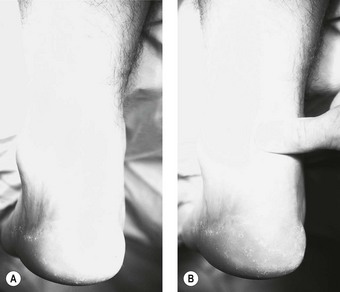
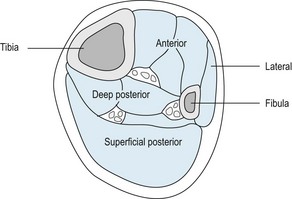
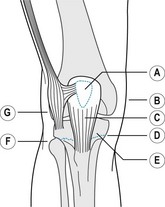
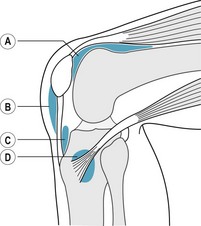
Box 13.5 An algorithmic approach to the treatment of heel spur syndrome
Initial visit
•
Ice massage for 20 minutes, 3 times daily
•
Contrast footbaths if swelling present, twice daily (see
Ch. 16)
•
In cases of extreme pain – cortisone injection followed by nerve stimulation/ultrasound treatment
•
Achilles tendon, calf muscle, hamstring stretching programme
•
Plantar fascia rest strapping and taping
•
Antipronation shoes if patient runs
•
Heel accommodation if plantar pain
•
Entrapment of the medial plantar nerve
Second visit
•
Continued physical therapy – nerve stimulation/ultrasound
•
Iontophoresis (cortisone) patch
•
NSAIDs, if needed, for 4–8 days
•
Cease all impact activity
•
Biomechanical evaluation/impression casting for orthotics
•
Plantar fascia rest strapping and taping
Third visit
•
Continue physical therapy
•
Iontophoresis (cortisone patch)
•
Evaluate shoe selection
If there is no significant improvement, or in cases where recurrence of symptoms has occurred due to activity:
Fourth visit
•
Continue physical therapy
•
Cam walker removable cast
If painful symptoms continue:
Fifth visit
•
Third and final cortisone injection
•
Below-knee fibreglass cast immobilisation
If symptoms continue with no significant improvement after 6 months to 1 year of conservative care:
Sixth visit
Surgical consultation
The initial conservative management consists of physical therapies at least three times a week to reduce the inflammation. These therapies are nerve stimulation, ultrasound, iontophoresis (cortisone patches), ice massage and cross-friction massage, and initial treatment with oral NSAIDs, and occasionally a therapeutic steroid injection directed towards the site of the inflammation or the infracalcaneal bursa. The injection consists of 0.5 ml (2 mg) dexamethadexasone acetate, 0.5 ml (2 mg) dexamethasone phosphate, 0.2 ml Wydase, 1.5 ml lidocaine (lignocaine) 1% plain, 1.5 ml bupivacaine 0.5% plain and 0.25 ml cyanocobalamin (vitamin B12). This has proven to be most useful with sports injury patients. On occasion, when the use of corticosteroids is not advisable or the limit of three injections has been reached, homeopathic anti-inflammatory medications such as Zeel (rhus toxicodendron) or Trameel (2.0 ml vials) may be employed. These medications can also be substituted into the ‘cocktail’ rather than using the cortisone, or for patients opposed to using cortisone (Bordelon 1993). One must be knowledgeable about the metabolism of these drugs and their potential side-effects before prescribing them. It should be emphasised that injection therapy for reduction of acute plantar fascial inflammation is only a temporary treatment and it must be combined with biomechanical orthotic control.
Rest from the activity is most important. Use of orthoses for the control of excessive pronation is essential; however, as the participant’s progressive pronation or compression of the orthotic appliance occurs, modifications and adjustments to the device may be necessary. The author advises re-casting the sports patient every 3 years, as the foot will change biomechanically and structurally. In many cases, orthotic control may not be sufficient to eliminate excessive pronation and any other underlying biomechanical factors. Therefore it is prudent that the athlete ceases all impact activity and cross-trains until they are asymptomatic. In cases where there is excessive pronation combined with a shortened gastrocnemius–soleus muscle complex, or tight heel cord, stretching and flexibility exercises are beneficial. Heel cord stretching with the knee both extended and flexed will help to isolate both the gastrocnemius–soleus and the Achilles and allow for reduced equinus in heel strike, and diminish plantar fascial strain. When conservative measures fail to produce a resolution to the complaints, a night splint (a posterior below-knee splint holds the foot at 90° to the leg, and extends the foot and plantar fascia) is used to help prevent contracture of the intrinsic plantar structures. This has been shown to be effective in reducing pain and stiffness when patients take their first steps out of bed in the morning. It is recommended that the night splint be worn at 5° of dorsiflexion for a minimum of 3 months while gradually weaning the patient off the splint in 2-week increments. On occasion, when night splints and aggressive physical therapy have been employed, and symptoms continue to be present, the author frequently applies a below-knee fibreglass cast or employs a below-knee Cam walker removable cast to rest the foot and extremity completely. It should be reiterated that at least 6 months to 1 year of conservative management should be attempted before surgery is even contemplated.
When surgery is indicated there are two approaches that may be employed, depending on the presence of a symptomatic infracalcaneal spur. The heel spur is not the offending problem, but rather the chronic inflammation and enthesopathy of the surrounding fascia. Therefore, plantar fasciotomy, with or without excision of the infracalcaneal spur, is the surgical procedure of choice for chronic unresolved heel pain. This author recommends only a release of the medial third (medial band) of the proximal plantar fascia, leaving the lateral two-thirds of the plantar fascia intact for cases involving pure plantar fasciitis. Endoscopic plantar fasciotomy has proved to be a viable alternative to open surgical plantar fasciotomy. It is generally agreed that there should be minimal invasion of the tissues in an athlete, and that releasing the entire plantar fascia will only destabilise the intrinsic structures and lead to compensatory complaints such as sinus tarsitis, calcaneocuboid joint syndrome, midtarsal joint pain, anterior tendinitis, ankle discomfort and metatarsalgia. In cases where scar tissue thickening of the fascia occurs it may be necessary to excise a section of the proximal plantar fascia.
When there is nerve compression, entrapment, neuritis or a ‘mini-compartment syndrome’, it is important to perform a decompression of the nerve simultaneously at the time of plantar fascia release. This is performed by dividing the abductor hallucis muscle and the fascia and freeing the medial calcaneal nerve (Baxter & Pfeffer 1992, Murphey & Baxter 1985). It is also suggested that if symptoms of a tarsal tunnel entrapment are present further lengthening of the incision is performed proximally, and the tarsal tunnel should be released (Stein et al 1989). If a heel spur is found to be projected into the flexor digitorum brevis muscle or the quadratus muscle, superior to the plantar fascia, then if large enough it should be removed. It is agreed, however, that the spur is indeed not the culprit, and that the spur does not have to be removed on all occasions. This should be explained to the patient fully preoperatively.
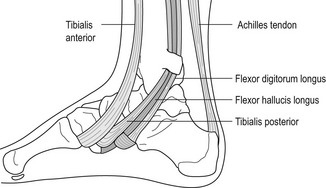
Although not a regularly occurring problem in the athlete, entrapment of the medial plantar nerve has been described as a ‘jogger’s foot’ (Murphey & Baxter 1985, Stein et al 1989). The aetiology of this condition involves the fascial covering which, if thickened, may break down or entrap the nerve. The abductor hallucis muscle may also compress the nerve. Tendinitis of the flexor hallucis longus and/or the flexor digitorum longus can also mimic neuritis of the medial plantar nerve.
The typical clinical presentation of the athlete with medial plantar nerve entrapment or neuritis will be similar to the medial calcaneal nerve entity, with burning, radiating sharp pain from the arch to the hallux or second toe, and shooting pain or numbness. Excessive pronation in sports, athletic shoes, ski boots or skating boots that have a high arch or a hard insole and/or rigid orthosis can also irritate the nerve.
Ankle equinus
Ankle equinus is defined as a limitation of ankle joint dorsiflexion to less than 10° of dorsiflexion of the neutral foot required for normal gait. Without the minimum 10° of dorsiflexion at the ankle, function of the foot will be altered, and compensation at the midtarsal joint will develop (Subotnick 1999e).
Equinus may be present due to either soft-tissue limitation or bony block at the ankle. It may also be a result of congenitally short gastrocnemius muscle, obliquity of the ankle joint or congenital osseous limitation. Previous ankle injury (sprains, fractures or direct trauma) can cause dorsal lipping at either the neck of the talus or the anterior–inferior portion of the tibia, which can prohibit freedom of movement within the ankle mortise. Rubbing, grinding and impingement may occur, causing degenerative changes in the articular surfaces of both the talus and tibia. Performance in sports that require free movement of the ankle mortise can be adversely affected.
Other clinical entities that can result in equinus are traumatic injuries to the posterior muscle groups and myositis, which may cause fibrosis, scar tissue formation and eventual shortening of the muscle belly itself. In cases of long-distance runners, when muscle groups are greatly fatigued and lactic acid levels increase, cramps or tears of the gastrocnemius or soleus muscles can occur, creating scar tissue as well as weakened or contracted muscle groups. There are also cases where children’s long bones literally outgrow muscle groups, creating short and underdeveloped muscles. Women wearing high-heeled shoes create an equinus, which can have severe ramifications when exercise is performed. The author recommends female patients to ‘kick off’ the high heels during the middle of the day and perform some simple stretching exercises. It is essential that they do the same before initiating their exercise routine, particularly if they exercise in the evening after a full day of wearing high-heeled shoes. Equinus can be seen in cases of generalised ligamentous laxity, which can result in gastrocnemius tightness and shortening.
To differentiate between a bony and soft-tissue limitation, the patient is examined in the prone position with the knee flexed. Once the knee is extended, the foot will then begin to plantar flex into an equinus position, creating a soft-tissue contracture of the superficial gastrocnemius. This contracture usually occurs during the last 20° when the knee is going from flexion to full extension. In some cases there may also be a contracture of the soleus muscle. If the limited ankle joint dorsiflexion occurs both when the knee is flexed and extended, then the problem is not a soft-tissue equinus but rather a bony block. This is referred to as anterior impingement exostosis. This can be seen on a lateral-projection radiograph with the foot stressed to maximum dorsiflexion.
Some of the clinical features that are seen in equinus involve a variety of gait adaptations. There is some transverse plane abduction of the feet with external femoral rotation at the hip, extended knee flexion throughout the gait cycle, early heel-off, which will aggravate the medial head of the gastrocnemius as well as the Achilles tendon. Other areas of compensation include a shortened stride, abductory twist of the foot and heel, excessive pronation, an elongated propulsive phase and forefoot subluxation, creating medial column prolapse. The ankle, now limited in its ability to dorsiflex, compensates by attempting to use the midtarsal joint. For the midtarsal joint to function efficiently the subtalar joint must be pronated to unlock the midtarsal joint. In addition, contracture of the gastrocnemius–soleus complex will pronate the foot further, which then compensates at the midtarsal joint. As a result, the athlete may have calf leg cramps, digital contractures and rearfoot pain. In addition, subluxation of the knee may also occur, leading to chronic knee pain.
Posterior muscle group equinus in the athlete is also secondary to a combination of tight gravity muscles and weak antigravity muscles. This imbalance between the two groups can lead to further contracture, and additional compensatory action. An aggressive stretching programme and flexibility training, often with a sports physical therapist, trainer and massage therapist, can help to alleviate this dynamic imbalance and afford better heel strike and an overall more efficient gait performance. When observing the wear pattern on the shoes, there will be minimal heel and lateral wear, while excessive wear will be seen at the forefoot and under the ball of the shoe.
In cases of anterior ankle impingement, or ankle bony block, the athlete will complain of pain at the anterior aspect of the ankle or in the Achilles tendon. This can also be seen with hyperostosis of the neck of talus, as the athlete attempts to maximally dorsiflex. The location of the bony block may also have a bearing on the heel strike of the athlete (Subotnick 1999e). An anterior lateral exostosis creates a supinated foot plant, while an anterior medial exostosis will create a pronated foot plant. As the foot and ankle reach a maximum point of dorsiflexion, tension and enthesitis of the Achilles tendon will occur, which will lead to distinct pain either in the tendon or at its insertion.
The conservative treatment for osseous deformity of the ankle is with the use of heel lifts. When conservative measures have been exhausted for a bony ankle equinus, surgical resection, either arthroscopically or via arthrotomy, may be required. Postoperatively the athlete is encouraged to passively remobilise the ankle.
Heel lifts, used concomitantly with a stretching routine, are very helpful for a soft-tissue equinus deformity. The stretching is imperative to prevent recurrence of the posterior muscle group contracture, including the tightening of the heel cord. Those athletes with hypertonicity will benefit from heat treatment.
Achilles tendon injuries
Achilles tendinitis, or paratendinitis, is a chronic condition seen in running and jumping sports. It is one of the most common injuries in athletes and has been estimated to afflict 6.5–20% of all runners (Clement et al 1984, James et al 1978, Krissoff & Ferris 1979, Subotnick & Roth 1988). Due to its structure as well as the functional demands, the Achilles tendon is susceptible to both acute and chronic injury. Repetitive loading that exceeds the ability of the Achilles tendon to repair may cause tendinitis, whereas the acute rapid loading of the tendon may cause traumatic rupture. Paratendinitis of the Achilles tendon accounts for 20% of all non-specific tenosynovitis or paratendinitis seen in the foot and ankle. Some of the aetiological factors of acute or chronic Achilles tendinitis are irritation of the heel against the counter of the shoe, excessive pronation, limb-length discrepancy and a tight gastrocnemius–soleus complex as a result of inadequate stretching. Also involved are conditions such as Haglund’s deformity and a short Achilles tendon. The repetitive loading seen in long-distance marathon running and the traction of the tendon–muscle unit due to jumping, hill running, or running on uneven or hard surfaces may also contribute. Similarly, an increase in running mileage, intensity of interval speed running and the start of a running or athletic programme after a prolonged period of inactivity can all be factors.
Other factors include running or athletic shoes that show excessive outer-sole wear, inner soles that are crushed down and heel counters that are distorted and create an unstable heel strike or midstance phase of gait. Biomechanical considerations that can contribute to Achilles tendinitis and paratendinitis include lower extremity malalignments, such as tibial varum, compensated gastrocnemius–soleus equinus or ankle block equinus, and a cavus foot with excessive supinated heel strike. These factors will lead to unusual lateral shoe wear, which then causes hyperpronation at midstance, creating higher levels of torque on the Achilles tendon.
Other compensatory biomechanical factors, such as rearfoot varus, forefoot varus, forefoot valgus with a plantar-flexed first ray, and varus condition of metatarsals two to five, also contribute to pathologies of the Achilles tendon.
Overuse injuries of the Achilles tendon result from the inflammatory process in the tendon tissue as well as the paratendon. Inflammation is the direct result of repetitive microtraumatic forces. The inflammatory process is a necessary component of the healing process. In many cases, acute inflammation is productive, whereas chronic inflammation can be destructive and disabling. The key is early treatment of this overuse disorder to prevent the injury from becoming irreversible.
After initial vasoconstriction and haemostasis, local vasodilatation takes place, leading to the release of capillary fluid. Prostaglandin production due to inflammation causes vasodilatation, which then produces oedema. Histological changes within the tendon constitute the underlying reason for the pain and pathological conditions in the tendon (Astom & Rausing 1995). In cases of chronic Achilles tendinitis, nodules comprised of mucoid degeneration will appear, together with longitudinal fissures within the tendon itself.
The areas most commonly involved in Achilles tendinitis are:
•
The myotendinous junction.
•
The area of the Achilles tendon approximately 8–10 cm proximal to the distal insertional region of the calcaneus and ankle joint.
Lagergren and Lindholm (1958) noted that the area 2–6 cm above the calcaneal insertion had the poorest blood supply, which substantiates the fact that most non-insertional Achilles tendon injuries occur in this region. For participants over the age of 35 years, overuse tendinopathy in this region increases dramatically. This is due to the blood flow by approximately 40% at this age.
Carr and Norris (1989) showed that there was a significant reduction in the number, as well as the mean percentage of area occupied by, blood vessels in this region.
•
The insertional region of the Achilles tendon into the posterior aspect of the calcaneus, with or without calcinosis.
Clain and Baxter (1992) categorised the entities into insertional and non-insertional tendinitis. Insertional tendinitis will usually involve the adjacent bursa, and includes anatomical changes within the tendon, such as thickening, microscopic tearing, calcinosis within the tendon body, and fraying as a result of a Haglund’s deformity or other enthesopathies.
The triceps surae combine to form the Achilles tendon. The Achilles is the largest and strongest tendon in the body. It is estimated that the tendon receives up to 7000 N of force (Clain & Baxter 1992). While running, the Achilles tendon is subjected to constant extreme forces with tensile loads of up to eight times the body weight. The medial head of the gastrocnemius is the main component during running, whereas the soleus, which lies deep to the gastrocnemius, is subject to early disuse atrophy secondary to undertraining and/or immobilisation. The Achilles tendon places the insertion of the soleus medial to that of the gastrocnemius on the calcaneus. In addition, there is a medial insertion of the Achilles tendon on the calcaneus. Because the tendons of the gastrocnemius and soleus do not have a parallel configuration, the theory is that the shear stress between the two tendons creates an area of potential weakness, and eventual rupture due to attrition (Christensen 1953).
The triceps surae are the main decelerators of the leg, a major supinator of the subtalar joint, a plantar flexor of the ankle and a stabiliser of the rearfoot. In sports such as gymnastics and ballet, the muscle complex assists in maintaining a variety of movements and positions.
The Achilles tendon does not have a synovial sheath, rather it is surrounded only by a peritenon. The peritenon as well as the tendon is subject to acute trauma, chronic overuse or disease entity. Peritendinitis of the Achilles tendon may occur as a result of athletic shoe counter irritation, a sudden increase in running or workout intensity, or prolonged running or walking. Microvascular studies of this body indicate that there is an area of relative avascularity just proximal to its insertion into the calcaneus. As a result, this region is highly vulnerable to Achilles tendinitis, peritendinitis and eventual rupture.
Chronic traction, irritation, and inflammation of the Achilles tendon will present as tenosynovitis, or a partial or complete rupture. Microscopically, an abnormal Achilles tendon of an athlete suffering from chronic tendinopathy differs from a normal tendon. First there is a loss in collagen continuity, and an increase in ground substance, vascularity and cellularity (fibroblasts and myofibroblasts). In those who suffer from chronic overuse pathologies of the tendons, inflammatory cells are absent (Khan et al 2000).
Although Achilles tendinitis is rare, acute primary tendinosis will be recognised as pain over the posterior aspect of the Achilles tendon. Paratenonitis is a more accurate term for this condition, which presents as an inflammation of the paratenon, whether lined by synovium or not. Chronic tenosynovitis will cause fibrosis of the paratenon, creating pain upon motion. This condition has been referred to as adhesive tendinopathy. It is associated with intratendinous degeneration (Jarvinen et al 1997) and produces the crepitus or, as Subotnick (1999d) refers to it, the ‘glue’ that forms between the tendon and paratenon. The clinician will feel swelling and inflammation in the paratenon. The tendon should be checked completely to rule out any small tears or ‘dells’ in the tendon. Any thickness or egg-shaped appearance of the tendon may be a clue as to pathology within the tendon itself, or partial rupture. When palpating the paratenon, localised oedema is more indicative of a tenosynovitis. Investigative studies such as magnetic resonance imaging (MRI), ultrasound or even xerograms may assist in making the correct diagnosis.
Paddue et al (1976) classified Achilles tendon pathology into three distinct entities based on the clinical and histological findings seen at the time of surgery:
•
peritendinitis with tendinosis
Peritendinitis is a pathology of the highly vascular paratenon. The condition has also been described by Kvist and Kvist (1980) as fibrin adhesions organised between the paratenon and the tendon itself. When peritendinitis and tendinosis is seen in combination, there will be a significant change in the tendon morphology itself. The Achilles tendon will become thicker, softer and yellowed. Paddue et al (1976) and Kvist and Kvist (1980) described cleavage planes as well as vascular budding from the paratenon invading the tendon. The third classification, referred to as pure tendinosis, is seen in cases of acute ruptures of the Achilles tendon. Paddue et al (1976) described mucinoid degeneration and lipomatous infiltration of the collagen fibres, with patients who had no prodromal symptoms prior to rupture. In those subjects who did suffer Achilles tendon pain prior to rupture, a specific zone of histiocytic infiltrate and capillary infiltration was seen.
Obtaining a thorough history from the patient will often help to determine the underlying cause of the injury and the level of activity (i.e. miles run, length of time involved in workouts, intensity and competitiveness). Subjective findings, such as the type of pain, its character and when it occurs (before, during or after activity), are also important. Previous treatment, particularly localised corticosteroid injections, should be noted.
The clinical examination should involve the evaluation of both Achilles tendons, as well as a comparison of both lower extremities. The clinician should examine for signs of swelling, erythema, thickness of the tendon or paratenon, nodules and any bony abnormalities. On occasion, ossification within the tendon body itself may occur. The ankle joint range of motion should be evaluated to rule out equinus. This examination should take place with the forefoot supinated and with the knee both flexed and extended. The difference between the symptomatic leg versus the contralateral asymptomatic leg should be noted. During the examination, the practitioner should look, feel and listen for any palpable or audible crepitus surrounding the tendon, while actively or passively putting the foot and ankle through the range of motion. The combination of an accurate history and thorough physical examination can help to determine whether the injury is an insertional or non-insertional tendinitis, or a combination of the two.
In the acute stage of Achilles paratendinitis, the complaint will be unilateral. Clancy et al (1992) defined tendinitis of less than 2 weeks duration as acute, 3–6 weeks duration as subacute, and more than 6 weeks duration as chronic. The symptoms are usually local; the affected tendon becomes two to three times its normal size, with soft-tissue swelling, crepitus and restricted pain upon movement. Any presence of nodular formation above the insertion may be indicative of microscopic tears or small ruptures of some of the tendon fibres. The pain will be most discrete above the tendon insertion, precipitated by overuse activity and relieved quickly by rest.
Treatment for the acute stage of Achilles tendinitis and paratendinitis consists of a decrease in activity or an attempt to eliminate the overuse.
When running or activity continues, reducing mileage, eliminating all hill and interval training, avoiding uneven running surfaces, and ceasing all jumping or bouncing repetitive sports is required. Ice or contrast whirlpools after activity, as well a consistent pre- and post-exercise stretching programme, should be adhered to.
Measures to reduce the inflammation include anti-inflammatory medications, icing (3–4 times daily of no more than 20 minutes duration), analgesics and heel lifts (except in cases of unilateral Achilles tendinosis and/or paratendinitis secondary to limb-length discrepancy, where only the short limb is raised). Physical therapy modalities consist of iontophoresis nerve stimulation of the muscle–tendon unit and ultrasound, together with biomechanical correction and prescription orthoses to reduce excessive pronation and pull upon the Achilles tendon. Shoe selection is also important, with a flexible athletic shoe and moulded Achilles pad to prevent irritation of the tendon. Homeopathic injectable medications in combination with local anaesthetic can be administered followed by deep soft-tissue cross-friction massage. This will assist in breaking up scar tissue and adhesions painlessly and improve circulation to the region. After a period of time, a mild stretching programme can be initiated, with strengthening of the anterior and posterior muscle groups.
All running and other physical impact athletic activity must cease for at least 4–6 weeks. A non-impact cross-training programme consisting of bicycling, elliptical trainer, swimming and deep water jogging can be substituted during the recuperative phase. Failure on the part of the athlete to adhere to the recommended rehabilitation programme can result in chronic adhesive inflammation, and ultimately focal degeneration of the tendon, which can lead to chronic tendinitis and partial or complete rupture of the tendon. In cases where the conservative measures have continued to be ineffective, the tendon and extremity can be rested by using a cast to immobilise the area or a removable Cam walker.
In the subacute stage, diffuse swelling along the tendon is indicative of thickening of the paratenon. Crepitus can be palpated upon movement of the tendon. The participant will relate symptoms of pain, particularly upon rapid acceleration. Fibrosis of the paratenon secondary to tenosynovitis will create pain upon motion. Treatment is similar to the acute phase, with aggressive physical therapy and cross-friction massage. On occasion, when this phase occurs, local anaesthetic injections with or without homeopathic medication can be administered to achieve lysis of the adhesions along the course of the paratenon. Adjunctive treatment with cast immobilisation should also be considered for this particular overuse injury (Box 13.6).
Acute complete rupture of the Achilles tendon
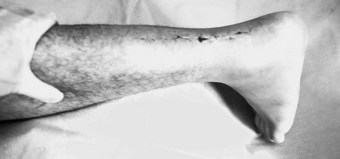
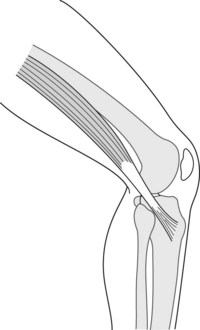
Acute Achilles tendon rupture usually occurs in poorly conditioned middle-aged men who quite often are the ‘weekend warriors’, not engaged in athletic activities on a consistent basis. On occasion, it may affect those participants who have taken oral or injectable corticosteroids. The most frequent site of the tendon rupture will be 2–6 cm from the calcaneus (Fig. 13.12). The injury will occur normally during a rapid eccentric loading (push-off), with the knee extended, as the foot and ankle are landing in dorsiflexion with a contracted soleus muscle. At 8% strain, the tendon fails and breaks the collagen cross-links (Soma & Mandlebaum 1994). The tendon is at great risk if tension is applied too rapidly, if the tendon is under tension before further loading, or if the tendon is weak compared to the muscle. Participants will often say they felt a pop in the back of the leg, and that it felt as if someone hit them in their calf or tendon. On occasion, a direct traumatic blow to the Achilles tendon can create the rupture. In the acute rupture, pain will be present but will not be the major presenting complaint, which will instead be the onset of swelling, ecchymosis, a palpable gap in the Achilles tendon, and a diminished or complete inability to plantar flex the foot (Fig. 13.13). The patient will be unable to continue play at the time of injury and will no longer be able to continue athletic activity. The Thompson test is used to determine whether there has been a complete rupture of the tendon. The test is performed by squeezing the gastrocnemius–soleus firmly in the prone position, where the normal reaction should be plantar flexion. When there is a complete rupture of the Achilles tendon, plantar flexion will not occur (Fig. 13.14). The patient may substitute the intact posterior tibial or fibular (peroneal) muscles or the flexor digitorum longus to plantar flex the ankle. However, they will be unable to perform the single heel raise test, indicating a marked reduction in strength due to the tendon injury.
A number of clinicians advocate closed treatment, with cast immobilisation in plantar flexion to reapproximate the frayed tendon ends to allow for healing (Lea & Smith 1968, 1972). They cite good functional results without the morbidity of surgical intervention, in addition to a more rapid return to activity, avoidance of the necessity for admission to hospital and lower healthcare costs (Ingles et al 1976, Mahan & Carter 1992). However, the closed cast treatment carries with it a higher rate of re-rupture, 10–29% being reported. This ultimately will reduce the ability to perform on the athletic court or field, and is not advised for the active athletic patient (Bradley & Tibone 1990, Carden et al 1989). Of even greater interest, Ingles et al (1976) found that, upon isokinetic testing, the non-surgical subjects in their study achieved only 62–67% of strength and endurance, compared to 88–100% in the surgically corrected group.
This conservative form of treatment is reserved for the patient who is not attempting to return to high levels of athletic activity or demanding functional performance. With these factors in mind, and the fact that the athletic patient who does not have surgery is at high risk for re-rupture, surgical primary repair is recommended. With improved surgical technique and postoperative rehabilitation, surgical repair now has reduced morbidity, and allows patients to return to their pre-injury level of participation (Soma & Mandlebaum 1994). For the athlete, many clinicians recommend primary surgical repair for complete ruptures.
The surgical procedure is combined with an aggressive postoperative active range of motion programme to recreate a level of functional performance that was present before injury. The complication rate from this surgery has been as high as 20% for minor incidents, and 12% for major incidents, with recent rates being as low as 2% (Willis et al 1986). The reported complications include infection, adhesions, sural neuroma, delayed wound healing with or without necrosis, re-rupture and continued pain. In the study by Soma and Mandlebaum (1994), 100% of patients returned to athletic participation 12 months after surgical repair and had no functional deficit on isokinetic testing. It has also been reported that, after surgical repair, approximately 75% of high-performance athletes and 90% or more of recreational athletes can be restored to competitive level activity (Singer & Jones 1986).
A number of surgical procedures for repair of the Achilles tendon have been described. Reapproximation of the torn tendon ends is the great challenge. The majority of these ruptures when operated on are discovered to be located just distal to the musculotendinous junction, and demonstrate a frayed appearance. Surgical repair may include the following:
•
open primary repair with direct suturing (
Nistor 1981)
Turco and Spinella (1987) identified five factors that challenge successful repair of the Achilles tendon:
1.
suturing of the shredded tendon
2.
re-establishment of physiological tension
3.
weakness associated with a lengthened tendon
4.
revitalising an ischaemic injured tendon
5.
difficulty obtaining secure fixation when the insertion is avulsed from the calcaneal tuberosity.
When attempting to secure the distal portion of the tendon or graft to the calcaneus this author has found the Mitek GII™ bone/tissue anchor with Mersaline, or the newer Mitek Panaloc™ bone/tissue anchor with Panacryl (Johnson & Johnson), to be useful. Both tissue anchors help to increase the pullout strength of the suture from the calcaneus to the tendon, and can help prevent re-rupture.
Postoperatively, the patient is placed in an above-the-knee non-weight-bearing, posterior splint cast, at a mild equinus, to allow for immediate postoperative swelling. Following the first week, the cast is removed and the joint taken through gentle passive and active range of motion exercises. After 2 weeks, when the sutures are removed, the patient is placed in a below-knee, posterior splint cast, which can be removed daily to allow for range of motion exercises. From weeks 3 to 5 a gentle return to progressive weight bearing is begun.
The patient can then be placed in a Cam walker removable cast, maintaining mild plantar flexion to neutral position. Physical therapy rehabilitation actively begins at the sixth week postoperatively, combining early range of motion with progressive resistance exercises. This has been shown to help attain a successful repair with maximum strength of the Achilles tendon unit, while minimising atrophy of the muscle and tendon, the key being early return to physical activity with minimal sequelae.
Insertional Achilles tendinitis and calcific tendinosis
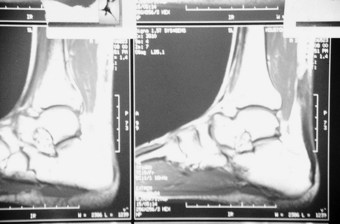
Many athletes involved in running, jumping sports, skiing and skating relate pain at the insertion of the Achilles tendon and its insertion into the calcaneus. This is normally associated with hypertrophy of the posterior portion of the calcaneus, a prominent posterosuperior angle of the calcaneus, retrocalcaneal bursitis, Haglund’s deformity, an insertional traction exostosis, with ossification or spurring at the site of the Achilles tendon, as well as calcification within the tendon body (Fig. 13.16). In cases where a retrocalcaneal exostosis or hypertrophied posterior aspect of the calcaneus is present, shortening of the Achilles tendon will occur, placing strain on the tendon, as well as chronic irritation, due to increased shoe pressure. Using a lateral-projection radiograph (see Ch. 22), a hyperostosis directed superiorly into the tendon may be seen (Fig. 13.17). Fracturing or fragmentation of the spur may occur as a result of chronic traction forces of the Achilles tendon. A violent impact of the foot on the ground while participating in a sporting event, or a forced eccentric contraction of the gastrocnemius–soleus and Achilles due to excessive dorsiflexion, may also contribute to the formation of fractures along this spur. Microavulsions at the level of the insertion due to excessive traction forces of the Achilles tendon may result in the same pathology (Fig. 13.18). Although the exact aetiology of the calcific tendinitis and tendinosis is not known, the condition is thought to be related to age, overuse, trauma and enthesopathies, and it has a high occurrence rate (Subotnick & Vogler 1999a).
Anatomically the posterosuperior prominence or the bursal projection of the calcaneus functions to lengthen the lever arm of the Achilles tendon, increasing the mechanical advantage of the gastrocnemius–soleus when the ankle is dorsiflexed. At the same time, the retrocalcaneal bursa protects the Achilles from the posterosuperior aspect of the calcaneus when the ankle is once again dorsiflexed.
It is estimated that the Achilles tendon is subject to forces as great as 900 kg during periods of intense physical activity. The same pathological changes as seen in the calcaneal origin of the plantar fascia are also seen within the tendon and at its insertion.
Microscopic changes include fibrinoid and myxomatous degeneration, fibrosis, and eventual metaplastic calcification with resultant thickening and nodularity of the tendon (Saxena 1996). Also of interest is the fact that after the third and fourth decades of life, blood flow to the tendon shows a significant decrease. The reduced blood flow primarily affects the region of the Achilles tendon 2–6 cm superior to its insertion, which relates to the most frequently ruptured site (DiStefano & Nuron 1972, Lagergren & Lindholm 1958). Rarely, distal tears of the Achilles tendon through areas of calcification, just proximal to the insertion, have been associated with a posterosuperior calcaneal prominence, referred to as a calcaneal step, which irritates the tendon upon ankle dorsiflexion (Fig. 13.19).
Upon physical examination, a retrocalcaneal exostosis at the insertion of the tendon, with or without calcification within the Achilles tendon, is noted. The patient will describe a dull aching soreness or pain, sometimes with radiating pain along the sural nerve tract. Tenderness will be localised to the area of the insertion with the periosteum surrounding the calcaneus. The practitioner should compare the two heel regions, with thickening of the Achilles tendon clearly seen at the insertion. Again, the participant will describe pain upon activity that can be reproduced upon active/passive ankle joint range of motion, as well as upon direct palpation. Schepsis et al (1994) noted that there will be a decrease in the range of ankle joint passive dorsiflexion on the afflicted side. On palpation, the practitioner may note discrete crepitus upon ankle joint range of motion as a result of chronic inflammatory infiltrate and fibrin deposition throughout the tendon.
Conservative management of the insertional tendinitis and calcific tendinosis is similar to a painful Haglund’s deformity. Physical therapy modalities consisting of ice massage, oral anti-inflammatory medications, nerve stimulation, ultrasound, iontophoresis, viscoelastic heel lifts and removable or hard below-knee cast immobilisation are highly recommended. Of even greater importance is a stretching and strengthening programme, with emphasis on the gastrocnemius–soleus complex,. If an inflamed retrocalcaneal or insertional bursa is present, a single injection of corticosteroid or homeopathic medication may be given, followed by cessation of all physical activity for 2 weeks. Athletic shoe modification with accommodative padding surrounding the posterior heel counter may be employed to lessen the friction and irritation to the posterior prominence of the calcaneus. An orthosis with a mild heel raise can neutralise the irritation to the heel and prevent it becoming chronic. If all conservative measures fail to relieve the pain and irritation to the insertional area of the tendon, surgical intervention may be the only option.
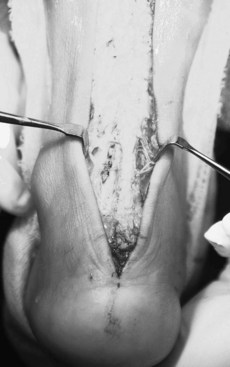
The surgical approach for repair of the posterior calcaneal exostosis and or insertional Achilles calcific tendinosis is dependent on the site, either medially, laterally, or both. Various authors have advocated that, with the patient in the prone position, a single longitudinal midlinear, two incision, medial and lateral linear approach, or a curvilinear and mildly oblique incision may be used. When the retrocalcaneal spurs are present at the insertion, then a midline tendon-splitting approach is advised, which allows for adequate exposure to the calcaneus, to resect the spur (Saxena 1996, Schepsis et al 1994) (Fig. 13.20).
In cases where the spur is central and the calcification is within the tendon and its insertion, the midline, tendon-splitting incision is best option. This approach minimises underscoring, and allows equal medial and lateral halves of the tendon to remain intact to the calcaneus distally. The medial and lateral bodies of tendon are then reflected, allowing for adequate inspection of the site and resection of any intratendinous calcification. For deeply inflamed retrocalcaneal bursae, paratendinosis and superior calcaneal steps, a ‘deepening split tenotomy’ may be required. After resection of the exostosis and intratendinous calcification, the two halves of the Achilles tendon are reattached using a Mitek-GII™ bone/tissue anchor with Mersaline non-absorbable suture, or the newer Mitek Panaloc™ bone/tissue anchor with absorbable Panacryl suture. To reinforce the repair of the tendon, additional absorbable 2-0 Vicryl is used to reinforce the anchoring of the tendon to the bone.
There are many opinions as to how long the patient should be non-weight bearing postoperatively, and to what degree the ankle should be rested with immobilisation. This author finds the first week to be most important, and in this time the patient is placed in a non-weight-bearing, posterior, splint cast with the ankle at 90° or slight equinus, moving with the assistance of crutches. This is followed in the second week with a semi-weight-bearing fibreglass cast, with the ankle held at neutral position, again moving with crutch assistance. At the end of the second week the sutures are removed and the patient may have the fibreglass cast repeated or be advanced to a removable Cam walker cast boot. Physical therapy modalities similar to those used in repair of the Achilles are encouraged, with active/passive range of motion of the ankle beginning in the third to fourth week postoperatively.
With the advanced use of tissue anchors for securing the Achilles tendon, athletic patients can progress at a much faster rate than before to their preoperative status.
Retrocalcaneal exostosis (Haglund’s deformity)
This refers to a hypertrophy or prominent posterosuperior–lateral border of the calcaneus, secondary to chronic mechanical irritation of the shoe heel counter. As the gastrocnemius–soleus complex acts to decelerate the body as it moves forward over the foot during the propulsive phase of gait, the heel is in contact with the counter of the shoe. This friction and irritation will then lead to the formation of an exostosis. Other secondary biomechanical causes of a retrocalcaneal exostosis include a compensated rearfoot varus, cavo varus, and frequently a forefoot varus, creating a supinated heel strike, which can also lead to irritation of the posterosuperior shelf of the calcaneus. Other factors that contribute to the formation of an exostosis include the inclination angle of the calcaneus, which determines the volume of the posterosuperior aspect of the calcaneus involved in shoe contact. The pitch or degree of adduction of the calcaneus may contribute to the formation of the exostosis more laterally. Due to chronic irritation, as well as the formation of a bursa, a superficial Achilles tendon bursitis or retrocalcaneal bursitis can occur. In some cases, a hyperkeratotic lesion or, when severe, an intractable plantar keratoma lesion can develop over the retrocalcaneal bursa and exostosis.
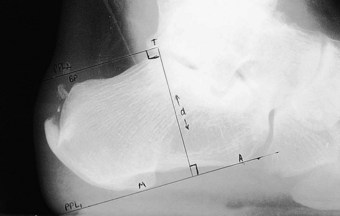
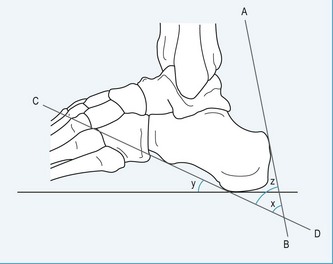
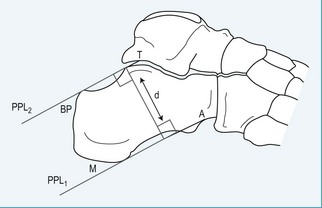
Using a lateral weight-bearing radiograph the practitioner can evaluate and determine the retrocalcaneal bursal projection, either by posterior calcaneal angle (Fig. 13.21) or by parallel pitch lines (Fig. 13.22). Keck and Kelly (1965) observed that an increase in the parallel pitch lines, and not in abnormal posterior calcaneal angle, determined the degree of posterior heel bursitis. Ruch (1974) pointed out the mechanical function of the posterior calcaneal projection that occurs with ankle joint dorsiflexion, and drew particular attention to the direct relationship this has with an increased calcaneal inclination on the posterosuperior prominence. Fowler and Philip (1945) described another radiographic assessment to determine the posterior bursal projection. It consists of evaluating a superior calcaneal angle, the x angle (Fig.13.21), which is subtended by lines drawn from the bursal projection to the posterior tuberosity (AB), and from the medial calcaneal tuberosity to the anterior calcaneal tuberosity (CD). They regarded an angle of greater than 75° to be pathological; however, the angle does not take into consideration the relationship between the calcaneus and the sole of the foot. Pavlov et al (1981) also described another set of criteria for evaluating the shape and pitch of the calcaneus. They used both the Fowler and Philip posterior calcaneal angle and the parallel pitch lines (PPL) to determine the prominence of the bursal projection and the pitch angle (calcaneal inclination angle). They describe a Haglund syndrome on a radiograph as:
•
a cortically intact bursal projection
•
loss of the retrocalcaneal recess, indicating a retrocalcaneal bursitis
•
thickening of the Achilles tendon
•
loss of the distinct interface between the Achilles tendon and the pre-Achilles fat pad, indicative of Achilles tendinitis.
The bursal sac contains a small amount of fluid. The normal retrocalcaneal bursa will accept 1–1.5 ml of fluid, and can be seen using bursography (Frey et al 1982). The chronic irritation, as previously described, will lead to an inflammation of the bursa, resulting in a thickening of the bursal wall, with effusion. The subcutaneous bursa is shaped like a horseshoe and is located between the skin and Achilles tendon. The purpose of the bursa is to protect the Achilles tendon and the underlying calcaneus from external pressures. Traction of the insertional region of the Achilles will also contribute to the calcification of the tendon, and with a vertically extended spur may be seen within the substance of the Achilles tendon, as it inserts into the calcaneus, leading to further inflammation of the bursa. This condition can lead to avulsions of the Achilles tendon and/or spur due to fractures and traction overloads. It is believed that the aetiology of retrocalcaneal exostosis or calcific tendinosis is age-related and due to overuse trauma, enthesopathy; it has a high occurrence rate (Fox et al 1975) (Fig. 13.23).
The development of a retrocalcaneal exostosis may be the result of a separate centre of ossification at the posterior angle of the calcaneum (Hoerr et al 1962). This independent ossification centre may be a small portion or fragment of the calcaneal apophysis and may grow separately from the calcaneal apophysis. Another possible aetiology involves traction apophysitis in adolescents. Repetitive traction of the Achilles tendon at its insertion to the calcaneal apophysis, combined with a compensated rearfoot varus, and heel counter irritation due to sports activity, can contribute to the hypertrophy of the retrocalcaneal and posterosuperior regions of the calcaneus. Chronic irritation can cause further hypertrophy and exostosis formation in later years.
Conservative treatment for symptomatic retrocalcaneal exostosis, similar to the treatment for Achilles paratenonitis, will provide temporary relief. Treatment consists of padding the shoe counter, softening or eliminating the counter via open-back shoes, and inserting a one-quarter to three-eighths inch (6–9.5 mm) heel lift (intended to raise the heel prominence above the counter to reduce shoe counter pressure). The heel height of the shoe has an important influence upon symptoms (Henegham & Pavlov 1984). Raising the heel in the shoe helps to decrease the calcaneal inclination angle, which then alters the position of the bursal projection away from the heel counter. Other forms of conservative care include NSAIDs, particularly during the acute inflammatory phase, ice massage, where the symptoms manifest, and stretching exercises, with special attention to the hamstrings, gastrocnemius–soleus complex and Achilles tendon. Orthoses are particularly helpful in controlling the imbalance in the rearfoot and preventing irritation between the heel and counter. Runners are advised against speed work, to reduce mileage and to avoid hill training. Although local steroid injections are contraindicated, in cases where a retrocalcaneal bursa is present an injection of short-term corticosteroid combined with local anaesthetic can be utilised. The injection should be performed very cautiously, perhaps once, exercising caution to avoid injecting into the tendon (Subotnick & Vogler 1999b). As it is known that local steroid injections can lead to rupture of the Achilles tendon, the injection should be well placed within the bursa and never within the Achilles tendon or its insertion. Following the injection, physical therapy including nerve stimulation, ultrasound, superficial massage and, in lieu of an injection, iontophoresis can be performed two or three times a week for 3–4 weeks. The athlete, and in particular the runner, should be advised to avoid all running, jumping, skiing or any impact activity for at least 2–3 weeks following the injection. They are also advised to participate in cross-training activities that will not cause tension on the Achilles or irritation of the bursal area or retrocalcaneal exostosis. In some severe cases, cast immobilisation or a removable Cam walker cast is recommended. The advantage of the removable cast is that it may be removed daily for access to physical therapy. These conservative measures will prove successful in the majority of cases; however, when all conservative treatment has been exhausted and symptoms continue to plague the athlete’s performance, surgical repair is recommended. The procedure may be performed either under general anaesthesia or under local anaesthetic with intravenous sedation. The procedure is similar to that performed for insertional tendinosis and calcific tendinitis. Postoperative care again parallels the previously described procedure.
ANKLE INJURIES
Acute sprains to the ankle are one of the most common injuries seen by the practitioner. The lateral ankle complex is the most frequently injured anatomical structure in athletes, comprising 38–45% of all injuries (Garrich 1982). The incidence of inversion injuries has been estimated at 1 per 10 000 persons per day (Brooks et al 1981, McCullock et al 1985).
Ankle sprains contribute to one-sixth of the sports injury loss time (Garrich 1982, Garrich & Requa 1973). Ankle sprains consist of 85% of all ankle injuries, with 85% of them being inversion sprains of the lateral collateral ligaments (Baldwin & Tezlaff 1982). Eversion mechanism sprains involving the deltoid ligament or medial collateral ligament constitute 5–6% of all ankle sprains, while syndesmosis injuries account for the remaining 10% (Baldwin & Tezlaff 1982). Frequently occurring ankle sprains can result from specific sport activity. The sports with the highest proportion of sprains at the ankle are volleyball with 82% and basketball 79%; football, racquetball and dance had more than 70%. Tennis, soccer and aerobic dance had more than 65% of the sprains reported. Sports with a lower frequency of ankle sprains were skiing, ballet and figure skating, each with less than 35%, and the sport with the fewest sprains among their ankle injuries was cycling, with 20% (Garrich & Requa 1988). There is no difference between men and women in the incidence of ankle sprains when comparing injuries sustained from engaging in similar activities (Garrich & Requa 1988). Recurring ankle sprains have always been a concern for the athlete. It has been shown that previous ankle sprains will create a higher potential for future injury (Glick et al 1976).
Many athletes will either ignore or self-treat the injury first, and seek attention only if the ankle continues to be swollen and painful and limits competitive participation. Many ankle injuries when seen are either undiagnosed, inadequately treated or, due to a lack of compliance, go on either to re-injury or chronic instability. Residual symptoms or recurrent sprains occurred in 42% of patients in one study (Bosien et al 1955). Ankle sprains in the athlete require proper and early diagnosis, as well as an extensive rehabilitation programme to return the athlete to his or her normal competitive status. Without such a treatment plan these injured ankles will be left weak and unstable and seriously subject to recurrent injury.
Anatomy
Three major ligament groups provide the support for the ankle group: the superficial and deep portions of the deltoid ligament, the tibiofibular ligaments, and the lateral ligament complex. The lateral ankle ligament complex of the ankle consists of three individual ligaments: the anterior talofibular ligament (ATFL), the calcaneofibular ligament (CFL) and the posterior talofibular ligament (PTFL). The other support ligaments of the lateral ankle region are the lateral talocalcaneal ligament (LTCL), the ankle syndesmosis, with its ligaments, and the subtalar joint and its ligaments. The collateral ligaments of the ankle are arranged anatomically to afford joint dorsiflexion and plantar flexion, while concomitantly not restricting subtalar joint inversion or eversion. In addition to supporting and stabilising the ankle, allowing for sagittal plane dorsiflexion and plantar flexion, they aid in proprioception.
The ATFL is the most anterior ligament of the ankle. It consists of an upper and lower band and is intracapsular and intra-articular. It lies in a transverse plane crossing from the anteroinferior surface of the fibula to the body and neck of the talus just anterior to the lateral malleolar articular facet. The medial articular surface of the talus or medial facet articulates with the opposite medial facet of the medial malleolus. The lateral articular surface of the talus or triangular lateral talar facet articulates with the analogous lateral facet of the fibular malleolus. The dorsal surface of the talus is also known as the ‘trochlear surface’, and the inferior surface of the tibia, which articulates with the trochlear surface of the talus, is referred to as the ‘tibial platform’. That space lying between the lateral articular surface of the talus and the medial articular surface of the fibular malleolus is referred to as the ‘lateral gutter’, and the opposite space between the medial malleolus and medial surface of the talus is called the ‘medial gutter’.
The ATFL is a flat quadrangular ligament that is closely developed within the joint capsule, and is the most frequently injured of the three. The ATFL runs parallel to the long axis of the talus when the ankle is in neutral or dorsiflexion, but more perpendicular to the long axis of the talus in equinus (Leonard 1949). This anatomical design leads to a very tight ligament throughout plantar flexion. The ATFL has been shown via biomechanical testing of the ankle ligaments to have the lowest yield force and ultimate load of the lateral collateral ligament complex (Siegler et al 1988). The ligaments are usually injured, with the anterior talofibular being first, followed by the CFL, and lastly the PTFL. The extent of the injury will depend on the level of plantar flexion and inversion forces, as well as the position of the ankle when the foot strikes the ground. In 20% of the population the ATFL is absent, leading to potential instability, and acute and recurrent sprains of the lateral ankle.
The CFL is a taut ligament that originates on the distal inferior surface of the fibula. It descends to an insertion on the tubercle of the lateral portion of the calcaneus. It inserts in a posteroinferior direction under the fibularis (peroneus) tendons. It lies in the sagittal plane approximately 90° inferior to the ATFL. The CFL is an extracapsular, round, cord-like structure that is finely attached to the joint capsule and to the medial surface of the fibular (peroneal) sheath. The ligament is separated from the joint capsule by a thin fatty layer. It possesses the highest elastic strength of the three collateral ligaments, having a higher yield force and ultimate load than the ATFL (Siegler et al 1988). The CFL crosses over the superior portion of the ankle as well as the subtalar joint, and lies perpendicular to the long axis of the talus when the ankle is in neutral or in dorsiflexion. The ATFL and CFL create an angle of 105° when the subtalar joint is in neutral position. The CFL has the greatest resistance to inversion, and in cases of inversion mechanism injury both the ligament and the attached fibularis (peroneus) tendon sheath will be involved.
The PTFL, the strongest of the three lateral collateral ankle ligaments, is the least commonly injured ligament. It is an intracapsular structure, is trapezoidal in shape, and originates proximally from the posterior aspect of the fibular malleolar fossa and attaches distally to the posterior surface of the talus, the lateral tubercle of the posterior process of the talus and to the os trigonum, when present. The PTL has the highest yield force and ultimate load of the three ligaments (Siegler et al 1988).
The lateral talocalcaneal ligament is a smaller ligament that crosses over the lateral aspect of the subtalar joint, and may also be torn in cases of inversion mechanism injuries of the ankle. Another important structure subject to injury is the syndesmosis, which consists of the anterior and posteroinferior tibiofibular ligaments, the interosseous ligament and the interosseous membrane. In external rotation injuries the syndesmosis is frequently injured. The syndesmosis is one of the initial structures to be damaged in either a supination eversion injury or a pronation eversion injury.
The ankle is most stable in dorsiflexion, allowing the talus to be securely locked in the ankle mortise while providing for additional stability against inversion stresses. When the ankle plantar flexes there is more anterior talar translation (drawer) and talar inversion (tilt) (Johnson & Markold 1983). Although the ATFL is the main talar stabiliser, when the ankle is in a plantar-flexed position the talus will be held less securely and will be more unstable in the ankle joint mortise. In this position the ATFL will be under greater tension and subject to higher risk of injury.
In addition to the ATFL being subject to higher loads with inversion and plantar flexion, the CFL may also be subject to injury during high loads in increased inversion. Occasionally, discrete tears in the CFL occur if the foot is forcefully dorsiflexed and inverted. The PTF rarely suffers from injury except in severe cases of total dislocation of the ankle joint. Brostrom (1964, 1966) showed that 20% of inversion ankle injuries involve both the ATFL and CFL.
Ankle ligament injuries may be classified into one of three grades according to pathology, function and instability:
•
Grade I. A grade I ankle sprain is indicative of an injury in which the ATFL is stretched and some microscopic ligament fibres are torn, but no macroscopic ligament tears are present and the ankle joint is stable. Patients present with mild swelling, mild or no haemorrhage or ecchymosis along the lateral margin of the ankle, pin-point tenderness overlying the injured ligament, mildly restricted range of motion and possible inability to fully weight bear.
•
Grade II. The grade II ankle sprain is a more moderate injury to the lateral collateral ligaments and consists of a definite complete tear of the ATFL, with a partial tear of the CFL and a mild to moderate ankle instability. Clinical examination reveals moderate swelling and tenderness along the anterolateral aspect of the ankle, restricted range of motion, haemorrhage, ecchymosis and mild instability.
•
Grade III. The grade III sprain is a severe injury signified by complete rupture of both the ATFL, with capsular tear, and the CFL. Clinical findings reveal marked swelling, haemorrhage with severe ecchymosis along the lateral margin of the ankle and heel, and discrete tenderness overlying the ATFL, CFL and the anterolateral capsule. There will also be moderate to severe laxity of the ankle, with typical anterior drawer or talar inversion tilt demonstrated on testing, indicative of the instability of the joint.
Special tests can be performed to determine ankle instability (Fig. 13.24). The anterior drawer and talar tilt (inversion stress) tests are manual stress tests designed to evaluate the integrity of the ATF and CFL. The anterior drawer test is performed with the patient’s knee flexed at least to 45° to relax the gastrocnemius. When patients extend the knee, this tends to alter the resistance to movement of the talus on the tibia. The patient’s heel is grasped posteriorly and the tibia is stabilised anteriorly with the other hand. As the calcaneus is pulled forward, a posterior force is placed on the tibia. This will allow the practitioner to translate the foot forward at the tibiotalar joint. A positive anterior drawer will reveal a so-called ‘suction sign’ overlying the anterolateral aspect of the ankle (between the lateral margin of the fibula and the talar trochlea), with greater than 4 mm of anterior displacement when compared to the contralateral uninjured ankle. This reveals incompetence of the ATFL (Anderson et al 1952, Schon & Ouzounian 1991). The talar tilt test is performed by grasping the lateral aspect of the calcaneus with one hand, while the medial aspect of the tibia is stabilised with the other hand.
CASE STUDY 13.6
LATERAL ANKLE INSTABILITY
A 28-year-old woman presented with a complaint of repeated right lateral ankle sprains and pain of several months duration. One month later she began describing pain along the medial aspect of her right arch and heel. The patient has had previous trauma to the right ankle, with a subsequent avulsion loose bone body of the medial malleolus.
The patient has been taking self-prescribed NSAIDs and applying ice to the ankle and arch/heel.
Temporary insoles were recommended with an NSAID and stretching exercises.
The patient wears Birkenstock sandals.
PAST MEDICAL HISTORY
The patient had a history of asthma and ocular toxoplasmosis. Her past surgical history included anterior cruciate ligament repair in the right knee, septorhinoplasty, T&A.
Injuries. Fracture left arm at 4 years old, repeated ankle sprains over the years.
Medicines. Orthotricycline, over-the-counter NSAIDs, vitamins.
Allergies. None known.
Social history. Married, works as a cardiac rehabilitation exercise physiologist, does not smoke, no special diet.
Family history. Mother had hallux abducto valgus, diabetes, heart problems.
TREATMENT PLAN
Two months after her initial visit the patient had a cast taken for Birkenstock inserts. The patient stated that her plantar fasciitis had improved but she continued to experience pain along the lateral aspect of her right ankle.
Four months later she still had recurrent pain to the plantar fascia and the abductor hallucis muscle, secondary to excessive pronation, and weakness of the longitudinal arch. She continued to have pain along the lateral column of the right foot in the area of the adductor digiti quinti.
A therapeutic steroid injection was given in the right heel.
Five months later she continued to have infracalcaneal heel pain in the right foot.
The injection was not very helpful, but a second injection was given with the addition of physiotherapy. A Cox-2 NSAID was prescribed, with ice treatment and stretching. The patient was told to cease all impact activity. She was about to leave on a vacation during which she would be walking often.
Six months after the initial visit the patient reported that the therapeutic steroid injection had been beneficial, but the pain had recurred and there seemed to be no improvement; however, compared to the first visit there was definite improvement.
On her vacation she walked excessively and now has significant pain and discomfort.
Physical therapy was given. The prescribed Cox-2 NSAID had not been effective, and the patient has begun to take over-the-counter NSAIDs. The patient was doing stretching exercises and applying ice, as well as receiving physiotherapy and cross-friction massage therapy.
An MRI scan was ordered to evaluate the ankle and heel; in addition, a night splint and neuromuscular stimulator were ordered. As a result of the review of the MRI a Cam-walker boot was ordered.
Seven months after the initial visit the patient continued to use the Cam-walker boot and continued to have infracalcaneal heel pain and ankle pain.
She continued to have physiotherapy treatment which seemed to produce a small improvement. Pain levels according to the therapist were 5/10, with a long gait, and right ankle plantar flexion was 4/5.
An attempt was made to increase strength, particularly with regard to right inversion and plantar flexion, and to improve prolonged standing and gait.
Seven and a half months after the initial visit the patient continued to have pain, which was increasing in severity and consistency. The patient had difficulty walking, with distinct pain in the right heel. A therapeutic steroid injection was attempted again (last of three).
Eight months after the initial visit the patient continued to have chronic plantar fasciitis, with no relief after exhaustive conservative care and physiotherapy. The patient had been cooperative, undertaking stretching, using ice, wearing orthotics, taking NSAIDs, and undergoing a series of three injections, with no improvement noted. Surgical intervention was recommended.
Nine months after the initial visit surgical correction was performed.
Procedures. Endoscopic plantar fasciotomy with decompression of the medial calcaneal nerve branch of the right heel. Arthroscopic evaluation of the right ankle. Partial synovectomy. Repair of the anterior talofibular ligament of the right ankle with Panaloc absorbable tissue anchor and Panacryl suture.
A below-knee fibreglass posterior splint cast was applied to the right leg.
POSTOPERATIVE COURSE
One week after the operation the post-splint cast was removed. The area was re-dressed and the patient was fitted with a below-knee fibreglass non-weight-bearing cast.
Two weeks after the operation there the patient reported no pain and the cast and the sutures were removed. There was minimal oedema and no erythema. The limb was placed in a below-knee Cam-walker boot and a CPM machine was ordered. Physiotherapy was to commence in one week.
Two months after the operation the incision had healed well, and the scar overlying the anterior talofibular ligament repair was reduced. The incision overlying the right heel had completely healed, but had left some scar tissue thickness. As a consequence of this there was some compensating gait to the forefoot and the lateral side of the foot. It was recommended that the patient wear a running shoe with an ankle brace. The range of motion of the ankle has improved and lateral ankle stability has been restored. There was mild discomfort in the right heel and arch following the operation. There was no pain in the right ankle. The patient was able to resume work on light duties.
Three months after the operation the patient’s gait was compensating, with a shortened heel strike, an extended forefoot contact phase, and to the lateral column. There was compensatory pain to the forefoot, along the fifth metatarsal head, and fifth metatarsal–cuboid joint.
A biomechanical evaluation was performed for a prescription sport orthotic device.
Heel pain has resolved and the scar is minimal.
The ankle is symptom free and is now stable.
With the ankle at 0° of dorsiflexion, the examiner inverts the calcaneus to its maximum. Under normal conditions, talus inversion is limited; however, when there is excessive excursion of the talus, the ATFL is suspected of rupture. Additional lateral dimpling is indicative of CFL injury or rupture. These tests should be performed with comparison with the contralateral side to rule out ligamentous laxity of an uninjured patient. In cases of acute injury local anaesthesia may be needed to perform the test adequately while preventing involuntary guarding by the patient.
Radiographic evaluation should be performed in addition to the anterior drawer and inversion talar tilt ankle joint stress views, and should include anteroposterior, lateral and mortise views of the ankle joint (Fig. 13.25). A stress inversion of 5–10° or greater of the injured versus uninjured side is considered to be pathological. An inversion stress of 18° or greater between the two sides is indicative of a double ligamentous injury (Pearlman et al 1992).
Karlsson et al (1989) found that anterior translation of the talus of 10 mm and talar tilt of 9° or more reliably indicate mechanical instability. The anterior drawer and inversion stress tests afford only a value in degrees and not a clinical picture. Ankles that have ligamentous laxity, or have higher than normal numerical values may indeed be normal and not show clinical signs of instability. Therefore, the clinician should not hold these study values to be a substitute for further investigation, nor should they be an empirical determinant for surgical intervention. An injury that produces syndesmosis can be evaluated using an external rotation stress radiograph. Widening of the tibiofibular clear space on the anteroposterior and mortise views of more than 6 mm indicates diastasis (Harper & Keller 1980).
Additionally, the extensor retinaculum and periosteal structures may also be involved in lateral ankle sprains. Upon radiographic evaluation the practitioner may notice an avulsion or osteochondral fracture associated with an inversion sprain. An avulsion fracture of the posterior aspect of the distal tibia can also be a sign of an injury causing syndesmosis.
There are a number of other tests that can be performed to determine ligamentous injury and ankle instability. These include:
•
Ankle arthrography, which radiographically reveals leakage of contrast dye from the lateral ankle joint and capsule. To obtain an accurate finding, this test should be performed within 48 hours of the injury to be truly effective. In addition, the exact location of the injury can also be determined. With rupture of the deltoid ligament, leakage of the dye will be inferior to the tip of the medial malleolus, radiating superiorly along the medial aspect of the ankle.
•
Peroneal tenography, which can be used to determine injury to the CFL. As mentioned earlier, the CFL is closely attached to the fibres of the peroneus tendon sheath, so that rupture of the CFL will show contrast dye extravasated from the tendon sheath into the lateroposterior recess of the ankle joint.
•
MRI examination, which has become the benchmark for non-invasive investigation of collateral ligament injury and instability. MRI can help to pinpoint specific soft-tissue injury
such as to the ligaments, capsuler or tendon, without exposing the patient to unnecessary radiation. MRI will determine the nature of both the medial or lateral ankle ligament injury by reflection of T1- and T2-weighted imaging. These studies will show oedema, haemorrhage, alteration of normal ligament contour (wavy) and interruption of ligament structure. A three-plane study can help to reveal rupture of the lateral collateral ligaments, deltoid ligament, peroneus or posterior tibial tendon. The clinician and radiologist can collaborate by using the history of the injury and MRI findings to determine the extent of the injury.
•
CT scans can be used to determine osseous injuries to the ankle that may not be seen on a radiograph. One of the advantages of CT scans is their precise recreation of minute injuries, such as osteochondritis dissicans, small avulsion fractures and loose joint bodies (
joint mice).
•
Arthroscopic evaluation of the injured ankle may be used as another investigative as well as therapeutic tool. With arthroscopic visualisation, excision of small bone fragments, hypertrophied synovium, chronic synovitis and osteochondral defects, as well as repair of soft-tissue and bony impingement syndromes can be performed, rendering the patient free of ankle pain (
Jarvin & Fercel 1994).
Initial conservative treatment for an acute lateral ankle injury, as well as chronic ankle instability, has proven to be most reliable. The focus of attention for the patient is on functional rehabilitation (range of motion, muscle strengthening and proprioceptive training). Specific areas of strengthening should include the fibular (peroneal) muscles, with stretching and flexibility of the Achilles and anterior tendon groups. Initial treatment should include rest, ice, compression and elevation (RICE), and NSAIDs. Initial conservative treatment may also include short-term cast or Cam walker immobilisation to allow the capsular and ligamentous structure to heal. On occasion, continued passive motion machine (CPM) treatment may help to restore the range of motion in the sagittal plane. High-top athletic shoes, taping before sporting events, air splints, ankle braces and orthoses may all aid in the prevention of recurrent ankle injury. Sports that involve side-to-side movement may predispose to recurrent injury, and thus may force the participant to cross-train during the rehabilitation programme (Box 13.7).
Box 13.7 Ankle sprains: treatment
Initial visit
•
History, mechanism of injury, determine severity of injury: radiographs if fracture is suspected – fifth metatarsal base, beak of calcaneus, os trigonum, fibular neck, fibular and tibial malleolus
•
Galvanic nerve stimulation if acute injury or swelling
•
Contrast foot baths, ultrasound if chronic swelling
•
Compression stocking or Unna boot for acute injury with surgical shoe
•
Ankle brace, air splint, or Cam walker removable cast for more severe ankle injuries
•
Weight bearing as tolerated, with crutches if able to be performed
Second visit
•
Continue contrast footbaths, Unna boot with compression wrap
•
Increase range of motion – therabands, towel
•
Continue physical therapy – nerve stimulation, ultrasound and iontophoresis – if swelling is present
•
Begin stretching exercises, Achilles, gastrocnemius–soleus, hamstrings, anterior groups
•
Gait analysis – check for compensation (correct when seen)
•
Check lateral shoe wear or lateral distortion of heel counter
•
When swelling is reduced, test for ankle instability (inversion stress, anterior drawer) and ankle strength
Third visit
•
Begin ankle strengthening (progressive programme) – fibularis (peroneus) and posterior tibial muscles
•
Increase ankle joint range of motion
•
Continue contrast footbaths
•
Continue physical therapy – nerve stimulation, ultrasound
•
Start toe raises, comparing affected and unaffected sides
•
Ankle strapping if needed
Fourth visit
•
Progressive ankle strengthening – fibularis (peroneus) muscles
•
Continue contrast footbaths, stretching range of motion
•
Teach strapping technique to patient
•
Encourage wearing of high-top athletic shoes, ankle brace, ankle strapping
•
Fabricate orthoses for biomechanical imbalances – rearfoot and forefoot varus
•
Begin proprioceptive exercises – roller-beam, side-to-side running, cutting drills
Subsequent visits
•
If chronic recurrent sprain, repeat ankle stress tests and order MRI evaluation
•
If ankle instability continues to be present consider stabilisation procedure
In some cases the athlete may experience residual pain and swelling for 6 weeks after the initial sprain due to injury of the ligaments and inflammation of the ankle joint. Pain on palpation may be elicited overlying the ATFL, sinus tarsi and CFL. Inversion of the subtalar joint may also elicit pain. In severe ankle sprains, ankle ligaments may not heal properly, there may be malalignment of the ankle joint, capsular tissues may heal with fibrosis and scarring, with associated hypertrophied synovitis. Chronic lateral ankle instability with recurrent sprain in the athlete is estimated to develop in approximately 20% of the injuries, regardless of the treatment (Moller-Larsen et al 1988, Rijke et al 1988). The athlete who suffers from chronic lateral ankle instability is subject to chronic pain, swelling, inflammation, recurrent injury and reduced functional stability of the ankle. Subotnick (1999c) states that this, in turn, creates a psychological fear of re-injury, impairing maximum performance as well as causing loss of training time. If, after investigative studies have been performed and exhaustive conservative care has been rendered, recurrent injury prevails and performance is hindered, surgical repair of the athlete’s unstable ankle may be required.
Surgical repair for chronic lateral ankle instability can be classified as either reparative or reconstructive. Reparative procedures involve re-establishing damaged ligamentous structures, whereas reconstructive procedures involve re-routing harvested tendons or grafts to substitute for failed ligaments (Schon & Ouzounian 1991). Reconstructive procedures can be divided according to the number of ligaments being strengthened. Single-ligament reconstruction will involve the ATFL, whereas double-ligament reconstruction (which is performed to correct anterolateral rotary instability) reconstructs the ATFL and CFL; a triple-ligament reconstruction rebuilds all three lateral collateral ligaments. Many surgical procedures have been described for repair of lateral ankle instability. The Brostrom (1965) procedure is one of the most popular, involving periosteal augmentation flaps to repair stretched out or torn ATF and CF ligaments. This repair is performed without fibularis (peroneus) tendon or other tissue harvesting, thus minimising surrounding ankle tissue damage while reconstructing the ligaments in their true physiological orientation.
Arthroscopic surgical repair of ruptured lateral collateral ankle ligaments with the use of soft-tissue anchors has been a significant advance in the treatment of chronic instability in the athlete. Although technically more difficult to perform than the open stabilisation procedures, this procedure has negated the need for tendon harvesting, minimises the disturbance of adjacent soft-tissue structures and enhances recovery by reducing recovery time. For the athlete, all these are very important factors.
This author is quite aggressive with the postoperative treatment of these delayed primary ligament repairs. Initially, a Jones compression dressing with a posterior splint is utilised to hold the foot and ankle in 0° ankle joint dorsiflexion to provide stability and control immediate postoperative oedema. Seven days postoperatively the patient returns for the first dressing change, and the splint is replaced by either a fibreglass cast or removable cast brace. The advantage of the cast brace is that it allows for gentle CPM to begin at 7–10 days postoperatively. This motion helps to prevent the occurrence of postoperative adhesions, capsulardesis and reduced ankle joint range. The Cam walker is worn for 4–6 weeks postoperatively, followed by a more aggressive physical therapy programme involving muscle strengthening, flexibility and proprioception exercises. After 2–3 months the patient is allowed to slowly return to activities. An ankle brace and orthoses are highly recommended to initially provide for additional lateral support and to help improve early restoration of proprioception.
LEG INJURIES
Chronic leg pain in the athlete
Chronic sports-related leg pain is a problem that many athletes experience. In the past, chronic lower leg pain was often regarded as ‘shin splints’ and recognised as an overuse injury due to excessive training or competitive-level participation. Today, the sports medicine specialist can target the underlying aetiology behind the development of the lower leg pain and determine the precise diagnosis. The goals for the specialist are specific: early and accurate diagnosis, a proper treatment plan, a specific rehabilitation programme, and prevention of recurrent injury.
There are many origins of sports-related lower leg pain. Tissues such as bone, muscle, tendon or insertion, ligament, nerves, arteries, veins and even skin can be the source of lower leg pain. The pain can be classified as traumatic or atraumatic in origin. Atraumatic exercise-induced injuries include medial tibial stress syndrome, stress fractures, gastrocnemius–soleus muscle strains, iliotibial band friction syndrome, tendinitis; compression syndromes (e.g. chronic compartment syndrome, nerve entrapment syndromes (fibularis (peroneus), tibial, popliteal)), arterial occlusion (popliteal artery syndrome) and radiculopathies, and muscle–fascial injuries, including fascial herniations, muscle soreness and muscle cramps. A number of authors have evaluated these conditions and have shown that a significant number of sufferers are athletes, particularly runners (Clanton & Schon 1993, Jones & Jones 1987, Orava & Puranen 1979, Styf 1989).
Tibial fasciitis – shin splints
Tibial fasciitis is an overuse inflammatory condition localised to the posteromedial crest of the tibia, with occasional involvement of the anterior crest of the tibia. Among runners and other athletes. This has been shown to be the most common cause of lower leg pain among runners and other athletes (Clanton & Schon 1993).
Tibial fasciitis is commonly referred to as posterior and anterior shin splints (Siocum 1967). The syndrome has been recognised as a medial tibial stress syndrome, resulting from overuse of the soleus fascia as it inserts on the posteromedial crest of the tibia, or the periosteal tissue beneath the posterior tibial muscle. Medial tibial stress syndrome may be due to either periostitis of the tibia or insertional fasciitis of the posterior tibial or soleus muscle; however, the clinician must exclude other possible entities, such as muscle strain of those same muscles, tibial stress fracture, deep posterior compartment syndrome and direct blunt injury to the muscle or bone. Detner (1986) developed a classification and management system for medial stress syndrome, dividing the overuse injury into three categories:
•
Type I represents a stress fracture or stress reaction of bone.
•
Type II identifies a chronic periosteal reaction due to excessive pulling of the soleus fascia.
•
Type III is associated with posterior tibial or posterior compartment syndrome. This is often seen with running as well as jumping sports.
Medial tibial stress syndrome (MTSS) is due to overuse or beginning an exercise programme too vigorously. There has been general agreement that certain underlying biomechanical factors contribute to the development of MTSS. Excessive pronation of the foot, as well as exercising on hard surfaces, may enhance eccentric contractions of leg muscles and contribute to the development of MTSS (Michael & Holder 1977, Richie et al 1993, Vitasalo & Kvist 1983). For runners who are excessive pronators, prolonged muscle strain and fatigue will develop. As pronation increases due to elongation of the running gait cycle, there will be a need for increased supination by the posterior tibial tendon. In the shin splint syndrome, abnormal pronation will lead to fatigue of the muscles and reduce shock absorption. With traction enthesopathy along the lower third of the tibia, pain will develop due to the periostitis at the muscle attachment. This will create increased pressure on the fascia by the tendon unit, focused on the fascia–periosteum interface along the tibial crest. Impact shock transmits with every running or jumping step that is taken. These ‘shock waves’ are transmitted along the tibia, interfering with healing of the ‘stress reaction’ along the fascia–periosteum attachment. This will lead to further injury of reparative bone cells, and prevent remodelling of damaged bone. Without adequate rest time the process continues, and can worsen to the point where a stress fracture may occur.
The duration of symptoms can be divided into:
•
less than 2 weeks (acute)
•
more than 6 weeks (chronic).
The location of the symptoms is divided into posteromedial, anterior and combination. The severity of the symptoms uses the functional pain scale grades 1 to 4:
•
Grade 1 represents pain upon palpation of the medial tibial crest, with no pain during activity or running.
•
Grade 2 is indicative of pain or discomfort after activity or running, but not during activity or running.
•
Grade 3 reveals pain both during running and residual discomfort after -activity or running.
•
Grade 4 demonstrates pain and discomfort while engaged in simple walking and the patient will be unable to run without symptoms.
Participants will describe recurrent pain along the posteromedial border of the middle and distal third of the tibia, which will have a gradual onset and is exacerbated by activity and running and relieved by rest. There will be no pain or symptoms in the foot or ankle. Mild swelling may be present. Physical examination reveals pain upon palpation along the posteromedial border and to a lesser degree along the anterior margin of the tibia.
The pain can range in severity from a dull ache to severe pain, particularly with prolonged activity. Neurovascular status is unaffected by MTSS.
Radiographic findings are usually negative for MTSS, except for mild localised thickening of the cortex. On occasion, the clinician may want to order a triphasic bone scan to differentiate between a simple shin splint and a stress fracture. Bone scans normally will reveal localised periostitis. Occasionally, MRI evaluation may help to pinpoint the exact location of the injury.
Treatment
In the majority of cases of MTSS early conservative treatment will lead to a successful outcome. All sporting activity should be ceased immediately, accompanied by icing, immobilisation and, if necessary, NSAIDs. The patient is advised not to resume activity until they walk without pain.
A four-phase treatment programme is recommended for patients suffering from MTSS:
•
Phase 1, the acute phase, is directed towards decreasing pain and inflammation. The treatment as described above is designed during the acute phase, and can last days to weeks.
•
Phase 2, the rehabilitation phase, is focused on decreasing the pain and swelling even further, while attempting to decrease or prevent the formation of scar tissue, strengthen the deep fascia–bone interface, and maintain flexibility of surrounding soft-tissue structures. Deep compartment muscle exercise can help to strengthen the deep fascia–bone interface, while reducing the tension force to the deep facial insertion.
•
Phase 3, the functional phase, is designed to strengthen the fascia–bone interface and to prevent the tibia from experiencing excessive tension forces. This may be accomplished via the use of orthotics, strapping and taping, as well as the use of neoprene sleeves.
•
Phase 4, the return-to-activity phase, is aimed at returning the athlete to his or her desired sport and level of participation.
The clinician should consult with the athlete as to the preventative measures that should be taken to protect them from further injury. This prevention programme can be designed by:
•
evaluating the patient biomechanically and observing their running gait
•
assistance in proper shoe selection
•
creating a strengthening programme for weak muscle groups
•
establishing flexibility and a stretching programme with the aim of improving range of motion in joints
•
following a reasonable training programme that is consistent with the athlete’s physical abilities and goals.
Box 13.8 suggests a treatment regimen for shin splints.
Box 13.8 Shin splints: treatment
Initial visit
•
Ice massage to painful shin areas
•
Radiograph, if chronic swelling
•
Biomechanical evaluation with gait analysis if not painful
•
Athletic shoe evaluation
•
Antipronation shoe selection if a runner
•
Varus heel wedge or temporary insole support
•
Ankle strapping, muscle strengthening exercises for rearfoot invertors
•
Rest with cross-training to avoid pain and further injury
Second visit
•
Two weeks of physical therapy – ultrasound, iontophoresis if needed
•
Radiographic evaluation if a stress fracture is suspected
•
Flexibility and strengthening home exercises
•
Consider functional orthoses if excessive subtalar joint pronation is noted
•
Continue to rest and cross-train (non-impact activities)
•
Ice massage with neoprene shin sleeve
Third visit
•
Dispense functional foot orthoses
•
Continue physical therapy modalities to help reduce pain, inflammation and swelling
•
Re-test muscle strength and consider manual resistance exercises, isotonic exercises to increase strength, and eccentric (plyometric) exercises to improve speed work
•
Order technetium-99m or triphasic bone scan to rule out stress fracture
•
If pain continues, consider walking with crutches with a Cam walker removable cast
•
Cross-training (swimming, water running, stationary bicycle)
Fourth visit
•
If pain continues, consider possible compartment syndrome
•
Repeat bone scan and evaluate radioactive uptake pattern
•
Check to ensure functional foot orthoses are properly controlling excessive pronation and abnormal motion with current shoes
•
Evaluate shoes and consider alternative shoe recommendation
•
Consider non-weight-bearing Cam walker or cast immobilisation and walking with crutches
Fifth visit
•
If pain continues, consider test for compartment syndrome
•
If test is positive, consider surgical intervention with fasciotomy
•
If bone continues to be negative, continue prolonged rest
•
Continue to treat any signs of pain, swelling and tenderness with physical therapy modalities
•
Check to see what cross-training activities are being followed and if patient is compliant
•
If bone scan is positive, non-weight-bearing cast immobilisation and walking with crutches should be employed
Stress fracture of the tibia and fibula
Stress fractures or fatigue fractures of the tibia and fibula are frequently seen in runners (Colt & Spyropoulos 1979, McBryde 1985, Markey 1987, Shelbourne et al 1988). The tibia is the bone most commonly involved (Belken 1980, Bennell et al 1996, Morris & Blickenstaff 1967). Morris and Blickenstaff (1967) used the term fatigue fracture to describe the application of mild forces or stress with eventual alteration or disruption of a material, such as bone. Therefore, a stress fracture is not the result of a single occurrence but rather an ongoing process. A fracture may be the end result, but it is the product of continued applied forces on the bone creating a weakness by resorbing bone in advance of the laying down of new bone. Stress fractures have been described in many sports (Morris & Blickenstaff 1967, Orava 1980a, Radel et al 1992).
There have been a number of explanations for the development of stress fractures. The first hypothesis (Stanitski et al 1978) contends that stress overload causes muscle fatigue, creating a loss in shock absorption and allowing excessive forces to be transmitted to the underlying bone. The second hypothesis (Taunton et al 1981) states that stress fractures occur secondary to repeated muscular forces acting on the bone. Cortical and cancellous bone each have intrinsic properties. When a force, either compressive or tensile, is applied to the bone, stress is generated within the bone. Strain is then produced by a relative change in length. The stress–strain relationship is linear until the yield length is reached. The linear portion represents the elasticity of the bone, whereas beyond the yield strength the bone is irreversibly deformed, until it reaches the breaking point. It is at that point that the bone collapses in compression and separates in tension. A stress or fatigue fracture can occur due to repetitive small loads, or from a single large load. During these load cycles, microfractures develop that are transmitted through the bone until loads increase sufficiently to cause microstress fractures, which produce symptoms or a fracture. Compression, tension and rotation are some of the forces applied to the bones by other bones, ligaments, and muscle origins and insertions. An example of this principle was shown by Devas and Sweetnam (1956) who showed that stress fractures of the fibula are due to the contraction of the calf muscles that pull the fibula toward the tibia. In this case, the tension above the distal fibula is maximised due to a rigidly attached fibular malleolus.
Wolff’s law prescribes that bone remodels to the stress placed upon it in fairly predictable patterns. When osteoclastic activity outweighs osteoblastic activity, the bone is in a weakened state. An athlete who continues to exercise is at higher risk of developing a stress fracture until bone remodelling is complete. Complete cessation of all athletic activity is essential to allow the bone to remodel and thus be able to withstand stress that will be applied at a later time. Markey (1987) described two ‘phases’ of stress fractures. The first phase, characterised by osteoclastic activity, and radiographically noted by its rarefaction or lucency, is referred to as the fracture phase. The second phase, described as the healing phase, is shown radiographically to have increased sclerosis, cortical thickening and/or callus formation. Another term used to describe the response of bone remodelling to stress prior to stress fracture is a stress reaction (Jones et al 1989).
Stress fracture of the tibia may occur either at the medial plateau or the shaft. Approximately half of all stress fractures in athletes occur in the shaft of the tibia (Morris & Blickenstaff 1967). In ballet dancers, the middle third of the tibia is involved (Miller et al 1975) and in runners the area most frequently affected is the middle and distal thirds of the tibial shaft (Devas 1958, Devas & Sweetnam 1956). Stress fractures may also occur at the medial malleolus due to distance running or basketball or American football (Shelbourne et al 1988).
Stress fractures of the fibula generally occur in the lower third of the bone (Barrows 1940, 1948, Devas & Sweetnam 1956). Stress fractures are also seen in the proximal third of the fibula (Blair & Manley 1980), and are thought to occur as a result of the powerful muscle forces of the soleus, posterior tibial, fibular (peroneal) or flexor hallucis longus across the fibula during jumping exercise (Symeonides 1980).
Usually the athlete will describe a significant change or increase in their workout schedule. For the runner, this will be demonstrated by a sudden increase in mileage, inadequate footwear or change of shoes, downhill running, harder running surfaces or greater intensity (speed).
The athlete may have just resumed a training programme after injury, or be unprepared at the commencement of a new training programme. There is a gradual, onset of pain that develops over several weeks. The pain will usually begin after stressful activity and then recede upon rest. In runners, pain will usually be described as occurring towards the end of the run, with the intensity increasing and ultimately reaching a point where the pain becomes so severe that the athlete has to cease running. With rest, the runner will ordinarily experience relief; however, on the resumption of running the pain will reoccur, to the point where it occurs during everyday walking. The key for the clinician is to recognise the onset of a stress fracture and advise the athlete to refrain from all activity, otherwise a return to training or competition could result in a season-ending injury.
Physical examination will reveal an area of well-localised tenderness overlying the tibia or fibula. There will be pain on palpation, percussion pain, increased warmth, oedema or erythema. The patient will be unable to perform the one-legged hopping test without pain, and when treated with ultrasound the pain will be elicited.
Initially after injury, radiographs will be negative; however, after 2–3 weeks of symptoms subtle changes may be evident. These changes will include a periosteal reaction, denoted by a cortical radiolucency indicating bone resorption, followed by an increase in radiodensity, indicating cortical bone formation and thickening. A technetium-99m bone scan is most helpful when plain radiographic films are negative and the clinician suspects a stress fracture. In cases of stress fracture there will be an increased local uptake of contrast medium, which can stay positive for over a year despite the fact that healing has taken place. A triphasic or triple-phase bone scan can offer additional insight into the nature of the injury – whether it is a soft-tissue injury or a fracture, or an acute or chronic injury (Rupani et al 1985).
Treatment includes rest for at least 6–8 weeks, and if severe and chronic, 12 weeks may be necessary to allow for adequate bone healing and to prevent further injury. With rest, symptoms will resolve, and substitute cross-training activities are recommended. In the case of competitive athletes, prolonged cast immobilisation and walking with crutches may be required. On occasion, for a delayed healing fracture or malunion, pulsating electromagnetic fields (bone stimulation) may be employed to accelerate the bone-healing process (Rettig et al 1988). After a sufficient period of rest the participant can then proceed to a period of supervised rehabilitation. Again, it is essential that the clinician emphasise to the athlete the consequences of too early a return to weight-bearing activities. A slow, progressive return to full-impact activity is advised, with periods of rest or cross-training to allow for adequate recovery. The type and location of the fracture will help to determine the length of time before return to competition. For some anterior midtibia fractures an entire year of non-impact activity may be required before a return to full competition standard (Clanton & Solcher 1994). The athlete is not encouraged to return to activity until they have no symptoms of tenderness, and have achieved full range of motion and near-normal strength from their rehabilitation programme. A semirigid pneumatic-type tibial brace that allows the athlete a speedier return to competition than conventional treatment has been used, and continues to be scrutinised (Box 13.9).
Box 13.9 Tibial stress fractures: treatment
Initial visit
•
Radiographic evaluation if pain has been present for 2–3 weeks
•
Technetium-99m or triphasic bone scan if radiographs are inconclusive, suspicion of fracture, or pain present less than 2 weeks
•
Check athletic shoes for wear pattern and shock absorption
•
Stop all running and all impact activity; rest for 2–3 months to allow fracture to heal
•
Local physical therapy techniques to help reduce swelling and muscle soreness during rest
•
Flexibility and range of motion exercises
•
Cross-training activities (swimming, water-running, stationary bicycle) to maintain training effect
•
Perform gait analysis if patient is able to perform without pain
•
Make a return appointment when the patient has been pain-free for at least 2 weeks to re-evaluate the condition and to analyse possible biomechanical aetiology that will cause inadequate shock absorption
Second visit
•
Biomechanical evaluation – check for poor mechanics and for inadequate shock absorption
•
Recommend shoes with better shock absorption and antipronation control
•
Check bone scan results, if performed
•
Consider Spenco, Sorbothane or Superfeet insoles for increased padding in shoes
•
Continue non-impact cross-training activity if patient is pain free
•
Outline gradual return to activity programme if patient is pain free
•
Have runner patients refrain from all uphill and downhill running and interval training, reduce mileage and choose proper athletic shoes
•
Continue physical therapy if any swelling or tenderness is present
•
If pain returns with activity, or patient is unable to perform one-legged hopping test, arrange for follow-up appointment
Third visit
•
Repeat radiograph to see if tibial or fibular stress fracture is not healing
•
Check for
dreaded black line•
Consider other sources of pain – compartment syndrome, shin splints, muscle strain, fibular (peroneal) nerve entrapment
•
Continue physical therapy programme if pain persists
•
If fracture is seen, cast immobilisation with non-weight-bearing walking with crutches
•
Treat the localised tenderness and swelling, and when asymptomatic begin return to walk/run programme
•
If pain persists with return to exercise activity, arrange for follow-up visit
Fourth visit
•
Consider testing for compartment syndrome
•
Consider repeat of the technetium-99m or triphasic bone scan and evaluate for fracture or delayed union
•
Consider possible radiculopathy with pain referred from lower back
•
Consider prolonged period of rest with non-impact activities
The more serious anterior midshaft tibia stress fracture initially described by Burrows (1956) commonly goes on to delayed or non-union (Green et al 1985, Rettig et al 1988) and may go on to complete fracture (Brahms et al 1980). Conservative treatment for this extreme case may prove difficult and may require surgical excision, with bone grafting, as well as percutaneous drilling and/or intramedullary rodding (Barrick & Jackson 1992).
The key to success for the athlete is prevention. Recognising the aetiological factors that would lead to this type of injury is essential – for instance, biomechanical considerations (leg-length discrepancy, high tibial varum, tibial torsion, excessive pronation or cavus foot type), muscle imbalances, distorted, worn-out athletic shoes, and inappropriate training programmes should all be evaluated.
CASE STUDY 13.7
TIBIAL STRESS FRACTURE
A 29-year-old female runner presented with shin splint pain of 2 years duration. In addition, she had an iliotibial band injury 6 months ago from running. She has been running for about 2 years and started training for marathons. She saw a podiatrist for this problem about 2 years ago and had prescription orthotics made. These helped but did not resolve the condition completely, and she continued to have posterior shin splint pain.
She stated that she could run up to 7 miles without pain, but over that distance the onset of pain begins. Physical therapy has been attempted in the past, along with a strengthening programme for weak anterior group muscles. During her gym workouts she noticed no significant improvement. She ran in a half-marathon, but states that she could hardly walk or run after she completed the race.
X-RAY ANALYSIS
Anteroposterior and lateral weight-bearing radiographs of both feet revealed pronation of the subtalar joint, bilateral adduction of the talus with accessory navicular bones, bilateral varus rotation of the fifth digits, bilateral mild metatarsus primus elevatus, and bilateral midtarsal joint prolapse.
BIOMECHANICAL EVALUATION AND GAIT ANALYSIS
Barefooted and running gait analysis revealed right foot abduction, excessive subtalar and midtarsal joint pronation, right foot > left foot, and an abductory twist in the right foot.
TREATMENT PLAN
Improve existing orthotic devices by refurbishing and increasing rearfoot posting correction.
Technetium-99m bone scan to rule out a stress fracture along the medial posterior aspect of the tibial crest.
Vioxx 25 mg once daily with food was prescribed, together with icing, stretching exercises and rest from running.
Repeated technetium-99m bone scan in 3 months.
Acute and chronic compartment syndrome
Chronic compartment syndrome (CCS), less common than the acute compartment syndrome (ACS), can sometimes be confusing and make the diagnosis rather difficult. CCS is a condition that results from abnormally high intramuscular pressure during exercise or shortly afterwards. It may also be defined as a condition in which increased pressure within a limited anatomical space compromises the circulation and function of the tissues within that space, resulting in temporary or permanent damage to muscles and nerves (Matson 1975). ACS is generally secondary to trauma, although rarely it may develop due to vigorous exercise. CCS is an exercise-induced condition, with recurring symptoms when exercise is resumed and reduced symptoms when exercise is ceased. In a study by Detner (1986) of the 100 consecutive operative cases of CCS approximately 70% involved runners.
Anatomy
The leg is divided into four compartments: the anterior, lateral, deep posterior and superficial posterior (Fig. 13.26). Each compartment is separated from the others by osseous and fascial boundaries, except for the superficial posterior compartment, which has no osseous boundary and is separated only by fascia. The nerves, blood vessels and muscles are encased in compartments surrounded by non-stretch structures. The anterior compartment fascia extends from the anterior fibular (peroneal) septa and encompasses the anterior crest of the tibia. The deep posterior compartment fascia inserts into the posteromedial crest of the tibia and generally intersects the deep transverse fascia, soleus fascia and superficial posterior compartment fascia to form the intermuscular septum.
Everyday activity will result in an increase in capillary filtration to supply much-needed blood to the muscles. The muscles consequently will expand by 20–25% of their normal resting volume. Anteriorly, the crural fascia can expand to permit this increase in tissue volume. Compartment syndrome is caused by an increase in tissue pressure to a critical level, resulting in compromised tissue perfusion (Ashton 1975, Matson 1975). Physiologically, circulation to the microvasculature is impeded, creating a compromised condition of the intracompartmental musculature. Intracellular and extracellular fluid accumulation within the fascial space occurs. Venous and lymphatic compromise contributes to increased tissue pressure, creating further vascular compromise.
The typical history of the athlete with CCS is one of induced pain during exercise, or immediately upon conclusion of exercise, overlying the involved compartment. Pain will dramatically decline upon rest in the early stages. An aching or cramping pain will be described, even during simple walking. In the runner, however, pain will develop after a certain distance, time or intensity. Other symptoms may include shooting pains and leg weakness, with an inability to dorsiflex the foot (foot-drop), numbness and tingling to the leg, dorsum and plantar aspect of the foot, burning of the compartmental nerve, pain upon stretching of the involved muscles or tendons, a lump formation, or muscle herniation of the leg. Four nerves, each with sensory components, are present within the four lower leg compartments. It is rare to see all four compartments affected simultaneously but it is common to see bilateral involvement. The two compartments most commonly involved are the anterior and deep posterior compartments. Rorabeck (1986) postulated that compartment syndrome of the tibialis posterior could be regarded as a fifth compartment. With signs and symptoms of compressive neuropathy of the superficial and/or deep fibular (peroneal) nerves, the clinician should pay particular attention to those compartments. Muscle herniations through fascial defects have been found at a higher rate in compartment syndrome patients (approximately 30–60%) and can be a source of chronic pain with or without related nerve entrapment or compartment syndrome (Clanton & Schon 1993).
Radiographic evaluation is helpful to rule out other conditions that may be present, such as stress fractures, periostitis, occult bone tumours or acute trauma. MRI is advantageous because it provides a non-invasive means of assessing intracompartmental pressures simultaneously in all compartments (Amendola et al 1990). Another entity that may mimic a chronic compartment syndrome is intermittent claudication, which refers to a complex of symptoms characterised by pain in the muscles of the lower extremity with exercise; therefore, vascular examination may be required to rule out arterial insufficiency to that limb.
The diagnosis of CCS is first made on clinical evidence; however, measuring the intracompartmental pressure provides reproducible, objective documentation that is needed to confirm the diagnosis of CCS. There are four requisites necessary before the diagnosis of compartment syndrome can be made:
•
a limiting anatomical envelope
•
an increase in tissue pressure
•
compromised circulation
•
neuromuscular dysfunction.
There are a number of measuring systems that can be used to record pressures at rest, before and after exercise, and during exercise. They include the needle manometer, wick catheter, Rorabeck’s slit catheter and McDermott’s solid state transducer intracompartmental (STIC) catheter (for dynamic measurement of intracompartmental pressure) (Detner et al 1985, Mibarak 1981, Pedowitz et al 1990). CCS has also been described as an exertional compartment syndrome. Mibarak (1981) and Pedowitz et al (1990) developed criteria for the diagnosis utilising the wick catheter measurement of compartment pressure as follows. Utilising a wick or slit catheter technique under sterile conditions, the catheter is inserted into the involved compartment using local anaesthesia, and compartment pressures are then monitored at rest and during exercise. The presence of the catheter creates an exercise environment that produces pain, and eventually causes the subject to stop the exercise. Three pressures are recorded: the resting pressure, the pressure during exercise and the pressure after exercise – (1) pre-exercise pressure greater than 15 mmHg, (2) 1 minute post-exercise pressure greater than 30 mmHg, (3) 5 minutes post-exercise pressure greater than 20 mmHg. After 5 minutes the pressure levels should return to their pre-exercise levels. Levels can be monitored and, if the pressure exceeds the resting value by two times during exercise, a diagnosis of recurrent compartment (ACS or CCS) syndrome can be made.
If the ACS or CCS is confirmed, the goals of the athlete must be considered. If the athlete can reduce activities to a tolerable symptom level, surgery is not indicated. Conservative measures at this point are of little benefit, and the treatment indicated is surgical decompression by means of a fasciotomy of the involved compartment. The athletic patient should be thoroughly informed that they are at risk of developing an acute exertional compartment syndrome and that surgery has its inherent risks. Adequate decompression of the involved compartment is essential, without injury to the neurovascular structures or muscles below. Surgical decompressive fasciotomy has a 90% probability of producing significant improvement in or resolution of the symptoms (Martins et al 1984, Rorabeck et al 1988, Styf 1988). After fasciotomy there can be a decrease in strength by up to 20% of the compartment’s muscles (Garfin et al 1981, Mozan & Keagy 1969). The trade-off is that muscle weakness is usually offset by the tremendous pain relief and improved performance that the athlete will later achieve.
Muscle soreness
There are a number of additional lower leg problems that are exercise-induced. They do not fit into the category of overuse injuries or claudication syndromes; however, they have just as important an effect on the athlete. They include muscle soreness or delayed-onset muscle soreness (DOMS) (Armstrong 1984). Symptoms of DOMS usually increase in intensity within the first 24 hours after exercise, and peak at 24–72 hours, and subside over the next few days. DOMS can result from an overexertion of skeletal muscles, and although many people may experience DOMS, few ever seek medical attention.
Muscle cramps
Muscle cramps may occur in both the athlete and non-athlete alike. With muscle cramps, there is pain, a tightening of the muscle group, or both. Cramp is usually due to muscle fatigue, and the accumulation of metabolites during strenuous exercise, such as marathon running, triathlon swimming, cycling or running, or any other sport that involves prolonged muscle use or intensity. In some cases muscle cramps may require further investigation regarding their cause. Limb-length discrepancies, muscle weakness and inadequate preparation are just some of the potential aetiologies of cramp.
Muscle herniations
Muscle may bulge or herniate through normal fascial openings, through congenital fascial defects or via traumatic openings. Normal openings occur at the lateral or anterior compartment and in the deep posterior compartment. Congenital and traumatic herniations occur in the anterior and lateral compartments, and rarely overlying the medial or posterior leg.
Posterior tibial tendon dysfunction in the athlete
The posterior tibial tendon (PTT) is one of the main dynamic stabilisers of the hindfoot against valgus (eversion) deformity, and as such it is subject to repetitive overuse injury such as peritendinitis and rupture (Plattner 1989). PTT dysfunction has been described frequently as a form of progressive degeneration that may develop due to biomechanical conditions, such as excessive pronation. Often, PTT dysfunction is due to an intrinsic abnormality of the tendon. In cases of PTT dysfunction in which chronic tenosynovitis is a common predisposing factor, rupture of the tendon may often be seen (Mueller 1984). This is a chronic condition ranging from early swelling and pain, to chronic inflammation, to ultimate rupture. When the condition continues and chronic peritendinitis develops, the inflammatory process can cause the tendon to degenerate, gradually elongate, develop interstitial tears, and eventually rupture (Johnson & Strom 1989, Plattner 1989).
PTT dysfunction may have its origins in overuse, secondary to biomechanical and structural weaknesses, or may result from traumatic injury. With the increased participation in exercise and sport by people of all ages, the incidence of PTT tendinopathy has increased tremendously, particularly in older participants and in sports such as tennis, aerobic dance and walk/jogging.
Anatomy
The tibialis posterior muscle arises from the interosseous membrane and the adjacent surfaces of the tibia and fibula in the proximal third of the leg. The PTT is formed in the distal third of the leg encased in its own tendon sheath by the gathering of large muscle units (Fig. 13.27). The PTT travels posterior to the medial malleolus and anterior to the flexor digitorum longus, posterior tibial artery, vein and nerve, and flexor hallucis longus tendon, beneath the flexor retinaculum.
The tibialis posterior has multiple insertion sites on the plantar–medial aspect of the foot (Fig. 13.28). The most important insertion site is into the navicular tuberosity, with additional extensions into all three cuneiform bones, as well as the bases of the second, third and fourth metatarsals. As a result of this complex anatomical design and insertional attachment, the PTT is regarded as a plantar flexor of the ankle as well as a dynamic inverter (stabiliser) of the foot. During gait, the contraction of the tibialis posterior muscle creates subtalar inversion, locking both the calcaneocuboid and talonavicular joints and creating a rigid lever for forward propulsion of the foot over the metatarsal heads.
When the tibialis posterior does not function properly, inversion of the rearfoot will be greatly diminished. This will enable the overpowering of the gastrocnemius–soleus muscle group and will act on the medial column, more specifically the talonavicular joint. With this loss of PTT function, the fibularis (peroneus) brevis muscle and tendon act independently, which then creates a dynamic abduction and eversion force. As these dynamic forces occur (the action of the gastrocnemius–soleus muscle on the talonavicular joint, and the unopposed pull of the fibular (peroneal) brevis muscle and tendon), a gradual attenuation of the medial static constraints of the longitudinal arch occurs (Mann & Thompson 1985). Disruption of the tendon can result in a loss of integrity of the secondary soft-tissue support structures, namely, the deltoid ligament, talonavicular capsule and spring ligament of the mid- and rearfoot. These secondary static and dynamic restraints then become weaker, developing less mechanical advantage than the posterior tibial tendon, which eventually fails with repeated stress. This deformity of the tendon will create an increase in the valgus deformity of the calcaneus. The progressive deformity results in a medial subluxation (plantar flexion and adduction of the talus), calcaneal valgus and Achilles tendon rotation. Eventually, as this deformity of the PTT progresses, tightening of the Achilles tendon and heel takes place, contributing to the creation of an equinus deformity. Over a period of time of PTT dysfunction, a chronic malalignment of the talonavicular joint, medial column and rearfoot will occur. This flexible deformity continues to become more rigid and fixed with time. The relative strength of the tibialis posterior muscle is a function of its large cross-sectional area, and is greater than two times the strength of its primary antagonist, the fibularis (peroneus) brevis muscle (Sutherland 1966). Because the PTT has a short excursion, elongation of just 1 cm makes the tendon ineffective as the primary dynamic restraint to the longitudinal arch (Sutherland 1966). The deformity usually progresses to a painful flatfoot deformity, with pain described along the longitudinal arch, heel, medial ankle and sinus tarsi (Ross 1997).
Aetiology
The appearance of a unilateral flatfoot in the adult is a principal manifestation of PTT dysfunction (Funk et al 1986, Johnson 1983, Trevino & Baumhauer 1992). The PTT sheath may become inflamed, producing pain, swelling and tenosynovitis along its course. Tibialis posterior tendinitis can be traced to a number of aetiological factors:
•
degenerative tendon disease
•
iatrogenic (steroid injections)
•
shoe distortion (breakdown).
Overuse injuries can be seen in the runner or in other sports that involve excessive pronation and rearfoot imbalance. Inflammation of the tendon and sheath is directly related with strenuous athletic activity, and may occur in all age groups. A clinical picture of tenosynovitis affecting the PTT is seen as an overuse injury. Trauma often involves pronation, external rotation with possible fracture, and complete rupture of the PTT. An os tibiale externum or accessory navicular may also predispose the athlete to posterior tibial tendinitis, in particular following eversion-mechanism ankle injuries.
CASE STUDY 13.8
POSTERIOR TIBIAL TENDINITIS
A 44-year-old woman complained of pain along the course of the right posterior tibial tendon, and a pinpoint of tenderness over the right navicular bone. She also complained of pain along the lateral aspect of the fifth metatarsal base of the right foot.
The patient has been a runner for many years, and first presented 7 years ago with chronic tenosynovitis of her right Achilles tendon. She had a thickened tendon and paratenon with scar tissue formation. The condition was diagnosed on MRI as chronic tendinitis/tendinopathy within the distal Achilles tendon, as well as fluid accumulation/bursitis in the pre-Achilles bursa.
TREATMENT PLAN
All running and impact activities were ceased and physiotherapy treatment was arranged for chronic posterior tibial tenosynovitis, together with the application of ice and stretching exercises. Cox-2 NSAIDs were prescribed.
The patient’s old prescription orthotics were evaluated and found to be worn. The rearfoot and forefoot posts needed to be replaced. The original orthotic was 7 years old and a new biomechanical evaluation for prescription orthotics was performed.
BIOMECHANICAL EVALUATION
Medium arched foot type on and off weight bearing; first ray hypermobile and plantar flexed to dorsiflexed on weight bearing. Dorsiflexion of the hallux was normal.
Ankle joint dorsiflexion was limited to normal.
Subtalar joint range of motion. Inversion: right 20°, left 20°. Eversion: right 3°, left 2°. Subtalar neutral: right 2°, left 2°. Forefoot: right 2° varus, left 2° varus.
Gait analysis. Early heel lift-off, with an extended forefoot contact phase. Supinated heel strike, right > left, with lateral column impact. Tibial varum with mild abduction of the right foot. Pronation of the subtalar joint at midstance to toe-off phase of gait, right > left.
RESOLUTION OF POSTERIOR TIBIAL TENDINITIS
The patient’s pain had resolved after physiotherapy, rest from running and the use of new prescription orthotics. She had resumed her running and activity programme, with no recurrence of her previous complaints. She experienced no further pain on the navicular, the lateral base of the fifth metatarsal or the posterior tibial tendon.
History, physical examination and clinical findings
The history, physical examination and clinical findings of PTT dysfunction in the athlete reveal an overuse condition that, if diagnosed early in its course, can be treated successfully without further sequelae. The athlete with PTT disruption presents with a progressive acquired deformity of the foot and will have a painful flatfoot and difficulty in running or walking normally. The majority of PTT patients describe a gradual onset of the symptoms and the deformity, with disruptions of the PTT most commonly developing in women over 40 years of age (Funk et al 1986, Johnson 1983). Although the cause of PTT dysfunction is not known absolutely, it is believed to be multifactorial, with overuse being the underlying cause, resulting in a progressive degeneration and attenuation, with eventual partial or total rupture occurring when that tensile threshold has been reached. It has been suggested that the zone of hypervascularity between the medial malleolus and the navicular tuberosity is one of the major contributing factors in the development of PTT dysfunction (Frey et al 1990). This is also regarded as the most frequent site of degeneration. An underlying biomechanical weakness can be detected, such as a hypermobile first ray, an unstable medial column and longitudinal arch, and a functional calcaneal valgus. A poor biomechanical design of the tendon, as in a flexible flatfoot, will also predispose the tendon to mechanical abrasion as it courses around the malleolar pulley. In addition, leg-length discrepancy with asymmetrical pronation creating compensation also may be another contributing factor to the development of PTT dysfunction. This condition can be seen clearly when runners who pronate excessively run on one side of the road and back on the other side, or who constantly run in one direction (e.g. clockwise) on a track. This in itself creates a compensated short limb, and with the combined pronatory effect and flexible flatfoot the ingredients for PTT overuse injury are just right. It is rare to see ruptures of the PTT in young patients; however, traumatic PTT have been reported in the young athlete (Conti 1994, Woods & Leach 1991).
A clinical picture of PTT tenosynovitis reveals diffuse swelling, tenderness, warmth and pain at the medial aspect of the ankle and along the course of the tendon, into the medial proximal calf region. Patients will notice a progressive collapse of their longitudinal arch, ambulating on their medial ankle. It is often noted that abnormal shoe wear occurs towards the medial aspect of the outer sole of the heel. The runner, or other athlete, will describe fatigue in the early part of their activity, and a reduction in their ability to withstand prolonged activity. In some cases the athlete may be unable to participate altogether due to the pain and weakness of the foot.
Pain will be elicited over the medial aspect of the ankle by active inversion of the foot against resistance. Genu valgum of the knee may be seen, which can contribute to the painful flatfoot. The symptomatic foot will be abducted and will have a prolapsed or collapsed longitudinal arch. The athlete who presents with overuse injury and degeneration of the PTT will usually complain of pain distal to the medial malleolus. Radiographs are rarely useful in the evaluation of tenosynovitis (Kettlekamp & Alexander 1969). In advanced cases of PTT dysfunction lateral recess impingement involving the calcaneofibular ligament will occur, creating possible fibularis (peroneus) tendon irritation. In addition, a sinus tarsitis can develop with PTT dysfunction simultaneously.
Approximately half the patients with PTT recall a history of localised trauma (Mann 1993). It is essential to determine the exact location of the injury. Traumatic injuries that create partial or complete ruptures of the tendon will have focal pain at the insertion of the navicular bone. A traumatic eversion injury will affect both the spring ligament and the talonavicular capsule. Avulsions of the PTT at the insertion site of the navicular are not uncommon. Frequently, symptoms are related to athletic activity, with gradual weakness, fatigue and loss of strength during the propulsive (push-off) phase of gait. As a result of the instability of the medial column, deltoid ligament or spring ligament (plantar calcaneocuboid) insufficiency may contribute to the development of PTT dysfunction.
When PTT dysfunction has reached the final stages, pes plano valgus deformity will progress, causing a lateral shift in pain due to calcaneofibular impingement. Quite often the medial foot and ankle pain will resolve (Myerson 1996). In cases where the PTT has ruptured, inflammatory symptoms may be absent, while pain may be the major presenting complaint (Banks & McGlamry 1987). This can develop into an acquired flatfoot deformity, with inversion weakness or an inability of the rearfoot to invert at all. Weakness of the PTT can be seen by having the patient attempt to invert the rearfoot against a held plantar-flexed and everted position. This test isolates the PTT and attempts to neutralise the effect of the flexor hallucis longus and anterior tibial tendons from inverting the foot. It is helpful to be able to palpate the PTT posterior to the medial malleolus and feel it contract during inversion. A palpable contraction of the tendon with an acquired flatfoot deformity usually indicates that there is no rupture of the tendon. Rather, tendinitis, a partial tear of the PTT, rupture of the deltoid ligament or rupture of the spring ligament may be present. In some cases, excessive hypermobility of the talonavicular joint may be due to attenuation of the medial capsule and spring ligament complex.
Other tests for confirming injury to the PTT are the single-heel raise test and the ‘too-many-toes sign (Kerr & Henry 1989). A classic triad of deformities on weight bearing presents on physical examination:
•
valgus deflection of the heel
•
loss in height of the medial longitudinal arch
•
abduction of the forefoot ((too-many-toes sign).
This triad reveals a lack of supination of the foot and inversion of the heel when attempting to rise up on the toes. Thus, an inability to lift the heel off the ground is indicative of PTT dysfunction.
Other symptoms may include midlongitudinal arch pain, secondary to increased stress on the PTT, creating a weakened condition and allowing for an overpulling of the fibularis (peroneus) tendons. A significant degree of tension is produced on the surrounding ligamentous tissue, thus creating a soft-tissue shear of the talonavicular, calcaneocuboid and Lisfranc’s joints. The end result, subluxation of the lateral midtarsal joint, then occurs, creating forefoot abduction. The tibialis anterior will then become the dominant overpowering force, allowing a forefoot supinatus to develop. This increased tension on the PTT causes a change in the vascularity and a weakening of the tendon as it courses behind the distal–posterior aspect of the medial malleolus. The most hypovascular region of the tendon begins at 40 mm from its insertion to the navicular tuberosity, 1–1.5 cm distal to the medial malleolus, and extending proximally for 14 mm (Frey et al 1990, Johnson 1983, Mann & Thompson 1985).
Radiographic evidence is not a necessity before making the diagnosis of PTT dysfunction (Funk et al 1986, Mann & Thompson 1985, Rey 1953, Teasdale & Johnson 1994); however, it is important in the staging of the deformity (Teasdale & Johnson 1994). Standard anteroposterior and lateral weight-bearing radiographs of both feet, including weight-bearing radiographs of the ankle, should be performed to evaluate the athlete who has developed an acquired flatfoot deformity secondary to PTT dysfunction.
Early in the process the radiographic findings will be normal to minimal changes in the angle. As the deformity progresses, anteroposterior views will reveal a lateral subluxation of the talonavicular joint as the navicular slips laterally upon the talar head (Johnson & Strom 1989). The lateral view shows plantar flexing of the talus, decreased height of the longitudinal arch, collapse of the medial column, and prolapse of the talonavicular, naviculocuneiform and metatarsal cuneiform joints. The axial view may reveal a valgus deformity of the calcaneus. Weight-bearing anteroposterior views of the ankle are also important, particularly in the later stages of the deformity, to determine whether there are any arthritic changes or whether talar tilt is present.
Tenography, CT and MRI have been used to determine the nature of the injury and the level of pathology to the PTT (Alexander et al 1987, Beltran & Moscure 1990, Hogan 1993, Rosenberg et al 1986). Tenography has been useful in the past to evaluate the PTT, but it is an invasive procedure and has not always had positive results (Alexander et al 1987, Funk et al 1986). It can be helpful, however, when there is a rupture of the tendon and the site cannot be located, or if adhesions and fibrosis have developed. However, injection of the contrast dye into the PTT sheath can often be a difficult procedure. CT is directed more towards identifying osseous lesions and has been used to identify severe soft-tissue injuries or abnormalities of tendons.
More recently, advances in the detection of the PTT injury and dysfunction have been achieved through the use of the MRI (Conte et al 1992, Rosenberg et al 1986). Due to its superior soft-tissue contrast resolution, MRI provides a more complete view of the pathological changes than does CT (Conte et al 1992). MRI is the method of choice in the determining of pathological changes of the PTT. An additional advantage of MRI is that there is no risk to the patient from exposure to ionising radiation.
A variety of staging and classification systems have been developed for specific paratendinous structures. Funk et al (1986) developed a classification of four types of pathology involved with PTT dysfunction:
1.
avulsion from its insertion along the navicular tuberosity
2.
a midsubstance tendon rupture
3.
discontinuity of tendon tears without complete rupture
4.
an isolated tenosynovitis of the PTT (no tear of the tendon).
Conte et al (1992) also advanced a classification system of tears (types 1 to 3) of the PTT based on MRI. The classification reflects structural features and abnormal signals within the substance of the tendon:
•
in type I, the magnetic resonance image reveals one or two fine, longitudinal splits in the tendon, without patterns of degeneration
•
in type II, a wider longitudinal split in the tendon is seen, with intramural degeneration
•
in type III, there is more diffuse swelling and uniform degeneration of the tendon; some strands of the tendon may remain intact, or the tendon may be replaced with scar tissue formation.
Johnson and Strom (1989) devised a classification system for the staging of PTT dysfunction which has been modified by Myerson (1996). They classified PTT dysfunction abnormalities into three distinct stages, based on the presence or absence of rearfoot or transverse tarsal deformities, and the ability to achieve flexible reduction of the involved articulation.
•
Stage I reveals a normal length PTT affected by inflammation and associated peritendinitis or tendinosis. There is usually chronic medial ankle pain and swelling. The single heel raise test can be performed as the tendon is intact (
Fig. 13.29). Only a mild weakness and minimal deformity are present in this stage.
•
Stage II is a progressive process in which inflammation and degeneration occur, resulting in an elongated, attenuated PTT. As a result, secondary deformities develop as the midfoot pronates and abducts at the transverse tarsal joint. In addition, there will be a compensatory forefoot varus deformity. The longitudinal arch begins to flatten, whereas the rearfoot articulations remain normal in the non-weight-bearing state.
•
Stage III is characterised by a condition in which a rigid rearfoot and forefoot deformity develops. The rearfoot will be rigidly everted, and the forefoot abducted.
•
Stage IV demonstrates a valgus angulation of the talus, with concomitant degeneration of the ankle.
Funk et al (1986) reported that if the inflammatory process of PTT dysfunction is not interrupted by either surgical or non-surgical means, a persistent tenosynovitis (stage I) will progress to stages II and III.
In cases of acute athletic injury to the PTT, conservative non-surgical treatment is valuable in the initial stages, particularly in the presence of various underlying medical conditions that might preclude surgical intervention. Although conservative treatment may be of great benefit in the early stages, there is no guarantee that it will arrest the degenerative process. The initial treatment for PTT injury includes rest, ice, elevation, compression, NSAIDs and various types of immobilisation. In the later stages of acute PTT tenosynovitis a cast is usually indicated for several weeks or even months. The patient may be permitted to walk while wearing the cast or removable boot. However, the more practical approach is to be non-weight bearing. A comprehensive biomechanical evaluation of the lower extremity and a gait analysis should be performed in patients who have developed a flatfoot deformity. A semirigid orthosis with medial control to decrease hyperpronation and eversion of the heel and subtalar joint during the midstance phase of gait or running is prescribed. In the later stages of PTT dysfunction, an articulated ankle–foot orthosis is recommended. Physical therapy has been used as an additional treatment in reducing pain, inflammation, oedema and crepitus in the tendon sheath. On occasion, corticosteroid injections into the sheath have proved helpful in the immediate reduction of symptoms, but these injections may cause focal necrosis and lead to spontaneous rupture of the tendon, and thus their general use is contraindicated (Fadale & Wiggins 1994, Myerson 1996).
Surgical intervention is indicated only in severe cases of chronic tenosynovitis or tendon disruption that have not responded to conservative treatment. In cases of chronic tenosynovitis, characterised by changes in the sheath with degenerative changes in the tendon (as in stage I), debridement is indicated, with incising of the tendon sheath and excising of hypertrophied synovium, and decompression. This will help to decrease painful symptoms and permit improved function. The same condition is seen in attenuation of the Achilles and fibularis (peroneus) tendons. If the tendon is hypertrophied, an elliptical resection with repair can be performed. In cases in which partial tears have occurred, debridement followed by repair is required. In some cases an enlargement of the osseous groove inferior to the medial malleolus may be indicated. The objectives of the procedure are to reduce the girth size of the tendon and to provide an enlargement of the fibrous osseous groove to improve function of the tendon. Proximal Z-lengthening, tendon grafting and the Cobb procedure (taking the distal half of the anterior tibial tendon) are other procedures for direct repair of the PTT.
Postoperatively, the foot is immobilised, first in a pressure dressing followed by application of a posterior splint cast. The foot is placed in a plantar-flexed attitude to reduce stress and traction on the tendon and to allow for early passive range of motion. The patient is immobilised for a period of 3 weeks, followed by mild dorsiflexion and plantar flexion, in addition to inversion–eversion, beginning with passive range of motion and advancing to active range of motion.
In cases of chronic tenosynovitis secondary to PTT malposition, with a hypertrophied and/or accessory navicular, a modified Kidner procedure (rerouting of the tendon, with resection of the hypertrophied navicular bone and/or accessory bone) can be done using a Mitek GII or Panaloc (absorbable anchor) with Panacryl (Surgical Products, Inc., Westwood, MA) tissue anchor. The use of one or two tissue anchors to support the tendon attachment against the denuded bone helps to secure the tendon and improve the mechanical advantage of the tendon. This procedure assists in decreasing both strain and traction on the PTT in an athletic patient who does not need a tendon transfer or arthrodesis procedure, and affords a much earlier return to competitive participation (Ross 1997).
In severe stage II cases, transfer of the flexor digitorum longus (FDL) tendon is utilised. The procedure involves excision of the incompetent tendon or ruptured remains. While positioning the foot in plantar flexion and inversion, a side-to-side anastomosis of the PTT to the FDL tendon is performed and then into the navicular, together with partial translocation of the tibialis anterior into the navicular. The pulley posterior to the malleolus is recreated by repair of the flexor retinaculum (Funk et al 1986, Sutherland 1966). The distal stump of the FDL is tenodesed to the adjacent flexor hallucis longus. Tenodesis of the more proximal segment of the FDL to the myotendinous junction of the PTT is indicated only if the posterior tibial muscle has a normal colour and good elasticity (Goldner et al 1974, Johnson 1983, Mann & Thompson 1985). Immobilisation after this procedure may vary from 8 to 12 weeks.
Although the triple arthrodesis is regarded as the procedure of choice for patients with a severe deformity of the rearfoot with associated posterior tibial tendon dysfunction, long-term studies show that the procedure can lead to degenerative joint disease of the ankle and to the distal unfused joints. This is not a procedure for the athletic or extremely demanding active individual.
Athletic patients who are properly diagnosed as having PTT dysfunction and who are treated early in the onset of the disease with aggressive conservative management have a much more favourable prognosis and are able to return to sports participation much sooner. Even in cases where surgical intervention is inevitable, conservative treatment can serve as a stopgap measure.
Fibular (peroneal) tendinitis and tenosynovitis
The fibularis (peroneus) tendons and their synovial components can develop various conditions that may result in inflammation, pain, swelling or instability of the lateral ankle and lower extremity (Hatch 1994). Some of these conditions are a result of overuse, faulty athletic shoes, inappropriate training methods or traumatic injuries from a variety of sports that involve side-to-side or torsional activity. The fibularis (peroneus) tendons lie in a common synovial sheath, inferior to the superior fibular (peroneal) retinaculum, and then distally separate into their own sheaths. A thin mesomembrane separates the two tendon sheaths in the region of the fibular (peroneal) sulcus. The floor of the common sheath shares a close anatomical relationship with the talocalcaneal and calcaneofibular ligaments. Tears of the fibular (peroneal) retinaculum can result in anterior dislocation or recurrent subluxation of the fibularis (peroneus) tendons during impact exercise.
The fibularis (peroneus) tendons act by everting and plantar flexing the foot. They are also the primary lateral stabilisers of the ankle joint. Three types of injury to the fibularis (peroneus) tendons can occur in the athlete: overuse tendinitis, chronic subluxation and acute rupture. Biomechanical considerations, such as a plantar-flexed lateral column, can develop into a chronic subluxation of the cuboid, a fibular (peroneal) cuboid syndrome, or the two together. A forced dorsiflexion–eversion mechanism injury to the foot and ankle can result in acute traumatic fibularis (peroneus) tendon subluxation. This condition is seen in medial ankle sprains and is characterised by pain, chronic fibular (peroneal) tendinitis and instability of the ankle.
Fibular (peroneal) tendinitis is related to the pulley action of the lateral malleolus on the fibularis (peroneus) tendon. Mechanical stresses acting on the fibularis (peroneus) tendons as they course through the retrofibular sulcus cause a decrease in vascularity, chronic inflammation and degenerative changes. The athlete who presents with chronic fibular (peroneal) tendinitis will have pain in the retromalleolar area in addition to oedema and tenderness overlying the course of the tendon. When the clinician attempts to evert the foot against resistance pain will be produced overlying the tendon complex and lateral ankle.
Patients with chronic fibular (peroneal) tendonitis will relate a history of postinversion ankle injury, which was not severe enough to require immediate treatment at the time of injury. In addition to pain and tenderness along the course of the tendons, there may also be synovial oedema in the region. The task for the clinician is to determine whether one or both of the tendons are involved in the postinversion fibular (peroneal) tendinitis.
Treatment of painful, tender fibular (peroneal) tendinitis includes decreased activity, NSAIDs, ice massage and physical therapy. In some more severe cases, cast immobilisation may be necessary. Biomechanical factors should be considered, with orthoses being used to correct excessive supinated heel-strike. Rearfoot varus posting with a flange along the lateral margin of the device will assist in limiting stress on the fibularis (peroneus) tendons and limiting their traction.
In cases of a subluxing cuboid or fibular (peroneal) cuboid syndrome, cuboid manipulation may be performed. When pain is elicited along the plantar aspect of the foot from the cuboid to the first metatarsal, fibular (peroneal) cuboid syndrome should be suspected. Accommodative orthoses are recommended, with a 3–6 mm heel lift and a soft pad with a dancer’s cut-out under the first metatarsal. This allows relaxation and lengthening of this short tendon, preventing repeated traction and irritation. In a more severe case of fibular (peroneal) cuboid syndrome, in addition to manipulation of the cuboid a below-knee cast is applied, followed by active physical therapy and manipulation.
MRI or sonography of the lateral ankle region can assist in diagnosing chronic fibular (peroneal) tenosynovitis, focal degeneration and attenuation of the fibularis (peroneus) tendons. Standard radiographs may show an enlarged calcaneal tubercle, which may be responsible for chronic irritation, impingement and chronic synovitis. A CT scan can help in defining the retrofibular sulcus and assist in the planning of a surgical procedure to help correct the chronic subluxation of the fibularis (peroneus) tendons.
Subluxing fibularis (peroneus) tendons
The retrofibular groove is a shallow sulcus within which the fibularis (peroneus) brevis sits. Although the fibularis (peroneus) longus shares a common synovial sheath with the brevis, it does not make contact with the sulcus. The fibular (peroneal) complex is maintained by superior perineal retinaculum. Excessive tension on the fibular (peroneal) tendons while the foot is dorsiflexed and everted creates a slippage of the fibularis (peroneus) brevis tendon laterally and anteriorly, forcing the longus tendon with it into the synovial sheath. In addition to the subluxation of the fibular (peroneal) tendons, a rupture of the superior retinaculum may also develop. Pain and tenderness will occur along the course of the tendons, and recreating the same movement will usually reproduce a subluxation of the fibular (peroneal) tendons laterally and anteriorly across the fibular malleolus. Chronic subluxation will be accompanied by an audible click or pop as the tendon displaces and then relocates into the groove. Athletes who suffer from this disorder will often describe a feeling of ankle instability, that their ankle will ‘give way’. There are two types of subluxation in fibular (peroneal) tendons: chronic recurring and traumatic. In the case of traumatic subluxation, the superior retinaculum is ruptured as a result of subluxation of both the brevis and the longus in a forward direction, applying a tearing force on the retinacular sheath. Sports such as skiing, basketball, soccer and ice skating will often be responsible for a forced dorsiflexion, inversion mechanism injury, resulting in a powerful reflex muscular contracture of the fibular (peroneal) tendons. Inadequate treatment at the time of injury can result in a chronic subluxation. Ankle instability usually accompanies subluxation of the fibular (peroneal) tendon, and the ankle mortise should be checked. An acute fibular (peroneal) tendon subluxation will cause rupture of the superior fibular (peroneal) retinaculum together with an avulsion ‘fleck fracture’ at its insertion along the lateral border of the fibular malleolus. Chronic subluxation seldom requires treatment, although acute traumatic subluxation does require conservative treatment, in the form of a non-weight-bearing below-knee cast held at 90° for at least 4 weeks. Stress examination with inversion talar tilt measurements will help to determine whether a concomitant ankle stabilisation procedure is required.
When conservative management fails after chronic fibular (peroneal) dislocation, surgical repair is required. A variety of surgical procedures have been described in addition to the repair of the superior fibular (peroneal) retinaculum. They include posterior fibular groove reconstruction utilising a periosteal flap (Zoellner & Clancy 1979), rerouting of the fibular (peroneal) tendons under the calcaneal–fibular ligament (Sarmiento & Worf 1975), and a number of bone block reconstruction procedures.
An os peroneum in the athlete in combination with chronic fibular (peroneal) tendinitis can be a painful limiting injury, requiring surgical excision of the ossicle. The os peroneum is located lateral or deep to the fibularis (peroneus) longus, and in some cases is an integral part of the syndosmosis or synchondrosis at the cuboid (Sarratian 1983). Particular care must be taken to avoid severing any of the fibres of the fibularis (peroneus) longus during surgery.
Although rupture of the fibular (peroneal) tendons is rare, a partial tear or rupture is more common in cases of recalcitrant fibular (peroneal) tendinopathy. This occurs as a result of collagen fibre breakdown, focal degeneration and attenuation of the tendon fibres. These ruptures occur between the cuboid and the lateral malleolus, and may extend proximally to the myotendinous junction. Primary surgical repair is recommended to prevent instability of the ankle and overpowering of the invertors, leading to a foot that is unable to evert and plantar flex normally.
Overuse knee injuries
Overuse knee injuries are commonly seen in the practice of sports medicine. They occur when alignment is altered and when normal synchronous movement of the foot and lower extremity are altered. Examples of the causes of injuries of this type are a change or breakdown of footwear, unrelenting hard running surfaces, training, competitive techniques, equipment and biomechanical factors.
The athlete’s knee is frequently injured as a result of both direct trauma and overuse injury. Some of the common causes of knee pain in the sport participant include meniscal tears, patellofemoral joint instability, patellofemoral maltracking, patellar tendinitis, ligamentous injury (ACL, MCL, PCL, LCL), osteochondral injury (cartilaginous), chondromalacia patellae, and degenerative joint disease in the older exerciser. Some less common conditions are apophysitis of the tibial epiphysis (Osgood–Schlatter syndrome), discoid meniscus and knee plica, and some less common sources of knee pain in the athlete include (medially) semimembranosus tendinitis, pes anserinus bursitis, tibial collateral ligament bursitis and saphenous nerve entrapment. Anteriorly, less common sources of knee pain include Hoffa’s disease (proliferation of the fat pad following injury to the knee joint) and, laterally, iliotibial band syndrome, popliteus tendinitis and instability proximally of the tibiofibular joint. As the number of participants in sports and exercise increases, the sports medicine specialist is seeing these less common entities more frequently.
Definition
An overuse knee injury is a chronic, atraumatic injury, portrayed by chronic knee pain secondary to participation in sport. The athlete will deny any history of acute trauma to the knee joint. The physical examination will not reveal any instability of the knee joint due to ligamentous injury, and there will not be any signs of the knee joint locking or marked oedema, which would indicate meniscal damage or loose bone bodies within the joint. On occasion, there might be an accumulation of fluid around the joint, with tenderness in various areas surrounding the knee. Radiographic or arthroscopic evaluation of the knee usually does not reveal any pathological changes.
Symptoms
Pain may be present in the knee, or may be localised to the medial or lateral collateral ligaments, superior or inferior patellar tendon or the retropatellar region. The location of the pain may vary according to the individual and may be intermittent in nature, fluctuating from day to day. Sporting activity may exacerbate this resting knee pain. The participant with overuse knee pain may describe a feeling of weakness, aching or stiffness, which may at times be sharp and quite severe. Pain will be diminished or even extinguished by rest but will begin again once activity is resumed. Many runners and other athletes will be able to participate in their sport pain free, although pain may be experienced in the later stages of the activity or immediately afterwards. In the history, the patient may remember a particular episode that contributed to the overuse injury. Seen particularly in running, aerobics and alpine skiing, the pain is often associated with a change in terrain or running surface, or with too quick a return to a normal training programme after a prolonged period of inactivity.
Signs
Physical examination will reveal some form of biomechanical malalignment of the lower extremity and feet. Such malalignments include femoral anteversion, genu valgum or varum, tibial varum, subtalar varus, rearfoot varus or valgus, forefoot varus or valgus, and excessive pronation (Fig. 13.30). Muscle imbalance or muscle weakness can also have a bearing on overuse injury. This may also include atrophy of the quadriceps muscle group, weakened or tight hamstring muscles, or a tight gastrocnemius–soleus complex, despite the fact that there are no signs of ligamentous injury, meniscal insufficiency, internal derangement or swelling of the knee joint. Often this condition does not reveal any true pathology of the knee joint. For this reason, the clinician must obtain the proper athletic training history and observe the gait thoroughly.
Treatment
When abnormal pronation is the underlying cause, the foot imbalance must be corrected to re-establish a more normal lower leg alignment and neutrality of the knee. In this fashion, during propulsion the knee will be properly aligned and stable in propulsion. To correct this lower leg and structural imbalance, a functional orthosis must be employed. This will help the athlete to compensate for the structural abnormality and achieve a more ‘normal’ efficient gait.
Biomechanical causes of knee injuries
Various overuse knee injuries occur when the foot has to compensate for structural deformities in the lower leg or foot. This compensation is in the form of abnormal pronation of the subtalar joint, increasing the speed, or altering the amount of pronation or modifying the point in the gait cycle at which it normally occurs. Changes in subtalar joint pronation may have an adverse effect on the function of the lower limb, and later develop into chronic knee pain. The knee is the most common site for running injuries (James et al 1978, Van Mechlen 1992).
The knee joint is recognised for its design as an integral part of the impact-absorbing mechanism during running (Wosk & Voloshin 1981). Indirect shock absorption by knee flexion is also dependent on subtalar joint pronation (Root et al 1977). The knee is a ginglymoarthroidal-type joint, and for it to change to a flexed position transverse rotation between the tibia and femur must occur. At heel strike the knee is fully extended, and it must flex quickly to absorb the shock generated by heel strike. McMahon et al (1987) concluded that vertical stiffness increased with running speed and that at any given speed this stiffness could be reduced in a controlled fashion by running with greater knee flexion than normal. They also determined that the transmission of mechanical shock due to impact was very sensitive to the degree of knee flexion. At 35–45% of the support phase of the gait cycle, maximum pronation occurs in addition to maximum knee flexion. It is at the maximum point of flexion that the knee joint has its greatest degree of mobility and instability. Internal knee joint structures such as the ligaments and the menisci are at great risk of injury with the knee in this position. As pronation of the subtalar joint and flexion of the knee are initiated by ground reactive forces, shearing forces of the tibia will increase, and these combined with femoral rotation lead to knee injuries, the incidence of which has increased in recent years (Van Mechlen 1992).
During the propulsive phase, knee extension creates a more stable knee joint, as now the knee is subject to increased amounts of stress secondary to an extended lever arm. Due to synchronised knee and hip extension, a more effective use of the hip muscles occurs. This allows for a more effective propulsive phase of gait.
As the leg prepares for propulsion, the pelvis and the femur begin to rotate externally in phase with each other. The tibia will also attempt to rotate externally; however, an excessively pronated foot will prevent this from occurring. Twisting of the knee will develop, with the proximal portion of the knee joint rotating externally, while the distal portion of the joint is prevented from externally rotating and therefore develops an internal rotation. These effects of torque on the knee joint are at their greatest and most damaging after the midstance phase of gait when the foot is maximally pronated.
The efficiency of the biarticular gastrocnemius muscle is highly dependent on the degree of flexion at the knee. During the time the knee is fully flexed or extended, the displacement of the origin of the muscle produces a relative lengthening or shortening of the muscle, which is equal to or exceeds its length of contraction (Bates & Stergiou 1999a). Therefore, during the time the knee is extended, the gastrocnemius is passively stretched and is at its maximum efficiency, creating a power transfer from the quadriceps to the ankle. Conversely, when the knee is flexed, the gastrocnemius is relaxed and loses most of its efficiency. In this scenario, the soleus is the only active muscle, and it is very difficult for this to function without a knee that is extended during the process.
Malalignment and torque will then contribute to strain or injury to the structures of the knee, both intra-articularly and/or extra-articularly, as the demands of the sport increase. Impact injuries to the knee are also seen in knee malalignment conditions, as well as being as a result of the surfaces upon which the athlete performs.
The patellofemoral joint
This joint is subject to mechanical overuse injuries. The patella acts to decrease the friction of the quadriceps mechanism as it passes over the distal femoral condyles (Ficat & Hungerfred 1977); the patella acts as the sesamoid of the knee (Fig. 13.31). It works in a pulley-like groove on the femur, and serves as a fulcrum for the action of the quadriceps muscles. It guides the quadriceps complex and centralises the various actions of the four muscles of the quadriceps. These forces are then transmitted to the patellar tendon. The purpose of the patella within the extensor apparatus is to protect the tendon from friction and allow the extensor apparatus to withstand high compressive loads. The patella lengthens the extensor arm, providing a mechanical advantage for the quadriceps (Kaufer 1971).
Stability of the patellofemoral joint is due to a number of factors, including patellofemoral congruency, static ligamentous stabilisers and dynamic quadriceps and hamstring muscle stabilisers (Goodfellow et al 1976, Kaufer 1971). The patellotibial and the patellofemoral medial and lateral ligaments maintain proper tracking and keep the patella in the femoral groove (Kaplan 1962). The failure of any one of these stabilising factors will cause a malalignment and allow for unfavourable patellofemoral articulation, which will subsequently lead to increased loading on the articular surfaces.
Patellofemoral problems in runners
Patellofemoral joint pain is one of the most common stress-related injuries experienced by runners. Running biomechanics will guarantee a relatively smooth tracking of the patella in the femoral groove, and therefore it is unusual for the runner to experience an acute traumatic injury to the joint.
‘Runner’s knee syndrome’ is a mild lateral subluxation of the patella, and should not be mistaken for chondromalacia patellae. Runner’s knee can often be caused by an increased Q angle, or be due to excessive pronation of the foot (Fig. 13.32). Lateral shearing forces due to a malposition of the vastus medialis will cause subluxation of the patella over a runner’s career, and consequently will establish a new position for the patella to sit, thus creating an uneven pressure on the lateral surface of the femoral condyle. The patella will change shape appropriately as stress acts on it, causing it to adapt to this new position. Excessive pronation in running causes internal rotation of the tibia, thus increasing the impact shock to the patellofemoral joint region, leading to runner’s knee pain.
Runner’s knee syndrome develops because of poor running mechanics, malalignment problems, an increased Q angle, tibial varum, internal tibial torsion, a weakened quadriceps muscle group, hard running surfaces and faulty shoes. Women, due to their increased Q angle secondary to an anatomically wider pelvis, are at greater risk of suffering from this disorder. The use of prescription orthoses can help realign the foot, thus reducing the Q angle and torque, torsion and stress to the knee joint.
Chondromalacia patellae
Chondromalacia patellae has been associated with abnormalities of patellar tracking as well as changes in the contact forces of the patella. Malalignment with recurrent subluxation of the patella is probably one of the most common causes of chondromalacia (Outerbridge & Dunlop 1975). In cases where recurrent subluxation of the patella occurs, there will be damage to the patellar articular cartilage. High Q angles create greater tensile stress on the medial and, more specifically, the odd facet of the patella. Tensile fatigue has a direct effect on the medial or odd facet, contributing to the formation of an ‘open’ chondromalacic lesion. A ‘closed’ lesion of the patella is a result of a high Q angle combined with normal compressive forces producing high shear stress. A third lesion located over the lateral facet consists of a very firm, sclerotic cartilage. High compressive contact stresses act on the lateral facet in this patellar tracking disorder.
Chondromalacia is caused by a combination of factors that eventually push the patella out of its groove on the femur. These factors include: weakness of the vastus medialis muscle; an increased Q angle, creating an overpowering of the vastus lateralis muscle; and malalignment of the lower limb, creating excessive foot pronation and leading to increased tibial torsion and increased stress on the knee.
Chondromalacia is a degenerative process on the retropatellar surface. This has been classified in stages, from an early onset to an advanced stage of arthritis (Key et al 1999):
•
Stage I – a softening or degeneration of the articular cartilage
•
Stage II – a cleaving of the articular cartilage
•
Stage III – cleaving and fronds of the articular cartilage
•
Stage IV – a wearing away of the articular cartilage to subchondral bone.
On physical examination, the runner will complain of generalised, deep knee pain. In some cases, pain may be associated with the tracking areas of the patella, or the undersurfaces of the femoral condyles. The knee may be swollen, with a chronic effusion of synovial fluid. In severe cases, the clinician may elicit a positive patellofemoral grinding test. As previously mentioned, a high Q angle may be seen, as well as a patella that is malaligned. Quadriceps muscle testing will reveal a weak vastus medialis and a high insertion into the patella. The most important radiographic view is the axial or ‘skyline’ view, also known as the ‘sunrise’ or ‘sunset’ views. The infrapatellar view will consistently reveal the inferior surface of the patella in relation to the femoral condyles, without other bone projections obscuring the view. By taking views of both knees simultaneously, the clinician can determine both the morphology and the position of the patella in relation to the trochlear facets (Fig. 13.33). Radiographs may reveal osteophytic ‘spurs’.
To make the correct diagnosis of chondromalacia patellae, several differential diagnoses should be eliminated. These include: chronic synovitis, meniscal lesions, fat pad syndrome, pre-patellar bursitis, retropatellar bursitis, infrapatellar tendon tendinitis, medial synovial plica syndrome, pes anserinus bursitis and sprain of the retinaculum.
Conservative treatment is the most prudent plan, consisting of salicylates, NSAIDs, patellar stabilising devices, icing, ultrasound, nerve stimulation, massage, and a change of running programme and running surfaces. Intra-articular steroid injections are not recommended, as collagen and protein synthesis is reduced. Quadriceps exercises and iliotibial band stretching are highly recommended, particularly if the patient is a runner. Realignment of the maltracking patella can be accomplished with the use of orthotic therapy. Steadman (1979) has stated that pronation of the foot increases the patellofemoral angle, creating pressure and symptoms in the patellofemoral joint, and that either soft or rigid orthotics can be helpful in this disorder.
Surgical intervention consists of a lateral release with a concomitant medial imbrication of the vastus medialis to achieve a proximal realignment. Arthroscopic local debridement of the articular lesion is helpful if confined to one facet or if the chondromalacia is grade II or III in severity (McCarroll et al 1983).
Knee plica
The synovial plica is an embryonic fold or septum traversing the anterior compartment of the knee, and separating the suprapatellar pouch from the main body of the knee. In the embryo, the kneecap is surrounded by a large bursa that differentiates into the prepatellar, suprapatellar and the infrapatellar bursae (Fig. 13.34). These bursae can completely resorb during adulthood and fall into folds superiorly, laterally and medially. Commonly found in runners, these lesions can be asymptomatic, or can cause a great deal of pain and discomfort. Early in the course of patellofemoral pain the clinician should examine for the presence of a synovial plica. In athletic activity, trauma, overuse or long-distance running can cause the plica to become inflamed, thickened and fibrosed. A large plica can fold under the patella and cause lateral displacement. A medially fibrous type plica may also mimic a meniscal tear. This same plica can extend transversely across the joint and can insert into the medial aspect of the infrapatellar fat pad. During running, when the knee flexes and extends, this fibrous plica will run across the medial femoral condyle and create a great deal of pain.
The examiner can feel a shelf while the knee is flexed at about 30–40° and the thumb is rolled over the medial side of the patella. This test, referred to as the ‘thumb roll’, will elicit a snap or click that will be painful and strongly guarded. Arthroscopic evaluation may determine that the meniscus is normal, while a thickening of the synovial fold indicates that a synovial plica is present. This lesion may be excised arthroscopically, and the procedure has been proven to be very successful in eliminating knee pain in the athlete.
Iliotibial band friction syndrome
Iliotibial band friction syndrome is a painful, debilitating overuse injury that affects runners, particularly long-distance runners such as marathon runners and ultra-runners, and cyclists. This inflammatory disorder is an overuse injury caused by excessive friction from the iliotibial band (ITB) as it passes over the lateral femoral excrescence and possibly at Gerdy’s tubercle (Fig. 13.35). The injury develops as the knee flexes and extends during running, creating a friction ‘rub’ over the lateral femoral condyle, which is responsible for the inflammatory response. As the iliotibial tract passes distally, it remains in contact with the lateral intermuscular septum of the quadriceps muscles and inserts into a tubercle on the lateral tibial condyle. Some suggest that the ITB acts as an anterolateral stabiliser to the tibia (Kaplan 1958, Terry et al 1986). In extension, the ITB lies anterior to the lateral epicondyle of the femur, and as flexion over 30° occurs, the ITB passes over the condyle. Another underlying factor is that of significant tightness of the ITB, which is prevalent among many athletes and runners. Those runners who suddenly increase their mileage, increase intensity or include hill training in their programme are subject to developing this overuse injury. Runners who have biomechanical abnormalities such as a high degree of tibial varum, who are excessive supinators on heel strike, or who have hyperpronation after midstance are also more prone to this overuse injury. The pain will often be most intense at heel strike, while attempting to decelerate the limb (Nobel 1980). At the onset of the disorder, pain may develop after an extended run. When runners warm up adequately, painful symptoms seem to diminish. However, in more severe or chronic cases, the lateral knee will hurt even while running – the longer the distance run, the more severe the pain – with residual post-run effects. Other aetiological factors involved in the disorder include genu varum, an abnormally prominent lateral femoral epicondyle, and internal tibial torsion.
The ITB is a thickening of the fascia lata that extends from the iliac crest to insert into the lateral tibial condyle. The band is developed by insertions from the tensor fascia lata and gluteus maximus muscles. The syndrome is due to the inflammation of the ITB and bursa as well as the underlying periosteum of the lateral femoral condyle. Other differential diagnoses include patellofemoral dysfunction, biceps femoris tendinitis, popliteus tendinitis, lateral plica, lateral meniscus injury, degenerative joint disease and stress fracture.
The clinical presentation of ITB friction syndrome is generally tenderness at the point where the ITB slides over the lateral excrescence or at Gerdy’s tubercle. Pain may also extend along the course of the ITB, and may radiate proximally or distally. On occasion, soft-tissue swelling may be present, and there may also be palpable crepitus present at the same site. A positive ITB test will confirm the diagnosis. The test is performed by the clinician placing a varus stress upon the knee, and then fully extending and fully flexing it. During extension the ITB slides anteriorly, and during flexion it passes posteriorly. It is this sliding over the femoral excrescence that reproduces the pain. Another way of reproducing the pain is by means of the Nobel test: the lateral femoral epicondyle is palpated by the thumb, where no pain had been elicited previously. On occasion, if needed, MRI or an ultrasound scan can be employed to confirm the diagnosis (Ekman et al 1994).
Treatment for the early stages of the condition includes adequate stretching and warm-up, with heat or topical rub before a run, followed by additional stretching and ice massage afterwards. An ITB stretching programme is highly recommended for athletes with ITB friction syndrome (Box 13.10).
Box 13.10 Iliotibial band stretching programme
Each exercise to be done __ times a day; __ repetitions for each exercise.
Hold each stretch for 5–10 seconds.
Hip abductor stretch
Stand with the legs straight, feet together. Bend at the waist toward the side opposite of the leg to be stretched. The unaffected knee may be bent.
Iliotibial band stretch
Stand with knees straight, cross the leg to be stretched behind the other as far as possible. Stretch to the side of the leg in front.
Iliotibial band stretch
Same stance as above. Slightly bend the back knee. Move the trunk toward the unaffected side and the hips toward the affected side. Stretch will be felt along the outside of the bent knee.
Iliotibial band stretch/hamstring stretch
Stand with knees straight. Cross the legs so that the affected knee rests against the back of the unaffected leg. Turn the trunk away from the affected side as far as possible, reaching and attempting to touch the heel of the affected leg.
Iliotibial band stretch
Lie on the unaffected side with your back a few inches from the table edge. Bend the unaffected hip to maintain balance. Straighten the affected knee and place the leg over the edge of the table so the leg hangs straight. Let gravity pull the leg down, causing the stretch.
Iliotibial band stretch
Lie on the affected side with the knee locked and the leg in a straight line with the trunk; bend the upper knee. With your hands placed directly under the shoulders to bear the weight of the trunk, push up, extending your arms as far as possible. The affected leg must be kept straight to get maximum stretch in the hip.
In addition to the ITB stretching, rest or altering activity (e.g. shortening the duration of the training activity, avoiding hills, shortening stride length, changing directions on a circular or banked track, and running back on the same side of a crowned street) is advised, with controlling the inflammation process being the ultimate goal. Cyclists may need to change the height of their seat or their foot position on the bicycle pedal (Holmes et al 1993). When pain persists when running, alternative training programmes should be instituted, such as swimming, aqua-running and weight training. Cycling may exacerbate the ITB pain, and therefore should not be done.
Symptomatic treatment may also include iontophoresis, with a cortisone preparation. Bursal cortisone injections may also be employed in more severe cases. Again, initially ice should be used, followed by heat, whirlpool, ultrasound and NSAIDs. If the patient cannot run or exercise without pain after a course of alternative exercise and treatment, total rest for 4–6 weeks is recommended. For the most part, the great majority of athletes will respond favourably to conservative care (Box 13.11). Correction of biomechanical abnormalities may require only a simple insole or longitudinal arch support. However, in cases where there is an uncompensated inverted rearfoot, a prescription orthosis may be needed to prevent an excessive supinated heel strike, thus reducing the traction and stress on the ITB.
Box 13.11 Iliotibial band syndrome: treatment
Initial visit
•
Physical examination – perform Nobel’s compression test
•
Check for excessive iliotibial band tightness – Ober’s test
•
Rule out meniscal disease and other differential diagnoses
•
Check for tibial varum, excessive rotation or varus stress
•
Perform walking and running gait analysis
•
Check shoe wear pattern and/or distortion
•
Check for limb-length discrepancy
•
Prescribe iliotibial band stretching programme
•
Quadriceps strengthening programme with adductor strengthening programme
•
Ice massage to injured area
•
Alter athletic activity
If not improving significantly or re-flare with activity:
Second visit
•
Begin physical therapy: ultrasound, iontophoresis, followed by ice massage
•
Test for extensive rotation with foot/ankle taping
•
Recommend stability/motion control shoes and heel lift for limb-length discrepancy if seen
•
Continue stretching programme
•
Rest from all running or cycling activities if pain persists
If re-flare with return to activity:
Third visit
•
Consider possible meniscal disorder or collateral ligament injury
After 12 months of unsuccessful conservative treatment, surgical intervention may be considered. Release of the ITB at its attachment to the patella is one particular procedure that may be employed, as may a bursectomy.
Popliteus tendinitis
The popliteus muscle originates from the lateral femoral condyle and has a wide insertion on the posterior tibia above the soleal line. It passes superolaterally and anteriorly under the arcuate ligament, forming a tendon about 1 cm distal to the joint line. The tendon functions by:
•
maintaining internal rotation of the tibia on the femur
•
aiding the posterior cruciate ligament in preventing forward displacement of the femur on the tibia
This muscle will help stabilise the femur against forward displacement on the fixed tibia, particularly when running downhill.
For runners who run on a track, or a banked track, pain may be experienced on the lateral aspect of the knee, due to the internal rotation of the tibia on the femur.
Popliteus tendinitis is an overuse injury that is commonly seen in athletic individuals, particularly downhill runners and skiers, and those with excessive pronation.
Physical examination will reveal localised tenderness overlying the tendinous origin and along the lateral femoral condyle.
Treatment includes NSAIDs and restricting all downhill running, application of ice for acute symptoms, followed by whirlpool or moist heat, ultrasound, knee stretching and strengthening exercises. Alleviating stress to the popliteus is imperative. This can be accomplished by changing the side of the road or direction of running on a banked track, and by running uphill, not downhill.
Hamstring tendinitis
Injuries to the hamstring are due to a short flexor group. An athlete who does not stretch routinely or adequately is prone to a hamstring strain. Therefore, it is imperative that before engaging in any athletic activity the hamstring muscle group should be properly stretched. This is of particular importance before sprinting, running or during pre-season training. Careful attention during running to overstriding and/or oversprinting can help to prevent a hamstring injury.
Injury to the hamstring can occur at any site along its course. A strain will occur as a result of a sudden overextension of a tight hamstring, and be located either at the hip or the knee. Once a hamstring has been injured it will always be vulnerable to re-injury due to its intrinsic weakness.
On clinical examination, the hamstring will appear to be tight, and in cases where a partial or complete rupture has occurred there will be swelling, ecchymosis and/or haematoma formation. Pain will be elicited at the site of the injury, which is most commonly at the midthigh, or at the ischial tuberosity. A marathon runner who had experienced a cramp early in the race may have gone on to a partial rupture in the later stage of the race. Consequently, the runner will have pain against resistance, and may be unable to bear weight without excruciating pain.
In addition to tight hamstring muscle groups, repeated hamstring ‘pulls’ should alert the sports practitioner to the possibility of biomechanical malalignment. Examples include excessive subtalar pronation, genu valgum, internal rotation of the tibia, short limb and pelvic tilt.
In cases of internal tibial torsion, the origin of the hamstring and the insertion will be stretched and twisted, particularly in the latter stages of the propulsive phase of running. This also can be an underlying cause of injury, but will not be apparent to the untrained eye.
In cases of recurrent strain or pulls, or in chronic hamstring tendinitis, a biomechanical evaluation is essential to rule out poor foot imbalance and lower limb malalignment. In addition, muscle testing is also important to determine whether, for instance, the quadriceps muscle group is stronger than and overpowering the hamstring group. Muscle strengthening and flexibility exercises are essential to prevent recurrent injury. In some cases certain forms of sport may need to be altered or eliminated to prevent further injury. A daily stretching exercise programme to maintain flexibility and a pre-exercise warm-up should be part of the normal routine.
Groin injury/strain
A groin strain can be an extremely painful and debilitating injury, and it is commonly seen in sports such as soccer, football, tennis, rugby, and in sprinters, long-distance runners and other track and field events. The term groin strain incorporates many other conditions that cause pain in the groin region. The groin pain may occur suddenly, or be brought on by a series of traumas secondary to overuse. The structures involved in this area include the adductor muscles, inguinal ligaments, the pubis symphysis and ramus, the gracilis, the iliopsoas and the piriformis. A groin injury may be a result of a sudden violent overstretching of the leg in abduction and external rotation, particularly when there is an opposing force.
A groin injury may develop suddenly or insidiously, and the associated pain may be sharp or dull. In many cases the structure(s) involved may be difficult to identify. The injury presents in the early stages with a mild ache, following activity, which is then relieved by rest, but quickly returns during the subsequent period of activity. As the injury becomes more chronic, the pain will increase in severity, begin earlier in the activity and take longer to subside, until it reaches a point where there is a constant dull ache or pain. At this stage even walking may be painful. To test for the site of the pain, passive and active assisted motion of the patient’s hip with the leg extended in all directions, particularly abduction and external rotation, followed by careful palpation of the groin structures to pinpoint the site of injury is required.
For the most part, unless the injury was attributed to a sudden traumatic event, one should suspect a biomechanical origin. The most common aetiology for this biomechanical injury is a limb-length discrepancy, where the short limb pronates for a longer period of time than the longer limb. The hyperpronated foot creates a scenario in which increased internal rotation of the limb occurs, tilting the pelvis forward on that side. This effect on the limb, including the adductor muscle group and the iliopsoas, is to function with a twist, as the pelvis tilts downward. The adductor serves an important purpose in providing stability for the pelvis, particularly in sprinting as well as long-distance running. Functional control of the abnormality is essential to prevent recurrent or further injury.
Rest is the key to allow for healing of the groin strain or injury. Once the athlete stops all activity, pain subsides and the condition will resolve. In addition to rest, physical therapy, anti-inflammatory medication, and a well-designed and properly supervised rehabilitation programme should be part of the athlete’s post-injury care to return to a competitive level. Another goal in the treatment of groin injuries is to re-establish biomechanical control to the foot and limb. Limiting excessive pronation of the foot will assist in eliminating the twist in the leg, and in addition help to reduce some of the adverse effects of the limb-length difference. The use of orthotic devices will help to achieve this goal, while simultaneously helping to correct the pelvic tilt. The addition of a 4–7 mm heel lift to correct that imbalance or leg-length discrepancy is advised. To prevent groin injuries, the pelvis and lower extremity must be conditioned to withstand forces generated by the patient’s particular sport. Strengthening the groin muscles with exercise performed against resistance, and repetitions to build strength as well as endurance will help prevent recurrent injury. To maintain range of motion, special stretching exercises of the groin incorporating proprioceptive neuromuscular facilitation techniques should be used (Pink 1981).
LEG-LENGTH DISCREPANCY
Anatomical leg-length discrepancies are a result of the overgrowth or undergrowth of long bones. Functional limb-length discrepancies develop from a malpositioning of the various joints of the lower extremity. When a muscle group of one limb is shorter or overpowers the contralateral side, a functional limb-length discrepancy can develop. Another example is a hyperpronated foot that is more pronated than the contralateral foot, which creates a functional limb-length discrepancy and a lateral pelvic tilt.
A leg-length difference can be either anatomical or physiological. An anatomical leg-length difference describes a true anatomical difference that exists between the two legs. There is no variation in its measurement. The leg-length difference is seen in both the neutral calcaneal stance position and the relaxed calcaneal stance position. On the other hand, a physiological leg-length difference is the result of some other structural area or deviation that has had an effect on the leg length. Examples of a physiological leg-length difference include muscle imbalance and scoliosis, as well as abnormal biomechanics of the foot, which may originate above the pelvis or below the ankle.
Functional leg-length discrepancy may occur in sports where an overdevelopment of one limb produces a difference. Unilateral conditions such as these include iliotibial band syndrome, unilateral patellofemoral joint syndrome or chondromalacia, unilateral shin splints, unilateral posterior tibial tendinitis or Achilles tendinitis. Chronic heel pain in one foot versus an asymptomatic opposite heel is a clear clue that the patient may have a limb-length difference. It is incumbent on the clinician to perform a full biomechanical evaluation to determine where the asymmetry lies.
The biomechanical evaluation should include a measurement of both limbs so that the examiner can quantify the difference between the two. The measurement is taken from the anterior superior iliac spine to the medial malleolus. The femoral component should be measured at the joint line of the femur and the tibia. Knowing the anatomical landmarks and being consistent when measuring the two limbs is important. Measurements of the limb and limb segments, in both neutral and relaxed stances, can eliminate the foot as the source of the limb discrepancy. When examining the foot, evaluate both the forefoot and the rearfoot to determine whether there has been an injury or previous surgical intervention that might have led to some asymmetry.
An excellent way to determine whether there is an asymmetry in body position or gait is to watch the patient stand and walk while wearing a pair of shorts or swim suit. Start at the head and continue the observation down from the shoulders while looking for any tilt. The tilting of a shoulder could be indicative of a short side where the shoulder is tilting.
Look to see if the neck is curved. If a double scoliosis is present, there will be no ‘shoulder drop’. Next, look at the hips and pelvis and observe once again any tilt that might be present. Observe the hands to see if they are symmetrical, or if one hand is lower than the other during stance and gait. Draw a line down the spines of the vertebrae and, using a goniometer, measure the degree of deviation. Compare the scapulae and the sacral region, and look for any dimples. Rotation and twisting of the body takes place in the transverse plane; women are more prone to scoliosis than men, and thus these rotations may be more prevalent in women. Arm swing should alert the clinician to an asymmetry of the pelvis, with the opposing arm swinging more with the shorter leg.
It is very common for compensations for leg-length difference to be seen in the feet. The shorter side will supinate and maintain weight bearing on the outside of the foot while attempting to lengthen the limb. It can also function in an equinus position to prevent shortening of the limb. Conversely, the opposite will occur on the long-limb side. This foot will pronate more than normally to shorten the limb, and this will be evidenced by excessive medial shoe wear. It is estimated that 4–6 mm of shortening can occur with pronation at the subtalar joint.
Observe the runner or athlete in his or her running gait, and determine whether the stride is equal in comparison from both limbs. A shoulder drop, bouncing of the body or a short gait on one side versus the other shows an equinus gait and a short limb.
When an athlete relates a unilateral complaint (i.e. heel pain or knee pain), immediately suspect a limb-length discrepancy and check for any underlying aetiology. In the case of the heel pain the underlying cause could be a medial or lateral collateral ligament strain, or a muscle strain of the pes ansersartorius muscle insertion. A tight Achilles tendon or gastrocnemius–soleus complex secondary to one limb pronating more than the other is also indicative of a short limb.