Chapter 2 Inflammation
Inflammation
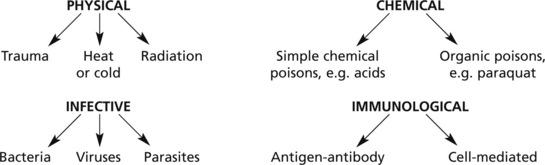
Inflammation is the dynamic process by which living tissues react to injury. They concern vascular and connective tissues particularly.
Various agents may kill or damage cells:
… and any other circumstance leading to tissue damage, e.g. vascular or hormonal disturbance.
The inflammatory reaction takes place in the surviving adjacent vascular and connective tissues; the specialised parenchymal cells do not directly participate.
The initial stages are known as the acute inflammatory reaction. Where the process is prolonged the inflammation may be subacute or chronic.
Acute Inflammation
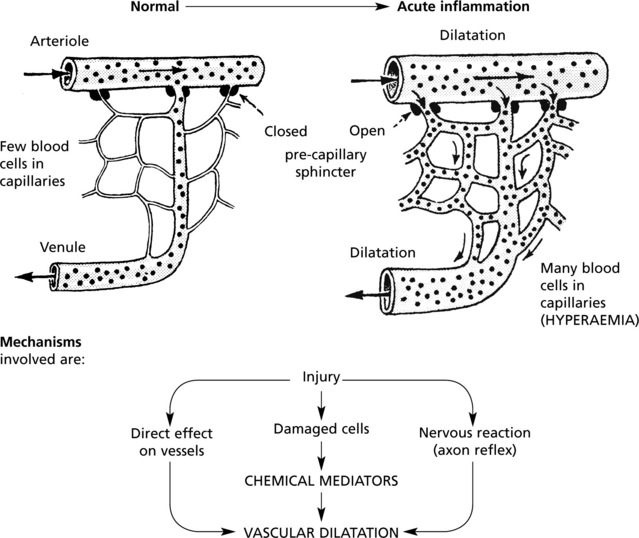
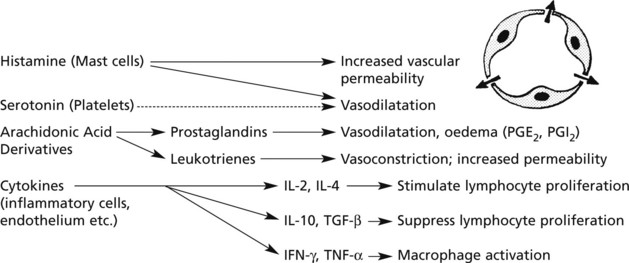
Hyperaemia
The hyperaemia in inflammation is associated with the well known microvascular changes which occur in Lewis’ triple response – a FLUSH, a FLARE and a WEAL. It occurs when a blunt instrument is drawn firmly across the skin and illustrates the vascular changes occurring in acute inflammation.
The stroke is marked momentarily by a white line due to vasoconstriction.
The flush, a dull red line, immediately follows and is due to capillary dilatation.
The flare, a bright red irregular surrounding zone, is due to arteriolar dilatation.
hyperaemia explains the classical signs of redness and heat.
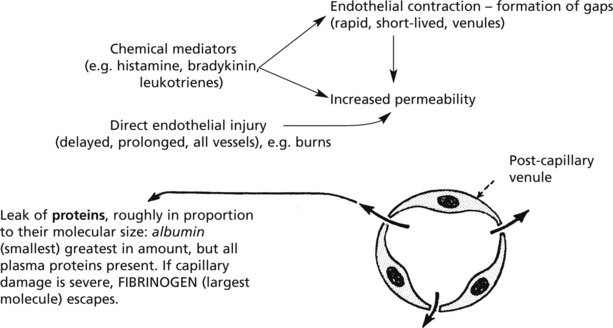
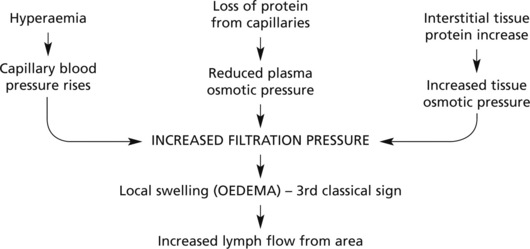
Exudation
Exudation is the increased passage of protein-rich fluid through the vessel wall into the interstitial tissue. It explains the weal in Lewis’ triple response.
| Advantageous results | Contents of fluid |
|---|---|
| Fluid increase | (a) Globulins → protective antibodies |
| ↓ | (b) Fibrin deposition → Helps to limit spread of bacteria |
| Dilution of toxins | (c) Various factors promoting subsequent healing |
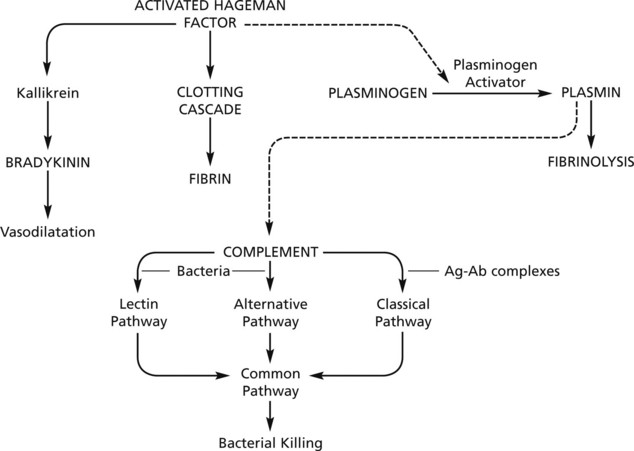
Emigration of Leucocytes
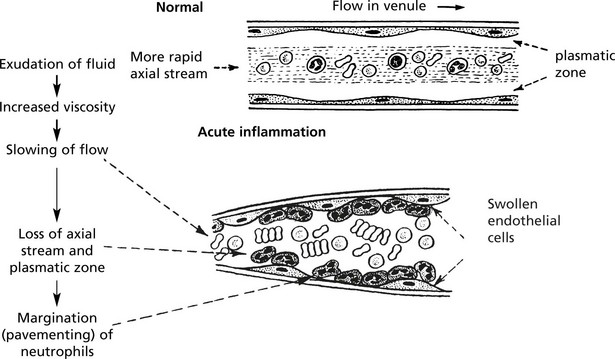
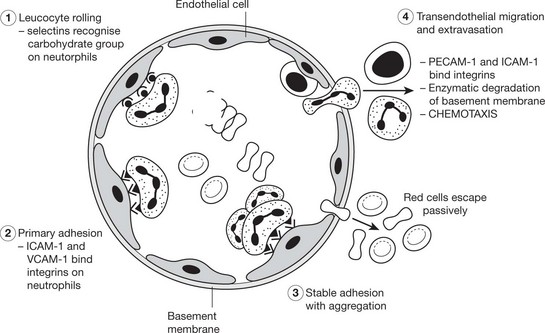
Neutrophils and mononuclears pass between the endothelial cell junctions by amoeboid movement through the venule wall into the tissue spaces. In this process both neutrophils and endothelial cells are activated and both express cell adhesion molecules, initially
Chemotaxis
The initial margination of neutrophils and mononuclears is potentiated by slowing of blood flow and by increased ‘stickiness’ of the endothelial surface.
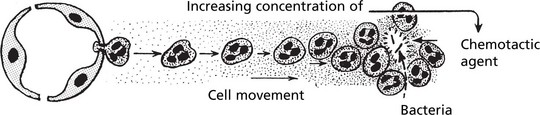
After penetration of the vessel wall, the subsequent movement of the leucocytes is controlled by chemotaxis. The cell moves in response to an increasing concentration gradient of the particular chemotactic agent, usually a protein or polypeptide.
Important examples of chemotactic agents are:
The leucocytes move by extension of an anterior pseudopod with attachment to extracellular matrix molecules such as fibronectin using cell adhesion molecules. The cell body is then pulled forward by actin and myosin filaments.
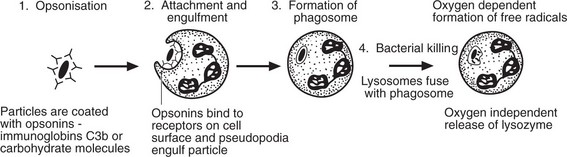
Phagocytosis
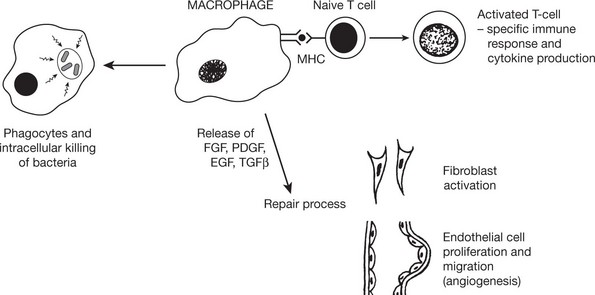
This is the process by which neutrophils and macrophages clear the injurious agent. It is an important defence mechanism in bacterial infections particularly.
There are 3 families of opsonin.
The opsonic activity is enhanced when it is confined within a solid organ or rigid medium such as a fibrin network; where conditions are looser and more fluid, activity is diminished.
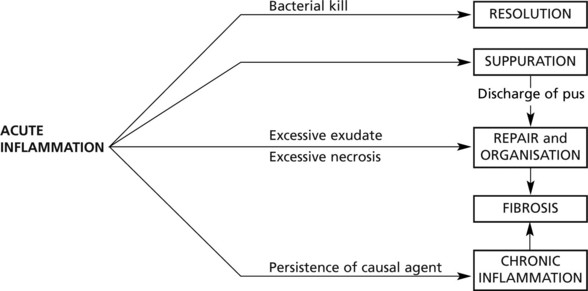
Sequels of Acute Inflammation
Resolution
This means the complete restoration of normal conditions after the acute inflammation. The three main features which potentiate this sequel are:
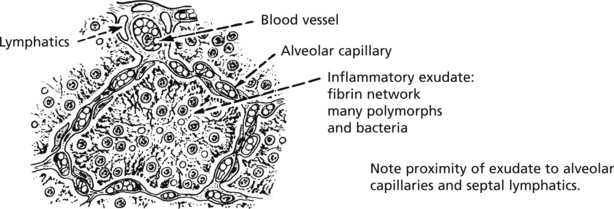
Resolution of lobar pneumonia (bacterial inflammation of lung alveoli) is a good example:
Suppuration
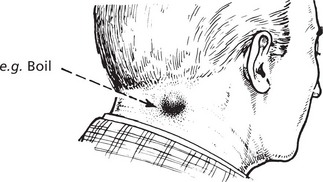
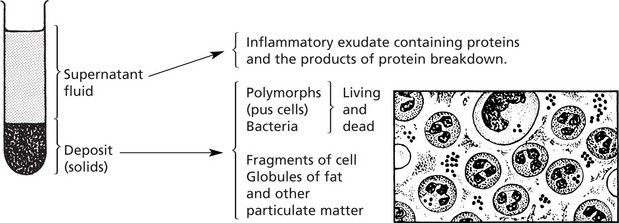
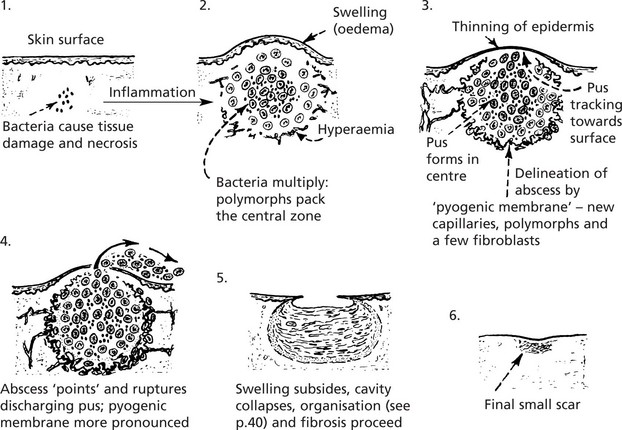
This means the formation of PUS; where pus accumulates an ABSCESS forms.
Infection by pyogenic (pus-forming) bacteria is the usual cause, e.g. staphylococcal abscess (or boil). The pus in this case is a thick, creamy yellow fluid which, on centrifugation, separates thus:
Organisation and Fibrosis
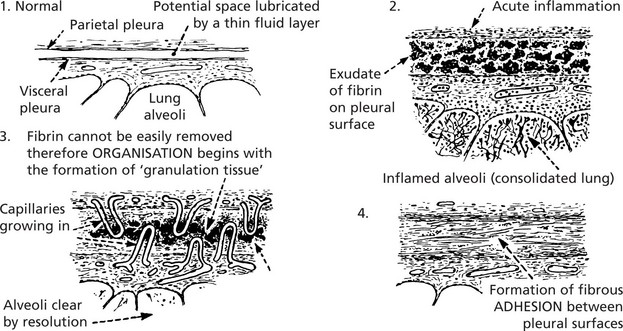
Organisation occurs when, during the acute inflammatory process, (a) there is excessive exudation or necrosis or (b) when local conditions are unfavourable for the removal of exudate and debris. The term also applies to the local reaction to the presence of thrombus and also the necrosis associated with infarction.
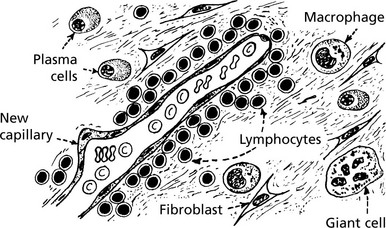
The changes are similar to those described in wound healing viz – the growth of new capillaries into the inert material (exudate or thrombus), the migration of macrophages and the proliferation of fibroblasts resulting in fibrosis.
A good example of organisation following acute inflammation is seen in the pleura overlying pneumonia. The inflammation of the lung tissue proper usually resolves completely (p.264); in contrast the pleural exudate is not easily removed and organisation takes place.
Other good examples of organisation are seen after infarction (see p.166).
Chronic Inflammation
Chronic Inflammation may (a) follow acute inflammation if the causal agent is not removed: or (b) be ‘primary’, i.e. there is no pre-existing acute stage.
Common causes of ‘primary’ chronic inflammation include:
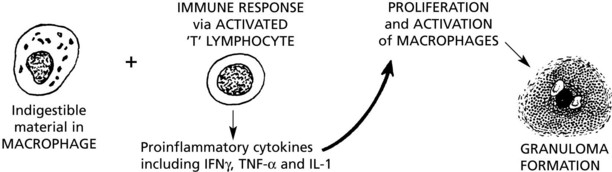
Granulomatous Inflammation
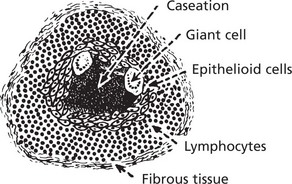
This is the term given to forms of chronic inflammation in which modified macrophages (epithelioid cells) accumulate in small clusters surrounded by lymphocytes. The small clusters are called granulomas. The basic lesion in tuberculosis is a good example.
Similar granulomas are seen in:
N.B. In all of these granulomatous diseases the basic lesion may be identical, but caseation only occurs in tuberculosis.
The epithelioid cells of the granulomas are modified macrophages, and giant cells are derived from macrophages usually by cell fusion but occasionally by nuclear division without cytoplasmic separation.
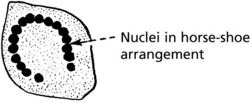
The Langhans’ giant cell – seen in chronic granulomata, e.g. tuberculosis and sarcoidosis.
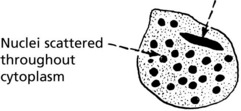
The foreign body giant cell – seen in association with particulate insoluble material.
Ulceration – Benign
ULCERATION is a complication of many disease processes.
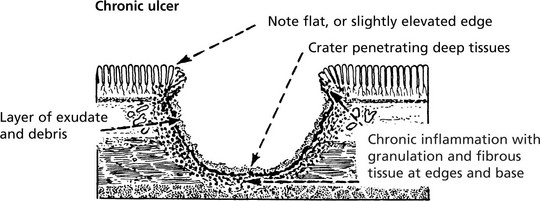
An ulcer is formed when the surface covering of an organ or tissue is lost due to necrosis and replaced by inflammatory tissue.
The most common sites are the alimentary tract and the skin.
Ulcers are divided into two main groups: 1. benign (inflammatory) and 2. malignant (cancerous).
The word ‘benign’ is used here in the limited sense of contrasting with ‘malignant’: ‘benign’ ulcers may have serious consequences.
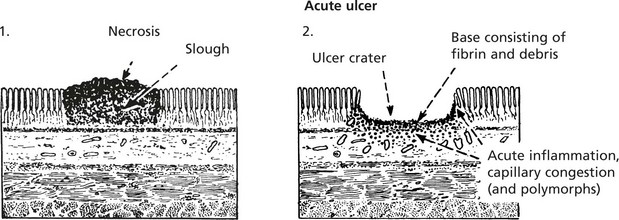
Evolution of a Benign Ulcer
Healing can occur at this stage with restoration to normal, but if irritation (e.g. bacterial action, slight trauma, digestive juices and acid) continues, a chronic ulcer forms.
Healing of a chronic ulcer may be impeded by the secondary obliterative changes in the blood vessels due to the chronic inflammation, and it is inevitably associated with a variable amount of scarring.
Ulceration – Malignant
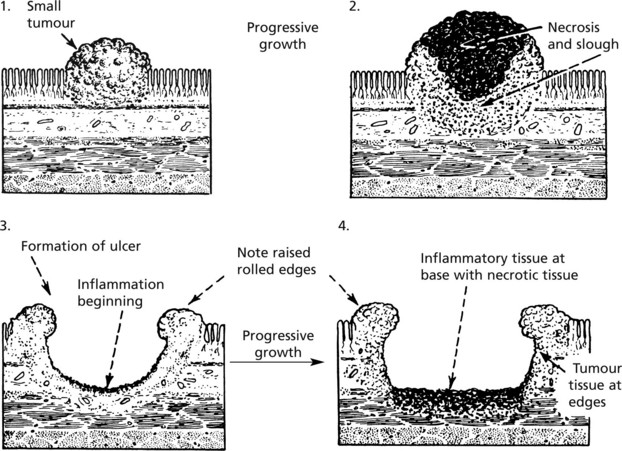
Evolution of a Malignant Ulcer (Ulcerated Tumour)
Such an ulcer is the result of the growth of a malignant tumour.
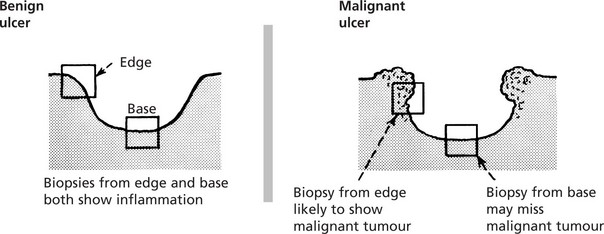
The differences between benign and malignant ulcers are most prominent at the edges from which a diagnostic biopsy should be taken. It is worth remembering that cancers often ulcerate but benign ulcers rarely undergo malignant change.
Inflammation – Anatomical Varieties
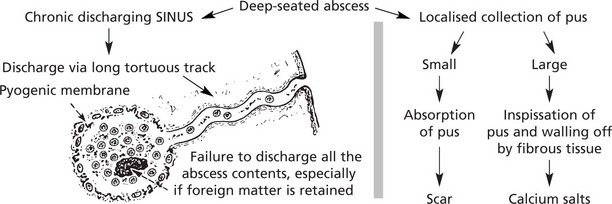
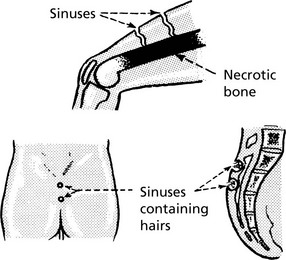
Sinus
A sinus is a tract lined usually by granulation tissue leading from a chronically inflamed cavity to a surface. In many cases the cause is the continuing presence of ‘foreign’ or necrotic material.
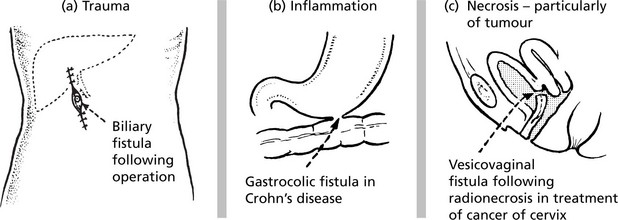
Fistula
A fistula is a track open at both ends, through which abnormal communication between two surfaces is established.
An EMPYEMA is a collection of pus in a body cavity or hollow organ. The term refers usually to the pleural cavity or the gall bladder.
CELLULITIS occurs when inflammation spreads in the connective tissue planes.