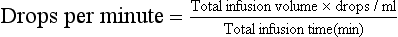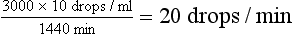
Small Animal Medical Nursing
When you have completed this chapter, you will be able to:
1 Describe general care of small animal patients, including bathing, grooming, ear cleaning, and nail trimming procedures.
2 Explain the special considerations in the care of recumbent, geriatric, and pediatric patients.
3 Describe the procedures for obtaining body temperature, blood pressure, pulse rate character, and respiratory rate.
4 Differentiate between sensible and insensible fluid losses and explain methods used to determine patient hydration status and calculate fluid requirements for rehydration of patients.
5 List routes of administration of fluid therapy treatments and describe monitoring procedures used for fluid therapy patients.
6 Describe the indications for and procedures used in blood transfusion and oxygen therapies.
7 List the canine and feline blood groups and describe procedures for blood typing and cross-matching.
8 List and describe the five methods of physical therapy used in small animal practice.
9 Describe the indications for and procedures used in respiratory and topical therapies in small animal practice.
10 List and describe common diseases of dogs and cats and provide an overview of small animal vaccines and vaccination protocols.
11 Define zoonosis and identify common zoonotic conditions and methods of control of zoonotic diseases.
12 List common diseases of the eyes and describe methods of diagnosis and treatment.
13 List and describe common cardiac and endocrine disorders of dogs and cats and describe methods of diagnosis and treatment.
14 List and describe common urogenital and gastrointestinal disorders of dogs and cats and describe methods of diagnosis and treatment.
15 List and describe common orthopedic disorders encountered in small animal practice.
Data interpretation by the veterinary technician consists of recognizing and correctly interpreting the observations that have been made. Stated differently, the technician must recognize and define the clinical problems. A clinical problem is anything that interferes with the well-being of the patient or anything that requires treatment or further diagnostic evaluation. Examples of clinical problems that might be recognized by the technician include diarrhea, vomiting, anorexia, and respiratory distress.
Once a problem is documented, a diagnostic or therapeutic plan is made. This may consist of repeating a clinical parameter such as a blood pressure reading or the patient's temperature before a more extensive plan is made. Once a clinical problem is positively identified, the attending veterinarian is consulted, and a diagnostic or therapeutic plan is implemented. For nursing to be optimally effective, a mechanism should exist for the ready exchange of information between technician and veterinarian. A team approach to animal health care is the ultimate goal, with veterinarian and technician each contributing their unique skills and abilities to the task of returning the patient to health. After a change of plan or treatment, continued observation of the patient is needed. The new plan or treatment may need to be altered again because of a changing clinical situation.
When implementing any diagnostic or therapeutic plan, it is important to remember that the quantity and nature of nursing care should always be individualized. One patient may readily accept a specific procedure, whereas another will resist to the point that the intended benefit is lost. Although excessive intervention may be detrimental to certain animals, this should not be construed as an excuse for medical neglect. The fundamental principle is that if a patient is not meeting a requirement for survival, the technician must promptly intervene. Certain animals require tremendous amounts of attention and affection from the technician simply to maintain the will to live during periods of separation from the owner.
Each technician and the head of every animal hospital should establish and maintain consistent standards of nursing care. Veterinary technicians have a professional and moral obligation to every patient to provide the following basic necessities:
• Clean, comfortable environment, as free of stress as possible
• Fresh food and clean water at all times unless restricted for medical reasons
• Adequate exercise and grooming care unless restricted for medical reasons
• Prompt and humane relief of suffering and pain
• Humane treatment of every patient with dignity at all times
GENERAL CARE
Grooming and bathing are aspects of the general care of the animal patient that are important for several reasons. First, a clean and well-groomed animal has an enhanced sense of well-being and potentially will recover from an illness more rapidly. Second, a clean animal is much less likely to develop severe contact dermatitis from urine scalding and fecal soiling of the skin, which, if it does occur, becomes another clinical problem to manage. Third, grooming and medicated baths are recommended for the prevention or treatment of many dermatologic problems. Bathing with shampoo that contains an insecticide is a useful adjunct in the control of ectoparasites. Finally, the cleanliness of the patient at the time of discharge is an indication to the owner of the overall quality of the health care provided.
Every animal hospital should have an adequate collection of grooming and bathing equipment and supplies (i.e., combs, brushes, scissors, towels for drying, electrical dryers, and a selection of shampoos appropriate for different situations). Care must be taken to prevent the spread of infections, such as dermatomycosis, from one animal to another via grooming instruments. These instruments should be thoroughly cleansed in an appropriate disinfectant solution after each use.
When clipping or removing hair from an animal for medical reasons, it is important to obtain the owner's permission, whenever possible. This is particularly important in animals used for show purposes. In certain breeds, such as the Afghan hound, regrowth of hair is extremely slow.
BATHING
The basic technique for bathing dogs and cats is to thoroughly wet the coat and then apply small amounts of shampoo starting at the head and working back to the tail. Rubbing the shampoo into the coat until a lather is produced, again starting from the head and working back to the tail is a generally accepted bathing method. The eyes should be protected from chemical injury by instilling a drop of mineral oil or a small amount of boric acid ophthalmic ointment in each eye before the bath. Care should be taken to prevent water from entering the external ear canal; this can be accomplished by placing a small piece of cotton in each ear. Remember to remove the cotton when the bath has been completed. Thermal injury from excessively hot water can be prevented by constantly monitoring the water temperature. Thorough rinsing with clean water prevents irritation of the skin from residual shampoo. The axillary and scrotal regions of long-haired dogs are particularly vulnerable to residual shampoo irritation. If a cage dryer is used, caution must be exercised to prevent overheating (hyperthermia). Shampoos containing insecticides should be used only with the approval of the attending veterinarian because of the possibility of cumulative toxicity or drug interactions with medications or other topically applied insecticides. If insecticidal dips are used, correct dilutions are necessary to prevent toxic reactions. If a complete immersion bath is contraindicated, localized soiling of the animal may be handled with a sponge bath. Orthopedic or neurologic patients may not be able to stand steady in the bath tub, and therefore a rubber mat should be placed in the tub to help reduce the risk of injury.
EXERCISE
Moderate exercise is beneficial for the general care of the animal patient. Exercise should take place in a secure, controlled, and safe environment so that injury or loss of the animal does not occur. Contraindications to exercise include many, but not all, respiratory, cardiovascular, and musculoskeletal problems. The decision whether to restrict exercise should be made after consultation with the attending veterinarian. Moderate exercise consists of taking the patient for a walk and can be considered the simplest and most basic form of physical therapy. It can be a useful means of reducing peripheral edema and improving muscle tone and strength.
FEEDING
The animal health technician plays a particularly pivotal role in ensuring that each patient remains in a positive energy balance, in which caloric intake exceeds metabolic requirements. The technician is in an excellent position to observe complete or partial anorexia and to take appropriate action to correct the situation. As long as the patient is not vomiting or the suppressed appetite is not due to a gastrointestinal problem, such as an ulcer or pancreatitis, there are a few things that should be tried to encourage the patient to eat. Substituting a more palatable food or texture such as canned food may solve the problem. Familiarity with the home feeding regimen will aid in the selection of palatable alternative diets. In certain instances, it may be advisable for the owner to prepare food at home and bring it to the hospital, such as chicken or hamburger and rice. It is helpful to stock a variety of types of food, such as canned, semimoist, and dry, in a variety of flavors to satisfy even the most discriminating patient. Personal attention at feeding time, such as talking to the patient and hand feeding the patient may work in some animals. Cats particularly may have an aversion to eating because they have lost the taste of food because of prolonged anorexia. Putting a small amount of canned food on the tongue or letting them lick it off the finger usually stimulates taste and interest in eating again. Force feeding, although not highly recommended, can be done in selected cases. This is done by mixing canned food with water for a slurry consistency and then administering the food with a syringe applied to the back of the animals mouth to stimulate the swallowing reflex. Care must be taken to avoid giving too much food at one time and to be sure that the animal is swallowing after each food bolus to prevent the patient from choking and/or aspirating food into the lungs causing life-threatening aspiration pneumonia. High-calorie density supplements, such as Nutrical (Evsco), may help to meet the caloric needs of the patient but by no means will meet the animal's daily requirement by themselves. Gastric gavage (stomach tubing) can be done in patients requiring force feedings for an extended period of time because it is less stressful to the patient and the technician. (The technique is discussed in Chapter 20.) More commonly, other methods of enteral feeding are being used with increasing frequency and include placement of nasoesophageal, esophagostomy, gastrostomy, and jejunostomy tubes. Specially tailored complete diets may be administered through these enteral tubes. All but the nasoesophageal tubes have large enough diameters to allow the use of commercially prepared prescription canned diets blended with water and strained to be easily administered through the tube. Only liquids (CliniCare diet, Abbott Laboratories) can pass through the nasoesophageal tube. Being able to use these complete diets ensures adequate nutritional requirements are being met in a variety of disease states, such as hepatic lipidosis in cats, and renal failure. Total or partial parenteral nutrition (TPN/PPN) may also be chosen and consists of administering a sterile liquid that contains a complete or partial nutritionally balanced diet and is given intravenously through a fresh and aseptically placed jugular catheter. Aseptic technique is needed for every feeding. This feeding option is very labor intensive and introduces the risk of sepsis to the patient if not administered properly. It is usually chosen for the most critically ill patients when other feeding options are not possible. Giving appetite stimulants to cats (does not work in the dog) is usually done when trying to get a cat to eat that has been off feed for quite a while and has no current illness, such as GI disease or nausea, from a metabolic disease to prevent them from eating. These drugs will increase interest in eating, but will not ensure adequate calorie intake by the patient.
NAIL TRIMMING
Nail trimming (pedicure) is an important general care technique. Excessive nail length results in altered gait and the potential accentuation of lameness problems. Excessively long nails are more likely to split or to be traumatically avulsed. Finally, untrimmed nails can become ingrown (usually into the footpads), resulting in cellulites or abscess formation.
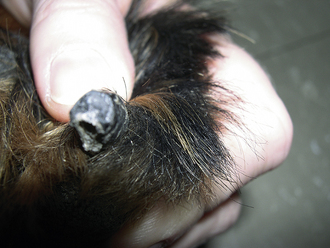
There are two common types of nail trimmers available (Whites and Resco; Figure 21-1). To avoid cutting pigmented (black) nails too short in the dog, the cutting surface of the nail trimmer should be held parallel to the palmar or plantar surface of the digital footpads, and the nail cut in this plane. In cats, the nails can be exposed by grasping the paw between the thumb and index finger and sliding the skin on the dorsum of the paw away from the nails (Figure 21-2). Once exposed, the nails can be trimmed as described for the dog. It should be noted that nails that have not been trimmed regularly have a “quick” or nail vein that extends further out into the claw than that of regularly trimmed nails. In this situation, one should be conservative with regard to how much nail is trimmed. The center of the nail takes on a fleshy, shining appearance in the region next to the quick (Figure 21-3). This is an indicator to trim no further. Because some animals vehemently resent handling of their feet for nail trimming, it is a good practice to routinely give a pedicure to any animal anesthetized or tranquilized for any procedure. If the blood vessel in the nail is inadvertently severed (the “quick” is cut), silver nitrate sticks can be used to stop the hemorrhage by means of chemical cautery. Other products available for chemical cautery include styptic powder and blood-stop powder, which are available from numerous companies. Owners can be instructed on the proper technique of nail trimming so that this routine task can be performed at home.
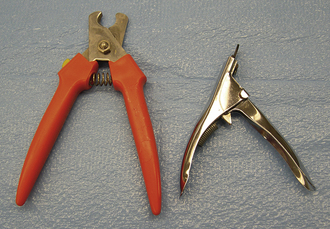
FIGURE 21-1 The two common types of nail trimmers, Whites (left) and Resco (right). The Whites nail trimmer is useful for very long nails that have curled back toward the footpad.
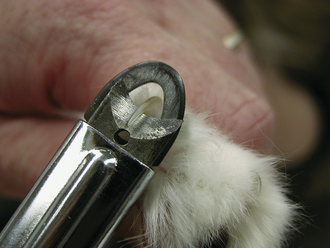
FIGURE 21-2 To trim the nails in cats, extend the claw by compressing the caudal part of the nail just in front of the footpad with the thumb and forefinger. At this point, one can visualize the vein or “quick” (pink area in claw), and the nail trimmer can be placed in front of the vein for trimming.
EAR CLEANING
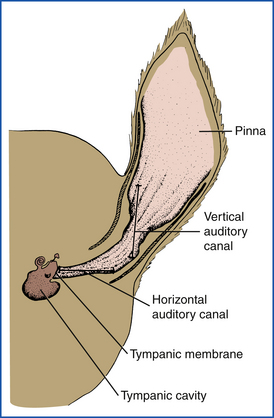
The external ear canal may accumulate cerumen, exudate, or cellular debris as a sequela to otitis externa or a foreign body (e.g., grass awn), which then requires cleaning. Certain breeds, notably poodles and terriers, may also accumulate excessive hair in the external ear canal. The initial and essential step in the treatment of any external ear problem is complete and thorough cleaning of the entire ear canal (Figure 21-4). Frequently, it is necessary to administer a short-acting general anesthetic or tranquilizer to properly clean the ears of patients that have painful ears and for patients that strongly resist ear cleanings. In some patients, it may be necessary to remove hair from the ear canals, whereas in others it may be left alone. This will depend on the severity of the ear infection, with the more severe inflamed ears responding to hair removal. A hemostat can be used to pull the hairs out, grabbing a small amount at a time. Excessive wax can be removed more easily if a ceruminolytic agent (i.e., dioctyl sodium succinate [Cerusol, Burns-Biotech labs]) is instilled first to soften the wax. Caution should be used before instilling ceruminolytics and certain ear cleaners that contain chlorhexidine when the integrity of the tympanic membrane is not known. Normal saline can be used for the initial cleaning agent until a proper ear canal examination can be performed to assess the tympanum. Using a soft rubber bulb syringe or a Luer-tipped catheter, excessive wax and debris can then be removed from the ear canals by gentle lavage with the chosen cleaning solution. Some practitioners advocate the use of pulsating streams of water from a dental hygiene apparatus (Water Pik, Teledyne Inc.) to clean the external ear canal. Approximately 5 ml of povidone-iodine (Betadine, Purdue-Frederick) or chlorhexidine solution (Nolvasan, Fort Dodge Laboratories) is added to approximately 236 to 384 ml of warm water. The stream of water should be applied in a rotating motion and directed parallel to the external ear canal. The excess water and debris can be collected in an ear irrigation basin or similar vessel. Avoid use of this method if the tympanum is not intact. Regardless of the irrigation system used, balls of cotton and cotton applicator sticks can then be used to gently wipe the wax from the external ear canal. It is important to remove only debris that is visible in the vertical canal so that debris is not pushed deep into the horizontal canal (Figure 21-4). Cleaning of the horizontal ear canal should be done only in the well-restrained patient and with caution to prevent damage to the tympanic membrane or the packing of debris deep into the horizontal canal (see Figure 21-4). A second otoscopic examination should be performed after the ear canals are cleaned to evaluate the completeness of the ear cleaning. Once the ear canal is sufficiently clean, the canal should be carefully dried with clean cotton swabs, and the initial dose of prescribed otic preparation instilled into each canal.
Before cleaning, some of the debris should be mixed in mineral oil and smeared on a microscope slide to be examined under low-power magnification for the presence of Otodectes (ear mites). A small amount of the debris should also be smeared on a dry microscope slide and stained with Diff-Quik solution for examination under high-power magnification for overgrowth of bacteria and yeast. If the ear canal contains purulent debris, a sample should be obtained for cytologic evaluation (smear), bacterial culture, and antibiotic sensitivity testing (sterile Culturette) before inserting instruments or cleaning solutions. Based on the cytology (yeast, bacteria) and mineral oil slides (mites), appropriate therapy can be initiated and then adjusted if necessary based on bacterial culture and sensitivity results when available in a few days.
ANAL SACS
The anal sacs are reservoirs for the secretions produced by the anal glands. The anal glands line the walls of the anal sacs and produce a foul-smelling fluid that varies from serous to pasty in consistency and is brown to off-white. The anal sacs are paired structures, approximately 1 cm in diameter, that lie between the internal and external anal sphincter muscles on either side of the anal canal. Each sac opens into the lateral margin of the anus by a single duct, at approximately the 4 and 8 o’clock positions of the anus (Figure 21-5).
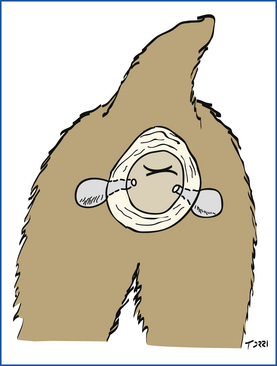
FIGURE 21-5 Schematic diagram of the anatomy of the canine anal sacs located approximately at the 4 o’clock and 8 o’clock positions.
Clinical signs associated with impacted anal sacs include excessive licking of the perineum; “scooting” or dragging the perineum on the floor; abnormal carriage of the tail; and vague indications of pain or discomfort in the perineal region.
The anal sacs are best emptied, or “expressed,” using the internal technique of inserting a lubricated, gloved forefinger into the rectum. The distended sacs are immobilized between the forefinger and thumb, which remains external to the anus. The sacs are generally found in a ventrolateral location. Gentle pressure is applied until the secretions are forced through the ducts. Because the ducts and the sac are occasionally compressed with this technique, if the sac cannot be expressed with gentle pressure, the finger and thumb are repositioned and pressure is reapplied. Paper towels, gauze, or cotton balls can be placed over the anus to collect the extremely unpleasant liquid from soiling the patient, environment, and the technician. External expression of the anal sacs is a technique that requires squeezing of the anal glands from the external anal sphincter. This technique is not recommended because of the frequent occluding of the ducts, inability to completely empty the sacs, and excessive pain it may cause the patient.
BEDDING
The optimal means of keeping an ambulatory dog clean is by the appropriate use of bedding and exercise runs. Several types of bedding are routinely used in small animal practice; they include newspaper, other types of paper products, blankets, towels, and lamb's wool products. It is important that the bedding material selected be either disposable or readily and effectively cleaned between uses. Because occasionally dogs will ingest their bedding, it is also important that the material be safe and nontoxic. Most dogs are extremely reluctant to urinate or defecate in their cage; therefore keeping the cage and patient clean is facilitated by the regular use of walks outside or use of an exercise run to allow them to urinate and defecate. Specifically, dogs should be walked or placed in the runs several times daily for an adequate period of time.
Generally, cats are easier to keep clean than dogs during periods of hospitalization. Cats will use litter pans and groom and clean themselves unless they are seriously ill. Litter should be changed daily, and pans or trays should be either disposable or constructed of materials that will allow thorough cleaning and disinfection between uses. To prevent litter from getting into open wounds or surgical incisions, newspaper shredded into long strips can be used in the litter pan in place of gravel litter. It is unnecessary to walk or place cats in exercise runs unless the hospital stay is unusually long.
DECUBITAL SORES
Prevention and management of decubital sores (bedsores) and urine scald are extremely important aspects of the care of recumbent patients. Animals with various neurologic or orthopedic problems can be recumbent for prolonged periods and require special care. Urine and fecal soiling can cause serious problems that can complicate recovery from the underlying condition. Scalding from urine or diarrhea can be prevented by a light topical application of a protective compound, such as Aquaphor (Beiersdorf, Inc.) or petrolatum (e.g., Vaseline) to susceptible perineal or inguinal areas.
Decubital sores not only complicate recovery, but can also be a source of sepsis, which can lead to the demise of the patient. The best treatment for decubital sores is prevention. Decubital sores develop over bony prominences as the result of continuous pressure and damage to the overlying skin. Various types of bedding have been advocated to reduce the frequency and severity of decubital sores; they include the use of air or water mattresses, foam padding, synthetic fleece, grids or grates, and straw. The material should either be disposable or have an impermeable surface that does not retain moisture or microorganisms and can be thoroughly cleaned. A potential problem with impermeable surfaces is that urine and moisture tend to remain in contact with the skin and can exacerbate the problem. Therefore care should be taken to keep the skin surface as dry as possible. This is why, for long-term management, straw is beneficial since adequate cushioning is available for the animal and urine drains through the straw and away from the patient.
Other routine measures that help to prevent decubital sores include frequent turning of the patient from side to side, intermittent use of slings or carts to prevent continuous pressure over the bony prominences, and frequent baths to keep the skin clean.
Once decubital sores have developed, they should be thoroughly cleaned with a surgical scrub. Surgical débridement of necrotic tissue may be necessary. After cleaning, the area should be completely dried. Soaking the affected area two to four times daily with a mild astringent will aid in keeping the decubital sore dry. A 1:40 astringent solution of aluminum acetate (Burow solution) may be made by dissolving one packet (Domeboro solution, Dome Laboratories) per pint of warm water. Ideally, the area of the decubital sore should be padded to prevent further pressure injury; however, the sore itself should remain exposed to the air to prevent retention of moisture. One way of accomplishing this is to fashion a “doughnut” from foam rubber and to fix this to the skin by means of adhesive tape. Unfortunately, it is difficult to maintain these pads in the proper location for long periods of time. Topical antimicrobial agents should be applied judiciously because many contain ointment or cream bases that form an occlusive dressing that will retain moisture. Further, it is questionable how beneficial they are in controlling an infected decubital sore.
GERIATRIC NURSING
With improved veterinary care, pets are enjoying an increased life span; consequently, the number of geriatric patients seen in small animal practices is increasing. The geriatric patient can be presented with a number of problems that directly influence the nursing process. These problems are generally related to or are secondary to degenerative diseases and other geriatric changes, such as arthritis, deafness, and blindness (see Chapter 37).
Dogs with arthritis or other degenerative diseases of the musculoskeletal system may be suffering from chronic pain. These animals are likely to react aggressively when an affected body part is touched or manipulated. Gentle handling when lifting or moving these patients and taking care to walk them at a slower pace than younger dogs is needed to prevent pain and fear in these patients. Dogs suffering from central nervous system disorders (e.g., a brain tumor or cerebral infarction) may also display aggressive behavior.
Deafness is another disorder that frequently accompanies old age. It is easy to surprise or startle a deaf, older dog, and certain dogs will instinctively respond by biting. When approaching a deaf dog, it is important that the patient is able to see you before you attempt to handle it or perform a procedure.
Blindness can occur in older dogs from cataracts, retinal degeneration, glaucoma, and other diseases. As is the case with deaf dogs, blind dogs should be approached cautiously. It is best to move slowly and speak while approaching the dog. Generally, elderly dogs and cats show less response to external stimuli. They appear to be less interested in their surroundings and frequently remain inactive for prolonged periods. In fact, they tend to resent any interference and react aggressively when disturbed. Some dogs forget previous training and may fail to respond to basic commands. Finally, the geriatric dog or cat is resistant to changes in daily routine. The stress of hospitalization alone can sometimes cause rapid deterioration. Obviously, it is impossible to correct or reverse many of the changes associated with aging; however, a willingness to provide gentle, compassionate nursing care is of paramount importance.
PEDIATRIC NURSING
The clinical situation that best illustrates the skills required in pediatric nursing is the hand rearing of orphaned puppies or kittens (see Chapter 10). The first step is to determine the caloric requirements of the puppy or kitten. During the first week of life, these requirements are approximately 27 cal/kg/day, 32 to 36 cal/kg/day during the second week, 36 to 41 cal/kg/day during the third week, and 41 to 45 cal/kg/day during the fourth week. A number of artificial milk replacers (Esbilac, Pet-Ag; Just Burn, Farnham) are available for use in puppies. KMR (Pet-Ag) is an artificial replacement for queen's milk. (See Table 21-1 for formula dosage.) The following formula can be used as a short-term emergency supplement in puppies: 8 oz of cow's milk mixed with two egg yolks and 1 tsp of corn oil. For an emergency formula in kittens, 4 oz of cow's milk can be mixed with two egg yolks and one drop of multivitamins. Once the total daily requirement has been calculated, this amount can be divided into four equal feedings. Frequent feedings are necessary to prevent overdistention of the stomach and subsequent emesis and aspiration pneumonia. Generally, it is faster and easier to use gavage via an orogastric tube than to bottle feed.
TABLE 21-1
Orphan Formula Dosage for Puppies and Kittens
| Age (wk) | Dosage∗ (ml/100 g Body wt/Day) |
| 1 | 13 |
| 2 | 17 |
| 3† | 20 |
| 4 | 22 |
The technique for gavage is to use a soft rubber feeding tube (Fr 8 to 16). The tube is marked with a marking pen or tape at a point equal to the distance between the tip of the nose and the eighth rib. The tube is advanced into the pharynx and down the esophagus to the level of the midthorax. A syringe can be used to inject the artificial milk replacer slowly. The stomach capacity of puppies and kittens can be calculated by using the following formula: body weight in grams times 5% equals the capacity of the stomach in milliliters. This milliliter amount should not be in a single feeding.
If the puppies or kittens are vigorous nursers, an alternative technique would be to use Pet Nursettes (Peg-Ag) or human premature baby bottle nipples. This technique is slower but may satisfy the pups and kittens more, so the incidence of litter mates nursing on each other will be reduced.
The neonatal puppy is essentially poikilothermic (body temperature varies with ambient temperature); therefore it is imperative that the ambient temperature of the whelping box be maintained between 30° C and 33° C. If hypothermia occurs, it will reduce feeding by the neonate and may enhance the pathogenicity of certain viruses, such as canine herpes. To detect hypothermia in neonates, it is desirable to use a low-reading clinical rectal thermometer.
A highly effective monitoring technique during the neonatal period is to weigh the neonates frequently. Newborn puppies and kittens should be weighed daily. Puppies should gain approximately 10% to 20% of their birth weight daily for the first week of life. Postage or food scales should be used to weigh each animal two or three times daily, especially during the first 2 weeks of life. Weight loss or failure to gain weight each day may be the first sign of illness. Puppies and kittens less than 2 weeks of age often do not defecate or urinate on their own. The mother stimulates these functions by gently licking the genitals and anus. This can be simulated by using a warm, wet cloth to gently wipe the genital and anal area a few times daily.
PRACTICAL NURSING PROCEDURES
In many veterinary practices, it is the responsibility of the veterinary technician to monitor the patient's vital signs (i.e., temperature, pulse, respirations).
TEMPERATURE
One routine method for determining the body temperature of a small animal is to use a standard mercury-in-glass clinical rectal thermometer. Veterinary thermometers differ from those used in humans in that the storage reservoir for the mercury is short and spherical rather than elongated. Human thermometers can be used in dogs and cats without difficulty. Thermometers can be calibrated in Fahrenheit or Celsius degrees. A Fahrenheit reading can be converted to Celsius by using the following formula: degrees C = (degrees F − 32) × 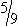 .
.
When taking the patient's temperature, one should first shake the thermometer so that the mercury is below the constriction in the glass tube. The thermometer is well lubricated with petrolatum, mineral oil, or a mild soap and inserted into the rectum with a gentle twisting motion. The thermometer is advanced into the rectum beyond the bulb and is held in place for the minimum period of time stated on the thermometer. The patient is restrained to prevent the thermometer from being broken. The thermometer is withdrawn, and the bulb and stem are wiped clean with an alcohol-soaked cotton swab. The thermometer is held horizontally and rotated until the magnified scale is clearly visible. Because of the constriction in the glass tube, the level of the mercury does not fall until it is shaken down. Finally, the thermometer should be stored in an antiseptic solution (e.g., benzalkonium chloride). Hot water should not be used for cleaning thermometers.
The more common and quicker method of obtaining a body temperature is with the use of digital thermometers (Figure 21-6). There are many brands available, and with most an auditory beep alerts the technicians when the reading is final.
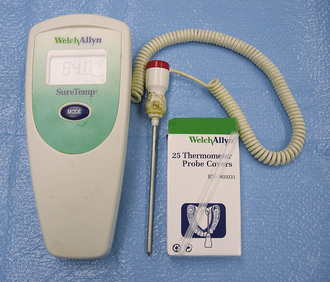
FIGURE 21-6 Digital electronic thermometer by Welch Allyn with removable and disposable plastic sheaths. These are very accurate and quick for the measurement of rectal temperature.
Certain diseases that produce fever display a diurnal pattern (i.e., the temperature fluctuates) during the day. If the patient's temperature is taken just once per day, the periods of fever may not be recognized. If this situation is suspected, a temperature chart may be kept by taking and recording the temperature at regular intervals, for example, every 4 hours.
The normal rectal temperature in the dog is 101.0° F to 102.5° F. The normal rectal temperature in the cat is 100.5° F to 102.5° F. Excitement or activity can elevate the temperature above these limits. In rare clinical situations (i.e., rectal laceration, rectal prolapse), it may not be possible to measure the rectal temperature. In these situations, the temperature may be taken in either the axilla or the external ear canal. The temperature recorded in these sites will be significantly lower than the simultaneous rectal temperature. In general, 1° F can be added to an axillary or ear canal temperature to approximate rectal temperature. These alternative techniques for determining the body temperature are useful when the same site is used serially in an individual patient, and the results are compared. The temperature is taken by placing the bulb of the thermometer deep in the axilla or ear canal for several minutes.
Recently, infrared thermometers have been developed that record accurate body core temperatures by focusing the infrared beam on the tympanic membrane. This thermometer is helpful in those patients with very low rectal temperatures or in those for which taking a rectal temperature is contraindicated (Ototemp Veterinary, Exergen Corp.).
PULSE
The rate and character of the pulse are valuable means of assessing the cardiovascular status of the patient. The pulse can be palpated in any artery located close to the body surface. Using an index finger to palpate the pulse is best for sensitivity with the thumb being the least sensitive. The pulse is most commonly felt in the femoral artery. The femoral artery is palpated on the medial aspect of the thigh, proximal to the stifle. Palpation of the femoral pulse requires practice and can be difficult in a trembling patient or in a patient with short, heavily muscled legs. Alternative sites for taking the pulse are the palmar aspect of the carpus and the ventral aspect of the base of the tail. The normal pulse rate in adult dogs is 60 to 160 beats/min, up to 180 beats/min in toy breeds, and 220 beats/min for puppies. The maximum rate in cats is 240 beats/min.
The heart rate can be counted by palpation or auscultation at the point of maximal intensity of the heartbeat. The point of maximal intensity is located at the costochondral junction between the left fourth and sixth intercostal spaces. If the pulse rate is taken at the same time as the heart rate and the pulse rate is less, this is called a pulse deficit. A pulse deficit generally indicates an abnormal heart rhythm.
The dog can have heart and pulse rates that are “regularly irregular.” Characteristically, the heart and pulse rates increase with inspiration and decrease with expiration. This normal variation is called sinus arrhythmia.
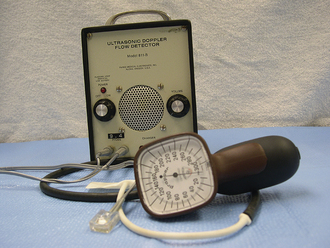
In addition to taking the pulse rate, it is beneficial to evaluate the pulse pressure and character of the pulse. Decreased pulse pressure may indicate systemic hypotension (drop in blood pressure) secondary to a process such as hypovolemic shock. Instrumentation has been developed for the noninvasive measurement of blood pressure in the dog and cat using the ultrasound Doppler method (Dinamap 8300, Critikon [Figure 21-7]; Parks 811-B, Parks Medical Electronics, Inc. [Figure 21-8]). Blood pressure readings are taken when the dog or cat has acclimated to the hospital environment to prevent falsely high readings. A neonatal cuff that has a width that is 40% the size of the limb circumference is placed over the medial tibial artery, which is found midway between the carpus and elbow or in the tibial area on a back leg. A small area is clipped free of hair distal to the cuff and transducer gel applied. The transducer is placed on top of the gel. Seven readings are taken for systolic and diastolic blood pressures. The highest and lowest values are omitted, and the remaining 5 values are averaged for the final value. The systolic readings are much more reliable than the diastolic readings with the Dinamap. The Parks ultrasound Doppler gives a more reliable reading in the cat. In the dog and cat, the normal systolic blood pressure is less than 160 mm Hg and normal diastolic blood pressure is less than 120 mm Hg.
RESPIRATION
The respiratory rate should be counted when the animal is at rest but not sleeping. Respiration involves both an inspiratory and expiratory phase. When counting the respiratory rate, it is necessary to count either inspirations or expirations but not both. The normal rate in the dog is between 15 and 30 breaths/min. Smaller breeds tend to have a more rapid rate of respiration than larger breeds. The rate in cats is between 20 and 30 breaths/min. In addition to determining the rate, it is important to characterize the respiratory status of the patient by inspection.
Several terms are used to describe respiratory function. Tachypnea refers to very rapid breathing. Hyperpnea indicates a condition in which the respiration is deeper and more rapid than normal. Depth of respiration indicates the volume of air inspired with each breath. Increased depth of respiration indicates a greater demand for oxygen. Shallow respiration can be caused by either metabolic derangement (e.g., acidosis) or mechanical injuries (e.g., fractured ribs). Dyspnea is a term used to indicate the subjective impression of increased difficulty or distress in breathing. Labored breathing is also used to describe difficulty in breathing and may include abdominal movements that occur simultaneously indicating the degree of increased effort to breathe by using abdominal muscles. Hyperventilation is seen as shallow, rapid breathing and occurs in severe metabolic acidosis and sometimes in severe respiratory disease.
All hospitalized patients should have their vital signs monitored at least once per day. Depending on the underlying problem and the status of the patient, it may be necessary to monitor the patient more frequently. The temperature, pulse, and respiration rate should be recorded in the medical record every time they are taken. This will facilitate recognition of abnormalities as early as possible. Further, serial observations will permit recognition of clinical trends.
ADMINISTRATION OF MEDICATIONS
It is important for the animal health technician to be familiar with several basic principles of clinical pharmacology. These principles are important when considering the route of administration of various drugs. Drugs can be administered parenterally (e.g., by injection), orally, or topically. The parenteral techniques routinely used in veterinary medicine include the intravenous, intramuscular, and subcutaneous routes. The specific techniques used to administer drugs by these various routes are discussed in Chapter 20. The discussion in this chapter is concerned with the selection of an appropriate route in various clinical situations.
In choosing the route of administration, a variety of factors must be considered. First, the pharmacologic properties of the drug should be considered. Certain drugs are not adequately absorbed when given by a certain route (e.g., gentamicin is poorly absorbed from the gastrointestinal tract). Similarly, insulin must be given by injection because it is destroyed in the gastrointestinal tract. Other drugs cannot be given by a certain route because they produce severe tissue reactions (e.g., thiamylal sodium causes sloughing of the skin if it is given subcutaneously). Another pharmacologic factor to consider is the rate of absorption. If an animal is critically ill, the route of administration that will provide the earliest onset of action is preferred. For example, an animal with a severe, overwhelming infection should receive an antibiotic intravenously rather than orally.
It is also important to consider the patient when considering the route of administration. For example, it is generally inadvisable to administer oral medications to a vomiting patient or to an animal with severe respiratory compromise or distress. The temperament of the patient should also be considered. In a fractious animal, it may be impossible to administer drugs topically, orally, or intravenously. Subcutaneous or intramuscular injections may be the only feasible routes of administration. Finally, convenience and compliance of the client will influence therapeutic decision. Obviously, the topical and oral routes are preferred for treatment at home.
The principal advantages of the oral route are convenience and reduced risk of infection or abscess caused by faulty injection technique. Disadvantages of the oral route include the potential for aspiration of liquid medications and the potential for animals to spit out the medication, so the prescribed dose is not absorbed.
Advantages of parenteral injections include, in general, more rapid absorption and greater assurance that the prescribed dose is accurately delivered.
The major advantage to topical medication is that systemic effects are reduced and safety is thus increased. The major disadvantage is that most systemic illnesses do not respond to topical medication alone.
Whenever any drug is administered, it is essential to record the treatment (drug, dose, time) and route of administration completely and accurately in the medical record. The notation should be made immediately after administering the medication. If this procedure is consistently followed, patient care will improve because it is less likely that treatments will be omitted or inadvertently repeated. In addition to improving the level of patient care, it should be remembered that this policy is important because the medical record is a legal document, and every treatment should be recorded in case of subsequent litigation.
It is also of utmost importance that all medications, either those used in the hospital or those dispensed for use at home, be labeled correctly (see Chapter 25). The dispensing label information should include the complete name of the drug, size or concentration of the drug, number of tablets or capsules or milliliters of drug dispensed, dose and frequency of administration, name of the client, and name of the hospital. If potentially toxic drugs are dispensed, childproof containers should be used, as determined by state and federal regulations.
FLUID THERAPY
The veterinary technician generally will not be called on to formulate a fluid order in a hospitalized patient without supervision of the attending veterinarian. However, familiarity with certain fundamental points will allow the technician to participate actively in this essential process.
The total volume of fluid required to treat an animal can be approximated by considering the volume of fluid needed to rehydrate the patient, volume of fluid needed for maintenance requirements, and volume of fluid needed to correct ongoing losses.
Sensible losses are roughly equivalent to urine output. Insensible losses represent the fluid lost in the feces and during respiration. These losses are considered as part of the daily maintenance requirements. Contemporary losses are due to ongoing problems (i.e., vomiting, diarrhea).
The hydration status, and thus the rehydration requirement, can be assessed by the following physical examination criteria: skin turgor, dryness of the mucous membranes, capillary refill time, and degree of sinkage of the eyes into the bony orbit. Several laboratory criteria are beneficial, particularly if they are followed serially; these include the hematocrit, total protein determination, and urine specific gravity (SG). Finally, serial body weights can be valuable in determining changes in hydration status. One pound of body weight is equivalent to 1 pt or 480 ml of fluid.
By using the physical examination findings mentioned, the degree of dehydration is estimated as a percentage of body weight (Table 21-2). Thus an animal that shows only a slight alteration in skin turgor is approximately 5% to 6% dehydrated. Skin turgor is evaluated by pinching a fold of the skin and subjectively assessing the rate at which it returns to its normal position. This is not a valid test in older animals or animals that have recently lost weight because of the increased skin turgor that develops due to decreased fat in the subcutaneous space. An animal that is 10% to 12% dehydrated will display pronounced changes in skin turgor; dry, tacky mucous membranes; prolonged capillary refill time; and eyes that are sunken into the orbits. The physical alterations associated with dehydration are a continuum, so an animal that is 8% dehydrated should have abnormalities midway between the end points described. It should be stressed that physical examination findings are at best very crude indicators of the degree of dehydration. The quantitative value of these parameters is improved if they are carefully and critically assessed over time.
TABLE 21-2
Diagnosis of Dehydration: Physical Examination Findings
| Dehydration (%) | Clinical Signs |
| <5 | Undetectable |
| 5-6 | Skin slightly doughy, inelastic consistency |
| 6-8 | Skin definitely inelastic; eyes very slightly sunken in orbits |
| 10-12 | Increased skin turgor; eyes sunken in orbits, prolonged refill time, dry mucous membranes |
| 12-15 | Shock and imminent death |
The laboratory criteria used to assess the degree of dehydration evaluate the extent of hemoconcentration. Thus the higher the hematocrit and the total protein determination, the more hemoconcentrated and thus dehydrated is the patient. These laboratory tests are useful in detecting relative changes and do not necessarily measure the absolute hydration status of the patient. If the concentrating ability of the kidneys is normal, a urine SG of more than 1.035 in the dog and 1.040 in the cat provides further evidence that the patient may be dehydrated.
Because changes in body weight over short periods are caused by changes in fluid balance rather than by the loss or gain of body mass, an accurate daily weight can also be helpful in assessing changes in the hydration status of the patient.
Once the degree of dehydration has been estimated, it can be used in calculating the volume of fluid needed to rehydrate the patient. The percent dehydration is multiplied by the body weight in kilograms and then by 1000. This is the number of milliliters needed to rehydrate the patient.
In addition to the volume required for rehydration, the maintenance requirement must be incorporated in the calculation of the daily fluid order. The maintenance requirement consists of estimates of both sensible and insensible losses.
As mentioned, sensible losses refer to the urine output. Insensible losses represent the fluid lost from the body via the gastrointestinal and respiratory tracts. Although sensible and insensible losses will vary somewhat depending on the clinical setting, a useful clinical approximation is 60 ml/kg/day (30 ml/lb/day). If the animal is not taking any liquid by mouth, a volume equivalent to the sensible and insensible losses (e.g., the maintenance requirement) should be included in the daily fluid order.
Most animals with problems that require fluid therapy do not have these problems resolve immediately on initiation of fluid therapy. Therefore contemporary or ongoing losses must also be considered in determining the daily fluid order. For example, if a patient has gastroenteritis, the volume of fluid lost with each episode of vomiting and diarrhea should be estimated and added to the rehydration and maintenance volumes. The volume of diarrhea and vomitus is frequently underestimated; therefore it has been recommended that the visual estimate be doubled to more accurately reflect the actual volume lost. Generally, the volume required to rehydrate the animal is not replaced immediately. Usually, the total volume is administered over the first 24 hours. Once rehydrated, maintenance requirement and ongoing losses are combined to calculate the fluid requirement for the next 24 hours and given over 24 hours (Box 21-1).
ROUTES OF FLUID ADMINISTRATION
Oral fluid administration is the preferred method because of reduced expense, ease of administration, and safety. Contraindications to oral fluid administration include vomiting and severe, life-threatening fluid imbalances that require immediate correction.
Many conditions respond well to subcutaneous administration of fluids. Fluids given subcutaneously should be warmed to body temperature and must be isotonic with extracellular fluid. Isotonic fluids have an osmotic pressure approximately equal to that of extracellular fluid. Never give subcutaneously dextrose solutions with a concentration of more than 2.5%; sloughing of skin and abscess formation are common sequelae. The volume and rate of subcutaneous fluid that can be given will vary from patient to patient. A rough guideline for total daily volume is approximately 60 ml/kg (30 ml/lb). Absorption of subcutaneous fluid will occur over 6 to 8 hours; therefore this total daily dose can be divided and given every 6 to 8 hours. It is necessary and desirable to administer this divided dose in as many sites as possible. Subcutaneous fluid administration is safe and easy; however, it is not the recommended route of administration when prompt correction of severe deficits is required. Intravenous fluid administration is indicated when a patient is severely compromised with dehydration, hypovolemia, electrolyte imbalances, hypoglycemia, and so forth. The intravenous route is the most common way to give fluid in the hospital and is indicated particularly for serious, life-threatening illness and vomiting patients. Aseptic technique is required to place an intravenous catheter into the cephalic vein, saphenous vein, or jugular vein. The catheter and the fluid drip set must be kept sterile and free of blood clots to allow long-term use (3 to 5 days maximum) of the intravenous line. Heparinized saline or sterile saline may be used to periodically flush the catheter to prevent blood clots from forming in the catheter. Intravenous fluid can be given at a continuous rate using a digital fluid pump, or they can be given intermittently using the free-flowing drip method (Box 21-2).
The intraperitoneal route is not a routine method of fluid administration because peritonitis and intraabdominal abscess formation may result from this form of fluid therapy. The rate of absorption of intraperitoneal fluids is roughly equivalent to the rate of absorption of subcutaneous fluid and therefore the intraperitoneal route is not adequate when prompt correction is needed. The exception to this is the use of intraperitoneal fluid administration in the neonate and wildlife neonate, where this route may be very effective.
Signs of volume overload include restlessness, hyperpnea (increased respiratory rate), serous (watery) nasal discharge, chemosis (edema of the ocular conjunctiva), and pitting edema. Volume overload can be caused by either an excessive total volume or an excessive rate of fluid administration. Decreased cardiac function or decreased plasma protein can predispose to a volume overload state. If volume overload is suspected, the lungs should be auscultated for evidence of pulmonary edema, and the central venous pressure should be determined. Before the development of pulmonary edema or elevated central venous pressure, weight gain may be seen. Therefore it is advisable to weigh the animal three times daily while intravenous fluid therapy is being used, especially in those patients who are less able to handle a fluid load (e.g., patients with cardiac or renal disease). The placement of an indwelling urinary catheter (Foley) and urinary outflow collection system will allow quantitation of urine production. This will allow a more accurate assessment of how much fluid is coming out and how much intravenous fluid the patient actually needs to prevent overzealous fluid therapy.
Fluid therapy is a dynamic process that must be reassessed at frequent intervals and adjusted to obtain the maximum results. The technician's role in clinically assessing the patient is important in making appropriate adjustments. The chance of inadvertent fluid overload can be reduced by using indwelling intravenous catheters and administering fluid over prolonged periods of time rather than using rapid bolus techniques. In addition, Minidrip (Travenol Laboratories, Inc.) and Buretrol (Travenol Laboratories, Inc.) administration sets can be used in cats and small dogs. Also, syringe pumps are useful in administering fluid to cats and very small dogs (Medfusion 2010 [Medex, Inc.] Syringe Pump; Figure 21-9).

FIGURE 21-9 Medfusion 2010 (Medex, Inc.) Syringe Pump used for the administration of small volumes and slow rates of fluid to the cat and small dog.
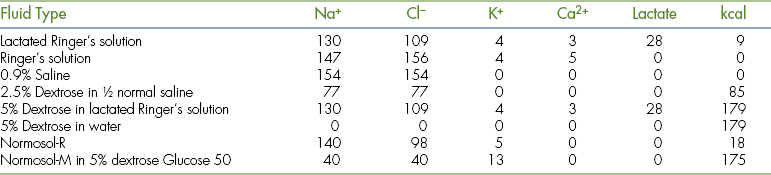
Several basic types of fluid are routinely used in small animal practice. They include physiologic (0.9%) saline, 5% dextrose in water, and extracellular fluid replacement solutions, such as lactated Ringer's solution or Ringer's solution. Combinations of these basic fluid types are also used. These basic parenteral fluid types can be supplemented with concentrated solutions of electrolytes and dextrose to produce the desired fluid composition appropriate for the specific clinical situation (Table 21-3).
Frequently, antimicrobials are added to intravenous fluid for administration. A number of the commonly used antimicrobials are incompatible with certain fluid (Table 21-4). The physical incompatibilities include precipitation of the drug out of solution and chemical inactivation. In addition to these incompatibilities, it has been noted that when certain drugs are mixed in infusion solutions, inactivation occurs. For example, when carbenicillin is added to a solution containing gentamicin, the gentamicin is inactivated. As a general rule, it is undesirable to mix multiple drugs in a syringe or intravenous fluid. Frequently, the interaction is visible on mixing, but other times it will not be observed before administration.
TABLE 21-4
Physical Incompatibilities of Antimicrobials in Intravenous Solutions
| Antimicrobial | Incompatible With |
| Amphotericin B | Normal saline |
| Cephalothin sodium | Lactated Ringer's solution, calcium gluconate, calcium chloride |
| Chloramphenicol sodium | Vitamin B complex with vitamin C succinate |
| Chlortetracycline hydrochloride, hydrochloride, tetracycline hydrochloride | Lactated Ringer's solution, oxytetracycline sodium bicarbonate, calcium chloride |
| Penicillins | Dextrose-containing solutions with pH >8 (i.e., added sodium bicarbonate) |
| Penicillin G potassium | Vitamin B complex with vitamin C |
CENTRAL VENOUS PRESSURE
The measurement of central venous pressure is a useful aid in evaluating the fluid status of a patient. When used and interpreted properly, it can substantially reduce the likelihood of excessive fluid administration. Measurement of the central venous pressure is a simple technique that can be performed in all veterinary practices.
To measure the central venous pressure, an indwelling intravenous catheter is placed in the cranial vena cava via the external jugular vein. It is very important that the catheter tip be located in the cranial vena cava. If the intravenous catheter is properly placed, a 2- to 5-mm fluctuation in central venous pressure will be noted with each respiration.
Next, a sterile three-way stopcock is attached to the intravenous catheter. The open line of the three-way stopcock is connected to the intravenous fluid source. The intravenous fluid is used to prime the manometer; that is, the manometer is filled to overflowing with the intravenous fluid. With the patient in lateral recumbency, the zero point of the manometer is positioned at the level of the sternum (Figure 21-10). The central venous pressure is equal to the level of intravenous fluid in the manometer once equilibrium has been established. To improve accuracy, this determination should be repeated a total of three times. If the pressure is high, prevent blood from entering the manometer because a blood clot may alter the measurements.
The following points are important considerations when measuring and interpreting central venous pressure measurements. Serial measurements should be performed with the same zero point and the patient in the same position. If the catheter is obstructed because of blood clots or kinking, the central venous pressure will be falsely elevated. Obstruction should be suspected if the level of the manometer does not fluctuate with respiration. Because continuous recording is not possible, pressure measurements are made intermittently. If intravenous fluid is not being administered between central venous pressure measurements, the catheter should be flushed with heparinized saline. Heparinized saline is prepared by adding 5 U of heparin per milliliter of saline. When evaluating the central venous pressure, it is better to evaluate trends rather than single measurements. Usually, changes of less than 3 cm of water are not significant. Using the sternum as the zero point, normal central venous pressure in the dog and cat varies between 0 and 5 cm of intravenous fluid. If the central venous pressure is consistently more than 8 to 10 cm of intravenous fluid, volume overload is suspected and fluid administration should be slowed or stopped.
BLOOD TRANSFUSION
See Emergency Nursing, Chapter 33, for transfusion therapy.
Blood Collection
The donor may be sedated if necessary but in most dogs this is not necessary once they are use to the routine. A surgical aseptic preparation of the collection site is performed. The collection site in the dog and cat is the jugular vein. Blood collection should be performed rapidly and without interruption, using a single venipuncture of the vein to prevent excessive activation of the clotting cascade and damage to the RBCs. If acid citrate dextrose (ACD Evacuated Blood Collection Bottle, Diamond Laboratories, Inc.) is being used, a separate collection set should be used. If citrate phosphate dextrose (CPD) plastic blood pack units (with Integral Donor Tube, Fenwall Laboratories, Inc.) are used, the attached needle should be used. If vacuum bottles are used, care should be taken not to lose the vacuum at the time of venipuncture. Use of glass bottle blood collection systems should be avoided since they are not closed systems and allow the blood to be exposed to room air. Glass bottles also cause platelet inactivation and clumping on contact with the glass surface.
In the cat, a 19-gauge butterfly needle (Travenol Laboratories) and a large syringe containing the desired anticoagulant can be used.
Several anticoagulants are available for routine collection of blood. Blood drawn in heparin or sodium citrate must be used within 24 to 48 hours because of the lack of an RBC preservative, which results in a major increase in pH and the subsequent decrease in red cell adenosine triphosphate. These chemical changes result in rigid red cells that do not deform and thus are rapidly removed from the recipient's circulation.
If blood is to be stored for longer than 48 hours, either acid citrate dextrose (ACD Evacuated Blood Collection Bottle) or citrate phosphate dextrose (CPD Blood Pack Units with Integral Donor Tube) must be used as the anticoagulant and the blood stored at 1° C to 6° C. The temperature cannot vary by more than 2° C, and if the blood is out of refrigeration long enough to warm to 10° C (approximately 30 minutes), it must be used immediately. During storage, the blood should be gently mixed periodically. When collected and stored as described, blood drawn in ACD has an effective storage life of approximately 14 days, and blood drawn in CPD has an effective storage life of approximately 21 days. Blood stored in CPDA-1, with the added RBC preservative adenosine, has an effective storage life of approximately 35 to 45 days.
Blood should be gradually warmed to approximately 37° C or room temperature before administration. Refrigerated blood can be warmed by placing the bag in a 40° C water bath. Care should be exercised to prevent excessive warming (more than 50° C). Excessive warming will cause hemolysis.
It is essential that strict asepsis be maintained during collection, storage, and administration of blood and blood products. Once a blood storage container has been entered, the stored blood should be used within 24 hours. Blood should be administered through a sterile blood administration kit (Blood Administration Set, Diamond Laboratories, Inc.). A micropore filter is suggested to reduce the transfusion of microemboli found in stored blood. Administration of blood and blood products can be given by the intravenous (the most common route), intraperitoneal, or interosseous routes (into the bone marrow). The intraperitoneal and intraosseous routes are used more in the neonate.
If the practice has a frequent demand for transfusion therapy, it is desirable to make optimal use of the available donors by separating blood into its components and administering only the needed component. Packed red cells can be produced by either centrifugation or by sedimentation of whole blood. Sedimented packed red cells are separated from plasma by gravity. The recovery of plasma is less efficient by this method; however, a centrifuge is not necessary. If collected in glass vacuum bottles, approximately 25% to 30% of the blood volume separates into plasma by 7 to 9 days, and 45% of the blood volume is available as plasma after 14 to 16 days. Plasma is harvested from the glass collection bottles with a sterile 17.5-cm needle and a sterile syringe. Blood in plastic packs separates more rapidly than blood in glass bottles. Plasma can be collected from plastic packs by means of either a sterile needle and syringe or a plasma transfer pack (Plasma Transfer Sets, Fenwall Laboratories) and a plasma extractor (Plasma Extractor, Fenwall Laboratories). The plasma transfer packs have attached tubing, adaptors, and sealable entry ports. Thus the plasma can be collected in a closed, sterile system. If the plasma is to be stored at refrigerator temperatures (18° C to 68° C) for longer than 24 hours, a closed system is essential. Plasma frozen at less than −208° C has a storage life of longer than 1 year. If frozen plasma is to be used to treat bleeding disorders, it should be frozen within a few hours of collection.
If the major indication for transfusion is decreased oxygen-carrying capability, the patient should receive packed red cells. Packed red cells can be administered rapidly with less risk of creating volume overload in a patient with compromised cardiovascular function. The use of packed red cells will also reduce the frequency of transfusion reactions caused by plasma protein incompatibility.
Plasma transfusions are used primarily to expand the extracellular fluid volume. Plasma is also used for its transient benefit in the management of hypoproteinemia. Fresh frozen plasma is a source of coagulation factors for the treatment of warfarin toxicity, DIC, and inherited coagulation factor deficiencies.
An alternative to packed RBCs is bovine hemoglobin solution (Oxyglobin, Biopure, Inc.) also referred to as an acellular oxygen-carrying replacement fluid. The advantages of bovine hemoglobin are no need for blood typing and cross-matching, no transfusion reactions and the convenience of having the product stored on the shelf up to 3 years. Caution is necessary in the cat because of possible pulmonary edema when given rapidly. Bovine plasma is an active colloid solution and can cause volume overload if given to a patient with heart failure or renal failure or to any patient if given rapidly or in large amount. Discoloration of serum, urine, and mucous membranes to a yellowish-brown is seen with bovine hemoglobin. Also, certain laboratory tests are affected by bovine hemoglobin in the serum.
Transfusion Reactions
Complications of blood transfusion can be both immunologic and nonimmunologic in origin. Immunologic reactions can result from the transfusion of incompatible blood. Incompatible RBCs in a previously unsensitized recipient will be destroyed 7 to 10 days after transfusion. If the recipient is subsequently exposed to incompatible blood, a more acute hemolytic reaction may occur. Clinical consequences of hemolytic transfusion reactions include the rapid development of tachycardia, hypotension, vomiting, salivation, and muscle tremors. Laboratory changes associated with significant acute hemolysis include hemoglobinemia, hemoglobinuria, and possible acquired coagulation disorders.
Delayed hemolytic reactions will sometimes occur following multiple transfusions. Delayed hemolysis should be suspected if the PCV drops unexpectedly 2 to 21 days after transfusion. The clinical and laboratory signs of acute hemolysis mentioned may not be detected in delayed hemolytic reactions. Transfusion reactions may also be caused by immunologic reactions caused by leukocyte, platelet, or plasma protein incompatibilities. Reactions between antigens and antibodies may activate the complement system and thus release vasoactive substances that may be responsible for trembling, vomiting, and urticaria (hives). Prior transfusion is not required for these reactions to occur. The use of antihistamines (diphenhydramine hydrochloride) approximately 30 minutes before transfusion may reduce these reactions.
Transfusion-induced fever is due to the response of the donor to foreign proteins. The initial step in controlling transfusion-induced fever is to slow the rate of transfusion. If no response is noted when the rate is reduced, the transfusion should be discontinued, and the patient observed closely for more severe signs of reaction. Bacterial contamination of the transfused blood will also produce fever and should be considered. Starting another transfusion after a period of time may eliminate the problem.
Nonimmunologic transfusion reactions are principally due to vascular overload. Signs of vascular overload include coughing, increased respiratory rate, respiratory distress, and vomiting. If there is evidence of preexisting cardiac dysfunction, the rate of administration of blood should be reduced to approximately 1 ml/kg/hr. Because vomiting is a potential adverse reaction to transfusion, food and water should be withheld from the patient during the transfusion and any medications scheduled to be given during this time.
OXYGEN THERAPY
The primary indication for oxygen therapy is hypoxia, which refers to a deficiency of oxygen at the tissue level. Tissue hypoxia may be caused by a reduction in perfusion (reduced blood flow) or a reduction in oxygen content of the blood. Hypoxia is probably more common than is recognized in veterinary medicine since a caged animal at rest will not show signs until the oxygen content of the blood is severely reduced.
Hypoxia can be manifested in a variety of ways, and the veterinary technician must be alert to identify these changes. Abnormalities that may be noted in the cardiovascular system include tachycardia or arrhythmias. An increased respiratory rate, open-mouthed breathing, and dyspnea may also be noted. Dyspnea is the term used to indicate subjective difficulty or distress in breathing. With severe hypoxia, central nervous system changes may be noted and include drowsiness, altered motor abilities, or increased excitability. Finally, cold extremities may indicate an inadequate supply of oxygen at the tissue level. Cyanosis is not a reliable indicator of hypoxia, especially if the animal is anemic. Cyanosis refers to dark bluish or purplish discoloration of the skin and mucous membranes.
Although the basic defect in hypoxia is decreased oxygen availability at the tissue level, it can occur by a variety of mechanisms. For example, it can result from lung disease, decreased cardiac output, or severe anemia.
In small animal practice, oxygen therapy is used primarily in the following clinical situations: pulmonary edema, severe bronchopneumonia, upper airway disease in brachycephalic breeds such as English bulldog and Boston terrier, pulmonary trauma, collapse of lung lobes, and shock. Measurement of hemoglobin saturation is performed with pulse oximetry (Figure 21-11). A pulse oximeter is used by applying a clip to nonpigmented skin or mucous membrane, such as the lip, tongue, pinnae, vulva, or prepuce to allow reading of hemoglobin saturation in the peripheral blood vessels. Hemoglobin saturation is an indirect way to monitor whether a patient has adequate peripheral arterial blood circulation. It is also a good indicator of hypoxemia due to decreased ventilation of air to the lungs. Direct measurement of oxygenation of arterial blood is monitored with the more invasive arterial blood gas. Arterial blood gas analysis determines the partial pressure of oxygen available in the bloodstream, which is a direct indicator of whether a patient can oxygenate blood in the lungs normally. An arterial blood sample is taken from the femoral artery to measure the arterial blood gas. Be careful not to incorporate air bubbles into the sample, and an immediate reading of the sample by a blood gas analyzer is imperative. A pulse oximeter reading less than 70% is considered decreased and an arterial blood gas PO2 less than 95 mm Hg is considered decreased.
Methods of oxygen therapy include oxygen cages, human pediatric incubators, masks, nasal catheters, endotracheal tubes, and intratracheal catheters.
Oxygen Cage
Oxygen cages for veterinary use are sold commercially. These cages permit control of not only the oxygen concentration but also temperature and humidity (Figure 21-12). These cages are useful in animals able to ventilate without assistance. However, they are expensive and consume large amounts of oxygen. Surplus human pediatric incubators are a less expensive means of providing similar therapy to small dogs, cats, or exotic animals. Oxygen cages and incubators should be flushed (filled) with oxygen after they have been opened. Some units are equipped with entry ports that allow access to the patient without excessive loss of oxygen.
An inspired oxygen concentration of 30% to 40% is adequate for animals requiring oxygen therapy. Excessively high oxygen concentrations can result in oxygen toxicity. Neonatal kittens appear to be particularly susceptible to retinal changes induced by oxygen toxicity.
Mask Induction
In certain circumstances, masks can be used to administer oxygen. Masks are available in a variety of sizes and shapes suitable for use in dogs and cats. If an oxygen mask is used, it is important to provide a high oxygen flow rate to prevent excessive accumulation of carbon dioxide. Administration of oxygen via a mask is suitable for short periods of time only and only in selected patients. Some patients will resist the use of an oxygen mask, and the resultant stress will negate any beneficial effect of the oxygen.
Intratracheal Catheter Induction
An alternative means of oxygen administration that is both inexpensive and effective is the intratracheal catheter. This technique is reserved for critically ill patients. The skin is aseptically prepared, and a local anesthetic is administered over the trachea in the midcervical area. An intravenous catheter (14, 16, or 18 gauge) is introduced into the trachea and advanced to a point craniad to the bifurcation of the trachea. The delivered oxygen should be humidified and administered at a flow rate of 0.5 to 4 L/min. The flow rate should be adjusted, depending on the size of the animal.
Nasal Catheter Induction
Nasal catheters can also be used to administer oxygen for brief periods to severely depressed animals. In this technique, a small (5 to 8 Fr) soft rubber feeding tube or urinary catheter is inserted through the external nares to the level of the caudal nasopharynx. The catheter can be coated with a topical anesthetic cream, or topical anesthetic drops can be instilled in the nostril to facilitate passage. Adhesive tape is attached to the catheter, and the tape is sutured to the forehead. An Elizabethan collar is used to prevent the patient from dislodging the catheter.
RESPIRATORY PHYSICAL THERAPY
Physical therapy of the respiratory system is a valuable adjunct to other forms of therapy for diseases of the lungs and airways. Appropriate physical therapy is also useful as a preventive measure in patients at high risk for the development of pulmonary disease. Secondary bronchopneumonia is a common complication in patients with lung lobe collapse. Stimulation of the cough reflex by compressing the trachea will expand the lungs maximally and help prevent lung collapse. Regular turning of recumbent patients will enhance drainage and circulation and thus prevent hypostatic congestion.
Percussion (coupage), also known as tapping or clapping, is a technique of striking the animal's chest to loosen bronchial secretion and thus facilitate drainage. The chest is struck with the hand held slightly cupped with fingers and thumb closed so that a cushion of air is trapped between the technician's hand and the chest wall. Best results come from using both hands alternately in rapid sequence for several seconds, moving from ventral to dorsal on the lung fields. When done properly, this is a noisy procedure; however, it is not painful to the patient. If the animal is ambulatory, a brief walk after coupage will aid in mobilization of respiratory secretions.
Whenever possible, animals with pulmonary problems should be maintained in an upright position (i.e., sternal recumbency). If necessary, slings or supports should be used to maintain this posture. When this is not practical, alternating sides of recumbency by turning the patient from one side to the other, every 2 hours, can prevent hypostatic congestion from developing.
TOPICAL THERAPY
Topical therapy plays an important role in the treatment of dermatologic disease. It can be used to treat a specific disease, such as sarcoptic mange. More frequently, however, topical therapy is used either in conjunction with systemic medications or as a form of symptomatic therapy when the diagnosis is unknown.
Plain tap water is one of the most effective topical agents. Depending on how water is used, it can either hydrate or dehydrate the skin. Frequent wetting of the skin will stimulate evaporation from the skin and thus cause dehydration. This approach can be useful in managing any acute moist dermatitis (“hot spot”). In contrast, if a film of oil (e.g., Alpha Keri, Westwood Pharmaceuticals, Inc.) is applied immediately after soaking with water, evaporation is slowed or stopped, and the skin remains moist.
Soaks
Soaks are an effective means of handling localized acute eruptions. Soaks can be applied with moist towels or by placing the animal in a water-filled basin or tub. Soaks for local acute dermatosis should be applied for 10 to 15 minutes three or more times daily. The involved area should be kept constantly moist, and the warm temperature of the soak should be maintained by adding hot water as needed. Some of the solutions commonly used for soaks in veterinary medicine include water, aluminum acetate (Burow's solution, Domeboro solution, Dome Laboratories), and magnesium sulfate or Epsom salts (1:65 solution in water, 1 tablespoonful per 1000 ml of water).
Astringents
Astringents precipitate proteins on the surface of an area of acute damage and form a beneficial covering. These agents do not penetrate deeply. Aluminum acetate is an excellent mild astringent. Another effective astringent is tannic acid. Tannic acid is combined with salicylic acid and alcohol in several products to form a potent astringent. These combination products are especially useful as part of the management of localized acute moist dermatitis; however, astringents should be applied only once to an involved area.
Baths
Cleansing baths are an important part of topical dermatologic therapy. Baths aid in the removal of dirt, debris, and scale. A variety of effective mild cleansing soaps or detergents are available. Mild dishwashing detergents or soaps (e.g., Joy, Palmolive Liquid) are effective and inexpensive. If a milder, less irritating product is desired, a balanced pH soap, such as Johnson's Baby Shampoo (Johnson & Johnson), can be used. If an even milder product is needed, vegetable oil soaps (coconut oil) are the most bland. Regardless of how mild the soap or detergent, it should always be thoroughly rinsed out of the coat with copious volumes of clean water.
A medicated bath can be applied as a shampoo or as a rinse applied to the animal after a routine cleansing bath. Medicated baths contain ingredients that enhance the actions of routine cleansing shampoos. Medicated shampoos should be lathered into the coat for 10 to 15 minutes. This allows the medicated component of the shampoo time for effect or limited absorption. Types of medicated baths used in small animal practice include colloidal oatmeal, tar-sulfur, sulfur-salicylic, and benzoyl peroxide products. Colloidal oatmeal (Aveeno, Cooper Care, Inc.; Epi-Soothe cream rinse, Allerderm, Inc.) baths are used for their soothing and antipruritic properties. Tar-sulfur shampoos (Lytar, Dermatologics for Veterinary Medicine, Inc.; Allerseb-T, Allerderm, Inc.) are used in the management of oily, flaky seborrheic conditions. Sulfur and salicylic shampoos (Sebalyte, Dermatologics for Veterinary Medicine, Inc.; Sebolux, Allerderm, Inc.) are used in the management of dry, flaky seborrheic conditions, and benzoyl peroxide shampoos (Oxydex, Dermatologics for Veterinary Medicine, Inc.; Pyoben, Allerderm, Inc.) are useful in the treatment of superficial pyoderma (bacterial skin infection), excessive crusting and debris problems, and oily seborrheic conditions. The underlying condition and the individual response to the medicated bath determine the required frequency of application.
Dips and Rinses
Dips or rinses use water as a means of delivering various antifungal or antiparasitic agents to the skin. Although applied to the skin, some of these agents have the potential to cause systemic toxicities. Clipping the hair and using cleansing baths help to obtain greater penetration in animals with excessive scale or crust. Dips that are useful in the treatment of dermatophytosis (ringworm) include dilute sodium hypochlorite solution, dilute Nolvasan solution (Fort Dodge Laboratories), dilute iodine solutions, or lime-sulfur solutions. Antiparasitic products used as dips or rinses include chlorpyrifos (Dursban), pyrethrins, pyrethroids, organophosphates (malathion), and carbamates. Amitraz (Mitaban, Upjohn Co.) is useful in the treatment of generalized demodectic mange.
Before using any topical agent the label should be checked to be sure it is safe to use in dogs, cats, puppies, and kittens. The age of young animals should be noted because some products are not recommended in the very young.
Powders
Powders are occasionally used in veterinary medicine as drying agents and vehicles for parasiticides and to reduce friction and irritation. When used as a drying agent, powders may be in the form of true powders, shake lotions, or pastes. Components that improve the drying action of various powdered products include talc, zinc oxide, cornstarch, and tannic acid. Carbaryl powders are a valuable part of flea control programs in the dog and cat. Labels should be checked carefully to be certain that the specific product is safe for dogs and cats. The powder must be worked down into the hair coat to increase the parasiticidal effect. This can be accomplished by rubbing the hair coat against the grain as the powder is applied. The powder should be applied to the entire body, excluding the face. Fractious or frightened cats can be treated by wrapping them in a thick bath towel and medicating small sections until the entire animal has been covered. Flea sprays can be used similarly.
Creams and Ointments
Creams and ointments are also used in the topical treatment of dermatologic problems. The area of treatment should be clipped, if not hairless, and protected from immediate removal by licking. For practical and economic reasons, the area to be treated should be relatively small. Ointments are thicker than creams and leave a greasy feeling when applied to the skin. Ointments and creams soften, lubricate, and protect the skin and aid in the removal of scale and crusts. Ointments and creams form an occlusive covering and therefore are not indicated for moist or oozing skin lesions.
Topical creams and ointments can be used to treat localized dermatophytosis (ringworm). They can be used as the sole type of therapy or as an adjunct to oral therapy or topical rinses. Creams and ointments must be restricted to small lesions because of expense and convenience. Effective topical fungicidal products used in veterinary medicine contain miconazole and thiabendazole. Because the use of ointments and creams alone is often insufficient to clear the infection or prevent reinfection, rinses or dips are important.
Otic Preparations
Most topical otic preparations contain various combinations of antibiotic, antiinflammatory, fungicidal, and parasiticidal agents. Topical antimicrobial agents are indicated whenever infection is present. Chloramphenicol, neomycin, polymyxin, and gentamicin are the commonly used antibiotics in these combination otic preparations. Neomycin and gentamicin have been reported to cause ototoxicity when used for prolonged periods in dogs with ruptured eardrums. (Gentamicin is inactivated by purulent exudate; therefore the ears must be thoroughly cleaned before use.)
Corticosteroids are used in these combination products because they decrease inflammation and the buildup of discharge and, consequently, decrease self-trauma by the animal. The antifungals are useful in treating dermatophytes and yeast organisms such as Malassezia pachydermatis (Pityrosporon). Thiabendazole and miconazole are effective topical antifungal agents.
Certain drugs owe their efficacy to their ability to alter the pH in the ear canal. Acetic acid (dilute vinegar solution) and Domeboro Otic (Dome Laboratories) are specific examples.
Products that contain rotenone in oil or thiabendazole are used to treat ear mites. It is essential that treatment for ear mites be continued for at least 3 weeks and that all animals in the household be treated. Otic instillation of ivermectin, as a one-time application (on occasion, two to four treatments may be needed), has also been shown to be effective in the treatment of ear mites.
INFECTIOUS DISEASES
This section will discuss a number of common medical problems of dogs and cats. It is not intended to be a comprehensive review of internal medicine; rather, several specific problems have been selected that illustrate or emphasize important aspects of medical nursing.
CANINE RESPIRATORY DISEASE COMPLEX
Synonyms for canine upper respiratory disease complex include kennel cough and infectious tracheobronchitis. This complex is composed of a number of different disease processes. Causative factors include viral and bacterial agents, and predisposing environmental factors. These factors may occur singly or in combination. Fever, coughing, ocular and nasal discharge, vomiting, diarrhea, lethargy, and depressed appetite may occur for 24 to 48 hours. The clinical signs are usually gone after 48 hours, except for a dry, hacking cough, which can linger up to 2 weeks. The diagnosis of this complex is usually based on historical and physical examination findings rather than on laboratory tests. This problem is most often self-limiting, and the duration of signs generally is no more than 2 weeks.
Treatment involves nursing care and the correction of any environmental factors that may have predisposed to the illness. The dog should be kept in a warm space that is well ventilated and free of drafts and should be fed a highly palatable diet. Appetite will be enhanced if eyes and nose are kept free of accumulated discharge. If appetite is suppressed, the patient should be encouraged to eat canned dog food or even selected table food, such as chicken and rice, for increased palatability. Intravenous or subcutaneous fluid therapy is occasionally necessary. Steam or vaporizer therapy may provide symptomatic relief of the dry cough. Steam therapy can be performed by placing the dog in a steam-filled bathroom several times per day. Alternatively, cold-mist vaporizers can be used several times daily.
The decision to use antitussive (cough suppressant) therapy should be based on the frequency of coughing and how prolonged the episodes are. If codeine-derivative cough suppressants are used to excess, depression and anorexia will result.
Treatment with antibiotics usually is not indicated unless there is evidence of lower respiratory or systemic involvement, for example, fever. The presence of a green- or yellow-colored nasal discharge may also warrant antibiotic therapy. If antibiotic therapy is instituted, a complete regimen of 10 to 14 days at full therapeutic doses should be completed. The selection of an antibiotic would ideally be based on the results of culture and sensitivity testing of a transtracheal wash. If these are not available, chloramphenicol, β-lactam penicillins, first-generation cephalosporins, or fluorinated quinolones are usually effective. The use of systemic products containing both antibiotics and corticosteroids is not indicated. Likewise, the intratracheal injection of any product is inappropriate therapy.
Because of the highly contagious nature of the causative organisms, an infected dog should be isolated from other hospitalized patients. If possible, hospitalization should be avoided. Once an outbreak occurs in a kennel or veterinary hospital, control is difficult. Ideally, the area should be kept vacant for approximately 2 weeks, and appropriate preventive measures should be instituted, consisting of the implementation of an effective vaccination protocol for every hospitalized patient. All dogs should preferably be vaccinated at least 10 days before exposure. At least every 3 years revaccination for the respiratory viruses of all patients over 1 year of age should be a consistent hospital policy. Vaccination with the intranasal vaccine for Bordetella bronchiseptica every 6 months is recommended for animals at high risk of exposure to the causative agents of infectious tracheobronchitis (e.g., frequent boarding or dog shows). The commonly used disinfectants, such as chlorhexidine (Novalsan) and benzalkonium (Roccal), effectively kill the causative bacteria and viruses.
FELINE RESPIRATORY DISEASE COMPLEX
The principal components of the feline respiratory disease complex are feline viral rhinotracheitis (herpesvirus) and feline calicivirus. Less frequently incriminated agents include feline pneumonitis (Chlamydia psittaci), Mycoplasma, and Bordetella bronchiseptica.
Clinical signs of this complex include fever, cough, paroxysms of sneezing, and hypersalivation. As the infection progresses, mucopurulent ocular and nasal discharge, lacrimation, and open-mouthed breathing can be seen. Ulceration of the tongue, hard palate, and nasal pad has been reported with feline calicivirus. The severity of signs and the mortality are greatest in young (less than 1 year of age), nonvaccinated cats and kittens. The severity of the clinical signs will vary widely from patient to patient. The variability results from a number of interacting factors, which include the virulence of the virus, infecting dose of virus, and general health and immune status of the infected cat.
Diagnosis is based primarily on history and clinical signs rather than on laboratory findings. Occasionally, laboratory confirmation of the diagnosis by means of virus isolation or the demonstration of serum antibodies is indicated. The additional expense of laboratory confirmation is justified only when dealing with groups of cats having a chronic history of feline respiratory disease complex.
Treatment will vary, depending on the severity of signs. Some cats will show only mild, transient signs, and they require no treatment. Secondary bacterial infection will occasionally be a sequelae to the feline respiratory disease complex, and therefore a broad-spectrum antibiotic may be indicated in the very young kitten (<12 weeks of age).
General nursing care is of much greater importance than antibiotics in typical cases. Whenever possible, infected cats should be treated at home rather than in the hospital.
A vital part of nursing care is to gently clean away accumulated ocular and nasal discharge. If the nostrils are kept patent, the cat is more likely to continue eating because of the cat's reliance on smell to encourage appetite. To ensure that this happens, the owner should indulge the pet and provide highly palatable food. Strongly flavored or odorous food (fish flavored) is more likely to stimulate the appetite of an anorectic cat. Steam therapy is frequently useful and can be achieved by placing the cat in a steam-filled bathroom or by using a vaporizer.
In cats that become completely anorectic, subcutaneous or intravenous fluid may be required until the appetite returns to normal. Repeated syringe feeding may be attempted; however, in certain cats, the associated stress may negate any beneficial effect. Alternatives that appear to be better tolerated include nasoesophageal or pharyngostomy tubes. These procedures should be reserved for severely cachectic cats.
The virus is usually transmitted through direct contact with an infected cat. Sneezing with subsequent aerosolization of the virus will spread the virus a distance of approximately 15 to 20 cm. Fomite transmission via hands, clothing, letterboxes, and food and water dishes is a more significant means of transmission than aerosolization in veterinary hospitals. The agents responsible for the feline respiratory disease complex are sensitive to hypochlorite disinfection.
The best way to prevent outbreaks of feline respiratory disease complex in hospitalized cats is to have an effective immunization protocol that requires vaccination of the respiratory viruses at least every 3 years in cats over 1 year of age. Adequate ventilation will reduce the likelihood that infection will spread within the hospital. The humidity should be maintained between 30% and 50%. Disposable food trays and litter pans and autoclavable water dishes should be used. Cats should not be moved from one cage to another unless absolutely necessary during an outbreak. Cages should be thoroughly cleansed with a dilute hypochlorite solution. Finally, because the infection can be spread via hands and clothing, meticulous hygiene on the part of all hospital personnel is essential. It is important to understand that up to 80% of the cats that develop this respiratory complex remain lifelong carriers of the organism or organisms. They can pose a risk to other cats or can experience a recrudescence of the complex in stressful situations.
CANINE DISTEMPER
Canine distemper is an important viral disease of dogs because of the ubiquitous nature of the virus and the mortality associated with infection. The severity of signs will vary from a transient, subclinical infection to a severe fatal disease that involves several different organ systems. This variability is due to the differing virulence of various virus strains and differences in host immunity.
The initial phase of the infection is associated with fever, transient anorexia, lethargy, and a mild serous ocular discharge after an approximate 9- to 14-day incubation period. Obviously, these signs are not specific for canine distemper. Later, as the virus spreads to the respiratory and gastrointestinal systems, mucopurulent ocular and nasal discharge, coughing, diarrhea, and, occasionally, vomiting are noted. Many dogs are anorectic at this point and become severely dehydrated. Involvement of the central nervous system may occur and can be the only signs manifested by some dogs. These dogs may develop seizures or other evidence of neurologic disease. Some dogs will seemingly recover from the severe respiratory and gastrointestinal signs, but weeks or months later they develop neurologic signs that either are fatal or require euthanasia because of their severity.
Although the virus may survive in the environment for weeks at near-freezing temperatures, it is susceptible to heat, drying, and ultraviolet light. Routine disinfection is usually effective in destroying the virus in a hospital or kennel. Patients suspected to have distemper should be housed separate from the rest of the hospital patients. An isolation ward or cat ward would be acceptable to prevent the spread of the virus to susceptible canine patients. Diligent washing of hands and preventing fomite transmission after handling distemper suspects is also necessary to prevent spread of the virus.
FELINE PANLEUKOPENIA
Feline panleukopenia is a potentially severe, highly contagious parvoviral disease of cats. Synonyms are feline distemper and infectious enteritis.
The typical clinical signs associated with feline panleukopenia include lethargy, anorexia, vomiting, and diarrhea after a 7-day incubation period. Characteristically, the feces are yellowish and semiformed to fluid in consistency; they may be blood tinged. Severe dehydration may be present. The temperature may be elevated or subnormal. Feline panleukopenia can be an acute disease. Rarely, development of signs is so rapid that the owner may suspect malicious poisoning. Kittens and young cats appear to be more severely affected.
Diagnosis of feline panleukopenia is based on the presence of the clinical signs described above in the presence of a low total leukocyte count (less than 2000 WBCs/mm3). The low total count is primarily due to low numbers of neutrophils. The diagnosis of feline panleukopenia can be confirmed by virus isolation and serologic and histopathologic characteristics.
Treatment is primarily supportive because specific antiviral drugs are not available. The cornerstone of successful therapy is the correction of fluid and electrolyte imbalances and prevention of sepsis by the use of broad-spectrum antibiotics. Symptomatic control of vomiting and diarrhea is usually indicated. Another complication the technician should be aware of is the development of hypoglycemia. This may be manifested by the development of extreme weakness, seizure activity, or both.
The prognosis for recovery is good if the cat survives the initial 3 to 6 days of severe clinical signs. The prognosis for kittens and young cats is guarded. A rising WBC count indicates a more favorable prognosis. During the recovery phase, the WBC count may exceed 50,000/mm3 and reveal a significant leftward shift. This should not be confused with the development of another infection because this can be a normal response.
If the queen is infected during pregnancy, fetal death or congenital defects in the kitten may result. The fetus is susceptible to the virus because most tissues have high cell-proliferation rates. If the fetus is infected just before or immediately after birth, the development of the cerebellum may be affected and hypocerebellum can occur. These kittens show balance and coordination problems beginning at about 3 to 4 weeks of age.
Fortunately, because of the availability of excellent vaccines, feline panleukopenia is currently an infrequent clinical problem.
FELINE LEUKEMIA VIRUS AND FELINE IMMUNODEFICIENCY VIRUS INFECTION
These two distinct retroviral infections in cats may cause similar clinical signs. Feline leukemia virus (FeLV) has been recognized for many years and may cause immunosuppression, neoplasia, or both. Lymphosarcoma and bone marrow disorders are the more common disorders associated with FeLV. The virus is transmitted between cats by direct contact through grooming, sharing food dishes, and fighting. The virus is easily killed in the environment, and isolation of an infected cat is adequate to prevent transmission to susceptible cats. Although most cats that are exposed to the virus successfully eliminate the infection, 1% to 3% of cats in single-cat households and up to 30% of cats in multiple-cat households will become persistently infected with the virus. These infected cats are then at risk for the development of the plethora of FeLV-related diseases. FeLV infection can be identified by an in-hospital test that detects viral antigen. There are many such in-hospital tests on the market and available to the practicing veterinarian. There are several vaccines available for the prevention of FeLV. These vaccines are not completely protective but do protect up to 70% of the vaccinated cat population.
Feline immunodeficiency virus (FIV), also called T-lymphotrophic T cell lentivirus (FTLV), is another virus that causes immunosuppression in the cat. Common clinical signs of infection with this virus include gingivitis, chronic diarrhea, generalized lymphadenopathy, fever, conjunctivitis, rhinitis, and dermatitis. It is notable that all these signs may be seen in cats infected with FeLV. FIV is found nationwide and, indeed, worldwide. Most cats infected with this virus will not become immune, which differs from FeLV infection. The disease is spread by inoculation of the virus through cat bites which allow blood transmission. Transmission of the virus by direct contact through grooming, sharing of food dishes, and close contact is less than that seen with FeLV. No treatment is available for this disease. Commercial kits detecting antibodies to this virus are available for in-hospital testing. A vaccine is currently available; however, the efficacy has not been established. Also, this vaccine will cause a positive FIV antibody test, complicating the ability to clearly document FIV infection in vaccinated cats.
ROUTINE IMMUNIZATION PROGRAM FOR DOGS AND CATS
One of the greatest areas of advancement in veterinary medicine in the past 50 years is in the prevention of infectious diseases. The purpose of any vaccination program is to prevent clinical disease by preventing or limiting infection. The vaccination program can also be the foundation of a complete well-animal health maintenance program. At the time of vaccination, owners should be counseled regarding nutrition, parasite control, and matters regarding reproduction. Chapter 9 provides a complete overview of canine and feline preventive health programs and vaccination recommendations.
A physical examination by the veterinarian at the time of vaccination is extremely important because a number of conditions will potentially influence the immunization procedure, such as pregnancy, debilitation, and fever.
Numerous factors influence the patient's ability to respond to vaccination. Factors that are of practical significance include colostral (maternal) antibodies, vaccine type, route of administration, age of the patient, nutritional status of the patient, and concurrent infection or drug therapy.
Colostral Antibodies
In puppies and kittens, approximately 95% of the circulating immunoglobulins come from absorption of colostrum (first milk) shortly after birth. These circulating immunoglobulins provide essential temporary protection, but they also have the ability to interfere with more permanent protection. Interference occurs because the vaccine does not reach the appropriate cells to stimulate the active immunity process. Consequently, it is necessary for the level of circulating immunoglobulins derived from the colostrum to be reduced before successful vaccination is possible. In puppies born to bitches that have received vaccinations against canine distemper and infectious canine hepatitis, this period of uncertain response to vaccination may extend to 14 weeks of age. Thus the last dose of vaccine should be administered at 14 to 16 weeks of age to optimize the success of the vaccination program. Colostral immunoglobulins to canine parvovirus may persist for at least 16 weeks in puppies; therefore the last dose of vaccine for parvovirus should be given no earlier than 16 weeks of age. In the Rottweiler and Doberman breeds, it is suggested that the last dose of parvovirus vaccine be given at 18 weeks of age.
An alternative technique to prevent or reduce the blocking effect of colostral antibodies on canine distemper vaccination is to use measles virus vaccine. Approximately 50% of puppies at 6 weeks of age will not respond to canine distemper virus vaccination, whereas the vast majority will respond to measles virus vaccine. The measles virus stimulates resistance against canine distemper in puppies regardless of circulating antibodies that the pup has acquired from the colostrum. Measles virus vaccine prevents clinical disease but does not prevent infection. Measles virus vaccine should be considered a temporary method of preventing canine distemper until the dog can respond to the canine distemper vaccine. There is no reason to use vaccines containing measles virus in dogs older than 16 weeks of age. There are no known public health dangers associated with the use of measles virus-containing vaccines. Measles virus vaccine does not provide protection against infectious canine hepatitis.
Methods of overcoming the effects of colostral (maternal) antibodies are not absolute. Therefore research is continuing in this area. Although colostral antibodies interfere with the immunization process, colostrum is extremely important for the protection of the neonate against a number of potentially harmful microorganisms. Puppies and kittens should never be deliberately deprived of colostrum.
Type of Vaccine
The type of vaccine is very important in formulating a successful vaccination program. Viral vaccines can be either inactivated or modified live virus vaccines. Because live virus vaccines depend on viral replication in the recipient animal to provide protection, the vaccine must be handled strictly according to the instructions supplied by the manufacturer. Inactivated vaccines are less labile; however, in general they must be administered several times to get an adequate protective response. It is impossible to state that one type of vaccine is categorically better than another; in the future, both inactivated and modified live virus types of vaccine will continue to be used.
To achieve the optimal response, the entire dose of vaccine should be given as recommended; the dose should not be split and given to more than one animal. Different vaccine products should not be mixed in the same syringe before administration. Frequently, vaccines contain preservatives that will interfere with another vaccine.
Route of Administration
The route of administration specified in the manufacturer's instructions should be followed. With certain viruses, significant differences in response occur, depending on the route of administration. For example, with measles virus and some rabies virus vaccines, the intramuscular route is much more effective than the subcutaneous route. The manufacturer's recommendations must be understood and followed for all vaccines.
With certain viruses (e.g., feline viral rhinotracheitis, calicivirus, feline infectious peritonitis) vaccines that produce local immunity have been developed. These vaccines are given by the intranasal and intraocular routes. An example of a bacterial disease for which an intranasal vaccine has been developed is Bordetella bronchiseptica. The basis for this approach is the concept that if the vaccine is administered by the same route that natural infection takes, greater local protection will be achieved. Unfortunately, these vaccines can produce mild clinical disease.
Because of the concern of development of feline sarcomas secondary to vaccination procedures, specific guidelines have been developed for vaccinating cats. Sarcomas have been associated more with rabies and feline leukemia virus vaccines than others. The suspected incidence of vaccine-induced sarcomas is approximately 1 in 1000 to 10,000 cases per year.
The suggested route of administration of rabies and feline leukemia vaccines is to give the rabies in the right rear leg (over the tibia) and the feline leukemia vaccine over the left tibia by the subcutaneous route. In this way, if a sarcoma does develop, amputation of the limb can be done to save the cat's life.
Age of Patient
The age of the animal is important, not only because of the persistence of colostral antibodies but also because of the relative immaturity of the immune response in the puppy and kitten during the first 2 weeks of life. This phenomenon is at least partially due to the hypothermia that exists during this period. Optimal functioning of the cells of the immune system depends on a normal body temperature. A puppy given vaccination at 8, 12, and 16 weeks and a kitten given vaccination at 9 and 12 weeks should be revaccinated at 1 year of age to ensure adequate response of the immune system to the vaccine.
Nutritional Status
An animal in poor nutritional condition may not respond adequately to vaccination. Generally, caution should be exercised in giving modified live virus vaccines to debilitated animals. However, a debilitated animal should be vaccinated if it is to be hospitalized. Although there is a chance the animal may not respond to the vaccination, it is also possible that the animal will be protected from infection with a virulent organism. If a debilitated dog or cat is vaccinated, vaccination should be repeated when the patient's nutritional status has improved so that immunity is more certain.
Concurrent Disease or Therapy
Occasionally, dogs and cats presented for vaccination are incubating an infectious disease. A detailed history of possible exposure to infected animals and a complete physical examination may suggest this situation. However, it is impossible to definitively diagnose most infections in the incubation stage. If there is a history of exposure to an infected animal, the owner should be informed that there is a risk of their animal developing disease despite vaccination.
Certain infections and diseases may be associated with alteration of the immune system and may interfere with successful response to vaccination; examples include dogs infected with demodectic mange and cats infected with feline leukemia virus or feline immunodeficiency virus.
It has been suggested that certain virus vaccines may increase the susceptibility of the recipient animal to the development of the disease for which one is vaccinating against, if the animal is incubating or infected with another virus simultaneously. For example, dogs infected with the canine parvovirus that are subsequently vaccinated with a modified live distemper vaccine may be prone to develop distemper encephalitis because of infection with the parvovirus.
Modified live virus vaccines are not recommended in dogs and cats receiving immunosuppressive agents. Drugs that suppress the immune system are frequently given to animals with cancer or autoimmune diseases, such as immune-mediated hemolytic anemia. Commonly used immunosuppressive agents include cyclophosphamide, azathioprine, methotrexate, and corticosteroids. When corticosteroids are used at antiinflammatory dose levels (less than 2 mg/kg of body weight), the response to virus vaccines is not altered.
Program Guidelines
When all the clinical factors discussed are considered, along with economic factors, it is safe to conclude that there is no single perfect vaccination program. Nonetheless, certain general guidelines are possible. Every veterinary hospital should establish a specific vaccination policy and protocol and adhere to it at all times. This will prevent errors of omission that could result if the vaccination policy is not clearly defined. Usually, the first vaccination should be administered when the animal is between 6 and 8 weeks of age. Animals should be revaccinated at 10 to 12 and 16 to 18 weeks of age. New vaccine guidelines recommend that revaccination should occur at 1 year of age or 1 year after the last puppy or kitten vaccine. Annual revaccination is unnecessary for most viral diseases, but for others it is of critical importance. Every 3 years vaccination is recommended for dogs and cats over 1 year of age that were properly vaccinated as puppies and kittens. Rabies vaccination could be given every 3 years as well, but must be given based on the local county regulations, which can be as often as yearly.
Vaccine Reactions
Anaphylactic reactions to vaccines can occur after vaccinations are given. Typically it will occur on the second or third vaccination in the puppy or kitten series. Severe anaphylactic reactions occur immediately and up to 30 minutes after the injection. Severe reactions cause cardiovascular shock and respiratory arrest that can lead to death if not treated immediately with intravenous (IV) corticosteroids, epinephrine, and fluids. Vomiting, diarrhea, and urticaria are early signs of a reaction and should be treated immediately with IV fluids and corticosteroids. Milder reactions of vomiting and diarrhea should be noted in the medical record as a possible vaccine reaction. In severe life-threatening reactions, the animal should never be vaccinated again. In milder cases, the animal should be premedicated with an antihistamine (diphenhydramine hydrochloride) 20 minutes before the vaccination and monitored for at least 30 minutes after the vaccination before leaving the clinic. Many puppies and kittens will be lethargic or sleep more than usual the day after their vaccinations, and this is normal. Neurologic signs such as seizure may occur in dogs within a few weeks after vaccinations and may be due to the distemper vaccine. This form of vaccine-related distemper is rarely fatal and usually resolves with treatment. Polyarthritis in cats may occur within weeks after vaccination and may be due to the calicivirus vaccine. Owners may notice a lump or hard nodule at the vaccination site, which may last for a few weeks. If it is present longer than 3 weeks, a biopsy should be taken to identify whether it is neoplasia or just a tissue inflammatory reaction.
PET-ASSOCIATED ZOONOSES
A zoonosis is a disease of animals that is transmissible to humans under natural conditions. The technician is frequently questioned by clients about the public health significance of animal diseases. Hospitalized animals may represent potential sources of zoonotic infection; thus these infections may be considered occupational diseases.
It is beyond the scope of this section to discuss all the pet-associated zoonoses, but several of the more important infections are described. It is important to stress that when questions about human medical care arise, a physician should be consulted.
Canine brucellosis rarely occurs in humans. Transmission from an infected dog to a human can occur by contact with blood, urine, semen, milk, and infected tissues. Vaginal discharges, aborted fetuses, and placental material after abortion contain large numbers of bacteria. Infection in humans can be an insidious, chronic disease that resembles infection with other strains of Brucella, or it can result in relatively mild flulike symptoms.
Toxoplasmosis can be acquired by human exposure to cat feces containing infective oocysts. Cats are an obligate host in the life cycle of Toxoplasma. Toxoplasma oocysts can remain viable in the environment for as long as 6 months under ideal conditions. The following recommendations to reduce the exposure hazard from toxoplasmosis-infected cats should be followed.
• Plastic gloves should be worn when cleaning litter pans or handling potentially contaminated soil.
• Children's sandboxes should be covered, and basic principles of sanitation should be followed.
• Immunodeficient people and women of child-bearing age should exercise extreme caution to reduce the risk of exposure.
• Women contemplating pregnancy should have their antibody status determined by a physician.
Those with a significant titer against toxoplasmosis are probably protected from reinfection. Antibody titers in cats are of little value because they indicate exposure to the organism and do not indicate that the cat is currently infected or is actively shedding infective oocysts. An enzyme-linked immunosorbent assay (ELISA), currently available through the University of Georgia and Colorado State University veterinary schools, identifies immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies in a cat's serum and may provide evidence for an acute or a recent infection in a cat. It should be stressed to the concerned client that eating raw or improperly cooked meat probably is the most common source of human toxoplasmosis.
Campylobacter and Salmonella are bacteria that can produce pet-associated zoonoses. Pets appear to be relatively infrequent sources of Campylobacter. When pets are incriminated, it is usually a stray or recently adopted puppy or kitten that has had recent diarrhea. The incidence of Salmonella infection acquired from pets is unknown. Animals can be asymptomatic shedders of this organism for an average of 6 weeks. Because the route of transmission is the fecal-oral route, good sanitation is important.
Reports of human leptospirosis attributed to vaccinated pets have appeared in medical literature. The Leptospira bacteria that are used for routine immunization may not protect against subclinical infection and shedding of the organisms in the urine. Because transmission is via infected urine, good sanitation is essential.
Visceral larva migrans and cutaneous larva migrans are caused by the migration of animal parasite larvae in human hosts. The technician plays an important role in prevention by educating clients about the risks posed by pets infected with intestinal parasites. Treatment of infected animals and reducing environmental contamination will reduce the incidence of these problems.
Plague (Yersinia pestis) is an infectious disease of animals that is transmitted to humans by the bite of an infected ectoparasite, usually the flea. Although the majority of cases in humans result from exposure to infected wild rodents, domestic cats have been associated with a number of infections in humans. Infections have been reported in persons employed in veterinary hospitals. Cats with suppurative lymphadenitis (infected draining lymph nodes) should be considered plague suspects, and caution should be exercised by the veterinary technician when handling exudates or treating draining wounds.
Cat-scratch disease is a disease of humans that usually is associated with cat scratches or close contact with cats. Rarely, exposure to cats has not occurred, and other injuries are incriminated, such as splinters, thorns, or dog scratches. The causative agent is Bartonella henselae. It is presumed that cats simply act as vectors for the disease because they are not ill. Multiple cases in the same household have occurred over a period of months or even years. In immunocompromised patients (e.g., humans infected with the human immunodeficiency virus), the disease can cause severe problems and therefore may pose a significant risk to these individuals. Usually, the disease in humans is a mild, self-limited problem.
Rabies is an acute, fatal viral disease of the central nervous system that affects all mammals. Rabies is transmitted by infected secretions, usually saliva. In the United States, the skunk and bat are the most important sources of human exposure. However, raccoons, foxes, and unimmunized dogs and cats may also represent a hazard. In most areas of the world, the dog is the most important vector of rabies.
If human exposure to rabies is suspected, a physician or public health official should be consulted immediately. Technicians should be familiar with local laws governing the handling of animals who have bitten humans. Veterinarians and technicians that have a high exposure to rabid animals should be vaccinated for rabies virus with the human vaccine and have their serum titers checked periodically to ensure adequate protection.
Animal bites can cause serious infectious complications, including cellulitis, lymphangitis, soft tissue abscesses, osteomyelitis, meningitis, and bacteremia. Humans who have undergone splenectomy are at particular risk of bacteremia and possibly death if the organism known as DF-2, isolated from the nasal and oral secretions of healthy dogs, is inoculated into tissues by a bite. Animal bites in the veterinary hospital should be washed thoroughly with a disinfectant solution (chlorhexidine) and examined by a physician for a prescription of antibiotics.
OPHTHALMOLOGY
Glaucoma is defined as an increase in intraocular pressure. Glaucoma may cause blindness, and there are certain breeds predisposed to primary glaucoma (Box 21-3).
The signs in early glaucoma are often subtle and can be variable. Acute glaucoma is a painful process; signs include tearing, sensitivity to bright light, and pawing at the eye. Inspection of the eye may reveal congested episcleral blood vessels, a dilated nonresponsive pupil, and a cloudy cornea. In chronic glaucoma, the major finding is an enlarged globe.
The diagnosis is made by documenting an increased intraocular pressure. Several methods are used to measure intraocular pressure. Tonometers are the most accurate, but some are expensive. The Schiotz tonometer is useful and costs approximately $300 to $400, which is well within the means of most veterinary practices (Figure 21-13). Tonopens are less cumbersome to use but can be cost prohibitive in smaller practices, costing approximately $1200.00 each (Figure 21-14).
FIGURE 21-13 Tonometer (Schiotz Tonometer) used for measurement of intraocular pressure. The tonometer is placed on the cornea to obtain the pressure reading.
FIGURE 21-14 Tono-pen XL (Mentor) used for measure of intraocular pressure. Improved tonometer that is less cumbersome to use.
Glaucoma is considered a medical emergency because delay in treatment may result in permanent damage to the eye. Several drugs are available to treat glaucoma, all of which work by either reducing aqueous production or increasing the opening at the drainage angle.
CATARACTS
A cataract is a focal or diffuse opacity within the lens and its capsule. Cataracts may be hereditary or nonhereditary. They should be differentiated from nuclear sclerosis, which is a normal aging change that decreases the clarity of the nucleus of the lens.
Inherited cataracts occur in many breeds and may be associated with other eye abnormalities. Different modes of inheritance have been reported in different breeds. Breeds reported to have inherited cataracts include the beagle, German shepherd, golden retriever, Labrador retriever, Afghan hound, American cocker spaniel, Boston terrier, poodle, and miniature schnauzer. Inherited cataracts have not been reported in the cat.
Cataracts can be the result of metabolic abnormalities, such as diabetes mellitus, inflammation, or trauma. Inflammatory diseases associated with cataracts include feline infectious peritonitis, feline leukemia virus, leptospirosis, and systemic mycoses.
There is no successful medical treatment for cataracts, but any associated inflammation should be treated. Medications that dilate the pupil may be helpful in improving vision in cases of immature or hypermature cataracts. Currently, the only effective therapy for cataracts is surgical removal of the lens.
CORNEAL ULCERS
Superficial corneal ulcers may result from trauma, decreased tear production (keratoconjunctivitis sicca), aberrant eyelashes (distichiasis, districhiasis), inward rolling of the eyelid (entropion), and inability to blink. Animals with superficial corneal ulcers experience a significant amount of pain. This pain is manifested as excessive tearing, sensitivity to bright light, and squinting (blepharospasm). Corneal ulcers are diagnosed by using fluorescein dye. Fluorescein is a water-soluble dye that will not stain the epithelial layer, but stains the underlying layers if the superficial epithelial layer is damaged.
Treatment should be directed first toward correcting the underlying cause. Once this has been accomplished, epithelialization of the ulcerated area is rapid and usually uncomplicated. Broad-spectrum antibiotics are generally used to eliminate infection. Antibiotic solutions and ointments that contain a corticosteroid should never be used with a corneal ulcer. Corticosteroids cause decreased healing with resultant deepening of the ulcer and possible corneal rupture. It has been shown that ointments may retard healing more than solutions; however, the difference in healing may not be clinically significant. One advantage to solutions is that the dose can be more easily controlled. Ophthalmic drops are applied by opening the upper and lower eyelid with one hand, tilting the patient's head slightly back, and then squeezing 1 to 2 drops of the solution into the eye with the other hand. Ophthalmic ointments are applied by the same restraint technique and then squeezing  inch of the ointment onto the cornea of the eye and then closing the eyelids shut for a moment to spread the medication. Systemic medications usually are not necessary with corneal ulcers. Occasionally, a surgical flap over the cornea or a special contact lens is necessary until epithelialization is complete.
inch of the ointment onto the cornea of the eye and then closing the eyelids shut for a moment to spread the medication. Systemic medications usually are not necessary with corneal ulcers. Occasionally, a surgical flap over the cornea or a special contact lens is necessary until epithelialization is complete.
DERMATOLOGY
Veterinary dermatology is an important part of small animal practice. Veterinary dermatology is a challenging discipline; although there are many causes of skin disease, there are only a limited number of ways in which the skin can react. Consequently, in many cases a specific etiologic diagnosis can be difficult to make.
It is beyond the scope of this chapter to consider all the common dermatologic diseases of dogs and cats.
CARDIOLOGY
Congestive heart failure is a clinical term used to describe the state when the heart is unable to maintain adequate cardiac output. Because of decreased cardiac output, the body's tissues do not receive sufficient blood supply for normal function. The decreased cardiac output and the resultant increase in pressure within the vessels entering the heart stimulate complex compensatory mechanisms that contribute to the clinical signs of congestive heart failure. The term congestive heart failure does not indicate a specific cause.
Tachycardia and cardiomegaly (heart enlargement) are general signs associated with congestive heart failure. However, depending on the principal site of involvement, signs of left or right ventricular failure will predominate.
Left ventricular failure results from dysfunction of the left atrioventricular valve (mitral valve), ventricle, or both. Clinical signs associated with left ventricular failure include cough, exertional dyspnea, orthopnea, and at times syncope. Characteristically, early in left ventricular failure, the cough occurs in paroxysms and at night or in the early morning. The cough in left ventricular failure is usually secondary to the development of pulmonary edema or occurs because the left atrium has enlarged and compressed the left main-stem bronchus. Exertional dyspnea refers to labored breathing associated with increased activity. This may be manifested as decreased exercise tolerance or reluctance to exercise. Orthopnea means difficult or labored breathing in the recumbent position. Pulmonary edema refers to the accumulation of abnormal fluid in the interstitial spaces and alveoli of the lungs. It can be detected by auscultating rales (crackles) in the lungs or by observing the characteristic pattern on chest radiographs. Syncope, or fainting, results from decreased cardiac output to the brain.
Right ventricular failure results from a pathologic condition of the right atrioventricular valve (tricuspid valve), right ventricle, or both. Clinical signs associated with right ventricular failure include hepatic enlargement, ascites, pleural effusion, and subcutaneous edema. Increased pressure in the abdominal veins results in congestion and enlargement of the liver. Increased hydrostatic pressure in capillaries results in leakage of fluid and the subsequent development of ascites, pleural effusion, and subcutaneous edema. Subcutaneous edema is a relatively rare sign in the dog and is seen late in the course of the condition. (Box 21-4 provides a list of signs seen with left and right ventricular failure.)
Certain cardiovascular problems result in both left and right ventricular failure. Obviously, the signs described are not specific for heart disease. Consequently, when evaluating a patient for cough or ascites, the conditions to rule out should include noncardiac problems.
MITRAL INSUFFICIENCY
Mitral insufficiency resulting from chronic mitral (left atrioventricular) valvular fibrosis is the most frequently diagnosed form of heart disease in the dog. It is followed in prevalence by chronic tricuspid (right atrioventricular) valvular fibrosis, which causes tricuspid insufficiency. Valvular insufficiency is a term used to indicate functional incompetence (leakage) of the valve with subsequent regurgitation (backward flow) of blood from the ventricle into the atrium during ventricular systole.
The signs associated with chronic mitral insufficiency are those of left ventricular failure (e.g., cough, exertional dyspnea, pulmonary edema). The specific cause of mitral valvular fibrosis is unknown; however, it appears to be associated with aging. Certain breeds appear to be predisposed, the majority of these being small breeds of dogs (e.g., miniature poodles). Mitral insufficiency as a cause of left ventricular failure is much less common in the cat. The diagnosis of chronic mitral insufficiency is based on the clinical history, auscultation of the heart and lungs, thoracic radiography, and electrocardiography. Although the traditional treatment for this condition has included the use of cardiac glycosides (e.g., digoxin), recent evidence indicates that cardiac contractility is normal to increased in the majority of these dogs, and therefore digoxin is not indicated until late in the course of the failure state.
Initially, the use of diuretics such as furosemide (Lasix), a sodium-restricted diet, and exercise restriction are the primary mode of therapy. Treatment with vasodilators, such as hydralazine (Apresoline), captopril (Capoten), and enalapril (Enacard), is also beneficial. These drugs work by decreasing the resistance against which the heart has to pump.
HEARTWORM DISEASE
Information on the life cycle and diagnostic procedures can be found in Chapter 17, Parasitology.
The treatment of heartworm disease can be divided into three phases. The first phase is to kill the adult heartworms (adulticidal therapy) that are present in the heart and blood vessels. The next phase is to eradicate the circulating microfilariae (microfilaricidal therapy). Finally, preventive medication (prophylactic therapy) is administered to those dogs at risk of developing heartworm disease. This would include any dog residing in or traveling to an endemic area.
Adulticide therapy consists of administering melarsomine dihydrochloride (Immiticide, Merial). It is an arsenical compound given by intramuscular injection into the epaxial muscles in the lumbar region. Two treatments are given 24 hours apart. If needed, a second treatment can be given 4 months later. In the clinically ill dogs, treatment to stabilize the disease is given, and then at a later date, the patient is treated with adulticide in two stages. One injection is given and then 1 month later two injections are given 24 hours apart. This allows a slower kill of heartworms with less chance of pulmonary reaction to dying worms.
The adult heartworms will die slowly over a 2- to 3-week period. Fever, coughing, and, in more severe cases, dyspnea and hemoptysis (coughing up blood) are the signs observed as the worms die and pass to the lungs (pulmonary thromboembolism). Prednisone therapy (1 mg/kg) is the accepted therapy for pulmonary thromboembolism. Administration of aspirin therapy (5 mg/kg once daily) is recommended in dogs with moderate to severe heart-worm disease to reduce thromboembolism. It may be started 1 week before treatment and continued for 4 to 6 weeks after treatment.
To minimize the development of clinical pulmonary thromboembolism, it is important to restrict exercise for 3 to 4 weeks after completion of adulticide therapy. If the signs associated with pulmonary thromboembolism are severe, hospitalization and the administration of bronchodilators, antiinflammatory drugs, and antibiotics are recommended. DIC may occur in dogs with severe clinical signs. Treatment of advanced DIC is usually unsuccessful.
Microfilaricidal therapy is begun 3 weeks after adulticide therapy. Ivermectin (Ivomec), 50 mg/kg orally once, is the current accepted method of treatment for microfilaria, even though it is not approved by the U.S. Food and Drug Administration (FDA) for this function.
Heartworm disease may be prevented with the use of one of several products. Some of these also have protective activity against some endoparasites. Table 21-5 lists the products currently available for heartworm prevention in the dog.
Feline heartworm disease is far less common than canine heartworm disease due to dogs being the natural host for heartworms. In endemic areas, occasionally cats are infected with heartworms. Clinical signs differ from the dog with mild reactions causing occasional vomiting. Severe reactions cause severe respiratory distress because of pulmonary thromboembolism. Most cats with the severe form do not survive. If they do survive, they are treated with corticosteroids for pulmonary thromboembolism and oxygen, and then put on feline heartworm prevention to prevent further infections in the future (see Table 21-5). Currently, there are no safe and effective treatments to eliminate heartworms in the cat. Because the cat is not the natural host, the worms die over a 2- to 3-year period as compared with a 5- to 7-year period in the dog. Preventing further infection during this time may allow the cat to become heartworm free. Diagnosing heartworm infections in the cat is much more difficult than in the dog because of the lower number of worms infecting the cat. In-house antigen tests used in the dog are usually not sensitive enough to pick up antigen in the cat. Thoracic radiographs, angiography, positive antibody titer, and clinical signs are used to diagnose feline heartworm disease.
CARDIOMYOPATHY
Cardiomyopathy is a general term that merely indicates that the basic pathologic lesion involves the heart muscle. Cardiomyopathies can be primary or secondary. Primary cardiomyopathies indicate that the myocardial disease is not due to any recurrent or preexisting cardiovascular or systemic disease. Primary cardiomyopathies in cats are further subdivided into hypertrophic, dilated, and restrictive forms. Secondary cardiomyopathies in dogs and cats are less frequent and are the result of diseases such as infection, metabolic disorders (e.g., uremia), endocrine problems (e.g., hyperthyroidism), and infiltrative processes (e.g., neoplasia).
Feline Cardiomyopathy
Hypertrophic cardiomyopathy is characterized by increased thickness of the myocardium and a small left ventricular lumen. Clinical signs are seen in middle-aged cats of all breeds. The most prominent sign is the sudden development of respiratory distress secondary to pulmonary edema. Hind-limb paresis (weakness) and severe pain may also be present. These hind-limb signs are caused by aortic thromboembolism (blood clots) disrupting the blood supply to the hind limbs. This problem can usually be diagnosed easily if femoral pulses are found to be poor or absent. Diagnosis of cardiomyopathy is based on history, physical examination, radiography, electrocardiography, and echocardiography. If echocardiography is not available, nonselective angiocardiography may be necessary for diagnosis. The basic initial therapeutic approach may include diuretics (e.g., Lasix, Hoechst-Roussel Pharmaceuticals), cage rest, oxygen therapy, β-adrenergic blockers such as propranolol (Inderal, Ayerst Laboratories), and calcium channel blockers such as diltiazem hydrochloride (Cardizem, Marion Merrell Dow). Long-term management consists of diuretics, beta blockers, calcium channel blockers, a sodium-restricted diet (feline H/D, Hill's), aspirin, and restricted activity. Aspirin is used to reduce the likelihood of aortic thromboembolism.
Dilated cardiomyopathy is characterized by extreme ventricular dilation and moderate atrial enlargement. This results in impaired pump function of the ventricle. This type of cardiomyopathy is also known as congestive cardiomyopathy. Signs of right ventricular failure usually predominate. In addition, cats may show a gradual onset of lethargy and anorexia and at times may be brought in dehydrated, hypothermic, and in cardiovascular shock. Respiratory distress secondary to pleural effusion and aortic thromboembolism resulting in hind-limb paresis is also occasionally seen. The basic therapeutic approach is to mechanically remove as much fluid as possible from the pleural cavity (thoracocentesis), administer digitalis (digoxin therapy), and administer diuretics. Aspirin is used as a preventive measure against aortic thromboembolism. Vasodilators, such as nitroglycerin ointment (Nitrol ointment, Kremers-Urban Co.), may have a role in the management of dilated cardiomyopathy.
Some cats with dilated cardiomyopathy have low plasma taurine levels, and cardiac function will increase with oral taurine supplementation of 250 to 500 mg daily. Cardiac function usually improves over a period of months, and if cats are placed on a diet containing ample taurine, cardiac drugs and taurine supplementation may eventually be discontinued. It should be stated that because this association of low taurine levels and cardiomyopathy in the cat has been made, almost all commercial and prescription diets have adequate levels of taurine, so low-taurine dilated cardiomyopathy is much less common than it used to be.
Restrictive cardiomyopathy is the least common form of primary feline cardiomyopathy. A synonym is endomyocardial fibrosis. Respiratory distress is the most common clinical sign. Diagnosis is similar to the other forms of primary cardiomyopathy. Response to therapy is generally poor.
Canine Cardiomyopathy
Primary cardiomyopathies in the dog are categorized as dilated (congestive), boxer cardiomyopathy, Doberman pinscher cardiomyopathy, and hypertrophic cardiomyopathy.
Dilated cardiomyopathy is most common in large and giant breed male dogs aged 4 to 6 years; however, English and American cocker spaniels are smaller-breed dogs that may be affected. Presenting signs often include weakness, lethargy, respiratory distress, cough, anorexia, weight loss, and possibly ascites and syncope. The left ventricle and atrium are dilated with decreased contractility. Diagnosis is confirmed by physical examination, radiography, electrocardiography, and echocardiography. Treatment consists of diuretics, a low sodium diet, arteriolar dilators, and positive inotropes, such as cardiac glycosides (digitalis, digoxin). The long-term prognosis is guarded in that most dogs with the dilated form of cardiomyopathy have an average life span of 6 to 8 months after the diagnosis has been made.
A specific cardiomyopathy occurs in boxers. These dogs may be asymptomatic or have syncope and episodic weakness. Arrhythmias are common and may cause sudden death. Diagnosis is confirmed by the same methods as those used in dogs with dilated cardiomyopathy. Treatment with diuretics and antiarrhythmics, such as propranolol, may be useful; however, prognosis is still poor.
Doberman pinschers may have a primary cardiomyopathy that is similar to congestive or dilated cardiomyopathy. Ventricular contractility is often severely compromised, and atrial arrhythmias are common. These dogs are often in fulminant congestive heart failure and require supportive care with oxygen, diuretics, positive inotropes, and vasodilators. Prognosis is poor.
Hypertrophic cardiomyopathy is the most uncommon primary cardiomyopathy in the dog. It is most often seen in German shepherd dogs and other large breeds. Presenting signs are referable to cardiac disease, and sudden death may occur. Treatment with diuretics and propranolol may improve cardiac output and clinical signs.
ENDOCRINOLOGY
Canine hyperadrenocorticism (Cushing syndrome) is a disorder that results from the excessive production of cortisol by the adrenal cortex. The clinical signs of canine hyperadrenocorticism include polyuria, polydipsia, abdominal distention, polyphagia, muscular weakness, dermatologic changes, and reproductive problems (anestrus, testicular atrophy). Cushing syndrome can result from excessive production of adrenocorticotropic hormone (ACTH) by the pituitary gland (pituitary-dependent hyperadrenocorticism) or from a functional tumor of the adrenal cortex. Pituitary-dependent hyperadrenocorticism is by far the most common, comprising approximately 80% of the cases. Diagnosis is based on measurements of the plasma cortisol levels after stimulation with ACTH or suppression with dexamethasone. Treatment is different for these two conditions. If a functional adrenal tumor is present, the recommended treatment is surgical removal. The drug used to treat Cushing syndrome caused by excessive ACTH production is mitotane (Lysodren, Bristol Laboratories). Side effects associated with the use of mitotane include anorexia, lethargy, vomiting, and depression. Hyperadrenocorticism is rare in the cat and usually is suspected because of the secondary consequences of the disease such as insulin resistance in a cat being treated for diabetes mellitus. Although there is much less known about this disease in the cat, diagnosis is similar to the dog. Treatment at this time is surgical removal of the adrenal glands. Medical therapy has not been as effective in the cat.
Canine hypoadrenocorticism (Addison's disease) is caused by a lack of glucocorticoid and/or mineralocorticoid levels and activity. It is generally seen in the middle-aged female, and the most common signs are gastrointestinal (vomiting, anorexia), weakness, depression, and collapse. These signs may have a waxing-waning course.
In an acute crisis these patients may present in acute collapse and in hypovolemic shock. The classic laboratory abnormalities are a low serum sodium (Na+) level and a high serum potassium (K+) level, resulting in a low Na+/K+ ratio (usually less than 25:1). These patients may also be azotemic and have a low urine specific gravity, which could be confused with renal failure.
The most accurate means of diagnosis is to perform an ACTH stimulation test and show that the patient has a very poor response to this drug because cortisol levels will not increase following ACTH administration.
The treatment consists of aggressive isotonic saline fluid therapy and supplementation with glucocorticoid and mineralocorticoid therapy. Prednisolone sodium succinate and desoxycorticosterone are the glucocorticoid and mineralocorticoid used to treat this disease. Addison's disease in the cat has not been reported.
HYPOGLYCEMIA
Canine hypoglycemia is a clinical problem associated with a variety of diseases rather than a specific diagnosis itself. The signs associated with hypoglycemia include weakness of the rear legs, generalized weakness, focal or diffuse muscle twitching, incoordination, blindness, generalized seizures, and behavioral changes. These behavioral changes include aggressive behavior and anxiety as evidenced by incessant running, barking, and loss of bowel and bladder control. These signs tend to be episodic, regardless of the cause of hypoglycemia. Hypoglycemia should be considered a differential diagnosis in any dog that is having seizures or is comatose.
The first step in evaluating a patient with suspected hypoglycemia is to verify or document that hypoglycemia exists. Improper handling of blood samples may result in falsely low blood glucose levels. The blood glucose level can be lowered if the serum is not removed from the clot or if the specimen is stored at room temperature for a prolonged period. It is preferable to remove serum from the clot within 10 to 15 minutes of drawing the blood sample. If this cannot be done, use of sodium fluoride tubes may be helpful.
Once hypoglycemia has been verified, the signalment, history, clinical findings, and further laboratory tests may be needed to reduce the long and rather diverse list of conditions that may cause hypoglycemia. Functional β-cell tumors (insulinomas of the pancreas), nonpancreatic tumors, hypoglycemia-ketonemia in pregnant bitches, glycogen storage diseases, septic shock, liver failure, juvenile and neonatal hypoglycemia, canine parvoviral diarrhea, and excessive insulin administration in diabetic patients are all examples of diseases that can cause hypoglycemia.
HYPOTHYROIDISM AND HYPERTHYROIDISM
Hypothyroidism is one of the most common endocrine disorders in the dog, but it is rare in the cat. Some of the common clinical signs include oily seborrhea, alopecia, thickened skin, weight gain, constipation, lethargy, and cold intolerance. There are some breeds with an apparent increased incidence of hypothyroidism (Box 21-5). The thyroid-stimulating hormone (TSH) stimulation test used to be the most accurate diagnostic test. TSH is no longer readily available; therefore a combination of three tests (total T4, free T4, and TSH levels) is used to diagnose the routine hypothyroid patient. Treatment of hypothyroidism consists of supplementation with thyroxine (T4).
Hyperthyroidism is the most common endocrinopathy affecting cats older than 5 years of age, but it is rare in the dog. The most common clinical signs of hyperthyroidism are weight loss despite a good appetite, restlessness, hyperactivity, and diarrhea. In many cases, a thyroid nodule can be palpated in the ventrocervical region of the neck. The diagnosis can usually be confirmed by documenting an elevated serum T4 level. Treatment may consist of medical therapy with methimazole (Tapazole, Eli Lilly and Co.), surgical removal of the thyroid nodule, and/or radioactive iodine (131I).
DIABETES MELLITUS
Diabetes mellitus is seen in the older dog and cat, and it is more common in the female dog and the male cat. Common clinical signs include excessive water intake (polydipsia), large volumes of urine (polyuria), weight loss in spite of a good appetite, and rapidly developing lens opacities (cataracts) in the dog. If the dog or cat is ketoacidotic, then weakness, vomiting, depression, and, possibly, coma may develop. The diagnosis of diabetes mellitus is made by documenting hyperglycemia, glucosuria, and, if the animal is ketoacidotic, ketonuria or ketonemia.
The technician's role in the treatment of patients with this endocrinopathy is twofold: (1) management of the ill ketoacidotic diabetic animal in the hospital and (2) education of clients concerning home management and treatment of their pets.
The ketoacidotic diabetic patient represents a true challenge for the veterinarian and technician alike, and it is important that they work in unison so that optimal patient care is achieved. The technician's role involves close monitoring of vital signs, ensuring fluids are given at the proper rate, frequent blood glucose determinations, and administering short-acting (regular/crystalline) insulin (see Table 21-6 for types of insulin). Because the ketoacidotic patient requires such close monitoring, the technician plays a major role in the minute-to-minute and hour-to-hour evaluation of the patient, so minor changes in the patient's condition can be recognized early and the veterinarian be informed. Because of the complexity of the ketoacidotic diabetic patient, all these functions should be done under the direct supervision of a veterinarian.
The second aspect of diabetic management involves the instruction of the client concerning home management of the pet. This can be a time-consuming function, and the technician who has a good understanding of diabetes management can be a tremendous asset to the veterinarian. Examples of areas in which the client should be instructed and/or shown include how to mix the insulin, read the syringes, draw up the insulin into the syringe, give the subcutaneous injection, and read urine test strips for urine glucose measurement. Having the owner practice giving injections to the pet by drawing up sterile saline in the syringe and giving saline injections is extremely helpful to the hesitant owner. In addition, the client needs to be instructed (1) about the type of diet to be fed and how much and when to feed, (2) not to give the insulin if the pet does not eat in the morning, and (3) to give the animal Karo syrup orally and call the hospital immediately if the pet has a seizure. All these items can be compiled into a handout that the technician can develop with the aid of the veterinarian. This handout can then be given to the client, who can refer to it as needed at home.
THERIOGENOLOGY
POSTPARTUM DISORDERS IN THE BITCH
The postpartum bitch may be brought to an animal hospital for a variety of serious problems after whelping. These problems include mastitis, metritis, and eclampsia. Mastitis refers to inflammation of one or more mammary glands. In severe cases, affected glands are hot and painful, and the patient is systemically ill. Bitches with septic mastitis are depressed, anorectic, and reluctant to care for the puppies. In less severe cases, the bitch may not be symptomatic; however, the puppies may fail to gain weight or may show signs of septicemia. Systemic antibiotics are used to treat mastitis. Because the affected glands produce abnormal milk, and the antibiotics excreted in the milk may be harmful to the puppies, it is recommended that the puppies be hand fed.
Severe mastitis may progress to abscess formation or gangrenous mastitis. Surgical drainage and treatment may be required in these cases.
Stasis of milk in the mammary glands can occasionally result in enlarged, painful mammary glands. Galactostasis may be observed during pseudopregnancy or at the time of weaning when the body is attempting to resorb milk. Unlike mastitis, dogs with galactostasis are not systemically ill. Treatment consists of application of cool towels and compresses to decrease inflammation. Care should be taken not to massage the glands because this can stimulate additional milk letdown.
Metritis is a uterine disease of the immediate postpartum period. Signs usually develop within the first week of whelping. Metritis is associated with retained placentae, retained fetuses, and dystocia. Clinical signs suggestive of metritis include fever, depression, and reduced interest in the puppies. A foul-smelling, brown or reddish-brown vaginal discharge may be present; the normal discharge after whelping is nonodorous and greenish. The diagnosis is based on history, clinical findings, and laboratory results. Laboratory tests that are useful include vaginal cytologic studies, CBCs, and bacterial cultures.
Initial therapy consists of replacing fluid deficits, treating shock, if present, and initiating antibiotic therapy after cultures have been obtained. Medical drainage of the uterus can be attempted in valuable breeding bitches. In severe cases, ovariohysterectomy may be indicated to save the bitch's life.
Hypocalcemia (eclampsia) usually occurs 2 to 3 weeks postpartum in small bitches with large litters but occasionally can occur before birth. Presenting signs include weakness and trembling and may proceed to tonic convulsions. The temperature is usually elevated during convulsions.
Diagnosis is based on clinical signs in a lactating female and low serum calcium levels. Treatment includes preventing the puppies from nursing on the dam, giving the dam intravenous 10% calcium gluconate, and ensuring the dam receives oral calcium lactate or calcium gluconate and vitamin D at home. The puppies are hand fed with nursing bottles and milk replacer until 4 weeks old. It is highly recommended that the dam have an ovariohysterectomy because of the high recurrence rate of eclampsia. See Chapter 14 for additional information on animal reproduction.
CANINE BRUCELLOSIS
Canine brucellosis is primarily an infection of the reproductive tract, although other organ systems may be involved. Brucella canis also has been isolated from dogs with discospondylitis and chronic recurrent fever. Brucellosis is a frequent cause of infertility and other reproductive problems in both males and females.
Definitive diagnosis requires demonstration of the organism by a culture of blood or body fluid. Serologic tests can be diagnostic as well. The rapid slide agglutination test is an easy, readily available test; however, false-positive results occur. The rapid slide agglutination can be used as a screen, with positive tests being confirmed using an alternative technique (e.g., agar gel immunodiffusion).
The mode of transmission is venereal. However, infection can also result from the ingestion of infected material, for example, aborted fetuses, placentae, and vaginal discharge. Because of these means of spread, brucellosis can quickly become a kennelwide problem.
Although a variety of antibiotic combinations have been recommended, therapeutic success cannot be guaranteed. After antibiotic therapy, some dogs will continue to harbor the organism and represent a risk to other dogs. Canine brucellosis is considered a possible zoonotic disease. For these reasons, some experts advocate removal of all infected dogs from the premises. Other experts feel that this position is extreme and instead recommend castration or ovariohysterectomy and antibiotic therapy for infected pet dogs.
Because treatment is not always successful, prevention is emphasized. All dogs should be tested before breeding or before introduction into a kennel.
PYOMETRITIS (PYOMETRA)
Pyometritis is a uterine disease that occurs during the luteal (approximately 1 to 2 months after estrus) phase of the reproductive cycle. It occurs in both bitches and queens. Pyometritis may be part of a complex that initially starts with cystic changes in the endometrium and endometrial hyperplasia. Prior estrogen therapy may predispose to pyometritis (see also Chapter 14).
Clinical signs are variable. A vaginal discharge may or may not be present, but, if present, the color of the discharge can be green, yellow, or reddish brown. Bitches with pyometritis frequently will be polydipsic and polyuric. Affected animals can be severely depressed and septic or clinically normal.
An enlarged uterus on radiographs and leukocytosis with a left shift are considered diagnostic. Fluid therapy to correct fluid and electrolyte deficits followed by emergency ovariohysterectomy is the treatment of choice in nonbreeding animals. In valuable breeding bitches, medical treatment with prostaglandin F2a has been advocated to preserve the breeding life of the patient. Treatment with prostaglandin F2a is expensive and potentially dangerous; therefore it should be strictly reserved for dogs of significant breeding value.
CANINE PROSTATIC DISEASE
Prostatic disease is occasionally seen in older intact male dogs. Clinical signs include straining to urinate (stranguria), painful urination (dysuria), blood in the urine (hematuria), and/or difficulty in defecation. The conditions that affect the prostate include benign prostatic hyperplasia, bacterial prostatitis, prostatic abscess, prostatic cyst, and prostatic neoplasia.
The following noninvasive techniques are used to evaluate the prostate: rectal palpation, routine radiology, sonography (ultrasound), urethrography, cytologic studies, and bacterial cultures of prostatic washes or the prostatic fraction of the ejaculate. Frequently it is difficult to differentiate neoplasia, infection, and hyperplasia with these noninvasive techniques. Consequently, surgical exploration and biopsy may be required to establish a definitive diagnosis.
Treatment varies, depending on the specific process. Dogs with benign prostatic hyperplasia respond to castration. Although estrogen therapy reduces the size of the prostate in benign prostatic hyperplasia, it is not recommended because of possible adverse reactions. Finasteride (Proscar, Merck & Co., Inc.) has been shown to be an effective medical treatment for reduction of prostatomegaly secondary to benign prostatic hyperplasia. Prostatic abscesses and cysts require surgical drainage. Bacterial prostatitis and prostatic abscesses are treated with antibiotics. Prostatic neoplasia is generally highly malignant, and treatment is directed toward palliation rather than cure. Some dogs with prostatic cancer may benefit from castration because the tumors possess testosterone receptors.
GASTROENTEROLOGY
Acute gastroenteritis is one of the more common problems seen in canine practice. Some examples of conditions that may cause this problem include dietary indiscretion, viral gastroenteritis, bacterial gastroenteritis, gastrointestinal foreign bodies, gastrointestinal parasites, intussusception, ingestion of toxins, acute pancreatitis, and hypoadrenocorticism. The clinical history, signalment, and physical examination may suggest the diagnosis. Frequently, response to symptomatic therapy is used to assess whether further diagnostic study is warranted. The intensity and degree of symptomatic and supportive care are determined by the severity of clinical signs.
The fundamental decision of whether to hospitalize the patient is based on a number of factors; they include the hydration status of the dog, severity and frequency of vomiting and diarrhea, presence or absence of blood in the vomitus or stool, and presence of fever or profound lethargy. Non–patient-related factors to be considered include the client's ability to provide adequate care for the patient at home and ability of the client to pay for hospitalized care.
Clinical management of outpatients consists primarily of dietary restriction, administration of locally acting gastrointestinal medications, and use of fluid therapy when indicated. Dietary restriction is the most important aspect of the symptomatic care of acute gastroenteritis. The objective is to rest the gastrointestinal tract. This is accomplished by withholding all food for 12 to 24 hours, depending on the details of the case. If vomiting is severe, water is also withheld. If diarrhea is present and vomiting has not occurred, warm electrolyte-containing solutions can be given by mouth. During this period of symptomatic therapy, it is imperative that the patient be observed closely to prevent ingestion of foreign material and detect any worsening of clinical signs.
After food has been withheld for the prescribed period, small, frequent, bland meals should be offered. These meals should be low in fat, low in fiber, and easily digested and absorbed. These criteria are met by prescription diets, such as Prescription Diet I-D (Hill's), and by homemade diets, such as cottage cheese and boiled rice. These diets should be warmed before feeding. These frequent, small, bland meals should be continued for 2 to 3 days. If the patient is doing well, the regular diet and feeding schedule can be gradually reintroduced over the next 3 to 5 days. If clinical signs recur during this process, the dog should be reevaluated. Further diagnosis, evaluation, and more intensive supportive therapy may be warranted.
Although a vast number of locally acting preparations are available for the treatment of acute gastroenteritis, most have not been proved effective in controlled clinical trials. An over-the-counter preparation containing bismuth subsalicylate (Pepto-Bismol) has been shown to shorten the duration of symptoms in humans with experimental viral enteritis. It is theorized that the beneficial response is not due to the coating action of the product but rather to the salicylate inhibiting prostaglandin synthesis. Prostaglandins play a role in diarrhea by affecting both motility and secretory activity of the gastrointestinal tract. The technician should be aware that Pepto-Bismol may cause the stool to be dark to black, giving the false impression that melena is present when it is not.
In animals that are slightly to mildly dehydrated, some form of fluid therapy is appropriate. Fluids can be administered by mouth if the patient is not vomiting. Commercial water and electrolyte solutions, such as Gatorade, can be used to restore hydration and correct electrolyte imbalances. Alternatively, a homemade solution can be prepared inexpensively. One formula that has been recommended consists of 3.5 g of sodium chloride, 2.5 g of sodium bicarbonate, 1.5 g of potassium chloride, and 20 g of glucose added to 1 L of water. Approximately 13.6 ml/kg/day of this solution will meet the maintenance requirements of the patient.
If the dog is mildly to moderately dehydrated or is vomiting, subcutaneous fluid is indicated. Lactated Ringer's solution or Normosol are the fluid of choice. If signs have been prolonged, the lactated Ringer's solution can be supplemented with potassium chloride. Generally, the dose of subcutaneous fluid is 4.5 to 9.0 ml/kg of body weight administered at multiple sites. This can be repeated if necessary.
Client education is an essential part of the symptomatic care for acute gastroenteritis. The client should be informed that a definitive diagnosis has not been established and that merely the symptoms are being treated. If the animal is getting worse or if the signs persist longer than 36 to 48 hours, the animal should be reevaluated. The technician should have a concerned, caring attitude during the outpatient visit so that if signs persist, the client will not hesitate to return or call for additional help. In many practices, it is standard procedure to telephone the client to receive follow-up progress reports. This ensures close client contact and thus improves the chances of successful management of the problem.
If initial clinical signs are severe or there is no response to symptomatic therapy, hospitalization is necessary. A major indication for hospitalization is the need for intravenous fluid therapy. Details about intravenous fluid therapy have been discussed.
Medications that alter the motility of the gastrointestinal tract may be indicated in cases of severe gastroenteritis. Improved understanding of the pathophysiology of intestinal motility has resulted in the more rational use of medications that are used to symptomatically treat vomiting and diarrhea. Anticholinergics cause hypomotility of the intestines and thus are of questionable efficacy in treating diarrhea. Antispasmodics are of minimal benefit as well.
Narcotics and narcotic-like drugs increase the rhythmic segmental contractions of the bowel, slow the passage of ingesta, and thus help to control diarrhea. These drugs should be used cautiously because of potential problems. Generally, they are reserved for more chronic or severe cases that are unresponsive to conservative therapy. A major disadvantage of the narcotic derivatives is that they can cause central nervous system depression. The decreased ingesta flow rate may result in increased absorption of toxins and altered bacterial flora in the gut. These compounds are contraindicated in the presence of intestinal obstruction.
Drugs used for the treatment of acute vomiting can be divided into several categories (Box 21-6).
Drugs used to decrease gastric acidity include antihistamines or H2 blockers, such as cimetidine (Tagamet, SK&F Lab Co.), ranitidine (Zantac,) and famotidine (Pepcid AC). Antacids do not decrease the secretion of acid; however, they neutralize the acid that is produced. Antacids must be given frequently because their duration of action is brief. Paradoxically, if antacids are not given frequently, total daily acid secretion increases. Antacids administered according to a schedule of two or three times per day are probably of no value and may, in fact, be harmful. In most practices, more frequent administration is not practical. Antidopaminergic drugs such as metoclopramide (Reglan) inhibit vomiting at the vomiting center in the central nervous system. Metoclopramide is contraindicated in intestinal obstruction because of its prokinetic or motility-stimulating activity. Phenothiazine-derivative tranquilizers, such as chlorpromazine, also work on the vomiting center of the central nervous system. These drugs are effective at controlling vomiting at much lower doses than the usual tranquilizer doses. These agents should be used with caution in dehydrated patients because of their blood pressure-lowering effects. Other antihistamines such as Dramamine act by inhibiting a neural center involved in vomiting called the chemoreceptor trigger zone. Vomiting induced by certain drugs, such as digoxin, is mediated by this center. Vomiting caused by motion sickness or vertigo may also respond to drugs in this group. Box 21-7 lists drugs used commonly for acute gastrointestinal disease.
When the patient has improved, oral fluid and frequent, small, bland meals can be instituted. After discharge from the hospital, the dog can be treated as already described under outpatient management.
CANINE VIRAL ENTERITIS
The most important cause of viral enteritis in the dog is canine parvovirus. Other viral agents can occasionally produce gastroenteritis; they include canine distemper and canine rotavirus.
Clinical signs vary from subtle lethargy and anorexia to severe, rapidly fatal hemorrhagic gastroenteritis. Dogs of any age can be affected; however, the more severe cases typically occur between 6 and 20 weeks of age. On physical examination, the pups are usually febrile, depressed, and dehydrated. Vomiting or diarrhea may be observed. The stool may be watery, watery with flecks of blood, or severely hemorrhagic. Occasionally, infected dogs will display abdominal tenderness or pain. The presence of fever is more commonly associated with parvovirus than with other enteric viruses. A history of vaccination does not rule out viral enteritis because maternal antibodies may have prevented a protective immune response to the vaccination.
Hemograms are usually normal with coronavirus enteritis but may be abnormal with parvovirus enteritis. Transient leukopenia is present in roughly one third to one half of dogs with parvovirus infections. Severely leukopenic patients may develop secondary infections because of a compromised immune system.
Plain abdominal radiographs do not reveal specific changes. Gastrointestinal contrast study changes may mimic small bowel obstruction. Abnormalities include dilated loops of bowel, tremendously prolonged passage time, and gas-capped fluid lines.
Definitive diagnosis is possible by several techniques. The viruses may be detected in the stool by electron microscopy. An ELISA performed on the feces can detect parvoviral antigen and can be used to demonstrate the virus in the feces during the period of active viral shedding. This period corresponds to the clinical illness. An easy-to-perform in-house test is available to check for parvovirus antigen in the stool (Probe-Canine Parvovirus Antigen test kit, Idexx Labs).
It should be stressed that the treatment of canine viral gastroenteritis is supportive because there are no effective antiviral agents. Treatment includes aggressive intravenous fluid therapy, antibiotics, injectable antiemetics, and keeping the animal clean and comfortable. One other complication seen with parvovirus infection, to which the technician should be alert, is the development of hypoglycemia. If profound weakness and/or seizures develop, a blood glucose level should be determined.
A myocardial form of canine parvovirus has been described in young pups. This form of the disease is characterized by sudden death in otherwise healthy pups; however, it is becoming less common. This may be because most pups have maternal antibodies at the critical period when they are susceptible to the myocardial form.
Canine parvovirus is highly contagious. The major route of the infection is fecal-oral. Dogs showing clinical signs will shed large numbers of viral particles for 1 to 2 weeks. The canine parvovirus is hardy; therefore once the environment is contaminated, infective virus will survive for prolonged periods. The virus has been shown to remain infectious in dog feces held at room temperature for longer than 6 months.
Good sanitation will reduce the numbers of infective virus in the hospital environment. Keeping infected patients isolated from other patients and wearing disposable gloves, gowns, and shoe covers every time the patient is handled will prevent spread of the virus within the hospital. Dilute hypochlorite (chlorine bleach and water, diluted to a ratio of 1:32) solutions have significant viricidal properties. However, because the virus is ubiquitous, the best means of prevention is an appropriate immunization program.
NEPHROLOGY AND UROLOGY
A urolith is a pathologic stone formed from mineral salts found in the urinary tract. Clinical signs depend on location, number, size, shape, and whether there is concurrent urinary tract infection. Urolith classification is generally based on the predominant mineral component, such as phosphate or urate. In the dog, more than 90% of uroliths are located in the bladder and urethra and fewer than 10% are located in the kidneys. Although uroliths can occur in any breed, some breeds suspected to be at greater risk include the miniature schnauzer, Dalmatian, dachshund, pug, English bulldog, Welsh corgi, basset hound, Pekingese, and Scottish terrier.
If the urolith is located in the bladder, there may be no clinical signs, but more commonly stranguria, increased frequency of urination (pollakiuria), and hematuria will be seen. If the urolith is in the urethra, there may be frequent attempts to urinate and dribbling of urine. If the urethra is completely obstructed by the stone or stones, abdominal distention, pain, anorexia, depression, and vomiting will be observed.
Laboratory findings generally are not specific for uroliths. Radiology, including contrast studies such as cystograms and pneumocystograms or ultrasound, may be necessary to establish the diagnosis. Generally speaking, uroliths are managed surgically. A prescription diet (S/D, Hill's) has been advocated as a means of medically treating phosphate uroliths. The diet is high in sodium and low in protein and phosphorus and has an acidifying effect on urine. Dissolution of the uroliths occurs over a period of weeks. Unfortunately, this medical approach has several important limitations. A prescription S/D diet is effective in the dissolution of only phosphate calculi and is not recommended as a long-term maintenance diet.
The overall recurrence rate for bladder stones is high, approximately 25%. Therefore efforts to reduce the chance of recurrence are very important. The first step is to analyze the mineral composition of the stone because different stone types are managed differently. It is also important to determine whether infection is present and, if so, which antibiotics are most likely to be effective.
Several preventive measures are appropriate regardless of the stone type. These include elimination of any infection and stimulation of increased urine output. The urine output can be increased by salting the diet and thereby increasing water intake.
Depending on the specific stone type, it may also be desirable to initiate dietary therapy and modify the urine pH. Ammonium chloride is commonly used to acidify the urine, and sodium bicarbonate is used to alkalinize it.
Because the recurrence rate for uroliths is high, client education is extremely important. First, long-term therapeutic compliance will be achieved only if the importance of these measures is stressed to the client. Second, the owner should be aware of signs that indicate recurrence of the problem.
FELINE LOWER URINARY TRACT DISEASE
Feline lower urinary tract disease (FLUTD) is the term used to describe a condition of unknown etiology in cats characterized by dysuria, hematuria, pollakiuria, urinating in uncommon places, and occasionally urethral obstruction. Urethral obstruction, if it occurs, is potentially fatal because of the associated severe metabolic derangements. The emergency treatment of feline urethral obstruction is covered in Chapter 33.
Because recurrence of the urethral obstruction is frequent, some clinicians prefer to routinely use indwelling urethral catheters for a brief period of time after relief of the obstruction. The justification for the use of indwelling catheters is to maintain urine flow without the trauma associated with recatheterization and manual compression of the bladder. Indwelling urethral catheters should be used judiciously because of the risk of ascending urinary tract infection and catheter-induced injury to the bladder or urethra. Complications associated with the use of indwelling catheters can be minimized if an appropriate catheter is selected. Commercially manufactured polypropylene catheters (Sovereign tomcat catheters and open-end tomcat catheters, Sherwood Medical Industries) can be either too short or too long. Therefore care should be taken to select a catheter with an appropriate length. Soft, flexible polyvinyl catheters, such as the Sovereign sterile disposable feeding tube and urethral catheter, are preferred because of decreased damage to the urethral and bladder mucosa. To pass these catheters, they are kept frozen until immediately before use. This will make the catheter sufficiently rigid to allow passage in a male cat. The catheter should be well lubricated before passage.
Indwelling urethral catheters are generally secured by suturing the catheter to the prepuce. Adhesive tape is attached longitudinally and transversely to the end of the catheter. If the catheter is wet when the tape is applied, it may not stick. Two simple interrupted sutures on either side of the prepuce penetrate the tape and thus prevent movement of the catheter. If analgesia is required to place the sutures, the prepuce can be numbed by applying an ice cube for 1 or 2 minutes. When the catheter is sutured in place, it should be done in such a way that there is no chance of kinking. An Elizabethan collar should be used to prevent the cat from removing the indwelling catheter.
To prevent ascending urinary tract infection, sterile technique is required when placing and maintaining the indwelling catheter. The collection apparatus should be a closed, sterile system. The entire system—catheter, plastic tubing, and collection bottle—must be sterile initially and must be kept sealed to prevent bacterial contamination. Povidone-iodine ointment should be applied several times daily at the point at which the catheter exits the urethra.
Indwelling urethral catheters should be used for as brief a time as possible. The prophylactic use of antimicrobials does not reduce infection. If infection does develop, it is frequently caused by an organism resistant to the prophylactic antimicrobial.
Because the recurrence rate for feline urologic syndrome is high, preventive measures are an important aspect of its medical management. Unfortunately, because the etiology of feline urologic syndrome is unknown, preventive measures are largely empirical. The most frequently recommended preventive measures include providing an ample supply of fresh, potable water, cleaning the litter pan frequently, and lightly salting the food to increase water intake and thus urine volume. The most common urinary crystals and stones are magnesium ammonia phosphate (struvite) and calcium oxalate. To prevent struvite crystals/stones, exclusive feeding of diets that contain 20 mg of magnesium per 100 kcal or less and that maintain a urine pH of 6.4 or less is the most important preventive measure. Certain diets, such as C/D or Feline Maintenance (Hill's), meet this requirement. Although urinary acidification with ammonium chloride has been recommended, it should be emphasized that some diets, such as the ones mentioned above, cause urinary acidification, and additional acidifiers are contraindicated. The basis of acidifying the urine is to increase the solubility of this crystalline material, which is incriminated as the cause of feline urologic syndrome.
If ammonium chloride is used with a nonacidifying diet, it should be thoroughly mixed with the food to improve palatability. It should also be administered with every meal. Any change in diet or introduction of a food additive, such as ammonium chloride or salt, should be done gradually over several days. This will reduce the chances of the cat rejecting the new or altered food. Enteric-coated ammonium chloride tablets are not effective in the cat.
In recent years, calcium oxalate bladder stones have become recognized in the cat as a new cause of FLUTD. Calcium oxalate stones are more likely to form in an acid urine, and therefore cats that eat an acidifying diet may be at risk for the formation of calcium oxalate stones. Cats at risk for forming both struvite and calcium oxalate stones can be managed on C/D Multicare (Hill's) diet.
In addition, some cats with FLUTD have no definable cause but may benefit from drugs, such as amitriptyline (Elavil) or glycosaminoglycans (Adequan).
CHRONIC RENAL FAILURE
Animals in renal failure should be fed diets containing reduced quantities of high-quality protein and adequate nonprotein calories. This can be accomplished by using prescription diets such as K/D (Hill's) or NF (Purina). These are moderate protein-restricted diets available for dogs in canned, semimoist, and dry forms. Feline K/D and NF canned and dry are products suitable for use in uremic cats.
If desired, homemade diets can be used. The following is a recipe for a moderately low protein diet for dogs:
The meat should be braised, retaining the fat, and thoroughly mixed with the other ingredients. This recipe will meet the daily requirements of a 13.5-kg dog.
The following is an example of a homemade protein-restricted diet for cats:
Dice and braise the liver, retaining fat. This recipe provides a total of 635 kcal/lb.
Many animals with renal failure are anorectic because of nausea and vomiting. Small, frequent meals are recommended to reduce the nausea. If the animal can tolerate food orally but is not eating, feeding by means of an orogastric tube is recommended. The diets described can be administered through a stomach tube if the ingredients are thoroughly mixed with water in a kitchen blender.
Supportive therapy for chronic renal failure includes the use of phosphorous binders, anabolic steroids, sodium bicarbonate, sodium chloride, calcium, and vitamin D metabolites. The use of these treatments should be based on documented abnormalities because the inappropriate or incorrect use of these agents can do more harm than good. Administration of subcutaneous fluid as a form of diuresis can be taught to the owner for use every other day or daily in advanced kidney failure. Surgically implanted subcutaneous catheters that do not require needle puncture for administration of fluid are a newer option for owners. Cats generally tolerate these catheters very well, and the owners can safely administer the fluid in a much shorter period of time because of the multiple fenestrations of the catheter allowing rapid fluid distribution without pocketing under the skin. This is also a less painful procedure for the patient.
ORTHOPEDICS
Hip dysplasia refers to a developmental problem of the canine coxofemoral joint. Subluxation of the femoral head leads to abnormal wear and eventual degenerative joint disease. The acetabulum is more shallow than normal, and the femoral head is flattened.
The cause of hip dysplasia is multifactorial. Genetics and environmental factors such as nutrition appear to be important. Hip dysplasia is seen in most large breeds and is inherited by a polygenic mode of inheritance. This means that many genes are responsible for its development. It is also quantitative in its expression. In other words, affected dogs can show slight or severe changes. As is characteristic for traits with a polygenic mode of inheritance, hip dysplasia is modified by environmental factors. For example, it has been suggested that dogs fed a high-calorie diet during growth have an increased incidence, whereas dogs fed a low-calorie diet have a decreased incidence.
The Orthopedic Foundation of America in Columbia, Mo., is an organization established to evaluate the hip radiographs of potential breeding dogs. Radiologists identify those dogs with radiographically normal hip joints. Unfortunately, because of the factors mentioned, breeding two radiographically normal dogs does not ensure normal progeny. It is better to evaluate entire families (siblings and progeny) when selecting dogs to be included in a breeding program to decrease the incidence of hip dysplasia. It is also important to recognize that good hip joints should not be the sole criterion for selection. Other traits, such as disposition, working ability, and conformation, should also be considered.
The clinical signs of hip dysplasia vary tremendously from occasional slight discomfort to a severe disabling disease. It should be remembered that the clinical signs of hip dysplasia do not always correlate with the severity of hip dysplasia detected radiographically.
Dogs with hip dysplasia will respond differently to varying levels of exercise. Some dogs are most comfortable with minimal activity, yet others do best with a regular regimen of moderate exercise. Swimming is an excellent form of exercise, since muscle tone is increased with the hip joints in a non−weight-bearing position. Any exercise program should be instituted gradually. Forced sudden activity, such as ball playing or rough play, should be discouraged. Severely affected dogs should be treated symptomatically with analgesics and antiinflammatory drugs.
Several surgical procedures have been advocated for the treatment of hip dysplasia. They include procedures such as pectineal myotomy, pelvic osteotomy, excision arthroplasty, and total hip prosthesis. A discussion of these surgical procedures is beyond the scope of this chapter.
INTERVERTEBRAL DISK DISEASE
Intervertebral disk disease is a relatively common problem affecting the spinal cord of chondrodystrophoid and other breeds. Breeds commonly affected include dachshunds, Pekingese, cocker spaniels, poodles, pugs, and beagles. The chondrodystrophoid breeds tend to develop signs at an earlier age than the nonchondrodystrophoid breeds.
The intervertebral disks are structures located between the vertebrae and function as a shock-absorbing system. The disk itself is composed of two parts: the firm fibrous outer annulus and the softer inner nucleus. In intervertebral disk disease, the annulus undergoes degeneration, and the nuclear material protrudes or is completely extruded. The result is compression of the spinal cord with the subsequent development of neurologic signs. These signs vary from simple pain to complete paralysis.
Intervertebral disk disease can be managed either conservatively with strict cage confinement and antiinflammatory drugs or more aggressively with neurosurgery. Management decisions are based on the history, neurologic signs, and wishes of the owner.
If conservative therapy is elected, the technician plays a vital role. Extreme care should be taken in handling the patient because movement may result in the extrusion of additional disk material and worsening of signs. To reduce handling, these patients should be placed in lower cages whenever possible. Because these patients are frequently in severe pain, gentle, compassionate care is essential. Many cases will benefit from some of the physical therapy techniques described earlier.
Dogs with intervertebral disk disease receiving antiinflammatory drugs, such as dexamethasone, may develop secondary problems, such as gastrointestinal hemorrhage or acute pancreatitis. Consequently, these patients should be observed closely for fever, anorexia, abdominal pain, hemorrhagic vomiting, and diarrhea.
Additional information regarding orthopedics is found in Chapter 30.
Feldman, E.C., Nelson, R.W. Canine and feline endocrinology and reproduction, ed 3. St Louis: WB Saunders; 2004.
Bonagura J.D., Twedt D., eds. Kirk's current veterinary therapy XIV. Philadelphia: WB Saunders, 2009.
Greene, C. Infectious diseases of the dog and cat, ed 3. St Louis: WB Saunders; 2006.
Hand, M.S., et al. Small animal clinical nutrition, ed 4. Marceline, Mo: Wadsworth Publishing; 2002.
Nelson, R., Couto, G. Small animal internal medicine, ed 4. St Louis: Mosby; 2009.
 TECHNICIAN NOTE
TECHNICIAN NOTE