Male Reproductive System
The reproductive system is arguably the most important in terms of survival of a species. In production animals, reproduction is essential for the continued supply of product, be it meat, fiber, milk, or many other products. Most production animals rely on a small number of males as breeding stock, so in addition to being 50% of the reproductive team, an infertile male or one carrying an undesirable trait has a major impact.
The male reproductive system usually receives little attention and time; with some exceptions, replacement of a breeding male is often more effective than diagnosis and treatment. Infertility in the male is difficult to reverse unless the cause can be readily found and corrected. This is where knowledge of the disease processes, responses to injury, and prognosis are so important.
Companion animals are not given the same amount of study as production animals, especially the bull and ram. The aim here is to emphasize the disorders of all species and highlight the mechanisms and reactions of the male reproductive tract by examining the disorders of each anatomic location.
Structure
The male reproductive tract can be divided into three major areas based not only on anatomic location but also on functional characteristics and important disease processes. These are the scrotum and its contents, the accessory genital glands, and the penis and prepuce.
The Scrotum and Contents
The purpose of the scrotum and its contents is to supply spermatozoa for transportation to the female. Although the general focus is on the testis and its germinal cells contained in the seminiferous tubules, other parts have important functional characteristics that allow this to happen. Testicular development is quiescent until puberty when spermatogenesis begins. The testes therefore are small until puberty, when they increase to their adult size. The testes are covered by a capsule (once called the tunica albuginea), which is relatively nonexpansile and usually maintains the testicular contents under slight pressure. Within the testis are the interstitial and intratubular regions. Within the interstitium are the interstitial endocrine (Leydig) cells, blood vessels, lymphatics, and macrophages, dendritic cells, mast cells, and T lymphocytes. Each seminiferous tubule has a lining of myoid cells and a limiting membrane. There is a basement membrane between these structures. The arrangement of the seminiferous tubules varies from species to species, but the end result is the formation of spermatozoa. After spermatogenesis, spermatozoa are transported through rete tubules into the efferent ductules and then into the epididymis, a single and extremely long duct. In doing so, the spermatozoa mature and are concentrated. The pathway out of the scrotal area is through the deferent duct.
The purpose of the scrotum, vaginal tunics, and spermatic cord (deferent duct, pampiniform plexus, and cremaster muscle) is to protect and maintain spermatogenesis at a temperature slightly lower than body temperature. The testes are raised or lowered, according to ambient temperature, by the cremaster muscle. There is a counter current vascular system that allows the testes to be at a lower temperature than body temperature; the pampiniform plexus assists this process. The pulsatile nature of arterial blood flow is altered to form a continuous and lower pressure system. In addition, the scrotal skin, which is thin, often hairless, and in most species has abundant apocrine sweat glands, helps to maintain a lower testicular and epididymal temperature. The vaginal tunic, which is an outpouching of the peritoneum, allows for free movement of the testes within the scrotal sac. The scrotum is composed of skin, dartos muscle, and scrotal fascia. It is fused with the parietal layer of the vaginal tunic.
Cell Types
The functions of the male reproductive system are the production of testosterone and the production and transportation of spermatozoa. Spermatozoa are formed from germ (stem) cells by a process of spermatogenesis. Spermatogenesis occurs in three stages: the proliferative, meiotic, and spermatogenic stages. The proliferative phase involves the mitotically active spermatogonia. They are present at the periphery of the seminiferous tubules on the basement membrane. The second phase is the meiotic phase, in which spermatocytes are formed. In the spermatogenic phase, spermatids and finally spermatozoa are formed. Spermatozoa have a head, body, and tail. The head contains the nucleus and an acrosome, which contains enzymes required for the penetration of the zona pellucida. The body or midpiece contains mitochondria, and the tail is a flagellum.
Sertoli cells provide support, nutrients, hormones, and cytokines to permit spermatogenesis. During spermatogenesis, the cells pass into a region that is separated from and external to the immune system of the body. The barrier is called the blood-testis barrier, and it is maintained by the Sertoli cells (Fig. 19-1). Control of spermatogenesis is achieved through a combination of both central (luteinizing hormone [LH] on the interstitial endocrine cells and follicle-stimulating hormone [FSH] on Sertoli cells) and local (paracrine and autocrine molecules, including testosterone) factors, and there is considerable crosstalk between germ cells, Sertoli cells, and interstitial endocrine cells. Local modification of spermatogenesis is achieved through increased or decreased apoptosis at any stage of development. Many of the disorders and conditions that affect spermatogenesis increase or decrease the apoptotic rate.
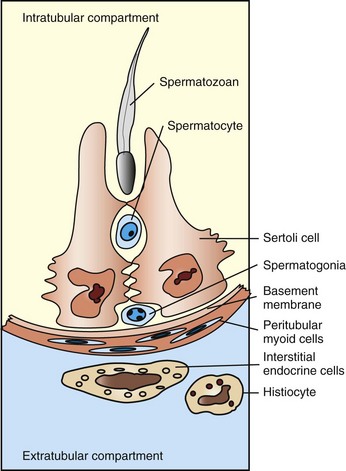
Fig. 19-1 Schematic diagram of the normal components of the testis.
The Sertoli cells, germ cells, and interstitial endocrine cells are closely integrated, and considerable messaging occurs between them. The blood-testis barrier is at the level of the Sertoli cells, with contributions from the myoid cells and basement membrane. Spermatogonia are on the interstitial side of the blood-testis barrier.
The cells lining the various ducts, including the rete tubules, efferent ductules, and epididymides, are epithelial cells that have a variety of functions, in addition to being a barrier. Absorption, phagocytosis, and secretion are all part of their physiologic role. Movement of spermatozoa along these ducts and tubules is achieved through smooth muscle contraction and the cilia.
In the accessory genital glands, storage of spermatozoa (particularly in the ampulla, where present) and secretion of various substances are the major roles for the epithelium of these glands. From the pelvic urethra and through the penile urethra, the lining cells are urothelial in type.
The Accessory Genital Glands
The purpose of the accessory glands is to provide nutrition and a transport medium for spermatozoa. There are four main accessory glands: the ampullae, vesicular glands, prostate, and bulbourethral glands. There is considerable species variation in the size, type, and number of these accessory glands. Horses and ruminants have all four glands, although the prostate of ruminants is either very small (bull) or is disbursed within the pelvic urethra (ram and buck). Pigs lack ampullae, dogs have only a prostate, and cats have a small prostate and bulbourethral glands. The secretion of the vesicular gland and prostate is serous, but the bulbourethral gland typically has a viscous mucoid product.
The Penis and Prepuce
There is considerable structural difference between the penis and prepuce of the various species. In prepubertal animals, the prepuce is attached to the penis, but they become separated at sexual maturity. The penis of the horse is erectile and is located within a prepuce that produces a thick, waxy material called smegma. Ruminants and pigs have a long fibrous penis that has some erectile tissue. To accommodate a penis of sufficient length, there is a series of bends called the sigmoid flexure, and there is a retractor muscle to hold the penis in the prepuce. There is an extension of the urethra in small ruminants that is called the vermiform appendage or the urethral process. During ejaculation, this appendage spins and sprays semen onto the cervix. The glans of the penis of the boar has a corkscrew shape that allows it to insert into the cervix. The penis of dogs and to a lesser extent cats is erectile and has an os penis. The cat has projections of penile epithelium called spines or barbs on its penis, and they are testosterone dependent.
Function
The overall function of the male reproductive system is to provide genetic material to the female for combination with female genetic material to produce an offspring with a combined and hopefully better genetic makeup. This is achieved by several separate areas—the scrotum and contents, accessory genital glands, and the penis and prepuce.
The Scrotum and Contents
The testes are the location of preparation of genetic material for transfer to the female. One-half of the chromosomes are produced by the process of meiosis in the seminiferous tubules in the process of spermatogenesis (see the section on cells of the male reproductive system). The testes also produce the hormone testosterone to ensure development of male phenotypic and behavioral characteristics. It also provides the signals that stimulate females to come into heat and become receptive. The testis also produces other substances for the regulation of spermatogenesis. Spermatozoa leave the testis in a high volume of fluid—the rete testis fluid—and enter the epididymis. The epididymis has the function of supporting and directing spermatozoal maturation and concentrating the spermatozoa for ejaculation. The function of the testis and epididymis depends on unique requirements, including maintenance of a temperature below body temperature, and a continuous nonpulsatile blood flow. The scrotum provides support and with the cremaster muscle in concert with the dartos muscle of the scrotal wall, thermoregulation by raising the testis and epididymis closer to the body wall. The pampiniform plexus is a countercurrent system that assists in maintaining a lower temperature, and the long and tortuous testicular artery removes the pulses of the arterial supply. The deferent duct is the conduit for taking the concentrated spermatozoa to the accessory genital glands.
The Accessory Genital Glands
The function of the accessory glands is to produce ejaculatory fluid to support, nourish, and protect spermatozoa as they are transferred to the female. The energy source of spermatozoa includes fructose produced by the accessory glands, usually the vesicular glands. Accessory genital glands also provide the fluid for territorial scent marking. The domestic species have a differing size and arrangement of accessory glands to do this. The amount and type of fluid varies according to the unique arrangements and mating behavior of the species. Coitus in ruminants is a rapid event, and they produce a small and highly concentrated ejaculate that is deposited around the external os of the cervix. On the other end of the scale are pigs, where coitus takes about 20 minutes, insemination is intrauterine, and the ejaculate is high volume (up to 500 mL). Pigs have large vesicular glands that produce a watery fluid and large bulbourethral glands that produce a viscous mucoid product. The prostate of the dog is the only gland, and it provides all the seminal fluid. All species except the dog and pig have ampullae of the deferent ducts; these provide secretion and a storage site for spermatozoa.
The Penis and Prepuce
The penis is designed to enter the vagina of the female and to deposit the ejaculate in a location unique to each species. Pigs and horses are intrauterine inseminators, whereas the others deposit semen around the cervix and proximal vagina. This ensures protection of spermatozoa, including the prevention of desiccation. There are also adaptations and functions that ensure the spermatozoa of the dominant male are the ones that fertilize the ova. This may explain why pigs and dogs have prolonged coitus. The penis of the cat has barbs that stimulate the vagina of the queen and induce ovulation. Small ruminants have a urethral process that sprays spermatozoa around the cervix, and the pig has a preputial diverticulum for scent production.
Responses to Injury
Injury to the male reproductive system comes in many different forms, and there are many potential targets, including the pituitary and endocrine control, interstitial endocrine cells, Sertoli cells, spermatogenic germ cells, and the various ducts. Apart from the obvious redundancy of having bilateral systems, the male reproductive tract has very little functional reserve and cannot undergo compensatory change to any significant degree. Some testicular compensatory hypertrophy is possible and is discussed later in this chapter.
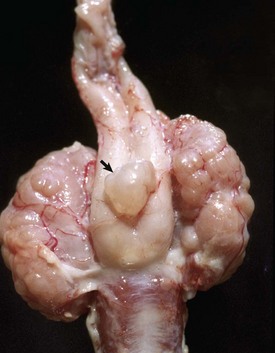
The formation of spermatic granulomas (Fig. 19-2) is one of the most dramatic and important responses of the male reproductive system to injury. Spermatic granulomas occur with rupture of a duct; spermiostasis and spermatocele are the usual preliminary stages. Spermatozoa are “foreign” to the body. Immunity to spermatozoa with the formation of antisperm antibody is a well-recognized phenomenon. The cell wall constituents and the high chromatin content make them resistant to degradation. Any injury that exposes spermatozoa to the tissue of the body results in severe inflammation, mostly of a granulomatous type. This can be a foreign body type response, an immunologic response, or both. The inflammation produced is usually chronic and results in severe scarring, which further obstructs tubules and ducts, leading to even more inflammation so that it becomes self-perpetuating. It is therefore critical to prevent such injury.
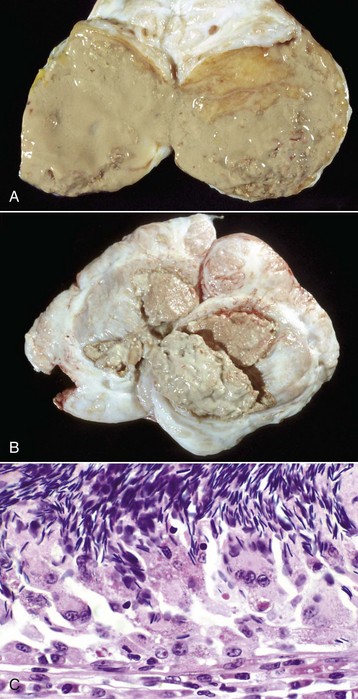
Fig. 19-2 Spermatic granulomas, tail of the epididymis, ram.
A, Most of the tail of the epididymis (bisected and reflected) has been replaced by a yellow-tan, semiliquid spermatic granuloma. These granulomas are any color from white to red. They are frequently and incorrectly called abscesses. B, Multiple encapsulated (chronic) spermatic granulomas with white caseous centers. C, A mass of spermatozoa free in the interstitium (upper half of image) is surrounded by epithelioid macrophages and multinucleated giant cells, some of which have phagocytosed spermatozoa. Lymphocytes, plasma cells, and fibrous connective tissue surround these granulomas (lower half of image). H&E stain. (A and B courtesy Drs. P. W. Ladds and R. A. Foster, James Cook University of North Queensland. C courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Injury to the testis can be the result of a primary attack on a target cell or may be secondary to interruption of hormonal regulation, whether systemic or local. The endpoint of an injury can therefore be far reaching. Regardless of the primary target, most injurious events result in germ cell degeneration and depletion. Germ cells are sensitive, but Sertoli cells are relatively resistant to injury. As a result, severe injury often results in seminiferous tubules containing only spermatogonia and Sertoli cells. As Sertoli cells degenerate, they become vacuolated and swollen. Exfoliation of normal germ cells can also occur. The spermatogenic epithelium responds to injury by increasing or decreasing apoptosis, resulting in spermatogenic arrest or a complete failure of spermatogenesis. As long as spermatogonia remain, spermatogenesis can restart. Failure of release of spermatozoa is an early indication of degeneration. The formation of multinucleated spermatid giant cells and phagocytosis of spermatozoa by Sertoli cells occur.
A major effect of injury to interstitial endocrine cells is the failure of the release of testosterone. This can effectively stop spermatogenesis by increasing apoptosis and by inhibiting maturation of spermatids. Direct damage to spermatozoa can affect motility and fertilizing capability. Recovery from injury, while taking time, is achieved by restarting spermatogenesis from the relatively resistant spermatogonia.
The testis is a specialized immunologic environment and has a reduced immune responsiveness, no doubt because of the importance of not developing an immunologic response to germ cells. Unfortunately, if an inflammatory response does occur within the testis, it is likely to be sustained.
Injury to the epididymis can have permanent and devastating effects. Injury affects not only the functions of the epididymides—including maturation and storage of spermatozoa, resorption of fluid, and secretion of materials—but severe injury also results in inflammation. Any constriction of the epididymal duct results in spermiostasis, potential rupture, and the formation of spermatic granulomas. Because the epididymis is very limited in its responses, alteration to the structure and function of the epididymis is often permanent and affects fertility in a dramatic fashion.
Injury to the accessory genital glands is not common, and they can recover, often with a reduced function. Functional reserve is often sufficient to maintain fertility.
The peritesticular tissues, in particular the vaginal tunics, are also prone to injury from peritoneal reactions secondary to epididymal disease or from a direct penetrating injury. The reaction of these tissues is identical to that of the peritoneum, and as such, fibrosis and adhesion are a common response. Adhesion can limit the movement of the testes and alter its ability to thermoregulate.
Portals of Entry
There are four main portals of entry of infectious and injurious agents to the male reproductive tract (Box 19-1). They include direct penetration and injury, ascending infection, hematogenous localization, and peritoneal spread.
The external location of the penis, prepuce, and scrotum makes them prone to direct penetrating injury and to blunt force trauma. Surprisingly, this is a relatively rare occurrence.
Ascending infection is relatively common. It occurs sporadically in adults but curiously is particularly a problem in pubertal animals when hormonal changes make the system more prone to infection from bacteria. This pubertal change was shown for Actinobacillus seminis and Histophilus somni, which are resident flora of the prepuce of sheep. The accessory genital glands in particular are targets, but there can be further ascension to the epididymis. It is exceedingly rare, because of the long length of the epididymis, for the testis to be involved in ascending infection. The male reproductive tract is also a target of pathogens that are spread by sexual activity. Spread from females or in some circumstances from other males is well recognized.
Hematogenous localization is a recognized portal of entry by specific pathogens, such as the various Brucella. The epididymis and the accessory genital glands are the main targets.
The vaginal tunic is an outpouching of the peritoneal cavity and any process affecting the peritoneum, whether infectious or neoplastic, affects the peritesticular tissues.
Defense Mechanisms
The male reproductive tract, and in particular the internal genitalia, is so exquisitely sensitive to injury that it is extremely important to prevent rather than respond to injury. The combined effect of the high antigenicity of spermatozoa and the exceedingly long and very narrow duct system means that there is little tolerance for inflammation, necrosis, or other injurious situations. Much of the reproductive system relies on isolation for protection and the prevention of infection or injury.
Innate Immunity
There are multiple factors that prevent infection and injury to the vulnerable tissues. The testes and epididymides are hidden from the external environment by their intrascrotal location and by the extremely long and narrow tube that connects them to the outside world. The thin luminal diameter and extreme length of the deferent duct makes ascending infection unlikely. There is almost continuous flow of fluid along the deferent duct and the epididymis, thus the flushing action is protective. Even the accessory genital glands are protected by the flushing action of urine and by the long length of the urethra in species, such as ruminants, and the pig.
Testicular and epididymal fluid has antibacterial properties. The high chlorine content may be partially responsible. Antimicrobial proteins in seminal plasma are numerous and include bovine seminal plasmin, lactoferrin, β-defensins, and the antibacterial CXC chemokine granulocyte chemotactic protein-2/CXCL6. Some of these are acquired in the epididymis, where they are bound to spermatozoa. There are also numerous cytokines normally produced in the tract. Epithelial cells of the reproductive tract have physical barrier proteins, including mucin, that prevent infection, and Toll-like receptors to stimulate inflammation.
Nonspecific cellular defenses are relatively few. Neutrophils are not normally present in seminal plasma but can infiltrate rapidly if required. Macrophages are present within tissue and particularly within the testes, where they are recruited and maintained by the interstitial endocrine cells. Their presence inhibits the immune response; the cell-mediated responses, such as natural killer cells, lymphocyte-activated killer cells, and cytotoxic T lymphocytes, are inhibited as part of immune privilege and tolerance.
Adaptive Immunity
Much of the reproductive tract can produce an adaptive immune response if called on to do so. Where it is important is in the more distal portions of the tract, particularly the accessory genital glands and the prepuce. Unfortunately, development of an adaptive response in the testis and the epididymis is often “too little, too late.” Also, development of adaptive responses in the testis is inhibited by local immunosuppressive factors. This does not, however, prevent adaptive immunity from developing in the testis. The epididymis and deferent duct do not have a developed mucosal immune system, but there is evidence that such a system exists in the accessory genital glands, particularly the ampulla and bulbourethral glands. When infection occurs, local immunity and transfer of serum immunoglobulin is present. Preputial immunity is also mediated by local humoral and cellular mechanisms. Immunoglobulin G (IgG)- and IgA-based systems are present. Much less is known of cell-mediated immune mechanisms in the male reproductive tract.
Using local immunity to protect against infectious disease, particularly those important sexually transmitted diseases, has had little success. Response to vaccination has been variable with some individuals having protective immunity, particularly with Campylobacter fetus infection in bulls. Systemic immunity to reproductive disease has also been of variable effectiveness. Although protecting against systemic infection, challenge to the reproductive tract has in some instances increased the response to the infection and caused more damage than would normally occur.
Inflammation
In general terms, inflammation of the male reproductive tract is similar to that of other systems. What is unique about the male genital tract is inflammation to spermatozoa. Spermatozoa and germ cells outside the blood-testis barrier are antigenic. There are also antigenic components within seminal fluid. Spermatozoa have antigens that attract immune cells and also nonspecifically bind immunoglobulin. These reactions can have a minimal effect on the tissues directly and act by agglutinating spermatozoa or by opsonization. In some instances, the effect is much more dramatic and immunization against spermatozoa can result in a severe inflammatory response. This inflammation can be local or spermatozoa can be “attacked” in those areas in which the tissue-spermatozoal barrier is the weakest. In many species, this is the region of the efferent ductules and the epididymis. This so-called autoimmune reaction to spermatozoa can be experimentally created, but a clinical correlate is infrequent. Local effects, however, are much more recognized. Direct damage to testicular parenchyma can result in granulomatous inflammation centered on the seminiferous tubules, the so-called intratubular orchitis. Where spermatozoa are exposed to the tissues of the body, the reaction is that of granulomatous inflammation. Macrophages and multinucleate giant cells are found adjacent to spermatozoa (see Fig. 19-2, C). Initially at least, CD4+ T lymphocytes are abundant. Immunoglobulin-producing cells, particularly IgG-containing cells, are found. Granulomas are formed with the characteristic appearance of layers of epithelioid macrophages with multinucleate giant cells and then lymphocytes and plasma cells with a surrounding of fibrous tissue. In advanced cases, inspissated spermatozoa are found within a fibrous capsule. The production of a fibrous capsule and resultant contraction leads to further obstruction to the various adjacent ducts and tubules, with the result that additional spermiostasis, spermatocele, and spermatic granuloma formation continues. This inflammation therefore has devastating effects on fertility. Further complications occur if spermatozoa are released into the cavity of the vaginal tunics because a severe periorchitis develops and results in fibrosis and the lack of ability to adequately thermoregulate the testes.
Disorders of Domestic Animals (Horses, Ruminants [Cattle, Sheep, and Goats], Pigs, Dogs, and Cats)
There are many different ways to approach disorders of the male reproductive system (see Appendix 19-1). Each approach has its own advantages and disadvantages. Some of the disorders affect more than one region, yet there are many disorders that have a primary manifestation in one anatomic location. Approaching disorders from a pathogenetic viewpoint is logical; however, it does not always account for clinical relevance or assist in the correlation between clinical signs and prognosis. Disorders are approached based on their anatomic location: scrotal contents, accessory genital glands, and the penis and prepuce—this approach is more clinically relevant. Before doing so, it is prudent to examine disorders of sexual development (DSD) because they fall in a distinct category.
Disorders of Sexual Development
There are a large number of anomalies of the male reproductive system (Box 19-2). Some have clinical relevance and others do not. Differentiating between these is very important. Some of the anomalies represent the most common disease of a particular species. Where this is the case, the disease is discussed under disorders of a particular anatomic location because this is the most clinically relevant place. We have attempted to divide the various anomalies into those that are major and minor based on effects on fertility and on the future breeding potential of the animal. Some anomalies have a genetic basis and such individuals should not be used as a stud animal, even though the animal may still be fertile.
Male Embryology
Normal males have an XY chromosomal sex. The differentiation of the bipotential fetal gonad to a testis depends on the presence of a gene—the sex-determining region of the Y chromosome (SRY) that codes for the testis-determining factor and on other genes that cause the germ cells to go into mitotic arrest. Supporting cells become the Sertoli cells, the steroid-producing cells become the interstitial endocrine cells (Leydig cells), and the mesenchyme develops the appearance of a testis. Further development requires the activation of many other genes that are not on the Y chromosome. The expression of SRY occurs briefly in the somatic cells of the indifferent gonads (or genital ridges). Because expression ceases shortly before Sertoli cells can be recognized, it is proposed that the functional gene product of SRY influences other genes, such as SOX9, that ensure the differentiation and maintenance of Sertoli cells. SOX9 is upregulated in XY individuals just before gonadal differentiation. Sertoli cells signal the other supporting cell precursor lines to differentiate along the male pathway. Early in sex differentiation, the embryo has a double set of ducts. The paramesonephric (Müllerian) ducts are female precursors, arising by invagination of the celomic cavity. The mesonephric (Wolffian) tubules and ducts are male precursors, arising from the primitive kidney, the mesonephros. The rete testis and efferent ductules are derived from mesonephric tubules. There are about 20 efferent ductules, although the number varies with species. The epididymis is derived from the part of the mesonephric duct within the mesonephros. The deferent duct, ampulla, and vesicular gland are derived from the distal part of the mesonephric duct, outside the mesonephros (Fig. 19-3).
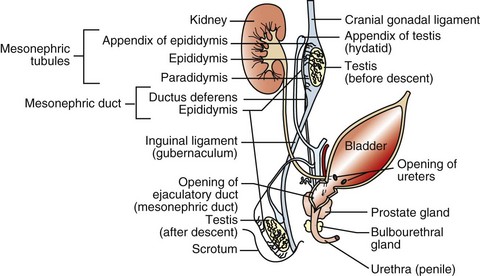
Fig. 19-3 Schematic diagram of the normal components of the male reproductive system and the embryonic structures, especially the mesonephric (Wolffian) duct and urogenital sinus and tubercle, from which they were derived.
The rete tubules and efferent ductules are formed from the mesonephric ductules; the epididymis, deferent duct, ampullae, and vesicular glands form from the mesonephric duct; the prostate and bulbourethral glands form from the urogenital sinus; and the penis, prepuce, and scrotum form from the genital tubercle and swellings.
After germ cells migrate from the yolk sac and the testes develop in the tissue of the genital ridge, the testes produce two hormones. Sertoli cells secrete the polypeptide hormone anti-Müllerian hormone (AMH, previously called Müllerian inhibitory substance) during embryonic development and at lower concentrations postnatally. AMH causes regression of the ipsilateral paramesonephric duct, indirectly through action on mesenchymal tissue. Sertoli cells stimulate differentiation of interstitial endocrine cells from the cells of the interstitium. Interstitial endocrine cells secrete the steroid hormone testosterone, which causes persistence and differentiation of the mesonephric ducts. Testosterone is likely transported down the mesonephric ducts rather than moving by simple diffusion. A third hormone, dihydrotestosterone, which is a metabolite of testosterone, is required for formation of the prostate gland, the closing of the urethral folds, and the formation of the penis and scrotum. The enzyme steroid 5α-reductase is produced by cells in the urogenital sinus, genital tubercle, and genital swellings and reduces testosterone to dihydrotestosterone. Functional androgen receptors on target tissues are necessary for androgen-dependent differentiation and growth. Species differences exist in whether the production of testosterone by interstitial endocrine cells is under the control of gonadotropin produced by the fetal pituitary gland or the placenta. Fetal interstitial endocrine cells are replaced by postnatal interstitial endocrine cells, which are relatively quiescent until puberty. The production of testosterone is regulated by luteinizing hormone (LH), which is under the control of gonadotropin-releasing hormone (GnRH) from the hypothalamus. Also, follicle-stimulating hormone (FSH) is controlled by GnRH; FSH is produced by the anterior pituitary gland. FSH regulates the activity of Sertoli cells and can thus influence AMH production. Sertoli cells stimulated by FSH produce a glycoprotein, androgen-binding protein, which fosters high testosterone concentration around the germ cells for the progression of spermatogenesis.
The external genitalia form when the genital tubercle is masculinized by the presence of androgens. Elongation of the tubercle forms the phallus; opposing urethral folds form the penile urethra; and the genital swellings fuse to form the scrotum.
Testicular Descent
The testes and epididymides undergo descent from their original location to the scrotum. There are three main phases in testicular descent: the abdominal translocation, the transinguinal migration and the inguinoscrotal migration. The developing gonads are held in place by cranial and caudal suspensory ligaments. The caudal ligament, the primitive gubernaculum testis, attaches the developing testis to the site of the inguinal canal. It develops into an intraabdominal and extraabdominal part that protrudes into the scrotum. Evagination of the peritoneum forms the inguinal canal and the vaginal tunic.
With the abdominal translocation phase particularly, stimulation of gubernaculum mesenchymal cells is controlled in rodents by the insulin-like peptide (INSL) hormone, INSL3. The receptor for INSL appears to be GREAT/LGR8. Other molecules are likely involved. The subsequent enlargement of the gubernaculum and progressive weakening of the cranial suspensory ligament anchors the testis so that with fetal growth, the testis retains its position near the inguinal canal.
Little is known of the transinguinal phase, apart from the lack of involvement of testosterone or INSL3. Dilation of the inguinal canal by the gubernaculum and then intraabdominal pressure is involved with movement of the testis through the inguinal canal to bring the testis and epididymis into a subcutaneous location. The third and inguinoscrotal phase of testicular descent is mediated by the hypothalamopituitary-induced production of gonadal androgens. Having said this, domestic animals with failure of testicular descent into the scrotum seldom have a deficiency of testosterone or a lack of the androgen receptor. The genitofemoral nerve and calcitonin gene–related proteins or binding sites are involved in rodents.
Major Anomalies
The most common and important major anomaly of the male reproductive system is failure of testicular descent or cryptorchidism. Testicular hypoplasia and segmental aplasia of the epididymis are a close second. These disorders and conditions are discussed further in the section on Disorders of the Scrotum and Contents. Much less common, but nevertheless important, are other DSD.
Disorders of Sexual Development: Errors of chromosomal, gonadal, or phenotypic sex are usually manifested by abnormalities in sexual dimorphism. Many of the abnormalities result in a female phenotype and are mentioned in more detail in Chapter 18. The concentration here is on those disorders that involve animals that have male gonads or are predominantly phenotypic males. One cannot always tell the underlying cause of sexual ambiguity by examining phenotype or even identifying the gonadal type. Complete definition of a DSD requires knowledge of all three components: chromosomal, gonadal, and phenotypic types.
Chromosomal Disorders of Sexual Development: Sex chromosomes can be abnormal in structure or number. Two examples of abnormal structure of the Y chromosome are deletion of the short arm and isochromosome formation (duplication of one arm and loss of the other). Affected individuals have a female phenotype and extremely hypoplastic gonads. A few cattle have been identified with an isochromosome Y. With duplication of one of the sex chromosomes in an individual with a Y chromosome (XYY or XXY), or a mosaic (as occurs with the calico or tricolor male cats), the external genitalia are male in character. Klinefelter syndrome (XXY) is discussed in the section on Testicular Hypoplasia. Chimeras, such as XX/XY, are phenotypically sexually ambiguous, and the degree depends on the relative amounts of each chromosome. Freemartins are chimeras and are discussed in Chapter 18.
XX Disorders of Sexual Development: Animals with an XX genotype, testicular tissue, and a male phenotype are called XX sex reversal individuals. They may be SRY positive or SRY negative. XX SRY-negative DSD is reported in many breeds of dogs, in goats, and in horses. Affected individuals have an ovotestis or testis. Those with ovotestes or a testis and an ovary are called hermaphrodites. Some ovotestes have an end-to-end arrangement of ovarian and testicular tissue, with clear demarcation between the two. Others have central testicular tissue with a periphery of ovarian structures. Bilateral hermaphrodites have an ovotestis on each side; unilateral hermaphrodites have an ovotestis on one side; and lateral hermaphrodites have a testis on one side and an ovary on the other. The genital tract in hermaphrodites is ambiguous and can be phenotypic male, female, or various combinations of the two, depending on the amount of hormones, including testosterone and AMH. It is assumed that XX SRY-negative individuals have a testis-determining region on another chromosome. XX SRY-positive DSD is not reported in domestic mammals.
XY Disorders of Sexual Development: There are three classifications of XY disorders of sexual development; those with disorders of gonadal development (gonadal dysgenesis, ovotestes, or aplasia), disorders of androgen synthesis or action, and miscellaneous types. They can be either XY SRY-positive or XY SRY-negative. XY DSD are identified in many species, including dogs and horses.
XY SRY-negative individuals usually have primitive and undifferentiated gonads, called gonadal dysgenesis, and a female phenotype. Their classification is therefore XY SRY-negative, gonadal dysgenesis DSD. XY SRY-positive individuals with a female phenotype usually have testes and are called male pseudohermaphrodites (Figs. 19-4 and 19-5). The differentiation of the genital tract can be slightly or greatly abnormal. In many cases, the mechanism for the abnormal differentiation is unknown, but there are well-recognized syndromes in which the underlying pathogenesis is known. Three such syndromes are persistent Müllerian duct syndrome (PMDS), androgen insensitivity, and steroid 5α-reductase deficiency.
Fig. 19-4 XY disorder of sexual development, male pseudohermaphrodite, external genitalia, boar.
This pig has a scrotum and intrascrotal testes, but the penis is small and clitoris-like and has a terminal urethral opening (ventral to the tail). (Courtesy Dr. D. Dodd; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
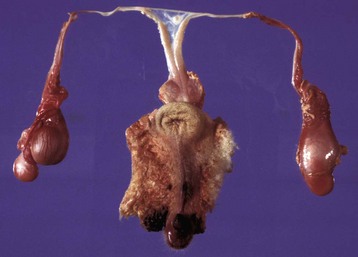
Fig. 19-5 XY disorder of sexual development, male pseudohermaphrodite (male feminization syndrome), reproductive tract, ram.
This sheep has testes, deferent ducts, and accessory genital glands but also a vulva and a prominent clitoris. An androgen receptor defect would explain these anomalies. (Courtesy RA Foster, Ontario Veterinary College, University of Guelph.)
PMDS is a rare disorder of AMH production or function. AMH can be absent or present in affected humans; the syndrome can be a result of either a mutation in the AMH gene or the AMH receptor. In animals, the syndrome is described in miniature schnauzer dogs and basset hounds with an autosomal recessive mode of inheritance and in a goat with an unknown mode of inheritance. Affected dogs have XY (or XXY) chromosomes and externally are normal males, with the common exception of unilateral or bilateral cryptorchidism. The testes, however, are attached to the cranial ends of uterine horns. When a testis has descended into the scrotum, the uterine horn passes through the inguinal ring. The deferent duct can be found microscopically within the myometrium. The cranial vagina and the prostate gland are often present. AMH is present in the testes of normal male dogs up to 143 days of age. AMH is present in the testes of young affected dogs also and is bioactive. A mutation in the structural gene for the AMH receptor results in AMH resistance in PMDS-affected dogs. Dogs with unilateral or bilateral scrotal testes can be fertile. Affected dogs may develop Sertoli cell tumor, hydrometra, and pyometra. Testicular abnormalities in PMDS, such as reduced spermatogenesis and tubular sclerosis, could be attributable to cryptorchidism. Cryptorchidism could be the result of interference with the MIS-controlled first phase of the function of the gubernaculums, the phase in which the testis migrates to the inguinal area.
Deficiency of 5α-reductase type 2, inherited as an autosomal recessive trait in humans, has not yet been documented in animals, but in all likelihood, it does exist. The enzyme converts testosterone to dihydrotestosterone, which is required for the masculinization of the urogenital sinus, tubercle, and genital swellings. Without dihydrotestosterone, these structures become caudal vagina, vestibule, clitoris, and vulva. Internally, mesonephric structures (deferent duct, epididymis) develop. The testes are likely to be retained.
Androgen receptor disorders are becoming increasingly recognized. Most cases are caused by a mutation in the androgen receptor gene, which is located on the X chromosome and thus in a single copy. Hundreds of mutations have been identified in other species, and the effects range from complete androgen insensitivity with female external genitalia (testicular feminization) (see Fig. 19-5) to mild male pseudohermaphroditism. The testes produce testosterone, but the stimulation of the mesonephric system is not normal because of defective androgen receptors. The normal androgen receptor has a hormone-binding and a DNA-binding domain; once activated by androgen, the domain changes shape and is able to bind to specific DNA sequences and regulate the transcription of other specific genes, leading to normal male differentiation. In humans with androgen insensitivity, the gene is rarely deleted, but numerous point mutations in the receptor gene have been identified, mostly in the hormone-binding and DNA-binding domains. Whether the result is partial or complete androgen insensitivity depends on the location of the mutation and the change in function of the receptor. Dihydrotestosterone binds to the androgen receptor with greater affinity than that of testosterone, and the receptor, when it acts as a transcriptional factor, might interact with different genes other than the testosterone-bound receptor. In domestic animals, complete androgen insensitivity is described in the equine, bovine, and feline species. The testes are often cryptorchid and are in the inguinal area. The first and second phases of testicular migration is normal. The inguinoscrotal third phase, which is under the control of androgens, does not occur. AMH produced by the testes causes regression of the paramesonephric ducts. External genitalia are female in complete androgen insensitivity, with a cranial blind end to the vagina, and neither the paramesonephric nor mesonephric ductal system is present.
Cryptorchidism is an XY SRY-positive DSD but is examined in detail as a separate entity in the discussion of the testis in the section on Disorders of the Scrotum and Contents.
Segmental Aplasia of Mesonephric Duct Derivatives: Segmental aplasia of the structures of mesonephric ductal origin (epididymis, deferent duct, ampulla, or vesicular gland) can involve any of the structures, but it most commonly involves the epididymis alone (Fig. 19-6) and less commonly other structures. Segmental aplasia is reported mainly in the bull and most commonly involves the body and tail of the epididymis and is unilateral. Its inheritance is thought to be autosomal recessive. Spermatozoa become impacted because the epididymal duct is blind ending; local dilation or rupture occurs secondarily, allowing escape of spermatozoa and formation of spermatic granulomas.
Ciliary Dyskinesia (Immotile Cilia Syndrome): One or more of several defects in the axoneme of cilia throughout the body and of the flagellum of spermatozoa causes ciliary dyskinesia, a rare disease identified in humans, dogs, pigs, mice, and rats. An autosomal recessive mode of inheritance is proposed. In dogs, heterogeneity of ultrastructural abnormalities of microtubule doublets and their dynein arms or central microtubules is reported. The effect on the reproductive system is immotile or hypomotile spermatozoa caused by flagellar lesions or to oligospermia (a subnormal concentration of spermatozoa) or azoospermia (no spermatozoa), presumably because of defective cilia in the epididymis and deferent duct. Because of defective cilia in the nasal mucosa, bronchial and bronchiolar mucosa, and ependyma, commonly associated lesions are rhinitis, bronchopneumonia, bronchiectasis, and hydrocephalus. Also associated is situs inversus, but the pathogenesis of the reversal of the normal left and right orientation of organs is unclear. Female infertility is related to defective function of cilia of the uterine tube.
Minor Anomalies
There are myriad minor anomalies that are of little consequence except when they are confused with other conditions that do affect fertility. Foremost of the minor anomalies are the various cysts that occur as a result of duplication or failure of regression of embryonic ducts and tubules.
Cysts of the Male Tract: It is often difficult to identify the origin of an individual cyst or group of cysts. In many instances, anatomic location is the determining factor. Many of the cysts are simply called inclusion cysts (Fig. 19-7). These have a wall of compressed collagen, a thin inner lining of flattened cells, and a clear fluid content. They are found where mesothelial cells are trapped adjacent to a serosal surface. Inclusion cysts attached to the head of the epididymis are good examples.
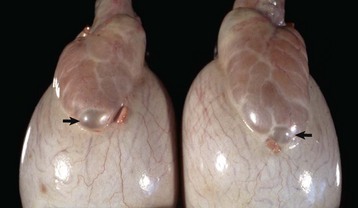
Fig. 19-7 Congenital inclusion cysts, testis and epididymis, ram.
These 7-mm cysts (arrows) in the tissue between the head of the epididymis and testis are incidental findings and are not significant. (Courtesy Drs. P. W. Ladds and R. A. Foster, James Cook University of North Queensland.)
The testis is derived from gonadal cords that form between the gonadal ridge on the medial side of the mesonephros and remnants of mesonephric tubules. The rete testis and efferent ductules are derived from mesonephric tubules. Within the mesonephros, many mesonephric tubules enter the single mesonephric duct. It is from the mesonephric part of the duct that the epididymis is derived. The deferent duct, ampulla, and vesicular gland are derived from the more caudal part of the mesonephric duct, outside the mesonephros. In the developed testis, about 20 efferent ductules are found, but the number varies with the species. Efferent ductules normally link in the head of the epididymis. Blind efferent ductules result when there is no connection to the epididymis and can be resorbed or persist and enlarge to form cysts or rupture. Blind-ending efferent ductules can be present in sufficient numbers to cause spermiostasis, spermatocele, and spermatic granulomas. The condition known as spermatic granuloma of the epididymal head (SGEH) is thus formed (see the section on Spermatic Granulomas and Epididymitis).
Unconnected with the lumen of the epididymal duct and efferent ductules, remnants of tubules of the mesonephros can form cysts adjacent to the head of the epididymis (external paradidymis) or within the head of the epididymis (internal paradidymis). Cysts, both connected and unconnected with the ductular system, are lined by ciliated columnar epithelium. They are clinically significant if they become large enough to cause stasis of spermatozoa in adjacent structures, or if they rupture and liberate spermatozoa into the surrounding tissue. The remnant of the paramesonephric duct, called the appendix testis, is not clinically significant and located on the cranial or cranioventral surface (depending on the species orientation of the testes) of the testis near the head of the epididymis. Sometimes, this small nodule of tissue can appear cystic. A similar cystic structure is found in the band of tissue between the ampullae. It is called the cystic uterus masculinus (Fig. 19-8). Some prostatic cysts of dogs have a similar origin.
Disorders of the Scrotum and Contents
Disease of the scrotal contents is based on a clinical approach in which the scrotal skin, vaginal tunics, testis and the epididymis are examined in turn. The most economically important disorders are those that affect the testis and epididymis, although the importance of disorders of the scrotal skin and tunics should not be forgotten. They directly affect thermoregulation and can be confused with disorders of the testis, epididymis, or spermatic cord.
The Scrotum
The fusion of the paired primordia of scrotal skin depends on androgens produced by cells of the gonad after it has differentiated into a testis. Altered androgen concentration or a lack of androgen receptors can lead to defects in the scrotum such as failure of fusion, cleft formation, or bifurcation. Defects can be local, confined to the scrotum and penis, or part of a wider range of defects in DSD. Hypospadias, in which the urethra opens on the ventral side of the penis, can be part of these anomalies.
Dermatitis of the scrotal skin is common. Often, it is nonspecific or involved in generalized dermatopathies. Dermatitis restricted to the scrotum can be the result of trauma, frostbite (Fig. 19-9), or exposure to environmental irritants such as cement dust. Some pathogens have the scrotum as a predilection site. These include Dermatophilus congolensis and Besnoitia besnoiti in the bull and Chorioptes bovis in the ram (Fig. 19-10). The heat generated in scrotal dermatitis can interfere with the thermoregulatory function of the scrotum, leading to testicular degeneration.
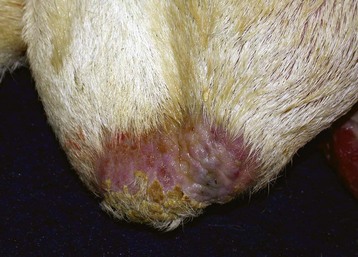
Fig. 19-9 Frostbite, scrotum, ram.
The skin of the lower portion of the scrotal sac has sloughed. The ventral scrotal skin is alopecic or covered by crusts from previous frostbite. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
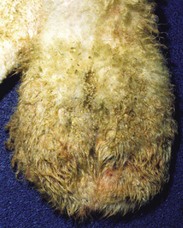
Fig. 19-10 Scrotal dermatitis (Chorioptes bovis), scrotum, ram.
There is extensive crusting and exudation of the skin in response to the chronic irritation and inflammation caused by the mites. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Neoplasms of any of the types that occur in the skin can occur in the scrotum but are much less common in the scrotal skin. Neoplasms occasionally encountered are papillomas in the boar and mast cell tumors and hemangiosarcomas in the dog. Testicular tumors, especially Sertoli cell tumors and interstitial cell tumors, occur in the scrotum of previously castrated male dogs and cats, respectively, presumably from cells inadvertently transplanted at surgery. Vascular abnormalities, often called hemangiomas and which are probably hamartomas, occur on the scrotum of the boar and dog. The scrotal veins of bulls sometimes become varicose.
Vaginal Tunic
The vaginal tunics are the extension of the peritoneum that lines the scrotal sac as the parietal layer and covers the testis, epididymis, and spermatic cord as the visceral layer. The cavity between the two layers is continuous with the peritoneal cavity. The tunics and the cavity thus are subject to all the disorders of the peritoneum and peritoneal cavity. Ascites therefore results in hydrocele or fluid accumulating within the tunics around the scrotal contents. Polyserositis in pigs especially and feline infectious peritonitis (FIP) in cats causes inflammation of the tunics and periorchitis. Neoplasia is uncommon; mesothelioma and peritoneal carcinomatosis are the most frequently encountered. Parasitic disease, especially the cysts of Cysticercus tenuicollis in rams, occurs periodically and can be confused with cysts or spermatic granulomas.
Inflammation of the vaginal tunics without initial inflammation of the abdominal peritoneum can be the result of trauma or local infection. The latter is likely to be an extension from orchitis or epididymitis. Especially well-known causes are trypanosomiasis in bulls, rams, and bucks, and Brucella ovis and Actinobacillus seminis in rams. Adhesions between the parietal and visceral vaginal tunic are fibrinous at first and later fibrous.
Testis and Epididymis
The testis and epididymis are inseparable, and disease of one usually results in disease of the other. Scrotal palpation is the main clinical method to identify abnormalities of this region, and the changes in size are the most obvious. We therefore use a size approach, beginning with those that cause small scrotal contents, then with those that result in enlargement of one or more of the intrascrotal structures (Box 19-3).
Reduced Testicular and Epididymal Size: Missing or aplastic components are rare. Segmental aplasia of the mesonephric duct, usually aplasia of the epididymal tail (see Fig. 19-6), is one example. It is described earlier under major anomalies. If one complete side of the scrotal contents is missing, either unilateral castration was performed or the affected animal was a unilateral cryptorchid. Sometimes, the undescended testis and epididymis subsequently descend, or the castration operator may have missed a testis and epididymis. Affected sheep, for example, often have the scrotum removed, and the testis is located beneath the underlying skin. A lack of both sides of the scrotal contents can represent castration or bilateral cryptorchidism and requires additional investigation, including measurements of hormonal concentrations, especially after treatment with GnRH, or surgical exploration. A search for secondary characteristics that are testosterone dependent, such as the barbs on the penis of the cat and the presence of a prostate in dogs, can assist in separating behavioral characteristics from similar behaviors in incompletely castrated males.
A small testicular size is of great importance, especially to production animals, as daily sperm output is correlated to testicular volume and weight. A small size indicates hypoplasia or atrophy. It can be extremely difficult to differentiate unless there is a history of size change. Hypoplasia is a congenital condition in which the testis does not increase to its full size at puberty. It is often seen as part of other syndromes, most commonly cryptorchidism.
Cryptorchidism: Cryptorchidism occurs when there is incomplete descent of the testis (normal testicular descent is discussed earlier). In most mammalian species, the testis normally descends into the scrotum by the time of birth. Cryptorchidism is more often unilateral than bilateral, with the side being somewhat species dependent. Many undescended testes are on the right side. In the horse, the distribution is equal left to right, and in the bull, the left side is usually affected. The undescended testis can be anywhere along its path from caudal to the kidney (Figs. 19-11 and 19-12, A) to the scrotum but is commonly within the abdomen near the internal inguinal ring, within the inguinal canal or in a subcutaneous location just outside the external inguinal ring. Epididymal development is coordinated with testicular development and consequently is slowed in cryptorchidism.
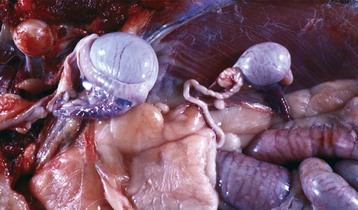
Fig. 19-11 Cryptorchid testis, intraabdominal, dog.
The retained testis and epididymis (right) are hypoplastic. The other descended testis (left) is normal. Intestines are below the right testis. (Courtesy Dr. Y. Niyo, College of Veterinary Medicine, Iowa State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
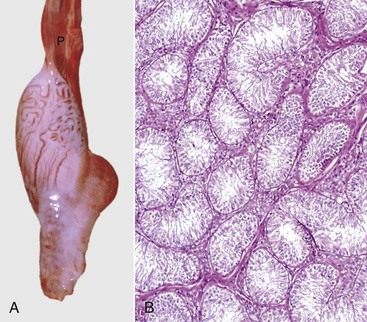
Fig. 19-12 Cryptorchid testis.
A, Cryptorchid testis and epididymis, bull calf. The testis and epididymis are hypoplastic and are barely larger than the pampiniform plexus above (P). There are no attachments to the vaginal tunic. B, Cryptorchid testis, dog. There is complete absence of spermatogenesis; however, the Sertoli cells are normal. H&E stain. (A courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph. B courtesy Dr. J.A. Ramos-Vara, College of Veterinary Medicine, Michigan State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Cryptorchidism is the most common disorder of sexual development (DSD). Most are XY SRY-positive with testes but with a disorder of phenotypic sex. It has a polygenetic basis and a reasonable working hypothesis is a sex-limited autosomal recessive mode of inheritance. It can be the result of a failure of normal production or regulation by one or more genes for the production of testosterone, androgen receptor, INSL3, INSL3 receptor, and/or calcitonin gene–related protein. Descriptions of cryptorchidism should include whether it is unilateral or bilateral and which stage of descent is abnormal (abdominal translocation, transinguinal migration, and inguinoscrotal migration).
In the horse, left and right testicular retention are almost equal in occurrence and the left retained testis is more likely to be abdominal than inguinal in location. Three breeds (Percheron, American saddle horse, and American quarter horse), ponies, and crossbred horses were significantly overrepresented in a large hospital-based study of cryptorchidism.
Abnormalities in the gubernaculum could cause cryptorchidism by its failure to develop, improper positioning, excessive growth, or failure to regress. In humans, some other predisposing or contributing factors for cryptorchidism include testicular hypoplasia, estrogen exposure in pregnancy, breech labor compromising blood supply to the testes, and late closing of the umbilicus, delaying the ability to increase abdominal pressure.
A cryptorchid testis remains small at puberty, probably because of its higher than optimal temperature. Superimposed atrophy occurs in the cryptorchid testis after puberty. The testis is small and fibrotic and has interstitial collagen deposition, hyaline thickening of the tubular basement membranes, and degeneration of germinal epithelium so that only a few spermatogonia remain with the normal complement of Sertoli cells (Fig. 19-12, B).
Cryptorchid testes are much more prone to develop neoplasia than scrotally placed ones. In the dog, Sertoli cell tumors are more likely to occur in the testes present in the abdomen (Fig. 19-13), whereas seminomas tend to develop more commonly in inguinally placed testes. The contralateral testis is also at increased risk for developing a neoplasm, even if that testis is located in the scrotum. A retained testis, especially if enlarged by a neoplasm, is prone to torsion.
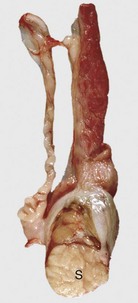
Fig. 19-13 Sertoli cell tumor, cryptorchid testis, dog.
The testicular parenchyma has been replaced by a white multilobular Sertoli cell tumor (S). The texture is firm, indicating fibrosis. This dog had bilaterally retained testes and epididymides and bilateral Sertoli cell tumors. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Cryptorchidism is a phenotypic DSD. Many animals with other DSD have cryptorchidism, and genetic testing can identify these disorders. Some of these individuals have ovotestes. The greater the ratio of testicular to ovarian tissue, the more likely an ovotestis has descended into the scrotum. A testis most often lies in the scrotum, and an ovary is in its normal position. The ovary or ovarian portion of an ovotestis is histologically normal, but the seminiferous tubules of a testis or ovotestis are abnormal because of a combination of cryptorchidism and the effects of estrogen produced by the ovarian tissue.
Testicular Hypoplasia: Hypoplasia of the testes is a common condition in which the testis is smaller than normal for the age of the animal, and it is often present in disorders such as cryptorchidism and other DSD. Hypoplasia of the testis and epididymis in an otherwise normal male is the specific focus here.
It is difficult to distinguish hypoplasia from testicular atrophy by using morphologic features. Both testicular hypoplasia and atrophy can occur alone, with no apparent contributing or influencing factors; either can also be associated with, secondary to, or part of some other lesion. Testicular and epididymal hypoplasia (Fig. 19-14, A and B) has been causally linked to poor general nutrition, zinc deficiency, specific genes in the Swedish red and white breed of cattle, and endocrine and cytogenetic abnormalities. Endocrine disturbances causing testicular hypoplasia are those that reduce production of either luteinizing hormone by the pituitary gland, which in turn influences testosterone production by the interstitial endocrine cells, or FSH by the pituitary gland, which stimulates the nurturing function of the Sertoli cells.
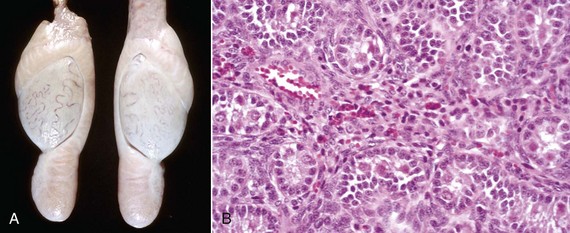
Fig. 19-14 Bilateral hypoplasia, testes, yearling ram.
A, Both testes and epididymides from this yearling ram are very small compared with normal age-matched controls. B, The seminiferous tubules are lined only by Sertoli cells, and there is no spermatogenesis. H&E stain. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
A wide range of cytogenetic abnormalities, from translocations and mosaics to nondisjunctions causing polysomies of sex chromosomes, result in testicular hypoplasia. The best known example of polysomy is the XXY karyotype of Klinefelter syndrome seen in stallions, bulls, boars, dogs, and tricolor cats. In cats, the syndrome is recognized in males with the tricolor, tortoise shell, or calico coat types. These cats can be XXY, XX/XXY, or more complex chimeras or mosaics with two or more X chromosomes and one or more Y chromosomes. The gene for black and the gene for orange are carried one per X chromosome, and so a normal male cat should not have hair of both colors.
Hypoplasia of the testis can theoretically occur when the number or length of seminiferous tubules is reduced, or when there are no or insufficient germ cells. Usually before puberty, the seminiferous tubules have only Sertoli cells and spermatogonia (see Fig. 19-14, B). At puberty, the tubules of hypoplastic testes undergo an irregular progressive sclerosis and become collagenous, presumably from concurrent degeneration. Interstitial endocrine cells appear to be more numerous and clustered.
Hypoplasia of the testes is not apparent until after puberty. Unilateral hypoplasia is more common than bilateral hypoplasia, but this difference in prevalence could be a reflection of the relative ease of recognizing a size abnormality when the normal contralateral testis is available for ready comparison. Unilateral hypoplasia is difficult to explain because most of the causes seem to act systemically and therefore should produce a bilateral effect.
The size range of a hypoplastic testis generally is from a prepubertal size (see Fig. 19-14, A) to almost normal. The consistency of a hypoplastic testis is near to normal. The severity of the hypoplasia can be graded histologically by the proportion of hypoplastic tubules scattered through the organ. Hypoplastic tubules have a small diameter and are lined only by Sertoli cells and sometimes a few spermatogonia. Interstitial endocrine cells appear proportionally more numerous, but this is only because the tubular area is reduced and the relative amount of interstitium is increased. In severe testicular hypoplasia, most or all of the tubules are abnormal; the tubules have a small diameter and a uniform microscopic appearance with only infrequent vacuolation of Sertoli cells and without a thickened basement membrane. In moderate hypoplasia, fewer tubules are small, those of normal size have some differentiation of the seminiferous epithelium, and a few tubules have complete spermatogenesis. In most tubules, however, when the stage of spermatocyte formation is reached, the spermatocytes undergo apoptosis or degeneration, leaving tubules lined by Sertoli cells with a vacuolated cytoplasm. The lumen of such tubules can contain cellular debris and multinucleate cells that are formed when dividing cells fail to separate. When hypoplasia is mild, only a few small tubules are lined only by Sertoli cells; most tubules have normal spermatogenesis. Mild hypoplasia is difficult to distinguish from testicular degeneration. The number of hypoplastic tubules would not be expected to increase with age because hypoplastic tubules do not arise after puberty. Mild hypoplasia is detected when scrotal circumference is just less than the accepted minimum.
Testicular Atrophy and Degeneration: Testes that reduce in size after puberty are macroscopically atrophic, and the microscopic change is degeneration of the seminiferous tubules. Testicular atrophy is a common lesion. Mild testicular degeneration can be detected only microscopically, but when it is severe and chronic, the testis is small and firm (Fig. 19-15). The causes are many, and in a particular individual, the specific cause is often unknown. Degeneration can be unilateral or bilateral, depending on whether the cause is local or systemic. In young growing males, the distinction between testicular degeneration and hypoplasia is often difficult to make using morphologic features. Both lesions are often present together because hypoplastic testes are prone to degeneration. Inflammation also can be superimposed on degeneration when there is obstruction, leading to back pressure, rupture of seminiferous tubules, and spermatic granuloma formation. Recovery of a testis with degeneration back to a normal testis is possible if the injurious agent is eliminated and damage is not too severe.
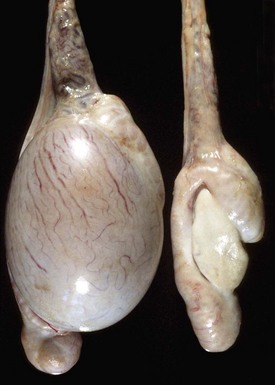
Fig. 19-15 Unilateral testicular atrophy and epididymitis, testis and epididymis, ram.
The affected testis (right) is small, and testicular veins are not visible on the capsule of the testis because of fibrosis and contraction of the connective tissue. The other testis (left) is bulbous, indicating hypertrophy. (Courtesy Drs. P. W. Ladds and R. A. Foster, James Cook University of North Queensland.)
Specific causes of testicular degeneration are numerous (Box 19-4). Increased apoptosis of germ cells is a common endpoint of many causes, regardless of whether the initial effect is on the hypothalamo-pituitary-gonadal endocrine axis or on the Sertoli cell–interstitial cell–germ cell axis. Fever or local heat from inflammation of the scrotal skin is a classic cause of degeneration. Obstruction of flow of spermatozoa causes testicular degeneration. Obstruction can be the result of developmental anomalies such as segmental aplasia of mesonephric duct derivatives, local injury, or inflammation of the epididymis. Vascular events, such as impairment from aging, torsion, or severe crushing of the spermatic cord, cause degeneration. Systemic injurious factors include nutritional deficiency, hormonal aberrations, toxins, irradiation, and oxidative stress. Hypovitaminosis A and zinc deficiency are specific nutritional deficiencies; general malnutrition also causes testicular degeneration. Interference with GnRH, LH and its control of androgen production by interstitial endocrine cells, or FSH and its effect on the production of androgen-binding protein by Sertoli cells can have a deleterious effect on the seminiferous epithelium. Such interference could happen, for example, when a neoplasm of the pituitary gland causes local compression of the pituitary gland, the hypothalamus, or both. Estrogen produced by Sertoli cell tumors induces testicular degeneration. Environmentally acquired endocrine disruptors are also implicated. Some therapeutic drugs, such as amphotericin B, gentamicin, and chemotherapy compounds, cause testicular degeneration.
Many toxins are capable of causing testicular degeneration, and most damage the spermatogonia and dividing primary spermatocytes, but some damage later stages and spermatocytes and spermatids or injure Sertoli cells.
A testis undergoing degeneration initially is softer than normal, and as the degeneration progresses, the testis becomes smaller. The cut surface of the normal testis and the acutely degenerated testis bulges slightly. After the acute phase, the testis becomes firmer and has small flecks or large areas of mineralization, especially in ruminants. Degeneration can be either generalized or regional, occurring in the ventral portion of the testis of bulls or in the dorsal part of the testis (near the head of the epididymis) in rams. If the degeneration is caused by ischemia after a vascular accident in the spermatic cord, small islands of parenchyma beneath the testicular capsule can survive the infarction because of diffusion of oxygen from vessels of the epididymis and capsule of the testis. Degeneration can occur locally around a lesion, such as a neoplasm, that expands and causes compression.
Microscopically, the initial change is spermatogenic arrest at one or more stages of the spermatogenic cycle. The seminiferous tubules have a smaller diameter. With further degeneration, there is a thickened basement membrane, decreased numbers of germinal cells, vacuolated Sertoli cells, intratubular multinucleated spermatids, and interstitial fibrosis (Fig. 19-16, A and B). A key lesion in the differentiation of testicular degeneration from testicular hypoplasia is the wavy basement membrane found in testicular degeneration because affected tubules had at some stage reached full size and then subsequently collapsed. At the end stage of testicular degeneration, Sertoli cells are the only lining cells remaining, but with time, these also disappear, leaving only the basement membranes. Mineralization can involve intratubular cellular debris, tubular basement membranes, or the interstitium.
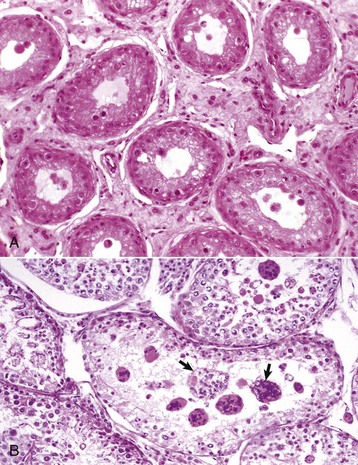
Fig. 19-16 Testicular degeneration, testis.
A, Ram. There is interstitial fibrosis that separates the seminiferous tubules. Spermatogenic arrest at the spermatocyte stage, vacuolation of Sertoli cells, and a wavy basement membrane caused by a reduction in tubular diameter are present. H&E stain. B, Dog. In addition to reduced spermatogenesis, there is formation of multinucleated spermatids (arrows) as a result of failure of spermatids to separate. This is a common change in testicular degeneration. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Degeneration of the epididymis is less well studied but does occur. In degenerative conditions, the epididymis is much less likely to be reduced in size. This lack of reduction in size can be helpful in differentiating testicular atrophy from hypoplasia macroscopically. In the former, the epididymis approximates adult size, whereas in hypoplasia the epididymis is small in size.
Testicular and Epididymal Enlargement: There are several disorders that result in testicular and epididymal enlargement. Foremost is inflammation, especially epididymitis and orchitis. Testicular neoplasia is a common cause in dogs.
Spermatic Granulomas and Epididymitis: Spermatic granuloma of the epididymal head is a unique congenital disease of most species. It is not an infectious condition, but inflammation dominates as a response to extravasated spermatozoa. It affects the efferent ductule region first, and then spreads to involve the head of the epididymis (Fig. 19-17, A). All efferent ductules should connect to the single epididymal duct in the head of the epididymis, but some are blind-ended. At puberty, the blind-ending ductules fill with spermatozoa and the resultant spermiostasis proceeds to spermatocele and then spermatic granulomas (Fig. 19-17, B). These progress over time and result in infertility because of obstruction of the epididymal duct. The back pressure produced by the spermatic granulomas causes dilation of the mediastinum of the testis and testicular atrophy.
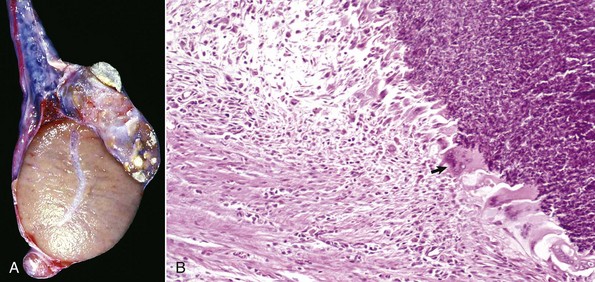
Fig. 19-17 Spermatic granuloma, head of the epididymis, dog.
A, The head of the epididymis is tremendously enlarged because of spermatic granulomas (white-yellow masses). The body and tail of the epididymis (ventral) are small, as the spermatic granulomas have obstructed the flow of spermatozoa from the testis to the epididymis. B, The mass of spermatozoa (right) in the interstitial connective tissue of the epididymis is surrounded by macrophages and multinucleated giant cells (arrow). H&E stain. (A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois; and Dr. J. King, College of Veterinary Medicine, Cornell University.)
Epididymitis is very important in rams (Fig. 19-18) and dogs (Fig. 19-19) and is rare in other species. The tail of the epididymis is almost always affected, allowing the majority of cases to be differentiated from spermatic granuloma of the epididymal head. Because the epididymis is only a single coiled duct, any lesion along its length has the potential to cause obstruction of spermatozoal flow and the formation of spermatic granulomas. Thus epididymitis is frequently accompanied by spermatic granulomas and also periorchitis. Epididymitis is most frequently encountered as a unilateral, chronic lesion of the tail of the epididymis and thus can be recognized by comparing the size and shape of the abnormal organ with the normal one. In acute inflammation, the epididymis is swollen and soft. In chronic inflammation, it is enlarged and firm because of abundant fibrous tissue and the presence of spermatic granulomas. Epididymitis is one of the causes of testicular degeneration, and macroscopically, the testis is atrophic. Focal fibrinous or fibrous adhesions occur between the visceral and parietal vaginal tunics over the epididymis (see Fig. 19-18). If a spermatic granuloma ruptures into the cavity of the vaginal tunic, diffuse inflammation can result (Fig. 19-20), followed by adhesions across the cavity. In some cases, fistulas discharge through the scrotum.
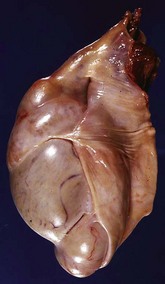
Fig. 19-18 Epididymitis (Brucella ovis), tunic adhesions, epididymis, ram.
Note the dramatic enlargement of the epididymis (left half of the image) and the adhesion of the parietal vaginal tunic to the visceral vaginal tunic around the affected epididymis. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois; and Dr. J. King, College of Veterinary Medicine, Cornell University.)
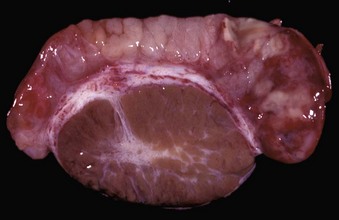
Fig. 19-19 Acute epididymitis, epididymis, dog.
The head (left) and tail (right) of the epididymis are grossly hyperemic and contain pale foci of suppurative exudate and spermatozoa. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois; and Dr. J. King, College of Veterinary Medicine, Cornell University.)
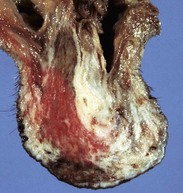
Fig. 19-20 Scrotal cellulitis and periorchitis, scrotum (testis has been removed), dog.
The vaginal tunic is thickened by inflammatory exudate, granulation tissue, and fibrous tissue. The inflammation extends into the skin. There is an ulcer in the ventral skin from self-mutilation. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Microscopically, the early lesions of epididymitis begin when bacteria are within the lumen of the duct. Neutrophils infiltrate, and their products combined with bacterial products, such as exotoxin and endotoxin, cause ductal and stromal necrosis, fibrin exudation, and edema. Spermatozoa extravasate, and spermatic granulomas form. With time, there will be neutrophils and macrophages within the tubules, epithelial hyperplasia, metaplasia, intraepithelial cavities, or lumina, and many lymphocytes and plasma cells within the interstitium. (Fig. 19-21).
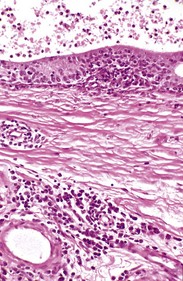
Fig. 19-21 Chronic epididymitis, epididymis, ram.
Note the intertubular fibrosis and large numbers of lymphocytes and plasma cells in the interstitium (lower half of image). The epithelium of the epididymal duct (top) is hyperplastic, and the lumen contains neutrophils and spermatozoa. H&E stain. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Noninfectious causes of epididymitis are exceedingly rare, and frequently, cases that have no recoverable agent have spermatic granulomas that remain after a previous infection is otherwise controlled.
Orchitis: True orchitis (inflammation of the testis) is much less common than epididymitis, probably because the testis is much further “upstream” than the epididymis and possibly because of its altered immunologic environment, which is antiinflammatory. Orchitis is, unfortunately, the clinical term for inflammation of the scrotal contents, even though most cases are epididymitis. Orchitis is usually accompanied by epididymitis; it may be an extension of epididymitis. Primary orchitis is usually hematogenous, with examples, including Brucella abortus in bulls, Corynebacterium pseudotuberculosis in rams, and Brucella suis in boars. It happens in several forms. Intratubular orchitis is centered on the seminiferous tubules, so it is assumed the agent and the inflammatory reaction begin there. Intratubular orchitis appears grossly as poorly defined, up to 1-cm yellow foci that become firm and white as the lesions become chronic. Initially, the affected tubules have acute inflammatory debris. The lining of the tubules is lost, but the tubular outlines remain. Spermatic granulomas frequently form. In the center of these granulomas, spermatozoa are free within the tissue and within macrophages. Macrophages and lymphocytes surround the spermatozoa and with time, collagen is formed at the edge of the lesion. When the lesion is predominantly in the interstitium, it is called interstitial orchitis (Fig. 19-22).
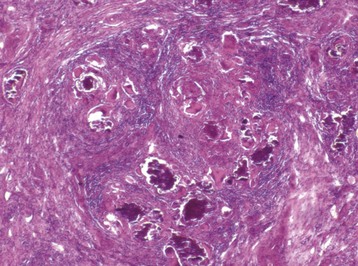
Fig. 19-22 Granulomatous interstitial orchitis, testis, ram.
There is granulomatous inflammation surrounding aggregates of spermatozoa and mineral that replaced the seminiferous tubules after they were destroyed. Lymphocytes and plasma cells predominate in the surrounding interstitium. H&E stain. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Necrotizing orchitis, such as is caused by Brucella abortus and Brucella suis, is the most severe form of orchitis. It is a more severe form of intratubular or interstitial orchitis, but in some cases the affected areas are so severely inflamed and the necrosis is so extensive that the original structures form a caseous mass. Gray-brown, initially soft and later firm, necrotic debris replaces an irregular but large portion of the testis. In a few extremely severe cases, a fistula develops through the scrotum. One manifestation of FIP is a fibrinous and necrotic orchitis (Fig. 19-23).
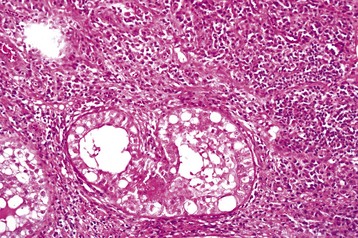
Fig. 19-23 Severe fibrinous interstitial orchitis, feline infectious peritonitis (FIP), testis, cat.
Note the severe orchitis with a mixture of fibrin and plasma cells in the interstitium. The tubules are degenerate and not directly involved. The testicular lesion was the first manifestation of FIP in this cat. H&E stain. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Granulomatous orchitis, especially tuberculous orchitis, is now very rare as countries eradicate Mycobacterium bovis. Mycotic orchitis caused by Blastomyces dermatitidis occurs sporadically in dogs in endemic areas.
Neoplasia: Testicular neoplasms are common in older dogs, much less frequent in horses, and rare in other species. They usually arise from germ cells, Sertoli cells, or interstitial endocrine cells. Occasionally, neoplasms of testicular mesenchymal structures or metastatic neoplasms are found. The three common primary testicular neoplasms are seminoma, Sertoli cell tumor and interstitial cell tumor; they occur singly or in combination. These primary neoplasms are almost always benign, and there are no features that indicate the likelihood of metastasis. Metastasis, when it does occur, is identified by nodules in the spermatic cord, scrotal lymph node, or beyond.
Germ cell neoplasms are seminoma, teratoma, and other less common types such as embryonal carcinoma. Seminomas are the most common testicular neoplasm in the aged stallion and the second most common canine testicular neoplasm (Fig. 19-24, A). They are more prevalent in cryptorchid testes than in descended testes. Multicentric origin within the testis and local invasiveness are characteristic, but metastasis is rare. The neoplasm is homogenous, white or pink-gray, and firm; bulges when cut; and has fine fibrous trabeculae. Microscopically, seminomas are either intratubular or diffuse, and the neoplastic cells are large round cells with scant cytoplasm and a large nucleus with a prominent nucleolus. Anisokaryosis is up to sixfold, but most cells are large and uniform in size. The mitotic rate is usually high. Giant cells, with either a single nucleus or multiple nuclei, are sometimes present (Fig. 19-24, B). Aggregates of CD8+ T lymphocytes are often present around blood vessels in seminomas and are a useful diagnostic feature, since they are not seen in other testicular neoplasms. Teratomas arise from totipotential primordial germ cells. They are uncommon but are best known in the young horse, especially in a cryptorchid testis. The neoplasms can be large, cystic, or polycystic and can contain recognizable hair, mucus, bone, or even teeth. Microscopically, derivatives of two of the three embryonic germ layers (ectoderm, mesoderm, and endoderm) are present. Most teratomas have well-differentiated tissue and are benign.
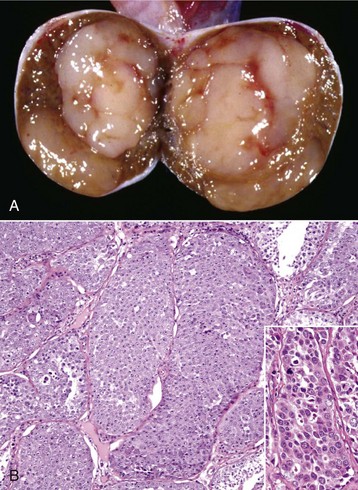
Fig. 19-24 Seminoma, testis, bisected and reflected section, dog.
A, Note the pale pink to beige circumscribed homogeneous mass. The cut surface has a gelatinous texture and has bulged slightly on incision. The contralateral testis was atrophic. B, Seminomas consist of round germinal cells with a high nuclear-to-cytoplasmic ratio and frequent mitoses (not shown here). Note how the cells have filled and expanded the seminiferous tubules. Inset: Higher magnification of the neoplastic cells. Note the mitoses. Despite this “malignant” appearance, most are behaviorally benign. H&E stain. (A courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy College of Veterinary Medicine, University of Illinois. Inset courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Interstitial cell tumor is the most common testicular neoplasm of the bull, dog, and cat. These neoplasms are almost always benign. They likely begin as regions of nodular hyperplasia. Some cases produce hormones, including estrogenic substances. They are readily identifiable grossly because they are spherical and well demarcated (Fig. 19-25, A), a tan-to-orange color, often with regions of hemorrhage. Microscopically, they are noninvasive and finely encapsulated. The neoplastic cells are arranged in solid sheets or packed into small groups by a fine fibrous stroma (Fig. 19-25, B). The cells of the bovine neoplasms vary little, but in the dog, the cells can be large, round, and polyhedral or spindle shaped. The cells have abundant cytoplasm that is often finely vacuolated and often has brown lipofuscin pigment. Nuclei are round, and anisokaryosis is usually minimal. Hemorrhage and necrosis is common.
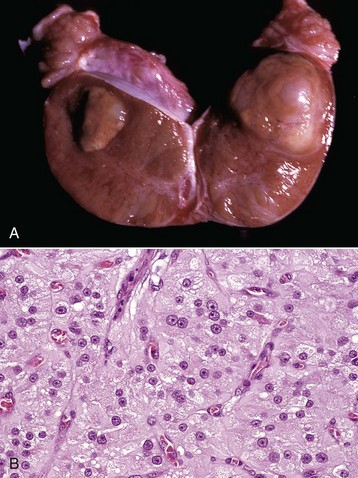
Fig. 19-25 Interstitial cell tumor, testis, bisected and reflected section, dog.
A, Note the well-demarcated, yellow-tan mass, which has bulged on incision. Such masses become hemorrhagic when they become large. Atrophy of an affected testis as the result of pressure is common when the tumor is large. B, Cells are arranged in packets surrounded by a fine fibrous stroma typical of endocrine cells. Their cytoplasm is pale, eosinophilic, and abundant and often has fine vacuoles. Mitoses are rare. H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Sertoli cell tumor is the third most common testicular neoplasm of the dog. It is rare in other species. In the dog, more than 50% of Sertoli cell tumors are located in retained testes. The neoplasm is well circumscribed, expansile, firm, white, and lobulated by fibrous bands and can cause dramatic enlargement of the affected testis (Fig. 19-26, A). Metastasis is rare but when present, is in the spermatic cord and occasionally spreads to the superficial inguinal (scrotal) lymph node. Metastases beyond the regional lymph node are reported but are very rare. Microscopically, the abundant fibrous tissue in Sertoli cell tumors distinguishes them from the other two common types of testicular neoplasm. Neoplastic Sertoli cells have an intratubular or a diffuse arrangement and tend to palisade along the fibrous stroma or form tubular structures (Fig. 19-26, B). They either resemble normal Sertoli cells or are pleomorphic. In addition to the effects of pressure and local invasion, about a third of Sertoli cell tumors produce a feminizing effect, causing gynecomastia (enlargement of the mammary glands), alopecia, hyperplasia, or squamous metaplasia of the acini of the prostate gland (Fig. 19-26, C). The amount of hormone produced is generally proportional to the size of the neoplasm. Although some produce estrogen, many produce inhibin, which inhibits GnRH secretion and subsequently LH and FSH release. This alters the balance between estrogen and testosterone production. A possibly life-threatening effect of hyperestrogenism is myelotoxicity, resulting in a poorly regenerative anemia, granulocytopenia, and thrombocytopenia.
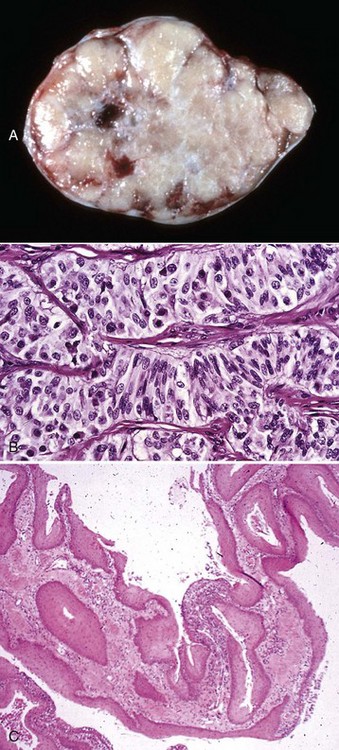
Fig. 19-26 Sertoli cell tumor, testis, dog.
A, Sertoli cell tumors are firm, white, and often lobulated, and the lobules are surrounded by fibrous bands. B, Histologically, Sertoli cell tumors have tubular structures lined by cells that resemble Sertoli cells and are supported by fine septa of fibrous tissue. H&E stain. C, Prostate, squamous metaplasia. Functional Sertoli cell tumors that secrete estrogen can induce hyperplasia and/or squamous metaplasia of the prostate gland. Normal epithelium of the prostatic ducts (transitional) and glandular acini (columnar) is replaced by stratified squamous keratinizing epithelium. H&E stain. (A courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois. B courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph. C, Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Mixed germ cell–stromal neoplasms are described also. The neoplasm-bearing testis is large and often cryptorchid. The testis is partially or completely replaced by a firm, gray-white to tan, single multilobed mass that is almost identical in appearance to the Sertoli cell tumor. Microscopically, germ cells intermingle with Sertoli cells in tubular structures of various sizes, thus it is a mixture of seminoma and Sertoli cell tumor.
Spermatic Cord
The spermatic cord is composed of the deferent duct, pampiniform plexus, and cremaster muscle. The scrotal lymph node and the inguinal canal are adjacent structures. The most common disease is varicocele or varicose dilation of veins of the pampiniform plexus (Fig. 19-27). This is seen most commonly in older rams and is described in the section on Disorders of Ruminants. It can be found in any species.
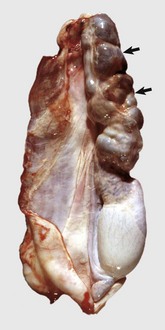
Fig. 19-27 Varicocele, pampiniform plexus, ram.
This extremely large varicocele (arrows) is larger than the testis. It is multinodular from large thromboses filling the dilated veins. (Courtesy Dr. P. W. Ladds, James Cook University of North Queensland.)
Torsion of the spermatic cord is seen in retained testes, especially when there is a testicular neoplasm present. Torsion also occurs periodically in the stallion and is a cause of colic. Torsion causes venous occlusion with a resultant venous infarction of the testis especially and also of the epididymis.
Inflammation of the spermatic cord (funisitis, scirrhous cord) occurs after the contamination of a castration wound. Sometimes the lesion is neutrophilic and necrotizing, but more often it is chronic and a scirrhous cord is encountered. Great enlargement of the distal part of the cord is caused by exuberant granulation tissue in which numerous small pockets of pus are scattered. Staphylococci are the bacteria frequently recovered from the pus in the horse. The term botryomycosis was used for these staphylococcal pyogranulomas. Radiating club-shaped eosinophilic deposits (Splendore-Hoeppli reaction) are present around the central clusters of bacteria. This is surrounded in turn by neutrophils and multinucleated giant cells and then by granulation and fibrous tissue.
Inguinal hernia is a differential diagnosis for masses or swellings in the region of the spermatic cord and scrotum. Stallions, older rams, and some Pietrain strains of pigs are particularly prone to inguinal hernia. It is an important cause of scrotal enlargement and colic in stallions. Little is known about the cause of inguinal hernia in domesticated animals. The herniated structures are usually a part of the intestine, and they can be free within the vaginal tunics or encased in a second fold of peritoneum. In stallions, the entrapped intestine undergoes venous infarction, causing life-threatening colic. In other species, intestinal infarcts are unusual.
Scrotal lymphadenopathy is another cause of swelling in the region of the spermatic cord. Caseous lymphadenitis in rams and lymphoma in dogs are causes of enlargement of the scrotal lymph nodes.
Disorders of the Accessory Genital Glands
The accessory genital glands include the ampullae, vesicular glands, prostate, and bulbourethral glands. The ampullae and vesicular glands are derived from the mesonephric duct, and the prostate and bulbourethral glands are derived from the urogenital sinus. Their hidden location within the pelvis means they are not routinely examined.
Ampulla
Disease of the ampullae is unusual, and the majority are microscopic. Bulls have variation in the patterns of insertion of ampullae at the seminal colliculus. The ampullae can be above or below the vesicular glands. The membrane between the ampullae is the location for the remnants of the paramesonephric duct—the cystic uterus masculinus—in bulls and rams especially. Hyperestrogenism can cause these to become greatly enlarged.
Ampullitis is a feature of infection with agents that cause epididymitis and vesicular adenitis, including but not restricted to Brucella abortus, Brucella ovis, Actinobacillus seminis, Histophilus somni, and many other agents. It may precede epididymitis as part of an ascending infection or occur when organisms and inflammatory products pass through the deferent duct from an infected epididymis.
Vesicular Glands
The major disease of the vesicular glands (seminal vesicles) is inflammation of the gland, and often, it is clinically silent, except in the bull where it is an important disease (see the section on Disorders of Ruminants).
Vesicular adenitis is occasionally seen in stallions and can be a part of lesions caused by Burkholderia pseudomallei in boars and the many Brucella species, especially Brucella ovis in sheep (Fig. 19-28).
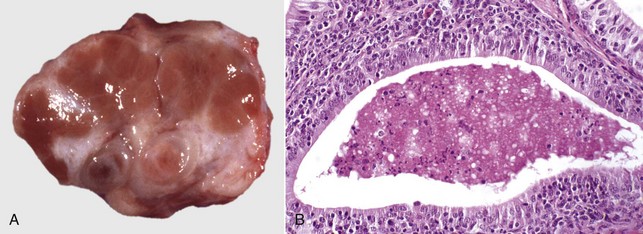
Fig. 19-28 Chronic vesicular adenitis, vesicular gland, cut surface, ram.
A, The normal lobulation of the vesicular gland is distorted by white-gray fibrous tissue that surrounds the glandular tissue. B, Chronic vesicular adenitis (Brucella ovis). Glandular acini are filled with degenerating neutrophils and debris, and neutrophils are migrating through the acinar epithelium. The interstitium is heavily infiltrated by plasma cells and lymphocytes. H&E stain. (Courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph.)
Prostate Gland
The prostate gland is derived from the urogenital sinus. Both estrogens and androgens are trophic to the prostate gland.
The only animal with any frequency of prostatic disease is the dog. There are three main disorders and their prevalence is, in descending order: hyperplasia (Fig. 19-29), neoplasia (Fig. 19-30), and prostatitis (Fig. 19-31). These disorders are discussed in the section on Disorders of Dogs and Cats.
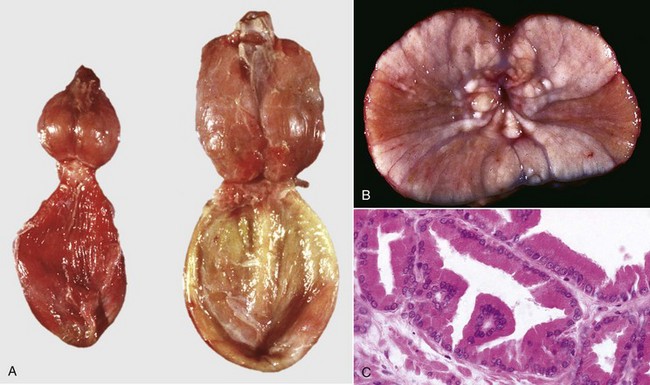
Fig. 19-29 Prostatic hyperplasia, prostate, dog.
A, Prostates of two dogs of different ages (oldest on the right [age-related growth]). Both prostates are bilaterally and symmetrically larger than normal postpubertal prostates. B, The lighter white tissues that in some areas bulge from the cut surface are areas of hyperplasia. A hyperplastic prostate is symmetrically enlarged and detectable on rectal palpation, ultrasonography, or by gross examination at necropsy. C, The prostatic acini are larger than normal, as the epithelium is hyperplastic and the cells enlarged (hypertrophy). Note the abundant granular apical cytoplasm and the uniform size and shape of the cells. Mitotic activity is very low. H&E stain. (A courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph. B courtesy Department of Veterinary Biosciences, The Ohio State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Dr. R.K. Myers, College of Veterinary Medicine, Iowa State University.)
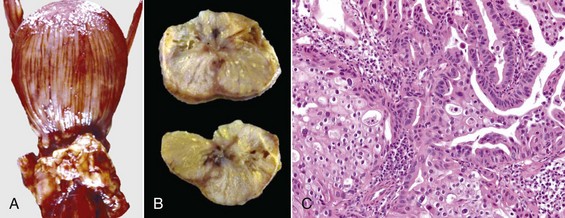
Fig. 19-30 Carcinoma, prostate, dog.
A, The adjacent pelvic tissues of this dog contain many coalescing nodules of metastatic carcinoma that have spread from the prostate, which is barely recognizable beneath the bladder (opened, upper half of image). Prostatic carcinomas are usually asymmetric and lobulated white-to-gray masses that expand the size of the gland and may compress the urethra (dysuria) and the colon (difficulty defecating, ribbon stools). B, Cross-section. Note the asymmetric enlargement. The focal white areas are regions of necrosis within a gland that is enlarged by neoplastic epithelial cells that induce the abundant fibrous tissue that is also present. C, Note the anaplastic prostatic epithelial cells arranged in acini (upper right) and solid lobules (lower left). Mitoses can be frequent in some cases. There also may be stromal or lymphatic invasion and desmoplasia (scirrhous response). H&E stain. (A courtesy Dr. M. Howard, College of Veterinary Medicine, Iowa State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. J. A. Ramos-Vara, College of Veterinary Medicine, Michigan State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
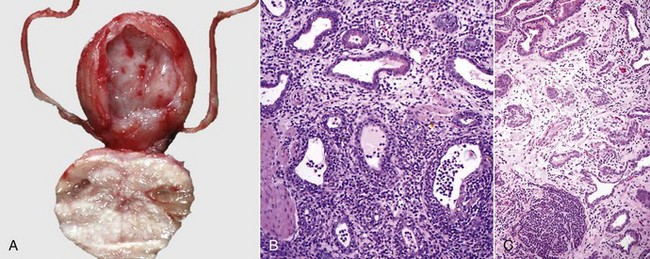
Fig. 19-31 Prostatitis, prostate, dog.
A, Acute prostatitis, cut surface. The prostate is enlarged with edema, and there are many white foci of inflammatory cells instead of the usually smooth red surface. Clinically, this condition is usually painful. B, Acute prostatitis. Note that the glands and interstitium contain large numbers of neutrophils. Most of these infections are of bacterial origin and develop after bacteria ascend the urethra. H&E stain. C, Chronic prostatitis. Note that the glands and interstitium contain large numbers of lymphocytes and macrophages and a large lymphoid nodule (bottom half of image). Follicular lymphoid hyperplasia is a common finding in chronically infected tissue. The abundant interstitial stroma is from fibroplasia and chronic inflammation. Most cases of chronic prostatitis have a bacterial origin. H&E stain. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Bulbourethral Glands
The bulbourethral gland, as with the prostate, is derived from the urogenital sinus. There are few lesions of the bulbourethral glands, no doubt because of their dense structure and lack of lumina to allow bacterial growth and persistence. The major disease of this gland is seen in castrated male sheep grazing clover pasture with a high estrogen content. The glands of these wethers become so large that they cause perineal swellings. The glands have massive hypertrophy, squamous metaplasia, and cyst formation.
Disorders of the Penis and Prepuce
Disorders of the penis and prepuce are relatively rare, yet infection is common and extremely important for food-producing animals. Many of the venereally transmitted organisms, such as Tritrichomonas foetus, Campylobacter fetus, herpesviruses, and papillomaviruses, are present in the prepuce and do not cause severe disease or have a mild and self-limiting course. The penis is protected from trauma and drying by the prepuce and this environment is permissive of many infections. Only some induce an immune reaction and/or inflammation.
Phimosis is the inability to extrude the penis, paraphimosis is the inability to retract the penis into the prepuce, and priapism is a persistent erection; all are seen occasionally.
Developmental Anomalies
Persistent frenulum is a band of tissue between the ventral raphe of the penis and the prepuce. The raphe of the penis and prepuce are remnants of the frenulum, a thin membrane ventral to the penis that normally ruptures at puberty, probably as the result of simple mechanical forces. Balanopreputial bands are similar but are located elsewhere on the penis. They are relatively common and minor anatomic abnormalities rather than serious defects, but they can have an important deleterious effect in limiting the extent to which the penis can be protruded from the sheath and in causing the erect penis to be curved instead of straight (Fig. 19-32). Persistent frenulum is important in bulls and boars. Judging by the frequent occurrence of large flaps and tags of tissue on the ventral raphe of the penis, transitory persistence of the frenulum is quite common.
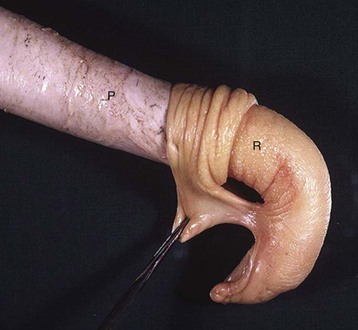
Fig. 19-32 Persistent frenulum, penis, bull.
A tag of tissue (forceps), a frenulum remnant, connects the ventral surface of the penis to the prepuce (R) at its reflection from the penis and has caused the penis (P) to grow curved ventrally as the animal has matured, thus making intromission impossible. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
The penis is subject to many abnormalities of size and form, such as congenital absence, hypoplasia, duplication, directional deviations, and in ruminants, absence of the sigmoid flexure and abnormal locations of the insertions of the retractor penis muscles. None of these lesions is common.
Hypospadias and epispadias are malformations of the urethral canal that create abnormal openings of the urethra on the ventral surface of the penis (hypospadias) or on the dorsal surface (epispadias). In hypospadias, the urinary opening is anywhere from the head to the shaft of the penis, penoscrotal junction, or the perineum. Their importance is in the potential for causing urinary obstruction and in interference with normal insemination.
Hemorrhage and Penile Hematoma
The penis is a highly vascular structure, and the presence of erectile tissue and high pressures during erection and coitus make the potential for severe or even fatal hemorrhage high (Fig. 19-33). Trauma to the penis is usually responsible; cuts, lacerations, and surgical incisions bleed profusely. Forced deviation of the penis in ruminants causes rupture and hemorrhage (see later) that can be fatal.
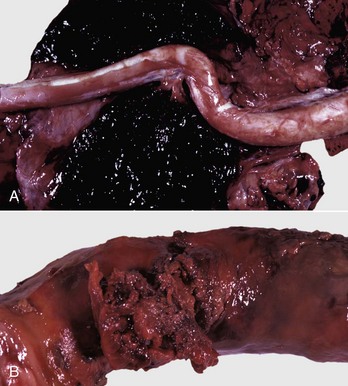
Fig. 19-33 Penile hematoma, penis, bull.
A, The large hemorrhage around the penis at the sigmoid flexure is a hematoma from rupture of the penis during forced deviation. B, Illustrated is the site of rupture of the penis with a blood clot filling the rupture site. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Inflammation
Inflammation of the penis is phallitis, of the head (glans) of the penis is balanitis, and of the prepuce is posthitis. Inflammation of the penis and prepuce (phaloposthitis or balanoposthitis) occurs mostly in castrated animals. This could be the result of alterations to structure because of a lack of testosterone and/or normal development and the tendency of these animals to urinate within their prepuce. Retention of urine in the preputial cavity creates an environment for the overgrowth of bacteria. Those that produce urease split urea to ammonia, a toxic molecule that damages the preputial epithelium, causing erosion and ulceration. Ovine posthitis (see later) is a classic disease.
Nonspecific posthitis occurs in all species and in the gelding, is believed to occur because of a lack of extrusion of the penis with a resultant buildup of the waxy smegma and bacterial overgrowth. A foul-smelling prepuce and posthitis is the result. Dogs commonly develop a nonspecific and purulent discharge of the prepuce.
Herpesviruses cause a multifocal phaloposthitis in several species. Equine herpesvirus 3 is the cause of equine coital exanthema, a disease of stallions and mares with a similarly short clinical course, but with larger (15 mm) ulcers with a predilection for the body rather than the head of the penis. Bovine herpesvirus 1 (BoHV-1) causes a phaloposthitis in the bull, progressing over the course of a few days from hyperemia and swelling to vesicles and pustules and then 1- to 2-mm ulcers, especially of the head of the penis (Fig. 19-34). In the vesiculopustular stage, intranuclear inclusion bodies are present briefly in epithelial cells of the penis and prepuce. Ulcerative balanoposthitis with acidophilic intranuclear inclusions has been observed in goats and is considered a result of caprine herpesvirus infection. Canine herpesvirus reportedly causes inflammation at the base of the penis and the reflection of the prepuce but does not cause the formation of pustules or ulcers. Resolution of these lesions in the affected species is rapid, leaving only hyperplastic mucosal lymphoid nodules and small areas of depigmented mucosa (see Chapter 18 for a detailed description). BoHV-1, suid herpesvirus 1 (pseudorabies virus) in pigs, caprine herpesvirus 1, and canine herpesvirus can persist in a latent state, capable of becoming reactivated by stress or treatment with corticosteroids.
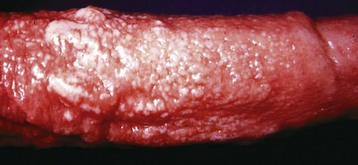
Fig. 19-34 Phaloposthitis, bovine herpesvirus 1, penis (free part), bull.
Note the vesicles and pustules of the mucosa. Bovine herpesvirus 1 causes hyperemia, swelling, vesicles, pustules, and 1- to 2-mm ulcers, especially of the prepuce. Intranuclear inclusion bodies are present microscopically, briefly in epithelial cells of the penis and prepuce during the vesiculopustular stage. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois; and Dr. J. King, College of Veterinary Medicine, Cornell University.)
Other organisms are capable of causing phaloposthitis. These include the larvae of Habronema spp. in the horse and Strongyloides papillosus in the bull. The well-known bovine venereally transmitted diseases of campylobacteriosis and trichomoniasis cause no lesions or minimal nonspecific lesions of the penis and prepuce, even though they reside there.
Foreign material sometimes is found in the prepuce; bulls, rams, bucks, and tomcats can have matted hair around their penis to form a constricting ring (colloquially called hair ring). This “ring” can cause a posthitis or even avascular penile necrosis. It is assumed the deposition of hair in this location is, at least in ruminants, the result of homosexual activity and the rubbing of the penis on the posterior of other animals. Dogs, especially those of the chondrodystrophic breeds with short legs, impact their prepuce with sand.
All species can have traumatic wounds of a variety of types and severity, resulting from mating injuries. Mating or attempting to mate through fences can cause lacerations of varying severities. Owners attempting to “untie” mating dogs can cause degloving injuries to the penis. Horses with penile laceration can develop exuberant granulation tissue (“proud flesh”) of the penis.
Many species develop preputial prolapse, but it is well known in the bull and is discussed later.
Papillomaviruses affect the penis of many species. Typical “warts” are produced in the dog, sarcoids are seen in horses, and the bull develops fibropapilloma (Fig. 19-35, A and B). The bovine disease is discussed in the section on Disorders of Ruminants.
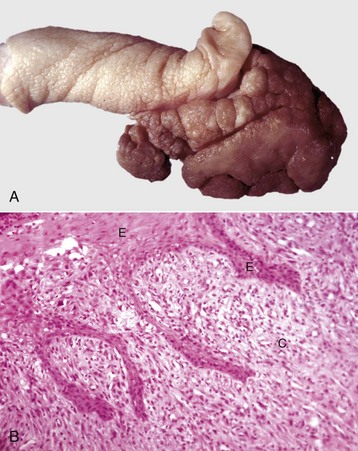
Fig. 19-35 Penile fibropapilloma, glans penis, bull.
A, There is a large exophytic papillary mass protruding from the penile epithelium. This large proliferative lesion on the penis is typical of a fibropapilloma. B, Note the abundant connective tissue (C) covered by hyperplastic stratified squamous epithelium (E) that is thickened and has elongated epidermal-dermal interdigitations that penetrate deeply into the connective tissue. H&E stain. (A courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph. B courtesy Dr. J. Simon, College of Veterinary Medicine, University of Illinois.)
Obstruction of the penile urethra, especially at the sigmoid flexure of all ruminants and the urethral process of small ruminants, is common with urolithiasis (see Chapter 11). The narrowest part of the urethra is the urethral process, and small uroliths that pass through the rest of the urethra become lodged near the tip. Depending on the degree of obstruction, necrosis, and rupture, penile and preputial necrosis can occur. Many animals die of bladder rupture before necrosis of the urethral process or penis occurs.
Neoplasms
Primary neoplasms of the penis and prepuce are mostly restricted to a limited number of types and species affected. Metastatic or multicentric neoplasms affect these tissues rarely. Dogs develop the xenotransplanted canine transmissible venereal tumor (CTVT; see the section on Disorders of Dogs and Cats). Papilloma and squamous cell carcinoma occur in the horse, bull, and dog. Horses develop sarcoids of the penis. The equine disease is described in more detail later.
Disorders of Horses
The most common disorders of horses are listed in Appendix 19-1. The common and important disorders of the scrotum and contents are cryptorchidism (as previously described), testicular and epididymal torsion, scirrhous cord, and inguinal hernia. Disorders of the accessory genital glands are rare. In the penis and prepuce, nonspecific posthitis in geldings, squamous cell carcinoma, habronemiasis, exuberant granulation tissue, and equine sarcoid are common. The disorders of the scrotum and contents and accessory genital glands are as previously described. Disorders of the penis and prepuce are described next.
Penile Squamous Cell Carcinoma
Both stallions and geldings develop squamous cell carcinomas of the glans penis (Fig. 19-36, A). Although exophytic masses do occur, the usual growth habit is infiltrative. These carcinomas induce abundant fibrous tissue, which results in an enlarged firm penis with focal ulcers. Microscopically, the neoplasm is well differentiated with the classic appearance of invasive nests, cords, and nodules of neoplastic epithelium with differentiation toward the stratum spinosum, often with well-developed keratin pearls (Fig. 19-36, B) or keratin squames mixed with neutrophils. The neoplastic cells are surrounded by fibrous tissue, as well as lymphocytes and plasma cells. Metastasis is to the superficial inguinal (scrotal), deep inguinal, and medial iliac lymph nodes. The prepuce often becomes edematous because of lymphatic obstruction, and the preputial cavity becomes distended by retained smegma, inflammatory debris, and urine.
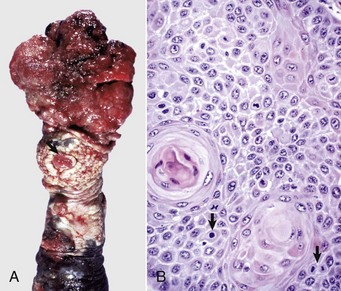
Fig. 19-36 Squamous cell carcinoma, penis, ventral surface, horse (gelding).
A, A very large ulcerated mass protrudes from the glans penis. The urethral opening is visible (arrow). B, Neoplastic squamous epithelial cells are often arranged around “keratin pearls.” Mitoses are frequent (arrows). H&E stain. (A courtesy Dr. R. A. Foster, Ontario Veterinary College, University of Guelph. B courtesy Dr. M. J. Abdy, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Penile Habronemiasis
Habronemiasis is the lesion caused by aberrant migration of the larvae of Habronema muscae and Draschia megastoma, which are deposited by infected flies on wounds on the penis or prepuce of horses. The gross lesion is an ulcerated, often exophytic mass on the penis or prepuce. It has the same appearance as exuberant granulation tissue or equine sarcoid. Microscopically, however, the lesions are multiple distinct nodules and tracts containing larvae and/or are filled with debris and eosinophils. Granulation and fibrous tissue surround the nodules and tracts.
Equine sarcoids and exuberant granulation tissue of the penis or prepuce are identical to their dermal counterparts (see Chapter 17). As with habronemiasis, these form ulcerated proliferative lesions on the penis and prepuce. Histologic evaluation is necessary to separate them.
Disorders of Ruminants (Cattle, Sheep, and Goats)
Disorders of the scrotum and contents are very similar between the bull, ram, and buck. All commonly develop cryptorchidism, testicular hypoplasia, and testicular atrophy/degeneration (described previously). Rams develop epididymitis commonly and varicocele (see next section). For the disorders of the accessory genital glands, vesicular adenitis is especially important in bulls. The disorders of the penis and prepuce of importance in the bull are penile-forced deviation and hematoma and in the ram, ovine posthitis and urolithiasis.
Epididymitis
Infectious epididymitis is most common and important in rams. It occurs in two main ways in rams: hematogenously by Brucella ovis and by ascending infection with bacteria such as Actinobacillus seminis, Histophilus somni, and Escherichia coli. Regardless of the causative agent, the lesion is similar and is dominated grossly and microscopically by swelling and the formation of spermatic granulomas. Macroscopically, the lesions are usually restricted to the tail of the epididymis, regardless of the causative bacterium. The tail of the epididymis is enlarged up to tenfold and is largest when spermatic granulomas form. Microscopically, the duct lumina contain a mixture of spermatozoa, neutrophils, and macrophages and multinucleate foreign body–type giant cells. The epithelium changes from simple columnar and ciliated to pseudostratified columnar and cuboidal with focal hyperplasia. These regions often develop a secondary lumen or intraepithelial lumina. Some of the epithelium becomes stratified squamous in type (squamous metaplasia). The smooth muscle wall of the duct and the interstitium contain many lymphocytes and plasma cells, plus edema and fibrin initially. Fibrous tissue rapidly develops with granulation tissue and eventually mature fibrous tissue (see Fig. 19-21). Interstitial abscesses and spermatic granulomas develop after either death of the tissue or rupture of the duct and development of a spermatocele. These often rupture into the cavity of the vaginal tunics and produce a periorchitis. With time and severity, the tunics become thickened with edema and fibrin deposition, followed by granulation tissue and finally fibrosis.
Varicocele
Varicocele is the local dilation of the spermatic vein in the pampiniform plexus. Older rams are most commonly affected, but the underlying defect is unknown. About half the cases are bilateral, and the unilateral cases are evenly divided between the left and right side. Thrombosis of affected vessels is common. The dilated veins are located near the inguinal ring; the complex distal part of the pampiniform plexus is not affected (see Fig. 19-27). The thrombosed and dilated vessels are up to 10 cm diameter and are so large that they interfere with thermoregulation, either by their sheer size or by interfering with the countercurrent system. Venous flow from the testis could theoretically be restricted and this could subsequently alter testicular oxygen tension and create oxidative stress, thus causing testicular degeneration, poor spermatozoal motility, and immature spermatozoa in the semen.
Vesicular Adenitis
Vesicular adenitis (seminal vesiculitis) is a significant disease because it reduces fertility, particularly in young bulls. Inflammation in the vesicular glands contributes inflammatory cells and mediators to the semen. It also reduces the ability of spermatozoa to survive freezing. Young bulls, in their first breeding season, are especially affected. The cause is most likely infectious; various organisms, including viruses, protozoa, Chlamydophila sp., Ureaplasma diversum, Mycoplasma sp., Brucella abortus, and other bacteria, have been investigated over the years. The pathogenesis is uncertain, but hypotheses include ascending infection, descending infection, hematogenous spread, congenital malformation preventing excretion of fluid and spermatozoa, and reflux of spermatozoa or urine into the glands.
The common form of vesicular adenitis is a chronic interstitial inflammation (see Fig. 19-28, A and B). It begins with an acute form where the glands are swollen with edema, and they are painful on palpation. Microscopically, neutrophils enter the lumen of glands and there is edema and fibrin in the interstitium. In the more chronic and interstitial form, the glands are enlarged and firm and have loss of lobulation. Glandular lumina contain neutrophils and debris, but there are many lymphocytes and plasma cells in the interstitium, and collagen is deposited between the acini (see Fig. 19-28, B). Metaplasia of glandular epithelium to a stratified squamous type is common.
Penile Forced Deviation and Hematoma
Penile hematoma (penile deviation; broken penis) after forced deviation is an important disease in bulls. A similar disease in the ram is reported. During mating, the penis probes for the vulva, and when appropriate, the copulatory thrust and ejaculation is done with great force. If the penis deviates from its intended target, lateral pressure is applied to the region of the sigmoid flexure and insertion of the retractor penis muscles, resulting in rupture and hemorrhage (see Fig. 19-33). The swelling of the hematoma is just cranial to the scrotum, and can be up to 50 cm diameter. In severe cases, hemorrhagic shock occurs. Small hematomas heal without complications, but depending on the extent of the injury and hematoma, granulation and scar tissue is formed and prevents extension of the penis (phimosis). Some hematomas become infected and result in a penile abscess.
Penile Fibropapilloma
Fibropapilloma occurs on the glans penis of young bulls (see Fig. 19-35, A and B). It is caused by bovine papillomavirus type 1 infection, but the pathogenesis is incompletely understood. Affected animals are young, usually in their first breeding season, and the lesions are self-limiting. Larger neoplasms can interfere with breeding by causing pain or by their physical size. Affected bulls can develop an aversion to mating. Macroscopically, the single or multiple warty masses have a papillary epithelial covering and a fibrous core. Surface ulceration is often extensive. They are histologically typical of fibropapillomas elsewhere, with epithelial and stromal hyperplasia and long projections of epithelium into the fibrous tissue. Intranuclear inclusion bodies are seen in some. The proportions of epithelial and fibrous tissue vary greatly from case to case.
Preputial Prolapse
Trauma is part of the pathogenesis of preputial prolapse in bulls. Bulls of the Bos indicus type have a pendulous prepuce, and many have a small or absent preputial retractor muscle. Temporary eversion of the prepuce for urination is common in bulls, but affected bulls have inadequate muscular control. The everted preputial mucosa is injured and becomes swollen with edema and inflammation. The prepuce remains everted (prolapsed), swells further, dries, and is further lacerated, leading to a cycle of injury and inflammation that is permanent. Affected bulls are unable to mate.
Ovine Posthitis
Ovine posthitis (pizzle rot) is a disease of wethers mostly and is caused by urease-producing Corynebacterium renale. When the diet is high in protein and the urine has a high concentration of urea, Corynebacterium renale cause the urea to be broken down to ammonia, which is cytotoxic. This produces ulceration of the prepuce near the orifice. Lack of testosterone (from castration) is also involved because the disease can be prevented by administration of androgens to wethers. If the preputial orifice becomes blocked by swelling, the disease becomes much more severe, spreading beyond the initial small ulcer on the hairless skin of the prepuce to diffusely involve the mucosa, with ulceration of the head of the penis and loss of the urethral process. Scarring and phimosis can result. In severe cases, the preputial orifice becomes blocked and the animals die from urinary retention.
Disorders of Pigs
Boars develop the full range of disorders that affect all species, especially cryptorchidism and testicular atrophy and degeneration. They do have a short list of unique conditions, including scrotal hemangiomas (described previously) and preputial diverticulitis.
Preputial diverticulitis
Boars are the only domesticated animal with a preputial diverticulum; it is located dorsal to the preputial orifice. Inflammation of the preputial diverticulum, preputial diverticulitis, has an unknown pathogenesis. Deflection of the penis into the diverticulum because of malformation or masturbation is suspected, and local infection occurs. Macroscopically, there is swelling above the preputial orifice and foul-smelling fluid and exudate can be expressed instead of the normal clear fluid. Histologically, the epithelium is eroded and covered with fibrin, debris, and neutrophils. Lymphocytes and plasma cells and granulation tissue are located in the wall. The diverticulum is also a location for papillomas to develop.
Disorders of Dogs and Cats
Dogs and cats have reproductive disorders that are common to all species, including cryptorchidism and testicular atrophy/degeneration. Dogs have a high prevalence of testicular neoplasia (described previously). The only accessory genital gland in the dog is the prostate, and dogs develop all of the disorders seen in humans, including prostatic hyperplasia, prostatic and paraprostatic cysts, prostatitis, and prostatic carcinoma. Of the many disorders of the penis and prepuce, canine transmissible venereal tumor is unique and outlined later.
Orchitis and Epididymitis
Infectious orchitis and epididymitis occur most commonly in mature dogs, and not just at puberty. The reason for this is unknown. Most cases are caused by Gram-negative bacteria such as Escherichia coli, and presumably by ascending infection. Orchitis is surprisingly common too, and hematogenous or local spread are both possible. In severe disease, dogs have a large painful and doughy scrotum plus endotoxemia and the systemic response to infection with anorexia, lethargy, and fever. The tail of the epididymis and vaginal tunics are usually affected, but in some cases, a severe necrotizing orchitis also occurs. Dogs will lick and chew at the affected scrotum and create a fistula to the scrotal contents (see Fig. 19-20). Brucella canis infection also causes epididymitis but by the hematogenous route. The lesions in the testis and epididymis (see Fig. 19-18) are identical to those described previously in the discussion of epididymitis in the section on Disorders of Ruminants. Testicular atrophy and degeneration is an invariable consequence of epididymitis, and many dogs also have prostatitis.
Prostate Gland (Prostatic) Hyperplasia
The dog is the only domestic animal species to spontaneously develop prostatic hyperplasia with age (see Fig. 19-29, A and B). The clinical consequence of prostatic enlargement includes constipation from the “ball valve” effect of a large prostate forced into the pelvis during attempted defecation. Although obstruction of the urethra is a feature of prostatic hyperplasia in humans, stenosis of the urethra only occasionally occurs in the canine disease. Enlargement of the prostate is hormone related, but the precise mechanisms are unknown. Enlargement of the gland does not occur in castrated dogs, and removal of androgens by castration of affected dogs causes atrophy. Administration of estrogens causes enlargement of the gland because estrogen and testosterone operate in concert. Estrogen is responsible for the fibromuscular hyperplasia and testosterone is mostly responsible for the epithelial hyperplasia (see Fig. 19-29, C). Enlargement of the gland is usually uniform. On occasion, the hyperplasia is cystic, and in extreme cases, a large single cyst or multiple small cysts are found. Microscopically, there is hyperplasia of the acinar epithelium and individual epithelial cells are larger than normal and their apical cytoplasm is expanded and filled with eosinophilic globules. Stromal hyperplasia causes a larger amount of the interlobular and to a lesser extent the intralobular fibromuscular stroma. Some acini are distended with fluid and have a flattened epithelium.
Estrogen-induced hypertrophy of the canine prostate gland is most often seen with a testicular Sertoli cell tumor. Hyperestrogenism also causes squamous metaplasia of acinar epithelium, ducts, prostatic urethra, and any uterus masculinus (see Fig. 19-26, C). Flattened keratinized epithelial cells are desquamated into the acini, and neutrophils and other inflammatory cells are present. Squamous metaplasia of the prostate gland in dogs is not preneoplastic.
Prostatic and Paraprostatic Cysts
Prostatic and paraprostatic cysts occur periodically in dogs, and their origin is debated. Intraprostatic cysts occur in prostatic hyperplasia, squamous metaplasia, and prostatitis. Paraprostatic cysts are outside the capsule of the prostate. Some are an enlarged cystic uterus masculinus (see Cysts of the Male Tract) and some are serosal inclusion cysts or pseudocysts that do not have an epithelial lining. Many are probably hyperplastic cysts formed when cystic acini extrude through the incomplete muscular layer of the prostatic capsule, in a manner similar to adenomyosis of the uterus. Paraprostatic cysts can attain giant proportions, as large as 30 cm in diameter. Some become infected and abscessed. They have a thin wall, and some have mineralization and ossification of the collagen in the capsule. Microscopically, they have an inner lining of either flattened cuboidal epithelium, cells resembling mesothelium, or granulation and fibrous tissue. They have a thin fibrous capsule. Those with mineralization and ossification have normal woven or lamellar bone within the capsule. Most have no inflammation.
Carcinoma of the Prostate Gland
Canine prostatic carcinoma is the only prostatic neoplasm of importance in domestic animals (see Fig. 19-30, A). A similar disease is exceedingly rare in the cat. The cause is not known, and castration is not protective. Prostatic hyperplasia and metaplasia appear not to precede neoplasia, although hyperplasia and carcinoma are found together in intact dogs. Prostatic intraepithelial neoplasia (PIN) is only found in a high-grade form in prostates that already have carcinoma—there is no recognized low and intermediate grade PIN to suggest progression from one to the next. Some of the clinical signs of carcinoma of the prostate are similar to those of prostatic hyperplasia because of the enlargement of the organ. Carcinoma and its metastases cause cachexia and locomotor abnormalities through pressure and invasion of nearby structures, including the bones of the spine and pelvis. Macroscopically, there are two general appearances. The most obvious is the type that causes asymmetric extreme enlargement (see Fig. 19-30, B). The prostate gland becomes very large and attached to other pelvic structures. The second type is mostly periurethral, with a necrotic and cystic cavity within the prostatic capsule. It causes minimal enlargement of the gland, but urinary obstruction and metastatic disease are present. Castrated dogs are usually affected with this latter form.
Much has been written and said about the prevalence of different types of neoplasia of the prostate. Some authors have divided them into adenocarcinoma and transitional cell carcinoma; the latter presumably arise from the pelvic urethra or from prostatic ducts. There is also an intermediate category of mixed glandular and transitional types. Attempts to accurately phenotype carcinomas by histochemical, immunohistochemical, and molecular methods are unsuccessful because there are no specific markers to separate acinar from urothelial types. This is probably because of the common origin of prostatic ducts and glands and for urothelium in general. Because of this, the term carcinoma of the prostate is a general term used to refer to adenocarcinoma, mixed carcinoma, squamous cell carcinoma, and transitional cell carcinoma when the histologic type is purely glandular, mixed, squamous, or urothelial. The appearance of a carcinoma reflects attempted differentiation toward glandular, urothelial, or epidermal tissues, or mixtures of two or more (see Fig. 19-30, C). There are varying degrees of stroma between the neoplastic epithelial cells. Some also contain sarcomatous tissue.
The prognosis for carcinoma of the prostate is generally poor. About 80% of affected dogs have metastases to a lymph node and/or lung at the time of diagnosis, and 20% of these have metastasized to bone.
Prostatitis
Prostatitis is seen periodically and can be clinically significant if toxemia accompanies the infection or if there is urinary obstruction. Prostatitis is found in older animals, often together with hyperplasia, or in young animals without hyperplasia. Although increased concentrations of zinc in prostatic fluid is associated with antimicrobial properties in humans and dogs, resistance to infection and resolution of infection are not correlated with zinc concentrations in prostatic tissue in the dog. Prostatitis can usefully be divided into acute, chronic, abscess formation, and specific (Brucella canis) forms. Organisms, such as Escherichia coli, Proteus vulgaris, and others, invade from the urethra. The affected organ can be diffusely or focally involved, swollen, red, and edematous (see Fig. 19-31, A). The early microscopic lesion is neutrophilic, and acini contain neutrophils and debris. The degree of necrosis varies and is extensive in some cases. Abscesses can form (see Fig. 19-31, B). The abscesses can persist or be replaced by fibrous tissue. With continued inflammation, lymphocytes and plasma cells are present in the interstitium in large numbers. Usually, fibrosis is an accompanying change (see Fig. 19-31, C). Prostatitis is part of the spectrum of lesions caused by Brucella canis in the dog, and the prostate can be the site of persistence of the bacterium.
Canine Transmissible Venereal Tumor
Canine transmissible venereal tumor (CTVT) is a type of xenotransplantation. Based on immunologic, cytogenetic, and nucleotide sequence studies, the neoplasm is thought to arise from a specific genetic alteration of canine histiocytes, followed by the transmission of abnormal cells from dog to dog via direct contact with the tumor (see Fig. 18-52). The primary neoplasm is usually on the external genitalia, but extragenital primaries and metastases also occur, particularly in stray dogs and those in poor health. The neoplasm is found on the penis, more often on the proximal parts, and not often on the prepuce (Fig. 19-37, A). The neoplasm can be single or multiple and up to 10 cm in diameter, with a red, ulcerated, multinodular surface. Microscopically, the neoplastic cells form a diffuse sheet with minimal stroma. They are large, round or oval, and uniform in size, thus resembling lymphocytes (Fig. 19-37, B). The cytoplasm is pale staining and may have peripheral vacuoles that are readily identified on cytologic preparations. This neoplasm can develop multifocal necrosis and spontaneously regress because of lymphocyte-mediated cytotoxicity.
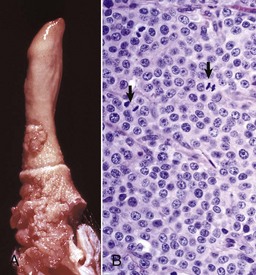
Fig. 19-37 Transmissible venereal tumor, penis, dog.
A, There is a large multinodular mass involving the penis and the prepuce at its reflection from the penis. B, Neoplastic cells are round, uniform in size, and often divided into packets by a fine fibrous stroma. Mitoses are frequent (arrows). H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. M. J. Abdy, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Amann, RP, Veeramachaneni, DNR. Cryptorchidism in common eutherian mammals. Reproduction. 2007;133:541–561.
Cornell, KK, Bostock, DG, Cooley, DM, et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: retrospective analysis of 76 cases. Prostate. 2000;45:173–183.
Fijak, M, Meinhardt, A. The testis in immune privilege. Immunol Reviews. 2006;21:66–81.
Foster, RA, Ladds, PW. The male genital system. In: Maxie G, ed. Jubb, Kennedy and Palmer’s pathology of domestic animals. San Diego: Academic Press, 2007.
Guazzone, VA, Jacobo, P, Theas, MS, et al. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech. 2009;72:620–628.
Hall, SH, Hamil, KG, French, FS. Host defense proteins of the male reproductive tract. J Androl. 2002;23:585–597.
Heindel, JJ, Treinen, KA. Physiology of the male reproductive system: endocrine, paracrine and autocrine regulation. Toxicol Pathol. 1989;17:411–445.
Hughes, IA, Acerini, CL. Factors controlling testis descent. European J Endocrinol. 2008;159:S75–S82.
LeRoy, BE, Northrup, N. Prostate cancer in dogs: comparative and clinical aspects. Vet J. 2009;180:149–162.
McEntee, K. Reproductive pathology of domestic mammals. New York: Academic Press; 1990.
Meyers-Wallen, VN. Review and update: genomic and molecular advances in sex determination and differentiation in small animals. Reprod Dom Anim. 2009;44:40–46.
Russell, LD, Ettlin, RA, Sinha Hikim, AP, et al. Histological and histopathological evaluation of the testis. Clearwater, Fla: Cache River Press; 1990.