Chapter 6 Neoplasia
Neoplasia
Cancer is the second commonest cause of death (25%) in the Western world after heart disease. Knowledge of non-neoplastic proliferation is helpful in understanding neoplasia.
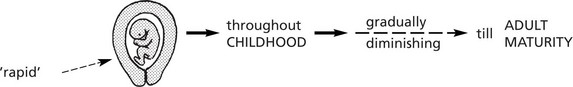
Physiological proliferation occurs:
ENLARGEMENT of an organ due to increase in the parenchymal cell mass may be due to:
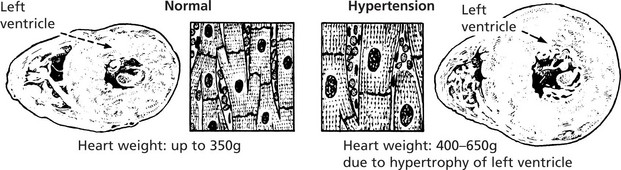
Physiological enlargement of organs is common and well illustrated by the increase in muscle bulk consequent upon training and the enlargement of uterus and breasts in pregnancy. Pathological enlargement is the result of disease processes.
Non-Neoplastic Proliferation
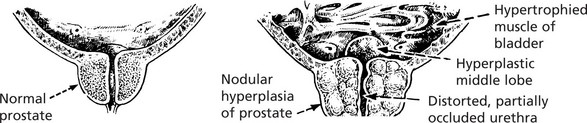
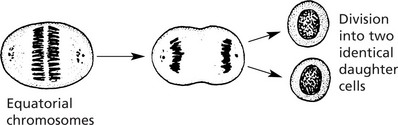
Hyperplasia
Note: If the abnormal stimulus is removed, the affected organ can return to normal.
Neoplastic Proliferation
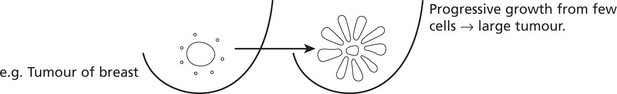
A tumour is a proliferation of cells which persists after the stimulus which initiated it has been withdrawn, i.e. it is autonomous. It is:
Neoplasms – Classification
Tumours may be classified in two ways: 1. clinical behaviour and 2. histological origin.
The tumour is classified according to its morbid anatomy and behaviour. Two main groups are recognised – benign (simple) and malignant. The contrast between these two groups is as follows:
| BENIGN | MALIGNANT | |
|---|---|---|
| Spread (the most important feature) | Remains localised | Cells transferred via lymphatics, blood vessels, tissue planes and serous cavities to set up satellite tumours (metastases) |
| Rate of Growth | Usually slow | Usually rapid |
| Boundaries | Circumscribed, often encapsulated | Irregular, ill-defined and non-encapsulated |
| Relationship to surrounding tissues | Compresses normal tissue | Invades and destroys normal tissues |
| Effects | Produced by pressure on vessels, tubes, nerves, organs, and by excess production of substances, e.g. hormones. Removal will alleviate these | Destroys structures, causes bleeding, forms strictures |
In practice, there is a spectrum of malignancy. Some tumours may grow locally and invade normal tissues but never produce metastases. Others will produce metastases only after a very considerable time while, at the other end of the spectrum, there are tumours which metastasise very early in their development.
Tumours may arise from any tissue in the body and they can be conveniently accommodated in five groups.
The commonest tumours arise from tissues which have a rapid turnover of cells and which are exposed to environmental mutagens, e.g. epithelium of mucous membranes, skin, breast and reproductive organs and lymphoid and haemopoietic tissues.
Malignant Tumours – Histology
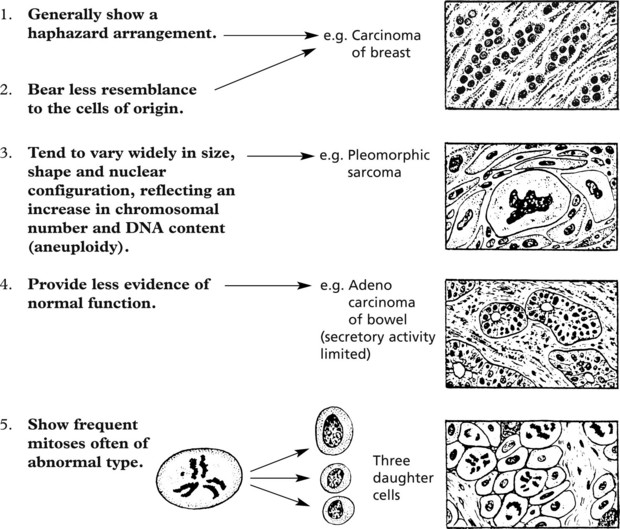
The cells of malignant tumours tend to be less well-differentiated than those of benign tumours.
There is a spectrum within malignant neoplasms from slowly growing well differentiated examples to rapidly growing undifferentiated highly malignant tumours.
Benign Epithelial Tumours
Benign epithelial tumours are essentially of two types: 1. papillomas and 2. adenomas.
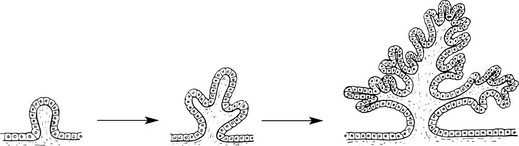
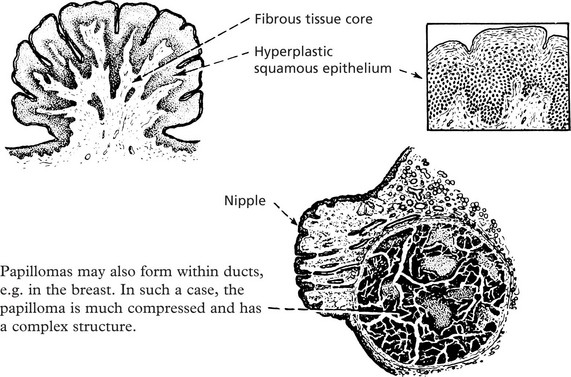
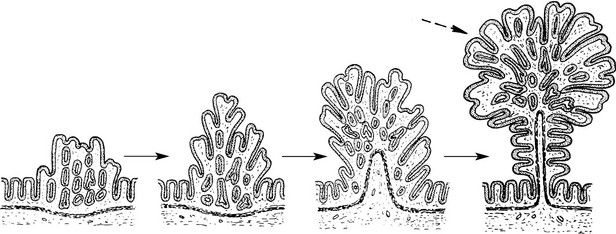
Papilloma
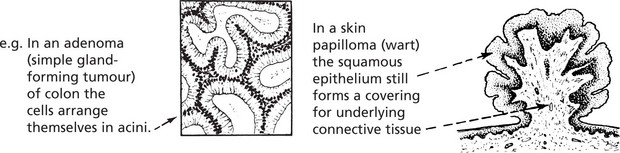
Papillomas take origin from an epithelial surface. As the epithelium proliferates it is thrown into folds which become increasingly complex.
The epithelial proliferation is accompanied by a corresponding growth of supporting connective tissue and blood vessels.
Typical examples are found in the skin, e.g. the common wart.
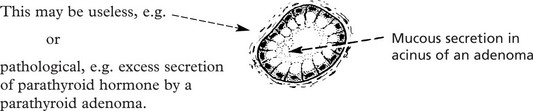
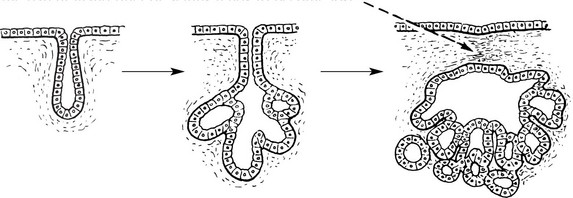
Adenoma
Adenomas are derived from the ducts and acini of glands, although the name is also used to cover simple tumours arising in solid epithelial organs.
Again the proliferation of epithelium of a gland causes the formation of tubules which ramify and become compound. The original communication with the parent gland duct or acinus tends to become lost.
In the case of a hollow viscus, such as the intestine, the adenomatous proliferation, instead of growing down into the underlying connective tissue, is usually pushed upwards into the lumen of the viscus.
The growth therefore combines the features of a papilloma and an adenoma. The term adenomatous polyp is often applied in such a case.
In the type which grows into the subjacent connective tissue, the progressive budding of the epithelium results in new acini which become nipped off from the parent acini.
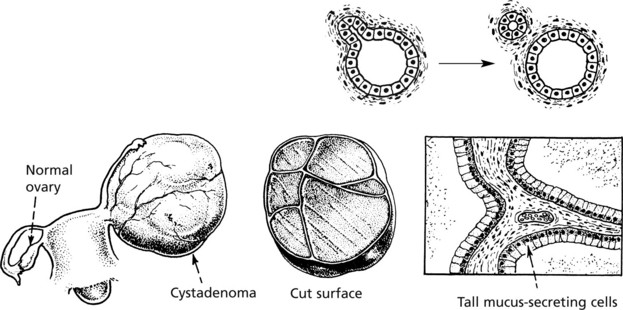
In cases in which retention of secretion is marked, a cyst forms and the tumour is then called a CYSTADENOMA which may reach an enormous size, e.g. some cystadenomas of the ovary may be 30–40 cm in diameter, particularly those which secrete mucus.
As in a hollow viscus, the proliferating epithelium may be heaped to form papillomas and the tumour then becomes a PAPILLARY CYSTADENOMA. These are also common in the ovary (see p.511).
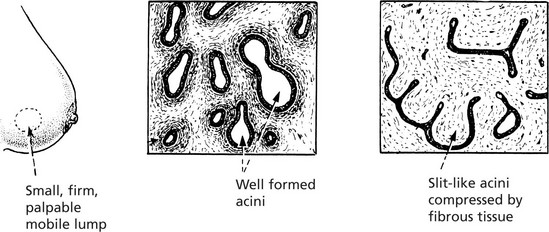
Fibroadenoma
In the breast the term is clinically useful for a small nodule consisting of a mixture of acinar elements and prominent supporting fibrous tissue. The histological appearances are variable depending on the distribution of the fibrous tissue (see also p.520). This is not now considered to be a true neoplasm.
Benign Connective Tissue Tumours
Benign connective tissue tumours are composed of mature connective tissues – fat, cartilage, bone and blood vessels. They tend to form encapsulated rounded or lobulated masses which compress the surrounding tissues.
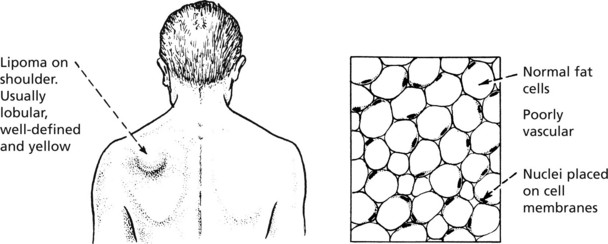
Lipoma
Circumscribed masses of fat are commonly found in the subcutaneous tissue of the arms, shoulders and buttocks. Less commonly they occur in the deep soft tissues of the limbs or retroperitoneum, but at these sites they must be carefully distinguished from low grade liposarcomas. Lipomas very rarely arise from the viscera.
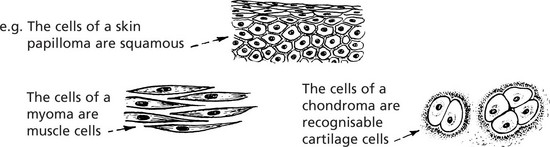
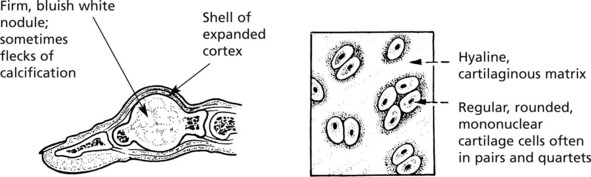
Chondroma
A chondroma usually arises within the medullary cavity of the tubular bones of the hands and feet; it grows slowly, causing gradual expansion of the bone. Less commonly it occurs in long bones. Rarely multiple enchondromas appear in children and are associated with deformity: they occasionally become malignant.
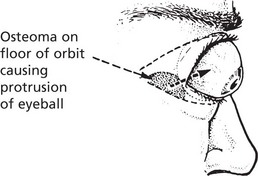
Osteoma
This tumour is mainly found in the bones of the skull, although it may occur in long bones. Osteomas are relatively small but may produce severe symptoms because of their situation.
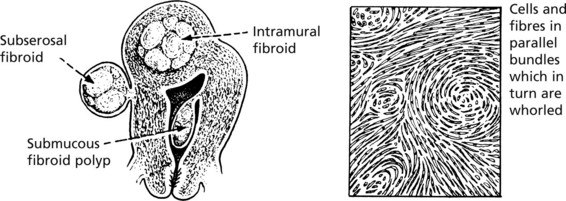
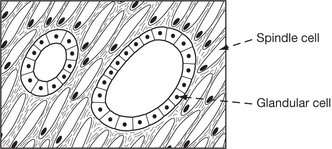
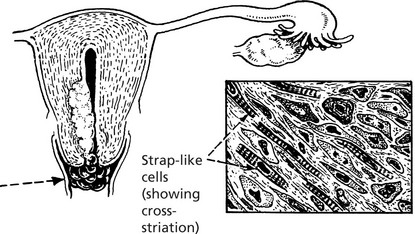
Leiomyoma (Fibroid)
The majority of benign smooth muscle tumours occur in the wall of the uterus where they are extremely common, but may lie within the uterine cavity or attached to the serosa. They are firm, rounded masses which usually begin in the myometrium.
Tumours of Fibrous Tissue
So-called fibromas are rare but tumour-like growths are fairly common. An example is a FIBROMATOSIS.
Palmar fibromatosis, at first nodular, causes flexion deformities of fingers (Dupuytren’s contracture) due to contraction of fibrous tissue within the palmar fascia. A similar condition may affect the plantar fascia. Deeply located fibromatoses behave in a locally aggressive manner.
Benign tumours of peripheral nerve and of vessels are described on pages 579 and 580.
Malignant Epithelial Tumours
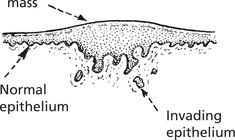
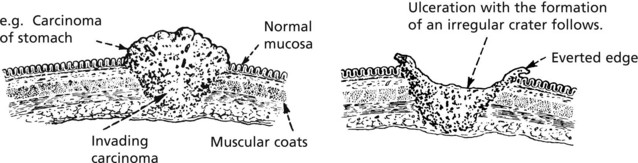
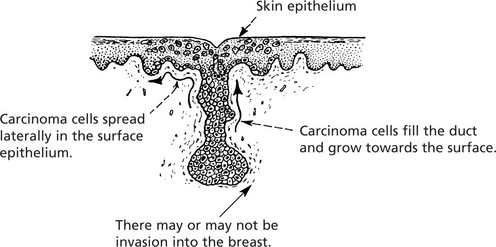
Malignant epithelial tumours are known as CARCINOMAS (Greek ‘karkinos’: a crab), referring to the typical irregular jagged shape. This is due to invasion into adjacent normal tissues (p.135).
Types of Carcinoma
Like benign epithelial tumours, carcinomas can arise from squamous or glandular epithelium.
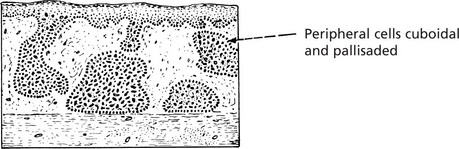
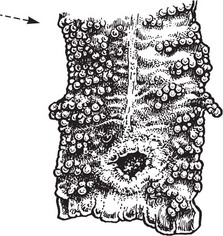
Squamous Cell Carcinoma
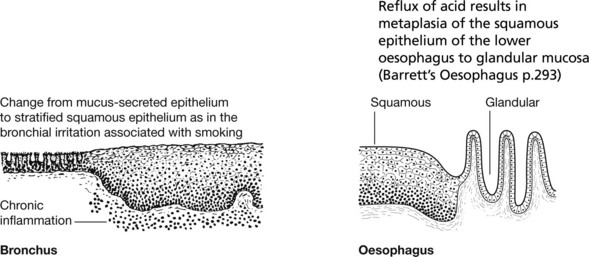
This is commonly found on the skin, especially exposed surfaces, but also develops in other sites covered by stratified squamous epithelium, e.g. lips, tongue, pharynx, oesophagus and vagina. In addition, it may occur on surfaces covered by glandular type epithelium through metaplastic transformation as in the bronchus, gall bladder and uterine cervix. It frequently arises from areas of carcinoma-in-situ (p.144).
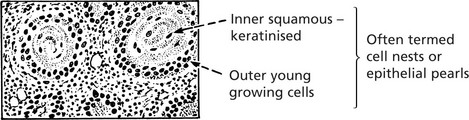
Histologically, it is composed of irregular strands and columns of invading epithelium which infiltrate the underlying connective tissue. If well differentiated, the central cells of the invading masses show conversion into eosinophilic keratin, while the outer layer consists of young basophilic cells. In cross-section, the appearance is typical.
Squamous carcinoma of skin is typically well differentiated and slow growing. In contrast, uterine cervical carcinomas are poorly differentiated and spread early to draining lymph nodes.
Types of Carcinoma
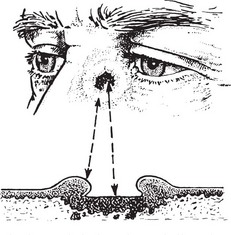
Basal Cell Carcinoma (Rodent Ulcer)
This tumour may arise in any part of the skin but is most common in the face, near the eyes and nose.
First Stage
It starts as a flattened papilloma which slowly enlarges over months or perhaps a year or two.
Second Stage
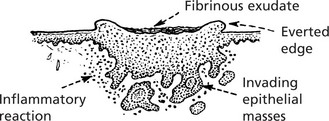
The surface breaks down and a shallow, ragged ulcer with pearly edges is formed.
Usually the malignant tissue spreads slowly but progressively, mainly in a lateral direction. It is composed of cells resembling those of the basal layer of the skin; it has a characteristic histological appearance.
There are many histological variants. Sometimes they show pseudoglandular structures: melanin deposition occurs in some tumours.
Basal cell carcinoma is a locally invasive growth which can be extremely destructive (hence its name: Rodent ulcer). It almost never metastasises. Thus the two processes of local invasion and distant metastases are not necessarily linked.
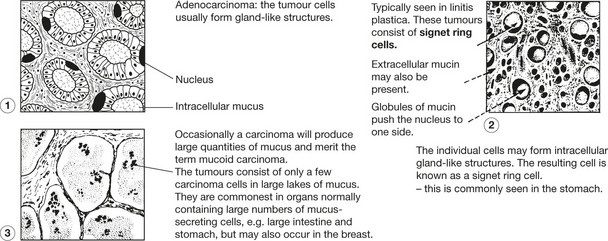
Carcinoma of Glandular Organs
These may take origin from gland acini, ducts or the glandular epithelium of mucous surfaces. The anatomical structure varies.
Histologically, carcinomas of glandular tissue have three basic forms.
Malignant Connective Tissue Tumours
Malignant connective tissue tumours are referred to as Sarcomas (Greek ‘sarkoma’: flesh). They arise in soft tissue, in bone and rarely in viscera.
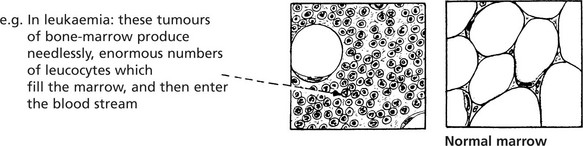
Sarcomas are far less common than carcinomas, but are second in frequency to leukaemias and lymphomas in childhood and early adult life.
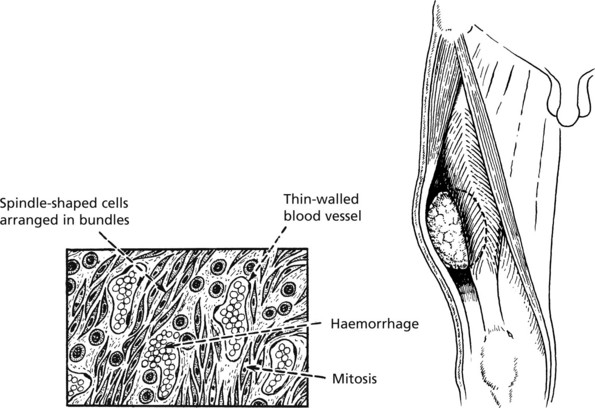
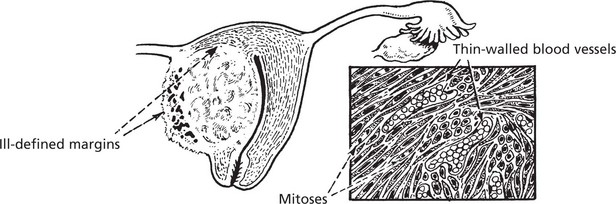
Unlike the ill-defined infiltrative carcinomas, sarcomas are large well defined fleshy tumours. Naked eye assessment suggests that they are encapsulated, but histology shows that this is a false impression. Malignant cells do infiltrate between normal tissues at the margin, so that surgical ‘shelling out’ is almost inevitably followed by local recurrence from aggregates of cells remaining in the tumour bed.
Most sarcomas consist of spindle shaped cells, although some are of round cell type. They are associated with the formation of many large thin walled blood vessels which are easily invaded by sarcoma cells. Blood borne metastases to lung are common. In contrast, lymph node involvement is rare in most types of sarcoma.
Sarcomas
Many different histological types of sarcoma have been described. The nomenclature is based on adding the suffix ‘sarcoma’ to the type of differentiation shown, e.g. chondrosarcoma, liposarcoma, leiomyosarcoma (cartilage, adipose tissue, smooth muscle).
In general, the grade of the tumour is of more prognostic importance than the precise histological type. Some of the commoner forms of sarcoma are described.
Liposarcoma
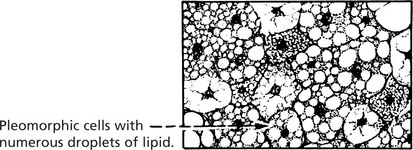
This tumour arises in soft tissues of the limbs and retroperitoneum. A number of sub types are described. Primitive fat containing cells (lipoblasts) are a feature.
Well differentiated liposarcoma has a good prognosis and rarely metastasises. Pleomorphic and round cell liposarcomas are highly aggressive tumours which metastasise early.
Myosarcoma (Sarcoma of Muscle)
These rare tumours arise in the skin, deep soft tissues and particularly in the uterus.
This is a rare tumour occurring mainly in children. It occurs very rarely in skeletal muscles. More common but still rare are polypoid tumours in the bladder, uterus and vagina, sometimes called sarcoma botryoides (grape-like).
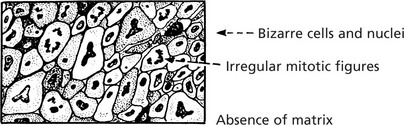
Pleomorphic or Anaplastic Sarcoma
Many highly malignant sarcomas are so poorly differentiated that their specific type cannot be determined.
Immunocytochemistry, electron microscopy and cytogenetic analysis (demonstrating typical chromosome translocations) all help in reaching more specific diagnoses than can be reached by conventional histology alone.
Sarcomas of bone are described on page 598.
Other Tumour Types
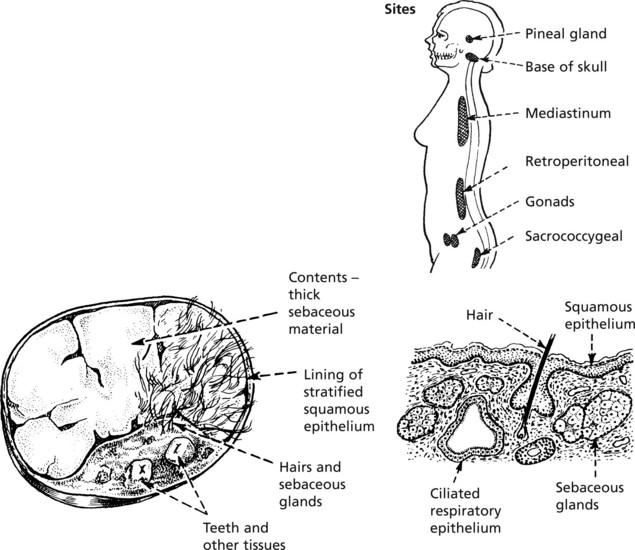
Teratoma
This is a tumour derived from totipotent germ cells. Most arise in the ovary (usually benign) and testis (almost always malignant): they may also occur at any site in the mid-line where germ cells have stopped in their migration to the gonads.
For example, Benign cystic teratoma is typically seen in the ovary.
It consists mainly of ectodermal structures such as skin and its appendages and neural tissue. Frequently respiratory epithelium, intestinal epithelium, bone and cartilage are present.
Testicular Teratomas are described on page 489.
Hamartoma
A hamartoma is a tumour-like but non-neoplastic malformation consisting of a mixture of tissues normally found at the particular site.
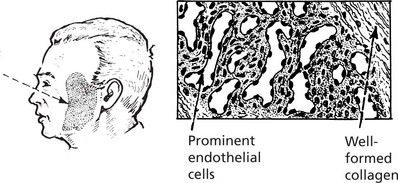
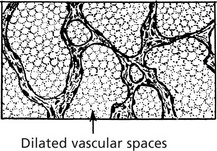
Haemangioma
These are common in the skin as ‘birth-marks’ which vary in size. Occasionally they may be found in internal organs. They are well defined, deep red or purple. A single artery provides a supply separate from the surrounding tissue.
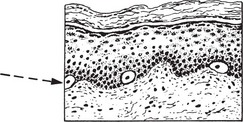
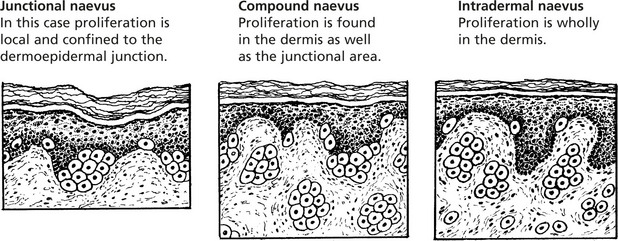
Benign Pigmented Naevus (Melanocytic Naevus or Mole)
Benign pigmented naevus is extremely common. The term ‘naevus’ means a birthmark, but most naevi are acquired in childhood and adolescence.
During fetal life melanin-pigment-forming neuroectodermal cells migrate to the skin and are found in small numbers in the basal layer of the skin.
The common congenital pigmented mole is the result of an abnormality in migration, proliferation and maturation of these neuroectodermal cells. A continuous layer of pigmented cells is found adjacent to the basal epidermal cells.
The resulting naevi vary in size and appearance, varying from small flat macular brown areas or smooth papular lesions to warty hairy excrescences. Pigmentation is variable.
Blue naevus is another variation in which melanocytes are arrested in migration in the dermis.
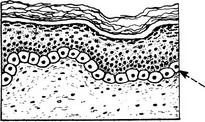
Active proliferative activity may extend into adult life, but the great majority of these lesions undergo a degree of involution. One feature which differentiates them from malignant change is that the deeper the cells penetrate the dermis the smaller they become and the less active.
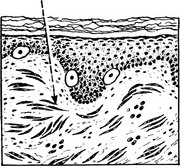
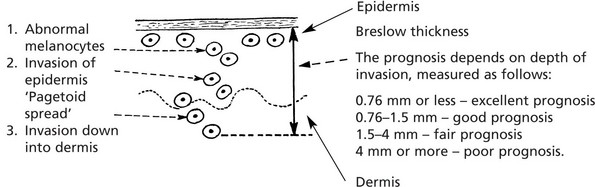
Malignant Melanoma
Malignant proliferation of melanocytes usually arises de novo but some melanomas arise from pre-existing naevi. Exposure to SUNLIGHT (the U.V. component) is the most important aetiological factor.
Sites
Some tumours are amelanotic (non-pigmented). The rate of growth is variable.
Four types of growth may occur:
Types 2, 3 and 4 occur in younger adults, and all can have an in situ stage. The features of malignant melanoma are:
Spread of Tumours
Tumours spread by several routes.
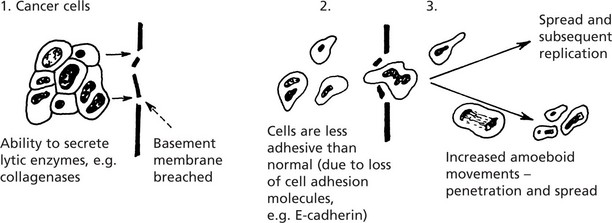
Local Spread
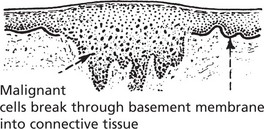
The proliferating cells break through normal barriers.
Further spread is by penetration between normal tissues.
An important principle is that these permeating tumour cells take the line of least physical resistance.
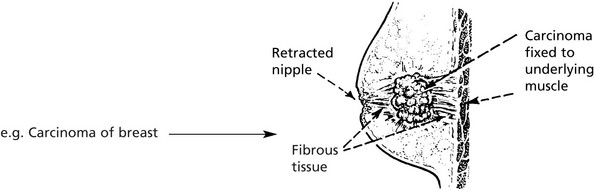
Tumours may stimulate the production of new collagen fibres which are sometimes converted into dense fibrous tissue → contracts and fixes the growth to surrounding structures
The following diagram illustrates the basic mechanisms of cancer cell invasion.
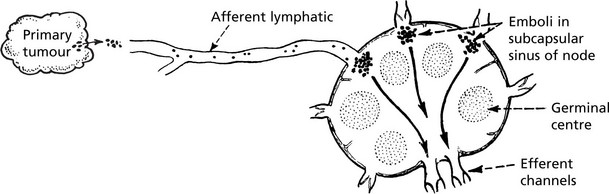
Lymphatic Spread
Lymphatic Spread
This is the commonest mode of spread of carcinomas and melanomas, but rarely of sarcomas. Malignant cells easily invade lymphatic channels from the tissue spaces.
Groups of cells form emboli in the lymph stream and are carried to the nearest node (the sentinel node).
The emboli appear first in the subcapsular sinus, and then progressively invade the tissues of the node. Eventually they reach the medulla and grow into the efferent channel and produce metastases in other nodes. Invaded nodes are enlarged, firm and white.
Histological examination of the sentinel node is increasingly carried out to select the patients who require extensive lymph node dissection.
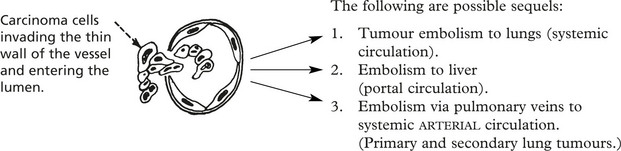
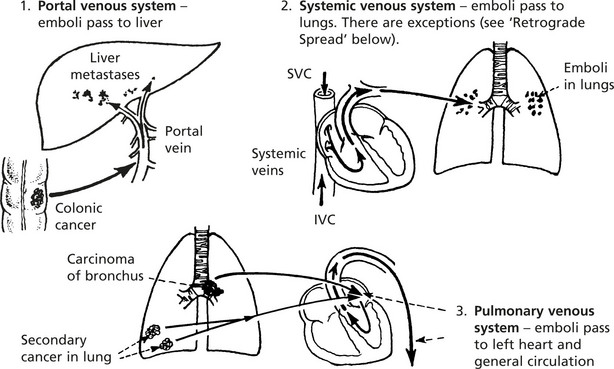
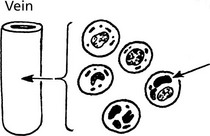
Blood Spread
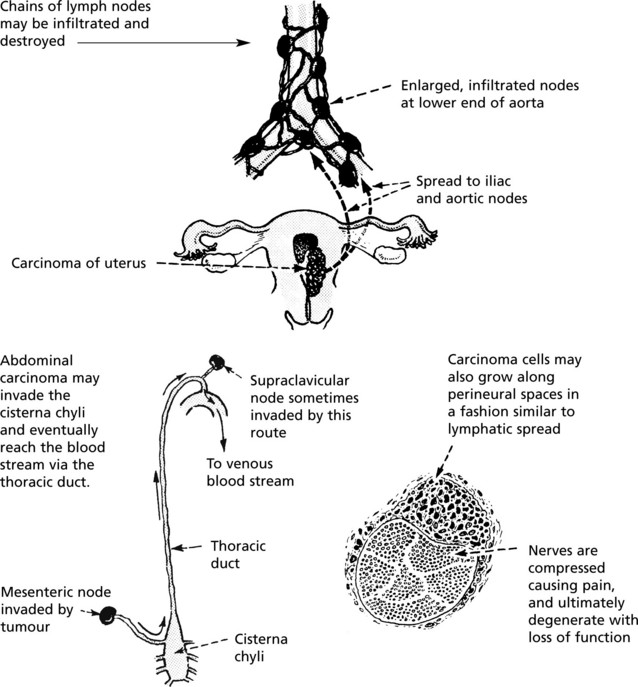
Both carcinomas and sarcomas spread by the blood stream. The entry of malignant cells into the blood is via invasion of VENULES and by lymphatic embolism through the thoracic duct into the subclavian vein.
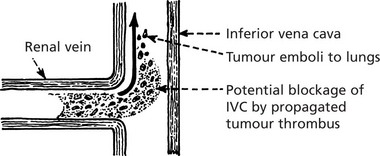
Via Larger Veins
Occasionally tumour thrombus is propagated from venules into larger veins. This is classically seen in RENAL CARCINOMA.
Rarely, a malignant process may be complicated by thrombosis of distant veins (thrombophlebitis migrans). This is not due to malignant invasion of the veins but is caused by the action of circulating thromboplastins formed by the tumour.
Arterial System
Direct invasion of arteries and arteriolar lumens is very rare because of the physical barrier provided by the thick muscular and elastic walls.
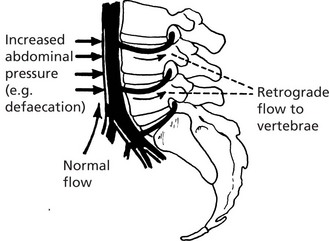
Retrograde Venous Spread
As in lymphatics, growth of tumour within a vein may cause reversal of blood flow. In addition, reversal of flow is apt to happen in certain areas of the body where veins form a rich plexus and are deficient in valves, e.g. in the pelvis and around vertebrae. Changes in intra-abdominal and intrathoracic pressures easily induce changes in blood flow in these channels. It is for this reason that secondary tumours are relatively common in vertebral bodies.
Fate of Carcinomatous Emboli
The distribution of carcinomatous emboli is determined in part by anatomy, but many complex factors both in the ‘seed’ (the cancer cell) and the ‘soil’ (the potential metastatic site) are at play in the establishment of metastases at particular sites. They include surface properties of the cancer cells e.g. increased expression of integrins and their receptors present in the endothelial cells at the metastatic site. Variation in the host IMMUNE RESPONSE is also important
Other Modes of Spread of Tumours
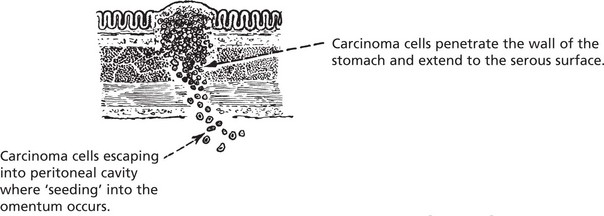
Transcoelomic Spread (via Serous Sacs)
This is an important and frequent route of spread in the peritoneal and pleural cavities. It also takes place in the pericardial sac.
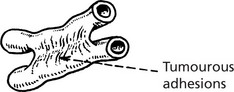
As malignant cells sink in the peritoneal cavity, they will settle in various sites. They may cause an inflammatory reaction with fibrin formation. This can cause adhesions between organs, e.g. loops of bowel.
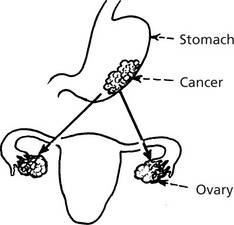
Gastric cancer can spread to both ovaries (so-called KRUKENBERG TUMOUR). An unusual but classic manifestation.
Effects of Tumours
Benign Tumours
(a) The localised tumour mass may compress neighbouring structures causing loss of function and (b) benign endocrine tumours may produce excess hormones.
Malignant Tumours
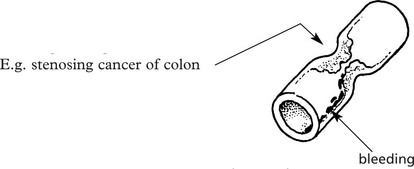
A tumour may narrow a hollow viscus e.g causing intestinal obstruction, ulceration and bleeding leading to anaemia.
Direct spread compresses adjacent viscera, blood vessels and nerves – LUNG CARCINOMA is illustrative (see p.277–278).
These are very numerous and variable. Two illustrative examples are given.
Non-Metastatic Complications
Patients with cancer often have severe weight loss (cachexia), loss of appetite and fever. These are probably due to release of cytokines (e.g. TUMOUR NECROSIS FACTOR) from tumour cells. Other complications include myopathy and neuropathy. Relapsing thrombosis of distant veins (thrombo-phlebitis migrans) is due to release of thromboplastins.
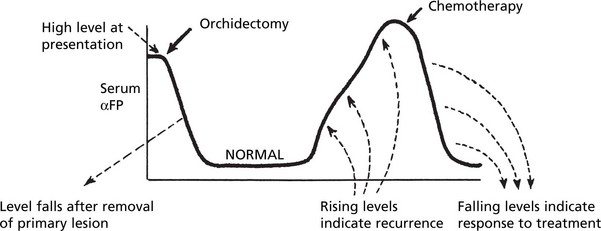
Tumour Markers
Tumour cells produce substances, many of which are proteins, which are helpful in diagnosis and monitoring of treatment.
| Tumour Marker | Tumour |
|---|---|
| Human chorionic gonadotrophin (HCG) | Choriocarcinoma, Teratoma of testis |
| αFeto-protein (αFP) | Hepatocellular carcinoma, Teratoma of testis |
| Prostate specific antigen, Prostatic acid phosphatase | Prostatic carcinoma |
| Carcino-embryonic antigen | Gastro-intestinal and other cancers |
| Calcitonin | Medullary thyroid carcinoma |
| 5-hydroxyindole-acetic acid (5HIAA) in URINE (metabolite of 5-hydroxytryptamine (5HT-SEROTONIN)) | Intestinal carcinoid |
Diagnosis of Tumours Immunocytochemistry
Diagnosis of tumours is made by combining clinical information with pathological information of several types:
Different immunocytochemical panels are used to help address specific histological dilemmas.
Thus, for a metastatic adenocarcinoma of unknown origin, the pathologist may request:
| Cytokeratin profiling | CK 7, 19, 20 |
| Transcription factors | Wilms tumour 1 (WT1) Thyroid transcription factor 1 (TTF-1) CDX2 |
| Hormone receptors | Oestogen (ER), progesterone (PR) receptors |
| ‘Tumour markers’ | Prostate specific antigen (PSA) Ca125 Carcinoembryonic antigen |
| An adenocarcinoma with this profile is likely to be of pulmonary origin | CK7 +, CK20 − TTF1 +ve, WT1 −ve, CDX2 −ve ER −ve, PR −ve Ca125 −ve PSA −ve |
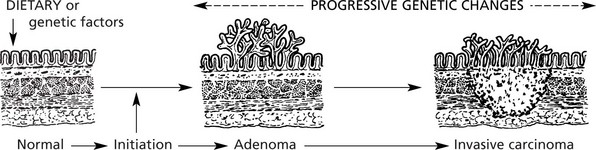
Pre-Malignancy
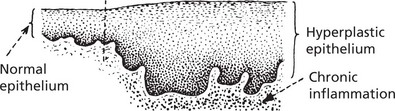
The pathological conditions which are associated with the development of malignancy fall into 3 groups: 1. Benign tumours, 2. Chronic inflammatory conditions and 3. Intraepithelial neoplasia.
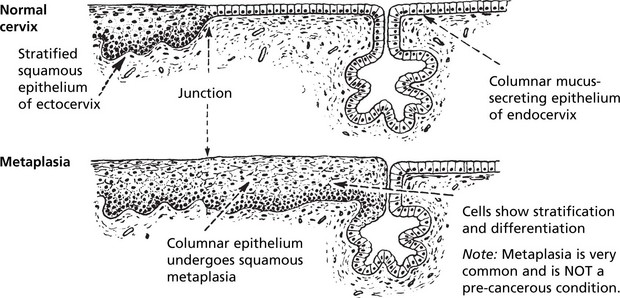
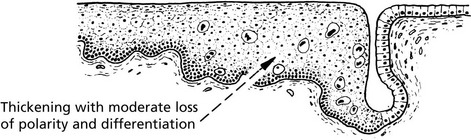
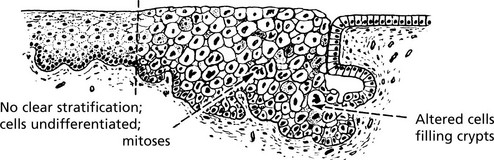
Carcinoma in situ (Intraepithelial Neoplasia)
This represents an intermediate stage in the production of a cancer. All the cytological features of malignancy are present, but the cells have not invaded the surrounding tissues. It is frequently found in the cervix uteri at the junction of ecto and endocervix.
In the cervix, the abbreviation CIN (cervical intraepithelial neoplasia) is used. There are 3 grades of severity.
These premalignant conditions may revert to normal, but most commonly they become truly malignant and invade the surrounding tissues.
The concept of progressive premalignant proliferation applies equally in other organs (e.g. breast, stomach, oesophagus, bronchus, prostate, mouth, vulva).
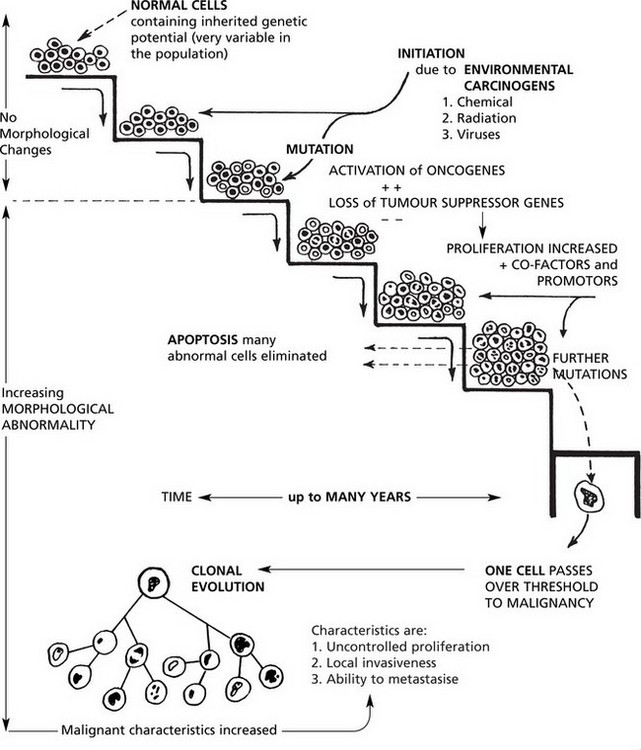
Carcinogenesis
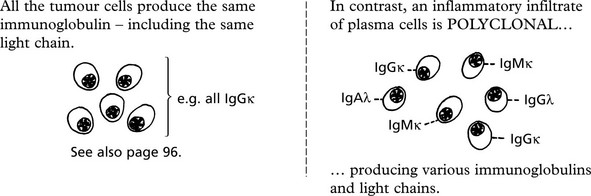
Malignant tumours are due to UNCONTROLLED PROLIFERATION of cells. Most tumours are MONOCLONAL, i.e. are derived from a single transformed cell.
This concept is well illustrated in MULTIPLE MYELOMA - a malignant tumour of plasma cells.
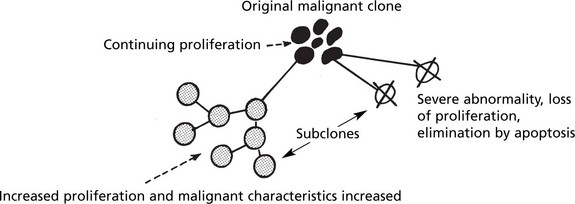
Clonal Evolution
In time, some cells undergo further mutations which are passed on to their progeny. This is called clonal evolution or progression and explains the morphological variations seen in tumours. Some of the subclones grow more rapidly and metastasise more readily while others are so abnormal that proliferation is not possible and the clone dies out.
Nuclear Morphology and DNA Content
In histological sections these abnormalities are indicated by variations in nuclear density (usually increased), size and shape (i.e. pleomorphism).
The term ‘polyploidy’ is used when the nuclear DNA is increased by exact multiples of the normal.
‘aneuploidy’ indicates irregular increases in DNA content.→
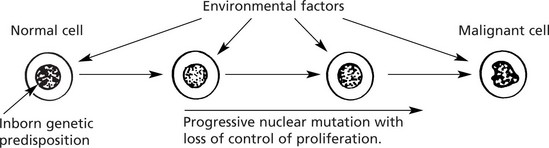
A number of factors, both environmental and genetic, contribute to a cell undergoing malignant change. This should be regarded as a multistep sequence (see p.154).
Environmental Factors
The 3 major environmental factors which induce tumours are 1. Chemical carcinogens, 2. Radiation and 3. Viruses.
Chemical Carcinogenesis
Historically, chemical agents were the first to be associated with cancer. Now, many are recognised in (a) industrial processes, (b) social habits and (c) diet.
| Industry | Tumour | Chemical Responsible |
|---|---|---|
| Aniline dyes | Bladder cancer | Naphthylamine |
| Insulation e.g. shipbuilding, building | Mesothelioma, lung, laryngeal cancer | Asbestos |
| Mineral oil and tar | Skin cancers | Benzpyrene and other hydrocarbons |
| Plastics | Angiosarcomas of liver | Vinyl chloride monomer |
| Wood dust | Cancer of nose and sinuses | ? |
Social Habits
Cigarette smoking is strongly associated with the development of many cancers, including lung, mouth, larynx and bladder. Chewing tobacco greatly increases the risk of oral cancer. It is likely that air pollution, e.g. combustion products of petrol and diesel, also contributes to carcinogenesis. Obesity is associated with an increased risk of cancer, e.g. of the uterus and colon.
Diet
Chemicals in food may be carcinogenic. Nitrosamines may be formed by the action of bacteria on ingested nitrites. AFLATOXINS are produced by fungi (Aspergillus flavus) and, by contaminating foodstuffs, may cause liver cancer.
Note: Chemical carcinogens may act in 2 ways:
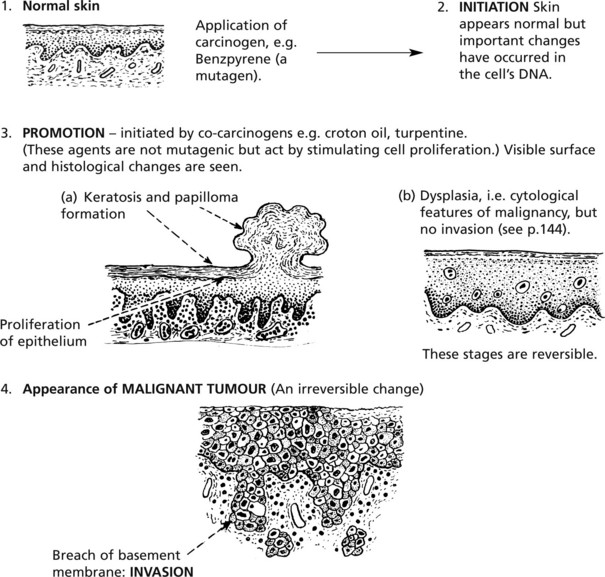
This is a multistep process of long duration.
The stages of initiation and promotion are important and were identified in classic experiments of skin carcinogenesis.
The order of application of these agents is important. No tumour follows application of promotor alone, or promotor followed by initiator.
In rodents, these changes take months; in humans the time scale is years and is exemplified in the skin, cervix uteri, bronchus, urinary bladder, colon and breast.
Radiant Energy
The potential dangers from irradiation give much cause for concern.
| Source of Radiation | Tumours | Type of Radiation |
|---|---|---|
| Sunlight | Melanoma, Carcinoma of skin | Ultraviolet (non-ionising) |
| Nuclear explosions (e.g. atom bombs, Chernobyl) | Leukaemia, carcinomas of lung, breast, thyroid | Ionising |
| Therapeutic irradiation | Various carcinomas, sarcomas, leukaemia | Ionising |
| Mining radioactive substances (e.g. uranium) | Lung carcinomas | Ionising |
| X-ray workers (historical) | Skin cancer, leukaemia | X-rays: Ionising |
Effects of Irradiation on Cells
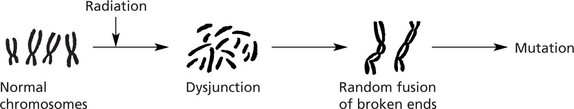
The ionising effect of radiation damages the cell’s DNA, especially during cell proliferation, ranging from single gene mutation to major chromosome damage, including breaks, deletions and translocations.
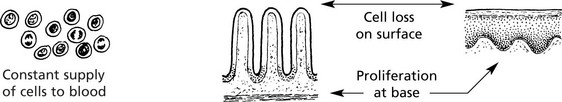
Proliferating cells are particularly vulnerable. Marrow and gastro-intestinal mucosa are highly sensitive.
Ultraviolet light is of low energy and mainly affects the skin. The ‘pyrimidine dimers’ formed by UV light are normally excised by DNA repair mechanisms. In xeroderma pigmentosum, these are deficient leading to numerous skin tumours.
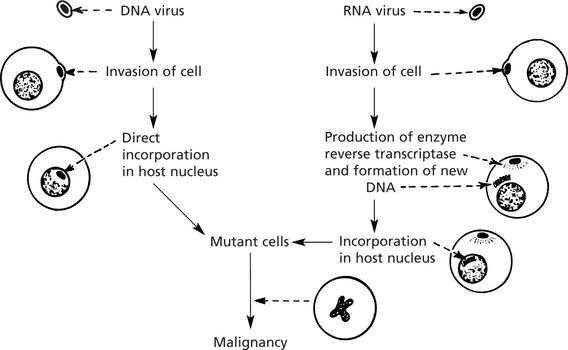
Carcinogenesis – Viruses
Viruses have long been known to cause cancer in animals (e.g. Rous sarcoma virus and the mouse mammary tumour virus – the Bittner milk factor).
In recent years viruses have been shown to contribute to the development of some human cancers.
| Virus | Type | Tumour type |
|---|---|---|
| Epstein-Barr (EBV) | DNA | Burkitt’s lymphoma, nasopharyngeal cancer, Hodgkin’s disease, post-transplantation lymphoma |
| Human herpes virus 8 (HHV-8) | DNA | Kaposi’s sarcoma |
| Hepatitis ‘B’ | DNA | Hepatocellular carcinomas |
| Human papilloma virus (HPV) | DNA | Cervical, penile, anal carcinoma |
| Human ‘T’ cell leukaemia virus (HTLV-1) | RNA (Retrovirus) | T-lymphoblastic leukaemia |
Carcinogenesis – Heredity
The inherited genetic influences in cancer are now well recognised.
Oncogenes and Tumour Suppressor Genes
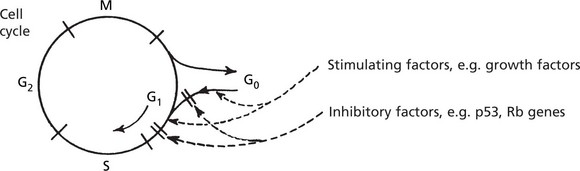
Cellular proto-oncogenes are NORMAL genes which STIMULATE cell division. Tumour suppressor genes are NORMAL genes which INHIBIT cell division. Their normal activity during somatic growth and in regeneration and repair takes place during the G0–G1 phase of the cell cycle and is strictly controlled.
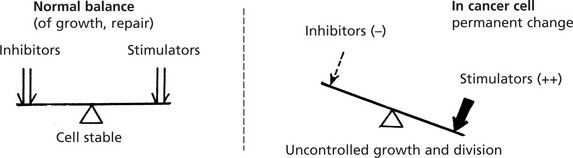
In cancer cells the normal controls are defective so that the balance between factors stimulating and inhibiting cell growth is permanently lost, resulting in increased proliferation.
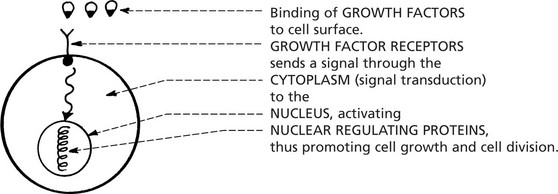
Cellular proto-oncogenes code for a number of proteins involved in cell proliferation:
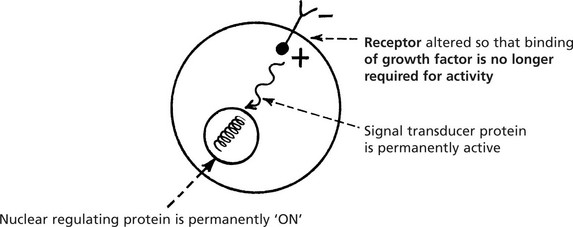
In the cancer cell, these normal genes are permanently changed to oncogenes and proliferation is uncontrolled.
Mutation or overexpression of genes which normally control apoptosis (p.4) are increasingly recognised in many tumours, e.g. overexpression of bcl-2 inhibits apoptosis in follicular lymphoma (p.432).
Oncogenes
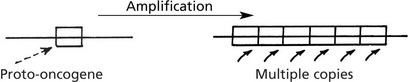
The production and activity of ONCOGENES is complex and there are many ways in which they are activated.
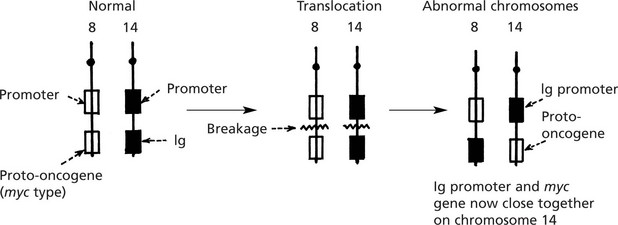
The myc oncogene is now under the influence of the Ig gene control and is permanently switched on.
A point mutation of the proto-oncogene produces a protein with an altered function so that over-stimulation occurs.
Tumour Suppressor Genes
Tumour suppressor genes are normal genes which switch off cell proliferation by acting on the cell cycle in G1. Their biological role is much broader than simply suppressing tumour function, but the name reflects how they have been discovered.
Loss of both copies of a tumour suppressor gene is required for cancer to develop. In contrast, only one copy of a given oncogene needs to be activated to cause excess proliferation.
| Tumour suppressor gene | Chromosome | Tumours |
|---|---|---|
| p53 | 17 | Carcinoma of lung and breast: sarcoma |
| Retinoblastoma (Rb) | 13 | Retinoblastoma, sarcoma: some carcinomas |
| NF1 | 17 | Neurofibromas, malignant peripheral nerve tumours |
| APC | 5 | Colonic carcinoma |
| WT1 | 11 | Wilms’ tumour, bladder cancer, |
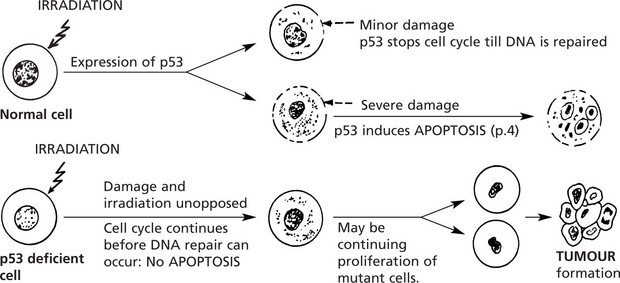
p53 gene: This gene codes for a nuclear phosphoprotein, which is expressed in increased amounts following cellular damage, e.g. irradiation.
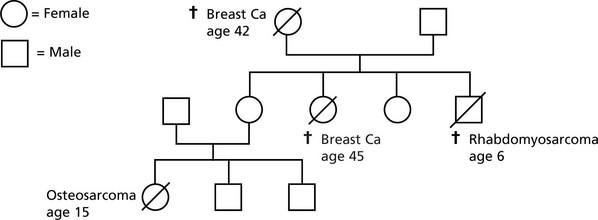
p53 has been called the ‘Guardian of the genome’. Abnormalities of p53 are found in at least 30% of all cancers.
Germ line p53 mutation is a feature of the Li-Fraumeni syndrome.