Female Reproductive System and Mammary Gland*
The reproductive system is arguably the most important system for the survival of a species. In production animals, reproduction is essential for the continued supply of product, whether it is meat, fiber, milk, or any of the many other by-products. Diseases of the reproductive system have not changed much in the last few decades, but our understanding of many of the processes has progressed dramatically and many of the accepted “dogmas” were challenged and/or modified. In addition, the traditional approach to diseases of the reproductive system focused on specific diseases for which information is known, rather than taking into consideration the overall significance in a clinical setting. In this chapter, each anatomic component or region is examined, and the relative importance of specific diseases or processes is emphasized. The traditional approach also concentrated on bovine and to a lesser extent equine reproductive diseases. In this chapter, diseases of all species, including companion animals, is included in more detail.
Structure
The female reproductive systems of domesticated animals, although diverse in anatomy, share many similarities in structure and function. Embryologic development of the female reproductive tract was thought to be the default when the male reproductive tract did not form, but now we know many unique genes and subsequent processes control it.
Genes initiate the pathways of ovarian differentiation and development. Dax1 is one gene that both promotes ovarian development and inhibits testicular development. In the development of the ovary, the germ cells undergo meiosis and the supporting cells surround the oocytes to become the granulosa and theca cells of the follicles. The differentiation of a female phenotype occurs with the development of the paramesonephric (Müllerian) ducts to form the uterine tube, uterus, and cranial vagina and for the urogenital sinus to develop into the caudal vagina and vulva.
The structural arrangement of the ovary is similar in all species except in the mare. The ovary itself has an outer layer of epithelium, which is of mesothelial origin. The cortex of the ovary contains follicles, stromal connective tissue, and blood vessels. The medulla has large blood vessels, lymphatic vessels, nerves, and loosely arranged connective tissue. Remnants of the mesonephric tubules, called the rete ovarii, are present in this region.
The follicles are where the ova develop and are named according to their stage of development: primordial, primary, secondary, and tertiary types. Each developing follicle has multiple layers of granulosa cells and peripheral theca cells. Ovulation occurs when the follicle ruptures, releasing the ovum and allowing the space to fill with blood and then with luteal cells to form the corpus hemorrhagicum and corpus luteum, respectively. Follicles that do not ovulate become atretic. In addition to these various ovarian structures, cats have interstitial glands that are cells of an endocrine type. The canine ovary has small ingrowths of the ovarian surface that are called subsurface epithelial structures and structures called granulosa cell rests that are aggregates of granulosa cells in a tubular arrangement.
Oogenesis is usually completed by birth. In most species, ovulation occurs through the outer surface of the ovary and the ovum is collected by the infundibulum.
The ovary of the mare differs from the other species in several ways. Equine fetal gonads undergo hypertrophy wherein interstitial endocrine cells, stimulated by equine chorionic gonadotropin (formerly called pregnant mare serum gonadotropin), expand in number and produce an extremely large gonad. These atrophy and disappear before birth. The ovary of the mare has a kidney shape with a depression called the ovulation fossa. The ovum is released from this depression. The follicles in the mare can attain a large size—up to 7 cm. Mares can therefore form a large corpus hemorrhagicum. On occasion, a corpus hemorrhagicum and a corpus luteum can be visible externally as structures that extend outward through the ovulation fossa.
The uterine tube has four regions—the infundibulum, ampulla, isthmus, and uterotubal junction. It is supported by a mesosalpinx. The mesosalpinx of the dog completely surrounds the ovary to form the bursa and has a large amount of fat; a small hole connects the interior aspect to the abdominal cavity. The infundibulum surrounds the ovary of each species, except in the horse where it only covers the ovulation fossa. The uterine tube is where fertilization occurs, and the conceptus then moves into the uterus.
All species have a bicornuate uterus with uterine horns and a uterine body. The uterus of the mare has longitudinal folds. Endometrial cups are present in the endometrium between 37 and 150 days of gestation and are the site of production of equine chorionic gonadotropin, formerly pregnant mare serum gonadotropin (Fig. 18-1). These typically form around the pregnant horn at the bifurcation. Their disappearance is an immune-mediated event. The placenta of the horse is diffuse and microcotyledonary. In ruminants, each uterine horn contains four rows of protuberances that become the caruncles. These may be pigmented in sheep. The placenta of ruminants is cotyledonary. The placenta of the pig is diffuse with small ridges. Dogs and cats have a zonary placenta with marginal hematomas.
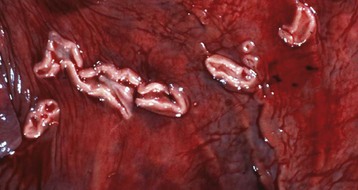
Fig. 18-1 Endometrial cups, uterus, mare.
Endometrial cups are plaque-like structures in the endometrium that form when trophoblasts invade the endometrium early in pregnancy. They are present between 37 and 150 days of pregnancy and secrete equine chorionic gonadotropin. The chorionic surface opposite each cup is called the chorioallantoic pouch and is avillous. (Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
The cervix is the structure that separates the external genitalia from the uterus and is an effective barrier from the external environment. Cervical mucus is viscous except during estrus when it becomes more plentiful and thinner. The cervix in the mare, dog, and cat does not have transverse folds as it does in the ruminants and sows. The cervix of the dog and cat opens on the dorsal aspect of the cranial vagina.
Cell Types
In general terms, much of the reproductive system has a barrier between the external environment and the internal environment that is based on epithelium and on an active mucosal immune system. Superimposed on this arrangement are the modifications that occur with the estrous cycle and with pregnancy.
The cell types of the ovary include the epithelium (surface epithelium, subsurface epithelial structures of the bitch, and the rete ovarii), the stroma, germ cells, and follicular cells. Lymphoid cells are usually absent. Control of ovarian function is from the hypothalamus and pituitary through release of gonadotropin-releasing hormone (GnRH), as well as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (see Fig. 12-3).
The uterus is a unique environment that is separated from the external environment by the cervix. Because it is designed to protect and nourish the fetus, there are some unique features of the endometrium. The endometrium has an epithelial lining of columnar and sometimes ciliated cells. There is also a stromal component of the endometrium. Inflammatory and immune cells are present, particularly during estrus, when the uterus is open to the external environment and to spermatozoa or semen (horses and pigs have intrauterine insemination). Mild inflammation is a “normal” part of the estrous cycle, especially in mares after breeding.
The “external” component of the female reproductive tract, which is the vulva, vagina, and part of the cervix, is lined by a stratified squamous epithelium that varies with the stage of cycle. This is best illustrated in the bitch and queen, in which vaginal cytology is a practical guide to determine the stage of cycle. During anestrus, the epithelium is predominantly of a basal type, with a large nucleus and a small amount of cytoplasm. With the progressive approach of estrus (i.e., during proestrus), the epithelium becomes more mature so that at estrus the majority of cells are superficial epithelial cells with either pyknotic nuclei or no nuclei. Lymphoid follicles beneath the epithelium are a normal part of the distal vagina.
Pregnancy brings about a world of change to the reproductive system. The maintenance of pregnancy and the exchange of nutrients between mother and fetus and the exchange of other products from fetus to mother depend on the interactions of the trophoblasts with the endometrium. During pregnancy, the trophoblasts are directly opposed to and in contact with the endometrial epithelium. In cattle, the maintenance of pregnancy depends on the inhibition of prostaglandin F2α (PGF2α) production in the endometrium so luteolysis does not occur. The trophoblast must avoid rejection by the mother and maintain a barrier yet provide a system for the exchange of nutrients and waste products. There is much to be learned about the enigma that is pregnancy.
Some of the cells that we would ordinarily consider “inflammatory” have specific and individual functions separate from their usual roles. Thus uterine macrophages, natural killer cells, and in some cases, neutrophils have separate and distinct functions. For example, macrophages are important in maintaining the size and shape of bovine caruncles, CD2+ T lymphocytes and natural killer–like cells are important in early pregnancy in pigs, and neutrophils are involved in cervical relaxation at parturition in sheep. Thus the usual function of inflammatory cells can be modified or used by the reproductive tract for specific but otherwise unexpected purposes that result in the maintenance of pregnancy.
Mammary Glands
Mammary Development, Lactation, and Involution
The ventrolateral ectoderm of the embryo becomes the mammary ridge and then the mammary complex. In development, mammary buds push into mesenchyme with their number equaling the number of mammae: mares 2, cows 4, ewes and does 2, sows 14, bitches 10, and queens 8. Sprouts form from each mammary bud, and the number equals the number of papillary ducts (and therefore mammary glands) per mamma: mares 2; cows, ewes, and does 1; sows 2; bitches 8 to 14; and queens 3 to 7. Mammae develop in male embryos, but in domesticated species, they only regress fully in the stallion.
As puberty approaches, there is branching of ducts mediated by prolactin, growth hormone, insulin-like growth factors, and many other factors. There is an intimate interaction between the mesenchyme and epithelium in the formation of ducts and alveoli. Mammary development is maximal at the onset of lactation. Milk flows from alveoli through the lactiferous ducts to a lactiferous sinus (in large animals) and with suckling, through the papillary duct or ducts.
There is variation between the species in the amount of regression that occurs when milking ceases. All species reduce the area of secretary epithelium and increase the relative amount of stroma of the gland. When secretion ceases completely, mammary fluid is resorbed. Bovine mammary glands do not regress as much as in other species, and they complete involution in about 2 weeks. Ewes take about 4 weeks to involute. Leukocytes, especially macrophages, infiltrate the involuting gland.
Cell Types
The mammary glands are sequestered from the outside world by the gatekeeper functions of the sphincter of the mammary papilla (teat) and the papillary duct and at least in the ruminant, its lining of keratinized squamous epithelium. The lactiferous sinus and ducts are lined by columnar epithelial cells, and the alveoli have the secretory epithelium. Secretion of milk components occurs in the alveoli. Mammary epithelial cells, specifically the alveolar cells, have receptors for immunoglobulin G (IgG); they are present for about 1 week before parturition, but the receptors disappear during lactation. Epithelial cells also allow for the transfer of IgA, which is produced locally in subepithelial plasma cells, into the alveolar lumens.
Lymphoid cells in the normal glands are derived from blood, and there is also homing of lymphocytes from the intestine (the enteromammary link) as part of the common mucosal immune system. Lymphocytes of the cellular immune system are present also but in low numbers.
Function
The overall function of the female reproductive tract is to provide a location for the conception, development, and eventual release of viable offspring. One offspring is sufficient for dairy cows, but the largest number possible is required for other production animals. Each anatomic site has its own particular function.
The function of the ovary is to develop and release an ovum or ova and to produce hormones, such as estrogen and progesterone, that influence animal behavior and affect other organs and tissue to maintain pregnancy.
The uterine tube acts as a transport system and storage site for spermatozoa. It also collects and transports the ovum or ova and provides a location for fertilization. The conceptus is nourished and eventually transported to the uterus for subsequent development.
The uterus provides a sterile and inert environment for the development of the conceptus. Exchange of nutrients and trophic factors, immunologic components such as immunoglobulin molecules, and waste products is a major function. This is achieved via placental attachment sites that increase the surface area of the interface between maternal and fetal tissue. At the appropriate time, the muscles of the uterus contribute to the release and birth of the developing individual.
The cervix functions as a gatekeeper by holding the products of conception within the uterus until the appointed time. It also provides a seal that prevents organisms and substances from entering the cranial vagina. Its dilation is an important part of parturition.
The vagina and vulva provide a passageway that allows semen to be deposited within an internal site, which protects the spermatozoa from desiccation and excessive contamination with organisms and materials that would excessively irritate or infect the uterus and uterine tube. The vagina also reduces contamination of the cervix, especially during pregnancy. It is also a portal for the fetus at parturition.
Mammary Glands
The mammary glands provide immunity and nutrients to neonates. Milk is the source of many defenses, including antimicrobial, antiinflammatory, and immune-modulating molecules and substances. In the immediate neonatal period, passive transfer of immunoglobulin via colostrum is an important means of providing immediate immunity to offspring of all domestic species. Most domestic species rely on colostrum ingested in the first 24 hours as the sole source of serum immunoglobulin. Dogs and cats are exceptions because there is some transplacental transfer. Components of the cellular immune system, such as lymphocytes and cytokines, are also transferred in milk. After the immediate postnatal period, substances in ingested milk (including immunoglobulin) provide some local protection against intestinal and respiratory pathogens.
The mammary glands of the modern dairy animal are expected to provide much more milk than is necessary for these functions, and the high-producing dairy cow is a special creature, developed to provide a product of good quality with a long shelf-life and modifications to the components of milk to suit consumers. This includes high production with a low somatic cell count.
Responses to Injury
Little is known about the response of the ovary to infection or insults. Observations of neutrophilic and in viral infections, lymphocytic inflammation indicates that the ovary is capable of inflammatory and immune responses as one would expect with other parts of the body. Hyperplasia of the surface epithelium is a common response, just as it is with mesothelium elsewhere.
The uterine tube is such a narrow structure that its function is altered readily with edema, inflammation, and scarring. Exocytosis of neutrophils from blood vessels via the interstitium can be rapid. In sufficient numbers, pus is formed. Local immune responses can develop and result in the presence of lymphocytes, plasma cells, and in some instances, lymphoid follicles. Granulation tissue formation in severe inflammatory conditions leads to scarring, and subsequent obstruction of the tube is followed by an accumulation of fluid (hydrosalpinx) or pus (pyosalpinx).
There are many studies of the response of the uterus to infection. Inflammation varies from mild, in postmating endometritis, to severe, in bacterial metritis and pyometra. In mild acute inflammation of the endometrium (endometritis) of species—apart from the dog and cat—neutrophils and macrophages migrate through the epithelium into the lumen, and the stratum compactum is edematous. This becomes more florid with an increased severity. Neutrophils and necrotic debris accumulate in the uterus until pyometra forms. Lymphocytes and plasma cells accumulate in the stroma of the endometrium. With chronicity and in severe situations, the epithelium becomes squamous, thus squamous metaplasia develops. Necrosis and erosion of the epithelium results in the formation of granulation tissue, and varying degrees of fibrosis are commonplace in severe infectious or inflammatory disease.
The canine endometrium in particular, as well as the feline endometrium, responds with cystic endometrial hyperplasia. Any injury or insult, whether inert foreign material or pathogenic infectious agents, stimulates this response, which occurs in diestrus. The luminal epithelium becomes papillated and can resemble a placental site.
In the vulva and vagina, inflammation and infection of the external part of the reproductive tract results in hyperplasia and keratinization of the stratified squamous epithelium. Exocytosis of inflammatory cells does occur, predominantly with neutrophils. These inflammatory cells do so with some difficulty because of the intercellular junctions between epithelial cells. The inflammatory response is typically lymphocytic and plasmacytic, and these cells can form a thick band of cells beneath the epithelium. Lymphoid follicles often form and give the affected region of the vagina a granular macroscopic appearance.
Reactions of the placenta are stereotyped and rely heavily on the maternal and to a lesser extent, the fetal immune systems. Some species variability is related to the types of placenta and the usual route of infection. The response of the fetus and placenta tends to be relatively rudimentary. Trophoblasts are phagocytic, and they will take up debris, blood, and infectious agents. Reactions of fluid exudation and connective tissue with granulation and fibrosis occur as elsewhere. The reactions of fetal macrophages and neutrophils are less obvious than is seen where maternal leukocytes are readily accessible. Placentitis occurs when there is sufficient time for a response; fetal loss can be rapid with fetal distress, and there can be insufficient time to mount an effective response. Chronic lesions are much more obvious and especially occur in ruminants. The cotyledonary placenta means that attachment of the placenta to the endometrium is restricted to a relatively small area in relation to what may appear to be the total placental area, but the extensive papillation of the components of the placentome produces a very large surface area. The intercotyledonary area is a potential space that can accumulate a large volume of exudate. Chronic inflammation in ruminants is common and identified by fibrosis of the placenta. The neutrophils are probably of maternal origin. Lymphoid follicles and plasma cells are a lesser component of the reaction, but lymphocytes do accumulate beneath the layer of trophoblasts and around blood vessels. In the equine placenta, placentitis involves a small area, usually around the cervical star. There is no potential space because of the diffuse microcotyledonary type of placenta, thus exudates and suppuration are much less prominent; chronic placentitis is rare. The inflammatory reaction in the pig, dog, and cat placenta is mild, and chronic placentitis is rare.
Mammary Glands
The mammary glands are usually sterile but can respond rapidly to infection or other irritants. The responses are stereotyped, however. The columnar epithelial cells can become hyperplastic, but the cells are not able to withstand injury to the same extent as stratified squamous epithelium, thus squamous metaplasia is a frequent occurrence when irritants, such as infection or intramammary preparations, are introduced. Necrosis of the epithelium is common in infectious disease, and granulation and scarring of the lining of the ducts and sinus are frequent.
Although the immune system of the normal mammary glands is quiescent, injury quickly results in recruitment of the various elements. Neutrophils and macrophages are rapidly recruited. Humoral and cellular immunity is typically seen in infectious conditions. The presence of large numbers of plasma cells with the development of a local immune system is an invariable component of the immune response in infection. Edema and subsequent fibrosis are part of the reaction to injury. The flow of milk is often halted by injury and exudates, thus the normal involutionary processes that result in resorption of secretion (macrophage and epithelial uptake) also operate.
Portals Of Entry
It is critical that infectious organisms are excluded from the uterus, otherwise fertility or pregnancy can be jeopardized. Portals of entry are listed in Box 18-1. Organisms that cause inflammation of the uterus can enter through the vulva (ascending infection) or can arrive via the blood (hematogenous infection). Reinfection of the external genitalia from nerves is a unique feature of infection with some herpesviruses.
Ascending infections occur at estrus, breeding, and parturition. At estrus, the cervix is open to admit spermatozoa. Contamination of the cranial vagina is very important in determining whether infection of the uterus occurs or not. Conformational and structural changes in the vulva and vagina are also important determinants of infection. These are discussed in more detail later. A subcategory of ascending infection is infection of semen by infectious agents. There are many agents, including bacteria, viruses, protozoa, and Ureaplasma and Mycoplasma spp., which can be acquired in this fashion. These are discussed further in the discussion on disorders of the uterus in the section on disorders of the Female Reproductive System. Ascending infection is the major portal by which the equine placenta becomes infected with bacteria or fungi. The cervix in the mare is “loose,” attains a much larger diameter, and can be readily opened with digital pressure. It is virtually impossible to penetrate the cervix of other species with a probe without creating severe trauma. Infection of the uterus and/or placenta by the ascending route with Streptococcus zooepidemicus is common in the mare, but most ascending infections in other species include a mixture of bacteria. This is particularly the case with postpartum infection.
Hematogenous infections are less common and are usually involved in specific infections, such as in brucellosis, salmonellosis, pestivirus, and herpesvirus infections, and they usually occur during pregnancy. Many of the fungal infections of the placenta occur via the hematogenous route.
There are some instances in which infections appear to descend from the ovary through the uterine tube. Some viral, chlamydial, and Ureaplasma infections can be descending.
Transaxonal infection of the distal reproductive tract occurs with some herpesviruses, where stressful events, such as parturition, cause a recrudescence. Neonates can be exposed and infected via this route, but clinical disease in the mother is unusual.
Mammary Glands
Portals of entry are listed in Box 18-2. Most infectious agents and foreign material (intramammary preparations) enter the gland in an ascending fashion via the papillary duct. Small (bacteria) and large (leaches) pathogens can enter the gland via this route. There are some instances in which organisms home to the mammary glands from systemic infection, but their number is small. Viruses, such as the retroviruses of caprine arthritis and encephalitis, ovine maedi-visna, and Mycoplasma spp., are good examples. Penetrating injury is rare.
Defense Mechanisms
Innate, nonimmune, or physical factors are very important in the defense of the reproductive system. An adaptive immune system occurs after these have failed. In many instances, failure of the physical factors that result in infection of the tract is too late for the fetus, and infertility and failure of pregnancy occurs. Defense mechanisms are listed in Box 18-3.
Innate Immunity
The reproductive tract requires a defense system that provides a sterile environment for the fetus but allows entry of antigenic and infectious materials (semen). It does this by providing specialized epithelium in the “contaminated” environment of the vulva and vagina, and then has a specialized structure, the cervix, to exclude most agents from the upper “sterile” regions. Vaginal epithelium has intercellular tight junctions that inhibit the transepithelial migration of agents and molecules, and it has molecules that detect pathogen-associated molecular patterns. These pattern recognition receptors, including Toll-like receptors and defensins, exclude or direct actions against many pathogens.
The anatomy and integrity of the cervix is very important in the exclusion of infectious agents from the uterus. Contamination of the cranial vagina is an additional factor, and conformation of the external genitalia is the physical factor that has received the most attention in relation to uterine infections. In older multiparous mares, the vulva is frequently higher than the floor of the pelvic canal and tends to become horizontal; air and contaminants, including feces, are sucked in or allowed entry into the vagina or even into the uterus. Urine can pool in the vagina of mares with defective function of the vestibule and vulval muscles. When contamination and pooling of urine occur, the vestibule and vagina become inflamed. Subsequently, the cervix and uterus also become affected, either from direct contact with environmental organisms or from local spread of the inflammation.
Muscular contractions of the uterus and gravitational drainage of secretions (mucus, lochia) from the uterus and vagina are physical factors that can flush out infectious organisms. Congenital malformations and anomalies, such as persistent hymen, can reduce outflow and increase pooling in the vagina and uterus. The altered environment of the vagina in spayed, obese bitches can predispose the vagina and vulva to infection.
After infection or irritation from semen, changes include hyperemia and edema of the uterine wall and lumen. These have the theoretical effect of diluting infectious or irritant substances. Recruitment of neutrophils from the blood occurs in response to chemotactic substances released by bacteria, complement, and inflammatory mediators from endometrium and leukocytes. Attracted neutrophils infiltrate the endometrium, enter the uterine lumen, and contribute additional amounts of inflammatory mediators, providing additional chemotactic stimuli. Complement activation, directly by bacteria via the alternate pathway or by specific antibody via the classical pathway, can eliminate bacteria, either by lysis after attack on their membranes or by phagocytosis enhanced by opsonization.
Adaptive Immunity
The reproductive tract is a unique environment because it must respond adequately to challenge from pathogens, yet tolerate the allogeneic spermatozoa and fetus. Adaptive immune responses, whether humoral or cellular, have to be carefully controlled. Control is achieved by differing cytokine expression of epithelial cells and their effects on regulatory T lymphocytes that make “decisions” as to the type and extent of the response. There is likely to be variation in the response, depending on the location. The response in the “sterile” compartments of the uterus and uterine tube are different from that of the “nonsterile” vagina and ectocervix.
Although the upper reproductive tract is part of the common mucosal immune system, it differs from intestinal and bronchial tissues because it does not have mucosal-associated lymphoid tissue (MALT) analogous to Peyer’s patches. This difference occurs because of the lack of continuous antigenic stimulation. Lymphoid follicles, however, are present in the vulva and distal vagina. The uterus also has the added potential influence of hormones to modify responses.
T lymphocytes are critical in determining whether the appropriate response is predominantly humoral or cell mediated. CD8+ T lymphocytes are the most common lymphocytes of the luminal endometrial epithelium and stroma (stratum compactum especially), although there is variation, depending on the location in the uterus. For example, CD4+ lymphocytes are more common in the horns of the uterus of mares, whereas CD8+ lymphocytes are more numerous in the body.
Little is known of the T lymphocyte and cell-mediated immune system of the reproductive tract; much more is known about its humoral immune system. It is generally believed that locally produced antibodies are more important in those diseases that are acquired by ascending infection, for example, Tritrichomonas foetus in cattle, whereas systemic immunity is more important in systemically or hematogenously acquired infections, such as Brucella sp. infection. The immunoglobulin isotype (IgA or IgG) and subisotype (IgG1 or IgG2) that is found in an individual infection is unique to that infection. The generalization that IgA is the main mucosal antibody is not always the case. The response to specific infectious agents is not uniform, and there are differing responses between species. Protection against Tritrichomonas foetus in cattle, for example, is mostly by IgG1. Local transfer of immunoglobulin occurs at all levels of the tract.
Leakage of serum into the uterine lumen from an inflamed endometrium also contributes to the antibody content of the uterine fluid. Opsonization of bacteria by antibodies, especially IgG, promotes more efficient phagocytosis by neutrophils and macrophages; thus they enhance the innate cellular response by phagocytes.
The influence of the estrous cycle on antibodies in the uterus is controversial, but data suggest that concentrations of luminal immunoglobulins and immunoglobulin-containing cells in the endometrium are not influenced by the stage of the estrous cycle.
Locally produced IgA interferes with attachment of bacteria to mucosal surfaces and can activate complement via the alternate pathway. It is not directly bactericidal and acts neither as an opsonin nor as a macrophage activator. Variations occur among species on the position in the tract where the concentration of IgA is greatest, but the sites correspond to the site of semen deposition (uterus in mares, vagina in cows).
Hormonal Influences on Innate and Adaptive Immunity
Infections of the uterus are more easily overcome at estrus than at other stages of the cycle. This increased response at estrus is probably a result at least in part to better drainage through an open cervix. Both estrogen and progesterone affect neutrophil and lymphocyte function, but there is variation in results obtained when the effects of hormones were studied. In some species, such as the mouse, estrogen induces an influx of neutrophils and macrophages (at estrus). Estrogen can also be involved in the upregulation of subsets of T lymphocytes. There is variation in the numbers of lymphocytes in the reproductive tract during the estrous cycle. Even so, there is evidence that CD4+ lymphocytes increase in number with increases in estrogen concentration. Progesterone, which dominates during the luteal phase and pregnancy, antagonizes the “proinflammatory” activity of estrogen. Studies in cattle and sheep indicate an upregulation of T and B lymphocyte responses during the follicular phase of the estrous cycle when estrogen dominates. Major histocompatibility complex II (MHC II) expression is enhanced at estrus in direct relationship with rising estrogen concentration. The influence of the estrous cycle on antibodies in the uterus is controversial, but data suggest that concentrations of luminal immunoglobulins and immunoglobulin-containing cells in the endometrium are not influenced by the stage of the estrous cycle. Generally, the uterus is more susceptible to infection during the progestational or luteal phase of the estrous cycle and during pregnancy. The nonpregnant uterus is highly resistant to infection. The mechanisms involved in the effect of sex hormones on neutrophils are unknown, and receptors for sex hormones have not been demonstrated in them.
Prostaglandins are normally produced by the epithelium of the endometrium. In most species (excluding the dog, cat, and primates), prostaglandins are responsible for lysis of the corpus luteum. In acute inflammation, prostaglandin production by the endometrium is increased, and lysis of the corpus luteum occurs. When there is epithelial and mucosal surface loss, production of prostaglandins is decreased, the corpus luteum persists, and the more susceptible progestational uterine environment is maintained.
Mammary Glands
As with the body in general, the mammary glands have a full range of mechanisms to prevent and control infectious disease. It relies heavily on isolation. The structure and function of the papillary duct of the teat and the keratin that accumulates there to form a plug prevent many potential pathogens from entering the gland. Secretions of the gland contain antimicrobial, antiinflammatory, and immune-modulating substances. Within the gland, there also are humoral and cellular defenses. Innate defense mechanisms are listed in Box 18-4.
Innate Immunity
Physical factors are very important in the resistance to infection. The teat orifice, with its sphincter, and the papillary duct offer mechanical resistance to the entry of organisms. The keratin and waxy nature of the inner aspect of the papillary duct can be protective by having bactericidal fatty acids, by adsorbing bacteria and desquamating when coated with bacteria, and by desiccating. Delays in the formation of the keratin plug at drying off or cracks in the teat ends increase the risk of intramammary infection. Regular milking of the lactating mammary glands probably is a natural defense mechanism because of the flushing of organisms and products of inflammation from the gland.
Soluble factors are numerous and contribute to resistance to infection. Lactoferrin, the major iron-binding protein of saliva and milk, is a nonspecific natural protective factor in milk. Mammary epithelial cells produce the bulk of lactoferrin in milk. Lactoferrin concentration is increased in acute mastitis and in the involuting gland. The binding of iron withholds this essential nutrient from pathogenic bacteria and thus has a bacteriostatic effect. The lactoperoxidase-thiocyanate-H2O2 system temporarily inhibits some streptococci and coliforms and Staphylococcus aureus. Lactoperoxidase is synthesized by mammary epithelium, thiocyanate is derived from certain green feeds, and H2O2 is produced by enzymic constituents of milk by streptococci or from an exogenous source. Hypothiocyanite produced by the lactoperoxidase system damages the inner bacterial membrane, killing the bacteria. Lysozyme, synthesized locally or from blood, destroys bacteria by lysis of cell wall peptidoglycan. Complement activated in mastitis by the alternate pathway in response to the presence of bacterial endotoxin can be important in bactericidal activity, opsonization, and promoting inflammation. Normal milk is antiinflammatory. Cytokines have a broad range of immunomodulation. Molecules of this general group include the interleukins (IL-2, especially), colony-stimulating factors (CSF), interferons (IFN), and tumor necrosis factor (TNF).
Cells that are not part of the adaptive or acquired immune system include macrophages, neutrophils, and natural killer cells. Macrophages are usually the most numerous leukocyte in mammary secretions. They phagocytose bacteria and act as antigen-presenting cells. Macrophages can be found free in the alveoli and the interstitium, as well as in the lamina propria of the lactiferous sinus and interlobular and intralobular lactiferous ducts. In a lactating cow, at least 500,000 phagocytes per milliliter of milk are necessary for defense of the mammary glands against invading bacteria. In uninfected bovine mammary glands, 50,000 to 200,000 neutrophils and macrophages per milliliter of milk are present, with the macrophages predominating. Lymphocytes represent about 10% of the leukocytes in lactation. Neutrophils are present only in low numbers unless there is bacterial infection or injury, when their influx can be dramatic. Neutrophils play an extremely important role in antibacterial action by phagocytosis and the release of antibacterial substances. Their function is inhibited in the periparturient period. Recruitment can be so rapid that neutrophils are the dominant cells as soon as 2 hours after infection. Cell counts in milk can average 700,000 per milliliter in subclinically infected quarters, and millions of neutrophils per milliliter are common in clinical infections.
Although neutrophils recruited from the blood are important in fighting infection in the mammary glands, they do not kill bacteria as well in milk as they do in blood. Milk seems to be a poor medium for the functioning of neutrophils. Some possible reasons include the absence of glucose in milk for the glycolytic metabolism of neutrophils, decreased amounts of glycogen in milk neutrophils, deficiency of opsonins and complement in milk, coating of the surface of neutrophils with casein, loss of neutrophil pseudopodia caused by phagocytosis of fat, and a decrease of hydrolytic enzymes within neutrophils after phagocytosis of casein and fat.
In experimental staphylococcal mastitis, the numbers of inflammatory cells (mostly neutrophils) in milk cycle up and down every several days, with a corresponding inverse cycling of the number of viable bacteria. When cell counts are at a peak, phagocytosis is optimal and bactericidal activity per cell is most efficient, by as much as 10,000-fold higher. The frequency and periodicity of the cycle, as well as the amplitude of the cell and bacterial counts, are independent for each infected quarter. The likely source of reinfection of the mammary glands is neutrophils that are inefficient at killing phagocytosed bacteria at the time of low cell count. As these cells undergo necrosis and lysis, their previously protected intracellular viable bacteria are released to multiply, and the inverse cycling of neutrophils and bacterial numbers continues.
In the first week after parturition, when neutrophils are most needed to deal with mammary infections, bovine blood neutrophils already are defective before they pass into the mammary glands. They have significantly impaired chemokinesis and decreased superoxide anion production, antibody-dependent cell-mediated cytotoxicity, and phagocytosis of bacteria. The causes are probably some combination of the effects of stress, energy, and protein demands of early lactation and the hormonal fluxes of this stage of the reproductive cycle. In the parturient period, the concentration of glucocorticoids is increased. This means that leukocyte function is less effective, because expression of l-selectin and CD18 on neutrophils are downregulated by glucocorticoids. This downregulates adhesion between neutrophils and vascular endothelium and transendothelial migration of neutrophils. Neutrophils are also important in creating bystander injury when the products of neutrophil granules, such as superoxide anions and enzymes, are released during phagocytosis and with neutrophil destruction.
Natural killer cells use perforin to kill bacteria in a major histocompatibility complex (MHC) independent way and is part of the nonspecific defenses of the gland.
Adaptive Immunity
The humoral immune system operates in the mammary glands in several ways apart from the transfer of immunoglobulin in colostrum and during lactation. Antibody concentration in normal bovine milk is small, about 1 mg/mL, and includes IgA, IgM, IgG1, and IgG2. IgA and IgM are synthesized locally in the stromal tissue of the acini of the mammary glands and may be part of the enteromammary link of the mucosal immune system, whereby lymphocytes from gut-associated lymphoid tissue (GALT) home to the gland. Most IgG is serum derived; IgG1 is selectively transferred into mammary secretion and is the major immunoglobulin class in milk obtained from healthy mammary glands. IgG2 is both serum derived and locally produced by resident plasma cells, especially in inflammation.
Particulate antigens, such as bacteria, stimulate an antibody response in the mammary glands of the cow, whereas soluble antigens do not. In colostrum and in milk from inflamed mammary glands, antibody concentration approaches 50 mg/mL. Early in inflammation, IgG1 and IgG2 opsonize bacteria to enhance phagocytosis by macrophages, but later the importance of IgG2 as an opsonin increases as neutrophils enter the gland. Neutrophils can transport IgG2 to the mammary glands as they move to the site of inflammation. IgM also functions as an opsonin. IgA does not opsonize but could prevent bacterial adherence to epithelium, inhibit bacterial multiplication, neutralize leukocyte-inhibiting bacterial toxins, and agglutinate bacteria. Concentrations of immunoglobulin are reduced in the periparturient period and may contribute to the increased susceptibility of the gland to infection.
Cell-mediated immunity in the gland is stimulated in infectious disease. Interleukins from mammary macrophages stimulate the immune system by activating T and B lymphocytes. Only a few B lymphocytes are present in the normal mammary glands and milk. T lymphocytes in normal mammary tissue and milk of cows and pigs are mostly CD8+ α/β T lymphocytes. The CD4+/CD8+ ratio is <1, which is reversed from the ratio in blood. The mammary glands thus have selective lymphocyte trafficking, favoring CD8+ lymphocytes, which have either cytotoxic or suppressor function. T lymphocytes that activate B lymphocytes, T lymphocytes, and macrophages are underrepresented in normal mammary tissue and milk. CD8+ T lymphocytes are found in the lactiferous duct and alveolar epithelium, whereas the lesser numbers of CD4+ lymphocytes and B lymphocytes are in clusters in the connective tissues. In early lactation, the CD8+ lymphocytes in milk function more as suppressor lymphocytes than cytotoxic lymphocytes, but the situation is reversed in mid and late lactation. Suppressor T lymphocytes control, modulate, or suppress the immune response, and cytotoxic T lymphocytes can act as scavengers, removing damaged mammary cells. CD4+ (T helper [TH]) lymphocytes predominate in goat mammary glands and in mastitis. In response to bacterial infection, an influx of CD4+ T lymphocytes occurs in milk, and these lymphocytes eventually outnumber CD8+ T lymphocytes. During the periparturient period, TH2 lymphocytes (cell-mediated response) secreting IL-4 and IL-10 predominate over TH1 lymphocytes (humoral-mediated response) secreting IL-2 and INF-γ. T lymphocytes may be cytotoxic, and they preferentially migrate to epithelial surfaces and can destroy altered epithelial cells. γ/δ T lymphocytes are present in greater numbers in mammary secretions and parenchyma when compared with blood. The percentage of γ/δ T lymphocytes of mammary parenchymal lymphocytes decreases in the postpartum period, a time of increased susceptibility of the mammary glands to disease, which suggests that γ/δ T lymphocytes can be important in defense against infection.
Disorders Of Domestic Animals (Horses, Ruminants [Cattle, Sheep, And Goats], Pigs, Dogs, And Cats)
Disorders of Sexual Development
An understanding of the development of the reproductive tract of domesticated animals is from studies in multiple species, including humans, laboratory rodents, and pigs. Sequencing of the genomes of animals now makes identification of genes and processes responsible for sexual development easier. Developmental anomalies and neoplasia are better understood, too. There is a pragmatic reason for examining embryology: it is easier to learn the mechanisms of how anomalies occur and to determine their significance rather than memorize every possible disorder.
Normal Sexual Development: Sexual development occurs in three sequential processes: (1) chromosomal sex is established at conception, (2) gonadal sex occurs early in fetal development, and (3) phenotypic sex soon follows. Germ cells migrate from the yolk sac to the genital ridge, and without germ cells the ovaries do not develop, and dysgenesis is the result. The undifferentiated and bipotential gonad acquires germ cells, mesenchymal cells, coelomic epithelial cells, and mesonephric epithelial cells. These form the major cell types in the developed gonad: germ cells; supporting, steroid-producing cells and unspecialized mesenchyme; and epithelium. Before differentiation into male and female, the embryo has a double set of ducts: the mesonephric (Wolffian) ducts and tubules and the paramesonephric (Müllerian) duct (Fig. 18-2). In individuals with a chromosomal sex of XX (female), without the sex-determining region of the Y chromosome (SRY negative), there is activation of genes and gene products so that a normal ovary develops. Development of a testis is inhibited. The tubular genitalia of the female develop from the paramesonephric ducts, and the mesonephric ducts disappear. The paramesonephric ducts are paired and join the urogenital sinus. They fuse to form the cranial vagina and the uterine body. The urogenital sinus forms the vulva and posterior part of the vagina. The external genital tubercle forms the clitoris. All stages of the development of the genitalia are under the control of genes and gene products; the female reproductive system is not a “default” system.
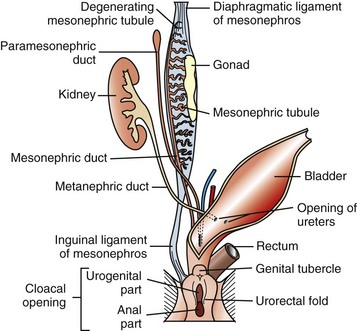
Fig. 18-2 Schematic diagram of the normal components of the female reproductive system and the embryonic structures, especially the paramesonephric (Müllerian) duct and urogenital sinus and tubercle from which they were derived.
The paired paramesonephric ducts fuse to form the body of the uterus, cervix, and cranial vagina. The mesonephric tubules remain as the microscopic rete ovarii, but the mesonephric ducts usually regress completely.
Major Anomalies: Major anomalies are those that result in dramatic abnormalities in sexual phenotype and usually result in infertility. Affected animals are phenotypically male, female, or ambiguous or altered. The exact defect cannot be reliably determined from phenotypic characteristics alone. There are a large number of individual steps involved in sexual development and differentiation, and missing or changing one step can have major effects on subsequent differentiation. Common disorders of sexual development (DSD) are listed in Table 18-1.
Abnormal location and size of the external genitalia creating phenotypic ambiguity is often an indication of a major underlying anomaly. A basic definition of syndromes and identifying the underlying defect and pathogenesis requires an assessment of the sex chromosomes, presence or absence of genes like SRY, gonadal type, and phenotype.
Sex chromosome DSD are those with an abnormal number and/or mixture of sex chromosomes, including XXY (Klinefelter syndrome), X_ (Turner syndrome), and XX/XY (chimerism). XY disorders of sexual development are those with disorders of testicular development, disorders of androgen synthesis or action, and miscellaneous conditions (see Chapter 19). XX DSD includes disorders of ovarian development, androgen excess, or miscellaneous disorders. The greater availability of tests for the SRY gene means a greater ability to define the underlying anomaly. XX disorders are now defined as XX SRY positive and XX SRY negative, and XY disorders are subdivided into XY SRY-positive and XY SRY-negative genotypes.
Gonadal anomalies are found at surgery or necropsy. Histologic assessment is necessary to differentiate between rudimentary gonads (gonadal dysgenesis), testis, ovary, and ovotestis or a combination of both male and female gonadal structures in a single gonad. In phenotypic females, the lack of an estrous cycle, an enlarged clitoris, and/or an increased distance from the anus to vulva may indicate an abnormality. Sex reversal, hermaphroditism, pseudohermaphroditism, and feminization are examples of clinical terms used to describe an abnormality. Descriptions of the phenotypic and gonadal anomalies are often done without the benefit of genotype, but it is preferable to describe the disorder completely as outlined next.
Sex Chromosome Disorders of Sexual Development: True chromosomal DSD is very rare. Cases of X_ (Turner syndrome) and XXY (Klinefelter syndrome) are reported. They usually have gonadal dysgenesis and a feminine phenotype.
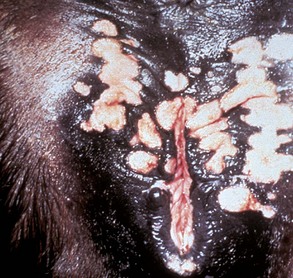
Chimerism is more common. Chimeras and mosaics have two or more somatic cell types, each with a different chromosomal constitution. Chimeras have two genetically distinct cell types that come from different individuals, whereas mosaicism is a different chromosomal constitution from altered mitosis. The most common chimera in domestic animals is the freemartin calf (Fig. 18-3). Vessels of the placentas from two different fetuses fuse and exchange blood between fetuses. Each fetus becomes a hematopoietic chimera. Anastomosis of placental vessels occurs most often in bovine species and less frequently in other ruminants and pigs. The freemartin is the female of a set of male and female twins. Gene products from the cells of the male fetus induce fetal Sertoli cells and seminiferous cordlike structures in the ovaries of the female twin. The ovaries are small and can have reduced number of or no germ cells or organs partially converted to testes. The paramesonephric (Müllerian) duct derivatives vary from almost normal to cordlike structures, but their lumens do not communication with the vagina. The vagina, vestibule, and vulva are hypoplastic. Vesicular glands are always present; other mesonephric (Wolffian) structures are present to varying degrees. Externally, the animal has a female phenotype, but the vestibule and vagina are short, the vulva is hypoplastic, and the clitoris is enlarged. The male twin is minimally affected.
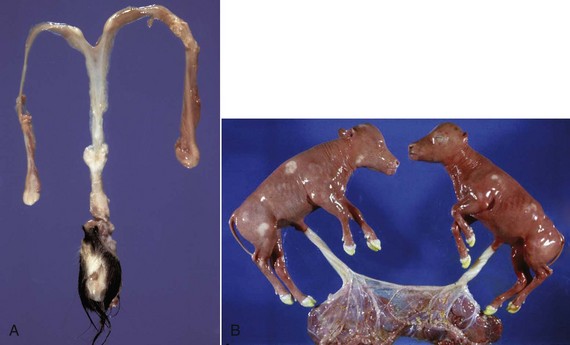
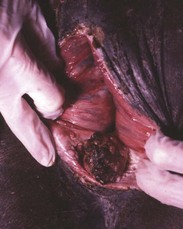
Fig. 18-3 Chromosomal disorder of sexual development, bovine freemartinism, cow.
A, Phenotypically female, reproductive tract, cow. The freemartin is the female of a set of male and female twins. Freemartins are chimeras. There is a vulva and vagina with a prominent clitoris. The internal genitalia consist of bulbourethral and vesicular glands, deferent duct, and short incomplete segments of uterus. The gonads are testis with epididymides attached. This major anomaly renders the cow infertile. B, Placenta, twin fetuses. Placental vascular anastomosis, which allows exchange of blood between fetuses, is a requirement for freemartinism. These anastomoses occur most often in the bovine species. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
XY Disorders of Sexual Development: XY DSD has a normal male chromosomal component (XY) and a female phenotype. They have abnormal gonadal development that drives phenotypic abnormalities, abnormal androgen synthesis, or lack androgen receptors. They are described according to the presence or absence of the SRY gene and the gonadal type. The most extreme example is XY DSD, SRY-positive with testes and female phenotype. These were called male pseudohermaphrodites, testicular feminization, or XY sex reversal (Fig. 18-4). They usually lack androgen receptors. Serum testosterone is present, but the genitalia are female. A mild form occurs in Miniature schnauzers with persistent Müllerian duct syndrome. They are XY males with a complete paramesonephric system, including uterine tube, uterus, and cranial portion of the vagina. They lack the anti-Müllerian hormone (AMH, previously called Müllerian inhibitory substance) or its receptor. XY DSD, SRY negative with gonadal dysgenesis and female phenotype is another category and is found in horses and other species. They have hypoplastic or undifferentiated gonads and a female phenotype.
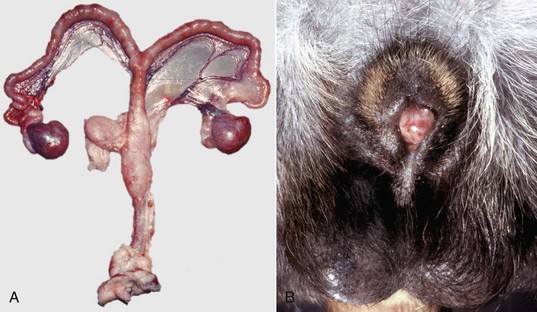
Fig. 18-4 Disorder of sexual development with testis, male pseudohermaphrodite, reproductive tract.
A, Pig. A testis and epididymis are present on each side. Note the well-developed uterus, cervix, and vagina. No ovarian tissue is present. B, Clitoral enlargement, dog. The clitoris protrudes between the labia of the vulva and is visible on the ventral floor of the vulva. Note the formation of a bifid scrotum ventral to the vulva. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
XX Disorders of Sexual Development: The only category of XX DSD reported in animals is XX DSD, SRY negative. Most are XX DSD, SRY negative with ovotestes and a female but ambiguous phenotype. They are usually true hermaphrodites with both male and female gonads (Fig. 18-5). They are phenotypically female, with masculinization, for example, an enlarged clitoris. American cocker spaniels and some other breeds of dog have this autosomal recessive trait. In goats, it is associated with the polled gene. Confirmation of this syndrome requires genotyping because in the case of goats, the presence of mammary development in bucks is not always an indication of an XX DSD.
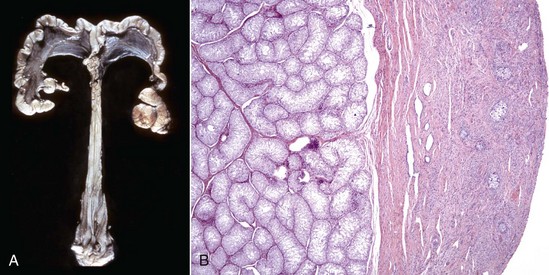
Fig. 18-5 Disorder of sexual development with ovotestis, true hermaphrodite, reproductive tract.
A, Gilt, an ovotestis is on the left and a testis on the right. Note the well-developed uterus, cervix, and vagina. B, Dog, ovotestis, at the periphery (right half of image) is the ovarian component with capsule and stroma. No active follicles are visible. The testicular component contains seminiferous tubules lined by Sertoli cells (left half of image). There is no spermatogenesis in these tubules. H&E stain. (A courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Anomalies with Normal Sexual Development: There are many different anomalies found in individuals with normal genotypic, gonadal, and phenotypic sex. They include failure of the normal maturation, hypoplasia, or aplasia of parts of the internal or external genitalia. Because normal development requires such intricate timing of events, including regression of some parts, the joining of tubes and tubules, migration of components from one site to another, the interaction of genes, and hormones and local factors, it is no wonder there are myriad anomalies.
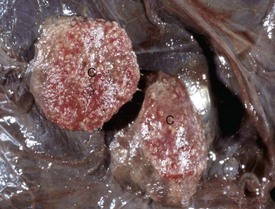
Segmental aplasia of the paramesonephric duct can affect any part and little is known of its pathogenesis. A genetic basis is implicated in shorthorn cattle, in which it is linked to the recessive gene for white coat color. The simplest form is failure of the paramesonephric duct to make a proper connection with the urogenital sinus, leaving a persistent hymen, a membrane at the site where the two precursor tissues join (Fig. 18-6). A perforated hymen sometimes persists and is not clinically significant. If the hymen is complete and there is no drainage of fluid from the uterus, the upstream portion of the vagina, cervix, and uterus distend with normal secretions. In the more severe forms of segmental aplasia, one or more segments of the vagina, cervix, uterine body, and uterine horns are absent or rudimentary. Aplasia of a segment of the uterus (Fig. 18-7) occurs in cattle. Prostaglandin can be synthesized and released from the blind ending uterine horn, just as is produced by a normally connected uterine horn. In those with a local utero-ovarian pathway for luteolysis, such as the cow, the absence of a segment of the uterus could result in insufficient PGF2α to cause regression of the corpus luteum. In the pig, where systemic transmission of PGF2α from the endometrium to the corpus luteum is important, prostaglandins from the blind uterine horn can have a lytic effect on the corpora lutea of pregnancy in the contralateral ovary. In the dog and cat, the uterus does not play a role in the regression of the corpus luteum.
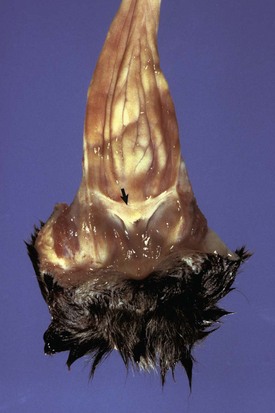
Fig. 18-6 Persistent hymen, vagina, and vulva, bitch.
The membrane (arrow) partially separates the vestibule from the vagina and is just cranial to the urethral opening. This minor anomaly is of little consequence and does not interfere with coitus or parturition. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
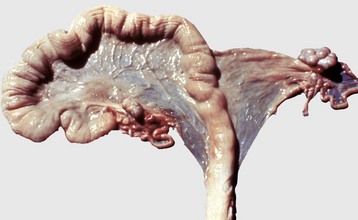
Fig. 18-7 Segmental aplasia of a uterine horn, uterus, pig.
The right uterine horn is completely missing. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Imperfect lateral fusion of the paired paramesonephric ducts results in anomalies. Normally the two ducts unite first at the cloacal end to form the vagina. The fusion moves cranially to form the cervix and the uterine body. Malformations caused by imperfect fusion are most common in and adjacent to the cervix. They range from a dorsoventral fibrous band in the cranial vagina, failure of fusion of the caudal cervix with bifurcation of the cervical canal, to complete duplication of the cervix and body of the uterus (uterus didelphys).
Hypoplasia (and its extreme, aplasia) of a portion of the reproductive tract apart from the tubular genitalia comes in many different degrees. Gonadal hypoplasia is common, especially in males, and these are discussed in the relevant sections.
Minor Anomalies: Minor or incidental anomalies are myriad in the reproductive tract. The same factors influencing the development of major anomalies in animals with normal sexual differentiation also operate here. Minor anomalies are incidental and not clinically significant apart from potentially being mistaken for lesions that do cause infertility, subfertility, or are life threatening. Foremost of these are the numerous cysts and tubular remnants. Periovarian cysts are extremely common and can be confused with cystic neoplasms. They can be derived from paramesonephric ducts, mesonephric ducts, and mesonephric tubules. Web Table 18-1 lists the location and names of common incidental cystic anomalies. They are discussed in more detail later.
Inclusion cysts of the reproductive tract are isolated cysts not derived from embryonic elements. The serosal inclusion cyst of the uterus in bitches is a common type (Fig. 18-8). It arises from a small group of mesothelial cells trapped beneath the serosal surface during involution of the uterus. These grapelike clusters of semitransparent thin-walled cysts are located on the serosal surface of the uterus. Subsequent distention and enlargement result in numerous cysts developing.
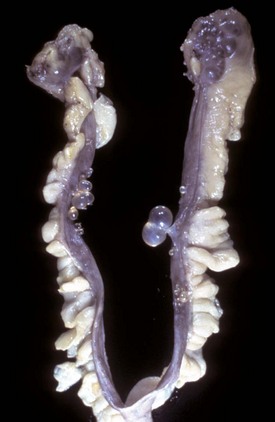
Fig. 18-8 Uterine serosal inclusion cysts, reproductive tract, bitch.
The cysts projecting from the serosal surface of the uterus are believed to arise from mesothelial cells trapped within serosal connective tissue. These cysts are an incidental finding at ovariohysterectomy. Note that there are also multiple thin-walled cysts around the right ovary. These cysts are remnants of embryonal ducts and are called periovarian cysts. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Common Syndromes
Many of the commonly seen syndromes of sexual development are listed in Table 18-1.
Developmental Anomalies: Agenesis, a total lack of ovarian tissue, can involve one or both ovaries. The entire reproductive tract also can be absent. In cases of bilateral agenesis, the tubular genitalia remain infantile.
Duplication of an ovary is a rare anomaly that arises by two different mechanisms: originating separately or splitting of an already developing ovary. The latter type is close to the normally located organ and may be connected to it. This anomaly is an uncommon cause of ovarian remnant syndrome, in which previously but incompletely spayed cats and dogs come into heat (estrus) again.
Hypoplasia of the ovaries occurs most commonly in cows. It occurs in Swedish Highland cattle as an autosomal recessive trait with incomplete penetrance. It is either unilateral or bilateral. The number of primordial follicles and the proportion of Graafian follicles are fewer than normal. Ovarian hypoplasia occurs with chromosomal DSD such as XXX and X_ chromosomes in mares and XXX DSD in cows. It is usually bilateral but not symmetric. The affected ovaries are small and lack follicles or surface scars from ovulation. Microscopically, cortical stroma and ova are absent or poorly developed. The tubular genitalia remain infantile after the expected time of puberty. Other causes of an infantile reproductive tract are malnutrition or other forms of debility. Ovaries in these animals, however, have numerous primordial follicles and can respond to gonadotropic hormones after removal of the debilitating factor.
Vascular hamartomas of the ovary are incidental findings in the mare, cow, and sow. They appear as a dark red mass on the surface of the ovary and consist of connective tissue and vascular channels lined by mature endothelial cells.
Cysts In and Around the Ovary: Periovarian (paraovarian) cysts are cysts that are external to the ovary. They are common findings in dogs and cats during ovariohysterectomy (see Web Table 18-1). Intraovarian cysts are the cysts within the ovary. These should be differentiated from cystic neoplasms (see next section).
Periovarian cysts: Periovarian cysts are usually cystic remnants of embryonic structures, either paramesonephric ducts or mesonephric tubules or ducts. Location of the cyst helps differentiate them. Cystic remnants of the paramesonephric ducts include the fimbrial cyst and the cystic accessory uterine tube. This is common in the mare and called the hydatid of Morgagni (Fig. 18-9). Hydatid of Morgagni measure up to several centimeters in diameter and are cranial to the ovary in the mesovarium. Cystic accessory uterine tubes are in the mesosalphynx. Histologically, they resemble the normal uterus and have a thin coat of muscle. There are cysts that arise from mesonephric remnants, either ducts or tubules. Cysts of the mesonephric duct are in the cranial or caudal mesovarium and histologically have a thick smooth muscle coat.
Intraovarian cysts: Intraovarian cysts are numerous and common. Many are derived from Graafian follicles but others are epithelial cysts from the surface epithelium or from the intraovarian rete ovarii, embryonic structures of mesonephric tubular origin. The most common in the mare is an epithelial inclusion cyst, and the most common in the dog and cat is the cystic rete ovarii (Fig. 18-10).
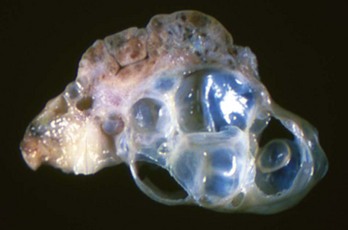
Fig. 18-10 Multiple cystic rete ovarii, ovary, bitch.
Note the multiple cysts (lower right half of image) within the ovary at the hilus. They are incidental findings in bitches and are of little consequence. They develop from the rete (mesonephric tubules) of the ovary and become cystically distended. In cats, they can be unilocular and very large and cause pressure atrophy of the ovary. They must be differentiated from cystadenomas and cystadenocarcinomas, histologically. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Epithelial inclusion cysts in mares can cause infertility (see Web Table 18-1). They are located in the ovary around the ovulation fossa. Epithelium from the surface of the ovary becomes pinched off and embedded in the stroma during ovulation. This epithelium produces fluid that causes the structure to enlarge and eventually reach several centimeters in diameter. They are identical in appearance to large follicles, but they do not appear and disappear as follicles should; histologic assessment is necessary for diagnosis. Their size and number may block ovulation. Epithelial inclusion cysts in other species, or cysts of subsurface epithelial structures in bitches form in a similar manner, but they are in the capsule of the ovary and are small and incidental.
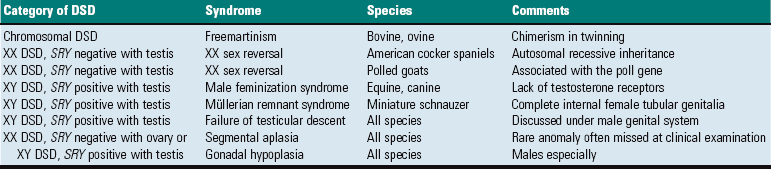
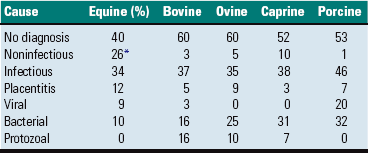
Cystic ovarian (Graafian) follicles, or follicular cysts, are defined as follicles that are larger than normal. They are especially important in cows and sows. The disease in cows is called cystic ovarian degeneration (COD). Bovine cystic follicles are 2.5 cm or more in diameter (Fig. 18-11) and persist for 10 or more days without the formation of a corpus luteum. The prolongation of the postpartum interval to first estrus (so called days-open) is the main consequence of cystic follicles. Ovulation does not occur. These cysts probably develop because of an abnormality of the hypothalamo-hypophyseal-ovarian axis that causes a deficiency of LH or of the LH receptor in the ovary, although the mechanism is not confirmed. Cystic ovarian degeneration is treated with GnRH, which causes release of LH in the pituitary, and with chorionic gonadotropin (high in LH). Evidence suggests that stress is involved wherein adrenocorticotropic hormone (ACTH) or cortisol inhibits GnRH release from the hypothalamus and prevents upregulation of LH receptors in the ovary. Higher concentrations of progesterone can have a similar effect. The end result is an inadequate LH surge and failure of ovulation. In a similar way, postpartum uterine infection can cause cystic follicles. Escherichia coli infection of the uterus increases concentrations of serum PGF2α metabolites and cortisol. It was proposed that bacterial endotoxins, or the prostaglandins produced because of damage caused by endotoxins, stimulate the adrenal cortical secretion of cortisol; cortisol excess suppresses the preovulatory release of LH, resulting in the development of cysts.
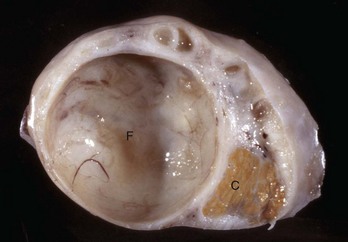
Fig. 18-11 Cystic Graafian follicle, ovary, cow (also called follicular cysts).
Follicular cysts (F) are larger than normal follicles and usually greater than 2.5 cm in diameter. They are the macroscopic lesions of cystic ovarian disease in the cow. They arise when ovulation of a normal follicle does not occur. C, Corpus luteum. (Courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph.)
Luteinized cysts are anovulatory luteinized follicular cysts. They develop from follicular cysts by delayed or insufficient release of LH and are therefore part of COD (see Web Table 18-1). It therefore occurs in cows and sows more often than in other species. Luteinized cells line the cystic cavity. Cystic follicles and luteinized cysts can occur in the same ovary.
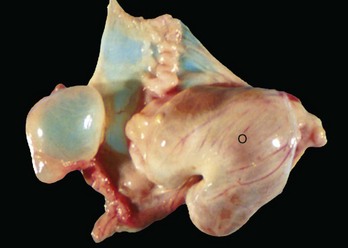
Cystic corpus luteum is a corpus luteum with a cystic center. It is unknown why a follicle fails to luteinize fully. The cystic center is larger than the normal small central cavity that occurs normally in some corpora lutea. Ovulation occurs in a normal follicle, but a large irregular cystic center develops (Fig. 18-12). The length of the estrous cycle is not affected, and most cysts are incidental.
Inflammation of the Ovary: Oophoritis, or inflammation of the ovary, is rare in domestic animals. Infectious bovine rhinotracheitis virus (bovine herpesvirus 1 [BoHV-1]) viremia in experimental studies can induce necrotizing oophoritis in the postestrus cow. A cloudy fibrinous fluid fills some follicles. Microscopically, the lesions in the corpus luteum range from focal necrosis and the presence of mononuclear cells to diffuse hemorrhage and necrosis. Most affected ovaries also have necrotic follicles and mononuclear cells in the stroma. Bovine viral diarrhea (BVD) virus, a vertically and horizontally transmitted virus responsible for mild-to-fatal enteric disease and reproductive failure, can localize in bovine oocytes and cumulus cells and cause chronic oophoritis. Infection of ovarian oocytes with BVD virus is one of several possible routes of transmission of the virus from cow to fetus. Bacterial oophoritis occasionally is found in cats and dogs. In cats, it must be differentiated from feline infectious peritonitis (FIP). The inflammation is around the ovary and within the uterine tube, suggesting that the causative bacteria ascended from the uterus.
Miscellaneous Diseases of the Ovary: Supernumerary follicles occur in bovine ovaries when FSH is used in doses to cause superovulation in preparation for embryo transfer. There may be more than a dozen well-developed ovarian follicles or corpora lutea.
Adhesions between infundibulum and ovary vary from thin bands to large sheets of fibrous tissue binding the walls together. The lesion is often bilateral in cows and results from ascending infection after postpartum metritis. Physical trauma from rectal palpation and manipulation of the ovary is another possible cause; infundibular adhesions are common in beef heifers. Adhesions can obstruct or cause retention of fluids and result in a cystic infundibulum.
A small amount of hemorrhage is normal at the time of ovulation in all species. The mare is an exception because the follicles are large and the cavity of the ovulated follicle fills with blood to form a large corpus hemorrhagicum. In rare cases, the hemorrhage can be extensive enough to form an ovarian hematoma or even hemoperitoneum. If the hematoma extends through the ovulation fossa, the corpus luteum develops external to the ovarian capsule. In the mare and cow, a focal area of serositis is detectable at the point of ovulation. Progression through a fibrinous to a fibrous stage is rapid, and an “ovulation tag” is formed. The manual expression of a persistent corpus luteum in the cow sometimes results in severe periovarian hemorrhage. Organization of the hematoma results in adhesions between the ovary and adjacent structures, such as infundibulum of the uterine tube, and causes infertility. Excessive hemorrhage into follicles is sometimes present in calves, and hemorrhage into cystic follicles occurs occasionally in the bitch.
Atretic follicles are those that become arrested at any stage of development and then regress. In any estrous cycle, only one or a small number of follicles is destined to mature, whereas the others undergo atresia at various stages of maturation. A similar process occurs in seasonal anestrus and in all domestic species during pregnancy, except for the mare. Follicular atresia is considered abnormal when it is a part of any disease process that interferes with the release of or the pituitary response to GnRH. Development of the follicle can be arrested at any stage, and after an undetermined amount of time, it degenerates. The ovum undergoes apoptosis first; then the granulosa cells become pyknotic, vacuolated, and desquamate. The follicle either persists as a cyst with a partial thin lining of granulosa cells or is replaced by macrophages, theca cells, and fibrous connective tissue, eventually becoming a small scar.
Neoplasms: There are three main groups of primary ovarian neoplasms in domesticated animals: germ cell, sex cord stromal, and epithelial neoplasms. Little is known of ovarian carcinogenesis. Neoplasms rarely metastasize to the ovary of domestic mammals.
Germ cell neoplasms: Neoplasms arising from germ cells can differentiate along either embryonic or extraembryonic lines and are benign or malignant. The majority of neoplasms of germ cells are benign and undifferentiated (dysgerminoma) or benign with somatic differentiation (teratoma).
Dysgerminoma is a rare neoplasm of all species. It is usually a white solid friable lobulated mass with areas of hemorrhage and necrosis. It is composed of large round cells with a high nuclear to cytoplasmic ratio and many mitoses. This neoplasm is identical to seminoma in the testis. Metastases are rare.
Ovarian teratomas are rare and usually well differentiated and benign. They have disorganized elements of at least two of the three embryonic germ layers: ectoderm, including neuroepithelium; mesoderm; and endoderm. Skin with hair is often present (Fig. 18-13). Bone, cartilage, nervous tissue, fat, and respiratory epithelium are frequently seen. Malignant teratomas occur less often, and they are usually poorly differentiated with primitive tissue types.
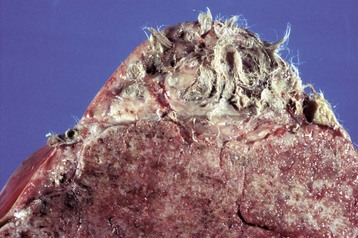
Fig. 18-13 Ovarian teratoma, ovary, bitch.
These tumors have cells that represent all three germ cell lines: ectodermal (epithelium, including neuroepithelium), mesodermal (mesenchymal tissue), and endodermal (intestine and respiratory tissues). The most common tissues seen macroscopically include hair, cartilage, and bone. This teratoma was 30 cm in diameter and surrounded by a bursa, but residual ovarian tissue was not found. Hair (top half of image) and bone are the main structures visible. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Sex cord stromal neoplasms: Sex cord stromal neoplasms have a phenotype that resembles the tissues derived from the sex cords and/follicles. Most have regions with any combination of granulosa cell, theca cell, luteal cell, Sertoli cell, or interstitial endocrine cell phenotypes. Granulosa cell phenotype usually predominates, thus most are called granulosa cell tumors. Most produce estrogens, androgens, and/or inhibin. The mare can have signs of anestrus (inhibin-producing), nymphomania (estrogen-producing), or stallion-like behavior (androgen-producing); the bitch is likely to have prolonged estrus and may develop pyometra.
Granulosa cell tumors: Granulosa cell tumors are the most common ovarian neoplasms reported in large animals. They are unilateral, smooth surfaced, and round and can be 20 to 30 cm in diameter. They can be solid, cystic, or polycystic (Fig. 18-14, A and B). The cysts can vary from microscopic size to several centimeters in diameter. Often, the fluid within the cysts is red-brown. Microscopically, the neoplastic cells resemble normal granulosa cells and often are arranged as they would be in normal Graafian follicles: in single or multiple rows of round to columnar cells lining fluid-filled spaces (Fig. 18-14, C). In less differentiated areas, the neoplastic cells are arranged in sheets. Sometimes, especially in queens, Call-Exner bodies are present. These are rosettes of granulosa cells around a central space. The stroma can be sparse or plentiful. Granulosa cell tumors in the mare and cow are usually benign, are sometimes malignant in the bitch, and are often malignant in the queen; prognostic criteria are not well established.
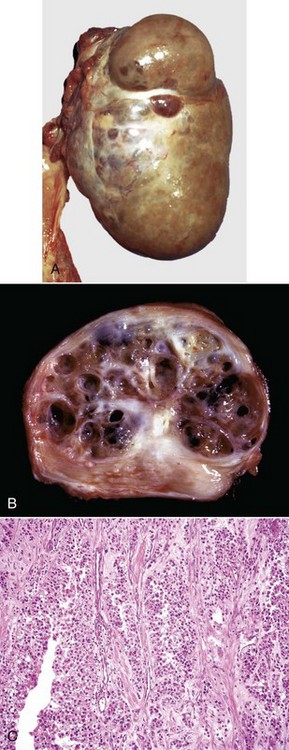
Fig. 18-14 Sex cord stromal neoplasm, granulosa cell tumor, ovary, cow.
A, This large, lobulated neoplasm has obliterated the normal structure of the ovary. They can be solid (as in this case) or multicystic. B, Multiple fluid-filled cysts and solid areas have caused the dramatic ovarian enlargement. Granulosa cell tumors are part of the group of neoplasms known as sex cord stromal tumors. C, This granulosa cell tumor has solid and cystic regions. The cysts are lined by cells that resemble granulosa cells of the follicle. H&E stain. (A courtesy College of Veterinary Medicine, University of Illinois. B and C courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Pure thecomas are sex cord stromal tumors with predominantly thecal differentiation. The cytoplasm of cells in a thecoma can contains lipid droplets, as do the cells of the internal theca. Areas of luteinization in sex cord stromal neoplasms occur, but pure luteomas, neoplasms with a uniform population of luteinized cells, are rare. Sertoli cell, Leydig cell, and interstitial cell tumors are also reported.
Epithelial neoplasms: The ovaries are covered by coelomic epithelium, the same layer of tissue that invaginates in early fetal life to form the lining of the internal tubular genitalia. Neoplasms of the ovarian surface epithelium can thus resemble the several neoplastic types of the endometrium. The cells overlying the ovaries, while contiguous with the mesothelium, are called ovarian epithelium. Neoplasms of the ovarian epithelium are adenoma and carcinoma, and these occur commonly in the bitch (Fig. 18-15, A). In dogs, they originate from the subsurface epithelial structures that are invaginations of the epithelium into the capsule of the ovary. Neoplasms also arise in the rete ovarii of the ovarian hilus but only very rarely. They are often multifocal, not from metastasis but instead from multiple de novo development. They also may be bilateral. Macroscopically, the affected ovary is large and multinodular and has a cystic or shaggy appearance. Histologically, they have a combination of papillary and cystic regions. When predominantly papillary, they are called papillary adenoma or adenocarcinoma, and if mostly cystic, they are called cystadenoma or cystadenocarcinoma. Malignant forms usually spread over the peritoneal surface by both lateral extension and seeding (Fig. 18-15, C), or they metastasize to lymph nodes and other organs. Ascites results from obstruction of the diaphragmatic lymph vessels, which reabsorb peritoneal fluid, and/or from excess fluid secretion by the neoplasm.
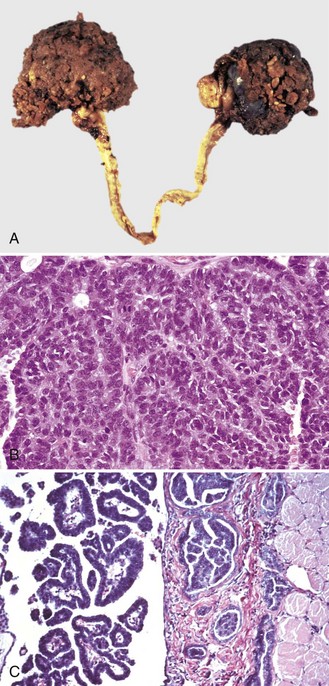
Fig. 18-15 Papillary ovarian carcinoma, ovary, bitch.
A, Both ovaries are enlarged by neoplastic epithelium that has formed papillary structures that give the masses a shaggy outer surface. These neoplasms are often malignant and bilateral, and they seed the abdomen, producing carcinomatosis. B, Neoplastic epithelial cells are arranged in cords and papillae, are pleomorphic, and have many mitoses. H&E stain. C, This papillary carcinoma has seeded the abdominal cavity, implanted on the peritoneum, invaded into the muscle of the diaphragm, and is in subserosal lymphatic vessels adjacent to the diaphragmatic skeletal muscle (right). H&E stain. (A courtesy Dr R.B. Miller, Ontario Veterinary College, University of Guelph. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. C courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Uterine Tubes: Lesions of the uterine tubes are either the result of current or previous infection or are incidental cystic remnants of embryonic ducts.
Salpingitis or inflammation of the uterine tube is the result of infection with bacteria. It accompanies endometritis, metritis, or pyometra in most species, thus it is the result of ascending infection and is often bilateral. Salpingitis is a rare lesion in the dog. Descending infection from the ovary may occur with viral infection. Direct hematogenous infection is possible. Macroscopic lesions are minimal. There may be hyperemia, thickening of the mucosa, and small amounts of luminal exudate. Inflammation of the infundibulum of the uterine tube accompanies perioophoritis. Microscopically, the inflammation ranges from mild to severe and from acute to chronic. Early mild lesions are loss of cilia and desquamation of epithelial cells at the tips of the mucosal folds. When severe, salpingitis involves other parts of the mucosa and sometimes the muscle layer. Exudate is present in the lumen. When adhesions form between the erosions and adjacent mucosa, the tube becomes cystic, reepithelializes, or is replaced by granulation tissue. Obstruction of the uterine tube combined with suppurative inflammation results in pyosalpinx, which is a dilated and pus-filled uterine tube. Neutrophils predominate and form large lakes in the lumen or in mucosal cysts derived from the glands of the tube. As with pyometra, the epithelium is missing or hyperplastic or develops squamous metaplasia.
Hydrosalpinx is a dilated and fluid-filled uterine tube. Obstruction of the uterine tube prevents normal fluid from exiting to the uterus. Obstruction results from previous salpingitis and scaring, from trauma in cattle after aggressive rectal palpation or manual expression of a corpus luteum, ovarian hematoma, or from cystic dilation of embryonic remnants or segmental aplasia. Macroscopically, there is dilation of the tube with clear fluid so that it becomes thin-walled, tortuous and appears to be longer than normal.
Lesions commonly encountered in dogs and cats are duct remnants in the mesosalpinx, more often of the mesonephric duct (simple tubular structures lined by low columnar to cuboidal cells) than paramesonephric duct (lined by a folded mucosa similar to the uterine tube mucosa) origin. Mesonephric and paramesonephric duct remnants are described in the discussion on periovarian cysts in the section on the Ovary.
Ectopic pregnancy of humans occurs when the conceptus develops in the Fallopian (uterine) tube. This is not reported in domesticated mammals. Ectopic pregnancy in dogs and cats occurs with traumatic rupture of the pregnant uterus and release of the fetus into the abdomen.
Inflammation: Uterine disease is a significant cause of infertility and mortality in some cases. Foremost of these are inflammatory diseases, usually the result of contamination of the uterus with bacteria.
Most uterine infections are the result of ascending infection that arises when the cervix is open—at estrus, parturition, or the postpartum period. Infection can descend from the ovary and uterine tube or via the hematogenous route, particularly in pregnancy when the uteroplacental interface is the site for preferential localization of organisms such as Coxiella, Chlamydia, and Ureaplasma spp. It is assumed that the placenta is the target and the endometrium is secondarily affected, but the interface of the two systems is a unique environment that is suitable for many infectious agents to multiply. Resistance of the uterus to infection is influenced by physical factors, the hormonal environment, and the innate and acquired immune mechanisms as discussed previously.
Endometritis is inflammation of the endometrium, usually to seminal fluid and bacterial infection in nonpregnant animals. In cows, herpesvirus infections are also a cause. In pregnancy, organisms that cause placentitis, fetal infection, and failure of pregnancy also cause inflammation of the endometrium. Postpartum endometritis occurs after a normal pregnancy and parturition, but endometritis is especially common and more severe after an abnormal parturition or failure of the uterus to involute. Lochia, the fluid and debris that is discharged from the uterus for a short time after parturition, must be an excellent nutrient medium for bacterial growth.
Mild cases of acute endometritis are not detectible grossly. In more severe cases of acute endometritis, the mucosa is swollen and has a corrugated surface, often with adherent fibrin and necrotic debris. At microscopic examination, neutrophils are found in the endometrial stroma and glands, with a range of surface changes from desquamation of a few surface epithelial cells to severe necrosis of the entire endometrium. Mild lesions resolve completely or incompletely with residual changes of cystic glands and periglandular fibrosis. Severe acute endometritis often becomes chronic, and necrotic endometrium is replaced by granulation tissue and subsequently fibrous tissue. Release of PGF2α in acute endometritis in large animals with 4 or 5 days of progesterone priming causes premature regression of the corpus luteum and shortening of the estrous cycle. The loss of endometrium (which produces PGF2α) in chronic endometritis decreases the amount of PGF2α, especially in the mare and the cow, and results in a persistent corpus luteum.
Persistent mating-induced endometritis is recognized in mares. Affected animals are unable to resolve acute endometritis that follows mating. It is normal for inflammation, presumably because of the local effects of seminal fluid, to last 24 to 36 hours in mares. Those mares with reduced uterine contractility fail to clear this inflammation and accumulate uterine and seminal fluid in the uterus and develop persistent edema. Reduced uterine contractility, which is the result of an intrinsic dysfunction or release of nitric oxide in the endometrium, is proposed as the cause. Greater susceptibility occurs with repeated pregnancies, loss of body condition, and genetics. The position of the uterus also plays a role, and normal mares have a more horizontal uterus that allows better drainage of fluid than the more drooped or vertical orientation of susceptible mares.
In the mare, endometrial biopsy is a breeding management tool because the severity of endometritis is correlated directly with the inability of the uterus to carry a fetus to term. To give a prognosis based on the features of the endometrium, the stage of cycle, number and presence of inflammatory cells, contents of the glands, and frequency and severity of periglandular fibrosis are scored (Fig. 18-16). The endometrium of individual mares is categorized as predicted high, reduced, or low likelihood of the birth of a live foal.
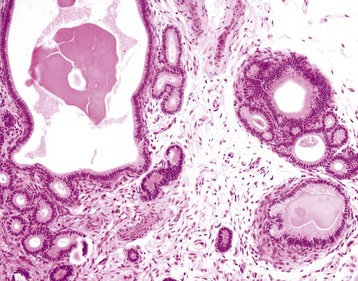
Fig. 18-16 Endometrial fibrosis, endometrial biopsy, mare.
Fibrosis from endometrial inflammation and edema results in endometrial glands forming nests (right) and cysts (left). This fibrosis results in reduced fertility due to a lack of attachment of the conceptus, or a failure of formation of normal microcotyledons and a reduced placental area. H&E stain. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Metritis is inflammation of all layers of the uterine wall (Fig. 18-17). It is a more severe and advanced form of endometritis. In its acute stage, the serosa is dull, finely granular, and has petechial hemorrhages and fine adhering strands of fibrin. Microscopically, there is edema and neutrophils in the endometrium, muscle layers, and the serosa.
Fig. 18-17 Postpartum metritis, uterus, cow.
The uterus is distended and filled with foul-smelling dark brown fluid. The endometrium was red-black and dull, indicating an endometritis secondary to bacterial infection. This cow developed a severe metritis immediately after calving. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Pyometra (accumulation of pus in the uterine lumen) occurs as a sequel to endometritis or metritis. It is an acute or chronic infection of the uterus with dilation and accumulation of pus in the lumen (Fig. 18-18). The closure of the cervix is not always complete, and if not, exudate is discharged into the vagina. In cows, endometritis and pyometra prevent prostaglandin release and the corpus luteum is retained thus mimicking pregnancy. In the bitch and queen, pyometra follows endometritis, which requires a corpus luteum for its development (see the discussion on cystic endometrial hyperplasia later in this chapter) (see Fig. 18-18, A and B). The color and consistency of the exudates vary with the infecting bacteria. Exudate is viscid and brown with Escherichia coli infection and creamy yellow with streptococcal infection. The uterus can be greatly distended but not necessarily uniformly. Macroscopically, necrotic, ulcerated, and hemorrhagic areas are present in the endometrium, with dry, white, thickened, finely cystic areas. Microscopically, the dry white areas have hyperplasia and squamous metaplasia, a common occurrence in chronic inflammation of any mucous membrane. The cystic areas are cystic endometrial hyperplasia (Fig. 18-19). Lesions outside the genital tract secondary to pyometra include widespread extramedullary hematopoiesis and immune complex glomerulopathy and are common in the bitch.
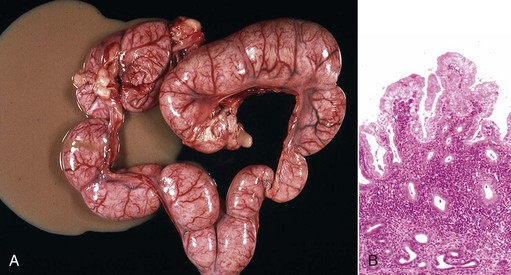
Fig. 18-18 Metritis-pyometra-endometrial hyperplasia, uterus, bitch.
A, Pyometra occurs several weeks after estrus. Bacteria grow in the uterus and induce a suppurative response. The uterus fills with pus and is distended. B, Endometritis and pyometra. There are large numbers of lymphocytes and plasma cells in the endometrial stroma. The surface epithelium is hyperplastic and papillary. The luminal epithelial cells are highly vacuolated. Pus in the lumen of the uterus was removed during processing of the histologic section. H&E stain. (A courtesy Dr. J. Wright, College of Veterinary Medicine, North Carolina State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
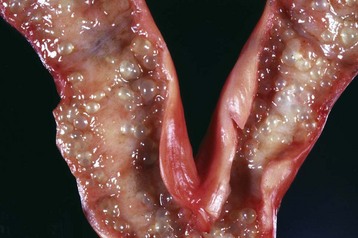
Fig. 18-19 Cystic endometrial hyperplasia, uterus, bitch.
Note the cysts in the mucosa of the endometrium. This change occurs under the influence of progesterone after estrus. Cystic hyperplasia may provide a suitable environment for bacteria to grow and cause pyometra, or alternatively, cystic hyperplasia may be secondary to uterine infection and endometritis. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Noninflammatory Diseases: Torsion of the uterus occurs in pregnant animals and most frequently in the cow. It occurs rarely in the pregnant bitch and queen (Fig. 18-20) but can occur when the uterus is enlarged by pyometra or mucometra (accumulation of mucus in the uterine lumen). The rotation is around the mesometrium and occurs at the level of the cervix in the cow and mare and at the junction of the uterine horn and body in the bitch and queen. Torsion results in circulatory compromise and venous infarction. The veins, which have a lower pressure and are thinner walled, are compressed and occluded before the arteries. The uterine wall and placenta become congested and edematous. The fetus dies and mummifies or if the cervix is open enough to allow the entrance of bacteria or fungi, putrefies. The uterine wall is friable and prone to rupture.
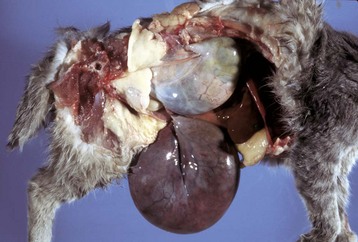
Fig. 18-20 Uterine torsion, cat.
The dark red black structure (bottom center) is a twisted uterine horn that contains a fetus. Its color is the result of venous infarction. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Rupture of the uterus occurs as a sequel to torsion, in severe dystocia and during treatment of uterine diseases by infusion of drugs and fluids. The torsion and dystocia-related ruptures are likely to be in the caudal part of the uterus and are often fatal because of hemorrhage or infection. The rupture that follows overvigorous infusion of medications into the uterus occurs at the lesser curvature of an infused horn, dissects beneath the serosa and into the mesometrium, and results in inflammation around the uterus (perimetritis).
Prolapse of the uterus after parturition is important in the cow, ewe, and sow. A flaccid uterus and excessive straining are predisposing conditions. Factors that cause uterine inertia, such as prolonged dystocia, hypocalcemia, and ingestion of estrogenic plants, usually contribute to prolapse of the uterus. The anatomic structures that are prolapsed can be restricted to the previously pregnant horn, cervix, or vagina or can include all of the uterus, the bladder, and sometimes some of the small intestine. As the structures prolapse, there is vascular compromise, and congestion and edema result (Fig. 18-21). Constriction by the vaginal and vulval tissues exacerbates this by further compressing blood vessels, and as edema develops, the tissues exposed to the outside environment continue to swell, hang down further, dry, and become traumatized. This further exacerbates the swelling. Continued straining and the effects of gravity on the prolapsed tissues continue to contribute to stretching of internal structures, including ligaments and blood vessels. Hemorrhage and shock can cause death even if the uterus is manually returned to its normal anatomic position in the abdominal cavity. If the animal survives, the intervening drying, trauma, venous infarction, and infection that occur may prevent future fertility.
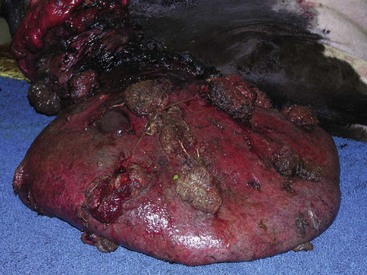
Fig. 18-21 Uterine prolapse, vulva, cow.
The uterus, cervix, and part of the vagina have prolapsed. The uterus has become swollen from dependent edema and from reduced venous outflow. The mucosae of the uterus and uterine caruncles are exteriorized and exposed to the environment, and thus they have become dehydrated and traumatized. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Retention of fetal membranes for longer than normal after parturition is common, especially in the cow. In cows, membranes are considered retained if not expelled by 24 hours postpartum and by 3 hours in the mare. In cattle, where this is studied the most, there are many processes and mechanisms thought to operate to cause the fetomaternal interface (cotyledon and endometrium) to separate. These include the following:
1. The effects of increasing relaxin and decreasing progesterone concentrations in increasing collagenase activity to favor enzymatic breakdown of collagen linkages.
2. Increased expression of MHC-1 by trophoblasts and a maternal immune response to allow leukocytes (especially neutrophils) and cytokines to promote separation.
3. Mechanical effects of uterine contraction induced by prostaglandin and estrogen upregulation of oxytocin receptors.
4. A turnover of maternal epithelial cells of the caruncle in normal pregnancy, where as parturition approaches, there is a gradual loss and flattening of the cells.
Trophoblasts compensate by hyperplasia. Normal separation of cotyledon from caruncle involves reduced cell proliferation and increased apoptosis of placental tissue. Parturition before there is complete “maturation” of the epithelium is believed to result in retention of placental membranes. Retention is common in cases in which cesarean section is medically necessary before the time of normal parturition because there may be insufficient time for complete maturation to occur. Infectious, nutritional, hormonal, circulatory, hereditary, and weather factors may inhibit the “maturation” process. Retained membranes can act as a nutrient medium for growth of contaminant bacteria and for the development of severe endometritis from the transient mild postparturient endometritis. Bacterial endometritis can even cause systemic disease, such as toxemia, septicemia, or disseminated intravascular coagulation (DIC).
Subinvolution of placental sites is a disease unique to the bitch. It is the longer than normal persistence of placental sites in the uterus after parturition beyond the normal 12 weeks. In the normal canine placenta, trophoblasts are found in the endometrium and around the blood vessels of the myometrium, but they rapidly degenerate in the postpartum period. Subinvolution of placental sites is identified clinically by an excessive bloody vaginal discharge that lasts for weeks or months after delivery instead of the normal 1 to 6 weeks. Grossly, subinvoluted placental sites are about twice as wide as normal sites for the same time after parturition, but their appearance is identical to normal except that fibrin adherent to the site is more prominent (Fig. 18-22). As such, multiple segmental thickenings of the walls of the uterine horns are visible from the serosal surface. The luminal surface of each site is a raised, rough, ragged, gray-to-brown plaque of hemorrhage and fibrin. Microscopically, the luminal part of the plaque consists of cell debris, hematoma, thrombus, and regenerating endometrium. In the deeper part of the site, the changes are an abundance of an eosinophilic matrix, hemorrhage, distention, and decreased density of endometrial glands. Trophoblasts appear to be more numerous in subinvolution sites than in normally involuting placental sites and are abundant at the deepest portion of the eosinophilic matrix; these cells can invade the myometrium and penetrate the full thickness of and perforate the wall of the uterus. Affected animals have a prolonged bloody discharge and can become anemic, and those dogs with coagulation disorders, such as von Willebrand’s disease, can exsanguinate. The uterus is prone to develop ascending infection, endometritis, and open pyometra.
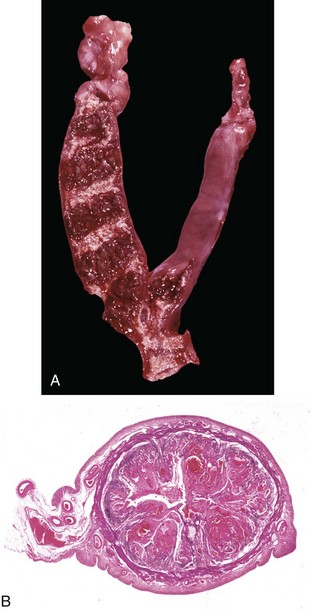
Fig. 18-22 Subinvolution of placental sites, uterus, bitch.
A, Incompletely involuted placental sites. The red transverse stripes are placental sites with hemorrhage, fibrin, and necrotic debris. They are larger than normal sites for the same stage after parturition and remain long after normal sites would have disappeared (12 to 15 weeks postpartum). B, Transverse section of the uterus at a subinvoluted placental site. The two pink outer layers are normal myometrium and underlying endometrial stroma. Most of the distended lumen is filled with large irregularly sized clots of blood, fibrin, and necrotic debris around which are endometrial epithelium and trophoblasts. H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Pseudopregnancy is an exaggerated form of a normal physiologic process. Every nonovariectomized female dog has a prolonged luteal phase of estrus, and this is called covert pseudopregnancy or physiologic pseudopregnancy. Some dogs, especially of the toy breeds, develop an exaggerated reaction. The mechanism is poorly understood, but prolactin or its receptors play a role. The presence of progesterone is necessary for the changes to occur. Overtly pseudopregnant dogs either have an increased concentration of prolactin, or they have increased sensitivity to prolactin. This can occur with a more rapid than usual decline in progesterone when dogs are spayed during diestrus. Hyperprolactinemia that occurs in response to visual stimuli of the presence of surrogate neonates results in the mammary development, lactation, maternal behavior, and other clinically apparent changes of pseudopregnancy. Uterine changes in pseudopregnancy can include the formation of structures that resemble placental sites (now called localized endometrial hyperplasia of pseudopregnancy or pseudoplacentation endometrial hyperplasia) and mucometra; obviously there are no fetuses.
Endometrial atrophy is usually the result of loss of ovarian function. It occurs (1) at anestrus, (2) with a debility such as malnutrition or cachexia, and (3) in DSD. Focal endometrial atrophy of unknown cause sometimes occurs in the mare. Atrophic endometrium is macroscopically thin. In the mare, the longitudinal folds are indistinct, and in the cow, the caruncles are flat. The uterus of the mare is the most studied microscopically because data from uterine biopsies is frequently used for the management of breeding. The endometrium of the uterus of the anestrous mare has cuboidal luminal and glandular epithelium, with short, straight glands.
Endometrial polyps are common lesions in older bitches and queens. The cause is unknown, but they usually occur with cystic endometrial hyperplasia. They are localized and often pedunculated hyperplastic nodules of endometrial stroma and glands that vary from microscopic up to several centimeters long (Fig. 18-23). They can cause obstruction of the uterine lumen and mucometra.
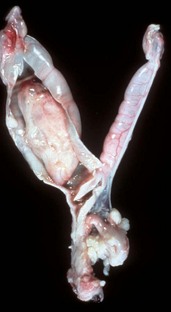
Fig. 18-23 Endometrial polyp, cat.
The uterine horn on the left is distended by a solid cylindrical polyp that is connected to the endometrium by a narrow stalk. (Courtesy Dr. G. Foley, Pfizer Inc.)
Endometrial hyperplasia can be localized or generalized; is an important lesion in the ewe, bitch, and queen; and is rare in the mare. Cystic follicles, granulosa cell tumors, and estrogens from plants are causes of endometrial hyperplasia in the cow. In ewes, it is caused by prolonged hyperestrogenism. Ingested estrogenic clover, such as subterranean (Trifolium subterraneum), and red (Trifolium pratense) clover is the most likely source of estrogen. In ewes, endometrial hyperplasia results in reduced fertility, dystocia, and uterine prolapse because of uterine hypotonicity. Glands of the endometrial type can develop in the cervical mucosa. Even when nonpregnant, ewes have mammary gland enlargement. Endometrial cysts develop beside and beneath the caruncles, and they are about 1 cm in diameter and are filled with clear fluid. The mycotoxin zearalenone obtained from moldy feed causes endometrial cysts in the sow. In the bitch and presumably the queen, cystic endometrial hyperplasia (CEH) is a common response of the uterus (see Fig. 18-19) that occurs in diestrus. The disease can be reproduced by estrogen priming of dogs followed by progesterone administration, but this may not be the physiologic mechanism. Bacteria are almost always present in the uterus of dogs with CEH and are probably the cause. Increased concentration of progesterone in late estrus or early diestrus and aberrant hormonal function may alter hormonal receptor expression. This may prime the uterus so that inflammation or irritation by bacteria (or other substances, such as suture material and oil) stimulates the uterus to undergo hyperplasia and the type of change seen in early pregnancy (the so-called decidual reaction).
Simple endometrial hyperplasia can be overlooked or recognizable macroscopically when the endometrium has patchy or diffuse thickening. When it is cystic, the lesion is readily recognized at surgery or at necropsy. Microscopically, the main component of endometrial hyperplasia is an increase in the size and number of glands with no change in the stroma except for edema. The glandular epithelium is progestational in appearance (i.e., the cells are columnar, hypertrophic, and hyperplastic and have a clear vacuolated cytoplasm) (see Fig. 18-18, B). As the glands become cystic with increased pressure of retained secretion the epithelium of the glands becomes flattened and simple squamous in type (compression atrophy). Mucometra and hydrometra are the accumulation of mucus and clear fluid, respectively, in the uterine lumen (Fig. 18-24, A and B). The cause is congenital or acquired obstruction of the outflow of the fluid and/or mucus that is produced by the endometrium and released when the cervix opens. However, hydrometra and mucometra can develop with excessive production in hyperestrogenism. Hydrometra and mucometra occur in pseudopregnancy, where it resolves spontaneously.
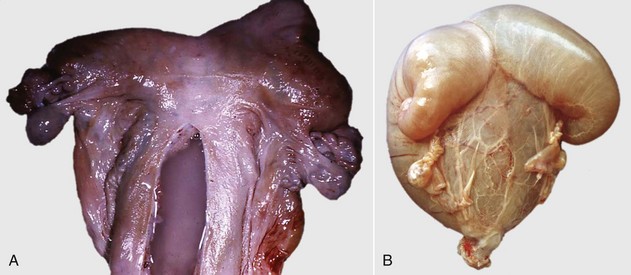
Fig. 18-24 Mucometra and hydrometra, uterus.
A, Mucometra, mare. Note the accumulation of mucus within the opened body of the distended uterus. B, Hydrometra, goat. The uterine horns and body are filled with clear watery fluid. (A courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois; and Dr. J. King, College of Veterinary Medicine, Cornell University. B courtesy Dr. P. W. Ladds, James Cook University of North Queensland.)
Adenomyosis is the presence of endometrium within the myometrium, and the effect generally is negligible in domestic animals, which do not menstruate. Adenomyosis is found in the cow, bitch, and queen. It is thought that endometrium is either forced into the myometrium by pressure of pregnancy or pyometra, or that the epithelium “migrates.” In primates, adenomyosis is considered part of endometriosis, which is a general term in which endometrium is found in ectopic locations. Endometriosis, in which endometrium is found on serosal surfaces, around the ovary, or in the chest, is not reported in domestic species. Macroscopic changes in adenomyosis are primarily localized thickening of the myometrium. In dramatic cases, there are cysts formed in the myometrium. Sometimes, in bitches the uterus undergoes diffuse symmetric or focal asymmetric enlargement near the cervix (Fig. 18-25). Microscopically, endometrial glands, stroma, or both are within the myometrium.
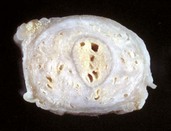
Fig. 18-25 Adenomyosis, uterine body, bitch. Formalin fixed specimen.
The myometrium (outer portion) is infiltrated and expanded by multiple cysts of endometrial stroma and glands. Many of these glands are filled with pus, and the endometrium is expanded as a result of inflammation secondary to bacterial infection. (Courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph.)
Neoplasms: Uterine neoplasms are uncommon in domestic animals. Carcinoma in the cow and leiomyoma in the bitch are the most frequently seen. Lymphoma, which affects multiples sites of the body, is a common neoplasm in the cow.
Endometrial carcinoma is well known in cattle and is found at the time of meat inspection. The cause is unknown. The early microscopic lesion is most often in the depths of the endometrial glands of the horns and less often in the body of the uterus. As it increases in size, the neoplasm thickens the uterine wall without altering the luminal epithelium. A scirrhous response, the deposition of large amounts of fibrous tissue, is a characteristic lesion and this makes the neoplasm firm and tough, and causes localized constriction bands on the serosal surface. The neoplasms can be small and annular or involve a large area of the uterine wall. Microscopically, the neoplasm is readily distinguished from normal endometrium by the increased size, pleomorphism, and disarray of the glandular epithelial cells and the accompanying scirrhous reaction. Metastases occur to the regional (iliac) lymph nodes and lungs, and they seed the serosal surfaces of the abdomen.
Lymphosarcoma in the cow in the enzootic form is caused by the bovine leukemia retrovirus and commonly affects a triad of organs, namely the heart, abomasum, and uterus, as well as the lymph nodes. In the uterus, as in other locations, the neoplastic cells can be focal, multifocal, or multifocally coalescing and up to 3 cm thick (Fig. 18-26). Affected areas are light yellow, slightly friable, and sometimes centrally necrotic and thicken or replace any or all layers of the uterine wall. An extensively involved uterus can support pregnancy, even to an advanced stage.
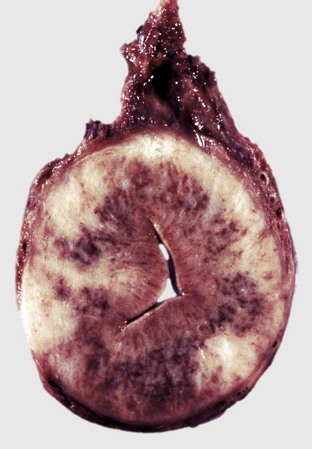
Fig. 18-26 Lymphoma (lymphosarcoma), uterus, cross-section, cow.
The mucosa, lamina propria, and myometrium are expanded by neoplastic lymphocytes. The dark-red to brown regions are areas of necrosis and hemorrhage. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Uterine leiomyomas in the bitch are often multiple neoplasms and can occur in the cervix and vagina (Fig. 18-27, A and B). Estrogens likely have a role in provoking and maintaining these neoplasms. In other domestic species, however, these neoplasms are rare and tend to be solitary. They are well demarcated, unencapsulated, spheric, and vary in size. If they are small, they are confined within the wall of the vagina, cervix, or uterus or can protrude into the lumen or project to the serosal surface of the uterus or into the pelvic canal. Some luminal neoplasms, especially those in the vagina, are pedunculated, making them liable to trauma or torsion. They are usually firm, pink or white, and occasionally calcified or edematous. The color is related to the amount of fibrous tissue present along with the whorled smooth muscle cells; in the macroscopically white neoplasms, fibrous tissue is the dominant component.
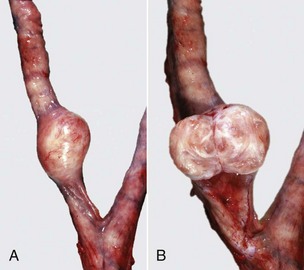
Fig. 18-27 Leiomyoma, uterus, bitch.
A, Note the well-circumscribed, firm mass in the left uterine horn. B, The cut section of the leiomyoma reveals gelatinous content and bands of smooth muscle and connective tissue. This mass is within and arising from the myometrium. (Courtesy Dr. D.D. Harrington, College of Veterinary Medicine, Purdue University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Failure of Pregnancy: Maintenance of pregnancy is one of those miraculous events that defy logic, and there is probably more that is not known than is known about pregnancy and embryonic and fetal development. The fetus is allogeneic and therefore foreign to the mother. Yet it survives, even though both fetus and mother could mount an immune response to each other. Tolerance or suppression of the maternal immune system is required, but the mechanisms are incompletely understood, and there is little agreement about how this actually occurs. Mechanisms in rodents and primates are studied in detail and involve several potential processes, including:
• An active role of systems like the Fas/Fas ligand system in which active immune cells undergo apoptosis when they contact trophoblasts expressing the Fas ligand.
• The suppression of material immunity in the endometrium by production of indolamine 2,3 dioxygenase (IDO), which inhibits tryptophan, an amino acid necessary in the growth and development of T lymphocytes.
• The lack of MHC expression on trophoblasts.
• Alteration of the balance of TH and suppressor lymphocytes.
Immunologic considerations are only part of maintenance of pregnancy. There are hormonal influences, especially maintenance of serum and uterine progesterone concentration by the corpus luteum and placenta. Stressors and systemic cytokines, such as prostaglandin, can cause luteolysis of the corpus luteum and terminate pregnancy during the time when pregnancy is dependent on the corpus luteum (all of pregnancy in cattle, goats, pigs and dogs, and up to 50 days of gestation in horses, sheep, and cats).
It is generally believed that fetuses initiate their own parturition. Disease conditions mimic normal parturition so that in conditions of fetal stress, such as maternal or fetal illness, hyperthermia, and hypoxia, the fetal pituitary secretes ACTH that results in glucocorticoid production by the fetal adrenal gland. Corticosteroids increase the synthesis of estrogens in the placenta, which causes upregulation of oxytocin receptors in the myometrium and the synthesis and release of PGF2α from the endometrium. PGF2α initiates myometrial contraction and causes luteolysis and a decreased progesterone production. Lysis of the corpus luteum results in relaxin secretion and a further decline in progesterone concentration. Relaxin secretion and reduced progesterone promotes placental separation from the endometrium by promoting collagenase activity. Initiation of birth by this process should result in a fresh (nonautolyzed) fetus, whereas rapid fetal death results in loss of pregnancy by other mechanisms, and the fetus, having spent additional time at body temperature, will be autolyzed.
The conceptus is the product of conception, and it is composed of the embryo, that part of the conceptus that gives rise to the adult, and the membranes. The time when embryos become fetuses is debated; some believe it is the time when the embryo develops features that allow their species and sex to be determined phenotypically and others consider it to be when the embryo begins spontaneous movement. This occurs at about 35 to 45 days of age in large animals.
Failure of pregnancy is divided into stages based on the fetus’s development and potential viability, as follows:
• Early embryonic mortality occurs at the embryonic stage, usually less than 35 to 45 days in large animals and about 20 days in dogs and cats.
• Fetal loss (abortion) occurs at the stage of fetal development, when the fetus is not independently viable.
The exact outcome of early embryonic mortality and fetal death is unpredictable and is influenced, among other things, by the cause of the failure of pregnancy, species, stage of gestation, and number of fetuses. The main outcomes are the following:
1. Embryonic death and return to estrus at the normal interval
2. Embryonic death and delayed return to service
Embryonic mortality occurs in all species, and the causes are outlined in the next section. Fetal loss with no autolysis is the norm in horses, sheep, and goats. In the bitch and queen, the lifespan of the corpus luteum is long in the nonpregnant animal and similar to pregnancy. When embryonic or fetal death occurs, the corpus luteum and products of conception may be retained until approximately the normal time of parturition. Fetal autolysis is therefore usual.
Mummification is one of the possible outcomes of fetal death. Rather than being expelled soon after death, the fetus is retained and progressively dehydrates to become a firm, dry mass, which is discolored by degraded hemoglobin to brown or black, and consists of leathery skin enclosing the other dehydrated organs (Fig. 18-28). The cause of death can be infectious or noninfectious, and organisms that promote lysis of dead tissue must be absent and the cervix must be closed to prevent the entry of putrefactive organisms. The situations in which mummification most commonly occurs are listed in Table 18-2. In twin pregnancy in the mare, the mummified fetus and the longer surviving fetus abort together before term. In parvovirus infection in the sow, mummified fetuses are retained and born at term along with live fetuses. In any species with a single fetus pregnancy, the mummified fetus can be expelled at any time or retained indefinitely.

Fig. 18-28 Failure of pregnancy, mummified fetus, pig.
This fetus died in utero, and the fluids were resorbed. Dehydration of a fetus in utero following death of the fetus usually takes longer than 1 week to occur. (Courtesy Ontario Veterinary College, University of Guelph.)
Maceration is when the fetus becomes liquefied (Fig. 18-29). This requires bacteria in the uterus, and these could be those that caused the fetal death or they could be putrefactive organisms that entered the uterus via the cervix. In addition to disintegration of the fetus (see Fig. 18-29), the uterus has lesions also. Endometritis or pyometra is present; the type of lesion depends on whether the cervix remains open or closed. Endometritis and pyometra tend to become severe and chronic. Emphysema occurs when the bacteria are gas forming, such as clostridia. Maternal toxemia and death are likely if the bacteria produce potent exotoxins. Fetal bones resist maceration, and if the uterus eventually regains some muscular tone, the bones can cause perforation.
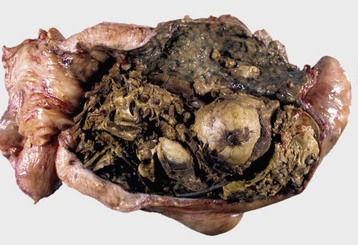
Fig. 18-29 Failure of pregnancy, macerated fetus, lamb.
Fetal bones, hair, and brown pasty material fill the uterus. The ewe was infertile. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
The general diagnostic rate published by veterinary diagnostic laboratories for determination of the cause of sporadic failure of pregnancy is about 50%. Identifying the cause of embryonic loss is very low because there are seldom embryonic or placental tissues to examine. The embryo is thought to dissolve, but the conceptus is so small that it may not be found. There are recognized infectious causes of early embryonic mortality, and the infection often occurs at or near the time of coitus or conception. Agents such as Campylobacter, Tritrichomonas, Ureaplasma, and nonspecific endometrial infections are among the common examples. It appears that chromosomal abnormalities account for the majority of noninfectious causes.
Although the overall diagnostic success rate in sporadic fetal loss (abortion) is about 50%, the success rate is greater in outbreaks. Many of the recognized causes are infectious because infections are usually easy to diagnose (Table 18-3). This has led to the general approach of determining if the loss of pregnancy is infectious or not.
Examination of fetus and placenta: Regardless of which species is involved, investigating failure of pregnancy requires a keen sense of what can be achieved. Depending on the situation, the investigation may have an economic impact, a zoonotic impact, or it may appeal to scientific curiosity. Maternal, fetal, and placental factors should be considered. There are infectious causes common to all species (see Table 18-2), causes that are species specific, and those that are geographically important (Table 18-4).
TABLE 18-4
Specific or Regionally Important Diseases that Cause Failure of Pregnancy
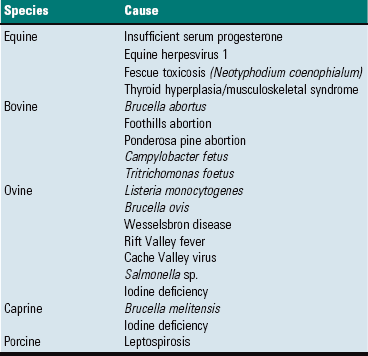
These are not the most common in all areas. Common causes are listed in Table 18-3.
Examination of the fetus and placenta and sampling of tissue are primarily to answer the question: Are there any fetal or placental abnormalities to explain the failure of pregnancy? Some of the lack of success in determining the cause is the failure to submit the appropriate specimens. Where feasible, send the whole fetus and membranes to the laboratory. When this is not feasible, success in ruling out fetal or placental factors depends on submission of the correct specimens, and this in part depends on knowing what specimens to send. Each diagnostic laboratory has a recommended list of samples to maximize diagnostic success.
Each species and breed of animal has an expected rate of development and an average size at birth. Variation from this normal development indicates an abnormality to explain. Fetal and placenta weight, fetal size including crown-rump length, and degree of development for gestation length are basic parameters and changes may indicate increased or inadequate maternal nutrition, concurrent disease, or placental sufficiency.
The degree of fetal autolysis and evidence of fetal distress provide useful information. When distressed and hypoxic, a fetus gasps and aspirates amniotic contents, and meconium is released into the amniotic fluid. Meconium staining of the skin can be seen grossly (Fig. 18-30). Keratin squames and meconium from the amniotic fluid are found in the lungs on histologic examination. A fetus caught in the birth canal for a sufficient period of time develops localized swellings such as a swollen tongue and face (see Fig. 18-30). Pressure from the birth canal restricts venous and lymphatic flow, and any fetal part trapped exterior to the birth canal is edematous. Fetal autolysis was studied in sheep and gives an approximation of the time of fetal death before expulsion. It is generally believed that an autolyzed fetus dies too quickly for it to initiate its own parturition, as would occur with septicemia or viremia. A fetus without autolysis initiated its own parturition.
Fig. 18-30 Dystocia, lamb.
This large lamb died during parturition. It was trapped in the birth canal by the shoulders and right foreleg, which has flexed (folded back). The lamb became hypoxic and defecated meconium, visible as a yellow deposit on the skin of the body caudal to the shoulder. The head and left foreleg became swollen and the skin dry. The skin of the rest of the body is moist from amniotic fluid (and meconium), indicating that this portion of the body was in the vagina and uterus. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
The fetus is similar to an adult in the general response to diseases (Fig. 18-31), particularly near parturition, but many of the responses are rudimentary, depending on the stage of gestation. The fetus is in a sterile environment and has no flora or fauna. Exposure to environmental or pathogenic agents occurs from contact with the external environment or with maternal infection. Many of the pathogens have an affinity for the reproductive tract and may infect and/or initiate failure of pregnancy in many species. Agents common to all species are listed in Table 18-2. Some of them are discussed next.
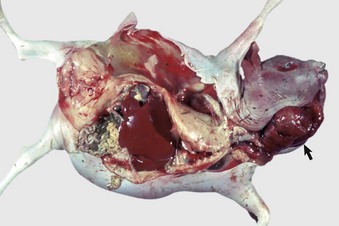
Fig. 18-31 Iodine deficiency, goiter, goat fetus.
This fetus has bilaterally extremely enlarged thyroid glands, alopecia (arrow), and myxedema evident subcutaneously over the thorax and abdomen, which are the classic lesions of severe hypothyroidism. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Failure of Pregnancy in Domestic Animals: Each species has common and known causes and profile of lesions and conditions that result in the failure of the pregnancy. Table 18-3 lists the likely laboratory diagnostic success and the types of diseases and conditions to expect.
In most species, the known causes of failure of pregnancy are infectious diseases and therefore one of the initial diagnostic processes is to separate the causes into infectious and noninfectious. Noninfectious causes affect the fetus or placenta, or both. Knowing the normal anatomy is therefore essential, and some of the unique structures of each species are listed next. Noninfectious fetal lesions are relatively uniform across the species. Anomalies affect the fetus primarily, unless they are sufficiently severe or large to cause dystocia and subsequent maternal death. Examples are hydrocephalus, hydrops fetus, and arthrogryposis. These are found sporadically in all species.
Many of the known causes of failure of pregnancy are infectious, and some of these infectious agents affect all species (see Table 18-2). Microorganisms of the same genus produce similar lesions in different species.
Viral diseases: The important viruses are the herpesviruses and pestiviruses. Herpesviruses cause failure of pregnancy in cows, mares, sows, and less frequently, other domestic species. Generally, the macroscopic lesions in the fetuses are multiple randomly distributed pale gray to white foci of acute necrosis, most commonly seen in the liver (Fig. 18-32, A). Often, the fetal liver is enlarged, and the necrotic foci are large enough to be visible as 1- to 2-mm white areas (Fig. 18-32, B). Foci can be red from hemorrhage. Similar lesions less frequently occur in other organs, including the lungs, kidneys, brain, or virtually any other organ. The placenta can be edematous, but in the majority of domestic species, there are no other changes. Pestiviruses in cattle (BVD virus), sheep (border disease virus), and pigs (classical swine fever virus) produce a similar spectrum of disease. They cause fetal death or malformation, depending on virus strain, fetal age, and stage of development of the fetal immune system. Placental and fetal lesions either are absent or are restricted to microscopic lymphocytic infiltrates in the heart and brain. Fetal malformations caused by these are discussed in the next section and in other chapters, especially of the nervous system.
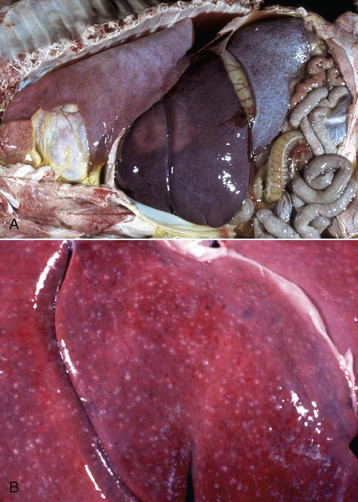
Fig. 18-32 Equine herpesvirus 1 infection, aborted equine fetus.
A, The changes typical of herpesvirus abortion include solid rubbery lungs and multiple foci of necrosis in the liver. B, Randomly distributed multiple 1-mm white foci in the liver are characteristic of necrosis caused by herpesvirus infection. This case is more florid than most. (A courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph. B courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
Bacterial diseases: Bacterial species causing inflammation of the placenta and fetal sepsis are numerous. Almost any organism that causes bacteremia and septicemia can infect the pregnant uterus; common examples are the Brucella, Salmonella, Listeria, and Campylobacter spp. Entry into the body is often after fecal-oral transmission and then hematogenous spread. Some gain entry by venereal transmission. The important genera are listed in Table 18-2. In all species of domestic animals, Salmonella, Campylobacter, and Listeria sp. cause intestinal disease, and if bacteremic, can cause placental and fetal infection. Some bacteria have a particular affinity for the reproductive tract, and there are genera that infect virtually every domestic animal species. Brucella, Leptospira, Chlamydophila, and Coxiella spp. are particularly important. Common or important bacterial causes of failure of pregnancy are outlined for those species in which they are especially important.
Protozoal diseases: The protozoa of importance are Toxoplasma gondii and Neospora caninum. Toxoplasma gondii can cause failure of pregnancy in virtually every species except cattle. Likewise, the list of domestic animals affected by Neospora caninum is growing. There are sporadic cases of Sarcocystis sp. abortion also. The lesions seen with these protozoa include multifocal placental necrosis and microscopic foci of necrosis and inflammation in many fetal organs, especially the brain.
Failure of Pregnancy in Horses:
Noninfectious failure of pregnancy: Any failure of pregnancy without an infectious cause is placed into the noninfectious failure of pregnancy category. The potential list is very long, and specific conditions vary with the domestic species. All domestic species have sporadic fetal anomalies, thyroidal hyperplasia and goiter, and dystocia.
Horses are unique in that they have a large number of noninfectious causes of failure of pregnancy (Box 18-5), related in some instances to the apparent lack of placental reserve and their microcotyledonary placentation.
Fetal anomalies are usually rare and sporadic in horses. In some regions, especially where feed quality during winter is inadequate, foals are lost because of thyroid hyperplasia and musculoskeletal disease (TH-MSD). The thyroid lesion is a microscopic hyperplasia, but there is no macroscopic enlargement of the glands. Routine histologic examination of the thyroid is therefore necessary to make this diagnosis. Musculoskeletal diseases seen in this syndrome include prognathia, flexural deformity, joint laxity, and tendon ruptures, presumably from hypothyroidism.
Examination of the placenta for lesions requires knowledge of normal anatomy, and there are many features and structures that are normal but have the appearance of being a lesion (Box 18-6). It is important to assess the equine placenta for the following:
• Anything that reduces placental area, such as avillous regions
• Lesions of the cervical star (Fig. 18-33)
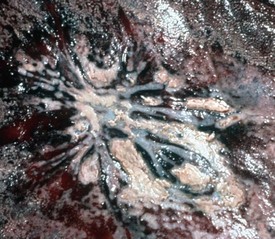
Fig. 18-33 Bacterial placentitis, cervical star, mare.
The chorion at the cervical star is thickened by edema, and there is fibrin, necrotic debris, and suppurative exudate on the surface. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
• Excessive length (normal is 36 to 83 cm) and torsion (Fig. 18-34) of the umbilical cord
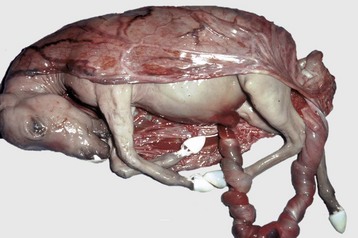
Fig. 18-34 Umbilical cord torsion, equine fetus.
This aborted fetus, wrapped in its amnion, has a very long and twisted umbilical cord. Twisted cords are often longer than 83 cm, a risk factor for torsion of the cord. (Courtesy College of Veterinary Medicine, University of Illinois.)
• Lesions of the amnion (amniotic plaques [Fig. 18-35, A])
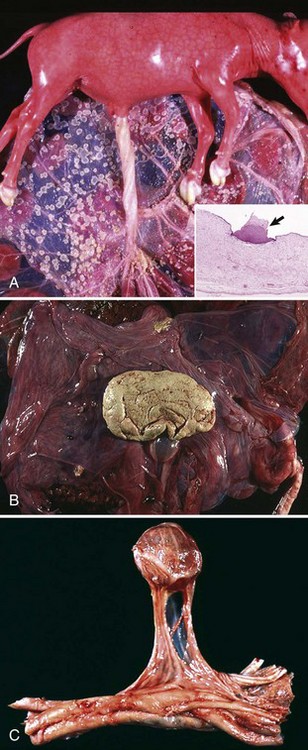
Fig. 18-35 Incidental structures, placentas.
A, Amniotic plaques, bovine fetus and placenta. Multiple white, raised circular plaques up to 1.5 cm in diameter are present on the fetal side of the amnion. They are normal incidental structures composed of stratified squamous keratinizing epithelium. Inset, An amniotic plaque (arrow). H&E stain. B, Hippomane, allantois, equine placenta. These rubbery flattened discs up to 10 cm in diameter are the end result of aggregation of sediments of allantoic fluid in the horse and other equids. They are incidental findings. C, Mineralized yolk sac remnant, umbilical cord, equine fetus. Yolk sac remnants seen on the allantoic portion of the umbilical cord are incidental structures. Note that in this case the yolk sac is connected by a stalk containing blood vessels and is located at the junction of the umbilical cord and the allantois. (A courtesy Department of Veterinary Biosciences, The Ohio State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. Inset courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. M. McCracken, College of Veterinary Medicine, University of Tennessee; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
Normally, chorionic villi develop where contact is made between the endometrium and the chorion. Small and insignificant regions of avillous placenta occur in the following:
• At the contact area between chorion and endocervix (cervical star).
• At sites of the endometrial cups (chorioallantoic pouches).
• Where there are folds of the chorion.
Two structures that often cause confusion are the hippomane and the yolk sac remnant. Almost all equine placentas have a hippomane in the allantoic cavity. These are rubbery concrements of allantoic precipitates that vary in color from white to tan to red (Fig. 18-35, B). The yolk sac remnant is the remaining tissue of the yolk sac that is a circular cystic structure found in the allantoic portion of the umbilical cord from its junction with the amnion to the chorioallantois. It may be surrounded by the umbilical vessels or extend from the cord with a stalk (Fig. 18-35, C). This remnant is often mineralized and has a fluid-filled center and an ossified wall. The outer surface is smooth, but the inner surface has a pattern that resembles the inner surface of the calvarium. It is sometimes mistaken for the skull of a twin or an amorphous globosus (see the section on Failure of Pregnancy in Ruminants; Fig. 18-36). The final common normal finding is allantoic pouches. These are outpouches of the allantois into the allantoic cavity to form a small polyp. Sometimes these have a long stalk.
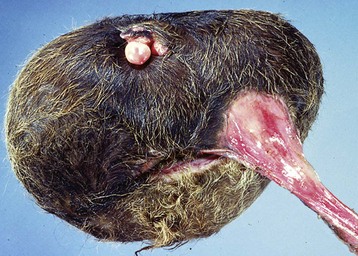
Fig. 18-36 Acardiac monster (bovine amorphous globosus).
This structure, covered with hair, is the remnant of a twin fetus and is attached to the placenta of the normal twin by a stalk. It is a rare finding in cattle and is usually of little consequence. (Courtesy Dr. J. Edwards, College of Veterinary Medicine, Texas A&M University; and Dr. J. King, College of Veterinary Medicine, Cornell University.)
Twinning is a common noninfectious cause of abortion in mares. Chorionic villi do not develop over the contact area between the two placentas. The combined functional area of the chorions of both twins is only slightly larger than that for a normal nontwin foal. Twin fetuses often have growth retardation. Aborted twin equine fetuses often appear to have died at different times. Death is thought to be from placental insufficiency. When the available space in the uterus is divided evenly, which is approximately 80% of cases, both twins usually die and are aborted in midgestation. In cases in which great disparity exists in the apportioning of space, the favored twin has a chance of survival, whereas the other dies and mummifies.
There are several abnormalities of the equine umbilical cord, inadequate or excessive length, and torsion. In torsion of the umbilical cord, the cord is usually longer than normal and excessively twisted (more than three complete turns). For a twisted cord to qualify as being functionally significant, there must be compromise of the umbilical vessels and urachus. The wall of the urachus is thinner and more pliable than the umbilical arteries or vein and thus is more easily constricted. Local distentions of the urachus occur between the twists and anywhere along its course in the cord from the umbilicus to the allantoic cavity. The cord is edematous and hemorrhagic, and red distended segments alternate with pale constricted twisted segments (see Fig. 18-34). Fibrin is sometimes present on the outer surface of affected areas of the cord.
In the mare, endometrial fibrosis, often the result of a previous endometritis, reduces the area of the endometrium available for the formation of the maternofetal interface (see Fig. 18-16). Chorionic villi–microcotyledons do not develop where there are no endometrium with glands. Thus in large areas of endometrial fibrosis, the chorion does not develop microcotyledons and their villi. Severely affected mares may become pregnant but do not carry the fetus to term because the functional area of the placenta is too small.
Premature placental separation in the mare has been described in two forms. One form occurs around the time of parturition, causing the chorioallantois to appear at the vulva with the cervical star intact. This is known as a “red bag” delivery because the exposed chorion is a bright red color. Although the caudal part of the chorioallantois is detached from the uterus, the cranial part remains in place, and there is tearing of the chorioallantois across the body of the placenta rather than at the cervical star. The other form occurs any time before parturition. Prematurely detached areas become brown and dehydrated.
Another equine placental disease is called body pregnancy. Fetal death and abortion occur as a result of placental insufficiency and fetal malnutrition. The initial site of fetal embryonic attachment is in the body of the uterus rather than at the bifurcation of the uterine horns. The fetus occupies the uterine body only rather than the body and horns, and the placenta is underdeveloped in the horns. Some fetuses expand in size so that the placenta extends through the cervix, where it is avillous and becomes infected with vaginal organisms.
Infectious failure of pregnancy:
Viruses: Equine herpesvirus 1 (EHV-1) is one of the most important causes of failure of pregnancy in mares. EHV-1 infects respiratory epithelium, but after lymph nodal involvement, the virus is transported to the uterus (and other tissues) in leukocytes where infection of uterine arteriolar endothelial cells occurs. Virus is also located between the maternofetal interface and in microcotyledons. The resultant microvascular damage leads to thrombosis, edema, hemorrhage, and infarction of microcotyledons. The endometrium has perivascular lymphocytes, neutrophils, and histiocytes. Fluid that escapes through the damaged endometrium separates the maternal and fetal layers, thus allowing virus from the endometrium to enter the placenta and then fetus. Fetal endothelial cells and the cells of most organs are then infected with the virus. The classic lesion, as with most herpesviruses, is focal hepatic necrosis. Focal necrosis occurs microscopically in many other organs also. Bronchiolar epithelial death and fibrin exudation produces a diffuse pneumonia. Fibrin casts in the trachea are formed in some cases, and this is a characteristic lesion. EHV-3 (equine coital exanthema) and EHV-4 produce a similar lesion but much less frequently.
Equine viral arteritis virus (an Arterivirus) in the mare causes failure of pregnancy, but in the majority of cases, there are no lesions in the fetus. The mechanism is probably fetal anoxia caused by compression of uterine blood vessels by virus-induced myometritis, although a few cases of arteritis in the chorion and myocardium of aborted fetuses are described.
Bacteria: In the mare, most bacterial pathogens enter the pregnant uterus through the cervix (see Fig. 18-33), and several bacteria are involved. These include hemolytic streptococci, especially Streptococcus zooepidemicus (frequently cultured from fetal organs, placentas, and uterine discharges), Escherichia coli, and other Gram-negative bacteria. Inflammation of the chorioallantois is most severe at the cervical star, opposite the cervix. Fetal expulsion and death occur because of a reduction in placental area caused by placentitis and/or fetal sepsis, which is probably more important. Because many cases have only a small area of placenta affected, the cervical star must be examined carefully for placentitis. Affected parts of the placenta are edematous and brown and covered by small amounts of fibrinonecrotic exudate. Macroscopic fetal lesions attributable to the infection are rare. Microscopically, the lesions include neutrophils in debris on the surface of affected microcotyledons, and severe inflammation of the stroma of microcotyledons and desquamation of trophoblasts. Microscopic fetal lesions are rare, despite the ease with which bacteria are recovered from many fetal organs.
Failure of Pregnancy in Ruminants (Cattle, Sheep, and Goats): The diagnostic process to determine the failure of pregnancy in ruminants is similar to that of other species. There are many similarities in the pathogenesis and morphology of the lesions of failure of pregnancy among ruminants because they all have a cotyledonary placenta. There are only several anatomically normal structures that are mistaken for lesions. These are adventitial placentation, mineralization of the membranes, and amniotic plaques.
Adventitial placentation in cattle is the formation of additional placentome (Fig. 18-37). More is seen in cows with a higher parity. It is also considered to be a hyperplastic response to inadequacy of existing placentome surface area. A reduction in the area of placentomes from the loss of caruncles can occur because of endometritis, removal of cotyledons during aggressive manipulations, removal of retained placental membranes, and chronic placentitis. Compensation for a reduced placentome area is also achieved by enlargement of the existing functional maternal caruncles. Adventitial placentation is also seen in hydroallantois and when fetuses are the result of cloning. These adventitious areas initially form near the normal placentomes but can expand to involve much of the chorionic surface.
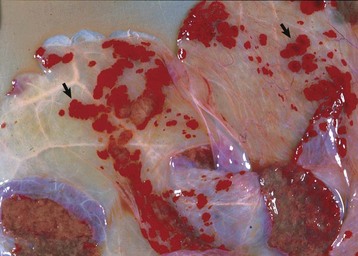
Fig. 18-37 Adventitial placentation, placenta, chorion, cow.
Additional sites of placentation are visible in the intercotyledonary chorion. They appear as red plaques (arrows), sometimes with villi, that extend from cotyledons. There is a corresponding change on the endometrium. (Courtesy Drs. W. Crowell and Tyler, College of Veterinary Medicine, University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, University of Georgia.)
All species have amniotic plaques (see Fig. 18-35, A). They are small raised plaques of epidermal tissue, and some may have hair growing from them. In ruminants, they are up to 2 cm in diameter and are only on the amnion, thus on the same side as the fetus. In particular, ruminants normally have mineralization of the placenta, which appears as a white lacy change, especially to the chorion.
Because the placentation of ruminants is cotyledonary, there is a large potential space between the placentomes in which exudates and agents can accumulate. Chronic placentitis involving the pericotyledonary areas and the intercotyledonary portions of the chorion is common. The lesions are stereotyped and include the following:
Most agents cause a similar change and the etiologic diagnosis depends on microbiologic examination. The list of possible infectious agents is long, although there are species of bacteria and fungi that are more prevalent in some geographic locations and in some ruminant species (see next).
Amorphous globosus is a rare and incidental finding, especially in bovine placentas. It is a type of acardiac monster and is a severely anomalous second fetus. Macroscopically, it is usually spheric, covered in hair, and attached to the placenta by a cord (see Fig. 18-36). Various organs can be identified within the structure, histologically.
Noninfectious failure of pregnancy: There is considerable geographic variation in the types of noninfectious failure of pregnancy. Fetal anomalies not related to infectious agents can take innumerable forms. Anomalies, such as aplasia or hypoplasia of the adrenal glands, or anomalies of the anterior pituitary cause prolonged gestation by altering the pituitary-adrenal axis. One classic example is prolonged gestation in ewes that ingest the plant Veratrum californicum on the fourteenth day of gestation. Along with cyclopia (Fig. 18-38) and holoprosencephaly, the pituitary may be absent.
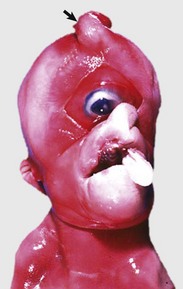
Fig. 18-38 Cyclopia, porcine fetus.
A defect of ocular and cranial development has resulted in fusion of the eyes (cyclops) and a proboscis (arrow) above the eye. Cyclopia can occur in the lambs of ewes that ingest the plant Veratrum californicum on the fourteenth day of gestation. (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
Hydramnios and hydroallantois refer to the excessive accumulation of fluid in the amniotic and allantoic sacs, respectively. These lesions occur mostly in the cow but could occur in any species. The volume and composition of placental fluids is mostly regulated by the membranes (amnion and allantois), and the nature of the dysregulation in hydramnios and hydroallantois is not known. Hydramnios also occurs with some fetal facial muscle and skeletal abnormalities in which impairment of the fetal drinking reflex reduces amniotic fluid removal by the fetus. Allantoic fluid is formed in part from fetal urine received through the urachus, thus excessive urination is implicated. In the cow, hydroallantois occurs in conjunction with adventitial placentation and in some twin pregnancies. The allantoic fluid composition changes from normal to that closely resembling maternal or fetal extracellular fluid. When these membranes are retained after delivery of a fetus, fluid sometimes continues to be produced.
Infectious failure of pregnancy:
Diseases of cattle: In cattle, Neospora caninum and bacteria are among the most common causes of failure of pregnancy, according to the results from diagnostic laboratories worldwide.
Bacterial diseases: Brucella abortus is eradicated from some countries but is a classic disease that is still important. Cattle are infected by exposure to infected placental fluids by several routes but most often through the alimentary tract. Infection via conjunctiva or inhalation are both possibilities. After exposure, the bacterium is found in macrophages in the draining lymph nodes. Bacteremia ensues, and systemic infection and localization in the pregnant uterus, testes (in bulls) and mammary gland occur. The trophism of Brucella abortus to the pregnant uterus is related to erythritol and steroid hormones. Bacteria enter the erythrophagocytic trophoblasts of the hemophagic organ of the placentome, at the base of the chorionic villi. They replicate in the rough endoplasmic reticulum of the periplacentomal and interplacentomal trophoblasts, a unique mechanism of intracellular parasitism. Macroscopic lesions of the placenta are those of a typical chronic placentitis and include thickening and edema of the intercotyledonary chorioallantois, a fibrinonecrotic coating of the intercotyledonary chorion, and cupping of the cotyledon. There are no unique lesions, but histologically, large numbers of bacteria are in trophoblasts and there is a vasculitis in both maternal and fetal tissues, a lesion that could be a response to endotoxin released from the Brucella organisms. Most fetuses develop pneumonia that ranges from minimal to severe. Microscopically, the pneumonia is either a chronic bronchopneumonia with numerous macrophages and lymphocytes and some neutrophils or a fibrinous pneumonia. Microscopic granulomas that include multinucleate giant cells occur in organs such as the lung, liver, spleen, and lymph nodes.
Other bacterial causes of placentitis produce similar and often identical lesions. Bacillus licheniformis is a common example. The pathogenesis of Bacillus licheniformis, based on experimental studies, suggests there is localization in the placentomes after bacteremia. Cotyledonary necrosis and suppurative inflammation occurs, with fetal infection from fetal bacteremia or by ingestion of contaminated amniotic fluid. Campylobacteriosis is a sporadic disease unless it is newly introduced into a herd. Campylobacter fetus var. venerealis can be a long-term inhabitant of the preputial cavity of bulls. It is transmitted venereally and can survive on the vaginal mucosa, but the cow must become pregnant for it to establish itself in the uterus. Early embryonic death is the most likely manifestation of campylobacteriosis and often the only clinical abnormalities are an irregular estrous cycle or the return to estrus of an animal thought to be pregnant. Much less frequently, abortion occurs at a later stage of pregnancy. Cows become resistant to subsequent infections by the bacterium. Gross and microscopic lesions in placentas are similar to those described in brucellosis (edema of the intercotyledonary chorioallantois and necrosis of cotyledons with microscopic inflammation of both) but are less severe and with fewer bacteria in the desquamated trophoblasts.
Leptospirosis is another disease where failure of pregnancy is a common outcome. Results of serologic studies indicate that a large percentage of cattle are infected, but most do not have clinical signs. Several different serovars are associated with abortion, especially Leptospira interrogans serovar Hardjo. In adult animals, the bacteria localize in the kidneys after the bacteremic phase. Pregnant cows abort weeks after the bacteremic phase, usually in the last trimester. Placental lesions are usually limited to edema, where fetal lesions often are mild and obscured by autolysis. Some dead fetuses expelled near term have the gross lesions of ascites and fibrinous peritonitis. Microscopic fetal lesions include interstitial nephritis and necrotizing hepatitis. Leptospires can be demonstrated in peritoneal or pleural cavity fluid by dark-field microscopic examination and in renal tubular lumens by silver stains or by immunohistochemical techniques. Measurement of the leptospiral antibody in fetal fluids (serum, cavity fluids) is a common method for diagnosis.
Epizootic bovine abortion (EBA), also known as foothill abortion, occurs in California and adjacent states. The causative agent, known as the agent of EBA, is carried by the tick Ornithodoros coriaceus. A novel Deltaproteobacterium is the suspected agent. The fetal disease is chronic with notable microscopic lesions 50 days after exposure of the dam to ticks. The lesions are sufficiently specific for a diagnosis in fetuses whose gestational age is greater than 100 days. Gross lesions include petechial hemorrhages of the conjunctiva and oral cavity, an enlarged nodular liver and ascites (both presumably from heart failure secondary to myocarditis), and enlarged lymph nodes and spleen. Lymphoid follicles are hyperplastic and have large numbers of histiocytes. The thymus is atrophic because of the loss of cortical lymphocytes. The thymic parenchyma and interstitium contain many histiocytes. Foci of acute necrosis are present in several organs, especially the lymph nodes and spleen. These foci frequently develop into pyogranulomas. Vasculitis occurs in several organs. Deposits of IgG and IgM are present in the vascular lesions.
Ureaplasma diversum causes failure of pregnancy at different times of gestation in cattle. The characteristic lesion in abortions is amnionitis, a change only occasionally seen in other bacterial or fungal infections. There is focal or diffuse reddening of the amnion especially and also the chorioallantois. There is thickening and yellow discoloration of the amnion (Fig. 18-39). The lesions are chronic and ongoing with fibrosis, edema, inflammation, and necrosis of the amnion occurring together with focal inflammation and necrosis of the cotyledons and the intercotyledonary chorioallantois. Many fetuses have a chronic bronchopneumonia with large lymphoid follicles near bronchi.
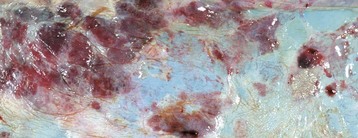
Fig. 18-39 Amnionitis, Ureaplasma diversum infection, cow.
The amnion has large, opaque, red and white areas of granulation tissue and fibrous tissue, respectively (left half of image). Amnionitis without placentitis in cattle is a good indicator of Ureaplasma sp. infection. (Courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph.)
Any bacterium with a bacteremic phase in cows could cause lesions in the placenta and fetus. Salmonella, Mannheimia, and Pasteurella spp. and Histophilus somni are therefore potential causes of placentitis, fetal pneumonia, hepatitis or other lesions, and failure of pregnancy.
Protozoal diseases: Neospora caninum is now recognized as a major cause of abortion in cows. Fetuses are 3 to 9 months’ gestational age and have no gross lesions, except in rare cases in which there is fetal heart failure from myocarditis. There are no macroscopic placental lesions. In the fetal brain, multiple foci of necrosis or groups of microglial cells are often adjacent to capillaries. Groups of Neospora caninum zoites in or around these foci are either extracellular or occur in glial or endothelial cells. In the heart, the lesions include multifocal epicarditis, myocarditis, endocarditis, and protozoal organisms in either myofibers or endothelial cells. Lymphocytic portal hepatitis, multifocal hepatocellular necrosis, and fibrin thrombi in hepatic sinusoids are also seen. Foci of lymphocytes are present in additional organs, including the placenta.
Tritrichomonas foetus causes transient vaginitis, cervicitis, and endometritis and therefore infertility. It can cause early embryonic mortality but only occasionally causes abortion. Macroscopic lesions are usually absent. Microscopically, there is placental edema and a mild lymphocytic and histiocytic chorionitis and focal necrosis of trophoblasts. Large numbers of trichomonads are present. Fetal pneumonia with intrabronchiolar neutrophils, macrophages, and some multinucleated giant cells may occur in one-half of cases. The diagnosis is confirmed when trichomonads are seen in the contents of the fetal abomasum or in fetal and placental tissues. Endometritis can be severe and result in pyometra.
Fungal diseases: Fungal abortion caused by Aspergillus spp. and the Zygomycetes (Absidia, Mortierella, Rhizopus, and Mucor) in cattle is a major cause in some geographic locales but is sporadic in most places. The fungi spread to the placenta hematogenously because the placentomes are involved first and there is multifocal involvement. In some cases, lesions occur in the placenta in the tip of the horn with the degree of fibrosis suggesting movement caudally; this suggests descending intrauterine spread. The placental changes are those of chronic necrotic placentitis as the cotyledons become enlarged, brown, and friable and the intercotyledonary chorioallantois becomes leathery and covered with a brown exudate (Fig. 18-40 and 18-41). In a small number of cases, the amnion may be thick, white, or yellow with leathery areas resembling the lesions caused by Ureaplasma diversum. Fetal dermatitis may be present wherein small, white, raised plaques are present on the skin of the fetus, often over the neck and shoulder. Microscopically, the lesions include large numbers of neutrophils, macrophages and lymphocytes in the amnion (if affected) and chorioallantois, and necrosis and desquamation of trophoblasts. The vessels at the base of the cotyledons have vasculitis and fungal vascular invasion. Lesions in the fetus may include a superficial perivascular and hyperkeratotic dermatitis and bronchopneumonia. Fungal hyphae, septate in Aspergillus and nonseptate in the Zygomycetes, are abundant in lesions of the placenta. Fungi can also be recovered from the fetal stomach, presumably from swallowed amniotic fluid.
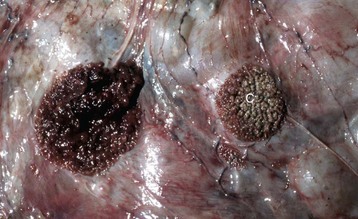
Fig. 18-40 Mycotic intercotyledonary placentitis, cow.
Marked edema, fibrosis, and thickening of the intercotyledonary placenta has caused it to be opaque. The cotyledon (C) on the right is necrotic. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
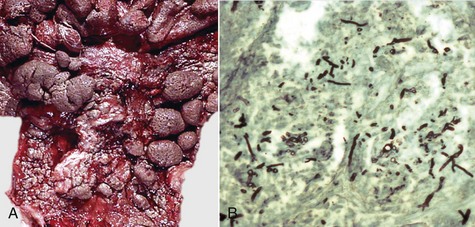
Fig. 18-41 Mycotic endometritis, postpartum uterus, cow.
A, The endometrial surface of the middle and lower portion of this uterus is irregularly thickened and corrugated. Caruncles are small or missing. B, The placenta is necrotic, and the numerous fungal hyphae (black) are irregular in diameter, do not have regular septation, and branch at odd angles, all features typical of the Aspergillus spp. and Zygomycetes. Gomori’s methenamine silver stain. (A courtesy Dr. J. King, College of Veterinary Medicine, Cornell University. B courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Viral diseases: The frequency of viral causes of failure of pregnancy in cattle is probably underreported, especially those caused by BVD virus. There are seldom macroscopic or microscopic lesions of BVD virus infection in the fetus and placenta, and the virus may not be found. The pathogenesis of BVD is outlined in Chapters 4 and 7.
Bovine herpesvirus 1 (BoHV-1) is a sporadic cause of bovine abortion. Fetal autolysis is usually present because of rapid death of the fetus. Fetal lesions are typical of herpesvirus infection in other species with multifocal necrosis and hemorrhage (see previous discussion). There are usually no macroscopic or microscopic placental lesions, although vascular endothelial necrosis and villous necrosis with neutrophils in the necrotic tissue occurs in experimental infection. In addition, BoHV-1 produces an acute necrotizing endometritis in the uterine body or caudal parts of the uterine horns of the cow, particularly in the postpartum period. Microscopically, the lesions range from mild focal lymphocytic endometritis to severe diffuse necrotizing metritis.
Bunyaviruses, such as Akabane virus, and the bluetongue viruses cause fetal infection and abortion and produce a range of lesions in the developing fetal central nervous system, including hydranencephaly, microencephaly, cerebellar hypoplasia, and a lack of spinal cord ventral horn neurons. The loss of ventral horn neurons causes denervation muscle atrophy that leads to arthrogryposis (fixation of limb joints) and skeletal deformities such as torticollis and scoliosis.
Bacterial diseases: In sheep, many cases of failure of pregnancy are caused by bacteria, including Chlamydophila abortus, Campylobacter fetus, and Brucella ovis.
Enzootic abortion of ewes is caused by Chlamydophila abortus and is one of the most common abortifacient agents in sheep. It occurs in all sheep-producing countries, producing late-term abortion. It occurs in two forms: outbreaks when first introduced into susceptible flocks or as an enzootic condition of ewe lambs. Infection is from placentas and fluids to the oral cavity, and ewes are immune to reinfection after the first abortion, but they may remain carriers. Ewes infected before 5 or 6 weeks of gestation abort in late gestation, but ewes infected after 5 or 6 weeks of gestation abort in the subsequent pregnancy. The pathogenesis of failure of pregnancy involves infection of susceptible animals and persistence of Chlamydophila abortus in an unknown site of the body. After about day 90 of pregnancy, there is proliferation of the Chlamydophila abortus in the placentome and then in trophoblasts in the intercotyledonary placenta. This leads to a cytokine and chemokine cascade, with inflammation and thrombosis of placental vessels and subsequent abortion. The placentitis is similar to that described in general terms in placentitis of cattle (see the discussion on bacterial disease in the section on Diseases of Cattle). Coxiella burnetii (see the section on Disease of Goats) and Brucella ovis infection induce an identical placentitis. The organisms distend the trophoblasts and are obvious with such special stains as modified Ziehl-Neelsen or Gimenez stains. In the fetus, foci of lymphocytes and macrophages may be present in the liver, lungs, and muscle.
Sheep develop coxiellosis, but it is more common in goats and is described in the section on Diseases of Goats.
Brucella ovis in sheep is probably transmitted venereally from a ram with epididymitis shedding large numbers of bacteria in the semen. Gross placental lesions are similar to those produced by Brucella abortus in cattle. There are no specific fetal lesions. Calcified plaques on the hooves are reported, but this is not a specific change. Microscopically, as with Brucella sp. infections in other species, large numbers of coccobacilli are found in trophoblasts and are free in the chorionic mesenchyme. There is a vasculitis that involves the larger chorionic vessels. Lesions in the fetus when present are bronchopneumonia, lymphadenitis, interstitial nephritis, and pericholangitis. In younger fetuses, the cells are monocytes and macrophages, whereas older fetuses have well-formed nodules of lymphocytes and plasma cells.
Campylobacter fetus ssp. fetus and Campylobacter jejuni are primarily intestinal inhabitants. These bacteria are transmitted fecal-orally or from infected placentas or fluid to the oral cavity. Failure of pregnancy occurs in outbreaks and continues throughout the flock as organisms spread from an aborting to an uninfected ewe. Infection of the pregnant uterus results in late-term abortion or the birth of live but sick lambs. Ewes become immune after the first infection. The placentitis results in an edematous intercotyledonary chorioallantois and friable, yellow cotyledons, as is seen in other cases of acute placentitis. At necropsy, about 25% of the fetuses have multiple, well-circumscribed, up to 20 mm in diameter yellow hepatic foci with red depressed centers, which are areas of necrosis. Microscopically, the lesions are those of an acute neutrophilic and necrotic placentitis, as occurs with other bacteria, including Brucella ovis. The chorioallantois and especially the cotyledon have necrotic neutrophils and trophoblasts along the surface. Campylobacter organisms are abundant among the inflammatory cells, within trophoblasts, and there may be dense emboli of bacteria in the capillaries of the chorionic villi. The hepatic lesion is a multifocal necrotizing hepatitis with abundant intralesional bacteria. Flexispira rappini causes an identical lesion in the ovine placenta and fetal liver, but infections are less common and sporadic.
Listeriosis caused by Listeria monocytogenes is a cause of sporadic abortions in many species, including cattle, sheep, and goats and of outbreaks of abortion in sheep. Although both nervous disease and reproductive disease occur in the same flock or herd, this is rare. Listerial abortions occur in the last trimester of pregnancy. Some aborting dams are septicemic and also have endometritis. The placental lesion, as with other bacterial placentitis, is severe diffuse necrotizing and suppurative placentitis of both the cotyledons and the intercotyledonary areas. There are multiple 1-mm yellow or white foci in many organs, but they are readily seen in large numbers in the liver. These foci are areas of acute multifocal necrotizing hepatitis in which the Gram-positive Listeria organisms are numerous. The microscopic lesions in the placenta are identical to those of campylobacteriosis and brucellosis, except that the trophoblasts, especially in the intercotyledonary areas, are filled with Gram-positive listerial bacilli.
Viral diseases: Bluetongue, border disease, Cache valley, and Akabane viruses are important causes of failure of pregnancy in some geographic regions. Bluetongue virus (Orbivirus) is an important viral infection of the ovine fetus in those areas in which bluetongue occurs. Fetal lambs with this infection can develop hydranencephaly. They have viral antigen in immature neural cells in areas of necrosis in the periventricular zone of the cerebral hemispheres. Bluetongue is discussed elsewhere. The pestivirus of sheep is border disease virus (BDV), and it produces a disease in pregnant sheep identical to that of BVD virus in pregnant cattle (see the section on Diseases of Cattle). Cache valley virus and Akabane virus produce an identical disease; Akabane viral disease is described in the section on Diseases of Cattle.
Protozoal diseases: Toxoplasma gondii is an important cause of abortion in ewes. Susceptible ewes eat food contaminated by cat feces that contain oocysts. Macroscopic lesions in the placenta are the characteristic white 1- to 2-mm foci of necrosis in the cotyledons (Fig. 18-42). There may be edema of the intercotyledonary chorioallantois. Microscopically, the cotyledonary lesions are distinctive and consist of multiple foci of necrosis with rare groups of Toxoplasma organisms within trophoblasts. Immunohistochemistry is often needed to detect them. A small percentage of fetuses aborted because of toxoplasmosis have cerebral leukoencephalomalacia, a nonspecific effect of fetal anoxia secondary to placentitis.
Diseases of goats: Coxiella burnetii infection is extremely important in goats especially but also in sheep and potentially in all species. Coxiella burnetii, the cause of Q fever in humans, causes abortion or the birth of dead or weak lambs or kids. Abortion occurs in newly exposed animals and repeat infection resulting in abortion is possible. Coxiella is acquired by ingestion or by inhalation. It is shed in vaginal discharges at parturition and in the milk. In affected placentas the intercotyledonary chorioallantois is thick, leathery, yellow, and covered with surface exudate (Fig. 18-43). There are no macroscopic fetal lesions. Microscopically, the placental lesions are most severe in the intercotyledonary areas where there is necrotic inflammatory debris on the chorionic surface and neutrophils, macrophages, and lymphocytes in the chorionic stroma. Hypertrophic trophoblasts contain myriad Coxiella organisms. Fetal lesions, if present, consist of peribronchiolar, renal medullary, and hepatic portal lymphocytic aggregates.
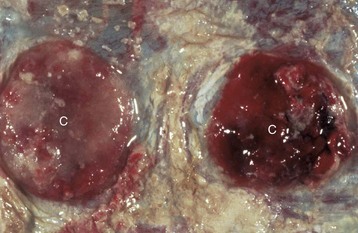
Fig. 18-43 Intercotyledonary placentitis (Coxiella burnetii), goat.
Note the opacity of the intercotyledonary placenta caused by thickening from inflammation and fibrosis. The cotyledons (C) have variable areas of gray discoloration, indicating necrosis and inflammatory exudates. The gross appearance of the lesions of placentitis tends to be similar, regardless of the etiologic agent identified by microbiological examination. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Goats also develop chlamydophilosis and campylobacteriosis as described for sheep. Brucella melitensis infection in goats (and sheep) occurs mainly in Mediterranean countries. There is an initial bacteremic phase, and subsequently, inflammation is localized to the mammary glands and pregnant uterus. Goats develop a more severe febrile disease and more severe mastitis than do sheep. The lesions are similar to those caused by Brucella abortus in cattle and Brucella ovis in sheep. Toxoplasmosis of goats is common but less so than in sheep.
Failure of Pregnancy in Pigs: The principles of diagnosis used in other species apply to pigs (disease of mother, fetus, or placenta; infectious and noninfectious disease); however, the approach in intensive pig production is different and more epidemiologic in nature. Strict biosecurity has eliminated many potential diseases.
For the majority of causes, there are no fetal or placental lesions and the testing for infectious agents is more microbiologic and molecular (i.e., polymerase chain reaction [PCR]) in nature.
Noninfectious failure of pregnancy: There are many potential noninfectious causes of failure of pregnancy. Fetal anomalies and goiter are readily identified, but most others are not. There are often no lesions in the mother, placenta, or fetus. Seasonal infertility wherein there is failure of pregnancy at a regular time each year is very common in large production units. It may be in the summer, when heat is implicated, or in the fall or with the onset of cold weather. This diagnosis is based on the appropriate history and a failure to identify other causes.
Infectious failure of pregnancy:
Viruses: Viral diseases are the most important infectious causes of reproductive failure in pigs. Porcine reproductive and respiratory syndrome (PRRS) virus, porcine parvovirus (PPV), pseudorabies virus (PRV), and porcine circovirus 2 (PCV-2) are the most important.
PRRS virus, an Arterivirus, is transmitted horizontally through body fluids and vertically to the fetuses of those sows without immunity. Transplacental infection of pig fetuses causes abortion, usually in the later stages of pregnancy, but there are seldom macroscopic and microscopic lesions in the fetuses or placentas. The diagnosis is based on evidence of herd exposure, maternal serology, and virus isolation. Lesions, when present, are segmental or diffuse hemorrhage in the umbilical cord as a result of necrotizing umbilical arteritis. Ascites, hydrothorax, and edema of perirenal tissue, splenic ligament, and mesentery are seen in some fetuses. Microscopically, a segmental arteritis occurs in the umbilicus and fetal lungs, heart, and kidneys. Alveolar septa in the lungs are thickened by lymphocytes and histiocytes, and proliferation of type 2 pneumocytes. Aggregates of lymphocytes, plasma cells, and macrophages are present in blood vessels in the heart, portal tracts, and cerebellar white matter. Endometritis and myometritis also occur with edema and lymphocytes and histiocytes in the interstitium and around uterine vessels.
PPV is an important cause of embryonic and fetal loss and causes death and mummification of affected fetal pigs (Fig. 18-44). Sows usually are not ill, but fetuses are infected and die at varying stages—all in the one pregnancy. This suggests fetus-to-fetus transmission within the uterus. The characteristic findings in fetuses therefore are some fresh fetuses, and then varying stages of autolysis and mummification, with the most mummified fetuses being the smallest. This indicates fetal death occurred at different stages of gestation. Microscopically, fetuses that were infected after immunocompetence had developed have widespread lymphocytes and plasma cells in the liver, lungs, kidneys, and cerebellum.
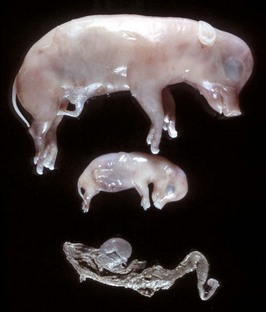
Fig. 18-44 Stillbirth, mummification, embryonic death and infertility (SMEDI), abortion, pig fetus.
Viruses, such as porcine parvovirus and porcine enteroviruses, induce SMEDI. These viruses affect the fetuses to differing degrees and at different stages of gestation. Those fetuses that die early in gestation are usually mummified (bottom fetus) or resorbed. (Courtesy College of Veterinary Medicine, University of Illinois.)
PCV-2 is capable of causing reproductive failure at any stage of gestation. The virus crosses the placenta and replicates in lymphoid tissues. Death of the fetus and expulsion occurs without macroscopic or microscopic lesions. Some affected litters have fresh, autolyzed, and mummified fetuses. Interstitial edema of the heart, a lymphocytic interstitial myocarditis, and ascites and hepatic congestion of heart failure may occur.
Porcine herpesvirus (PHV) is eradicated from many jurisdictions. Although it can cause typical herpesviral lesions in fetuses, it is one of the few where typical inclusion bodies are seen in the chorion.
Bacteria: Numerous bacteria are implicated in causing reproductive failure. Any bacteria that become bacteremic could localize in the placenta and cause failure of pregnancy; they are listed in Table 18-2. The bacteria that are especially important are Brucella suis and Leptospira sp.
Brucella suis causes failure of pregnancy, but the disease in pigs differs in several respects from brucellosis in ruminants. Suppurative endometritis, focal granulomas, and multiple hyperplastic lymphoid nodules may occur in the endometrium of nonpregnant sows. Endometrial glands become distended with neutrophils, and the luminal epithelium is eroded and focally has squamous metaplasia. In pregnancy, the lumen of the uterus between placentas has mucopurulent exudate in which trophoblasts contain intracellular bacteria. The chorioallantois has edema and some focal hemorrhage. Microscopically, there are many neutrophils in the tissue, necrotic debris on the chorionic surface, and loss of trophoblasts.
Leptospirosis is an important cause of pregnancy failure. The pathogenesis is similar to that of cattle and is discussed in Chapters 4 and 11. Leptospira interrogans serovar pomona is commonly isolated. Sows abort after a bacteremic phase, and there are no placental lesions. Fetuses become septicemic, die, and are autolytic. Some develop nephritis and/or neutrophils may be found in the peritoneal serosa.
Failure of Pregnancy in Dogs: Much less is written about failure of pregnancy in dogs than with production animals, in which the economic importance of pregnancy failure provides an incentive to identify the cause and prevent further disease. The following includes some traditional causes.
Infectious failure of pregnancy:
Viruses: Canine herpesvirus is capable of causing failure of pregnancy, although it is far more likely to cause mortality of puppies up to 8 weeks of age. The lesions of herpesvirus infection of pups are similar to the stereotypical changes of herpesviruses discussed in other species. In pups, infection is acquired in the perinatal period from recrudescence of the virus in the bitch. Chilling of the puppies and the subsequent lower body temperature facilitates viral replication and viremic spread. Multifocal hemorrhage of the kidney is a characteristic change, and microscopically, there is widespread focal necrosis and hemorrhage with typical intranuclear herpesviral inclusion bodies.
Bacteria: Brucella canis, as with other brucella, is acquired by the dog either through ingestion or venereally. Dogs acquiring the bacterium by ingestion initially develop lymphadenitis of the head and neck and bacteremia. Epididymitis and testicular degeneration are the lesions in male dogs, and pregnant females develop placentitis and fetal endocarditis, pneumonia, and hepatitis. Microscopically, trophoblasts of the marginal hematoma and zonary placenta are packed with Brucella organisms and there may be neutrophils within the chorion. Other possible fetal lesions include renal hemorrhage, inflammation of the pelvic connective tissue, and lymphadenitis.
There are reports of Salmonella sp., Campylobacter jejuni, Streptococcus canis, and Leptospira serovars causing abortion in dogs. Maternal illness and bacteremia results in infection of the placenta, but the lesions are mild and often are limited to a neutrophilic placentitis.
Protozoa: Toxoplasma gondii and Neospora caninum both cause failure of pregnancy, although it is uncommon. Macroscopic lesions in fetuses are absent, and microscopically, there may be focal necrosis of all tissues, but often there are protozoa that can only be found by immunohistochemistry.
Leishmania infantum is a reported cause of failure of pregnancy. Placental lesions are large numbers of amastigotes within trophoblasts of the zonary portion of the placenta. No lesions occur in other tissues.
Failure of Pregnancy in Cats: The causes of failure of pregnancy of cats include many of the important general diseases of cats, and little is known of the mechanisms of action. Lesions are seldom seen or reported unless there are obvious fetal anomalies. Feline herpesvirus, feline leukemia virus (FeLV), feline panleukopenia virus, and FIP virus are all implicated, either directly or epidemiologically, in pregnancy failure. Cases are from maternal illness rather than from placental or fetal lesions. Bacteria implicated in disease are those that cause failure of pregnancy in a wide range of species, including Coxiella burnetii, Salmonella, and Mycoplasma.
Cervix: Diseases involving only the cervix are rare in domestic mammals because most are extensions of uterine disease. There is considerable variation between species in the anatomy of the cervix, and this has an impact on uterine and placental health. The equine cervix is relatively loose, whereas the cervix of cattle and dogs is not. The responses are identical to those of the uterus because the cervix is a part of the uterus. Anatomic features and responses to injury are detailed earlier in this chapter. Humans and some primates develop papillomavirus infection and subsequently cervical carcinoma; domesticated species do not.
Noninflammatory Disease: Anomalies are rare, except in the cow where there can be hypertrophy or hypoplasia of the whole structure, aplasia of one or more of the usual five rugae, tortuosity, and dilation or diverticulum formation of the cervical canal. Two entire cervices or a single bifurcated cervix is recognized. This can occur as the only lesion or it is part of a more extensive failure of proper fusion of the paired paramesonephric ducts and can be found with a divided vagina and two compete uteri.
Ewes exposed to estrogenic substances in subterranean and red clover develop a permanent infertility because of alteration of the cervix. Affected animals have a cervix that has fused cervical folds and uterine-like glands that produce less viscous mucus. Alteration of cervical mucus affects spermatozoal migration and results in reduced fertility.
Neoplastic disease of the cervix of domestic animals is very rare and unlike humans, in which cervical dysplasia and neoplasia linked to human papillomavirus types is well recognized.
Inflammatory Disease: Cervicitis usually occurs as a minor lesion concurrently with more severe endometritis or vaginitis. This is observed in specific infectious diseases, such as contagious equine metritis caused by Taylorella equigenitalis, and in nonspecific postparturient endometritis, metritis, cervicitis, and vaginitis. Inflammation restricted to the cervix can result from poorly performed traumatic artificial insemination. In cows with acute cervicitis, the caudal rugae are edematous and prolapse into the vagina. Inflammatory exudate covers the cervical simple cuboidal epithelium and collects in the vagina. The cervical mucosa is thin with epithelium and a small amount of stroma, and the underlying fibromuscular tissue is relatively impervious to infection; thus most cases of cervicitis resolve readily. Those infections that follow traumatic dystocia are likely to involve the muscle layer from the onset and can produce severe lesions, including paracervical abscesses, local peritonitis with granulating tracts into the connective tissue of the pelvic canal, stenosis with adhesions across the lumen of the cervix in areas denuded of epithelium, or cervical glandular cysts filled with mucus or neutrophils.
Vulva and Vagina: There are few diseases that are unique to or commonly affect one domesticated species, and these are indicated next. For the majority of diseases, lesions occur sporadically and the disease in one species is similar in appearance to that of a different species. The two main categories of disease of the vagina and vulva are (1) infectious (and inflammatory) disease and (2) noninflammatory conditions, including anomalies, hyperplasia, and neoplasia.
Disorders of Domestic Animals:
Inflammatory diseases: Postparturient vulvitis and vaginitis occur when they are lacerated during dystocia and become infected. Trauma unrelated to parturition, such as during coitus, artificial insemination, embryo transfer or other human intervention, also could progress similarly. Inflammation of the cervix and cranial vagina after dystocia can have a more serious outcome because of local spread of infection into and through the wall into the peritoneal cavity.
Granular vulvitis describes the clinical appearance of the vulva and vagina in inflammatory diseases. Inflammation causes red coloration (hyperemia) and exudates, and the granular appearance is usually the result of development and hyperplasia of lymphoid follicles (Fig. 18-45). Thus any infectious agent can be a cause if there is sufficient time for cell-mediated or humoral immunity.
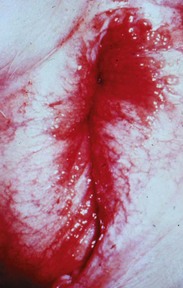
Fig. 18-45 Granular vulvitis, vulva, cow.
Granular vulvitis is a nonspecific condition resulting from development and hyperplasia of subepithelial lymphoid follicles of the vulva and vestibule. Inflammation of the vulva, in the initial stages, causes hyperemia. There is subsequent hyperplasia of lymphoid tissue, visible as 1 to 2 mm raised white nodules on the vulval mucosa. It is commonly seen in cows with infection of the vulva or vagina with Ureaplasma diversum and results in a granular appearance of the mucosa. (Courtesy Dr. R.B. Miller, Ontario Veterinary College, University of Guelph.)
Genital herpesvirus infection occurs in most species. The virus is venereally spread and causes multifocal epithelial necrosis and apoptosis and erosion at the first exposure. As with the general pathogenesis of herpesvirus infection, the virus enters the nerves and remains in neuronal cell bodies and ganglia in a latent state until there is recrudescence and shedding. Macroscopic lesions begin as 1 to 2 mm white, raised foci that soon erode (Fig. 18-46). Vesicle formation is unusual. In some species, the lesions will coalesce to form large ulcers, up to several centimeters diameter (Fig. 18-47). Affected pigmented skin loses pigmentation and remains as white regions of depigmentation (Fig. 18-48).
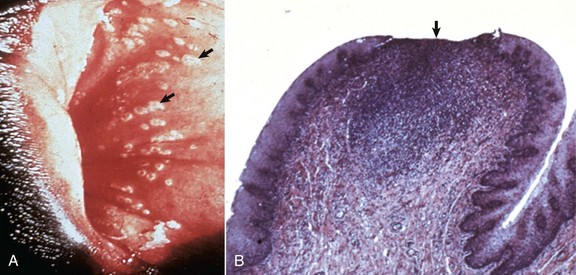
Fig. 18-46 Infectious pustular vulvovaginitis, bovine herpesvirus 1 infection, cow.
A, Focal ulceration of mucosa of the vestibule. The multiple white regions (arrows) are areas of necrotic epithelium and ulceration. B, Ulceration of mucosa of the vestibule. Note the ulcer (arrow) with loss of the epithelium over an aggregate of lymphocytes. H&E stain. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
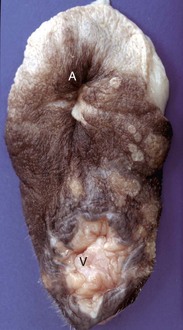
Fig. 18-47 Ulcerative vulvitis, caprine herpesvirus infection, goat.
The vulva (V) has numerous vesicles on the mucosa. The skin of the perineal region around the anus (A) and vulva has multiple circular regions of epithelial necrosis and erosion. Herpesviral infection of the genital tract has the classic lesions of vesicles that rupture to form ulcers, which are irregularly distributed on the affected areas. (Courtesy Dr. P.W. Ladds, James Cook University of North Queensland.)
Noninflammatory disease: Swelling of the vulva is normal during estrus. Excessive or persistent swelling is abnormal and occurs commonly in dogs (see below). It also occurs in hyperestrogenism, such as with estrogen-producing ovarian neoplasms or exposure to estrogenic substances (Fig. 18-49). The vaginal mucosa also swells with edema and protrudes through the vulva, exposing the mucosa.
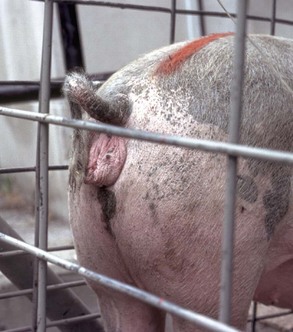
Fig. 18-49 Vulva hypertrophy and edema, estrogenic effect, sow.
The vulva of this sow is swollen with edema. This swelling is typical of hyperestrogenism secondary to mycotoxicosis. (Courtesy Dr. J. Simon, College of Veterinary Medicine, University of Illinois.)
Vaginal polyps occur in most species, but they are particularly common in dogs (Fig. 18-50, also see Disorders of Dogs). They begin as local edema because their stroma is either normal vaginal stroma swollen with edema fluid or there is diffuse fibrosis from longstanding edema. They may protrude from the vulva and frequently ulcerate.
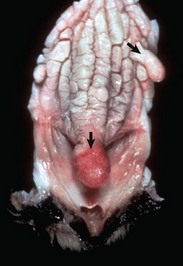
Fig. 18-50 Polyps, vagina, bitch.
There are several vaginal polyps (arrows) arising from the wall of the vagina. The larger caudal polyp adjacent to the urethra protrudes caudally through the labia of the vulva and is ulcerated. (Courtesy Dr. K. McEntee, Reproductive Pathology Collection, University of Illinois.)
Cysts in the vaginal wall arise from remnants of the mesonephric ducts (Gartner’s ducts) or vestibular (Bartholin’s) glands. Causes of cyst formation include inflammation of the lining of the duct or gland and hyperestrogenism, in which edema caused by estrogen stimulation is prolonged. The mesonephric ducts do not normally open into the vagina. When they become cystic, they form single or multiple cysts or a tortuous tube in the lateral floor of the vagina between the cervix and near the urethral opening. Major vestibular glands are found on the ventral and lateral walls of the vestibule and become cystic when edema, inflammation, or scar tissue obstructs their openings. Other anomalies of the vagina and vestibule include persistent hymen and vaginal septum (double vagina). Stricture or stenosis of the vagina or vestibule occurs as a congenital anomaly or follows traumatic injury at parturition.
The most common neoplasm of the vagina and vulva is the squamous cell carcinoma of the vulva. Leiomyoma and carcinoma of the vagina occur sporadically in all species but are most common in dogs. They are discussed later.
Squamous cell carcinoma of the vulva occurs in all species but especially in the cow, ewe, and mare (Fig. 18-51). Exposure to sunlight is a known cause. Ewes subjected to the Mules operation (surgery of the perineum and inguinal areas intended to prevent urine wetting the wool) and to short tail docking have a greater sun exposure of the vulva and a greater incidence of squamous cell carcinoma of the vulva. The lack of pigmentation of the vulva is an additional risk factor. Squamous cell carcinoma originates on the hairless and less pigmented skin of the vulva and has the appearance and biologic behavior of squamous cell carcinomas of the eye and conjunctiva, skin, and other sites. The neoplasm metastasizes late in the course of the disease to the iliac lymph nodes.
Disorders of Horses: Equine herpesvirus 3 (EHV-3) is the cause of equine coital exanthema. It is a typical genital herpesviral disease of the vulva, and the virus is spread venereally, results in transient vesicles and erosions of the external genitalia of both mares and stallions (see Fig. 18-48). Depigmentation of pigmented skin of the vulva occurs.
Disorders of Ruminants (Cattle, Sheep, and Goats): Granular vulvitis in cattle occurs with many different agents, and it is not a specific diagnosis. It simply indicates a local immune reaction to antigen. It can begin as an acute vulvitis or as a subclinical disease. Ureaplasma diversum is a classic cause of granular vulvitis. In the acute stage, there is a purulent vulvar discharge and a hyperemic vulvar mucosa with 2-mm raised granules (see Fig. 18-45). The disease can become chronic, and lesions resolve within 3 months. In about 10% of infected cows, discrete, raised, white nodules of follicular lymphoid hyperplasia, 2 to 5 mm in diameter, occur in rows or clusters on the dorsolateral wall of the vulva. Infection is usually self-limiting, but herds can have a persistent infection. The prepuce of bulls and their semen can remain infected and spread the infection during mating or artificial insemination. Reduced fertility with return to service or abortion occurs with pathogenic strains of the organism as a sequel to infection of the embryo or placenta.
Infectious pustular vulvovaginitis of cattle is caused by BoHV-1, which is similar to the BoHV-1 that is the cause of infectious bovine rhinotracheitis (IBR). The two diseases behave as separate entities, but their occurrence can overlap in a herd or in an individual animal. Vulvovaginitis is transmitted by coitus, artificial insemination, and possibly nose-to-vulva contact. The first evidence of the disease includes hyperemia and edema of the vagina and vulva; this is followed by petechial hemorrhages and slight nodularity of the mucosal surfaces because of edema within epithelial cells. A rapidly coalescing multifocal erosion of the mucosa follows (see Fig. 18-46). Microscopically, changes are detected in the epithelium, the lamina propria, and the lymphoid nodules. The epithelium develops eosinophilic intranuclear inclusions and undergoes ballooning degeneration or apoptosis, followed by desquamation, but usually without discrete stages of vesicle or pustule formation. The lamina propria is hyperemic and edematous. The subepithelial lymphoid nodules become prominent and hyperplastic. Resolution of the disease is rapid and by 7 and 10 days, the lesions are a slightly thickened epithelium and hyperplastic lymphoid nodules. Lesions are similar on the penis of affected bulls. An identical disease occurs in goats and rarely in sheep. Caprine herpesvirus 1 causes lesions in goats similar to those of bovine vulvovaginitis (see Fig. 18-47).
Disorders of Pigs: Toxicosis of pigs by the mycotoxin zearalenone found in Fusarium sp.–infected grain and corn is a cause of vaginal and vulval hypertrophy, particularly in prepubertal gilts (see Fig. 18-49). The toxin is a nonsteroidal estrogen that binds to the estrogen receptor. The vaginal and vulval lesions are stromal edema. Other effects of the mycotoxin are altered time of first estrus, both early and late, reduced numbers of live embryos, endometrial hyperplasia, and precocious mammary development.
Disorders of Dogs: In bitches, so-called nonspecific vaginitis or vulvitis is common. The lesions range from acute vaginitis to chronic granular vulvitis (see previous section).
Vaginal hyperplasia, hypertrophy, and/or prolapse of bitches are common diseases seen during the follicular stage (proestrus) of the first to third estrus periods in young animals, particularly of the brachiocephalic breeds. An increased sensitivity to estrogen is assumed, and there is excessive edema of the submucosal tissues of the vagina. The vaginal mucosa swells and interferes with coitus. Dramatic swelling can, if severe, result in vaginal tissue protruding from the vulva that becomes excoriated and ulcerated. Spontaneous regression during diestrus is the norm.
Vaginal polyps are relatively common in older bitches (see Fig. 18-50). They are often solitary, up to several centimeters in diameter, and have a thin stalk of attachment to the vaginal wall. Most are located on the ventral floor of the vagina. They are indistinguishable from leiomyomas macroscopically. Excision is usually curative.
Canine transmissible venereal tumor (CTVT) of dogs is transmitted at coitus by the transfer of intact neoplastic cells. CTVT cells have 59 chromosomes compared with the normal canine number of 78. Immunohistochemical evaluation suggests that these tumors have a histiocytic origin. Both sexes are affected. The neoplasm begins as a nodule beneath the vaginal or vestibular mucosa and when enlarged, breaks through the overlying mucosa. The lesion often begins in the dorsal wall of the vagina at the junction with the vestibule. It bulges into the lumen of the vagina and can protrude through the vulva as an ulcerated, friable mass (Fig. 18-52, A). Microscopically the neoplastic cells are large, round or oval, and uniform in size (Fig. 18-52, B) but with occasional large, bizarre nuclei. The cytoplasm is pale staining and may have peripheral vacuoles. This neoplasm can develop multifocal necrosis and spontaneously regress. Lymphocyte-mediated cytotoxicity occurs in some and results in regression of tumors. This neoplasm is particularly sensitive to vincristine. In countries with stray dogs and dogs in poor health, metastases to other sites, especially the skin, are relatively common.
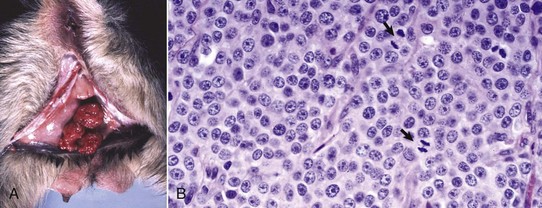
Fig. 18-52 Canine transmissible venereal tumor (CTVT), vulva and vagina, bitch.
A, The multinodular tumors markedly distend the lumens of the vagina and vestibule and are grossly characteristic of CTVT. B, Neoplastic cells are round and often divided into packets by a fine fibrous stroma. Mitoses are frequent (arrows). H&E stain. (A courtesy Dr. J. King, College of Veterinary Medicine, Cornell University. B courtesy Dr. M. J. Abdy, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Carcinoma of the vagina in bitches is a recognized entity. Some clearly arise from the urethra, especially when they extend from the urethral orifice. Others arise from vaginal epithelium, but little is known of their cause. They are phenotypically similar to transitional cell carcinomas. The long-term prognosis is poor, but the prevalence of metastatic disease varies and urethral obstruction often is the complication that is difficult to control.
Bitches develop single or multiple leiomyomas of the vagina. These usually only occur in entire bitches, so a hormonal link is proposed. Ovariohysterectomy may be curative, further supporting a hormonal dependence. The common differential diagnosis of leiomyoma is vaginal polyps (see previous discussion). These have a similar macroscopic appearance, and histologic examination is usually required to differentiate them because each is a well-circumscribed nodule up to several centimeters in diameter arising from the vaginal wall (see Fig. 18-50).
Mammary Glands
Mastitis and mammary neoplasia are the major diseases of the mammary glands. Mastitis is inflammation of mammary glands. Most cases of mastitis begin with galactophoritis, which is inflammation of the mammary ducts. Mastitis is the most economically important disease of the mammary glands, and although it occurs in all species, it is a particular problem in animals used for milk production.
Galactostasis is milk retention, and there is no systemic illness, even though the glands become engorged, hot, and painful. It occurs after weaning or in pseudopregnancy and is thought to result from inadequate oxytocin release because of fear, stress, or lack of mammary stimulation.
Agalactia is a failure of milk production and is rare. It is one of the manifestations of caprine arthritis and encephalitis virus (CAEV) infection in goats and maedi-visna virus infection of ewes. The udders are hard, thus it is called hard udder and no milk is produced. There is usually microscopic interstitial inflammation present.
Galactorrhea (also called inappropriate lactation and precocious lactation) is also unusual. It is seen in male goats of high milk production lines. In bitches, it is part of pseudopregnancy and occurs at the termination of diestrus when there is a prolactin surge in response to a reduction in progesterone concentration. It also occasionally occurs after ovariohysterectomy during diestrus.
Disorders of Horses
Mammary disease is sporadic in mares, although the full gamut of changes of other species does occur. Mastitis is the more prevalent disease of those reported. It is assumed that the pathogenesis of mastitis in mares is similar to that of other species, especially as the agents are similar, at least in type. Streptococcus zooepidemicus is the most prevalent, with Gram-negative species second. Early weaning of foals and seasonal insect feeding is implicated in predisposing a mare to mastitis. Mastitis occurs at any stage of lactation, is usually unilateral, and may just affect one mammary gland (mares have two or three per mamma), and results in local pain, swelling, and pyrexia in about 50% of cases.
Mammary carcinoma is also sporadic and very rare. No cause is known. Case reports indicate they are often metastatic.
Disorders of Ruminants (Cattle, Sheep, and Goats)
Disorders of Cattle: Mastitis in dairy cattle is an extremely important disease. Most of the organisms responsible for mastitis are bacteria, and the number and range of lesions they cause is large. The majority of cases are caused by Staphylococcus aureus and are of subclinical or moderate clinical forms.
Mammary pathogens can be divided into groups based on the source of infection. In one group are those organisms, such as Streptococcus agalactiae, Staphylococcus aureus, and Mycoplasma sp., for which the mammary glands are the principal site of persistence or reservoir. Coliform organisms are in the group acquired from the external environment, such as fecal matter, soil, water, or bedding. An overlap group has members, such as Streptococcus uberis and Streptococcus dysgalactiae, capable of persisting in either location. Cow-to-cow transmission is important for the mammary reservoir group, whereas contamination of the teat end is important for disease caused by the environmental group. The rates of new mammary gland infections in dairy cows caused by environmental pathogens are greatest during the first and the last 2 weeks of a 60-day nonlactating period. Coliform and streptococcal infections that are established during the nonlactating period with organisms from the environment are present at the time of parturition and cause clinical mastitis soon afterward.
Another way to group the pathogenic organisms is by the disease they cause. The three main groups, based on their virulence and effects, including systemic disease, are the Gram-negative pathogens, Gram-positive pathogens that cause acute severe necrotizing disease, and Gram-positive pathogens that cause chronic suppurative mastitis. Gram-negative organisms, particularly Escherichia coli, can cause severe mastitis and systemic effects because of endotoxin release. Gram-positive pathogens cause disease that ranges from subclinical mastitis to gangrenous mastitis. Many induce a chronic suppurative disease.
Gram-negative bacteria gain access to the glands and release endotoxins, and cytokine release results in necrosis and severe vascular leakage (Fig. 18-53). A systemic acute-phase reaction induced by endotoxins and cytokines causes fever, anorexia, leukopenia, hyperfibrinogenemia, and hypocalcemia. This latter feature can be mistaken for primary milk fever. Edema of the mamma and surrounding areas is often prominent. Local changes include death and sequestration of glandular tissue, wherein regions of the gland become dry, friable, and surrounded by a red border of hyperemia and hemorrhage (Fig. 18-54). The massive outpouring of edema fluid causes the gland to be dramatically swollen and hard and the “milk” to be watery and/or contain fibrin. Fibrin can obstruct the ducts and sinuses. Not only is this type of mastitis potentially life threatening because of endotoxemia, but also the severe effects on the gland often lead to reduced defenses to other pathogens so that secondary, pus-forming pathogens also proliferate.
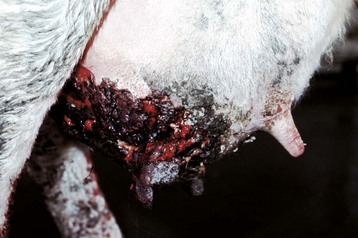
Fig. 18-53 Severe necrotic mastitis, coliform mastitis, mammary gland, cow.
Serum oozes through the dead skin of the affected right rear quarter. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
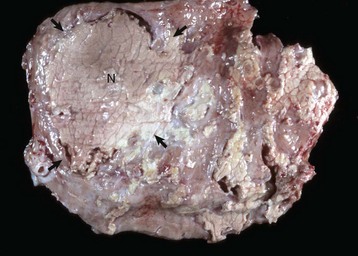
Fig. 18-54 Necrotizing mastitis, coliform mastitis, mammary gland, transverse section of quarter, cow.
Note the dry and pale gray-white area, typical of coagulative necrosis, to the left of center (delineated by arrows). The necrotic tissue (N) is partially surrounded by a zone of edema and by a thin light-gray band of fibrous tissue. Encapsulated necrotic tissue is designated a “sequestrum.” (Courtesy College of Veterinary Medicine, University of Illinois.)
Severe acute mastitis is caused when necrotizing Gram-positive bacteria, including virulent Staphylococcus aureus and streptococci, gain entry to the glands. With severe staphylococcal mastitis, neutrophils enter the tissue within minutes to hours, and the products of neutrophils contribute to the death of the glandular tissue. Cell surface–associated (adhesions, protein A, and capsular polysaccharides) and extracellular secretory products (leukotoxins, extracellular enzymes, and coagulase) of these organisms contribute to the damaging effect of these bacteria. The combined result is hemorrhage and death of the gland, with the result that the gland or part of the gland becomes gangrenous, hard, dry, and red-black. When severe, systemic effects of the acute-phase response caused by systemic cytokines induce fever, anorexia, weight loss, leukopenia, and hyperfibrinogenemia.
Chronic suppurative mastitis occurs when pus-forming bacteria do not induce the degree of necrosis, vascular effects, or systemic effects of the first two groups. These bacteria invoke a neutrophilic response that dominates the lesion and results in a buildup of necrotic leukocytic debris that typifies the suppurative response. The lesions they induce are centered on lactiferous ducts and sinus, and filling these with suppurative exudate (Fig. 18-55). Arcanobacterium pyogenes, Mycoplasma bovis, Streptococcus dysgalactiae, and various aerobes and anaerobes are prime candidates. Nocardial mastitis can be included in this group as well. Infection with these organisms, especially Arcanobacterium pyogenes, can occur with long-acting intramammary preparations used in the nonlactating or dry cow period. The occurrence in dry cows, initially recognized in the summer months, led to the name “summer mastitis.” Careful culture reveals up to five or six different species of the Arcanobacterium, Streptococcus, Bacteroides, Peptostreptococcus, and Fusobacterium genera. Because these are found in other pyogenic infections, it is assumed that they are environmental contaminates. Dry cows are not usually closely monitored, so the mastitis is typically chronic, with thick intramammary exudates and fibrosis. Arcanobacterium pyogenes causes mastitis in lactating, nonlactating, and even immature bovine mammary glands with abscesses in the small and large lactiferous ducts. Abscesses range from those microscopic in size to those grossly visible. Fistulas from the abscesses can form at the base of the teat. Fibrosis of the abscess walls can result in loss of small unaffected ducts and involution and fibrosis of the parenchyma they drain.
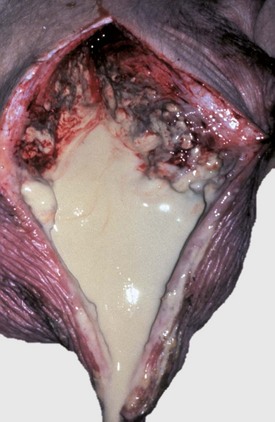
Fig. 18-55 Suppurative mastitis, mammary quarter, cow.
The lactiferous sinus and ducts are filled with viscous yellow pus. (Courtesy College of Veterinary Medicine, University of Illinois.)
Streptococcal Mastitis: Streptococcus agalactiae was the most important pathogen of the bovine mammae in the era before adequate mamma hygiene and efficient antibacterial drugs. Resistance of cows to mastitis caused by this organism is subject to great individual variation; in general, resistance decreases with age. The mamma is the only organ affected by this organism. Streptococcus agalactiae does not persist long in the environment. Once a cow is infected, however, the organism persists in the lactiferous sinus, with periodic waves of multiplication, increase in virulence, and tissue invasion. The initial response to invasion of Streptococcus agalactiae is interstitial edema and an influx of neutrophils into the interstitium and alveoli. The alveolar epithelium undergoes either brief hyperplasia or vacuolation and then desquamates. Macrophages quickly become a component of the cell population of infected alveoli, and fibrosis rapidly obliterates the lumen of these alveoli. Edema, cellular infiltration, and fibrosis are lesions found in infected and adjacent alveoli, so that pressure is increased within the lobule and within adjacent lobules. The increased pressure causes stagnation of milk flow, thereby initiating premature involution of a portion of the gland. After the acute phase, periductal fibrosis occurs and granulation tissue replaces part of the normal cuboidal to columnar epithelium of smaller ducts. Fibrous polyps can completely obstruct milk flow. Regeneration of ductal epithelium can occur after the granulation tissue has matured and contracted. The lactiferous ducts and sinuses, with their normally two-layered columnar epithelium, are similarly but less severely affected, often going through a phase of squamous metaplasia of the epithelium.
The macroscopic appearance depends on the stage of the disease; different stages occur in different areas. Usually more than one mamma is involved. In the acute stage, there is hyperemia of the lactiferous sinus. Milk quality is altered, and strands or clumps of debris are present in the milk, or the milk is transformed into pus. The areas of parenchymal edema and cellular infiltration are gray and turgid. Groups of alveoli, in which the secretion is retained because of obstruction of the duct by granulation tissue, resemble small abscesses. Involuting parenchyma and fibrotic parenchyma appear similar to one another and can be difficult to differentiate grossly. The lactiferous sinus becomes granular and thickened because of underlying projecting areas of granulation tissue and surrounding fibrosis.
Staphylococcal Mastitis: Staphylococcus aureus has a greater propensity than Streptococcus agalactiae to invade the interstitial tissue between alveoli, and thus can induce a more severe disease. Isolates of Staphylococcus aureus obtained from bovine mammary glands range from nonpathogenic to highly pathogenic. The severest form of staphylococcal mastitis is the gangrenous form (Fig. 18-56), usually seen shortly after parturition and involving a variable proportion of the udder. Severe acute inflammation, with classic heat, redness, swelling, and pain, progresses to coldness of the affected area, a blue-black color, and fluid exudation, indicating tissue death. Microscopically, during the first 48 hours after infection with toxigenic Staphylococcus aureus, the tissue has severe interstitial edema that increases the interalveolar stromal area. Progressive swelling, vacuolation, and focal erosion of epithelial cells occur throughout the ducts and are prominent near the junction of stratified squamous epithelium and columnar epithelium of the papillary duct. The bacteria attach to epithelial cells, cause focal damage, and later can be seen on, within, and below ductal and alveolar epithelia The cellular phase of the inflammatory response is rapid, with neutrophils initially in the subepithelial tissues of the duct system, then within the epithelium, and later in the lumen of alveoli.
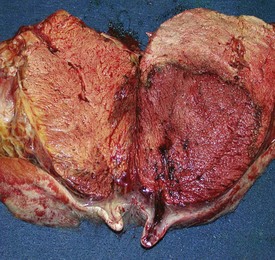
Fig. 18-56 Gangrenous mastitis (Clostridium sp.), mammary gland, transverse section of udder, cow.
Most of the right quarter and some of the adjacent left quarter are dark red with hemorrhage. A well-demarcated hyperemic (darker red) border has formed at its junction with the adjacent normal gland (right udder). There is also marked subcutaneous edema between the gland and the skin. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
The less severe form follows a course similar to that of streptococcal mastitis. Initially, damage occurs to the epithelium of the lactiferous sinus and larger lactiferous ducts. Bacteria expand rapidly along the ducts and produce acute inflammation in groups of adjacent terminal alveoli. In chronically infected quarters, macrophages are the principal cell type in the epithelial lining, in lumens, and especially in the glandular interstitium. Lymphocytes increase in number, but some investigators have reported a lack of increase in plasma cells. These observations suggest that mammary lymphocytes could become hyporesponsive to antigenic stimulation in chronically infected mammary glands. Some data indicate that mammary lymphocyte function is compromised; blastogenesis is depressed in lymphocytes recovered from infected mammary glands. Studies have documented that plasma cells are prevalent in the stroma of mammary glands with chronic Staphylococcus aureus mastitis; more cells produce IgA than IgG antibody, and IgA-containing cells increase in number as the disease progresses. The extent of regeneration is uncertain; it is unclear whether damaged alveoli redevelop secretory tissue or remaining healthy tissue undergoes compensatory hypertrophy, or whether both processes occur.
In chronic suppurative staphylococcal mastitis, abscess formation follows acute disease. Abscesses, scattered and often coalescing, vary in size from those that are microscopic to those grossly visible. Sometimes staphylococcal bacteria are surrounded by rosettes of club-shaped material (the term botryomycosis was applied to such lesions). An equally important and parallel set of events occurs in the lobules not invaded by bacteria as the obstruction of milk flow by granulation tissue and pressure from surrounding fibrosis result in involution. Multiple, small, soft cream to pink nodules, some containing pus, are separated by bands of fibrous tissue. Disease caused by less pathogenic strains of staphylococci, such as nonhemolytic coagulase-negative strains, progresses less dramatically and not necessarily with obvious abscess formation. However, the same components of granulation tissue and fibrosis are present, causing obstruction and pressure, which in turn cause atrophy of adjacent lobules.
Coliform Mastitis: Coliform mastitis occurs when bacteria from the environment contaminate the opening of the papillary duct and ascend. The most common bacteria are Escherichia coli, Enterobacter aerogenes, and Klebsiella pneumoniae. Coliform bacteria probably exert their damaging effect via endotoxin acting on the vasculature. In the acute form of the disease, the lesions are hyperemia, hemorrhage, and edema of the affected areas centered on the lactiferous ducts. The fluid in the lactiferous sinus is cloudy and blood stained and has clumps of fibrin (Fig. 18-57). Microscopically, interlobular septa are edematous and fibrin thrombi are in lymph vessels. Epithelium of ducts and alveoli is necrotic, and only low numbers of inflammatory cells are within them. Coliform bacteria are numerous within both the alveoli and the epithelium. The severity of the disease in postparturient cows is attributed to a delay in the influx of neutrophils. The cow’s response to endotoxin is influenced by the stage of the reproductive cycle. The nonlactating mammary glands are much less sensitive to the effects of endotoxin than are the lactating ones.
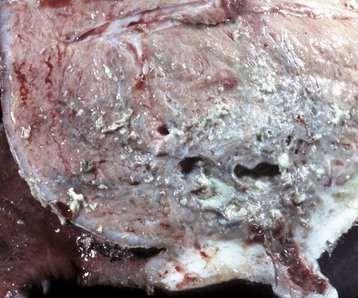
Fig. 18-57 Coliform mastitis, mammary glands, cow.
There is marked thickening of the walls of the lactiferous ducts. White to yellow fibrin and pus have collected in the lactiferous ducts and the upper portion of the lactiferous sinus. (Courtesy College of Veterinary Medicine, University of Illinois.)
If the cow survives endotoxemia, the necrotic mammary tissue, which can be a large portion of a mamma, separates from the viable tissue and is sequestered or eventually sloughs (Fig. 18-58). Cows in early lactation with less severe coliform mastitis often develop chronic mastitis that has alterations of hyperplasia, disorganization, and filiform processes of the epithelial lining of the papillary duct and lactiferous sinus.
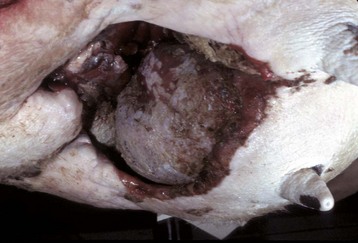
Fig. 18-58 Sloughed quarter, mammary gland, necrotizing mastitis, cow.
The necrotic right rear quarter has recently sloughed, leaving a large ulcerated area covered by a thin gray layer of exudate. The surface of the ulcer is finely granular, indicating the formation of granulation tissue. (Courtesy College of Veterinary Medicine, University of Illinois.)
Mycoplasmal Mastitis: Mycoplasma mastitis in cows occurs as individual sporadic cases or in outbreaks. Several mycoplasmas are capable of causing bovine mastitis, but Mycoplasma bovis is the most prevalent. The disease caused by Mycoplasma bovis can affect one or all mammae; mycoplasmas inoculated into one mamma often spread to all. Hematogenous spread and contamination of the teat are therefore routes by which the gland becomes infected. Affected quarters initially are enlarged, firm, and light brown and have a nodular parenchyma. The nodules are abscesses that can be up to 10 cm in diameter. Large numbers of neutrophils are found in the lobular interstitium and alveoli in the early stages. This changes with chronicity to include lymphocytes and macrophages. Early vacuolation and degeneration of alveolar epithelium is followed by hyperplasia and then by metaplasia to a relatively undifferentiated multilayered epithelial lining. Focal erosions of ductal epithelium are replaced by granulation tissue. Aggregates of lymphocytes occur in the lobular interstitium and around ducts. Interstitial fibrosis and lobular atrophy occur in the late stages. Spread of the organism to calves and resultant otitis, arthritis, and pneumonia can occur.
Granulomatous Mastitis: Granulomatous mastitis in the cow occurs when drugs for the treatment or prevention of mastitis are introduced through the teat and are contaminated with Nocardia asteroides, Cryptococcus neoformans, atypical Mycobacterium sp. (other than Mycobacterium bovis), or Candida spp. These infectious agents can also cause spontaneous mammary disease. Nocardial mastitis is the best known of these diseases because it occurs in outbreaks. Severely affected cows develop pyrexia that may last for several weeks. Cows become lethargic and lose weight, as would be expected with systemic cytokine release. The glands are hot and swollen and may have multiple abscesses or granulomas. Small white particles may be found in the exudate. Lesions in the mammary glands are centered on the lactiferous ducts and sinuses because galactophoritis is the prominent finding. Because the infection is chronic and ascending, lobules are affected to a varying degree. Granulomas and pyogranulomas predominate microscopically. These are usually surrounded by fibrous tissue, and extensive involvement results in the gland being replaced by a framework of fibrous tissue surrounding pockets of inflammatory cells and central necrotic debris (Fig. 18-59). An udder affected with cryptococcal mastitis has the same yellow gelatinous material that is typical of cryptococcal lesions elsewhere.
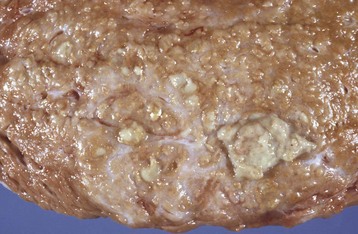
Fig. 18-59 Chronic mastitis and galactophoritis (Nocardia spp.), mammary glands, transverse section, cow.
Chronic inflammation of the lactiferous ducts and adjacent mammary glands has resulted in the replacement of most of this gland by pyogranulomas and abscesses containing yellow pus. The adjacent normal glandular tissue has involuted. This cow was infected when the dry cow medication was contaminated by Nocardia spp. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Disorders of Sheep and Goats: There are two main agents recovered from the mammary glands of sheep: Mannheimia haemolytica and Staphylococcus aureus. In many sheep flocks, the main manifestation of infection with these agents is unexpected death because these bacteria are responsible for an acute necrotizing or gangrenous mastitis. It is difficult to be certain, but the morbidity can be about 5% and mortality 20%. Mastitis in goats is similar, and as with the disease in sheep, is assumed to have a similar pathogenesis to the disease in cattle. For mycoplasmal mastitis, Mycoplasma agalactiae or Mycoplasma mycoides ssp. mycoides are usually the causative agents.
Goats infected with CAEV can develop a “hard udder.” The udder is hard, and little milk can be expressed from the teat. Recovery occurs, but milk production is reduced. Histologically, there are large numbers of lymphocytes and lymphoid follicles in the interstitium between the glands. The virus grows in mammary epithelium and is present in milk. The newborn is infected through infected milk. A similar disease in sheep is seen with infection with maedi-visna virus. The pathogenesis is provided in more detail in the chapter of respiratory disease; briefly the virus is spread through respiratory secretions rather than milk, the virus replicates in macrophages and results in slowly progressive lesions with large numbers of lymphocytes and plasma cells in many organs, the mammary glands being one.
Disorders of Dogs
Neoplasia of the mammary glands is common in the dog, and it has the highest incidence of all domesticated species (Fig. 18-60). Most canine mammary tumors are clinically benign. There has been much interest in canine mammary neoplasia both from the view-point of prognosis and treatment, from a logical pathogenetic viewpoint, and as a tool in comparative oncogenesis. Although the phenomenon of mammary neoplasia is well recognized, the cause is not. Ovariohysterectomy after the second estrus dramatically increases the prevalence of this disease. The “window of susceptibility” is up to 2 years of age. A high-protein diet decreases susceptibility, whereas treatment with medroxyprogesterone acetate and being a purebred increases it.
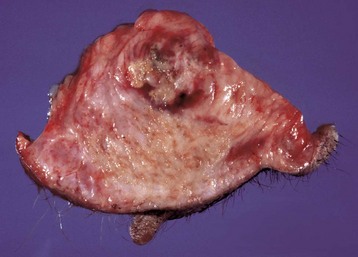
Fig. 18-60 Mammary carcinoma, mammary gland, bitch.
This mammary carcinoma has infiltrated and replaced normal mammary glands and contiguous soft tissue. The upper nodule is the neoplasm, and the lower and surrounding white tissues are composed of infiltrating neoplastic cells and fibrous tissue, the result of a desmoplastic response. (Courtesy Dr. R.A. Foster, Ontario Veterinary College, University of Guelph.)
Mammary neoplasms are a diverse group that are dominated by epithelial and combined epithelial and myoepithelial tumors. Sarcomas, such as fibrosarcoma and osteosarcoma, are much less common but are particularly aggressive and metastatic. The embryology of the mammary glands involves a close association between the epithelium and mesenchyme, so it is not surprising that tumors are often combinations of stroma and epithelium with the so-called complex adenomas and carcinomas, with a mixture of epithelial and myoepithelial components, and the mixed mammary tumor with those elements plus cartilage and bone.
The development of mammary epithelial neoplasia can proceed from ductal or lobular hyperplasia to dysplasia and on to neoplasia and subsequent progression from benign adenoma to noninvasive carcinoma and to invasive forms (Fig. 18-61). Once progression begins, an individual will often continue to develop neoplasia, and the development of multiple mammary masses is likely. The prognosis for each subsequent mass is not dependent on the previous one, so that many different types of neoplasia can be found in one animal.
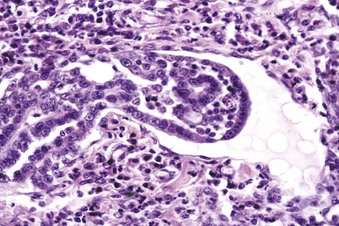
Fig. 18-61 Adenocarcinoma, invasion into lymphatic vessels, bitch.
Note the neoplastic cells infiltrating through the wall of the lymphatic (above left). Microscopic invasion by mammary carcinoma into a lymphatic, as depicted here, is an indicator of a poor prognosis. In this case, there are lymphocytes and plasma cells in the perilymphatic tissues. H&E stain. (Courtesy Dr. M. Domingo, Autonomous University of Barcelona; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Predicting behavior of mammary neoplasms is an inexact science. From a pragmatic and simplistic view, invasion is an indicator of a poor prognosis. It is the reason that excisional biopsy is the best procedure for diagnosis in dogs; cytology can be useful in identifying inflammation and sarcomas but can give a false impression of malignancy in epithelial tumors. The neoplasms that arise are variable in their phenotypes and as such, there are many different classification schemes.
General principles of neoplasia apply, and in dogs, those that are locally invasive are more likely to be life threatening. The threat increases with the lack of differentiation, and there is an increasing risk of metastasis with tubulopapillary, solid, and anaplastic carcinomas, respectively. Those with metastasis to the local lymph node have an especially poor prognosis. Because there is a potential for progression from benign to malignant, neoplasms (either because of rapid growth or having additional time to develop) larger than 5 cm in diameter have a poorer prognosis.
In dogs, mastitis caused by bacteria or Mycoplasma sp. occurs early in lactation or pseudopregnancy. Staphylococci, streptococci, and Escherichia coli are major isolates. It is assumed that the pathogenetic pathways of mastitis in dogs are the same as for dairy cows. Infection of fissures in nipples and adjacent skin spreads via the lactiferous ducts into the gland and results in suppurative inflammation and/or abscesses. The glands become swollen, large, firm, and edematous because the toxins and tissue destruction by the agents or the cytokine and contents of inflammatory cell granules cause acute inflammation. It may be superimposed on mammary hyperplasia or mammary neoplasia, especially on tumors of ducts. Systemic illness is often seen.
Disorders of Cats
Fibroadenomatous hyperplasia (mammary hypertrophy) is highly prevalent and is the most common disease; it occurs in young intact female cats. Those less than 2 years old are most likely to develop mammary enlargement, which usually occurs in the spring and with the first several estrous cycles. Much of what is known about this disease is based on its coinciding with the luteal phase of estrus, early in pregnancy, or after progestin therapy. High concentrations of progesterone or progesterone-like substances are the common link. As expected, megestrol acetate treatment of an old neutered male or female can induce this also. What is difficult to explain is the distribution of the lesion, as all mammary glands, only one mamma, or parts of one mammary gland may be as well. The lesion is proliferation of mammary ducts and adjacent stroma (Fig. 18-62). This overstimulation of an otherwise normal process is likely to be the result of a local dysregulation of tissue growth with the stimulation of receptors by progesterone. Some have theorized an exaggerated response to prolactin as well. Hemorrhages, coagulative necrosis, and/or ulceration of affected areas can occur. In young females, resolution is spontaneous or ovariohysterectomy is effective. Older neutered animals on progestin require drug withdrawal and sometimes mastectomy.
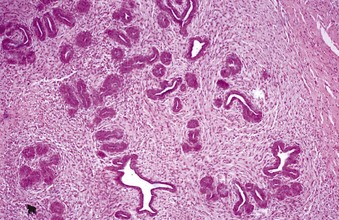
Fig. 18-62 Mammary hypertrophy (fibroadenomatous hyperplasia), mammary gland, cat.
Mammary ducts have proliferated and are surrounded by abundant loosely arranged stromal tissues. Typically, cats with mammary hypertrophy are young and develop enlargement of one or several mammary glands in the spring. H&E stain. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Mammary neoplasia in cats is relatively uncommon, so studies of causal factors are limited. There is not the same relationship with early desexing as there is with dogs. Progression from focal hyperplasia to adenoma to carcinoma is recognized, but this progression is presumably rapid, as many of the carcinomas are small.
The majority of neoplasms in cats are carcinomas and they metastasize. They are usually single and occur in the tissue adjacent to the nipple; 75% to 96% are adenocarcinomas and show rapid growth (Fig. 18-63). They metastasize to regional lymph nodes (axillary or the superficial inguinal nodes), lungs, or other mammary glands. The average age of affected animals is 11 years, and there is a 7- to 9-year-old risk plateau. Intact animals are at a slightly greater risk, but there is no beneficial effect of early spaying. The poorest prognosis is in older cats with neoplasms that are greater than 3 cm in diameter, with the presence of regional lymph node involvement, with increased number of mitoses, and with the presence of necrosis. Well-differentiated tumors have a better prognosis. The interval between diagnosis and death is often short, less than 1 year, but the range is wide. There is no or only a weak correlation between histologic features, including proliferation indices and prognosis, except perhaps size.
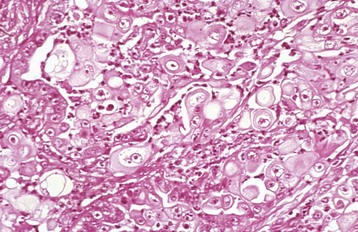
Fig. 18-63 Mammary carcinoma, mammary gland, cat.
The cells of this anaplastic neoplasm do not resemble those of normal epithelium but are large and round. They are arranged either in clusters or as individual cells within a fibrous stroma. Inflammatory cells, including neutrophils and lymphocytes, are also present. H&E stain. (Courtesy College of Veterinary Medicine, University of Illinois.)
Alves, D, McEwen, B, Hazlett, M, et al. Trends in bovine abortions submitted to the Ontario Ministry of Agriculture, Food and Rural Affairs, 1993-1995. Can Vet J. 1996;37:287–288.
Beagley, JC, Whitman, KJ, Baptiste, KE, et al. Physiology and treatment of retained fetal membranes in cattle. J Vet Intern Med. 2010;24:261–268.
Boos, A, Janssen, V, Mulling, C. Proliferation and apoptosis in bovine placentomes during pregnancy and around induced and spontaneous parturition as well as in cows retaining the fetal membranes. Reproduction. 2003;126:469–480.
Carvalho Neta, AV, Mol, JPS, Xavier, MX, et al. Pathogenesis of bovine brucellosis. Vet J. 2010;184:146–155.
Corbeil, LB, BonDurant, RH. Immunity to bovine reproductive infections. Vet Clin North Am Food Anim Pract. 2001;17:567–583.
Dillman, RC, Dennis, SM. Sequential sterile autolysis in the ovine fetus: macroscopic changes. Am J Vet Res. 1976;37:403–407.
Dunne, HW. Abortion, stillbirth, fetal death and infectious infertility. In Leman AD, ed.: Diseases of swine, ed 4, Ames: Iowa State University Press, 1975.
Feldman, EC, Nelson, RW. Canine female reproduction in canine and feline endocrinology and reproduction, ed 3. St Louis: Saunders; 2004.
Giles, RC, Donahue, JM, Hong, CB, et al. Causes of abortion, stillbirth and perinatal death in horses: 3527 cases (1986-1991). J Am Vet Med Assoc. 1993;203(8):1170–1175.
Gobello, C, Concannon, PQ, Verstegen, J, Canine pseudopregnancy: a review. Concannon PW, England G, Verstegen J, eds. Recent advances in small animal reproduction. International Veterinary Information Service: Ithaca, NY, 2001 www.ivis.org
Grooms, DL. Reproductive losses caused by bovine viral diarrhea virus and leptospirosis. Theriogenology. 2006;66:624–628.
Hughes, IA. Disorders of sex development: a new definition and classification. Best Pract Res Clin Endocrinol Metabol. 2008;22:119–134.
Jamakluddin, AA, Case, JT, Hird, DW, et al. Dairy cattle abortion in California: evaluation of diagnostic laboratory data. J Vet Diagn Invest. 1996;8:210–218.
Kawate, N. Studies on the regulation of expression of luteinizing hormone receptor in the ovary and the mechanism of follicular cyst formation in ruminants. J Reprod Develop. 2004;50:1–8.
Kerr, K, Entrican, G, McKeever, D, et al. Immunopathology of Chlamydophila abortus infection in sheep and mice. Res Vet Sci. 2005;78:1–7.
Kirkbride, CA. Etiologic agents detected in a 10 year study of bovine abortions and stillbirths. J Vet Diagn Invest. 1992;4:175–180.
Kirkbride, CA. Diagnoses in 1,784 ovine abortions and stillbirths. J Vet Diagn Invest. 1993;5:398–402.
Leblanc, MM, Persistent mating induced endometritis in the mare: pathogenesis, diagnosis, and treatment. Ball BA, ed. Recent advances in equine reproduction. International Veterinary Information Service: Ithaca, NY, 2003 www.ivis.org
Lunn, DP, Davis-Poynter, N, Flaminio, MJBF, et al. Equine Herpesvirus-1 Consensus Statement. J Vet Intern Med. 2009;23:450–461.
Meyers-Wallen, VN. Review and update: genomic and molecular advances in sex determination and differentiation in small animals. Reprod Dom Anim. 2009;44:40–46.
McEntee, K. Reproductive pathology of domestic mammals. New York: Academic Press; 1990.
Noakes, DE, Dhaliwal, GK, England, GCW. Cystic endometrial hyperplasia/pyometra in dogs: a review of the causes and pathogenesis. J Reprod Fertil Suppl. 2001;57:395–406.
Peter, AT. An update on cystic ovarian degeneration in cattle. Reprod Dom Anim. 2004;39:1–7.
Quayle, AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57:61–79.
Ramadan, AA, Johnson, GL, III., Lewis, GS. Regulation of uterine immune function during the estrus cycle and in response to infectious bacteria in sheep. J Anim Sci. 1997;75:1621–1632.
Robertson, SA. Control of the immunological environment of the uterus. Rev Reprod. 2000;54:164–174.
Sordillo, LM, Nickerson, SC. Morphologic changes in the bovine mammae during involution and lactogenesis. Am J Vet Res. 1988;49:1112–1120.
Sordillo, LM, Streicher, KL. Mammae immunity and mastitis susceptibility. J Mammae Biol Neoplasia. 2002;7:135–146.
Tengelsen, LA, Yamini, B, Mullany, TP, et al. A 12-year retrospective study of equine abortion in Michigan. J Vet Diagn Invest. 1997;9:303–306.
Thatcher, WW, Guiszeloglu, A, Mattos, R, et al. Uterine-conceptus interactions and reproductive the failure in cattle. Theriogenology. 2001;56(9):1435–1450.
Whitwell, KE. Morphology and pathology of the equine umbilical cord. J Reprod Fertil Suppl. 1975;23:599–603.
Whitwell, KE, Jeffcott, LB. Morphological studies on the fetal membranes of the normal singleton foal at term. Res Vet Sci. 1975;19:44–55.
*Dr. Helen M. Acland made contributions to this chapter in the third edition.