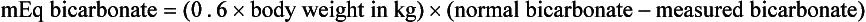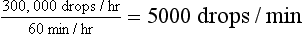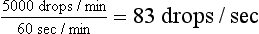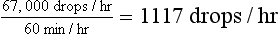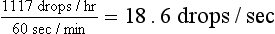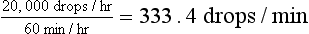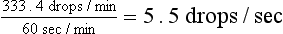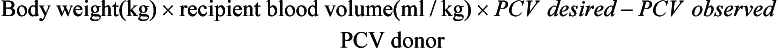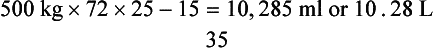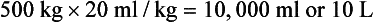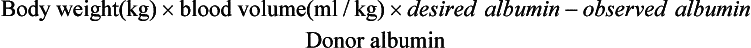INTRAVENOUS FLUID THERAPY
Horses coming to the hospital for emergency treatment frequently are suffering from varying degrees of dehydration and circulatory (hypovolemic) shock. Dehydration is defined as the excessive loss of total body water. In many cases, electrolyte abnormalities accompany this loss of water and contribute to the clinical signs exhibited by the patient. When dehydration is mild, compensatory mechanisms, including peripheral vasoconstriction, movement of fluid from the interstitial to the intravascular space, increased heart rate and contractility, and retention of water and sodium by the kidney, serve to increase the circulating plasma volume, maintain cardiac output, and therefore tissue perfusion. In these cases, there are often only subtle signs of dehydration. In other cases, these compensatory mechanisms are overwhelmed, or the loss of fluids and electrolytes is continuous leading to dehydration and hypovolemic shock. With shock there is a decreased cardiac output leading to decreased tissue perfusion and hypoxia at the cellular level. Under conditions of hypoxia, cellular energy stores are rapidly depleted leading to derangement in the normal cellular metabolic pathways. If untreated, organ dysfunction ensues progressing from the least perfused organs, the gastrointestinal tract, kidneys, and liver, to the organs demanding the greatest perfusion, such as the heart and brain. Eventually the perfusion of the brain and heart fall to critical levels, compensatory mechanisms fail, and complete circulatory collapse leads to death of the patient.
Clinical Signs of Dehydration
The percent dehydration is a subjective estimate of the percentage of body mass lost in fluid. Subjective estimations of hydration are not highly accurate, but they serve as a starting point for treatment that requires frequent reassessment. In horses, signs of dehydration are usually not obvious until the horse is at least 5% dehydrated. At this level, signs of dehydration are mild and may include depression, tachycardia, and decreased pulse quality. As dehydration worsens, additional signs of dehydration may include delayed recovery of skin tenting, tacky mucous membranes, prolonged CRT (greater than 3 seconds), and poor jugular distensibility.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Subjective estimations of hydration are not highly accurate, but they serve as a starting point for treatment that requires frequent reassessment.
A more accurate clinical assessment of dehydration is the change in body mass measured by weight before, during, and after fluid therapy. Although this is a highly accurate measure of hydration, it is impractical in most practice settings. In addition to physical examination findings, hematology findings consistent with a presumptive diagnosis of dehydration include an elevated PCV and plasma TP concentration. This is a simple test that can be performed quickly with equipment available in most private practices and can be used to measure response to fluid therapy. In addition, results of a biochemistry profile that are consistent with dehydration include an elevated creatinine and BUN level. Elevation in BUN and creatinine are collectively termed azotemia and may be the result of dehydration (prerenal azotemia), kidney failure (renal azotemia), and urethral obstruction (postrenal azotemia).
Catheter Location
The location of venous access is determined by the goals of therapy. If the catheter is placed to deliver large quantities of IV fluids, blood or blood products, or if fluids of high osmolarity, such as parenteral nutrition solutions, are to be delivered through the catheter, a larger peripheral vein, such as the jugular vein, or central venous access is recommended. This allows for a larger-size catheter to be placed and as a result of the laminar flow characteristics of these veins decreases the likelihood of thrombophlebitis that occurs when fluids of high osmolarity are administered IV. Administration of antibiotics can be accomplished via jugular catheterization; however, there may be instances in which catheterization of the jugular vein is not desirable. In those cases, catheterization of the accessory cephalic, saphenous, or lateral thoracic veins are reasonable alternatives.
Catheter Selection
IV catheters have become a mainstay in large animal emergency nursing. Advances in emergency and critical care of large animals have resulted in the need for short- and long-term venous access for the delivery of large volumes of IV fluids, medications, such as sedation and analgesics, antibiotics, antiinflammatory drugs, and unfortunately, on occasion euthanasia solutions. In addition, the more frequent use of blood, blood products, and parenteral nutrition has resulted in the need for specialty catheters and delivery systems to minimize catheter-related complications. There are now a wide variety of choices for catheter materials, size, mechanisms for introduction into the vein, and lumen number available. Initially, human catheters were adapted to veterinary use; however, since the emergence of companies, such as Mila International, basic and specialty catheters are now manufactured specifically for veterinary use. Factors that must be considered when selecting the size and type of catheter to be used include the size of the patient, vein to be catheterized, the condition of the patient at presentation, the fluid or medication to be administered through the catheter, the desired rate of administration, expected duration of catheterization, and what is readily available at the time of emergency.
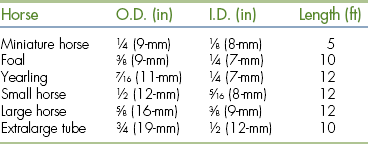
Commercially available IV catheters for large animal use range in size from 10 to 25 gauge and lengths from 1 to 6 inches. The gauge of a catheter is a measurement of its outside diameter. Smaller-gauge catheters have larger outside diameters, which correspond to a larger inside diameter and a higher rate of flow. The size used is dictated by the size of the patient and the treatment to be administered. Large-diameter catheters (10 to 14 gauge × 5.25 inches) are most commonly used for the administration of IV fluids and medication to adult horses. Foals, ponies, and miniature horses are typically catheterized with smaller 16- to 18-gauge catheters ranging from 3.5 to 5.25 inches in length. In addition to size, fluids with higher viscosity, such as blood or plasma, are more easily administered through large-diameter catheters.
Catheters with a large internal diameter permit more rapid administration of fluid, but have the disadvantage of being more damaging to the vein. Catheters of small diameter, 18 to 25 gauge, are reserved for special uses, such as arterial blood pressure monitoring, where they are placed in small peripheral arteries, such as the facial, transverse facial, or metatarsal artery.
Catheter material also affects the choice of catheters. Commonly used materials for manufacture of catheters include polypropylene, PVC, polytetrafluoroethylene (Teflon), nylon, silicone rubber (Silastic), polyurethane, and polyether block amide (Pebax). Different materials result in catheters of different internal diameters, lengths, and stiffness. The differences in material characteristics and size of catheters results in differences in their potential to damage the vessel wall and form a thrombus (thrombogenicity). Traditionally, large-diameter catheters have been manufactured using polypropylene and Teflon. These catheters are stiff with large outside diameters and typically contact the vessel wall over their entire length. This contact damages the vascular endothelial lining exposing collagen and initiates the clotting cascade. This can result in the formation of a blood clot (thrombus) or fibrin sheaths that extend the length of the catheter. Contamination of this thrombus with bacteria can cause the development of inflammation or infection of the thrombus and vein termed thrombophlebitis. Catheters manufactured from silicone (Silastic), polyurethane, or Pebax have the advantage of being softer than Teflon or polypropylene and tend to float in the lumen of the vein. This decreases the thrombogenic potential of the catheter. A disadvantage of Silastic catheters is their porosity that predisposed them to bacterial adherence. For this reason, Silastic catheters must be placed under strict aseptic conditions. As a general rule, large-diameter catheters made of polypropylene or Teflon should only be used for short duration (12 to 24 hours) and are inappropriate for use in small patients, such as foals. Catheters manufactured from Silastic, polyurethane, or Pebax can be maintained as long as 2 to 3 weeks depending on the manufacturer's recommendations.
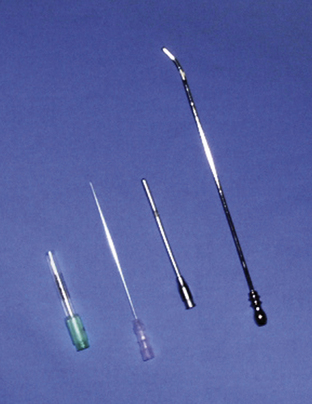
The process of introduction of the catheter into the vein is also variable depending on the size and material the catheter is made from. Short-term Teflon and polypropylene catheters and 14- and 16-gauge polyurethane catheters are most commonly purchased as over-the-needle catheters (Figure 33-108). These have the advantage of simple insertion into the vein with minimal training necessary and low cost. Silastic catheters and certain styles of polyurethane catheters used in foals are of an over-the-wire design. For these catheters, a flexible wire is inserted into the vein before catheterization. The wire acts as a guide for the flexible catheter to follow to ensure proper placement without kinking (Figure 33-109). These catheters are less thrombogenic and have fewer catheter-related complications, such as kinking or breaking. Their disadvantages include expense, the need for added training and practice for correct insertion, and they require absolute sterile preparation for administration. Peel-away introducers are also available that allow catheter placement through a preplaced introducer. After passage of the catheter through the introducer, it is split into two halves for easy removal from the vein.
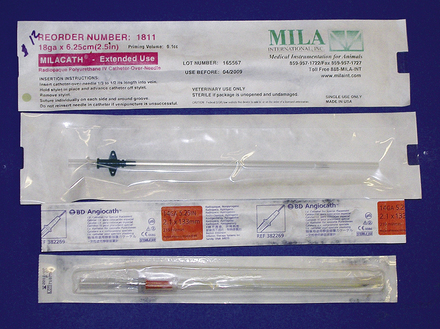
FIGURE 33-108 Example of over-the-needle catheters

FIGURE 33-109 Example of an over-the-wire catheter
Specialized catheters for the simultaneous administration of IV fluid, total parenteral nutrition (TPN), blood products, and medication are also available. These catheters come in double- or triple-lumen configurations with over-the-wire or peel-away introducers for ease of insertion. Multiport catheters must maintain continuous flow of fluids through each lumen. Stagnation of fluid flow enhances proliferation of bacteria that may have gained access to the ports. Double- or triple-lumen catheters used when additional ports are no longer needed should be replaced with a single-lumen catheter.
In emergency situations, adult horses frequently arrive at the hospital seriously dehydrated and in shock. These patients require large volumes of fluid to be administered in a short period of time. These horses are best treated by insertion of a large catheter, such as a 10-gauge Teflon catheter for initial fluid volume replacement. Because of their size and the material used to manufacture these catheters, they cause significant damage to the vein and predispose the horse to catheter-related complications. Therefore they should be used for only short periods of time (12 to 24 hours) and removed once the fluid deficit has been replaced. An alternative plan is to place multiple catheters. This may be accomplished in several ways. First, a 10-gauge catheter can be placed alone and replaced with a smaller 14- to 16-gauge catheter once the initial volume deficit has been replaced. This is frequently accomplished by passing a guidewire through the original catheter, removing the 10-gauge catheter by passing it over the guidewire, and replacing it with a smaller catheter placed over the wire. This technique has also been demonstrated to be successful at treating catheter-associated infection without jeopardizing additional veins. Alternatively a 10-gauge catheter may be positioned opposite to a smaller catheter and removed once fluid deficits are corrected. Finally, multiple smaller catheters, usually 14- to 16-gauge, may be placed in multiple veins or stacked in the same vein if large-diameter catheters are not available (Figure 33-110). Fourteen- to 16-gauge catheters are most commonly employed once fluid deficits have been replaced and are used primarily for continued maintenance fluids and the administration of other therapeutic agents, such as antibiotics (Figure 33-111).
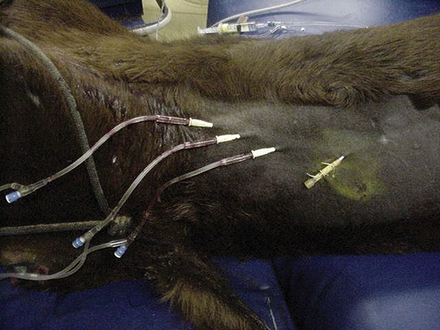
FIGURE 33-110 IV catheters stacked in a single vein for rapid delivery of large volumes of IV fluids
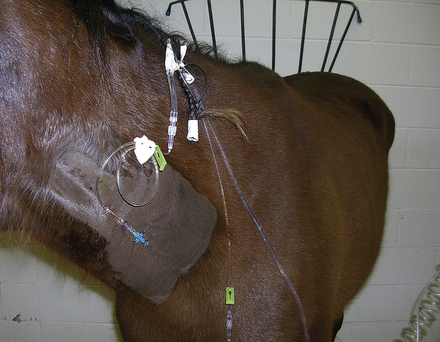
FIGURE 33-111 IV fluid administration through a 14-gauge catheter
Catheter Maintenance
Regardless of the type of catheter and the duration of use, the catheter and surrounding skin should be examined at least two times per day for evidence of heat surrounding the insertion site, swelling around the catheter or a cordlike swelling of the catheterized vein, pain on palpation, or drainage around the catheter insertion site. These may be signs of inflammation or infection of the subcutaneous tissue surrounding the catheter or thrombophlebitis. Catheters with a continuous flow of fluids do not require regular flushing unless the fluids are discontinued. Catheters that are not removed following discontinuation of fluids, those used for the administration of IV medication, and unused lumens of multilumen catheters should be flushed with heparinized saline solutions two to four times per day. Flushing ensures that the catheter remains patent and prevents stagnant flow in the unused catheter lumen and bacterial colonization. If signs of subcutaneous infection around the catheter or thrombophlebitis arise, the catheter should be removed immediately and the catheter tip aseptically collected and submitted for bacterial culture and sensitivity.
 TECHNICIAN NOTE
TECHNICIAN NOTE
IV catheters and the surrounding skin should be examined at least two times per day for evidence of heat, swelling around the catheter or a cordlike swelling of the catheterized vein, pain on palpation, or drainage from the catheter insertion site.
Fluid Therapy Plan
Fluid therapy can be performed by the administration of oral or IV fluids in the horse. In most emergency situations, fluid administration is for the purpose of replacing the extracellular fluid lost to gastrointestinal disease; therefore oral administration of fluid for replacement is often contraindicated. IV fluids are employed to correct acute dehydration and to maintain the circulating plasma volume in the face of ongoing fluid loss (diarrhea, gastric reflux, ileus, etc.). Three basic questions must be answered when designing a fluid therapy plan:
1. What type of fluid is to be administered?
2. How much fluid will be administered?
3. At what rate will the fluid be administered?
In most cases, isotonic crystalloid fluids are used for the initial replacement of fluid in horses with dehydration and hypovolemic shock. Two broad classifications of replacement fluids are available for use in horses: normal saline (0.9% sodium chloride) and balanced polyionic electrolyte solutions (LRS, Normasol-R, and Plasma-Lyte). Normal saline is much higher in sodium and chloride than plasma, but contains no other electrolytes. Its use is primarily as a replacement solution in conditions where plasma sodium levels are less than 125 mEq/L or in disease conditions where potassium-free solutions are desired, such as HYPP, urinary bladder rupture in foals, or renal failure.
The electrolyte composition of most commercially available balanced electrolyte fluids for horses is essentially the same as that found in plasma. The use of balanced electrolyte solutions is to replace the fluid volume and electrolytes lost from the extracellular space acutely. Once the fluid to be administered is decided, additional electrolytes can be added to the stock solution to correct specific electrolyte deficiencies and to correct acid-base deficits. Potassium, calcium, and magnesium are frequently supplemented intravenously in horses that are unable to ingest food or water because of gastrointestinal disease. These electrolytes are rapidly depleted in patients without oral intake of food or water and are essential for normal cardiac and smooth muscle function. If electrolyte solutions or other medications are added to stock solutions of fluid, the bags should be clearly labeled with the additive, the concentration, amount added, and the date the fluid was mixed.
Bicarbonate is supplemented in patients suffering from severe metabolic acidosis, most commonly foals. Dehydration leads to decreased tissue perfusion and a change from aerobic to anaerobic metabolism at the cellular level. The end product of anaerobic metabolism is lactic acid that causes a decrease in plasma pH and lactic acidemia. In most cases, the administration of IV fluid rapidly improves tissue perfusion, and lactic acidemia is corrected without specific therapy. In patients where plasma pH is less than 7.2, bicarbonate supplementation is required. The dose of bicarbonate to be replaced is calculated as:
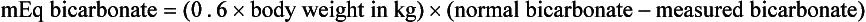
0.6 represents an estimation of volume of total body fluid. This number is different for foals and adults. For foals, 0.6 is the correct estimation because a greater percentage of body mass is made up of water. In adults needing bicarbonate supplementation, 0.3 is used as an estimate of volume of total body fluid.
Sodium bicarbonate solutions are mixed in sterile water or saline for administration. Bicarbonate cannot be administered with fluids containing calcium. Mixing of bicarbonate with calcium-containing fluid will result in the formation of an insoluble precipitate. Patients receiving IV bicarbonate must have normal respiratory function for excess carbon dioxide to be expired or acidemia will worsen. One half of the calculated dose is replaced over 4 to 6 hours with the remainder administered over 12 to 24 hours.
Once the type of fluid to be administered is determined, the volume to be administered is calculated. When developing an initial fluid therapy plan, three components must be accounted for to adequately correct and maintain hydration:
1. The volume to be replaced
2. The volume required for maintenance
3. The volume of continued loss
To determine the volume of fluid to be replaced, an estimation of the percent dehydration must be made. Dehydration is a subjective estimation that requires frequent reevaluation to determine if dehydration is being corrected. The simplest estimation is based on a scale of mild, moderate, and severe dehydration (Table 33-4).
TABLE 33-4
Estimation of the Percentage Dehydration in Horses
| Severity of Dehydration |
% Dehydration |
Clinical Signs |
| Mild |
5-6 |
Normal mucous membranes, normal to slightly prolonged skin tent, CRT 1-2 sec |
| Moderate |
7-9 |
Tacky mucous membranes, mildly prolonged skin tent, CRT 2-4 sec |
| Severe |
>9 |
Dry mucous membranes, prolonged skin tent, CRT >4 sec |
The percent dehydration is multiplied by the estimated body weight of the patient to determine the replacement fluid volume.
Maintenance fluid needs are relatively straightforward. The average adult horse requires 50 to 60 ml/kg/24-hr period. Foals have a higher total body water percentage and therefore a higher maintenance requirement usually 100 ml/kg/24 hr.
Continued losses can be roughly estimated or measured directly, such as volume of gastric reflux collected over a 24-hour period. Estimation of 1 to 4 L is not uncommon, or continued loss can be estimated as a multiplication of maintenance (1.5 to two times maintenance).
These volumes are added together to give an estimation of the volume of fluid to be administered to a patient over a 24-hour period. Usually the replacement volume is administered over 4 to 6 hours with the remainder over the next 18 to 24 hours. A typical fluid therapy plane is illustrated in Box 33-4 for a 500-kg horse. This example will be used for the remainder of this chapter.
BOX 33-4 A Typical Fluid Therapy Plan for a 500-kg Horse That is 8% Dehydrated
| 500-kg horse |
|
| Estimated dehydration: 8% |
|
| 1. Rehydration: |
|
| % estimated dehydration × weight in kg |
|
| 0.08 × 500 kg = 40 L |
40 L |
| 2. Maintenance: |
|
| Adult: 50 ml/kg/day |
|
| 50 ml/kg/24 hr × 500 kg = 25,000 ml = 25 L |
25 L/24 hr |
| 3. Continued loss: |
|
| Estimate volume: 2-5 L/hr |
|
| 2 L/hr × 12 hr = 24 L |
24 L |
| OR |
|
| Multiple of maintenance 1.5-3 × maintenance |
|
| 2 × 25 L = 50 L/24 hr = 25 L/12 hr |
___ |
| Total |
89 L |
Frequent reassessment of the patient's physical condition and monitoring of PCV, TP, BUN, creatinine, and electrolytes are necessary to adjust the volume and rate of fluid administration and any additives.
Rate of Administration
Although the rate of fluid delivery can be determined by simple calculation, the volume of fluid delivered to equine patients makes estimating an accurate rate difficult. Most horses seen in the hospital on an emergency basis are initially administered a “shock” dose of fluids equivalent to 60 to 90 ml/kg/hr. Fluid rate is usually calculated as drops per second. As can be seen in the example in Box 33-5, the number of calculated drops per second cannot be accurately measured and is essentially equal to a fluid rate that is a constant wide open stream. In these cases, the volume of fluid delivered should be estimated by the graduations marked on the fluid bag. The volume should be recorded in the medical record every 1 to 2 hours and the rate adjusted as needed to deliver the desired amount of fluid.
BOX 33-5 Calculation for Delivery Rate of Replacement Fluid
Initial Fluid Delivery Rate
Example 1 60 ml/hr (shock dose)
500 kg × 60 ml/kg/hr = 30,000 ml/hr (30 L/hr)
30,000 ml × 10 drops/ml = 300,000 drops/hr
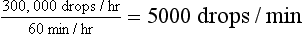
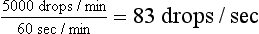
Example 2 Replacement volume of 40 L to be replaced in 6 hr
40 L/6 hr = 6.7 L/hr
6.7 L × 1000 ml/L = 6700 ml/hr
6700 ml/hr × 10 drops/ml = 67,000 drops/hr
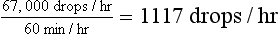
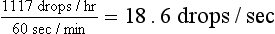
Once the initial fluid deficit has been replaced, the fluid rate should be slowed to meet the requirements for maintenance and continued loss. This rate can be calculated as in Box 33-6.
BOX 33-6 Fluid Rate for Maintenance and Continued Losses
Maintenance and Continued Loss Fluid Rate
49 L/24 hr = 2 L/hr
2 L × 1000 ml/L = 2000 ml
2000 ml/hr ×10 drops = 20,000 drops/hr
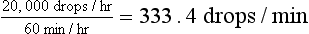
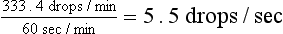
This can be counted as 55 drops/10 sec.
Also note that this approximates two times maintenance as described previously.
Hypertonic Saline Solution
Hypertonic saline solutions used in equine veterinary medicine consist of a 7.2% solution of sodium chloride usually packaged in 1-L bottles. Hypertonic saline has an almost immediate, though short-lived, effect on the circulating plasma by drawing fluid from the interstitial and intracellular space into the vasculature. Plasma volume expansion results in increased cardiac output, improved blood pressure, better oxygen delivery to and better oxygen use by the tissues. Hypertonic saline is administered at a dose of 4 ml/kg over 5 to 10 minutes. It rapidly expands circulating plasma volume; however, its effects last for approximately 60 minutes.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Hypertonic saline is administered at a dose of 4 ml/kg over 5 to 10 minutes.
Colloids
Hydroxyethyl starches, also known as synthetic colloids, are high-molecular-weight molecules that function similar to the natural colloid albumin in the circulatory system. When administered intravenously to horses suffering from dehydration and hypovolemic shock, they exert oncotic pressure that serves to maintain plasma volume and peripheral perfusion. The advantages of synthetic colloids over albumin include lower antigenicity, and because of their larger molecular weight, they tend to remain in the circulation for a longer period of time. In conditions where albumin is lost from the circulation as a result of increased capillary permeability, IV administration of albumin will only result in further redistribution of albumin to the interstitial space. Synthetic colloid will remain in the intravascular space longer, exerting its oncotic effect for up to 120 hours. Hetastarch is the most commonly used synthetic colloid in the United States. It is administered at a dose of 10 ml/kg of a 6% solution. Disadvantages of hetastarch include expense, the association of bleeding disorders with its use, and the inability to measure the oncotic effect of colloids by refractometer.
BLOOD AND BLOOD PRODUCTS
Blood loss and anemia in horses is caused by one of three mechanisms: whole blood loss, erythrocyte destruction, or the failure to produce erythrocytes. Whole blood loss is the most common cause for anemia in horses and presents most frequently as an emergency. Significant blood loss in horses is most commonly associated with wounds involving the neurovascular supply of the distal limb, middle uterine artery rupture in mares, guttural pouch mycosis, hemolytic anemias, and neonatal isoerythrolysis in foals.
On average, blood constitutes approximately 8% of the horse's body weight. This means that an average 500-kg horse has a blood volume of approximately 40 L. This estimate varies depending on the age, breed, and use of the horse and can range from 7% to 15%. The horse can lose approximately 20% of its total circulating blood volume without demonstrating clinical signs. When acute blood loss occurs, the immediate response by the body is to ensure the delivery of oxygen to the vital organs, such as the heart and brain. Hemorrhage of greater than one third of the circulating blood volume can result in irreversible shock and death. This is the result of inadequate oxygen delivery to the tissues. Therefore the goal of treatment of acute anemia or blood loss is to maintain appropriate oxygen delivery to the tissue.
 TECHNICIAN NOTE
TECHNICIAN NOTE
On average, blood constitutes approximately 8% of the horse's body weight that is equivalent to 40 L in a 500-kg horse.
Oxygen delivery to the tissue is affected by two primary mechanisms: (1) cardiac output and (2) oxygen content of the blood reaching the tissues. The oxygen content of the blood is dependent on hemoglobin concentration and is directly related to the RBC mass. Whole blood loss affects oxygen delivery not only by the loss of erythrocytes and hemoglobin, but also the loss of plasma. This decreased plasma volume causes a decrease in the amount of blood pumped by each contraction of the heart (stroke volume) and therefore the total amount of blood pumped by the heart to the tissues (cardiac output). The loss of RBCs and hemoglobin decreases the oxygen-carrying capacity of the blood.
In response to blood loss, the body has several compensatory mechanisms to maintain cardiac output and oxygen delivery. Immediate compensatory responses include peripheral vasoconstriction, splenic contraction (the spleen can maintain up to 30% of the circulating erythrocyte mass), and a shift of fluid from the interstitial space to the intravascular space.
The decision to perform a blood transfusion is often difficult and should not be taken lightly. Blood transfusion is an invasive procedure requiring the catheterization and collection of blood from the donor potentially creating a situation of acute blood loss and the administration of the collected blood to the recipient. This process is time and labor intensive and may add significant cost to the therapy. In addition, undesirable transfusion reactions that range in severity from mild urticaria to acute anaphylaxis and death have been reported in horses. Compensatory mechanisms and the loss of equivalent concentrations of fluid, cells, and protein with whole blood loss can make the decision to perform a blood transfusion based on hematologic values potentially inaccurate. Hematologic measurements, such as PCV and TP concentrations, may take up to 24 hours after the initial blood loss to change significantly. Therefore the decision to perform a blood transfusion should always be based on the nature of the illness or injury, a history of known large volume blood loss, clinical signs, and hematologic measurements (Box 33-7).
BOX 33-7 General Recommendations for Blood Transfusion
PCV <20% following acute hemorrhage
PCV <12%-14% following chronic hemorrhage
Clinical signs consistent with hypovolemia following acute hemorrhage
The clinical signs exhibited by the horse following acute hemorrhage are the result of a combination of hypovolemia and decreased erythrocyte mass. These signs are consistently observed in horses with anemia regardless of the underlying cause and can include tachycardia, tachypnea, lethargy, inappetence, depression, colic, sweating, cold extremities, and pale mucous membranes.
Clinicopathologic changes are dependent on the time between presentation and changes in signs because of the previously described compensatory mechanisms. In a recent review of 31 horses treated by blood transfusion, only 11 of 18 (61%) horses with acute blood loss had PCV or hemoglobin levels less than the normal reference range. When present, clinicopathologic abnormalities may include decreased PCV and TP, increased creatinine and BUN levels, and decreased oxygen saturation.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Clinical signs consistent with blood loss in horses regardless of the underlying cause can include tachycardia, tachypnea, lethargy, inappetence, depression, colic, sweating, cold extremities, and pale mucous membranes.
Blood Transfusion
Transfusion of whole blood to the anemic patient has been demonstrated to result in a significant improvement in PCV, creatinine, and oxygen saturation and a significant improvement in clinical signs. An organized well-thought-out plan must be established and personnel trained to proceed through the process in a systematic fashion. This will minimize the cost, materials used, and time needed to safely collect the necessary volume of blood from the donor and promptly transfuse the blood to the patient.
Blood Donors: A minimum of two blood donors should be available at all times. These may be horses housed at the practice or client-owned horses in which special arrangements have been made for their use as donors. Unlike humans that have three blood types (A, B, and O), horses have seven different blood types represented by capital letters A, C, D, P, K, Q, and U and up to 30 factors in each type designated by lower case letters. Also unlike humans, there is no universal equine blood donor. Identification of the most appropriate donor should be made well in advance of the need for transfusion. Blood can be collected from potential donors and sent to a number of veterinary laboratories for typing (Box 33-8).
BOX 33-8 Blood Typing Laboratories
Equine Blood Typing Research Laboratory
University of Kentucky
Department of Veterinary Science
Lexington, KY 40546
859-257-3022
Serology Laboratory
University of California
Davis, CA 95616
530-752-9284
Stormont Laboratory
1237 E. Beamer St. Suite D
Woodland, CA 95776
530-661-3078
Mann Equitest, Inc.
335 Laird Rd. Unit 4
Guelph, Ontario N1H 6J3
Canada
519-836-2400
The most desirable donors are geldings with no history of receiving a blood transfusion and Aa and Oa negative because these are the most immunogenic erythrocyte antigens. Donors should have a complete well-maintained health record and should receive regularly scheduled vaccinations, deworming, an annual enzyme immunoassay (EIA) (Coggins) test, and have routine dental and foot care performed.
Larger blood donors are desirable to allow a greater volume of blood to be collected. Although 450 kg is a minimum weight, horses greater than 550 kg are more desirable. Mares and horses with a history of previous transfusion have a greater risk for antierythrocyte antibodies (Box 33-9).
BOX 33-9 Desirable Blood Donor Characteristics
>450 kg
Gelding
Free of blood-borne diseases
In good health
No previous transfusion or pregnancy if a mare
Aa, Oa, and hemolysin negative
PCV >35 and TP >6.0
A PCV and TP should be performed on all donor horses before blood collection. Donors with PCV less than 35 or a TP of less than 6 should not be used. A healthy donor 500 kg in body weight with a PCV of 35% to 40% can safely donate up to 8 L (20% of blood volume) of blood every 30 days.
 TECHNICIAN NOTE
TECHNICIAN NOTE
At the time of blood collection, donor horses should have a PCV of at least 35 and a TP of at least 6.0 mg/dl.
Blood Collection: A blood collection kit containing all necessary supplies can be organized and stored for ready use. Technical staff should be adequately trained in the process of blood collection and the process rehearsed to ensure that it is carried out efficiently.
Blood can be collected into a number of containers, including sterilized glass jars, commercial blood collection bottles containing acid-citrate-dextrose anticoagulant (ACD bottle), specially designed bags for blood collection, and gas-sterilized fluid bags. The container determines the type of anticoagulant used. ACD bottles have the advantage of a vacuum that allows rapid collection of blood; however, the disadvantage when used for blood collection in horses is their size, damage to the erythrocytes from impact on the bottle, inactivation of platelets by the glass, and risk of breaking the glass (Figure 33-112). ACD bottles also require additional tubing for the collection process. Commercially available plastic blood bags for blood collection in horses have the advantage of preservation of platelet function, less erythrocyte damage, the ability to collect large volumes in a single container, and no risk of breakage (Figure 33-113). Their disadvantage is that collection is by gravity flow and can be time consuming.

FIGURE 33-112 ACD glass bottles for the collection of blood

FIGURE 33-113 Commercially available blood collection bags for horses
A crew composed of three individuals is recommended for safe, efficient blood collection. One person handles the horse, one manages the catheter, and one controls the blood collection device. The area over the donor's jugular vein is aseptically prepared and 2 to 5 ml of local anesthetic are locally infused over the proposed catheter or needle site. Blood can be collected safely through a larger needle (14-gauge × 1.5-inch), a specialized blood collection trochar (8- to 11-gauge × 75-mm), or IV catheter (14-gauge × 5.5-inch). Venous access should be directed in the opposite direction of normal blood flow to speed collection. The vein may need to be occluded continuously to distend the vein and speed collection. The blood must be collected into the chosen collection device under strict asepsis.
Standard ACD bottles contain enough ACD to collect 450 ml of blood. The amount of ACD in bags is dependent on the volume of the bag. When using a sterilized glass bottle, enough ACD should be added to the bottle to produce a ratio of 9 parts blood: 1 part ACD (2000 ml total volume = 200 ml ACD: 1800 ml whole blood). Although not commonly used as an anticoagulant for blood transfusions, in an emergency situation heparin can be added to sterile glass bottles for immediate collection and administration. When using heparin as an anticoagulant, 625 IU heparin is used per 50 ml of blood collected, and the blood should be administered immediately. The bottle or bag should be gently swirled or rocked during the collection to ensure proper mixing of the blood and anticoagulant. If collecting blood into bottles, once the bottle is full, the tubing should be clamped near the bottle and the needle removed from the stopper. To maintain strict asepsis, needles should be changed in between bottles before resuming collection. The tubing attached to bags should be stripped of residual blood, tied in three knots, or clamped and cut below the clamp. Following blood collection, donors from whom 20% of the blood volume has been removed should receive 5 to 20 L crystalloid fluids to prevent signs consistent with hypovolemia. They should also receive 1 to 3 lb of complete feed and free-choice water. The catheter site should be examined two times per day for signs of heat, pain, or swelling for the next 5 to 7 days.
Crossmatching: Crossmatching is an in vitro screening test used to assist in the detection of incompatibilities between donor and recipient blood. The incompatibilities are the result of antibodies present in the serum of the donor or recipient to the other's erythrocytes. Incompatibilities are recognized by clumping (agglutination) of erythrocytes following mixing of donor and recipient erythrocytes and plasma or hemolysis after the addition of rabbit complement to this mixture. The two components of this screening test are the major and minor crossmatch. The major is highly recommended by most authors and involves the mixing of recipient plasma or serum with donor erythrocytes. A minor crossmatch is considered optional in situations where the donor has no known exposure to the blood of other individuals and consists of mixing donor plasma or serum with recipient erythrocytes. Box 33-10 provides step-by-step instructions for performing major and minor crossmatching.
BOX 33-10 Crossmatch Procedure
Materials
1. EDTA anticoagulated blood and serum form recipient and donor
2. Pipette and tips accurate up to 0.05-0.1 ml
3. 37° C water bath or incubator
4. Microscope
Procedure
1. Into appropriately labeled donor and recipient tubes pipette 2-4 drops of whole blood from each donor and recipient and fill to within 1 cm of the top with normal saline.
2. Cover the tubes with parafilm (wax paper) film and mix thoroughly.
3. In a tabletop centrifuge, centrifuge for 1 min at maximum speed and decant the saline supernatant. There will be some residual saline in the bottom of the tube.
4. Gently strum the bottom of the tube to resuspend the pellet, fill again with saline, and repeat steps 2 and 3.
5. Gently resuspend the cells in the saline at the bottom of the tube and add approximately 4.7 ml of additional saline. The residual volume is approximately 0.3 ml. Addition of 4.7 ml will result in a solution of erythrocytes of approximately 6%.
6. Label 1 tube autocontrol. For each donor, label two tubes major/donor ID and minor/donor ID.
7. To each tube labeled major, add 0.1 ml recipient serum, and then add 0.05 ml of the 6% donor cell suspension to the corresponding major/donor ID tube.
8. To each tube labeled minor, add 0.1 ml of donor serum from the appropriate donor, then add 0.5 ml recipient 6% cell suspension.
9. To the tube labeled autocontrol, add 0.1 ml recipient serum and 0.05 ml recipient cell suspension.
10. Mix all tubes well and centrifuge at maximum speed for 30 seconds.
11. Gently shake the cells to slowly loosen them from the pellet and observe grossly for agglutination or hemolysis (red supernatant).
12. Centrifuge tube again 30 seconds at maximum speed and repeat step 11. Place a drop of suspension on a microscope slide and observe at 10× power for agglutination.
Although highly recommended, the limitations of equine crossmatching must be understood. False-positive and false-negative reactions are commonly observed as a result of spontaneous rouleaux formation, which must be distinguished from agglutination. Rouleau is described as the microscopic appearance of linear stacks of erythrocytes similar to stacks of coins. Agglutination is the irregular spherical clumping of erythrocytes observed under the microscope. Agglutination can be confirmed by mixing a small quantity of blood with normal saline. If present, agglutination will persist after mixing, and rouleaux will disperse. In addition, various amounts of agglutination are present in all crossmatch reactions, and it is difficult to determine at what level agglutination is considered incompatible. Therefore incompatibilities may only be recognized with large amounts of agglutination. Finally the most serious transfusion reactions occur as a result of erythrocyte hemolysis rather than agglutination. Lysis is only observed following the addition of complement, a procedure that is impractical in most practice settings and usually only performed in blood typing laboratories. Therefore crossmatching has its greatest value when reaction between donor and recipient blood is severe (large amounts of agglutination) indicating an obvious incompatibility. Transfusion reaction may still occur even after blood has been determined to be compatible by crossmatching. In most cases, naturally occurring antibodies to equine erythrocytes are uncommon, and a transfusion can be safely administered without crossmatching. Blood should initially be administered slowly and the recipient observed for clinical signs of a transfusion reaction and the transfusion slowed or discontinued if signs increase in severity or persist.
Administration: The recipient should have an IV catheter placed aseptically. Infusion should be performed only through a specialized blood delivery infusion set with an in-line filter (Figure 33-114). Some authors recommend replacing the infusion set after every 4 L of blood administered. Although seldom are crystalloid fluids and blood administered simultaneously, if this is practiced, normal saline (0.9% NaCl) is the only crystalloid fluid that can be safely administered with blood. Most replacement fluids, such as LRS, contain calcium, which can initiate blood clotting, and hypotonic fluids, such as dextrose, can cause hemolysis.
FIGURE 33-114 Blood delivery set with an in-line filter
The volume of blood to be transfused can be estimated by two methods:
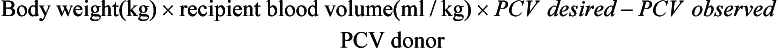
This calculation is based on a normal blood volume in the adult horse of 72 ml/kg and of 151 ml/kg in the neonate.
By this calculation, an average 500-kg adult with a PCV of 15 and a desired PCV of 25 would require 10.28 L.
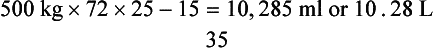
An alternative is simply to calculate 10 to 20 ml of blood/kg body weight
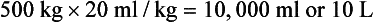
A recent retrospective study in horses demonstrated a 4% increase in PCV and significant improvement in clinical signs of horses with acute blood loss after the administration of 15 ml/kg whole blood.
The administration of blood should be closely monitored for signs of transfusion reactions. There are many published recommendations regarding the frequency of monitoring. The author has used a rate of 5 ml/kg/hr as an initial infusion rate with monitoring of the recipient's temperature, pulse, and respiration every 5 minutes for the first 15 minutes. If no signs of a transfusion reaction develop, the rate is increased to 10 to 25 ml/kg/hr while observing the patient continuously and monitoring and recording temperature, pulse, and respiration every 30 minutes. Signs of a transfusion reaction are variable and may include urticaria, dyspnea, tachypnea, tachycardia, fever, restlessness, muscle fasciculations, sudden recumbency, anaphylactic shock, and death. Depending on the severity of the signs, if observed, the transfusion should be slowed or discontinued.
Plasma Transfusion
Plasma is the cell-free portion of blood. Its constituents include colloids, such as albumin and other small protein; electrolytes; immunoglobulin (antibodies); antibacterial protein, such as complement; and clotting factors. The indications for plasma administration in horses are numerous and may include hypoproteinemia or hypoalbuminemia, failure of passive transfer, septicemia or endotoxemia, DIC, warfarin toxicity, and the treatment and prevention of specific disease for which antitoxic and passive immunization plasma have been developed. Although equine plasma readily separates from erythrocytes without the aid or expensive plasmapheresis equipment, collection of this plasma is both labor and time consuming and must be performed under the strictest of aseptic conditions. The collection of quantities large enough to treat most equine patients cannot be accomplished economically in most practice settings. Equine plasma is available commercially from several manufacturers worldwide (Box 33-11). These companies maintain herds of horses that are specifically for the production of various highly specific plasma products (Box 33-12) that can be shipped and stored frozen for extended periods of time.
BOX 33-11 Sources of Equine Plasma
BOX 33-12 Indications for Types of Equine Plasma
Normal
Replacement of albumin, complement clotting factors, and immunoglobulin in adults and foals
Hyper/Polyimmune
Failure of passive transfer in neonatal foals
E. coli J5/Salmonella
Treatment of septicemia and endotoxemia in foals and adults
Rhodococcus equi
Passive immunity and prophylaxis before exposure to R. equi
Clostridium botulinum Type B Antitoxin
Prophylaxis following exposure to C. botulinum by ingestion and antitoxin in clinically affected patients
West Nile Virus
Passive immunity and treatment of clinical cases
Streptococcus equi
Passive immunity and treatment of clinical cases
Plasma requires thawing before administration. The manufacturer's directions for storage, thawing, and administration of specific plasma products should be consulted before administration. Routinely, plasma is stored at −18° C (the same temperature as a standard refrigerator freezer) and thawed immediately before administration. The most convenient method for thawing is to place the bag in water that is approximately 40° C (approximately 104° F). The plasma should be mixed periodically and cool water replaced until approximately body temperature. Thawing in water that is too hot should be avoided because this can result in the destruction of plasma protein and immunoglobulin. Plasma that is thawed to room temperature or above should not be refrozen for later use. Administration should always be performed through a filtered blood delivery set.
 TECHNICIAN NOTE
TECHNICIAN NOTE
Commercially available FP should be thawed by placing the bag in water that is approximately 104° F.
The volume of plasma to be administered is dependent on the manufacture's recommendation for treatment and prevention of the specific condition for which the plasma is administered or the calculated need based on hypoproteinemia. Plasma transfusion is indicated in horses in which the plasma TP is less than 4 g/L or albumin concentrations are less than 2 g/L. In horses that are hypoproteinemic, the volume of plasma to be administered can be estimated by the following formula:
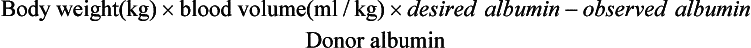
This calculation is based on an estimated plasma volume in the adult horse of 48 ml/kg, 95 ml/kg in the neonate, 62 ml/kg in foals from 1 to 4 weeks of age, and 53 ml/kg in foals from 4 to 12 weeks of age.
Following plasma transfusion, the observed increase in plasma albumin concentration is less than usually expected as a result of redistribution of the albumin to the extravascular space. The volume necessary to significantly increase the TP and albumin concentrations of the blood are large. A minimum of 6 L of normal plasma is required to increase the recipient's albumin concentration 1 to 2 g/L. Although the concentrations do not change appreciably, a dramatic clinical improvement is often observed.
Plasma administration is similar to whole blood and should be performed through a blood delivery set with an in-line filter, and the patient should be carefully observed for signs of transfusion reaction and the transfusion slowed or discontinued if signs are observed.
Blood Substitutes
Oxyglobin (Biopure Corp., Cambridge, Mass.) is an ultrapurified, polymerized bovine hemoglobin in a modified LRS solution that contains13.8 g/dl of hemoglobin. Oxyglobin has been approved for use in dogs and has been used extralabel in foals with neonatal isoerythrolysis as a hemoglobin replacement. This solution increases the oxygen-carrying capacity in anemic patients. Oxyglobin has been used in horses at a dose range between 10 to 30 ml/kg delivered at a rate of 10 to 20 ml/kg/hr. The large hemoglobin molecule also acts as a colloid solution. It is a cell-free hemoglobin solution that lacks erythrocytes and erythrocyte antigens. Therefore crossmatching is not necessary before administration. Other advantages of oxyglobin include a long shelf life (36 months) and no preadministration preparation (off-the-shelf administration) and no need for specialized administration sets.
In limited use in horses, oxyglobin has been used in the treatment of a miniature horse with chronic ovarian bleeding and in foals with NI as a means of controlling clinical signs while blood is collected and processed. Disadvantages of oxyglobin include too high of cost to be used in adult horses, a short plasma half-life thus requiring additional blood products be administered, and evidence that suggests oxyglobin may decrease cardiac output.
CASE PRESENTATION 33-2
History and Signalment
A 12-year-old quarter horse gelding came to the hospital with a 2-day history of moderate to severe abdominal pain. A presumptive diagnosis of a small colon impaction was made by the referring veterinarian, and he was treated with IV fluids and flunixin meglumine for pain. There has been no response to therapy.
Initial Physical Examination
On presentation, the horse was mildly depressed and exhibited intermittent episodes of mild to moderate abdominal pain.
His temperature was 99, pulse 48, and respiration of 16 breaths per minute.
Mucous membranes were pale pink and tacky, and his CRT was 3 seconds. The skin over the eyelids remained persistently tented for 5 to 6 seconds.
He was determined to be moderately (7% to 8%) dehydrated based on physical examination.
This was confirmed by the results of a CBC and chemistry profile that revealed an elevated PCV (42) and TP (7) and what was suspected to be a prerenal azotemia (creatinine: 2.5, normal: 1.4 to 1.7).
His weight was estimated to be approximately 1000 lb (450 kg).
There was no gastric reflux, and a rectal examination revealed an extensive small colon impaction.
Initial Fluid Therapy
1. Replacement Fluids
(450 kg) (0.08) = 36 L to be administered over 4 hours
36 L/4 hr = 9 L/hr
9 L = 9000 ml
9000 ml/60 min/hr = 150 ml/min
150 ml/min/60 sec/min = 2.5 ml/sec
2.5 ml/sec × 10 drops/ml = 25 drops/sec, too fast to count; therefore the rate can only be monitored using the graduations on the fluid bag and adjusting the rate as needed.
2. Maintenance and continued loss
(450 kg) (50 ml/kg/24 hr) = 22,500 ml or 22.5 L/24 hr
22.5 L/24 hr = 937 ml/hr
937 ml/hr/60 min/hr = 15.6 ml/min
15.6 ml × 10 drops/ml = 156 drops/min
156 drops/min/60 sec/min = 2.5 drops/sec or 25 drops/10 sec
Continued loss was considered small and estimated to be 1.5 times maintenance.
15.6 ml/min × 1.5 = 23.4 ml/min
23.4 ml/min × 10 drops/ml = 234 drops/min
234 drops/min/60 sec/min = 3.9 or roughly 4 drops/sec
3. Continued therapy
As time went on, the horse experienced more pain. Approximately 10 L of gastric reflux was collected, he began to exhibit abdominal distention, and his PCV increased to 48.
The abnormal rectal examination, gastric reflux, and systemic deterioration were indications for abdominal exploratory surgery.
The horse was anesthetized with a ventral midline celiotomy, and abdominal exploratory was performed and the small colon impaction relieved.
4. Anesthetic recovery
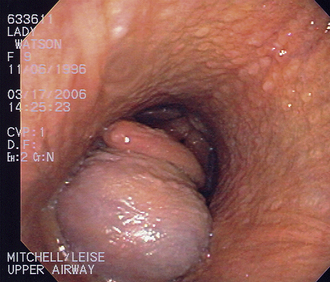
The horse stood unassisted approximately 45 minutes after removal from gas anesthesia. Within 5 minutes of standing, he began to exhibit signs of discomfort initially thought to be abdominal pain. These signs progressed to flaring of the nostrils, exaggerated inspiratory efforts, inspiratory stridor, and severe anxiety.
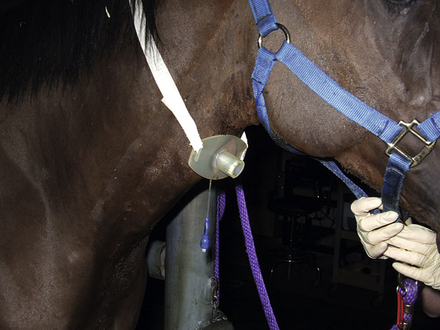
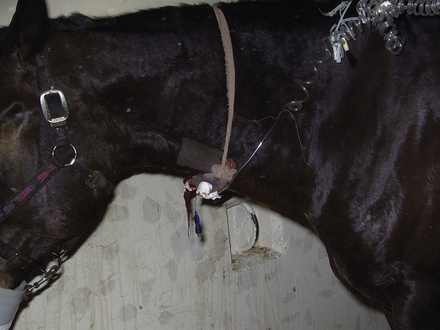
A standing emergency tracheotomy was performed, and a temporary tracheotomy tube was positioned and fixed in place with gauze. Oxygen insufflation through the tracheotomy tube was initiated at 15 L/min, and the respiratory signs resolved within minutes. Oxygen insufflation was discontinued after 1 hour. The presumptive diagnosis was acute upper respiratory obstruction resulting from postanesthetic laryngeal spasm.
5. Postoperative care
The horse remained on IV fluids for 48 hours following surgery. Food was introduced at 24 hours and gradually increased to free-choice hay over 5 days. The tracheotomy tube was intermittently occluded to determine if the laryngeal spasm persisted. The horse was able to breathe normally after 5 days. The tube was removed, and the horse was discharged 7 days after surgery.
 TECHNICIAN NOTE
TECHNICIAN NOTE