 CHAPTER 66 Congenital Infections
CHAPTER 66 Congenital Infections
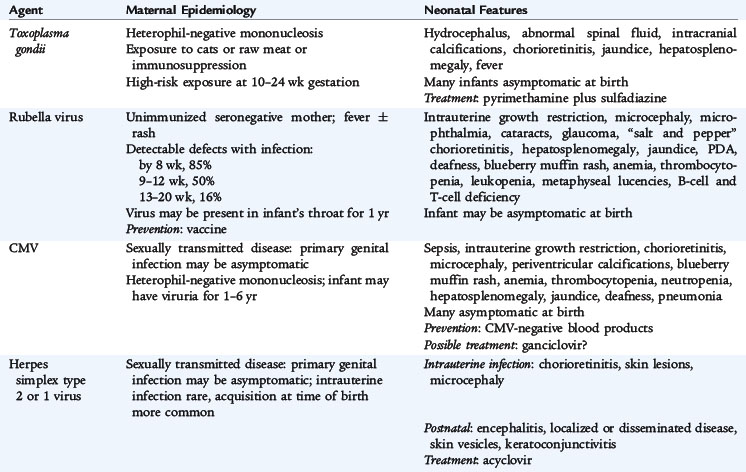
An infection acquired transplacentally during gestation is a congenital infection. Numerous pathogens that produce mild or subclinical disease in older infants and children can cause severe disease in neonates who acquire such infections prenatally or perinatally. Sepsis, meningitis, pneumonia, and other infections caused by numerous perinatally acquired pathogens are the cause of significant neonatal morbidity and mortality. Congenital infections include a well-known group of fungal, bacterial, and viral pathogens: toxoplasmosis, rubella, cytomegalovirus (CMV), herpes simplex virus (HSV), varicella-zoster virus, congenital syphilis, parvovirus, human immunodeficiency virus (HIV), hepatitis B, Neisseria gonorrhoeae, Chlamydia, and Mycobacterium tuberculosis.
Many of the clinical manifestations of congenital infections are similar, including intrauterine growth restriction, nonimmune hydrops, anemia, thrombocytopenia, jaundice, hepatosplenomegaly, chorioretinitis, and congenital malformations. Some unique manifestations and epidemiologic characteristics of these infections are listed in Table 66-1. Evaluation of patients thought to have a congenital infection should include attempts to isolate the organism by culture (for rubella, CMV, HSV, gonorrhea, and M. tuberculosis), to identify the antigen of the pathogen (for hepatitis B and Chlamydia trachomatis), to identify the pathogen's genome with polymerase chain reaction (PCR), and to identify specific fetal production of antibodies (IgM or increasing titer of IgG for Toxoplasma, syphilis, parvovirus, HIV, or Borrelia).
Treatment is not always available, specific, or effective. Nonetheless, some encouraging results have been reported for preventing the disease and for specifically treating the infant when the correct diagnosis is made (see Table 66-1).
TOXOPLASMOSIS
Vertical transmission of Toxoplasma gondii occurs by transplacental transfer of the organism from the mother to the fetus after an acute maternal infection. Fetal infection rarely can occur after reactivation of disease in an immunocompromised pregnant mother. Transmission from an acutely infected mother to her fetus occurs in about 30% to 40% of cases, but the rate varies directly with gestational age. Transmission rates and the timing of fetal infection correlate directly with placental blood flow; the risk of infection increases throughout gestation to 90% or greater near term, and the time interval between maternal and fetal infection decreases.
The severity of fetal disease varies inversely with the gestational age at which maternal infection occurs. Most infants have subclinical infection with no overt disease at birth; however, specific ophthalmologic and central nervous system (CNS) evaluations may reveal abnormalities. The classic findings of hydrocephalus, chorioretinitis, and intracerebral calcifications suggest the diagnosis of congenital toxoplasmosis. Affected infants tend to be small for gestational age, develop early-onset jaundice, have hepatosplenomegaly, and present with a generalized maculopapular rash. Seizures are common, and skull films may reveal diffuse cortical calcifications in contrast to the periventricular pattern observed with CMV. These infants are at increased risk for long-term neurologic and neurodevelopmental complications.
Serologic tests are the primary means of diagnosis. IgG-specific antibodies achieve a peak concentration 1 to 2 months after infection and remain positive indefinitely. For infants with seroconversion or a fourfold increase in IgG titers, specific IgM antibody determinations should be performed in patients to confirm disease. Especially for congenital infections, measurement of IgA and IgE antibodies can be useful to confirm the disease. Thorough ophthalmologic, auditory, and neurologic evaluations (head computed tomography and cerebrospinal fluid [CSF] examination) are indicated.
For symptomatic and asymptomatic congenital infections, initial therapy should include pyrimethamine (supplemented with folic acid) combined with sulfadiazine. Duration of therapy is often prolonged even up to 1 year. Optimal dosages of medications and duration of therapy should be determined in consultation with appropriate specialists.
RUBELLA
With the widespread use of vaccination, congenital rubella is rare in developed countries. Acquired in utero during early gestation, rubella can cause severe neonatal consequences. The occurrence of congenital defects approaches 85% if infection is acquired during the first 4 weeks of gestation; close to 40% spontaneously abort or are stillborn. If infection occurs during weeks 13 to 16, 35% of infants can have abnormalities. Infection after 4 months’ gestation does not seem to cause disease.
The most common characteristic abnormalities associated with congenital rubella include ophthalmologic (cataracts, retinopathy, and glaucoma), cardiac (patent ductus arteriosus and peripheral pulmonary artery stenosis), auditory (sensorineural hearing loss), and neurologic (behavioral disorders, meningoencephalitis, and mental retardation) conditions. Additionally, infants can present with growth retardation, hepatosplenomegaly, early-onset jaundice, thrombocytopenia, radiolucent bone disease, and purpuric skin lesions (“blueberry muffin” appearance from dermal erythropoiesis).
Detection of rubella-specific IgM antibody usually indicates recent infection. Additionally, measurement of rubella-specific IgG over several months can be confirmatory. Rubella virus can be isolated from blood, urine, CSF, and throat swab specimens. Infants with congenital rubella are chronically and persistently infected and tend to shed live virus in urine, stools, and respiratory secretions for 1 year. Infants should be isolated while in the hospital and kept away from susceptible pregnant women when sent home.
CYTOMEGALOVIRUS
CMV is the most common congenital infection and the leading cause of sensorineural hearing loss, mental retardation, retinal disease, and cerebral palsy. Congenital CMV occurs in about 0.5% to 1.5% of births. When primary infection occurs in mothers during a pregnancy, the virus is transmitted to the fetus in approximately 35% of cases. Rates of CMV infection are three to seven times greater in infants born to adolescent mothers. The risk of transmission of CMV to the fetus is independent of gestational age at the time of maternal infection. The earlier in gestation that the primary maternal infection occurs, the more symptomatic the infant will be at birth. The most common sources of CMV for primary infections occurring in mothers during pregnancy are sexual contacts and contact with young children. It is well known that CMV can be transmitted to the fetus even when maternal infection occurred long before conception. This transmission can occur as the result of virus reactivation, chronic infection, or reinfection with a new strain.
More than 90% of infants who have congenital CMV infection exhibit no clinical evidence of disease at birth. Approximately 10% of infected infants are small for gestational age and have symptoms at birth. Findings include microcephaly, thrombocytopenia, hepatosplenomegaly, hepatitis, intracranial calcifications, chorioretinitis, and hearing abnormalities. Some infants can present with a blueberry muffin appearance as the result of dermal erythropoiesis. Skull films may reveal periventricular calcifications. An additional 10% of infected infants may not present until later in infancy or early childhood, when they are found to have sensorineural hearing loss and developmental delays. Mortality is 10% to 15% in symptomatic newborns. Perinatal CMV infection acquired during birth or from mother’s milk is not associated with newborn illness or CNS sequelae.
Congenital CMV infection is diagnosed by detection of virus in the urine or saliva. Detection is often accomplished by traditional virus culture methods but can take several weeks to obtain a result. Rapid culture methods using centrifugation to enhance infectivity and monoclonal antibody to detect early antigens in infected tissue culture can give results in 24 hours. PCR also can be used to detect small amounts of CMV DNA in the urine. Detection of CMV within the first 3 weeks after birth is considered proof of congenital CMV infection.
There are no antiviral agents currently approved for the treatment of congenital CMV infection. Trial studies in severely symptomatic newborns of the antiviral agent, ganciclovir, have shown a lack of progression of hearing loss.
HERPES SIMPLEX VIRUS
HSV-2 accounts for 90% of primary genital herpes. About 70% to 85% of neonatal herpes simplex infections are caused by HSV-2. Most commonly, neonatal infections are acquired from the mother shortly before (ascending infection) or during passage through the birth canal at delivery. The incidence of neonatal HSV is estimated to range from 1 in 3000 to 20,000 live births. Infants with HSV infections are more likely to be born prematurely (40% of affected infants are <36 weeks’ gestation). The risk of infection at delivery in an infant born vaginally to a mother with primary genital herpes is about 33% to 50%. The risk to an infant born to a mother with a reactivated infection is less than 5%. More than 75% of infants who acquire HSV infection are born to mothers who have no previous history or clinical findings consistent with HSV infection.
Most infants are normal at birth, and symptoms of infection develop at 5 to 10 days of life. Symptoms of neonatal HSV infection include disseminated disease involving multiple organ systems, most notably the liver and lungs; localized infection to the CNS; or localized infection to the skin, eyes, and mouth. Symptoms may overlap, and in many cases of disseminated disease, skin lesions are a late finding. Disseminated infection should be considered in any infant with symptoms of sepsis, liver dysfunction, and negative bacteriologic cultures. HSV infection also should be suspected in any neonate who presents with fever, irritability, abnormal CSF findings, and seizures. Initial symptoms can occur anytime between birth and 4 weeks of age, although disseminated disease usually occurs during the first week of life. HSV infections are often severe, and a delay in treatment can result in significant morbidity and mortality.
For the diagnosis of neonatal HSV infection, specimens for culture should be obtained from any skin vesicle, nasopharynx, eyes, urine, blood, CSF, stool, or rectum. Positive cultures obtained from these sites more than 48 hours after birth indicate intrapartum exposure. PCR is a sensitive method for detecting HSV DNA in blood, urine, and CSF.
Parenteral acyclovir is the treatment of choice for neonatal HSV infections. Acyclovir should be administered to all infants suspected to have infection or diagnosed with HSV. The most benign outcome with regard to morbidity and mortality is observed in infants with disease limited to the skin, eyes, and mouth.
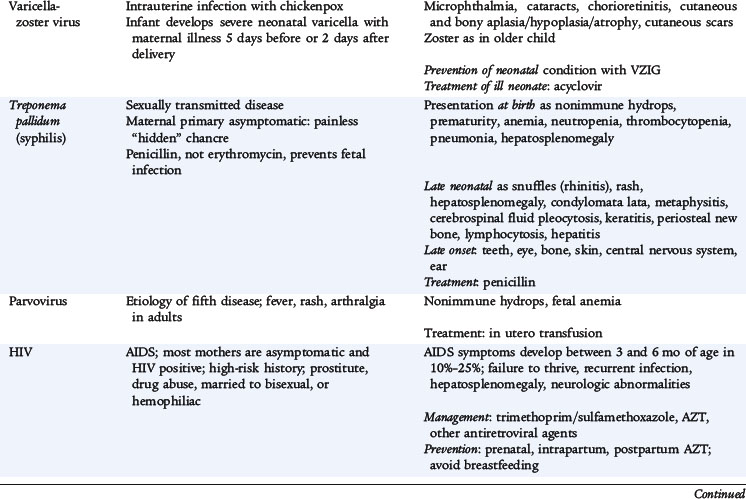
VARICELLA-ZOSTER VIRUS
The incidence of congenital infection among infants born to mothers who have varicella is about 2% when infection occurs within the first 20 weeks of gestation. Fetal infection after maternal varicella can result in varicella embryopathy, which is characterized by zigzag skin scarring and limb atrophy. CNS manifestations (hydrocephalus and microcephaly) and eye abnormalities (cataracts and chorioretinitis) also may occur.
Varicella infection can be fatal for the infant of a mother who develops varicella 5 days before to 2 days after delivery. Clinical features include severe rash, pneumonia, hepatitis, and death in 20% to 30% of cases. Infants born to mothers who are infected before 5 days prior to delivery have less severe disease secondary to the placental transfer of varicella-specific IgG antibody.
Varicella-zoster virus can be isolated from scrapings of a vesicle base during the first 3 to 4 days of the eruptions, but rarely from secretions from other sites, such as the respiratory tract. A significant increase in serum varicella IgG antibody can confirm the diagnosis. PCR is a sensitive method for detecting varicella DNA in any body fluid or tissue.
CONGENITAL SYPHILIS
Congenital syphilis most commonly results from transplacental infection of the fetus, although the fetus can acquire infection by contact with a chancre at birth. Additionally, hematogenous infection can occur throughout pregnancy. The longer the time elapsed between the mother’s infection and pregnancy, the less likely she is to transmit the disease to the fetus.
Intrauterine infection can result in stillbirth, hydrops fetalis, or prematurity. Clinical symptoms vary but include hepatosplenomegaly, snuffles, lymphadenopathy, mucocutaneous lesions, osteochondritis, rash, hemolytic anemia, and thrombocytopenia. Untreated infants, regardless of whether they manifest symptoms at birth, may develop late symptoms, which usually appear after 2 years of age and involve the CNS, bones, joints, teeth, eyes, and skin. Some manifestations of disease may not become apparent until many years after birth, such as interstitial keratitis, eighth cranial nerve deafness, Hutchinson teeth, bowing of the skins, frontal bossing, mulberry molars, saddle nose, rhagades, and Clutton joints. The combination of interstitial keratitis, eighth cranial nerve deafness, and Hutchinson teeth is commonly referred to as the Hutchinson triad.
Many infants are asymptomatic at the time of diagnosis. If untreated, most infants develop symptoms within the first 5 weeks of life. The most striking lesions affect the mucocutaneous tissues and bones. Early signs of infection may be poor feeding and snuffles (syphilitic rhinitis). Snuffles are more severe and persistent than the common cold and are often bloody. A maculopapular desquamative rash develops over the palms and soles and around the mouth and anus. The rash may progress to become vesicular with bullae. Severely ill infants may be born with hydrops and have profound anemia. Severe consolidated pneumonia may be present at birth, and there may be laboratory findings consistent with a glomerulonephritis. CSF evaluation may reveal a pleocytosis and elevated protein. More than 90% of symptomatic infants exhibit radiographic abnormalities of the long bones consistent with osteochondritis and perichondritis.
No newborn should be discharged from the hospital without knowledge or determination of the mother’s serologic status for syphilis. All infants born to seropositive mothers require a careful examination and a quantitative nontreponemal syphilis test. Darkfield examination of direct fluorescent antibody staining of organisms obtained by scraping a skin or mucous membrane lesion is the quickest and most direct method of diagnosis. More commonly, serologic testing is used. The nontreponemal reaginic antibody assays—the Venereal Disease Research Laboratory (VDRL) and the rapid plasma reagin—are helpful as indicators of disease. All infants born to seropositive mothers require a careful examination and a quantitative nontreponemal syphilis test. The test performed on the infant should be the same as that performed on the mother to enable comparison of results. An infant should be evaluated further if the maternal titer has increased fourfold, if the infant’s titer is fourfold greater than the mother’s titer, if the infant is symptomatic, or if the mother has inadequately treated syphilis. A mother infected later in pregnancy may deliver an infant who is incubating active disease. The mother and infant may have negative serologic testing at birth. When clinical or serologic tests suggest congenital syphilis, CSF should be examined microscopically, and a CSF VDRL test should be performed. An increased CSF white blood cell count and protein concentration suggests neurosyphilis; a positive CSF VDRL is diagnostic.
Parenteral penicillin is the preferred drug of choice for treatment of syphilis. Penicillin G for 10 to 14 days is the only documented effective therapy for infants who have congenital syphilis and neurosyphilis. Infants should have repeat nontreponemal antibody titers repeated at 3, 6, and 12 months to document falling titers. Infants with neurosyphilis must be followed carefully with serologic testing and CSF determinations every 6 months for at least 3 years or until CSF findings are normal.
HUMAN PARVOVIRUS B19
Infection with parvovirus B19 is recognized most often as erythema infectiosum, which is characterized by mild systemic symptoms, fever, and commonly a distinctive “slapped cheek” facial rash. Approximately 30% to 60% of adults are seropositive for human parvovirus B19. A significant proportion of childbearing women is potentially susceptible to infection.
Parvovirus B19 selectively infects erythropoietic precursors and inhibits their growth by inducing cell cycle arrest and apoptosis. Parvovirus infections often are associated with mild neutropenia and thrombocytopenia, but instances of transient pancytopenia also have been reported. Although the actual risk is probably low, the virus can infect the fetus, leading to fetal anemia, nonimmune hydrops, and fetal demise. Pathologic studies of parvovirus B19 human fetuses also have suggested that myocardial inflammation and subendocardial fibroelastosis may contribute to fetal hydrops.
Detection of serum parvovirus B19–specific IgM antibody is the preferred diagnostic test. A positive IgM test result indicates that infection probably occurred within the past 2 to 4 months. Antenatal treatment of parvovirus B19–infected fetuses with hydrops has included serial maternal ultrasound, fetal intrauterine blood transfusion, and maternal digitalization. Spontaneous resolution of fetal hydrops with normal outcome has been reported. Treatment for the infant after birth is mainly supportive and centered on the management of hydrops. Infants with aplastic crisis may require transfusions of blood products.
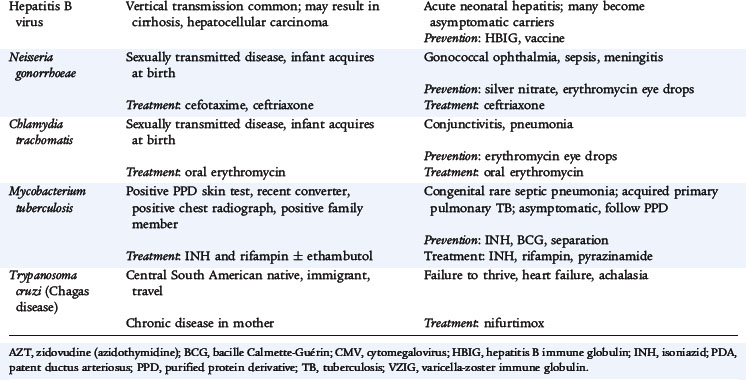
NEISSERIA GONORRHOEAE
N. gonorrhoeae infection in a newborn usually involves the eyes (ophthalmia neonatorum). Other sites of infection include scalp abscesses (often associated with fetal monitoring with scalp electrodes), vaginitis, and disseminated disease with bacteremia, arthritis, or meningitis. Transmission to the infant usually occurs during passage through the birth canal when mucous membranes come in contact with infected secretions.
Infection usually is present within the first 5 days of life and is characterized initially by a clear, watery discharge, which rapidly becomes purulent. There is marked conjunctival hyperemia and chemosis. Infection tends to be bilateral; however, one eye may be clinically worse than the other. Untreated infections can spread to the cornea (keratitis) and anterior chamber of the eye. This extension can result in corneal perforation and blindness.
Recommended treatment for isolated infection, such as ophthalmia neonatorum, is one intramuscular dose of ceftriaxone. Infants with gonococcal ophthalmia should receive eye irrigations with saline solution at frequent intervals before discharge. Topical antibiotic therapy alone is inadequate and is unnecessary when recommended systemic antimicrobial therapy is given. Infants with gonococcal ophthalmia should be hospitalized and evaluated for disseminated disease (sepsis, arthritis, meningitis). Disseminated disease should be treated with antimicrobial therapy (ceftriaxone or cefotaxime) for 7 days. Cefotaxime can be used in infants with hyperbilirubinemia. If documented, infants with meningitis should be treated for 10 to 14 days.
Tests for concomitant infection with C. trachomatis, congenital syphilis, and HIV should be performed. Results of the maternal test for hepatitis B surface antigen should be confirmed. Topical prophylaxis with silver nitrate, erythromycin, or tetracycline is recommended for all newborns for the prevention of gonococcal ophthalmia.
CHLAMYDIA
C. trachomatis is the most common reportable sexually transmitted infection, with a high rate of infection among sexually active adolescents and young adults. Prevalence of the organism in pregnant women ranges from 6% to 12% and can be 40% in adolescents. Chlamydia can be transmitted from the genital tract of infected mothers to their newborns. Acquisition occurs in about 50% of infants born vaginally to infected mothers. Transmission also has been reported in some infants delivered by cesarean section with intact membranes. In infected infants, the risk of conjunctivitis is 25% to 50%, and the risk of pneumonia is 5% to 20%. The nasopharynx is the most commonly infected anatomic site.
Neonatal chlamydial conjunctivitis is characterized by ocular congestion, edema, and discharge developing 5 to 14 days to several weeks after birth and lasting for 1 to 2 weeks. Clinical manifestations vary from mild conjunctivitis to intense inflammation and swelling. Both eyes are almost always involved; however, one eye may appear to be more swollen and infected than the other. The cornea is rarely involved and preauricular adenopathy is rare.
Pneumonia in a young infant can occur between 2 and 19 weeks of age and is characterized by an afebrile illness with a repetitive staccato cough, tachypnea, and rales. Wheezing is uncommon. Hyperinflation with diffuse infiltrates can be seen on chest radiograph. Nasal stuffiness and otitis media can occur.
Diagnosis can be made by scraping the conjunctiva and culturing the material. Giemsa staining of the conjunctival scrapings revealing the presence of blue-stained intracytoplasmic inclusions within the epithelial cells is diagnostic. PCR is also available. Infants with conjunctivitis and pneumonia are treated with oral erythromycin for 14 days. Topical treatment of conjunctivitis is ineffective and unnecessary. The recommended topical prophylaxis with silver nitrate, erythromycin, or tetracycline for all newborns for the prevention of gonococcal ophthalmia does not prevent neonatal chlamydial conjunctivitis.
De Almeida M.F.B., Draque C.M. Neonatal jaundice and breastfeeding. NeoReviews. 2007;8:e282-e288.
Frankovich J., Sandborg C., Barnes P., et al. Neonatal lupus and related autoimmune disorders of infants. NeoReviews. 2008;9:e207-e217.
HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002.
Hoffman J.D., Estrella E.A. Newborn presentation of connective tissue disorders. NeoReviews. 2007;8:e110-e119.
Kates E.H., Kates J.S. Anemia and polycythemia in the newborn. Pediatr Rev. 2007;28:33-34.
Phillip A.G.S. Neonatal meningitis in the new millennium. NeoReviews. 2003;4:c73-c80.
Steinhorn R.H., Farrow K.N. Pulmonary hypertension in the neonate. NeoReviews. 2007;8:e14-e21.
Tumbaga P.F., Philip A.G.S. Perinatal group B streptococcal infections and the new guidelines: an update. NeoReviews. 2006;7:e524-e530.
Wong R.J., Stevenson D.K., Ahlfors C.E., et al. Neonatal jaundice: bilirubin physiology and clinical chemistry. NeoReviews. 2007;8:e58-e67.
 SEE
SEE