it can be administered over 24 hours. Maintenance fluid requirements are calculated by well-established formulas. Most formulas are based on body weight (e.g., maintenance fluid dose = 60 ml/kg/day), although some believe that fluid requirements are best approximated by the basal energy requirement (e.g., fluid dose in ml/day = (30 × kg body weight) + 70). Ongoing fluid losses from vomiting, diarrhea, hemorrhage, or fluid effusion may occur in hospitalized patients. Staff members should record and estimate the volume of such losses so that the veterinarian can devise an appropriate maintenance fluid therapy plan that takes such losses into consideration.
Calculated fluid rates are only a starting point, and animals receiving fluid therapy must be closely monitored for both dehydration and fluid overload.
Relying solely on calculated fluid rates places the animal at risk for either inadequate fluid therapy or “overhydration.” Clinical signs and subjective parameters associated with overhydration include coughing, tachypnea, respiratory distress, nasal discharge, conjunctival edema (also called chemosis [Figure 33-10]), and peripheral edema (Figure 33-11). Abnormal lung sounds may be due to pulmonary edema caused by overhydration. Objective parameters used to evaluate fluid therapy include hematocrit (or PCV)/total protein (TP) (PCV/TP), body weight, central venous pressure (CVP), blood lactate, urine specific gravity, and urine output. The PCV/TP should be monitored frequently as shock and replacement fluid therapy is administered. If the hematocrit falls below 20% or if the TP decreases by 50% or more of the initial value, the veterinarians may consider changes in type of fluid or rate of administration. In addition, catheters and catheter sites should be routinely evaluated to check for catheter patency and cleanliness and to guard against catheter-associated inflammation.
STANDARDS OF CARE AND EMERGENCY PROTOCOLS
Standardized procedures and patient care protocols are helpful in dealing with common clinical presentations and emergency situations. A standardized approach helps maintain an expected standard of care, allows the veterinary team to respond rapidly to a crisis, and minimizes confusion among staff members during an emergency. Written procedure manuals can aid in the training of new staff members and serve as a reference for review by experienced team members. In developing a procedures manual, minimal standards of care should be agreed upon. If a group of practices have shared staff or clientele (i.e., an emergency clinic that serves a community of veterinary practices), agreed-upon procedures or protocols enhance patient care and facilitate communication. Certain situations (trauma, shock, CPR) are common scenarios in all veterinary facilities and merit individual discussion in this chapter.
TRIAGE OF THE TRAUMA PATIENT
Traumatic injuries in small animals require rapid, accurate assessment and special monitoring to ensure good care and to guard against the secondary complications of trauma. The patient with multiple body-system trauma is at a greater risk for complications as a result of the additive effects of each injury. Secondary complications of trauma include but are not limited to disseminated intravascular coagulation (DIC), sepsis, multiorgan failure, and distress caused by pain.
“Triage” is the process of determining the priority of need and the proper order of treatment when evaluating a clinical situation. Triage may be used to identify which patients in a group of animals require immediate treatment. In addition, standard triage protocols are used to identify which problems and which body systems should be evaluated first in a patient with multiple abnormalities. Most veterinarians are familiar with mnemonics dictating the principles of triage. These mnemonics are useful in managing cases and in instructing staff members in the approach to emergency situations and cardiopulmonary arrest. Initially the “ABCs of cardiopulmonary resuscitation” illustrate a good strategy for assessing trauma victims and animals with cardiopulmonary arrest. In this protocol, priority is given to treating respiratory, cardiac, and vascular problems. Other body systems are subsequently evaluated in a systematic manner. Following the ABC protocol, the letter “A” reminds us to consider arterial bleeding and to rapidly establish an airway. The letter “B” directs our attention to breathing assistance and manual ventilation (if needed). Subsequently the letter “C” prompts evaluation of cardiac and circulatory problems, such as hypotension and dehydration. Following the ABC strategy ensures support of vital body systems. Box 33-1 describes an alternative mnemonic, A CRASH PLAN, that refers to treatment priorities over an extended spectrum of body systems. The diverse nature of biology and medicine leaves room for debating the exact priorities in any given case. The real value of these schematic plans lies in their ability to standardize treatment and encourage rational stepwise thought during otherwise chaotic emergency situations.
Arterial bleeding is a serious priority for animals in an emergency. In part, this problem is uncommon because animals with significant arterial hemorrhage may not survive long enough to reach a veterinary hospital. If arterial hemorrhage is present, direct pressure should be applied to the wound immediately and continued until definitive control of bleeding may be attempted. If the vessel is easily visualized, the vessel can be clamped. However, placement of ligatures is time consuming and is not an immediate priority during the initial emergency assessment. A tourniquet may be applied to a limb to control hemorrhage if the distal limb cannot be salvaged (Figure 33-12).
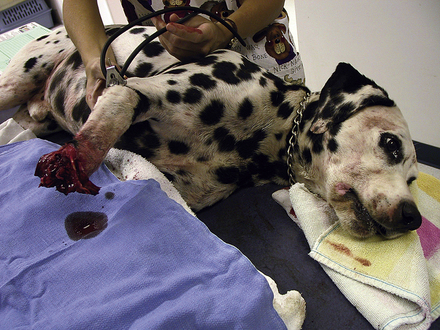
FIGURE 33-12 Tourniquet placement can control arterial bleeding from the extremities when the distal limb is not salvageable. (Photo courtesy of Dr. John Mauterer, Mandeville, La.)
The respiratory system is the next priority. Evaluation can be done quickly and efficiently by observation and auscultation. Visual assessment of respiratory pattern and mucous membrane color can be combined with thorough auscultation. Oxygen supplementation should be provided if the patient is in respiratory distress or is not hemodynamically stable (as judged by pale or cyanotic mucous membranes, weak pulses, rapid heart rate, or presence of an irregular heartbeat). Techniques for supplementation of oxygen include face mask (Figure 33-13) or blowby technique (Figure 33-14), nasal cannula (Figure 33-15), and placement in an oxygen cage (Figure 33-16) or oxygen canopy (Figure 33-17). Lastly, intubation with manual or mechanical ventilation may be used to supply 100% oxygen.
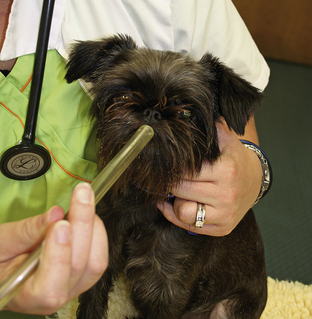
FIGURE 33-14 Oxygen flow from an oxygen source can be administered by the “blowby” technique to provide temporary noninvasive oxygen supplementation
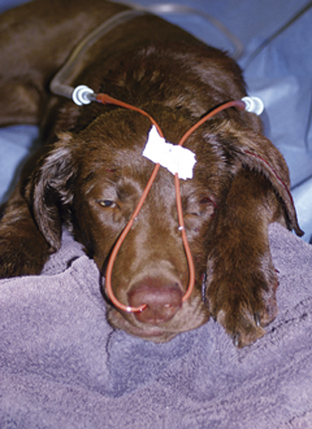
FIGURE 33-15 Bilateral nasal canulas can be attached to an oxygen source for comfortable and durable oxygen supplementation

FIGURE 33-17 An oxygen canopy can be constructed from an Elizabethan collar and used in most veterinary practices
Veterinarians and technicians should be alert to common and severe respiratory problems associated with trauma, including upper airway trauma or rupture, pneumothorax, hemothorax, pulmonary contusions, diaphragmatic hernia, and flail chest. Signs of upper airway trauma may include bloody respiratory discharge, increased respiratory effort, subcutaneous emphysema, and increased upper airway noise. In animals with pneumothorax and hemothorax, air or blood becomes trapped between the body wall and lung, resulting in collapse or compression of the lung. Clinical signs of hemothorax or pneumothorax include rapid shallow breathing (a restrictive breathing pattern) and respiratory distress. Flail chest results from two or more consecutive ribs that are broken in two places. This results in an independently moveable segment of the chest wall with paradoxical motion during respirations (i.e., a flail chest segment collapses during inhalation and expands during exhalation). Pain associated with flail chest segments further inhibits normal breathing. Rapid recognition of these problems is imperative because additional emergency procedures including thoracocentesis and thoracic drain placement may be required for animals with these conditions. Thoracocentesis is a diagnostic and therapeutic procedure that can be the difference between life and death until a thoracic drain can be placed.
Additional diagnostics may include thoracic radiographs, pulse oximetry, and arterial blood gas analysis. Respiratory injuries often benefit from oxygen supplementation, but severe injuries may require mechanical ventilation.
The cardiovascular system is clearly a priority system and is often assessed in combination with the respiratory system. Thoracic auscultation combined with assessment of mucous membrane color (CRT) and femoral artery pulse quality provides a quick evaluation of the cardiovascular system. An ECG can be used to detect the presence of heart rhythm disturbances. Often monitoring equipment can be set up during the animal's initial assessment so that the ECG is performed as an extension of the physical examination.
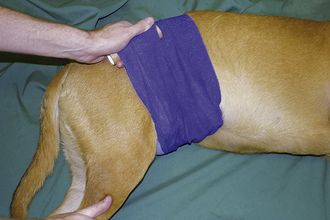
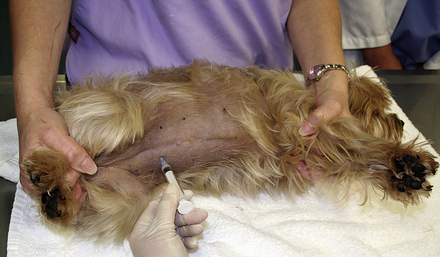
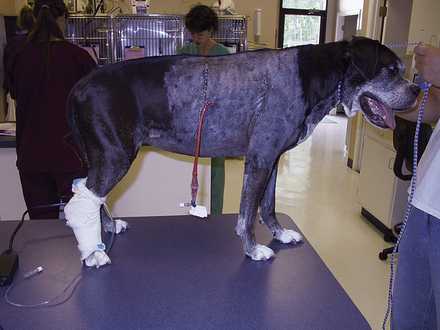
During triage of the trauma patient, blood loss must be evaluated and addressed immediately. Mucous membrane color, CRT, and hematocrit with total plasma solids should be evaluated as soon as possible. If outward hemorrhage is apparent, this must be addressed, and fluid therapy and/or blood products should be considered. Remember that internal hemorrhage may be occurring in sites that are not easily observed, such as the pleural space and the peritoneal cavity. An abdominal pressure bandage may be placed if abdominal or pelvic injuries (i.e., femur or pelvic fractures, road rash) are outwardly evident. Application of such a bandage may help preempt a worsening hemoabdomen and prevent further cardiovascular decompensation. Proper application of abdominal pressure bandages is important. First, a folded gauze pad or a rolled towel is placed on midline (Figures 33-18 and 33-19) and secured with gauze cling (Figure 33-20). Further tension may be applied with cohesive bandage material (Figure 33-21). Care is taken not to secure the bandage so tightly that the animal has discomfort or impaired respiration. Thoracocentesis and abdominocentesis may be considered if patient monitoring suggests the presence of ongoing unrecognized hemorrhage. Abdominocentesis may also be used to detect uroabdomen (urine in the abdomen from rupture of the bladder or ureters).
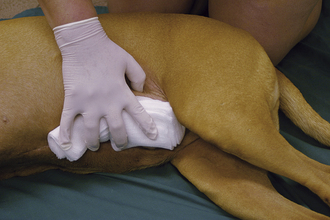
FIGURE 33-18 In the initial step of applying an abdominal pressure bandage, folded gauze is placed on midline to provide a padded site of pressure
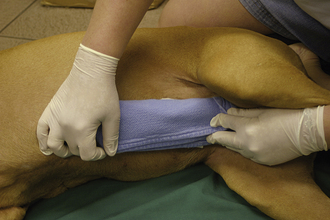
FIGURE 33-19 A rolled towel may be placed on top of the gauze pad to add to the cushion and further focus the bandage pressure

FIGURE 33-20 Gauze cling is used to secure the bandage material on midline and apply appropriate tension
Neurologic evaluation of the trauma patient is difficult. Recognizing severe head trauma and changes in intracranial pressure should be a priority. Increased intracranial pressure results from hemorrhage, edema, and inflammation. Signs of increased intracranial pressure include changes in mentation and level of consciousness along with changes in pupillary light response (PLR) and pupillary size. Although decompressive surgeries are sometimes used in people with increased intracranial pressure, most veterinary cases of head trauma are medically managed. This difference may in part be due to the availability of specialized trauma centers and emergency rooms equipped with cross-sectional imaging that treat people. Management of head trauma is complicated by controversy and debate among veterinarians. Veterinarians sometimes have concerns that overzealous fluid administration could worsen cerebral edema. However, in systemically unstable animals with head trauma, treating hypotension and hypovolemia is still a priority. Restriction of fluid to prevent cerebral edema in the face of hypotension should be avoided because hypotension and dehydration often worsen cerebral ischemia and hypoxia.
In head trauma patients, volume expansion with colloids may be more beneficial than the use of crystalloids because smaller volumes can be used. These smaller fluid volumes pose less risk for volume overload and cerebral edema. Veterinarians often disagree on the use of various drugs for head trauma. Corticosteroids reduce intracranial inflammation, but may have other harmful side effects. At this time, the consensus in the veterinary profession is against the use of steroids in head trauma. Mannitol and furosemide are diuretics that can reduce cerebral edema. Mannitol and furosemide are important drugs for head trauma patients. However, mannitol is contraindicated in animals with either active intracranial bleeding or hypovolemia.
Spinal injuries should be assessed via thorough palpation of the spine and critical evaluation of extremity pain sensation and tendon reflexes. However, a complete neurologic examination is usually postponed until after other triage priorities have been managed. A syndrome of “spinal shock” may interfere with interpretation of the neurologic examination for up to 24 hours.
Orthopedic injuries should be stabilized whenever possible. A splint placed on bone fractures must incorporate the joint above and the joint below the fracture site to provide adequate stabilization of the injury.
Early closure of contaminated soft tissue injuries is not a priority in animals with concurrent internal injury and circulatory compromise. Instead, sterile bandages may be applied to keep the wounds clean and moist until they can be dealt with safely.
Although emergency trauma cases can be stressful, it is important to follow a systematic approach to the patient. It is imperative that the patient be thoroughly examined and triaged when first seen and closely monitored during hospitalization.
TRIAGE OF THE CRITICAL CARE PATIENT
The principles of triage can be applied to many cases outside the realm of trauma and cardiac arrest. After all, stepwise management of treatment priorities is the basis for providing quality medical care. Severely ill animals often have a limited or vague clinical history. However, a methodical review of body systems (including a triagelike review of basic life support systems) will document a stoic animal's true clinical condition and help determine the nature and extent of disease. Application of standardized (triagelike) protocols also helps in the provision of good nursing care. For example, a recumbent animal with gray mucous membranes, tachycardia, weak pulses, poor CRT, and hypothermia will benefit from oxygen supplementation, IV fluid therapy, and external warming, irrespective of the final diagnosis. The value of a team approach cannot be overstated. Appropriate triage of body systems during daily interactions with the patient (including serial physical examinations) facilitates the recognition of new problems and important clinical changes. Nursing and technical staff often have a unique insight into the health of hospitalized animals by virtue of the time spent with each patient. Veterinarians can take advantage of this insight by listening to their staff and requesting additional clinical information. For these reasons, it may be beneficial to review the principles of triage with staff members in the context of critical care.
SECONDARY COMPLICATIONS
Secondary complications of trauma and critical illness are common in veterinary medicine. Although technicians are not called upon to make a diagnosis or formulate treatment plans, a detailed understanding of such complications can be beneficial. For example, well-trained staff members can prepare for and anticipate complications by knowing appropriate clinical signs and monitoring techniques. This knowledge enables experienced staff members, who are familiar with commonly performed diagnostic and treatment strategies, to better assist the veterinarian.
PAIN
Detection and assessment of pain is often a challenge in veterinary patients because animals cannot directly communicate their physical condition and there are no pathognomonic signs of pain. Common signs frequently associated with pain include vocalization, depression, anorexia, tachypnea, tachycardia, hypertension, hypotension, pale mucous membranes, aggression, abnormal postures, excess salivation, and dilated pupils. Abdominal pain in particular may be expressed by a classic “praying” or “play bowing” position in which the forequarters are crouched with the abdomen and hindquarters elevated from the ground (Figure 33-22). It is important to note that animals who do not exhibit any of these signs are not necessarily pain free. All trauma victims should be assumed to experience some degree of pain. Many critical illnesses, such as pancreatitis, meningitis, and cancer, are clearly painful problems. Obtunded animals, such as those with severe head trauma, may be unaware of or simply unable to express their pain. Pain management is an important component of veterinary care. Untreated pain causes stress and harmful physiologic changes that prolong recovery.
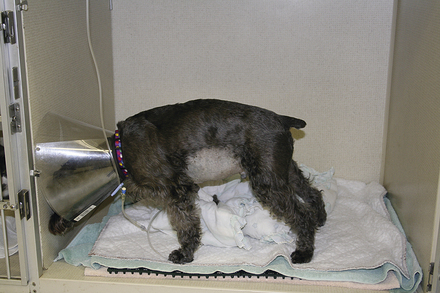
FIGURE 33-22 The classic posture of abdominal pain (demonstrated by this schnauzer with pancreatitis) consists of a raised and guarded abdomen in combination with reluctance to lie down
Staff members trained in the recognition and treatment of pain can help ensure that appropriate analgesia is provided in a compassionate and preemptive manner as part of sound medical care. Many analgesic drugs (i.e., opioids) have cardiac and respiratory depressant effects, which can be dangerous in systemically unstable animals. Nonsteroidal antiinflammatory drugs (NSAIDs) have little effect on the cardiopulmonary system, but may have side effects that affect the gastrointestinal and renal systems. With few exceptions, the systemic administration of analgesics may be safely considered. Regional or local analgesia techniques are useful and should also be considered. For instance, flail chest segments may be treated with a local anesthetic nerve block to reduce pain and allow comfortable breathing.
DISSEMINATED INTRAVASCULAR COAGULATION
Trauma causes tissue and/or vessel injury that is a normal trigger for coagulation (blood clot formation). In most cases, the body's natural homeostatic mechanisms prevent widespread abnormal clotting by balancing clot formation with clot resolution. However, in animals with massive injuries and severe inflammation, the natural balance between clot formation and clot prevention and resolution may be disrupted. When this happens, massive activation of coagulation overwhelms the body's normal regulatory functions and systemic clot formation begins on a widespread scale (instead of confined to a small site of injury). This phenomenon of systemic clot formation and loss of regulatory control is called disseminated intravascular coagulation. In DIC, microclots form throughout the body's capillary network disrupting blood flow to vital organs and causing organ failure (especially in the kidneys, brain, heart, and lungs). Widespread and uncontrolled clotting and clot lysis consume platelets, clotting factors, and regulatory protein, which paradoxically leads to bleeding tendency. DIC is often described as a vicious cycle where clotting and bleeding are occurring spontaneously and simultaneously. Importantly, DIC is always a secondary complication of some other severe disease. Common veterinary causes of DIC include trauma, pancreatitis, heatstroke, cancer, liver disease, immune-mediated hemolytic anemia, and snake envenomation. Clinical signs of DIC are often masked by those of the primary disease or trauma.
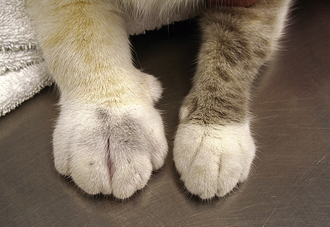
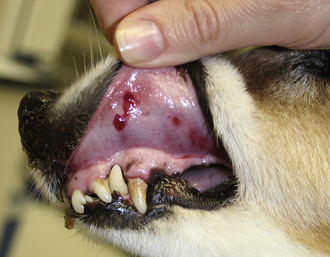
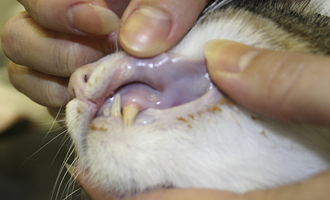
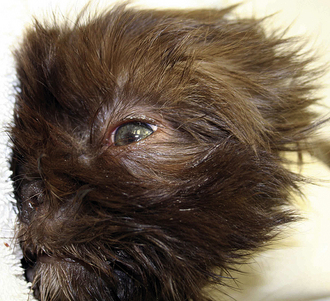
In the early stages of DIC, the body is clotting excessively (hypercoagulable), and signs of thrombosis and poor blood flow are prevalent. These signs include unexplained edema, cold extremities, tachypnea, pale mucous membranes, hypotension, and neurologic signs. In later stages of DIC (once clotting factors have been consumed), bleeding tendencies are prevalent. In this phase, clinical signs include unexplained hemorrhage or bruising (hematoma, intraocular bleeding, hemoabdomen or thorax, and excessive bleeding from venipuncture sites). Tiny pinpoint bruises, known as petechiae, commonly appear on the skin (especially along the ventrum and inguinal areas [Figure 33-23]) and mucous membranes (especially the gums and sclera [Figure 33-24]). Large petechial hemorrhages, known as ecchymoses, may form in similar areas (Figure 33-25).

FIGURE 33-23 The bleeding tendency associated with DIC often results in small hemorrhages called petechiae, which are often seen on the thinly haired skin of the ventral abdomen
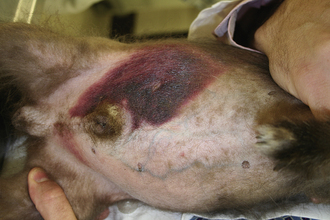
FIGURE 33-25 Larger hemorrhages, such as the one on this dog's abdomen, are sometimes called ecchymoses
There is no single specific sign or test for the diagnosis of DIC. Instead, the diagnosis is based on supportive lab findings and clinical signs in animals with severe underlying diseases. Decreased platelet count (as a result of platelet consumption) is a consistent and early finding in DIC. Another reliable indicator of DIC is red blood cell (RBC) morphology. Schistocytes or fragmented RBCs are often seen in cases of DIC because fibrin strands span small blood vessels and “rough up” the red cells. This results in distorted borders and red cell fragments that may be seen on the blood smear.
In later stages of DIC, a coagulation profile may detect clotting factor deficiency. A coagulation profile often includes several tests of clotting function, including the prothrombin time (PT), partial thromboplastin time (PTT), and the activated clotting time (ACT). The advent of in-house coagulation time analyzers allows practitioners to conveniently monitor trends in all bleeding times. Measurement of anticoagulant protein (i.e., antithrombin levels) and by-products of clot breakdown (i.e., fibrin degradation products and d-dimers) are also used in the diagnosis of DIC. In cases of DIC, antithrombin levels decrease, whereas fibrin degradation products (FDP) and d-dimers increase. These changes occur as a result of consumption of regulatory anticoagulant protein and accumulation of products of clot breakdown.
Successful treatment of DIC requires resolution of the primary disease. Supportive care with fluid therapy and oxygen supplementation are important. Adjunctive therapy with blood products and anticoagulants may interrupt the self-propagating cycle of coagulation and blood loss. In addition, plasma products and fresh whole blood transfusions help replenish depleted clotting factors, provide anticoagulant regulatory protein, and manage anemia caused by blood loss. Whole blood may also provide some fresh platelets, but it should be noted that these platelets are generally few in number and survive only a short time. Heparin is an anticoagulant commonly used in conjunction with plasma. If heparin is used at moderate to high doses, it should be tapered before discontinuing therapy. Unfortunately, treatment of DIC is often unsuccessful because many underlying conditions cannot be rapidly resolved and it is exceedingly difficult to restore the body to a state of healthy equilibrium once compensatory mechanisms are overwhelmed. DIC carries a poor to grave prognosis.
SHOCK AND THE SYSTEMIC INFLAMMATORY RESPONSE SYNDROME
Animals with poor blood flow and impaired oxygen delivery to tissues are said to be in a state of “shock.” This clinical syndrome is caused by circulatory failure (despite its name, “shock” has no association with electrocution). Untreated shock is rapidly fatal because imbalance between tissue oxygen demand and oxygen delivery causes tissue injury, organ failure, and death.
In the early stages of shock, impaired perfusion triggers natural compensatory mechanisms (vasoconstriction, increased heart rate, increased cardiac contractility) that maintain blood pressure and increase cardiac output. This early or compensated phase of shock is known as the hyperdynamic phase or compensatory phase. Clinical signs during this phase relate more to adaptive physiologic responses than to perfusion failure. The clinical signs in this phase include increased heart rate and respiratory rate, rapid CRT, injected mucous membranes, and increased pulse pressure. These findings can be subjective and easily missed. During this phase, the weakness, depression, and altered consciousness associated with later stages of shock are either absent or mild. The most recognizable clinical signs of hyperdynamic shock include brick red mucous membranes and bounding pulses.
Close monitoring performed by alert staff members is important because early recognition and treatment may prevent further progression of shock.
Uncompensated or hypodynamic shock ensues if there is progressive underlying disease or if compensatory mechanisms and treatment fail to restore normal blood flow and oxygen delivery to the body. During uncompensated shock, cardiac output and systemic blood pressure are inadequate. Blood flow is preferentially distributed to vital organs (brain, heart) at the expense of other tissues. This shunting of blood exacerbates the oxygen deficit and fluid imbalance in other tissues and can lead to organ failure. The commonly recognized clinical signs of uncompensated shock are associated with circulatory failure and include hypotension (low blood pressure), rapid heart rate, weak pulses, prolonged CRT, pale mucous membranes, hypothermia, overt weakness, depression, and loss of consciousness. Eventually, prolonged hypoxia results in vascular paralysis, systemic vasodilation, and fulminant cardiovascular collapse. This terminal phase of shock is irreversible and rapidly fatal.
Shock is a state of emergency that is associated with many causes. When subdivided according to underlying cause, general categories of shock are hypovolemic shock, distributive shock, cardiogenic shock (including obstructive shock), and septic shock. Hypovolemic shock is the most common form of shock in small animals. In this form of shock, perfusion failure results from a reduction in circulating blood volume caused by bleeding, dehydration, or effusive fluid loss (i.e., abdominal fluid accumulation). Distributive shock is associated with maldistribution of blood flow associated with pathologic vasodilation. In this syndrome, pooling of blood in capillaries and veins results in a decrease in effective blood volume (regardless of intravascular volume or cardiac output). Common causes of distributive shock include trauma, heatstroke, envenomation, and anaphylaxis. As its name implies, cardiogenic shock is associated with decreased cardiac output. Cardiogenic shock can occur from heart failure resulting from many primary heart diseases, such as cardiomyopathy, valvular disease, and cardiac arrhythmias. A subset of cardiogenic shock known as obstructive shock is associated with obstruction of blood flow. Common causes of obstructive shock include pericardial disease, heartworm disease, pulmonary hypertension, and pulmonary thromboembolism.
Shock that is caused by infection is referred to as septic shock or sepsis. Septic shock can be triggered by primary infectious diseases, but it can also occur with opportunistic infections. Extensive tissue damage associated with severe disease (such as trauma, heatstroke, envenomations, and pancreatitis) creates areas of poorly perfused and devitalized tissue that provide a setting for opportunistic bacterial growth. Infection in such areas is difficult to combat because disruptions in blood flow prevent systemically administered antibiotics from reaching the site of infection. The local inflammatory response triggered by some bacterial toxins may help clear infections. However, in animals with widespread injury or tissue damage, an exaggerated inflammatory response develops that leads to uncontrolled systemic inflammation. This systemic inflammation precipitates a state of shock by inducing vasodilation, vascular permeability, poor cardiac function, and activation of coagulation. Hyperglycemia may occur in the early phase of septic shock as a result of the effects of stress hormones on metabolism. In later stages, hypoglycemia is predominant as glucose is consumed by both bacteria and body demands.
In the absence of infection, a systemic inflammatory response syndrome (SIRS), which parallels septic shock, can be triggered by any critical illness where systemic inflammation is a problem. As is the case with other forms of shock, SIRS patients may go through an early hyperdynamic phase followed by an uncompensated or hypodynamic phase. In the hyperdynamic phase, circulatory collapse is temporarily held at bay by compensatory mechanisms that increase cardiac output and maintain blood pressure. During this phase, bounding pulses and brick red mucous membranes may be noted. Clinical manifestations of SIRS include circulatory changes, thermoregulatory dysfunction (fever or hypothermia), depression, tachypnea, and DIC. Recognition of septic shock and SIRS in individual patients is based on clinical findings, supportive history, and laboratory data. Fulminant septic shock and SIRS are both associated with a syndrome of multiple-organ dysfunction (MODS). Kidney failure and liver failure are particularly common.
TREATMENT OF SHOCK
Treatment of underlying diseases is important in the management of animals in shock. For instance, animals with septic shock should receive appropriate antibiotics, and animals with traumatic bleeding may need a blood transfusion. Unfortunately, management of underlying conditions takes time and is often only partially effective. Therefore animals diagnosed with SIRS or other states of shock should receive treatment based on triage priorities similar to those animals in cardiac arrest. Treatments are focused on restoring oxygen delivery and perfusion to the tissues. Oxygen supplementation should be provided immediately via face mask during initial resuscitation efforts. This allows a staff member to continually monitor the patient during treatment. Nasal oxygen catheters or an oxygen cage (or canopy) may also be used. Preventing circulatory collapse is the highest treatment priority during management of shock syndromes. This is accomplished primarily with aggressive fluid therapy to restore effective vascular volume and blood pressure. Shock dosages of crystalloid solutions are 90 ml/kg/hr in the dog and 45 to 60 ml/kg/hr in the cat. When using crystalloid therapy in the dog, shock doses of fluid can be administered in quarter-dose increments. For dogs, a quick formula to calculate a “quarter shock dose” is to take the body weight in pounds and multiply by 10. For example, a volume of 400 ml is an appropriate quarter shock dose for a 40-lb dog. This “quarter shock dose” is given over 15 minutes, and the animal is reevaluated. For cats, the same formula can be used; however, you must divide the dose in half (or use the body weight in kilograms instead of pounds).
A pressure bag may be helpful for administering large fluid volumes over a short period of time (Figure 33-26). Colloid fluids are also used to restore vascular volume to patients in shock. Colloid doses depend on the type of colloid used. Blood products, such as whole blood and plasma, may also be used in some cases during resuscitation efforts. Hemoglobin-based oxygen carrying solutions made from polymerized bovine hemoglobin sometimes have limited commercial availability. When administered IV, these solutions act like a colloid, but also have oxygen-carrying capacity. These features make them popular resuscitation fluids for some clinicians.
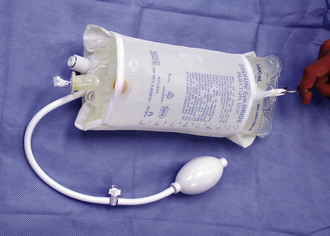
FIGURE 33-26 IV fluid bags can be placed within a pressure bag to increase the rate of fluid administration
As noted before in the section on fluid therapy, all fluid doses are simply guidelines. Fluid therapy should be administered “to effect,” which means that shock fluids are administered until monitoring parameters indicate that treatment has had the desired effect. Monitoring may indicate that higher or lower doses may be necessary. Appropriate fluid therapy often results in normalization of heart rate, blood pressure, and mucous membrane color. Additional monitoring parameters include CVP, urine output, blood lactate levels, hematocrit, and TP. It should be noted that unusually large crystalloid and colloid fluid volumes may be necessary to maintain vascular volume in unstable (“shocky”) animals. Intensive patient monitoring is critical.
Animals with refractory hypotension, despite appropriate fluid therapy, may be candidates for use of vasopressor drugs (dopamine, dobutamine).
Veterinarians generally give these drugs as a constant rate infusion to increase vascular tone and cardiac output. At low dose levels in dogs, dopamine selectively increases renal perfusion, which can be helpful in restoring urine output. At moderate doses, beneficial effects on systemic blood pressure are prevalent. However, at high doses, dopamine has an adverse effect on renal perfusion and may worsen kidney failure. Dobutamine primarily increases blood pressure by enhancing cardiac contractility and cardiac output. Both dopamine and dobutamine can be associated with cardiac arrhythmias, and ECG monitoring and careful auscultation may be helpful.
PERFUSION FAILURE AND REPERFUSION INJURY
Reperfusion injury is a cellular injury that develops as blood flow returns to an area or tissue previously deprived of perfusion. During cardiopulmonary arrest and shock, oxygen-starved tissues develop an anaerobic metabolism and are depleted of cellular energy stores. These conditions alter certain enzyme systems and destabilize white blood cell (WBC) membranes. Upon reestablishment of oxygenation and perfusion (as occurs with successful resuscitation and fluid therapy), the altered enzyme systems generate harmful molecules called oxygen free radicals. At the same time, membrane-damaged WBCs release inflammatory mediators that contribute to the reactive environment. The oxygen free radicals and inflammatory mediators cause inflammation and vessel injury leading to thrombosis and edema. These effects are collectively called “reperfusion injury.” Reperfusion injury may result in systemic disorders, such as DIC, SIRS, and multiorgan dysfunction. Following resuscitation from shock or cardiopulmonary arrest, all vital organ systems can be affected by reperfusion injury and inflammation. For this reason, all systems must be monitored closely and supported, with special attention paid to basic life support systems.
CARDIOPULMONARY ARREST
Cardiopulmonary arrest is defined as the cessation of breathing and effective blood circulation. In most veterinary patients, cardiopulmonary arrest occurs in dying animals as the terminal stage of an advanced disease. However, arrest can occur as a complication of any critical illness and even in healthy patients undergoing anesthesia. Resuscitation efforts are commonly referred to as cardiopulmonary resuscitation or more properly cardiopulmonary cerebrovascular resuscitation (CPCR). The acronym CPCR emphasizes the importance of maintaining perfusion and oxygen delivery to the central nervous system during and after an arrest.
This is important so that resuscitation offers a chance to return an animal to full function rather than simply returning cardiopulmonary function to a brain dead animal.
In an arrest, time is crucial if resuscitation is going to be successful. Therefore preparation and trained personnel are essential for management of these situations. Specific equipment and facility recommendations have been addressed previously in this chapter. In addition, one of the most important aspects of emergency preparedness involves knowing which patients are likely to arrest. These patients include those with heart disease, respiratory disease, hypothermia, multiorgan failure, trauma, and shock. Contributing factors also include hypoxia, heightened vagus nerve stimulation (vagal tone), acid-base disturbances, electrolyte abnormalities, and anesthesia. Common diseases associated with heightened vagal tone include gastrointestinal disease, respiratory disease, neurologic disease, and ophthalmic disease. The most commonly recognized source of vagus-mediated arrest occurs in weak and vomiting animals. Vomiting is accompanied by a reflex slowing of the heart rate (bradycardia) mediated by the vagus nerve. In susceptible animals, this slowing of the heart rate can be extreme and lead to cardiac arrest. Other stimuli for vagus-mediated arrest include urination and defecation. There are many factors involved in an arrest scenario because each individual animal deals with disease, traumatic insult, or stress differently. Sudden changes in the animal's physical status can be warning signs of impending arrest. In all high-risk patients, it is important to frequently monitor respirations, pulse rate/character, mucous membranes color (for pallor or cyanosis), and body temperature. Anesthetized patients should also be monitored for unexplained changes in anesthetic depth. Recognizing an impending arrest and alerting other team members to the crisis are the first steps in resuscitation.
CARDIOPULMONARY CEREBROVASCULAR RESUSCITATION
Resuscitation efforts (CPCR) may be divided into two phases: basic life support and advanced life support. As noted in the preceding discussion of triage, the steps of resuscitation may be correlated with letters of the alphabet to assist with training and remembering the order of steps. Basic life support involves the important first steps or ABCs of resuscitation. If these steps are not successful, subsequent efforts at resuscitation are futile.
BASIC LIFE SUPPORT
In basic life support, A is for airway. Staff members responding to a potentially arrested animal should note if the animal is breathing. If respirations are absent or weak, the mouth should be opened and the oropharynx examined for possible obstruction. Common sources of airway obstruction include respiratory secretions, aspirated vomitus, blood, ingested foreign material, and mass lesions (hematomas, neoplasia, etc). If obstruction is noted, the airway should be cleared with suction or manual removal of foreign material. Caution is indicated to prevent being bitten, although most animals in partial or full states of arrest have limited or no capacity to bite. A sponge forceps and gauze may be helpful in clearing some exudates. Once the airway is cleared, staff members should note whether these steps have stimulated the animal to breathe.
If the animal does not begin to breathe, the patient must receive ventilation assistance. B is for breathing. Mouth-to-nose resuscitation may be performed by sealing the lip margins and blowing into the animal's nose. This method requires no special equipment and will deliver about 16% oxygen. This level of oxygenation is inadequate and should only be done temporarily until a higher supply of oxygen can be provided. Mouth-to-nose resuscitation efforts carry some risk to caregivers treating animals with potentially zoonotic diseases. Endotracheal intubation and ventilation with an Ambu bag in room air provides 21% oxygen. The best method of assisted ventilation is endotracheal intubation and delivery of 100% oxygen from an oxygen source. Ideally, animals should be intubated in lateral recumbency to prevent elevation of the head and positional changes that impair cerebral blood flow in arrested animals. Many times, however, intubation is performed more rapidly and accurately using a laryngoscope with the animal in sternal recumbency. Rapid intubation is imperative, and delays or failure can be catastrophic to further resuscitation. Following intubation, the tube should be secured with a gauze tie. If intubation is not possible, a narrow orotracheal catheter or transtracheal cannula is sometimes useful. However, a large-bore airway is preferred and may require a surgical tracheostomy. During assisted ventilation, the first two breaths administered should be long breaths lasting a full 2 seconds, followed by patient assessment. In some instances, restoring an open airway will lead to recovery of spontaneous respirations by the patient. If not, the animal should be manually ventilated at a rate slightly higher than the expected normal. Assisted ventilation should expand the chest by 30%, with a slightly longer expiration than inspiration. If the breathing circuit contains a manometer (i.e., most anesthetic machines), a pressure of 10 to 20 cm of water should be obtained with each breath.
Acupuncture is a method that can be attempted to treat respiratory arrest when other efforts have failed. The acupuncture point is Governor Vessel 26 (VG 26) of Jen Chung. This point is located at the nasal philtrum at the level of the ventral edge of the nares (Figure 33-27). A 25-gauge needle is applied to the bone at this point and then twirled to induce breathing.
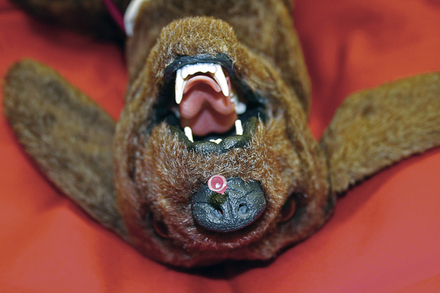
FIGURE 33-27 Jen Chung acupuncture to stimulate breathing is demonstrated in a canine resuscitation model
C is for circulation. Once the airway is established and ventilation provided, circulation must be assessed by palpation of pulses (or apex heartbeat) and auscultation of the heart. Peripheral pulses are nonpalpable when the mean blood pressure is less than 60 mm Hg. An apex heartbeat may be indistinguishable when the pressure is less than 40 mm Hg. It is important to note that some animals suffer respiratory arrest without cardiac arrest. Improper chest compressions can stress the patient and precipitate cardiac arrest in some of these cases. However, once cardiac arrest has been confirmed, chest compressions should be started immediately.
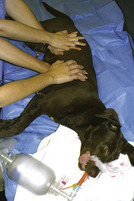
Positioning of the animal during compressions depends on the animal's size, the shape of the chest (barrel chest versus deep and narrow chest), and the caregiver's ability to deliver adequate compressions. There are two theoretic models to explain forward motion of blood during CPCR. In small animals or those with a narrow chest conformation, chest compressions (during closed-chest CPCR) and direct cardiac massage (during open-chest CPCR) apply forces to the heart that mimic the normal heart mechanics. This is known as the “cardiac pump.” In large animals and those with barrel chests, changes in chest conformation limit the direct effect of chest compressions on the heart. In these cases, increased intrathoracic pressure during compression results in forward blood flow from the heart, which serves as a passive blood reservoir. This is referred to as the “thoracic pump” model and is thought to play a significant role in medium- to large-size animals during CPCR. To optimize the cardiac and thoracic pumps, animals less than 15 lb (7 kg) should be placed in lateral recumbency. Animals greater than 15 lb may be placed in either lateral or dorsal recumbency. The point of compression (hand placement) for the cardiac pump is located directly over the heart (Figure 33-28). For the thoracic pump, the point of compression is located at the widest part of the chest.

FIGURE 33-28 Correct placement of hands on an animal less than 15 lb for the administration of chest compression, which simulates cardiac contractions (the cardiac pump)
Effectiveness of CPCR should be assessed by palpating for a pulse and evaluating the mucous membrane color. If available, an ECG can be extremely beneficial at this point to assess the heart and also to evaluate the effectiveness of resuscitation efforts. Traditionally a pulse and an electrical waveform on ECG should be generated with each compression. If available, end-tidal carbon dioxide (capnography) is a reliable monitor of ventilation and perfusion. Other monitoring equipment (ECG, pulse oximeter, venous blood gas analysis, and lactate levels) can provide useful quantitative information. If monitoring suggests the CPCR effort is inadequate, a change in technique may be required to improve effectiveness. Common changes to the CPCR effort include changing places with another team member, changing the animal's position, and/or altering your compression technique. Ventilation and chest compressions may be interposed or administered simultaneously. Administering ventilations and chest compressions simultaneously enhances the thoracic pump by increasing intrathoracic pressure during the compression (systole) and increasing venous return and atrial filling during relaxation (diastole). Once compressions have begun, a solid rhythm can develop if the breaths are administered simultaneously to the compressions. This rate should be 120/min for animals less than 15 lb and 80 to 100/min for animals greater than 15 lb. Interposed abdominal compressions assist in directing blood in the lower half of the body back toward the heart (via increased intraabdominal pressure) and may be administered by another team member (Figure 33-29).
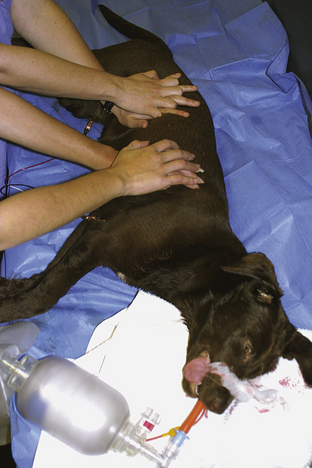
FIGURE 33-29 Abdominal compressions may be interposed with chest compressions to increase blood return to the heart during CPCR
Open-chest CPCR is mostly indicated in animals with chest trauma (flail chest, pneumothorax, and diaphragmatic hernias) because of the interference of such injuries on closed-chest compressions. In this method of CPCR, the chest is surgically opened on the left side at the fifth intercostal space, and compressions are applied to the heart from the apex to the base. Care should be taken not to twist the heart, which can occlude major vessels. Nontraumatic occlusion of the descending aorta during open-chest CPCR may improve coronary and cerebral blood flow. Open-chest CPCR is only beneficial if initiated early in the resuscitation effort. The decision to perform an emergency thoracotomy for open-chest CPCR should be made within 2 minutes of cardiopulmonary arrest.
ADVANCED LIFE SUPPORT
Advanced life support includes interpretation of an ECG and administration of drugs based on cardiac output, blood pressure, and the presence of arrhythmias. These steps in resuscitation are important only after basic life support has been established (i.e., the animal is being ventilated, and adequate circulation is provided). If the animal has responded to resuscitation efforts and has a perfusing rhythm, advanced life support may not be necessary. Unfortunately, in many cases, life-threatening arrhythmias and hypotension are common and require treatment.
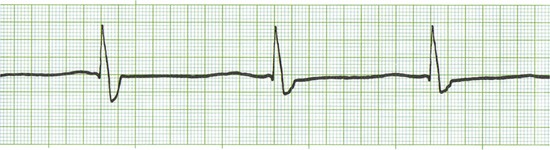
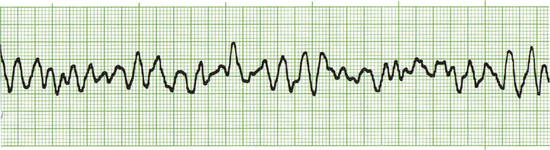
Common drugs used in CPCR include atropine, epinephrine, naloxone, lidocaine, and magnesium chloride or sulfate. Proper use of an ECG allows recognition of specific arrhythmias so that appropriate drugs may be administered and patient response to therapy can be gauged. There are three basic arrhythmias seen during an arrest. These include asystole (“flat line”) (Figure 33-30), nonperfusing rhythms (electromechanical dissociation or pulseless electrical activity) (Figure 33-31), and ventricular fibrillation (Figure 33-32). IV drug doses are listed in Table 33-1. In many cases, asystole and nonperfusing rhythms are preceded by progressive bradycardia. Bradycardia can be a sign of imminent arrest and should prompt notification of other staff members. Sinus bradycardia may be treated with atropine as the animal is monitored. During asystole, both electrical and mechanical cardiac activity has stopped. Asystole is treated with atropine and/or epinephrine with repeated doses administered if no response is observed. Electromechanical dissociation (EMD) is a common terminal arrhythmia in cats and dogs. The hallmark of this arrhythmia is the presence of ECG complexes with no cardiac contractions to generate a pulse (hence the synonym, pulseless electrical activity [PEA]). This rhythm can have a diverse appearance, but often mimics a ventricular arrhythmia with wide bizarre QRS complexes occurring at a slow rate. EMD is treated with naloxone, megadose atropine, or epinephrine.
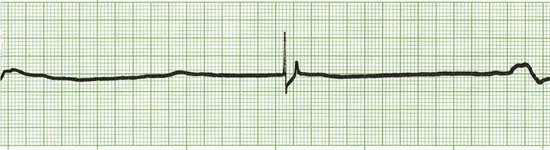
FIGURE 33-30 This ECG is from an arrested animal in asystole (flat line). A single “escape beat” is also present
Ventricular fibrillation is a common arrhythmia in people suffering myocardial infarction. It also occurs in cats and dogs during cardiopulmonary arrest. In dogs, ventricular fibrillation may be preceded by rapid ventricular tachycardia (Figure 33-33), especially when multifocal ventricular beats or R on T phenomenon are present. When ventricular fibrillation is diagnosed, it must be converted as soon as possible for resuscitation to be successful. Conversion of this arrhythmia may be attempted before initiation of basic life support. Treatment of choice for this arrhythmia is electrical defibrillation using an electrical defibrillator. If this is not available, chemical defibrillation may be attempted using drugs such as magnesium chloride. A strong precordial thump is potentially effective as a last resort. Electrical defibrillators should only be used by specially trained personnel (Figure 33-34). Tips for appropriate use of an electrical defibrillator include:
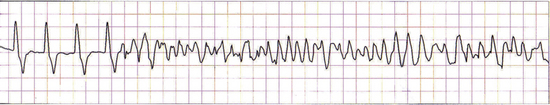
FIGURE 33-33 Ventricular tachycardia (on the left of the ECG) suddenly degenerates into ventricular fibrillation (on the right side of the ECG)
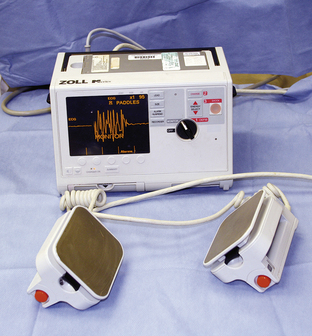
FIGURE 33-34 An electrical defibrillator and ECG should be located on top of the crash cart for treatment of ventricular fibrillation during cardiac arrest
1. Apply adequate pressure to the chest with the paddles.
2. Use the largest paddle surface area.
3. Use a proper conducting gel or saline solution-soaked gauze.
4. Make sure that all staff members are clear of the patient and table. (This includes the person operating the defibrillator.)
Alcohol should never be used near defibrillator paddles because of the risk for fire. The recommended dose is 2 to 4 J/kg. Initially, use a setting at the lower end of the dose range, and repeat or double the dose if no response is seen. Open-chest defibrillation requires specific paddles and a modified dose (usually one tenth of the transthoracic dose).
Drugs administered during CPCR may be ineffective as a result of poor perfusion and failure of the drugs to reach their target tissues (primarily the heart). A central vein catheter (i.e., jugular catheter [Figure 33-35]) is the CPCR drug administration route of preference during closed-chest CPCR. These catheters facilitate delivery of the drug(s) directly to the heart or its close proximity.
The next best route is intratracheal administration. This route of administration takes advantage of alveolar membranes, which have a large surface area and receive a high blood flow separated by a narrow diffusion barrier. An acronym to remember which drugs can be administered by the intratracheal route is LEAN (lidocaine, epinephrine, atropine, and naloxone). Intratracheal drugs are administered via a catheter passed through the endotracheal tube (Figure 33-36). Insertion of drug into the intratracheal catheter must be followed by a flush of air or saline to ensure drug deposition into the airways. A deep breath administered via manual ventilation helps to further distribute the drug. Drug doses administered by the intratracheal route may be rapidly estimated by doubling the IV dose. Small drug volumes may require dilution for effective administration. Drug uptake by the pulmonary circulation is impaired by conditions such as pulmonary edema, which negates this as a suitable route. Good communication between team members performing CPCR is imperative because a vigorous chest compression delivered at an inopportune moment may result in exhalation of medications administered intratracheally.
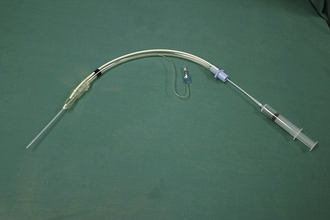
FIGURE 33-36 A polypropylene catheter passed through an endotracheal tube can be used for the intratracheal administration of some drugs during CPCR
Drugs may be administered via a peripheral IV catheter (i.e., cephalic or saphenous vein catheters) if a central line or intratracheal access is unavailable or contraindicated. All drugs administered peripherally must be followed with a good flush of saline solution to ensure delivery into the circulation and toward the heart. Intraosseous catheters and intralingual injection are another means of peripheral administration. Placement of an intraosseous catheter (or intralingual injection) in the arrested patient can be rapid and does not need to interrupt the CPCR attempt. Intralingual drug doses are usually double the standard IV drug dose. The last route for drug administration is intracardiac. This is chosen last because of the challenge of hitting a flaccid heart, the need to stop CPCR to administer drugs, and the risk of damaging the heart. However, in open-chest CPCR, intracardiac drug administration is preferred. Usually, one tenth of the IV dose is injected directly into the left ventricle.
Crystalloid fluids or colloids may be beneficial if hypovolemia was a predisposing factor of the arrest or to compete with peripheral vasodilation and support blood pressure. However, fluid support of peripheral circulation is not a high priority during CPCR. Fluids may be contraindicated during CPCR because fluid volumes are poorly tolerated by a failed cardiac pump and volume overload or overhydration easily develops.
The decision to initiate CPCR is made on a case-by-case basis according to the wishes of an informed pet owner. Many critically ill animals face an already grave prognosis. Following an arrest and successful resuscitation, the risk of rearrest and subsequent death is high. “Do not attempt resuscitation” orders may be indicated after discussion with the clinician and pet owner. Assessing the patient's risk of arrest and addressing the desires of the client are important early on. If a patient does not respond to CPCR within 20 minutes, continuation of the resuscitation effort is unlikely to succeed. Successful resuscitation rates in veterinary medicine are approximately 10%. Fortunately, certain patients do respond to basic and advanced life support. Many of these cases have a reversible disease process and/or a treatable cause of arrest. A written record of everything done during the CPCR should be made for the team to learn from and for the client record.
Regardless, the real work begins following successful resuscitation.
PROLONGED LIFE SUPPORT
Proper postresuscitation management has two primary focuses. First, primary factors leading up to the arrest should be identified and treated. Second, problems caused by the arrest and the trauma of the resuscitation effort should be recognized and managed.
The central nervous system is particularly sensitive to injury during states of shock or cardiopulmonary arrest. In health, cerebral blood flow is locally controlled and maintained by reflexes that maintain blood flow and protect the brain from hypertension and volume overload. This autoregulation of cerebral blood flow is lost during arrest, leaving the central nervous system susceptible to further injury during and after resuscitation. Cerebral ischemia and reperfusion injury resulting in nerve cell death is a serious complication of cardiopulmonary arrest and resuscitation. Initially, neurologic examinations should be done hourly. PLR, responsiveness to stimulation, respiratory pattern, motor responses, and motor postures should be noted. Normal pupil size and PLRs are positive signs. Slow PLRs, anisocoria, and pinpoint pupils that are nonresponsive to light are progressively guarded neurologic indicators. Nonresponsive bilaterally dilated pupils indicate severe brain damage and a poor prognosis. Recent administration of atropine during the arrest should be ruled out as a cause of dilated nonresponsive pupils. Brainstem damage should be suspected in patients lacking a corneal reflex or swallow, or gag, reflex. Breathing patterns also reflect brainstem function, and erratic breathing patterns and periods of apnea (breathlessness) are poor prognostic indicators.
The cardiovascular system is clearly “ground zero” during cardiac arrest. In addition, abnormalities associated with other organ failures may further impact heart rhythm and blood pressure. Changes in heart rhythm, vascular tone, and cardiac output predispose to systemic hypotension and rearrest. Consequently, it is imperative to continuously monitor the ECG and blood pressure in the postresuscitation period. Accurate blood pressure measurements may be obtained using direct and indirect methods. Direct blood pressure measurement is ideal, but requires an arterial catheter and specialized equipment. Indirect blood pressure measurements may be obtained with Doppler or oscillometric methods. Arrhythmias causing clinical signs or hemodynamic compromise should be treated. Oxygen therapy has relatively few complications in the short term and is a useful treatment.
Acute kidney failure is a common problem associated with states of shock and cardiac arrest because the kidneys are particularly susceptible to damage caused by hypovolemia and hypotension. Consequently, monitoring of kidney and electrolyte parameters should be performed frequently in the postresuscitation period. Decreased urine production is associated with severe kidney failure. Urine output should be monitored hourly for at least 24 hours after an arrest.
Strict attention should be paid to maintaining adequate hydration and blood pressure. Hypotension is a common complication, and the mean arterial blood pressure should be maintained within normal limits to guarantee adequate kidney blood flow. Veterinarians sometimes use an “ins and outs” fluid therapy plan to maintain fluid balance when kidney function is in question. In such cases, administered fluid doses are balanced with calculated fluid losses. Appropriate fluid therapy will maintain hydration without causing volume overload. CVP, repeated PCV/TP, and body weight measurements are useful indicators of volume status. Hemodialysis and peritoneal dialysis are available at certain specialized treatment centers.
Primary respiratory disease is a common factor leading up to cardiopulmonary arrest. Respiratory complications of arrest and resuscitation include pulmonary edema as a result of congestive heart failure, noncardiogenic edema associated with hypoxia, and pulmonary thromboembolism. Vigorous chest compressions during resuscitation efforts may also result in pulmonary contusions, rib fractures, atelectasis, and/or edema. These injuries must be addressed in the postresuscitation treatment plan. Optimal respiratory therapy may require ongoing oxygen supplementation, ventilation support, and monitoring of arterial blood gas analysis. If blood gas analysis is not available, monitoring should include pulse oximetry and/or capnography.
Blood glucose concentrations should be monitored frequently because many patients that arrest develop hypoglycemia. Normal blood glucose concentrations should be maintained by adequate supplementation when indicated. Hyperglycemia should be prevented.
MEDICAL TREATMENTS IN THE POSTARREST PERIOD
Many variables affect the outcome and management of an arrested patient. These variables include the underlying diseases and reasons for arrest and the experience and equipment available to the resuscitation team. Difficulties facing the arrested patient and the syndromes associated with the postarrest period are well known. However, disagreement exists regarding treatment methods, treatment priorities, and which therapies result in the best outcome. All therapies of postarrest patients are somewhat controversial because of an inability to create a standardized model for conclusive studies. Many drugs may be useful in the postresuscitation patient, and the list continues to grow. The following is a brief review of medical therapies and the rationale behind their use.
Mannitol is an osmotic diuretic sometimes used in the management of acute renal failure and cerebral edema. The mannitol molecule resides in the vascular space and draws water from the interstitial spaces between cells, thereby decreasing edema and expanding the vascular volume. As mannitol is excreted by the kidneys, its osmotic effects “pull” water with it into the urine. Mannitol is also a free-radical scavenger, which may aid in the treatment of reperfusion injury. Some caution is advised in the use of mannitol because it can exacerbate volume overload. At high doses, mannitol may be nephrotoxic, and it is contraindicated in hypovolemic animals.
Furosemide (Lasix) is a potent diuretic that is commonly used in the treatment of pulmonary edema and acute kidney failure. The diuretic effects of furosemide increase urine output and may enhance the effects of mannitol. In cases of pulmonary edema, furosemide causes volume contraction, which decreases edema formation and hastens resolution of edema.
Glucocorticosteroids (i.e., dexamethasone sodium phosphate, prednisolone sodium succinate, and methylprednisolone sodium succinate) are extremely controversial as to appropriate use. They may be beneficial in stabilizing cellular membranes, thereby decreasing the release of membrane-derived inflammatory mediators. Methylprednisolone sodium succinate is a free-radical scavenger. As such, it is one of a few drugs capable of rapid action against the oxygen free radicals created during reperfusion injury.
Dobutamine, a synthetic catecholamine, improves the contractility of heart muscle and may be administered to maintain mean arterial blood pressure. The related drug, dopamine, can be used to increase renal perfusion in canine patients at low doses and to increase systemic blood pressure at higher dosages. The use of dopamine in feline patients is controversial and limited to the systemic blood pressure effects of the drug.
Sodium bicarbonate is used in the treatment of severe life-threatening acidosis. However, caution is warranted because overzealous bicarbonate therapy is both harmful and easy to accomplish. Unpredictable acid-base disturbances and other problems can be attributed to overdoses. Blood pH must be monitored via serial venous or arterial blood gas analyses.
Lidocaine is an antiarrhythmic drug used to treat ventricular tachycardia. Care should be taken to accurately interpret the ECG tracing because this drug may be contraindicated in ventricular escape and isolated premature ventricular complexes. The latter are common arrhythmias observed in the immediate postresuscitation period. Although abnormal, these rhythms do provide functional blood perfusion in many cases. Abolishing a perfusing ventricular rhythm with lidocaine may result in development of a nonperfusing rhythm and death.
Because all body systems are affected by cardiopulmonary arrest, a whole body approach to monitoring and therapy is required. For this reason, successfully resuscitated animals are among the most critical and labor-intensive patients. The prognosis for long-term survival is often poor. Those patients successfully resuscitated require constant monitoring and support for several days.
TRANSFUSION MEDICINE
In many private veterinary practices, blood for transfusion is obtained from healthy dogs and cats owned by veterinarians, staff, and informed clients. Large dogs and cats are preferred as blood donors because of ease of venipuncture and ability to donate complete blood units. Preferably, cats that donate blood should live exclusively indoors to limit their exposure to transmissible infections. For purposes of blood donation, cats should weigh more than 10 lb, and dogs should weigh more than 60 lb. A well-organized volunteer blood donor program can provide a safe and adequate blood supply for most veterinary practices.
Animals who donate blood for transfusion should be regularly screened for transmissible infectious diseases.
Unfortunately, the number of potential pathogens greatly outnumbers the available resources for most veterinary practice blood donor programs. In 2005, the American College of Veterinary Internal Medicine published a consensus statement on canine and feline blood donor screening for infectious disease. Readers are referred to this document (Wardrop K et al: J Vet Intern Med Vol 19:1:135-142, 2005.) for more information on this topic.
In general, cats who donate blood should have a negative test result for the following diseases:
• Hemotrophic feline mycoplasma (formally hemobartonella)
• Feline leukemia virus (FeLV)
In general, dogs who donate blood should have a negative test result for the following diseases:
• Common tick-borne pathogens (Ehrlichia, anaplasmosis, neorickettsiosis)
Additional infectious disease testing may be indicated for canine and feline blood donors depending upon regional factors.
Collection of blood from donor animals is described in Chapter 21. Many veterinarians use commercial veterinary blood banks as a convenient alternative for supplying safe blood products. Commercial veterinary blood banks may obtain blood from healthy community volunteer pets or from healthy animals maintained at the blood bank facility. Infectious disease screening is generally the responsibility of the blood bank, but veterinarians should scrutinize the screening and safety policies of the blood bank products that they use.
Just as in humans, dogs and cats have blood types that are important in determining the compatibility of blood donors and transfusion recipients. Blood typing systems use RBC surface antigens to identify separate blood types. In these systems, animals may be positive (have the blood type antigen) or negative (do not have the antigen).
Most cats have natural preexisting antibodies to foreign blood types. These antibodies will react to incompatible blood types causing severe and potentially fatal transfusion reactions. Obviously, cats donating or receiving transfusions should be tested to ensure blood type compatibility. Many dogs, however, do not have preexisting antibodies to other blood groups. Therefore most dogs tolerate their first transfusion extremely well. During transfusion, however, exposure to foreign RBCs may trigger antibodies that precipitate future transfusion reactions. Administering blood type compatible transfusions in dogs helps reduce the risk of current and future transfusion reactions. Blood typing in veterinary medicine is complicated by numerous antigen groups and species differences. Blood typing kits using a card agglutination test are commercially available to make pretransfusion testing convenient (Figures 33-37 and 33-38).
FIGURE 33-37 A feline blood typing card (Rapid Vet-H, DMS Laboratories) is shown. Note the agglutination indicating this cat is blood type B
FIGURE 33-38 A canine blood typing card (Rapid Vet-H, DMS Laboratories) is shown. The patient sample does not agglutinate, indicating that the pet is DEA 1.1 negative
The most commonly used canine blood typing system in the United States is the dog erythrocyte antigen (DEA) system that can be used to identify eight separate blood types. Despite this high number of blood antigens, most clinically significant transfusion reactions are associated with only a few major antigens (DEA 1.1 and 1.2). DEA 1.1 is the most antigenic blood type. Therefore blood donors ideally will be DEA 1.1 negative.
Blood typing in cats is much more straightforward when compared with dogs. Cats have three blood types (type A, type B, and type AB). The feline AB-blood type system is specific to cats, and the antigens have no relation to the ABO blood types used in people. Worldwide, most cats are blood type A, whereas blood type B is uncommon, and blood type AB is rare. The distribution of blood types varies between geographic regions and between breeds. More than 90% of cats in the United States are blood type A. Blood type B is more common in certain purebred cats. Although type A is the most common feline blood type, any individual cat (mixed breed or domestic shorthair included) may have type B or type AB blood. Cats that are blood type A may have a low level of natural preexisting antibodies against type B blood antigens. Therefore administering type B blood to a feline patient that is type A may result in a transfusion reaction, even if it is the patient's first transfusion. These initial reactions tend to be mild because the level of these preexisting antibodies is relatively low in type A cats. Risk for severe transfusion reaction increases with subsequent mismatched transfusions because the patient has been further sensitized against the foreign blood type. Cats that are blood type B always have naturally high antibody titers against type A blood antigens. Therefore administering type A blood to a feline patient that is type B will invariably result in a rapid, severe, and potentially fatal allergic reaction.
This reaction manifests as anaphylactic shock and is characterized by circulatory collapse, respiratory distress, and vomiting. Cats who are type AB express antigens from both blood groups (A and B) on the surface of their RBCs. These cats do not develop antibodies against either blood group and therefore may receive transfusions from any feline donor.
Clearly, blood typing is an important consideration for blood donors and transfusion patients. However, blood type also plays a role in “neonatal isoerythrolysis,” a cause of death in kittens borne to type B mothers. Because both male and female parents contribute to the genetic blood type of their offspring, kittens within a litter may be type A, type B, or type AB. Type B mothers have strong anti-A antibody titers. These maternal anti-A antibodies can be absorbed from colostrum by nursing kittens and will attack the blood cells of type A or type AB kittens resulting in severe hemolytic anemia. This condition is one cause of the so-called “fading kitten syndrome,” where healthy kittens unexpectedly become ill and die.
Transfusion reactions may occur even when animals are administered blood of a compatible type. Crossmatching is used to further document compatibility between donor and recipient.
In this test, components of donor and recipient blood samples are mixed, and the technician looks for agglutination. Agglutination, which indicates incompatibility, is the clumping of RBCs into grossly or microscopically visible aggregates. When performing a “major crossmatch,” the donor cells are mixed with the recipient plasma, whereas a “minor crossmatch” evaluates recipient cells mixed with donor plasma. In some diseases (most notably immune-mediated hemolytic anemia), patient blood samples may demonstrate “autoagglutination,” where agglutination of the patient's RBCs occurs spontaneously (Figure 33-39). This spontaneous agglutination makes interpretation of a crossmatch nearly impossible.
FIGURE 33-39 Autoagglutination can be detected by mixing a drop of saline with a drop of patient's blood on a slide. Note the spontaneous clumping or agglutination of blood in this dog with immune-mediated hemolytic anemia
Blood is a complex biologic fluid containing a mixture of blood cells, protein, and fluid. RBCs contain hemoglobin, and their primary function is carrying oxygen. WBCs are immune system cells, and their primary function is to mediate inflammation and fight infection. Platelets are small cellular particles that are active during blood clotting. Plasma is the fluid component of nonclotted blood and is rich in protein. Plasma contains clotting factors plus albumin and globulin protein. Serum is the fluid component of blood after clotting has occurred. Serum and plasma are similar fluids, but have different protein contents. Additional information about blood cells is found in Chapter 16.
Whole blood and packed red blood cell (pRBC) units are ideal transfusion products for animals with anemia. Importantly, transfusion is almost always a symptomatic therapy. Even with successful transfusion, the original cause of anemia must be determined and corrected for treatment to be successful.
Whole blood units are popular for transfusion in private veterinary practices because whole blood is readily available, contains all blood components, and requires minimal processing. The primary disadvantage of whole blood use is a relatively short storage life (14 to 45 days depending upon the anticoagulant preservative solution used). Advances in animal blood banking and veterinary medicine allow the veterinary team to extend the useful life of whole blood donations by separating the blood into components for individual use. Refined blood banking practices also reduce the risk of transfusion because separated blood components contain less reactive substances. One whole blood unit may be separated into pRBCs and plasma components that may be administered to separate patients (Figure 33-40). Although packed red cells have a similar storage life to whole blood, the plasma components may be frozen for long periods of time. Selective use of blood components reduces the physical demands on blood donors, allows veterinary practices to use fewer donors, and reduces the cost of blood banking programs. In addition, pRBCs allow clinicians to give the same amount of RBCs in a smaller volume. This strategy can be particularly helpful in small animals or animals with complicated fluid needs (i.e., those with kidney disease or heart disease).
FIGURE 33-40 One whole blood unit may be separated into pRBCs and plasma for individual use of these blood components
Plasma is a complex biologic fluid. Plasma is separated from whole blood in a centrifuge designed for blood products (Figures 33-41 and 33-42). Special care is taken to prevent contamination of the collected plasma with blood cells or hemolysis (Figures 33-43 and 33-44). Plasma that is frozen within 6 hours of collection is termed “fresh frozen plasma” (FFP). FFP contains all clotting factors plus albumin and globulin protein. FFP may be stored frozen for up to 1 year without a change in quality or stability of its protein. Plasma that is frozen after 6 hours of collection loses some of the unstable clotting factors and is simply termed “frozen plasma” (FP). After 1 year, FFP also loses the more labile clotting factors. Thus after 1 year, FFP reverts to FP. Importantly, FP still retains the majority of clotting factors (including the vitamin K–dependent factors) and albumin and globulin. FP may be stored safely for up to 5 years. Plasma is usually administered to patients with known or anticipated coagulation abnormalities (i.e., rodenticide ingestion, DIC, etc.).

FIGURE 33-41 A technician places blood into a centrifuge, which will separate the blood and plasma contained in the whole unit
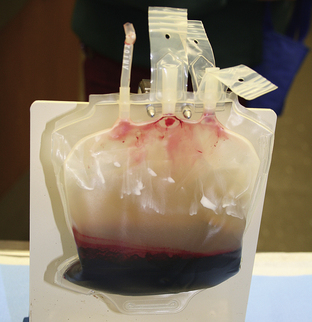
FIGURE 33-43 After centrifugation, blood cells have settled to the bottom of the bag, and plasma remains on top
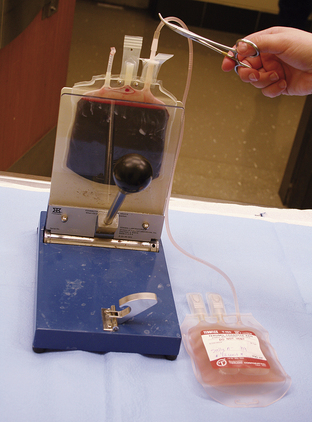
FIGURE 33-44 A plasma press is used to transfer the plasma into a separate collection bag for storage
Cryoprecipitate is a specialized plasma product that is rich in select clotting factors (VII, von Willebrand's factor, and fibrinogen). Cryoprecipitate is created by partially thawing FFP at 1° C to 6° C. The partially thawed plasma is centrifuged, and the solid cold-insoluble plasma component is collected. Cryoprecipitate is able to provide a concentrated source of factors in a small volume. Cryoprecipitate may be specifically indicated for use in dogs with von Willebrand's disease or with isolated clotting factor VII deficiency. The supernatant (cryopoor plasma) has fewer active components, but is safe for transfusion for up to 5 years.
Patients with von Willebrand's disease are deficient in a specific blood clotting factor known as von Willebrand's factor. This factor is important in platelet adhesion and is integral to normal blood clotting. Cryoprecipitate may be a good blood product for animals with this disease, but many veterinary practices are not able to make this specialized plasma product. The drug desmopressin (DDAVP) has been reported to cause release of stored von Willebrand's factor. Administration of DDAVP to a blood donor may raise the concentration of von Willebrand's factor in a harvested unit of whole blood or plasma. The resulting blood product is sometimes referred to as von Willebrand's loaded plasma.
Blood products are essentially biologic drugs. They have indications, side effects, and usual dosages. Exactly when to consider transfusion is a clinical judgment call that varies between veterinarians and between diseases. Generally, hematocrit values less than 20% will prompt consideration of a transfusion, but there is no magic number that triggers transfusion. Most clinicians base transfusion need on clinical signs. Clinical signs of anemia that may prompt transfusion include weakness, malaise, tachycardia, tachypnea, and syncope. Sudden anemia is generally much more difficult for animals and may require transfusion at a relatively higher hematocrit when compared with chronic anemia. In chronic anemia, animals may have time to adapt and compensate for the disease and hence may be more stable even at lower hematocrits.
Many blood products are given to effect (i.e., they are given in flexible dose ranges until a target is reached). In many cases of anemia, our objective is to eliminate clinical signs rather than to achieve a predetermined hematocrit. A dose of whole blood for dogs may be calculated from the recipient's hematocrit, the donor's hematocrit, and the desired hematocrit of the patient after transfusion.
This traditional formula has been used with good success, but is cumbersome to use. A simpler guideline for transfusion follows: administering 1 ml/lb of whole blood raises the patient hematocrit by 1%. This simple rule of thumb is easy to calculate on a stat basis and has proven to be clinically accurate in dogs. A standard unit of canine whole blood contains 450 ml. A standard unit of feline whole blood is 50 to 60 ml. Most cats receive one unit of whole blood and are reassessed after each transfusion. A standard dose of plasma is 15 to 20 ml/kg for both cats and dogs. This may be a one-time dose or may be repeated. There is no maximum limit to the amount of blood products that may be given to a single patient. However, repetitive blood or plasma transfusion raises the risk of side effects (including transfusion reaction and volume overload).
Blood and plasma must both be administered through a blood filter to prevent administration of clots. Commercially available blood administration sets with an in-line filter are ideal for this purpose (Figure 33-45). Blood products should be administered in 4 hours or less to reduce the chances of contaminant bacterial growth. Initially a slow rate of delivery is selected to allow for monitoring of adverse events at the start of transfusion. During the first 30 minutes of transfusion, the initial infusion rate should be 50% of the intended rate of delivery. For instance, a dog receiving one full unit of blood (450 ml) over 4 hours would have a total delivery rate of 150 ml/hr, but should be started at 75 ml/hr. Infusion rates vary depending upon the volume status of the patient. Animals with congestive heart failure or those at risk for volume overload should be closely monitored and administered blood at conservative rates. Infusion pumps rated for use with blood products are helpful in delivering specific doses of transfusion products (Figure 33-46). Pumps that are not rated for blood should not be used because they may deliver erroneous volumes or damage RBCs (hemolysis) during infusion. When low infusion rates are required, blood units may be divided into separate syringes and administered one at a time. Syringes to be administered later should be kept in a refrigerator to preserve the viability and sterility of the blood. In this manner, patients can receive their full blood dose at slower rates.

FIGURE 33-45 Commercially available blood administration sets are available with in-line filters to prevent inadvertent infusion of clots

FIGURE 33-46 Blood products are administered in syringes on a syringe pump. Note the square-shaped blood filter attached to the syringe
Any adverse events should be recorded. Vital signs (temperature, pulse, respiration, mucous membrane color, or CRT) should also be recorded every 15 minutes during transfusion. Standardized monitoring forms for the medical record encourage compliance and help document transfusion reactions. If a transfusion reaction is noted, administration of the blood product should stop, and the veterinarian should be notified. Transfusion reactions may result from allergic reactions to foreign cells and protein. Adverse inflammatory reactions may also be provoked by blood products that are hemolyzed or contaminated with microorganisms. Reactions to plasma products may occur, but are much less common when compared with RBC products. Transfusion reactions may have classic features of allergic reactions, such as erythema (redness [Figure 33-47]), urticaria (hives), and pruritus (itching). Other allergic signs may include anxiety, fever, vomiting, diarrhea, and nausea. These types of allergic reaction are often reversible and may be treated by cessation of transfusion and administration of corticosteroids and/or antihistamines. Lysis of transfused RBCs is a common adverse reaction, which may be acute or delayed. Signs of hemolysis include progressive anemia, hemoglobinemia, and hemoglobinuria. Delayed hemolytic reactions sometimes go unnoticed because they may occur days or weeks later (after the patient has been discharged from the hospital). Severe transfusion reactions may precipitate acute renal failure. Anaphylactic shock may occur with subsequent tachycardia, hypotension, collapse, and death.
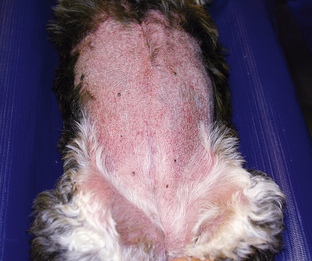
FIGURE 33-47 Transfusion reactions can include many signs, including the cutaneous erythema pictured here
Once a transfusion has been completed, the patient's hematocrit and TP should be reassessed after fluid and RBC volumes have been fully distributed (usually 30 minutes to an hour after the end of transfusion).
This step is often regretfully overlooked. Posttransfusion data is important in documenting the end result of transfusion and in the recognition of transfusion reactions.
PATIENT MONITORING
The primary objectives of critical care monitoring include (1) evaluation of current status, (2) evaluating the response to therapy, and (3) detecting new problems. Continuous 24-hour monitoring should be provided to patients that are critical or unstable, whereas 8-hour monitoring intervals may be acceptable in improved or stable patients. Both subjective parameters and objective parameters are used to provide the most complete evaluation of patient progress. Subjective parameters include hydration status and mentation (attitude, alertness, appetite). Objective parameters include body weight, urine production, vital signs (i.e., temperature, pulse, respirations), and lab results. Special techniques are often used to monitor, diagnose, and treat critically ill veterinary patients. The most important tools in monitoring any patient are serial physical examinations and assessment by a trained and attentive caregiver.
Expensive equipment may be helpful in some situations, but will never replace a hands-on nursing approach. Likewise, continuity of care is important so that dedicated caregivers can recognize patient trends, alert the veterinary team, and respond appropriately. A rational approach to patient monitoring can be loosely organized around the principles of triage and body system anatomy.
RESPIRATORY SYSTEM
Care should be taken to minimize stress in animals with respiratory difficulty (dyspnea). Oxygen supplementation is an important treatment in some animals and can be provided via blowby, nasal cannulae, face mask, oxygen canopy, or oxygen cage or incubator. In all patients (especially those with known respiratory disease), it is important to note the rate, pattern, and effort of breathing. Obstructive airway disease (i.e., laryngeal paralysis or tracheal foreign material) is often characterized by a slow and deep respiratory pattern with harsh or whistling upper airway noise. Restrictive respiratory disease (i.e., pleural effusion, chest wall injuries) often manifests as rapid and shallow breathing. Respiratory disease may be detected by monitoring respiratory rate, mucous membrane color, and respiratory effort. A significant abdominal component or effort during inspiration is abnormal. All lung fields and the upper airways should be ausculted. Additional sounds with inspiration or expiration should be noted and significant changes recorded in the medical record and reported to the veterinarian. Fluid and air in the pleural space often result in muffled heart and lung sounds. Percussing the thoracic wall during auscultation may aid in determining whether air or fluid is in the pleural space. Certain lower airway disorders, such as pneumonia and pulmonary edema, are characterized by crackles during each phase of respiration.
Monitoring equipment, such as a pulse oximeter (Figure 33-48) and capnograph, can provide additional information to the veterinary team. These monitors are frequently used for anesthesia monitoring, but can also be used for continuous monitoring of critically ill patients. Pulse oximetry uses an infrared sensor to count the pulse rate and provide a noninvasive measure of arterial hemoglobin-oxygen saturation (usually in percent). Infrared sensors are made to clip onto the tongue, lip margin, or flank. Rectal probes (with sanitary covers) for recumbent patients are also available. It is important to note that the pulse oximeter reading is a hemoglobin-oxygen saturation value and is not identical to the blood oxygen content or arterial oxygen partial pressure (PaO2). The structure and biochemistry of hemoglobin allows saturation to remain high despite some significant changes in blood oxygen content. The pulse oximeter is a good crisis prevention tool, but does not provide fine details about the oxygenation and ventilation status of the patient.
FIGURE 33-48 A pulse oximeter provides a convenient and reliable means to monitor oxygenation in critical or anesthetized animals. Note the pulse waveform, pulse rate, and SPO2 reported on the monitor
If the pulse oximeter detects a problem with oxygen saturation, a blood gas analysis and oxygen supplementation may be ordered. In normal patients, the oxygen saturation should remain above 95%. Poor perfusion at the probe site, hypothermia, and movement will impede the sensor's ability to obtain an accurate reading. If the pulse oximeter sensor triggers an alarm, manual palpation of the pulse, auscultation of the heart, and check of the mucous membranes should be performed immediately before adjusting the equipment.
Capnography also uses infrared technology to estimate the carbon dioxide concentration of expired air. By incorporating a capnometer into the breathing circuit of intubated animals, the end-tidal carbon dioxide (carbon dioxide concentration at the end of expiration) can be measured (Figure 33-49). The end-tidal carbon dioxide is an estimate of PaCO2 (arterial carbon dioxide levels), which can be useful in monitoring the efficiency of mechanical ventilation. Capnometry can also be used to confirm endotracheal intubation and to detect airway occlusion. If an endotracheal tube is misplaced into the esophagus or occluded by exudates, end-tidal carbon dioxide will be negligible. Additionally, capnographs have been used to assess the efficacy of resuscitation efforts during CPR. Successful resuscitation is expected to result in progressively increased end-tidal carbon dioxide values as forward blood flow returns carbon dioxide from the tissues.
FIGURE 33-49 A capnometer attached to the breathing circuit of intubated animals can be used to measure the end-tidal carbon dioxide (carbon dioxide concentration at the end of expiration)
Arterial blood gas analysis provides specific data on the oxygenation, ventilation, and acid-base status of the pet. Normal ranges for arterial blood gas values are published elsewhere. Values for oxygen and carbon dioxide are expressed as a pressure (PaO2 or PaCO2) in units of millimeters of mercury (mm Hg). Arterial blood samples may be drawn from the femoral artery, dorsal pedal artery, or lingual artery by palpating the pulse and guiding the needle into the artery by tactile sensation (Figure 33-50). The femoral artery and dorsal pedal arteries are the most common sites of arterial blood sampling. Lingual artery samples may be obtained in anesthetized or comatose animals, but they should be avoided because of the risk for inducing a large oral hematoma, which can obstruct the airway and interfere with swallowing. Acquiring arterial blood samples may be too stressful for some patients. By definition, ventilation is determined by the carbon dioxide level (PCO2) on an arterial blood gas analysis. Hypoventilation (failure to “blow off” carbon dioxide) causes an increase in blood carbon dioxide. Hypoventilation occurs with certain respiratory problems and can cause acidosis. Hyperventilation (“blowing off carbon dioxide”) results in decreased carbon dioxide. This situation may occur with respiratory disease or as a compensatory response to metabolic acidosis. Oxygenation is indicated by the PaO2, and a low value indicates hypoxemia. Signs of hypoxemia include cyanotic (blue) mucous membranes (Figure 33-51), weakness, rapid heart rate, increased respiratory rate, and increased respiratory effort.

FIGURE 33-50 The femoral pulse is palpated before arterial blood sample collection from the femoral artery of a dog
The difference between alveolar (lung) oxygenation and arterial oxygenation is a calculated indicator of the efficiency of oxygen transfer to the blood by the lung. In patients that are hypoxemic, this alveolar-arterial gradient (A-a gradient) calculation can provide a single value to assess and monitor the animal's oxygen exchange. The alveolar oxygen content (A) is calculated by the alveolar gas equation: A = (BP − 47) 0.21 − PCO2/0.8, where BP is the barometric pressure (760 mm Hg at sea level), 47 is the vaporization pressure of water (a constant physical property of water), and 0.21 is the oxygen percent of room air. This formula is only used when the patient is breathing room air. In the formula above, PCO2 is obtained from the arterial blood gas analysis, and 0.8 is the respiratory exchange quotient (a mathematic constant). At elevations near sea level, the formula simplifies to A = 150 − PCO2/0.8. The arterial oxygen content (a) is the PaO2 obtained from the arterial blood gas analysis (in mm Hg). To finish calculating the A-a gradient, the arterial oxygen content (a) is subtracted from the alveolar oxygen content (A). In animals with normal oxygen exchange, the A-a gradient is less than 10 mm Hg. As a general rule, an A-a gradient more than 30 mm Hg is an indication for oxygen therapy.
To determine how responsive the patient is to oxygen therapy, an arterial sample is collected while the patient is on supplemental oxygen, and a second arterial blood gas analysis is performed. The original A-a calculation is invalid for patients receiving oxygen therapy. Instead, oxygenation is assessed by evaluating the ratio of arterial oxygen to inspired oxygen (PaO2/FIO2). PaO2 is obtained from the blood gas analysis. FIO2 is the fraction (percent) of inspired oxygen being supplemented in decimal form. For example, a dog receiving 100% oxygen would have an FIO2 of 1.0. Most of the time, animals receiving oxygen therapy by mask or nasal catheters have an FIO2 of close to 40% (FIO2 = 0.4). By definition an animal's disease is responsive to oxygen if the PaO2/FIO2 value is greater than 250. As a general rule of thumb, the PaO2 should be approximately five times the FIO2.
CARDIOVASCULAR SYSTEM AND PERFUSION/HYDRATION
Monitoring of common physical examination findings can be key to detecting serious health problems. Technicians should be comfortable using a stethoscope and familiar with the basic principles of cardiac auscultation and pulse palpation. The detection of a new heart murmur, irregular heartbeats (arrhythmia), and/or changes in heart or lung sounds can be signs of an impending crisis. For example, animals that are “overhydrated” may develop crackles characteristic of pulmonary edema. Factors that decrease sound intensity include obesity, pleural or pericardial effusion, and hypovolemia. Likewise, pulse character and quality are important clues to the hydration status and stability of the critically ill pet. If the mean arterial blood pressure is less than 60 mm Hg, pulse strength will be diminished and it will be difficult to palpate pulses. Common sites to palpate a pulse are the femoral, dorsal pedal, or lingual arteries. Pulse rates should parallel the heart rate, rhythm, and quality. Comparing the pulse rate and heart rate during auscultation can aid in detecting “pulse deficits” that may be produced by irregular heartbeats.
Monitoring of hydration status and peripheral perfusion is crucial in sick animals. Helpful monitoring parameters include texture and color of mucous membranes, skin turgor (Figure 33-52), CRT, quantitation or close estimation of urine output, thoracic auscultation, pulse rate and character, serial body weights, and temperature (both core body temperature and peripheral limb temperature). Physical signs of overhydration include serous nasal discharge, chemosis (conjunctival edema; see Figure 33-10), peripheral edema, body cavity effusion, and weight gain. Noninvasive monitoring equipment, such as an ECG and indirect blood pressure equipment, can provide additional information regarding the pet's cardiovascular status. Patients at greater risk for fluid imbalance, such as those with heart disease, pneumonia, and renal disease, may require more invasive monitoring techniques. Invasive monitoring techniques include PCV, TP, direct blood pressure, and CVP measurement. Trends in observations of both subjective and objective parameters provide the most information. Diligent record keeping and consistency in monitoring techniques are imperative.
FIGURE 33-52 Well-hydrated animals have normal skin elasticity and turgor, but this animal demonstrates poor skin elasticity associated with severe dehydration
Blood Pressure
In humans, blood pressure is often expressed as a fraction, with systolic pressure as the numerator and diastolic pressure as the denominator. Mean arterial pressure is calculated from the systolic and diastolic values. A similar fraction can be used in cats and dogs. Normal systolic blood pressure varies between species, but generally ranges from 100 to 150 mm Hg. An ideal mean blood pressure ranges from 75 to 90 mm Hg. Arterial blood pressure can be monitored directly or indirectly and provides more information than simple subjective assessments of pulse character. Systolic values more than 90 mm Hg and diastolic values greater than 60 mm Hg are required to maintain adequate perfusion of vital organs (namely the kidneys and brain). A systolic blood pressure greater than 175 mm Hg indicates hypertension. However, stress of illness and anxiety associated with hospital visits can transiently increase the blood pressure in nervous animals. In cats and dogs, pulses should be palpable if the mean arterial blood pressure is greater than 60 mm Hg.
Indirect blood pressure is obtained using either oscillometric or Doppler equipment. Oscillometric blood pressure monitors often come in combination with an ECG and pulse oximeter and are popularly used in monitoring anesthetized or sedentary animals. These monitors use a blood pressure cuff to determine systolic, diastolic, and mean arterial pressures and can be programmed to take readings at regular intervals. To ensure an accurate measurement, cuff size should be proportionate to the size of the animal. As a general rule, the diameter of the cuff should approximate 40% of the circumference of the limb at the site of cuff placement.
Common sites of cuff placement include the metacarpus, metatarsus, and tail. Doppler blood pressure equipment uses an ultrasound crystal and monitor to audibly locate the arterial pulse (Figure 33-53). Hair is clipped over an artery (usually the ventral digital artery or tail artery) to enhance detection of the pulse. The ultrasound crystal with ultrasound gel is positioned over the palpable pulse so that the Doppler elicits a clear sound with each pulse. Subsequently a blood pressure cuff is applied to the patient and a sphygmomanometer is used to inflate the cuff until the audible pulse can no longer be heard. Finally, pressure is released in the cuff until the pulse can be heard again. The pressure at which the pulse is again detected is equivalent to the systolic blood pressure. This technique is more labor intensive for monitoring because this procedure must be manually performed. In addition, mean and diastolic blood pressures are not reliably obtained by this technique. However, many clinicians prefer Doppler equipment for blood pressure measurement because the audible pulse is reassuring and allows a subjective assessment of the accuracy of data.
FIGURE 33-53 Indirect blood pressure measurement using ultrasound Doppler equipment provides an accurate reading of the systolic blood pressure
Direct blood pressure measurement is more invasive because it requires catheterization of an artery and specialized equipment. This method is considered the most accurate method of blood pressure measurement. Since most arteries cannot be directly visualized at the time of arterial puncture, arterial catheters are placed using digital palpation of the pulse as a guide. Common arteries for catheterization include the dorsal pedal artery and femoral artery. Once in place, the arterial catheter is connected to a monitor via commercially available transducer equipment. The monitor displays a pulse waveform and reads out a systolic, diastolic, and mean blood pressure. Comparing the pulse waveform deflection with an ECG allows staff members to note the pulse pressure created by each cardiac contraction. This can be helpful in determining the effects of transient arrhythmias. Staff members should be trained in the use and care of arterial catheters. No medications should be administered via intraarterial injection, and arterial catheters must be appropriately labeled to prevent confusion.
Arterial catheters are flushed slowly and regularly to prevent clot formation. Arterial catheters must be properly secured to prevent animal movement from disconnecting equipment. If equipment becomes detached from the catheter hub, an open arterial catheter can result in rapid severe blood loss, which is particularly dangerous in small animals.
Central Venous Pressure
Blood flows unidirectionally from arteries to veins and back to the heart in part because of the natural pressure gradient between these areas. Venous pressures are maintained lower than arterial pressures to facilitate forward blood flow. Most discussions of blood pressure refer to systemic arterial blood pressure. However, venous blood pressures are also important. Central venous pressure refers to the blood pressure in central veins, such as the thoracic vena cava.
Because the veins hold a large volume of fluid, CVP is used to monitor hydration and the efficacy of fluid therapy. A normal CVP is 0 to 5 cm H2O. Values less than zero indicate hypovolemia, dehydration, or inadequate fluid therapy. Values trending upward to 8 or 10 indicate an increase in vascular volume and adequate fluid therapy. Sudden increases in CVP or values above 10 may indicate venous congestion, increased thoracic pressure, and volume overload. Patient status should be closely reviewed. CVP values are usually used in conjunction with other subjective measures of hydration (e.g., heart rate, mucous membrane appearance, skin turgor).
Measurement of CVP requires placement of a long catheter in a central vein. Most commonly, jugular vein catheters are placed that reach into the thoracic vena cava. Equipment for measuring CVP can be constructed from a three-way stopcock that separates a manometer (in cm H2O) and a saline-filled syringe from an extension set (Figure 33-54). The extension set and manometer are flushed with normal saline, and the extension set is connected to the patient's central line catheter. The animal is placed in sternal or lateral recumbency, and the apparatus is held so that the zero mark of the manometer is in the approximate position of the distal catheter tip (Figure 33-55). In most small animals, this position is approximated by the location of the heart (i.e., the sternum in laterally recumbent animals and the point of the elbow during sternal recumbency). After flushing the catheter to ensure smooth flow of blood, the stopcock is opened between the patient and the manometer. After several seconds, the saline column of the manometer will equilibrate with the pressure at the end of the catheter reflecting the CVP, which can be measured on the manometer (Figure 33-56).
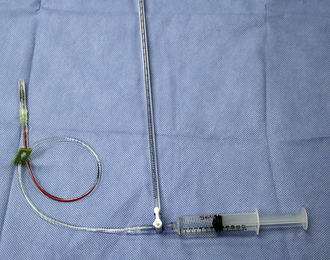
FIGURE 33-54 Equipment for measuring CVP can be constructed from a three-way stopcock that separates a manometer (in cm H2O) and a saline-filled syringe from an extension set
COAGULATION STATUS
Assessment of an animal's coagulation status may be helpful in assessing unexplained bleeding and detecting DIC. Evaluation of a blood smear may detect RBC morphology changes and allow an estimation of platelet numbers. The presence of schistocytes or fragmented RBCs along with a decrease in platelet numbers may be seen with DIC, splenic tumors, and severe heartworm disease. A high percentage of echinocytes can be seen in animals suffering from snake envenomation.
A normal platelet count is between 200,000 and 500,000 platelets per microliter in most species. Although a complete platelet count is advised, rapid assessment or confirmation of platelet numbers may be obtained by estimating the platelet numbers on a blood smear. This can be done by counting the number of platelets per high-power field and multiplying by 15,000. A better estimate is obtained if several high-power fields are assessed and averaged. Platelet clumping, which is common at the edge of blood smears, can make platelet estimates invalid.
The buccal mucosal bleeding time (BMBT) evaluates platelet function and is often used as an in-house screening test for von Willebrand's disease (an inherited disease effecting platelet function). A commercially available lancet device (Figure 33-57) is used to make a standardized incision (specific size and depth) into the buccal mucosa on the inside of the cheek (Figure 33-58). Following this incision, the blood flowing from the cut is blotted away with filter or absorbent paper to permit visualization of the site without disturbing the incision (Figure 33-59). The time required until bleeding stops is the BMBT, which represents the time for formation of an initial platelet plug. Normal time for the dog is less than 4 minutes. Following completion of the test, renewed bleeding at the site can occur if the incision is disturbed or if the animal has other coagulation problems, but such bleeding does not affect the reported time.
RENAL/URINARY SYSTEM
Ideally, all hospitalized animals should have a urine sample obtained for urinalysis. Urine specific gravity is important information in determining urinary tract health and in interpreting changes in kidney parameters and the urine sediment. Because urine specific gravity and blood values for blood urine nitrogen (BUN) and creatinine can be affected by fluid therapy and medications (diuretics, such as furosemide and mannitol); pretreatment blood and urine samples should be obtained. However, if such samples cannot be readily obtained, fluid therapy should not be delayed, particularly if the animal is dehydrated or unstable. Urine output should be estimated in all animals and recorded in the medical record. In critical cases, close monitoring of urine production may be performed via urine collection through indwelling urinary catheters or specifically designed cages that allow urine to drain through a grate to be collected. An indwelling urinary catheter attached to a sterile urine collecting bag is a comfortable, clean, and accurate way to monitor urine output (Figure 33-60). Because catheters may clog, kink, or change position, urine collection equipment should be regularly inspected and replaced if faulty. Minimum urine production is 2 to 4 ml/kg/hr. Inadequate urine production may be an indication for changes in fluid therapy and should be interpreted with other patient data (i.e., body weight, CVP, etc.) and reported to the veterinarian.
CENTRAL NERVOUS SYSTEM
Unfortunately, few objective parameters are available to monitor the central nervous system. Serial physical and neurologic examinations are perhaps the best means of detecting new or ongoing problems. Changes in mentation, responsiveness, level of consciousness, and respiratory patterns may indicate changes in neurologic status and should be reported.
Likewise, pupillary size and responsiveness to light are important signs to monitor. Increased intracranial pressure results in a syndrome of brainstem and cerebral herniation whereby intracranial pressure changes cause compression of the brain against solid connective tissue and bone. Early signs of increased intracranial pressure include mental dullness, tachypnea, tachycardia, and dilated pupils. Later signs include bradycardia, fixed pinpoint pupils (Figure 33-61), seizures, coma, and death. Early recognition and intervention are key to the management of this syndrome. Animals at risk for brain herniation include those with head trauma, hydrocephalus, brain tumors, and encephalitis. Herniation can also be a complication of some diagnostic procedures, such as spinal fluid collection and myelogram.
ABDOMINAL CAVITY
Each patient should receive a daily physical examination. Palpation of the abdomen may detect abdominal distention resulting from fluid accumulation, organ enlargement, and intestinal gas. Abdominal pain can be detected by the presence of discomfort, splinting, or vocalization. Animals with unexplained abdominal pain or distention warrant further investigation and monitoring.
Abdominocentesis is a procedure to confirm the presence of abdominal fluid and to collect samples for fluid analysis. During this procedure, the abdomen is clipped of hair and aseptically cleaned with surgical scrub. A needle attached to a syringe is inserted into the abdomen near the umbilicus, and gentle suction is applied to withdraw fluid (Figure 33-62). Care is taken not to redirect the needle blindly within the abdomen because this may result in unnecessary pain and potential injury to abdominal organs. Fluid collected via abdominocentesis may be submitted for culture and cytology. Abdominocentesis is commonly performed to detect active hemorrhage, infection (peritonitis), ascites, uroabdomen, and neoplastic effusions. If only a small amount of fluid is suspected, a four-quadrant tap may be performed in which the four areas of the abdomen centered around the umbilicus are sampled. If no sample is obtained and abdominal fluid is still suspected, a diagnostic peritoneal lavage (DPL) may be performed. The DPL is a modified procedure for collecting small amounts of abdominal fluid. In this procedure, the animal is prepared as described earlier. Before fluid withdrawal, a volume of 10 to 20 ml/kg of sterile warm isotonic saline is instilled into the abdomen. After a brief moment to adjust the animal's position and allow the instilled fluid to mix with abdominal contents, standard abdominocentesis is performed. The added fluid may dislodge adherent debris and mix with isolated fluid pockets that can then be collected during standard abdominocentesis.
THORACIC CAVITY
Animals with cardiac and respiratory disease often have similar clinical signs (namely, coughing, labored breathing, exercise intolerance, and collapse). Unfortunately, these signs can occur with many different causes, including upper airway obstruction, pulmonary edema, pleural effusion, pneumonia, primary heart disease, and thoracic neoplasia. A thorough physical examination often directs diagnostic tests to differentiate respiratory from cardiac problems. Muffled heart and respiratory sounds often indicate fluid or air in the pleural space (the space between the lungs and chest wall). The presence of pleural fluid or air causes respiratory problems as a result of physical interference with normal lung expansion. Crackles heard during the different phases of breathing usually indicate pulmonary disease, such as pneumonia or pulmonary edema. In general, crackles indicate relatively severe pulmonary disease.
Chest x-rays are commonly used to confirm, classify, and document the presence of cardiac and respiratory diseases. If thoracic fluid is documented on x-rays or clinically suggested during examination, thoracocentesis to remove the fluid is indicated. Thoracocentesis is usually performed with the animal comfortably restrained in sternal recumbency. During this procedure, one or both sides of the chest are clipped of hair (in the area of rib spaces 7 through 9) and cleaned with surgical scrub. A butterfly catheter (or needle with extension set) is attached to a three-way stopcock and syringe (Figure 33-63). The needle is advanced into the chest along the cranial aspect of a rib to prevent discomfort and bleeding associated with nerve and vessel bundles located behind each rib. As the needle is inserted into the chest, an assistant controls the syringe and maintains the stopcock in the closed position to prevent environmental air from entering the chest during inspiration. Once the needle is positioned in the pleural space, the stopcock is opened to allow the assistant to suction fluid from the pleural space into the syringe. If a large amount of fluid must be removed, the stopcock is closed to the patient as the syringe is repeatedly emptied. Samples for culture, cytology, and fluid analysis are often obtained early during the procedure. Thoracocentesis may be performed as an emergency diagnostic procedure in animals with severe respiratory distress and suspected pleural fluid (Figure 33-64). Air can also accumulate in the pleural space. This condition is referred to as a pneumothorax and often results from traumatic rupture of airways or from severe lung disease. In such cases, air leaks outside of the lung, but is trapped inside the chest. Thoracocentesis (as described earlier) may be used to remove air from the pleural space. If fluid or air continues to accumulate in the chest, “chest drains,” or thoracostomy tubes, may be placed that allow repetitive or continuous drainage of air and/or fluid from the pleural space (Figure 33-65).
COMMON EMERGENCIES
Urethral obstruction is a serious and common emergency in both cats and dogs. Causes of such obstruction include urinary stones, tumors, trauma, and/or inflammation. Animals that are unable to urinate develop metabolic abnormalities quickly and may develop secondary kidney damage. In some cases, unrecognized urinary obstruction results in permanent kidney failure and even rupture of the urinary system and leakage of urine into the abdomen. Consequently, untreated urinary obstruction is fatal.
Diagnosis of urinary obstruction is based on history, physical examination, laboratory tests, and diagnostic imaging (x-rays or ultrasound). Complete urinary obstruction is often preceded by signs of lower urinary tract disease, such as straining to urinate, painful urination, blood in the urine, or lack of urination. Obstruction of urine flow results in a large, firm, swollen, and painful bladder that can be palpated during physical examination. Affected animals often have pelvic or abdominal pain, lethargy, and dehydration. Veterinarians and technicians should be familiar with the historical and physical examination findings associated with urethral obstruction so that a rapid diagnosis and intervention may be made.
Failure to excrete urine results in rapid onset of severe fluid and electrolyte imbalances. Common laboratory abnormalities include elevated potassium (hyperkalemia), elevated kidney parameters (azotemia; increased BUN and creatinine) and metabolic acidosis. Acidosis in animals with urinary obstruction is due to the accumulation of lactic acid (from poor perfusion during dehydration) and from accumulation of uremic acids (metabolic by-products usually excreted by the kidneys). Metabolic acidosis may be recognized by closely scrutinizing the blood pH, bicarbonate levels, and total carbon dioxide levels in lab reports. Venous blood gas analysis is helpful in determining the acid-base status and may be needed to obtain some of this information. Hyperkalemia is often severe and can be acutely life threatening because it triggers cardiac arrhythmias and can be a cause of cardiac arrest. Significant elevation of serum potassium results in fairly unique ECG changes that may allow early recognition of the problem. These changes include: (1) bradycardia, (2) “spiked” T waves, (3) shortened R-wave amplitude, (4) blunted or missing P waves, and (5) prolonged QRS interval (wide, short complexes). Astute veterinarians and technicians should be aware of these classic ECG features that may allow for early recognition of hyperkalemia (Figure 33-66).
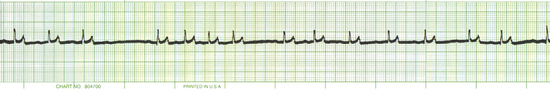
FIGURE 33-66 Classic ECG features associated with hyperkalemia include bradycardia, “spiked” T waves, shortened R-wave amplitude, blunted or missing P waves, and prolonged QRS interval (wide, short complexes)
X-rays and ultrasound can be used to confirm the presence of urinary obstruction, but are more important for determining the cause of obstruction. Care should be taken to include all of the urinary tract in the x-ray study. In male animals in particular, the x-rays should include the perineal and penile urethra.
X-rays are a common and readily available means to detect most stones in the bladder or urethra. X-rays must be of sufficient quality to detect small stones. However, not all stones are easily detected by radiography because some stones appear less opaque than others. In some cases, nonradiopaque (“lucent”) stones may only be detected by contrast x-ray procedures or ultrasound. Ultrasound provides a noninvasive detailed evaluation of the urinary tract. In particular, ultrasound is useful in the detection of bladder masses and in the evaluation of the upper urinary tract. Evaluation of the upper urinary tract (kidneys and ureters) is important because stones and tumors may occur in this area. In addition, ultrasound may detect congestion and dilation of the ureters (hydroureter) and the urine collecting system of the kidneys (hydronephrosis), both of which may be caused by lower urinary obstruction.
Regardless of the cause, emergency treatment of urinary obstruction has two priorities: (1) relieve the urinary obstruction and (2) correct the fluid and electrolyte changes.
Relief of urinary obstruction generally requires passage of a urinary catheter. Urinary catheterization can be difficult in obstructed patients. Repetitively draining urine via cystocentesis should be avoided or used as a therapy of last resort because the turgid and inflamed urinary bladder is at risk for rupture. In some instances, cystocentesis may be chosen as an emergency procedure to relieve the immediate tension on the bladder. This temporary relief may facilitate later catheterization. In patients where urinary catheterization is not successful, urinary diversion via urethrostomy or a cystostomy tube may be considered.
Placement of urinary catheters in male and female dogs and cats is described later. Proper urinary catheterization generally requires two persons. One person places the catheter in a sterile manner while an assistant is present to restrain and properly position the animal. A catheter of appropriate length and diameter should be selected. The catheter must be long enough to reach the bladder. Generally a large diameter catheter is desirable to allow for higher flow rates during drainage or flushing of the catheter. In some instances, a small diameter catheter may be chosen to bypass an area of partial urinary obstruction.
Urinary Catheterization of Dogs
In the male dog, the patient is restrained in lateral recumbency with the hind limbs retracted caudally. The assistant extrudes the penis from the prepuce by retracting the skin sheath while stabilizing the penis at the base. The end of the penis is cleansed with an antiseptic solution (Betadine or chlorhexidine). Before placement, the catheter should be lubricated with sterile lubricant. The catheter is inserted into the urethral orifice and advanced into the bladder. Resistance to catheterization is sometimes encountered in anatomic areas where the urethra narrows (at the os penis, at the ischial arch, and in the prostatic urethra). These areas also correspond to common sites of obstruction because stones tend to lodge in these narrow areas. If a stone is encountered, there may be a gritty or solid resistance to advancing the catheter. Most stones that are encountered in this manner may be “retropulsed” into the bladder by flushing sterile saline into the catheter in a pulsatile manner.
This flushing procedure dilates the urethra surrounding the stone and helps propel the stone back into the urinary bladder. Retropulsion of stones may sometimes be assisted by massaging the urethra at the site of obstruction during digital rectal palpation. Care must be taken not to traumatize or tear the urethra during this procedure. Once the catheter is in place, urine should flow freely from the bladder. This urine should be collected for analysis, and the total urine volume should be measured and recorded. Depending upon the cause, a urinary catheter may be left in place (indwelling catheter) for a period of time. Indwelling catheters should be connected to a sterile and “closed” collection system.
This allows for hygienic urine drainage and collection and reduces the risk of infection.
In the female dog, urinary obstruction by stones is less common because the urethra is relatively short and wide compared with the male urethra. The female urethral anatomy more readily allows for the passage of urinary stones. In certain circumstances, however, catheterization of the female urinary tract may still be indicated. Passing a urinary catheter in female dogs is easier if one is familiar with the genitourinary anatomy. Anatomically the reproductive tract (vagina) and urinary system (urethra) converge into a shared mucous membrane cavity called the vestibule. The vestibule is contained within the vulva (external genitalia). The urethral orifice is palpable as a ridge of tissue known as the urethral papilla on the ventral surface of the vestibule. Dorsal to the urethral papilla is the opening of the vagina. Failed attempts at urinary catheterization often result in passage of a catheter into the vagina. Proper placement into the urethra may be performed by either digital palpation of anatomic landmarks or by direct visualization of the urethral orifice. The authors prefer to pass female urinary catheters in dogs via palpation alone because this seems most comfortable for our patients and generally is met with a high degree of success. Using this technique, the urethral papilla is palpated within the vestibule using a gloved and lubricated finger. The dorsal opening to the vagina is located and occluded with the index finger that also is used to guide the urinary catheter into the urethra. Proper placement of the catheter is confirmed by urine drainage via the catheter or by palpation. As an alternative to this procedure, some clinicians prefer to use a vaginal speculum to retract the external vulva (labia) and directly visualize the urethral orifice. This technique is successful, but more often requires sedation to ensure patient comfort during the procedure.
Urinary Catheterization of Cats
Urinary catheterization is a challenge in cats because of their small size, but feline urinary catheterization procedures are roughly parallel to the procedures in dogs. The feline urethra is quite narrow and requires selection of small catheters (usually 3 to 5 French catheters). Digital palpation of the urethral orifice is usually not possible in female cats because of their small size. However, catheterization may be successfully performed blindly or via visualization. The urethral orifice in female cats is located at midline on the floor of the vestibule approximately 1 cm from the opening of the external vulva. With the cat positioned in lateral or sternal recumbency, an experienced technician may pass a catheter blindly along the floor of the vestibule and into the urethra. Sedation and visualization of the urethral orifice may be required for success.
Urinary obstruction is particularly common in male cats, where obstruction is a common manifestation of idiopathic feline lower urinary tract disease (FLUTD). Previously known simply as feline urologic syndrome (FUS), this disease is characterized by clinical signs of straining to urinate, blood in the urine, and pain. Early signs of urinary tract disease may go unnoticed in outdoor or multicat households where less observation of urinary habits allows these common signs to be overlooked. In fact, urinary signs may go completely unnoticed, and cats with this problem may be seen for unexplained abdominal or hindquarter pain. When obstruction occurs, clinical signs of systemic illness are related to severe fluid and electrolyte imbalances. The cause of the FLUTD is unknown, but sterile inflammation develops within the bladder and lower urinary tract of affected cats. This inflammation results in concretions of protein, mucus, and crystals that frequently obstruct the narrow urethra of male cats (Figure 33-67). Importantly, urinary tract infection is usually not present initially. Affected cats may have multiple or recurrent episodes of urinary problems throughout life.

FIGURE 33-67 Urine from a cat with urinary tract obstruction shows a heavy sediment of blood and inflammation
In male cats, urethral catheterization may be assisted by positioning the cat in a “perineal position.” In this position, the cat may be in lateral or dorsal recumbency. The hind limbs are retracted cranially, and the tail is retracted caudally to expose the perineal region. The male penis is extruded by an assistant, and an effort is made to keep the penile urethra straight by positioning it in an alignment that roughly parallels the colon. Such positioning facilitates urethral catheterization and minimizes trauma to the urethra during the procedure. Urethral catheterization in cats may be further facilitated by sedation or administration of a muscle relaxant. Importantly, many cats with urethral obstruction are medically unstable and are poor candidates for sedation. These sick cats often will tolerate urethral catheterization with minimal or no sedation. Many clinicians prefer to pass a rigid polypropylene “tomcat” catheter during the initial attempts to unobstruct the urethra. These rigid catheters are uncomfortable and may be traumatic to the bladder and urethra. Therefore when an indwelling urinary catheter is required, one should attempt to replace the rigid catheters with a soft flexible catheter as soon as the urethra is free of obstruction (Figure 33-68). As in all species, indwelling urinary catheters should be secured in place and connected to a closed sterile urine collection system.

FIGURE 33-68 A soft, flexible, indwelling urinary catheter is secured in place. Note the hemorrhagic urine (associated with recent urinary obstruction) that is removed from the bladder
The goals of fluid therapy following urinary obstruction include: (1) correction of dehydration, (2) correction of electrolyte abnormalities, and (3) correction of acidosis.
Fluids should be administered via the IV route. As a general rule, isotonic sodium chloride is the fluid of choice to correct dehydration without exacerbating potassium abnormalities. However, a balanced electrolyte and pH-buffered fluid is also a good choice for patients with acidosis. Fluid rate calculations should replace the fluid deficit and provide maintenance fluid requirements. Following relief of urinary obstruction, the kidneys respond by temporarily increasing urine production. This “postobstructive diuresis” increases the fluid needs of the patient. Careful monitoring of urine output following relief of obstruction assists in tailoring fluid therapy to the individual patient.
Fluid, electrolyte, and acid-base abnormalities usually correct quickly with aggressive fluid therapy alone, but hyperkalemia and acidosis sometimes require additional therapy. Adjunctive treatment of these disorders is strongly based on clinical judgments. In some animals with severe or persistent acidosis, bicarbonate therapy may be indicated. Bicarbonate therapy is generally pursued with caution to avoid “overtreatment” that may result in metabolic alkalosis or unpredictable acid-base shifts. When bicarbonate is used, the dose is calculated and usually administered in parts. Close monitoring of the acid-base status via repetitive venous blood gas analysis is commonly performed when bicarbonate therapy is used.
In addition to standard fluid therapy, veterinarians may treat hyperkalemia with insulin and dextrose infusions. Physiologically, insulin drives both glucose and potassium into cells and can be used to lower serum potassium in animals with life-threatening hyperkalemia. Rapid-acting regular insulin is administered IV along with a bolus of dextrose. The dextrose helps prevent hypoglycemia and may stimulate further endogenous insulin release. Insulin-dextrose therapy has an onset of action within 15 to 30 minutes, and the effects may last for a few hours. Veterinarians also treat life-threatening hyperkalemia with calcium gluconate infusions. Nerve and muscle cell activity is reduced in states of hyperkalemia because of the effect of high potassium on the cellular membrane potential. These effects result in cardiac arrhythmias, including bradycardia and cardiac arrest. Calcium gluconate may be administered IV to counteract these effects and transiently normalize the membrane potential and restore normal cell excitability. Calcium gluconate has an almost immediate effect (within minutes) on cardiac conductance and protects from potassium-related arrhythmias. However, animals receiving calcium gluconate should have continuous ECG monitoring because rapid calcium infusion can also result in cardiac arrhythmia. Calcium gluconate infusions may also cause hypercalcemia. For these reasons, calcium infusions are given “to effect” using the lowest effective dose during careful monitoring.
TOXIN INGESTION
Dogs are well known for randomly ingesting things in their environment during play or from curiosity. Cats are normally more discriminate and fastidious in their eating habits, but will still ingest noxious substances in their environment. These behaviors make toxin ingestion a common presenting complaint or telephone call to veterinary offices. See also Chapter 35. A brief clinical review of common toxin ingestions seen in emergency practice is presented here.
Veterinary technicians are often the first point of contact when pet owners call about toxin ingestion. Although we should be knowledgeable about the most common toxins, we should also be careful about what information and advice is presented by telephone. Information gleaned from telephone conversations is notoriously incomplete, and the consequences of error can be severe. When in question, the best advice to pet owners is to bring the pet to the veterinary practice for examination. Consequently the real purpose of a client telephone call is to gather information relevant to the animal's treatment before an appointment. The following information should be obtained:
1. What did the animal ingest?
2. When did the animal ingest it?
3. Is the animal showing any clinical signs?
4. Was the ingestion witnessed or suspected?
5. Are there other animals or children who could also be exposed?
Manufacturer labels contain important information and should be saved and brought to the veterinary practice with the pet.
The product label may be used to identify any harmful ingredients and may have instructions to follow after unintended ingestion or overdose. Many products have a toll-free number for questions. This type of information helps the veterinary team anticipate the needs of the patient and plan for the animal's arrival. Additional information may be obtained from local government-sponsored poison control centers. Information from these centers is species specific for humans and may not be entirely applicable to animals. Because of similarities in mammalian physiology, however, information about human poison exposure is still helpful in most cases of small animal toxin ingestion. Fortunately, animal-specific poison control centers are readily available and are a wealth of species-specific information. Animal poison control centers often charge a reasonable fee for this service.
Induction of vomiting (also called emesis) is a common treatment for the ingestion of harmful substances. Timely induction of vomiting may eliminate the toxin from the body before it can be absorbed and exert any harmful effects. In most instances, vomiting should be induced within 4 hours of ingestion. This is a flexible time limit that roughly corresponds to normal gastric emptying time. Gastric emptying time may be prolonged in some instances. Once a substance has exited the stomach and is being absorbed in the small intestine, induction of vomiting is unlikely to have a therapeutic benefit.
Importantly, induction of vomiting is not a cure-all for toxin ingestion and should be cautiously considered like any other treatment. As a general rule, ingestion of caustic substances and petroleum products should not be treated by vomiting because of the risk of further injury or aspiration. Emesis is contraindicated after ingestion of many household products, including bleach, lye, gasoline, and oil (and many other acids and strong alkali agents). In addition, induction of vomiting is strongly contraindicated in animals with altered awareness because sedated, comatose, or tranquilized animals are at high risk for developing aspiration pneumonia. Vomiting may be induced by a variety of means. In dogs, apomorphine is a potent emetic agent that directly stimulates the vomiting centers of the brain to cause vomiting. The drug can be compounded into a variety of forms. The drug is absorbed across the conjunctival membranes of the eye, and small tablets of the drug may be placed in the conjunctival sac until vomiting has occurred. Subsequently the drug can be flushed from the conjunctiva to terminate the vomiting episode. Apomorphine is not effective in cats. Because they do not respond to apomorphine, induction of vomiting in cats is challenging. Xylazine (a sedative drug) often stimulates vomiting in cats. This emetic effect must be balanced with the risk of sedation-related aspiration when xylazine or similar drugs are used for this purpose. A number of household remedies, such as hydrogen peroxide and syrup of ipecac, have been used to induce vomiting in both cats and dogs. Hydrogen peroxide administered orally will reliably result in vomiting as a result of bitter taste and gastric irritation. The widespread use of hydrogen peroxide as a topical antiseptic makes it a convenient over-the-counter and generally available emetic. However, hydrogen peroxide can be dangerous in sensitive or overdosed animals because careless use of hydrogen peroxide can result in severe hemorrhagic gastroenteritis. Syrup of ipecac induces vomiting by inciting gastric irritation and by stimulating the vomiting centers of the brain. This once common home remedy has become less popular because induction of vomiting at home has some risk and may delay other more appropriate medical treatments.
When vomiting is not possible but gastric emptying is desired, gastric lavage is sometimes an option. Unfortunately, gastric lavage does not always result in effective or timely gastric emptying and carries some inherent risks. Therefore whenever possible, gastric emptying via induction of vomiting is nearly always preferred over gastric lavage. For the gastric lavage procedure, animals are sedated and an endotracheal tube is placed to secure the airway and prevent aspiration. Subsequently a stomach tube is placed, and the stomach contents are removed via lavage and drainage. This procedure is time consuming (which may delay other treatments), and the sedation required for gastric lavage adds risk. Additionally, residual lavage fluid sometimes merely adds to gastric volume and raises the risk of vomiting and aspiration. If gastric lavage is indicated, it is best performed by experienced personnel.
In addition to gastric emptying, treatments for nonspecific toxin ingestion may also include administration of adsorbents and cathartics. Adsorbents bind toxins to prevent absorption and facilitate their excretion. Cathartics are medications that affect GI transit and speed fecal elimination of toxins. The most common adsorbent is activated charcoal. Activated charcoal comes in a variety of commercially available formulations. Oral suspension liquid preparations are easy to administer directly by mouth (alone or mixed with food) or by stomach tube (Figures 33-69 and 33-70). Activated charcoal may bind intestinal nutrients and other orally administered medications, but does not appear to have any common clinically significant side effects. Activated charcoal is excreted in the feces and will result in dark or black discolored stool that may be mistaken for melena. Some formulations of activated charcoal contain sorbitol as a cathartic. Sorbitol is a sugar alcohol that draws water into the large bowel, resulting in a laxative effect.
The treatment and clinical evaluation of specific toxins is beyond the scope of this chapter. However, a few common toxicities warrant brief discussion.
Ethylene Glycol Ingestion: Ethylene glycol is the active ingredient of most automotive antifreeze solutions and is highly toxic. Animals most frequently come into contact with antifreeze solution that has spilled during vehicle maintenance. Although ethylene glycol exposure may be associated with cold climates or occur seasonally in the winter, the universal use of antifreeze in automobiles makes it a common toxicity in all parts of the country and at all times of the year. Ethylene glycol is reportedly sweet and palatable, and animals willingly ingest it. Unfortunately the palatability and high toxicity of antifreeze make it a common source in malicious animal poisoning.
Ethylene glycol ingestion results in a fairly classic progression of clinical signs. “Early” signs of ethylene glycol poisoning occur within 30 minutes to 12 hours postingestion and include vomiting, lethargy, excessive drinking and urination, and neurologic signs (ataxia, proprioceptive deficits, seizures, etc.). These initial neurologic signs often diminish as ethylene glycol is metabolized, leading to a false sense of recovery. However, the metabolites of ethylene glycol soon cause fulminant renal failure in both cats and dogs. Kidney failure generally develops within 12 to 24 hours in cats, and somewhat later (48 to 72 hours) in dogs. This kidney failure is responsible for the “late” clinical signs of ethylene glycol poisoning that include renewed neurologic signs (e.g., seizures, coma, and death) and other manifestations of severe kidney injury (e.g., oliguria or anuria, severe lethargy, dehydration, and vomiting). The progression and severity of these clinical signs is dose dependent. Cats are much more susceptible than dogs to ethylene glycol and develop lethal toxicity at much lower doses. Without treatment, ethylene glycol ingestion is almost uniformly fatal.
Early detection of ethylene glycol toxicity is incredibly important if treatment is to be attempted. Unexplained or transient neurologic signs should prompt consideration of this toxicity. Definitive diagnosis requires either witnessed ingestion or demonstration of ethylene glycol in blood or urine. Ethylene glycol assays are commonly performed at veterinary reference laboratories and at human hospitals, and test kits for veterinary cage-side testing are reliable. When testing blood samples, it is important to interpret the results in context of the time of ingestion. Ethylene glycol blood levels peak at 12 hours postingestion in dogs and 3 to 6 hours postingestion in cats and then diminish rapidly. Thereafter, it may be more prudent to investigate ethylene glycol levels in the urine, where the compound is excreted. Isosthenuria and calcium oxalate monohydrate crystals in the urine may be seen within hours of ingestion and can be important supporting evidence in the diagnosis of toxicity (Figure 33-71). Veterinarians and technicians should be able to identify these characteristic crystals. Remember that azotemia and other changes associated with acute renal failure have a more delayed onset and may not be present early on.
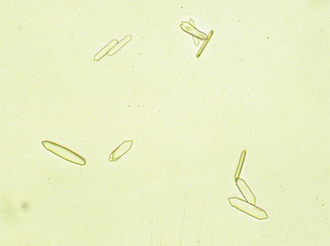
FIGURE 33-71 Calcium oxalate monohydrate crystals are associated with ethylene glycol intoxication and have a characteristic appearance. (Photo courtesy Dr. Steve Gaunt, Baton Rouge, La.)
Treatment of ethylene glycol toxicity varies with the level of available care and the timing of diagnosis. Cases of witnessed ingestion should be managed with standard detoxification procedures, including induction of vomiting and administration of activated charcoal. Remember that ethylene glycol makes animals sick, but it is the toxic metabolites of ethylene glycol that ultimately result in death (by causing kidney failure). Therefore standard treatment is focused on preventing metabolism of the toxin. In dogs, metabolism of ethylene glycol may be inhibited by administration of fomepizole (Antizol-Vet, Orphan Medical) according to the manufacturer's recommendations. Historically, fomepizole was ineffective in cats, and medical grade ethanol was used to competitively inhibit the enzymes responsible for ethylene glycol metabolism. More recent information suggests that fomepizole (at a dose specific for cats) may be effective for the treatment of cats with ethylene glycol ingestion. Some specialty referral centers are able to employ dialysis in the management of ethylene glycol toxicity (Figure 33-72). In the early stages (before kidney failure), dialysis is able to remove the toxin from the blood circulation before kidney injury occurs. In these cases (of early recognition and treatment), the prognosis is good. However, cases that are recognized late may incur permanent kidney damage. The prognosis for animals with ethylene glycol–induced acute renal failure is poor, but in some cases, dialysis may provide enough support for regeneration to occur.
Rodenticide Ingestion (Rat Poison): The most common rodenticides are anticoagulant compounds that cause death by inducing life-threatening hemorrhage. These anticoagulant rodenticides are vitamin K antagonists. Vitamin K1 is required for the final production step of numerous clotting factors made by the liver. The anticoagulant rodenticides interfere with clotting factor synthesis and cause clinical signs of bleeding within 24 to 72 hours of ingestion.
Common anticoagulant rodenticides include warfarin, brodifacoum, bromadiolone, diphacinone, and chlorophacinone.
Diagnosis of rodenticide ingestion generally consists of documenting possible exposure, observing abnormal bleeding (Figure 33-73), and identifying prolongation of clotting times. In general, both PT and activated partial thromboplastin time (APTT) become prolonged. Measurement of these clotting times is usually performed on citrated whole blood samples. Countertop analyzers are available for rapid in-house assessment of clotting times.
FIGURE 33-73 Rodenticide ingestion often results in spontaneous bruising and bleeding. Note the fresh blood flowing from the right nostril of this dog
Witnessed ingestion of rodenticide compounds should be treated with induction of vomiting and administration of activated charcoal. Such animals may avoid life-threatening toxicity by such early intervention. However, monitoring of coagulation parameters or prophylactic vitamin K1 supplementation is still recommended. Coagulation abnormalities, if present, usually start to correct within 12 hours of oral vitamin K1 supplementation. Vitamin K1 therapy is often initiated with a subcutaneous injection. IV injection of vitamin K is contraindicated because of the risk of anaphylaxis. Intramuscular injection is avoided because of the risk of hematoma formation. Animals receiving subcutaneous vitamin K1 may develop allergic reactions similar to a vaccine reaction. These reactions include facial swelling, itching, and generalized hives (Figure 33-74). If such a reaction is noted, the animal should be treated with an antihistamine and corticosteroid therapy before switching to oral supplementation. Allergic reactions to orally administered vitamin K1 are uncommon. Duration of vitamin K1 therapy depends upon the specific compound ingested, but most cases are treated for approximately 1 month.
FIGURE 33-74 Facial swelling, itching, and hives may be seen during allergic reactions to vitamin K injections
Animals with unwitnessed ingestion present a more difficult challenge because these animals usually are not diagnosed until abnormal bleeding is observed. Common sites of blood loss include nose (Figure 33-73) and oral cavity, spontaneous bruising of the skin and sclera, bleeding into body cavities (hemothorax, hemoabdomen), and intestinal blood loss. Essentially, anticoagulant rodenticides can result in bleeding anywhere. Affected animals risk dying from blood loss anemia or may have secondary problems, such as respiratory compromise from intrapulmonary hemorrhage. In addition to vitamin K1 supplementation, treatment of these critical cases also requires correction of blood loss and replacement of active clotting factors via blood or plasma transfusion.
Permethrin Toxicity: Many effective and convenient flea and tick control products are available for over-the-counter purchase by today's pet owner. Some of the most popular products are topically applied to the skin, and the active ingredients are taken up and distributed over the pet within natural skin oils. Examples of these products include fipronil (Frontline, Merial, Duluth, Ga.), imidacloprid (Advantage, Bayer Animal Health, Shawnee Mission, Kan.), methoprene, and permethrin. Fipronil is effective in killing adult fleas and ticks. Imidacloprid is effective against adult and larval fleas, but is not effective against ticks. Methoprene is an insect growth regulator that prevents larval fleas from maturing into adult fleas. Methoprene does not affect mammals and is considered quite safe. Permethrin is a synthetic pyrethroid compound with insecticidal properties used in the control of fleas and ticks. Although these products are available without a prescription, veterinarians are still the best source of information on the proper selection, application, and safety of these products. Many pet owners misread labels or make assumptions about product safety. Products that are safe to use in dogs may not be safe to use in cats (even in small doses).
Permethrin's insecticidal properties are derived from neurotoxic effects that cause paralysis and death in insects. Cats appear uniquely predisposed to the toxic effects of permethrin, which can cause generalized tremors, muscle fasciculations, and seizures in cats. Permethrin toxicity has become one of the most common causes of seizures in cats as a result of the misapplication of products intended for dogs. Cats exposed to permethrin compounds develop dramatic clinical signs within 1 to 2 hours of application. In addition to the clinical signs noted earlier, affected cats often have hyperthermia, hypersalivation, dyspnea, ataxia, hyperesthesia, aggression, and gastrointestinal signs. Death may occur in severe intoxication, in sensitive animals, or when treatment is delayed.
Diagnosis of permethrin toxicity requires a history of exposure and subsequent development of clinical signs. Diagnostic tests to rule out other causes of seizures may be needed in some cases. Treatment of affected cats should include decontamination of the skin via bathing. Bathing symptomatic cats is difficult, but is important in preventing further uptake of the toxin. Muscle relaxants (such as methocarbamol), anticonvulsants, and sedative drugs (such as diazepam) can be useful in the symptomatic treatment of seizures, tremors, and muscle fasciculation. Fluid therapy is indicated to correct dehydration that develops from hypersalivation and protracted muscle activity. In addition, fluid therapy and bathing are helpful in managing hyperthermia. The overall prognosis for cats that receive prompt treatment is good. Cats should be monitored in the hospital during recovery for at least 24 hours.
CONCLUSION
Emergency and critical care veterinary hospitals treat the sickest and often the most unpredictable animals. These unique challenges emphasize the importance of basic patient care and advanced monitoring techniques. Providing comfort and basic needs should be a nursing priority. Technicians must be aware of common clinical problems, anticipate the needs of the patient, and be alert to new problems and impending crises. Familiarity with a variety of monitoring techniques and diagnostic procedures will allow for the best possible care. The best monitoring plans use multiple parameters and a standardized approach to interpreting and responding to problems during emergencies or standard patient care, triage, and CPR.
LARGE ANIMAL EMERGENCY NURSING
An emergency is defined as a sudden, urgent, usually unexpected event or occurrence requiring immediate action. In equine practice, an emergency is usually the result of the patient's response to an environmental stimulus (fight or flight), the curious nature of horses, obstacles in the environment, or the unique nature of the patient's anatomy (natural areas of narrowing of the horse's gastrointestinal tract). Less commonly a veterinary emergency may be the result of human malice, negligence, or neglect. When an emergency arises, the veterinarian and the staff must act with the appropriate amount of care, compassion, and in an expeditious manner. In some cases, such as long bone fractures or severe abdominal pain, the decision to treat or to humanely euthanize the patient is the first issue to be addressed. When treatment is attempted, it is often initiated with minimal objective data regarding the patient's condition. These are circumstances where the veterinarian and the staff must react rather than ponder the situation. Guess and reassess is often the way in which emergencies are initially approached until the patient, client, and situation are stabilized. An experienced, well-trained veterinary technician is essential in the organization of instruments and material, initial assessment, and triage of the horse seen on an emergency basis. Depending on the presenting complaint and the patient's condition, several criteria must be assessed and met:
• First and foremost, the owner or client, patient, veterinarian, and staff should be protected from injury.
• Relief of the patient's pain and anxiety. In no circumstance is it appropriate to withhold pain medication from a patient that is obviously in need.
• Transport the patient for definitive care.
• If a fracture or breakdown injury has occurred, stabilization if the affected limb is an immediate concern, particularly if surgical treatment is to be attempted.
• Protect from contamination and initially treat soft tissue trauma.
• Control hemorrhage if present.
• Be sure that the patient has an appropriate airway and is able to breath.
• Fluid resuscitation and correction of electrolyte abnormalities.
Each of these criteria will be addressed in detail repeatedly throughout the remainder of this chapter.
The purpose of this chapter is to review the veterinary technician's role in the management of emergencies specific to equine veterinary medicine. This includes the initial examination and determination of the patient's condition, the equipment, instruments, and materials needed to manage equine emergencies, and finally, this chapter will review the use of IV fluids, blood, and blood products in equine emergency medicine.
GENERAL CONSIDERATIONS
The chief complaint or reason the patient is seen will dictate what preparations are made before the patient's arrival or what equipment is to be carried into the field if examination is undertaken in an ambulatory situation. It is essential that all needed equipment be organized before arrival of the patient. This includes needles, syringes, catheters, IV fluids, fluid delivery systems, oxygen tanks and regulators, IV fluids, and any medications, such as NSAIDs and sedation. In addition, any equipment that may need to warm up, such as x-ray processes, should be turned on before arrival of the patient. Emergency and first-aid equipment can be stored conveniently in already prepared carts or emergency kits that can be organized based on emergency needs (Figure 33-75), or the material can be organized onto carts before arrival of the patient (Figure 33-76).
Emergency drugs that are stored in carts or tots should be arranged in alphabetic order, and a list of the drugs, their indications, dosage, and typical volumes administered to an average 450-kg adult or 50-kg foal can be laminated and stored with the drugs for quick reference (Figure 33-77). Table 33-2 is a list of emergency drugs commonly used in equine practice.
TRANSPORT OF THE INJURED OR CRITICALLY ILL HORSE
Considerable misconceptions exist as a result of the lack of specific published recommendations on the transport of critically ill or injured equine patients. Too often veterinarians are reluctant to recommend and clients are apprehensive about transporting patients to the hospital for definitive care because of the fear of injury during transport. These fears are often unfounded, and the risk of transport seldom if ever outweighs the benefit of appropriate treatment. Although trailering is an athletic event with the horse needing to balance its weight, sometimes on three legs through turns and sudden stops, most horses withstand transport well as long as appropriately prepared and hauled.
Before Loading
Preparation for transport is dependent on the type of emergency. Owners should be instructed at the time of the initial call as to the safest, most appropriate means of hauling their horse. If referring an emergency to another hospital, the referral hospital should be contacted before transport and any specific instructions for transport obtained.
It is vitally important that the person hauling the sick or injured horse be provided with accurate, easy-to-follow directions to the hospital, and contact telephone numbers should be provided in the event that the driver becomes lost, the horse's condition deteriorates, or the horse dies in route. The driver should also be instructed to call the practice when they are estimated to be within 15 to 30 minutes of the practice or if the horse dies. The following are recommendations
for the transport of horses suffering from specific emergency conditions.
Abdominal Pain
Clients often are fearful of transporting patients with severe abdominal pain. In particular those patients that frequently lie down. Although there are risks in hauling these patients, even the most painful and sickest horses usually endure the trailer ride. The horse's concentration is often drawn away from its abdominal pain by the necessity to maintain balance. Patients suffering from dehydration or shock will benefit from the administration of IV crystalloid fluids and hypertonic saline solutions before loading on the trailer. These patients are best hauled in a slant load or two-horse trailer with chest and rear bars to allow the horse to lean as desired. Horses with severe abdominal pain can also be hauled in an open stock trailer to allow them to lie down, if desired. Horses can be mildly sedated and NSAIDs administered to relieve pain. If the trailer ride will last longer than the expected action of the sedation, the hauler may be provided with additional doses of a medication, such as xylazine, that can be administered intramuscularly. The hauler can stop at set intervals to check on the condition of the horse and readminister sedation if needed. In extreme cases, IV fluids may be provided to horses during transport. Fluids may be hung from the ceiling of the trailer; however, a plan for regularly scheduled stops must be made to ensure that the fluids do not run out and allow blood to back up and obstruct the catheter. Colic horses should also have a nasogastric tube passed and gastric reflux removed immediately before loading.
 TECHNICIAN NOTE
TECHNICIAN NOTE