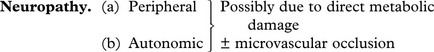Chapter 16 Endocrine System
Pituitary Hyperfunction
624Adrenal Cortex – Overactivity
634Adrenal Cortex – Hypofunction
635Adrenal Cortex and Medulla
636Multiple Endocrine Neoplasia Syndromes (MENS)
643
Endocrine Diseases
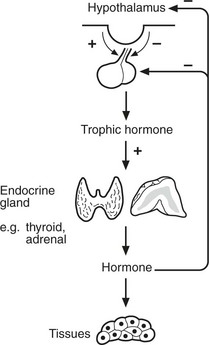
Most endocrine glands are controlled by hormones produced in the anterior pituitary, themselves under control of substances produced in the hypothalamus. A variety of stimuli control pituitary and hypothalamic hormone release, especially feedback control from hormone levels from the target glands. Levels of pituitary hormones show a circadian rhythm.
Endocrine diseases can be broadly classified as:
Hormone excess
–
primary overproduction by gland
–
secondary to excessive trophic hormone
Hormone deficiency
–
primary underproduction by gland, e.g. due to inflammation, post surgery
–
secondary to insufficient trophic hormones
Hormone resistance
–
target organ resistance
–
failure to activate hormone
Effects of non-functioning tumours
–
local pressure and invasion
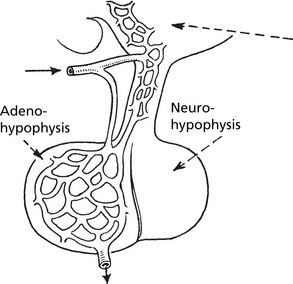
Pituitary Gland
The pituitary has two parts; the anterior (adenohypophysis) and the posterior (neurohypophysis).
Anterior Pituitary (Adenohypophysis)
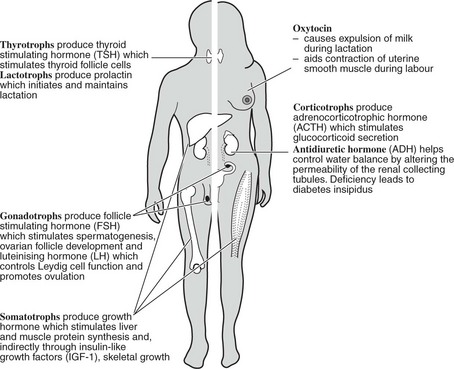
The blood supply is via a portal system from the hypothalamus bringing peptides which stimulate or inhibit pituitary hormone production. The cells producing each hormone can be identified by immunohistochemistry (for stored hormone) or in situ hybridisation (for mRNA).
Posterior Pituitary
This is composed of neural tissue and secretes two hormones made in the hypothalamus (oxytocin and antidiuretic hormone).
Note
1.
ACTH (39 amino acids long) is synthesised as part of a large molecule proopiomelanocortin (POMC) from which other molecules including melanocyte stimulating hormone (MSH) are derived.
2.
Hypothalamic releasing hormones have been found for TSH (TRH), LH/FSH (GnRH), ACTH (CRH) and GH (GHRH); somatostatin inhibits release of GH.
Pituitary Hyperfunction
In most cases, this is associated with pituitary adenoma.
Pituitary adenomas are divided on the basis of size into macroadenomas (>10 mm) and microadenomas (<10 mm).
Small tumours present only if they produce excess hormones, while larger tumours may cause pressure effects (e.g. on optic chiasma, page 141) or with hypopituitarism due to destruction of normal pituitary. Occasionally haemorrhage into a pituitary adenoma causes raised intracranial pressure (pituitary apoplexy).
Gigantism and Acromegaly
These are both the result of excess production of growth hormone by a somatotroph (acidophil) adenoma. Gigantism arises in children before the epiphyses have fused; acromegaly occurs in adult life.
Gigantism
The excess growth hormone induces skeletal growth, the bones retaining their normal shape and relative proportions. Fusion of epiphyses is delayed, but eventually occurs and the features of acromegaly appear.
Acromegaly
There is overgrowth of bone and soft tissue.
Clinical signs
Features coarsened: nose enlarged.
Prognathic (projecting jaw).
Irregular bone formation – interferes with joint function – leads to osteoarthritis.
Pain due to nerve compression is common. Glucose tolerance is diminished and diabetes occurs in 10%. High blood pressure with cardiac hypertrophy and extensive atheroma is common, and the patient may die in cardiac failure. There is a 2–3 fold increased risk of colonic cancer.
Hyperprolactinaemia
Prolactin secreting adenomas are often small. They cause amenorrhoea, infertility and occasionally galactorrhoea in younger women, but are usually asymptomatic in older women and men.
CUSHING’S SYNDROME is frequently due to an ACTH secreting adenoma when it is known as Cushing’s disease. The effects are described on p.634.
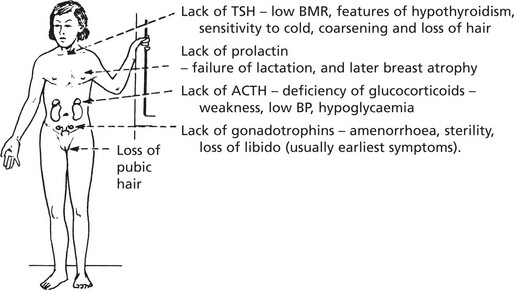
Hypopituitarism
Failure of pituitary secretion may affect one or several hormones. Causes include:
(b)
Pituitary surgery/cranial irradiation.
(d)
Hypothalamic dysfunction, including craniopharyngioma.
(e)
Sheehan’s syndrome: is used as an example. It is still an important cause where obstetric services are poor.
This syndrome occurs in women and is due to ischaemic necrosis of the pituitary following post-partum haemorrhage. The normally low pressure in the pituitary portal vascular supply increases the susceptibility of the gland. The results vary with the extent of necrosis and may include the following:
In time, the peripheral endocrine organs - thyroid, adrenals, ovaries - show atrophy.
In childhood, growth hormone deficiency is a cause of dwarfism. Skeletal growth is diminished with retarded sexual development but normal intelligence. Hypothalamic GHRH deficiency may be responsible.
Note: Some individuals treated with human growth hormone have developed Creutzfeld–Jacob disease (p.555).
In adults, GH deficiency leads to lethargy, diminished muscle mass, obesity and premature atheroma.
Inflammation of the Pituitary Gland
Apart from involvement in acute meningitis, inflammation, acute or chronic, is uncommon. Tuberculosis, syphilis and sarcoidosis all rarely occur.
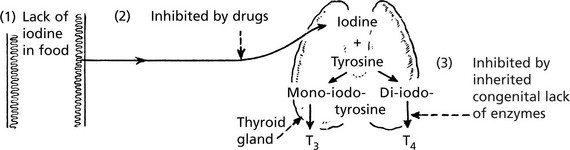
Thyroid Gland – Underactivity
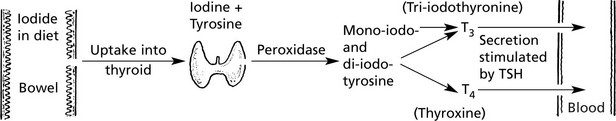
The thyroid gland is under the control of the pituitary thyroid-stimulating hormone (TSH p.623).
T4 can be regarded as a prohormone, which is converted to active T3 by deiodination in liver, muscle and kidney.
Thyroid Hypofunction
Insufficient thyroid hormone is produced, resulting in myxoedema in adults and cretinism in children.
Adults – Myxoedema
Clinical signs
Basal metabolic rate reduced, weight gain.
Body temperature falls, cold intolerance.
Respiratory and heart rates reduced.
Skin thickened, non-pitting oedema due to increase in mucopolysaccharide ground substance.
Hair brittle, dry and falls out.
Blood cholesterol is raised.
TSH secretion is increased.
T
3 and T
4 blood levels low.
Causes
1.
Autoimmune thyroiditis
(a)
Atrophic form – so-called
primary myxoedema – the commonest cause of hypothyroidism
2.
Severe iodine deficiency.
3.
Dyshormonogenesis – inborn errors in the formation of thyroid hormones.
4.
Anti-thyroid drugs, e.g. lithium
5.
Excessive surgical resection of thyroid gland.
6.
Treatment with radioiodine.
7.
Hypopituitarism → reduced TSH.
Pathological changes in primary myxoedema
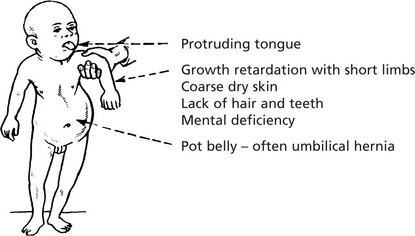
Children – Cretinism
Infants normal at birth, but abnormality appears within weeks.
Clinical Signs
Changes are irreversible unless treatment is given early. Two forms are recognised:
Thyroid Gland – Overactivity
Endemic Cretinism
This occurs in districts where goitre is common due to iodine deficiency. The infantile thyroid is usually enlarged and nodular. Histologically, there are hyperplastic foci containing colloid which compress the intervening tissue. The incidence of this disorder has reduced following addition of iodine to salt.
Sporadic Cretinism
This is usually due to congenital hypoplasia or absence of the thyroid. Deaf mutism is often present.
Dyshormonogenesis
In this condition, cretinism is due to a congenital familial recessive enzyme defect leading to inability to complete the formation of thyroid hormone. The thyroid gland is enlarged and shows epithelial hyperplasia. TSH is increased.
Thyroid Hyperfunction
Excessive quantities of circulating thyroid hormone (T3 and T4) cause thyrotoxicosis. Three types of thyroid lesion can give rise to thyrotoxicosis.
1.
Graves’ disease (exophthalmic goitre) >80%.
2.
Toxic nodular goitre 10%.
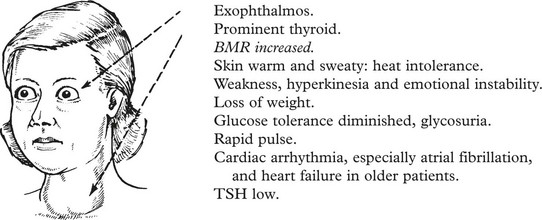
Graves’ Disease (Exophthalmic Goitre)
Clinical signs:
Graves’ Disease
In some cases there are foci of thyroiditis with lymphocytes and plasma cells.
Other changes:
1.
Patches of myxoedema – usually on pre-tibial aspect of legs.
2.
Exophthalmos, due to autoimmune damage to the eye muscles.
Aetiology:
1.
Usually in females, peak 20–40 years.
2.
More common in families showing high incidence of autoimmune disease, e.g. thyroiditis, pernicious anaemia.
3.
The stimulation of the thyroid is due to an autoantibody (thyroid stimulating immunoglobulin/TSH antibody also known as TRAb) which reacts with and activates the surface receptor for TSH on thyroid epithelium. Cyclic AMP is formed and this stimulates hyperplasia of the epithelium and increased formation of thyroid hormone. With the increase in hormone, the blood TSH falls.
Toxic Adenoma
Most adenomas are non-active; only a small proportion (1%) give rise to toxic symptoms. Most patients are women over 40 years.
There is usually only one large adenoma present. The histological features are of follicles which may be small or of normal size. Increased production of thyroid hormone by the adenoma, which is autonomous, causes a fall in TSH, and the remainder of the thyroid is inactive.
Toxic Nodular Goitre
This develops in some cases of non-toxic nodular goitre. Nodules of hyperplasia are interspersed with inactive tissue. The condition is more common over the age of 50 especially in women. Exophthalmos is absent. Cardiac arrhythmias and heart failure is common.
Thyroid Gland
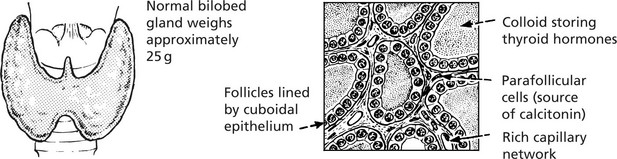
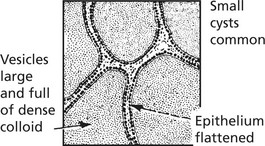
Non-Toxic Goitre
This is a simple enlargement of the thyroid gland, not associated with increased secretion of thyroid hormone.
The gland is enlarged and pale pink. Two phases can be recognised:
(a)
Diffuse hyperplasia: The gland consists mainly of small closely packed acini lined by columnar epithelium and containing a small amount of poorly stained colloid. Occasional intra-acinar papilliform epithelial projections may be seen.
(b)
Nodular hyperplasia: This is a later stage. Areas of marked hyperplasia cause atrophy of intervening parenchyma. It appears to be related to continuing severity of iodine deficiency.
Sometimes the enlarged gland is translucent and brown due to the large amount of stored colloid (colloid goitre).
This is common in women and appears at puberty or during pregnancy. There are usually no symptoms, but pressure symptoms develop if the thyroid is retrosternal, e.g. pressure on trachea causing stridor, pressure on recurrent laryngeal nerve → hoarseness.
Aetiology. In most regions the aetiology is unknown. Goitre is endemic in central areas of the world, mountainous regions remote from the sea – Switzerland, Himalayas, Andes, etc. There is a lack of iodine in the soil, hence in the food. Addition of iodine to salt has lowered the prevalence.
Mechanism of goitre production
Autoimmune Thyroiditis
This type of disease is associated with the appearance of thyroid antibodies in the blood and inflammation with lymphocytes and plasma cells in the thyroid gland. There is an associated risk of development of non-Hodgkin’s lymphoma in each.
1.
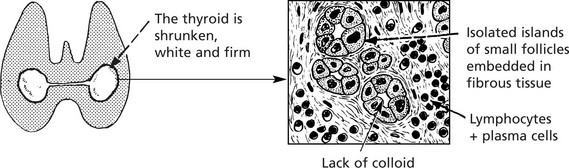
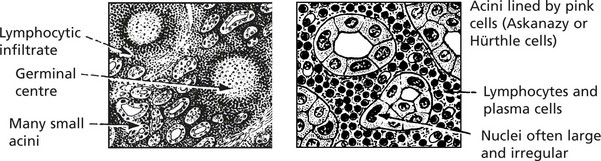
Hashimoto’s thyroiditis (lymphadenoid goitre)
This is the most distinctive type of autoimmune thyroid disease, and the changes are widespread.
Clinical effects: The patient may be euthyroid but eventually may develop hypothyroidism.
Any attempt to reduce the size of the goitre by surgery inevitably causes hypothyroidism.
In a small proportion of cases the patient has thyrotoxicosis in the early stages of the disease due to overactivity of the gland.
2.
Primary Myxoedema
Antibodies to thyroid hormones and epithelium are found in this disease (p.626).
3.
Focal thyroiditis
This extremely common form of autoimmune disease is usually asymptomatic, but partial thyroidectomy may precipitate hypothyroidism. The concentration of antibodies in the plasma is always low.
Patients with autoimmune thyroiditis often have other organ-specific antibodies, e.g. gastric, adrenal etc., and pernicious anaemia and adrenal insufficiency may occur.
4.
Graves’ disease (p.628).
Tumours of Thyroid
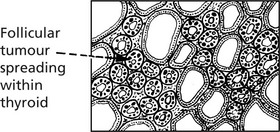
Follicular Adenoma
This fairly common benign tumour is usually single and is encapsulated with compression of the surrounding gland. Very occasionally there is hypersecretion with thyrotoxicosis. Degenerative changes including haemorrhage into the tumour are common.
MALIGNANT TUMOURS are common. Five forms are recognised:
1.
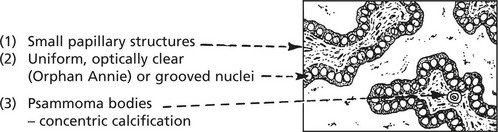
Papillary carcinoma 60–70%. This affects particularly young women: the tumours are usually small and may be multiple within the thyroid. They metastasise readily to local lymph nodes and the first clinical sign may be an enlarged cervical lymph node containing metastatic papillary tumour. Remote spread is unusual.
The histological appearances are typical:
2.
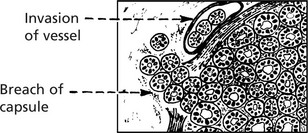
Follicular carcinoma 15–20%. These tumours have their highest incidence in women over middle age. They present in 2 forms:
(a)
Minimally invasive, well encapsulated and can only be differentiated from adenoma by invasion of the capsule and/or veins.
(b)
As a tumour of varying degrees of follicular differentiation, which spreads widely in the thyroid and invades venules.
Blood spread particularly to bones and lungs is usual in follicular carcinoma.
3.
Medullary carcinoma 5–10%. This is a rare tumour arising from the calcitonin-producing cells of the thyroid. Blood calcitonin is high. In addition, there may be production of other hormones resulting in a carcinoid or Cushing’s syndrome. There is a familial form in which there are multiple tumours of a number of endocrine organs (MEN 2, p.643).
4.
Anaplastic carcinoma This occurs in the elderly and may cause stridor. Distant metastases are common. The prognosis is very poor.
5.
Lymphoma B cell lymphoma occasionally arises in long-standing autoimmune thyroiditis (particularly Hashimoto’s disease).
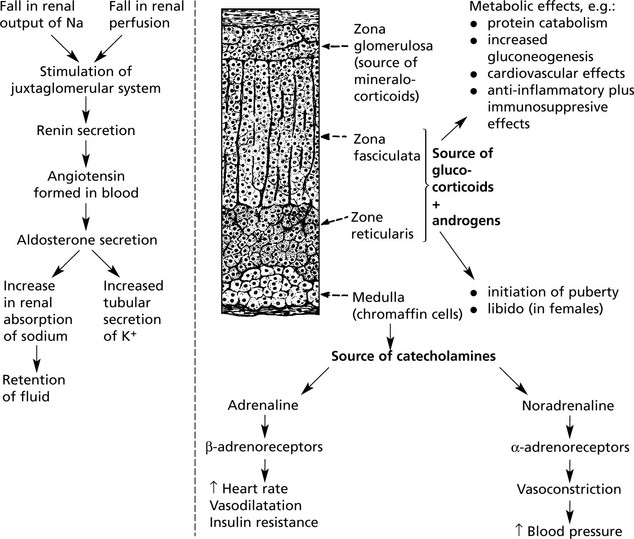
Adrenal Gland
The adrenal gland has two parts and several functions.
There are:
Mineralocorticoids
The main one is aldosterone. It is involved with the renin-angiotensin system and ADH in the maintenance of blood volume. The mechanism is as follows:
Adrenal Cortex – Overactivity
Overactivity manifests itself in three ways:
1.
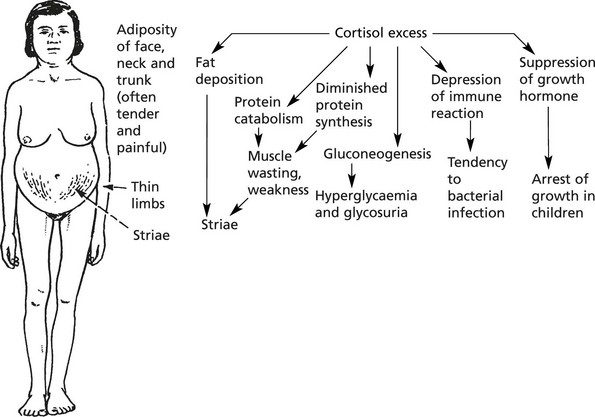
Cushing’s syndrome (hypersecretion of cortisol) – CORTICOSTEROID EXCESS This condition is commonest in women, but occurs also in men and rarely in children.
Clinical Effects
In addition:
1.
Osteoporosis → kyphosis
3.
Degree of virilism common in women.
Causes
1.
Excessive secretion of ACTH by pituitary adenoma, 70%.
2.
Adenoma or carcinoma of adrenal cortex, 20%.
3.
Inappropriate secretion of ACTH e.g. by bronchial carcinoma, carcinoids, pancreatic tumours.
4.
Prolonged administration of glucocorticoids or ACTH as therapy.
2.
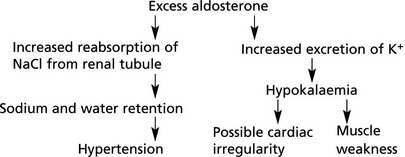
Primary hyperaldosteronism (Conn’s syndrome) – MINERLOCORTICOID EXCESS This is due to (˜60%) or an adenoma (˜35%) or hyperplasia of the zona glomerulosa.
Effects
Note: Secondary hyperaldosteronism is a consequence of any high renin state, e.g. renal ischaemia.
3.
Excessive sex hormone secretion – ANDROGEN EXCESS
This can occur occasionally with an adrenal cortical adenoma; usually excessive androgens are secreted. (a) In children it causes precocious puberty; (b) In adult females – virilism, oligomenorrhoea.
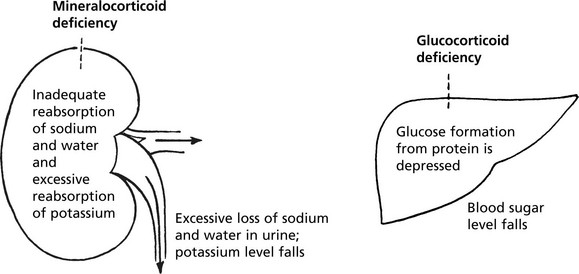
Adrenal Cortex – Hypofunction
Adrenal cortical insufficiency may be due to panhypopituitarism or destruction of about 90% of the adrenal cortex. Acute adrenal failure is usually due to septicaemia, especially meningococcal (Waterhouse-Friderichsen syndrome).
Chronic Hypofunction
Causes
1.
Addison’s disease. This is an autoimmune adrenalitis with humoral or cell mediated damage to the adrenal gland. It is responsible for at least 75% of cases.
2.
Tuberculous destruction of adrenals – remains an important cause, especially in developing countries.
Other autoimmune diseases affecting other endocrine glands are commonly associated with Addison’s disease, e.g. thyroid, parathyroid disease, diabetes.
Other Features
Muscular weakness and wasting.
Gastrointestinal upsets (vomiting, diarrhoea).
Pigmentation of exposed and pressure areas of skin (↑ MSH).
Crises occur especially if acute infection complicates the condition.
Administration of adrenal hormones restores individual to normal.
Note: In adrenal gland failure, a high level of ACTH results. Melanocyte stimulating hormone is derived from the same precursor pro-opiomelanocortin.
Adrenal Cortex And Medulla
Congenital Adrenocortical Enzyme Defects
These are rare, inherited conditions due to autosomal recessive traits.
Each transformation step in the pathways of steroid formation in the adrenal cortex requires the activity of one or more enzymes. Deficiency of any enzyme will interfere with production of the end product.
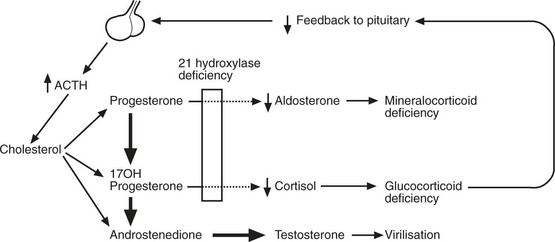
21 Hydroxylase Deficiency
This is the commonest type, and the lack of this enzyme prevents the production of cortisol and aldosterone. The low blood cortisol activates ACTH secretion. Adrenal hyperplasia follows and, although cortisol is not formed, other steroids are produced in excess. The resulting clinical syndrome varies with the severity of the defect.
1.
Symptoms of Addison’s disease.
2.
Lesser degrees of adrenal failure plus symptoms due to excess of sex steroids.
(a)
Pseudohermaphroditism in females; precocious puberty in males.
3.
No signs of adrenal failure but sex disturbance, e.g. amenorrhoea, hirsutism.
Adrenal Medulla
Excess production of catecholamines may occur with some tumours of the medulla. Three are described:
1.
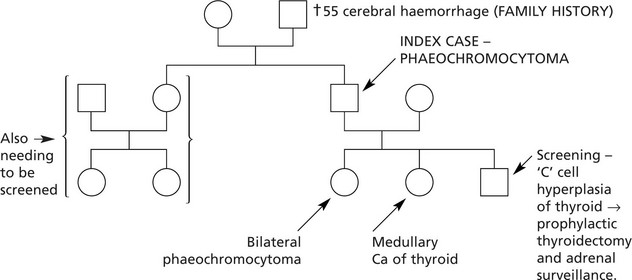
Phaeochromocytoma. This tumour is composed of chromaffin cells. Symptoms are due to paroxysmal overproduction of the amines with hypertension, raised metabolic rate and blood sugar. Cerebral haemorrhage may occur. About 25% of phaeochromocytomas are familial, 10% are bilateral and 10% are malignant. It is very difficult to predict behaviour on histological grounds.
Familial cases may be associated with:
(c)
Von Hippel–Lindau syndrome.
(d)
Germ line mutations of succinate dehydrogenase genes.
2.
Ganglioneuroma: a benign tumour, composed of well-differentiated ganglion cells (
p.577).
3.
Neuroblastoma: a very malignant tumour of primitive nerve cells, occurring in children.
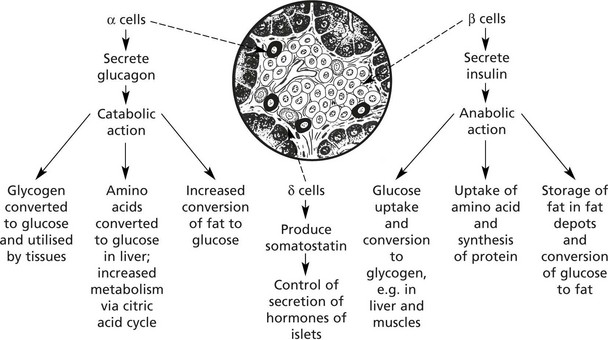
Endocrine Pancreas
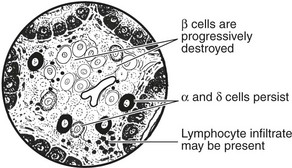
The islets of Langerhans form 1–2% of the pancreatic tissue. Four types of cell make up the islets. The majority are β cells.
Insulin also stimulates reabsorption of glucose from the renal glomerular filtrate
Insulin and glucagon have virtually opposite actions. The action of insulin is also opposed by growth hormone and glucocorticoids.
The fourth type of cell is the pancreatic polypeptide (PP) cell, found in highest concentration in the head of the pancreas.
Diabetes Mellitus
This condition is due to an absolute or relative lack of insulin activity. The American Diabetic Association classification of diabetes includes 10 categories.
There are 2 main types
Type 1 – immune mediated β cell destruction → absolute insulin deficiency.
Type 2 – adult onset due to insulin resistance, and β cell dysfunction and a range of rarer causes including genetic defects of β cell function and insulin receptors, diseases of the exocrine pancreas, and gestational diabetes.
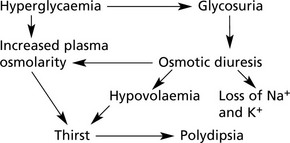
Biochemical Changes and Clinical Effects
The main results of insulin lack are:
1.
Inability to control carbohydrate metabolism, causing:
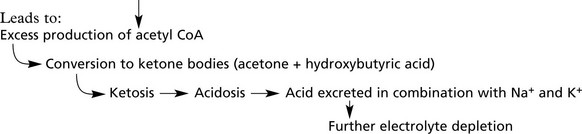
2.
Increased fat catabolism
3.
Increased catabolism of amino acids prevents proper protein synthesis and this, together with (1) and (2) above, leads to loss of weight despite polyphagia.
Types of Diabetes
Primary Forms
Type 1 Diabetes
This form is due to destruction of β cells in the islets of Langerhans.
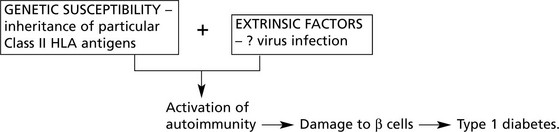
The onset is acute and the peak of incidence is around 13 years. Factors of importance in the aetiology:
1.
There is a familial incidence and in 80% of cases there is an association with Class II HLA antigens (particularly HLA DR3, DR4).
2.
Environmental factors, e.g. coxsackie B virus, may trigger islet cell destruction.
3.
Cell-mediated immunity against islet antigens and humoral antibodies are present in most cases.
This has given rise to a theory of pathogenesis:
Other forms of auto-antibody, e.g. gastric, thyroid, adrenal, have been demonstrated.
Primary Forms
Type 2 Diabetes Mellitus
This is the commonest
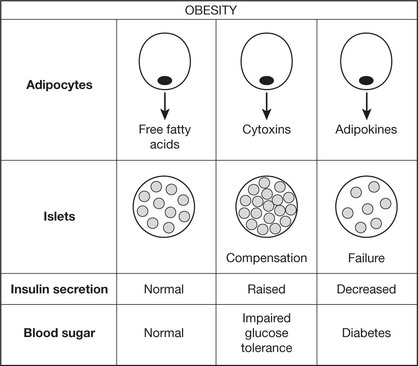
form of diabetes affecting 10% of adults over 65 in Western society. It is commoner in Asians and Afro-Caribbeans within Western societies. It is more frequent in females and the incidence increases with age. In contrast to Type 1, the onset is slow and the changes in glucose metabolism mild. (Diet restriction to reduce obesity and oral hypoglycaemic drugs usually control the blood sugar.) Clinical presentation is often due to complications, particularly vascular. Often the disorder is detected by biochemical screening.
Aetiology
This is a multifactorial disorder involving environmental and strong genetic factors. The basic mechanism is prolonged insulin resistance in the tissues leading eventually to inadequate secretion of insulin by β cells. The combination of obesity, Type 2 diabetes and hyperlipidaemia leads to an increased risk of cardiovascular disease.
The following theory accommodates the known facts:
Note 1: The concordance rate for identical twins is up to 60% in some studies.
Note 2: β cells secrete islet amyloid protein along with insulin. Amyloid is deposited in islets in Type 2 diabetes probably reflecting the prolonged β cell activity.
Secondary forms (Type 3)
Diabetes may complicate:
1.
A number of endocrine diseases (acromegaly, Cushing’s syndrome, phaeochromocytoma)
2.
Metabolic diseases (haemochromatosis)
3.
Drug therapy (steroids, thiazide diuretics)
4.
Pancreatic inflammation, etc. (chronic pancreatitis, mumps, cystic fibrosis).
Gestational Diabetes (Type 4)
This is associated with glycosuria during pregnancy and the birth of overweight babies. Control of the maternal blood sugar reduces birth weight to normal. Permanent diabetes is apt to develop at a later date.
Complications of Diabetes
1.
Diabetic Coma. 2 forms occur:
(a)
Keto-acidotic coma. This is common in Type 1 diabetes. Hyperosmolarity, hypovolaemia, acidosis and loss of electrolytes if unchecked lead to coma.
(b)
Hyperosmolar non-ketotic coma. This develops slowly in Type 2 diabetes. Hyperglycaemia builds up and produces profound dehydration.
2.
Hypoglycaemic coma. This complication of treatment occurs when insulin intake is excessive for the amount of food consumed.
3.
Cardiovascular lesions
(a)
Atheroma develops at an earlier age and with increased severity. Coronary thrombosis is common.
(b)
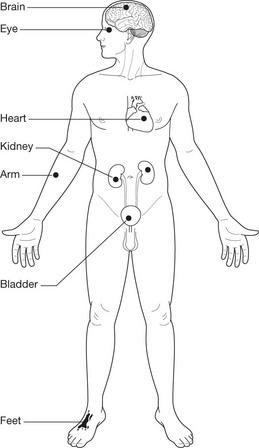
Microangiopathy causing occlusion of arterioles and capillaries. These vascular lesions are responsible for many of the clinical lesions e.g. cardiac failure, retinopathy, neuropathy, gangrene of limbs, Kimmelstiel–Wilson lesions in the kidney.
The frequency of cardiovascular lesions can be minimised by tight control of blood glucose levels (usually by monitoring glycated haemoglobin) and blood pressure.
4.
Renal failure is common. It may be due to glomerulosclerosis, but pyelonephritis and renal papillary necrosis are other causes.
5.
Infections. There is an increased susceptibility to sepsis, fungal infections and tuberculosis.
6.
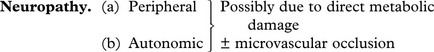
Pancreatic Endocrine Tumours (Islet Cell Tumours)
These are uncommon. Most are benign and are asymptomatic unless they secrete excess hormones. 10% are malignant. The clinical effects vary with the hormone produced.
1.
INSULINOMAS arise from β cells and produce attacks of hypoglycaemia.
2.
GASTRINOMAS cause multiple peptic ulcers (Zollinger-Ellison syndrome).
3.
Glucagonomas and somatostatinomas induce diabetes.
4.
Other hormones secreted, e.g. serotin and ACTH leading to carcinoid syndrome, Cushing’s syndrome, etc.
These tumours may form part of multiple endocrine neoplasia syndromes (p.643).
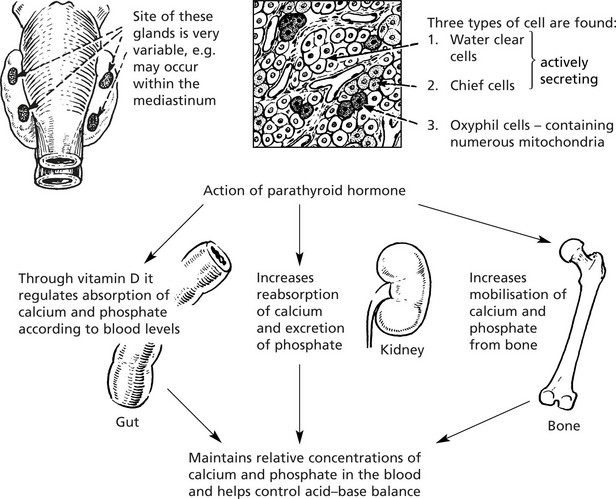
Parathyroid Glandsr
The parathyroids are four small glands lying posterior to the thyroid gland.
Hyperfunction (see P.591)
There are 3 forms:
1.
Primary
This is due to adenoma (> 80%), hyperplasia (approx. 15%) and carcinoma (approx. 2%).
The blood calcium is raised.
The effects are:
(a)
Formation of renal calculi sometimes leading to renal failure.
(b)
Parathyroid bone disease, now uncommon
(c)
General muscle weakness.
(d)
Metastatic calcification.
2.
Secondary
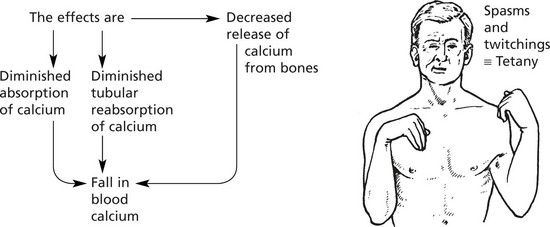
Parathyroid hyperplasia is a response to the low blood calcium from various causes as follows:
3.
Tertiary
In a few cases of secondary hyperparathyroidism an autonomous nodule develops in the hyperplastic gland and HYPERCALCAEMIA results.
Note:
Humoral Hypercalcaemia of Malignancy.
Carcinomas, particularly of the lung and kidney may produce
Parathyroid hormone related peptide (PTHrP) which causes
hypercalcaemia.
This is not related to parathyroid disease.
Hypoparathyroidism
This occurs in 3 circumstances:
1.
Surgical removal, sometimes accidentally during thyroidectomy
2.
Auto-immune disease (very rare).
3.
Congenital deficiency (e.g. DiGeorge syndrome).
Multiple Endocrine Neoplasia Syndromes (MENS)
This is a group of familial conditions (autosomal dominant inheritance) characterised by multiple endocrine tumours.
The main syndromes are:
MEN 1 (WERMER Syndrome) – Parathyroid adenoma, hyperplasia,
Pancreatic endocrine neoplasms,
Pituitary adenomas, especially prolactinomas.
This is due to mutation of the MEN 1 gene on chromosome 11, which encodes menin, a nuclear protein.
MEN 2A (SIPPLE Syndrome ) – Medullary carcinoma of thyroid,
Parathyroid adenoma or hyperplasia.
MEN 2B – Medullary carcinoma of thyroid (poor prognosis),
Ganglioneuromas of gut and skin,
Marfanoid habitus.
These are due to mutations activating the ret oncogene on chromosome 10 which encodes a cell surface receptor with tyrosine kinase activity.
Their importance is that family screening may pick up tumours at an early stage, indeed at the stage of hyperplasia which often precedes the development of tumours.
Illustrative family history: