Chapter 5 Immunity
Immunity
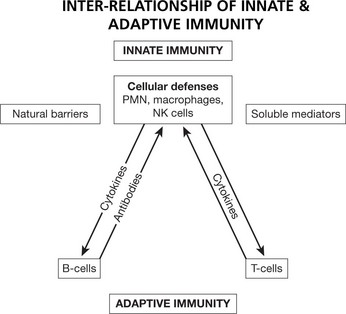
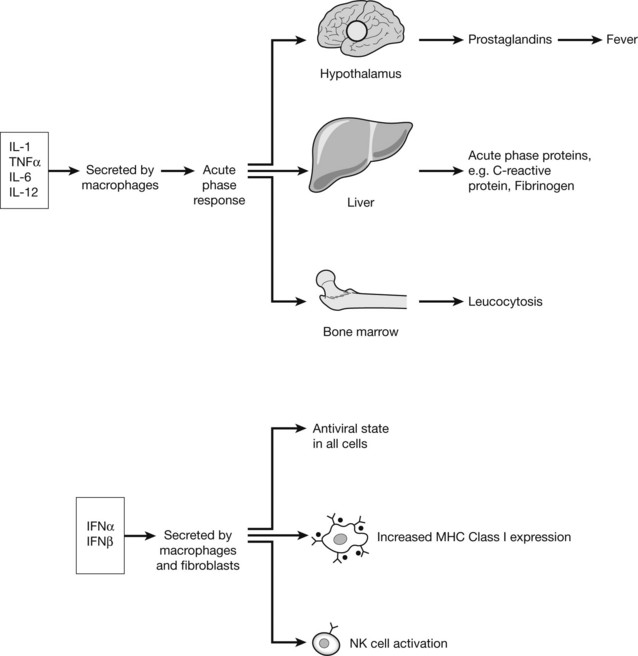
The immune system protects us from invading pathogenic microorganisms and cancer. Immunity – the state of protection from infectious disease – has both a less specific or INNATE and a more specific or ADAPTIVE component.
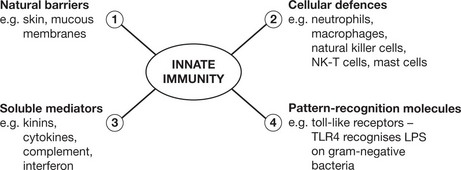
Innate Immunity
This provides the first line of defence against infection. It is a rapid response (minutes); it is not specific to a particular pathogen. It has no memory and does not confer long-lasting immunity to the host. It has 4 main components and is found in all classes of plant and animal life.
Adaptive Immunity
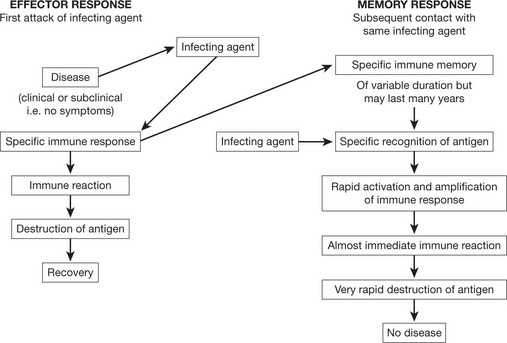
This provides a specific immune response directed at an invading pathogen. Following exposure to a foreign organism there is an initial EFFECTOR RESPONSE that eliminates or neutralizes a pathogen. Later re-exposure to the same foreign organism induces a MEMORY RESPONSE with a more rapid immune reaction that eliminates the pathogen and prevents disease. This response is found only in vertebrates.
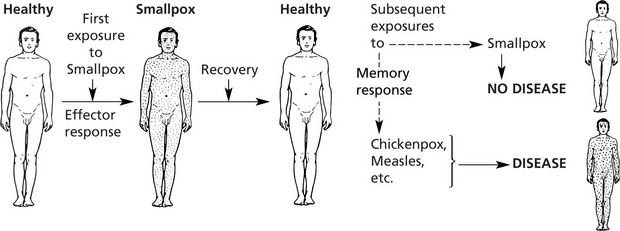
It has been known from historical times that a person who has recovered from an infectious disease, e.g. smallpox, is most unlikely to suffer from it again – even when exposed maximally – although he would remain susceptible to other infections.
That is, during the recovery period he has acquired specific immunity to smallpox but not to other unrelated infections.
Note: This immunity may extend to related infections: the use of the immunity against smallpox conferred by world-wide vaccination with cowpox (pioneered by Jenner in the 18th century) has completely eliminated smallpox throughout the world.
The Adaptive Immune Response
Adaptive Immune Response
ANTIGENS – Substances that can be recognised by the immunoglobulin receptor of B cells or the T cell receptor when complexed with MHC are called ANTIGENS. They are usually part of infectious agents such as bacteria and some viruses although other foreign materials are also antigenic. Most antigens are proteins but some large carbohydrate molecules such as lipopolysaccharides will induce antibody formation.
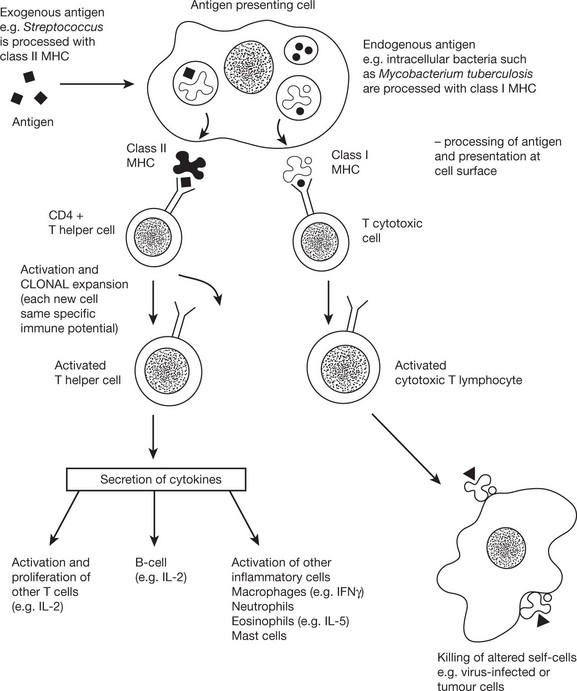
EPITOPES are the immunologically active regions of an antigen that bind to specific membrane receptors on lymphocytes or to secreted antibodies. B and T cells recognise different epitopes on the same antigenic molecule. B cells usually recognise soluble antigen (exogenous). Epitopes recognised by T cells are often internal peptides that are exposed by processing with antigen-presenting cells (endogenous).
Cellular Basis of the Adaptive Immune Response
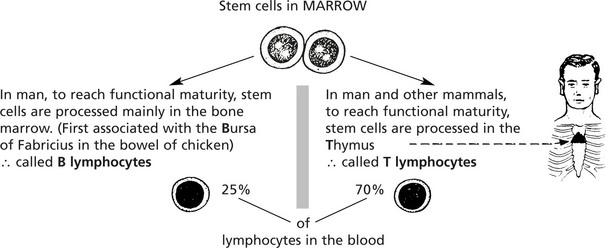
The main cells of the adaptive immune response are the lymphocytes. They are indistinguishable by light microscopy using conventional stains but can be separated by the presence of different surface proteins on T and B cells.
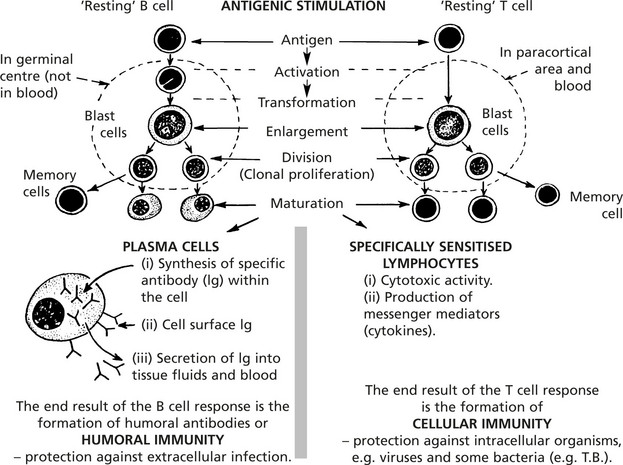
The introduction of an antigen results in activation and proliferation of these two lymphocyte populations. This activity takes place in the lymphoid tissues.
Genetic Influences on the Immune Response
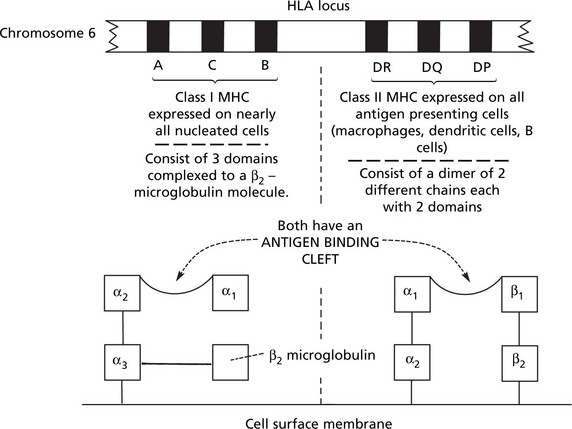
The major histocompatibility complex, also known as the human leucocyte antigen (HLA), has an important role in the immune response and disease.
The genes controlling the system are in 2 major groups (Class I and Class II) on chromosome 6: each group has 3 genes which are highly polymorphic (i.e. show great variation within individual members of the same animal species). The multiple alleles of each gene encode for surface membrane glycoproteins.
The important roles of HLA are:
Cellular Immunity
Activation of T cells requires the involvement of antigen presenting cells. Within these cells, foreign antigen is proteolytically digested to short peptides and then presented with HLA molecules to the T cell which possesses a specific receptor for that antigen.
Cytokines
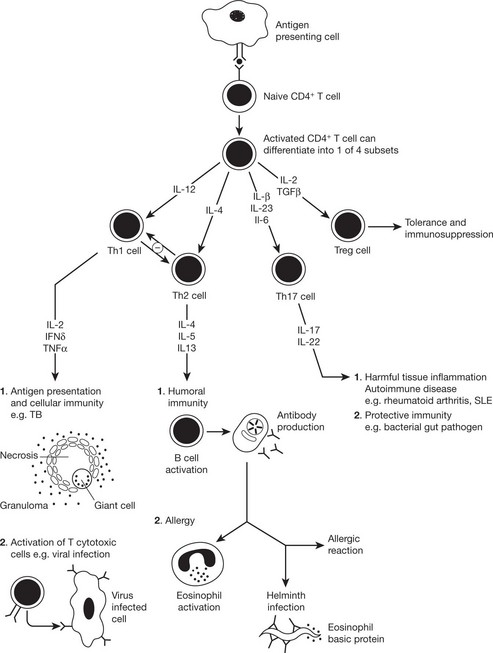
The chemical messengers of immune system are known as CYTOKINES. These small proteins are produced by virtually all cells of the innate and adaptive immune system, particularly CD4+ lymphocytes. The quantity and type of cytokine can positively and negatively regulate cells and cytokine effect is in turn controlled by expression or down-regulation of cytokine receptors on the cell surface.
Cytokines Involved in Adaptive Immunity
The immune response to a particular pathogen must induce an appropriate set of effector functions that can eliminate the pathogen from the host. Differences in cytokine secretion patterns among T helper cells are determinants of the type of immune response made to a particular antigenic challenge. Accordingly the cytokines secreting T helper cells are currently divided into 4 subsets:
The balance between the two main subsets determines disease outcome, e.g. tuberculoid leprosy is characterised by a TH1 response (destructive granulomas, few parasites). In lepromatous leprosy there is a TH2 response (disseminated disease, many parasites).
Humoral Immunity
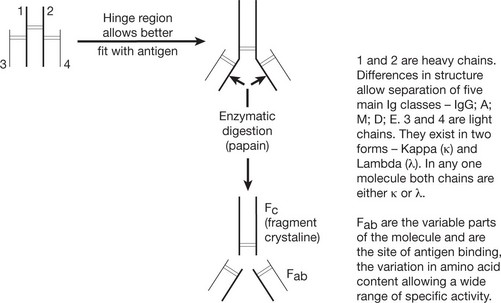
Basic Structure of Immunoglobulin
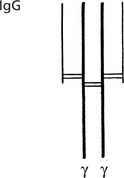
The basic immunoglobulin is a protein molecule consisting of 2 identical LIGHT chains and two identical HEAVY chains. Each light chain is bound to a heavy chain by a disulphide bond. Similarly disulphide bridges link the two heavy and light chain combinations to form the basic four chain structure.
Antibody Mediated Effector Functions
Fc receptors on macrophages and neutrophils can bind the Fc of Ig molecule resulting in phagocytosis of the antigen-antibody complex.
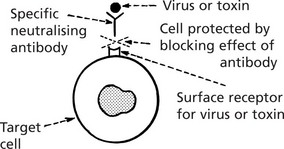
The linking of antibody bound to target cells (e.g. virus infected cells) with the Fc receptors of natural killer (NK) cells, enables the NK cell to recognise and kill the target cell.
Immunoglobulins
Antibody Classes and Activities
These groups are named according to the composition of the heavy chains.
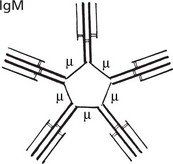
IgM (heavy chain = μ) is a polymer, joined by J chains, of five identical Ig molecules, therefore called macroglobulin.
Although in low concentration in the blood, 0.5–2 mg/ml, it is the main Ig on the surface of B lymphocytes (before conversion to plasma cells). It is the first immunoglobulin class produced and is active in the primary response. It neutralises viruses. In combination with complement, it is actively bactericidal and is especially effective in bacteraemia.
Note: The numerous (10) antigenbinding sites increase its efficiency.
The natural blood group antibodies, anti-A and anti-B are M globulins.
IgG (heavy chain = γ) is a single molecule with two antigen binding sites.
This is the most abundant class of immunoglobulin, with a concentration in blood of 8–16 mg/ml. There are 4 subclasses IgG1, IgG2, IgG3 and IgG4. Of its several activities the important ones are:
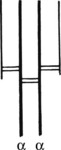
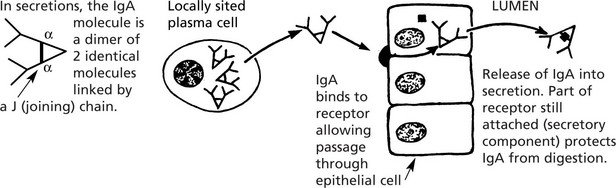
IgA (heavy chain = α) occurs in the blood (1.5–4 mg/ml) where its function is unknown.
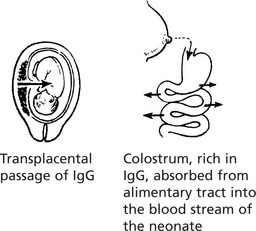
It is the predominant immunoglobulin in secretions of eyes, nose, mouth, bronchi and gut. Its function is to protect mucous surfaces from antigenic attack and prevent access of foreign substances to the circulation and general immune system. It may neutralise toxins and prevent binding to mucous surfaces. IgA is a single molecule in the serum.
The diagram illustrates the local production of IgA in the gut.
| IgD | (heavy chain Δ) of uncertain function: concentration in blood very low probably because it is not secreted by plasma cells. Like IgM it is present on the surface of B lymphocytes prior to transformation. |
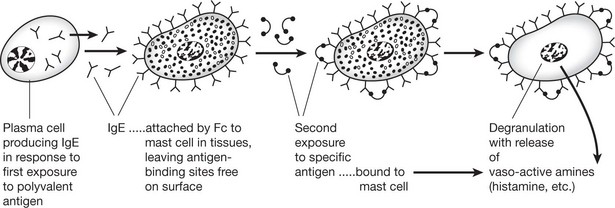
| IgE | (heavy chain ε) – a single molecule similar to IgG and IgA. It is sometimes called REAGIN: concentration in blood is low (20–500 ng/ml). The serum level is raised in worm infestation and is probably protective. Its main activity is mediated by MAST CELLS (or BASOPHILS); it is the principal mediator of atopic and anaphylactic disease. |
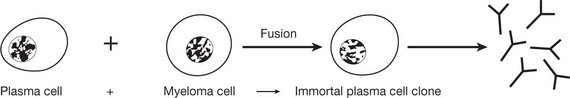
The range of antibodies is immense. B cells produce this vast number by rearranging the genes from the immunoglobulin light and heavy chains. The appropriate clone of plasma cells is stimulated by binding of antigen to the cell surface receptor (so-called clonal selection).
Immune Reactions
Antigen-Antibody Interactions
When an antigen (Ag) binds with an antibody (Ab) an Ag/Ab complex is formed. This reaction is reversible in varying degrees and depends on the affinity of the antibody for an individual antigen and the avidity (strength when multiple epitopes on an antigen interact with multiple binding sites).
The consequences of Ag/Ab interaction include:
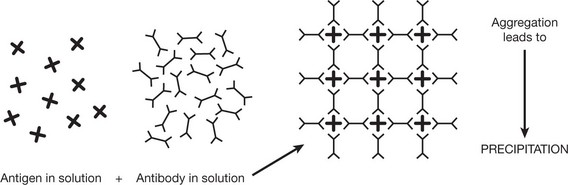
A soluble antigen is rendered insoluble by aggregation of the Ag/Ab complexes into a lattice.
This reaction is the basis of immunoelectrophoresis, used e.g. to identify a monoclonal band in myeloma.
Particulate antigen, e.g. bacteria and red blood cells, are aggregated in the same way as in the precipitation reaction and the process is called agglutination. Agglutination reactions are routinely performed to type red cells (ABO typing).
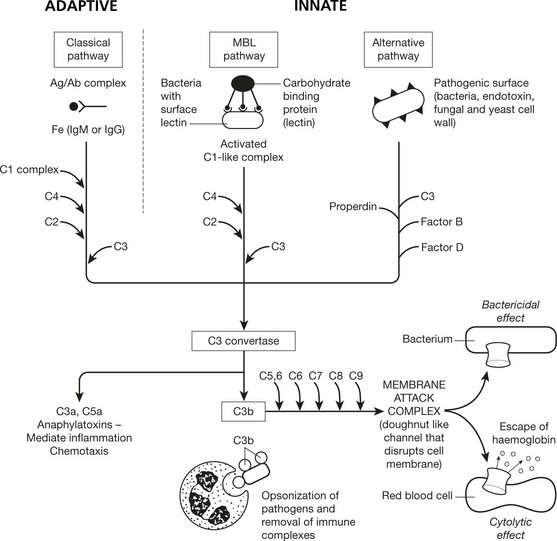
Complement System
Complement consists of nine main protein components present in inactive form in the blood. There are 3 pathways of complement activation. Activation results in the formation of multi-molecular enzymes that activate further components in a cascade ultimately generating a membrane attack complex that is capable of causing cell lysis.
The mannose-binding lectin (MBL) and alternative pathways do not require antibody for activation and are therefore a component of the innate immune system.
Activation of C by Ag/Ab complex is called fixation of complement. Using red cells as markers, this fixation can detect the presence of antigen or antibody in serum.
E.g. Test serum + antigen + C.
If serum contains antibody, C is fixed, otherwise it remains free.
Add sensitised RBC (coated with Ab).
Lysis of RBC = complement still free ∴ no Ab in original serum.
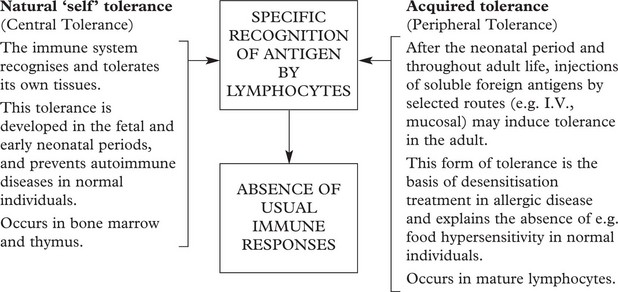
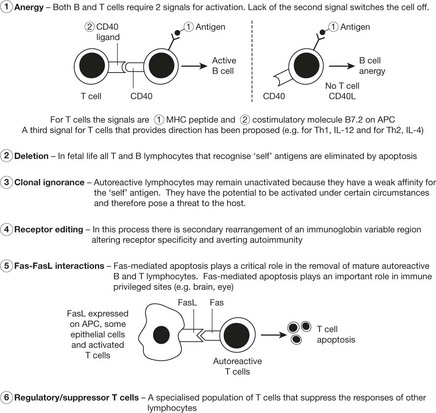
Tolerance
Humoral and cell-mediated immunity are specific active immune responses with a protective function.
In certain circumstances an antigen does not evoke these active responses. This is because the lymphocytes, although recognising the antigen, do not react – this is TOLERANCE.
Tolerance is an important protective mechanism. When it breaks down, the serious effects give rise to autoimmune disease (see p.107).
Immunopathology
The complicated and delicately balanced immune mechanisms clearly have been developed to protect against antigens, particularly infections. When these immune reactions are upset, the protective mechanism can itself be a source of disease states.
There are three main categories: 1. hypersensitivity states, 2. immune deficiency states and 3. autoimmune diseases.
Hypersensitivity Reactions
These consist of an inappropriate response by an individual to an antigen, following a previous exposure. They differ from the protective immune response in that they are exaggerated, inappropriate or damaging to the host. Depending on the main type of immune response concerned, these are classified as follows:
This classification is to some extent artificial. Hypersensitivity may start as an immediate humoral reaction but end in a mixed state with both humoral and cellular activities. In the 1960s, Coombs and Gell divided hypersensitivity reactions into four types that are still used today.
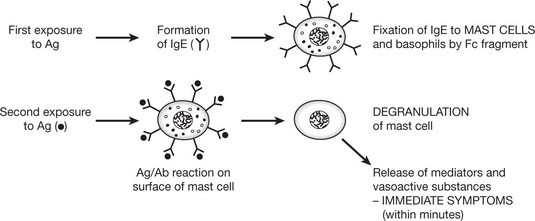
Type 1 Anaphylaxis, Atopy, Allergy
This occurs within 20–60 minutes of exposure to antigen and is often referred to as ‘immediate type hypersensitivity’.
All 3 terms have been used for this reaction. The basic mechanism is as follows.
Antigens And ‘Allergens’
The most potent allergens are usually large molecules with molecular weights up to 40 000. Examples of common ‘allergens’ are pollen, house mite dust, cat fur and penicillin. Food allergy, e.g. to peanuts and milk, is uncommon.
Hypersensitivity States
Clinical Examples of Type I Reaction
Eosinophils are a common finding in the tissues of patients with allergies, attracted by eosinophil chemotactic factor released by mast cells. Allergic conditions are often familial with a genetic basis. Several candidate loci have been proposed including loci linked to cytokine genes and a gene for the IgE receptor on mast cells.
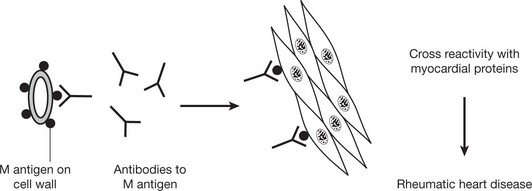
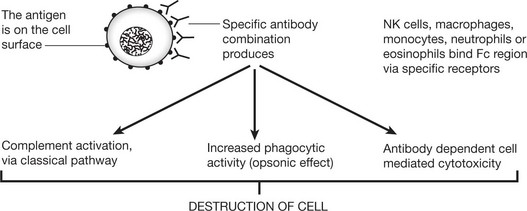
Type II – Cytotoxic Type
This cytotoxic reaction causes some forms of haemolytic anaemia (e.g. autoimmune HA (p.392): Rhesus incompatibility (p.393)) and some blood transfusion reactions. Certain drugs (e.g. penicillins) can also cause autoimmune HA by acting as a hapten. This is a small molecule that only becomes immunogenic when combined with a host protein carrier.
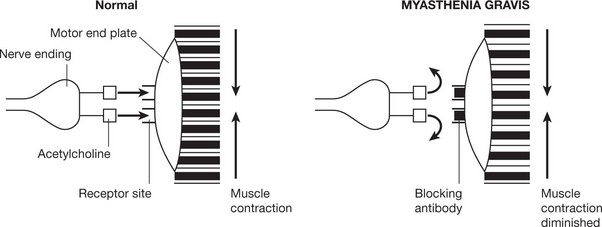
In some auto-immune disorders antibodies of this type are directed against specialised cell surface receptors, e.g. Graves’ disease or myasthenia gravis (see p.109).
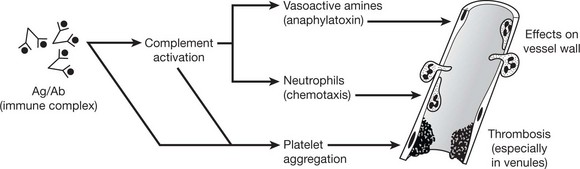
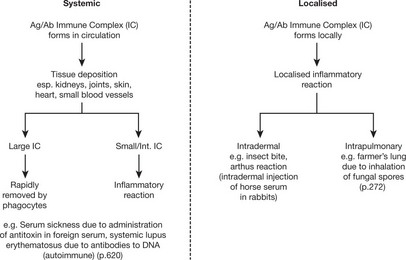
Type III – Immune Complex (Arthus) Type
The reaction is due to the consequences of specific direct antigen/antibody combination particularly complement activation (p.99) and platelet aggregation. The antibodies involved are IgG or IgM.
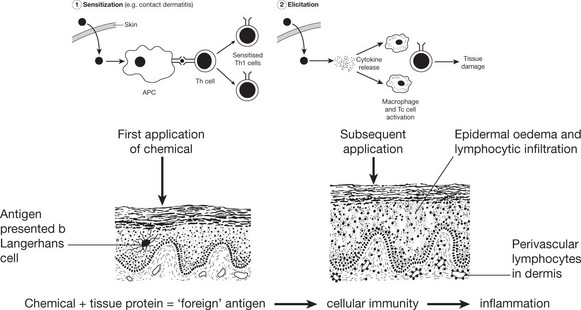
Type IV – (Delayed) Type
This reaction is an antigen elicited cellular immune response which produces tissue injury independently of the presence of antibody. The reaction is usually delayed taking 24–72 hours to develop. Classic examples include the tubercle follicle, graft rejection and contact dermatitis.
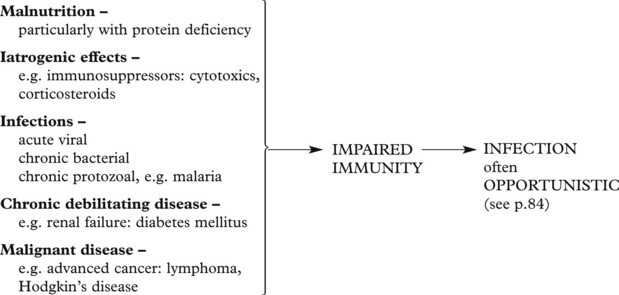
Immune Deficiency States
Failure in any one alters the immune response. The deficiencies may be of a primary nature, commonly genetic in origin, or secondary to some other disease or circumstance. Equally, the alteration in any system may be quantitative or qualitative. In many of these deficiencies there is a failure in more than one system.
Primary (inherited) deficiencies of the specific system are rare. They include B cell, T cell and combined B and T cell deficits. B cell deficiency leads to infection by pyogenic bacteria. T cell deficits lead to infection by viruses, fungi and intracellular bacteria.
Secondary deficiencies of the specific system are common. Usually T cell activity is affected, resulting in deficient cellular immunity and, later, B cell deficit occurs.
The acquired immunodeficiency syndrome (AIDS) is now a major world-wide public health problem. Other predisposing conditions and diseases include:
Deficiencies of the innate immune system are rare. The disorders of neutrophil function are described on page 408.
Immune Deficiency States – Aids
The acquired immune deficiency syndrome (AIDS) is a world-wide epidemic with large numbers of cases in sub-Saharan Africa and South East Asia. Globally, an estimated 40 million people are infected with the virus.
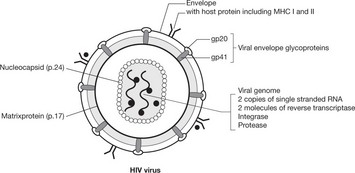
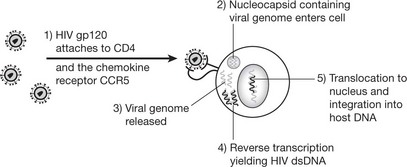
The virus (human immunodeficiency virus (HIV)) is of the retrovirus group: it infects and destroys CD4 T-lymphocytes (macrophages, monocytes and dendritic cells are also infected). There are 2 strains of HIV: HIV1 which is more virulent and HIV2 which predominates in West Africa.
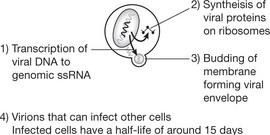
The disease is slowly progressive and untreated is usually ultimately fatal. Recently highly active antiretroviral therapy (HAART), usually a combination of drugs such as nucleoside analogues and proteases, has been shown to reduce viral load to undetectable levels and has decreased the incidence of opportunistic infections and the death rate in the USA. However these drugs are expensive and have significant side effects.
| Infection | Latent period | AIDS |
|---|---|---|
| CD4 > 500 × 106 | CD4 – 200–500 × 106 | CD4 < 200 × 106 |
| Asymptomatic or a short febrile illness. Although the patient’s cells contain HIV, tests for antibodies may be negative for up to several months. | Virus present in lymphocytes: may be persistent lymph node enlargement and fever. | Infections and tumours |
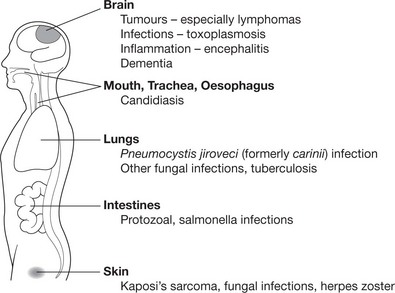
The whole range of opportunistic infection (see p.84), including disseminated virus infection (e.g. herpes simplex and cytomegalovirus), occurs.
The diagram shows the more common AIDS-associated diseases and sites:
Blood Changes
Antibodies – Specific antibodies appear up to 6 months after infection and form the basis of the diagnostic test for HIV. The antibody titre may fall greatly late in the disease.
Immunoglobulins are usually elevated in the early stages.
CD4 lymphocytes may be severely reduced, producing a lymphopenia (see above).
Epidemiology and Transmission
Although the virus may be present in many body fluids and secretions, transmission is by the parenteral route, usually by 1. sexual contact, 2. infected blood or 3. from mother to infant.
Transmission does not occur with normal social contact and there is a very low risk to medical or nursing personnel using normal procedures.
Autoimmune Diseases
Autoimmune diseases result from, or are associated with, an immune response against the individual’s own cells, or in some cases cell products. Although both humoral and cellular immunity are involved, it is thought that changes in the latter are of primary importance. In autoimmunity, something occurs to destroy integrity of self tolerance. The aetiology of autoimmunity is not fully established. Potential causes include:
Autoimmune diseases were traditionally classified as organ specific and non-organ specific. However, since there is some crossover between these two groups, they can be classified by the predominant effector mechanism leading to organ damage.
PREDOMINANTLY ANTIBODY-MEDIATED AUTOIMMUNE DISEASE
| Autoantigen | Target Organ | Disease |
|---|---|---|
| Red blood cells | Red blood cells | Haemolytic anaemia |
| Acetylcholine receptor | Voluntary muscle | Myasthenia gravis |
| TSH receptor | Thyroid | Graves’ disease |
| Nuclear constituents e.g. DNA, | Many – Kidney, skin, blood vessels, joint, heart | Systemic lupus erythematosus |
PREDOMINANTLY T CELL MEDIATED AUTOIMMUNE DISEASE
| Autoantigen | Target Organ | Disease |
|---|---|---|
| Myelin basic protein | Central nervous system | Multiple sclerosis |
| β-islet cells | Pancreas | Type 1 insulin dependent diabetes mellitus |
| Thyroglobulin Microsomal antigens Thyroid peroxidase |
Thyroid | Hashimoto’s thyroiditis |
| IgG | Synovium/joints | Rheumatoid arthritis |
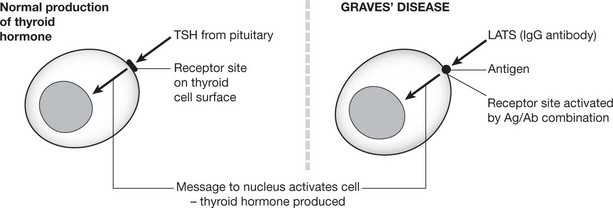
Primary thyrotoxicosis (Graves’ disease) and myasthenia gravis are of particular interest. In these diseases specific autoimmune antibodies combine with antigen on the cell surface and either stimulate or block the action of the physiological agent which would normally turn on the activity of the cell.
Stimulatory Effect
In Graves’ disease, excessive amounts of thyroid hormone are produced. LATS (long acting thyroid stimulator), an IgG auto-antibody, combines with antigen on the thyroid cell surface and produces changes mimicking those produced by TSH (thyroid stimulating hormone) physiologically manufactured by the pituitary.
Applied Immunology
Immunohistochemical Identification
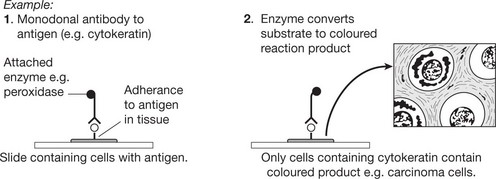
Immunohistochemistry is now well established and allows identification of specific substances in histological sections.
This or similar techniques are used to identify a wide range of substances. Immunohistochemistry is now a major ancillary technique used in the pathology laboratory to subtype cancers.
Flow Cytometry
In this technique, cells in suspension are labelled by fluorescent markers, excited by a laser and counted electronically by passing them through a flow cytometer. This is the technique employed, among others, for monitoring CD4+ counts in HIV.
Prophylaxis and Treatment of Infections
Applied Immunology – Tissue Transplantation
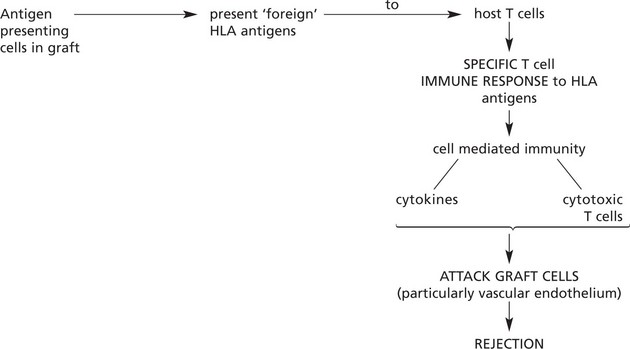
In the past, allografting (transplanting of tissues or organs from one individual to another of the same species) inevitably led to rejection of the graft by the host. Understanding of the mechanisms has allowed intervention so that nowadays successful survival of grafts is usual (e.g. 90% survival of renal transplants after 1 year).
Rejection may also occur, very rapidly, if pre-existing antibodies are present (e.g. due to previous incompatible blood transfusion or pregnancy) – leading to activation of complement with haemolysis.
Usually rejection takes weeks while the cell mediated immune response is building up.
The remarkable improvement in transplant prognosis is due to:
Paradoxically the pre-transplant transfusion of compatible blood improves the chances of graft survival.
Graft Versus Host Disease (GVHD)
When immunosuppressed individuals (e.g. leukaemia patients) undergo bone marrow transplantation, T cells from the graft proliferate in the recipient (host) and establish an immune response against the host tissues: the main target organs are the skin, the liver and the alimentary tract.